Biomaterials Translational ›› 2021, Vol. 2 ›› Issue (4): 323-342.doi: 10.12336/biomatertransl.2021.04.001
• REVIEW • Previous Articles Next Articles
Qiang Wei1, Shenghao Wang1, Feng Han1, Huan Wang1, Weidong Zhang1, Qifan Yu1, Changjiang Liu2, Luguang Ding2, Jiayuan Wang2, Lili Yu2, Caihong Zhu2,*( ), Bin Li1,2,3,*(
), Bin Li1,2,3,*( )
)
Received:2021-04-05
Revised:2021-06-13
Accepted:2021-07-10
Online:2021-12-28
Published:2021-12-28
Contact:
Caihong Zhu,Bin Li
E-mail:zhucaihong@suda.edu.cn;binli@suda.edu.cn
About author:Caihong Zhu, zhucaihong@suda.edu.cn; Bin Li, binli@suda.edu.cn.Wei, Q.; Wang, S.; Han, F.; Wang, H.; Zhang, W.; Yu, Q.; Liu, C.; Ding, L.; Wang, J.; Yu, L.; Zhu, C.; Li, B. Cellular modulation by the mechanical cues from biomaterials for tissue engineering. Biomater Transl. 2021, 2(4), 323-342.
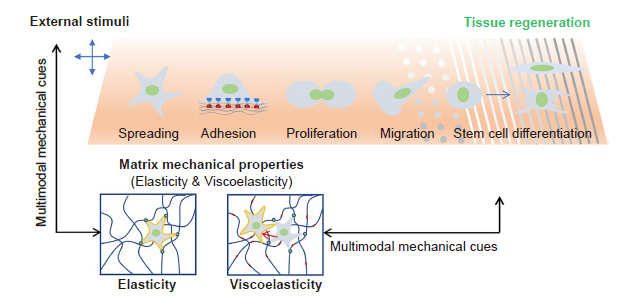
Figure 1. Schematic illustration of matrix mechanical cues that regulate cell behaviours. In general, the matrix mechanical cues include elasticity, viscoelasticity, topography, fibre stiffness, and external stimuli, all of which can regulate many cellular behaviours, including cell adhesion, spreading, proliferation, migration and differentiation. However, a single mechanical stimulus is generally insufficient to induce stem cell differentiation and achieve tissue regeneration; instead, multimodal mechanical factors including elasticity, viscoelasticity, topography and external mechanical stimuli would have a synergistic effect in guiding cell behaviours to promote tissue regeneration.
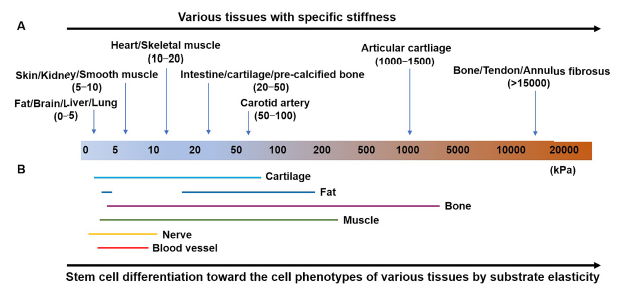
Figure 2. (A, B) Distinct moduli of human tissues suggesting tissue-specific stiffness (A) and substrate elasticity (B) used to direct stem cell differentiation toward the cell phenotypes of various tissues. Figure 2A was adapted from Handorf et al.8 by using some of its data in combination with other data source13-15 and Figure 2B was reprinted from Han et al.18 Copyright © Royal Society of Chemistry.
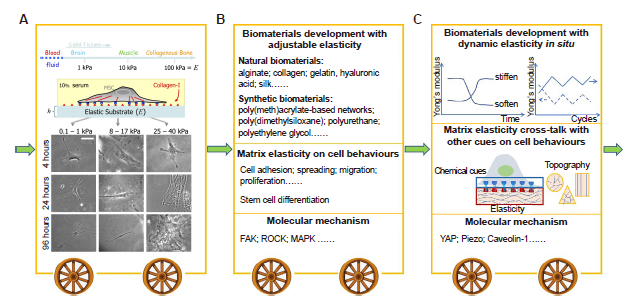
Figure 3. Stages in the development of research showing how matrix elasticity regulates cell behaviours. (A) The pioneering study demonstrates that human BMSCs were effectively induced to differentiate into neuronal, muscle or bone lineages when they were cultured on soft, medium, or stiff substrates. Stiffness was then considered as one of the most important mechanical cues in tissue engineering. Reprint from Engler et al.20 Copyright 2006, with permission from Elsevier. (B) In the following years, numerous biomaterials have been developed to explore cell behaviours including stem cell fate affected by substrate elasticity, and its underlying molecular mechanism. (C) In recent years, many studies began to focus on the interplay between substrate elasticity and other cues (such as topography, geometry, growth factors, etc.) and its effect on cell behaviours. In addition, to simulate dynamic changes in stiffness in vivo, biomaterials with dynamic elasticity in situ have been designed to explore mechanobiological pathways that may differ from those under static cell culture. Further, new mechanosensitive proteins have been found to be involved in the cellular responses toward matrix elasticity, including YAP, piezo, caveolin-1, etc. BMSCs: bone marrow mesenchymal stem cells; FAK: focal adhesion kinase; MAPK: mitogen-activated protein kinase; ROCK: RHO-related protein kinase 1; YAP: yes-associated protein.
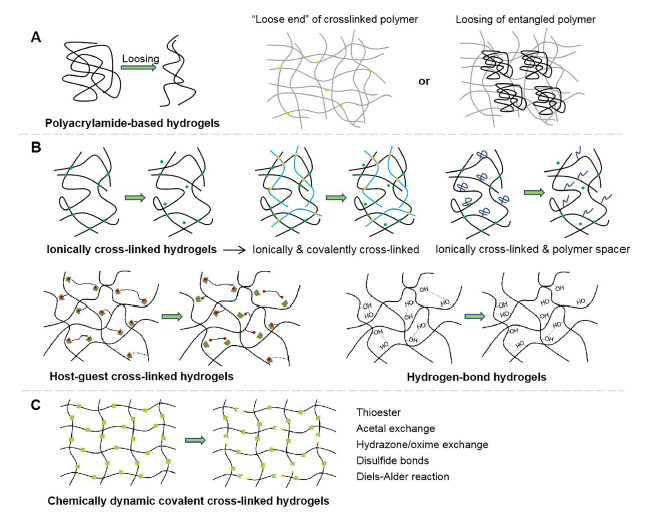
Figure 4. Schematic diagram showing the molecular mechanism of the changes undergone by viscoelastic hydrogels when subjected to an external force. (A) Polyacrylamide-based hydrogels with different loss moduli varied through the movement of loose ends of polymer chains, or the loosing of entangled linear polyacrylamide. (B) Physically cross-linked hydrogels with varying viscoelasticity through the breaking of ionic interactions, hydrogen bonding, guest-host interactions, etc. In particular, for ionically cross-linked hydrogels, the viscoelasticity can also be tuned by incorporating covalent cross-linkers and polymer spacers. (C) Chemically-dynamic cross-linked hydrogels which change through the dissociation of chemical covalent bonds.
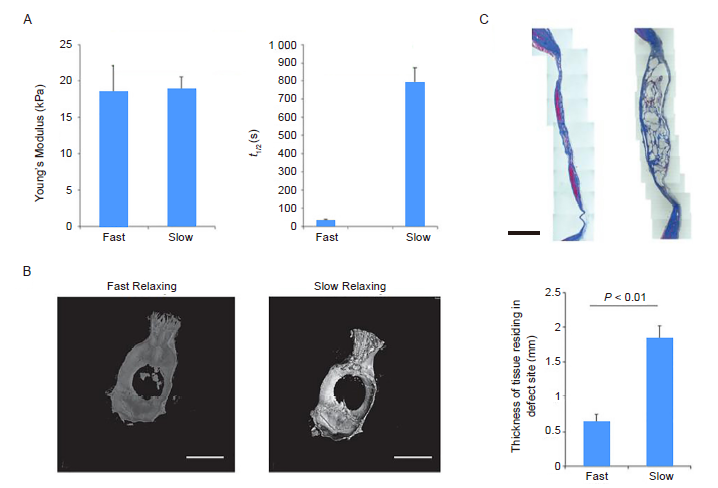
Figure 5. Substrate stress-relaxation regulates scaffold remodelling and bone formation in vivo. (A) Young’s modulus and stress relaxation of slow- and fast-relaxing alginate hydrogels. (B) Representative micro-computed tomography renderings of rat calvaria 3 months post-injury. (C) Masson’s trichrome staining of the defect site in fast-relaxing and slow-relaxing gel conditions. Scale bars: 1 cm in B and 2 mm in C. Data are expressed as mean ± SD (n = 8–10) and were analysed by Student’s t-test. τ1/2: stress relaxation rate. Reproduced with the permission of Darnell et al.94 Copyright Wiley-VCH Verlag GmbH & Co. KGaA.
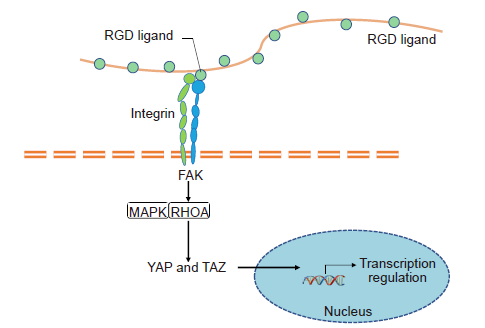
Figure 6. YAP/TAZ mediated pathway through mechano-transduction. A cell probes its ECM mechanical environment via membrane receptors (e.g. integrins) to transmit force which regulates the stability of focal adhesion complexes containing focal adhesion kinase (FAK). FAK phosphorylates and activates mechanoresponsive signalling elements, such as mitogen-activated protein kinase (MAPK) and transforming protein Ras homolog gene family member A (RHOA). Simultaneously, the intracellular force regulates the nuclear translocation of transcription regulator such as yes-associated protein (YAP)/transcriptional co-activator with PDZ-binding motif (TAZ).
| Material | Fabrication method | Elasticity | Effects on cell behaviours | Cell source | Reference | |||
|---|---|---|---|---|---|---|---|---|
| Alginate | Alginate microspheres prepared by microfluidic technology with different elasticities and microarchitectures,controlled by calcium ion concentrations. | 18, 32 kPa | Elasticity and porosity regulated the fate of encapsulated MSCs through modulation of the nuclear factor-κB pathway | MSCs | ||||
| Chitosan-hyaluronic acid | Porous chitosan-hyaluronic acid scaffolds of varied stiffness were fabricated using a phase separation method | 1.41–27.7 kPa | Increased matrix stiffness resulted in increased drug resistance of glioblastoma multiforme cells, and elevated expression of drug resistance-,hypoxia-, and invasion-related genes | Glioblastoma multiforme cells | ||||
| Dynamic protein hydrogels | A Ru2+-mediated photochemical strategy was used to crosslink an aqueous solution of FGR(G-MEP-R)2 into a chemically-crosslinked protein hydrogel | 6–20 kPa | Human lung fibroblasts dynamically responded to changes of hydrogel mechanics in a reversible fashion, regulated by redox state | Human lung fibroblasts | ||||
| Fibrin-alginate | Mechanical properties were tuneable via calcium chloride crosslinking | 0.6–3.8 kPa | Spreading of MSCs and endothelial cells was a function of alginate crosslinking density | MSCs, endothelial cells | ||||
| Hyaluronic acid | Methacrylated hyaluronic acid was synthesized to allow for crosslinking via Michael addition using the crosslinker dithiothreitol | 0.2–4.5 kPa | Human breast cancer cell (MDA-MB-231Br) adhesion, spreading, proliferation and migration were tightly regulated by the hydrogel stiffness | MDA-MB-231Br | ||||
| Polyacrylamide | Stiffness of polyacrylamide gels was adjusted using different monomer-to-crosslinker formulations | 2–32 kPa | Cytoskeleton assembly and cell morphology were efficiently regulated by substrate stiffness | HeLa cells | ||||
| Poly (dimethylsiloxane) | Poly(dimethylsiloxane) was used as the base material in which iron particles were embedded to create a magnetorheological elastomer, whose elasticity was controlled by the spacer distances between the magnet and the samples | 10–55 kPa | The softer substrates yielded more organised sarcomeres,and sarcomere formation was positively correlated with the degree of myocyte enrichment when using human-derived induced pluripotent stem cell cardiomyocytes | Human-derived induced pluripotent stem cell cardiomyocytes, cardiac fibroblasts | ||||
| Polyurethane | Controlling the crosslinking of tri-block copolymer and polycaprolactone triol yielded polyurethanes of varying elasticity | 45.0–244.8 kPa | Scaffolds with different stiffnesses stimulated the proliferation of different types of cells | 3T3 fibroblasts, MG63 cells | ||||
| Silk fibroin | Developed by introducing inert silk fibroin nanofibres within an enzyme crosslinked system of silk fibroin | 9–60 kPa | MSCs differentiated into endothelial, myoblast and osteoblast cells on the different elastic substrates | MSCs | ||||
| Silk fibroin-collagen | The concentrations of both proteins was changed gradually while maintaining the ratio at 1:7, which resulted in a gradual change in stiffness at a fixed composition | 0.1–20 kPa | High rigidity allowed human MSCs to preserve all-directional spreading with polygonal shape. Soft substrates might not maintain the polygonal shape | Human MSCs | ||||
| Poly(ether carbonate urethane)urea | Young’s modulus of scaffolds was tuned by adjusting the molecular weight of polydiol (soft segment) as well as the feed ratios of hard molecular segment to soft molecular segment | 2.5–13.4 MPa | Annulus fibrosus-derived stem cells showed strong tendencies to differentiate into various types of annulus fibrosus-like cells depending on the substrate elasticity | Annulus fibrosus-derived stem cells | ||||
| PEG | Stiffness was adjusted by adding various PEG monomers and the photoinitiator lithium phenyl-2,4,6-trimethylbenzoylphosphinate | 1.5–12.6 kPa | The functional and molecular outputs of adult mouse ventricular myocytes were dependent on the PEG hydrogel stiffness | Adult mouse ventricular myocytes | ||||
| Poly(L-lactide-co-caprolactone)/poly(L-lactic acid) | Fibre stiffness was controlled by altering the flow rates of the poly(L-lactic acid)-core and poly(L-lactide-co-caprolactone)-shell solutions. | 14.7–2141.7 MPa | Higher stiffness of the aligned fibrous substrates was found to significantly encourage the proliferation and migration of human umbilical artery smooth muscle cells | Human umbilical arterial smooth muscle cells | ||||
| GelMA hydrogels | Prepared by photocrosslinking methacrylate gelatine and adjusting the stiffness by varying the concentration | 3–180 kPa | PC12 cell viability, adhesion, spreading and average neurite length were influenced by stiffness | PC12 cells | ||||
| GelMA/PEGDA hydrogels | Prepared by photocrosslinking methacrylate gelatine and adjusting the stiffness with the crosslinker PEGDA | 4, 40 kPa | Increased matrix stiffness promoted osteogenic differentiation of MSCs | MSCs | ||||
| GelMA/Collagen hydrogels | Prepared by mixing collagen and GelMA to form an interpenetrating network | 2–12 kPa | With the increase of matrix stiffness, the invasion and sprouting of the two cells decreased regardless of fibre content | MDA-MB-231Br and endothelial cells | ||||
| Alginate/GelMA hydrogels | Prepared by mixing alginate and GelMA | 6–13 kPa | The expression level of MSC osteogenesis markers was enhanced with the increase in the matrix elastic modulus | MSCs | ||||
Additional Table 1. Commonly-used biomaterials with various elasticities and their effects on cells.
| Material | Fabrication method | Elasticity | Effects on cell behaviours | Cell source | Reference | |||
|---|---|---|---|---|---|---|---|---|
| Alginate | Alginate microspheres prepared by microfluidic technology with different elasticities and microarchitectures,controlled by calcium ion concentrations. | 18, 32 kPa | Elasticity and porosity regulated the fate of encapsulated MSCs through modulation of the nuclear factor-κB pathway | MSCs | ||||
| Chitosan-hyaluronic acid | Porous chitosan-hyaluronic acid scaffolds of varied stiffness were fabricated using a phase separation method | 1.41–27.7 kPa | Increased matrix stiffness resulted in increased drug resistance of glioblastoma multiforme cells, and elevated expression of drug resistance-,hypoxia-, and invasion-related genes | Glioblastoma multiforme cells | ||||
| Dynamic protein hydrogels | A Ru2+-mediated photochemical strategy was used to crosslink an aqueous solution of FGR(G-MEP-R)2 into a chemically-crosslinked protein hydrogel | 6–20 kPa | Human lung fibroblasts dynamically responded to changes of hydrogel mechanics in a reversible fashion, regulated by redox state | Human lung fibroblasts | ||||
| Fibrin-alginate | Mechanical properties were tuneable via calcium chloride crosslinking | 0.6–3.8 kPa | Spreading of MSCs and endothelial cells was a function of alginate crosslinking density | MSCs, endothelial cells | ||||
| Hyaluronic acid | Methacrylated hyaluronic acid was synthesized to allow for crosslinking via Michael addition using the crosslinker dithiothreitol | 0.2–4.5 kPa | Human breast cancer cell (MDA-MB-231Br) adhesion, spreading, proliferation and migration were tightly regulated by the hydrogel stiffness | MDA-MB-231Br | ||||
| Polyacrylamide | Stiffness of polyacrylamide gels was adjusted using different monomer-to-crosslinker formulations | 2–32 kPa | Cytoskeleton assembly and cell morphology were efficiently regulated by substrate stiffness | HeLa cells | ||||
| Poly (dimethylsiloxane) | Poly(dimethylsiloxane) was used as the base material in which iron particles were embedded to create a magnetorheological elastomer, whose elasticity was controlled by the spacer distances between the magnet and the samples | 10–55 kPa | The softer substrates yielded more organised sarcomeres,and sarcomere formation was positively correlated with the degree of myocyte enrichment when using human-derived induced pluripotent stem cell cardiomyocytes | Human-derived induced pluripotent stem cell cardiomyocytes, cardiac fibroblasts | ||||
| Polyurethane | Controlling the crosslinking of tri-block copolymer and polycaprolactone triol yielded polyurethanes of varying elasticity | 45.0–244.8 kPa | Scaffolds with different stiffnesses stimulated the proliferation of different types of cells | 3T3 fibroblasts, MG63 cells | ||||
| Silk fibroin | Developed by introducing inert silk fibroin nanofibres within an enzyme crosslinked system of silk fibroin | 9–60 kPa | MSCs differentiated into endothelial, myoblast and osteoblast cells on the different elastic substrates | MSCs | ||||
| Silk fibroin-collagen | The concentrations of both proteins was changed gradually while maintaining the ratio at 1:7, which resulted in a gradual change in stiffness at a fixed composition | 0.1–20 kPa | High rigidity allowed human MSCs to preserve all-directional spreading with polygonal shape. Soft substrates might not maintain the polygonal shape | Human MSCs | ||||
| Poly(ether carbonate urethane)urea | Young’s modulus of scaffolds was tuned by adjusting the molecular weight of polydiol (soft segment) as well as the feed ratios of hard molecular segment to soft molecular segment | 2.5–13.4 MPa | Annulus fibrosus-derived stem cells showed strong tendencies to differentiate into various types of annulus fibrosus-like cells depending on the substrate elasticity | Annulus fibrosus-derived stem cells | ||||
| PEG | Stiffness was adjusted by adding various PEG monomers and the photoinitiator lithium phenyl-2,4,6-trimethylbenzoylphosphinate | 1.5–12.6 kPa | The functional and molecular outputs of adult mouse ventricular myocytes were dependent on the PEG hydrogel stiffness | Adult mouse ventricular myocytes | ||||
| Poly(L-lactide-co-caprolactone)/poly(L-lactic acid) | Fibre stiffness was controlled by altering the flow rates of the poly(L-lactic acid)-core and poly(L-lactide-co-caprolactone)-shell solutions. | 14.7–2141.7 MPa | Higher stiffness of the aligned fibrous substrates was found to significantly encourage the proliferation and migration of human umbilical artery smooth muscle cells | Human umbilical arterial smooth muscle cells | ||||
| GelMA hydrogels | Prepared by photocrosslinking methacrylate gelatine and adjusting the stiffness by varying the concentration | 3–180 kPa | PC12 cell viability, adhesion, spreading and average neurite length were influenced by stiffness | PC12 cells | ||||
| GelMA/PEGDA hydrogels | Prepared by photocrosslinking methacrylate gelatine and adjusting the stiffness with the crosslinker PEGDA | 4, 40 kPa | Increased matrix stiffness promoted osteogenic differentiation of MSCs | MSCs | ||||
| GelMA/Collagen hydrogels | Prepared by mixing collagen and GelMA to form an interpenetrating network | 2–12 kPa | With the increase of matrix stiffness, the invasion and sprouting of the two cells decreased regardless of fibre content | MDA-MB-231Br and endothelial cells | ||||
| Alginate/GelMA hydrogels | Prepared by mixing alginate and GelMA | 6–13 kPa | The expression level of MSC osteogenesis markers was enhanced with the increase in the matrix elastic modulus | MSCs | ||||
| Biomaterials | Fabrication method | Viscoelasticity | Effects on cell behaviours | Cell source | Reference |
|---|---|---|---|---|---|
| Alginate hydrogels | Prepared by ionic crosslinking of alginate | Obtained by covalently or ionically crosslinking alginate gels with the same initial Young’s modulus by adjusting the concentration of crosslinker | Both computational modelling and experimental studies revealed that spreading of cells cultured on soft substrates that exhibit stress relaxation is greater than cell spreading on elastic substrates of the same modulus, but similar to that of cells spreading on stiffer elastic substrates | U2OS & 3T3 fibroblasts | |
| Prepared by ionic crosslinking of alginate | The time for the initial stress of the material to be relaxed to half its value during a stress relaxation test (τ1/2) was modulated from ~1 minute to ~1 hour by controlling the molecular weight of alginate | Cell spreading, proliferation, and osteogenic differentiation of MSCs were all enhanced in cells cultured in gels with faster relaxation | MSCs | ||
| Alginate-PEG hydrogels | Prepared by ionic crosslinking of PEG-functionalised alginate | PEG acts as a spacer to provide a steric spacing of crosslinking zones in alginate. Increased concentration and molecular weight of the PEG resulted in faster stress relaxation, a high loss modulus, and increased creep | The hydrogels can be used for 3D culture. Faster relaxation led to increased spreading and proliferation of fibroblasts, and enhanced osteogenic differentiation of MSCs | 3T3 fibroblasts; MSCs | |
| Alginate interpenetrating network as an artificial ECM | Prepared by a combination of ionic and covalent cross-linking of click-functionalised alginate, interpenetrating with fibrillar collagen type I | Varying the mode and magnitude of crosslinking enables tuneable stiffness and viscoelasticity | MSC expression of immunomodulatory markers was differentially impacted by the viscoelasticity and stiffness of the matrix | MSCs | |
| Boronate ester hydrogel | Prepared by reversible boronate esterification of boronic acid with vic-diols | Viscoelasticity increased as a function of the boronic acid and vicinal diol concentration, and also increased with decreasing cross-linker concentration, where the maximal loss tangent achieved was 0.55 at 0.1 rad/s | The cell area and nuclear area, focal adhesion tension, and subcellular location of YAP/TAZ were found to be lower for cells cultured on viscoelastic hydrogels compared to elastic hydrogels with a similar storage modulus | NIH-3T3 cells | |
| Boronate-based hydrogels | Based on reversible boronate bonds | Relaxation time constants on the order of seconds or less | Fast relaxation matrix mechanics are found to promote cell-matrix interactions, leading to spreading and an increase in nuclear volume, and induce YAP/TAZ binding domain nuclear localization at longer times | MSCs | |
| Collagen gels | Fabricated by adjusting pH | Strain-enhanced stress relaxation of collagen gels arises from force-dependent unbinding of weak bonds between collagen fibres | - | - | |
| Hyaluronic acid hydrogels | Crosslinked via photo-responsive guest-host pairing of azobenzene to β-cyclodextrin | Relaxation time from 6 seconds to minutes | The hydrogels maintained a high level of viability after 3 days of culture | NIH 3T3 cells | |
| Hyaluronic acid | Combined light-mediated covalent and supramolecular crosslinking was used to afford spatiotemporal control of the viscoelastic network | Significantly higher loss moduli compared to elastic group. Photopatterning enabled presentation of dynamic, heterogeneous viscoelastic properties | LX-2 cells respond to the viscoelastic hydrogel by displaying reductions in spread area, MRTF-A nuclear translation, and organisation of actin stress fibres | LX-2 stellate cells | |
| Hyaluronic acid-collagen hydrogels | Interpenetrating network based on HA crosslinked with dynamic hydrazone bonds with collagen type I | The time for the initial stress of the material to be relaxed to half its value during a stress relaxation test (τ1/2) was modulated from ~233 seconds to > 18000 seconds | Faster relaxation promotes cell spreading, fibre remodelling, and focal adhesion formation in 3D culture | Human MSCs | |
| Oxime cross-linked alginate hydrogels | Formed by mixing alkoxyamine-containing alginate with aldehyde-containing alginate | Stress-relaxation was tuneable by varying the composition or environmental factors | The gels showed very nice short-term cytocompatibility with the encapsulated cells. Growth and migration benefited from the stress relaxation capability | 2PK3 cells | |
| PEG hydrogels | Crosslinked by reversible hydrazone bonds | τ1/2 could be varied from 5–6000 seconds by changing the number of PEG or by changing the ratio of benzaldehyde to aliphatic aldehyde crosslinkers | Covalently-adaptable hydrogels allowed for the development of physiologically-relevant morphologies, whereas non-adaptable gels prevented cytoskeletal rearrangement and extension | C2C12 myoblasts | |
| Thioester hydrogel | Photopolymerisation between PEG-SH and thioester-containing divinyl crosslinker | Through control of pH, gel stoichiometry, and crosslinker structure, viscoelastic properties were modulated across several orders of magnitude | MSCs encapsulated in the thioester hydrogels were able to elongate in 3D and display increased proliferation relative to those in static hydrogels | MSCs | |
| Hyaluronic acid and PEG | A DN was formed based on the combination of supramolecular GH hyaluronic acid networks with covalent networks from the photocrosslinking of PEG-fibrinogen and PEG-diacrylate | Dependent on the polymer concentration the GH network | The increase of GH concentration led to the enhancement of the viscosity of the DN hydrogel and the enhancement of cell spreading and proliferation | MSCs |
Additional Table 2. Scaffolds with tuneable viscoelasticity through various crosslinkers and their effects on cells.
| Biomaterials | Fabrication method | Viscoelasticity | Effects on cell behaviours | Cell source | Reference |
|---|---|---|---|---|---|
| Alginate hydrogels | Prepared by ionic crosslinking of alginate | Obtained by covalently or ionically crosslinking alginate gels with the same initial Young’s modulus by adjusting the concentration of crosslinker | Both computational modelling and experimental studies revealed that spreading of cells cultured on soft substrates that exhibit stress relaxation is greater than cell spreading on elastic substrates of the same modulus, but similar to that of cells spreading on stiffer elastic substrates | U2OS & 3T3 fibroblasts | |
| Prepared by ionic crosslinking of alginate | The time for the initial stress of the material to be relaxed to half its value during a stress relaxation test (τ1/2) was modulated from ~1 minute to ~1 hour by controlling the molecular weight of alginate | Cell spreading, proliferation, and osteogenic differentiation of MSCs were all enhanced in cells cultured in gels with faster relaxation | MSCs | ||
| Alginate-PEG hydrogels | Prepared by ionic crosslinking of PEG-functionalised alginate | PEG acts as a spacer to provide a steric spacing of crosslinking zones in alginate. Increased concentration and molecular weight of the PEG resulted in faster stress relaxation, a high loss modulus, and increased creep | The hydrogels can be used for 3D culture. Faster relaxation led to increased spreading and proliferation of fibroblasts, and enhanced osteogenic differentiation of MSCs | 3T3 fibroblasts; MSCs | |
| Alginate interpenetrating network as an artificial ECM | Prepared by a combination of ionic and covalent cross-linking of click-functionalised alginate, interpenetrating with fibrillar collagen type I | Varying the mode and magnitude of crosslinking enables tuneable stiffness and viscoelasticity | MSC expression of immunomodulatory markers was differentially impacted by the viscoelasticity and stiffness of the matrix | MSCs | |
| Boronate ester hydrogel | Prepared by reversible boronate esterification of boronic acid with vic-diols | Viscoelasticity increased as a function of the boronic acid and vicinal diol concentration, and also increased with decreasing cross-linker concentration, where the maximal loss tangent achieved was 0.55 at 0.1 rad/s | The cell area and nuclear area, focal adhesion tension, and subcellular location of YAP/TAZ were found to be lower for cells cultured on viscoelastic hydrogels compared to elastic hydrogels with a similar storage modulus | NIH-3T3 cells | |
| Boronate-based hydrogels | Based on reversible boronate bonds | Relaxation time constants on the order of seconds or less | Fast relaxation matrix mechanics are found to promote cell-matrix interactions, leading to spreading and an increase in nuclear volume, and induce YAP/TAZ binding domain nuclear localization at longer times | MSCs | |
| Collagen gels | Fabricated by adjusting pH | Strain-enhanced stress relaxation of collagen gels arises from force-dependent unbinding of weak bonds between collagen fibres | - | - | |
| Hyaluronic acid hydrogels | Crosslinked via photo-responsive guest-host pairing of azobenzene to β-cyclodextrin | Relaxation time from 6 seconds to minutes | The hydrogels maintained a high level of viability after 3 days of culture | NIH 3T3 cells | |
| Hyaluronic acid | Combined light-mediated covalent and supramolecular crosslinking was used to afford spatiotemporal control of the viscoelastic network | Significantly higher loss moduli compared to elastic group. Photopatterning enabled presentation of dynamic, heterogeneous viscoelastic properties | LX-2 cells respond to the viscoelastic hydrogel by displaying reductions in spread area, MRTF-A nuclear translation, and organisation of actin stress fibres | LX-2 stellate cells | |
| Hyaluronic acid-collagen hydrogels | Interpenetrating network based on HA crosslinked with dynamic hydrazone bonds with collagen type I | The time for the initial stress of the material to be relaxed to half its value during a stress relaxation test (τ1/2) was modulated from ~233 seconds to > 18000 seconds | Faster relaxation promotes cell spreading, fibre remodelling, and focal adhesion formation in 3D culture | Human MSCs | |
| Oxime cross-linked alginate hydrogels | Formed by mixing alkoxyamine-containing alginate with aldehyde-containing alginate | Stress-relaxation was tuneable by varying the composition or environmental factors | The gels showed very nice short-term cytocompatibility with the encapsulated cells. Growth and migration benefited from the stress relaxation capability | 2PK3 cells | |
| PEG hydrogels | Crosslinked by reversible hydrazone bonds | τ1/2 could be varied from 5–6000 seconds by changing the number of PEG or by changing the ratio of benzaldehyde to aliphatic aldehyde crosslinkers | Covalently-adaptable hydrogels allowed for the development of physiologically-relevant morphologies, whereas non-adaptable gels prevented cytoskeletal rearrangement and extension | C2C12 myoblasts | |
| Thioester hydrogel | Photopolymerisation between PEG-SH and thioester-containing divinyl crosslinker | Through control of pH, gel stoichiometry, and crosslinker structure, viscoelastic properties were modulated across several orders of magnitude | MSCs encapsulated in the thioester hydrogels were able to elongate in 3D and display increased proliferation relative to those in static hydrogels | MSCs | |
| Hyaluronic acid and PEG | A DN was formed based on the combination of supramolecular GH hyaluronic acid networks with covalent networks from the photocrosslinking of PEG-fibrinogen and PEG-diacrylate | Dependent on the polymer concentration the GH network | The increase of GH concentration led to the enhancement of the viscosity of the DN hydrogel and the enhancement of cell spreading and proliferation | MSCs |
| 1. | Wolff, J. Concept of the Law of Bone Remodelling. In The Law of Bone Remodelling, Wolff, J., ed. Springer Berlin Heidelberg: Berlin, Heidelberg. 1986; p 1. |
| 2. |
Doyle, A. D.; Carvajal, N.; Jin, A.; Matsumoto, K.; Yamada, K. M. Local 3D matrix microenvironment regulates cell migration through spatiotemporal dynamics of contractility-dependent adhesions. Nat Commun. 2015, 6, 8720.
doi: 10.1038/ncomms9720 URL |
| 3. |
Hadden, W. J.; Young, J. L.; Holle, A. W.; McFetridge, M. L.; Kim, D. Y.; Wijesinghe, P.; Taylor-Weiner, H.; Wen, J. H.; Lee, A. R.; Bieback, K.; Vo, B. N.; Sampson, D. D.; Kennedy, B. F.; Spatz, J. P.; Engler, A. J.; Choi, Y. S. Stem cell migration and mechanotransduction on linear stiffness gradient hydrogels. Proc Natl Acad Sci U S A. 2017, 114, 5647-5652.
doi: 10.1073/pnas.1618239114 URL |
| 4. |
Mao, A. S.; Shin, J. W.; Mooney, D. J. Effects of substrate stiffness and cell-cell contact on mesenchymal stem cell differentiation. Biomaterials. 2016, 98, 184-191.
doi: 10.1016/j.biomaterials.2016.05.004 URL |
| 5. |
Nisenholz, N.; Rajendran, K.; Dang, Q.; Chen, H.; Kemkemer, R.; Krishnan, R.; Zemel, A. Active mechanics and dynamics of cell spreading on elastic substrates. Soft Matter. 2014, 10, 7234-7246.
doi: 10.1039/C4SM00780H URL |
| 6. |
Shin, J. W.; Mooney, D. J. Extracellular matrix stiffness causes systematic variations in proliferation and chemosensitivity in myeloid leukemias. Proc Natl Acad Sci U S A. 2016, 113, 12126-12131.
doi: 10.1073/pnas.1611338113 URL |
| 7. | Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014, 15, 786-801. |
| 8. |
Handorf, A. M.; Zhou, Y.; Halanski, M. A.; Li, W. J. Tissue stiffness dictates development, homeostasis, and disease progression. Organogenesis. 2015, 11, 1-15.
doi: 10.1080/15476278.2015.1019687 URL |
| 9. |
Chaudhuri, O. Viscoelastic hydrogels for 3D cell culture. Biomater Sci. 2017, 5, 1480-1490.
doi: 10.1039/C7BM00261K URL |
| 10. | Yang, Y.; Wang, K.; Gu, X.; Leong, K. W. Biophysical regulation of cell behavior-cross talk between substrate stiffness and nanotopography. Engineering (Beijing). 2017, 3, 36-54. |
| 11. |
Panciera, T.; Azzolin, L.; Cordenonsi, M.; Piccolo, S. Mechanobiology of YAP and TAZ in physiology and disease. Nat Rev Mol Cell Biol. 2017, 18, 758-770.
doi: 10.1038/nrm.2017.87 URL |
| 12. |
Chaudhuri, O.; Cooper-White, J.; Janmey, P. A.; Mooney, D. J.; Shenoy, V. B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature. 2020, 584, 535-546.
doi: 10.1038/s41586-020-2612-2 URL |
| 13. |
Levental, I.; Georges, P. C.; Janmey, P. A. Soft biological materials and their impact on cell function. Soft Matter. 2007, 3, 299-306.
doi: 10.1039/B610522J URL |
| 14. |
Vining, K. H.; Mooney, D. J. Mechanical forces direct stem cell behaviour in development and regeneration. Nat Rev Mol Cell Biol. 2017, 18, 728-742.
doi: 10.1038/nrm.2017.108 URL |
| 15. |
Sharabi, M.; Wertheimer, S.; Wade, K. R.; Galbusera, F.; Benayahu, D.; Wilke, H. J.; Haj-Ali, R. Towards intervertebral disc engineering: Bio-mimetics of form and function of the annulus fibrosus lamellae. J Mech Behav Biomed Mater. 2019, 94, 298-307.
doi: 10.1016/j.jmbbm.2019.03.023 URL |
| 16. |
Kleemann, R. U.; Krocker, D.; Cedraro, A.; Tuischer, J.; Duda, G. N. Altered cartilage mechanics and histology in knee osteoarthritis: relation to clinical assessment (ICRS Grade). Osteoarthritis Cartilage. 2005, 13, 958-963.
doi: 10.1016/j.joca.2005.06.008 URL |
| 17. |
Zhang, X.; Cai, D.; Zhou, F.; Yu, J.; Wu, X.; Yu, D.; Zou, Y.; Hong, Y.; Yuan, C.; Chen, Y.; Pan, Z.; Bunpetch, V.; Sun, H.; An, C.; Yi-Chin, T.; Ouyang, H.; Zhang, S. Targeting downstream subcellular YAP activity as a function of matrix stiffness with Verteporfin-encapsulated chitosan microsphere attenuates osteoarthritis. Biomaterials. 2020, 232, 119724.
doi: 10.1016/j.biomaterials.2019.119724 URL |
| 18. |
Han, F.; Zhu, C.; Guo, Q.; Yang, H.; Li, B. Cellular modulation by the elasticity of biomaterials. J Mater Chem B. 2016, 4, 9-26.
doi: 10.1039/C5TB02077H URL |
| 19. |
Sherratt, M. J. Tissue elasticity and the ageing elastic fibre. Age (Dordr). 2009, 31, 305-325.
doi: 10.1007/s11357-009-9103-6 URL |
| 20. |
Engler, A. J.; Sen, S.; Sweeney, H. L.; Discher, D. E. Matrix elasticity directs stem cell lineage specification. Cell. 2006, 126, 677-689.
doi: 10.1016/j.cell.2006.06.044 URL |
| 21. | Erickson, A. E.; Lan Levengood, S. K.; Sun, J.; Chang, F. C.; Zhang, M. Fabrication and characterization of chitosan-hyaluronic acid scaffolds with varying stiffness for glioblastoma cell culture. Adv Healthc Mater. 2018, 7, e1800295. |
| 22. |
Vorwald, C. E.; Gonzalez-Fernandez, T.; Joshee, S.; Sikorski, P.; Leach, J. K. Tunable fibrin-alginate interpenetrating network hydrogels to support cell spreading and network formation. Acta Biomater. 2020, 108, 142-152.
doi: 10.1016/j.actbio.2020.03.014 URL |
| 23. |
Narkhede, A. A.; Crenshaw, J. H.; Manning, R. M.; Rao, S. S. The influence of matrix stiffness on the behavior of brain metastatic breast cancer cells in a biomimetic hyaluronic acid hydrogel platform. J Biomed Mater Res A. 2018, 106, 1832-1841.
doi: 10.1002/jbm.a.v106.7 URL |
| 24. |
Sun, Y.; Zhang, K.; Deng, R.; Ren, X.; Wu, C.; Li, J. Tunable stiffness of graphene oxide/polyacrylamide composite scaffolds regulates cytoskeleton assembly. Chem Sci. 2018, 9, 6516-6522.
doi: 10.1039/C8SC02100G URL |
| 25. |
Corbin, E. A.; Vite, A.; Peyster, E. G.; Bhoopalam, M.; Brandimarto, J.; Wang, X.; Bennett, A. I.; Clark, A. T.; Cheng, X.; Turner, K. T.; Musunuru, K.; Margulies, K. B. Tunable and reversible substrate stiffness reveals a dynamic mechanosensitivity of cardiomyocytes. ACS Appl Mater Interfaces. 2019, 11, 20603-20614.
doi: 10.1021/acsami.9b02446 URL |
| 26. |
Mi, H. Y.; Jing, X.; Yilmaz, G.; Hagerty, B. S.; Enriquez, E.; Turng, L. S. In situ synthesis of polyurethane scaffolds with tunable properties by controlled crosslinking of tri-block copolymer and polycaprolactone triol for tissue regeneration. Chem Eng J. 2018, 348, 786-798.
doi: 10.1016/j.cej.2018.04.198 URL |
| 27. |
Buitrago, J. O.; Patel, K. D.; El-Fiqi, A.; Lee, J. H.; Kundu, B.; Lee, H. H.; Kim, H. W. Silk fibroin/collagen protein hybrid cell-encapsulating hydrogels with tunable gelation and improved physical and biological properties. Acta Biomater. 2018, 69, 218-233.
doi: 10.1016/j.actbio.2017.12.026 URL |
| 28. |
Crocini, C.; Walker, C. J.; Anseth, K. S.; Leinwand, L. A. Three-dimensional encapsulation of adult mouse cardiomyocytes in hydrogels with tunable stiffness. Prog Biophys Mol Biol. 2020, 154, 71-79.
doi: 10.1016/j.pbiomolbio.2019.04.008 URL |
| 29. |
Yi, B.; Shen, Y.; Tang, H.; Wang, X.; Li, B.; Zhang, Y. Stiffness of aligned fibers regulates the phenotypic expression of vascular smooth muscle cells. ACS Appl Mater Interfaces. 2019, 11, 6867-6880.
doi: 10.1021/acsami.9b00293 URL |
| 30. | Wu, Y.; Xiang, Y.; Fang, J.; Li, X.; Lin, Z.; Dai, G.; Yin, J.; Wei, P.; Zhang, D. The influence of the stiffness of GelMA substrate on the outgrowth of PC12 cells. Biosci Rep. 2019, 39, BSR20181748. |
| 31. |
Jiang, P.; Mao, Z.; Gao, C. Combinational effect of matrix elasticity and alendronate density on differentiation of rat mesenchymal stem cells. Acta Biomater. 2015, 19, 76-84.
doi: 10.1016/j.actbio.2015.03.018 URL |
| 32. |
Berger, A. J.; Linsmeier, K. M.; Kreeger, P. K.; Masters, K. S. Decoupling the effects of stiffness and fiber density on cellular behaviors via an interpenetrating network of gelatin-methacrylate and collagen. Biomaterials. 2017, 141, 125-135.
doi: 10.1016/j.biomaterials.2017.06.039 URL |
| 33. |
Ansari, S.; Sarrion, P.; Hasani-Sadrabadi, M. M.; Aghaloo, T.; Wu, B. M.; Moshaverinia, A. Regulation of the fate of dental-derived mesenchymal stem cells using engineered alginate-GelMA hydrogels. J Biomed Mater Res A. 2017, 105, 2957-2967.
doi: 10.1002/jbm.a.36148 URL |
| 34. |
Ansari, S.; Chen, C.; Hasani-Sadrabadi, M. M.; Yu, B.; Zadeh, H. H.; Wu, B. M.; Moshaverinia, A. Hydrogel elasticity and microarchitecture regulate dental-derived mesenchymal stem cell-host immune system cross-talk. Acta Biomater. 2017, 60, 181-189.
doi: 10.1016/j.actbio.2017.07.017 URL |
| 35. |
Wang, X.; Ding, Z.; Wang, C.; Chen, X.; Xu, H.; Lu, Q.; Kaplan, D. L. Bioactive silk hydrogels with tunable mechanical properties. J Mater Chem B. 2018, 6, 2739-2746.
doi: 10.1039/C8TB00607E URL |
| 36. |
Zhu, C.; Li, J.; Liu, C.; Zhou, P.; Yang, H.; Li, B. Modulation of the gene expression of annulus fibrosus-derived stem cells using poly(ether carbonate urethane)urea scaffolds of tunable elasticity. Acta Biomater. 2016, 29, 228-238.
doi: 10.1016/j.actbio.2015.09.039 URL |
| 37. |
Günay, K. A.; Ceccato, T. L.; Silver, J. S.; Bannister, K. L.; Bednarski, O. J.; Leinwand, L. A.; Anseth, K. S. PEG-anthracene hydrogels as an on-demand stiffening matrix to study mechanobiology. Angew Chem Int Ed Engl. 2019, 58, 9912-9916.
doi: 10.1002/anie.v58.29 URL |
| 38. |
Young, J. L.; Engler, A. J. Hydrogels with time-dependent material properties enhance cardiomyocyte differentiation in vitro. Biomaterials. 2011, 32, 1002-1009.
doi: 10.1016/j.biomaterials.2010.10.020 URL |
| 39. |
Fu, L.; Haage, A.; Kong, N.; Tanentzapf, G.; Li, H. Dynamic protein hydrogels with reversibly tunable stiffness regulate human lung fibroblast spreading reversibly. Chem Commun (Camb). 2019, 55, 5235-5238.
doi: 10.1039/C9CC01276A URL |
| 40. |
Zhang, D.; Zhou, C.; Wang, Q.; Cai, L.; Du, W.; Li, X.; Zhou, X.; Xie, J. Extracellular matrix elasticity regulates osteocyte gap junction elongation: involvement of paxillin in intracellular signal transduction. Cell Physiol Biochem. 2018, 51, 1013-1026.
doi: 10.1159/000495482 URL |
| 41. |
Xie, J.; Zhang, Q.; Zhu, T.; Zhang, Y.; Liu, B.; Xu, J.; Zhao, H. Substrate stiffness-regulated matrix metalloproteinase output in myocardial cells and cardiac fibroblasts: implications for myocardial fibrosis. Acta Biomater. 2014, 10, 2463-2472.
doi: 10.1016/j.actbio.2014.01.031 URL |
| 42. |
Yeung, T.; Georges, P. C.; Flanagan, L. A.; Marg, B.; Ortiz, M.; Funaki, M.; Zahir, N.; Ming, W.; Weaver, V.; Janmey, P. A. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 2005, 60, 24-34.
doi: 10.1002/(ISSN)1097-0169 URL |
| 43. |
Hansen, T. D.; Koepsel, J. T.; Le, N. N.; Nguyen, E. H.; Zorn, S.; Parlato, M.; Loveland, S. G.; Schwartz, M. P.; Murphy, W. L. Biomaterial arrays with defined adhesion ligand densities and matrix stiffness identify distinct phenotypes for tumorigenic and nontumorigenic human mesenchymal cell types. Biomater Sci. 2014, 2, 745-756.
doi: 10.1039/C3BM60278H URL |
| 44. |
Melica, M. E.; La Regina, G.; Parri, M.; Peired, A. J.; Romagnani, P.; Lasagni, L. Substrate stiffness modulates renal progenitor cell properties via a ROCK-mediated mechanotransduction Mechanism. Cells. 2019, 8, 1561.
doi: 10.3390/cells8121561 URL |
| 45. |
Peyton, S. R.; Putnam, A. J. Extracellular matrix rigidity governs smooth muscle cell motility in a biphasic fashion. J Cell Physiol. 2005, 204, 198-209.
doi: 10.1002/(ISSN)1097-4652 URL |
| 46. |
Bangasser, B. L.; Shamsan, G. A.; Chan, C. E.; Opoku, K. N.; Tüzel, E.; Schlichtmann, B. W.; Kasim, J. A.; Fuller, B. J.; McCullough, B. R.; Rosenfeld, S. S.; Odde, D. J. Shifting the optimal stiffness for cell migration. Nat Commun. 2017, 8, 15313.
doi: 10.1038/ncomms15313 URL |
| 47. |
Lo, C. M.; Wang, H. B.; Dembo, M.; Wang, Y. L. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000, 79, 144-152.
doi: 10.1016/S0006-3495(00)76279-5 URL |
| 48. |
Okimura, C.; Sakumura, Y.; Shimabukuro, K.; Iwadate, Y. Sensing of substratum rigidity and directional migration by fast-crawling cells. Phys Rev E. 2018, 97, 052401.
doi: 10.1103/PhysRevE.97.052401 URL |
| 49. |
Hartman, C. D.; Isenberg, B. C.; Chua, S. G.; Wong, J. Y. Vascular smooth muscle cell durotaxis depends on extracellular matrix composition. Proc Natl Acad Sci U S A. 2016, 113, 11190-11195.
doi: 10.1073/pnas.1611324113 URL |
| 50. |
Liu, N.; Zhou, M.; Zhang, Q.; Yong, L.; Zhang, T.; Tian, T.; Ma, Q.; Lin, S.; Zhu, B.; Cai, X. Effect of substrate stiffness on proliferation and differentiation of periodontal ligament stem cells. Cell Prolif. 2018, 51, e12478.
doi: 10.1111/cpr.2018.51.issue-5 URL |
| 51. | Ansardamavandi, A.; Tafazzoli-Shadpour, M.; Shokrgozar, M. A. Behavioral remodeling of normal and cancerous epithelial cell lines with differing invasion potential induced by substrate elastic modulus. Cell Adh Migr. 2018, 12, 472-488. |
| 52. |
Leipzig, N. D.; Shoichet, M. S. The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials. 2009, 30, 6867-6878.
doi: 10.1016/j.biomaterials.2009.09.002 URL |
| 53. | Robinson, K. G.; Nie, T.; Baldwin, A. D.; Yang, E. C.; Kiick, K. L.; Akins, R. E., Jr. Differential effects of substrate modulus on human vascular endothelial, smooth muscle, and fibroblastic cells. J Biomed Mater Res A. 2012, 100, 1356-1367. |
| 54. |
Eroshenko, N.; Ramachandran, R.; Yadavalli, V. K.; Rao, R. R. Effect of substrate stiffness on early human embryonic stem cell differentiation. J Biol Eng. 2013, 7, 7.
doi: 10.1186/1754-1611-7-7 URL |
| 55. |
Arshi, A.; Nakashima, Y.; Nakano, H.; Eaimkhong, S.; Evseenko, D.; Reed, J.; Stieg, A. Z.; Gimzewski, J. K.; Nakano, A. Rigid microenvironments promote cardiac differentiation of mouse and human embryonic stem cells. Sci Technol Adv Mater. 2013, 14, 025003.
doi: 10.1088/1468-6996/14/2/025003 URL |
| 56. |
Macrí-Pellizzeri, L.; Pelacho, B.; Sancho, A.; Iglesias-García, O.; Simón-Yarza, A. M.; Soriano-Navarro, M.; González-Granero, S.; García-Verdugo, J. M.; De-Juan-Pardo, E. M.; Prosper, F. Substrate stiffness and composition specifically direct differentiation of induced pluripotent stem cells. Tissue Eng Part A. 2015, 21, 1633-1641.
doi: 10.1089/ten.tea.2014.0251 URL |
| 57. |
Fu, J.; Chuah, Y. J.; Liu, J.; Tan, S. Y.; Wang, D. A. Respective effects of gelatin-coated polydimethylsiloxane (PDMS) substrates on self-renewal and cardiac differentiation of induced pluripotent stem cells (iPSCs). ACS Biomater Sci Eng. 2018, 4, 4321-4330.
doi: 10.1021/acsbiomaterials.8b00993 URL |
| 58. |
Janmey, P. A.; Fletcher, D. A.; Reinhart-King, C. A. Stiffness sensing by cells. Physiol Rev. 2020, 100, 695-724.
doi: 10.1152/physrev.00013.2019 URL |
| 59. |
Lantoine, J.; Grevesse, T.; Villers, A.; Delhaye, G.; Mestdagh, C.; Versaevel, M.; Mohammed, D.; Bruyère, C.; Alaimo, L.; Lacour, S. P.; Ris, L.; Gabriele, S. Matrix stiffness modulates formation and activity of neuronal networks of controlled architectures. Biomaterials. 2016, 89, 14-24.
doi: 10.1016/j.biomaterials.2016.02.041 URL |
| 60. |
Chen, G.; Dong, C.; Yang, L.; Lv, Y. 3D scaffolds with different stiffness but the same microstructure for bone tissue engineering. ACS Appl Mater Interfaces. 2015, 7, 15790-15802.
doi: 10.1021/acsami.5b02662 URL |
| 61. |
Zhang, Q.; Yu, Y.; Zhao, H. The effect of matrix stiffness on biomechanical properties of chondrocytes. Acta Biochim Biophys Sin (Shanghai). 2016, 48, 958-965.
doi: 10.1093/abbs/gmw087 URL |
| 62. |
Sun, A. X.; Lin, H.; Fritch, M. R.; Shen, H.; Alexander, P. G.; DeHart, M.; Tuan, R. S. Chondrogenesis of human bone marrow mesenchymal stem cells in 3-dimensional, photocrosslinked hydrogel constructs: Effect of cell seeding density and material stiffness. Acta Biomater. 2017, 58, 302-311.
doi: 10.1016/j.actbio.2017.06.016 URL |
| 63. |
Sarem, M.; Arya, N.; Heizmann, M.; Neffe, A. T.; Barbero, A.; Gebauer, T. P.; Martin, I.; Lendlein, A.; Shastri, V. P. Interplay between stiffness and degradation of architectured gelatin hydrogels leads to differential modulation of chondrogenesis in vitro and in vivo. Acta Biomater. 2018, 69, 83-94.
doi: 10.1016/j.actbio.2018.01.025 URL |
| 64. |
Zhan, X. Effect of matrix stiffness and adhesion ligand density on chondrogenic differentiation of mesenchymal stem cells. J Biomed Mater Res A. 2020, 108, 675-683.
doi: 10.1002/jbm.a.v108.3 URL |
| 65. |
Takaza, M.; Moerman, K. M.; Gindre, J.; Lyons, G.; Simms, C. K. The anisotropic mechanical behaviour of passive skeletal muscle tissue subjected to large tensile strain. J Mech Behav Biomed Mater. 2013, 17, 209-220.
doi: 10.1016/j.jmbbm.2012.09.001 URL |
| 66. |
Asbach, P.; Klatt, D.; Hamhaber, U.; Braun, J.; Somasundaram, R.; Hamm, B.; Sack, I. Assessment of liver viscoelasticity using multifrequency MR elastography. Magn Reson Med. 2008, 60, 373-379.
doi: 10.1002/mrm.v60:2 URL |
| 67. |
Balleyguier, C.; Canale, S.; Ben Hassen, W.; Vielh, P.; Bayou, E. H.; Mathieu, M. C.; Uzan, C.; Bourgier, C.; Dromain, C. Breast elasticity: principles, technique, results: an update and overview of commercially available software. Eur J Radiol. 2013, 82, 427-434.
doi: 10.1016/j.ejrad.2012.03.001 URL |
| 68. |
Kearney, S. P.; Khan, A.; Dai, Z.; Royston, T. J. Dynamic viscoelastic models of human skin using optical elastography. Phys Med Biol. 2015, 60, 6975-6990.
doi: 10.1088/0031-9155/60/17/6975 URL |
| 69. |
Yang, X.; Muthukumaran, P.; DasDe, S.; Teoh, S. H.; Choi, H.; Lim, S. K.; Lee, T. Positive alterations of viscoelastic and geometric properties in ovariectomized rat femurs with concurrent administration of ibandronate and PTH. Bone. 2013, 52, 308-317.
doi: 10.1016/j.bone.2012.09.039 URL |
| 70. |
Cameron, A. R.; Frith, J. E.; Cooper-White, J. J. The influence of substrate creep on mesenchymal stem cell behaviour and phenotype. Biomaterials. 2011, 32, 5979-5993.
doi: 10.1016/j.biomaterials.2011.04.003 URL |
| 71. |
Charrier, E. E.; Pogoda, K.; Wells, R. G.; Janmey, P. A. Control of cell morphology and differentiation by substrates with independently tunable elasticity and viscous dissipation. Nat Commun. 2018, 9, 449.
doi: 10.1038/s41467-018-02906-9 URL |
| 72. |
Chaudhuri, O.; Gu, L.; Darnell, M.; Klumpers, D.; Bencherif, S. A.; Weaver, J. C.; Huebsch, N.; Mooney, D. J. Substrate stress relaxation regulates cell spreading. Nat Commun. 2015, 6, 6364.
doi: 10.1038/ncomms7364 URL |
| 73. |
Chaudhuri, O.; Gu, L.; Klumpers, D.; Darnell, M.; Bencherif, S. A.; Weaver, J. C.; Huebsch, N.; Lee, H. P.; Lippens, E.; Duda, G. N.; Mooney, D. J. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat Mater. 2016, 15, 326-334.
doi: 10.1038/nmat4489 URL |
| 74. |
Nam, S.; Stowers, R.; Lou, J.; Xia, Y.; Chaudhuri, O. Varying PEG density to control stress relaxation in alginate-PEG hydrogels for 3D cell culture studies. Biomaterials. 2019, 200, 15-24.
doi: 10.1016/j.biomaterials.2019.02.004 URL |
| 75. |
Vining, K. H.; Stafford, A.; Mooney, D. J. Sequential modes of crosslinking tune viscoelasticity of cell-instructive hydrogels. Biomaterials. 2019, 188, 187-197.
doi: 10.1016/j.biomaterials.2018.10.013 URL |
| 76. |
Nam, S.; Hu, K. H.; Butte, M. J.; Chaudhuri, O. Strain-enhanced stress relaxation impacts nonlinear elasticity in collagen gels. Proc Natl Acad Sci U S A. 2016, 113, 5492-5497.
doi: 10.1073/pnas.1523906113 URL |
| 77. |
Rosales, A. M.; Rodell, C. B.; Chen, M. H.; Morrow, M. G.; Anseth, K. S.; Burdick, J. A. Reversible control of network properties in azobenzene-containing hyaluronic acid-based hydrogels. Bioconjug Chem. 2018, 29, 905-913.
doi: 10.1021/acs.bioconjchem.7b00802 URL |
| 78. |
Marozas, I. A.; Anseth, K. S.; Cooper-White, J. J. Adaptable boronate ester hydrogels with tunable viscoelastic spectra to probe timescale dependent mechanotransduction. Biomaterials. 2019, 223, 119430.
doi: 10.1016/j.biomaterials.2019.119430 URL |
| 79. | Tang, S.; Ma, H.; Tu, H. C.; Wang, H. R.; Lin, P. C.; Anseth, K. S. Adaptable fast relaxing boronate-based hydrogels for probing cell-matrix interactions. Adv Sci (Weinh). 2018, 5, 1800638. |
| 80. |
Hui, E.; Gimeno, K. I.; Guan, G.; Caliari, S. R. Spatiotemporal control of viscoelasticity in phototunable hyaluronic acid hydrogels. Biomacromolecules. 2019, 20, 4126-4134.
doi: 10.1021/acs.biomac.9b00965 URL |
| 81. |
Lou, J.; Stowers, R.; Nam, S.; Xia, Y.; Chaudhuri, O. Stress relaxing hyaluronic acid-collagen hydrogels promote cell spreading, fiber remodeling, and focal adhesion formation in 3D cell culture. Biomaterials. 2018, 154, 213-222.
doi: 10.1016/j.biomaterials.2017.11.004 URL |
| 82. |
Sánchez-Morán, H.; Ahmadi, A.; Vogler, B.; Roh, K. H. Oxime cross-linked alginate hydrogels with tunable stress relaxation. Biomacromolecules. 2019, 20, 4419-4429.
doi: 10.1021/acs.biomac.9b01100 URL |
| 83. |
McKinnon, D. D.; Domaille, D. W.; Cha, J. N.; Anseth, K. S. Biophysically defined and cytocompatible covalently adaptable networks as viscoelastic 3D cell culture systems. Adv Mater. 2014, 26, 865-872.
doi: 10.1002/adma.v26.6 URL |
| 84. |
Brown, T. E.; Carberry, B. J.; Worrell, B. T.; Dudaryeva, O. Y.; McBride, M. K.; Bowman, C. N.; Anseth, K. S. Photopolymerized dynamic hydrogels with tunable viscoelastic properties through thioester exchange. Biomaterials. 2018, 178, 496-503.
doi: 10.1016/j.biomaterials.2018.03.060 URL |
| 85. |
Loebel, C.; Ayoub, A.; Galarraga, J. H.; Kossover, O.; Simaan-Yameen, H.; Seliktar, D.; Burdick, J. A. Tailoring supramolecular guest-host hydrogel viscoelasticity with covalent fibrinogen double networks. J Mater Chem B. 2019, 7, 1753-1760.
doi: 10.1039/C8TB02593B URL |
| 86. |
Zhao, X.; Huebsch, N.; Mooney, D. J.; Suo, Z. Stress-relaxation behavior in gels with ionic and covalent crosslinks. J Appl Phys. 2010, 107, 63509.
doi: 10.1063/1.3343265 URL |
| 87. |
Lee, H. P.; Gu, L.; Mooney, D. J.; Levenston, M. E.; Chaudhuri, O. Mechanical confinement regulates cartilage matrix formation by chondrocytes. Nat Mater. 2017, 16, 1243-1251.
doi: 10.1038/nmat4993 URL |
| 88. |
Ryan, A. J.; O’Brien, F. J. Insoluble elastin reduces collagen scaffold stiffness, improves viscoelastic properties, and induces a contractile phenotype in smooth muscle cells. Biomaterials. 2015, 73, 296-307.
doi: 10.1016/j.biomaterials.2015.09.003 URL |
| 89. |
Bauer, A.; Gu, L.; Kwee, B.; Li, W. A.; Dellacherie, M.; Celiz, A. D.; Mooney, D. J. Hydrogel substrate stress-relaxation regulates the spreading and proliferation of mouse myoblasts. Acta Biomater. 2017, 62, 82-90.
doi: 10.1016/j.actbio.2017.08.041 URL |
| 90. |
Zheng, J. Y.; Han, S. P.; Chiu, Y. J.; Yip, A. K.; Boichat, N.; Zhu, S. W.; Zhong, J.; Matsudaira, P. Epithelial monolayers coalesce on a viscoelastic substrate through redistribution of vinculin. Biophys J. 2017, 113, 1585-1598.
doi: 10.1016/j.bpj.2017.07.027 URL |
| 91. |
Sommerfeld, S. D.; Elisseeff, J. H. Time to relax: mechanical stress release guides stem cell responses. Cell Stem Cell. 2016, 18, 166-167.
doi: 10.1016/j.stem.2016.01.020 URL |
| 92. |
Ghosh, P.; Rameshbabu, A. P.; Dhara, S. Citrate cross-linked gels with strain reversibility and viscoelastic behavior accelerate healing of osteochondral defects in a rabbit model. Langmuir. 2014, 30, 8442-8451.
doi: 10.1021/la500698v URL |
| 93. |
Ghosh, P.; Rameshbabu, A. P.; Das, D.; Francis, N. K.; Pawar, H. S.; Subramanian, B.; Pal, S.; Dhara, S. Covalent cross-links in polyampholytic chitosan fibers enhances bone regeneration in a rabbit model. Colloids Surf B Biointerfaces. 2015, 125, 160-169.
doi: 10.1016/j.colsurfb.2014.11.031 URL |
| 94. |
Darnell, M.; Young, S.; Gu, L.; Shah, N.; Lippens, E.; Weaver, J.; Duda, G.; Mooney, D. Substrate stress-relaxation regulates scaffold remodeling and bone formation in vivo. Adv Healthc Mater. 2017, 6, 1601185.
doi: 10.1002/adhm.v6.1 URL |
| 95. |
Richardson, B. M.; Wilcox, D. G.; Randolph, M. A.; Anseth, K. S. Hydrazone covalent adaptable networks modulate extracellular matrix deposition for cartilage tissue engineering. Acta Biomater. 2019, 83, 71-82.
doi: 10.1016/j.actbio.2018.11.014 URL |
| 96. |
Li, W.; Wu, D.; Hu, D.; Zhu, S.; Pan, C.; Jiao, Y.; Li, L.; Luo, B.; Zhou, C.; Lu, L. Stress-relaxing double-network hydrogel for chondrogenic differentiation of stem cells. Mater Sci Eng C Mater Biol Appl. 2020, 107, 110333.
doi: 10.1016/j.msec.2019.110333 URL |
| 97. |
Mandal, K.; Gong, Z.; Rylander, A.; Shenoy, V. B.; Janmey, P. A. Opposite responses of normal hepatocytes and hepatocellular carcinoma cells to substrate viscoelasticity. Biomater Sci. 2020, 8, 1316-1328.
doi: 10.1039/C9BM01339C URL |
| 98. |
Chen, J.; Wright, K. E.; Birch, M. A. Nanoscale viscoelastic properties and adhesion of polydimethylsiloxane for tissue engineering. Acta Mech Sin. 2014, 30, 2-6.
doi: 10.1007/s10409-014-0022-0 URL |
| 99. |
Trappmann, B.; Gautrot, J. E.; Connelly, J. T.; Strange, D. G.; Li, Y.; Oyen, M. L.; Cohen Stuart, M. A.; Boehm, H.; Li, B.; Vogel, V.; Spatz, J. P.; Watt, F. M.; Huck, W. T. Extracellular-matrix tethering regulates stem-cell fate. Nat Mater. 2012, 11, 642-649.
doi: 10.1038/nmat3339 URL |
| 100. |
Wen, J. H.; Vincent, L. G.; Fuhrmann, A.; Choi, Y. S.; Hribar, K. C.; Taylor-Weiner, H.; Chen, S.; Engler, A. J. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nat Mater. 2014, 13, 979-987.
doi: 10.1038/nmat4051 URL |
| 101. |
Sadtler, K.; Wolf, M. T.; Ganguly, S.; Moad, C. A.; Chung, L.; Majumdar, S.; Housseau, F.; Pardoll, D. M.; Elisseeff, J. H. Divergent immune responses to synthetic and biological scaffolds. Biomaterials. 2019, 192, 405-415.
doi: 10.1016/j.biomaterials.2018.11.002 URL |
| 102. |
Chu, G.; Yuan, Z.; Zhu, C.; Zhou, P.; Wang, H.; Zhang, W.; Cai, Y.; Zhu, X.; Yang, H.; Li, B. Substrate stiffness- and topography-dependent differentiation of annulus fibrosus-derived stem cells is regulated by Yes-associated protein. Acta Biomater. 2019, 92, 254-264.
doi: 10.1016/j.actbio.2019.05.013 URL |
| 103. |
Shvedova, A. A.; Kisin, E. R.; Mercer, R.; Murray, A. R.; Johnson, V. J.; Potapovich, A. I.; Tyurina, Y. Y.; Gorelik, O.; Arepalli, S.; Schwegler-Berry, D.; Hubbs, A. F.; Antonini, J.; Evans, D. E.; Ku, B. K.; Ramsey, D.; Maynard, A.; Kagan, V. E.; Castranova, V.; Baron, P. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am J Physiol Lung Cell Mol Physiol. 2005, 289, L698-708.
doi: 10.1152/ajplung.00084.2005 URL |
| 104. | Suki, B.; Sato, S.; Parameswaran, H.; Szabari, M. V.; Takahashi, A.; Bartolák-Suki, E. Emphysema and mechanical stress-induced lung remodeling. Physiology (Bethesda). 2013, 28, 404-413. |
| 105. |
Nguyen, A. T.; Sathe, S. R.; Yim, E. K. From nano to micro: topographical scale and its impact on cell adhesion, morphology and contact guidance. J Phys Condens Matter. 2016, 28, 183001.
doi: 10.1088/0953-8984/28/18/183001 URL |
| 106. |
Wu, S.; Duan, B.; Qin, X.; Butcher, J. T. Living nano-micro fibrous woven fabric/hydrogel composite scaffolds for heart valve engineering. Acta Biomater. 2017, 51, 89-100.
doi: 10.1016/j.actbio.2017.01.051 URL |
| 107. |
Wang, S.; Zhong, S.; Lim, C. T.; Nie, H. Effects of fiber alignment on stem cells-fibrous scaffold interactions. J Mater Chem B. 2015, 3, 3358-3366.
doi: 10.1039/C5TB00026B URL |
| 108. |
Fu, X.; Wang, H. Spatial arrangement of polycaprolactone/collagen nanofiber scaffolds regulates the wound healing related behaviors of human adipose stromal cells. Tissue Eng Part A. 2012, 18, 631-642.
doi: 10.1089/ten.tea.2011.0069 URL |
| 109. |
Moffa, M.; Sciancalepore, A. G.; Passione, L. G.; Pisignano, D. Combined nano- and micro-scale topographic cues for engineered vascular constructs by electrospinning and imprinted micro-patterns. Small. 2014, 10, 2439-2450.
doi: 10.1002/smll.v10.12 URL |
| 110. | Yan, J.; Qiang, L.; Gao, Y.; Cui, X.; Zhou, H.; Zhong, S.; Wang, Q.; Wang, H. Effect of fiber alignment in electrospun scaffolds on keratocytes and corneal epithelial cells behavior. J Biomed Mater Res A. 2012, 100, 527-535. |
| 111. |
Stevens, M. M.; George, J. H. Exploring and engineering the cell surface interface. Science. 2005, 310, 1135-1138.
doi: 10.1126/science.1106587 URL |
| 112. |
Dalby, M. J.; Gadegaard, N.; Oreffo, R. O. Harnessing nanotopography and integrin-matrix interactions to influence stem cell fate. Nat Mater. 2014, 13, 558-569.
doi: 10.1038/nmat3980 URL |
| 113. |
Hou, Y.; Yu, L.; Xie, W.; Camacho, L. C.; Zhang, M.; Chu, Z.; Wei, Q.; Haag, R. Surface roughness and substrate stiffness synergize to drive cellular mechanoresponse. Nano Lett. 2020, 20, 748-757.
doi: 10.1021/acs.nanolett.9b04761 URL |
| 114. |
Jahanmard, F.; Baghban Eslaminejad, M.; Amani-Tehran, M.; Zarei, F.; Rezaei, N.; Croes, M.; Amin Yavari, S. Incorporation of F-MWCNTs into electrospun nanofibers regulates osteogenesis through stiffness and nanotopography. Mater Sci Eng C Mater Biol Appl. 2020, 106, 110163.
doi: 10.1016/j.msec.2019.110163 URL |
| 115. |
Qu, F.; Guilak, F.; Mauck, R. L. Cell migration: implications for repair and regeneration in joint disease. Nat Rev Rheumatol. 2019, 15, 167-179.
doi: 10.1038/s41584-018-0151-0 URL |
| 116. |
Liu, W.; Thomopoulos, S.; Xia, Y. Electrospun nanofibers for regenerative medicine. Adv Healthc Mater. 2012, 1, 10-25.
doi: 10.1002/adhm.201100021 URL |
| 117. | Song, K. H.; Heo, S. J.; Peredo, A. P.; Davidson, M. D.; Mauck, R. L.; Burdick, J. A. Influence of fiber stiffness on meniscal cell migration into dense fibrous networks. Adv Healthc Mater. 2020, 9, e1901228. |
| 118. |
Davidson, M. D.; Song, K. H.; Lee, M. H.; Llewellyn, J.; Du, Y.; Baker, B. M.; Wells, R. G.; Burdick, J. A. Engineered fibrous networks to investigate the influence of fiber mechanics on myofibroblast differentiation. ACS Biomater Sci Eng. 2019, 5, 3899-3908.
doi: 10.1021/acsbiomaterials.8b01276 URL |
| 119. |
Northey, J. J.; Przybyla, L.; Weaver, V. M. Tissue force programs cell fate and tumor aggression. Cancer Discov. 2017, 7, 1224-1237.
doi: 10.1158/2159-8290.CD-16-0733 URL |
| 120. |
Brusatin, G.; Panciera, T.; Gandin, A.; Citron, A.; Piccolo, S. Biomaterials and engineered microenvironments to control YAP/TAZ-dependent cell behaviour. Nat Mater. 2018, 17, 1063-1075.
doi: 10.1038/s41563-018-0180-8 URL |
| 121. |
Hove, J. R.; Köster, R. W.; Forouhar, A. S.; Acevedo-Bolton, G.; Fraser, S. E.; Gharib, M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature. 2003, 421, 172-177.
doi: 10.1038/nature01282 URL |
| 122. |
Yang, Y.; Beqaj, S.; Kemp, P.; Ariel, I.; Schuger, L. Stretch-induced alternative splicing of serum response factor promotes bronchial myogenesis and is defective in lung hypoplasia. J Clin Invest. 2000, 106, 1321-1330.
doi: 10.1172/JCI8893 URL |
| 123. | Lefebvre, V.; Bhattaram, P. Vertebrate skeletogenesis. Curr Top Dev Biol. 2010, 90, 291-317. |
| 124. |
Ni, S.; Ling, Z.; Wang, X.; Cao, Y.; Wu, T.; Deng, R.; Crane, J. L.; Skolasky, R.; Demehri, S.; Zhen, G.; Jain, A.; Wu, P.; Pan, D.; Hu, B.; Lyu, X.; Li, Y.; Chen, H.; Qi, H.; Guan, Y.; Dong, X.; Wan, M.; Zou, X.; Lu, H.; Hu, J.; Cao, X. Sensory innervation in porous endplates by Netrin-1 from osteoclasts mediates PGE2-induced spinal hypersensitivity in mice. Nat Commun. 2019, 10, 5643.
doi: 10.1038/s41467-019-13476-9 URL |
| 125. |
Lacroix, D.; Prendergast, P. J. A mechano-regulation model for tissue differentiation during fracture healing: analysis of gap size and loading. J Biomech. 2002, 35, 1163-1171.
doi: 10.1016/S0021-9290(02)00086-6 URL |
| 126. |
Meza, D.; Musmacker, B.; Steadman, E.; Stransky, T.; Rubenstein, D. A.; Yin, W. Endothelial cell biomechanical responses are dependent on both fluid shear stress and tensile strain. Cell Mol Bioeng. 2019, 12, 311-325.
doi: 10.1007/s12195-019-00585-0 URL |
| 127. |
Gayer, C. P.; Basson, M. D. The effects of mechanical forces on intestinal physiology and pathology. Cell Signal. 2009, 21, 1237-1244.
doi: 10.1016/j.cellsig.2009.02.011 URL |
| 128. |
Madhavan, S.; Anghelina, M.; Rath-Deschner, B.; Wypasek, E.; John, A.; Deschner, J.; Piesco, N.; Agarwal, S. Biomechanical signals exert sustained attenuation of proinflammatory gene induction in articular chondrocytes. Osteoarthritis Cartilage. 2006, 14, 1023-1032.
doi: 10.1016/j.joca.2006.03.016 URL |
| 129. |
Sowa, G.; Agarwal, S. Cyclic tensile stress exerts a protective effect on intervertebral disc cells. Am J Phys Med Rehabil. 2008, 87, 537-544.
doi: 10.1097/PHM.0b013e31816197ee URL |
| 130. |
Branski, R. C.; Perera, P.; Verdolini, K.; Rosen, C. A.; Hebda, P. A.; Agarwal, S. Dynamic biomechanical strain inhibits IL-1beta-induced inflammation in vocal fold fibroblasts. J Voice. 2007, 21, 651-660.
doi: 10.1016/j.jvoice.2006.06.005 URL |
| 131. |
Zhao, R.; Liu, W.; Xia, T.; Yang, L. Disordered mechanical stress and tissue engineering therapies in intervertebral disc degeneration. Polymers (Basel). 2019, 11, 1151.
doi: 10.3390/polym11071151 URL |
| 132. |
Pratsinis, H.; Papadopoulou, A.; Neidlinger-Wilke, C.; Brayda-Bruno, M.; Wilke, H. J.; Kletsas, D. Cyclic tensile stress of human annulus fibrosus cells induces MAPK activation: involvement in proinflammatory gene expression. Osteoarthritis Cartilage. 2016, 24, 679-687.
doi: 10.1016/j.joca.2015.11.022 URL |
| 133. |
Yurube, T.; Hirata, H.; Kakutani, K.; Maeno, K.; Takada, T.; Zhang, Z.; Takayama, K.; Matsushita, T.; Kuroda, R.; Kurosaka, M.; Nishida, K. Notochordal cell disappearance and modes of apoptotic cell death in a rat tail static compression-induced disc degeneration model. Arthritis Res Ther. 2014, 16, R31.
doi: 10.1186/ar4460 URL |
| 134. |
Chang, S. H.; Mori, D.; Kobayashi, H.; Mori, Y.; Nakamoto, H.; Okada, K.; Taniguchi, Y.; Sugita, S.; Yano, F.; Chung, U. I.; Kim-Kaneyama, J. R.; Yanagita, M.; Economides, A.; Canalis, E.; Chen, D.; Tanaka, S.; Saito, T. Excessive mechanical loading promotes osteoarthritis through the gremlin-1-NF-κB pathway. Nat Commun. 2019, 10, 1442.
doi: 10.1038/s41467-019-09491-5 URL |
| 135. |
Yanoshita, M.; Hirose, N.; Okamoto, Y.; Sumi, C.; Takano, M.; Nishiyama, S.; Asakawa-Tanne, Y.; Horie, K.; Onishi, A.; Yamauchi, Y.; Mitsuyoshi, T.; Kunimatsu, R.; Tanimoto, K. Cyclic tensile strain upregulates pro-inflammatory cytokine expression via FAK-MAPK signaling in chondrocytes. Inflammation. 2018, 41, 1621-1630.
doi: 10.1007/s10753-018-0805-8 URL |
| 136. |
Hirose, N.; Okamoto, Y.; Yanoshita, M.; Asakawa, Y.; Sumi, C.; Takano, M.; Nishiyama, S.; Su, S. C.; Mitsuyoshi, T.; Kunimatsu, R.; Tanne, K.; Tanimoto, K. Protective effects of cilengitide on inflammation in chondrocytes under excessive mechanical stress. Cell Biol Int. 2020, 44, 966-974.
doi: 10.1002/cbin.v44.4 URL |
| 137. |
Hunt, B. J.; Jurd, K. M. Endothelial cell activation. A central pathophysiological process. BMJ. 1998, 316, 1328-1329.
doi: 10.1136/bmj.316.7141.1328 URL |
| 138. |
Chatterjee, S.; Browning, E. A.; Hong, N.; DeBolt, K.; Sorokina, E. M.; Liu, W.; Birnbaum, M. J.; Fisher, A. B. Membrane depolarization is the trigger for PI3K/Akt activation and leads to the generation of ROS. Am J Physiol Heart Circ Physiol. 2012, 302, H105-114.
doi: 10.1152/ajpheart.00298.2011 URL |
| 139. |
Hynes, R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992, 69, 11-25.
doi: 10.1016/0092-8674(92)90115-S URL |
| 140. | Guan, J. L. Integrin signaling through FAK in the regulation of mammary stem cells and breast cancer. IUBMB Life. 2010, 62, 268-276. |
| 141. | Bolós, V.; Gasent, J. M.; López-Tarruella, S.; Grande, E. The dual kinase complex FAK-Src as a promising therapeutic target in cancer. Onco Targets Ther. 2010, 3, 83-97. |
| 142. |
Liu, C.; Luo, J. W.; Liang, T.; Lin, L. X.; Luo, Z. P.; Zhuang, Y. Q.; Sun, Y. L. Matrix stiffness regulates the differentiation of tendon-derived stem cells through FAK-ERK1/2 activation. Exp Cell Res. 2018, 373, 62-70.
doi: 10.1016/j.yexcr.2018.08.023 URL |
| 143. |
Lee, H. J.; Li, N.; Evans, S. M.; Diaz, M. F.; Wenzel, P. L. Biomechanical force in blood development: extrinsic physical cues drive pro-hematopoietic signaling. Differentiation. 2013, 86, 92-103.
doi: 10.1016/j.diff.2013.06.004 URL |
| 144. |
Biggs, M. J.; Richards, R. G.; Gadegaard, N.; Wilkinson, C. D.; Oreffo, R. O.; Dalby, M. J. The use of nanoscale topography to modulate the dynamics of adhesion formation in primary osteoblasts and ERK/MAPK signalling in STRO-1+ enriched skeletal stem cells. Biomaterials. 2009, 30, 5094-5103.
doi: 10.1016/j.biomaterials.2009.05.049 URL |
| 145. |
Teo, B. K.; Wong, S. T.; Lim, C. K.; Kung, T. Y.; Yap, C. H.; Ramagopal, Y.; Romer, L. H.; Yim, E. K. Nanotopography modulates mechanotransduction of stem cells and induces differentiation through focal adhesion kinase. ACS Nano. 2013, 7, 4785-4798.
doi: 10.1021/nn304966z URL |
| 146. |
Khatiwala, C. B.; Kim, P. D.; Peyton, S. R.; Putnam, A. J. ECM compliance regulates osteogenesis by influencing MAPK signaling downstream of RhoA and ROCK. J Bone Miner Res. 2009, 24, 886-898.
doi: 10.1359/jbmr.081240 URL |
| 147. |
Halder, G.; Dupont, S.; Piccolo, S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat Rev Mol Cell Biol. 2012, 13, 591-600.
doi: 10.1038/nrm3416 URL |
| 148. |
Dasgupta, I.; McCollum, D. Control of cellular responses to mechanical cues through YAP/TAZ regulation. J Biol Chem. 2019, 294, 17693-17706.
doi: 10.1074/jbc.REV119.007963 URL |
| 149. |
Du, J.; Chen, X.; Liang, X.; Zhang, G.; Xu, J.; He, L.; Zhan, Q.; Feng, X. Q.; Chien, S.; Yang, C. Integrin activation and internalization on soft ECM as a mechanism of induction of stem cell differentiation by ECM elasticity. Proc Natl Acad Sci U S A. 2011, 108, 9466-9471.
doi: 10.1073/pnas.1106467108 URL |
| 150. |
Coste, B.; Mathur, J.; Schmidt, M.; Earley, T. J.; Ranade, S.; Petrus, M. J.; Dubin, A. E.; Patapoutian, A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010, 330, 55-60.
doi: 10.1126/science.1193270 URL |
| 151. |
Pethő, Z.; Najder, K.; Bulk, E.; Schwab, A. Mechanosensitive ion channels push cancer progression. Cell Calcium. 2019, 80, 79-90.
doi: 10.1016/j.ceca.2019.03.007 URL |
| 152. |
Caliari, S. R.; Vega, S. L.; Kwon, M.; Soulas, E. M.; Burdick, J. A. Dimensionality and spreading influence MSC YAP/TAZ signaling in hydrogel environments. Biomaterials. 2016, 103, 314-323.
doi: 10.1016/j.biomaterials.2016.06.061 URL |
| 153. |
Cameron, A. R.; Frith, J. E.; Gomez, G. A.; Yap, A. S.; Cooper-White, J. J. The effect of time-dependent deformation of viscoelastic hydrogels on myogenic induction and Rac1 activity in mesenchymal stem cells. Biomaterials. 2014, 35, 1857-1868.
doi: 10.1016/j.biomaterials.2013.11.023 URL |
| 154. |
Yim, E. K.; Darling, E. M.; Kulangara, K.; Guilak, F.; Leong, K. W. Nanotopography-induced changes in focal adhesions, cytoskeletal organization, and mechanical properties of human mesenchymal stem cells. Biomaterials. 2010, 31, 1299-1306.
doi: 10.1016/j.biomaterials.2009.10.037 URL |
| 155. |
Mascharak, S.; Benitez, P. L.; Proctor, A. C.; Madl, C. M.; Hu, K. H.; Dewi, R. E.; Butte, M. J.; Heilshorn, S. C. YAP-dependent mechanotransduction is required for proliferation and migration on native-like substrate topography. Biomaterials. 2017, 115, 155-166.
doi: 10.1016/j.biomaterials.2016.11.019 URL |
| 156. |
Cui, Y.; Hameed, F. M.; Yang, B.; Lee, K.; Pan, C. Q.; Park, S.; Sheetz, M. Cyclic stretching of soft substrates induces spreading and growth. Nat Commun. 2015, 6, 6333.
doi: 10.1038/ncomms7333 URL |
| 157. |
Wang, L.; Luo, J. Y.; Li, B.; Tian, X. Y.; Chen, L. J.; Huang, Y.; Liu, J.; Deng, D.; Lau, C. W.; Wan, S.; Ai, D.; Mak, K. K.; Tong, K. K.; Kwan, K. M.; Wang, N.; Chiu, J. J.; Zhu, Y.; Huang, Y. Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature. 2016, 540, 579-582.
doi: 10.1038/nature20602 URL |
| 158. |
Pathak, M. M.; Nourse, J. L.; Tran, T.; Hwe, J.; Arulmoli, J.; Le, D. T.; Bernardis, E.; Flanagan, L. A.; Tombola, F. Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc Natl Acad Sci U S A. 2014, 111, 16148-16153.
doi: 10.1073/pnas.1409802111 URL |
| 159. |
Niepel, M. S.; Ekambaram, B. K.; Schmelzer, C. E. H.; Groth, T. Polyelectrolyte multilayers of poly (l-lysine) and hyaluronic acid on nanostructured surfaces affect stem cell response. Nanoscale. 2019, 11, 2878-2891.
doi: 10.1039/C8NR05529G URL |
| 160. |
Yao, S.; Liu, X.; Yu, S.; Wang, X.; Zhang, S.; Wu, Q.; Sun, X.; Mao, H. Co-effects of matrix low elasticity and aligned topography on stem cell neurogenic differentiation and rapid neurite outgrowth. Nanoscale. 2016, 8, 10252-10265.
doi: 10.1039/C6NR01169A URL |
| 161. |
Frank, V.; Kaufmann, S.; Wright, R.; Horn, P.; Yoshikawa, H. Y.; Wuchter, P.; Madsen, J.; Lewis, A. L.; Armes, S. P.; Ho, A. D.; Tanaka, M. Frequent mechanical stress suppresses proliferation of mesenchymal stem cells from human bone marrow without loss of multipotency. Sci Rep. 2016, 6, 24264.
doi: 10.1038/srep24264 URL |
| 162. |
Tan, S.; Fang, J. Y.; Yang, Z.; Nimni, M. E.; Han, B. The synergetic effect of hydrogel stiffness and growth factor on osteogenic differentiation. Biomaterials. 2014, 35, 5294-5306.
doi: 10.1016/j.biomaterials.2014.02.040 URL |
| 163. |
Grinnell, F.; Ho, C. H. The effect of growth factor environment on fibroblast morphological response to substrate stiffness. Biomaterials. 2013, 34, 965-974.
doi: 10.1016/j.biomaterials.2012.10.036 URL |
| 164. |
Chen, C.; Xie, J.; Deng, L.; Yang, L. Substrate stiffness together with soluble factors affects chondrocyte mechanoresponses. ACS Appl Mater Interfaces. 2014, 6, 16106-16116.
doi: 10.1021/am504135b URL |
| 165. |
Chang, H.; Liu, X. Q.; Hu, M.; Zhang, H.; Li, B. C.; Ren, K. F.; Boudou, T.; Albiges-Rizo, C.; Picart, C.; Ji, J. Substrate stiffness combined with hepatocyte growth factor modulates endothelial cell behavior. Biomacromolecules. 2016, 17, 2767-2776.
doi: 10.1021/acs.biomac.6b00318 URL |
| 166. |
Wang, J.; Tian, L.; Chen, N.; Ramakrishna, S.; Mo, X. The cellular response of nerve cells on poly-L-lysine coated PLGA-MWCNTs aligned nanofibers under electrical stimulation. Mater Sci Eng C Mater Biol Appl. 2018, 91, 715-726.
doi: 10.1016/j.msec.2018.06.025 URL |
| 167. |
Li, Q.; Zhang, B.; Kasoju, N.; Ma, J.; Yang, A.; Cui, Z.; Wang, H.; Ye, H. Differential and interactive effects of substrate topography and chemistry on human mesenchymal stem cell gene expression. Int J Mol Sci. 2018, 19, 2344.
doi: 10.3390/ijms19082344 URL |
| 168. |
Yamamoto, S.; Okada, K.; Sasaki, N.; Chang, A. C.; Yamaguchi, K.; Nakanishi, J. Photoactivatable hydrogel interfaces for resolving the interplay of chemical, mechanical, and geometrical regulation of collective cell migration. Langmuir. 2019, 35, 7459-7468.
doi: 10.1021/acs.langmuir.8b02371 URL |
| 169. |
Li, J.; Kwiatkowska, B.; Lu, H.; Voglstätter, M.; Ueda, E.; Grunze, M.; Sleeman, J.; Levkin, P. A.; Nazarenko, I. Collaborative action of surface chemistry and topography in the regulation of mesenchymal and epithelial markers and the shape of cancer cells. ACS Appl Mater Interfaces. 2016, 8, 28554-28565.
doi: 10.1021/acsami.6b11338 URL |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||