Biomaterials Translational ›› 2021, Vol. 2 ›› Issue (4): 361-375.doi: 10.12336/biomatertransl.2021.04.009
• REVIEW • Previous Articles
Ge Yan, Yuqi Liu, Minghui Xie, Jiawei Shi, Weihua Qiao*( ), Nianguo Dong*(
), Nianguo Dong*( )
)
Received:2021-08-30
Revised:2021-11-26
Accepted:2021-12-03
Online:2021-12-28
Published:2021-12-28
Contact:
Weihua Qiao,Nianguo Dong
E-mail:weihua_qiao@hust.edu.cn;dongnianguo@hotmail.com
About author:Weihua Qiao, weihua_qiao@hust.edu.cn; Nianguo Dong, dongnianguo@hotmail.com.# Author Equally.
Yan, G.; Liu, Y.; Xie, M.;Shi, J.; Qiao, W.; Dong, N. Experimental and computational models for tissue-engineered heart valves: a narrative review Biomater Transl. 2021, 2(4), 361-375.
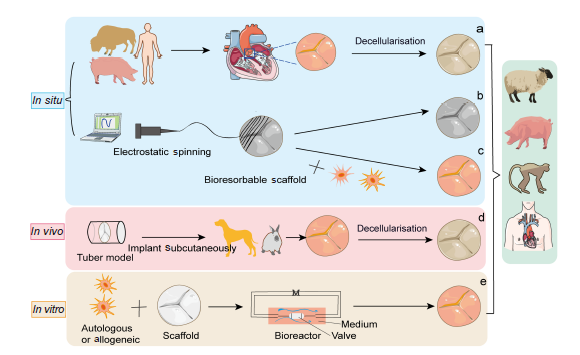
Figure 1. Schematic illustration showing tissue engineered heart valves strategies. (a) In situ, tissue engineered heart valve approaches are derived from decellularised homograft or xenograft scaffolds and can be reshaped by various cells and tissue sources. (b) Bioresorbable polymeric scaffolds can be manufactured by the method of electrostatic spinning and implanted in vivo without cell growth. (c) Bioresorbable polymeric scaffolds are pre-seeded with autologous cells. (d) In vivo, a non-degradable valve scaffold is implanted subcutaneously in an animal to induce the formation of fibrous tissue. (e) In vitro, autologous or allogeneic cells are cultured on a bioresorbable synthetic scaffold in a bioreactor system with a culture medium (M). Created with smart.server.com and ChemDraw.
| Species | TEHV manufacture | Surgical method | Functionality assessment and post-mortem analysis | Reference |
|---|---|---|---|---|
| Small animal model | ||||
| Mouse | Sulfonic polymer hybrid extracellular matrix | Subdermal | Histological and immunohistochemical analysis | |
| Rat | Glut-fixed porcine aortic valve | Subdermal | Histological and quantitative analysis | |
| Electrospinning polycaprolactone scaffold | Subdermal | Scanning electron microscopic and histological analysis | ||
| Allogeneic aortic conduit grafts | End-to-side anastomosis with infrarenal aorta | Histological and immunohistochemical analysis, quantitative real-time polymerase chain reaction | ||
| Rabbit | Poly urethane scaffold | Subdermal | Macroscopic and histological analysis | |
| Large animal model | ||||
| Adult sheep | Poly(glycolic acid) scaffold | Pulmonary valve replacement via transcatheter-based jugular | Intracardiac echocardiography, cardiac magnetic resonance imaging, computed tomography, histological and immunohistochemical analysis | |
| Polyvinylidene fluoride scaffold | Left anterolateral thoracotomy | Inspected for thrombotic deposits, light microscopic analysis, electron microscopic analysis or processed for extracellular matrix assay | ||
| Juvenile sheep (13–17 kg) | Tissue-engineered porcine pulmonary valved conduit | Off-pump cardiopulmonary bypass/left anterolateral thoracotomy | Transthoracic ultrasound echocardiography, mortality, operating time | |
| Foetal ovine | PCL scaffold | Transcatheter pulmonary valve replacement | Ultrasound (pulmonary valve annulus), foetal outcome | |
| Yorkshire pigs (80 kg) | AZ31 magnesium alloy biodegradable stent frame scaffold-based polycarbonate urethane urea TEHV | Cardiopulmonary bypass in the pulmonary position | Echocardiography, biaxial mechanical testing, scanning electron microscopy | |
| Porcine | Decellularised porcine pulmonary heart valves | Median sternotomy or limited lateral thoracotomy, then injection of TEHV | Echocardiographic examination, invasive pressure, angiographic measurements | |
| Vietnamese pigs | Decellularised porcine aortic valves conduit | Xypho‐jugular incision, followed by median longitudinal sternotomy | Echocardiography, macroscopic inspection, metabolic labelling | |
| Canine | Decellularised porcine pulmonary heart valves | Left anterolateral thoracotomy | Echocardiography, histology (haematoxylin and eosin, Masson) and immunohistochemistry, transmission electron microscopy | |
| Non-human primate (Chacma Baboon) | Poly(glycolic acid) scaffold | A mini-sternotomy using an antegrade transapical approach | Transesophageal echocardiography, epicardial echocardiography, Pulmonary artery pressure measurement, biochemical analysis | |
Table 1 Animal models used in TEHV studies.
| Species | TEHV manufacture | Surgical method | Functionality assessment and post-mortem analysis | Reference |
|---|---|---|---|---|
| Small animal model | ||||
| Mouse | Sulfonic polymer hybrid extracellular matrix | Subdermal | Histological and immunohistochemical analysis | |
| Rat | Glut-fixed porcine aortic valve | Subdermal | Histological and quantitative analysis | |
| Electrospinning polycaprolactone scaffold | Subdermal | Scanning electron microscopic and histological analysis | ||
| Allogeneic aortic conduit grafts | End-to-side anastomosis with infrarenal aorta | Histological and immunohistochemical analysis, quantitative real-time polymerase chain reaction | ||
| Rabbit | Poly urethane scaffold | Subdermal | Macroscopic and histological analysis | |
| Large animal model | ||||
| Adult sheep | Poly(glycolic acid) scaffold | Pulmonary valve replacement via transcatheter-based jugular | Intracardiac echocardiography, cardiac magnetic resonance imaging, computed tomography, histological and immunohistochemical analysis | |
| Polyvinylidene fluoride scaffold | Left anterolateral thoracotomy | Inspected for thrombotic deposits, light microscopic analysis, electron microscopic analysis or processed for extracellular matrix assay | ||
| Juvenile sheep (13–17 kg) | Tissue-engineered porcine pulmonary valved conduit | Off-pump cardiopulmonary bypass/left anterolateral thoracotomy | Transthoracic ultrasound echocardiography, mortality, operating time | |
| Foetal ovine | PCL scaffold | Transcatheter pulmonary valve replacement | Ultrasound (pulmonary valve annulus), foetal outcome | |
| Yorkshire pigs (80 kg) | AZ31 magnesium alloy biodegradable stent frame scaffold-based polycarbonate urethane urea TEHV | Cardiopulmonary bypass in the pulmonary position | Echocardiography, biaxial mechanical testing, scanning electron microscopy | |
| Porcine | Decellularised porcine pulmonary heart valves | Median sternotomy or limited lateral thoracotomy, then injection of TEHV | Echocardiographic examination, invasive pressure, angiographic measurements | |
| Vietnamese pigs | Decellularised porcine aortic valves conduit | Xypho‐jugular incision, followed by median longitudinal sternotomy | Echocardiography, macroscopic inspection, metabolic labelling | |
| Canine | Decellularised porcine pulmonary heart valves | Left anterolateral thoracotomy | Echocardiography, histology (haematoxylin and eosin, Masson) and immunohistochemistry, transmission electron microscopy | |
| Non-human primate (Chacma Baboon) | Poly(glycolic acid) scaffold | A mini-sternotomy using an antegrade transapical approach | Transesophageal echocardiography, epicardial echocardiography, Pulmonary artery pressure measurement, biochemical analysis | |
| Species & bioreactors | Advantages | Disadvantages |
|---|---|---|
| Small animal model | ||
| Mouse | Low cost of maintenance, ease of gene-editing and surgical manipulation, multiple types of antibodies | Not-suitable for in situ studies for small size and mismatched anatomy and physiology |
| Rat | Low cost of maintenance, ease of surgical approach, access for implantation into the systemic circulation | Not-suitable for in situ studies |
| Rabbit | Low cost of upkeep, larger size for larger graft implantation | Especially suitable for ectopic studies (subcutaneous implantation) |
| Large animal model | ||
| Sheep | The golden standard for translational studies, the similarity of body size to human, easy access for transcatheter replacement of tissue-engineered heart valves, suitable growing speed for growing model | Higher cost of purchase and upkeep, special facilities required for housing |
| Pig | Resemblance to humans in terms of cardiovascular anatomy and physiology, access to transgenic models | Higher cost of purchase and upkeep, special facilities required for housing, higher risk of post-operation infection, the possibility of chronic arterial occlusion and cardiac death after CPB, unsuitable growing speed compared with sheep |
| Dog | Docile character, thin skin for implantation of catheters and convenient imaging, lower risk of post-operative infection | Higher cost of purchase and upkeep, special facilities required for housing, difficulty in getting approval |
| Non-human primate | The best model for translational clinical research for anatomical, physiological, genetic, and immune similarity | The highest cost of purchase and upkeep, special facilities required for housing and social needs of primates, special equipment, and training required for surgical approach |
| Bioreactors | ||
| Pulse-flow bioreactors | The anatomy is more similar to the physiology | Generate complex and ill-defined mechanical conditioning, which cannot be readily controlled |
| Single mechanical stimulus bioreactor | The type and size of the mechanical stimulation can be finely adjusted | Only single stimulation can be made, a good cultural environment is needed and high cost |
| Multi-mechanical stimulus bioreactor | Know the synergistic effect of different combinations of mechanical stimuli on cells and tissues | May exist mutual interference that led to inconsistent results |
| Computer-regulated bioreactor | Predictable, easy to understand hydrodynamic parameters | Only mechanical simulations, not chemical and biological ones |
Table 2 Advantages and disadvantages of animal models and bioreactors used in tissue-engineered heart valve studies.
| Species & bioreactors | Advantages | Disadvantages |
|---|---|---|
| Small animal model | ||
| Mouse | Low cost of maintenance, ease of gene-editing and surgical manipulation, multiple types of antibodies | Not-suitable for in situ studies for small size and mismatched anatomy and physiology |
| Rat | Low cost of maintenance, ease of surgical approach, access for implantation into the systemic circulation | Not-suitable for in situ studies |
| Rabbit | Low cost of upkeep, larger size for larger graft implantation | Especially suitable for ectopic studies (subcutaneous implantation) |
| Large animal model | ||
| Sheep | The golden standard for translational studies, the similarity of body size to human, easy access for transcatheter replacement of tissue-engineered heart valves, suitable growing speed for growing model | Higher cost of purchase and upkeep, special facilities required for housing |
| Pig | Resemblance to humans in terms of cardiovascular anatomy and physiology, access to transgenic models | Higher cost of purchase and upkeep, special facilities required for housing, higher risk of post-operation infection, the possibility of chronic arterial occlusion and cardiac death after CPB, unsuitable growing speed compared with sheep |
| Dog | Docile character, thin skin for implantation of catheters and convenient imaging, lower risk of post-operative infection | Higher cost of purchase and upkeep, special facilities required for housing, difficulty in getting approval |
| Non-human primate | The best model for translational clinical research for anatomical, physiological, genetic, and immune similarity | The highest cost of purchase and upkeep, special facilities required for housing and social needs of primates, special equipment, and training required for surgical approach |
| Bioreactors | ||
| Pulse-flow bioreactors | The anatomy is more similar to the physiology | Generate complex and ill-defined mechanical conditioning, which cannot be readily controlled |
| Single mechanical stimulus bioreactor | The type and size of the mechanical stimulation can be finely adjusted | Only single stimulation can be made, a good cultural environment is needed and high cost |
| Multi-mechanical stimulus bioreactor | Know the synergistic effect of different combinations of mechanical stimuli on cells and tissues | May exist mutual interference that led to inconsistent results |
| Computer-regulated bioreactor | Predictable, easy to understand hydrodynamic parameters | Only mechanical simulations, not chemical and biological ones |
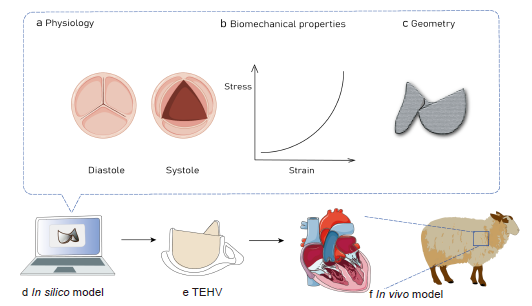
Figure 3. Schematic representation showing that the computational modelling mimics the physiology (a), biomechanical properties (b) and geometry (c) of heart valves in silico (d) to clarify the role of these parameters in the development of TEHVs to enable better use of in vivo models (f). TEHV: tissue-engineered heart valve. Created with Biorender.com and smart.server.com and ChemDraw.
| Model | Application | Objective | Year | Reference |
|---|---|---|---|---|
| Computational modelling | Tissue-engineered heart valve | Guiding tissue-engineered heart valve design for long-term in vivo performance in a translational sheep model | 2018 | |
| Tissue-engineered vascular graft | Identifying optimal design parameters to save development time and costs while improving clinical outcomes | 2019 | ||
| Bioprosthetic heart valve | Investigating the impacts of bovine and porcine pericardium tissues with different thicknesses and tissue mechanical properties in bioprosthetic heart valve applications | 2019 2017 2016 | ||
| Tissue-engineered heart valve | Integrating computational simulation into tissue-engineering approaches can lead to more successful and predictable clinical outcomes | 2018 | ||
| Biomechanical model | Aortic stenosis | Providing the results of the numerical simulation of the valve function | 2020 | |
| Finite element models | Congenital bicuspid aortic valve | Quantifying aortic valve and root biomechanical alterations associated with bicuspid geometry | 2010 | |
| Calcific aortic valve disease | Studying the calcification progression in aortic valves | 2017 | ||
| Bioprosthetic heart valve | Comparing tensile properties of xenopericardium to choose tissue more appropriate for bioprosthetic heart valve tissue | 2020 | ||
| Three-dimensional bioprinting | Heart valve | Using computational fluid dynamics, digital image processing, artificial intelligence, and continuum mechanics during their optimisation and implementation to mimic the original and understand valvular problems | 2019 2018 | |
| Geometric model | Functional tri-leaflet aortic valves | Establishing a list of geometric guidelines to ensure safe operation of the valve during the cardiac cycle | 2006 | |
| Numeric model | Aortic root | Studying the correlation between intraoperative effective height and diastolic coaptation | 2013 | |
| Neural network material model | Simulation of the aortic heart valve | Providing an efficient computational analysis framework with increased physical and functional realism for the simulation of native and replacement tri-leaflet heart valves | 2021 2020 |
Table 3 Advantages and disadvantages of animal models and bioreactors used in tissue-engineered heart valve studies.
| Model | Application | Objective | Year | Reference |
|---|---|---|---|---|
| Computational modelling | Tissue-engineered heart valve | Guiding tissue-engineered heart valve design for long-term in vivo performance in a translational sheep model | 2018 | |
| Tissue-engineered vascular graft | Identifying optimal design parameters to save development time and costs while improving clinical outcomes | 2019 | ||
| Bioprosthetic heart valve | Investigating the impacts of bovine and porcine pericardium tissues with different thicknesses and tissue mechanical properties in bioprosthetic heart valve applications | 2019 2017 2016 | ||
| Tissue-engineered heart valve | Integrating computational simulation into tissue-engineering approaches can lead to more successful and predictable clinical outcomes | 2018 | ||
| Biomechanical model | Aortic stenosis | Providing the results of the numerical simulation of the valve function | 2020 | |
| Finite element models | Congenital bicuspid aortic valve | Quantifying aortic valve and root biomechanical alterations associated with bicuspid geometry | 2010 | |
| Calcific aortic valve disease | Studying the calcification progression in aortic valves | 2017 | ||
| Bioprosthetic heart valve | Comparing tensile properties of xenopericardium to choose tissue more appropriate for bioprosthetic heart valve tissue | 2020 | ||
| Three-dimensional bioprinting | Heart valve | Using computational fluid dynamics, digital image processing, artificial intelligence, and continuum mechanics during their optimisation and implementation to mimic the original and understand valvular problems | 2019 2018 | |
| Geometric model | Functional tri-leaflet aortic valves | Establishing a list of geometric guidelines to ensure safe operation of the valve during the cardiac cycle | 2006 | |
| Numeric model | Aortic root | Studying the correlation between intraoperative effective height and diastolic coaptation | 2013 | |
| Neural network material model | Simulation of the aortic heart valve | Providing an efficient computational analysis framework with increased physical and functional realism for the simulation of native and replacement tri-leaflet heart valves | 2021 2020 |
| 1. |
Fioretta, E. S.; Motta, S. E.; Lintas, V.; Loerakker, S.; Parker, K. K.; Baaijens, F. P. T.; Falk, V.; Hoerstrup, S. P.; Emmert, M. Y. Next-generation tissue-engineered heart valves with repair, remodelling and regeneration capacity. Nat Rev Cardiol. 2021, 18, 92-116.
doi: 10.1038/s41569-020-0422-8 URL |
| 2. |
Eoh, J. H.; Shen, N.; Burke, J. A.; Hinderer, S.; Xia, Z.; Schenke-Layland, K.; Gerecht, S. Enhanced elastin synthesis and maturation in human vascular smooth muscle tissue derived from induced-pluripotent stem cells. Acta Biomater. 2017, 52, 49-59.
doi: 10.1016/j.actbio.2017.01.083 URL |
| 3. |
Henaine, R.; Roubertie, F.; Vergnat, M.; Ninet, J. Valve replacement in children: a challenge for a whole life. Arch Cardiovasc Dis. 2012, 105, 517-528.
doi: 10.1016/j.acvd.2012.02.013 URL |
| 4. |
Kaneko, T.; Cohn, L. H.; Aranki, S. F. Tissue valve is the preferred option for patients aged 60 and older. Circulation. 2013, 128, 1365-1371.
doi: 10.1161/CIRCULATIONAHA.113.002584 URL |
| 5. |
Emmert, M. Y.; Hoerstrup, S. P. Challenges in translating tissue engineered heart valves into clinical practice. Eur Heart J. 2017, 38, 619-621.
doi: 10.1093/eurheartj/ehx075 URL |
| 6. |
Zhang, B. L.; Bianco, R. W.; Schoen, F. J. Preclinical assessment of cardiac valve substitutes: current status and considerations for engineered tissue heart valves. Front Cardiovasc Med. 2019, 6, 72.
doi: 10.3389/fcvm.2019.00072 URL |
| 7. |
Chester, A. H.; Grande-Allen, K. J. Which biological properties of heart valves are relevant to tissue engineering? Front Cardiovasc Med. 2020, 7, 63.
doi: 10.3389/fcvm.2020.00063 URL |
| 8. |
Quint, C.; Kondo, Y.; Manson, R. J.; Lawson, J. H.; Dardik, A.; Niklason, L. E. Decellularized tissue-engineered blood vessel as an arterial conduit. Proc Natl Acad Sci U S A. 2011, 108, 9214-9219.
doi: 10.1073/pnas.1019506108 URL |
| 9. | Dahl, S. L.; Kypson, A. P.; Lawson, J. H.; Blum, J. L.; Strader, J. T.; Li, Y.; Manson, R. J.; Tente, W. E.; DiBernardo, L.; Hensley, M. T.; Carter, R.; Williams, T. P.; Prichard, H. L.; Dey, M. S.; Begelman, K. G.; Niklason, L. E. Readily available tissue-engineered vascular grafts. Sci Transl Med. 2011, 3, 68ra69. |
| 10. |
Majid, Q. A.; Fricker, A. T. R.; Gregory, D. A.; Davidenko, N.; Hernandez Cruz, O.; Jabbour, R. J.; Owen, T. J.; Basnett, P.; Lukasiewicz, B.; Stevens, M.; Best, S.; Cameron, R.; Sinha, S.; Harding, S. E.; Roy, I. Natural biomaterials for cardiac tissue engineering: a highly biocompatible solution. Front Cardiovasc Med. 2020, 7, 554597.
doi: 10.3389/fcvm.2020.554597 URL |
| 11. |
Xue, Y.; Sant, V.; Phillippi, J.; Sant, S. Biodegradable and biomimetic elastomeric scaffolds for tissue-engineered heart valves. Acta Biomater. 2017, 48, 2-19.
doi: 10.1016/j.actbio.2016.10.032 URL |
| 12. |
Mirani, B.; Parvin Nejad, S.; Simmons, C. A. Recent progress toward clinical translation of tissue-engineered heart valves. Can J Cardiol. 2021, 37, 1064-1077.
doi: 10.1016/j.cjca.2021.03.022 URL |
| 13. |
Hayashida, K.; Kanda, K.; Yaku, H.; Ando, J.; Nakayama, Y. Development of an in vivo tissue-engineered, autologous heart valve (the biovalve): preparation of a prototype model. J Thorac Cardiovasc Surg. 2007, 134, 152-159.
doi: 10.1016/j.jtcvs.2007.01.087 URL |
| 14. | Yamanami, M.; Yahata, Y.; Uechi, M.; Fujiwara, M.; Ishibashi-Ueda, H.; Kanda, K.; Watanabe, T.; Tajikawa, T.; Ohba, K.; Yaku, H.; Nakayama, Y. Development of a completely autologous valved conduit with the sinus of Valsalva using in-body tissue architecture technology: a pilot study in pulmonary valve replacement in a beagle model. Circulation. 2010, 122, S100-106. |
| 15. |
Motta, S. E.; Lintas, V.; Fioretta, E. S.; Dijkman, P. E.; Putti, M.; Caliskan, E.; Rodriguez Cetina Biefer, H.; Lipiski, M.; Sauer, M.; Cesarovic, N.; Hoerstrup, S. P.; Emmert, M. Y. Human cell-derived tissue-engineered heart valve with integrated Valsalva sinuses: towards native-like transcatheter pulmonary valve replacements. NPJ Regen Med. 2019, 4, 14.
doi: 10.1038/s41536-019-0077-4 URL |
| 16. |
Schmitt, B.; Spriestersbach, H.; D, O. H. I.; Radtke, T.; Bartosch, M.; Peters, H.; Sigler, M.; Frese, L.; Dijkman, P. E.; Baaijens, F. P.; Hoerstrup, S. P.; Berger, F. Percutaneous pulmonary valve replacement using completely tissue-engineered off-the-shelf heart valves: six-month in vivo functionality and matrix remodelling in sheep. EuroIntervention. 2016, 12, 62-70.
doi: 10.4244/EIJV12I1A12 URL |
| 17. |
Capulli, A. K.; Emmert, M. Y.; Pasqualini, F. S.; Kehl, D.; Caliskan, E.; Lind, J. U.; Sheehy, S. P.; Park, S. J.; Ahn, S.; Weber, B.; Goss, J. A.; Hoerstrup, S. P.; Parker, K. K. JetValve: Rapid manufacturing of biohybrid scaffolds for biomimetic heart valve replacement. Biomaterials. 2017, 133, 229-241.
doi: 10.1016/j.biomaterials.2017.04.033 URL |
| 18. |
Guo, G.; Jin, L.; Wu, B.; He, H.; Yang, F.; Xu, L.; Lei, Y.; Wang, Y. A method for simultaneously crosslinking and functionalizing extracellular matrix-based biomaterials as bioprosthetic heart valves with enhanced endothelialization and reduced inflammation. Acta Biomater. 2021, 119, 89-100.
doi: 10.1016/j.actbio.2020.10.029 URL |
| 19. |
Lovekamp, J. J.; Simionescu, D. T.; Mercuri, J. J.; Zubiate, B.; Sacks, M. S.; Vyavahare, N. R. Stability and function of glycosaminoglycans in porcine bioprosthetic heart valves. Biomaterials. 2006, 27, 1507-1518.
doi: 10.1016/j.biomaterials.2005.08.003 URL |
| 20. |
Jana, S.; Franchi, F.; Lerman, A. Trilayered tissue structure with leaflet-like orientations developed through in vivo tissue engineering. Biomed Mater. 2019, 15, 015004.
doi: 10.1088/1748-605X/ab52e2 URL |
| 21. |
Soares, A. L.; Oomens, C. W.; Baaijens, F. P. A computational model to describe the collagen orientation in statically cultured engineered tissues. Comput Methods Biomech Biomed Engin. 2014, 17, 251-262.
doi: 10.1080/10255842.2012.680192 URL |
| 22. | Emmert, M. Y.; Schmitt, B. A.; Loerakker, S.; Sanders, B.; Spriestersbach, H.; Fioretta, E. S.; Bruder, L.; Brakmann, K.; Motta, S. E.; Lintas, V.; Dijkman, P. E.; Frese, L.; Berger, F.; Baaijens, F. P. T.; Hoerstrup, S. P. Computational modeling guides tissue-engineered heart valve design for long-term in vivo performance in a translational sheep model. Sci Transl Med. 2018, 10, eaan4587. |
| 23. |
Flanagan, T. C.; Sachweh, J. S.; Frese, J.; Schnöring, H.; Gronloh, N.; Koch, S.; Tolba, R. H.; Schmitz-Rode, T.; Jockenhoevel, S. In vivo remodeling and structural characterization of fibrin-based tissue-engineered heart valves in the adult sheep model. Tissue Eng Part A. 2009, 15, 2965-2976.
doi: 10.1089/ten.tea.2009.0018 URL |
| 24. | Wu, H.; Xu, Z. W.; Liu, X. M.; Gong, D.; Wan, J. Y.; Xu, X. F.; Zhou, Z. F.; Li, W. B. An in vivo model of in situ implantation using pulmonary valved conduit in large animals under off-pump condition. Chin Med J (Engl). 2013, 126, 4540-4544. |
| 25. | Zakko, J.; Blum, K. M.; Drews, J. D.; Wu, Y. L.; Hatoum, H.; Russell, M.; Gooden, S.; Heitkemper, M.; Conroy, O.; Kelly, J.; Carey, S.; Sacks, M.; Texter, K.; Ragsdale, E.; Strainic, J.; Bocks, M.; Wang, Y.; Dasi, L. P.; Armstrong, A. K.; Breuer, C. Development of tissue engineered heart valves for percutaneous transcatheter delivery in a fetal ovine model. JACC Basic Transl Sci. 2020, 5, 815-828. |
| 26. |
Coyan, G. N.; D’Amore, A.; Matsumura, Y.; Pedersen, D. D.; Luketich, S. K.; Shanov, V.; Katz, W. E.; David, T. E.; Wagner, W. R.; Badhwar, V. In vivo functional assessment of a novel degradable metal and elastomeric scaffold-based tissue engineered heart valve. J Thorac Cardiovasc Surg. 2019, 157, 1809-1816.
doi: 10.1016/j.jtcvs.2018.09.128 URL |
| 27. |
Schlegel, F.; Salameh, A.; Oelmann, K.; Halling, M.; Dhein, S.; Mohr, F. W.; Dohmen, P. M. Injectable tissue engineered pulmonary heart valve implantation into the pig model: A feasibility study. Med Sci Monit Basic Res. 2015, 21, 135-140.
doi: 10.12659/MSMBR.894838 URL |
| 28. |
Bianco, R. W.; St Cyr, J. A.; Schneider, J. R.; Rasmussen, T. M.; Clack, R. M.; Shim, H. S.; Sandstad, J.; Rysavy, J.; Foker, J. E. Canine model for long-term evaluation of prosthetic mitral valves. J Surg Res. 1986, 41, 134-140.
doi: 10.1016/0022-4804(86)90018-1 URL |
| 29. |
Ye, X.; Bhushan, B.; Zhou, M.; Lei, W. The surface microstructure of cusps and leaflets in rabbit and mouse heart valves. Beilstein J Nanotechnol. 2014, 5, 622-629.
doi: 10.3762/bjnano.5.73 URL |
| 30. |
Weber, B.; Dijkman, P. E.; Scherman, J.; Sanders, B.; Emmert, M. Y.; Grünenfelder, J.; Verbeek, R.; Bracher, M.; Black, M.; Franz, T.; Kortsmit, J.; Modregger, P.; Peter, S.; Stampanoni, M.; Robert, J.; Kehl, D.; van Doeselaar, M.; Schweiger, M.; Brokopp, C. E.; Wälchli, T.; Falk, V.; Zilla, P.; Driessen-Mol, A.; Baaijens, F. P.; Hoerstrup, S. P. Off-the-shelf human decellularized tissue-engineered heart valves in a non-human primate model. Biomaterials. 2013, 34, 7269-7280.
doi: 10.1016/j.biomaterials.2013.04.059 URL |
| 31. |
Smith, M. R.; Wood, W. B., Jr. An experimental analysis of the curative action of penicillin in acute bacterial infections. III. The effect of suppuration upon the antibacterial action of the drug. J Exp Med. 1956, 103, 509-522.
doi: 10.1084/jem.103.4.509 URL |
| 32. |
Kim, M. S.; Jeong, S.; Lim, H. G.; Kim, Y. J. Differences in xenoreactive immune response and patterns of calcification of porcine and bovine tissues in α-Gal knock-out and wild-type mouse implantation models. Eur J Cardiothorac Surg. 2015, 48, 392-399.
doi: 10.1093/ejcts/ezu501 URL |
| 33. |
Christ, T.; Dohmen, P. M.; Holinski, S.; Schönau, M.; Heinze, G.; Konertz, W. Suitability of the rat subdermal model for tissue engineering of heart valves. Med Sci Monit Basic Res. 2014, 20, 194-199.
doi: 10.12659/MSMBR.893088 URL |
| 34. |
Assmann, A.; Delfs, C.; Munakata, H.; Schiffer, F.; Horstkötter, K.; Huynh, K.; Barth, M.; Stoldt, V. R.; Kamiya, H.; Boeken, U.; Lichtenberg, A.; Akhyari, P. Acceleration of autologous in vivo recellularization of decellularized aortic conduits by fibronectin surface coating. Biomaterials. 2013, 34, 6015-6026.
doi: 10.1016/j.biomaterials.2013.04.037 URL |
| 35. |
Rashid, S. T.; Salacinski, H. J.; Hamilton, G.; Seifalian, A. M. The use of animal models in developing the discipline of cardiovascular tissue engineering: a review. Biomaterials. 2004, 25, 1627-1637.
doi: 10.1016/S0142-9612(03)00522-2 URL |
| 36. |
Driessen-Mol, A.; Emmert, M. Y.; Dijkman, P. E.; Frese, L.; Sanders, B.; Weber, B.; Cesarovic, N.; Sidler, M.; Leenders, J.; Jenni, R.; Grünenfelder, J.; Falk, V.; Baaijens, F. P. T.; Hoerstrup, S. P. Transcatheter implantation of homologous “off-the-shelf” tissue-engineered heart valves with self-repair capacity: long-term functionality and rapid in vivo remodeling in sheep. J Am Coll Cardiol. 2014, 63, 1320-1329.
doi: 10.1016/j.jacc.2013.09.082 URL |
| 37. | Shinoka, T.; Ma, P. X.; Shum-Tim, D.; Breuer, C. K.; Cusick, R. A.; Zund, G.; Langer, R.; Vacanti, J. P.; Mayer, J. E., Jr. Tissue-engineered heart valves. Autologous valve leaflet replacement study in a lamb model. Circulation. 1996, 94, II164-168. |
| 38. |
Schroeder, F.; Polzer, S.; Slažanský, M.; Man, V.; Skácel, P. Predictive capabilities of various constitutive models for arterial tissue. J Mech Behav Biomed Mater. 2018, 78, 369-380.
doi: 10.1016/j.jmbbm.2017.11.035 URL |
| 39. |
Swindle, M. M.; Makin, A.; Herron, A. J.; Clubb, F. J., Jr.; Frazier, K. S. Swine as models in biomedical research and toxicology testing. Vet Pathol. 2012, 49, 344-356.
doi: 10.1177/0300985811402846 URL |
| 40. | Grehan, J. F.; Hilbert, S. L.; Ferrans, V. J.; Droel, J. S.; Salerno, C. T.; Bianco, R. W. Development and evaluation of a swine model to assess the preclinical safety of mechanical heart valves. J Heart Valve Dis. 2000, 9, 710-719; discussion 719-720. |
| 41. |
Canty, J. M., Jr.; Suzuki, G.; Banas, M. D.; Verheyen, F.; Borgers, M.; Fallavollita, J. A. Hibernating myocardium: chronically adapted to ischemia but vulnerable to sudden death. Circ Res. 2004, 94, 1142-1149.
doi: 10.1161/01.RES.0000125628.57672.CF URL |
| 42. |
Gallo, M.; Poser, H.; Bottio, T.; Bonetti, A.; Franci, P.; Naso, F.; Buratto, E.; Zanella, F.; Perona, G.; Dal Lin, C.; Bianco, R.; Spina, M.; Busetto, R.; Marchini, M.; Ortolani, F.; Iop, L.; Gerosa, G. The Vietnamese pig as a translational animal model to evaluate tissue engineered heart valves: promising early experience. Int J Artif Organs. 2017, 40, 142-149.
doi: 10.5301/ijao.5000568 URL |
| 43. |
Gallo, M.; Bianco, R.; Bottio, T.; Naso, F.; Franci, P.; Zanella, F.; Perona, G.; Busetto, R.; Spina, M.; Gandaglia, A.; Gerosa, G. Tissue-engineered heart valves: intra-operative protocol. J Cardiovasc Transl Res. 2013, 6, 660-661.
doi: 10.1007/s12265-013-9480-1 URL |
| 44. |
Gallo, M.; Naso, F.; Poser, H.; Rossi, A.; Franci, P.; Bianco, R.; Micciolo, M.; Zanella, F.; Cucchini, U.; Aresu, L.; Buratto, E.; Busetto, R.; Spina, M.; Gandaglia, A.; Gerosa, G. Physiological performance of a detergent decellularized heart valve implanted for 15 months in Vietnamese pigs: surgical procedure, follow-up, and explant inspection. Artif Organs. 2012, 36, E138-150.
doi: 10.1111/aor.2012.36.issue-6 URL |
| 45. |
Boudjemline, Y.; Agnoletti, G.; Bonnet, D.; Behr, L.; Borenstein, N.; Sidi, D.; Bonhoeffer, P. Steps toward the percutaneous replacement of atrioventricular valves an experimental study. J Am Coll Cardiol. 2005, 46, 360-365.
doi: 10.1016/j.jacc.2005.01.063 URL |
| 46. |
Dewey, T. M.; Walther, T.; Doss, M.; Brown, D.; Ryan, W. H.; Svensson, L.; Mihaljevic, T.; Hambrecht, R.; Schuler, G.; Wimmer-Greinecker, G.; Mohr, F. W.; Mack, M. J. Transapical aortic valve implantation: an animal feasibility study. Ann Thorac Surg. 2006, 82, 110-116.
doi: 10.1016/j.athoracsur.2006.02.035 URL |
| 47. |
Lutter, G.; Kuklinski, D.; Berg, G.; Von Samson, P.; Martin, J.; Handke, M.; Uhrmeister, P.; Beyersdorf, F. Percutaneous aortic valve replacement: an experimental study. I. Studies on implantation. J Thorac Cardiovasc Surg. 2002, 123, 768-776.
doi: 10.1067/mtc.2002.121157 URL |
| 48. |
Walther, T.; Dewey, T.; Wimmer-Greinecker, G.; Doss, M.; Hambrecht, R.; Schuler, G.; Mohr, F. W.; Mack, M. Transapical approach for sutureless stent-fixed aortic valve implantation: experimental results. Eur J Cardiothorac Surg. 2006, 29, 703-708.
doi: 10.1016/j.ejcts.2006.01.062 URL |
| 49. |
Kokozidou, M.; Katsargyris, A.; Verhoeven, E. L. G.; Schulze-Tanzil, G. Vascular access animal models used in research. Ann Anat. 2019, 225, 65-75.
doi: 10.1016/j.aanat.2019.06.002 URL |
| 50. | Camacho, P.; Fan, H.; Liu, Z.; He, J. Q. Large mammalian animal models of heart disease. J Cardiovasc Dev Dis. 2016, 3, 30. |
| 51. |
Iwai, S.; Torikai, K.; Coppin, C. M.; Sawa, Y. Minimally immunogenic decellularized porcine valve provides in situ recellularization as a stentless bioprosthetic valve. J Artif Organs. 2007, 10, 29-35.
doi: 10.1007/s10047-006-0360-1 URL |
| 52. |
Yang, H.; Shao, N.; Holmström, A.; Zhao, X.; Chour, T.; Chen, H.; Itzhaki, I.; Wu, H.; Ameen, M.; Cunningham, N. J.; Tu, C.; Zhao, M. T.; Tarantal, A. F.; Abilez, O. J.; Wu, J. C. Transcriptome analysis of non human primate-induced pluripotent stem cell-derived cardiomyocytes in 2D monolayer culture vs. 3D engineered heart tissue. Cardiovasc Res. 2021, 117, 2125-2136.
doi: 10.1093/cvr/cvaa281 URL |
| 53. |
Rahman, M. A.; Robert-Guroff, M. Accelerating HIV vaccine development using non-human primate models. Expert Rev Vaccines. 2019, 18, 61-73.
doi: 10.1080/14760584.2019.1557521 URL |
| 54. |
Brok, H. P.; Bauer, J.; Jonker, M.; Blezer, E.; Amor, S.; Bontrop, R. E.; Laman, J. D.; t Hart, B. A. Non-human primate models of multiple sclerosis. Immunol Rev. 2001, 183, 173-185.
doi: 10.1034/j.1600-065x.2001.1830114.x URL |
| 55. |
Lu, T.; Yang, B.; Wang, R.; Qin, C. Xenotransplantation: current status in preclinical research. Front Immunol. 2019, 10, 3060.
doi: 10.3389/fimmu.2019.03060 URL |
| 56. |
Anderson, J. M.; Rodriguez, A.; Chang, D. T. Foreign body reaction to biomaterials. Semin Immunol. 2008, 20, 86-100.
doi: 10.1016/j.smim.2007.11.004 URL |
| 57. |
Raasch, M.; Rennert, K.; Jahn, T.; Peters, S.; Henkel, T.; Huber, O.; Schulz, I.; Becker, H.; Lorkowski, S.; Funke, H.; Mosig, A. Microfluidically supported biochip design for culture of endothelial cell layers with improved perfusion conditions. Biofabrication. 2015, 7, 015013.
doi: 10.1088/1758-5090/7/1/015013 URL |
| 58. |
Rath, S.; Salinas, M.; Villegas, A. G.; Ramaswamy, S. Differentiation and distribution of marrow stem cells in flex-flow environments demonstrate support of the valvular phenotype. PLoS One. 2015, 10, e0141802.
doi: 10.1371/journal.pone.0141802 URL |
| 59. |
Flanagan, T. C.; Cornelissen, C.; Koch, S.; Tschoeke, B.; Sachweh, J. S.; Schmitz-Rode, T.; Jockenhoevel, S. The in vitro development of autologous fibrin-based tissue-engineered heart valves through optimised dynamic conditioning. Biomaterials. 2007, 28, 3388-3397.
doi: 10.1016/j.biomaterials.2007.04.012 URL |
| 60. |
Deb, N.; Ali, M. S.; Mathews, A.; Chang, Y. W.; Lacerda, C. M. Shear type and magnitude affect aortic valve endothelial cell morphology, orientation, and differentiation. Exp Biol Med (Maywood). 2021, 246, 2278-2289.
doi: 10.1177/15353702211023359 URL |
| 61. |
Li, A.; Tan, L.; Zhang, S.; Tao, J.; Wang, Z.; Wei, D. Low shear stress-induced endothelial mesenchymal transformation via the down-regulation of TET2. Biochem Biophys Res Commun. 2021, 545, 20-26.
doi: 10.1016/j.bbrc.2021.01.062 URL |
| 62. |
Bazan, O.; Simbara, M. M. O.; Ortiz, J. P.; Malmonge, S. M.; Andrade, A.; Yanagihara, J. I. In vitro hydrodynamic evaluation of a scaffold for heart valve tissue engineering. Artif Organs. 2019, 43, 195-198.
doi: 10.1111/aor.2019.43.issue-2 URL |
| 63. |
Tefft, B. J.; Choe, J. A.; Young, M. D.; Hennessy, R. S.; Morse, D. W.; Bouchard, J. A.; Hedberg, H. J.; Consiglio, J. F.; Dragomir-Daescu, D.; Simari, R. D.; Lerman, A. Cardiac valve bioreactor for physiological conditioning and hydrodynamic performance assessment. Cardiovasc Eng Technol. 2019, 10, 80-94.
doi: 10.1007/s13239-018-00382-2 URL |
| 64. |
Kim, J.; Lee, Y.; Choi, S.; Ha, H. Pulsatile flow pump based on an iterative controlled piston pump actuator as an in-vitro cardiovascular flow model. Med Eng Phys. 2020, 77, 118-124.
doi: 10.1016/j.medengphy.2019.10.020 URL |
| 65. |
Qian, T.; Gil, D. A.; Contreras Guzman, E.; Gastfriend, B. D.; Tweed, K. E.; Palecek, S. P.; Skala, M. C. Adaptable pulsatile flow generated from stem cell-derived cardiomyocytes using quantitative imaging-based signal transduction. Lab Chip. 2020, 20, 3744-3756.
doi: 10.1039/D0LC00546K URL |
| 66. |
Posmantur, R.; Hayes, R. L.; Dixon, C. E.; Taft, W. C. Neurofilament 68 and neurofilament 200 protein levels decrease after traumatic brain injury. J Neurotrauma. 1994, 11, 533-545.
doi: 10.1089/neu.1994.11.533 URL |
| 67. |
Hildebrand, D. K.; Wu, Z. J.; Mayer, J. E., Jr.; Sacks, M. S. Design and hydrodynamic evaluation of a novel pulsatile bioreactor for biologically active heart valves. Ann Biomed Eng. 2004, 32, 1039-1049.
doi: 10.1114/B:ABME.0000036640.11387.4b URL |
| 68. |
Gandaglia, A.; Bagno, A.; Naso, F.; Spina, M.; Gerosa, G. Cells, scaffolds and bioreactors for tissue-engineered heart valves: a journey from basic concepts to contemporary developmental innovations. Eur J Cardiothorac Surg. 2011, 39, 523-531.
doi: 10.1016/j.ejcts.2010.07.030 URL |
| 69. |
Ramaswamy, S.; Boronyak, S. M.; Le, T.; Holmes, A.; Sotiropoulos, F.; Sacks, M. S. A novel bioreactor for mechanobiological studies of engineered heart valve tissue formation under pulmonary arterial physiological flow conditions. J Biomech Eng. 2014, 136, 121009.
doi: 10.1115/1.4028815 URL |
| 70. |
Nguemgo Kouam, P.; Bühler, H.; Hero, T.; Adamietz, I. A. The increased adhesion of tumor cells to endothelial cells after irradiation can be reduced by FAK-inhibition. Radiat Oncol. 2019, 14, 25.
doi: 10.1186/s13014-019-1230-3 URL |
| 71. |
Hampel, U.; Garreis, F.; Burgemeister, F.; Eßel, N.; Paulsen, F. Effect of intermittent shear stress on corneal epithelial cells using an in vitro flow culture model. Ocul Surf. 2018, 16, 341-351.
doi: 10.1016/j.jtos.2018.04.005 URL |
| 72. |
Helle, E.; Ampuja, M.; Antola, L.; Kivelä, R. Flow-induced transcriptomic remodeling of endothelial cells derived from human induced pluripotent stem cells. Front Physiol. 2020, 11, 591450.
doi: 10.3389/fphys.2020.591450 URL |
| 73. |
Moon du, G.; Christ, G.; Stitzel, J. D.; Atala, A.; Yoo, J. J. Cyclic mechanical preconditioning improves engineered muscle contraction. Tissue Eng Part A. 2008, 14, 473-482.
doi: 10.1089/tea.2007.0104 URL |
| 74. | Sun, L.; Qu, L.; Zhu, R.; Li, H.; Xue, Y.; Liu, X.; Fan, J.; Fan, H. Effects of mechanical stretch on cell proliferation and matrix formation of mesenchymal stem cell and anterior cruciate ligament fibroblast. Stem Cells Int. 2016, 2016, 9842075. |
| 75. |
Ku, C. H.; Johnson, P. H.; Batten, P.; Sarathchandra, P.; Chambers, R. C.; Taylor, P. M.; Yacoub, M. H.; Chester, A. H. Collagen synthesis by mesenchymal stem cells and aortic valve interstitial cells in response to mechanical stretch. Cardiovasc Res. 2006, 71, 548-556.
doi: 10.1016/j.cardiores.2006.03.022 URL |
| 76. |
Syedain, Z. H.; Tranquillo, R. T. Controlled cyclic stretch bioreactor for tissue-engineered heart valves. Biomaterials. 2009, 30, 4078-4084.
doi: 10.1016/j.biomaterials.2009.04.027 URL |
| 77. |
Engelmayr, G. C., Jr.; Rabkin, E.; Sutherland, F. W.; Schoen, F. J.; Mayer, J. E., Jr.; Sacks, M. S. The independent role of cyclic flexure in the early in vitro development of an engineered heart valve tissue. Biomaterials. 2005, 26, 175-187.
doi: 10.1016/j.biomaterials.2004.02.035 URL |
| 78. |
Engelmayr, G. C., Jr.; Hildebrand, D. K.; Sutherland, F. W.; Mayer, J. E., Jr.; Sacks, M. S. A novel bioreactor for the dynamic flexural stimulation of tissue engineered heart valve biomaterials. Biomaterials. 2003, 24, 2523-2532.
doi: 10.1016/S0142-9612(03)00051-6 URL |
| 79. |
Kutikhin, A. G.; Sinitsky, M. Y.; Yuzhalin, A. E.; Velikanova, E. A. Shear stress: an essential driver of endothelial progenitor cells. J Mol Cell Cardiol. 2018, 118, 46-69.
doi: 10.1016/j.yjmcc.2018.03.007 URL |
| 80. |
Yang, Z.; Xia, W. H.; Zhang, Y. Y.; Xu, S. Y.; Liu, X.; Zhang, X. Y.; Yu, B. B.; Qiu, Y. X.; Tao, J. Shear stress-induced activation of Tie2-dependent signaling pathway enhances reendothelialization capacity of early endothelial progenitor cells. J Mol Cell Cardiol. 2012, 52, 1155-1163.
doi: 10.1016/j.yjmcc.2012.01.019 URL |
| 81. |
Campinho, P.; Vilfan, A.; Vermot, J. Blood flow forces in shaping the vascular system: a focus on endothelial cell behavior. Front Physiol. 2020, 11, 552.
doi: 10.3389/fphys.2020.00552 URL |
| 82. |
da Silva, R. A.; Fernandes, C.; Feltran, G. D. S.; Gomes, A. M.; de Camargo Andrade, A. F.; Andia, D. C.; Peppelenbosch, M. P.; Zambuzzi, W. F. Laminar shear stress-provoked cytoskeletal changes are mediated by epigenetic reprogramming of TIMP1 in human primary smooth muscle cells. J Cell Physiol. 2019, 234, 6382-6396.
doi: 10.1002/jcp.v234.5 URL |
| 83. |
Gonzalez, B. A.; Perez-Nevarez, M.; Mirza, A.; Perez, M. G.; Lin, Y. M.; Hsu, C. D.; Caobi, A.; Raymond, A.; Gomez Hernandez, M. E.; Fernandez-Lima, F.; George, F.; Ramaswamy, S. Physiologically relevant fluid-induced oscillatory shear stress stimulation of mesenchymal stem cells enhances the engineered valve matrix phenotype. Front Cardiovasc Med. 2020, 7, 69.
doi: 10.3389/fcvm.2020.00069 URL |
| 84. |
Li, J.; He, Y.; Bu, H.; Wang, M.; Yu, J.; Li, L.; Li, H.; Zhang, X.; Cui, X.; Cheng, M. Oscillating shear stress mediates mesenchymal transdifferentiation of EPCs by the Kir2.1 channel. Heart Vessels. 2020, 35, 1473-1482.
doi: 10.1007/s00380-020-01625-w URL |
| 85. |
Gao, Y.; Cui, X.; Wang, M.; Zhang, Y.; He, Y.; Li, L.; Li, H.; Zhang, X.; Cheng, M. Oscillatory shear stress induces the transition of EPCs into mesenchymal cells through ROS/PKCζ/p53 pathway. Life Sci. 2020, 253, 117728.
doi: 10.1016/j.lfs.2020.117728 URL |
| 86. |
Converse, G. L.; Buse, E. E.; Neill, K. R.; McFall, C. R.; Lewis, H. N.; VeDepo, M. C.; Quinn, R. W.; Hopkins, R. A. Design and efficacy of a single-use bioreactor for heart valve tissue engineering. J Biomed Mater Res B Appl Biomater. 2017, 105, 249-259.
doi: 10.1002/jbm.v105.2 URL |
| 87. |
Jockenhoevel, S.; Zund, G.; Hoerstrup, S. P.; Schnell, A.; Turina, M. Cardiovascular tissue engineering: a new laminar flow chamber for in vitro improvement of mechanical tissue properties. ASAIO J. 2002, 48, 8-11.
doi: 10.1097/00002480-200201000-00003 URL |
| 88. |
Engelmayr, G. C., Jr.; Sales, V. L.; Mayer, J. E., Jr.; Sacks, M. S. Cyclic flexure and laminar flow synergistically accelerate mesenchymal stem cell-mediated engineered tissue formation: Implications for engineered heart valve tissues. Biomaterials. 2006, 27, 6083-6095.
doi: 10.1016/j.biomaterials.2006.07.045 URL |
| 89. |
Ramaswamy, S.; Gottlieb, D.; Engelmayr, G. C., Jr.; Aikawa, E.; Schmidt, D. E.; Gaitan-Leon, D. M.; Sales, V. L.; Mayer, J. E., Jr.; Sacks, M. S. The role of organ level conditioning on the promotion of engineered heart valve tissue development in-vitro using mesenchymal stem cells. Biomaterials. 2010, 31, 1114-1125.
doi: 10.1016/j.biomaterials.2009.10.019 URL |
| 90. |
Mongkoldhumrongkul, N.; Latif, N.; Yacoub, M. H.; Chester, A. H. Effect of side-specific valvular shear stress on the content of extracellular matrix in aortic valves. Cardiovasc Eng Technol. 2018, 9, 151-157.
doi: 10.1007/s13239-016-0280-z URL |
| 91. |
Engelmayr, G. C., Jr.; Soletti, L.; Vigmostad, S. C.; Budilarto, S. G.; Federspiel, W. J.; Chandran, K. B.; Vorp, D. A.; Sacks, M. S. A novel flex-stretch-flow bioreactor for the study of engineered heart valve tissue mechanobiology. Ann Biomed Eng. 2008, 36, 700-712.
doi: 10.1007/s10439-008-9447-6 URL |
| 92. |
Vozzi, F.; Bianchi, F.; Ahluwalia, A.; Domenici, C. Hydrostatic pressure and shear stress affect endothelin-1 and nitric oxide release by endothelial cells in bioreactors. Biotechnol J. 2014, 9, 146-154.
doi: 10.1002/biot.v9.1 URL |
| 93. |
VeDepo, M. C.; Buse, E. E.; Paul, A.; Converse, G. L.; Hopkins, R. A. Non-physiologic bioreactor processing conditions for heart valve tissue engineering. Cardiovasc Eng Technol. 2019, 10, 628-637.
doi: 10.1007/s13239-019-00438-x URL |
| 94. |
Hutmacher, D. W.; Singh, H. Computational fluid dynamics for improved bioreactor design and 3D culture. Trends Biotechnol. 2008, 26, 166-172.
doi: 10.1016/j.tibtech.2007.11.012 URL |
| 95. |
Williams, A.; Nasim, S.; Salinas, M.; Moshkforoush, A.; Tsoukias, N.; Ramaswamy, S. A “sweet-spot” for fluid-induced oscillations in the conditioning of stem cell-based engineered heart valve tissues. J Biomech. 2017, 65, 40-48.
doi: 10.1016/j.jbiomech.2017.09.035 URL |
| 96. |
Salinas, M.; Ramaswamy, S. Computational simulations predict a key role for oscillatory fluid shear stress in de novo valvular tissue formation. J Biomech. 2014, 47, 3517-3523.
doi: 10.1016/j.jbiomech.2014.08.028 URL |
| 97. | Lichtenberg, A.; Tudorache, I.; Cebotari, S.; Suprunov, M.; Tudorache, G.; Goerler, H.; Park, J. K.; Hilfiker-Kleiner, D.; Ringes-Lichtenberg, S.; Karck, M.; Brandes, G.; Hilfiker, A.; Haverich, A. Preclinical testing of tissue-engineered heart valves re-endothelialized under simulated physiological conditions. Circulation. 2006, 114, I559-565. |
| 98. |
Lichtenberg, A.; Tudorache, I.; Cebotari, S.; Ringes-Lichtenberg, S.; Sturz, G.; Hoeffler, K.; Hurscheler, C.; Brandes, G.; Hilfiker, A.; Haverich, A. In vitro re-endothelialization of detergent decellularized heart valves under simulated physiological dynamic conditions. Biomaterials. 2006, 27, 4221-4229.
doi: 10.1016/j.biomaterials.2006.03.047 URL |
| 99. |
Santoro, R.; Venkateswaran, S.; Amadeo, F.; Zhang, R.; Brioschi, M.; Callanan, A.; Agrifoglio, M.; Banfi, C.; Bradley, M.; Pesce, M. Acrylate-based materials for heart valve scaffold engineering. Biomater Sci. 2017, 6, 154-167.
doi: 10.1039/C7BM00854F URL |
| 100. |
Best, C. A.; Szafron, J. M.; Rocco, K. A.; Zbinden, J.; Dean, E. W.; Maxfield, M. W.; Kurobe, H.; Tara, S.; Bagi, P. S.; Udelsman, B. V.; Khosravi, R.; Yi, T.; Shinoka, T.; Humphrey, J. D.; Breuer, C. K. Differential outcomes of venous and arterial tissue engineered vascular grafts highlight the importance of coupling long-term implantation studies with computational modeling. Acta Biomater. 2019, 94, 183-194.
doi: 10.1016/j.actbio.2019.05.063 URL |
| 101. |
Hsu, M. C.; Kamensky, D.; Xu, F.; Kiendl, J.; Wang, C.; Wu, M. C.; Mineroff, J.; Reali, A.; Bazilevs, Y.; Sacks, M. S. Dynamic and fluid-structure interaction simulations of bioprosthetic heart valves using parametric design with T-splines and Fung-type material models. Comput Mech. 2015, 55, 1211-1225.
doi: 10.1007/s00466-015-1166-x URL |
| 102. |
Martin, C.; Sun, W. Simulation of long-term fatigue damage in bioprosthetic heart valves: effects of leaflet and stent elastic properties. Biomech Model Mechanobiol. 2014, 13, 759-770.
doi: 10.1007/s10237-013-0532-x URL |
| 103. |
Szafron, J. M.; Ramachandra, A. B.; Breuer, C. K.; Marsden, A. L.; Humphrey, J. D. Optimization of tissue-engineered vascular graft design using computational modeling. Tissue Eng Part C Methods. 2019, 25, 561-570.
doi: 10.1089/ten.tec.2019.0086 URL |
| 104. |
Sulejmani, F.; Caballero, A.; Martin, C.; Pham, T.; Sun, W. Evaluation of transcatheter heart valve biomaterials: Computational modeling using bovine and porcine pericardium. J Mech Behav Biomed Mater. 2019, 97, 159-170.
doi: 10.1016/j.jmbbm.2019.05.020 URL |
| 105. |
Zakerzadeh, R.; Hsu, M. C.; Sacks, M. S. Computational methods for the aortic heart valve and its replacements. Expert Rev Med Devices. 2017, 14, 849-866.
doi: 10.1080/17434440.2017.1389274 URL |
| 106. |
Abbasi, M.; Barakat, M. S.; Vahidkhah, K.; Azadani, A. N. Characterization of three-dimensional anisotropic heart valve tissue mechanical properties using inverse finite element analysis. J Mech Behav Biomed Mater. 2016, 62, 33-44.
doi: 10.1016/j.jmbbm.2016.04.031 URL |
| 107. | Fernández-Ruiz, I. Computer modelling to personalize bioengineered heart valves. Nat Rev Cardiol. 2018, 15, 440-441. |
| 108. | Butcher, J. T. The root problem of heart valve engineering. Sci Transl Med. 2018, 10, eaat5850. |
| 109. |
Simmons, C. A. Taking bioengineered heart valves from faulty to functional. Nature. 2018, 559, 42-43.
doi: 10.1038/d41586-018-05566-3 URL |
| 110. |
Loureiro-Ga, M.; Veiga, C.; Fdez-Manin, G.; Jimenez, V. A.; Calvo-Iglesias, F.; Iñiguez, A. A biomechanical model of the pathological aortic valve: simulation of aortic stenosis. Comput Methods Biomech Biomed Engin. 2020, 23, 303-311.
doi: 10.1080/10255842.2020.1720001 URL |
| 111. |
Conti, C. A.; Della Corte, A.; Votta, E.; Del Viscovo, L.; Bancone, C.; De Santo, L. S.; Redaelli, A. Biomechanical implications of the congenital bicuspid aortic valve: a finite element study of aortic root function from in vivo data. J Thorac Cardiovasc Surg. 2010, 140, 890-896, 896.e1-2.
doi: 10.1016/j.jtcvs.2010.01.016 URL |
| 112. |
Arzani, A.; Mofrad, M. R. K. A strain-based finite element model for calcification progression in aortic valves. J Biomech. 2017, 65, 216-220.
doi: 10.1016/j.jbiomech.2017.10.014 URL |
| 113. |
Rassoli, A.; Fatouraee, N.; Guidoin, R.; Zhang, Z. Comparison of tensile properties of xenopericardium from three animal species and finite element analysis for bioprosthetic heart valve tissue. Artif Organs. 2020, 44, 278-287.
doi: 10.1111/aor.v44.3 URL |
| 114. |
Vashistha, R.; Kumar, P.; Dangi, A. K.; Sharma, N.; Chhabra, D.; Shukla, P. Quest for cardiovascular interventions: precise modeling and 3D printing of heart valves. J Biol Eng. 2019, 13, 12.
doi: 10.1186/s13036-018-0132-5 URL |
| 115. |
van der Valk, D. C.; van der Ven, C. F. T.; Blaser, M. C.; Grolman, J. M.; Wu, P. J.; Fenton, O. S.; Lee, L. H.; Tibbitt, M. W.; Andresen, J. L.; Wen, J. R.; Ha, A. H.; Buffolo, F.; van Mil, A.; Bouten, C. V. C.; Body, S. C.; Mooney, D. J.; Sluijter, J. P. G.; Aikawa, M.; Hjortnaes, J.; Langer, R.; Aikawa, E. Engineering a 3D-bioprinted model of human heart valve disease using nanoindentation-based biomechanics. Nanomaterials (Basel). 2018, 8, 296.
doi: 10.3390/nano8050296 URL |
| 116. |
Labrosse, M. R.; Beller, C. J.; Robicsek, F.; Thubrikar, M. J. Geometric modeling of functional trileaflet aortic valves: development and clinical applications. J Biomech. 2006, 39, 2665-2672.
doi: 10.1016/j.jbiomech.2005.08.012 URL |
| 117. |
Marom, G.; Haj-Ali, R.; Rosenfeld, M.; Schäfers, H. J.; Raanani, E. Aortic root numeric model: correlation between intraoperative effective height and diastolic coaptation. J Thorac Cardiovasc Surg. 2013, 145, 303-304.
doi: 10.1016/j.jtcvs.2012.08.043 URL |
| 118. | Zhang, W.; Rossini, G.; Kamensky, D.; Bui-Thanh, T.; Sacks, M. S. Isogeometric finite element-based simulation of the aortic heart valve: Integration of neural network structural material model and structural tensor fiber architecture representations. Int J Numer Method Biomed Eng. 2021, 37, e3438. |
| 119. |
Li, Q.; Wang, J.; Tao, H.; Zhou, Q.; Chen, J.; Fu, B.; Qin, W.; Li, D.; Hou, J.; Chen, J.; Zhang, W. H. The prediction model of warfarin individual maintenance dose for patients undergoing heart valve replacement, based on the back propagation neural network. Clin Drug Investig. 2020, 40, 41-53.
doi: 10.1007/s40261-019-00850-0 URL |
| 120. |
Obbink-Huizer, C.; Oomens, C. W.; Loerakker, S.; Foolen, J.; Bouten, C. V.; Baaijens, F. P. Computational model predicts cell orientation in response to a range of mechanical stimuli. Biomech Model Mechanobiol. 2014, 13, 227-236.
doi: 10.1007/s10237-013-0501-4 URL |
| 121. |
Loerakker, S.; Obbink-Huizer, C.; Baaijens, F. P. A physically motivated constitutive model for cell-mediated compaction and collagen remodeling in soft tissues. Biomech Model Mechanobiol. 2014, 13, 985-1001.
doi: 10.1007/s10237-013-0549-1 URL |
| 122. |
Ristori, T.; Bouten, C. V. C.; Baaijens, F. P. T.; Loerakker, S. Predicting and understanding collagen remodeling in human native heart valves during early development. Acta Biomater. 2018, 80, 203-216.
doi: 10.1016/j.actbio.2018.08.040 URL |
| 123. |
Loerakker, S.; Ristori, T.; Baaijens, F. P. T. A computational analysis of cell-mediated compaction and collagen remodeling in tissue-engineered heart valves. J Mech Behav Biomed Mater. 2016, 58, 173-187.
doi: 10.1016/j.jmbbm.2015.10.001 URL |
| 124. | Soares, J. S.; T, B. L.; Sotiropoulos, F.; M, S. S. Modeling the role of oscillator flow and dynamic mechanical conditioning on dense connective tissue formation in mesenchymal stem cell-derived heart valve tissue engineering. J Med Device. 2013, 7, 0409271-0409272. |
| 125. |
Soares, J. S.; Sacks, M. S. A triphasic constrained mixture model of engineered tissue formation under in vitro dynamic mechanical conditioning. Biomech Model Mechanobiol. 2016, 15, 293-316.
doi: 10.1007/s10237-015-0687-8 URL |
| 126. |
Sanders, B.; Loerakker, S.; Fioretta, E. S.; Bax, D. J.; Driessen-Mol, A.; Hoerstrup, S. P.; Baaijens, F. P. Improved geometry of decellularized tissue engineered heart valves to prevent leaflet retraction. Ann Biomed Eng. 2016, 44, 1061-1071.
doi: 10.1007/s10439-015-1386-4 URL |
| 127. |
Loerakker, S.; Argento, G.; Oomens, C. W.; Baaijens, F. P. Effects of valve geometry and tissue anisotropy on the radial stretch and coaptation area of tissue-engineered heart valves. J Biomech. 2013, 46, 1792-1800.
doi: 10.1016/j.jbiomech.2013.05.015 URL |
| 128. |
Motta, S. E.; Fioretta, E. S.; Lintas, V.; Dijkman, P. E.; Hilbe, M.; Frese, L.; Cesarovic, N.; Loerakker, S.; Baaijens, F. P. T.; Falk, V.; Hoerstrup, S. P.; Emmert, M. Y. Geometry influences inflammatory host cell response and remodeling in tissue-engineered heart valves in-vivo. Sci Rep. 2020, 10, 19882.
doi: 10.1038/s41598-020-76322-9 URL |
| [1] | Xirui Jing, Qiuyue Ding, Qinxue Wu, Weijie Su, Keda Yu, Yanlin Su, Bing Ye, Qing Gao, Tingfang Sun, Xiaodong Guo. Magnesium-based materials in orthopaedics: material properties and animal models [J]. Biomaterials Translational, 2021, 2(3): 197-213. |
| [2] | Yizhong Peng, Xiangcheng Qing, Hongyang Shu, Shuo Tian, Wenbo Yang, Songfeng Chen, Hui Lin, Xiao Lv, Lei Zhao, Xi Chen, Feifei Pu, Donghua Huang, Xu Cao, Zengwu Shao. Proper animal experimental designs for preclinical research of biomaterials for intervertebral disc regeneration [J]. Biomaterials Translational, 2021, 2(2): 91-142. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||