Biomaterials Translational ›› 2022, Vol. 3 ›› Issue (1): 55-64.doi: 10.12336/biomatertransl.2022.01.006
• REVIEW • Previous Articles Next Articles
Received:2022-01-24
Revised:2022-03-06
Accepted:2022-03-10
Online:2022-03-28
Published:2022-03-28
Contact:
Quan Yuan
E-mail:yaunquan@scu.edu.cn
About author:Quan Yuan, yaunquan@scu.edu.cn.
Cao, S.; Yuan, Q. An update of nanotopographical surfaces in modulating stem cell fate: a narrative review. Biomater Transl. 2022, 3(1), 55-64.
| Nanotopographical surfaces | Structure features | Fabrication technique | Cellular effect |
|---|---|---|---|
| Static patterned surfaces | Nanopillar | Ultraviolet-lithography, injection molding | Promote cells elongation and differentiation |
| Nanopits | Colloidal lithography | Provide large surface traction forces to promote cell adhesion | |
| Nanopore | Anodization | Prohibit cell attachment and limit cell migration | |
| Nanospike | Photolithography | Enhance stem cell differentiation, secretion of growth factors | |
| Grooved surfaces | Argon ion plasma, molding | Promote cell adhesion and proliferation | |
| Dynamic patterned surfaces | Electro responsive, nanotubes to nanotips | Electrochemical polymerization | Dynamic attachment and detachment to mesenchymal stem cells |
| Ultraviolet responsive, flat to rigid | Spin coating | Induce cyclic cellular and nuclear stretches | |
| Thermoresponsive, flat to wrinkle | Ultraviolet polymerization and spin coating | Dynamic response of focal adhesion | |
| Roughness | Gradient: 0.77–1.09 µm | Molding | Cellular attachment, F-actin arrangement |
| High: 14.3 nm, low: 71 nm | Electrospinning | Cell morphology, metabolic activity | |
| Gradient: 200 nm–1.2 μm | Soft lithography | Enhance cell mechanosensing and osteogenic differentiation of mesenchymal stem cells |
Table 1 Nanotopogrphical features and their cellular effect on stem cells
| Nanotopographical surfaces | Structure features | Fabrication technique | Cellular effect |
|---|---|---|---|
| Static patterned surfaces | Nanopillar | Ultraviolet-lithography, injection molding | Promote cells elongation and differentiation |
| Nanopits | Colloidal lithography | Provide large surface traction forces to promote cell adhesion | |
| Nanopore | Anodization | Prohibit cell attachment and limit cell migration | |
| Nanospike | Photolithography | Enhance stem cell differentiation, secretion of growth factors | |
| Grooved surfaces | Argon ion plasma, molding | Promote cell adhesion and proliferation | |
| Dynamic patterned surfaces | Electro responsive, nanotubes to nanotips | Electrochemical polymerization | Dynamic attachment and detachment to mesenchymal stem cells |
| Ultraviolet responsive, flat to rigid | Spin coating | Induce cyclic cellular and nuclear stretches | |
| Thermoresponsive, flat to wrinkle | Ultraviolet polymerization and spin coating | Dynamic response of focal adhesion | |
| Roughness | Gradient: 0.77–1.09 µm | Molding | Cellular attachment, F-actin arrangement |
| High: 14.3 nm, low: 71 nm | Electrospinning | Cell morphology, metabolic activity | |
| Gradient: 200 nm–1.2 μm | Soft lithography | Enhance cell mechanosensing and osteogenic differentiation of mesenchymal stem cells |
| Stem cell type | Scaffold | Topographical features | Application |
|---|---|---|---|
| Mesenchymal stem cell | Polyesters | Nanograting or nanopillars | Cartilage regenerationNeurogenic differentiation |
| Fibrin hydrogel | Hierarchical aligned | ||
| Neural stem cell | Indium tin oxide-coated glass | Nanopore | Neuronal differentiation |
| Silicon oxide surface | Nanopillar arrays | ||
| Polydimethylsiloxane | Nanowrinkle | ||
| Induced pluripotent stem cell | Glass surface | Random nanoscale structures | Neuronal differentiation |
| Silk fibroin substrates | Anisotropic patterned | Cardiac regeneration | |
| Multielectrode arrays | Nanoarrays | Preclinical analysis of excitable cell function |
Table 2 Examples from the literature of nanotopography controls stem cell fate
| Stem cell type | Scaffold | Topographical features | Application |
|---|---|---|---|
| Mesenchymal stem cell | Polyesters | Nanograting or nanopillars | Cartilage regenerationNeurogenic differentiation |
| Fibrin hydrogel | Hierarchical aligned | ||
| Neural stem cell | Indium tin oxide-coated glass | Nanopore | Neuronal differentiation |
| Silicon oxide surface | Nanopillar arrays | ||
| Polydimethylsiloxane | Nanowrinkle | ||
| Induced pluripotent stem cell | Glass surface | Random nanoscale structures | Neuronal differentiation |
| Silk fibroin substrates | Anisotropic patterned | Cardiac regeneration | |
| Multielectrode arrays | Nanoarrays | Preclinical analysis of excitable cell function |
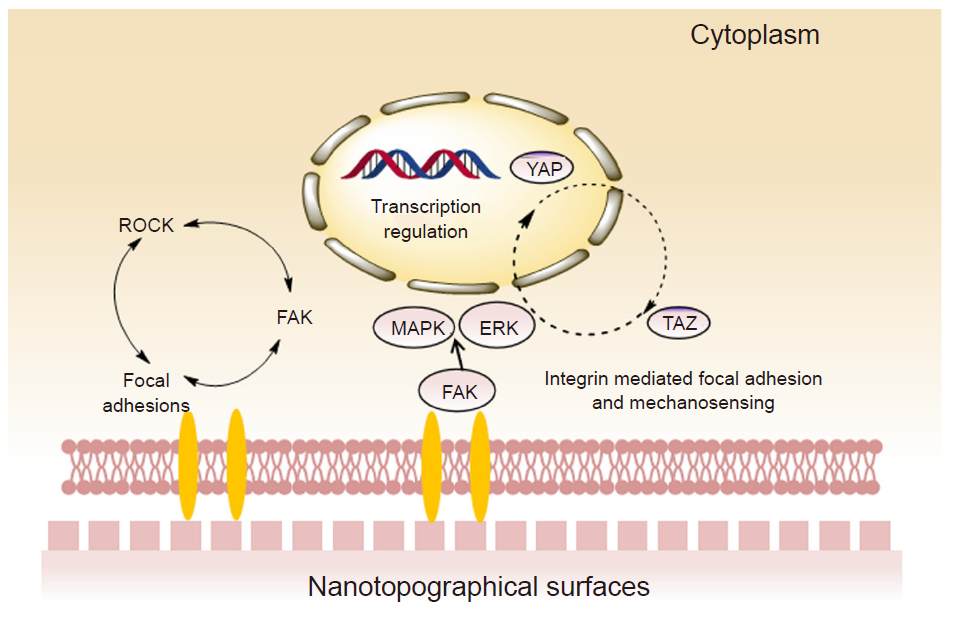
Figure 1. Schematic illustration of cellular response to nanotopographical cues and relevant mechanotransduction. External nanotopographical cues exerting on cell-nanotopograpy interface mediates the subsequent mechanosensing and focal adhesion, which regulated the downstream molecular expression corresponding to different cell behaviors. FAK: focal adhesion kinase; ERK: extracellular signal regulated kinase; MAPK: mitogen activated protein kinase; MEK: mitogen activated protein kinase; ROCK: Rho-associated protein kinase; YAP: yes-associated protein; TAZ: transcriptional co-activator with PDZ-binding motif.
| 1. |
Bianco, P.; Robey, P. G. Stem cells in tissue engineering. Nature. 2001, 414, 118-121.
doi: 10.1038/35102181 URL |
| 2. | Frazier, T.; Hamel, K.; Wu, X.; Rogers, E.; Lassiter, H.; Robinson, J.; Mohiuddin, O.; Henderson, M.; Gimble, J. Adipose-derived cells: building blocks of three-dimensional microphysiological systems. Biomater Transl. 2021, 2, 301-306. |
| 3. | Mahla, R. S. Stem cells applications in regenerative medicine and disease therapeutics. Int J Cell Biol. 2016, 2016, 6940283. |
| 4. | Jones, D. L.; Wagers, A. J. No place like home: anatomy and function of the stem cell niche. Nat Rev Mol Cell Biol. 2008, 9, 11-21. |
| 5. |
Morrison, S. J.; Spradling, A. C. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008, 132, 598-611.
doi: 10.1016/j.cell.2008.01.038 URL |
| 6. |
Ding, S.; Kingshott, P.; Thissen, H.; Pera, M.; Wang, P. Y. Modulation of human mesenchymal and pluripotent stem cell behavior using biophysical and biochemical cues: A review. Biotechnol Bioeng. 2017, 114, 260-280.
doi: 10.1002/bit.26075 URL |
| 7. |
Li, J.; Liu, Y.; Zhang, Y.; Yao, B.; Enhejirigala; Li, Z.; Song, W.; Wang, Y.; Duan, X.; Yuan, X.; Fu, X.; Huang, S. Biophysical and biochemical cues of biomaterials guide mesenchymal stem cell behaviors. Front Cell Dev Biol. 2021, 9, 640388.
doi: 10.3389/fcell.2021.640388 URL |
| 8. |
Lou, H. Y.; Zhao, W.; Li, X.; Duan, L.; Powers, A.; Akamatsu, M.; Santoro, F.; McGuire, A. F.; Cui, Y.; Drubin, D. G.; Cui, B. Membrane curvature underlies actin reorganization in response to nanoscale surface topography. Proc Natl Acad Sci U S A. 2019, 116, 23143-23151.
doi: 10.1073/pnas.1910166116 URL |
| 9. |
Wan, X.; Liu, Z.; Li, L. Manipulation of stem cells fates: the master and multifaceted roles of biophysical cues of biomaterials. Adv Funct Mater. 2021, 31, 2010626.
doi: 10.1002/adfm.v31.23 URL |
| 10. | Wang, S.; Hashemi, S.; Stratton, S.; Arinzeh, T. L. The effect of physical cues of biomaterial scaffolds on stem cell behavior. Adv Healthc Mater. 2021, 10, e2001244. |
| 11. |
Hou, Y.; Xie, W.; Fan, X.; Tang, P.; Yu, L.; Haag, R. “Raspberry” hierarchical topographic features regulate human mesenchymal stem cell adhesion and differentiation via enhanced mechanosensing. ACS Appl Mater Interfaces. 2021, 13, 54840-54849.
doi: 10.1021/acsami.1c18722 URL |
| 12. | Yu, L.; Tang, P.; Nie, C.; Hou, Y.; Haag, R. Well-defined nanostructured biointerfaces: strengthened cellular interaction for circulating tumor cells isolation. Adv Healthc Mater. 2021, 10, e2002202. |
| 13. |
Zhang, M.; Sun, Q.; Liu, Y.; Chu, Z.; Yu, L.; Hou, Y.; Kang, H.; Wei, Q.; Zhao, W.; Spatz, J. P.; Zhao, C.; Cavalcanti-Adam, E. A. Controllable ligand spacing stimulates cellular mechanotransduction and promotes stem cell osteogenic differentiation on soft hydrogels. Biomaterials. 2021, 268, 120543.
doi: 10.1016/j.biomaterials.2020.120543 URL |
| 14. | Qiang, W.; Shenghao, W.; Feng, H.; Huan, W.; Weidong, Z.; Qifan, Y.; Changjiang, L.; Luguang, D.; Jiayuan, W.; Lili, Y.; Caihong, Z.; Bin, L. Cellular modulation by the mechanical cues from biomaterials for tissue engineering. Biomater Transl. 2021, 2, 323-342. |
| 15. |
Chen, W.; Shao, Y.; Li, X.; Zhao, G.; Fu, J. Nanotopographical surfaces for stem cell fate control: engineering mechanobiology from the bottom. Nano Today. 2014, 9, 759-784.
doi: 10.1016/j.nantod.2014.12.002 URL |
| 16. |
Firkowska-Boden, I.; Helbing, C.; Dauben, T. J.; Pieper, M.; Jandt, K. D. How nanotopography-induced conformational changes of fibrinogen affect platelet adhesion and activation. Langmuir. 2020, 36, 11573-11580.
doi: 10.1021/acs.langmuir.0c02094 URL |
| 17. |
Dalby, M. J.; Gadegaard, N.; Oreffo, R. O. Harnessing nanotopography and integrin-matrix interactions to influence stem cell fate. Nat Mater. 2014, 13, 558-569.
doi: 10.1038/nmat3980 URL |
| 18. |
Kartikasari, N.; Yamada, M.; Watanabe, J.; Tiskratok, W.; He, X.; Kamano, Y.; Egusa, H. Titanium surface with nanospikes tunes macrophage polarization to produce inhibitory factors for osteoclastogenesis through nanotopographic cues. Acta Biomater. 2022, 137, 316-330.
doi: 10.1016/j.actbio.2021.10.019 URL |
| 19. |
Damiati, L. A.; Tsimbouri, M. P.; Hernandez, V. L.; Jayawarna, V.; Ginty, M.; Childs, P.; Xiao, Y.; Burgess, K.; Wells, J.; Sprott, M. R.; Meek, R. M. D.; Li, P.; Oreffo, R. O. C.; Nobbs, A.; Ramage, G.; Su, B.; Salmeron-Sanchez, M.; Dalby, M. J. Materials-driven fibronectin assembly on nanoscale topography enhances mesenchymal stem cell adhesion, protecting cells from bacterial virulence factors and preventing biofilm formation. Biomaterials. 2022, 280, 121263.
doi: 10.1016/j.biomaterials.2021.121263 URL |
| 20. |
Chen, Z.; Ni, S.; Han, S.; Crawford, R.; Lu, S.; Wei, F.; Chang, J.; Wu, C.; Xiao, Y. Nanoporous microstructures mediate osteogenesis by modulating the osteo-immune response of macrophages. Nanoscale. 2017, 9, 706-718.
doi: 10.1039/C6NR06421C URL |
| 21. |
Ripamonti, U. Functionalized surface geometries induce: “bone: formation by autoinduction”. Front Physiol. 2017, 8, 1084.
doi: 10.3389/fphys.2017.01084 URL |
| 22. | Donnelly, H.; Dalby, M. J.; Salmeron-Sanchez, M.; Sweeten, P. E. Current approaches for modulation of the nanoscale interface in the regulation of cell behavior. Nanomedicine. 2018, 14, 2455-2464. |
| 23. |
Ankam, S.; Teo, B. K. K.; Pohan, G.; Ho, S. W. L.; Lim, C. K.; Yim, E. K. F. Temporal changes in nucleus morphology, lamin A/C and histone methylation during nanotopography-induced neuronal differentiation of stem cells. Front Bioeng Biotechnol. 2018, 6, 69.
doi: 10.3389/fbioe.2018.00069 URL |
| 24. |
Jiao, A.; Moerk, C. T.; Penland, N.; Perla, M.; Kim, J.; Smith, A. S. T.; Murry, C. E.; Kim, D. H. Regulation of skeletal myotube formation and alignment by nanotopographically controlled cell-secreted extracellular matrix. J Biomed Mater Res A. 2018, 106, 1543-1551.
doi: 10.1002/jbm.a.v106.6 URL |
| 25. |
Xia, Y.; Whitesides, G. M. Soft lithography. Annu Rev Mater Sci. 1998, 28, 153-184.
doi: 10.1146/matsci.1998.28.issue-1 URL |
| 26. |
Griffin, M. F.; Butler, P. E.; Seifalian, A. M.; Kalaskar, D. M. Control of stem cell fate by engineering their micro and nanoenvironment. World J Stem Cells. 2015, 7, 37-50.
doi: 10.4252/wjsc.v7.i1.37 URL |
| 27. |
Higuchi, A.; Ling, Q. D.; Chang, Y.; Hsu, S. T.; Umezawa, A. Physical cues of biomaterials guide stem cell differentiation fate. Chem Rev. 2013, 113, 3297-3328.
doi: 10.1021/cr300426x URL |
| 28. |
Kilian, K. A.; Bugarija, B.; Lahn, B. T.; Mrksich, M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc Natl Acad Sci U S A. 2010, 107, 4872-4877.
doi: 10.1073/pnas.0903269107 URL |
| 29. |
van Dorp, W. F.; Zhang, X.; Feringa, B. L.; Hansen, T. W.; Wagner, J. B.; De Hosson, J. T. Molecule-by-molecule writing using a focused electron beam. ACS Nano. 2012, 6, 10076-10081.
doi: 10.1021/nn303793w URL |
| 30. |
Basnar, B.; Willner, I. Dip-pen-nanolithographic patterning of metallic, semiconductor, and metal oxide nanostructures on surfaces. Small. 2009, 5, 28-44.
doi: 10.1002/smll.v5:1 URL |
| 31. |
Norman, J. J.; Desai, T. A. Methods for fabrication of nanoscale topography for tissue engineering scaffolds. Ann Biomed Eng. 2006, 34, 89-101.
doi: 10.1007/s10439-005-9005-4 URL |
| 32. | Higgins, S. G.; Becce, M.; Belessiotis-Richards, A.; Seong, H.; Sero, J. E.; Stevens, M. M. High-aspect-ratio nanostructured surfaces as biological metamaterials. Adv Mater. 2020, 32, e1903862. |
| 33. |
Bucaro, M. A.; Vasquez, Y.; Hatton, B. D.; Aizenberg, J. Fine-tuning the degree of stem cell polarization and alignment on ordered arrays of high-aspect-ratio nanopillars. ACS Nano. 2012, 6, 6222-6230.
doi: 10.1021/nn301654e URL |
| 34. | Kim, J. S.; Kuk, E.; Yu, K. N.; Kim, J. H.; Park, S. J.; Lee, H. J.; Kim, S. H.; Park, Y. K.; Park, Y. H.; Hwang, C. Y.; Kim, Y. K.; Lee, Y. S.; Jeong, D. H.; Cho, M. H. Antimicrobial effects of silver nanoparticles. Nanomedicine. 2007, 3, 95-101. |
| 35. |
Rasmussen, C. H.; Reynolds, P. M.; Petersen, D. R.; Hansson, M.; McMeeking, R. M.; Dufva, M.; Gadegaard, N. Enhanced differentiation of human embryonic stem cells toward definitive endoderm on ultrahigh aspect ratio nanopillars. Adv Funct Mater. 2016, 26, 815-823.
doi: 10.1002/adfm.v26.6 URL |
| 36. |
Kim, J. H.; Kim, H. W.; Cha, K. J.; Han, J.; Jang, Y. J.; Kim, D. S.; Kim, J. H. Nanotopography promotes pancreatic differentiation of human embryonic stem cells and induced pluripotent stem cells. ACS Nano. 2016, 10, 3342-3355.
doi: 10.1021/acsnano.5b06985 URL |
| 37. |
Karazisis, D.; Omar, O.; Petronis, S.; Thomsen, P.; Rasmusson, L. Molecular response to nanopatterned implants in the human jaw bone. ACS Biomater Sci Eng. 2021, 7, 5878-5889.
doi: 10.1021/acsbiomaterials.1c00861 URL |
| 38. |
Seo, C. H.; Jeong, H.; Feng, Y.; Montagne, K.; Ushida, T.; Suzuki, Y.; Furukawa, K. S. Micropit surfaces designed for accelerating osteogenic differentiation of murine mesenchymal stem cells via enhancing focal adhesion and actin polymerization. Biomaterials. 2014, 35, 2245-2252.
doi: 10.1016/j.biomaterials.2013.11.089 URL |
| 39. |
Kim, H. J Regulation of neural stem cell fate by natural products. Biomol Ther (Seoul). 2019, 27, 15-24.
doi: 10.4062/biomolther.2018.184 URL |
| 40. |
Park, S.; Park, H. H.; Sun, K.; Gwon, Y.; Seong, M.; Kim, S.; Park, T. E.; Hyun, H.; Choung, Y. H.; Kim, J.; Jeong, H. E. Hydrogel nanospike patch as a flexible anti-pathogenic scaffold for regulating stem cell behavior. ACS Nano. 2019, 13, 11181-11193.
doi: 10.1021/acsnano.9b04109 URL |
| 41. | Armstrong, J. P. K.; Puetzer, J. L.; Serio, A.; Guex, A. G.; Kapnisi, M.; Breant, A.; Zong, Y.; Assal, V.; Skaalure, S. C.; King, O.; Murty, T.; Meinert, C.; Franklin, A. C.; Bassindale, P. G.; Nichols, M. K.; Terracciano, C. M.; Hutmacher, D. W.; Drinkwater, B. W.; Klein, T. J.; Perriman, A. W.; Stevens, M. M. Engineering anisotropic muscle tissue using acoustic cell patterning. Adv Mater. 2018, 30, e1802649. |
| 42. |
Kim, C. S.; Kim, J. H.; Kim, B.; Park, Y. S.; Kim, H. K.; Tran, H. T.; Kim, S. H.; Jeon, H.; Kim, S.; Sim, J. H.; Shin, H. M.; Kim, G.; Baik, Y. J.; Lee, K. J.; Kim, H. Y.; Yun, T. J.; Kim, Y. S.; Kim, H. R. A specific groove pattern can effectively induce osteoblast differentiation. Adv Funct Mater. 2017, 27, 1703569.
doi: 10.1002/adfm.v27.44 URL |
| 43. |
Baek, J.; Jung, W. B.; Cho, Y.; Lee, E.; Yun, G. T.; Cho, S. Y.; Jung, H. T.; Im, S. G. Facile fabrication of high-definition hierarchical wrinkle structures for investigating the geometry-sensitive fate commitment of human neural stem cells. ACS Appl Mater Interfaces. 2019, 11, 17247-17255.
doi: 10.1021/acsami.9b03479 URL |
| 44. |
Bjørge, I. M.; Choi, I. S.; Correia, C. R.; Mano, J. F. Nanogrooved microdiscs for bottom-up modulation of osteogenic differentiation. Nanoscale. 2019, 11, 16214-16221.
doi: 10.1039/C9NR06267J URL |
| 45. |
Leclech, C.; Villard, C. Cellular and subcellular contact guidance on microfabricated substrates. Front Bioeng Biotechnol. 2020, 8, 551505.
doi: 10.3389/fbioe.2020.551505 URL |
| 46. |
Abagnale, G.; Sechi, A.; Steger, M.; Zhou, Q.; Kuo, C. C.; Aydin, G.; Schalla, C.; Müller-Newen, G.; Zenke, M.; Costa, I. G.; van Rijn, P.; Gillner, A.; Wagner, W. Surface topography guides morphology and spatial patterning of induced pluripotent stem cell colonies. Stem Cell Reports. 2017, 9, 654-666.
doi: 10.1016/j.stemcr.2017.06.016 URL |
| 47. |
Lee, M. R.; Kwon, K. W.; Jung, H.; Kim, H. N.; Suh, K. Y.; Kim, K.; Kim, K. S. Direct differentiation of human embryonic stem cells into selective neurons on nanoscale ridge/groove pattern arrays. Biomaterials. 2010, 31, 4360-4366.
doi: 10.1016/j.biomaterials.2010.02.012 URL |
| 48. |
De Martino, S.; Zhang, W.; Klausen, L.; Lou, H. Y.; Li, X.; Alfonso, F. S.; Cavalli, S.; Netti, P. A.; Santoro, F.; Cui, B. Dynamic manipulation of cell membrane curvature by light-driven reshaping of azopolymer. Nano Lett. 2020, 20, 577-584.
doi: 10.1021/acs.nanolett.9b04307 URL |
| 49. | Nickmans, K.; van der Heijden, D. A. C.; Schenning, A. P. H. J. Photonic shape memory chiral nematic polymer coatings with changing surface topography and color. Adv Funct Mater. 2019, 7, 1900592. |
| 50. |
Wei, Y.; Mo, X.; Zhang, P.; Li, Y.; Liao, J.; Li, Y.; Zhang, J.; Ning, C.; Wang, S.; Deng, X.; Jiang, L. Directing stem cell differentiation via electrochemical reversible switching between nanotubes and nanotips of polypyrrole array. ACS Nano. 2017, 11, 5915-5924.
doi: 10.1021/acsnano.7b01661 URL |
| 51. | De Martino, S.; Cavalli, S.; Netti, P. A. Photoactive interfaces for spatio-temporal guidance of mesenchymal stem cell fate. Adv Healthc Mater. 2020, 9, e2000470. |
| 52. | Shi, H.; Wu, X.; Sun, S.; Wang, C.; Vangelatos, Z.; Ash-Shakoor, A.; Grigoropoulos, C. P.; Mather, P. T.; Henderson, J. H.; Ma, Z. Profiling the responsiveness of focal adhesions of human cardiomyocytes to extracellular dynamic nano-topography. Bioact Mater. 2022, 10, 367-377. |
| 53. |
Hou, H.; Yin, J.; Jiang, X. Smart patterned surface with dynamic wrinkles. Acc Chem Res. 2019, 52, 1025-1035.
doi: 10.1021/acs.accounts.8b00623 URL |
| 54. |
Zhang, S.; Ma, B.; Liu, F.; Duan, J.; Wang, S.; Qiu, J.; Li, D.; Sang, Y.; Liu, C.; Liu, D.; Liu, H. Polylactic acid nanopillar array-driven osteogenic differentiation of human adipose-derived stem cells determined by pillar diameter. Nano Lett. 2018, 18, 2243-2253.
doi: 10.1021/acs.nanolett.7b04747 URL |
| 55. |
Das Ghosh, L.; Hasan, J.; Jain, A.; Sundaresan, N. R.; Chatterjee, K. A nanopillar array on black titanium prepared by reactive ion etching augments cardiomyogenic commitment of stem cells. Nanoscale. 2019, 11, 20766-20776.
doi: 10.1039/C9NR03424B URL |
| 56. |
Yang, W.; Han, W.; He, W.; Li, J.; Wang, J.; Feng, H.; Qian, Y. Surface topography of hydroxyapatite promotes osteogenic differentiation of human bone marrow mesenchymal stem cells. Mater Sci Eng C Mater Biol Appl. 2016, 60, 45-53.
doi: 10.1016/j.msec.2015.11.012 URL |
| 57. |
Chen, H.; Huang, X.; Zhang, M.; Damanik, F.; Baker, M. B.; Leferink, A.; Yuan, H.; Truckenmüller, R.; van Blitterswijk, C.; Moroni, L. Tailoring surface nanoroughness of electrospun scaffolds for skeletal tissue engineering. Acta Biomater. 2017, 59, 82-93.
doi: 10.1016/j.actbio.2017.07.003 URL |
| 58. |
Sun, S.; Shi, H.; Moore, S.; Wang, C.; Ash-Shakoor, A.; Mather, P. T.; Henderson, J. H.; Ma, Z. Progressive myofibril reorganization of human cardiomyocytes on a dynamic nanotopographic substrate. ACS Appl Mater Interfaces. 2020, 12, 21450-21462.
doi: 10.1021/acsami.0c03464 URL |
| 59. |
Hou, Y.; Yu, L.; Xie, W.; Camacho, L. C.; Zhang, M.; Chu, Z.; Wei, Q.; Haag, R. Surface roughness and substrate stiffness synergize to drive cellular mechanoresponse. Nano Lett. 2020, 20, 748-757.
doi: 10.1021/acs.nanolett.9b04761 URL |
| 60. |
Park, J.; Bauer, S.; von der Mark, K.; Schmuki, P. Nanosize and vitality: TiO2 nanotube diameter directs cell fate. Nano Lett. 2007, 7, 1686-1691.
doi: 10.1021/nl070678d URL |
| 61. |
Oh, S.; Brammer, K. S.; Li, Y. S.; Teng, D.; Engler, A. J.; Chien, S.; Jin, S. Stem cell fate dictated solely by altered nanotube dimension. Proc Natl Acad Sci U S A. 2007, 106, 2130-2135.
doi: 10.1073/pnas.0813200106 URL |
| 62. |
Lee, M. S.; Lee, D. H.; Jeon, J.; Oh, S. H.; Yang, H. S. Topographically defined, biodegradable nanopatterned patches to regulate cell fate and acceleration of bone regeneration. ACS Appl Mater Interfaces. 2018, 10, 38780-38790.
doi: 10.1021/acsami.8b14745 URL |
| 63. | Dehghan-Baniani, D.; Mehrjou, B.; Chu, P. K.; Wu, H. A Biomimetic nano-engineered platform for functional tissue engineering of cartilage superficial zone. Adv Healthc Mater. 2021, 10, e2001018. |
| 64. |
Wu, Y.; Yang, Z.; Denslin, V.; Ren, X.; Lee, C. S.; Yap, F. L.; Lee, E. H. Repair of osteochondral defects with predifferentiated mesenchymal stem cells of distinct phenotypic character derived from a nanotopographic platform. Am J Sports Med. 2020, 48, 1735-1747.
doi: 10.1177/0363546520907137 URL |
| 65. |
Yao, S.; Liu, X.; Yu, S.; Wang, X.; Zhang, S.; Wu, Q.; Sun, X.; Mao, H. Co-effects of matrix low elasticity and aligned topography on stem cell neurogenic differentiation and rapid neurite outgrowth. Nanoscale. 2016, 8, 10252-10265.
doi: 10.1039/C6NR01169A URL |
| 66. |
Poudineh, M.; Wang, Z.; Labib, M.; Ahmadi, M.; Zhang, L.; Das, J.; Ahmed, S.; Angers, S.; Kelley, S. O. Three-dimensional nanostructured architectures enable efficient neural differentiation of mesenchymal stem cells via mechanotransduction. Nano Lett. 2018, 18, 7188-7193.
doi: 10.1021/acs.nanolett.8b03313 URL |
| 67. |
Lim, M. S.; Ko, S. H.; Kim, M. S.; Lee, B.; Jung, H. S.; Kim, K.; Park, C. H. Hybrid nanofiber scaffold-based direct conversion of neural precursor cells/dopamine neurons. Int J Stem Cells. 2019, 12, 340-346.
doi: 10.15283/ijsc18123 URL |
| 68. |
Yang, S. S.; Cha, J.; Cho, S. W.; Kim, P. Time-dependent retention of nanotopographical cues in differentiated neural stem cells. ACS Biomater Sci Eng. 2019, 5, 3802-3807.
doi: 10.1021/acsbiomaterials.8b01057 URL |
| 69. |
Cho, Y. W.; Kim, D. S.; Suhito, I. R.; Han, D. K.; Lee, T.; Kim, T. H. Enhancing neurogenesis of neural stem cells using homogeneous nanohole pattern-modified conductive platform. Int J Mol Sci. 2019, 21, 191.
doi: 10.3390/ijms21010191 URL |
| 70. |
Simitzi, C.; Karali, K.; Ranella, A.; Stratakis, E. Controlling the outgrowth and functions of neural stem cells: the effect of surface topography. Chemphyschem. 2018, 19, 1143-1163.
doi: 10.1002/cphc.v19.10 URL |
| 71. |
Lee, J. M.; Kang, W. S.; Lee, K. G.; Cho, H. Y.; Conley, B.; Ahrberg, C. D.; Lim, J. H.; Mo, S. J.; Mun, S. G.; Kim, E. J.; Choi, J. W.; Lee, K. B.; Lee, S. J.; Chung, B. G. Combinatorial biophysical cue sensor array for controlling neural stem cell fate. Biosens Bioelectron. 2020, 156, 112125.
doi: 10.1016/j.bios.2020.112125 URL |
| 72. | Peter, W. A. Human pluripotent stem cells: tools for regenerative medicine. Biomater Transl. 2021, 2, 294-300. |
| 73. |
Tsui, J. H.; Ostrovsky-Snider, N. A.; Yama, D. M. P.; Donohue, J. D.; Choi, J. S.; Chavanachat, R.; Larson, J. D.; Murphy, A. R.; Kim, D. H. Conductive silk-polypyrrole composite scaffolds with bioinspired nanotopographic cues for cardiac tissue engineering. J Mater Chem B. 2018, 6, 7185-7196.
doi: 10.1039/C8TB01116H URL |
| 74. |
Macgregor, M.; Williams, R.; Downes, J.; Bachhuka, A.; Vasilev, K. The role of controlled surface topography and chemistry on mouse embryonic stem cell attachment, growth and self-renewal. Materials (Basel). 2017, 10, 1081.
doi: 10.3390/ma10091081 URL |
| 75. |
Kim, J. H.; Park, B. G.; Kim, S. K.; Lee, D. H.; Lee, G. G.; Kim, D. H.; Choi, B. O.; Lee, K. B.; Kim, J. H. Nanotopographical regulation of pancreatic islet-like cluster formation from human pluripotent stem cells using a gradient-pattern chip. Acta Biomater. 2019, 95, 337-347.
doi: 10.1016/j.actbio.2018.12.011 URL |
| 76. |
Ko, J. Y.; Oh, H. J.; Lee, J.; Im, G. I. Nanotopographic influence on the in vitro behavior of induced pluripotent stem cells. Tissue Eng Part A. 2018, 24, 595-606.
doi: 10.1089/ten.tea.2017.0144 URL |
| 77. |
Chen, W.; Han, S.; Qian, W.; Weng, S.; Yang, H.; Sun, Y.; Villa-Diaz, L. G.; Krebsbach, P. H.; Fu, J. Nanotopography regulates motor neuron differentiation of human pluripotent stem cells. Nanoscale. 2018, 10, 3556-3565.
doi: 10.1039/C7NR05430K URL |
| 78. |
Smith, A. S. T.; Choi, E.; Gray, K.; Macadangdang, J.; Ahn, E. H.; Clark, E. C.; Laflamme, M. A.; Wu, J. C.; Murry, C. E.; Tung, L.; Kim, D. H. NanoMEA: a tool for high-throughput, electrophysiological phenotyping of patterned excitable cells. Nano Lett. 2020, 20, 1561-1570.
doi: 10.1021/acs.nanolett.9b04152 URL |
| 79. |
Pennacchio, F. A.; Caliendo, F.; Iaccarino, G.; Langella, A.; Siciliano, V.; Santoro, F. Three-dimensionally patterned scaffolds modulate the biointerface at the nanoscale. Nano Lett. 2019, 19, 5118-5123.
doi: 10.1021/acs.nanolett.9b01468 URL |
| 80. |
Hansel, C. S.; Crowder, S. W.; Cooper, S.; Gopal, S.; João Pardelha da Cruz, M.; de Oliveira Martins, L.; Keller, D.; Rothery, S.; Becce, M.; Cass, A. E. G.; Bakal, C.; Chiappini, C.; Stevens, M. M. Nanoneedle-mediated stimulation of cell mechanotransduction machinery. ACS Nano. 2019, 13, 2913-2926.
doi: 10.1021/acsnano.8b06998 URL |
| 81. |
Salsmann, A.; Schaffner-Reckinger, E.; Kieffer, N. RGD, the Rho’d to cell spreading. Eur J Cell Biol. 2006, 85, 249-254.
doi: 10.1016/j.ejcb.2005.08.003 URL |
| 82. |
Berrier, A. L.; Yamada, K. M. Cell-matrix adhesion. J Cell Physiol. 2007, 213, 565-573.
doi: 10.1002/(ISSN)1097-4652 URL |
| 83. |
Wehrle-Haller, B. Structure and function of focal adhesions. Curr Opin Cell Biol. 2012, 24, 116-124.
doi: 10.1016/j.ceb.2011.11.001 URL |
| 84. |
Dumbauld, D. W.; Lee, T. T.; Singh, A.; Scrimgeour, J.; Gersbach, C. A.; Zamir, E. A.; Fu, J.; Chen, C. S.; Curtis, J. E.; Craig, S. W.; García, A. J. How vinculin regulates force transmission. Proc Natl Acad Sci U S A. 2013, 110, 9788-9793.
doi: 10.1073/pnas.1216209110 URL |
| 85. | Roca-Cusachs, P.; del Rio, A.; Puklin-Faucher, E.; Gauthier, N. C.; Biais, N.; Sheetz, M. P. Integrin-dependent force transmission to the extracellular matrix by α-actinin triggers adhesion maturation. Proc Natl Acad Sci U S A. 2013, 110, E1361-1370. |
| 86. |
Lv, H.; Li, L.; Sun, M.; Zhang, Y.; Chen, L.; Rong, Y.; Li, Y. Mechanism of regulation of stem cell differentiation by matrix stiffness. Stem Cell Res Ther. 2015, 6, 103.
doi: 10.1186/s13287-015-0083-4 URL |
| 87. |
Randriantsilefisoa, R.; Hou, Y.; Pan, Y.; Camacho, J. L. C.; Kulka, M. W.; Zhang, J.; Haag, R. Interaction of human mesenchymal stem cells with soft nanocomposite hydrogels based on polyethylene glycol and dendritic polyglycerol. Adv Funct Mater. 2020, 30, 1905200.
doi: 10.1002/adfm.v30.1 URL |
| 88. | Lundin, V.; Sugden, W. W.; Theodore, L. N.; Sousa, P. M.; Han, A.; Chou, S.; Wrighton, P. J.; Cox, A. G.; Ingber, D. E.; Goessling, W.; Daley, G. Q.; North, T. E. YAP regulates hematopoietic stem cell formation in response to the biomechanical forces of blood flow. Dev Cell. 2020, 52, 446-460.e5. |
| 89. |
González-García, C.; Sousa, S. R.; Moratal, D.; Rico, P.; Salmerón-Sánchez, M. Effect of nanoscale topography on fibronectin adsorption, focal adhesion size and matrix organisation. Colloids Surf B Biointerfaces. 2010, 77, 181-190.
doi: 10.1016/j.colsurfb.2010.01.021 URL |
| 90. | Ross, E. A.; Turner, L. A.; Saeed, A.; Burgess, K. V.; Blackburn, G.; Reynolds, P.; Wells, J. A.; Mountford, J.; Gadegaard, N.; Salmeron-Sanchez, M.; Oreffo, R. O.; Dalby, M. J. Nanotopography reveals metabolites that maintain the immunosuppressive phenotype of mesenchymal stem cells. bioRxiv. 2019, 603332. |
| 91. |
Ngandu Mpoyi, E.; Cantini, M.; Reynolds, P. M.; Gadegaard, N.; Dalby, M. J.; Salmerón-Sánchez, M. Protein adsorption as a key mediator in the nanotopographical control of cell behavior. ACS Nano. 2016, 10, 6638-6647.
doi: 10.1021/acsnano.6b01649 URL |
| 92. |
Luo, J.; Walker, M.; Xiao, Y.; Donnelly, H.; Dalby, M. J.; Salmeron-Sanchez, M. The influence of nanotopography on cell behaviour through interactions with the extracellular matrix - a review. Bioact Mater. 2021. doi: 10.1016/j.bioactmat.2021.11.024.
doi: 10.1016/j.bioactmat.2021.11.024 URL |
| 93. |
Lei, R.; Kumar, S. Getting the big picture of cell-matrix interactions: High-throughput biomaterial platforms and systems-level measurements. Curr Opin Solid State Mater Sci. 2020, 24, 100871.
doi: 10.1016/j.cossms.2020.100871 URL |
| 94. | Yang, L.; Jurczak, K. M.; Ge, L.; van Rijn, P. High-throughput screening and hierarchical topography-mediated neural differentiation of mesenchymal stem cells. Adv Healthc Mater. 2020, 9, e2000117. |
| [1] | Xuechen Zhang, Ana Justo Caetano, Paul T. Sharpe, Ana Angelova Volponi. Oral stem cells, decoding and mapping the resident cells populations [J]. Biomaterials Translational, 2022, 3(1): 24-30. |
| [2] | Deepika Arora, Pamela Gehron Robey. Recent updates on the biological basis of heterogeneity in bone marrow stromal cells/skeletal stem cells [J]. Biomaterials Translational, 2022, 3(1): 3-16. |
| [3] | Suzanne M. Watt. The long and winding road: homeostatic and disordered haematopoietic microenvironmental niches: a narrative review [J]. Biomaterials Translational, 2022, 3(1): 31-54. |
| [4] | Emma Steijvers, Armaan Ghei, Zhidao Xia. Manufacturing artificial bone allografts: a perspective [J]. Biomaterials Translational, 2022, 3(1): 65-80. |
| [5] | Ke Hu, Yuxuan Li, Zunxiang Ke, Hongjun Yang, Chanjun Lu, Yiqing Li, Yi Guo, Weici Wang. History, progress and future challenges of artificial blood vessels: a narrative review [J]. Biomaterials Translational, 2022, 3(1): 81-98. |
| [6] | Peter W. Andrews. Human pluripotent stem cells: tools for regenerative medicine [J]. Biomaterials Translational, 2021, 2(4): 294-300. |
| [7] | Trivia P. Frazier, Katie Hamel, Xiying Wu, Emma Rogers, Haley Lassiter, Jordan Robinson, Omair Mohiuddin, Michael Henderson, Jeffrey M. Gimble. Adipose-derived cells: building blocks of three-dimensional microphysiological systems [J]. Biomaterials Translational, 2021, 2(4): 301-306. |
| [8] | Arnold I. Caplan. Mesenchymal stem cells and COVID-19: the process of discovery and of translation [J]. Biomaterials Translational, 2021, 2(4): 307-311. |
| [9] | Yizhong Peng, Jinye Li, Hui Lin, Shuo Tian, Sheng Liu, Feifei Pu, Lei Zhao, Kaige Ma, Xiangcheng Qing, Zengwu Shao. Endogenous repair theory enriches construction strategies for orthopaedic biomaterials: a narrative review [J]. Biomaterials Translational, 2021, 2(4): 343-360. |
| [10] | Kamolrat Metavarayuth, Esteban Villarreal, Hui Wang, Qian Wang. Surface topography and free energy regulate osteogenesis of stem cells: effects of shape-controlled gold nanoparticles [J]. Biomaterials Translational, 2021, 2(2): 165-173. |
| [11] | Yizhong Peng, Xiangcheng Qing, Hongyang Shu, Shuo Tian, Wenbo Yang, Songfeng Chen, Hui Lin, Xiao Lv, Lei Zhao, Xi Chen, Feifei Pu, Donghua Huang, Xu Cao, Zengwu Shao. Proper animal experimental designs for preclinical research of biomaterials for intervertebral disc regeneration [J]. Biomaterials Translational, 2021, 2(2): 91-142. |
| [12] | Pingli Wu, Yangyang Liang, Guoming Sun. Engineering immune-responsive biomaterials for skin regeneration [J]. Biomaterials Translational, 2021, 2(1): 61-71. |
| [13] | Yiqing Wang, Xiangyu Chu, Bing Wang. Recombinant adeno-associated virus-based gene therapy combined with tissue engineering for musculoskeletal regenerative medicine [J]. Biomaterials Translational, 2021, 2(1): 19-29. |
| [14] | Isak Jatoi, Jingyu Fan. A biomaterials viewpoint for the 2020 SARS-CoV-2 vaccine development [J]. Biomaterials Translational, 2021, 2(1): 30-42. |
| [15] | Xing Yang, Yuanyuan Li, Xujie Liu, Wei He, Qianli Huang, Qingling Feng. Nanoparticles and their effects on differentiation of mesenchymal stem cells [J]. Biomaterials Translational, 2020, 1(1): 58-68. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
