Biomaterials Translational ›› 2020, Vol. 1 ›› Issue (1): 46-57.doi: 10.3877/cma.j.issn.2096-112X.2020.01.005
• REVIEW • Previous Articles Next Articles
Ronghua Tan, Ying Wan*( ), Xiangliang Yang*(
), Xiangliang Yang*( )
)
Received:2020-08-01
Revised:2020-09-02
Accepted:2020-09-11
Online:2020-12-28
Published:2020-12-28
Contact:
Ying Wan,Xiangliang Yang
E-mail:ying_wan@hust.edu.cn;yangxl@hust.edu.cn
Tan, R.; Wan, Y.; Yang, X. Hydroxyethyl starch and its derivatives as nanocarriers for delivery of diagnostic and therapeutic agents towards cancers. Biomater Transl. 2020, 1(1), 46-57.
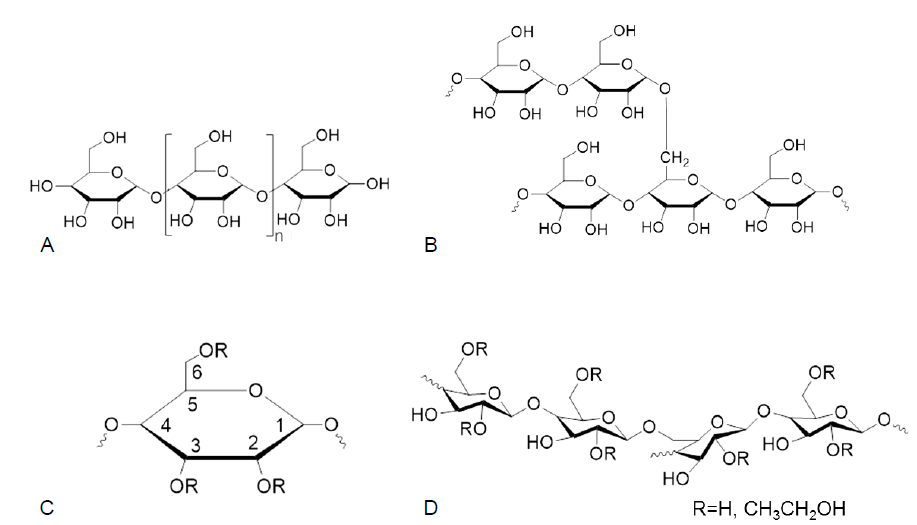
Figure 1. (A, B) Chemical structures of amylose (A) and amylopectin (B). (C) Possible substitution patterns of hydroxyethyl modification in the glucosyl unit of starch. (D) Chemical structures of HES.
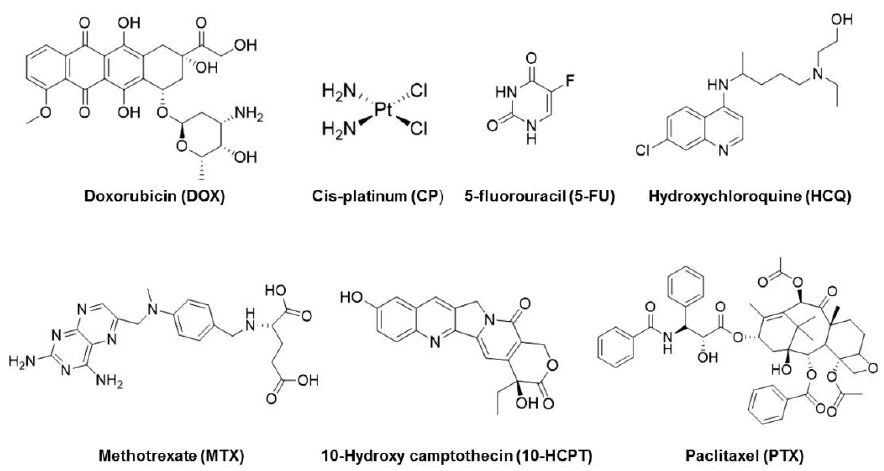
Figure 2. Chemical structures of several kinds of chemotherapeutic anticancer drugs used for constructing HES-based prodrugs. HES: hydroxyethyl starch.
| Name of prodrug | Name of drug | Responsiveness | Linkage | Strength | Reference |
|---|---|---|---|---|---|
| HES-SS-DOX | DOX | Redox | Disulphide bond | Responsive release; Improved efficiency; Reduced side effects | Hu et al. |
| HES-SS-PTX | PTX | Enzyme/Redox | Disulphide bond | Responsive release; Enhanced penetration; Improved efficiency; Reduced side effects | Li et al. |
| HES-FUAC | 5-FU | - | Ester bond | Improved efficiency; Reduced side effects | Luo et al. |
| 10-HCPT-HES | 10-HCPT | - | Amide bond | Improved efficiency; Reduced side effects | Li et al. |
| HES-MTX | MTX | - | Ester bond | Improved efficiency; Reduced side effects | Goszczyński et al. |
| CQ-HES | HCQ | - | Ester bond | Improved efficiency; Reduced side effects | Sleightholm et al. |
| HES-Hyd-DOX | DOX | pH | Hydrazine bond | Responsive release | Zhu et al. |
| HES=DOX | DOX | pH | Imine bond | Targeting; Improved efficiency; Reduced side effects | Li et al. |
| HES=DOX/cRGD | DOX | pH | Imine bond | Targeting; Improved efficiency; Reduced side effects | Li et al. |
| HES-DOX/LHRH | DOX | pH | Imine bond | Targeting; Improved efficiency; Reduced side effects | Zhao et al. |
| LA-HES-Pt | Pt | - | Ester bond | Improved efficiency; Reduced side effects | Xiao et al. |
Table 1 HES-based polymeric prodrugs and their characteristics
| Name of prodrug | Name of drug | Responsiveness | Linkage | Strength | Reference |
|---|---|---|---|---|---|
| HES-SS-DOX | DOX | Redox | Disulphide bond | Responsive release; Improved efficiency; Reduced side effects | Hu et al. |
| HES-SS-PTX | PTX | Enzyme/Redox | Disulphide bond | Responsive release; Enhanced penetration; Improved efficiency; Reduced side effects | Li et al. |
| HES-FUAC | 5-FU | - | Ester bond | Improved efficiency; Reduced side effects | Luo et al. |
| 10-HCPT-HES | 10-HCPT | - | Amide bond | Improved efficiency; Reduced side effects | Li et al. |
| HES-MTX | MTX | - | Ester bond | Improved efficiency; Reduced side effects | Goszczyński et al. |
| CQ-HES | HCQ | - | Ester bond | Improved efficiency; Reduced side effects | Sleightholm et al. |
| HES-Hyd-DOX | DOX | pH | Hydrazine bond | Responsive release | Zhu et al. |
| HES=DOX | DOX | pH | Imine bond | Targeting; Improved efficiency; Reduced side effects | Li et al. |
| HES=DOX/cRGD | DOX | pH | Imine bond | Targeting; Improved efficiency; Reduced side effects | Li et al. |
| HES-DOX/LHRH | DOX | pH | Imine bond | Targeting; Improved efficiency; Reduced side effects | Zhao et al. |
| LA-HES-Pt | Pt | - | Ester bond | Improved efficiency; Reduced side effects | Xiao et al. |
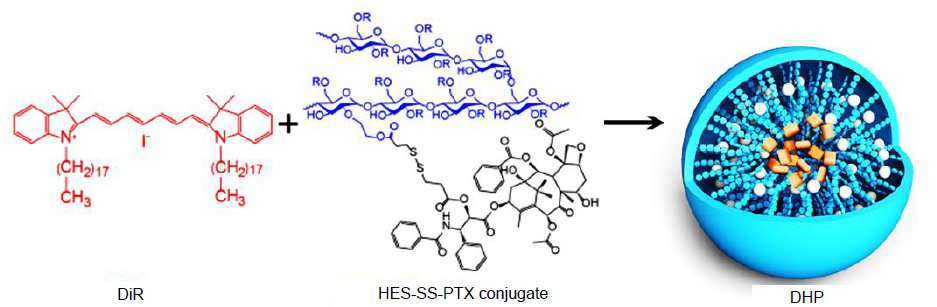
Figure 3. Schematic illustration for the construction of DiR/HES-SS-PTX NPs (DHP). DiR: 1,1-dioctadecyl-3,3,3,3-tetramethyl indotricarbocyanine iodide; HES: hydroxyethyl starch; NPs: nanoparticles; PTX: paclitaxel.
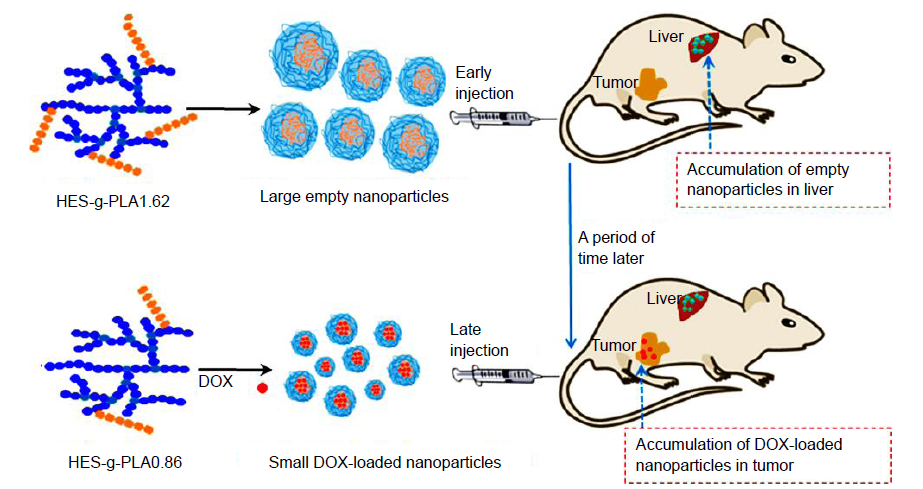
Figure 4. Schematic illustration showing the delivery of DOX toward tumours using HES-g-PLA partner nanocarriers. DOX: doxorubicin; HES-g-PLA: hydroxyethyl starch-grafted-polylactide.
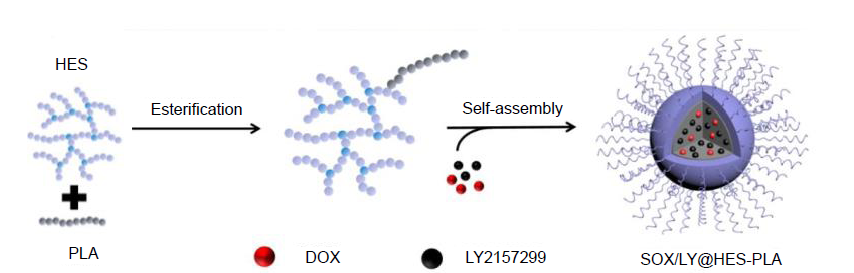
Figure 5. Schematic representation showing co-loading of DOX and LY2157299 into HES-g-PLA NPs. DOX: doxorubicin; HES-g-PLA: hydroxyethyl starch-grafted-polylactide; LY2157299: a transforming growth factor-β receptor inhibitor.
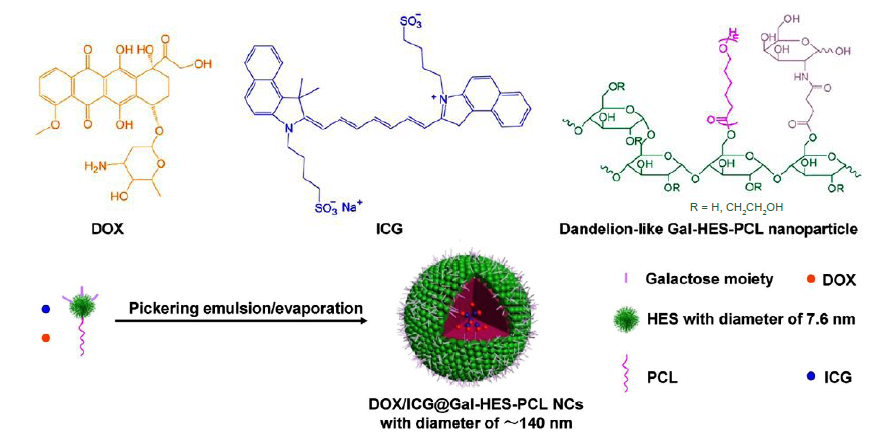
Figure 6. Schematic illustration of the structure of DOX/ICG-loaded Gal-HES-PCL nanocolloidosomes and their pickering emulsion formation. DOX: doxorubicin; Gal: galactose; HES: hydroxyethyl starch; ICG: indocyanine green; PCL: polycaprolactone.
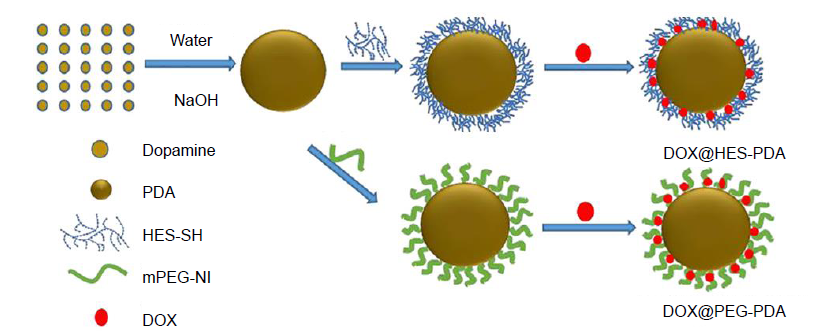
Figure 7. Schematic illustration showing the preparation of DOX@HES-PDA NPs and DOX@PEG-PDA NPs. DOX: doxorubicin; HES: hydroxyethyl starch; mPEG: methoxy poly(ethylene glycol); NPs: nanoparticles; PDA: polydopamine; PEG: poly(ethylene glycol).
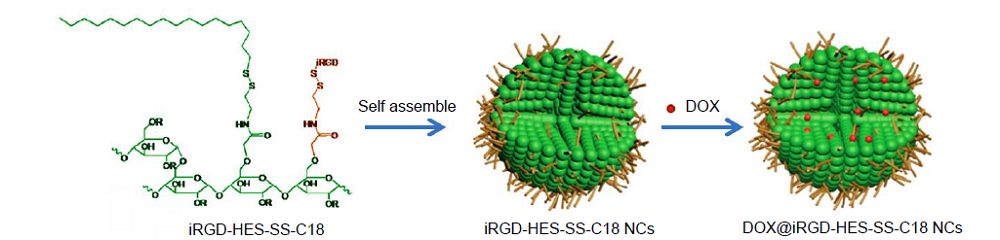
Figure 8. Schematic illustration of the fabrication of DOX@iRGD-HES-SS-C18 NCs. C18: 1-octadecanethiol; DOX: doxorubicin; HES: hydroxyethyl starch; iRGD: 9-amino acid cyclic peptide; NC: nanoclusters.
| Name of nanoparticles | Name of drug | Responsiveness | Strength | Reference |
|---|---|---|---|---|
| DiR/HES-SS-PTX | PTX | Redox/Radiation | Combination therapy; Imaging | Li et al. |
| HES-SS-DOX@ICG | DOX | Redox/Radiation | Combination therapy; Imaging | Yu et al. |
| DOX@HES-g-PLA | DOX | - | RES blockade | Yu et al. |
| DOX/LY@HES-g-PLA | DOX | - | Overcoming metastasis | Zhou et al. |
| DOX/ICG@Gal-HES-PCL | DOX | Radiation | Targeting; Combination therapy; Imaging | Hu et al. |
| DOX@HES-PDA | DOX | - | HESylation comparison | Wu et al. |
| DOX@iRGD-HES-SS-C18 | DOX | Redox | Targeting | Hu et al. |
| ICG@HES-OA | PEITC | Radiation | Photodynamic therapy;Combination therapy | Hu et al. |
| PyHES-NAC | DOX | pH | Oral delivery | Jong et al. |
| HES-CUR | CUR | - | Improved efficiency | Chen et al. |
| HES-TG100-115-CDM-PEG | Sorafenib | - | Combination therapy | Li and Zhao |
Table 2 HES-based nanoparticles and their characteristics.
| Name of nanoparticles | Name of drug | Responsiveness | Strength | Reference |
|---|---|---|---|---|
| DiR/HES-SS-PTX | PTX | Redox/Radiation | Combination therapy; Imaging | Li et al. |
| HES-SS-DOX@ICG | DOX | Redox/Radiation | Combination therapy; Imaging | Yu et al. |
| DOX@HES-g-PLA | DOX | - | RES blockade | Yu et al. |
| DOX/LY@HES-g-PLA | DOX | - | Overcoming metastasis | Zhou et al. |
| DOX/ICG@Gal-HES-PCL | DOX | Radiation | Targeting; Combination therapy; Imaging | Hu et al. |
| DOX@HES-PDA | DOX | - | HESylation comparison | Wu et al. |
| DOX@iRGD-HES-SS-C18 | DOX | Redox | Targeting | Hu et al. |
| ICG@HES-OA | PEITC | Radiation | Photodynamic therapy;Combination therapy | Hu et al. |
| PyHES-NAC | DOX | pH | Oral delivery | Jong et al. |
| HES-CUR | CUR | - | Improved efficiency | Chen et al. |
| HES-TG100-115-CDM-PEG | Sorafenib | - | Combination therapy | Li and Zhao |
| 1. |
Aslam, M.; Naveed, S.; Ahmed, A.; Abbas, Z.; Gull, I.; Athar, M. Side effects of chemotherapy in cancer patients and evaluation of patients opinion about starvation based differential chemotherapy. J Cancer Ther. 2014,5, 817-822.
doi: 10.4236/jct.2014.58089 URL |
| 2. |
Volk-Draper, L.; Hall, K.; Griggs, C.; Rajput, S.; Kohio, P.; DeNardo, D.; Ran, S. Paclitaxel therapy promotes breast cancer metastasis in a TLR4-dependent manner. Cancer Res. 2014,74, 5421-5434.
doi: 10.1158/0008-5472.CAN-14-0067 URL pmid: 25274031 |
| 3. |
Wang, A. Z.; Langer, R.; Farokhzad, O. C. Nanoparticle delivery of cancer drugs. Annu Rev Med. 2012,63, 185-198.
doi: 10.1146/annurev-med-040210-162544 URL pmid: 21888516 |
| 4. |
Fang, R.; Liu, M.; Jiang, L. Design of nanoparticle systems by controllable assembly and temporal/spatial regulation. Adv Funct Mater. 2020,30, 1903351.
doi: 10.1002/adfm.v30.2 URL |
| 5. |
Harada, A.; Kataoka, K. Supramolecular assemblies of block copolymers in aqueous media as nanocontainers relevant to biological applications. Prog Polym Sci. 2006,31, 949-982.
doi: 10.1016/j.progpolymsci.2006.09.004 URL |
| 6. |
Tao, R.; Gao, M.; Liu, F.; Guo, X.; Fan, A.; Ding, D.; Kong, D.; Wang, Z.; Zhao, Y. Alleviating the liver toxicity of chemotherapy via ph-responsive hepatoprotective prodrug micelles. ACS Appl Mater Interfaces. 2018,10, 21836-21846.
doi: 10.1021/acsami.8b04192 URL pmid: 29897226 |
| 7. |
Wang, Y.; Wang, X.; Deng, F.; Zheng, N.; Liang, Y.; Zhang, H.; He, B.; Dai, W.; Wang, X.; Zhang, Q. The effect of linkers on the self-assembling and anti-tumor efficacy of disulfide-linked doxorubicin drug-drug conjugate nanoparticles. J Control Release. 2018,279, 136-146.
doi: 10.1016/j.jconrel.2018.04.019 URL pmid: 29655991 |
| 8. |
An, X.; Zhu, A.; Luo, H.; Ke, H.; Chen, H.; Zhao, Y. Rational design of multi-stimuli-responsive nanoparticles for precise cancer therapy. ACS Nano. 2016,10, 5947-5958.
doi: 10.1021/acsnano.6b01296 URL pmid: 27285378 |
| 9. |
Meng, X.; Gao, M.; Deng, J.; Lu, D.; Fan, A.; Ding, D.; Kong, D.; Wang, Z.; Zhao, Y. Self-immolative micellar drug delivery: The linker matters. Nano Res. 2018,11, 6177-6189.
doi: 10.1007/s12274-018-2134-5 URL |
| 10. |
Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015,33, 941-951.
doi: 10.1038/nbt.3330 URL pmid: 26348965 |
| 11. |
Deng, C.; Jiang, Y.; Cheng, R.; Meng, F.; Zhong, Z. Biodegradable polymeric micelles for targeted and controlled anticancer drug delivery: Promises, progress and prospects. Nano Today. 2012,7, 467-480.
doi: 10.1016/j.nantod.2012.08.005 URL |
| 12. |
Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv Drug Deliv Rev. 2008,60, 1650-1662.
doi: 10.1016/j.addr.2008.09.001 URL pmid: 18848591 |
| 13. |
Goodarzi, N.; Varshochian, R.; Kamalinia, G.; Atyabi, F.; Dinarvand, R. A review of polysaccharide cytotoxic drug conjugates for cancer therapy. Carbohydr Polym. 2013,92, 1280-1293.
doi: 10.1016/j.carbpol.2012.10.036 URL pmid: 23399156 |
| 14. |
Westphal, M.; James, M. F.; Kozek-Langenecker, S.; Stocker, R.; Guidet, B.; Van Aken, H. Hydroxyethyl starches: different products--different effects. Anesthesiology. 2009,111, 187-202.
doi: 10.1097/ALN.0b013e3181a7ec82 URL pmid: 19512862 |
| 15. |
Li, D.; Ding, J.; Zhuang, X.; Chen, L.; Chen, X. Drug binding rate regulates the properties of polysaccharide prodrugs. J Mater Chem B. 2016,4, 5167-5177.
doi: 10.1039/c6tb00991c URL pmid: 32263515 |
| 16. |
Paleos, C. M.; Sideratou, Z.; Tsiourvas, D. Drug delivery systems based on hydroxyethyl starch. Bioconjug Chem. 2017,28, 1611-1624.
doi: 10.1021/acs.bioconjchem.7b00186 URL pmid: 28431209 |
| 17. |
Goszczyński, T. M.; Filip-Psurska, B.; Kempińska, K.; Wietrzyk, J.; Boratyński, J. Hydroxyethyl starch as an effective methotrexate carrier in anticancer therapy. Pharmacol Res Perspect. 2014,2, e00047.
doi: 10.1002/prp2.47 URL pmid: 25505592 |
| 18. |
Xie, F.; Pollet, E.; Halley, P. J.; Avérous, L. Starch-based nano-biocomposites. Prog Polym Sci. 2013,38, 1590-1628.
doi: 10.1016/j.progpolymsci.2013.05.002 URL |
| 19. |
Chen, Q.; Yu, H.; Wang, L.; ul Abdin, Z.; Chen, Y.; Wang, J.; Zhou, W.; Yang, X.; Khan, R. U.; Zhang, H.; Chen, X. Recent progress in chemical modification of starch and its applications. RSC Adv. 2015,5, 67459-67474.
doi: 10.1039/C5RA10849G URL |
| 20. | Glover, P. A.; Rudloff, E.; Kirby, R. Hydroxyethyl starch: a review of pharmacokinetics, pharmacodynamics, current products, and potential clinical risks, benefits, and use. J Vet Emerg Crit Care (San Antonio). 2014,24, 642-661. |
| 21. |
Li, W.; Xiao, X.; Zhang, W.; Zheng, J.; Luo, Q.; Ouyang, S.; Zhang, G. Compositional, morphological, structural and physicochemical properties of starches from seven naked barley cultivars grown in China. Food Res Int. 2014,58, 7-14.
doi: 10.1016/j.foodres.2014.01.053 URL |
| 22. |
Ai, Y.; Jane, J. L. Gelatinization and rheological properties of starch. Starke. 2015,67, 213-224.
doi: 10.1002/star.201400201 URL |
| 23. | Gosch, C. I.; Haase, T.; Wolf, B. A.; Kulicke, W. M. Molar mass distribution and size of hydroxyethyl starch fractions obtained by continuous polymer fractionation. Starke. 2002,54, 375-384. |
| 24. |
Boldt, J. Modern rapidly degradable hydroxyethyl starches: current concepts. Anesth Analg. 2009,108, 1574-1582.
doi: 10.1213/ane.0b013e31819e9e6c URL pmid: 19372338 |
| 25. |
Metcalf, W.; Papadopoulos, A.; Tufaro, R.; Barth, A. A clinical physiologic study of hydroxyethyl starch. Surg Gynecol Obstet. 1970,131, 255-267.
URL pmid: 5433843 |
| 26. |
Besheer, A.; Hause, G.; Kressler, J.; Mäder, K. Hydrophobically modified hydroxyethyl starch: synthesis, characterization, and aqueous self-assembly into nano-sized polymeric micelles and vesicles. Biomacromolecules. 2007,8, 359-367.
URL pmid: 17256901 |
| 27. |
Yu, C.; Zhou, Q.; Xiao, F.; Li, Y.; Hu, H.; Wan, Y.; Li, Z.; Yang, X. Enhancing doxorubicin delivery toward tumor by hydroxyethyl starch-g-polylactide partner nanocarriers. ACS Appl Mater Interfaces. 2017,9, 10481-10493.
doi: 10.1021/acsami.7b00048 URL pmid: 28266842 |
| 28. |
Li, Y.; Wu, Y.; Chen, J.; Wan, J.; Xiao, C.; Guan, J.; Song, X.; Li, S.; Zhang, M.; Cui, H.; Li, T.; Yang, X.; Li, Z.; Yang, X. A simple glutathione-responsive turn-on theranostic nanoparticle for dual-modal imaging and chemo-photothermal combination therapy. Nano Lett. 2019,19, 5806-5817.
doi: 10.1021/acs.nanolett.9b02769 URL pmid: 31331172 |
| 29. |
Li, Y.; Hu, H.; Zhou, Q.; Ao, Y.; Xiao, C.; Wan, J.; Wan, Y.; Xu, H.; Li, Z.; Yang, X. α-Amylase- and redox-responsive nanoparticles for tumor-targeted drug delivery. ACS Appl Mater Interfaces. 2017,9, 19215-19230.
doi: 10.1021/acsami.7b04066 URL pmid: 28513132 |
| 30. |
Hu, H.; Li, Y.; Zhou, Q.; Ao, Y.; Yu, C.; Wan, Y.; Xu, H.; Li, Z.; Yang, X. Redox-sensitive hydroxyethyl starch-doxorubicin conjugate for tumor targeted drug delivery. ACS Appl Mater Interfaces. 2016,8, 30833-30844.
doi: 10.1021/acsami.6b11932 URL pmid: 27791359 |
| 31. |
Hu, H.; Wan, J.; Huang, X.; Tang, Y.; Xiao, C.; Xu, H.; Yang, X.; Li, Z. iRGD-decorated reduction-responsive nanoclusters for targeted drug delivery. Nanoscale. 2018,10, 10514-10527.
doi: 10.1039/c8nr02534g URL pmid: 29799599 |
| 32. |
Xiao, C.; Hu, H.; Yang, H.; Li, S.; Zhou, H.; Ruan, J.; Zhu, Y.; Yang, X.; Li, Z. Colloidal hydroxyethyl starch for tumor-targeted platinum delivery. Nanoscale Adv. 2019,1, 1002-1012.
doi: 10.1039/C8NA00271A URL |
| 33. |
Wu, H.; Hu, H.; Wan, J.; Li, Y.; Wu, Y.; Tang, Y.; Xiao, C.; Xu, H.; Yang, X.; Li, Z. Hydroxyethyl starch stabilized polydopamine nanoparticles for cancer chemotherapy. Chem Eng J. 2018,349, 129-145.
doi: 10.1016/j.cej.2018.05.082 URL |
| 34. |
Liu, Q.; Yang, X.; Xu, H.; Pan, K.; Yang, Y. Novel nanomicelles originating from hydroxyethyl starch-g-polylactide and their release behavior of docetaxel modulated by the PLA chain length. Eur Polym J. 2013,49, 3522-3529.
doi: 10.1016/j.eurpolymj.2013.08.012 URL |
| 35. |
Zhou, Q.; Li, Y.; Zhu, Y.; Yu, C.; Jia, H.; Bao, B.; Hu, H.; Xiao, C.; Zhang, J.; Zeng, X.; Wan, Y.; Xu, H.; Li, Z.; Yang, X. Co-delivery nanoparticle to overcome metastasis promoted by insufficient chemotherapy. J Control Release. 2018,275, 67-77.
doi: 10.1016/j.jconrel.2018.02.026 URL pmid: 29471038 |
| 36. |
Larson, N.; Ghandehari, H. Polymeric conjugates for drug delivery. Chem Mater. 2012,24, 840-853.
doi: 10.1021/cm2031569 URL pmid: 22707853 |
| 37. |
Zhou, P.; Li, Z.; Chau, Y. Synthesis, characterization, and in vivo evaluation of poly(ethylene oxide-co-glycidol)-platinate conjugate. Eur J Pharm Sci. 2010,41, 464-472.
doi: 10.1016/j.ejps.2010.07.014 URL pmid: 20709170 |
| 38. | Lipinski, C. Poor aqueous solubility-an industry wide problem in drug discovery. Am Pharm Rev. 2002,5, 82-85. |
| 39. |
Zhu, C.; Liu, L.; Yang, Q.; Lv, F.; Wang, S. Water-soluble conjugated polymers for imaging, diagnosis, and therapy. Chem Rev. 2012,112, 4687-4735.
doi: 10.1021/cr200263w URL pmid: 22670807 |
| 40. |
Luo, Q.; Wang, P.; Miao, Y.; He, H.; Tang, X. A novel 5-fluorouracil prodrug using hydroxyethyl starch as a macromolecular carrier for sustained release. Carbohydr Polym. 2012,87, 2642-2647.
doi: 10.1016/j.carbpol.2011.11.039 URL |
| 41. |
Zhou, Z.; Ma, X.; Jin, E.; Tang, J.; Sui, M.; Shen, Y.; Van Kirk, E. A.; Murdoch, W. J.; Radosz, M. Linear-dendritic drug conjugates forming long-circulating nanorods for cancer-drug delivery. Biomaterials. 2013,34, 5722-5735.
doi: 10.1016/j.biomaterials.2013.04.012 URL pmid: 23639529 |
| 42. |
Jana, S.; Mandlekar, S.; Marathe, P. Prodrug design to improve pharmacokinetic and drug delivery properties: challenges to the discovery scientists. Curr Med Chem. 2010,17, 3874-3908.
doi: 10.2174/092986710793205426 URL pmid: 20858214 |
| 43. |
Dong, Z.; Li, Q.; Guo, D.; Shu, Y.; Polli, J. E. Synjournal and evaluation of bile acid-ribavirin conjugates as prodrugs to target the liver. J Pharm Sci. 2015,104, 2864-2876.
doi: 10.1002/jps.24375 URL pmid: 25645375 |
| 44. |
Lelieveldt, L.; Kristyanto, H.; Pruijn, G. J. M.; Scherer, H. U.; Toes, R. E. M.; Bonger, K. M. Sequential prodrug strategy to target and eliminate ACPA-selective autoreactive B cells. Mol Pharm. 2018,15, 5565-5573.
doi: 10.1021/acs.molpharmaceut.8b00741 URL pmid: 30289723 |
| 45. |
Li, D.; Feng, X.; Chen, L.; Ding, J.; Chen, X. One-step synthesis of targeted acid-labile polysaccharide prodrug for efficiently intracellular drug delivery. ACS Biomater Sci Eng. 2018,4, 539-546.
doi: 10.1021/acsbiomaterials.7b00856 URL |
| 46. |
Zhao, K.; Li, D.; Xu, W.; Ding, J.; Jiang, W.; Li, M.; Wang, C.; Chen, X. Targeted hydroxyethyl starch prodrug for inhibiting the growth and metastasis of prostate cancer. Biomaterials. 2017,116, 82-94.
doi: 10.1016/j.biomaterials.2016.11.030 URL pmid: 27914269 |
| 47. |
Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986,46, 6387-6392.
URL pmid: 2946403 |
| 48. |
Zhou, Q.; Shao, S.; Wang, J.; Xu, C.; Xiang, J.; Piao, Y.; Zhou, Z.; Yu, Q.; Tang, J.; Liu, X.; Gan, Z.; Mo, R.; Gu, Z.; Shen, Y. Enzyme-activatable polymer-drug conjugate augments tumour penetration and treatment efficacy. Nat Nanotechnol. 2019,14, 799-809.
doi: 10.1038/s41565-019-0485-z URL pmid: 31263194 |
| 49. |
Li, G.; Li, Y.; Tang, Y.; Zhang, Y.; Zhang, Y.; Yin, T.; Xu, H.; Cai, C.; Tang, X. Hydroxyethyl starch conjugates for improving the stability, pharmacokinetic behavior and antitumor activity of 10-hydroxy camptothecin. Int J Pharm. 2014,471, 234-244.
doi: 10.1016/j.ijpharm.2014.05.038 URL pmid: 24861941 |
| 50. |
Li, G.; Zhao, M.; Zhao, L. Well-defined hydroxyethyl starch-10-hydroxy camptothecin super macromolecule conjugate: cytotoxicity, pharmacodynamics research, tissue distribution test and intravenous injection safety assessment. Drug Deliv. 2016,23, 2860-2868.
doi: 10.3109/10717544.2015.1110844 URL pmid: 26836216 |
| 51. | Zhu, Y.; Yao, X.; Chen, X.; Chen, L. pH-sensitive hydroxyethyl starch-doxorubicin conjugates as antitumor prodrugs with enhanced anticancer efficacy. J Appl Polym Sci. 2015,132, 42778. |
| 52. |
Sleightholm, R.; Yang, B.; Yu, F.; Xie, Y.; Oupický, D. Chloroquine-modified hydroxyethyl starch as a polymeric drug for cancer therapy. Biomacromolecules. 2017,18, 2247-2257.
doi: 10.1021/acs.biomac.7b00023 URL pmid: 28708385 |
| 53. |
Kuppusamy, P.; Li, H.; Ilangovan, G.; Cardounel, A. J.; Zweier, J. L.; Yamada, K.; Krishna, M. C.; Mitchell, J. B. Noninvasive imaging of tumor redox status and its modification by tissue glutathione levels. Cancer Res. 2002,62, 307-312.
URL pmid: 11782393 |
| 54. |
Liu, S. V.; Liu, S.; Pinski, J. Luteinizing hormone-releasing hormone receptor targeted agents for prostate cancer. Expert Opin Investig Drugs. 2011,20, 769-778.
URL pmid: 21449823 |
| 55. |
Kunath, K.; von Harpe, A.; Fischer, D.; Kissel, T. Galactose-PEI-DNA complexes for targeted gene delivery: degree of substitution affects complex size and transfection efficiency. J Control Release. 2003,88, 159-172.
doi: 10.1016/s0168-3659(02)00458-3 URL pmid: 12586513 |
| 56. |
Li, Y.; Liu, G.; Ma, J.; Lin, J.; Lin, H.; Su, G.; Chen, D.; Ye, S.; Chen, X.; Zhu, X.; Hou, Z. Chemotherapeutic drug-photothermal agent co-self-assembling nanoparticles for near-infrared fluorescence and photoacoustic dual-modal imaging-guided chemo-photothermal synergistic therapy. J Control Release. 2017,258, 95-107.
doi: 10.1016/j.jconrel.2017.05.011 URL pmid: 28501673 |
| 57. |
Yu, C.; Liu, C.; Wang, S.; Li, Z.; Hu, H.; Wan, Y.; Yang, X. Hydroxyethyl starch-based nanoparticles featured with redox-sensitivity and chemo-photothermal therapy for synergized tumor eradication. Cancers (Basel). 2019,11, 207.
doi: 10.3390/cancers11020207 URL |
| 58. |
Hu, H.; Xiao, C.; Wu, H.; Li, Y.; Zhou, Q.; Tang, Y.; Yu, C.; Yang, X.; Li, Z. Nanocolloidosomes with selective drug release for active tumor-targeted imaging-guided photothermal/chemo combination therapy. ACS Appl Mater Interfaces. 2017,9, 42225-42238.
doi: 10.1021/acsami.7b14796 URL pmid: 29124920 |
| 59. |
Veronese, F. M. Peptide and protein PEGylation: a review of problems and solutions. Biomaterials. 2001,22, 405-417.
doi: 10.1016/s0142-9612(00)00193-9 URL pmid: 11214751 |
| 60. |
Nichols, J. W.; Bae, Y. H. Odyssey of a cancer nanoparticle: from injection site to site of action. Nano Today. 2012,7, 606-618.
doi: 10.1016/j.nantod.2012.10.010 URL pmid: 23243460 |
| 61. |
Lemarchand, C.; Gref, R.; Couvreur, P. Polysaccharide-decorated nanoparticles. Eur J Pharm Biopharm. 2004,58, 327-341.
doi: 10.1016/j.ejpb.2004.02.016 URL pmid: 15296959 |
| 62. |
Baier, G.; Baumann, D.; Siebert, J. M.; Musyanovych, A.; Mailänder, V.; Landfester, K. Suppressing unspecific cell uptake for targeted delivery using hydroxyethyl starch nanocapsules. Biomacromolecules. 2012,13, 2704-2715.
doi: 10.1021/bm300653v URL pmid: 22844871 |
| 63. |
Noga, M.; Edinger, D.; Kläger, R.; Wegner, S. V.; Spatz, J. P.; Wagner, E.; Winter, G.; Besheer, A. The effect of molar mass and degree of hydroxyethylation on the controlled shielding and deshielding of hydroxyethyl starch-coated polyplexes. Biomaterials. 2013,34, 2530-2538.
doi: 10.1016/j.biomaterials.2012.12.025 URL pmid: 23312901 |
| 64. |
Liebner, R.; Mathaes, R.; Meyer, M.; Hey, T.; Winter, G.; Besheer, A. Protein HESylation for half-life extension: synjournal, characterization and pharmacokinetics of HESylated anakinra. Eur J Pharm Biopharm. 2014,87, 378-385.
doi: 10.1016/j.ejpb.2014.03.010 URL pmid: 24681396 |
| 65. |
Liu, Y.; Ai, K.; Lu, L. Polydopamine and its derivative materials: synthesis and promising applications in energy, environmental, and biomedical fields. Chem Rev. 2014,114, 5057-5115.
doi: 10.1021/cr400407a URL pmid: 24517847 |
| 66. |
Hu, H.; Chen, J.; Yang, H.; Huang, X.; Wu, H.; Wu, Y.; Li, F.; Yi, Y.; Xiao, C.; Li, Y.; Tang, Y.; Li, Z.; Zhang, B.; Yang, X. Potentiating photodynamic therapy of ICG-loaded nanoparticles by depleting GSH with PEITC. Nanoscale. 2019,11, 6384-6393.
doi: 10.1039/c9nr01306g URL pmid: 30888375 |
| 67. |
Jong, K.; Ju, B.; Zhang, S. Synjournal of pH-responsive N-acetyl-cysteine modified starch derivatives for oral delivery. J Biomater Sci Polym Ed. 2017,28, 1525-1537.
doi: 10.1080/09205063.2017.1333698 URL pmid: 28532282 |
| 68. |
Besheer, A.; Vogel, J.; Glanz, D.; Kressler, J.; Groth, T.; Mäder, K. Characterization of PLGA nanospheres stabilized with amphiphilic polymers: hydrophobically modified hydroxyethyl starch vs pluronics. Mol Pharm. 2009,6, 407-415.
doi: 10.1021/mp800119h URL pmid: 19718794 |
| 69. |
Chen, S.; Wu, J.; Tang, Q.; Xu, C.; Huang, Y.; Huang, D.; Luo, F.; Wu, Y.; Yan, F.; Weng, Z.; Wang, S. Nano-micelles based on hydroxyethyl starch-curcumin conjugates for improved stability, antioxidant and anticancer activity of curcumin. Carbohydr Polym. 2020,228, 115398.
doi: 10.1016/j.carbpol.2019.115398 URL pmid: 31635734 |
| 70. |
Naksuriya, O.; Okonogi, S.; Schiffelers, R. M.; Hennink, W. E. Curcumin nanoformulations: a review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials. 2014,35, 3365-3383.
doi: 10.1016/j.biomaterials.2013.12.090 URL pmid: 24439402 |
| 71. |
Serban, D.; Leng, J.; Cheresh, D. H-ras regulates angiogenesis and vascular permeability by activation of distinct downstream effectors. Circ Res. 2008,102, 1350-1358.
doi: 10.1161/CIRCRESAHA.107.169664 URL pmid: 18467631 |
| 72. |
Li, G.; Zhao, L. Sorafenib-loaded hydroxyethyl starch-TG100-115 micelles for the treatment of liver cancer based on synergistic treatment. Drug Deliv. 2019,26, 756-764.
doi: 10.1080/10717544.2019.1642418 URL pmid: 31357893 |
| 73. |
Kang, B.; Okwieka, P.; Schöttler, S.; Seifert, O.; Kontermann, R. E.; Pfizenmaier, K.; Musyanovych, A.; Meyer, R.; Diken, M.; Sahin, U.; Mailänder, V.; Wurm, F. R.; Landfester, K. Tailoring the stealth properties of biocompatible polysaccharide nanocontainers. Biomaterials. 2015,49, 125-134.
URL pmid: 25725561 |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||