Biomaterials Translational ›› 2020, Vol. 1 ›› Issue (1): 89-98.doi: 10.3877/cma.j.issn.2096-112X.2020.01.009
• RESEARCH ARTICLE • Previous Articles
Jishan Yuan1, Panita Maturavongsadit2,3, Zhihui Zhou1, Bin Lv1, Yuan Lin4, Jia Yang2, Jittima Amie Luckanagul5,6,*( )
)
Received:2020-09-11
Revised:2020-10-13
Accepted:2020-10-14
Online:2020-12-28
Published:2020-12-28
Contact:
Jittima Amie Luckanagul
E-mail:jittima.luck@gmail.com
Yuan, J.; Maturavongsadit, P.; Zhou, Z.; Lv, B.; Lin, Y.; Yang, J.; Luckanagul, J. Hyaluronic acid-based hydrogels with tobacco mosaic virus containing cell adhesive peptide induce bone repair in normal and osteoporotic rats. Biomater Transl. 2020, 1(1), 89-98.
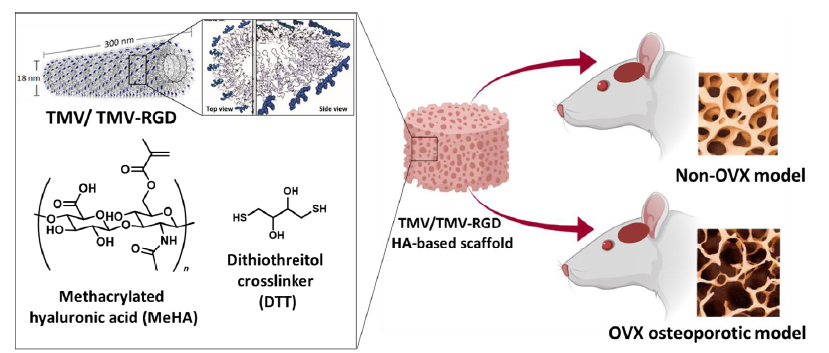
Figure 1. Schematic illustration of our study design. Methacrylated hyaluronic acid (MeHA) was synthesised and used with incorporated virus particles as a hydrogel structural platform to fill skull defects in osteoporotic and normal Sprague-Dawley rats. The RGD mutant TMV graphic was generated from PyMol with coordinates 2TMV from the Protein Data Bank (www.rcsb.org) and created with BioRender.com. OVX: ovariectomised; RGD: arginyl-glycyl-aspartic acid; TMV: tobacco mosaic virus.
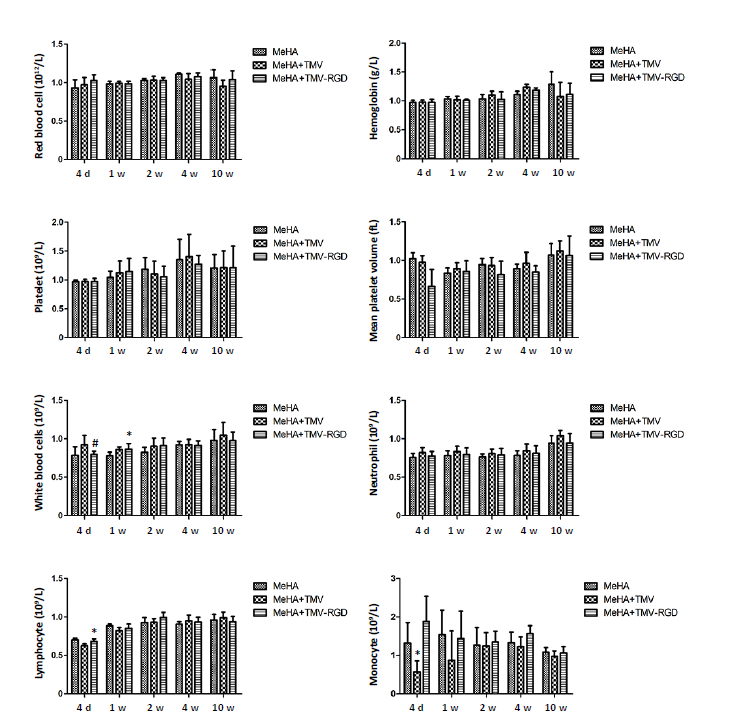
Figure 2. Haematological analysis of non-OVX animals treated with three different hydrogel materials (MeHA, MeHA + TMV, and MeHA + TMV-RGD) to fill their skull defects. Total blood counts were performed at five time points (4 days (d), 1, 2, 4, and 10 weeks (w)). Data are expressed as the mean ± SD (n = 3). *P < 0.05, vs. MeHA group; #P < 0.05, vs. MeHA + TMV group (one-way analysis of variance). MeHA: methacrylated hyaluronic acid; OVX: ovariectomised; RGD: arginyl-glycyl-aspartic acid; TMV: tobacco mosaic virus.
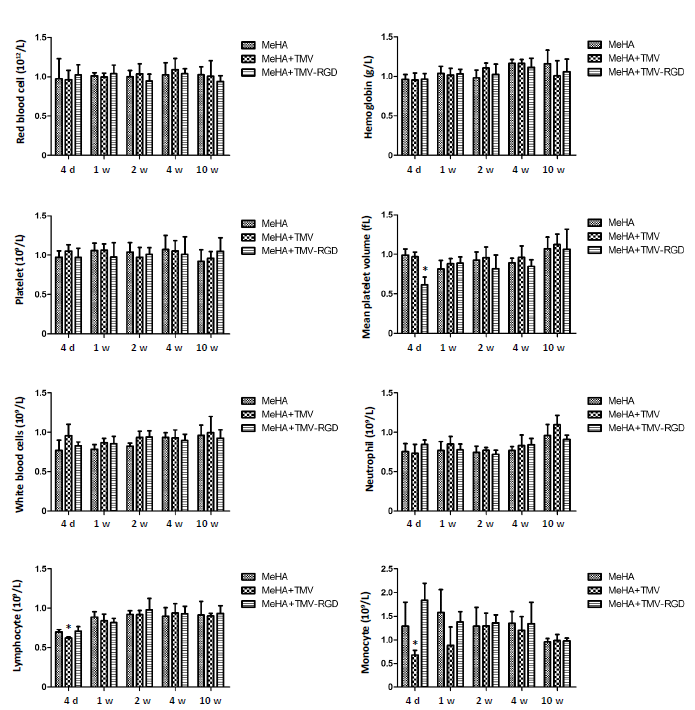
Figure 3. Haematological analysis of OVX animals treated with three different materials (MeHA, MeHA + TMV, and MeHA + TMV-RGD) to fill their skull defects. Total blood counts were performed at five time points (4 days (d), 1, 2, 4, and 10 weeks (w)). Data are expressed as the mean ± SD (n = 3). **P < 0.05, vs. MeHA group (one-way analysis of variance). MeHA: methacrylated hyaluronic acid; OVX: ovariectomised; RGD: arginyl-glycyl-aspartic acid; TMV: tobacco mosaic virus.
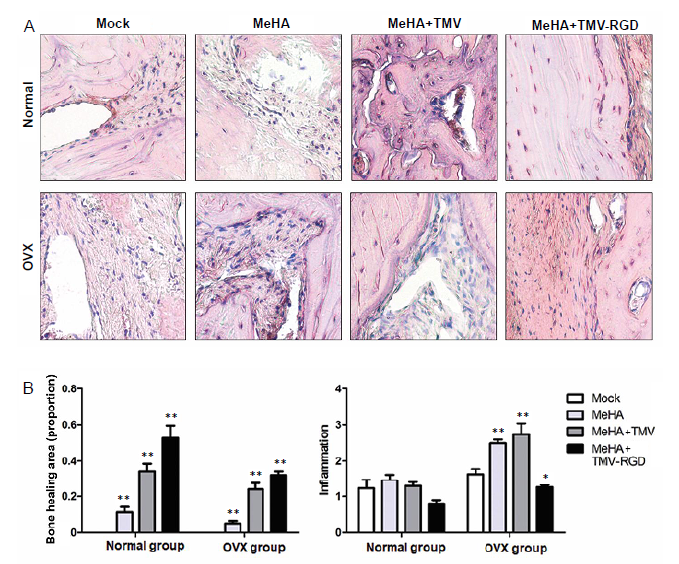
Figure 4. Inflammatory cell infiltration observed by hematoxylin and eosin staining. (A) Photomicrographs of hematoxylin and eosin-stained sections from skull defects implanted with each type of hydrogel (MeHA, MeHA + TMV, and MeHA + TMV-RGD) (original magnification, 40×). The new bone is visible as a compact structure with a pink colour. The connective tissue can be seen as a structured network of cells in a purple colour. (B) Hematoxylin and eosin histological scoring of the three types of hydrogels confirm the difference in proportion of bone healing area and degree of inflammation (arbitrary scoring). Data are expressed as the mean ± SD (n = 3). *P < 0.05, **P < 0.01, vs. sham group (one-way analysis of variance). MeHA: methacrylated hyaluronic acid; OVX: ovariectomised; RGD: arginyl-glycyl-aspartic acid; TMV: tobacco mosaic virus.
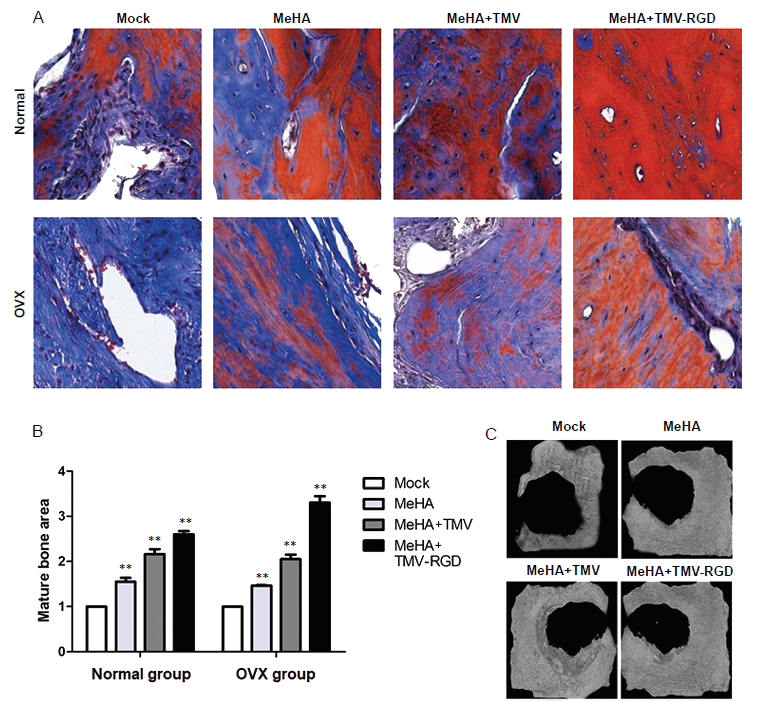
Figure 5. Histopathological analysis of bone substitute hydrogel implants stained with Masson’s trichrome. (A) Photomicrographs of corresponding Masson’s trichrome-stained sections from each type of hydrogel (original magnification, 40×). In general, Masson’s trichrome stains mature bone with osteoid formation red, whilst blue stain indicates developing calcified bone. (B) Histological scoring of the three types of hydrogels in tissue sections show the different amounts of mature bone area. Data are expressed as the mean ± SD (n = 3). *P < 0.05, **P < 0.01, vs. sham group (one-way analysis of variance). (C) Micro CT images of the cranial-defected bones dissected from non-OVX groups with different types of hydrogel implants at 10 weeks post-surgery. MeHA: methacrylated hyaluronic acid; OVX: ovariectomised; RGD: arginyl-glycyl-aspartic acid; TMV: tobacco mosaic virus.
| 1. |
Kaur, G.; Valarmathi, M. T.; Potts, J. D.; Jabbari, E.; Sabo-Attwood, T.; Wang, Q. Regulation of osteogenic differentiation of rat bone marrow stromal cells on 2D nanorod substrates. Biomaterials. 2010,31, 1732-1741.
doi: 10.1016/j.biomaterials.2009.11.041 URL pmid: 20022632 |
| 2. |
Kaur, G.; Wang, C.; Sun, J.; Wang, Q. The synergistic effects of multivalent ligand display and nanotopography on osteogenic differentiation of rat bone marrow stem cells. Biomaterials. 2010,31, 5813-5824.
doi: 10.1016/j.biomaterials.2010.04.017 URL pmid: 20452665 |
| 3. |
Maturavongsadit, P.; Luckanagul, J. A.; Metavarayuth, K.; Zhao, X.; Chen, L.; Lin, Y.; Wang, Q. Promotion of in vitro chondrogenesis of mesenchymal stem cells using in situ hyaluronic hydrogel functionalized with rod-like viral nanoparticles. Biomacromolecules. 2016,17, 1930-1938.
doi: 10.1021/acs.biomac.5b01577 URL pmid: 26999064 |
| 4. | Metavarayuth, K.; Sitasuwan, P.; Luckanagul, J. A.; Feng, S.; Wang, Q. Virus nanoparticles mediated osteogenic differentiation of bone derived mesenchymal stem cells. Adv Sci (Weinh). 2015,2, 1500026. |
| 5. |
Sitasuwan, P.; Lee, L. A.; Bo, P.; Davis, E. N.; Lin, Y.; Wang, Q. A plant virus substrate induces early upregulation of BMP2 for rapid bone formation. Integr Biol (Camb). 2012,4, 651-660.
doi: 10.1039/c2ib20041d URL |
| 6. |
Zhao, X.; Lin, Y.; Wang, Q. Virus-based scaffolds for tissue engineering applications. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015,7, 534-547.
doi: 10.1002/wnan.1327 URL pmid: 25521747 |
| 7. |
Nguyen, H. G.; Metavarayuth, K.; Wang, Q. Upregulation of osteogenesis of mesenchymal stem cells with virus-based thin films. Nanotheranostics. 2018,2, 42-58.
doi: 10.7150/ntno.19974 URL pmid: 29291162 |
| 8. |
Griffith, L. G.; Naughton, G. Tissue engineering--current challenges and expanding opportunities. Science. 2002,295, 1009-1014.
doi: 10.1126/science.1069210 URL pmid: 11834815 |
| 9. |
Khademhosseini, A.; Langer, R.; Borenstein, J.; Vacanti, J. P. Microscale technologies for tissue engineering and biology. Proc Natl Acad Sci U S A. 2006,103, 2480-2487.
doi: 10.1073/pnas.0507681102 URL pmid: 16477028 |
| 10. |
Khademhosseini, A.; Vacanti, J. P.; Langer, R. Progress in tissue engineering. Sci Am. 2009,300, 64-71.
doi: 10.1038/scientificamerican0109-64 URL pmid: 19186751 |
| 11. |
Khademhosseini, A.; Langer, R. Microengineered hydrogels for tissue engineering. Biomaterials. 2007,28, 5087-5092.
doi: 10.1016/j.biomaterials.2007.07.021 URL pmid: 17707502 |
| 12. |
Zhu, J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials. 2010,31, 4639-4656.
doi: 10.1016/j.biomaterials.2010.02.044 URL pmid: 20303169 |
| 13. |
Bichara, D. A.; Zhao, X.; Bodugoz-Senturk, H.; Ballyns, F. P.; Oral, E.; Randolph, M. A.; Bonassar, L. J.; Gill, T. J.; Muratoglu, O. K. Porous poly(vinyl alcohol)-hydrogel matrix-engineered biosynthetic cartilage. Tissue Eng Part A. 2011,17, 301-309.
doi: 10.1089/ten.TEA.2010.0322 URL pmid: 20799889 |
| 14. |
Elisseeff, J. Injectable cartilage tissue engineering. Expert Opin Biol Ther. 2004,4, 1849-1859.
doi: 10.1517/14712598.4.12.1849 URL pmid: 15571448 |
| 15. |
Aldaye, F. A.; Senapedis, W. T.; Silver, P. A.; Way, J. C. A structurally tunable DNA-based extracellular matrix. J Am Chem Soc. 2010,132, 14727-14729.
doi: 10.1021/ja105431h URL pmid: 20925350 |
| 16. |
Kloxin, A. M.; Kasko, A. M.; Salinas, C. N.; Anseth, K. S. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science. 2009,324, 59-63.
doi: 10.1126/science.1169494 URL pmid: 19342581 |
| 17. |
Luckanagul, J.; Lee, L. A.; Nguyen, Q. L.; Sitasuwan, P.; Yang, X.; Shazly, T.; Wang, Q. Porous alginate hydrogel functionalized with virus as three-dimensional scaffolds for bone differentiation. Biomacromolecules. 2012,13, 3949-3958.
doi: 10.1021/bm301180c URL pmid: 23148483 |
| 18. |
Luckanagul, J. A.; Lee, L. A.; You, S.; Yang, X.; Wang, Q. Plant virus incorporated hydrogels as scaffolds for tissue engineering possess low immunogenicity in vivo. J Biomed Mater Res A. 2015,103, 887-895.
doi: 10.1002/jbm.a.35227 URL pmid: 24829052 |
| 19. |
Luckanagul, J. A.; Metavarayuth, K.; Feng, S.; Maneesaay, P.; Clark, A. Y.; Yang, X.; García, A. J.; Wang, Q. Tobacco mosaic virus functionalized alginate hydrogel scaffolds for bone regeneration in rats with cranial defect. ACS Biomater Sci Eng. 2016,2, 606-615.
doi: 10.1021/acsbiomaterials.5b00561 URL |
| 20. |
Mahajan, H. S.; Gattani, S. In situ gels of Metoclopramide Hydrochloride for intranasal delivery: in vitro evaluation and in vivo pharmacokinetic study in rabbits. Drug Deliv. 2010,17, 19-27.
doi: 10.3109/10717540903447194 URL pmid: 19958151 |
| 21. |
Burdick, J. A.; Prestwich, G. D. Hyaluronic acid hydrogels for biomedical applications. Adv Mater. 2011,23, H41-56.
doi: 10.1002/adma.201003963 URL pmid: 21394792 |
| 22. |
Maturavongsadit, P.; Bi, X.; Metavarayuth, K.; Luckanagul, J. A.; Wang, Q. Influence of cross-linkers on the in vitro chondrogenesis of mesenchymal stem cells in hyaluronic acid hydrogels. ACS Appl Mater Interfaces. 2017,9, 3318-3329.
doi: 10.1021/acsami.6b12437 URL pmid: 28025887 |
| 23. |
Ananthanarayanan, B.; Kim, Y.; Kumar, S. Elucidating the mechanobiology of malignant brain tumors using a brain matrix-mimetic hyaluronic acid hydrogel platform. Biomaterials. 2011,32, 7913-7923.
doi: 10.1016/j.biomaterials.2011.07.005 URL pmid: 21820737 |
| 24. |
Jakob, F.; Ebert, R.; Ignatius, A.; Matsushita, T.; Watanabe, Y.; Groll, J.; Walles, H. Bone tissue engineering in osteoporosis. Maturitas. 2013,75, 118-124.
doi: 10.1016/j.maturitas.2013.03.004 URL pmid: 23562167 |
| 25. |
Lelovas, P. P.; Xanthos, T. T.; Thoma, S. E.; Lyritis, G. P.; Dontas, I. A. The laboratory rat as an animal model for osteoporosis research. Comp Med. 2008,58, 424-430.
URL pmid: 19004367 |
| 26. |
Wronski, T. J.; Cintrón, M.; Dann, L. M. Temporal relationship between bone loss and increased bone turnover in ovariectomized rats. Calcif Tissue Int. 1988,43, 179-183.
doi: 10.1007/BF02571317 URL pmid: 3141020 |
| 27. |
Wronski, T. J.; Dann, L. M.; Scott, K. S.; Cintrón, M. Long-term effects of ovariectomy and aging on the rat skeleton. Calcif Tissue Int. 1989,45, 360-366.
doi: 10.1007/BF02556007 URL pmid: 2509027 |
| 28. |
Xin, Z.; Jin, C.; Chao, L.; Zheng, Z.; Liehu, C.; Panpan, P.; Weizong, W.; Xiao, Z.; Qingjie, Z.; Honggang, H.; Longjuan, Q.; Xiao, C.; Jiacan, S. A matrine derivative M54 suppresses osteoclastogenesis and prevents ovariectomy-induced bone loss by targeting ribosomal protein S5. Front Pharmacol. 2018,9, 22.
doi: 10.3389/fphar.2018.00022 URL pmid: 29441015 |
| 29. |
Zhou, L.; Liu, Q.; Yang, M.; Wang, T.; Yao, J.; Cheng, J.; Yuan, J.; Lin, X.; Zhao, J.; Tickner, J.; Xu, J. Dihydroartemisinin, an anti-malaria drug, suppresses estrogen deficiency-induced osteoporosis, osteoclast formation, and RANKL-induced signaling pathways. J Bone Miner Res. 2016,31, 964-974.
doi: 10.1002/jbmr.2771 URL pmid: 26684711 |
| 30. |
Mardas, N.; Stavropoulos, A.; Karring, T. Calvarial bone regeneration by a combination of natural anorganic bovine-derived hydroxyapatite matrix coupled with a synthetic cell-binding peptide (PepGen): an experimental study in rats. Clin Oral Implants Res. 2008,19, 1010-1015.
doi: 10.1111/j.1600-0501.2008.01572.x URL pmid: 18828817 |
| 31. |
Durão, S. F.; Gomes, P. S.; Colaço, B. J.; Silva, J. C.; Fonseca, H. M.; Duarte, J. R.; Felino, A. C.; Fernandes, M. H. The biomaterial-mediated healing of critical size bone defects in the ovariectomized rat. Osteoporos Int. 2014,25, 1535-1545.
doi: 10.1007/s00198-014-2656-y URL pmid: 24573401 |
| 32. |
Namkung-Matthai, H.; Appleyard, R.; Jansen, J.; Hao Lin, J.; Maastricht, S.; Swain, M.; Mason, R. S.; Murrell, G. A.; Diwan, A. D.; Diamond, T. Osteoporosis influences the early period of fracture healing in a rat osteoporotic model. Bone. 2001,28, 80-86.
doi: 10.1016/s8756-3282(00)00414-2 URL pmid: 11165946 |
| 33. |
Hao, Y. J.; Zhang, G.; Wang, Y. S.; Qin, L.; Hung, W. Y.; Leung, K.; Pei, F. X. Changes of microstructure and mineralized tissue in the middle and late phase of osteoporotic fracture healing in rats. Bone. 2007,41, 631-638.
doi: 10.1016/j.bone.2007.06.006 URL pmid: 17652051 |
| 34. |
Oberg, S.; Johansson, C.; Rosenquist, J. B. Bone formation after implantation of autolysed antigen extracted allogeneic bone in ovariectomized rabbits. Int J Oral Maxillofac Surg. 2003,32, 628-632.
doi: 10.1054/ijom.2003.0428 URL pmid: 14636614 |
| [1] | Xirui Jing, Qiuyue Ding, Qinxue Wu, Weijie Su, Keda Yu, Yanlin Su, Bing Ye, Qing Gao, Tingfang Sun, Xiaodong Guo. Magnesium-based materials in orthopaedics: material properties and animal models [J]. Biomaterials Translational, 2021, 2(3): 197-213. |
| [2] | Xiaowen Xu, Jie Song. Segmental long bone regeneration guided by degradable synthetic polymeric scaffolds [J]. Biomaterials Translational, 2020, 1(1): 33-45. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||