Biomaterials Translational ›› 2021, Vol. 2 ›› Issue (2): 143-150.doi: 10.12336/biomatertransl.2021.02.004
• RESEARCH ARTICLE • Previous Articles Next Articles
Wendy Rachel Francis1,#, Zhao Liu1,2,#, Sian E Owens1, Xiao Wang1,3, Huaming Xue4, Alex Lord1, Venkateswarlu Kanamarlapudi1, Zhidao Xia1,*( )
)
Received:2021-01-26
Revised:2021-06-07
Accepted:2021-06-09
Online:2021-06-28
Published:2021-06-28
Contact:
Zhidao Xia
E-mail:z.xia@swansea.ac.uk
About author:#Author Equally.
Francis, W. R.; Liu, Z.; Owens, S. E.; Wang, X.; Xue, H.; Lord, A.; Kanamarlapudi, V.; Xia, Z. Role of hypoxia inducible factor 1α in cobalt nanoparticle induced cytotoxicity of human THP-1 macrophages. Biomater Transl. 2021, 2(2), 143-150.
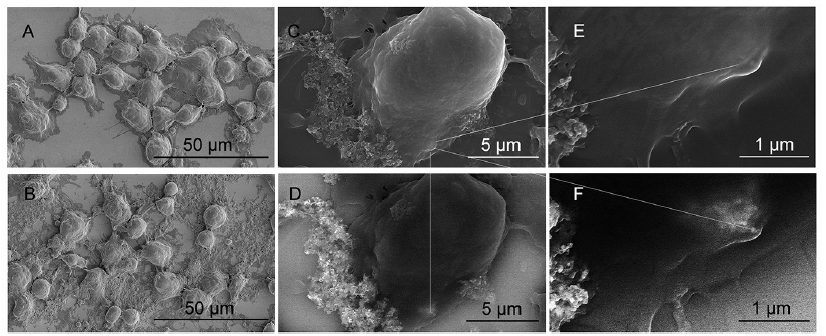
Figure 1. Micrographs of cryo-SEM illustrates THP-1 macrophages and their phagocytosis of CoNP. (A) Cell morphology of control THP-1 cells. (B) Cell morphology of THP-1 cells at 24 hours after CoNP (100 μg/mL). (C) High magnification of normal SEM showing a macrophage in contact with CoNPs. (D) Backscatter SEM of the same cells in C, revealing high electron density particles, in particular one cluster of CoNPs not showing in C. (E) Highlighted area demonstrating the cluster of CoNP not showing by normal SEM. (F) Highlighted area demonstrating phagocytosis of the cluster of CoNPs that were engulfed and submerged within the macrophage cell membrane. Scale bars: 50 μm in A, B; 5 μm in C, D; 1 μm in E, F. CoNP: cobalt nanoparticle; SEM: scanning electron microscope.
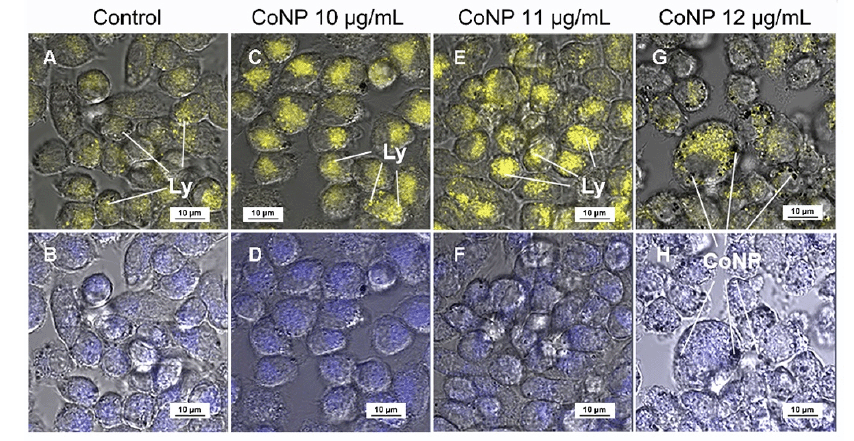
Figure 2. The pH within THP-1 macrophages at 24 hours after CoNP incubation was measured using LysoSensor Yellow/Blue DND 160 probes. (A-H) The blue fluorescence indicates neutral pH within the cell (A, C, E, G), whilst the yellow fluorescence shows the acidity of the lysosomes following the engulfment of CoNP (B, D, F, H). With increasing CoNP dose, the numbers and acidity of lysosomes within the cell increased whilst the pH within the cell remained neutral. CoNP: cobalt nanoparticle; Ly: lysosomes.
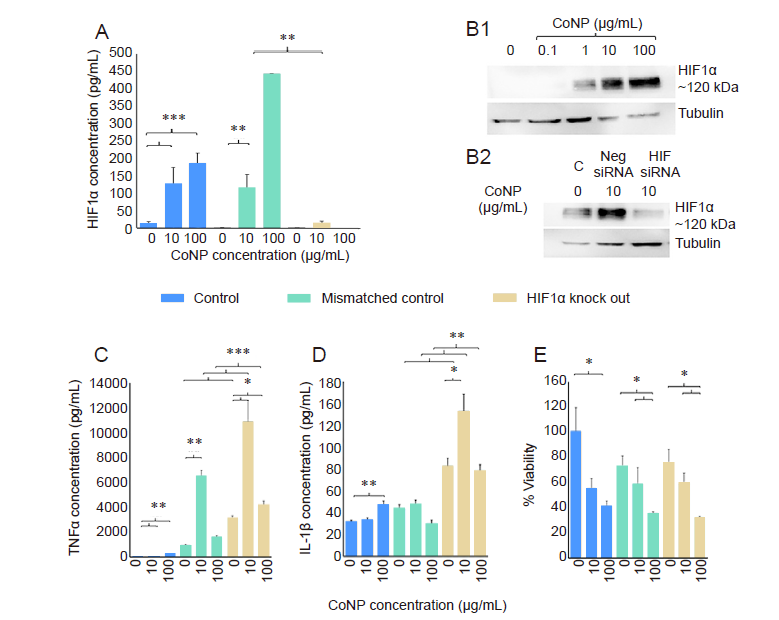
Figure 3. Analysis of HIF1α expression and the effect of HIF1α knockout on cytotoxicity and inflammatory factors (TNFα and IL-1β). (A) THP-1 cells were lysed for protein extraction 24 hours following treatment with CoNP (10 and 100 μg/mL). HIF1α concentration was measured by ELISA Duoset?. (B) Western blot of HIF1α expression. (B1) HIF1α concentration was increased with CoNP dose. (B2) Knockdown of HIF1α using siRNA showed a significantly reduced production of HIF1α protein in response to CoNP. (C, D) TNFα (C) and IL-1β (D) concentration in THP-1 cells with CoNP treatment were measured in the culture supernatants using enzyme-linked immunosorbent assay Duoset? 24 hours following CoNP treatment (0, 10, 100 μg/mL). Both TNFα (C) and IL-1β (D) secretion increased with CoNP dose in the healthy and transfected macrophages. Both TNFα and IL 1β increased significantly following transfection in comparison to the control. Cultures transfected with HIF1α siRNA showed significant up-regulation of TNFα and IL-1β in comparison to the mismatched control. (E) Percentage viability of healthy and transfected THP-1 cultures treated with CoNP (0, 10, 100 μg/mL) as measured by neutral red staining. Viability significantly reduced with increasing CoNP dose within the control, mismatched control and HIF1α knock out. There were no significant differences between the mismatched control and cultures transfected with HIF1α small interfering RNA. Data are expressed as mean ± SE. All experiments were repeated twice or three times with consistent results. *P < 0.05, *P < 0.01 (Wilcoxon matched pairs test). CoNP: cobalt nanoparticle; ELISA: enzyme-linked immunosorbent assay; HIF1α: hypoxia inducible transcription factor 1α; IL-1β: interleukin 1β; TNFα: tumour necrosis factor α.
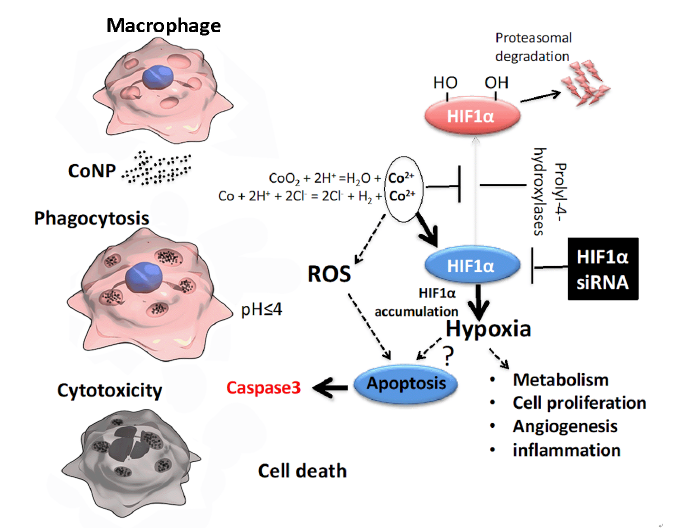
Figure 4. Schematic diagram. CoNP: cobalt nanoparticle; HIF1α: hypoxia inducible transcription factor 1α; ROS: reactive oxygen species; siRNA: small interfering RNA.
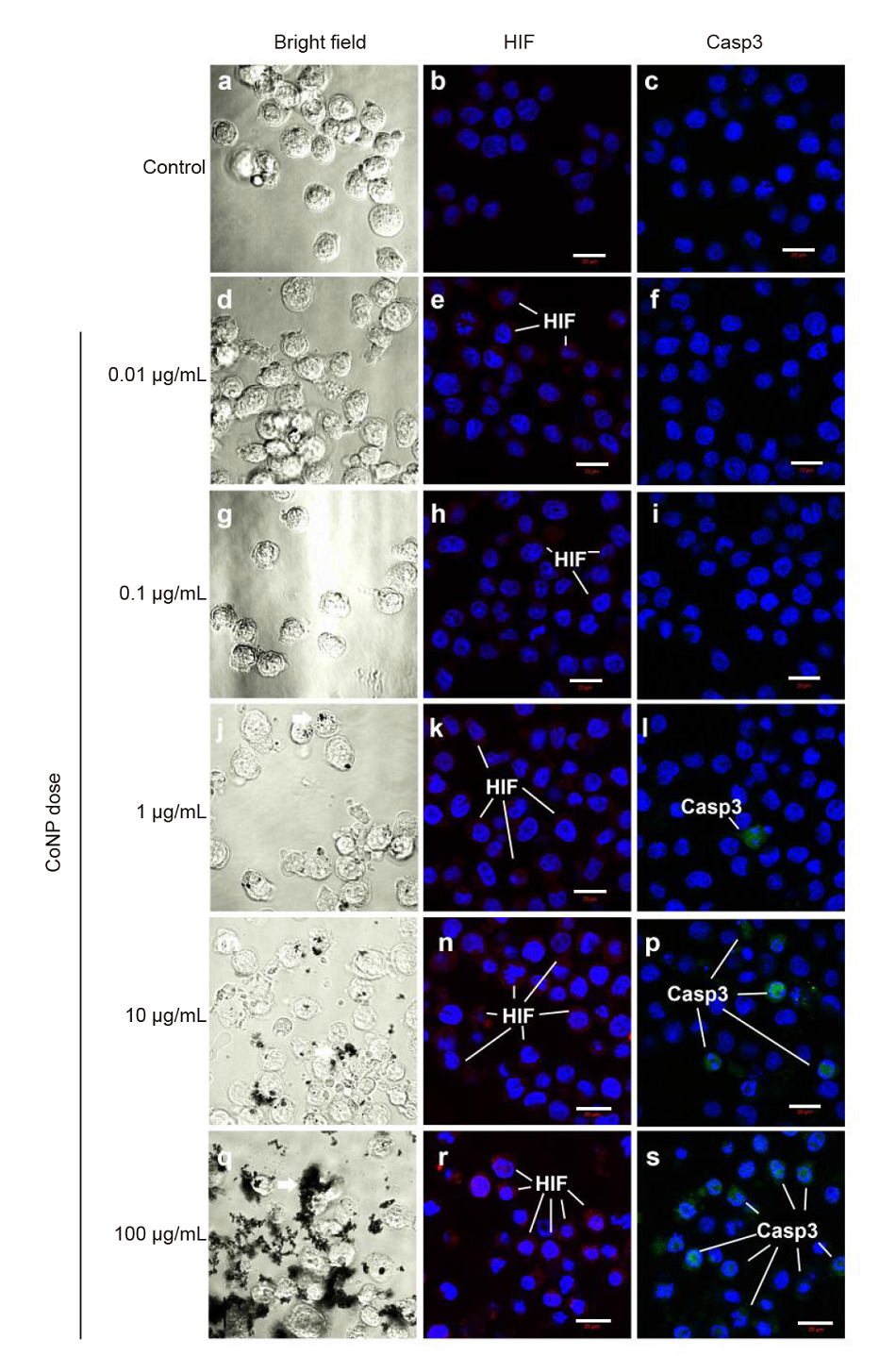
Additional Figure 1. Bright field and confocal images illustrate the phagocytosis and the immunocytochemical staining of hypoxia inducible transcription factor 1α (HIF1α) and caspase 3 (Casp3) of THP-1 macrophages in response to different dose of cobalt nanoparticles (CoNPs). It shows a dose dependent increases of HIF1α staining (arrows to the red coloured staining) with the increases of CoNP doses, and Casp3 staining (arrows to the green coloured staining) is in correlation with HIF1α. However, Casp3 is less pronanced when the doses of CoNPs are lower than 1 μg/mL. Scale bars: 20 μm.
| 1. |
Learmonth, I. D.; Young, C.; Rorabeck, C. The operation of the century: total hip replacement. Lancet. 2007, 370, 1508-1519.
doi: 10.1016/S0140-6736(07)60457-7 URL |
| 2. |
O’Boyle, C. A.; McGee, H.; Hickey, A.; O’Malley, K.; Joyce, C. R. Individual quality of life in patients undergoing hip replacement. Lancet. 1992, 339, 1088-1091.
doi: 10.1016/0140-6736(92)90673-Q URL |
| 3. |
Samelko, L.; Caicedo, M. S.; Lim, S. J.; Della-Valle, C.; Jacobs, J.; Hallab, N. J. Cobalt-alloy implant debris induce HIF-1α hypoxia associated responses: a mechanism for metal-specific orthopedic implant failure. PLoS One. 2013, 8, e67127.
doi: 10.1371/journal.pone.0067127 URL |
| 4. | Schoon, J.; Hesse, B.; Rakow, A.; Ort, M. J.; Lagrange, A.; Jacobi, D.; Winter, A.; Huesker, K.; Reinke, S.; Cotte, M.; Tucoulou, R.; Marx, U.; Perka, C.; Duda, G. N.; Geissler, S. Metal-specific biomaterial accumulation in human peri-implant bone and bone marrow. Adv Sci (Weinh). 2020, 7, 2000412. |
| 5. | Xia, Z.; Kwon, Y. M.; Mehmood, S.; Downing, C.; Jurkschat, K.; Murray, D. W. Characterization of metal-wear nanoparticles in pseudotumor following metal-on-metal hip resurfacing. Nanomedicine. 2011, 7, 674-681. |
| 6. |
Junnila, M.; Seppänen, M.; Mokka, J.; Virolainen, P.; Pölönen, T.; Vahlberg, T.; Mattila, K.; Tuominen, E. K.; Rantakokko, J.; Äärimaa, V.; Itälä, A.; Mäkelä, K. T. Adverse reaction to metal debris after Birmingham hip resurfacing arthroplasty. Acta Orthop. 2015, 86, 345-350.
doi: 10.3109/17453674.2014.1004015 URL |
| 7. |
Lainiala, O.; Eskelinen, A.; Elo, P.; Puolakka, T.; Korhonen, J.; Moilanen, T. Adverse reaction to metal debris is more common in patients following MoM total hip replacement with a 36 mm femoral head than previously thought: results from a modern MoM follow-up programme. Bone Joint J. 2014, 96-b, 1610-1617.
doi: 10.1302/0301-620X.96B12.33742 URL |
| 8. | Langton, D. J.; Joyce, T. J.; Jameson, S. S.; Lord, J.; Van Orsouw, M.; Holland, J. P.; Nargol, A. V.; De Smet, K. A. Adverse reaction to metal debris following hip resurfacing: the influence of component type, orientation and volumetric wear. J Bone Joint Surg Br. 2011, 93, 164-171. |
| 9. | Xia, Z.; Ricciardi, B. F.; Liu, Z.; von Ruhland, C.; Ward, M.; Lord, A.; Hughes, L.; Goldring, S. R.; Purdue, E.; Murray, D.; Perino, G. Nano-analyses of wear particles from metal-on-metal and non-metal-on-metal dual modular neck hip arthroplasty. Nanomedicine. 2017, 13, 1205-1217. |
| 10. |
Kwon, Y. M.; Xia, Z.; Glyn-Jones, S.; Beard, D.; Gill, H. S.; Murray, D. W. Dose-dependent cytotoxicity of clinically relevant cobalt nanoparticles and ions on macrophages in vitro. Biomed Mater. 2009, 4, 025018.
doi: 10.1088/1748-6041/4/2/025018 URL |
| 11. |
Simonsen, L. O.; Harbak, H.; Bennekou, P. Cobalt metabolism and toxicology--a brief update. Sci Total Environ. 2012, 432, 210-215.
doi: 10.1016/j.scitotenv.2012.06.009 URL |
| 12. |
Lauwerys, R.; Lison, D. Health risks associated with cobalt exposure--an overview. Sci Total Environ. 1994, 150, 1-6.
doi: 10.1016/0048-9697(94)90125-2 URL |
| 13. |
Lison, D.; Lauwerys, R. Cobalt bioavailability from hard metal particles. Further evidence that cobalt alone is not responsible for the toxicity of hard metal particles. Arch Toxicol. 1994, 68, 528-531.
doi: 10.1007/s002040050108 URL |
| 14. |
Wang, S.; Liu, F.; Zeng, Z.; Yang, H.; Jiang, H. The protective effect of bafilomycin a1 against cobalt nanoparticle-induced cytotoxicity and aseptic inflammation in macrophages in vitro. Biol Trace Elem Res. 2016, 169, 94-105.
doi: 10.1007/s12011-015-0381-9 URL |
| 15. |
Catelas, I.; Petit, A.; Vali, H.; Fragiskatos, C.; Meilleur, R.; Zukor, D. J.; Antoniou, J.; Huk, O. L. Quantitative analysis of macrophage apoptosis vs. necrosis induced by cobalt and chromium ions in vitro. Biomaterials. 2005, 26, 2441-2453.
doi: 10.1016/j.biomaterials.2004.08.004 URL |
| 16. |
Salib, C. G.; Lewallen, E. A.; Paradise, C. R.; Tibbo, M. E.; Robin, J. X.; Trousdale, W. H.; Morrey, L. M.; Xiao, J.; Turner, T. W.; Limberg, A. K.; Jay, A. G.; Thaler, R.; Dudakovic, A.; Sanchez-Sotelo, J.; Morrey, M. E.; Berry, D. J.; Lewallen, D. G.; van Wijnen, A. J.; Abdel, M. P. Molecular pathology of adverse local tissue reaction caused by metal-on-metal implants defined by RNA-seq. Genomics. 2019, 111, 1404-1411.
doi: 10.1016/j.ygeno.2018.09.013 URL |
| 17. |
Vengellur, A.; LaPres, J. J. The role of hypoxia inducible factor 1alpha in cobalt chloride induced cell death in mouse embryonic fibroblasts. Toxicol Sci. 2004, 82, 638-646.
doi: 10.1093/toxsci/kfh278 URL |
| 18. |
Ke, Q.; Costa, M. Hypoxia-inducible factor-1 (HIF-1). Mol Pharmacol. 2006, 70, 1469-1480.
doi: 10.1124/mol.106.027029 URL |
| 19. |
Dai, Z. J.; Gao, J.; Ma, X. B.; Yan, K.; Liu, X. X.; Kang, H. F.; Ji, Z. Z.; Guan, H. T.; Wang, X. J. Up-regulation of hypoxia inducible factor-1α by cobalt chloride correlates with proliferation and apoptosis in PC-2 cells. J Exp Clin Cancer Res. 2012, 31, 28.
doi: 10.1186/1756-9966-31-28 URL |
| 20. |
Nyga, A.; Hart, A.; Tetley, T. D. Importance of the HIF pathway in cobalt nanoparticle-induced cytotoxicity and inflammation in human macrophages. Nanotoxicology. 2015, 9, 905-917.
doi: 10.3109/17435390.2014.991430 URL |
| 21. |
DePedro, H. M.; Urayama, P. Using LysoSensor Yellow/Blue DND-160 to sense acidic pH under high hydrostatic pressures. Anal Biochem. 2009, 384, 359-361.
doi: 10.1016/j.ab.2008.10.007 URL |
| 22. |
Hart, A. J.; Quinn, P. D.; Lali, F.; Sampson, B.; Skinner, J. A.; Powell, J. J.; Nolan, J.; Tucker, K.; Donell, S.; Flanagan, A.; Mosselmans, J. F. Cobalt from metal-on-metal hip replacements may be the clinically relevant active agent responsible for periprosthetic tissue reactions. Acta Biomater. 2012, 8, 3865-3873.
doi: 10.1016/j.actbio.2012.05.003 URL |
| 23. |
Williams, A.; Flynn, K. J.; Xia, Z.; Dunstan, P. R. Multivariate spectral analysis of pH SERS probes for improved sensing capabilities. J Raman Spectrosc. 2016, 47, 819-827.
doi: 10.1002/jrs.v47.7 URL |
| 24. |
Xia, Z.; Triffitt, J. T. A review on macrophage responses to biomaterials. Biomed Mater. 2006, 1, R1-9.
doi: 10.1088/1748-6041/1/1/R01 URL |
| 25. | Liu, M.; Bell, S.; Segarra, M.; Steven Tay, N. H.; Will, G.; Saman, W.; Bruno, F. A eutectic salt high temperature phase change material: Thermal stability and corrosion of SS316 with respect to thermal cycling. Sol Energy Mater Sol Cells. 2017, 170, 1-7. |
| 26. |
Jeong, J.; Han, Y.; Poland, C. A.; Cho, W. S. Response-metrics for acute lung inflammation pattern by cobalt-based nanoparticles. Part Fibre Toxicol. 2015, 12, 13.
doi: 10.1186/s12989-015-0089-1 URL |
| 27. | Czarnek, K.; Terpiłowska, S.; Siwicki, A. K. Selected aspects of the action of cobalt ions in the human body. Cent Eur JImmunol. 2015, 40, 236-242. |
| 28. |
Paukkeri, E. L.; Korhonen, R.; Hämäläinen, M.; Pesu, M.; Eskelinen, A.; Moilanen, T.; Moilanen, E. The inflammatory phenotype in failed metal-on-metal hip arthroplasty correlates with blood metal concentrations. PLoS One. 2016, 11, e0155121.
doi: 10.1371/journal.pone.0155121 URL |
| 29. |
Majmundar, A. J.; Wong, W. J.; Simon, M. C. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010, 40, 294-309.
doi: 10.1016/j.molcel.2010.09.022 URL |
| 30. |
Matsuura, H.; Ichiki, T.; Ikeda, J.; Takeda, K.; Miyazaki, R.; Hashimoto, T.; Narabayashi, E.; Kitamoto, S.; Tokunou, T.; Sunagawa, K. Inhibition of prolyl hydroxylase domain-containing protein downregulates vascular angiotensin II type 1 receptor. Hypertension. 2011, 58, 386-393.
doi: 10.1161/HYPERTENSIONAHA.110.167106 URL |
| 31. |
Salnikow, K.; Donald, S. P.; Bruick, R. K.; Zhitkovich, A.; Phang, J. M.; Kasprzak, K. S. Depletion of intracellular ascorbate by the carcinogenic metals nickel and cobalt results in the induction of hypoxic stress. J Biol Chem. 2004, 279, 40337-40344.
doi: 10.1074/jbc.M403057200 URL |
| 32. |
Saini, Y.; Greenwood, K. K.; Merrill, C.; Kim, K. Y.; Patial, S.; Parameswaran, N.; Harkema, J. R.; LaPres, J. J. Acute cobalt-induced lung injury and the role of hypoxia-inducible factor 1alpha in modulating inflammation. Toxicol Sci. 2010, 116, 673-681.
doi: 10.1093/toxsci/kfq155 URL |
| 33. | Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. Biomed Res Int. 2013, 2013, 942916. |
| 34. |
Risom, L.; Møller, P.; Loft, S. Oxidative stress-induced DNA damage by particulate air pollution. Mutat Res. 2005, 592, 119-137.
doi: 10.1016/j.mrfmmm.2005.06.012 URL |
| 35. |
Papageorgiou, I.; Brown, C.; Schins, R.; Singh, S.; Newson, R.; Davis, S.; Fisher, J.; Ingham, E.; Case, C. P. The effect of nano- and micron-sized particles of cobalt-chromium alloy on human fibroblasts in vitro. Biomaterials. 2007, 28, 2946-2958.
doi: 10.1016/j.biomaterials.2007.02.034 URL |
| 36. |
Catelas, I.; Petit, A.; Zukor, D. J.; Antoniou, J.; Huk, O. L. TNF-alpha secretion and macrophage mortality induced by cobalt and chromium ions in vitro-qualitative analysis of apoptosis. Biomaterials. 2003, 24, 383-391.
doi: 10.1016/S0142-9612(02)00351-4 URL |
| 37. | Xu, J. W.; Konttinen, Y. T.; Lassus, J.; Natah, S.; Ceponis, A.; Solovieva, S.; Aspenberg, P.; Santavirta, S. Tumor necrosis factor-alpha (TNF-alpha) in loosening of total hip replacement (THR). Clin Exp Rheumatol. 1996, 14, 643-648. |
| 38. |
Cummins, E. P.; Berra, E.; Comerford, K. M.; Ginouves, A.; Fitzgerald, K. T.; Seeballuck, F.; Godson, C.; Nielsen, J. E.; Moynagh, P.; Pouyssegur, J.; Taylor, C. T. Prolyl hydroxylase-1 negatively regulates IkappaB kinase-beta, giving insight into hypoxia-induced NFkappaB activity. Proc Natl Acad Sci U S A. 2006, 103, 18154-18159.
doi: 10.1073/pnas.0602235103 URL |
| 39. |
Rius, J.; Guma, M.; Schachtrup, C.; Akassoglou, K.; Zinkernagel, A. S.; Nizet, V.; Johnson, R. S.; Haddad, G. G.; Karin, M. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008, 453, 807-811.
doi: 10.1038/nature06905 URL |
| 40. |
Cramer, T.; Yamanishi, Y.; Clausen, B. E.; Förster, I.; Pawlinski, R.; Mackman, N.; Haase, V. H.; Jaenisch, R.; Corr, M.; Nizet, V.; Firestein, G. S.; Gerber, H. P.; Ferrara, N.; Johnson, R. S. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003, 112, 645-657.
doi: 10.1016/S0092-8674(03)00154-5 URL |
| 41. | Bandarra, D.; Biddlestone, J.; Mudie, S.; Müller, H. A.; Rocha, S. HIF-1α restricts NF-κB-dependent gene expression to control innate immunity signals. Dis Model Mech. 2015, 8, 169-181. |
| 42. |
Catelas, I.; Petit, A.; Zukor, D. J.; Huk, O. L. Cytotoxic and apoptotic effects of cobalt and chromium ions on J774 macrophages - Implication of caspase-3 in the apoptotic pathway. J Mater Sci Mater Med. 2001, 12, 949-953.
doi: 10.1023/A:1012800813662 URL |
| 43. |
Shi, Y.; Chang, M.; Wang, F.; Ouyang, X.; Jia, Y.; Du, H. Role and mechanism of hypoxia-inducible factor-1 in cell growth and apoptosis of breast cancer cell line MDA-MB-231. Oncol Lett. 2010, 1, 657-662.
doi: 10.3892/ol_00000115 URL |
| 44. |
Greijer, A. E.; van der Wall, E. The role of hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J Clin Pathol. 2004, 57, 1009-1014.
doi: 10.1136/jcp.2003.015032 URL |
| 45. |
Palazon, A.; Goldrath, A. W.; Nizet, V.; Johnson, R. S. HIF transcription factors, inflammation, and immunity. Immunity. 2014, 41, 518-528.
doi: 10.1016/j.immuni.2014.09.008 URL |
| [1] | Yunsong Shi, Ruijun He, Xiangyu Deng, Zengwu Shao, Davide Deganello, Chunze Yan, Zhidao Xia. Three-dimensional biofabrication of an aragonite-enriched self-hardening bone graft substitute and assessment of its osteogenicity in vitro and in vivo [J]. Biomaterials Translational, 2020, 1(1): 69-81. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||