Biomaterials Translational ›› 2022, Vol. 3 ›› Issue (2): 152-161.doi: 10.12336/biomatertransl.2022.02.007
• RESEARCH ARTICLE • Previous Articles Next Articles
Panita Maturavongsadit1, Weiwei Wu2, Jingyu Fan1, Igor B. Roninson3, Taixing Cui2,*( ), Qian Wang1,*(
), Qian Wang1,*( )
)
Received:2022-04-11
Revised:2022-05-16
Accepted:2022-05-30
Online:2022-06-28
Published:2022-06-28
Contact:
Taixing Cui,Qian Wang
E-mail:Taixing.Cui@uscmed.sc.edu;wang263@mailbox.sc.edu
About author:Qian Wang, wang263@mailbox.sc.edu.
Maturavongsadit, P.; Wu, W.; Fan, J.; Roninson, I. B.; Cui, T.; Wang, Q. Graphene-incorporated hyaluronic acid-based hydrogel as a controlled Senexin A delivery system. Biomater Transl. 2022, 3(2), 152-161.

Figure 1. (A) Schematic illustrating the synthesis of Senexin A-loaded graphene (GO-SenA) hyaluronic acid (HA)-based hydrogels via Michael addition reaction. (B) GO-SenA was first prepared and then in-situ encapsulated to form a GO-SenA-incorporated HA-based hydrogel. RT: room temperature.
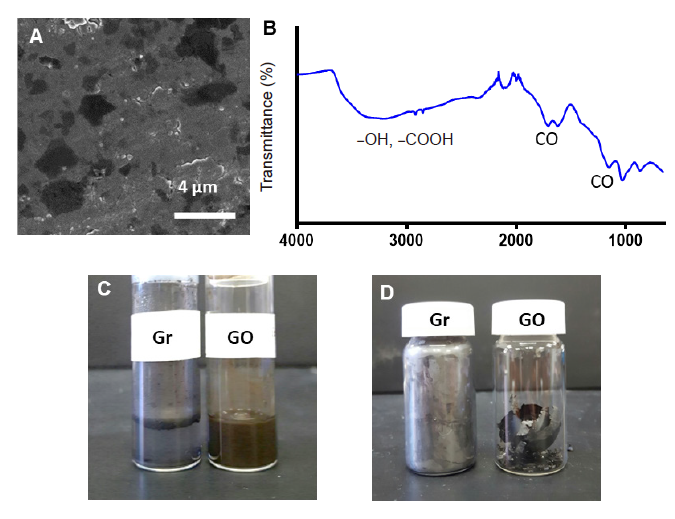
Figure 2. Characterisation of the synthesised GO. (A) Scanning electron microscopic image of the synthesised GO after sonication for 1 hour. Scale bar: 4 μm. (B) Fourier transform infrared spectroscopic spectrum of the synthesised GO. (C) Physical appearances of graphene and the synthesised GO dispersed in phosphate-buffered saline. (D) Physical appearances of graphene and the synthesised GO after drying. GO: graphene oxide nanosheet; Gr: graphene flakes.
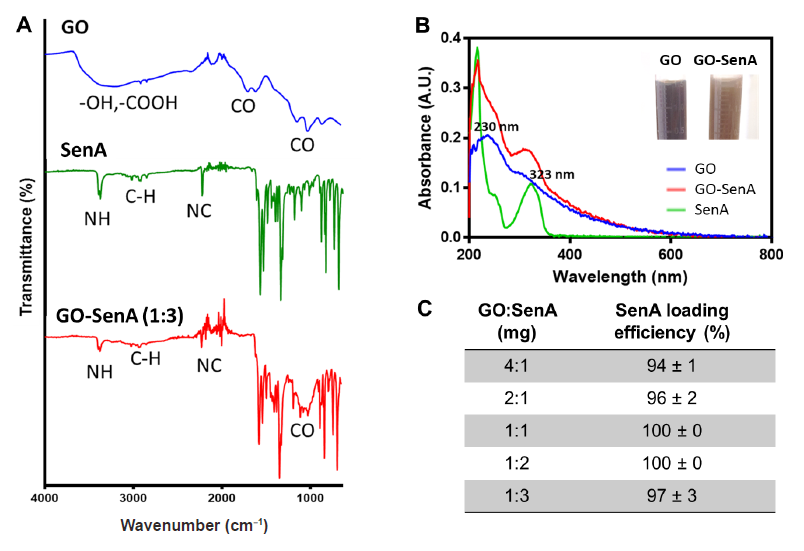
Figure 3. Preparation and characterisation of GO-SenA. (A) Fourier transform infrared spectra of GO, SenA, and GO-SenA. (B) Ultraviolet-visible spectra of GO, SenA, and GO-SenA. (C) Loading efficiency of SenA onto GO at different weight ratios (n = 3). A.U.: absorbance unit; GO: graphene oxide nanosheet; GO-SenA: Senexin A-loaded graphene oxide nanosheet; SenA: Senexin A.
| Formulation | MeHA (%w/v) | 0.5 M DTT-Crosslinker (μL/100 μL) | GO-SenA (1:3) (%w/v) | Gelation time (min) |
|---|---|---|---|---|
| 1 | 3 | 2 | 0.1 | 35-48 |
| 2 | 3 | 2 | 0.3 | 50-96 |
| 3 | 3 | 4 | 0.1 | 30-35 |
| 4 | 3 | 4 | 0.3 | 40-50 |
| 5 | 3 | 6 | 0.1 | 8-12 |
| 6 | 3 | 6 | 0.3 | 6-10 |
Table 1. Gelation times of different GO-SenA loaded HA hydrogels which were determined by the tilting method
| Formulation | MeHA (%w/v) | 0.5 M DTT-Crosslinker (μL/100 μL) | GO-SenA (1:3) (%w/v) | Gelation time (min) |
|---|---|---|---|---|
| 1 | 3 | 2 | 0.1 | 35-48 |
| 2 | 3 | 2 | 0.3 | 50-96 |
| 3 | 3 | 4 | 0.1 | 30-35 |
| 4 | 3 | 4 | 0.3 | 40-50 |
| 5 | 3 | 6 | 0.1 | 8-12 |
| 6 | 3 | 6 | 0.3 | 6-10 |
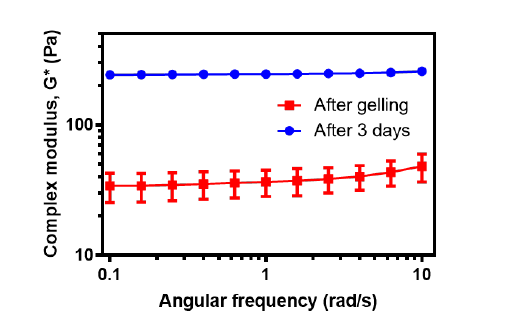
Figure 4. Mechanical properties of the optimised GO-SenA-loaded HA hydrogels immediately after gelation, and 3 days after gelation. The values are expressed as mean ± SD (n = 3). GO: graphene oxide nanosheet; HA: hyaluronic acid; SenA: Senexin A.
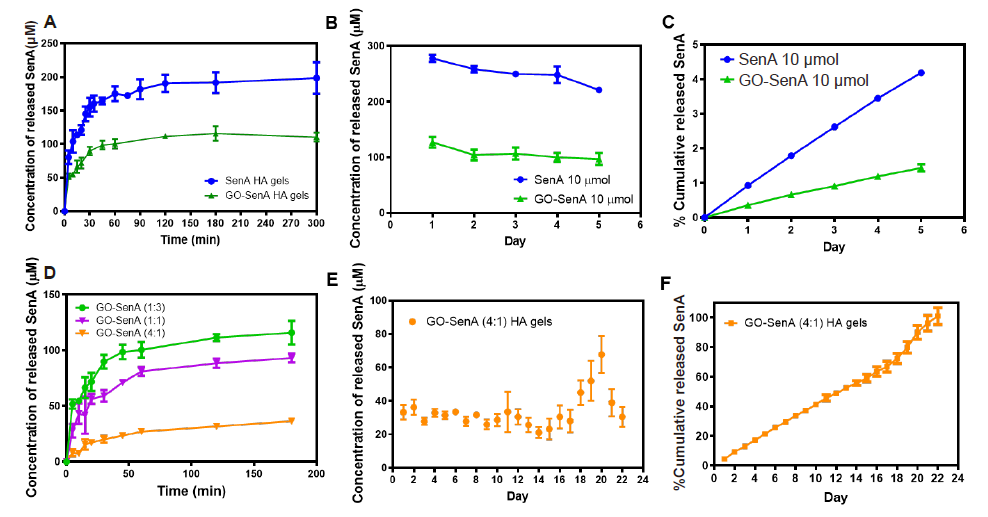
Figure 5. In vitro SenA release profiles of GO-SenA-loaded HA hydrogels. (A) In vitro SenA release profiles of GO-SenA HA hydrogels compared to SenA loaded HA hydrogels over the first 5 hours of incubation in phosphate-buffered saline at 37°C. (B) In vitro SenA release profiles of GO-SenA HA hydrogels and SenA loaded HA hydrogels over 5 days in phosphate-buffered saline at 37°C. (C) In vitro cumulative release profile of SenA from GO-SenA HA and SenA-loaded HA hydrogels calculated from B. SenA concentration loaded into both GO-SenA HA and SenA-loaded HA hydrogels were equal at 10 µmol per hydrogel. (D) In vitro SenA release profiles of GO-SenA HA hydrogels using different loading ratios of GO:SenA (1:3, 1:1, and 4:1). (E) In vitro SenA release profiles of GO-SenA HA hydrogels using 4:1 loading ratio of GO:SenA. (F) The cumulative release profile of SenA from GO-SenA HA hydrogels shown in E. All values are expressed as mean ± SD (n = 3). GO: graphene oxide nanosheet; HA: hyaluronic acid; SenA: Senexin A.
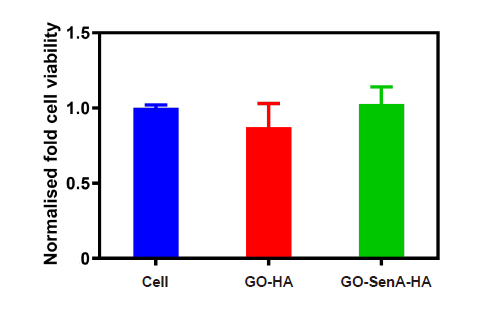
Figure 6. In vitro cytocompatibility of GO-SenA-loaded HA hydrogels. (A) Cell viability of vascular smooth muscle cells after culturing in primary medium with GO-HA hydrogels or GO-SenA-loaded HA hydrogels or without any hydrogel for 24 hours using CellTiter-Blue assay. The data were normalised to the cell viability intensity of cell alone (positive control). The values expressed are mean ± SD (n= 3) from two repeated experiments. GO: graphene oxide nanosheet; HA: hyaluronic acid; SenA: Senexin A.
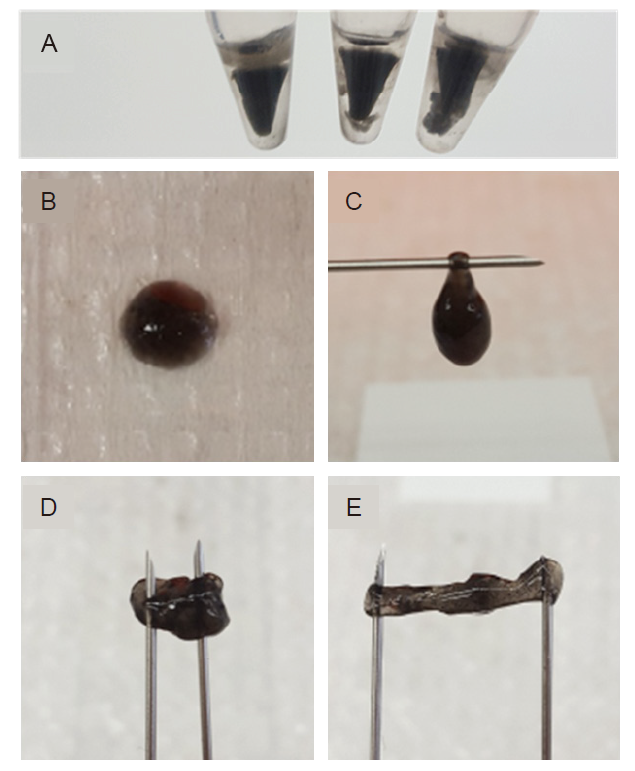
Additional Figure 1. The formation and physical appearances of rigid graphene scaffold. (A) The formation of reduced GO hydrogels when using high DTT, 6 µL/100 µL. (B, C) Physical appearances of Senexin A-loaded graphene HA hydrogels immediately after gelling (D, E) Physical appearances of Senexin A-loaded graphene HA hydrogels after gelling for 3 days. DTT: dithiothreitol; GO: graphene oxide nanosheet; HA: hyaluronic acid.
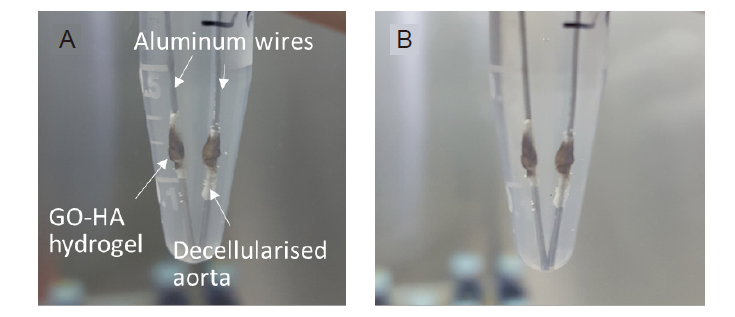
Additional Figure 2. (A, B) The attachment of Senexin A-loaded graphene HA hydrogels to the decellularised scaffolds in phosphate-buffered saline immediately after gelling (A) and 1 day later (B). GO: graphene oxide nanosheet; HA: hyaluronic acid.
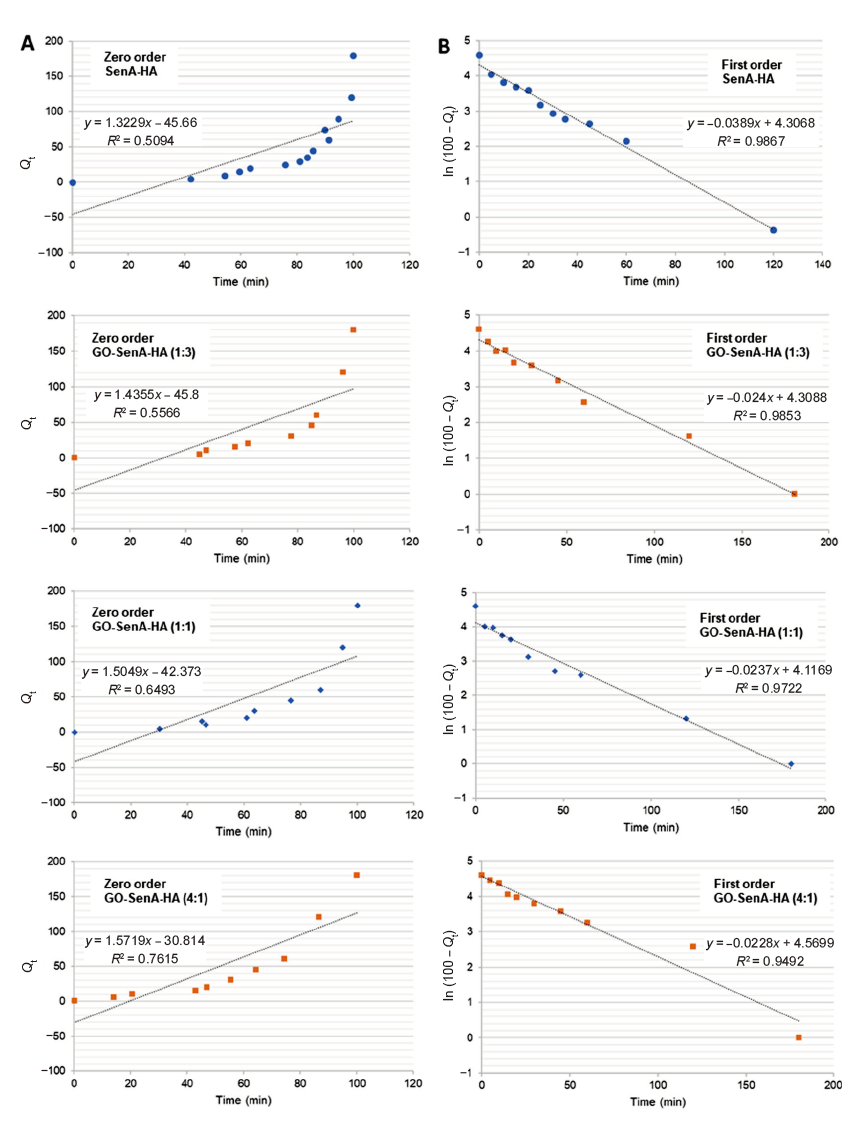
Additional Figure 3. (A, B) Fitting of the data for SenA release from the SenA-loaded HA hydrogels (SenA-HA) and GO-SenA HA hydrogels having different loading ratios of GO:SenA (1:3, 1:1, and 4:1) into phosphate-buffered saline (pH 7.4) to the zero order (A) and first order kinetics (B). GO: graphene oxide nanosheet; HA: hyaluronic acid; SenA: Senexin A.
| Formulation | MeHA (% w/v) | 0.5 M DTT- Crosslinker (μL/100 μL) | Gelation time (min) | Gelation time (min) |
|---|---|---|---|---|
| 1 | 3 | 2 | 60–70 | 35–48 |
| 2 | 3 | 2.8 | 40–45 | 50–96 |
| 3 | 3 | 3.4 | 30–35 | 30–35 |
| 4 | 4 | 2.8 | 10–15 | 40–50 |
| 5 | 4 | 3.7 | 10–15 | |
| 6 | 4 | 4.6 | 5–7 | |
| 7 | 5 | 3.4 | 3–5 | |
| 8 | 5 | 4.6 | 3–4 | 8–12 |
| 9 | 5 | 5.7 | 2–3 | 6–10 |
Additional Table 1. Screening gelation times of HA-based hydrogels without SenA and GO incorporation
| Formulation | MeHA (% w/v) | 0.5 M DTT- Crosslinker (μL/100 μL) | Gelation time (min) | Gelation time (min) |
|---|---|---|---|---|
| 1 | 3 | 2 | 60–70 | 35–48 |
| 2 | 3 | 2.8 | 40–45 | 50–96 |
| 3 | 3 | 3.4 | 30–35 | 30–35 |
| 4 | 4 | 2.8 | 10–15 | 40–50 |
| 5 | 4 | 3.7 | 10–15 | |
| 6 | 4 | 4.6 | 5–7 | |
| 7 | 5 | 3.4 | 3–5 | |
| 8 | 5 | 4.6 | 3–4 | 8–12 |
| 9 | 5 | 5.7 | 2–3 | 6–10 |
| Formulation | Zero order | First order | Higuchi | Korsmeyer-Peppas | ||||
|---|---|---|---|---|---|---|---|---|
| K0 | R2 | K1 | R2 | Kh | R2 | n | R2 | |
| SenA-HA | 1.322 | 0.509 | 0.389 | 0.987 | 0.116 | 0.787 | 0.546 | 0.943 |
| GO-SenA-HA (1:3) | 1.435 | 0.557 | 0.240 | 0.972 | 0.121 | 0.817 | 0.276 | 0.946 |
| GO-SenA-HA (1:1) | 1.504 | 0.649 | 0.237 | 0.985 | 0.122 | 0.884 | 0.368 | 0.950 |
| GO-SenA-HA (4:1) | 1.572 | 0.762 | 0.228 | 0.949 | 0.122 | 0.938 | 0.545 | 0.905 |
Additional Table 2. Correlation coefficient (R2), rate constant (K), and release exponent (n) values obtained by fitting the data of the release of SenA from different hydrogel formulations into phosphate-buffered saline at pH 7.4
| Formulation | Zero order | First order | Higuchi | Korsmeyer-Peppas | ||||
|---|---|---|---|---|---|---|---|---|
| K0 | R2 | K1 | R2 | Kh | R2 | n | R2 | |
| SenA-HA | 1.322 | 0.509 | 0.389 | 0.987 | 0.116 | 0.787 | 0.546 | 0.943 |
| GO-SenA-HA (1:3) | 1.435 | 0.557 | 0.240 | 0.972 | 0.121 | 0.817 | 0.276 | 0.946 |
| GO-SenA-HA (1:1) | 1.504 | 0.649 | 0.237 | 0.985 | 0.122 | 0.884 | 0.368 | 0.950 |
| GO-SenA-HA (4:1) | 1.572 | 0.762 | 0.228 | 0.949 | 0.122 | 0.938 | 0.545 | 0.905 |
| 1. |
Sanders, W. G.; Hogrebe, P. C.; Grainger, D. W.; Cheung, A. K.; Terry, C. M. A biodegradable perivascular wrap for controlled, local and directed drug delivery. J Control Release. 2012, 161, 81-89.
doi: 10.1016/j.jconrel.2012.04.029 URL |
| 2. |
Katare, R.; Riu, F.; Rowlinson, J.; Lewis, A.; Holden, R.; Meloni, M.; Reni, C.; Wallrapp, C.; Emanueli, C.; Madeddu, P. Perivascular delivery of encapsulated mesenchymal stem cells improves postischemic angiogenesis via paracrine activation of VEGF-A. Arterioscler Thromb Vasc Biol. 2013, 33, 1872-1880.
doi: 10.1161/ATVBAHA.113.301217 URL |
| 3. |
Masaki, T.; Rathi, R.; Zentner, G.; Leypoldt, J. K.; Mohammad, S. F.; Burns, G. L.; Li, L.; Zhuplatov, S.; Chirananthavat, T.; Kim, S. J.; Kern, S.; Holman, J.; Kim, S. W.; Cheung, A. K. Inhibition of neointimal hyperplasia in vascular grafts by sustained perivascular delivery of paclitaxel. Kidney Int. 2004, 66, 2061-2069.
doi: 10.1111/j.1523-1755.2004.00985.x URL |
| 4. |
Mylonaki, I.; Allémann, É.; Saucy, F.; Haefliger, J. A.; Delie, F.; Jordan, O. Perivascular medical devices and drug delivery systems: Making the right choices. Biomaterials. 2017, 128, 56-68.
doi: 10.1016/j.biomaterials.2017.02.028 URL |
| 5. | Wu, B.; Werlin, E. C.; Chen, M.; Mottola, G.; Chatterjee, A.; Lance, K. D.; Bernards, D. A.; Sansbury, B. E.; Spite, M.; Desai, T. A.; Conte, M. S. Perivascular delivery of resolvin D1 inhibits neointimal hyperplasia in a rabbit vein graft model. J Vasc Surg. 2018, 68:188S-200S.e4. |
| 6. |
Lee, J.; Jang, E. H.; Kim, J. H.; Park, S.; Kang, Y.; Park, S.; Lee, K.; Kim, J. H.; Youn, Y. N.; Ryu, W. Highly flexible and porous silk fibroin microneedle wraps for perivascular drug delivery. J Control Release. 2021, 340, 125-135.
doi: 10.1016/j.jconrel.2021.10.024 URL |
| 7. |
Uman, S.; Dhand, A.; Burdick, J. A. Recent advances in shear-thinning and self-healing hydrogels for biomedical applications. J Appl Polym Sci. 2020, 137, 48668.
doi: 10.1002/app.48668 URL |
| 8. | Dovedytis, M.; Liu, Z. J.; Bartlett, S. Hyaluronic acid and its biomedical applications: A review. Engineered Regen. 2020, 1, 102-113. |
| 9. |
Tiwari, S.; Bahadur, P. Modified hyaluronic acid based materials for biomedical applications. Int J Biol Macromol. 2019, 121, 556-571.
doi: 10.1016/j.ijbiomac.2018.10.049 URL |
| 10. |
Trombino, S.; Servidio, C.; Curcio, F.; Cassano, R. Strategies for hyaluronic acid-based hydrogel design in drug delivery. Pharmaceutics. 2019, 11, 407.
doi: 10.3390/pharmaceutics11080407 URL |
| 11. |
Ghasemiyeh, P.; Mohammadi-Samani, S. Hydrogels as drug delivery systems; pros and cons. Trends Pharm Sci. 2019, 5, 7-24.
doi: 10.1016/0165-6147(84)90349-3 URL |
| 12. | Fu, S.; Xiao, X.; Dong, H.; Zhang, Z.; Zhang, X.; Zhong, Z.; Zhuo, R. An injectable hyaluronic acid/PEG hydrogel produced via copper-free click chemistry for drug delivery. J Control Release. 2017, 259, e123-e124. |
| 13. |
França, C. G.; Plaza, T.; Naveas, N.; Andrade Santana, M. H.; Manso-Silván, M.; Recio, G.; Hernandez-Montelongo, J. Nanoporous silicon microparticles embedded into oxidized hyaluronic acid/adipic acid dihydrazide hydrogel for enhanced controlled drug delivery. Microporous Mesoporous Mater. 2021, 310, 110634.
doi: 10.1016/j.micromeso.2020.110634 URL |
| 14. |
Merino, S.; Martín, C.; Kostarelos, K.; Prato, M.; Vázquez, E. Nanocomposite hydrogels: 3D polymer-nanoparticle synergies for on-demand drug delivery. ACS Nano. 2015, 9, 4686-4697.
doi: 10.1021/acsnano.5b01433 URL |
| 15. |
Yegappan, R.; Selvaprithiviraj, V.; Mohandas, A.; Jayakumar, R. Nano polydopamine crosslinked thiol-functionalized hyaluronic acid hydrogel for angiogenic drug delivery. Colloids Surf B Biointerfaces. 2019, 177, 41-49.
doi: 10.1016/j.colsurfb.2019.01.035 URL |
| 16. |
Min, Q.; Liu, J.; Zhang, Y.; Yang, B.; Wan, Y.; Wu, J. Dual network hydrogels incorporated with bone morphogenic protein-7-loaded hyaluronic acid complex nanoparticles for inducing chondrogenic differentiation of synovium-derived mesenchymal stem cells. Pharmaceutics. 2020, 12, 613.
doi: 10.3390/pharmaceutics12070613 URL |
| 17. | Asadi, H.; Ghaee, A.; Nourmohammadi, J.; Mashak, A. Electrospun zein/graphene oxide nanosheet composite nanofibers with controlled drug release as antibacterial wound dressing. Int J Polymeric Mater Polymeric Biomater. 2020, 69, 173-185. |
| 18. |
Ghawanmeh, A. A.; Ali, G. A. M.; Algarni, H.; Sarkar, S. M.; Chong, K. F. Graphene oxide-based hydrogels as a nanocarrier for anticancer drug delivery. Nano Res. 2019, 12, 973-990.
doi: 10.1007/s12274-019-2300-4 URL |
| 19. |
Kolanthai, E.; Sindu, P. A.; Khajuria, D. K.; Veerla, S. C.; Kuppuswamy, D.; Catalani, L. H.; Mahapatra, D. R. Graphene oxide-A tool for the preparation of chemically crosslinking free alginate-chitosan-collagen scaffolds for bone tissue engineering. ACS Appl Mater Interfaces. 2018, 10, 12441-12452.
doi: 10.1021/acsami.8b00699 URL |
| 20. |
Choe, G.; Oh, S.; Seok, J. M.; Park, S. A.; Lee, J. Y. Graphene oxide/alginate composites as novel bioinks for three-dimensional mesenchymal stem cell printing and bone regeneration applications. Nanoscale. 2019, 11, 23275-23285.
doi: 10.1039/C9NR07643C URL |
| 21. |
Yildiz, G.; Bolton-Warberg, M.; Awaja, F. Graphene and graphene oxide for bio-sensing: General properties and the effects of graphene ripples. Acta Biomater. 2021, 131, 62-79.
doi: 10.1016/j.actbio.2021.06.047 URL |
| 22. | Gosai, A.; Khondakar, K. R.; Ma, X.; Ali, M. A. Application of functionalized graphene oxide based biosensors for health monitoring: simple graphene derivatives to 3D printed platforms. Biosensors (Basel). 2021, 11, 384. |
| 23. |
Geim, A. K.; Novoselov, K. S. The rise of graphene. Nat Mater. 2007, 6, 183-191.
doi: 10.1038/nmat1849 URL |
| 24. |
Kim, K. S.; Zhao, Y.; Jang, H.; Lee, S. Y.; Kim, J. M.; Kim, K. S.; Ahn, J. H.; Kim, P.; Choi, J. Y.; Hong, B. H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature. 2009, 457, 706-710.
doi: 10.1038/nature07719 URL |
| 25. |
Service, R. F. Materials science. Carbon sheets an atom thick give rise to graphene dreams. Science. 2009, 324, 875-877.
doi: 10.1126/science.324_875 URL |
| 26. |
Zhou, X.; Liu, Z. A scalable, solution-phase processing route to graphene oxide and graphene ultralarge sheets. Chem Commun (Camb). 2010, 46, 2611-2613.
doi: 10.1039/b914412a URL |
| 27. |
Pandey, H.; Parashar, V.; Parashar, R.; Prakash, R.; Ramteke, P. W.; Pandey, A. C. Controlled drug release characteristics and enhanced antibacterial effect of graphene nanosheets containing gentamicin sulfate. Nanoscale. 2011, 3, 4104-4108.
doi: 10.1039/c1nr10661a URL |
| 28. |
Miao, W.; Shim, G.; Lee, S.; Lee, S.; Choe, Y. S.; Oh, Y. K. Safety and tumor tissue accumulation of pegylated graphene oxide nanosheets for co-delivery of anticancer drug and photosensitizer. Biomaterials. 2013, 34, 3402-3410.
doi: 10.1016/j.biomaterials.2013.01.010 URL |
| 29. |
Porter, D. C.; Farmaki, E.; Altilia, S.; Schools, G. P.; West, D. K.; Chen, M.; Chang, B. D.; Puzyrev, A. T.; Lim, C. U.; Rokow-Kittell, R.; Friedhoff, L. T.; Papavassiliou, A. G.; Kalurupalle, S.; Hurteau, G.; Shi, J.; Baran, P. S.; Gyorffy, B.; Wentland, M. P.; Broude, E. V.; Kiaris, H.; Roninson, I. B. Cyclin-dependent kinase 8 mediates chemotherapy-induced tumor-promoting paracrine activities. Proc Natl Acad Sci U S A. 2012, 109, 13799-13804.
doi: 10.1073/pnas.1206906109 URL |
| 30. |
Yu, H.; Zhang, B.; Bulin, C.; Li, R.; Xing, R. High-efficient synthesis of graphene oxide based on improved hummers method. Sci Rep. 2016, 6, 36143.
doi: 10.1038/srep36143 URL |
| 31. |
Marcano, D. C.; Kosynkin, D. V.; Berlin, J. M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L. B.; Lu, W.; Tour, J. M. Improved synthesis of graphene oxide. ACS Nano. 2010, 4, 4806-4814.
doi: 10.1021/nn1006368 URL |
| 32. |
Maturavongsadit, P.; Bi, X.; Metavarayuth, K.; Luckanagul, J. A.; Wang, Q. Influence of cross-linkers on the in vitro chondrogenesis of mesenchymal stem cells in hyaluronic acid hydrogels. ACS Appl Mater Interfaces. 2017, 9, 3318-3329.
doi: 10.1021/acsami.6b12437 URL |
| 33. |
Maturavongsadit, P.; Luckanagul, J. A.; Metavarayuth, K.; Zhao, X.; Chen, L.; Lin, Y.; Wang, Q. Promotion of in vitro chondrogenesis of mesenchymal stem cells using in situ hyaluronic hydrogel functionalized with rod-like viral nanoparticles. Biomacromolecules. 2016, 17, 1930-1938.
doi: 10.1021/acs.biomac.5b01577 URL |
| 34. |
Hiemstra, C.; Zhou, W.; Zhong, Z.; Wouters, M.; Feijen, J. Rapidly in situ forming biodegradable robust hydrogels by combining stereocomplexation and photopolymerization. J Am Chem Soc. 2007, 129, 9918-9926.
doi: 10.1021/ja072113p URL |
| 35. | Dash, S.; Murthy, P. N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol Pharm. 2010, 67, 217-223. |
| 36. |
Kilkenny, C.; Browne, W. J.; Cuthill, I. C.; Emerson, M.; Altman, D. G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412.
doi: 10.1371/journal.pbio.1000412 URL |
| 37. | Gurunathan, S.; Han, J. W.; Kim, E. S.; Park, J. H.; Kim, J. H. Reduction of graphene oxide by resveratrol: a novel and simple biological method for the synthesis of an effective anticancer nanotherapeutic molecule. Int J Nanomedicine. 2015, 10, 2951-2969. |
| 38. |
Korsmeyer, R. W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N. A. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm. 1983, 15, 25-35.
doi: 10.1016/0378-5173(83)90064-9 URL |
| 39. | Wang, H.; Gu, W.; Xiao, N.; Ye, L.; Xu, Q. Chlorotoxin-conjugated graphene oxide for targeted delivery of an anticancer drug. Int J Nanomedicine. 2014, 9, 1433-1442. |
| 40. |
Depan, D.; Shah, J.; Misra, R. D. K. Controlled release of drug from folate-decorated and graphene mediated drug delivery system: Synthesis, loading efficiency, and drug release response. Mater Sci Eng C. 2011, 31, 1305-1312.
doi: 10.1016/j.msec.2011.04.010 URL |
| 41. |
Lv, Y.; Tao, L.; Annie Bligh, S. W.; Yang, H.; Pan, Q.; Zhu, L. Targeted delivery and controlled release of doxorubicin into cancer cells using a multifunctional graphene oxide. Mater Sci Eng C Mater Biol Appl. 2016, 59, 652-660.
doi: 10.1016/j.msec.2015.10.065 URL |
| 42. |
Yang, D.; Gao, S.; Fang, Y.; Lin, X.; Jin, X.; Wang, X.; Ke, L.; Shi, K. The π-π stacking-guided supramolecular self-assembly of nanomedicine for effective delivery of antineoplastic therapies. Nanomedicine (Lond). 2018, 13, 3159-3177.
doi: 10.2217/nnm-2018-0288 URL |
| 43. |
Song, J.; Cui, N.; Sun, S.; Lu, X.; Wang, Y.; Shi, H.; Lee, E. S.; Jiang, H. B. Controllability of graphene oxide doxorubicin loading capacity based on density functional theory. Nanomaterials (Basel). 2022, 12, 479.
doi: 10.3390/nano12030479 URL |
| 44. |
Bonacucina, G.; Cespi, M.; Palmieri, G. F. Evaluation of dissolution kinetics of hydrophilic polymers by use of acoustic spectroscopy. Int J Pharm. 2009, 377, 153-158.
doi: 10.1016/j.ijpharm.2009.04.043 URL |
| 45. |
Siepmann, J.; Göpferich, A. Mathematical modeling of bioerodible, polymeric drug delivery systems. Adv Drug Deliv Rev. 2001, 48, 229-247.
doi: 10.1016/S0169-409X(01)00116-8 URL |
| 46. |
Sanchez, V. C.; Jachak, A.; Hurt, R. H.; Kane, A. B. Biological interactions of graphene-family nanomaterials: an interdisciplinary review. Chem Res Toxicol. 2012, 25, 15-34.
doi: 10.1021/tx200339h URL |
| 47. |
Girish, C. M.; Sasidharan, A.; Gowd, G. S.; Nair, S.; Koyakutty, M. Confocal Raman imaging study showing macrophage mediated biodegradation of graphene in vivo. Adv Healthc Mater. 2013, 2, 1489-1500.
doi: 10.1002/adhm.201200489 URL |
| 48. |
Chen, G. Y.; Yang, H. J.; Lu, C. H.; Chao, Y. C.; Hwang, S. M.; Chen, C. L.; Lo, K. W.; Sung, L. Y.; Luo, W. Y.; Tuan, H. Y.; Hu, Y. C. Simultaneous induction of autophagy and toll-like receptor signaling pathways by graphene oxide. Biomaterials. 2012, 33, 6559-6569.
doi: 10.1016/j.biomaterials.2012.05.064 URL |
| 49. |
Zhou, H.; Zhao, K.; Li, W.; Yang, N.; Liu, Y.; Chen, C.; Wei, T. The interactions between pristine graphene and macrophages and the production of cytokines/chemokines via TLR- and NF-κB-related signaling pathways. Biomaterials. 2012, 33, 6933-6942.
doi: 10.1016/j.biomaterials.2012.06.064 URL |
| 50. |
Russier, J.; Treossi, E.; Scarsi, A.; Perrozzi, F.; Dumortier, H.; Ottaviano, L.; Meneghetti, M.; Palermo, V.; Bianco, A. Evidencing the mask effect of graphene oxide: a comparative study on primary human and murine phagocytic cells. Nanoscale. 2013, 5, 11234-11247.
doi: 10.1039/c3nr03543c URL |
| 51. |
Yue, H.; Wei, W.; Yue, Z.; Wang, B.; Luo, N.; Gao, Y.; Ma, D.; Ma, G.; Su, Z. The role of the lateral dimension of graphene oxide in the regulation of cellular responses. Biomaterials. 2012, 33, 4013-4021.
doi: 10.1016/j.biomaterials.2012.02.021 URL |
| 52. | Aviles-Olmos, I.; Dickson, J.; Kefalopoulou, Z.; Djamshidian, A.; Kahan, J.; Ell, P.; Whitton, P.; Wyse, R.; Isaacs, T.; Lees, A.; Limousin, P.; Foltynie, T. Motor and cognitive advantages persist 12 months after exenatide exposure in Parkinson’s disease. J Parkinsons Dis. 2014, 4, 337-344. |
| 53. | Zhu, W.; Masaki, T.; Bae, Y. H.; Rathi, R.; Cheung, A. K.; Kern, S. E. Development of a sustained-release system for perivascular delivery of dipyridamole. J Biomed Mater Res B Appl Biomater. 2006, 77, 135-143. |
| 54. |
Edelman, E. R.; Nathan, A.; Katada, M.; Gates, J.; Karnovsky, M. J. Perivascular graft heparin delivery using biodegradable polymer wraps. Biomaterials. 2000, 21, 2279-2286.
doi: 10.1016/S0142-9612(00)00154-X URL |
| 55. |
Schachner, T.; Zou, Y.; Oberhuber, A.; Tzankov, A.; Mairinger, T.; Laufer, G.; Bonatti, J. O. Local application of rapamycin inhibits neointimal hyperplasia in experimental vein grafts. Ann Thorac Surg. 2004, 77, 1580-1585.
doi: 10.1016/j.athoracsur.2003.10.008 URL |
| 56. |
Okada, T.; Bark, D. H.; Mayberg, M. R. Localized release of perivascular heparin inhibits intimal proliferation after endothelial injury without systemic anticoagulation. Neurosurgery. 1989, 25, 892-898.
doi: 10.1227/00006123-198912000-00007 URL |
| 57. |
Lipke, E. A.; West, J. L. Localized delivery of nitric oxide from hydrogels inhibits neointima formation in a rat carotid balloon injury model. Acta Biomater. 2005, 1, 597-606.
doi: 10.1016/j.actbio.2005.07.010 URL |
| 58. |
Masters, K. S.; Lipke, E. A.; Rice, E. E.; Liel, M. S.; Myler, H. A.; Zygourakis, C.; Tulis, D. A.; West, J. L. Nitric oxide-generating hydrogels inhibit neointima formation. J Biomater Sci Polym Ed. 2005, 16, 659-672.
doi: 10.1163/1568562053783722 URL |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||