During the coronavirus disease 2019 (COVID-19) pandemic in early 2020, Thailand, like many other countries around the world, experienced a lack of personal protective equipment (PPE) for frontline medical staff. This was not only due to the scarcity of PPE, but also because some of the available PPE did not meet the standard to properly protect frontline medical staff from the virus. During that time, the industrial sector worked closely with academic and governmental sectors to develop and produce appropriate isolation gowns, coveralls and masks.
In mid-March 2020, a polyester fabric-producing factory which is familiar with medical textiles realized that the lack of PPE in Thailand was quite severe. Therefore, the company worked closely with a garment company to develop an isolation gown for medics. Within two months, a reusable isolation gown which passed ANSI/AAMI PB70 Level 2,1 “Rao Soo” (which means we fight) was successfully developed. The Rao Soo isolation gown can be washed and reused up to 20 times. In addition, it is produced from recycled polyethylene terephthalate water bottles. The Government Pharmaceutical Organization has reported that since May 2020, production capacity has reached more than one million isolation gowns per month.
Because some tasks performed by staff on the medical frontline may cause them to come into contact with fluid containing the virus, a coverall with an infection protection level which can protect the wearer from blood containing the virus has also been developed. The target specification for the coverall gowns was to provide high biohazard performance as well as physical performance. Even though we have never previously produced high-performance fabric for PPE in Thailand, we considered various types of fabric that we were able to produce. There are several spunbonded nonwoven fabric producers in Thailand. Consequently, this type of fabric was chosen to provide the mechanical strength of the fabric. However, spunbonded nonwoven fabric would allow blood and virus particles to penetrate. Therefore, a layer of fabric was needed that was able to withstand both blood and virus particles. First, plastic film is known to withstand the passage of both blood and virus; however, plastic film can be easily torn. In addition, the water vapor transmission of plastic film is quite poor, making it uncomfortable to wear. Fortunately, in Thailand there is also a factory producing breathable plastic film which is used to produce diapers and sanitary napkins. Therefore, they provided the breathable film to be laminated with the spunbonded nonwoven fabric.
While the fabric producers developed a fabric with suitable biohazard and physical performance, the garment factories tried to develop seams which would be able to withstand penetration of the blood and virus. After three months of hard work, a PPE gown that passed ANSI/AAMI PB70 Level 4 was successfully developed. This has been named “Rao Chana” which means, in Thai, “we win”.
Overview and definition of PPE: Health-care workers (HCWs) are among the group at highest risk of infection by the spread of infectious diseases such as COVID-19, Ebola virus disease and severe acute respiratory syndrome (SARS).2, 3 When HCWs are the frontline personnel who diagnose and care for the infected patients, the probability of HCWs catching a contagious virus is markedly increased compared to other occupations. Aerosol droplets and transfer of contaminants from hands to mucous membranes are considered major routes of infection for COVID-19.4 One of the important tools in limiting the transmissibility of the virus to this group of people is by using PPE for the prevention of direct exposure to contaminated fluids from patients’ blood and droplets created by activities such as talking, coughing, and sneezing.5 In 2004, the Occupational Safety and Health Administration of the U.S. Department of Labor identified the broad meaning of PPE that helps establish and maintain a safe and healthy work environment to be categorized as 1) eye and face protection 2) head protection 3) foot and leg protection 4) hand and arm protection 5) body protection and 6) hearing protection. For HCWs, the importance of PPE in preventing microbial infection highlights the equipment that covers body protection (such as, aprons, gowns or coveralls), respiratory tract protection by masks or respirators, and eye protection by goggles.3, 6 In this viewpoint, the focus lies on protective clothing for body protection. After gloves, the reported second most frequently-used PPE in the healthcare setting was protective gowns.7 Appropriate selection of PPE gowns has been suggested in many guidelines.8-10 The Association for the Advancement of Medical Instrumentation (AAMI) suggested that isolation gowns should be used to prevent the transfer of contaminated fluids within the healthcare environment in high-risk situations of patient isolation.11 Also, a comparable recommendation on the use of isolation gowns has been produced by The U.S. Food and Drug Administration giving the definition of a PPE gown as “a gown intended to protect healthcare patients and personnel from the transfer of microorganisms, body fluids, and particulate material”. The specific definition of PPE gowns includes coverage of the torso including arms and exposed body areas, clothing and garments that create appropriate blockage of the microorganisms and other contagious secretions and excretions.12
The U.S. Food and Drug Administration listed the barrier protection levels of gowns and other types of PPE for their levels of protection as follows:13
Level 1: Minimal risk, to be used, for example, during basic care, standard isolation, as cover gowns for visitors, or in a standard medical unit;
Level 2: Low risk, to be used, for example, during blood draw, suturing, in the intensive care unit (ICU), or a pathology lab.;
Level 3: Moderate risk, to be used, for example, during arterial blood draw, inserting an intravenous (IV) line, in the emergency room, or for trauma cases;
Level 4: High risk, to be used, for example, during long, fluid-intense procedures, surgery, when pathogen resistance is needed or infectious diseases are suspected (non-airborne).
There are differences in the definitions between isolation gowns and surgical gowns. A surgical gowns’ critical zones are defined by the surgical procedures to cover the front of the body from the top of the shoulders to the knees and the arms from the wrist cuff to above the elbow. They are designed to protect both health-care personnel during surgical procedures and the patient from any transmissible matter. While surgical gowns are applicable for any risk level (Levels 1-4), isolation gowns are used when there is a medium to high risk of contamination and a need for larger critical zones. For isolation gowns, critical zones include all areas except bindings, cuffs, and hems that must reach the highest liquid barrier protection level of the performance standards. This protection level applies to all seams as well as the rest of the gown. Moreover, it is recommended that the materials of the isolation gown should cover as much of the body as is appropriate for the intended use. The Centers for Disease Control and Prevention’s Guideline for Isolation Precautions suggests that HCWs should wear the isolation gowns during procedures and patient-care activities.14 In the technical compliance with relevant performance standards options, depending on risks, for isolation gowns to be used with COVID-19, the World Health Organization (WHO) 2020 currently recommends either AAMI PB70 (Level 1-3)1 and ASTM F3352 (U.S.),15 EN 13034 - Type PB [6] (stitched gown), with a minimum hydrostatic head of 50 cmH2O (E.U.),16 AAMI PB70 Level 4 and ASTM F3352 (U.S.)1 or ISO 16604 Class 5 (E.U.)17 for providing viral penetration resistance, or an alternative equivalent set of standards. However, the standard options for surgical gowns are listed by the WHO, 2020 for AAMI PB701 and ASTM F2407 (U.S.),18 EN 13795 (E.U.),19 EN 13034 - Type PB [6] (stitched gown), with minimum hydrostatic head of 50 cmH2O (E.U.), YY/T 0506 (China)20 or alternative equivalent set of standards, EN 556 (E.U.),21 if sterile, or alternative equivalent set of standards.22 Another type of PPE body protection is a coverall, classified only in E.U. standard (EN 13688).23 A coverall with EN 1412624 is certified against infective agents. It is designed to protect the entire body, from head to ankle. For the performance tests, a coverall is required to pass fabric, seam and whole garment tests.
Despite the substantial performance of PPE confirmed by standard test methods, the use of such equipment is considered only one of the infection prevention and control measures. HCWs should not rely on PPE as the primary prevention strategy. Without effective administrative and engineering controls, PPE can be inadequate to control the spread of diseases, as described in the WHO’s publication ‘Infection prevention and control of epidemic- and pandemic-prone acute respiratory infections in health care’.9 With the extensive use of and demand for PPE, shortage of these medical supplies is an extremely critical issue which arose around the world during this pandemic situation. The safety and efficacy of the PPE supplied to HCWs were of serious concern. In some areas, supplies of PPE failed the quality requirement for the standard used to handle COVID-19. The unmet need for safer and more effective PPE is still the major challenge across the globe, even with all the recommendations formulated from various organizations such as the WHO, the National Health Service (U.K.), and the Centers for Disease Control and Prevention in the U.S. concerning the specification and use of PPE.25 These issues severely affected Thailand’s healthcare system, where the supply of PPE gowns previously relied entirely on imports and no standard testing has ever been developed before.
Standards and test methods focusing on the synthetic blood and virus penetration test method: In order to control and prevent infection during the COVID-19 pandemic, the use of personal protective clothing has dramatically increased worldwide. One possible means of exposure to biological fluid contaminated with COVID-19 is by penetration through protective clothing. Hence, healthcare professionals must wear suitable and effective protective apparel when caring for patients with suspected or confirmed COVID-19 infection. There are two major regulatory standards: (i) EN14126:200324 and (ii) ANSI/AAMI PB 70:2012,1 to evaluate the penetration resistance of protective clothing. The European Union commonly uses the EN14126:2003 standard, while the United States of America generally uses the ANSI/AAMI PB 70:2012 standard. The EN14126:2003 consists of five test methods to assess the barrier performance of protective clothing against infective agents and biological fluids (Table 1). The EN14126:2003 uses various types of micro-organisms in different test methods to evaluate the resistance of a garment against penetration by infectious agents; and uses synthetic blood as a surrogate for biological fluid (ISO 16603).26 The ANSI/AAMI PB 70:2012 uses four test methods to evaluate liquid barrier performance and to classify protective apparel intended for use in a healthcare setting (Table 2). Among the series of test methods from both standards, the synthetic blood penetration test (ISO 16603 and ASTM F1670)27 and viral penetration test (ISO 16604 and ASTM F1671) are the critical assays to evaluate the efficacy of protective clothing for protection against COVID-19.
Table 1 Test methods for EN14126:2003
| Test method | Test description | Types of contaminations | Approximate contaminant size |
|---|---|---|---|
| ISO 16603 | Determination of the resistance of protective clothing materials to penetration by blood and body fluids | Synthetic blood | - |
| ISO 16604 | Determination of the resistance of protective clothing materials to penetration by blood-borne pathogens | Phi-X 174 bacteriophage | 0.03 mm28 |
| ISO 22610 | Determination of the resistance to wet bacterial penetration | Bacillus atrophaeus contaminated liquid | 1.0-1.6 mm (length); 0.6-0.9 mm (diameter)29 |
| ISO/DIS 22611 | Determination of the resistance to penetration by biologically contaminated aerosols | Staphylococcus aureus contaminated aerosols | 1 mm30 |
| ISO 22612 | Determination of resistance to dry microbial penetration | Bacillus subtilis contaminated talcum powder | 0.9-1.5 mm (length); 0.4-0.7 mm (diameter)29 |
Table 2 Test methods for ANSI/AAMI PB 70:2012
| Test method | Test description | Challenge liquid |
|---|---|---|
| AATCC 42 | Water resistance: impact penetration | Water |
| AATCC 127 | Water resistance: hydrostatic pressure | Water |
| ASTM F1670 | Determination of the resistance of protective clothing materials to penetration by synthetic blood | Synthetic blood |
| ASTM F1671 | Determination of the resistance of protective clothing materials to penetration by blood-borne pathogens | Phi-X 174 bacteriophage |
ISO 16603 and ASTM F1670 are screening assays that evaluate the barrier performance of clothing material against penetration by synthetic blood solution. The key factor for these tests is the surface tension of the challenge liquid. Liquids with high surface tension tend to stay on the surface of the material, while liquids with lower surface tension are more likely to penetrate through a garment. The surface tension of synthetic blood solution (≈ 42 mN/m) used in these assays is close to that of blood (≈ 50 mN/m),31, 32 sweat (≈ 40 mN/m)33 and saliva (≈ 40 mN/m),33 while water has higher surface tension than biological fluids (≈ 70 mN/m). Hence, test methods using water as the challenge solution are not appropriate to be used for this purpose.
ISO 1660417 and ASTM F167127 are the assays used for evaluation of the resistance of protective clothing to viral penetration. These two tests are similar to ISO 16603 and ASTM F1670; however, they use Phi-X 174 bacteriophage suspension as the challenge liquid, rather than a solution of synthetic blood. For ISO 16604 and ASTM F1671, after exposure to a bacteriophage suspension at a certain pressure and duration, the opposite side of the tested garment is rinsed with an assay fluid; this assay fluid is further cultured with host bacteria, Escherichia coli, to investigate plaque formation. If the tested garment is resistant to penetration by bacteriophages, no liquid is observed to penetrate the tested specimen and no plaques are formed in the Escherichia coli cultures treated with the assay fluid.
The choice of virus used in the challenge solution is a critical factor for the viral penetration test. Due to its size, the Phi-X 174 bacteriophage is a good surrogate microbe for evaluation of the penetration resistance of protective clothing against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus. The Phi-X 174 bacteriophage is a non-pathogenic virus with diameter of 0.03 mm, less than one third the size of the SARS-CoV-2 virus (SARS-CoV-2 ≈ 0.1 mm in diameter34). In the other standard tests, e.g. ISO 22610, ISO/DIS 22611 and ISO 22612, the size of the micro-organisms used in these assays are much larger than the SARS-CoV-2 virus (Table 2). Hence, only ISO 16604 and ASTM F1671 tests provide appropriate conditions for investigation of the barrier performance of a material against SARS-CoV-2.
The pressure level used in the test is another important factor for the viral penetration assay. The major difference between the ISO vs. ASTM tests is the amount of pressure applied in performing the tests. In ASTM F1670 and ASTM F1671, tests are conducted with only one pressure level (13.8 kPa (2 psi)). Hence, the ASTM test can provide clear delineation regarding whether a material is protective or not, under specified conditions; however, it is a poor approach to categorize the barrier performance of materials against viral penetration.35 To overcome this limitation, ISO adopted and modified the ASTM F1670 and ASTM F1671 tests to create ISO 16603 and ISO 16604 by using a series of pressure levels (from 0 kPa to 20 kPa) in the test procedure. Hence, ISO 16603 and ISO 16604 can be used to rank the barrier performance of a tested garment into classes (Table 3). Note that 14 kPa in ISO 16603 and ISO 16604 is the closest equivalent pressure to the ASTM methods.
Table 3 ANSI/AAMI PB70 and EN14126:2003 classification of the level of barrier performance
| Level | Test methods | Criteria |
|---|---|---|
| ANSI/AAMI PB70 standard | ||
| 1 | AATCC 42 | ≤ 4.5 g |
| 2 | AATCC 42 | ≤ 1.0 g |
| AATCC 127 | ≥ 20 cm | |
| 3 | AATCC 42 | ≤ 1.0 g |
| AATCC 127 | ≥ 50 cm | |
| 4 | ASTM F1670 | No penetration at 13.8 kPa |
| ASTM F1671 | No penetration at 13.8 kPa | |
| EN14126:2003 standard | ||
| 1 | ISO 16603 & ISO 16604 | 0.0 kPa |
| 2 | ISO 16603 & ISO 16604 | 1.75 kPa |
| 3 | ISO 16603 & ISO 16604 | 3.5 kPa |
| 4 | ISO 16603 & ISO 16604 | 7.0 kPa |
| 5 | ISO 16603 & ISO 16604 | 14.0 kPa |
| 6 | ISO 16603 & ISO 16604 | 20.0 kPa |
Lesson for development of a standard test from first principles; development of adaptive equipment: as a result of the COVID-19 pandemic in early 2020, the demand for PPE for medical personnel increased exponentially. Most countries suffered particularly from a lack of medical gowns. In Thailand, an urgent plan for medical gown production was established by cooperation among governmental and non-governmental sections. At the outset, the most significant issue was the selection of textile and/or fabric material for the gowns. Clothing materials must be tested according to standards such as ANSI/AAMI PB70 (2012) and BS EN14126 (2003) which evaluate their resistance to synthetic blood penetration. Unfortunately, the testing equipment referred to in the ISO standard (ISO16603, 2004), known as “penetration test apparatus”, is very hard to find or invent during an urgent period. An ad hoc device was therefore developed as a substitute for the penetration test apparatus specified in the ISO standard. In this section, the details of the test equipment developed in-house will be described.
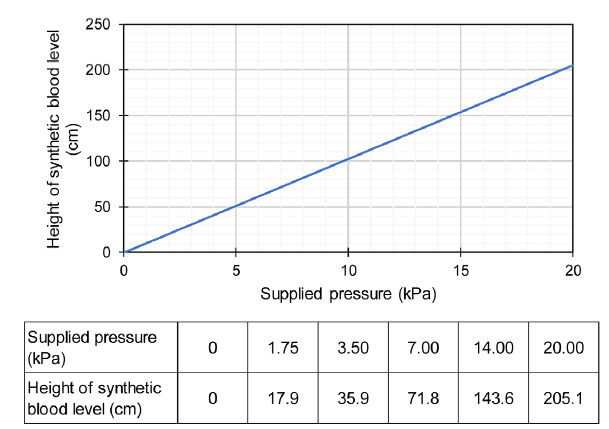
The equipment consisted of two main parts, namely a penetration test cell and a pressure supply unit. The penetration test cell was made from two 20-mm thick transparent acrylic plates. A large opening was made in the top plate to investigate blood penetration while a circular groove was provided on the surface of the bottom plate to install an O-ring. A small hole was drilled in the middle of the bottom plate to supply synthetic blood under pressure. This small hole was connected to the pressure supply unit through a control valve. To test a material, a sample was cut of approximately the same size as the acrylic plate and installed on top of the O-ring. During testing, the specimen was sandwiched between the top and bottom plates and screws at the four corners of the plates were tightened to hold it in position. The pressure supply unit consisted of a cylindrical tank to hold the synthetic blood and apply pressure to the penetration test cell. The pressure was controlled by adjusting the elevation of the tank, which is similar to hydrostatic pressure. The pressure supply unit could generate a head of pressure up to approximately 2 m of the synthetic blood level, which was sufficient for the penetration test with a controlled pressure range of 0-20 kPa. The resolution of the supplied pressure could be controlled at 1 mm of the synthetic blood level (approximately 10 Pa). By comparing with readings from a pressure gauge, it was verified that the generated pressure could be calculated by multiplication of the weight of the synthetic blood unit and the synthetic blood level. The relationship between the height of the synthetic blood level and the supplied pressure is presented in Figure 1.
Figure 1.
Figure 1.
Relationship between height of synthetic blood level and supplied pressure.
The obvious advantages of this adaptive testing equipment were its simplicity and low production cost, meaning that it could be easily reproduced for widespread utilisation. The pressure supply unit was able to provide a precise and stable pressure throughout the tested specimen especially in the low-pressure range. The use of transparent acrylic plates for the penetration test cell provided clear visibility during testing. The acrylic plates were also easy to clean after each test. This adaptive equipment could also be further modified for multiple testing. Moreover, the results of synthetic blood penetration tests based on the adaptive equipment were compared with the results of samples tested using the penetration test apparatus specified in the ISO standard and no significant difference was found between the results.
Conclusion and perspectives: Due to the recent COVID-19 outbreak, an increase in the number of cases of infection among frontline HCWs has been reported all over the world.36 Before the availability of a safe and effective vaccination programme, implementation of preventive measures, particularly regarding the use of PPE (e.g. gloves, masks, face/eye protectors and gowns), remained the only option to reduce the risk SARS-CoV-2 infection in these particular groups. However, the global shortage of PPE and resultant price rises during the first wave of COVID-19 became a major problem especially in many developing and low-income countries.37 This raises an important issue as well as self-awareness regarding the national security of the supply of medical products in each country. Therefore, strengthening of the clothing and garment industries to increase their capabilities regarding production of medical textiles is one of the strategies adopted in many countries including Thailand. The Thai Food and Drug Administration together with the Medical Products Consortium of Thailand and other partners set up a national platform to promote the development of medical textiles and medical devices. Such implementation not only supported the self-sufficiency of the country but also opened up the possibility of launching their products onto the global markets. This strategy is in-line with the estimation that the global demand for PPE is 100 times the normal level.38
From the technological viewpoint of PPE testing, it has been documented that the surgical and isolation gowns available in the global marketplace varied remarkably in terms of their resistance to blood and viral penetration, which depended on the fabric used, the design of the gown and the interface.5 Most protective gowns have been classified into different levels and tested according to international standards (ASTM F2407, ANSI/AAMI PB70, EN 13795 and EN 14126) as described in the main text. For the viral penetration test, international standards such as ASTM 1671 and ISO 16604 recommend use of the bacteriophage Phi-X-174 as the test model. However, it is questionable whether the use of this bacteriophage is appropriate to ensure the resistance of clothing material against SARS-CoV-2 penetration due to the disparity in shape, size and polarity.39 According to our experience, the application of other types of virus such as the influenza virus may be a better model, more representative of SARS-CoV-2 in the viral penetration test. Supportive evidence can be found in the N95 filtering test using viable H1N1 influenza virus (ATCC VR-95).40 Moreover, additional testing such as antiviral activity of the modified fabric used in protective gown development may be recommended by international standards in the near future.
Author contributions
Conceptualisation: JAL; validation: CI; formal analysis, data curation, and writing—original draft preparation: VB, AS, SL, TB, CI, JAL; investigation: SL, TB; resources: AS, SL, TB, JAL; manuscript review and editing: JAL; supervision: AS, CI; funding acquisition: AS, JAL. All authors have read and agreed to the published version of the manuscript.
Financial support
This work was supported by the Faculty of Engineering, Chulalongkorn University.
Acknowledgement
The help and service of the Pharmaceutical Research Instrument Centre, Faculty of Pharmaceutical Sciences, Chulalongkorn University was appreciated. The authors would like to extend our gratitude to Dr. Kanit Tapasa, the Department of Science Service: DSS, Ministry of Higher Education, Science, Research and Innovation for hosting and coordinating the PPE development project and to the Thai-Food and Drug Administration for constructive advice. The graphical abstract was created with BioRender.com
Conflicts of interest statement
The authors declare no competing financial interests.
Data sharing statement
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Reference
Liquid barrier performance and classification ofprotective apparel and drapes intended for use in health care facilities
China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019
DOI:10.1056/NEJMoa2001017
URL
PMID:31978945
[Cited within: 1]

In December 2019, a cluster of patients with pneumonia of unknown cause was linked to a seafood wholesale market in Wuhan, China. A previously unknown betacoronavirus was discovered through the use of unbiased sequencing in samples from patients with pneumonia. Human airway epithelial cells were used to isolate a novel coronavirus, named 2019-nCoV, which formed a clade within the subgenus sarbecovirus, Orthocoronavirinae subfamily. Different from both MERS-CoV and SARS-CoV, 2019-nCoV is the seventh member of the family of coronaviruses that infect humans. Enhanced surveillance and further investigation are ongoing. (Funded by the National Key Research and Development Program of China and the National Major Project for Control and Prevention of Infectious Disease in China.).
Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff
DOI:10.1002/14651858.CD011621.pub2
URL
PMID:27093058
[Cited within: 2]

BACKGROUND: In epidemics of highly infectious diseases, such as Ebola Virus Disease (EVD) or SARS, healthcare workers (HCW) are at much greater risk of infection than the general population, due to their contact with patients' contaminated body fluids. Contact precautions by means of personal protective equipment (PPE) can reduce the risk. It is unclear which type of PPE protects best, what is the best way to remove PPE, and how to make sure HCWs use PPE as instructed. OBJECTIVES: To evaluate which type or component of full-body PPE and which method of donning or removing (doffing) PPE have the least risk of self-contamination or infection for HCWs, and which training methods most increase compliance with PPE protocols. SEARCH METHODS: We searched MEDLINE (PubMed up to 8 January 2016), Cochrane Central Register of Trials (CENTRAL up to 20 January 2016), EMBASE (embase.com up to 8 January 2016), CINAHL (EBSCOhost up to 20 January 2016), and OSH-Update up to 8 January 2016. We also screened reference lists of included trials and relevant reviews, and contacted NGOs and manufacturers of PPE. SELECTION CRITERIA: We included all eligible controlled studies that compared the effect of types or components of PPE in HCWs exposed to highly infectious diseases with serious consequences, such as EVD and SARS, on the risk of infection, contamination, or noncompliance with protocols. This included studies that simulated contamination with fluorescent markers or a non-pathogenic virus.We also included studies that compared the effect of various ways of donning or removing PPE, and the effects of various types of training in PPE use on the same outcomes. DATA COLLECTION AND ANALYSIS: Two authors independently selected studies, extracted data and assessed risk of bias in included trials. We intended to perform meta-analyses but we did not find sufficiently similar studies to combine their results. MAIN RESULTS: We included nine studies with 1200 participants evaluating ten interventions. Of these, eight trials simulated the exposure with a fluorescent marker or virus or bacteria containing fluids. Five studies evaluated different types of PPE against each other but two did not report sufficient data. Another two studies compared different types of donning and doffing and three studies evaluated the effect of different types of training.None of the included studies reported a standardised classification of the protective properties against viral penetration of the PPE, and only one reported the brand of PPE used. None of the studies were conducted with HCWs exposed to EVD but in one study participants were exposed to SARS. Different types of PPE versus each otherIn simulation studies, contamination rates varied from 25% to 100% of participants for all types of PPE. In one study, PPE made of more breathable material did not lead to a statistically significantly different number of spots with contamination but did have greater user satisfaction (Mean Difference (MD) -0.46 (95% Confidence Interval (CI) -0.84 to -0.08, range 1 to 5, very low quality evidence). In another study, gowns protected better than aprons. In yet another study, the use of a powered air-purifying respirator protected better than a now outdated form of PPE. There were no studies on goggles versus face shields, on long- versus short-sleeved gloves, or on the use of taping PPE parts together. Different methods of donning and doffing procedures versus each otherTwo cross-over simulation studies (one RCT, one CCT) compared different methods for donning and doffing against each other. Double gloving led to less contamination compared to single gloving (Relative Risk (RR) 0.36; 95% CI 0.16 to 0.78, very low quality evidence) in one simulation study, but not to more noncompliance with guidance (RR 1.08; 95% CI 0.70 to 1.67, very low quality evidence). Following CDC recommendations for doffing led to less contamination in another study (very low quality evidence). There were no studies on the use of disinfectants while doffing. Different types of training versus each otherIn one study, the use of additional computer simulation led to less errors in doffing (MD -1.2, 95% CI -1.6 to -0.7) and in another study additional spoken instruction led to less errors (MD -0.9, 95% CI -1.4 to -0.4). One retrospective cohort study assessed the effect of active training - defined as face-to-face instruction - versus passive training - defined as folders or videos - on noncompliance with PPE use and on noncompliance with doffing guidance. Active training did not considerably reduce noncompliance in PPE use (Odds Ratio (OR) 0.63; 95% CI 0.31 to 1.30) but reduced noncompliance with doffing procedures (OR 0.45; 95% CI 0.21 to 0.98, very low quality evidence). There were no studies on how to retain the results of training in the long term or on resource use.The quality of the evidence was very low for all comparisons because of high risk of bias in studies, indirectness of evidence, and small numbers of participants. This means that it is likely that the true effect can be substantially different from the one reported here. AUTHORS' CONCLUSIONS: We found very low quality evidence that more breathable types of PPE may not lead to more contamination, but may have greater user satisfaction. We also found very low quality evidence that double gloving and CDC doffing guidance appear to decrease the risk of contamination and that more active training in PPE use may reduce PPE and doffing errors more than passive training. However, the data all come from single studies with high risk of bias and we are uncertain about the estimates of effects.We need simulation studies conducted with several dozens of participants, preferably using a non-pathogenic virus, to find out which type and combination of PPE protects best, and what is the best way to remove PPE. We also need randomised controlled studies of the effects of one type of training versus another to find out which training works best in the long term. HCWs exposed to highly infectious diseases should have their use of PPE registered and should be prospectively followed for their risk of infection.
A review of isolation gowns in healthcare: fabric and gown properties
URL
PMID:26989351
[Cited within: 2]

The threat of emerging infectious diseases including Ebola hemorrhagic fever, pandemic influenza, avian influenza, Hepatitis B, Hepatitis C, and SARS has highlighted the need for effective personal protective equipment (PPE) to protect healthcare workers (HCWs), patients, and visitors. PPE is a critical component in the hierarchy of controls used to protect HCWs from infectious hazards. HCW PPE may include gowns, respirators, face masks, gloves, eye protection, face shields, and head and shoe coverings. Important research has been conducted in certain areas, such as respirators and protective masks, but studies in other areas, particularly gowns, are scarce. Gowns are identified as the second-most-used piece of PPE, following gloves, in the healthcare setting. According to the Centers for Disease Control and Prevention's Guideline for Isolation Precautions, isolation gowns should be worn to protect HCWs' arms and exposed body areas during procedures and patient-care activities when anticipating contact with clothing, blood, bodily fluids, secretions and excretions. Isolation gowns currently available on the marketplace offer varying resistance to blood and other bodily fluids depending on the type of the material, its impermeability, and wear and tear. While some studies show no benefit of the routine use of isolation gowns, others demonstrate that its use is associated with a reduced infection rate. This paper reviews isolation gowns in healthcare settings, including the fabrics used, gown design and interfaces, as well as critical parameters that affect microorganism and liquid transmission through fabrics.
Using personal protective equipment (PPE)
.https://www.cdc.gov/coronavirus/2019-ncov/hcp/using-ppe.html. Accessed by February 21,
Taking cover: single-use vs. reusable gowns and drapes
Australian Guidelines for the Prevention and Control of Infection in Healthcare (2010)
https://www.nhmrc.gov.au/about-us/publications/australian-guidelines-prevention-and-control-infection-healthcare-2010#block-views-block-file-attachments-content-block-1. Accessed by February 2,
Rational use of personal protective equipment for coronavirus disease (COVID-19) and considerations during severe shortages: interim guidance
https://www.who.int/publications/i/item/rational-use-of-personal-protective-equipment-for-coronavirus-disease-(covid-19)-and-considerations-during-severe-shortages. Accessed by February 2,
Safe use of personal protective equipment in the treatment of infectious diseases of high consequence
. Accessed by February 2,
A bacteriologically occlusive clothing system for use in the operating room
DOI:10.1302/0301-620X.65B4.6874723
URL
PMID:6874723
[Cited within: 1]

A comparison was made in a laminar-flow operating room between total-body exhaust gowns and a clothing system made from Fabric 450. This disposable clothing was found to be much more comfortable and convenient than the total-body exhaust gowns. The average airborne bacterial counts obtained during total hip replacement operations from each of the clothing systems were identical when the downflow method of ventilation was used (0.7 per cubic metre) and no significant difference could be demonstrated when the crossflow system was used (2.2 per cubic metre with the total-body exhaust gowns and 3.1 per cubic metre with the disposable clothing). Tests in a dispersal chamber were carried out to find the effectiveness of each item of the disposable clothing in reducing bacterial dispersion. These tests demonstrated the relative ineffectiveness of wearing a surgical gown as compared with wearing the complete system. It was confirmed bacteriologically that the downflow system of ventilation was more efficient than the crossflow type; the importance of this observation with respect to clothing and sepsis is discussed in this paper.
Behaviours and rituals in the operating theatre. A report from the Hospital Infection Society Working Party on infection control in operating theatres
DOI:10.1053/jhin.2002.1220 URL PMID:12183138 [Cited within: 1]
Medical Gowns
https://www.fda.gov/medical-devices/personal-protective-equipment-infection-control/medical-gowns. Accessed by February 21,
Guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings
https://www.cdc.gov/infectioncontrol/guidelines/isolation/index.html. Accessed by February 2,
Standard specification for isolation gowns intended for use in healthcare facilities
Protective clothing against liquid chemicals - Performance requirements for chemical protective clothing offering limited protective performance against liquid chemicals (Type 6 and Type PB [6] equipment)
Clothing for protection against contact with blood and body fluids — Determination of resistance of protective clothing materials to penetration by blood-borne pathogens — Test method using Phi-X 174 bacteriophage
Standard specification for surgical gowns intended for use in healthcare facilities
Surgical clothing and drapes - Requirements and test methods - Part 1: Surgical drapes and gowns
Patients, staff and equipment with the surgical drapes, gowns and clean air suits - Part 2: Performance requirements and test methods
Sterilization of medical devices - Requirements for medical devices to be designated “STERILE” - Part 1: Requirements for terminally sterilized medical devices
Technical specifications of personal protective equipment for COVID-19: interim guidance
https://apps.who.int/iris/handle/10665/336622. Accessed by February 21,
Protective clothing — general requirements. International Organization for Standardization: Vernier,SO 13688:2013
Protective clothing - Performance requirements and test methods for protective clothing against infective agents
Sustainable personal protective clothing for healthcare applications: a review
DOI:10.1021/acsnano.0c05537
URL
PMID:32866368
[Cited within: 1]

Personal protective equipment (PPE) is critical to protect healthcare workers (HCWs) from highly infectious diseases such as COVID-19. However, hospitals have been at risk of running out of the safe and effective PPE including personal protective clothing needed to treat patients with COVID-19, due to unprecedented global demand. In addition, there are only limited manufacturing facilities of such clothing available worldwide, due to a lack of available knowledge about relevant technologies, ineffective supply chains, and stringent regulatory requirements. Therefore, there remains a clear unmet need for coordinating the actions and efforts from scientists, engineers, manufacturers, suppliers, and regulatory bodies to develop and produce safe and effective protective clothing using the technologies that are locally available around the world. In this review, we discuss currently used PPE, their quality, and the associated regulatory standards. We survey the current state-of-the-art antimicrobial functional finishes on fabrics to protect the wearer against viruses and bacteria and provide an overview of protective medical fabric manufacturing techniques, their supply chains, and the environmental impacts of current single-use synthetic fiber-based protective clothing. Finally, we discuss future research directions, which include increasing efficiency, safety, and availability of personal protective clothing worldwide without conferring environmental problems.
Clothing for protection against contact with blood and body fluids — Determination of the resistance of protective clothing materials to penetration by blood and body fluids — Test method using synthetic blood
Standard test method for resistance of materials used in protective clothing to penetration by synthetic blood
Structural changes of tailless bacteriophage ΦX174 during penetration of bacterial cell walls
DOI:10.1073/pnas.1716614114
URL
PMID:29229840
[Cited within: 1]

Unlike tailed bacteriophages, which use a preformed tail for transporting their genomes into a host bacterium, the ssDNA bacteriophage PhiX174 is tailless. Using cryo-electron microscopy and time-resolved small-angle X-ray scattering, we show that lipopolysaccharides (LPS) form bilayers that interact with PhiX174 at an icosahedral fivefold vertex and induce single-stranded (ss) DNA genome ejection. The structures of PhiX174 complexed with LPS have been determined for the pre- and post-ssDNA ejection states. The ejection is initiated by the loss of the G protein spike that encounters the LPS, followed by conformational changes of two polypeptide loops on the major capsid F proteins. One of these loops mediates viral attachment, and the other participates in making the fivefold channel at the vertex contacting the LPS.
Difference between the spore sizes of Bacillus anthracis and other Bacillus species
DOI:10.1111/j.1365-2672.2006.03111.x
URL
PMID:17241334
[Cited within: 2]

AIMS: To determine the size distribution of the spores of Bacillus anthracis, and compare its size with other Bacillus species grown and sporulated under similar conditions. METHODS AND RESULTS: Spores from several Bacillus species, including seven strains of B. anthracis and six close neighbours, were prepared and studied using identical media, protocols and instruments. Here, we report the spore length and diameter distributions, as determined by transmission electron microscopy (TEM). We calculated the aspect ratio and volume of each spore. All the studied strains of B. anthracis had similar diameter (mean range between 0.81 +/- 0.08 microm and 0.86 +/- 0.08 microm). The mean lengths of the spores from different B. anthracis strains fell into two significantly different groups: one with mean spore lengths 1.26 +/- 0.13 microm or shorter, and another group of strains with mean spore lengths between 1.49 and 1.67 microm. The strains of B. anthracis that were significantly shorter also sporulated with higher yield at relatively lower temperature. The grouping of B. anthracis strains by size and sporulation temperature did not correlate with their respective virulence. CONCLUSIONS: The spores of Bacillus subtilis and Bacillus atrophaeus (previously named Bacillus globigii), two commonly used simulants of B. anthracis, were considerably smaller in length, diameter and volume than all the B. anthracis spores studied. Although rarely used as simulants, the spores of Bacillus cereus and Bacillus thuringiensis had dimensions similar to those of B. anthracis. SIGNIFICANCE AND IMPACT OF THE STUDY: Spores of nonvirulent Bacillus species are often used as simulants in the development and testing of countermeasures for biodefence against B. anthracis. The data presented here should help in the selection of simulants that better resemble the properties of B. anthracis, and thus, more accurately represent the performance of collectors, detectors and other countermeasures against this threat agent.
The giant protein Ebh is a determinant of Staphylococcus aureus cell size and complement resistance
DOI:10.1128/JB.01366-13
URL
PMID:24363342
[Cited within: 1]

Staphylococcus aureus USA300, the clonal type associated with epidemic community-acquired methicillin-resistant S. aureus (MRSA) infections, displays the giant protein Ebh on its surface. Mutations that disrupt the ebh reading frame increase the volume of staphylococcal cells and alter the cross wall, a membrane-enclosed peptidoglycan synthesis and assembly compartment. S. aureus ebh variants display increased sensitivity to oxacillin (methicillin) as well as susceptibility to complement-mediated killing. Mutations in ebh are associated with reduced survival of mutant staphylococci in blood and diminished virulence in mice. We propose that Ebh, following its secretion into the cross wall, contributes to the characteristic cell growth and envelope assembly pathways of S. aureus, thereby enabling complement resistance and the pathogenesis of staphylococcal infections.
Surface tension measurement of normal human blood samples by pendant drop method
DOI:10.1080/03091902.2020.1770348
URL
PMID:32589070
[Cited within: 1]

The surface tension of blood plays an important role not only in the birth and decompression sickness but also in other functionality of the organism. It also provides capillary action during blood flow process. In this article, a simple and low-cost device is designed and fabricated for measuring the surface tension of blood by pendant drop method. In this device, a droplet of blood is formed in a closed chamber on tip of an 18-gauge blunt needle and it is photographed by a camera in very humid conditions (RH = 99%) to minimise the evaporation. A wetted wick is provided at the bottom of the chamber for maintaining constant relative humidity in chamber. Surface tension of blood is inferred using drop shape factor method and image analysis technique at various experimental conditions. This device is validated and calibrated with surface tension measurements of water and silicone oil. Its measurements are in good agreement against data reported in literature. Post-validation, surface tensions of blood samples with and without anticoagulant of healthy persons at various temperatures (range from 20 to 40 degrees C) was measured. It was found that the surface tension of normal blood samples strongly correlates with blood temperature. The surface tension of female blood was remarkably different from same of male blood. However, the effect of age (21-60 year) on the surface tension was negligible for all practical purposes. Increased percentage of anticoagulant in blood increases its surface tension. This research specifies a baseline for surface tension of normal blood samples at various conditions which in turn provides new insights to pathologists in identifying various disease conditions.
Surface tension of blood
URL
PMID:9728499
[Cited within: 1]

The surface tension of blood assessed in a group of 71 healthy subjects (24 men and 47 women) by the drop method at a temperature of 22 degrees C was 55.89 x 10(-3) N x m(-1), S.D.=3.57 x 10(-3) N x m(-1). It did not correlate with age or sex of the examined subjects nor with any of the following variables: red cell sedimentation rate, blood haemoglobin levels, number of erythrocytes, total serum cholesterol, total serum triacylglycerols, creatinine blood levels, ALT and AST activity. The surface tension of blood and other body fluids can play an important part not only in the genesis and development of decompression sickness but also in other processes in the organism.
Dynamic surface tension of saliva: general relationships and application in medical diagnostics
URL PMID:19577904 [Cited within: 2]
SARS-CoV-2 (COVID-19) by the numbers
DOI:10.7554/eLife.57309
URL
PMID:32228860
[Cited within: 1]

The COVID-19 pandemic is a harsh reminder of the fact that, whether in a single human host or a wave of infection across continents, viral dynamics is often a story about the numbers. In this article we provide a one-stop, curated graphical source for the key numbers (based mostly on the peer-reviewed literature) about the SARS-CoV-2 virus that is responsible for the pandemic. The discussion is framed around two broad themes: i) the biology of the virus itself; ii) the characteristics of the infection of a single human host.
A new approach to measure the resistance of fabric to liquid and viral penetration
Healthcare worker infections and deaths due to COVID-19: A survey from 37 nations and a call for WHO to post national data on their website
DOI:10.1016/j.ijid.2020.10.064 URL PMID:33130210 [Cited within: 1]
Global shortage of personal protective equipment
DOI:10.1016/S1473-3099(20)30501-6 URL PMID:32592673 [Cited within: 1]
Coronavirus: global stocks of protective gear are depleted, with demand at “100 times” normal level, WHO warns
DOI:10.1136/bmj.m543 URL PMID:32041691 [Cited within: 1]
Isolation gowns in health care settings: Laboratory studies, regulations and standards, and potential barriers of gown selection and use
DOI:10.1016/j.ajic.2015.07.042
URL
PMID:26391468
[Cited within: 1]

Although they play an important role in infection prevention and control, textile materials and personal protective equipment (PPE) used in health care settings are known to be one of the sources of cross-infection. Gowns are recommended to prevent transmission of infectious diseases in certain settings; however, laboratory and field studies have produced mixed results of their efficacy. PPE used in health care is regulated as either class I (low risk) or class II (intermediate risk) devices in the United States. Many organizations have published guidelines for the use of PPE, including isolation gowns, in health care settings. In addition, the Association for the Advancement of Medical Instrumentation published a guidance document on the selection of gowns and a classification standard on liquid barrier performance for both surgical and isolation gowns. However, there is currently no existing standard specific to isolation gowns that considers not only the barrier resistance but also a wide array of end user desired attributes. As a result, infection preventionists and purchasing agents face several difficulties in the selection process, and end users have limited or no information on the levels of protection provided by isolation gowns. Lack of knowledge about the performance of protective clothing used in health care became more apparent during the 2014 Ebola epidemic. This article reviews laboratory studies, regulations, guidelines and standards pertaining to isolation gowns, characterization problems, and other potential barriers of isolation gown selection and use.
Challenge of N95 filtering facepiece respirators with viable H1N1 influenza aerosols
DOI:10.1086/670225
URL
PMID:23571366
[Cited within: 1]

OBJECTIVE. Specification of appropriate personal protective equipment for respiratory protection against influenza is somewhat controversial. In a clinical environment, N95 filtering facepiece respirators (FFRs) are often recommended for respiratory protection against infectious aerosols. This study evaluates the ability of N95 FFRs to capture viable H1N1 influenza aerosols. METHODs. Five N95 FFR models were challenged with aerosolized viable H1N1 influenza and inert polystyrene latex particles at continuous flow rates of 85 and 170 liters per minute. Virus was assayed using Madin-Darby canine kidney cells to determine the median tissue culture infective dose (TCID50). Aerosols were generated using a Collison nebulizer containing H1N1 influenza virus at 1 x 10(8) TCID50/mL. To determine filtration efficiency, viable sampling was performed upstream and downstream of the FFR. RESULTS. N95 FFRs filtered 0.8-mum particles of both H1N1 influenza and inert origins with more than 95% efficiency. With the exception of 1 model, no statistically significant difference in filtration performance was observed between influenza and inert particles of similar size. Although statistically significant differences were observed for 2 models when comparing the 2 flow rates, the differences have no significance to protection. CONCLUSIONS. This study empirically demonstrates that a National Institute for Occupational Safety and Health-approved N95 FFR captures viable H1N1 influenza aerosols as well as or better than its N95 rating, suggesting that a properly fitted FFR reduces inhalation exposure to airborne influenza virus. This study also provides evidence that filtration efficiency is based primarily on particle size rather than the nature of the particle's origin.