Biomaterials Translational ›› 2021, Vol. 2 ›› Issue (3): 236-247.doi: 10.12336/biomatertransl.2021.03.06
• REVIEW • Previous Articles Next Articles
Jialin Niu1, Hua Huang1, Jia Pei1, Zhaohui Jin1, Shaokang Guan2, Guangyin Yuan1,*( )
)
Received:2021-06-03
Revised:2021-08-25
Accepted:2021-09-10
Online:2021-09-28
Published:2021-09-28
Contact:
Guangyin Yuan
E-mail:gyyuan@sjtu.edu.cn
About author:Guangyin Yuan, gyyuan@sjtu.edu.cn.Niu, J.; Huang, H.; Pei, J.; Jin, Z.; Guan, S.; Yuan, G. Research and development strategy for biodegradable magnesium-based vascular stents: a review. Biomater Transl. 2021, 2(3), 236-247.
| Alloying Element | Chemical symbol | Biocompatibility | Reference |
|---|---|---|---|
| Calcium | Ca | The essential element and the most abundant cation in the human body. Ca mainly exists in bones and teeth, and is regulated and metabolised through the kidney and intestine. | |
| Zinc | Zn | Zn is a trace element with a concentration of 12.4-17.4 µM in serum. Zn plays an important role in the immune system, bone and cartilage, and participates in the metabolism of nucleic acids and energy. Excessive Zn is neurotoxic and could lead to hypertension, coronary heart disease and other diseases. | |
| Aluminium | Al | The normal concentration of Al in human serum is 2.1-4.8 g/L. Al has potential neurotoxicity and could trigger Alzheimer’s disease. | |
| Manganese | Mn | Mn is one of the essential trace elements in the human body, and participates in the synthesis and metabolism of lipids, amino acids and sugars, while also playing an important role in the immune system, bone growth, coagulation function and neurotransmission. Excessive Mn content is neurotoxic, leading to manganese poisoning, body disorders, Parkinson's disease and myocardial infarction. | |
| Strontium | Sr | Sr is a trace element in the human body that is mainly present in bone and teeth. Sr promotes bone formation, inhibits bone resorption, and improves bone strength and density. | |
| Rare earth | NA | Y, Gd, Nd, Dy and Eu show low cytotoxicity, while La and Ce inhibit cell activity. The mechanism of their toxicity remains to be studied. | |
| Zirconium | Zr | No toxicity or carcinogenicity. Zr is a commonly-used dental and joint replacement material in the clinic. | |
| Silicon | Si | Si is the third most abundant trace element in the human body, and is present in bone, skin, blood vessels and other tissues. Lack of Si affects the synthesis of glycosaminoglycans and collagen, leading to disorders including bone deformity and tooth dysplasia. |
Table 1 A brief summary of the biological function of alloying elements in magnesium alloys.
| Alloying Element | Chemical symbol | Biocompatibility | Reference |
|---|---|---|---|
| Calcium | Ca | The essential element and the most abundant cation in the human body. Ca mainly exists in bones and teeth, and is regulated and metabolised through the kidney and intestine. | |
| Zinc | Zn | Zn is a trace element with a concentration of 12.4-17.4 µM in serum. Zn plays an important role in the immune system, bone and cartilage, and participates in the metabolism of nucleic acids and energy. Excessive Zn is neurotoxic and could lead to hypertension, coronary heart disease and other diseases. | |
| Aluminium | Al | The normal concentration of Al in human serum is 2.1-4.8 g/L. Al has potential neurotoxicity and could trigger Alzheimer’s disease. | |
| Manganese | Mn | Mn is one of the essential trace elements in the human body, and participates in the synthesis and metabolism of lipids, amino acids and sugars, while also playing an important role in the immune system, bone growth, coagulation function and neurotransmission. Excessive Mn content is neurotoxic, leading to manganese poisoning, body disorders, Parkinson's disease and myocardial infarction. | |
| Strontium | Sr | Sr is a trace element in the human body that is mainly present in bone and teeth. Sr promotes bone formation, inhibits bone resorption, and improves bone strength and density. | |
| Rare earth | NA | Y, Gd, Nd, Dy and Eu show low cytotoxicity, while La and Ce inhibit cell activity. The mechanism of their toxicity remains to be studied. | |
| Zirconium | Zr | No toxicity or carcinogenicity. Zr is a commonly-used dental and joint replacement material in the clinic. | |
| Silicon | Si | Si is the third most abundant trace element in the human body, and is present in bone, skin, blood vessels and other tissues. Lack of Si affects the synthesis of glycosaminoglycans and collagen, leading to disorders including bone deformity and tooth dysplasia. |
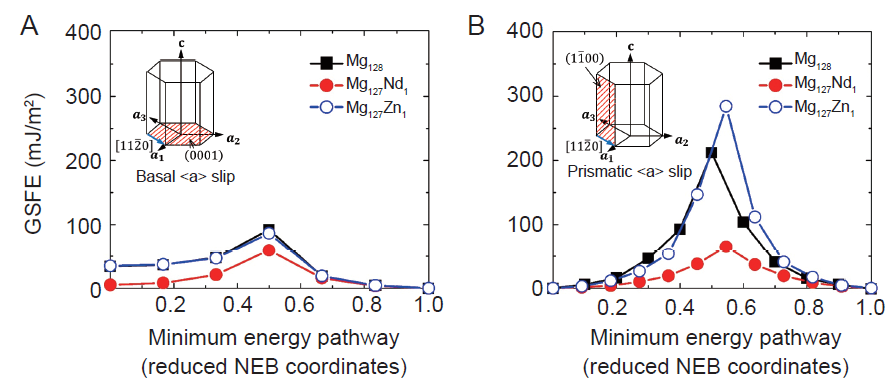
Figure 2. GSFE data show that the alloying element Nd plays essentials roles in basal (A) and non-basal (B) <a> slips. GSFE: generalized stacking fault energy; Nd: neodymium; NEB: nudged elastic band.
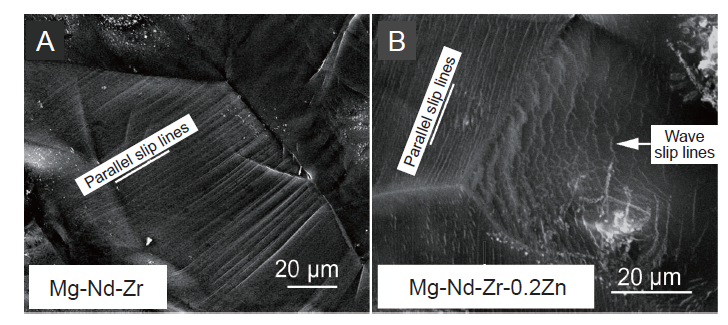
Figure 3. Fracture surface morphology of tensile samples of (A) Mg-Nd-Zr and (B) Mg-Nd-Zr-0.2Zn. Parallel slip lines show the basal slip, while wave slip lines indicate the non-basal slip. Scale bars: 20 μm. Reprinted from Fu et al.73 Copyright with Trans Tech Publications, Ltd.
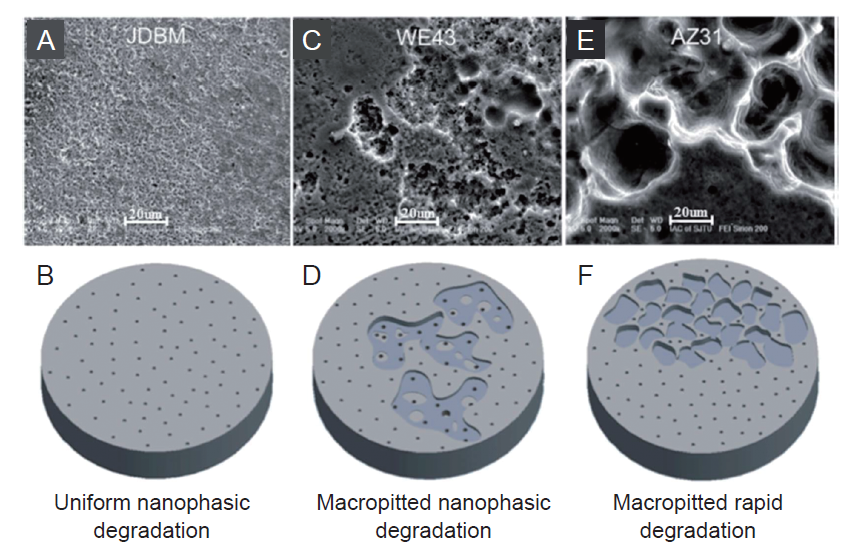
Figure 4. Surface morphology and schematic diagram of the degradation of JDBM (A, B), WE43 (C, D) and AZ31 (E, F) alloys. The surface of JDBM sample is uniformly distributed with nano-sized corrosion pits, while WE43 and AZ31 alloys show excessive localized corrosion with macroscopic pitting or delamination. Scale bars: 20 μm. JDBM: Mg-Nd-Zn-Zr alloy. Reprinted with permission from Mao et al.44 Copyright 2013 American Chemical Society.
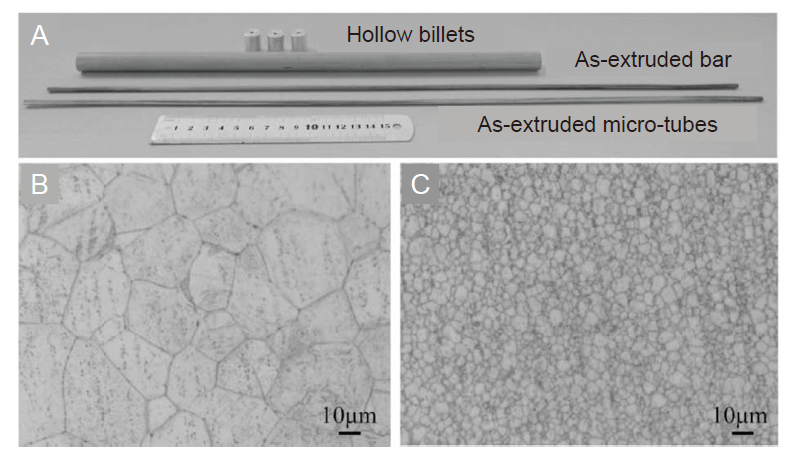
Figure 5. (A) Photographs of as-extruded bar, hollow billets and as-extruded microtubes. (B, C) Optical microstructure of as-extruded bar (B) and microtubes (C) of JDBM alloy. The average grain size of as-extruded bar is 14 μm, while that of as-extruded microtubes is about 2 μm. Scale bars: 10 μm. JDBM: Mg-Nd-Zn-Zr alloy. Reprinted from Lu et al.81 Copyright 2019, with permission from Elsevier.
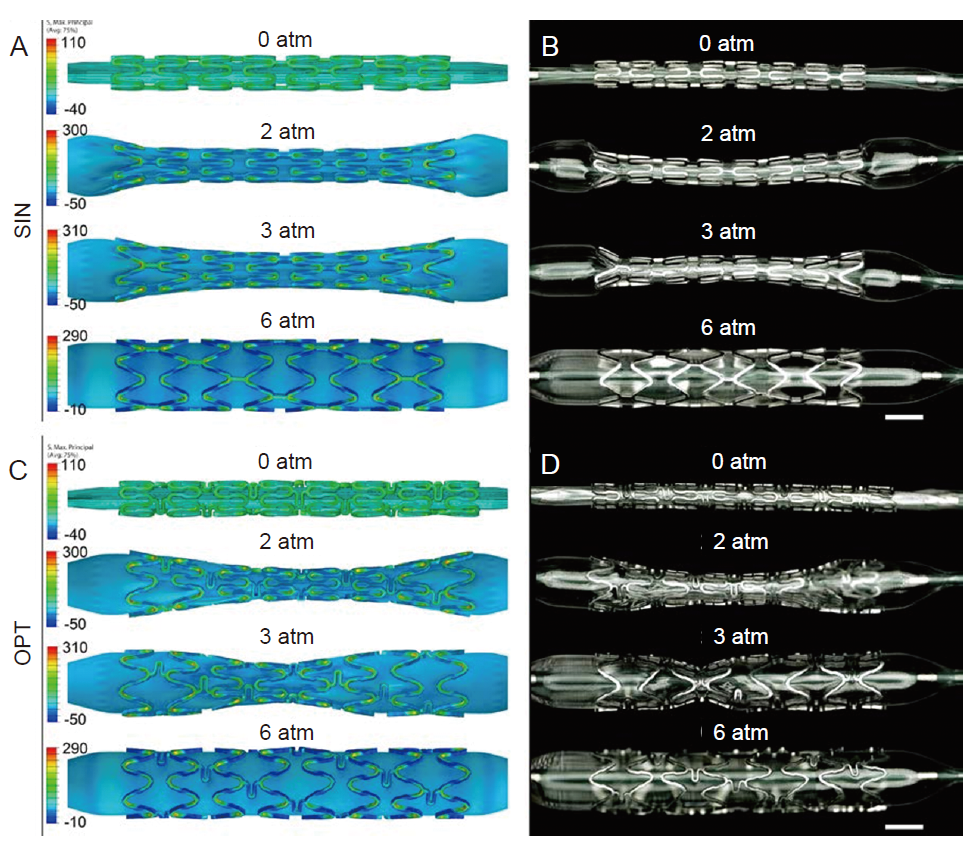
Figure 6. Simulated of the maximum principal stress distribution during expansion process and the experimental validation for SIN stent (A, B) and OPT stent (C, D). During the expansion process, the “dog bone effect” of SIN stent lasts longer than OPT stent, and is more likely to cause local stress concentration. At 3 atm (1 atm = 101.325 kPa) of the balloon pressure, the maximum principal stress of SIN stent is 308.1 MPa, which is 15.7% higher than that of OPT stent. The finite element simulated result is consistent with the experimental observation. Scale bars: 1 mm. OPT: optimized; SIN: sine-wave. Reprinted from Chen et al.92 Copyright 2019, with permission from Elsevier.
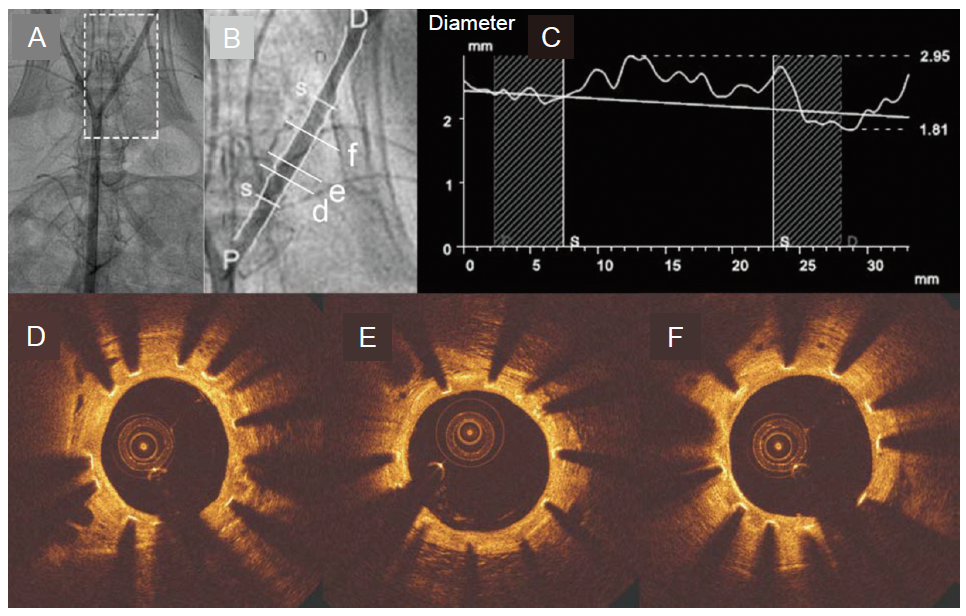
Figure 7. Quantitative coronary angiography and OCT result at 3 months post-operation. (A, B) Angiography shows the location of the stent. B is the enlargement of the box in A. (C) The lumen diameter along the iliac artery. The horizontal axis is the distance along the iliac artery, and vertical axis is the lumen diameter. (D-F) The OCT images. OCT: optical coherence tomography; D: distal; P: proximal. Reprinted from Chen et al.92 Copyright 2019, with permission from Elsevier.
| [1] | Virani, S. S.; Alonso, A.; Benjamin, E. J.; Bittencourt, M. S.; Callaway, C. W.; Carson, A. P.; Chamberlain, A. M.; Chang, A. R.; Cheng, S.; Delling, F. N.; Djousse, L.; Elkind, M. S. V.; Ferguson, J. F.; Fornage, M.; Khan, S. S.; Kissela, B. M.; Knutson, K. L.; Kwan, T. W.; Lackland, D. T.; Lewis, T. T.; Lichtman, J. H.; Longenecker, C. T.; Loop, M. S.; Lutsey, P. L.; Martin, S. S.; Matsushita, K.; Moran, A. E.; Mussolino, M. E.; Perak, A. M.; Rosamond, W. D.; Roth, G. A.; Sampson, U. K. A.; Satou, G. M.; Schroeder, E. B.; Shah, S. H.; Shay, C. M.; Spartano, N. L.; Stokes, A.; Tirschwell, D. L.; VanWagner, L. B.; Tsao, C. W.; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2020 Update: a report from theAmerican Heart Association. Circulation. 2020, 141, e139-e596. |
| [2] |
Dotter, C. T.; Judkins, M. P. Transluminal treatment of arteriosclerotic obstruction. Description of a new technic and a preliminary report of its application. 1964. Radiology. 1989, 172, 904-920.
doi: 10.1148/172.3.904 URL |
| [3] |
Deb, S.; Wijeysundera, H. C.; Ko, D. T.; Tsubota, H.; Hill, S.; Fremes, S. E. Coronary artery bypass graft surgery vs percutaneous interventions in coronary revascularization: a systematic review. JAMA. 2013, 310, 2086-2095.
doi: 10.1001/jama.2013.281718 URL |
| [4] | Sousa, J. E.; Costa, M. A.; Farb, A.; Abizaid, A.; Sousa, A.; Seixas, A. C.; da Silva, L. M.; Feres, F.; Pinto, I.; Mattos, L. A.; Virmani, R. Images in cardiovascular medicine. Vascular healing 4 years after the implantation of sirolimus-eluting stent in humans: a histopathological examination. Circulation. 2004, 110, e5-6. |
| [5] |
Guagliumi, G.; Farb, A.; Musumeci, G.; Valsecchi, O.; Tespili, M.; Motta, T.; Virmani, R. Images in cardiovascular medicine. Sirolimus-eluting stent implanted in human coronary artery for 16 months: pathological findings. Circulation. 2003, 107, 1340-1341.
doi: 10.1161/01.CIR.0000062700.42060.6F URL |
| [6] |
Camenzind, E.; Steg, P. G.; Wijns, W. Stent thrombosis late after implantation of first-generation drug-eluting stents: a cause for concern. Circulation. 2007, 115, 1440-1455; discussion 1455.
doi: 10.1161/CIRCULATIONAHA.106.666800 URL |
| [7] |
Kuchulakanti, P. K.; Chu, W. W.; Torguson, R.; Ohlmann, P.; Rha, S. W.; Clavijo, L. C.; Kim, S. W.; Bui, A.; Gevorkian, N.; Xue, Z.; Smith, K.; Fournadjieva, J.; Suddath, W. O.; Satler, L. F.; Pichard, A. D.; Kent, K. M.; Waksman, R. Correlates and long-term outcomes of angiographically proven stent thrombosis with sirolimus- and paclitaxel-eluting stents. Circulation. 2006, 113, 1108-1113.
doi: 10.1161/CIRCULATIONAHA.105.600155 URL |
| [8] |
Kerner, A.; Gruberg, L.; Kapeliovich, M.; Grenadier, E. Late stent thrombosis after implantation of a sirolimus-eluting stent. Catheter Cardiovasc Interv. 2003, 60, 505-508.
doi: 10.1002/(ISSN)1522-726X URL |
| [9] |
Im, S. H.; Jung, Y.; Kim, S. H. Current status and future direction of biodegradable metallic and polymeric vascular scaffolds for next-generation stents. Acta Biomater. 2017, 60, 3-22.
doi: 10.1016/j.actbio.2017.07.019 URL |
| [10] |
Mostaed, E.; Sikora-Jasinska, M.; Drelich, J. W.; Vedani, M. Zinc-based alloys for degradable vascular stent applications. Acta Biomater. 2018, 71, 1-23.
doi: 10.1016/j.actbio.2018.03.005 URL |
| [11] |
Bowen, P. K.; Shearier, E. R.; Zhao, S.; Guillory, R. J., 2nd; Zhao, F., Goldman, J., Drelich, J. W. Biodegradable metals for cardiovascular stents: from clinical concerns to recent Zn-alloys. Adv Healthc Mater. 2016, 5, 1121-1140.
doi: 10.1002/adhm.v5.10 URL |
| [12] |
Stack, R. S.; Califf, R. M.; Phillips, H. R.; Pryor, D. B.; Quigley, P. J.; Bauman, R. P.; Tcheng, J. E.; Greenfield, J. C., Jr, . Interventional cardiac catheterization at Duke Medical Center. Am J Cardiol. 1988, 62, 3f-24f.
doi: 10.1016/0002-9149(88)91529-9 URL |
| [13] |
Wiebe, J.; Nef, H. M.; Hamm, C. W. Current status of bioresorbable scaffolds in the treatment of coronary artery disease. J Am Coll Cardiol. 2014, 64, 2541-2551.
doi: 10.1016/j.jacc.2014.09.041 URL |
| [14] |
Onuma, Y.; Serruys, P. W.; Gomez, J.; de Bruyne, B.; Dudek, D.; Thuesen, L.; Smits, P.; Chevalier, B.; McClean, D.; Koolen, J.; Windecker, S.; Whitbourn, R.; Meredith, I.; Garcia-Garcia, H.; Ormiston, J. A. Comparison of in vivo acute stent recoil between the bioresorbable everolimus-eluting coronary scaffolds (revision 1.0 and 1.1) and the metallic everolimus-eluting stent. Catheter Cardiovasc Interv. 2011, 78, 3-12.
doi: 10.1002/ccd.v78.1 URL |
| [15] |
Serruys, P. W.; Chevalier, B.; Dudek, D.; Cequier, A.; Carrié, D.; Iniguez, A.; Dominici, M.; van der Schaaf, R. J.; Haude, M.; Wasungu, L.; Veldhof, S.; Peng, L.; Staehr, P.; Grundeken, M. J.; Ishibashi, Y.; Garcia-Garcia, H. M.; Onuma, Y. A bioresorbable everolimus-eluting scaffold versus a metallic everolimus-eluting stent for ischaemic heart disease caused by de-novo native coronary artery lesions (ABSORB II): an interim 1-year analysis of clinical and procedural secondary outcomes from a randomised controlled trial. Lancet. 2015, 385, 43-54.
doi: 10.1016/S0140-6736(14)61455-0 URL |
| [16] |
Ellis, S. G.; Kereiakes, D. J.; Metzger, D. C.; Caputo, R. P.; Rizik, D. G.; Teirstein, P. S.; Litt, M. R.; Kini, A.; Kabour, A.; Marx, S. O.; Popma, J. J.; McGreevy, R.; Zhang, Z.; Simonton, C.; Stone, G. W.; ABSORB III Investigators. Everolimus-eluting bioresorbable scaffolds for coronary artery disease. N Engl J Med. 2015, 373, 1905-1915.
doi: 10.1056/NEJMoa1509038 URL |
| [17] |
Ali, Z. A.; Serruys, P. W.; Kimura, T.; Gao, R.; Ellis, S. G.; Kereiakes, D. J.; Onuma, Y.; Simonton, C.; Zhang, Z.; Stone, G. W. 2-year outcomes with the Absorb bioresorbable scaffold for treatment of coronary artery disease: a systematic review and meta-analysis of seven randomised trials with an individual patient data substudy. Lancet. 2017, 390, 760-772.
doi: 10.1016/S0140-6736(17)31470-8 URL |
| [18] |
Lipinski, M. J.; Escarcega, R. O.; Baker, N. C.; Benn, H. A.; Gaglia, M. A., Jr, ., Torguson, R., Waksman, R. Scaffold thrombosis after percutaneous coronary intervention with ABSORB bioresorbable vascular scaffold: a systematic review and meta-analysis. JACC Cardiovasc Interv. 2016, 9, 12-24.
doi: 10.1016/j.jcin.2015.09.024 URL |
| [19] |
Serruys, P. W.; Ormiston, J. A.; Onuma, Y.; Regar, E.; Gonzalo, N.; Garcia-Garcia, H. M.; Nieman, K.; Bruining, N.; Dorange, C.; Miquel-Hébert, K.; Veldhof, S.; Webster, M.; Thuesen, L.; Dudek, D. A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methods. Lancet. 2009, 373, 897-910.
doi: 10.1016/S0140-6736(09)60325-1 URL |
| [20] |
Ge, J. Bioresorbable vascular scaffold for the treatment of coronary in-stent restenosis: New dawn or frost on snow? Catheter Cardiovasc Interv. 2018, 92, 678-679.
doi: 10.1002/ccd.27880 URL |
| [21] |
Laires, M. J.; Monteiro, C. P.; Bicho, M. Role of cellular magnesium in health and human disease. Front Biosci. 2004, 9, 262-276.
doi: 10.2741/1223 URL |
| [22] |
Byrd, R. P., Jr, ., Roy, T. M. Magnesium: its proven and potential clinical significance. South Med J. 2003, 96, 104.
doi: 10.1097/01.SMJ.0000049846.49028.8F URL |
| [23] |
Vormann, J. Magnesium: nutrition and metabolism. Mol Aspects Med. 2003, 24, 27-37.
doi: 10.1016/S0098-2997(02)00089-4 URL |
| [24] | Zheng, Y. F.; Gu, X. N.; Witte, F. Biodegradable metals. Mater Sci Eng RRep. 2014, 77, 1-34. |
| [25] |
Ang, H. Y.; Huang, Y. Y.; Lim, S. T.; Wong, P.; Joner, M.; Foin, N. Mechanical behavior of polymer-based vs. metallic-based bioresorbable stents. J Thorac Dis. 2017, 9, S923-S934.
doi: 10.21037/jtd URL |
| [26] |
Heublein, B.; Rohde, R.; Kaese, V.; Niemeyer, M.; Hartung, W.; Haverich, A. Biocorrosion of magnesium alloys: a new principle in cardiovascular implant technology? Heart. 2003, 89, 651-656.
doi: 10.1136/heart.89.6.651 URL |
| [27] |
Erbel, R.; Di Mario, C.; Bartunek, J.; Bonnier, J.; de Bruyne, B.; Eberli, F. R.; Erne, P.; Haude, M.; Heublein, B.; Horrigan, M.; Ilsley, C.; Böse, D.; Koolen, J.; Lüscher, T. F.; Weissman, N.; Waksman, R.; PROGRESS-AMS (Clinical Performance and Angiographic Results of Coronary Stenting with Absorbable Metal Stents) Investigators. Temporary scaffolding of coronary arteries with bioabsorbable magnesium stents: a prospective, non-randomised multicentre trial. Lancet. 2007, 369, 1869-1875.
doi: 10.1016/S0140-6736(07)60853-8 URL |
| [28] |
Peeters, P.; Bosiers, M.; Verbist, J.; Deloose, K.; Heublein, B. Preliminary results after application of absorbable metal stents in patients with critical limb ischemia. J Endovasc Ther. 2005, 12, 1-5.
doi: 10.1583/04-1349R.1 URL |
| [29] |
Zartner, P.; Cesnjevar, R.; Singer, H.; Weyand, M. First successful implantation of a biodegradable metal stent into the left pulmonary artery of a preterm baby. Catheter Cardiovasc Interv. 2005, 66, 590-594.
doi: 10.1002/(ISSN)1522-726X URL |
| [30] |
Waksman, R.; Erbel, R.; Di Mario, C.; Bartunek, J.; de Bruyne, B.; Eberli, F. R.; Erne, P.; Haude, M.; Horrigan, M.; Ilsley, C.; Böse, D.; Bonnier, H.; Koolen, J.; Lüscher, T. F.; Weissman, N. J.; PROGRESS-AMS (Clinical Performance Angiographic Results of Coronary Stenting with Absorbable Metal Stents) Investigators. Early- and long-term intravascular ultrasound and angiographic findings after bioabsorbable magnesium stent implantation in human coronary arteries. JACC Cardiovasc Interv. 2009, 2, 312-320.
doi: 10.1016/j.jcin.2008.09.015 URL |
| [31] |
Haude, M.; Erbel, R.; Erne, P.; Verheye, S.; Degen, H.; Böse, D.; Vermeersch, P.; Wijnbergen, I.; Weissman, N.; Prati, F.; Waksman, R.; Koolen, J. Safety and performance of the drug-eluting absorbable metal scaffold (DREAMS) in patients with de-novo coronary lesions: 12 month results of the prospective, multicentre, first-in-man BIOSOLVE-I trial. Lancet. 2013, 381, 836-844.
doi: 10.1016/S0140-6736(12)61765-6 URL |
| [32] |
Haude, M.; Ince, H.; Abizaid, A.; Toelg, R.; Lemos, P. A.; von Birgelen, C.; Christiansen, E. H.; Wijns, W.; Neumann, F. J.; Kaiser, C.; Eeckhout, E.; Lim, S. T.; Escaned, J.; Garcia-Garcia, H. M.; Waksman, R. Safety and performance of the second-generation drug-eluting absorbable metal scaffold in patients with de-novo coronary artery lesions (BIOSOLVE-II): 6 month results of a prospective, multicentre, non-randomised, first-in-man trial. Lancet. 2016, 387, 31-39.
doi: 10.1016/S0140-6736(15)00447-X URL |
| [33] |
Haude, M.; Ince, H.; Abizaid, A.; Toelg, R.; Lemos, P. A.; von Birgelen, C.; Christiansen, E. H.; Wijns, W.; Neumann, F. J.; Kaiser, C.; Eeckhout, E.; Lim, S. T.; Escaned, J.; Onuma, Y.; Garcia-Garcia, H. M.; Waksman, R. Sustained safety and performance of the second-generation drug-eluting absorbable metal scaffold in patients with de novo coronary lesions: 12-month clinical results and angiographic findings of the BIOSOLVE-II first-in-man trial. Eur Heart J. 2016, 37, 2701-2709.
doi: 10.1093/eurheartj/ehw196 URL |
| [34] |
Wu, W.; Chen, S.; Gastaldi, D.; Petrini, L.; Mantovani, D.; Yang, K.; Tan, L.; Migliavacca, F. Experimental data confirm numerical modeling of the degradation process of magnesium alloys stents. Acta Biomater. 2013, 9, 8730-8739.
doi: 10.1016/j.actbio.2012.10.035 URL |
| [35] |
Grogan, J. A.; O’Brien, B. J.; Leen, S. B.; McHugh, P. E. A corrosion model for bioabsorbable metallic stents. Acta Biomater. 2011, 7, 3523-3533.
doi: 10.1016/j.actbio.2011.05.032 URL |
| [36] |
Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C. J.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials. 2005, 26, 3557-3563.
doi: 10.1016/j.biomaterials.2004.09.049 URL |
| [37] | Kong, X.; Wang, L.; Li, G.; Qu, X.; Niu, J.; Tang, T.; Dai, K.; Yuan, G.; Hao, Y. Mg-based bone implants show promising osteoinductivity and controllable degradation: A long-term study in a goat femoral condyle fracture model. Mater Sci Eng CMater Biol Appl. 2018, 86, 42-47. |
| [38] |
Kuhlmann, J.; Bartsch, I.; Willbold, E.; Schuchardt, S.; Holz, O.; Hort, N.; Höche, D.; Heineman, W. R.; Witte, F. Fast escape of hydrogen from gas cavities around corroding magnesium implants. Acta Biomater. 2013, 9, 8714-8721.
doi: 10.1016/j.actbio.2012.10.008 URL |
| [39] |
Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007, 13, 688-694.
doi: 10.1038/nm1577 URL |
| [40] | Lu, H. T.; Sun, X. J. Hydrogen medicine: research advance, controversy and challenges. Dier Junyi Daxue Xuebao. 2018, 39, 1181-1187. |
| [41] |
Qin, S. Role of Hydrogen in Atherosclerotic Disease: From Bench to Bedside. Curr Pharm Des. 2021, 27, 713-722.
doi: 10.2174/1381612826666201124112152 URL |
| [42] |
Mani, G.; Feldman, M. D.; Patel, D.; Agrawal, C. M. Coronary stents: a materials perspective. Biomaterials. 2007, 28, 1689-1710.
doi: 10.1016/j.biomaterials.2006.11.042 URL |
| [43] | Zhang, X. B.; Mao, L.; Yuan, G. Y.; Wang, Z. Z. Performances of biodegradable Mg-Nd-Zn-Zr magnesium alloy for cardiovascular stent. Xiyou Jinshu Cailiao yuGongcheng. 2013, 42, 1300-1305. |
| [44] |
Mao, L.; Shen, L.; Niu, J.; Zhang, J.; Ding, W.; Wu, Y.; Fan, R.; Yuan, G. Nanophasic biodegradation enhances the durability and biocompatibility of magnesium alloys for the next-generation vascular stents. Nanoscale. 2013, 5, 9517-9522.
doi: 10.1039/c3nr02912c URL |
| [45] |
Zong, Y.; Yuan, G.; Zhang, X.; Mao, L.; Niu, J.; Ding, W. Comparison of biodegradable behaviors of AZ31 and Mg-Nd-Zn-Zr alloys in Hank’s physiological solution. Mater Sci Eng B. 2012, 177, 395-401.
doi: 10.1016/j.mseb.2011.09.042 URL |
| [46] |
Zhang, X.; Yuan, G.; Niu, J.; Fu, P.; Ding, W. Microstructure, mechanical properties, biocorrosion behavior, and cytotoxicity of as-extruded Mg-Nd-Zn-Zr alloy with different extrusion ratios. J Mech Behav Biomed Mater. 2012, 9, 153-162.
doi: 10.1016/j.jmbbm.2012.02.002 URL |
| [47] |
Zhang, X.; Yuan, G.; Mao, L.; Niu, J.; Fu, P.; Ding, W. Effects of extrusion and heat treatment on the mechanical properties and biocorrosion behaviors of a Mg-Nd-Zn-Zr alloy. J Mech Behav Biomed Mater. 2012, 7, 77-86.
doi: 10.1016/j.jmbbm.2011.05.026 URL |
| [48] |
Zhang, X.; Wang, Z.; Yuan, G.; Xue, Y. Improvement of mechanical properties and corrosion resistance of biodegradable Mg-Nd-Zn-Zr alloys by double extrusion. Mater Sci Eng B. 2012, 177, 1113-1119.
doi: 10.1016/j.mseb.2012.05.020 URL |
| [49] |
Mao, L.; Yuan, G.; Wang, S.; Niu, J.; Wu, G.; Ding, W. A novel biodegradable Mg-Nd-Zn-Zr alloy with uniform corrosion behavior in artificial plasma. Mater Lett. 2012, 88, 1-4.
doi: 10.1016/j.matlet.2012.08.012 URL |
| [50] |
Bondy, S. C. The neurotoxicity of environmental aluminum is still an issue. Neurotoxicology. 2010, 31, 575-581.
doi: 10.1016/j.neuro.2010.05.009 URL |
| [51] |
Verstraeten, S. V.; Aimo, L.; Oteiza, P. I. Aluminium and lead: molecular mechanisms of brain toxicity. Arch Toxicol. 2008, 82, 789-802.
doi: 10.1007/s00204-008-0345-3 URL |
| [52] |
El-Rahman, S. S. Neuropathology of aluminum toxicity in rats (glutamate and GABA impairment). Pharmacol Res. 2003, 47, 189-194.
doi: 10.1016/S1043-6618(02)00336-5 URL |
| [53] |
Peacock, M. Calcium metabolism in health and disease. Clin J Am Soc Nephrol. 2010, 5 Suppl 1, S23-30.
doi: 10.2215/CJN.05910809 URL |
| [54] |
Cashman, K. D. Calcium intake, calcium bioavailability and bone health. Br J Nutr. 2002, 87 Suppl 2, S169-177.
doi: 10.1079/BJN/2002534 URL |
| [55] |
Renkema, K. Y.; Alexander, R. T.; Bindels, R. J.; Hoenderop, J. G. Calcium and phosphate homeostasis: concerted interplay of new regulators. Ann Med. 2008, 40, 82-91.
doi: 10.1080/07853890701689645 URL |
| [56] |
Chasapis, C. T.; Loutsidou, A. C.; Spiliopoulou, C. A.; Stefanidou, M. E. Zinc and human health: an update. Arch Toxicol. 2012, 86, 521-534.
doi: 10.1007/s00204-011-0775-1 URL |
| [57] | Frederickson, C. J.; Koh, J. Y.; Bush, A. I. The neurobiology of zinc in health and disease. Nat Rev Neurosci. 2005, 6, 449-462. |
| [58] |
Aschner, M.; Guilarte, T. R.; Schneider, J. S.; Zheng, W. Manganese: recent advances in understanding its transport and neurotoxicity. Toxicol Appl Pharmacol. 2007, 221, 131-147.
doi: 10.1016/j.taap.2007.03.001 URL |
| [59] |
Erikson, K. M.; Aschner, M. Manganese neurotoxicity and glutamate-GABA interaction. Neurochem Int. 2003, 43, 475-480.
doi: 10.1016/S0197-0186(03)00037-8 URL |
| [60] |
Erikson, K. M.; Syversen, T.; Aschner, J. L.; Aschner, M. Interactions between excessive manganese exposures and dietary iron-deficiency in neurodegeneration. Environ Toxicol Pharmacol. 2005, 19, 415-421.
doi: 10.1016/j.etap.2004.12.053 URL |
| [61] |
Pors Nielsen, S, The biological role of strontium. Bone. 2004, 35, 583-588.
doi: 10.1016/j.bone.2004.04.026 URL |
| [62] |
Marie, P. J.; Ammann, P.; Boivin, G.; Rey, C. Mechanisms of action and therapeutic potential of strontium in bone. Calcif Tissue Int. 2001, 69, 121-129.
doi: 10.1007/s002230010055 URL |
| [63] | Jugdaohsingh, R. Silicon and bone health. J Nutr Health Aging. 2007, 11, 99-110. |
| [64] | Pérez-Granados, A. M.; Vaquero, M. P. Silicon, aluminium, arsenic and lithium: essentiality and human health implications. J Nutr Health Aging. 2002, 6, 154-162. |
| [65] | Seaborn, C. D.; Nielsen, F. H. Silicon: A Nutritional Beneficence for Bones, Brains and Blood Vessels? Nutr Today. 1993, 28, 13-18. |
| [66] |
Chevalier, J. What future for zirconia as a biomaterial? Biomaterials. 2006, 27, 535-543.
doi: 10.1016/j.biomaterials.2005.07.034 URL |
| [67] |
Denry, I.; Kelly, J. R. State of the art of zirconia for dental applications. Dent Mater. 2008, 24, 299-307.
doi: 10.1016/j.dental.2007.05.007 URL |
| [68] |
Feyerabend, F.; Fischer, J.; Holtz, J.; Witte, F.; Willumeit, R.; Drücker, H.; Vogt, C.; Hort, N. Evaluation of short-term effects of rare earth and other elements used in magnesium alloys on primary cells and cell lines. Acta Biomater. 2010, 6, 1834-1842.
doi: 10.1016/j.actbio.2009.09.024 URL |
| [69] |
Nakamura, Y.; Tsumura, Y.; Tonogai, Y.; Shibata, T.; Ito, Y. Differences in behavior among the chlorides of seven rare earth elements administered intravenously to rats. Fundam Appl Toxicol. 1997, 37, 106-116.
doi: 10.1006/faat.1997.2322 URL |
| [70] | Drynda, A.; Deinet, N.; Braun, N.; Peuster, M. Rare earth metals used in biodegradable magnesium-based stents do not interfere with proliferation of smooth muscle cells but do induce the upregulation of inflammatory genes. J Biomed Mater Res A. 2009, 91, 360-369. |
| [71] |
Willbold, E.; Gu, X.; Albert, D.; Kalla, K.; Bobe, K.; Brauneis, M.; Janning, C.; Nellesen, J.; Czayka, W.; Tillmann, W.; Zheng, Y.; Witte, F. Effect of the addition of low rare earth elements (lanthanum, neodymium, cerium) on the biodegradation and biocompatibility of magnesium. Acta Biomater. 2015, 11, 554-562.
doi: 10.1016/j.actbio.2014.09.041 URL |
| [72] |
Hurley, M. F.; Efaw, C. M.; Davis, P. H.; Croteau, J. R.; Graugnard, E.; Birbilis, N. Volta potentials measured by scanning kelvin probe force microscopy as relevant to corrosion of magnesium alloys. Corrosion. 2014, 71, 160-170.
doi: 10.5006/1432 URL |
| [73] |
Fu, P. H.; Peng, L. M.; Nie, J. F.; Jiang, H. Y.; Ma, L.; Bourgeois, L. Ductility improvement of Mg-Nd-Zr cast alloy by trace addition of Zn. Mater Sci Forum. 2011, 690, 230-233.
doi: 10.4028/www.scientific.net/MSF.690 URL |
| [74] |
Zhang, X.; Yuan, G.; Wang, Z. Mechanical properties and biocorrosion resistance of Mg-Nd-Zn-Zr alloy improved by cyclic extrusion and compression. Mater Lett. 2012, 74, 128-131.
doi: 10.1016/j.matlet.2012.01.086 URL |
| [75] |
Wu, Q.; Zhu, S.; Wang, L.; Liu, Q.; Yue, G.; Wang, J.; Guan, S. The microstructure and properties of cyclic extrusion compression treated Mg-Zn-Y-Nd alloy for vascular stent application. J Mech Behav Biomed Mater. 2012, 8, 1-7.
doi: 10.1016/j.jmbbm.2011.12.011 URL |
| [76] |
Ralston, K. D.; Birbilis, N.; Davies, C. H. J. Revealing the relationship between grain size and corrosion rate of metals. Scripta Mater. 2010, 63, 1201-1204.
doi: 10.1016/j.scriptamat.2010.08.035 URL |
| [77] | Cao, C. Principles of electrochemistry of corrosion. Chemistry Industry Press: Beijing, 2008. |
| [78] |
Chen, L.; Bin, Y.; Zou, W.; Wang, X.; Li, W. The influence of Sr on the microstructure, degradation and stress corrosion cracking of the Mg alloys - ZK40xSr. J Mech Behav Biomed Mater. 2017, 66, 187-200.
doi: 10.1016/j.jmbbm.2016.11.014 URL |
| [79] | Werkhoven, R. J.; Sillekens, W. H.; van Lieshout, J. B. J. M. Processing aspects of magnesium alloy stent tube. In Magnesium technology 2011, Sillekens, W. H.; Agnew, S. R.; Neelameggham, N. R.; Mathaudhu, S. N., eds.; Springer International Publishing: Cham, 2016; pp 419-424. |
| [80] |
Serruys, P. W.; Kutryk, M. J.; Ong, A. T. Coronary-artery stents. N Engl J Med. 2006, 354, 483-495.
doi: 10.1056/NEJMra051091 URL |
| [81] |
Lu, W.; Yue, R.; Miao, H.; Pei, J.; Huang, H.; Yuan, G. Enhanced plasticity of magnesium alloy micro-tubes for vascular stents by double extrusion with large plastic deformation. Mater Lett. 2019, 245, 155-157.
doi: 10.1016/j.matlet.2019.02.114 URL |
| [82] | Fang, G.; Ai, W. J.; Leeflang, S.; Duszczyk, J.; Zhou, J. Multipass cold drawing of magnesium alloy minitubes for biodegradable vascular stents. Mater Sci Eng CMater Biol Appl. 2013, 33, 3481-3488. |
| [83] |
Yoshida, K.; Koiwa, A. Cold drawing of magnesium alloy tubes for medical. J Solid Mech Mater Eng. 2011, 5, 1071-1078.
doi: 10.1299/jmmp.5.1071 URL |
| [84] | Hanada, K.; Matsuzaki, K.; Huang, X.; Chino, Y. Fabrication of Mg alloy tubes for biodegradable stent application. Mater Sci Eng CMater Biol Appl. 2013, 33, 4746-4750. |
| [85] | Liu, F.; Chen, C.; Niu, J.; Pei, J.; Zhang, H.; Huang, H.; Yuan, G. The processing of Mg alloy micro-tubes for biodegradable vascular stents. Mater Sci Eng CMater Biol Appl. 2015, 48, 400-407. |
| [86] |
Karanasiou, G. S.; Papafaklis, M. I.; Conway, C.; Michalis, L. K.; Tzafriri, R.; Edelman, E. R.; Fotiadis, D. I. Stents: biomechanics, biomaterials, and insights from computational modeling. Ann Biomed Eng. 2017, 45, 853-872.
doi: 10.1007/s10439-017-1806-8 URL |
| [87] |
Bressloff, N. W.; Ragkousis, G.; Curzen, N. Design optimisation of coronary artery stent systems. Ann Biomed Eng. 2016, 44, 357-367.
doi: 10.1007/s10439-015-1373-9 URL |
| [88] |
Pant, S.; Limbert, G.; Curzen, N. P.; Bressloff, N. W. Multiobjective design optimisation of coronary stents. Biomaterials. 2011, 32, 7755-7773.
doi: 10.1016/j.biomaterials.2011.07.059 URL |
| [89] |
Grogan, J. A.; Leen, S. B.; McHugh, P. E. A physical corrosion model for bioabsorbable metal stents. Acta Biomater. 2014, 10, 2313-2322.
doi: 10.1016/j.actbio.2013.12.059 URL |
| [90] |
Wu, W.; Gastaldi, D.; Yang, K.; Tan, L.; Petrini, L.; Migliavacca, F. Finite element analyses for design evaluation of biodegradable magnesium alloy stents in arterial vessels. Mater Sci Eng B. 2011, 176, 1733-1740.
doi: 10.1016/j.mseb.2011.03.013 URL |
| [91] |
Grogan, J. A.; Leen, S. B.; McHugh, P. E. Optimizing the design of a bioabsorbable metal stent using computer simulation methods. Biomaterials. 2013, 34, 8049-8060.
doi: 10.1016/j.biomaterials.2013.07.010 URL |
| [92] |
Chen, C.; Chen, J.; Wu, W.; Shi, Y.; Jin, L.; Petrini, L.; Shen, L.; Yuan, G.; Ding, W.; Ge, J.; Edelman, E. R.; Migliavacca, F. In vivo and in vitro evaluation of a biodegradable magnesium vascular stent designed by shape optimization strategy. Biomaterials. 2019, 221, 119414.
doi: 10.1016/j.biomaterials.2019.119414 URL |
| [93] | Shi, Y.; Zhang, L.; Chen, J.; Zhang, J.; Yuan, F.; Shen, L.; Chen, C.; Pei, J.; Li, Z.; Tan, J.; Yuan, G. In vitro and in vivo degradation of rapamycin-eluting Mg-Nd-Zn-Zr alloy stents in porcine coronary arteries. Mater Sci Eng CMater Biol Appl. 2017, 80, 1-6. |
| [94] |
Shi, Y.; Pei, J.; Zhang, L.; Lee, B. K.; Yun, Y.; Zhang, J.; Li, Z.; Gu, S.; Park, K.; Yuan, G. Understanding the effect of magnesium degradation on drug release and anti-proliferation on smooth muscle cells for magnesium-based drug eluting stents. Corros Sci. 2017, 123, 297-309.
doi: 10.1016/j.corsci.2017.04.016 URL |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||