Biomaterials Translational ›› 2022, Vol. 3 ›› Issue (3): 221-233.doi: 10.12336/biomatertransl.2022.03.006
• RESEARCH ARTICLE • Previous Articles
Monchupa Kingsak1, Panita Maturavongsadit1, Hong Jiang2, Qian Wang1,*( )
)
Received:2022-08-26
Revised:2022-09-07
Accepted:2022-09-17
Online:2022-09-28
Published:2022-09-28
Contact:
Qian Wang
E-mail:Wang263@mailbox.sc.edu
About author:Qian Wang,Wang263@mailbox.sc.edu.
Kingsak, M.; Maturavongsadit, P.; Jiang, H.; Wang, Q. Cellular responses to nanoscale substrate topography of TiO2 nanotube arrays: cell morphology and adhesion. Biomater Transl. 2022, 3(3), 221-233.
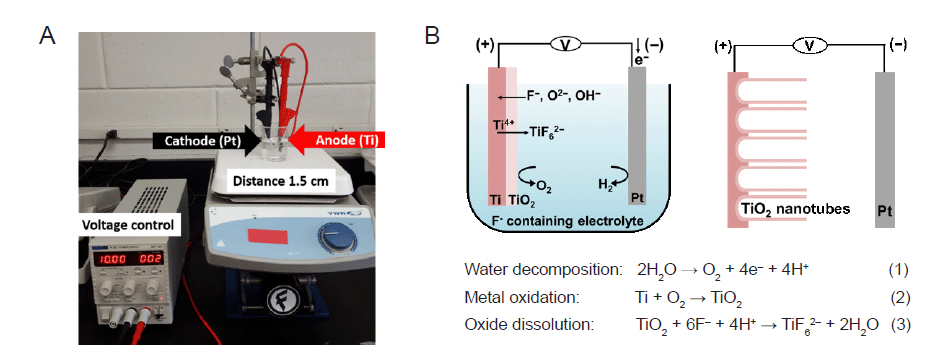
Figure 1. The synthesis of TNA by electrochemical anodization. (A) The experimental setup for the anodization of Ti. Ti was used as the anode and Pt was used as the cathode. The distance between Ti and Pt was controlled at 1.5 cm. (B) Schematic illustration showing the reactions occurring during anodization of the Ti sheet. Three chemical reactions (water decomposition, metal oxidation, and oxide dissolution) occur simultaneously to create nanotube–like structures during the anodization process. Figure 1B has been redrawn based on an original figure by Regonini et al.44 Pt: platinum; Ti: titanium; TiO2: titanium dioxide; TNA: TiO2 nanotube array.
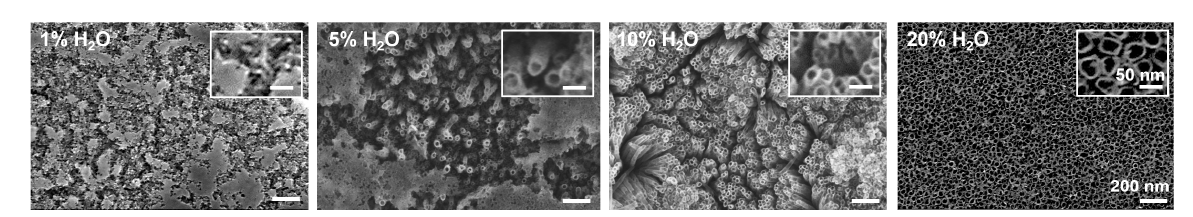
Figure 2. The effect of differences in water content in the electrolyte on tube geometry. Scanning electron microscopy images of anodized TiO2 nanotubes prepared by anodizing Ti at 10 V for 3 hours in DEG electrolytes containing 0.5 wt% NH4F with different concentrations of 1%, 5%, 10% and 20% (v/v) H2O. Increasing the water content from 1% to 20% markedly reduced the time required to dissolve the native oxide during TNA formation. The insets show enlarged images of the anodized substrate prepared with different water contents in the electrolyte. All images and insets share the same scale bars at 200 nm and 50 nm, respectively. DEG: diethylene glycol; NH4F: ammonium fluoride; Ti: titanium; TiO2: titanium dioxide; TNA: TiO2 nanotube array.
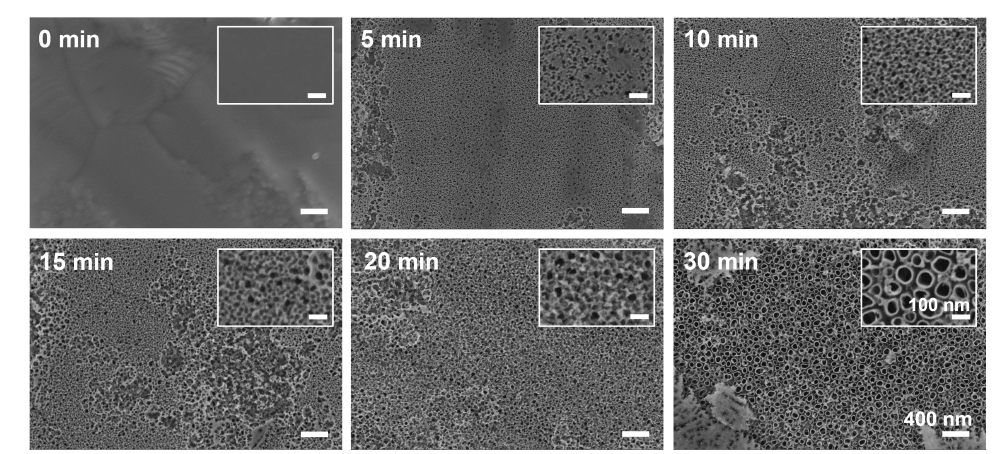
Figure 3. The effect of anodization times on nanotube development and structure. Scanning electron microscopy images show the evolution of nanotube formation during different anodizing times at 20 V for 5, 10, 15, 20, or 30 minutes in DEG–based electrolyte containing 0.5 wt% NH4F and 20% (v/v) H2O. Nanopits were created at 5 minutes, nanoporous layer was generated over the surface at 10–20 minutes, and nanotubes were developed at 30 minutes of anodizing times. The insets show enlarged images of the anodized substrate at different anodizing times. All images and insets share the same scale bars at 400 nm and 100 nm, respectively. DEG: diethylene glycol; NH4F: ammonium fluoride.
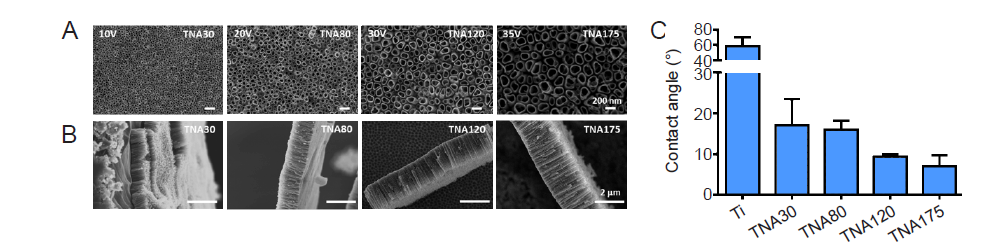
Figure 4. Characterization of sample nanostructure and surface wetting. (A, B) Top–view (A) and side–view (B) scanning electron microscopy images of TNAs prepared by anodizing Ti in DEG–based electrolyte containing 0.5 wt% NH4F and 20% (v/v) H2O at 10, 20, 30, and 35 V for 3 hours. The tube diameter and length were increased respectively by raising the applied voltage of anodization. Scale bars: 200 nm in A and 2 μm in B. (C) Surface wettability of TNAs at different diameters of pore size (30, 80, 120, and 175 nm). Data are expressed as mean ± SD (n = 6). DEG: diethylene glycol; NH4F: ammonium fluoride; Ti; titanium; TNA: titanium dioxide nanotube array.
| Ti | TNA30 (10 V) | TNA80 (20 V) | TNA120 (30 V) | TNA175 (35 V) | |
|---|---|---|---|---|---|
| Element (weight%) | |||||
| O | – | 23.75 | 23.09 | 22.48 | 22.74 |
| F | – | 6.57 | 6.47 | 6.28 | 6.31 |
| Ti | 100 | 69.68 | 70.44 | 71.24 | 70.96 |
| Diameter (nm) | – | 31.1±5.0 | 78.3±16.8 | 120.9±27.5 | 174.2±31.4 |
| Length (µm) | – | 0.6±0.1 | 1.0±0.1 | 1.7±0.1 | 2.3±0.4 |
Table 1. The chemical components, diameters and lengths of the anodised TNAs using 0.5% NH4F with 20% (v/v) H2O in DEG–based electrolyte under different voltages
| Ti | TNA30 (10 V) | TNA80 (20 V) | TNA120 (30 V) | TNA175 (35 V) | |
|---|---|---|---|---|---|
| Element (weight%) | |||||
| O | – | 23.75 | 23.09 | 22.48 | 22.74 |
| F | – | 6.57 | 6.47 | 6.28 | 6.31 |
| Ti | 100 | 69.68 | 70.44 | 71.24 | 70.96 |
| Diameter (nm) | – | 31.1±5.0 | 78.3±16.8 | 120.9±27.5 | 174.2±31.4 |
| Length (µm) | – | 0.6±0.1 | 1.0±0.1 | 1.7±0.1 | 2.3±0.4 |
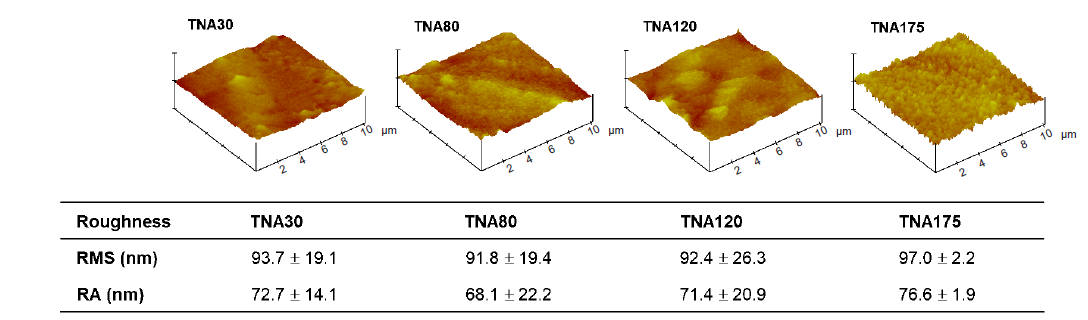
Figure 5. Summary of the surface roughness of TNAs with different diameters (30, 80, 120, 175 nm). The roughness was characterized by atomic force microscopy. The data are expressed as mean ± SD (n = 4). No significant change was observed in any comparisons based on one–way analysis of variance followed by Tukey’s multiple comparisons test. Ra: mean roughness; RMS: root mean square roughness; TNA: titanium dioxide nanotube array.
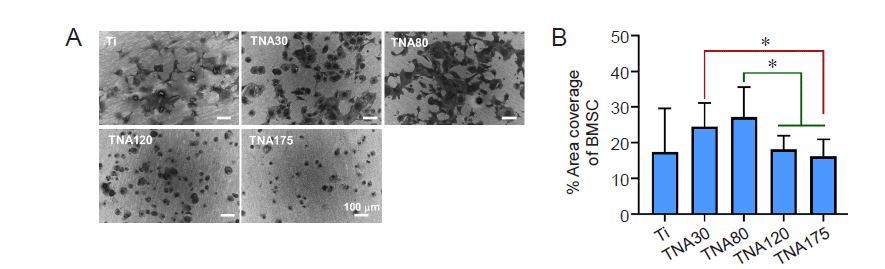
Figure 6. Morphology of BMSCs cultured on Ti or TNA substrates in two–dimensional cell culture without foetal bovine serum. TNA30 and TNA80 enhanced cell spreading and percentage cell area coverage of BMSCs. (A) SEM images show the morphology of BMSCs cultured on Ti or TNA substrates prepared by anodizing Ti in a DEG–based electrolyte at 10, 20, 30, or 35 V for 3 hours. The cells cultured on TNA30 and TNA80 exhibited good spreading all over the surface, while cells on TNA120 and TNA175 adopted a rounded shape with poor spreading. All scale bars share a length of 100 µm. (B) Percentage area coverage of BMSCs on Ti or TNA substrates, calculated from SEM images using ImageJ. Data are expressed as mean ± SD (n = 3). *P < 0.05 (Student’s t–test or one–way analysis of variance followed by Tukey’s multiple comparisons test). BMSC: bone marrow–derived mesenchymal stem cell; DEG: diethylene glycol; SEM: scanning electron microscopy; Ti; titanium; TNA: titanium dioxide nanotube array.
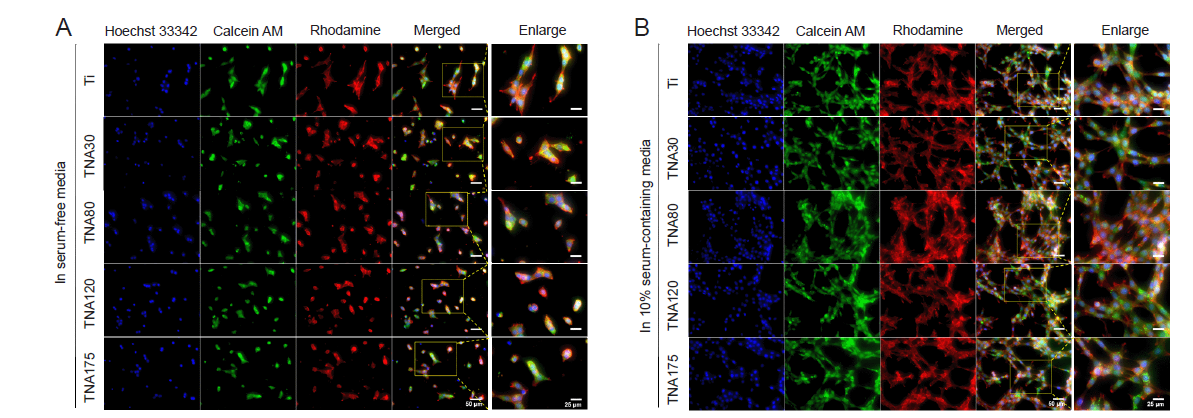
Figure 7. Morphology of NIH3T3 cells cultured on Ti or TNA substrates in two–dimensional cell culture with and without FBS. FBS significantly affected cell morphology and proliferation. (A, B) Confocal laser scanning microscopy images of NIH3T3 cells cultured on Ti or TNA substrates after 1 day of incubation in serum–free medium (A), and in 10% serum–containing medium (B). The morphology of NIH3T3 cells cultured in serum–containing medium showed good spreading, while the cells cultured in serum–free medium showed poor spreading and fewer cells. Cells were fixed and stained with Hoechst 33342 (blue fluorescence), Calcein AM (green fluorescence), and Rhodamine phalloidin (red fluorescence). The scale bars indicate a length of 50 µm for all images and 25 µm for the enlarged images. FBS: foetal bovine serum; Ti; titanium; TNA: titanium dioxide nanotube array.
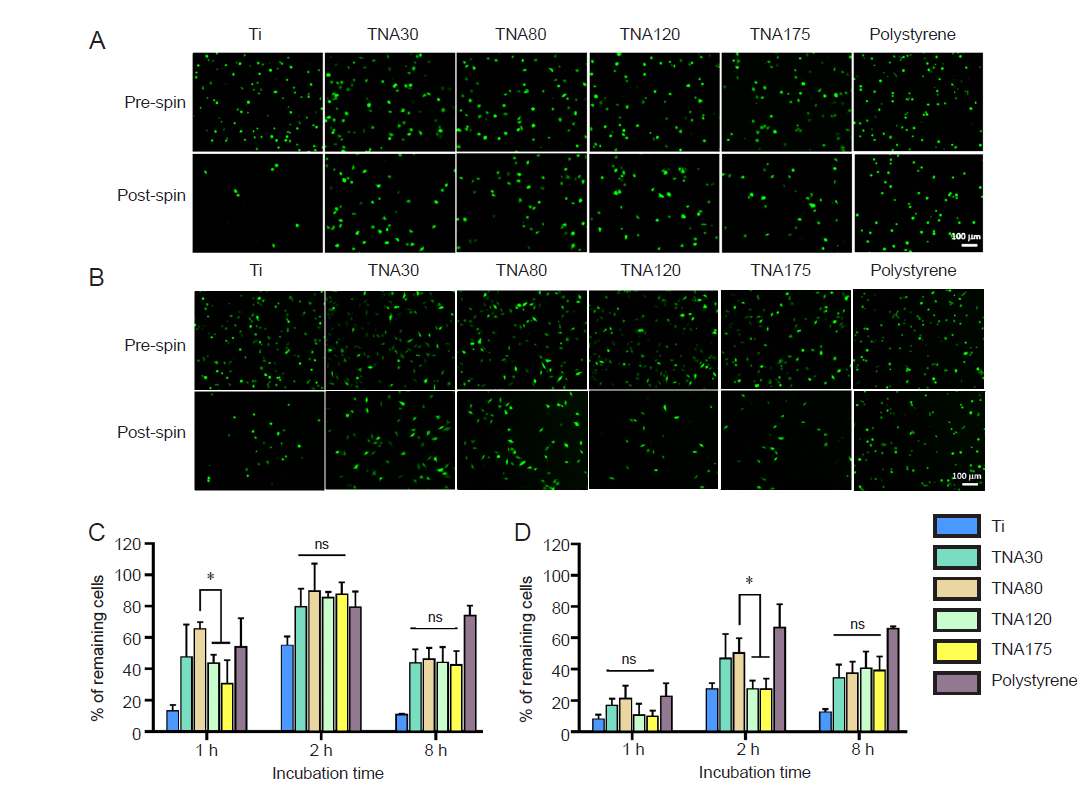
Figure 8. Assessment of the adhesion of NIH3T3 and BHK–21 cells by centrifugation assay. TNA80 promoted enhanced cell adhesion in the early stage of cell attachment. (A, B) Fluorescent images show NIH3T3 cells (A) and BHK–21 cells (B) attached on Ti, TNAs, and tissue culture plate (polystyrene) and incubated in serum–free medium for 1 and 2 hours, respectively, before and after spinning. After post spin, NIH3T3 and BHK–21 cells cultured on TNA80 detached from the substrate fewer than those on TNA120 and TNA175 after 1 and 2 hours of incubation time, respectively. The cells were stained with Calcein AM. The scale bar indicates a length of 100 µm. (C, D) The percentage of remaining NIH3T3 cells (C) and BHK–21 cells (D) after centrifugation following different incubation times. Data are expressed as mean ± SD (n = 3). *P < 0.05 (Student’s t–test and one–way analysis of variance followed by Tukey’s multiple comparisons test). ns: not significant; Ti; titanium; TNA: titanium dioxide nanotube array.
| Physical techniques | Chemical techniques | Coating techniques | Others |
|---|---|---|---|
| Grit blasting | Anodization | Sol–Gel coating | Lithography |
| Nanoparticle compaction | Acid treatment | Self–assembly of monolayers | Ultraviolet photofunctionalization |
| Alkali treatment | Discrete crystalline deposition | ||
| Hydrogen peroxide treatment | Plasma spray | ||
| Chemical vapor deposition | Ion implantation | ||
| Sputtering | |||
| Pulsed laser deposition | |||
| Electron beam evaporation |
Table 2. Various techniques used to generate nanotopographical features and surface roughness on implant materials
| Physical techniques | Chemical techniques | Coating techniques | Others |
|---|---|---|---|
| Grit blasting | Anodization | Sol–Gel coating | Lithography |
| Nanoparticle compaction | Acid treatment | Self–assembly of monolayers | Ultraviolet photofunctionalization |
| Alkali treatment | Discrete crystalline deposition | ||
| Hydrogen peroxide treatment | Plasma spray | ||
| Chemical vapor deposition | Ion implantation | ||
| Sputtering | |||
| Pulsed laser deposition | |||
| Electron beam evaporation |
| 1. |
Gibon, E.; Amanatullah, D. F.; Loi, F.; Pajarinen, J.; Nabeshima, A.; Yao, Z.; Hamadouche, M.; Goodman, S. B. The biological response to orthopaedic implants for joint replacement: Part I: Metals. J Biomed Mater Res B Appl Biomater. 2017, 105, 2162-2173.
doi: 10.1002/jbm.b.33734 URL |
| 2. | Cherubino, P.; Ratti, C.; Fagetti, A.; Binda, T. Total hip arthroplasty and bone fragility. Aging Clin Exp Res. 2011, 23, 76-77. |
| 3. | Etkin, C. D.; Springer, B. D. The American Joint Replacement Registry-the first 5 years. Arthroplast Today. 2017, 3, 67-69. |
| 4. |
Saini, M.; Singh, Y.; Arora, P.; Arora, V.; Jain, K. Implant biomaterials: a comprehensive review. World J Clin Cases. 2015, 3, 52-57.
doi: 10.12998/wjcc.v3.i1.52 URL |
| 5. | Guo, X.; Wang, Q. Magnesium-based biodegradable metal materials: past, present and future. Biomater Transl. 2021, 2, 175-176. |
| 6. | Jing, X.; Ding, Q.; Wu, Q.; Su, W.; Yu, K.; Su, Y.; Ye, B.; Gao, Q.; Sun, T.; Guo, X. Magnesium-based materials in orthopaedics: material properties and animal models. Biomater Transl. 2021, 2, 197-213. |
| 7. |
Eliaz, N. Corrosion of metallic biomaterials: a review. Materials (Basel). 2019, 12, 407.
doi: 10.3390/ma12030407 URL |
| 8. |
Shokeen, B.; Zamani, L.; Zadmehr, S.; Pouraghaie, S.; Ozawa, R.; Yilmaz, B.; Lilak, S.; Sharma, S.; Ogawa, T.; Moshaverinia, A.; Lux, R. Surface characterization and assessment of biofilm formation on two titanium-based implant coating materials. Front Dent Med. 2021, 2, 695417.
doi: 10.3389/fdmed.2021.695417 URL |
| 9. |
Arciola, C. R.; Campoccia, D.; Montanaro, L. Implant infections: adhesion, biofilm formation and immune evasion. Nat Rev Microbiol. 2018, 16, 397-409.
doi: 10.1038/s41579-018-0019-y URL |
| 10. |
Kim, K. T.; Eo, M. Y.; Nguyen, T. T. H.; Kim, S. M. General review of titanium toxicity. Int J Implant Dent. 2019, 5, 10.
doi: 10.1186/s40729-019-0162-x URL |
| 11. |
Gittens, R. A.; Olivares-Navarrete, R.; Schwartz, Z.; Boyan, B. D. Implant osseointegration and the role of microroughness and nanostructures: lessons for spine implants. Acta Biomater. 2014, 10, 3363-3371.
doi: 10.1016/j.actbio.2014.03.037 URL |
| 12. | Schwarz, F.; Wieland, M.; Schwartz, Z.; Zhao, G.; Rupp, F.; Geis-Gerstorfer, J.; Schedle, A.; Broggini, N.; Bornstein, M. M.; Buser, D.; Ferguson, S. J.; Becker, J.; Boyan, B. D.; Cochran, D. L. Potential of chemically modified hydrophilic surface characteristics to support tissue integration of titanium dental implants. J Biomed Mater Res B Appl Biomater. 2009, 88, 544-557. |
| 13. |
Kam, K. R.; Walsh, L. A.; Bock, S. M.; Ollerenshaw, J. D.; Ross, R. F.; Desai, T. A. The effect of nanotopography on modulating protein adsorption and the fibrotic response. Tissue Eng Part A. 2014, 20, 130-138.
doi: 10.1089/ten.tea.2012.0772 URL |
| 14. |
Kaur, G.; Valarmathi, M. T.; Potts, J. D.; Jabbari, E.; Sabo-Attwood, T.; Wang, Q. Regulation of osteogenic differentiation of rat bone marrow stromal cells on 2D nanorod substrates. Biomaterials. 2010, 31, 1732-1741.
doi: 10.1016/j.biomaterials.2009.11.041 URL |
| 15. |
Kaur, G.; Valarmathi, M. T.; Potts, J. D.; Wang, Q. The promotion of osteoblastic differentiation of rat bone marrow stromal cells by a polyvalent plant mosaic virus. Biomaterials. 2008, 29, 4074-4081.
doi: 10.1016/j.biomaterials.2008.06.029 URL |
| 16. |
Kaur, G.; Wang, C.; Sun, J.; Wang, Q. The synergistic effects of multivalent ligand display and nanotopography on osteogenic differentiation of rat bone marrow stem cells. Biomaterials. 2010, 31, 5813-5824.
doi: 10.1016/j.biomaterials.2010.04.017 URL |
| 17. |
Lin, Y.; Su, Z.; Niu, Z.; Li, S.; Kaur, G.; Lee, L. A.; Wang, Q. Layer-by-layer assembly of viral capsid for cell adhesion. Acta Biomater. 2008, 4, 838-843.
doi: 10.1016/j.actbio.2008.02.026 URL |
| 18. | Sitasuwan, P.; Lee, L. A.; Li, K.; Nguyen, H. G.; Wang, Q. RGD-conjugated rod-like viral nanoparticles on 2D scaffold improve bone differentiation of mesenchymal stem cells. Front Chem. 2014, 2, 31. |
| 19. |
Metavarayuth, K.; Maturavongsadit, P.; Chen, X.; Sitasuwan, P.; Lu, L.; Su, J.; Wang, Q. Nanotopographical cues mediate osteogenesis of stem cells on virus substrates through BMP-2 intermediate. Nano Lett. 2019, 19, 8372-8380.
doi: 10.1021/acs.nanolett.9b02001 URL |
| 20. | Metavarayuth, K.; Villarreal, E.; Wang, H.; Wang, Q. Surface topography and free energy regulate osteogenesis of stem cells: effects of shape-controlled gold nanoparticles. Biomater Transl. 2021, 2, 165-173. |
| 21. | Robin, A.; Bernardes de Almeida Ribeiro, M.; Luiz Rosa, J.; Zenhei Nakazato, R.; Borges Silva, M. Formation of TiO2 nanotube layer by anodization of titanium in ethylene Glycol-H2O electrolyte. J Surf Eng Mater Adv Technol. 2014, 4, 123-130. |
| 22. |
Brammer, K. S.; Frandsen, C. J.; Jin, S. TiO2 nanotubes for bone regeneration. Trends Biotechnol. 2012, 30, 315-322.
doi: 10.1016/j.tibtech.2012.02.005 URL |
| 23. |
Kulkarni, M.; Mazare, A.; Gongadze, E.; Perutkova, Š.; Kralj-Iglič, V.; Milošev, I.; Schmuki, P.; Iglič, A.; Mozetič, M. Titanium nanostructures for biomedical applications. Nanotechnology. 2015, 26, 062002.
doi: 10.1088/0957-4484/26/6/062002 URL |
| 24. | Strnad, G. ; Petrovan, C. ; Russu, O. ; Jakab-Farkas, L. TiO2 nanostructured surfaces for biomedical applications developed by electrochemical anodization. IOP Conference Series: Materials Science and Engineering. 2016, 161, 012051. |
| 25. |
Dalby, M. J.; Gadegaard, N.; Oreffo, R. O. Harnessing nanotopography and integrin-matrix interactions to influence stem cell fate. Nat Mater. 2014, 13, 558-569.
doi: 10.1038/nmat3980 URL |
| 26. |
Wang, N.; Butler, J. P.; Ingber, D. E. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993, 260, 1124-1127.
doi: 10.1126/science.7684161 URL |
| 27. |
Ravichandran, R.; Liao, S.; Ng, C.; Chan, C. K.; Raghunath, M.; Ramakrishna, S. Effects of nanotopography on stem cell phenotypes. World J Stem Cells. 2009, 1, 55-66.
doi: 10.4252/wjsc.v1.i1.55 URL |
| 28. |
Oh, S.; Brammer, K. S.; Li, Y. S.; Teng, D.; Engler, A. J.; Chien, S.; Jin, S. Stem cell fate dictated solely by altered nanotube dimension. Proc Natl Acad Sci U S A. 2009, 106, 2130-2135.
doi: 10.1073/pnas.0813200106 URL |
| 29. |
Park, J. H.; Gu, L.; von Maltzahn, G.; Ruoslahti, E.; Bhatia, S. N.; Sailor, M. J. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nat Mater. 2009, 8, 331-336.
doi: 10.1038/nmat2398 URL |
| 30. |
Park, J.; Bauer, S.; Schmuki, P.; von der Mark, K. Narrow window in nanoscale dependent activation of endothelial cell growth and differentiation on TiO2 nanotube surfaces. Nano Lett. 2009, 9, 3157-3164.
doi: 10.1021/nl9013502 URL |
| 31. | Yu, W. Q.; Jiang, X. Q.; Zhang, F. Q.; Xu, L. The effect of anatase TiO2 nanotube layers on MC3T3-E1 preosteoblast adhesion, proliferation, and differentiation. J Biomed Mater Res A. 2010, 94, 1012-1022. |
| 32. |
Zhao, L.; Liu, L.; Wu, Z.; Zhang, Y.; Chu, P. K. Effects of micropitted/nanotubular titania topographies on bone mesenchymal stem cell osteogenic differentiation. Biomaterials. 2012, 33, 2629-2641.
doi: 10.1016/j.biomaterials.2011.12.024 URL |
| 33. |
Lai, M.; Cai, K.; Zhao, L.; Chen, X.; Hou, Y.; Yang, Z. Surface functionalization of TiO2 nanotubes with bone morphogenetic protein 2 and its synergistic effect on the differentiation of mesenchymal stem cells. Biomacromolecules. 2011, 12, 1097-1105.
doi: 10.1021/bm1014365 URL |
| 34. |
Smith, B. S.; Yoriya, S.; Johnson, T.; Popat, K. C. Dermal fibroblast and epidermal keratinocyte functionality on titania nanotube arrays. Acta Biomater. 2011, 7, 2686-2696.
doi: 10.1016/j.actbio.2011.03.014 URL |
| 35. | Zhang, Y.; Luo, R.; Tan, J.; Wang, J.; Lu, X.; Qu, S.; Weng, J.; Feng, B. Osteoblast behaviors on titania nanotube and mesopore layers. Regen Biomater. 2017, 4, 81-87. |
| 36. |
Park, J.; Bauer, S.; von der Mark, K.; Schmuki, P. Nanosize and vitality: TiO2 nanotube diameter directs cell fate. Nano Lett. 2007, 7, 1686-1691.
doi: 10.1021/nl070678d URL |
| 37. |
Boussu, K.; Van der Bruggen, B.; Volodin, A.; Snauwaert, J.; Van Haesendonck, C.; Vandecasteele, C. Roughness and hydrophobicity studies of nanofiltration membranes using different modes of AFM. J Colloid Interface Sci. 2005, 286, 632-638.
doi: 10.1016/j.jcis.2005.01.095 URL |
| 38. |
Lee, L. A.; Nguyen, Q. L.; Wu, L.; Horvath, G.; Nelson, R. S.; Wang, Q. Mutant plant viruses with cell binding motifs provide differential adhesion strengths and morphologies. Biomacromolecules. 2012, 13, 422-431.
doi: 10.1021/bm2014558 URL |
| 39. |
Nadri, S.; Soleimani, M. Comparative analysis of mesenchymal stromal cells from murine bone marrow and amniotic fluid. Cytotherapy. 2007, 9, 729-737.
doi: 10.1080/14653240701656061 URL |
| 40. | Ito, H.; Uchida, T.; Makita, K. Interactions between rat alveolar epithelial cells and bone marrow-derived mesenchymal stem cells: an in vitro co-culture model. Intensive Care Med Exp. 2015, 3, 53. |
| 41. | Schneider, C. A.; Rasband, W. S.; Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012, 9, 671-675. |
| 42. |
Doube, M.; Kłosowski, M. M.; Arganda-Carreras, I.; Cordelières, F. P.; Dougherty, R. P.; Jackson, J. S.; Schmid, B.; Hutchinson, J. R.; Shefelbine, S. J. BoneJ: free and extensible bone image analysis in ImageJ. Bone. 2010, 47, 1076-1079.
doi: 10.1016/j.bone.2010.08.023 URL |
| 43. |
Guan, D.; Wang, Y. Synthesis and growth mechanism of multilayer TiO2 nanotube arrays. Nanoscale. 2012, 4, 2968-2977.
doi: 10.1039/c2nr30315a URL |
| 44. |
Regonini, D.; Bowen, C. R.; Jaroenworaluck, A.; Stevens, R. A review of growth mechanism, structure and crystallinity of anodized TiO2 nanotubes. Mater Sci Eng R Rep. 2013, 74, 377-406.
doi: 10.1016/j.mser.2013.10.001 URL |
| 45. | Brammer, K. S.; Oh, S.; Frandsen, C. J.; Jin, S. Biomaterials and biotechnology schemes utilizing TiO2 nanotube arrays. In Biomaterials science and engineering, IntechOpen: London, United Kingdom, 2011. |
| 46. | Zamudio Torres, I.; Pérez Bueno, J. J.; Meas Vong, Y. Process of growth TiO2 nanotubes by anodization in an organic media. In Microscopy: advances in scientific research and education, Méndez-Vilas, A., Ed. Formatex: 2014; pp 887-893. |
| 47. |
Ozkan, S.; Nguyen, N. T.; Mazare, A.; Cerri, I.; Schmuki, P. Controlled spacing of self-organized anodic TiO2 nanotubes. Electrochem Commun. 2016, 69, 76-79.
doi: 10.1016/j.elecom.2016.06.004 URL |
| 48. |
Yoriya, S.; Mor, G. K.; Sharma, S.; Grimes, C. A. Synthesis of ordered arrays of discrete, partially crystalline titania nanotubes by Ti anodization using diethylene glycol electrolytes. J Mater Chem. 2008, 18, 3332-3336.
doi: 10.1039/b802463d URL |
| 49. |
Valota, A.; LeClere, D. J.; Skeldon, P.; Curioni, M.; Hashimoto, T.; Berger, S.; Kunze, J.; Schmuki, P.; Thompson, G. E. Influence of water content on nanotubular anodic titania formed in fluoride/glycerol electrolytes. Electrochim Acta. 2009, 54, 4321-4327.
doi: 10.1016/j.electacta.2009.02.098 URL |
| 50. |
Ferraris, S.; Spriano, S.; Pan, G.; Venturello, A.; Bianchi, C. L.; Chiesa, R.; Faga, M. G.; Maina, G.; Vernè, E. Surface modification of Ti-6Al-4V alloy for biomineralization and specific biological response: Part I, inorganic modification. J Mater Sci Mater Med. 2011, 22, 533-545.
doi: 10.1007/s10856-011-4246-2 URL |
| 51. |
Albu, S. P.; Roy, P.; Virtanen, S.; Schmuki, P. Self-organized TiO2 nanotube arrays: critical effects on morphology and growth. Isr J Chem. 2010, 50, 453-467.
doi: 10.1002/ijch.201000059 URL |
| 52. |
Bauer, S.; Kleber, S.; Schmuki, P. TiO2 nanotubes: Tailoring the geometry in H3PO4/HF electrolytes. Electrochem Commun. 2006, 8, 1321-1325.
doi: 10.1016/j.elecom.2006.05.030 URL |
| 53. |
Yin, H.; Liu, H.; Shen, W. Z. The large diameter and fast growth of self-organized TiO2 nanotube arrays achieved via electrochemical anodization. Nanotechnology. 2010, 21, 035601.
doi: 10.1088/0957-4484/21/3/035601 URL |
| 54. |
Bauer, S.; Park, J.; Faltenbacher, J.; Berger, S.; von der Mark, K.; Schmuki, P. Size selective behavior of mesenchymal stem cells on ZrO(2) and TiO(2) nanotube arrays. Integr Biol (Camb). 2009, 1, 525-532.
doi: 10.1039/b908196h URL |
| 55. |
Sánchez-Tovar, R.; Lee, K.; García-Antón, J.; Schmuki, P. Formation of anodic TiO2 nanotube or nanosponge morphology determined by the electrolyte hydrodynamic conditions. Electrochem Commun. 2013, 26, 1-4.
doi: 10.1016/j.elecom.2012.09.041 URL |
| 56. |
Law, K. Y. Definitions for hydrophilicity, hydrophobicity, and superhydrophobicity: getting the basics right. J Phys Chem Lett. 2014, 5, 686-688.
doi: 10.1021/jz402762h URL |
| 57. |
Lamour, G.; Hamraoui, A.; Buvailo, A.; Xing, Y.; Keuleyan, S.; Prakash, V.; Eftekhari-Bafrooei, A.; Borguet, E. Contact angle measurements using a simplified experimental setup. J Chem Educ. 2010, 87, 1403-1407.
doi: 10.1021/ed100468u URL |
| 58. |
Liu, G.; Du, K.; Wang, K. Surface wettability of TiO2 nanotube arrays prepared by electrochemical anodization. Appl Surf Sci. 2016, 388, 313-320.
doi: 10.1016/j.apsusc.2016.01.010 URL |
| 59. |
Ozcan, M.; Allahbeickaraghi, A.; Dündar, M. Possible hazardous effects of hydrofluoric acid and recommendations for treatment approach: a review. Clin Oral Investig. 2012, 16, 15-23.
doi: 10.1007/s00784-011-0636-6 URL |
| 60. |
Everett, E. T. Fluoride’s effects on the formation of teeth and bones, and the influence of genetics. J Dent Res. 2011, 90, 552-560.
doi: 10.1177/0022034510384626 URL |
| 61. |
Zareidoost, A.; Yousefpour, M.; Ghaseme, B.; Amanzadeh, A. The relationship of surface roughness and cell response of chemical surface modification of titanium. J Mater Sci Mater Med. 2012, 23, 1479-1488.
doi: 10.1007/s10856-012-4611-9 URL |
| 62. |
Metavarayuth, K.; Sitasuwan, P.; Zhao, X.; Lin, Y.; Wang, Q. Influence of surface topographical cues on the differentiation of mesenchymal stem cells in vitro. ACS Biomater Sci Eng. 2016, 2, 142-151.
doi: 10.1021/acsbiomaterials.5b00377 URL |
| 63. |
Gstraunthaler, G.; Lindl, T.; van der Valk, J. A plea to reduce or replace fetal bovine serum in cell culture media. Cytotechnology. 2013, 65, 791-793.
doi: 10.1007/s10616-013-9633-8 URL |
| 64. |
Heger, J. I.; Froehlich, K.; Pastuschek, J.; Schmidt, A.; Baer, C.; Mrowka, R.; Backsch, C.; Schleußner, E.; Markert, U. R.; Schmidt, A. Human serum alters cell culture behavior and improves spheroid formation in comparison to fetal bovine serum. Exp Cell Res. 2018, 365, 57-65.
doi: 10.1016/j.yexcr.2018.02.017 URL |
| 65. | Fang, C. Y.; Wu, C. C.; Fang, C. L.; Chen, W. Y.; Chen, C. L. Long-term growth comparison studies of FBS and FBS alternatives in six head and neck cell lines. PLoS One. 2017, 12, e0178960. |
| 66. | Bratengeier, C. Mechanisms of mechanically induced Osteoclastogenesis: in a novel in vitro model for bone implant loosening. Linköping University: Norrköping. 2019. |
| 67. |
Li, P. P.; Gu, C.; Liang, B. Y.; Wang, L.; Zhou, Y.; Tan, W. S. A serum-free medium suitable for maintaining cell morphology and liver-specific function in induced human hepatocytes. Cytotechnology. 2019, 71, 329-344.
doi: 10.1007/s10616-018-0289-2 URL |
| 68. |
Khalili, A. A.; Ahmad, M. R. A review of cell adhesion studies for biomedical and biological applications. Int J Mol Sci. 2015, 16, 18149-18184.
doi: 10.3390/ijms160818149 URL |
| 69. |
Chen, S.; Guo, Y.; Liu, R.; Wu, S.; Fang, J.; Huang, B.; Li, Z.; Chen, Z.; Chen, Z. Tuning surface properties of bone biomaterials to manipulate osteoblastic cell adhesion and the signaling pathways for the enhancement of early osseointegration. Colloids Surf B Biointerfaces. 2018, 164, 58-69.
doi: 10.1016/j.colsurfb.2018.01.022 URL |
| 70. |
Xi, A.; Bothun, G. D. Centrifugation-based assay for examining nanoparticle-lipid membrane binding and disruption. Analyst. 2014, 139, 973-981.
doi: 10.1039/c3an01601c URL |
| 71. | Franco-Barraza, J.; Beacham, D. A.; Amatangelo, M. D.; Cukierman, E. Preparation of extracellular matrices produced by cultured and primary fibroblasts. Curr Protoc Cell Biol. 2016, 71, 10.19.11-10.19.34. |
| 72. |
Khan, R. S.; Newsome, P. N. A comparison of phenotypic and functional properties of mesenchymal stromal cells and multipotent adult progenitor cells. Front Immunol. 2019, 10, 1952.
doi: 10.3389/fimmu.2019.01952 URL |
| 73. |
Hong, S. H.; Lee, M. H.; Koo, M. A.; Seon, G. M.; Park, Y. J.; Kim, D.; Park, J. C. Stem cell passage affects directional migration of stem cells in electrotaxis. Stem Cell Res. 2019, 38, 101475.
doi: 10.1016/j.scr.2019.101475 URL |
| 74. |
Zheng, H.; Martin, J. A.; Duwayri, Y.; Falcon, G.; Buckwalter, J. A. Impact of aging on rat bone marrow-derived stem cell chondrogenesis. J Gerontol A Biol Sci Med Sci. 2007, 62, 136-148.
doi: 10.1093/gerona/62.2.136 URL |
| 75. |
Higuchi, A.; Shimmura, S.; Takeuchi, T.; Suematsu, M.; Tsubota, K. Elucidation of apoptosis induced by serum deprivation in cultured conjunctival epithelial cells. Br J Ophthalmol. 2006, 90, 760-764.
doi: 10.1136/bjo.2005.088203 URL |
| 76. | Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular biology of the cell. 4th ed. Garland Science: New York, 2002. |
| 77. | Schwartz, M. A. Integrins and extracellular matrix in mechanotransduction. Cold Spring Harb Perspect Biol. 2010, 2, a005066. |
| 78. | Wozniak, M. A.; Modzelewska, K.; Kwong, L.; Keely, P. J. Focal adhesion regulation of cell behavior. Biochim Biophys Acta. 2004, 1692, 103-119. |
| 79. |
Mirbagheri, M.; Adibnia, V.; Hughes, B. R.; Waldman, S. D.; Banquy, X.; Hwang, D. K. Advanced cell culture platforms: a growing quest for emulating natural tissues. Mater Horiz. 2019, 6, 45-71.
doi: 10.1039/C8MH00803E URL |
| 80. |
Arnold, M.; Cavalcanti-Adam, E. A.; Glass, R.; Blümmel, J.; Eck, W.; Kantlehner, M.; Kessler, H.; Spatz, J. P. Activation of integrin function by nanopatterned adhesive interfaces. Chemphyschem. 2004, 5, 383-388.
doi: 10.1002/cphc.200301014 URL |
| 81. |
Kim, D. H.; Provenzano, P. P.; Smith, C. L.; Levchenko, A. Matrix nanotopography as a regulator of cell function. J Cell Biol. 2012, 197, 351-360.
doi: 10.1083/jcb.201108062 URL |
| 82. |
Reyes, C. D.; Petrie, T. A.; Burns, K. L.; Schwartz, Z.; García, A. J. Biomolecular surface coating to enhance orthopaedic tissue healing and integration. Biomaterials. 2007, 28, 3228-3235.
doi: 10.1016/j.biomaterials.2007.04.003 URL |
| 83. |
Wojtowicz, A. M.; Shekaran, A.; Oest, M. E.; Dupont, K. M.; Templeman, K. L.; Hutmacher, D. W.; Guldberg, R. E.; García, A. J. Coating of biomaterial scaffolds with the collagen-mimetic peptide GFOGER for bone defect repair. Biomaterials. 2010, 31, 2574-2582.
doi: 10.1016/j.biomaterials.2009.12.008 URL |
| 84. | Hanawa, T. Titanium-tissue interface reaction and its control with surface treatment. Front Bioeng Biotechnol. 2019, 7, 170. |
| 85. | Tapscott, D. C.; Wottowa, C. Orthopedic implant materials. In In StatPearls, StatPearls Publishing: Treasure Island (FL), 2022. |
| 86. | Wang, W.; Poh, C. K. Titanium alloys in orthopaedics. In Titanium alloys - advances in properties control, Sieniawski, J.; Ziaja, W., eds.; IntechOpen: London, 2013. |
| 87. |
Kim, Y. H.; Choi, M.; Kim, J. W. Are titanium implants actually safe for magnetic resonance imaging examinations? Arch Plast Surg. 2019, 46, 96-97.
doi: 10.5999/aps.2018.01466 URL |
| 88. |
Mendonça, G.; Mendonça, D. B.; Aragão, F. J.; Cooper, L. F. Advancing dental implant surface technology--from micron- to nanotopography. Biomaterials. 2008, 29, 3822-3835.
doi: 10.1016/j.biomaterials.2008.05.012 URL |
| 89. |
Pachauri, P.; Bathala, L. R.; Sangur, R. Techniques for dental implant nanosurface modifications. J Adv Prosthodont. 2014, 6, 498-504.
doi: 10.4047/jap.2014.6.6.498 URL |
| 90. |
Bressan, E.; Sbricoli, L.; Guazzo, R.; Tocco, I.; Roman, M.; Vindigni, V.; Stellini, E.; Gardin, C.; Ferroni, L.; Sivolella, S.; Zavan, B. Nanostructured surfaces of dental implants. Int J Mol Sci. 2013, 14, 1918-1931.
doi: 10.3390/ijms14011918 URL |
| 91. | Baena, R. R. y.; Rizzo, S.; Manzo, L.; Lupi, S. M.; Andersson, M. Nanofeatured titanium surfaces for dental implantology: biological effects, biocompatibility, and safety. J Nanomaterials. 2017, 2017, 18. |
| 92. |
Roy, P.; Berger, S.; Schmuki, P. TiO2 nanotubes: synthesis and applications. Angew Chem Int Ed Engl. 2011, 50, 2904-2939.
doi: 10.1002/anie.201001374 URL |
| 93. |
Lee, W.; Scholz, R.; Gösele, U. A continuous process for structurally well-defined Al2O3 nanotubes based on pulse anodization of aluminum. Nano Lett. 2008, 8, 2155-2160.
doi: 10.1021/nl080280x URL |
| 94. |
Minagar, S.; Berndt, C. C.; Wang, J.; Ivanova, E.; Wen, C. A review of the application of anodization for the fabrication of nanotubes on metal implant surfaces. Acta Biomater. 2012, 8, 2875-2888.
doi: 10.1016/j.actbio.2012.04.005 URL |
| 95. | Mohan, L.; Kar, S.; Nandhini, B.; Kumar, S. S. D.; Nagai, M.; Santra, T. S. Formation of nanostructures on magnesium alloy by anodization for potential biomedical applications. Mater Today Commun. 2020, 25, 101403. |
| 96. |
Rani, R. A.; Zoolfakar, A. S.; Ou, J. Z.; Kadir, R. A.; Nili, H.; Latham, K.; Sriram, S.; Bhaskaran, M.; Zhuiykov, S.; Kaner, R. B.; Kalantar-zadeh, K. Reduced impurity-driven defect states in anodized nanoporous Nb2O5: the possibility of improving performance of photoanodes. Chem Commun (Camb). 2013, 49, 6349-6351.
doi: 10.1039/c3cc42998a URL |
| 97. |
Dobosz, I. Influence of the anodization conditions and chemical treatment on the formation of alumina membranes with defined pore diameters. J Porous Mater. 2021, 28, 1011-1022.
doi: 10.1007/s10934-021-01052-w URL |
| 98. |
Quinn, J.; McFadden, R.; Chan, C. W.; Carson, L. Titanium for orthopedic applications: an overview of surface modification to improve biocompatibility and prevent bacterial biofilm formation. iScience. 2020, 23, 101745.
doi: 10.1016/j.isci.2020.101745 URL |
| 99. |
Mansoorianfar, M.; Tavoosi, M.; Mozafarinia, R.; Ghasemi, A.; Doostmohammadi, A. Preparation and characterization of TiO2 nanotube arrays on Ti6Al4V surface for enhancement of cell treatment. Surf Coat Technol. 2017, 321, 409-415.
doi: 10.1016/j.surfcoat.2017.05.016 URL |
| 100. |
Popat, K. C.; Leoni, L.; Grimes, C. A.; Desai, T. A. Influence of engineered titania nanotubular surfaces on bone cells. Biomaterials. 2007, 28, 3188-3197.
doi: 10.1016/j.biomaterials.2007.03.020 URL |
| 101. |
Li, Y.; Li, B.; Fu, X.; Li, J.; Li, C.; Li, H.; Li, H.; Liang, C.; Wang, H.; Zhou, L.; Xin, S. Anodic oxidation modification improve bioactivity and biocompatibility of titanium implant surface. J Hard Tissue Biol. 2013, 22, 351-358.
doi: 10.2485/jhtb.22.351 URL |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||