Biomaterials Translational ›› 2023, Vol. 4 ›› Issue (1): 18-26.doi: 10.12336/biomatertransl.2023.01.004
• REVIEW • Previous Articles Next Articles
Rob Jess1,2#, Tao Ling3,#,†, Yi Xiong3,*( ), Chris J. Wright1, Feihu Zhao1,2,*(
), Chris J. Wright1, Feihu Zhao1,2,*( )
)
Received:2022-12-27
Revised:2023-01-17
Accepted:2023-02-27
Online:2023-03-28
Published:2023-03-28
Contact:
* Feihu Zhao,About author:Feihu Zhao,feihu.zhao@swansea.ac.uk;Yi Xiong,xiongy3@sustech.edu.cn.Jess, R.; Ling, T.; Xiong, Y.; Wright, C. J.; Zhao, F. Mechanical environment for in vitro cartilage tissue engineering assisted by in silico models. Biomater Transl. 2023, 4(1), 18-26.
| Chondrocytes resource | Substrate material & stiffness | Biological responses | Reference |
|---|---|---|---|
| Porcine | Hydrogel: (i) 3.7 kPa (ii) 53 kPa | ? Type I and II collagen: no difference ? Cell number and ECM amount: (i) > (ii) | |
| Sheep | Hydrogel: (i) 5 kPa (ii) 10 kPa (iii) 20 kPa | ? Aggrecan, Col2a1, and Sox9 levels: (i) > (ii) or (iii) ? Organisation of actin cytoskeleton (co–related to loss of chondrocyte phenotype): (i) < (ii) or (iii) | |
| Bovine | Hydrogel: (i) 3.8 kPa (ii) 17.1 kPa (iii) 29.9 kPa | ? Round cell morphology and decreased actin cytoskeletal organisation: (iii) > (ii) > (i) ? sGAG/DNA, Col2a1 and aggrecan expressions: (iii) > (ii) > (i) | |
| Bovine | Hydrogel: (i) 1 kPa (ii) 15 kPa (iii) 30 kPa | ? sGAG and type II collagen expressions: (iii) > (ii) > (i) | |
| Murine | Type II collagen-coated PAM: 0.2 – 1.1 MPa | ? Proteoglycan deposition, and Sox9, Col2a1, and aggrecan mRNA expressions are greatest under the substrate stiffness of 0.5 MPa ? mRNA level: no difference | |
| Bovine | PAM gel coated with type I collagen: (i) 4 kPa (ii) 10 kPa (iii) 40 kPa (iv) 100 kPa | ? Differentiated chondrocyte phenotype: (i) > (ii) – (iv) ? Type II collagen and aggrecan genes: (i) > (ii) - (iv) ? Type I collagen: (i) < (ii) – (iv) | |
| Human | (i) PDMS: 4.8 MPa (ii) PS: 2.9 GPa | ? Sox9 and type II collagen expressions: (i) > (ii) ? Proliferation: no difference |
Table 1. Chondrocytes respond to substrates with different stiffness in cell culturing
| Chondrocytes resource | Substrate material & stiffness | Biological responses | Reference |
|---|---|---|---|
| Porcine | Hydrogel: (i) 3.7 kPa (ii) 53 kPa | ? Type I and II collagen: no difference ? Cell number and ECM amount: (i) > (ii) | |
| Sheep | Hydrogel: (i) 5 kPa (ii) 10 kPa (iii) 20 kPa | ? Aggrecan, Col2a1, and Sox9 levels: (i) > (ii) or (iii) ? Organisation of actin cytoskeleton (co–related to loss of chondrocyte phenotype): (i) < (ii) or (iii) | |
| Bovine | Hydrogel: (i) 3.8 kPa (ii) 17.1 kPa (iii) 29.9 kPa | ? Round cell morphology and decreased actin cytoskeletal organisation: (iii) > (ii) > (i) ? sGAG/DNA, Col2a1 and aggrecan expressions: (iii) > (ii) > (i) | |
| Bovine | Hydrogel: (i) 1 kPa (ii) 15 kPa (iii) 30 kPa | ? sGAG and type II collagen expressions: (iii) > (ii) > (i) | |
| Murine | Type II collagen-coated PAM: 0.2 – 1.1 MPa | ? Proteoglycan deposition, and Sox9, Col2a1, and aggrecan mRNA expressions are greatest under the substrate stiffness of 0.5 MPa ? mRNA level: no difference | |
| Bovine | PAM gel coated with type I collagen: (i) 4 kPa (ii) 10 kPa (iii) 40 kPa (iv) 100 kPa | ? Differentiated chondrocyte phenotype: (i) > (ii) – (iv) ? Type II collagen and aggrecan genes: (i) > (ii) - (iv) ? Type I collagen: (i) < (ii) – (iv) | |
| Human | (i) PDMS: 4.8 MPa (ii) PS: 2.9 GPa | ? Sox9 and type II collagen expressions: (i) > (ii) ? Proliferation: no difference |
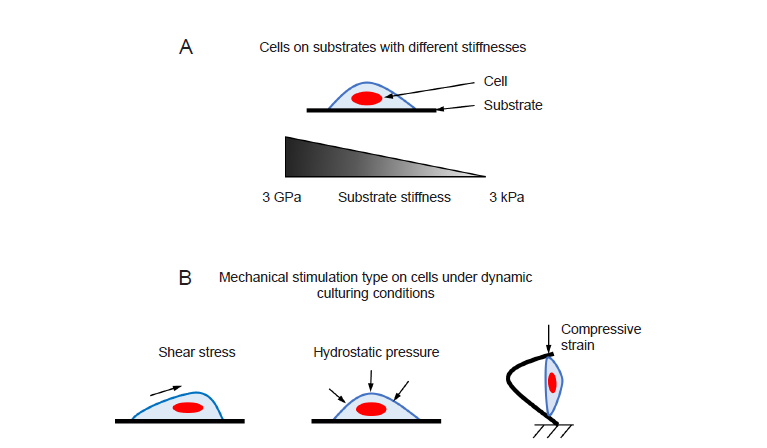
Figure 1. (A) In static culturing condition, cells are cultured on substrates with different stiffnesses. (B) Different types of mechanical stimulations have been applied to cells in dynamic culturing conditions.
| 1 | Jovic, T. H.; Jessop, Z. M.; Al-Sabah, A.; Whitaker, I. S. 12 - The clinical need for 3D printed tissue in reconstructive surgery. In 3D bioprinting for reconstructive surgery, Thomas, D. J.; Jessop, Z. M.; Whitaker, I. S., Eds.; Woodhead Publishing: 2018; pp 235-244. |
| 2 |
Francis, S. L.; Di Bella, C.; Wallace, G. G.; Choong, P. F. M. Cartilage tissue engineering using stem cells and bioprinting technology-barriers to clinical translation. Front Surg. 2018, 5, 70.
doi: 10.3389/fsurg.2018.00070 URL |
| 3 |
Kessler, M. W.; Grande, D. A. Tissue engineering and cartilage. Organogenesis. 2008, 4, 28-32.
doi: 10.4161/org.6116 URL |
| 4 | Fahy, N.; Alini, M.; Stoddart, M. J. Mechanical stimulation of mesenchymal stem cells: Implications for cartilage tissue engineering. J Orthop Res. 2018, 36, 52-63. |
| 5 | Schuh, E.; Hofmann, S.; Stok, K.; Notbohm, H.; Müller, R.; Rotter, N. Chondrocyte redifferentiation in 3D: the effect of adhesion site density and substrate elasticity. J Biomed Mater Res A. 2012, 100, 38-47. |
| 6 | Smith, R. L.; Rusk, S. F.; Ellison, B. E.; Wessells, P.; Tsuchiya, K.; Carter, D. R.; Caler, W. E.; Sandell, L. J.; Schurman, D. J. In vitro stimulation of articular chondrocyte mRNA and extracellular matrix synthesis by hydrostatic pressure. J Orthop Res. 1996, 14, 53-60. |
| 7 | O’Conor, C. J.; Case, N.; Guilak, F. Mechanical regulation of chondrogenesis. Stem Cell Res Ther. 2013, 4, 61. |
| 8 |
Ghasemi-Mobarakeh, L.; Prabhakaran, M. P.; Tian, L.; Shamirzaei-Jeshvaghani, E.; Dehghani, L.; Ramakrishna, S. Structural properties of scaffolds: Crucial parameters towards stem cells differentiation. World J Stem Cells. 2015, 7, 728-744.
doi: 10.4252/wjsc.v7.i4.728 URL |
| 9 |
Zhao, F.; Vaughan, T. J.; McNamara, L. M. Quantification of fluid shear stress in bone tissue engineering scaffolds with spherical and cubical pore architectures. Biomech Model Mechanobiol. 2016, 15, 561-577.
doi: 10.1007/s10237-015-0710-0 URL |
| 10 |
O’Brien, F. J. Biomaterials & scaffolds for tissue engineering. Mater Today. 2011, 14, 88-95.
doi: 10.1016/S1369-7021(11)70058-X URL |
| 11 |
Irawan, V.; Sung, T. C.; Higuchi, A.; Ikoma, T. Collagen scaffolds in cartilage tissue engineering and relevant approaches for future development. Tissue Eng Regen Med. 2018, 15, 673-697.
doi: 10.1007/s13770-018-0135-9 |
| 12 |
Zhao, F.; van Rietbergen, B.; Ito, K.; Hofmann, S. Flow rates in perfusion bioreactors to maximise mineralisation in bone tissue engineering in vitro. J Biomech. 2018, 79, 232-237.
doi: 10.1016/j.jbiomech.2018.08.004 URL |
| 13 |
Breuls, R. G.; Jiya, T. U.; Smit, T. H. Scaffold stiffness influences cell behavior: opportunities for skeletal tissue engineering. Open Orthop J. 2008, 2, 103-109.
doi: 10.2174/1874325000802010103 URL |
| 14 |
Olivares-Navarrete, R.; Lee, E. M.; Smith, K.; Hyzy, S. L.; Doroudi, M.; Williams, J. K.; Gall, K.; Boyan, B. D.; Schwartz, Z. Substrate stiffness controls osteoblastic and chondrocytic differentiation of mesenchymal stem cells without exogenous stimuli. PLoS One. 2017, 12, e0170312.
doi: 10.1371/journal.pone.0170312 URL |
| 15 |
Selig, M.; Lauer, J. C.; Hart, M. L.; Rolauffs, B. Mechanotransduction and stiffness-sensing: mechanisms and opportunities to control multiple molecular aspects of cell phenotype as a design cornerstone of cell-instructive biomaterials for articular cartilage repair. Int J Mol Sci. 2020, 21, 5399.
doi: 10.3390/ijms21155399 URL |
| 16 |
Jiang, C.; Sun, Z. M.; Zhu, D. C.; Guo, Q.; Xu, J. J.; Lin, J. H.; Chen, Z. X.; Wu, Y. S. Inhibition of Rac1 activity by NSC23766 prevents cartilage endplate degeneration via Wnt/β-catenin pathway. J Cell Mol Med. 2020, 24, 3582-3592.
doi: 10.1111/jcmm.v24.6 URL |
| 17 | Dobrokhotov, O.; Samsonov, M.; Sokabe, M.; Hirata, H. Mechanoregulation and pathology of YAP/TAZ via Hippo and non-Hippo mechanisms. Clin Transl Med. 2018, 7, 23. |
| 18 |
Sanz-Ramos, P.; Mora, G.; Vicente-Pascual, M.; Ochoa, I.; Alcaine, C.; Moreno, R.; Doblaré, M.; Izal-Azcárate, I. Response of sheep chondrocytes to changes in substrate stiffness from 2 to 20 Pa: effect of cell passaging. Connect Tissue Res. 2013, 54, 159-166.
doi: 10.3109/03008207.2012.762360 URL |
| 19 |
Li, X.; Chen, S.; Li, J.; Wang, X.; Zhang, J.; Kawazoe, N.; Chen, G. 3D culture of chondrocytes in gelatin hydrogels with different stiffness. Polymers (Basel). 2016, 8, 269.
doi: 10.3390/polym8080269 URL |
| 20 |
Bachmann, B.; Spitz, S.; Schädl, B.; Teuschl, A. H.; Redl, H.; Nürnberger, S.; Ertl, P. Stiffness matters: fine-tuned hydrogel elasticity alters chondrogenic redifferentiation. Front Bioeng Biotechnol. 2020, 8, 373.
doi: 10.3389/fbioe.2020.00373 URL |
| 21 |
Allen, J. L.; Cooke, M. E.; Alliston, T. ECM stiffness primes the TGFβ pathway to promote chondrocyte differentiation. Mol Biol Cell. 2012, 23, 3731-3742.
doi: 10.1091/mbc.e12-03-0172 URL |
| 22 |
Schuh, E.; Kramer, J.; Rohwedel, J.; Notbohm, H.; Müller, R.; Gutsmann, T.; Rotter, N. Effect of matrix elasticity on the maintenance of the chondrogenic phenotype. Tissue Eng Part A. 2010, 16, 1281-1290.
doi: 10.1089/ten.tea.2009.0614 URL |
| 23 |
Bergholt, N. L.; Foss, M.; Saeed, A.; Gadegaard, N.; Lysdahl, H.; Lind, M.; Foldager, C. B. Surface chemistry, substrate, and topography guide the behavior of human articular chondrocytes cultured in vitro. J Biomed Mater Res A. 2018, 106, 2805-2816.
doi: 10.1002/jbm.a.v106.11 URL |
| 24 |
Ronan, W.; Deshpande, V. S.; McMeeking, R. M.; McGarry, J. P. Cellular contractility and substrate elasticity: a numerical investigation of the actin cytoskeleton and cell adhesion. Biomech Model Mechanobiol. 2014, 13, 417-435.
doi: 10.1007/s10237-013-0506-z URL |
| 25 |
McEvoy, E.; Deshpande, V. S.; McGarry, P. Free energy analysis of cell spreading. J Mech Behav Biomed Mater. 2017, 74, 283-295.
doi: 10.1016/j.jmbbm.2017.06.006 URL |
| 26 |
Ristori, T.; Vigliotti, A.; Baaijens, F. P. T.; Loerakker, S.; Deshpande, V. S. Prediction of cell alignment on cyclically strained grooved substrates. Biophys J. 2016, 111, 2274-2285.
doi: 10.1016/j.bpj.2016.09.052 URL |
| 27 |
Ristori, T.; Notermans, T. M. W.; Foolen, J.; Kurniawan, N. A.; Bouten, C. V. C.; Baaijens, F. P. T.; Loerakker, S. Modelling the combined effects of collagen and cyclic strain on cellular orientation in collagenous tissues. Sci Rep. 2018, 8, 8518.
doi: 10.1038/s41598-018-26989-y |
| 28 |
Deshpande, V. S.; McMeeking, R. M.; Evans, A. G. A bio-chemo-mechanical model for cell contractility. Proc Natl Acad Sci U S A. 2006, 103, 14015-14020.
doi: 10.1073/pnas.0605837103 URL |
| 29 |
Ronan, W.; Pathak, A.; Deshpande, V. S.; McMeeking, R. M.; McGarry, J. P. Simulation of the mechanical response of cells on micropost substrates. J Biomech Eng. 2013, 135, 101012.
doi: 10.1115/1.4025114 URL |
| 30 |
Deshpande, V. S.; Mrksich, M.; McMeeking, R. M.; Evans, A. G. A bio-mechanical model for coupling cell contractility with focal adhesion formation. J Mech Phys Solids. 2008, 56, 1484-1510.
doi: 10.1016/j.jmps.2007.08.006 URL |
| 31 |
O’Reilly, A.; Kelly, D. J. A computational model of osteochondral defect repair following implantation of stem cell-laden multiphase scaffolds. Tissue Eng Part A. 2017, 23, 30-42.
doi: 10.1089/ten.tea.2016.0175 URL |
| 32 |
Burke, D. P.; Kelly, D. J. Substrate stiffness and oxygen as regulators of stem cell differentiation during skeletal tissue regeneration: a mechanobiological model. PLoS One. 2012, 7, e40737.
doi: 10.1371/journal.pone.0040737 URL |
| 33 |
Guo, T.; Yu, L.; Lim, C. G.; Goodley, A. S.; Xiao, X.; Placone, J. K.; Ferlin, K. M.; Nguyen, B. N.; Hsieh, A. H.; Fisher, J. P. Effect of dynamic culture and periodic compression on human mesenchymal stem cell proliferation and chondrogenesis. Ann Biomed Eng. 2016, 44, 2103-2113.
doi: 10.1007/s10439-015-1510-5 URL |
| 34 |
Hwang, N. S.; Zhang, C.; Hwang, Y. S.; Varghese, S. Mesenchymal stem cell differentiation and roles in regenerative medicine. Wiley Interdiscip Rev Syst Biol Med. 2009, 1, 97-106.
doi: 10.1002/wsbm.v1:1 URL |
| 35 |
Ravalli, S.; Szychlinska, M. A.; Lauretta, G.; Musumeci, G. New insights on mechanical stimulation of mesenchymal stem cells for cartilage regeneration. Appl Sci. 2020, 10, 2927.
doi: 10.3390/app10082927 URL |
| 36 |
Ouyang, X.; Xie, Y.; Wang, G. Mechanical stimulation promotes the proliferation and the cartilage phenotype of mesenchymal stem cells and chondrocytes co-cultured in vitro. Biomed Pharmacother. 2019, 117, 109146.
doi: 10.1016/j.biopha.2019.109146 URL |
| 37 |
Nebelung, S.; Gavenis, K.; Rath, B.; Tingart, M.; Ladenburger, A.; Stoffel, M.; Zhou, B.; Mueller-Rath, R. Continuous cyclic compressive loading modulates biological and mechanical properties of collagen hydrogels seeded with human chondrocytes. Biorheology. 2011, 48, 247-261.
doi: 10.3233/BIR-2012-0597 URL |
| 38 |
Shahin, K.; Doran, P. M. Tissue engineering of cartilage using a mechanobioreactor exerting simultaneous mechanical shear and compression to simulate the rolling action of articular joints. Biotechnol Bioeng. 2012, 109, 1060-1073.
doi: 10.1002/bit.24372 URL |
| 39 |
Engstrøm, A.; Gillesberg, F. S.; Bay Jensen, A. C.; Karsdal, M. A.; Thudium, C. S. Dynamic compression inhibits cytokine-mediated type II collagen degradation. Osteoarthr Cartil Open. 2022, 4, 100292.
doi: 10.1016/j.ocarto.2022.100292 URL |
| 40 |
Sawatjui, N.; Limpaiboon, T.; Schrobback, K.; Klein, T. Biomimetic scaffolds and dynamic compression enhance the properties of chondrocyte- and MSC-based tissue-engineered cartilage. J Tissue Eng Regen Med. 2018, 12, 1220-1229.
doi: 10.1002/term.v12.5 URL |
| 41 |
Grogan, S. P.; Sovani, S.; Pauli, C.; Chen, J.; Hartmann, A.; Colwell, C. W., Jr.; Lotz, M. K.; D’Lima, D. D. Effects of perfusion and dynamic loading on human neocartilage formation in alginate hydrogels. Tissue Eng Part A. 2012, 18, 1784-1792.
doi: 10.1089/ten.tea.2011.0506 URL |
| 42 |
Engstrøm, A.; Gillesberg, F. S.; Groen, S. S.; Frederiksen, P.; Bay-Jensen, A. C.; Karsdal, M. A.; Thudium, C. S. Intermittent dynamic compression confers anabolic effects in articular cartilage. Appl Sci. 2021, 11, 7469.
doi: 10.3390/app11167469 URL |
| 43 |
Capuana, E.; Marino, D.; Di Gesù, R.; La Carrubba, V.; Brucato, V.; Tuan, R. S.; Gottardi, R. A high-throughput mechanical activator for cartilage engineering enables rapid screening of in vitro response of tissue models to physiological and supra-physiological loads. Cells Tissues Organs. 2022, 211, 670-688.
doi: 10.1159/000514985 URL |
| 44 |
Vetsch, J. R.; Betts, D. C.; Müller, R.; Hofmann, S. Flow velocity-driven differentiation of human mesenchymal stromal cells in silk fibroin scaffolds: A combined experimental and computational approach. PLoS One. 2017, 12, e0180781.
doi: 10.1371/journal.pone.0180781 URL |
| 45 |
Jin, M.; Frank, E. H.; Quinn, T. M.; Hunziker, E. B.; Grodzinsky, A. J. Tissue shear deformation stimulates proteoglycan and protein biosynthesis in bovine cartilage explants. Arch Biochem Biophys. 2001, 395, 41-48.
doi: 10.1006/abbi.2001.2543 URL |
| 46 |
Waldman, S. D.; Spiteri, C. G.; Grynpas, M. D.; Pilliar, R. M.; Kandel, R. A. Long-term intermittent shear deformation improves the quality of cartilaginous tissue formed in vitro. J Orthop Res. 2003, 21, 590-596.
doi: 10.1016/S0736-0266(03)00009-3 URL |
| 47 |
Gooch, K. J.; Kwon, J. H.; Blunk, T.; Langer, R.; Freed, L. E.; Vunjak-Novakovic, G. Effects of mixing intensity on tissue-engineered cartilage. Biotechnol Bioeng. 2001, 72, 402-407.
doi: 10.1002/(ISSN)1097-0290 URL |
| 48 |
Smith, R. L.; Donlon, B. S.; Gupta, M. K.; Mohtai, M.; Das, P.; Carter, D. R.; Cooke, J.; Gibbons, G.; Hutchinson, N.; Schurman, D. J. Effects of fluid-induced shear on articular chondrocyte morphology and metabolism in vitro. J Orthop Res. 1995, 13, 824-831.
doi: 10.1002/(ISSN)1554-527X URL |
| 49 | Akmal, M.; Anand, A.; Anand, B.; Wiseman, M.; Goodship, A. E.; Bentley, G. The culture of articular chondrocytes in hydrogel constructs within a bioreactor enhances cell proliferation and matrix synthesis. J Bone Joint Surg Br. 2006, 88, 544-553. |
| 50 |
Nazempour, A.; Quisenberry, C. R.; Abu-Lail, N. I.; Van Wie, B. J. Combined effects of oscillating hydrostatic pressure, perfusion and encapsulation in a novel bioreactor for enhancing extracellular matrix synthesis by bovine chondrocytes. Cell Tissue Res. 2017, 370, 179-193.
doi: 10.1007/s00441-017-2651-7 |
| 51 | Scherer, K.; Schünke, M.; Sellckau, R.; Hassenpflug, J.; Kurz, B. The influence of oxygen and hydrostatic pressure on articular chondrocytes and adherent bone marrow cells in vitro. Biorheology. 2004, 41, 323-333. |
| 52 |
Ikenoue, T.; Trindade, M. C.; Lee, M. S.; Lin, E. Y.; Schurman, D. J.; Goodman, S. B.; Smith, R. L. Mechanoregulation of human articular chondrocyte aggrecan and type II collagen expression by intermittent hydrostatic pressure in vitro. J Orthop Res. 2003, 21, 110-116.
doi: 10.1016/S0736-0266(02)00091-8 URL |
| 53 |
Correia, C.; Pereira, A. L.; Duarte, A. R.; Frias, A. M.; Pedro, A. J.; Oliveira, J. T.; Sousa, R. A.; Reis, R. L. Dynamic culturing of cartilage tissue: the significance of hydrostatic pressure. Tissue Eng Part A. 2012, 18, 1979-1991.
doi: 10.1089/ten.tea.2012.0083 URL |
| 54 |
Olivares, A. L.; Marsal, E.; Planell, J. A.; Lacroix, D. Finite element study of scaffold architecture design and culture conditions for tissue engineering. Biomaterials. 2009, 30, 6142-6149.
doi: 10.1016/j.biomaterials.2009.07.041 URL |
| 55 | Melke, J.; Zhao, F.; van Rietbergen, B.; Ito, K.; Hofmann, S. Localisation of mineralised tissue in a complex spinner flask environment correlates with predicted wall shear stress level localisation. Eur Cell Mater. 2018, 36, 57-68. |
| 56 | Zhao, F.; Lacroix, D.; Ito, K.; van Rietbergen, B.; Hofmann, S. Changes in scaffold porosity during bone tissue engineering in perfusion bioreactors considerably affect cellular mechanical stimulation for mineralization. Bone Rep. 2020, 12, 100265. |
| 57 |
Papantoniou, I.; Guyot, Y.; Sonnaert, M.; Kerckhofs, G.; Luyten, F. P.; Geris, L.; Schrooten, J. Spatial optimization in perfusion bioreactors improves bone tissue-engineered construct quality attributes. Biotechnol Bioeng. 2014, 111, 2560-2570.
doi: 10.1002/bit.v111.12 URL |
| 58 |
Guyot, Y.; Papantoniou, I.; Luyten, F. P.; Geris, L. Coupling curvature-dependent and shear stress-stimulated neotissue growth in dynamic bioreactor cultures: a 3D computational model of a complete scaffold. Biomech Model Mechanobiol. 2016, 15, 169-180.
doi: 10.1007/s10237-015-0753-2 URL |
| 59 |
Guyot, Y.; Luyten, F. P.; Schrooten, J.; Papantoniou, I.; Geris, L. A three-dimensional computational fluid dynamics model of shear stress distribution during neotissue growth in a perfusion bioreactor. Biotechnol Bioeng. 2015, 112, 2591-2600.
doi: 10.1002/bit.25672 URL |
| 60 |
Shakhawath Hossain, M.; Bergstrom, D. J.; Chen, X. B. A mathematical model and computational framework for three-dimensional chondrocyte cell growth in a porous tissue scaffold placed inside a bi-directional flow perfusion bioreactor. Biotechnol Bioeng. 2015, 112, 2601-2610.
doi: 10.1002/bit.25678 URL |
| 61 |
Nava, M. M.; Raimondi, M. T.; Pietrabissa, R. A multiphysics 3D model of tissue growth under interstitial perfusion in a tissue-engineering bioreactor. Biomech Model Mechanobiol. 2013, 12, 1169-1179.
doi: 10.1007/s10237-013-0473-4 URL |
| 62 |
Brunelli, M.; Perrault, C. M.; Lacroix, D. Short bursts of cyclic mechanical compression modulate tissue formation in a 3D hybrid scaffold. J Mech Behav Biomed Mater. 2017, 71, 165-174.
doi: 10.1016/j.jmbbm.2017.03.008 URL |
| 63 |
Zhao, F.; Mc Garrigle, M. J.; Vaughan, T. J.; McNamara, L. M. In silico study of bone tissue regeneration in an idealised porous hydrogel scaffold using a mechano-regulation algorithm. Biomech Model Mechanobiol. 2018, 17, 5-18.
doi: 10.1007/s10237-017-0941-3 URL |
| 64 |
Wang, L.; Shi, Q.; Cai, Y.; Chen, Q.; Guo, X.; Li, Z. Mechanical-chemical coupled modeling of bone regeneration within a biodegradable polymer scaffold loaded with VEGF. Biomech Model Mechanobiol. 2020, 19, 2285-2306.
doi: 10.1007/s10237-020-01339-y |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||