Biomaterials Translational ›› 2023, Vol. 4 ›› Issue (1): 51-61.doi: 10.12336/biomatertransl.2023.01.007
• RESEARCH ARTICLE • Previous Articles
Xiaodan Wang1,2,*( ), Qinmei Li1, Huawei Yang2,3,*(
), Qinmei Li1, Huawei Yang2,3,*( )
)
Received:2023-01-05
Revised:2023-02-17
Accepted:2023-03-09
Online:2023-03-29
Published:2023-03-28
Contact:
* Xiaodan Wang,About author:Xiaodan Wang,wxd@hygeamed.com;Wang, X.; Li, Q.; Yang, H. Effect of radiation sterilisation on the structure and antibacterial properties of antimicrobial peptides. Biomater Transl. 2023, 4(1), 51-61.
| Sample | Arm No. | Designed Lys content (%) | Actual Lys contenta (%) | Mn designed molecular weight (Da) | Mn determined molecular weight (Da)b | ? determined molecular weightb |
|---|---|---|---|---|---|---|
| LP1 | 1 | 70 | 70.8 | 6219.9 | 6200 | 1.07 |
| LP2 | 1 | 60 | 61.4 | 5976.6 | 6000 | 1.14 |
| LP3 | 1 | 50 | 51.2 | 5733.2 | 5700 | 1.24 |
| G2–P1 | 16 | 70 | 71 | 101155.6 | 111200 | 1.05 |
| G2–P2 | 16 | 60 | 60 | 97262.3 | 97300 | 1.08 |
| G2–P3 | 16 | 50 | 48.2 | 93369 | 93400 | 1.12 |
| LV1 | 1 | 70 | 68.6 | 5787.5 | 5800 | 1.11 |
| LV2 | 1 | 60 | 59.3 | 5400 | 5500 | 1.09 |
| LV3 | 1 | 50 | 49 | 5012.5 | 5000 | 1.18 |
| G2–V1 | 16 | 70 | 72.3 | 94236.4 | 94300 | 1.02 |
| G2–V2 | 16 | 60 | 59.1 | 88036.7 | 88000 | 1.07 |
| G2–V3 | 16 | 50 | 50 | 81837 | 81900 | 1.14 |
| LL30 | 1 | 100 | 100 | 6949.9 | 6900 | 1.05 |
| G2–L30 | 16 | 100 | 100 | 112835.4 | 113000 | 1.13 |
Table 1. Detailed molecular parameters of the antimicrobial peptides
| Sample | Arm No. | Designed Lys content (%) | Actual Lys contenta (%) | Mn designed molecular weight (Da) | Mn determined molecular weight (Da)b | ? determined molecular weightb |
|---|---|---|---|---|---|---|
| LP1 | 1 | 70 | 70.8 | 6219.9 | 6200 | 1.07 |
| LP2 | 1 | 60 | 61.4 | 5976.6 | 6000 | 1.14 |
| LP3 | 1 | 50 | 51.2 | 5733.2 | 5700 | 1.24 |
| G2–P1 | 16 | 70 | 71 | 101155.6 | 111200 | 1.05 |
| G2–P2 | 16 | 60 | 60 | 97262.3 | 97300 | 1.08 |
| G2–P3 | 16 | 50 | 48.2 | 93369 | 93400 | 1.12 |
| LV1 | 1 | 70 | 68.6 | 5787.5 | 5800 | 1.11 |
| LV2 | 1 | 60 | 59.3 | 5400 | 5500 | 1.09 |
| LV3 | 1 | 50 | 49 | 5012.5 | 5000 | 1.18 |
| G2–V1 | 16 | 70 | 72.3 | 94236.4 | 94300 | 1.02 |
| G2–V2 | 16 | 60 | 59.1 | 88036.7 | 88000 | 1.07 |
| G2–V3 | 16 | 50 | 50 | 81837 | 81900 | 1.14 |
| LL30 | 1 | 100 | 100 | 6949.9 | 6900 | 1.05 |
| G2–L30 | 16 | 100 | 100 | 112835.4 | 113000 | 1.13 |
| Sample | Before radiation sterilisation | After radiation sterilisation | |||
|---|---|---|---|---|---|
| PBS buffer | PBS buffer | PBS/AcOH | HFP | ||
| LP1 | + | + | + | + | |
| LP2 | + | + | + | + | |
| LP3 | + | + | + | + | |
| G2–P1 | + | – | ± | + | |
| G2–P2 | + | – | – | + | |
| G2–P3 | + | – | – | + | |
| LV1 | + | + | + | + | |
| LV2 | + | + | + | + | |
| LV3 | + | + | + | + | |
| G2–V1 | + | – | – | ± | |
| G2–V2 | + | – | – | ± | |
| G2–V3 | + | – | – | ± | |
| LL30 | + | + | + | + | |
| G2–L30 | + | – | ± | + | |
Table 2. Solubility of the antimicrobial peptides before and after radiation sterilisation
| Sample | Before radiation sterilisation | After radiation sterilisation | |||
|---|---|---|---|---|---|
| PBS buffer | PBS buffer | PBS/AcOH | HFP | ||
| LP1 | + | + | + | + | |
| LP2 | + | + | + | + | |
| LP3 | + | + | + | + | |
| G2–P1 | + | – | ± | + | |
| G2–P2 | + | – | – | + | |
| G2–P3 | + | – | – | + | |
| LV1 | + | + | + | + | |
| LV2 | + | + | + | + | |
| LV3 | + | + | + | + | |
| G2–V1 | + | – | – | ± | |
| G2–V2 | + | – | – | ± | |
| G2–V3 | + | – | – | ± | |
| LL30 | + | + | + | + | |
| G2–L30 | + | – | ± | + | |
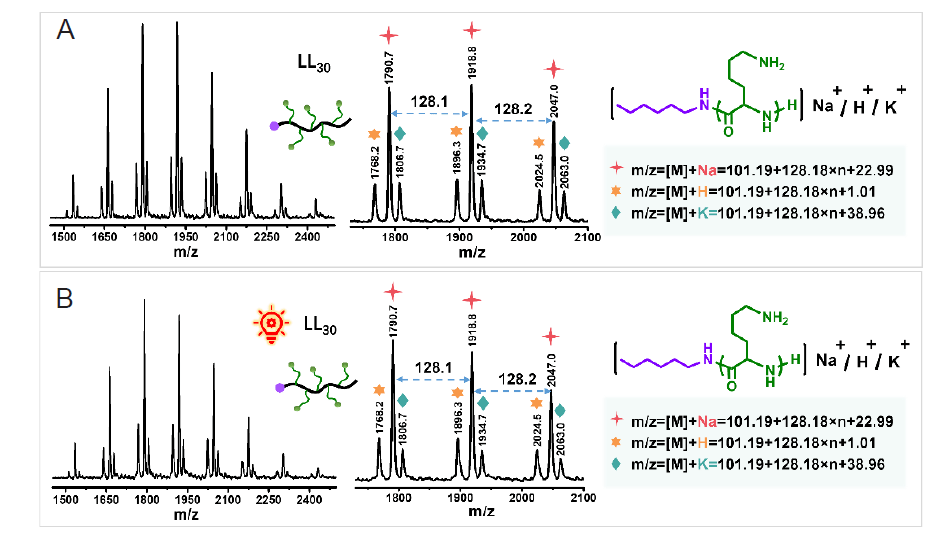
Figure 3. The spectra of LL30 before (A) and after (B) irradiation under matrix–assisted laser desorption/ionisation time of flight (MALDI–TOF) mass spectrometry.
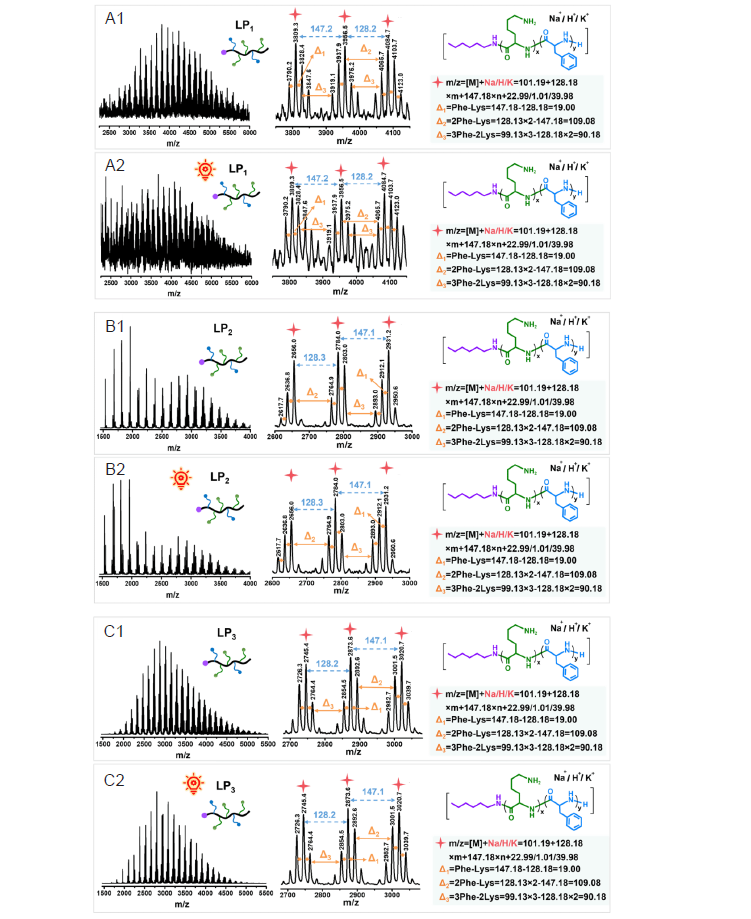
Figure 4. The spectra of LP1–LP3 before (A1–C1) and after (A2–C2) irradiation under matrix–assisted laser desorption/ionisation time of flight (MALDI–TOF) mass spectrometry.
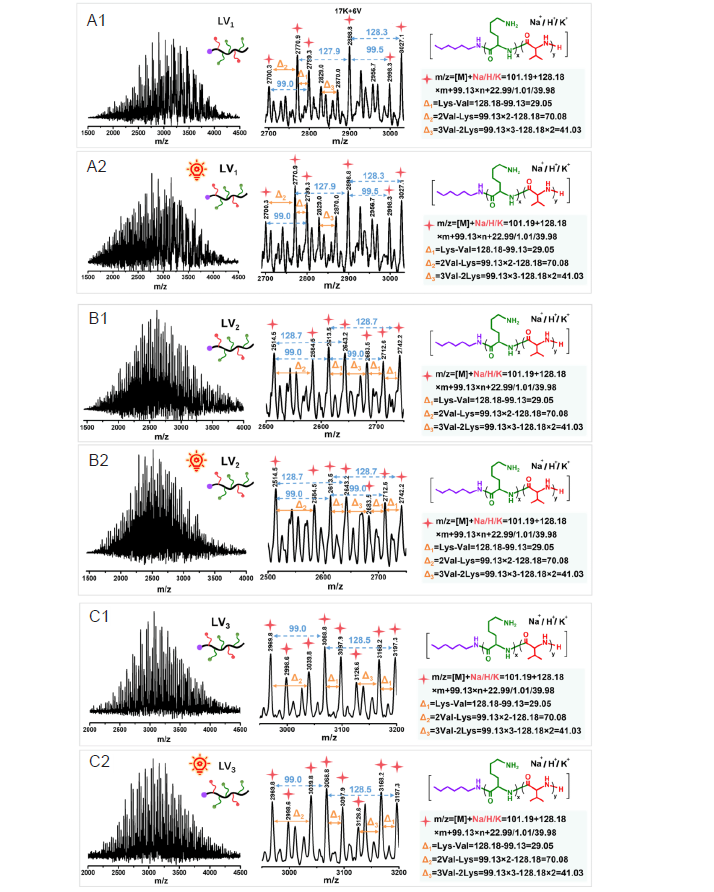
Figure 5. The spectra of LV1–LV3 before (A1–C1) and after (A2–C2) irradiation under matrix–assisted laser desorption/ionisation time of flight (MALDI–TOF) mass spectrometry.
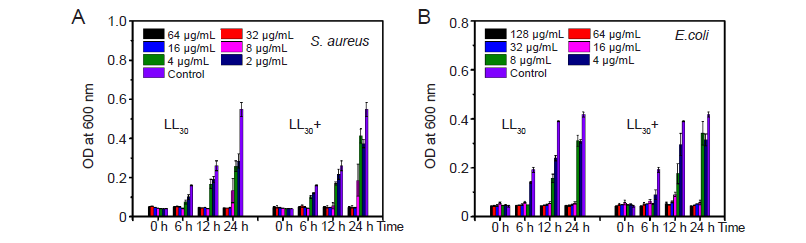
Figure 6. The minimum inhibitory concentrations of LL30 before and after irradiation. (A) Staphylococcus aureus (S. aureus). (B) Escherichia coli (E. coli). OD: optical density.
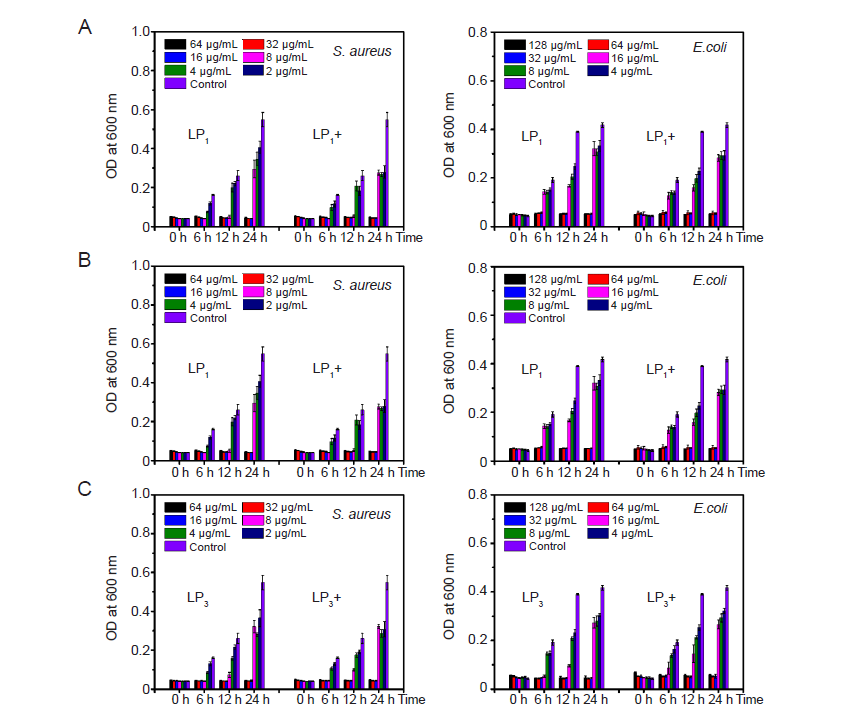
Figure 7. The minimum inhibitory concentrations of LP1–LP3 (A–C) before and after irradiation. E. coli: Escherichia coli; OD: optical density; S. aureus: Staphylococcus aureus.
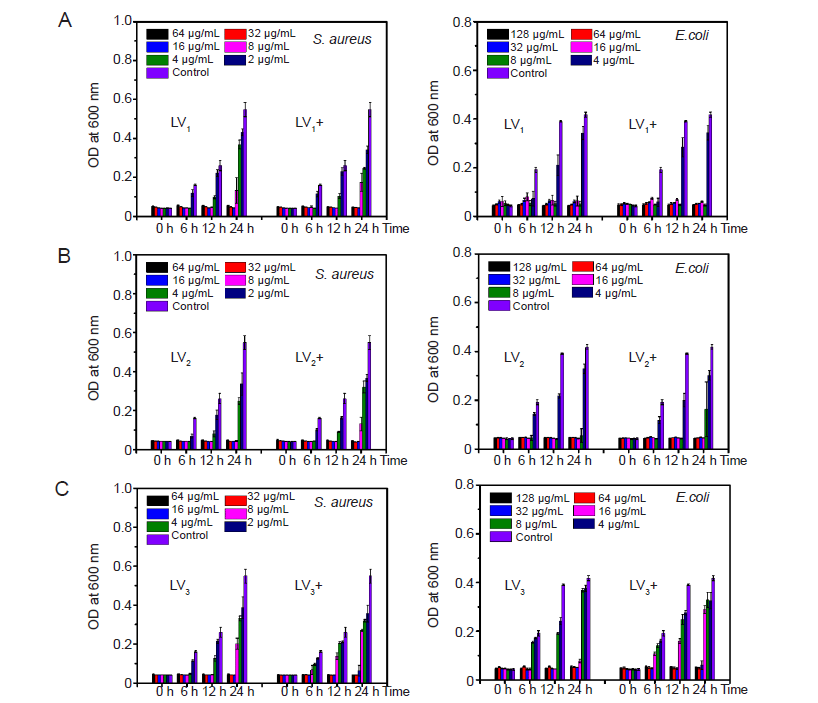
Figure 8. The minimum inhibitory concentrations of LV1–LV3 (A–C) before and after irradiation. E. coli: Escherichia coli; OD: optical density; S. aureus: Staphylococcus aureus.
| S. aureus | E. coli | |
|---|---|---|
| LL30 | ||
| Before irradiation | 16 | 16 |
| After irradiation | 16 | 16 |
| LP2 | ||
| Before irradiation | 16 | 32 |
| After irradiation | 16 | 32 |
| LP2 | ||
| Before irradiation | 16 | 32 |
| After irradiation | 16 | 32 |
| LP3 | ||
| Before irradiation | 16 | 32 |
| After irradiation | 16 | 32 |
| LV1 | ||
| Before irradiation | 16 | 8 |
| After irradiation | 16 | 8 |
| LV2 | ||
| Before irradiation | 8 | 16 |
| After irradiation | 16 | 16 |
| LV3 | ||
| Before irradiation | 16 | 32 |
| After irradiation | 32 | 64 |
Table 3. The minimum inhibitory concentrations (μg/mL) of antimicrobial peptides against S. aureus and E. coli before and after irradiation
| S. aureus | E. coli | |
|---|---|---|
| LL30 | ||
| Before irradiation | 16 | 16 |
| After irradiation | 16 | 16 |
| LP2 | ||
| Before irradiation | 16 | 32 |
| After irradiation | 16 | 32 |
| LP2 | ||
| Before irradiation | 16 | 32 |
| After irradiation | 16 | 32 |
| LP3 | ||
| Before irradiation | 16 | 32 |
| After irradiation | 16 | 32 |
| LV1 | ||
| Before irradiation | 16 | 8 |
| After irradiation | 16 | 8 |
| LV2 | ||
| Before irradiation | 8 | 16 |
| After irradiation | 16 | 16 |
| LV3 | ||
| Before irradiation | 16 | 32 |
| After irradiation | 32 | 64 |
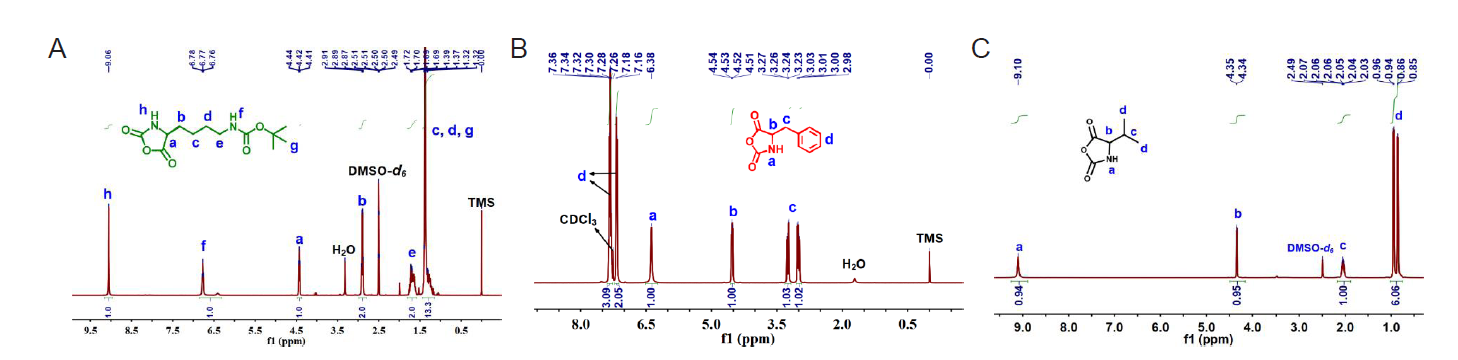
Figure 1. 1H nuclear magnetic resonance spectroscopy of Nε–tert–butyloxycarbonyl–L–lysine N–carboxyanhydride (Boc–L–Lys–NCA) (A), D–phenylalanine N–carboxyanhydride (D–Phe–NCA) (B) and D, L–valine N–carboxyanhydride (D, L–Val–NCA) (C).
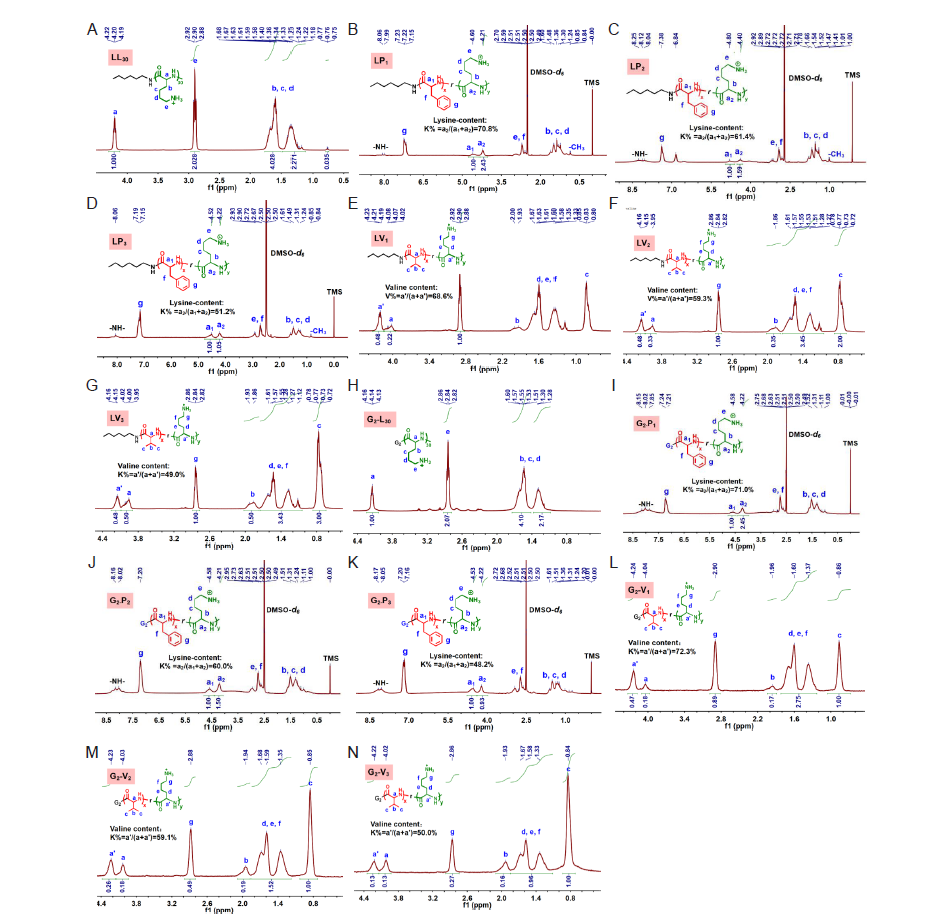
Figure 2. 1H nuclear magnetic resonance spectroscopy of LL30 (A), LP1 (B), LP2 (C), LP3 (D), LV1 (E), LV2 (F), LV3 (G), G2–L30 (H), G2–P1 (I), G2–P2 (J), G2–P3 (K), G2–L30 (L), G2–L30 (M) and G2–L30 (N).
| 1 |
Deusenbery, C.; Wang, Y.; Shukla, A. Recent innovations in bacterial infection detection and treatment. ACS Infect Dis. 2021, 7, 695-720.
doi: 10.1021/acsinfecdis.0c00890 URL |
| 2 |
Yu, Q.; Wu, Z.; Chen, H. Dual-function antibacterial surfaces for biomedical applications. Acta Biomater. 2015, 16, 1-13.
doi: 10.1016/j.actbio.2015.01.018 URL |
| 3 | Bai, D.; Chen, J.; Li, P.; Huang, W. Perspectives on biomaterial-associated infection: pathogenesis and current clinical demands. In Racing for the surface: pathogenesis of implant infection and advanced antimicrobial strategies, Li, B.; Moriarty, T. F.; Webster, T.; Xing, M., eds.; Springer International Publishing: Cham, 2020; pp 75-93. |
| 4 | Lai, N. M.; Chaiyakunapruk, N.; Lai, N. A.; O’Riordan, E.; Pau, W. S.; Saint, S. Catheter impregnation, coating or bonding for reducing central venous catheter-related infections in adults. Cochrane Database Syst Rev. 2016, 3, CD007878. |
| 5 |
Cloutier, M.; Mantovani, D.; Rosei, F. Antibacterial coatings: challenges, perspectives, and opportunities. Trends Biotechnol. 2015, 33, 637-652.
doi: 10.1016/j.tibtech.2015.09.002 URL |
| 6 |
Campoccia, D.; Montanaro, L.; Arciola, C. R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials. 2013, 34, 8533-8554.
doi: 10.1016/j.biomaterials.2013.07.089 URL |
| 7 |
Chen, Y. M.; Dai, A. P.; Shi, Y.; Liu, Z. J.; Gong, M. F.; Yin, X. B. Effectiveness of silver-impregnated central venous catheters for preventing catheter-related blood stream infections: a meta-analysis. Int J Infect Dis. 2014, 29, 279-286.
doi: 10.1016/j.ijid.2014.09.018 URL |
| 8 |
Rupp, M. E.; Fitzgerald, T.; Marion, N.; Helget, V.; Puumala, S.; Anderson, J. R.; Fey, P. D. Effect of silver-coated urinary catheters: efficacy, cost-effectiveness, and antimicrobial resistance. Am J Infect Control. 2004, 32, 445-450.
doi: 10.1016/j.ajic.2004.05.002 URL |
| 9 |
Singh, R.; Hokenstad, E. D.; Wiest, S. R.; Kim-Fine, S.; Weaver, A. L.; McGree, M. E.; Klingele, C. J.; Trabuco, E. C.; Gebhart, J. B. Randomized controlled trial of silver-alloy-impregnated suprapubic catheters versus standard suprapubic catheters in assessing urinary tract infection rates in urogynecology patients. Int Urogynecol J. 2019, 30, 779-787.
doi: 10.1007/s00192-018-3726-z |
| 10 |
Saint, S.; Elmore, J. G.; Sullivan, S. D.; Emerson, S. S.; Koepsell, T. D. The efficacy of silver alloy-coated urinary catheters in preventing urinary tract infection: a meta-analysis. Am J Med. 1998, 105, 236-241.
doi: 10.1016/S0002-9343(98)00240-X URL |
| 11 |
Safdar, N.; O’Horo, J. C.; Ghufran, A.; Bearden, A.; Didier, M. E.; Chateau, D.; Maki, D. G. Chlorhexidine-impregnated dressing for prevention of catheter-related bloodstream infection: a meta-analysis. Crit Care Med. 2014, 42, 1703-1713.
doi: 10.1097/CCM.0000000000000319 URL |
| 12 |
Lorente, L. Review: chlorhexidine-impregnated dressings reduce risk of colonisation of central venous catheters and risk of catheter-related bloodstream infection. Evid Based Nurs. 2015, 18, 91.
doi: 10.1136/eb-2014-101959 URL |
| 13 |
Srisang, S.; Nasongkla, N. Spray coating of foley urinary catheter by chlorhexidine-loadedpoly(ε-caprolactone) nanospheres: effect of lyoprotectants, characteristics, and antibacterial activity evaluation. Pharm Dev Technol. 2019, 24, 402-409.
doi: 10.1080/10837450.2018.1502317 URL |
| 14 |
Bayston, R.; Fisher, L. E.; Weber, K. An antimicrobial modified silicone peritoneal catheter with activity against both Gram-positive and Gram-negative bacteria. Biomaterials. 2009, 30, 3167-3173.
doi: 10.1016/j.biomaterials.2009.02.028 URL |
| 15 |
Luther, E. M.; Schmidt, M. M.; Diendorf, J.; Epple, M.; Dringen, R. Upregulation of metallothioneins after exposure of cultured primary astrocytes to silver nanoparticles. Neurochem Res. 2012, 37, 1639-1648.
doi: 10.1007/s11064-012-0767-4 URL |
| 16 |
Blair, J. M.; Webber, M. A.; Baylay, A. J.; Ogbolu, D. O.; Piddock, L. J. Molecular mechanisms of antibiotic resistance. Nat Rev Microbiol. 2015, 13, 42-51.
doi: 10.1038/nrmicro3380 |
| 17 |
Tan, P.; Fu, H.; Ma, X. Design, optimization, and nanotechnology of antimicrobial peptides: From exploration to applications. Nano Today. 2021, 39, 101229.
doi: 10.1016/j.nantod.2021.101229 URL |
| 18 |
Rasines Mazo, A.; Allison-Logan, S.; Karimi, F.; Chan, N. J.; Qiu, W.; Duan, W.; O’Brien-Simpson, N. M.; Qiao, G. G. Ring opening polymerization of α-amino acids: advances in synthesis, architecture and applications of polypeptides and their hybrids. Chem Soc Rev. 2020, 49, 4737-4834.
doi: 10.1039/C9CS00738E URL |
| 19 |
Luong, H. X.; Thanh, T. T.; Tran, T. H. Antimicrobial peptides - advances in development of therapeutic applications. Life Sci. 2020, 260, 118407.
doi: 10.1016/j.lfs.2020.118407 URL |
| 20 |
Hancock, R. E.; Sahl, H. G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol. 2006, 24, 1551-1557.
doi: 10.1038/nbt1267 |
| 21 |
Ageitos, J. M.; Sánchez-Pérez, A.; Calo-Mata, P.; Villa, T. G. Antimicrobial peptides (AMPs): Ancient compounds that represent novel weapons in the fight against bacteria. Biochem Pharmacol. 2017, 133, 117-138.
doi: 10.1016/j.bcp.2016.09.018 URL |
| 22 |
Mowery, B. P.; Lee, S. E.; Kissounko, D. A.; Epand, R. F.; Epand, R. M.; Weisblum, B.; Stahl, S. S.; Gellman, S. H. Mimicry of antimicrobial host-defense peptides by random copolymers. J Am Chem Soc. 2007, 129, 15474-15476.
doi: 10.1021/ja077288d URL |
| 23 |
Deming, T. J. Synthesis of side-chain modified polypeptides. Chem Rev. 2016, 116, 786-808.
doi: 10.1021/acs.chemrev.5b00292 URL |
| 24 |
Wang, X.; Yang, F.; Yang, H.; Zhang, X.; Tang, H.; Luan, S. Preparation of antibacterial polypeptides with different topologies and their antibacterial properties. Biomater Sci. 2022, 10, 834-845.
doi: 10.1039/D1BM01620B URL |
| 25 | Raza, S.; Iqbal, Y.; Ullah, I.; Mubarak, M. S.; Hameed, M. U.; Raza, M. Effects of gamma irradiation on the physico-chemical and biological properties of levofloxacin. Pak J Pharm Sci. 2018, 31, 181-186. |
| 26 |
Gomes, A. D.; de Oliveira, A. A. R.; Houmard, M.; Nunes, E. H. M. Gamma sterilization of collagen/hydroxyapatite composites: Validation and radiation effects. Appl Radiat Isot. 2021, 174, 109758.
doi: 10.1016/j.apradiso.2021.109758 URL |
| 27 |
Domańska, I. M.; Oledzka, E.; Sobczak, M. Sterilization process of polyester based anticancer-drug delivery systems. Int J Pharm. 2020, 587, 119663.
doi: 10.1016/j.ijpharm.2020.119663 URL |
| 28 |
Maturana, P.; Gonçalves, S.; Martinez, M.; Espeche, J. C.; Santos, N. C.; Semorile, L.; Maffia, P. C.; Hollmann, A. Interactions of “de novo” designed peptides with bacterial membranes: Implications in the antimicrobial activity. Biochim Biophys Acta Biomembr. 2020, 1862, 183443.
doi: 10.1016/j.bbamem.2020.183443 URL |
| 29 |
Yang, Z.; Xi, Y.; Bai, J.; Jiang, Z.; Wang, S.; Zhang, H.; Dai, W.; Chen, C.; Gou, Z.; Yang, G.; Gao, C. Covalent grafting of hyperbranched poly-L-lysine on Ti-based implants achieves dual functions of antibacteria and promoted osteointegration in vivo. Biomaterials. 2021, 269, 120534.
doi: 10.1016/j.biomaterials.2020.120534 URL |
| 30 |
Bargh, S.; Silindir-Gunay, M.; Ozer, A. Y.; Colak, S.; Kutlu, B.; Nohutcu, R. The effects of gamma and microwave sterilization on periodontological grafts. Chemical Physics Impact. 2021, 3, 100046.
doi: 10.1016/j.chphi.2021.100046 URL |
| 31 | Sharma, A.; Anup, N.; Tekade, R. K. Chapter 21 - Achieving sterility in biomedical and pharmaceutical products (part-II): radiation sterilization. In The future of pharmaceutical product development and research, Tekade, R. K., ed. Academic Press: 2020; pp 789-848. |
| 32 |
Nguyen, H.; Cassady, A. I.; Bennett, M. B.; Gineyts, E.; Wu, A.; Morgan, D. A.; Forwood, M. R. Reducing the radiation sterilization dose improves mechanical and biological quality while retaining sterility assurance levels of bone allografts. Bone. 2013, 57, 194-200.
doi: 10.1016/j.bone.2013.07.036 URL |
| 33 |
De Guzman, Z. M.; Cervancia, C. R.; Dimasuay, K. G.; Tolentino, M. M.; Abrera, G. B.; Cobar, M. L.; Fajardo, A. C., Jr.; Sabino, N. G.; Manila-Fajardo, A. C.; Feliciano, C. P. Radiation inactivation of Paenibacillus larvae and sterilization of American Foul Brood (AFB) infected hives using Co-60 gamma rays. Appl Radiat Isot. 2011, 69, 1374-1379.
doi: 10.1016/j.apradiso.2011.05.032 URL |
| 34 |
B.G. Porto, K. M.; Napolitano, C. M.; Borrely, S. I. Gamma radiation effects in packaging for sterilization of health products and their constituents paper and plastic film. Radiat Phys Chem. 2018, 142, 23-28.
doi: 10.1016/j.radphyschem.2016.12.019 URL |
| 35 |
Liu, H.; Zhang, X.; Zhao, Z.; Yang, F.; Xue, R.; Yin, L.; Song, Z.; Cheng, J.; Luan, S.; Tang, H. Efficient synthesis and excellent antimicrobial activity of star-shaped cationic polypeptides with improved biocompatibility. Biomater Sci. 2021, 9, 2721-2731.
doi: 10.1039/D0BM02151B URL |
| 36 |
Wiegand, I.; Hilpert, K.; Hancock, R. E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc. 2008, 3, 163-175.
doi: 10.1038/nprot.2007.521 |
| 37 |
Li, P.; Poon, Y. F.; Li, W.; Zhu, H. Y.; Yeap, S. H.; Cao, Y.; Qi, X.; Zhou, C.; Lamrani, M.; Beuerman, R. W.; Kang, E. T.; Mu, Y.; Li, C. M.; Chang, M. W.; Leong, S. S.; Chan-Park, M. B. A polycationic antimicrobial and biocompatible hydrogel with microbe membrane suctioning ability. Nat Mater. 2011, 10, 149-156.
doi: 10.1038/nmat2915 |
| 38 |
Wang, X.; Shi, H.; Tang, H.; Yu, H.; Yan, Q.; Yang, H.; Zhang, X.; Luan, S. Electrostatic assembly functionalization of poly (γ-glutamic acid) for biomedical antibacterial applications. J Mater Sci Technol. 2020, 59, 14-25.
doi: 10.1016/j.jmst.2020.05.017 URL |
| 39 |
Haji-Saeid, M.; Sampa, M. H. O.; Chmielewski, A. G. Radiation treatment for sterilization of packaging materials. Radiat Phys Chem. 2007, 76, 1535-1541.
doi: 10.1016/j.radphyschem.2007.02.068 URL |
| 40 | Liscano, Y.; Salamanca, C. H.; Vargas, L.; Cantor, S.; Laverde-Rojas, V.; Oñate-Garzón, J. Increases in hydrophilicity and charge on the polar face of alyteserin 1c helix change its selectivity towards Gram-positive bacteria. Antibiotics (Basel). 2019, 8, 238. |
| 41 |
Palermo, E. F.; Lienkamp, K.; Gillies, E. R.; Ragogna, P. J. Antibacterial activity of polymers: discussions on the nature of amphiphilic balance. Angew Chem Int Ed Engl. 2019, 58, 3690-3693.
doi: 10.1002/anie.v58.12 URL |
| 42 |
Judzewitsch, P. R.; Nguyen, T. K.; Shanmugam, S.; Wong, E. H. H.; Boyer, C. Towards sequence-controlled antimicrobial polymers: effect of polymer block order on antimicrobial activity. Angew Chem Int Ed Engl. 2018, 57, 4559-4564.
doi: 10.1002/anie.v57.17 URL |
| 43 |
Lam, S. J.; O’Brien-Simpson, N. M.; Pantarat, N.; Sulistio, A.; Wong, E. H.; Chen, Y. Y.; Lenzo, J. C.; Holden, J. A.; Blencowe, A.; Reynolds, E. C.; Qiao, G. G. Combating multidrug-resistant Gram-negative bacteria with structurally nanoengineered antimicrobial peptide polymers. Nat Microbiol. 2016, 1, 16162.
doi: 10.1038/nmicrobiol.2016.162 |
| 44 |
Osmanoğlu, Y. E.; Sütçü, K. EPR studies of the free radicals generated in gamma irradiated amino acid derivatives. J Mol Struct. 2017, 1145, 240-243.
doi: 10.1016/j.molstruc.2017.05.086 URL |
| 45 | Duliu, O. G.; Bercu, V. Chapter 2 - ESr investigation of the free radicals in irradiated foods. In Electron spin resonance in food science, Shukla, A. K., ed. Academic Press: 2017; pp 17-32. |
| 46 |
Atrous, H.; Benbettaieb, N.; Hosni, F.; Danthine, S.; Blecker, C.; Attia, H.; Ghorbel, D. Effect of γ-radiation on free radicals formation, structural changes and functional properties of wheat starch. Int J Biol Macromol. 2015, 80, 64-76.
doi: 10.1016/j.ijbiomac.2015.06.014 URL |
| 47 |
Liu, W.; Wang, M.; Xing, Z.; Wu, G. The free radical species in polyacrylonitrile fibers induced by γ-radiation and their decay behaviors. Radiat Phys Chem. 2012, 81, 835-839.
doi: 10.1016/j.radphyschem.2012.03.017 URL |
| 48 |
Zhao, Y.; Wang, M.; Tang, Z.; Wu, G. ESR study of free radicals in UHMW-PE fiber irradiated by gamma rays. Radiat Phys Chem. 2010, 79, 429-433.
doi: 10.1016/j.radphyschem.2009.11.007 URL |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||