Introduction
The coronavirus disease 2019 (COVID-19) pandemic has been causing great loss of lives and negative impacts on the global economy. People across the world have been struggling to contain the spread of COVID-19.1 The general public is guided to wear face masks and adopt social distancing and enhanced handwashing measures.2 Doctors and healthcare workers are working on the front line to provide tests on possibly positive cases and take care of infected patients. Biomedical researchers are actively developing vaccines3 and seeking anti-viral drugs for the treatment of COVID-19. Epidemiologists are collecting a large amount of data and constructing statistical models to predict the trend of the pandemic.1, 4
Researchers in physical sciences can and should make contributions to the ongoing combat against COVID-19.5 In addition to the design of new personal protective equipment (PPE) with improved performance by optimizing materials and structures,6 researchers in physical sciences can take part in revealing the transmission mechanisms of the virus. While the microscopic process of virus infection is a topic for molecular biology,7 the transmission of a virus from person to person and the ultimate outbreak of a pandemic is an intrinsically interdisciplinary multi-scale problem that requires concerted efforts of researchers in different fields including physical sciences. Below we first provide an overview of the transmission pathways of respiratory tract viruses, the structure and composition of the COVID-19 virus particles and the virus-laden respiratory droplets, and then discuss research opportunities for physical scientists.
Overview of Transmission Pathways
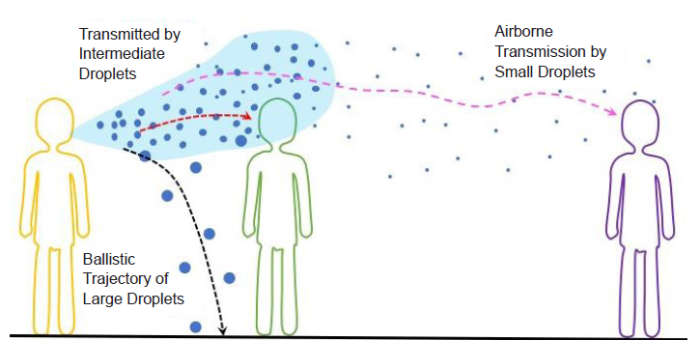
It is well established that the transmission route of respiratory tract viruses including that causing COVID-19 begins with the release of respiratory droplets by an infected person through respiratory activities such as breathing, speaking, coughing, and sneezing.8, 9 These droplets are polydisperse with sizes ranging from about 1 μm to about 2 mm.10-14 The size of a droplet plays an important role in determining its fate, as illustrated in Figure 1. (1) Large droplets (≥ 100 μm) fall to the ground or any open surface, such as the surfaces of door knobs, desks, handrails, and touchscreens, because of gravity. Viruses in these large droplets are transmitted to a healthy person if the person touches the surface contaminated by the droplets or the desiccated remains of the droplets.15, 16 (2) Intermediate droplets (~10-100 μm) can defy gravity and be carried by an airflow. The droplets can then be inhaled by a healthy person, completing the droplet transmission of viruses. The reduction of droplet size resulting from the evaporation of water, which is the major liquid content of a respiratory droplet, further facilitates the droplet transmission.17 (3) Small droplets (< 5 μm) can dry quickly. However, small droplets and droplet nuclei resulting from desiccation can be suspended in air for an extended period and form the so-called aerosol.16, 18-21 Viruses in an aerosol can be transmitted to a healthy person through direct inhalation. This process can occur over a long distance (~5 m) from the source of droplets, in contrast to the short-range transmission caused by intermediate and large droplets, which typically takes place close (1 to 2 m) to the source.
Figure 1.
Figure 1.
Schematic illustration of the different transmission routes of respiratory tract viruses via large (≥ 100 μm), intermediate (~10-100 μm), and small (< 5 μm) droplets.
In order to cause a new infection, viruses need to get into the body of a healthy person. Touching infectious virus-laden droplets or droplet nuclei deposited on an open surface followed by touching face immediately brings the viruses in contact with eyes, nose, and mouth. Droplets or droplet nuclei inhaled by a person can continue to travel inside the respiratory tract.22 Larger ones are deposited in the upper respiratory tract, such as the nasal cavity and throat. Smaller droplets can reach the lower respiratory tract, travel through the hierarchy of the human lung structure, and eventually arrive at the alveoli. During this process, water may condense onto the inhaled droplets, enlarging them and facilitating their deposition on the surface of the human air pathway. No matter how and where the viruses are deposited, the viruses in the new host go through the mucus layer and subsequently penetrate the mucous membranes to enter healthy cells and multiply themselves.23, 24 The new viruses generated then infect nearby healthy cells, migrate to other parts of the body, and ultimately lead to various symptoms.
Brief Description of Coronavirus Particles
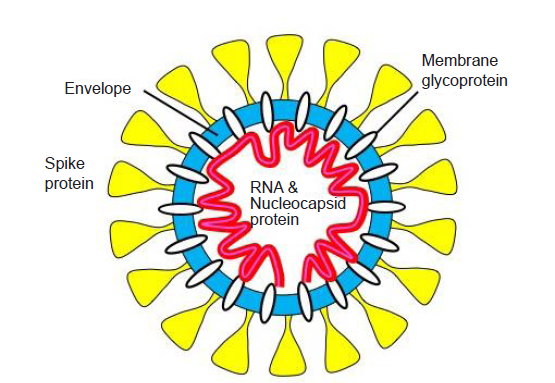
A coronavirus particle possesses a core-shell structure,7 as shown in Figure 2. At the core, there is a single-strand RNA enclosed by nucleocapsid proteins, which adopts the conformation of a tightly packed coil. The core is enveloped by a lipid bilayer membrane decorated with various proteins, including the “corona” formed by spike proteins. The diameter of a coronavirus particle can vary from about 60 nm to about 140 nm, with a typical value about 100 nm. After a virus particle enters a host cell, it releases its genetic materials, which then exploit the machinery of the host cell to make components needed to form new virus particles through a biological self-assembly process. To maintain infectivity, the lipid layer of a virus particle must remain intact through the entire transmission process before it invades a host cell. The lipid layer may be compromised by chemical sanitization using alcohol or through physical processes such as heating and desiccation, thus deactivating the virus. Therefore, a deeper understanding is needed on how the structure and activity of a virus particle are affected by the physical processes occurring during the transmission of respiratory droplets.
Figure 2.
Figure 2.
Structure of a coronavirus particle.
Composition of Respiratory Droplets
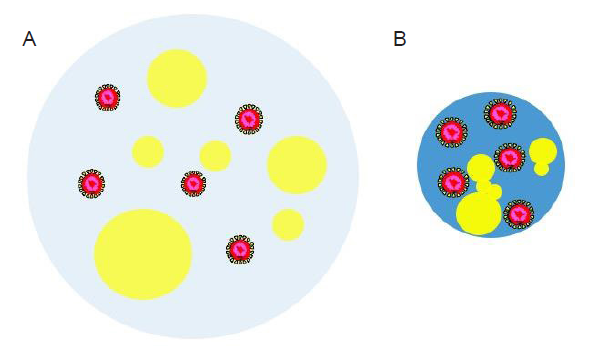
A respiratory droplet is a complex, multicomponent soft matter system, as shown in Figure 3. It is overly simplified by treating a respiratory droplet exhaled by an infectious patient as an aqueous solution of virus particles. Instead, various nonaqueous components are present in the droplet.5, 25-28 Some representative nonaqueous components are listed and described in Table 1. The mass fraction of the nonaqueous components in a respiratory droplet is typically a few percent, but far exceeds that of the virus particles. The presence of these nonaqueous components has a strong influence on the drying behavior of the droplet in an airflow and on various surfaces, as well as the viability and transmissibility of the viruses contained in the droplet.
Figure 3.
Figure 3.
Schematic illustration of a virus-laden respiratory droplet after being released by an infected person (A) and after the loss of some water content through evaporation (B). The water part of the droplet is colored light blue in A while blue in B. Yellow “bubbles” of various sizes indicate the nonaqueous components such as salt, proteins, and surfactants. The virus particles are drawn as core-shell structures with the core colored in red.
Table 1 List of representative nonaqueous components in a respiratory droplet
| Nonaqueous components | Brief description |
|---|---|
| Salt and lactate | Soluble, present as charged ions |
| Cholesterol | Insoluble, non-polar (except for the hydroxyl group at one end) |
| Proteins and enzymes | Amphiphilic, net charge depends on the sequence of amino acids and pH |
| Lung surfactants | Amphiphilic mixture of phospholipids and proteins |
| Cell debris | Organic waste from epithelial and immune system cells |
| Bacteria | Most bacterial cell walls carry negative charges |
| Viruses | pH-dependent surface charges |
Evaporation of Respiratory Droplets
Water evaporation is a basic physical process during the person-to-person transmission of virus-laden respiratory droplets. As illustrated in Figure 3, water evaporation directly affects the size of a droplet and thereby the probabilities of it being carried by an airflow, falling on an open surface, and depositing on the surface of a respiratory tract.22, 29, 30 Moreover, evaporation affects the composition and structure of the droplet and hence the microenvironment of the viruses within.31-33 Virus particles only occupy a small fraction of the droplet. The infectivity of the virus is directly affected by the pH and salt concentration,34-36 which are changed by the loss of water during evaporation.
Trajectories of Respiratory Droplets
The person-to-person transmission of virus-laden droplets involves their trajectories in an airflow. A droplet trajectory is affected by multiple factors including the droplet size, the initial velocity of the droplet at the source, gravity, and the drag force experienced by the droplet in the airflow.30, 37, 38 Turbulent flow with the irregular and chaotic movement of gas molecules is ubiquitous in respiratory activities as well as in aerodynamics.39-42 Turbulence affects both the initial conditions of the droplets generated by respiratory activities and their trajectories afterward in the airflow. It can greatly extend the range traveled by a droplet. The stochastic nature of turbulent flow gives rise to the murky and sometimes inconsistent size criteria for dividing the droplets into small, intermediate, and large categories and associating droplet size to different transmission mechanisms. Additionally, because of the stochasticity of the droplet trajectories, the 2-m or 6-feet social distancing is an empirical measure that allows the majority but not all large droplets to fall out of an airflow. Further studies of the role of turbulence can help optimize the barriers in PPE for droplets of various sizes and improve the rules guiding social distancing. It can also help design effective equipment and procedures to filter potentially contaminated air for indoor air circulation.
Suggestion for Future Research
Physicochemical characterization of respiratory droplets
To understand the role of respiratory droplets in transmitting the COVID-19 virus (and many other respiratory viruses), it is crucial to have reliable methods to characterize respiratory droplets at various stages after they are released into air. Many physical attributes need to be measured for a droplet, such as size, drying rate, composition, internal pressure, pH value, internal distribution and structure of nonvolatile substances including virus particles, and diffusivity in air. These attributes largely determine the fate of a droplet and thus the transmission pathway.
Various characterization methods,43, 44 such as fluorescent microscopy and spectroscopy, surface tensiometry, X-ray and neutron scattering, cryo-transmission electron microscope, and atomic force microscope, can be used to measure the physicochemical attributes of respiratory droplets. For example, droplet size and its distribution may be measured by droplet deposition analysis and light scattering method.45 The attributes of a droplet are strongly correlated and the correlations can be used to deduce certain attributes from others. For example, a charged droplet can be placed in an electric field and its size can be deduced by measuring the mobility of the droplet. Droplet velocity in air can also be measured with an aerodynamic particle sizer that utilizes laser beams separated by a small distance to measure the time needed by a droplet to travel that distance.45 The measured velocity can then be used to deduce the aerodynamic diameter of the droplet. The diffusivity of a droplet in air is determined by its size, air viscosity, and temperature. The variation of droplet size over time can be used to deduce its drying rate, which in turn is affected by the distribution of surfactant molecules in the droplet. Such a distribution sets the surface tension of a droplet, which together with the droplet size determines the internal pressure of the droplet through the Young-Laplace equation. As the size and composition of a droplet change with time, its pH value varies accordingly. The measurement of the structure and distribution of nonvolatile substances in droplets is more challenging and typically requires collection of exhaled droplets followed by chemical analysis through various spectrometric methods. A broad spectrum of characterization tools, such as those from colloidal science43 and physical chemistry,46 can be harnessed to measure the physicochemical properties of respiratory droplets and the results can be combined to yield a complete picture of their dynamics and structural evolution in an airflow, which will provide valuable insight into their capability of transmitting the COVID-19 virus.
Evaporation of droplets in airflows
The evaporation of droplets is driven by the difference between the partial vapor pressure at the droplet surface and the ambient vapor pressure. Both the rate of evaporation and the ultimate droplet size after evaporation are affected by the relative humidity (RH) and temperature of the ambient environment.40, 47 The dependence of droplet evaporation on ambient conditions is the key to understanding the seasonality and variation by geographic regions of the outbreak of respiratory virus diseases. Evidence has shown that an outbreak is more likely to peak in winter and in regions with cold and dry climates.48 Inside the respiratory tract, RH and temperature affect the evaporation of an inhaled droplet and the inverse process of water condensation onto the droplet,22, 49 thus influencing its probability of depositing onto the surface of the respiratory airway.
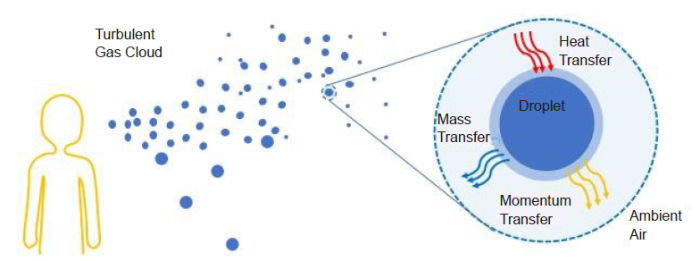
Evaporation of a liquid droplet at quiescent conditions has been studied both experimentally and theoretically. However, evaporation of droplets in a laminar or turbulent airflow, both of which occur along the transmission path of viruses (Figure 4), need to be systematically studied. For experiments, an airflow mimicking that along the trajectories of droplets at various conditions needs to be introduced around the suspended liquid droplets. For theoretical research, an airflow may be incorporated as the boundary conditions for the droplet undergoing evaporation. A theoretical study of evaporation in turbulent flow would be more challenging than that in laminar flow, as the theoretical description of turbulent flow relies on an appropriate turbulence model and thus is more complex. Nevertheless, the study including a turbulent airflow is significant given the ubiquity of turbulence along the droplet trajectory. Investigating the physics of evaporation in an airflow, in particular, the dependence of the evaporation rate and droplet size on RH and temperature, is critical to the efforts of reducing virus transmission by controlling indoor conditions, such as in the setting of passenger airplanes.
Figure 4.
Figure 4.
Schematic illustration of the evaporation of a respiratory droplet in a turbulent airflow.
Evaporation of droplets on surfaces
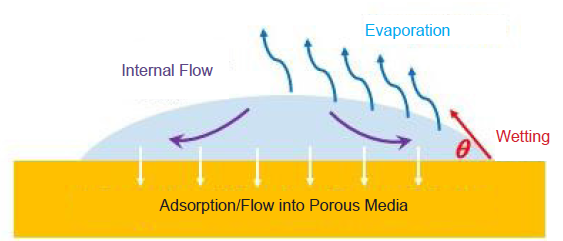
A liquid droplet deposited on a superhydrophobic surface has been used in the experimental study of droplet evaporation.50 An extensive study of droplet evaporation on various open surfaces is much needed. Evaporation reduces water from the droplet and affects the time during which the viruses remain infectious on the surface, as the infectivity of the viruses relies on a certain amount of water. Apart from the airflow surrounding the droplet, the rate of evaporation depends on the interaction between the droplet and the surface (wetting behavior) and also the structural features of the surface (Figure 5). Elucidating the drying behavior of the deposited droplets may shed light on the origin of the varying virus lifetime on different surfaces.6 It has been shown that the virus lifetime is shorter on rough and porous surfaces, such as the surfaces of textiles and paper, compared with that on smooth and less permeable surfaces, such as the surfaces of stainless steel, glass, and plastics.16, 51 One conjecture is that larger surface roughness enhances the rate of evaporation and the surface porosity provides a pathway for water adsorption, both accelerating the desiccation of the droplet and hence shortening the virus lifetime. Thorough experimental and theoretical research is needed to evaluate the conjecture. A recent study also showed that the charge state of a surface also strongly affects the deactivation time of the virus in the deposited droplet.6 Understanding the relation between virus lifetime and droplet evaporation on a surface can guide the improvement of the surfaces of PPE as well as the design of a self-sanitizing surface, including those that can slowly release anti-viral chemicals to disinfect the viruses in the deposited droplets.
Figure 5.
Figure 5.
Schematic illustration of the evaporation of a droplet on the surface of a porous material.
Evaporation of complex fluid droplets
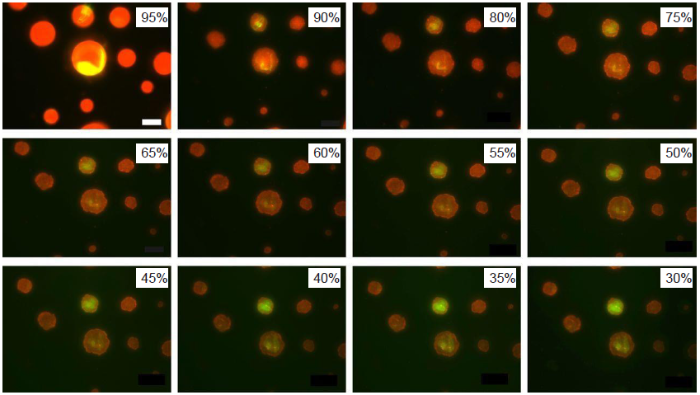
Respiratory droplets are composed of a complex fluid containing multiple components. A surrogate model of the droplet50 has been used to study the water evaporation rate and the size and internal structure of the droplet nuclei after evaporation (Figure 6). The model droplet contains water and three other components including NaCl, mucin, and 1,2-dihexadecanoyl-sn-glycero-3-phosphocholine, which is one of the most abundant lung surfactants. Morphological changes including liquid-liquid phase separations and the crystallization of salts and surfactants during evaporation are revealed by optical and fluorescence microscopies. Since the Laplace pressure depends on the droplet size, the pressure inside the droplet thus varies as the droplet undergoes evaporation and changes its size. The changes of the internal pressure and structure inside a droplet may potentially deform and damage the virus particles, specifically the lipid membranes. Future work is needed to reveal the effects of morphological changes on the integrity and thus the infectivity of the virus particles. It has also been shown that the presence of surfactants retards the evaporation process and inhibits the rehydration of non-volatile components in a dry droplet.50 Presumably, the presence of surfactants is related to the reduction of surface tension. However, during evaporation the surfactants can accumulate at the surface of the droplet and form a skin layer, thus slowing down the drying process. This is another topic for future research. Ongoing and future experiments also call for a systematic study of the evaporation of a complex fluid droplet on the theoretical side.
Figure 6.
Figure 6.
Fluorescence images of model droplets exposed to decreasing relative humidity. A droplet contains water, NaCl, mucin (red), and DDPC (green). DDPC: 1,2-dihexadecanoyl-sn-glycero-3-phosphocholine. Reprinted with permission from Vejerano and Marr.50 Copyright (2018) The Royal Society.
Future experimental and theoretical studies should include the evaporation of a complex fluid droplet in a more realistic scenario with an airflow. Identifying the effects of different environmental factors on evaporation and thereby the virus infectivity may help engineer the composition of respiratory droplets at the source, such as using specialized chewing gum and inhalable surfactants, for the containment of virus transmission.
Distribution of viruses in a droplet
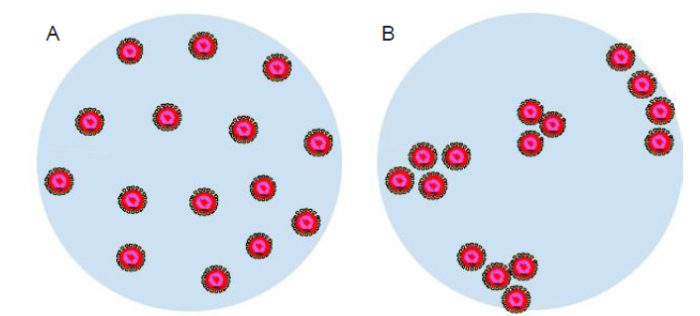
A respiratory droplet of an infected person typically contains multiple virus particles. The infectivity of the viruses is possibly related to the distribution of these particles in the droplet. One conjecture is that a cluster of virus particles would increase the effectiveness of the infection of healthy human cells. At quiescent conditions, the viruses may be uniformly distributed across the droplet or aggregated to form clusters, as illustrated in Figure 7. Factors affecting the distribution of the virus particles inside a droplet may include the concentration of viruses, the interactions between viruses and other components of the droplet, such as proteins and surfactants, and the interactions among viruses themselves as well. Besides, if liquid-liquid phase separation occurs in the droplet, the viruses may be preferentially distributed in certain parts of the resulting internal structure. In experiments, the distribution of virus particles may be studied using fluorescence microscopy where a suitable fluorophore is used to label the virus particles and pinpoint their locations in the droplet.50 In theory, the thermodynamics framework (e.g., the Flory-Huggins theory) developed for the phase behavior of synthetic nanoparticles in a matrix of synthetic polymers52 may provide hints for a new theoretical description. On the modeling side, particle-based approaches such molecular dynamics simulations may be used to reveal how the virus particle distribution is affected by the presence of other components in the droplet and how this distribution evolves as the droplets undergoes evaporation.
Figure 7.
Figure 7.
Schematic illustration of a respiratory droplet with uniformly distributed virus particles (A) and aggregated clusters of virus particles (B). The virus particles are drawn as core-shell structures with the core colored in red.
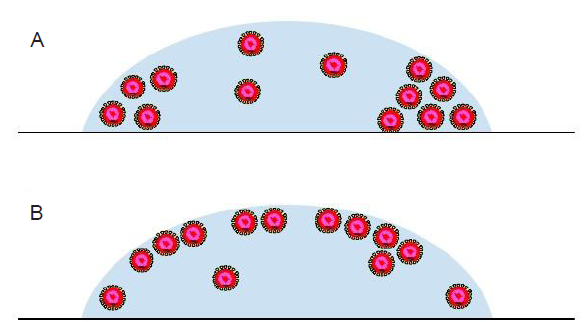
At conditions out of equilibrium, in particular, during the evaporation of droplets in an airflow, the distribution of virus particles may be altered compared to that at quiescence. Morphological changes of the droplet itself induced by liquid-liquid phase separation and crystallization of certain components may directly change the distribution of virus particles. Internal flows of water molecules induced during evaporation may re-distribute the virus particles as well, as shown in Figure 8. One intriguing question is whether the virus particles tend to aggregate at the edge of a deposited droplet (Figure 8A), similar to the “coffee ring” effect53 for a puddle of particle-laden liquid after drying, or accumulate at the droplet-air interface (Figure 8B),54 or form stratified structures during evaporation as observed in drying colloidal suspensions and colloid/polymer mixtures.55-57 The aggregation of virus particles on the droplet-air interface may facilitate the transport of the viruses after they are taken into the body of a healthy person. Experimentally, fluorescence microscopy may be used to track the evolution of the virus particle distribution at controlled evaporation conditions. Theoretically, the physics underlying the “coffee ring” effect and the stratification process (i.e., the physics of particle motion under various gradients) may be invoked to study the conjectured flow-induced redistribution of viruses.
Figure 8.
Figure 8.
The distribution of virus particles in a droplet on a surface is conjectured to be affected by the evaporation dynamics and internal flow. The virus particles can possibly aggregate at the edge of the deposited droplet (A) or the droplet-air interface (B). The virus particles are drawn as core-shell structures with the core colored in red.
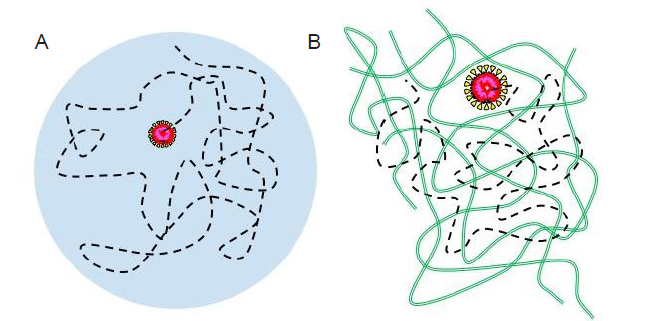
Dynamics of virus particles in a droplet
The motion of virus particles in a respiratory droplet, as illustrated in Figure 9A, is important to their distribution in the droplet and also the transport of the viruses to human cells after the inhalation of the droplet. While the biosynthesis of a virus particle is not a passive process, the motion of a virus particle in a droplet is passively agitated by thermal fluctuation. The complex fluid in a respiratory droplet exhibits dynamic viscoelasticity that provides the background for the passive motion of embedded virus particles. The coupling of the virus particles and the viscoelastic background gives rise to a random motion, the mobility of which is quantified by the diffusion coefficient. On the side of the virus particle, the coupling depends on its size and shape. Favorable attractions of the virus particle to certain components of the droplet act to slow down the diffusion. An attraction stronger than the thermal energy may even bind a virus particle to nearby molecules and result in a combined motion. The mobility of virus particles can be measured by tracking them using fluorescence microscopy. A theoretical model for virus mobility may be developed based on recent theoretical research on the mobility of synthetical particles in a viscoelastic polymer matrix.58-60
Figure 9.
Figure 9.
Schematic illustration of the trajectory (black dashed lines) of an individual virus particle in a respiratory droplet (A) and a mucus layer (green chains) (B). The virus particles are drawn as core-shell structures with the core colored in red.
The abovementioned study may be generalized to investigate the motion of virus particles in a mucus layer (Figure 9B), which is a protective fluid layer that covers the mucous membrane lining, eyes, nose, and lungs.23 This layer is also a multi-component soft matter system exhibiting interesting viscoelastic properties. The virus mobility in this layer is critical to the interpenetration through the mucus layer for virus infection. While mucus is a complex fluid different from the materials making up a respiratory droplet, the same experimental and theoretical tools discussed previously may be used to conduct the research. A more general study of nanoscale objects in a complex viscoelastic liquid can also benefit the design of nanocarriers that can penetrate the respiratory liquid or mucus layer to deliver anti-viral chemicals.
Summary
As of the publication of this article, the COVID-19 pandemic is far from being contained. An important question to the researchers in physical sciences is: What non-medical, non-clinical-care research can be performed to address the real-world challenge posed by the COVID-19 virus? In light of the imperative need, this article presents an overview of virus-laden respiratory droplets, which play a crucial role in COVID-19 transmission, and further offers a list of suggestions for future research. The authors hope that this article would encourage physical scientists to navigate the literature, design new studies, inform policymakers, and educate the general public about the science of virus transmission.
Author contributions
TG initiated and formulated the overall structure of this review. TG prepared the figures with suggestions by SC. Both TG and SC contributed to the final version of this manuscript.
Financial support
This work was supported by the National Science Foundation (No. DMR-1944887).
Acknowledgement
Ting Ge is supported by the start-up funds from the University of South Carolina. The material prepared by Shengfeng Cheng is based upon work supported by the National Science Foundation under Grant DMR-1944887.
Conflicts of interest statement
The authors declare no competing financial interest.
Data sharing statement
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Reference
Coronavirus disease (COVID-19) advice for the public
https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public. Accessed by January 3,
Coronavirus vaccine tracker
https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html. Accessed by January 3,
Diverse Computer Simulation Models Provide Unified Lessons on University Operation during a Pandemic
DOI:10.1093/biosci/biaa064 URL PMID:32665736 [Cited within: 1]
COVID-19: A call for physical scientists and engineers
DOI:10.1021/acsnano.0c02618
URL
PMID:32267678
[Cited within: 2]

The COVID-19 pandemic is one of those global challenges that transcends territorial, political, ideological, religious, cultural, and certainly academic boundaries. Public health and healthcare workers are at the frontline, working to contain and to mitigate the spread of this disease. Although intervening biological and immunological responses against viral infection may seem far from the physical sciences and engineering that typically work with inanimate objects, there actually is much that can-and should-be done to help in this global crisis. In this Perspective, we convert the basics of infectious respiratory diseases and viruses into physical sciences and engineering intuitions, and through this exercise, we present examples of questions, hypotheses, and research needs identified based on clinicians' experiences. We hope researchers in the physical sciences and engineering will proactively study these challenges, develop new hypotheses, define new research areas, and work with biological researchers, healthcare, and public health professionals to create user-centered solutions and to inform the general public, so that we can better address the many challenges associated with the transmission and spread of infectious respiratory diseases.
A surface coating that rapidly inactivates SARS-CoV-2
DOI:10.1021/acsami.0c11425
URL
PMID:32657566
[Cited within: 3]

SARS-CoV-2, the virus that causes the disease COVID-19, remains viable on solids for periods of up to 1 week, so one potential route for human infection is via exposure to an infectious dose from a solid. We have fabricated and tested a coating that is designed to reduce the longevity of SARS-CoV-2 on solids. The coating consists of cuprous oxide (Cu2O) particles bound with polyurethane. After 1 h on coated glass or stainless steel, the viral titer was reduced by about 99.9% on average compared to the uncoated sample. An advantage of a polyurethane-based coating is that polyurethane is already used to coat a large number of everyday objects. Our coating adheres well to glass and stainless steel as well as everyday items that people may fear to touch during a pandemic, such as a doorknob, a pen, and a credit card keypad button. The coating performs well in the cross-hatch durability test and remains intact and active after 13 days of being immersed in water or after exposure to multiple cycles of exposure to the virus and disinfection.
The molecular biology of coronaviruses
DOI:10.1016/S0065-3527(06)66005-3
URL
PMID:16877062
[Cited within: 2]

Coronaviruses are large, enveloped RNA viruses of both medical and veterinary importance. Interest in this viral family has intensified in the past few years as a result of the identification of a newly emerged coronavirus as the causative agent of severe acute respiratory syndrome (SARS). At the molecular level, coronaviruses employ a variety of unusual strategies to accomplish a complex program of gene expression. Coronavirus replication entails ribosome frameshifting during genome translation, the synthesis of both genomic and multiple subgenomic RNA species, and the assembly of progeny virions by a pathway that is unique among enveloped RNA viruses. Progress in the investigation of these processes has been enhanced by the development of reverse genetic systems, an advance that was heretofore obstructed by the enormous size of the coronavirus genome. This review summarizes both classical and contemporary discoveries in the study of the molecular biology of these infectious agents, with particular emphasis on the nature and recognition of viral receptors, viral RNA synthesis, and the molecular interactions governing virion assembly.
Inactivation of influenza A viruses in the environment and modes of transmission: a critical review
DOI:10.1016/j.jinf.2008.08.013
URL
PMID:18848358
[Cited within: 1]

OBJECTIVES: The relative importance of airborne, droplet and contact transmission of influenza A virus and the efficiency of control measures depends among other factors on the inactivation of viruses in different environmental media. METHODS: We systematically review available information on the environmental inactivation of influenza A viruses and employ information on infectious dose and results from mathematical models to assess transmission modes. RESULTS: Daily inactivation rate constants differ by several orders of magnitude: on inanimate surfaces and in aerosols daily inactivation rates are in the order of 1-10(2), on hands in the order of 10(3). Influenza virus can survive in aerosols for several hours, on hands for a few minutes. Nasal infectious dose of influenza A is several orders of magnitude larger than airborne infectious dose. CONCLUSIONS: The airborne route is a potentially important transmission pathway for influenza in indoor environments. The importance of droplet transmission has to be reassessed. Contact transmission can be limited by fast inactivation of influenza virus on hands and is more so than airborne transmission dependent on behavioral parameters. However, the potentially large inocula deposited in the environment through sneezing and the protective effect of nasal mucus on virus survival could make contact transmission a key transmission mode.
Transmission of SARS-CoV-2: implications for infection prevention precautions
https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions. Accessed by January 3,
The numbers and the sites of origin of the droplets expelled during expiratory activities
URL PMID:21009905 [Cited within: 1]
Relation between the airborne diameters of respiratory droplets and the diameter of the stains left after recovery
Cough-generated aerosols of Mycobacterium tuberculosis: a new method to study infectiousness
DOI:10.1164/rccm.200308-1101OC
URL
PMID:14656754

The concentration and size distribution of infectious aerosols produced by patients with pulmonary tuberculosis (TB) has never been directly measured. We aimed to assess the feasibility of a method that we developed to collect and quantify culturable cough-generated aerosols of Mycobacterium tuberculosis. Subjects were recruited from a referral hospital and most had multidrug-resistant TB. They coughed into a chamber containing microbial air samplers while cough frequency was measured during two 5-minute sessions. Cough-generated aerosol cultures were positive in 4 of 16 subjects (25%) with smear-positive pulmonary TB. There was a rapid decrease in the cough-generated aerosol cultures within the first 3 weeks of effective treatment. Culture-positive cough aerosols were associated with lack of treatment during the previous week (p = 0.007), and there was a trend in the association with cough frequency (p = 0.08). The size distributions of these aerosols were variable, but most particle sizes were in the respirable range. Quantification of viable cough-generated aerosols is feasible and offers a new approach to study infectiousness and transmission of M. tuberculosis and other airborne pathogens.
Characterization of expiration air jets and droplet size distributions immediately at the mouth opening
DOI:10.1016/j.jaerosci.2008.10.003
URL
PMID:32287373

Size distributions of expiratory droplets expelled during coughing and speaking and the velocities of the expiration air jets of healthy volunteers were measured. Droplet size was measured using the interferometric Mie imaging (IMI) technique while the particle image velocimetry (PIV) technique was used for measuring air velocity. These techniques allowed measurements in close proximity to the mouth and avoided air sampling losses. The average expiration air velocity was 11.7 m/s for coughing and 3.9 m/s for speaking. Under the experimental setting, evaporation and condensation effects had negligible impact on the measured droplet size. The geometric mean diameter of droplets from coughing was 13.5 mum and it was 16.0 mum for speaking (counting 1-100). The estimated total number of droplets expelled ranged from 947 to 2085 per cough and 112-6720 for speaking. The estimated droplet concentrations for coughing ranged from 2.4 to 5.2 cm(-3) per cough and 0.004-0.223 cm(-3) for speaking.
Exhaled droplets due to talking and coughing
DOI:10.1098/rsif.2009.0388.focus
URL
PMID:19812073
[Cited within: 1]

Respiratory infections can be spread via 'contact' with droplets from expiratory activities such as talking, coughing and sneezing, and also from aerosol-generating clinical procedures. Droplet sizes predominately determine the times they can remain airborne, the possibility of spread of infectious diseases and thus the strategies for controlling the infections. While significant inconsistencies exist between the existing measured data on respiratory droplets generated during expiratory activities, a food dye was used in the mouth during measurements of large droplets, which made the expiratory activities 'unnatural'. We carried out a series of experiments using glass slides and a microscope as well as an aerosol spectrometer to measure the number and size of respiratory droplets produced from the mouth of healthy individuals during talking and coughing with and without a food dye. The total mass of respiratory droplets was measured using a mask, plastic bag with tissue and an electronic balance with a high precision. Considerable subject variability was observed and the average size of droplets captured using glass slides and microscope was about 50-100 microm. Smaller droplets were also detected by the aerosol spectrometer. More droplets seemed to be generated when a food dye was used.
Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination
DOI:10.1016/j.jhin.2015.08.027
URL
PMID:26597631
[Cited within: 1]

Viruses with pandemic potential including H1N1, H5N1, and H5N7 influenza viruses, and severe acute respiratory syndrome (SARS)/Middle East respiratory syndrome (MERS) coronaviruses (CoV) have emerged in recent years. SARS-CoV, MERS-CoV, and influenza virus can survive on surfaces for extended periods, sometimes up to months. Factors influencing the survival of these viruses on surfaces include: strain variation, titre, surface type, suspending medium, mode of deposition, temperature and relative humidity, and the method used to determine the viability of the virus. Environmental sampling has identified contamination in field-settings with SARS-CoV and influenza virus, although the frequent use of molecular detection methods may not necessarily represent the presence of viable virus. The importance of indirect contact transmission (involving contamination of inanimate surfaces) is uncertain compared with other transmission routes, principally direct contact transmission (independent of surface contamination), droplet, and airborne routes. However, influenza virus and SARS-CoV may be shed into the environment and be transferred from environmental surfaces to hands of patients and healthcare providers. Emerging data suggest that MERS-CoV also shares these properties. Once contaminated from the environment, hands can then initiate self-inoculation of mucous membranes of the nose, eyes or mouth. Mathematical and animal models, and intervention studies suggest that contact transmission is the most important route in some scenarios. Infection prevention and control implications include the need for hand hygiene and personal protective equipment to minimize self-contamination and to protect against inoculation of mucosal surfaces and the respiratory tract, and enhanced surface cleaning and disinfection in healthcare settings.
Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1
DOI:10.1056/NEJMc2004973 URL PMID:32182409 [Cited within: 3]
Infection prevention and control of epidemic-and pandemic-prone acute respiratory infections in health care
https://apps.who.int/iris/bitstream/handle/10665/112656/9789241507134_eng.pdf;jsessionid=41AA684FB64571CE8D8A453C4F2B2096?sequence=1. Accessed by January 3,
Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals
DOI:10.1038/s41586-020-2271-3
URL
PMID:32340022
[Cited within: 1]

The ongoing outbreak of coronavirus disease 2019 (COVID-19) has spread rapidly on a global scale. Although it is clear that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is transmitted through human respiratory droplets and direct contact, the potential for aerosol transmission is poorly understood(1-3). Here we investigated the aerodynamic nature of SARS-CoV-2 by measuring viral RNA in aerosols in different areas of two Wuhan hospitals during the outbreak of COVID-19 in February and March 2020. The concentration of SARS-CoV-2 RNA in aerosols that was detected in isolation wards and ventilated patient rooms was very low, but it was higher in the toilet areas used by the patients. Levels of airborne SARS-CoV-2 RNA in the most public areas was undetectable, except in two areas that were prone to crowding; this increase was possibly due to individuals infected with SARS-CoV-2 in the crowd. We found that some medical staff areas initially had high concentrations of viral RNA with aerosol size distributions that showed peaks in the submicrometre and/or supermicrometre regions; however, these levels were reduced to undetectable levels after implementation of rigorous sanitization procedures. Although we have not established the infectivity of the virus detected in these hospital areas, we propose that SARS-CoV-2 may have the potential to be transmitted through aerosols. Our results indicate that room ventilation, open space, sanitization of protective apparel, and proper use and disinfection of toilet areas can effectively limit the concentration of SARS-CoV-2 RNA in aerosols. Future work should explore the infectivity of aerosolized virus.
Toward understanding the risk of secondary airborne infection: emission of respirable pathogens
DOI:10.1080/15459620590918466
URL
PMID:15764538

Certain respiratory tract infections are transmitted through air. Coughing and sneezing by an infected person can emit pathogen-containing particles with diameters less than 10 microm that can reach the alveolar region. Based on our analysis of the sparse literature on respiratory aerosols, we estimated that emitted particles quickly decrease in diameter due to water loss to one-half the initial values, and that in one cough the volume in particles with initial diameters less than 20 microm is 60 x 10(-8) mL. The pathogen emission rate from a source case depends on the frequency of expiratory events, the respirable particle volume, and the pathogen concentration in respiratory fluid. Viable airborne pathogens are removed by exhaust ventilation, particle settling, die-off, and air disinfection methods; each removal mechanism can be assigned a first-order rate constant. The pathogen concentration in well-mixed room air depends on the emission rate, the size distribution of respirable particles carrying pathogens, and the removal rate constants. The particle settling rate and the alveolar deposition fraction depend on particle size. Given these inputs plus a susceptible person's breathing rate and exposure duration to room air, an expected alveolar dosemicrois estimated. If the infectious dose is one organism, as appears to be true for tuberculosis, infection risk is estimated by the expression: R = 1-exp(-micro). Using published tuberculosis data concerning cough frequency, bacilli concentration in respiratory fluid, and die-off rate, we illustrate the model via a plausible scenario for a person visiting the room of a pulmonary tuberculosis case. We suggest that patients termed
Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities
Airborne transmission of SARS-CoV-2: The world should face the reality
An adaptable model for growth and/or shrinkage of droplets in the respiratory tract during inhalation of aqueous particles
The interaction between respiratory pathogens and mucus
DOI:10.1016/j.chom.2016.01.001
URL
PMID:26867175
[Cited within: 2]

The interaction between respiratory pathogens and their hosts is complex and incompletely understood. This is particularly true when pathogens encounter the mucus layer covering the respiratory tract. The mucus layer provides an essential first host barrier to inhaled pathogens that can prevent pathogen invasion and subsequent infection. Respiratory mucus has numerous functions and interactions, both with the host and with pathogens. This review summarizes the current understanding of respiratory mucus and its interactions with the respiratory pathogens Pseudomonas aeruginosa, respiratory syncytial virus and influenza viruses, with particular focus on influenza virus transmissibility and host-range specificity. Based on current findings we propose that respiratory mucus represents an understudied host-restriction factor for influenza virus.
High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa
DOI:10.1038/s41368-020-0074-x
URL
PMID:32094336
[Cited within: 1]

It has been reported that ACE2 is the main host cell receptor of 2019-nCoV and plays a crucial role in the entry of virus into the cell to cause the final infection. To investigate the potential route of 2019-nCov infection on the mucosa of oral cavity, bulk RNA-seq profiles from two public databases including The Cancer Genome Atlas (TCGA) and Functional Annotation of The Mammalian Genome Cap Analysis of Gene Expression (FANTOM5 CAGE) dataset were collected. RNA-seq profiling data of 13 organ types with para-carcinoma normal tissues from TCGA and 14 organ types with normal tissues from FANTOM5 CAGE were analyzed in order to explore and validate the expression of ACE2 on the mucosa of oral cavity. Further, single-cell transcriptomes from an independent data generated in-house were used to identify and confirm the ACE2-expressing cell composition and proportion in oral cavity. The results demonstrated that the ACE2 expressed on the mucosa of oral cavity. Interestingly, this receptor was highly enriched in epithelial cells of tongue. Preliminarily, those findings have explained the basic mechanism that the oral cavity is a potentially high risk for 2019-nCoV infectious susceptibility and provided a piece of evidence for the future prevention strategy in dental clinical practice as well as daily life.
Dilution of respiratory solutes in exhaled condensates
DOI:10.1164/ajrccm.165.5.2101018
URL
PMID:11874811
[Cited within: 1]

Most exhaled water is produced as gaseous water vapor, which can be collected in cooled condensers. The presence of nonvolatile solutes in these condensates suggests that droplets of respiratory fluid (RF) have also been collected. However, calculation of RF solute concentrations from condensates requires estimation of the dilution of RF droplets by water vapor. We used condensate electrolyte concentrations to calculate the dilution of RF droplets in condensates from 20 normal subjects. The total ionic concentration (conductivity) was 497 plus minus 68 (mean plus minus SEM) muM. Of this, 229 plus minus 43 muM was NH(4)(+), but little NH(4)(+) was collected from subjects with tracheostomies, indicating oral formation. The Na+ concentration in condensate ([Na+](cond)) averaged 242 plus minus 43 muM. Large variations in [Na(+)](cond) correlated well with variations of K+ in condensate ([K+](cond)) and Cl-) in condensate ([Cl-](cond)), and were attributed to differences in respiratory droplet dilution. Dividing condensate values of ([Na+] + [K+] ) by those of plasma indicated that RF represented between 0.01% and 2.00% of condensate volumes. Calculated values for Na+, K+, Cl-, lactate, and protein in RF were [Na+](RF) = 91 +/- 8 mM, [K+](RF) = 60 +/- 11 mM, [Cl-](RF) = 102 +/- 17 mM, [lactate](RF) = 44 +/- 17 mM, and [protein](RF) = 7.63 +/- 1.82 g/dl, respectively.
A novel method for assessing dissolution of aerosol inhaler products
DOI:10.1016/s0378-5173(03)00091-7
URL
PMID:12672613

Glucocorticoids administered by inhalation remain a first-line treatment of patients with asthma allergic rhinitis and advanced chronic obstructive pulmonary disease. Budesonide (BD), fluticasone propionate (FP) and triamcinolone acetonide (TA) have high hepatic first-pass inactivation of the swallowed fraction of the inhaled dose, whereas there is no first-pass metabolism in the lung. Hence, the lung bioavailability will determine the overall systemic absorption and the systemic bioactivity. Efficacy of inhaled agents in the respiratory tract depends on the site of deposition and physicochemical properties of the drug, which dictates rate of dissolution, absorption, metabolism and elimination. However, to date no official method exists for testing dissolution rates from inhalation aerosols. An in vitro flow through dissolution method may be useful to provide information on rate of release and determine formulation differences between products or in product development. After administration of three glucocorticoids into a cascade impactor they underwent dissolution in a flow through cell utilising water, simulated lung fluid (SLF) and modified SLF with L-alpha-phosphatidylcholine (DPPC) as a dissolution medium, at constant flow and temperature. Modified SLF significantly increased the dissolution rate compared with SLF alone. This novel technique appears to be a useful method of evaluating dissolution of these glucocorticoids and may also be applied to other respiratory products administered via aerosols.
Lipids in human saliva
DOI:10.1016/0003-9969(95)00077-1
URL
PMID:8833598

A simple and reproducible method of determining the quality and quantity of neutral lipids in human saliva was tested. Parotid, submandibular and whole stimulated saliva were collected from 10 healthy adults. The lipids were extracted by the Folch method. A special method for extraction of glycolipids was also tested but gave no additional recovery. Thin-layer chromatography was used for separating the different lipid classes. The concentrations of total lipids in parotid, submandibular and whole stimulated saliva were 0.2, 0.9 and 1.3 mg/dl, respectively. Cholesteryl esters, cholesterol, triglycerides, diglycerides, monoglycerides and free fatty acids accounted for 96-99 percent of the total salivary lipids. Thus, polar lipids such as phospholipids contributed only a minor fraction, indicating that the lipids are not primarily of membrane origin. Ultracentrifugation of saliva samples at d = 1.21 g ml(-1) showed that the salivary lipids did not float like blood plasma lipoproteins. Therefore, they must be in a different state of aggregation from lipids in blood or lymph. No significant lipase activity of the type that acts on plasma lipoproteins was found in parotid or submandibular saliva. The content of free fatty acids and partial glycerides was high.
A new collection method for the evaluation of nasal mucus proteins
DOI:10.1046/j.1365-2222.1998.00312.x
URL
PMID:9720823
[Cited within: 1]

BACKGROUND: Different sampling techniques for collecting nasal mucus have been used. The eosinophil and its granule toxin proteins, especially the eosinophil cationic protein (ECP), is an important inflammatory cell. OBJECTIVE: In the healthy subjects (with no acute or chronic disease of the respiratory tract), no detailed quantitative data on mucus nasal proteins have been reported, so the aim of the study is to demonstrate a simple and reliable method for the collection of nasal mucus and for measuring nasal ECP. METHODS: Our method consists in collecting two specimens of mucus from each nasal fossa, using a square of sterile pre-humidified gauze, centrifuged at 2000g for 20 min and stored at -20 degrees C until tested. To evaluate the reproducibility of the technique, 30 healthy subjects were retested after 24 h. RESULTS: The amounts of secretion gathered in the 89 collections did not show any statistical difference between the sexes. In the pooled subjects (n=59), the mean levels of ECP were 108 ng/mL and 325 ng/g and total protein (TP) levels were 701 mg/dL and 2.5 mg/g (expressed in absolute concentration and concentration related to the weight in grams). In the classes, according to age, no statistical differences were found on analysis of the groups by: sex, recovery rate, and ECP and TP concentrations. The Reproducibility Index was very good for the recovery rate (0.78), ECP (ng/mL=0.95, ng/g=0.83) and TP (mg/dL=0.89, mg/g=0.76). CONCLUSION: With our method, the sample collections were taken with minimal stimulation of the mucosa and it was well tolerated by the subjects; the low known dilution factor is also adequate for analysing small quantities of mucus recovered, and the protein concentration can be measured as an absolute value.
How far droplets can move in indoor environments--revisiting the Wells evaporation-falling curve
URL PMID:17542834 [Cited within: 1]
Modeling the role of respiratory droplets in Covid-19 type pandemics
Images reveal that atmospheric particles can undergo liquid-liquid phase separations
Variation in pH of model secondary organic aerosol during liquid-liquid phase separation
DOI:10.1021/acs.jpca.6b00275
URL
PMID:27082856

The majority of atmospheric aerosols consist of both organic and inorganic components. At intermediate relative humidity (RH), atmospheric aerosol can undergo liquid-liquid phase separation (LLPS) in which the organic and inorganic fractions segregate from each other. We have extended the study of LLPS to the effect that phase separation has on the pH of the overall aerosols and the pH of the individual phases. Using confocal microscopy and pH sensitive dyes, the pH of internally mixed model aerosols consisting of polyethylene glycol 400 and ammonium sulfate as well as the pH of the organic fraction during LLPS have been directly measured. During LLPS, the pH of the organic fraction was observed to increase to 4.2 +/- 0.2 from 3.8 +/- 0.1 under high RH when the aerosol was internally mixed. In addition, the high spatial resolution of the confocal microscope allowed us to characterize the composition of each of the phases, and we have observed that during LLPS the organic shell still contains large quantities of water and should be characterized as an aqueous organic-rich phase rather than simply an organic phase.
Drying kinetics of salt solution droplets: Water evaporation rates and crystallization
DOI:10.1021/acs.jpcb.8b09584
URL
PMID:30550715
[Cited within: 1]

Drying and crystallization of solution droplets is a problem of broad relevance, determining the microstructures of particles formed in spray-drying, the phase of particles delivered by, for example, aerosol formulations for inhalation therapies, and the impact of aerosols on radiative forcing and climate. The ephemeral nature of free droplets, particularly when considering the drying kinetics of droplets with highly volatile constituents, has often precluded the accurate measurement of transient properties such as droplet size and composition, preventing the robust assessment of predictive models of droplet-drying rates, nucleation, and crystallization. Here, we report novel measurements of the drying kinetics of individual aqueous sodium chloride solution droplets using an electrodynamic balance to isolate and trap single aerosol droplets (radius approximately 25 mum). The initial solution droplet size and composition are shown to be highly reproducible in terms of drying rate and crystallization time when examined over hundreds of identical evaporating droplets. We introduce a numerical model that determines the concentration gradient across the radial profile of the droplet as it dries, considering both the surface recession because of evaporation and the diffusion of components within the droplet. Drying-induced crystallization is shown to be fully determined for this system, with nucleation and instantaneous crystallization occurring once a critical supersaturation level of 2.04 +/- 0.02 is achieved at the surface of the evaporating droplet. This phenomenological model provides a consistent account of the timescale and surface concentration of free-droplet crystallization on drying for the different drying conditions studied, a necessary step in progress toward achieving control over rates of crystallization and the competitive formation of amorphous particles.
Some factors affecting the survival of airborne viruses
DOI:10.1099/0022-1317-10-3-209 URL PMID:4324730 [Cited within: 1]
Rigidification of neutral lipid bilayers in the presence of salts
DOI:10.1529/biophysj.107.112615
URL
PMID:17586572

We studied the influence of sodium and calcium chloride on the global and local membrane properties of fluid palmitoyl-oleoyl phosphatidylcholine bilayers, applying synchrotron small-angle x-ray diffraction, spin-labeling electron paramagnetic resonance spectroscopy, and differential scanning calorimetry, as well as simultaneous density and acoustic measurements. The salt concentration was varied over a wide range from 0 to 5 M. We found that NaCl leads to a continuous swelling of the bilayers, whereas the behavior of the bilayer separation dW in the presence of CaCl2 is more complex, showing an initial large dW value, which decreased upon further addition of salt and finally increased again in the high concentration regime. This can be understood by a change of balance between electrostatic and van der Waals interactions. We were further able to show that both salts lead to a significant increase of order within the lipid bilayer, leading to a decrease of bilayer elasticity and shift of main phase transition temperature. This effect is more pronounced for Ca2+, and occurs mainly in the high salt-concentration regime. Thus, we were able to reconcile previous controversies between molecular dynamics simulations and x-ray diffraction experiments regarding the effect of salts on neutral lipid bilayers.
Mechanisms by which ambient humidity may affect viruses in aerosols
DOI:10.1128/AEM.01658-12
URL
PMID:22820337
[Cited within: 1]

Many airborne viruses have been shown to be sensitive to ambient humidity, yet the mechanisms responsible for this phenomenon remain elusive. We review multiple hypotheses, including water activity, surface inactivation, and salt toxicity, that may account for the association between humidity and viability of viruses in aerosols. We assess the evidence and limitations for each hypothesis based on findings from virology, aerosol science, chemistry, and physics. In addition, we hypothesize that changes in pH within the aerosol that are induced by evaporation may trigger conformational changes of the surface glycoproteins of enveloped viruses and subsequently compromise their infectivity. This hypothesis may explain the differing responses of enveloped viruses to humidity. The precise mechanisms underlying the relationship remain largely unverified, and attaining a complete understanding of them will require an interdisciplinary approach.
The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission
DOI:10.1073/pnas.2006874117
URL
PMID:32404416
[Cited within: 1]

Speech droplets generated by asymptomatic carriers of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are increasingly considered to be a likely mode of disease transmission. Highly sensitive laser light scattering observations have revealed that loud speech can emit thousands of oral fluid droplets per second. In a closed, stagnant air environment, they disappear from the window of view with time constants in the range of 8 to 14 min, which corresponds to droplet nuclei of ca. 4 mum diameter, or 12- to 21-mum droplets prior to dehydration. These observations confirm that there is a substantial probability that normal speaking causes airborne virus transmission in confined environments.
The flow physics of COVID-19
Violent expiratory events: on coughing and sneezing
DOI:10.1017/jfm.2014.88 URL [Cited within: 1]
Dynamics of airborne influenza A viruses indoors and dependence on humidity
DOI:10.1371/journal.pone.0021481
URL
PMID:21731764
[Cited within: 1]

Convective patterns of flow during inspiration
Turbulent gas clouds and respiratory pathogen emissions: Potential implications for reducing transmission of COVID-19
URL PMID:32215590 [Cited within: 1]
Characterization methods of colloids - Part I
Characterization methods of colloids - Part II
Exhaled particles and small airways
DOI:10.1186/s12931-019-0970-9
URL
PMID:30634967
[Cited within: 2]

Characterization methods: Physical and chemical characterization techniques
Evaporation and dispersion of respiratory droplets from coughing
DOI:10.1111/ina.12297
URL
PMID:26945674
[Cited within: 1]

Understanding how respiratory droplets become droplet nuclei and their dispersion is essential for understanding the mechanisms and control of disease transmission via droplet-borne and airborne routes. A theoretical model was developed to estimate the size of droplet nuclei and their dispersion as a function of the ambient humidity and droplet composition. The model-predicted dried droplet nuclei size was 32% of the original diameter, which agrees with the maximum residue size in the classic study by Duguid, 1946, Edinburg Med. J., 52, 335 and the validation experiment in this study, but is smaller than the 50% size predicted by Nicas et al., 2005, J. Occup. Environ. Hyg., 2, 143. The droplet nuclei size at a relative humidity of 90% (25 degrees C) could be 30% larger than the size of the same droplet at a relative humidity of less than 67.3% (25 degrees C). The trajectories of respiratory droplets in a cough jet are significantly affected by turbulence, which promotes the wide dispersion of droplets. We found that medium-sized droplets (e.g., 60 mum) are more influenced by humidity than are smaller and larger droplets, while large droplets (>/=100 mum), whose travel is less influenced by humidity, quickly settle out of the jet.
Effects of temperature and humidity on the spread of COVID-19: A systematic review
Effect of environmental conditions on SARS-CoV-2 stability in human nasal mucus and sputum
Physico-chemical characteristics of evaporating respiratory fluid droplets
DOI:10.1098/rsif.2017.0939
URL
PMID:29491178
[Cited within: 5]

The detailed physico-chemical characteristics of respiratory droplets in ambient air, where they are subject to evaporation, are poorly understood. Changes in the concentration and phase of major components in a droplet-salt (NaCl), protein (mucin) and surfactant (dipalmitoylphosphatidylcholine)-may affect the viability of any pathogens contained within it and thus may affect the efficiency of transmission of infectious disease by droplets and aerosols. The objective of this study is to investigate the effect of relative humidity (RH) on the physico-chemical characteristics of evaporating droplets of model respiratory fluids. We labelled these components in model respiratory fluids and observed evaporating droplets suspended on a superhydrophobic surface using optical and fluorescence microscopy. When exposed to continuously decreasing RH, droplets of different model respiratory fluids assumed different morphologies. Loss of water induced phase separation as well as indication of a decrease in pH. The presence of surfactant inhibited the rapid rehydration of the non-volatile components. An enveloped virus, varphi6, that has been proposed as a surrogate for influenza virus appeared to be homogeneously distributed throughout the dried droplet. We hypothesize that the increasing acidity and salinity in evaporating respiratory droplets may affect the structure of the virus, although at low enough RH, crystallization of the droplet components may eliminate their harmful effects.
Prolonged infectivity of SARS-CoV-2 in fomites
Perspective: Outstanding theoretical questions in polymer-nanoparticle hybrids
DOI:10.1063/1.4990501
URL
PMID:28711055
[Cited within: 1]

This topical review discusses the theoretical progress made in the field of polymer nanocomposites, i.e., hybrid materials created by mixing (typically inorganic) nanoparticles (NPs) with organic polymers. It primarily focuses on the outstanding issues in this field and is structured around five separate topics: (i) the synthesis of functionalized nanoparticles; (ii) their phase behavior when mixed with a homopolymer matrix and their assembly into well-defined superstructures; (iii) the role of processing on the structures realized by these hybrid materials and the role of the mobilities of the different constituents; (iv) the role of external fields (electric, magnetic) in the active assembly of the NPs; and (v) the engineering properties that result and the factors that control them. While the most is known about topic (ii), we believe that significant progress needs to be made in the other four topics before the practical promise offered by these materials can be realized. This review delineates the most pressing issues on these topics and poses specific questions that we believe need to be addressed in the immediate future.
A review on suppression and utilization of the coffee-ring effect
DOI:10.1016/j.cis.2017.12.008
URL
PMID:29310771
[Cited within: 1]

Evaporation of sessile droplets containing non-volatile solutes dispersed in a volatile solvent leaves behind ring-like solid stains. As the volatile species evaporates, pinning of the contact line gives rise to capillary flows that transport non-volatile solutes to the contact line. This phenomenon, called the coffee-ring effect, compromises the overall performance of industrially relevant manufacturing processes involving evaporation such as printing, biochemical analysis, manufacturing of nano-structured materials through colloidal and macromolecular patterning. Various approaches have been developed to suppress this phenomenon, which is otherwise difficult to avoid. The coffee-ring effect has also been leveraged to prepare new materials through convection induced assembly. This review underlines not only the strategies developed to suppress the coffee-ring effect but also sheds light on approaches to arrive at novel processes and materials. Working principles and applicability of these strategies are discussed together with a critical comparison.
Kinetically driven self assembly of highly ordered nanoparticle monolayers
URL PMID:16547519 [Cited within: 1]
A critical and quantitative review of the stratification of particles during the drying of colloidal films
DOI:10.1039/c8sm01025k
URL
PMID:30024010
[Cited within: 1]

For a wide range of applications, films are deposited from colloidal particles suspended in a volatile liquid. There is burgeoning interest in stratifying colloidal particles into separate layers within the final dry film to impart properties at the surface different to the interior. Here, we outline the mechanisms by which colloidal mixtures can stratify during the drying process. The problem is considered here as a three-way competition between evaporation of the continuous liquid, sedimentation of particles, and their Brownian diffusion. In particle mixtures, the sedimentation of larger or denser particles offers one means of stratification. When the rate of evaporation is fast relative to diffusion, binary mixtures of large and small particles can stratify with small particles on the top, according to physical models and computer simulations. We compare experimental results found in the scientific literature to the predictions of several recent models in a quantitative way. Although there is not perfect agreement between them, some general trends emerge in the experiments, simulations and models. The stratification of small particles on the top of a film is favoured when the colloidal suspension is dilute but when both the concentration of the small particles and the solvent evaporation rate are sufficiently high. A higher particle size ratio also favours stratification by size. This review points to ways that microstructures can be designed and controlled in colloidal materials to achieve desired properties.
Dispersing nanoparticles in a polymer film via solvent evaporation
Stratification in drying films containing bidisperse mixtures of nanoparticles
URL PMID:29792029 [Cited within: 1]
Dynamics of polymer segments, polymer chains, and nanoparticles in polymer nanocomposite melts: A review
Mobility of polymer-tethered nanoparticles in unentangled polymer melts
DOI:10.1021/acs.macromol.8b02138
URL
PMID:30956355

tau ( *) is approximated as the MSD Deltar (2)(t) tail of monomers in a free tail, whereas Deltar (2)(t) of a multi-tail NP for t > tau ( *) is approximated as the MSD Deltar (2)(t) star of the branch point of a star polymer. The time dependence of Deltar (2)(t) in a tail-dominated regime exhibits two qualitatively different sub-diffusive regimes. The first sub-diffusive regime for t tau ( *) occurs as the particle participates in the dynamics of the tails. For NPs with loosely grafted chains, there is a Gaussian brush region surrounding the NP, where the chain strands in Gaussian conformations undergo Rouse dynamics with no hydrodynamic coupling. The crossover time tau ( *) for loosely grafted multi-tail NPs in a tail-dominated regime decreases as the number of tails increases. For NPs with densely grafted chains, the tails are hydrodynamically coupled to each other. The hydrodynamic radii for the diffusion of densely grafted multi-tail NPs are approximated by the sum of the particle and tail sizes.]]>
Effects of tethered polymers on dynamics of nanoparticles in unentangled polymer melts