Biomaterials Translational ›› 2021, Vol. 2 ›› Issue (1): 10-18.doi: 10.3877/cma.j.issn.2096-112X.2021.01.003
• REVIEW • Previous Articles Next Articles
Ting Ge1,*( ), Shengfeng Cheng2,*(
), Shengfeng Cheng2,*( )
)
Received:2020-10-26
Revised:2020-12-24
Accepted:2020-12-29
Online:2021-03-31
Published:2021-03-28
Contact:
Ting Ge,Shengfeng Cheng
E-mail:tingg@mailbox.sc.edu;chengsf@vt.edu
Ge, T.; Cheng, S. Physicochemical properties of respiratory droplets and their role in COVID-19 pandemics: a critical review. Biomater Transl. 2021, 2(1), 10-18.
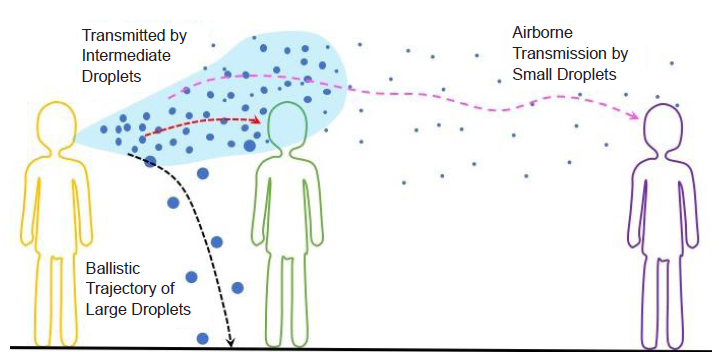
Figure 1. Schematic illustration of the different transmission routes of respiratory tract viruses via large (≥ 100 μm), intermediate (~10-100 μm), and small (< 5 μm) droplets.
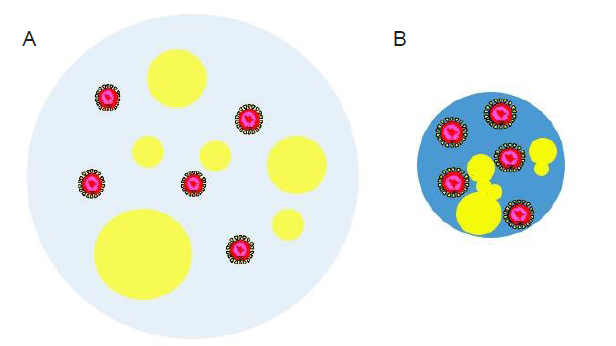
Figure 3. Schematic illustration of a virus-laden respiratory droplet after being released by an infected person (A) and after the loss of some water content through evaporation (B). The water part of the droplet is colored light blue in A while blue in B. Yellow “bubbles” of various sizes indicate the nonaqueous components such as salt, proteins, and surfactants. The virus particles are drawn as core-shell structures with the core colored in red.
| Nonaqueous components | Brief description |
|---|---|
| Salt and lactate | Soluble, present as charged ions |
| Cholesterol | Insoluble, non-polar (except for the hydroxyl group at one end) |
| Proteins and enzymes | Amphiphilic, net charge depends on the sequence of amino acids and pH |
| Lung surfactants | Amphiphilic mixture of phospholipids and proteins |
| Cell debris | Organic waste from epithelial and immune system cells |
| Bacteria | Most bacterial cell walls carry negative charges |
| Viruses | pH-dependent surface charges |
Table 1 List of representative nonaqueous components in a respiratory droplet
| Nonaqueous components | Brief description |
|---|---|
| Salt and lactate | Soluble, present as charged ions |
| Cholesterol | Insoluble, non-polar (except for the hydroxyl group at one end) |
| Proteins and enzymes | Amphiphilic, net charge depends on the sequence of amino acids and pH |
| Lung surfactants | Amphiphilic mixture of phospholipids and proteins |
| Cell debris | Organic waste from epithelial and immune system cells |
| Bacteria | Most bacterial cell walls carry negative charges |
| Viruses | pH-dependent surface charges |
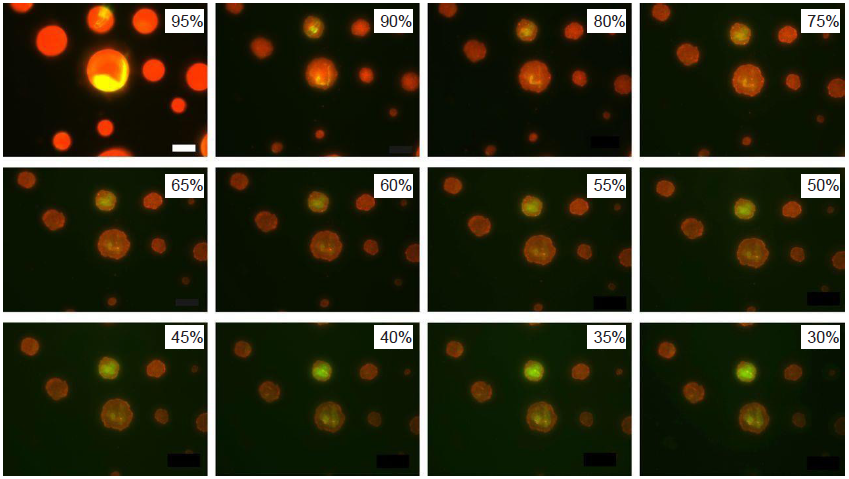
Figure 6. Fluorescence images of model droplets exposed to decreasing relative humidity. A droplet contains water, NaCl, mucin (red), and DDPC (green). DDPC: 1,2-dihexadecanoyl-sn-glycero-3-phosphocholine. Reprinted with permission from Vejerano and Marr.50 Copyright (2018) The Royal Society.
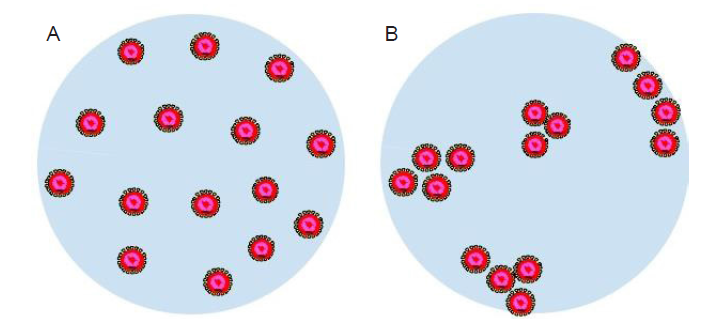
Figure 7. Schematic illustration of a respiratory droplet with uniformly distributed virus particles (A) and aggregated clusters of virus particles (B). The virus particles are drawn as core-shell structures with the core colored in red.
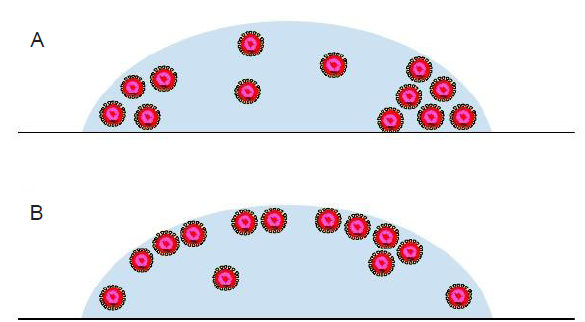
Figure 8. The distribution of virus particles in a droplet on a surface is conjectured to be affected by the evaporation dynamics and internal flow. The virus particles can possibly aggregate at the edge of the deposited droplet (A) or the droplet-air interface (B). The virus particles are drawn as core-shell structures with the core colored in red.
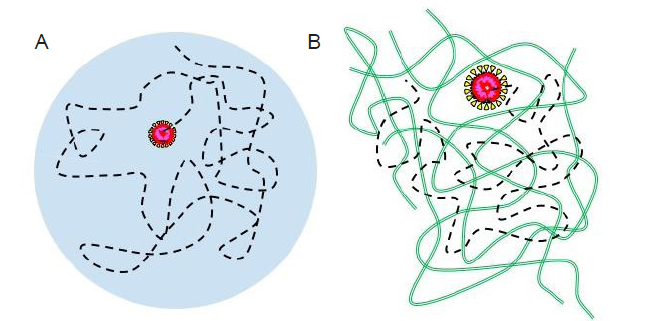
Figure 9. Schematic illustration of the trajectory (black dashed lines) of an individual virus particle in a respiratory droplet (A) and a mucus layer (green chains) (B). The virus particles are drawn as core-shell structures with the core colored in red.
| 1. | Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu. Accessed by January 3, 2021. |
| 2. | World Health Organization. Coronavirus disease (COVID-19) advice for the public. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public. Accessed by January 3, 2021. |
| 3. | Zimmer, C.; Corum, J.; Wee, S. L. Coronavirus vaccine tracker. https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html. Accessed by January 3, 2021. |
| 4. |
Ghaffarzadegan, N.; Childs, L. M.; Täuber, U. C. Diverse Computer Simulation Models Provide Unified Lessons on University Operation during a Pandemic. Bioscience. 2020. doi: 10.1093/biosci/biaa122.
doi: 10.1093/biosci/biaa064 URL pmid: 32665736 |
| 5. |
Huang, H.; Fan, C.; Li, M.; Nie, H. L.; Wang, F. B.; Wang, H.; Wang, R.; Xia, J.; Zheng, X.; Zuo, X.; Huang, J. COVID-19: A call for physical scientists and engineers. ACS Nano. 2020, 14, 3747-3754.
doi: 10.1021/acsnano.0c02618 URL pmid: 32267678 |
| 6. |
Behzadinasab, S.; Chin, A.; Hosseini, M.; Poon, L.; Ducker, W. A. A surface coating that rapidly inactivates SARS-CoV-2. ACS Appl Mater Interfaces. 2020, 12, 34723-34727.
doi: 10.1021/acsami.0c11425 URL pmid: 32657566 |
| 7. |
Masters, P. S. The molecular biology of coronaviruses. Adv Virus Res. 2006, 66, 193-292.
doi: 10.1016/S0065-3527(06)66005-3 URL pmid: 16877062 |
| 8. |
Weber, T. P.; Stilianakis, N. I. Inactivation of influenza A viruses in the environment and modes of transmission: a critical review. J Infect. 2008, 57, 361-373.
doi: 10.1016/j.jinf.2008.08.013 URL pmid: 18848358 |
| 9. | World Health Organization. Transmission of SARS-CoV-2: implications for infection prevention precautions. https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions. Accessed by January 3, 2021. |
| 10. |
Duguid, J. P. The numbers and the sites of origin of the droplets expelled during expiratory activities. Edinb Med J. 1945, 52, 385-401.
URL pmid: 21009905 |
| 11. | Loudon, R. G.; Roberts, R. M. Relation between the airborne diameters of respiratory droplets and the diameter of the stains left after recovery. Nature. 1967, 213, 95-96. |
| 12. |
Fennelly, K. P.; Martyny, J. W.; Fulton, K. E.; Orme, I. M.; Cave, D. M.; Heifets, L. B. Cough-generated aerosols of Mycobacterium tuberculosis: a new method to study infectiousness. Am J Respir Crit Care Med. 2004, 169, 604-609.
doi: 10.1164/rccm.200308-1101OC URL pmid: 14656754 |
| 13. |
Chao, C. Y. H.; Wan, M. P.; Morawska, L.; Johnson, G. R.; Ristovski, Z. D.; Hargreaves, M.; Mengersen, K.; Corbett, S.; Li, Y.; Xie, X.; Katoshevski, D. Characterization of expiration air jets and droplet size distributions immediately at the mouth opening. J Aerosol Sci. 2009, 40, 122-133.
doi: 10.1016/j.jaerosci.2008.10.003 URL pmid: 32287373 |
| 14. |
Xie, X.; Li, Y.; Sun, H.; Liu, L. Exhaled droplets due to talking and coughing. J R Soc Interface. 2009, 6 Suppl 6, S703-714.
doi: 10.1098/rsif.2009.0388.focus URL pmid: 19812073 |
| 15. |
Otter, J. A.; Donskey, C.; Yezli, S.; Douthwaite, S.; Goldenberg, S. D.; Weber, D. J. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J Hosp Infect. 2016, 92, 235-250.
doi: 10.1016/j.jhin.2015.08.027 URL pmid: 26597631 |
| 16. |
van Doremalen N.; Bushmaker, T.; Morris, D. H.; Holbrook, M. G.; Gamble, A.; Williamson, B. N.; Tamin, A.; Harcourt, J. L.; Thornburg, N. J.; Gerber, S. I.; Lloyd-Smith, J. O.; de Wit, E.; Munster, V. J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020, 382, 1564-1567.
doi: 10.1056/NEJMc2004973 URL pmid: 32182409 |
| 17. | World Health Organization. Infection prevention and control of epidemic-and pandemic-prone acute respiratory infections in health care. https://apps.who.int/iris/bitstream/handle/10665/112656/9789241507134_eng.pdf;jsessionid=41AA684FB64571CE8D8A453C4F2B2096?sequence=1. Accessed by January 3, 2021. |
| 18. |
Liu, Y.; Ning, Z.; Chen, Y.; Guo, M.; Liu, Y.; Gali, N. K.; Sun, L.; Duan, Y.; Cai, J.; Westerdahl, D.; Liu, X.; Xu, K.; Ho, K. F.; Kan, H.; Fu, Q.; Lan, K. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020, 582, 557-560.
doi: 10.1038/s41586-020-2271-3 URL pmid: 32340022 |
| 19. |
Nicas, M.; Nazaroff, W. W.; Hubbard, A. Toward understanding the risk of secondary airborne infection: emission of respirable pathogens. J Occup Environ Hyg. 2005, 2, 143-154.
doi: 10.1080/15459620590918466 URL pmid: 15764538 |
| 20. | Morawska, L.; Johnson, G. R.; Ristovski, Z. D.; Hargreaves, M.; Mengersen, K.; Corbett, S.; Chao, C. Y. H.; Li, Y.; Katoshevski, D. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. J Aerosol Sci. 2009, 40, 256-269. |
| 21. | Morawska, L.; Cao, J. Airborne transmission of SARS-CoV-2: The world should face the reality. Environ Int. 2020, 139, 105730. |
| 22. | Grasmeijer, N.; Frijlink, H. W.; Hinrichs, W. L. J. An adaptable model for growth and/or shrinkage of droplets in the respiratory tract during inhalation of aqueous particles. J Aerosol Sci. 2016, 93, 21-34. |
| 23. |
Zanin, M.; Baviskar, P.; Webster, R.; Webby, R. The interaction between respiratory pathogens and mucus. Cell Host Microbe. 2016, 19, 159-168.
doi: 10.1016/j.chom.2016.01.001 URL pmid: 26867175 |
| 24. |
Xu, H.; Zhong, L.; Deng, J.; Peng, J.; Dan, H.; Zeng, X.; Li, T.; Chen, Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020, 12, 8.
doi: 10.1038/s41368-020-0074-x URL pmid: 32094336 |
| 25. |
Effros, R. M.; Hoagland, K. W.; Bosbous, M.; Castillo, D.; Foss, B.; Dunning, M.; Gare, M.; Lin, W.; Sun, F. Dilution of respiratory solutes in exhaled condensates. Am J Respir Crit Care Med. 2002, 165, 663-669.
doi: 10.1164/ajrccm.165.5.2101018 URL pmid: 11874811 |
| 26. |
Davies, N. M.; Feddah, M. R. A novel method for assessing dissolution of aerosol inhaler products. Int J Pharm. 2003, 255, 175-187.
doi: 10.1016/s0378-5173(03)00091-7 URL pmid: 12672613 |
| 27. |
Larsson, B.; Olivecrona, G.; Ericson, T. Lipids in human saliva. Arch Oral Biol. 1996, 41, 105-110.
doi: 10.1016/0003-9969(95)00077-1 URL pmid: 8833598 |
| 28. |
Ruocco, L.; Fattori, B.; Romanelli, A.; Martelloni, M.; Casani, A.; Samolewska, M.; Rezzonico, R. A new collection method for the evaluation of nasal mucus proteins. Clin Exp Allergy. 1998, 28, 881-888.
doi: 10.1046/j.1365-2222.1998.00312.x URL pmid: 9720823 |
| 29. |
Xie, X.; Li, Y.; Chwang, A. T.; Ho, P. L.; Seto, W. H. How far droplets can move in indoor environments--revisiting the Wells evaporation-falling curve. Indoor Air. 2007, 17, 211-225.
URL pmid: 17542834 |
| 30. | Chaudhuri, S.; Basu, S.; Kabi, P.; Unni, V. R.; Saha, A. Modeling the role of respiratory droplets in Covid-19 type pandemics. Phys Fluids (1994). 2020, 32, 063309. |
| 31. | You, Y.; Renbaum-Wolff, L.; Carreras-Sospedra, M.; Hanna, S. J.; Hiranuma, N.; Kamal, S.; Smith, M. L.; Zhang, X.; Weber, R. J.; Shilling, J. E.; Dabdub, D.; Martin, S. T.; Bertram, A. K. Images reveal that atmospheric particles can undergo liquid-liquid phase separations. Proc Natl Acad Sci U S A. 2012, 109, 13188-13193. |
| 32. |
Dallemagne, M. A.; Huang, X. Y.; Eddingsaas, N. C. Variation in pH of model secondary organic aerosol during liquid-liquid phase separation. J Phys Chem A. 2016, 120, 2868-2876.
doi: 10.1021/acs.jpca.6b00275 URL pmid: 27082856 |
| 33. |
Gregson, F. K. A. Robinson, J. F.; Miles, R. E. H.; Royall, C. P.; Reid, J. P. Drying kinetics of salt solution droplets: Water evaporation rates and crystallization. J Phys Chem B. 2019, 123, 266-276.
doi: 10.1021/acs.jpcb.8b09584 URL pmid: 30550715 |
| 34. |
Benbough, J. E. Some factors affecting the survival of airborne viruses. J Gen Virol. 1971, 10, 209-220.
doi: 10.1099/0022-1317-10-3-209 URL pmid: 4324730 |
| 35. |
Pabst, G.; Hodzic, A.; Strancar, J.; Danner, S.; Rappolt, M.; Laggner, P. Rigidification of neutral lipid bilayers in the presence of salts. Biophys J. 2007, 93, 2688-2696.
doi: 10.1529/biophysj.107.112615 URL pmid: 17586572 |
| 36. |
Yang, W.; Marr, L. C. Mechanisms by which ambient humidity may affect viruses in aerosols. Appl Environ Microbiol. 2012, 78, 6781-6788.
doi: 10.1128/AEM.01658-12 URL pmid: 22820337 |
| 37. |
Stadnytskyi, V.; Bax, C. E.; Bax, A.; Anfinrud, P. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. Proc Natl Acad Sci U S A. 2020, 117, 11875-11877.
doi: 10.1073/pnas.2006874117 URL pmid: 32404416 |
| 38. | Mittal, R.; Ni, R.; Seo, J. H. The flow physics of COVID-19. J Fluid Mech. 2020, 894, F2. |
| 39. |
Bourouiba, L.; Dehandschoewercker, E.; Bush, John W. M. Violent expiratory events: on coughing and sneezing. J Fluid Mech. 2014, 745, 537-563.
doi: 10.1017/jfm.2014.88 URL |
| 40. |
Yang, W.; Marr, L. C. Dynamics of airborne influenza A viruses indoors and dependence on humidity. PLoS One. 2011, 6, e21481.
doi: 10.1371/journal.pone.0021481 URL pmid: 21731764 |
| 41. |
Olson, D. E.; Sudlow, M. F.; Horsfield, K.; Filley, G. F. Convective patterns of flow during inspiration. Arch Intern Med. 1973, 131, 51-57.
URL pmid: 4682063 |
| 42. |
Bourouiba, L. Turbulent gas clouds and respiratory pathogen emissions: Potential implications for reducing transmission of COVID-19. JAMA. 2020, 323, 1837-1838.
URL pmid: 32215590 |
| 43. | Kontogeorgis, G. M.; Kiil, S. Characterization methods of colloids - Part I. In Introduction to applied colloid and surface chemistry, Kontogeorgis, G. M.; Kiil, S., eds.; John Wiley & Sons, Ltd.: Hoboken, 2016; pp 185-201. |
| 44. | Kontogeorgis, G. M.; Kiil, S. Characterization methods of colloids - Part II. In Introduction to applied colloid and surface chemistry, Kontogeorgis, G. M.; Kiil, S., eds.; John Wiley & Sons, Inc.: Hoboken, 2016; pp 202-210. |
| 45. |
Bake, B.; Larsson, P.; Ljungkvist, G.; Ljungström, E.; Olin, A. C. Exhaled particles and small airways. Respir Res. 2019, 20, 8.
doi: 10.1186/s12931-019-0970-9 URL pmid: 30634967 |
| 46. | Cornier, J.; Owen, A.; Kwade, A.; Van de Voorde, M. Characterization methods: Physical and chemical characterization techniques. In Pharmaceutical Nanotechnology: Innovation and Production, Cornier, J.; Owen, A.; Kwade, A.; Voorde, M. V. D., eds.; John Wiley & Sons, Inc.: Hoboken, 2017; pp 135-156. |
| 47. |
Liu, L.; Wei, J.; Li, Y.; Ooi, A. Evaporation and dispersion of respiratory droplets from coughing. Indoor Air. 2017, 27, 179-190.
doi: 10.1111/ina.12297 URL pmid: 26945674 |
| 48. | Mecenas, P.; Bastos, R.; Vallinoto, A. C. R.; Normando, D. Effects of temperature and humidity on the spread of COVID-19: A systematic review. PLoS One. 2020, 15, e0238339. |
| 49. | Matson, M. J.; Yinda, C. K.; Seifert, S. N.; Bushmaker, T.; Fischer, R. J.; van Doremalen, N.; Lloyd-Smith, J. O.; Munster, V. J. Effect of environmental conditions on SARS-CoV-2 stability in human nasal mucus and sputum. Emerg Infect Dis. 2020, 26, 2276-2278. |
| 50. |
Vejerano, E. P.; Marr, L. C. Physico-chemical characteristics of evaporating respiratory fluid droplets. J R Soc Interface. 2018, 15, 20170939.
doi: 10.1098/rsif.2017.0939 URL pmid: 29491178 |
| 51. | Pastorino, B.; Touret, F.; Gilles, M.; de Lamballerie, X.; Charrel, R. N. Prolonged infectivity of SARS-CoV-2 in fomites. Emerg Infect Dis. 2020, 26, 2256-2257. |
| 52. |
Kumar, S. K.; Ganesan, V.; Riggleman, R. A. Perspective: Outstanding theoretical questions in polymer-nanoparticle hybrids. J Chem Phys. 2017, 147, 020901.
doi: 10.1063/1.4990501 URL pmid: 28711055 |
| 53. |
Mampallil, D.; Eral, H. B. A review on suppression and utilization of the coffee-ring effect. Adv Colloid Interface Sci. 2018, 252, 38-54.
doi: 10.1016/j.cis.2017.12.008 URL pmid: 29310771 |
| 54. |
Bigioni, T. P.; Lin, X. M.; Nguyen, T. T.; Corwin, E. I.; Witten, T. A.; Jaeger, H. M. Kinetically driven self assembly of highly ordered nanoparticle monolayers. Nat Mater. 2006, 5, 265-270.
URL pmid: 16547519 |
| 55. |
Schulz, M.; Keddie, J. L. A critical and quantitative review of the stratification of particles during the drying of colloidal films. Soft Matter. 2018, 14, 6181-6197.
doi: 10.1039/c8sm01025k URL pmid: 30024010 |
| 56. | Cheng, S.; Grest, G. S. Dispersing nanoparticles in a polymer film via solvent evaporation. ACS Macro Lett. 2016, 5, 694-698. |
| 57. |
Tang, Y.; Grest, G. S.; Cheng, S. Stratification in drying films containing bidisperse mixtures of nanoparticles. Langmuir. 2018, 34, 7161-7170.
URL pmid: 29792029 |
| 58. | Bailey, E. J.; Winey, K. I. Dynamics of polymer segments, polymer chains, and nanoparticles in polymer nanocomposite melts: A review. Prog Polym Sci. 2020, 105, 101242. |
| 59. |
Ge, T.; Rubinstein, M. Mobility of polymer-tethered nanoparticles in unentangled polymer melts. Macromolecules. 2019, 52, 1536-1545.
doi: 10.1021/acs.macromol.8b02138 URL pmid: 30956355 |
| 60. | Ge, T.; Rubinstein, M.; Grest, G. S. Effects of tethered polymers on dynamics of nanoparticles in unentangled polymer melts. Macromolecules. 2020, 53, 6898-6906. |
| [1] | Isak Jatoi, Jingyu Fan. A biomaterials viewpoint for the 2020 SARS-CoV-2 vaccine development [J]. Biomaterials Translational, 2021, 2(1): 30-42. |
| [2] | Dahae Seong, Monchupa Kingsak, Yuan Lin, Qian Wang, Shamia Hoque. Fate and transport of enveloped viruses in indoor built spaces - through understanding vaccinia virus and surface interactions [J]. Biomaterials Translational, 2021, 2(1): 50-60. |
| [3] | Kaewta Rattanapisit, Gorawit Yusakul, Balamurugan Shanmugaraj, Kittinop Kittirotruji, Phassorn Suwatsrisakul, Eakachai Prompetchara, Suthira Taychakhoonavud, Waranyoo Phoolcharoen. Plant-produced recombinant SARS-CoV-2 receptor-binding domain; an economical, scalable biomaterial source for COVID-19 diagnosis [J]. Biomaterials Translational, 2021, 2(1): 43-49. |
| [4] | Jishan Yuan, Panita Maturavongsadit, Zhihui Zhou, Bin Lv, Yuan Lin, Jia Yang, Jittima Amie Luckanagul. Hyaluronic acid-based hydrogels with tobacco mosaic virus containing cell adhesive peptide induce bone repair in normal and osteoporotic rats [J]. Biomaterials Translational, 2020, 1(1): 89-98. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||