Ten Cate’s oral histology: development, structure, and function
6
2012
... Teeth are ectodermal organs that are formed by reciprocal and sequential interaction between the oral epithelial cells (ectoderm) and cranial neural crest-derived mesenchymal cells, during the embryonic development. The epithelial cells give rise to the enamel-forming ameloblasts, which are highly specialized cells that undergo apoptosis following the production of enamel, and the mesenchymal cells give rise to differentiated cells that contribute to form the majority of dental tissues, such as dentine-forming odontoblasts, other diverse populations of dental pulp cells, periodontal ligament and cementum.1 ...
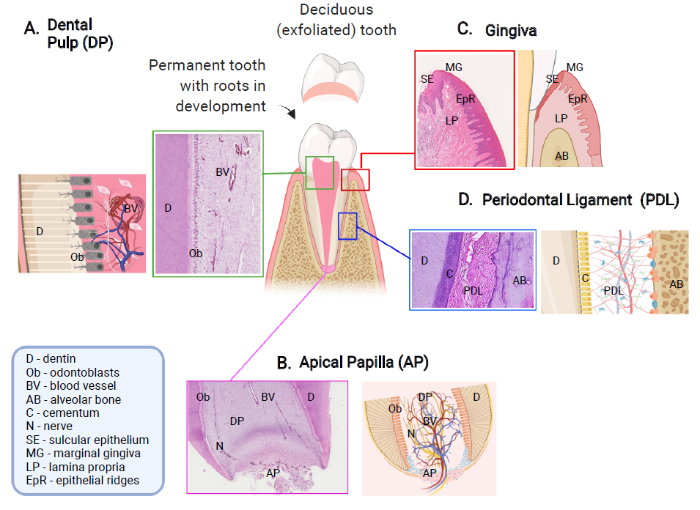
... Teeth that are still undergoing development of their roots harbour a unique population of cells located in the apical papilla region that have been shown to exhibit stem cells properties, termed stem cells from apical papilla (SCAP).28, 29 The apical papilla region plays an important role in root development and formation of different dental tissues during this physiological process1 (Figure 1B). ...
... The oral mucosa is the first line of defence against oral diseases. One of the most rapidly dividing tissues in our body, it exhibits remarkable healing properties of foetal-like, scarless wound healing phenotype.40 The tissue that directly surrounds the tooth and attaches to the tooth surface by a junctional epithelium, providing a seal, is known as gingiva (Figure 1C). Gingiva is part of a complex of tissues, known as periodontium, along with the alveolar socket, periodontal ligament and the cementum, that provide attachment of the tooth and play role in its homeostasis.1, 41 It is particularly important structure, providing defence against microorganisms, during plaque formation and providing resistance to mechanical forces during mastication. ...
... The gingiva is composed of epithelium, derived from embryonic ectoderm, and underlying connective tissue that originates from neural crest cells and mesoderm.1 Gingival tissue is easily accessible during routine dental procedures and very often remnants of the tissue are found following tooth extraction, making it a very attractive source for isolation of different stem cells.7-10 Cells derived from the connective tissue compartment of human gingiva have been characterised by expression of MSC markers, such as Oct-4 (octamer-binding transcription factor 4), SSEA-4 (stage-specific embryonic antigen-4) and STRO-142, 43 and undergo multilineage differentiation under in vitro conditions.43 The cells from the epithelial compartment and derived from oral mucosa were also characterised by expression of stem cells markers in vitro and used in ocular surface reconstruction when grown as oral mucosal epithelial sheets in rabbit44 and human,45 offering promising results for future regenerative therapies. ...
... The periodontal ligament is a connective tissue that provides the anchorage to the tooth within the alveolar bone socket and takes the mechanical pressure during dental function and can be defined as a fibrous joint (Figure 1D).1 The periodontal ligament develops from the dental follicle, which is a fibrous sac that surrounds the developing tooth bud.1 In vivo lineage-tracing experiments suggested that the dental follicle contains mesenchymal progenitor cells expressing parathyroid hormone-related protein, which give rise to cells forming the periodontal attachment apparatus in a manner regulated by autocrine signalling through the parathyroid hormone/parathyroid hormone-related protein receptor.46 ...
... 1 In vivo lineage-tracing experiments suggested that the dental follicle contains mesenchymal progenitor cells expressing parathyroid hormone-related protein, which give rise to cells forming the periodontal attachment apparatus in a manner regulated by autocrine signalling through the parathyroid hormone/parathyroid hormone-related protein receptor.46 ...
Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo
4
2000
... Cells with stem cells properties have been derived and characterized from different dental/oral tissues,2-6 making the teeth and the supporting oral tissues attractive and accessible sources of stem cells.7-10 ...
... The isolation of cells that exhibited stem cell properties from adult human dental pulps was reported by Gronthos and colleagues2, 3 who described population of cells, DPSCs obtained from extracted permanent third molars, showing high proliferation and high colony-forming properties, being able to produce sporadic, densely calcified nodules. These cells, when transplanted in vivo into immunocompromised mice, demonstrated ability to generate dentin/pulp-like complexes.2, 3 Further characterization revealed that DPSCs have multilineage differentiation capacity in vitro.2-9 ...
... 2, 3 Further characterization revealed that DPSCs have multilineage differentiation capacity in vitro.2-9 ...
... 2-9 ...
Stem cell properties of human dental pulp stem cells
2
2002
... The isolation of cells that exhibited stem cell properties from adult human dental pulps was reported by Gronthos and colleagues2, 3 who described population of cells, DPSCs obtained from extracted permanent third molars, showing high proliferation and high colony-forming properties, being able to produce sporadic, densely calcified nodules. These cells, when transplanted in vivo into immunocompromised mice, demonstrated ability to generate dentin/pulp-like complexes.2, 3 Further characterization revealed that DPSCs have multilineage differentiation capacity in vitro.2-9 ...
... , 3 Further characterization revealed that DPSCs have multilineage differentiation capacity in vitro.2-9 ...
Isolation and characterization of postnatal stem cells from human dental tissues
0
2007
Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine
3
2009
... When isolated, SCAP have been characterised by their capacity for multilineage differentiation, following induction in vitro.5 In comparison to DPSCs, these cells express higher proliferation and cell migration capacity5, 33 and have a higher expression of antiapoptotic protein, longer telomere length, and greater telomerase activity associated with cellular lifespan and cell proliferation than DPSCs.34 All different stem cells isolated from dental/oral tissues have shown to be able to undergo osteogenic differentiation and produce minerals with different chemical composition in vitro, when analysed with Raman spectroscopy.35 A possible downregulation of RUNX2 has been suggested when SCAP cells undergo diversion from osteoblastic to odontoblastic differentiation route.36 ...
... 5, 33 and have a higher expression of antiapoptotic protein, longer telomere length, and greater telomerase activity associated with cellular lifespan and cell proliferation than DPSCs.34 All different stem cells isolated from dental/oral tissues have shown to be able to undergo osteogenic differentiation and produce minerals with different chemical composition in vitro, when analysed with Raman spectroscopy.35 A possible downregulation of RUNX2 has been suggested when SCAP cells undergo diversion from osteoblastic to odontoblastic differentiation route.36 ...
... The secretome analysis of SCAP cells indicates that they secrete significantly more chemokines, neurotrophins and proteins involved in metabolic processes and transcription, compared to bone marrow MSCs.37 Being located at the very tip of the developing root, enables the region of the apical papilla to have accessibility to a collateral circulation,5, 38 which might affect the diverse dynamic of the cellular components and processes of the region and therefore the SCAP population of cells. ...
Characterization of stem and progenitor cells in the dental pulp of erupted and unerupted murine molars
1
2010
... Cells with stem cells properties have been derived and characterized from different dental/oral tissues,2-6 making the teeth and the supporting oral tissues attractive and accessible sources of stem cells.7-10 ...
Stem cell-based biological tooth repair and regeneration
4
2010
... Cells with stem cells properties have been derived and characterized from different dental/oral tissues,2-6 making the teeth and the supporting oral tissues attractive and accessible sources of stem cells.7-10 ...
... Cells with stem cell properties were also isolated from the remnants of human deciduous teeth dental pulp (Figure 1), termed “stem cells from human deciduous teeth”.19 These cells can be easily obtained, making the deciduous, physiologically replaced teeth, a highly accessible, otherwise discarded, source for MSCs.7-10 Stem cells from human deciduous teeth were also shown to be highly proliferative, clonogenic cells that have capacity for multilineage differentiation with higher proliferation rates and increased population doublings, when compared to DPSCs isolated from permanent teeth. These cells show osteo-inductive capacity and were able to generate dentine-like structures when transplanted in vivo.19-24 ...
... Stem cells from human deciduous teeth lack expression of major histocompatibility complex-II and have been shown to express hypo-immunogenic phenotype, inhibiting T-cell function through conserved induction of cellular stress.25 Moreover, these immunomodulatory properties have been shown to be conserved in vitro and follow the common strategy of immunoregulation that takes place in vivo, during the process of tissue repair.26 These important immunomodulatory properties, in addition to their neural crest origin27 and ability to keep their stem cell properties after a long-term period cryopreservation, as well as their accessibility, (from usually discarded tissues, such as 20 deciduous teeth in our lifetime), make them a particularly attractive source for MSCs and target for a therapeutic strategy.7-10 ...
... The gingiva is composed of epithelium, derived from embryonic ectoderm, and underlying connective tissue that originates from neural crest cells and mesoderm.1 Gingival tissue is easily accessible during routine dental procedures and very often remnants of the tissue are found following tooth extraction, making it a very attractive source for isolation of different stem cells.7-10 Cells derived from the connective tissue compartment of human gingiva have been characterised by expression of MSC markers, such as Oct-4 (octamer-binding transcription factor 4), SSEA-4 (stage-specific embryonic antigen-4) and STRO-142, 43 and undergo multilineage differentiation under in vitro conditions.43 The cells from the epithelial compartment and derived from oral mucosa were also characterised by expression of stem cells markers in vitro and used in ocular surface reconstruction when grown as oral mucosal epithelial sheets in rabbit44 and human,45 offering promising results for future regenerative therapies. ...
The tooth -- a treasure chest of stem cells
0
2013
Tooth repair and regeneration
2
2018
... In shallow enamel and enamel/dentinal damage, resident odontoblasts, polarised and highly specialised cells, located on the dentin-pulp border are activated, protecting the dental pulp via formation of reactionary dentine.13, 14 If the damage advances, resident odontoblasts may not survive and this initiates a cascade of stem cell activation, proliferation, and differentiation into new odontoblast-like cells that engage in reparative dentine secretion.9, 15 ...
... The isolation of cells that exhibited stem cell properties from adult human dental pulps was reported by Gronthos and colleagues2, 3 who described population of cells, DPSCs obtained from extracted permanent third molars, showing high proliferation and high colony-forming properties, being able to produce sporadic, densely calcified nodules. These cells, when transplanted in vivo into immunocompromised mice, demonstrated ability to generate dentin/pulp-like complexes.2, 3 Further characterization revealed that DPSCs have multilineage differentiation capacity in vitro.2-9 ...
Tooth bioengineering and regenerative dentistry
4
2019
... Cells with stem cells properties have been derived and characterized from different dental/oral tissues,2-6 making the teeth and the supporting oral tissues attractive and accessible sources of stem cells.7-10 ...
... Cells with stem cell properties were also isolated from the remnants of human deciduous teeth dental pulp (Figure 1), termed “stem cells from human deciduous teeth”.19 These cells can be easily obtained, making the deciduous, physiologically replaced teeth, a highly accessible, otherwise discarded, source for MSCs.7-10 Stem cells from human deciduous teeth were also shown to be highly proliferative, clonogenic cells that have capacity for multilineage differentiation with higher proliferation rates and increased population doublings, when compared to DPSCs isolated from permanent teeth. These cells show osteo-inductive capacity and were able to generate dentine-like structures when transplanted in vivo.19-24 ...
... Stem cells from human deciduous teeth lack expression of major histocompatibility complex-II and have been shown to express hypo-immunogenic phenotype, inhibiting T-cell function through conserved induction of cellular stress.25 Moreover, these immunomodulatory properties have been shown to be conserved in vitro and follow the common strategy of immunoregulation that takes place in vivo, during the process of tissue repair.26 These important immunomodulatory properties, in addition to their neural crest origin27 and ability to keep their stem cell properties after a long-term period cryopreservation, as well as their accessibility, (from usually discarded tissues, such as 20 deciduous teeth in our lifetime), make them a particularly attractive source for MSCs and target for a therapeutic strategy.7-10 ...
... The gingiva is composed of epithelium, derived from embryonic ectoderm, and underlying connective tissue that originates from neural crest cells and mesoderm.1 Gingival tissue is easily accessible during routine dental procedures and very often remnants of the tissue are found following tooth extraction, making it a very attractive source for isolation of different stem cells.7-10 Cells derived from the connective tissue compartment of human gingiva have been characterised by expression of MSC markers, such as Oct-4 (octamer-binding transcription factor 4), SSEA-4 (stage-specific embryonic antigen-4) and STRO-142, 43 and undergo multilineage differentiation under in vitro conditions.43 The cells from the epithelial compartment and derived from oral mucosa were also characterised by expression of stem cells markers in vitro and used in ocular surface reconstruction when grown as oral mucosal epithelial sheets in rabbit44 and human,45 offering promising results for future regenerative therapies. ...
Induction and regulation of crown dentinogenesis: embryonic events as a template for dental tissue repair?
1
2001
... The tooth has an ability of limited repair by production of newly formed layer of dentin, deposited by odontoblast-like cells observed and reported by Smith and Lesot,11 and Smith et al.12 suggesting that within the dental pulp, there is a pool of cells with mesenchymal stem characteristics, able to be recruited and activated when the need for repair occurs (Figure 1A). ...
Recruitment of dental pulp cells by dentine and pulp extracellular matrix components
1
2012
... The tooth has an ability of limited repair by production of newly formed layer of dentin, deposited by odontoblast-like cells observed and reported by Smith and Lesot,11 and Smith et al.12 suggesting that within the dental pulp, there is a pool of cells with mesenchymal stem characteristics, able to be recruited and activated when the need for repair occurs (Figure 1A). ...
Reactionary dentinogenesis
1
1995
... In shallow enamel and enamel/dentinal damage, resident odontoblasts, polarised and highly specialised cells, located on the dentin-pulp border are activated, protecting the dental pulp via formation of reactionary dentine.13, 14 If the damage advances, resident odontoblasts may not survive and this initiates a cascade of stem cell activation, proliferation, and differentiation into new odontoblast-like cells that engage in reparative dentine secretion.9, 15 ...
Reactionary dentinogenesis and neuroimmune response in dental caries
1
2014
... In shallow enamel and enamel/dentinal damage, resident odontoblasts, polarised and highly specialised cells, located on the dentin-pulp border are activated, protecting the dental pulp via formation of reactionary dentine.13, 14 If the damage advances, resident odontoblasts may not survive and this initiates a cascade of stem cell activation, proliferation, and differentiation into new odontoblast-like cells that engage in reparative dentine secretion.9, 15 ...
1
2000
... In shallow enamel and enamel/dentinal damage, resident odontoblasts, polarised and highly specialised cells, located on the dentin-pulp border are activated, protecting the dental pulp via formation of reactionary dentine.13, 14 If the damage advances, resident odontoblasts may not survive and this initiates a cascade of stem cell activation, proliferation, and differentiation into new odontoblast-like cells that engage in reparative dentine secretion.9, 15 ...
Dual origin of mesenchymal stem cells contributing to organ growth and repair
4
2011
... When addressing the question on the origin of the dental pulp stem cells (DPSCs), Feng et al.16 by using genetic lineage tracing, reported a dual origin of the dental pulp cells. Pericytes were shown to differentiate into specialized tooth mesenchyme-derived cells, odontoblasts, during tooth growth and in response to damage in vivo.16, 17 Nevertheless, this contribution to odontoblast differentiation does not account for all cell differentiation, and an additional source, displaying mesenchymal stem cell (MSC)-like properties, was identified within the dental pulp resident cells that migrate toward areas of tissue damage and differentiate into odontoblasts, when stimulated.16 A significant population of MSCs during development, self-renewal and repair of a tooth were also suggested to be derived from peripheral nerve-associated glial cells that generate multipotent MSCs and further differentiate into pulp cells and highly specialized odontoblasts, shown in a mouse incisor as a model that exhibits continuous growth.18 ...
... 16, 17 Nevertheless, this contribution to odontoblast differentiation does not account for all cell differentiation, and an additional source, displaying mesenchymal stem cell (MSC)-like properties, was identified within the dental pulp resident cells that migrate toward areas of tissue damage and differentiate into odontoblasts, when stimulated.16 A significant population of MSCs during development, self-renewal and repair of a tooth were also suggested to be derived from peripheral nerve-associated glial cells that generate multipotent MSCs and further differentiate into pulp cells and highly specialized odontoblasts, shown in a mouse incisor as a model that exhibits continuous growth.18 ...
... 16 A significant population of MSCs during development, self-renewal and repair of a tooth were also suggested to be derived from peripheral nerve-associated glial cells that generate multipotent MSCs and further differentiate into pulp cells and highly specialized odontoblasts, shown in a mouse incisor as a model that exhibits continuous growth.18 ...
... When teeth are damaged, the local odontoblast cells upregulate their activity, but if damage persists these local odontoblasts cannot cope and their death provides a trigger for proliferation of resident pericytes and glial cells located in the vicinity of the damage. These cells have stem cell properties and differentiate into new odontoblast-like cells, capable to engage in reparative processes.16-18 These studies importantly indicated the different origins of the resident, mesenchyme derived stem cells in the dental pulp. ...
αSMA-expressing perivascular cells represent dental pulp progenitors in vivo
1
2017
... When addressing the question on the origin of the dental pulp stem cells (DPSCs), Feng et al.16 by using genetic lineage tracing, reported a dual origin of the dental pulp cells. Pericytes were shown to differentiate into specialized tooth mesenchyme-derived cells, odontoblasts, during tooth growth and in response to damage in vivo.16, 17 Nevertheless, this contribution to odontoblast differentiation does not account for all cell differentiation, and an additional source, displaying mesenchymal stem cell (MSC)-like properties, was identified within the dental pulp resident cells that migrate toward areas of tissue damage and differentiate into odontoblasts, when stimulated.16 A significant population of MSCs during development, self-renewal and repair of a tooth were also suggested to be derived from peripheral nerve-associated glial cells that generate multipotent MSCs and further differentiate into pulp cells and highly specialized odontoblasts, shown in a mouse incisor as a model that exhibits continuous growth.18 ...
Glial origin of mesenchymal stem cells in a tooth model system
3
2014
... When addressing the question on the origin of the dental pulp stem cells (DPSCs), Feng et al.16 by using genetic lineage tracing, reported a dual origin of the dental pulp cells. Pericytes were shown to differentiate into specialized tooth mesenchyme-derived cells, odontoblasts, during tooth growth and in response to damage in vivo.16, 17 Nevertheless, this contribution to odontoblast differentiation does not account for all cell differentiation, and an additional source, displaying mesenchymal stem cell (MSC)-like properties, was identified within the dental pulp resident cells that migrate toward areas of tissue damage and differentiate into odontoblasts, when stimulated.16 A significant population of MSCs during development, self-renewal and repair of a tooth were also suggested to be derived from peripheral nerve-associated glial cells that generate multipotent MSCs and further differentiate into pulp cells and highly specialized odontoblasts, shown in a mouse incisor as a model that exhibits continuous growth.18 ...
... When teeth are damaged, the local odontoblast cells upregulate their activity, but if damage persists these local odontoblasts cannot cope and their death provides a trigger for proliferation of resident pericytes and glial cells located in the vicinity of the damage. These cells have stem cell properties and differentiate into new odontoblast-like cells, capable to engage in reparative processes.16-18 These studies importantly indicated the different origins of the resident, mesenchyme derived stem cells in the dental pulp. ...
... During feeding, abrasion occurs at the tips of mouse incisor, causing a continuous need for compensation to maintain the sharpness of the teeth. The loss of cells at the tips is compensated for by continuous cell production at the apical end where distinct MSC and epithelial stem cell niches reside.18, 52, 53 ...
SHED: stem cells from human exfoliated deciduous teeth
2
2003
... Cells with stem cell properties were also isolated from the remnants of human deciduous teeth dental pulp (Figure 1), termed “stem cells from human deciduous teeth”.19 These cells can be easily obtained, making the deciduous, physiologically replaced teeth, a highly accessible, otherwise discarded, source for MSCs.7-10 Stem cells from human deciduous teeth were also shown to be highly proliferative, clonogenic cells that have capacity for multilineage differentiation with higher proliferation rates and increased population doublings, when compared to DPSCs isolated from permanent teeth. These cells show osteo-inductive capacity and were able to generate dentine-like structures when transplanted in vivo.19-24 ...
... 19-24 ...
The efficacy of mesenchymal stem cells to regenerate and repair dental structures
1
2005
... Stem cells from human deciduous teeth lack expression of major histocompatibility complex-II and have been shown to express hypo-immunogenic phenotype, inhibiting T-cell function through conserved induction of cellular stress.25 Moreover, these immunomodulatory properties have been shown to be conserved in vitro and follow the common strategy of immunoregulation that takes place in vivo, during the process of tissue repair.26 These important immunomodulatory properties, in addition to their neural crest origin27 and ability to keep their stem cell properties after a long-term period cryopreservation, as well as their accessibility, (from usually discarded tissues, such as 20 deciduous teeth in our lifetime), make them a particularly attractive source for MSCs and target for a therapeutic strategy.7-10 ...
SHED differentiate into functional odontoblasts and endothelium
0
2010
Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth
0
2008
Stem cells from human-exfoliated deciduous teeth can differentiate into dopaminergic neuron-like cells
0
2010
Stem cell proliferation pathways comparison between human exfoliated deciduous teeth and dental pulp stem cells by gene expression profile from promising dental pulp
1
2009
... Cells with stem cell properties were also isolated from the remnants of human deciduous teeth dental pulp (Figure 1), termed “stem cells from human deciduous teeth”.19 These cells can be easily obtained, making the deciduous, physiologically replaced teeth, a highly accessible, otherwise discarded, source for MSCs.7-10 Stem cells from human deciduous teeth were also shown to be highly proliferative, clonogenic cells that have capacity for multilineage differentiation with higher proliferation rates and increased population doublings, when compared to DPSCs isolated from permanent teeth. These cells show osteo-inductive capacity and were able to generate dentine-like structures when transplanted in vivo.19-24 ...
Mesenchymal stem cells inhibit T-cell function through conserved induction of cellular stress
1
2019
... Stem cells from human deciduous teeth lack expression of major histocompatibility complex-II and have been shown to express hypo-immunogenic phenotype, inhibiting T-cell function through conserved induction of cellular stress.25 Moreover, these immunomodulatory properties have been shown to be conserved in vitro and follow the common strategy of immunoregulation that takes place in vivo, during the process of tissue repair.26 These important immunomodulatory properties, in addition to their neural crest origin27 and ability to keep their stem cell properties after a long-term period cryopreservation, as well as their accessibility, (from usually discarded tissues, such as 20 deciduous teeth in our lifetime), make them a particularly attractive source for MSCs and target for a therapeutic strategy.7-10 ...
Immune modulation by apoptotic dental pulp stem cells in vivo
1
2018
... Stem cells from human deciduous teeth lack expression of major histocompatibility complex-II and have been shown to express hypo-immunogenic phenotype, inhibiting T-cell function through conserved induction of cellular stress.25 Moreover, these immunomodulatory properties have been shown to be conserved in vitro and follow the common strategy of immunoregulation that takes place in vivo, during the process of tissue repair.26 These important immunomodulatory properties, in addition to their neural crest origin27 and ability to keep their stem cell properties after a long-term period cryopreservation, as well as their accessibility, (from usually discarded tissues, such as 20 deciduous teeth in our lifetime), make them a particularly attractive source for MSCs and target for a therapeutic strategy.7-10 ...
Human deciduous teeth stem cells (SHED) display neural crest signature characters
1
2017
... Stem cells from human deciduous teeth lack expression of major histocompatibility complex-II and have been shown to express hypo-immunogenic phenotype, inhibiting T-cell function through conserved induction of cellular stress.25 Moreover, these immunomodulatory properties have been shown to be conserved in vitro and follow the common strategy of immunoregulation that takes place in vivo, during the process of tissue repair.26 These important immunomodulatory properties, in addition to their neural crest origin27 and ability to keep their stem cell properties after a long-term period cryopreservation, as well as their accessibility, (from usually discarded tissues, such as 20 deciduous teeth in our lifetime), make them a particularly attractive source for MSCs and target for a therapeutic strategy.7-10 ...
The hidden treasure in apical papilla: the potential role in pulp/dentin regeneration and bioroot engineering
2
2008
... Teeth that are still undergoing development of their roots harbour a unique population of cells located in the apical papilla region that have been shown to exhibit stem cells properties, termed stem cells from apical papilla (SCAP).28, 29 The apical papilla region plays an important role in root development and formation of different dental tissues during this physiological process1 (Figure 1B). ...
... These cells have been characterized by their plasticity, expression of stem cell markers, such as STRO-1 and CD146 as well as CD73, CD90, and CD105; typical stem cell markers.28-30 One surface marker for pluripotency, CD24, has been found to be directly correlated to SCAP; where it was found to be exclusively expressed in SCAP.31 CD24a+ cells could be detected in primary dental papilla in mice and humans, and marked as unique multipotent stem cells from the dental pulp with enhanced osteogenic/odontogenic differentiation capabilities to form dentin and neurovascular-like structures.32 ...
Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study
1
2008
... Teeth that are still undergoing development of their roots harbour a unique population of cells located in the apical papilla region that have been shown to exhibit stem cells properties, termed stem cells from apical papilla (SCAP).28, 29 The apical papilla region plays an important role in root development and formation of different dental tissues during this physiological process1 (Figure 1B). ...
The angiogenic potential of DPSCs and SCAPs in an in vivo model of dental pulp regeneration
1
2017
... These cells have been characterized by their plasticity, expression of stem cell markers, such as STRO-1 and CD146 as well as CD73, CD90, and CD105; typical stem cell markers.28-30 One surface marker for pluripotency, CD24, has been found to be directly correlated to SCAP; where it was found to be exclusively expressed in SCAP.31 CD24a+ cells could be detected in primary dental papilla in mice and humans, and marked as unique multipotent stem cells from the dental pulp with enhanced osteogenic/odontogenic differentiation capabilities to form dentin and neurovascular-like structures.32 ...
Long-term exposure to pro-inflammatory cytokines inhibits the osteogenic/dentinogenic differentiation of stem cells from the apical papilla
1
2016
... These cells have been characterized by their plasticity, expression of stem cell markers, such as STRO-1 and CD146 as well as CD73, CD90, and CD105; typical stem cell markers.28-30 One surface marker for pluripotency, CD24, has been found to be directly correlated to SCAP; where it was found to be exclusively expressed in SCAP.31 CD24a+ cells could be detected in primary dental papilla in mice and humans, and marked as unique multipotent stem cells from the dental pulp with enhanced osteogenic/odontogenic differentiation capabilities to form dentin and neurovascular-like structures.32 ...
Regeneration of pulpo-dentinal-like complex by a group of unique multipotent CD24a(+) stem cells
1
2020
... These cells have been characterized by their plasticity, expression of stem cell markers, such as STRO-1 and CD146 as well as CD73, CD90, and CD105; typical stem cell markers.28-30 One surface marker for pluripotency, CD24, has been found to be directly correlated to SCAP; where it was found to be exclusively expressed in SCAP.31 CD24a+ cells could be detected in primary dental papilla in mice and humans, and marked as unique multipotent stem cells from the dental pulp with enhanced osteogenic/odontogenic differentiation capabilities to form dentin and neurovascular-like structures.32 ...
Stem cells from the apical papilla (SCAP) as a tool for endogenous tissue regeneration
1
2018
... When isolated, SCAP have been characterised by their capacity for multilineage differentiation, following induction in vitro.5 In comparison to DPSCs, these cells express higher proliferation and cell migration capacity5, 33 and have a higher expression of antiapoptotic protein, longer telomere length, and greater telomerase activity associated with cellular lifespan and cell proliferation than DPSCs.34 All different stem cells isolated from dental/oral tissues have shown to be able to undergo osteogenic differentiation and produce minerals with different chemical composition in vitro, when analysed with Raman spectroscopy.35 A possible downregulation of RUNX2 has been suggested when SCAP cells undergo diversion from osteoblastic to odontoblastic differentiation route.36 ...
Comparative analysis of telomere length, telomerase and reverse transcriptase activity in human dental stem cells
1
2011
... When isolated, SCAP have been characterised by their capacity for multilineage differentiation, following induction in vitro.5 In comparison to DPSCs, these cells express higher proliferation and cell migration capacity5, 33 and have a higher expression of antiapoptotic protein, longer telomere length, and greater telomerase activity associated with cellular lifespan and cell proliferation than DPSCs.34 All different stem cells isolated from dental/oral tissues have shown to be able to undergo osteogenic differentiation and produce minerals with different chemical composition in vitro, when analysed with Raman spectroscopy.35 A possible downregulation of RUNX2 has been suggested when SCAP cells undergo diversion from osteoblastic to odontoblastic differentiation route.36 ...
Composition of mineral produced by dental mesenchymal stem cells
1
2015
... When isolated, SCAP have been characterised by their capacity for multilineage differentiation, following induction in vitro.5 In comparison to DPSCs, these cells express higher proliferation and cell migration capacity5, 33 and have a higher expression of antiapoptotic protein, longer telomere length, and greater telomerase activity associated with cellular lifespan and cell proliferation than DPSCs.34 All different stem cells isolated from dental/oral tissues have shown to be able to undergo osteogenic differentiation and produce minerals with different chemical composition in vitro, when analysed with Raman spectroscopy.35 A possible downregulation of RUNX2 has been suggested when SCAP cells undergo diversion from osteoblastic to odontoblastic differentiation route.36 ...
Angiogenic potential and secretome of human apical papilla mesenchymal stem cells in various stress microenvironments
1
2015
... When isolated, SCAP have been characterised by their capacity for multilineage differentiation, following induction in vitro.5 In comparison to DPSCs, these cells express higher proliferation and cell migration capacity5, 33 and have a higher expression of antiapoptotic protein, longer telomere length, and greater telomerase activity associated with cellular lifespan and cell proliferation than DPSCs.34 All different stem cells isolated from dental/oral tissues have shown to be able to undergo osteogenic differentiation and produce minerals with different chemical composition in vitro, when analysed with Raman spectroscopy.35 A possible downregulation of RUNX2 has been suggested when SCAP cells undergo diversion from osteoblastic to odontoblastic differentiation route.36 ...
Profiling the secretome of human stem cells from dental apical papilla
1
2016
... The secretome analysis of SCAP cells indicates that they secrete significantly more chemokines, neurotrophins and proteins involved in metabolic processes and transcription, compared to bone marrow MSCs.37 Being located at the very tip of the developing root, enables the region of the apical papilla to have accessibility to a collateral circulation,5, 38 which might affect the diverse dynamic of the cellular components and processes of the region and therefore the SCAP population of cells. ...
Microbial modulation of stem cells and future directions in regenerative endodontics
1
2017
... The secretome analysis of SCAP cells indicates that they secrete significantly more chemokines, neurotrophins and proteins involved in metabolic processes and transcription, compared to bone marrow MSCs.37 Being located at the very tip of the developing root, enables the region of the apical papilla to have accessibility to a collateral circulation,5, 38 which might affect the diverse dynamic of the cellular components and processes of the region and therefore the SCAP population of cells. ...
Conversion of stem cells from apical papilla into endothelial cells by small molecules and growth factors
1
2021
... Through a process of transdifferentiation, SCAP cells were successfully converted into endothelial cells by small molecules and growth factors and shown to generate vascular structures using the in vivo Matrigel plug angiogenesis assay in an immune-deficient mouse model.39 ...
A scarless healing tale: comparing homeostasis and wound healing of oral mucosa with skin and oesophagus
3
2021
... The oral mucosa is the first line of defence against oral diseases. One of the most rapidly dividing tissues in our body, it exhibits remarkable healing properties of foetal-like, scarless wound healing phenotype.40 The tissue that directly surrounds the tooth and attaches to the tooth surface by a junctional epithelium, providing a seal, is known as gingiva (Figure 1C). Gingiva is part of a complex of tissues, known as periodontium, along with the alveolar socket, periodontal ligament and the cementum, that provide attachment of the tooth and play role in its homeostasis.1, 41 It is particularly important structure, providing defence against microorganisms, during plaque formation and providing resistance to mechanical forces during mastication. ...
... The remarkable way that oral wounds heal with negligible complications compared to cutaneous wounds, has always triggered interest among researchers. Various mechanisms, such as differential proliferative and differentiation cell programs, epithelial remodelling, reduced inflammatory response, and presence of a distinct modulation of adult stem cells were suggested to contribute to this remarkable wound-healing property.40 The homeostasis of gingiva requires coordinated interactions between different populations of epithelial, mesenchymal, and immune cells.50 ...
... The cells derived from the underlying mesenchyme that reside in the subepithelial compartment are characterised by high plasticity and respond to signals sent by the overlying epithelial layer.40 Single-cell RNA-sequencing of the epithelial basal layer, adjacent to the subepithelial population of mesenchymal cells has elucidated the oral mucosa epithelial stem cell niche and suggested their role in the process of healing.50 ...
1
2008
... The oral mucosa is the first line of defence against oral diseases. One of the most rapidly dividing tissues in our body, it exhibits remarkable healing properties of foetal-like, scarless wound healing phenotype.40 The tissue that directly surrounds the tooth and attaches to the tooth surface by a junctional epithelium, providing a seal, is known as gingiva (Figure 1C). Gingiva is part of a complex of tissues, known as periodontium, along with the alveolar socket, periodontal ligament and the cementum, that provide attachment of the tooth and play role in its homeostasis.1, 41 It is particularly important structure, providing defence against microorganisms, during plaque formation and providing resistance to mechanical forces during mastication. ...
Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis
1
2009
... The gingiva is composed of epithelium, derived from embryonic ectoderm, and underlying connective tissue that originates from neural crest cells and mesoderm.1 Gingival tissue is easily accessible during routine dental procedures and very often remnants of the tissue are found following tooth extraction, making it a very attractive source for isolation of different stem cells.7-10 Cells derived from the connective tissue compartment of human gingiva have been characterised by expression of MSC markers, such as Oct-4 (octamer-binding transcription factor 4), SSEA-4 (stage-specific embryonic antigen-4) and STRO-142, 43 and undergo multilineage differentiation under in vitro conditions.43 The cells from the epithelial compartment and derived from oral mucosa were also characterised by expression of stem cells markers in vitro and used in ocular surface reconstruction when grown as oral mucosal epithelial sheets in rabbit44 and human,45 offering promising results for future regenerative therapies. ...
Comparison of mesenchymal stem cells derived from gingival tissue and periodontal ligament in different incubation conditions
2
2013
... The gingiva is composed of epithelium, derived from embryonic ectoderm, and underlying connective tissue that originates from neural crest cells and mesoderm.1 Gingival tissue is easily accessible during routine dental procedures and very often remnants of the tissue are found following tooth extraction, making it a very attractive source for isolation of different stem cells.7-10 Cells derived from the connective tissue compartment of human gingiva have been characterised by expression of MSC markers, such as Oct-4 (octamer-binding transcription factor 4), SSEA-4 (stage-specific embryonic antigen-4) and STRO-142, 43 and undergo multilineage differentiation under in vitro conditions.43 The cells from the epithelial compartment and derived from oral mucosa were also characterised by expression of stem cells markers in vitro and used in ocular surface reconstruction when grown as oral mucosal epithelial sheets in rabbit44 and human,45 offering promising results for future regenerative therapies. ...
... 43 The cells from the epithelial compartment and derived from oral mucosa were also characterised by expression of stem cells markers in vitro and used in ocular surface reconstruction when grown as oral mucosal epithelial sheets in rabbit44 and human,45 offering promising results for future regenerative therapies. ...
Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders
1
2004
... The gingiva is composed of epithelium, derived from embryonic ectoderm, and underlying connective tissue that originates from neural crest cells and mesoderm.1 Gingival tissue is easily accessible during routine dental procedures and very often remnants of the tissue are found following tooth extraction, making it a very attractive source for isolation of different stem cells.7-10 Cells derived from the connective tissue compartment of human gingiva have been characterised by expression of MSC markers, such as Oct-4 (octamer-binding transcription factor 4), SSEA-4 (stage-specific embryonic antigen-4) and STRO-142, 43 and undergo multilineage differentiation under in vitro conditions.43 The cells from the epithelial compartment and derived from oral mucosa were also characterised by expression of stem cells markers in vitro and used in ocular surface reconstruction when grown as oral mucosal epithelial sheets in rabbit44 and human,45 offering promising results for future regenerative therapies. ...
Long-term results of autologous cultivated oral mucosal epithelial transplantation in the scar phase of severe ocular surface disorders
1
2011
... The gingiva is composed of epithelium, derived from embryonic ectoderm, and underlying connective tissue that originates from neural crest cells and mesoderm.1 Gingival tissue is easily accessible during routine dental procedures and very often remnants of the tissue are found following tooth extraction, making it a very attractive source for isolation of different stem cells.7-10 Cells derived from the connective tissue compartment of human gingiva have been characterised by expression of MSC markers, such as Oct-4 (octamer-binding transcription factor 4), SSEA-4 (stage-specific embryonic antigen-4) and STRO-142, 43 and undergo multilineage differentiation under in vitro conditions.43 The cells from the epithelial compartment and derived from oral mucosa were also characterised by expression of stem cells markers in vitro and used in ocular surface reconstruction when grown as oral mucosal epithelial sheets in rabbit44 and human,45 offering promising results for future regenerative therapies. ...
Single-cell transcriptomic analysis reveals developmental relationships and specific markers of mouse periodontium cellular subsets
4
2021
... The periodontal ligament is a connective tissue that provides the anchorage to the tooth within the alveolar bone socket and takes the mechanical pressure during dental function and can be defined as a fibrous joint (Figure 1D).1 The periodontal ligament develops from the dental follicle, which is a fibrous sac that surrounds the developing tooth bud.1 In vivo lineage-tracing experiments suggested that the dental follicle contains mesenchymal progenitor cells expressing parathyroid hormone-related protein, which give rise to cells forming the periodontal attachment apparatus in a manner regulated by autocrine signalling through the parathyroid hormone/parathyroid hormone-related protein receptor.46 ...
... Similarly to other dental tissues, single-cell RNA sequencing studies revealed fundamental cellular heterogeneity of the mouse periodontium.46 Three major cell types were identified, including mesenchymal cells, endothelial cells, and immune cells, with some (periodontal ligament) mesenchymal populations, such as Axin2 and CD90/Thy1 that form cementoblasts.57 Cells expressing Scleraxis (Scx+), basic helix-loop-helix transcriptional factor, that is abundantly expressed by tendons and ligaments, have been suggested to give rise to osteoblasts and other fibroblasts.46 These recent studies map the lineage hierarchy in the periodontium at a single-cell level, largely contributing to understanding of development of periodontium, as well as understanding the intercellular interactions of cells during health and disease.46, 57 ...
... 46 These recent studies map the lineage hierarchy in the periodontium at a single-cell level, largely contributing to understanding of development of periodontium, as well as understanding the intercellular interactions of cells during health and disease.46, 57 ...
... 46, 57 ...
Immunomodulatory properties of human periodontal ligament stem cells
1
2009
... Cells with stem cell properties have been isolated and characterised, showing multilineage differentiation and expressing common MSC markers such as STRO-1, STRO-4, CD29, CD73, CD90 (Thy1), CD106 (vascular cell adhesion molecule 1) and CD146 (MUC18).47, 48 Cultured human periodontal ligament stem cells were also shown to be positive for pericyte markers such as CD146, neural/glial antigen 2 and CD140b,49 suggesting perivascular origin. Periodontal ligament stem cells, as all other dental stem cells, can be easily acquired from extracted teeth or during clinical procedure when the root surface is exposed. Interestingly, periodontal ligament stem cells retain similar immunophenotypic characteristics, when isolated from healthy and inflamed tissue,50 pointing at a possible usage of inflamed tissue, regularly exported during routine periodontal surgery, as a source to isolate cells with MSCs properties. ...
Mesenchymal stem cells in teeth
1
2020
... Cells with stem cell properties have been isolated and characterised, showing multilineage differentiation and expressing common MSC markers such as STRO-1, STRO-4, CD29, CD73, CD90 (Thy1), CD106 (vascular cell adhesion molecule 1) and CD146 (MUC18).47, 48 Cultured human periodontal ligament stem cells were also shown to be positive for pericyte markers such as CD146, neural/glial antigen 2 and CD140b,49 suggesting perivascular origin. Periodontal ligament stem cells, as all other dental stem cells, can be easily acquired from extracted teeth or during clinical procedure when the root surface is exposed. Interestingly, periodontal ligament stem cells retain similar immunophenotypic characteristics, when isolated from healthy and inflamed tissue,50 pointing at a possible usage of inflamed tissue, regularly exported during routine periodontal surgery, as a source to isolate cells with MSCs properties. ...
Periodontal ligament stem cells possess the characteristics of pericytes
1
2013
... Cells with stem cell properties have been isolated and characterised, showing multilineage differentiation and expressing common MSC markers such as STRO-1, STRO-4, CD29, CD73, CD90 (Thy1), CD106 (vascular cell adhesion molecule 1) and CD146 (MUC18).47, 48 Cultured human periodontal ligament stem cells were also shown to be positive for pericyte markers such as CD146, neural/glial antigen 2 and CD140b,49 suggesting perivascular origin. Periodontal ligament stem cells, as all other dental stem cells, can be easily acquired from extracted teeth or during clinical procedure when the root surface is exposed. Interestingly, periodontal ligament stem cells retain similar immunophenotypic characteristics, when isolated from healthy and inflamed tissue,50 pointing at a possible usage of inflamed tissue, regularly exported during routine periodontal surgery, as a source to isolate cells with MSCs properties. ...
Defining human mesenchymal and epithelial heterogeneity in response to oral inflammatory disease
6
2021
... Cells with stem cell properties have been isolated and characterised, showing multilineage differentiation and expressing common MSC markers such as STRO-1, STRO-4, CD29, CD73, CD90 (Thy1), CD106 (vascular cell adhesion molecule 1) and CD146 (MUC18).47, 48 Cultured human periodontal ligament stem cells were also shown to be positive for pericyte markers such as CD146, neural/glial antigen 2 and CD140b,49 suggesting perivascular origin. Periodontal ligament stem cells, as all other dental stem cells, can be easily acquired from extracted teeth or during clinical procedure when the root surface is exposed. Interestingly, periodontal ligament stem cells retain similar immunophenotypic characteristics, when isolated from healthy and inflamed tissue,50 pointing at a possible usage of inflamed tissue, regularly exported during routine periodontal surgery, as a source to isolate cells with MSCs properties. ...
... The remarkable way that oral wounds heal with negligible complications compared to cutaneous wounds, has always triggered interest among researchers. Various mechanisms, such as differential proliferative and differentiation cell programs, epithelial remodelling, reduced inflammatory response, and presence of a distinct modulation of adult stem cells were suggested to contribute to this remarkable wound-healing property.40 The homeostasis of gingiva requires coordinated interactions between different populations of epithelial, mesenchymal, and immune cells.50 ...
... The cells derived from the underlying mesenchyme that reside in the subepithelial compartment are characterised by high plasticity and respond to signals sent by the overlying epithelial layer.40 Single-cell RNA-sequencing of the epithelial basal layer, adjacent to the subepithelial population of mesenchymal cells has elucidated the oral mucosa epithelial stem cell niche and suggested their role in the process of healing.50 ...
... Fully mapping the identity and organisation of the tissue architecture on a single-cell level is essential to understand their roles in homeostasis and disease. A recent study revealed a well-orchestrated remodelling of the gingival tissue, with changes in actions between epithelial, mesenchymal, and immune cells, when transition happens from health and mild to severe disease (periodontitis). At a transcriptomic level, it was shown that there is a corresponding shift in cellular proportions.50 In health, there were low numbers of follicular and plasma B cells with a marked progressive increase from mild to severe. Memory B cells showed distinctive increase at disease onset with a subsequent decrease in the severe sample.50 A novel, mesenchymal population characterised by adipocyte enhancer binding protein 1 expression was also identified. This study raised intriguing questions about the role of certain cell types in response to inflammatory processes offering venues for translating this knowledge into clinical strategies for drug development for the wide range of chronic inflammatory diseases.50 ...
... 50 A novel, mesenchymal population characterised by adipocyte enhancer binding protein 1 expression was also identified. This study raised intriguing questions about the role of certain cell types in response to inflammatory processes offering venues for translating this knowledge into clinical strategies for drug development for the wide range of chronic inflammatory diseases.50 ...
... 50 ...
1
2022
... Mapping the cellular composition of different organs and tissues offers understanding of mechanisms of growth and repair. Recent studies, using cell sequencing approaches shed light on the cellular architecture of different dental tissues, offering a deeper view of the populations of cells and understanding the underlying mechanisms that drive the homeostasis, repair, and regeneration. These studies greatly contribute to the mission stated by the Human Cell Atlas project as “To create comprehensive reference maps of all human cells—the fundamental units of life—as a basis for both understanding human health and diagnosing, monitoring, and treating disease”.51 ...
A quiescent cell population replenishes mesenchymal stem cells to drive accelerated growth in mouse incisors
2
2018
... During feeding, abrasion occurs at the tips of mouse incisor, causing a continuous need for compensation to maintain the sharpness of the teeth. The loss of cells at the tips is compensated for by continuous cell production at the apical end where distinct MSC and epithelial stem cell niches reside.18, 52, 53 ...
... Differentiated and progenitor cells were described in stem-cell niches using bulk RNA-sequence, revealing a transcriptional complexity within the dental tissues.54 In a recent study, using single-cell sequencing, the whole population of ameloblasts in a mouse incisor was mapped, suggesting that epithelial progeny appear in diverse, characteristic patches.55 They suggested that the progenitor area of these cells, previously noted for their plasticity56 might rely on functional diversification of different stem cells with a stemness gradation in a continuously growing model, such as the mouse incisor.52-56 ...
Regulation of mesenchymal stem to transit-amplifying cell transition in the continuously growing mouse incisor
1
2018
... During feeding, abrasion occurs at the tips of mouse incisor, causing a continuous need for compensation to maintain the sharpness of the teeth. The loss of cells at the tips is compensated for by continuous cell production at the apical end where distinct MSC and epithelial stem cell niches reside.18, 52, 53 ...
Resolving stem and progenitor cells in the adult mouse incisor through gene co-expression analysis
1
2017
... Differentiated and progenitor cells were described in stem-cell niches using bulk RNA-sequence, revealing a transcriptional complexity within the dental tissues.54 In a recent study, using single-cell sequencing, the whole population of ameloblasts in a mouse incisor was mapped, suggesting that epithelial progeny appear in diverse, characteristic patches.55 They suggested that the progenitor area of these cells, previously noted for their plasticity56 might rely on functional diversification of different stem cells with a stemness gradation in a continuously growing model, such as the mouse incisor.52-56 ...
Dental cell type atlas reveals stem and differentiated cell types in mouse and human teeth
4
2020
... Differentiated and progenitor cells were described in stem-cell niches using bulk RNA-sequence, revealing a transcriptional complexity within the dental tissues.54 In a recent study, using single-cell sequencing, the whole population of ameloblasts in a mouse incisor was mapped, suggesting that epithelial progeny appear in diverse, characteristic patches.55 They suggested that the progenitor area of these cells, previously noted for their plasticity56 might rely on functional diversification of different stem cells with a stemness gradation in a continuously growing model, such as the mouse incisor.52-56 ...
... When analysing human teeth and comparing the apical papilla region of a human tooth with developing roots and comparing this with the dental pulp of a fully developed tooth, it was found that these differ substantially and form at least several transcriptionally distinct subpopulations.55 ...
... In the same study Krivanek et al, compared the pulp of human nongrowing molars to the mouse nongrowing molars and found that human molars contained a pulp subpopulation that was localized in the peri-odontoblastic layer (cell-free and cell-rich zones) which are absent in mouse molars.55 Interestingly, the results from the study showed that populations of Smoc2– and Smoc2+ human pulp subtypes expressed maturation hierarchy like that in mouse continuously growing incisor. Smoc2+ human subtype cells were in the apical papilla region, around the Hertwig epithelial root sheath, region where the SCAP cells are derived from.55 ...
... 55 ...
Stem cells in tooth development, growth, repair, and regeneration
2
2015
... Differentiated and progenitor cells were described in stem-cell niches using bulk RNA-sequence, revealing a transcriptional complexity within the dental tissues.54 In a recent study, using single-cell sequencing, the whole population of ameloblasts in a mouse incisor was mapped, suggesting that epithelial progeny appear in diverse, characteristic patches.55 They suggested that the progenitor area of these cells, previously noted for their plasticity56 might rely on functional diversification of different stem cells with a stemness gradation in a continuously growing model, such as the mouse incisor.52-56 ...
... -56 ...
Stem cell contributions to cementoblast differentiation in healthy periodontal ligament and periodontitis
2
2021
... Similarly to other dental tissues, single-cell RNA sequencing studies revealed fundamental cellular heterogeneity of the mouse periodontium.46 Three major cell types were identified, including mesenchymal cells, endothelial cells, and immune cells, with some (periodontal ligament) mesenchymal populations, such as Axin2 and CD90/Thy1 that form cementoblasts.57 Cells expressing Scleraxis (Scx+), basic helix-loop-helix transcriptional factor, that is abundantly expressed by tendons and ligaments, have been suggested to give rise to osteoblasts and other fibroblasts.46 These recent studies map the lineage hierarchy in the periodontium at a single-cell level, largely contributing to understanding of development of periodontium, as well as understanding the intercellular interactions of cells during health and disease.46, 57 ...
... , 57 ...