Osteoarthritis is mainly characterized by articular cartilage progressive loss, osteophyte formation, synovial membrane inflammation and thickening of the subchondral bone, which leads to the generation of osteochondral (OC) defects with limited self-healing capacity. In order to repair defects located in a joint and restore its function, one possible approach is the use of tissue-engineered OC scaffolds, which are intended to delay or eliminate the need for joint replacement. Although they have been established for the repair of small OC defects, no products to date have demonstrated the appropriate biomechanical properties required to promote successful long-lasting regeneration of large OC defects. Biomaterials and Additive Manufacturing: Osteochondral Scaffold (BAMOS) project addresses this challenge in osteoarthritis treatment1 by bringing together five internationally leading research organisations (Universidad de Las Palmas de Gran Canaria, Spain; University of Minho, Portugal; University College London, UK; Xi’an Jiaotong University and Zhejiang University, China) and two healthcare providers (Royal National Orthopaedic Hospital, UK; and Saúde Atlântica-Gestão Hospitalar, S.A., Portugal) to work on the development, manufacturing and marketing of OC scaffolds for the repair of large cartilage damages in osteoarthritis patients. The following specific objectives of BAMOS derive from this main objective: a) Define clinical specifications for OC scaffolds, b) develop new OC scaffolds biomaterials, c) develop innovative additive manufacturing techniques to produce patient-specific OC scaffolds, d) assess the OC scaffold in both in vitro and in vivo, and e) equip early-stage researchers with the advanced knowledge and experience to address society’s grand healthcare challenges.
After a complete physicochemical and in vitro characterization, the efficacy of different OC scaffolds developed in the context of BAMOS project was evaluated using clinical animal models. The OC scaffolds tested in vivo, which are reviewed in the following sections, included bilayered and trilayered three-dimensional structures.
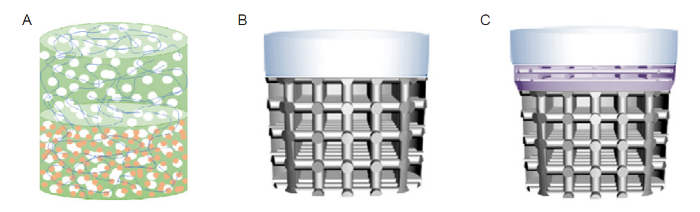
Bilayered OC scaffolds: A rabbit knee critical size OC defect model was used for assessing in vivo OC regeneration when implanting a horseradish peroxidase cross-linked silk fibroin-based (HRP-SF) scaffold2 (Figure 1A). These three-dimensional structures were prepared by combining two distinct layers: an HRP-SF layer that served as cartilage of the OC scaffold; and a subchondral bone-like layer (also based on HRP-SF) containing ion-doped beta-tricalcium phosphate (β-TCP) particles (HRP-SF/ZnSr-β-TCP).3 Two OC defects were created in each rabbit by manual drilling: one of them was used for the implantation of the scaffold, while the other one was left empty to serve as a control. After 8 weeks of implantation, the hierarchical scaffolds showed good integration (with no signs of inflammatory reactions), cartilage tissue regeneration and calcified tissue formation. Histological analyses confirmed the formation of collagen type II and glycosaminoglycans in the HRP-SF layer, while bone ingrowth and blood vessel infiltration were observed in the bone-like layer.2 These OC bilayered scaffolds have also shown to possess sufficient structural integrity, memory-shape properties, and suitable mechanical and in vitro biological properties, even preventing bacterial biofilm formation,3 which in sum confirms the potential of these structures to be used in OC tissue engineering applications.
Figure 1.
Figure 1.
Scaffolds intended for osteochondral regeneration developed in Biomaterials and Additive Manufacturing: Osteochondral Scaffold (BAMOS) project and tested in vivo. (A) Enzymatically cross-linked silk fibroin-based bilayered scaffold. (B) Titanium-collagen/poly(lactic-co-glycolic acid) bilayered scaffold. (C) Titanium-polylactic acid-collagen/poly(lactic-co-glycolic acid trilayered scaffold.
Also using a rabbit model, bilayered scaffolds were implanted into OC defects created at the distal femoral trochlea of New Zealand white male rabbits and tested for 24 weeks.4 These scaffolds were composed of a titanium (Ti) matrix that served as a bone layer, and a collagen/poly(lactic-co-glycolic acid) layer intended for cartilage regeneration (Figure 1B). The experimental group (n = 12) was compared to a control group in which only the collagen/poly(lactic-co-glycolic acid) layer was implanted into the OC defect (n = 9), and another one in which the drill was left empty. We concluded that the mechanical support provided by the Ti layer promoted subchondral bone formation and new tissue integration, which led to better cartilage regeneration.
·Casting and freeze-drying methods were used to obtain a collagen/poly(lactic-co-glycolic acid) composite layer to act as a cartilage-like layer.
·Material extrusion of polymers from a heated nozzle (MEX-TRB/P), commonly referred as fused deposition modelling, was used to manufacture a polylactic acid-based two-part junction layer that served as calcified cartilage of the hierarchical scaffold.
·Powder bed fusion of metal (PBF-LB/M) was used to produce a porous Ti matrix intended for bone regeneration.
Aiming to assess the short-term performance of the proposed trilayered scaffolds when treating large OC defects, a 12-week in vivo evaluation was carried out using a sheep stifle condyle model.6 Results showed a stable mechanical fixation of the three-dimensional structure on the implantation site with no adverse effects observed on the surrounding tissues. Improved bone ingrowth into the Ti matrix, as well as enhanced formation of hyaline-like cartilage tissue, were reported after histological examinations. The up-regulation of the chondrogenic-related markers aggrecan and collagen type II confirmed the capacity of the proposed three-dimensional structures to regenerate cartilage tissue. In summary, the results obtained showed the potential of these cell-free scaffolds to be applied in the treatment of large OC defects.
Other in vivo tests carried out in BAMOS: Interestingly, the incorporation of bone marrow concentrate into the developed trilayered scaffolds has led to a non-significant improvement in bone regeneration when treating OC defects.7 In this case, an ovine stifle condyle model was used during a 6-month test. Despite obtaining no significantly higher quantity of newly formed bone when using the Ti-polylactic acid-collagen/poly(lactic-co-glycolic acid) scaffold, the results suggested that enhanced bone homogeneity and biomechanical durability were obtained when implanting the trilayered scaffolds (seeded with bone marrow concentrate), thus producing a higher quality of new subchondral bone tissue. Similarly, no statistically significant differences in terms of OC regeneration were obtained between collagen/hydroxyapatite scaffolds with or without bone marrow concentrate when tested in vivo using an ovine femoral condyle model for 6 months.8
An ovine condyle model was also used to validate a novel numerical model developed in the context of BAMOS project, which is intended for optimization of both scaffold design and material properties, but also for prediction of the scaffold’s biological performance.9 The simulated cell distribution in the scaffold matched well with the in vivo regenerated bone tissue distribution. Therefore, the proposed model could serve as a tool to reduce the number of preliminary time- and cost-consuming in vivo and in vitro tests needed to optimize the scaffold design.
Although adequate implant-tissue interaction and scaffold resorption have yet to be confirmed in vivo in the long term
(> 6 months of efficacy evaluation), the results herein presented suggest that the bilayered and trilayered scaffolds developed in BAMOS have potential to effectively treat large OC defects in the short term. Thus, these three-dimensional structures could be used by clinicians as a one-step surgical procedure, providing a viable treatment option to avoid or delay joint replacement. Furthermore, their hierarchical three-dimensional structure and demonstrated capacity to favour cell-material interaction make the proposed scaffolds suitable candidates to be tested as osteoarthritis in vitro models for pathological investigation and therapeutic compound screening.
Author contributions
Project administration, and funding acquisition: MM; resources: MM, CL, JMO; visualization: RD; manuscript draft: MM, RD; manuscript review and editing: MT, VR, CL, JMO. All authors approved the final version of this manuscript.
Financial support
This work is part of the developments carried out in BAMOS project, funded from the European Union’s Horizon 2020 Research and Innovation Programme under grant agreement No. 734156.
Acknowledgement
Not applicable.
Conflicts of interest statement
The authors have no competing interests to declare.
Editor note: Chaozong Liu is an Editorial Board member of Biomaterials Translational. He was blinded from reviewing or making decisions on the manuscript. The article was subject to the journal’s standard procedures, with peer review handled independently of this Editorial Board member and his research group.
Open access statement
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Reference
Biomaterials and additive manufacturing: osteochondral scaffold innovation applied to osteoarthritis (BAMOS project)
DOI:10.1631/jzus.A18NW001 URL [Cited within: 1]
In vivo performance of hierarchical HRP-crosslinked silk fibroin/β-TCP scaffolds for osteochondral tissue regeneration
Enzymatically cross-linked silk fibroin-based hierarchical scaffolds for osteochondral regeneration
DOI:10.1021/acsami.8b21259 URL [Cited within: 2]
Bilayered scaffold with 3D printed stiff subchondral bony compartment to provide constant mechanical support for long-term cartilage regeneration
DOI:10.1016/j.jot.2021.09.001 URL [Cited within: 1]
Improved bone and cartilage regeneration in a rapid-manufactured functionally-graded osteochondral
In vivo evaluation of additively manufactured multi-layered scaffold for the repair of large osteochondral defects
Micro-computed tomography analysis of subchondral bone regeneration using osteochondral scaffolds in an ovine condyle model
DOI:10.3390/app11030891 URL [Cited within: 1]
Sheep condyle model evaluation of bone marrow cell concentrate combined with a scaffold for repair of large osteochondral defects
DOI:10.1302/2046-3758.1010.BJR-2020-0504.R1 URL [Cited within: 1]
Determination of an initial stage of the bone tissue ingrowth into titanium matrix by cell adhesion model
DOI:10.3389/fbioe.2021.736063 URL [Cited within: 1]