Biomaterials Translational ›› 2021, Vol. 2 ›› Issue (2): 151-164.doi: 10.12336/biomatertransl.2021.02.005
• RESEARCH ARTICLE • Previous Articles Next Articles
Huoyan Hong1,#, Xiaoyun Wang2,#, Xinran Song1, Gomaa El Fawal1,3, Kaili Wang1, Di Jiang1, Yifei Pei1, Zhe Wang1, Hongsheng Wang1,*( )
)
Received:2021-04-17
Revised:2021-06-04
Accepted:2021-06-07
Online:2021-06-28
Published:2021-06-28
Contact:
Hongsheng Wang
E-mail:whs@dhu.edu.cn
About author:#Author equally.
Hong, H.; Wang, X.; Song, X.; El Fawal, G.; Wang, K.; Jiang, D.; Pei, Y.; Wang, Z.; Wang, H. Transdermal delivery of interleukin-12 gene targeting dendritic cells enhances the anti-tumour effect of programmed cell death protein 1 monoclonal antibody. Biomater Transl. 2021, 2(2), 151-164.
| Gene | Sequence (5′ -3′) |
|---|---|
| β-Actin | Forward: GGC TGT ATT CCC CTC CAT CG |
| Reverse: CCA GTT GGT AAC AAT GCC ATG T | |
| IL-12a | Forward: TCC AGC AGC TCC TCT CAG TG |
| Reverse: ACT GGC TAA GAC ACC TGG CA | |
| IL-12b | Forward: GGC TGG ACT GCA TGA TAG CG |
| Reverse: GCC AGG ATG TCT CTG CTC CT |
Table 1 Primer sequences for real-time fluorescence quantitative polymerase chain reaction
| Gene | Sequence (5′ -3′) |
|---|---|
| β-Actin | Forward: GGC TGT ATT CCC CTC CAT CG |
| Reverse: CCA GTT GGT AAC AAT GCC ATG T | |
| IL-12a | Forward: TCC AGC AGC TCC TCT CAG TG |
| Reverse: ACT GGC TAA GAC ACC TGG CA | |
| IL-12b | Forward: GGC TGG ACT GCA TGA TAG CG |
| Reverse: GCC AGG ATG TCT CTG CTC CT |
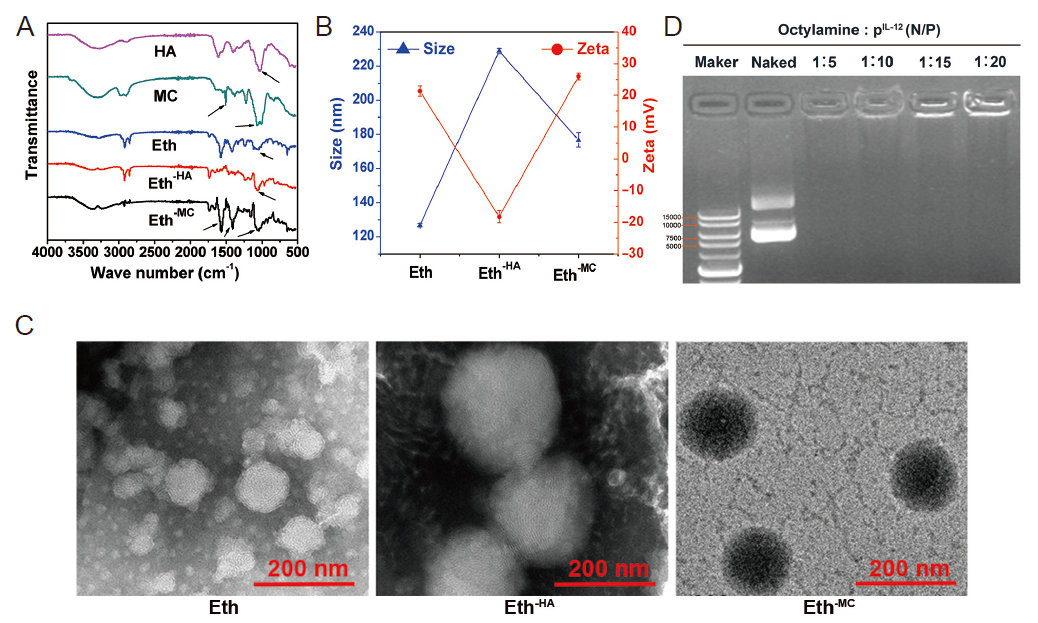
Figure 1. Characterization of Eths. (A) Infrared spectra of the materials. Arrows indicate the characteristic peaks. (B) Particle size and electric potential. Data are expressed as mean ± SD. The experiments were repeated by three times. (C) Transmission electron microscopy images of Eths. The adsorption of HA made the particle size of Eth increase significantly while the potential greatly reduced. When MC is further adsorbed on the surface of HA, the particle size decreases but is still larger than bare Eth, and the potential increased slightly. Scale bars: 200 nm. (D) Image of agarose gel electrophoresis at different octadecylamine:pIL-12 ratios. Eth: ethosome; HA: hyaluronic acid; MC: mannosylated chitosan; pIL-12: plasmid containing IL-12 gene.
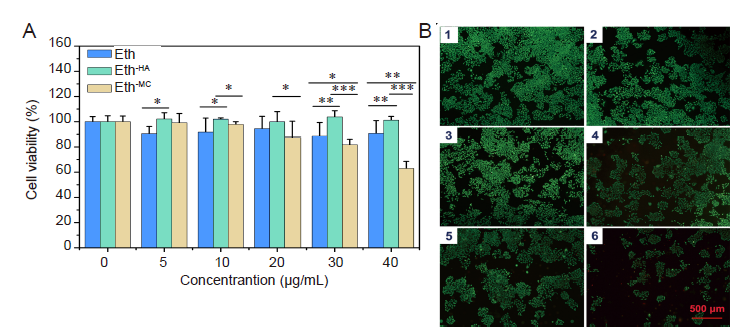
Figure 2. Cytotoxicity of Eths with different modifications as evaluated by Cell Counting Kit-8. (A) Cell viability. Data are expressed as mean ± SD. The experiments were repeated by three times. *P < 0.05, **P < 0.01, ***P < 0.001 (one-way analysis of variance followed with Tukey’s post hoc test). (B) Live and dead staining of Eth-MC-treated cells. There was no significant difference in cell morphology among different groups, but cell proliferation was inhibited to a certain extent when the concentration of Eth-MC is higher than 30 μg/mL. B1-6: 0, 5, 10, 20, 30, and 40 μg/mL. The green fluorescence indicates live cells. Scale bars: 500 μm. Eth: ethosome; HA: hyaluronic acid; MC: mannosylated chitosan.
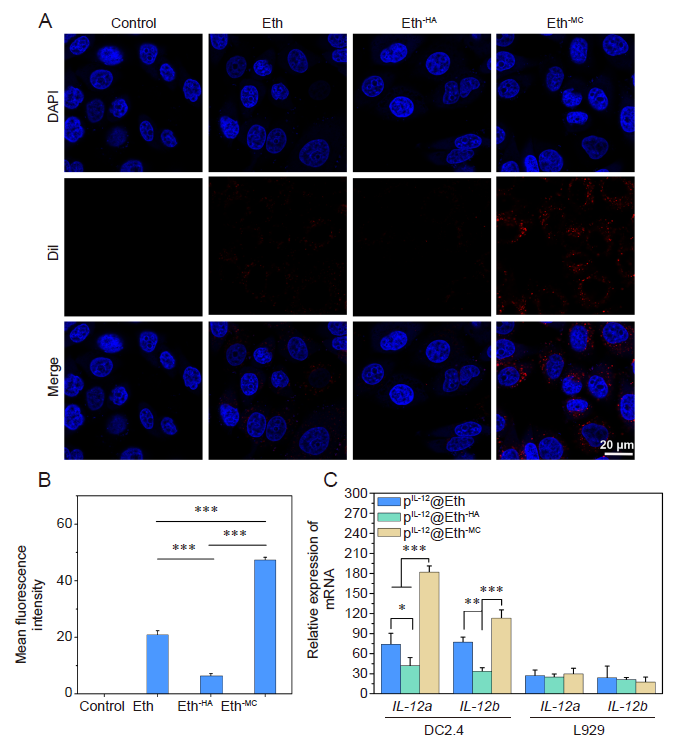
Figure 3. Performance of Eths targeting dendritic cells. (A) Confocal laser scanning microscopy images of dendritic cells phagocytosing DiI (red)-labelled Eths. The phagocytosis efficiency of Eth-MC was significantly higher than that of others. Scale bars: 20 μm. (B) Fluorescent intensity analysis of A. (C) Relative mRNA expression of IL-12 in different cells transfected with pIL-12@Eths detected by real-time polymerase chain reaction. Data are expressed as mean ± SD. The experiments were repeated by three times. *P < 0.05, **P < 0.01, ***P < 0.001 (one-way analysis of variance followed with Tukey’s post hoc test). DAPI: 4,6-diamino-2-phenylindole dihydrochloride; DiI: 1,1’-dioctadecyl-3,3,3’,3’-tetramethylindocarbocyanine perchlorate; Eth: ethosome; HA: hyaluronic acid; IL-12: interleukin-12; MC: mannosylated chitosan; pIL-12: plasmid containing IL-12 gene.
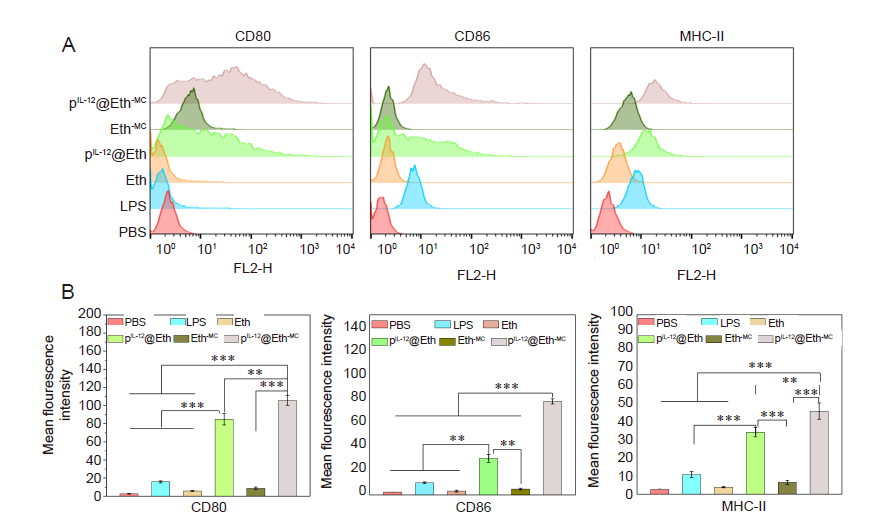
Figure 4. IL-12 gene-loaded Eths stimulate DCs expression of CD80, CD86 and MHC-II. (A) Flow cytometry histograms. (B) Quantitative analysis of CD80, CD86 and MHC-II. Data are expressed as mean ± SD. The experiments were repeated by three times. **P < 0.01, ***P < 0.001 (one-way analysis of variance followed with Tukey’s post hoc test). Eth: ethosome; HA: hyaluronic acid; IL-12: interleukin-12; LPS: lipopolysaccharide; MC: mannosylated chitosan; MHC-II: major histocompatibility complex-II; PBS: phosphate-buffered saline; pIL-12: plasmid containing IL-12 gene.
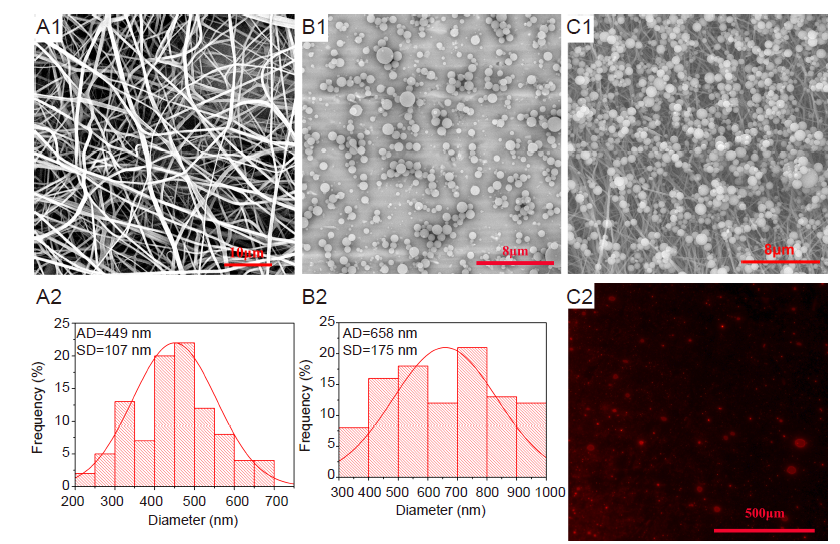
Figure 5. Morphology of SF-PVA nanofibrous mats (A1), microspheres (B1) and TCI patch (C1). (A2) Distribution of diameters in A1. (B2) Distribution of diameters in B1. (C2) Fluorescence micrograph of TCI patch. Red represents DiI-labeled Eth-MC. Scale bars: 10 μm in A1, 8 μm in B1 and C1, 500 μm in C2. AD: average diameter; Eth-MC: mannosylated chitosan-modified ethosome; PVA: polyvinyl alcohol; SF: silk fibroin; TCI: transcutaneous immunization.
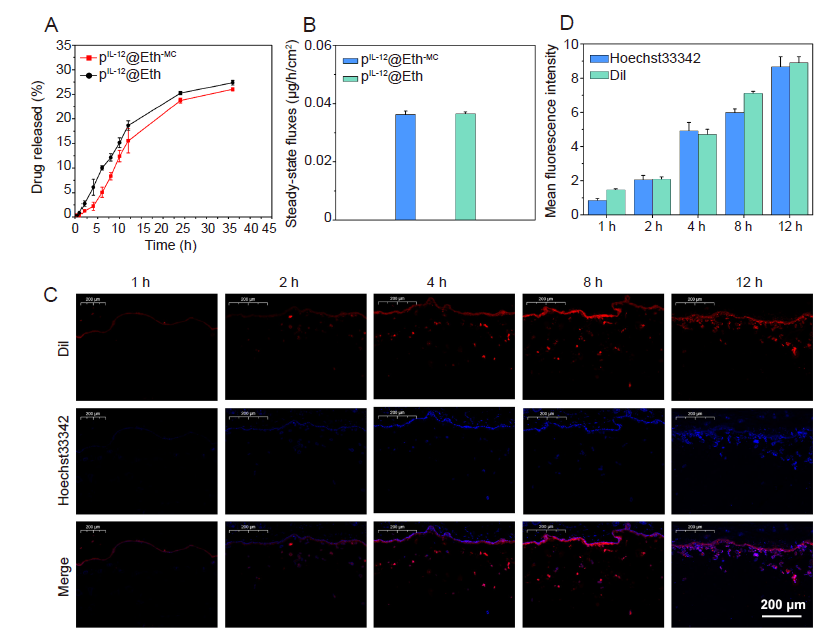
Figure 6. (A, B) In vitro cumulative transdermal drug release curve (A) and its Steady-state release flux (B). (C, D) Fluorescence images of skin sections (C) and their fluorescent intensity analysis (D) after transdermal administration with pIL-12@Eth-MC. The amount of Eth and DNA that penetrate into the skin tissue increases with time. Scale bar: 200 μm. Data are expressed as mean ± SD. The experiments were repeated by three times. DiI: 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate; Eth: ethosome; IL-12: interleukin-12; MC: mannosylated chitosan; pIL-12: plasmid containing IL-12 gene.
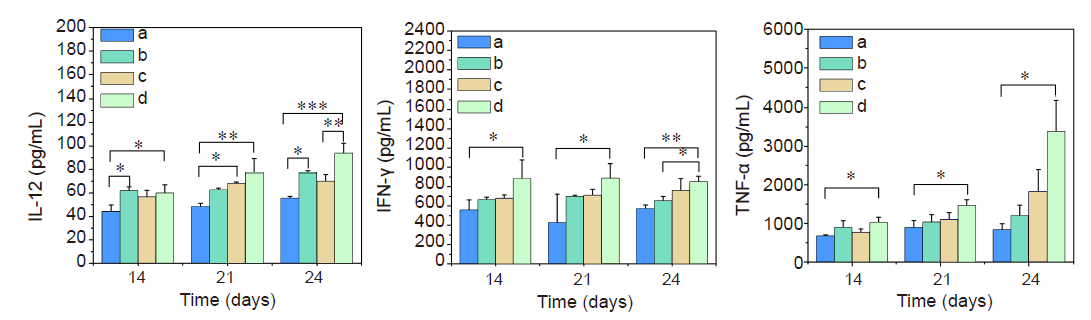
Figure 7. Expression of cytokines in the blood of melanoma-bearing mice given TCI and/or aPD-1 monotherapy treatment. a﹣d: Control, TCI monotherapy, aPD-1 monotherapy, and TCI + aPD-1 groups. Data are expressed as mean ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001 (one-way analysis of variance followed with Tukey’s post hoc test). aPD-1: programmed cell death protein 1 monoclonal antibody; IFN-γ: interferon-γ; IL-12: interleukin-12; TCI: transcutaneous immunization; TNF-α: tumour necrosis factor-α.
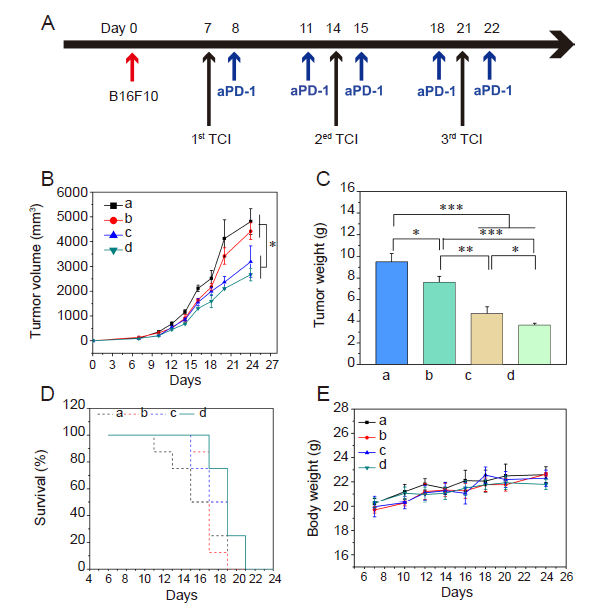
Figure 8. Schematic diagram of animal experiment protocol (A), tumour volume change curves (B), tumour weight at day 24 (C), survival rate (D) and Body weight change curves (E) in melanoma-bearing mice with TCI and/or aPD-1 treatment. a-d: Control, TCI monotherapy, aPD-1 monotherapy, and TCI + aPD-1 groups. Data are expressed as mean ± SD (n = 4). *P < 0.05, **P < 0.01, ***P < 0.001 (one-way analysis of variance followed with Tukey’s post hoc test). aPD-1: programmed cell death protein 1 monoclonal antibody; TCI: transcutaneous immunization.
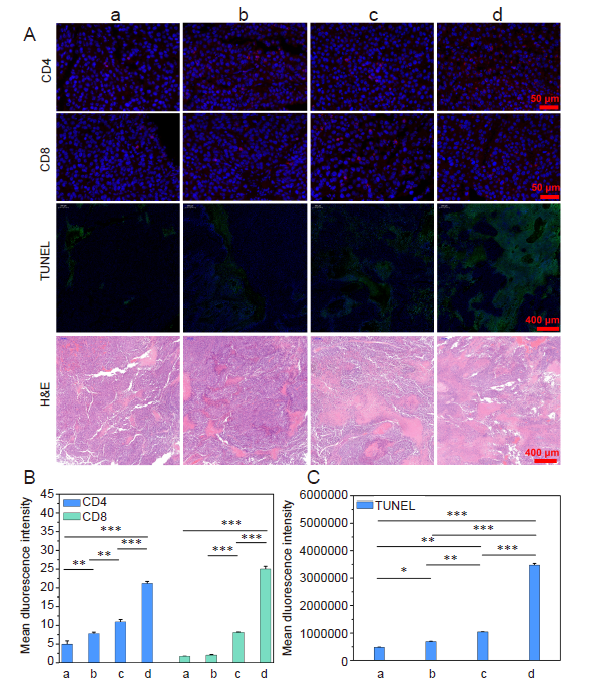
Figure 9. (A) Different staining images of tumour tissue from the melanoma-bearing mice after receiving different treatments. (B, C) Fluorescent intensity analysis of anti-CD4/CD8 or TUNEL staining. The treatment groups had more tumour cell apoptosis and more cytotoxic T cell infiltration than the control, and the combined treatment group was far better than the other groups. Scale bars: 50 and 400 μm. a-d: Control, TCI monotherapy, aPD-1 monotherapy, and TCI + aPD-1 groups. Data are expressed as mean ± SD (n = 4). *P < 0.05, **P < 0.01, ***P < 0.001 (one-way analysis of variance followed with Tukey’s post hoc test). aPD-1: programmed cell death protein 1 monoclonal antibody; H&E: hematoxylin-eosin; TCI: transcutaneous immunization; TUNEL: terminal deoxynucleotidyl transferase dUTP nick-end labelling.
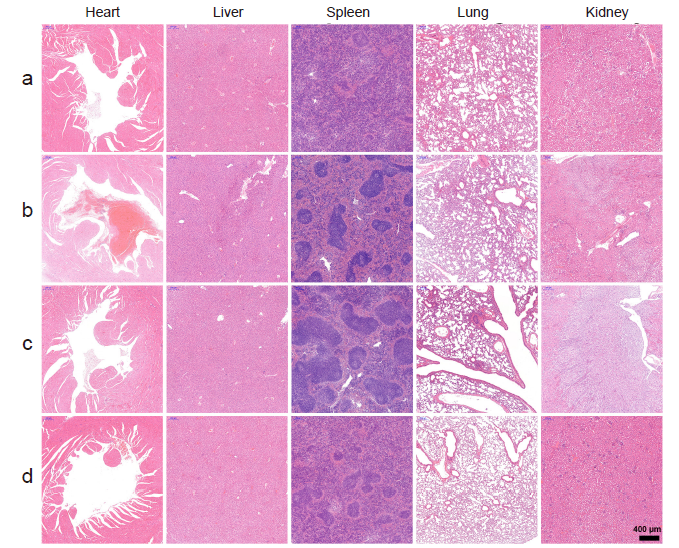
Figure 10. Histological characterization (hematoxylin-eosin staining) of the major organs from the melanoma-bearing mice after receiving different treatments. The organs showed little difference among the groups. a-d: Control, TCI monotherapy, aPD-1 monotherapy, and TCI + aPD-1 groups. Scale bar: 400 μm. aPD-1: programmed cell death protein 1 monoclonal antibody; TCI: transcutaneous immunization.
| 1. |
Tikkanen, A.; Iivanainen, S.; Koivunen, J. P. Treatment discontinuation and re-initiation of anti-PD-(L)1 agents in metastatic cancers. J Cancer Res Clin Oncol. 2020, 146, 2153-2160.
doi: 10.1007/s00432-020-03217-7 URL |
| 2. |
Aspeslagh, S.; Chabanon, R. M.; Champiat, S.; Postel-Vinay, S. Understanding genetic determinants of resistance to immune checkpoint blockers. Semin Cancer Biol. 2020, 65, 123-139.
doi: 10.1016/j.semcancer.2019.12.020 URL |
| 3. | Fu, J.; Kanne, D. B.; Leong, M.; Glickman, L. H.; McWhirter, S. M.; Lemmens, E.; Mechette, K.; Leong, J. J.; Lauer, P.; Liu, W.; Sivick, K. E.; Zeng, Q.; Soares, K. C.; Zheng, L.; Portnoy, D. A.; Woodward, J. J.; Pardoll, D. M.; Dubensky, T. W.; Jr.; Kim, Y. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci Transl Med. 2015, 7, 283ra252. |
| 4. |
Segovia, M.; Russo, S.; Jeldres, M.; Mahmoud, Y. D.; Perez, V.; Duhalde, M.; Charnet, P.; Rousset, M.; Victoria, S.; Veigas, F.; Louvet, C.; Vanhove, B.; Floto, R. A.; Anegon, I.; Cuturi, M. C.; Girotti, M. R.; Rabinovich, G. A.; Hill, M. Targeting TMEM176B enhances antitumor immunity and augments the efficacy of immune checkpoint blockers by unleashing inflammasome activation. Cancer Cell. 2019, 35, 767-781.e6.
doi: 10.1016/j.ccell.2019.04.003 URL |
| 5. | Nakao, S.; Arai, Y.; Tasaki, M.; Yamashita, M.; Murakami, R.; Kawase, T.; Amino, N.; Nakatake, M.; Kurosaki, H.; Mori, M.; Takeuchi, M Nakamura, T. Intratumoral expression of IL-7 and IL-12 using an oncolytic virus increases systemic sensitivity to immune checkpoint blockade. Sci Transl Med. 2020, 12, eaax7992. |
| 6. |
Wang, M.; Liu, Y.; Cheng, Y.; Wei, Y.; Wei, X. Immune checkpoint blockade and its combination therapy with small-molecule inhibitors for cancer treatment. Biochim Biophys Acta Rev Cancer. 2019, 1871, 199-224.
doi: 10.1016/j.bbcan.2018.12.002 URL |
| 7. | Ruan, H.; Bu, L.; Hu, Q.; Cheng, H.; Lu, W.; Gu, Z. Strategies of combination drug delivery for immune checkpoint blockades. Adv Healthc Mater. 2019, 8, e1801099. |
| 8. |
Kos, S.; Lopes, A.; Preat, V.; Cemazar, M.; Lampreht Tratar, U.; Ucakar, B.; Vanvarenberg, K.; Sersa, G.; Vandermeulen, G. Intradermal DNA vaccination combined with dual CTLA-4 and PD-1 blockade provides robust tumor immunity in murine melanoma. PLoS One. 2019, 14, e0217762.
doi: 10.1371/journal.pone.0217762 URL |
| 9. |
Garris, C. S.; Arlauckas, S. P.; Kohler, R. H.; Trefny, M. P.; Garren, S.; Piot, C.; Engblom, C.; Pfirschke, C.; Siwicki, M.; Gungabeesoon, J.; Freeman, G. J.; Warren, S. E.; Ong, S.; Browning, E.; Twitty, C. G.; Pierce, R. H.; Le, M. H.; Algazi, A. P.; Daud, A. I.; Pai, S. I.; Zippelius, A.; Weissleder, R.; Pittet, M. J. Successful anti-PD-1 cancer immunotherapy requires T cell-dendritic cell crosstalk involving the cytokines IFN-γ and IL-12. Immunity. 2018, 49,1148-1161.e7.
doi: 10.1016/j.immuni.2018.09.024 URL |
| 10. | Yao, W.; Li, Y.; Zeng, L.; Zhang, X.; Zhou, Z.; Zheng, M.; Wan, H. Intratumoral injection of dendritic cells overexpressing interleukin-12 inhibits melanoma growth. Oncol Rep. 2019, 42, 370-376. |
| 11. |
Bussio, J. I.; Molina-Perea, C.; González-Aramundiz, J. V. Lower-sized chitosan nanocapsules for transcutaneous antigen delivery. Nanomaterials (Basel). 2018, 8, 659.
doi: 10.3390/nano8090659 URL |
| 12. | Sugita, K.; Kabashima, K.; Atarashi, K.; Shimauchi, T.; Kobayashi, M.; Tokura, Y. Innate immunity mediated by epidermal keratinocytes promotes acquired immunity involving Langerhans cells and T cells in the skin. Clin Exp Immunol. 2007, 147, 176-183. |
| 13. |
Mutyambizi, K.; Berger, C. L.; Edelson, R. L. The balance between immunity and tolerance: the role of Langerhans cells. Cell Mol Life Sci. 2009, 66, 831-840.
doi: 10.1007/s00018-008-8470-y URL |
| 14. |
Yang, X.; Wang, X.; Hong, H.; Elfawal, G.; Lin, S.; Wu, J.; Jiang, Y.; He, C.; Mo, X.; Kai, G.; Wang, H. Galactosylated chitosan-modified ethosomes combined with silk fibroin nanofibers is useful in transcutaneous immunization. J Control Release. 2020, 327, 88-99.
doi: 10.1016/j.jconrel.2020.07.047 URL |
| 15. |
Anantaworasakul, P.; Chaiyana, W.; Michniak-Kohn, B. B.; Rungseevijitprapa, W.; Ampasavate, C. Enhanced transdermal delivery of concentrated capsaicin from chili extract-loaded lipid nanoparticles with reduced skin irritation. Pharmaceutics. 2020, 12, 463.
doi: 10.3390/pharmaceutics12050463 URL |
| 16. |
Touitou, E.; Dayan, N.; Bergelson, L.; Godin, B.; Eliaz, M. Ethosomes - novel vesicular carriers for enhanced delivery: characterization and skin penetration properties. J Control Release. 2000, 65, 403-418.
doi: 10.1016/S0168-3659(99)00222-9 URL |
| 17. | Mezei, M.; Gulasekharam, V. Liposomes--a selective drug delivery system for the topical route of administration. Lotion dosage form. Life Sci. 1980, 26, 1473-1477. |
| 18. |
Di Blasio, S.; Wortel, I. M.; van Bladel, D. A.; de Vries, L. E.; Duiveman-de Boer, T.; Worah, K.; de Haas, N.; Buschow, S. I.; de Vries, I. J.; Figdor, C. G.; Hato, S. V. Human CD1c(+) DCs are critical cellular mediators of immune responses induced by immunogenic cell death. Oncoimmunology. 2016, 5, e1192739.
doi: 10.1080/2162402X.2016.1192739 URL |
| 19. |
Unger, W. W.; van Kooyk, Y. ‘Dressed for success’ C-type lectin receptors for the delivery of glyco-vaccines to dendritic cells. Curr Opin Immunol. 2011, 23, 131-137.
doi: 10.1016/j.coi.2010.11.011 URL |
| 20. |
Ikehara, Y.; Shiuchi, N.; Kabata-Ikehara, S.; Nakanishi, H.; Yokoyama, N.; Takagi, H.; Nagata, T.; Koide, Y.; Kuzushima, K.; Takahashi, T.; Tsujimura, K.; Kojima, N. Effective induction of anti-tumor immune responses with oligomannose-coated liposome targeting to intraperitoneal phagocytic cells. Cancer Lett. 2008, 260, 137-145.
doi: 10.1016/j.canlet.2007.10.038 URL |
| 21. |
Gao, H.; Gonçalves, C.; Gallego, T.; François-Heude, M.; Malard, V.; Mateo, V.; Lemoine, F.; Cendret, V.; Djedaini-Pilard, F.; Moreau, V.; Pichon, C.; Midoux, P. Comparative binding and uptake of liposomes decorated with mannose oligosaccharides by cells expressing the mannose receptor or DC-SIGN. Carbohydr Res. 2020, 487, 107877.
doi: 10.1016/j.carres.2019.107877 URL |
| 22. |
Ye, J.; Yang, Y.; Dong, W.; Gao, Y.; Meng, Y.; Wang, H.; Li, L.; Jin, J.; Ji, M.; Xia, X.; Chen, X.; Jin, Y.; Liu, Y. Drug-free mannosylated liposomes inhibit tumor growth by promoting the polarization of tumor-associated macrophages. Int J Nanomedicine. 2019, 14, 3203-3220.
doi: 10.2147/IJN URL |
| 23. | Mata-Espinosa, D. A.; Francisco-Cruz, A.; Marquina-Castillo, B.; Barrios-Payan, J.; Ramos-Espinosa, O.; Bini, E. I.; Xing, Z.; Hernández-Pando, R. Immunotherapeutic effects of recombinant adenovirus encoding interleukin 12 in experimental pulmonary tuberculosis. Scand J Immunol. 2019, 89, e12743. |
| 24. |
Luo, M.; Liang, X.; Luo, S. T.; Wei, X. W.; Liu, T.; Ren, J.; Ma, C. C.; Yang, Y. H.; Wang, B. L.; Liu, L.; Song, X. R.; He, Z. Y.; Wei, Y. Q. Folate-modified lipoplexes delivering the interleukin-12 gene for targeting colon cancer immunogene therapy. J Biomed Nanotechnol. 2015, 11, 2011-2023.
doi: 10.1166/jbn.2015.2136 URL |
| 25. |
Takemoto, N.; Intlekofer, A. M.; Northrup, J. T.; Wherry, E. J.; Reiner, S. L. Cutting edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J Immunol. 2006, 177, 7515-7519.
doi: 10.4049/jimmunol.177.11.7515 URL |
| 26. | Portielje, J. E.; Lamers, C. H.; Kruit, W. H.; Sparreboom, A.; Bolhuis, R. L.; Stoter, G.; Huber, C.; Gratama, J. W. Repeated administrations of interleukin (IL)-12 are associated with persistently elevated plasma levels of IL-10 and declining IFN-gamma, tumor necrosis factor-alpha, IL-6, and IL-8 responses. Clin Cancer Res. 2003, 9, 76-83. |
| 27. |
Wang, P.; Li, X.; Wang, J.; Gao, D.; Li, Y.; Li, H.; Chu, Y.; Zhang, Z.; Liu, H.; Jiang, G.; Cheng, Z.; Wang, S.; Dong, J.; Feng, B.; Chard, L. S.; Lemoine, N. R.; Wang, Y. Re-designing Interleukin-12 to enhance its safety and potential as an anti-tumor immunotherapeutic agent. Nat Commun. 2017, 8, 1395.
doi: 10.1038/s41467-017-01385-8 URL |
| 28. |
Yamasaki, S.; Sakuma, W.; Yasui, H.; Daicho, K.; Saito, T.; Fujisawa, S.; Isogai, A.; Kanamori, K. Nanocellulose xerogels with high porosities and large specific surface areas. Front Chem. 2019, 7, 316.
doi: 10.3389/fchem.2019.00316 URL |
| 29. |
Rask, M. B.; Knopp, M. M.; Olesen, N. E.; Holm, R.; Rades, T. Influence of PVP/VA copolymer composition on drug-polymer solubility. Eur J Pharm Sci. 2016, 85, 10-17.
doi: 10.1016/j.ejps.2016.01.026 URL |
| 30. |
Kheradvar, S. A.; Nourmohammadi, J.; Tabesh, H.; Bagheri, B. Starch nanoparticle as a vitamin E-TPGS carrier loaded in silk fibroin-poly(vinyl alcohol)-Aloe vera nanofibrous dressing. Colloids Surf B Biointerfaces. 2018, 166, 9-16.
doi: 10.1016/j.colsurfb.2018.03.004 URL |
| 31. |
Chaubey, P.; Mishra, B. Mannose-conjugated chitosan nanoparticles loaded with rifampicin for the treatment of visceral leishmaniasis. Carbohydr Polym. 2014, 101, 1101-1108.
doi: 10.1016/j.carbpol.2013.10.044 URL |
| 32. |
Gao, M.; Zhu, X.; Wu, L.; Qiu, L. Cationic polyphosphazene vesicles for cancer immunotherapy by efficient in vivo cytokine IL-12 plasmid delivery. Biomacromolecules. 2016, 17, 2199-2209.
doi: 10.1021/acs.biomac.6b00433 URL |
| 33. |
Wang, Y.; Lin, Y. X.; Qiao, S. L.; An, H. W.; Ma, Y.; Qiao, Z. Y.; Rajapaksha, R. P.; Wang, H. Polymeric nanoparticles promote macrophage reversal from M2 to M1 phenotypes in the tumor microenvironment. Biomaterials. 2017, 112, 153-163.
doi: 10.1016/j.biomaterials.2016.09.034 URL |
| 34. |
Junyaprasert, V. B.; Boonme, P.; Wurster, D. E.; Rades, T. Aerosol OT microemulsions as carriers for transdermal delivery of hydrophobic and hydrophilic local anesthetics. Drug Deliv. 2008, 15, 323-330.
doi: 10.1080/10717540802035319 URL |
| 35. |
Chaubey, P.; Mishra, B.; Mudavath, S. L.; Patel, R. R.; Chaurasia, S.; Sundar, S.; Suvarna, V.; Monteiro, M. Mannose-conjugated curcumin-chitosan nanoparticles: Efficacy and toxicity assessments against Leishmania donovani. Int J Biol Macromol. 2018, 111, 109-120.
doi: 10.1016/j.ijbiomac.2017.12.143 URL |
| 36. | Tsepilov, R. N.; Beloded, A. V.; Samoĭlenko, II. Optimization of Streptococcus equi subsp. zooepidemicus cultivation process--producer of hyaluronic acid. Zh Mikrobiol Epidemiol Immunobiol. 2013, 12-20. |
| 37. |
Bodade, S. S.; Shaikh, K. S.; Kamble, M. S.; Chaudhari, P. D. A study on ethosomes as mode for transdermal delivery of an antidiabetic drug. Drug Deliv. 2013, 20, 40-46.
doi: 10.3109/10717544.2012.752420 URL |
| 38. |
Lu, J.; Guo, T.; Fan, Y.; Li, Z.; He, Z.; Yin, S.; Feng, N. Recent developments in the principles, modification and application prospects of functionalized ethosomes for topical delivery. Curr Drug Deliv. 2020. doi: 10.2174/1567201817666200826093102.
doi: 10.2174/1567201817666200826093102 URL |
| 39. |
Singh, B.; Maharjan, S.; Sindurakar, P.; Cho, K. H.; Choi, Y. J.; Cho, C. S. Needle-free immunization with chitosan-based systems. Int J Mol Sci. 2018, 19, 3639.
doi: 10.3390/ijms19113639 URL |
| 40. |
Zha, F.; Chen, W.; Zhang, L.; Yu, D. Electrospun natural polymer and its composite nanofibrous scaffolds for nerve tissue engineering. J Biomater Sci Polym Ed. 2020, 31, 519-548.
doi: 10.1080/09205063.2019.1697170 URL |
| 41. |
Laubach, J.; Joseph, M.; Brenza, T.; Gadhamshetty, V.; Sani, R. K. Exopolysaccharide and biopolymer-derived films as tools for transdermal drug delivery. J Control Release. 2021, 329, 971-987.
doi: 10.1016/j.jconrel.2020.10.027 URL |
| 42. | Tahara, H.; Lotze, M. T. Antitumor effects of interleukin-12 (IL-12): applications for the immunotherapy and gene therapy of cancer. Gene Ther. 1995, 2, 96-106. |
| 43. |
Alsaieedi, A.; Holler, A.; Velica, P.; Bendle, G.; Stauss, H. J. Safety and efficacy of Tet-regulated IL-12 expression in cancer-specific T cells. Oncoimmunology. 2019, 8, 1542917.
doi: 10.1080/2162402X.2018.1542917 URL |
| 44. |
Razi Soofiyani, S.; Kazemi, T.; Lotfipour, F.; Mohammad Hosseini, A.; Shanehbandi, D.; Hallaj-Nezhadi, S.; Baradaran, B. Gene therapy with IL-12 induced enhanced anti-tumor activity in fibrosarcoma mouse model. Artif Cells Nanomed Biotechnol. 2016, 44, 1988-1993.
doi: 10.3109/21691401.2015.1129618 URL |
| 45. |
Kobelt, D.; Zhang, C.; Clayton-Lucey, I. A.; Glauben, R.; Voss, C.; Siegmund, B.; Stein, U. Pro-inflammatory TNF-α and IFN-γ promote tumor growth and metastasis via induction of MACC1. Front Immunol. 2020, 11, 980.
doi: 10.3389/fimmu.2020.00980 URL |
| 46. |
Huang, J. H.; Zhang, S. N.; Choi, K. J.; Choi, I. K.; Kim, J. H.; Lee, M. G.; Kim, H.; Yun, C. O. Therapeutic and tumor-specific immunity induced by combination of dendritic cells and oncolytic adenovirus expressing IL-12 and 4-1BBL. Mol Ther. 2010, 18, 264-274.
doi: 10.1038/mt.2009.205 URL |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||