Biomaterials Translational ›› 2021, Vol. 2 ›› Issue (4): 287-293.doi: 10.12336/biomatertransl.2021.04.003
• VIEWPOINT • Previous Articles Next Articles
Received:2021-12-12
Revised:2021-12-17
Accepted:2021-12-20
Online:2021-12-28
Published:2021-12-28
Contact:
James T. Triffitt
E-mail:james.triffitt@ndorms.ox.ac.uk
About author:James T. Triffitt, james.triffitt@ndorms.ox.ac.uk.
Triffitt, J. A brief history of the development of stromal stem cells (stem cells of the skeleton). Biomater Transl. 2021, 2(4), 287-293.
| Author | Nomenclature |
|---|---|
| Arthur W. Ham | Osteogenic cells |
| Numerous authors | Connective tissue stem cells |
| Alexander J. Friedenstein | Marrow stromal cells |
| Maureen E. Owen | Stromal stem cells |
| James T. Triffitt | Stromal fibroblastic cells |
| Arnold I. Caplan | Mesenchymal stem cells or medicinal signalling cells (MSCs) |
| Paul C. Schiller | Marrow isolated adult multilineage inducible cells (MIAMI cells) |
| Catherine M. Verfaillie | Multipotent adult progenitor cells (MAPCs) |
| Pamela G. Robey | Skeletal stem cells |
Table 1 Some examples of the nomenclature for the stromal cell preparations of bone marrow used by the named scientific investigators over many years.
| Author | Nomenclature |
|---|---|
| Arthur W. Ham | Osteogenic cells |
| Numerous authors | Connective tissue stem cells |
| Alexander J. Friedenstein | Marrow stromal cells |
| Maureen E. Owen | Stromal stem cells |
| James T. Triffitt | Stromal fibroblastic cells |
| Arnold I. Caplan | Mesenchymal stem cells or medicinal signalling cells (MSCs) |
| Paul C. Schiller | Marrow isolated adult multilineage inducible cells (MIAMI cells) |
| Catherine M. Verfaillie | Multipotent adult progenitor cells (MAPCs) |
| Pamela G. Robey | Skeletal stem cells |
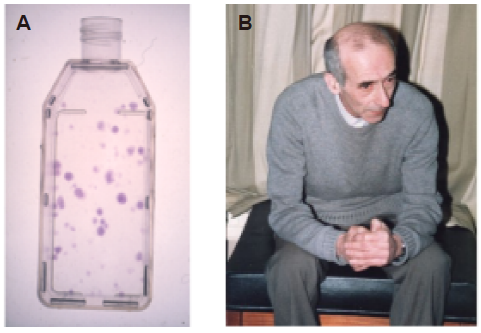
Figure 1. (A) Culture flask of adherent rabbit bone marrow stromal fibroblasts growing as single colonies from single-cell suspensions of marrow stroma; these called colony forming units fibroblastic (CFU-F). (B) Alexander Friedenstein (1924–1998) in research discussions whilst visiting the MRC Bone Research Laboratory, Oxford, UK in the early 1980s.

Figure 2. Leaders of the bone field and eminent attendees of the meeting held under the auspices of the UK Bone and Tooth Society at Keble College in Oxford, UK in July 1993, in honour of the retirement of Dr. Maureen E. Owen. Front row (from left): Alexander Friedenstein (Russia), Hari Reddi (USA), Alberta Zambonine-Zallone (Italy), Maureen Owen (UK), Peter Nijweide (Holland). Second row (from left): Larry Raisz (USA), Clarke Anderson (USA), Gidean Rodan (USA), John Termine (USA), Arnie Kahn (USA), Rolfe Howlett (Australia). Back row (from left): Greg Mundy (USA/Australia), Jack Martin (Australia), Steve Krane (USA), Herbie Fleisch (Switzerland), Gastone Marotti (Italy).
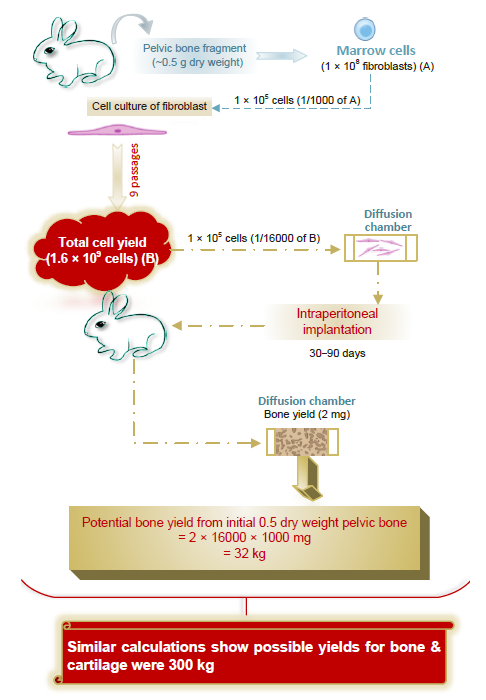
Figure 3. Osteogenic capacity of young rabbit bone marrow from a small fragment (0.5 g dry weight) of pelvic bone, calculated by proportion as osteogenic tissue (bone or bone and cartilage) and determined by tissue formation in isolated diffusion chambers implanted in vivo.
| 1. | Gowers, E. The Complete Plain Words. Her Majesty’s Stationery Office: London. 1954. |
| 2. | Lajtha, L. G. Haemopoietic stem cells: concept and definitions. Blood Cells. 1979, 5, 447-455. |
| 3. | Lajtha, L. G. Cellular kinetics of haemopoiesis. In Blood and its disorders, 2nd ed.; Hardisty, R. M.; Weatherall, D. J., eds.; Blackwell Scientific Publications: Oxford. 1982; pp 57-74. |
| 4. | Schofield, R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978, 4, 7-25. |
| 5. |
Bianco, P. “Mesenchymal” stem cells. Annu Rev Cell Dev Biol. 2014, 30, 677-704.
doi: 10.1146/cellbio.2014.30.issue-1 URL |
| 6. |
Bianco, P. Stem cells and bone: a historical perspective. Bone. 2015, 70, 2-9.
doi: 10.1016/j.bone.2014.08.011 URL |
| 7. | Aubin, J.; Triffitt, J. Mesenchymal stem cells and osteoblast differentiation. In Principles of bone biology (2nd edition) Volume 1 (of 2). Bilezikian, J. P.; Raisz, L. G.; Rodan, G. A.; eds; Academic Press, San Diego, California, USA. 2002. |
| 8. | Triffitt, J. The stem cell of the osteoblast. In Principles of bone biology. Bilezikian, J. P.; Raisz, L. G.; Rodan, G. A.; eds; Academic Press, Inc.: San Diego, CA, USA. 1996. |
| 9. | Ham, A.; Leeson, T. Histology. Pitman Medical Publishing Co., Ltd.: London. 1961. |
| 10. | McLean, F. C.; Urist, M. R. Bone, an introduction to the physiology of skeletal tissue. The Univerisity of Chicago Press: Chicago, USA, 1955. |
| 11. | Stump, C. W. The histogenesis of bone. J Anat. 1925, 59, 136-154. |
| 12. | Schäfer, E. A. Essentials of histology. Hospital (Lond 1886). 1907, 42, 166. |
| 13. |
Neumann, P. E.; Neumann, E. E. General histological woes: Definition and classification of tissues. Clin Anat. 2021, 34, 794-801.
doi: 10.1002/ca.v34.5 URL |
| 14. | Marchand, F. Der Process der Wundheiling. Verlag von Ferdinand Enke: Stuttgart. 1901. |
| 15. | Keith, A. Menders of the maimed. Henry Frowde Oxford University Press: London. 1919. |
| 16. | Bloom, W.; Fawcett, D. A textbook of histology. W.B. Saunders Company: Philadelphia-London-Toronto. 1968. |
| 17. | Ham, A. W. A histological study of the early phases of bone repair. J Bone Joint Surg Am. 1930, 12, 827-844. |
| 18. | Pritchard, J. J. The osteoblast. In The biochemistry and physiology of bone, Bourne, G. H., ed. Academic Press: Cambridge. 1972; pp 21-43. |
| 19. | Rasmussen, H.; Bordier, P. The physiological and cellular basis of metabolic bone disease. Waverly Press, Inc.: Baltimore, MD, USA. 1974. |
| 20. |
Chambers, T. J. The pathobiology of the osteoclast. J Clin Pathol. 1985, 38, 241-252.
doi: 10.1136/jcp.38.3.241 URL |
| 21. | Ash, P.; Loutit, J. F.; Townsend, K. M. Osteoclasts derive from hematopoietic stem cells according to marker, giant lysosomes of beige mice. Clin Orthop Relat Res. 1981, 249-258. |
| 22. |
Ash, P.; Loutit, J. F.; Townsend, K. M. Osteoclasts derived from haematopoietic stem cells. Nature. 1980, 283, 669-670.
doi: 10.1038/283669a0 URL |
| 23. |
Walker, D. G. Osteopetrosis cured by temporary parabiosis. Science. 1973, 180, 875.
doi: 10.1126/science.180.4088.875 URL |
| 24. |
Walker, D. G. Spleen cells transmit osteopetrosis in mice. Science. 1975, 190, 785-787.
doi: 10.1126/science.1198094 URL |
| 25. |
Loutit, J. F.; Nisbet, N. W. The origin of osteoclasts. Immunobiology. 1982, 161, 193-203.
doi: 10.1016/S0171-2985(82)80074-0 URL |
| 26. |
Nisbet, N. W.; Menage, J.; Loutit, J. F. Osteogenesis in osteopetrotic mice. Calcif Tissue Int. 1982, 34, 37-42.
doi: 10.1007/BF02411206 URL |
| 27. |
Teitelbaum, S. L. Bone resorption by osteoclasts. Science. 2000, 289, 1504-1508.
doi: 10.1126/science.289.5484.1504 URL |
| 28. |
Waller, E. K.; Olweus, J.; Lund-Johansen, F.; Huang, S.; Nguyen, M.; Guo, G. R.; Terstappen, L. The “common stem cell” hypothesis reevaluated: human fetal bone marrow contains separate populations of hematopoietic and stromal progenitors. Blood. 1995, 85, 2422-2435.
doi: 10.1182/blood.V85.9.2422.bloodjournal8592422 URL |
| 29. |
Triffitt, J. T. Initiation and enhancement of bone formation. A review. Acta Orthop Scand. 1987, 58, 673-684.
doi: 10.3109/17453678709146514 URL |
| 30. |
Tonna, E. A.; Cronkite, E. P. Autoradiographic studies of cell proliferation in the periosteum of intact and fractured femora of mice utilizing DNA labeling with H3-thymidine. Proc Soc Exp Biol Med. 1961, 107, 719-721.
doi: 10.3181/00379727-107-26733 URL |
| 31. |
Tonna, E. A.; Cronkite, E. P. An autoradiographic study of periosteal cell proliferation with tritiated thymidine. Lab Invest. 1962, 11, 455-462.
doi: 10.1038/jid.1948.115 URL |
| 32. | Tonna, E. A.; Cronkite, E. P. The periosteum. Autoradiographic studies on cellular proliferation and transformation utilizing tritiated thymidine. Clin Orthop Relat Res. 1963, 30, 218-233. |
| 33. |
Owen, M. Cell population kinetics of an osteogenic tissue. I. J Cell Biol. 1963, 19, 19-32.
doi: 10.1083/jcb.19.1.19 URL |
| 34. |
Owen, M.; Macpherson, S. Cell population kinetics of an osteogenic tissue. II. J Cell Biol. 1963, 19, 33-44.
doi: 10.1083/jcb.19.1.33 URL |
| 35. | Owen, M. The origin of bone cells. Int Rev Cytol. 1970, 28, 213-238. |
| 36. | Young, R. W. Autoradiographic studies on cell proliferation and differentiation in bones of young rats injected with thymidine-H3. Anat Rec. 1962, 142, 293-294. |
| 37. |
Young, R. W. Cell proliferation and specialization during endochondral osteogenesis in young rats. J Cell Biol. 1962, 14, 357-370.
doi: 10.1083/jcb.14.3.357 URL |
| 38. |
Göthlin, G.; Ericsson, J. L. On the histogenesis of the cells in fracture callus. Electron microscopic autoradiographic observations in parabiotic rats and studies on labeled monocytes. Virchows Arch B Cell Pathol. 1973, 12, 318-329.
doi: 10.1007/BF02894009 URL |
| 39. |
Feehan, J.; Kassem, M.; Pignolo, R. J.; Duque, G. Bone from blood: characteristics and clinical implications of circulating osteogenic progenitor (COP) cells. J Bone Miner Res. 2021, 36, 12-23.
doi: 10.1002/jbmr.v36.1 URL |
| 40. |
Pignolo, R. J.; Kassem, M. Circulating osteogenic cells: implications for injury, repair, and regeneration. J Bone Miner Res. 2011, 26, 1685-1693.
doi: 10.1002/jbmr.370 URL |
| 41. |
Feehan, J.; Nurgali, K.; Apostolopoulos, V.; Al Saedi, A.; Duque, G. Circulating osteogenic precursor cells: Building bone from blood. EBioMedicine. 2019, 39, 603-611.
doi: 10.1016/j.ebiom.2018.11.051 URL |
| 42. | Maximow, A. Cultures of blood leucocytes. From lymphocyte and monocyte to connective tissue. Arch Exp Zellforsch. 1928, 5, 169-268. |
| 43. |
Luria, E. A.; Panasyuk, A. F.; Friedenstein, A. Y. Fibroblast colony formation from monolayer cultures of blood cells. Transfusion. 1971, 11, 345-349.
doi: 10.1111/trf.1971.11.issue-6 URL |
| 44. |
Kuznetsov, S. A.; Mankani, M. H.; Leet, A. I.; Ziran, N.; Gronthos, S.; Robey, P. G. Circulating connective tissue precursors: extreme rarity in humans and chondrogenic potential in guinea pigs. Stem Cells. 2007, 25, 1830-1839.
doi: 10.1634/stemcells.2007-0140 URL |
| 45. |
Kuznetsov, S. A.; Mankani, M. H.; Gronthos, S.; Satomura, K.; Bianco, P.; Robey, P. G. Circulating skeletal stem cells. J Cell Biol. 2001, 153, 1133-1140.
doi: 10.1083/jcb.153.5.1133 URL |
| 46. | Afanasyev, B. V.; Elstner, E. E.; Zander, A. R. A. J. Friedenstein, founder of the mesenchymal stem cell concept. Transplant Cell Ther. 2009, 1, 35-38. |
| 47. |
Maximow, A. A. Relation of blood cells to connective tissues and endothelium. Physiol Rev. 1924, 4, 533-563.
doi: 10.1152/physrev.1924.4.4.533 URL |
| 48. |
Moore, M. A.; Sheridan, A. P.; Allen, T. D.; Dexter, T. M. Prolonged hematopoiesis in a primate bone marrow culture system: characteristics of stem cell production and the hematopoietic microenvironment. Blood. 1979, 54, 775-793.
doi: 10.1182/blood.V54.4.775.775 URL |
| 49. | Brockbank, K. G.; de Jong, J. P.; Piersma, A. H.; Voerman, J. S. Hemopoiesis on purified bone-marrow-derived reticular fibroblast in vitro. Exp Hematol. 1986, 14, 386-394. |
| 50. |
Tavassoli, M.; Friedenstein, A. Hemopoietic stromal microenvironment. Am J Hematol. 1983, 15, 195-203.
doi: 10.1002/(ISSN)1096-8652 URL |
| 51. | Friedenstein, A. J.; Latzinik, N. W.; Grosheva, A. G.; Gorskaya, U. F. Marrow microenvironment transfer by heterotopic transplantation of freshly isolated and cultured cells in porous sponges. Exp Hematol. 1982, 10, 217-227. |
| 52. | Friedenstein, A. J.; Ivanov-Smolenski, A. A.; Chajlakjan, R. K.; Gorskaya, U. F.; Kuralesova, A. I.; Latzinik, N. W.; Gerasimow, U. W. Origin of bone marrow stromal mechanocytes in radiochimeras and heterotopic transplants. Exp Hematol. 1978, 6, 440-444. |
| 53. | Friedenstein, A. J. Precursor cells of mechanocytes. Int Rev Cytol. 1976, 47, 327-359. |
| 54. |
Friedenstein, A.; Kuralesova, A. I. Osteogenic precursor cells of bone marrow in radiation chimeras. Transplantation. 1971, 12, 99-108.
doi: 10.1097/00007890-197108000-00001 URL |
| 55. | Friedenstein, A. J.; Chailakhjan, R. K.; Lalykina, K. S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970, 3, 393-403. |
| 56. | Friedenstein, A. J. Stromal mechanisms of bone marrow: cloning in vitro and retransplantation in vivo. Haematol Blood Transfus. 1980, 25, 19-29. |
| 57. |
Friedenstein, A. J.; Chailakhyan, R. K.; Latsinik, N. V.; Panasyuk, A. F.; Keiliss-Borok, I. V. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974, 17, 331-340.
doi: 10.1097/00007890-197404000-00001 URL |
| 58. | Friedenstein, A. J.; Deriglasova, U. F.; Kulagina, N. N.; Panasuk, A. F.; Rudakowa, S. F.; Luriá, E. A.; Ruadkow, I. A. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 1974, 2, 83-92. |
| 59. | Friedenstein, A. J.; Chailakhyan, R. K.; Gerasimov, U. V. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987, 20, 263-272. |
| 60. |
Quastler, H.; Sherman, F. G. Cell population kinetics in the intestinal epithelium of the mouse. Exp Cell Res. 1959, 17, 420-438.
doi: 10.1016/0014-4827(59)90063-1 URL |
| 61. |
Hughes, W. L.; Bond, V. P.; Brecher, G.; Cronkite, E. P.; Painter, R. B.; Quastler, H.; Sherman, F. G. Cellular proliferation in the mouse as revealed by autoradiography with tritiated thymidine. Proc Natl Acad Sci U S A. 1958, 44, 476-483.
doi: 10.1073/pnas.44.5.476 URL |
| 62. | Luria, E. A.; Owen, M. E.; Friedenstein, A. J.; Morris, J. F.; Kuznetsow, S. A. Bone formation in organ cultures of bone marrow. Cell Tissue Res. 1987, 248, 449-454. |
| 63. | Luria, E. A.; Owen, M.; Friedenstein, A. J.; Morris, J.; Kuznetsow, S. A.; Joyner, C. Bone formation in organ culture of marrow pieces. Bone. 1986, 7, 313. |
| 64. | Owen, M.; Friedenstein, A. J. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp. 1988, 136, 42-60. |
| 65. | Owen, M. Lineage of osteogenic cells and their relationship to the stromal system. In Bone and Mineral Research, Peck, W. A., ed. Elsevier Science Publishers B.V.: Amsterdam. 1985; pp 1-25. |
| 66. |
Caplan, A. I. Mesenchymal stem cells. J Orthop Res. 1991, 9, 641-650.
doi: 10.1002/(ISSN)1554-527X URL |
| 67. |
Urist, M. R.; DeLange, R. J.; Finerman, G. A. Bone cell differentiation and growth factors. Science. 1983, 220, 680-686.
doi: 10.1126/science.6403986 URL |
| 68. |
Urist, M. R. Bone: formation by autoinduction. Science. 1965, 150, 893-899.
doi: 10.1126/science.150.3698.893 URL |
| 69. | Wozney, J. M.; Rosen, V.; Byrne, M.; Celeste, A. J.; Moutsatsos, I.; Wang, E. A. Growth factors influencing bone development. J Cell Sci Suppl. 1990, 13, 149-156. |
| 70. |
Wozney, J. M.; Rosen, V.; Celeste, A. J.; Mitsock, L. M.; Whitters, M. J.; Kriz, R. W.; Hewick, R. M.; Wang, E. A. Novel regulators of bone formation: molecular clones and activities. Science. 1988, 242, 1528-1534.
doi: 10.1126/science.3201241 URL |
| 71. | Friedenstein, A. J. Determined and inducible osteogenic precursor cells. In Ciba Foundation Symposium 11 (new series), Associated Scientific Publishers: Amsterdam. 1973. |
| 72. |
Pittenger, M. F.; Discher, D. E.; Péault, B. M.; Phinney, D. G.; Hare, J. M.; Caplan, A. I. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019, 4, 22.
doi: 10.1038/s41536-019-0083-6 URL |
| 73. |
Triffitt, J. T. Stem cells and the philosopher’s stone. J Cell Biochem Suppl. 2002, 38, 13-19.
doi: 10.1002/(ISSN)1097-4644 URL |
| 74. |
Wagers, A. J.; Weissman, I. L. Plasticity of adult stem cells. Cell. 2004, 116, 639-648.
doi: 10.1016/S0092-8674(04)00208-9 URL |
| 75. |
Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006, 126, 663-676.
doi: 10.1016/j.cell.2006.07.024 URL |
| 76. | Brockbank, K. G.; de Jong, J. P. Hemopoiesis on skin-derived fibroblasts in vitro. Leukemia. 1987, 1, 609-612. |
| 77. |
Uccelli, A.; Moretta, L.; Pistoia, V. Immunoregulatory function of mesenchymal stem cells. Eur J Immunol. 2006, 36, 2566-2573.
doi: 10.1002/(ISSN)1521-4141 URL |
| 78. |
Caplan, A. I. Mesenchymal stem cells: time to change the name! Stem Cells Transl Med. 2017, 6, 1445-1451.
doi: 10.1002/sctm.17-0051 URL |
| 79. | U.S. Food and Drug Administration. Important patient and consumer information about regenerative medicine therapies. https://www.fda.gov/vaccines-blood-biologics/consumers-biologics/important-patient-and-consumer-information-about-regenerative-medicine-therapies. Accessed July 9, 2021. |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
