Biomaterials Translational ›› 2022, Vol. 3 ›› Issue (1): 3-16.doi: 10.12336/biomatertransl.2022.01.002
• REVIEW • Previous Articles Next Articles
Deepika Arora1,2,3, Pamela Gehron Robey1,*( )
)
Received:2022-01-20
Revised:2022-03-17
Accepted:2022-03-20
Online:2022-03-28
Published:2022-03-28
Contact:
Pamela Gehron Robey
E-mail:pamela.robey@nih.gov
About author:Pamela Gehron Robey, pamela.robey@nih.gov.Arora, D.; Robey, P. G. Recent updates on the biological basis of heterogeneity in bone marrow stromal cells/skeletal stem cells. Biomater Transl. 2022, 3(1), 3-16.
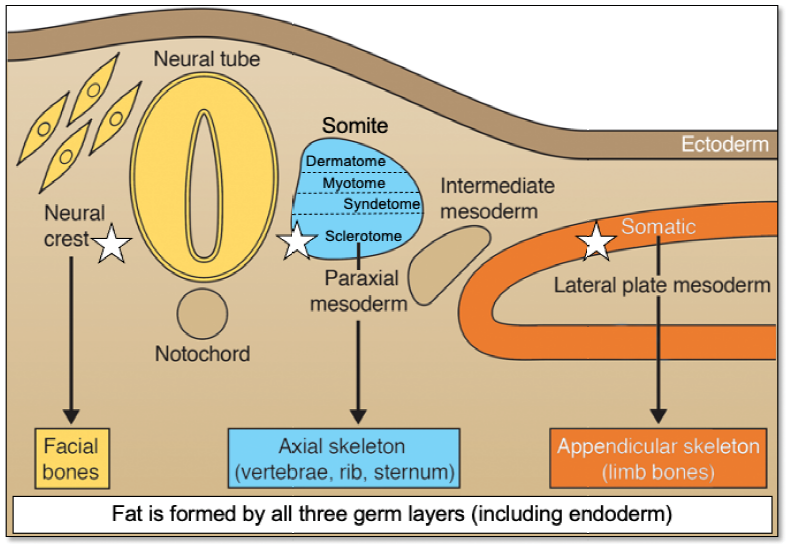
Figure 1. Developmental origins of different connective tissues that have been reported to contain “MSCs”. Of note, bone originates from three different embryonic specifications (noted by a white star). Skin, muscle, tendons and ligaments, and bone arise from different specifications of the sclerotome. Bone marrow adipose tissue originates from paraxial mesoderm, lateral plate mesoderm and neural crest. While bone marrow adipose tissue arises from mesoderm and neural crest, other forms of fat originate from all three germ layers. “MSCs” are not a lineage. MSC: mesenchymal stem/stromal cell. Adapted from Bianco and Robey.2
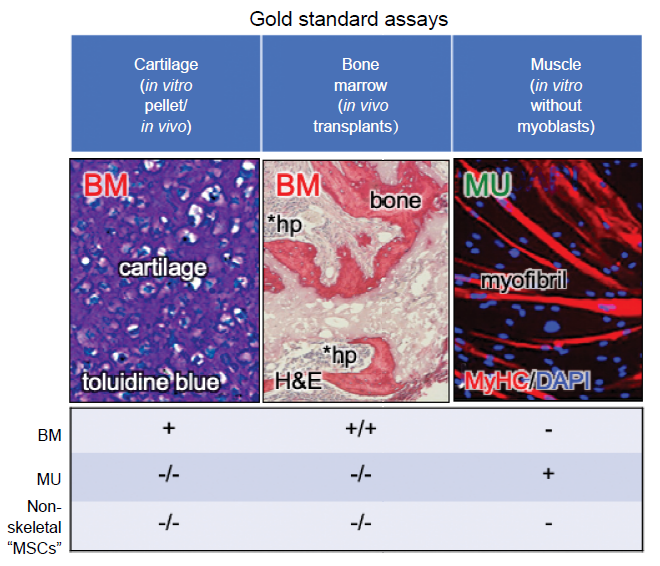
Figure 2. Gold standard assays by which to assess differentiation capacity. Many of the currently used “standard” assays of differentiation are prone to artifact or misinterpretation. However, there are assays that can faithfully report differentiation capacity: 1) the in vitro cartilage pellet assay, whereby one can see chondrocytes lying in lacunae surrounded by extracellular matrix that stains purple with toluidine blue, 2) the in vivo transplantation assay whereby donor cells are able to make bone matrix, osteocytes, osteoblasts, and in some cases, support haematopoiesis and formation of marrow adipocytes (the latter two properties are not shared by all forms of skeletal stem cells), and 3) the in vitro myogenic assay, whereby myotubes are formed in the absence of exogenous myoblasts (which will spontaneously fuse with any fibroblastic population). Adapted in part from Sacchetti et al.30 BM: bone marrow; DAPI: diamidino-2-phenylindole; H&E: hematoxylin and eosin; hp: haematopoiesis; MSC: mesenchymal stem/stromal cell; MU: muscle; MyHC: myosin heavy chain.
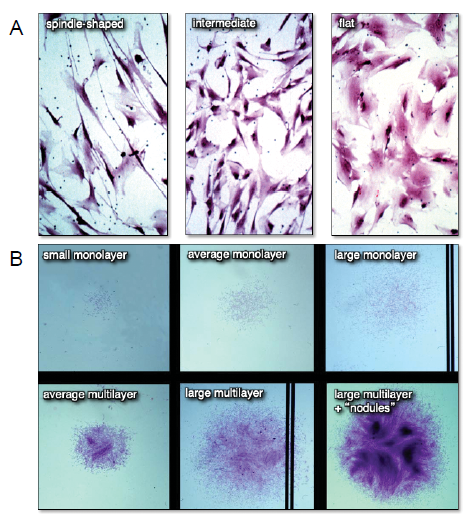
Figure 3. Differences in cell morphology (A) and colony size and habit (B) of freshly isolated bone marrow stromal cell suspensions plated at clonal density. When individual colonies with different cell shapes and colony habits are expanded ex vivo and transplanted in vivo, neither parameter correlated with the formation of a bone/marrow organ (a measure of multipotency). Adapted from Satomura et al.43
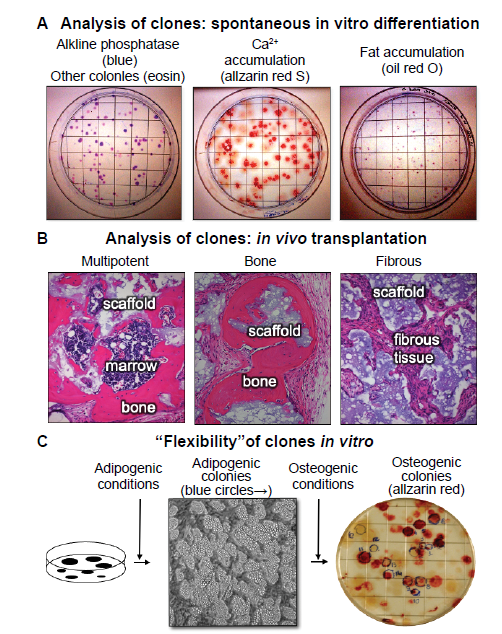
Figure 4. Clonal analysis - an essential step in the determination of stem cell potency. (A) When colonies are allowed to grow beyond the 10–14 days usually used for colony forming efficiency, the individual colonies begin to spontaneously differentiate. The vast majority of the colonies are alkaline phosphatase positive (right panel), indicative of osteogenic and pre-adipogenic cells. Approximately 50% of the colonies were Alizarin Red positive (centre panel) and approximately 10% stained with oil red O (right panel, unpublished data). (B) When individual colonies were isolated, expanded ex vivo, and transplanted subcutaneously into immunocompromised mice with an hydroxyapatite/tricalcium phosphate scaffold, ~10% of the single colony-derived strains made a complete bone/marrow organ (multipotent), whereas ~50% formed only bone (unipotent), and the remainder formed only fibrous tissue. Adapted from Sworder et al.13 (C) In studies where colonies were first incubated with adipogenic medium, colonies that accumulated fat identifiable by inverted light microscopy were marked with a blue circle. When the medium was changed to an osteogenic medium, a number of the adipogenic colonies also became alizarin red positive, indicating that the original CFU-F was able to give rise to adipogenic cells, and then osteogenic cells; an indication of “flexibility” (unpublished data). CFU-F: colony forming units-fibroblast.
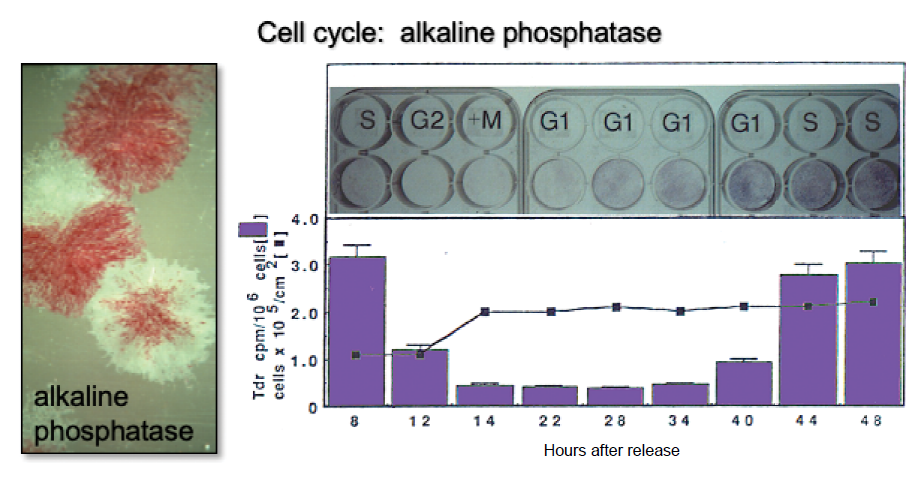
Figure 5. Changes in alkaline phosphatase based on position on the cell cycle. It has long been recognized that BMSC/SSC colonies are heterogeneous with respect to alkaline phosphatase activity (left panel, slide given to PGR by Alexander Friedenstein). Later, it was determined that when cultures of osteogenic cells are synchronized using an amphidicolin protocol, cells in S phase have high levels of activity. During G2 + M phase, alkaline phosphatase is cleaved from the cell surface and released into the medium. Cell surface activity is restored during the following G1 and S phases. Adapted in part from Fedarko et al.53 BMSC: bone marrow stromal cells; SSC: skeletal stem cell.
| 1. | Bianco, P.; Robey, P. G. Skeletal stem cells. In Handbook of adult and fetal stem cells, Lanza, R. P., ed. Academic Press: San Diego, 2004; pp 415-424. |
| 2. |
Bianco, P.; Robey, P. G. Skeletal stem cells. Development. 2015, 142, 1023-1027.
doi: 10.1242/dev.102210 URL |
| 3. |
Robey, P. G. Cell sources for bone regeneration: the good, the bad, and the ugly (but promising). Tissue Eng Part B Rev. 2011, 17, 423-430.
doi: 10.1089/ten.teb.2011.0199 URL |
| 4. |
Ambrosi, T. H.; Longaker, M. T.; Chan, C. K. F. A revised perspective of skeletal stem cell biology. Front Cell Dev Biol. 2019, 7, 189.
doi: 10.3389/fcell.2019.00189 URL |
| 5. |
Sacchetti, B.; Funari, A.; Michienzi, S.; Di Cesare, S.; Piersanti, S.; Saggio, I.; Tagliafico, E.; Ferrari, S.; Robey, P. G.; Riminucci, M.; Bianco, P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007, 131, 324-336.
doi: 10.1016/j.cell.2007.08.025 URL |
| 6. | Friedenstein, A. J.; Chailakhjan, R. K.; Lalykina, K. S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970, 3, 393-403. |
| 7. | Owen, M. E.; Cavé, J.; Joyner,, C. J. Clonal analysis in vitro of osteogenic differentiation of marrow CFU-F. J Cell Sci. 1987, 87 ( Pt 5), 731-738. |
| 8. | Friedenstein, A. J. Precursor cells of mechanocytes. Int Rev Cytol. 1976, 47, 327-359. |
| 9. | Owen, M.; Friedenstein, A. J. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp. 1988, 136, 42-60. |
| 10. |
Johnstone, B.; Hering, T. M.; Caplan, A. I.; Goldberg, V. M.; Yoo, J. U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998, 238, 265-272.
doi: 10.1006/excr.1997.3858 URL |
| 11. |
Gronthos, S.; Zannettino, A. C.; Hay, S. J.; Shi, S.; Graves, S. E.; Kortesidis, A.; Simmons, P. J. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci. 2003, 116, 1827-1835.
doi: 10.1242/jcs.00369 URL |
| 12. |
Kuznetsov, S. A.; Krebsbach, P. H.; Satomura, K.; Kerr, J.; Riminucci, M.; Benayahu, D.; Robey, P. G. Single-colony derived strains of human marrow stromal fibroblasts form bone after transplantation in vivo. J Bone Miner Res. 1997, 12, 1335-1347.
doi: 10.1359/jbmr.1997.12.9.1335 URL |
| 13. |
Sworder, B. J.; Yoshizawa, S.; Mishra, P. J.; Cherman, N.; Kuznetsov, S. A.; Merlino, G.; Balakumaran, A.; Robey, P. G. Molecular profile of clonal strains of human skeletal stem/progenitor cells with different potencies. Stem Cell Res. 2015, 14, 297-306.
doi: 10.1016/j.scr.2015.02.005 URL |
| 14. |
Caplan, A. I. Mesenchymal stem cells. J Orthop Res. 1991, 9, 641-650.
doi: 10.1002/(ISSN)1554-527X URL |
| 15. |
Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006, 8, 315-317.
doi: 10.1080/14653240600855905 URL |
| 16. | MacCord, K. “Mesenchyme”. Embryo Project Encyclopedia (2012-09-14). ISSN: 1940-5030 http://embryo.asu.edu/handle/10776/3941. |
| 17. |
Olsen, B. R.; Reginato, A. M.; Wang, W. Bone development. Annu Rev Cell Dev Biol. 2000, 16, 191-220.
doi: 10.1146/cellbio.2000.16.issue-1 URL |
| 18. |
Minasi, M. G.; Riminucci, M.; De Angelis, L.; Borello, U.; Berarducci, B.; Innocenzi, A.; Caprioli, A.; Sirabella, D.; Baiocchi, M.; De Maria, R.; Boratto, R.; Jaffredo, T.; Broccoli, V.; Bianco, P.; Cossu, G. The meso-angioblast: a multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Development. 2002, 129, 2773-2783.
doi: 10.1242/dev.129.11.2773 URL |
| 19. | Gilbert, S. F. Developmental Biology. 10th ed.; Sinauer Associates, Inc.: Sunderland, 2014. |
| 20. |
Ren, J.; Jin, P.; Sabatino, M.; Balakumaran, A.; Feng, J.; Kuznetsov, S. A.; Klein, H. G.; Robey, P. G.; Stroncek, D. F. Global transcriptome analysis of human bone marrow stromal cells (BMSC) reveals proliferative, mobile and interactive cells that produce abundant extracellular matrix proteins, some of which may affect BMSC potency. Cytotherapy. 2011, 13, 661-674.
doi: 10.3109/14653249.2010.548379 URL |
| 21. |
da Silva Meirelles, L.; Chagastelles, P. C.; Nardi, N. B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006, 119, 2204-2213.
doi: 10.1242/jcs.02932 URL |
| 22. |
Bianco, P.; Robey, P. G.; Simmons, P. J. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008, 2, 313-319.
doi: 10.1016/j.stem.2008.03.002 URL |
| 23. |
Yang, Y. K.; Ogando, C. R.; Wang See, C.; Chang, T. Y.; Barabino, G. A. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res Ther. 2018, 9, 131.
doi: 10.1186/s13287-018-0876-3 URL |
| 24. |
Rojewski, M. T.; Weber, B. M.; Schrezenmeier, H. Phenotypic characterization of mesenchymal stem cells from various tissues. Transfus Med Hemother. 2008, 35, 168-184.
doi: 10.1159/000129013 URL |
| 25. |
Sotiropoulou, P. A.; Perez, S. A.; Salagianni, M.; Baxevanis, C. N.; Papamichail, M. Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells. 2006, 24, 462-471.
doi: 10.1634/stemcells.2004-0331 URL |
| 26. |
Urist, M. R. Bone: formation by autoinduction. Science. 1965, 150, 893-899.
doi: 10.1126/science.150.3698.893 URL |
| 27. |
Friedenstein, A. J.; Lalykina, K. S. Thymus cells are inducible to osteogenesis. Eur J Immunol. 1972, 2, 602-603.
doi: 10.1002/(ISSN)1521-4141 URL |
| 28. |
Bonewald, L. F.; Harris, S. E.; Rosser, J.; Dallas, M. R.; Dallas, S. L.; Camacho, N. P.; Boyan, B.; Boskey, A. von Kossa staining alone is not sufficient to confirm that mineralization in vitro represents bone formation. Calcif Tissue Int. 2003, 72, 537-547.
doi: 10.1007/s00223-002-1057-y URL |
| 29. |
Diascro, D. D., Jr.; Vogel, R. L.; Johnson, T. E.; Witherup, K. M.; Pitzenberger, S. M.; Rutledge, S. J.; Prescott, D. J.; Rodan, G. A.; Schmidt, A. High fatty acid content in rabbit serum is responsible for the differentiation of osteoblasts into adipocyte-like cells. J Bone Miner Res. 1998, 13, 96-106.
doi: 10.1359/jbmr.1998.13.1.96 URL |
| 30. |
Sacchetti, B.; Funari, A.; Remoli, C.; Giannicola, G.; Kogler, G.; Liedtke, S.; Cossu, G.; Serafini, M.; Sampaolesi, M.; Tagliafico, E.; Tenedini, E.; Saggio, I.; Robey, P. G.; Riminucci, M.; Bianco, P. No identical “mesenchymal stem cells” at different times and sites: human committed progenitors of distinct origin and differentiation potential are incorporated as adventitial cells in microvessels. Stem Cell Reports. 2016, 6, 897-913.
doi: 10.1016/j.stemcr.2016.05.011 URL |
| 31. | Robey, P. “Mesenchymal stem cells”: fact or fiction, implications in their therapeutic use. F1000Res. 2017, 6, F1000 Faculty Rev-1524. |
| 32. |
Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P. G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000, 97, 13625-13630.
doi: 10.1073/pnas.240309797 URL |
| 33. |
Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L. W.; Robey, P. G.; Shi, S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003, 100, 5807-5812.
doi: 10.1073/pnas.0937635100 URL |
| 34. |
Seo, B. M.; Miura, M.; Gronthos, S.; Bartold, P. M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P. G.; Wang, C. Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004, 364, 149-155.
doi: 10.1016/S0140-6736(04)16627-0 URL |
| 35. |
Sherwood, R. I.; Christensen, J. L.; Conboy, I. M.; Conboy, M. J.; Rando, T. A.; Weissman, I. L.; Wagers, A. J. Isolation of adult mouse myogenic progenitors: functional heterogeneity of cells within and engrafting skeletal muscle. Cell. 2004, 119, 543-554.
doi: 10.1016/j.cell.2004.10.021 URL |
| 36. | Liu, X.; Rui, T.; Zhang, S.; Ding, Z. Heterogeneity of MSC: origin, molecular identities, and functionality. Stem Cells Int. 2019, 2019, 9281520. |
| 37. | Nemeth, K.; Mayer, B.; Sworder, B. J.; Kuznetsov, S. A.; Mezey, E. A practical guide to culturing mouse and human bone marrow stromal cells. Curr Protoc Immunol. 2013, 102, 22F.12.1-22F.12.13. |
| 38. |
Chu, D. T.; Phuong, T. N. T.; Tien, N. L. B.; Tran, D. K.; Thanh, V. V.; Quang, T. L.; Truong, D. T.; Pham, V. H.; Ngoc, V. T. N.; Chu-Dinh, T.; Kushekhar, K. An update on the progress of isolation, culture, storage, and clinical application of human bone marrow mesenchymal stem/stromal cells. Int J Mol Sci. 2020, 21, 708.
doi: 10.3390/ijms21030708 URL |
| 39. |
Coleman, C. M.; Curtin, C.; Barry, F. P.; O’Flatharta, C.; Murphy, J. M. Mesenchymal stem cells and osteoarthritis: remedy or accomplice? Hum Gene Ther. 2010, 21, 1239-1250.
doi: 10.1089/hum.2010.138 URL |
| 40. |
Caplan, A. I.; Hariri, R. Body management: mesenchymal stem cells control the internal regenerator. Stem Cells Transl Med. 2015, 4, 695-701.
doi: 10.5966/sctm.2014-0291 URL |
| 41. |
Haniffa, M. A.; Collin, M. P.; Buckley, C. D.; Dazzi, F. Mesenchymal stem cells: the fibroblasts’ new clothes? Haematologica. 2009, 94, 258-263.
doi: 10.3324/haematol.13699 URL |
| 42. |
Galipeau, J.; Sensébé, L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018, 22, 824-833.
doi: 10.1016/j.stem.2018.05.004 URL |
| 43. |
Satomura, K.; Derubeis, A. R.; Fedarko, N. S.; Ibaraki-O’Connor, K.; Kuznetsov, S. A.; Rowe, D. W.; Young, M. F.; Gehron Robey, P. Receptor tyrosine kinase expression in human bone marrow stromal cells. J Cell Physiol. 1998, 177, 426-438.
doi: 10.1002/(ISSN)1097-4652 URL |
| 44. |
Rennerfeldt, D. A.; Raminhos, J. S.; Leff, S. M.; Manning, P.; Van Vliet, K. J. Emergent heterogeneity in putative mesenchymal stem cell colonies: Single-cell time lapsed analysis. PLoS One. 2019, 14, e0213452.
doi: 10.1371/journal.pone.0213452 URL |
| 45. |
Akintoye, S. O.; Lam, T.; Shi, S.; Brahim, J.; Collins, M. T.; Robey, P. G. Skeletal site-specific characterization of orofacial and iliac crest human bone marrow stromal cells in same individuals. Bone. 2006, 38, 758-768.
doi: 10.1016/j.bone.2005.10.027 URL |
| 46. |
Kidwai, F.; Mui, B. W. H.; Arora, D.; Iqbal, K.; Hockaday, M.; de Castro Diaz, L. F.; Cherman, N.; Martin, D.; Myneni, V. D.; Ahmad, M.; Futrega, K.; Ali, S.; Merling, R. K.; Kaufman, D. S.; Lee, J.; Robey, P. G. Lineage-specific differentiation of osteogenic progenitors from pluripotent stem cells reveals the FGF1-RUNX2 association in neural crest-derived osteoprogenitors. Stem Cells. 2020, 38, 1107-1123.
doi: 10.1002/stem.3206 URL |
| 47. |
Debnath, S.; Yallowitz, A. R.; McCormick, J.; Lalani, S.; Zhang, T.; Xu, R.; Li, N.; Liu, Y.; Yang, Y. S.; Eiseman, M.; Shim, J. H.; Hameed, M.; Healey, J. H.; Bostrom, M. P.; Landau, D. A.; Greenblatt, M. B. Discovery of a periosteal stem cell mediating intramembranous bone formation. Nature. 2018, 562, 133-139.
doi: 10.1038/s41586-018-0554-8 URL |
| 48. |
Sivaraj, K. K.; Jeong, H. W.; Dharmalingam, B.; Zeuschner, D.; Adams, S.; Potente, M.; Adams, R. H. Regional specialization and fate specification of bone stromal cells in skeletal development. Cell Rep. 2021, 36, 109352.
doi: 10.1016/j.celrep.2021.109352 URL |
| 49. |
Ambrosi, T. H.; Sinha, R.; Steininger, H. M.; Hoover, M. Y.; Murphy, M. P.; Koepke, L. S.; Wang, Y.; Lu, W. J.; Morri, M.; Neff, N. F.; Weissman, I. L.; Longaker, M. T.; Chan, C. K. Distinct skeletal stem cell types orchestrate long bone skeletogenesis. eLife. 2021, 10, e66063.
doi: 10.7554/eLife.66063 URL |
| 50. |
Tormin, A.; Li, O.; Brune, J. C.; Walsh, S.; Schütz, B.; Ehinger, M.; Ditzel, N.; Kassem, M.; Scheding, S. CD146 expression on primary nonhematopoietic bone marrow stem cells is correlated with in situ localization. Blood. 2011, 117, 5067-5077.
doi: 10.1182/blood-2010-08-304287 URL |
| 51. |
Boudin, E.; Fijalkowski, I.; Hendrickx, G.; Van Hul, W. Genetic control of bone mass. Mol Cell Endocrinol. 2016, 432, 3-13.
doi: 10.1016/j.mce.2015.12.021 URL |
| 52. |
Ren, J.; Stroncek, D. F.; Zhao, Y.; Jin, P.; Castiello, L.; Civini, S.; Wang, H.; Feng, J.; Tran, K.; Kuznetsov, S. A.; Robey, P. G.; Sabatino, M. Intra-subject variability in human bone marrow stromal cell (BMSC) replicative senescence: molecular changes associated with BMSC senescence. Stem cell research. 2013, 11, 1060-1073.
doi: 10.1016/j.scr.2013.07.005 URL |
| 53. |
Fedarko, N. S.; Bianco, P.; Vetter, U.; Robey, P. G. Human bone cell enzyme expression and cellular heterogeneity: correlation of alkaline phosphatase enzyme activity with cell cycle. J Cell Physiol. 1990, 144, 115-121.
doi: 10.1002/(ISSN)1097-4652 URL |
| 54. |
Galindo, M.; Kahler, R. A.; Teplyuk, N. M.; Stein, J. L.; Lian, J. B.; Stein, G. S.; Westendorf, J. J.; van Wijnen, A. J. Cell cycle related modulations in Runx2 protein levels are independent of lymphocyte enhancer-binding factor 1 (Lef1) in proliferating osteoblasts. J Mol Histol. 2007, 38, 501-506.
doi: 10.1007/s10735-007-9143-0 URL |
| 55. |
Oh, J.; Lee, Y. D.; Wagers, A. J. Stem cell aging: mechanisms, regulators and therapeutic opportunities. Nat Med. 2014, 20, 870-880.
doi: 10.1038/nm.3651 URL |
| 56. | Ambrosi, T. H.; Goodnough, L. H.; Steininger, H. M.; Hoover, M. Y.; Kim, E.; Koepke, L. S.; Marecic, O.; Zhao, L.; Seita, J.; Bishop, J. A.; Gardner, M. J.; Chan, C. K. F. Geriatric fragility fractures are associated with a human skeletal stem cell defect. Aging Cell. 2020, 19, e13164. |
| 57. |
Sahin, E.; Depinho, R. A. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature. 2010, 464, 520-528.
doi: 10.1038/nature08982 URL |
| 58. |
Ambrosi, T. H.; Marecic, O.; McArdle, A.; Sinha, R.; Gulati, G. S.; Tong, X.; Wang, Y.; Steininger, H. M.; Hoover, M. Y.; Koepke, L. S.; Murphy, M. P.; Sokol, J.; Seo, E. Y.; Tevlin, R.; Lopez, M.; Brewer, R. E.; Mascharak, S.; Lu, L.; Ajanaku, O.; Conley, S. D.; Seita, J.; Morri, M.; Neff, N. F.; Sahoo, D.; Yang, F.; Weissman, I. L.; Longaker, M. T.; Chan, C. K. F. Aged skeletal stem cells generate an inflammatory degenerative niche. Nature. 2021, 597, 256-262.
doi: 10.1038/s41586-021-03795-7 URL |
| 59. |
Josephson, A. M.; Bradaschia-Correa, V.; Lee, S.; Leclerc, K.; Patel, K. S.; Muinos Lopez, E.; Litwa, H. P.; Neibart, S. S.; Kadiyala, M.; Wong, M. Z.; Mizrahi, M. M.; Yim, N. L.; Ramme, A. J.; Egol, K. A.; Leucht, P. Age-related inflammation triggers skeletal stem/progenitor cell dysfunction. Proc Natl Acad Sci U S A. 2019, 116, 6995-7004.
doi: 10.1073/pnas.1810692116 URL |
| 60. |
Sarkar, T. J.; Quarta, M.; Mukherjee, S.; Colville, A.; Paine, P.; Doan, L.; Tran, C. M.; Chu, C. R.; Horvath, S.; Qi, L. S.; Bhutani, N.; Rando, T. A.; Sebastiano, V. Transient non-integrative expression of nuclear reprogramming factors promotes multifaceted amelioration of aging in human cells. Nat Commun. 2020, 11, 1545.
doi: 10.1038/s41467-020-15174-3 URL |
| 61. |
Saul, D.; Monroe, D. G.; Rowsey, J. L.; Kosinsky, R. L.; Vos, S. J.; Doolittle, M. L.; Farr, J. N.; Khosla, S. Modulation of fracture healing by the transient accumulation of senescent cells. eLife. 2021, 10, e69958.
doi: 10.7554/eLife.69958 URL |
| 62. |
Cakouros, D.; Gronthos, S. Epigenetic regulators of mesenchymal stem/stromal cell lineage determination. Curr Osteoporos Rep. 2020, 18, 597-605.
doi: 10.1007/s11914-020-00616-0 URL |
| 63. |
Buisman, S. C.; de Haan, G. Epigenetic changes as a target in aging haematopoietic stem cells and age-related malignancies. Cells. 2019, 8, 868.
doi: 10.3390/cells8080868 URL |
| 64. |
Cakouros, D.; Gronthos, S. The changing epigenetic landscape of mesenchymal stem/stromal cells during aging. Bone. 2020, 137, 115440.
doi: 10.1016/j.bone.2020.115440 URL |
| 65. |
Tsai, C. C.; Hung, S. C. Functional roles of pluripotency transcription factors in mesenchymal stem cells. Cell Cycle. 2012, 11, 3711-3712.
doi: 10.4161/cc.22048 URL |
| 66. |
Li, Z.; Liu, C.; Xie, Z.; Song, P.; Zhao, R. C.; Guo, L.; Liu, Z.; Wu, Y. Epigenetic dysregulation in mesenchymal stem cell aging and spontaneous differentiation. PLoS One. 2011, 6, e20526.
doi: 10.1371/journal.pone.0020526 URL |
| 67. |
So, A. Y.; Jung, J. W.; Lee, S.; Kim, H. S.; Kang, K. S. DNA methyltransferase controls stem cell aging by regulating BMI1 and EZH2 through microRNAs. PLoS One. 2011, 6, e19503.
doi: 10.1371/journal.pone.0019503 URL |
| 68. |
Robey, P. G.; Kuznetsov, S. A.; Ren, J.; Klein, H. G.; Sabatino, M.; Stroncek, D. F. Generation of clinical grade human bone marrow stromal cells for use in bone regeneration. Bone. 2015, 70, 87-92.
doi: 10.1016/j.bone.2014.07.020 URL |
| 69. |
Mabuchi, Y.; Okawara, C.; Méndez-Ferrer, S.; Akazawa, C. Cellular heterogeneity of mesenchymal stem/stromal cells in the bone marrow. Front Cell Dev Biol. 2021, 9, 689366.
doi: 10.3389/fcell.2021.689366 URL |
| 70. |
Placzek, M. R.; Chung, I. M.; Macedo, H. M.; Ismail, S.; Mortera Blanco, T.; Lim, M.; Cha, J. M.; Fauzi, I.; Kang, Y.; Yeo, D. C.; Ma, C. Y.; Polak, J. M.; Panoskaltsis, N.; Mantalaris, A. Stem cell bioprocessing: fundamentals and principles. J R Soc Interface. 2009, 6, 209-232.
doi: 10.1098/rsif.2008.0442 URL |
| 71. |
Wilson, A.; Webster, A.; Genever, P. Nomenclature and heterogeneity: consequences for the use of mesenchymal stem cells in regenerative medicine. Regen Med. 2019, 14, 595-611.
doi: 10.2217/rme-2018-0145 URL |
| 72. |
Liu, S.; de Castro, L. F.; Jin, P.; Civini, S.; Ren, J.; Reems, J. A.; Cancelas, J.; Nayak, R.; Shaw, G.; O’Brien, T.; McKenna, D. H.; Armant, M.; Silberstein, L.; Gee, A. P.; Hei, D. J.; Hematti, P.; Kuznetsov, S. A.; Robey, P. G.; Stroncek, D. F. Manufacturing differences affect human bone marrow stromal cell characteristics and function: comparison of production methods and products from multiple centers. Sci Rep. 2017, 7, 46731.
doi: 10.1038/srep46731 URL |
| 73. |
Iaquinta, M. R.; Mazzoni, E.; Manfrini, M.; D’Agostino, A.; Trevisiol, L.; Nocini, R.; Trombelli, L.; Barbanti-Brodano, G.; Martini, F.; Tognon, M. Innovative biomaterials for bone regrowth. Int J Mol Sci. 2019, 20, 618.
doi: 10.3390/ijms20030618 URL |
| 74. |
Zhang, C.; Zhang, L.; Liu, L.; Lv, L.; Gao, L.; Liu, N.; Wang, X.; Ye, J. Mechanical behavior of a titanium alloy scaffold mimicking trabecular structure. J Orthop Surg Res. 2020, 15, 40.
doi: 10.1186/s13018-019-1489-y URL |
| 75. | Barbanti Brodano, G.; Mazzoni, E.; Tognon, M.; Griffoni, C.; Manfrini, M. Human mesenchymal stem cells and biomaterials interaction: a promising synergy to improve spine fusion. Eur Spine J. 2012, 21 Suppl 1, S3-9. |
| 76. |
Hay, S. B.; Ferchen, K.; Chetal, K.; Grimes, H. L.; Salomonis, N. The Human Cell Atlas bone marrow single-cell interactive web portal. Exp Hematol. 2018, 68, 51-61.
doi: 10.1016/j.exphem.2018.09.004 URL |
| 77. | Zheng, S.; Papalexi, E.; Butler, A.; Stephenson, W.; Satija, R. Molecular transitions in early progenitors during human cord blood hematopoiesis. Mol Syst Biol. 2018, 14, e8041. |
| 78. |
Dahlin, J. S.; Hamey, F. K.; Pijuan-Sala, B.; Shepherd, M.; Lau, W. W. Y.; Nestorowa, S.; Weinreb, C.; Wolock, S.; Hannah, R.; Diamanti, E.; Kent, D. G.; Göttgens, B.; Wilson, N. K. A single-cell hematopoietic landscape resolves 8 lineage trajectories and defects in Kit mutant mice. Blood. 2018, 131, e1-e11.
doi: 10.1182/blood-2017-12-821413 URL |
| 79. | Chan, C. K. F.; Gulati, G. S.; Sinha, R.; Tompkins, J. V.; Lopez, M.; Carter, A. C.; Ransom, R. C.; Reinisch, A.; Wearda, T.; Murphy, M.; Brewer, R. E.; Koepke, L. S.; Marecic, O.; Manjunath, A.; Seo, E. Y.; Leavitt, T.; Lu, W. J.; Nguyen, A.; Conley, S. D.; Salhotra, A.; Ambrosi, T. H.; Borrelli, M. R.; Siebel, T.; Chan, K.; Schallmoser, K.; Seita, J.; Sahoo, D.; Goodnough, H.; Bishop, J.; Gardner, M.; Majeti, R.; Wan, D. C.; Goodman, S.; Weissman, I. L.; Chang, H. Y.; Longaker, M. T. Identification of the human skeletal stem cell. Cell. 2018, 175, 43-56.e21. |
| 80. |
Tikhonova, A. N.; Dolgalev, I.; Hu, H.; Sivaraj, K. K.; Hoxha, E.; Cuesta-Domínguez, Á.; Pinho, S.; Akhmetzyanova, I.; Gao, J.; Witkowski, M.; Guillamot, M.; Gutkin, M. C.; Zhang, Y.; Marier, C.; Diefenbach, C.; Kousteni, S.; Heguy, A.; Zhong, H.; Fooksman, D. R.; Butler, J. M.; Economides, A.; Frenette, P. S.; Adams, R. H.; Satija, R.; Tsirigos, A.; Aifantis, I. The bone marrow microenvironment at single-cell resolution. Nature. 2019, 569, 222-228.
doi: 10.1038/s41586-019-1104-8 URL |
| 81. | Baryawno, N.; Przybylski, D.; Kowalczyk, M. S.; Kfoury, Y.; Severe, N.; Gustafsson, K.; Kokkaliaris, K. D.; Mercier, F.; Tabaka, M.; Hofree, M.; Dionne, D.; Papazian, A.; Lee, D.; Ashenberg, O.; Subramanian, A.; Vaishnav, E. D.; Rozenblatt-Rosen, O.; Regev, A.; Scadden, D. T. A cellular taxonomy of the bone marrow stroma in homeostasis and leukemia. Cell. 2019, 177, 1915-1932.e16. |
| 82. | Wolock, S. L.; Krishnan, I.; Tenen, D. E.; Matkins, V.; Camacho, V.; Patel, S.; Agarwal, P.; Bhatia, R.; Tenen, D. G.; Klein, A. M.; Welner, R. S. Mapping distinct bone marrow niche populations and their differentiation paths. Cell Rep. 2019, 28, 302-311.e5. |
| 83. |
Liu, S.; Stroncek, D. F.; Zhao, Y.; Chen, V.; Shi, R.; Chen, J.; Ren, J.; Liu, H.; Bae, H. J.; Highfill, S. L.; Jin, P. Single cell sequencing reveals gene expression signatures associated with bone marrow stromal cell subpopulations and time in culture. J Transl Med. 2019, 17, 23.
doi: 10.1186/s12967-018-1766-2 URL |
| 84. |
Baldarelli, R. M.; Hill, D. P.; Blake, J. A.; Adachi, J.; Furuno, M.; Bradt, D.; Corbani, L. E.; Cousins, S.; Frazer, K. S.; Qi, D.; Yang, L.; Ramachandran, S.; Reed, D.; Zhu, Y.; Kasukawa, T.; Ringwald, M.; King, B. L.; Maltais, L. J.; McKenzie, L. M.; Schriml, L. M.; Maglott, D.; Church, D. M.; Pruitt, K.; Eppig, J. T.; Richardson, J. E.; Kadin, J. A.; Bult, C. J. Connecting sequence and biology in the laboratory mouse. Genome Res. 2003, 13, 1505-1519.
doi: 10.1101/gr.991003 URL |
| 85. |
Sun, M.; Schwalb, B.; Schulz, D.; Pirkl, N.; Etzold, S.; Larivière, L.; Maier, K. C.; Seizl, M.; Tresch, A.; Cramer, P. Comparative dynamic transcriptome analysis (cDTA) reveals mutual feedback between mRNA synthesis and degradation. Genome Res. 2012, 22, 1350-1359.
doi: 10.1101/gr.130161.111 URL |
| 86. |
Tung, P. Y.; Blischak, J. D.; Hsiao, C. J.; Knowles, D. A.; Burnett, J. E.; Pritchard, J. K.; Gilad, Y. Batch effects and the effective design of single-cell gene expression studies. Sci Rep. 2017, 7, 39921.
doi: 10.1038/srep39921 URL |
| 87. |
Chen, J.; Cheung, F.; Shi, R.; Zhou, H.; Lu, W.; CHI Consortium. PBMC fixation and processing for Chromium single-cell RNA sequencing. J Transl Med. 2018, 16, 198.
doi: 10.1186/s12967-018-1578-4 URL |
| 88. |
Banfi, A.; Muraglia, A.; Dozin, B.; Mastrogiacomo, M.; Cancedda, R.; Quarto, R. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: Implications for their use in cell therapy. Exp Hematol. 2000, 28, 707-715.
doi: 10.1016/S0301-472X(00)00160-0 URL |
| 89. |
Post, S.; Abdallah, B. M.; Bentzon, J. F.; Kassem, M. Demonstration of the presence of independent pre-osteoblastic and pre-adipocytic cell populations in bone marrow-derived mesenchymal stem cells. Bone. 2008, 43, 32-39.
doi: 10.1016/j.bone.2008.03.011 URL |
| 90. | Elsafadi, M.; Manikandan, M.; Atteya, M.; Hashmi, J. A.; Iqbal, Z.; Aldahmash, A.; Alfayez, M.; Kassem, M.; Mahmood, A. Characterization of cellular and molecular heterogeneity of bone marrow stromal cells. Stem Cells Int. 2016, 2016, 9378081. |
| 91. |
Xiang, Y.; Wu, C.; Wu, J.; Quan, W.; Cheng, C.; Zhou, J.; Chen, L.; Xiang, L.; Li, F.; Zhang, K.; Ran, Q.; Zhang, Y.; Li, Z. In vitro expansion affects the response of human bone marrow stromal cells to irradiation. Stem Cell Res Ther. 2019, 10, 82.
doi: 10.1186/s13287-019-1191-3 URL |
| 92. |
Oetjen, K. A.; Lindblad, K. E.; Goswami, M.; Gui, G.; Dagur, P. K.; Lai, C.; Dillon, L. W.; McCoy, J. P.; Hourigan, C. S. Human bone marrow assessment by single-cell RNA sequencing, mass cytometry, and flow cytometry. JCI insight. 2018, 3, e124928.
doi: 10.1172/jci.insight.124928 URL |
| 93. | Wolock, S. L.; Krishnan, I.; Tenen, D. E.; Matkins, V.; Camacho, V.; Patel, S.; Agarwal, P.; Bhatia, R.; Tenen, D. G.; Klein, A. M.; Welner, R. S. Mapping distinct bone marrow niche populations and their differentiation paths. Cell Rep. 2019, 28, 302-311. e5. |
| [1] | Suzanne M. Watt. The long and winding road: homeostatic and disordered haematopoietic microenvironmental niches: a narrative review [J]. Biomaterials Translational, 2022, 3(1): 31-54. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||