Biomaterials Translational ›› 2023, Vol. 4 ›› Issue (1): 5-17.doi: 10.12336/biomatertransl.2023.01.003
• REVIEW • Previous Articles Next Articles
Yehao Zhang, Cong Wang, Wenhui Zhang, Xinming Li*( )
)
Received:2022-11-17
Revised:2023-02-02
Accepted:2023-03-10
Online:2023-03-28
Published:2023-03-28
Contact:
* Xinming Li,About author:Xinming Li,xinmingli@suda.edu.cn.Zhang, Y.; Wang, C.; Zhang, W.; Li X. Bioactive peptides for anticancer therapies. Biomater Transl. 2023, 4(1), 5-17.
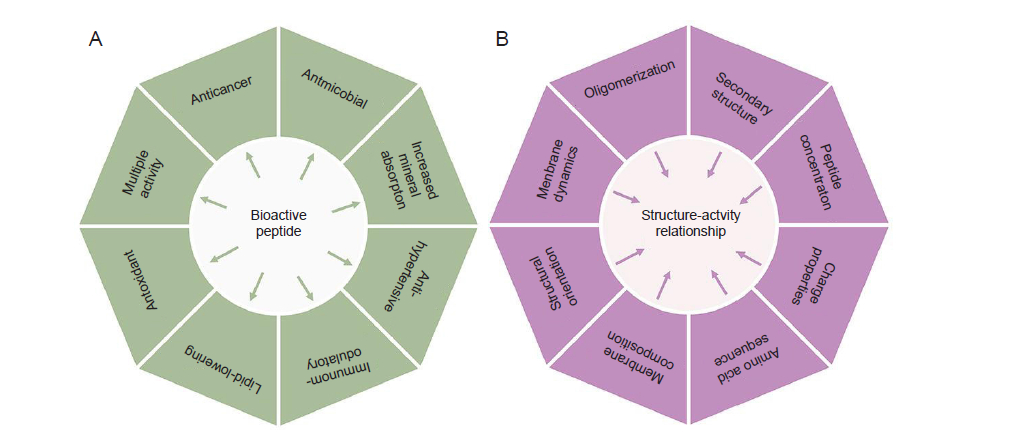
Figure 1. (A) Multipurpose bioactivities exhibited by peptides from natural resources, (B) Physicochemical and physiological factors of bioactive peptides that determine their anticancer activities.
| Amino acid residue | Amino acid properties | Action on cancer cells | References |
|---|---|---|---|
| Effect on cell membrane interactions | |||
| Lysine | Positively charged, polar and hydrophilic | Disrupt cell membrane integrity and penetrate cell membrane, leading to cancer cell cytotoxicity | |
| Arginine | |||
| Histidine | Induce cancer cytotoxicity via membrane permeability under acidic condition | ||
| Glutamic acid | Negatively charged, polar and hydrophilic | Antiproliferative activities on tumour cells | |
| Aspartic acid | |||
| Effect on cancer cell structure | |||
| Cysteine | Polar, non–charged | Interact with numerous cell surface receptors for stabilizing and maintaining extracellular motif/domain structure | |
| Proline | Non–polar, aliphatic | Membrane interaction and conformational flexibility of peptide chains | |
| Glycine | Membrane interaction and conformational flexibility | ||
| Phenylalanine | Aromatic | Enhance the affinity with cancer cell membrane | |
| Effect on cancer cell metabolism | |||
| Methionine | Polar, non–charged | Reduced methionine will arrest cancer cell proliferation | |
| Tyrosine | Aromatic | increase cytotoxic activity | |
| Tryptophan | Aromatic | binding at the major groove of nuclear DNA | |
Table 1. Effects of amino acid residues in ACPs on cancer cells
| Amino acid residue | Amino acid properties | Action on cancer cells | References |
|---|---|---|---|
| Effect on cell membrane interactions | |||
| Lysine | Positively charged, polar and hydrophilic | Disrupt cell membrane integrity and penetrate cell membrane, leading to cancer cell cytotoxicity | |
| Arginine | |||
| Histidine | Induce cancer cytotoxicity via membrane permeability under acidic condition | ||
| Glutamic acid | Negatively charged, polar and hydrophilic | Antiproliferative activities on tumour cells | |
| Aspartic acid | |||
| Effect on cancer cell structure | |||
| Cysteine | Polar, non–charged | Interact with numerous cell surface receptors for stabilizing and maintaining extracellular motif/domain structure | |
| Proline | Non–polar, aliphatic | Membrane interaction and conformational flexibility of peptide chains | |
| Glycine | Membrane interaction and conformational flexibility | ||
| Phenylalanine | Aromatic | Enhance the affinity with cancer cell membrane | |
| Effect on cancer cell metabolism | |||
| Methionine | Polar, non–charged | Reduced methionine will arrest cancer cell proliferation | |
| Tyrosine | Aromatic | increase cytotoxic activity | |
| Tryptophan | Aromatic | binding at the major groove of nuclear DNA | |
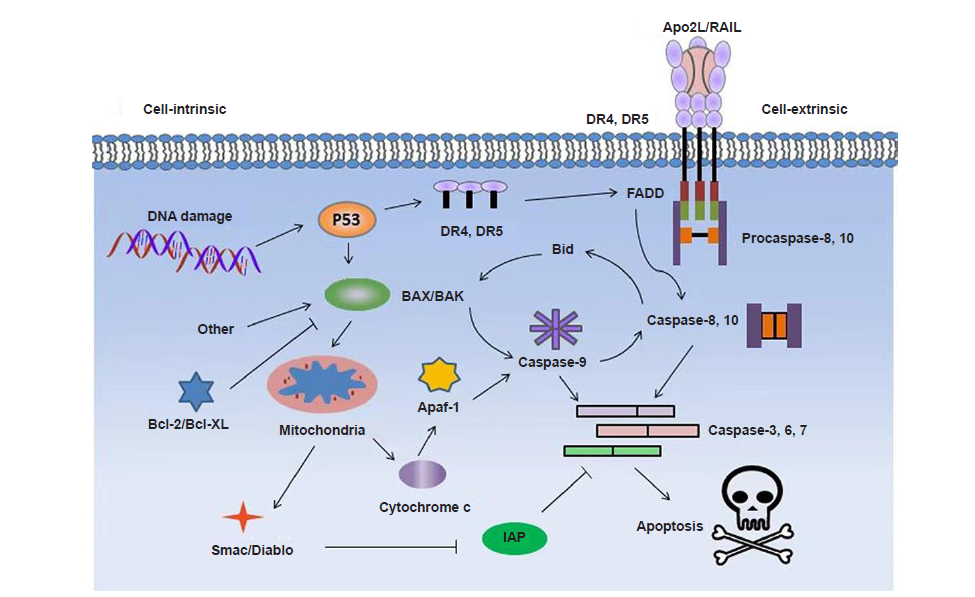
Figure 2. Proposed signaling pathways for induced apoptosis of cancer cells by pro–apoptotic peptides. Apaf–1: apoptotic protease activating factor 1; Apo2L: Apo2 ligand; Diablo: direct IAP binding protein with low pI; DR: death receptor; FADD: Fas associated via death domain; IAP: inhibitor of apoptosis protein; TRAIL: tumour necrosis factor–related apoptosis–inducing ligand.
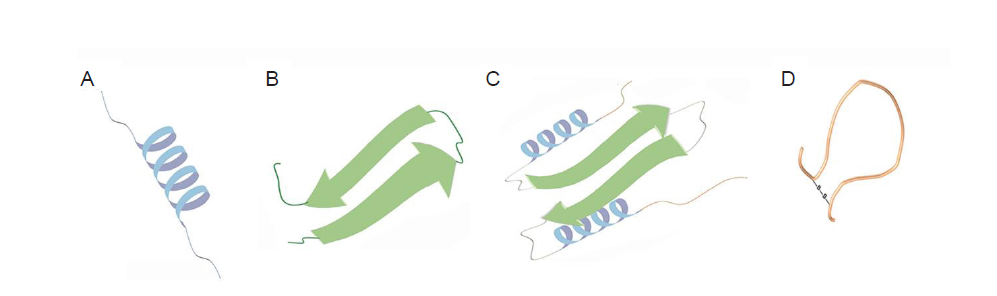
Figure 3. Schematic illustration of ACP structures containing α–helices (A), β–sheets (B), extended structures (C) or cyclic loops (D). ACP: anticancer peptide.
| No. | ACP | Cancer type | Mechanism | Molecular sequence | Reference |
|---|---|---|---|---|---|
| 1 | LL–37 | Human oral squamous cell, carcinoma cells | Toroidal pore mechanism | LLGDFFRKSKEKIGKEFKRI VQRIKDFLRNLVPRTES | |
| 2 | α–Defensins | Human myeloid leukaemia cell line (U937) | Cytolytic activity | ACYCRIPACIAGERRYGTCI YQGRLWAFCC | |
| 3 | β–Defensin–3 | HeLa, Jurkat and U937 cancer cell lines | Binding to cell membrane to cause cytolysis | GIINTLQKYYCRVRGGRCA VLSCLPKEEQIGKCSTRGRK CCRRKK | |
| 4 | Bovine lactoferricin | Drug–resistant and drug–sensitive cancer cells | Cytolysis and immunogenicity | FKCRRWQWRMKKLGAP SITCVRRAF | |
| 5 | Gomesin | Murine and human cancer cell lines along with melanoma and leukaemia | Carpet model for destroying the membrane | QCRRLCYKQRCVTYCRGR | |
| 6 | Cecropin B1 | NSCLC cell line | Tumour growth inhibition using pore formation and apoptosis | KWKIFKKIEKVGRNIRNG IIKAGPAVAVLGEAKAL | |
| 7 | Magainin 2 | Human lung cancer cells A59 and in | Formation of pores on cell membranes | GIGKFLHSAKKFGKAFVG EIMNS | |
| 8 | Brevinin 2R | Breast adenocarcinoma MCF–7, and lung carcinoma A549 cell | Lysosomal death pathway and autophagy–like cell death | KLKNFAKGVAQSLLNKAS CKLSGQC | |
| 9 | Bufforin IIb | Leukaemia, breast, prostate, and colon cancer | Mitochondrial apoptosis | TRSSRAGLQFPVGRVHRLL RK | |
| 10 | Brevinvin | Lung cancer H460, melanoma cell, glioblastoma U251MG, colon cancer HCT116 cell lines | Penetrating into the lipidic bilayer causing cell death | FLPLAVSLAANFLPK LFCKI TKKC | |
| 11 | Phylloseptin–PHa | Breast cancer cells MCF–7, breast epithelial cells MCF10A | Penetrating into the lipidic bilayer causing cell death | FLSLIPAAISAVSALANHF | |
| 12 | Ranatuerin–2PLx | Prostate cancer cell PC–3 | Cell apoptosis | GIMDTVKNAAKNLAGQLL DKLKCSITAC | |
| 13 | Dermaseptins | Prostate cancer cell PC–3 | Pore formation one the lipid bilayer | GLWSKIKEVGKEAAKAAAK AAGKAALGAVSEAV | |
| 14 | Chrysophsin–1, –2 and –3 | Human fibrosarcoma HT–1080, histiocytic lymphoma U937, and cervical carcinoma HeLa cell lines | Disrupt the plasma membrane | FFGWLIKGAIHAGKAIHG LIHRRRH | |
| 15 | D–K6L9 | Breast and prostate cancer cell lines | Reduce neovascularization | LKLLKKLLKKLLKLL |
Table 2. Mechanism of some anticancer peptides from different origins for cancer treatment
| No. | ACP | Cancer type | Mechanism | Molecular sequence | Reference |
|---|---|---|---|---|---|
| 1 | LL–37 | Human oral squamous cell, carcinoma cells | Toroidal pore mechanism | LLGDFFRKSKEKIGKEFKRI VQRIKDFLRNLVPRTES | |
| 2 | α–Defensins | Human myeloid leukaemia cell line (U937) | Cytolytic activity | ACYCRIPACIAGERRYGTCI YQGRLWAFCC | |
| 3 | β–Defensin–3 | HeLa, Jurkat and U937 cancer cell lines | Binding to cell membrane to cause cytolysis | GIINTLQKYYCRVRGGRCA VLSCLPKEEQIGKCSTRGRK CCRRKK | |
| 4 | Bovine lactoferricin | Drug–resistant and drug–sensitive cancer cells | Cytolysis and immunogenicity | FKCRRWQWRMKKLGAP SITCVRRAF | |
| 5 | Gomesin | Murine and human cancer cell lines along with melanoma and leukaemia | Carpet model for destroying the membrane | QCRRLCYKQRCVTYCRGR | |
| 6 | Cecropin B1 | NSCLC cell line | Tumour growth inhibition using pore formation and apoptosis | KWKIFKKIEKVGRNIRNG IIKAGPAVAVLGEAKAL | |
| 7 | Magainin 2 | Human lung cancer cells A59 and in | Formation of pores on cell membranes | GIGKFLHSAKKFGKAFVG EIMNS | |
| 8 | Brevinin 2R | Breast adenocarcinoma MCF–7, and lung carcinoma A549 cell | Lysosomal death pathway and autophagy–like cell death | KLKNFAKGVAQSLLNKAS CKLSGQC | |
| 9 | Bufforin IIb | Leukaemia, breast, prostate, and colon cancer | Mitochondrial apoptosis | TRSSRAGLQFPVGRVHRLL RK | |
| 10 | Brevinvin | Lung cancer H460, melanoma cell, glioblastoma U251MG, colon cancer HCT116 cell lines | Penetrating into the lipidic bilayer causing cell death | FLPLAVSLAANFLPK LFCKI TKKC | |
| 11 | Phylloseptin–PHa | Breast cancer cells MCF–7, breast epithelial cells MCF10A | Penetrating into the lipidic bilayer causing cell death | FLSLIPAAISAVSALANHF | |
| 12 | Ranatuerin–2PLx | Prostate cancer cell PC–3 | Cell apoptosis | GIMDTVKNAAKNLAGQLL DKLKCSITAC | |
| 13 | Dermaseptins | Prostate cancer cell PC–3 | Pore formation one the lipid bilayer | GLWSKIKEVGKEAAKAAAK AAGKAALGAVSEAV | |
| 14 | Chrysophsin–1, –2 and –3 | Human fibrosarcoma HT–1080, histiocytic lymphoma U937, and cervical carcinoma HeLa cell lines | Disrupt the plasma membrane | FFGWLIKGAIHAGKAIHG LIHRRRH | |
| 15 | D–K6L9 | Breast and prostate cancer cell lines | Reduce neovascularization | LKLLKKLLKKLLKLL |
| Phase | Biological peptides | Cancer type | Mechanisms |
|---|---|---|---|
| Early phase I | MUC–1 peptide vaccine | Breast cancer | Positive anti–MUC1 antibody responses |
| HER–2/neu peptide vaccine | Breast cancer | Specific interferon–γ and IL–5 producing T–cell responses | |
| GAA/TT–peptide vaccine and poly–ICLC | Astrocytoma, oligoastrocytoma and glioma | GAA–specific T–cell responses | |
| Phase I | Gag:267–274 peptide vaccine | Melanoma | Cytotoxic T–cell lymphocyte responses |
| HPV16 E7 peptide–pulsed autologous DCs | Cervical cancer | Pulsed autologous DC immunotherapy | |
| LY6K, VEGFR1, VEGFR2 | Esophageal cancer | Immune responses including LY6K, VEGFR1 and VEGFR2 specific T–cells | |
| Antiangiogenic peptide vaccine | Hepatocellular carcinoma | Cytotoxic T–cell lymphocyte responses | |
| HLA–A*0201 or HLA–A*0206–restricted URLC10 peptides | Non–small cell lung cancer | Cytotoxic T–cell lymphocyte responses, antigen cascade, regulatory T–cells, cancer antigens and human leukocyte antigen levels | |
| Phase I/II | MAGE–3.A1 peptide and CpG 7909 | Malignant melanoma | Cytotoxic T–cell lymphocyte responses |
| VEGFR1–1084, VEGFR2–169 | Pancreatic cancer | Cytotoxic T–cell lymphocyte responses | |
| HER–2/neu peptide vaccine | Breast cancer | Human epidermal growth factor receptor 2–specific T–cell response | |
| Phase II | gp100:209–217(210M), HPV 16 E7:12–20 | Melanoma | T–cell immunity |
| WT1 126–134 peptide | Acute myeloid leukaemia | T–cell response | |
| G250 peptide | Metastatic renal cell carcinoma | Cytotoxic T–cell lymphocyte responses | |
| Phase III | PR1 leukaemia peptide vaccine | Leukaemia | Immune response |
| Phase IV | Degarelix | Prostatic neoplasms | Binds to GnRH receptors |
Table 3. Lists of anticancer peptides in clinical trials
| Phase | Biological peptides | Cancer type | Mechanisms |
|---|---|---|---|
| Early phase I | MUC–1 peptide vaccine | Breast cancer | Positive anti–MUC1 antibody responses |
| HER–2/neu peptide vaccine | Breast cancer | Specific interferon–γ and IL–5 producing T–cell responses | |
| GAA/TT–peptide vaccine and poly–ICLC | Astrocytoma, oligoastrocytoma and glioma | GAA–specific T–cell responses | |
| Phase I | Gag:267–274 peptide vaccine | Melanoma | Cytotoxic T–cell lymphocyte responses |
| HPV16 E7 peptide–pulsed autologous DCs | Cervical cancer | Pulsed autologous DC immunotherapy | |
| LY6K, VEGFR1, VEGFR2 | Esophageal cancer | Immune responses including LY6K, VEGFR1 and VEGFR2 specific T–cells | |
| Antiangiogenic peptide vaccine | Hepatocellular carcinoma | Cytotoxic T–cell lymphocyte responses | |
| HLA–A*0201 or HLA–A*0206–restricted URLC10 peptides | Non–small cell lung cancer | Cytotoxic T–cell lymphocyte responses, antigen cascade, regulatory T–cells, cancer antigens and human leukocyte antigen levels | |
| Phase I/II | MAGE–3.A1 peptide and CpG 7909 | Malignant melanoma | Cytotoxic T–cell lymphocyte responses |
| VEGFR1–1084, VEGFR2–169 | Pancreatic cancer | Cytotoxic T–cell lymphocyte responses | |
| HER–2/neu peptide vaccine | Breast cancer | Human epidermal growth factor receptor 2–specific T–cell response | |
| Phase II | gp100:209–217(210M), HPV 16 E7:12–20 | Melanoma | T–cell immunity |
| WT1 126–134 peptide | Acute myeloid leukaemia | T–cell response | |
| G250 peptide | Metastatic renal cell carcinoma | Cytotoxic T–cell lymphocyte responses | |
| Phase III | PR1 leukaemia peptide vaccine | Leukaemia | Immune response |
| Phase IV | Degarelix | Prostatic neoplasms | Binds to GnRH receptors |
| 1 |
Poston, G. J. Global cancer surgery: the Lancet Oncology review. Eur J Surg Oncol. 2015, 41, 1559-1561.
doi: 10.1016/j.ejso.2015.09.004 URL |
| 2 |
Norouzi, P.; Mirmohammadi, M.; Houshdar Tehrani, M. H. Anticancer peptides mechanisms, simple and complex. Chem Biol Interact. 2022, 368, 110194.
doi: 10.1016/j.cbi.2022.110194 URL |
| 3 | Sung, H.; Ferlay, J.; Siegel, R. L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021, 71, 209-249. |
| 4 |
Hanahan, D.; Weinberg, R. A. The hallmarks of cancer. Cell. 2000, 100, 57-70.
doi: 10.1016/S0092-8674(00)81683-9 URL |
| 5 |
Cai, Z.; Yin, Y.; Shen, C.; Wang, J.; Yin, X.; Chen, Z.; Zhou, Y.; Zhang, B. Comparative effectiveness of preoperative, postoperative and perioperative treatments for resectable gastric cancer: A network meta-analysis of the literature from the past 20 years. Surg Oncol. 2018, 27, 563-574.
doi: 10.1016/j.suronc.2018.07.011 URL |
| 6 |
Yu, W. D.; Sun, G.; Li, J.; Xu, J.; Wang, X. Mechanisms and therapeutic potentials of cancer immunotherapy in combination with radiotherapy and/or chemotherapy. Cancer Lett. 2019, 452, 66-70.
doi: 10.1016/j.canlet.2019.02.048 URL |
| 7 |
Aljabery, F.; Shabo, I.; Gimm, O.; Jahnson, S.; Olsson, H. The expression profile of p14, p53 and p21 in tumour cells is associated with disease-specific survival and the outcome of postoperative chemotherapy treatment in muscle-invasive bladder cancer. Urol Oncol. 2018, 36, 530.e7-530.e18.
doi: 10.1016/j.urolonc.2018.05.025 URL |
| 8 | Rajalakshmi, M.; Suveena, S.; Vijayalakshmia, P.; Indu, S.; Roy, A.; Ludas, A. DaiCee: A database for anti-cancer compounds with targets and side effect profiles. Bioinformation. 2020, 16, 843-848. |
| 9 |
Shimizu, C. Side effects of anticancer treatment and the needs for translational research on toxicity: a clinician’s perspective. Nihon Yakurigaku Zasshi. 2015, 146, 72-75.
doi: 10.1254/fpj.146.72 URL |
| 10 |
Pérez-Tomás, R. Multidrug resistance: retrospect and prospects in anti-cancer drug treatment. Curr Med Chem. 2006, 13, 1859-1876.
doi: 10.2174/092986706777585077 URL |
| 11 |
Hoekstra, R.; Verweij, J.; Eskens, F. A. Clinical trial design for target specific anticancer agents. Invest New Drugs. 2003, 21, 243-250.
doi: 10.1023/A:1023581731443 |
| 12 |
Vulfovich, M.; Saba, N. Molecular biological design of novel antineoplastic therapies. Expert Opin Investig Drugs. 2004, 13, 577-607.
doi: 10.1517/13543784.13.6.577 URL |
| 13 |
Wang, L.; Qu, L.; Lin, S.; Yang, Q.; Zhang, X.; Jin, L.; Dong, H.; Sun, D. Biological functions and applications of antimicrobial peptides. Curr Protein Pept Sci. 2022, 23, 226-247.
doi: 10.2174/1389203723666220519155942 URL |
| 14 | Hilchie, A. L.; Hoskin, D. W.; Power Coombs, M. R. Anticancer activities of natural and synthetic peptides. Adv Exp Med Biol. 2019, 1117, 131-147. |
| 15 |
Soon, T. N.; Chia, A. Y. Y.; Yap, W. H.; Tang, Y. Q. Anticancer mechanisms of bioactive peptides. Protein Pept Lett. 2020, 27, 823-830.
doi: 10.2174/0929866527666200409102747 URL |
| 16 |
Marqus, S.; Pirogova, E.; Piva, T. J. Evaluation of the use of therapeutic peptides for cancer treatment. J Biomed Sci. 2017, 24, 21.
doi: 10.1186/s12929-017-0328-x URL |
| 17 |
Chiangjong, W.; Chutipongtanate, S.; Hongeng, S. Anticancer peptide: Physicochemical property, functional aspect and trend in clinical application (Review). Int J Oncol. 2020, 57, 678-696.
doi: 10.3892/ijo URL |
| 18 |
Vlieghe, P.; Lisowski, V.; Martinez, J.; Khrestchatisky, M. Synthetic therapeutic peptides: science and market. Drug Discov Today. 2010, 15, 40-56.
doi: 10.1016/j.drudis.2009.10.009 URL |
| 19 | Thundimadathil, J. Cancer treatment using peptides: current therapies and future prospects. J Amino Acids. 2012, 2012, 967347. |
| 20 |
Zhang, D.; He, Y.; Ye, Y.; Ma, Y.; Zhang, P.; Zhu, H.; Xu, N.; Liang, S. Little antimicrobial peptides with big therapeutic roles. Protein Pept Lett. 2019, 26, 564-578.
doi: 10.2174/1573406415666190222141905 URL |
| 21 | Juretić, D. Designed multifunctional peptides for intracellular targets. Antibiotics (Basel). 2022, 11, 1196. |
| 22 |
Basith, S.; Manavalan, B.; Shin, T. H.; Lee, D. Y.; Lee, G. Evolution of machine learning algorithms in the prediction and design of anticancer peptides. Curr Protein Pept Sci. 2020, 21, 1242-1250.
doi: 10.2174/1389203721666200117171403 URL |
| 23 |
Palomo, J. M. Solid-phase peptide synthesis: an overview focused on the preparation of biologically relevant peptides. RSC Adv. 2014, 4, 32658-32672.
doi: 10.1039/C4RA02458C URL |
| 24 |
Dai, Y.; Cai, X.; Shi, W.; Bi, X.; Su, X.; Pan, M.; Li, H.; Lin, H.; Huang, W.; Qian, H. Pro-apoptotic cationic host defense peptides rich in lysine or arginine to reverse drug resistance by disrupting tumor cell membrane. Amino Acids. 2017, 49, 1601-1610.
doi: 10.1007/s00726-017-2453-y URL |
| 25 |
Navarro, S.; Aleu, J.; Jiménez, M.; Boix, E.; Cuchillo, C. M.; Nogués, M. V. The cytotoxicity of eosinophil cationic protein/ribonuclease 3 on eukaryotic cell lines takes place through its aggregation on the cell membrane. Cell Mol Life Sci. 2008, 65, 324-337.
doi: 10.1007/s00018-007-7499-7 URL |
| 26 |
Midoux, P.; Kichler, A.; Boutin, V.; Maurizot, J. C.; Monsigny, M. Membrane permeabilization and efficient gene transfer by a peptide containing several histidines. Bioconjug Chem. 1998, 9, 260-267.
doi: 10.1021/bc9701611 URL |
| 27 |
Yamaguchi, Y.; Yamamoto, K.; Sato, Y.; Inoue, S.; Morinaga, T.; Hirano, E. Combination of aspartic acid and glutamic acid inhibits tumor cell proliferation. Biomed Res. 2016, 37, 153-159.
doi: 10.2220/biomedres.37.153 URL |
| 28 |
Oancea, E.; Teruel, M. N.; Quest, A. F.; Meyer, T. Green fluorescent protein (GFP)-tagged cysteine-rich domains from protein kinase C as fluorescent indicators for diacylglycerol signaling in living cells. J Cell Biol. 1998, 140, 485-498.
doi: 10.1083/jcb.140.3.485 URL |
| 29 |
Shamova, O.; Orlov, D.; Stegemann, C.; Czihal, P.; Hoffmann, R.; Brogden, K.; Kolodkin, N.; Sakuta, G.; Tossi, A.; Sahl, H.-G.; Kokryakov, V.; Lehrer, R. I. ChBac3.4: a novel proline-rich antimicrobial peptide from goat leukocytes. Int J Pept Res Ther. 2009, 15, 31-42.
doi: 10.1007/s10989-008-9159-7 URL |
| 30 |
Dennison, S. R.; Whittaker, M.; Harris, F.; Phoenix, D. A. Anticancer alpha-helical peptides and structure/function relationships underpinning their interactions with tumour cell membranes. Curr Protein Pept Sci. 2006, 7, 487-499.
doi: 10.2174/138920306779025611 URL |
| 31 |
Kawaguchi, K.; Han, Q.; Li, S.; Tan, Y.; Igarashi, K.; Kiyuna, T.; Miyake, K.; Miyake, M.; Chmielowski, B.; Nelson, S. D.; Russell, T. A.; Dry, S. M.; Li, Y.; Singh, A. S.; Eckardt, M. A.; Unno, M.; Eilber, F. C.; Hoffman, R. M. Targeting methionine with oral recombinant methioninase (o-rMETase) arrests a patient-derived orthotopic xenograft (PDOX) model of BRAF-V600E mutant melanoma: implications for chronic clinical cancer therapy and prevention. Cell Cycle. 2018, 17, 356-361.
doi: 10.1080/15384101.2017.1405195 URL |
| 32 | Ahmaditaba, M. A.; Houshdar Tehrani, M. H.; Zarghi, A.; Shahosseini, S.; Daraei, B. Design, synthesis and biological evaluation of novel peptide-like analogues as selective COX-2 inhibitors. Iran J Pharm Res. 2018, 17, 87-92. |
| 33 |
Bhunia, D.; Mondal, P.; Das, G.; Saha, A.; Sengupta, P.; Jana, J.; Mohapatra, S.; Chatterjee, S.; Ghosh, S. Spatial position regulates power of tryptophan: discovery of a major-groove-specific nuclear-localizing, cell-penetrating tetrapeptide. J Am Chem Soc. 2018, 140, 1697-1714.
doi: 10.1021/jacs.7b10254 URL |
| 34 | Hoskin, D. W.; Ramamoorthy, A. Studies on anticancer activities of antimicrobial peptides. Biochim Biophys Acta. 2008, 1778, 357-375. |
| 35 |
Szlasa, W.; Zendran, I.; Zalesińska, A.; Tarek, M.; Kulbacka, J. Lipid composition of the cancer cell membrane. J Bioenerg Biomembr. 2020, 52, 321-342.
doi: 10.1007/s10863-020-09846-4 |
| 36 |
Schweizer, F. Cationic amphiphilic peptides with cancer-selective toxicity. Eur J Pharmacol. 2009, 625, 190-194.
doi: 10.1016/j.ejphar.2009.08.043 URL |
| 37 |
Sok, M.; Sentjurc, M.; Schara, M. Membrane fluidity characteristics of human lung cancer. Cancer Lett. 1999, 139, 215-220.
doi: 10.1016/S0304-3835(99)00044-0 URL |
| 38 |
Zwaal, R. F.; Schroit, A. J. Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood. 1997, 89, 1121-1132.
doi: 10.1182/blood.V89.4.1121 URL |
| 39 | Mai, J. C.; Mi, Z.; Kim, S. H.; Ng, B.; Robbins, P. D. A proapoptotic peptide for the treatment of solid tumors. Cancer Res. 2001, 61, 7709-7712. |
| 40 |
Li, H.; Kolluri, S. K.; Gu, J.; Dawson, M. I.; Cao, X.; Hobbs, P. D.; Lin, B.; Chen, G.; Lu, J.; Lin, F.; Xie, Z.; Fontana, J. A.; Reed, J. C.; Zhang, X. Cytochrome c release and apoptosis induced by mitochondrial targeting of nuclear orphan receptor TR3. Science. 2000, 289, 1159-1164.
doi: 10.1126/science.289.5482.1159 URL |
| 41 |
Bouchet, S.; Tang, R.; Fava, F.; Legrand, O.; Bauvois, B. The CNGRC-GG-D(KLAKLAK)2 peptide induces a caspase-independent, Ca2+-dependent death in human leukemic myeloid cells by targeting surface aminopeptidase N/CD13. Oncotarget. 2016, 7, 19445-19467.
doi: 10.18632/oncotarget.v7i15 URL |
| 42 |
Li, Y.; Yu, J. Research progress in structure-activity relationship of bioactive peptides. J Med Food. 2015, 18, 147-156.
doi: 10.1089/jmf.2014.0028 URL |
| 43 |
Chalamaiah, M.; Yu, W.; Wu, J. Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: a review. Food Chem. 2018, 245, 205-222.
doi: 10.1016/j.foodchem.2017.10.087 URL |
| 44 | Sharma, S. V. Melittin-induced hyperactivation of phospholipase A2 activity and calcium influx in ras-transformed cells. Oncogene. 1993, 8, 939-947. |
| 45 |
Quintal-Bojórquez, N.; Segura-Campos, M. R. Bioactive peptides as therapeutic adjuvants for cancer. Nutr Cancer. 2021, 73, 1309-1321.
doi: 10.1080/01635581.2020.1813316 URL |
| 46 | Lee, H. T.; Lee, C. C.; Yang, J. R.; Lai, J. Z.; Chang, K. Y. A large-scale structural classification of antimicrobial peptides. Biomed Res Int. 2015, 2015, 475062. |
| 47 | Libério, M. S.; Joanitti, G. A.; Fontes, W.; Castro, M. S. Anticancer peptides and proteins: a panoramic view. Protein Pept Lett. 2013, 20, 380-391. |
| 48 |
Rothbard, J. B.; Jessop, T. C.; Lewis, R. S.; Murray, B. A.; Wender, P. A. Role of membrane potential and hydrogen bonding in the mechanism of translocation of guanidinium-rich peptides into cells. J Am Chem Soc. 2004, 126, 9506-9507.
doi: 10.1021/ja0482536 URL |
| 49 |
Guidotti, G.; Brambilla, L.; Rossi, D. Cell-penetrating peptides: from basic research to clinics. Trends Pharmacol Sci. 2017, 38, 406-424.
doi: 10.1016/j.tips.2017.01.003 URL |
| 50 | Oelkrug, C.; Hartke, M.; Schubert, A. Mode of action of anticancer peptides (ACPs) from amphibian origin. Anticancer Res. 2015, 35, 635-643. |
| 51 |
Gabernet, G.; Müller, A. T.; Hiss, J. A.; Schneider, G. Membranolytic anticancer peptides. MedChemComm. 2016, 7, 2232-2245.
doi: 10.1039/C6MD00376A URL |
| 52 |
Huang, Y. B.; Wang, X. F.; Wang, H. Y.; Liu, Y.; Chen, Y. Studies on mechanism of action of anticancer peptides by modulation of hydrophobicity within a defined structural framework. Mol Cancer Ther. 2011, 10, 416-426.
doi: 10.1158/1535-7163.MCT-10-0811 URL |
| 53 |
Lehrer, R. I.; Lichtenstein, A. K.; Ganz, T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol. 1993, 11, 105-128.
doi: 10.1146/immunol.1993.11.issue-1 URL |
| 54 |
Kumar, P.; Kizhakkedathu, J. N.; Straus, S. K. Antimicrobial peptides: diversity, mechanism of action and strategies to improve the activity and biocompatibility in vivo. Biomolecules. 2018, 8, 4.
doi: 10.3390/biom8010004 URL |
| 55 |
Veldhuizen, E. J.; Schneider, V. A.; Agustiandari, H.; van Dijk, A.; Tjeerdsma-van Bokhoven, J. L.; Bikker, F. J.; Haagsman, H. P. Antimicrobial and immunomodulatory activities of PR-39 derived peptides. PLoS One. 2014, 9, e95939.
doi: 10.1371/journal.pone.0095939 URL |
| 56 |
Chan, Y. R.; Gallo, R. L. PR-39, a syndecan-inducing antimicrobial peptide, binds and affects p130(Cas). J Biol Chem. 1998, 273, 28978-28985.
doi: 10.1074/jbc.273.44.28978 URL |
| 57 |
Bae, S.; Oh, K.; Kim, H.; Kim, Y.; Kim, H. R.; Hwang, Y. I.; Lee, D. S.; Kang, J. S.; Lee, W. J. The effect of alloferon on the enhancement of NK cell cytotoxicity against cancer via the up-regulation of perforin/granzyme B secretion. Immunobiology. 2013, 218, 1026-1033.
doi: 10.1016/j.imbio.2012.12.002 URL |
| 58 |
Ramalho, S. D.; Pinto, M. E. F.; Ferreira, D.; Bolzani, V. S. Biologically active orbitides from the euphorbiaceae family. Planta Med. 2018, 84, 558-567.
doi: 10.1055/s-0043-122604 URL |
| 59 | Hu, E.; Wang, D.; Chen, J.; Tao, X. Novel cyclotides from Hedyotis diffusa induce apoptosis and inhibit proliferation and migration of prostate cancer cells. Int J Clin Exp Med. 2015, 8, 4059-4065. |
| 60 |
Zhang, G.; Liu, S.; Liu, Y.; Wang, F.; Ren, J.; Gu, J.; Zhou, K.; Shan, B. A novel cyclic pentapeptide, H-10, inhibits B16 cancer cell growth and induces cell apoptosis. Oncol Lett. 2014, 8, 248-252.
doi: 10.3892/ol.2014.2121 URL |
| 61 |
Sable, R.; Parajuli, P.; Jois, S. Peptides, peptidomimetics, and polypeptides from marine sources: a wealth of natural sources for pharmaceutical applications. Mar Drugs. 2017, 15, 124.
doi: 10.3390/md15040124 URL |
| 62 |
Chakrabarti, S.; Guha, S.; Majumder, K. Food-derived bioactive peptides in human health: challenges and opportunities. Nutrients. 2018, 10, 1738.
doi: 10.3390/nu10111738 URL |
| 63 |
Burton, M. F.; Steel, P. G. The chemistry and biology of LL-37. Nat Prod Rep. 2009, 26, 1572-1584.
doi: 10.1039/b912533g URL |
| 64 |
Mader, J. S.; Mookherjee, N.; Hancock, R. E.; Bleackley, R. C. The human host defense peptide LL-37 induces apoptosis in a calpain- and apoptosis-inducing factor-dependent manner involving Bax activity. Mol Cancer Res. 2009, 7, 689-702.
doi: 10.1158/1541-7786.MCR-08-0274 URL |
| 65 |
Ganz, T.; Selsted, M. E.; Szklarek, D.; Harwig, S. S.; Daher, K.; Bainton, D. F.; Lehrer, R. I. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest. 1985, 76, 1427-1435.
doi: 10.1172/JCI112120 URL |
| 66 |
McKeown, S. T.; Lundy, F. T.; Nelson, J.; Lockhart, D.; Irwin, C. R.; Cowan, C. G.; Marley, J. J. The cytotoxic effects of human neutrophil peptide-1 (HNP1) and lactoferrin on oral squamous cell carcinoma (OSCC) in vitro. Oral Oncol. 2006, 42, 685-690.
doi: 10.1016/j.oraloncology.2005.11.005 URL |
| 67 |
Jang, A.; Jo, C.; Kang, K.-S.; Lee, M. Antimicrobial and human cancer cell cytotoxic effect of synthetic angiotensin-converting enzyme (ACE) inhibitory peptides. Food Chem. 2008, 107, 327-336.
doi: 10.1016/j.foodchem.2007.08.036 URL |
| 68 | Su, L.; Xu, G.; Shen, J.; Tuo, Y.; Zhang, X.; Jia, S.; Chen, Z.; Su, X. Anticancer bioactive peptide suppresses human gastric cancer growth through modulation of apoptosis and the cell cycle. Oncol Rep. 2010, 23, 3-9. |
| 69 |
Yu, L.; Yang, L.; An, W.; Su, X. Anticancer bioactive peptide-3 inhibits human gastric cancer growth by suppressing gastric cancer stem cells. J Cell Biochem. 2014, 115, 697-711.
doi: 10.1002/jcb.24711 URL |
| 70 |
Suarez-Jimenez, G. M.; Burgos-Hernandez, A.; Ezquerra-Brauer, J. M. Bioactive peptides and depsipeptides with anticancer potential: sources from marine animals. Mar Drugs. 2012, 10, 963-986.
doi: 10.3390/md10050963 URL |
| 71 |
Conlon, J. M.; Mechkarska, M.; Lukic, M. L.; Flatt, P. R. Potential therapeutic applications of multifunctional host-defense peptides from frog skin as anti-cancer, anti-viral, immunomodulatory, and anti-diabetic agents. Peptides. 2014, 57, 67-77.
doi: 10.1016/j.peptides.2014.04.019 URL |
| 72 |
Kim, M. K.; Kang, N.; Ko, S. J.; Park, J.; Park, E.; Shin, D. W.; Kim, S. H.; Lee, S. A.; Lee, J. I.; Lee, S. H.; Ha, E. G.; Jeon, S. H.; Park, Y. Antibacterial and antibiofilm activity and mode of action of magainin 2 against drug-resistant acinetobacter baumannii. Int J Mol Sci. 2018, 19, 3041.
doi: 10.3390/ijms19103041 URL |
| 73 |
Lehmann, J.; Retz, M.; Sidhu, S. S.; Suttmann, H.; Sell, M.; Paulsen, F.; Harder, J.; Unteregger, G.; Stöckle, M. Antitumor activity of the antimicrobial peptide magainin II against bladder cancer cell lines. Eur Urol. 2006, 50, 141-147.
doi: 10.1016/j.eururo.2005.12.043 URL |
| 74 |
Hsu, K. C.; Li-Chan, E. C. Y.; Jao, C. L. Antiproliferative activity of peptides prepared from enzymatic hydrolysates of tuna dark muscle on human breast cancer cell line MCF-7. Food Chem. 2011, 126, 617-622.
doi: 10.1016/j.foodchem.2010.11.066 URL |
| 75 |
Roy, M. K.; Watanabe, Y.; Tamai, Y. Induction of apoptosis in HL-60 cells by skimmed milk digested with a proteolytic enzyme from the yeast Saccharomyces cerevisiae. J Biosci Bioeng. 1999, 88, 426-432.
doi: 10.1016/S1389-1723(99)80221-7 URL |
| 76 | Steijns, J. M.; van Hooijdonk, A. C. Occurrence, structure, biochemical properties and technological characteristics of lactoferrin. Br J Nutr. 2000, 84 Suppl 1, S11-17. |
| 77 |
Mader, J. S.; Salsman, J.; Conrad, D. M.; Hoskin, D. W. Bovine lactoferricin selectively induces apoptosis in human leukemia and carcinoma cell lines. Mol Cancer Ther. 2005, 4, 612-624.
doi: 10.1158/1535-7163.MCT-04-0077 URL |
| 78 |
Yin, C. M.; Wong, J. H.; Xia, J.; Ng, T. B. Studies on anticancer activities of lactoferrin and lactoferricin. Curr Protein Pept Sci. 2013, 14, 492-503.
doi: 10.2174/13892037113149990066 URL |
| 79 |
Wang, Z.; Zhang, X. Isolation and identification of anti-proliferative peptides from Spirulina platensis using three-step hydrolysis. J Sci Food Agric. 2017, 97, 918-922.
doi: 10.1002/jsfa.2017.97.issue-3 URL |
| 80 |
Chi, C.-F.; Hu, F.-Y.; Wang, B.; Li, T.; Ding, G.-F. Antioxidant and anticancer peptides from the protein hydrolysate of blood clam (Tegillarca granosa) muscle. J Funct Foods. 2015, 15, 301-313.
doi: 10.1016/j.jff.2015.03.045 URL |
| 81 |
Fernández-Tomé, S.; Sanchón, J.; Recio, I.; Hernández-Ledesma, B. Transepithelial transport of lunasin and derived peptides: Inhibitory effects on the gastrointestinal cancer cells viability. J Food Compost Anal. 2018, 68, 101-110.
doi: 10.1016/j.jfca.2017.01.011 URL |
| 82 |
Luna Vital, D. A.; González de Mejía, E.; Dia, V. P.; Loarca-Piña, G. Peptides in common bean fractions inhibit human colorectal cancer cells. Food Chem. 2014, 157, 347-355.
doi: 10.1016/j.foodchem.2014.02.050 URL |
| 83 |
Soares, A. M.; Zuliani, J. P. Toxins of animal venoms and inhibitors: molecular and biotechnological tools useful to human and animal health. Curr Top Med Chem. 2019, 19, 1868-1871.
doi: 10.2174/156802661921191024114842 URL |
| 84 |
Havas, L. J. Effect of bee venom on colchicine-induced tumours. Nature. 1950, 166, 567-568.
doi: 10.1038/166567a0 |
| 85 | Kerkis, I.; Hayashi, M. A.; Prieto da Silva, A. R.; Pereira, A.; De Sá Júnior, P. L.; Zaharenko, A. J.; Rádis-Baptista, G.; Kerkis, A.; Yamane, T. State of the art in the studies on crotamine, a cell penetrating peptide from South American rattlesnake. Biomed Res Int. 2014, 2014, 675985. |
| 86 |
Pereira, A.; Kerkis, A.; Hayashi, M. A.; Pereira, A. S.; Silva, F. S.; Oliveira, E. B.; Prieto da Silva, A. R.; Yamane, T.; Rádis-Baptista, G.; Kerkis, I. Crotamine toxicity and efficacy in mouse models of melanoma. Expert Opin Investig Drugs. 2011, 20, 1189-1200.
doi: 10.1517/13543784.2011.602064 URL |
| 87 |
Bakare, O. O.; Gokul, A.; Wu, R.; Niekerk, L. A.; Klein, A.; Keyster, M. Biomedical relevance of novel anticancer peptides in the sensitive treatment of cancer. Biomolecules. 2021, 11, 1120.
doi: 10.3390/biom11081120 URL |
| 88 |
Aghazadeh, H.; Memariani, H.; Ranjbar, R.; Pooshang Bagheri, K. The activity and action mechanism of novel short selective LL-37-derived anticancer peptides against clinical isolates of Escherichia coli. Chem Biol Drug Des. 2019, 93, 75-83.
doi: 10.1111/cbdd.2019.93.issue-1 URL |
| 89 |
Fruitwala, S.; El-Naccache, D. W.; Chang, T. L. Multifaceted immune functions of human defensins and underlying mechanisms. Semin Cell Dev Biol. 2019, 88, 163-172.
doi: 10.1016/j.semcdb.2018.02.023 URL |
| 90 |
Liu, S.; Zhou, L.; Li, J.; Suresh, A.; Verma, C.; Foo, Y. H.; Yap, E. P.; Tan, D. T.; Beuerman, R. W. Linear analogues of human beta-defensin 3: concepts for design of antimicrobial peptides with reduced cytotoxicity to mammalian cells. ChemBioChem. 2008, 9, 964-973.
doi: 10.1002/(ISSN)1439-7633 URL |
| 91 |
Zweytick, D. LTX-315 - a promising novel antitumor peptide and immunotherapeutic agent. Cell Stress. 2019, 3, 328-329.
doi: 10.15698/cst URL |
| 92 |
Jeyamogan, S.; Khan, N. A.; Sagathevan, K.; Siddiqui, R. Sera/organ lysates of selected animals living in polluted environments exhibit cytotoxicity against cancer cell lines. Anticancer Agents Med Chem. 2019, 19, 2251-2268.
doi: 10.2174/1871520619666191011161314 URL |
| 93 |
Brady, D.; Grapputo, A.; Romoli, O.; Sandrelli, F. Insect cecropins, antimicrobial peptides with potential therapeutic applications. Int J Mol Sci. 2019, 20, 5862.
doi: 10.3390/ijms20235862 URL |
| 94 |
Pinto, I. B.; dos Santos Machado, L.; Meneguetti, B. T.; Nogueira, M. L.; Espínola Carvalho, C. M.; Roel, A. R.; Franco, O. L. Utilization of antimicrobial peptides, analogues and mimics in creating antimicrobial surfaces and bio-materials. Biochem Eng J. 2019, 150, 107237.
doi: 10.1016/j.bej.2019.107237 URL |
| 95 |
Li, B.; Lyu, P.; Xie, S.; Qin, H.; Pu, W.; Xu, H.; Chen, T.; Shaw, C.; Ge, L.; Kwok, H. F. LFB: A Novel Antimicrobial Brevinin-Like Peptide from the Skin Secretion of the Fujian Large Headed Frog, Limnonectes fujianensi. Biomolecules. 2019, 9, 242.
doi: 10.3390/biom9060242 URL |
| 96 |
Zahedifard, F.; Lee, H.; No, J. H.; Salimi, M.; Seyed, N.; Asoodeh, A.; Rafati, S. Anti-leishmanial activity of Brevinin 2R and its Lauric acid conjugate type against L. major: In vitro mechanism of actions and in vivo treatment potentials. PLoS Negl Trop Dis. 2019, 13, e0007217.
doi: 10.1371/journal.pntd.0007217 URL |
| 97 |
Liu, Y.; Tavana, O.; Gu, W. p 53 modifications: exquisite decorations of the powerful guardian. J Mol Cell Biol. 2019, 11, 564-577.
doi: 10.1093/jmcb/mjz060 URL |
| 98 |
Chen, X.; Zhang, L.; Ma, C.; Zhang, Y.; Xi, X.; Wang, L.; Zhou, M.; Burrows, J. F.; Chen, T. A novel antimicrobial peptide, Ranatuerin-2PLx, showing therapeutic potential in inhibiting proliferation of cancer cells. Biosci Rep. 2018, 38, BSR20180710.
doi: 10.1042/BSR20180710 URL |
| 99 |
Tornesello, A. L.; Borrelli, A.; Buonaguro, L.; Buonaguro, F. M.; Tornesello, M. L. Antimicrobial peptides as anticancer agents: functional properties and biological activities. Molecules. 2020, 25, 2850.
doi: 10.3390/molecules25122850 URL |
| 100 |
Tripathi, A. K.; Kumari, T.; Harioudh, M. K.; Yadav, P. K.; Kathuria, M.; Shukla, P. K.; Mitra, K.; Ghosh, J. K. Identification of GXXXXG motif in Chrysophsin-1 and its implication in the design of analogs with cell-selective antimicrobial and anti-endotoxin activities. Sci Rep. 2017, 7, 3384.
doi: 10.1038/s41598-017-03576-1 |
| 101 |
Hansen, I.; Isaksson, J.; Poth, A. G.; Hansen, K.; Andersen, A. J. C.; Richard, C. S. M.; Blencke, H. M.; Stensvåg, K.; Craik, D. J.; Haug, T. Isolation and characterization of antimicrobial peptides with unusual disulfide connectivity from the colonial ascidian synoicum turgens. Mar Drugs. 2020, 18, 51.
doi: 10.3390/md18010051 URL |
| 102 |
Oren, Z.; Shai, Y. Mode of action of linear amphipathic alpha-helical antimicrobial peptides. Biopolymers. 1998, 47, 451-463.
doi: 10.1002/(SICI)1097-0282(1998)47:6<>1.0.CO;2-W URL |
| 103 | Shai, Y. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by alpha-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim Biophys Acta. 1999, 1462, 55-70. |
| 104 |
Matsuzaki, K.; Murase, O.; Fujii, N.; Miyajima, K. An antimicrobial peptide, magainin 2, induced rapid flip-flop of phospholipids coupled with pore formation and peptide translocation. Biochemistry. 1996, 35, 11361-11368.
doi: 10.1021/bi960016v URL |
| 105 |
Garay, H.; Espinosa, L. A.; Perera, Y.; Sánchez, A.; Diago, D.; Perea, S. E.; Besada, V.; Reyes, O.; González, L. J. Characterization of low-abundance species in the active pharmaceutical ingredient of CIGB-300: A clinical-grade anticancer synthetic peptide. J Pept Sci. 2018, 24, e3081.
doi: 10.1002/psc.v24.6 URL |
| 106 |
Perea, S. E.; Reyes, O.; Baladron, I.; Perera, Y.; Farina, H.; Gil, J.; Rodriguez, A.; Bacardi, D.; Marcelo, J. L.; Cosme, K.; Cruz, M.; Valenzuela, C.; López-Saura, P. A.; Puchades, Y.; Serrano, J. M.; Mendoza, O.; Castellanos, L.; Sanchez, A.; Betancourt, L.; Besada, V.; Silva, R.; López, E.; Falcón, V.; Hernández, I.; Solares, M.; Santana, A.; Díaz, A.; Ramos, T.; López, C.; Ariosa, J.; González, L. J.; Garay, H.; Gómez, D.; Gómez, R.; Alonso, D. F.; Sigman, H.; Herrera, L.; Acevedo, B. CIGB-300, a novel proapoptotic peptide that impairs the CK2 phosphorylation and exhibits anticancer properties both in vitro and in vivo. Mol Cell Biochem. 2008, 316, 163-167.
doi: 10.1007/s11010-008-9814-5 URL |
| 107 |
Rodríguez-Ulloa, A.; Ramos, Y.; Gil, J.; Perera, Y.; Castellanos-Serra, L.; García, Y.; Betancourt, L.; Besada, V.; González, L. J.; Fernández-de-Cossio, J.; Sanchez, A.; Serrano, J. M.; Farina, H.; Alonso, D. F.; Acevedo, B. E.; Padrón, G.; Musacchio, A.; Perea, S. E. Proteomic profile regulated by the anticancer peptide CIGB-300 in non-small cell lung cancer (NSCLC) cells. J Proteome Res. 2010, 9, 5473-5483.
doi: 10.1021/pr100728v URL |
| 108 | Hirabayashi, K.; Yanagisawa, R.; Saito, S.; Higuchi, Y.; Koya, T.; Sano, K.; Koido, S.; Okamoto, M.; Sugiyama, H.; Nakazawa, Y.; Shimodaira, S. Feasibility and immune response of WT1 peptide vaccination in combination with OK-432 for paediatric solid tumors. Anticancer Res. 2018, 38, 2227-2234. |
| 109 | Yanagisawa, R.; Koizumi, T.; Koya, T.; Sano, K.; Koido, S.; Nagai, K.; Kobayashi, M.; Okamoto, M.; Sugiyama, H.; Shimodaira, S. WT1-pulsed dendritic cell vaccine combined with chemotherapy for resected pancreatic cancer in a phase I study. Anticancer Res. 2018, 38, 2217-2225. |
| 110 | Ohno, S.; Takano, F.; Ohta, Y.; Kyo, S.; Myojo, S.; Dohi, S.; Sugiyama, H.; Ohta, T.; Inoue, M. Frequency of myeloid dendritic cells can predict the efficacy of Wilms’ tumor 1 peptide vaccination. Anticancer Res. 2011, 31, 2447-2452. |
| 111 |
Ishikawa, H.; Imano, M.; Shiraishi, O.; Yasuda, A.; Peng, Y. F.; Shinkai, M.; Yasuda, T.; Imamoto, H.; Shiozaki, H. Phase I clinical trial of vaccination with LY6K-derived peptide in patients with advanced gastric cancer. Gastric Cancer. 2014, 17, 173-180.
doi: 10.1007/s10120-013-0258-6 URL |
| 112 | Vasef, M. A.; Ross, J. S.; Cohen, M. B. Telomerase activity in human solid tumors. Diagnostic utility and clinical applications. Am J Clin Pathol. 1999, 112, S68-75. |
| 113 |
Bernhardt, S. L.; Gjertsen, M. K.; Trachsel, S.; Møller, M.; Eriksen, J. A.; Meo, M.; Buanes, T.; Gaudernack, G. Telomerase peptide vaccination of patients with non-resectable pancreatic cancer: A dose escalating phase I/II study. Br J Cancer. 2006, 95, 1474-1482.
doi: 10.1038/sj.bjc.6603437 |
| 114 | Kokhaei, P.; Palma, M.; Hansson, L.; Osterborg, A.; Mellstedt, H.; Choudhury, A. Telomerase (hTERT 611-626) serves as a tumor antigen in B-cell chronic lymphocytic leukemia and generates spontaneously antileukemic, cytotoxic T cells. Exp Hematol. 2007, 35, 297-304. |
| 115 |
Aspeslagh, S.; Awada, A.; A, S. M. P.; Aftimos, P.; Bahleda, R.; Varga, A.; Soria, J. C. Phase I dose-escalation study of plitidepsin in combination with bevacizumab in patients with refractory solid tumors. Anticancer Drugs. 2016, 27, 1021-1027.
doi: 10.1097/CAD.0000000000000409 URL |
| 116 |
Engel, J. B.; Tinneberg, H. R.; Rick, F. G.; Berkes, E.; Schally, A. V. Targeting of peptide cytotoxins to LHRH receptors for treatment of cancer. Curr Drug Targets. 2016, 17, 488-494.
doi: 10.2174/138945011705160303154717 URL |
| 117 | Noguchi, M.; Matsumoto, K.; Uemura, H.; Arai, G.; Eto, M.; Naito, S.; Ohyama, C.; Nasu, Y.; Tanaka, M.; Moriya, F.; Suekane, S.; Matsueda, S.; Komatsu, N.; Sasada, T.; Yamada, A.; Kakuma, T.; Itoh, K. An open-label, randomized phase II trial of personalized peptide vaccination in patients with bladder cancer that progressed after platinum-based chemotherapy. Clin Cancer Res. 2016, 22, 54-60. |
| 118 |
Brown, T. A.; Byrd, K.; Vreeland, T. J.; Clifton, G. T.; Jackson, D. O.; Hale, D. F.; Herbert, G. S.; Myers, J. W.; Greene, J. M.; Berry, J. S.; Martin, J.; Elkas, J. C.; Conrads, T. P.; Darcy, K. M.; Hamilton, C. A.; Maxwel, G. L.; Peoples, G. E. Final analysis of a phase I/IIa trial of the folate-binding protein-derived E39 peptide vaccine to prevent recurrence in ovarian and endometrial cancer patients. Cancer Med. 2019, 8, 4678-4687.
doi: 10.1002/cam4.v8.10 URL |
| 119 |
Schwartzentruber, D. J.; Lawson, D. H.; Richards, J. M.; Conry, R. M.; Miller, D. M.; Treisman, J.; Gailani, F.; Riley, L.; Conlon, K.; Pockaj, B.; Kendra, K. L.; White, R. L.; Gonzalez, R.; Kuzel, T. M.; Curti, B.; Leming, P. D.; Whitman, E. D.; Balkissoon, J.; Reintgen, D. S.; Kaufman, H.; Marincola, F. M.; Merino, M. J.; Rosenberg, S. A.; Choyke, P.; Vena, D.; Hwu, P. gp 100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011, 364, 2119-2127.
doi: 10.1056/NEJMoa1012863 URL |
| 120 |
Mikecin, A. M.; Walker, L. R.; Kuna, M.; Raucher, D. Thermally targeted p21 peptide enhances bortezomib cytotoxicity in androgen-independent prostate cancer cell lines. Anticancer Drugs. 2014, 25, 189-199.
doi: 10.1097/CAD.0000000000000036 URL |
| 121 |
Xie, M.; Liu, D.; Yang, Y. Anti-cancer peptides: classification, mechanism of action, reconstruction and modification. Open Biol. 2020, 10, 200004.
doi: 10.1098/rsob.200004 URL |
| 122 |
Hu, X.; Liu, S. Recent advances towards the fabrication and biomedical applications of responsive polymeric assemblies and nanoparticle hybrid superstructures. Dalton Trans. 2015, 44, 3904-3922.
doi: 10.1039/C4DT03609C URL |
| 123 |
Hwang, J. S.; Kim, S. G.; Shin, T. H.; Jang, Y. E.; Kwon, D. H.; Lee, G. Development of anticancer peptides using artificial intelligence and combinational therapy for cancer therapeutics. Pharmaceutics. 2022, 14, 997.
doi: 10.3390/pharmaceutics14050997 URL |
| 124 |
Regberg, J.; Srimanee, A.; Langel, U. Applications of cell-penetrating peptides for tumor targeting and future cancer therapies. Pharmaceuticals (Basel). 2012, 5, 991-1007.
doi: 10.3390/ph5090991 URL |
| 125 |
Chatzisideri, T.; Leonidis, G.; Sarli, V. Cancer-targeted delivery systems based on peptides. Future Med Chem. 2018, 10, 2201-2226.
doi: 10.4155/fmc-2018-0174 URL |
| 126 |
Kim, B. J.; Xu, B. Enzyme-instructed self-assembly for cancer therapy and imaging. Bioconjug Chem. 2020, 31, 492-500.
doi: 10.1021/acs.bioconjchem.0c00025 URL |
| 127 |
Shi, J.; Xu, B. Nanoscale assemblies of small molecules control the fate of cells. Nano Today. 2015, 10, 615-630.
doi: 10.1016/j.nantod.2015.09.001 URL |
| 128 |
Liu, X.; Wu, F.; Ji, Y.; Yin, L. Recent advances in anti-cancer protein/peptide delivery. Bioconjug Chem. 2019, 30, 305-324.
doi: 10.1021/acs.bioconjchem.8b00750 URL |
| 129 |
Conibear, A. C.; Schmid, A.; Kamalov, M.; Becker, C. F. W.; Bello, C. Recent advances in peptide-based approaches for cancer treatment. Curr Med Chem. 2020, 27, 1174-1205.
doi: 10.2174/0929867325666171123204851 URL |
| [1] | Xiaodan Wang, Qinmei Li, Huawei Yang. Effect of radiation sterilisation on the structure and antibacterial properties of antimicrobial peptides [J]. Biomaterials Translational, 2023, 4(1): 51-61. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||