1
1954
... In all aspects of communication the meanings of words and definitions are of paramount importance for clear understanding and transmission of ideas. However, these meanings may change with time so that words and phrases take on different interpretations. Furthermore, the way words are used in writing significantly affects the conveyance of ideas from one mind to another.1 In the long history of the study of the histogenesis of bone we see some of these changes in meaning and understanding of concepts that have perhaps contributed to serious misinterpretation of the ideas of some investigators in the field. It is hoped that this brief historical perspective may explain and clarify at least some of the problems that have resulted. ...
Haemopoietic stem cells: concept and definitions
1
1979
... This manuscript outlines a brief history of the idea that osteogenic cells are present postnatally in the adult skeleton. Furthermore, these primitive cells are important for the supply of committed progenitor cells for bone formation and the regeneration of this tissue after traumatic injury throughout life. Over the years, many names have been given to the cells that have osteogenic potential and a selection of these are given in Table 1. In terms of study of cell populations and cellular kinetics the formation of the cells of the blood in the process of haematopoiesis is most appropriate for scientific investigation. It is the system most extensively studied as in normal physiology and under abnormal stress it shows rapid cell proliferation and differentiation. In general terms, the most primitive cell of any tissue is the stem cell, which is conventionally defined by findings from haematology studies as an undifferentiated cell that can renew indefinitely and give rise to differentiated progeny.2 It has thus been defined as “a cell type which, in the adult organism, can maintain its own numbers in spite of physiological or artificial removal of cells from the population.”3 Also it is important to realise that tissue environments have been shown to exist that are essential to maintain the stem cell state; the so-called stem cell niche.4 ...
Cellular kinetics of haemopoiesis
1
1982
... This manuscript outlines a brief history of the idea that osteogenic cells are present postnatally in the adult skeleton. Furthermore, these primitive cells are important for the supply of committed progenitor cells for bone formation and the regeneration of this tissue after traumatic injury throughout life. Over the years, many names have been given to the cells that have osteogenic potential and a selection of these are given in Table 1. In terms of study of cell populations and cellular kinetics the formation of the cells of the blood in the process of haematopoiesis is most appropriate for scientific investigation. It is the system most extensively studied as in normal physiology and under abnormal stress it shows rapid cell proliferation and differentiation. In general terms, the most primitive cell of any tissue is the stem cell, which is conventionally defined by findings from haematology studies as an undifferentiated cell that can renew indefinitely and give rise to differentiated progeny.2 It has thus been defined as “a cell type which, in the adult organism, can maintain its own numbers in spite of physiological or artificial removal of cells from the population.”3 Also it is important to realise that tissue environments have been shown to exist that are essential to maintain the stem cell state; the so-called stem cell niche.4 ...
The relationship between the spleen colony-forming cell and the haemopoietic stem cell
1
1978
... This manuscript outlines a brief history of the idea that osteogenic cells are present postnatally in the adult skeleton. Furthermore, these primitive cells are important for the supply of committed progenitor cells for bone formation and the regeneration of this tissue after traumatic injury throughout life. Over the years, many names have been given to the cells that have osteogenic potential and a selection of these are given in Table 1. In terms of study of cell populations and cellular kinetics the formation of the cells of the blood in the process of haematopoiesis is most appropriate for scientific investigation. It is the system most extensively studied as in normal physiology and under abnormal stress it shows rapid cell proliferation and differentiation. In general terms, the most primitive cell of any tissue is the stem cell, which is conventionally defined by findings from haematology studies as an undifferentiated cell that can renew indefinitely and give rise to differentiated progeny.2 It has thus been defined as “a cell type which, in the adult organism, can maintain its own numbers in spite of physiological or artificial removal of cells from the population.”3 Also it is important to realise that tissue environments have been shown to exist that are essential to maintain the stem cell state; the so-called stem cell niche.4 ...
“Mesenchymal” stem cells
1
2014
... This report represents the author’s view of some of the significant early steps in understanding regarding the existence of skeletal stem cells, together with the salient concepts that have resulted in massive expansion of interest and the current development of stromal stem cells from a number of tissues for clinical and commercial exploitation. Not all aspects of relevance to this field are considered here however and more extensive assessments of the important developments and problems encountered in progress in the area of knowledge concerning the bone-forming progenitors are seen in previous accounts.5-8 ...
Stem cells and bone: a historical perspective
0
2015
Mesenchymal stem cells and osteoblast differentiation
0
2002
The stem cell of the osteoblast
1
1996
... This report represents the author’s view of some of the significant early steps in understanding regarding the existence of skeletal stem cells, together with the salient concepts that have resulted in massive expansion of interest and the current development of stromal stem cells from a number of tissues for clinical and commercial exploitation. Not all aspects of relevance to this field are considered here however and more extensive assessments of the important developments and problems encountered in progress in the area of knowledge concerning the bone-forming progenitors are seen in previous accounts.5-8 ...
2
1961
... Generally histologists have noted that there are four basic types of tissues in the human body; epithelial tissue, connective tissue, muscular tissue and nervous tissue.9 As noted by McLean and Urist,10 in embryonic or post-foetal development bone is observed to arise from a transformation of connective tissue, which derives from the mesenchyme. This had been discussed many years ago in the works of Schäfer and many others.11, 12 However, even the histological classification of human adult tissues and this general, miscellaneous term of ‘connective tissue’ is now under consideration for revision, with proposed definitions of all tissues being better characterized by both structure and function to fit in with newer knowledge.13 ...
... In growing bone tissue three distinct types of cells are easily distinguished.9, 16, 17 These are: cuboidal osteoblasts, seen to be actively synthesizing the tissue on the advancing front; osteocytes that derive from matrix-encapsulated osteoblasts and come to reside within the calcified bone matrix; and osteoclasts, giant multinucleated cells associated directly with areas of bone resorption. An extensive review of osteoblasts and their functional significance up to the 1970s is given by Pritchard.18 The fact that osteoblasts are never observed to divide indicated that they must be recruited from other local cells which proliferate to give the potential for production of these bone-forming cells. ...
1
1955
... Generally histologists have noted that there are four basic types of tissues in the human body; epithelial tissue, connective tissue, muscular tissue and nervous tissue.9 As noted by McLean and Urist,10 in embryonic or post-foetal development bone is observed to arise from a transformation of connective tissue, which derives from the mesenchyme. This had been discussed many years ago in the works of Schäfer and many others.11, 12 However, even the histological classification of human adult tissues and this general, miscellaneous term of ‘connective tissue’ is now under consideration for revision, with proposed definitions of all tissues being better characterized by both structure and function to fit in with newer knowledge.13 ...
The histogenesis of bone
3
1925
... Generally histologists have noted that there are four basic types of tissues in the human body; epithelial tissue, connective tissue, muscular tissue and nervous tissue.9 As noted by McLean and Urist,10 in embryonic or post-foetal development bone is observed to arise from a transformation of connective tissue, which derives from the mesenchyme. This had been discussed many years ago in the works of Schäfer and many others.11, 12 However, even the histological classification of human adult tissues and this general, miscellaneous term of ‘connective tissue’ is now under consideration for revision, with proposed definitions of all tissues being better characterized by both structure and function to fit in with newer knowledge.13 ...
... With regard to the histogenesis of bone, this subject has been of great interest and some contention for numerous biologists and clinicians for well over a century11, 14, 15 and yet still some present controversy exists. One major aspect of concern has been for many years the identification, characterization and differentiation potentials of the early precursors of the bone-tissue forming cells, the osteoblasts, and in particular, to be considered here, that of putative stem cells that are proposed to be involved in skeletal regeneration as required throughout life. ...
... Until about 40 years ago it was widely assumed, mainly from histological studies, that the two major cellular systems of bone formation and bone resorption present in bone tissue were part of the same cell lineage derived from a common progenitor. Histological evidence seemed to suggest that there was transformation of one cell to another and this represented differing functional states of the same cell type.11, 16, 19 The bone-forming osteoblastic cells and the bone-destroying osteoclastic cells thus having the same progenitor cell origins. ...
Essentials of histology
1
1907
... Generally histologists have noted that there are four basic types of tissues in the human body; epithelial tissue, connective tissue, muscular tissue and nervous tissue.9 As noted by McLean and Urist,10 in embryonic or post-foetal development bone is observed to arise from a transformation of connective tissue, which derives from the mesenchyme. This had been discussed many years ago in the works of Schäfer and many others.11, 12 However, even the histological classification of human adult tissues and this general, miscellaneous term of ‘connective tissue’ is now under consideration for revision, with proposed definitions of all tissues being better characterized by both structure and function to fit in with newer knowledge.13 ...
General histological woes: Definition and classification of tissues
1
2021
... Generally histologists have noted that there are four basic types of tissues in the human body; epithelial tissue, connective tissue, muscular tissue and nervous tissue.9 As noted by McLean and Urist,10 in embryonic or post-foetal development bone is observed to arise from a transformation of connective tissue, which derives from the mesenchyme. This had been discussed many years ago in the works of Schäfer and many others.11, 12 However, even the histological classification of human adult tissues and this general, miscellaneous term of ‘connective tissue’ is now under consideration for revision, with proposed definitions of all tissues being better characterized by both structure and function to fit in with newer knowledge.13 ...
1
1901
... With regard to the histogenesis of bone, this subject has been of great interest and some contention for numerous biologists and clinicians for well over a century11, 14, 15 and yet still some present controversy exists. One major aspect of concern has been for many years the identification, characterization and differentiation potentials of the early precursors of the bone-tissue forming cells, the osteoblasts, and in particular, to be considered here, that of putative stem cells that are proposed to be involved in skeletal regeneration as required throughout life. ...
1
1919
... With regard to the histogenesis of bone, this subject has been of great interest and some contention for numerous biologists and clinicians for well over a century11, 14, 15 and yet still some present controversy exists. One major aspect of concern has been for many years the identification, characterization and differentiation potentials of the early precursors of the bone-tissue forming cells, the osteoblasts, and in particular, to be considered here, that of putative stem cells that are proposed to be involved in skeletal regeneration as required throughout life. ...
2
1968
... In growing bone tissue three distinct types of cells are easily distinguished.9, 16, 17 These are: cuboidal osteoblasts, seen to be actively synthesizing the tissue on the advancing front; osteocytes that derive from matrix-encapsulated osteoblasts and come to reside within the calcified bone matrix; and osteoclasts, giant multinucleated cells associated directly with areas of bone resorption. An extensive review of osteoblasts and their functional significance up to the 1970s is given by Pritchard.18 The fact that osteoblasts are never observed to divide indicated that they must be recruited from other local cells which proliferate to give the potential for production of these bone-forming cells. ...
... Until about 40 years ago it was widely assumed, mainly from histological studies, that the two major cellular systems of bone formation and bone resorption present in bone tissue were part of the same cell lineage derived from a common progenitor. Histological evidence seemed to suggest that there was transformation of one cell to another and this represented differing functional states of the same cell type.11, 16, 19 The bone-forming osteoblastic cells and the bone-destroying osteoclastic cells thus having the same progenitor cell origins. ...
A histological study of the early phases of bone repair
1
1930
... In growing bone tissue three distinct types of cells are easily distinguished.9, 16, 17 These are: cuboidal osteoblasts, seen to be actively synthesizing the tissue on the advancing front; osteocytes that derive from matrix-encapsulated osteoblasts and come to reside within the calcified bone matrix; and osteoclasts, giant multinucleated cells associated directly with areas of bone resorption. An extensive review of osteoblasts and their functional significance up to the 1970s is given by Pritchard.18 The fact that osteoblasts are never observed to divide indicated that they must be recruited from other local cells which proliferate to give the potential for production of these bone-forming cells. ...
1
1972
... In growing bone tissue three distinct types of cells are easily distinguished.9, 16, 17 These are: cuboidal osteoblasts, seen to be actively synthesizing the tissue on the advancing front; osteocytes that derive from matrix-encapsulated osteoblasts and come to reside within the calcified bone matrix; and osteoclasts, giant multinucleated cells associated directly with areas of bone resorption. An extensive review of osteoblasts and their functional significance up to the 1970s is given by Pritchard.18 The fact that osteoblasts are never observed to divide indicated that they must be recruited from other local cells which proliferate to give the potential for production of these bone-forming cells. ...
1
1974
... Until about 40 years ago it was widely assumed, mainly from histological studies, that the two major cellular systems of bone formation and bone resorption present in bone tissue were part of the same cell lineage derived from a common progenitor. Histological evidence seemed to suggest that there was transformation of one cell to another and this represented differing functional states of the same cell type.11, 16, 19 The bone-forming osteoblastic cells and the bone-destroying osteoclastic cells thus having the same progenitor cell origins. ...
The pathobiology of the osteoclast
1
1985
... However, growing experimental data indicated that this was not the true state of affairs. A number of studies including transplants of spleen and bone marrow, parabiosis and cell tracking by using quail-chick chimaeras indicated that osteoclasts were derived from myeloid progenitors and that these cells were circulatory.20 Elegant experiments both in vivo and in vitro lead to the conclusion that osteoclasts were part of the haemopoietic stem cell lineage and were formed by fusion of mononuclear precursors of the monocyte-macrophage system.21-27 Additionally, from further critical experiments it was later concluded that there was no evidence that supported the idea that there was a single common progenitor for both stromal and hemopoietic lineages within foetal or adult bone marrow.28 Thus the osteoblasts and osteocytes were of a separate cell lineage to the osteoclast. ...
Osteoclasts derive from hematopoietic stem cells according to marker, giant lysosomes of beige mice
1
1981
... However, growing experimental data indicated that this was not the true state of affairs. A number of studies including transplants of spleen and bone marrow, parabiosis and cell tracking by using quail-chick chimaeras indicated that osteoclasts were derived from myeloid progenitors and that these cells were circulatory.20 Elegant experiments both in vivo and in vitro lead to the conclusion that osteoclasts were part of the haemopoietic stem cell lineage and were formed by fusion of mononuclear precursors of the monocyte-macrophage system.21-27 Additionally, from further critical experiments it was later concluded that there was no evidence that supported the idea that there was a single common progenitor for both stromal and hemopoietic lineages within foetal or adult bone marrow.28 Thus the osteoblasts and osteocytes were of a separate cell lineage to the osteoclast. ...
Osteoclasts derived from haematopoietic stem cells
0
1980
Osteopetrosis cured by temporary parabiosis
0
1973
Spleen cells transmit osteopetrosis in mice
0
1975
The origin of osteoclasts
0
1982
Osteogenesis in osteopetrotic mice
0
1982
Bone resorption by osteoclasts
1
2000
... However, growing experimental data indicated that this was not the true state of affairs. A number of studies including transplants of spleen and bone marrow, parabiosis and cell tracking by using quail-chick chimaeras indicated that osteoclasts were derived from myeloid progenitors and that these cells were circulatory.20 Elegant experiments both in vivo and in vitro lead to the conclusion that osteoclasts were part of the haemopoietic stem cell lineage and were formed by fusion of mononuclear precursors of the monocyte-macrophage system.21-27 Additionally, from further critical experiments it was later concluded that there was no evidence that supported the idea that there was a single common progenitor for both stromal and hemopoietic lineages within foetal or adult bone marrow.28 Thus the osteoblasts and osteocytes were of a separate cell lineage to the osteoclast. ...
The “common stem cell” hypothesis reevaluated: human fetal bone marrow contains separate populations of hematopoietic and stromal progenitors
1
1995
... However, growing experimental data indicated that this was not the true state of affairs. A number of studies including transplants of spleen and bone marrow, parabiosis and cell tracking by using quail-chick chimaeras indicated that osteoclasts were derived from myeloid progenitors and that these cells were circulatory.20 Elegant experiments both in vivo and in vitro lead to the conclusion that osteoclasts were part of the haemopoietic stem cell lineage and were formed by fusion of mononuclear precursors of the monocyte-macrophage system.21-27 Additionally, from further critical experiments it was later concluded that there was no evidence that supported the idea that there was a single common progenitor for both stromal and hemopoietic lineages within foetal or adult bone marrow.28 Thus the osteoblasts and osteocytes were of a separate cell lineage to the osteoclast. ...
Initiation and enhancement of bone formation. A review
1
1987
... The progenitor cells of the osteoblast were observed to be locally-derived cells that were non-circulatory and residing close to all bone surfaces.29 This local origin was classically demonstrated by autoradiographic studies of cell proliferation in active tissue-forming areas as determined by tritiated thymidine uptake into the nuclei of proliferating cells.30-37 At centres of intramembranous and cartilaginous bone formation similar evidence of local supply of progenitor cells were shown to occur. The non-circulatory nature of osteogenic precursors in skeletal healing was indicated by parabiosis experiments, in which the blood circulatory systems of two individual animals are joined together. In these studies it was observed that, after a skeletal fracture was created in one individual parabiont, no bone matrix-synthesising osteoblast precursors were supplied through the circulation from radioactively-labelled cells of a tritiated thymidine-labelled animal to the regenerating fracture site in the unlabelled animal.38 More recently claims of support for the phenomenon of circulating osteogenic progenitors have been made and to some extent reviewed.39-41 But it is this author’s view that the supportive studies have alternative possible explanations for the results mentioned therein. It may be concluded that any contribution of such progenitors is unlikely to be of any direct relevance to skeletal regeneration and for any normal tissue reconstruction. That fibroblasts can be grown from blood samples from a number of species is not questioned and has been documented by many after Maximow et al.’s original claims.42-45 However, their involvement in normal physiological healing of the skeleton is still unproven despite extensive research and the significance of the presence in blood is still considered to be unknown. ...
Autoradiographic studies of cell proliferation in the periosteum of intact and fractured femora of mice utilizing DNA labeling with H3-thymidine
1
1961
... The progenitor cells of the osteoblast were observed to be locally-derived cells that were non-circulatory and residing close to all bone surfaces.29 This local origin was classically demonstrated by autoradiographic studies of cell proliferation in active tissue-forming areas as determined by tritiated thymidine uptake into the nuclei of proliferating cells.30-37 At centres of intramembranous and cartilaginous bone formation similar evidence of local supply of progenitor cells were shown to occur. The non-circulatory nature of osteogenic precursors in skeletal healing was indicated by parabiosis experiments, in which the blood circulatory systems of two individual animals are joined together. In these studies it was observed that, after a skeletal fracture was created in one individual parabiont, no bone matrix-synthesising osteoblast precursors were supplied through the circulation from radioactively-labelled cells of a tritiated thymidine-labelled animal to the regenerating fracture site in the unlabelled animal.38 More recently claims of support for the phenomenon of circulating osteogenic progenitors have been made and to some extent reviewed.39-41 But it is this author’s view that the supportive studies have alternative possible explanations for the results mentioned therein. It may be concluded that any contribution of such progenitors is unlikely to be of any direct relevance to skeletal regeneration and for any normal tissue reconstruction. That fibroblasts can be grown from blood samples from a number of species is not questioned and has been documented by many after Maximow et al.’s original claims.42-45 However, their involvement in normal physiological healing of the skeleton is still unproven despite extensive research and the significance of the presence in blood is still considered to be unknown. ...
An autoradiographic study of periosteal cell proliferation with tritiated thymidine
0
1962
The periosteum. Autoradiographic studies on cellular proliferation and transformation utilizing tritiated thymidine
0
1963
Cell population kinetics of an osteogenic tissue. I
1
1963
... In the 1970s, Friedenstein’s work had come to the attention of Maureen Owen (Figure 2) in Oxford after she became interested in cell origins in the skeleton following a brief sojourn at the Brookhaven National Laboratory, New York when accompanying her husband who was on sabbatical from the University of Oxford. There Quastler and others were continuing to develop methods to study dynamic equilibria of cell populations by using the then newly created tritiated thymidine compound. This was administered to animals to trace proliferating cells by autoradiographic techniques.60, 61 Upon her return to Oxford in Dame Janet Vaughan’s ‘MRC Bone-seeking Isotopes Unit’, Owen et al.33, 34 applied this method to study bone cell kinetics in the rabbit periosteum. In subsequent years, Owen corresponded extensively with Friedenstein and after a number of abortive attempts he was able to visit Oxford in the early 1980s. With other leaders in the bone field he later attended a meeting held in Keble College under the auspices of the Bone and Tooth Society in honour of Maureen Owen’s retirement in 1993 (Figure 2). Their closer collaboration during this period resulted in widespread recognition of their concepts of the origins of bone-forming cells and to a number of significant joint publications regarding the osteogenic cell lineages derived from bone marrow.62-64 This lead to the idea that similar to bone marrow the connective tissue stroma of many organs throughout the body contained stem cells, stromal stem cells, that were presumed to differentiate into the mature stromal cell lines of the tissues from which they originated.65 This concept was embraced by many researchers and has led to investigations on the differentiation potentials of these stromal connective tissue cells obtained from a wide variety of tissues. These ideas were consolidated by the extensive work of Arnold Caplan on skeletal development that culminated in his proposal in 1991 for use of the term ‘mesenchymal stem cells’ (MSCs) for such cells.66 It also threw light on the known ability of connective tissue cells throughout the body that could be induced into bone formation by osteoinductive factors.67-70 These connective tissue cells had been previously designated as ‘inducible osteogenic precursor cells’ (IOPC) by Friedenstein,71 as opposed to the term ‘determined osteogenic precursor cells’ (DOPC), which are cells close to bone surfaces that are committed to osteogenesis without any inductive stimulus being required. ...
Cell population kinetics of an osteogenic tissue. II
1
1963
... In the 1970s, Friedenstein’s work had come to the attention of Maureen Owen (Figure 2) in Oxford after she became interested in cell origins in the skeleton following a brief sojourn at the Brookhaven National Laboratory, New York when accompanying her husband who was on sabbatical from the University of Oxford. There Quastler and others were continuing to develop methods to study dynamic equilibria of cell populations by using the then newly created tritiated thymidine compound. This was administered to animals to trace proliferating cells by autoradiographic techniques.60, 61 Upon her return to Oxford in Dame Janet Vaughan’s ‘MRC Bone-seeking Isotopes Unit’, Owen et al.33, 34 applied this method to study bone cell kinetics in the rabbit periosteum. In subsequent years, Owen corresponded extensively with Friedenstein and after a number of abortive attempts he was able to visit Oxford in the early 1980s. With other leaders in the bone field he later attended a meeting held in Keble College under the auspices of the Bone and Tooth Society in honour of Maureen Owen’s retirement in 1993 (Figure 2). Their closer collaboration during this period resulted in widespread recognition of their concepts of the origins of bone-forming cells and to a number of significant joint publications regarding the osteogenic cell lineages derived from bone marrow.62-64 This lead to the idea that similar to bone marrow the connective tissue stroma of many organs throughout the body contained stem cells, stromal stem cells, that were presumed to differentiate into the mature stromal cell lines of the tissues from which they originated.65 This concept was embraced by many researchers and has led to investigations on the differentiation potentials of these stromal connective tissue cells obtained from a wide variety of tissues. These ideas were consolidated by the extensive work of Arnold Caplan on skeletal development that culminated in his proposal in 1991 for use of the term ‘mesenchymal stem cells’ (MSCs) for such cells.66 It also threw light on the known ability of connective tissue cells throughout the body that could be induced into bone formation by osteoinductive factors.67-70 These connective tissue cells had been previously designated as ‘inducible osteogenic precursor cells’ (IOPC) by Friedenstein,71 as opposed to the term ‘determined osteogenic precursor cells’ (DOPC), which are cells close to bone surfaces that are committed to osteogenesis without any inductive stimulus being required. ...
The origin of bone cells
0
1970
Autoradiographic studies on cell proliferation and differentiation in bones of young rats injected with thymidine-H3
0
1962
Cell proliferation and specialization during endochondral osteogenesis in young rats
1
1962
... The progenitor cells of the osteoblast were observed to be locally-derived cells that were non-circulatory and residing close to all bone surfaces.29 This local origin was classically demonstrated by autoradiographic studies of cell proliferation in active tissue-forming areas as determined by tritiated thymidine uptake into the nuclei of proliferating cells.30-37 At centres of intramembranous and cartilaginous bone formation similar evidence of local supply of progenitor cells were shown to occur. The non-circulatory nature of osteogenic precursors in skeletal healing was indicated by parabiosis experiments, in which the blood circulatory systems of two individual animals are joined together. In these studies it was observed that, after a skeletal fracture was created in one individual parabiont, no bone matrix-synthesising osteoblast precursors were supplied through the circulation from radioactively-labelled cells of a tritiated thymidine-labelled animal to the regenerating fracture site in the unlabelled animal.38 More recently claims of support for the phenomenon of circulating osteogenic progenitors have been made and to some extent reviewed.39-41 But it is this author’s view that the supportive studies have alternative possible explanations for the results mentioned therein. It may be concluded that any contribution of such progenitors is unlikely to be of any direct relevance to skeletal regeneration and for any normal tissue reconstruction. That fibroblasts can be grown from blood samples from a number of species is not questioned and has been documented by many after Maximow et al.’s original claims.42-45 However, their involvement in normal physiological healing of the skeleton is still unproven despite extensive research and the significance of the presence in blood is still considered to be unknown. ...
On the histogenesis of the cells in fracture callus. Electron microscopic autoradiographic observations in parabiotic rats and studies on labeled monocytes
1
1973
... The progenitor cells of the osteoblast were observed to be locally-derived cells that were non-circulatory and residing close to all bone surfaces.29 This local origin was classically demonstrated by autoradiographic studies of cell proliferation in active tissue-forming areas as determined by tritiated thymidine uptake into the nuclei of proliferating cells.30-37 At centres of intramembranous and cartilaginous bone formation similar evidence of local supply of progenitor cells were shown to occur. The non-circulatory nature of osteogenic precursors in skeletal healing was indicated by parabiosis experiments, in which the blood circulatory systems of two individual animals are joined together. In these studies it was observed that, after a skeletal fracture was created in one individual parabiont, no bone matrix-synthesising osteoblast precursors were supplied through the circulation from radioactively-labelled cells of a tritiated thymidine-labelled animal to the regenerating fracture site in the unlabelled animal.38 More recently claims of support for the phenomenon of circulating osteogenic progenitors have been made and to some extent reviewed.39-41 But it is this author’s view that the supportive studies have alternative possible explanations for the results mentioned therein. It may be concluded that any contribution of such progenitors is unlikely to be of any direct relevance to skeletal regeneration and for any normal tissue reconstruction. That fibroblasts can be grown from blood samples from a number of species is not questioned and has been documented by many after Maximow et al.’s original claims.42-45 However, their involvement in normal physiological healing of the skeleton is still unproven despite extensive research and the significance of the presence in blood is still considered to be unknown. ...
Bone from blood: characteristics and clinical implications of circulating osteogenic progenitor (COP) cells
1
2021
... The progenitor cells of the osteoblast were observed to be locally-derived cells that were non-circulatory and residing close to all bone surfaces.29 This local origin was classically demonstrated by autoradiographic studies of cell proliferation in active tissue-forming areas as determined by tritiated thymidine uptake into the nuclei of proliferating cells.30-37 At centres of intramembranous and cartilaginous bone formation similar evidence of local supply of progenitor cells were shown to occur. The non-circulatory nature of osteogenic precursors in skeletal healing was indicated by parabiosis experiments, in which the blood circulatory systems of two individual animals are joined together. In these studies it was observed that, after a skeletal fracture was created in one individual parabiont, no bone matrix-synthesising osteoblast precursors were supplied through the circulation from radioactively-labelled cells of a tritiated thymidine-labelled animal to the regenerating fracture site in the unlabelled animal.38 More recently claims of support for the phenomenon of circulating osteogenic progenitors have been made and to some extent reviewed.39-41 But it is this author’s view that the supportive studies have alternative possible explanations for the results mentioned therein. It may be concluded that any contribution of such progenitors is unlikely to be of any direct relevance to skeletal regeneration and for any normal tissue reconstruction. That fibroblasts can be grown from blood samples from a number of species is not questioned and has been documented by many after Maximow et al.’s original claims.42-45 However, their involvement in normal physiological healing of the skeleton is still unproven despite extensive research and the significance of the presence in blood is still considered to be unknown. ...
Circulating osteogenic cells: implications for injury, repair, and regeneration
0
2011
Circulating osteogenic precursor cells: Building bone from blood
1
2019
... The progenitor cells of the osteoblast were observed to be locally-derived cells that were non-circulatory and residing close to all bone surfaces.29 This local origin was classically demonstrated by autoradiographic studies of cell proliferation in active tissue-forming areas as determined by tritiated thymidine uptake into the nuclei of proliferating cells.30-37 At centres of intramembranous and cartilaginous bone formation similar evidence of local supply of progenitor cells were shown to occur. The non-circulatory nature of osteogenic precursors in skeletal healing was indicated by parabiosis experiments, in which the blood circulatory systems of two individual animals are joined together. In these studies it was observed that, after a skeletal fracture was created in one individual parabiont, no bone matrix-synthesising osteoblast precursors were supplied through the circulation from radioactively-labelled cells of a tritiated thymidine-labelled animal to the regenerating fracture site in the unlabelled animal.38 More recently claims of support for the phenomenon of circulating osteogenic progenitors have been made and to some extent reviewed.39-41 But it is this author’s view that the supportive studies have alternative possible explanations for the results mentioned therein. It may be concluded that any contribution of such progenitors is unlikely to be of any direct relevance to skeletal regeneration and for any normal tissue reconstruction. That fibroblasts can be grown from blood samples from a number of species is not questioned and has been documented by many after Maximow et al.’s original claims.42-45 However, their involvement in normal physiological healing of the skeleton is still unproven despite extensive research and the significance of the presence in blood is still considered to be unknown. ...
Cultures of blood leucocytes. From lymphocyte and monocyte to connective tissue
1
1928
... The progenitor cells of the osteoblast were observed to be locally-derived cells that were non-circulatory and residing close to all bone surfaces.29 This local origin was classically demonstrated by autoradiographic studies of cell proliferation in active tissue-forming areas as determined by tritiated thymidine uptake into the nuclei of proliferating cells.30-37 At centres of intramembranous and cartilaginous bone formation similar evidence of local supply of progenitor cells were shown to occur. The non-circulatory nature of osteogenic precursors in skeletal healing was indicated by parabiosis experiments, in which the blood circulatory systems of two individual animals are joined together. In these studies it was observed that, after a skeletal fracture was created in one individual parabiont, no bone matrix-synthesising osteoblast precursors were supplied through the circulation from radioactively-labelled cells of a tritiated thymidine-labelled animal to the regenerating fracture site in the unlabelled animal.38 More recently claims of support for the phenomenon of circulating osteogenic progenitors have been made and to some extent reviewed.39-41 But it is this author’s view that the supportive studies have alternative possible explanations for the results mentioned therein. It may be concluded that any contribution of such progenitors is unlikely to be of any direct relevance to skeletal regeneration and for any normal tissue reconstruction. That fibroblasts can be grown from blood samples from a number of species is not questioned and has been documented by many after Maximow et al.’s original claims.42-45 However, their involvement in normal physiological healing of the skeleton is still unproven despite extensive research and the significance of the presence in blood is still considered to be unknown. ...
Fibroblast colony formation from monolayer cultures of blood cells
0
1971
Circulating connective tissue precursors: extreme rarity in humans and chondrogenic potential in guinea pigs
0
2007
Circulating skeletal stem cells
1
2001
... The progenitor cells of the osteoblast were observed to be locally-derived cells that were non-circulatory and residing close to all bone surfaces.29 This local origin was classically demonstrated by autoradiographic studies of cell proliferation in active tissue-forming areas as determined by tritiated thymidine uptake into the nuclei of proliferating cells.30-37 At centres of intramembranous and cartilaginous bone formation similar evidence of local supply of progenitor cells were shown to occur. The non-circulatory nature of osteogenic precursors in skeletal healing was indicated by parabiosis experiments, in which the blood circulatory systems of two individual animals are joined together. In these studies it was observed that, after a skeletal fracture was created in one individual parabiont, no bone matrix-synthesising osteoblast precursors were supplied through the circulation from radioactively-labelled cells of a tritiated thymidine-labelled animal to the regenerating fracture site in the unlabelled animal.38 More recently claims of support for the phenomenon of circulating osteogenic progenitors have been made and to some extent reviewed.39-41 But it is this author’s view that the supportive studies have alternative possible explanations for the results mentioned therein. It may be concluded that any contribution of such progenitors is unlikely to be of any direct relevance to skeletal regeneration and for any normal tissue reconstruction. That fibroblasts can be grown from blood samples from a number of species is not questioned and has been documented by many after Maximow et al.’s original claims.42-45 However, their involvement in normal physiological healing of the skeleton is still unproven despite extensive research and the significance of the presence in blood is still considered to be unknown. ...
Friedenstein, founder of the mesenchymal stem cell concept
2
2009
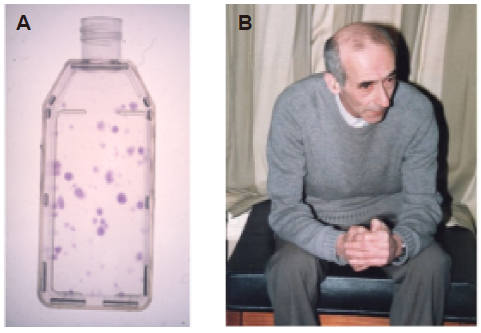
... The modern era that heralded the extensive work on the osteogenic stem cell system was initiated by the now classical work from the late 1960s onwards of Alexander Friedenstein (1924–1998) (Figures 1 and 2). Friedenstein was a Russian scientist46 who championed the fundamental ideas of his compatriot and aristocratic medical predecessor, Alexander Maximow (1874–1928). Maximow was a leader in his era of studies on bone marrow haematopoiesis together with the relationship of this process to the supporting connective tissue, the marrow stroma. In general terms ‘stroma’ refers to the supporting tissue of any organ that supports the active cells with specific functions, the parenchyma. From his careful histological observations he introduced the concept of marrow-resident haemopoietic stem cells, from which all cells in the blood were produced.47 Maximow’s scientific contributions however, were discredited in his own country upon his escape to the USA in 1922. Maximow proposed that in the bone marrow the development of the precursors of the blood cells, produced from haemopoetic stem cells, to be closely dependent on local factors from cells in the marrow stroma that supported them. These marrow stromal cells and the relationship to bone and cartilage formation were central features of Friedenstein’s life works.46 ...
... 46 ...
Relation of blood cells to connective tissues and endothelium
1
1924
... The modern era that heralded the extensive work on the osteogenic stem cell system was initiated by the now classical work from the late 1960s onwards of Alexander Friedenstein (1924–1998) (Figures 1 and 2). Friedenstein was a Russian scientist46 who championed the fundamental ideas of his compatriot and aristocratic medical predecessor, Alexander Maximow (1874–1928). Maximow was a leader in his era of studies on bone marrow haematopoiesis together with the relationship of this process to the supporting connective tissue, the marrow stroma. In general terms ‘stroma’ refers to the supporting tissue of any organ that supports the active cells with specific functions, the parenchyma. From his careful histological observations he introduced the concept of marrow-resident haemopoietic stem cells, from which all cells in the blood were produced.47 Maximow’s scientific contributions however, were discredited in his own country upon his escape to the USA in 1922. Maximow proposed that in the bone marrow the development of the precursors of the blood cells, produced from haemopoetic stem cells, to be closely dependent on local factors from cells in the marrow stroma that supported them. These marrow stromal cells and the relationship to bone and cartilage formation were central features of Friedenstein’s life works.46 ...
Prolonged hematopoiesis in a primate bone marrow culture system: characteristics of stem cell production and the hematopoietic microenvironment
1
1979
... Maximow’s views had led to the concept of a microenvironment being required in the bone marrow for haematopoiesis to occur and that the reticular cells, the stromal fibroblasts, in marrow were important contributors to this environment. These concepts were proven by later work showing that in vitro cultures of stromal underlayers established this environment48 and that reticular fibroblasts contributed importantly to this role in the maintenance of pluripotent stem cells and in the proliferation and differentiation of cells committed to the granulocyte-monocyte lineage.49 ...
Hemopoiesis on purified bone-marrow-derived reticular fibroblast in vitro
1
1986
... Maximow’s views had led to the concept of a microenvironment being required in the bone marrow for haematopoiesis to occur and that the reticular cells, the stromal fibroblasts, in marrow were important contributors to this environment. These concepts were proven by later work showing that in vitro cultures of stromal underlayers established this environment48 and that reticular fibroblasts contributed importantly to this role in the maintenance of pluripotent stem cells and in the proliferation and differentiation of cells committed to the granulocyte-monocyte lineage.49 ...
Hemopoietic stromal microenvironment
1
1983
... Furthermore transplantation of marrow cells to heterotopic sites was shown to produce a bony ossicle containing haemopoietic marrow, with the latter originating from cells of the recipient and with the stromal elements being derived from the donor.50-53 Marrow from these ossicles could be transplanted repeatedly into immunocompatible hosts to yield a similar result.54 Also if single-cell suspensions of bone marrow were plated in culture flasks at low density, individual colonies of fibroblasts were produced, the so called colony forming units fibroblastic (CFU-F) (Figure 1). Each of these CFU-F colonies was shown to derive from a single cell.55, 56 After many passages of cultures from these colonies, if these cells were implanted in vivo heterotopically, they retained the capability to produce a bone marrow ossicle. Similar cells derived from spleen did not however57, 58 and thus the marrow fibroblasts transferred the required haematopietic environment for blood formation, as Maximow had earlier suggested, as well as being cells that give rise to cartilage and bone tissues; that is they are skeletal osteogenic progenitors. ...
Marrow microenvironment transfer by heterotopic transplantation of freshly isolated and cultured cells in porous sponges
0
1982
Origin of bone marrow stromal mechanocytes in radiochimeras and heterotopic transplants
0
1978
Precursor cells of mechanocytes
1
1976
... Furthermore transplantation of marrow cells to heterotopic sites was shown to produce a bony ossicle containing haemopoietic marrow, with the latter originating from cells of the recipient and with the stromal elements being derived from the donor.50-53 Marrow from these ossicles could be transplanted repeatedly into immunocompatible hosts to yield a similar result.54 Also if single-cell suspensions of bone marrow were plated in culture flasks at low density, individual colonies of fibroblasts were produced, the so called colony forming units fibroblastic (CFU-F) (Figure 1). Each of these CFU-F colonies was shown to derive from a single cell.55, 56 After many passages of cultures from these colonies, if these cells were implanted in vivo heterotopically, they retained the capability to produce a bone marrow ossicle. Similar cells derived from spleen did not however57, 58 and thus the marrow fibroblasts transferred the required haematopietic environment for blood formation, as Maximow had earlier suggested, as well as being cells that give rise to cartilage and bone tissues; that is they are skeletal osteogenic progenitors. ...
Osteogenic precursor cells of bone marrow in radiation chimeras
1
1971
... Furthermore transplantation of marrow cells to heterotopic sites was shown to produce a bony ossicle containing haemopoietic marrow, with the latter originating from cells of the recipient and with the stromal elements being derived from the donor.50-53 Marrow from these ossicles could be transplanted repeatedly into immunocompatible hosts to yield a similar result.54 Also if single-cell suspensions of bone marrow were plated in culture flasks at low density, individual colonies of fibroblasts were produced, the so called colony forming units fibroblastic (CFU-F) (Figure 1). Each of these CFU-F colonies was shown to derive from a single cell.55, 56 After many passages of cultures from these colonies, if these cells were implanted in vivo heterotopically, they retained the capability to produce a bone marrow ossicle. Similar cells derived from spleen did not however57, 58 and thus the marrow fibroblasts transferred the required haematopietic environment for blood formation, as Maximow had earlier suggested, as well as being cells that give rise to cartilage and bone tissues; that is they are skeletal osteogenic progenitors. ...
The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells
1
1970
... Furthermore transplantation of marrow cells to heterotopic sites was shown to produce a bony ossicle containing haemopoietic marrow, with the latter originating from cells of the recipient and with the stromal elements being derived from the donor.50-53 Marrow from these ossicles could be transplanted repeatedly into immunocompatible hosts to yield a similar result.54 Also if single-cell suspensions of bone marrow were plated in culture flasks at low density, individual colonies of fibroblasts were produced, the so called colony forming units fibroblastic (CFU-F) (Figure 1). Each of these CFU-F colonies was shown to derive from a single cell.55, 56 After many passages of cultures from these colonies, if these cells were implanted in vivo heterotopically, they retained the capability to produce a bone marrow ossicle. Similar cells derived from spleen did not however57, 58 and thus the marrow fibroblasts transferred the required haematopietic environment for blood formation, as Maximow had earlier suggested, as well as being cells that give rise to cartilage and bone tissues; that is they are skeletal osteogenic progenitors. ...
Stromal mechanisms of bone marrow: cloning in vitro and retransplantation in vivo
1
1980
... Furthermore transplantation of marrow cells to heterotopic sites was shown to produce a bony ossicle containing haemopoietic marrow, with the latter originating from cells of the recipient and with the stromal elements being derived from the donor.50-53 Marrow from these ossicles could be transplanted repeatedly into immunocompatible hosts to yield a similar result.54 Also if single-cell suspensions of bone marrow were plated in culture flasks at low density, individual colonies of fibroblasts were produced, the so called colony forming units fibroblastic (CFU-F) (Figure 1). Each of these CFU-F colonies was shown to derive from a single cell.55, 56 After many passages of cultures from these colonies, if these cells were implanted in vivo heterotopically, they retained the capability to produce a bone marrow ossicle. Similar cells derived from spleen did not however57, 58 and thus the marrow fibroblasts transferred the required haematopietic environment for blood formation, as Maximow had earlier suggested, as well as being cells that give rise to cartilage and bone tissues; that is they are skeletal osteogenic progenitors. ...
Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo
1
1974
... Furthermore transplantation of marrow cells to heterotopic sites was shown to produce a bony ossicle containing haemopoietic marrow, with the latter originating from cells of the recipient and with the stromal elements being derived from the donor.50-53 Marrow from these ossicles could be transplanted repeatedly into immunocompatible hosts to yield a similar result.54 Also if single-cell suspensions of bone marrow were plated in culture flasks at low density, individual colonies of fibroblasts were produced, the so called colony forming units fibroblastic (CFU-F) (Figure 1). Each of these CFU-F colonies was shown to derive from a single cell.55, 56 After many passages of cultures from these colonies, if these cells were implanted in vivo heterotopically, they retained the capability to produce a bone marrow ossicle. Similar cells derived from spleen did not however57, 58 and thus the marrow fibroblasts transferred the required haematopietic environment for blood formation, as Maximow had earlier suggested, as well as being cells that give rise to cartilage and bone tissues; that is they are skeletal osteogenic progenitors. ...
Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method
1
1974
... Furthermore transplantation of marrow cells to heterotopic sites was shown to produce a bony ossicle containing haemopoietic marrow, with the latter originating from cells of the recipient and with the stromal elements being derived from the donor.50-53 Marrow from these ossicles could be transplanted repeatedly into immunocompatible hosts to yield a similar result.54 Also if single-cell suspensions of bone marrow were plated in culture flasks at low density, individual colonies of fibroblasts were produced, the so called colony forming units fibroblastic (CFU-F) (Figure 1). Each of these CFU-F colonies was shown to derive from a single cell.55, 56 After many passages of cultures from these colonies, if these cells were implanted in vivo heterotopically, they retained the capability to produce a bone marrow ossicle. Similar cells derived from spleen did not however57, 58 and thus the marrow fibroblasts transferred the required haematopietic environment for blood formation, as Maximow had earlier suggested, as well as being cells that give rise to cartilage and bone tissues; that is they are skeletal osteogenic progenitors. ...
Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers
2
1987
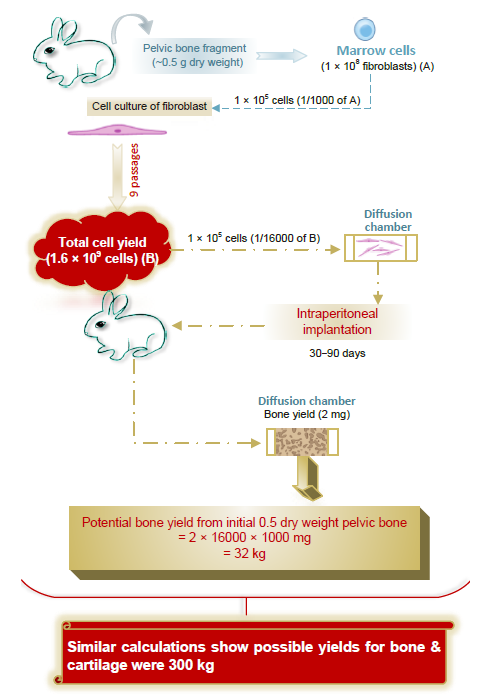
... Friedenstein showed in his extensive work that the cultured cells produced many growth factors and also demonstrated a great variety of characteristics in their resultant proliferation and differentiation. In particular, when grafted heterotopically only a small number of individual CFU-F colonies, around 10–15%, formed a bony ossicle with associated marrow. In in vitro culture around one-third of these individual CFU-F colonies, each derived from a single cell, were highly proliferative. After twenty to thirty cell doublings they still retained osteogenicity when assayed by culture within isolated diffusion chambers.59 Calculations by extrapolation of the proportional yields of osteogenic tissue from the pelvic marrow of young rabbits indicated that over 30 kg bone tissue or 300 kg of bone and cartilage tissue could potentially be produced from a small amount of marrow weighing just 0.5 g dry weight (Figure 3).59 This indicated extensive capacity for bone formation and expansion of osteogenic units and was the first real evidence that osteogenic stem cells existed in the bone marrow. ...
... 59 This indicated extensive capacity for bone formation and expansion of osteogenic units and was the first real evidence that osteogenic stem cells existed in the bone marrow. ...
Cell population kinetics in the intestinal epithelium of the mouse
1
1959
... In the 1970s, Friedenstein’s work had come to the attention of Maureen Owen (Figure 2) in Oxford after she became interested in cell origins in the skeleton following a brief sojourn at the Brookhaven National Laboratory, New York when accompanying her husband who was on sabbatical from the University of Oxford. There Quastler and others were continuing to develop methods to study dynamic equilibria of cell populations by using the then newly created tritiated thymidine compound. This was administered to animals to trace proliferating cells by autoradiographic techniques.60, 61 Upon her return to Oxford in Dame Janet Vaughan’s ‘MRC Bone-seeking Isotopes Unit’, Owen et al.33, 34 applied this method to study bone cell kinetics in the rabbit periosteum. In subsequent years, Owen corresponded extensively with Friedenstein and after a number of abortive attempts he was able to visit Oxford in the early 1980s. With other leaders in the bone field he later attended a meeting held in Keble College under the auspices of the Bone and Tooth Society in honour of Maureen Owen’s retirement in 1993 (Figure 2). Their closer collaboration during this period resulted in widespread recognition of their concepts of the origins of bone-forming cells and to a number of significant joint publications regarding the osteogenic cell lineages derived from bone marrow.62-64 This lead to the idea that similar to bone marrow the connective tissue stroma of many organs throughout the body contained stem cells, stromal stem cells, that were presumed to differentiate into the mature stromal cell lines of the tissues from which they originated.65 This concept was embraced by many researchers and has led to investigations on the differentiation potentials of these stromal connective tissue cells obtained from a wide variety of tissues. These ideas were consolidated by the extensive work of Arnold Caplan on skeletal development that culminated in his proposal in 1991 for use of the term ‘mesenchymal stem cells’ (MSCs) for such cells.66 It also threw light on the known ability of connective tissue cells throughout the body that could be induced into bone formation by osteoinductive factors.67-70 These connective tissue cells had been previously designated as ‘inducible osteogenic precursor cells’ (IOPC) by Friedenstein,71 as opposed to the term ‘determined osteogenic precursor cells’ (DOPC), which are cells close to bone surfaces that are committed to osteogenesis without any inductive stimulus being required. ...
Cellular proliferation in the mouse as revealed by autoradiography with tritiated thymidine
1
1958
... In the 1970s, Friedenstein’s work had come to the attention of Maureen Owen (Figure 2) in Oxford after she became interested in cell origins in the skeleton following a brief sojourn at the Brookhaven National Laboratory, New York when accompanying her husband who was on sabbatical from the University of Oxford. There Quastler and others were continuing to develop methods to study dynamic equilibria of cell populations by using the then newly created tritiated thymidine compound. This was administered to animals to trace proliferating cells by autoradiographic techniques.60, 61 Upon her return to Oxford in Dame Janet Vaughan’s ‘MRC Bone-seeking Isotopes Unit’, Owen et al.33, 34 applied this method to study bone cell kinetics in the rabbit periosteum. In subsequent years, Owen corresponded extensively with Friedenstein and after a number of abortive attempts he was able to visit Oxford in the early 1980s. With other leaders in the bone field he later attended a meeting held in Keble College under the auspices of the Bone and Tooth Society in honour of Maureen Owen’s retirement in 1993 (Figure 2). Their closer collaboration during this period resulted in widespread recognition of their concepts of the origins of bone-forming cells and to a number of significant joint publications regarding the osteogenic cell lineages derived from bone marrow.62-64 This lead to the idea that similar to bone marrow the connective tissue stroma of many organs throughout the body contained stem cells, stromal stem cells, that were presumed to differentiate into the mature stromal cell lines of the tissues from which they originated.65 This concept was embraced by many researchers and has led to investigations on the differentiation potentials of these stromal connective tissue cells obtained from a wide variety of tissues. These ideas were consolidated by the extensive work of Arnold Caplan on skeletal development that culminated in his proposal in 1991 for use of the term ‘mesenchymal stem cells’ (MSCs) for such cells.66 It also threw light on the known ability of connective tissue cells throughout the body that could be induced into bone formation by osteoinductive factors.67-70 These connective tissue cells had been previously designated as ‘inducible osteogenic precursor cells’ (IOPC) by Friedenstein,71 as opposed to the term ‘determined osteogenic precursor cells’ (DOPC), which are cells close to bone surfaces that are committed to osteogenesis without any inductive stimulus being required. ...
Bone formation in organ cultures of bone marrow
1
1987
... In the 1970s, Friedenstein’s work had come to the attention of Maureen Owen (Figure 2) in Oxford after she became interested in cell origins in the skeleton following a brief sojourn at the Brookhaven National Laboratory, New York when accompanying her husband who was on sabbatical from the University of Oxford. There Quastler and others were continuing to develop methods to study dynamic equilibria of cell populations by using the then newly created tritiated thymidine compound. This was administered to animals to trace proliferating cells by autoradiographic techniques.60, 61 Upon her return to Oxford in Dame Janet Vaughan’s ‘MRC Bone-seeking Isotopes Unit’, Owen et al.33, 34 applied this method to study bone cell kinetics in the rabbit periosteum. In subsequent years, Owen corresponded extensively with Friedenstein and after a number of abortive attempts he was able to visit Oxford in the early 1980s. With other leaders in the bone field he later attended a meeting held in Keble College under the auspices of the Bone and Tooth Society in honour of Maureen Owen’s retirement in 1993 (Figure 2). Their closer collaboration during this period resulted in widespread recognition of their concepts of the origins of bone-forming cells and to a number of significant joint publications regarding the osteogenic cell lineages derived from bone marrow.62-64 This lead to the idea that similar to bone marrow the connective tissue stroma of many organs throughout the body contained stem cells, stromal stem cells, that were presumed to differentiate into the mature stromal cell lines of the tissues from which they originated.65 This concept was embraced by many researchers and has led to investigations on the differentiation potentials of these stromal connective tissue cells obtained from a wide variety of tissues. These ideas were consolidated by the extensive work of Arnold Caplan on skeletal development that culminated in his proposal in 1991 for use of the term ‘mesenchymal stem cells’ (MSCs) for such cells.66 It also threw light on the known ability of connective tissue cells throughout the body that could be induced into bone formation by osteoinductive factors.67-70 These connective tissue cells had been previously designated as ‘inducible osteogenic precursor cells’ (IOPC) by Friedenstein,71 as opposed to the term ‘determined osteogenic precursor cells’ (DOPC), which are cells close to bone surfaces that are committed to osteogenesis without any inductive stimulus being required. ...
Bone formation in organ culture of marrow pieces
0
1986
Stromal stem cells: marrow-derived osteogenic precursors
1
1988
... In the 1970s, Friedenstein’s work had come to the attention of Maureen Owen (Figure 2) in Oxford after she became interested in cell origins in the skeleton following a brief sojourn at the Brookhaven National Laboratory, New York when accompanying her husband who was on sabbatical from the University of Oxford. There Quastler and others were continuing to develop methods to study dynamic equilibria of cell populations by using the then newly created tritiated thymidine compound. This was administered to animals to trace proliferating cells by autoradiographic techniques.60, 61 Upon her return to Oxford in Dame Janet Vaughan’s ‘MRC Bone-seeking Isotopes Unit’, Owen et al.33, 34 applied this method to study bone cell kinetics in the rabbit periosteum. In subsequent years, Owen corresponded extensively with Friedenstein and after a number of abortive attempts he was able to visit Oxford in the early 1980s. With other leaders in the bone field he later attended a meeting held in Keble College under the auspices of the Bone and Tooth Society in honour of Maureen Owen’s retirement in 1993 (Figure 2). Their closer collaboration during this period resulted in widespread recognition of their concepts of the origins of bone-forming cells and to a number of significant joint publications regarding the osteogenic cell lineages derived from bone marrow.62-64 This lead to the idea that similar to bone marrow the connective tissue stroma of many organs throughout the body contained stem cells, stromal stem cells, that were presumed to differentiate into the mature stromal cell lines of the tissues from which they originated.65 This concept was embraced by many researchers and has led to investigations on the differentiation potentials of these stromal connective tissue cells obtained from a wide variety of tissues. These ideas were consolidated by the extensive work of Arnold Caplan on skeletal development that culminated in his proposal in 1991 for use of the term ‘mesenchymal stem cells’ (MSCs) for such cells.66 It also threw light on the known ability of connective tissue cells throughout the body that could be induced into bone formation by osteoinductive factors.67-70 These connective tissue cells had been previously designated as ‘inducible osteogenic precursor cells’ (IOPC) by Friedenstein,71 as opposed to the term ‘determined osteogenic precursor cells’ (DOPC), which are cells close to bone surfaces that are committed to osteogenesis without any inductive stimulus being required. ...
Lineage of osteogenic cells and their relationship to the stromal system
1
1985
... In the 1970s, Friedenstein’s work had come to the attention of Maureen Owen (Figure 2) in Oxford after she became interested in cell origins in the skeleton following a brief sojourn at the Brookhaven National Laboratory, New York when accompanying her husband who was on sabbatical from the University of Oxford. There Quastler and others were continuing to develop methods to study dynamic equilibria of cell populations by using the then newly created tritiated thymidine compound. This was administered to animals to trace proliferating cells by autoradiographic techniques.60, 61 Upon her return to Oxford in Dame Janet Vaughan’s ‘MRC Bone-seeking Isotopes Unit’, Owen et al.33, 34 applied this method to study bone cell kinetics in the rabbit periosteum. In subsequent years, Owen corresponded extensively with Friedenstein and after a number of abortive attempts he was able to visit Oxford in the early 1980s. With other leaders in the bone field he later attended a meeting held in Keble College under the auspices of the Bone and Tooth Society in honour of Maureen Owen’s retirement in 1993 (Figure 2). Their closer collaboration during this period resulted in widespread recognition of their concepts of the origins of bone-forming cells and to a number of significant joint publications regarding the osteogenic cell lineages derived from bone marrow.62-64 This lead to the idea that similar to bone marrow the connective tissue stroma of many organs throughout the body contained stem cells, stromal stem cells, that were presumed to differentiate into the mature stromal cell lines of the tissues from which they originated.65 This concept was embraced by many researchers and has led to investigations on the differentiation potentials of these stromal connective tissue cells obtained from a wide variety of tissues. These ideas were consolidated by the extensive work of Arnold Caplan on skeletal development that culminated in his proposal in 1991 for use of the term ‘mesenchymal stem cells’ (MSCs) for such cells.66 It also threw light on the known ability of connective tissue cells throughout the body that could be induced into bone formation by osteoinductive factors.67-70 These connective tissue cells had been previously designated as ‘inducible osteogenic precursor cells’ (IOPC) by Friedenstein,71 as opposed to the term ‘determined osteogenic precursor cells’ (DOPC), which are cells close to bone surfaces that are committed to osteogenesis without any inductive stimulus being required. ...
Mesenchymal stem cells
1
1991
... In the 1970s, Friedenstein’s work had come to the attention of Maureen Owen (Figure 2) in Oxford after she became interested in cell origins in the skeleton following a brief sojourn at the Brookhaven National Laboratory, New York when accompanying her husband who was on sabbatical from the University of Oxford. There Quastler and others were continuing to develop methods to study dynamic equilibria of cell populations by using the then newly created tritiated thymidine compound. This was administered to animals to trace proliferating cells by autoradiographic techniques.60, 61 Upon her return to Oxford in Dame Janet Vaughan’s ‘MRC Bone-seeking Isotopes Unit’, Owen et al.33, 34 applied this method to study bone cell kinetics in the rabbit periosteum. In subsequent years, Owen corresponded extensively with Friedenstein and after a number of abortive attempts he was able to visit Oxford in the early 1980s. With other leaders in the bone field he later attended a meeting held in Keble College under the auspices of the Bone and Tooth Society in honour of Maureen Owen’s retirement in 1993 (Figure 2). Their closer collaboration during this period resulted in widespread recognition of their concepts of the origins of bone-forming cells and to a number of significant joint publications regarding the osteogenic cell lineages derived from bone marrow.62-64 This lead to the idea that similar to bone marrow the connective tissue stroma of many organs throughout the body contained stem cells, stromal stem cells, that were presumed to differentiate into the mature stromal cell lines of the tissues from which they originated.65 This concept was embraced by many researchers and has led to investigations on the differentiation potentials of these stromal connective tissue cells obtained from a wide variety of tissues. These ideas were consolidated by the extensive work of Arnold Caplan on skeletal development that culminated in his proposal in 1991 for use of the term ‘mesenchymal stem cells’ (MSCs) for such cells.66 It also threw light on the known ability of connective tissue cells throughout the body that could be induced into bone formation by osteoinductive factors.67-70 These connective tissue cells had been previously designated as ‘inducible osteogenic precursor cells’ (IOPC) by Friedenstein,71 as opposed to the term ‘determined osteogenic precursor cells’ (DOPC), which are cells close to bone surfaces that are committed to osteogenesis without any inductive stimulus being required. ...
Bone cell differentiation and growth factors
1
1983
... In the 1970s, Friedenstein’s work had come to the attention of Maureen Owen (Figure 2) in Oxford after she became interested in cell origins in the skeleton following a brief sojourn at the Brookhaven National Laboratory, New York when accompanying her husband who was on sabbatical from the University of Oxford. There Quastler and others were continuing to develop methods to study dynamic equilibria of cell populations by using the then newly created tritiated thymidine compound. This was administered to animals to trace proliferating cells by autoradiographic techniques.60, 61 Upon her return to Oxford in Dame Janet Vaughan’s ‘MRC Bone-seeking Isotopes Unit’, Owen et al.33, 34 applied this method to study bone cell kinetics in the rabbit periosteum. In subsequent years, Owen corresponded extensively with Friedenstein and after a number of abortive attempts he was able to visit Oxford in the early 1980s. With other leaders in the bone field he later attended a meeting held in Keble College under the auspices of the Bone and Tooth Society in honour of Maureen Owen’s retirement in 1993 (Figure 2). Their closer collaboration during this period resulted in widespread recognition of their concepts of the origins of bone-forming cells and to a number of significant joint publications regarding the osteogenic cell lineages derived from bone marrow.62-64 This lead to the idea that similar to bone marrow the connective tissue stroma of many organs throughout the body contained stem cells, stromal stem cells, that were presumed to differentiate into the mature stromal cell lines of the tissues from which they originated.65 This concept was embraced by many researchers and has led to investigations on the differentiation potentials of these stromal connective tissue cells obtained from a wide variety of tissues. These ideas were consolidated by the extensive work of Arnold Caplan on skeletal development that culminated in his proposal in 1991 for use of the term ‘mesenchymal stem cells’ (MSCs) for such cells.66 It also threw light on the known ability of connective tissue cells throughout the body that could be induced into bone formation by osteoinductive factors.67-70 These connective tissue cells had been previously designated as ‘inducible osteogenic precursor cells’ (IOPC) by Friedenstein,71 as opposed to the term ‘determined osteogenic precursor cells’ (DOPC), which are cells close to bone surfaces that are committed to osteogenesis without any inductive stimulus being required. ...
Bone: formation by autoinduction
0
1965
Growth factors influencing bone development
0
1990
Novel regulators of bone formation: molecular clones and activities
1
1988
... In the 1970s, Friedenstein’s work had come to the attention of Maureen Owen (Figure 2) in Oxford after she became interested in cell origins in the skeleton following a brief sojourn at the Brookhaven National Laboratory, New York when accompanying her husband who was on sabbatical from the University of Oxford. There Quastler and others were continuing to develop methods to study dynamic equilibria of cell populations by using the then newly created tritiated thymidine compound. This was administered to animals to trace proliferating cells by autoradiographic techniques.60, 61 Upon her return to Oxford in Dame Janet Vaughan’s ‘MRC Bone-seeking Isotopes Unit’, Owen et al.33, 34 applied this method to study bone cell kinetics in the rabbit periosteum. In subsequent years, Owen corresponded extensively with Friedenstein and after a number of abortive attempts he was able to visit Oxford in the early 1980s. With other leaders in the bone field he later attended a meeting held in Keble College under the auspices of the Bone and Tooth Society in honour of Maureen Owen’s retirement in 1993 (Figure 2). Their closer collaboration during this period resulted in widespread recognition of their concepts of the origins of bone-forming cells and to a number of significant joint publications regarding the osteogenic cell lineages derived from bone marrow.62-64 This lead to the idea that similar to bone marrow the connective tissue stroma of many organs throughout the body contained stem cells, stromal stem cells, that were presumed to differentiate into the mature stromal cell lines of the tissues from which they originated.65 This concept was embraced by many researchers and has led to investigations on the differentiation potentials of these stromal connective tissue cells obtained from a wide variety of tissues. These ideas were consolidated by the extensive work of Arnold Caplan on skeletal development that culminated in his proposal in 1991 for use of the term ‘mesenchymal stem cells’ (MSCs) for such cells.66 It also threw light on the known ability of connective tissue cells throughout the body that could be induced into bone formation by osteoinductive factors.67-70 These connective tissue cells had been previously designated as ‘inducible osteogenic precursor cells’ (IOPC) by Friedenstein,71 as opposed to the term ‘determined osteogenic precursor cells’ (DOPC), which are cells close to bone surfaces that are committed to osteogenesis without any inductive stimulus being required. ...
Determined and inducible osteogenic precursor cells
1
1973
... In the 1970s, Friedenstein’s work had come to the attention of Maureen Owen (Figure 2) in Oxford after she became interested in cell origins in the skeleton following a brief sojourn at the Brookhaven National Laboratory, New York when accompanying her husband who was on sabbatical from the University of Oxford. There Quastler and others were continuing to develop methods to study dynamic equilibria of cell populations by using the then newly created tritiated thymidine compound. This was administered to animals to trace proliferating cells by autoradiographic techniques.60, 61 Upon her return to Oxford in Dame Janet Vaughan’s ‘MRC Bone-seeking Isotopes Unit’, Owen et al.33, 34 applied this method to study bone cell kinetics in the rabbit periosteum. In subsequent years, Owen corresponded extensively with Friedenstein and after a number of abortive attempts he was able to visit Oxford in the early 1980s. With other leaders in the bone field he later attended a meeting held in Keble College under the auspices of the Bone and Tooth Society in honour of Maureen Owen’s retirement in 1993 (Figure 2). Their closer collaboration during this period resulted in widespread recognition of their concepts of the origins of bone-forming cells and to a number of significant joint publications regarding the osteogenic cell lineages derived from bone marrow.62-64 This lead to the idea that similar to bone marrow the connective tissue stroma of many organs throughout the body contained stem cells, stromal stem cells, that were presumed to differentiate into the mature stromal cell lines of the tissues from which they originated.65 This concept was embraced by many researchers and has led to investigations on the differentiation potentials of these stromal connective tissue cells obtained from a wide variety of tissues. These ideas were consolidated by the extensive work of Arnold Caplan on skeletal development that culminated in his proposal in 1991 for use of the term ‘mesenchymal stem cells’ (MSCs) for such cells.66 It also threw light on the known ability of connective tissue cells throughout the body that could be induced into bone formation by osteoinductive factors.67-70 These connective tissue cells had been previously designated as ‘inducible osteogenic precursor cells’ (IOPC) by Friedenstein,71 as opposed to the term ‘determined osteogenic precursor cells’ (DOPC), which are cells close to bone surfaces that are committed to osteogenesis without any inductive stimulus being required. ...
Mesenchymal stem cell perspective: cell biology to clinical progress
2
2019
... The easy growth in culture of these stromal fibroblastic cell lines from numerous tissue sources and isolation by their simple plastic substrate-adhesive properties lead to the explosion of interest from that time to the present day.72 However the original concepts of Owen, Friedenstein and others of stromal cell lineage progression put forward by analogy to the supply of blood cells by the haemopoietic stem cell seem to have be overlooked when cultures of these stromal cells were obtained. In the haemopoietic system the descendants of a potentially self-renewing stem cell are considered to pass through a continuum of cell progression to committed progenitors leading to fully differentiated end cells. The term “mesenchymal stem cells” however was applied to these integrated, heterogenous cultured stromal cell populations when obviously these contained infinitesimal numbers of stem cells, if any at all. That the culture may have been derived from a stem cell, or perhaps derived from an early progenitor therefrom, was feasible, but the majority of cells so produced more than likely represented a variety of more committed progenitors. Hence the blanket term ‘mesenchymal stem cells’ for such heterogenous cultures was inappropriate and erroneous at the time. ...
... As all cell populations are known to secrete bioactive factors, it is not surprising that this phenomenon would become apparent in the development of the commercial use of cultured stromal cell populations for cell therapy as part of tissue regenerative strategies. Indeed the microenvironment created by the marrow stroma is critical for normal haematopoieis. Also, fibroblastic cultures from skin were reported earlier to have some similar hemopoietic regulatory properties as those attributed to bone marrow-derived fibroblasts suggesting that even these are perhaps not unique to fibroblasts derived from haemopoietic tissues.76 Significant effects of MSCs on immune regulatory function in particular being recognised,77 has meant that many clinical trials have been conducted testing MSCs from a variety of sources to investigate any beneficial, therapeutic, immune modulation in inflammatory conditions and for tissue regeneration in a number of situations.72 ...
Stem cells and the philosopher’s stone
1
2002
... Possibly resulting from this attractive, evocative nomenclature these proliferative cell cultures were considered by many to represent tissue-retained, putative embryonic-like stem cells, with multipotency and the capacity to regenerate many tissues outwith the classical osteoblast-chondrocyte-adipocyte lineage of the marrow stromal fibroblastic cells. So for example skin, muscle, brain, intestine, heart and pancreatic tissue differentiation from MSCs were claimed to be possible in animals after parenteral administration of these cells for use in regenerative therapies. Although studied extensively over many years in in vitro culture studies in which cellular expression of biochemical cell markers of these tissue types were detected, none of these functional cell transformations have been proven to occur in vivo.73, 74 As all somatic cells retain genetic information it suggests that they can be manipulated artificially under appropriate conditions to activate transcription of a few non-lineage markers in cell populations, which may suggest possible transdifferentiation to another tissue type. Indeed, induced pluripotential stem cell technology that modifies chromatin has questioned whether such stem cells could be generated from differentiated somatic cells.75 But in vivo normal physiological environmental controls are supreme in maintaining and restricting phenotypic identity in the natural state. This restricts determination of cell fate and cell lineages are preserved. But this does not preclude that under diverse pathological conditions such controls may be altered. It is apparent that much more knowledge is required of the inductive mechanisms that may change cell lineage phenotypes. ...
Plasticity of adult stem cells
1
2004
... Possibly resulting from this attractive, evocative nomenclature these proliferative cell cultures were considered by many to represent tissue-retained, putative embryonic-like stem cells, with multipotency and the capacity to regenerate many tissues outwith the classical osteoblast-chondrocyte-adipocyte lineage of the marrow stromal fibroblastic cells. So for example skin, muscle, brain, intestine, heart and pancreatic tissue differentiation from MSCs were claimed to be possible in animals after parenteral administration of these cells for use in regenerative therapies. Although studied extensively over many years in in vitro culture studies in which cellular expression of biochemical cell markers of these tissue types were detected, none of these functional cell transformations have been proven to occur in vivo.73, 74 As all somatic cells retain genetic information it suggests that they can be manipulated artificially under appropriate conditions to activate transcription of a few non-lineage markers in cell populations, which may suggest possible transdifferentiation to another tissue type. Indeed, induced pluripotential stem cell technology that modifies chromatin has questioned whether such stem cells could be generated from differentiated somatic cells.75 But in vivo normal physiological environmental controls are supreme in maintaining and restricting phenotypic identity in the natural state. This restricts determination of cell fate and cell lineages are preserved. But this does not preclude that under diverse pathological conditions such controls may be altered. It is apparent that much more knowledge is required of the inductive mechanisms that may change cell lineage phenotypes. ...
Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors
1
2006
... Possibly resulting from this attractive, evocative nomenclature these proliferative cell cultures were considered by many to represent tissue-retained, putative embryonic-like stem cells, with multipotency and the capacity to regenerate many tissues outwith the classical osteoblast-chondrocyte-adipocyte lineage of the marrow stromal fibroblastic cells. So for example skin, muscle, brain, intestine, heart and pancreatic tissue differentiation from MSCs were claimed to be possible in animals after parenteral administration of these cells for use in regenerative therapies. Although studied extensively over many years in in vitro culture studies in which cellular expression of biochemical cell markers of these tissue types were detected, none of these functional cell transformations have been proven to occur in vivo.73, 74 As all somatic cells retain genetic information it suggests that they can be manipulated artificially under appropriate conditions to activate transcription of a few non-lineage markers in cell populations, which may suggest possible transdifferentiation to another tissue type. Indeed, induced pluripotential stem cell technology that modifies chromatin has questioned whether such stem cells could be generated from differentiated somatic cells.75 But in vivo normal physiological environmental controls are supreme in maintaining and restricting phenotypic identity in the natural state. This restricts determination of cell fate and cell lineages are preserved. But this does not preclude that under diverse pathological conditions such controls may be altered. It is apparent that much more knowledge is required of the inductive mechanisms that may change cell lineage phenotypes. ...
Hemopoiesis on skin-derived fibroblasts in vitro
1
1987
... As all cell populations are known to secrete bioactive factors, it is not surprising that this phenomenon would become apparent in the development of the commercial use of cultured stromal cell populations for cell therapy as part of tissue regenerative strategies. Indeed the microenvironment created by the marrow stroma is critical for normal haematopoieis. Also, fibroblastic cultures from skin were reported earlier to have some similar hemopoietic regulatory properties as those attributed to bone marrow-derived fibroblasts suggesting that even these are perhaps not unique to fibroblasts derived from haemopoietic tissues.76 Significant effects of MSCs on immune regulatory function in particular being recognised,77 has meant that many clinical trials have been conducted testing MSCs from a variety of sources to investigate any beneficial, therapeutic, immune modulation in inflammatory conditions and for tissue regeneration in a number of situations.72 ...
Immunoregulatory function of mesenchymal stem cells
1
2006
... As all cell populations are known to secrete bioactive factors, it is not surprising that this phenomenon would become apparent in the development of the commercial use of cultured stromal cell populations for cell therapy as part of tissue regenerative strategies. Indeed the microenvironment created by the marrow stroma is critical for normal haematopoieis. Also, fibroblastic cultures from skin were reported earlier to have some similar hemopoietic regulatory properties as those attributed to bone marrow-derived fibroblasts suggesting that even these are perhaps not unique to fibroblasts derived from haemopoietic tissues.76 Significant effects of MSCs on immune regulatory function in particular being recognised,77 has meant that many clinical trials have been conducted testing MSCs from a variety of sources to investigate any beneficial, therapeutic, immune modulation in inflammatory conditions and for tissue regeneration in a number of situations.72 ...
Mesenchymal stem cells: time to change the name!
1
2017
... Nevertheless, now because of perhaps harmful connotations in patient-commercial interactions, the erroneous term that identified all the cell populations produced as being stem cells has been proposed to be finally rescinded.78 The proposal has been put forward that the name of ‘mesenchymal stem cells’ be changed to ‘medicinal signalling cells’, as any MSC preparations when applied to a patient may supply bioactive factors that may stimulate regeneration of tissue by the resident stem cells. ...
1
2021
... Whilst it is agreed that marrow stromal fibroblasts cells have long known to secrete bioactive cell factors shown to affect immune cells, to a critical observer this change in terminology for MSCs may appear to be more for conservation of the acronym MSC than being of any selective nomenclature benefit. It is noted that as all cells secrete bioactive factors any could be considered potentially medicinal in particular situations. Importantly, none of these stromal cell preparations have been proven to have acceptable clinical healing qualities as medicines despite extensive clinical trials. This is evident as to date “the only stem cell products that are FDA-approved for use in the United States consist of blood-forming stem cells (also known as hematopoietic progenitor cells) that are derived from umbilical cord blood.”79 ...