Management of hyperglycemia in older adults with type 2 diabetes
1
2022
... With changes in modern lifestyles, the incidence of diabetes is gradually increasing, which brings a heavy economic burden to society and patients. Diabetes is a metabolic disease marked by hyperglycaemia, which causes angiogenic disorders in tissues, leading to complications such as diabetes–related cardiovascular disease, renal failure, amputation, and blindness.1,2 Hyperglycaemia in diabetic patients is an important cause of vascular dysfunction, and persistent hyperglycaemia causes cellular dysfunction by producing glycation end products, increasing oxidative stress levels, and causing mitochondrial dysfunction.3 Diabetic wounds are a frequent complication in diabetic patients. They are mainly chronic wounds caused by peripheral nerve damage and vascular dysfunction. Because they are prone to develop into local ulcers, wound gangrene and even require amputation, they cause patients enormous psychological and economic burdens. These chronic wounds have therefore become a difficult problem that clinicians need to solve urgently.4,5 Factors affecting the healing of diabetic wounds mainly include decreased proliferation and migration of fibroblasts, infection, persistent inflammatory response, and decreased angiogenesis.6,7 At present, the main goal of diabetic wound treatment is to restore blood supply to the wound and reduce ischaemia at the site.6,8 The current clinical wound treatment methods mainly include wound accessories, negative pressure drainage, skin grafting and surgical treatment.9,10 However, due to the many factors affecting the healing of diabetic wounds, there is currently no effective treatment to promote diabetic wound healing. ...
Vascular complication in adolescents with diabetes mellitus
1
2020
... With changes in modern lifestyles, the incidence of diabetes is gradually increasing, which brings a heavy economic burden to society and patients. Diabetes is a metabolic disease marked by hyperglycaemia, which causes angiogenic disorders in tissues, leading to complications such as diabetes–related cardiovascular disease, renal failure, amputation, and blindness.1,2 Hyperglycaemia in diabetic patients is an important cause of vascular dysfunction, and persistent hyperglycaemia causes cellular dysfunction by producing glycation end products, increasing oxidative stress levels, and causing mitochondrial dysfunction.3 Diabetic wounds are a frequent complication in diabetic patients. They are mainly chronic wounds caused by peripheral nerve damage and vascular dysfunction. Because they are prone to develop into local ulcers, wound gangrene and even require amputation, they cause patients enormous psychological and economic burdens. These chronic wounds have therefore become a difficult problem that clinicians need to solve urgently.4,5 Factors affecting the healing of diabetic wounds mainly include decreased proliferation and migration of fibroblasts, infection, persistent inflammatory response, and decreased angiogenesis.6,7 At present, the main goal of diabetic wound treatment is to restore blood supply to the wound and reduce ischaemia at the site.6,8 The current clinical wound treatment methods mainly include wound accessories, negative pressure drainage, skin grafting and surgical treatment.9,10 However, due to the many factors affecting the healing of diabetic wounds, there is currently no effective treatment to promote diabetic wound healing. ...
Regulation of endothelial progenitor cell functions during hyperglycemia: new therapeutic targets in diabetic wound healing
1
2022
... With changes in modern lifestyles, the incidence of diabetes is gradually increasing, which brings a heavy economic burden to society and patients. Diabetes is a metabolic disease marked by hyperglycaemia, which causes angiogenic disorders in tissues, leading to complications such as diabetes–related cardiovascular disease, renal failure, amputation, and blindness.1,2 Hyperglycaemia in diabetic patients is an important cause of vascular dysfunction, and persistent hyperglycaemia causes cellular dysfunction by producing glycation end products, increasing oxidative stress levels, and causing mitochondrial dysfunction.3 Diabetic wounds are a frequent complication in diabetic patients. They are mainly chronic wounds caused by peripheral nerve damage and vascular dysfunction. Because they are prone to develop into local ulcers, wound gangrene and even require amputation, they cause patients enormous psychological and economic burdens. These chronic wounds have therefore become a difficult problem that clinicians need to solve urgently.4,5 Factors affecting the healing of diabetic wounds mainly include decreased proliferation and migration of fibroblasts, infection, persistent inflammatory response, and decreased angiogenesis.6,7 At present, the main goal of diabetic wound treatment is to restore blood supply to the wound and reduce ischaemia at the site.6,8 The current clinical wound treatment methods mainly include wound accessories, negative pressure drainage, skin grafting and surgical treatment.9,10 However, due to the many factors affecting the healing of diabetic wounds, there is currently no effective treatment to promote diabetic wound healing. ...
Targeting epigenetic mechanisms in diabetic wound healing
1
2019
... With changes in modern lifestyles, the incidence of diabetes is gradually increasing, which brings a heavy economic burden to society and patients. Diabetes is a metabolic disease marked by hyperglycaemia, which causes angiogenic disorders in tissues, leading to complications such as diabetes–related cardiovascular disease, renal failure, amputation, and blindness.1,2 Hyperglycaemia in diabetic patients is an important cause of vascular dysfunction, and persistent hyperglycaemia causes cellular dysfunction by producing glycation end products, increasing oxidative stress levels, and causing mitochondrial dysfunction.3 Diabetic wounds are a frequent complication in diabetic patients. They are mainly chronic wounds caused by peripheral nerve damage and vascular dysfunction. Because they are prone to develop into local ulcers, wound gangrene and even require amputation, they cause patients enormous psychological and economic burdens. These chronic wounds have therefore become a difficult problem that clinicians need to solve urgently.4,5 Factors affecting the healing of diabetic wounds mainly include decreased proliferation and migration of fibroblasts, infection, persistent inflammatory response, and decreased angiogenesis.6,7 At present, the main goal of diabetic wound treatment is to restore blood supply to the wound and reduce ischaemia at the site.6,8 The current clinical wound treatment methods mainly include wound accessories, negative pressure drainage, skin grafting and surgical treatment.9,10 However, due to the many factors affecting the healing of diabetic wounds, there is currently no effective treatment to promote diabetic wound healing. ...
Recent advancements in biopolymer and metal nanoparticle-based materials in diabetic wound healing management
1
2019
... With changes in modern lifestyles, the incidence of diabetes is gradually increasing, which brings a heavy economic burden to society and patients. Diabetes is a metabolic disease marked by hyperglycaemia, which causes angiogenic disorders in tissues, leading to complications such as diabetes–related cardiovascular disease, renal failure, amputation, and blindness.1,2 Hyperglycaemia in diabetic patients is an important cause of vascular dysfunction, and persistent hyperglycaemia causes cellular dysfunction by producing glycation end products, increasing oxidative stress levels, and causing mitochondrial dysfunction.3 Diabetic wounds are a frequent complication in diabetic patients. They are mainly chronic wounds caused by peripheral nerve damage and vascular dysfunction. Because they are prone to develop into local ulcers, wound gangrene and even require amputation, they cause patients enormous psychological and economic burdens. These chronic wounds have therefore become a difficult problem that clinicians need to solve urgently.4,5 Factors affecting the healing of diabetic wounds mainly include decreased proliferation and migration of fibroblasts, infection, persistent inflammatory response, and decreased angiogenesis.6,7 At present, the main goal of diabetic wound treatment is to restore blood supply to the wound and reduce ischaemia at the site.6,8 The current clinical wound treatment methods mainly include wound accessories, negative pressure drainage, skin grafting and surgical treatment.9,10 However, due to the many factors affecting the healing of diabetic wounds, there is currently no effective treatment to promote diabetic wound healing. ...
A review of diabetic wound models-Novel insights into diabetic foot ulcer
2
2021
... With changes in modern lifestyles, the incidence of diabetes is gradually increasing, which brings a heavy economic burden to society and patients. Diabetes is a metabolic disease marked by hyperglycaemia, which causes angiogenic disorders in tissues, leading to complications such as diabetes–related cardiovascular disease, renal failure, amputation, and blindness.1,2 Hyperglycaemia in diabetic patients is an important cause of vascular dysfunction, and persistent hyperglycaemia causes cellular dysfunction by producing glycation end products, increasing oxidative stress levels, and causing mitochondrial dysfunction.3 Diabetic wounds are a frequent complication in diabetic patients. They are mainly chronic wounds caused by peripheral nerve damage and vascular dysfunction. Because they are prone to develop into local ulcers, wound gangrene and even require amputation, they cause patients enormous psychological and economic burdens. These chronic wounds have therefore become a difficult problem that clinicians need to solve urgently.4,5 Factors affecting the healing of diabetic wounds mainly include decreased proliferation and migration of fibroblasts, infection, persistent inflammatory response, and decreased angiogenesis.6,7 At present, the main goal of diabetic wound treatment is to restore blood supply to the wound and reduce ischaemia at the site.6,8 The current clinical wound treatment methods mainly include wound accessories, negative pressure drainage, skin grafting and surgical treatment.9,10 However, due to the many factors affecting the healing of diabetic wounds, there is currently no effective treatment to promote diabetic wound healing. ...
... 6,8 The current clinical wound treatment methods mainly include wound accessories, negative pressure drainage, skin grafting and surgical treatment.9,10 However, due to the many factors affecting the healing of diabetic wounds, there is currently no effective treatment to promote diabetic wound healing. ...
Cutaneous innervation in impaired diabetic wound healing
2
2021
... With changes in modern lifestyles, the incidence of diabetes is gradually increasing, which brings a heavy economic burden to society and patients. Diabetes is a metabolic disease marked by hyperglycaemia, which causes angiogenic disorders in tissues, leading to complications such as diabetes–related cardiovascular disease, renal failure, amputation, and blindness.1,2 Hyperglycaemia in diabetic patients is an important cause of vascular dysfunction, and persistent hyperglycaemia causes cellular dysfunction by producing glycation end products, increasing oxidative stress levels, and causing mitochondrial dysfunction.3 Diabetic wounds are a frequent complication in diabetic patients. They are mainly chronic wounds caused by peripheral nerve damage and vascular dysfunction. Because they are prone to develop into local ulcers, wound gangrene and even require amputation, they cause patients enormous psychological and economic burdens. These chronic wounds have therefore become a difficult problem that clinicians need to solve urgently.4,5 Factors affecting the healing of diabetic wounds mainly include decreased proliferation and migration of fibroblasts, infection, persistent inflammatory response, and decreased angiogenesis.6,7 At present, the main goal of diabetic wound treatment is to restore blood supply to the wound and reduce ischaemia at the site.6,8 The current clinical wound treatment methods mainly include wound accessories, negative pressure drainage, skin grafting and surgical treatment.9,10 However, due to the many factors affecting the healing of diabetic wounds, there is currently no effective treatment to promote diabetic wound healing. ...
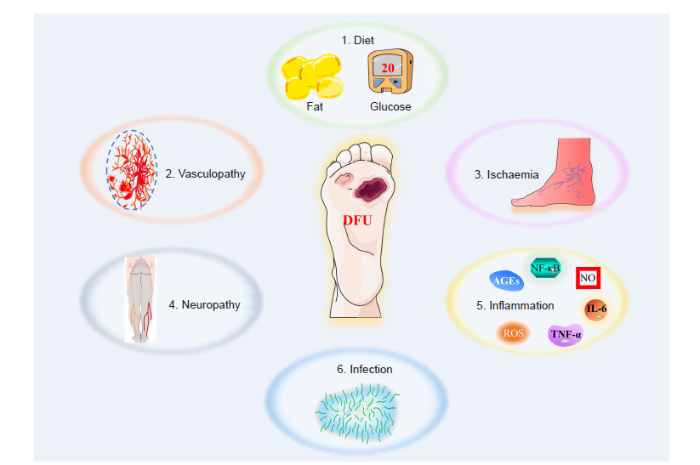
... Both sensory and autonomic nerves populate skin tissues. Cutaneous sensory nerves predominate and are widely distributed in the skin, including extending into the upper epidermis where they come into contact with the external environment. In contrast, autonomic nerves are distributed in the dermis of the skin and mainly regulate lymphatic function, blood circulation, and appendageal function.7 Nowadays, there is a growing appreciation that neuropathy is a major factor in impaired skin integrity in diabetes.63 Diabetic neuropathological changes preferentially damage sensory neurons, and the unmyelinated small–diameter sensory axons are especially susceptible.79 Evidence of sensory neuropathy is decreased or absent sense of vibration and superficial sensitivity including pressure, haptics, and subjective paresthesias. Consequently, the sensation of pain is substantially decreased and the risk of trauma is significantly higher. Due to the loss of pain sensation, serious ulcerations are underestimated and injuries are thus often not noticed in patients with diabetes mellitus.74 Autonomic nerve damage leads to arterial sclerosis, vasomotor paralysis, arteriovenous shunts in the microvascular network, dysfunctional sudomotor function, and neuropathic oedemas in damaged skin. As a result, affected skin in patients with diabetes mellitus has a reduced protective function and an increased risk of injury.77 The combination of these peripheral nerve problems leads to abnormal foot pressures in the form of an elevated plantar pressure load, accompanied by the development of hyperkeratosis and finally diabetic foot ulcers.80,81 ...
Wound healing and diabetes mellitus
1
2003
... With changes in modern lifestyles, the incidence of diabetes is gradually increasing, which brings a heavy economic burden to society and patients. Diabetes is a metabolic disease marked by hyperglycaemia, which causes angiogenic disorders in tissues, leading to complications such as diabetes–related cardiovascular disease, renal failure, amputation, and blindness.1,2 Hyperglycaemia in diabetic patients is an important cause of vascular dysfunction, and persistent hyperglycaemia causes cellular dysfunction by producing glycation end products, increasing oxidative stress levels, and causing mitochondrial dysfunction.3 Diabetic wounds are a frequent complication in diabetic patients. They are mainly chronic wounds caused by peripheral nerve damage and vascular dysfunction. Because they are prone to develop into local ulcers, wound gangrene and even require amputation, they cause patients enormous psychological and economic burdens. These chronic wounds have therefore become a difficult problem that clinicians need to solve urgently.4,5 Factors affecting the healing of diabetic wounds mainly include decreased proliferation and migration of fibroblasts, infection, persistent inflammatory response, and decreased angiogenesis.6,7 At present, the main goal of diabetic wound treatment is to restore blood supply to the wound and reduce ischaemia at the site.6,8 The current clinical wound treatment methods mainly include wound accessories, negative pressure drainage, skin grafting and surgical treatment.9,10 However, due to the many factors affecting the healing of diabetic wounds, there is currently no effective treatment to promote diabetic wound healing. ...
Effectiveness of honey dressing in the treatment of diabetic foot ulcers: A systematic review and meta-analysis
1
2019
... With changes in modern lifestyles, the incidence of diabetes is gradually increasing, which brings a heavy economic burden to society and patients. Diabetes is a metabolic disease marked by hyperglycaemia, which causes angiogenic disorders in tissues, leading to complications such as diabetes–related cardiovascular disease, renal failure, amputation, and blindness.1,2 Hyperglycaemia in diabetic patients is an important cause of vascular dysfunction, and persistent hyperglycaemia causes cellular dysfunction by producing glycation end products, increasing oxidative stress levels, and causing mitochondrial dysfunction.3 Diabetic wounds are a frequent complication in diabetic patients. They are mainly chronic wounds caused by peripheral nerve damage and vascular dysfunction. Because they are prone to develop into local ulcers, wound gangrene and even require amputation, they cause patients enormous psychological and economic burdens. These chronic wounds have therefore become a difficult problem that clinicians need to solve urgently.4,5 Factors affecting the healing of diabetic wounds mainly include decreased proliferation and migration of fibroblasts, infection, persistent inflammatory response, and decreased angiogenesis.6,7 At present, the main goal of diabetic wound treatment is to restore blood supply to the wound and reduce ischaemia at the site.6,8 The current clinical wound treatment methods mainly include wound accessories, negative pressure drainage, skin grafting and surgical treatment.9,10 However, due to the many factors affecting the healing of diabetic wounds, there is currently no effective treatment to promote diabetic wound healing. ...
Dysregulation of wound healing mechanisms in diabetes and the importance of negative pressure wound therapy (NPWT)
1
2017
... With changes in modern lifestyles, the incidence of diabetes is gradually increasing, which brings a heavy economic burden to society and patients. Diabetes is a metabolic disease marked by hyperglycaemia, which causes angiogenic disorders in tissues, leading to complications such as diabetes–related cardiovascular disease, renal failure, amputation, and blindness.1,2 Hyperglycaemia in diabetic patients is an important cause of vascular dysfunction, and persistent hyperglycaemia causes cellular dysfunction by producing glycation end products, increasing oxidative stress levels, and causing mitochondrial dysfunction.3 Diabetic wounds are a frequent complication in diabetic patients. They are mainly chronic wounds caused by peripheral nerve damage and vascular dysfunction. Because they are prone to develop into local ulcers, wound gangrene and even require amputation, they cause patients enormous psychological and economic burdens. These chronic wounds have therefore become a difficult problem that clinicians need to solve urgently.4,5 Factors affecting the healing of diabetic wounds mainly include decreased proliferation and migration of fibroblasts, infection, persistent inflammatory response, and decreased angiogenesis.6,7 At present, the main goal of diabetic wound treatment is to restore blood supply to the wound and reduce ischaemia at the site.6,8 The current clinical wound treatment methods mainly include wound accessories, negative pressure drainage, skin grafting and surgical treatment.9,10 However, due to the many factors affecting the healing of diabetic wounds, there is currently no effective treatment to promote diabetic wound healing. ...
Wound repair and regeneration
1
2008
... Wound repair is a complex process requiring activation of multiple biological signals and the coordination of cells that together regulate the wound repair process.11 After creation of a wound, the internal and external factors in the wound influence the repair process. As trauma research continues to develop, cutting–edge treatment modalities include stem cell therapy, gene therapy and tissue engineering.12,13 The normal process of wound healing can be classified into four stages, including haemostasis, inflammation, proliferation and remodelling.14⇓–16 The haemostatic phase is the period immediately following cutaneous trauma, when the body initiates the haemostatic process, with vasoconstriction and thrombus formation promoting blood clotting. The process involves a number of pro–vasoconstrictive factors such as thromboxane, epinephrine and complement. The inflammatory phase is a series of inflammatory reactions of the body to trauma. The major objective of this phase is to remove pathogenic bacteria and harmful tissue debris from the wounded area. In addition, in this phase, blood vessels will dilate and become more permeable. Chemotactic factors induce the accumulation of macrophages, neutrophils and other inflammatory cells in the wound and secretion of cytokines, promoting the production of granulation tissue. The proliferation phase is the stage of cell proliferation and differentiation, where fibroblasts in the wound migrate and proliferate. Extensive extracellular matrix is synthesised and collagen is produced. The proliferative phase is the stage of cell migration, proliferation, extracellular matrix synthesis, collagen production, inflammatory cell release, cell migration and granulation tissue formation creating new vessels. The remodelling phase is the dynamic balance between tissue regeneration and fibrosis. Fibroblasts partially differentiate into myofibroblasts to contract the wound, while the deposited collagen fibres are reconstituted into regularly–arranged and mature fibres on the basis of glycosaminoglycans and proteoglycans. The granulation tissue gradually degrades into mature, non–vascular, non–cellular scar tissue.17⇓–19 Therefore, in this review, we summarise the different animal experimental models for preclinical evaluation of diabetic wounds and further discuss the promising future of hydrogel biomaterials in diabetic wound healing. ...
Skin tissue engineering: wound healing based on stem-cell-based therapeutic strategies
1
2019
... Wound repair is a complex process requiring activation of multiple biological signals and the coordination of cells that together regulate the wound repair process.11 After creation of a wound, the internal and external factors in the wound influence the repair process. As trauma research continues to develop, cutting–edge treatment modalities include stem cell therapy, gene therapy and tissue engineering.12,13 The normal process of wound healing can be classified into four stages, including haemostasis, inflammation, proliferation and remodelling.14⇓–16 The haemostatic phase is the period immediately following cutaneous trauma, when the body initiates the haemostatic process, with vasoconstriction and thrombus formation promoting blood clotting. The process involves a number of pro–vasoconstrictive factors such as thromboxane, epinephrine and complement. The inflammatory phase is a series of inflammatory reactions of the body to trauma. The major objective of this phase is to remove pathogenic bacteria and harmful tissue debris from the wounded area. In addition, in this phase, blood vessels will dilate and become more permeable. Chemotactic factors induce the accumulation of macrophages, neutrophils and other inflammatory cells in the wound and secretion of cytokines, promoting the production of granulation tissue. The proliferation phase is the stage of cell proliferation and differentiation, where fibroblasts in the wound migrate and proliferate. Extensive extracellular matrix is synthesised and collagen is produced. The proliferative phase is the stage of cell migration, proliferation, extracellular matrix synthesis, collagen production, inflammatory cell release, cell migration and granulation tissue formation creating new vessels. The remodelling phase is the dynamic balance between tissue regeneration and fibrosis. Fibroblasts partially differentiate into myofibroblasts to contract the wound, while the deposited collagen fibres are reconstituted into regularly–arranged and mature fibres on the basis of glycosaminoglycans and proteoglycans. The granulation tissue gradually degrades into mature, non–vascular, non–cellular scar tissue.17⇓–19 Therefore, in this review, we summarise the different animal experimental models for preclinical evaluation of diabetic wounds and further discuss the promising future of hydrogel biomaterials in diabetic wound healing. ...
Stem cell dynamics, migration and plasticity during wound healing
1
2019
... Wound repair is a complex process requiring activation of multiple biological signals and the coordination of cells that together regulate the wound repair process.11 After creation of a wound, the internal and external factors in the wound influence the repair process. As trauma research continues to develop, cutting–edge treatment modalities include stem cell therapy, gene therapy and tissue engineering.12,13 The normal process of wound healing can be classified into four stages, including haemostasis, inflammation, proliferation and remodelling.14⇓–16 The haemostatic phase is the period immediately following cutaneous trauma, when the body initiates the haemostatic process, with vasoconstriction and thrombus formation promoting blood clotting. The process involves a number of pro–vasoconstrictive factors such as thromboxane, epinephrine and complement. The inflammatory phase is a series of inflammatory reactions of the body to trauma. The major objective of this phase is to remove pathogenic bacteria and harmful tissue debris from the wounded area. In addition, in this phase, blood vessels will dilate and become more permeable. Chemotactic factors induce the accumulation of macrophages, neutrophils and other inflammatory cells in the wound and secretion of cytokines, promoting the production of granulation tissue. The proliferation phase is the stage of cell proliferation and differentiation, where fibroblasts in the wound migrate and proliferate. Extensive extracellular matrix is synthesised and collagen is produced. The proliferative phase is the stage of cell migration, proliferation, extracellular matrix synthesis, collagen production, inflammatory cell release, cell migration and granulation tissue formation creating new vessels. The remodelling phase is the dynamic balance between tissue regeneration and fibrosis. Fibroblasts partially differentiate into myofibroblasts to contract the wound, while the deposited collagen fibres are reconstituted into regularly–arranged and mature fibres on the basis of glycosaminoglycans and proteoglycans. The granulation tissue gradually degrades into mature, non–vascular, non–cellular scar tissue.17⇓–19 Therefore, in this review, we summarise the different animal experimental models for preclinical evaluation of diabetic wounds and further discuss the promising future of hydrogel biomaterials in diabetic wound healing. ...
The biological processes during wound healing
1
2021
... Wound repair is a complex process requiring activation of multiple biological signals and the coordination of cells that together regulate the wound repair process.11 After creation of a wound, the internal and external factors in the wound influence the repair process. As trauma research continues to develop, cutting–edge treatment modalities include stem cell therapy, gene therapy and tissue engineering.12,13 The normal process of wound healing can be classified into four stages, including haemostasis, inflammation, proliferation and remodelling.14⇓–16 The haemostatic phase is the period immediately following cutaneous trauma, when the body initiates the haemostatic process, with vasoconstriction and thrombus formation promoting blood clotting. The process involves a number of pro–vasoconstrictive factors such as thromboxane, epinephrine and complement. The inflammatory phase is a series of inflammatory reactions of the body to trauma. The major objective of this phase is to remove pathogenic bacteria and harmful tissue debris from the wounded area. In addition, in this phase, blood vessels will dilate and become more permeable. Chemotactic factors induce the accumulation of macrophages, neutrophils and other inflammatory cells in the wound and secretion of cytokines, promoting the production of granulation tissue. The proliferation phase is the stage of cell proliferation and differentiation, where fibroblasts in the wound migrate and proliferate. Extensive extracellular matrix is synthesised and collagen is produced. The proliferative phase is the stage of cell migration, proliferation, extracellular matrix synthesis, collagen production, inflammatory cell release, cell migration and granulation tissue formation creating new vessels. The remodelling phase is the dynamic balance between tissue regeneration and fibrosis. Fibroblasts partially differentiate into myofibroblasts to contract the wound, while the deposited collagen fibres are reconstituted into regularly–arranged and mature fibres on the basis of glycosaminoglycans and proteoglycans. The granulation tissue gradually degrades into mature, non–vascular, non–cellular scar tissue.17⇓–19 Therefore, in this review, we summarise the different animal experimental models for preclinical evaluation of diabetic wounds and further discuss the promising future of hydrogel biomaterials in diabetic wound healing. ...
Recent advances on the development of wound dressings for diabetic foot ulcer treatment--a review
1
2013
... Wound repair is a complex process requiring activation of multiple biological signals and the coordination of cells that together regulate the wound repair process.11 After creation of a wound, the internal and external factors in the wound influence the repair process. As trauma research continues to develop, cutting–edge treatment modalities include stem cell therapy, gene therapy and tissue engineering.12,13 The normal process of wound healing can be classified into four stages, including haemostasis, inflammation, proliferation and remodelling.14⇓–16 The haemostatic phase is the period immediately following cutaneous trauma, when the body initiates the haemostatic process, with vasoconstriction and thrombus formation promoting blood clotting. The process involves a number of pro–vasoconstrictive factors such as thromboxane, epinephrine and complement. The inflammatory phase is a series of inflammatory reactions of the body to trauma. The major objective of this phase is to remove pathogenic bacteria and harmful tissue debris from the wounded area. In addition, in this phase, blood vessels will dilate and become more permeable. Chemotactic factors induce the accumulation of macrophages, neutrophils and other inflammatory cells in the wound and secretion of cytokines, promoting the production of granulation tissue. The proliferation phase is the stage of cell proliferation and differentiation, where fibroblasts in the wound migrate and proliferate. Extensive extracellular matrix is synthesised and collagen is produced. The proliferative phase is the stage of cell migration, proliferation, extracellular matrix synthesis, collagen production, inflammatory cell release, cell migration and granulation tissue formation creating new vessels. The remodelling phase is the dynamic balance between tissue regeneration and fibrosis. Fibroblasts partially differentiate into myofibroblasts to contract the wound, while the deposited collagen fibres are reconstituted into regularly–arranged and mature fibres on the basis of glycosaminoglycans and proteoglycans. The granulation tissue gradually degrades into mature, non–vascular, non–cellular scar tissue.17⇓–19 Therefore, in this review, we summarise the different animal experimental models for preclinical evaluation of diabetic wounds and further discuss the promising future of hydrogel biomaterials in diabetic wound healing. ...
Wound healing: an overview
1
2006
... Wound repair is a complex process requiring activation of multiple biological signals and the coordination of cells that together regulate the wound repair process.11 After creation of a wound, the internal and external factors in the wound influence the repair process. As trauma research continues to develop, cutting–edge treatment modalities include stem cell therapy, gene therapy and tissue engineering.12,13 The normal process of wound healing can be classified into four stages, including haemostasis, inflammation, proliferation and remodelling.14⇓–16 The haemostatic phase is the period immediately following cutaneous trauma, when the body initiates the haemostatic process, with vasoconstriction and thrombus formation promoting blood clotting. The process involves a number of pro–vasoconstrictive factors such as thromboxane, epinephrine and complement. The inflammatory phase is a series of inflammatory reactions of the body to trauma. The major objective of this phase is to remove pathogenic bacteria and harmful tissue debris from the wounded area. In addition, in this phase, blood vessels will dilate and become more permeable. Chemotactic factors induce the accumulation of macrophages, neutrophils and other inflammatory cells in the wound and secretion of cytokines, promoting the production of granulation tissue. The proliferation phase is the stage of cell proliferation and differentiation, where fibroblasts in the wound migrate and proliferate. Extensive extracellular matrix is synthesised and collagen is produced. The proliferative phase is the stage of cell migration, proliferation, extracellular matrix synthesis, collagen production, inflammatory cell release, cell migration and granulation tissue formation creating new vessels. The remodelling phase is the dynamic balance between tissue regeneration and fibrosis. Fibroblasts partially differentiate into myofibroblasts to contract the wound, while the deposited collagen fibres are reconstituted into regularly–arranged and mature fibres on the basis of glycosaminoglycans and proteoglycans. The granulation tissue gradually degrades into mature, non–vascular, non–cellular scar tissue.17⇓–19 Therefore, in this review, we summarise the different animal experimental models for preclinical evaluation of diabetic wounds and further discuss the promising future of hydrogel biomaterials in diabetic wound healing. ...
Epidermal stem cells in wound healing and their clinical applications
1
2019
... Wound repair is a complex process requiring activation of multiple biological signals and the coordination of cells that together regulate the wound repair process.11 After creation of a wound, the internal and external factors in the wound influence the repair process. As trauma research continues to develop, cutting–edge treatment modalities include stem cell therapy, gene therapy and tissue engineering.12,13 The normal process of wound healing can be classified into four stages, including haemostasis, inflammation, proliferation and remodelling.14⇓–16 The haemostatic phase is the period immediately following cutaneous trauma, when the body initiates the haemostatic process, with vasoconstriction and thrombus formation promoting blood clotting. The process involves a number of pro–vasoconstrictive factors such as thromboxane, epinephrine and complement. The inflammatory phase is a series of inflammatory reactions of the body to trauma. The major objective of this phase is to remove pathogenic bacteria and harmful tissue debris from the wounded area. In addition, in this phase, blood vessels will dilate and become more permeable. Chemotactic factors induce the accumulation of macrophages, neutrophils and other inflammatory cells in the wound and secretion of cytokines, promoting the production of granulation tissue. The proliferation phase is the stage of cell proliferation and differentiation, where fibroblasts in the wound migrate and proliferate. Extensive extracellular matrix is synthesised and collagen is produced. The proliferative phase is the stage of cell migration, proliferation, extracellular matrix synthesis, collagen production, inflammatory cell release, cell migration and granulation tissue formation creating new vessels. The remodelling phase is the dynamic balance between tissue regeneration and fibrosis. Fibroblasts partially differentiate into myofibroblasts to contract the wound, while the deposited collagen fibres are reconstituted into regularly–arranged and mature fibres on the basis of glycosaminoglycans and proteoglycans. The granulation tissue gradually degrades into mature, non–vascular, non–cellular scar tissue.17⇓–19 Therefore, in this review, we summarise the different animal experimental models for preclinical evaluation of diabetic wounds and further discuss the promising future of hydrogel biomaterials in diabetic wound healing. ...
Chronic wound healing: a review of current management and treatments
1
2017
... Wound repair is a complex process requiring activation of multiple biological signals and the coordination of cells that together regulate the wound repair process.11 After creation of a wound, the internal and external factors in the wound influence the repair process. As trauma research continues to develop, cutting–edge treatment modalities include stem cell therapy, gene therapy and tissue engineering.12,13 The normal process of wound healing can be classified into four stages, including haemostasis, inflammation, proliferation and remodelling.14⇓–16 The haemostatic phase is the period immediately following cutaneous trauma, when the body initiates the haemostatic process, with vasoconstriction and thrombus formation promoting blood clotting. The process involves a number of pro–vasoconstrictive factors such as thromboxane, epinephrine and complement. The inflammatory phase is a series of inflammatory reactions of the body to trauma. The major objective of this phase is to remove pathogenic bacteria and harmful tissue debris from the wounded area. In addition, in this phase, blood vessels will dilate and become more permeable. Chemotactic factors induce the accumulation of macrophages, neutrophils and other inflammatory cells in the wound and secretion of cytokines, promoting the production of granulation tissue. The proliferation phase is the stage of cell proliferation and differentiation, where fibroblasts in the wound migrate and proliferate. Extensive extracellular matrix is synthesised and collagen is produced. The proliferative phase is the stage of cell migration, proliferation, extracellular matrix synthesis, collagen production, inflammatory cell release, cell migration and granulation tissue formation creating new vessels. The remodelling phase is the dynamic balance between tissue regeneration and fibrosis. Fibroblasts partially differentiate into myofibroblasts to contract the wound, while the deposited collagen fibres are reconstituted into regularly–arranged and mature fibres on the basis of glycosaminoglycans and proteoglycans. The granulation tissue gradually degrades into mature, non–vascular, non–cellular scar tissue.17⇓–19 Therefore, in this review, we summarise the different animal experimental models for preclinical evaluation of diabetic wounds and further discuss the promising future of hydrogel biomaterials in diabetic wound healing. ...
The exosome - a naturally secreted nanoparticle and its application to wound healing
1
2016
... Wound repair is a complex process requiring activation of multiple biological signals and the coordination of cells that together regulate the wound repair process.11 After creation of a wound, the internal and external factors in the wound influence the repair process. As trauma research continues to develop, cutting–edge treatment modalities include stem cell therapy, gene therapy and tissue engineering.12,13 The normal process of wound healing can be classified into four stages, including haemostasis, inflammation, proliferation and remodelling.14⇓–16 The haemostatic phase is the period immediately following cutaneous trauma, when the body initiates the haemostatic process, with vasoconstriction and thrombus formation promoting blood clotting. The process involves a number of pro–vasoconstrictive factors such as thromboxane, epinephrine and complement. The inflammatory phase is a series of inflammatory reactions of the body to trauma. The major objective of this phase is to remove pathogenic bacteria and harmful tissue debris from the wounded area. In addition, in this phase, blood vessels will dilate and become more permeable. Chemotactic factors induce the accumulation of macrophages, neutrophils and other inflammatory cells in the wound and secretion of cytokines, promoting the production of granulation tissue. The proliferation phase is the stage of cell proliferation and differentiation, where fibroblasts in the wound migrate and proliferate. Extensive extracellular matrix is synthesised and collagen is produced. The proliferative phase is the stage of cell migration, proliferation, extracellular matrix synthesis, collagen production, inflammatory cell release, cell migration and granulation tissue formation creating new vessels. The remodelling phase is the dynamic balance between tissue regeneration and fibrosis. Fibroblasts partially differentiate into myofibroblasts to contract the wound, while the deposited collagen fibres are reconstituted into regularly–arranged and mature fibres on the basis of glycosaminoglycans and proteoglycans. The granulation tissue gradually degrades into mature, non–vascular, non–cellular scar tissue.17⇓–19 Therefore, in this review, we summarise the different animal experimental models for preclinical evaluation of diabetic wounds and further discuss the promising future of hydrogel biomaterials in diabetic wound healing. ...
Clinically relevant experimental rodent models of diabetic foot ulcer
4
2022
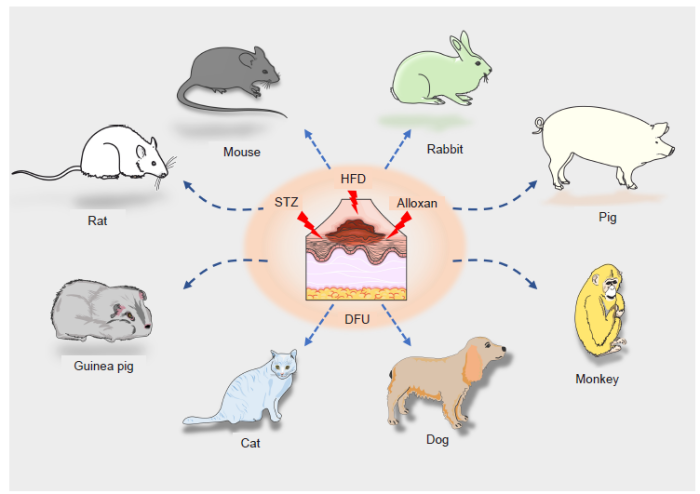
... Although no animal model fully simulates the pathophysiological mechanisms of human diabetic wounds, the exploration of using animal models to simulate the disease in preclinical studies and basic research is of utmost importance. In an animal, diabetes can be induced by different mechanisms including viral, genetic and chemical induction, and spontaneous autoimmune hyperglycemia.20 Streptozotocin (STZ) and alloxan are the most widely–used agents in chemical diabetic induction. It has been reported that STZ induces disease characteristics and similar responses in different animal models. Species including mice, rabbits, guinea pigs, monkeys, dogs and cats have been reported to be successfully used for the induction of diabetes by STZ, as indicated by the clinical features and pathological changes that resemble those of human diabetes (Figure 1).21 A vast array of in vivo experiments has demonstrated that STZ–induced diabetes and the subsequent changes constitute a reliable animal model.22,23 Goyal et al.21 and Szkudelski24 systematically summarised the mechanisms that caused diabetogenic changes following induction by the chemical STZ. These diabetogenic mechanisms included reactive oxygen species (ROS) generation, DNA damage, oxidative stress and glucose overloading, thereby causing pancreatic β cell damage.21,24 The diabetogenic action is characterised by selective death of β cells, insulin dysfunction, hyperglycaemia, and symptoms of polyuria, polydipsia and weight loss, which resemble human signs of diabetes mellitus. As for alloxan, it generates ROS and generates superoxide radicals and hydroxyl radicals. These hydroxyl radicals ultimately contribute to the death of β cells. As a thiol reagent, alloxan also selectively inhibits high glucose–induced insulin secretion by inhibiting the β cell glucose sensor glucokinase. The most significant disadvantage of using chemicals such as STZ and alloxan is perhaps the chemical toxicity to organs of the body.20 However, they have been widely used in various animal models. In this review, we focus on the animal models of diabetic wound healing involving rats, mice, rabbits, and pigs. ...
... 20 However, they have been widely used in various animal models. In this review, we focus on the animal models of diabetic wound healing involving rats, mice, rabbits, and pigs. ...
... Rabbits were also monitored for clinical signs including changes in behaviour, activity, diet, urination/defecation and blood glucose levels.46,47 The rabbit ear model of diabetic wounds overcomes the contraction issues and has the advantage of the ability to create multiple wounds.20 Zhang et al.48 studied T2D models using New Zealand rabbits. Rabbits were first fed a HFD for 2 months. At the end of this period, the rabbits were injected every 3 days with alloxan monohydrate (50 mg/kg) via the ear following 6 hours of fasting. Rabbits with fasting blood glucose level > 11.1 mM were then used in the experiment. After the induction of T2D, a 10 mm × 10 mm full–thickness section of skin was excised and a radial debridement of the wound was conducted. The wounds in the treatment group were treated with epidermal growth factor (EGF) once a day for 1 month then compared with the control group. In the treatment group, endogenous EGF mRNA was significantly higher than in the control group, which indicated that exogenous EGF treatment accelerated the healing of diabetic wounds by upregulating the expression of EGF mRNA in newly–generated tissues. In another alloxan–induced diabetic rabbit ear ulcer model,49 alloxan at 150 mg/kg in 30 mL of saline was administered intravenously via an ear vein. After drug induction, glucose and molasses were provided for 24 hours to avoid hypoglycaemia. Blood glucose levels were checked weekly using blood from the marginal ear vein after the blood sugar level had stabilised. Four weeks after alloxan treatment, rabbits were anaesthetised and full–thickness punch biopsy wounds with a diameter of 6 mm were created on each of the hyperglycaemic rabbits. They found that autologous circulating angiogenic cells treated with osteopontin could promote diabetic wounds healing. By using the same diabetic models with alloxan, collagen seeded with allogeneic mesenchymal stem cells resulted in increased angiogenesis and accelerated diabetic wound healing.50 ...
... Pigs also have the advantage of a similar skin wound healing process to humans. The courses of re–epithelialisation and granulation tissue formation closely resemble those in humans.20 Pig diabetic ulcer models have been successfully established.51,52 The chemical drug STZ was also used to cause systematic hyperglycaemia in pigs at a dose of 150 mg/kg body weight, then serum glucose concentration was measured, and short–acting and long–acting insulin was used to keep the glucose level between 350 and 550 mg/dL for 14 days. After induction, the full thickness of pig was created. By detecting glucose concentration in serum and wound fluid, they found that delayed wound healing in diabetic pigs is not induced by local high glucose concentration. They further found that diabetic pigs showed impaired healing accompanied by reduction of insulin–like growth factor 1 level in the healing wound.51 In another pig in vivo ulcer model,53 pathogen–free pigs were injured using a modified electro–keratome set to obtain partial–thickness wounds of 10 mm × 7 mm × 0.5 mm. Wounds were treated within 20 minutes with mevastatin. The results showed that mevastatin selectively triggered an anti–proliferative, pro–migratory phenotype and restored EGF sensitivity in the diabetic wound, resulting in healing of chronic wounds.53 However, pigs are difficult to anaesthetise due to their large size. The use of pigs as diabetic models is largely limited because of limitations including the long period needed to raise them and to the expense of maintaining them. ...
Challenges and issues with streptozotocin-induced diabetes - A clinically relevant animal model to understand the diabetes pathogenesis and evaluate therapeutics
5
2016
... Although no animal model fully simulates the pathophysiological mechanisms of human diabetic wounds, the exploration of using animal models to simulate the disease in preclinical studies and basic research is of utmost importance. In an animal, diabetes can be induced by different mechanisms including viral, genetic and chemical induction, and spontaneous autoimmune hyperglycemia.20 Streptozotocin (STZ) and alloxan are the most widely–used agents in chemical diabetic induction. It has been reported that STZ induces disease characteristics and similar responses in different animal models. Species including mice, rabbits, guinea pigs, monkeys, dogs and cats have been reported to be successfully used for the induction of diabetes by STZ, as indicated by the clinical features and pathological changes that resemble those of human diabetes (Figure 1).21 A vast array of in vivo experiments has demonstrated that STZ–induced diabetes and the subsequent changes constitute a reliable animal model.22,23 Goyal et al.21 and Szkudelski24 systematically summarised the mechanisms that caused diabetogenic changes following induction by the chemical STZ. These diabetogenic mechanisms included reactive oxygen species (ROS) generation, DNA damage, oxidative stress and glucose overloading, thereby causing pancreatic β cell damage.21,24 The diabetogenic action is characterised by selective death of β cells, insulin dysfunction, hyperglycaemia, and symptoms of polyuria, polydipsia and weight loss, which resemble human signs of diabetes mellitus. As for alloxan, it generates ROS and generates superoxide radicals and hydroxyl radicals. These hydroxyl radicals ultimately contribute to the death of β cells. As a thiol reagent, alloxan also selectively inhibits high glucose–induced insulin secretion by inhibiting the β cell glucose sensor glucokinase. The most significant disadvantage of using chemicals such as STZ and alloxan is perhaps the chemical toxicity to organs of the body.20 However, they have been widely used in various animal models. In this review, we focus on the animal models of diabetic wound healing involving rats, mice, rabbits, and pigs. ...
... 21 and Szkudelski24 systematically summarised the mechanisms that caused diabetogenic changes following induction by the chemical STZ. These diabetogenic mechanisms included reactive oxygen species (ROS) generation, DNA damage, oxidative stress and glucose overloading, thereby causing pancreatic β cell damage.21,24 The diabetogenic action is characterised by selective death of β cells, insulin dysfunction, hyperglycaemia, and symptoms of polyuria, polydipsia and weight loss, which resemble human signs of diabetes mellitus. As for alloxan, it generates ROS and generates superoxide radicals and hydroxyl radicals. These hydroxyl radicals ultimately contribute to the death of β cells. As a thiol reagent, alloxan also selectively inhibits high glucose–induced insulin secretion by inhibiting the β cell glucose sensor glucokinase. The most significant disadvantage of using chemicals such as STZ and alloxan is perhaps the chemical toxicity to organs of the body.20 However, they have been widely used in various animal models. In this review, we focus on the animal models of diabetic wound healing involving rats, mice, rabbits, and pigs. ...
... 21,24 The diabetogenic action is characterised by selective death of β cells, insulin dysfunction, hyperglycaemia, and symptoms of polyuria, polydipsia and weight loss, which resemble human signs of diabetes mellitus. As for alloxan, it generates ROS and generates superoxide radicals and hydroxyl radicals. These hydroxyl radicals ultimately contribute to the death of β cells. As a thiol reagent, alloxan also selectively inhibits high glucose–induced insulin secretion by inhibiting the β cell glucose sensor glucokinase. The most significant disadvantage of using chemicals such as STZ and alloxan is perhaps the chemical toxicity to organs of the body.20 However, they have been widely used in various animal models. In this review, we focus on the animal models of diabetic wound healing involving rats, mice, rabbits, and pigs. ...
... A diabetic skin ulcer is one of the most commonly–used models for the study of diabetic complications, and the model has been well explored and widely studied.25⇓⇓⇓⇓–30 Rats are perhaps the first choice for diabetic animal models.22,31 Given that the STZ model of diabetes induces clinical features in animals resembling those in humans, chemical STZ models serve as a perfect platform for developing diabetic wounds.21,22 To simulate human diabetic wounds, Yang et al.32 administered STZ to male Sprague–Dawley rats to generate diabetes. They prepared a 1% STZ solution, with a dose of 50 mg/kg, which was then intraperitoneally injected into Sprague–Dawley rats. After injection, a wound healing model was created and monitored. Using this model in an earlier study,27 a dorsal full–thickness ulcer was induced after 5 weeks of intraperitoneal injection of 60 mg/kg STZ. Another study involved administration of 65 mg/kg of STZ to maintain the fasting blood glucose concentration of Sprague–Dawley rats above 250 mg/dL, and then circular full–thickness skin excisions of 2 cm in diameter were created on the back of these rats.23 Zhang et al.33 also employed a chemically–induced diabetic model using 60 mg/kg STZ, and achieved fasting glucose levels of more than 16.7 mM, and full–thickness wounds with a diameter of 2 cm were then created on the backs of the rats. Liu and coworkers34 created 2 cm full–thickness wounds on STZ–generated diabetic Sprague–Dawley rats with fasting glucose levels over 11.1 mM. In one final example, a full–thickness dorsal wound was created after intraperitoneal administration of STZ (55 mg/kg) for 3 days.35 Collectively, these studies show that STZ at a dose of 50–65 mg/kg causes dysfunction of glucose regulation, which is a feasible method for imitating the human diabetic state and serves as a perfect platform for subsequent diabetic wound models. ...
... Mouse models are clinically relevant and have the advantages of testing potential therapeutic effects. There are two types of diabetes in mice, type 1 diabetic (T1D) models include the nonobese diabetic (NOD) mice and STZ–induced mice; type 2 diabetic (T2D) models include obese ob/ob mice and db/db mice.21 T1D is a disease caused by the destruction of β cells.36 Nowadays, the NOD mouse still serves as the best spontaneous model for T1D because the disease spontaneously develops and has many immune pathogenic characteristics in common with human T1D.36⇓–38 For instance, the NGL construct containing four tandem copies of the 5′ human immunodeficiency virus–long terminal repeat enhancer was used to generate transgenic NOD mice. Female mice with blood glucose > 15 mM are regarded as diabetic mice.39 STZ can also be used in T1D models. For instance, STZ–induced T1D models were generated on 8–week–old db/db mice by administering a dose of 70 mg/kg for 12 weeks and glucose beyond 300 mg/dL was considered as a diabetic state.40 ...
Evaluating STZ-induced impaired wound healing in rats
3
2018
... Although no animal model fully simulates the pathophysiological mechanisms of human diabetic wounds, the exploration of using animal models to simulate the disease in preclinical studies and basic research is of utmost importance. In an animal, diabetes can be induced by different mechanisms including viral, genetic and chemical induction, and spontaneous autoimmune hyperglycemia.20 Streptozotocin (STZ) and alloxan are the most widely–used agents in chemical diabetic induction. It has been reported that STZ induces disease characteristics and similar responses in different animal models. Species including mice, rabbits, guinea pigs, monkeys, dogs and cats have been reported to be successfully used for the induction of diabetes by STZ, as indicated by the clinical features and pathological changes that resemble those of human diabetes (Figure 1).21 A vast array of in vivo experiments has demonstrated that STZ–induced diabetes and the subsequent changes constitute a reliable animal model.22,23 Goyal et al.21 and Szkudelski24 systematically summarised the mechanisms that caused diabetogenic changes following induction by the chemical STZ. These diabetogenic mechanisms included reactive oxygen species (ROS) generation, DNA damage, oxidative stress and glucose overloading, thereby causing pancreatic β cell damage.21,24 The diabetogenic action is characterised by selective death of β cells, insulin dysfunction, hyperglycaemia, and symptoms of polyuria, polydipsia and weight loss, which resemble human signs of diabetes mellitus. As for alloxan, it generates ROS and generates superoxide radicals and hydroxyl radicals. These hydroxyl radicals ultimately contribute to the death of β cells. As a thiol reagent, alloxan also selectively inhibits high glucose–induced insulin secretion by inhibiting the β cell glucose sensor glucokinase. The most significant disadvantage of using chemicals such as STZ and alloxan is perhaps the chemical toxicity to organs of the body.20 However, they have been widely used in various animal models. In this review, we focus on the animal models of diabetic wound healing involving rats, mice, rabbits, and pigs. ...
... A diabetic skin ulcer is one of the most commonly–used models for the study of diabetic complications, and the model has been well explored and widely studied.25⇓⇓⇓⇓–30 Rats are perhaps the first choice for diabetic animal models.22,31 Given that the STZ model of diabetes induces clinical features in animals resembling those in humans, chemical STZ models serve as a perfect platform for developing diabetic wounds.21,22 To simulate human diabetic wounds, Yang et al.32 administered STZ to male Sprague–Dawley rats to generate diabetes. They prepared a 1% STZ solution, with a dose of 50 mg/kg, which was then intraperitoneally injected into Sprague–Dawley rats. After injection, a wound healing model was created and monitored. Using this model in an earlier study,27 a dorsal full–thickness ulcer was induced after 5 weeks of intraperitoneal injection of 60 mg/kg STZ. Another study involved administration of 65 mg/kg of STZ to maintain the fasting blood glucose concentration of Sprague–Dawley rats above 250 mg/dL, and then circular full–thickness skin excisions of 2 cm in diameter were created on the back of these rats.23 Zhang et al.33 also employed a chemically–induced diabetic model using 60 mg/kg STZ, and achieved fasting glucose levels of more than 16.7 mM, and full–thickness wounds with a diameter of 2 cm were then created on the backs of the rats. Liu and coworkers34 created 2 cm full–thickness wounds on STZ–generated diabetic Sprague–Dawley rats with fasting glucose levels over 11.1 mM. In one final example, a full–thickness dorsal wound was created after intraperitoneal administration of STZ (55 mg/kg) for 3 days.35 Collectively, these studies show that STZ at a dose of 50–65 mg/kg causes dysfunction of glucose regulation, which is a feasible method for imitating the human diabetic state and serves as a perfect platform for subsequent diabetic wound models. ...
... ,22 To simulate human diabetic wounds, Yang et al.32 administered STZ to male Sprague–Dawley rats to generate diabetes. They prepared a 1% STZ solution, with a dose of 50 mg/kg, which was then intraperitoneally injected into Sprague–Dawley rats. After injection, a wound healing model was created and monitored. Using this model in an earlier study,27 a dorsal full–thickness ulcer was induced after 5 weeks of intraperitoneal injection of 60 mg/kg STZ. Another study involved administration of 65 mg/kg of STZ to maintain the fasting blood glucose concentration of Sprague–Dawley rats above 250 mg/dL, and then circular full–thickness skin excisions of 2 cm in diameter were created on the back of these rats.23 Zhang et al.33 also employed a chemically–induced diabetic model using 60 mg/kg STZ, and achieved fasting glucose levels of more than 16.7 mM, and full–thickness wounds with a diameter of 2 cm were then created on the backs of the rats. Liu and coworkers34 created 2 cm full–thickness wounds on STZ–generated diabetic Sprague–Dawley rats with fasting glucose levels over 11.1 mM. In one final example, a full–thickness dorsal wound was created after intraperitoneal administration of STZ (55 mg/kg) for 3 days.35 Collectively, these studies show that STZ at a dose of 50–65 mg/kg causes dysfunction of glucose regulation, which is a feasible method for imitating the human diabetic state and serves as a perfect platform for subsequent diabetic wound models. ...
Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway
2
2020
... Although no animal model fully simulates the pathophysiological mechanisms of human diabetic wounds, the exploration of using animal models to simulate the disease in preclinical studies and basic research is of utmost importance. In an animal, diabetes can be induced by different mechanisms including viral, genetic and chemical induction, and spontaneous autoimmune hyperglycemia.20 Streptozotocin (STZ) and alloxan are the most widely–used agents in chemical diabetic induction. It has been reported that STZ induces disease characteristics and similar responses in different animal models. Species including mice, rabbits, guinea pigs, monkeys, dogs and cats have been reported to be successfully used for the induction of diabetes by STZ, as indicated by the clinical features and pathological changes that resemble those of human diabetes (Figure 1).21 A vast array of in vivo experiments has demonstrated that STZ–induced diabetes and the subsequent changes constitute a reliable animal model.22,23 Goyal et al.21 and Szkudelski24 systematically summarised the mechanisms that caused diabetogenic changes following induction by the chemical STZ. These diabetogenic mechanisms included reactive oxygen species (ROS) generation, DNA damage, oxidative stress and glucose overloading, thereby causing pancreatic β cell damage.21,24 The diabetogenic action is characterised by selective death of β cells, insulin dysfunction, hyperglycaemia, and symptoms of polyuria, polydipsia and weight loss, which resemble human signs of diabetes mellitus. As for alloxan, it generates ROS and generates superoxide radicals and hydroxyl radicals. These hydroxyl radicals ultimately contribute to the death of β cells. As a thiol reagent, alloxan also selectively inhibits high glucose–induced insulin secretion by inhibiting the β cell glucose sensor glucokinase. The most significant disadvantage of using chemicals such as STZ and alloxan is perhaps the chemical toxicity to organs of the body.20 However, they have been widely used in various animal models. In this review, we focus on the animal models of diabetic wound healing involving rats, mice, rabbits, and pigs. ...
... A diabetic skin ulcer is one of the most commonly–used models for the study of diabetic complications, and the model has been well explored and widely studied.25⇓⇓⇓⇓–30 Rats are perhaps the first choice for diabetic animal models.22,31 Given that the STZ model of diabetes induces clinical features in animals resembling those in humans, chemical STZ models serve as a perfect platform for developing diabetic wounds.21,22 To simulate human diabetic wounds, Yang et al.32 administered STZ to male Sprague–Dawley rats to generate diabetes. They prepared a 1% STZ solution, with a dose of 50 mg/kg, which was then intraperitoneally injected into Sprague–Dawley rats. After injection, a wound healing model was created and monitored. Using this model in an earlier study,27 a dorsal full–thickness ulcer was induced after 5 weeks of intraperitoneal injection of 60 mg/kg STZ. Another study involved administration of 65 mg/kg of STZ to maintain the fasting blood glucose concentration of Sprague–Dawley rats above 250 mg/dL, and then circular full–thickness skin excisions of 2 cm in diameter were created on the back of these rats.23 Zhang et al.33 also employed a chemically–induced diabetic model using 60 mg/kg STZ, and achieved fasting glucose levels of more than 16.7 mM, and full–thickness wounds with a diameter of 2 cm were then created on the backs of the rats. Liu and coworkers34 created 2 cm full–thickness wounds on STZ–generated diabetic Sprague–Dawley rats with fasting glucose levels over 11.1 mM. In one final example, a full–thickness dorsal wound was created after intraperitoneal administration of STZ (55 mg/kg) for 3 days.35 Collectively, these studies show that STZ at a dose of 50–65 mg/kg causes dysfunction of glucose regulation, which is a feasible method for imitating the human diabetic state and serves as a perfect platform for subsequent diabetic wound models. ...
Streptozotocin-nicotinamide-induced diabetes in the rat. Characteristics of the experimental model
2
2012
... Although no animal model fully simulates the pathophysiological mechanisms of human diabetic wounds, the exploration of using animal models to simulate the disease in preclinical studies and basic research is of utmost importance. In an animal, diabetes can be induced by different mechanisms including viral, genetic and chemical induction, and spontaneous autoimmune hyperglycemia.20 Streptozotocin (STZ) and alloxan are the most widely–used agents in chemical diabetic induction. It has been reported that STZ induces disease characteristics and similar responses in different animal models. Species including mice, rabbits, guinea pigs, monkeys, dogs and cats have been reported to be successfully used for the induction of diabetes by STZ, as indicated by the clinical features and pathological changes that resemble those of human diabetes (Figure 1).21 A vast array of in vivo experiments has demonstrated that STZ–induced diabetes and the subsequent changes constitute a reliable animal model.22,23 Goyal et al.21 and Szkudelski24 systematically summarised the mechanisms that caused diabetogenic changes following induction by the chemical STZ. These diabetogenic mechanisms included reactive oxygen species (ROS) generation, DNA damage, oxidative stress and glucose overloading, thereby causing pancreatic β cell damage.21,24 The diabetogenic action is characterised by selective death of β cells, insulin dysfunction, hyperglycaemia, and symptoms of polyuria, polydipsia and weight loss, which resemble human signs of diabetes mellitus. As for alloxan, it generates ROS and generates superoxide radicals and hydroxyl radicals. These hydroxyl radicals ultimately contribute to the death of β cells. As a thiol reagent, alloxan also selectively inhibits high glucose–induced insulin secretion by inhibiting the β cell glucose sensor glucokinase. The most significant disadvantage of using chemicals such as STZ and alloxan is perhaps the chemical toxicity to organs of the body.20 However, they have been widely used in various animal models. In this review, we focus on the animal models of diabetic wound healing involving rats, mice, rabbits, and pigs. ...
... ,24 The diabetogenic action is characterised by selective death of β cells, insulin dysfunction, hyperglycaemia, and symptoms of polyuria, polydipsia and weight loss, which resemble human signs of diabetes mellitus. As for alloxan, it generates ROS and generates superoxide radicals and hydroxyl radicals. These hydroxyl radicals ultimately contribute to the death of β cells. As a thiol reagent, alloxan also selectively inhibits high glucose–induced insulin secretion by inhibiting the β cell glucose sensor glucokinase. The most significant disadvantage of using chemicals such as STZ and alloxan is perhaps the chemical toxicity to organs of the body.20 However, they have been widely used in various animal models. In this review, we focus on the animal models of diabetic wound healing involving rats, mice, rabbits, and pigs. ...
Bone marrow mesenchymal stem cells preconditioned with nitric-oxide-releasing chitosan/PVA hydrogel accelerate diabetic wound healing in rabbits
1
2021
... A diabetic skin ulcer is one of the most commonly–used models for the study of diabetic complications, and the model has been well explored and widely studied.25⇓⇓⇓⇓–30 Rats are perhaps the first choice for diabetic animal models.22,31 Given that the STZ model of diabetes induces clinical features in animals resembling those in humans, chemical STZ models serve as a perfect platform for developing diabetic wounds.21,22 To simulate human diabetic wounds, Yang et al.32 administered STZ to male Sprague–Dawley rats to generate diabetes. They prepared a 1% STZ solution, with a dose of 50 mg/kg, which was then intraperitoneally injected into Sprague–Dawley rats. After injection, a wound healing model was created and monitored. Using this model in an earlier study,27 a dorsal full–thickness ulcer was induced after 5 weeks of intraperitoneal injection of 60 mg/kg STZ. Another study involved administration of 65 mg/kg of STZ to maintain the fasting blood glucose concentration of Sprague–Dawley rats above 250 mg/dL, and then circular full–thickness skin excisions of 2 cm in diameter were created on the back of these rats.23 Zhang et al.33 also employed a chemically–induced diabetic model using 60 mg/kg STZ, and achieved fasting glucose levels of more than 16.7 mM, and full–thickness wounds with a diameter of 2 cm were then created on the backs of the rats. Liu and coworkers34 created 2 cm full–thickness wounds on STZ–generated diabetic Sprague–Dawley rats with fasting glucose levels over 11.1 mM. In one final example, a full–thickness dorsal wound was created after intraperitoneal administration of STZ (55 mg/kg) for 3 days.35 Collectively, these studies show that STZ at a dose of 50–65 mg/kg causes dysfunction of glucose regulation, which is a feasible method for imitating the human diabetic state and serves as a perfect platform for subsequent diabetic wound models. ...
Nitric oxide therapy for diabetic wound healing
1
2019
... A diabetic skin ulcer is one of the most commonly–used models for the study of diabetic complications, and the model has been well explored and widely studied.25⇓⇓⇓⇓–30 Rats are perhaps the first choice for diabetic animal models.22,31 Given that the STZ model of diabetes induces clinical features in animals resembling those in humans, chemical STZ models serve as a perfect platform for developing diabetic wounds.21,22 To simulate human diabetic wounds, Yang et al.32 administered STZ to male Sprague–Dawley rats to generate diabetes. They prepared a 1% STZ solution, with a dose of 50 mg/kg, which was then intraperitoneally injected into Sprague–Dawley rats. After injection, a wound healing model was created and monitored. Using this model in an earlier study,27 a dorsal full–thickness ulcer was induced after 5 weeks of intraperitoneal injection of 60 mg/kg STZ. Another study involved administration of 65 mg/kg of STZ to maintain the fasting blood glucose concentration of Sprague–Dawley rats above 250 mg/dL, and then circular full–thickness skin excisions of 2 cm in diameter were created on the back of these rats.23 Zhang et al.33 also employed a chemically–induced diabetic model using 60 mg/kg STZ, and achieved fasting glucose levels of more than 16.7 mM, and full–thickness wounds with a diameter of 2 cm were then created on the backs of the rats. Liu and coworkers34 created 2 cm full–thickness wounds on STZ–generated diabetic Sprague–Dawley rats with fasting glucose levels over 11.1 mM. In one final example, a full–thickness dorsal wound was created after intraperitoneal administration of STZ (55 mg/kg) for 3 days.35 Collectively, these studies show that STZ at a dose of 50–65 mg/kg causes dysfunction of glucose regulation, which is a feasible method for imitating the human diabetic state and serves as a perfect platform for subsequent diabetic wound models. ...
Fibrin improves skin wound perfusion in a diabetic rat model
2
2017
... A diabetic skin ulcer is one of the most commonly–used models for the study of diabetic complications, and the model has been well explored and widely studied.25⇓⇓⇓⇓–30 Rats are perhaps the first choice for diabetic animal models.22,31 Given that the STZ model of diabetes induces clinical features in animals resembling those in humans, chemical STZ models serve as a perfect platform for developing diabetic wounds.21,22 To simulate human diabetic wounds, Yang et al.32 administered STZ to male Sprague–Dawley rats to generate diabetes. They prepared a 1% STZ solution, with a dose of 50 mg/kg, which was then intraperitoneally injected into Sprague–Dawley rats. After injection, a wound healing model was created and monitored. Using this model in an earlier study,27 a dorsal full–thickness ulcer was induced after 5 weeks of intraperitoneal injection of 60 mg/kg STZ. Another study involved administration of 65 mg/kg of STZ to maintain the fasting blood glucose concentration of Sprague–Dawley rats above 250 mg/dL, and then circular full–thickness skin excisions of 2 cm in diameter were created on the back of these rats.23 Zhang et al.33 also employed a chemically–induced diabetic model using 60 mg/kg STZ, and achieved fasting glucose levels of more than 16.7 mM, and full–thickness wounds with a diameter of 2 cm were then created on the backs of the rats. Liu and coworkers34 created 2 cm full–thickness wounds on STZ–generated diabetic Sprague–Dawley rats with fasting glucose levels over 11.1 mM. In one final example, a full–thickness dorsal wound was created after intraperitoneal administration of STZ (55 mg/kg) for 3 days.35 Collectively, these studies show that STZ at a dose of 50–65 mg/kg causes dysfunction of glucose regulation, which is a feasible method for imitating the human diabetic state and serves as a perfect platform for subsequent diabetic wound models. ...
... 27 a dorsal full–thickness ulcer was induced after 5 weeks of intraperitoneal injection of 60 mg/kg STZ. Another study involved administration of 65 mg/kg of STZ to maintain the fasting blood glucose concentration of Sprague–Dawley rats above 250 mg/dL, and then circular full–thickness skin excisions of 2 cm in diameter were created on the back of these rats.23 Zhang et al.33 also employed a chemically–induced diabetic model using 60 mg/kg STZ, and achieved fasting glucose levels of more than 16.7 mM, and full–thickness wounds with a diameter of 2 cm were then created on the backs of the rats. Liu and coworkers34 created 2 cm full–thickness wounds on STZ–generated diabetic Sprague–Dawley rats with fasting glucose levels over 11.1 mM. In one final example, a full–thickness dorsal wound was created after intraperitoneal administration of STZ (55 mg/kg) for 3 days.35 Collectively, these studies show that STZ at a dose of 50–65 mg/kg causes dysfunction of glucose regulation, which is a feasible method for imitating the human diabetic state and serves as a perfect platform for subsequent diabetic wound models. ...
Topical α-gal nanoparticles accelerate diabetic wound healing
1
2020
... A diabetic skin ulcer is one of the most commonly–used models for the study of diabetic complications, and the model has been well explored and widely studied.25⇓⇓⇓⇓–30 Rats are perhaps the first choice for diabetic animal models.22,31 Given that the STZ model of diabetes induces clinical features in animals resembling those in humans, chemical STZ models serve as a perfect platform for developing diabetic wounds.21,22 To simulate human diabetic wounds, Yang et al.32 administered STZ to male Sprague–Dawley rats to generate diabetes. They prepared a 1% STZ solution, with a dose of 50 mg/kg, which was then intraperitoneally injected into Sprague–Dawley rats. After injection, a wound healing model was created and monitored. Using this model in an earlier study,27 a dorsal full–thickness ulcer was induced after 5 weeks of intraperitoneal injection of 60 mg/kg STZ. Another study involved administration of 65 mg/kg of STZ to maintain the fasting blood glucose concentration of Sprague–Dawley rats above 250 mg/dL, and then circular full–thickness skin excisions of 2 cm in diameter were created on the back of these rats.23 Zhang et al.33 also employed a chemically–induced diabetic model using 60 mg/kg STZ, and achieved fasting glucose levels of more than 16.7 mM, and full–thickness wounds with a diameter of 2 cm were then created on the backs of the rats. Liu and coworkers34 created 2 cm full–thickness wounds on STZ–generated diabetic Sprague–Dawley rats with fasting glucose levels over 11.1 mM. In one final example, a full–thickness dorsal wound was created after intraperitoneal administration of STZ (55 mg/kg) for 3 days.35 Collectively, these studies show that STZ at a dose of 50–65 mg/kg causes dysfunction of glucose regulation, which is a feasible method for imitating the human diabetic state and serves as a perfect platform for subsequent diabetic wound models. ...
Murine model of wound healing
1
2013
... A diabetic skin ulcer is one of the most commonly–used models for the study of diabetic complications, and the model has been well explored and widely studied.25⇓⇓⇓⇓–30 Rats are perhaps the first choice for diabetic animal models.22,31 Given that the STZ model of diabetes induces clinical features in animals resembling those in humans, chemical STZ models serve as a perfect platform for developing diabetic wounds.21,22 To simulate human diabetic wounds, Yang et al.32 administered STZ to male Sprague–Dawley rats to generate diabetes. They prepared a 1% STZ solution, with a dose of 50 mg/kg, which was then intraperitoneally injected into Sprague–Dawley rats. After injection, a wound healing model was created and monitored. Using this model in an earlier study,27 a dorsal full–thickness ulcer was induced after 5 weeks of intraperitoneal injection of 60 mg/kg STZ. Another study involved administration of 65 mg/kg of STZ to maintain the fasting blood glucose concentration of Sprague–Dawley rats above 250 mg/dL, and then circular full–thickness skin excisions of 2 cm in diameter were created on the back of these rats.23 Zhang et al.33 also employed a chemically–induced diabetic model using 60 mg/kg STZ, and achieved fasting glucose levels of more than 16.7 mM, and full–thickness wounds with a diameter of 2 cm were then created on the backs of the rats. Liu and coworkers34 created 2 cm full–thickness wounds on STZ–generated diabetic Sprague–Dawley rats with fasting glucose levels over 11.1 mM. In one final example, a full–thickness dorsal wound was created after intraperitoneal administration of STZ (55 mg/kg) for 3 days.35 Collectively, these studies show that STZ at a dose of 50–65 mg/kg causes dysfunction of glucose regulation, which is a feasible method for imitating the human diabetic state and serves as a perfect platform for subsequent diabetic wound models. ...
A small molecule HIF-1α stabilizer that accelerates diabetic wound healing
1
2021
... A diabetic skin ulcer is one of the most commonly–used models for the study of diabetic complications, and the model has been well explored and widely studied.25⇓⇓⇓⇓–30 Rats are perhaps the first choice for diabetic animal models.22,31 Given that the STZ model of diabetes induces clinical features in animals resembling those in humans, chemical STZ models serve as a perfect platform for developing diabetic wounds.21,22 To simulate human diabetic wounds, Yang et al.32 administered STZ to male Sprague–Dawley rats to generate diabetes. They prepared a 1% STZ solution, with a dose of 50 mg/kg, which was then intraperitoneally injected into Sprague–Dawley rats. After injection, a wound healing model was created and monitored. Using this model in an earlier study,27 a dorsal full–thickness ulcer was induced after 5 weeks of intraperitoneal injection of 60 mg/kg STZ. Another study involved administration of 65 mg/kg of STZ to maintain the fasting blood glucose concentration of Sprague–Dawley rats above 250 mg/dL, and then circular full–thickness skin excisions of 2 cm in diameter were created on the back of these rats.23 Zhang et al.33 also employed a chemically–induced diabetic model using 60 mg/kg STZ, and achieved fasting glucose levels of more than 16.7 mM, and full–thickness wounds with a diameter of 2 cm were then created on the backs of the rats. Liu and coworkers34 created 2 cm full–thickness wounds on STZ–generated diabetic Sprague–Dawley rats with fasting glucose levels over 11.1 mM. In one final example, a full–thickness dorsal wound was created after intraperitoneal administration of STZ (55 mg/kg) for 3 days.35 Collectively, these studies show that STZ at a dose of 50–65 mg/kg causes dysfunction of glucose regulation, which is a feasible method for imitating the human diabetic state and serves as a perfect platform for subsequent diabetic wound models. ...
The use of animal models in diabetes research
1
2012
... A diabetic skin ulcer is one of the most commonly–used models for the study of diabetic complications, and the model has been well explored and widely studied.25⇓⇓⇓⇓–30 Rats are perhaps the first choice for diabetic animal models.22,31 Given that the STZ model of diabetes induces clinical features in animals resembling those in humans, chemical STZ models serve as a perfect platform for developing diabetic wounds.21,22 To simulate human diabetic wounds, Yang et al.32 administered STZ to male Sprague–Dawley rats to generate diabetes. They prepared a 1% STZ solution, with a dose of 50 mg/kg, which was then intraperitoneally injected into Sprague–Dawley rats. After injection, a wound healing model was created and monitored. Using this model in an earlier study,27 a dorsal full–thickness ulcer was induced after 5 weeks of intraperitoneal injection of 60 mg/kg STZ. Another study involved administration of 65 mg/kg of STZ to maintain the fasting blood glucose concentration of Sprague–Dawley rats above 250 mg/dL, and then circular full–thickness skin excisions of 2 cm in diameter were created on the back of these rats.23 Zhang et al.33 also employed a chemically–induced diabetic model using 60 mg/kg STZ, and achieved fasting glucose levels of more than 16.7 mM, and full–thickness wounds with a diameter of 2 cm were then created on the backs of the rats. Liu and coworkers34 created 2 cm full–thickness wounds on STZ–generated diabetic Sprague–Dawley rats with fasting glucose levels over 11.1 mM. In one final example, a full–thickness dorsal wound was created after intraperitoneal administration of STZ (55 mg/kg) for 3 days.35 Collectively, these studies show that STZ at a dose of 50–65 mg/kg causes dysfunction of glucose regulation, which is a feasible method for imitating the human diabetic state and serves as a perfect platform for subsequent diabetic wound models. ...
Umbilical cord-derived mesenchymal stem cell-derived exosomes combined pluronic F127 hydrogel promote chronic diabetic wound healing and complete skin regeneration
1
2020
... A diabetic skin ulcer is one of the most commonly–used models for the study of diabetic complications, and the model has been well explored and widely studied.25⇓⇓⇓⇓–30 Rats are perhaps the first choice for diabetic animal models.22,31 Given that the STZ model of diabetes induces clinical features in animals resembling those in humans, chemical STZ models serve as a perfect platform for developing diabetic wounds.21,22 To simulate human diabetic wounds, Yang et al.32 administered STZ to male Sprague–Dawley rats to generate diabetes. They prepared a 1% STZ solution, with a dose of 50 mg/kg, which was then intraperitoneally injected into Sprague–Dawley rats. After injection, a wound healing model was created and monitored. Using this model in an earlier study,27 a dorsal full–thickness ulcer was induced after 5 weeks of intraperitoneal injection of 60 mg/kg STZ. Another study involved administration of 65 mg/kg of STZ to maintain the fasting blood glucose concentration of Sprague–Dawley rats above 250 mg/dL, and then circular full–thickness skin excisions of 2 cm in diameter were created on the back of these rats.23 Zhang et al.33 also employed a chemically–induced diabetic model using 60 mg/kg STZ, and achieved fasting glucose levels of more than 16.7 mM, and full–thickness wounds with a diameter of 2 cm were then created on the backs of the rats. Liu and coworkers34 created 2 cm full–thickness wounds on STZ–generated diabetic Sprague–Dawley rats with fasting glucose levels over 11.1 mM. In one final example, a full–thickness dorsal wound was created after intraperitoneal administration of STZ (55 mg/kg) for 3 days.35 Collectively, these studies show that STZ at a dose of 50–65 mg/kg causes dysfunction of glucose regulation, which is a feasible method for imitating the human diabetic state and serves as a perfect platform for subsequent diabetic wound models. ...
Huangbai Liniment accelerated wound healing by activating Nrf2 signaling in diabetes
1
2020
... A diabetic skin ulcer is one of the most commonly–used models for the study of diabetic complications, and the model has been well explored and widely studied.25⇓⇓⇓⇓–30 Rats are perhaps the first choice for diabetic animal models.22,31 Given that the STZ model of diabetes induces clinical features in animals resembling those in humans, chemical STZ models serve as a perfect platform for developing diabetic wounds.21,22 To simulate human diabetic wounds, Yang et al.32 administered STZ to male Sprague–Dawley rats to generate diabetes. They prepared a 1% STZ solution, with a dose of 50 mg/kg, which was then intraperitoneally injected into Sprague–Dawley rats. After injection, a wound healing model was created and monitored. Using this model in an earlier study,27 a dorsal full–thickness ulcer was induced after 5 weeks of intraperitoneal injection of 60 mg/kg STZ. Another study involved administration of 65 mg/kg of STZ to maintain the fasting blood glucose concentration of Sprague–Dawley rats above 250 mg/dL, and then circular full–thickness skin excisions of 2 cm in diameter were created on the back of these rats.23 Zhang et al.33 also employed a chemically–induced diabetic model using 60 mg/kg STZ, and achieved fasting glucose levels of more than 16.7 mM, and full–thickness wounds with a diameter of 2 cm were then created on the backs of the rats. Liu and coworkers34 created 2 cm full–thickness wounds on STZ–generated diabetic Sprague–Dawley rats with fasting glucose levels over 11.1 mM. In one final example, a full–thickness dorsal wound was created after intraperitoneal administration of STZ (55 mg/kg) for 3 days.35 Collectively, these studies show that STZ at a dose of 50–65 mg/kg causes dysfunction of glucose regulation, which is a feasible method for imitating the human diabetic state and serves as a perfect platform for subsequent diabetic wound models. ...
Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway
1
2020
... A diabetic skin ulcer is one of the most commonly–used models for the study of diabetic complications, and the model has been well explored and widely studied.25⇓⇓⇓⇓–30 Rats are perhaps the first choice for diabetic animal models.22,31 Given that the STZ model of diabetes induces clinical features in animals resembling those in humans, chemical STZ models serve as a perfect platform for developing diabetic wounds.21,22 To simulate human diabetic wounds, Yang et al.32 administered STZ to male Sprague–Dawley rats to generate diabetes. They prepared a 1% STZ solution, with a dose of 50 mg/kg, which was then intraperitoneally injected into Sprague–Dawley rats. After injection, a wound healing model was created and monitored. Using this model in an earlier study,27 a dorsal full–thickness ulcer was induced after 5 weeks of intraperitoneal injection of 60 mg/kg STZ. Another study involved administration of 65 mg/kg of STZ to maintain the fasting blood glucose concentration of Sprague–Dawley rats above 250 mg/dL, and then circular full–thickness skin excisions of 2 cm in diameter were created on the back of these rats.23 Zhang et al.33 also employed a chemically–induced diabetic model using 60 mg/kg STZ, and achieved fasting glucose levels of more than 16.7 mM, and full–thickness wounds with a diameter of 2 cm were then created on the backs of the rats. Liu and coworkers34 created 2 cm full–thickness wounds on STZ–generated diabetic Sprague–Dawley rats with fasting glucose levels over 11.1 mM. In one final example, a full–thickness dorsal wound was created after intraperitoneal administration of STZ (55 mg/kg) for 3 days.35 Collectively, these studies show that STZ at a dose of 50–65 mg/kg causes dysfunction of glucose regulation, which is a feasible method for imitating the human diabetic state and serves as a perfect platform for subsequent diabetic wound models. ...
The ameliorative effect of bromelain on STZ-induced type 1 diabetes in rats through Oxi-LDL/LPA/LPAR1 pathway
1
2021
... A diabetic skin ulcer is one of the most commonly–used models for the study of diabetic complications, and the model has been well explored and widely studied.25⇓⇓⇓⇓–30 Rats are perhaps the first choice for diabetic animal models.22,31 Given that the STZ model of diabetes induces clinical features in animals resembling those in humans, chemical STZ models serve as a perfect platform for developing diabetic wounds.21,22 To simulate human diabetic wounds, Yang et al.32 administered STZ to male Sprague–Dawley rats to generate diabetes. They prepared a 1% STZ solution, with a dose of 50 mg/kg, which was then intraperitoneally injected into Sprague–Dawley rats. After injection, a wound healing model was created and monitored. Using this model in an earlier study,27 a dorsal full–thickness ulcer was induced after 5 weeks of intraperitoneal injection of 60 mg/kg STZ. Another study involved administration of 65 mg/kg of STZ to maintain the fasting blood glucose concentration of Sprague–Dawley rats above 250 mg/dL, and then circular full–thickness skin excisions of 2 cm in diameter were created on the back of these rats.23 Zhang et al.33 also employed a chemically–induced diabetic model using 60 mg/kg STZ, and achieved fasting glucose levels of more than 16.7 mM, and full–thickness wounds with a diameter of 2 cm were then created on the backs of the rats. Liu and coworkers34 created 2 cm full–thickness wounds on STZ–generated diabetic Sprague–Dawley rats with fasting glucose levels over 11.1 mM. In one final example, a full–thickness dorsal wound was created after intraperitoneal administration of STZ (55 mg/kg) for 3 days.35 Collectively, these studies show that STZ at a dose of 50–65 mg/kg causes dysfunction of glucose regulation, which is a feasible method for imitating the human diabetic state and serves as a perfect platform for subsequent diabetic wound models. ...
Advances in our understanding of the pathophysiology of Type 1 diabetes: lessons from the NOD mouse
2
2014
... Mouse models are clinically relevant and have the advantages of testing potential therapeutic effects. There are two types of diabetes in mice, type 1 diabetic (T1D) models include the nonobese diabetic (NOD) mice and STZ–induced mice; type 2 diabetic (T2D) models include obese ob/ob mice and db/db mice.21 T1D is a disease caused by the destruction of β cells.36 Nowadays, the NOD mouse still serves as the best spontaneous model for T1D because the disease spontaneously develops and has many immune pathogenic characteristics in common with human T1D.36⇓–38 For instance, the NGL construct containing four tandem copies of the 5′ human immunodeficiency virus–long terminal repeat enhancer was used to generate transgenic NOD mice. Female mice with blood glucose > 15 mM are regarded as diabetic mice.39 STZ can also be used in T1D models. For instance, STZ–induced T1D models were generated on 8–week–old db/db mice by administering a dose of 70 mg/kg for 12 weeks and glucose beyond 300 mg/dL was considered as a diabetic state.40 ...
... 36⇓–38 For instance, the NGL construct containing four tandem copies of the 5′ human immunodeficiency virus–long terminal repeat enhancer was used to generate transgenic NOD mice. Female mice with blood glucose > 15 mM are regarded as diabetic mice.39 STZ can also be used in T1D models. For instance, STZ–induced T1D models were generated on 8–week–old db/db mice by administering a dose of 70 mg/kg for 12 weeks and glucose beyond 300 mg/dL was considered as a diabetic state.40 ...
Mouse models for the study of autoimmune type 1 diabetes: a NOD to similarities and differences to human disease
1
2011
... Mouse models are clinically relevant and have the advantages of testing potential therapeutic effects. There are two types of diabetes in mice, type 1 diabetic (T1D) models include the nonobese diabetic (NOD) mice and STZ–induced mice; type 2 diabetic (T2D) models include obese ob/ob mice and db/db mice.21 T1D is a disease caused by the destruction of β cells.36 Nowadays, the NOD mouse still serves as the best spontaneous model for T1D because the disease spontaneously develops and has many immune pathogenic characteristics in common with human T1D.36⇓–38 For instance, the NGL construct containing four tandem copies of the 5′ human immunodeficiency virus–long terminal repeat enhancer was used to generate transgenic NOD mice. Female mice with blood glucose > 15 mM are regarded as diabetic mice.39 STZ can also be used in T1D models. For instance, STZ–induced T1D models were generated on 8–week–old db/db mice by administering a dose of 70 mg/kg for 12 weeks and glucose beyond 300 mg/dL was considered as a diabetic state.40 ...
Animal models of spontaneous autoimmune disease: type 1 diabetes in the nonobese diabetic mouse
1
2007
... Mouse models are clinically relevant and have the advantages of testing potential therapeutic effects. There are two types of diabetes in mice, type 1 diabetic (T1D) models include the nonobese diabetic (NOD) mice and STZ–induced mice; type 2 diabetic (T2D) models include obese ob/ob mice and db/db mice.21 T1D is a disease caused by the destruction of β cells.36 Nowadays, the NOD mouse still serves as the best spontaneous model for T1D because the disease spontaneously develops and has many immune pathogenic characteristics in common with human T1D.36⇓–38 For instance, the NGL construct containing four tandem copies of the 5′ human immunodeficiency virus–long terminal repeat enhancer was used to generate transgenic NOD mice. Female mice with blood glucose > 15 mM are regarded as diabetic mice.39 STZ can also be used in T1D models. For instance, STZ–induced T1D models were generated on 8–week–old db/db mice by administering a dose of 70 mg/kg for 12 weeks and glucose beyond 300 mg/dL was considered as a diabetic state.40 ...
NF-κB is weakly activated in the NOD mouse model of type 1 diabetes
1
2018
... Mouse models are clinically relevant and have the advantages of testing potential therapeutic effects. There are two types of diabetes in mice, type 1 diabetic (T1D) models include the nonobese diabetic (NOD) mice and STZ–induced mice; type 2 diabetic (T2D) models include obese ob/ob mice and db/db mice.21 T1D is a disease caused by the destruction of β cells.36 Nowadays, the NOD mouse still serves as the best spontaneous model for T1D because the disease spontaneously develops and has many immune pathogenic characteristics in common with human T1D.36⇓–38 For instance, the NGL construct containing four tandem copies of the 5′ human immunodeficiency virus–long terminal repeat enhancer was used to generate transgenic NOD mice. Female mice with blood glucose > 15 mM are regarded as diabetic mice.39 STZ can also be used in T1D models. For instance, STZ–induced T1D models were generated on 8–week–old db/db mice by administering a dose of 70 mg/kg for 12 weeks and glucose beyond 300 mg/dL was considered as a diabetic state.40 ...
Mefunidone ameliorates diabetic kidney disease in STZ and db/db mice
1
2021
... Mouse models are clinically relevant and have the advantages of testing potential therapeutic effects. There are two types of diabetes in mice, type 1 diabetic (T1D) models include the nonobese diabetic (NOD) mice and STZ–induced mice; type 2 diabetic (T2D) models include obese ob/ob mice and db/db mice.21 T1D is a disease caused by the destruction of β cells.36 Nowadays, the NOD mouse still serves as the best spontaneous model for T1D because the disease spontaneously develops and has many immune pathogenic characteristics in common with human T1D.36⇓–38 For instance, the NGL construct containing four tandem copies of the 5′ human immunodeficiency virus–long terminal repeat enhancer was used to generate transgenic NOD mice. Female mice with blood glucose > 15 mM are regarded as diabetic mice.39 STZ can also be used in T1D models. For instance, STZ–induced T1D models were generated on 8–week–old db/db mice by administering a dose of 70 mg/kg for 12 weeks and glucose beyond 300 mg/dL was considered as a diabetic state.40 ...
Therapeutic effects of adipose stem cells from diabetic mice for the treatment of type 2 diabetes
3
2018
... For the most common T2D model in mice, studies showed that daily STZ administration generated T2D models in C57BL/6 mice given 40 mg/kg for 3 consecutive days41 or in 8–week–old db/+ mice given 50 mg/kg for 4 consecutive days.42 Many other researchers instead used a straightforward high–fat diet (HFD) to model human T2D because a HFD is a dependable model that provides an appropriate aetiological, pathological, and treatment option for T2D.43 C57BL/6 mice were provided with a rodent diet containing 60 kcal% fat.44 The glucose and insulin levels were used to evaluate T2D induction at 20 weeks. In addition to single diet–induced diabetes, use of a HFD combined with multiple doses of STZ has also been used in a mouse model. Wang et al.41 combined a HFD with chemical STZ treatment to induce T2D. Specifically, 5–week–old C57BL/6 mice were fed with a HFD (60% of calories from fat) for 24 weeks. After HFD feeding for 23 weeks, mice were injected with 40 mg/kg STZ daily for 3 consecutive days to generate T2D.41 In a similar experiment, C57BL/6 mice were fed a HFD for 8 weeks, and then given an intraperitoneal injection of 100 mg/kg STZ at weeks 9 and 11. After treatment, 4 hour–fasting blood glucose over 16.7 mM in the tail vein was considered T2D. The mouse model is clinically relevant and has the benefit that therapeutic agents have been tested.45 Therefore, it is essential that the appropriate model is chosen when considering the type of wound healing. ...
... 41 combined a HFD with chemical STZ treatment to induce T2D. Specifically, 5–week–old C57BL/6 mice were fed with a HFD (60% of calories from fat) for 24 weeks. After HFD feeding for 23 weeks, mice were injected with 40 mg/kg STZ daily for 3 consecutive days to generate T2D.41 In a similar experiment, C57BL/6 mice were fed a HFD for 8 weeks, and then given an intraperitoneal injection of 100 mg/kg STZ at weeks 9 and 11. After treatment, 4 hour–fasting blood glucose over 16.7 mM in the tail vein was considered T2D. The mouse model is clinically relevant and has the benefit that therapeutic agents have been tested.45 Therefore, it is essential that the appropriate model is chosen when considering the type of wound healing. ...
... 41 In a similar experiment, C57BL/6 mice were fed a HFD for 8 weeks, and then given an intraperitoneal injection of 100 mg/kg STZ at weeks 9 and 11. After treatment, 4 hour–fasting blood glucose over 16.7 mM in the tail vein was considered T2D. The mouse model is clinically relevant and has the benefit that therapeutic agents have been tested.45 Therefore, it is essential that the appropriate model is chosen when considering the type of wound healing. ...
Proximal tubule autophagy differs in type 1 and 2 diabetes
1
2019
... For the most common T2D model in mice, studies showed that daily STZ administration generated T2D models in C57BL/6 mice given 40 mg/kg for 3 consecutive days41 or in 8–week–old db/+ mice given 50 mg/kg for 4 consecutive days.42 Many other researchers instead used a straightforward high–fat diet (HFD) to model human T2D because a HFD is a dependable model that provides an appropriate aetiological, pathological, and treatment option for T2D.43 C57BL/6 mice were provided with a rodent diet containing 60 kcal% fat.44 The glucose and insulin levels were used to evaluate T2D induction at 20 weeks. In addition to single diet–induced diabetes, use of a HFD combined with multiple doses of STZ has also been used in a mouse model. Wang et al.41 combined a HFD with chemical STZ treatment to induce T2D. Specifically, 5–week–old C57BL/6 mice were fed with a HFD (60% of calories from fat) for 24 weeks. After HFD feeding for 23 weeks, mice were injected with 40 mg/kg STZ daily for 3 consecutive days to generate T2D.41 In a similar experiment, C57BL/6 mice were fed a HFD for 8 weeks, and then given an intraperitoneal injection of 100 mg/kg STZ at weeks 9 and 11. After treatment, 4 hour–fasting blood glucose over 16.7 mM in the tail vein was considered T2D. The mouse model is clinically relevant and has the benefit that therapeutic agents have been tested.45 Therefore, it is essential that the appropriate model is chosen when considering the type of wound healing. ...
An overview of murine high fat diet as a model for type 2 diabetes mellitus
1
2016
... For the most common T2D model in mice, studies showed that daily STZ administration generated T2D models in C57BL/6 mice given 40 mg/kg for 3 consecutive days41 or in 8–week–old db/+ mice given 50 mg/kg for 4 consecutive days.42 Many other researchers instead used a straightforward high–fat diet (HFD) to model human T2D because a HFD is a dependable model that provides an appropriate aetiological, pathological, and treatment option for T2D.43 C57BL/6 mice were provided with a rodent diet containing 60 kcal% fat.44 The glucose and insulin levels were used to evaluate T2D induction at 20 weeks. In addition to single diet–induced diabetes, use of a HFD combined with multiple doses of STZ has also been used in a mouse model. Wang et al.41 combined a HFD with chemical STZ treatment to induce T2D. Specifically, 5–week–old C57BL/6 mice were fed with a HFD (60% of calories from fat) for 24 weeks. After HFD feeding for 23 weeks, mice were injected with 40 mg/kg STZ daily for 3 consecutive days to generate T2D.41 In a similar experiment, C57BL/6 mice were fed a HFD for 8 weeks, and then given an intraperitoneal injection of 100 mg/kg STZ at weeks 9 and 11. After treatment, 4 hour–fasting blood glucose over 16.7 mM in the tail vein was considered T2D. The mouse model is clinically relevant and has the benefit that therapeutic agents have been tested.45 Therefore, it is essential that the appropriate model is chosen when considering the type of wound healing. ...
Metformin strongly affects gut microbiome composition in high-fat diet-induced type 2 diabetes mouse model of both sexes
1
2021
... For the most common T2D model in mice, studies showed that daily STZ administration generated T2D models in C57BL/6 mice given 40 mg/kg for 3 consecutive days41 or in 8–week–old db/+ mice given 50 mg/kg for 4 consecutive days.42 Many other researchers instead used a straightforward high–fat diet (HFD) to model human T2D because a HFD is a dependable model that provides an appropriate aetiological, pathological, and treatment option for T2D.43 C57BL/6 mice were provided with a rodent diet containing 60 kcal% fat.44 The glucose and insulin levels were used to evaluate T2D induction at 20 weeks. In addition to single diet–induced diabetes, use of a HFD combined with multiple doses of STZ has also been used in a mouse model. Wang et al.41 combined a HFD with chemical STZ treatment to induce T2D. Specifically, 5–week–old C57BL/6 mice were fed with a HFD (60% of calories from fat) for 24 weeks. After HFD feeding for 23 weeks, mice were injected with 40 mg/kg STZ daily for 3 consecutive days to generate T2D.41 In a similar experiment, C57BL/6 mice were fed a HFD for 8 weeks, and then given an intraperitoneal injection of 100 mg/kg STZ at weeks 9 and 11. After treatment, 4 hour–fasting blood glucose over 16.7 mM in the tail vein was considered T2D. The mouse model is clinically relevant and has the benefit that therapeutic agents have been tested.45 Therefore, it is essential that the appropriate model is chosen when considering the type of wound healing. ...
Research techniques made simple: animal models of wound healing
1
2018
... For the most common T2D model in mice, studies showed that daily STZ administration generated T2D models in C57BL/6 mice given 40 mg/kg for 3 consecutive days41 or in 8–week–old db/+ mice given 50 mg/kg for 4 consecutive days.42 Many other researchers instead used a straightforward high–fat diet (HFD) to model human T2D because a HFD is a dependable model that provides an appropriate aetiological, pathological, and treatment option for T2D.43 C57BL/6 mice were provided with a rodent diet containing 60 kcal% fat.44 The glucose and insulin levels were used to evaluate T2D induction at 20 weeks. In addition to single diet–induced diabetes, use of a HFD combined with multiple doses of STZ has also been used in a mouse model. Wang et al.41 combined a HFD with chemical STZ treatment to induce T2D. Specifically, 5–week–old C57BL/6 mice were fed with a HFD (60% of calories from fat) for 24 weeks. After HFD feeding for 23 weeks, mice were injected with 40 mg/kg STZ daily for 3 consecutive days to generate T2D.41 In a similar experiment, C57BL/6 mice were fed a HFD for 8 weeks, and then given an intraperitoneal injection of 100 mg/kg STZ at weeks 9 and 11. After treatment, 4 hour–fasting blood glucose over 16.7 mM in the tail vein was considered T2D. The mouse model is clinically relevant and has the benefit that therapeutic agents have been tested.45 Therefore, it is essential that the appropriate model is chosen when considering the type of wound healing. ...
Streptozotocin induced acute clinical effects in rabbits (Oryctolagus cuniculus)
1
2015
... Rabbits were also monitored for clinical signs including changes in behaviour, activity, diet, urination/defecation and blood glucose levels.46,47 The rabbit ear model of diabetic wounds overcomes the contraction issues and has the advantage of the ability to create multiple wounds.20 Zhang et al.48 studied T2D models using New Zealand rabbits. Rabbits were first fed a HFD for 2 months. At the end of this period, the rabbits were injected every 3 days with alloxan monohydrate (50 mg/kg) via the ear following 6 hours of fasting. Rabbits with fasting blood glucose level > 11.1 mM were then used in the experiment. After the induction of T2D, a 10 mm × 10 mm full–thickness section of skin was excised and a radial debridement of the wound was conducted. The wounds in the treatment group were treated with epidermal growth factor (EGF) once a day for 1 month then compared with the control group. In the treatment group, endogenous EGF mRNA was significantly higher than in the control group, which indicated that exogenous EGF treatment accelerated the healing of diabetic wounds by upregulating the expression of EGF mRNA in newly–generated tissues. In another alloxan–induced diabetic rabbit ear ulcer model,49 alloxan at 150 mg/kg in 30 mL of saline was administered intravenously via an ear vein. After drug induction, glucose and molasses were provided for 24 hours to avoid hypoglycaemia. Blood glucose levels were checked weekly using blood from the marginal ear vein after the blood sugar level had stabilised. Four weeks after alloxan treatment, rabbits were anaesthetised and full–thickness punch biopsy wounds with a diameter of 6 mm were created on each of the hyperglycaemic rabbits. They found that autologous circulating angiogenic cells treated with osteopontin could promote diabetic wounds healing. By using the same diabetic models with alloxan, collagen seeded with allogeneic mesenchymal stem cells resulted in increased angiogenesis and accelerated diabetic wound healing.50 ...
Spontaneous diabetes mellitus in the New Zealand white rabbit: physiologic characteristics
1
1981
... Rabbits were also monitored for clinical signs including changes in behaviour, activity, diet, urination/defecation and blood glucose levels.46,47 The rabbit ear model of diabetic wounds overcomes the contraction issues and has the advantage of the ability to create multiple wounds.20 Zhang et al.48 studied T2D models using New Zealand rabbits. Rabbits were first fed a HFD for 2 months. At the end of this period, the rabbits were injected every 3 days with alloxan monohydrate (50 mg/kg) via the ear following 6 hours of fasting. Rabbits with fasting blood glucose level > 11.1 mM were then used in the experiment. After the induction of T2D, a 10 mm × 10 mm full–thickness section of skin was excised and a radial debridement of the wound was conducted. The wounds in the treatment group were treated with epidermal growth factor (EGF) once a day for 1 month then compared with the control group. In the treatment group, endogenous EGF mRNA was significantly higher than in the control group, which indicated that exogenous EGF treatment accelerated the healing of diabetic wounds by upregulating the expression of EGF mRNA in newly–generated tissues. In another alloxan–induced diabetic rabbit ear ulcer model,49 alloxan at 150 mg/kg in 30 mL of saline was administered intravenously via an ear vein. After drug induction, glucose and molasses were provided for 24 hours to avoid hypoglycaemia. Blood glucose levels were checked weekly using blood from the marginal ear vein after the blood sugar level had stabilised. Four weeks after alloxan treatment, rabbits were anaesthetised and full–thickness punch biopsy wounds with a diameter of 6 mm were created on each of the hyperglycaemic rabbits. They found that autologous circulating angiogenic cells treated with osteopontin could promote diabetic wounds healing. By using the same diabetic models with alloxan, collagen seeded with allogeneic mesenchymal stem cells resulted in increased angiogenesis and accelerated diabetic wound healing.50 ...
Therapeutic effect of the epidermal growth factor on diabetic foot ulcer and the underlying mechanisms
1
2019
... Rabbits were also monitored for clinical signs including changes in behaviour, activity, diet, urination/defecation and blood glucose levels.46,47 The rabbit ear model of diabetic wounds overcomes the contraction issues and has the advantage of the ability to create multiple wounds.20 Zhang et al.48 studied T2D models using New Zealand rabbits. Rabbits were first fed a HFD for 2 months. At the end of this period, the rabbits were injected every 3 days with alloxan monohydrate (50 mg/kg) via the ear following 6 hours of fasting. Rabbits with fasting blood glucose level > 11.1 mM were then used in the experiment. After the induction of T2D, a 10 mm × 10 mm full–thickness section of skin was excised and a radial debridement of the wound was conducted. The wounds in the treatment group were treated with epidermal growth factor (EGF) once a day for 1 month then compared with the control group. In the treatment group, endogenous EGF mRNA was significantly higher than in the control group, which indicated that exogenous EGF treatment accelerated the healing of diabetic wounds by upregulating the expression of EGF mRNA in newly–generated tissues. In another alloxan–induced diabetic rabbit ear ulcer model,49 alloxan at 150 mg/kg in 30 mL of saline was administered intravenously via an ear vein. After drug induction, glucose and molasses were provided for 24 hours to avoid hypoglycaemia. Blood glucose levels were checked weekly using blood from the marginal ear vein after the blood sugar level had stabilised. Four weeks after alloxan treatment, rabbits were anaesthetised and full–thickness punch biopsy wounds with a diameter of 6 mm were created on each of the hyperglycaemic rabbits. They found that autologous circulating angiogenic cells treated with osteopontin could promote diabetic wounds healing. By using the same diabetic models with alloxan, collagen seeded with allogeneic mesenchymal stem cells resulted in increased angiogenesis and accelerated diabetic wound healing.50 ...
Autologous circulating angiogenic cells treated with osteopontin and delivered via a collagen scaffold enhance wound healing in the alloxan-induced diabetic rabbit ear ulcer model
1
2013
... Rabbits were also monitored for clinical signs including changes in behaviour, activity, diet, urination/defecation and blood glucose levels.46,47 The rabbit ear model of diabetic wounds overcomes the contraction issues and has the advantage of the ability to create multiple wounds.20 Zhang et al.48 studied T2D models using New Zealand rabbits. Rabbits were first fed a HFD for 2 months. At the end of this period, the rabbits were injected every 3 days with alloxan monohydrate (50 mg/kg) via the ear following 6 hours of fasting. Rabbits with fasting blood glucose level > 11.1 mM were then used in the experiment. After the induction of T2D, a 10 mm × 10 mm full–thickness section of skin was excised and a radial debridement of the wound was conducted. The wounds in the treatment group were treated with epidermal growth factor (EGF) once a day for 1 month then compared with the control group. In the treatment group, endogenous EGF mRNA was significantly higher than in the control group, which indicated that exogenous EGF treatment accelerated the healing of diabetic wounds by upregulating the expression of EGF mRNA in newly–generated tissues. In another alloxan–induced diabetic rabbit ear ulcer model,49 alloxan at 150 mg/kg in 30 mL of saline was administered intravenously via an ear vein. After drug induction, glucose and molasses were provided for 24 hours to avoid hypoglycaemia. Blood glucose levels were checked weekly using blood from the marginal ear vein after the blood sugar level had stabilised. Four weeks after alloxan treatment, rabbits were anaesthetised and full–thickness punch biopsy wounds with a diameter of 6 mm were created on each of the hyperglycaemic rabbits. They found that autologous circulating angiogenic cells treated with osteopontin could promote diabetic wounds healing. By using the same diabetic models with alloxan, collagen seeded with allogeneic mesenchymal stem cells resulted in increased angiogenesis and accelerated diabetic wound healing.50 ...
Topical administration of allogeneic mesenchymal stromal cells seeded in a collagen scaffold augments wound healing and increases angiogenesis in the diabetic rabbit ulcer
1
2013
... Rabbits were also monitored for clinical signs including changes in behaviour, activity, diet, urination/defecation and blood glucose levels.46,47 The rabbit ear model of diabetic wounds overcomes the contraction issues and has the advantage of the ability to create multiple wounds.20 Zhang et al.48 studied T2D models using New Zealand rabbits. Rabbits were first fed a HFD for 2 months. At the end of this period, the rabbits were injected every 3 days with alloxan monohydrate (50 mg/kg) via the ear following 6 hours of fasting. Rabbits with fasting blood glucose level > 11.1 mM were then used in the experiment. After the induction of T2D, a 10 mm × 10 mm full–thickness section of skin was excised and a radial debridement of the wound was conducted. The wounds in the treatment group were treated with epidermal growth factor (EGF) once a day for 1 month then compared with the control group. In the treatment group, endogenous EGF mRNA was significantly higher than in the control group, which indicated that exogenous EGF treatment accelerated the healing of diabetic wounds by upregulating the expression of EGF mRNA in newly–generated tissues. In another alloxan–induced diabetic rabbit ear ulcer model,49 alloxan at 150 mg/kg in 30 mL of saline was administered intravenously via an ear vein. After drug induction, glucose and molasses were provided for 24 hours to avoid hypoglycaemia. Blood glucose levels were checked weekly using blood from the marginal ear vein after the blood sugar level had stabilised. Four weeks after alloxan treatment, rabbits were anaesthetised and full–thickness punch biopsy wounds with a diameter of 6 mm were created on each of the hyperglycaemic rabbits. They found that autologous circulating angiogenic cells treated with osteopontin could promote diabetic wounds healing. By using the same diabetic models with alloxan, collagen seeded with allogeneic mesenchymal stem cells resulted in increased angiogenesis and accelerated diabetic wound healing.50 ...
Impaired wound healing in an acute diabetic pig model and the effects of local hyperglycemia
2
2008
... Pigs also have the advantage of a similar skin wound healing process to humans. The courses of re–epithelialisation and granulation tissue formation closely resemble those in humans.20 Pig diabetic ulcer models have been successfully established.51,52 The chemical drug STZ was also used to cause systematic hyperglycaemia in pigs at a dose of 150 mg/kg body weight, then serum glucose concentration was measured, and short–acting and long–acting insulin was used to keep the glucose level between 350 and 550 mg/dL for 14 days. After induction, the full thickness of pig was created. By detecting glucose concentration in serum and wound fluid, they found that delayed wound healing in diabetic pigs is not induced by local high glucose concentration. They further found that diabetic pigs showed impaired healing accompanied by reduction of insulin–like growth factor 1 level in the healing wound.51 In another pig in vivo ulcer model,53 pathogen–free pigs were injured using a modified electro–keratome set to obtain partial–thickness wounds of 10 mm × 7 mm × 0.5 mm. Wounds were treated within 20 minutes with mevastatin. The results showed that mevastatin selectively triggered an anti–proliferative, pro–migratory phenotype and restored EGF sensitivity in the diabetic wound, resulting in healing of chronic wounds.53 However, pigs are difficult to anaesthetise due to their large size. The use of pigs as diabetic models is largely limited because of limitations including the long period needed to raise them and to the expense of maintaining them. ...
... 51 In another pig in vivo ulcer model,53 pathogen–free pigs were injured using a modified electro–keratome set to obtain partial–thickness wounds of 10 mm × 7 mm × 0.5 mm. Wounds were treated within 20 minutes with mevastatin. The results showed that mevastatin selectively triggered an anti–proliferative, pro–migratory phenotype and restored EGF sensitivity in the diabetic wound, resulting in healing of chronic wounds.53 However, pigs are difficult to anaesthetise due to their large size. The use of pigs as diabetic models is largely limited because of limitations including the long period needed to raise them and to the expense of maintaining them. ...
Staphylococcus aureus triggers induction of miR-15B-5P to Diminish DNA repair and deregulate inflammatory response in diabetic foot ulcers
1
2018
... Pigs also have the advantage of a similar skin wound healing process to humans. The courses of re–epithelialisation and granulation tissue formation closely resemble those in humans.20 Pig diabetic ulcer models have been successfully established.51,52 The chemical drug STZ was also used to cause systematic hyperglycaemia in pigs at a dose of 150 mg/kg body weight, then serum glucose concentration was measured, and short–acting and long–acting insulin was used to keep the glucose level between 350 and 550 mg/dL for 14 days. After induction, the full thickness of pig was created. By detecting glucose concentration in serum and wound fluid, they found that delayed wound healing in diabetic pigs is not induced by local high glucose concentration. They further found that diabetic pigs showed impaired healing accompanied by reduction of insulin–like growth factor 1 level in the healing wound.51 In another pig in vivo ulcer model,53 pathogen–free pigs were injured using a modified electro–keratome set to obtain partial–thickness wounds of 10 mm × 7 mm × 0.5 mm. Wounds were treated within 20 minutes with mevastatin. The results showed that mevastatin selectively triggered an anti–proliferative, pro–migratory phenotype and restored EGF sensitivity in the diabetic wound, resulting in healing of chronic wounds.53 However, pigs are difficult to anaesthetise due to their large size. The use of pigs as diabetic models is largely limited because of limitations including the long period needed to raise them and to the expense of maintaining them. ...
Mevastatin promotes healing by targeting caveolin-1 to restore EGFR signaling
2
2019
... Pigs also have the advantage of a similar skin wound healing process to humans. The courses of re–epithelialisation and granulation tissue formation closely resemble those in humans.20 Pig diabetic ulcer models have been successfully established.51,52 The chemical drug STZ was also used to cause systematic hyperglycaemia in pigs at a dose of 150 mg/kg body weight, then serum glucose concentration was measured, and short–acting and long–acting insulin was used to keep the glucose level between 350 and 550 mg/dL for 14 days. After induction, the full thickness of pig was created. By detecting glucose concentration in serum and wound fluid, they found that delayed wound healing in diabetic pigs is not induced by local high glucose concentration. They further found that diabetic pigs showed impaired healing accompanied by reduction of insulin–like growth factor 1 level in the healing wound.51 In another pig in vivo ulcer model,53 pathogen–free pigs were injured using a modified electro–keratome set to obtain partial–thickness wounds of 10 mm × 7 mm × 0.5 mm. Wounds were treated within 20 minutes with mevastatin. The results showed that mevastatin selectively triggered an anti–proliferative, pro–migratory phenotype and restored EGF sensitivity in the diabetic wound, resulting in healing of chronic wounds.53 However, pigs are difficult to anaesthetise due to their large size. The use of pigs as diabetic models is largely limited because of limitations including the long period needed to raise them and to the expense of maintaining them. ...
... 53 However, pigs are difficult to anaesthetise due to their large size. The use of pigs as diabetic models is largely limited because of limitations including the long period needed to raise them and to the expense of maintaining them. ...
A model of type 2 diabetes in the guinea pig using sequential diet-induced glucose intolerance and streptozotocin treatment
1
2017
... Despite advances in the methods of creating wound healing models, there is still a lack of an optimal preclinical model capable of completely recapitulating human diabetic wounds. However, the pace of exploration has not slowed. Hyperglycaemia was evident in 25–100% of treated guinea pigs within 48 hours, at doses of 200 or 250 mg/kg of STZ.54 Especially when HFD–fed guinea pigs were treated with STZ, glucose intolerance and fasting hyperglycaemia could persist for more than 21 days. Moreover, diabetes and insulin resistance models can also be induced by genetic manipulation. A novel strain of mice expressing the human HLA–DR4 gene was created, which was characterised by diverse leucocyte infiltration and the formation of anti–proinsulin auto–antibodies that was akin to human T1D.55 The more exploration and attempts are carried out to create preclinical models of diabetic wound healing, the more these lab findings can be translated into clinical treatments for diabetic wounds. ...
Proinsulin-mediated induction of type 1 diabetes in HLA-DR4-transgenic mice
1
2018
... Despite advances in the methods of creating wound healing models, there is still a lack of an optimal preclinical model capable of completely recapitulating human diabetic wounds. However, the pace of exploration has not slowed. Hyperglycaemia was evident in 25–100% of treated guinea pigs within 48 hours, at doses of 200 or 250 mg/kg of STZ.54 Especially when HFD–fed guinea pigs were treated with STZ, glucose intolerance and fasting hyperglycaemia could persist for more than 21 days. Moreover, diabetes and insulin resistance models can also be induced by genetic manipulation. A novel strain of mice expressing the human HLA–DR4 gene was created, which was characterised by diverse leucocyte infiltration and the formation of anti–proinsulin auto–antibodies that was akin to human T1D.55 The more exploration and attempts are carried out to create preclinical models of diabetic wound healing, the more these lab findings can be translated into clinical treatments for diabetic wounds. ...
Current therapeutic strategies in diabetic foot ulcers
2
2019
... High–glucose–induced peripheral neuropathy, peripheral arterial occlusive disease, infection, immunosuppression and increased plantar stress are all important factors of diabetic wounds.56,57 However, diabetic wounds do not follow the normal dynamic process of wound healing: haemostasis, inflammation, proliferation, and remodelling. These complex internal and external factors make patients more vulnerable to problems developing at many levels (Figure 2).58,59 ...
... DFU are a serious complication of diabetes resulting in significant morbidity, poor quality of life, worse psychological endurance, and a high mortality rate.90,91 The wound–healing process of these ulcers often becomes refractory, especially when the underlying causes are unclear and the patient does not receive holistic professional treatments. In diabetic patients, reduced fibrinolysis, poor formation of extracellular matrix, aberrant secretion of inflammatory cytokines, diminution of angiogenesis, reduction of fibroblasts and infection are all important factors that contribute to the problem of deficient wound closure. The current standard diabetic wound treatments comprise four main principles: (1) debridement, (2) infection management, (3) offloading and (4) revascularisation (Figure 3).92 Despite these standards, many diabetic wounds persist. Strategies for the management of patients with a diabetic wound are multidisciplinary approaches and involved in multifactorial processes including glycaemic control.56 Therefore, new approaches are warranted for these complex wounds. ...
Managing diabetic foot ulcers: pharmacotherapy for wound healing
1
2021
... High–glucose–induced peripheral neuropathy, peripheral arterial occlusive disease, infection, immunosuppression and increased plantar stress are all important factors of diabetic wounds.56,57 However, diabetic wounds do not follow the normal dynamic process of wound healing: haemostasis, inflammation, proliferation, and remodelling. These complex internal and external factors make patients more vulnerable to problems developing at many levels (Figure 2).58,59 ...
The diabetic foot ulcer
1
2020
... High–glucose–induced peripheral neuropathy, peripheral arterial occlusive disease, infection, immunosuppression and increased plantar stress are all important factors of diabetic wounds.56,57 However, diabetic wounds do not follow the normal dynamic process of wound healing: haemostasis, inflammation, proliferation, and remodelling. These complex internal and external factors make patients more vulnerable to problems developing at many levels (Figure 2).58,59 ...
Prevention and treatment of diabetic foot ulcers
1
2017
... High–glucose–induced peripheral neuropathy, peripheral arterial occlusive disease, infection, immunosuppression and increased plantar stress are all important factors of diabetic wounds.56,57 However, diabetic wounds do not follow the normal dynamic process of wound healing: haemostasis, inflammation, proliferation, and remodelling. These complex internal and external factors make patients more vulnerable to problems developing at many levels (Figure 2).58,59 ...
11. Microvascular complications and foot care: standards of medical care in diabetes-2020
1
2020
... Diabetes mellitus is often associated with a series of changes in macro– and micro–vessels that manifest as multiple complications. A spectrum of lesions associated with microvascular outcomes in patients include chronic kidney disease, diabetic retinopathy, cardiovascular disorders and foot ulcers.60⇓⇓–63 In diabetic kidney disease, glomerular vasculopathy damage drives the development of diabetic kidney disease which leads to glomerular hyperfiltration, progressive albuminuria, declining glomerular filtration rate, followed by progressive failure of renal function.64⇓–66 Diabetic retinopathy includes changes in vascular permeability, capillary degeneration, capillary microaneurysms, and excessive neovascularisation within the retina, which contribute to changes in the retina blood vessels, and lead to visual impairment and eventually cause premature blindness.63,67 Diabetic heart disease encompasses a series of disorders ranging from diabetic myocardial infarction to cardiomyopathy, heart failure and sudden cardiac death. Long–term hyperglycaemia leads to an increased risk of atherosclerosis of coronary vessels and differential remodelling of myocardial arterioles and capillaries,62 which is thought to be induced by the increased advanced–glycation end products, activation of the renin–angiotensin–aldosterone system, impaired microcirculation, abnormal mitochondrial function and aberrant calcium signaling.68,69 ...
Diabetic microvascular complications
1
2018
... Diabetes mellitus is often associated with a series of changes in macro– and micro–vessels that manifest as multiple complications. A spectrum of lesions associated with microvascular outcomes in patients include chronic kidney disease, diabetic retinopathy, cardiovascular disorders and foot ulcers.60⇓⇓–63 In diabetic kidney disease, glomerular vasculopathy damage drives the development of diabetic kidney disease which leads to glomerular hyperfiltration, progressive albuminuria, declining glomerular filtration rate, followed by progressive failure of renal function.64⇓–66 Diabetic retinopathy includes changes in vascular permeability, capillary degeneration, capillary microaneurysms, and excessive neovascularisation within the retina, which contribute to changes in the retina blood vessels, and lead to visual impairment and eventually cause premature blindness.63,67 Diabetic heart disease encompasses a series of disorders ranging from diabetic myocardial infarction to cardiomyopathy, heart failure and sudden cardiac death. Long–term hyperglycaemia leads to an increased risk of atherosclerosis of coronary vessels and differential remodelling of myocardial arterioles and capillaries,62 which is thought to be induced by the increased advanced–glycation end products, activation of the renin–angiotensin–aldosterone system, impaired microcirculation, abnormal mitochondrial function and aberrant calcium signaling.68,69 ...
Diabetes, cardiovascular disease and the microcirculation
2
2018
... Diabetes mellitus is often associated with a series of changes in macro– and micro–vessels that manifest as multiple complications. A spectrum of lesions associated with microvascular outcomes in patients include chronic kidney disease, diabetic retinopathy, cardiovascular disorders and foot ulcers.60⇓⇓–63 In diabetic kidney disease, glomerular vasculopathy damage drives the development of diabetic kidney disease which leads to glomerular hyperfiltration, progressive albuminuria, declining glomerular filtration rate, followed by progressive failure of renal function.64⇓–66 Diabetic retinopathy includes changes in vascular permeability, capillary degeneration, capillary microaneurysms, and excessive neovascularisation within the retina, which contribute to changes in the retina blood vessels, and lead to visual impairment and eventually cause premature blindness.63,67 Diabetic heart disease encompasses a series of disorders ranging from diabetic myocardial infarction to cardiomyopathy, heart failure and sudden cardiac death. Long–term hyperglycaemia leads to an increased risk of atherosclerosis of coronary vessels and differential remodelling of myocardial arterioles and capillaries,62 which is thought to be induced by the increased advanced–glycation end products, activation of the renin–angiotensin–aldosterone system, impaired microcirculation, abnormal mitochondrial function and aberrant calcium signaling.68,69 ...
... 62 which is thought to be induced by the increased advanced–glycation end products, activation of the renin–angiotensin–aldosterone system, impaired microcirculation, abnormal mitochondrial function and aberrant calcium signaling.68,69 ...
Mechanisms of diabetic complications
3
2013
... Diabetes mellitus is often associated with a series of changes in macro– and micro–vessels that manifest as multiple complications. A spectrum of lesions associated with microvascular outcomes in patients include chronic kidney disease, diabetic retinopathy, cardiovascular disorders and foot ulcers.60⇓⇓–63 In diabetic kidney disease, glomerular vasculopathy damage drives the development of diabetic kidney disease which leads to glomerular hyperfiltration, progressive albuminuria, declining glomerular filtration rate, followed by progressive failure of renal function.64⇓–66 Diabetic retinopathy includes changes in vascular permeability, capillary degeneration, capillary microaneurysms, and excessive neovascularisation within the retina, which contribute to changes in the retina blood vessels, and lead to visual impairment and eventually cause premature blindness.63,67 Diabetic heart disease encompasses a series of disorders ranging from diabetic myocardial infarction to cardiomyopathy, heart failure and sudden cardiac death. Long–term hyperglycaemia leads to an increased risk of atherosclerosis of coronary vessels and differential remodelling of myocardial arterioles and capillaries,62 which is thought to be induced by the increased advanced–glycation end products, activation of the renin–angiotensin–aldosterone system, impaired microcirculation, abnormal mitochondrial function and aberrant calcium signaling.68,69 ...
... 63,67 Diabetic heart disease encompasses a series of disorders ranging from diabetic myocardial infarction to cardiomyopathy, heart failure and sudden cardiac death. Long–term hyperglycaemia leads to an increased risk of atherosclerosis of coronary vessels and differential remodelling of myocardial arterioles and capillaries,62 which is thought to be induced by the increased advanced–glycation end products, activation of the renin–angiotensin–aldosterone system, impaired microcirculation, abnormal mitochondrial function and aberrant calcium signaling.68,69 ...
... Both sensory and autonomic nerves populate skin tissues. Cutaneous sensory nerves predominate and are widely distributed in the skin, including extending into the upper epidermis where they come into contact with the external environment. In contrast, autonomic nerves are distributed in the dermis of the skin and mainly regulate lymphatic function, blood circulation, and appendageal function.7 Nowadays, there is a growing appreciation that neuropathy is a major factor in impaired skin integrity in diabetes.63 Diabetic neuropathological changes preferentially damage sensory neurons, and the unmyelinated small–diameter sensory axons are especially susceptible.79 Evidence of sensory neuropathy is decreased or absent sense of vibration and superficial sensitivity including pressure, haptics, and subjective paresthesias. Consequently, the sensation of pain is substantially decreased and the risk of trauma is significantly higher. Due to the loss of pain sensation, serious ulcerations are underestimated and injuries are thus often not noticed in patients with diabetes mellitus.74 Autonomic nerve damage leads to arterial sclerosis, vasomotor paralysis, arteriovenous shunts in the microvascular network, dysfunctional sudomotor function, and neuropathic oedemas in damaged skin. As a result, affected skin in patients with diabetes mellitus has a reduced protective function and an increased risk of injury.77 The combination of these peripheral nerve problems leads to abnormal foot pressures in the form of an elevated plantar pressure load, accompanied by the development of hyperkeratosis and finally diabetic foot ulcers.80,81 ...
Diabetic kidney disease: challenges, progress, and possibilities
1
2017
... Diabetes mellitus is often associated with a series of changes in macro– and micro–vessels that manifest as multiple complications. A spectrum of lesions associated with microvascular outcomes in patients include chronic kidney disease, diabetic retinopathy, cardiovascular disorders and foot ulcers.60⇓⇓–63 In diabetic kidney disease, glomerular vasculopathy damage drives the development of diabetic kidney disease which leads to glomerular hyperfiltration, progressive albuminuria, declining glomerular filtration rate, followed by progressive failure of renal function.64⇓–66 Diabetic retinopathy includes changes in vascular permeability, capillary degeneration, capillary microaneurysms, and excessive neovascularisation within the retina, which contribute to changes in the retina blood vessels, and lead to visual impairment and eventually cause premature blindness.63,67 Diabetic heart disease encompasses a series of disorders ranging from diabetic myocardial infarction to cardiomyopathy, heart failure and sudden cardiac death. Long–term hyperglycaemia leads to an increased risk of atherosclerosis of coronary vessels and differential remodelling of myocardial arterioles and capillaries,62 which is thought to be induced by the increased advanced–glycation end products, activation of the renin–angiotensin–aldosterone system, impaired microcirculation, abnormal mitochondrial function and aberrant calcium signaling.68,69 ...
Kidney disease in diabetes: from mechanisms to clinical presentation and treatment strategies
1
2021
... Diabetes mellitus is often associated with a series of changes in macro– and micro–vessels that manifest as multiple complications. A spectrum of lesions associated with microvascular outcomes in patients include chronic kidney disease, diabetic retinopathy, cardiovascular disorders and foot ulcers.60⇓⇓–63 In diabetic kidney disease, glomerular vasculopathy damage drives the development of diabetic kidney disease which leads to glomerular hyperfiltration, progressive albuminuria, declining glomerular filtration rate, followed by progressive failure of renal function.64⇓–66 Diabetic retinopathy includes changes in vascular permeability, capillary degeneration, capillary microaneurysms, and excessive neovascularisation within the retina, which contribute to changes in the retina blood vessels, and lead to visual impairment and eventually cause premature blindness.63,67 Diabetic heart disease encompasses a series of disorders ranging from diabetic myocardial infarction to cardiomyopathy, heart failure and sudden cardiac death. Long–term hyperglycaemia leads to an increased risk of atherosclerosis of coronary vessels and differential remodelling of myocardial arterioles and capillaries,62 which is thought to be induced by the increased advanced–glycation end products, activation of the renin–angiotensin–aldosterone system, impaired microcirculation, abnormal mitochondrial function and aberrant calcium signaling.68,69 ...
The presence of diabetes mellitus further impairs structural and functional capillary density in patients with chronic kidney disease
1
2021
... Diabetes mellitus is often associated with a series of changes in macro– and micro–vessels that manifest as multiple complications. A spectrum of lesions associated with microvascular outcomes in patients include chronic kidney disease, diabetic retinopathy, cardiovascular disorders and foot ulcers.60⇓⇓–63 In diabetic kidney disease, glomerular vasculopathy damage drives the development of diabetic kidney disease which leads to glomerular hyperfiltration, progressive albuminuria, declining glomerular filtration rate, followed by progressive failure of renal function.64⇓–66 Diabetic retinopathy includes changes in vascular permeability, capillary degeneration, capillary microaneurysms, and excessive neovascularisation within the retina, which contribute to changes in the retina blood vessels, and lead to visual impairment and eventually cause premature blindness.63,67 Diabetic heart disease encompasses a series of disorders ranging from diabetic myocardial infarction to cardiomyopathy, heart failure and sudden cardiac death. Long–term hyperglycaemia leads to an increased risk of atherosclerosis of coronary vessels and differential remodelling of myocardial arterioles and capillaries,62 which is thought to be induced by the increased advanced–glycation end products, activation of the renin–angiotensin–aldosterone system, impaired microcirculation, abnormal mitochondrial function and aberrant calcium signaling.68,69 ...
Incidence and progression of diabetic retinopathy: a systematic review
1
2019
... Diabetes mellitus is often associated with a series of changes in macro– and micro–vessels that manifest as multiple complications. A spectrum of lesions associated with microvascular outcomes in patients include chronic kidney disease, diabetic retinopathy, cardiovascular disorders and foot ulcers.60⇓⇓–63 In diabetic kidney disease, glomerular vasculopathy damage drives the development of diabetic kidney disease which leads to glomerular hyperfiltration, progressive albuminuria, declining glomerular filtration rate, followed by progressive failure of renal function.64⇓–66 Diabetic retinopathy includes changes in vascular permeability, capillary degeneration, capillary microaneurysms, and excessive neovascularisation within the retina, which contribute to changes in the retina blood vessels, and lead to visual impairment and eventually cause premature blindness.63,67 Diabetic heart disease encompasses a series of disorders ranging from diabetic myocardial infarction to cardiomyopathy, heart failure and sudden cardiac death. Long–term hyperglycaemia leads to an increased risk of atherosclerosis of coronary vessels and differential remodelling of myocardial arterioles and capillaries,62 which is thought to be induced by the increased advanced–glycation end products, activation of the renin–angiotensin–aldosterone system, impaired microcirculation, abnormal mitochondrial function and aberrant calcium signaling.68,69 ...
The Changing landscape of pharmacotherapy for diabetes mellitus: a review of cardiovascular outcomes
1
2019
... Diabetes mellitus is often associated with a series of changes in macro– and micro–vessels that manifest as multiple complications. A spectrum of lesions associated with microvascular outcomes in patients include chronic kidney disease, diabetic retinopathy, cardiovascular disorders and foot ulcers.60⇓⇓–63 In diabetic kidney disease, glomerular vasculopathy damage drives the development of diabetic kidney disease which leads to glomerular hyperfiltration, progressive albuminuria, declining glomerular filtration rate, followed by progressive failure of renal function.64⇓–66 Diabetic retinopathy includes changes in vascular permeability, capillary degeneration, capillary microaneurysms, and excessive neovascularisation within the retina, which contribute to changes in the retina blood vessels, and lead to visual impairment and eventually cause premature blindness.63,67 Diabetic heart disease encompasses a series of disorders ranging from diabetic myocardial infarction to cardiomyopathy, heart failure and sudden cardiac death. Long–term hyperglycaemia leads to an increased risk of atherosclerosis of coronary vessels and differential remodelling of myocardial arterioles and capillaries,62 which is thought to be induced by the increased advanced–glycation end products, activation of the renin–angiotensin–aldosterone system, impaired microcirculation, abnormal mitochondrial function and aberrant calcium signaling.68,69 ...
Cardiovascular safety trials for all new diabetes mellitus drugs
1
2019
... Diabetes mellitus is often associated with a series of changes in macro– and micro–vessels that manifest as multiple complications. A spectrum of lesions associated with microvascular outcomes in patients include chronic kidney disease, diabetic retinopathy, cardiovascular disorders and foot ulcers.60⇓⇓–63 In diabetic kidney disease, glomerular vasculopathy damage drives the development of diabetic kidney disease which leads to glomerular hyperfiltration, progressive albuminuria, declining glomerular filtration rate, followed by progressive failure of renal function.64⇓–66 Diabetic retinopathy includes changes in vascular permeability, capillary degeneration, capillary microaneurysms, and excessive neovascularisation within the retina, which contribute to changes in the retina blood vessels, and lead to visual impairment and eventually cause premature blindness.63,67 Diabetic heart disease encompasses a series of disorders ranging from diabetic myocardial infarction to cardiomyopathy, heart failure and sudden cardiac death. Long–term hyperglycaemia leads to an increased risk of atherosclerosis of coronary vessels and differential remodelling of myocardial arterioles and capillaries,62 which is thought to be induced by the increased advanced–glycation end products, activation of the renin–angiotensin–aldosterone system, impaired microcirculation, abnormal mitochondrial function and aberrant calcium signaling.68,69 ...
The ulcerated leg: when to revascularize
1
2012
... Microcirculatory changes are noted in the diabetic foot skin and ulcers. Chronic diabetic ulcers are developed and worsened by functional and structural microvascular dysfunction, arteriovenous shunting, microcirculatory ischaemia and different degrees of occlusive arterial lesions.70,71 Functionally, the skin microvasculature is altered with severely reduced capillary circulation, and occlusive hyperaemic response. It is known that the microvasculature of diabetic patients fails to respond appropriately to stress and injury.72 The rational mechanism is that blood is shunted away from the nutritional capillaries via subpapillary arteriovenous shunts, which are innervated by diabetic–damaged nerves that lead to the opening of these shunts and much lower resistance compared to nutritional capillaries. Structurally, the most obvious changes are the thickening of the capillary basement membrane, diminished capillary size, and pericyte degeneration and increased shunting of blood through arteriovenous channels.73 ...
Role of the microcirculation in diabetic foot ulceration
1
2006
... Microcirculatory changes are noted in the diabetic foot skin and ulcers. Chronic diabetic ulcers are developed and worsened by functional and structural microvascular dysfunction, arteriovenous shunting, microcirculatory ischaemia and different degrees of occlusive arterial lesions.70,71 Functionally, the skin microvasculature is altered with severely reduced capillary circulation, and occlusive hyperaemic response. It is known that the microvasculature of diabetic patients fails to respond appropriately to stress and injury.72 The rational mechanism is that blood is shunted away from the nutritional capillaries via subpapillary arteriovenous shunts, which are innervated by diabetic–damaged nerves that lead to the opening of these shunts and much lower resistance compared to nutritional capillaries. Structurally, the most obvious changes are the thickening of the capillary basement membrane, diminished capillary size, and pericyte degeneration and increased shunting of blood through arteriovenous channels.73 ...
Diabetic foot ulcers
2
2019
... Microcirculatory changes are noted in the diabetic foot skin and ulcers. Chronic diabetic ulcers are developed and worsened by functional and structural microvascular dysfunction, arteriovenous shunting, microcirculatory ischaemia and different degrees of occlusive arterial lesions.70,71 Functionally, the skin microvasculature is altered with severely reduced capillary circulation, and occlusive hyperaemic response. It is known that the microvasculature of diabetic patients fails to respond appropriately to stress and injury.72 The rational mechanism is that blood is shunted away from the nutritional capillaries via subpapillary arteriovenous shunts, which are innervated by diabetic–damaged nerves that lead to the opening of these shunts and much lower resistance compared to nutritional capillaries. Structurally, the most obvious changes are the thickening of the capillary basement membrane, diminished capillary size, and pericyte degeneration and increased shunting of blood through arteriovenous channels.73 ...
... Neural damage is termed “neuropathy”. According to epidemiological data, approximately 50% of the cases of diabetic foot syndrome can be accounted for by neuropathy while peripheral angiopathy is responsible for just 15%.74 Moreover, diabetic neuropathy can cause a series of complications including long–term chronic pain, foot ulcers, foot infections, and even catastrophic events including amputations.75,76 The pathophysiological mechanisms involved in diabetic neuropathy have been comprehensively and extensively reviewed.72 Diabetic peripheral neuropathy is caused by nerve dysfunction and neurocyte death as a consequence of oxidative stress, mitochondrial dysfunction, metabolic disorder and inflammation.75,77 Hyperglycaemia and insulin resistance collectively cause an imbalance in the mitochondrial redox state and excess formation of ROS, thereby contributing to dysregulation of metabolic pathways, a loss of stored energy and axonal injury, promoting diabetic neuropathy.78 ...
Microvascular dysfunction in diabetic foot disease and ulceration
1
2009
... Microcirculatory changes are noted in the diabetic foot skin and ulcers. Chronic diabetic ulcers are developed and worsened by functional and structural microvascular dysfunction, arteriovenous shunting, microcirculatory ischaemia and different degrees of occlusive arterial lesions.70,71 Functionally, the skin microvasculature is altered with severely reduced capillary circulation, and occlusive hyperaemic response. It is known that the microvasculature of diabetic patients fails to respond appropriately to stress and injury.72 The rational mechanism is that blood is shunted away from the nutritional capillaries via subpapillary arteriovenous shunts, which are innervated by diabetic–damaged nerves that lead to the opening of these shunts and much lower resistance compared to nutritional capillaries. Structurally, the most obvious changes are the thickening of the capillary basement membrane, diminished capillary size, and pericyte degeneration and increased shunting of blood through arteriovenous channels.73 ...
Neuropathy and diabetic foot syndrome
2
2016
... Neural damage is termed “neuropathy”. According to epidemiological data, approximately 50% of the cases of diabetic foot syndrome can be accounted for by neuropathy while peripheral angiopathy is responsible for just 15%.74 Moreover, diabetic neuropathy can cause a series of complications including long–term chronic pain, foot ulcers, foot infections, and even catastrophic events including amputations.75,76 The pathophysiological mechanisms involved in diabetic neuropathy have been comprehensively and extensively reviewed.72 Diabetic peripheral neuropathy is caused by nerve dysfunction and neurocyte death as a consequence of oxidative stress, mitochondrial dysfunction, metabolic disorder and inflammation.75,77 Hyperglycaemia and insulin resistance collectively cause an imbalance in the mitochondrial redox state and excess formation of ROS, thereby contributing to dysregulation of metabolic pathways, a loss of stored energy and axonal injury, promoting diabetic neuropathy.78 ...
... Both sensory and autonomic nerves populate skin tissues. Cutaneous sensory nerves predominate and are widely distributed in the skin, including extending into the upper epidermis where they come into contact with the external environment. In contrast, autonomic nerves are distributed in the dermis of the skin and mainly regulate lymphatic function, blood circulation, and appendageal function.7 Nowadays, there is a growing appreciation that neuropathy is a major factor in impaired skin integrity in diabetes.63 Diabetic neuropathological changes preferentially damage sensory neurons, and the unmyelinated small–diameter sensory axons are especially susceptible.79 Evidence of sensory neuropathy is decreased or absent sense of vibration and superficial sensitivity including pressure, haptics, and subjective paresthesias. Consequently, the sensation of pain is substantially decreased and the risk of trauma is significantly higher. Due to the loss of pain sensation, serious ulcerations are underestimated and injuries are thus often not noticed in patients with diabetes mellitus.74 Autonomic nerve damage leads to arterial sclerosis, vasomotor paralysis, arteriovenous shunts in the microvascular network, dysfunctional sudomotor function, and neuropathic oedemas in damaged skin. As a result, affected skin in patients with diabetes mellitus has a reduced protective function and an increased risk of injury.77 The combination of these peripheral nerve problems leads to abnormal foot pressures in the form of an elevated plantar pressure load, accompanied by the development of hyperkeratosis and finally diabetic foot ulcers.80,81 ...
Epidemiology of peripheral neuropathy and lower extremity disease in diabetes
2
2019
... Neural damage is termed “neuropathy”. According to epidemiological data, approximately 50% of the cases of diabetic foot syndrome can be accounted for by neuropathy while peripheral angiopathy is responsible for just 15%.74 Moreover, diabetic neuropathy can cause a series of complications including long–term chronic pain, foot ulcers, foot infections, and even catastrophic events including amputations.75,76 The pathophysiological mechanisms involved in diabetic neuropathy have been comprehensively and extensively reviewed.72 Diabetic peripheral neuropathy is caused by nerve dysfunction and neurocyte death as a consequence of oxidative stress, mitochondrial dysfunction, metabolic disorder and inflammation.75,77 Hyperglycaemia and insulin resistance collectively cause an imbalance in the mitochondrial redox state and excess formation of ROS, thereby contributing to dysregulation of metabolic pathways, a loss of stored energy and axonal injury, promoting diabetic neuropathy.78 ...
... 75,77 Hyperglycaemia and insulin resistance collectively cause an imbalance in the mitochondrial redox state and excess formation of ROS, thereby contributing to dysregulation of metabolic pathways, a loss of stored energy and axonal injury, promoting diabetic neuropathy.78 ...
A synoptic overview of neurovascular interactions in the foot
1
2020
... Neural damage is termed “neuropathy”. According to epidemiological data, approximately 50% of the cases of diabetic foot syndrome can be accounted for by neuropathy while peripheral angiopathy is responsible for just 15%.74 Moreover, diabetic neuropathy can cause a series of complications including long–term chronic pain, foot ulcers, foot infections, and even catastrophic events including amputations.75,76 The pathophysiological mechanisms involved in diabetic neuropathy have been comprehensively and extensively reviewed.72 Diabetic peripheral neuropathy is caused by nerve dysfunction and neurocyte death as a consequence of oxidative stress, mitochondrial dysfunction, metabolic disorder and inflammation.75,77 Hyperglycaemia and insulin resistance collectively cause an imbalance in the mitochondrial redox state and excess formation of ROS, thereby contributing to dysregulation of metabolic pathways, a loss of stored energy and axonal injury, promoting diabetic neuropathy.78 ...
2
2019
... Neural damage is termed “neuropathy”. According to epidemiological data, approximately 50% of the cases of diabetic foot syndrome can be accounted for by neuropathy while peripheral angiopathy is responsible for just 15%.74 Moreover, diabetic neuropathy can cause a series of complications including long–term chronic pain, foot ulcers, foot infections, and even catastrophic events including amputations.75,76 The pathophysiological mechanisms involved in diabetic neuropathy have been comprehensively and extensively reviewed.72 Diabetic peripheral neuropathy is caused by nerve dysfunction and neurocyte death as a consequence of oxidative stress, mitochondrial dysfunction, metabolic disorder and inflammation.75,77 Hyperglycaemia and insulin resistance collectively cause an imbalance in the mitochondrial redox state and excess formation of ROS, thereby contributing to dysregulation of metabolic pathways, a loss of stored energy and axonal injury, promoting diabetic neuropathy.78 ...
... Both sensory and autonomic nerves populate skin tissues. Cutaneous sensory nerves predominate and are widely distributed in the skin, including extending into the upper epidermis where they come into contact with the external environment. In contrast, autonomic nerves are distributed in the dermis of the skin and mainly regulate lymphatic function, blood circulation, and appendageal function.7 Nowadays, there is a growing appreciation that neuropathy is a major factor in impaired skin integrity in diabetes.63 Diabetic neuropathological changes preferentially damage sensory neurons, and the unmyelinated small–diameter sensory axons are especially susceptible.79 Evidence of sensory neuropathy is decreased or absent sense of vibration and superficial sensitivity including pressure, haptics, and subjective paresthesias. Consequently, the sensation of pain is substantially decreased and the risk of trauma is significantly higher. Due to the loss of pain sensation, serious ulcerations are underestimated and injuries are thus often not noticed in patients with diabetes mellitus.74 Autonomic nerve damage leads to arterial sclerosis, vasomotor paralysis, arteriovenous shunts in the microvascular network, dysfunctional sudomotor function, and neuropathic oedemas in damaged skin. As a result, affected skin in patients with diabetes mellitus has a reduced protective function and an increased risk of injury.77 The combination of these peripheral nerve problems leads to abnormal foot pressures in the form of an elevated plantar pressure load, accompanied by the development of hyperkeratosis and finally diabetic foot ulcers.80,81 ...
Cutaneous manifestations of diabetes mellitus: a review
1
2017
... Neural damage is termed “neuropathy”. According to epidemiological data, approximately 50% of the cases of diabetic foot syndrome can be accounted for by neuropathy while peripheral angiopathy is responsible for just 15%.74 Moreover, diabetic neuropathy can cause a series of complications including long–term chronic pain, foot ulcers, foot infections, and even catastrophic events including amputations.75,76 The pathophysiological mechanisms involved in diabetic neuropathy have been comprehensively and extensively reviewed.72 Diabetic peripheral neuropathy is caused by nerve dysfunction and neurocyte death as a consequence of oxidative stress, mitochondrial dysfunction, metabolic disorder and inflammation.75,77 Hyperglycaemia and insulin resistance collectively cause an imbalance in the mitochondrial redox state and excess formation of ROS, thereby contributing to dysregulation of metabolic pathways, a loss of stored energy and axonal injury, promoting diabetic neuropathy.78 ...
Diabetes and peripheral nerve disease
1
2021
... Both sensory and autonomic nerves populate skin tissues. Cutaneous sensory nerves predominate and are widely distributed in the skin, including extending into the upper epidermis where they come into contact with the external environment. In contrast, autonomic nerves are distributed in the dermis of the skin and mainly regulate lymphatic function, blood circulation, and appendageal function.7 Nowadays, there is a growing appreciation that neuropathy is a major factor in impaired skin integrity in diabetes.63 Diabetic neuropathological changes preferentially damage sensory neurons, and the unmyelinated small–diameter sensory axons are especially susceptible.79 Evidence of sensory neuropathy is decreased or absent sense of vibration and superficial sensitivity including pressure, haptics, and subjective paresthesias. Consequently, the sensation of pain is substantially decreased and the risk of trauma is significantly higher. Due to the loss of pain sensation, serious ulcerations are underestimated and injuries are thus often not noticed in patients with diabetes mellitus.74 Autonomic nerve damage leads to arterial sclerosis, vasomotor paralysis, arteriovenous shunts in the microvascular network, dysfunctional sudomotor function, and neuropathic oedemas in damaged skin. As a result, affected skin in patients with diabetes mellitus has a reduced protective function and an increased risk of injury.77 The combination of these peripheral nerve problems leads to abnormal foot pressures in the form of an elevated plantar pressure load, accompanied by the development of hyperkeratosis and finally diabetic foot ulcers.80,81 ...
Global disability burdens of diabetes-related lower-extremity complications in 1990 and 2016
1
2020
... Both sensory and autonomic nerves populate skin tissues. Cutaneous sensory nerves predominate and are widely distributed in the skin, including extending into the upper epidermis where they come into contact with the external environment. In contrast, autonomic nerves are distributed in the dermis of the skin and mainly regulate lymphatic function, blood circulation, and appendageal function.7 Nowadays, there is a growing appreciation that neuropathy is a major factor in impaired skin integrity in diabetes.63 Diabetic neuropathological changes preferentially damage sensory neurons, and the unmyelinated small–diameter sensory axons are especially susceptible.79 Evidence of sensory neuropathy is decreased or absent sense of vibration and superficial sensitivity including pressure, haptics, and subjective paresthesias. Consequently, the sensation of pain is substantially decreased and the risk of trauma is significantly higher. Due to the loss of pain sensation, serious ulcerations are underestimated and injuries are thus often not noticed in patients with diabetes mellitus.74 Autonomic nerve damage leads to arterial sclerosis, vasomotor paralysis, arteriovenous shunts in the microvascular network, dysfunctional sudomotor function, and neuropathic oedemas in damaged skin. As a result, affected skin in patients with diabetes mellitus has a reduced protective function and an increased risk of injury.77 The combination of these peripheral nerve problems leads to abnormal foot pressures in the form of an elevated plantar pressure load, accompanied by the development of hyperkeratosis and finally diabetic foot ulcers.80,81 ...
New perspective in diabetic neuropathy: from the periphery to the brain, a call for early detection, and precision medicine
1
2019
... Both sensory and autonomic nerves populate skin tissues. Cutaneous sensory nerves predominate and are widely distributed in the skin, including extending into the upper epidermis where they come into contact with the external environment. In contrast, autonomic nerves are distributed in the dermis of the skin and mainly regulate lymphatic function, blood circulation, and appendageal function.7 Nowadays, there is a growing appreciation that neuropathy is a major factor in impaired skin integrity in diabetes.63 Diabetic neuropathological changes preferentially damage sensory neurons, and the unmyelinated small–diameter sensory axons are especially susceptible.79 Evidence of sensory neuropathy is decreased or absent sense of vibration and superficial sensitivity including pressure, haptics, and subjective paresthesias. Consequently, the sensation of pain is substantially decreased and the risk of trauma is significantly higher. Due to the loss of pain sensation, serious ulcerations are underestimated and injuries are thus often not noticed in patients with diabetes mellitus.74 Autonomic nerve damage leads to arterial sclerosis, vasomotor paralysis, arteriovenous shunts in the microvascular network, dysfunctional sudomotor function, and neuropathic oedemas in damaged skin. As a result, affected skin in patients with diabetes mellitus has a reduced protective function and an increased risk of injury.77 The combination of these peripheral nerve problems leads to abnormal foot pressures in the form of an elevated plantar pressure load, accompanied by the development of hyperkeratosis and finally diabetic foot ulcers.80,81 ...
Diabetic foot syndrome: immune-inflammatory features as possible cardiovascular markers in diabetes
1
2015
... The onset of diabetic foot ulcers (DFU) is a complex process and the pathogenesis is multifactorial with several factors working together. Pathogenic factors that are able to generate DFU are multifactorial.82 It is known that diabetes mellitus is characterised by a persistent low–grade inflammatory state. A pathophysiological diabetic state involves enhanced–proinflammatory responses and suppressed anti–inflammatory responses, as indicated by the excessive oxidative stress and increase in advanced glycation end products, NF–κB activation, nitric oxide blocking, DNA damage, and proinflammatory cytokines. The increased levels of pro–inflammatory cytokines such as interleukin 1, interleukin 6 and tumour necrosis factor–α in diabetic patients indicates a predisposition to suffer DFU.83,84 ...
Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: An update on glucose toxicity
1
2018
... The onset of diabetic foot ulcers (DFU) is a complex process and the pathogenesis is multifactorial with several factors working together. Pathogenic factors that are able to generate DFU are multifactorial.82 It is known that diabetes mellitus is characterised by a persistent low–grade inflammatory state. A pathophysiological diabetic state involves enhanced–proinflammatory responses and suppressed anti–inflammatory responses, as indicated by the excessive oxidative stress and increase in advanced glycation end products, NF–κB activation, nitric oxide blocking, DNA damage, and proinflammatory cytokines. The increased levels of pro–inflammatory cytokines such as interleukin 1, interleukin 6 and tumour necrosis factor–α in diabetic patients indicates a predisposition to suffer DFU.83,84 ...
Dysfunctional wound healing in diabetic foot ulcers: new crossroads
1
2018
... The onset of diabetic foot ulcers (DFU) is a complex process and the pathogenesis is multifactorial with several factors working together. Pathogenic factors that are able to generate DFU are multifactorial.82 It is known that diabetes mellitus is characterised by a persistent low–grade inflammatory state. A pathophysiological diabetic state involves enhanced–proinflammatory responses and suppressed anti–inflammatory responses, as indicated by the excessive oxidative stress and increase in advanced glycation end products, NF–κB activation, nitric oxide blocking, DNA damage, and proinflammatory cytokines. The increased levels of pro–inflammatory cytokines such as interleukin 1, interleukin 6 and tumour necrosis factor–α in diabetic patients indicates a predisposition to suffer DFU.83,84 ...
Wound bioburden and infection-related complications in diabetic foot ulcers
1
2008
... Diabetic patients are at higher risk of developing infections than persons without diabetes.85 Hyperglycemia disrupts the integrity of small blood vessels, and the immune response is inadequate to protect against local infection, thereby resulting in ulceration.86 Moreover, diabetic foot infections typically begin within a site of trauma or ulceration. Infection is an invasion by microorganisms and their reactions to the host immune system, which induce inflammatory responses, immunopathy, and subsequent tissue destruction.87 Microbial load, diversity of microbial spectrum, and the synergistic association of microbes are responsible for the development of diabetic foot infection.88 Since several factors predispose diabetic patients to developing an infection, an accurate and timely microbial assessment is one of the essential and effective regimes for preventing DFU infection.88,89 ...
Novel biological therapies for the treatment of diabetic foot ulcers
3
2017
... Diabetic patients are at higher risk of developing infections than persons without diabetes.85 Hyperglycemia disrupts the integrity of small blood vessels, and the immune response is inadequate to protect against local infection, thereby resulting in ulceration.86 Moreover, diabetic foot infections typically begin within a site of trauma or ulceration. Infection is an invasion by microorganisms and their reactions to the host immune system, which induce inflammatory responses, immunopathy, and subsequent tissue destruction.87 Microbial load, diversity of microbial spectrum, and the synergistic association of microbes are responsible for the development of diabetic foot infection.88 Since several factors predispose diabetic patients to developing an infection, an accurate and timely microbial assessment is one of the essential and effective regimes for preventing DFU infection.88,89 ...
... In the general therapeutic policy for diabetic wounds, it is widely recommended that good blood glucose control improves wound healing and limits adverse effects. As hyperglycaemia is a major factor impeding normal wound healing, restoration of normoglycaemia should always be part of the treatment plan.90,113 One of the most effective controls and preventative measures for diabetic wounds is to maintain blood glucose at an appropriate level with anti–diabetic drugs and appropriate diet.86 Treatment by intravenous injection of insulin has been proven to significantly increase the wound–healing rate to 30.3% compared to 9.8% in those without drug treatment.114 Maintenance of normoglycaemia with continuous subcutaneous insulin for four weeks resulted in up to 88.1% of DFU patients achieving a relatively good wound–healing effect.115 Furthermore, an animal model of diabetic wounds showed that topical application of insulin cream markedly accelerated healing and shortened the healing time by activating the protein kinase B (AKT) and extracellular regulated protein kinases pathways.116 It was concluded that intralesional administration of insulin had a double benefit, which not only improved vascularisation and granulation, but also prevented infection as an anabolic peptide.86 In addition to local injection, insulin has also been used to intermittently irrigate diabetic wounds combined with continuous drainage via vacuum sealing drainage.117 This combined treatment reduced inflammatory reaction, promoted formation of granulation tissue, improved tissue recovery function, and speeded up the wound healing rate, although it had no effects on blood glucose levels. Dipeptidyl peptidase–4 inhibitors were beneficial for diabetic wound healing because they regulated endothelial progenitor cells, thereby facilitating angiogenesis, promoting the formation of granular tissue and regulating the immune response to hypoxia in diabetic wounds.118 Metformin, another conventional antidiabetic drug, was demonstrated to promote diabetic wound healing by increasing transcription and protein levels of endocan, boosting the angiogenic potential of endothelial cells and improving nitric oxide generation under high–glucose conditions.119 Exendin–4 is a glucagon–like peptide–1 receptor agonist. Topical application of exendin–4 itself was effective for the treatment of diabetic skin wounds and in combination with the addition of adipose–derived stem cells further expedited diabetic wound healing.120 ...
... 86 In addition to local injection, insulin has also been used to intermittently irrigate diabetic wounds combined with continuous drainage via vacuum sealing drainage.117 This combined treatment reduced inflammatory reaction, promoted formation of granulation tissue, improved tissue recovery function, and speeded up the wound healing rate, although it had no effects on blood glucose levels. Dipeptidyl peptidase–4 inhibitors were beneficial for diabetic wound healing because they regulated endothelial progenitor cells, thereby facilitating angiogenesis, promoting the formation of granular tissue and regulating the immune response to hypoxia in diabetic wounds.118 Metformin, another conventional antidiabetic drug, was demonstrated to promote diabetic wound healing by increasing transcription and protein levels of endocan, boosting the angiogenic potential of endothelial cells and improving nitric oxide generation under high–glucose conditions.119 Exendin–4 is a glucagon–like peptide–1 receptor agonist. Topical application of exendin–4 itself was effective for the treatment of diabetic skin wounds and in combination with the addition of adipose–derived stem cells further expedited diabetic wound healing.120 ...
Diabetic foot infections: a comprehensive overview
1
2019
... Diabetic patients are at higher risk of developing infections than persons without diabetes.85 Hyperglycemia disrupts the integrity of small blood vessels, and the immune response is inadequate to protect against local infection, thereby resulting in ulceration.86 Moreover, diabetic foot infections typically begin within a site of trauma or ulceration. Infection is an invasion by microorganisms and their reactions to the host immune system, which induce inflammatory responses, immunopathy, and subsequent tissue destruction.87 Microbial load, diversity of microbial spectrum, and the synergistic association of microbes are responsible for the development of diabetic foot infection.88 Since several factors predispose diabetic patients to developing an infection, an accurate and timely microbial assessment is one of the essential and effective regimes for preventing DFU infection.88,89 ...
Classification, microbiology and treatment of diabetic foot infections
4
2018
... Diabetic patients are at higher risk of developing infections than persons without diabetes.85 Hyperglycemia disrupts the integrity of small blood vessels, and the immune response is inadequate to protect against local infection, thereby resulting in ulceration.86 Moreover, diabetic foot infections typically begin within a site of trauma or ulceration. Infection is an invasion by microorganisms and their reactions to the host immune system, which induce inflammatory responses, immunopathy, and subsequent tissue destruction.87 Microbial load, diversity of microbial spectrum, and the synergistic association of microbes are responsible for the development of diabetic foot infection.88 Since several factors predispose diabetic patients to developing an infection, an accurate and timely microbial assessment is one of the essential and effective regimes for preventing DFU infection.88,89 ...
... 88,89 ...
... Wound infection is a known predictor of poor wound healing and amputation.90,98 The enhanced recognition of infection and appropriate treatment in diabetic wound infection is imperative to improve outcomes. Empirical identifications and regimens are usually selected for the initial infection. However, inappropriate treatments with antibiotics are often associated with several adverse effects, including antibacterial resistance.90,99,100 Therefore, knowledge about the microbial aetiology and understanding of antibiotic resistance in diabetic foot infections is of the great importance to the effective management of these infected wounds.88 Nowadays, a set of common principles may provide comprehensive classification systems for diabetic foot infections, and a comprehensive description of microbiology.88,101 According to a Cochrane review, the use of an antimicrobial dressing instead of a non–antimicrobial dressing increased the healing of DFUs over a medium–term follow–up period. Moreover, their analyses showed that there was little difference in the risk of adverse events related to treatment between systemic and topical antimicrobial treatments.89 Another recommendation suggested that mild tissue infections should be treated for 1–2 weeks and more severe cases for about 3 weeks. Wounds complicated with bone infections should receive prolonged and upgraded antibiotic therapy with more broad–spectrum agents.102 However, antibiotics given to treat infection do not actually heal the wound itself. Therefore, therapies should always be combined with other diabetic wound treatments.92 ...
... 88,101 According to a Cochrane review, the use of an antimicrobial dressing instead of a non–antimicrobial dressing increased the healing of DFUs over a medium–term follow–up period. Moreover, their analyses showed that there was little difference in the risk of adverse events related to treatment between systemic and topical antimicrobial treatments.89 Another recommendation suggested that mild tissue infections should be treated for 1–2 weeks and more severe cases for about 3 weeks. Wounds complicated with bone infections should receive prolonged and upgraded antibiotic therapy with more broad–spectrum agents.102 However, antibiotics given to treat infection do not actually heal the wound itself. Therefore, therapies should always be combined with other diabetic wound treatments.92 ...
Topical antimicrobial agents for treating foot ulcers in people with diabetes
2
2017
... Diabetic patients are at higher risk of developing infections than persons without diabetes.85 Hyperglycemia disrupts the integrity of small blood vessels, and the immune response is inadequate to protect against local infection, thereby resulting in ulceration.86 Moreover, diabetic foot infections typically begin within a site of trauma or ulceration. Infection is an invasion by microorganisms and their reactions to the host immune system, which induce inflammatory responses, immunopathy, and subsequent tissue destruction.87 Microbial load, diversity of microbial spectrum, and the synergistic association of microbes are responsible for the development of diabetic foot infection.88 Since several factors predispose diabetic patients to developing an infection, an accurate and timely microbial assessment is one of the essential and effective regimes for preventing DFU infection.88,89 ...
... Wound infection is a known predictor of poor wound healing and amputation.90,98 The enhanced recognition of infection and appropriate treatment in diabetic wound infection is imperative to improve outcomes. Empirical identifications and regimens are usually selected for the initial infection. However, inappropriate treatments with antibiotics are often associated with several adverse effects, including antibacterial resistance.90,99,100 Therefore, knowledge about the microbial aetiology and understanding of antibiotic resistance in diabetic foot infections is of the great importance to the effective management of these infected wounds.88 Nowadays, a set of common principles may provide comprehensive classification systems for diabetic foot infections, and a comprehensive description of microbiology.88,101 According to a Cochrane review, the use of an antimicrobial dressing instead of a non–antimicrobial dressing increased the healing of DFUs over a medium–term follow–up period. Moreover, their analyses showed that there was little difference in the risk of adverse events related to treatment between systemic and topical antimicrobial treatments.89 Another recommendation suggested that mild tissue infections should be treated for 1–2 weeks and more severe cases for about 3 weeks. Wounds complicated with bone infections should receive prolonged and upgraded antibiotic therapy with more broad–spectrum agents.102 However, antibiotics given to treat infection do not actually heal the wound itself. Therefore, therapies should always be combined with other diabetic wound treatments.92 ...
Update on management of diabetic foot ulcers
7
2018
... DFU are a serious complication of diabetes resulting in significant morbidity, poor quality of life, worse psychological endurance, and a high mortality rate.90,91 The wound–healing process of these ulcers often becomes refractory, especially when the underlying causes are unclear and the patient does not receive holistic professional treatments. In diabetic patients, reduced fibrinolysis, poor formation of extracellular matrix, aberrant secretion of inflammatory cytokines, diminution of angiogenesis, reduction of fibroblasts and infection are all important factors that contribute to the problem of deficient wound closure. The current standard diabetic wound treatments comprise four main principles: (1) debridement, (2) infection management, (3) offloading and (4) revascularisation (Figure 3).92 Despite these standards, many diabetic wounds persist. Strategies for the management of patients with a diabetic wound are multidisciplinary approaches and involved in multifactorial processes including glycaemic control.56 Therefore, new approaches are warranted for these complex wounds. ...
... Cells at the nonhealing edge of chronic diabetic wounds exhibit a pathogenic and proinflammatory phenotype that inhibits and prolongs wound healing.93 Debridement is regarded as an effective intervention to speed up ulcer healing and is widely practiced in diabetic foot care.94 Surgical debridement involves the removal of all devitalised, infected, or festering and necrotic tissue within the wound bed or adjacent to the wound until surrounding healthy tissue is exposed.90 It is the fastest and most effective treatment for progressive and recalcitrant ulcers. The most traditional form is mechanical debridement with the application of moist and wet dressings.95 Enzymatic debridement uses chemical agents such as collagenase and streptokinase. It selectively targets necrotic tissue and is applicable to ischaemic wounds.92,95,96 It has been reported that collagenase in combination with serial sharp debridement appeared more beneficial over standard care alone.96 Calluses in diabetes mellitus are hyperkeratotic lesions caused by pressure, which are a component cause of ulceration. It has been reported that the surgical debridement of hyperkeratoses can reduce peak plantar pressure by 26%.97 Debridement by different means aided in granulation tissue formation and re–epithelialisation and promoted wound healing.93 Wound debridement involves removal of calluses. ...
... Wound infection is a known predictor of poor wound healing and amputation.90,98 The enhanced recognition of infection and appropriate treatment in diabetic wound infection is imperative to improve outcomes. Empirical identifications and regimens are usually selected for the initial infection. However, inappropriate treatments with antibiotics are often associated with several adverse effects, including antibacterial resistance.90,99,100 Therefore, knowledge about the microbial aetiology and understanding of antibiotic resistance in diabetic foot infections is of the great importance to the effective management of these infected wounds.88 Nowadays, a set of common principles may provide comprehensive classification systems for diabetic foot infections, and a comprehensive description of microbiology.88,101 According to a Cochrane review, the use of an antimicrobial dressing instead of a non–antimicrobial dressing increased the healing of DFUs over a medium–term follow–up period. Moreover, their analyses showed that there was little difference in the risk of adverse events related to treatment between systemic and topical antimicrobial treatments.89 Another recommendation suggested that mild tissue infections should be treated for 1–2 weeks and more severe cases for about 3 weeks. Wounds complicated with bone infections should receive prolonged and upgraded antibiotic therapy with more broad–spectrum agents.102 However, antibiotics given to treat infection do not actually heal the wound itself. Therefore, therapies should always be combined with other diabetic wound treatments.92 ...
... 90,99,100 Therefore, knowledge about the microbial aetiology and understanding of antibiotic resistance in diabetic foot infections is of the great importance to the effective management of these infected wounds.88 Nowadays, a set of common principles may provide comprehensive classification systems for diabetic foot infections, and a comprehensive description of microbiology.88,101 According to a Cochrane review, the use of an antimicrobial dressing instead of a non–antimicrobial dressing increased the healing of DFUs over a medium–term follow–up period. Moreover, their analyses showed that there was little difference in the risk of adverse events related to treatment between systemic and topical antimicrobial treatments.89 Another recommendation suggested that mild tissue infections should be treated for 1–2 weeks and more severe cases for about 3 weeks. Wounds complicated with bone infections should receive prolonged and upgraded antibiotic therapy with more broad–spectrum agents.102 However, antibiotics given to treat infection do not actually heal the wound itself. Therefore, therapies should always be combined with other diabetic wound treatments.92 ...
... Procedures to reduce abnormally elevated pressure, mechanical stress and shear stress at the site of the ulcer constitute probably one of the most important interventions to promote diabetic healing.103 Long–term pressure at the ulcer site can cause deformities of foot anatomy, delay ulcer healing, increase the risk of recurrence after healing, and seriously escalate the probability of amputation. A surgical procedure is important to stabilise and harmonise the foot for long term off–loading. However, the common situation is that surgical offloading is only used in high–risk patients for whom conservative management has failed.90 More recently, it has been suggested that surgical off–loading should not be reserved only for those cases that are recalcitrant, but extended to ulcer prevention and remission in diabetic wounds.104 In addition to surgical methods, off–loading can be achieved with the aid of devices, including modified shoes, boots, and orthotic walkers, which have proven to be the ideal form of adequate offloading.90,105,106 ...
... 90,105,106 ...
... In the general therapeutic policy for diabetic wounds, it is widely recommended that good blood glucose control improves wound healing and limits adverse effects. As hyperglycaemia is a major factor impeding normal wound healing, restoration of normoglycaemia should always be part of the treatment plan.90,113 One of the most effective controls and preventative measures for diabetic wounds is to maintain blood glucose at an appropriate level with anti–diabetic drugs and appropriate diet.86 Treatment by intravenous injection of insulin has been proven to significantly increase the wound–healing rate to 30.3% compared to 9.8% in those without drug treatment.114 Maintenance of normoglycaemia with continuous subcutaneous insulin for four weeks resulted in up to 88.1% of DFU patients achieving a relatively good wound–healing effect.115 Furthermore, an animal model of diabetic wounds showed that topical application of insulin cream markedly accelerated healing and shortened the healing time by activating the protein kinase B (AKT) and extracellular regulated protein kinases pathways.116 It was concluded that intralesional administration of insulin had a double benefit, which not only improved vascularisation and granulation, but also prevented infection as an anabolic peptide.86 In addition to local injection, insulin has also been used to intermittently irrigate diabetic wounds combined with continuous drainage via vacuum sealing drainage.117 This combined treatment reduced inflammatory reaction, promoted formation of granulation tissue, improved tissue recovery function, and speeded up the wound healing rate, although it had no effects on blood glucose levels. Dipeptidyl peptidase–4 inhibitors were beneficial for diabetic wound healing because they regulated endothelial progenitor cells, thereby facilitating angiogenesis, promoting the formation of granular tissue and regulating the immune response to hypoxia in diabetic wounds.118 Metformin, another conventional antidiabetic drug, was demonstrated to promote diabetic wound healing by increasing transcription and protein levels of endocan, boosting the angiogenic potential of endothelial cells and improving nitric oxide generation under high–glucose conditions.119 Exendin–4 is a glucagon–like peptide–1 receptor agonist. Topical application of exendin–4 itself was effective for the treatment of diabetic skin wounds and in combination with the addition of adipose–derived stem cells further expedited diabetic wound healing.120 ...
Diabetic foot ulcers: Part I. Pathophysiology and prevention
1
2014
... DFU are a serious complication of diabetes resulting in significant morbidity, poor quality of life, worse psychological endurance, and a high mortality rate.90,91 The wound–healing process of these ulcers often becomes refractory, especially when the underlying causes are unclear and the patient does not receive holistic professional treatments. In diabetic patients, reduced fibrinolysis, poor formation of extracellular matrix, aberrant secretion of inflammatory cytokines, diminution of angiogenesis, reduction of fibroblasts and infection are all important factors that contribute to the problem of deficient wound closure. The current standard diabetic wound treatments comprise four main principles: (1) debridement, (2) infection management, (3) offloading and (4) revascularisation (Figure 3).92 Despite these standards, many diabetic wounds persist. Strategies for the management of patients with a diabetic wound are multidisciplinary approaches and involved in multifactorial processes including glycaemic control.56 Therefore, new approaches are warranted for these complex wounds. ...
Diabetic foot ulcers: appraising standard of care and reviewing new trends in management
3
2020
... DFU are a serious complication of diabetes resulting in significant morbidity, poor quality of life, worse psychological endurance, and a high mortality rate.90,91 The wound–healing process of these ulcers often becomes refractory, especially when the underlying causes are unclear and the patient does not receive holistic professional treatments. In diabetic patients, reduced fibrinolysis, poor formation of extracellular matrix, aberrant secretion of inflammatory cytokines, diminution of angiogenesis, reduction of fibroblasts and infection are all important factors that contribute to the problem of deficient wound closure. The current standard diabetic wound treatments comprise four main principles: (1) debridement, (2) infection management, (3) offloading and (4) revascularisation (Figure 3).92 Despite these standards, many diabetic wounds persist. Strategies for the management of patients with a diabetic wound are multidisciplinary approaches and involved in multifactorial processes including glycaemic control.56 Therefore, new approaches are warranted for these complex wounds. ...
... Cells at the nonhealing edge of chronic diabetic wounds exhibit a pathogenic and proinflammatory phenotype that inhibits and prolongs wound healing.93 Debridement is regarded as an effective intervention to speed up ulcer healing and is widely practiced in diabetic foot care.94 Surgical debridement involves the removal of all devitalised, infected, or festering and necrotic tissue within the wound bed or adjacent to the wound until surrounding healthy tissue is exposed.90 It is the fastest and most effective treatment for progressive and recalcitrant ulcers. The most traditional form is mechanical debridement with the application of moist and wet dressings.95 Enzymatic debridement uses chemical agents such as collagenase and streptokinase. It selectively targets necrotic tissue and is applicable to ischaemic wounds.92,95,96 It has been reported that collagenase in combination with serial sharp debridement appeared more beneficial over standard care alone.96 Calluses in diabetes mellitus are hyperkeratotic lesions caused by pressure, which are a component cause of ulceration. It has been reported that the surgical debridement of hyperkeratoses can reduce peak plantar pressure by 26%.97 Debridement by different means aided in granulation tissue formation and re–epithelialisation and promoted wound healing.93 Wound debridement involves removal of calluses. ...
... Wound infection is a known predictor of poor wound healing and amputation.90,98 The enhanced recognition of infection and appropriate treatment in diabetic wound infection is imperative to improve outcomes. Empirical identifications and regimens are usually selected for the initial infection. However, inappropriate treatments with antibiotics are often associated with several adverse effects, including antibacterial resistance.90,99,100 Therefore, knowledge about the microbial aetiology and understanding of antibiotic resistance in diabetic foot infections is of the great importance to the effective management of these infected wounds.88 Nowadays, a set of common principles may provide comprehensive classification systems for diabetic foot infections, and a comprehensive description of microbiology.88,101 According to a Cochrane review, the use of an antimicrobial dressing instead of a non–antimicrobial dressing increased the healing of DFUs over a medium–term follow–up period. Moreover, their analyses showed that there was little difference in the risk of adverse events related to treatment between systemic and topical antimicrobial treatments.89 Another recommendation suggested that mild tissue infections should be treated for 1–2 weeks and more severe cases for about 3 weeks. Wounds complicated with bone infections should receive prolonged and upgraded antibiotic therapy with more broad–spectrum agents.102 However, antibiotics given to treat infection do not actually heal the wound itself. Therefore, therapies should always be combined with other diabetic wound treatments.92 ...
The role of surgical debridement in healing of diabetic foot ulcers
2
2010
... Cells at the nonhealing edge of chronic diabetic wounds exhibit a pathogenic and proinflammatory phenotype that inhibits and prolongs wound healing.93 Debridement is regarded as an effective intervention to speed up ulcer healing and is widely practiced in diabetic foot care.94 Surgical debridement involves the removal of all devitalised, infected, or festering and necrotic tissue within the wound bed or adjacent to the wound until surrounding healthy tissue is exposed.90 It is the fastest and most effective treatment for progressive and recalcitrant ulcers. The most traditional form is mechanical debridement with the application of moist and wet dressings.95 Enzymatic debridement uses chemical agents such as collagenase and streptokinase. It selectively targets necrotic tissue and is applicable to ischaemic wounds.92,95,96 It has been reported that collagenase in combination with serial sharp debridement appeared more beneficial over standard care alone.96 Calluses in diabetes mellitus are hyperkeratotic lesions caused by pressure, which are a component cause of ulceration. It has been reported that the surgical debridement of hyperkeratoses can reduce peak plantar pressure by 26%.97 Debridement by different means aided in granulation tissue formation and re–epithelialisation and promoted wound healing.93 Wound debridement involves removal of calluses. ...
... 93 Wound debridement involves removal of calluses. ...
Debridement of diabetic foot ulcers
1
2010
... Cells at the nonhealing edge of chronic diabetic wounds exhibit a pathogenic and proinflammatory phenotype that inhibits and prolongs wound healing.93 Debridement is regarded as an effective intervention to speed up ulcer healing and is widely practiced in diabetic foot care.94 Surgical debridement involves the removal of all devitalised, infected, or festering and necrotic tissue within the wound bed or adjacent to the wound until surrounding healthy tissue is exposed.90 It is the fastest and most effective treatment for progressive and recalcitrant ulcers. The most traditional form is mechanical debridement with the application of moist and wet dressings.95 Enzymatic debridement uses chemical agents such as collagenase and streptokinase. It selectively targets necrotic tissue and is applicable to ischaemic wounds.92,95,96 It has been reported that collagenase in combination with serial sharp debridement appeared more beneficial over standard care alone.96 Calluses in diabetes mellitus are hyperkeratotic lesions caused by pressure, which are a component cause of ulceration. It has been reported that the surgical debridement of hyperkeratoses can reduce peak plantar pressure by 26%.97 Debridement by different means aided in granulation tissue formation and re–epithelialisation and promoted wound healing.93 Wound debridement involves removal of calluses. ...
Management of diabetic foot ulcers
2
2012
... Cells at the nonhealing edge of chronic diabetic wounds exhibit a pathogenic and proinflammatory phenotype that inhibits and prolongs wound healing.93 Debridement is regarded as an effective intervention to speed up ulcer healing and is widely practiced in diabetic foot care.94 Surgical debridement involves the removal of all devitalised, infected, or festering and necrotic tissue within the wound bed or adjacent to the wound until surrounding healthy tissue is exposed.90 It is the fastest and most effective treatment for progressive and recalcitrant ulcers. The most traditional form is mechanical debridement with the application of moist and wet dressings.95 Enzymatic debridement uses chemical agents such as collagenase and streptokinase. It selectively targets necrotic tissue and is applicable to ischaemic wounds.92,95,96 It has been reported that collagenase in combination with serial sharp debridement appeared more beneficial over standard care alone.96 Calluses in diabetes mellitus are hyperkeratotic lesions caused by pressure, which are a component cause of ulceration. It has been reported that the surgical debridement of hyperkeratoses can reduce peak plantar pressure by 26%.97 Debridement by different means aided in granulation tissue formation and re–epithelialisation and promoted wound healing.93 Wound debridement involves removal of calluses. ...
... ,95,96 It has been reported that collagenase in combination with serial sharp debridement appeared more beneficial over standard care alone.96 Calluses in diabetes mellitus are hyperkeratotic lesions caused by pressure, which are a component cause of ulceration. It has been reported that the surgical debridement of hyperkeratoses can reduce peak plantar pressure by 26%.97 Debridement by different means aided in granulation tissue formation and re–epithelialisation and promoted wound healing.93 Wound debridement involves removal of calluses. ...
Enzymatic debridement with collagenase in wounds and ulcers: a systematic review and meta-analysis
2
2017
... Cells at the nonhealing edge of chronic diabetic wounds exhibit a pathogenic and proinflammatory phenotype that inhibits and prolongs wound healing.93 Debridement is regarded as an effective intervention to speed up ulcer healing and is widely practiced in diabetic foot care.94 Surgical debridement involves the removal of all devitalised, infected, or festering and necrotic tissue within the wound bed or adjacent to the wound until surrounding healthy tissue is exposed.90 It is the fastest and most effective treatment for progressive and recalcitrant ulcers. The most traditional form is mechanical debridement with the application of moist and wet dressings.95 Enzymatic debridement uses chemical agents such as collagenase and streptokinase. It selectively targets necrotic tissue and is applicable to ischaemic wounds.92,95,96 It has been reported that collagenase in combination with serial sharp debridement appeared more beneficial over standard care alone.96 Calluses in diabetes mellitus are hyperkeratotic lesions caused by pressure, which are a component cause of ulceration. It has been reported that the surgical debridement of hyperkeratoses can reduce peak plantar pressure by 26%.97 Debridement by different means aided in granulation tissue formation and re–epithelialisation and promoted wound healing.93 Wound debridement involves removal of calluses. ...
... 96 Calluses in diabetes mellitus are hyperkeratotic lesions caused by pressure, which are a component cause of ulceration. It has been reported that the surgical debridement of hyperkeratoses can reduce peak plantar pressure by 26%.97 Debridement by different means aided in granulation tissue formation and re–epithelialisation and promoted wound healing.93 Wound debridement involves removal of calluses. ...
Preventing foot ulcers in patients with diabetes
1
2005
... Cells at the nonhealing edge of chronic diabetic wounds exhibit a pathogenic and proinflammatory phenotype that inhibits and prolongs wound healing.93 Debridement is regarded as an effective intervention to speed up ulcer healing and is widely practiced in diabetic foot care.94 Surgical debridement involves the removal of all devitalised, infected, or festering and necrotic tissue within the wound bed or adjacent to the wound until surrounding healthy tissue is exposed.90 It is the fastest and most effective treatment for progressive and recalcitrant ulcers. The most traditional form is mechanical debridement with the application of moist and wet dressings.95 Enzymatic debridement uses chemical agents such as collagenase and streptokinase. It selectively targets necrotic tissue and is applicable to ischaemic wounds.92,95,96 It has been reported that collagenase in combination with serial sharp debridement appeared more beneficial over standard care alone.96 Calluses in diabetes mellitus are hyperkeratotic lesions caused by pressure, which are a component cause of ulceration. It has been reported that the surgical debridement of hyperkeratoses can reduce peak plantar pressure by 26%.97 Debridement by different means aided in granulation tissue formation and re–epithelialisation and promoted wound healing.93 Wound debridement involves removal of calluses. ...
Meta-analysis of risk factors for amputation in diabetic foot infections
1
2019
... Wound infection is a known predictor of poor wound healing and amputation.90,98 The enhanced recognition of infection and appropriate treatment in diabetic wound infection is imperative to improve outcomes. Empirical identifications and regimens are usually selected for the initial infection. However, inappropriate treatments with antibiotics are often associated with several adverse effects, including antibacterial resistance.90,99,100 Therefore, knowledge about the microbial aetiology and understanding of antibiotic resistance in diabetic foot infections is of the great importance to the effective management of these infected wounds.88 Nowadays, a set of common principles may provide comprehensive classification systems for diabetic foot infections, and a comprehensive description of microbiology.88,101 According to a Cochrane review, the use of an antimicrobial dressing instead of a non–antimicrobial dressing increased the healing of DFUs over a medium–term follow–up period. Moreover, their analyses showed that there was little difference in the risk of adverse events related to treatment between systemic and topical antimicrobial treatments.89 Another recommendation suggested that mild tissue infections should be treated for 1–2 weeks and more severe cases for about 3 weeks. Wounds complicated with bone infections should receive prolonged and upgraded antibiotic therapy with more broad–spectrum agents.102 However, antibiotics given to treat infection do not actually heal the wound itself. Therefore, therapies should always be combined with other diabetic wound treatments.92 ...
The microbiology of diabetic foot infections: a meta-analysis
1
2021
... Wound infection is a known predictor of poor wound healing and amputation.90,98 The enhanced recognition of infection and appropriate treatment in diabetic wound infection is imperative to improve outcomes. Empirical identifications and regimens are usually selected for the initial infection. However, inappropriate treatments with antibiotics are often associated with several adverse effects, including antibacterial resistance.90,99,100 Therefore, knowledge about the microbial aetiology and understanding of antibiotic resistance in diabetic foot infections is of the great importance to the effective management of these infected wounds.88 Nowadays, a set of common principles may provide comprehensive classification systems for diabetic foot infections, and a comprehensive description of microbiology.88,101 According to a Cochrane review, the use of an antimicrobial dressing instead of a non–antimicrobial dressing increased the healing of DFUs over a medium–term follow–up period. Moreover, their analyses showed that there was little difference in the risk of adverse events related to treatment between systemic and topical antimicrobial treatments.89 Another recommendation suggested that mild tissue infections should be treated for 1–2 weeks and more severe cases for about 3 weeks. Wounds complicated with bone infections should receive prolonged and upgraded antibiotic therapy with more broad–spectrum agents.102 However, antibiotics given to treat infection do not actually heal the wound itself. Therefore, therapies should always be combined with other diabetic wound treatments.92 ...
Diabetic foot infection: Antibiotic therapy and good practice recommendations
1
2017
... Wound infection is a known predictor of poor wound healing and amputation.90,98 The enhanced recognition of infection and appropriate treatment in diabetic wound infection is imperative to improve outcomes. Empirical identifications and regimens are usually selected for the initial infection. However, inappropriate treatments with antibiotics are often associated with several adverse effects, including antibacterial resistance.90,99,100 Therefore, knowledge about the microbial aetiology and understanding of antibiotic resistance in diabetic foot infections is of the great importance to the effective management of these infected wounds.88 Nowadays, a set of common principles may provide comprehensive classification systems for diabetic foot infections, and a comprehensive description of microbiology.88,101 According to a Cochrane review, the use of an antimicrobial dressing instead of a non–antimicrobial dressing increased the healing of DFUs over a medium–term follow–up period. Moreover, their analyses showed that there was little difference in the risk of adverse events related to treatment between systemic and topical antimicrobial treatments.89 Another recommendation suggested that mild tissue infections should be treated for 1–2 weeks and more severe cases for about 3 weeks. Wounds complicated with bone infections should receive prolonged and upgraded antibiotic therapy with more broad–spectrum agents.102 However, antibiotics given to treat infection do not actually heal the wound itself. Therefore, therapies should always be combined with other diabetic wound treatments.92 ...
2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections
1
2012
... Wound infection is a known predictor of poor wound healing and amputation.90,98 The enhanced recognition of infection and appropriate treatment in diabetic wound infection is imperative to improve outcomes. Empirical identifications and regimens are usually selected for the initial infection. However, inappropriate treatments with antibiotics are often associated with several adverse effects, including antibacterial resistance.90,99,100 Therefore, knowledge about the microbial aetiology and understanding of antibiotic resistance in diabetic foot infections is of the great importance to the effective management of these infected wounds.88 Nowadays, a set of common principles may provide comprehensive classification systems for diabetic foot infections, and a comprehensive description of microbiology.88,101 According to a Cochrane review, the use of an antimicrobial dressing instead of a non–antimicrobial dressing increased the healing of DFUs over a medium–term follow–up period. Moreover, their analyses showed that there was little difference in the risk of adverse events related to treatment between systemic and topical antimicrobial treatments.89 Another recommendation suggested that mild tissue infections should be treated for 1–2 weeks and more severe cases for about 3 weeks. Wounds complicated with bone infections should receive prolonged and upgraded antibiotic therapy with more broad–spectrum agents.102 However, antibiotics given to treat infection do not actually heal the wound itself. Therefore, therapies should always be combined with other diabetic wound treatments.92 ...
Executive summary: 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections
1
2012
... Wound infection is a known predictor of poor wound healing and amputation.90,98 The enhanced recognition of infection and appropriate treatment in diabetic wound infection is imperative to improve outcomes. Empirical identifications and regimens are usually selected for the initial infection. However, inappropriate treatments with antibiotics are often associated with several adverse effects, including antibacterial resistance.90,99,100 Therefore, knowledge about the microbial aetiology and understanding of antibiotic resistance in diabetic foot infections is of the great importance to the effective management of these infected wounds.88 Nowadays, a set of common principles may provide comprehensive classification systems for diabetic foot infections, and a comprehensive description of microbiology.88,101 According to a Cochrane review, the use of an antimicrobial dressing instead of a non–antimicrobial dressing increased the healing of DFUs over a medium–term follow–up period. Moreover, their analyses showed that there was little difference in the risk of adverse events related to treatment between systemic and topical antimicrobial treatments.89 Another recommendation suggested that mild tissue infections should be treated for 1–2 weeks and more severe cases for about 3 weeks. Wounds complicated with bone infections should receive prolonged and upgraded antibiotic therapy with more broad–spectrum agents.102 However, antibiotics given to treat infection do not actually heal the wound itself. Therefore, therapies should always be combined with other diabetic wound treatments.92 ...
New developments in the biomechanics of the diabetic foot
1
... Procedures to reduce abnormally elevated pressure, mechanical stress and shear stress at the site of the ulcer constitute probably one of the most important interventions to promote diabetic healing.103 Long–term pressure at the ulcer site can cause deformities of foot anatomy, delay ulcer healing, increase the risk of recurrence after healing, and seriously escalate the probability of amputation. A surgical procedure is important to stabilise and harmonise the foot for long term off–loading. However, the common situation is that surgical offloading is only used in high–risk patients for whom conservative management has failed.90 More recently, it has been suggested that surgical off–loading should not be reserved only for those cases that are recalcitrant, but extended to ulcer prevention and remission in diabetic wounds.104 In addition to surgical methods, off–loading can be achieved with the aid of devices, including modified shoes, boots, and orthotic walkers, which have proven to be the ideal form of adequate offloading.90,105,106 ...
Diabetic foot off loading and ulcer remission: Exploring surgical off-loading
1
2021
... Procedures to reduce abnormally elevated pressure, mechanical stress and shear stress at the site of the ulcer constitute probably one of the most important interventions to promote diabetic healing.103 Long–term pressure at the ulcer site can cause deformities of foot anatomy, delay ulcer healing, increase the risk of recurrence after healing, and seriously escalate the probability of amputation. A surgical procedure is important to stabilise and harmonise the foot for long term off–loading. However, the common situation is that surgical offloading is only used in high–risk patients for whom conservative management has failed.90 More recently, it has been suggested that surgical off–loading should not be reserved only for those cases that are recalcitrant, but extended to ulcer prevention and remission in diabetic wounds.104 In addition to surgical methods, off–loading can be achieved with the aid of devices, including modified shoes, boots, and orthotic walkers, which have proven to be the ideal form of adequate offloading.90,105,106 ...
Optimizing footwear for the diabetic foot: Data-driven custom-made footwear concepts and their effect on pressure relief to prevent diabetic foot ulceration
1
2020
... Procedures to reduce abnormally elevated pressure, mechanical stress and shear stress at the site of the ulcer constitute probably one of the most important interventions to promote diabetic healing.103 Long–term pressure at the ulcer site can cause deformities of foot anatomy, delay ulcer healing, increase the risk of recurrence after healing, and seriously escalate the probability of amputation. A surgical procedure is important to stabilise and harmonise the foot for long term off–loading. However, the common situation is that surgical offloading is only used in high–risk patients for whom conservative management has failed.90 More recently, it has been suggested that surgical off–loading should not be reserved only for those cases that are recalcitrant, but extended to ulcer prevention and remission in diabetic wounds.104 In addition to surgical methods, off–loading can be achieved with the aid of devices, including modified shoes, boots, and orthotic walkers, which have proven to be the ideal form of adequate offloading.90,105,106 ...
Foot structure and footwear prescription in diabetes mellitus
1
2008
... Procedures to reduce abnormally elevated pressure, mechanical stress and shear stress at the site of the ulcer constitute probably one of the most important interventions to promote diabetic healing.103 Long–term pressure at the ulcer site can cause deformities of foot anatomy, delay ulcer healing, increase the risk of recurrence after healing, and seriously escalate the probability of amputation. A surgical procedure is important to stabilise and harmonise the foot for long term off–loading. However, the common situation is that surgical offloading is only used in high–risk patients for whom conservative management has failed.90 More recently, it has been suggested that surgical off–loading should not be reserved only for those cases that are recalcitrant, but extended to ulcer prevention and remission in diabetic wounds.104 In addition to surgical methods, off–loading can be achieved with the aid of devices, including modified shoes, boots, and orthotic walkers, which have proven to be the ideal form of adequate offloading.90,105,106 ...
The diabetic foot: The never-ending challenge
1
2016
... One study identified that more than 40% of DFU patients suffered from peripheral arterial disease (PAD), and PAD was the strongest prognostic factor for non–healing wounds and amputation.107 Diabetic angiopathy predominantly affects the infrapopliteal blood vessels.108 Impaired circulation and ischaemic vessels are the indications for revascularisation. One single–centre retrospective analysis showed that successful revascularisation of DFU patients has a significant impact on limb salvage rate and wound healing.109 A literature review showed the outcomes of 1–year limb salvage rates in endovascular revascularisation (a median of 85%) were broadly similar to those treated by open bypass surgery (a median of 78%).110 Data from 187 lower–extremity revascularisation procedures supported the use of the wound, ischaemia, and foot infection classification system to predict the revascularisation benefit in chronic limb–threatening ischaemia. Limbs with less benefit from revascularisation will more frequently require amputation despite the aggressive revascularisation.111 Although studies have shown that aggressive and timely revascularisation results in surprising progress that markedly reduced amputation rates, those DFU patients with higher–grade PAD tended to have higher rates of amputation even after revascularisation.112 ...
Current concepts for the evaluation and management of diabetic foot ulcers
1
2018
... One study identified that more than 40% of DFU patients suffered from peripheral arterial disease (PAD), and PAD was the strongest prognostic factor for non–healing wounds and amputation.107 Diabetic angiopathy predominantly affects the infrapopliteal blood vessels.108 Impaired circulation and ischaemic vessels are the indications for revascularisation. One single–centre retrospective analysis showed that successful revascularisation of DFU patients has a significant impact on limb salvage rate and wound healing.109 A literature review showed the outcomes of 1–year limb salvage rates in endovascular revascularisation (a median of 85%) were broadly similar to those treated by open bypass surgery (a median of 78%).110 Data from 187 lower–extremity revascularisation procedures supported the use of the wound, ischaemia, and foot infection classification system to predict the revascularisation benefit in chronic limb–threatening ischaemia. Limbs with less benefit from revascularisation will more frequently require amputation despite the aggressive revascularisation.111 Although studies have shown that aggressive and timely revascularisation results in surprising progress that markedly reduced amputation rates, those DFU patients with higher–grade PAD tended to have higher rates of amputation even after revascularisation.112 ...
Successful revascularization has a significant impact on limb salvage rate and wound healing for patients with diabetic foot ulcers: single-centre retrospective analysis with a multidisciplinary approach
1
2020
... One study identified that more than 40% of DFU patients suffered from peripheral arterial disease (PAD), and PAD was the strongest prognostic factor for non–healing wounds and amputation.107 Diabetic angiopathy predominantly affects the infrapopliteal blood vessels.108 Impaired circulation and ischaemic vessels are the indications for revascularisation. One single–centre retrospective analysis showed that successful revascularisation of DFU patients has a significant impact on limb salvage rate and wound healing.109 A literature review showed the outcomes of 1–year limb salvage rates in endovascular revascularisation (a median of 85%) were broadly similar to those treated by open bypass surgery (a median of 78%).110 Data from 187 lower–extremity revascularisation procedures supported the use of the wound, ischaemia, and foot infection classification system to predict the revascularisation benefit in chronic limb–threatening ischaemia. Limbs with less benefit from revascularisation will more frequently require amputation despite the aggressive revascularisation.111 Although studies have shown that aggressive and timely revascularisation results in surprising progress that markedly reduced amputation rates, those DFU patients with higher–grade PAD tended to have higher rates of amputation even after revascularisation.112 ...
Effectiveness of revascularization of the ulcerated foot in patients with diabetes and peripheral artery disease: a systematic review
1
2016
... One study identified that more than 40% of DFU patients suffered from peripheral arterial disease (PAD), and PAD was the strongest prognostic factor for non–healing wounds and amputation.107 Diabetic angiopathy predominantly affects the infrapopliteal blood vessels.108 Impaired circulation and ischaemic vessels are the indications for revascularisation. One single–centre retrospective analysis showed that successful revascularisation of DFU patients has a significant impact on limb salvage rate and wound healing.109 A literature review showed the outcomes of 1–year limb salvage rates in endovascular revascularisation (a median of 85%) were broadly similar to those treated by open bypass surgery (a median of 78%).110 Data from 187 lower–extremity revascularisation procedures supported the use of the wound, ischaemia, and foot infection classification system to predict the revascularisation benefit in chronic limb–threatening ischaemia. Limbs with less benefit from revascularisation will more frequently require amputation despite the aggressive revascularisation.111 Although studies have shown that aggressive and timely revascularisation results in surprising progress that markedly reduced amputation rates, those DFU patients with higher–grade PAD tended to have higher rates of amputation even after revascularisation.112 ...
Evaluation of revascularization benefit quartiles using the Wound, Ischemia, and foot Infection classification system for diabetic patients with chronic limb-threatening ischemia
1
2021
... One study identified that more than 40% of DFU patients suffered from peripheral arterial disease (PAD), and PAD was the strongest prognostic factor for non–healing wounds and amputation.107 Diabetic angiopathy predominantly affects the infrapopliteal blood vessels.108 Impaired circulation and ischaemic vessels are the indications for revascularisation. One single–centre retrospective analysis showed that successful revascularisation of DFU patients has a significant impact on limb salvage rate and wound healing.109 A literature review showed the outcomes of 1–year limb salvage rates in endovascular revascularisation (a median of 85%) were broadly similar to those treated by open bypass surgery (a median of 78%).110 Data from 187 lower–extremity revascularisation procedures supported the use of the wound, ischaemia, and foot infection classification system to predict the revascularisation benefit in chronic limb–threatening ischaemia. Limbs with less benefit from revascularisation will more frequently require amputation despite the aggressive revascularisation.111 Although studies have shown that aggressive and timely revascularisation results in surprising progress that markedly reduced amputation rates, those DFU patients with higher–grade PAD tended to have higher rates of amputation even after revascularisation.112 ...
Society for Vascular Surgery limb stage and patient risk correlate with outcomes in an amputation prevention program
1
2016
... One study identified that more than 40% of DFU patients suffered from peripheral arterial disease (PAD), and PAD was the strongest prognostic factor for non–healing wounds and amputation.107 Diabetic angiopathy predominantly affects the infrapopliteal blood vessels.108 Impaired circulation and ischaemic vessels are the indications for revascularisation. One single–centre retrospective analysis showed that successful revascularisation of DFU patients has a significant impact on limb salvage rate and wound healing.109 A literature review showed the outcomes of 1–year limb salvage rates in endovascular revascularisation (a median of 85%) were broadly similar to those treated by open bypass surgery (a median of 78%).110 Data from 187 lower–extremity revascularisation procedures supported the use of the wound, ischaemia, and foot infection classification system to predict the revascularisation benefit in chronic limb–threatening ischaemia. Limbs with less benefit from revascularisation will more frequently require amputation despite the aggressive revascularisation.111 Although studies have shown that aggressive and timely revascularisation results in surprising progress that markedly reduced amputation rates, those DFU patients with higher–grade PAD tended to have higher rates of amputation even after revascularisation.112 ...
Pathogenesis and treatment of impaired wound healing in diabetes mellitus: new insights
1
2014
... In the general therapeutic policy for diabetic wounds, it is widely recommended that good blood glucose control improves wound healing and limits adverse effects. As hyperglycaemia is a major factor impeding normal wound healing, restoration of normoglycaemia should always be part of the treatment plan.90,113 One of the most effective controls and preventative measures for diabetic wounds is to maintain blood glucose at an appropriate level with anti–diabetic drugs and appropriate diet.86 Treatment by intravenous injection of insulin has been proven to significantly increase the wound–healing rate to 30.3% compared to 9.8% in those without drug treatment.114 Maintenance of normoglycaemia with continuous subcutaneous insulin for four weeks resulted in up to 88.1% of DFU patients achieving a relatively good wound–healing effect.115 Furthermore, an animal model of diabetic wounds showed that topical application of insulin cream markedly accelerated healing and shortened the healing time by activating the protein kinase B (AKT) and extracellular regulated protein kinases pathways.116 It was concluded that intralesional administration of insulin had a double benefit, which not only improved vascularisation and granulation, but also prevented infection as an anabolic peptide.86 In addition to local injection, insulin has also been used to intermittently irrigate diabetic wounds combined with continuous drainage via vacuum sealing drainage.117 This combined treatment reduced inflammatory reaction, promoted formation of granulation tissue, improved tissue recovery function, and speeded up the wound healing rate, although it had no effects on blood glucose levels. Dipeptidyl peptidase–4 inhibitors were beneficial for diabetic wound healing because they regulated endothelial progenitor cells, thereby facilitating angiogenesis, promoting the formation of granular tissue and regulating the immune response to hypoxia in diabetic wounds.118 Metformin, another conventional antidiabetic drug, was demonstrated to promote diabetic wound healing by increasing transcription and protein levels of endocan, boosting the angiogenic potential of endothelial cells and improving nitric oxide generation under high–glucose conditions.119 Exendin–4 is a glucagon–like peptide–1 receptor agonist. Topical application of exendin–4 itself was effective for the treatment of diabetic skin wounds and in combination with the addition of adipose–derived stem cells further expedited diabetic wound healing.120 ...
Effect of systemic insulin treatment on diabetic wound healing
1
2017
... In the general therapeutic policy for diabetic wounds, it is widely recommended that good blood glucose control improves wound healing and limits adverse effects. As hyperglycaemia is a major factor impeding normal wound healing, restoration of normoglycaemia should always be part of the treatment plan.90,113 One of the most effective controls and preventative measures for diabetic wounds is to maintain blood glucose at an appropriate level with anti–diabetic drugs and appropriate diet.86 Treatment by intravenous injection of insulin has been proven to significantly increase the wound–healing rate to 30.3% compared to 9.8% in those without drug treatment.114 Maintenance of normoglycaemia with continuous subcutaneous insulin for four weeks resulted in up to 88.1% of DFU patients achieving a relatively good wound–healing effect.115 Furthermore, an animal model of diabetic wounds showed that topical application of insulin cream markedly accelerated healing and shortened the healing time by activating the protein kinase B (AKT) and extracellular regulated protein kinases pathways.116 It was concluded that intralesional administration of insulin had a double benefit, which not only improved vascularisation and granulation, but also prevented infection as an anabolic peptide.86 In addition to local injection, insulin has also been used to intermittently irrigate diabetic wounds combined with continuous drainage via vacuum sealing drainage.117 This combined treatment reduced inflammatory reaction, promoted formation of granulation tissue, improved tissue recovery function, and speeded up the wound healing rate, although it had no effects on blood glucose levels. Dipeptidyl peptidase–4 inhibitors were beneficial for diabetic wound healing because they regulated endothelial progenitor cells, thereby facilitating angiogenesis, promoting the formation of granular tissue and regulating the immune response to hypoxia in diabetic wounds.118 Metformin, another conventional antidiabetic drug, was demonstrated to promote diabetic wound healing by increasing transcription and protein levels of endocan, boosting the angiogenic potential of endothelial cells and improving nitric oxide generation under high–glucose conditions.119 Exendin–4 is a glucagon–like peptide–1 receptor agonist. Topical application of exendin–4 itself was effective for the treatment of diabetic skin wounds and in combination with the addition of adipose–derived stem cells further expedited diabetic wound healing.120 ...
Insulin pump for the treatment of diabetes in combination with ulcerative foot infections
1
2016
... In the general therapeutic policy for diabetic wounds, it is widely recommended that good blood glucose control improves wound healing and limits adverse effects. As hyperglycaemia is a major factor impeding normal wound healing, restoration of normoglycaemia should always be part of the treatment plan.90,113 One of the most effective controls and preventative measures for diabetic wounds is to maintain blood glucose at an appropriate level with anti–diabetic drugs and appropriate diet.86 Treatment by intravenous injection of insulin has been proven to significantly increase the wound–healing rate to 30.3% compared to 9.8% in those without drug treatment.114 Maintenance of normoglycaemia with continuous subcutaneous insulin for four weeks resulted in up to 88.1% of DFU patients achieving a relatively good wound–healing effect.115 Furthermore, an animal model of diabetic wounds showed that topical application of insulin cream markedly accelerated healing and shortened the healing time by activating the protein kinase B (AKT) and extracellular regulated protein kinases pathways.116 It was concluded that intralesional administration of insulin had a double benefit, which not only improved vascularisation and granulation, but also prevented infection as an anabolic peptide.86 In addition to local injection, insulin has also been used to intermittently irrigate diabetic wounds combined with continuous drainage via vacuum sealing drainage.117 This combined treatment reduced inflammatory reaction, promoted formation of granulation tissue, improved tissue recovery function, and speeded up the wound healing rate, although it had no effects on blood glucose levels. Dipeptidyl peptidase–4 inhibitors were beneficial for diabetic wound healing because they regulated endothelial progenitor cells, thereby facilitating angiogenesis, promoting the formation of granular tissue and regulating the immune response to hypoxia in diabetic wounds.118 Metformin, another conventional antidiabetic drug, was demonstrated to promote diabetic wound healing by increasing transcription and protein levels of endocan, boosting the angiogenic potential of endothelial cells and improving nitric oxide generation under high–glucose conditions.119 Exendin–4 is a glucagon–like peptide–1 receptor agonist. Topical application of exendin–4 itself was effective for the treatment of diabetic skin wounds and in combination with the addition of adipose–derived stem cells further expedited diabetic wound healing.120 ...
Topical insulin accelerates wound healing in diabetes by enhancing the AKT and ERK pathways: a double-blind placebo-controlled clinical trial
1
2012
... In the general therapeutic policy for diabetic wounds, it is widely recommended that good blood glucose control improves wound healing and limits adverse effects. As hyperglycaemia is a major factor impeding normal wound healing, restoration of normoglycaemia should always be part of the treatment plan.90,113 One of the most effective controls and preventative measures for diabetic wounds is to maintain blood glucose at an appropriate level with anti–diabetic drugs and appropriate diet.86 Treatment by intravenous injection of insulin has been proven to significantly increase the wound–healing rate to 30.3% compared to 9.8% in those without drug treatment.114 Maintenance of normoglycaemia with continuous subcutaneous insulin for four weeks resulted in up to 88.1% of DFU patients achieving a relatively good wound–healing effect.115 Furthermore, an animal model of diabetic wounds showed that topical application of insulin cream markedly accelerated healing and shortened the healing time by activating the protein kinase B (AKT) and extracellular regulated protein kinases pathways.116 It was concluded that intralesional administration of insulin had a double benefit, which not only improved vascularisation and granulation, but also prevented infection as an anabolic peptide.86 In addition to local injection, insulin has also been used to intermittently irrigate diabetic wounds combined with continuous drainage via vacuum sealing drainage.117 This combined treatment reduced inflammatory reaction, promoted formation of granulation tissue, improved tissue recovery function, and speeded up the wound healing rate, although it had no effects on blood glucose levels. Dipeptidyl peptidase–4 inhibitors were beneficial for diabetic wound healing because they regulated endothelial progenitor cells, thereby facilitating angiogenesis, promoting the formation of granular tissue and regulating the immune response to hypoxia in diabetic wounds.118 Metformin, another conventional antidiabetic drug, was demonstrated to promote diabetic wound healing by increasing transcription and protein levels of endocan, boosting the angiogenic potential of endothelial cells and improving nitric oxide generation under high–glucose conditions.119 Exendin–4 is a glucagon–like peptide–1 receptor agonist. Topical application of exendin–4 itself was effective for the treatment of diabetic skin wounds and in combination with the addition of adipose–derived stem cells further expedited diabetic wound healing.120 ...
Effects of intermittent irrigation of insulin solution combined with continuous drainage of vacuum sealing drainage in chronic diabetic lower limb ulcers
1
2015
... In the general therapeutic policy for diabetic wounds, it is widely recommended that good blood glucose control improves wound healing and limits adverse effects. As hyperglycaemia is a major factor impeding normal wound healing, restoration of normoglycaemia should always be part of the treatment plan.90,113 One of the most effective controls and preventative measures for diabetic wounds is to maintain blood glucose at an appropriate level with anti–diabetic drugs and appropriate diet.86 Treatment by intravenous injection of insulin has been proven to significantly increase the wound–healing rate to 30.3% compared to 9.8% in those without drug treatment.114 Maintenance of normoglycaemia with continuous subcutaneous insulin for four weeks resulted in up to 88.1% of DFU patients achieving a relatively good wound–healing effect.115 Furthermore, an animal model of diabetic wounds showed that topical application of insulin cream markedly accelerated healing and shortened the healing time by activating the protein kinase B (AKT) and extracellular regulated protein kinases pathways.116 It was concluded that intralesional administration of insulin had a double benefit, which not only improved vascularisation and granulation, but also prevented infection as an anabolic peptide.86 In addition to local injection, insulin has also been used to intermittently irrigate diabetic wounds combined with continuous drainage via vacuum sealing drainage.117 This combined treatment reduced inflammatory reaction, promoted formation of granulation tissue, improved tissue recovery function, and speeded up the wound healing rate, although it had no effects on blood glucose levels. Dipeptidyl peptidase–4 inhibitors were beneficial for diabetic wound healing because they regulated endothelial progenitor cells, thereby facilitating angiogenesis, promoting the formation of granular tissue and regulating the immune response to hypoxia in diabetic wounds.118 Metformin, another conventional antidiabetic drug, was demonstrated to promote diabetic wound healing by increasing transcription and protein levels of endocan, boosting the angiogenic potential of endothelial cells and improving nitric oxide generation under high–glucose conditions.119 Exendin–4 is a glucagon–like peptide–1 receptor agonist. Topical application of exendin–4 itself was effective for the treatment of diabetic skin wounds and in combination with the addition of adipose–derived stem cells further expedited diabetic wound healing.120 ...
Wound healing effects of dipeptidyl peptidase-4 inhibitors: An emerging concept in management of diabetic foot ulcer-A review
1
2016
... In the general therapeutic policy for diabetic wounds, it is widely recommended that good blood glucose control improves wound healing and limits adverse effects. As hyperglycaemia is a major factor impeding normal wound healing, restoration of normoglycaemia should always be part of the treatment plan.90,113 One of the most effective controls and preventative measures for diabetic wounds is to maintain blood glucose at an appropriate level with anti–diabetic drugs and appropriate diet.86 Treatment by intravenous injection of insulin has been proven to significantly increase the wound–healing rate to 30.3% compared to 9.8% in those without drug treatment.114 Maintenance of normoglycaemia with continuous subcutaneous insulin for four weeks resulted in up to 88.1% of DFU patients achieving a relatively good wound–healing effect.115 Furthermore, an animal model of diabetic wounds showed that topical application of insulin cream markedly accelerated healing and shortened the healing time by activating the protein kinase B (AKT) and extracellular regulated protein kinases pathways.116 It was concluded that intralesional administration of insulin had a double benefit, which not only improved vascularisation and granulation, but also prevented infection as an anabolic peptide.86 In addition to local injection, insulin has also been used to intermittently irrigate diabetic wounds combined with continuous drainage via vacuum sealing drainage.117 This combined treatment reduced inflammatory reaction, promoted formation of granulation tissue, improved tissue recovery function, and speeded up the wound healing rate, although it had no effects on blood glucose levels. Dipeptidyl peptidase–4 inhibitors were beneficial for diabetic wound healing because they regulated endothelial progenitor cells, thereby facilitating angiogenesis, promoting the formation of granular tissue and regulating the immune response to hypoxia in diabetic wounds.118 Metformin, another conventional antidiabetic drug, was demonstrated to promote diabetic wound healing by increasing transcription and protein levels of endocan, boosting the angiogenic potential of endothelial cells and improving nitric oxide generation under high–glucose conditions.119 Exendin–4 is a glucagon–like peptide–1 receptor agonist. Topical application of exendin–4 itself was effective for the treatment of diabetic skin wounds and in combination with the addition of adipose–derived stem cells further expedited diabetic wound healing.120 ...
Metformin effect on endocan biogenesis in human endothelial cells under diabetic condition
1
2019
... In the general therapeutic policy for diabetic wounds, it is widely recommended that good blood glucose control improves wound healing and limits adverse effects. As hyperglycaemia is a major factor impeding normal wound healing, restoration of normoglycaemia should always be part of the treatment plan.90,113 One of the most effective controls and preventative measures for diabetic wounds is to maintain blood glucose at an appropriate level with anti–diabetic drugs and appropriate diet.86 Treatment by intravenous injection of insulin has been proven to significantly increase the wound–healing rate to 30.3% compared to 9.8% in those without drug treatment.114 Maintenance of normoglycaemia with continuous subcutaneous insulin for four weeks resulted in up to 88.1% of DFU patients achieving a relatively good wound–healing effect.115 Furthermore, an animal model of diabetic wounds showed that topical application of insulin cream markedly accelerated healing and shortened the healing time by activating the protein kinase B (AKT) and extracellular regulated protein kinases pathways.116 It was concluded that intralesional administration of insulin had a double benefit, which not only improved vascularisation and granulation, but also prevented infection as an anabolic peptide.86 In addition to local injection, insulin has also been used to intermittently irrigate diabetic wounds combined with continuous drainage via vacuum sealing drainage.117 This combined treatment reduced inflammatory reaction, promoted formation of granulation tissue, improved tissue recovery function, and speeded up the wound healing rate, although it had no effects on blood glucose levels. Dipeptidyl peptidase–4 inhibitors were beneficial for diabetic wound healing because they regulated endothelial progenitor cells, thereby facilitating angiogenesis, promoting the formation of granular tissue and regulating the immune response to hypoxia in diabetic wounds.118 Metformin, another conventional antidiabetic drug, was demonstrated to promote diabetic wound healing by increasing transcription and protein levels of endocan, boosting the angiogenic potential of endothelial cells and improving nitric oxide generation under high–glucose conditions.119 Exendin–4 is a glucagon–like peptide–1 receptor agonist. Topical application of exendin–4 itself was effective for the treatment of diabetic skin wounds and in combination with the addition of adipose–derived stem cells further expedited diabetic wound healing.120 ...
Exendin-4 in combination with adipose-derived stem cells promotes angiogenesis and improves diabetic wound healing
1
2017
... In the general therapeutic policy for diabetic wounds, it is widely recommended that good blood glucose control improves wound healing and limits adverse effects. As hyperglycaemia is a major factor impeding normal wound healing, restoration of normoglycaemia should always be part of the treatment plan.90,113 One of the most effective controls and preventative measures for diabetic wounds is to maintain blood glucose at an appropriate level with anti–diabetic drugs and appropriate diet.86 Treatment by intravenous injection of insulin has been proven to significantly increase the wound–healing rate to 30.3% compared to 9.8% in those without drug treatment.114 Maintenance of normoglycaemia with continuous subcutaneous insulin for four weeks resulted in up to 88.1% of DFU patients achieving a relatively good wound–healing effect.115 Furthermore, an animal model of diabetic wounds showed that topical application of insulin cream markedly accelerated healing and shortened the healing time by activating the protein kinase B (AKT) and extracellular regulated protein kinases pathways.116 It was concluded that intralesional administration of insulin had a double benefit, which not only improved vascularisation and granulation, but also prevented infection as an anabolic peptide.86 In addition to local injection, insulin has also been used to intermittently irrigate diabetic wounds combined with continuous drainage via vacuum sealing drainage.117 This combined treatment reduced inflammatory reaction, promoted formation of granulation tissue, improved tissue recovery function, and speeded up the wound healing rate, although it had no effects on blood glucose levels. Dipeptidyl peptidase–4 inhibitors were beneficial for diabetic wound healing because they regulated endothelial progenitor cells, thereby facilitating angiogenesis, promoting the formation of granular tissue and regulating the immune response to hypoxia in diabetic wounds.118 Metformin, another conventional antidiabetic drug, was demonstrated to promote diabetic wound healing by increasing transcription and protein levels of endocan, boosting the angiogenic potential of endothelial cells and improving nitric oxide generation under high–glucose conditions.119 Exendin–4 is a glucagon–like peptide–1 receptor agonist. Topical application of exendin–4 itself was effective for the treatment of diabetic skin wounds and in combination with the addition of adipose–derived stem cells further expedited diabetic wound healing.120 ...
A composite hydrogel of chitosan/heparin/poly (γ-glutamic acid) loaded with superoxide dismutase for wound healing
1
2018
... Hydrogel is a promising wound–healing material with properties of good breathability, moisture retention, the possibility of loading bioactive substances and the ability to absorb wound exudate and cool the wound surface, thus relieving the patient’s pain.121,122 Biomedical hydrogels have been extensively investigated as a scaffolding material for efficient drug/cell delivery in wound healing and functional reconstruction of skin tissue.123⇓⇓–126 The typical three–dimensional cross–linked structure of hydrogels allows high drug/cell encapsulation and local drug/cell persistence.127 The ideal wound hydrogel materials should have a wide range of functional properties including pore structure, antimicrobial activity, appropriate mechanical properties, haemostatic ability, controlled biodegradation, sustained release of bioactive molecules and good biocompatibility.128⇓⇓–131 ...
A biodegradable hydrogel system containing curcumin encapsulated in micelles for cutaneous wound healing
1
2013
... Hydrogel is a promising wound–healing material with properties of good breathability, moisture retention, the possibility of loading bioactive substances and the ability to absorb wound exudate and cool the wound surface, thus relieving the patient’s pain.121,122 Biomedical hydrogels have been extensively investigated as a scaffolding material for efficient drug/cell delivery in wound healing and functional reconstruction of skin tissue.123⇓⇓–126 The typical three–dimensional cross–linked structure of hydrogels allows high drug/cell encapsulation and local drug/cell persistence.127 The ideal wound hydrogel materials should have a wide range of functional properties including pore structure, antimicrobial activity, appropriate mechanical properties, haemostatic ability, controlled biodegradation, sustained release of bioactive molecules and good biocompatibility.128⇓⇓–131 ...
Grape seed-inspired smart hydrogel scaffolds for melanoma therapy and wound healing
1
2019
... Hydrogel is a promising wound–healing material with properties of good breathability, moisture retention, the possibility of loading bioactive substances and the ability to absorb wound exudate and cool the wound surface, thus relieving the patient’s pain.121,122 Biomedical hydrogels have been extensively investigated as a scaffolding material for efficient drug/cell delivery in wound healing and functional reconstruction of skin tissue.123⇓⇓–126 The typical three–dimensional cross–linked structure of hydrogels allows high drug/cell encapsulation and local drug/cell persistence.127 The ideal wound hydrogel materials should have a wide range of functional properties including pore structure, antimicrobial activity, appropriate mechanical properties, haemostatic ability, controlled biodegradation, sustained release of bioactive molecules and good biocompatibility.128⇓⇓–131 ...
Photo-inspired antibacterial activity and wound healing acceleration by hydrogel embedded with Ag/Ag@AgCl/ZnO nanostructures
1
2017
... Hydrogel is a promising wound–healing material with properties of good breathability, moisture retention, the possibility of loading bioactive substances and the ability to absorb wound exudate and cool the wound surface, thus relieving the patient’s pain.121,122 Biomedical hydrogels have been extensively investigated as a scaffolding material for efficient drug/cell delivery in wound healing and functional reconstruction of skin tissue.123⇓⇓–126 The typical three–dimensional cross–linked structure of hydrogels allows high drug/cell encapsulation and local drug/cell persistence.127 The ideal wound hydrogel materials should have a wide range of functional properties including pore structure, antimicrobial activity, appropriate mechanical properties, haemostatic ability, controlled biodegradation, sustained release of bioactive molecules and good biocompatibility.128⇓⇓–131 ...
Development of a UV crosslinked biodegradable hydrogel containing adipose derived stem cells to promote vascularization for skin wounds and tissue engineering
1
2017
... Hydrogel is a promising wound–healing material with properties of good breathability, moisture retention, the possibility of loading bioactive substances and the ability to absorb wound exudate and cool the wound surface, thus relieving the patient’s pain.121,122 Biomedical hydrogels have been extensively investigated as a scaffolding material for efficient drug/cell delivery in wound healing and functional reconstruction of skin tissue.123⇓⇓–126 The typical three–dimensional cross–linked structure of hydrogels allows high drug/cell encapsulation and local drug/cell persistence.127 The ideal wound hydrogel materials should have a wide range of functional properties including pore structure, antimicrobial activity, appropriate mechanical properties, haemostatic ability, controlled biodegradation, sustained release of bioactive molecules and good biocompatibility.128⇓⇓–131 ...
A mussel-inspired conductive, self-adhesive, and self-healable tough hydrogel as cell stimulators and implantable bioelectronics
1
2017
... Hydrogel is a promising wound–healing material with properties of good breathability, moisture retention, the possibility of loading bioactive substances and the ability to absorb wound exudate and cool the wound surface, thus relieving the patient’s pain.121,122 Biomedical hydrogels have been extensively investigated as a scaffolding material for efficient drug/cell delivery in wound healing and functional reconstruction of skin tissue.123⇓⇓–126 The typical three–dimensional cross–linked structure of hydrogels allows high drug/cell encapsulation and local drug/cell persistence.127 The ideal wound hydrogel materials should have a wide range of functional properties including pore structure, antimicrobial activity, appropriate mechanical properties, haemostatic ability, controlled biodegradation, sustained release of bioactive molecules and good biocompatibility.128⇓⇓–131 ...
Adaptable hydrogels mediate cofactor-assisted activation of biomarker-responsive drug delivery via positive feedback for enhanced tissue regeneration
1
2018
... Hydrogel is a promising wound–healing material with properties of good breathability, moisture retention, the possibility of loading bioactive substances and the ability to absorb wound exudate and cool the wound surface, thus relieving the patient’s pain.121,122 Biomedical hydrogels have been extensively investigated as a scaffolding material for efficient drug/cell delivery in wound healing and functional reconstruction of skin tissue.123⇓⇓–126 The typical three–dimensional cross–linked structure of hydrogels allows high drug/cell encapsulation and local drug/cell persistence.127 The ideal wound hydrogel materials should have a wide range of functional properties including pore structure, antimicrobial activity, appropriate mechanical properties, haemostatic ability, controlled biodegradation, sustained release of bioactive molecules and good biocompatibility.128⇓⇓–131 ...
Efficient angiogenesis-based diabetic wound healing/skin reconstruction through bioactive antibacterial adhesive ultraviolet shielding nanodressing with exosome release
2
2019
... Hydrogel is a promising wound–healing material with properties of good breathability, moisture retention, the possibility of loading bioactive substances and the ability to absorb wound exudate and cool the wound surface, thus relieving the patient’s pain.121,122 Biomedical hydrogels have been extensively investigated as a scaffolding material for efficient drug/cell delivery in wound healing and functional reconstruction of skin tissue.123⇓⇓–126 The typical three–dimensional cross–linked structure of hydrogels allows high drug/cell encapsulation and local drug/cell persistence.127 The ideal wound hydrogel materials should have a wide range of functional properties including pore structure, antimicrobial activity, appropriate mechanical properties, haemostatic ability, controlled biodegradation, sustained release of bioactive molecules and good biocompatibility.128⇓⇓–131 ...
... In recent years, Guo et al.132 developed a specially–designed hydrogel with smart targeting of refractory wound characteristics and synthesised an injectable, inflammation–responsive, self–healing hydrogel to treat diabetic wounds. They found that hydrogels loaded with zinc oxide nanoparticles (nZnO), and micelles@paeoniflorin exhibited excellent structural integrity and rheological properties while remaining injectable. In addition the hydrogel released nZnO and paeoniflorin in response to an inflammatory microenvironment. They also found that their hydrogel exhibited good cytocompatibility, and robust haemostatic and angiogenic properties, which promoted healing of chronically–infected wounds in a rat model of diabetes. Wang et al.128 reported that they fabricated an injectable adhesive thermosensitive multifunctional polysaccharide–based hydrogel dressing. They found that the hydrogel dressing possessed multifunctional properties including antibacterial activity, fast haemostatic ability, self–healing behaviour, tissue–adhesive and good ultraviolet–shielding functions. It was also able to maintain the bioactivity of exosomes and promote the proliferation, migration and angiogenic ability of human umbilical vein endothelial cells. The results of an in vivo animal experiment indicated that the hydrogel promoted formation of granulation tissue, collagen deposition, remodelling, re–epithelialisation and further accelerated diabetic wound healing by enhancing angiogenesis. Further, some researchers used the Schiff base reaction to prepare hydrogels containing polyethylene glycol, carboxymethyl chitosan, and basic fibroblast growth factor. They found that the hydrogels had good wet–tissue adhesion, antibacterial properties, excellent biocompatibility, fast haemostatic capacity and self–repair abilities. The results demonstrated that these carboxymethyl chitosan–based hydrogels significantly accelerated full–thickness diabetic wound healing by increasing epithelialisation and collagen production and promoting the formation of hair follicles as well as improving neovascularisation by upregulating CD31.133 Recently, other researchers reported that an injected thermosensitive hydrogel incorporating Prussian blue nanoparticles enhanced angiogenesis, and reduced interleukin 6 and tumour necrosis factor–α levels. They also found that thermosensitive hydrogels improved diabetic wound healing by scavenging ROS and restoring mitochondrial function.134 ...
Cobalt-mediated multi-functional dressings promote bacteria-infected wound healing
1
2019
... Hydrogel is a promising wound–healing material with properties of good breathability, moisture retention, the possibility of loading bioactive substances and the ability to absorb wound exudate and cool the wound surface, thus relieving the patient’s pain.121,122 Biomedical hydrogels have been extensively investigated as a scaffolding material for efficient drug/cell delivery in wound healing and functional reconstruction of skin tissue.123⇓⇓–126 The typical three–dimensional cross–linked structure of hydrogels allows high drug/cell encapsulation and local drug/cell persistence.127 The ideal wound hydrogel materials should have a wide range of functional properties including pore structure, antimicrobial activity, appropriate mechanical properties, haemostatic ability, controlled biodegradation, sustained release of bioactive molecules and good biocompatibility.128⇓⇓–131 ...
Engineering a sprayable and elastic hydrogel adhesive with antimicrobial properties for wound healing
1
2017
... Hydrogel is a promising wound–healing material with properties of good breathability, moisture retention, the possibility of loading bioactive substances and the ability to absorb wound exudate and cool the wound surface, thus relieving the patient’s pain.121,122 Biomedical hydrogels have been extensively investigated as a scaffolding material for efficient drug/cell delivery in wound healing and functional reconstruction of skin tissue.123⇓⇓–126 The typical three–dimensional cross–linked structure of hydrogels allows high drug/cell encapsulation and local drug/cell persistence.127 The ideal wound hydrogel materials should have a wide range of functional properties including pore structure, antimicrobial activity, appropriate mechanical properties, haemostatic ability, controlled biodegradation, sustained release of bioactive molecules and good biocompatibility.128⇓⇓–131 ...
Novel mussel-inspired injectable self-healing hydrogel with anti-biofouling property
1
2015
... Hydrogel is a promising wound–healing material with properties of good breathability, moisture retention, the possibility of loading bioactive substances and the ability to absorb wound exudate and cool the wound surface, thus relieving the patient’s pain.121,122 Biomedical hydrogels have been extensively investigated as a scaffolding material for efficient drug/cell delivery in wound healing and functional reconstruction of skin tissue.123⇓⇓–126 The typical three–dimensional cross–linked structure of hydrogels allows high drug/cell encapsulation and local drug/cell persistence.127 The ideal wound hydrogel materials should have a wide range of functional properties including pore structure, antimicrobial activity, appropriate mechanical properties, haemostatic ability, controlled biodegradation, sustained release of bioactive molecules and good biocompatibility.128⇓⇓–131 ...
Development of a microenvironment-responsive hydrogel promoting chronically infected diabetic wound healing through sequential hemostatic, antibacterial, and angiogenic activities
1
2022
... In recent years, Guo et al.132 developed a specially–designed hydrogel with smart targeting of refractory wound characteristics and synthesised an injectable, inflammation–responsive, self–healing hydrogel to treat diabetic wounds. They found that hydrogels loaded with zinc oxide nanoparticles (nZnO), and micelles@paeoniflorin exhibited excellent structural integrity and rheological properties while remaining injectable. In addition the hydrogel released nZnO and paeoniflorin in response to an inflammatory microenvironment. They also found that their hydrogel exhibited good cytocompatibility, and robust haemostatic and angiogenic properties, which promoted healing of chronically–infected wounds in a rat model of diabetes. Wang et al.128 reported that they fabricated an injectable adhesive thermosensitive multifunctional polysaccharide–based hydrogel dressing. They found that the hydrogel dressing possessed multifunctional properties including antibacterial activity, fast haemostatic ability, self–healing behaviour, tissue–adhesive and good ultraviolet–shielding functions. It was also able to maintain the bioactivity of exosomes and promote the proliferation, migration and angiogenic ability of human umbilical vein endothelial cells. The results of an in vivo animal experiment indicated that the hydrogel promoted formation of granulation tissue, collagen deposition, remodelling, re–epithelialisation and further accelerated diabetic wound healing by enhancing angiogenesis. Further, some researchers used the Schiff base reaction to prepare hydrogels containing polyethylene glycol, carboxymethyl chitosan, and basic fibroblast growth factor. They found that the hydrogels had good wet–tissue adhesion, antibacterial properties, excellent biocompatibility, fast haemostatic capacity and self–repair abilities. The results demonstrated that these carboxymethyl chitosan–based hydrogels significantly accelerated full–thickness diabetic wound healing by increasing epithelialisation and collagen production and promoting the formation of hair follicles as well as improving neovascularisation by upregulating CD31.133 Recently, other researchers reported that an injected thermosensitive hydrogel incorporating Prussian blue nanoparticles enhanced angiogenesis, and reduced interleukin 6 and tumour necrosis factor–α levels. They also found that thermosensitive hydrogels improved diabetic wound healing by scavenging ROS and restoring mitochondrial function.134 ...
Carboxymethyl chitosan-based hydrogels containing fibroblast growth factors for triggering diabetic wound healing
1
2022
... In recent years, Guo et al.132 developed a specially–designed hydrogel with smart targeting of refractory wound characteristics and synthesised an injectable, inflammation–responsive, self–healing hydrogel to treat diabetic wounds. They found that hydrogels loaded with zinc oxide nanoparticles (nZnO), and micelles@paeoniflorin exhibited excellent structural integrity and rheological properties while remaining injectable. In addition the hydrogel released nZnO and paeoniflorin in response to an inflammatory microenvironment. They also found that their hydrogel exhibited good cytocompatibility, and robust haemostatic and angiogenic properties, which promoted healing of chronically–infected wounds in a rat model of diabetes. Wang et al.128 reported that they fabricated an injectable adhesive thermosensitive multifunctional polysaccharide–based hydrogel dressing. They found that the hydrogel dressing possessed multifunctional properties including antibacterial activity, fast haemostatic ability, self–healing behaviour, tissue–adhesive and good ultraviolet–shielding functions. It was also able to maintain the bioactivity of exosomes and promote the proliferation, migration and angiogenic ability of human umbilical vein endothelial cells. The results of an in vivo animal experiment indicated that the hydrogel promoted formation of granulation tissue, collagen deposition, remodelling, re–epithelialisation and further accelerated diabetic wound healing by enhancing angiogenesis. Further, some researchers used the Schiff base reaction to prepare hydrogels containing polyethylene glycol, carboxymethyl chitosan, and basic fibroblast growth factor. They found that the hydrogels had good wet–tissue adhesion, antibacterial properties, excellent biocompatibility, fast haemostatic capacity and self–repair abilities. The results demonstrated that these carboxymethyl chitosan–based hydrogels significantly accelerated full–thickness diabetic wound healing by increasing epithelialisation and collagen production and promoting the formation of hair follicles as well as improving neovascularisation by upregulating CD31.133 Recently, other researchers reported that an injected thermosensitive hydrogel incorporating Prussian blue nanoparticles enhanced angiogenesis, and reduced interleukin 6 and tumour necrosis factor–α levels. They also found that thermosensitive hydrogels improved diabetic wound healing by scavenging ROS and restoring mitochondrial function.134 ...
Thermosensitive hydrogel incorporating prussian blue nanoparticles promotes diabetic wound healing via ROS scavenging and mitochondrial function restoration
1
2022
... In recent years, Guo et al.132 developed a specially–designed hydrogel with smart targeting of refractory wound characteristics and synthesised an injectable, inflammation–responsive, self–healing hydrogel to treat diabetic wounds. They found that hydrogels loaded with zinc oxide nanoparticles (nZnO), and micelles@paeoniflorin exhibited excellent structural integrity and rheological properties while remaining injectable. In addition the hydrogel released nZnO and paeoniflorin in response to an inflammatory microenvironment. They also found that their hydrogel exhibited good cytocompatibility, and robust haemostatic and angiogenic properties, which promoted healing of chronically–infected wounds in a rat model of diabetes. Wang et al.128 reported that they fabricated an injectable adhesive thermosensitive multifunctional polysaccharide–based hydrogel dressing. They found that the hydrogel dressing possessed multifunctional properties including antibacterial activity, fast haemostatic ability, self–healing behaviour, tissue–adhesive and good ultraviolet–shielding functions. It was also able to maintain the bioactivity of exosomes and promote the proliferation, migration and angiogenic ability of human umbilical vein endothelial cells. The results of an in vivo animal experiment indicated that the hydrogel promoted formation of granulation tissue, collagen deposition, remodelling, re–epithelialisation and further accelerated diabetic wound healing by enhancing angiogenesis. Further, some researchers used the Schiff base reaction to prepare hydrogels containing polyethylene glycol, carboxymethyl chitosan, and basic fibroblast growth factor. They found that the hydrogels had good wet–tissue adhesion, antibacterial properties, excellent biocompatibility, fast haemostatic capacity and self–repair abilities. The results demonstrated that these carboxymethyl chitosan–based hydrogels significantly accelerated full–thickness diabetic wound healing by increasing epithelialisation and collagen production and promoting the formation of hair follicles as well as improving neovascularisation by upregulating CD31.133 Recently, other researchers reported that an injected thermosensitive hydrogel incorporating Prussian blue nanoparticles enhanced angiogenesis, and reduced interleukin 6 and tumour necrosis factor–α levels. They also found that thermosensitive hydrogels improved diabetic wound healing by scavenging ROS and restoring mitochondrial function.134 ...
Innovations in gene and growth factor delivery systems for diabetic wound healing
1
2018
... There is also a growing body of evidence suggesting that hydrogel biomaterials can be considered as an ideal sustained–release carrier of drugs or growth factors to release clinically significant doses of therapeutic agents in the process of wound repair.135 For example, Niu et al.136 prepared an injectable, thermosensitive and ROS–scavenging hydrogel as a release carrier of recombinant human MG53 protein and found that the hydrogel allowed for sustained release of recombinant human MG53 and enhanced diabetic wound healing and hair follicle development, which indicated the potential therapeutic prospects of using ROS–scavenging gel in combination with recombinant human MG53 to treat diabetic wounds. Further, Lee and Lin137 developed a chitosan/polyvinyl acetate hetero–composite hydrogel loading EGF. Their results showed that the hydrogels sustainably released EGF and polyhexamethylene biguanide in a microenvironment to generate antibacterial effects and promote cell growth for wound repair. They further found that the hydrogel promoted faster collagen deposition and re–epithelisation and reduced the inflammatory response, which accelerated diabetic wound healing. Recent research has revealed that Wu et al.138 developed a conductive and intrinsically–antibacterial hydrogel with pH responsiveness using quaternised chitosan, polyaniline and polyethylene glycol as delivery carrier of deferoxamine which is a pro–angiogenic drug. Their results illustrated that the hydrogels smartly released deferoxamine and enhanced vascularisation by increasing the expression of vascular endothelial growth factor and improving the proliferation and migration of endothelial cells. The results of in vivo animal experiments revealed that the hydrogels combined with electrical stimulation enhanced angiogenesis and significantly accelerated infected diabetic wound healing.138 We used cryogenic three–dimensional printing technology to create a small intestinal submucosa/mesoporous bioactive glass hydrogel scaffold as a release carrier of bioactive exosomes. The results showed that the hydrogel scaffolds permit sustained release of bioactive exosomes with adequate biocompatibility and good haemostatic ability, and enhanced the angiogenic ability of human umbilical vein endothelial cells. We also found that the hydrogel increased blood perfusion of wounds, promoted collagen deposition, and accelerated diabetic wound healing by enhancing angiogenesis.139 ...
Sustained delivery of rhMG53 promotes diabetic wound healing and hair follicle development
1
2022
... There is also a growing body of evidence suggesting that hydrogel biomaterials can be considered as an ideal sustained–release carrier of drugs or growth factors to release clinically significant doses of therapeutic agents in the process of wound repair.135 For example, Niu et al.136 prepared an injectable, thermosensitive and ROS–scavenging hydrogel as a release carrier of recombinant human MG53 protein and found that the hydrogel allowed for sustained release of recombinant human MG53 and enhanced diabetic wound healing and hair follicle development, which indicated the potential therapeutic prospects of using ROS–scavenging gel in combination with recombinant human MG53 to treat diabetic wounds. Further, Lee and Lin137 developed a chitosan/polyvinyl acetate hetero–composite hydrogel loading EGF. Their results showed that the hydrogels sustainably released EGF and polyhexamethylene biguanide in a microenvironment to generate antibacterial effects and promote cell growth for wound repair. They further found that the hydrogel promoted faster collagen deposition and re–epithelisation and reduced the inflammatory response, which accelerated diabetic wound healing. Recent research has revealed that Wu et al.138 developed a conductive and intrinsically–antibacterial hydrogel with pH responsiveness using quaternised chitosan, polyaniline and polyethylene glycol as delivery carrier of deferoxamine which is a pro–angiogenic drug. Their results illustrated that the hydrogels smartly released deferoxamine and enhanced vascularisation by increasing the expression of vascular endothelial growth factor and improving the proliferation and migration of endothelial cells. The results of in vivo animal experiments revealed that the hydrogels combined with electrical stimulation enhanced angiogenesis and significantly accelerated infected diabetic wound healing.138 We used cryogenic three–dimensional printing technology to create a small intestinal submucosa/mesoporous bioactive glass hydrogel scaffold as a release carrier of bioactive exosomes. The results showed that the hydrogel scaffolds permit sustained release of bioactive exosomes with adequate biocompatibility and good haemostatic ability, and enhanced the angiogenic ability of human umbilical vein endothelial cells. We also found that the hydrogel increased blood perfusion of wounds, promoted collagen deposition, and accelerated diabetic wound healing by enhancing angiogenesis.139 ...
Chitosan/PVA hetero-composite hydrogel containing antimicrobials, perfluorocarbon nanoemulsions, and growth factor-loaded nanoparticles as a multifunctional dressing for diabetic wound healing: synthesis, characterization, and in vitro/in vivo evaluation
1
2022
... There is also a growing body of evidence suggesting that hydrogel biomaterials can be considered as an ideal sustained–release carrier of drugs or growth factors to release clinically significant doses of therapeutic agents in the process of wound repair.135 For example, Niu et al.136 prepared an injectable, thermosensitive and ROS–scavenging hydrogel as a release carrier of recombinant human MG53 protein and found that the hydrogel allowed for sustained release of recombinant human MG53 and enhanced diabetic wound healing and hair follicle development, which indicated the potential therapeutic prospects of using ROS–scavenging gel in combination with recombinant human MG53 to treat diabetic wounds. Further, Lee and Lin137 developed a chitosan/polyvinyl acetate hetero–composite hydrogel loading EGF. Their results showed that the hydrogels sustainably released EGF and polyhexamethylene biguanide in a microenvironment to generate antibacterial effects and promote cell growth for wound repair. They further found that the hydrogel promoted faster collagen deposition and re–epithelisation and reduced the inflammatory response, which accelerated diabetic wound healing. Recent research has revealed that Wu et al.138 developed a conductive and intrinsically–antibacterial hydrogel with pH responsiveness using quaternised chitosan, polyaniline and polyethylene glycol as delivery carrier of deferoxamine which is a pro–angiogenic drug. Their results illustrated that the hydrogels smartly released deferoxamine and enhanced vascularisation by increasing the expression of vascular endothelial growth factor and improving the proliferation and migration of endothelial cells. The results of in vivo animal experiments revealed that the hydrogels combined with electrical stimulation enhanced angiogenesis and significantly accelerated infected diabetic wound healing.138 We used cryogenic three–dimensional printing technology to create a small intestinal submucosa/mesoporous bioactive glass hydrogel scaffold as a release carrier of bioactive exosomes. The results showed that the hydrogel scaffolds permit sustained release of bioactive exosomes with adequate biocompatibility and good haemostatic ability, and enhanced the angiogenic ability of human umbilical vein endothelial cells. We also found that the hydrogel increased blood perfusion of wounds, promoted collagen deposition, and accelerated diabetic wound healing by enhancing angiogenesis.139 ...
Injectable conductive and angiogenic hydrogels for chronic diabetic wound treatment
2
2022
... There is also a growing body of evidence suggesting that hydrogel biomaterials can be considered as an ideal sustained–release carrier of drugs or growth factors to release clinically significant doses of therapeutic agents in the process of wound repair.135 For example, Niu et al.136 prepared an injectable, thermosensitive and ROS–scavenging hydrogel as a release carrier of recombinant human MG53 protein and found that the hydrogel allowed for sustained release of recombinant human MG53 and enhanced diabetic wound healing and hair follicle development, which indicated the potential therapeutic prospects of using ROS–scavenging gel in combination with recombinant human MG53 to treat diabetic wounds. Further, Lee and Lin137 developed a chitosan/polyvinyl acetate hetero–composite hydrogel loading EGF. Their results showed that the hydrogels sustainably released EGF and polyhexamethylene biguanide in a microenvironment to generate antibacterial effects and promote cell growth for wound repair. They further found that the hydrogel promoted faster collagen deposition and re–epithelisation and reduced the inflammatory response, which accelerated diabetic wound healing. Recent research has revealed that Wu et al.138 developed a conductive and intrinsically–antibacterial hydrogel with pH responsiveness using quaternised chitosan, polyaniline and polyethylene glycol as delivery carrier of deferoxamine which is a pro–angiogenic drug. Their results illustrated that the hydrogels smartly released deferoxamine and enhanced vascularisation by increasing the expression of vascular endothelial growth factor and improving the proliferation and migration of endothelial cells. The results of in vivo animal experiments revealed that the hydrogels combined with electrical stimulation enhanced angiogenesis and significantly accelerated infected diabetic wound healing.138 We used cryogenic three–dimensional printing technology to create a small intestinal submucosa/mesoporous bioactive glass hydrogel scaffold as a release carrier of bioactive exosomes. The results showed that the hydrogel scaffolds permit sustained release of bioactive exosomes with adequate biocompatibility and good haemostatic ability, and enhanced the angiogenic ability of human umbilical vein endothelial cells. We also found that the hydrogel increased blood perfusion of wounds, promoted collagen deposition, and accelerated diabetic wound healing by enhancing angiogenesis.139 ...
... 138 We used cryogenic three–dimensional printing technology to create a small intestinal submucosa/mesoporous bioactive glass hydrogel scaffold as a release carrier of bioactive exosomes. The results showed that the hydrogel scaffolds permit sustained release of bioactive exosomes with adequate biocompatibility and good haemostatic ability, and enhanced the angiogenic ability of human umbilical vein endothelial cells. We also found that the hydrogel increased blood perfusion of wounds, promoted collagen deposition, and accelerated diabetic wound healing by enhancing angiogenesis.139 ...
Cryogenic 3D printed hydrogel scaffolds loading exosomes accelerate diabetic wound healing
1
2021
... There is also a growing body of evidence suggesting that hydrogel biomaterials can be considered as an ideal sustained–release carrier of drugs or growth factors to release clinically significant doses of therapeutic agents in the process of wound repair.135 For example, Niu et al.136 prepared an injectable, thermosensitive and ROS–scavenging hydrogel as a release carrier of recombinant human MG53 protein and found that the hydrogel allowed for sustained release of recombinant human MG53 and enhanced diabetic wound healing and hair follicle development, which indicated the potential therapeutic prospects of using ROS–scavenging gel in combination with recombinant human MG53 to treat diabetic wounds. Further, Lee and Lin137 developed a chitosan/polyvinyl acetate hetero–composite hydrogel loading EGF. Their results showed that the hydrogels sustainably released EGF and polyhexamethylene biguanide in a microenvironment to generate antibacterial effects and promote cell growth for wound repair. They further found that the hydrogel promoted faster collagen deposition and re–epithelisation and reduced the inflammatory response, which accelerated diabetic wound healing. Recent research has revealed that Wu et al.138 developed a conductive and intrinsically–antibacterial hydrogel with pH responsiveness using quaternised chitosan, polyaniline and polyethylene glycol as delivery carrier of deferoxamine which is a pro–angiogenic drug. Their results illustrated that the hydrogels smartly released deferoxamine and enhanced vascularisation by increasing the expression of vascular endothelial growth factor and improving the proliferation and migration of endothelial cells. The results of in vivo animal experiments revealed that the hydrogels combined with electrical stimulation enhanced angiogenesis and significantly accelerated infected diabetic wound healing.138 We used cryogenic three–dimensional printing technology to create a small intestinal submucosa/mesoporous bioactive glass hydrogel scaffold as a release carrier of bioactive exosomes. The results showed that the hydrogel scaffolds permit sustained release of bioactive exosomes with adequate biocompatibility and good haemostatic ability, and enhanced the angiogenic ability of human umbilical vein endothelial cells. We also found that the hydrogel increased blood perfusion of wounds, promoted collagen deposition, and accelerated diabetic wound healing by enhancing angiogenesis.139 ...