Biomaterials Translational ›› 2022, Vol. 3 ›› Issue (3): 188-200.doi: 10.12336/biomatertransl.2022.03.003
• REVIEW • Previous Articles Next Articles
Yiqiang Hu1,2,#, Yuan Xiong1,2,#, Ranyang Tao1,2, Hang Xue1,2, Lang Chen1,2, Ze Lin1,2, Adriana C. Panayi3, Bobin Mi1,2,*( ), Guohui Liu1,2,*(
), Guohui Liu1,2,*( )
)
Received:2022-05-01
Revised:2022-05-27
Accepted:2022-08-26
Online:2022-09-28
Published:2022-09-28
Contact:
Bobin Mi, E-mail:mibobin@hust.edu.cn; Guohui Liu, liuguohui@hust.edu.cn
About author:Bobin Mi, mibobin@hust.edu.cn;Hu, Y.; Xiong, Y.; Tao, R.; Xue, H.; Chen, L.; Lin, Z.; Panayi, A.; Mi, B.; Liu, G. Advances and perspective on animal models and hydrogel biomaterials for diabetic wound healing. Biomater Transl. 2022, 3(3), 188-200.
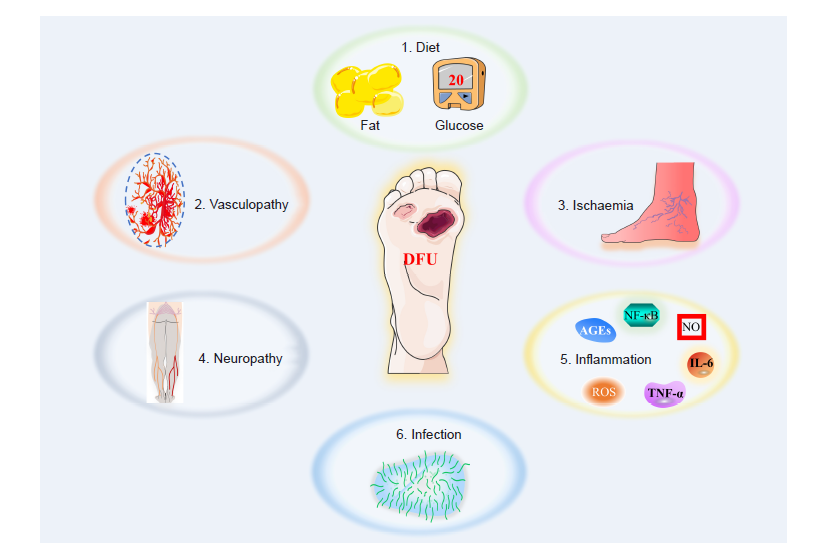
Figure 2. The main factors that contribute to the formation of a diabetic wound. AGE: advanced glycation end products; DFU: diabetic foot ulcers; IL–6: interleukin 6; NF–κB: nuclear factor kappa–B; NO: nitric oxide; ROS: reactive oxygen species; TNF–α: tumour necrosis factor–α.
| 1. |
Gandhi, G. Y.; Mooradian, A. D. Management of hyperglycemia in older adults with type 2 diabetes. Drugs Aging. 2022, 39, 39-58.
doi: 10.1007/s40266-021-00910-1 URL |
| 2. |
Graves, L. E.; Donaghue, K. C. Vascular complication in adolescents with diabetes mellitus. Front Endocrinol (Lausanne). 2020, 11, 370.
doi: 10.3389/fendo.2020.00370 URL |
| 3. |
Wan, G.; Chen, Y.; Chen, J.; Yan, C.; Wang, C.; Li, W.; Mao, R.; Machens, H. G.; Yang, X.; Chen, Z. Regulation of endothelial progenitor cell functions during hyperglycemia: new therapeutic targets in diabetic wound healing. J Mol Med (Berl). 2022, 100, 485-498.
doi: 10.1007/s00109-021-02172-1 URL |
| 4. |
den Dekker, A.; Davis, F. M.; Kunkel, S. L.; Gallagher, K. A. Targeting epigenetic mechanisms in diabetic wound healing. Transl Res. 2019, 204, 39-50.
doi: 10.1016/j.trsl.2018.10.001 URL |
| 5. |
Vijayakumar, V.; Samal, S. K.; Mohanty, S.; Nayak, S. K. Recent advancements in biopolymer and metal nanoparticle-based materials in diabetic wound healing management. Int J Biol Macromol. 2019, 122, 137-148.
doi: 10.1016/j.ijbiomac.2018.10.120 URL |
| 6. |
Phang, S. J.; Arumugam, B.; Kuppusamy, U. R.; Fauzi, M. B.; Looi, M. L. A review of diabetic wound models-Novel insights into diabetic foot ulcer. J Tissue Eng Regen Med. 2021, 15, 1051-1068.
doi: 10.1002/term.3246 URL |
| 7. |
Nowak, N. C.; Menichella, D. M.; Miller, R.; Paller, A. S. Cutaneous innervation in impaired diabetic wound healing. Transl Res. 2021, 236, 87-108.
doi: 10.1016/j.trsl.2021.05.003 URL |
| 8. |
Greenhalgh, D. G. Wound healing and diabetes mellitus. Clin Plast Surg. 2003, 30, 37-45.
doi: 10.1016/S0094-1298(02)00066-4 URL |
| 9. |
Wang, C.; Guo, M.; Zhang, N.; Wang, G. Effectiveness of honey dressing in the treatment of diabetic foot ulcers: A systematic review and meta-analysis. Complement Ther Clin Pract. 2019, 34, 123-131.
doi: 10.1016/j.ctcp.2018.09.004 URL |
| 10. | Khamaisi, M.; Balanson, S. Dysregulation of wound healing mechanisms in diabetes and the importance of negative pressure wound therapy (NPWT). Diabetes Metab Res Rev. 2017, 33, e2929. |
| 11. |
Gurtner, G. C.; Werner, S.; Barrandon, Y.; Longaker, M. T. Wound repair and regeneration. Nature. 2008, 453, 314-321.
doi: 10.1038/nature07039 URL |
| 12. |
Nourian Dehkordi, A.; Mirahmadi Babaheydari, F.; Chehelgerdi, M.; Raeisi Dehkordi, S. Skin tissue engineering: wound healing based on stem-cell-based therapeutic strategies. Stem Cell Res Ther. 2019, 10, 111.
doi: 10.1186/s13287-019-1212-2 URL |
| 13. |
Dekoninck, S.; Blanpain, C. Stem cell dynamics, migration and plasticity during wound healing. Nat Cell Biol. 2019, 21, 18-24.
doi: 10.1038/s41556-018-0237-6 URL |
| 14. |
Yang, F.; Bai, X.; Dai, X.; Li, Y. The biological processes during wound healing. Regen Med. 2021, 16, 373-390.
doi: 10.2217/rme-2020-0066 URL |
| 15. |
Moura, L. I.; Dias, A. M.; Carvalho, E.; de Sousa, H. C. Recent advances on the development of wound dressings for diabetic foot ulcer treatment--a review. Acta Biomater. 2013, 9, 7093-7114.
doi: 10.1016/j.actbio.2013.03.033 URL |
| 16. | Broughton, G., 2nd; Janis, J. E.; Attinger, C. E. Wound healing: an overview. Plast Reconstr Surg. 2006, 117, 1e-S-32e-S. |
| 17. |
Yang, R.; Liu, F.; Wang, J.; Chen, X.; Xie, J.; Xiong, K. Epidermal stem cells in wound healing and their clinical applications. Stem Cell Res Ther. 2019, 10, 229.
doi: 10.1186/s13287-019-1312-z URL |
| 18. |
Han, G.; Ceilley, R. Chronic wound healing: a review of current management and treatments. Adv Ther. 2017, 34, 599-610.
doi: 10.1007/s12325-017-0478-y URL |
| 19. |
Rani, S.; Ritter, T. The exosome - a naturally secreted nanoparticle and its application to wound healing. Adv Mater. 2016, 28, 5542-5552.
doi: 10.1002/adma.201504009 URL |
| 20. |
Rai, V.; Moellmer, R.; Agrawal, D. K. Clinically relevant experimental rodent models of diabetic foot ulcer. Mol Cell Biochem. 2022, 477, 1239-1247.
doi: 10.1007/s11010-022-04372-w URL |
| 21. |
Goyal, S. N.; Reddy, N. M.; Patil, K. R.; Nakhate, K. T.; Ojha, S.; Patil, C. R.; Agrawal, Y. O. Challenges and issues with streptozotocin-induced diabetes - A clinically relevant animal model to understand the diabetes pathogenesis and evaluate therapeutics. Chem Biol Interact. 2016, 244, 49-63.
doi: 10.1016/j.cbi.2015.11.032 URL |
| 22. | Ansell, D. M.; Marsh, C.; Walker, L.; Hardman, M. J.; Holden, K. Evaluating STZ-induced impaired wound healing in rats. J Invest Dermatol. 2018, 138, 994-997. |
| 23. |
Yu, M.; Liu, W.; Li, J.; Lu, J.; Lu, H.; Jia, W.; Liu, F. Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway. Stem Cell Res Ther. 2020, 11, 350.
doi: 10.1186/s13287-020-01824-2 URL |
| 24. |
Szkudelski, T. Streptozotocin-nicotinamide-induced diabetes in the rat. Characteristics of the experimental model. Exp Biol Med (Maywood). 2012, 237, 481-490.
doi: 10.1258/ebm.2012.011372 URL |
| 25. |
Ahmed, R.; Afreen, A.; Tariq, M.; Zahid, A. A.; Masoud, M. S.; Ahmed, M.; Ali, I.; Akram, Z.; Hasan, A. Bone marrow mesenchymal stem cells preconditioned with nitric-oxide-releasing chitosan/PVA hydrogel accelerate diabetic wound healing in rabbits. Biomed Mater. 2021, 16, 035014.
doi: 10.1088/1748-605X/abc28b URL |
| 26. | Malone-Povolny, M. J.; Maloney, S. E.; Schoenfisch, M. H. Nitric oxide therapy for diabetic wound healing. Adv Healthc Mater. 2019, 8, e1801210. |
| 27. |
Hoppenbrouwers, T.; Tuk, B.; Fijneman, E. M.; de Maat, M. P.; van Neck, J. W. Fibrin improves skin wound perfusion in a diabetic rat model. Thromb Res. 2017, 151, 36-40.
doi: 10.1016/j.thromres.2017.01.002 URL |
| 28. |
Kaymakcalan, O. E.; Abadeer, A.; Goldufsky, J. W.; Galili, U.; Karinja, S. J.; Dong, X.; Jin, J. L.; Samadi, A.; Spector, J. A. Topical α-gal nanoparticles accelerate diabetic wound healing. Exp Dermatol. 2020, 29, 404-413.
doi: 10.1111/exd.14084 URL |
| 29. | Dunn, L.; Prosser, H. C.; Tan, J. T.; Vanags, L. Z.; Ng, M. K.; Bursill, C. A. Murine model of wound healing. J Vis Exp. 2013, e50265. |
| 30. |
Li, G.; Ko, C. N.; Li, D.; Yang, C.; Wang, W.; Yang, G. J.; Di Primo, C.; Wong, V. K. W.; Xiang, Y.; Lin, L.; Ma, D. L.; Leung, C. H. A small molecule HIF-1α stabilizer that accelerates diabetic wound healing. Nat Commun. 2021, 12, 3363.
doi: 10.1038/s41467-021-23448-7 URL |
| 31. |
King, A. J. The use of animal models in diabetes research. Br J Pharmacol. 2012, 166, 877-894.
doi: 10.1111/j.1476-5381.2012.01911.x URL |
| 32. |
Yang, J.; Chen, Z.; Pan, D.; Li, H.; Shen, J. Umbilical cord-derived mesenchymal stem cell-derived exosomes combined pluronic F127 hydrogel promote chronic diabetic wound healing and complete skin regeneration. Int J Nanomedicine. 2020, 15, 5911-5926.
doi: 10.2147/IJN.S249129 URL |
| 33. | Zhang, J.; Zhou, R.; Xiang, C.; Jia, Q.; Wu, H.; Yang, H. Huangbai Liniment accelerated wound healing by activating Nrf2 signaling in diabetes. Oxid Med Cell Longev. 2020, 2020, 4951820. |
| 34. |
Liu, W.; Yu, M.; Xie, D.; Wang, L.; Ye, C.; Zhu, Q.; Liu, F.; Yang, L. Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res Ther. 2020, 11, 259.
doi: 10.1186/s13287-020-01756-x URL |
| 35. |
Abo El-Magd, N. F.; Ramadan, N. M.; Eraky, S. M. The ameliorative effect of bromelain on STZ-induced type 1 diabetes in rats through Oxi-LDL/LPA/LPAR1 pathway. Life Sci. 2021, 285, 119982.
doi: 10.1016/j.lfs.2021.119982 URL |
| 36. |
Jayasimhan, A.; Mansour, K. P.; Slattery, R. M. Advances in our understanding of the pathophysiology of Type 1 diabetes: lessons from the NOD mouse. Clin Sci (Lond). 2014, 126, 1-18.
doi: 10.1042/CS20120627 URL |
| 37. |
Driver, J. P.; Serreze, D. V.; Chen, Y. G. Mouse models for the study of autoimmune type 1 diabetes: a NOD to similarities and differences to human disease. Semin Immunopathol. 2011, 33, 67-87.
doi: 10.1007/s00281-010-0204-1 URL |
| 38. | Giarratana, N.; Penna, G.; Adorini, L. Animal models of spontaneous autoimmune disease: type 1 diabetes in the nonobese diabetic mouse. Methods Mol Biol. 2007, 380, 285-311. |
| 39. |
Irvin, A. E.; Jhala, G.; Zhao, Y.; Blackwell, T. S.; Krishnamurthy, B.; Thomas, H. E.; Kay, T. W. H. NF-κB is weakly activated in the NOD mouse model of type 1 diabetes. Sci Rep. 2018, 8, 4217.
doi: 10.1038/s41598-018-22738-3 URL |
| 40. | Jiang, Y.; Xie, F.; Lv, X.; Wang, S.; Liao, X.; Yu, Y.; Dai, Q.; Zhang, Y.; Meng, J.; Hu, G.; Peng, Z.; Tao, L. Mefunidone ameliorates diabetic kidney disease in STZ and db/db mice. FASEB J. 2021, 35, e21198. |
| 41. |
Wang, M.; Song, L.; Strange, C.; Dong, X.; Wang, H. Therapeutic effects of adipose stem cells from diabetic mice for the treatment of type 2 diabetes. Mol Ther. 2018, 26, 1921-1930.
doi: 10.1016/j.ymthe.2018.06.013 URL |
| 42. |
Sakai, S.; Yamamoto, T.; Takabatake, Y.; Takahashi, A.; Namba-Hamano, T.; Minami, S.; Fujimura, R.; Yonishi, H.; Matsuda, J.; Hesaka, A.; Matsui, I.; Matsusaka, T.; Niimura, F.; Yanagita, M.; Isaka, Y. Proximal tubule autophagy differs in type 1 and 2 diabetes. J Am Soc Nephrol. 2019, 30, 929-945.
doi: 10.1681/ASN.2018100983 URL |
| 43. | Heydemann, A. An overview of murine high fat diet as a model for type 2 diabetes mellitus. J Diabetes Res. 2016, 2016, 2902351. |
| 44. |
Silamiķele, L.; Silamiķelis, I.; Ustinova, M.; Kalniņa, Z.; Elbere, I.; Petrovska, R.; Kalniņa, I.; Kloviņš, J. Metformin strongly affects gut microbiome composition in high-fat diet-induced type 2 diabetes mouse model of both sexes. Front Endocrinol (Lausanne). 2021, 12, 626359.
doi: 10.3389/fendo.2021.626359 URL |
| 45. |
Grada, A.; Mervis, J.; Falanga, V. Research techniques made simple: animal models of wound healing. J Invest Dermatol. 2018, 138:2095-2105.e1.
doi: 10.1016/j.jid.2018.08.005 URL |
| 46. | Saleem Mir, M.; Maqbool Darzi, M.; Khalil Baba, O.; Khan, H. M.; Kamil, S. A.; Sofi, A. H.; Wani, S. A. Streptozotocin induced acute clinical effects in rabbits (Oryctolagus cuniculus). Iran J Pathol. 2015, 10, 206-213. |
| 47. |
Conaway, H. H.; Faas, F. H.; Smith, S. D.; Sanders, L. L. Spontaneous diabetes mellitus in the New Zealand white rabbit: physiologic characteristics. Metabolism. 1981, 30, 50-56.
doi: 10.1016/0026-0495(81)90218-3 URL |
| 48. | Zhang, J.; Hu, W.; Diao, Q.; Wang, Z.; Miao, J.; Chen, X.; Xue, Z. Therapeutic effect of the epidermal growth factor on diabetic foot ulcer and the underlying mechanisms. Exp Ther Med. 2019, 17, 1643-1648. |
| 49. |
O’Loughlin, A.; Kulkarni, M.; Vaughan, E. E.; Creane, M.; Liew, A.; Dockery, P.; Pandit, A.; O’Brien, T. Autologous circulating angiogenic cells treated with osteopontin and delivered via a collagen scaffold enhance wound healing in the alloxan-induced diabetic rabbit ear ulcer model. Stem Cell Res Ther. 2013, 4, 158.
doi: 10.1186/scrt388 URL |
| 50. |
O’Loughlin, A.; Kulkarni, M.; Creane, M.; Vaughan, E. E.; Mooney, E.; Shaw, G.; Murphy, M.; Dockery, P.; Pandit, A.; O’Brien, T. Topical administration of allogeneic mesenchymal stromal cells seeded in a collagen scaffold augments wound healing and increases angiogenesis in the diabetic rabbit ulcer. Diabetes. 2013, 62, 2588-2594.
doi: 10.2337/db12-1822 URL |
| 51. |
Velander, P.; Theopold, C.; Hirsch, T.; Bleiziffer, O.; Zuhaili, B.; Fossum, M.; Hoeller, D.; Gheerardyn, R.; Chen, M.; Visovatti, S.; Svensson, H.; Yao, F.; Eriksson, E. Impaired wound healing in an acute diabetic pig model and the effects of local hyperglycemia. Wound Repair Regen. 2008, 16, 288-293.
doi: 10.1111/j.1524-475X.2008.00367.x URL |
| 52. |
Ramirez, H. A.; Pastar, I.; Jozic, I.; Stojadinovic, O.; Stone, R. C.; Ojeh, N.; Gil, J.; Davis, S. C.; Kirsner, R. S.; Tomic-Canic, M. Staphylococcus aureus triggers induction of miR-15B-5P to Diminish DNA repair and deregulate inflammatory response in diabetic foot ulcers. J Invest Dermatol. 2018, 138, 1187-1196.
doi: 10.1016/j.jid.2017.11.038 URL |
| 53. | Sawaya, A. P.; Jozic, I.; Stone, R. C.; Pastar, I.; Egger, A. N.; Stojadinovic, O.; Glinos, G. D.; Kirsner, R. S.; Tomic-Canic, M. Mevastatin promotes healing by targeting caveolin-1 to restore EGFR signaling. JCI Insight. 2019, 4, e129320. |
| 54. | Podell, B. K.; Ackart, D. F.; Richardson, M. A.; DiLisio, J. E.; Pulford, B.; Basaraba, R. J. A model of type 2 diabetes in the guinea pig using sequential diet-induced glucose intolerance and streptozotocin treatment. Dis Model Mech. 2017, 10, 151-162. |
| 55. |
Verhagen, J.; Smith, E. L.; Whettlock, E. M.; Macintyre, B.; Peakman, M. Proinsulin-mediated induction of type 1 diabetes in HLA-DR4-transgenic mice. Sci Rep. 2018, 8, 14106.
doi: 10.1038/s41598-018-32546-4 URL |
| 56. | Perez-Favila, A.; Martinez-Fierro, M. L.; Rodriguez-Lazalde, J. G.; Cid-Baez, M. A.; Zamudio-Osuna, M. J.; Martinez-Blanco, M. D. R.; Mollinedo-Montaño, F. E.; Rodriguez-Sanchez, I. P.; Castañeda-Miranda, R.; Garza-Veloz, I. Current therapeutic strategies in diabetic foot ulcers. Medicina (Kaunas). 2019, 55, 714. |
| 57. |
Dixon, D.; Edmonds, M. Managing diabetic foot ulcers: pharmacotherapy for wound healing. Drugs. 2021, 81, 29-56.
doi: 10.1007/s40265-020-01415-8 URL |
| 58. |
Reardon, R.; Simring, D.; Kim, B.; Mortensen, J.; Williams, D.; Leslie, A. The diabetic foot ulcer. Aust J Gen Pract. 2020, 49, 250-255.
doi: 10.31128/AJGP-11-19-5161 URL |
| 59. |
Lim, J. Z.; Ng, N. S.; Thomas, C. Prevention and treatment of diabetic foot ulcers. J R Soc Med. 2017, 110, 104-109.
doi: 10.1177/0141076816688346 URL |
| 60. | American Diabetes Association. 11. Microvascular complications and foot care: standards of medical care in diabetes-2020. Diabetes Care. 2020, 43, S135-S151. |
| 61. | Migdalis, I.; Czupryniak, L.; Lalic, N.; Leslie, R. D.; Papanas, N.; Valensi, P. Diabetic microvascular complications. Int J Endocrinol. 2018, 2018, 5683287. |
| 62. |
Strain, W. D.; Paldánius, P. M. Diabetes, cardiovascular disease and the microcirculation. Cardiovasc Diabetol. 2018, 17, 57.
doi: 10.1186/s12933-018-0703-2 URL |
| 63. |
Forbes, J. M.; Cooper, M. E. Mechanisms of diabetic complications. Physiol Rev. 2013, 93, 137-188.
doi: 10.1152/physrev.00045.2011 URL |
| 64. |
Alicic, R. Z.; Rooney, M. T.; Tuttle, K. R. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017, 12, 2032-2045.
doi: 10.2215/CJN.11491116 URL |
| 65. |
Ricciardi, C. A.; Gnudi, L. Kidney disease in diabetes: from mechanisms to clinical presentation and treatment strategies. Metabolism. 2021, 124, 154890.
doi: 10.1016/j.metabol.2021.154890 URL |
| 66. | Schoina, M.; Loutradis, C.; Theodorakopoulou, M.; Dimitroulas, T.; Triantafillidou, E.; Doumas, M.; Karagiannis, A.; Garyfallos, A.; Papagianni, A.; Sarafidis, P. The presence of diabetes mellitus further impairs structural and functional capillary density in patients with chronic kidney disease. Microcirculation. 2021, 28, e12665. |
| 67. |
Sabanayagam, C.; Banu, R.; Chee, M. L.; Lee, R.; Wang, Y. X.; Tan, G.; Jonas, J. B.; Lamoureux, E. L.; Cheng, C. Y.; Klein, B. E. K.; Mitchell, P.; Klein, R.; Cheung, C. M. G.; Wong, T. Y. Incidence and progression of diabetic retinopathy: a systematic review. Lancet Diabetes Endocrinol. 2019, 7, 140-149.
doi: 10.1016/S2213-8587(18)30128-1 URL |
| 68. |
Wu, L.; Gunton, J. E. The Changing landscape of pharmacotherapy for diabetes mellitus: a review of cardiovascular outcomes. Int J Mol Sci. 2019, 20, 5853.
doi: 10.3390/ijms20235853 URL |
| 69. |
Low Wang, C. C.; Everett, B. M.; Burman, K. D.; Wilson, P. W. F. Cardiovascular safety trials for all new diabetes mellitus drugs? Circulation. 2019, 139, 1741-1743.
doi: 10.1161/CIRCULATIONAHA.118.038771 URL |
| 70. | Apelqvist, J. A.; Lepäntalo, M. J. The ulcerated leg: when to revascularize. Diabetes Metab Res Rev. 2012, 28 Suppl 1, 30-35. |
| 71. |
Korzon-Burakowska, A.; Edmonds, M. Role of the microcirculation in diabetic foot ulceration. Int J Low Extrem Wounds. 2006, 5, 144-148.
doi: 10.1177/1534734606292037 URL |
| 72. |
Grennan, D. Diabetic foot ulcers. JAMA. 2019, 321, 114.
doi: 10.1001/jama.2018.18323 URL |
| 73. |
Chao, C. Y.; Cheing, G. L. Microvascular dysfunction in diabetic foot disease and ulceration. Diabetes Metab Res Rev. 2009, 25, 604-614.
doi: 10.1002/dmrr.1004 URL |
| 74. |
Volmer-Thole, M.; Lobmann, R. Neuropathy and diabetic foot syndrome. Int J Mol Sci. 2016, 17, 917.
doi: 10.3390/ijms17060917 URL |
| 75. |
Hicks, C. W.; Selvin, E. Epidemiology of peripheral neuropathy and lower extremity disease in diabetes. Curr Diab Rep. 2019, 19, 86.
doi: 10.1007/s11892-019-1212-8 URL |
| 76. |
Balasubramanian, G.; Vas, P.; Chockalingam, N.; Naemi, R. A synoptic overview of neurovascular interactions in the foot. Front Endocrinol (Lausanne). 2020, 11, 308.
doi: 10.3389/fendo.2020.00308 URL |
| 77. |
Lechleitner, M.; Abrahamian, H.; Francesconi, C.; Kofler, M.; Sturm, W.; Köhler, G. Diabetic neuropathy and diabetic foot syndrome (Update 2019). Wien Klin Wochenschr. 2019, 131, 141-150.
doi: 10.1007/s00508-019-1487-4 pmid: 30980143 |
| 78. |
Lima, A. L.; Illing, T.; Schliemann, S.; Elsner, P. Cutaneous manifestations of diabetes mellitus: a review. Am J Clin Dermatol. 2017, 18, 541-553.
doi: 10.1007/s40257-017-0275-z URL |
| 79. |
Zilliox, L. A. Diabetes and peripheral nerve disease. Clin Geriatr Med. 2021, 37, 253-267.
doi: 10.1016/j.cger.2020.12.001 URL |
| 80. |
Zhang, Y.; Lazzarini, P. A.; McPhail, S. M.; van Netten, J. J.; Armstrong, D. G.; Pacella, R. E. Global disability burdens of diabetes-related lower-extremity complications in 1990 and 2016. Diabetes Care. 2020, 43, 964-974.
doi: 10.2337/dc19-1614 URL |
| 81. |
Yang, H.; Sloan, G.; Ye, Y.; Wang, S.; Duan, B.; Tesfaye, S.; Gao, L. New perspective in diabetic neuropathy: from the periphery to the brain, a call for early detection, and precision medicine. Front Endocrinol (Lausanne). 2019, 10, 929.
doi: 10.3389/fendo.2019.00929 URL |
| 82. |
Tuttolomondo, A.; Maida, C.; Pinto, A. Diabetic foot syndrome: immune-inflammatory features as possible cardiovascular markers in diabetes. World J Orthop. 2015, 6, 62-76.
doi: 10.5312/wjo.v6.i1.62 URL |
| 83. |
Giri, B.; Dey, S.; Das, T.; Sarkar, M.; Banerjee, J.; Dash, S. K. Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: An update on glucose toxicity. Biomed Pharmacother. 2018, 107, 306-328.
doi: 10.1016/j.biopha.2018.07.157 URL |
| 84. |
Davis, F. M.; Kimball, A.; Boniakowski, A.; Gallagher, K. Dysfunctional wound healing in diabetic foot ulcers: new crossroads. Curr Diab Rep. 2018, 18, 2.
doi: 10.1007/s11892-018-0970-z URL |
| 85. |
Gardner, S. E.; Frantz, R. A. Wound bioburden and infection-related complications in diabetic foot ulcers. Biol Res Nurs. 2008, 10, 44-53.
doi: 10.1177/1099800408319056 URL |
| 86. |
Adeghate, J.; Nurulain, S.; Tekes, K.; Fehér, E.; Kalász, H.; Adeghate, E. Novel biological therapies for the treatment of diabetic foot ulcers. Expert Opin Biol Ther. 2017, 17, 979-987.
doi: 10.1080/14712598.2017.1333596 URL |
| 87. | Pitocco, D.; Spanu, T.; Di Leo, M.; Vitiello, R.; Rizzi, A.; Tartaglione, L.; Fiori, B.; Caputo, S.; Tinelli, G.; Zaccardi, F.; Flex, A.; Galli, M.; Pontecorvi, A.; Sanguinetti, M. Diabetic foot infections: a comprehensive overview. Eur Rev Med Pharmacol Sci. 2019, 23, 26-37. |
| 88. |
Ghotaslou, R.; Memar, M. Y.; Alizadeh, N. Classification, microbiology and treatment of diabetic foot infections. J Wound Care. 2018, 27, 434-441.
doi: 10.12968/jowc.2018.27.7.434 URL |
| 89. | Dumville, J. C.; Lipsky, B. A.; Hoey, C.; Cruciani, M.; Fiscon, M.; Xia, J. Topical antimicrobial agents for treating foot ulcers in people with diabetes. Cochrane Database Syst Rev. 2017, 6, CD011038. |
| 90. |
Everett, E.; Mathioudakis, N. Update on management of diabetic foot ulcers. Ann N Y Acad Sci. 2018, 1411, 153-165.
doi: 10.1111/nyas.13569 URL |
| 91. |
Alavi, A.; Sibbald, R. G.; Mayer, D.; Goodman, L.; Botros, M.; Armstrong, D. G.; Woo, K.; Boeni, T.; Ayello, E. A.; Kirsner, R. S. Diabetic foot ulcers: Part I. Pathophysiology and prevention. J Am Acad Dermatol. 2014, 70, 1.e1-18; quiz 19-20.
doi: 10.1016/j.jaad.2013.08.019 URL |
| 92. |
Aldana, P. C.; Khachemoune, A. Diabetic foot ulcers: appraising standard of care and reviewing new trends in management. Am J Clin Dermatol. 2020, 21, 255-264.
doi: 10.1007/s40257-019-00495-x URL |
| 93. |
Lebrun, E.; Tomic-Canic, M.; Kirsner, R. S. The role of surgical debridement in healing of diabetic foot ulcers. Wound Repair Regen. 2010, 18, 433-438.
doi: 10.1111/j.1524-475X.2010.00619.x URL |
| 94. | Edwards, J.; Stapley, S. Debridement of diabetic foot ulcers. Cochrane Database Syst Rev. 2010, 2010, CD003556. |
| 95. |
Alexiadou, K.; Doupis, J. Management of diabetic foot ulcers. Diabetes Ther. 2012, 3, 4.
doi: 10.1007/s13300-012-0004-9 URL |
| 96. |
Patry, J.; Blanchette, V. Enzymatic debridement with collagenase in wounds and ulcers: a systematic review and meta-analysis. Int Wound J. 2017, 14, 1055-1065.
doi: 10.1111/iwj.12760 URL |
| 97. |
Singh, N.; Armstrong, D. G.; Lipsky, B. A. Preventing foot ulcers in patients with diabetes. JAMA. 2005, 293, 217-228.
doi: 10.1001/jama.293.2.217 URL |
| 98. | Sen, P.; Demirdal, T.; Emir, B. Meta-analysis of risk factors for amputation in diabetic foot infections. Diabetes Metab Res Rev. 2019, 35, e3165. |
| 99. |
Macdonald, K. E.; Boeckh, S.; Stacey, H. J.; Jones, J. D. The microbiology of diabetic foot infections: a meta-analysis. BMC Infect Dis. 2021, 21, 770.
doi: 10.1186/s12879-021-06516-7 URL |
| 100. | Barwell, N. D.; Devers, M. C.; Kennon, B.; Hopkinson, H. E.; McDougall, C.; Young, M. J.; Robertson, H. M. A.; Stang, D.; Dancer, S. J.; Seaton, A.; Leese, G. P.; Scottish Diabetes Foot Action Group. Diabetic foot infection: Antibiotic therapy and good practice recommendations. Int J Clin Pract. 2017, 71, e13006. |
| 101. |
Lipsky, B. A.; Berendt, A. R.; Cornia, P. B.; Pile, J. C.; Peters, E. J.; Armstrong, D. G.; Deery, H. G.; Embil, J. M.; Joseph, W. S.; Karchmer, A. W.; Pinzur, M. S.; Senneville, E.; Infectious Diseases Society of America. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012, 54, e132-173.
doi: 10.1093/cid/cis346 URL |
| 102. |
Lipsky, B. A.; Berendt, A. R.; Cornia, P. B.; Pile, J. C.; Peters, E. J.; Armstrong, D. G.; Deery, H. G.; Embil, J. M.; Joseph, W. S.; Karchmer, A. W.; Pinzur, M. S.; Senneville, E.; Infectious Diseases Society of America. Executive summary: 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012, 54, 1679-1684.
doi: 10.1093/cid/cis460 URL |
| 103. | Cavanagh, P. R.; Ulbrecht, J. S.; Caputo, G. M. New developments in the biomechanics of the diabetic foot. Diabetes Metab Res Rev. 2000, 16 Suppl 1, S6-S10. |
| 104. | Ahluwalia, R.; Maffulli, N.; Lázaro-Martínez, J. L.; Kirketerp-Møller, K.; Reichert, I. Diabetic foot off loading and ulcer remission: Exploring surgical off-loading. Surgeon. 2021, 19, e526-e535. |
| 105. | Zwaferink, J. B. J.; Custers, W.; Paardekooper, I.; Berendsen, H. A.; Bus, S. A. Optimizing footwear for the diabetic foot: Data-driven custom-made footwear concepts and their effect on pressure relief to prevent diabetic foot ulceration. PLoS One. 2020, 15, e0224010. |
| 106. | Bus, S. A. Foot structure and footwear prescription in diabetes mellitus. Diabetes Metab Res Rev. 2008, 24 Suppl 1, S90-95. |
| 107. | Peter-Riesch, B. The diabetic foot: The never-ending challenge. Endocr Dev. 2016, 31, 108-134. |
| 108. |
Mavrogenis, A. F.; Megaloikonomos, P. D.; Antoniadou, T.; Igoumenou, V. G.; Panagopoulos, G. N.; Dimopoulos, L.; Moulakakis, K. G.; Sfyroeras, G. S.; Lazaris, A. Current concepts for the evaluation and management of diabetic foot ulcers. EFORT Open Rev. 2018, 3, 513-525.
doi: 10.1302/2058-5241.3.180010 URL |
| 109. |
Caetano, A. P.; Conde Vasco, I.; Veloso Gomes, F.; Costa, N. V.; Luz, J. H.; Spaepen, E.; Formiga, A.; Coimbra, É.; Neves, J.; Bilhim, T. Successful revascularization has a significant impact on limb salvage rate and wound healing for patients with diabetic foot ulcers: single-centre retrospective analysis with a multidisciplinary approach. Cardiovasc Intervent Radiol. 2020, 43, 1449-1459.
doi: 10.1007/s00270-020-02604-4 URL |
| 110. | Hinchliffe, R. J.; Brownrigg, J. R.; Andros, G.; Apelqvist, J.; Boyko, E. J.; Fitridge, R.; Mills, J. L.; Reekers, J.; Shearman, C. P.; Zierler, R. E.; Schaper, N. C.; International Working Group on the Diabetic Foot. Effectiveness of revascularization of the ulcerated foot in patients with diabetes and peripheral artery disease: a systematic review. Diabetes Metab Res Rev. 2016, 32 Suppl 1, 136-144. |
| 111. |
Hicks, C. W.; Canner, J. K.; Sherman, R. L.; Black, J. H., 3rd; Lum, Y. W.; Abularrage, C. J. Evaluation of revascularization benefit quartiles using the Wound, Ischemia, and foot Infection classification system for diabetic patients with chronic limb-threatening ischemia. J Vasc Surg. 2021, 74, 1232-1239.e3.
doi: 10.1016/j.jvs.2021.03.017 URL |
| 112. |
Causey, M. W.; Ahmed, A.; Wu, B.; Gasper, W. J.; Reyzelman, A.; Vartanian, S. M.; Hiramoto, J. S.; Conte, M. S. Society for Vascular Surgery limb stage and patient risk correlate with outcomes in an amputation prevention program. J Vasc Surg. 2016, 63, 1563-1573.e2.
doi: 10.1016/j.jvs.2016.01.011 URL |
| 113. |
Baltzis, D.; Eleftheriadou, I.; Veves, A. Pathogenesis and treatment of impaired wound healing in diabetes mellitus: new insights. Adv Ther. 2014, 31, 817-836.
doi: 10.1007/s12325-014-0140-x URL |
| 114. |
Vatankhah, N.; Jahangiri, Y.; Landry, G. J.; Moneta, G. L.; Azarbal, A. F. Effect of systemic insulin treatment on diabetic wound healing. Wound Repair Regen. 2017, 25, 288-291.
doi: 10.1111/wrr.12514 URL |
| 115. | Fan, H. J.; Yu, J. H.; Cui, G. M.; Zhang, W. Y.; Yang, X.; Dong, Q. J. Insulin pump for the treatment of diabetes in combination with ulcerative foot infections. J Biol Regul Homeost Agents. 2016, 30, 465-470. |
| 116. | Lima, M. H.; Caricilli, A. M.; de Abreu, L. L.; Araújo, E. P.; Pelegrinelli, F. F.; Thirone, A. C.; Tsukumo, D. M.; Pessoa, A. F.; dos Santos, M. F.; de Moraes, M. A.; Carvalheira, J. B.; Velloso, L. A.; Saad, M. J. Topical insulin accelerates wound healing in diabetes by enhancing the AKT and ERK pathways: a double-blind placebo-controlled clinical trial. PLoS One. 2012, 7, e36974. |
| 117. | Sun, Y.; Fan, W.; Yang, W.; Wang, G.; Yu, G.; Zhang, D.; Wang, Y. Effects of intermittent irrigation of insulin solution combined with continuous drainage of vacuum sealing drainage in chronic diabetic lower limb ulcers. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2015, 29, 812-817. |
| 118. |
Saboo, A.; Rathnayake, A.; Vangaveti, V. N.; Malabu, U. H. Wound healing effects of dipeptidyl peptidase-4 inhibitors: An emerging concept in management of diabetic foot ulcer-A review. Diabetes Metab Syndr. 2016, 10, 113-119.
doi: 10.1016/j.dsx.2015.04.006 URL |
| 119. | Zolali, E.; Rezabakhsh, A.; Nabat, E.; Jaberi, H.; Rahbarghazi, R.; Garjani, A. Metformin effect on endocan biogenesis in human endothelial cells under diabetic condition. Arch Med Res. 2019, 50, 304-314. |
| 120. |
Seo, E.; Lim, J. S.; Jun, J. B.; Choi, W.; Hong, I. S.; Jun, H. S. Exendin-4 in combination with adipose-derived stem cells promotes angiogenesis and improves diabetic wound healing. J Transl Med. 2017, 15, 35.
doi: 10.1186/s12967-017-1145-4 URL |
| 121. |
Zhang, L.; Ma, Y.; Pan, X.; Chen, S.; Zhuang, H.; Wang, S. A composite hydrogel of chitosan/heparin/poly (γ-glutamic acid) loaded with superoxide dismutase for wound healing. Carbohydr Polym. 2018, 180, 168-174.
doi: 10.1016/j.carbpol.2017.10.036 URL |
| 122. |
Gong, C.; Wu, Q.; Wang, Y.; Zhang, D.; Luo, F.; Zhao, X.; Wei, Y.; Qian, Z. A biodegradable hydrogel system containing curcumin encapsulated in micelles for cutaneous wound healing. Biomaterials. 2013, 34, 6377-6387.
doi: 10.1016/j.biomaterials.2013.05.005 URL |
| 123. |
Ma, H.; Zhou, Q.; Chang, J.; Wu, C. Grape seed-inspired smart hydrogel scaffolds for melanoma therapy and wound healing. ACS Nano. 2019, 13, 4302-4311.
doi: 10.1021/acsnano.8b09496 URL |
| 124. |
Mao, C.; Xiang, Y.; Liu, X.; Cui, Z.; Yang, X.; Yeung, K. W. K.; Pan, H.; Wang, X.; Chu, P. K.; Wu, S. Photo-inspired antibacterial activity and wound healing acceleration by hydrogel embedded with Ag/Ag@AgCl/ZnO nanostructures. ACS Nano. 2017, 11, 9010-9021.
doi: 10.1021/acsnano.7b03513 URL |
| 125. |
Eke, G.; Mangir, N.; Hasirci, N.; MacNeil, S.; Hasirci, V. Development of a UV crosslinked biodegradable hydrogel containing adipose derived stem cells to promote vascularization for skin wounds and tissue engineering. Biomaterials. 2017, 129, 188-198.
doi: 10.1016/j.biomaterials.2017.03.021 URL |
| 126. |
Han, L.; Lu, X.; Wang, M.; Gan, D.; Deng, W.; Wang, K.; Fang, L.; Liu, K.; Chan, C. W.; Tang, Y.; Weng, L. T.; Yuan, H. A mussel-inspired conductive, self-adhesive, and self-healable tough hydrogel as cell stimulators and implantable bioelectronics. Small. 2017, 13, 1601916.
doi: 10.1002/smll.201601916 URL |
| 127. | Zhang, K.; Jia, Z.; Yang, B.; Feng, Q.; Xu, X.; Yuan, W.; Li, X.; Chen, X.; Duan, L.; Wang, D.; Bian, L. Adaptable hydrogels mediate cofactor-assisted activation of biomarker-responsive drug delivery via positive feedback for enhanced tissue regeneration. Adv Sci (Weinh). 2018, 5, 1800875. |
| 128. |
Wang, M.; Wang, C.; Chen, M.; Xi, Y.; Cheng, W.; Mao, C.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; Guo, Y.; Lei, B. Efficient angiogenesis-based diabetic wound healing/skin reconstruction through bioactive antibacterial adhesive ultraviolet shielding nanodressing with exosome release. ACS Nano. 2019, 13, 10279-10293.
doi: 10.1021/acsnano.9b03656 URL |
| 129. |
Shi, Q.; Luo, X.; Huang, Z.; Midgley, A. C.; Wang, B.; Liu, R.; Zhi, D.; Wei, T.; Zhou, X.; Qiao, M.; Zhang, J.; Kong, D.; Wang, K. Cobalt-mediated multi-functional dressings promote bacteria-infected wound healing. Acta Biomater. 2019, 86, 465-479.
doi: 10.1016/j.actbio.2018.12.048 URL |
| 130. |
Annabi, N.; Rana, D.; Shirzaei Sani, E.; Portillo-Lara, R.; Gifford, J. L.; Fares, M. M.; Mithieux, S. M.; Weiss, A. S. Engineering a sprayable and elastic hydrogel adhesive with antimicrobial properties for wound healing. Biomaterials. 2017, 139, 229-243.
doi: 10.1016/j.biomaterials.2017.05.011 URL |
| 131. |
Li, L.; Yan, B.; Yang, J.; Chen, L.; Zeng, H. Novel mussel-inspired injectable self-healing hydrogel with anti-biofouling property. Adv Mater. 2015, 27, 1294-1299.
doi: 10.1002/adma.201405166 URL |
| 132. |
Guo, C.; Wu, Y.; Li, W.; Wang, Y.; Kong, Q. Development of a microenvironment-responsive hydrogel promoting chronically infected diabetic wound healing through sequential hemostatic, antibacterial, and angiogenic activities. ACS Appl Mater Interfaces. 2022, 14, 30480-30492.
doi: 10.1021/acsami.2c02725 URL |
| 133. |
Hao, Y.; Zhao, W.; Zhang, H.; Zheng, W.; Zhou, Q. Carboxymethyl chitosan-based hydrogels containing fibroblast growth factors for triggering diabetic wound healing. Carbohydr Polym. 2022, 287, 119336.
doi: 10.1016/j.carbpol.2022.119336 URL |
| 134. |
Xu, Z.; Liu, Y.; Ma, R.; Chen, J.; Qiu, J.; Du, S.; Li, C.; Wu, Z.; Yang, X.; Chen, Z.; Chen, T. Thermosensitive hydrogel incorporating prussian blue nanoparticles promotes diabetic wound healing via ROS scavenging and mitochondrial function restoration. ACS Appl Mater Interfaces. 2022, 14, 14059-14071.
doi: 10.1021/acsami.1c24569 URL |
| 135. | Laiva, A. L.; O’Brien, F. J.; Keogh, M. B. Innovations in gene and growth factor delivery systems for diabetic wound healing. J Tissue Eng Regen Med. 2018, 12, e296-e312. |
| 136. | Niu, H.; Li, H.; Guan, Y.; Zhou, X.; Li, Z.; Zhao, S. L.; Chen, P.; Tan, T.; Zhu, H.; Bergdall, V.; Xu, X.; Ma, J.; Guan, J. Sustained delivery of rhMG53 promotes diabetic wound healing and hair follicle development. Bioact Mater. 2022, 18, 104-115. |
| 137. |
Lee, Y. H.; Lin, S. J. Chitosan/PVA hetero-composite hydrogel containing antimicrobials, perfluorocarbon nanoemulsions, and growth factor-loaded nanoparticles as a multifunctional dressing for diabetic wound healing: synthesis, characterization, and in vitro/in vivo evaluation. Pharmaceutics. 2022, 14: 537.
doi: 10.3390/pharmaceutics14030537 URL |
| 138. |
Wu, C.; Long, L.; Zhang, Y.; Xu, Y.; Lu, Y.; Yang, Z.; Guo, Y.; Zhang, J.; Hu, X.; Wang, Y. Injectable conductive and angiogenic hydrogels for chronic diabetic wound treatment. J Control Release. 2022, 344, 249-260.
doi: 10.1016/j.jconrel.2022.03.014 URL |
| 139. |
Hu, Y.; Wu, B.; Xiong, Y.; Tao, R.; Panayi, A. C.; Chen, L.; Tian, W.; Xue, H.; Shi, L.; Zhang, X.; Xiong, L.; Mi, B.; Liu, G. Cryogenic 3D printed hydrogel scaffolds loading exosomes accelerate diabetic wound healing. Chem Eng J. 2021, 426, 130634.
doi: 10.1016/j.cej.2021.130634 URL |
| [1] | Ke Hu, Yuxuan Li, Zunxiang Ke, Hongjun Yang, Chanjun Lu, Yiqing Li, Yi Guo, Weici Wang. History, progress and future challenges of artificial blood vessels: a narrative review [J]. Biomaterials Translational, 2022, 3(1): 81-98. |
| [2] | Xirui Jing, Qiuyue Ding, Qinxue Wu, Weijie Su, Keda Yu, Yanlin Su, Bing Ye, Qing Gao, Tingfang Sun, Xiaodong Guo. Magnesium-based materials in orthopaedics: material properties and animal models [J]. Biomaterials Translational, 2021, 2(3): 197-213. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||