Biomaterials Translational ›› 2021, Vol. 2 ›› Issue (4): 312-322.doi: 10.12336/biomatertransl.2021.04.007
• RESEARCH ARTICLE • Previous Articles Next Articles
Yang Zhao, Qing Sun, Bo Huo*( )
)
Received:2021-09-30
Revised:2021-11-29
Accepted:2021-12-10
Online:2021-12-28
Published:2021-12-28
Contact:
Bo Huo
E-mail:huobo@bit.edu.cn
About author:Bo Huo, huobo@bit.edu.cn.Zhao, Y.; Sun, Q.; Huo, B. Focal adhesion regulates osteogenic differentiation of mesenchymal stem cells and osteoblasts. Biomater Transl. 2021, 2(4), 312-322.
| Pattern | Diameter (μm) | Area (μm2) | Spacing (μm) | Pattern area/total area (%) |
|---|---|---|---|---|
| LL | 12 | 113 | 36 | 9 |
| SS | 8 | 50 | 24 | 9 |
| SL | 8 | 50 | 36 | 4 |
Table 1 Geometric parameters of the three patterns.
| Pattern | Diameter (μm) | Area (μm2) | Spacing (μm) | Pattern area/total area (%) |
|---|---|---|---|---|
| LL | 12 | 113 | 36 | 9 |
| SS | 8 | 50 | 24 | 9 |
| SL | 8 | 50 | 36 | 4 |
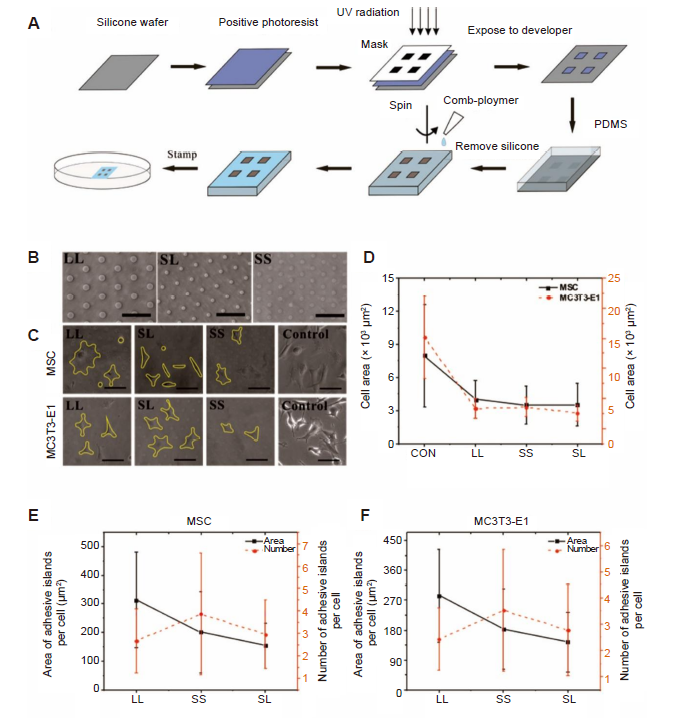
Figure 1. Micropatterned substrate and cell culture. (A) The process of micropatterning. (B) Bright-field images of micropatterned substrates. (C) Bright-field images of cells cultured on the micropatterned or blank substrates for 3 days. The yellow lines show the outline of the cells. The free-spreading cells showed polygons, while the spreading area of the patterned cells decreased. Scale bars: 50 μm. (D) Spread area at 3 days of cell culture. (E, F) Statistical analysis of the area and number of adhesive islands after 3 days of culture. Data are presented as mean ± SD (n = 3), and were analysed by one-way analysis of variance followed by Tukey’s post hoc analysis. CON: control (freely spreading group); LL: large circles with large spacing; MSC: mesenchymal stem cell; PDMS: polydimethylsiloxane; SL: small circles with large spacing; SS: small circles with small spacing; UV: ultraviolet.
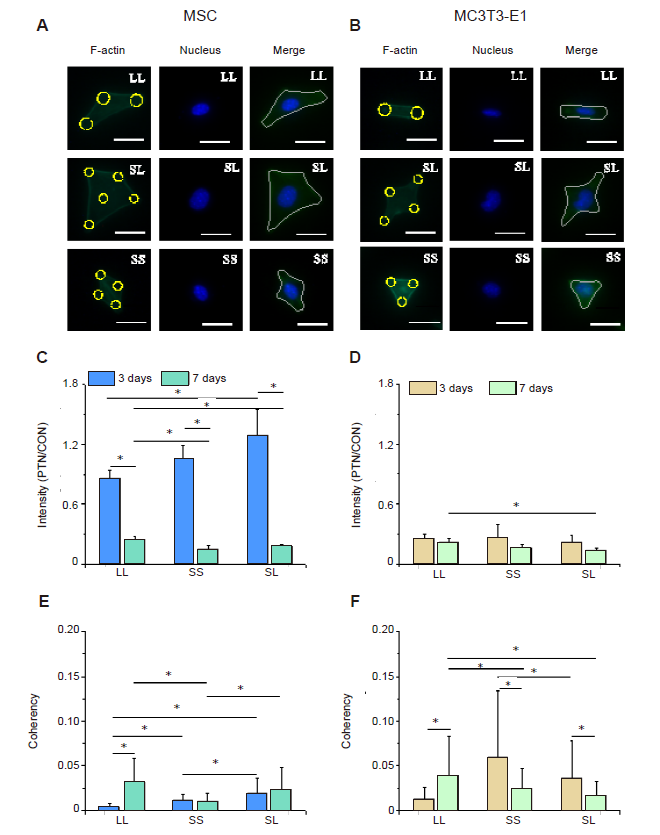
Figure 2. F-actin distribution in cells cultured on micropatterned substrates. (A, B) Fluorescent images of individual MSCs (A) and MC3T3-E1 cells (B) stained with phalloidin-labelled F-actin (green) with Hoechst 33342-stained nuclei (blue) at 3 days after seeding. Cells could be spread out into triangles, dumbbells, etc. The yellow circles represent the location of micropatterned islands. The gray shape represents the spreading shape of cells. Scale bars: 50 μm. (C, D) Statistical results of F-actin in MSCs (C) and MC3T3-E1 cells (D). (E, F) Statistical results of F-actin coherency in MSCs (E) and MC3T3-E1 cells (F). The larger the value, the more ordered the actin, and the more consistent the direction of stress fibres. Data are presented as mean ± SD (n = 3). *P < 0.05 (one-way analysis of variance followed by Tukey’s post hoc analysis). CON: control (freely spreading group); LL: large circles with large spacing; MSC: mesenchymal stem cell; PTN: pattern; SL: small circles with large spacing; SS: small circles with small spacing.
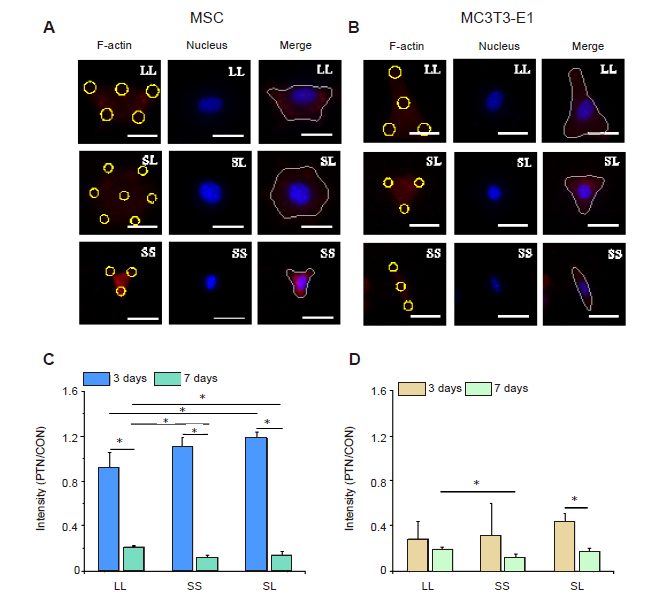
Figure 3. P-MLC2 in cells cultured on micropatterned substrates. (A, B) Fluorescent images of individual MSCs (A) and MC3T3-E1 cells (B) stained with TRITC-labeled P-MLC2 (red) with Hoechst 33342-stained nuclei (blue) at 3 days after seeding. The yellow circles represent the location of micropatterned islands. The gray shape represents the spreading shape of cells. Scale bars: 50 μm. (C, D) Statistical results of P-MLC2 in MSCs (C) and MC3T3-E1 cells (D). Data are presented as mean ± SD (n = 3). *P < 0.05 (one-way analysis of variance followed by Tukey’s post hoc analysis). CON: control (freely spreading group); LL: large circles with large spacing; MSC: mesenchymal stem cell; P-MLC2: phosphorylated myosin light chain 2; PTN: pattern; SL: small circles with large spacing; SS: small circles with small spacing; TRITC: tetraethyl rhodamine isothiocyanate.
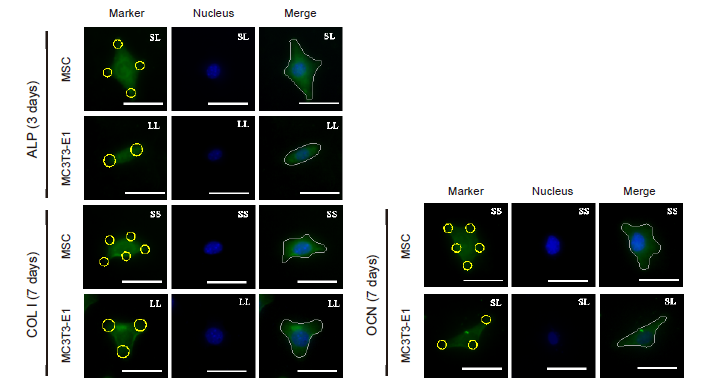
Figure 4. FITC-labeled fluorescent images of the osteogenic differentiation markers ALP, COL I, and OCN in MSCs and MC3T3-E1 cells cultured on a micropatterned substrate after 3 (ALP) or 7 (COL I, OCN) days of culture. Cells might spread out into triangles, dumbbells, etc. The yellow circles indicate the location of micropatterned islands. The gray shape represents the spreading shape of cells. Scale bars: 50 μm. ALP: alkaline phosphatase; COL I: type I collagen; FITC: fluorescein isothiocyanate; MSC: mesenchymal stem cell; OCN: osteocalcin.
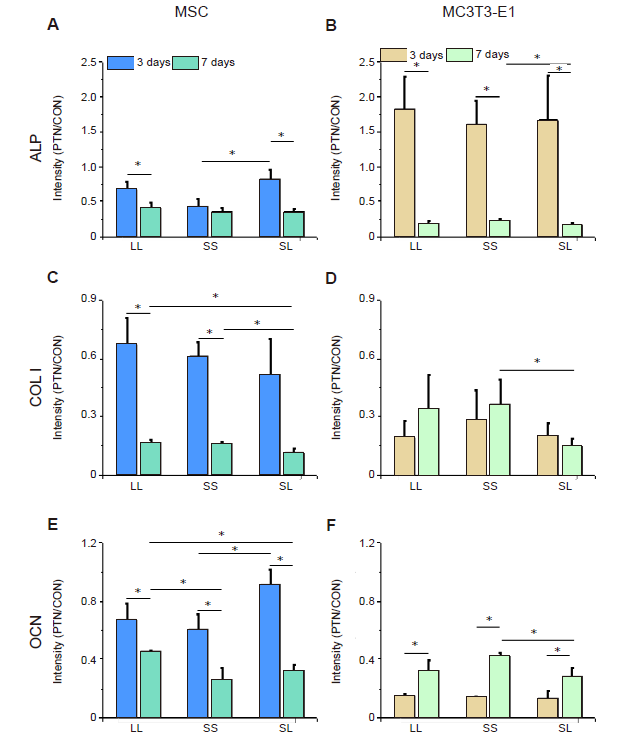
Figure 5. Intensity of osteogenic differentiation markers in cells cultured on a micropatterned substrate. (A-F) Relative fluorescent intensity of ALP, COL I, and OCN in MSCs (A, C, E) and MC3T3-E1 cells (B, D, F). Data are presented as mean ± SD (n = 3). *P < 0.05 (one-way analysis of variance followed by Tukey’s post hoc analysis). ALP: alkaline phosphatase; COL I: type I collagen; CON: control (freely spreading group); LL: large circles with large spacing; MSC: mesenchymal stem cell; OCN: osteocalcin; PTN: pattern; SL: small circles with large spacing; SS: small circles with small spacing.
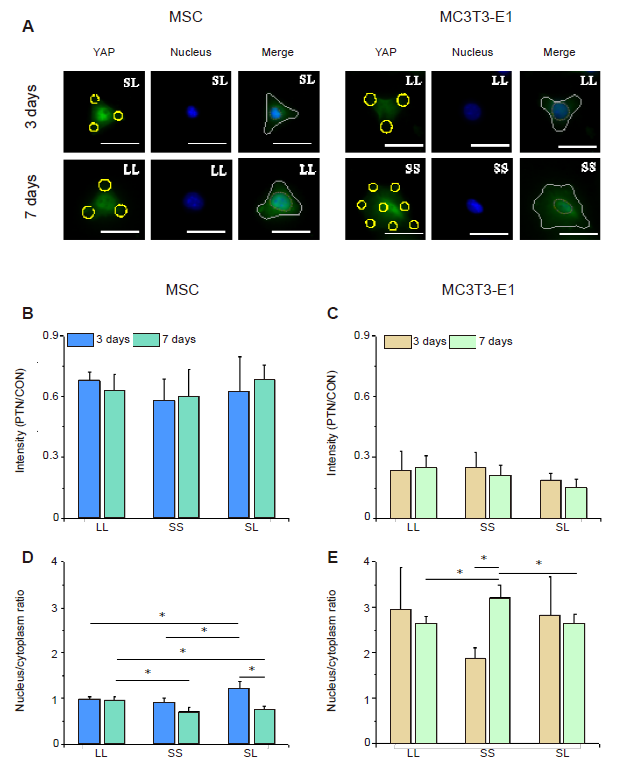
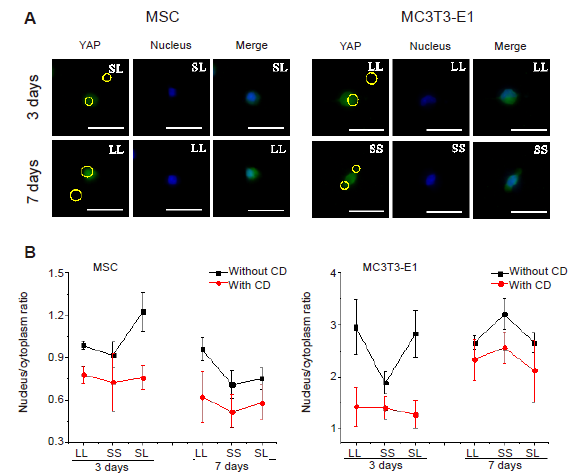
Figure 6. YAP staining in cells cultured on a micropatterned substrate. (A) Fluorescent images of individual cells stained with fluorescein isothiocyanate-labeled YAP (green) with Hoechst 33342-stained nuclei (blue) after 3 or 7 days of culture on the micropatterned substrate. Yellow circles indicate the location of micropatterned islands, and yellow dotted circles show the outlines of nuclei. The gray shape represents the spreading shape of cells. Scale bars: 50 μm. (B, C) Statistical analyses of the fluorescence intensity of YAP in MSCs and MC3T3-E1 cells, respectively. (D, E) Statistical analyses of the nuclear/cytoplasmic ratio of YAP in MSCs and MC3T3-E1 cells, respectively. Data are presented as mean ± SD (n = 3). *P < 0.05 (one-way analysis of variance followed by Tukey’s post hoc analysis). CON: control (free spreading group); LL: large circles with large spacing; MSC: mesenchymal stem cell; PTN: pattern; SL: small circles with large spacing; SS: small circles with small spacing; YAP: yes-associated proteins.
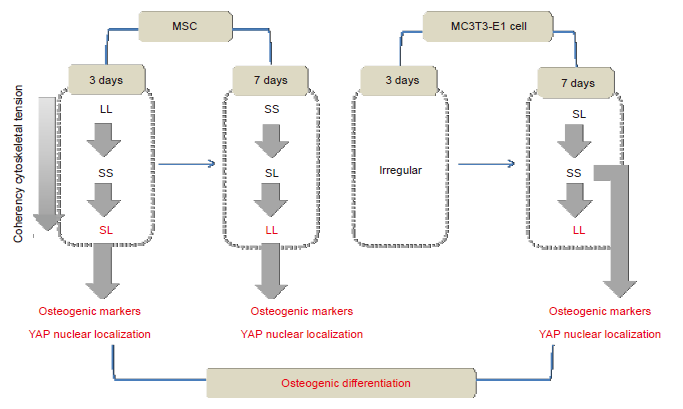
Figure 7. Summary of the mechanism via which focal adhesion distribution regulates osteogenic differentiation. LL: large circles with large spacing; MSC: mesenchymal stem cell; SL: small circles with large spacing; SS: small circles with small spacing; YAP: yes-associated proteins.
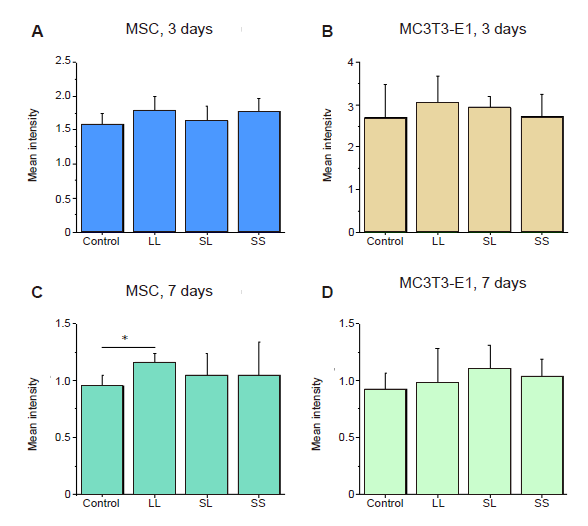
Additional Figure 1. Statistical analysis of the fluorescence intensity of TUNEL-stained cells cultured on micropatterned substrates. (A-D) MSCs and MC3T3-E1 cells cultured for 3 or 7 days. Data are presented as mean ± SD (n = 3). *P < 0.05 (one-way analysis of variance followed by Tukey’s post hoc analysis). LL: large circles with large spacing; MSC: mesenchymal stem cell; SL: small circles with large spacing; SS: small circles with small spacing; TUNEL: terminal deoxynucleotidyl transferase dUTP nick end labelling.
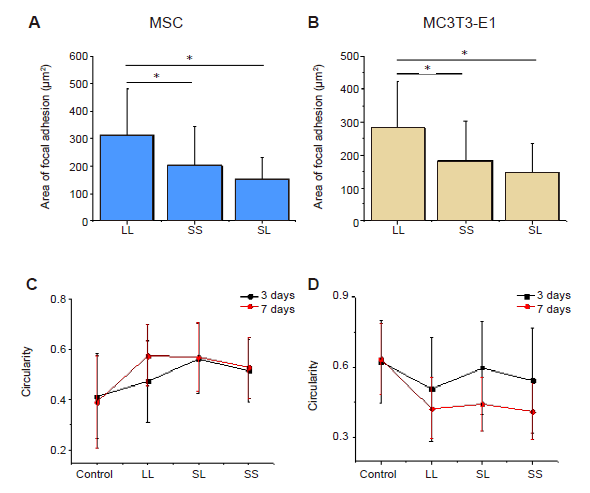
Additional Figure 2. Statistical analysis of the area and circularity of focal adhesions. (A) Area of focal adhesion of MSCs. (B) Area of focal adhesion of MC3T3-E1 cells. (C) Circularity of MSCs. (D) Circularity of MC3T3-E1 cells. Data are presented as mean ± SD (n = 3). *P < 0.05 (one-way analysis of variance followed by Tukey’s post hoc analysis). CON: control; LL: large circles with large spacing; MSC: mesenchymal stem cell; SL: small circles with large spacing; SS: small circles with small spacing.
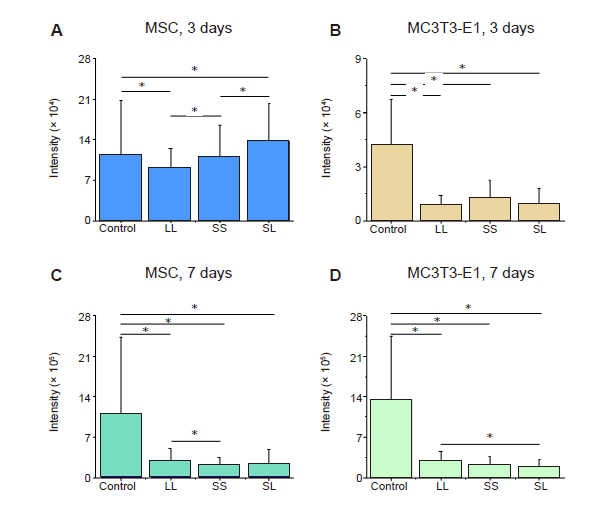
Additional Figure 3. Statistical results of fluorescence intensity of F-actin. (A-D) MSCs and MC3T3-E1 cells cultured for 3 or 7 days. Data are presented as mean ± SD (n = 3). *P < 0.05 (one-way analysis of variance followed by Tukey’s post hoc analysis). LL: large circles with large spacing; MSC: mesenchymal stem cell; SL: small circles with large spacing; SS: small circles with small spacing.
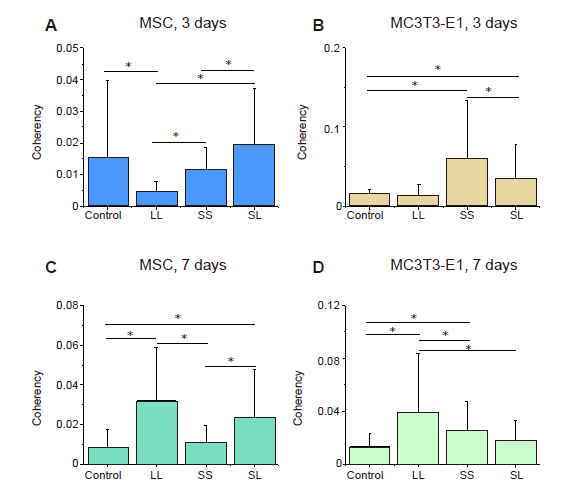
Additional Figure 4. Statistical results of F-actin coherency. (A-D) MSCs and MC3T3-E1 cells cultured for 3 or 7 days. The larger the value, the more ordered the actin, and the more consistent the direction of stress fibres. Data are presented as mean ± SD (n = 3). *P < 0.05 (one-way analysis of variance followed by Tukey’s post hoc analysis). LL: large circles with large spacing; MSC: mesenchymal stem cell; SL: small circles with large spacing; SS: small circles with small spacing.
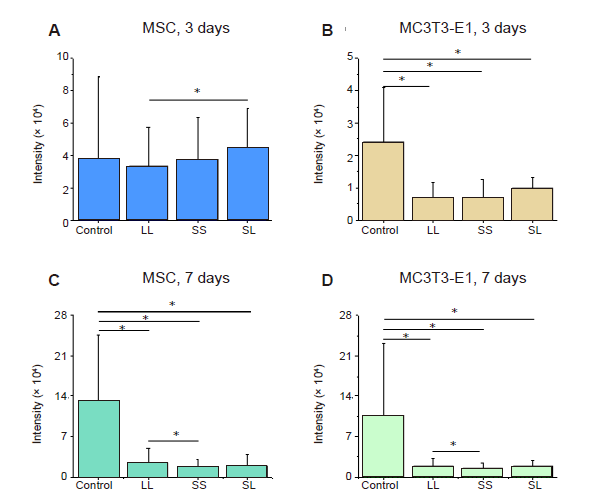
Additional Figure 5. Statistical results of fluorescence intensity of P-MLC2. (A-D) MSCs and MC3T3-E1 cells cultured for 3 or 7 days. Data are presented as mean ± SD (n = 3). *P < 0.05 (one-way analysis of variance followed by Tukey’s post hoc analysis). LL: large circles with large spacing; MSC: mesenchymal stem cell; P-MLC2: phosphorylated myosin light chain 2; SL: small circles with large spacing; SS: small circles with small spacing.
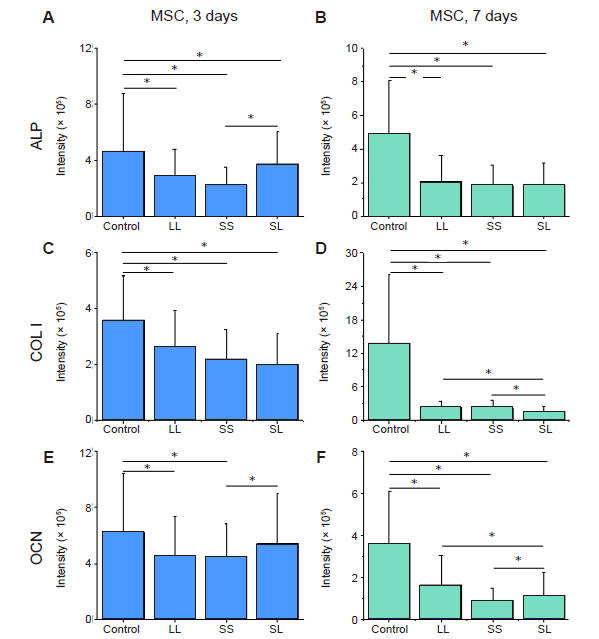
Additional Figure 6. Fluorescence intensity of osteogenic differentiation markers in MSCs cultured on micropatterned substrates. (A-F) Statistical results of fluorescent intensity of ALP, COL I, and OCN, respectively, after culture for 3 days (A, C, E) or 7 days (B, D, F). Data are presented as mean ± SD (n = 3). *P < 0.05 (one-way analysis of variance followed by Tukey’s post hoc analysis). ALP: alkaline phosphatase; COL I: type I collagen; LL: large circles with large spacing; MSC: mesenchymal stem cell; OCN: osteocalcin; SL: small circles with large spacing; SS: small circles with small spacing.
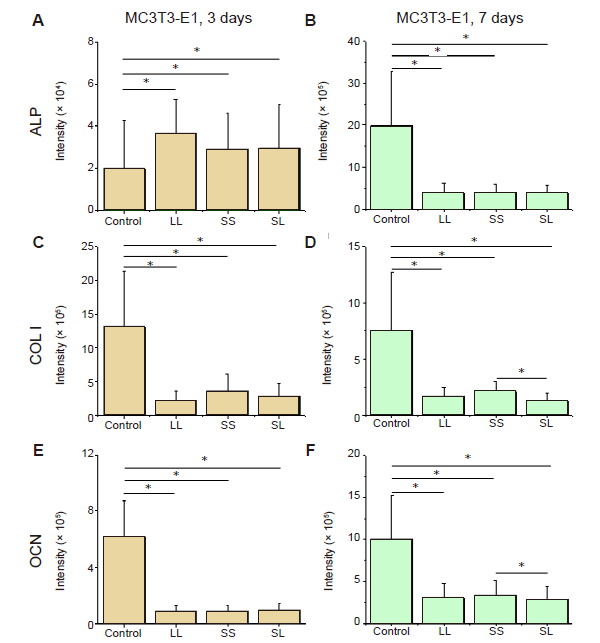
Additional Figure 7. Fluorescence intensity of osteogenic differentiation markers in MC3T3-E1 cells cultured on micropatterned substrates. (A-F) Statistical results of fluorescent intensity of ALP, COL I, and OCN in cells cultured for 3 days (A, C, E) or 7 days (B, D, F), respectively. Data are presented as mean ± SD (n = 3). *P < 0.05 (one-way analysis of variance followed by Tukey’s post hoc analysis). ALP: alkaline phosphatase; COL I: type I collagen; LL: large circles with large spacing; OCN: osteocalcin; SL: small circles with large spacing; SS: small circles with small spacing.
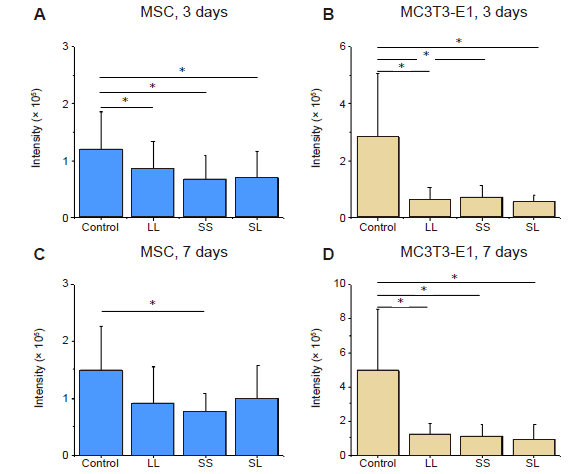
Additional Figure 8. Statistical results of fluorescence intensity of YAP. (A-D) MSCs and MC3T3-E1 cells cultured for 3 or 7 days. Data are presented as mean ± SD (n = 3). *P < 0.05 (one-way analysis of variance followed by Tukey’s post hoc analysis). LL: large circles with large spacing; MSC: mesenchymal stem cell; SL: small circles with large spacing; SS: small circles with small spacing; YAP: yes-associated proteins.
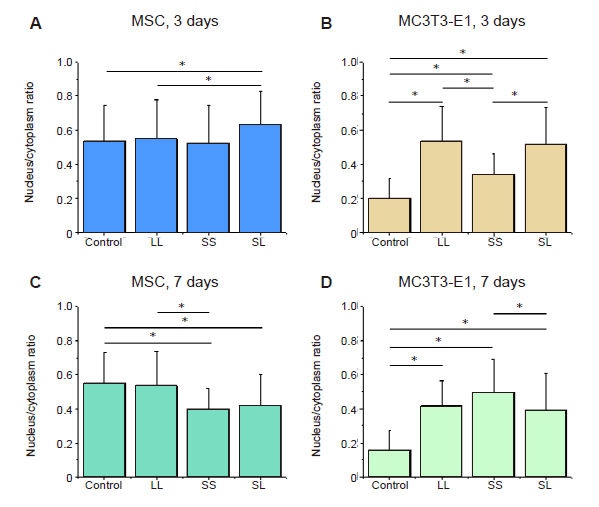
Additional Figure 9. Statistical analyses of the nuclear/cytoplasmic ratio of YAP. (A-D) MSCs and MC3T3-E1 cells cultured for 3 or 7 days. Data are presented as mean ± SD (n = 3). *P < 0.05 (one-way analysis of variance followed by Tukey’s post hoc analysis). LL: large circles with large spacing; MSC: mesenchymal stem cell; SL: small circles with large spacing; SS: small circles with small spacing; YAP: yes-associated proteins.
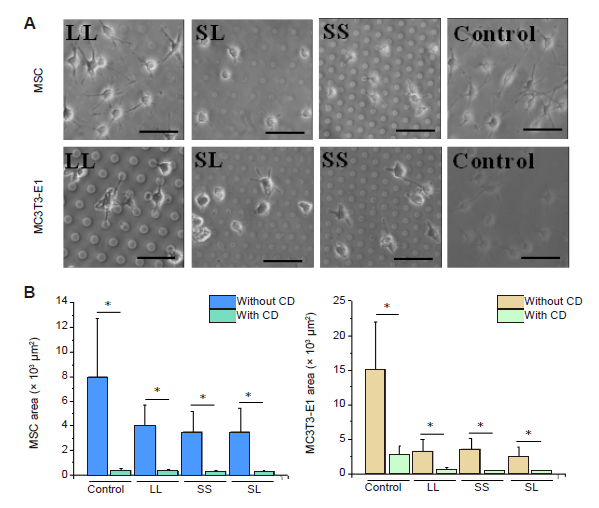
Additional Figure 10. Cell phenotypes and area after CD treatment for 24 hours. (A) Bright-field images of the cell on the micropatterned substrates. Cells were shrunk obviously. Scale bars: 50 μm. (B) Statistical analysis of the spreading area of the cells. Data are presented as mean ± SD (n = 3). *P < 0.05 (one-way analysis of variance followed by Tukey’s post hoc analysis). CD: cytochalasin D; LL: large circles with large spacing; MSC: mesenchymal stem cell; SL: small circles with large spacing; SS: small circles with small spacing.
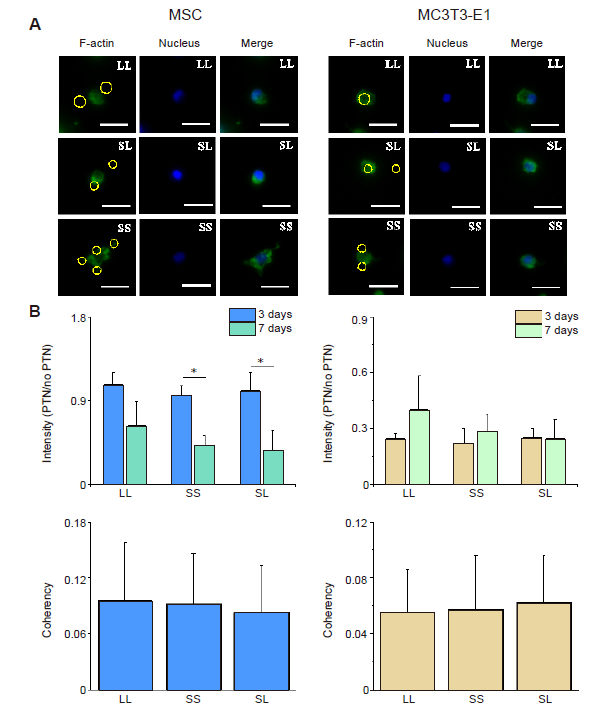
Additional Figure 11. SStatistical results of F-actin staining after CD treatment for 24 hours. (A) Fluorescent images of F-actin in shrunk cells. Scale bars: 50 μm. (B) Statistical results of fluorescence intensity of F-actin. (C) Statistical results of F-actin coherency. The larger the value, the better the order of actin, and the more consistent the direction of stress fibre. Data are presented as mean ± SD (n = 3). *P < 0.05 (one-way analysis of variance followed by Tukey’s post hoc analysis). CD: cytochalasin D; LL: large circles with large spacing; MSC: mesenchymal stem cell; SL: small circles with large spacing; SS: small circles with small spacing.
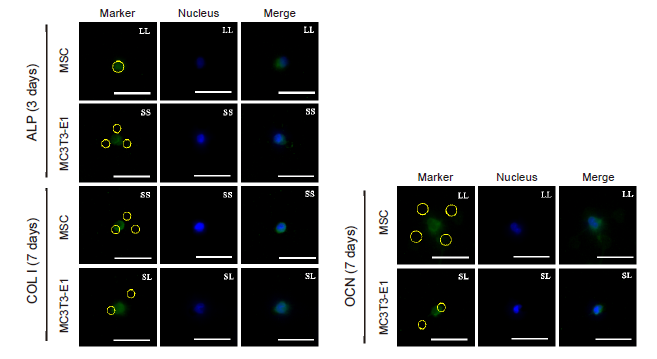
Additional Figure 12. Fluorescent images of osteogenic differentiation markers (green, FITC-labeled) in individual cells treated with CD and cultured on the micropatterned substrates for 3 (ALP) or 7 days (COL I, OCN). The yellow circles represent the location of micropatterned islands. Scale bars: 50 μm. ALP: alkaline phosphatase; CD: cytochalasin D; COL I: type I collagen; FITC: fluorescein isothiocyanate; MSC: mesenchymal stem cell; OCN: osteocalcin.
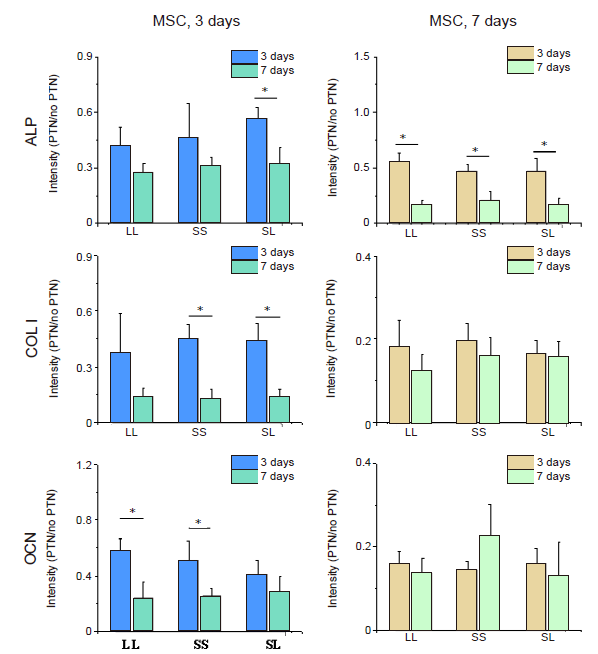
Additional Figure 13. Fluorescence intensity of osteogenic markers in cells cultured on micropatterned substrates after CD treatment. Data are presented as mean ± SD (n = 3). *P < 0.05 (one-way analysis of variance followed by Tukey’s post hoc analysis). ALP: alkaline phosphatase; CD: cytochalasin D; COL I: type I collagen; LL: large circles with large spacing; MSC: mesenchymal stem cell; OCN: osteocalcin; PTN: pattern; SL: small circles with large spacing; SS: small circles with small spacing.
Additional Figure 14. Statistical results of YAP localisation after CD treatment. (A) Fluorescent images of YAP. Nuclear transfer of YAP could not be observed. The yellow circles represent the location of micropatterned islands. Scale bars: 50 μm. (B) Statistical analyses of the nuclear/cytoplasmic ratio of YAP in MSCs and MC3T3-E1 cells. Data are presented as mean ± SD (n = 3), and were analysed by one-way analysis of variance followed by Tukey’s post hoc analysis. CD: cytochalasin D; LL: large circles with large spacing; MSC: mesenchymal stem cell; SL: small circles with large spacing; SS: small circles with small spacing; YAP: yes-associated proteins.
| 1. |
Zachar, L.; Bačenková, D.; Rosocha, J. Activation, homing, and role of the mesenchymal stem cells in the inflammatory environment. J Inflamm Res. 2016, 9, 231-240.
doi: 10.2147/JIR URL |
| 2. |
Qiu, J.; Guo, J.; Geng, H.; Qian, W.; Liu, X. Three-dimensional porous graphene nanosheets synthesized on the titanium surface for osteogenic differentiation of rat bone mesenchymal stem cells. Carbon. 2017, 125, 227-235.
doi: 10.1016/j.carbon.2017.09.064 URL |
| 3. |
Shahrousvand, M.; Sadeghi, G. M. M.; Shahrousvand, E.; Ghollasi, M.; Salimi, A. Superficial physicochemical properties of polyurethane biomaterials as osteogenic regulators in human mesenchymal stem cells fates. Colloids Surf B Biointerfaces. 2017, 156, 292-304.
doi: 10.1016/j.colsurfb.2017.04.059 URL |
| 4. |
Yang, W.; Han, W.; He, W.; Li, J.; Wang, J.; Feng, H.; Qian, Y. Surface topography of hydroxyapatite promotes osteogenic differentiation of human bone marrow mesenchymal stem cells. Mater Sci Eng C Mater Biol Appl. 2016, 60, 45-53.
doi: 10.1016/j.msec.2015.11.012 URL |
| 5. |
Faia-Torres, A. B.; Guimond-Lischer, S.; Rottmar, M.; Charnley, M.; Goren, T.; Maniura-Weber, K.; Spencer, N. D.; Reis, R. L.; Textor, M.; Neves, N. M. Differential regulation of osteogenic differentiation of stem cells on surface roughness gradients. Biomaterials. 2014, 35, 9023-9032.
doi: 10.1016/j.biomaterials.2014.07.015 URL |
| 6. |
Bajpai, I.; Rukini, A.; Jung, K. J.; Song, I. H.; Kim, S. Surface morphological influence on the in vitro bioactivity and response of mesenchymal stem cells. Mater Technol. 2017, 32, 535-542.
doi: 10.1080/10667857.2017.1317062 URL |
| 7. |
Liu, X.; Liu, R.; Cao, B.; Ye, K.; Li, S.; Gu, Y.; Pan, Z.; Ding, J. Subcellular cell geometry on micropillars regulates stem cell differentiation. Biomaterials. 2016, 111, 27-39.
doi: 10.1016/j.biomaterials.2016.09.023 URL |
| 8. |
Carvalho, A.; Pelaez-Vargas, A.; Hansford, D. J.; Fernandes, M. H.; Monteiro, F. J. Effects of line and pillar array microengineered SiO2 thin films on the osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. Langmuir. 2016, 32, 1091-1100.
doi: 10.1021/acs.langmuir.5b03955 URL |
| 9. |
Li, S.; Kuddannaya, S.; Chuah, Y. J.; Bao, J.; Zhang, Y.; Wang, D. Combined effects of multi-scale topographical cues on stable cell sheet formation and differentiation of mesenchymal stem cells. Biomater Sci. 2017, 5, 2056-2067.
doi: 10.1039/C7BM00134G URL |
| 10. | Kaivosoja, E.; Suvanto, P.; Barreto, G.; Aura, S.; Soininen, A.; Franssila, S.; Konttinen, Y. T. Cell adhesion and osteogenic differentiation on three-dimensional pillar surfaces. J Biomed Mater Res A. 2013, 101, 842-852. |
| 11. |
Li, J.; Li, J. J.; Zhang, J.; Wang, X.; Kawazoe, N.; Chen, G. Gold nanoparticle size and shape influence on osteogenesis of mesenchymal stem cells. Nanoscale. 2016, 8, 7992-8007.
doi: 10.1039/C5NR08808A URL |
| 12. |
Li, J.; Zhang, J.; Chen, Y.; Kawazoe, N.; Chen, G. TEMPO-conjugated gold nanoparticles for reactive oxygen species scavenging and regulation of stem cell differentiation. ACS Appl Mater Interfaces. 2017, 9, 35683-35692.
doi: 10.1021/acsami.7b12486 URL |
| 13. |
Peng, R.; Yao, X.; Cao, B.; Tang, J.; Ding, J. The effect of culture conditions on the adipogenic and osteogenic inductions of mesenchymal stem cells on micropatterned surfaces. Biomaterials. 2012, 33, 6008-6019.
doi: 10.1016/j.biomaterials.2012.05.010 URL |
| 14. |
Wang, X.; Hu, X.; Dulińska-Molak, I.; Kawazoe, N.; Yang, Y.; Chen, G. Discriminating the independent influence of cell adhesion and spreading area on stem cell fate determination using micropatterned surfaces. Sci Rep. 2016, 6, 28708.
doi: 10.1038/srep28708 URL |
| 15. |
McBeath, R.; Pirone, D. M.; Nelson, C. M.; Bhadriraju, K.; Chen, C. S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004, 6, 483-495.
doi: 10.1016/S1534-5807(04)00075-9 URL |
| 16. |
Both, S. K.; van der Muijsenberg, A. J.; van Blitterswijk, C. A.; de Boer, J.; de Bruijn, J. D. A rapid and efficient method for expansion of human mesenchymal stem cells. Tissue Eng. 2007, 13, 3-9.
doi: 10.1089/ten.2005.0513 URL |
| 17. |
Kilian, K. A.; Bugarija, B.; Lahn, B. T.; Mrksich, M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc Natl Acad Sci U S A. 2010, 107, 4872-4877.
doi: 10.1073/pnas.0903269107 URL |
| 18. |
Naganuma, T. The relationship between cell adhesion force activation on nano/micro-topographical surfaces and temporal dependence of cell morphology. Nanoscale. 2017, 9, 13171-13186.
doi: 10.1039/C7NR04785A URL |
| 19. |
Yourek, G.; Hussain, M. A.; Mao, J. J. Cytoskeletal changes of mesenchymal stem cells during differentiation. ASAIO J. 2007, 53, 219-228.
doi: 10.1097/MAT.0b013e31802deb2d URL |
| 20. |
Rodríguez, J. P.; González, M.; Ríos, S.; Cambiazo, V. Cytoskeletal organization of human mesenchymal stem cells (MSC) changes during their osteogenic differentiation. J Cell Biochem. 2004, 93, 721-731.
doi: 10.1002/jcb.v93:4 URL |
| 21. |
Cao, B.; Peng, Y.; Liu, X.; Ding, J. Effects of functional groups of materials on nonspecific adhesion and chondrogenic induction of mesenchymal stem cells on free and micropatterned surfaces. ACS Appl Mater Interfaces. 2017, 9, 23574-23585.
doi: 10.1021/acsami.7b08339 URL |
| 22. |
Chang, B.; Ma, C.; Liu, X. Nanofibers regulate single bone marrow stem cell osteogenesis via FAK/RhoA/YAP1 pathway. ACS Appl Mater Interfaces. 2018, 10, 33022-33031.
doi: 10.1021/acsami.8b11449 URL |
| 23. |
Fu, R.; Liu, Q.; Song, G.; Baik, A.; Hu, M.; Sun, S.; Guo, X. E.; Long, M.; Huo, B. Spreading area and shape regulate apoptosis and differentiation of osteoblasts. Biomed Mater. 2013, 8, 055005.
doi: 10.1088/1748-6041/8/5/055005 URL |
| 24. |
Huo, B.; Lu, X. L.; Costa, K. D.; Xu, Q.; Guo, X. E. An ATP-dependent mechanism mediates intercellular calcium signaling in bone cell network under single cell nanoindentation. Cell Calcium. 2010, 47, 234-241.
doi: 10.1016/j.ceca.2009.12.005 URL |
| 25. |
Huo, B.; Lu, X. L.; Hung, C. T.; Costa, K. D.; Xu, Q.; Whitesides, G. M.; Guo, X. E. Fluid Flow Induced Calcium Response in Bone Cell Network. Cell Mol Bioeng. 2008, 1, 58-66.
doi: 10.1007/s12195-008-0011-0 URL |
| 26. |
Lu, X. L.; Huo, B.; Chiang, V.; Guo, X. E. Osteocytic network is more responsive in calcium signaling than osteoblastic network under fluid flow. J Bone Miner Res. 2012, 27, 563-574.
doi: 10.1002/jbmr.1474 URL |
| 27. |
He, S.; Liu, C.; Li, X.; Ma, S.; Huo, B.; Ji, B. Dissecting Collective Cell Behavior in Polarization and Alignment on Micropatterned Substrates. Biophys J. 2015, 109, 489-500.
doi: 10.1016/j.bpj.2015.06.058 URL |
| 28. |
Ma, H.; Hyun, J.; Zhang, Z.; Beebe Jr, T. P.; Chilkoti, A. Fabrication of biofunctionalized quasi-three-dimensional microstructures of a nonfouling comb polymer using soft lithography. Adv Funct Mater. 2005, 15, 529-540.
doi: 10.1002/(ISSN)1616-3028 URL |
| 29. | Pantic, I.; Nesic, D.; Basailovic, M.; Cetkovic, M.; Mazic, S.; Suzic-Lazic, J.; Popevic, M. Chromatin fractal organization, textural patterns, and circularity of nuclear envelope in adrenal zona fasciculata cells. Microsc Microanal. 2016, 22, 1120-1127. |
| 30. | Schneider, C. A.; Rasband, W. S.; Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012, 9, 671-675. |
| 31. |
Rezakhaniha, R.; Agianniotis, A.; Schrauwen, J. T.; Griffa, A.; Sage, D.; Bouten, C. V.; van de Vosse, F. N.; Unser, M.; Stergiopulos, N. Experimental investigation of collagen waviness and orientation in the arterial adventitia using confocal laser scanning microscopy. Biomech Model Mechanobiol. 2012, 11, 461-473.
doi: 10.1007/s10237-011-0325-z URL |
| 32. | Ohashi, K.; Fujiwara, S.; Mizuno, K. Roles of the cytoskeleton, cell adhesion and rho signalling in mechanosensing and mechanotransduction. J Biochem. 2017, 161, 245-254. |
| 33. |
Liu, C.; He, S.; Li, X.; Huo, B.; Ji, B. Mechanics of cell mechanosensing on patterned substrate. J Appl Mech. 2016, 83, 051014.
doi: 10.1115/1.4032907 URL |
| 34. |
Wang, X.; Li, S.; Yan, C.; Liu, P.; Ding, J. Fabrication of RGD micro/nanopattern and corresponding study of stem cell differentiation. Nano Lett. 2015, 15, 1457-1467.
doi: 10.1021/nl5049862 URL |
| 35. |
Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; Elvassore, N.; Piccolo, S. Role of YAP/TAZ in mechanotransduction. Nature. 2011, 474, 179-183.
doi: 10.1038/nature10137 URL |
| 36. | Bertrand, A. T.; Ziaei, S.; Ehret, C.; Duchemin, H.; Mamchaoui, K.; Bigot, A.; Mayer, M.; Quijano-Roy, S.; Desguerre, I.; Lainé, J.; Ben Yaou, R.; Bonne, G.; Coirault, C. Cellular microenvironments reveal defective mechanosensing responses and elevated YAP signaling in LMNA-mutated muscle precursors. J Cell Sci. 2014, 127, 2873-2884. |
| 37. |
Zhao, Y.; Sun, Q.; Wang, S.; Huo, B. Spreading shape and area regulate the osteogenesis of mesenchymal stem cells. Tissue Eng Regen Med. 2019, 16, 573-583.
doi: 10.1007/s13770-019-00213-y URL |
| 38. |
Chen, C. S.; Alonso, J. L.; Ostuni, E.; Whitesides, G. M.; Ingber, D. E. Cell shape provides global control of focal adhesion assembly. Biochem Biophys Res Commun. 2003, 307, 355-361.
doi: 10.1016/S0006-291X(03)01165-3 URL |
| 39. |
Kim, D. H.; Wirtz, D. Focal adhesion size uniquely predicts cell migration. FASEB J. 2013, 27, 1351-1361.
doi: 10.1096/fsb2.v27.4 URL |
| 40. |
Bilem, I.; Chevallier, P.; Plawinski, L.; Sone, E. D.; Durrieu, M. C.; Laroche, G. Interplay of geometric cues and RGD/BMP-2 crosstalk in directing stem cell fate. ACS Biomater Sci Eng. 2017, 3, 2514-2523.
doi: 10.1021/acsbiomaterials.7b00279 URL |
| 41. |
Gao, L.; McBeath, R.; Chen, C. S. Stem cell shape regulates a chondrogenic versus myogenic fate through Rac1 and N-cadherin. Stem Cells. 2010, 28, 564-572.
doi: 10.1002/stem.v28:3 URL |
| 42. |
Wada, K.; Itoga, K.; Okano, T.; Yonemura, S.; Sasaki, H. Hippo pathway regulation by cell morphology and stress fibers. Development. 2011, 138, 3907-3914.
doi: 10.1242/dev.070987 URL |
| 43. | Zhang, Y. Y.; Sun, Y.; Huang, Y.; Gong, H. Effect of cell geometry on yap localization of mesenchymal stem cells on micropatterned surfaces. Zhongguo Ke Xue. 2016, 46, 321-329. |
| 44. |
Lee, S. W.; Lee, H. J.; Lee, J. W.; Kim, K. H.; Kang, J. H.; Lee, M. H.; Lee, S. C. Surface functionalization of microgrooved titanium with dual growth factor-releasing nanoparticles for synergistic osteogenic differentiation of human mesenchymal stem cells. Colloids Surf B Biointerfaces. 2015, 135, 565-574.
doi: 10.1016/j.colsurfb.2015.08.011 URL |
| [1] | Qingchuan Wang, Weidan Wang, Yanfang Li, Weirong Li, Lili Tan, Ke Yang. Biofunctional magnesium coating of implant materials by physical vapour deposition [J]. Biomaterials Translational, 2021, 2(3): 248-256. |
| [2] | Kamolrat Metavarayuth, Esteban Villarreal, Hui Wang, Qian Wang. Surface topography and free energy regulate osteogenesis of stem cells: effects of shape-controlled gold nanoparticles [J]. Biomaterials Translational, 2021, 2(2): 165-173. |
| [3] | Yunsong Shi, Ruijun He, Xiangyu Deng, Zengwu Shao, Davide Deganello, Chunze Yan, Zhidao Xia. Three-dimensional biofabrication of an aragonite-enriched self-hardening bone graft substitute and assessment of its osteogenicity in vitro and in vivo [J]. Biomaterials Translational, 2020, 1(1): 69-81. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||