Biomaterials Translational ›› 2021, Vol. 2 ›› Issue (2): 165-173.doi: 10.12336/biomatertransl.2021.02.006
• RESEARCH ARTICLE • Previous Articles
Kamolrat Metavarayuth, Esteban Villarreal, Hui Wang, Qian Wang*( )
)
Received:2021-04-05
Revised:2021-06-07
Accepted:2021-06-09
Online:2021-06-28
Published:2021-06-28
Contact:
Qian Wang
E-mail:Wang263@mailbox.sc.edu
Metavarayuth, K.; Villarreal, E.; Wang, H.; Wang Q. Surface topography and free energy regulate osteogenesis of stem cells: effects of shape-controlled gold nanoparticles. Biomater Transl. 2021, 2(2), 165-173.
| AuNP | L-ascorbic acid | Au seed | 0.1 M (1-hexadecyl)trimethylammonium chloride | 0.5 mM HAuCl4 (2 mL) addition rate |
|---|---|---|---|---|
| Quasi-spherical nanoparticles | 0.01 M, 130 μL | 10 μL | 2 mL | 2 mL/h using a syringe pump |
| Nanotrioctahedra | 0.1 M, 100 μL | 10 μL | 2 mL | 1 shot injection (stir simultaneously) |
| Porous nanoparticles | 0.1 M, 10 μL | 10 μL | 2 mL | 1 shot injection (no stir) |
| Concave nanocube particles | 0.1 M, 130 μL | 10 μL | 2 mL | 2 mL/h using a syringe pump |
Table 1 The composition of growth solution mixture and HAuCl4 addition rate for each AuNP
| AuNP | L-ascorbic acid | Au seed | 0.1 M (1-hexadecyl)trimethylammonium chloride | 0.5 mM HAuCl4 (2 mL) addition rate |
|---|---|---|---|---|
| Quasi-spherical nanoparticles | 0.01 M, 130 μL | 10 μL | 2 mL | 2 mL/h using a syringe pump |
| Nanotrioctahedra | 0.1 M, 100 μL | 10 μL | 2 mL | 1 shot injection (stir simultaneously) |
| Porous nanoparticles | 0.1 M, 10 μL | 10 μL | 2 mL | 1 shot injection (no stir) |
| Concave nanocube particles | 0.1 M, 130 μL | 10 μL | 2 mL | 2 mL/h using a syringe pump |
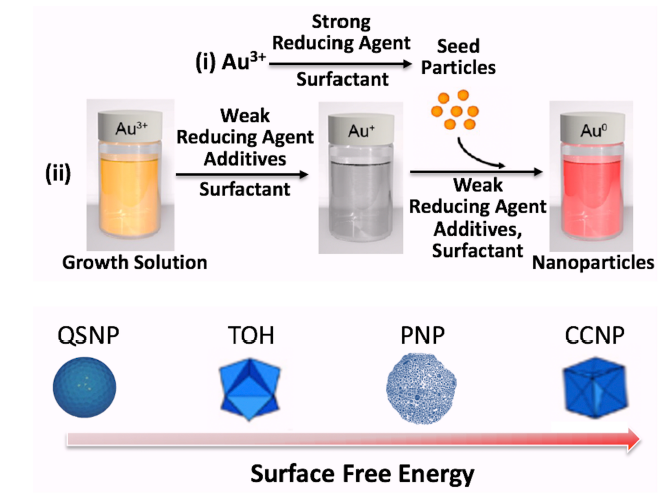
Figure 1. Seed-mediated synthesis of AuNPs with different shapes. Seed particles were first prepared by reduction of Au3+ in a strong reducing agent as the first step. Then the Au3+ growth solution was reduced in weak reducing agent and surfactant to Au+ which was kinetically controlled to grow on seed particles from the first step into gold nanoparticles with different shapes. Slower reduction rates produced AuNPs that were more stable or had lower surface energies such as quasi-nanospheres enclosed by {111} and {100} facets, while faster reaction rates produced high index faceting Au QSNPs with higher surface energies, such as TOH, PNPs, and CCNPs.31, 32 AuNPs: gold nanoparticles; CCNPs: concave nanocube particles; PNPs: porous nanoparticles; QSNPs: quasi-spherical nanoparticles; TOH: nanotrioctahedra.
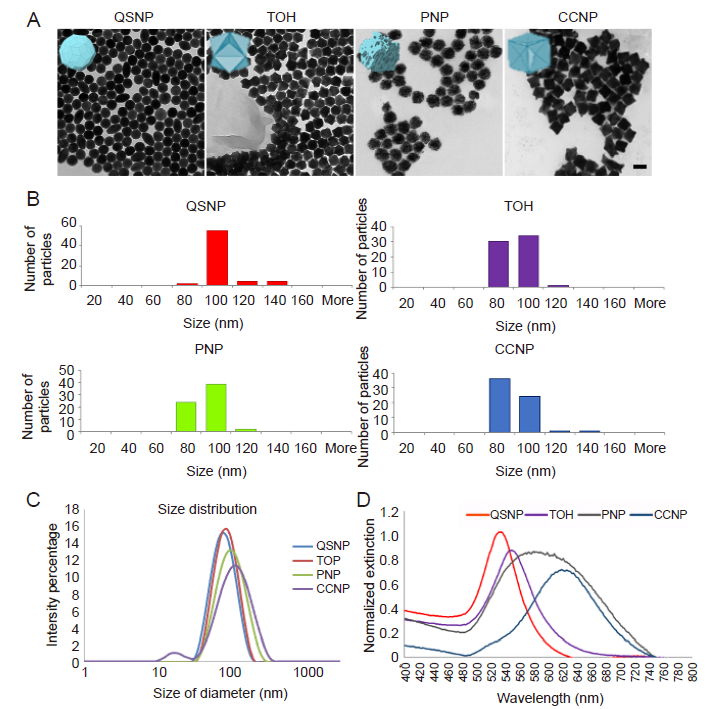
Figure 2. Characterisation of different-shaped AuNPs. (A) Transmission electron microscopic images of different-shaped AuNPs. The images show that AuNPs were similar in size and monodisperse in term of shape. Scale bar: 100 nm. (B) Particle size analysis of images obtained by transmission electron microscopy. AuNPs: gold nanoparticles; CCNPs: concave nanocube particles; PNPs: porous nanoparticles; QSNPs: quasi-spherical nanoparticles; TOH: nanotrioctahedra. (C) Size distribution of the different-shaped AuNPs analysed by dynamic light scattering. The results of dynamic light scattering showed a monodisperse peak of each shape of AuNPs with similar diameter, approximately 90 nm. (D) Optical extinction spectra of AuNPs reflecting differences in localised surface plasmon resonance.
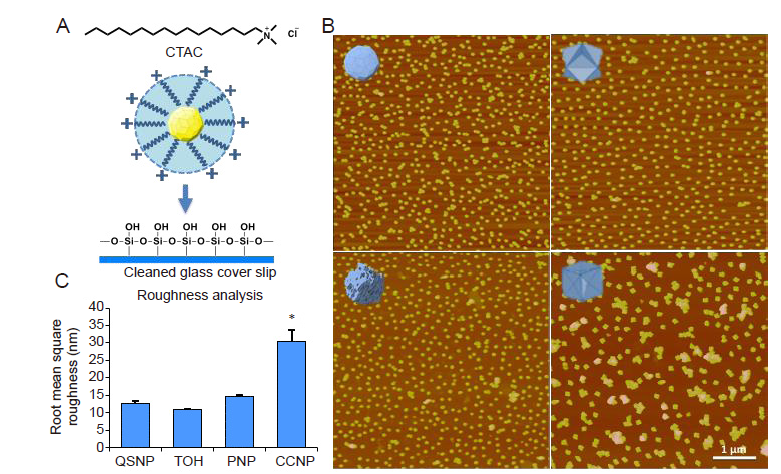
Figure 3. AuNP-coated substrate analysis. (A) AuNPs were deposited onto ‘piranha’-treated glass cover-slips by electrostatic interaction between positively-charged quaternary amine function groups on AuNP ligands and negatively-charged ‘piranha’-treated glass cover-slips. (B) Atomic force microscopic images show the different shapes of AuNP-coated substrates. The AuNP-coated glass cover-slips exhibited similar coverage of AuNPs on the substrate which was approximately 40﹣50%. (C) Root mean square (Ra) roughness from data collected from atomic force microscopic images. The Ra result revealed no significant difference in microscale roughness among QSNP-, TOH-, and PNP-coated substrates. CCNP-coated substrates showed significantly higher roughness compared to the other surfaces. Data are expressed as mean ± SD. *P < 0.05 based on one-way analysis of variance. AuNPs: gold nanoparticles; CCNPs: concave nanocube particles; CTAC: (1-hexadecyl)trimethylammonium chloride; PNPs: porous nanoparticles; QSNPs: quasi-spherical nanoparticles; TOH: nanotrioctahedra.
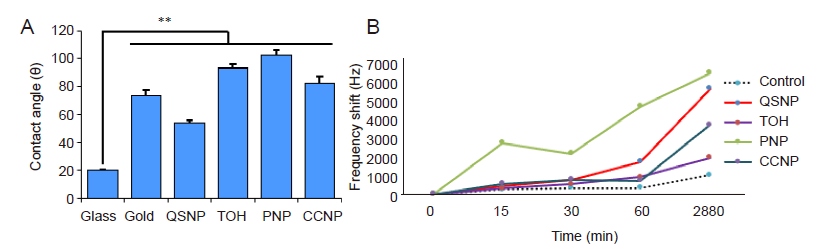
Figure 4. AuNP-coated substrate characterisation by contact angle measurement and QCM measurement. (A) AuNP-coated surfaces exhibit higher contact angles compared to an uncoated glass surface. QSNP is the most hydrophilic AuNP-coated surface, followed by gold sputtered surface, TOH-, CCNP-, and PNP-coated substrates respectively. (B) Frequency shifts measured by QCM after incubation of the AuNP-coated QCM probe with cell culture medium. Gold sputtered quartz probe was used as control. Data are expressed as mean (± SD). **P ≤ 0.01 based on one-way analysis of variance. AuNPs: gold nanoparticles; CCNPs: concave nanocube particles; PNPs: porous nanoparticles; QCM: quartz crystal microbalance; QSNPs: quasi-spherical nanoparticles; TOH: nanotrioctahedra.
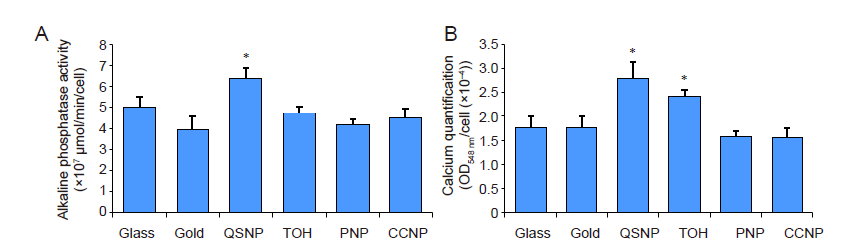
Figure 5. Cytochemical analysis of the osteogenic differentiation process of rat bone marrow stem cells on control glass, gold-sputtered substrates, and gold nanoparticle-coated substrates at day 2 after incubated in osteogenic media. (A) Alkaline phosphatase activity of cells cultured on different substrates. (B) Optical density at 548 nm of solubilised Alizarin red S staining normalised to cell number indicates the relative deposited calcium quantity at day 5. The mineralisation of cells on QSNP and TOH substrates was significantly higher than that of control glass, gold-sputtered, PNP and CCNP substrates. Data are expressed as mean ± SD (n = 3). *P < 0.05 (one-way analysis of variance followed by multiple comparisons). CCNPs: concave nanocube particles; PNPs: porous nanoparticles; QSNPs: quasi-spherical nanoparticles; TOH: nanotrioctahedra.
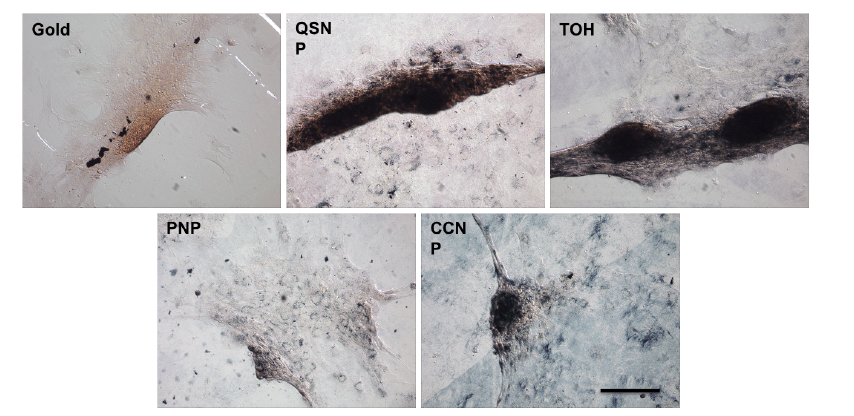
Figure 6. Micrographic images of bone marrow stem cells stained with lizarin red S staining of each sample at day 5. Cells on QSNP and TOH substrates are obviously stained for calcium deposition, as illustrated by the deep red colour for calcium of large cell nodules, whereas much weaker staining was observed on gold-sputtered and PNP-coated substrates. The Alizarin red S staining confirms calcium mineralisation of cells on the two substrates. Notably, cells on CCNP substrate only formed small nodules that were also stained with Alizarin red S. Scale bar: 200 μm. CCNP: concave nanocube particle; PNP: porous nanoparticle; QSNP: quasi-spherical nanoparticle; TOH: nanotrioctahedra.
| 1. |
Higuchi, A.; Ling, Q. D.; Chang, Y.; Hsu, S. T.; Umezawa, A. Physical cues of biomaterials guide stem cell differentiation fate. Chem Rev. 2013, 113, 3297-3328.
doi: 10.1021/cr300426x URL |
| 2. |
Metavarayuth, K.; Sitasuwan, P.; Zhao, X.; Lin, Y.; Wang, Q. Influence of surface topographical cues on the differentiation of mesenchymal stem cells in vitro. ACS Biomater Sci Eng. 2016, 2, 142-151.
doi: 10.1021/acsbiomaterials.5b00377 URL |
| 3. |
Badami, A. S.; Kreke, M. R.; Thompson, M. S.; Riffle, J. S.; Goldstein, A. S. Effect of fiber diameter on spreading, proliferation, and differentiation of osteoblastic cells on electrospun poly(lactic acid) substrates. Biomaterials. 2006, 27, 596-606.
doi: 10.1016/j.biomaterials.2005.05.084 URL |
| 4. |
Miller, D. C.; Thapa, A.; Haberstroh, K. M.; Webster, T. J. Endothelial and vascular smooth muscle cell function on poly(lactic-co-glycolic acid) with nano-structured surface features. Biomaterials. 2004, 25, 53-61.
doi: 10.1016/S0142-9612(03)00471-X URL |
| 5. |
Rahmati, M.; Silva, E. A.; Reseland, J. E.; C, A. H.; Haugen, H. J. Biological responses to physicochemical properties of biomaterial surface. Chem Soc Rev. 2020, 49, 5178-5224.
doi: 10.1039/D0CS00103A URL |
| 6. |
Kaur, G.; Valarmathi, M. T.; Potts, J. D.; Wang, Q. The promotion of osteoblastic differentiation of rat bone marrow stromal cells by a polyvalent plant mosaic virus. Biomaterials. 2008, 29, 4074-4081.
doi: 10.1016/j.biomaterials.2008.06.029 URL |
| 7. |
Metavarayuth, K.; Maturavongsadit, P.; Chen, X.; Sitasuwan, P.; Lu, L.; Su, J.; Wang, Q. Nanotopographical cues mediate osteogenesis of stem cells on virus substrates through BMP-2 intermediate. Nano Lett. 2019, 19, 8372-8380.
doi: 10.1021/acs.nanolett.9b02001 URL |
| 8. | Metavarayuth, K.; Sitasuwan, P.; Luckanagul, J. A.; Feng, S.; Wang, Q. Virus nanoparticles mediated osteogenic differentiation of bone derived mesenchymal stem cells. Adv Sci (Weinh). 2015, 2, 1500026. |
| 9. |
Sitasuwan, P.; Lee, L. A.; Bo, P.; Davis, E. N.; Lin, Y.; Wang, Q. A plant virus substrate induces early upregulation of BMP2 for rapid bone formation. Integr Biol (Camb). 2012, 4, 651-660.
doi: 10.1039/c2ib20041d URL |
| 10. | Yuan, J.; Maturavongsadit, P.; Zhou, Z.; Lv, B.; Lin, Y.; Yang, J.; Luckanagul, J. Hyaluronic acid-based hydrogels with tobacco mosaic virus containing cell adhesive peptide induce bone repair in normal and osteoporotic rats. Biomater Transl. 2020, 1, 89-98. |
| 11. |
Maturavongsadit, P.; Luckanagul, J. A.; Metavarayuth, K.; Zhao, X.; Chen, L.; Lin, Y.; Wang, Q. Promotion of in vitro chondrogenesis of mesenchymal stem cells using in situ hyaluronic hydrogel functionalized with rod-like viral nanoparticles. Biomacromolecules. 2016, 17, 1930-1938.
doi: 10.1021/acs.biomac.5b01577 URL |
| 12. |
Gentleman, M. M.; Gentleman, E. The role of surface free energy in osteoblast-biomaterial interactions. Int Mater Rev. 2014, 59, 417-429.
doi: 10.1179/1743280414Y.0000000038 URL |
| 13. |
Yang, D.; Lü, X.; Hong, Y.; Xi, T.; Zhang, D. The molecular mechanism of mediation of adsorbed serum proteins to endothelial cells adhesion and growth on biomaterials. Biomaterials. 2013, 34, 5747-5758.
doi: 10.1016/j.biomaterials.2013.04.028 URL |
| 14. |
Wang, J.; Chen, X.; Guo, B.; Yang, X.; Zhou, Y.; Zhu, X.; Zhang, K.; Fan, Y.; Tu, C.; Zhang, X. A serum protein adsorption profile on BCP ceramics and influence of the elevated adsorption of adhesive proteins on the behaviour of MSCs. J Mater Chem B. 2018, 6, 7383-7395.
doi: 10.1039/C8TB02283F URL |
| 15. |
Wang, Z.; Lin, S. The impact of low-surface-energy functional groups on oil fouling resistance in membrane distillation. J Membr Sci. 2017, 527, 68-77.
doi: 10.1016/j.memsci.2016.12.063 URL |
| 16. |
Rosales, J. I.; Marshall, G. W.; Marshall, S. J.; Watanabe, L. G.; Toledano, M.; Cabrerizo, M. A.; Osorio, R. Acid-etching and hydration influence on dentin roughness and wettability. J Dent Res. 1999, 78, 1554-1559.
doi: 10.1177/00220345990780091001 URL |
| 17. | Lu, Z.; Wu, Y.; Cong, Z.; Qian, Y.; Wu, X.; Shao, N.; Qiao, Z.; Zhang, H.; She, Y.; Chen, K.; Xiang, H.; Sun, B.; Yu, Q.; Yuan, Y.; Lin, H.; Zhu, M.; Liu, R. Effective and biocompatible antibacterial surfaces via facile synthesis and surface modification of peptide polymers. Bioact Mater. 2021, 6, 4531-4541. |
| 18. | Zhao, G.; Schwartz, Z.; Wieland, M.; Rupp, F.; Geis-Gerstorfer, J Cochran, D. L Boyan, B. D. High surface energy enhances cell response to titanium substrate microstructure. J Biomed Mater Res A. 2005, 74, 49-58. |
| 19. |
Feng, B.; Weng, J.; Yang, B. C.; Qu, S. X.; Zhang, X. D. Characterization of surface oxide films on titanium and adhesion of osteoblast. Biomaterials. 2003, 24, 4663-4670.
doi: 10.1016/S0142-9612(03)00366-1 URL |
| 20. |
Nakamura, M.; Hori, N.; Ando, H.; Namba, S.; Toyama, T.; Nishimiya, N.; Yamashita, K. Surface free energy predominates in cell adhesion to hydroxyapatite through wettability. Mater Sci Eng C Mater Biol Appl. 2016, 62, 283-292.
doi: 10.1016/j.msec.2016.01.037 URL |
| 21. |
Razafiarison, T.; Holenstein, C. N.; Stauber, T.; Jovic, M.; Vertudes, E.; Loparic, M.; Kawecki, M.; Bernard, L.; Silvan, U.; Snedeker, J. G. Biomaterial surface energy-driven ligand assembly strongly regulates stem cell mechanosensitivity and fate on very soft substrates. Proc Natl Acad Sci U S A. 2018, 115, 4631-4636.
doi: 10.1073/pnas.1704543115 URL |
| 22. | Faghihi, S.; Azari, F.; Szpunar, J. A.; Vali, H.; Tabrizian, M. Titanium crystal orientation as a tool for the improved and regulated cell attachment. J Biomed Mater Res A. 2009, 91, 656-662. |
| 23. |
Dykman, L.; Khlebtsov, N. Gold nanoparticles in biomedical applications: recent advances and perspectives. Chem Soc Rev. 2012, 41, 2256-2282.
doi: 10.1039/C1CS15166E URL |
| 24. |
Huang, D.; Liao, F.; Molesa, S.; Redinger, D.; Subramanian, V. Plastic-compatible low resistance printable gold nanoparticle conductors for flexible electronics. J Electrochem Soc. 2003, 150, G412.
doi: 10.1149/1.1582466 URL |
| 25. |
Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab J Chem. 2019, 12, 908-931.
doi: 10.1016/j.arabjc.2017.05.011 URL |
| 26. |
Tiwari, P. M.; Vig, K.; Dennis, V. A.; Singh, S. R. Functionalized gold nanoparticles and their biomedical applications. Nanomaterials (Basel). 2011, 1, 31-63.
doi: 10.3390/nano1010031 URL |
| 27. |
Yáñez-Sedeño, P.; Pingarrón, J. M. Gold nanoparticle-based electrochemical biosensors. Anal Bioanal Chem. 2005, 382, 884-886.
doi: 10.1007/s00216-005-3221-5 URL |
| 28. |
Sousa, L. M.; Vilarinho, L. M.; Ribeiro, G. H.; Bogado, A. L.; Dinelli, L. R. An electronic device based on gold nanoparticles and tetraruthenated porphyrin as an electrochemical sensor for catechol. R Soc Open Sci. 2017, 4, 170675.
doi: 10.1098/rsos.170675 URL |
| 29. |
Holec, D.; Dumitraschkewitz, P.; Vollath, D.; Fischer, F. D. Surface energy of au nanoparticles depending on their size and shape. Nanomaterials (Basel). 2020, 10, 484.
doi: 10.3390/nano10030484 URL |
| 30. |
Grzelczak, M.; Pérez-Juste, J.; Mulvaney, P.; Liz-Marzán, L. M. Shape control in gold nanoparticle synthesis. Chem Soc Rev. 2008, 37, 1783-1791.
doi: 10.1039/b711490g URL |
| 31. | Zhang, Q.; Large, N.; Nordlander, P.; Wang, H. Porous Au nanoparticles with tunable plasmon resonances and intense field enhancements for single-particle SERS. J Phys Chem Lett. 2014, 5, 370-374. |
| 32. |
Zhang, Q.; Large, N.; Wang, H. Gold nanoparticles with tipped surface structures as substrates for single-particle surface-enhanced Raman spectroscopy: concave nanocubes, nanotrisoctahedra, and nanostars. ACS Appl Mater Interfaces. 2014, 6, 17255-17267.
doi: 10.1021/am505245z URL |
| 33. |
Zhang, Q.; Zhou, Y.; Villarreal, E.; Lin, Y.; Zou, S.; Wang, H. Faceted Gold Nanorods: Nanocuboids, Convex Nanocuboids, and Concave Nanocuboids. Nano Lett. 2015, 15, 4161-4169.
doi: 10.1021/acs.nanolett.5b01286 URL |
| 34. |
Petryayeva, E.; Krull, U. J. Localized surface plasmon resonance: nanostructures, bioassays and biosensing--a review. Anal Chim Acta. 2011, 706, 8-24.
doi: 10.1016/j.aca.2011.08.020 URL |
| 35. |
Orendorff, C. J.; Sau, T. K.; Murphy, C. J. Shape-dependent plasmon-resonant gold nanoparticles. Small. 2006, 2, 636-639.
doi: 10.1002/(ISSN)1613-6829 URL |
| 36. |
Malmsten, M. Formation of adsorbed protein layers. J Colloid Interface Sci. 1998, 207, 186-199.
doi: 10.1006/jcis.1998.5763 URL |
| 37. |
Wertz, C. F.; Santore, M. M. Effect of surface hydrophobicity on adsorption and relaxation kinetics of albumin and fibrinogen: single-species and competitive behavior. Langmuir. 2001, 17, 3006-3016.
doi: 10.1021/la0017781 URL |
| 38. | Latour, R. A. Biomaterials: protein-surface interactions. In Encyclopedia of Biomaterials and Biomedical Engineering, Wnek, G.; Bowlin, G., eds.; CRC Press: 2008; pp 270-284. |
| 39. |
Deng, Z. J.; Mortimer, G.; Schiller, T.; Musumeci, A.; Martin, D.; Minchin, R. F. Differential plasma protein binding to metal oxide nanoparticles. Nanotechnology. 2009, 20, 455101.
doi: 10.1088/0957-4484/20/45/455101 URL |
| 40. |
Zernik, J.; Twarog, K.; Upholt, W. B. Regulation of alkaline phosphatase and alpha 2(I) procollagen synthesis during early intramembranous bone formation in the rat mandible. Differentiation. 1990, 44, 207-215.
doi: 10.1111/j.1432-0436.1990.tb00619.x URL |
| 41. |
Igarashi, M.; Kamiya, N.; Hasegawa, M.; Kasuya, T.; Takahashi, T.; Takagi, M. Inductive effects of dexamethasone on the gene expression of Cbfa1, Osterix and bone matrix proteins during differentiation of cultured primary rat osteoblasts. J Mol Histol. 2004, 35, 3-10.
doi: 10.1023/B:HIJO.0000020883.33256.fe URL |
| 42. |
Li, Y.; Kim, J. H.; Choi, E. H.; Han, I. Promotion of osteogenic differentiation by non-thermal biocompatible plasma treated chitosan scaffold. Sci Rep. 2019, 9, 3712.
doi: 10.1038/s41598-019-40371-6 URL |
| 43. |
Lim, E. K.; Keem, J. O.; Yun, H. S.; Jung, J.; Chung, B. H. Smart nanoprobes for the detection of alkaline phosphatase activity during osteoblast differentiation. Chem Commun (Camb). 2015, 51, 3270-3272.
doi: 10.1039/C4CC09620G URL |
| 44. | Yuan, J.; Maturavongsadit, P.; Metavarayuth, K.; Luckanagul, J. A.; Wang, Q. Enhanced bone defect repair by polymeric substitute fillers of multiarm polyethylene glycol-crosslinked hyaluronic acid hydrogels. Macromol Biosci. 2019, 19, e1900021. |
| 45. |
Yang, Y. K.; Ogando, C. R.; Wang See, C.; Chang, T. Y.; Barabino, G. A. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res Ther. 2018, 9, 131.
doi: 10.1186/s13287-018-0876-3 URL |
| 46. |
Neuhuber, B.; Swanger, S. A.; Howard, L.; Mackay, A.; Fischer, I. Effects of plating density and culture time on bone marrow stromal cell characteristics. Exp Hematol. 2008, 36, 1176-1185.
doi: 10.1016/j.exphem.2008.03.019 URL |
| 47. |
Parhi, P.; Golas, A.; Vogler, E. A. Role of proteins and water in the initial attachment of mammalian cells to biomedical surfaces: A Review. J Adhes Sci Technol. 2010, 24, 853-888.
doi: 10.1163/016942409X12598231567907 URL |
| 48. | Lim, J. Y.; Liu, X.; Vogler, E. A.; Donahue, H. J. Systematic variation in osteoblast adhesion and phenotype with substratum surface characteristics. J Biomed Mater Res A. 2004, 68, 504-512. |
| 49. |
Liu, X.; Lim, J. Y.; Donahue, H. J.; Dhurjati, R.; Mastro, A. M.; Vogler, E. A. Influence of substratum surface chemistry/energy and topography on the human fetal osteoblastic cell line hFOB 1.19: Phenotypic and genotypic responses observed in vitro. Biomaterials. 2007, 28, 4535-4550.
doi: 10.1016/j.biomaterials.2007.06.016 URL |
| 50. |
Baier, R. E.; Meyer, A. E.; Natiella, J. R.; Natiella, R. R.; Carter, J. M. Surface properties determine bioadhesive outcomes: methods and results. J Biomed Mater Res. 1984, 18, 337-355.
doi: 10.1002/(ISSN)1097-4636 URL |
| 51. |
Groth, T.; Altankov, G. Studies on cell-biomaterial interaction: role of tyrosine phosphorylation during fibroblast spreading on surfaces varying in wettability. Biomaterials. 1996, 17, 1227-1234.
doi: 10.1016/0142-9612(96)84943-X URL |
| 52. |
Schakenraad, J. M.; Busscher, H. J.; Wildevuur, C. R.; Arends, J. Thermodynamic aspects of cell spreading on solid substrata. Cell Biophys. 1988, 13, 75-91.
doi: 10.1007/BF02797367 URL |
| 53. |
Tamada, Y.; Ikada, Y. Fibroblast growth on polymer surfaces and biosynjournal of collagen. J Biomed Mater Res. 1994, 28, 783-789.
doi: 10.1002/(ISSN)1097-4636 URL |
| [1] | Xuechen Zhang, Ana Justo Caetano, Paul T. Sharpe, Ana Angelova Volponi. Oral stem cells, decoding and mapping the resident cells populations [J]. Biomaterials Translational, 2022, 3(1): 24-30. |
| [2] | Deepika Arora, Pamela Gehron Robey. Recent updates on the biological basis of heterogeneity in bone marrow stromal cells/skeletal stem cells [J]. Biomaterials Translational, 2022, 3(1): 3-16. |
| [3] | Suzanne M. Watt. The long and winding road: homeostatic and disordered haematopoietic microenvironmental niches: a narrative review [J]. Biomaterials Translational, 2022, 3(1): 31-54. |
| [4] | Shuqin Cao, Quan Yuan. An update of nanotopographical surfaces in modulating stem cell fate: a narrative review [J]. Biomaterials Translational, 2022, 3(1): 55-64. |
| [5] | Emma Steijvers, Armaan Ghei, Zhidao Xia. Manufacturing artificial bone allografts: a perspective [J]. Biomaterials Translational, 2022, 3(1): 65-80. |
| [6] | Ke Hu, Yuxuan Li, Zunxiang Ke, Hongjun Yang, Chanjun Lu, Yiqing Li, Yi Guo, Weici Wang. History, progress and future challenges of artificial blood vessels: a narrative review [J]. Biomaterials Translational, 2022, 3(1): 81-98. |
| [7] | Trivia P. Frazier, Katie Hamel, Xiying Wu, Emma Rogers, Haley Lassiter, Jordan Robinson, Omair Mohiuddin, Michael Henderson, Jeffrey M. Gimble. Adipose-derived cells: building blocks of three-dimensional microphysiological systems [J]. Biomaterials Translational, 2021, 2(4): 301-306. |
| [8] | Yang Zhao, Qing Sun, Bo Huo. Focal adhesion regulates osteogenic differentiation of mesenchymal stem cells and osteoblasts [J]. Biomaterials Translational, 2021, 2(4): 312-322. |
| [9] | Yizhong Peng, Jinye Li, Hui Lin, Shuo Tian, Sheng Liu, Feifei Pu, Lei Zhao, Kaige Ma, Xiangcheng Qing, Zengwu Shao. Endogenous repair theory enriches construction strategies for orthopaedic biomaterials: a narrative review [J]. Biomaterials Translational, 2021, 2(4): 343-360. |
| [10] | Qingchuan Wang, Weidan Wang, Yanfang Li, Weirong Li, Lili Tan, Ke Yang. Biofunctional magnesium coating of implant materials by physical vapour deposition [J]. Biomaterials Translational, 2021, 2(3): 248-256. |
| [11] | Yizhong Peng, Xiangcheng Qing, Hongyang Shu, Shuo Tian, Wenbo Yang, Songfeng Chen, Hui Lin, Xiao Lv, Lei Zhao, Xi Chen, Feifei Pu, Donghua Huang, Xu Cao, Zengwu Shao. Proper animal experimental designs for preclinical research of biomaterials for intervertebral disc regeneration [J]. Biomaterials Translational, 2021, 2(2): 91-142. |
| [12] | Pingli Wu, Yangyang Liang, Guoming Sun. Engineering immune-responsive biomaterials for skin regeneration [J]. Biomaterials Translational, 2021, 2(1): 61-71. |
| [13] | Isak Jatoi, Jingyu Fan. A biomaterials viewpoint for the 2020 SARS-CoV-2 vaccine development [J]. Biomaterials Translational, 2021, 2(1): 30-42. |
| [14] | Yunsong Shi, Ruijun He, Xiangyu Deng, Zengwu Shao, Davide Deganello, Chunze Yan, Zhidao Xia. Three-dimensional biofabrication of an aragonite-enriched self-hardening bone graft substitute and assessment of its osteogenicity in vitro and in vivo [J]. Biomaterials Translational, 2020, 1(1): 69-81. |
| [15] | Xing Yang, Yuanyuan Li, Xujie Liu, Wei He, Qianli Huang, Qingling Feng. Nanoparticles and their effects on differentiation of mesenchymal stem cells [J]. Biomaterials Translational, 2020, 1(1): 58-68. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||