Biomaterials Translational ›› 2021, Vol. 2 ›› Issue (3): 197-213.doi: 10.12336/biomatertransl.2021.03.004
• REVIEW • Previous Articles Next Articles
Xirui Jing1, Qiuyue Ding1, Qinxue Wu2, Weijie Su1, Keda Yu1, Yanlin Su1, Bing Ye1, Qing Gao1, Tingfang Sun1, Xiaodong Guo1,*( )
)
Received:2021-07-16
Revised:2021-08-16
Accepted:2021-09-10
Online:2021-09-28
Published:2021-09-28
Contact:
Xiaodong Guo
E-mail:xiaodongguo@hust.edu.cn
About author:Xiaodong Guo, xiaodongguo@hust.edu.cn.
Jing, X.; Ding, Q.; Wu, Q.; Su, W.; Yu, K.; Su, Y.; Ye, B.; Gao, Q.; Sun, T.; Guo, X. Magnesium-based materials in orthopaedics: material properties and animal models. Biomater Transl. 2021, 2(3), 197-213.
| Property | Natural bone | Magnesium alloys | Titanium alloys | Stainless steels |
|---|---|---|---|---|
| Density (g/cm3) | 1.8﹣2.1 | 1.74﹣2.0 | 4.4﹣4.5 | 7.9﹣8.1 |
| Elastic modulus (GPa) | 3﹣20 | 41﹣45 | 110﹣117 | 189﹣205 |
| Yield strength (MPa) | 130﹣180 | 85﹣190 | 758﹣1117 | 170﹣310 |
Table 1 Mechanical properties of various metallic implants compared with natural bone.
| Property | Natural bone | Magnesium alloys | Titanium alloys | Stainless steels |
|---|---|---|---|---|
| Density (g/cm3) | 1.8﹣2.1 | 1.74﹣2.0 | 4.4﹣4.5 | 7.9﹣8.1 |
| Elastic modulus (GPa) | 3﹣20 | 41﹣45 | 110﹣117 | 189﹣205 |
| Yield strength (MPa) | 130﹣180 | 85﹣190 | 758﹣1117 | 170﹣310 |
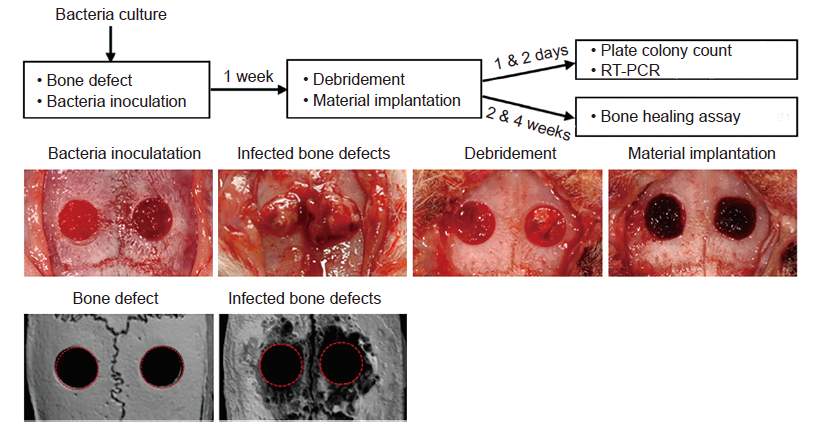
Figure 4. Creation of infected cranial defects. Adapted from Dong et al.88 Copyright 2017, with permission from Elsevier. RT-PCR: real-time polymerase chain reaction.
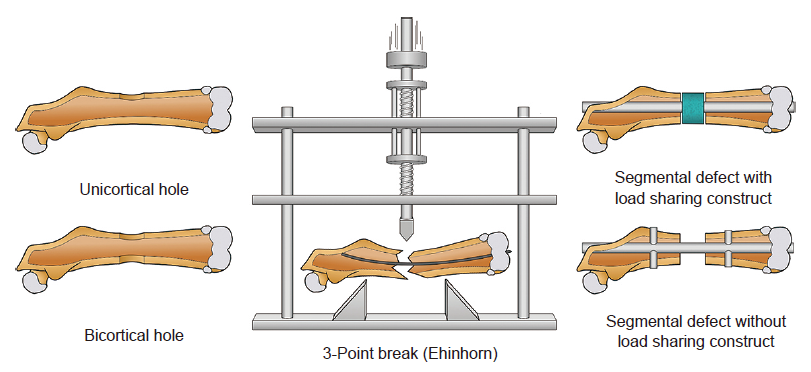
Figure 5. Illustration of bone healing models in the femoral diaphysis. Reprinted from Gunderson et al.90 Copyright 2020, with permission from Elsevier.
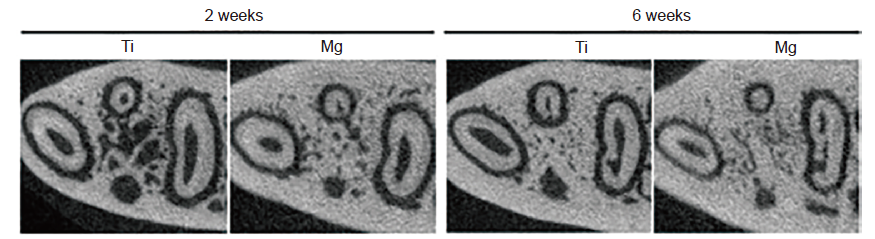
Figure 8. Mg rod for mandibular repair in rats. Mg: magnesium; Ti: titanium. Reprinted from He et al.123 Copyright 2020, with permission from American Academy of Periodontology.
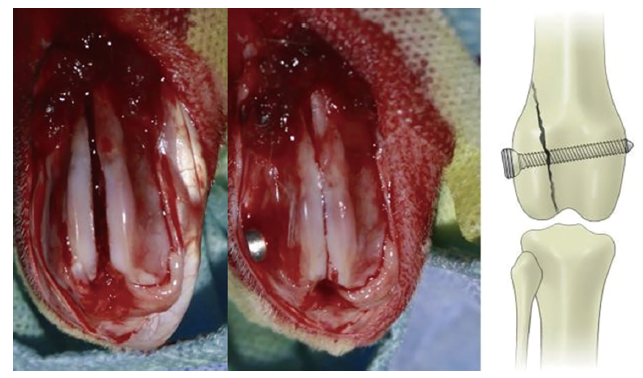
Figure 10. Magnesium screws for repair of distal femoral fractures in rabbits. Reprinted from Han et al.128 Copyright 2015, with permission from Elsevier.
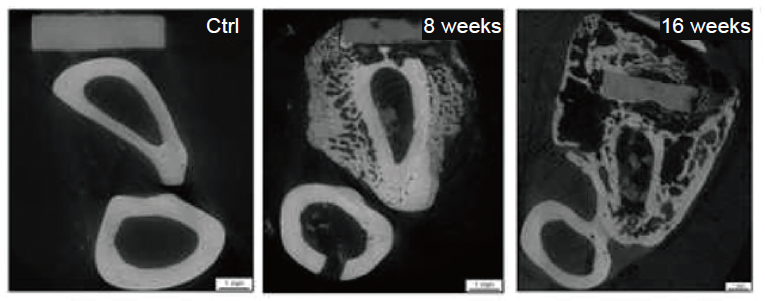
Figure 11. Magnesium-based plates and screws for the treatment of ulnar fractures in rabbits. Scale bars: 1 mm. Reprinted from Hung et al.129 Copyright 2019, with permission from Elsevier Ltd. on behalf of Acta Materialia Inc.
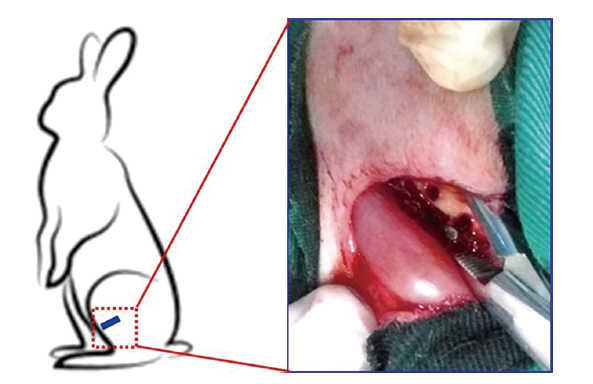
Figure 12. Schematic diagram showing the surgical process in the rabbit femoral defect model. Reprinted from Jiang et al.132 Copyright 2017 Wiley Periodicals, Inc. Reproduced with permission.
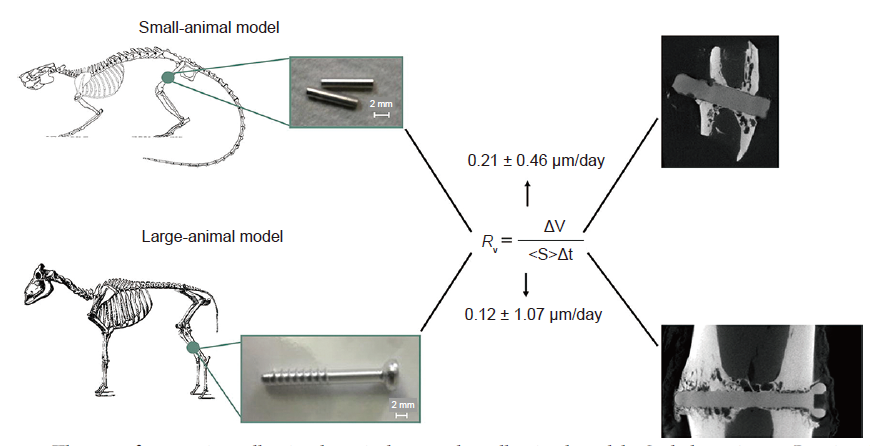
Figure 13. The use of magnesium alloy implants in large and small animal models. Scale bars: 2 mm. Reprinted with permission from Grün et al.133 Copyright 2018, Acta Materialia Inc.
| [1] |
Karinkanta, S.; Piirtola, M.; Sievänen, H.; Uusi-Rasi, K.; Kannus, P. Physical therapy approaches to reduce fall and fracture risk among older adults. Nat Rev Endocrinol. 2010, 6, 396-407.
doi: 10.1038/nrendo.2010.70 URL |
| [2] |
Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current status on clinical applications of magnesium-based orthopaedic implants: A review from clinical translational perspective. Biomaterials. 2017, 112, 287-302.
doi: 10.1016/j.biomaterials.2016.10.017 URL |
| [3] |
Sha, M.; Guo, Z.; Fu, J.; Li, J.; Yuan, C. F.; Shi, L.; Li, S. J. The effects of nail rigidity on fracture healing in rats with osteoporosis. Acta Orthop. 2009, 80, 135-138.
doi: 10.1080/17453670902807490 URL |
| [4] |
Singhvi, M. S.; Zinjarde, S. S.; Gokhale, D. V. Polylactic acid: synjournal and biomedical applications. J Appl Microbiol. 2019, 127, 1612-1626.
doi: 10.1111/jam.v127.6 URL |
| [5] | Zhao, D.; Zhu, T.; Li, J.; Cui, L.; Zhang, Z.; Zhuang, X.; Ding, J. Poly(lactic-co-glycolic acid)-based composite bone-substitute materials. Bioact Mater. 2021, 6, 346-360. |
| [6] | Wang, J. L.; Xu, J. K.; Hopkins, C.; Chow, D. H.; Qin, L. Biodegradable magnesium-based implants in orthopedics-a general review and perspectives. Adv Sci (Weinh). 2020, 7, 1902443. |
| [7] | Navarro, M.; Michiardi, A.; Castaño, O.; Planell, J. A. Biomaterials in orthopaedics. J RSoc Interface. 2008, 5, 1137-1158. |
| [8] |
Barber, F. A.; Dockery, W. D. Long-term absorption of poly-L-lactic Acid interference screws. Arthroscopy. 2006, 22, 820-826.
doi: 10.1016/j.arthro.2006.04.096 URL |
| [9] | Cheung, W. H.; Miclau, T.; Chow, S. K.; Yang, F. F.; Alt, V. Fracture healing in osteoporotic bone. Injury. 2016, 47 Suppl 2, S21-26. |
| [10] |
Zheng, N.; Tang, N.; Qin, L. Atypical femoral fractures and current management. J Orthop Translat. 2016, 7, 7-22.
doi: 10.1016/j.jot.2016.06.029 URL |
| [11] |
Farraro, K. F.; Sasaki, N.; Woo, S. L.; Kim, K. E.; Tei, M. M.; Speziali, A.; McMahon, P. J. Magnesium ring device to restore function of a transected anterior cruciate ligament in the goat stifle joint. J Orthop Res. 2016, 34, 2001-2008.
doi: 10.1002/jor.23210 URL |
| [12] |
Xia, J.; Chen, H.; Yan, J.; Wu, H.; Wang, H.; Guo, J.; Zhang, X.; Zhang, S.; Zhao, C.; Chen, Y. High-purity magnesium staples suppress inflammatory response in rectal anastomoses. ACS Appl Mater Interfaces. 2017, 9, 9506-9515.
doi: 10.1021/acsami.7b00813 URL |
| [13] | Naujokat, H.; Ruff, C. B.; Klüter, T.; Seitz, J. M.; Açil, Y.; Wiltfang, J. Influence of surface modifications on the degradation of standard-sized magnesium plates and healing of mandibular osteotomies in miniature pigs. Int J Oral Maxillofac Surg. 2020, 49, 272-283. |
| [14] | Krämer, M.; Schilling, M.; Eifler, R.; Hering, B.; Reifenrath, J.; Besdo, S.; Windhagen, H.; Willbold, E.; Weizbauer, A. Corrosion behavior, biocompatibility and biomechanical stability of a prototype magnesium-based biodegradable intramedullary nailing system. Mater Sci Eng CMater Biol Appl. 2016, 59, 129-135. |
| [15] |
Erbel, R.; Di Mario, C.; Bartunek, J.; Bonnier, J.; de Bruyne, B.; Eberli, F. R.; Erne, P.; Haude, M.; Heublein, B.; Horrigan, M.; Ilsley, C.; Böse, D.; Koolen, J.; Lüscher, T. F.; Weissman, N.; Waksman, R.; Temporary scaffolding of coronary arteries with bioabsorbable magnesium stents: a prospective, n.-r. m. t. Temporary scaffolding of coronary arteries with bioabsorbable magnesium stents: a prospective, non-randomised multicentre trial. Lancet. 2007, 369, 1869-1875.
doi: 10.1016/S0140-6736(07)60853-8 URL |
| [16] |
Wu, J.; Lee, B.; Saha, P.; P, N. K. A feasibility study of biodegradable magnesium-aluminum-zinc-calcium-manganese (AZXM) alloys for tracheal stent application. J Biomater Appl. 2019, 33, 1080-1093.
doi: 10.1177/0885328218824775 URL |
| [17] |
Schaller, B.; Saulacic, N.; Imwinkelried, T.; Beck, S.; Liu, E. W.; Gralla, J.; Nakahara, K.; Hofstetter, W.; Iizuka, T. In vivo degradation of magnesium plate/screw osteosynjournal implant systems: Soft and hard tissue response in a calvarial model in miniature pigs. J Craniomaxillofac Surg. 2016, 44, 309-317.
doi: 10.1016/j.jcms.2015.12.009 URL |
| [18] |
Guo, X.; Xu, H.; Zhang, F.; Lu, F. Bioabsorbable high-purity magnesium interbody cage: degradation, interbody fusion, and biocompatibility from a goat cervical spine model. Ann Transl Med. 2020, 8, 1054.
doi: 10.21037/atm URL |
| [19] |
Zhao, Y.; Yu, S.; Wu, X.; Dai, H.; Liu, W.; Tu, R.; Goto, T. Construction of macroporous magnesium phosphate-based bone cement with sustained drug release. Mater Des. 2021, 200, 109466.
doi: 10.1016/j.matdes.2021.109466 URL |
| [20] |
Lai, Y.; Li, Y.; Cao, H.; Long, J.; Wang, X.; Li, L.; Li, C.; Jia, Q.; Teng, B.; Tang, T.; Peng, J.; Eglin, D.; Alini, M.; Grijpma, D. W.; Richards, G.; Qin, L. Osteogenic magnesium incorporated into PLGA/TCP porous scaffold by 3D printing for repairing challenging bone defect. Biomaterials. 2019, 197, 207-219.
doi: 10.1016/j.biomaterials.2019.01.013 URL |
| [21] |
Zhang, D.; Ni, N.; Su, Y.; Miao, H.; Tang, Z.; Ji, Y.; Wang, Y.; Gao, H.; Ju, Y.; Sun, N.; Sun, H.; Yuan, G.; Wang, Y.; Zhou, H.; Huang, H.; Gu, P.; Fan, X. Targeting local osteogenic and ancillary cells by mechanobiologically optimized magnesium scaffolds for orbital bone reconstruction in canines. ACS Appl Mater Interfaces. 2020, 12, 27889-27904.
doi: 10.1021/acsami.0c00553 URL |
| [22] |
Chakraborty Banerjee, P.; Al-Saadi, S.; Choudhary, L.; Harandi, S. E.; Singh, R. Magnesium implants: prospects and challenges. Materials (Basel). 2019, 12, 136.
doi: 10.3390/ma12010136 URL |
| [23] | Agarwal, S.; Curtin, J.; Duffy, B.; Jaiswal, S. Biodegradable magnesium alloys for orthopaedic applications: A review on corrosion, biocompatibility and surface modifications. Mater Sci Eng CMater Biol Appl. 2016, 68, 948-963. |
| [24] |
Zhao, D.; Huang, S.; Lu, F.; Wang, B.; Yang, L.; Qin, L.; Yang, K.; Li, Y.; Li, W.; Wang, W.; Tian, S.; Zhang, X.; Gao, W.; Wang, Z.; Zhang, Y.; Xie, X.; Wang, J.; Li, J. Vascularized bone grafting fixed by biodegradable magnesium screw for treating osteonecrosis of the femoral head. Biomaterials. 2016, 81, 84-92.
doi: 10.1016/j.biomaterials.2015.11.038 URL |
| [25] |
Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C. J.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials. 2005, 26, 3557-3563.
doi: 10.1016/j.biomaterials.2004.09.049 URL |
| [26] |
Zhang, J.; Shang, Z.; Jiang, Y.; Zhang, K.; Li, X.; Ma, M.; Li, Y.; Ma, b. Biodegradable metals for bone fracture repair in animal models: a systematic review. Regen Biomater. 2021, 8, rbaa047.
doi: 10.1093/rb/rbaa047 URL |
| [27] |
Seitz, J. M.; Eifler, R.; Bach, F. W.; Maier, H. J. Magnesium degradation products: effects on tissue and human metabolism. J Biomed Mater Res A. 2014, 102, 3744-3753.
doi: 10.1002/jbm.a.35023 URL |
| [28] | Gonzalez, J.; Hou, R. Q.; Nidadavolu, E. P. S.; Willumeit-Römer, R.; Feyerabend, F. Magnesium degradation under physiological conditions - Best practice. Bioact Mater. 2018, 3, 174-185. |
| [29] | Walker, J.; Shadanbaz, S.; Woodfield, T. B.; Staiger, M. P.; Dias, G. J. Magnesium biomaterials for orthopedic application: a review from a biological perspective. J Biomed Mater Res BAppl Biomater. 2014, 102, 1316-1331. |
| [30] |
Oshibe, N.; Marukawa, E.; Yoda, T.; Harada, H. Degradation and interaction with bone of magnesium alloy WE43 implants: A long-term follow-up in vivo rat tibia study. J Biomater Appl. 2019, 33, 1157-1167.
doi: 10.1177/0885328218822050 URL |
| [31] |
Holweg, P.; Berger, L.; Cihova, M.; Donohue, N.; Clement, B.; Schwarze, U.; Sommer, N. G.; Hohenberger, G.; van den Beucken, J.; Seibert, F.; Leithner, A.; Löffler, J. F.; Weinberg, A. M. A lean magnesium-zinc-calcium alloy ZX00 used for bone fracture stabilization in a large growing-animal model. Acta Biomater. 2020, 113, 646-659.
doi: 10.1016/j.actbio.2020.06.013 URL |
| [32] | Han, H. S.; Jun, I.; Seok, H. K.; Lee, K. S.; Lee, K.; Witte, F.; Mantovani, D.; Kim, Y. C.; Glyn-Jones, S.; Edwards, J. R. Biodegradable magnesium alloys promote angio-osteogenesis to enhance bone repair. Adv Sci (Weinh). 2020, 7, 2000800. |
| [33] | Gao, J.; Su, Y.; Qin, Y. X. Calcium phosphate coatings enhance biocompatibility and degradation resistance of magnesium alloy: Correlating in vitro and in vivo studies. Bioact Mater. 2021, 6, 1223-1229. |
| [34] |
Liu, W.; Li, T.; Yang, C.; Wang, D.; He, G.; Cheng, M.; Wang, Q.; Zhang, X. Lithium-incorporated nanoporous coating formed by micro arc oxidation (MAO) on magnesium alloy with improved corrosion resistance, angiogenesis and osseointegration. J Biomed Nanotechnol. 2019, 15, 1172-1184.
doi: 10.1166/jbn.2019.2767 URL |
| [35] |
Helmholz, H.; Will, O.; Penate-Medina, T.; Humbert, J.; Damm, T.; Luthringer-Feyerabend, B.; Willumeit-Römer, R.; Glüer, C. C.; Penate-Medina, O. Tissue responses after implantation of biodegradable Mg alloys evaluated by multimodality 3D micro-bioimaging in vivo. J Biomed Mater Res A. 2021, 109, 1521-1529.
doi: 10.1002/jbm.a.v109.8 URL |
| [36] |
Grada, A.; Mervis, J.; Falanga, V. Research techniques made simple: animal models of wound healing. J Invest Dermatol. 2018, 138, 2095-2105.e1.
doi: 10.1016/j.jid.2018.08.005 URL |
| [37] |
Ribitsch, I.; Baptista, P. M.; Lange-Consiglio, A.; Melotti, L.; Patruno, M.; Jenner, F.; Schnabl-Feichter, E.; Dutton, L. C.; Connolly, D. J.; van Steenbeek, F. G.; Dudhia, J.; Penning, L. C. Large animal models in regenerative medicine and tissue engineering: to do or not to do. Front Bioeng Biotechnol. 2020, 8, 972.
doi: 10.3389/fbioe.2020.00972 URL |
| [38] | Al Alawi, A. M.; Majoni, S. W.; Falhammar, H. Magnesium and human health: perspectives and research directions. Int J Endocrinol. 2018, 2018, 9041694. |
| [39] |
Romani, A. M. Cellular magnesium homeostasis. Arch Biochem Biophys. 2011, 512, 1-23.
doi: 10.1016/j.abb.2011.05.010 URL |
| [40] |
Wolf, F. I.; Trapani, V. Cell (patho)physiology of magnesium. Clin Sci (Lond). 2008, 114, 27-35.
doi: 10.1042/CS20070129 URL |
| [41] |
de Baaij, J. H.; Hoenderop, J. G.; Bindels, R. J. Magnesium in man: implications for health and disease. Physiol Rev. 2015, 95, 1-46.
doi: 10.1152/physrev.00012.2014 URL |
| [42] |
Jahnen-Dechent, W.; Ketteler, M. Magnesium basics. Clin Kidney J. 2012, 5, i3-i14.
doi: 10.1093/ndtplus/sfr163 URL |
| [43] | Elin, R. J. Assessment of magnesium status for diagnosis and therapy. Magnes Res. 2010, 23, S194-198. |
| [44] |
Razzaque, M. S. Magnesium: are we consuming enough? Nutrients. 2018, 10, 1863.
doi: 10.3390/nu10121863 URL |
| [45] |
Yamanaka, R.; Shindo, Y.; Oka, K. Magnesium is a key player in neuronal maturation and neuropathology. Int J Mol Sci. 2019, 20, 3439.
doi: 10.3390/ijms20143439 URL |
| [46] |
Yoshizawa, S.; Brown, A.; Barchowsky, A.; Sfeir, C. Magnesium ion stimulation of bone marrow stromal cells enhances osteogenic activity, simulating the effect of magnesium alloy degradation. Acta Biomater. 2014, 10, 2834-2842.
doi: 10.1016/j.actbio.2014.02.002 URL |
| [47] |
Belluci, M. M.; de Molon, R. S.; Rossa, C., Jr.; Tetradis, S.; Giro, G.; Cerri, P. S.; Marcantonio, E., Jr, ., Orrico, S. R. P. Severe magnesium deficiency compromises systemic bone mineral density and aggravates inflammatory bone resorption. J Nutr Biochem. 2020, 77, 108301.
doi: 10.1016/j.jnutbio.2019.108301 URL |
| [48] |
Ciosek, Ż.; Kot, K.; Kosik-Bogacka, D.; Łanocha-Arendarczyk, N.; Rotter, I. The effects of calcium, magnesium, phosphorus, fluoride, and lead on bone tissue. Biomolecules. 2021, 11, 506.
doi: 10.3390/biom11040506 URL |
| [49] |
Zhai, Z.; Qu, X.; Li, H.; Yang, K.; Wan, P.; Tan, L.; Ouyang, Z.; Liu, X.; Tian, B.; Xiao, F.; Wang, W.; Jiang, C.; Tang, T.; Fan, Q.; Qin, A.; Dai, K. The effect of metallic magnesium degradation products on osteoclast-induced osteolysis and attenuation of NF-κB and NFATc1 signaling. Biomaterials. 2014, 35, 6299-6310.
doi: 10.1016/j.biomaterials.2014.04.044 URL |
| [50] |
Xie, H.; Cui, Z.; Wang, L.; Xia, Z.; Hu, Y.; Xian, L.; Li, C.; Xie, L.; Crane, J.; Wan, M.; Zhen, G.; Bian, Q.; Yu, B.; Chang, W.; Qiu, T.; Pickarski, M.; Duong, L. T.; Windle, J. J.; Luo, X.; Liao, E.; Cao, X. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat Med. 2014, 20, 1270-1278.
doi: 10.1038/nm.3668 URL |
| [51] |
Maier, J. A.; Castiglioni, S.; Locatelli, L.; Zocchi, M.; Mazur, A. Magnesium and inflammation: advances and perspectives. Semin Cell Dev Biol. 2021, 115, 37-44.
doi: 10.1016/j.semcdb.2020.11.002 URL |
| [52] | Zheng, Z.; Chen, Y.; Hong, H.; Shen, Y.; Wang, Y.; Sun, J.; Wang, X. The “Yin and Yang” of immunomodulatory magnesium-enriched graphene oxide nanoscrolls decorated biomimetic scaffolds in promoting bone regeneration. Adv Healthc Mater. 2021, 10, e2000631. |
| [53] |
Chen, Z.; Mao, X.; Tan, L.; Friis, T.; Wu, C.; Crawford, R.; Xiao, Y. Osteoimmunomodulatory properties of magnesium scaffolds coated with β-tricalcium phosphate. Biomaterials. 2014, 35, 8553-8565.
doi: 10.1016/j.biomaterials.2014.06.038 URL |
| [54] | Libako, P.; Nowacki, W.; Castiglioni, S.; Mazur, A.; Maier, J. A. Extracellular magnesium and calcium blockers modulate macrophage activity. Magnes Res. 2016, 29, 11-21. |
| [55] |
Li, Z.; Meyers, C. A.; Chang, L.; Lee, S.; Li, Z.; Tomlinson, R.; Hoke, A.; Clemens, T. L.; James, A. W. Fracture repair requires TrkA signaling by skeletal sensory nerves. J Clin Invest. 2019, 129, 5137-5150.
doi: 10.1172/JCI128428 URL |
| [56] |
Zhang, Y.; Xu, J.; Ruan, Y. C.; Yu, M. K.; O’Laughlin, M.; Wise, H.; Chen, D.; Tian, L.; Shi, D.; Wang, J.; Chen, S.; Feng, J. Q.; Chow, D. H.; Xie, X.; Zheng, L.; Huang, L.; Huang, S.; Leung, K.; Lu, N.; Zhao, L.; Li, H.; Zhao, D.; Guo, X.; Chan, K.; Witte, F.; Chan, H. C.; Zheng, Y.; Qin, L. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat Med. 2016, 22, 1160-1169.
doi: 10.1038/nm.4162 URL |
| [57] |
Michailova, A. P.; Belik, M. E.; Belik, M. E.; McCulloch, A. D. Effects of magnesium on cardiac excitation-contraction coupling. J Am Coll Nutr. 2004, 23, 514s-517s.
doi: 10.1080/07315724.2004.10719392 URL |
| [58] | Teragawa, H.; Matsuura, H.; Chayama, K.; Oshima, T. Mechanisms responsible for vasodilation upon magnesium infusion in vivo: clinical evidence. Magnes Res. 2002, 15, 241-246. |
| [59] | Teragawa, H.; Kato, M.; Yamagata, T.; Matsuura, H.; Kajiyama, G. Magnesium causes nitric oxide independent coronary artery vasodilation in humans. Heart. 2001, 86, 212-216. |
| [60] |
Cochrane, D. E.; Douglas, W. W. Histamine release by exocytosis from rat mast cells on reduction of extracellular sodium: a secretory response inhibited by calcium, strontium, barium or magnesium. J Physiol. 1976, 257, 433-448.
doi: 10.1113/jphysiol.1976.sp011377 URL |
| [61] |
Komaki, F.; Akiyama, T.; Yamazaki, T.; Kitagawa, H.; Nosaka, S.; Shirai, M. Effects of intravenous magnesium infusion on in vivo release of acetylcholine and catecholamine in rat adrenal medulla. Auton Neurosci. 2013, 177, 123-128.
doi: 10.1016/j.autneu.2013.03.004 URL |
| [62] |
Hashimoto, Y.; Nishimura, Y.; Maeda, H.; Yokoyama, M. Assessment of magnesium status in patients with bronchial asthma. J Asthma. 2000, 37, 489-496.
doi: 10.3109/02770900009055475 URL |
| [63] | Amin, M.; Abdel-Fattah, M.; Zaghloul, S. S. Magnesium concentration in acute asthmatic children. Iran J Pediatr. 2012, 22, 463-467. |
| [64] |
Hashim Ali Hussein, S.; Nielsen, L. P.; Konow Bøgebjerg Dolberg, M.; Dahl, R. Serum magnesium and not vitamin D is associated with better QoL in COPD: A cross-sectional study. Respir Med. 2015, 109, 727-733.
doi: 10.1016/j.rmed.2015.03.005 URL |
| [65] | Gumus, A.; Haziroglu, M.; Gunes, Y. Association of serum magnesium levels with frequency of acute exacerbations in chronic obstructive pulmonary disease: a prospective study. Pulm Med. 2014, 2014, 329476. |
| [66] |
Niinomi, M. Metallic biomaterials. J Artif Organs. 2008, 11, 105-110.
doi: 10.1007/s10047-008-0422-7 URL |
| [67] |
Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888-3903.
doi: 10.1016/j.actbio.2012.06.037 URL |
| [68] |
Chen, J.; Tan, L.; Yu, X.; Etim, I. P.; Ibrahim, M.; Yang, K. Mechanical properties of magnesium alloys for medical application: a review. J Mech Behav Biomed Mater. 2018, 87, 68-79.
doi: 10.1016/j.jmbbm.2018.07.022 URL |
| [69] |
Staiger, M. P.; Pietak, A. M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: a review. Biomaterials. 2006, 27, 1728-1734.
doi: 10.1016/j.biomaterials.2005.10.003 URL |
| [70] | Liu, C.; Ren, Z.; Xu, Y.; Pang, S.; Zhao, X.; Zhao, Y. Biodegradable magnesium alloys developed as bone repair materials: a review. Scanning. 2018, 2018, 9216314. |
| [71] |
Ding, W. Opportunities and challenges for the biodegradable magnesium alloys as next-generation biomaterials. Regen Biomater. 2016, 3, 79-86.
doi: 10.1093/rb/rbw003 URL |
| [72] |
Kirkland, N. T.; Birbilis, N.; Staiger, M. P. Assessing the corrosion of biodegradable magnesium implants: a critical review of current methodologies and their limitations. Acta Biomater. 2012, 8, 925-936.
doi: 10.1016/j.actbio.2011.11.014 URL |
| [73] |
Kawasaki, H.; Guan, J.; Tamama, K. Hydrogen gas treatment prolongs replicative lifespan of bone marrow multipotential stromal cells in vitro while preserving differentiation and paracrine potentials. Biochem Biophys Res Commun. 2010, 397, 608-613.
doi: 10.1016/j.bbrc.2010.06.009 URL |
| [74] |
Kamrani, S.; Fleck, C. Biodegradable magnesium alloys as temporary orthopaedic implants: a review. Biometals. 2019, 32, 185-193.
doi: 10.1007/s10534-019-00170-y URL |
| [75] |
Jang, Y.; Collins, B.; Sankar, J.; Yun, Y. Effect of biologically relevant ions on the corrosion products formed on alloy AZ31B: an improved understanding of magnesium corrosion. Acta Biomater. 2013, 9, 8761-8770.
doi: 10.1016/j.actbio.2013.03.026 URL |
| [76] |
Wagener, V.; Faltz, A. S.; Killian, M. S.; Schmuki, P.; Virtanen, S. Protein interactions with corroding metal surfaces: comparison of Mg and Fe. Faraday Discuss. 2015, 180, 347-360.
doi: 10.1039/C4FD00253A URL |
| [77] | Bobby Kannan, M.; Singh Raman, R. K.; Witte, F.; Blawert, C.; Dietzel, W. Influence of circumferential notch and fatigue crack on the mechanical integrity of biodegradable magnesium-based alloy in simulated body fluid. J Biomed Mater Res BAppl Biomater. 2011, 96, 303-309. |
| [78] |
Kuhlmann, J.; Bartsch, I.; Willbold, E.; Schuchardt, S.; Holz, O.; Hort, N.; Höche, D.; Heineman, W. R.; Witte, F. Fast escape of hydrogen from gas cavities around corroding magnesium implants. Acta Biomater. 2013, 9, 8714-8721.
doi: 10.1016/j.actbio.2012.10.008 URL |
| [79] |
Yang, J.; Koons, G. L.; Cheng, G.; Zhao, L.; Mikos, A. G.; Cui, F. A review on the exploitation of biodegradable magnesium-based composites for medical applications. Biomed Mater. 2018, 13, 022001.
doi: 10.1088/1748-605X/aa8fa0 URL |
| [80] |
Taguchi, T.; Lopez, M. J. An overview of de novo bone generation in animal models. J Orthop Res. 2021, 39, 7-21.
doi: 10.1002/jor.v39.1 URL |
| [81] |
Bigham-Sadegh, A.; Oryan, A. Selection of animal models for pre-clinical strategies in evaluating the fracture healing, bone graft substitutes and bone tissue regeneration and engineering. Connect Tissue Res. 2015, 56, 175-194.
doi: 10.3109/03008207.2015.1027341 URL |
| [82] |
Pfeiffenberger, M.; Damerau, A.; Lang, A.; Buttgereit, F.; Hoff, P.; Gaber, T. Fracture healing research-shift towards in vitro modeling? Biomedicines. 2021, 9, 748.
doi: 10.3390/biomedicines9070748 URL |
| [83] |
Spicer, P. P.; Kretlow, J. D.; Young, S.; Jansen, J. A.; Kasper, F. K.; Mikos, A. G. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat Protoc. 2012, 7, 1918-1929.
doi: 10.1038/nprot.2012.113 URL |
| [84] |
Dubey, N.; Ferreira, J. A.; Malda, J.; Bhaduri, S. B.; Bottino, M. C. Extracellular matrix/amorphous magnesium phosphate bioink for 3D bioprinting of craniomaxillofacial bone tissue. ACS Appl Mater Interfaces. 2020, 12, 23752-23763.
doi: 10.1021/acsami.0c05311 URL |
| [85] |
Yuan, Z.; Wei, P.; Huang, Y.; Zhang, W.; Chen, F.; Zhang, X.; Mao, J.; Chen, D.; Cai, Q.; Yang, X. Injectable PLGA microspheres with tunable magnesium ion release for promoting bone regeneration. Acta Biomater. 2019, 85, 294-309.
doi: 10.1016/j.actbio.2018.12.017 URL |
| [86] | Gomes, P. S.; Fernandes, M. H. Rodent models in bone-related research: the relevance of calvarial defects in the assessment of bone regeneration strategies. Lab Anim. 2011, 45, 14-24. |
| [87] |
Fang, B.; Qiu, P.; Xia, C.; Cai, D.; Zhao, C.; Chen, Y.; Wang, H.; Liu, S.; Cheng, H.; Tang, Z.; Wang, B.; Fan, S.; Lin, X. Extracellular matrix scaffold crosslinked with vancomycin for multifunctional antibacterial bone infection therapy. Biomaterials. 2021, 268, 120603.
doi: 10.1016/j.biomaterials.2020.120603 URL |
| [88] | Dong, Y.; Liu, W.; Lei, Y.; Wu, T.; Zhang, S.; Guo, Y.; Liu, Y.; Chen, D.; Yuan, Q.; Wang, Y. Effect of gelatin sponge with colloid silver on bone healing in infected cranial defects. Mater Sci Eng CMater Biol Appl. 2017, 70, 371-377. |
| [89] |
Reichert, J. C.; Saifzadeh, S.; Wullschleger, M. E.; Epari, D. R.; Schütz, M. A.; Duda, G. N.; Schell, H.; van Griensven, M.; Redl, H.; Hutmacher, D. W. The challenge of establishing preclinical models for segmental bone defect research. Biomaterials. 2009, 30, 2149-2163.
doi: 10.1016/j.biomaterials.2008.12.050 URL |
| [90] |
Gunderson, Z. J.; Campbell, Z. R.; McKinley, T. O.; Natoli, R. M.; Kacena, M. A. A comprehensive review of mouse diaphyseal femur fracture models. Injury. 2020, 51, 1439-1447.
doi: 10.1016/j.injury.2020.04.011 URL |
| [91] |
den Boer, F. C.; Patka, P.; Bakker, F. C.; Wippermann, B. W.; van Lingen, A.; Vink, G. Q.; Boshuizen, K.; Haarman, H. J. New segmental long bone defect model in sheep: quantitative analysis of healing with dual energy x-ray absorptiometry. J Orthop Res. 1999, 17, 654-660.
doi: 10.1002/(ISSN)1554-527X URL |
| [92] | Christou, C.; Oliver, R. A.; Pelletier, M. H.; Walsh, W. R. Ovine model for critical-size tibial segmental defects. Comp Med. 2014, 64, 377-385. |
| [93] |
Gugala, Z.; Lindsey, R. W.; Gogolewski, S. New Approaches in the treatment of critical-size segmental defects in long bones. Macromol Symp. 2007, 253, 147-161.
doi: 10.1002/(ISSN)1521-3900 URL |
| [94] |
McKinley, T.; O.; Natoli, R. M.; Fischer, J. P.; Rytlewski, J. D.; Scofield, D. C.; Usmani, R.; Kuzma, A.; Griffin, K. S.; Jewell, E.; Childress, P.; Shively, K. D.; Chu, T. G.; Anglen, J. O.; Kacena, M. A. Internal fixation construct and defect size affect healing of a translational porcine diaphyseal tibial segmental bone defect. Mil Med. 2020. doi: 10.1093/milmed/usaa516.
doi: 10.1093/milmed/usaa516 URL |
| [95] |
Yavari, S. A.; van der Stok, J.; Ahmadi, S. M.; Wauthle, R.; Schrooten, J.; Weinans, H.; Zadpoor, A. A. Mechanical analysis of a rodent segmental bone defect model: the effects of internal fixation and implant stiffness on load transfer. J Biomech. 2014, 47, 2700-2708.
doi: 10.1016/j.jbiomech.2014.05.006 URL |
| [96] |
Perren, S. M. Evolution of the internal fixation of long bone fractures. The scientific basis of biological internal fixation: choosing a new balance between stability and biology. J Bone Joint Surg Br. 2002, 84, 1093-1110.
doi: 10.1302/0301-620X.84B8.0841093 URL |
| [97] |
Bottagisio, M.; Coman, C.; Lovati, A. B. Animal models of orthopaedic infections. A review of rabbit models used to induce long bone bacterial infections. J Med Microbiol. 2019, 68, 506-537.
doi: 10.1099/jmm.0.000952 URL |
| [98] |
Roux, K. M.; Cobb, L. H.; Seitz, M. A.; Priddy, L. B. Innovations in osteomyelitis research: A review of animal models. Animal Model Exp Med. 2021, 4, 59-70.
doi: 10.1002/ame2.12149 URL |
| [99] |
Couly, G. F.; Coltey, P. M.; Le Douarin, N. M. The triple origin of skull in higher vertebrates: a study in quail-chick chimeras. Development. 1993, 117, 409-429.
doi: 10.1242/dev.117.2.409 URL |
| [100] |
Reichert, J. C.; Gohlke, J.; Friis, T. E.; Quent, V. M.; Hutmacher, D. W. Mesodermal and neural crest derived ovine tibial and mandibular osteoblasts display distinct molecular differences. Gene. 2013, 525, 99-106.
doi: 10.1016/j.gene.2013.04.026 URL |
| [101] |
Liu, G.; Guo, Y.; Zhang, L.; Wang, X.; Liu, R.; Huang, P.; Xiao, Y.; Chen, Z.; Chen, Z. A standardized rat burr hole defect model to study maxillofacial bone regeneration. Acta Biomater. 2019, 86, 450-464.
doi: 10.1016/j.actbio.2018.12.049 URL |
| [102] |
Waksman, R.; Erbel, R.; Di Mario, C.; Bartunek, J.; de Bruyne, B.; Eberli, F. R.; Erne, P.; Haude, M.; Horrigan, M.; Ilsley, C.; Böse, D.; Bonnier, H.; Koolen, J.; Lüscher, T. F.; Weissman, N. J.; PROGRESS-AMS (clinical Performance angiographic results of coronary stenting with absorbable metal stents) Investigators. Early- and long-term intravascular ultrasound and angiographic findings after bioabsorbable magnesium stent implantation in human coronary arteries. JACC Cardiovasc Interv. 2009, 2, 312-320.
doi: 10.1016/j.jcin.2008.09.015 URL |
| [103] |
Cui, H. K.; Li, F. B.; Guo, Y. C.; Zhao, Y. L.; Yan, R. F.; Wang, W.; Li, Y. D.; Wang, Y. L.; Yuan, G. Y. Intermediate analysis of magnesium alloy covered stent for a lateral aneurysm model in the rabbit common carotid artery. Eur Radiol. 2017, 27, 3694-3702.
doi: 10.1007/s00330-016-4715-6 URL |
| [104] |
Field, J. R.; Ruthenbeck, G. R. Qualitative and quantitative radiological measures of fracture healing. Vet Comp Orthop Traumatol. 2018, 31, 1-9.
doi: 10.3415/VCOT-17-03-0042 URL |
| [105] |
Bissinger, O.; Kirschke, J. S.; Probst, F. A.; Stauber, M.; Wolff, K. D.; Haller, B.; Götz, C.; Plank, C.; Kolk, A. Micro-CT vs. whole body multirow detector CT for analysing bone regeneration in an Animal model. PLoS One. 2016, 11, e0166540.
doi: 10.1371/journal.pone.0166540 URL |
| [106] |
Pennington, Z.; Ehresman, J.; Lubelski, D.; Cottrill, E.; Schilling, A.; Ahmed, A. K.; Feghali, J.; Witham, T. F.; Sciubba, D. M. Assessing underlying bone quality in spine surgery patients: a narrative review of dual-energy X-ray absorptiometry (DXA) and alternatives. Spine J. 2021, 21, 321-331.
doi: 10.1016/j.spinee.2020.08.020 URL |
| [107] |
Messina, C.; Sconfienza, L. M.; Bandirali, M.; Guglielmi, G.; Ulivieri, F. M. Adult dual-energy X-ray absorptiometry in clinical practice: how I report it. Semin Musculoskelet Radiol. 2016, 20, 246-253.
doi: 10.1055/s-0036-1592370 URL |
| [108] |
Schwarzenberg, P.; Darwiche, S.; Yoon, R. S.; Dailey, H. L. Imaging modalities to assess fracture healing. Curr Osteoporos Rep. 2020, 18, 169-179.
doi: 10.1007/s11914-020-00584-5 URL |
| [109] |
Martín-Badosa, E.; Amblard, D.; Nuzzo, S.; Elmoutaouakkil, A.; Vico, L.; Peyrin, F. Excised bone structures in mice: imaging at three-dimensional synchrotron radiation micro CT. Radiology. 2003, 229, 921-928.
doi: 10.1148/radiol.2293020558 URL |
| [110] |
Irie, M. S.; Rabelo, G. D.; Spin-Neto, R.; Dechichi, P.; Borges, J. S.; Soares, P. B. F. Use of micro-computed tomography for bone evaluation in dentistry. Braz Dent J. 2018, 29, 227-238.
doi: 10.1590/0103-6440201801979 URL |
| [111] |
Só, B. B.; Silveira, F. M.; Llantada, G. S.; Jardim, L. C.; Calcagnotto, T.; Martins, M. A. T.; Martins, M. D. Effects of osteoporosis on alveolar bone repair after tooth extraction: a systematic review of preclinical studies. Arch Oral Biol. 2021, 125, 105054.
doi: 10.1016/j.archoralbio.2021.105054 URL |
| [112] |
Rousselle, S. D.; Wicks, J. R.; Tabb, B. C.; Tellez, A.; O’Brien, M. Histology strategies for medical implants and interventional device studies. Toxicol Pathol. 2019, 47, 235-249.
doi: 10.1177/0192623319827288 URL |
| [113] |
Lim, S.; Kim, J. A.; Lee, T.; Lee, D.; Nam, S. H.; Lim, J.; Park, E. K. Stimulatory effects of KPR-A148 on osteoblast differentiation and bone regeneration. Tissue Eng Regen Med. 2019, 16, 405-413.
doi: 10.1007/s13770-019-00200-3 URL |
| [114] |
Jeong, J. H.; Jin, E. S.; Kim, J. Y.; Lee, B.; Min, J.; Jeon, S. R.; Lee, M.; Choi, K. H. The effect of biocomposite screws on bone regeneration in a rat osteoporosis model. World Neurosurg. 2017, 106, 964-972.
doi: 10.1016/j.wneu.2017.07.083 URL |
| [115] |
Hu, J.; Zhou, J.; Wu, J.; Chen, Q.; Du, W.; Fu, F.; Yu, H.; Yao, S.; Jin, H.; Tong, P.; Chen, D.; Wu, C.; Ruan, H. Loganin ameliorates cartilage degeneration and osteoarthritis development in an osteoarthritis mouse model through inhibition of NF-κB activity and pyroptosis in chondrocytes. J Ethnopharmacol. 2020, 247, 112261.
doi: 10.1016/j.jep.2019.112261 URL |
| [116] | Huang, Y. Combined treatment of vitamin K and teriparatide on bone metabolism and biomechanics in rats with osteoporosis. Exp Ther Med. 2018, 15, 315-319. |
| [117] |
Friedemann, M. C.; Mehta, N. A.; Jessen, S. L.; Charara, F. H.; Ginn-Hedman, A. M.; Kaulfus, C. N.; Brocklesby, B. F.; Robinson, C. B.; Jokerst, S.; Glowczwski, A.; Clubb, F. J.; Jr, .; Weeks, B. R. Introduction to currently applied device pathology. Toxicol Pathol. 2019, 47, 221-234.
doi: 10.1177/0192623319826585 URL |
| [118] |
Jackson, N.; Assad, M.; Vollmer, D.; Stanley, J.; Chagnon, M. Histopathological evaluation of orthopedic medical devices: the state-of-the-art in animal Models, imaging, and histomorphometry techniques. Toxicol Pathol. 2019, 47, 280-296.
doi: 10.1177/0192623318821083 URL |
| [119] | Hamushan, M.; Cai, W.; Zhang, Y.; Ren, Z.; Du, J.; Zhang, S.; Zhao, C.; Cheng, P.; Zhang, X.; Shen, H.; Han, P. High-purity magnesium pin enhances bone consolidation in distraction osteogenesis via regulating Ptch protein activating Hedgehog-alternative Wnt signaling. Bioact Mater. 2021, 6, 1563-1574. |
| [120] |
Darouiche, R. O. Treatment of infections associated with surgical implants. N Engl J Med. 2004, 350, 1422-1429.
doi: 10.1056/NEJMra035415 URL |
| [121] |
Robinson, D. A.; Griffith, R. W.; Shechtman, D.; Evans, R. B.; Conzemius, M. G. In vitro antibacterial properties of magnesium metal against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. Acta Biomater. 2010, 6, 1869-1877.
doi: 10.1016/j.actbio.2009.10.007 URL |
| [122] |
Li, Y.; Liu, G.; Zhai, Z.; Liu, L.; Li, H.; Yang, K.; Tan, L.; Wan, P.; Liu, X.; Ouyang, Z.; Yu, Z.; Tang, T.; Zhu, Z.; Qu, X.; Dai, K. Antibacterial properties of magnesium in vitro and in an in vivo model of implant-associated methicillin-resistant Staphylococcus aureus infection. Antimicrob Agents Chemother. 2014, 58, 7586-7591.
doi: 10.1128/AAC.03936-14 URL |
| [123] |
He, W.; Zhang, H.; Qiu, J. Osteogenic effects of bioabsorbable magnesium implant in rat mandibles and in vitro. J Periodontol. 2021, 92, 1181-1191.
doi: 10.1002/jper.v92.8 URL |
| [124] | Berglund, I. S.; Jacobs, B. Y.; Allen, K. D.; Kim, S. E.; Pozzi, A.; Allen, J. B.; Manuel, M. V. Peri-implant tissue response and biodegradation performance of a Mg-1.0Ca-0.5Sr alloy in rat tibia. Mater Sci Eng CMater Biol Appl. 2016, 62, 79-85. |
| [125] | Tie, D.; Feyerabend, F.; Müller, W. D.; Schade, R.; Liefeith, K.; Kainer, K. U.; Willumeit, R. Antibacterial biodegradable Mg-Ag alloys. Eur Cell Mater. 2013, 25, 284-298; discussion 298. |
| [126] |
Jähn, K.; Saito, H.; Taipaleenmäki, H.; Gasser, A.; Hort, N.; Feyerabend, F.; Schlüter, H.; Rueger, J. M.; Lehmann, W.; Willumeit-Römer, r.; Hesse, E. Intramedullary Mg2Ag nails augment callus formation during fracture healing in mice. Acta Biomater. 2016, 36, 350-360.
doi: 10.1016/j.actbio.2016.03.041 URL |
| [127] |
Yoshizawa, S.; Chaya, A.; Verdelis, K.; Bilodeau, E. A.; Sfeir, C. An in vivo model to assess magnesium alloys and their biological effect on human bone marrow stromal cells. Acta Biomater. 2015, 28, 234-239.
doi: 10.1016/j.actbio.2015.08.037 URL |
| [128] |
Han, P.; Cheng, P.; Zhang, S.; Zhao, C.; Ni, J.; Zhang, Y.; Zhong, W.; Hou, P.; Zhang, X.; Zheng, Y.; Chai, Y. In vitro and in vivo studies on the degradation of high-purity Mg (99.99wt.%) screw with femoral intracondylar fractured rabbit model. Biomaterials. 2015, 64, 57-69.
doi: 10.1016/j.biomaterials.2015.06.031 URL |
| [129] |
Hung, C. C.; Chaya, A.; Liu, K.; Verdelis, K.; Sfeir, C. The role of magnesium ions in bone regeneration involves the canonical Wnt signaling pathway. Acta Biomater. 2019, 98, 246-255.
doi: 10.1016/j.actbio.2019.06.001 URL |
| [130] |
Wang, J.; Xu, J.; Song, B.; Chow, D. H.; Shu-Hang Yung, P.; Qin, l. Magnesium (Mg) based interference screws developed for promoting tendon graft incorporation in bone tunnel in rabbits. Acta Biomater. 2017, 63, 393-410.
doi: 10.1016/j.actbio.2017.09.018 URL |
| [131] |
Li, Y.; Liu, L.; Wan, P.; Zhai, Z.; Mao, Z.; Ouyang, Z.; Yu, D.; Sun, Q.; Tan, L.; Ren, L.; Zhu, Z.; Hao, Y.; Qu, X.; Yang, K.; Dai, K. Biodegradable Mg-Cu alloy implants with antibacterial activity for the treatment of osteomyelitis: In vitro and in vivo evaluations. Biomaterials. 2016, 106, 250-263.
doi: 10.1016/j.biomaterials.2016.08.031 URL |
| [132] |
Jiang, Y.; Wang, B.; Jia, Z.; Lu, X.; Fang, L.; Wang, K.; Ren, F. Polydopamine mediated assembly of hydroxyapatite nanoparticles and bone morphogenetic protein-2 on magnesium alloys for enhanced corrosion resistance and bone regeneration. J Biomed Mater Res A. 2017, 105, 2750-2761.
doi: 10.1002/jbm.a.v105.10 URL |
| [133] |
Grün, N. G.; Holweg, P.; Tangl, S.; Eichler, J.; Berger, L.; van den Beucken, J.; Löffler, J. F.; Klestil, T.; Weinberg, A. M. Comparison of a resorbable magnesium implant in small and large growing-animal models. Acta Biomater. 2018, 78, 378-386.
doi: 10.1016/j.actbio.2018.07.044 URL |
| [134] | Marukawa, E.; Tamai, M.; Takahashi, Y.; Hatakeyama, I.; Sato, M.; Higuchi, Y.; Kakidachi, H.; Taniguchi, H.; Sakamoto, T.; Honda, J.; Omura, K.; Harada, H. Comparison of magnesium alloys and poly-l-lactide screws as degradable implants in a canine fracture model. J Biomed Mater Res BAppl Biomater. 2016, 104, 1282-1289. |
| [135] |
Wang, S.; Liu, Y.; Fang, D.; Shi, S. The miniature pig: a useful large animal model for dental and orofacial research. Oral Dis. 2007, 13, 530-537.
doi: 10.1111/odi.2007.13.issue-6 URL |
| [136] | Echeverry-Rendon, M.; Allain, J. P.; Robledo, S. M.; Echeverria, F.; Harmsen, M. C. Coatings for biodegradable magnesium-based supports for therapy of vascular disease: A general view. Mater Sci Eng CMater Biol Appl. 2019, 102, 150-163. |
| [137] |
Zartner, P. A.; Schranz, D.; Mini, N.; Schneider, M. B.; Schneider, K. Acute treatment of critical vascular stenoses with a bioabsorbable magnesium scaffold in infants with CHDs. Cardiol Young. 2020, 30, 493-499.
doi: 10.1017/S1047951120000384 URL |
| [138] |
Blachutzik, F.; Achenbach, S.; Tröbs, M.; Marwan, M.; Weissner, M.; Nef, H.; Schlundt, C. Effect of non-compliant balloon postdilatation on magnesium-based bioresorbable vascular scaffolds. Catheter Cardiovasc Interv. 2019, 93, 202-207.
doi: 10.1002/ccd.v93.2 URL |
| [139] |
Li, H.; Zhong, H.; Xu, K.; Yang, K.; Liu, J.; Zhang, B.; Zheng, F.; Xia, Y.; Tan, L.; Hong, D. Enhanced efficacy of sirolimus-eluting bioabsorbable magnesium alloy stents in the prevention of restenosis. J Endovasc Ther. 2011, 18, 407-415.
doi: 10.1583/10-3353.1 URL |
| [140] |
Bowen, P. K.; Drelich, A.; Drelich, J.; Goldman, J. Rates of in vivo (arterial) and in vitro biocorrosion for pure magnesium. J Biomed Mater Res A. 2015, 103, 341-349.
doi: 10.1002/jbm.a.v103.1 URL |
| [141] |
Waksman, R.; Pakala, R.; Kuchulakanti, P. K.; Baffour, R.; Hellinga, D.; Seabron, R.; Tio, F. O.; Wittchow, E.; Hartwig, S.; Harder, C.; Rohde, R.; Heublein, B.; Andreae, A.; Waldmann, K. H.; Haverich, A. Safety and efficacy of bioabsorbable magnesium alloy stents in porcine coronary arteries Catheter Cardiovasc Interv. 2006, 68, 607-617; discussion 618-619.
doi: 10.1002/(ISSN)1522-726X URL |
| [142] |
Heublein, B.; Rohde, R.; Kaese, V.; Niemeyer, M.; Hartung, W.; Haverich, A. Biocorrosion of magnesium alloys: a new principle in cardiovascular implant technology? Heart. 2003, 89, 651-656.
doi: 10.1136/heart.89.6.651 URL |
| [143] |
Zhu, J.; Zhang, X.; Niu, J.; Shi, Y.; Zhu, Z.; Dai, D.; Chen, C.; Pei, J.; Yuan, G.; Zhang, R. Biosafety and efficacy evaluation of a biodegradable magnesium-based drug-eluting stent in porcine coronary artery. Sci Rep. 2021, 11, 7330.
doi: 10.1038/s41598-021-86803-0 URL |
| [144] | Shi, Y.; Zhang, L.; Chen, J.; Zhang, J.; Yuan, F.; Shen, L.; Chen, C.; Pei, J.; Li, Z.; Tan, J.; Yuan, G. In vitro and in vivo degradation of rapamycin-eluting Mg-Nd-Zn-Zr alloy stents in porcine coronary arteries. Mater Sci Eng CMater Biol Appl. 2017, 80, 1-6. |
| [145] |
Zhang, J.; Li, H.; Wang, W.; Huang, H.; Pei, J.; Qu, H.; Yuan, G.; Li, Y. The degradation and transport mechanism of a Mg-Nd-Zn-Zr stent in rabbit common carotid artery: a 20-month study. Acta Biomater. 2018, 69, 372-384.
doi: 10.1016/j.actbio.2018.01.018 URL |
| [146] |
O’Loughlin, P. F.; Morr, S.; Bogunovic, L.; Kim, A. D.; Park, B.; Lane, J. M. Selection and development of preclinical models in fracture-healing research. J Bone Joint Surg Am. 2008, 90 Suppl 1, 79-84.
doi: 10.2106/JBJS.G.01585 URL |
| [147] |
Lang, A.; Schulz, A.; Ellinghaus, A.; Schmidt-Bleek, K. Osteotomy models - the current status on pain scoring and management in small rodents. Lab Anim. 2016, 50, 433-441.
doi: 10.1177/0023677216675007 URL |
| [148] |
Haffner-Luntzer, M.; Hankenson, K. D.; Ignatius, A.; Pfeifer, R.; Khader, B. A.; Hildebrand, F.; van Griensven, M.; Pape, H. C.; Lehmicke, M. Review of animal models of comorbidities in fracture-healing research. J Orthop Res. 2019, 37, 2491-2498.
doi: 10.1002/jor.v37.12 URL |
| [149] | Decker, S.; Reifenrath, J.; Omar, M.; Krettek, C.; Müller, C. W. Non-osteotomy and osteotomy large animal fracture models in orthopedic trauma research. Orthop Rev (Pavia). 2014, 6, 5575. |
| [150] | Sun, Y.; Wu, H.; Wang, W.; Zan, R.; Peng, H.; Zhang, S.; Zhang, X. Translational status of biomedical Mg devices in China. Bioact Mater. 2019, 4, 358-365. |
| [1] | Yiqiang Hu, Yuan Xiong, Ranyang Tao, Hang Xue, Lang Chen, Ze Lin, Adriana C. Panayi, Bobin Mi, Guohui Liu. Advances and perspective on animal models and hydrogel biomaterials for diabetic wound healing [J]. Biomaterials Translational, 2022, 3(3): 188-200. |
| [2] | Chavee Laomeephol, Helena Ferreira, Sorada Kanokpanont, Jittima Amie Luckanagul, Nuno M Neves, Siriporn Damrongsakkul. Osteogenic differentiation of encapsulated cells in dexamethasone–loaded phospholipid–induced silk fibroin hydrogels [J]. Biomaterials Translational, 2022, 3(3): 213-220. |
| [3] | Ricardo Donate, Maryam Tamaddon, Viviana Ribeiro, Mario Monzón, J. Miguel Oliveira, Chaozong Liu. Translation through collaboration: practice applied in BAMOS project in in vivo testing of innovative osteochondral scaffolds [J]. Biomaterials Translational, 2022, 3(2): 102-104. |
| [4] | Melika Sahranavard, Soulmaz Sarkari, SeyedehMina Safavi, Farnaz Ghorbani. Three-dimensional bio-printing of decellularized extracellular matrix-based bio-inks for cartilage regeneration: a systematic review [J]. Biomaterials Translational, 2022, 3(2): 105-115. |
| [5] | Emma Steijvers, Armaan Ghei, Zhidao Xia. Manufacturing artificial bone allografts: a perspective [J]. Biomaterials Translational, 2022, 3(1): 65-80. |
| [6] | Ke Hu, Yuxuan Li, Zunxiang Ke, Hongjun Yang, Chanjun Lu, Yiqing Li, Yi Guo, Weici Wang. History, progress and future challenges of artificial blood vessels: a narrative review [J]. Biomaterials Translational, 2022, 3(1): 81-98. |
| [7] | Yizhong Peng, Jinye Li, Hui Lin, Shuo Tian, Sheng Liu, Feifei Pu, Lei Zhao, Kaige Ma, Xiangcheng Qing, Zengwu Shao. Endogenous repair theory enriches construction strategies for orthopaedic biomaterials: a narrative review [J]. Biomaterials Translational, 2021, 2(4): 343-360. |
| [8] | Ying Luo, Jue Wang, Michael Tim Yun Ong, Patrick Shu-hang Yung, Jiali Wang, Ling Qin. Update on the research and development of magnesium-based biodegradable implants and their clinical translation in orthopaedics [J]. Biomaterials Translational, 2021, 2(3): 188-196. |
| [9] | Yu Lu, Subodh Deshmukh, Ian Jones, Yu-Lung Chiu. Biodegradable magnesium alloys for orthopaedic applications [J]. Biomaterials Translational, 2021, 2(3): 214-235. |
| [10] | Jialin Niu, Hua Huang, Jia Pei, Zhaohui Jin, Shaokang Guan, Guangyin Yuan. Research and development strategy for biodegradable magnesium-based vascular stents: a review [J]. Biomaterials Translational, 2021, 2(3): 236-247. |
| [11] | Qingchuan Wang, Weidan Wang, Yanfang Li, Weirong Li, Lili Tan, Ke Yang. Biofunctional magnesium coating of implant materials by physical vapour deposition [J]. Biomaterials Translational, 2021, 2(3): 248-256. |
| [12] | Aditya Joshi, George Dias, Mark P. Staiger. In silico modelling of the corrosion of biodegradable magnesium-based biomaterials: modelling approaches, validation and future perspectives [J]. Biomaterials Translational, 2021, 2(3): 257-271. |
| [13] | Jing Long, Bin Teng, Wei Zhang, Long Li, Ming Zhang, Yingqi Chen, Zhenyu Yao, Xiangbo Meng, Xinluan Wang, Ling Qin, Yuxiao Lai. Preclinical evaluation of acute systemic toxicity of magnesium incorporated poly(lactic-co-glycolic acid) porous scaffolds by three-dimensional printing [J]. Biomaterials Translational, 2021, 2(3): 272-284. |
| [14] | Yiqing Wang, Xiangyu Chu, Bing Wang. Recombinant adeno-associated virus-based gene therapy combined with tissue engineering for musculoskeletal regenerative medicine [J]. Biomaterials Translational, 2021, 2(1): 19-29. |
| [15] | Maryam Tamaddon, Helena Gilja, Ling Wang, J. Miguel Oliveira, Xiaodan Sun, Rongwei Tan, Chaozong Liu. Osteochondral scaffolds for early treatment of cartilage defects in osteoarthritic joints: from bench to clinic [J]. Biomaterials Translational, 2020, 1(1): 3-17. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||