Biomaterials Translational ›› 2022, Vol. 3 ›› Issue (1): 81-98.doi: 10.12336/biomatertransl.2022.01.008
• REVIEW • Previous Articles
Ke Hu1,#, Yuxuan Li1,#, Zunxiang Ke2, Hongjun Yang3, Chanjun Lu1, Yiqing Li1, Yi Guo1,4,*( ), Weici Wang1,*(
), Weici Wang1,*( )
)
Received:2022-01-22
Revised:2022-02-24
Accepted:2022-03-01
Online:2022-03-28
Published:2022-03-28
Contact:
Yi Guo,hxshuyun@hotmail.com;Weici Wang,weiciwang@gmail.com.
About author:Yi Guo, hxshuyun@hotmail.com;Hu, K.; Li, Y.; Ke, Z.; Yang, H.; Lu, C.; Li, Y.; Guo, Y.; Wang, W. History, progress and future challenges of artificial blood vessels: a narrative review. Biomater Transl. 2022, 3(1), 81-98.
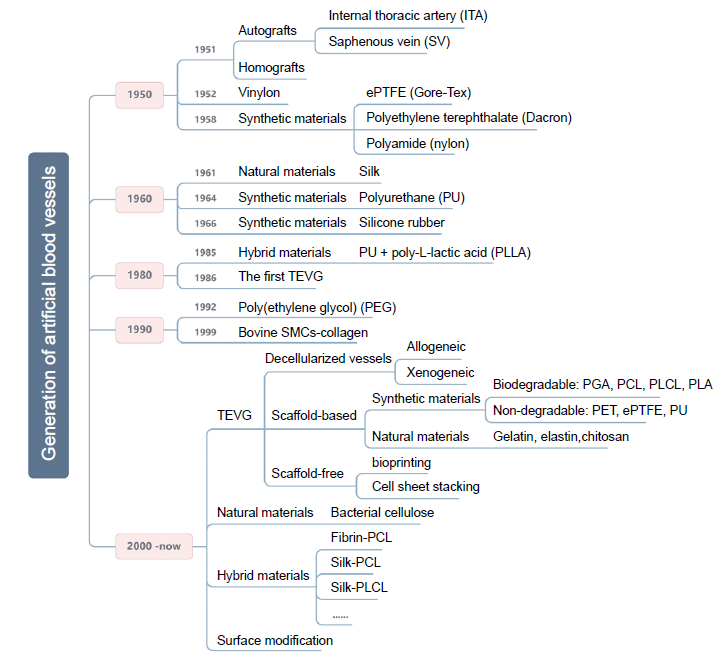
Figure 1. The generation of artificial blood vessels. A few significant time points in the generation of artificial blood vessels and the main research areas are shown. ePTFE: expanded polytetrafluoroethylene; PCL: polycaprolactone; PGA: poly(glycolic acid); PLA: polylactic acid; PLCL: poly(L-lactide-co-caprolactone). Created with Biorender.com.
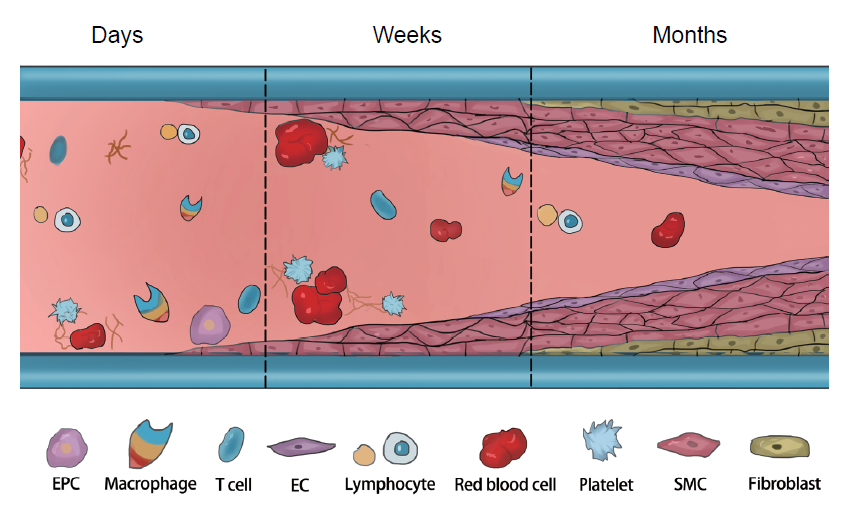
Figure 3. The challenges after vascular graft implantation. After implantation, insufficient endothelialisation and aggressive proliferation of SMCs over time can lead to IH and thrombosis. Inflammatory cells play an important role in regulating the functions of ECs and SMCs. EC: endothelial cell; EPC: endothelial progenitor cell; IH: intimal hyperplasia; SMC: smooth muscle cells. Created with Biorender.com.
| Vessel type | Materials | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Synthetic polymers | ePTFE (Gore-Tex), PET (Dacron), PU, PCL, PLCL, PLA, PGS. | Excellent mechanical properties. Easy availability. Mass production. Easy surgical suturing. Preventing vascular burst. Can be stored for off-the-shelf use. | Causes thrombosis, intimal hyperplasia, calcification, and chronic inflammation. No growth potential. Poor haemocompatibility. Compliance mismatch. | |
| Natural biomaterials | Silk fibroin, collagen, elastin, chitosan, bacterial cellulose | Excellent biocompatibility. Enhanced biological signalling. Tunable mechanical properties. | Weak mechanical strength. Causes vascular graft dilation and aneurysms. Easy degradation. Overly complex designs. Difficulty in translation. | |
| Decellularised vessels | Animal artery, umbilical artery, umbilical vein | Low immunogenicity. Preserved extracellular matrix, meso- and microvasculature. | Increased thrombogenicity. Host immune response. Difficulty in precise recellularisation. Calcification. |
Table 1 Advantages and disadvantages of artificial blood vessels in different materials
| Vessel type | Materials | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Synthetic polymers | ePTFE (Gore-Tex), PET (Dacron), PU, PCL, PLCL, PLA, PGS. | Excellent mechanical properties. Easy availability. Mass production. Easy surgical suturing. Preventing vascular burst. Can be stored for off-the-shelf use. | Causes thrombosis, intimal hyperplasia, calcification, and chronic inflammation. No growth potential. Poor haemocompatibility. Compliance mismatch. | |
| Natural biomaterials | Silk fibroin, collagen, elastin, chitosan, bacterial cellulose | Excellent biocompatibility. Enhanced biological signalling. Tunable mechanical properties. | Weak mechanical strength. Causes vascular graft dilation and aneurysms. Easy degradation. Overly complex designs. Difficulty in translation. | |
| Decellularised vessels | Animal artery, umbilical artery, umbilical vein | Low immunogenicity. Preserved extracellular matrix, meso- and microvasculature. | Increased thrombogenicity. Host immune response. Difficulty in precise recellularisation. Calcification. |
| Artery diameter | Usage sites | Indications | Main challenges | Commercial materials | |
|---|---|---|---|---|---|
| Large | > 8 mm | Aortoiliac arteries. | Open aortic aneurysm repair. Coarctation of the aorta. | Weak mechanical durability. Compliance mismatch. Aneurysmal-like dilation. | Non-degradable materials: PET, ePTFE |
| Medium | 6–8 mm | Carotid artery. Femoral artery. | Similar to large | Similar to large | Similar to large |
| Small | < 6 mm | Coronary arteries. Infrainguinal arteries (below the inguinal ligament). Infrageniculate arteries (below the knee). Coronary artery bypass. | Arteriovenous shunts.Coronary artery bypass. Peripheral arterial occlusive disease. | Stenosis/occlusion caused by thrombosis or intimal hyperplasia. Low haemocompatibility. | Autologous vessels: ITA, SV |
Table 2 Artificial blood vessels of different diameters
| Artery diameter | Usage sites | Indications | Main challenges | Commercial materials | |
|---|---|---|---|---|---|
| Large | > 8 mm | Aortoiliac arteries. | Open aortic aneurysm repair. Coarctation of the aorta. | Weak mechanical durability. Compliance mismatch. Aneurysmal-like dilation. | Non-degradable materials: PET, ePTFE |
| Medium | 6–8 mm | Carotid artery. Femoral artery. | Similar to large | Similar to large | Similar to large |
| Small | < 6 mm | Coronary arteries. Infrainguinal arteries (below the inguinal ligament). Infrageniculate arteries (below the knee). Coronary artery bypass. | Arteriovenous shunts.Coronary artery bypass. Peripheral arterial occlusive disease. | Stenosis/occlusion caused by thrombosis or intimal hyperplasia. Low haemocompatibility. | Autologous vessels: ITA, SV |
| Burst pressure (mmHg) | Compliance (%/100 mmHg) | |
|---|---|---|
| Values for artificial blood vessels | >1000 | 10-20 |
| Dog femoral artery | 2895 ± 263 | 10.3 ± 2.3 |
| Human internal mammary artery | 3196 ± 1264 | 11.5 ± 3.9 |
| Biodegradable chitosan vascular grafts (with 4 mm inner diameter) | 1688 ± 236 | 5.7 ± 1.3 |
| Tissue-engineered blood vessels | 3490 ± 892 | 3.4 ± 1.6 |
| Expanded polytetrafluoroethylene grafts | – | 0.51 |
| Silk fibroin grafts | – | 1.90 |
Table 3 Mechanical properties of natural vessels and some artificial blood vessels.
| Burst pressure (mmHg) | Compliance (%/100 mmHg) | |
|---|---|---|
| Values for artificial blood vessels | >1000 | 10-20 |
| Dog femoral artery | 2895 ± 263 | 10.3 ± 2.3 |
| Human internal mammary artery | 3196 ± 1264 | 11.5 ± 3.9 |
| Biodegradable chitosan vascular grafts (with 4 mm inner diameter) | 1688 ± 236 | 5.7 ± 1.3 |
| Tissue-engineered blood vessels | 3490 ± 892 | 3.4 ± 1.6 |
| Expanded polytetrafluoroethylene grafts | – | 0.51 |
| Silk fibroin grafts | – | 1.90 |
| Animal model | Characteristics | Application inner diameter (mm) | Common length (mm) | Longest implantation period | Implantation site | References |
|---|---|---|---|---|---|---|
| Sheep | Similar cardiovascular physiology, endothelialisation mechanisms and thrombogenicity mechanisms to humans. Suitable size and long-term studies possible.Higher incidence of hypercoagulability. | 4–6 | 80–100 | 9 months | Carotid artery | |
| 4–6 | 60–100 | 3 months | Arteriovenous graft | |||
| Pig | Similar vascular physiology and anatomy to humans. Well established as a model for assessing vascular grafts. Mount an extensive immune response to implanted tissues. | 3–6 | 30–100 | 6 months | Iliac artery | |
| 3–6 | 10–100 | 4 weeks | Carotid artery | |||
| Dog | Lack of spontaneous endothelialisation and immune response restricts study lengths. Ease of accessing vessel due to thin skin. Thrombogenicity mechanisms and vessel viscoelastic properties differ from humans. | 3–6 | 30–50 | 6 months | Abdominal artery | |
| 3–6 | 30–50 | 1 year | Carotid artery | |||
| Baboon | Physiology and cardiovascular anatomy are the most similar to humans. Suitable for a wide range of non-invasive imaging techniques adapted from humans. High cost and ethical concerns associated with using primates in medical research. | 3–6 | 30–50 | 6 months | Arteriovenous graft | |
| Rabbit | Similar endothelialisation rates and thrombogenicity mechanisms to humans. Demanding higher anticoagulant function. Limited to short-term studies. | 1–4 | 5–30 | 12 months | Carotid artery | |
| 1–4 | 5–30 | 2 weeks | Femoral artery | |||
| 1–4 | 5–30 | 3 months | Abdominal artery | |||
| Rat | Large sample size. Wide variety of transgenic lines. Allows exploration of genetic/molecular mechanisms. Ideal for biocompatibility and cell infiltration studies. | 1–3 | 5–30 | 18 months | Abdominal artery | |
| 1–3 | 5–10 | 12 weeks | Carotid artery | |||
| Mouse | 0.5–1 | 3–10 | 6 months | Carotid artery |
Table 4 Common animal models for evaluation of artificial blood vessels in vivo.
| Animal model | Characteristics | Application inner diameter (mm) | Common length (mm) | Longest implantation period | Implantation site | References |
|---|---|---|---|---|---|---|
| Sheep | Similar cardiovascular physiology, endothelialisation mechanisms and thrombogenicity mechanisms to humans. Suitable size and long-term studies possible.Higher incidence of hypercoagulability. | 4–6 | 80–100 | 9 months | Carotid artery | |
| 4–6 | 60–100 | 3 months | Arteriovenous graft | |||
| Pig | Similar vascular physiology and anatomy to humans. Well established as a model for assessing vascular grafts. Mount an extensive immune response to implanted tissues. | 3–6 | 30–100 | 6 months | Iliac artery | |
| 3–6 | 10–100 | 4 weeks | Carotid artery | |||
| Dog | Lack of spontaneous endothelialisation and immune response restricts study lengths. Ease of accessing vessel due to thin skin. Thrombogenicity mechanisms and vessel viscoelastic properties differ from humans. | 3–6 | 30–50 | 6 months | Abdominal artery | |
| 3–6 | 30–50 | 1 year | Carotid artery | |||
| Baboon | Physiology and cardiovascular anatomy are the most similar to humans. Suitable for a wide range of non-invasive imaging techniques adapted from humans. High cost and ethical concerns associated with using primates in medical research. | 3–6 | 30–50 | 6 months | Arteriovenous graft | |
| Rabbit | Similar endothelialisation rates and thrombogenicity mechanisms to humans. Demanding higher anticoagulant function. Limited to short-term studies. | 1–4 | 5–30 | 12 months | Carotid artery | |
| 1–4 | 5–30 | 2 weeks | Femoral artery | |||
| 1–4 | 5–30 | 3 months | Abdominal artery | |||
| Rat | Large sample size. Wide variety of transgenic lines. Allows exploration of genetic/molecular mechanisms. Ideal for biocompatibility and cell infiltration studies. | 1–3 | 5–30 | 18 months | Abdominal artery | |
| 1–3 | 5–10 | 12 weeks | Carotid artery | |||
| Mouse | 0.5–1 | 3–10 | 6 months | Carotid artery |
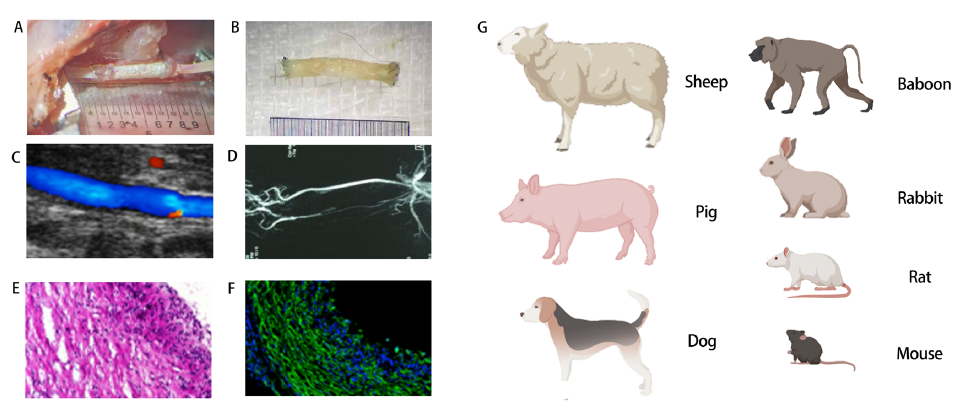
Figure 4. Evaluations of artificial blood vessels in vivo. (A) Macroscopic views of grafts upon implantation. (B) Macroscopic views of grafts post-operation. (C) Ultrasound image of graft implanted into rat common carotid artery. (D) Representative image of recorded angiogram showing graft patency. (E) Haematoxylin & eosin cross-sectional image. (F) Immunofluorescence image of the middle section of a vascular graft. (G) Common animal models for evaluation in vivo. EVG: elastic Van Gieson (Verhoeff ′s Van Gieson); GRAFT: graft. Created with Biorender.com.
| Intervention/treatment | Number of patients | Condition/disease | Years | Trial ID/phase | Testing status |
|---|---|---|---|---|---|
| Device: Synthetic vascular grafts | 207 | Peripheral arterial occlusive disease | 2010–2013 | NCT01113892/NA | Completed |
| Device: HAVG graft implantation | 40 | End-stage renal disease Kidney failure, chronic | 2012–2016 | NCT01744418/NA | Active, not recruiting |
| Device: FUSION Vascular Graft | 117 | Peripheral arterial occlusive disease (PAOD) | 2009–2013 | NCT01601496/NA | Terminated |
| Device: POSS-PCU vascular graft | 30 | Renal insufficiency | 2021–2025 | NCT02301312/NA | Not yet recruiting |
| Combination Product: Tissue Engineered Vascular Grafts | 4 | Single ventricle cardiac anomaly | 2009–2017 | NCT01034007/Phase 1 | Completed |
| Device: ProEndoTecc Vascular Graft | 33 | Peripheral arterial disease Peripheral vascular disease | 2010–2012 | NCT01095237/NA | Terminated |
| Device: ASC coated ePTFE vascular graft Device: Propaten graft | 60 | Lower limb ischemia | 2011–2022 | NCT01305863/NA | Active, not recruiting |
| Biological: natural human collagen arteriovenous graft for haemodialysis access | 10 | End stage renal disease | 2021–2022 | NCT04905511/Phase 1 | Recruiting |
| Device: POSS-PCU vascular graft | 30 | Renal insufficiency | 2021–2025 | NCT02301312/NA | Not yet recruiting |
| Procedure: Revascularisation using a BIOPROTEC graft | 45 | Peripheral artery disease | 2018–2023 | NCT04018846/NA | Recruiting |
| Device: Expanded polytetrafluoroethylene graft Device: Bovine carotid artery graft | 10 | End stage renal disease Haemolysis Arteriovenous graft | 2015–2018 | NCT03300024/NA | Terminated (Funding ended) |
| Device: Expedial vascular access graft | 172 | End stage renal disease | 2004–2006 | NCT00131872/Phase 2 | Terminated |
| Device: Covera vascular covered stent | 100 | Arteriovenous fistula | 2020–2023 | NCT04261686/NA | Enrolling by invitation |
| Device: Endovascular revascularisation of peripheral arteries | 150 | Vascular diseases, peripheral | 2021–2022 | NCT04765566/NA | Active, not recruiting |
| Device: InnAVasc arteriovenous graft surgical implant | 26 | Kidney failure, chronicRenal dialysis | 2019–2021 | NCT03645681/NA | Active, not recruiting |
| Procedure: blood sampling procedurethe vascular prosthesis manufactured by electrospinning | 120 | Arterial occlusive disease | 2014–2015 | NCT02255188/NA | Completed |
| Procedure: revascularisation Device: Propaten® Device: Crude PTFE | 228 | Ischemia lesions | 2018–2023 | NCT03430076/NA | Recruiting |
| Device: Paclitaxel-eluting graft | 20 | Haemodialysis access failure | 2018–2023 | NCT04285073/NA | Recruiting |
| Device: GORE PROPATEN vascular graft Procedure: Disadvantaged autologous vein graft | 31 | Peripheral arterial occlusive disease | 2007–2010 | NCT00617279/Phase 4 | Terminated (study terminated due to low enrolment) |
| Device: EvoCit Device: EvoHep | 38 | Kidney diseases Haemodialysis complication End stage renal disease | 2018–2019 | NCT03887468/ | Completed |
| Combination product: Tissue engineered vascular grafts | 24 | Cardiovascular diseases | 2020–2025 | NCT04467671/Phase 2 | Recruiting |
Table 5 Clinical trials of various vascular grafts. This table aims to be representative rather than comprehensive.
| Intervention/treatment | Number of patients | Condition/disease | Years | Trial ID/phase | Testing status |
|---|---|---|---|---|---|
| Device: Synthetic vascular grafts | 207 | Peripheral arterial occlusive disease | 2010–2013 | NCT01113892/NA | Completed |
| Device: HAVG graft implantation | 40 | End-stage renal disease Kidney failure, chronic | 2012–2016 | NCT01744418/NA | Active, not recruiting |
| Device: FUSION Vascular Graft | 117 | Peripheral arterial occlusive disease (PAOD) | 2009–2013 | NCT01601496/NA | Terminated |
| Device: POSS-PCU vascular graft | 30 | Renal insufficiency | 2021–2025 | NCT02301312/NA | Not yet recruiting |
| Combination Product: Tissue Engineered Vascular Grafts | 4 | Single ventricle cardiac anomaly | 2009–2017 | NCT01034007/Phase 1 | Completed |
| Device: ProEndoTecc Vascular Graft | 33 | Peripheral arterial disease Peripheral vascular disease | 2010–2012 | NCT01095237/NA | Terminated |
| Device: ASC coated ePTFE vascular graft Device: Propaten graft | 60 | Lower limb ischemia | 2011–2022 | NCT01305863/NA | Active, not recruiting |
| Biological: natural human collagen arteriovenous graft for haemodialysis access | 10 | End stage renal disease | 2021–2022 | NCT04905511/Phase 1 | Recruiting |
| Device: POSS-PCU vascular graft | 30 | Renal insufficiency | 2021–2025 | NCT02301312/NA | Not yet recruiting |
| Procedure: Revascularisation using a BIOPROTEC graft | 45 | Peripheral artery disease | 2018–2023 | NCT04018846/NA | Recruiting |
| Device: Expanded polytetrafluoroethylene graft Device: Bovine carotid artery graft | 10 | End stage renal disease Haemolysis Arteriovenous graft | 2015–2018 | NCT03300024/NA | Terminated (Funding ended) |
| Device: Expedial vascular access graft | 172 | End stage renal disease | 2004–2006 | NCT00131872/Phase 2 | Terminated |
| Device: Covera vascular covered stent | 100 | Arteriovenous fistula | 2020–2023 | NCT04261686/NA | Enrolling by invitation |
| Device: Endovascular revascularisation of peripheral arteries | 150 | Vascular diseases, peripheral | 2021–2022 | NCT04765566/NA | Active, not recruiting |
| Device: InnAVasc arteriovenous graft surgical implant | 26 | Kidney failure, chronicRenal dialysis | 2019–2021 | NCT03645681/NA | Active, not recruiting |
| Procedure: blood sampling procedurethe vascular prosthesis manufactured by electrospinning | 120 | Arterial occlusive disease | 2014–2015 | NCT02255188/NA | Completed |
| Procedure: revascularisation Device: Propaten® Device: Crude PTFE | 228 | Ischemia lesions | 2018–2023 | NCT03430076/NA | Recruiting |
| Device: Paclitaxel-eluting graft | 20 | Haemodialysis access failure | 2018–2023 | NCT04285073/NA | Recruiting |
| Device: GORE PROPATEN vascular graft Procedure: Disadvantaged autologous vein graft | 31 | Peripheral arterial occlusive disease | 2007–2010 | NCT00617279/Phase 4 | Terminated (study terminated due to low enrolment) |
| Device: EvoCit Device: EvoHep | 38 | Kidney diseases Haemodialysis complication End stage renal disease | 2018–2019 | NCT03887468/ | Completed |
| Combination product: Tissue engineered vascular grafts | 24 | Cardiovascular diseases | 2020–2025 | NCT04467671/Phase 2 | Recruiting |
| Company | Product name | Material | Details and modification | Indications for use |
|---|---|---|---|---|
| Atrium Medical Corporation | Flixene IFG Vascular graft | ePTFE | Very strong and durable with three layers | Arterial vascular reconstruction/segmental bypass/arteriovenous vascular access |
| Bard Peripheral Vascular, Inc. | VenafloTM II Vascular graft | ePTFE | Cuffed to promote good hemodynamic performance | Subcutaneous arteriovenous conduits for blood access only |
| Edwards Life Sciences | Edwards Lifespan reinforced expanded PTFE vascular graft | ePTFE | Higher crush and kink resistance | Bypass or reconstruction of diseased or occluded blood vessels/arteriovenous shunts |
| Maquet Cardiovascular, LLC | FUSION Vascular Graft | ePTFE | Two layers fused with a proprietary polycarbonate-urethane adhesive | Peripheral artery repair or replacement/vascular access |
| FUSIONTM and FUSIONTM Bioline Vascular Grafts | ePTFE and PET | Two layers with heparin/albumin coating on the interior surface | Peripheral artery repair or replacement | |
| EXXCELTM Soft ePTFE Vascular Grafts | ePTFE | featuring a GUIDELINE® stripe to facilitate proper graft alignment. | Peripheral arteries (iliac, femoral, popliteal, infrageniculate vessels, axillary, renal) repair or replacement/vascular access | |
| InterVascular SAS | InterGard Heparin | PET | Heparin bonded collagen coating | Peripheral artery replacement |
| PECA Labs, Inc. | ExGraft and ExGraft Carbon EPTFE Vascular Graft | ePTFE | A radiopaque ink applied to the surface and the exGraft Carbon ePTFE vascular graft coating with carbon | Peripheral artery repair or replacement/dialysis access |
| Vascular Flow Technologies Ltd. | Spiral FlowTM Peripheral Vascular Graft | ePTFE | Propagating spiral flow through the graft and into the distal circulation, reinforced | Bypass or reconstruction of occluded or diseased peripheral arterial blood vessels above or below the knee |
| Vascutek Ltd. | Vascutek Gelsoft Plus ERS Vascular Graft | PET | External polypropylene support, gelatine-sealed, knitted polyester grafts | Indicated for extra anatomical vascular repair, primarily for axillo-femoral/bi-femoral bypass and femoropopliteal reconstruction |
| Vascutek GelsealTM Vascular Grafts | PET | Knitted, gelatine impregnated | Indicated for replacement or bypass of abdominal arteries afflicted with aneurysmal or occlusive disease | |
| Vascutek GelsoftTM Vascular Grafts | PET | Knitted, gelatine impregnated, zero porosity | Indicated for abdominal and peripheral vascular repair | |
| Vascutek GelsoftTM Plus Vascular Grafts | PET | Knitted, gelatine impregnated, dilation resistant | Indicated exclusively for abdominal and peripheral vascular repair | |
| W.L. Gore & Associates, Inc. | Gore-Tex | ePTFE | Unmodified | Vascular access |
| Gore-Tex Stretch | ePTFE | Stretch | ||
| Gore Propaten | ePTFE | Reduced thrombogenicity through covalently binding to bioactive heparin |
Table 6 Commercially-available artificial blood vessels in clinical use.
| Company | Product name | Material | Details and modification | Indications for use |
|---|---|---|---|---|
| Atrium Medical Corporation | Flixene IFG Vascular graft | ePTFE | Very strong and durable with three layers | Arterial vascular reconstruction/segmental bypass/arteriovenous vascular access |
| Bard Peripheral Vascular, Inc. | VenafloTM II Vascular graft | ePTFE | Cuffed to promote good hemodynamic performance | Subcutaneous arteriovenous conduits for blood access only |
| Edwards Life Sciences | Edwards Lifespan reinforced expanded PTFE vascular graft | ePTFE | Higher crush and kink resistance | Bypass or reconstruction of diseased or occluded blood vessels/arteriovenous shunts |
| Maquet Cardiovascular, LLC | FUSION Vascular Graft | ePTFE | Two layers fused with a proprietary polycarbonate-urethane adhesive | Peripheral artery repair or replacement/vascular access |
| FUSIONTM and FUSIONTM Bioline Vascular Grafts | ePTFE and PET | Two layers with heparin/albumin coating on the interior surface | Peripheral artery repair or replacement | |
| EXXCELTM Soft ePTFE Vascular Grafts | ePTFE | featuring a GUIDELINE® stripe to facilitate proper graft alignment. | Peripheral arteries (iliac, femoral, popliteal, infrageniculate vessels, axillary, renal) repair or replacement/vascular access | |
| InterVascular SAS | InterGard Heparin | PET | Heparin bonded collagen coating | Peripheral artery replacement |
| PECA Labs, Inc. | ExGraft and ExGraft Carbon EPTFE Vascular Graft | ePTFE | A radiopaque ink applied to the surface and the exGraft Carbon ePTFE vascular graft coating with carbon | Peripheral artery repair or replacement/dialysis access |
| Vascular Flow Technologies Ltd. | Spiral FlowTM Peripheral Vascular Graft | ePTFE | Propagating spiral flow through the graft and into the distal circulation, reinforced | Bypass or reconstruction of occluded or diseased peripheral arterial blood vessels above or below the knee |
| Vascutek Ltd. | Vascutek Gelsoft Plus ERS Vascular Graft | PET | External polypropylene support, gelatine-sealed, knitted polyester grafts | Indicated for extra anatomical vascular repair, primarily for axillo-femoral/bi-femoral bypass and femoropopliteal reconstruction |
| Vascutek GelsealTM Vascular Grafts | PET | Knitted, gelatine impregnated | Indicated for replacement or bypass of abdominal arteries afflicted with aneurysmal or occlusive disease | |
| Vascutek GelsoftTM Vascular Grafts | PET | Knitted, gelatine impregnated, zero porosity | Indicated for abdominal and peripheral vascular repair | |
| Vascutek GelsoftTM Plus Vascular Grafts | PET | Knitted, gelatine impregnated, dilation resistant | Indicated exclusively for abdominal and peripheral vascular repair | |
| W.L. Gore & Associates, Inc. | Gore-Tex | ePTFE | Unmodified | Vascular access |
| Gore-Tex Stretch | ePTFE | Stretch | ||
| Gore Propaten | ePTFE | Reduced thrombogenicity through covalently binding to bioactive heparin |
| 1. |
Blais, C.; Rochette, L.; Ouellet, S.; Huynh, T. Complex evolution of epidemiology of vascular diseases, including increased disease burden: from 2000 to 2015. Can J Cardiol. 2014, 36, 740-746.
doi: 10.1016/j.cjca.2019.10.021 URL |
| 2. | Zoghbi, W. A.; Duncan, T.; Antman, E.; Barbosa, M.; Champagne, B.; Chen, D.; Gamra, H.; Harold, J. G.; Josephson, S.; Komajda, M.; Logstrup, S.; Jur, C.; Mayosi, B. M.; Mwangi, J.; Ralston, J.; Sacco, R. L.; Sim, K. H.; Smith, S. C., Jr.; Vardas, P. E.; Wood, D. A. Sustainable Development Goals and the future of cardiovascular health. A statement from the Global Cardiovascular Disease Taskforce. Eur Heart J. 2014, 35, 3238-3239. |
| 3. |
Nelson, J. A.; Fischer, J. P.; Grover, R.; Kovach, S. J.; Low, D. W.; Kanchwala, S. K.; Levin, L. S.; Serletti, J. M.; Wu, L. C. Vein grafting your way out of trouble: examining the utility and efficacy of vein grafts in microsurgery. J Plast Reconstr Aesthet Surg. 2015, 68, 830-836.
doi: 10.1016/j.bjps.2015.02.008 URL |
| 4. |
Caliskan, E.; de Souza, D. R.; Böning, A.; Liakopoulos, O. J.; Choi, Y. H.; Pepper, J.; Gibson, C. M.; Perrault, L. P.; Wolf, R. K.; Kim, K. B.; Emmert, M. Y. Saphenous vein grafts in contemporary coronary artery bypass graft surgery. Nat Rev Cardiol. 2020, 17, 155-169.
doi: 10.1038/s41569-019-0249-3 URL |
| 5. |
Kaiser, E.; Jaganathan, S. K.; Supriyanto, E.; Ayyar, M. Fabrication and characterization of chitosan nanoparticles and collagen-loaded polyurethane nanocomposite membrane coated with heparin for atrial septal defect (ASD) closure. 3 Biotech. 2017, 7, 174.
doi: 10.1007/s13205-017-0830-6 URL |
| 6. |
Biancari, F.; Railo, M.; Lundin, J.; Albäck, A.; Kantonen, I.; Lehtola, A.; Lepäntalo, M. Redo bypass surgery to the infrapopliteal arteries for critical leg ischaemia. Eur J Vasc Endovasc Surg. 2001, 21, 137-142.
doi: 10.1053/ejvs.2000.1290 URL |
| 7. | Cai, Q.; Liao, W.; Xue, F.; Wang, X.; Zhou, W.; Li, Y.; Zeng, W. Selection of different endothelialization modes and different seed cells for tissue-engineered vascular graft. Bioact Mater. 2021, 6, 2557-2568. |
| 8. |
Tara, S.; Kurobe, H.; Maxfield, M. W.; Rocco, K. A.; Yi, T.; Naito, Y.; Breuer, C. K.; Shinoka, T. Evaluation of remodeling process in small-diameter cell-free tissue-engineered arterial graft. J Vasc Surg. 2015, 62, 734-743.
doi: 10.1016/j.jvs.2014.03.011 URL |
| 9. | Filipe, E. C.; Santos, M.; Hung, J.; Lee, B. S. L.; Yang, N.; Chan, A. H. P.; Ng, M. K. C.; Rnjak-Kovacina, J.; Wise, S. G. Rapid endothelialization of off-the-shelf small diameter silk vascular grafts. JACC Basic Transl Sci. 2018, 3, 38-53. |
| 10. |
Moore, M. J.; Tan, R. P.; Yang, N.; Rnjak-Kovacina, J.; Wise, S. G. Bioengineering artificial blood vessels from natural materials. Trends Biotechnol. 2021. doi: 10.1016/j.tibtech.2021.11.003.
doi: 10.1016/j.tibtech.2021.11.003 URL |
| 11. |
Brown, B. N.; Haschak, M. J.; Lopresti, S. T.; Stahl, E. C. Effects of age-related shifts in cellular function and local microenvironment upon the innate immune response to implants. Semin Immunol. 2017, 29, 24-32.
doi: 10.1016/j.smim.2017.05.001 URL |
| 12. |
Stekelenburg, M.; Rutten, M. C.; Snoeckx, L. H.; Baaijens, F. P. Dynamic straining combined with fibrin gel cell seeding improves strength of tissue-engineered small-diameter vascular grafts. Tissue Eng Part A. 2009, 15, 1081-1089.
doi: 10.1089/ten.tea.2008.0183 URL |
| 13. |
Konig, G.; McAllister, T. N.; Dusserre, N.; Garrido, S. A.; Iyican, C.; Marini, A.; Fiorillo, A.; Avila, H.; Wystrychowski, W.; Zagalski, K.; Maruszewski, M.; Jones, A. L.; Cierpka, L.; de la Fuente, L. M.; L’Heureux, N. Mechanical properties of completely autologous human tissue engineered blood vessels compared to human saphenous vein and mammary artery. Biomaterials. 2009, 30, 1542-1550.
doi: 10.1016/j.biomaterials.2008.11.011 URL |
| 14. |
Gu, Y.; Tian, C.; Qin, Y.; Sun, Y.; Liu, S.; Li, H.; Duan, X.; Shu, C.; Ouyang, C. The novel hybrid polycarbonate polyurethane / polyester three-layered large-diameter artificial blood vessel. J Biomater Appl. 2022, 36, 965-975.
doi: 10.1177/08853282211033415 URL |
| 15. |
Dubost, C.; Allary, M.; Oeconomos, N. Resection of an aneurysm of the abdominal aorta: reestablishment of the continuity by a preserved human arterial graft, with result after five months. AMA Arch Surg. 1952, 64, 405-408.
doi: 10.1001/archsurg.1952.01260010419018 URL |
| 16. |
Blakemore, A. H.; Voorhees, A. B., Jr. The use of tubes constructed from vinyon N cloth in bridging arterial defects; experimental and clinical. Ann Surg. 1954, 140, 324-334.
doi: 10.1097/00000658-195409000-00008 URL |
| 17. | De Bakey, M. E.; Cooley, D. A.; Crawford, E. S.; Morris, G. C, Jr. Clinical application of a new flexible knitted dacron arterial substitute. Am Surg. 1958, 24, 862-869. |
| 18. |
Ravi, S.; Chaikof, E. L. Biomaterials for vascular tissue engineering. Regen Med. 2010, 5, 107-120.
doi: 10.2217/rme.09.77 URL |
| 19. |
Whittemore, A. D.; Kent, K. C.; Donaldson, M. C.; Couch, N. P.; Mannick, J. A.; What is the proper role of polytetrafluoroethylene grafts in infrainguinal reconstruction? J Vasc Surg. 1989, 10, 299-305.
doi: 10.1016/0741-5214(89)90445-X URL |
| 20. |
van der Lei, B.; Darius, H.; Schrör, K.; Nieuwenhuis, P.; Molenaar, I.; Wildevuur, C. R. Arterial wall regeneration in small-caliber vascular grafts in rats. Neoendothelial healing and prostacyclin production. J Thorac Cardiovasc Surg. 1985, 90, 378-386.
doi: 10.1016/S0022-5223(19)38593-9 URL |
| 21. |
Weinberg, C. B.; Bell, E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986, 231, 397-400.
doi: 10.1126/science.2934816 URL |
| 22. |
Bobadilla, J. L. From ebers to EVARs: a historical perspective on aortic surgery. Aorta (Stamford). 2013, 1, 89-95.
doi: 10.12945/j.aorta.2013.13-004 URL |
| 23. |
Niklason, L. E. Techview: medical technology. Replacement a.rteries made to order. Science. 1999, 286, 1493-1494.
doi: 10.1126/science.286.5444.1493 URL |
| 24. |
Niklason, L. E.; Gao, J.; Abbott, W. M.; Hirschi, K. K.; Houser, S.; Marini, R.; Langer, R. Functional arteries grown in vitro. Science. 1999, 284, 489-493.
doi: 10.1126/science.284.5413.489 URL |
| 25. |
Skovrind, I.; Harvald, E. B.; Juul Belling, H.; Jørgensen, C. D.; Lindholt, J. S.; Andersen, D. C. Concise review: patency of small-diameter tissue-engineered vascular grafts: a meta-analysis of preclinical trials. Stem Cells Transl Med. 2019, 8, 671-680.
doi: 10.1002/sctm.18-0287 URL |
| 26. |
Lawson, J. H.; Glickman, M. H.; Ilzecki, M.; Jakimowicz, T.; Jaroszynski, A.; Peden, E. K.; Pilgrim, A. J.; Prichard, H. L.; Guziewicz, M.; Przywara, S.; Szmidt, J.; Turek, J.; Witkiewicz, W.; Zapotoczny, N.; Zubilewicz, T.; Niklason, L. E. Bioengineered human acellular vessels for dialysis access in patients with end-stage renal disease: two phase 2 single-arm trials. Lancet. 2016, 387, 2026-2034.
doi: 10.1016/S0140-6736(16)00557-2 URL |
| 27. |
Lee, A. Y.; Mahler, N.; Best, C.; Lee, Y. U.; Breuer, C. K. Regenerative implants for cardiovascular tissue engineering. Transl Res. 2014, 163, 321-341.
doi: 10.1016/j.trsl.2014.01.014 URL |
| 28. |
Klinkert, P.; Post, P. N.; Breslau, P. J.; van Bockel, J. H. Saphenous vein versus PTFE for above-knee femoropopliteal bypass. A review of the literature. Eur J Vasc Endovasc Surg. 2004, 27, 357-362.
doi: 10.1016/j.ejvs.2003.12.027 URL |
| 29. |
Spadaccio, C.; Rainer, A.; Barbato, R.; Chello, M.; Meyns, B. The fate of large-diameter Dacron® vascular grafts in surgical practice: are we really satisfied? Int J Cardiol. 2013, 168, 5028-5029.
doi: 10.1016/j.ijcard.2013.07.165 URL |
| 30. |
Conte, M. S. The ideal small arterial substitute: a search for the Holy Grail? FASEB J. 1998, 12, 43-45.
doi: 10.1096/fsb2.v12.1 URL |
| 31. |
Akoh, J. A.; Patel, N. Infection of hemodialysis arteriovenous grafts. J Vasc Access. 2010, 11, 155-158.
doi: 10.1177/112972981001100213 URL |
| 32. |
Knox, W. G. Aneurysm occurring in a femoral artery Dacron prosthesis five and one-half years after insertion. Ann Surg. 1962, 156, 827-830.
doi: 10.1097/00000658-196211000-00018 URL |
| 33. |
Eslami, M. H.; Gangadharan, S. P.; Belkin, M.; Donaldson, M. C.; Whittemore, A. D.; Conte, M. S. Monocyte adhesion to human vein grafts: a marker for occult intraoperative injury? J Vasc Surg. 2001, 34, 923-929.
doi: 10.1067/mva.2001.118590 URL |
| 34. |
Kohler, T. R.; Kirkman, T. R.; Kraiss, L. W.; Zierler, B. K.; Clowes, A. W. Increased blood flow inhibits neointimal hyperplasia in endothelialized vascular grafts. Circ Res. 1991, 69, 1557-1565.
doi: 10.1161/01.RES.69.6.1557 URL |
| 35. |
Greenwald, S. E.; Berry, C. L. Improving vascular grafts: the importance of mechanical and haemodynamic properties. J Pathol. 2000, 190, 292-299.
doi: 10.1002/(ISSN)1096-9896 URL |
| 36. |
Haruguchi, H.; Teraoka, S. Intimal hyperplasia and hemodynamic factors in arterial bypass and arteriovenous grafts: a review. J Artif Organs. 2003, 6, 227-235.
doi: 10.1007/s10047-003-0232-x URL |
| 37. | Davies, M. G.; Hagen, P. O. Reprinted article “Pathophysiology of vein graft failure: a review”. Eur J Vasc Endovasc Surg. 2011, 42 Suppl 1, S19-29. |
| 38. |
FitzGibbon, G. M.; Leach, A. J.; Kafka, H. P.; Keon, W. J. Coronary bypass graft fate: long-term angiographic study. J Am Coll Cardiol. 1991, 17, 1075-1080.
doi: 10.1016/0735-1097(91)90834-V URL |
| 39. |
van der Wal, A. C.; Becker, A. E.; Elbers, J. R.; Das, P. K. An immunocytochemical analysis of rapidly progressive atherosclerosis in human vein grafts. Eur J Cardiothorac Surg. 1992, 6, 469-473.; discussion 474.
doi: 10.1016/1010-7940(92)90242-P URL |
| 40. | Chiesa, R.; Astore, D.; Frigerio, S.; Garriboli, L.; Piccolo, G.; Castellano, R.; Scalamogna, M.; Odero, A.; Pirrelli, S.; Biasi, G.; Mingazzini, P.; Biglioli, P.; Polvani, G.; Guarino, A.; Agrifoglio, G.; Tori, A.; Spina, G. Vascular prosthetic graft infection: epidemiology, bacteriology, pathogenesis and treatment. Acta Chir Belg. 2002, 102, 238-247. |
| 41. | Zetrenne, E.; McIntosh, B. C.; McRae, M. H.; Gusberg, R.; Evans, G. R.; Narayan, D. Prosthetic vascular graft infection: a multi-center review of surgical management. Yale J Biol Med. 2007, 80, 113-121. |
| 42. |
Padberg, F. T., Jr.; Calligaro, K. D.; Sidawy, A. N. Complications of arteriovenous hemodialysis access: recognition and management. J Vasc Surg. 2008, 48, 55s-80s.
doi: 10.1016/j.jvs.2008.08.067 URL |
| 43. |
Wu, W.; Allen, R. A.; Wang, Y. Fast-degrading elastomer enables rapid remodeling of a cell-free synthetic graft into a neoartery. Nat Med. 2012, 18, 1148-1153.
doi: 10.1038/nm.2821 URL |
| 44. |
Yokota, T.; Ichikawa, H.; Matsumiya, G.; Kuratani, T.; Sakaguchi, T.; Iwai, S.; Shirakawa, Y.; Torikai, K.; Saito, A.; Uchimura, E.; Kawaguchi, N.; Matsuura, N.; Sawa, Y. In situ tissue regeneration using a novel tissue-engineered, small-caliber vascular graft without cell seeding. J Thorac Cardiovasc Surg. 2008, 136, 900-907.
doi: 10.1016/j.jtcvs.2008.02.058 URL |
| 45. |
Ross, R.; Glomset, J. A. Atherosclerosis and the arterial smooth muscle cell: Proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science. 1973, 180, 1332-1339.
doi: 10.1126/science.180.4093.1332 URL |
| 46. |
Wang, Z.; Liu, C.; Zhu, D.; Gu, X.; Xu, Y.; Qin, Q.; Dong, N.; Zhang, S.; Wang, J. Untangling the co-effects of oriented nanotopography and sustained anticoagulation in a biomimetic intima on neovessel remodeling. Biomaterials. 2020, 231, 119654.
doi: 10.1016/j.biomaterials.2019.119654 URL |
| 47. |
Gong, W.; Lei, D.; Li, S.; Huang, P.; Qi, Q.; Sun, Y.; Zhang, Y.; Wang, Z.; You, Z.; Ye, X.; Zhao, Q. Hybrid small-diameter vascular grafts: Anti-expansion effect of electrospun poly ε-caprolactone on heparin-coated decellularized matrices. Biomaterials. 2016, 76, 359-370.
doi: 10.1016/j.biomaterials.2015.10.066 URL |
| 48. |
Jalaie, H.; Steitz, J.; Afify, M.; Barbati, M. E.; Hoeft, K.; Assar, M. A. M.; Hermanns-Sachweh, B.; Tolba, R. H.; Jacobs, M. J.; Schleimer, K. In vivo endothelialization and neointimal hyperplasia assessment after angioplasty of sheep carotid artery with a novel polycarbonate polyurethane patch. J Biomater Appl. 2019, 34, 208-218.
doi: 10.1177/0885328219849368 URL |
| 49. | Torikai, K.; Ichikawa, H.; Hirakawa, K.; Matsumiya, G.; Kuratani, T.; Iwai, S.; Saito, A.; Kawaguchi, N.; Matsuura, N.; Sawa, Y. A self-renewing, tissue-engineered vascular graft for arterial reconstruction. J Thorac Cardiovasc Surg. 2008, 136:37-45, 45.e1. |
| 50. |
Pan, Y.; Zhou, X.; Wei, Y.; Zhang, Q.; Wang, T.; Zhu, M.; Li, W.; Huang, R.; Liu, R.; Chen, J.; Fan, G.; Wang, K.; Kong, D.; Zhao, Q. Small-diameter hybrid vascular grafts composed of polycaprolactone and polydioxanone fibers. Sci Rep. 2017, 7, 3615.
doi: 10.1038/s41598-017-03851-1 URL |
| 51. | Hedin, U. Long-term results of PTFE grafts. J Vasc Access. 2015, 16Suppl 9, S87-92. |
| 52. |
Alessandrino, A.; Chiarini, A.; Biagiotti, M.; Dal Prà, I.; Bassani, G. A.; Vincoli, V.; Settembrini, P.; Pierimarchi, P.; Freddi, G.; Armato, U. Three-layered silk fibroin tubular scaffold for the repair and regeneration of small caliber blood vessels: from design to in vivo pilot tests. Front Bioeng Biotechnol. 2019, 7, 356.
doi: 10.3389/fbioe.2019.00356 URL |
| 53. |
Zhang, F.; Bambharoliya, T.; Xie, Y.; Liu, L.; Celik, H.; Wang, L.; Akkus, O.; King, M. W. A hybrid vascular graft harnessing the superior mechanical properties of synthetic fibers and the biological performance of collagen filaments. Mater Sci Eng C Mater Biol Appl. 2021, 118, 111418.
doi: 10.1016/j.msec.2020.111418 URL |
| 54. |
Shelah, O.; Wertheimer, S.; Haj-Ali, R.; Lesman, A. Coral-derived collagen fibers for engineering aligned tissues. Tissue Eng Part A. 2021, 27, 187-200.
doi: 10.1089/ten.tea.2020.0116 URL |
| 55. |
Dastagir, K.; Dastagir, N.; Limbourg, A.; Reimers, K.; Strauß, S.; Vogt, P. M. In vitro construction of artificial blood vessels using spider silk as a supporting matrix. J Mech Behav Biomed Mater. 2020, 101, 103436.
doi: 10.1016/j.jmbbm.2019.103436 URL |
| 56. |
Joyce, K.; Fabra, G. T.; Bozkurt, Y.; Pandit, A. Bioactive potential of natural biomaterials: identification, retention and assessment of biological properties. Signal Transduct Target Ther. 2021, 6, 122.
doi: 10.1038/s41392-021-00512-8 URL |
| 57. |
Vasconcelos, A.; Gomes, A. C.; Cavaco-Paulo, A. Novel silk fibroin/elastin wound dressings. Acta Biomater. 2012, 8, 3049-3060.
doi: 10.1016/j.actbio.2012.04.035 URL |
| 58. |
Daamen, W. F.; Hafmans, T.; Veerkamp, J. H.; van Kuppevelt, T. H. Isolation of intact elastin fibers devoid of microfibrils. Tissue Eng. 2005, 11, 1168-1176.
doi: 10.1089/ten.2005.11.1168 URL |
| 59. |
Scherner, M.; Reutter, S.; Klemm, D.; Sterner-Kock, A.; Guschlbauer, M.; Richter, T.; Langebartels, G.; Madershahian, N.; Wahlers, T.; Wippermann, J. In vivo application of tissue-engineered blood vessels of bacterial cellulose as small arterial substitutes: proof of concept? J Surg Res. 2014, 189, 340-347.
doi: 10.1016/j.jss.2014.02.011 URL |
| 60. |
Blanco Parte, F. G.; Santoso, S. P.; Chou, C. C.; Verma, V.; Wang, H. T.; Ismadji, S.; Cheng, K. C. Current progress on the production, modification, and applications of bacterial cellulose. Crit Rev Biotechnol. 2020, 40, 397-414.
doi: 10.1080/07388551.2020.1713721 URL |
| 61. | Weber, C.; Reinhardt, S.; Eghbalzadeh, K.; Wacker, M.; Guschlbauer, M.; Maul, A.; Sterner-Kock, A.; Wahlers, T.; Wippermann, J.; Scherner, M. Patency and in vivo compatibility of bacterial nanocellulose grafts as small-diameter vascular substitute. J Vasc Surg. 2018, 68:177S-187S.e1. |
| 62. |
Li, Y.; Jiang, K.; Feng, J.; Liu, J.; Huang, R.; Chen, Z.; Yang, J.; Dai, Z.; Chen, Y.; Wang, N.; Zhang, W.; Zheng, W.; Yang, G.; Jiang, X. Construction of small-diameter vascular graft by shape-memory and self-rolling bacterial cellulose membrane. Adv Healthc Mater. 2017, 6, 1601343.
doi: 10.1002/adhm.v6.11 URL |
| 63. |
Bao, L.; Tang, J.; Hong, F. F.; Lu, X.; Chen, L. Physicochemical properties and in vitro biocompatibility of three bacterial nanocellulose conduits for blood vessel applications. Carbohydr Polym. 2020, 239, 116246.
doi: 10.1016/j.carbpol.2020.116246 URL |
| 64. |
Bao, L.; Hong, F. F.; Li, G.; Hu, G.; Chen, L. Implantation of air-dried bacterial nanocellulose conduits in a small-caliber vascular prosthesis rabbit model. Mater Sci Eng C Mater Biol Appl. 2021, 122, 111922.
doi: 10.1016/j.msec.2021.111922 URL |
| 65. |
Tanaka, T.; Abe, Y.; Cheng, C. J.; Tanaka, R.; Naito, A.; Asakura, T. Development of small-diameter elastin-silk fibroin vascular grafts. Front Bioeng Biotechnol. 2020, 8, 622220.
doi: 10.3389/fbioe.2020.622220 URL |
| 66. |
Hasan, A.; Memic, A.; Annabi, N.; Hossain, M.; Paul, A.; Dokmeci, M. R.; Dehghani, F.; Khademhosseini, A. Electrospun scaffolds for tissue engineering of vascular grafts. Acta Biomater. 2014, 10, 11-25.
doi: 10.1016/j.actbio.2013.08.022 URL |
| 67. | Pellegata, A. F.; Asnaghi, M. A.; Stefani, I.; Maestroni, A.; Maestroni, S.; Dominioni, T.; Zonta, S.; Zerbini, G.; Mantero, S. Detergent-enzymatic decellularization of swine blood vessels: insight on mechanical properties for vascular tissue engineering. Biomed Res Int. 2013, 2013, 918753. |
| 68. |
Englberger, L.; Noti, J.; Immer, F. F.; Stalder, M.; Eckstein, F. S.; Carrel, T. P. The Shelhigh No-React bovine internal mammary artery: a questionable alternative conduit in coronary bypass surgery? Eur J Cardiothorac Surg. 2008, 33, 222-224.
doi: 10.1016/j.ejcts.2007.11.006 URL |
| 69. | Leoce, B. M.; Montoya, M.; Dardik, H.; Bernik, T. R. Rapid degradation and subsequent endovascular salvage of upper extremity cryogenic allograft bypass. Ann Vasc Surg. 2019, 57:276.e5-276.e8. |
| 70. |
Soletti, L.; Hong, Y.; Guan, J.; Stankus, J. J.; El-Kurdi, M. S.; Wagner, W. R.; Vorp, D. A. A bilayered elastomeric scaffold for tissue engineering of small diameter vascular grafts. Acta Biomater. 2010, 6, 110-122.
doi: 10.1016/j.actbio.2009.06.026 URL |
| 71. |
Wu, T.; Huang, C.; Li, D.; Yin, A.; Liu, W.; Wang, J.; Chen, J.; Ei-Hamshary, H.; Al-Deyab, S. S.; Mo, X. A multi-layered vascular scaffold with symmetrical structure by bi-directional gradient electrospinning. Colloids Surf B Biointerfaces. 2015, 133, 179-188.
doi: 10.1016/j.colsurfb.2015.05.048 URL |
| 72. |
Ju, Y. M.; Choi, J. S.; Atala, A.; Yoo, J. J.; Lee, S. J. Bilayered scaffold for engineering cellularized blood vessels. Biomaterials. 2010, 31, 4313-4321.
doi: 10.1016/j.biomaterials.2010.02.002 URL |
| 73. |
de Valence, S.; Tille, J. C.; Mugnai, D.; Mrowczynski, W.; Gurny, R.; Möller, M.; Walpoth, B. H. Long term performance of polycaprolactone vascular grafts in a rat abdominal aorta replacement model. Biomaterials. 2012, 33, 38-47.
doi: 10.1016/j.biomaterials.2011.09.024 URL |
| 74. |
Fukunishi, T.; Best, C. A.; Sugiura, T.; Shoji, T.; Yi, T.; Udelsman, B.; Ohst, D.; Ong, C. S.; Zhang, H.; Shinoka, T.; Breuer, C. K.; Johnson, J.; Hibino, N. Tissue-engineered small diameter arterial vascular grafts from cell-free nanofiber PCL/chitosan scaffolds in a sheep model. PLoS One. 2016, 11, e0158555.
doi: 10.1371/journal.pone.0158555 URL |
| 75. |
Xing, Y.; Gu, Y.; Guo, L.; Guo, J.; Xu, Z.; Xiao, Y.; Fang, Z.; Wang, C.; Feng, Z. G.; Wang, Z. Gelatin coating promotes in situ endothelialization of electrospun polycaprolactone vascular grafts. J Biomater Sci Polym Ed. 2021, 32, 1161-1181.
doi: 10.1080/09205063.2021.1909413 URL |
| 76. |
Stollwerck, P. L.; Kozlowski, B.; Sandmann, W.; Grabitz, K.; Pfeiffer, T. Long-term dilatation of polyester and expanded polytetrafluoroethylene tube grafts after open repair of infrarenal abdominal aortic aneurysms. J Vasc Surg. 2011, 53, 1506-1513.
doi: 10.1016/j.jvs.2011.02.028 URL |
| 77. | Salata, K.; Hussain, M. A.; de Mestral, C.; Greco, E.; Awartani, H.; Aljabri, B. A.; Mamdani, M.; Forbes, T. L.; Bhatt, D. L.; Verma, S.; Al-Omran, M. Population-based long-term outcomes of open versus endovascular aortic repair of ruptured abdominal aortic aneurysms. J Vasc Surg. 2020, 71:1867-1878.e8. |
| 78. |
Steuer, J.; Lachat, M.; Veith, F. J.; Wanhainen, A. Endovascular grafts for abdominal aortic aneurysm. Eur Heart J. 2016, 37, 145-151.
doi: 10.1093/eurheartj/ehv593 URL |
| 79. |
Seib, F. P.; Herklotz, M.; Burke, K. A.; Maitz, M. F.; Werner, C.; Kaplan, D. L. Multifunctional silk-heparin biomaterials for vascular tissue engineering applications. Biomaterials. 2014, 35, 83-91.
doi: 10.1016/j.biomaterials.2013.09.053 URL |
| 80. |
Saberianpour, S.; Heidarzadeh, M.; Geranmayeh, M. H.; Hosseinkhani, H.; Rahbarghazi, R.; Nouri, M. Tissue engineering strategies for the induction of angiogenesis using biomaterials. J Biol Eng. 2018, 12, 36.
doi: 10.1186/s13036-018-0133-4 URL |
| 81. | Dahl, S. L.; Kypson, A. P.; Lawson, J. H.; Blum, J. L.; Strader, J. T.; Li, Y.; Manson, R. J.; Tente, W. E.; DiBernardo, L.; Hensley, M. T.; Carter, R.; Williams, T. P.; Prichard, H. L.; Dey, M. S.; Begelman, K. G.; Niklason, L. E. Readily available tissue-engineered vascular grafts. Sci Transl Med. 2011, 3, 68ra69. |
| 82. |
McAllister, T. N.; Maruszewski, M.; Garrido, S. A.; Wystrychowski, W.; Dusserre, N.; Marini, A.; Zagalski, K.; Fiorillo, A.; Avila, H.; Manglano, X.; Antonelli, J.; Kocher, A.; Zembala, M.; Cierpka, L.; de la Fuente, L. M.; L’Heureux, N. Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: a multicentre cohort study. Lancet. 2009, 373, 1440-1446.
doi: 10.1016/S0140-6736(09)60248-8 URL |
| 83. |
Eitan, Y.; Sarig, U.; Dahan, N.; Machluf, M. Acellular cardiac extracellular matrix as a scaffold for tissue engineering: in vitro cell support, remodeling, and biocompatibility. Tissue Eng Part C Methods. 2010, 16, 671-683.
doi: 10.1089/ten.tec.2009.0111 URL |
| 84. |
Badylak, S. F.; Freytes, D. O.; Gilbert, T. W. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009, 5, 1-13.
doi: 10.1016/j.actbio.2008.09.013 URL |
| 85. |
Swinehart, I. T.; Badylak, S. F. Extracellular matrix bioscaffolds in tissue remodeling and morphogenesis. Dev Dyn. 2016, 245, 351-360.
doi: 10.1002/dvdy.v245.3 URL |
| 86. |
Lin, C. H.; Hsia, K.; Ma, H.; Lee, H.; Lu, J. H. In vivo performance of decellularized vascular grafts: a review article. Int J Mol Sci. 2018, 19, 2101.
doi: 10.3390/ijms19072101 URL |
| 87. |
Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: methods, materials, and applications. Chem Rev. 2019, 119, 5298-5415.
doi: 10.1021/acs.chemrev.8b00593 URL |
| 88. |
Yin, A.; Luo, R.; Li, J.; Mo, X.; Wang, Y.; Zhang, X. Coaxial electrospinning multicomponent functional controlled-release vascular graft: Optimization of graft properties. Colloids Surf B Biointerfaces. 2017, 152, 432-439.
doi: 10.1016/j.colsurfb.2017.01.045 URL |
| 89. |
Rodriguez, M.; Kluge, J. A.; Smoot, D.; Kluge, M. A.; Schmidt, D. F.; Paetsch, C. R.; Kim, P. S.; Kaplan, D. L. Fabricating mechanically improved silk-based vascular grafts by solution control of the gel-spinning process. Biomaterials. 2020, 230, 119567.
doi: 10.1016/j.biomaterials.2019.119567 URL |
| 90. |
Kuang, H.; Wang, Y.; Shi, Y.; Yao, W.; He, X.; Liu, X.; Mo, X.; Lu, S.; Zhang, P. Construction and performance evaluation of Hep/silk-PLCL composite nanofiber small-caliber artificial blood vessel graft. Biomaterials. 2020, 259, 120288.
doi: 10.1016/j.biomaterials.2020.120288 URL |
| 91. | L’Heureux, N.; Dusserre, N.; Konig, G.; Victor, B.; Keire, P.; Wight, T. N.; Chronos, N. A.; Kyles, A. E.; Gregory, C. R.; Hoyt, G.; Robbins, R. C.; McAllister, T. N. Human tissue-engineered blood vessels for adult arterial revascularization. Nat Med. 2006, 12, 361-365. |
| 92. |
Kimicata, M.; Swamykumar, P.; Fisher, J. P. Extracellular matrix for small-diameter vascular grafts. Tissue Eng Part A. 2020, 26, 1388-1401.
doi: 10.1089/ten.tea.2020.0201 URL |
| 93. |
Ainslie, K. M.; Bachelder, E. M.; Borkar, S.; Zahr, A. S.; Sen, A.; Badding, J. V.; Pishko, M. V. Cell adhesion on nanofibrous polytetrafluoroethylene (nPTFE). Langmuir. 2007, 23, 747-754.
doi: 10.1021/la060948s URL |
| 94. |
Zilla, P.; Bezuidenhout, D.; Human, P. Prosthetic vascular grafts: wrong models, wrong questions and no healing. Biomaterials. 2007, 28, 5009-5027.
doi: 10.1016/j.biomaterials.2007.07.017 URL |
| 95. |
Ashraf, R.; Sofi, H. S.; Malik, A.; Beigh, M. A.; Hamid, R.; Sheikh, F. A. Recent Trends in the Fabrication of Starch Nanofibers: Electrospinning and Non-electrospinning Routes and Their Applications in Biotechnology. Appl Biochem Biotechnol. 2019, 187, 47-74.
doi: 10.1007/s12010-018-2797-0 URL |
| 96. | Kudo, F. A.; Nishibe, T.; Miyazaki, K.; Flores, J.; Yasuda, K. Albumin-coated knitted Dacron aortic prosthses. Study of postoperative inflammatory reactions. Int Angiol. 2002, 21, 214-217. |
| 97. |
Radenkovic, D.; Solouk, A.; Seifalian, A. Personalized development of human organs using 3D printing technology. Med Hypotheses. 2016, 87, 30-33.
doi: 10.1016/j.mehy.2015.12.017 URL |
| 98. |
Melchiorri, A. J.; Hibino, N.; Best, C. A.; Yi, T.; Lee, Y. U.; Kraynak, C. A.; Kimerer, L. K.; Krieger, A.; Kim, P.; Breuer, C. K.; Fisher, J. P. 3D-printed biodegradable polymeric vascular grafts. Adv Healthc Mater. 2016, 5, 319-325.
doi: 10.1002/adhm.v5.3 URL |
| 99. | Mosadegh, B.; Xiong, G.; Dunham, S.; Min, J. K. Current progress in 3D printing for cardiovascular tissue engineering. Biomed Mater. 2015, 10, 034002. |
| 100. |
Lee, S. J.; Heo, D. N.; Park, J. S.; Kwon, S. K.; Lee, J. H.; Lee, J. H.; Kim, W. D.; Kwon, I. K.; Park, S. A. Characterization and preparation of bio-tubular scaffolds for fabricating artificial vascular grafts by combining electrospinning and a 3D printing system. Phys Chem Chem Phys. 2015, 17, 2996-2999.
doi: 10.1039/C4CP04801F URL |
| 101. |
Sakaguchi, K.; Shimizu, T.; Horaguchi, S.; Sekine, H.; Yamato, M.; Umezu, M.; Okano, T. In vitro engineering of vascularized tissue surrogates. Sci Rep. 2013, 3, 1316.
doi: 10.1038/srep01316 URL |
| 102. |
Sekine, H.; Shimizu, T.; Sakaguchi, K.; Dobashi, I.; Wada, M.; Yamato, M.; Kobayashi, E.; Umezu, M.; Okano, T. In vitro fabrication of functional three-dimensional tissues with perfusable blood vessels. Nat Commun. 2013, 4, 1399.
doi: 10.1038/ncomms2406 URL |
| 103. |
Scott, R. A.; Panitch, A. Macromolecular approaches to prevent thrombosis and intimal hyperplasia following percutaneous coronary intervention. Biomacromolecules. 2014, 15, 2825-2832.
doi: 10.1021/bm5007757 URL |
| 104. |
Wang, Z.; Lu, Y.; Qin, K.; Wu, Y.; Tian, Y.; Wang, J.; Zhang, J.; Hou, J.; Cui, Y.; Wang, K.; Shen, J.; Xu, Q.; Kong, D.; Zhao, Q. Enzyme-functionalized vascular grafts catalyze in-situ release of nitric oxide from exogenous NO prodrug. J Control Release. 2015, 210, 179-188.
doi: 10.1016/j.jconrel.2015.05.283 URL |
| 105. |
Ranjan, A. K.; Kumar, U.; Hardikar, A. A.; Poddar, P.; Nair, P. D.; Hardikar, A. A. Human blood vessel-derived endothelial progenitors for endothelialization of small diameter vascular prosthesis. PLoS One. 2009, 4, e7718.
doi: 10.1371/journal.pone.0007718 URL |
| 106. |
Kang, I. K.; Kwon, B. K.; Lee, J. H.; Lee, H. B. Immobilization of proteins on poly(methyl methacrylate) films. Biomaterials. 1993, 14, 787-792.
doi: 10.1016/0142-9612(93)90045-4 URL |
| 107. |
Arepally, G. M.; Cines, D. B. Pathogenesis of heparin-induced thrombocytopenia. Transl Res. 2020, 225, 131-140.
doi: 10.1016/j.trsl.2020.04.014 URL |
| 108. |
More, R. S.; Brack, M. J.; Gershlick, A. H. Heparin after angioplasty: an unresolved issue? Eur Heart J. 1993, 14, 1543-1547.
doi: 10.1093/eurheartj/14.11.1543 URL |
| 109. |
Bezon, E.; Khalifa, A. A.; Le Gal, G.; Choplain, J. N.; Mansourati, J.; Barra, J. A. Use of arterial patch to improve re-endothelialization in a sheep model of open carotid endarterectomy. An incentive to use internal thoracic artery as an on-lay patch following coronary endarterecomy? Interact Cardiovasc Thorac Surg. 2009, 8, 543-547.
doi: 10.1510/icvts.2008.198317 URL |
| 110. |
Patel, S. D.; Waltham, M.; Wadoodi, A.; Burnand, K. G.; Smith, A. The role of endothelial cells and their progenitors in intimal hyperplasia. Ther Adv Cardiovasc Dis. 2010, 4, 129-141.
doi: 10.1177/1753944710362903 URL |
| 111. |
Melchiorri, A. J.; Hibino, N.; Fisher, J. P. Strategies and techniques to enhance the in situ endothelialization of small-diameter biodegradable polymeric vascular grafts. Tissue Eng Part B Rev. 2013, 19, 292-307.
doi: 10.1089/ten.teb.2012.0577 URL |
| 112. |
Nguyen, T. U.; Shojaee, M.; Bashur, C. A.; Kishore, V. Electrochemical fabrication of a biomimetic elastin-containing bi-layered scaffold for vascular tissue engineering. Biofabrication. 2018, 11, 015007.
doi: 10.1088/1758-5090/aaeab0 URL |
| 113. |
Bates, N. M.; Heidenreich, H. E.; Fallon, M. E.; Yao, Y.; Yim, E. K. F.; Hinds, M. T.; Anderson, D. E. J. Bioconjugation of a collagen-mimicking peptide onto poly(vinyl alcohol) encourages endothelialization while minimizing thrombosis. Front Bioeng Biotechnol. 2020, 8, 621768.
doi: 10.3389/fbioe.2020.621768 URL |
| 114. |
Do, T. M.; Yang, Y.; Deng, A. Porous bilayer vascular grafts fabricated from electrospinning of the recombinant human collagen (RHC) peptide-based blend. Polymers. 2021, 13, 4042.
doi: 10.3390/polym13224042 URL |
| 115. |
Adipurnama, I.; Yang, M. C.; Ciach, T.; Butruk-Raszeja, B. Surface modification and endothelialization of polyurethane for vascular tissue engineering applications: a review. Biomater Sci. 2016, 5, 22-37.
doi: 10.1039/C6BM00618C URL |
| 116. |
Issa Bhaloo, S.; Wu, Y.; Le Bras, A.; Yu, B.; Gu, W.; Xie, Y.; Deng, J.; Wang, Z.; Zhang, Z.; Kong, D.; Hu, Y.; Qu, A.; Zhao, Q.; Xu, Q. Binding of Dickkopf-3 to CXCR7 enhances vascular progenitor cell migration and degradable graft regeneration. Circ Res. 2018, 123, 451-466.
doi: 10.1161/CIRCRESAHA.118.312945 URL |
| 117. | Pan, Y.; Yang, J.; Wei, Y.; Wang, H.; Jiao, R.; Moraga, A.; Zhang, Z.; Hu, Y.; Kong, D.; Xu, Q.; Zeng, L.; Zhao, Q. Histone deacetylase 7-derived peptides play a vital role in vascular repair and regeneration. Adv Sci (Weinh). 2018, 5, 1800006. |
| 118. | Kong, X.; Han, B.; Wang, H.; Li, H.; Xu, W.; Liu, W. Mechanical properties of biodegradable small-diameter chitosan artificial vascular prosthesis. J Biomed Mater Res A. 2012, 100, 1938-1945. |
| 119. |
Aytemiz, D.; Sakiyama, W.; Suzuki, Y.; Nakaizumi, N.; Tanaka, R.; Ogawa, Y.; Takagi, Y.; Nakazawa, Y.; Asakura, T. Small-diameter silk vascular grafts (3 mm diameter) with a double-raschel knitted silk tube coated with silk fibroin sponge. Adv Healthc Mater. 2013, 2, 361-368.
doi: 10.1002/adhm.v2.2 URL |
| 120. |
Meinel, L.; Kaplan, D. L. Silk constructs for delivery of musculoskeletal therapeutics. Adv Drug Deliv Rev. 2012, 64, 1111-1122.
doi: 10.1016/j.addr.2012.03.016 URL |
| 121. |
Peichev, M.; Naiyer, A. J.; Pereira, D.; Zhu, Z.; Lane, W. J.; Williams, M.; Oz, M. C.; Hicklin, D. J.; Witte, L.; Moore, M. A.; Rafii, S. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000, 95, 952-958.
doi: 10.1182/blood.V95.3.952.003k27_952_958 URL |
| 122. |
Rhodes, N. P.; Williams, D. F. Plasma recalcification as a measure of contact phase activation and heparinization efficacy after contact with biomaterials. Biomaterials. 1994, 15, 35-37.
doi: 10.1016/0142-9612(94)90194-5 URL |
| 123. |
Jin, D.; Hu, J.; Xia, D.; Liu, A.; Kuang, H.; Du, J.; Mo, X.; Yin, M. Evaluation of a simple off-the-shelf bi-layered vascular scaffold based on poly(L-lactide-co-ε-caprolactone)/silk fibroin in vitro and in vivo. Int J Nanomedicine. 2019, 14, 4261-4276.
doi: 10.2147/IJN URL |
| 124. |
Zheng, W.; Wang, Z.; Song, L.; Zhao, Q.; Zhang, J.; Li, D.; Wang, S.; Han, J.; Zheng, X. L.; Yang, Z.; Kong, D. Endothelialization and patency of RGD-functionalized vascular grafts in a rabbit carotid artery model. Biomaterials. 2012, 33, 2880-2891.
doi: 10.1016/j.biomaterials.2011.12.047 URL |
| 125. |
Yang, X.; Wei, J.; Lei, D.; Liu, Y.; Wu, W. Appropriate density of PCL nano-fiber sheath promoted muscular remodeling of PGS/PCL grafts in arterial circulation. Biomaterials. 2016, 88, 34-47.
doi: 10.1016/j.biomaterials.2016.02.026 URL |
| 126. |
Syedain, Z.; Reimer, J.; Lahti, M.; Berry, J.; Johnson, S.; Tranquillo, R. T. Tissue engineering of acellular vascular grafts capable of somatic growth in young lambs. Nat Commun. 2016, 7, 12951.
doi: 10.1038/ncomms12951 URL |
| 127. |
Swartz, D. D.; Andreadis, S. T. Animal models for vascular tissue-engineering. Curr Opin Biotechnol. 2013, 24, 916-925.
doi: 10.1016/j.copbio.2013.05.005 URL |
| 128. |
Byrom, M. J.; Bannon, P. G.; White, G. H.; Ng, M. K. Animal models for the assessment of novel vascular conduits. J Vasc Surg. 2010, 52, 176-195.
doi: 10.1016/j.jvs.2009.10.080 URL |
| 129. | Riboldi, S. A.; Tozzi, M.; Bagardi, M.; Ravasio, G.; Cigalino, G.; Crippa, L.; Piccolo, S.; Nahal, A.; Spandri, M.; Catto, V.; Tironi, M.; Greco, F. G.; Remuzzi, A.; Acocella, F. A novel hybrid silk fibroin/polyurethane arteriovenous graft for hemodialysis: proof-of-concept animal study in an ovine model. Adv Healthc Mater. 2020, 9, e2000794. |
| 130. |
Koens, M. J.; Krasznai, A. G.; Hanssen, A. E.; Hendriks, T.; Praster, R.; Daamen, W. F.; van der Vliet, J. A.; van Kuppevelt, T. H. Vascular replacement using a layered elastin-collagen vascular graft in a porcine model: one week patency versus one month occlusion. Organogenesis. 2015, 11, 105-121.
doi: 10.1080/15476278.2015.1038448 URL |
| 131. |
Yamamoto, S.; Okamoto, H.; Haga, M.; Shigematsu, K.; Miyata, T.; Watanabe, T.; Ogawa, Y.; Takagi, Y.; Asakura, T. Rapid endothelialization and thin luminal layers in vascular grafts using silk fibroin. J Mater Chem B. 2016, 4, 938-946.
doi: 10.1039/C5TB02528A URL |
| 132. |
Li, H.; Wang, Y.; Sun, X.; Tian, W.; Xu, J.; Wang, J. Steady-state behavior and endothelialization of a silk-based small-caliber scaffold in vivo transplantation. Polymers. 2019, 11, 1303.
doi: 10.3390/polym11081303 URL |
| 133. | Cutiongco, M. F.; Kukumberg, M.; Peneyra, J. L.; Yeo, M. S.; Yao, J. Y.; Rufaihah, A. J.; Le Visage, C.; Ho, J. P.; Yim, E. K. Submillimeter diameter poly(vinyl alcohol) vascular graft patency in rabbit model. Front Bioeng Biotechnol. 2016, 4, 44. |
| 134. |
Zhang, J.; Huang, H.; Ju, R.; Chen, K.; Li, S.; Wang, W.; Yan, Y. In vivo biocompatibility and hemocompatibility of a polytetrafluoroethylene small diameter vascular graft modified with sulfonated silk fibroin. Am J Surg. 2017, 213, 87-93.
doi: 10.1016/j.amjsurg.2016.04.005 URL |
| 135. |
Li, W.; Chen, J.; Xu, P.; Zhu, M.; Wu, Y.; Wang, Z.; Zhao, T.; Cheng, Q.; Wang, K.; Fan, G.; Zhu, Y.; Kong, D. Long-term evaluation of vascular grafts with circumferentially aligned microfibers in a rat abdominal aorta replacement model. J Biomed Mater Res B Appl Biomater. 2018, 106, 2596-2604.
doi: 10.1002/jbm.b.34076 URL |
| 136. |
Fu, J.; Ding, X.; Stowell, C. E. T.; Wu, Y. L.; Wang, Y. Slow degrading poly(glycerol sebacate) derivatives improve vascular graft remodeling in a rat carotid artery interposition model. Biomaterials. 2020, 257, 120251.
doi: 10.1016/j.biomaterials.2020.120251 URL |
| 137. |
Sugiura, T.; Tara, S.; Nakayama, H.; Kurobe, H.; Yi, T.; Lee, Y. U.; Lee, A. Y.; Breuer, C. K.; Shinoka, T. Novel bioresorbable vascular graft with sponge-type scaffold as a small-diameter arterial graft. Ann Thorac Surg. 2016, 102, 720-727.
doi: 10.1016/j.athoracsur.2016.01.110 URL |
| 138. |
Weber, B.; Emmert, M. Y.; Schoenauer, R.; Brokopp, C.; Baumgartner, L.; Hoerstrup, S. P. Tissue engineering on matrix: future of autologous tissue replacement. Semin Immunopathol. 2011, 33, 307-315.
doi: 10.1007/s00281-011-0258-8 URL |
| 139. |
Itoh, M.; Mukae, Y.; Kitsuka, T.; Arai, K.; Nakamura, A.; Uchihashi, K.; Toda, S.; Matsubayashi, K.; Oyama, J. I.; Node, K.; Kami, D.; Gojo, S.; Morita, S.; Nishida, T.; Nakayama, K.; Kobayashi, E. Development of an immunodeficient pig model allowing long-term accommodation of artificial human vascular tubes. Nat Commun. 2019, 10, 2244.
doi: 10.1038/s41467-019-10107-1 URL |
| 140. |
Rohren, E. M.; Kliewer, M. A.; Carroll, B. A.; Hertzberg, B. S. A spectrum of Doppler waveforms in the carotid and vertebral arteries. AJR Am[J] Roentgenol. 2003, 181, 1695-1704.
doi: 10.2214/ajr.181.6.1811695 URL |
| 141. |
Fang, S.; Ellman, D. G.; Andersen, D. C. Review: tissue engineering of small-diameter vascular grafts and their in vivo evaluation in large animals and humans. Cells. 2021, 10, 713.
doi: 10.3390/cells10030713 URL |
| 142. |
Fayon, A.; Menu, P.; El Omar, R. Cellularized small-caliber tissue-engineered vascular grafts: looking for the ultimate gold standard. NPJ Regen Med. 2021, 6, 46.
doi: 10.1038/s41536-021-00155-x URL |
| 143. | Ren, X.; Feng, Y.; Guo, J.; Wang, H.; Li, Q.; Yang, J.; Hao, X.; Lv, J.; Ma, N.; Li, W. Surface modification and endothelialization of biomaterials as potential scaffolds for vascular tissue engineering applications. Chem Soc Rev. 2015, 44, 5680-5742. |
| 144. |
Kang, H. W.; Lee, S. J.; Ko, I. K.; Kengla, C.; Yoo, J. J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol. 2016, 34, 312-319.
doi: 10.1038/nbt.3413 URL |
| 145. |
Li, X.; Liu, L.; Zhang, X.; Xu, T. Research and development of 3D printed vasculature constructs. Biofabrication. 2018, 10, 032002.
doi: 10.1088/1758-5090/aabd56 URL |
| 146. |
Wang, D.; Liu, H.; Fan, Y. Silk fibroin for vascular regeneration. Microsc Res Tech. 2017, 80, 280-290.
doi: 10.1002/jemt.v80.3 URL |
| 147. | Duijvelshoff, R.; Cabrera, M. S.; Sanders, B.; Dekker, S.; Smits, A.; Baaijens, F. P. T.; Bouten, C. V. C. Transcatheter-delivered expandable bioresorbable polymeric graft with stenting capacity induces vascular regeneration. JACC Basic Transl Sci. 2020, 5, 1095-1110. |
| [1] | Shuqin Cao, Quan Yuan. An update of nanotopographical surfaces in modulating stem cell fate: a narrative review [J]. Biomaterials Translational, 2022, 3(1): 55-64. |
| [2] | Emma Steijvers, Armaan Ghei, Zhidao Xia. Manufacturing artificial bone allografts: a perspective [J]. Biomaterials Translational, 2022, 3(1): 65-80. |
| [3] | Yizhong Peng, Jinye Li, Hui Lin, Shuo Tian, Sheng Liu, Feifei Pu, Lei Zhao, Kaige Ma, Xiangcheng Qing, Zengwu Shao. Endogenous repair theory enriches construction strategies for orthopaedic biomaterials: a narrative review [J]. Biomaterials Translational, 2021, 2(4): 343-360. |
| [4] | Xirui Jing, Qiuyue Ding, Qinxue Wu, Weijie Su, Keda Yu, Yanlin Su, Bing Ye, Qing Gao, Tingfang Sun, Xiaodong Guo. Magnesium-based materials in orthopaedics: material properties and animal models [J]. Biomaterials Translational, 2021, 2(3): 197-213. |
| [5] | Kamolrat Metavarayuth, Esteban Villarreal, Hui Wang, Qian Wang. Surface topography and free energy regulate osteogenesis of stem cells: effects of shape-controlled gold nanoparticles [J]. Biomaterials Translational, 2021, 2(2): 165-173. |
| [6] | Yizhong Peng, Xiangcheng Qing, Hongyang Shu, Shuo Tian, Wenbo Yang, Songfeng Chen, Hui Lin, Xiao Lv, Lei Zhao, Xi Chen, Feifei Pu, Donghua Huang, Xu Cao, Zengwu Shao. Proper animal experimental designs for preclinical research of biomaterials for intervertebral disc regeneration [J]. Biomaterials Translational, 2021, 2(2): 91-142. |
| [7] | Pingli Wu, Yangyang Liang, Guoming Sun. Engineering immune-responsive biomaterials for skin regeneration [J]. Biomaterials Translational, 2021, 2(1): 61-71. |
| [8] | Yiqing Wang, Xiangyu Chu, Bing Wang. Recombinant adeno-associated virus-based gene therapy combined with tissue engineering for musculoskeletal regenerative medicine [J]. Biomaterials Translational, 2021, 2(1): 19-29. |
| [9] | Isak Jatoi, Jingyu Fan. A biomaterials viewpoint for the 2020 SARS-CoV-2 vaccine development [J]. Biomaterials Translational, 2021, 2(1): 30-42. |
| [10] | Maryam Tamaddon, Helena Gilja, Ling Wang, J. Miguel Oliveira, Xiaodan Sun, Rongwei Tan, Chaozong Liu. Osteochondral scaffolds for early treatment of cartilage defects in osteoarthritic joints: from bench to clinic [J]. Biomaterials Translational, 2020, 1(1): 3-17. |
| [11] | Xing Yang, Yuanyuan Li, Xujie Liu, Wei He, Qianli Huang, Qingling Feng. Nanoparticles and their effects on differentiation of mesenchymal stem cells [J]. Biomaterials Translational, 2020, 1(1): 58-68. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||