Biomaterials Translational ›› 2022, Vol. 3 ›› Issue (4): 280-294.doi: 10.12336/biomatertransl.2022.04.007
• REVIEW • Previous Articles
Guixin Yuan1,2,3, Zan Li4, Xixi Lin1,2,3, Na Li1,2,3,*( ), Ren Xu1,2,3,*(
), Ren Xu1,2,3,*( )
)
Received:2022-11-09
Revised:2022-11-29
Accepted:2022-12-19
Online:2022-12-29
Published:2022-12-28
Contact:
Na Li, Email: xuren526@xmu.edu.cn; Ren Xu, Email: lina0924@xmu.edu.cn
About author:Na Li, lina0924@xmu.edu.cn.;#Author Equally.
Yuan, G.; Li, Z.; Lin, X.; Li, N.; Xu, R. New perspective of skeletal stem cells. Biomater Transl. 2022, 3(4), 280-294.
| PDPN | CD146 | CD73 | CD164 | THY1 | AlphaV | Thy | 6C3 | CD105 | CD200 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| hSSC | + | – | + | + | mSSCs | + | – | – | – | ||
| hBCSP | + | + | Pre–BCSP | + | – | – | – | – | |||
| hOP–1 | – | + | hi | BCSP | + | – | – | + | |||
| hOP–2 | – | + | – | – | lo | PCP | + | – | – | + | + |
| hCP–1 | + | – | – | – | Thy | + | + | – | + | ||
| hCP–2 | + | – | – | + | BLSP | + | + | – | – | ||
| hCP–3 | + | – | + | + | 6C3 | + | – | + | + | ||
| HEC | + | – | – | – |
Table 1. Differences in surface markers between hSSCs and mSSCs
| PDPN | CD146 | CD73 | CD164 | THY1 | AlphaV | Thy | 6C3 | CD105 | CD200 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| hSSC | + | – | + | + | mSSCs | + | – | – | – | ||
| hBCSP | + | + | Pre–BCSP | + | – | – | – | – | |||
| hOP–1 | – | + | hi | BCSP | + | – | – | + | |||
| hOP–2 | – | + | – | – | lo | PCP | + | – | – | + | + |
| hCP–1 | + | – | – | – | Thy | + | + | – | + | ||
| hCP–2 | + | – | – | + | BLSP | + | + | – | – | ||
| hCP–3 | + | – | + | + | 6C3 | + | – | + | + | ||
| HEC | + | – | – | – |
| Gene | Transgenes | Source | Function | References |
|---|---|---|---|---|
| Col2a1 | Col2a1a–CreER | Bone/cartilage | Contribute to osteoblasts and chondrocytes and the formation of CD31+ blood vessels | |
| Gli1 | Gli1–CreERT | Bone/cartilage | Expressed in the quiescent zone of the long bone growth plate in postnatal mice | |
| PTHrP | PTHrP–CreER | Bone/cartilage | Generate chondrocytes, osteoblasts, and mature cortical osteocytes during the repair of femoral fracture | |
| Sox9 | Sox9–CreERT | cartilage | Differentiate into chondrocytes | |
| FoxA2 | FoxA2–CreER | Bone/cartilage | Promote growth plate tissue regeneration, exhibit higher clonogenicity, and longevity | |
| mTert | mTert–rtTA | Cartilage | Contribute to endochondral osteogenesis as chondrogenic osteoprogenitor cells |
Table 2. Markers of skeletal stem cells in long bone growth plates
| Gene | Transgenes | Source | Function | References |
|---|---|---|---|---|
| Col2a1 | Col2a1a–CreER | Bone/cartilage | Contribute to osteoblasts and chondrocytes and the formation of CD31+ blood vessels | |
| Gli1 | Gli1–CreERT | Bone/cartilage | Expressed in the quiescent zone of the long bone growth plate in postnatal mice | |
| PTHrP | PTHrP–CreER | Bone/cartilage | Generate chondrocytes, osteoblasts, and mature cortical osteocytes during the repair of femoral fracture | |
| Sox9 | Sox9–CreERT | cartilage | Differentiate into chondrocytes | |
| FoxA2 | FoxA2–CreER | Bone/cartilage | Promote growth plate tissue regeneration, exhibit higher clonogenicity, and longevity | |
| mTert | mTert–rtTA | Cartilage | Contribute to endochondral osteogenesis as chondrogenic osteoprogenitor cells |
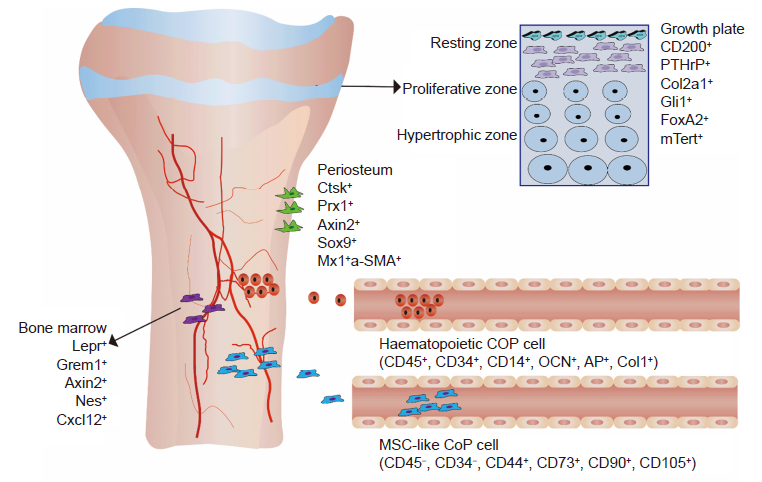
Figure 1. The distribution of SSCs in the growth plate, periosteum, bone marrow, and peripheral circulation shows different cell surface markers. The cell surface markers of SSCs on the growth plate are CD200+, PTHrP+ Col2a1+, Gli1+, FoxA2+, and mTert+. The cell surface markers of PSCs are Ctsk+, Prx1+ Axin2+, Sox9+, Mx1+, and α–SMA+. The cell surface markers of SSCs in the bone marrow cavity are Lepr+, Nestin+, Nes+, Cxcl12+, and Grem1+. The cell surface markers of circulating osteogenic cells in the peripheral circulation are haematopoietic COP cells (CD45+, CD34+, CD14+, OCN+, AP+, and Col1+) and MSC–like COP cells (CD45–, CD34–, CD44+, CD73+, CD90+, and CD105+). Axin2: axis inhibition protein 2; AP: alkaline phosphatase; OCN: osteocalcin; Col1: type 1 collagen; Col2a1: type 2 collagen alpha 1 chain; COP: circulating osteogenic cell; Ctsk: cathepsin K; Cxcl12: chemokine (C–X–C motif) ligand 12; FoxA2: forkhead box A2; Gli1: GLI–Kruppel family member GLI1; Grem1: gremlin 1, DAN family BMP antagonist; Lepr: leptin receptor; MSC: mesenchymal stem cell; mTert: mouse Telomerase; Mx1: MX dynamin like GTPase 1; Nes: nestin; Prx1: paired related homeobox 1; PSC: periosteal stem cell; PTHrP: parathyroid–associated protein; Sox9: SRY (sex determining region Y)–box 9; SSCs: skeletal stem cells; α–SMA: α–smooth muscle actin.
| Gene | Transgenes | Source | Function | References |
|---|---|---|---|---|
| Ctsk | Ctsk–Cre | Bone/cartilage/adipose | A marker of osteoclasts; distinguishing periosteal osteoprogenitor cell types | |
| Prx1 | Prrx1–Cre | Bone/cartilage | In the repair of cranial defects | |
| Sox9 | Sox9–CreERT | Bone/cartilage | Generate chondrocytes, osteoblasts, and mature cortical osteocytes during the repair of femoral fracture | |
| Mx1 | Mx1–Cre αSMA–CreERT αSMA–GFP | Bone/cartilage | Repairing new periosteum | |
| αSMA | ||||
| Gli1 | Gli1–CreERT | Bone/cartilage | Labels skeletal stem cells of the growth plate | |
| Hoxa11 | Hoxa11–CreERT2 Hoxa11–EGFP | Bone/cartilage | Regulating differentiation of Hox–expressing skeletal stem cells into the osteolineage |
Table 3. Markers of skeletal stem cells in the periosteum of long bones
| Gene | Transgenes | Source | Function | References |
|---|---|---|---|---|
| Ctsk | Ctsk–Cre | Bone/cartilage/adipose | A marker of osteoclasts; distinguishing periosteal osteoprogenitor cell types | |
| Prx1 | Prrx1–Cre | Bone/cartilage | In the repair of cranial defects | |
| Sox9 | Sox9–CreERT | Bone/cartilage | Generate chondrocytes, osteoblasts, and mature cortical osteocytes during the repair of femoral fracture | |
| Mx1 | Mx1–Cre αSMA–CreERT αSMA–GFP | Bone/cartilage | Repairing new periosteum | |
| αSMA | ||||
| Gli1 | Gli1–CreERT | Bone/cartilage | Labels skeletal stem cells of the growth plate | |
| Hoxa11 | Hoxa11–CreERT2 Hoxa11–EGFP | Bone/cartilage | Regulating differentiation of Hox–expressing skeletal stem cells into the osteolineage |
| Gene | Transgenes | Source | Function | References |
|---|---|---|---|---|
| Axin2 | Axin2–CreER | Bone/cartilage/adipose | Osteoblasts, mesenchymal cells | |
| Cxcl12 | Cxcl12–CreER | Bone/cartilage | Reactivate to form osteoblasts after injury | |
| Mx1 | Mx1–CreER | Bone/cartilage | Differentiate into cartilage and adipose tissue, respond to bone fractures | |
| Nestin | Nestin–CreER | Bone/cartilage | Nestin+ mesenchymal stem cells can self–renew and expand in transplantation experiments | |
| Lepr | Lepr–Cre | Bone/cartilage/adipose | Regulation of adipogenesis and osteogenesis | |
| Grem1 | Grem1–CreER | Bone/cartilage | Mark osteochondroreticular stem cells, bone repair |
Table 4. Markers of skeletal stem cells in the bone marrow
| Gene | Transgenes | Source | Function | References |
|---|---|---|---|---|
| Axin2 | Axin2–CreER | Bone/cartilage/adipose | Osteoblasts, mesenchymal cells | |
| Cxcl12 | Cxcl12–CreER | Bone/cartilage | Reactivate to form osteoblasts after injury | |
| Mx1 | Mx1–CreER | Bone/cartilage | Differentiate into cartilage and adipose tissue, respond to bone fractures | |
| Nestin | Nestin–CreER | Bone/cartilage | Nestin+ mesenchymal stem cells can self–renew and expand in transplantation experiments | |
| Lepr | Lepr–Cre | Bone/cartilage/adipose | Regulation of adipogenesis and osteogenesis | |
| Grem1 | Grem1–CreER | Bone/cartilage | Mark osteochondroreticular stem cells, bone repair |
| Craniofacial bone | Gene | Transgenes | Source | Function | References |
|---|---|---|---|---|---|
| Periosteum of the cranium | Ctsk | Ctsk–Cre | Bone/cartilage | Intramembranous osteogenesis | |
| Mx1 | Mx1–Cre | Bone/cartilage | Participate in bone healing | ||
| SMA | αSMA–CreERT | Bone/cartilage | |||
| Calvarial sutures | Gli1 | Gli1–CreER | Bone/cartilage | Cause premature craniosynostosis | |
| Axin2 | Axin2–CreERT | Bone/cartilage | Respond to orthodontic tension force | ||
| Prx1 | Prx1–Cre | Bone/cartilage | Scattered distribution in calvaria sutures | ||
| Teeth and periodontal tissue | Gli1 | Gli1–CreER | Bone/cartilage | Regulated by Wnt pathway | |
| Axin2 | Axin2–CreERT | Bone/cartilage | Primary progenitor cells of cementoblasts | ||
| Prx1 | Prx1–Cre | Bone/cartilage | Participate in angiogenesis. | ||
| Jaw bone | Ctsk | Ctsk–Cre | Bone/cartilage | Present on the periosteum of the jaw | |
| Ly6a | Bone/cartilage |
Table 5. Markers of skeletal stem cells in craniofacial bone
| Craniofacial bone | Gene | Transgenes | Source | Function | References |
|---|---|---|---|---|---|
| Periosteum of the cranium | Ctsk | Ctsk–Cre | Bone/cartilage | Intramembranous osteogenesis | |
| Mx1 | Mx1–Cre | Bone/cartilage | Participate in bone healing | ||
| SMA | αSMA–CreERT | Bone/cartilage | |||
| Calvarial sutures | Gli1 | Gli1–CreER | Bone/cartilage | Cause premature craniosynostosis | |
| Axin2 | Axin2–CreERT | Bone/cartilage | Respond to orthodontic tension force | ||
| Prx1 | Prx1–Cre | Bone/cartilage | Scattered distribution in calvaria sutures | ||
| Teeth and periodontal tissue | Gli1 | Gli1–CreER | Bone/cartilage | Regulated by Wnt pathway | |
| Axin2 | Axin2–CreERT | Bone/cartilage | Primary progenitor cells of cementoblasts | ||
| Prx1 | Prx1–Cre | Bone/cartilage | Participate in angiogenesis. | ||
| Jaw bone | Ctsk | Ctsk–Cre | Bone/cartilage | Present on the periosteum of the jaw | |
| Ly6a | Bone/cartilage |
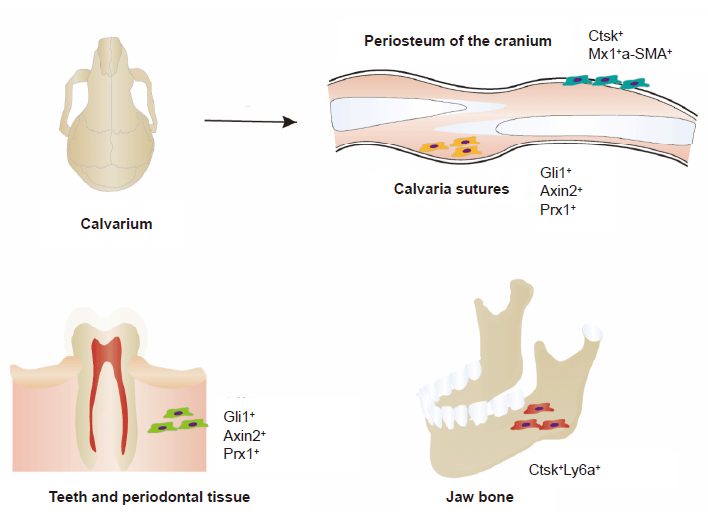
Figure 2. Distribution of SSCs in craniofacial bone, with different cell surfaces shown. The cell surface markers of SSCs in the cranial periosteum are Ctsk+ and Mx1+αSMA+. The cell surface markers of SSCs in the calvarial sutures are Gli1+, Prx1+, and Axin2+. The cell surface markers of SSCs in the teeth and periodontal tissue are Gli1+, Prx1+, and Axin2+. The cell surface markers of SSCs in the jaw bone are Ctsk+ and Ly6a+. Axin2: axis inhibition protein 2; Ctsk: cathepsin K; Gli1: GLI–Kruppel family member GLI1; Ly6a: lymphocyte antigen 6 complex, locus A; Mx1: MX dynamin like GTPase 1; Prx1: paired related homeobox 1; SSC: skeletal stem cell; αSMA: α–smooth muscle actin.
| Cell type | Markers | Function | References |
|---|---|---|---|
| Haematopoietic COP cells | CD45+CD34+CD14+OCN+AP+Col1+ | Maintain a stable level in the peripheral circulation | |
| MSC–like COP cells | CD45–CD34–CD44+CD73+CD90+CD105+ | Possess characteristics of mesenchymal stem cells |
Table 6. Markers of circulating osteogenic cells in the peripheral circulation
| Cell type | Markers | Function | References |
|---|---|---|---|
| Haematopoietic COP cells | CD45+CD34+CD14+OCN+AP+Col1+ | Maintain a stable level in the peripheral circulation | |
| MSC–like COP cells | CD45–CD34–CD44+CD73+CD90+CD105+ | Possess characteristics of mesenchymal stem cells |
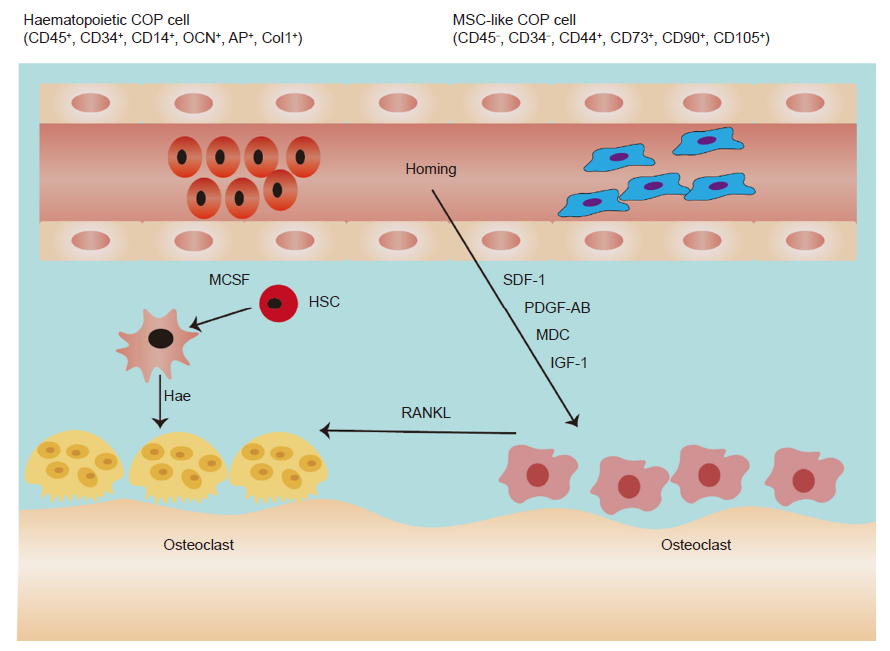
Figure 3. Circulating osteogenic cells contribute to bone formation through stem cell homing to receive IGF–1, PDGF–AB, SDF–1, and MDC. M–CSF stimulates HSCs to differentiate into monocytes and macrophages and then differentiate into osteoclasts to receive RANKL. COP: circulating osteogenic precursor cell; HSC: haematopoietic stem cell; IGF–1: insulin–like growth factor 1; M–CSF: macrophage colony–stimulating factor; MDC: macrophage–derived chemokine; MSC: mesenchymal stem cell; PDGF–AB: platelet derived growth factor AB; RANKL: receptor activator of nuclear factor kappa–Β ligand; SDF–1: stromal cell–derived factor–1.
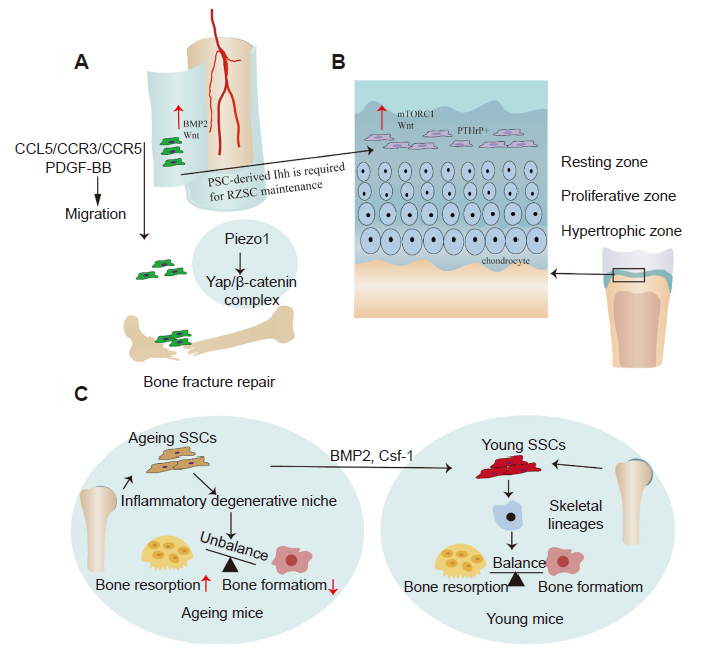
Figure 4. (A) Wnt and BMP2 are enriched in PSCs. PDGF–BB and CCL5/CCR3/CCR5 promote the migration of SSCs, while activation of Piezo1 promotes the expression and nuclear localisation of Yap in PSCs, and forms a transcriptional Yap/β–catenin complex which promotes fracture healing. (B) The Wnt and mTORC1 signalling pathways regulate SSCs of the growth plate. PSCs control growth plate SSCs through the Ihh signalling pathway. (C) Aged mice develop a pro–inflammatory microenvironment that disrupts the osteoclast–osteoblast balance. A combination of BMP2 and CSF1 antagonists reverses this change and activates aging SSCs in mice, returning them to a younger state. BMP2: bone morphogenetic protein 2; CCL5: chemokine (C–C motif) ligand 5; CCR3: C–C motif chemokine receptor 3; CCR5: C–C motif chemokine receptor 5; CSF1: colony stimulating factor 1; mTORC1: mechanistic target of rapamycin complex; PDGF–BB: platelet derived growth factor BB; PSC: periosteal stem cell; PTHrP: parathyroid hormone–related protein; Wnt: wingless–related integration site; Yap: yes–related protein.
| 1. |
Zheng, C.; Chen, J.; Liu, S.; Jin, Y. Stem cell-based bone and dental regeneration: a view of microenvironmental modulation. Int J Oral Sci. 2019, 11, 23.
doi: 10.1038/s41368-019-0060-3 URL |
| 2. |
Zhu, L.; Luo, D.; Liu, Y. Effect of the nano/microscale structure of biomaterial scaffolds on bone regeneration. Int J Oral Sci. 2020, 12, 6.
doi: 10.1038/s41368-020-0073-y URL |
| 3. |
Su, N.; Yang, J.; Xie, Y.; Du, X.; Chen, H.; Zhou, H.; Chen, L. Bone function, dysfunction and its role in diseases including critical illness. Int J Biol Sci. 2019, 15, 776-787.
doi: 10.7150/ijbs.27063 URL |
| 4. |
Khosla, S.; Farr, J. N.; Tchkonia, T.; Kirkland, J. L. The role of cellular senescence in ageing and endocrine disease. Nat Rev Endocrinol. 2020, 16, 263-275.
doi: 10.1038/s41574-020-0335-y URL |
| 5. |
Henkel, J.; Woodruff, M. A.; Epari, D. R.; Steck, R.; Glatt, V.; Dickinson, I. C.; Choong, P. F.; Schuetz, M. A.; Hutmacher, D. W. Bone regeneration based on tissue engineering conceptions - a 21st century perspective. Bone Res. 2013, 1, 216-248.
doi: 10.4248/BR201303002 URL |
| 6. |
Gao, C.; Peng, S.; Feng, P.; Shuai, C. Bone biomaterials and interactions with stem cells. Bone Res. 2017, 5, 17059.
doi: 10.1038/boneres.2017.59 URL |
| 7. |
Anthony, B. A.; Link, D. C. Regulation of hematopoietic stem cells by bone marrow stromal cells. Trends Immunol. 2014, 35, 32-37.
doi: 10.1016/j.it.2013.10.002 URL |
| 8. |
Ng, A. P.; Alexander, W. S. Haematopoietic stem cells: past, present and future. Cell Death Discov. 2017, 3, 17002.
doi: 10.1038/cddiscovery.2017.2 URL |
| 9. |
Chan, C. K.; Seo, E. Y.; Chen, J. Y.; Lo, D.; McArdle, A.; Sinha, R.; Tevlin, R.; Seita, J.; Vincent-Tompkins, J.; Wearda, T.; Lu, W. J.; Senarath-Yapa, K.; Chung, M. T.; Marecic, O.; Tran, M.; Yan, K. S.; Upton, R.; Walmsley, G. G.; Lee, A. S.; Sahoo, D.; Kuo, C. J.; Weissman, I. L.; Longaker, M. T. Identification and specification of the mouse skeletal stem cell. Cell. 2015, 160, 285-298.
doi: 10.1016/j.cell.2014.12.002 URL |
| 10. |
Chan, C. K. F.; Gulati, G. S.; Sinha, R.; Tompkins, J. V.; Lopez, M.; Carter, A. C.; Ransom, R. C.; Reinisch, A.; Wearda, T.; Murphy, M.; Brewer, R. E.; Koepke, L. S.; Marecic, O.; Manjunath, A.; Seo, E. Y.; Leavitt, T.; Lu, W. J.; Nguyen, A.; Conley, S. D.; Salhotra, A.; Ambrosi, T. H.; Borrelli, M. R.; Siebel, T.; Chan, K.; Schallmoser, K.; Seita, J.; Sahoo, D.; Goodnough, H.; Bishop, J.; Gardner, M.; Majeti, R.; Wan, D. C.; Goodman, S.; Weissman, I. L.; Chang, H. Y.; Longaker, M. T. Identification of the human skeletal stem cell. Cell. 2018, 175, 43-56.e21.
doi: 10.1016/j.cell.2018.07.029 URL |
| 11. |
Kretzschmar, K.; Watt, F. M. Lineage tracing. Cell. 2012, 148, 33-45.
doi: 10.1016/j.cell.2012.01.002 URL |
| 12. |
Gulati, G. S.; Murphy, M. P.; Marecic, O.; Lopez, M.; Brewer, R. E.; Koepke, L. S.; Manjunath, A.; Ransom, R. C.; Salhotra, A.; Weissman, I. L.; Longaker, M. T.; Chan, C. K. F. Isolation and functional assessment of mouse skeletal stem cell lineage. Nat Protoc. 2018, 13, 1294-1309.
doi: 10.1038/nprot.2018.041 URL |
| 13. |
Roelofs, A. J.; Kania, K.; Rafipay, A. J.; Sambale, M.; Kuwahara, S. T.; Collins, F. L.; Smeeton, J.; Serowoky, M. A.; Rowley, L.; Wang, H.; Gronewold, R.; Kapeni, C.; Méndez-Ferrer, S.; Little, C. B.; Bateman, J. F.; Pap, T.; Mariani, F. V.; Sherwood, J.; Crump, J. G.; De Bari, C. Identification of the skeletal progenitor cells forming osteophytes in osteoarthritis. Ann Rheum Dis. 2020, 79, 1625-1634.
doi: 10.1136/annrheumdis-2020-218350 URL |
| 14. |
Debnath, S.; Yallowitz, A. R.; McCormick, J.; Lalani, S.; Zhang, T.; Xu, R.; Li, N.; Liu, Y.; Yang, Y. S.; Eiseman, M.; Shim, J. H.; Hameed, M.; Healey, J. H.; Bostrom, M. P.; Landau, D. A.; Greenblatt, M. B. Discovery of a periosteal stem cell mediating intramembranous bone formation. Nature. 2018, 562, 133-139.
doi: 10.1038/s41586-018-0554-8 URL |
| 15. |
Han, X.; Zhang, Z.; He, L.; Zhu, H.; Li, Y.; Pu, W.; Han, M.; Zhao, H.; Liu, K.; Li, Y.; Huang, X.; Zhang, M.; Jin, H.; Lv, Z.; Tang, J.; Wang, J.; Sun, R.; Fei, J.; Tian, X.; Duan, S.; Wang, Q. D.; Wang, L.; He, B.; Zhou, B. A suite of new Dre recombinase drivers markedly expands the ability to perform intersectional genetic targeting. Cell Stem Cell. 2021, 28, 1160-1176.e7.
doi: 10.1016/j.stem.2021.01.007 URL |
| 16. |
He, L.; Li, Y.; Li, Y.; Pu, W.; Huang, X.; Tian, X.; Wang, Y.; Zhang, H.; Liu, Q.; Zhang, L.; Zhao, H.; Tang, J.; Ji, H.; Cai, D.; Han, Z.; Han, Z.; Nie, Y.; Hu, S.; Wang, Q. D.; Sun, R.; Fei, J.; Wang, F.; Chen, T.; Yan, Y.; Huang, H.; Pu, W. T.; Zhou, B. Enhancing the precision of genetic lineage tracing using dual recombinases. Nat Med. 2017, 23, 1488-1498.
doi: 10.1038/nm.4437 URL |
| 17. |
Wagner, D. E.; Klein, A. M. Lineage tracing meets single-cell omics: opportunities and challenges. Nat Rev Genet. 2020, 21, 410-427.
doi: 10.1038/s41576-020-0223-2 URL |
| 18. |
Hsu, Y. C. Theory and practice of lineage tracing. Stem Cells. 2015, 33, 3197-3204.
doi: 10.1002/stem.2123 URL |
| 19. |
Zomer, A.; Steenbeek, S. C.; Maynard, C.; van Rheenen, J. Studying extracellular vesicle transfer by a Cre-loxP method. Nat Protoc. 2016, 11, 87-101.
doi: 10.1038/nprot.2015.138 URL |
| 20. |
Kristianto, J.; Johnson, M. G.; Zastrow, R. K.; Radcliff, A. B.; Blank, R. D. Spontaneous recombinase activity of Cre-ERT2 in vivo. Transgenic Res. 2017, 26, 411-417.
doi: 10.1007/s11248-017-0018-1 URL |
| 21. |
Muzumdar, M. D.; Tasic, B.; Miyamichi, K.; Li, L.; Luo, L. A global double-fluorescent Cre reporter mouse. Genesis. 2007, 45, 593-605.
doi: 10.1002/dvg.20335 URL |
| 22. | Madisen, L.; Zwingman, T. A.; Sunkin, S. M.; Oh, S. W.; Zariwala, H. A.; Gu, H.; Ng, L. L.; Palmiter, R. D.; Hawrylycz, M. J.; Jones, A. R.; Lein, E. S.; Zeng, H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010, 13, 133-140. |
| 23. |
Soriano, P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999, 21, 70-71.
doi: 10.1038/5007 URL |
| 24. | Matsushita, Y.; Ono, W.; Ono, N. Bone regeneration via skeletal cell lineage plasticity: All hands mobilized for emergencies: quiescent mature skeletal cells can be activated in response to injury and robustly participate in bone regeneration through cellular plasticity. Bioessays. 2021, 43, e2000202. |
| 25. | Ono, N.; Balani, D. H.; Kronenberg, H. M. Stem and progenitor cells in skeletal development. Curr Top Dev Biol. 2019, 133, 1-24. |
| 26. |
Jeffery, E. C.; Mann, T. L. A.; Pool, J. A.; Zhao, Z.; Morrison, S. J. Bone marrow and periosteal skeletal stem/progenitor cells make distinct contributions to bone maintenance and repair. Cell Stem Cell. 2022, 29, 1547-1561.e6.
doi: 10.1016/j.stem.2022.10.002 URL |
| 27. |
Bianco, P.; Robey, P. G. Skeletal stem cells. Development. 2015, 142, 1023-1027.
doi: 10.1242/dev.102210 URL |
| 28. | Bianco, P.; Kuznetsov, S. A.; Riminucci, M.; Gehron Robey, P. Postnatal skeletal stem cells. Methods Enzymol. 2006, 419, 117-148. |
| 29. |
Bianco, P. “Mesenchymal” stem cells. Annu Rev Cell Dev Biol. 2014, 30, 677-704.
doi: 10.1146/annurev-cellbio-100913-013132 URL |
| 30. |
Bianco, P.; Cao, X.; Frenette, P. S.; Mao, J. J.; Robey, P. G.; Simmons, P. J.; Wang, C. Y. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013, 19, 35-42.
doi: 10.1038/nm.3028 URL |
| 31. |
He, J.; Yan, J.; Wang, J.; Zhao, L.; Xin, Q.; Zeng, Y.; Sun, Y.; Zhang, H.; Bai, Z.; Li, Z.; Ni, Y.; Gong, Y.; Li, Y.; He, H.; Bian, Z.; Lan, Y.; Ma, C.; Bian, L.; Zhu, H.; Liu, B.; Yue, R. Dissecting human embryonic skeletal stem cell ontogeny by single-cell transcriptomic and functional analyses. Cell Res. 2021, 31, 742-757.
doi: 10.1038/s41422-021-00467-z URL |
| 32. |
Zhao, H.; Zhou, W.; Yao, Z.; Wan, Y.; Cao, J.; Zhang, L.; Zhao, J.; Li, H.; Zhou, R.; Li, B.; Wei, G.; Zhang, Z.; French, C. A.; Dekker, J. D.; Yang, Y.; Fisher, S. E.; Tucker, H. O.; Guo, X. Foxp1/2/4 regulate endochondral ossification as a suppresser complex. Dev Biol. 2015, 398, 242-254.
doi: 10.1016/j.ydbio.2014.12.007 URL |
| 33. | Ambrosi, T. H.; Goodnough, L. H.; Chan, C. K. F. Human skeletal stem cell aging. Aging (Albany NY). 2020, 12, 16669-16671. |
| 34. | Hallett, S. A.; Matsushita, Y.; Ono, W.; Sakagami, N.; Mizuhashi, K.; Tokavanich, N.; Nagata, M.; Zhou, A.; Hirai, T.; Kronenberg, H. M.; Ono, N. Chondrocytes in the resting zone of the growth plate are maintained in a Wnt-inhibitory environment. Elife. 2021, 10, e64513. |
| 35. |
Wang, Y.; Middleton, F.; Horton, J. A.; Reichel, L.; Farnum, C. E.; Damron, T. A. Microarray analysis of proliferative and hypertrophic growth plate zones identifies differentiation markers and signal pathways. Bone. 2004, 35, 1273-1293.
doi: 10.1016/j.bone.2004.09.009 URL |
| 36. |
Li, X.; Yang, S.; Yuan, G.; Jing, D.; Qin, L.; Zhao, H.; Yang, S. Type II collagen-positive progenitors are important stem cells in controlling skeletal development and vascular formation. Bone Res. 2022, 10, 46.
doi: 10.1038/s41413-022-00214-z URL |
| 37. |
Mizuhashi, K.; Ono, W.; Matsushita, Y.; Sakagami, N.; Takahashi, A.; Saunders, T. L.; Nagasawa, T.; Kronenberg, H. M.; Ono, N. Resting zone of the growth plate houses a unique class of skeletal stem cells. Nature. 2018, 563, 254-258.
doi: 10.1038/s41586-018-0662-5 URL |
| 38. |
Shi, Y.; He, G.; Lee, W. C.; McKenzie, J. A.; Silva, M. J.; Long, F. Gli1 identifies osteogenic progenitors for bone formation and fracture repair. Nat Commun. 2017, 8, 2043.
doi: 10.1038/s41467-017-02171-2 URL |
| 39. |
Carlone, D. L.; Riba-Wolman, R. D.; Deary, L. T.; Tovaglieri, A.; Jiang, L.; Ambruzs, D. M.; Mead, B. E.; Shah, M. S.; Lengner, C. J.; Jaenisch, R.; Breault, D. T. Telomerase expression marks transitional growth-associated skeletal progenitor/stem cells. Stem Cells. 2021, 39, 296-305.
doi: 10.1002/stem.3318 URL |
| 40. |
Muruganandan, S.; Pierce, R.; Teguh, D. A.; Perez, R. F.; Bell, N.; Nguyen, B.; Hohl, K.; Snyder, B. D.; Grinstaff, M. W.; Alberico, H.; Woods, D.; Kong, Y.; Sima, C.; Bhagat, S.; Ho, K.; Rosen, V.; Gamer, L.; Ionescu, A. M. A FoxA2+ long-term stem cell population is necessary for growth plate cartilage regeneration after injury. Nat Commun. 2022, 13, 2515.
doi: 10.1038/s41467-022-30247-1 URL |
| 41. |
Newton, P. T.; Li, L.; Zhou, B.; Schweingruber, C.; Hovorakova, M.; Xie, M.; Sun, X.; Sandhow, L.; Artemov, A. V.; Ivashkin, E.; Suter, S.; Dyachuk, V.; El Shahawy, M.; Gritli-Linde, A.; Bouderlique, T.; Petersen, J.; Mollbrink, A.; Lundeberg, J.; Enikolopov, G.; Qian, H.; Fried, K.; Kasper, M.; Hedlund, E.; Adameyko, I.; Sävendahl, L.; Chagin, A. S. A radical switch in clonality reveals a stem cell niche in the epiphyseal growth plate. Nature. 2019, 567, 234-238.
doi: 10.1038/s41586-019-0989-6 URL |
| 42. | Ambrosi, T. H.; Sinha, R.; Steininger, H. M.; Hoover, M. Y.; Murphy, M. P.; Koepke, L. S.; Wang, Y.; Lu, W. J.; Morri, M.; Neff, N. F.; Weissman, I. L.; Longaker, M. T.; Chan, C. K. Distinct skeletal stem cell types orchestrate long bone skeletogenesis. Elife. 2021, 10, e66063. |
| 43. |
Matic, I.; Matthews, B. G.; Wang, X.; Dyment, N. A.; Worthley, D. L.; Rowe, D. W.; Grcevic, D.; Kalajzic, I. Quiescent bone lining cells are a major source of osteoblasts during adulthood. Stem Cells. 2016, 34, 2930-2942.
doi: 10.1002/stem.2474 URL |
| 44. |
Duchamp de Lageneste, O.; Julien, A.; Abou-Khalil, R.; Frangi, G.; Carvalho, C.; Cagnard, N.; Cordier, C.; Conway, S. J.; Colnot, C. Periosteum contains skeletal stem cells with high bone regenerative potential controlled by Periostin. Nat Commun. 2018, 9, 773.
doi: 10.1038/s41467-018-03124-z URL |
| 45. |
Berendsen, A. D.; Olsen, B. R. Bone development. Bone. 2015, 80, 14-18.
doi: 10.1016/j.bone.2015.04.035 URL |
| 46. |
Allen, M. R.; Hock, J. M.; Burr, D. B. Periosteum: biology, regulation, and response to osteoporosis therapies. Bone. 2004, 35, 1003-1012.
doi: 10.1016/j.bone.2004.07.014 URL |
| 47. |
He, X.; Bougioukli, S.; Ortega, B.; Arevalo, E.; Lieberman, J. R.; McMahon, A. P. Sox9 positive periosteal cells in fracture repair of the adult mammalian long bone. Bone. 2017, 103, 12-19.
doi: 10.1016/j.bone.2017.06.008 URL |
| 48. |
Ortinau, L. C.; Wang, H.; Lei, K.; Deveza, L.; Jeong, Y.; Hara, Y.; Grafe, I.; Rosenfeld, S. B.; Lee, D.; Lee, B.; Scadden, D. T.; Park, D. Identification of functionally distinct Mx1+αSMA+ periosteal skeletal stem cells. Cell Stem Cell. 2019, 25, 784-796.e5.
doi: 10.1016/j.stem.2019.11.003 URL |
| 49. | Xia, C.; Ge, Q.; Fang, L.; Yu, H.; Zou, Z.; Zhang, P.; Lv, S.; Tong, P.; Xiao, L.; Chen, D.; Wang, P. E.; Jin, H. TGF-β/Smad2 signalling regulates enchondral bone formation of Gli1(+) periosteal cells during fracture healing. Cell Prolif. 2020, 53, e12904. |
| 50. |
Song, J. Y.; Pineault, K. M.; Dones, J. M.; Raines, R. T.; Wellik, D. M. Hox genes maintain critical roles in the adult skeleton. Proc Natl Acad Sci U S A. 2020, 117, 7296-7304.
doi: 10.1073/pnas.1920860117 URL |
| 51. |
Pineault, K. M.; Song, J. Y.; Kozloff, K. M.; Lucas, D.; Wellik, D. M. Hox11 expressing regional skeletal stem cells are progenitors for osteoblasts, chondrocytes and adipocytes throughout life. Nat Commun. 2019, 10, 3168.
doi: 10.1038/s41467-019-11100-4 URL |
| 52. |
Lotinun, S.; Ishihara, Y.; Nagano, K.; Kiviranta, R.; Carpentier, V. T.; Neff, L.; Parkman, V.; Ide, N.; Hu, D.; Dann, P.; Brooks, D.; Bouxsein, M. L.; Wysolmerski, J.; Gori, F.; Baron, R. Cathepsin K-deficient osteocytes prevent lactation-induced bone loss and parathyroid hormone suppression. J Clin Invest. 2019, 129, 3058-3071.
doi: 10.1172/JCI122936 URL |
| 53. |
Yang, W.; Wang, J.; Moore, D. C.; Liang, H.; Dooner, M.; Wu, Q.; Terek, R.; Chen, Q.; Ehrlich, M. G.; Quesenberry, P. J.; Neel, B. G. Ptpn11 deletion in a novel progenitor causes metachondromatosis by inducing hedgehog signalling. Nature. 2013, 499, 491-495.
doi: 10.1038/nature12396 URL |
| 54. |
Han, Y.; Feng, H.; Sun, J.; Liang, X.; Wang, Z.; Xing, W.; Dai, Q.; Yang, Y.; Han, A.; Wei, Z.; Bi, Q.; Ji, H.; Kang, T.; Zou, W. Lkb1 deletion in periosteal mesenchymal progenitors induces osteogenic tumors through mTORC1 activation. J Clin Invest. 2019, 129, 1895-1909.
doi: 10.1172/JCI124590 URL |
| 55. |
Ouyang, Z.; Chen, Z.; Ishikawa, M.; Yue, X.; Kawanami, A.; Leahy, P.; Greenfield, E. M.; Murakami, S. Prx1 and 3.2kb Col1a1 promoters target distinct bone cell populations in transgenic mice. Bone. 2014, 58, 136-145.
doi: 10.1016/j.bone.2013.10.016 URL |
| 56. |
Esposito, A.; Wang, L.; Li, T.; Miranda, M.; Spagnoli, A. Role of Prx1-expressing skeletal cells and Prx1-expression in fracture repair. Bone. 2020, 139, 115521.
doi: 10.1016/j.bone.2020.115521 URL |
| 57. |
Wilk, K.; Yeh, S. A.; Mortensen, L. J.; Ghaffarigarakani, S.; Lombardo, C. M.; Bassir, S. H.; Aldawood, Z. A.; Lin, C. P.; Intini, G. Postnatal calvarial skeletal stem cells expressing PRX1 reside exclusively in the calvarial sutures and are required for bone regeneration. Stem Cell Reports. 2017, 8, 933-946.
doi: 10.1016/j.stemcr.2017.03.002 URL |
| 58. |
Murao, H.; Yamamoto, K.; Matsuda, S.; Akiyama, H. Periosteal cells are a major source of soft callus in bone fracture. J Bone Miner Metab. 2013, 31, 390-398.
doi: 10.1007/s00774-013-0429-x URL |
| 59. |
van Gastel, N.; Stegen, S.; Eelen, G.; Schoors, S.; Carlier, A.; Daniëls, V. W.; Baryawno, N.; Przybylski, D.; Depypere, M.; Stiers, P. J.; Lambrechts, D.; Van Looveren, R.; Torrekens, S.; Sharda, A.; Agostinis, P.; Lambrechts, D.; Maes, F.; Swinnen, J. V.; Geris, L.; Van Oosterwyck, H.; Thienpont, B.; Carmeliet, P.; Scadden, D. T.; Carmeliet, G. Lipid availability determines fate of skeletal progenitor cells via SOX9. Nature. 2020, 579, 111-117.
doi: 10.1038/s41586-020-2050-1 URL |
| 60. |
Ortinau, L.; Lei, K.; Jeong, Y.; Park, D. Real-time imaging of CCL5-induced migration of periosteal skeletal stem cells in mice. J Vis Exp. 2020, 10.3791/61162.
doi: 10.3791/61162 |
| 61. |
Rux, D. R.; Song, J. Y.; Swinehart, I. T.; Pineault, K. M.; Schlientz, A. J.; Trulik, K. G.; Goldstein, S. A.; Kozloff, K. M.; Lucas, D.; Wellik, D. M. Regionally restricted hox function in adult bone marrow multipotent mesenchymal stem/stromal cells. Dev Cell. 2016, 39, 653-666.
doi: 10.1016/j.devcel.2016.11.008 URL |
| 62. |
Tournaire, G.; Stegen, S.; Giacomini, G.; Stockmans, I.; Moermans, K.; Carmeliet, G.; van Gastel, N. Nestin-GFP transgene labels skeletal progenitors in the periosteum. Bone. 2020, 133, 115259.
doi: 10.1016/j.bone.2020.115259 URL |
| 63. |
Tsukasaki, M.; Komatsu, N.; Negishi-Koga, T.; Huynh, N. C.; Muro, R.; Ando, Y.; Seki, Y.; Terashima, A.; Pluemsakunthai, W.; Nitta, T.; Nakamura, T.; Nakashima, T.; Ohba, S.; Akiyama, H.; Okamoto, K.; Baron, R.; Takayanagi, H. Periosteal stem cells control growth plate stem cells during postnatal skeletal growth. Nat Commun. 2022, 13, 4166.
doi: 10.1038/s41467-022-31592-x URL |
| 64. |
Abbuehl, J. P.; Tatarova, Z.; Held, W.; Huelsken, J. Long-term engraftment of primary bone marrow stromal cells repairs niche damage and improves hematopoietic stem cell transplantation. Cell Stem Cell. 2017, 21, 241-255.e6.
doi: 10.1016/j.stem.2017.07.004 URL |
| 65. | Shi, Y.; Kang, X.; Wang, Y.; Bian, X.; He, G.; Zhou, M.; Tang, K. Exosomes derived from bone marrow stromal cells (BMSCs) enhance tendon-bone healing by regulating macrophage polarization. Med Sci Monit. 2020, 26, e923328. |
| 66. | Friedenstein, A. J. ; Piatetzky, S., II; Petrakova, K. V. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966, 16, 381-390. |
| 67. |
Caplan, A. I. Mesenchymal stem cells. J Orthop Res. 1991, 9, 641-650.
doi: 10.1002/jor.1100090504 URL |
| 68. |
Horwitz, E. M.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F. C.; Deans, R. J.; Krause, D. S.; Keating, A.; International Society for Cellular Therapy. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005, 7, 393-395.
doi: 10.1080/14653240500319234 URL |
| 69. | Prieto González, E. A. Heterogeneity in adipose stem cells. Adv Exp Med Biol. 2019, 1123, 119-150. |
| 70. |
De Micheli, A. J.; Laurilliard, E. J.; Heinke, C. L.; Ravichandran, H.; Fraczek, P.; Soueid-Baumgarten, S.; De Vlaminck, I.; Elemento, O.; Cosgrove, B. D. Single-cell analysis of the muscle stem cell hierarchy identifies heterotypic communication signals involved in skeletal muscle regeneration. Cell Rep. 2020, 30, 3583-3595.e5.
doi: 10.1016/j.celrep.2020.02.067 URL |
| 71. |
De Bari, C.; Dell’Accio, F.; Vanlauwe, J.; Eyckmans, J.; Khan, I. M.; Archer, C. W.; Jones, E. A.; McGonagle, D.; Mitsiadis, T. A.; Pitzalis, C.; Luyten, F. P. Mesenchymal multipotency of adult human periosteal cells demonstrated by single-cell lineage analysis. Arthritis Rheum. 2006, 54, 1209-1221.
doi: 10.1002/art.21753 URL |
| 72. | Bartold, M.; Gronthos, S.; Haynes, D.; Ivanovski, S. Mesenchymal stem cells and biologic factors leading to bone formation. J Clin Periodontol. 2019, 46 Suppl 21, 12-32. |
| 73. | McLeod, C. M.; Mauck, R. L. On the origin and impact of mesenchymal stem cell heterogeneity: new insights and emerging tools for single cell analysis. Eur Cell Mater. 2017, 34, 217-231. |
| 74. |
Usami, Y.; Gunawardena, A. T.; Francois, N. B.; Otsuru, S.; Takano, H.; Hirose, K.; Matsuoka, M.; Suzuki, A.; Huang, J.; Qin, L.; Iwamoto, M.; Yang, W.; Toyosawa, S.; Enomoto-Iwamoto, M. Possible contribution of Wnt-responsive chondroprogenitors to the postnatal murine growth plate. J Bone Miner Res. 2019, 34, 964-974.
doi: 10.1002/jbmr.3658 URL |
| 75. |
Maruyama, T.; Jeong, J.; Sheu, T. J.; Hsu, W. Stem cells of the suture mesenchyme in craniofacial bone development, repair and regeneration. Nat Commun. 2016, 7, 10526.
doi: 10.1038/ncomms10526 URL |
| 76. |
Matsushita, Y.; Nagata, M.; Kozloff, K. M.; Welch, J. D.; Mizuhashi, K.; Tokavanich, N.; Hallett, S. A.; Link, D. C.; Nagasawa, T.; Ono, W.; Ono, N. A Wnt-mediated transformation of the bone marrow stromal cell identity orchestrates skeletal regeneration. Nat Commun. 2020, 11, 332.
doi: 10.1038/s41467-019-14029-w URL |
| 77. |
Ono, N.; Ono, W.; Mizoguchi, T.; Nagasawa, T.; Frenette, P. S.; Kronenberg, H. M. Vasculature-associated cells expressing nestin in developing bones encompass early cells in the osteoblast and endothelial lineage. Dev Cell. 2014, 29, 330-339.
doi: 10.1016/j.devcel.2014.03.014 URL |
| 78. |
Yue, R.; Zhou, B. O.; Shimada, I. S.; Zhao, Z.; Morrison, S. J. Leptin receptor promotes adipogenesis and reduces osteogenesis by regulating mesenchymal stromal cells in adult bone marrow. Cell Stem Cell. 2016, 18, 782-796.
doi: 10.1016/j.stem.2016.02.015 URL |
| 79. |
Zhou, B. O.; Yue, R.; Murphy, M. M.; Peyer, J. G.; Morrison, S. J. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell. 2014, 15, 154-168.
doi: 10.1016/j.stem.2014.06.008 URL |
| 80. |
Méndez-Ferrer, S.; Michurina, T. V.; Ferraro, F.; Mazloom, A. R.; Macarthur, B. D.; Lira, S. A.; Scadden, D. T.; Ma’ayan, A.; Enikolopov, G. N.; Frenette, P. S. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010, 466, 829-834.
doi: 10.1038/nature09262 URL |
| 81. |
Park, D.; Spencer, J. A.; Koh, B. I.; Kobayashi, T.; Fujisaki, J.; Clemens, T. L.; Lin, C. P.; Kronenberg, H. M.; Scadden, D. T. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell. 2012, 10, 259-272.
doi: 10.1016/j.stem.2012.02.003 URL |
| 82. |
Worthley, D. L.; Churchill, M.; Compton, J. T.; Tailor, Y.; Rao, M.; Si, Y.; Levin, D.; Schwartz, M. G.; Uygur, A.; Hayakawa, Y.; Gross, S.; Renz, B. W.; Setlik, W.; Martinez, A. N.; Chen, X.; Nizami, S.; Lee, H. G.; Kang, H. P.; Caldwell, J. M.; Asfaha, S.; Westphalen, C. B.; Graham, T.; Jin, G.; Nagar, K.; Wang, H.; Kheirbek, M. A.; Kolhe, A.; Carpenter, J.; Glaire, M.; Nair, A.; Renders, S.; Manieri, N.; Muthupalani, S.; Fox, J. G.; Reichert, M.; Giraud, A. S.; Schwabe, R. F.; Pradere, J. P.; Walton, K.; Prakash, A.; Gumucio, D.; Rustgi, A. K.; Stappenbeck, T. S.; Friedman, R. A.; Gershon, M. D.; Sims, P.; Grikscheit, T.; Lee, F. Y.; Karsenty, G.; Mukherjee, S.; Wang, T. C. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell. 2015, 160, 269-284.
doi: 10.1016/j.cell.2014.11.042 URL |
| 83. |
Pérez-Lozano, M. L.; Sudre, L.; van Eegher, S.; Citadelle, D.; Pigenet, A.; Lafage-Proust, M. H.; Pastoureau, P.; De Ceuninck, F.; Berenbaum, F.; Houard, X. Gremlin-1 and BMP-4 overexpressed in osteoarthritis drive an osteochondral-remodeling program in osteoblasts and hypertrophic chondrocytes. Int J Mol Sci. 2022, 23, 2084.
doi: 10.3390/ijms23042084 URL |
| 84. |
Sivaraj, K. K.; Jeong, H. W.; Dharmalingam, B.; Zeuschner, D.; Adams, S.; Potente, M.; Adams, R. H. Regional specialization and fate specification of bone stromal cells in skeletal development. Cell Rep. 2021, 36, 109352.
doi: 10.1016/j.celrep.2021.109352 URL |
| 85. | Zhou, X.; von der Mark, K.; Henry, S.; Norton, W.; Adams, H.; de Crombrugghe, B. Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice. PLoS Genet. 2014, 10, e1004820. |
| 86. |
Shu, H. S.; Liu, Y. L.; Tang, X. T.; Zhang, X. S.; Zhou, B.; Zou, W.; Zhou, B. O. Tracing the skeletal progenitor transition during postnatal bone formation. Cell Stem Cell. 2021, 28, 2122-2136.e3.
doi: 10.1016/j.stem.2021.08.010 URL |
| 87. |
Jing, D.; Chen, Z.; Men, Y.; Yi, Y.; Wang, Y.; Wang, J.; Yi, J.; Wan, L.; Shen, B.; Feng, J. Q.; Zhao, Z.; Zhao, H.; Li, C. Response of Gli1(+) suture stem cells to mechanical force upon suture expansion. J Bone Miner Res. 2022, 37, 1307-1320.
doi: 10.1002/jbmr.4561 URL |
| 88. |
Men, Y.; Wang, Y.; Yi, Y.; Jing, D.; Luo, W.; Shen, B.; Stenberg, W.; Chai, Y.; Ge, W. P.; Feng, J. Q.; Zhao, H. Gli1+ periodontium stem cells are regulated by osteocytes and occlusal force. Dev Cell. 2020, 54, 639-654.e6.
doi: 10.1016/j.devcel.2020.06.006 URL |
| 89. |
Wang, K.; Xu, C.; Xie, X.; Jing, Y.; Chen, P. J.; Yadav, S.; Wang, Z.; Taylor, R. W.; Wang, J.; Feng, J. Q. Axin2+ PDL cells directly contribute to new alveolar bone formation in response to orthodontic tension force. J Dent Res. 2022, 101, 695-703.
doi: 10.1177/00220345211062585 URL |
| 90. |
Gong, X.; Zhang, H.; Xu, X.; Ding, Y.; Yang, X.; Cheng, Z.; Tao, D.; Hu, C.; Xiang, Y.; Sun, Y. Tracing PRX1(+) cells during molar formation and periodontal ligament reconstruction. Int J Oral Sci. 2022, 14, 5.
doi: 10.1038/s41368-021-00155-z URL |
| 91. |
Ding, Y.; Mo, C.; Geng, J.; Li, J.; Sun, Y. Identification of periosteal osteogenic progenitors in jawbone. J Dent Res. 2022, 101, 1101-1109.
doi: 10.1177/00220345221084200 URL |
| 92. |
Lattanzi, W.; Barba, M.; Novegno, F.; Massimi, L.; Tesori, V.; Tamburrini, G.; Galgano, S.; Bernardini, C.; Caldarelli, M.; Michetti, F.; Di Rocco, C. Lim mineralization protein is involved in the premature calvarial ossification in sporadic craniosynostoses. Bone. 2013, 52, 474-484.
doi: 10.1016/j.bone.2012.09.004 URL |
| 93. |
Zhao, H.; Feng, J.; Ho, T. V.; Grimes, W.; Urata, M.; Chai, Y. The suture provides a niche for mesenchymal stem cells of craniofacial bones. Nat Cell Biol. 2015, 17, 386-396.
doi: 10.1038/ncb3139 URL |
| 94. | Maruyama, T.; Stevens, R.; Boka, A.; DiRienzo, L.; Chang, C.; Yu, H. I.; Nishimori, K.; Morrison, C.; Hsu, W. BMPR1A maintains skeletal stem cell properties in craniofacial development and craniosynostosis. Sci Transl Med. 2021, 13, eabb4416. |
| 95. |
Maruyama, T.; Jiang, M.; Abbott, A.; Yu, H. I.; Huang, Q.; Chrzanowska-Wodnicka, M.; Chen, E. I.; Hsu, W. Rap1b is an effector of Axin2 regulating crosstalk of signaling pathways during skeletal development. J Bone Miner Res. 2017, 32, 1816-1828.
doi: 10.1002/jbmr.3171 URL |
| 96. |
Xie, X.; Xu, C.; Zhao, L.; Wu, Y.; Feng, J. Q.; Wang, J. Axin2-expressing cells in the periodontal ligament are regulated by bone morphogenetic protein signalling and play a pivotal role in periodontium development. J Clin Periodontol. 2022, 49, 945-956.
doi: 10.1111/jcpe.13666 URL |
| 97. |
Ransom, R. C.; Carter, A. C.; Salhotra, A.; Leavitt, T.; Marecic, O.; Murphy, M. P.; Lopez, M. L.; Wei, Y.; Marshall, C. D.; Shen, E. Z.; Jones, R. E.; Sharir, A.; Klein, O. D.; Chan, C. K. F.; Wan, D. C.; Chang, H. Y.; Longaker, M. T. Mechanoresponsive stem cells acquire neural crest fate in jaw regeneration. Nature. 2018, 563, 514-521.
doi: 10.1038/s41586-018-0650-9 URL |
| 98. |
Fernández, M.; Simon, V.; Herrera, G.; Cao, C.; Del Favero, H.; Minguell, J. J. Detection of stromal cells in peripheral blood progenitor cell collections from breast cancer patients. Bone Marrow Transplant. 1997, 20, 265-271.
doi: 10.1038/sj.bmt.1700890 URL |
| 99. |
Kuznetsov, S. A.; Mankani, M. H.; Gronthos, S.; Satomura, K.; Bianco, P.; Robey, P. G. Circulating skeletal stem cells. J Cell Biol. 2001, 153, 1133-1140.
doi: 10.1083/jcb.153.5.1133 URL |
| 100. |
Eghbali-Fatourechi, G. Z.; Lamsam, J.; Fraser, D.; Nagel, D.; Riggs, B. L.; Khosla, S. Circulating osteoblast-lineage cells in humans. N Engl J Med. 2005, 352, 1959-1966.
doi: 10.1056/NEJMoa044264 URL |
| 101. |
Feehan, J.; Nurgali, K.; Apostolopoulos, V.; Al Saedi, A.; Duque, G. Circulating osteogenic precursor cells: building bone from blood. EBioMedicine. 2019, 39, 603-611.
doi: 10.1016/j.ebiom.2018.11.051 URL |
| 102. |
Kumagai, K.; Vasanji, A.; Drazba, J. A.; Butler, R. S.; Muschler, G. F. Circulating cells with osteogenic potential are physiologically mobilized into the fracture healing site in the parabiotic mice model. J Orthop Res. 2008, 26, 165-175.
doi: 10.1002/jor.20477 URL |
| 103. |
Kelly, R. R.; McDonald, L. T.; Pellegrini, V. D.; Cray, J. J.; Larue, A. C. Identification of circulating murine CD34(+)OCN(+) cells. Cytotherapy. 2018, 20, 1371-1380.
doi: 10.1016/j.jcyt.2018.07.004 URL |
| 104. |
Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006, 8, 315-317.
doi: 10.1080/14653240600855905 URL |
| 105. |
Alm, J. J.; Koivu, H. M.; Heino, T. J.; Hentunen, T. A.; Laitinen, S.; Aro, H. T. Circulating plastic adherent mesenchymal stem cells in aged hip fracture patients. J Orthop Res. 2010, 28, 1634-1642.
doi: 10.1002/jor.21167 URL |
| 106. | Zvaifler, N. J.; Marinova-Mutafchieva, L.; Adams, G.; Edwards, C. J.; Moss, J.; Burger, J. A.; Maini, R. N. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res. 2000, 2, 477-488. |
| 107. |
Suda, R. K.; Billings, P. C.; Egan, K. P.; Kim, J. H.; McCarrick-Walmsley, R.; Glaser, D. L.; Porter, D. L.; Shore, E. M.; Pignolo, R. J. Circulating osteogenic precursor cells in heterotopic bone formation. Stem Cells. 2009, 27, 2209-2219.
doi: 10.1002/stem.150 URL |
| 108. | Hong, H. S.; Lee, J.; Lee, E.; Kwon, Y. S.; Lee, E.; Ahn, W.; Jiang, M. H.; Kim, J. C.; Son, Y. A new role of substance P as an injury-inducible messenger for mobilization of CD29(+) stromal-like cells. Nat Med. 2009, 15, 425-435. |
| 109. |
Rochefort, G. Y.; Delorme, B.; Lopez, A.; Hérault, O.; Bonnet, P.; Charbord, P.; Eder, V.; Domenech, J. Multipotential mesenchymal stem cells are mobilized into peripheral blood by hypoxia. Stem Cells. 2006, 24, 2202-2208.
doi: 10.1634/stemcells.2006-0164 URL |
| 110. |
Rubin, M. R.; Manavalan, J. S.; Dempster, D. W.; Shah, J.; Cremers, S.; Kousteni, S.; Zhou, H.; McMahon, D. J.; Kode, A.; Sliney, J.; Shane, E.; Silverberg, S. J.; Bilezikian, J. P. Parathyroid hormone stimulates circulating osteogenic cells in hypoparathyroidism. J Clin Endocrinol Metab. 2011, 96, 176-186.
doi: 10.1210/jc.2009-2682 URL |
| 111. |
Pignolo, R. J.; Kassem, M Circulating osteogenic cells: implications for injury, repair, and regeneration. J Bone Miner Res. 2011, 26, 1685-1693.
doi: 10.1002/jbmr.370 URL |
| 112. | Eggenhofer, E.; Luk, F.; Dahlke, M. H.; Hoogduijn, M. J. The life and fate of mesenchymal stem cells. Front Immunol. 2014, 5, 148. |
| 113. |
Kyoizumi, S.; Kubo, Y.; Misumi, M.; Kajimura, J.; Yoshida, K.; Hayashi, T.; Imai, K.; Ohishi, W.; Nakachi, K.; Young, L. F.; Shieh, J. H.; Moore, M. A.; van den Brink, M. R.; Kusunoki, Y. Circulating hematopoietic stem and progenitor cells in aging atomic bomb survivors. Radiat Res. 2016, 185, 69-76.
doi: 10.1667/RR14209.1 URL |
| 114. |
Lo Sicco, C.; Tasso, R.; Reverberi, D.; Cilli, M.; Pfeffer, U.; Cancedda, R. Identification of a new cell population constitutively circulating in healthy conditions and endowed with a homing ability toward injured sites. Sci Rep. 2015, 5, 16574.
doi: 10.1038/srep16574 URL |
| 115. |
Lo Sicco, C.; Reverberi, D.; Villa, F.; Pfeffer, U.; Quarto, R.; Cancedda, R.; Tasso, R. Circulating healing (CH) cells expressing BST2 are functionally activated by the injury-regulated systemic factor HGFA. Stem Cell Res Ther. 2018, 9, 300.
doi: 10.1186/s13287-018-1056-1 URL |
| 116. |
Kawakami, Y.; Matsumoto, T.; Mifune, Y.; Fukui, T.; Patel, K. G.; Walker, G. N.; Kurosaka, M.; Kuroda, R. Therapeutic potential of endothelial progenitor cells in the field of orthopaedics. Curr Stem Cell Res Ther. 2017, 12, 3-13.
doi: 10.2174/1574888X11666160810102945 URL |
| 117. |
Kuroda, R.; Matsumoto, T.; Kawakami, Y.; Fukui, T.; Mifune, Y.; Kurosaka, M. Clinical impact of circulating CD34-positive cells on bone regeneration and healing. Tissue Eng Part B Rev. 2014, 20, 190-199.
doi: 10.1089/ten.teb.2013.0511 URL |
| 118. |
Ma, X. L.; Sun, X. L.; Wan, C. Y.; Ma, J. X.; Tian, P. Significance of circulating endothelial progenitor cells in patients with fracture healing process. J Orthop Res. 2012, 30, 1860-1866.
doi: 10.1002/jor.22134 URL |
| 119. |
Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008, 8, 726-736.
doi: 10.1038/nri2395 URL |
| 120. | Gattazzo, F.; Urciuolo, A.; Bonaldo, P. Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochim Biophys Acta. 2014, 1840, 2506-2519. |
| 121. |
Ambrosi, T. H.; Marecic, O.; McArdle, A.; Sinha, R.; Gulati, G. S.; Tong, X.; Wang, Y.; Steininger, H. M.; Hoover, M. Y.; Koepke, L. S.; Murphy, M. P.; Sokol, J.; Seo, E. Y.; Tevlin, R.; Lopez, M.; Brewer, R. E.; Mascharak, S.; Lu, L.; Ajanaku, O.; Conley, S. D.; Seita, J.; Morri, M.; Neff, N. F.; Sahoo, D.; Yang, F.; Weissman, I. L.; Longaker, M. T.; Chan, C. K. F. Aged skeletal stem cells generate an inflammatory degenerative niche. Nature. 2021, 597, 256-262.
doi: 10.1038/s41586-021-03795-7 URL |
| 122. |
Josephson, A. M.; Bradaschia-Correa, V.; Lee, S.; Leclerc, K.; Patel, K. S.; Muinos Lopez, E.; Litwa, H. P.; Neibart, S. S.; Kadiyala, M.; Wong, M. Z.; Mizrahi, M. M.; Yim, N. L.; Ramme, A. J.; Egol, K. A.; Leucht, P. Age-related inflammation triggers skeletal stem/progenitor cell dysfunction. Proc Natl Acad Sci U S A. 2019, 116, 6995-7004.
doi: 10.1073/pnas.1810692116 URL |
| 123. |
Oh, J.; Lee, Y. D.; Wagers, A. J. Stem cell aging: mechanisms, regulators and therapeutic opportunities. Nat Med. 2014, 20, 870-880.
doi: 10.1038/nm.3651 URL |
| 124. |
Ransom, R. C.; Hunter, D. J.; Hyman, S.; Singh, G.; Ransom, S. C.; Shen, E. Z.; Perez, K. C.; Gillette, M.; Li, J.; Liu, B.; Brunski, J. B.; Helms, J. A. Axin2-expressing cells execute regeneration after skeletal injury. Sci Rep. 2016, 6, 36524.
doi: 10.1038/srep36524 URL |
| 125. | Tevlin, R.; Seo, E. Y.; Marecic, O.; McArdle, A.; Tong, X.; Zimdahl, B.; Malkovskiy, A.; Sinha, R.; Gulati, G.; Li, X.; Wearda, T.; Morganti, R.; Lopez, M.; Ransom, R. C.; Duldulao, C. R.; Rodrigues, M.; Nguyen, A.; Januszyk, M.; Maan, Z.; Paik, K.; Yapa, K. S.; Rajadas, J.; Wan, D. C.; Gurtner, G. C.; Snyder, M.; Beachy, P. A.; Yang, F.; Goodman, S. B.; Weissman, I. L.; Chan, C. K.; Longaker, M. T. Pharmacological rescue of diabetic skeletal stem cell niches. Sci Transl Med. 2017, 9, eaag2809. |
| 126. |
Rosen, V. BMP2 signaling in bone development and repair. Cytokine Growth Factor Rev. 2009, 20, 475-480.
doi: 10.1016/j.cytogfr.2009.10.018 URL |
| 127. |
Lowery, J. W.; Pazin, D.; Intini, G.; Kokabu, S.; Chappuis, V.; Capelo, L. P.; Rosen, V. The role of BMP2 signaling in the skeleton. Crit Rev Eukaryot Gene Expr. 2011, 21, 177-185.
doi: 10.1615/CritRevEukarGeneExpr.v21.i2.60 URL |
| 128. | Salazar, V. S.; Capelo, L. P.; Cantù, C.; Zimmerli, D.; Gosalia, N.; Pregizer, S.; Cox, K.; Ohte, S.; Feigenson, M.; Gamer, L.; Nyman, J. S.; Carey, D. J.; Economides, A.; Basler, K.; Rosen, V. Reactivation of a developmental Bmp2 signaling center is required for therapeutic control of the murine periosteal niche. Elife. 2019, 8, e42386. |
| 129. |
Liu, Y.; Tian, H.; Hu, Y.; Cao, Y.; Song, H.; Lan, S.; Dai, Z.; Chen, W.; Zhang, Y.; Shao, Z.; Liu, Y.; Tong, W. Mechanosensitive Piezo1 is crucial for periosteal stem cell-mediated fracture healing. Int J Biol Sci. 2022, 18, 3961-3980.
doi: 10.7150/ijbs.71390 URL |
| 130. |
Gao, B.; Deng, R.; Chai, Y.; Chen, H.; Hu, B.; Wang, X.; Zhu, S.; Cao, Y.; Ni, S.; Wan, M.; Yang, L.; Luo, Z.; Cao, X. Macrophage-lineage TRAP+ cells recruit periosteum-derived cells for periosteal osteogenesis and regeneration. J Clin Invest. 2019, 129, 2578-2594.
doi: 10.1172/JCI98857 URL |
| 131. |
Gan, Y.; He, J.; Zhu, J.; Xu, Z.; Wang, Z.; Yan, J.; Hu, O.; Bai, Z.; Chen, L.; Xie, Y.; Jin, M.; Huang, S.; Liu, B.; Liu, P. Spatially defined single-cell transcriptional profiling characterizes diverse chondrocyte subtypes and nucleus pulposus progenitors in human intervertebral discs. Bone Res. 2021, 9, 37.
doi: 10.1038/s41413-021-00163-z URL |
| [1] | Deepika Arora, Pamela Gehron Robey. Recent updates on the biological basis of heterogeneity in bone marrow stromal cells/skeletal stem cells [J]. Biomaterials Translational, 2022, 3(1): 3-16. |
| [2] | Suzanne M. Watt. The long and winding road: homeostatic and disordered haematopoietic microenvironmental niches: a narrative review [J]. Biomaterials Translational, 2022, 3(1): 31-54. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||