Biomaterials Translational ›› 2023, Vol. 4 ›› Issue (4): 199-212.doi: 10.12336/biomatertransl.2023.04.002
• REVIEW • Previous Articles Next Articles
Han Liu1,2,3,4, Jiacan Su1,2,3,4,*( )
)
Received:2023-09-30
Revised:2023-11-24
Accepted:2023-12-05
Online:2023-12-27
Published:2023-12-28
Contact:
Jiacan Su, drsujiacan@163.com.
Figure 1. Schematic illustration of organoid extracellular vesicle (OEV)-based bone disease treatment strategy. OEVs have emerging as promising cell-free nanocarriers for bone therapy due to their vigoroso physiological effects, significant biological functions, stable loading capacity, and great biocompatibility. Created with BioRender.com.
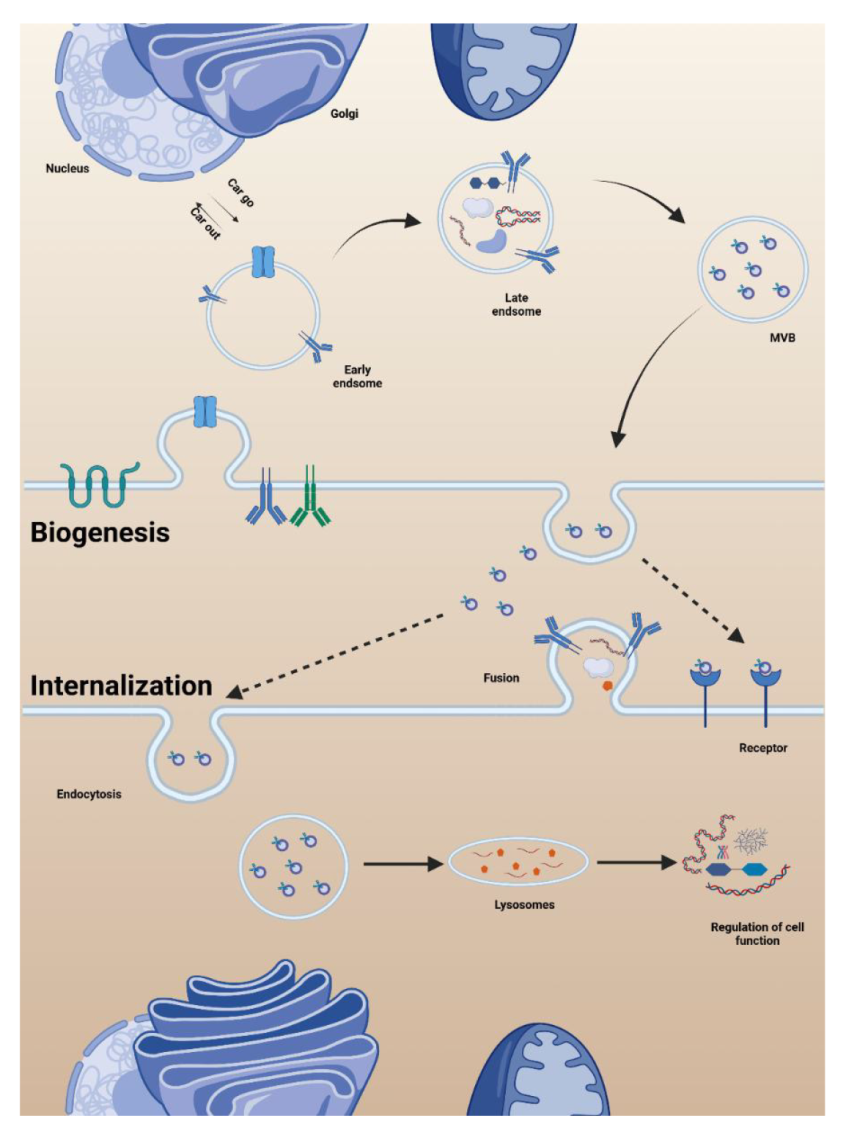
Figure 2. Biogenesis and internalisation of organoid extracellular vesicles (OEVs). The early endosome was formed by the absorption of extracellular proteins by the plasma membrane through endocytosis. The early endosome gradually matured into the late endosome by exchanging goods with the endoplasmic reticulum and Golgi apparatus. In the late endosomes, a second plasma membrane invasion occurs to form multivesicular body (MVB), which is finally selected to fuse with the plasma membrane of the cell to release OEVs or to fuse with lysosomes with the participation of sorting proteins. The free OEVs arrive at the recipient cell and are absorbed by endocytosis or receptor ligand binding. Created with BioRender.com.
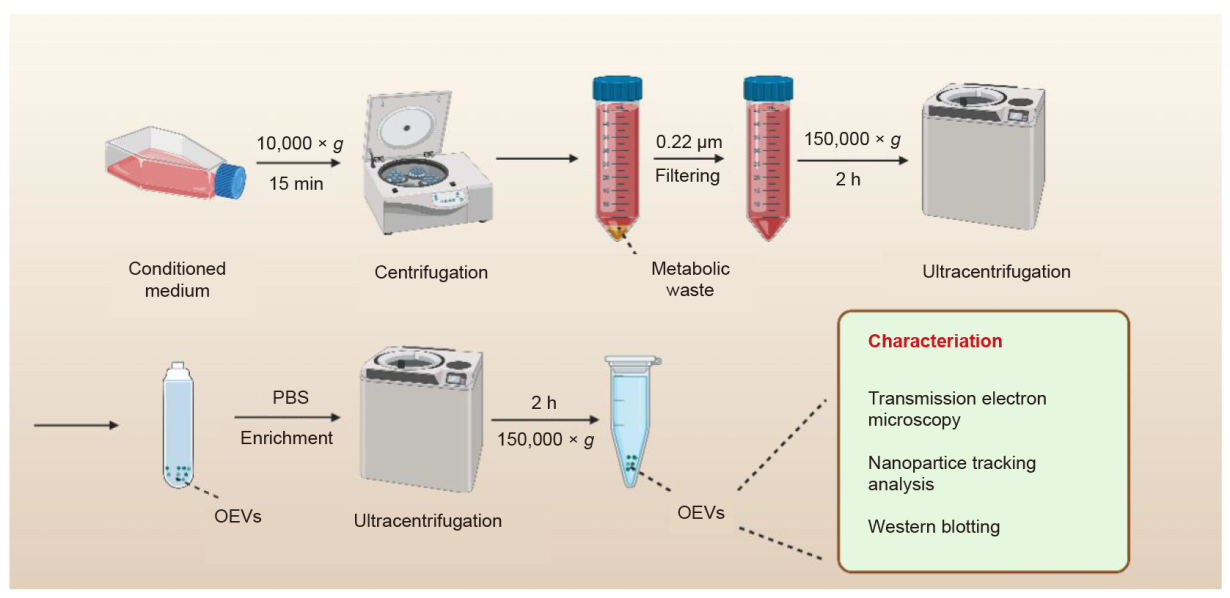
Figure 3. Isolation of organoid extracellular vesicles (OEVs). After three-dimensional (3D) cultivation, the organoid culture is collected and centrifuged at 10,000 × g for 15 minutes at 4°C. The supernatant is then filtered by 0.22 μm sterile filter to remove impurities. Subsequently, OEVs precipitate are collected by the ultracentrifugation for 2 hours at 150,000 × g. The collected OEVs are purified with phosphate buffered saline (PBS) and ultracentrifuged at 150,000 × g for 2 hours. The obtained OEVs can be characterised and verified using nanoparticle tracking analysis, transmission electron microscopy, and Western blotting to represent the size, shape, concentration, and specific markers of OEVs. The collected OEVs are used immediately or stored at –80°C until use. Created with BioRender.com.
| Method | Principle | Advantage | Disadvantage | Reference |
|---|---|---|---|---|
| Gradient ultrafast centrifugation | Different settlement coefficient | High purity; Separable subgroup | Time-consuming; High equipment requirements | |
| Volume exclusion chromatography | Different particle size | High purity; Fast preparation | Expensive; Low output | |
| Immunoaffinity capture | Specific binding | High purity; Specific exosomes | Expensive; Need to optimise ligand; Low yield | |
| Microfluidic technology | Immunoaffinity, particle size and density | High efficiency; No chemical pollution | Low yield; Expensive | |
| EVs extraction kit | Immune magnetic bead capture | Simple method | Low output; Expensive | - |
| Sucrose density gradient centrifugation method | Centrifugal force | High purity | Low output; Long time; Tedious process |
Table 1. The extraction methods of organoid extracellular vesicles
| Method | Principle | Advantage | Disadvantage | Reference |
|---|---|---|---|---|
| Gradient ultrafast centrifugation | Different settlement coefficient | High purity; Separable subgroup | Time-consuming; High equipment requirements | |
| Volume exclusion chromatography | Different particle size | High purity; Fast preparation | Expensive; Low output | |
| Immunoaffinity capture | Specific binding | High purity; Specific exosomes | Expensive; Need to optimise ligand; Low yield | |
| Microfluidic technology | Immunoaffinity, particle size and density | High efficiency; No chemical pollution | Low yield; Expensive | |
| EVs extraction kit | Immune magnetic bead capture | Simple method | Low output; Expensive | - |
| Sucrose density gradient centrifugation method | Centrifugal force | High purity | Low output; Long time; Tedious process |
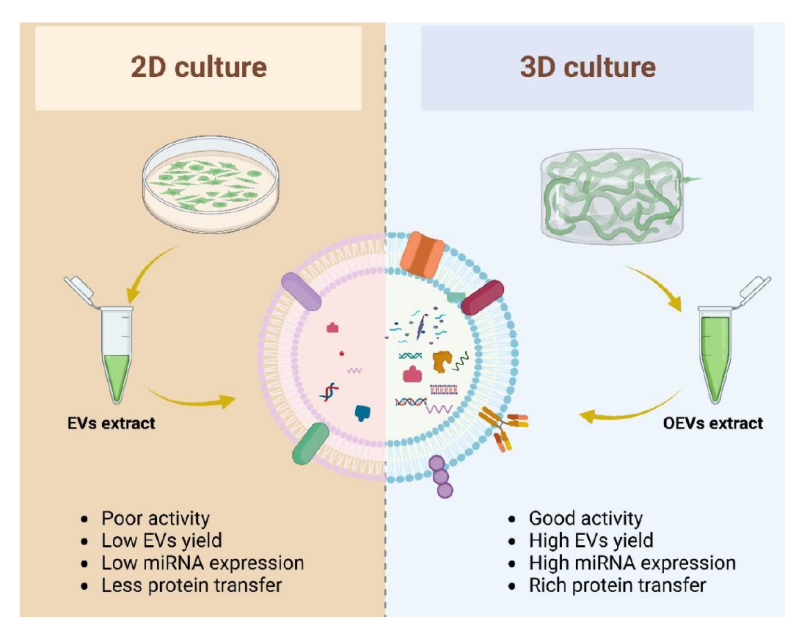
Figure 4. The differences between organoid extracellular vesicles (OEVs) and traditional extracellular vesicles (EVs). Two-dimensional (2D) cultured cells produced fewer EVs, poor bioactivity, and less protein and nucleic acid (left), while three-dimensional (3D) cultured cells produced more OEVs, more active, and more protein and nucleic acid (right). Created with BioRender.com. miRNA: microRNA.
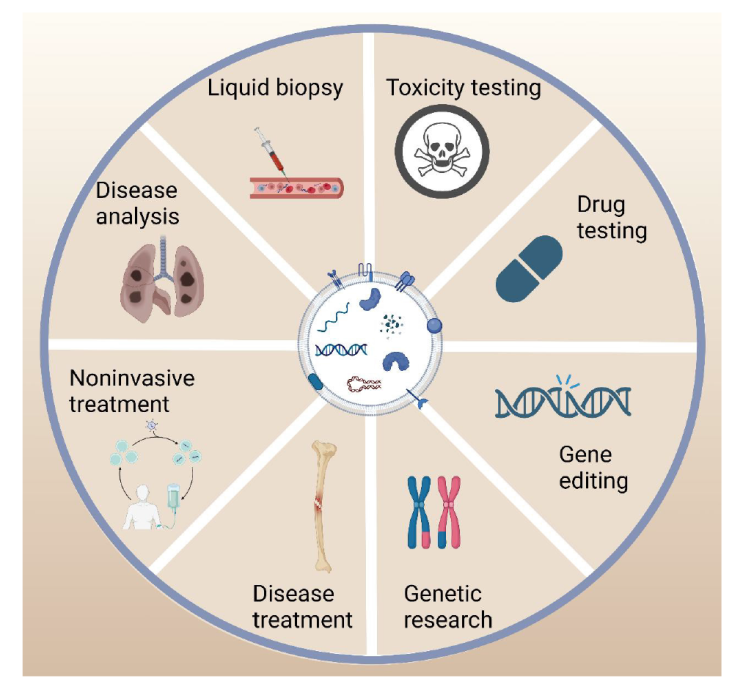
Figure 5. The application diagram of organoid extracellular vesicles (OEVs). OEVs have a wide range of applications, including liquid biopsy, pharmacological testing, toxicity testing, disease treatment, customised personalised medicine, genetic research. Created with BioRender.com.
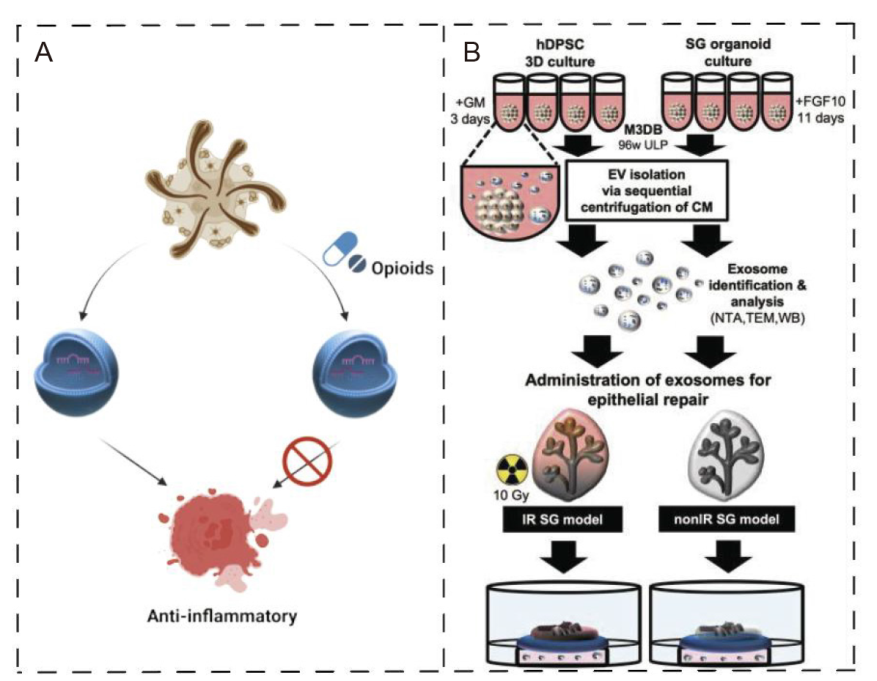
Figure 6. Organoid extracellular vesicles (OEVs) for disease treatment. (A) OEVs secreted by intestinal organoids can exert anti-inflammatory effects, while the anti-inflammatory effects of secreted OEVs are lost after the use of opioids acting on organoids. Created with BioRender.com. (B) Cultivation and collection of SG-like organ (SGO) by magnetic 3D bio-assembly (M3DB) system for the treatment of radiation-induced epithelial damage. Reprinted from Chansaenroj et al.79 3D: three-dimensional; 96w ULP: 96-well ultra-low attachment plate; CM: conditioned media; EV: extracellular vesicle; FGF10: fibroblast growth factor 10; GM: growth media; hDPSC: human dental pulp stem cell; IR: irradiated; NTA: nanoparticle tracking analysis; SG: salivary gland; TEM: transmission electron microscopy; WB: Western blotting.
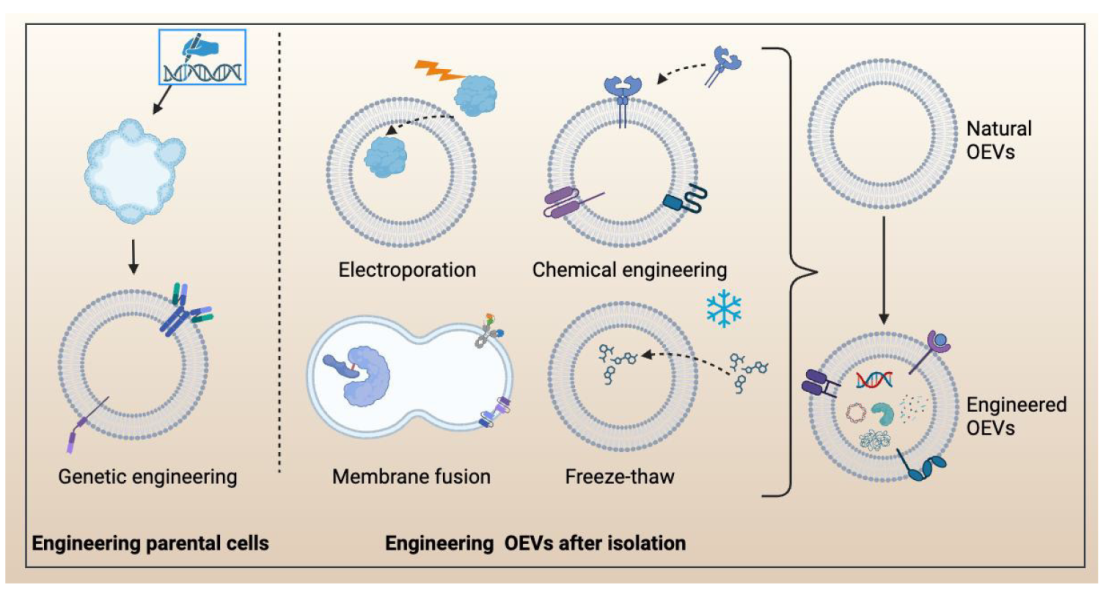
Figure 7. The engineering approaches for modifying organoid extracellular vesicles (OEVs), including the engineering parental cells and engineering OEVs after isolation. Engineering parental cells, such as using clustered regularly interspaced short palindromic repeats-CRISPR-associated protein 9 (CRISPR-Cas9) to modify cells to obtain engineered organoids. Engineering OEVs after isolation are mainly Electroporation, chemical engineering, membrane fusion, and freeze thaw. Created with BioRender.com.
| Engineering approach | Strategy | Method | Purpose | Reference |
|---|---|---|---|---|
| Engineering parental cells | Genetic engineering | Direct modification of parent cells | The protein was displayed on the surface of extracellular vesicles to enrich its physiological function | |
| Synthetic biology | Shuttle plasmid | Giving new functionality to bacterial extracellular vesicles | ||
| Engineering after isolation | Membrane fusion | Co-incubation | Loading of exogenous cargo into the membrane | |
| Chemical engineering | Non-covalent reaction | Increased extracellular vesicles targeting | ||
| Chemical engineering | Click chemistry | Loading of azides onto the membrane surface | ||
| Freeze-thaw | Freeze-thaw cycle | Loading extracellular vesicles with exogenous substances and ensuring normal morphology | ||
| Electroporation technique | High voltage electric field | Transfer of DNA, or/and RNA into extracellular vesicles |
Table 2. Engineered retrofit solutions for organoid extracellular vesicles
| Engineering approach | Strategy | Method | Purpose | Reference |
|---|---|---|---|---|
| Engineering parental cells | Genetic engineering | Direct modification of parent cells | The protein was displayed on the surface of extracellular vesicles to enrich its physiological function | |
| Synthetic biology | Shuttle plasmid | Giving new functionality to bacterial extracellular vesicles | ||
| Engineering after isolation | Membrane fusion | Co-incubation | Loading of exogenous cargo into the membrane | |
| Chemical engineering | Non-covalent reaction | Increased extracellular vesicles targeting | ||
| Chemical engineering | Click chemistry | Loading of azides onto the membrane surface | ||
| Freeze-thaw | Freeze-thaw cycle | Loading extracellular vesicles with exogenous substances and ensuring normal morphology | ||
| Electroporation technique | High voltage electric field | Transfer of DNA, or/and RNA into extracellular vesicles |
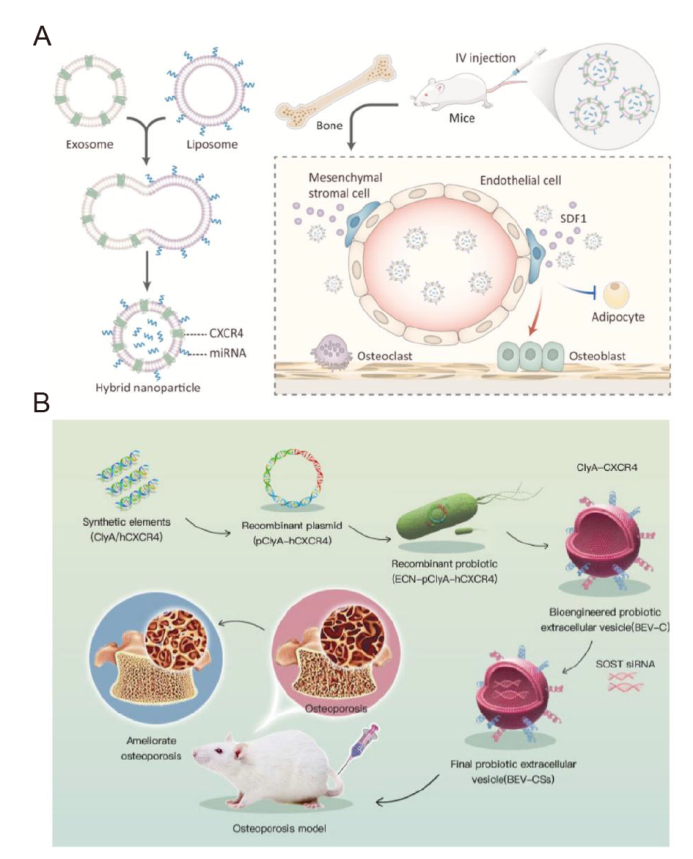
Figure 8. Engineering parental cells to endow their extracellular vesicles (EVs) with powerful functions. (A) Schematic illustration of exosome-guided microRNA (miRNA) blocking. Reprinted from Hu et al.53 (B) Schematic illustration of the construction of bioengineered bacterial EVs (BEVs). Reprinted from Liu et al.11 Copyright 2023, with permission from Elsevier. BEV: bacterial extracellular vesicle; BEV-C: BEVs-hCXCR4; BEV-CS: BEVs-hCXCR4-SOST siRNA; ClyA: A bacterial surface protein; CXCR4: C-X-C motif chemokine receptor 4; ECN: construct probiotic Escherichia coli Nissle 1917; hCXCR4: human C-X-C motif chemokine receptor 4; IV: intravenous; p: plasmid; SDF1: stromal cell-derived factor 1; siRNA: small interfering RNA; SOST: sclerostin.
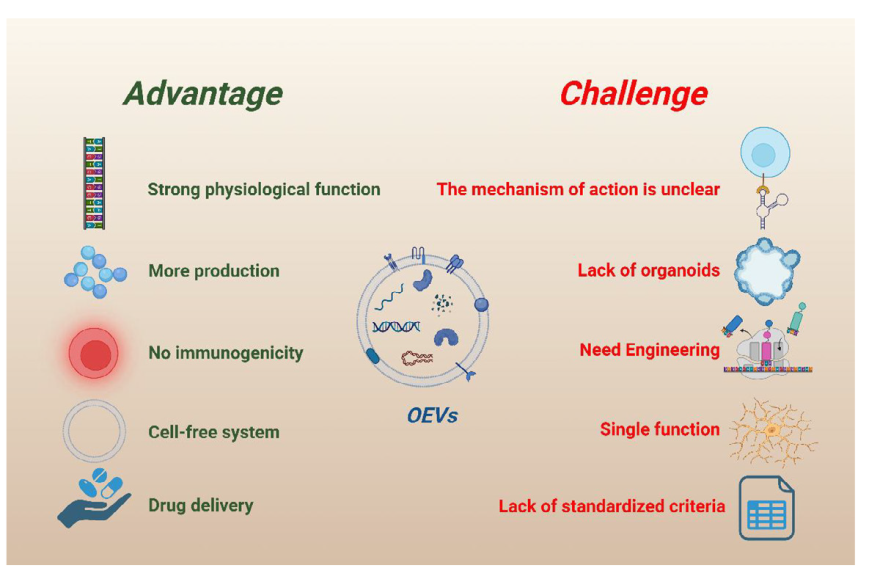
Figure 9. Advantages and challenges of organoid extracellular vesicles (OEVs). OEVs have the advantages of strong physiological function, high yield, low immunogenicity, cell-free system, and good delivery potential. At the same time, OEVs also have several obstacles, including unknown functional mechanism, lack of source, need engineering transformation, single function, and lack of standardised extraction course. Created with BioRender.com.
| 1. |
Farr, J. N.; Khosla, S. Cellular senescence in bone. Bone. 2019, 121, 121-133.
doi: 10.1016/j.bone.2019.01.015 URL |
| 2. | Metavarayuth, K.; Villarreal, E.; Wang, H.; Wang, Q. Surface topography and free energy regulate osteogenesis of stem cells: effects of shape-controlled gold nanoparticles. Biomater Transl. 2021, 2, 165-173. |
| 3. |
Wang, T.; Huang, S.; He, C. Senescent cells: a therapeutic target for osteoporosis. Cell Prolif. 2022, 55, e13323.
doi: 10.1111/cpr.v55.12 URL |
| 4. |
Brown, C. Osteoporosis: staying strong. Nature. 2017, 550, S15-S17.
doi: 10.1038/550S15a URL |
| 5. |
Coryell, P. R.; Diekman, B. O.; Loeser, R. F. Mechanisms and therapeutic implications of cellular senescence in osteoarthritis. Nat Rev Rheumatol. 2021, 17, 47-57.
doi: 10.1038/s41584-020-00533-7 |
| 6. |
Loeser, R. F.; Collins, J. A.; Diekman, B. O. Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016, 12, 412-420.
doi: 10.1038/nrrheum.2016.65 |
| 7. | Yuan, J.; Maturavongsadit, P.; Zhou, Z.; Lv, B.; Lin, Y.; Yang, J.; Luckanagul, J. A. Hyaluronic acid-based hydrogels with tobacco mosaic virus containing cell adhesive peptide induce bone repair in normal and osteoporotic rats. Biomater Transl. 2020, 1, 89-98. |
| 8. | Day, R. O.; Graham, G. G. Non-steroidal anti-inflammatory drugs (NSAIDs). BMJ. 2013, 346, f3195. |
| 9. |
Katz, J. N.; Arant, K. R.; Loeser, R. F. Diagnosis and treatment of hip and knee osteoarthritis: a review. JAMA. 2021, 325, 568-578.
doi: 10.1001/jama.2020.22171 URL |
| 10. | Arora, D.; Robey, P. G. Recent updates on the biological basis of heterogeneity in bone marrow stromal cells/skeletal stem cells. Biomater Transl. 2022, 3, 3-16. |
| 11. |
Liu, H.; Zhang, H.; Wang, S.; Cui, J.; Weng, W.; Liu, X.; Tang, H.; Hu, Y.; Li, X.; Zhang, K.; Zhou, F.; Jing, Y.; Su, J. Bone-targeted bioengineered bacterial extracellular vesicles delivering siRNA to ameliorate osteoporosis. Compos B Eng. 2023, 255, 110610.
doi: 10.1016/j.compositesb.2023.110610 URL |
| 12. |
Liu, H.; Li, M.; Zhang, T.; Liu, X.; Zhang, H.; Geng, Z.; Su, J. Engineered bacterial extracellular vesicles for osteoporosis therapy. Chem Eng J. 2022, 450, 138309.
doi: 10.1016/j.cej.2022.138309 URL |
| 13. |
Liu, H.; Geng, Z.; Su, J. Engineered mammalian and bacterial extracellular vesicles as promising nanocarriers for targeted therapy. Extracell Vesicles Circ Nucleic Acids. 2022, 3, 63-86.
doi: 10.20517/evcna URL |
| 14. |
Liu, H.; Zhang, H.; Han, Y.; Hu, Y.; Geng, Z.; Su, J. Bacterial extracellular vesicles-based therapeutic strategies for bone and soft tissue tumors therapy. Theranostics. 2022, 12, 6576-6594.
doi: 10.7150/thno.78034 URL |
| 15. | Guo, J.; Wang, F.; Hu, Y.; Luo, Y.; Wei, Y.; Xu, K.; Zhang, H.; Liu, H.; Bo, L.; Lv, S.; Sheng, S.; Zhuang, X.; Zhang, T.; Xu, C.; Chen, X.; Su, J. Exosome-based bone-targeting drug delivery alleviates impaired osteoblastic bone formation and bone loss in inflammatory bowel diseases. Cell Rep Med. 2023, 4, 100881. |
| 16. |
Song, H.; Li, X.; Zhao, Z.; Qian, J.; Wang, Y.; Cui, J.; Weng, W.; Cao, L.; Chen, X.; Hu, Y.; Su, J. Reversal of osteoporotic activity by endothelial cell-secreted bone targeting and biocompatible exosomes. Nano Lett. 2019, 19, 3040-3048.
doi: 10.1021/acs.nanolett.9b00287 URL |
| 17. | Wang, J.; Li, X.; Wang, S.; Cui, J.; Ren, X.; Su, J. Bone-targeted exosomes: strategies and applications. Adv Healthc Mater. 2023, 12, e2203361. |
| 18. | Pang, L.; Jin, H.; Lu, Z.; Xie, F.; Shen, H.; Li, X.; Zhang, X.; Jiang, X.; Wu, L.; Zhang, M.; Zhang, T.; Zhai, Y.; Zhang, Y.; Guan, H.; Su, J.; Li, M.; Gao, J. Treatment with mesenchymal stem cell-derived nanovesicle-containing gelatin methacryloyl hydrogels alleviates osteoarthritis by modulating chondrogenesis and macrophage polarization. Adv Healthc Mater. 2023, 12, e2300315. |
| 19. | Liu, H.; Zhang, Q.; Wang, S.; Weng, W.; Jing, Y.; Su, J. Bacterial extracellular vesicles as bioactive nanocarriers for drug delivery: Advances and perspectives. Bioact Mater. 2022, 14, 169-181. |
| 20. | Wang, Z. X.; Luo, Z. W.; Li, F. X.; Cao, J.; Rao, S. S.; Liu, Y. W.; Wang, Y. Y.; Zhu, G. Q.; Gong, J. S.; Zou, J. T.; Wang, Q.; Tan, Y. J.; Zhang, Y.; Hu, Y.; Li, Y. Y.; Yin, H.; Wang, X. K.; He, Z. H.; Ren, L.; Liu, Z. Z.; Hu, X. K.; Yuan, L. Q.; Xu, R.; Chen, C. Y.; Xie, H. Aged bone matrix-derived extracellular vesicles as a messenger for calcification paradox. Nat Commun. 2022, 13, 1453. |
| 21. |
Jiang, Y.; Li, J.; Xue, X.; Yin, Z.; Xu, K.; Su, J. Engineered extracellular vesicles for bone therapy. Nano Today. 2022, 44, 101487.
doi: 10.1016/j.nantod.2022.101487 URL |
| 22. | Liu, J. H.; Yue, T.; Luo, Z. W.; Cao, J.; Yan, Z. Q.; Jin, L.; Wan, T. F.; Shuai, C. J.; Wang, Z. G.; Zhou, Y.; Xu, R.; Xie, H. Akkermansia muciniphila promotes type H vessel formation and bone fracture healing by reducing gut permeability and inflammation. Dis Model Mech. 2020, 13, dmm043620. |
| 23. |
Shan, S. K.; Lin, X.; Li, F.; Xu, F.; Zhong, J. Y.; Guo, B.; Wang, Y.; Zheng, M. H.; Wu, F.; Yuan, L. Q. Exosomes and bone disease. Curr Pharm Des. 2019, 25, 4536-4549.
doi: 10.2174/1381612825666191127114054 URL |
| 24. |
Mi, B.; Chen, L.; Xiong, Y.; Yang, Y.; Panayi, A. C.; Xue, H.; Hu, Y.; Yan, C.; Hu, L.; Xie, X.; Lin, Z.; Zhou, W.; Cao, F.; Xiao, X.; Feng, Q.; Liu, G. Osteoblast/osteoclast and immune cocktail therapy of an exosome/drug delivery multifunctional hydrogel accelerates fracture repair. ACS Nano. 2022, 16, 771-782.
doi: 10.1021/acsnano.1c08284 URL |
| 25. | Zeng, Z. L.; Xie, H. Mesenchymal stem cell-derived extracellular vesicles: a possible therapeutic strategy for orthopaedic diseases: a narrative review. Biomater Transl. 2022, 3, 175-187. |
| 26. | Chen, S.; Chen, X.; Geng, Z.; Su, J. The horizon of bone organoid: a perspective on construction and application. Bioact Mater. 2022, 18, 15-25. |
| 27. |
Keshara, R.; Kim, Y. H.; Grapin-Botton, A. Organoid imaging: seeing development and function. Annu Rev Cell Dev Biol. 2022, 38, 447-466.
doi: 10.1146/cellbio.2022.38.issue-1 URL |
| 28. |
Liu, H.; Sun, J.; Wang, M.; Wang, S.; Su, J.; Xu, C. Intestinal organoids and organoids extracellular vesicles for inflammatory bowel disease treatment. Chem Eng J. 2023, 465, 142842.
doi: 10.1016/j.cej.2023.142842 URL |
| 29. |
Liu, H.; Su, J. Organoid and organoid extracellular vesicles for osteoporotic fractures therapy: current status and future perspectives. Interdiscip Med. 2023, 1, e20230011.
doi: 10.1002/inmd.v1.3 URL |
| 30. |
Bock, C.; Boutros, M.; Camp, J. G.; Clarke, L.; Clevers, H.; Knoblich, J. A.; Liberali, P.; Regev, A.; Rios, A. C.; Stegle, O.; Stunnenberg, H. G.; Teichmann, S. A.; Treutlein, B.; Vries, R. G. J.; Human cell atlas ‘biological network’ organoids. The organoid cell atlas. Nat Biotechnol. 2021, 39, 13-17.
doi: 10.1038/s41587-020-00762-x |
| 31. |
Garreta, E.; Kamm, R. D.; Chuva de Sousa Lopes, S. M.; Lancaster, M. A.; Weiss, R.; Trepat, X.; Hyun, I.; Montserrat, N. Rethinking organoid technology through bioengineering. Nat Mater. 2021, 20, 145-155.
doi: 10.1038/s41563-020-00804-4 |
| 32. |
Brandenberg, N.; Hoehnel, S.; Kuttler, F.; Homicsko, K.; Ceroni, C.; Ringel, T.; Gjorevski, N.; Schwank, G.; Coukos, G.; Turcatti, G.; Lutolf, M. P. High-throughput automated organoid culture via stem-cell aggregation in microcavity arrays. Nat Biomed Eng. 2020, 4, 863-874.
doi: 10.1038/s41551-020-0565-2 |
| 33. |
Lancaster, M. A.; Knoblich, J. A. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014, 345, 1247125.
doi: 10.1126/science.1247125 URL |
| 34. | Zha, Q. B.; Yao, Y. F.; Ren, Z. J.; Li, X. J.; Tang, J. H. Extracellular vesicles: an overview of biogenesis, function, and role in breast cancer. Tumour Biol. 2017, 39, 1010428317691182. |
| 35. |
van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018, 19, 213-228.
doi: 10.1038/nrm.2017.125 URL |
| 36. |
Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019, 21, 9-17.
doi: 10.1038/s41556-018-0250-9 |
| 37. | Jin, S.; Wang, Y.; Wu, X.; Li, Z.; Zhu, L.; Niu, Y.; Zhou, Y.; Liu, Y. Young exosome bio-nanoparticles restore aging-impaired tendon stem/progenitor cell function and reparative capacity. Adv Mater. 2023, 35, e2211602. |
| 38. | Record, M.; Carayon, K.; Poirot, M.; Silvente-Poirot, S. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim Biophys Acta. 2014, 1841, 108-120. |
| 39. |
Nguyen, A.; Yaffe, M. B. Proteomics and systems biology approaches to signal transduction in sepsis. Crit Care Med. 2003, 31, S1-6.
doi: 10.1097/00003246-200301001-00001 URL |
| 40. | Murphy, D. E.; de Jong, O. G.; Brouwer, M.; Wood, M. J.; Lavieu, G.; Schiffelers, R. M.; Vader, P. Extracellular vesicle-based therapeutics: natural versus engineered targeting and trafficking. Exp Mol Med. 2019, 51, 1-12. |
| 41. |
Pegtel, D. M.; Gould, S. J. Exosomes. Annu Rev Biochem. 2019, 88, 487-514.
doi: 10.1146/annurev-biochem-013118-111902 URL |
| 42. | Zhang, Q.; Wang, L.; Wang, S.; Cheng, H.; Xu, L.; Pei, G.; Wang, Y.; Fu, C.; Jiang, Y.; He, C.; Wei, Q. Signaling pathways and targeted therapy for myocardial infarction. Signal Transduct Target Ther. 2022, 7, 78. |
| 43. | Zhong, L.; Liao, D.; Li, J.; Liu, W.; Wang, J.; Zeng, C.; Wang, X.; Cao, Z.; Zhang, R.; Li, M.; Jiang, K.; Zeng, Y. X.; Sui, J.; Kang, T. Rab22a-NeoF1 fusion protein promotes osteosarcoma lung metastasis through its secretion into exosomes. Signal Transduct Target Ther. 2021, 6, 59. |
| 44. |
Dutta, D.; Heo, I.; Clevers, H. Disease modeling in stem cell-derived 3D organoid systems. Trends Mol Med. 2017, 23, 393-410.
doi: 10.1016/j.molmed.2017.02.007 URL |
| 45. |
Shao, H.; Im, H.; Castro, C. M.; Breakefield, X.; Weissleder, R.; Lee, H. New technologies for analysis of extracellular vesicles. Chem Rev. 2018, 118, 1917-1950.
doi: 10.1021/acs.chemrev.7b00534 URL |
| 46. | Rong, Y.; Wang, Z.; Tang, P.; Wang, J.; Ji, C.; Chang, J.; Zhu, Y.; Ye, W.; Bai, J.; Liu, W.; Yin, G.; Yu, L.; Zhou, X.; Cai, W. Engineered extracellular vesicles for delivery of siRNA promoting targeted repair of traumatic spinal cord injury. Bioact Mater. 2023, 23, 328-342. |
| 47. |
Zhang, Q.; Jeppesen, D. K.; Higginbotham, J. N.; Franklin, J. L.; Coffey, R. J. Comprehensive isolation of extracellular vesicles and nanoparticles. Nat Protoc. 2023, 18, 1462-1487.
doi: 10.1038/s41596-023-00811-0 |
| 48. | Lai, J. J.; Chau, Z. L.; Chen, S. Y.; Hill, J. J.; Korpany, K. V.; Liang, N. W.; Lin, L. H.; Lin, Y. H.; Liu, J. K.; Liu, Y. C.; Lunde, R.; Shen, W. T. Exosome processing and characterization approaches for research and technology development. Adv Sci (Weinh). 2022, 9, e2103222. |
| 49. |
Takov, K.; Yellon, D. M.; Davidson, S. M. Comparison of small extracellular vesicles isolated from plasma by ultracentrifugation or size-exclusion chromatography: yield, purity and functional potential. J Extracell Vesicles. 2019, 8, 1560809.
doi: 10.1080/20013078.2018.1560809 URL |
| 50. |
Chattrairat, K.; Yasui, T.; Suzuki, S.; Natsume, A.; Nagashima, K.; Iida, M.; Zhang, M.; Shimada, T.; Kato, A.; Aoki, K.; Ohka, F.; Yamazaki, S.; Yanagida, T.; Baba, Y. All-in-one nanowire assay system for capture and analysis of extracellular vesicles from an ex vivo brain tumor model. ACS Nano. 2023, 17, 2235-2244.
doi: 10.1021/acsnano.2c08526 URL |
| 51. |
Dong, L.; Zieren, R. C.; Horie, K.; Kim, C. J.; Mallick, E.; Jing, Y.; Feng, M.; Kuczler, M. D.; Green, J.; Amend, S. R.; Witwer, K. W.; de Reijke, T. M.; Cho, Y. K.; Pienta, K. J.; Xue, W. Comprehensive evaluation of methods for small extracellular vesicles separation from human plasma, urine and cell culture medium. J Extracell Vesicles. 2020, 10, e12044.
doi: 10.1002/jev2.v10.2 URL |
| 52. |
Foers, A. D.; Chatfield, S.; Dagley, L. F.; Scicluna, B. J.; Webb, A. I.; Cheng, L.; Hill, A. F.; Wicks, I. P.; Pang, K. C. Enrichment of extracellular vesicles from human synovial fluid using size exclusion chromatography. J Extracell Vesicles. 2018, 7, 1490145.
doi: 10.1080/20013078.2018.1490145 URL |
| 53. | Hu, Y.; Li, X.; Zhang, Q.; Gu, Z.; Luo, Y.; Guo, J.; Wang, X.; Jing, Y.; Chen, X.; Su, J. Exosome-guided bone targeted delivery of Antagomir-188 as an anabolic therapy for bone loss. Bioact Mater. 2021, 6, 2905-2913. |
| 54. |
Xu, X.; Liang, Y.; Li, X.; Ouyang, K.; Wang, M.; Cao, T.; Li, W.; Liu, J.; Xiong, J.; Li, B.; Xia, J.; Wang, D.; Duan, L. Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration. Biomaterials. 2021, 269, 120539.
doi: 10.1016/j.biomaterials.2020.120539 URL |
| 55. |
Szvicsek, Z.; Oszvald, Á.; Szabó, L.; Sándor, G. O.; Kelemen, A.; Soós, A.; Pálóczi, K.; Harsányi, L.; Tölgyes, T.; Dede, K.; Bursics, A.; Buzás, E. I.; Zeöld, A.; Wiener, Z. Extracellular vesicle release from intestinal organoids is modulated by Apc mutation and other colorectal cancer progression factors. Cell Mol Life Sci. 2019, 76, 2463-2476.
doi: 10.1007/s00018-019-03052-1 |
| 56. |
Abdollahi, S. Extracellular vesicles from organoids and 3D culture systems. Biotechnol Bioeng. 2021, 118, 1029-1049.
doi: 10.1002/bit.v118.3 URL |
| 57. |
Wu, W.; Zhou, W.; Jiang, J.; Wang, M.; Zhang, J.; Yang, J.; Tang, Q.; Liu, H.; Liu, D.; Xu, W.; Zhong, J. L.; Yang, L.; Lei, M. Mechanical stimuli-induced CCL2 restores adult mouse cells to regenerate hair follicles. Mol Ther Nucleic Acids. 2023, 32, 94-110.
doi: 10.1016/j.omtn.2023.03.002 URL |
| 58. | Rocha, S.; Carvalho, J.; Oliveira, P.; Voglstaetter, M.; Schvartz, D.; Thomsen, A. R.; Walter, N.; Khanduri, R.; Sanchez, J. C.; Keller, A.; Oliveira, C.; Nazarenko, I. 3D cellular architecture affects microrna and protein cargo of extracellular vesicles. Adv Sci (Weinh). 2019, 6, 1800948. |
| 59. |
Yuan, X.; Sun, L.; Jeske, R.; Nkosi, D.; York, S. B.; Liu, Y.; Grant, S. C.; Meckes, D. G., Jr.; Li, Y. Engineering extracellular vesicles by three-dimensional dynamic culture of human mesenchymal stem cells. J Extracell Vesicles. 2022, 11, e12235.
doi: 10.1002/jev2.v11.6 URL |
| 60. | Liu, C.; Chen, X.; Liu, Y.; Sun, L.; Yu, Z.; Ren, Y.; Zeng, C.; Li, Y. Engineering extracellular matrix-bound nanovesicles secreted by three-dimensional human mesenchymal stem cells. Adv Healthc Mater. 2023, 12, e2301112. |
| 61. |
Zhang, Y.; Chopp, M.; Zhang, Z. G.; Katakowski, M.; Xin, H.; Qu, C.; Ali, M.; Mahmood, A.; Xiong, Y. Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2D and 3D conditions improves functional recovery in rats after traumatic brain injury. Neurochem Int. 2017, 111, 69-81.
doi: 10.1016/j.neuint.2016.08.003 URL |
| 62. |
Jalilian, E.; Massoumi, H.; Bigit, B.; Amin, S.; Katz, E. A.; Guaiquil, V. H.; Anwar, K. N.; Hematti, P.; Rosenblatt, M. I.; Djalilian, A. R. Bone marrow mesenchymal stromal cells in a 3D system produce higher concentration of extracellular vesicles (EVs) with increased complexity and enhanced neuronal growth properties. Stem Cell Res Ther. 2022, 13, 425.
doi: 10.1186/s13287-022-03128-z |
| 63. |
Ural, E. E.; Toomajian, V.; Hoque Apu, E.; Veletic, M.; Balasingham, I.; Ashammakhi, N.; Kanada, M.; Contag, C. H. Visualizing extracellular vesicles and their function in 3D tumor microenvironment models. Int J Mol Sci. 2021, 22, 4784.
doi: 10.3390/ijms22094784 URL |
| 64. |
He, C.; Zheng, S.; Luo, Y.; Wang, B. Exosome theranostics: biology and translational medicine. Theranostics. 2018, 8, 237-255.
doi: 10.7150/thno.21945 URL |
| 65. | Yang, B.; Chen, Y.; Shi, J. Exosome biochemistry and advanced nanotechnology for next-generation theranostic platforms. Adv Mater. 2019, 31, e1802896. |
| 66. |
Cully, M. Exosome-based candidates move into the clinic. Nat Rev Drug Discov. 2021, 20, 6-7.
doi: 10.1038/d41573-020-00220-y |
| 67. | Zinger, A.; Cvetkovic, C.; Sushnitha, M.; Naoi, T.; Baudo, G.; Anderson, M.; Shetty, A.; Basu, N.; Covello, J.; Tasciotti, E.; Amit, M.; Xie, T.; Taraballi, F.; Krencik, R. Humanized biomimetic nanovesicles for neuron targeting. Adv Sci (Weinh). 2021, 8, e2101437. |
| 68. |
Tauro, B. J.; Greening, D. W.; Mathias, R. A.; Mathivanan, S.; Ji, H.; Simpson, R. J. Two distinct populations of exosomes are released from LIM1863 colon carcinoma cell-derived organoids. Mol Cell Proteomics. 2013, 12, 587-598.
doi: 10.1074/mcp.M112.021303 URL |
| 69. |
Jurj, A.; Pasca, S.; Braicu, C.; Rusu, I.; Korban, S. S.; Berindan-Neagoe, I. Focus on organoids: cooperation and interconnection with extracellular vesicles - Is this the future of in vitro modeling? Semin Cancer Biol. 2022, 86, 367-381.
doi: 10.1016/j.semcancer.2021.12.002 URL |
| 70. |
Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J. J.; Lötvall, J. O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007, 9, 654-659.
doi: 10.1038/ncb1596 |
| 71. |
Théry, C.; Duban, L.; Segura, E.; Véron, P.; Lantz, O.; Amigorena, S. Indirect activation of naïve CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol. 2002, 3, 1156-1162.
doi: 10.1038/ni854 |
| 72. |
Zhang, Y.; Yan, Y.; Meng, J.; Girotra, M.; Ramakrishnan, S.; Roy, S. Immune modulation mediated by extracellular vesicles of intestinal organoids is disrupted by opioids. Mucosal Immunol. 2021, 14, 887-898.
doi: 10.1038/s41385-021-00392-9 URL |
| 73. |
Roush, S.; Slack, F. J. The let-7 family of microRNAs. Trends Cell Biol. 2008, 18, 505-516.
doi: 10.1016/j.tcb.2008.07.007 URL |
| 74. |
Lee, H.; Han, S.; Kwon, C. S.; Lee, D. Biogenesis and regulation of the let-7 miRNAs and their functional implications. Protein Cell. 2016, 7, 100-113.
doi: 10.1007/s13238-015-0212-y URL |
| 75. |
Watanabe, S.; Kobayashi, S.; Ogasawara, N.; Okamoto, R.; Nakamura, T.; Watanabe, M.; Jensen, K. B.; Yui, S. Transplantation of intestinal organoids into a mouse model of colitis. Nat Protoc. 2022, 17, 649-671.
doi: 10.1038/s41596-021-00658-3 |
| 76. |
Vissink, A.; Mitchell, J. B.; Baum, B. J.; Limesand, K. H.; Jensen, S. B.; Fox, P. C.; Elting, L. S.; Langendijk, J. A.; Coppes, R. P.; Reyland, M. E. Clinical management of salivary gland hypofunction and xerostomia in head-and-neck cancer patients: successes and barriers. Int J Radiat Oncol Biol Phys. 2010, 78, 983-991.
doi: 10.1016/j.ijrobp.2010.06.052 URL |
| 77. |
Sun, B. K.; Siprashvili, Z.; Khavari, P. A. Advances in skin grafting and treatment of cutaneous wounds. Science. 2014, 346, 941-945.
doi: 10.1126/science.1253836 URL |
| 78. |
Adine, C.; Ng, K. K.; Rungarunlert, S.; Souza, G. R.; Ferreira, J. N. Engineering innervated secretory epithelial organoids by magnetic three-dimensional bioprinting for stimulating epithelial growth in salivary glands. Biomaterials. 2018, 180, 52-66.
doi: 10.1016/j.biomaterials.2018.06.011 URL |
| 79. | Chansaenroj, A.; Adine, C.; Charoenlappanit, S.; Roytrakul, S.; Sariya, L.; Osathanon, T.; Rungarunlert, S.; Urkasemsin, G.; Chaisuparat, R.; Yodmuang, S.; Souza, G. R.; Ferreira, J. N. Magnetic bioassembly platforms towards the generation of extracellular vesicles from human salivary gland functional organoids for epithelial repair. Bioact Mater. 2022, 18, 151-163. |
| 80. |
Liang, Y.; Duan, L.; Lu, J.; Xia, J. Engineering exosomes for targeted drug delivery. Theranostics. 2021, 11, 3183-3195.
doi: 10.7150/thno.52570 URL |
| 81. |
Liang, Y.; Xu, X.; Li, X.; Xiong, J.; Li, B.; Duan, L.; Wang, D.; Xia, J. Chondrocyte-targeted microRNA delivery by engineered exosomes toward a cell-free osteoarthritis therapy. ACS Appl Mater Interfaces. 2020, 12, 36938-36947.
doi: 10.1021/acsami.0c10458 URL |
| 82. |
Zou, J.; Shi, M.; Liu, X.; Jin, C.; Xing, X.; Qiu, L.; Tan, W. Aptamer-functionalized exosomes: elucidating the cellular uptake mechanism and the potential for cancer-targeted chemotherapy. Anal Chem. 2019, 91, 2425-2430.
doi: 10.1021/acs.analchem.8b05204 URL |
| 83. |
Wen, M.; Wang, J.; Ou, Z.; Nie, G.; Chen, Y.; Li, M.; Wu, Z.; Xiong, S.; Zhou, H.; Yang, Z.; Long, G.; Su, J.; Liu, H.; Jing, Y.; Wen, Z.; Fu, Y.; Zhou, T.; Xie, H.; Guan, W.; Sun, X.; Wang, Z.; Wang, J.; Chen, X.; Jiang, L.; Qin, X.; Xue, Y.; Huang, M.; Huang, X.; Pan, R.; Zhen, H.; Du, Y.; Li, Q.; Huang, X.; Wu, Y.; Wang, P.; Zhao, K.; Situ, B.; Hu, X.; Zheng, L. Bacterial extracellular vesicles: A position paper by the microbial vesicles task force of the Chinese society for extracellular vesicles. Interdiscip Med. 2023, 1, e20230017.
doi: 10.1002/inmd.v1.3 URL |
| 84. |
Lin, L.; Guo, Z.; He, E.; Long, X.; Wang, D.; Zhang, Y.; Guo, W.; Wei, Q.; He, W.; Wu, W.; Li, J.; Wo, L.; Hong, D.; Zheng, J.; He, M.; Zhao, Q. SIRT2 regulates extracellular vesicle-mediated liver-bone communication. Nat Metab. 2023, 5, 821-841.
doi: 10.1038/s42255-023-00803-0 |
| 85. | Wang, L.; Wang, D.; Ye, Z.; Xu, J. Engineering extracellular vesicles as delivery systems in therapeutic applications. Adv Sci (Weinh). 2023, 10, e2300552. |
| 86. |
Ji, N.; Wang, F.; Wang, M.; Zhang, W.; Liu, H.; Su, J. Engineered bacterial extracellular vesicles for central nervous system diseases. J Control Release. 2023, 364, 46-60.
doi: 10.1016/j.jconrel.2023.10.027 URL |
| 87. | Lin, Y.; Wu, J.; Gu, W.; Huang, Y.; Tong, Z.; Huang, L.; Tan, J. Exosome-liposome hybrid nanoparticles deliver CRISPR/Cas9 system in MSCs. Adv Sci (Weinh). 2018, 5, 1700611. |
| 88. | Cui, Y.; Guo, Y.; Kong, L.; Shi, J.; Liu, P.; Li, R.; Geng, Y.; Gao, W.; Zhang, Z.; Fu, D. A bone-targeted engineered exosome platform delivering siRNA to treat osteoporosis. Bioact Mater. 2022, 10, 207-221. |
| 89. |
Smyth, T.; Petrova, K.; Payton, N. M.; Persaud, I.; Redzic, J. S.; Graner, M. W.; Smith-Jones, P.; Anchordoquy, T. J. Surface functionalization of exosomes using click chemistry. Bioconjug Chem. 2014, 25, 1777-1784.
doi: 10.1021/bc500291r URL |
| 90. |
Shi, Y.; Guo, S.; Liang, Y.; Liu, L.; Wang, A.; Sun, K.; Li, Y. Construction and evaluation of liraglutide delivery system based on milk exosomes: a new idea for oral peptide delivery. Curr Pharm Biotechnol. 2022, 23, 1072-1079.
doi: 10.2174/1389201022666210820114236 URL |
| 91. |
Cheng, K.; Zhao, R.; Li, Y.; Qi, Y.; Wang, Y.; Zhang, Y.; Qin, H.; Qin, Y.; Chen, L.; Li, C.; Liang, J.; Li, Y.; Xu, J.; Han, X.; Anderson, G. J.; Shi, J.; Ren, L.; Zhao, X.; Nie, G. Bioengineered bacteria-derived outer membrane vesicles as a versatile antigen display platform for tumor vaccination via plug-and-display technology. Nat Commun. 2021, 12, 2041.
doi: 10.1038/s41467-021-22308-8 |
| 92. |
Li, Y.; Zhao, R.; Cheng, K.; Zhang, K.; Wang, Y.; Zhang, Y.; Li, Y.; Liu, G.; Xu, J.; Xu, J.; Anderson, G. J.; Shi, J.; Ren, L.; Zhao, X.; Nie, G. Bacterial outer membrane vesicles presenting programmed death 1 for improved cancer immunotherapy via immune activation and checkpoint inhibition. ACS Nano. 2020, 14, 16698-16711.
doi: 10.1021/acsnano.0c03776 URL |
| 93. |
Yang, Y.; Hong, Y.; Nam, G. H.; Chung, J. H.; Koh, E.; Kim, I. S. Virus-mimetic fusogenic exosomes for direct delivery of integral membrane proteins to target cell membranes. Adv Mater. 2017, 29, 1605604.
doi: 10.1002/adma.v29.13 URL |
| 94. |
Chatterjee, M.; Özdemir, S.; Kunadt, M.; Koel-Simmelink, M.; Boiten, W.; Piepkorn, L.; Pham, T. V.; Chiasserini, D.; Piersma, S. R.; Knol, J. C.; Möbius, W.; Mollenhauer, B.; van der Flier, W. M.; Jimenez, C. R.; Teunissen, C. E.; Jahn, O.; Schneider, A. C1q is increased in cerebrospinal fluid-derived extracellular vesicles in Alzheimer’s disease: A multi-cohort proteomics and immuno-assay validation study. Alzheimers Dement. 2023, 19, 4828-4840.
doi: 10.1002/alz.v19.11 URL |
| 95. |
Piffoux, M.; Silva, A. K. A.; Wilhelm, C.; Gazeau, F.; Tareste, D. Modification of extracellular vesicles by fusion with liposomes for the design of personalized biogenic drug delivery systems. ACS Nano. 2018, 12, 6830-6842.
doi: 10.1021/acsnano.8b02053 URL |
| 96. | Chen, Q.; Huang, G.; Wu, W.; Wang, J.; Hu, J.; Mao, J.; Chu, P. K.; Bai, H.; Tang, G. A hybrid eukaryotic-prokaryotic nanoplatform with photothermal modality for enhanced antitumor vaccination. Adv Mater. 2020, 32, e1908185. |
| 97. |
Yi, K.; Rong, Y.; Huang, L.; Tang, X.; Zhang, Q.; Wang, W.; Wu, J.; Wang, F. Aptamer-exosomes for tumor theranostics. ACS Sens. 2021, 6, 1418-1429.
doi: 10.1021/acssensors.0c02237 URL |
| 98. | Sun, S.; Liu, H.; Hu, Y.; Wang, Y.; Zhao, M.; Yuan, Y.; Han, Y.; Jing, Y.; Cui, J.; Ren, X.; Chen, X.; Su, J. Selection and identification of a novel ssDNA aptamer targeting human skeletal muscle. Bioact Mater. 2023, 20, 166-178. |
| 99. |
Haney, M. J.; Klyachko, N. L.; Zhao, Y.; Gupta, R.; Plotnikova, E. G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A. V.; Batrakova, E. V. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release. 2015, 207, 18-30.
doi: 10.1016/j.jconrel.2015.03.033 URL |
| 100. |
Hajipour, H.; Farzadi, L.; Roshangar, L.; Latifi, Z.; Kahroba, H.; Shahnazi, V.; Hamdi, K.; Ghasemzadeh, A.; Fattahi, A.; Nouri, M. A human chorionic gonadotropin (hCG) delivery platform using engineered uterine exosomes to improve endometrial receptivity. Life Sci. 2021, 275, 119351.
doi: 10.1016/j.lfs.2021.119351 URL |
| 101. |
Zha, Y.; Li, Y.; Lin, T.; Chen, J.; Zhang, S.; Wang, J. Progenitor cell-derived exosomes endowed with VEGF plasmids enhance osteogenic induction and vascular remodeling in large segmental bone defects. Theranostics. 2021, 11, 397-409.
doi: 10.7150/thno.50741 URL |
| 102. |
Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M. J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011, 29, 341-345.
doi: 10.1038/nbt.1807 |
| 103. |
Chen, C. Y.; Rao, S. S.; Yue, T.; Tan, Y. J.; Yin, H.; Chen, L. J.; Luo, M. J.; Wang, Z.; Wang, Y. Y.; Hong, C. G.; Qian, Y. X.; He, Z. H.; Liu, J. H.; Yang, F.; Huang, F. Y.; Tang, S. Y.; Xie, H. Glucocorticoid-induced loss of beneficial gut bacterial extracellular vesicles is associated with the pathogenesis of osteonecrosis. Sci Adv. 2022, 8, eabg8335.
doi: 10.1126/sciadv.abg8335 URL |
| 104. | Liu, J. H.; Chen, C. Y.; Liu, Z. Z.; Luo, Z. W.; Rao, S. S.; Jin, L.; Wan, T. F.; Yue, T.; Tan, Y. J.; Yin, H.; Yang, F.; Huang, F. Y.; Guo, J.; Wang, Y. Y.; Xia, K.; Cao, J.; Wang, Z. X.; Hong, C. G.; Luo, M. J.; Hu, X. K.; Liu, Y. W.; Du, W.; Luo, J.; Hu, Y.; Zhang, Y.; Huang, J.; Li, H. M.; Wu, B.; Liu, H. M.; Chen, T. H.; Qian, Y. X.; Li, Y. Y.; Feng, S. K.; Chen, Y.; Qi, L. Y.; Xu, R.; Tang, S. Y.; Xie, H. Extracellular vesicles from child gut microbiota enter into bone to preserve bone mass and strength. Adv Sci (Weinh). 2021, 8, 2004831. |
| 105. |
Liu, H.; Wu, Y.; Wang, F.; Wang, S.; Ji, N.; Wang, M.; Zhou, G.; Han, R.; Liu, X.; Weng, W.; Tan, H.; Jing, Y.; Zhang, W.; Zhang, H.; Shi, Z.; Su, J. Bone-targeted engineered bacterial extracellular vesicles delivering miRNA to treat osteoporosis. Compos B Eng. 2023, 267, 111047.
doi: 10.1016/j.compositesb.2023.111047 URL |
| 106. |
Kalluri, R.; McAndrews, K. M. The role of extracellular vesicles in cancer. Cell. 2023, 186, 1610-1626.
doi: 10.1016/j.cell.2023.03.010 URL |
| 107. |
Eastlake, K.; Wang, W.; Jayaram, H.; Murray-Dunning, C.; Carr, A. J. F.; Ramsden, C. M.; Vugler, A.; Gore, K.; Clemo, N.; Stewart, M.; Coffey, P.; Khaw, P. T.; Limb, G. A. Phenotypic and functional characterization of Müller glia isolated from induced pluripotent stem cell-derived retinal organoids: improvement of retinal ganglion cell function upon transplantation. Stem Cells Transl Med. 2019, 8, 775-784.
doi: 10.1002/sctm.18-0263 URL |
| 108. |
Eastlake, K.; Lamb, W. D. B.; Luis, J.; Khaw, P. T.; Jayaram, H.; Limb, G. A. Prospects for the application of Müller glia and their derivatives in retinal regenerative therapies. Prog Retin Eye Res. 2021, 85, 100970.
doi: 10.1016/j.preteyeres.2021.100970 URL |
| 109. | Xu, X.; Song, J. Segmental long bone regeneration guided by degradable synthetic polymeric scaffolds. Biomater Transl. 2020, 1, 33-45. |
| 110. | Yuan, G.; Li, Z.; Lin, X.; Li, N.; Xu, R. New perspective of skeletal stem cells. Biomater Transl. 2022, 3, 280-294. |
| 111. |
Arthur, P.; Kandoi, S.; Sun, L.; Kalvala, A.; Kutlehria, S.; Bhattacharya, S.; Kulkarni, T.; Nimma, R.; Li, Y.; Lamba, D. A.; Singh, M. Biophysical, molecular and proteomic profiling of human retinal organoid-derived exosomes. Pharm Res. 2023, 40, 801-816.
doi: 10.1007/s11095-022-03350-7 |
| 112. |
Liu, C.; Helsper, S.; Marzano, M.; Chen, X.; Muok, L.; Esmonde, C.; Zeng, C.; Sun, L.; Grant, S. C.; Li, Y. Human forebrain organoid-derived extracellular vesicle labeling with iron oxides for in vitro magnetic resonance imaging. Biomedicines. 2022, 10, 3060.
doi: 10.3390/biomedicines10123060 URL |
| 113. | Wang, Y.; Chu, X.; Wang, B. Recombinant adeno-associated virus-based gene therapy combined with tissue engineering for musculoskeletal regenerative medicine. Biomater Transl. 2021, 2, 19-29. |
| [1] | Xiaoxiang Ren, Ruixue Xu, Chenjie Xu, Jiacan Su. Harnessing exosomes for targeted therapy: strategy and application [J]. Biomaterials Translational, 2024, 5(1): 46-58. |
| [2] | Zhao–Lin Zeng, Hui Xie. Mesenchymal stem cell–derived extracellular vesicles: a possible therapeutic strategy for orthopaedic diseases: a narrative review [J]. Biomaterials Translational, 2022, 3(3): 175-187. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||