Biomaterials Translational ›› 2023, Vol. 4 ›› Issue (4): 270-279.doi: 10.12336/biomatertransl.2023.04.006
• RESEARCH ARTICLE • Previous Articles Next Articles
Shunshu Deng1,2,3, Fuwei Zhu1,2,3, Kai Dai1,2,3,4,*( ), Jing Wang1,3,4,*(
), Jing Wang1,3,4,*( ), Changsheng Liu2,3,4,5,*(
), Changsheng Liu2,3,4,5,*( )
)
Received:2023-09-21
Revised:2023-11-14
Accepted:2023-11-30
Online:2023-12-27
Published:2023-12-28
Contact:
Kai Dai, daikai233@foxmail.com;Jing Wang, biomatwj@163.com;Changsheng Liu, liucs@ecust.edu.cn.
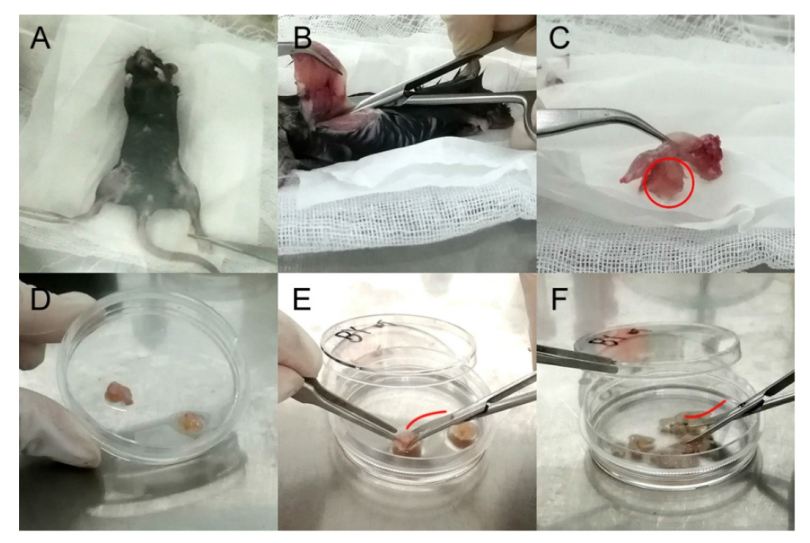
Figure 1. Illustrations of osteo-organoid cell separation procedures. (A) The mouse body soaked in 75% (v/v) ethanol was placed in biosafety cabinet. (B) After removing skin and muscle, a hindlimb was completely cut off, so that the femur and tibia could be used for extraction of bone marrow-derived mesenchymal stem cells (BM-MSCs). (C) The osteo-organoid was hidden in the swollen part of the muscle (red circle) between femur and calf. (D) Osteo-organoids covered with periosteum-like tissue were separated in a 60-mm cell culture dish. (E) The surface of the blade was convex (red curve) to cut the osteo-organoids. (F) The surface of the blade was concave (red curve) to shred the osteo-organoids.
| Experiment | Culture vessel | Volume of medium | Seeding density | Other suggestions |
|---|---|---|---|---|
| Colony forming unit-fibroblast | 6-well plate | 2 mL per well | 5 × 105 cells per well | Replace α-MEM with DMEM to prepare the complete medium |
| Purification | 100-mm dish | 15 mL per dish | 1 × 107 cells per dish | Culture under the condition of 5% O2 atmosphere |
| Differentiation | 6-well plate | 2 mL per well | 2 × 105 cells per well | Use α-MEM containing 10% fetal bovine serum and 1% penicillin-streptomycin as the medium before differentiation assays |
| Proliferation | 60-mm dish | 5 mL per dish | 2 × 105 cells per well | Incubate the MSC for 2 hours in a medium containing 50 μM EdU |
Table 1. Details of cell culture in different experiments
| Experiment | Culture vessel | Volume of medium | Seeding density | Other suggestions |
|---|---|---|---|---|
| Colony forming unit-fibroblast | 6-well plate | 2 mL per well | 5 × 105 cells per well | Replace α-MEM with DMEM to prepare the complete medium |
| Purification | 100-mm dish | 15 mL per dish | 1 × 107 cells per dish | Culture under the condition of 5% O2 atmosphere |
| Differentiation | 6-well plate | 2 mL per well | 2 × 105 cells per well | Use α-MEM containing 10% fetal bovine serum and 1% penicillin-streptomycin as the medium before differentiation assays |
| Proliferation | 60-mm dish | 5 mL per dish | 2 × 105 cells per well | Incubate the MSC for 2 hours in a medium containing 50 μM EdU |
| Characteristics | Operations |
|---|---|
| Adhesion of MSCs are earlier than other cells from marrow | Wash cells using Dulbecco’s phosphate-buffered saline after seeding overnight |
| MSCs are easy to be digested and suspended | Time of digestion during the cell passage is not recommended to exceed 2 minutes |
| Low oxygen pressure increases the proliferation and reduces spontaneous differentiation of MSCs | Expand cells under the hypoxic condition (5% O2) |
Table 2. Purification operations are based on the characteristics of MSCs
| Characteristics | Operations |
|---|---|
| Adhesion of MSCs are earlier than other cells from marrow | Wash cells using Dulbecco’s phosphate-buffered saline after seeding overnight |
| MSCs are easy to be digested and suspended | Time of digestion during the cell passage is not recommended to exceed 2 minutes |
| Low oxygen pressure increases the proliferation and reduces spontaneous differentiation of MSCs | Expand cells under the hypoxic condition (5% O2) |
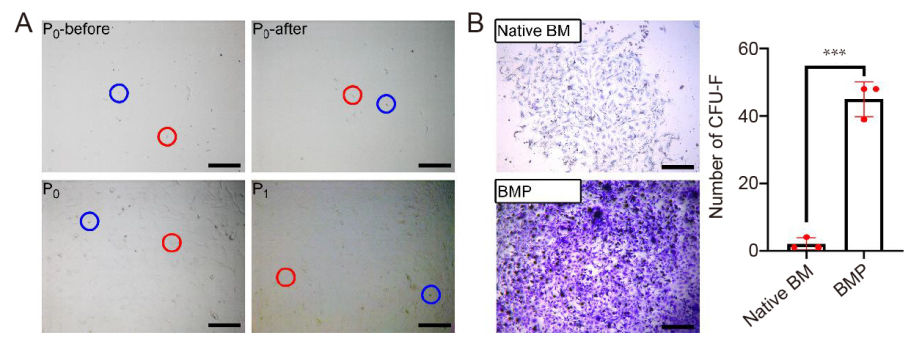
Figure 2. Morphology of MSCs and CFU-F assay. (A) Primary cells before (P0-before) and after (P0-after) the first refreshing of the medium, and MSCs at 80% confluence at P0 and P1. Red and blue circles indicate spindle-shaped and round cells. (B) Left: Primary cells from osteo-organoids (constructed with recombinant human bone morphogenetic protein 2-loaded gelatin sponge scaffolds) formed larger colonies than that from bone marrow (native BM). Scale bars: 200 μm. Right: The number of colonies in each well of a standard 6-well plate. Data are expressed as mean ± SD (n = 3). ***P < 0.001 (Student’s t-test). CFU-F: colony forming unit-fibroblast; MSCs: mesenchymal stem cells; P: passage.
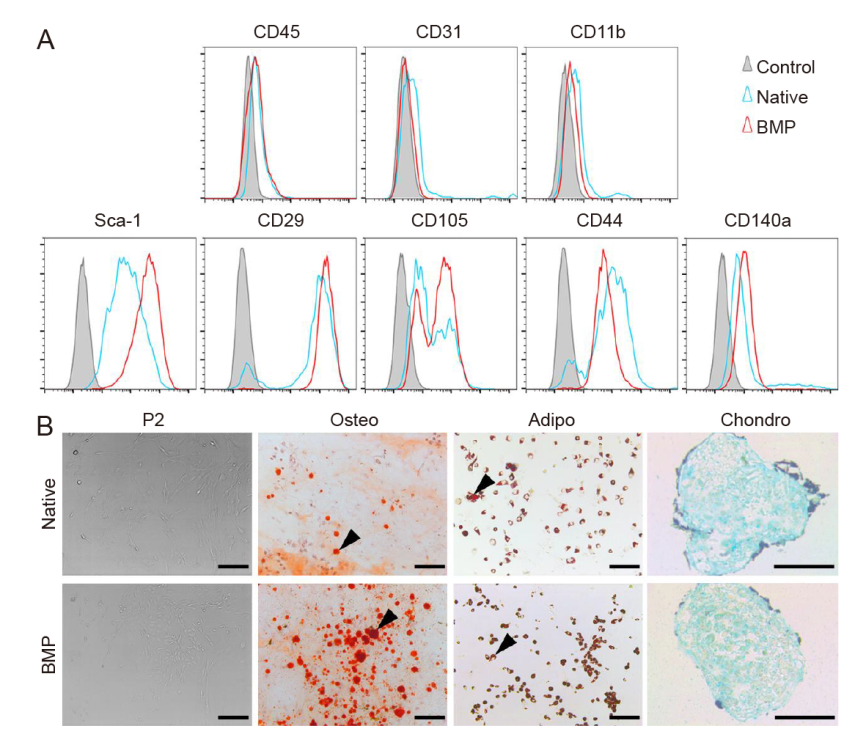
Figure 3. Cell surface phenotype and multilineage differentiation of MSCs at P2. (A) Flow cytometry of odMSCs (constructed with recombinant human bone morphogenetic protein 2-loaded gelatin sponge scaffolds; BMP) and bone marrow (native). (B) Alizarin red, oil red O or Alcian blue staining after differentiation under the osteogenic, adipogenic or chondrogenic induction. The osteogenic and adipogenic differentiation of odMSCs were more obvious than that of BM-MSCs. Black triangles indicate calcified nodules and mature adipocytes. Scale bars: 200 μm. BM-MSCs: bone marrow-derived mesenchymal stem cells; MSCs: mesenchymal stem cells; odMSCs: osteo-organoid-derived mesenchymal stem cells; P2: passage 2; Sca-1: stem cell antigen-1.
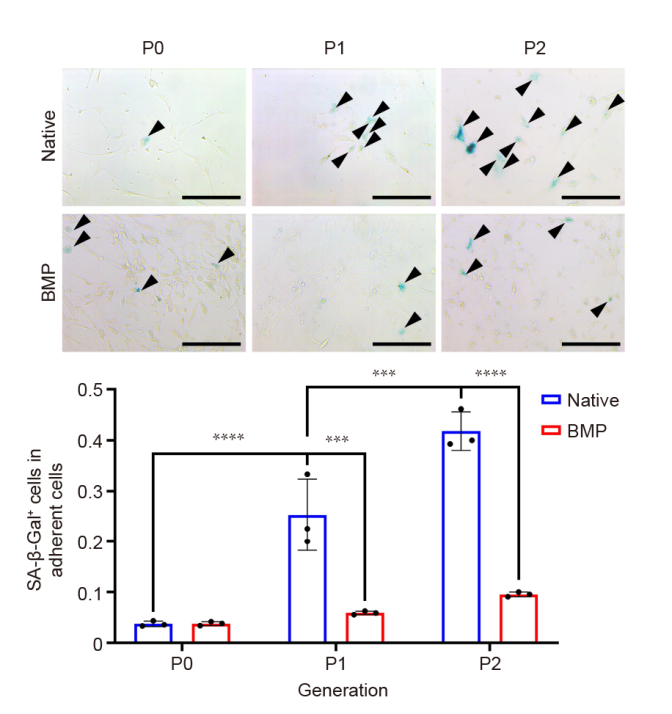
Figure 4. Upper: Ageing process for MSCs from P0 to P2. Black triangles indicate SA-β-Gal positive cells. Scale bars: 200 μm. Lower: The proportions of SA-β-Gal positive cells. The proportion of cell senescence in the native BM-MSCs group (native) increased with passage, while it remained basically unchanged in the odMSCs (constructed with recombinant human bone morphogenetic protein 2-loaded gelatin sponge scaffolds; BMP). Data are expressed as mean ± SD (n = 3). ***P < 0.001, ****P < 0.0001 (two-way analysis of variance followed by Bonferroni’s multiple comparison test). BM-MSCs: bone marrow-derived mesenchymal stem cells; odMSCs: osteo-organoid-derived mesenchymal stem cells; P: passage; SA-β-Gal: senescence-associated β-galactosidase.
| Source | P0 | P1 | Total |
|---|---|---|---|
| Osteo-organoid | 3 days | 3 days | 6 days |
| Native bone marrow | 8 days | 4 days | 12 days |
Table 3. Time of mesenchymal stem cell passage from the osteo-organoid vs. native bone marrow
| Source | P0 | P1 | Total |
|---|---|---|---|
| Osteo-organoid | 3 days | 3 days | 6 days |
| Native bone marrow | 8 days | 4 days | 12 days |
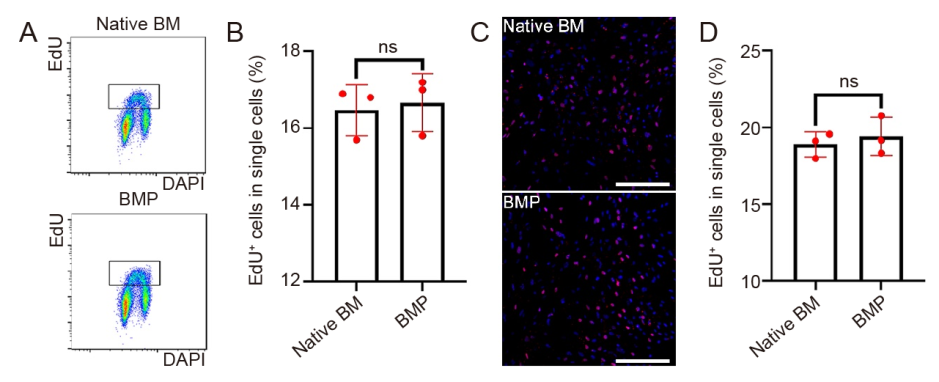
Figure 5. Cell proliferation of MSCs at P2. (A, B) Scatter diagrams (A) and content of EdU+ cells (B) by flow cytometric analysis. (C, D) Fluorescence confocal image (blue: DAPI; red: EdU; C) and statistical analysis (D). Scale bars: 200 μm. Data are expressed as mean ± SD (n = 3), and were analyzed by Student’s t-test. BM: native bone marrow-derived mesenchymal stem cells; BMP: osteo-organoid-derived mesenchymal stem cells (constructed with recombinant human bone morphogenetic protein 2-loaded gelatin sponge scaffolds); DAPI: 4′,6-diamidino-2-phenylindole; EdU: 5-ethynyl-2′-deoxyuridine; MSCs: mesenchymal stem cells; ns: not significant; P2: passage 2.
| 1. |
Zhu, H.; Guo, Z. K.; Jiang, X. X.; Li, H.; Wang, X. Y.; Yao, H. Y.; Zhang, Y.; Mao, N. A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nat Protoc. 2010, 5, 550-560.
doi: 10.1038/nprot.2009.238 |
| 2. |
Li, W. Y.; Choi, Y. J.; Lee, P. H.; Huh, K.; Kang, Y. M.; Kim, H. S.; Ahn, Y. H.; Lee, G.; Bang, O. Y. Mesenchymal stem cells for ischemic stroke: changes in effects after ex vivo culturing. Cell Transplant. 2008, 17, 1045-1059.
doi: 10.3727/096368908786991551 URL |
| 3. |
Kurth, T. B.; Dell’accio, F.; Crouch, V.; Augello, A.; Sharpe, P. T.; De Bari, C. Functional mesenchymal stem cell niches in adult mouse knee joint synovium in vivo. Arthritis Rheum. 2011, 63, 1289-1300.
doi: 10.1002/art.v63.5 URL |
| 4. |
Huang, S.; Xu, L.; Sun, Y.; Wu, T.; Wang, K.; Li, G. An improved protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. J Orthop Translat. 2015, 3, 26-33.
doi: 10.1016/j.jot.2014.07.005 URL |
| 5. |
Rossi, C. A.; Flaibani, M.; Blaauw, B.; Pozzobon, M.; Figallo, E.; Reggiani, C.; Vitiello, L.; Elvassore, N.; De Coppi, P. In vivo tissue engineering of functional skeletal muscle by freshly isolated satellite cells embedded in a photopolymerizable hydrogel. FASEB J. 2011, 25, 2296-2304.
doi: 10.1096/fsb2.v25.7 URL |
| 6. |
Zhai, W.; Yong, D.; El-Jawhari, J. J.; Cuthbert, R.; McGonagle, D.; Win Naing, M.; Jones, E. Identification of senescent cells in multipotent mesenchymal stromal cell cultures: current methods and future directions. Cytotherapy. 2019, 21, 803-819.
doi: 10.1016/j.jcyt.2019.05.001 URL |
| 7. |
Karnoub, A. E.; Dash, A. B.; Vo, A. P.; Sullivan, A.; Brooks, M. W.; Bell, G. W.; Richardson, A. L.; Polyak, K.; Tubo, R.; Weinberg, R. A. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007, 449, 557-563.
doi: 10.1038/nature06188 |
| 8. |
Salazar-Noratto, G. E.; Luo, G.; Denoeud, C.; Padrona, M.; Moya, A.; Bensidhoum, M.; Bizios, R.; Potier, E.; Logeart-Avramoglou, D.; Petite, H. Understanding and leveraging cell metabolism to enhance mesenchymal stem cell transplantation survival in tissue engineering and regenerative medicine applications. Stem Cells. 2020, 38, 22-33.
doi: 10.1002/stem.3079 URL |
| 9. |
Wang, Y.; Zhang, W.; Yao, Q. Copper-based biomaterials for bone and cartilage tissue engineering. J Orthop Translat. 2021, 29, 60-71.
doi: 10.1016/j.jot.2021.03.003 URL |
| 10. |
Uezumi, A.; Fukada, S.; Yamamoto, N.; Takeda, S.; Tsuchida, K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 2010, 12, 143-152.
doi: 10.1038/ncb2014 |
| 11. |
Tidu, F.; De Zuani, M.; Jose, S. S.; Bendíčková, K.; Kubala, L.; Caruso, F.; Cavalieri, F.; Forte, G.; Frič, J. NFAT signaling in human mesenchymal stromal cells affects extracellular matrix remodeling and antifungal immune responses. iScience. 2021, 24, 102683.
doi: 10.1016/j.isci.2021.102683 URL |
| 12. |
Wang, G.; Cao, K.; Liu, K.; Xue, Y.; Roberts, A. I.; Li, F.; Han, Y.; Rabson, A. B.; Wang, Y.; Shi, Y. Kynurenic acid, an IDO metabolite, controls TSG-6-mediated immunosuppression of human mesenchymal stem cells. Cell Death Differ. 2018, 25, 1209-1223.
doi: 10.1038/s41418-017-0006-2 |
| 13. |
Kfoury, Y.; Scadden, D. T. Mesenchymal cell contributions to the stem cell niche. Cell Stem Cell. 2015, 16, 239-253.
doi: 10.1016/j.stem.2015.02.019 URL |
| 14. |
McCullen, S. D.; Chow, A. G.; Stevens, M. M. In vivo tissue engineering of musculoskeletal tissues. Curr Opin Biotechnol. 2011, 22, 715-720.
doi: 10.1016/j.copbio.2011.05.001 URL |
| 15. |
Li, Z.; Niu, S.; Guo, B.; Gao, T.; Wang, L.; Wang, Y.; Wang, L.; Tan, Y.; Wu, J.; Hao, J. Stem cell therapy for COVID-19, ARDS and pulmonary fibrosis. Cell Prolif. 2020, 53, e12939.
doi: 10.1111/cpr.v53.12 URL |
| 16. |
Hejcl, A.; Sedý, J.; Kapcalová, M.; Toro, D. A.; Amemori, T.; Lesný, P.; Likavcanová-Mašínová, K.; Krumbholcová, E.; Prádný, M.; Michálek, J.; Burian, M.; Hájek, M.; Jendelová, P.; Syková, E. HPMA-RGD hydrogels seeded with mesenchymal stem cells improve functional outcome in chronic spinal cord injury. Stem Cells Dev. 2010, 19, 1535-1546.
doi: 10.1089/scd.2009.0378 URL |
| 17. |
Maruyama, M.; Pan, C. C.; Moeinzadeh, S.; Storaci, H. W.; Guzman, R. A.; Lui, E.; Ueno, M.; Utsunomiya, T.; Zhang, N.; Rhee, C.; Yao, Z.; Takagi, M.; Goodman, S. B.; Yang, Y. P. Effect of porosity of a functionally-graded scaffold for the treatment of corticosteroid-associated osteonecrosis of the femoral head in rabbits. J Orthop Translat. 2021, 28, 90-99.
doi: 10.1016/j.jot.2021.01.002 URL |
| 18. |
Sun, L.; Akiyama, K.; Zhang, H.; Yamaza, T.; Hou, Y.; Zhao, S.; Xu, T.; Le, A.; Shi, S. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells. 2009, 27, 1421-1432.
doi: 10.1002/stem.68 URL |
| 19. |
Cao, X.; Duan, L.; Hou, H.; Liu, Y.; Chen, S.; Zhang, S.; Liu, Y.; Wang, C.; Qi, X.; Liu, N.; Han, Z.; Zhang, D.; Han, Z. C.; Guo, Z.; Zhao, Q.; Li, Z. IGF-1C hydrogel improves the therapeutic effects of MSCs on colitis in mice through PGE(2)-mediated M2 macrophage polarization. Theranostics. 2020, 10, 7697-7709.
doi: 10.7150/thno.45434 URL |
| 20. |
Soleimani, M.; Nadri, S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc. 2009, 4, 102-106.
doi: 10.1038/nprot.2008.221 |
| 21. |
Houlihan, D. D.; Mabuchi, Y.; Morikawa, S.; Niibe, K.; Araki, D.; Suzuki, S.; Okano, H.; Matsuzaki, Y. Isolation of mouse mesenchymal stem cells on the basis of expression of Sca-1 and PDGFR-α. Nat Protoc. 2012, 7, 2103-2111.
doi: 10.1038/nprot.2012.125 |
| 22. |
Lin, W.; Xu, L.; Lin, S.; Shi, L.; Wang, B.; Pan, Q.; Lee, W. Y. W.; Li, G. Characterisation of multipotent stem cells from human peripheral blood using an improved protocol. J Orthop Translat. 2019, 19, 18-28.
doi: 10.1016/j.jot.2019.02.003 URL |
| 23. |
Matsuda, K.; Falkenberg, K. J.; Woods, A. A.; Choi, Y. S.; Morrison, W. A.; Dilley, R. J. Adipose-derived stem cells promote angiogenesis and tissue formation for in vivo tissue engineering. Tissue Eng Part A. 2013, 19, 1327-1335.
doi: 10.1089/ten.tea.2012.0391 URL |
| 24. |
Lin, W.; Xu, L.; Li, G. A novel protocol for isolation and culture of multipotent progenitor cells from human urine. J Orthop Translat. 2019, 19, 12-17.
doi: 10.1016/j.jot.2019.02.005 URL |
| 25. | Xu, Y.; Zhang, T.; Chen, Y.; Shi, Q.; Li, M.; Qin, T.; Hu, J.; Lu, H.; Liu, J.; Chen, C. Isolation and characterization of multipotent canine urine-derived stem cells. Stem Cells Int. 2020, 2020, 8894449. |
| 26. |
Huang, R. L.; Kobayashi, E.; Liu, K.; Li, Q. Bone graft prefabrication following the in vivo bioreactor principle. EBioMedicine. 2016, 12, 43-54.
doi: 10.1016/j.ebiom.2016.09.016 URL |
| 27. |
Yin, J. Q.; Zhu, J.; Ankrum, J. A. Manufacturing of primed mesenchymal stromal cells for therapy. Nat Biomed Eng. 2019, 3, 90-104.
doi: 10.1038/s41551-018-0325-8 |
| 28. |
Mauney, J. R.; Nguyen, T.; Gillen, K.; Kirker-Head, C.; Gimble, J. M.; Kaplan, D. L. Engineering adipose-like tissue in vitro and in vivo utilizing human bone marrow and adipose-derived mesenchymal stem cells with silk fibroin 3D scaffolds. Biomaterials. 2007, 28, 5280-5290.
doi: 10.1016/j.biomaterials.2007.08.017 URL |
| 29. |
Cui, L.; Xiang, S.; Chen, D.; Fu, R.; Zhang, X.; Chen, J.; Wang, X. A novel tissue-engineered bone graft composed of silicon-substituted calcium phosphate, autogenous fine particulate bone powder and BMSCs promotes posterolateral spinal fusion in rabbits. J Orthop Translat. 2021, 26, 151-161.
doi: 10.1016/j.jot.2020.06.003 URL |
| 30. |
Dai, K.; Deng, S.; Yu, Y.; Zhu, F.; Wang, J.; Liu, C. Construction of developmentally inspired periosteum-like tissue for bone regeneration. Bone Res. 2022, 10, 1.
doi: 10.1038/s41413-021-00166-w |
| 31. |
Dai, K.; Shen, T.; Yu, Y.; Deng, S.; Mao, L.; Wang, J.; Liu, C. Generation of rhBMP-2-induced juvenile ossicles in aged mice. Biomaterials. 2020, 258, 120284.
doi: 10.1016/j.biomaterials.2020.120284 URL |
| 32. |
Dai, K.; Zhang, Q.; Deng, S.; Yu, Y.; Zhu, F.; Zhang, S.; Pan, Y.; Long, D.; Wang, J.; Liu, C. A BMP-2-triggered in vivo osteo-organoid for cell therapy. Sci Adv. 2023, 9, eadd1541.
doi: 10.1126/sciadv.add1541 URL |
| 33. | Dey, D.; Bagarova, J.; Hatsell, S. J.; Armstrong, K. A.; Huang, L.; Ermann, J.; Vonner, A. J.; Shen, Y.; Mohedas, A. H.; Lee, A.; Eekhoff, E. M.; van Schie, A.; Demay, M. B.; Keller, C.; Wagers, A. J.; Economides, A. N.; Yu, P. B. Two tissue-resident progenitor lineages drive distinct phenotypes of heterotopic ossification. Sci Transl Med. 2016, 8, 366ra163. |
| 34. |
Smith, E.; Yang, J.; McGann, L.; Sebald, W.; Uludag, H. RGD-grafted thermoreversible polymers to facilitate attachment of BMP-2 responsive C2C12 cells. Biomaterials. 2005, 26, 7329-7338.
doi: 10.1016/j.biomaterials.2005.05.060 URL |
| 35. |
Zhou, B. O.; Yue, R.; Murphy, M. M.; Peyer, J. G.; Morrison, S. J. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell. 2014, 15, 154-168.
doi: 10.1016/j.stem.2014.06.008 URL |
| 36. |
Leong, D. J.; Sun, H. B. Mesenchymal stem cells in tendon repair and regeneration: basic understanding and translational challenges. Ann N Y Acad Sci. 2016, 1383, 88-96.
doi: 10.1111/nyas.2016.1383.issue-1 URL |
| 37. |
Wagner, W.; Horn, P.; Castoldi, M.; Diehlmann, A.; Bork, S.; Saffrich, R.; Benes, V.; Blake, J.; Pfister, S.; Eckstein, V.; Ho, A. D. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS One. 2008, 3, e2213.
doi: 10.1371/journal.pone.0002213 URL |
| 38. |
Dimri, G. P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E. E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O.; et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995, 92, 9363-9367.
doi: 10.1073/pnas.92.20.9363 URL |
| 39. |
Julien, A.; Kanagalingam, A.; Martínez-Sarrà, E.; Megret, J.; Luka, M.; Ménager, M.; Relaix, F.; Colnot, C. Direct contribution of skeletal muscle mesenchymal progenitors to bone repair. Nat Commun. 2021, 12, 2860.
doi: 10.1038/s41467-021-22842-5 |
| 40. |
Wang, X.; Matthews, B. G.; Yu, J.; Novak, S.; Grcevic, D.; Sanjay, A.; Kalajzic, I. PDGF modulates BMP2-induced osteogenesis in periosteal progenitor cells. JBMR Plus. 2019, 3, e10127.
doi: 10.1002/jbm4.v3.5 URL |
| 41. |
Lees-Shepard, J. B.; Yamamoto, M.; Biswas, A. A.; Stoessel, S. J.; Nicholas, S. E.; Cogswell, C. A.; Devarakonda, P. M.; Schneider, M. J., Jr.; Cummins, S. M.; Legendre, N. P.; Yamamoto, S.; Kaartinen, V.; Hunter, J. W.; Goldhamer, D. J. Activin-dependent signaling in fibro/adipogenic progenitors causes fibrodysplasia ossificans progressiva. Nat Commun. 2018, 9, 471.
doi: 10.1038/s41467-018-02872-2 |
| 42. | Zhang, Q.; Liu, Y.; Li, J.; Wang, J.; Liu, C. Recapitulation of growth factor-enriched microenvironment via BMP receptor activating hydrogel. Bioact Mater. 2023, 20, 638-650. |
| 43. |
Fernández-Santos, M. E.; Garcia-Arranz, M.; Andreu, E. J.; García-Hernández, A. M.; López-Parra, M.; Villarón, E.; Sepúlveda, P.; Fernández-Avilés, F.; García-Olmo, D.; Prosper, F.; Sánchez-Guijo, F.; Moraleda, J. M.; Zapata, A. G. Optimization of mesenchymal stromal cell (MSC) manufacturing processes for a better therapeutic outcome. Front Immunol. 2022, 13, 918565.
doi: 10.3389/fimmu.2022.918565 URL |
| 44. | Saha, D.; Hofmann, N.; Mueller, T.; Niemann, H.; Glasmacher, B. C-2014: Investigation of genetic and epigenetic changes of cryopreserved mesenchymal stem cells. Cryobiology. 2014, 69, 519. |
| 45. |
Shu, Z.; Heimfeld, S.; Gao, D. Hematopoietic SCT with cryopreserved grafts: adverse reactions after transplantation and cryoprotectant removal before infusion. Bone Marrow Transplant. 2014, 49, 469-476.
doi: 10.1038/bmt.2013.152 |
| 46. | Lagonda, C. A.; Tjahjadi, F. B.; Fauza, D.; Kusnadi, Y. Hypoxia increases vegf secretion in multiple sources of mesenchymal stem cell. Cytotherapy. 2018, 20, S44-S45. |
| 47. |
Wang, X.; Li, F.; Xie, L.; Crane, J.; Zhen, G.; Mishina, Y.; Deng, R.; Gao, B.; Chen, H.; Liu, S.; Yang, P.; Gao, M.; Tu, M.; Wang, Y.; Wan, M.; Fan, C.; Cao, X. Inhibition of overactive TGF-β attenuates progression of heterotopic ossification in mice. Nat Commun. 2018, 9, 551.
doi: 10.1038/s41467-018-02988-5 |
| [1] | Zhao–Lin Zeng, Hui Xie. Mesenchymal stem cell–derived extracellular vesicles: a possible therapeutic strategy for orthopaedic diseases: a narrative review [J]. Biomaterials Translational, 2022, 3(3): 175-187. |
| [2] | Arnold I. Caplan. Mesenchymal stem cells and COVID-19: the process of discovery and of translation [J]. Biomaterials Translational, 2021, 2(4): 307-311. |
| [3] | Xing Yang, Yuanyuan Li, Xujie Liu, Wei He, Qianli Huang, Qingling Feng. Nanoparticles and their effects on differentiation of mesenchymal stem cells [J]. Biomaterials Translational, 2020, 1(1): 58-68. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||