Biomaterials Translational ›› 2020, Vol. 1 ›› Issue (1): 33-45.doi: 10.3877/cma.j.issn.2096-112X.2020.01.004
• REVIEW • Previous Articles Next Articles
Received:2020-08-31
Revised:2020-10-26
Accepted:2020-10-29
Online:2020-12-28
Published:2020-12-28
Contact:
Jie Song
E-mail:Jie.Song@umassmed.edu
Xu, X.; Song, J. Segmental long bone regeneration guided by degradable synthetic polymeric scaffolds. Biomater Transl. 2020, 1(1), 33-45.
| Graft composition | Animal | Segmental defect | Therapeutics | Regeneration outcomes | Limitations |
|---|---|---|---|---|---|
| 3D printed PCL/β-TCP composite | SheeP | 3 cm tibial | 3.5 mg rhBMP-7 | Radiographic union; mechanical restoration | Slow graft resorption |
| PLGA microparticles | SheeP | 2.5 cm femoral | 4-mg rhBMP-2 | Radiographic union (no mechanical testing) | Tendency of PLGA breakage |
| PLGA-coated gelatine sponge | Dog | 2.5 cm tibial | 0.4 mg/mL rhBMP-2 | Radiographic union; mechanical restoration | Small sample size; Bone resorption |
| Porous PLA-PEG/HAP | Rabbit | 1.5 cm radial | 5-20 μg rhBMP-2 | Radiographic union (no mechanical testing) | Slow graft resorption |
| PLA-DX-PEG/b-TCP | Rabbit | 1.5 cm femoral | 50 mg rhBMP-2 | Radiographic union; mechanical restoration; full graft resorption | Graft distortion within defect |
| 3D-printed PELGA/HAP | Rat | 5 mm femoral | 400 ng rhBMP-2/7 | Facile & stable graft fixation; rapid union, full graft resorption & mechanical restoration | Larger animal translation unknown |
| Solid PPF rod/porous sleeve with PLGA microparticle | Rat | 5 mm femoral | 2-8 μg rhBMP-2 | Improved defect fixation by solid rod; improved bone formation | Regeneration impeded by solid rod; no union |
| Crosslinked PPF/PPF diacrylate with PLGA microparticle | Rabbit | 1.5 cm radial | 200 μg TP508 | Improved osteointegration | No union; slow graft resorption |
| Salicylic acid-based poly(anhydride-ester)/PCL membrane | Rat | 5 mm femoral | 12 μg rhBMP-2 | Ectopic bone formation suppressed; long bone regeneration improved (no mechanical testing) | Poor graft mechanical property; long-term remodelling unclear |
| Tyrosine-derived polycarbonate/CP | Rabbit | 1.5 cm radial | 17-35 μg rhBMP-2 | Improved bone formation (no mechanical testing) | No union |
| pHEMA-HAp composite | Rat | 5 mm femoral | 400 ng rhBMP-2/7 | Radiographic union; mechanical restoration | Slow graft resorption |
| MMP-sensitive 4-arm PEG hydrogel with integrin binding GFOGER | Murine | 2.5 mm radial | 30 ng rhBMP-2 | Radiographic union; mechanical restoration; MMP-responsive degradation | Potentially high manufacturing cost |
Table 1 Degradable synthetic polymeric scaffolds for long bone segmental defects
| Graft composition | Animal | Segmental defect | Therapeutics | Regeneration outcomes | Limitations |
|---|---|---|---|---|---|
| 3D printed PCL/β-TCP composite | SheeP | 3 cm tibial | 3.5 mg rhBMP-7 | Radiographic union; mechanical restoration | Slow graft resorption |
| PLGA microparticles | SheeP | 2.5 cm femoral | 4-mg rhBMP-2 | Radiographic union (no mechanical testing) | Tendency of PLGA breakage |
| PLGA-coated gelatine sponge | Dog | 2.5 cm tibial | 0.4 mg/mL rhBMP-2 | Radiographic union; mechanical restoration | Small sample size; Bone resorption |
| Porous PLA-PEG/HAP | Rabbit | 1.5 cm radial | 5-20 μg rhBMP-2 | Radiographic union (no mechanical testing) | Slow graft resorption |
| PLA-DX-PEG/b-TCP | Rabbit | 1.5 cm femoral | 50 mg rhBMP-2 | Radiographic union; mechanical restoration; full graft resorption | Graft distortion within defect |
| 3D-printed PELGA/HAP | Rat | 5 mm femoral | 400 ng rhBMP-2/7 | Facile & stable graft fixation; rapid union, full graft resorption & mechanical restoration | Larger animal translation unknown |
| Solid PPF rod/porous sleeve with PLGA microparticle | Rat | 5 mm femoral | 2-8 μg rhBMP-2 | Improved defect fixation by solid rod; improved bone formation | Regeneration impeded by solid rod; no union |
| Crosslinked PPF/PPF diacrylate with PLGA microparticle | Rabbit | 1.5 cm radial | 200 μg TP508 | Improved osteointegration | No union; slow graft resorption |
| Salicylic acid-based poly(anhydride-ester)/PCL membrane | Rat | 5 mm femoral | 12 μg rhBMP-2 | Ectopic bone formation suppressed; long bone regeneration improved (no mechanical testing) | Poor graft mechanical property; long-term remodelling unclear |
| Tyrosine-derived polycarbonate/CP | Rabbit | 1.5 cm radial | 17-35 μg rhBMP-2 | Improved bone formation (no mechanical testing) | No union |
| pHEMA-HAp composite | Rat | 5 mm femoral | 400 ng rhBMP-2/7 | Radiographic union; mechanical restoration | Slow graft resorption |
| MMP-sensitive 4-arm PEG hydrogel with integrin binding GFOGER | Murine | 2.5 mm radial | 30 ng rhBMP-2 | Radiographic union; mechanical restoration; MMP-responsive degradation | Potentially high manufacturing cost |
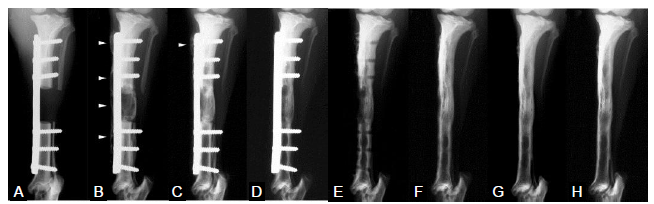
Figure 1. Radiographs of a defect treated with polymer-coated gelatin sponge impregnated with recombinant bone morphogenetic protein 2 (0.4 mg/cm3) (anteroposterior view). (A-H) Radiographs were taken at 0 (A), 4 (B), 8 (C), 16 (D and E; before and after plate removal, respectively), 32 (F), 52 (G) and 104 (H) weeks postoperatively. Arrowheads in B and C indicate the hypertrophic bone beyond the metal plate. Reproduced from Kokubo et al.39 with permission from Elsevier.
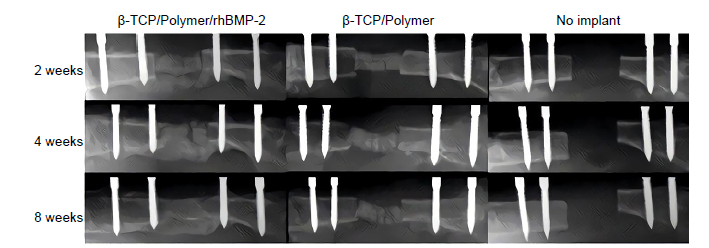
Figure 2. Representative femoral radiographs. From left, implanted with β-TCP with PLA-DX-PEG and rhBMP-2, β-TCP with PLA-DX-PEG without rhBMP-2, and critical size bone defect without implantation (sham surgery). Sequential radiographs show bone repair at 2, 4, and 8 weeks after implantation in the experimental group. Reproduced from Yoneda et al.47 with permission from Elsevier. DX: p-dioxanone; PEG: poly(ethylene glycol); PLA: poly(lactic acid); rhBMP-2: recombinant bone morphogenetic protein 2; β-TCP: β-tricalcium phosphate.
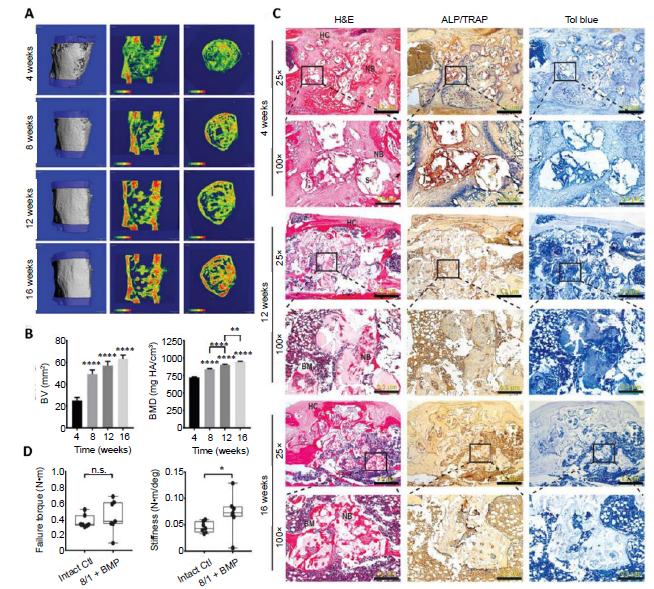
Figure 3. Accelerated healing of 5-mm rat femoral segmental defects by 25% HAp-PELGA(8/1) grafts preabsorbed with 400-ng rhBMP-2/7. (A) 3D μCT images and BMD colour maps (centre sagittal and axial slices) of the ROI showing maturing regenerated bone within the defect over time. Global thresholding was applied to exclude bone densities below 518.2 mg HAp/cm3 (HAp-PELGA graft invisible at this threshold). (B) Longitudinal μCT quantification of BV and BMD (n ≥ 12) within the ROI over time. Data are presented as means ± SEM. **P < 0.01, ****P < 0.0001 (one-way analysis of variance with Tukey’s post-hoc test). The global lower threshold of 518.2 mg HAp/cm3 was applied for all quantifications. (C) Histological micrographs of H&E-, ALP/TRAP-, and Tol blue-stained sections of explanted graft-filled femurs over time. Scale bars: 1.2 mm (25× magnification) and 300 μm (100× magnification). Boxed regions shown at higher magnification in bottom rows. (D) Boxplots of failure torque and stiffness of intact (control) versus regenerated femur (8/1 + BMP) 16 weeks after being treated with HAp-PELGA(8/1) grafts preloaded with 400-ng rhBMP-2/7 (n = 7). *P < 0.05 (Wilcoxon-Mann-Whitney rank sum test). Reprinted from Zhang et al.54 with permission from AAAS. 3D: three-dimensional; ALP: alkaline phosphatase; BM: bone marrow; BMD: bone mineral density; BMP: bone morphogenetic protein; BV: bone volume; Ctl: control; H&E: haematoxylin and eosin; HAp: hydroxyapatite; HC: healing callus; n.s.: P > 0.05; NB: new bone; PELGA: poly(lactic-co-glycolic acid)-b-poly(ethylene glycol)-b-poly(lactic-co-glycolic acid); rhBMP: human recombinant bone morphogenetic protein; ROI: region of interest; S: scaffold; TRAP: tartrate-resistant acid phosphatase; μCT: micro-computed tomography.
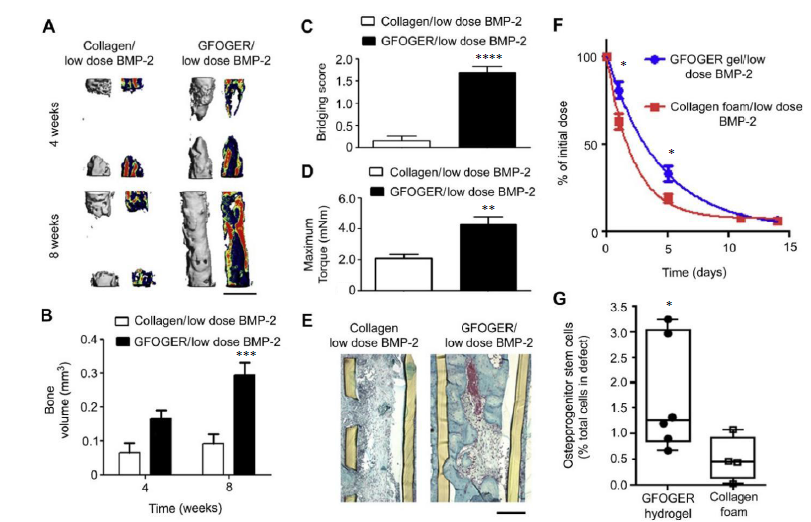
Figure 4. BMP-2 delivery from GFOGER-functionalized gels improves bone regeneration compared to collagen foams. (A) 3D μCT reconstructions of radii (left) and mineral density sagittal sections (right). Scale bar: 1 mm. (B) μCT measures of bone volume in radial defects. (C) Bridging score at 8 weeks post-implantation (n = 13). (D) Maximum torque values for 8 weeks radial samples subjected to torsion mechanical testing to failure (n = 5-9). (E) Sections of 8 weeks radial samples stained with Safranin-O/Fast Green. Scale bar: 200 μm. (F) Retention of infrared dye-labelled BMP-2 at implanted defect sites in vivo (n = 6). (G) Quantification of CD45-/CD90+ osteoprogenitor cells present in the defects 7 days post-implantation (n = 4-6). *P < 0.05, ***P < 0.001, ****P < 0.0001, vs. collagen foam/low dose BMP-2. Reproduced from Shekaran et al.126 with permission from Elsevier. 3D: three-dimensional; BMP-2: bone morphogenetic protein 2; GFOGER: α2β1 integrin-specific hexapeptide sequence Gly-Phe-Hyp-Gly-Glu-Ar; μCT: micro-computed tomography.
| 1. |
Bauer, T. W.; Muschler, G. F. Bone graft materials. An overview of the basic science. Clin Orthop Relat Res. 2000, 10-27.
doi: 10.1097/00003086-199611000-00003 URL pmid: 8913140 |
| 2. |
Beekley, A. C.; Starnes, B. W.; Sebesta, J. A. Lessons learned from modern military surgery. Surg Clin North Am. 2007, 87, 157-184, vii.
doi: 10.1016/j.suc.2006.09.008 URL pmid: 17127127 |
| 3. |
Brown, K. V.; Guthrie, H. C.; Ramasamy, A.; Kendrew, J. M.; Clasper, J. Modern military surgery: lessons from Iraq and Afghanistan. J Bone Joint Surg Br. 2012,94, 536-543.
doi: 10.1302/0301-620X.94B4.28602 URL pmid: 22434472 |
| 4. |
DeCoster, T. A.; Gehlert, R. J.; Mikola, E. A.; Pirela-Cruz, M. A. Management of posttraumatic segmental bone defects. J Am Acad Orthop Surg. 2004,12, 28-38.
doi: 10.5435/00124635-200401000-00005 URL pmid: 14753795 |
| 5. |
Mauffrey, C.; Barlow, B. T.; Smith, W. Management of segmental bone defects. J Am Acad Orthop Surg. 2015,23, 143-153.
doi: 10.5435/JAAOS-D-14-00018 URL pmid: 25716002 |
| 6. |
Winkler, T.; Sass, F. A.; Duda, G. N.; Schmidt-Bleek, K. A review of biomaterials in bone defect healing, remaining shortcomings and future opportunities for bone tissue engineering: The unsolved challenge. Bone Joint Res. 2018,7, 232-243.
doi: 10.1302/2046-3758.73.BJR-2017-0270.R1 URL pmid: 29922441 |
| 7. |
Lauthe, O.; Soubeyrand, M.; Babinet, A.; Dumaine, V.; Anract, P.; Biau, D. J. The indications and donor-site morbidity of tibial cortical strut autografts in the management of defects in long bones. Bone Joint J. 2018, 100-B, 667-674.
doi: 10.1302/0301-620X.100B5.BJJ-2017-0577.R2 URL pmid: 29701102 |
| 8. |
Hopp, S. G.; Dahners, L. E.; Gilbert, J. A. A study of the mechanical strength of long bone defects treated with various bone autograft substitutes: an experimental investigation in the rabbit. J Orthop Res. 1989,7, 579-584.
doi: 10.1002/jor.1100070416 URL pmid: 2544712 |
| 9. |
Chiarello, E.; Cadossi, M.; Tedesco, G.; Capra, P.; Calamelli, C.; Shehu, A.; Giannini, S. Autograft, allograft and bone substitutes in reconstructive orthopedic surgery. Aging Clin Exp Res. 2013,25 Suppl 1, S101-103.
doi: 10.1007/s40520-013-0088-8 URL pmid: 24046051 |
| 10. |
Bostrom, M. P.; Seigerman, D. A. The clinical use of allografts, demineralized bone matrices, synthetic bone graft substitutes and osteoinductive growth factors: a survey study. HSS J. 2005,1, 9-18.
doi: 10.1007/s11420-005-0111-5 URL pmid: 18751803 |
| 11. |
Betz, R. R. Limitations of autograft and allograft: new synthetic solutions. Orthopedics. 2002,25, s561-570.
URL pmid: 12038843 |
| 12. |
Sorger, J. I.; Hornicek, F. J.; Zavatta, M.; Menzner, J. P.; Gebhardt, M. C.; Tomford, W. W.; Mankin, H. J. Allograft fractures revisited. Clin Orthop Relat Res. 2001, 66-74.
URL pmid: 9678034 |
| 13. |
Finkemeier, C. G. Bone-grafting and bone-graft substitutes. J Bone Joint Surg Am. 2002,84, 454-464.
doi: 10.2106/00004623-200203000-00020 URL pmid: 11886919 |
| 14. |
Boyce, T.; Edwards, J.; Scarborough, N. Allograft bone. The influence of processing on safety and performance. Orthop Clin North Am. 1999,30, 571-581.
doi: 10.1016/s0030-5898(05)70110-3 URL pmid: 10471762 |
| 15. |
Kuttappan, S.; Mathew, D.; Nair, M. B. Biomimetic composite scaffolds containing bioceramics and collagen/gelatin for bone tissue engineering - A mini review. Int J Biol Macromol. 2016,93, 1390-1401.
URL pmid: 27316767 |
| 16. |
Cobos, J. A.; Lindsey, R. W.; Gugala, Z. The cylindrical titanium mesh cage for treatment of a long bone segmental defect: description of a new technique and report of two cases. J Orthop Trauma. 2000,14, 54-59.
doi: 10.1097/00005131-200001000-00011 URL pmid: 10630804 |
| 17. |
Attias, N.; Lindsey, R. W. Case reports: management of large segmental tibial defects using a cylindrical mesh cage. Clin Orthop Relat Res. 2006,450, 259-266.
doi: 10.1097/01.blo.0000223982.29208.a4 URL pmid: 16702918 |
| 18. |
Marcacci, M.; Kon, E.; Moukhachev, V.; Lavroukov, A.; Kutepov, S.; Quarto, R.; Mastrogiacomo, M.; Cancedda, R. Stem cells associated with macroporous bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue Eng. 2007,13, 947-955.
doi: 10.1089/ten.2006.0271 URL pmid: 17484701 |
| 19. |
Kasten, P.; Vogel, J.; Geiger, F.; Niemeyer, P.; Luginbühl, R.; Szalay, K. The effect of platelet-rich plasma on healing in critical-size long-bone defects. Biomaterials. 2008,29, 3983-3992.
doi: 10.1016/j.biomaterials.2008.06.014 URL pmid: 18614227 |
| 20. |
Geiger, M.; Li, R. H.; Friess, W. Collagen sponges for bone regeneration with rhBMP-2. Adv Drug Deliv Rev. 2003,55, 1613-1629.
doi: 10.1016/j.addr.2003.08.010 URL pmid: 14623404 |
| 21. |
Raina, D. B.; Qayoom, I.; Larsson, D.; Zheng, M. H.; Kumar, A.; Isaksson, H.; Lidgren, L.; Tägil, M. Guided tissue engineering for healing of cancellous and cortical bone using a combination of biomaterial based scaffolding and local bone active molecule delivery. Biomaterials. 2019,188, 38-49.
doi: 10.1016/j.biomaterials.2018.10.004 URL pmid: 30321863 |
| 22. |
Lissenberg-Thunnissen, S. N.; de Gorter, D. J.; Sier, C. F.; Schipper, I. B. Use and efficacy of bone morphogenetic proteins in fracture healing. Int OrthoP. 2011,35, 1271-1280.
URL pmid: 21698428 |
| 23. |
Zara, J. N.; Siu, R. K.; Zhang, X.; Shen, J.; Ngo, R.; Lee, M.; Li, W.; Chiang, M.; Chung, J.; Kwak, J.; Wu, B. M.; Ting, K.; Soo, C. High doses of bone morphogenetic protein 2 induce structurally abnormal bone and inflammation in vivo. Tissue Eng Part A. 2011,17, 1389-1399.
doi: 10.1089/ten.TEA.2010.0555 URL pmid: 21247344 |
| 24. |
Tazaki, J.; Murata, M.; Akazawa, T.; Yamamoto, M.; Ito, K.; Arisue, M.; Shibata, T.; Tabata, Y. BMP-2 release and dose-response studies in hydroxyapatite and beta-tricalcium phosphate. Biomed Mater Eng. 2009,19, 141-146.
doi: 10.3233/BME-2009-0573 URL pmid: 19581707 |
| 25. |
Mines, D.; Gu, Y.; Kou, T. D.; Cooper, G. S. Recombinant human bone morphogenetic protein-2 and pancreatic cancer: a retrospective cohort study. Pharmacoepidemiol Drug Saf. 2011,20, 111-118.
doi: 10.1002/pds.2057 URL pmid: 21254281 |
| 26. |
Lee, J.; Byun, H.; Madhurakkat Perikamana, S. K.; Lee, S.; Shin, H. Current advances in immunomodulatory biomaterials for bone regeneration. Adv Healthc Mater. 2019,8, e1801106.
doi: 10.1002/adhm.201801106 URL pmid: 30328293 |
| 27. |
Chocholata, P.; Kulda, V.; Babuska, V. Fabrication of scaffolds for bone-tissue regeneration. Materials (Basel). 2019,12, 568.
doi: 10.3390/ma12040568 URL |
| 28. |
Zhang, B.; Song, J. 3D-printed biomaterials for guided tissue regeneration. Small Methods. 2018,2, 1700306.
doi: 10.1002/smtd.v2.9 URL |
| 29. |
Jang, J. H.; Castano, O.; Kim, H. W. Electrospun materials as potential platforms for bone tissue engineering. Adv Drug Deliv Rev. 2009,61, 1065-1083.
doi: 10.1016/j.addr.2009.07.008 URL pmid: 19646493 |
| 30. |
Moutos, F. T.; Freed, L. E.; Guilak, F. A biomimetic three-dimensional woven composite scaffold for functional tissue engineering of cartilage. Nat Mater. 2007,6, 162-167.
URL pmid: 17237789 |
| 31. |
Moutos, F. T.; Glass, K. A.; Compton, S. A.; Ross, A. K.; Gersbach, C. A.; Guilak, F.; Estes, B. T. Anatomically shaped tissue-engineered cartilage with tunable and inducible anticytokine delivery for biological joint resurfacing. Proc Natl Acad Sci USA. 2016,113, E4513-4522.
doi: 10.1073/pnas.1601639113 URL pmid: 27432980 |
| 32. |
Kanczler, J. M.; Oreffo, R. O. Osteogenesis and angiogenesis: the potential for engineering bone. Eur Cell Mater. 2008,15, 100-114.
doi: 10.22203/ecm.v015a08 URL pmid: 18454418 |
| 33. | Smith, R. Biodegradable Polymers for Industrial Applications. Woodhead Publishing: 2005. |
| 34. | Cortizo, M. S.; Belluzo, M. S. Biodegradable polymers for bone tissue engineering. In Industrial applications of renewable biomass products: past, present and future, Goyanes, S. N.; D’Accorso, N. B., eds.; Springer International Publishing: Cham, 2017; pp 47-74. |
| 35. |
Webb, A. R.; Yang, J.; Ameer, G. A. Biodegradable polyester elastomers in tissue engineering. Expert Opin Biol Ther. 2004,4, 801-812.
doi: 10.1517/14712598.4.6.801 URL pmid: 15174963 |
| 36. |
Narayanan, G.; Vernekar, V. N.; Kuyinu, E. L.; Laurencin, C. T. Poly (lactic acid)-based biomaterials for orthopaedic regenerative engineering. Adv Drug Deliv Rev. 2016,107, 247-276.
doi: 10.1016/j.addr.2016.04.015 URL pmid: 27125191 |
| 37. |
Temenoff, J. S.; Mikos, A. G. Injectable biodegradable materials for orthopedic tissue engineering. Biomaterials. 2000,21, 2405-2412.
doi: 10.1016/s0142-9612(00)00108-3 URL pmid: 11055288 |
| 38. |
Kirker-Head, C. A.; Gerhart, T. N.; Armstrong, R.; Schelling, S. H.; Carmel, L. A. Healing bone using recombinant human bone morphogenetic protein 2 and copolymer. Clin Orthop Relat Res. 1998, 205-217.
URL pmid: 10627737 |
| 39. |
Kokubo, S.; Mochizuki, M.; Fukushima, S.; Ito, T.; Nozaki, K.; Iwai, T.; Takahashi, K.; Yokota, S.; Miyata, K.; Sasaki, N. Long-term stability of bone tissues induced by an osteoinductive biomaterial, recombinant human bone morphogenetic protein-2 and a biodegradable carrier. Biomaterials. 2004,25, 1795-1803.
doi: 10.1016/j.biomaterials.2003.08.030 URL pmid: 14738843 |
| 40. |
Nancollas, G. H.; Henneman, Z. J. Calcium oxalate: calcium phosphate transformations. Urol Res. 2010,38, 277-280.
doi: 10.1007/s00240-010-0292-3 URL pmid: 20625892 |
| 41. |
Chung, W. J.; Kwon, K. Y.; Song, J.; Lee, S. W. Evolutionary screening of collagen-like peptides that nucleate hydroxyapatite crystals. Langmuir. 2011,27, 7620-7628.
doi: 10.1021/la104757g URL pmid: 21291244 |
| 42. |
Godavitarne, C.; Robertson, A.; Peters, J.; Rogers, B. Biodegradable materials. Orthop Trauma. 2017,31, 316-320.
doi: 10.1016/j.mporth.2017.07.011 URL |
| 43. |
Cancedda, R.; Giannoni, P.; Mastrogiacomo, M. A tissue engineering approach to bone repair in large animal models and in clinical practice. Biomaterials. 2007,28, 4240-4250.
doi: 10.1016/j.biomaterials.2007.06.023 URL pmid: 17644173 |
| 44. | Reichert, J. C.; Cipitria, A.; Epari, D. R.; Saifzadeh, S.; Krishnakanth, P.; Berner, A.; Woodruff, M. A.; Schell, H.; Mehta, M.; Schuetz, M. A.; Duda, G. N.; Hutmacher, D. W. A tissue engineering solution for segmental defect regeneration in load-bearing long bones. Sci Transl Med. 2012,4, 141ra193. |
| 45. |
Jakus, A. E.; Rutz, A. L.; Jordan, S. W.; Kannan, A.; Mitchell, S. M.; Yun, C.; Koube, K. D.; Yoo, S. C.; Whiteley, H. E.; Richter, C. P.; Galiano, R. D.; Hsu, W. K.; Stock, S. R.; Hsu, E. L.; Shah, R. N. Hyperelastic “bone”: A highly versatile, growth factor-free, osteoregenerative, scalable, and surgically friendly biomaterial. Sci Transl Med. 2016,8, 358ra127.
doi: 10.1126/scitranslmed.aaf7704 URL pmid: 27683552 |
| 46. |
Kaito, T.; Myoui, A.; Takaoka, K.; Saito, N.; Nishikawa, M.; Tamai, N.; Ohgushi, H.; Yoshikawa, H. Potentiation of the activity of bone morphogenetic protein-2 in bone regeneration by a PLA-PEG/hydroxyapatite composite. Biomaterials. 2005,26, 73-79.
doi: 10.1016/j.biomaterials.2004.02.010 URL pmid: 15193882 |
| 47. |
Yoneda, M.; Terai, H.; Imai, Y.; Okada, T.; Nozaki, K.; Inoue, H.; Miyamoto, S.; Takaoka, K. Repair of an intercalated long bone defect with a synthetic biodegradable bone-inducing implant. Biomaterials. 2005,26, 5145-5152.
doi: 10.1016/j.biomaterials.2005.01.054 URL pmid: 15792541 |
| 48. |
Zhang, B.; Filion, T. M.; Kutikov, A. B.; Song, J. Facile stem cell delivery to bone grafts enabled by smart shape recovery and stiffening of degradable synthetic periosteal membranes. Adv Funct Mater. 2017,27, 1604784.
doi: 10.1002/adfm.v27.5 URL |
| 49. |
Zhang, B.; DeBartolo, J. E.; Song, J. Shape recovery with concomitant mechanical strengthening of amphiphilic shape memory polymers in warm water. ACS Appl Mater Interfaces. 2017,9, 4450-4456.
doi: 10.1021/acsami.6b14167 URL pmid: 28125208 |
| 50. |
Kutikov, A. B.; Gurijala, A.; Song, J. Rapid prototyping amphiphilic polymer/hydroxyapatite composite scaffolds with hydration-induced self-fixation behavior. Tissue Eng Part C Methods. 2015,21, 229-241.
doi: 10.1089/ten.TEC.2014.0213 URL pmid: 25025950 |
| 51. |
Kutikov, A. B.; Song, J. An amphiphilic degradable polymer/hydroxyapatite composite with enhanced handling characteristics promotes osteogenic gene expression in bone marrow stromal cells. Acta Biomater. 2013,9, 8354-8364.
doi: 10.1016/j.actbio.2013.06.013 URL pmid: 23791675 |
| 52. |
Kutikov, A. B.; Reyer, K. A.; Song, J. Shape memory performance of thermoplastic amphiphilic triblock copolymer poly(D,L-lactic acid-co-ethylene glycol-co-D,L-lactic acid) (PELA)/hydroxyapatite composites. Macromol Chem Phys. 2014,215, 2482-2490.
doi: 10.1002/macp.201400340 URL pmid: 26457046 |
| 53. |
Kutikov, A. B.; Skelly, J. D.; Ayers, D. C.; Song, J. Templated repair of long bone defects in rats with bioactive spiral-wrapped electrospun amphiphilic polymer/hydroxyapatite scaffolds. ACS Appl Mater Interfaces. 2015,7, 4890-4901.
URL pmid: 25695310 |
| 54. |
Zhang, B.; Skelly, J. D.; Maalouf, J. R.; Ayers, D. C.; Song, J. Multifunctional scaffolds for facile implantation, spontaneous fixation, and accelerated long bone regeneration in rodents. Sci Transl Med. 2019,11, eaau7411.
doi: 10.1126/scitranslmed.aau7411 URL pmid: 31341064 |
| 55. |
Peter, S. J.; Yaszemski, M. J.; Suggs, L. J.; Payne, R. G.; Langer, R.; Hayes, W. C.; Unroe, M. R.; Alemany, L. B.; Engel, P. S.; Mikos, A. G. Characterization of partially saturated poly(propylene fumarate) for orthopaedic application. J Biomater Sci Polym Ed. 1997,8, 893-904.
URL pmid: 9342654 |
| 56. |
Temenoff, J. S.; Mikos, A. G. Review: tissue engineering for regeneration of articular cartilage. Biomaterials. 2000,21, 431-440.
doi: 10.1016/s0142-9612(99)00213-6 URL pmid: 10674807 |
| 57. |
Domb, A. J.; Manor, N.; Elmalak, O. Biodegradable bone cement compositions based on acrylate and epoxide terminated poly(propylene fumarate) oligomers and calcium salt compositions. Biomaterials. 1996,17, 411-417.
doi: 10.1016/0142-9612(96)89657-8 URL pmid: 8938235 |
| 58. |
Peter, S. J.; Kim, P.; Yasko, A. W.; Yaszemski, M. J.; Mikos, A. G. Crosslinking characteristics of an injectable poly(propylene fumarate)/beta-tricalcium phosphate paste and mechanical properties of the crosslinked composite for use as a biodegradable bone cement. J Biomed Mater Res. 1999,44, 314-321.
URL pmid: 10397934 |
| 59. |
Lee, K. W.; Wang, S.; Lu, L.; Jabbari, E.; Currier, B. L.; Yaszemski, M. J. Fabrication and characterization of poly(propylene fumarate) scaffolds with controlled pore structures using 3-dimensional printing and injection molding. Tissue Eng. 2006,12, 2801-2811.
doi: 10.1089/ten.2006.12.2801 URL pmid: 17518649 |
| 60. |
Henslee, A. M.; Spicer, P. P.; Yoon, D. M.; Nair, M. B.; Meretoja, V. V.; Witherel, K. E.; Jansen, J. A.; Mikos, A. G.; Kasper, F. K. Biodegradable composite scaffolds incorporating an intramedullary rod and delivering bone morphogenetic protein-2 for stabilization and bone regeneration in segmental long bone defects. Acta Biomater. 2011,7, 3627-3637.
doi: 10.1016/j.actbio.2011.06.043 URL pmid: 21757034 |
| 61. |
Hedberg, E. L.; Kroese-Deutman, H. C.; Shih, C. K.; Crowther, R. S.; Carney, D. H.; Mikos, A. G.; Jansen, J. A. Effect of varied release kinetics of the osteogenic thrombin peptide TP508 from biodegradable, polymeric scaffolds on bone formation in vivo. J Biomed Mater Res A. 2005,72, 343-353.
doi: 10.1002/jbm.a.30265 URL pmid: 15666357 |
| 62. |
Kumar, N.; Langer, R. S.; Domb, A. J. Polyanhydrides: an overview. Adv Drug Deliv Rev. 2002,54, 889-910.
doi: 10.1016/s0169-409x(02)00050-9 URL pmid: 12384314 |
| 63. |
Basu, A.; Domb, A. J. Recent advances in polyanhydride based biomaterials. Adv Mater. 2018,30, e1706815.
doi: 10.1002/adma.201706815 URL pmid: 29707879 |
| 64. |
Katti, D. S.; Lakshmi, S.; Langer, R.; Laurencin, C. T. Toxicity, biodegradation and elimination of polyanhydrides. Adv Drug Deliv Rev. 2002,54, 933-961.
doi: 10.1016/s0169-409x(02)00052-2 URL pmid: 12384316 |
| 65. |
Subramanian, S.; Mitchell, A.; Yu, W.; Snyder, S.; Uhrich, K.; O’Connor, J. P. Salicylic acid-based polymers for guided bone regeneration using bone morphogenetic protein-2. Tissue Eng Part A. 2015,21, 2013-2024.
URL pmid: 25813520 |
| 66. |
Giannoudis, P. V.; MacDonald, D. A.; Matthews, S. J.; Smith, R. M.; Furlong, A. J.; De Boer, P. Nonunion of the femoral diaphysis. The influence of reaming and non-steroidal anti-inflammatory drugs. J Bone Joint Surg Br. 2000,82, 655-658.
doi: 10.1302/0301-620x.82b5.9899 URL pmid: 10963160 |
| 67. |
Goodman, S.; Ma, T.; Trindade, M.; Ikenoue, T.; Matsuura, I.; Wong, N.; Fox, N.; Genovese, M.; Regula, D.; Smith, R. L. COX-2 selective NSAID decreases bone ingrowth in vivo. J Orthop Res. 2002,20, 1164-1169.
doi: 10.1016/S0736-0266(02)00079-7 URL pmid: 12472224 |
| 68. |
Simon, A. M.; O’Connor, J. P. Dose and time-dependent effects of cyclooxygenase-2 inhibition on fracture-healing. J Bone Joint Surg Am. 2007,89, 500-511.
doi: 10.2106/JBJS.F.00127 URL pmid: 17332098 |
| 69. |
Hauenstein, O.; Agarwal, S.; Greiner, A. Bio-based polycarbonate as synthetic toolbox. Nat Commun. 2016,7, 11862.
doi: 10.1038/ncomms11862 URL pmid: 27302694 |
| 70. | Legrand, D. G.; Bendler, J. T. Handbook of polycarbonate science and technology. CRC Press: Boca Raton, 2000. |
| 71. |
Calafat, A. M.; Weuve, J.; Ye, X.; Jia, L. T.; Hu, H.; Ringer, S.; Huttner, K.; Hauser, R. Exposure to bisphenol A and other phenols in neonatal intensive care unit premature infants. Environ Health Perspect. 2009,117, 639-644.
doi: 10.1289/ehp.0800265 URL pmid: 19440505 |
| 72. |
Kim, J. G. Chemical recycling of poly(bisphenol A carbonate). Polym Chem. 2020,11, 4830-4849.
doi: 10.1039/C9PY01927H URL |
| 73. |
Pêgo, A. P.; Poot, A. A.; Grijpma, D. W.; Feijen, J. Copolymers of trimethylene carbonate and epsilon-caprolactone for porous nerve guides: synjournal and properties. J Biomater Sci Polym Ed. 2001,12, 35-53.
doi: 10.1163/156856201744434 URL pmid: 11334188 |
| 74. |
Pêgo, A. P.; Poot, A. A.; Grijpma, D. W.; Feijen, J. In vitro degradation of trimethylene carbonate based (co)polymers. Macromol Biosci. 2002,2, 411-419.
doi: 10.1002/mabi.200290000 URL |
| 75. |
Rokicki, G. Aliphatic cyclic carbonates and spiroorthocarbonates as monomers. Prog Polym Sci. 2000,25, 259-342.
doi: 10.1016/S0079-6700(00)00006-X URL |
| 76. |
Bat, E.; Kothman, B. H.; Higuera, G. A.; van Blitterswijk, C. A.; Feijen, J.; Grijpma, D. W. Ultraviolet light crosslinking of poly(trimethylene carbonate) for elastomeric tissue engineering scaffolds. Biomaterials. 2010,31, 8696-8705.
doi: 10.1016/j.biomaterials.2010.07.102 URL pmid: 20739060 |
| 77. | Dankers, P. Y. W.; Zhang, Z.; Wisse, E.; Grijpma, D. W.; Sijbesma, R. P.; Feijen, J. Oligo(trimethylene carbonate)-based supramolecular biomaterials. MacromoleculesMacromolecules. 2006,39, 8763-8771. |
| 78. |
Zhu, K. J.; Hendren, R. W.; Jensen, K.; Pitt, C. G. Synthesis, properties, and biodegradation of poly(1,3-trimethylene carbonate). Macromolecules. 1991,24, 1736-1740.
doi: 10.1021/ma00008a008 URL |
| 79. |
Feng, J.; Zhuo, R.-X.; Zhang, X.-Z. Construction of functional aliphatic polycarbonates for biomedical applications. Prog Polym Sci. 2012,37, 211-236.
doi: 10.1016/j.progpolymsci.2011.07.008 URL |
| 80. |
Xia, Y.; Yao, J.; Shao, C. H.; Shen, X. Y.; Xie, L. Z.; Chen, G.; Peng, S. S.; Zhang, F. M.; Gu, N. Biodegradable poly(butylene-carbonate) porous membranes for guided bone regeneration: In vitro and in vivo studies. J Bioact Compatible Polym. 2013,28, 621-636.
doi: 10.1177/0883911513509471 URL |
| 81. |
van Leeuwen, A. C.; Yuan, H.; Passanisi, G.; van der Meer, J. W.; de Bruijn, J. D.; van Kooten, T. G.; Grijpma, D. W.; Bos, R. R. Poly(trimethylene carbonate) and biphasic calcium phosphate composites for orbital floor reconstruction: a feasibility study in sheeP. Eur Cell Mater. 2014,27, 81-96; discussion 96-97.
doi: 10.22203/ecm.v027a07 URL pmid: 24488822 |
| 82. |
Han, Y.; Shi, Q.; Hu, J.; Du, Q.; Chen, X.; Jing, X. Grafting BSA onto poly[(L-lactide)-co-carbonate] microspheres by click chemistry. Macromol Biosci. 2008,8, 638-644.
doi: 10.1002/mabi.200700306 URL pmid: 18401865 |
| 83. |
Xu, J.; Prifti, F.; Song, J. A versatile monomer for preparing well-defined functional polycarbonates and poly(ester-carbonates). Macromolecules. 2011,44, 2660-2667.
doi: 10.1021/ma200021m URL pmid: 21686053 |
| 84. |
Chen, W.; Yang, H.; Wang, R.; Cheng, R.; Meng, F.; Wei, W.; Zhong, Z. Versatile synthesis of functional biodegradable polymers by combining ring-opening polymerization and postpolymerization modification via michael-type addition reaction. Macromolecules. 2010,43, 201-207.
doi: 10.1021/ma901897y URL |
| 85. |
Kolb, H. C.; Finn, M. G.; Sharpless, K. B. Click chemistry: diverse chemical function from a few good reactions. Angew Chem Int Ed Engl. 2001,40, 2004-2021.
doi: 10.1002/1521-3773(20010601)40:11<2004::aid-anie2004>3.3.co;2-x URL pmid: 11433435 |
| 86. |
Agard, N. J.; Prescher, J. A.; Bertozzi, C. R. A strain-promoted [3 + 2] azide-alkyne cycloaddition for covalent modification of biomolecules in living systems. J Am Chem Soc. 2004,126, 15046-15047.
doi: 10.1021/ja044996f URL pmid: 15547999 |
| 87. |
Tangpasuthadol, V.; Pendharkar, S. M.; Peterson, R. C.; Kohn, J. Hydrolytic degradation of tyrosine-derived polycarbonates, a class of new biomaterials. Part II: 3-yr study of polymeric devices. Biomaterials. 2000,21, 2379-2387.
doi: 10.1016/s0142-9612(00)00105-8 URL pmid: 11055285 |
| 88. |
Kohn, J.; Langer, R. Polymerization reactions involving the side chains of .alpha.-L-amino acids. J Am Chem Soc. 1987,109, 817-820.
doi: 10.1021/ja00237a030 URL |
| 89. |
Pyhältö, T.; Lapinsuo, M.; Pätiälä, H.; Pelto, M.; Törmälä, P.; Rokkanen, P. Fixation of distal femoral osteotomies with self-reinforced poly(desamino tyrosyl-tyrosine ethyl ester carbonate) rods: an experimental study on rats. J Orthop Sci. 2002,7, 549-556.
doi: 10.1007/s007760200098 URL pmid: 12355129 |
| 90. | Abramson, S. D.; Seyda, A.; Sit, P. S.; Kohn, J. Characterization of degradable polymers for orthopedic application. In Polymer based systems on tissue engineering, replacement and regeneration, Reis, R. L.; Cohn, D., Eds.; Springer Netherlands: Dordrecht, 2002; pp 125-138. |
| 91. |
Hooper, K. A.; Macon, N. D.; Kohn, J. Comparative histological evaluation of new tyrosine-derived polymers and poly (L-lactic acid) as a function of polymer degradation. J Biomed Mater Res. 1998,41, 443-454.
doi: 10.1002/(sici)1097-4636(19980905)41:3<443::aid-jbm14>3.0.co;2-j URL pmid: 9659614 |
| 92. |
Choueka, J.; Charvet, J. L.; Koval, K. J.; Alexander, H.; James, K. S.; Hooper, K. A.; Kohn, J. Canine bone response to tyrosine-derived polycarbonates and poly(L-lactic acid). J Biomed Mater Res. 1996,31, 35-41.
doi: 10.1002/(SICI)1097-4636(199605)31:1<35::AID-JBM5>3.0.CO;2-R URL pmid: 8731147 |
| 93. |
Magno, M. H. R.; Kim, J.; Srinivasan, A.; McBride, S.; Bolikal, D.; Darr, A.; Hollinger, J. O.; Kohn, J. Synjournal, degradation and biocompatibility of tyrosine-derived polycarbonate scaffolds. J Mater Chem. 2010,20, 8885-8893.
doi: 10.1039/c0jm00868k URL |
| 94. |
Saxena, S.; Chang, W.; Fakhrzadeh, A.; Murthy, N. S.; Zhang, W.; Kohn, J.; Yelick, P. C. Calcium phosphate enriched synthetic tyrosine-derived polycarbonate - dicalcium phosphate dihydrate polymer scaffolds for enhanced bone regeneration. Materialia. 2020,9, 100616.
doi: 10.1016/j.mtla.2020.100616 URL pmid: 32968719 |
| 95. |
Asikainen, A. J.; Noponen, J.; Mesimäki, K.; Laitinen, O.; Peltola, J.; Pelto, M.; Kellomäki, M.; Ashammakhi, N.; Lindqvist, C.; Suuronen, R. Tyrosine derived polycarbonate membrane is useful for guided bone regeneration in rabbit mandibular defects. J Mater Sci Mater Med. 2005,16, 753-758.
doi: 10.1007/s10856-005-2613-6 URL pmid: 15965746 |
| 96. |
Asikainen, A. J.; Noponen, J.; Lindqvist, C.; Pelto, M.; Kellomäki, M.; Juuti, H.; Pihlajamäki, H.; Suuronen, R. Tyrosine-derived polycarbonate membrane in treating mandibular bone defects. An experimental study. J R Soc Interface. 2006,3, 629-635.
doi: 10.1098/rsif.2006.0119 URL pmid: 16971331 |
| 97. |
Kim, J.; Magno, M. H.; Waters, H.; Doll, B. A.; McBride, S.; Alvarez, P.; Darr, A.; Vasanji, A.; Kohn, J.; Hollinger, J. O. Bone regeneration in a rabbit critical-sized calvarial model using tyrosine-derived polycarbonate scaffolds. Tissue Eng Part A. 2012,18, 1132-1139.
doi: 10.1089/ten.TEA.2011.0582 URL pmid: 22220747 |
| 98. |
Zhang, W.; Zhang, Z.; Chen, S.; Macri, L.; Kohn, J.; Yelick, P. C. Mandibular jaw bone regeneration using human dental cell-seeded tyrosine-derived polycarbonate scaffolds. Tissue Eng Part A. 2016,22, 985-993.
doi: 10.1089/ten.TEA.2016.0166 URL pmid: 27369635 |
| 99. |
Kim, J.; McBride, S.; Donovan, A.; Darr, A.; Magno, M. H.; Hollinger, J. O. Tyrosine-derived polycarbonate scaffolds for bone regeneration in a rabbit radius critical-size defect model. Biomed Mater. 2015,10, 035001.
doi: 10.1088/1748-6041/10/3/035001 URL pmid: 25953950 |
| 100. |
Tangpasuthadol, V.; Pendharkar, S. M.; Kohn, J. Hydrolytic degradation of tyrosine-derived polycarbonates, a class of new biomaterials. Part I: study of model compounds. Biomaterials. 2000,21, 2371-2378.
doi: 10.1016/s0142-9612(00)00104-6 URL pmid: 11055284 |
| 101. |
Han, S. H.; Jung, S. H.; Lee, J. H. Preparation of beta-tricalcium phosphate microsphere-hyaluronic acid-based powder gel composite as a carrier for rhBMP-2 injection and evaluation using long bone segmental defect model. J Biomater Sci Polym Ed. 2019,30, 679-693.
doi: 10.1080/09205063.2019.1601871 URL pmid: 30939993 |
| 102. |
Kolambkar, Y. M.; Dupont, K. M.; Boerckel, J. D.; Huebsch, N.; Mooney, D. J.; Hutmacher, D. W.; Guldberg, R. E. An alginate-based hybrid system for growth factor delivery in the functional repair of large bone defects. Biomaterials. 2011,32, 65-74.
doi: 10.1016/j.biomaterials.2010.08.074 URL pmid: 20864165 |
| 103. |
Boerckel, J. D.; Kolambkar, Y. M.; Dupont, K. M.; Uhrig, B. A.; Phelps, E. A.; Stevens, H. Y.; García, A. J.; Guldberg, R. E. Effects of protein dose and delivery system on BMP-mediated bone regeneration. Biomaterials. 2011,32, 5241-5251.
doi: 10.1016/j.biomaterials.2011.03.063 URL pmid: 21507479 |
| 104. |
Krebs, M. D.; Salter, E.; Chen, E.; Sutter, K. A.; Alsberg, E. Calcium phosphate-DNA nanoparticle gene delivery from alginate hydrogels induces in vivo osteogenesis. J Biomed Mater Res A. 2010,92, 1131-1138.
doi: 10.1002/jbm.a.32441 URL pmid: 19322877 |
| 105. |
Oest, M. E.; Dupont, K. M.; Kong, H. J.; Mooney, D. J.; Guldberg, R. E. Quantitative assessment of scaffold and growth factor-mediated repair of critically sized bone defects. J Orthop Res. 2007,25, 941-950.
doi: 10.1002/jor.20372 URL pmid: 17415756 |
| 106. |
Kanczler, J. M.; Ginty, P. J.; White, L.; Clarke, N. M.; Howdle, S. M.; Shakesheff, K. M.; Oreffo, R. O. The effect of the delivery of vascular endothelial growth factor and bone morphogenic protein-2 to osteoprogenitor cell populations on bone formation. Biomaterials. 2010,31, 1242-1250.
doi: 10.1016/j.biomaterials.2009.10.059 URL pmid: 19926128 |
| 107. |
Boerckel, J. D.; Dupont, K. M.; Kolambkar, Y. M.; Lin, A. S.; Guldberg, R. E. In vivo model for evaluating the effects of mechanical stimulation on tissue-engineered bone repair. J Biomech Eng. 2009,131, 084502.
doi: 10.1115/1.3148472 URL pmid: 19604025 |
| 108. |
Priddy, L. B.; Chaudhuri, O.; Stevens, H. Y.; Krishnan, L.; Uhrig, B. A.; Willett, N. J.; Guldberg, R. E. Oxidized alginate hydrogels for bone morphogenetic protein-2 delivery in long bone defects. Acta Biomater. 2014,10, 4390-4399.
doi: 10.1016/j.actbio.2014.06.015 URL pmid: 24954001 |
| 109. |
Shuang, F.; Hou, S. X.; Zhao, Y. T.; Zhong, H. B.; Xue, C.; Zhu, J. L.; Bu, G. Y.; Cao, Z. Characterization of an injectable chitosan-demineralized bone matrix hybrid for healing critical-size long-bone defects in a rabbit model. Eur Rev Med Pharmacol Sci. 2014,18, 740-752.
URL pmid: 24668718 |
| 110. |
Kim, S.; Bedigrew, K.; Guda, T.; Maloney, W. J.; Park, S.; Wenke, J. C.; Yang, Y. P. Novel osteoinductive photo-cross-linkable chitosan-lactide-fibrinogen hydrogels enhance bone regeneration in critical size segmental bone defects. Acta Biomater. 2014,10, 5021-5033.
doi: 10.1016/j.actbio.2014.08.028 URL pmid: 25174669 |
| 111. |
Luca, L.; Rougemont, A. L.; Walpoth, B. H.; Boure, L.; Tami, A.; Anderson, J. M.; Jordan, O.; Gurny, R. Injectable rhBMP-2-loaded chitosan hydrogel composite: osteoinduction at ectopic site and in segmental long bone defect. J Biomed Mater Res A. 2011,96, 66-74.
doi: 10.1002/jbm.a.32957 URL pmid: 21105153 |
| 112. |
Song, J.; Saiz, E.; Bertozzi, C. R. A new approach to mineralization of biocompatible hydrogel scaffolds: an efficient process toward 3-dimensional bonelike composites. J Am Chem Soc. 2003,125, 1236-1243.
doi: 10.1021/ja028559h URL pmid: 12553825 |
| 113. |
Song, J.; Malathong, V.; Bertozzi, C. R. Mineralization of synthetic polymer scaffolds: a bottom-up approach for the development of artificial bone. J Am Chem Soc. 2005,127, 3366-3372.
doi: 10.1021/ja043776z URL pmid: 15755154 |
| 114. |
Song, J.; Xu, J.; Filion, T.; Saiz, E.; Tomsia, A. P.; Lian, J. B.; Stein, G. S.; Ayers, D. C.; Bertozzi, C. R. Elastomeric high-mineral content hydrogel-hydroxyapatite composites for orthopedic applications. J Biomed Mater Res A. 2009,89, 1098-1107.
doi: 10.1002/jbm.a.32110 URL pmid: 18546185 |
| 115. |
Xu, J.; Li, X.; Lian, J. B.; Ayers, D. C.; Song, J. Sustained and localized in vitro release of BMP-2/7, RANKL, and tetracycline from FlexBone, an elastomeric osteoconductive bone substitute. J Orthop Res. 2009,27, 1306-1311.
doi: 10.1002/jor.20890 URL pmid: 19350632 |
| 116. |
Filion, T. M.; Li, X.; Mason-Savas, A.; Kreider, J. M.; Goldstein, S. A.; Ayers, D. C.; Song, J. Elastomeric osteoconductive synthetic scaffolds with acquired osteoinductivity expedite the repair of critical femoral defects in rats. Tissue Eng Part A. 2011,17, 503-511.
doi: 10.1089/ten.TEA.2010.0274 URL pmid: 20818999 |
| 117. |
Sonnet, C.; Simpson, C. L.; Olabisi, R. M.; Sullivan, K.; Lazard, Z.; Gugala, Z.; Peroni, J. F.; Weh, J. M.; Davis, A. R.; West, J. L.; Olmsted-Davis, E. A. Rapid healing of femoral defects in rats with low dose sustained BMP2 expression from PEGDA hydrogel microspheres. J Orthop Res. 2013,31, 1597-1604.
doi: 10.1002/jor.22407 URL pmid: 23832813 |
| 118. |
Almany, L.; Seliktar, D. Biosynthetic hydrogel scaffolds made from fibrinogen and polyethylene glycol for 3D cell cultures. Biomaterials. 2005,26, 2467-2477.
doi: 10.1016/j.biomaterials.2004.06.047 URL pmid: 15585249 |
| 119. |
Zhang, Z.; Feng, S. S. The drug encapsulation efficiency, in vitro drug release, cellular uptake and cytotoxicity of paclitaxel-loaded poly(lactide)-tocopheryl polyethylene glycol succinate nanoparticles. Biomaterials. 2006,27, 4025-4033.
doi: 10.1016/j.biomaterials.2006.03.006 URL pmid: 16564085 |
| 120. |
Xu, J.; Filion, T. M.; Prifti, F.; Song, J. Cytocompatible poly(ethylene glycol)-co-polycarbonate hydrogels cross-linked by copper-free, strain-promoted click chemistry. Chem Asian J. 2011,6, 2730-2737.
doi: 10.1002/asia.201100411 URL pmid: 21954076 |
| 121. |
Xu, J.; Feng, E.; Song, J. Bioorthogonally cross-linked hydrogel network with precisely controlled disintegration time over a broad range. J Am Chem Soc. 2014,136, 4105-4108.
doi: 10.1021/ja4130862 URL pmid: 24597638 |
| 122. |
Hubbell, J. A. Materials as morphogenetic guides in tissue engineering. Curr Opin Biotechnol. 2003,14, 551-558.
doi: 10.1016/j.copbio.2003.09.004 URL pmid: 14580588 |
| 123. |
Culver, J. C.; Hoffmann, J. C.; Poché, R. A.; Slater, J. H.; West, J. L.; Dickinson, M. E. Three-dimensional biomimetic patterning in hydrogels to guide cellular organization. Adv Mater. 2012,24, 2344-2348.
doi: 10.1002/adma.201200395 URL pmid: 22467256 |
| 124. |
Lei, Y.; Segura, T. DNA delivery from matrix metalloproteinase degradable poly(ethylene glycol) hydrogels to mouse cloned mesenchymal stem cells. Biomaterials. 2009,30, 254-265.
doi: 10.1016/j.biomaterials.2008.09.027 URL pmid: 18838159 |
| 125. |
Sridhar, B. V.; Brock, J. L.; Silver, J. S.; Leight, J. L.; Randolph, M. A.; Anseth, K. S. Development of a cellularly degradable PEG hydrogel to promote articular cartilage extracellular matrix deposition. Adv Healthc Mater. 2015,4, 702-713.
doi: 10.1002/adhm.201400695 URL pmid: 25607633 |
| 126. |
Shekaran, A.; García, J. R.; Clark, A. Y.; Kavanaugh, T. E.; Lin, A. S.; Guldberg, R. E.; García, A. J. Bone regeneration using an alpha 2 beta 1 integrin-specific hydrogel as a BMP-2 delivery vehicle. Biomaterials. 2014,35, 5453-5461.
doi: 10.1016/j.biomaterials.2014.03.055 URL pmid: 24726536 |
| 127. |
Rosales, A. M.; Anseth, K. S. The design of reversible hydrogels to capture extracellular matrix dynamics. Nature reviews Materials. 2016,1, 15012.
doi: 10.1038/natrevmats.2015.12 URL pmid: 29214058 |
| 128. |
Chaudhuri, O.; Gu, L.; Klumpers, D.; Darnell, M.; Bencherif, S. A.; Weaver, J. C.; Huebsch, N.; Lee, H. P.; Lippens, E.; Duda, G. N.; Mooney, D. J. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat Mater. 2016,15, 326-334.
doi: 10.1038/nmat4489 URL pmid: 26618884 |
| 129. |
Tan, Y.; Huang, H.; Ayers, D. C.; Song, J. Modulating viscoelasticity, stiffness, and degradation of synthetic cellular niches via stoichiometric tuning of covalent versus dynamic noncovalent cross-linking. ACS Cent Sci. 2018,4, 971-981.
doi: 10.1021/acscentsci.8b00170 URL pmid: 30159394 |
| 130. |
Fonseca, K. B.; Granja, P. L.; Barrias, C. C. Engineering proteolytically-degradable artificial extracellular matrices. Prog Polym Sci. 2014,39, 2010-2029.
doi: 10.1016/j.progpolymsci.2014.07.003 URL |
| 131. |
Chen, Y.; Zhang, J.; Liu, X.; Wang, S.; Tao, J.; Huang, Y.; Wu, W.; Li, Y.; Zhou, K.; Wei, X.; Chen, S.; Li, X.; Xu, X.; Cardon, L.; Qian, Z.; Gou, M. Noninvasive in vivo 3D bioprinting. Sci Adv. 2020,6, eaba7406.
doi: 10.1126/sciadv.aba7406 URL pmid: 32537512 |
| 132. |
Urciuolo, A.; Poli, I.; Brandolino, L.; Raffa, P.; Scattolini, V.; Laterza, C.; Giobbe, G. G.; Zambaiti, E.; Selmin, G.; Magnussen, M.; Brigo, L.; De Coppi, P.; Salmaso, S.; Giomo, M.; Elvassore, N. Intravital three-dimensional bioprinting. Nat Biomed Eng. 2020,4, 901-915.
doi: 10.1038/s41551-020-0568-z URL pmid: 32572195 |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
