Introduction
Despite the inherent healing capacity of bone, regenerative reconstruction of critical-size long bone segmental defects (LBSDs) resulting from traumatic injuries, osteoporotic fractures among the elderly, or tumour resections remains a formidable clinical challenge due to inadequacies of existing bone grafting technologies.1-6 Gold standard cancellous autografts retrieved from the non-weight-bearing region of the patient’s own skeleton (e.g. iliac crest, ribs) are known for rapid resorptions in vivo and often result in poor unions in LBSD reconstruction.7 Although autologous cortical bone grafts (e.g. fibular segments) can achieve higher union rates and superior mechanical strength restoration in LBSD reconstruction,8 their limited availability and associated donor site morbidity present major hurdles for widespread clinical use.9 Meanwhile, devitalized allogenic long bone grafts harvested from donor cadavers, although less limited by supplies, are known for notoriously high failure rates when used for LBSD reconstruction due to their structural instability and poor vascularization.10, 11 For instance, 18% and 46% of traumatic long bone injury patients in a retrospective study experienced structural allograft failures in the first 3 years and the longer term, respectively.12 These limitations associated with autografting and allografting have driven the demands for viable synthetic bone graft alternatives.13
Current clinically-used synthetic bone graft substitutes such as bioceramics, collagen sponges/hydrogels, demineralized bone matrix paste/putty, or their coarse combinations14, 15 are known for their tendency to break and their inadequate/inconsistent in vivo performances. For LBSD repair, auxiliary metallic mesh cages are often used in addition to conventional fixations to help locally retain these mechanically-inferior formulations within the defect.16, 17 In addition, the slow resorption of calcium apatite-based ceramic grafts, known to persist for years in vivo,18 presents a hurdle to the timely restoration of the mechanical integrity of the long bone.19 Although human recombinant bone morphogenetic proteins 2 and 7 (rhBMP-2 and rhBMP-7) have been clinically used to improve bone graft osteointegration and are approved by the United States Food and Drug Administration for certain indications,20-22 the supraphysiological clinical doses (e.g. milligrams scale) required are associated with local or systemic complications ranging from ectopic bone formation to death.23-25 Overall, synthetic bone grafts combining attractive surgical handling characteristics, structural stability, suitable degradability and safe doses of biotherapeutics expediting osteointegration are still lacking for limb salvage applications.
Recent progress in understanding the cellular and molecular processes governing long bone regeneration has offered new clues for the design of next-generation synthetic bone grafts. LBSDs are known to disrupt multiple tissue compartments including the bone, bone marrow, periosteum, endosteum, vasculature and surrounding muscles and nerves. Accordingly, its regenerative healing is governed by tightly-orchestrated signalling pathways involving a large number of cells (e.g. immune cells, blood cells, mesenchymal and hematopoietic stem cells, musculoskeletal cells), starting with acute inflammation and ending with the remodelling of the regenerated bone.6 Synthetic bone grafts implanted into LBSDs directly interact with the myriad of endogenous cells recruited to the defect site, impacting their cross-talk during the dynamic competition between the processes of tissue regeneration vs. degeneration. Strategies for modulating immune responses, osteogenesis, vascularization, and bone remodelling through the manipulation of biomaterial hydrophilicity, surface charge, microroughness, porosities26 and/or temporally-controlled delivery of biotherapeutics6, 26 have been actively explored. Varied successes of these approaches, although not always generalizable across a broad spectrum of biomaterials, point to a broad range of means to augment the performance of synthetic bone grafts for LBSD reconstruction.
The past two decades have also witnessed rapid developments and popularization of a range of rapid prototyping/three-dimensional (3D) printing technologies, particularly fused deposition modelling and bioprinting, for the fabrication of biomaterial scaffolds to guide tissue regenerations.27, 28 Compared to more conventional porous scaffold fabrication techniques such as gas foaming, particulate leaching, thermally-induced phase separation, freeze drying and freeze casting,27, 28 these 3D-printing techniques have the distinct advantage of precise spatial and geometrical controls over scaffold micro/macro-porosities and, in the case of bioprinting, co-delivery of biotherapeutics/cells. Combined with electrospinning29 and 3D weaving,30, 31 these enabling tools have made it possible to recapitulate complex mesoscale structural features of skeletal tissues in biomimetic synthetic bone grafts to promote vascularization, osteointegration and effective bone remodelling.
In this review, we highlight recent synthetic bone grafts fabricated from degradable synthetic polymer/mineral composites, particularly polyesters, polyanhydrides, and polycarbonates, as well as degradable poly(ethylene glycol) (PEG)-based hydrogels for the regenerative repair of critical-size LBSDs (Table 1). An electronic search of Google Scholar, PubMed, Medline, EMBASE, and Cochrane Library for literature describing “critical-size long bone defect regeneration”, published in English between 2000 and 2020, was performed. The results were then screened by title and abstract to only include those involving degradable synthetic polymers. Finally, we further narrowed down the list by excluding animal studies that employed neither proper controls nor quantitative outcome measures. Some of these grafts were fabricated by 3D printing and will be pointed out accordingly. Whenever possible, the guided bone regeneration outcomes including the radiographic union, degree of new bone formation, synthetic graft degradation/resorption, and the functional properties of regenerated bone are compared to those achieved with current grafting standards or healthy controls. Although angiogenesis is known to be tightly coupled to osteogenesis and an important parameter of functional bone regeneration,32 the highly varied (or lack of) quantitative assessments of angiogenesis in most studies makes head-to-head comparisons difficult. Accordingly, we choose to focus on restoration of the mechanical properties of the regenerated long bone as a key indicator of functional long bone regeneration as its success requires sufficient vascularization and integration with the host bone. The varied successes and limitations of these synthetic bone grafts will be appreciated from their physiochemical properties, degradation characteristics and the dose of their integrated biotherapeutics. Most examples highlighted involve the delivery of osteogenic proteins and peptides rather than exogenous therapeutic cells that might represent a higher translational barrier in terms of regulatory approvals.
Table 1 Degradable synthetic polymeric scaffolds for long bone segmental defects
| Graft composition | Animal | Segmental defect | Therapeutics | Regeneration outcomes | Limitations |
|---|---|---|---|---|---|
| 3D printed PCL/β-TCP composite | SheeP44 | 3 cm tibial | 3.5 mg rhBMP-7 | Radiographic union; mechanical restoration | Slow graft resorption |
| PLGA microparticles | SheeP38 | 2.5 cm femoral | 4-mg rhBMP-2 | Radiographic union (no mechanical testing) | Tendency of PLGA breakage |
| PLGA-coated gelatine sponge | Dog39 | 2.5 cm tibial | 0.4 mg/mL rhBMP-2 | Radiographic union; mechanical restoration | Small sample size; Bone resorption |
| Porous PLA-PEG/HAP | Rabbit46 | 1.5 cm radial | 5-20 μg rhBMP-2 | Radiographic union (no mechanical testing) | Slow graft resorption |
| PLA-DX-PEG/b-TCP | Rabbit47 | 1.5 cm femoral | 50 mg rhBMP-2 | Radiographic union; mechanical restoration; full graft resorption | Graft distortion within defect |
| 3D-printed PELGA/HAP | Rat54 | 5 mm femoral | 400 ng rhBMP-2/7 | Facile & stable graft fixation; rapid union, full graft resorption & mechanical restoration | Larger animal translation unknown |
| Solid PPF rod/porous sleeve with PLGA microparticle | Rat60 | 5 mm femoral | 2-8 μg rhBMP-2 | Improved defect fixation by solid rod; improved bone formation | Regeneration impeded by solid rod; no union |
| Crosslinked PPF/PPF diacrylate with PLGA microparticle | Rabbit61 | 1.5 cm radial | 200 μg TP508 | Improved osteointegration | No union; slow graft resorption |
| Salicylic acid-based poly(anhydride-ester)/PCL membrane | Rat65 | 5 mm femoral | 12 μg rhBMP-2 | Ectopic bone formation suppressed; long bone regeneration improved (no mechanical testing) | Poor graft mechanical property; long-term remodelling unclear |
| Tyrosine-derived polycarbonate/CP | Rabbit99 | 1.5 cm radial | 17-35 μg rhBMP-2 | Improved bone formation (no mechanical testing) | No union |
| pHEMA-HAp composite | Rat116 | 5 mm femoral | 400 ng rhBMP-2/7 | Radiographic union; mechanical restoration | Slow graft resorption |
| MMP-sensitive 4-arm PEG hydrogel with integrin binding GFOGER | Murine126 | 2.5 mm radial | 30 ng rhBMP-2 | Radiographic union; mechanical restoration; MMP-responsive degradation | Potentially high manufacturing cost |
Note: 3D: three-dimensional; CP: calcium phosphate; DX: p-dioxanone; GFOGER: α2β1 integrin-specific hexapeptide sequence Gly-Phe-Hyp-Gly-Glu-Arg; HAp: hydroxyapatite; MMP: matrix metalloproteinase; PCL: polycaprolactone; PEG: poly(ethylene glycol); PELGA: poly(lactic-co-glycolic acid)-b-poly(ethylene glycol)-b-poly(lactic-co-glycolic acid); pHEMA: poly(2-hydroxyethyl methacrylate); PLA: poly(lactic acid); PLGA: poly(D,L-lactic-co-glycolic acid); PPF: poly(propylene fumarate); rhBMP: recombinant human bone morphogenetic protein; TP508: Chrysalin, a 23-amino acid peptide representing amino acids 508-530 of human prothrombin; β-TCP: β-tricalcium phosphate.
Biodegradable Synthetic Polymer-Based Composites for Critical-Size Long Bone Segmental Defect Regeneration
According to the definition of the American Society for Testing and Materials, biodegradability refers to the susceptibility of a material to be decomposed into carbon dioxide, methane, water, and/or inorganic compounds as well as biomass.33 Here we focus on synthetic biodegradable polymers capable of undergoing decomposition in humans and vertebrate animals into fragments that can be further metabolized and readily removed from the body through natural pathways (e.g., excretion or metabolism).34 The most commonly-used biodegradable polymers for orthopaedic applications include polyesters (conventional and amphiphilic polylactides, poly(propylene fumarate) (PPF)), polyanhydrides, and polycarbonates. Here we discuss recent applications of synthetic bone grafts composed of these degradable polymers and other structural additives such as the osteoconductive biominerals hydroxyapatite (HAp) and β-tricalcium phosphate (β-TCP) for the regenerative repair of LBSDs.
Polyester-based composite bone grafts
Conventional polylactide-based composites
Polyesters remain the most popular and widely used biodegradable polymers for medical uses.35 Of them, polylactides are the most extensively investigated, with poly(lactic acid) (PLA), polyglycolic acid, poly(D,L-lactic-co-glycolic acid) (PLGA), polycaprolactone (PCL) and their copolymers cleared by the United States Food and Drug Administration for various medical applications ranging from resorbable sutures, drug delivery formulations to orthopaedic applications.36 These polymers can be prepared by ring-opening polymerization or copolymerization of glycolide, lactide, and/or ε-caprolactone. They undergo biodegradation via nonspecific hydrolytic scissions of the ester linkages at varied rates. End degradation products such as lactic acid, a natural metabolite, can be transported to the liver for metabolic conversions.37
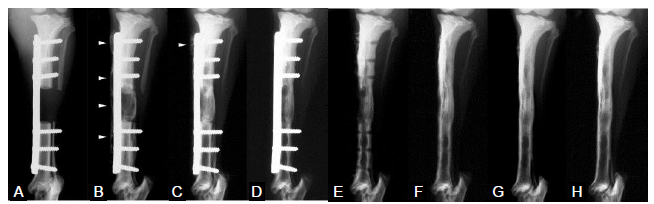
The most common use of polylactides for LBSD reconstruction is to exploit their degradability for the in vivo delivery of osteogenic therapeutic factors. An earlier study delivering rhBMP-2 and autologous blood via PLGA microparticles to 25-mm, critical-size femoral segmental defects in sheeP38 demonstrated the efficacy of 2- and 4-mg rhBMP-2 or autologous blood in improving new bone formation within the LBSD compared to the empty PLGA microparticle carriers. With the delivery of therapeutics, new bone mineral content within the LBSD was found to reach that of the intact femur by 4 months while the recanalization of the intramedullary canal approached completion by 12 months. However, the tendency of the weak PLGA to break in situ was noted and the biomechanical restoration of the regenerated bone was not evaluated. PLGA was also used to coat gelatine sponge grafts for the delivery of 0.4 mg/mL rhBMP-2 into 2.5-cm tibial segmental defects in dogs.39 Unlike the defect treated with the polymer-coated gelatine sponge scaffold alone that failed to bridge by 4 months, the defects treated with the polymer-coated gelatine sponge grafts impregnated with rhBMP-2 achieved radiographic union by 4 months (Figure 1A-E). Upon removal of the fixation plate, the regenerated bone continued to be remodelled over 2 years (Figure 1F-H), with the torsional stiffness exceeding that of the intact tibiae at 8 months and returning to the level of intact tibiae at 2 years.
Figure 1.
Figure 1.
Radiographs of a defect treated with polymer-coated gelatin sponge impregnated with recombinant bone morphogenetic protein 2 (0.4 mg/cm3) (anteroposterior view). (A-H) Radiographs were taken at 0 (A), 4 (B), 8 (C), 16 (D and E; before and after plate removal, respectively), 32 (F), 52 (G) and 104 (H) weeks postoperatively. Arrowheads in B and C indicate the hypertrophic bone beyond the metal plate. Reproduced from Kokubo et al.39 with permission from Elsevier.
The osteoconductive minerals β-TCP and HAp, known for their varying in vivo resorption rates,40 abilities to absorb a wide range of protein factors41 and to buffer acidic degradation products of polylactides, have long been used to fabricate degradable polymer-mineral composite bone graft substitutes.42, 43 A successful use of 3D-printed macroporous PCL/β-TCP composite scaffold for the repair of 3 cm, critical-size tibial segmental defects in sheep was demonstrated with the incorporation/delivery of 1.75 or 3.5 mg rhBMP-7.44 Bony callus bridged over the critical-size LBSDs by 3 months, with the torsional strength of the regenerated bone in both groups reaching the level achieved by the autologous cancellous bone grafting control. By 1 year, the mechanical restoration resulting from the PCL/β-TCP/3.5-mg rhBMP-7 treatment transcended those treated with the autologous cancellous bone graft control, although the synthetic bone graft was not fully resorbed, likely due to the slow degradation of PCL.
It should also be noted that the milligram scale of recombinant bone morphogenetic protein (BMP) protein therapeutics used in combination with the polylactide-based scaffolds in these large animal LBSD repair studies resembled the high human clinical doses known for adverse local and systemic health risks. More recently, 3D-printed elastic composites of HAp/PCL or HAp/PLGA with mineral contents as high as 90 wt% were developed as BMP delivery carriers for spinal fusion and calvarial bone repair applcations.45 It remains to be seen whether these high-mineral content polylactide composites may facilitate the functional regeneration of LBSDs, especially with substantially reduced effective loading doses of BMP therapeutics.
Amphiphilic polylactide-based composites
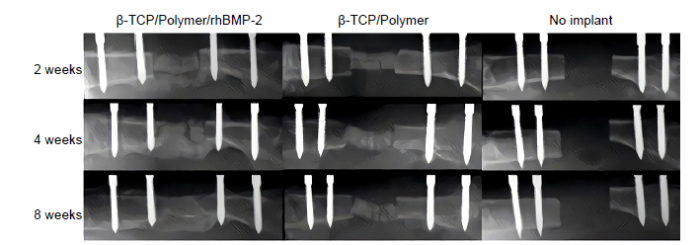
To improve the aqueous wettability, enhance the interfacial bonding with hydrophilic biominerals, and expedite the hydrolytic degradation of polylactide-based composite grafts, amphiphilic block copolymers containing both hydrophilic PEG block and hydrophobic PLA, PGLA or PCL block have been developed. A number of studies have explored the use of these amphiphilic polylactide-mineral composites for the repair of critical-size LBSDs. Porous amphiphilic copolymer PLA-PEG/HAp composites in combination with 5 or 20 μg rhBMP-2 were shown to enable bony callus formation bridging over 15-mm radial segmental defects in rabbits within 2 months.46 The composite grafts, however, were not fully resorbed by 2 months, and it was unclear how the mechanical integrity of the repaired defect compared to that of intact controls. Similarly, composite grafts composed of amphiphilic random copolymers consisting of PLA, p-dioxanone and PEG (PLA-DX-PEG) and β-TCP were used to deliver 50 μg of rhBMP-2 for the repair of 15-mm femoral segmental defects in rabbits.47 Bony callus bridged over the defects at 2 months (Figure 2), restoring the bending stiffness to 40% of the intact controls. The implant was completely resorbed by 6 months, accompanied by the restoration of mechanical integrity and natural anatomical structure of the regenerated femur through continued remodelling of the new bone.
Figure 2.
Figure 2.
Representative femoral radiographs. From left, implanted with β-TCP with PLA-DX-PEG and rhBMP-2, β-TCP with PLA-DX-PEG without rhBMP-2, and critical size bone defect without implantation (sham surgery). Sequential radiographs show bone repair at 2, 4, and 8 weeks after implantation in the experimental group. Reproduced from Yoneda et al.47 with permission from Elsevier. DX: p-dioxanone; PEG: poly(ethylene glycol); PLA: poly(lactic acid); rhBMP-2: recombinant bone morphogenetic protein 2; β-TCP: β-tricalcium phosphate.
We have developed multi-functional shape memory composite bone grafts based on the amphiphilic copolymers PLA-PEG-PLA or PLGA-PEG-PLGA with HAp, and elucidated how useful physical handling characteristics and biological performances may be engineered to enhance LBSD regeneration outcomes through a series of studies.48-54 With the hydrophobic PLA or PLGA blocks providing tunable degradability while the hydrophilic central PEG block enables strong bonding with HAp, the amphiphilic composites exhibited enhanced elasticity and aqueous wettability.48, 50, 51 Compared to conventional polylactide-HAp composites, the well-integrated HAp in the amphiphilic composites more effectively promoted osteochondral lineage commitment of bone marrow-derived stromal cells in unstimulated culture and supported far more potent osteogenesis upon in vitro osteogenic induction.51 The well-dispersed HAp distribution within the amphiphilic composites, maximizing mineral surface area for protein absorption, also enabled the sustained release of BMP protein therapeutics from both electrospun fibrous meshes53 and 3D-printed macroporous scaffolds54 for guided long bone regeneration. By controlling the block length and the ratio of hydrophobic PLA/PLGA vs. hydrophilic PEG, we also programmed hydration-induced stiffening behaviour, driven by microphase separation and PEG crystallization, to enable stable self-fixation of the amphiphilic composites within confined defects.49, 50 Finally, taking advantage of the respective thermal transitions of the amphiphilic blocks, we programmed shape memory behaviours that enable facile temporary shape programming at ambient temperature and shape recovery at safe physiological temperatures.48-50, 52
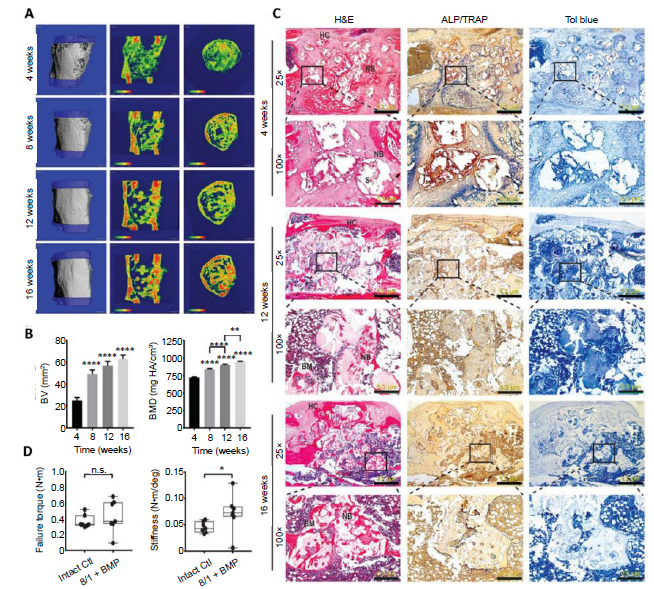
The successful translation of these multifunctional amphiphilic degradable shape memory composites for safer and more effective regenerative LBSD repair was recently demonstrated in rodents.54 Specifically, the macroporous 25% Hap-PLGA-PEG-PLGA(8/1) grafts incorporating PLGA-PEG-PLGA triblock copolymers, with a lactide to gylcolide ratio of 8:1, and 25 wt% Hap, were 3D printed to fit 5-mm, critical-size femoral segmental defects in rats. The graft could be compressed into a short cylinder at the time of surgery for convenient placement within the LBSD, and then underwent shape recovery at body temperature and spontaneous swelling and stiffening upon contact with bodily fluid. These unique graft characteristics translated into much shorter surgery time compared to the placement of the weak collagen sponge controls, and resulted in superior graft fixation as demonstrated by the substantially-higher graft fixation force measured (> 2 orders of magnitude higher than that of collagen sponge) and the 100% graft stability achieved in vivo. Importantly, when the graft was loaded with 400 ng of a rhBMP-2/7 heterodimer, (equivalent to ~13 μg in a 60-kg human), it led to the formation of bridging bony callus as early as 1 month (Figure 3A). Continuous remodelling led to steady increases in bone volume and bone mineral density (Figure 3B), the recanalization of regenerated bone, and the full resorption of the bone graft by 3 months (Figures 3A and C), culminating in the restoration of torsional strength to the level of intact controls by 4 months (Figure 3D). It should be noted that such a functional LBSD regeneration was achieved with a recombinant BMP protein therapeutic dose > 2 orders of magnitude lower than those typically required with collagen sponge carriers. Indeed, the significantly lower effective BMP loading dose on the amphiphilic composite graft, along with the excellent graft fixation stability, translated into the complete elimination of ectopic bone formation that was consistently observed in LBSDs treated with collagen/BMP controls. It remains to be seen whether this exciting shape memory bone graft technology may translate into safer and more effective limb salvage in larger animals and humans.
Figure 3.
Figure 3.
Accelerated healing of 5-mm rat femoral segmental defects by 25% HAp-PELGA(8/1) grafts preabsorbed with 400-ng rhBMP-2/7. (A) 3D μCT images and BMD colour maps (centre sagittal and axial slices) of the ROI showing maturing regenerated bone within the defect over time. Global thresholding was applied to exclude bone densities below 518.2 mg HAp/cm3 (HAp-PELGA graft invisible at this threshold). (B) Longitudinal μCT quantification of BV and BMD (n ≥ 12) within the ROI over time. Data are presented as means ± SEM. **P < 0.01, ****P < 0.0001 (one-way analysis of variance with Tukey’s post-hoc test). The global lower threshold of 518.2 mg HAp/cm3 was applied for all quantifications. (C) Histological micrographs of H&E-, ALP/TRAP-, and Tol blue-stained sections of explanted graft-filled femurs over time. Scale bars: 1.2 mm (25× magnification) and 300 μm (100× magnification). Boxed regions shown at higher magnification in bottom rows. (D) Boxplots of failure torque and stiffness of intact (control) versus regenerated femur (8/1 + BMP) 16 weeks after being treated with HAp-PELGA(8/1) grafts preloaded with 400-ng rhBMP-2/7 (n = 7). *P < 0.05 (Wilcoxon-Mann-Whitney rank sum test). Reprinted from Zhang et al.54 with permission from AAAS. 3D: three-dimensional; ALP: alkaline phosphatase; BM: bone marrow; BMD: bone mineral density; BMP: bone morphogenetic protein; BV: bone volume; Ctl: control; H&E: haematoxylin and eosin; HAp: hydroxyapatite; HC: healing callus; n.s.: P > 0.05; NB: new bone; PELGA: poly(lactic-co-glycolic acid)-b-poly(ethylene glycol)-b-poly(lactic-co-glycolic acid); rhBMP: human recombinant bone morphogenetic protein; ROI: region of interest; S: scaffold; TRAP: tartrate-resistant acid phosphatase; μCT: micro-computed tomography.
Poly(propylene fumarate)-based composites
Poly(propylene fumarate) (PPF), an unsaturated linear polyester used for orthopaedic applications,55, 56 can be prepared by the transesterification of di-(2-hydroxypropyl) fumarate. Fumaric acid, the main degradation product of PPF, is one of the essential Kreb’s cycle acid intermediates and is widely used in the food industry. The fumarate double bonds in PPF allow the polymer to be further crosslinked in situ for applications ranging from injectable biodegradable bone cements57, 58 to fabricating macroporous scaffolds crosslinked in 3D-printed negative moulds.59 By altering the composition and crosslinking of the polymers, PPF with compressive strength ranging from tens to hundreds of megapascals can be prepared. Solid PPF intramedullary rods, with or without a porous PPF sleeve for encouraging osteointegration, and with rhBMP-2 delivery via embedded PLGA microparticles, were examined for the stabilization and repair of 5-mm, critical-size femoral segmental defects in rats.60 The solid PPF intramedullary rod, applied in addition to plate fixation, improved defect fixation. Unfortunately, although the porous coating incorporating 2 or 8 μg of rhBMP-2 promoted new bone formation, complete union was not achieved in any treatment groups examined, suggesting that the solid PPF rod impeded long bone regeneration.
Porous, thermally-crosslinked PPF/PPF diacrylate composite scaffolds containing PLGA microparticles loaded with the osteogenic peptide TP508 were also examined for guided regeneration of 15 mm critical-size radial segmental defects in rabbits.61 Whereas the composite scaffold containing 100 or 0 μg of TP508 led to minimal bone formation (< 10% bridging), the scaffolds bearing 200 μg of TP508 via the PLGA microparticles improved the osteointegration (up to 80% bridging). Unfortunately, the PPF scaffold remained largely undegraded and the defect failed to be fully bridged by 12 weeks. These studies point to the limitation of slowly degrading PPF scaffolds for functional long bone regeneration.
Polyanhydride-based composites
Polyanhydrides, another class of biodegradable polymers frequently used for drug delivery,62, 63 can be prepared by ring-opening polymerization, melt polycondensation, dehydrochlorination and dehydrative coupling. The carboxylic acid degradation products resulting from the hydrolytic cleavage of the anhydride linkages should be carefully chosen for in vivo applications, to minimize mutagenicity or cytotoxicity.64 Of note, polyanhydrides that can be hydrolysed into therapeutic acids such as salicylic acid, a nonsteroidal anti-inflammatory drug, have been explored for limiting undesired ectopic bone formation associated with LBSD repair when high doses of rhBMP-2 are delivered via collagen sponge carriers.65 The idea was to utilize the ability of nonsteroidal anti-inflammatory drugs to suppress bone formation via the inhibition of cyclooxygenase-266-68 to counter the excessive release of rhBMP-2 into the tissues surrounding the LBSD. Specifically, salicylic acid-based poly(anhydride-ester) (SAPAE) was electrospun with PCL into thin membranes capable of fast degradation (FD-SAPAE) or slow degradation (SD-SAPAE). Collagen sponges loaded with 12-μg BMP-2 were placed within 5-mm rat femoral segmental defects with or without FD-SAPAE, SD-SAPAE or PCL control membranes.65 Whereas massive ectopic bone formation was observed in the groups without any membrane wrapping or those wrapped with PCL control or SD-SAPAE by 4 weeks, the treatment with FD-SAPAE membrane improved bone formation within the LBSD while suppressing ectopic bone formation. The study, however, did not investigate the longer-term bone remodelling outcome or the mechanical integrity of regenerated bone as a function of salicylic acid release kinetics and membrane degradations. It should be noted that the poor mechanical properties of the polyanhydride precludes its use as a standalone bone graft for LBSD repair, and the complex dynamics and interplay of the biological actions of BMP-2 and salicylic acid could present barriers to the clinical translation of this strategy.
Polycarbonate-based composite bone grafts
Polycarbonates69 are a class of thermoplastics that have broad-ranging applications from construction materials, digital storage media to containers and automobile parts. Poly(bisphenol A carbonate), prepared from the condensation of bisphenol A with phosgene or diphenyl carbonate, is a leading example due to its high impact resistance, ductility, optical transparency and low production costs.70 Unfortunately, the oestrogen-like behaviour of bisphenol A71 causes major concerns for its use in applications such as food containers as well as biomedically in vivo.72 Aliphatic polycarbonates that are degradable into non-xenoestrogenic alkyl alcohols and carbon dioxide under physiological conditions have thus attracted attention for biomedical uses.73-79 For guided bone regenerations, porous poly(butylene carbonate) membranes80 and poly(trimethylene-carbonate) barrier films81 were examined for treating non-weight-bearing calvarial and mandibular defects, respectively; they were found to perform comparably in terms of bone regeneration outcome to the respective PCL and polytetrafluoroethylene controls. These aliphatic polycarbonates, mechanically inferior to PCL, have not been applied to the regenerative repair of weight-bearing LBSDs. Meanwhile, to enable the introduction of functionalities and hydrophilicity desired for potential therapeutics delivery and osteointegration, carbonate monomers with “clickable” functionalities including alkyne-,82 azide-83 and (methyl)acrylate84 have been prepared. For instance, we developed an azido-substituted cyclic trimethylene carbonate monomer that can be used for controlled homopolymerization and copolymerization with lactides,82 and demonstrated the facile functionalization of the resulting polycarbonates and poly(ester-carbonates) via either copper-catalyzed85 or copper-free, strain-promoted86 azido-alkyne cycloaddition “click” chemistries. Whether and how mineral composites prepared with these functional polycarbonates translate into the regenerative LBSD repair remains to be determined.
Tyrosine-derived polycarbonates (Tyr-PCs), with hydrolytically-labile carbonate linkages and ester linkages along the main chain and connecting the side chains, respectively, were first developed by Kohn and Langer in 1987 as structural analogues of conventional poly(amino acids).87-89 Being mouldable,90 biocompatible and exhibiting good bone apposition,91-93 this relatively new class of degradable polycarbonates have been explored for orthopaedic applications ranging from fixation rods89 to guided craniomaxillofacial bone regeneration.94-98 A Tyr-PC terpolymer, polymerized from 89 mol% desaminotyrosyl-tyrosine alkyl ester and 10 mol% desaminotyrosyl-tyrosine and incorporating 1 mol% 1000-Da PEG, was explored for the repair of 15-mm, critical-size radial segmental defects in rabbits, with the polymer scaffold coated with calcium phosphate and 0-, 17- or 35-μg rhBMP-2.99 Whereas the Trp-PC + calcium phosphate scaffold alone induced minimal bone formation (< 2.5%), the loading of 17- or 35-μg rhBMP-2 led to significant increases in new bone formation within the LBSDs at 4 and 8 weeks. However, the defects were not fully restored at 8 weeks and the mechanical integrity of the regenerated bone was not evaluated.
As scaffold degradation kinetics and the immunogenicity of degradation products directly impact immune responses including macrophage polarization and the efficiency of osteogenesis, osteointegration and remodelling, the hydrolytically-degradable carbonate and ester linkages in Tyr-PCs provide a unique opportunity to modulate the osteoimmunological responses. For example, by manipulating side chain chemistry, Tyr-PCs can be engineered to achieve faster hydrolysis of the carbonate linkages, thereby delaying/mitigating the acute inflammatory responses often observed with the acidic degradation products of polyester-based scaffolds.100 Long-term local and systemic safety profiles, however, will have to be established after any chemical modifications under the context of specific in vivo applications.
Synthetic Biodegradable Polyethylene Glycol-Based Hydrogels for Critical-Size Long Bone Segmental Defect Regeneration
Limitations of polysaccharide-based hydrogels
Naturally-occurring polysaccharide-based hydrogels such as hyaluronic acid,101 alginate,102-108 and chitosan109-111 have been explored for LBSD repairs with varying degrees of success, with the polysaccharides often chemically modified with cell-adhesive peptides/proteins and/or loaded with osteogenic/angiogenic factors. Due to the intrinsically weak mechanical properties, these hydrogel formulations were often augmented with other structural components including osteoconductive minerals, or delivered within a secondary containment to the site of LBSDs. The microgram-scale rhBMP-2 delivered via most of these scaffolds for treating rodent critical-size LBSDs, when scaled to human, is unlikely to address the safety concerns associated with the current clinical doses delivered with collagen sponge carriers. Finally, covalent modifications of naturally-occurring polysaccharides in a regioselective manner and with reproducible stoichiometric control can be challenging.
Limitations of non-degradable crosslinked synthetic hydrogel composites
In comparison, wholly synthetic hydrogels crosslinked from well-defined building blocks present unique advantages in addressing some of the challenges associated with naturally occurring polysaccharide-based hydrogels. For instance, poly(2-hydroxyethyl methacrylate)-based hydrogels bearing biomimetic mineral-binding ligands can be readily prepared by copolymerizing functional methacrylate monomers.112, 113 We demonstrated that high-mineral content (up to 70 wt%) poly(2-hydroxyethyl methacrylate)-HA composite with outstanding structural integrity, interfacial adhesion and compressive elasticity can be prepared for bone tissue engineering applications.114, 115 When the poly(2-hydroxyethyl methacrylate)-mineral composite grafts containing 50 wt% HA and 400-ng rhBMP-2/7 were press-fit within 5-mm, critical-size femoral segmental defects in rats, they led to robust bridging bony callus formation by 6 weeks, with the torsional integrity of the remodelled new bone comparable to that of healthy controls.116 However, due to the non-hydrolytically degradable nature of the polymethacrylate network, the composite graft remained sandwiched within the new bone, likely taking a very long time for the graft to be resorbed. This is also a limitation of conventional photo-crosslinked polyethylene glycol di(meth)acrylate-based hydrogels as synthetic bone grafts. Although lower molecular mass PEG oligomers can be excreted through the urine, the slow degradation of the crosslinked system could impede timely tissue integration and graft resorption for LBSD reconstruction.117 Accordingly, there is a need for PEG-based hydrogels with more controlled degradation and bio-functionalities (e.g. cell-adhesiveness to overcome its bioinert nature118) for LBSD reconstructions.
Integrating controlled degradation to crosslinked polyethylene glycol-based hydrogels
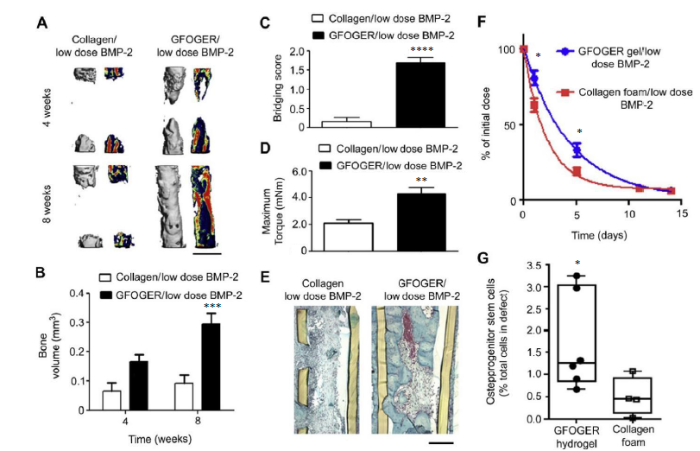
To expedite the hydrolytic degradation of crosslinked PEG-based hydrogels, degradable polymer segments (e.g. polylactide,119 polycarbonate120) could be covalently integrated within the 3D network. Alternatively, we showed that when isolated degradable ester linkages were strategically placed near the strain-promoted azide-alkyne cycloaddition crosslinking site of well-structured PEG hydrogels, a broad range of degradation rates (from days to years) predicted by first-order hydrolytic degradation kinetics could be engineered.121 Finally, PEG hydrogels crosslinked by matrix metalloproteinase-sensitive peptide crosslinkers122 have seen numerous applications for bone and cartilage tissue engineering where the neotissue integration benefited from timely, environmentally-responsive scaffold degradation.123-125 For instance, Shekaran et al.126 crosslinked four-arm PEG-maleimides bearing the pro-osteogenic α2β1 integrin-specific hexapeptide Gly-Phe-Hyp-Gly-Glu-Ar (GFOGER) with matrix metalloproteinase-sensitive crosslinkers. These hydrogels were loaded with varying doses of rhBMP-2 for the repair of 2.5-mm murine radial segmental defects. The GFOGER-functionalized hydrogel alone was shown to result in substantial new bone formation within the defect, outperforming those tethered with the more commonly used cell adhesive RGD peptides. Furthermore, with the delivery of a low dose of 30-ng rhBMP-2, the GFOGER-modified, matrix metalloproteinase-responsive hydrogel underwent timely in vivo degradation and BMP-2 release, effectively repairing the LBSD with bridging new bone that fully restored its torsional integrity by 2 months (Figure 4). By contrast, the collagen control absorbed with the same dose of rhBMP-2 was unable to fully bridge the defect within the same timeframe. This well-designed scaffold has the potential to be a more effective and safer BMP therapeutics carrier for a range of orthopaedic applications.
Figure 4.
Figure 4.
BMP-2 delivery from GFOGER-functionalized gels improves bone regeneration compared to collagen foams. (A) 3D μCT reconstructions of radii (left) and mineral density sagittal sections (right). Scale bar: 1 mm. (B) μCT measures of bone volume in radial defects. (C) Bridging score at 8 weeks post-implantation (n = 13). (D) Maximum torque values for 8 weeks radial samples subjected to torsion mechanical testing to failure (n = 5-9). (E) Sections of 8 weeks radial samples stained with Safranin-O/Fast Green. Scale bar: 200 μm. (F) Retention of infrared dye-labelled BMP-2 at implanted defect sites in vivo (n = 6). (G) Quantification of CD45-/CD90+ osteoprogenitor cells present in the defects 7 days post-implantation (n = 4-6). *P < 0.05, ***P < 0.001, ****P < 0.0001, vs. collagen foam/low dose BMP-2. Reproduced from Shekaran et al.126 with permission from Elsevier. 3D: three-dimensional; BMP-2: bone morphogenetic protein 2; GFOGER: α2β1 integrin-specific hexapeptide sequence Gly-Phe-Hyp-Gly-Glu-Ar; μCT: micro-computed tomography.
Outlook
In the past two decades, a wide range of degradable synthetic polymeric bone grafts have been developed to promote the regenerative repair of LBSDs, some already yielding exciting outcomes in preclinical animal models. For eventual successful clinical translations, further enhancements of the efficacy and safety while ensuring reproducible, scalable and affordable manufacturing of these grafts will be necessary. It is also critical that preclinical studies are more rigorously designed to include functional outcome evaluations such as mechanical property assessment of regenerated long bone against current grafting standards and healthy controls. The assessment of longer-term local tissue responses and systemic safety of the synthetic bone grafts and their degradation products should also be encouraged within the bone tissue engineering community.
Emerging strategies for modulating osteoimmune responses during scaffold-guided bone regeneration such as macrophage polarization and osteoclast-mediated bone remodelling through the manipulation of physiochemical properties of biomaterial scaffolds26 could lead to more effective regenerative repair of LBSDs. For instance, pro- vs. anti-inflammatory cytokines6, 26 may be tethered to the synthetic bone graft to provide environmentally-responsive, temporally-controlled release to promote regenerative rather than degenerative repair processes. Meanwhile, recent advances in designing viscoelastic synthetic biomaterials127-129 that better recapitulate the dynamic tissue mechanics including stress relaxation properties130 may enable better control over the fate and function of exogenously-delivered cell therapeutics or the myriad of endogenous cells recruited to the site of LBSDs. Furthermore, the engineering of multifunctional synthetic bone graft properties such as shape memory and in situ stiffening has the potential to improve the efficiency and precision of the surgical delivery and fixation of personalized bone grafts. Finally, the continued innovation of materials fabrication techniques such as intravital bioprinting131, 132 may open the door for longitudinal delivery of therapeutics to the surface of autogenic, allogenic and synthetic bone grafts in a spatially-defined manner.
Author contributions
XX and JS searched the literature and wrote the review. Both authors approved the final version of this manuscript.
Financial support
This work is supported by an Alex Lemonade Stand Foundation Innovation Grant and a BRIDGE Award from the University of Massachusetts Medical School.
Acknowledgement
None.
Conflicts of interest statement
The authors declare no competing financial interest.
Data sharing statement
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Reference
Bone graft materials. An overview of the basic science
DOI:10.1097/00003086-199611000-00003
URL
PMID:8913140
[Cited within: 1]

Contemporary management of Grade IIIB open tibial fractures has evolved to include intravenous antibiotics, thorough interval surgical debridement, rigid skeletal fixation, early local or free tissue myoplasty, and liberal use of autogenous bone graft beneath a clean, stable wound. External fixation has been the skeletal stabilization of choice with the lowest reported deep sepsis rates. Pin tract infection, malunion, and nonunion have complicated its use. Static unreamed locked nailing is an alternative treatment that has been successfully used in lower grade open tibial fractures. A metaanalysis of the literature was undertaken to determine whether there was evidence favoring 1 method of skeletal fixation. Inclusion criteria were restricted to studies that were randomized to either external fixation or unreamed intramedullary nail methods and that used a strict definition of Grade IIIB to include muscle transfer for soft tissue coverage. Two studies were identified and combined to show no difference in deep sepsis rate. Intramedullary nailing significantly shortened union time whereas external fixation showed a trend toward a higher incidence of malunion and superficial sepsis. More well designed randomized studies would add to this initial effort and yield more compelling evidence for either form of fixation.
Lessons learned from modern military surgery
DOI:10.1016/j.suc.2006.09.008
URL
PMID:17127127

The era of global terrorism and asymmetric warfare heralded by the September 11, 2001 attacks on the United States have blurred the traditional lines between civilian and military trauma. The lessons learned by physicians in the theaters of war, particularly regarding the response to mass casualties, blast and fragmentation injuries, and resuscitation of casualties in austere environments, likely resonate strongly with civilian trauma surgeons in the current era. The evolution of a streamlined trauma system in the theaters of operations, the introduction of an in-theater institution review board process, and dedicated personnel to collect combat casualty data have resulted in improved data capture and realtime, on-the-scene research.
Modern military surgery: lessons from Iraq and Afghanistan
DOI:10.1302/0301-620X.94B4.28602
URL
PMID:22434472
[Cited within: 1]

The types of explosive devices used in warfare and the pattern of war wounds have changed in recent years. There has, for instance, been a considerable increase in high amputation of the lower limb and unsalvageable leg injuries combined with pelvic trauma. The conflicts in Iraq and Afghanistan prompted the Department of Military Surgery and Trauma in the United Kingdom to establish working groups to promote the development of best practice and act as a focus for research. In this review, we present lessons learnt in the initial care of military personnel sustaining major orthopaedic trauma in the Middle East.
Management of posttraumatic segmental bone defects
DOI:10.5435/00124635-200401000-00005
URL
PMID:14753795

Because of difficulty in managing posttraumatic segmental bone defects and the resultant poor outcomes, amputation historically was the preferred treatment. Massive cancellous bone autograft has been the principal alternative to amputation. Primary shortening or use of the adjacent fibula as a graft also has been used to attempt limb salvage. Of more recent methods of management, bone transport with distraction osteogenesis has been suggested as the leading option for defects of 2 to 10 cm, but problems include delayed union at the docking site and prolonged treatment time. Free vascularized bone transfer has been suggested as the leading option for defects of 5 to 12 cm, but hypertrophy of the graft is unreliable and late fracture, common. Bone graft substitutes continue to be developed, but they have not yet reached clinical efficacy for posttraumatic segmental bone defects. Although each of the new techniques has shown some limited success, complications remain common.
Management of segmental bone defects
DOI:10.5435/JAAOS-D-14-00018
URL
PMID:25716002

Segmental bone defects cause significant disability in patients. Modern orthopaedic surgical techniques have proved to be reliable for reconstruction of these defects. Autogenous bone graft remains the standard of care for reconstruction of small defects (<5 cm). Induced membrane technique and distraction osteogenesis are contemporary strategies of choice for reconstruction of larger bony defects. The use of vascularized fibular grafts has waned in popularity because of donor site morbidity and the success of alternative methods. Complications are ubiquitous with all methods of reconstruction for segmental bone defects but can be limited with careful surgical judgment and technique. In most cases, the rehabilitation period is prolonged, although some treatment options are shorter and enable a more active recovery than do others.
A review of biomaterials in bone defect healing, remaining shortcomings and future opportunities for bone tissue engineering: The unsolved challenge
DOI:10.1302/2046-3758.73.BJR-2017-0270.R1
URL
PMID:29922441
[Cited within: 4]

Despite its intrinsic ability to regenerate form and function after injury, bone tissue can be challenged by a multitude of pathological conditions. While innovative approaches have helped to unravel the cascades of bone healing, this knowledge has so far not improved the clinical outcomes of bone defect treatment. Recent findings have allowed us to gain in-depth knowledge about the physiological conditions and biological principles of bone regeneration. Now it is time to transfer the lessons learned from bone healing to the challenging scenarios in defects and employ innovative technologies to enable biomaterial-based strategies for bone defect healing. This review aims to provide an overview on endogenous cascades of bone material formation and how these are transferred to new perspectives in biomaterial-driven approaches in bone regeneration. Cite this article: T. Winkler, F. A. Sass, G. N. Duda, K. Schmidt-Bleek. A review of biomaterials in bone defect healing, remaining shortcomings and future opportunities for bone tissue engineering: The unsolved challenge. Bone Joint Res 2018;7:232-243. DOI: 10.1302/2046-3758.73.BJR-2017-0270.R1.
The indications and donor-site morbidity of tibial cortical strut autografts in the management of defects in long bones
DOI:10.1302/0301-620X.100B5.BJJ-2017-0577.R2
URL
PMID:29701102
[Cited within: 1]

Aims: The primary aim of this study was to determine the morbidity of a tibial strut autograft and characterize the rate of bony union following its use. Patients and Methods: We retrospectively assessed a series of 104 patients from a single centre who were treated with a tibial strut autograft of > 5 cm in length. A total of 30 had a segmental reconstruction with continuity of bone, 27 had a segmental reconstruction without continuity of bone, 29 had an arthrodesis and 18 had a nonunion. Donor-site morbidity was defined as any event that required a modification of the postoperative management. Union was assessed clinically and radiologically at a median of 36 months (IQR, 14 to 74). Results: Donor-site morbidity occurred in four patients (4%; 95% confidence interval (CI) 1 to 10). One patient had a stress fracture of the tibia, which healed with a varus deformity, requiring an osteotomy. Two patients required evacuation of a haematoma and one developed anterior compartment syndrome which required fasciotomies. The cumulative probability of union was 90% (95% CI 80 to 96) at five years. The type of reconstruction (p = 0.018), continuity of bone (p = 0.006) and length of tibial graft (p = 0.037) were associated with the time to union. Conclusion: The tibial strut autograft has a low risk of morbidity and provides adequate bone stock for treating various defects of long bones. Cite this article: Bone Joint J 2018;100-B:667-74.
A study of the mechanical strength of long bone defects treated with various bone autograft substitutes: an experimental investigation in the rabbit
DOI:10.1002/jor.1100070416
URL
PMID:2544712
[Cited within: 1]

This study was designed to determine which of several bone grafting materials would be the most efficacious substitute for autogenous bone graft in the treatment of segmental long bone defects. The experimental model was a 1-cm defect in the rabbit ulna. The control group had nothing implanted in the defect. The six grafts tested were: (a) autogenous iliac crest bone, (b) autogenous cortical bone (ulna), (c) hydroxylapatite, (d) hydroxylapatite-demineralized bone matrix (allograft) composite graft, (e) freeze-dried bone (allograft), and (f) demineralized bone matrix (allograft). At 6 weeks postoperatively, the ulnas were harvested, examined radiographically, and tested mechanically in torsion. The radiographic examination proved to be of little value because some materials were radiodense at the time of implantation. The rates (percentage) of union, torques at failure, and energy to failure values were statistically significantly higher than control for all groups except hydroxylapatite. We concluded that demineralized bone matrix and hydroxylapatite-demineralized bone matrix composite graft compare favorably with cortical replacement (autograft) in mechanical strength and rate of union and therefore may be satisfactory substitutes for bone grafting. Freeze-dried bone did not appear to be as satisfactory because of its low mean energy to failure, but statistical analysis failed to confirm this opinion. Hydroxylapatite graft, when used alone, does not appear to be a suitable material for grafting segmental bone defects.
Autograft, allograft and bone substitutes in reconstructive orthopedic surgery
DOI:10.1007/s40520-013-0088-8
URL
PMID:24046051
[Cited within: 1]

Reconstruction of bone defects is a challenge for all orthopedic surgeons worldwide; to overcome this problem there are different options: the use of autografts, allografts and bone substitutes (BSs) to enhance and accelerate bone repair. Autografts have excellent biological properties but are associated with morbidity of the donor site and are restricted in volume. Allografts are available in adequate quantity but concerns still remain about the risk of infections, moreover they do not have osteogenetic properties. Bone substitutes have different indications and are very attractive for orthopedic surgeons. The present paper briefly reviews the advantages and disadvantages of autografts, allografts and BSs for bone reconstruction.
The clinical use of allografts, demineralized bone matrices, synthetic bone graft substitutes and osteoinductive growth factors: a survey study
DOI:10.1007/s11420-005-0111-5
URL
PMID:18751803
[Cited within: 1]

The emergence of new bone grafting options and alternatives has led to significant uncertainty when determining the most appropriate product for surgical procedures requiring bone graft in orthopedics. Allografts, demineralized bone matrices, synthetic bone graft substitutes, and osteoinductive growth factors are all viable options, yet there is a lack of data reporting clinical usage of these products. This correspondence reports on the use of bone grafting products at the Hospital for Special Surgery for a 27-month period and makes recommendations based on surgical usage, safety, and cost. Approximately half (48.6%) of all bone graft substitutes were implanted during spinal surgery. Arthroplasty, trauma, and foot/hand cases all used considerable amounts of bone grafting products as well (20.1%, 19.0%, 12.1%, respectively). Considerable differences were noticed in usage of bone grafting products among each orthopedic discipline. Of all bone graft substitutes used in arthroplasty, 14.4% were demineralized bone matrices, whereas 56.8% were allografts. Demineralized bone matrix grafts were used in 82% of trauma surgery and 89% of foot/hand cases. An increase in synthetic bone graft alternatives was noticed near the end of our investigation period.
Limitations of autograft and allograft: new synthetic solutions
URL
PMID:12038843
[Cited within: 1]

Autogenous cancellous bone is widely regarded as an ideal construct for graft procedures, supplying osteoinductive growth factors, osteogenic cells, and a structural scaffold. However, procurement morbidity and constraints on obtainable quantities limit its use. Allograft is the next best alternative at present; however, minor immunogenic rejection and risk of disease transmission are unresolved issues. Although synthetic grafting materials eliminate these risks, these materials do not transfer osteoinductive or osteogenic elements to the host site. To offer the advantages of autograft and allograft, a composite graft may be considered. Such a graft can combine a synthetic scaffold with biologic elements to stimulate cell infiltration and new bone formation.
Allograft fractures revisited
URL
PMID:9678034
[Cited within: 1]

Recent developments in dual xray absorptiometry have made it possible to quantify bone mineral density changes adjacent to total hip arthroplasty. Even small changes in local bone mass that are not visible with conventional radiographs can be detected using dual xray absorptiometry. Commonly there is a loss of 10% to 45% of the periprosthetic bone mass during the first years after total hip arthroplasty. Recent studies have suggested that this bone loss is not necessarily progressive and some degree of restoration of bone density around implants may occur. Current data suggest that there is active bone remodeling in the proximal femur in response to prosthetic implantation. Such response differs between different stem designs and type of fixation.
Bone-grafting and bone-graft substitutes
DOI:10.2106/00004623-200203000-00020 URL PMID:11886919 [Cited within: 1]
Allograft bone. The influence of processing on safety and performance
DOI:10.1016/s0030-5898(05)70110-3
URL
PMID:10471762
[Cited within: 1]

Advances in tissue processing technology have been important for the successful use of bone allografts. The challenge is to prepare allografts that are well cleaned, sterile, and free of viruses while still preserving the natural biologic and biomechanical properties of the tissue. This article discusses how processing techniques aimed at achieving safety and sterility can affect the properties vital for graft incorporation and healing.
Biomimetic composite scaffolds containing bioceramics and collagen/gelatin for bone tissue engineering - A mini review
URL PMID:27316767 [Cited within: 1]
The cylindrical titanium mesh cage for treatment of a long bone segmental defect: description of a new technique and report of two cases
DOI:10.1097/00005131-200001000-00011
URL
PMID:10630804
[Cited within: 1]

This report describes a new technique for treatment of a segmental defect in long bones that uses a cylindrical titanium mesh cage, in combination with cancellous bone allograft and demineralized bone matrix putty (Grafton), stabilized with a statically locked intramedullary nail. Two clinical cases of tibia defects treated with this technique are presented. At the one-year follow-up, radiographically both cases demonstrated excellent limb alignment, stability, and bony healing. Immediate full weight-bearing was initiated in each case, and early limb functional recovery was achieved. Preliminary data suggest that this technique may be a reasonable alternative to currently used methods for management of select long bone segmental defects.
Case reports: management of large segmental tibial defects using a cylindrical mesh cage
DOI:10.1097/01.blo.0000223982.29208.a4
URL
PMID:16702918
[Cited within: 1]

We report a case series of three patients who sustained open Gustilo-Anderson Type IIIB tibia fractures associated with extensive segmental bone and soft tissue loss. The patients initially were treated with serial wound irrigations, debridements, and external fixation. After the soft tissue envelope was reconstructed successfully, each large segmental bone defect was reconstructed with a cylindrical titanium mesh cage packed with a composite of cancellous allograft and demineralized bone matrix putty and stabilized with a statically locked intramedullary nail. The mean segmental bone loss was 12.2 cm, and all patients had a minimum 1-year followup. One year after reconstruction, radiographs showed stable, well-aligned, healed constructs, and computed tomography images verified the presence of bony ingrowth throughout the cages. All patients were able to ambulate with full weightbearing, and had good ipsilateral knee, hip, and ankle range of motion. This technique seems to be a reasonable alternative for treating large segmental tibial bone defects.
Stem cells associated with macroporous bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study
DOI:10.1089/ten.2006.0271
URL
PMID:17484701
[Cited within: 1]

Extensive bone loss is still a major problem in orthopedics. A number of different therapeutic approaches have been developed and proposed, but so far none have proven to be fully satisfactory. We used a new tissue engineering approach to treat four patients with large bone diaphysis defects and poor therapeutic alternatives. To obtain implantable three-dimensional (3D) living constructs, cells isolated from the patients' bone marrow stroma were expanded in culture and seeded onto porous hydroxyapatite (HA) ceramic scaffolds designed to match the bone deficit in terms of size and shape. During the surgical session, an Ilizarov apparatus or a monoaxial external fixator was positioned on the patient's affected limb and the ceramic cylinder seeded with cells was placed in the bone defect. Patients were evaluated at different postsurgery time intervals by conventional radiographs and computed tomography (CT) scans. In one patient, an angiographic evaluation was performed at 6.5 years follow-up. In this study we analyze the long-term outcome of these patients following therapy. No major complications occurred in the early or late postoperative periods, nor were major complaints reported by the patients. No signs of pain, swelling, or infection were observed at the implantation site. Complete fusion between the implant and the host bone occurred 5 to 7 months after surgery. In all patients at the last follow-up (6 to 7 years postsurgery in patients 1 to 3), a good integration of the implants was maintained. No late fractures in the implant zone were observed. The present study shows the long-term durability of bone regeneration achieved by a bone engineering approach. We consider the obtained results very promising and propose the use of culture-expanded osteoprogenitor cells in conjunction with porous bioceramics as a real and significant improvement in the repair of critical-sized long bone defects.
The effect of platelet-rich plasma on healing in critical-size long-bone defects
DOI:10.1016/j.biomaterials.2008.06.014
URL
PMID:18614227
[Cited within: 1]

The role of platelet-rich plasma (PRP) as a promoter of bone healing remains controversial. The hypothesis investigated was that PRP improves bone healing of a critical-size diaphyseal radius defect in a rabbit model. The bone defect was filled with a high-surface ceramic scaffold, calcium-deficient hydroxyapatite (CDHA), with the addition of allogenic PRP, mesenchymal stem cells (MSC) or both. PRP yielded better bone formation than the empty CDHA scaffold as determined by both histology and micro-computer tomography (P<0.05) after 16 weeks, whereas no difference was observed on biomechanical testing. Similar behavior was found in samples with MSC; however, the combination of MSC and PRP did not further improve bone healing. Furthermore, the resorption of CDHA was improved by the addition of PRP, MSC and MSC/PRP, but there were no differences between the groups. The areas of bone formation were greater in areas adjacent to the bone resection areas and towards the intact ulna. In conclusion, PRP improves bone healing in a diaphyseal rabbit model on CDHA and the combination of CDHA. This study supports the allogenic use of PRP for bone healing as an off-the-shelf therapy.
Collagen sponges for bone regeneration with rhBMP-2
DOI:10.1016/j.addr.2003.08.010
URL
PMID:14623404
[Cited within: 1]

In the US alone, approximately 500,000 patients annually undergo surgical procedures to treat bone fractures, alleviate severe back pain through spinal fusion procedures, or promote healing of non-unions. Many of these procedures involve the use of bone graft substitutes. An alternative to bone grafts are the bone morphogenetic proteins (BMPs), which have been shown to induce bone formation. For optimal effect, BMPs must be combined with an adequate matrix, which serves to prolong the residence time of the protein and, in some instances, as support for the invading osteoprogenitor cells. Several factors involved in the preparation of adequate matrices, specifically collagen sponges, were investigated in order to test the performance in a new role as an implant providing local delivery of an osteoinductive differentiation factor. Another focus of this review is the current system consisting of a combination of recombinant human BMP-2 (rhBMP-2) and an absorbable collagen sponge (ACS). The efficacy and safety of the combination has been clearly proven in both animal and human trials.
Guided tissue engineering for healing of cancellous and cortical bone using a combination of biomaterial based scaffolding and local bone active molecule delivery
DOI:10.1016/j.biomaterials.2018.10.004
URL
PMID:30321863

A metaphyseal bone defect due to infection, tumor or fracture leads to loss of cancellous and cortical bone. An animal model separating the cancellous and cortical healing was used with a combination of a macroporous gelatin-calcium sulphate-hydroxyapatite (Gel-CaS-HA) biomaterial as a cancellous defect filler, and a thin collagen membrane (CM) guiding cortical bone regeneration. The membrane was immobilized with bone morphogenic protein-2 (rhBMP-2) to enhance the osteoinductive properties. The Gel-CaS-HA cancellous defect filler contained both rhBMP-2 and a bisphosphonate, (zoledronate = ZA) to prevent premature callus resorption induced by the pro-osteoclast effect of rhBMP-2 alone. In the first part of the study, the CM delivering both rhBMP-2 and ZA was tested in a muscle pouch model in rats and the co-delivery of rhBMP-2 and ZA via the CM resulted in higher amounts of bone compared to rhBMP-2 alone. Secondly, an established tibia defect model in rats was used to study cortical and cancellous bone regeneration. The defect was left empty, filled with Gel-CaS-HA alone, Gel-CaS-HA immobilized with ZA or Gel-CaS-HA immobilized with rhBMP-2+ZA. Functionalization of the Gel-CaS-HA scaffold with bioactive molecules produced significantly more bone in the cancellous defect and its surroundings but cortical defect healing was delayed likely due to the protrusion of the Gel-CaS-HA into the cortical bone. To guide cortical regeneration, the cortical defect was sealed endosteally by a CM with or without rhBMP-2. Subsequently, the cancellous defect was filled with Gel-CaS-HA containing ZA and rhBMP-2+ZA. In the groups where the CM was doped with rhBMP-2, significantly higher number of cortices bridged. The approach to guide cancellous as well as cortical bone regeneration separately in a metaphyseal defect using two bioactive molecule immobilized biomaterials is promising and could improve the clinical care of patients with metaphyseal defects.
Use and efficacy of bone morphogenetic proteins in fracture healing
URL PMID:21698428 [Cited within: 1]
High doses of bone morphogenetic protein 2 induce structurally abnormal bone and inflammation in vivo
DOI:10.1089/ten.TEA.2010.0555
URL
PMID:21247344
[Cited within: 1]

The major Food and Drug Association-approved osteoinductive factors in wide clinical use are bone morphogenetic proteins (BMPs). Although BMPs can promote robust bone formation, they also induce adverse clinical effects, including cyst-like bone formation and significant soft tissue swelling. In this study, we evaluated multiple BMP2 doses in a rat femoral segmental defect model and in a minimally traumatic rat femoral onlay model to determine its dose-dependent effects. Results of our femoral segmental defect model established a low BMP2 concentration range (5 and 10 mug/mL, total dose 0.375 and 0.75 mug in 75 mug total volume) unable to induce defect fusion, a mid-range BMP2 concentration range able to fuse the defect without adverse effects (30 mug/mL, total dose 2.25 mug in 75 mug total volume), and a high BMP2 concentration range (150, 300, and 600 mug/mL, total dose 11.25, 22.5, and 45 mug in 75 mug total volume) able to fuse the defect, but with formation of cyst-like bony shells filled with histologically confirmed adipose tissue. In addition, compared to control, 4 mg/mL BMP2 also induced significant tissue inflammatory infiltrates and exudates in the femoral onlay model that was accompanied by increased numbers of osteoclast-like cells at 3, 7, and 14 days. Overall, we consistently reproduced BMP2 side effects of cyst-like bone and soft tissue swelling using high BMP2 concentration approaching the typical human 1500 mug/mL.
BMP-2 release and dose-response studies in hydroxyapatite and beta-tricalcium phosphate
DOI:10.3233/BME-2009-0573
URL
PMID:19581707

The purpose of this study is to compare in vivo retention of BMP-2 and bone induction in HAp (porosity: 60-80%, pore size: 100-600 mum, sintering temperature: 800 degrees C, surface area: 1 m(2)/g) and beta-TCP (porosity: 75%, pore size: 100-400 mum, sintering temperature: 1050 degrees C, surface area: 4 m(2)/g). We estimated the in vivo release profile of (125)I-labeled BMP-2 and bone induction of hard tissues histologically. The amount of BMP-2 remaining in the beta-TCP at 1 day after implantation was 49.6%, while the amount was 34.0% in the HAp. Furthermore, the HAp and beta-TCP containing 0.0, 0.05, 0.1, 0.3, 0.5, 1.0, 5.0 microg of BMP-2 were implanted into the back subcutis of 4-week old Wistar rats. At 3 weeks after implantation, the ceramics were explanted and evaluated histologically. The HAp/BMP-2 (5.0 microg) system showed 3.0% in the total volume of bone at 3 weeks, while only in the beta-TCP/BMP-2 (5.0 microg) system showed 32.5%. These results indicate that the absorbable beta-TCP block may be an effective bioceramic for bone induction to deliver BMP-2 to the site of action.
Recombinant human bone morphogenetic protein-2 and pancreatic cancer: a retrospective cohort study
DOI:10.1002/pds.2057
URL
PMID:21254281
[Cited within: 1]

PURPOSE: To assess whether use of recombinant human bone morphogenetic protein-2 (rhBMP-2) during lumbar spinal fusion surgery affects subsequent risk of pancreatic cancer. METHODS: Using US Medicare claims data, we performed a retrospective cohort study of patients who underwent lumbar spinal fusion surgery between October 2003 and December 2005. The study population, all >66 years, was identified from procedure codes for lumbar fusion. Claims for a bone morphogenetic protein (BMP) served as a proxy for rhBMP-2 exposure (another BMP product shared the same code). Pancreatic cancer was identified from claims indicating this diagnosis and cancer-specific therapy. We used Cox proportional hazard regression to estimate hazard ratios (HRs) and 95%CIs. RESULTS: Of the 93,654 patients in the study, the mean age was 75 years, and 16.5% had claims for BMP. During a mean 1.4 years of follow-up, 91 patients were diagnosed with pancreatic cancer (eight in the BMP- and 83 in the non-BMP cohort). Consistent with previous research, pancreatic cancer was associated with older age, male gender, black race, and diabetes mellitus. Compared to those who did not receive BMP, patients exposed to BMP were not at increased risk of pancreatic cancer (adjusted HR=0.70, 95%CI: 0.34-1.45). A chart review substudy validated the exposure measure; 52/55 patients with claims for BMP received rhBMP-2. CONCLUSIONS: In this large study of elderly patients who underwent lumbar fusion surgery, exposure to BMP was not associated with an increased risk of pancreatic cancer.
Current advances in immunomodulatory biomaterials for bone regeneration
DOI:10.1002/adhm.201801106
URL
PMID:30328293
[Cited within: 4]

Biomaterials with suitable surface modification strategies are contributing significantly to the rapid development of the field of bone tissue engineering. Despite these encouraging results, utilization of biomaterials is poorly translated to human clinical trials potentially due to lack of knowledge about the interaction between biomaterials and the body defense mechanism, the
Fabrication of scaffolds for bone-tissue regeneration
DOI:10.3390/ma12040568 URL [Cited within: 2]
3D-printed biomaterials for guided tissue regeneration
DOI:10.1002/smtd.v2.9 URL [Cited within: 2]
Electrospun materials as potential platforms for bone tissue engineering
DOI:10.1016/j.addr.2009.07.008
URL
PMID:19646493
[Cited within: 1]

Nanofibrous materials produced by electrospinning processes have attracted considerable interest in tissue regeneration, including bone reconstruction. A range of novel materials and processing tools have been developed to mimic the native bone extracellular matrix for potential applications as tissue engineering scaffolds and ultimately to restore degenerated functions of the bone. Degradable polymers, bioactive inorganics and their nanocomposites/hybrids nanofibers with suitable mechanical properties and bone bioactivity for osteoblasts and progenitor/stem cells have been produced. The surface functionalization with apatite minerals and proteins/peptides as well as drug encapsulation within the nanofibers is a promising strategy for achieving therapeutic functions with nanofibrous materials. Recent attempts to endow a 3D scaffolding technique to the electrospinning regime have shown some promise for engineering 3D tissue constructs. With the improvement in knowledge and techniques of bone-targeted nanofibrous matrices, bone tissue engineering is expected to be realized in the near future.
A biomimetic three-dimensional woven composite scaffold for functional tissue engineering of cartilage
URL PMID:17237789 [Cited within: 1]
Anatomically shaped tissue-engineered cartilage with tunable and inducible anticytokine delivery for biological joint resurfacing
DOI:10.1073/pnas.1601639113
URL
PMID:27432980
[Cited within: 1]

Biological resurfacing of entire articular surfaces represents an important but challenging strategy for treatment of cartilage degeneration that occurs in osteoarthritis. Not only does this approach require anatomically sized and functional engineered cartilage, but the inflammatory environment within an arthritic joint may also inhibit chondrogenesis and induce degradation of native and engineered cartilage. The goal of this study was to use adult stem cells to engineer anatomically shaped, functional cartilage constructs capable of tunable and inducible expression of antiinflammatory molecules, specifically IL-1 receptor antagonist (IL-1Ra). Large (22-mm-diameter) hemispherical scaffolds were fabricated from 3D woven poly(epsilon-caprolactone) (PCL) fibers into two different configurations and seeded with human adipose-derived stem cells (ASCs). Doxycycline (dox)-inducible lentiviral vectors containing eGFP or IL-1Ra transgenes were immobilized to the PCL to transduce ASCs upon seeding, and constructs were cultured in chondrogenic conditions for 28 d. Constructs showed biomimetic cartilage properties and uniform tissue growth while maintaining their anatomic shape throughout culture. IL-1Ra-expressing constructs produced nearly 1 microg/mL of IL-1Ra upon controlled induction with dox. Treatment with IL-1 significantly increased matrix metalloprotease activity in the conditioned media of eGFP-expressing constructs but not in IL-1Ra-expressing constructs. Our findings show that advanced textile manufacturing combined with scaffold-mediated gene delivery can be used to tissue engineer large anatomically shaped cartilage constructs that possess controlled delivery of anticytokine therapy. Importantly, these cartilage constructs have the potential to provide mechanical functionality immediately upon implantation, as they will need to replace a majority, if not the entire joint surface to restore function.
Osteogenesis and angiogenesis: the potential for engineering bone
DOI:10.22203/ecm.v015a08
URL
PMID:18454418
[Cited within: 1]

The repair of large bone defects remains a major clinical orthopaedic challenge. Bone is a highly vascularised tissue reliant on the close spatial and temporal connection between blood vessels and bone cells to maintain skeletal integrity. Angiogenesis thus plays a pivotal role in skeletal development and bone fracture repair. Current procedures to repair bone defects and to provide structural and mechanical support include the use of grafts (autologous, allogeneic) or implants (polymeric or metallic). These approaches face significant limitations due to insufficient supply, potential disease transmission, rejection, cost and the inability to integrate with the surrounding host tissue. The engineering of bone tissue offers new therapeutic strategies to aid musculoskeletal healing. Various scaffold constructs have been employed in the development of tissue-engineered bone; however, an active blood vessel network is an essential pre-requisite for these to survive and integrate with existing host tissue. Combination therapies of stem cells and polymeric growth factor release scaffolds tailored to promote angiogenesis and osteogenesis are under evaluation and development actively to stimulate bone regeneration. An understanding of the cellular and molecular interactions of blood vessels and bone cells will enhance and aid the successful development of future vascularised bone scaffold constructs, enabling survival and integration of bioengineered bone with the host tissue. The role of angiogenic and osteogenic factors in the adaptive response and interaction of osteoblasts and endothelial cells during the multi step process of bone development and repair will be highlighted in this review, with consideration of how some of these key mechanisms can be combined with new developments in tissue engineering to enable repair and growth of skeletal fractures. Elucidation of the processes of angiogenesis, osteogenesis and tissue engineering strategies offer exciting future therapeutic opportunities for skeletal repair and regeneration in orthopaedics.
Biodegradable polyester elastomers in tissue engineering
DOI:10.1517/14712598.4.6.801
URL
PMID:15174963
[Cited within: 1]

Tissue engineering often makes use of biodegradable scaffolds to guide and promote controlled cellular growth and differentiation in order to generate new tissue. There has been significant research regarding the effects of scaffold surface chemistry and degradation rate on tissue formation and the importance of these parameters is widely recognised. Nevertheless, studies describing the role of mechanical stimuli during tissue development and function suggest that the mechanical properties of the scaffold will also be important. In particular, scaffold mechanics should be taken into account if mechanical stimulation, such as cyclic strain, will be incorporated into strategies to grow improved tissues or the target tissue to be replaced has elastomeric properties. Biodegradable polyesters, such as polyglycolide, polylactide and poly(lactide-co-glycolide), although commonly used in tissue engineering, undergo plastic deformation and failure when exposed to long-term cyclic strain, limiting their use in engineering elastomeric tissues. This review will cover the latest advances in the development of biodegradable polyester elastomers for use as scaffolds to engineer tissues, such as heart valves and blood vessels.
Poly (lactic acid)-based biomaterials for orthopaedic regenerative engineering
DOI:10.1016/j.addr.2016.04.015
URL
PMID:27125191
[Cited within: 1]

Regenerative engineering converges tissue engineering, advanced materials science, stem cell science, and developmental biology to regenerate complex tissues such as whole limbs. Regenerative engineering scaffolds provide mechanical support and nanoscale control over architecture, topography, and biochemical cues to influence cellular outcome. In this regard, poly (lactic acid) (PLA)-based biomaterials may be considered as a gold standard for many orthopaedic regenerative engineering applications because of their versatility in fabrication, biodegradability, and compatibility with biomolecules and cells. Here we discuss recent developments in PLA-based biomaterials with respect to processability and current applications in the clinical and research settings for bone, ligament, meniscus, and cartilage regeneration.
Injectable biodegradable materials for orthopedic tissue engineering
DOI:10.1016/s0142-9612(00)00108-3
URL
PMID:11055288
[Cited within: 1]

The large number of orthopedic procedures performed each year, including many performed arthroscopically, have led to great interest in injectable biodegradable materials for regeneration of bone and cartilage. A variety of materials have been developed for these applications, including ceramics, naturally derived substances and synthetic polymers. These materials demonstrate overall biocompatibility and appropriate mechanical properties, as well as promote tissue formation, thus providing an important step towards minimally invasive orthopedic procedures. This review provides a comparison of these materials based on mechanical properties, biocompatibility and regeneration efficacy. Advantages and disadvantages of each material are explained and design criteria for injectable biodegradable systems are provided.
Healing bone using recombinant human bone morphogenetic protein 2 and copolymer
URL
PMID:10627737
[Cited within: 2]

Sixty patients with acetabular fractures were treated surgically. All fractures were a result of high energy trauma, most with significant associated injuries. Fifty-three of the patients were followed up for at least 2 years. Clinical outcome was analyzed clinically using the Harris hip score and radiographically. In 41 (77.4%) of the patients, the surgical procedure was judged successful (Harris hip score greater than 80 points). Three factors were found to be statistically significant predictors of such an outcome: patient age younger than 40 years; simple fractures based on the classification of Letournel and Judet; and absence of damage to the femoral head. Possible influential factors that were not found to be statistically significant in this population included additional injuries, immediate complications, quality of reduction, heterotopic ossification, ipsilateral femoral fracture, and sciatic nerve damage. Open reduction and internal fixation of the displaced acetabular fracture, although a demanding procedure, can result in a satisfactory clinical outcome given a consistent approach with a dedicated team.
Long-term stability of bone tissues induced by an osteoinductive biomaterial, recombinant human bone morphogenetic protein-2 and a biodegradable carrier
DOI:10.1016/j.biomaterials.2003.08.030
URL
PMID:14738843
[Cited within: 3]

The long-term stability of bone tissues induced by recombinant human bone morphogenetic protein-2 (rhBMP-2) and poly[L-lactide-co-glycolide] copolymer-coated gelatin sponge (PGS) was examined. In 16 dogs, 2.5 cm unilateral bone defects were created in the left tibial diaphyses. Tibia was fixed with metal plate, and PGS impregnated with (0.4 mg/cm(3)) or without rhBMP-2 was implanted into 15 or one defects, respectively. The metal plates of rhBMP-2-treated limbs were removed 16 weeks after the implantation. The bilateral tibiae of five animals each of the rhBMP-2-treated group were harvested at 32, 52 or 104 weeks, and served for biomechanical testing and histology. Although the defect that received PGS alone resulted in nonunion at 16 weeks, all defects treated with rhBMP-2 achieved radiographic bony union by 8 weeks. Biomechanical properties of the regenerated bones restored to the levels of intact tibiae at 32 weeks, but torsional stiffness was significantly higher. No statistical significances were detected in all parameters between regenerated and intact tibiae at 104 weeks. No radiographic and histological findings suggesting enhanced resorption to the regenerated bones were observed. These results suggest the long-term stability of the bone tissues induced by rhBMP-2, and the usefulness of rhBMP-2-impregnated PGS as a biomaterial for long bone defect filling.
Calcium oxalate: calcium phosphate transformations
DOI:10.1007/s00240-010-0292-3
URL
PMID:20625892

Knowledge of the physical-chemical mechanisms responsible for the crystal growth and dissolution events involved in stone formation might enable the manipulation of thermodynamics in such a way as to increase the solubility of sparingly soluble phases (such as calcium oxalates and phosphates), thereby reducing the driving force for stone formation. This may be accomplished through modification of pH, reduction of supersaturation with respect to nucleating phases, and the presence of key inhibitors. If these modifications are made during the initial stages of crystallite nucleation, they could potentially reduce the participation of phases such as Randall's plaques in stone formation.
Evolutionary screening of collagen-like peptides that nucleate hydroxyapatite crystals
DOI:10.1021/la104757g
URL
PMID:21291244
[Cited within: 1]

The biogenesis of inorganic/organic composite materials such as bone typically involves the process of templated mineralization. Biomimetic synthesis of bone-like materials therefore requires the development of organic scaffolds that mediate mineralization of hydroxyapatite (HAP), the major inorganic component of bone. Using phage display, we identified a 12-residue peptide that bound to single-crystal HAP and templated the nucleation and growth of crystalline HAP mineral in a sequence- and composition-dependent manner. The sequence responsible for the mineralizing activity resembled the tripeptide repeat (Gly-Pro-Hyp) of type I collagen, a major component of bone extracellular matrix. Using a panel of synthetic peptides, we defined the structural features required for mineralizing activity. The results support a model for the cooperative noncovalent interaction of the peptide with HAP and suggest that native collagen may have a mineral-templating function in vivo. We expect this short HAP-binding peptide to be useful in the synthesis of three-dimensional bone-like materials.
Biodegradable materials
DOI:10.1016/j.mporth.2017.07.011 URL [Cited within: 1]
A tissue engineering approach to bone repair in large animal models and in clinical practice
DOI:10.1016/j.biomaterials.2007.06.023
URL
PMID:17644173
[Cited within: 1]

The repair of large segmental bone defects due to trauma, inflammation and tumor surgery remains a major clinical problem. Animal models were developed to test bone repair by tissue engineering approaches, mimicking real clinical situations. Studies differed with regard to animals (dog, sheep, goat), treated bone (femur, tibia, mandible), chemistry and structure of the scaffolds. Still, an advantage in the bone formation and in the healing of the segmental defect was always observed when scaffolds were seeded with bone marrow derived stromal cells (BMSCs). In the year 1998 was performed the first implantation of a porous ceramic construct in a bone segmental defect of a patient; it was the first construct seeded with cultured autologous osteogenic cells. Since then, only few other similar cases were treated by the same approach. However, in other fields, such as oral and maxillofacial surgery, injectable cells/platelet-rich plasma composites have been used as grafting materials for maxillary sinus floor augmentation and/or onlay plasty. More recently, the reconstruction of a human mandible was also reported by means of a bone-muscle-flap in vivo prefabrication technique, where the patient served as his own bioreactor. Indeed continuous implementations test and provide new means of defects treatment and cure. However, based on results so far obtained in animal models and pilot clinical studies, one can affirm that the bone tissue engineering approaches, although successful in most cases, need further validation before a wide application in clinics. In particular, the supply of oxygen and nutrients to the cells in the inner part of the implanted scaffolds remains a major concern, requiring additional investigations.
A tissue engineering solution for segmental defect regeneration in load-bearing long bones
Hyperelastic “bone”: A highly versatile, growth factor-free, osteoregenerative, scalable, and surgically friendly biomaterial
DOI:10.1126/scitranslmed.aaf7704
URL
PMID:27683552
[Cited within: 1]

Despite substantial attention given to the development of osteoregenerative biomaterials, severe deficiencies remain in current products. These limitations include an inability to adequately, rapidly, and reproducibly regenerate new bone; high costs and limited manufacturing capacity; and lack of surgical ease of handling. To address these shortcomings, we generated a new, synthetic osteoregenerative biomaterial, hyperelastic
Potentiation of the activity of bone morphogenetic protein-2 in bone regeneration by a PLA-PEG/hydroxyapatite composite
DOI:10.1016/j.biomaterials.2004.02.010
URL
PMID:15193882
[Cited within: 2]

Bone morphogenetic proteins (BMPs) are biologically active molecules capable of inducing new bone formation, and show potential for clinical use in bone defect repair. However, an ideal system for delivering BMPs that can potentiate their bone-inducing ability and provide initial mechanical strength and scaffold for bone ingrowth has not yet been developed. In this study, to construct a carrier/scaffold system for BMPs, we combined two biomaterials: interconnected-porous calcium hydroxyapatite ceramics (IP-CHA), and the synthetic biodegradable polymer poly D,L,-lactic acid-polyethyleneglycol block co-polymer (PLA-PEG). We used a rabbit radii model to evaluate the bone-regenerating efficacy of rhBMP-2/PLA-PEG/IP-CHA composite. At 8 weeks after implantation, all bone defects in groups treated with 5 or 20 microg of BMP were completely repaired with sufficient strength. Furthermore, using this carrier scaffold system, we reduced the amount of BMP necessary for such results to about a tenth of the amount needed in previous studies, probably due to the superior osteoconduction ability of IP-CHA and the optimal drug delivery system provided by PLA-PEG, inducing new bone formation in the interconnected pores. The present findings indicate that the synthetic biodegradable polymer/IP-CHA composite is an excellent combination carrier/scaffold delivery system for rhBMP-2, and that it strongly promotes the clinical effects of rhBMP-2 in bone tissue regeneration.
Repair of an intercalated long bone defect with a synthetic biodegradable bone-inducing implant
DOI:10.1016/j.biomaterials.2005.01.054
URL
PMID:15792541
[Cited within: 3]

Recombinant human bone morphogenetic protein (rhBMP)-2 in a block copolymer composed of poly-D,L-lactic acid with randomly inserted p-dioxanone and polyethylene glycol (PLA-DX-PEG) as a carrier and porous beta-tricalcium phosphate (beta-TCP) blocks were used to generate a new fully absorbable osteogenic biomaterial. The bone regenerability of the rhBMP-2/PLA-DX-PEG/beta-TCP composite was studied in a critical-sized rabbit bone defect model. In an initial study, a composite of PLA-DX-PEG (250 mg) and beta-TCP (300 mg) loaded with or without rhBMP2 (50 microg) was implanted into a 1.5 cm intercalated bone defect created in a rabbit femur. Defects were assessed by biweekly radiography until 8 weeks postoperatively. The bony union of the defect was recognized only in the BMP-loaded group. To obtain further data on biomechanical and remodeling properties, another BMP-loaded composites group was made and observed up to 24 weeks. All defects were completely repaired without residual traces of implants. Anatomical and mechanical properties of the repaired bone examined by histology, 3-dimensional CT (3D-CT) and mechanical testing were essentially equivalent to the nonoperated-on femur at 24 weeks. These experimental results indicate that fully absorbable rhBMP-2/PLA-DX-PEG/beta-TCP is a promising composite having osteogenicity efficient enough for repairing large bone defects.
Facile stem cell delivery to bone grafts enabled by smart shape recovery and stiffening of degradable synthetic periosteal membranes
DOI:10.1002/adfm.v27.5 URL [Cited within: 3]
Shape recovery with concomitant mechanical strengthening of amphiphilic shape memory polymers in warm water
DOI:10.1021/acsami.6b14167
URL
PMID:28125208
[Cited within: 1]

Maintaining adequate or enhancing mechanical properties of shape memory polymers (SMPs) after shape recovery in an aqueous environment are greatly desired for biomedical applications of SMPs as self-fitting tissue scaffolds or minimally invasive surgical implants. Here we report stable temporary shape fixing and facile shape recovery of biodegradable triblock amphiphilic SMPs containing a poly(ethylene glycol) (PEG) center block and flanking poly(lactic acid) or poly(lactic-co-glycolic acid) blocks in warm water, accompanied by concomitant enhanced mechanical strengths. Differential scanning calorimetry (DSC), wide-angle X-ray diffraction (WXRD), and small-angle X-ray scattering (SAXS) analyses revealed that the unique stiffening of the amphiphilic SMPs upon hydration was due to hydration-driven microphase separation and PEG crystallization. We further demonstrated that the chemical composition of degradable blocks in these SMPs could be tailored to affect the persistence of hydration-induced stiffening upon subsequent dehydration. These properties combined open new horizons for these amphiphilic SMPs for smart weight-bearing in vivo applications (e.g., as self-fitting intervertebral discs). This study also provides a new material design strategy to strengthen polymers in aqueous environment in general.
Rapid prototyping amphiphilic polymer/hydroxyapatite composite scaffolds with hydration-induced self-fixation behavior
DOI:10.1089/ten.TEC.2014.0213
URL
PMID:25025950
[Cited within: 3]

Two major factors hampering the broad use of rapid prototyped biomaterials for tissue engineering applications are the requirement for custom-designed or expensive research-grade three-dimensional (3D) printers and the limited selection of suitable thermoplastic biomaterials exhibiting physical characteristics desired for facile surgical handling and biological properties encouraging tissue integration. Properly designed thermoplastic biodegradable amphiphilic polymers can exhibit hydration-dependent hydrophilicity changes and stiffening behavior, which may be exploited to facilitate the surgical delivery/self-fixation of the scaffold within a physiological tissue environment. Compared to conventional hydrophobic polyesters, they also present significant advantages in blending with hydrophilic osteoconductive minerals with improved interfacial adhesion for bone tissue engineering applications. Here, we demonstrated the excellent blending of biodegradable, amphiphilic poly(D,L-lactic acid)-poly(ethylene glycol)-poly(D,L-lactic acid) (PLA-PEG-PLA) (PELA) triblock co-polymer with hydroxyapatite (HA) and the fabrication of high-quality rapid prototyped 3D macroporous composite scaffolds using an unmodified consumer-grade 3D printer. The rapid prototyped HA-PELA composite scaffolds and the PELA control (without HA) swelled (66% and 44% volume increases, respectively) and stiffened (1.38-fold and 4-fold increases in compressive modulus, respectively) in water. To test the hypothesis that the hydration-induced physical changes can translate into self-fixation properties of the scaffolds within a confined defect, a straightforward in vitro pull-out test was designed to quantify the peak force required to dislodge these scaffolds from a simulated cylindrical defect at dry versus wet states. Consistent with our hypothesis, the peak fixation force measured for the PELA and HA-PELA scaffolds increased 6-fold and 15-fold upon hydration, respectively. Furthermore, we showed that the low-fouling 3D PELA inhibited the attachment of NIH3T3 fibroblasts or bone marrow stromal cells while the HA-PELA readily supported cellular attachment and osteogenic differentiation. Finally, we demonstrated the feasibility of rapid prototyping biphasic PELA/HA-PELA scaffolds for potential guided bone regeneration where an osteoconductive scaffold interior encouraging osteointegration and a nonadhesive surface discouraging fibrous tissue encapsulation is desired. This work demonstrated that by combining facile and readily translatable rapid prototyping approaches with unique biomaterial designs, biodegradable composite scaffolds with well-controlled macroporosities, spatially defined biological microenvironment, and useful handling characteristics can be developed.
An amphiphilic degradable polymer/hydroxyapatite composite with enhanced handling characteristics promotes osteogenic gene expression in bone marrow stromal cells
DOI:10.1016/j.actbio.2013.06.013
URL
PMID:23791675
[Cited within: 2]

Electrospun polymer/hydroxyapatite (HA) composites combining biodegradability with osteoconductivity are attractive for skeletal tissue engineering applications. However, most biodegradable polymers such as poly(lactic acid) (PLA) are hydrophobic and do not blend with adequate interfacial adhesion with HA, compromising the structural homogeneity, mechanical integrity and biological performance of the composite. To overcome this challenge, we combined a hydrophilic polyethylene glycol (PEG) block with poly(d,l-lactic acid) to improve the adhesion of the degradable polymer with HA. The amphiphilic triblock copolymer PLA-PEG-PLA (PELA) improved the stability of HA-PELA suspension at 25wt.% HA content, which was readily electrospun into HA-PELA composite scaffolds with uniform fiber dimensions. HA-PELA was highly extensible (failure strain>200% vs. <40% for HA-PLA), superhydrophilic ( approximately 0 degrees water contact angle vs. >100 degrees for HA-PLA), and exhibited an 8-fold storage modulus increase (unlike deterioration for HA-PLA) upon hydration, owing to the favorable interaction between HA and PEG. HA-PELA also better promoted osteochondral lineage commitment of bone marrow stromal cells in unstimulated culture and supported far more potent osteogenic gene expression upon induction than HA-PLA. We demonstrate that the chemical incorporation of PEG is an effective strategy to improve the performance of degradable polymer/HA composites for bone tissue engineering applications.
Shape memory performance of thermoplastic amphiphilic triblock copolymer poly(D,L-lactic acid-co-ethylene glycol-co-D,L-lactic acid) (PELA)/hydroxyapatite composites
DOI:10.1002/macp.201400340
URL
PMID:26457046
[Cited within: 1]

Biodegradable polymer/hydroxyapatite (HA) composites are desired for skeletal tissue engineering. When engineered with thermal-responsive shape memory properties, they may be delivered in a minimally invasive temporary shape and subsequently triggered to conform to a tissue defect. Here we report the shape memory properties of thermoplastic amphiphilic poly(D,L-lactic acid-co-ethylene glycol-co-D,L-lactic acid) (PELA, 120 kDa) and HA-PELA composites. These materials can be cold-deformed and stably fixed into temporary shapes at room temperature and undergo rapid shape recovery (< 3 s) at 50 degrees C. Stable fixation (>99% fixing ratio) of large deformations is achieved at -20 degrees C. While the shape recovery from tensile deformations slows with higher HA contents, all composites (up to 20 wt% HA) achieve high shape recovery (>90%) upon 10-min equilibration at 50 degrees C. The permanent shapes of HA-PELA can be reprogramed at 50 degrees C, and macroporous shape memory scaffolds can be fabricated by rapid prototyping.
Templated repair of long bone defects in rats with bioactive spiral-wrapped electrospun amphiphilic polymer/hydroxyapatite scaffolds
URL PMID:25695310 [Cited within: 1]
Multifunctional scaffolds for facile implantation, spontaneous fixation, and accelerated long bone regeneration in rodents
DOI:10.1126/scitranslmed.aau7411
URL
PMID:31341064
[Cited within: 5]

Graft-guided regenerative repair of critical long bone defects achieving facile surgical delivery, stable graft fixation, and timely restoration of biomechanical integrity without excessive biotherapeutics remains challenging. Here, we engineered hydration-induced swelling/stiffening and thermal-responsive shape-memory properties into scalable, three-dimensional-printed amphiphilic degradable polymer-osteoconductive mineral composites as macroporous, non-load-bearing, resorbable synthetic grafts. The distinct physical properties of the grafts enabled straightforward surgical insertion into critical-size rat femoral segmental defects. Grafts rapidly recovered their precompressed shape, stiffening and swelling upon warm saline rinse to result in 100% stable graft fixation. The osteoconductive macroporous grafts guided bone formation throughout the defect as early as 4 weeks after implantation; new bone remodeling correlated with rates of scaffold composition-dependent degradation. A single dose of 400-ng recombinant human bone morphogenetic protein-2/7 heterodimer delivered via the graft accelerated bone regeneration bridging throughout the entire defect by 4 weeks after delivery. Full restoration of torsional integrity and complete scaffold resorption were achieved by 12 to 16 weeks after surgery. This biomaterial platform enables personalized bone regeneration with improved surgical handling, in vivo efficacy and safety.
Characterization of partially saturated poly(propylene fumarate) for orthopaedic application
URL PMID:9342654 [Cited within: 1]
Review: tissue engineering for regeneration of articular cartilage
DOI:10.1016/s0142-9612(99)00213-6
URL
PMID:10674807
[Cited within: 1]

Joint pain due to cartilage degeneration is a serious problem, affecting people of all ages. Although many techniques, often surgical, are currently employed to treat this affliction, none have had complete success. Recent advances in biology and materials science have pushed tissue engineering to the forefront of new cartilage repair techniques. This review seeks to condense information for the biomaterialist interested in developing materials for this application. Articular cartilage anatomy, types of injury, and current repair methods are explained. The need for biomaterials, current commonly used materials for tissue-engineered cartilage, and considerations in scale-up of cell-biomaterial constructs are summarized.
Biodegradable bone cement compositions based on acrylate and epoxide terminated poly(propylene fumarate) oligomers and calcium salt compositions
DOI:10.1016/0142-9612(96)89657-8
URL
PMID:8938235
[Cited within: 1]

The synthesis of biodegradable bone cement compositions is presented. These bone cement compositions can be applied as a putty-like mixture and harden to a strong material in a bone fracture. They degrade from the site of application to allow the ingrowth of new bone for complete healing of the bone fracture. The bone cement is composed of a solid particulate phase dispersed in an initially liquid polymeric phase, which can be hardened by cross-linking. The polymeric phase is a low-molecular-weight liquid poly(propylene fumarate) (PPF) containing double bonds available for cross-linking. The solid particulate phase consists of calcium carbonate and tricalcium phosphate. PPF oligomers of Mw = 1800 and Mn = 750 were prepared from the condensation of non-volatile bis(2-hydroxypropyl fumarate) and propylene-bis(hydrogen maleate) trimers. PPF terminated divinyl and diepoxide derivatives were obtained from the reactions between PPF diol and acryloyl chloride or epichlorhydrin, respectively. Putty-like cement compositions were prepared from a mixture of 30 wt% polymer phase containing benzoyl peroxide-dimethyl toluidine as polymerization catalyst and 70 wt% calcium salts. The divinyl and diepoxide terminated PPF oligomers provided a high strength composition of between 30 and 129 MPa which is suitable for bone cement applications. In vitro hydrolysis of the composites showed little weight loss with the compressive strength remaining above 20 MPa after 4 weeks in buffer solution. Compositions of the PPF oligomers cross-linked without calcium salts showed a gradual weight loss (10-65 wt% after 4 weeks) when placed in buffer solution followed by high water absorption (18-200 wt% after 4 weeks), with the epoxide terminated PPF being the least to degrade or absorb water.
Crosslinking characteristics of an injectable poly(propylene fumarate)/beta-tricalcium phosphate paste and mechanical properties of the crosslinked composite for use as a biodegradable bone cement
URL PMID:10397934 [Cited within: 1]
Fabrication and characterization of poly(propylene fumarate) scaffolds with controlled pore structures using 3-dimensional printing and injection molding
DOI:10.1089/ten.2006.12.2801
URL
PMID:17518649
[Cited within: 1]

Poly(propylene fumarate) (PPF) is an injectable, biodegradable polymer that has been used for fabricating preformed scaffolds in tissue engineering applications because of in situ crosslinking characteristics. Aiming for understanding the effects of pore structure parameters on bone tissue ingrowth, 3-dimensional (3D) PPF scaffolds with controlled pore architecture have been produced in this study from computer-aided design (CAD) models. We have created original scaffold models with 3 pore sizes (300, 600, and 900 microm) and randomly closed 0%, 10%, 20%, or 30% of total pores from the original models in 3 planes. PPF scaffolds were fabricated by a series steps involving 3D printing of support/build constructs, dissolving build materials, injecting PPF, and dissolving support materials. To investigate the effects of controlled pore size and interconnectivity on scaffolds, we compared the porosities between the models and PPF scaffolds fabricated thereby, examined pore morphologies in surface and cross-section using scanning electron microscopy, and measured permeability using the falling head conductivity test. The thermal properties of the resulting scaffolds as well as uncrosslinked PPF were determined by differential scanning calorimetry and thermogravimetric analysis. Average pore sizes and pore shapes of PPF scaffolds with 600- and 900-microm pores were similar to those of CAD models, but they depended on directions in those with 300-microm pores. Porosity and permeability of PPF scaffolds decreased as the number of closed pores in original models increased, particularly when the pore size was 300 microm as the result of low porosity and pore occlusion. These results show that 3D printing and injection molding technique can be applied to crosslinkable polymers to fabricate 3D porous scaffolds with controlled pore structures, porosity, and permeability using their CAD models.
Biodegradable composite scaffolds incorporating an intramedullary rod and delivering bone morphogenetic protein-2 for stabilization and bone regeneration in segmental long bone defects
DOI:10.1016/j.actbio.2011.06.043
URL
PMID:21757034
[Cited within: 2]

In this study, a two-part bone tissue engineering scaffold was investigated. The scaffold consists of a solid poly(propylene fumarate) (PPF) intramedullary rod for mechanical support surrounded by a porous PPF sleeve for osseointegration and delivery of poly(dl-lactic-co-glycolic acid) (PLGA) microspheres with adsorbed recombinant human bone morphogenetic protein-2 (rhBMP-2). Scaffolds were implanted into critical size rat segmental femoral defects with internal fixation for 12 weeks. Bone formation was assessed throughout the study via radiography, and following euthanasia, via microcomputed tomography and histology. Mechanical stabilization was evaluated further via torsional testing. Experimental implant groups included the PPF rod alone and the rod with a porous PPF sleeve containing PLGA microspheres with 0, 2 or 8 mug of rhBMP-2 adsorbed onto their surface. Results showed that presence of the scaffold increased mechanical stabilization of the defect, as evidenced by the increased torsional stiffness of the femurs by the presence of a rod compared to the empty defect. Although the presence of a rod decreased bone formation, the presence of a sleeve combined with a low or high dose of rhBMP-2 increased the torsional stiffness to 2.06 +/- 0.63 and 1.68 +/- 0.56 N.mm, respectively, from 0.56 +/- 0.24 N.mm for the rod alone. The results indicate that, while scaffolds may provide structural support to regenerating tissues and increase their mechanical properties, the presence of scaffolds within defects may hinder overall bone formation if they interfere with cellular processes.
Effect of varied release kinetics of the osteogenic thrombin peptide TP508 from biodegradable, polymeric scaffolds on bone formation in vivo
DOI:10.1002/jbm.a.30265
URL
PMID:15666357
[Cited within: 2]

This study was designed to assess the influence of varied release kinetics of the osteogenic thrombin peptide TP508 from osteoconductive poly(propylene fumarate)-based (PPF) composite scaffolds on bone formation in vivo. Four classes of scaffolds were constructed with different TP508 dosages (200, 100, or 0 microg) and release kinetics (large burst release, minimal burst release, or no release) and implanted in 15.0 mm segmental defects in rabbit radii. The animals were euthanized at 12 weeks and the implants were analyzed by light microscopy, histological scoring analysis, and histomorphometric analysis. Samples from all classes displayed bone growth within the pores of the scaffold near the edges of the defect. In areas where bone was not observed, the pores were filled with mostly fibrous tissue and exhibited minimal inflammatory response for all classes. In contrast to other scaffold classes, scaffolds containing a total dose of 200 microg TP508 and exhibiting a large burst release profile showed statistically more bone formation guided along the surface of the scaffold, with these scaffolds averaging 80% of the defect length bridged with bone compared to 10% or less bridged for the other scaffold classes. These results demonstrate that the extent of in vivo bone formation in response to controlled release from PPF-composite scaffolds is determined by the release kinetics of the incorporated osteogenic peptide.
Polyanhydrides: an overview
DOI:10.1016/s0169-409x(02)00050-9
URL
PMID:12384314
[Cited within: 1]

Polyanhydrides have been considered to be useful biomaterials as carriers of drugs to various organs of the human body such as brain, bone, blood vessels, and eyes. They can be prepared easily from available, low cost resources and can be manipulated to meet desirable characteristics. Polyanhydrides are biocompatible and degrade in vivo into non-toxic diacid counterparts that are eliminated from the body as metabolites. Owing to their usefulness, this review focuses on the development, synthesis methods, structures and characterization of polyanhydrides, which will provide an overview for the researchers in the field. Their in vitro and in vivo degradability, toxicity, biocompatibility and applications are discussed in the subsequent chapters of this special issue on polyanhydrides and poly(ortho esters).
Recent advances in polyanhydride based biomaterials
DOI:10.1002/adma.201706815
URL
PMID:29707879
[Cited within: 1]

This review focusses on recent developments of polyanhydrides, a class of degradable synthetic biopolymers. Polyanhydrides have been used as carriers for controlled delivery of drugs. A polyanhydride copolymer of carboxyphenoxy propane and sebacic acid has been used in Gliadel brain tumor implants for the controlled delivery of carmustine or bis-chloroethylnitrosourea. They are easy and inexpensive to synthesize (especially scale up). However, polyanhydrides possess a short shelf-life. Hydrolytic cleavage and anhydride interchanges lower their molecular weights during storage. One of the highlights in recent developments of polyanhydride chemistry is the discovery of alternating copolymers having extended shelf-life. Other highlights include their applications in biomedical electronics, vaccine delivery, and nano/micro particulate delivery systems. This review examines approaches for polyanhydride synthesis followed by their recent developments in biomedical applications.
Toxicity, biodegradation and elimination of polyanhydrides
DOI:10.1016/s0169-409x(02)00052-2
URL
PMID:12384316
[Cited within: 1]

Although originally developed for the textile industry, polyanhydrides have found extensive use in biomedical applications due to their biodegradability and excellent biocompatibility. Polyanhydrides are most commonly synthesized from diacid monomers by polycondensation. Efficient control over various physicochemical properties, such as biodegradability and biocompatibility, can be achieved for this class of polymers, due to the availability of a wide variety of diacid monomers as well as by copolymerization of these monomers. Biodegradation of these polymers takes place by the hydrolysis of the anhydride bonds and the polymer undergoes predominantly surface erosion, a desired property to attain near zero-order drug release profile. This review examines the mode of degradation and elimination of these polyanhydrides in vivo as well as the biocompatibility and toxicological aspects of various polyanhydrides.
Salicylic acid-based polymers for guided bone regeneration using bone morphogenetic protein-2
URL PMID:25813520 [Cited within: 3]
Nonunion of the femoral diaphysis. The influence of reaming and non-steroidal anti-inflammatory drugs
DOI:10.1302/0301-620x.82b5.9899
URL
PMID:10963160
[Cited within: 1]

We assessed factors which may affect union in 32 patients with nonunion of a fracture of the diaphysis of the femur and 67 comparable patients whose fracture had united. These included gender, age, smoking habit, the use of non-steroidal anti-inflammatory drugs (NSAIDs) the type of fracture (AO classification), soft-tissue injury (open or closed), the type of nail, the mode of locking, reaming nu non-reaming, infection, failure of the implant, distraction at the fracture site, and the time to full weight-bearing. Patients with severe head injuries were excluded. Both groups were comparable with regard to gender, Injury Severity Score and soft-tissue injury. There was no relationship between the rate of union and the type of implant, mode of locking, reaming, distraction or smoking. There were fewer cases of nonunion in more comminuted fractures (type C) and in patients who were able to bear weight early. There was a marked association between nonunion and the use of NSAIDs after injury (p = 0.000001) and delayed healing was noted in patients who took NSAIDs and whose fractures had united.
COX-2 selective NSAID decreases bone ingrowth in vivo
DOI:10.1016/S0736-0266(02)00079-7
URL
PMID:12472224

Whether non-steroidal anti-inflammatory drug (NSAID)-induced suppression of bone ingrowth is due to cyclooxygenase-1 (COX-1) inhibition, cyclooxygenase-2 (COX-2) inhibition, or through a yet unidentified pathway is unknown. In this study, the effects of a non-specific COX-1 and COX-2 inhibitor, versus a specific COX-2 inhibitor on bone ingrowth and tissue differentiation are examined in vivo. Harvest chambers were implanted unilaterally in the tibiae of eight mature, New Zealand white rabbits. After a 6-week period for osseointegration of the chamber, the following oral treatments were given for 4 weeks each, followed by a harvest in each case: drinking water with no NSAID (control 1), Naproxen sodium--a COX-1 and COX-2 non-specific inhibitor at a dose of 110 mg/kg/day in the drinking water, drinking water with no NSAID (control 2), and Rofecoxib-a COX-2 inhibitor at a dose of 12.5 mg/day inserted directly into the rabbit's mouth. Harvested specimens were snap frozen, cut into serial 6 microm sections and stained with hematoxylin and eosin for general morphological characterization, and alkaline phosphatase (osteoblast marker). Sections were also processed for immunoperoxidase staining using monoclonal antibodies to identify cells expressing the vitronectin receptor (osteoclast-like cells). With drinking water alone, the percentage of bone ingrowth averaged 24.8 +/- 2.9% and 29.9 +/- 4.5% respectively. Naproxen sodium in the drinking water and oral Rofecoxib decreased bone ingrowth significantly (15.9 +/- 3.3%. p = 0.031 and 18.5 +/- 2+/-4%, p = 0.035 compared to drinking water respectively). Both Naproxen sodium (p = 0.026) and Rofecoxib (p = 0.02) decreased the number of CD51 positive osteoclast-like cells per section compared with drinking water alone. Rofecoxib decreased the area of osteoblasts per section area (p = 0.014) compared to controls, although the value for Naproxen sodium did not reach statistical significance. The results of the present study suggest that bone formation is suppressed by oral administration of an NSAID which contains a COX-2 inhibitor. COX-2 inhibitors currently taken for arthritis and other conditions may potentially delay fracture healing and bone ingrowth.
Dose and time-dependent effects of cyclooxygenase-2 inhibition on fracture-healing
DOI:10.2106/JBJS.F.00127
URL
PMID:17332098
[Cited within: 1]

BACKGROUND: Fracture-healing is impaired in mice lacking a functional cyclooxygenase-2 (COX-2) gene or in rats continuously treated with COX-2 inhibitors. These observations indicate that COX-2 is a critical regulator of fracture repair. Nonsteroidal anti-inflammatory drugs are commonly used to treat pain associated with musculoskeletal trauma and disease. Nonsteroidal anti-inflammatory drugs inhibit COX-2 function and in so doing can impair fracture-healing. The goal of the present study was to determine how variations in nonsteroidal anti-inflammatory drug therapy ultimately affect fracture-healing. METHODS: Closed femoral fractures were made in female Sprague-Dawley rats. The rats were treated with different doses of celecoxib (a COX-2-selective nonsteroidal anti-inflammatory drug) or were treated for different periods before or after fracture with celecoxib. Eight weeks after the fracture, healing was assessed with radiography and destructive torsional mechanical testing. The effect of celecoxib treatment on fracture callus prostaglandin E2 and F(2alpha) levels was determined as a measure of cyclooxygenase activity. RESULTS: Celecoxib doses as small as 2 mg/kg/day reduced fracture callus mechanical properties and caused a significant increase in the proportion of nonunions. Similarly, treatment with celecoxib at a dose of 4 mg/kg/day for just five days reduced fracture callus mechanical properties and significantly increased the proportion of nonunions. Conversely, celecoxib therapy prior to fracture or initiated fourteen days after fracture did not significantly increase the proportion of nonunions. Celecoxib treatment at a dose of 4 mg/kg/day reduced fracture callus prostaglandin E2 and F(2alpha) levels by >60%. CONCLUSIONS: COX-2-selective nonsteroidal anti-inflammatory drug therapy during the early stages of fracture repair significantly reduced fracture callus mechanical properties at later stages of healing and increased the proportion of nonunions in this animal model.
Bio-based polycarbonate as synthetic toolbox
DOI:10.1038/ncomms11862
URL
PMID:27302694
[Cited within: 1]

Completely bio-based poly(limonene carbonate) is a thermoplastic polymer, which can be synthesized by copolymerization of limonene oxide (derived from limonene, which is found in orange peel) and CO2. Poly(limonene carbonate) has one double bond per repeating unit that can be exploited for further chemical modifications. These chemical modifications allow the tuning of the properties of the aliphatic polycarbonate in nearly any direction. Here we show synthetic routes to demonstrate that poly(limonene carbonate) is the perfect green platform polymer, from which many functional materials can be derived. The relevant examples presented in this study are the transformation from an engineering thermoplastic into a rubber, addition of permanent antibacterial activity, hydrophilization and even pH-dependent water solubility of the polycarbonate. Finally, we show a synthetic route to yield the completely saturated counterpart that exhibits improved heat processability due to lower reactivity.
Exposure to bisphenol A and other phenols in neonatal intensive care unit premature infants
DOI:10.1289/ehp.0800265
URL
PMID:19440505
[Cited within: 1]

OBJECTIVE: We previously demonstrated that exposure to polyvinyl chloride plastic medical devices containing di(2-ethylhexyl) phthalate (DEHP) was associated with higher urinary concentrations of several DEHP metabolites in 54 premature infants in two neonatal intensive care units than in the general population. For 42 of these infants, we evaluated urinary concentrations of several phenols, including bisphenol A (BPA), in association with the use of the same medical devices. MEASUREMENTS: We measured the urinary concentrations of free and total (free plus conjugated) species of BPA, triclosan, benzophenone-3, methyl paraben, and propyl paraben. RESULTS: The percentage of BPA present as its conjugated species was > 90% in more than three-quarters of the premature infants. Intensity of use of products containing DEHP was strongly associated with BPA total concentrations but not with any other phenol. Adjusting for institution and sex, BPA total concentrations among infants in the group of high use of DEHP-containing products were 8.75 times as high as among infants in the low use group (p < 0.0001). Similarly, after adjusting for sex and DEHP-containing product use category, BPA total concentrations among infants in Institution A were 16.6 times as high as those among infants in Institution B (p < 0.0001). CONCLUSION: BPA geometric mean urinary concentration (30.3 microg/L) among premature infants undergoing intensive therapeutic medical interventions was one order of magnitude higher than that among the general population. Conjugated species were the primary urinary metabolites of BPA, suggesting that premature infants have some capacity to metabolize BPA. The differences in exposure to BPA by intensity of use of DEHP-containing medical products highlight the need for further studies to determine the specific source(s) of exposure to BPA.
Chemical recycling of poly(bisphenol A carbonate)
DOI:10.1039/C9PY01927H URL [Cited within: 1]
Copolymers of trimethylene carbonate and epsilon-caprolactone for porous nerve guides: synjournal and properties
DOI:10.1163/156856201744434
URL
PMID:11334188
[Cited within: 1]

Copolymers of trimethylene carbonate and epsilon-caprolactone were synthesized and characterized with the aim of assessing their potential in the development of a flexible and slowly degrading artificial nerve guide for the bridging of large nerve defects. The effect of the monomer ratio on the physical properties of the polymers and its influence on the processability of the materials was investigated. Under the applied polymerization conditions (130 degrees C, 3 days using stannous octoate as a catalyst) high molecular weight polymers (Mn above 93 000) were obtained. All copolymers had glass transition temperatures below room temperature. At trimethylene carbonate contents higher than 25 mol% no crystallinity was detected. A decrease in crystallinity resulted in the loss of strength and decrease in toughness, as well as in an increased polymer wettability. Amorphous poly(trimethylene carbonate), however, showed excellent ultimate mechanical properties due to strain-induced crystallization (Tm = 36 degrees C). Low crystallinity copolymers could be processed into dimensionally stable porous structures by means of immersion precipitation and by combination of this technique with the use of porosifying agents. Porous membranes of poly(trimethylene carbonate) could be prepared when blended with small amounts of high molecular weight poly(ethylene oxide). Poly(trimethylene carbonate) and poly(trimethylene carbonate-co-epsilon-caprolactone) copolymers with high epsilon-caprolactone content possess good physical properties and are processable into porous structures. These materials are most suitable for the preparation of porous artificial nerve guides.
In vitro degradation of trimethylene carbonate based (co)polymers
DOI:10.1002/mabi.200290000 URL
Aliphatic cyclic carbonates and spiroorthocarbonates as monomers
DOI:10.1016/S0079-6700(00)00006-X URL
Ultraviolet light crosslinking of poly(trimethylene carbonate) for elastomeric tissue engineering scaffolds
DOI:10.1016/j.biomaterials.2010.07.102
URL
PMID:20739060

A practical method of photocrosslinking high molecular weight poly(trimethylene carbonate)(PTMC) is presented. Flexible, elastomeric and biodegradable networks could be readily prepared by UV irradiating PTMC films containing pentaerythritol triacrylate (PETA) and a photoinitiator. The network characteristics, mechanical properties, wettability, and in vitro enzymatic erosion of the photocrosslinked PTMC films were investigated. Densely crosslinked networks with gel contents up to 98% could be obtained in this manner. Upon photocrosslinking, flexible and tough networks with excellent elastomeric properties were obtained. To illustrate the ease with which the properties of the networks can be tailored, blends of PTMC with mPEG-PTMC or with PTMC-PCL-PTMC were also photocrosslinked. The wettability and the enzymatic erosion rate of the networks could be tuned by blending with block copolymers. Tissue engineering scaffolds were also fabricated using these flexible photocrosslinkable materials. After crosslinking, the fabricated PTMC-based scaffolds showed inter-connected pores and extensive microporosity. Human mesenchymal stem cell (hMSC) culturing studies showed that the photocrosslinked scaffolds prepared from PTMC and PTMC/PTMC-PCL-PTMC blends are well-suited for tissue engineering applications.
Oligo(trimethylene carbonate)-based supramolecular biomaterials
Synthesis, properties, and biodegradation of poly(1,3-trimethylene carbonate)
Construction of functional aliphatic polycarbonates for biomedical applications
DOI:10.1016/j.progpolymsci.2011.07.008 URL [Cited within: 1]
Biodegradable poly(butylene-carbonate) porous membranes for guided bone regeneration: In vitro and in vivo studies
DOI:10.1177/0883911513509471 URL [Cited within: 1]
Poly(trimethylene carbonate) and biphasic calcium phosphate composites for orbital floor reconstruction: a feasibility study in sheeP
DOI:10.22203/ecm.v027a07
URL
PMID:24488822
[Cited within: 1]

In the treatment of orbital floor fractures, bone is ideally regenerated. The materials currently used for orbital floor reconstruction do not lead to the regeneration of bone. Our objective was to render polymeric materials based on poly(trimethylene carbonate) (PTMC) osteoinductive, and to evaluate their suitability for use in orbital floor reconstruction. For this purpose, osteoinductive biphasic calcium phosphate (BCP) particles were introduced into a polymeric PTMC matrix. Composite sheets containing 50 wt% BCP particles were prepared. Also laminates with poly(D,L-lactide) (PDLLA) were prepared by compression moulding PDLLA films onto the composite sheets. After sterilisation by gamma irradiation, the sheets were used to reconstruct surgically-created orbital floor defects in sheep. The bone inducing potential of the different implants was assessed upon intramuscular implantation. The performance of the implants in orbital floor reconstruction was assessed by cone beam computed tomography (CBCT). Histological evaluation revealed that in the orbital and intramuscular implantations of BCP containing specimens, bone formation could be seen after 3 and 9 months. Analysis of the CBCT scans showed that the composite PTMC sheets and the laminated composite sheets performed well in orbital floor reconstruction. It is concluded that PTMC/BCP composites and PTMC/BCP composites laminated with PDLLA have osteoinductive properties and seem suitable for use in orbital floor reconstruction.
Grafting BSA onto poly[(L-lactide)-co-carbonate] microspheres by click chemistry
DOI:10.1002/mabi.200700306
URL
PMID:18401865
[Cited within: 2]

Model protein bovine serum albumin (BSA) was covalently grafted onto poly[(L-lactide)-co-carbonate] microsphere surfaces by
A versatile monomer for preparing well-defined functional polycarbonates and poly(ester-carbonates)
DOI:10.1021/ma200021m
URL
PMID:21686053
[Cited within: 1]

Despite the increasing demands for functional degradable biomaterials, strategies for generating materials with modular compositions and well-defined functionalities from common building blocks are still lacking. Here we report an azido-functionalized cyclic carbonate monomer, AzDXO, that exhibited controlled/
Versatile synthesis of functional biodegradable polymers by combining ring-opening polymerization and postpolymerization modification via michael-type addition reaction
DOI:10.1021/ma901897y URL
Click chemistry: diverse chemical function from a few good reactions
DOI:10.1002/1521-3773(20010601)40:11<2004::aid-anie2004>3.3.co;2-x
URL
PMID:11433435
[Cited within: 1]

Examination of nature's favorite molecules reveals a striking preference for making carbon-heteroatom bonds over carbon-carbon bonds-surely no surprise given that carbon dioxide is nature's starting material and that most reactions are performed in water. Nucleic acids, proteins, and polysaccharides are condensation polymers of small subunits stitched together by carbon-heteroatom bonds. Even the 35 or so building blocks from which these crucial molecules are made each contain, at most, six contiguous C-C bonds, except for the three aromatic amino acids. Taking our cue from nature's approach, we address here the development of a set of powerful, highly reliable, and selective reactions for the rapid synthesis of useful new compounds and combinatorial libraries through heteroatom links (C-X-C), an approach we call
A strain-promoted [3 + 2] azide-alkyne cycloaddition for covalent modification of biomolecules in living systems
DOI:10.1021/ja044996f
URL
PMID:15547999
[Cited within: 1]

Selective chemical reactions that are orthogonal to the diverse functionality of biological systems have become important tools in the field of chemical biology. Two notable examples are the Staudinger ligation of azides and phosphines and the Cu(I)-catalyzed [3 + 2] cycloaddition of azides and alkynes (
Hydrolytic degradation of tyrosine-derived polycarbonates, a class of new biomaterials. Part II: 3-yr study of polymeric devices
DOI:10.1016/s0142-9612(00)00105-8
URL
PMID:11055285
[Cited within: 1]

The kinetics and mechanisms of in vitro degradation of tyrosine-derived polycarbonates, a new class of polymeric biomaterials, were studied extensively at 37 degrees C. These polymers carry an alkyl ester pendent chain that allows the fine-tuning of the polymer's material properties, its biological interactions with cells and tissue, and its degradation behavior. The polymer carrying an ethyl ester pendent chain, poly(DTE carbonate), has been established as a promising orthopedic implant material, exhibiting bone apposition when in contact with hard tissue. Tyrosine-derived polycarbonates are relatively stable and degrade only very slowly in vitro. Therefore, accelerated studies were conducted at 50 and 65 degrees C to observe the behavior of polymers during the later stages of degradation. Varying the pendent chain length affected the rate of water uptake, initial degradation rate, and physical stability of the polymeric devices. During the 3-yr study, the polymer degraded by random chain cleavage of the carbonate bonds, accompanied by a relatively small amount of pendent chain de-esterification. No mass loss was observed during this period at 37 degrees C, but mass loss was readily evident during the accelerated studies at 50 and 65 degrees C. Thus, it is reasonable to assume that mass loss will occur also at 37 degrees C, albeit only after extensive backbone carbonate cleavage and pendent chain ester hydrolysis. The dimension and surface area of the devices influenced the initial degradation rate, but did not significantly affect the overall rate of degradation. No evidence of
Polymerization reactions involving the side chains of .alpha.-L-amino acids
Fixation of distal femoral osteotomies with self-reinforced poly(desamino tyrosyl-tyrosine ethyl ester carbonate) rods: an experimental study on rats
DOI:10.1007/s007760200098
URL
PMID:12355129
[Cited within: 2]

Self-reinforced poly(desamino tyrosyl-tyrosine ethyl ester carbonate) poly(DTE carbonate) rods (diameter, 2 mm; length, 26 mm) were implanted into the dorsal subcutaneous tissue of 16 rats. Osteotomies of the distal femur were fixed with these rods (2 mm by 15 mm) in 64 other rats. The follow-up times varied from 1 week to 1 year. After sacrifice, three-point bending and shear tests and molecular weight measurements were performed for subcutaneously placed rods. Radiological, histological, histomorphometrical, microradiographic, and oxytetracycline-fluorescence studies of the osteotomized and intact control femurs were performed. At 36 weeks, the bending strength of the rods was nearly at the same level as the initial value, and the shear strength was decreased to about one quarter of the initial value. One of the 64 evaluated osteotomies showed signs of infection at 24 weeks, and there were five failures of fixation. Fifty-eight osteotomies healed uneventfully. No gross signs of inflammatory or foreign-body reactions were observed. The present investigation showed that the mechanical strength and fixation properties of SR-poly(DTE carbonate) rods are suitable for fixation of cancellous bone osteotomies in rats. The present article is the first report on successful application of SR-poly(DTE carbonate) rods for fixation of cancellous bone osteotomies.
Characterization of degradable polymers for orthopedic application
In Polymer based systems on tissue engineering, replacement and regeneration, Reis, R. L.; Cohn, D., Eds.;
Comparative histological evaluation of new tyrosine-derived polymers and poly (L-lactic acid) as a function of polymer degradation
DOI:10.1002/(sici)1097-4636(19980905)41:3<443::aid-jbm14>3.0.co;2-j
URL
PMID:9659614
[Cited within: 1]

Previous studies demonstrated that poly(DTE carbonate) and poly (DTE adipate), two tyrosine-derived polymers, have suitable properties for use in biomedical applications. This study reports the evaluation of the in vivo tissue response to these polymers in comparison to poly(L-lactic acid) (PLLA). Typically, the biocompatibility of a material is determined through histological evaluations as a function of implantation time in a suitable animal model. However, due to changes that can occur in the tissue response at different stages of the degradation process, a fixed set of time points is not ideal for comparative evaluations of materials having different rates of degradation. Therefore the tissue response elicited by poly(DTE carbonate), poly(DTE adipate), and PLLA was evaluated as a function of molecular weight. This allowed the tissue response to be compared at corresponding stages of degradation. Poly(DTE adipate) consistently elicited the mildest tissue response, as judged by the width and lack of cellularity of the fibrous capsule formed around the implant. The tissue response to poly(DTE carbonate) was mild throughout the 570 day study. However, the response to PLLA fluctuated as a function of the degree of degradation, exhibiting an increase in the intensity of inflammation as the implant began to lose mass. At the completion of the study, tissue ingrowth into the degrading and disintegrating poly(DTE adipate) implant was evident while no comparative ingrowth of tissue was seen for PLLA. The similarity of the in vivo and in vitro degradation rates of each polymer confirmed the absence of enzymatic involvement in the degradation process. A comparison of molecular weight retention, water uptake, and mass loss in vivo with two commonly used in vitro systems [phosphate-buffered saline (PBS) and simulated body fluid (SBF)] demonstrated that for the two tyrosine-derived polymers the in vivo results were equally well simulated in vitro with PBS and SBF. However, for PLLA the in vivo results were better simulated in vitro using PBS.
Canine bone response to tyrosine-derived polycarbonates and poly(L-lactic acid)
DOI:10.1002/(SICI)1097-4636(199605)31:1<35::AID-JBM5>3.0.CO;2-R
URL
PMID:8731147

Tyrosine-derived polycarbonates are a new class of degradable polymers developed for orthopedic applications. In this study the long-term (48 week) in vivo degradation kinetics and host bone response to poly(DTE carbonate) and poly(DTH carbonate) were investigated using a canine bone chamber model. Poly(L-lactic acid) (PLA) served as a control material. Two chambers of each test material were retrieved at 6-, 12-, 24-, and 48-week time points. Tyrosine-derived polycarbonates were found to exhibit degradation kinetics comparable to PLA. Each test material lost approximately 50% of its initial molecular weight (Mw) over the 48-week test period. Poly(DTE carbonate) and poly(DTH carbonate) test chambers were characterized by sustained bone ingrowth throughout the 48 weeks. In contrast, bone ingrowth into the PLA chambers peaked at 24 weeks and dropped by half at the 48-week time point. A fibrous tissue layer was found surrounding the PLA implants at all time points. This fibrous tissue layer was notably absent at the interface between bone and the tyrosine-derived polycarbonates. Histologic sections revealed intimate contact between bone and tyrosine-derived polycarbonates. From a degradation-biocompatibility perspective, the tyrosine-derived polycarbonates appear to be comparable, if not superior, to PLA in this canine bone chamber model.
Synjournal, degradation and biocompatibility of tyrosine-derived polycarbonate scaffolds
DOI:10.1039/c0jm00868k URL [Cited within: 1]
Calcium phosphate enriched synthetic tyrosine-derived polycarbonate - dicalcium phosphate dihydrate polymer scaffolds for enhanced bone regeneration
DOI:10.1016/j.mtla.2020.100616
URL
PMID:32968719
[Cited within: 1]

Optimal repair of large craniomaxillofacial (CMF) defects caused by trauma or disease requires the development of new, synthetic osteoconductive materials in combination with cell-based therapies, to overcome the limitations of traditionally used bone graft substitutes. In this study, tyrosine-derived polycarbonate, E1001(1k) scaffolds were fabricated to incorporate the osteoinductive coating, Dicalcium phosphate dihydrate (DCPD). The biocompatibility of E1001(1k)-DCPD, E1001(1k)-betaTCP and E1001(1k) scaffolds was compared using in vitro culture with human dental pulp stem cells (hDPSCs). We found that the DCPD coating was converted to carbonated hydroxyapatite over time in in vitro culture in Osteogenic Media, while the betaTCP did not. hDPSCs exhibited slow initial attachment and proliferation on DCPD E1001(1k) scaffolds, but subsequently improved over time in culture, and promoted osteogenic differentiation. To the best of our knowledge, this study highlights for the first time the effects of Osteogenic Media on phase changes of DCPD, and on DCPD scaffold cytocompatibility with hDPSCs. DCPD showed similar hDPSC biocompatibility and osteoconductivity as compared to betaTCP, and osteogenic differentiation of seeded hDPSCs. These studies suggest that E1001(1k)-DCPD scaffolds are a superior tool for craniofacial bone regeneration and provide the foundation for future in vivo testing.
Tyrosine derived polycarbonate membrane is useful for guided bone regeneration in rabbit mandibular defects
DOI:10.1007/s10856-005-2613-6
URL
PMID:15965746

Standardized bilateral through-and-through defects (12x6 mm) were created extraorally in the mandibular angle of 18 New Zealand White rabbits. Animals were divided in to three groups (n=6) according to the intended healing time. On the left side, defects were covered with a poly(desaminotyrosyl-tyrosine-ethyl ester carbonate) (PDTE carbonate) membrane wrapped around the inferior border of the mandible and fixed with bioabsorbable sutures. On the right side, the defects were filled with a mesh made of bioactive glass 13-93 and 3 wt% chitosan. The defects were covered with the same membranes. Periosteal flap was sutured over the membrane. Radiographically, bone ingrowth was seen in all specimens at 12 weeks postoperatively. At 24 weeks, completely ossified area remained approximately at the same level as at 12 weeks, but the non-ossified area decreased to almost zero. However, the bioactive glass mesh did not improve the results. Nevertheless, enveloping the defect with PDTE carbonate membrane seemed to play a crucial role in new bone formation. Based on these results, we conclude that tyrosine polycarbonate is a promising new material for guided bone regeneration.
Tyrosine-derived polycarbonate membrane in treating mandibular bone defects. An experimental study
DOI:10.1098/rsif.2006.0119
URL
PMID:16971331

This study was designed to evaluate the suitability of a novel bioabsorbable material in treating bone defects. A poly(desaminotyrosyl-tyrosine-ethyl ester carbonate) (PDTE carbonate) membrane (thickness 0.2-0.3 mm) was implanted into the mandibular angle of 20 New Zealand White rabbits to cover a through-and-through defect (12 x 6 mm). In group 1, the defects were left unfilled but covered with membrane and in group 2 the defects were filled with bioactive glass mesh and covered with membrane, too. Controls were left uncovered and unfilled. The animals were followed for 6, 12, 24 and 52 weeks, respectively. The material was evaluated by qualitative analysis of histological reactions and newly formed bone. We found that PDTE carbonate elicited a modest foreign body reaction in the tissues, which was uniform throughout the study. New bone formation was seen in all samples after six weeks. Group 1 had more new bone formation until 24 weeks and after this the difference settled. Based on findings of this study it was concluded that PDTE carbonate membranes have good biocompatibility and are sufficient to enhance bone growth without additional supportive matrix.
Bone regeneration in a rabbit critical-sized calvarial model using tyrosine-derived polycarbonate scaffolds
DOI:10.1089/ten.TEA.2011.0582
URL
PMID:22220747

Porous three-dimensional tyrosine-derived polycarbonate (TyrPC) scaffolds with a bimodal pore distribution were fabricated to mimic bone architecture using a combination of salt-leaching and phase separation techniques. TyrPC scaffolds degraded in register with bone regeneration during the 6-week study period and compressive moduli of the scaffolds were maintained >0.5 MPa at 6 weeks of incubation in PBS at 37 degrees C. The TyrPC scaffolds either unsupplemented or supplemented with recombinant human bone morphogenetic protein-2 (rhBMP-2) were implanted in a rabbit calvarial critical-sized defect (CSD) model and the TyrPC scaffolds treated with rhBMP-2 or TyrPC coated with calcium phosphate scaffold alone promoted bone regeneration in a rabbit calvarial CSD at 6 weeks postimplantation. A synthetic TyrPC polymeric scaffold either without a biological supplement or with a minimal dose of rhBMP-2 induced bone regeneration comparable to a commercially available bone graft substitute in a nonrodent CSD animal model.
Mandibular jaw bone regeneration using human dental cell-seeded tyrosine-derived polycarbonate scaffolds
DOI:10.1089/ten.TEA.2016.0166
URL
PMID:27369635
[Cited within: 1]

Here we present a new model for alveolar jaw bone regeneration, which uses human dental pulp cells (hDPCs) combined with tyrosine-derived polycarbonate polymer scaffolds [E1001(1k)] containing beta-tricalcium phosphate (beta-TCP) [E1001(1k)/beta-TCP]. E1001(1k)/beta-TCP scaffolds (5 mm diameter x 1 mm thickness) were fabricated to fit a 5 mm rat mandibular ramus critical bone defect. Five experimental groups were examined in this study: (1) E1001(1k)/beta-TCP scaffolds seeded with a high density of hDPCs, 5.0 x 10(5) hDPCs/scaffold (CH); (2) E1001(1k)/beta-TCP scaffolds seeded with a lower density of hDPCs, 2.5 x 10(5) hDPCs/scaffold (CL); (3) acellular E1001(1k)/beta-TCP scaffolds (SA); (4) acellular E1001(1k)/beta-TCP scaffolds supplemented with 4 mug recombinant human bone morphogenetic protein-2 (BMP); and (5) empty defects (EDs). Replicate hDPC-seeded and acellular E1001(1k)/beta-TCP scaffolds were cultured in vitro in osteogenic media for 1 week before implantation for 3 and 6 weeks. Live microcomputed tomography (muCT) imaging at 3 and 6 weeks postimplantation revealed robust bone regeneration in the BMP implant group. CH and CL groups exhibited similar uniformly distributed mineralized tissue coverage throughout the defects, but less than the BMP implants. In contrast, SA-treated defects exhibited sparse areas of mineralized tissue regeneration. The ED group exhibited slightly reduced defect size. Histological analyses revealed no indication of an immune response. In addition, robust expression of dentin and bone differentiation marker expression was observed in hDPC-seeded scaffolds, whereas, in contrast, BMP and SA implants exhibited only bone and not dentin differentiation marker expression. hDPCs were detected in 3-week but not in 6-week hDPC-seeded scaffold groups, indicating their survival for at least 3 weeks. Together, these results show that hDPC-seeded E1001(1k)/beta-TCP scaffolds support the rapid regeneration of osteo-dentin-like mineralized jaw tissue, suggesting a promising new therapy for alveolar jaw bone repair and regeneration.
Tyrosine-derived polycarbonate scaffolds for bone regeneration in a rabbit radius critical-size defect model
DOI:10.1088/1748-6041/10/3/035001
URL
PMID:25953950
[Cited within: 2]

The aim of the study was to determine bone regeneration in a rabbit radius critical-size defect (CSD) model using a specific polymer composition (E1001(1k)) from a library of tyrosine-derived polycarbonate scaffolds coated with a calcium phosphate (CP) formulation (E1001(1k) + CP) supplemented with recombinant human bone morphogenetic protein-2 (rhBMP-2). Specific doses of rhBMP-2 (0, 17, and 35 mug/scaffold) were used. E1001(1k) + CP scaffolds were implanted in unilateral segmental defects (15 mm length) in the radial diaphyses of New Zealand White rabbits. At 4 and 8 weeks post-implantation, bone regeneration was determined using micro-computed tomography (microCT), histology, and histomorphometry. The quantitative outcome data suggest that E1001(1k) + CP scaffolds with rhBMP-2 were biocompatible and promoted bone regeneration in segmental bone defects. Histological examination of the implant sites showed that scaffolds made of E1001(1k) + CP did not elicit adverse cellular or tissue responses throughout test periods up to 8 weeks. Noteworthy is that the incorporation of a very small amount of rhBMP-2 into the scaffolds (as low as 17 mug/defect site) promoted significant bone regeneration compared to scaffolds consisting of E1001(1k) + CP alone. This finding indicates that E1001(1k) + CP may be an effective platform for bone regeneration in a critical size rabbit radius segmental defect model, requiring only a minimal dose of rhBMP-2.
Hydrolytic degradation of tyrosine-derived polycarbonates, a class of new biomaterials. Part I: study of model compounds
DOI:10.1016/s0142-9612(00)00104-6
URL
PMID:11055284
[Cited within: 1]

Tyrosine-derived polycarbonates have been identified as promising, degradable polymers for use in orthopedic applications. These polymers are non-toxic, biocompatible, and exhibit good bone apposition when in contact with hard tissue. Tyrosine-derived polycarbonates were designed to incorporate two hydrolytically labile bonds in each repeat unit, a carbonate bond that connects the monomer units and an ester bond connecting a pendent chain. The relative hydrolysis rate of the two bonds will determine the type of degradation products and the degradation pathway of the polymers. In order to study the degradation mechanism of these polycarbonates in more detail, a series of small model compounds were designed that mimic the repeat unit of the polymer. Results obtained from the use of these model compounds suggested that the backbone carbonate bond is hydrolyzed at a faster rate than the pendent chain ester bond. Increasing the length of the alkyl pendent chain lowered the hydrolysis rates of both hydrolyzable linkages, possibly by hindering the access of water molecules to those sites. The hydrolysis rates of both linkages were pH dependent with the lowest rate at pH about 5. The results from this study can be used to explain the degradation behavior of the corresponding polycarbonates as well as their degradation mechanisms. This information is essential when evaluating the utility of tyrosine-derived polycarbonates as degradable medical implant materials.
Preparation of beta-tricalcium phosphate microsphere-hyaluronic acid-based powder gel composite as a carrier for rhBMP-2 injection and evaluation using long bone segmental defect model
DOI:10.1080/09205063.2019.1601871
URL
PMID:30939993
[Cited within: 1]

The specific objective of this study was to evaluate whether rhBMP-2-loaded bio-scaffolds can be used as effective rhBMP-2 carriers in the implantation of bone defect sites or poor bone quality in host bone. The rhBMP-2 release pattern test showed slow results in both groups, and a 1:9 ratio composition with a high water-absorption rate was selected for in vivo study. All animals euthanized after 9 weeks. The new bone formation and bone quantity and quality of fibular samples were examined. The results showed that the rhBMP-2 powder gel composite improved the new bone formation in the cortical bone and the marrow space. The length of new bone formation ratio of the rhBMP-2 loaded composite group was significantly higher than the powder gel group. The composite of powder gel seems to be a nice carrier, and slow release of rhBMP-2 can promote new bone formation in a segmental cortical bone defect after implantation.
An alginate-based hybrid system for growth factor delivery in the functional repair of large bone defects
DOI:10.1016/j.biomaterials.2010.08.074
URL
PMID:20864165
[Cited within: 1]

The treatment of challenging fractures and large osseous defects presents a formidable problem for orthopaedic surgeons. Tissue engineering/regenerative medicine approaches seek to solve this problem by delivering osteogenic signals within scaffolding biomaterials. In this study, we introduce a hybrid growth factor delivery system that consists of an electrospun nanofiber mesh tube for guiding bone regeneration combined with peptide-modified alginate hydrogel injected inside the tube for sustained growth factor release. We tested the ability of this system to deliver recombinant bone morphogenetic protein-2 (rhBMP-2) for the repair of critically-sized segmental bone defects in a rat model. Longitudinal mu-CT analysis and torsional testing provided quantitative assessment of bone regeneration. Our results indicate that the hybrid delivery system resulted in consistent bony bridging of the challenging bone defects. However, in the absence of rhBMP-2, the use of nanofiber mesh tube and alginate did not result in substantial bone formation. Perforations in the nanofiber mesh accelerated the rhBMP-2 mediated bone repair, and resulted in functional restoration of the regenerated bone. mu-CT based angiography indicated that perforations did not significantly affect the revascularization of defects, suggesting that some other interaction with the tissue surrounding the defect such as improved infiltration of osteoprogenitor cells contributed to the observed differences in repair. Overall, our results indicate that the hybrid alginate/nanofiber mesh system is a promising growth factor delivery strategy for the repair of challenging bone injuries.
Effects of protein dose and delivery system on BMP-mediated bone regeneration
DOI:10.1016/j.biomaterials.2011.03.063
URL
PMID:21507479

Delivery of recombinant proteins is a proven therapeutic strategy to promote endogenous repair mechanisms and tissue regeneration. Bone morphogenetic protein-2 (rhBMP-2) has been used to promote spinal fusion and repair of challenging bone defects; however, the current clinically-used carrier, absorbable collagen sponge, requires high doses and has been associated with adverse complications. We evaluated the hypothesis that the relationship between protein dose and regenerative efficacy depends on delivery system. First, we determined the dose-response relationship for rhBMP-2 delivered to 8-mm rat bone defects in a hybrid nanofiber mesh/alginate delivery system at six doses ranging from 0 to 5 mug. Next, we directly compared the hybrid delivery system to the collagen sponge at 0.1 and 1.0 mug. Finally, we compared the in vivo protein release properties of the two delivery methods. In the hybrid delivery system, bone volume, connectivity and mechanical properties increased in a dose-dependent manner to rhBMP-2. Consistent bridging of the defect was observed for doses of 1.0 mug and greater. Compared to collagen sponge delivery at the same 1.0 mug dose, the hybrid system yielded greater connectivity by week 4 and 2.5-fold greater bone volume by week 12. These differences may be explained by the significantly greater protein retention in the hybrid system compared to collagen sponge. This study demonstrates a clear dose-dependent effect of rhBMP-2 delivered using a hybrid nanofiber mesh/alginate delivery system. Furthermore, the effective dose was found to vary with delivery system, demonstrating the importance of biomaterial carrier properties in the delivery of recombinant proteins.
Calcium phosphate-DNA nanoparticle gene delivery from alginate hydrogels induces in vivo osteogenesis
DOI:10.1002/jbm.a.32441
URL
PMID:19322877

There is a significant need for improved therapy for bone regeneration. The delivery of recombinant bone morphogenetic proteins has been approved for clinical use to promote osteogenesis, but still has limitations such as expense, degradation of the proteins in vivo and difficulties retaining protein at the site of injury. Localized gene delivery is a promising alternative therapy, as it would allow sustained expression of specific osteoinductive growth factors by cells near the damaged site. We have engineered an injectable system for localized, sustained nonviral gene delivery from alginate hydrogels containing preosteoblastic cells and calcium phosphate-DNA nanoparticles. The nanoparticles utilized in this report are stable, on the order of 100 nm, and have a high DNA incorporation efficiency (>66%). When the nanoparticles were incorporated in alginate hydrogels, sustained release of DNA was observed. Furthermore, MC3T3-E1 preosteoblast cells exhibited the capacity to form bony tissue in as little as two and half weeks when mixed with DNA nanoparticles encoding for BMP-2 into the alginate hydrogels and injected subcutaneously in the backs of mice. This injectable, minimally invasive gene delivery system may be efficacious in bone regeneration applications.
Quantitative assessment of scaffold and growth factor-mediated repair of critically sized bone defects
DOI:10.1002/jor.20372
URL
PMID:17415756

An 8-mm rat segmental defect model was used to evaluate quantitatively the ability of longitudinally oriented poly(L-lactide-co-D,L-lactide) scaffolds with or without growth factors to promote bone healing. BMP-2 and TGF-beta3, combined with RGD-alginate hydrogel, were co-delivered to femoral defects within the polymer scaffolds at a dose previously shown to synergistically induce ectopic mineralization. A novel modular composite implant design was used to achieve reproducible stable fixation, provide a window for longitudinal in vivo micro-CT monitoring of 3D bone ingrowth, and allow torsional biomechanical testing of functional integration. Sequential micro-CT analysis showed that bone ingrowth increased significantly between 4 and 16 weeks for the scaffold-treated defects with or without growth factors, but no increase with time was observed in empty defect controls. Treatment with scaffold alone improved defect stability at 16 weeks compared to nontreatment, but did not achieve bone union or restoration of mechanical function. Augmentation of scaffolds with BMP-2 and TGF-beta3 significantly increased bone formation at both 4 and 16 weeks compared to nontreatment, but only produced bone bridging of the defect region in two of six cases. Histological evaluation indicated that bone formed first at the periphery of the scaffolds, followed by more limited mineral deposition within the scaffold interior, suggesting that the cells participating in the initial healing response were primarily derived from periosteum. This study introduces a challenging segmental defect model that facilitates quantitative evaluation of strategies to repair critically sized bone defects. Healing of the defect region was improved by implanting structural polymeric scaffolds infused with growth factors incorporated within RGD-alginate. However, functional integration of the constructs appeared limited by continued presence of slow-degrading scaffolds and suboptimal dose or delivery of osteoinductive signals.
The effect of the delivery of vascular endothelial growth factor and bone morphogenic protein-2 to osteoprogenitor cell populations on bone formation
DOI:10.1016/j.biomaterials.2009.10.059
URL
PMID:19926128

Regenerating bone tissue involves complex, temporal and coordinated signal cascades of which bone morphogenic protein-2 (BMP-2) and vascular endothelial growth factor (VEGF(165)) play a prominent role. The aim of this study was to determine if the delivery of human bone marrow stromal cells (HBMSC) seeded onto VEGF(165)/BMP-2 releasing composite scaffolds could enhance the bone regenerative capability in a critical sized femur defect. Alginate-VEGF(165)/P(DL)LA-BMP-2 scaffolds were fabricated using a supercritical CO(2) mixing technique and an alginate entrapment protocol. Increased release of VEGF(165) (750.4+/-596.8 rho g/ml) compared to BMP-2 (136.9+/-123.4 r hog/ml) was observed after 7-days in culture. Thereafter, up till 28 days, an increased rate of release of BMP-2 compared to VEGF(165) was observed. The alginate-VEGF(165)/P(DL)LA-BMP-2+HBMSC group showed a significant increase in the quantity of regenerated bone compared to the alginate-VEGF(165)/P(DL)LA-BMP-2 and alginate/P(DL)LA groups respectively in a critical sized femur defect study as indices measured by microCT. Histological examination confirmed significant new endochondral bone matrix in the HBMSC seeded alginate-VEGF(165)/P(DL)LA-BMP-2 defect group in comparison to the other groups. These studies demonstrate the ability to deliver a combination of HBMSC with angiogenic and osteogenic factors released from biodegradable scaffold composites enhances the repair and regeneration of critical sized bone defects.
In vivo model for evaluating the effects of mechanical stimulation on tissue-engineered bone repair
DOI:10.1115/1.3148472
URL
PMID:19604025

It has long been known that the bone adapts according to the local mechanical environment. To date, however, a model for studying the effects of functional mechanical loading on tissue-engineered bone repair in vivo has not yet been established. We have developed a rat femoral defect model, in which ambulatory loads are transduced through the implanted tissue-engineered construct to elucidate the role of the mechanical environment in functional restoration of a large bone defect. This model uses compliant fixation plates with integrated elastomeric segments, which allow transduction of ambulatory loads. Multiaxially and uniaxially compliant plates were characterized by mechanical testing and evaluated using in vivo pilot studies. In the first study, experimental limbs were implanted with multiaxial plates, which have a low stiffness in multiple loading modes. In the second study, experimental limbs were stabilized by a uniaxial plate, which allowed only axial deformation of the defect. X-ray scans and mechanical testing revealed that the multiaxial plates were insufficient to stabilize the defect and prevent fracture under ambulatory loads as a result of low flexural and torsional stiffness. The uniaxial plates, however, maintained integrity of the defect when implanted over a 12 week period. Postmortem microCT scans revealed a 19% increase in bone volume in the axially loaded limb compared with the contralateral standard control, and postmortem mechanical testing indicated that torsional strength and stiffness were increased 25.6- and 3.9-fold, respectively, compared with the control. Finite element modeling revealed high strain gradients in the soft tissue adjacent to the newly formed bone within the implanted construct. This study introduces an in vivo model for studying the effects of physiological mechanical loading on tissue-engineered bone repair. Preliminary results using this new in vivo model with the uniaxially compliant plate showed positive effects of load-bearing on functional defect repair.
Oxidized alginate hydrogels for bone morphogenetic protein-2 delivery in long bone defects
DOI:10.1016/j.actbio.2014.06.015
URL
PMID:24954001
[Cited within: 1]

Autograft treatment of large bone defects and fracture non-unions is complicated by limited tissue availability and donor site morbidity. Polymeric biomaterials such as alginate hydrogels provide an attractive tissue engineering alternative due to their biocompatibility, injectability, and tunable degradation rates. Irradiated RGD-alginate hydrogels have been used to deliver proteins such as bone morphogenetic protein-2 (BMP-2), to promote bone regeneration and restoration of function in a critically sized rat femoral defect model. However, slow degradation of irradiated alginate hydrogels may impede integration and remodeling of the regenerated bone to its native architecture. Oxidation of alginate has been used to promote degradation of alginate matrices. The objective of this study was to evaluate the effects of alginate oxidation on BMP-2 release and bone regeneration. We hypothesized that oxidized-irradiated alginate hydrogels would elicit an accelerated release of BMP-2, but degrade faster in vivo, facilitating the formation of higher quality, more mature bone compared to irradiated alginate. Indeed, oxidation of irradiated alginate did accelerate in vitro BMP-2 release. Notably, the BMP-2 retained within both constructs was bioactive at 26days, as observed by induction of alkaline phosphatase activity and positive Alizarin Red S staining of MC3T3-E1 cells. From the in vivo study, robust bone regeneration was observed in both groups through 12weeks by radiography, micro-computed tomography analyses, and biomechanical testing. Bone mineral density was significantly greater for the oxidized-irradiated alginate group at 8weeks. Histological analyses of bone defects revealed enhanced degradation of oxidized-irradiated alginate and suggested the presence of more mature bone after 12weeks of healing.
Characterization of an injectable chitosan-demineralized bone matrix hybrid for healing critical-size long-bone defects in a rabbit model
URL
PMID:24668718
[Cited within: 1]

BACKGROUND: The effect of injectable demineralized bone matrix (DBM) on bone repair is not known. Here, we tested the hypothesis that injectable DBM can heal a critical-size diaphyseal radius defect in a rabbit model. MATERIALS AND METHODS: The bone defect was filled with DBM powder, injectable DBM or powdered, freeze-dried powdered allografts. Radiological determination, gross evaluation, histology, and micro-computer tomography was carried out 4, 8, and 12 weeks after the surgery, respectively. RESULTS: The injectable DBM group yielded better when compared with the freeze-dried powder group (p < 0.05). Moreover, biomechanical functionality was restored comparable to normal levels in the injectable DBM group. CONCLUSIONS: The injectable DBM was as effective in structurally and functionally repairing bone defects as the DBM powder and more effective than the freeze-dried bone powder. Thus, our study supports the use of injectable DBM for bone healing.
Novel osteoinductive photo-cross-linkable chitosan-lactide-fibrinogen hydrogels enhance bone regeneration in critical size segmental bone defects
DOI:10.1016/j.actbio.2014.08.028
URL
PMID:25174669

The purpose of this study was to develop and characterize a novel photo-cross-linkable chitosan-lactide-fibrinogen (CLF) hydrogel and evaluate the efficacy of bone morphogenetic protein-2 (BMP-2) containing a CLF hydrogel for osteogenesis in vitro and in vivo. We synthesized the CLF hydrogels and characterized their chemical structure, degradation rate, compressive modulus and in vitro BMP-2 release kinetics. We evaluated bioactivities of the BMP-2 containing CLF hydrogels (0, 50, 100 and 500ngml(-1)) in vitro using W-20-17 preosteoblast mouse bone marrow stromal cells and C2C12 mouse myoblast cells. The effect of BMP-2 containing CLF gels (0, 0.5, 1, 2 and 5mug) on bone formation was evaluated using rat critical size segmental bone defects for 4weeks. Fourier transform infrared spectroscopy spectra and scanning electron microscopy images showed chemical and structural changes by the addition of fibrinogen into the chitosan-lactide copolymer. The incorporation of fibrinogen molecules significantly increased the compressive modulus of the hydrogels. The in vitro BMP-2 release study showed initial burst releases from the CLF hydrogels followed by sustained releases, regardless of the concentration of the BMP-2 over 4weeks. Cells in all groups were viable in the presence of the hydrogels regardless of BMP-2 doses, indicating non-cytotoxicity of hydrogels. Alkaline phosphate activity and mineralization of cells exhibited dose dependence on BMP-2 containing CLF hydrogels. Radiography, microcomputed tomography and histology confirmed that the BMP-2 containing CLF hydrogels prompted neo-osteogenesis and accelerated healing of the defects in a dose-dependent manner. Thus the CLF hydrogel is a promising delivery system of growth factors for bone regeneration.
Injectable rhBMP-2-loaded chitosan hydrogel composite: osteoinduction at ectopic site and in segmental long bone defect
DOI:10.1002/jbm.a.32957
URL
PMID:21105153
[Cited within: 1]

Carriers for bone morphogenetic protein-2 (BMP-2) used in clinical practice still suffer from limitations such as insufficient protein retention. In addition, there is a clinical need for injectable carriers. The main objective of this study was to assess bone forming ability of rhBMP-2 combined either with chitosan hydrogel (rhBMP-2/CH) or chitosan hydrogel containing beta-tricalcium phosphate (beta-TCP) (rhBMP-2/CH/TCP). Formulations were first compared in a rat ectopic intramuscular bone formation model, and the optimal formulation was further evaluated in healing of 15-mm critical size defect in the radius of a rabbit. Three weeks after injection ectopically formed bone was analyzed by microcomputerized tomography (micro-CT) and histology. Significantly higher (4.7-fold) mineralized bone formation was observed in the rhBMP-2/CH/TCP group compared to rhBMP-2/CH group. In a pilot study, defect in a rabbit radius treated with rhBMP-2/CH/TCP showed incomplete regeneration at 8 weeks with composite leakage from the defect, indicating the need for formulation refinement when segmental defect repair is foreseen.
A new approach to mineralization of biocompatible hydrogel scaffolds: an efficient process toward 3-dimensional bonelike composites
DOI:10.1021/ja028559h
URL
PMID:12553825
[Cited within: 1]

As a first step toward the design and fabrication of biomimetic bonelike composite materials, we have developed a template-driven nucleation and mineral growth process for the high-affinity integration of hydroxyapatite with a poly(2-hydroxyethyl methacrylate) (pHEMA) hydrogel scaffold. A mineralization technique was developed that exposes carboxylate groups on the surface of cross-linked pHEMA, promoting high-affinity nucleation and growth of calcium phosphate on the surface, along with extensive calcification of the hydrogel interior. Robust surface mineral layers a few microns thick were obtained. The same mineralization technique, when applied to a hydrogel that is less prone to surface hydrolysis, led to distinctly different mineralization patterns, in terms of both the extent of mineralization and the crystallinity of the apatite grown on the hydrogel surface. This template-driven mineralization technique provides an efficient approach toward bonelike composites with high mineral-hydrogel interfacial adhesion strength.
Mineralization of synthetic polymer scaffolds: a bottom-up approach for the development of artificial bone
DOI:10.1021/ja043776z
URL
PMID:15755154
[Cited within: 1]

The controlled integration of organic and inorganic components confers natural bone with superior mechanical properties. Bone biogenesis is thought to occur by templated mineralization of hard apatite crystals by an elastic protein scaffold, a process we sought to emulate with synthetic biomimetic hydrogel polymers. Cross-linked polymethacrylamide and polymethacrylate hydrogels were functionalized with mineral-binding ligands and used to template the formation of hydroxyapatite. Strong adhesion between the organic and inorganic materials was achieved for hydrogels functionalized with either carboxylate or hydroxy ligands. The mineral-nucleating potential of hydroxyl groups identified here broadens the design parameters for synthetic bonelike composites and suggests a potential role for hydroxylated collagen proteins in bone mineralization.
Elastomeric high-mineral content hydrogel-hydroxyapatite composites for orthopedic applications
DOI:10.1002/jbm.a.32110
URL
PMID:18546185
[Cited within: 1]

The design of synthetic bone grafts that mimic the structure and composition of bone and possess good surgical handling characteristics remains a major challenge. We report the development of poly(2-hydroxyethyl methacrylate) (pHEMA)-hydroxyapatite (HA) composites termed
Sustained and localized in vitro release of BMP-2/7, RANKL, and tetracycline from FlexBone, an elastomeric osteoconductive bone substitute
DOI:10.1002/jor.20890
URL
PMID:19350632

We tested the hypothesis that synthetic composites containing a high percentage of osteoconductive biominerals well-integrated with a hydrophilic polymer matrix can be engineered to provide both the structural and biochemical framework of a viable synthetic bone substitute. FlexBone, an elastic hydrogel-mineral composite exhibiting excellent structural integration was prepared by crosslinking poly(2-hydroxyethyl methacrylate) hydrogel in the presence of 25 wt% nanocrystalline hydroxyapatite and 25 wt% tricalcium phosphate. Biologically active factors tetracycline, BMP-2/7, and RANKL that stimulate bone formation and remodeling were encapsulated into FlexBone during polymerization or via postpolymerization adsorption. SEM and dynamic mechanical analyses showed that the encapsulation of tetracycline (5.0 wt%) did not compromise the structural integrity and compressive behavior of FlexBone, which could withstand repetitive megapascal-compressive loadings and be securely press-fitted into critical femoral defects. Dose-dependent, sustained in vitro release of tetracycline was characterized by spectroscopy and bacterial inhibition. A single dose of 40 ng BMP-2/7 or 10 ng RANKL pre-encapsulated with 50 mg FlexBone, released over 1 week, was able to induce local osteogenic differentiation of myoblast C2C12 cells and osteoclastogenesis of macrophage RAW264.7 cells, respectively. With a bonelike structural composition, useful surgical handling characteristics, and tunable biochemical microenvironment, FlexBone provides an exciting opportunity for the treatment of hard-to-heal skeletal defects with minimal systemic side effects.
Elastomeric osteoconductive synthetic scaffolds with acquired osteoinductivity expedite the repair of critical femoral defects in rats
DOI:10.1089/ten.TEA.2010.0274
URL
PMID:20818999
[Cited within: 2]

Regenerative medicine aspires to reduce reliance on or overcome limitations associated with donor tissue-mediated repair. Structural bone allografts are commonly used in orthopedic surgery, with a high percentage of graft failure due to poor tissue integration. This problem is aggravated among elderly, those suffering from metabolic conditions, or those undergoing cancer therapies that compromise graft healing. Toward this end, we developed a synthetic graft named FlexBone, in which nanocrystalline hydroxyapatite (50 wt%) was structurally integrated with crosslinked poly(hydroxyethyl methacrylate) hydrogel, which provides dimensional stability and elasticity. It recapitulates the essential role of nanocrystalline hydroxyapatite in defining the osteoconductivity and biochemical microenvironment of bone because of its affinity for biomolecules. Here, we demonstrate that FlexBone effectively absorbed endogenously secreted signaling molecules associated with the inflammation/graft healing cascade upon being press-fit into a 5-mm rat femoral segmental defect. Further, when preabsorbed with a single dose of 400 ng recombinant human (rh) bone morphogenetic protein-2/7 heterodimer, it enabled the functional repair of the critical-sized defect by 8-12 weeks. FlexBone was stably encapsulated by the bridging bony callus and the FlexBone-callus interface was continuously remodeled. In summary, FlexBone combines the dimensional stability and osteoconductivity of structural bone allografts with desirable surgical compressibility and acquired osteoinductivity in an easy-to-fabricate and scalable synthetic biomaterial.
Rapid healing of femoral defects in rats with low dose sustained BMP2 expression from PEGDA hydrogel microspheres
DOI:10.1002/jor.22407
URL
PMID:23832813
[Cited within: 1]

Current strategies for bone regeneration after traumatic injury often fail to provide adequate healing and integration. Here, we combined the poly (ethylene glycol) diacrylate (PEGDA) hydrogel with allogeneic
Biosynthetic hydrogel scaffolds made from fibrinogen and polyethylene glycol for 3D cell cultures
DOI:10.1016/j.biomaterials.2004.06.047
URL
PMID:15585249
[Cited within: 1]

Tissue engineering scaffolds are fabricated from either biological materials, which provide biofunctional signals and interact well with cells, or from synthetic polymers, which provide precise control over their structural properties. We describe a biosynthetic hybrid scaffold comprised of a fibrinogen backbone and crosslinked with difunctional polyethylene glycol (PEG) side chains. Denatured fibrinogen fragments are PEGylated with PEG-diacrylates, mixed with photoinitiator and exposed to UV light to form a hydrogel material in the presence of a cell suspension. This unique hydrogel material provides a distinct advantage over other scaffold materials because its mechanical properties are highly malleable while the biological functionality is maintained by the backbone of the polymeric network. The elastic modulus of the PEG-fibrinogen hydrogel is dependent on the molecular weight of the PEG constituent and proportional to the percent polymeric composition. The biological domains in the fibrinogen backbone provide attachment motifs for endothelial cell and smooth muscle cell adhesion as well as proteolytic sensitivity for biodegradation. Smooth muscle cells demonstrate the ability to proteolytically penetrate through the hydrogel material and form interconnecting networks of cells. Our efforts to develop novel biodegradable scaffolds for cultivating cells in a 3D environment are beneficial for tissue regeneration therapies.
The drug encapsulation efficiency, in vitro drug release, cellular uptake and cytotoxicity of paclitaxel-loaded poly(lactide)-tocopheryl polyethylene glycol succinate nanoparticles
DOI:10.1016/j.biomaterials.2006.03.006
URL
PMID:16564085
[Cited within: 1]

Paclitaxel is one of the most effective antineoplastic drugs. Its current clinical administration is formulated in Cremophor EL, which causes serious side effects. Nanoparticle (NP) technology may provide a solution for such poisonous adjuvant problems and promote a sustained chemotherapy, in which biodegradable polymers play a key role. Our group has successfully synthesized novel poly(lactide)-tocopheryl polyethylene glycol succinate (TPGS) (PLA-TPGS) copolymers of desired hydrophobic-hydrophilic balance for NP formulation of anticancer drugs. The present work is focused on effects of the PLA:TPGS composition ratio on drug encapsulation efficiency, in vitro drug release, in vitro cellular uptake and viability of the PLA-TPGS NP formulation of paclitaxel. The PLA-TPGS copolymers of various PLA:TPGS ratios were synthesized by the ring-opening polymerization method and characterized by GPC and (1)H NMR for their molecular structure. Paclitaxel-loaded PLA-TPGS NPs were prepared by a modified solvent extraction/evaporation method and characterized by laser light scattering for size and size distribution, scanning electron microscopy for surface morphology and zeta potential for surface charge. High performance liquid chromatography was used to measure the drug encapsulation efficiency and in vitro drug release profile. Cancer cell lines HT-29 and Caco-2 were used to image and measure the cellular uptake of fluorescent PLA-TPGS NPs. Cancer cell viability of the drug-loaded PLA-TPGS was measured by MTT assay. It was found that the PLA:TPGS composition ratio has little effects on the particle size and size distribution. However, the PLA-TPGS NPs of 89:11 PLA:TPGS ratio achieved the best effects on the drug encapsulation efficiency, the cellular uptake and the cancer cell mortality of the drug-loaded PLA-TPGS NPs. This research was also carried out in close comparison with the drug-loaded PLGA NPs.
Cytocompatible poly(ethylene glycol)-co-polycarbonate hydrogels cross-linked by copper-free, strain-promoted click chemistry
DOI:10.1002/asia.201100411
URL
PMID:21954076
[Cited within: 1]

Strategies to encapsulate cells in cytocompatible three-dimensional hydrogels with tunable mechanical properties and degradability without harmful gelling conditions are highly desired for regenerative medicine applications. Here we reported a method for preparing poly(ethylene glycol)-co-polycarbonate hydrogels through copper-free, strain-promoted azide-alkyne cycloaddition (SPAAC) click chemistry. Hydrogels with varying mechanical properties were formed by
Bioorthogonally cross-linked hydrogel network with precisely controlled disintegration time over a broad range
DOI:10.1021/ja4130862
URL
PMID:24597638
[Cited within: 1]

Hydrogels with predictable degradation are highly desired for biomedical applications where timely disintegration of the hydrogel (e.g., drug delivery, guided tissue regeneration) is required. However, precisely controlling hydrogel degradation over a broad range in a predictable manner is challenging due to limited intrinsic variability in the degradation rate of liable bonds and difficulties in modeling degradation kinetics for complex polymer networks. More often than not, empirical tuning of the degradation profile results in undesired changes in other properties. Here we report a simple but versatile hydrogel platform that allows us to formulate hydrogels with predictable disintegration time from 2 to >250 days yet comparable macroscopic physical properties. This platform is based on a well-defined network formed by two pairs of four-armed polyethylene glycol macromers terminated with azide and dibenzocyclooctyl groups, respectively, via labile or stable linkages. The high-fidelity bioorthogonal reaction between the symmetric hydrophilic macromers enables robust cross-linking in water, phosphate-buffered saline, and cell culture medium to afford tough hydrogels capable of withstanding >90% compressive strain. Strategic placement of labile ester linkages near the cross-linking site within this superhydrophilic network, accomplished by adjustments of the ratio of the macromers used, enables broad tuning of the disintegration rates precisely matching with the theoretical predictions based on first-order linkage cleavage kinetics. This platform can be exploited for applications where a precise degradation rate is targeted.
Materials as morphogenetic guides in tissue engineering
DOI:10.1016/j.copbio.2003.09.004
URL
PMID:14580588

Within native tissues cells are held within the extracellular matrix (ECM), which has a role in maintaining homeostasis, guiding development and directing regeneration. Efforts in tissue engineering have aimed to mimick the ECM to help guide morphogenesis and tissue repair. Studies have not only looked at ways to mimick the structure and characteristics of the ECM, but have also considered ways to reproduce its molecular properties including its bioadhesive character, proteolytic susceptibility and ability to bind growth factors.
Three-dimensional biomimetic patterning in hydrogels to guide cellular organization
DOI:10.1002/adma.201200395
URL
PMID:22467256
[Cited within: 1]

An image-guided micropatterning method is demonstrated for generating biomimetic hydrogel scaffolds with two-photon laser scanning photolithography. This process utilizes computational methods to directly translate three-dimensional cytoarchitectural features from labeled tissues into material structures. We use this method to pattern hydrogels that guide cellular organization by structurally and biochemically recapitulating complex vascular niche microenvironments with high pattern fidelity at the microscale.
DNA delivery from matrix metalloproteinase degradable poly(ethylene glycol) hydrogels to mouse cloned mesenchymal stem cells
DOI:10.1016/j.biomaterials.2008.09.027
URL
PMID:18838159

The ability to genetically modify mesenchymal stem cells (MSCs) seeded inside synthetic hydrogel scaffolds would offer an alternative approach to guide MSC differentiation and to study molecular pathways in three dimensions than protein delivery. In this report, we explored gene transfer to infiltrating MSCs into matrix metalloproteinase (MMP) degradable hydrogels that were loaded with DNA/poly(ethylene imine) (PEI) polyplexes. DNA/PEI polyplexes were encapsulated inside poly(ethylene glycol) (PEG) hydrogels crosslinked with MMP-degradable peptides via Michael addition chemistry. A large fraction of encapsulated polyplexes remained active after encapsulation (65%) and the mechanical properties of the hydrogels were unchanged by the encapsulation of the polyplexes. Cells were seeded inside the hydrogel scaffolds using two different approaches: clustered and homogeneous. The viability of MSCs was similar in hydrogels with and without polyplexes. Transgene expression was characterized with time using a secreted reporter gene and showed different profiles for clustered and homogeneously seeded cells. Clustered cells resulted in cumulative transgene expression that increased through the 21-day incubation, while homogeneously seeded cells resulted in cumulative transgene expression that plateaued after 7 days of culture. The use of hydrogel scaffolds that allow cellular infiltration to deliver DNA may result in long lasting signals in vivo, which are essential for the regeneration of functional tissues.
Development of a cellularly degradable PEG hydrogel to promote articular cartilage extracellular matrix deposition
DOI:10.1002/adhm.201400695
URL
PMID:25607633

Healing articular cartilage remains a significant clinical challenge because of its limited self-healing capacity. While delivery of autologous chondrocytes to cartilage defects has received growing interest, combining cell-based therapies with scaffolds that capture aspects of native tissue and promote cell-mediated remodeling could improve outcomes. Currently, scaffold-based therapies with encapsulated chondrocytes permit matrix production; however, resorption of the scaffold does not match the rate of production by cells leading to generally low extracellular matrix outputs. Here, a poly (ethylene glycol) (PEG) norbornene hydrogel is functionalized with thiolated transforming growth factor (TGF-beta1) and cross-linked by an MMP-degradable peptide. Chondrocytes are co-encapsulated with a smaller population of mesenchymal stem cells, with the goal of stimulating matrix production and increasing bulk mechanical properties of the scaffold. The co-encapsulated cells cleave the MMP-degradable target sequence more readily than either cell population alone. Relative to non-degradable gels, cellularly degraded materials show significantly increased glycosaminoglycan and collagen deposition over just 14 d of culture, while maintaining high levels of viability and producing a more widely-distributed matrix. These results indicate the potential of an enzymatically degradable, peptide-functionalized PEG hydrogel to locally influence and promote cartilage matrix production over a short period. Scaffolds that permit cell-mediated remodeling may be useful in designing treatment options for cartilage tissue engineering applications.
Bone regeneration using an alpha 2 beta 1 integrin-specific hydrogel as a BMP-2 delivery vehicle
DOI:10.1016/j.biomaterials.2014.03.055
URL
PMID:24726536
[Cited within: 3]

Non-healing bone defects present tremendous socioeconomic costs. Although successful in some clinical settings, bone morphogenetic protein (BMP) therapies require supraphysiological dose delivery for bone repair, raising treatment costs and risks of complications. We engineered a protease-degradable poly(ethylene glycol) (PEG) synthetic hydrogel functionalized with a triple helical, alpha2beta1 integrin-specific peptide (GFOGER) as a BMP-2 delivery vehicle. GFOGER-functionalized hydrogels lacking BMP-2 directed human stem cell differentiation and produced significant enhancements in bone repair within a critical-sized bone defect compared to RGD hydrogels or empty defects. GFOGER functionalization was crucial to the BMP-2-dependent healing response. Importantly, these engineered hydrogels outperformed the current clinical carrier in repairing non-healing bone defects at low BMP-2 doses. GFOGER hydrogels provided sustained in vivo release of encapsulated BMP-2, increased osteoprogenitor localization in the defect site, enhanced bone formation and induced defect bridging and mechanically robust healing at low BMP-2 doses which stimulated almost no bone regeneration when delivered from collagen sponges. These findings demonstrate that GFOGER hydrogels promote bone regeneration in challenging defects with low delivered BMP-2 doses and represent an effective delivery vehicle for protein therapeutics with translational potential.
The design of reversible hydrogels to capture extracellular matrix dynamics. Nature reviews
DOI:10.1038/natrevmats.2015.12
URL
PMID:29214058
[Cited within: 1]

The extracellular matrix (ECM) is a dynamic environment that constantly provides physical and chemical cues to embedded cells. Much progress has been made in engineering hydrogels that can mimic the ECM, but hydrogel properties are, in general, static. To recapitulate the dynamic nature of the ECM, many reversible chemistries have been incorporated into hydrogels to regulate cell spreading, biochemical ligand presentation and matrix mechanics. For example, emerging trends include the use of molecular photoswitches or biomolecule hybridization to control polymer chain conformation, thereby enabling the modulation of the hydrogel between two states on demand. In addition, many non-covalent, dynamic chemical bonds have found increasing use as hydrogel crosslinkers or tethers for cell signalling molecules. These reversible chemistries will provide greater temporal control of adhered cell behaviour, and they allow for more advanced in vitro models and tissue-engineering scaffolds to direct cell fate.
Hydrogels with tunable stress relaxation regulate stem cell fate and activity
DOI:10.1038/nmat4489
URL
PMID:26618884

Natural extracellular matrices (ECMs) are viscoelastic and exhibit stress relaxation. However, hydrogels used as synthetic ECMs for three-dimensional (3D) culture are typically elastic. Here, we report a materials approach to tune the rate of stress relaxation of hydrogels for 3D culture, independently of the hydrogel's initial elastic modulus, degradation, and cell-adhesion-ligand density. We find that cell spreading, proliferation, and osteogenic differentiation of mesenchymal stem cells (MSCs) are all enhanced in cells cultured in gels with faster relaxation. Strikingly, MSCs form a mineralized, collagen-1-rich matrix similar to bone in rapidly relaxing hydrogels with an initial elastic modulus of 17 kPa. We also show that the effects of stress relaxation are mediated by adhesion-ligand binding, actomyosin contractility and mechanical clustering of adhesion ligands. Our findings highlight stress relaxation as a key characteristic of cell-ECM interactions and as an important design parameter of biomaterials for cell culture.
Modulating viscoelasticity, stiffness, and degradation of synthetic cellular niches via stoichiometric tuning of covalent versus dynamic noncovalent cross-linking
DOI:10.1021/acscentsci.8b00170
URL
PMID:30159394
[Cited within: 1]

Viscoelasticity, stiffness, and degradation of tissue matrices regulate cell behavior, yet predictive synergistic tuning of these properties in synthetic cellular niches remains elusive. We hypothesize that reversible physical cross-linking can be quantitatively introduced to synthetic hydrogels to accelerate stress relaxation and enhance network stiffness, while strategic placement of isolated labile linkages near cross-linking sites can predict hydrogel degradation, both of which are essential for creating adaptive cellular niches. To test these hypotheses, chondrocytes were encapsulated in hydrogels formed by biorthogonal covalent and noncovalent physical cross-linking of a pair of hydrophilic building blocks. The stiffer and more viscoelastic hydrogels with DBCO-DBCO physical cross-links facilitated proliferation and chondrogenic ECM deposition of encapsulated cells by dissipating stress imposed by expanding cell mass/ECM via dynamic disruption/reformation of physical cross-links. Degradation of labile linkages near covalent cross-linkers further facilitated cell proliferation and timed cell release while maintaining chondrogenic phenotype. This work presents new chemical tools for engineering permissive synthetic niches for cell encapsulation, 3D expansion, and release.
Engineering proteolytically-degradable artificial extracellular matrices
DOI:10.1016/j.progpolymsci.2014.07.003 URL [Cited within: 1]
Noninvasive in vivo 3D bioprinting
DOI:10.1126/sciadv.aba7406
URL
PMID:32537512
[Cited within: 1]

Three-dimensional (3D) printing technology has great potential in advancing clinical medicine. Currently, the in vivo application strategies for 3D-printed macroscale products are limited to surgical implantation or in situ 3D printing at the exposed trauma, both requiring exposure of the application site. Here, we show a digital near-infrared (NIR) photopolymerization (DNP)-based 3D printing technology that enables the noninvasive in vivo 3D bioprinting of tissue constructs. In this technology, the NIR is modulated into customized pattern by a digital micromirror device, and dynamically projected for spatially inducing the polymerization of monomer solutions. By ex vivo irradiation with the patterned NIR, the subcutaneously injected bioink can be noninvasively printed into customized tissue constructs in situ. Without surgery implantation, a personalized ear-like tissue constructs with chondrification and a muscle tissue repairable cell-laden conformal scaffold were obtained in vivo. This work provides a proof of concept of noninvasive in vivo 3D bioprinting.
Intravital three-dimensional bioprinting
DOI:10.1038/s41551-020-0568-z
URL
PMID:32572195
[Cited within: 1]

Fabrication of three-dimensional (3D) structures and functional tissues directly in live animals would enable minimally invasive surgical techniques for organ repair or reconstruction. Here, we show that 3D cell-laden photosensitive polymer hydrogels can be bioprinted across and within tissues of live mice, using bio-orthogonal two-photon cycloaddition and crosslinking of the polymers at wavelengths longer than 850 nm. Such intravital 3D bioprinting-which does not create by-products and takes advantage of commonly available multiphoton microscopes for the accurate positioning and orientation of the bioprinted structures into specific anatomical sites-enables the fabrication of complex structures inside tissues of live mice, including the dermis, skeletal muscle and brain. We also show that intravital 3D bioprinting of donor-muscle-derived stem cells under the epimysium of hindlimb muscle in mice leads to the de novo formation of myofibres in the mice. Intravital 3D bioprinting could serve as an in vivo alternative to conventional bioprinting.