Introduction
The surprising discovery, first reported in 2008, that tobacco mosaic virus (TMV) accelerates bone differentiation of mesenchymal stem cells (MSCs), and curiosity regarding this effect, have been inspiring extensive research in our group.1-7 We demonstrated that a TMV substrate promoted MSC osteogenesis by upregulating bone morphogenetic protein-2 (BMP-2) gene expression.4, 5 With the well-known clinically-proven role of BMP-2 in bone repair, and the discovery that it is highly upregulated on a two-dimensional virus-coated cell culture surface, the search was on for more clinically-compliant three-dimensional (3D) systems incorporating the virus and its bio-functional derivatives which could be designed and tested for tissue engineering applications.3, 6, 7 3D hydrogels provide an excellent environment for cell-cell and cell-matrix interaction. Moreover, hydrogel systems can be directly used in subsequent tissue-engineering applications. Owing to the hydrophilic polymer network with high water content, and solid-like mechanical properties, hydrogels can be ideal systems for providing a tailored microenvironment supporting microvasculature and tissue growth.8-12 In addition, the exchange of electrolytes, small molecules and cytokines along with cell migration are allowed by the porous structure that is essential for cell viability and tissue growth.13, 14 A hydrogel system must be able to accommodate and present bio-functionality synergising desirable physical, chemical, and cell-responsive properties to serve different biomedical applications.15, 16
We have previously reported our creation of a porous sponge-like scaffold based on alginate hydrogel pre-cast with TMV and its mutant RGD (TMV-RGD) for enhanced osteogenesis of 3D-cultured MSCs.17 In addition, we have created a hybrid TMV scaffold in a simple injectable form, using thiol-ene “click” chemistry, to promote the differentiation of MSCs to cartilage.3 Methacrylated hyaluronic acid (MeHA) polymer was cross-linked with cysteine-inserted TMV mutants (TMV1cys) through thiol-ene reaction, with the hydrogel structured under physiological conditions. Using this TMV-hydrogel hybrid system as 3D scaffold, enhanced collagen accumulation and BMP-2 upregulation were observed, resulting in promotion of the in vitro chondrogenesis of MSCs. In another model hydrogel scaffold, incorporation of RGD-inserted TMV mutants (TMV-RGD) into the polysaccharide-based hydrogel further promoted the in vitro differentiation of BMSCs, while also demonstrating an increase in collagen production.17 These results suggested that TMV and TMV-RGD could play critical roles in directing MSC chondrogenesis in a hydrogel microenvironment.
These continuing efforts have been focusing on a pre-clinical series of in vivo studies. We have tested the effects of TMV and TMV-RGD mutant in a 3D sponge-like alginate hydrogel based on their toxicity in both mouse and rat models and their efficacy regarding bone regeneration potential in a cranial defect rat model. TMV, as a plant virus nano-scaffolding material, might have raised concerns regarding its effect on the humoral immune response, which could be a potential drawback of using plant virus materials in clinical tissue-engineering applications. The antigenicity of TMV and its RGD mutant in the hydrogel implant was evaluated and the results demonstrated that the virus was biocompatible in the form of a hydrogel implant using a BALB/c mouse model. Immune response towards TMV was compromised by the immobilisation and slow release of the virus from the bulk hydrogels.18 In another study using normal rat model with cranial bone removal, inflammation and healing of confined critical size cranial bone defects when treated with porous alginate hydrogels carrying TMV and TMV-RGD were comparatively investigated. Both types of scaffolds permitted the healing of critical size defects (CSDs) in the cranial segmental bone and demonstrated superiority in bone remodelling and maturation compared to the blank carrier control, as shown by histological data.19 In correlation with the mouse model, no systemic inflammatory reactions nor severe immunogenic adverse effects were observed.18
Hyaluronic acid (HA) is a polysaccharide commonly found in the human body which is a component of the connective tissue microenvironment important for cell survival, motility, differentiation and proliferation. It has many biological roles in the body such as tissue organisation, wound healing, and angiogenesis. HA is a linear chain polysaccharide composed of repeating disaccharides, glucuronic acid and N-acetylglucosamine. HA possesses negative charges that can attract water molecules conferring the ability to form hydrogels suitable as extracellular matrix surrogates. One of the most interesting polymer-based delivery systems, which can form gels after application to the delivery site, is the “in situ gel system”. Such a system can also offer a variety of mechanical features similar to a solid-like material, but, on the other hand, can be administered in a liquid form that could be beneficial for clinical operation.20 With this in situ gel character, the system allows simultaneous incorporation of other therapeutics such as proteins, cytokines, nanoparticles, and cells, for which personalised dosing may be critical during the administration operation. Moreover, the material has the potential to fill the irregular spaces of a defect area. The sol-to-gel transition of an in situ gel can be induced by various mechanisms, for example; ultraviolet light is one popular example of a stimulant for in situ photo-crosslinking of hydrogels. We here utilised a hydrogel carrier system with a chemical trigger. The sol-to-gel transition occurs at a pre-defined time after the chemical crosslinker is added into the formulation mixture containing modified HA as the gel-forming polymer. This system has been well-characterised in vitro in terms of its physical properties, microstructure, mechanical properties, and cyto-compatibility. This hydrogel formulation was developed from medium molecular weight HA, modified with a methacrylate group, as a major scaffold component of the hydrogel. The in situ crosslinking with dithiothreitol was induced via a Michael addition reaction under physiological conditions.21, 22 Gelation time, porosity and mechanical properties of the hydrogel can be tailored by altering the degree of modification of HA (methacrylation of HA), the concentration of HA, and the ratio of crosslinkers.23 However, for our proof-of-concept study focusing on the in vivo efficacy of the TMV and TMV-RGD incorporated into an HA hydrogel carrier for use in bone repair, the in situ gel was pre-cast in a mould and implanted into the defect site.
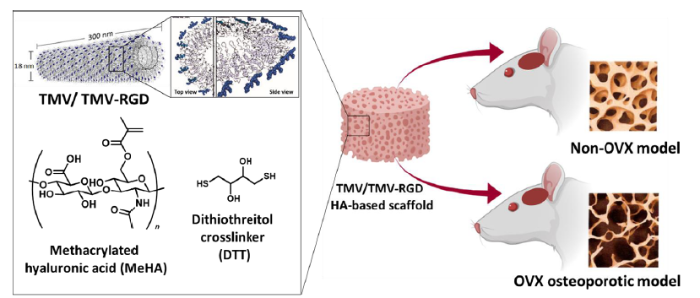
Osteoporosis can be caused by many factors with multiple gene interactions. Patients with this non-communicable disease are prone to suffer fragility fractures of vertebrae, hips and radii. A critical concern for this group of patients is the complications, which they can experience following serious fractures that may occur due to accidents and trauma. The delays in healing, regeneration and recovery can be significant due to aging and the pre-existing genetic background. The public apprehension of disease is tremendous and continuously growing, globally, while average life expectancy is also on the upsurge. The clinical need for bone repair, systemic or locally-induced bone regeneration and bone tissue engineering has increased and become a challenge for health-care systems.24 A study suggested that osteoporosis is a major problem contributing to the increasing cost of medical care in the European Union in which mortality and morbidity rates are rising in the elderly with the disease. The incidence of osteoporotic fractures is higher in women compared to men, since women after menopause experience hormonal alterations which can cause a serious loss of bone mass.25 One of the most commonly-used animal models in research on postmenopausal osteoporosis is the ovariectomised (OVX) rat model. It has been evidenced that statistically-significant bone loss is observed in the proximal tibial metaphysis in just 14 days after the OVX induction of normal rats. When rats were subjected to ovary removal, bone resorption exceeded bone formation initially, causing bone loss. After a certain period, bone remodelling eventually reached a steady state, with a balance between resorption and formation, for example; a 90-day interval was required for the proximal tibial metaphysis to reach a steady state.26, 27 In this report, we explored the effect of TMV and its RGD mutant embedded within an in situ HA-based hydrogel on bone regeneration in both normal and osteoporotic model rats as shown in Figure 1.
Figure 1.
Figure 1.
Schematic illustration of our study design. Methacrylated hyaluronic acid (MeHA) was synthesised and used with incorporated virus particles as a hydrogel structural platform to fill skull defects in osteoporotic and normal Sprague-Dawley rats. The RGD mutant TMV graphic was generated from PyMol with coordinates 2TMV from the Protein Data Bank (www.rcsb.org) and created with BioRender.com. OVX: ovariectomised; RGD: arginyl-glycyl-aspartic acid; TMV: tobacco mosaic virus.
Methods
Preparation of virus-functionalised MeHA hydrogel and synthesis of MeHA
MeHA was synthesized following the published protocol.3 In this study, HA with molecular weight between 40-60 kDa was used. The reaction was one step and was carried out at a pH maintained above 8.0. Methacrylate anhydride was added twice during the entire process. Briefly, HA was dissolved at 1 wt% in potassium phosphate buffer, pH 8, and methacrylic anhydride, at a 6- to 10-fold molar excess relative to the HA disaccharide repeat unit, was added dropwise to the solution at 4°C. The pH of the two-phase reaction mixture was adjusted to 8.0 with 5 M aqueous NaOH, and the reaction was continued for 24 hours at 4°C with frequent readjustment of the solution pH to keep it above 8.0. The product was dialyzed against milli-Q water for at least 48 hours, followed by centrifugation to obtain the precipitate, which was then flash-frozen in liquid nitrogen and then lyophilised, resulting in a dry product that was further analysed and the degree of modification calculated by proton nuclear magnetic resonance. The expected degree of modification was 40-50% of total functional HA monomer molecules.
Hydrogel fabrication and functionalisation with virus particles
In general, to fabricate the HA hydrogels, MeHA polymers with a degree of modification of 40-50% were dissolved in phosphate-buffered saline at a concentration of 3 wt%, followed by the cross-linking agent, dithiothreitol, that was added at a molar ratio of thiol/ene = 2:1. The pH of the mixture was adjusted to 8.0 with 2 M aqueous NaOH, and gelation took place at the predicted time. To form the TMV-based HA hydrogels, the same procedure was adopted with incorporation of wild-type TMV or TMV-RGD mutant suspended in potassium phosphate buffer to make a final virus concentration of 0.1 wt% in the pre-gel solution before the addition of dithiothreitol.
Animals, experimental design and treatments
The animal model protocols of this study were approved by the Ethics Committee on Animal Use of the Zhenjiang Affiliated First People’s Hospital affiliated to Jiangsu University. Twelve-week-old female Sprague-Dawley rats were purchased from Cavens (Changzhou, China). Twenty-four rats were randomly divided into an osteoporosis group, in which osteoporosis was established by ovariectomy (OVX group), and a normal group, with twelve rats in each group. The OVX surgery was carried out using the same procedure as previously described in a mouse model.28, 29 Briefly, rats were anesthetised with 1% pentobarbital (40 mg/kg). then placed in a prone position on a fixed table, and the limbs and head were fixed. Conventional skin preparation was conducted with a 1.5 cm radius, the operative area was disinfected, and covered with sterile drapes. A 1.5 cm dorsal incision was made at the posterior midline of the back with a scalpel, below the costal margin of both sides as the centre, and below the costal margin of both sides as the radius. After ligation, complete removal of the ovary was confirmed by careful observation and the animal was checked for any bleeding in the uterine horn. After dressing, the muscle and fascia were sutured, then the skin was closed using discontinuous sutures. The contralateral ovary was excised in the same way and the wound was closed. Sham operation of the control group was performed following the same procedure as that of the experimental group, but the ovary was preserved and adipose tissue around the ovary of equal weight was removed.
At 12 weeks post-OVX, a skull defect 8 mm in diameter was created in all animals. Within each group (normal and OVX groups), three rats were treated as a mock group without hydrogel treatment, while the remaining nine rats constituted the treatment groups. In the treatment groups, each rat was randomly assigned to treatment with one of three different types of hydrogel (n = 3 per treatment) to replace the defect. The hydrogels were: 1) blank MeHA, 2) wild-type TMV-incorporated MeHA, and 3) TMV-RGD mutant-incorporated MeHA.
Disk-shaped hydrogels were implanted to fill the cranial bone defects generated. Rats were given 2-4 mg/kg of subcutaneous carprofen 2-4 hours before surgery for analgesia. Procaine penicillin (60,000 IU) was given subcutaneously for infection prophylaxis. Instruments were autoclaved prior to use to minimise the risk of post-surgical infection and cross-contamination. The animals were anesthetised with 3% isoflurane in oxygen. After the appropriate level of anaesthesia was obtained, the fur over the cranium was shaved, and the skin was cleansed with normal saline to remove loose hair. A dry heating pad was used to maintain the animal’s body temperature during surgery. A midline incision was made from the middle of the nasofrontal area to the external occipital protuberance utilising a sterile No. 15 scalpel blade. Full-thickness skin flaps were reflected laterally with a periosteal elevator to expose the calvaria. An 8-mm craniotomy was performed in all animals utilising a dental handpiece system at low speed and with copious sterile saline irrigation to remove loose debris and to avoid the generation of frictional heat at the surgical site. The bone defects were standardised using an 8-mm diameter trephine bur to outline the treatment area. A PTFE barrier membrane (kindly provided by Osteogenics Biomedical, Lubbock, TX, USA) was inserted to form a barrier on top of the dura. Each hydrogel was carefully placed into the defect, and a second PTFE membrane was placed over the defect and under the periosteum. The periosteum was repositioned and the defect was then closed with continuous interlocking sutures using nylon suture material. Postoperative analgesia to relieve pain (carprofen, 2-4 mg/kg) was given orally at least once every 24 hours for 48 hours. The animals were weighed before and after surgery then daily for the first 2 days and weekly until the end of the experiment. Signs of pain or distress were monitored daily after surgery.
Haematological assay
A complete blood count was performed immediately after each blood withdrawal. A 20 μL blood sample was transferred into a 1 mL microtainer tube with dipotassium ethylenediamine tetraacetic acid. Each tube was analysed in an automated blood counter machine (Shanghai Jill Biochemical Co. Ltd., Shanghai, China). The numbers of total white blood cells, haemoglobin, lymphocytes (LYM), mean platelet volume (MPV), monocytes (MON), neutrophils, platelets and red blood cells were evaluated.
Histological analysis
The cranial tissues of the experimental group and the control group were collected and fixed in 10% formalin. The treated tissue was embedded in paraffin and 5 µm paraffin sections were prepared. Hematoxylin and eosin (H&E) and Masson’s trichrome staining were then performed on the tissue sections following standard procedures. The degree of tissue inflammation was graded as 0, 1, 2, or 3 according to the number of inflammatory cells (mainly LYMs, neutrophils, macrophages, etc.), while the degree of fibroconnective tissue and of vascularisation were observed in or around the implant centre. The bone tissue formation in the Masson’s trichrome staining was graded using an ordinal scoring system by the pathologist expert in scoring experimental animal tissue sections. At least three sections of each sample of excised animal tissue were read and evaluated. In this experiment, the degree of tissue inflammation was scored as 0 (normal or none); 1 (rarely seen); 2 (slightly increased, 5 to 20 per 40× field); or 3 (obviously increased, > 20/40× field). Bone regeneration was also evaluated and scored according to the degree of new bone formation as follows: 1, 2, 3, and 4 for none, rarely seen; ≤ 2/40× field, few or moderate; 3-10/40× field, and dense; > 10/40× field, respectively.
Bone structure analysis
The skull structure of the operated animals was analysed using micro-computed tomography (Micro CT, Skyscan1172, Antwerp, Belgium). The analysis conditions were 80 kV, 124 μA and resolution was 8 μm. Structural parameters of the skull were analysed using the built-in software. Total bone mineral density was measured, and the trabecular parameters of bone volume/total volume, trabecular number, trabecular pattern factor, and bone surface area per unit total volume were evaluated. Two-dimensional and 3D bone structure image slices were reconstructed.
Statistical analysis
Data are expressed as the mean ± standard deviation (SD). Differences between groups were analysed by one-way analysis of variance using GraphPad Prism 5 (GraphPad Software Inc., La Jolla, CA, USA) and Origin 6.1 (OriginLab Corporation, Northampton, MA, USA). A P value less than 0.05 was considered statistically significant.
Results
Description of animal behaviour
The skull defect surgery caused the OVX animals some weaknesses. The OVX animals with bone removed required a longer period to recover from the skull surgery with a longer period of persistent hair yellowing indicating the lack of self-cleaning and self-grooming activities. At 1-4 weeks after skull defect creation, the OVX animals showed some behavioural changes, with reduced drinking and eating compared to the normal rats. After week 4, all rats performed the same activities with no major differences between the groups. In general, the animals recovered from the surgery and the wounds healed within 10 weeks. Both OVX and normal rats with skull defects, however, exhibited a slight behavioural deviation with their heads occasionally rubbed into the bedding material. This behaviour continued up to the end of the experiment at 10 weeks post-operatively.
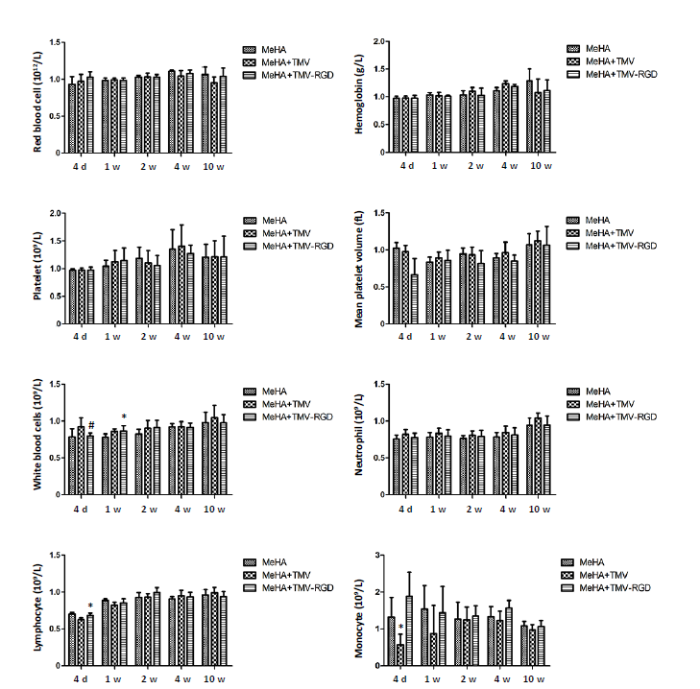
Routine blood tests in animals
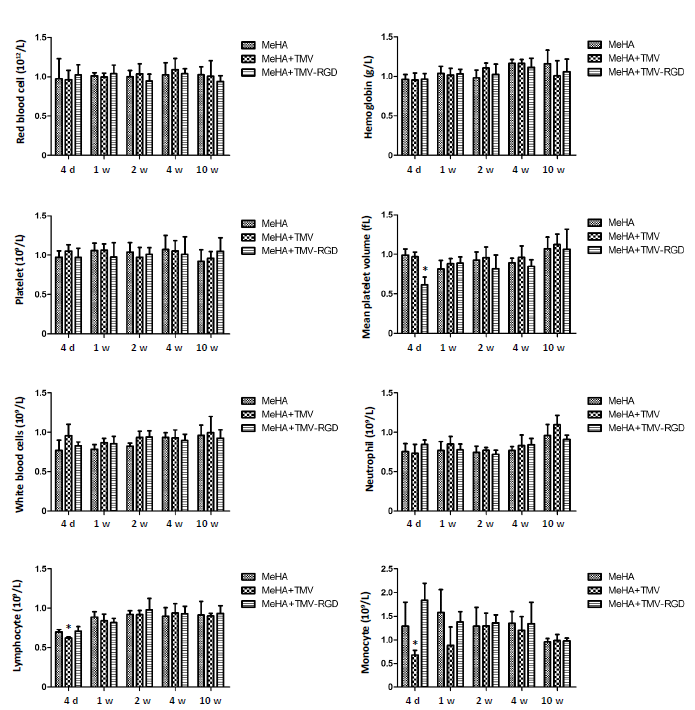
Total blood counts were routinely performed in both OVX rats and normal rats in order to rule out any systemic effect from the treatments. Blood samples from each group of rats at 4 days and at 1, 2, 4 and 10 weeks were analysed. The parameters analysed included numbers of total white blood cells, haemoglobin, LYM, MPV, MON, neutrophils, platelets and red blood cells. In the control group, haemoglobin, neutrophil, red blood cell and white blood cell numbers were comparable between each type of material treatment and remained relatively constant across all time-points (Figure 2). There was no significant difference in the number of LYM between groups except for the result on day 4 showing lower number with the MeHA + TMV group. Slight increases of LYM were observed in all groups during the experimental period up to week 2. The number of MPV, MON and platelets showed no significant difference between the different groups. However, the MON count on day 4 showed a decrease in cell numbers in the group that was implanted with TMV-incorporated MeHA gel (MeHA + TMV). Figure 3 shows the blood test results from OVX rats. Similar trends were observed across all types of blood cells. No significant differences were observed between treatment groups except for the LYM, MPV, and MON. The LYM and MON numbers from the TMV-incorporated MeHA gel were less than the other groups, a finding which was in agreement with the results observed in the normal rats. MPV showed significant reductions in the OVX rats treated with TMV-RGD-incorporated MeHA gel (MeHA + TMV-RGD). However, within 2 weeks, regular blood counts were restored in all rats in both control and OVX groups with no significant differences among any of the types of hydrogel treatment.
Figure 2.
Figure 2.
Haematological analysis of non-OVX animals treated with three different hydrogel materials (MeHA, MeHA + TMV, and MeHA + TMV-RGD) to fill their skull defects. Total blood counts were performed at five time points (4 days (d), 1, 2, 4, and 10 weeks (w)). Data are expressed as the mean ± SD (n = 3). *P < 0.05, vs. MeHA group; #P < 0.05, vs. MeHA + TMV group (one-way analysis of variance). MeHA: methacrylated hyaluronic acid; OVX: ovariectomised; RGD: arginyl-glycyl-aspartic acid; TMV: tobacco mosaic virus.
Figure 3.
Figure 3.
Haematological analysis of OVX animals treated with three different materials (MeHA, MeHA + TMV, and MeHA + TMV-RGD) to fill their skull defects. Total blood counts were performed at five time points (4 days (d), 1, 2, 4, and 10 weeks (w)). Data are expressed as the mean ± SD (n = 3). **P < 0.05, vs. MeHA group (one-way analysis of variance). MeHA: methacrylated hyaluronic acid; OVX: ovariectomised; RGD: arginyl-glycyl-aspartic acid; TMV: tobacco mosaic virus.
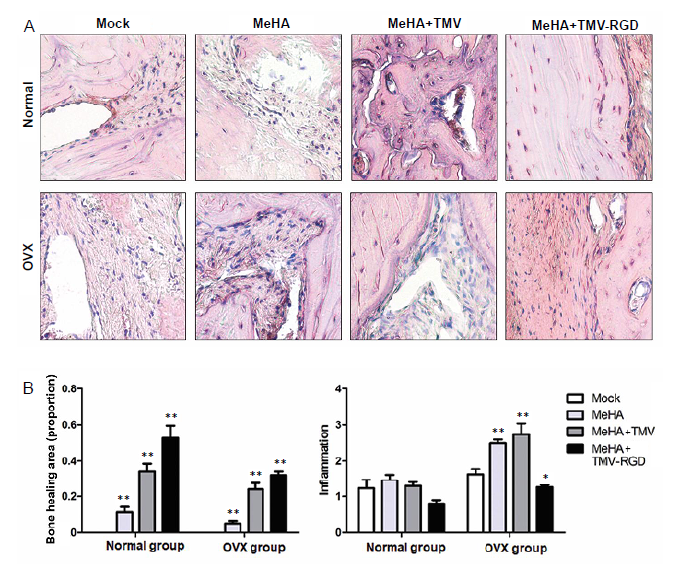
Hematoxylin and eosin staining of inflammatory cell infiltration
After 10 weeks, histological assessment of inflammatory cells around the implant sites revealed that inflammatory cell infiltration was significantly increased in the OVX rats in comparison to the normal rats with all types of hydrogel implant, but no difference was observed between the sham groups. Within the normal rats, MeHA + TMV-RGD treatment led to a significant reduction of inflammation around the implant site (mean score for degree of inflammation = 0.8 ± 0.10), compared to the sham control without hydrogel (MeHA) and the MeHA + TMV groups (average score = 1.2 ± 0.25, 1.5 ± 0.15, and 1.3 ± 0.10, respectively). For the OVX rats, the minimal inflammation score of 1.3 ± 0.06 was also observed in the MeHA + TMV-RGD treatment groups while the MeHA and MeHA + TMV groups showed significantly higher inflammation scores (average score = 2.5 ± 0.10 and 2.7 ± 0.31, respectively) compared to the sham control (1.6 ± 0.15). Interestingly, these results suggest that TMV-RGD could compromise the local inflammatory response caused by the surgical skull defect. These data show that neither the virus incorporated into the hydrogel nor the hydrogel itself induced excessive inflammation or any adverse tissue reactions in the rat cranial defect model.
Bone healing was also evaluated in the H&E-stained sections (Figure 4). The void spots in the histological sections represented the defect area without any new tissue formation, and their sizes were calculated as a percentage of the bone healing area. Figure 4 shows significant increases in the area of bone tissue formation from all types of implants in the normal rats, with the highest score given to the group with implanted TMV-RGD (mean score = 0.5 ± 0.06), followed by the TMV (mean score = 0.3 ± 0.04), and the blank MeHA hydrogel (mean score = 0.1 ± 0.02). The same trend was also observed in the OVX model, with the most intense bone tissue formation scoring a mean of 0.3 ± 0.02 for the sections from TMV-RGD MeHA hydrogel-implanted rats. Overall, the bone formation scores given to each type of implant were significantly lower in the OVX rats compared to the normal groups. This is expected because OVX causes impaired bone regeneration. Altogether, both evaluations from H&E staining indicated that all types of hydrogels had a positive effect in inducing bone formation, with the TMV-RGD hydrogel implant having the greatest bone induction potential and the best in vivo compatibility.
Figure 4.
Figure 4.
Inflammatory cell infiltration observed by hematoxylin and eosin staining. (A) Photomicrographs of hematoxylin and eosin-stained sections from skull defects implanted with each type of hydrogel (MeHA, MeHA + TMV, and MeHA + TMV-RGD) (original magnification, 40×). The new bone is visible as a compact structure with a pink colour. The connective tissue can be seen as a structured network of cells in a purple colour. (B) Hematoxylin and eosin histological scoring of the three types of hydrogels confirm the difference in proportion of bone healing area and degree of inflammation (arbitrary scoring). Data are expressed as the mean ± SD (n = 3). *P < 0.05, **P < 0.01, vs. sham group (one-way analysis of variance). MeHA: methacrylated hyaluronic acid; OVX: ovariectomised; RGD: arginyl-glycyl-aspartic acid; TMV: tobacco mosaic virus.
Masson’s trichrome staining
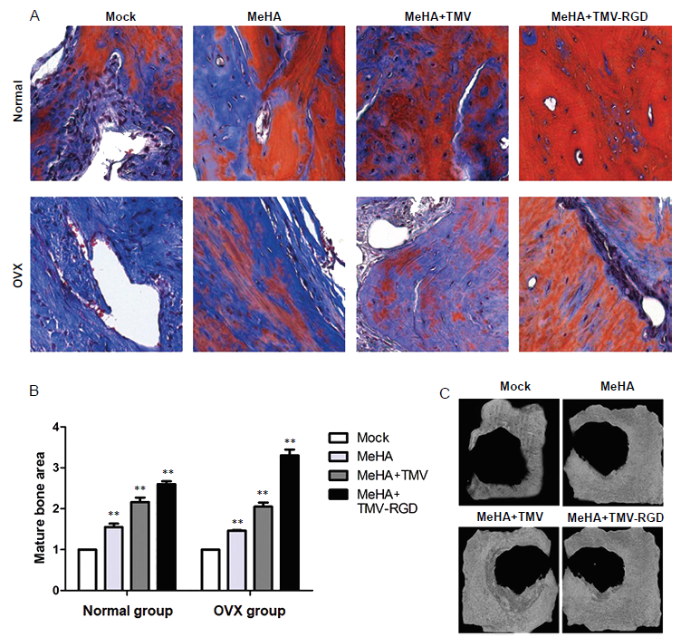
Masson’s trichrome staining was used to confirm the results of H&E staining in terms of bone tissue regeneration potential induced by the implanted hydrogels in the cranial defects of rats in both control and OVX groups at 10 weeks post-surgery to identify the amount of new calcified bone in the defect area.
As shown in Figure 5A, no or only minimal new bone was observed in the control group at 10 weeks post-surgery, especially in the OVX rat group. The degree of bone injury in the OVX group was more serious than that in the normal group. In both rat models, the formation of new bone in the defect area treated with any of the three types of MeHA-based hydrogel was pronounced. The sham groups showed highly intense blue staining with less red stain indicating lower density of bone tissue. Consistent with H&E analysis, all three implant materials showed increased red staining, among which the TMV-RGD material group had the most intense red stain, indicating remarkably increased bone tissue repair.
Figure 5.
Figure 5.
Histopathological analysis of bone substitute hydrogel implants stained with Masson’s trichrome. (A) Photomicrographs of corresponding Masson’s trichrome-stained sections from each type of hydrogel (original magnification, 40×). In general, Masson’s trichrome stains mature bone with osteoid formation red, whilst blue stain indicates developing calcified bone. (B) Histological scoring of the three types of hydrogels in tissue sections show the different amounts of mature bone area. Data are expressed as the mean ± SD (n = 3). *P < 0.05, **P < 0.01, vs. sham group (one-way analysis of variance). (C) Micro CT images of the cranial-defected bones dissected from non-OVX groups with different types of hydrogel implants at 10 weeks post-surgery. MeHA: methacrylated hyaluronic acid; OVX: ovariectomised; RGD: arginyl-glycyl-aspartic acid; TMV: tobacco mosaic virus.
Figure 5B shows the mean scores corresponding to the mature bone area observed in the implanted groups when normalised to the sham group within each particular model. The same trend was shared between the two animal models (normal and OVX) with the highest bone formation in the TMV-RGD MeHA implant group. However, the OVX rats appeared to show only a minimal response towards all treatments as expected.
The skull defect area from each treatment group, in both control and OVX rats, was subjected to micro-CT. In the normal rats, the treatment groups showed slight differences in the area of the defect, correlating with the histological score.
As shown in Figure 5C, the unmodified hydrogel induced minimal growth from the circular edge of the defect over the calvarial CSDs in the sham control rats, since intramembranous bone formation starts only from the defect margins and progresses centripetally to eventually fill the original defect.30 The penetration of new bone tissue formation into the CSDs seemed to be enhanced progressively in respect to the hydrogel implants with TMV and TMV-RGD1. However, with the induction of osteoporosis by OVX, micro-CT analysis showed no observable regenerated hard bone structure in response to any treatments within the observation period. A possible explanation is that the 10-week implantation period before animal sacrifice was not long enough to allow the complete formation of calcified hard bone tissue due to compromised performance of bone regeneration in the model animals. A previous study demonstrated significant impairment of the amount of newly-formed bone observed in OVX animals at three and six months of healing, compared with the sham-operated animals.31 This could indicate that the critical period for observable healing would need to be longer after OVX. In addition, the experimental challenge for bone defects used in this study involved the combination of creation of a defect (calvarial CSD) and osteoporosis induction. A pre-clinical report from OVX animals demonstrated that osteoporosis may reduce bone mechanical properties and retard post-fracture bone healing.32, 33
Reduced bone formation and lower bone quality have also been reported in grafted bony defects, in calvarial CSDs treated with guided bone regeneration and a graft.31, 34 As calvarial CSDs are known to be relatively more challenging compared to other research models, for example, infrabony or alveolar defects, where all the bony surfaces surrounding the defects may facilitate the regeneration process and act as a reservoir of osteoprogenitor cells,30 this may have been responsible for the low levels of bone regeneration observed in our study.
Discussion
This study was designed to investigate two major aspects. First, to produce proof-of-concept findings regarding whether the cellular response towards TMV could be implemented under physiological conditions. This study was considered part of the continuing effort to emphasise the concept of utilising plant viruses in the biomaterial platform to innovate molecular designs featuring the material’s nanostructure for application in tissue regeneration by manipulation of cell interactions to induce a cellular response. Second, the experiment was designed as a step closer towards medical use of the plant virus in the hydrogel platform for regenerative medicine, for the treatment of tissue damage that could arise due to aging or trauma. The materials were used as cranial defect bone fillers and were tested in both normal rats as well as in an osteoporosis model. The result showed that bone healing was induced by the hydrogel scaffolds modified with TMV or with the TMV-RGD mutant. Bone formation was enhanced by incorporation of the RGD peptide onto the surface of the TMV particle by genetic modification of TMV. This virus-functionalisation approach could potentially be a method of choice for the modification of biocompatible materials used in biomedical treatments.
Author contributions
Conceptualisation: JYuan, JAL; methodology: JYuan, JAL, PM; software: JYuan, ZZ, BL, JYang; validation: ZZ, BL, YL; formal analysis, data curation, and writing—original draft preparation: JYuan, JAL; investigation: JYuan, JYang, PM; resources: JYuan, JAL; writing—review and editing, YL, PM; visualisation: JAL, PM; supervision: JAL; project administration:, JYuan, ZZ, BL, JYang; funding acquisition: JYuan, YL, JAL. All authors have read and agreed to the published version of the manuscript.
Financial support
This research was supported by the Thailand Research Fund and Office of the Higher Education Commission (No. MRG6180264), Chulalongkorn University, and the National Natural Science Foundation of China (No. 21750110445).
Acknowledgement
The corresponding author (JAL) would like to thank Professor Prasit Pavasant, Faculty of Dentistry at Chulalongkorn University, for his advice. All authors would like to thank Professor Zhaohui Su for the access to instruments and facilities used in this research at Changchun Institute of Applied Chemistry (CIAC), Changchun, China. Graphical abstract and Figure 1 were Created with BioRender.com.
Conflicts of interest statement
The authors declare no conflict of interest.
Data sharing statement
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Reference
Regulation of osteogenic differentiation of rat bone marrow stromal cells on 2D nanorod substrates
DOI:10.1016/j.biomaterials.2009.11.041
URL
PMID:20022632
[Cited within: 1]

Bone marrow stromal cells (BMSCs) possess multi-lineage differentiation potential and can be induced to undergo differentiation into various cell types with the correct combination of chemical and environmental factors. Although, they have shown great prospects in therapeutic and medical applications, less is known about their behavior on nanosurfaces mimicking the extra cellular matrix (ECM). In this report we have employed 2D substrates coated with tobacco mosaic virus (TMV) nanorods to study the differentiation process of BMSCs into osteoblast like cells. TMV is a rod-shaped plant virus with an average length of 300 nm and diameter of 18 nm. The osteogenic differentiation of BMSCs on TMV was studied over time points of 7, 14 and 21 days. We examined the temporal gene expression changes during these time points by real-time quantitative PCR (RT-qPCR) analysis. As expected, osteo-specific genes (osteocalcin, osteopontin and osteonectin) were upregulated and showed a maximum change in expression on TMV at 14 days which was 7 days earlier than on tissue culture plastic (TCP). Based on the genes expression profile generated by RT-qPCR experiments, we proposed that the early interaction of cells with TMV triggers on signaling pathways which regulate speedy expression of osteocalcin in turn, resulting in early mineralization of the cells. To further investigate these regulating factors we studied global changes in gene expression (DNA microarray analyses) during osteogenic differentiation on the nanosubstrate. Multitudes of genes were affected by culturing cells on nanorod substrate, which corroborated our initial PCR findings. Microarray analysis further revealed additional targets influenced by the presence of nanorods on the surface, of which, the expression of bone morphogenetic protein 2 (BMP2) was of particular interests. Further investigation into the temporal change of BMP2, revealed that it acts as a major promoter in signaling the early regulation of osteocalcin on TMV coated substrates.
The synergistic effects of multivalent ligand display and nanotopography on osteogenic differentiation of rat bone marrow stem cells
DOI:10.1016/j.biomaterials.2010.04.017
URL
PMID:20452665

Cell-substrate interactions play a vital role in governing crucial cell functions such as adhesion, proliferation and differentiation. Surface topography and chemical properties can initiate signaling cascades modulating cell behavior. However, mimicking extracellular environment to direct cell functions through cell-surface interactions is challenging. In this report, we employed tobacco mosaic virus (TMV) as a model system to present nanotopographic features along with multivalent ligand display to study osteogenic differentiation of bone marrow stem cells (BMSCs). TMV is a rod shaped plant virus which is 300 nm in length and 18 nm in diameter. A single TMV rod comprises of 2130 identical coat proteins which assemble into the rod-like helical structure around the single strand of RNA. For the present study TMV was chemically modified with phosphate to induce calcium incorporation. Gene regulation during BMSC differentiation on TMV and TMV-phosphate (TMV-Phos) was studied over time points of 7, 14 and 21 days. We examined changes in gene expression of osteospecific genes (osteocalcin, osteopontin and runx2) which indicate that nanofeatures functionalized with phosphate groups exhibited significantly higher up regulation of osteospecific genes. Furthermore, we studied the gene regulation by coating Ti substrates with TMV and TMV-Phos. TMV-Phos coated substrates displayed higher expression of the studied genes as compared to Ti substrates. Our results imply that the differentiation capacity of BMSCs can be significantly enhanced through simple multivalent interactions with simple functional units rather than using complex biomolecules.
Promotion of in vitro chondrogenesis of mesenchymal stem cells using in situ hyaluronic hydrogel functionalized with rod-like viral nanoparticles
DOI:10.1021/acs.biomac.5b01577
URL
PMID:26999064
[Cited within: 3]

This study focuses on the development of injectable hydrogels to mimic the cartilage microenvironment using hyaluronic acid (HA) derivatives as starting materials. Cysteine-inserted Tobacco mosaic virus (TMV) mutants (TMV1cys) could be cross-linked to methacrylated hyaluronic acid (MeHA) polymers by thiol-ene
Virus nanoparticles mediated osteogenic differentiation of bone derived mesenchymal stem cells
A plant virus substrate induces early upregulation of BMP2 for rapid bone formation
DOI:10.1039/c2ib20041d URL [Cited within: 1]
Virus-based scaffolds for tissue engineering applications
DOI:10.1002/wnan.1327
URL
PMID:25521747
[Cited within: 1]

One of the major research directions of tissue engineering is to develop artificial scaffolds that can mimic extracellular matrix (ECM) and support the growth of functional cells for the repair of damaged tissues and organs. Recently, virus particles have expanded as nanosized building blocks for materials applications. Viruses represent monodispersed supramolecular assemblies with organized three-dimensional architecture, which can be isolated in high yield and purity with batch-to-batch consistency. In addition, virus particles can be re-engineered by chemical and genetic modification to incorporate multivalent functional ligands with high density and ordered arrangement. In this review, we highlight that the self-assembly of the reengineered viruses can form two-dimensional and three-dimensional scaffolds, which can be employed to support cell growth and regulate cellular functions such as adhesion, spreading and proliferation. In particular, the application of virus-based scaffolds for directed differentiation of pluripotent stem cells for bone and neural regeneration is discussed. Finally, the in vivo behaviors of virus nanoparticles will be discussed for the consideration of tissue engineering applications.
Upregulation of osteogenesis of mesenchymal stem cells with virus-based thin films
DOI:10.7150/ntno.19974
URL
PMID:29291162
[Cited within: 2]

A major aim of tissue engineering is to develop biomimetic scaffolding materials that can guide the proliferation, self-renewal and differentiation of multipotent stem cells into specific lineages. Cellular functions can be controlled by the interactions between cells and biomaterials. Therefore, the surface chemistry and topography of support materials play a pivotal role in modulating cell behaviors at many stages of cell growth and development. Due to their highly ordered structure and programmable surface chemistries, which provide unique topography as biomaterials, viral nanoparticles have been utilized as building blocks for targeted cell growth and differentiation. This review article discusses the fabrication of two-dimensional virus-based thin film on substrates and highlights the study of the effect of chemical and physical cues introduced by plant virus nanoparticle thin films on the promotion of osteogenic differentiation of BMSCs.
Tissue engineering--current challenges and expanding opportunities
DOI:10.1126/science.1069210
URL
PMID:11834815
[Cited within: 1]

Tissue engineering can be used to restore, maintain, or enhance tissues and organs. The potential impact of this field, however, is far broader-in the future, engineered tissues could reduce the need for organ replacement, and could greatly accelerate the development of new drugs that may cure patients, eliminating the need for organ transplants altogether.
Microscale technologies for tissue engineering and biology
DOI:10.1073/pnas.0507681102
URL
PMID:16477028

Microscale technologies are emerging as powerful tools for tissue engineering and biological studies. In this review, we present an overview of these technologies in various tissue engineering applications, such as for fabricating 3D microfabricated scaffolds, as templates for cell aggregate formation, or for fabricating materials in a spatially regulated manner. In addition, we give examples of the use of microscale technologies for controlling the cellular microenvironment in vitro and for performing high-throughput assays. The use of microfluidics, surface patterning, and patterned cocultures in regulating various aspects of cellular microenvironment is discussed, as well as the application of these technologies in directing cell fate and elucidating the underlying biology. Throughout this review, we will use specific examples where available and will provide trends and future directions in the field.
Progress in tissue engineering
DOI:10.1038/scientificamerican0109-64 URL PMID:19186751
Microengineered hydrogels for tissue engineering
DOI:10.1016/j.biomaterials.2007.07.021
URL
PMID:17707502

Hydrogels have been extensively used in various biomedical applications such as drug delivery and biosensing. More recently the ability to engineer the size and shape of biologically relevant hydrogels has generated new opportunities in addressing challenges in tissue engineering such as vascularization, tissue architecture and cell seeding. Here, we discuss the use of microengineered hydrogels for tissue engineering applications. We will initially provide an overview of the various approaches that can be used to synthesize hydrogels with controlled features and will subsequently discuss the emerging applications of these hydrogels.
Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering
DOI:10.1016/j.biomaterials.2010.02.044
URL
PMID:20303169
[Cited within: 1]

In this review, we explore different approaches for introducing bioactivity into poly(ethylene glycol) (PEG) hydrogels. Hydrogels are excellent scaffolding materials for repairing and regenerating a variety of tissues because they can provide a highly swollen three-dimensional (3D) environment similar to soft tissues. Synthetic hydrogels like PEG-based hydrogels have advantages over natural hydrogels, such as the ability for photopolymerization, adjustable mechanical properties, and easy control of scaffold architecture and chemical compositions. However, PEG hydrogels alone cannot provide an ideal environment to support cell adhesion and tissue formation due to their bio-inert nature. The natural extracellular matrix (ECM) has been an attractive model for the design and fabrication of bioactive scaffolds for tissue engineering. ECM-mimetic modification of PEG hydrogels has emerged as an important strategy to modulate specific cellular responses. To tether ECM-derived bioactive molecules (BMs) to PEG hydrogels, various strategies have been developed for the incorporation of key ECM biofunctions, such as specific cell adhesion, proteolytic degradation, and signal molecule-binding. A number of cell types have been immobilized on bioactive PEG hydrogels to provide fundamental knowledge of cell/scaffold interactions. This review addresses the recent progress in material designs and fabrication approaches leading to the development of bioactive hydrogels as tissue engineering scaffolds.
Porous poly(vinyl alcohol)-hydrogel matrix-engineered biosynthetic cartilage
DOI:10.1089/ten.TEA.2010.0322
URL
PMID:20799889
[Cited within: 1]

The objective of this study was to fabricate hydrogel matrix-engineered biosynthetic cartilage using a porous poly(vinyl alcohol) hydrogel (PVA-H) and articular chondrocytes. Chondrocytes were suspended in fibrin gel (FG) or saline carriers and injected into porous PVA-H discs and three-layered constructs (PVA-H between devitalized cartilage). After implantation in nude mice, PVA discs were explanted at 6 weeks and subjected to creep testing for a 20 h period. The three-layered constructs were explanted at 12 weeks and subjected to tensile testing to determine the strength of the interface between the engineered hydrogel and devitalized cartilage. Histological analysis revealed PVA-H porous channels occupied by chondrocytes. Extracellular matrix was identified by Safranin-O and toluidine blue stains. Immunohistochemical analysis revealed a positive stain for COL II and scant staining for COL I. Creep and relaxation response of PVA-FG-chondrocyte constructs was similar to that of native cartilage. The presence of cells and FG significantly enhanced the integration strength of layered constructs (p < 0.05). These results demonstrate that porous PVA-H in combination with FG and chondrocytes provides a favorable microenvironment for tissue engineering of articular cartilage, creating a biosynthetic construct that can adhere to native devitalized articular cartilage utilizing hydrogel matrix-engineered technology.
Injectable cartilage tissue engineering
DOI:10.1517/14712598.4.12.1849
URL
PMID:15571448
[Cited within: 1]

Cartilage is the tissue that lines the surface of bones in articulating joints, allowing painless joint movement. Cartilage loss is an increasingly significant problem, particularly with the ageing of active baby boomers, with few efficacious treatments available at present. Tissue engineering is a field that has evolved over recent years to combat tissue loss by providing a living tissue equivalent or substitute that can mimic the properties of the lost tissue. The general strategy of tissue engineering is to place cells on a biomaterial scaffold that is designed to promote cell function and form new tissue. This review describes the status of materials that are available as injectable scaffolds for tissue engineering and the numerous cell types that can be applied to cartilage repair, including cells derived from cartilage and stem cells. The current state of injectable cartilage tissue engineering and the hurdles that remain for widespread clinical application are discussed.
A structurally tunable DNA-based extracellular matrix
DOI:10.1021/ja105431h
URL
PMID:20925350
[Cited within: 1]

The principles of DNA nanotechnology and protein engineering have been combined to generate a new class of artificial extracellular matrices. The potential of this material for ex vivo cellular scaffolding was demonstrated using experiments in which human cervical cancer cells were found to adhere strongly, stay alive, and grow with high migration rates. The use of DNA in our DNA/protein-based matrices makes these structures inherently amenable to structural tunability. By engineering single-stranded domains into the DNA portions, we were able to fine-tune the scaffold's persistence length and stiffness as perceived by cells. This was used to direct the outcome of the cell's cytoskeletal arrangement and overall shape, the status of its signal transduction protein p-FAK, and the localization of its intracellular transcription factors FOXO1a. This contribution lays the groundwork for the facile and modular construction of programmable extracellular matrices that can bring about the systematic study and replication of the naturally occurring extracellular niche.
Photodegradable hydrogels for dynamic tuning of physical and chemical properties
DOI:10.1126/science.1169494
URL
PMID:19342581
[Cited within: 1]

We report a strategy to create photodegradable poly(ethylene glycol)-based hydrogels through rapid polymerization of cytocompatible macromers for remote manipulation of gel properties in situ. Postgelation control of the gel properties was demonstrated to introduce temporal changes, creation of arbitrarily shaped features, and on-demand pendant functionality release. Channels photodegraded within a hydrogel containing encapsulated cells allow cell migration. Temporal variation of the biochemical gel composition was used to influence chondrogenic differentiation of encapsulated stem cells. Photodegradable gels that allow real-time manipulation of material properties or chemistry provide dynamic environments with the scope to answer fundamental questions about material regulation of live cell function and may affect an array of applications from design of drug delivery vehicles to tissue engineering systems.
Porous alginate hydrogel functionalized with virus as three-dimensional scaffolds for bone differentiation
DOI:10.1021/bm301180c
URL
PMID:23148483
[Cited within: 2]

In regenerative medicine, a synthetic extracellular matrix is crucial for supporting stem cells during its differentiation process to integrate into surrounding tissues. Hydrogels are used extensively in biomaterials as synthetic matrices to support the cells. However, to mimic the biological niche of a functional tissue, various chemical functionalities are necessary. We present here, a method of functionalizing a highly porous hydrogel with functional groups by mixing the hydrogel with a plant virus, tobacco mosaic virus (TMV), and its mutant. The implication of this process resides with the three important features of TMV: its well-defined genetic/chemical modularity, its multivalency (TMV capsid is composed of 2130 copies of identical subunits), and its well-defined structural features. Previous studies utilizing the native TMV on two-dimensional supports accelerated mesenchymal stem cell differentiation, and surfaces modified with genetically modified viral particles further enhanced cell attachment and differentiation. Herein we demonstrate that functionalization of a porous alginate scaffold can be achieved by the addition of viral particles with minimal processing and downstream purifications, and the cell attachment and differentiation within the macroporous scaffold can be effectively manipulated by altering the peptide or small molecule displayed on the viral particles.
Plant virus incorporated hydrogels as scaffolds for tissue engineering possess low immunogenicity in vivo
DOI:10.1002/jbm.a.35227
URL
PMID:24829052
[Cited within: 2]

Viruses are no longer recognized purely for being ubiquitous pathogens, but have served as building blocks for material chemistry and nanotechnology. Thousands of coat protein subunits of a viral particle can be modified chemically and/or genetically. We have previously shown that the three-dimensional porous hydrogels can easily be functionalized by Tobacco mosaic virus (TMV), a rod-like plant virus, using its mutant, RGD-TMV. RGD-TMV hosted bioadhesive peptide (RGD) in the hydrogel, which was shown to enhance cell attachment and promote osteogenic differentiation of cultured stem cell. To translate this technology to potential clinical applications, we sought to study the biocompatibility of the hydrogel. In this paper, the hydrogels were implanted in vivo and assessed for their immunogenicity, toxicity, and biodegradability. Immune response for TMV substantially decreased when incorporated in the hydrogel implants. The implanted TMV hydrogels exhibited no apparent toxicity and were degradable in mice. The results highlighted the feasibility of using TMV incorporated hydrogels as scaffolding materials for regenerative medicine in terms of biocompatibility and biodegradability.
Tobacco mosaic virus functionalized alginate hydrogel scaffolds for bone regeneration in rats with cranial defect
DOI:10.1021/acsbiomaterials.5b00561 URL [Cited within: 1]
In situ gels of Metoclopramide Hydrochloride for intranasal delivery: in vitro evaluation and in vivo pharmacokinetic study in rabbits
DOI:10.3109/10717540903447194
URL
PMID:19958151
[Cited within: 1]

Intranasal (IN) administration is a promising approach for rapid-onset delivery of medications and to circumvent their first-pass elimination when taken orally. Metoclopramide Hydrochloride (MET HCl) is a potent antiemetic, effective even for preventing emesis induced by cancer chemotherapy. The feasibility of developing an efficacious intranasal formulation of metoclopramide has been undertaken in this study. Formulations were modulated so as to have gelation at physiological ion content after intranasal administration. Gelation was determined by physical appearance. The mucoadhesive force in terms of detachment stress, determined using sheep nasal mucosal membrane, increased with increasing concentration of carbopol. The results of in vitro drug permeation studies across sheep nasal mucosa indicate that effective permeation could be significantly increased by using in situ gelling formulation with carbopol concentration 0.15% or greater. Histological examination did not detect any damage during in vitro permeation studies. Finally, the bioavailability study in rabbits revealed that the absolute bioavailability of MET HCl was significantly increased from 40.67% in the case of the oral drug solution to 54.61% in the case of the nasal in situ gel. This study points to the potential of mucoadhesive nasal in situ gel in terms of ease of administration, accuracy of dosing, prolonged nasal residence and improved drug bioavailability.
Hyaluronic acid hydrogels for biomedical applications
DOI:10.1002/adma.201003963
URL
PMID:21394792
[Cited within: 1]

Hyaluronic acid (HA), an immunoneutral polysaccharide that is ubiquitous in the human body, is crucial for many cellular and tissue functions and has been in clinical use for over thirty years. When chemically modified, HA can be transformed into many physical forms-viscoelastic solutions, soft or stiff hydrogels, electrospun fibers, non-woven meshes, macroporous and fibrillar sponges, flexible sheets, and nanoparticulate fluids-for use in a range of preclinical and clinical settings. Many of these forms are derived from the chemical crosslinking of pendant reactive groups by addition/condensation chemistry or by radical polymerization. Clinical products for cell therapy and regenerative medicine require crosslinking chemistry that is compatible with the encapsulation of cells and injection into tissues. Moreover, an injectable clinical biomaterial must meet marketing, regulatory, and financial constraints to provide affordable products that can be approved, deployed to the clinic, and used by physicians. Many HA-derived hydrogels meet these criteria, and can deliver cells and therapeutic agents for tissue repair and regeneration. This progress report covers both basic concepts and recent advances in the development of HA-based hydrogels for biomedical applications.
Influence of cross-linkers on the in vitro chondrogenesis of mesenchymal stem cells in hyaluronic acid hydrogels
DOI:10.1021/acsami.6b12437
URL
PMID:28025887
[Cited within: 1]

This study aims to investigate the effect of the structures of cross-linkers on the in vitro chondrogenic differentiation of bone mesenchymal stem cells (BMSCs) in hyaluronic acid (HA)-based hydrogels. The hydrogels were prepared by the covalent cross-linking of methacrylated HA with different types of thiol-tailored molecules, including dithiothreitol (DTT), 4-arm poly(ethylene glycol) (PEG), and multiarm polyamidoamine (PAMAM) dendrimer using thiol-ene
Elucidating the mechanobiology of malignant brain tumors using a brain matrix-mimetic hyaluronic acid hydrogel platform
DOI:10.1016/j.biomaterials.2011.07.005
URL
PMID:21820737
[Cited within: 1]

Glioblastoma multiforme (GBM) is a malignant brain tumor characterized by diffuse infiltration of single cells into the brain parenchyma, which is a process that relies in part on aberrant biochemical and biophysical interactions between tumor cells and the brain extracellular matrix (ECM). A major obstacle to understanding ECM regulation of GBM invasion is the absence of model matrix systems that recapitulate the distinct composition and physical structure of brain ECM while allowing independent control of adhesive ligand density, mechanics, and microstructure. To address this need, we synthesized brain-mimetic ECMs based on hyaluronic acid (HA) with a range of stiffnesses that encompasses normal and tumorigenic brain tissue and functionalized these materials with short Arg-Gly-Asp (RGD) peptides to facilitate cell adhesion. Scanning electron micrographs of the hydrogels revealed a dense, sheet-like microstructure with apparent nanoscale porosity similar to brain extracellular space. On flat hydrogel substrates, glioma cell spreading area and actin stress fiber assembly increased strongly with increasing density of RGD peptide. Increasing HA stiffness under constant RGD density produced similar trends and increased the speed of random motility. In a three-dimensional (3D) spheroid paradigm, glioma cells invaded HA hydrogels with morphological patterns distinct from those observed on flat surfaces or in 3D collagen-based ECMs but highly reminiscent of those seen in brain slices. This material system represents a brain-mimetic model ECM with tunable ligand density and stiffness amenable to investigations of the mechanobiological regulation of brain tumor progression.
Bone tissue engineering in osteoporosis
DOI:10.1016/j.maturitas.2013.03.004
URL
PMID:23562167
[Cited within: 1]

Osteoporosis is a polygenetic, environmentally modifiable disease, which precipitates into fragility fractures of vertebrae, hip and radius and also confers a high risk of fractures in accidents and trauma. Aging and the genetic molecular background of osteoporosis cause delayed healing and impair regeneration. The worldwide burden of disease is huge and steadily increasing while the average life expectancy is also on the rise. The clinical need for bone regeneration applications, systemic or in situ guided bone regeneration and bone tissue engineering, will increase and become a challenge for health care systems. Apart from in situ guided tissue regeneration classical ex vivo tissue engineering of bone has not yet reached the level of routine clinical application although a wealth of scaffolds and growth factors has been developed. Engineering of complex bone constructs in vitro requires scaffolds, growth and differentiation factors, precursor cells for angiogenesis and osteogenesis and suitable bioreactors in various combinations. The development of applications for ex vivo tissue engineering of bone faces technical challenges concerning rapid vascularization for the survival of constructs in vivo. Recent new ideas and developments in the fields of bone biology, materials science and bioreactor technology will enable us to develop standard operating procedures for ex vivo tissue engineering of bone in the near future. Once prototyped such applications will rapidly be tailored for compromised conditions like vitamin D and sex hormone deficiencies, cellular deficits and high production of regeneration inhibitors, as they are prevalent in osteoporosis and in higher age.
The laboratory rat as an animal model for osteoporosis research
URL
PMID:19004367
[Cited within: 1]

Osteoporosis is an important systemic disorder, affecting mainly Caucasian women, with a diverse and multifactorial etiology. A large variety of animal species, including rodents, rabbits, dogs, and primates, have been used as animal models in osteoporosis research. Among these, the laboratory rat is the preferred animal for most researchers. Its skeleton has been studied extensively, and although there are several limitations to its similarity to the human condition, these can be overcome through detailed knowledge of its specific traits or with certain techniques. The rat has been used in many experimental protocols leading to bone loss, including hormonal interventions (ovariectomy, orchidectomy, hypophysectomy, parathyroidectomy), immobilization, and dietary manipulations. The aim of the current review is not only to present the ovariectomized rat and its advantages as an appropriate model for the research of osteoporosis, but also to provide information about the most relevant age and bone site selection according to the goals of each experimental protocol. In addition, several methods of bone mass evaluation are assessed, such as biochemical markers, densitometry, histomorphometry, and bone mechanical testing, that are used for monitoring and evaluation of this animal model in preventive or therapeutic strategies for osteoporosis.
Temporal relationship between bone loss and increased bone turnover in ovariectomized rats
DOI:10.1007/BF02571317
URL
PMID:3141020
[Cited within: 1]

To characterize osteopenic changes in ovariectomized (OVX) rats as a function of time, female Sprague Dawley rats (240 g body weight, 90 days old) were subjected to bilateral ovariectomy or sham surgery and killed at various times from 14-180 days postovariectomy. The proximal tibial metaphysis was processed undecalcified for quantitative bone histomorphometry. Osteopenia and increased indices of bone resorption and formation were detected in OVX rats as early as 14 days. Longitudinal bone growth was also significantly increased by ovariectomy at 14 days, but returned to control levels at all later times. In OVX rats, osteopenia became progressively more pronounced with time up to 100 days postovariectomy, after which trabecular bone volume appeared to stabilize at the markedly reduced level of 5%. Changes in osteoclast surface, osteoblast surface, and fluoro-chrome-based indices of bone formation in OVX rats followed a similar time course. The maximal increase in these parameters occurred during the first several months postovariectomy followed by a gradual decline toward control levels. Our results indicate that the initial rapid phase of bone loss in OVX rats is coincident with the maximal increase in bone turnover. At later times postovariectomy, bone loss and bone turnover both subside. These findings emphasize the close temporal association between the development of osteopenia and increased bone turnover in OVX rats.
Long-term effects of ovariectomy and aging on the rat skeleton
DOI:10.1007/BF02556007
URL
PMID:2509027
[Cited within: 1]

The long-term skeletal effects of ovariectomy and aging were studied in female Sprague-Dawley rats sacrificed at 270, 370, and 540 days after bilateral ovariectomy (OVX) or sham surgery at 90 days of age. The proximal tibia was processed undecalcified for quantitative bone histomorphometry. For continuity, data from these late time points were combined with previously published data from earlier time points (0-180 days). A biphasic pattern of cancellous bone loss was detected in the proximal tibial metaphysis of OVX rats. An initial, rapid phase of bone loss out to 100 days was followed by an intermediate period of relative stabilization of cancellous bone volume at the markedly osteopenic level of 5-7%. After 270 days, a slow phase of bone loss occurred during which cancellous bone volume declined to 1-2%. Both the initial, rapid phase and the late, slow phase of bone loss in OVX rats were associated with increased bone turnover. In control rats, cancellous bone volume remained constant at 25-30% out to 270 days (12 months of age), then decreased to approximately 10% by 540 days (21 months of age). This age-related bone loss was also associated with increased bone turnover. It is interesting to note that the proximal tibial growth plates were closed in approximately a quarter of the control rats by 15-21 months of age. Our data indicate that a slow rate of bone loss and increased bone turnover persist in OVX rats during the later stages of estrogen deficiency. Therefore, the development of osteopenia is coincident with increased bone turnover in OVX rats as well as in aged, control rats.
A matrine derivative M54 suppresses osteoclastogenesis and prevents ovariectomy-induced bone loss by targeting ribosomal protein S5
DOI:10.3389/fphar.2018.00022
URL
PMID:29441015
[Cited within: 1]

Post-menopausal osteoporosis (PMOP) is a metabolic bone disorder characterized by low bone mass and micro-architectural deterioration of bone tissue. The over-activated osteoclastogenesis, which plays an important role in osteoporosis, has become an important therapeutic target. M54 was a bioactive derivative of the Chinese traditional herb matrine. We found that M54 could suppress RANKL-induced osteoclastogenesis in bone marrow mononuclear cells and RAW264.7 cells through suppressing NF-kappaB, PI3K/AKT, and MAPKs pathways activity in vitro, and prevent ovariectomy-induced bone loss in vivo. Our previous study has proved that ribosomal protein S5 (RPS5) was a direct target of M19, based on which M54 was synthesized. Thus we deduced that M54 also targeted RPS5. During osteoclastogenesis, the RPS5 level in RAW264.7 cells was significantly down-regulated while M54 could maintain its level. After RPS5 was silenced, the inhibitory effects of M54 on osteoclastogenesis were partially compromised, indicating that M54 took effects through targeting RPS5. In summary, M54 was a potential clinical medicine for post-menopause osteoporosis treatment, and RPS5 is a possible key protein in PMOP.
Dihydroartemisinin, an anti-malaria drug, suppresses estrogen deficiency-induced osteoporosis, osteoclast formation, and RANKL-induced signaling pathways
DOI:10.1002/jbmr.2771
URL
PMID:26684711

Osteoporosis is an osteolytic disease that features enhanced osteoclast formation and bone resorption. Identification of agents that can inhibit osteoclast formation and function is important for the treatment of osteoporosis. Dihydroartemisinin is a natural compound used to treat malaria but its role in osteoporosis is not known. Here, we found that dihydroartemisinin can suppress RANKL-induced osteoclastogenesis and bone resorption in a dose-dependent manner. Dihydroartemisinin inhibited the expression of osteoclast marker genes such as cathepsin K, calcitonin receptor, and tartrate-resistant acid phosphatase (TRAcP). Furthermore, dihydroartemisinin inhibited RANKL-induced NF-kappaB and NFAT activity. In addition, using an in vivo ovariectomized mouse model, we show that dihydroartemisinin is able to reverse the bone loss caused by ovariectomy. Together, this study shows that dihydroartemisinin attenuates bone loss in ovariectomized mice through inhibiting RANKL-induced osteoclast formation and function. This indicates that dihydroartemisinin, the first physiology or medicine nobel prize discovery of China, is a potential treatment option against osteolytic bone disease. (c) 2015 American Society for Bone and Mineral Research.
Calvarial bone regeneration by a combination of natural anorganic bovine-derived hydroxyapatite matrix coupled with a synthetic cell-binding peptide (PepGen): an experimental study in rats
DOI:10.1111/j.1600-0501.2008.01572.x
URL
PMID:18828817
[Cited within: 2]

OBJECTIVES: The aim of this study was to evaluate histologically the effect of natural anorganic bovine-derived hydroxyapatite matrix (ABM) coupled with a synthetic cell-binding peptide on the healing of critical size calvarial defects in rats. MATERIAL AND METHODS: Sixteen 4-month-old rats were used in the study. A 5 mm trephine defect was created in each parietal bone of every animal. One defect was left untreated (control) while the contralateral defect was treated with a natural ABM coupled with a synthetic cell-binding peptide (test). At 60 and 120 days post-operatively, groups of eight animals were sacrificed and 7-10-microm-thick decalcified sections were produced from both test and control sides. Three sections, 100 mum apart, representing the central area of each defect were selected for the histometric analysis. RESULTS: Histological analysis showed limited bone formation in both control and test defects at both observation periods. The control defects healed with fibrous connective tissue occupying the midportion of the defect and minimal new bone formation at the periphery. In the test defects, the major part of the defect was occupied by graft particles embedded in connective tissue. After 60 days of healing the residual defects accounted up to 94.6% of the original defect dimensions in the control specimens and 90.6% in the test specimens. The differences between test and control defects were not statistically significant (P=0.06). After 120 days of healing, the residual defects accounted up 89.9% of the original defect dimensions in the control specimens and 85% in the test specimens. The difference was not statistically significant (P=0.33). CONCLUSION: The ABM coupled with a synthetic cell-binding peptide failed to substantially promote new bone formation in rat calvarial defects.
The biomaterial-mediated healing of critical size bone defects in the ovariectomized rat
DOI:10.1007/s00198-014-2656-y
URL
PMID:24573401
[Cited within: 2]

UNLABELLED: This study demonstrated an impaired biomaterial-mediated bone regeneration in a critical sized calvarial defect established within an ovariectomized rat model. Histological and microtomographic evidences were supported by an impaired osteoblastic gene expression and altered expression of estrogen receptors and adipogenic markers. INTRODUCTION: This work aims to address the bone regeneration process in the ovariectomized rat model, by assessing a calvarial critical size defect implanted with a biocompatible bovine bone mineral graft. METHODS: Animals were randomly divided into two groups: Ovx (bilateral ovariectomy) and Sham (control surgery). Following 8 weeks, all animals were submitted to a surgical bicortical craniotomy (5-mm circular critical size defect), which was filled with a biocompatible mineral graft. Animals were euthanized at 1, 3, and 6 months following graft implantation (n = 10), and results on the orthotopic bone regeneration process were blindly evaluated by radiographic, microtomographic, histological, histomorphometric, and gene expression techniques. RESULTS: In the attained model, in both Sham and Ovx groups, the bone regenerative process was found to occur in a slow-paced manner. Likewise, a qualitative evaluation of the microtomographic and histological analysis, as well as quantitative data from histomorphometric indexes, revealed reduced bone regeneration in Ovx animals, at the assayed time points. Significant differences were attained at the 3 and 6 months. Gene expression analysis revealed a reduced expression of osteoblastic-related genes and an altered expression of estrogen receptors and adipogenic markers, within the regenerating bone of Ovx animals. CONCLUSIONS: Due to the similarities between the osteoporotic animal model and the human condition of postmenopausal osteoporosis, it might be relevant to consider the potential clinical implication of the osteoporotic condition in the biomaterial-mediated bone tissue healing/regeneration process.
Osteoporosis influences the early period of fracture healing in a rat osteoporotic model
DOI:10.1016/s8756-3282(00)00414-2
URL
PMID:11165946
[Cited within: 1]

Osteoporotic fractures commonly occur in the elderly. Although current therapies are aimed at the prevention and treatment of osteoporotic fractures, studies examing the fracture healing process in osteoporotic bone are limited. We produced an osteoporotic rat model by ovariectomy (ovx) and maintained a low calcium diet (LCD) in order to evaluate the influence of osteoporosis on fracture healing. Callus formation and strength was monitored over a 3 week period by histological and biomechanical assessment. Data collected simultaneously on a group of rats undergoing sham surgery (sx) were used for comparison. A 40% reduction in fracture callus cross-sectional area and a 23% reduction in bone mineral density in the healing femur of the ovx rats was observed on day 21 following fracture as compared with the sx group (p < 0.01). Biomechanical data from the healing femur of the ovx rats revealed a fivefold decrease in the energy required to break the fracture callus, a threefold decrease in peak failure load, a twofold decrease in stiffness and a threefold decrease in stress as compared with the sx group (p < 0.01, respectively). Histomorphological analysis revealed a delay in fracture callus healing with poor development of mature bone in the ovx rats. This study provides physical evidence of altered fracture healing in osteoporotic bone, which may have important implications in evaluating the effects of new treatments for osteoporosis on fracture healing.
Changes of microstructure and mineralized tissue in the middle and late phase of osteoporotic fracture healing in rats
DOI:10.1016/j.bone.2007.06.006
URL
PMID:17652051
[Cited within: 1]

BACKGROUND: With osteoporosis emerged as one of the most important health issues, more and more investigations are focusing on osteoporotic fracture healing. However, there are few studies on the changes of microstructure and mineralized tissue of newly formed callus. OBJECTIVE: We established an osteoporotic fracture rat model to evaluate the changes of microstructure and mineralized tissue during osteoporotic fracture healing. MATERIALS AND METHODS: A mid-shaft femur fracture model was established 12 weeks after ovariectomy as an osteoporotic fracture group (OPF group). Femurs were then harvested at 4 weeks, 8 weeks and 12 weeks after fracture for peripheral quantitative computed tomography (pQCT), micro-computed tomography (MicroCT), histology and biomechanical test. A sham-operated group was used for comparison, i.e. the normal fracture group (NF group). RESULTS: The pQCT-derived total external callus area in the OPF group was smaller than that in the NF group at 4 weeks after fracture (P<0.05), whereas it was 21% larger in the OPF group than that in the NF group at 12 weeks after fracture (P<0.01). The pQCT-derived bone mineral density in the OPF group was significantly inferior to the NF group at all the time points (P<0.05 for all the time points, respectively). MicroCT data, at 12 weeks after fracture, showed the total callus, bony callus, and newly formed bone was approximately 20% lower in the OPF group than that in the NP group, and the total connectivity was 56% lower in the OPF group as compared to the NF group. Biomechanical test data, at 12 weeks after fracture, showed that the failure load of the left femur of OPF group was 17% less compared to that of the NF group (P<0.01), and 15% lower bending stiffness (P<0.05), 20% lower bending stress (P<0.01), and 28% lower energy at failure (P<0.01) were observed in the OPF group as compared to the NF group. CONCLUSION: The decrease in mineralized tissue and the not well connected microstructure in newly formed callus may explain the decline of mechanical impairment of fracture healing in the ovariectomized rats.
Bone formation after implantation of autolysed antigen extracted allogeneic bone in ovariectomized rabbits
DOI:10.1054/ijom.2003.0428
URL
PMID:14636614
[Cited within: 1]

This study was undertaken to evaluate the bone formation response to AAA bone in healthy and oestrogen deficient animals. Seventeen young healthy New Zealand female rabbits were used. Nine rabbits were subjected to ovariectomy and the remaining eight were sham-operated. Four weeks after ovariectomy standardized round cavities, 5mm in diameter, were made medially in the cortical part of each proximal tibia. To half of the cavities autolysed antigen-extracted allogeneic AAA bone granules were added. After another 8 weeks the animals were sacrificed and sections of the tibial experimental areas were obtained. These were studied in light microscopy and the bone and non-bone areas were measured with computer support. The study showed that the addition of a bone inductive substance such as AAA bone enhances bone formation also in oestrogen deficient animals.