Next-generation tissue-engineered heart valves with repair, remodelling and regeneration capacity
1
2021
... Valvular heart disease affects an increasing number of patients in both developed and developing countries. Since the use of mechanical or bioprosthetic valves, the number of valve replacement procedures performed each year has been constantly increasing and is expected to reach 850,000 implantations worldwide by 2050.1, 2 As a particularly vulnerable patient population, children with congenital heart malformations often require multiple reoperations because current repair materials lack inherent growth, and this is associated with an exponentially increased risk of surgical complications.3 In addition, mechanical valves are prone to inflammation, infection and thrombosis, while bioprosthetic valves experience calcification which has both stiffening and thickening effects on the valve cusps, eventually leading to insufficient valve closure and leakage. Consequently, many of the patients implanted with these prosthetic heart valves are exposed to a lifelong risk of valve prosthesis-related morbidity and mortality.4 ...
Enhanced elastin synthesis and maturation in human vascular smooth muscle tissue derived from induced-pluripotent stem cells
2
2017
... Valvular heart disease affects an increasing number of patients in both developed and developing countries. Since the use of mechanical or bioprosthetic valves, the number of valve replacement procedures performed each year has been constantly increasing and is expected to reach 850,000 implantations worldwide by 2050.1, 2 As a particularly vulnerable patient population, children with congenital heart malformations often require multiple reoperations because current repair materials lack inherent growth, and this is associated with an exponentially increased risk of surgical complications.3 In addition, mechanical valves are prone to inflammation, infection and thrombosis, while bioprosthetic valves experience calcification which has both stiffening and thickening effects on the valve cusps, eventually leading to insufficient valve closure and leakage. Consequently, many of the patients implanted with these prosthetic heart valves are exposed to a lifelong risk of valve prosthesis-related morbidity and mortality.4 ...
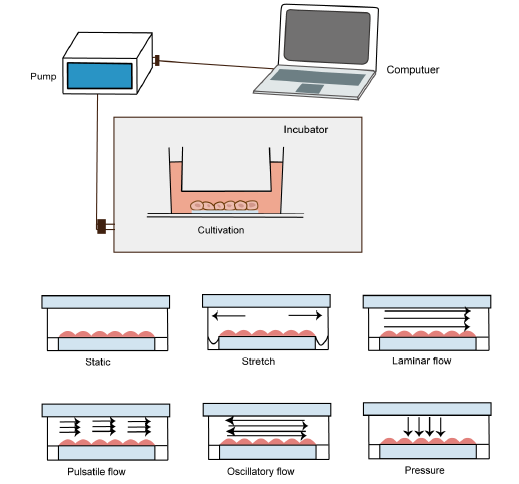
... Heart valves in our bodies encounter a variety of complex mechanical forces. During each cardiac cycle, native valves are continuously subjected to highly-complex tension, flexure, pressure, and shear stress forces as a result of blood flow, for example, aortic valve leaflets experience peak fluid-induced shear stresses of approximately 64-71 dyn/cm2 during systole.57 To better understand each component’s contribution to cellular behaviour and implementation, these mechanical forces must be separated. In an engineered heart valve tissue context, mechanical stimuli, particularly those that incorporate fluid-induced shear stress, have been shown to enhance progenitor cell differentiation pathways,58 regulate ECM remodelling,59 impact the behaviour of valvular endothelial cells behaviour60 or endothelial mesenchymal transformation,61 and induce morphological remodelling in cells cytoskeleton.57 ...
Valve replacement in children: a challenge for a whole life
1
2012
... Valvular heart disease affects an increasing number of patients in both developed and developing countries. Since the use of mechanical or bioprosthetic valves, the number of valve replacement procedures performed each year has been constantly increasing and is expected to reach 850,000 implantations worldwide by 2050.1, 2 As a particularly vulnerable patient population, children with congenital heart malformations often require multiple reoperations because current repair materials lack inherent growth, and this is associated with an exponentially increased risk of surgical complications.3 In addition, mechanical valves are prone to inflammation, infection and thrombosis, while bioprosthetic valves experience calcification which has both stiffening and thickening effects on the valve cusps, eventually leading to insufficient valve closure and leakage. Consequently, many of the patients implanted with these prosthetic heart valves are exposed to a lifelong risk of valve prosthesis-related morbidity and mortality.4 ...
Tissue valve is the preferred option for patients aged 60 and older
1
2013
... Valvular heart disease affects an increasing number of patients in both developed and developing countries. Since the use of mechanical or bioprosthetic valves, the number of valve replacement procedures performed each year has been constantly increasing and is expected to reach 850,000 implantations worldwide by 2050.1, 2 As a particularly vulnerable patient population, children with congenital heart malformations often require multiple reoperations because current repair materials lack inherent growth, and this is associated with an exponentially increased risk of surgical complications.3 In addition, mechanical valves are prone to inflammation, infection and thrombosis, while bioprosthetic valves experience calcification which has both stiffening and thickening effects on the valve cusps, eventually leading to insufficient valve closure and leakage. Consequently, many of the patients implanted with these prosthetic heart valves are exposed to a lifelong risk of valve prosthesis-related morbidity and mortality.4 ...
Challenges in translating tissue engineered heart valves into clinical practice
1
2017
... The design of tissue-engineered heart valves (TEHVs) with self-repair, remodelling and regeneration capacity allows the replacement of whole or part of diseased tissues or organs with biomimetic replicates to address the limitations of current mechanical and bioprosthetic valves, and may become a promising therapeutic alternative for patients with valvular disease, particularly paediatric and elderly patients.5 ...
Preclinical assessment of cardiac valve substitutes: current status and considerations for engineered tissue heart valves
1
2019
... To make better use of TEHVs in clinical patients, the regulatory framework governing the safety and efficacy of medical devices can be considered in three distinct phases of product study: preclinical studies, clinical studies, and post-market monitoring. Among them, pre-clinical studies comprise in-vitro (i.e., engineering and material characterisation) and in-vivo components (i.e., animal models). Animal testing of cardiovascular devices can provide invaluable information on their safety, but there are still many uncertainties in the anatomical and physiological structure of the animal model. In addition the evaluation of large animals is difficult, and requires a lot of financial and human investment, consequently it is very important to select an appropriate model for performance evaluation in the construction stage.6 ...
Which biological properties of heart valves are relevant to tissue engineering?
1
2020
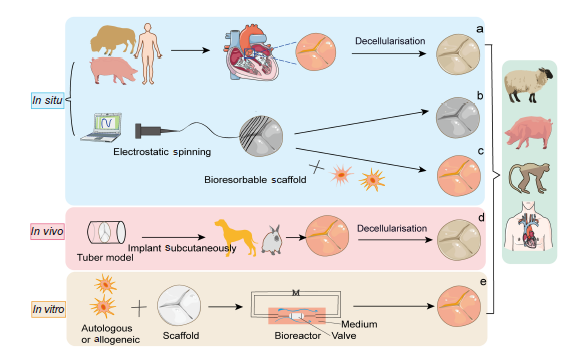
... Four essential elements needed for the synthesis of functional TEHVs are scaffolds from natural or synthetic biomaterials, cells encapsulated within scaffolds, signalling molecules to regulate cell responses, and models to test the implantability of TEHVs.7 There are three types of scaffold available: (1) Decellularised ECM: obtained by physical agitation, chemical surfactant removal, and enzymatic digestion to remove antigenic materials and allogeneic cells from the graft,8 so that the decellularised scaffold can be seeded with cells derived from the intended recipient.9 (2) Natural biodegradable materials: derived from natural matrix materials and displaying robust biocompatibility, including collagen, hyaluronate, gelatine, glycosaminoglycan, chitosan, alginate, fibrin, silk, dextran, and Matrigel.10 (3) Synthetic biodegradable materials: including poly(glycolic acid) (PGA), polylactic acid, polylactic-co-glycolic acid, poly(L-lactic acid), poly-ε-caprolactone, polyethylene glycol, polyvinyl alcohol, polypropylene fumarate, polyacrylic acid, and others.11 Various tissue-engineering approaches have been used: (1) In vitro: autologous or allogenic cells are isolated and seeded onto a bioresorbable scaffold and then cultured in a bioreactor system until the new composite scaffold has sufficient mechanical elasticity and strength for implantation.12 (2) In vivo: this strategy relies on fibrotic encapsulation of valvular moulds implanted subcutaneously with autologous ECM.13-15 (3) In situ: decellularised homograft or xenograft materials are implanted to grow cells and remodel the ECM15-17 (Figure 1). ...
Decellularized tissue-engineered blood vessel as an arterial conduit
1
2011
... Four essential elements needed for the synthesis of functional TEHVs are scaffolds from natural or synthetic biomaterials, cells encapsulated within scaffolds, signalling molecules to regulate cell responses, and models to test the implantability of TEHVs.7 There are three types of scaffold available: (1) Decellularised ECM: obtained by physical agitation, chemical surfactant removal, and enzymatic digestion to remove antigenic materials and allogeneic cells from the graft,8 so that the decellularised scaffold can be seeded with cells derived from the intended recipient.9 (2) Natural biodegradable materials: derived from natural matrix materials and displaying robust biocompatibility, including collagen, hyaluronate, gelatine, glycosaminoglycan, chitosan, alginate, fibrin, silk, dextran, and Matrigel.10 (3) Synthetic biodegradable materials: including poly(glycolic acid) (PGA), polylactic acid, polylactic-co-glycolic acid, poly(L-lactic acid), poly-ε-caprolactone, polyethylene glycol, polyvinyl alcohol, polypropylene fumarate, polyacrylic acid, and others.11 Various tissue-engineering approaches have been used: (1) In vitro: autologous or allogenic cells are isolated and seeded onto a bioresorbable scaffold and then cultured in a bioreactor system until the new composite scaffold has sufficient mechanical elasticity and strength for implantation.12 (2) In vivo: this strategy relies on fibrotic encapsulation of valvular moulds implanted subcutaneously with autologous ECM.13-15 (3) In situ: decellularised homograft or xenograft materials are implanted to grow cells and remodel the ECM15-17 (Figure 1). ...
Readily available tissue-engineered vascular grafts
1
2011
... Four essential elements needed for the synthesis of functional TEHVs are scaffolds from natural or synthetic biomaterials, cells encapsulated within scaffolds, signalling molecules to regulate cell responses, and models to test the implantability of TEHVs.7 There are three types of scaffold available: (1) Decellularised ECM: obtained by physical agitation, chemical surfactant removal, and enzymatic digestion to remove antigenic materials and allogeneic cells from the graft,8 so that the decellularised scaffold can be seeded with cells derived from the intended recipient.9 (2) Natural biodegradable materials: derived from natural matrix materials and displaying robust biocompatibility, including collagen, hyaluronate, gelatine, glycosaminoglycan, chitosan, alginate, fibrin, silk, dextran, and Matrigel.10 (3) Synthetic biodegradable materials: including poly(glycolic acid) (PGA), polylactic acid, polylactic-co-glycolic acid, poly(L-lactic acid), poly-ε-caprolactone, polyethylene glycol, polyvinyl alcohol, polypropylene fumarate, polyacrylic acid, and others.11 Various tissue-engineering approaches have been used: (1) In vitro: autologous or allogenic cells are isolated and seeded onto a bioresorbable scaffold and then cultured in a bioreactor system until the new composite scaffold has sufficient mechanical elasticity and strength for implantation.12 (2) In vivo: this strategy relies on fibrotic encapsulation of valvular moulds implanted subcutaneously with autologous ECM.13-15 (3) In situ: decellularised homograft or xenograft materials are implanted to grow cells and remodel the ECM15-17 (Figure 1). ...
Natural biomaterials for cardiac tissue engineering: a highly biocompatible solution
1
2020
... Four essential elements needed for the synthesis of functional TEHVs are scaffolds from natural or synthetic biomaterials, cells encapsulated within scaffolds, signalling molecules to regulate cell responses, and models to test the implantability of TEHVs.7 There are three types of scaffold available: (1) Decellularised ECM: obtained by physical agitation, chemical surfactant removal, and enzymatic digestion to remove antigenic materials and allogeneic cells from the graft,8 so that the decellularised scaffold can be seeded with cells derived from the intended recipient.9 (2) Natural biodegradable materials: derived from natural matrix materials and displaying robust biocompatibility, including collagen, hyaluronate, gelatine, glycosaminoglycan, chitosan, alginate, fibrin, silk, dextran, and Matrigel.10 (3) Synthetic biodegradable materials: including poly(glycolic acid) (PGA), polylactic acid, polylactic-co-glycolic acid, poly(L-lactic acid), poly-ε-caprolactone, polyethylene glycol, polyvinyl alcohol, polypropylene fumarate, polyacrylic acid, and others.11 Various tissue-engineering approaches have been used: (1) In vitro: autologous or allogenic cells are isolated and seeded onto a bioresorbable scaffold and then cultured in a bioreactor system until the new composite scaffold has sufficient mechanical elasticity and strength for implantation.12 (2) In vivo: this strategy relies on fibrotic encapsulation of valvular moulds implanted subcutaneously with autologous ECM.13-15 (3) In situ: decellularised homograft or xenograft materials are implanted to grow cells and remodel the ECM15-17 (Figure 1). ...
Biodegradable and biomimetic elastomeric scaffolds for tissue-engineered heart valves
1
2017
... Four essential elements needed for the synthesis of functional TEHVs are scaffolds from natural or synthetic biomaterials, cells encapsulated within scaffolds, signalling molecules to regulate cell responses, and models to test the implantability of TEHVs.7 There are three types of scaffold available: (1) Decellularised ECM: obtained by physical agitation, chemical surfactant removal, and enzymatic digestion to remove antigenic materials and allogeneic cells from the graft,8 so that the decellularised scaffold can be seeded with cells derived from the intended recipient.9 (2) Natural biodegradable materials: derived from natural matrix materials and displaying robust biocompatibility, including collagen, hyaluronate, gelatine, glycosaminoglycan, chitosan, alginate, fibrin, silk, dextran, and Matrigel.10 (3) Synthetic biodegradable materials: including poly(glycolic acid) (PGA), polylactic acid, polylactic-co-glycolic acid, poly(L-lactic acid), poly-ε-caprolactone, polyethylene glycol, polyvinyl alcohol, polypropylene fumarate, polyacrylic acid, and others.11 Various tissue-engineering approaches have been used: (1) In vitro: autologous or allogenic cells are isolated and seeded onto a bioresorbable scaffold and then cultured in a bioreactor system until the new composite scaffold has sufficient mechanical elasticity and strength for implantation.12 (2) In vivo: this strategy relies on fibrotic encapsulation of valvular moulds implanted subcutaneously with autologous ECM.13-15 (3) In situ: decellularised homograft or xenograft materials are implanted to grow cells and remodel the ECM15-17 (Figure 1). ...
Recent progress toward clinical translation of tissue-engineered heart valves
1
2021
... Four essential elements needed for the synthesis of functional TEHVs are scaffolds from natural or synthetic biomaterials, cells encapsulated within scaffolds, signalling molecules to regulate cell responses, and models to test the implantability of TEHVs.7 There are three types of scaffold available: (1) Decellularised ECM: obtained by physical agitation, chemical surfactant removal, and enzymatic digestion to remove antigenic materials and allogeneic cells from the graft,8 so that the decellularised scaffold can be seeded with cells derived from the intended recipient.9 (2) Natural biodegradable materials: derived from natural matrix materials and displaying robust biocompatibility, including collagen, hyaluronate, gelatine, glycosaminoglycan, chitosan, alginate, fibrin, silk, dextran, and Matrigel.10 (3) Synthetic biodegradable materials: including poly(glycolic acid) (PGA), polylactic acid, polylactic-co-glycolic acid, poly(L-lactic acid), poly-ε-caprolactone, polyethylene glycol, polyvinyl alcohol, polypropylene fumarate, polyacrylic acid, and others.11 Various tissue-engineering approaches have been used: (1) In vitro: autologous or allogenic cells are isolated and seeded onto a bioresorbable scaffold and then cultured in a bioreactor system until the new composite scaffold has sufficient mechanical elasticity and strength for implantation.12 (2) In vivo: this strategy relies on fibrotic encapsulation of valvular moulds implanted subcutaneously with autologous ECM.13-15 (3) In situ: decellularised homograft or xenograft materials are implanted to grow cells and remodel the ECM15-17 (Figure 1). ...
Development of an in vivo tissue-engineered, autologous heart valve (the biovalve): preparation of a prototype model
4
2007
... Four essential elements needed for the synthesis of functional TEHVs are scaffolds from natural or synthetic biomaterials, cells encapsulated within scaffolds, signalling molecules to regulate cell responses, and models to test the implantability of TEHVs.7 There are three types of scaffold available: (1) Decellularised ECM: obtained by physical agitation, chemical surfactant removal, and enzymatic digestion to remove antigenic materials and allogeneic cells from the graft,8 so that the decellularised scaffold can be seeded with cells derived from the intended recipient.9 (2) Natural biodegradable materials: derived from natural matrix materials and displaying robust biocompatibility, including collagen, hyaluronate, gelatine, glycosaminoglycan, chitosan, alginate, fibrin, silk, dextran, and Matrigel.10 (3) Synthetic biodegradable materials: including poly(glycolic acid) (PGA), polylactic acid, polylactic-co-glycolic acid, poly(L-lactic acid), poly-ε-caprolactone, polyethylene glycol, polyvinyl alcohol, polypropylene fumarate, polyacrylic acid, and others.11 Various tissue-engineering approaches have been used: (1) In vitro: autologous or allogenic cells are isolated and seeded onto a bioresorbable scaffold and then cultured in a bioreactor system until the new composite scaffold has sufficient mechanical elasticity and strength for implantation.12 (2) In vivo: this strategy relies on fibrotic encapsulation of valvular moulds implanted subcutaneously with autologous ECM.13-15 (3) In situ: decellularised homograft or xenograft materials are implanted to grow cells and remodel the ECM15-17 (Figure 1). ...
... The rapid development of TEHVs and in situ tissue-engineering triggered the studies on animals. Although animal models are essential to determine the clinical potential of TEHVs, because they are complete organisms that can mimic human physiology to some extent, there are still no ideal animal models or international consensus on standards. To further verify the safety and efficacy of TEHVs, several appropriate pre-clinical animal models, including large and small animals whose hearts are similar to that of humans, were translated into the preclinic. Briefly, small animals are used for in vivo assessments such as subcutaneous implantation,13, 18-21 while large animal models are used to test functionality and re-cellularisation in situ22-30 (Table 1). ...
... Animal models used in TEHV studies.
| Species | TEHV manufacture | Surgical method | Functionality assessment and post-mortem analysis | Reference |
| Small animal model |
| Mouse | Sulfonic polymer hybrid extracellular matrix | Subdermal | Histological and immunohistochemical analysis | 18 |
| Rat | Glut-fixed porcine aortic valve | Subdermal | Histological and quantitative analysis | 19 |
| Electrospinning polycaprolactone scaffold | Subdermal | Scanning electron microscopic and histological analysis | 20 |
| Allogeneic aortic conduit grafts | End-to-side anastomosis with infrarenal aorta | Histological and immunohistochemical analysis, quantitative real-time polymerase chain reaction | 21 |
| Rabbit | Poly urethane scaffold | Subdermal | Macroscopic and histological analysis | 13 |
| Large animal model |
| Adult sheep | Poly(glycolic acid) scaffold | Pulmonary valve replacement via transcatheter-based jugular | Intracardiac echocardiography, cardiac magnetic resonance imaging, computed tomography, histological and immunohistochemical analysis | 22 |
| Polyvinylidene fluoride scaffold | Left anterolateral thoracotomy | Inspected for thrombotic deposits, light microscopic analysis, electron microscopic analysis or processed for extracellular matrix assay | 23 |
Juvenile sheep
(13–17 kg) | Tissue-engineered porcine pulmonary valved conduit | Off-pump cardiopulmonary bypass/left anterolateral thoracotomy | Transthoracic ultrasound echocardiography, mortality, operating time | 24 |
| Foetal ovine | PCL scaffold | Transcatheter pulmonary valve replacement | Ultrasound (pulmonary valve annulus), foetal outcome | 25 |
| Yorkshire pigs (80 kg) | AZ31 magnesium alloy biodegradable stent frame scaffold-based polycarbonate urethane urea TEHV | Cardiopulmonary bypass in the pulmonary position | Echocardiography, biaxial mechanical testing, scanning electron microscopy | 26 |
| Porcine | Decellularised porcine pulmonary heart valves | Median sternotomy or limited lateral thoracotomy, then injection of TEHV | Echocardiographic examination, invasive pressure, angiographic measurements | 27 |
| Vietnamese pigs | Decellularised porcine aortic valves conduit | Xypho‐jugular incision, followed by median longitudinal sternotomy | Echocardiography, macroscopic inspection, metabolic labelling | 28 |
| Canine | Decellularised porcine pulmonary heart valves | Left anterolateral thoracotomy | Echocardiography, histology (haematoxylin and eosin, Masson) and immunohistochemistry, transmission electron microscopy | 29 |
| Non-human primate (Chacma Baboon) | Poly(glycolic acid) scaffold | A mini-sternotomy using an antegrade transapical approach | Transesophageal echocardiography, epicardial echocardiography, Pulmonary artery pressure measurement, biochemical analysis | 30 |
Note: TEHV: tissue-engineered heart valve. ...
... The rabbit is the largest of small animal models, so that larger grafts can be implanted for study. Ye and colleagues29 used scanning electron microscopy to characterise the surface of rabbit heart valves. Based on the results, they developed a microstructural model of a heart valve to investigate the best geometric parameters of the rough surface of the valves to improve the replacement of heart valves.29 In addition, like rats, rabbit subdermal models have been used in TEHV studies. Hayashida et al.13 manufactured an autologous heart valve using a poly-urethane scaffold and implanted it in a rabbit subdermal model for 4 weeks, after which the graft was harvested for macroscopic and histologic analyses to evaluate its mechanical properties and microstructure. The results proved the good biocompatibility of the polymer scaffold. ...
Development of a completely autologous valved conduit with the sinus of Valsalva using in-body tissue architecture technology: a pilot study in pulmonary valve replacement in a beagle model
0
2010
Human cell-derived tissue-engineered heart valve with integrated Valsalva sinuses: towards native-like transcatheter pulmonary valve replacements
2
2019
... Four essential elements needed for the synthesis of functional TEHVs are scaffolds from natural or synthetic biomaterials, cells encapsulated within scaffolds, signalling molecules to regulate cell responses, and models to test the implantability of TEHVs.7 There are three types of scaffold available: (1) Decellularised ECM: obtained by physical agitation, chemical surfactant removal, and enzymatic digestion to remove antigenic materials and allogeneic cells from the graft,8 so that the decellularised scaffold can be seeded with cells derived from the intended recipient.9 (2) Natural biodegradable materials: derived from natural matrix materials and displaying robust biocompatibility, including collagen, hyaluronate, gelatine, glycosaminoglycan, chitosan, alginate, fibrin, silk, dextran, and Matrigel.10 (3) Synthetic biodegradable materials: including poly(glycolic acid) (PGA), polylactic acid, polylactic-co-glycolic acid, poly(L-lactic acid), poly-ε-caprolactone, polyethylene glycol, polyvinyl alcohol, polypropylene fumarate, polyacrylic acid, and others.11 Various tissue-engineering approaches have been used: (1) In vitro: autologous or allogenic cells are isolated and seeded onto a bioresorbable scaffold and then cultured in a bioreactor system until the new composite scaffold has sufficient mechanical elasticity and strength for implantation.12 (2) In vivo: this strategy relies on fibrotic encapsulation of valvular moulds implanted subcutaneously with autologous ECM.13-15 (3) In situ: decellularised homograft or xenograft materials are implanted to grow cells and remodel the ECM15-17 (Figure 1). ...
... 15-17 (Figure 1). ...
Percutaneous pulmonary valve replacement using completely tissue-engineered off-the-shelf heart valves: six-month in vivo functionality and matrix remodelling in sheep
0
2016
JetValve: Rapid manufacturing of biohybrid scaffolds for biomimetic heart valve replacement
1
2017
... Four essential elements needed for the synthesis of functional TEHVs are scaffolds from natural or synthetic biomaterials, cells encapsulated within scaffolds, signalling molecules to regulate cell responses, and models to test the implantability of TEHVs.7 There are three types of scaffold available: (1) Decellularised ECM: obtained by physical agitation, chemical surfactant removal, and enzymatic digestion to remove antigenic materials and allogeneic cells from the graft,8 so that the decellularised scaffold can be seeded with cells derived from the intended recipient.9 (2) Natural biodegradable materials: derived from natural matrix materials and displaying robust biocompatibility, including collagen, hyaluronate, gelatine, glycosaminoglycan, chitosan, alginate, fibrin, silk, dextran, and Matrigel.10 (3) Synthetic biodegradable materials: including poly(glycolic acid) (PGA), polylactic acid, polylactic-co-glycolic acid, poly(L-lactic acid), poly-ε-caprolactone, polyethylene glycol, polyvinyl alcohol, polypropylene fumarate, polyacrylic acid, and others.11 Various tissue-engineering approaches have been used: (1) In vitro: autologous or allogenic cells are isolated and seeded onto a bioresorbable scaffold and then cultured in a bioreactor system until the new composite scaffold has sufficient mechanical elasticity and strength for implantation.12 (2) In vivo: this strategy relies on fibrotic encapsulation of valvular moulds implanted subcutaneously with autologous ECM.13-15 (3) In situ: decellularised homograft or xenograft materials are implanted to grow cells and remodel the ECM15-17 (Figure 1). ...
A method for simultaneously crosslinking and functionalizing extracellular matrix-based biomaterials as bioprosthetic heart valves with enhanced endothelialization and reduced inflammation
4
2021
... The rapid development of TEHVs and in situ tissue-engineering triggered the studies on animals. Although animal models are essential to determine the clinical potential of TEHVs, because they are complete organisms that can mimic human physiology to some extent, there are still no ideal animal models or international consensus on standards. To further verify the safety and efficacy of TEHVs, several appropriate pre-clinical animal models, including large and small animals whose hearts are similar to that of humans, were translated into the preclinic. Briefly, small animals are used for in vivo assessments such as subcutaneous implantation,13, 18-21 while large animal models are used to test functionality and re-cellularisation in situ22-30 (Table 1). ...
... Animal models used in TEHV studies.
| Species | TEHV manufacture | Surgical method | Functionality assessment and post-mortem analysis | Reference |
| Small animal model |
| Mouse | Sulfonic polymer hybrid extracellular matrix | Subdermal | Histological and immunohistochemical analysis | 18 |
| Rat | Glut-fixed porcine aortic valve | Subdermal | Histological and quantitative analysis | 19 |
| Electrospinning polycaprolactone scaffold | Subdermal | Scanning electron microscopic and histological analysis | 20 |
| Allogeneic aortic conduit grafts | End-to-side anastomosis with infrarenal aorta | Histological and immunohistochemical analysis, quantitative real-time polymerase chain reaction | 21 |
| Rabbit | Poly urethane scaffold | Subdermal | Macroscopic and histological analysis | 13 |
| Large animal model |
| Adult sheep | Poly(glycolic acid) scaffold | Pulmonary valve replacement via transcatheter-based jugular | Intracardiac echocardiography, cardiac magnetic resonance imaging, computed tomography, histological and immunohistochemical analysis | 22 |
| Polyvinylidene fluoride scaffold | Left anterolateral thoracotomy | Inspected for thrombotic deposits, light microscopic analysis, electron microscopic analysis or processed for extracellular matrix assay | 23 |
Juvenile sheep
(13–17 kg) | Tissue-engineered porcine pulmonary valved conduit | Off-pump cardiopulmonary bypass/left anterolateral thoracotomy | Transthoracic ultrasound echocardiography, mortality, operating time | 24 |
| Foetal ovine | PCL scaffold | Transcatheter pulmonary valve replacement | Ultrasound (pulmonary valve annulus), foetal outcome | 25 |
| Yorkshire pigs (80 kg) | AZ31 magnesium alloy biodegradable stent frame scaffold-based polycarbonate urethane urea TEHV | Cardiopulmonary bypass in the pulmonary position | Echocardiography, biaxial mechanical testing, scanning electron microscopy | 26 |
| Porcine | Decellularised porcine pulmonary heart valves | Median sternotomy or limited lateral thoracotomy, then injection of TEHV | Echocardiographic examination, invasive pressure, angiographic measurements | 27 |
| Vietnamese pigs | Decellularised porcine aortic valves conduit | Xypho‐jugular incision, followed by median longitudinal sternotomy | Echocardiography, macroscopic inspection, metabolic labelling | 28 |
| Canine | Decellularised porcine pulmonary heart valves | Left anterolateral thoracotomy | Echocardiography, histology (haematoxylin and eosin, Masson) and immunohistochemistry, transmission electron microscopy | 29 |
| Non-human primate (Chacma Baboon) | Poly(glycolic acid) scaffold | A mini-sternotomy using an antegrade transapical approach | Transesophageal echocardiography, epicardial echocardiography, Pulmonary artery pressure measurement, biochemical analysis | 30 |
Note: TEHV: tissue-engineered heart valve. ...
... Due to their small size, assessment of TEHVs in mice is much more difficult than in other animal models. This animal model is typically used to evaluate the development of cardiac tissue and differentiation of valves, but ways of appraising the functionality of TEHVs in mice is challenging. Subdermal implantation models are the most commonly-used models at present. Mice have unique advantages including convenience in gene editing, availability of a great variety of immunodeficient strains, and multiple types of antibodies. Kim et al.32 implanted porcine and bovine pericardium, aortic valve and aortic wall into the subcutaneous tissue of wild-type and α-gal knock-out mice. Enzyme-linked immunosorbent assay analyses were used to measure anti-α-gal antibody titres before and after implantation. Histopathology and quantification of calcification revealed that immunoreaction to α-gal may cause more severe calcification in α-gal knock-out mice.32 Bioprosthetic heart valves (BHVs) have also been subcutaneously implanted in mouse models. Guo and his colleagues18 used sulphonic monomers to crosslink with decellularised ECM to obtain hybrid ECM. Then a mouse subdermal model was used to evaluate immune responses and calcification. The results indicated that BHVs crosslinked with sulphonic polymer hybrid ECM exhibited better biocompatibility compared with those crosslinked with glutaraldehyde.18 These studies reveal a potential mechanism of BHV calcification and provide novel perspectives in TEHV design. Although, mice models have unique advantages above, the single subdermal approach and difference of cardiovascular physiology still limit the use of mice. Otherwise, we discover that the back area of mice is so small that if multi-type grafts are subcutaneously implanted in mouse models, the grafts may affect each other, which reduces the veracity of the result. ...
... 18 These studies reveal a potential mechanism of BHV calcification and provide novel perspectives in TEHV design. Although, mice models have unique advantages above, the single subdermal approach and difference of cardiovascular physiology still limit the use of mice. Otherwise, we discover that the back area of mice is so small that if multi-type grafts are subcutaneously implanted in mouse models, the grafts may affect each other, which reduces the veracity of the result. ...
Stability and function of glycosaminoglycans in porcine bioprosthetic heart valves
3
2006
... Animal models used in TEHV studies.
| Species | TEHV manufacture | Surgical method | Functionality assessment and post-mortem analysis | Reference |
| Small animal model |
| Mouse | Sulfonic polymer hybrid extracellular matrix | Subdermal | Histological and immunohistochemical analysis | 18 |
| Rat | Glut-fixed porcine aortic valve | Subdermal | Histological and quantitative analysis | 19 |
| Electrospinning polycaprolactone scaffold | Subdermal | Scanning electron microscopic and histological analysis | 20 |
| Allogeneic aortic conduit grafts | End-to-side anastomosis with infrarenal aorta | Histological and immunohistochemical analysis, quantitative real-time polymerase chain reaction | 21 |
| Rabbit | Poly urethane scaffold | Subdermal | Macroscopic and histological analysis | 13 |
| Large animal model |
| Adult sheep | Poly(glycolic acid) scaffold | Pulmonary valve replacement via transcatheter-based jugular | Intracardiac echocardiography, cardiac magnetic resonance imaging, computed tomography, histological and immunohistochemical analysis | 22 |
| Polyvinylidene fluoride scaffold | Left anterolateral thoracotomy | Inspected for thrombotic deposits, light microscopic analysis, electron microscopic analysis or processed for extracellular matrix assay | 23 |
Juvenile sheep
(13–17 kg) | Tissue-engineered porcine pulmonary valved conduit | Off-pump cardiopulmonary bypass/left anterolateral thoracotomy | Transthoracic ultrasound echocardiography, mortality, operating time | 24 |
| Foetal ovine | PCL scaffold | Transcatheter pulmonary valve replacement | Ultrasound (pulmonary valve annulus), foetal outcome | 25 |
| Yorkshire pigs (80 kg) | AZ31 magnesium alloy biodegradable stent frame scaffold-based polycarbonate urethane urea TEHV | Cardiopulmonary bypass in the pulmonary position | Echocardiography, biaxial mechanical testing, scanning electron microscopy | 26 |
| Porcine | Decellularised porcine pulmonary heart valves | Median sternotomy or limited lateral thoracotomy, then injection of TEHV | Echocardiographic examination, invasive pressure, angiographic measurements | 27 |
| Vietnamese pigs | Decellularised porcine aortic valves conduit | Xypho‐jugular incision, followed by median longitudinal sternotomy | Echocardiography, macroscopic inspection, metabolic labelling | 28 |
| Canine | Decellularised porcine pulmonary heart valves | Left anterolateral thoracotomy | Echocardiography, histology (haematoxylin and eosin, Masson) and immunohistochemistry, transmission electron microscopy | 29 |
| Non-human primate (Chacma Baboon) | Poly(glycolic acid) scaffold | A mini-sternotomy using an antegrade transapical approach | Transesophageal echocardiography, epicardial echocardiography, Pulmonary artery pressure measurement, biochemical analysis | 30 |
Note: TEHV: tissue-engineered heart valve. ...
... Rat subdermal models have been proven to be an ideal model for assessment of immunoreaction and biological compatibility after implantation.33 Lovekamp and colleagues19 implanted tissue-engineered valve samples in juvenile rats through a small dorsal incision and subdermal pocket to evaluate calcification in vivo. Histological analysis and quantitative results showed extensive areas of calcification in tissue samples.19 Jana et al.20 employed a tri-layered nanofibrous substrate manufactured through electrospinning to imitate the structure of a heart valve. Then the TEHV sample was implanted subcutaneously into a rat model for 2 months. The results showed that cells infiltrated into the structure and glycosaminoglycans and elastin were observed, demonstrating good regeneration.20 ...
... 19 Jana et al.20 employed a tri-layered nanofibrous substrate manufactured through electrospinning to imitate the structure of a heart valve. Then the TEHV sample was implanted subcutaneously into a rat model for 2 months. The results showed that cells infiltrated into the structure and glycosaminoglycans and elastin were observed, demonstrating good regeneration.20 ...
Trilayered tissue structure with leaflet-like orientations developed through in vivo tissue engineering
3
2019
... Animal models used in TEHV studies.
| Species | TEHV manufacture | Surgical method | Functionality assessment and post-mortem analysis | Reference |
| Small animal model |
| Mouse | Sulfonic polymer hybrid extracellular matrix | Subdermal | Histological and immunohistochemical analysis | 18 |
| Rat | Glut-fixed porcine aortic valve | Subdermal | Histological and quantitative analysis | 19 |
| Electrospinning polycaprolactone scaffold | Subdermal | Scanning electron microscopic and histological analysis | 20 |
| Allogeneic aortic conduit grafts | End-to-side anastomosis with infrarenal aorta | Histological and immunohistochemical analysis, quantitative real-time polymerase chain reaction | 21 |
| Rabbit | Poly urethane scaffold | Subdermal | Macroscopic and histological analysis | 13 |
| Large animal model |
| Adult sheep | Poly(glycolic acid) scaffold | Pulmonary valve replacement via transcatheter-based jugular | Intracardiac echocardiography, cardiac magnetic resonance imaging, computed tomography, histological and immunohistochemical analysis | 22 |
| Polyvinylidene fluoride scaffold | Left anterolateral thoracotomy | Inspected for thrombotic deposits, light microscopic analysis, electron microscopic analysis or processed for extracellular matrix assay | 23 |
Juvenile sheep
(13–17 kg) | Tissue-engineered porcine pulmonary valved conduit | Off-pump cardiopulmonary bypass/left anterolateral thoracotomy | Transthoracic ultrasound echocardiography, mortality, operating time | 24 |
| Foetal ovine | PCL scaffold | Transcatheter pulmonary valve replacement | Ultrasound (pulmonary valve annulus), foetal outcome | 25 |
| Yorkshire pigs (80 kg) | AZ31 magnesium alloy biodegradable stent frame scaffold-based polycarbonate urethane urea TEHV | Cardiopulmonary bypass in the pulmonary position | Echocardiography, biaxial mechanical testing, scanning electron microscopy | 26 |
| Porcine | Decellularised porcine pulmonary heart valves | Median sternotomy or limited lateral thoracotomy, then injection of TEHV | Echocardiographic examination, invasive pressure, angiographic measurements | 27 |
| Vietnamese pigs | Decellularised porcine aortic valves conduit | Xypho‐jugular incision, followed by median longitudinal sternotomy | Echocardiography, macroscopic inspection, metabolic labelling | 28 |
| Canine | Decellularised porcine pulmonary heart valves | Left anterolateral thoracotomy | Echocardiography, histology (haematoxylin and eosin, Masson) and immunohistochemistry, transmission electron microscopy | 29 |
| Non-human primate (Chacma Baboon) | Poly(glycolic acid) scaffold | A mini-sternotomy using an antegrade transapical approach | Transesophageal echocardiography, epicardial echocardiography, Pulmonary artery pressure measurement, biochemical analysis | 30 |
Note: TEHV: tissue-engineered heart valve. ...
... Rat subdermal models have been proven to be an ideal model for assessment of immunoreaction and biological compatibility after implantation.33 Lovekamp and colleagues19 implanted tissue-engineered valve samples in juvenile rats through a small dorsal incision and subdermal pocket to evaluate calcification in vivo. Histological analysis and quantitative results showed extensive areas of calcification in tissue samples.19 Jana et al.20 employed a tri-layered nanofibrous substrate manufactured through electrospinning to imitate the structure of a heart valve. Then the TEHV sample was implanted subcutaneously into a rat model for 2 months. The results showed that cells infiltrated into the structure and glycosaminoglycans and elastin were observed, demonstrating good regeneration.20 ...
... 20 ...
A computational model to describe the collagen orientation in statically cultured engineered tissues
3
2014
... The rapid development of TEHVs and in situ tissue-engineering triggered the studies on animals. Although animal models are essential to determine the clinical potential of TEHVs, because they are complete organisms that can mimic human physiology to some extent, there are still no ideal animal models or international consensus on standards. To further verify the safety and efficacy of TEHVs, several appropriate pre-clinical animal models, including large and small animals whose hearts are similar to that of humans, were translated into the preclinic. Briefly, small animals are used for in vivo assessments such as subcutaneous implantation,13, 18-21 while large animal models are used to test functionality and re-cellularisation in situ22-30 (Table 1). ...
... Animal models used in TEHV studies.
| Species | TEHV manufacture | Surgical method | Functionality assessment and post-mortem analysis | Reference |
| Small animal model |
| Mouse | Sulfonic polymer hybrid extracellular matrix | Subdermal | Histological and immunohistochemical analysis | 18 |
| Rat | Glut-fixed porcine aortic valve | Subdermal | Histological and quantitative analysis | 19 |
| Electrospinning polycaprolactone scaffold | Subdermal | Scanning electron microscopic and histological analysis | 20 |
| Allogeneic aortic conduit grafts | End-to-side anastomosis with infrarenal aorta | Histological and immunohistochemical analysis, quantitative real-time polymerase chain reaction | 21 |
| Rabbit | Poly urethane scaffold | Subdermal | Macroscopic and histological analysis | 13 |
| Large animal model |
| Adult sheep | Poly(glycolic acid) scaffold | Pulmonary valve replacement via transcatheter-based jugular | Intracardiac echocardiography, cardiac magnetic resonance imaging, computed tomography, histological and immunohistochemical analysis | 22 |
| Polyvinylidene fluoride scaffold | Left anterolateral thoracotomy | Inspected for thrombotic deposits, light microscopic analysis, electron microscopic analysis or processed for extracellular matrix assay | 23 |
Juvenile sheep
(13–17 kg) | Tissue-engineered porcine pulmonary valved conduit | Off-pump cardiopulmonary bypass/left anterolateral thoracotomy | Transthoracic ultrasound echocardiography, mortality, operating time | 24 |
| Foetal ovine | PCL scaffold | Transcatheter pulmonary valve replacement | Ultrasound (pulmonary valve annulus), foetal outcome | 25 |
| Yorkshire pigs (80 kg) | AZ31 magnesium alloy biodegradable stent frame scaffold-based polycarbonate urethane urea TEHV | Cardiopulmonary bypass in the pulmonary position | Echocardiography, biaxial mechanical testing, scanning electron microscopy | 26 |
| Porcine | Decellularised porcine pulmonary heart valves | Median sternotomy or limited lateral thoracotomy, then injection of TEHV | Echocardiographic examination, invasive pressure, angiographic measurements | 27 |
| Vietnamese pigs | Decellularised porcine aortic valves conduit | Xypho‐jugular incision, followed by median longitudinal sternotomy | Echocardiography, macroscopic inspection, metabolic labelling | 28 |
| Canine | Decellularised porcine pulmonary heart valves | Left anterolateral thoracotomy | Echocardiography, histology (haematoxylin and eosin, Masson) and immunohistochemistry, transmission electron microscopy | 29 |
| Non-human primate (Chacma Baboon) | Poly(glycolic acid) scaffold | A mini-sternotomy using an antegrade transapical approach | Transesophageal echocardiography, epicardial echocardiography, Pulmonary artery pressure measurement, biochemical analysis | 30 |
Note: TEHV: tissue-engineered heart valve. ...
... Although computational methods have proven to have high potential, the application and advancement of computational models in cardiovascular tissue engineering applications have presented some challenges. For example, the increased incorporation of features of biological growth and remodelling in computational models usually lead to increased model complexity. To obtain a model that can replicate the physiological problem more closely, the development of numerical methods to a number of models is essential for the optimisation for scaffold properties and for the prediction of TEHV function and adaptation.21 ...
Computational modeling guides tissue-engineered heart valve design for long-term in vivo performance in a translational sheep model
8
2018
... The rapid development of TEHVs and in situ tissue-engineering triggered the studies on animals. Although animal models are essential to determine the clinical potential of TEHVs, because they are complete organisms that can mimic human physiology to some extent, there are still no ideal animal models or international consensus on standards. To further verify the safety and efficacy of TEHVs, several appropriate pre-clinical animal models, including large and small animals whose hearts are similar to that of humans, were translated into the preclinic. Briefly, small animals are used for in vivo assessments such as subcutaneous implantation,13, 18-21 while large animal models are used to test functionality and re-cellularisation in situ22-30 (Table 1). ...
... Animal models used in TEHV studies.
| Species | TEHV manufacture | Surgical method | Functionality assessment and post-mortem analysis | Reference |
| Small animal model |
| Mouse | Sulfonic polymer hybrid extracellular matrix | Subdermal | Histological and immunohistochemical analysis | 18 |
| Rat | Glut-fixed porcine aortic valve | Subdermal | Histological and quantitative analysis | 19 |
| Electrospinning polycaprolactone scaffold | Subdermal | Scanning electron microscopic and histological analysis | 20 |
| Allogeneic aortic conduit grafts | End-to-side anastomosis with infrarenal aorta | Histological and immunohistochemical analysis, quantitative real-time polymerase chain reaction | 21 |
| Rabbit | Poly urethane scaffold | Subdermal | Macroscopic and histological analysis | 13 |
| Large animal model |
| Adult sheep | Poly(glycolic acid) scaffold | Pulmonary valve replacement via transcatheter-based jugular | Intracardiac echocardiography, cardiac magnetic resonance imaging, computed tomography, histological and immunohistochemical analysis | 22 |
| Polyvinylidene fluoride scaffold | Left anterolateral thoracotomy | Inspected for thrombotic deposits, light microscopic analysis, electron microscopic analysis or processed for extracellular matrix assay | 23 |
Juvenile sheep
(13–17 kg) | Tissue-engineered porcine pulmonary valved conduit | Off-pump cardiopulmonary bypass/left anterolateral thoracotomy | Transthoracic ultrasound echocardiography, mortality, operating time | 24 |
| Foetal ovine | PCL scaffold | Transcatheter pulmonary valve replacement | Ultrasound (pulmonary valve annulus), foetal outcome | 25 |
| Yorkshire pigs (80 kg) | AZ31 magnesium alloy biodegradable stent frame scaffold-based polycarbonate urethane urea TEHV | Cardiopulmonary bypass in the pulmonary position | Echocardiography, biaxial mechanical testing, scanning electron microscopy | 26 |
| Porcine | Decellularised porcine pulmonary heart valves | Median sternotomy or limited lateral thoracotomy, then injection of TEHV | Echocardiographic examination, invasive pressure, angiographic measurements | 27 |
| Vietnamese pigs | Decellularised porcine aortic valves conduit | Xypho‐jugular incision, followed by median longitudinal sternotomy | Echocardiography, macroscopic inspection, metabolic labelling | 28 |
| Canine | Decellularised porcine pulmonary heart valves | Left anterolateral thoracotomy | Echocardiography, histology (haematoxylin and eosin, Masson) and immunohistochemistry, transmission electron microscopy | 29 |
| Non-human primate (Chacma Baboon) | Poly(glycolic acid) scaffold | A mini-sternotomy using an antegrade transapical approach | Transesophageal echocardiography, epicardial echocardiography, Pulmonary artery pressure measurement, biochemical analysis | 30 |
Note: TEHV: tissue-engineered heart valve. ...
... The ovine model is the gold standard animal model in translational research into TEHV replacement that satisfies the requirements of the U.S. Food and Drug Administration.35 The similarity of the size of the heart valves and the animal’s cardiovascular physiology to those of the human make the sheep an ideal model for use in TEHV studies. These species also have the advantages of a long neck, which means that the carotid artery and jugular vein—the most common surgical approach for transcatheter replacement of TEHVs—are easily accessible. In addition, sheep are fast-maturing animals and adult individuals do not grow so that they can be used in assessment of long-term functionality and durability of the grafts. TEHVs manufactured from polymer or decellularised scaffolds have been used in adult sheep models. Emmert and colleagues22 used computer modelling to design PGA polymer scaffold TEHVs, and implanted them into the pulmonary valves of sheep using minimally-invasive percutaneous trans-jugular access, then used intracardiac echocardiography, computed tomography (CT), and magnetic resonance imaging to evaluate the functionality of the graft. After sacrifice, immunohistochemistry and other methods were used to assess the remodelling of the valve.22 The outcome suggested that TEHVs based on computer modelling can effectively overcome valve insufficiency due to the contraction of leaflets and adverse tissue remodelling.36 Shinoka et al.37 constructed TEHVs from PGA biodegradable scaffold seeded with fibroblasts and endothelial cells and implanted them into pulmonary valves of sheep. After 8 weeks, transplanted autologous cells generated ECM on the scaffold, and showed good regenerative ability.37 ...
... 22 The outcome suggested that TEHVs based on computer modelling can effectively overcome valve insufficiency due to the contraction of leaflets and adverse tissue remodelling.36 Shinoka et al.37 constructed TEHVs from PGA biodegradable scaffold seeded with fibroblasts and endothelial cells and implanted them into pulmonary valves of sheep. After 8 weeks, transplanted autologous cells generated ECM on the scaffold, and showed good regenerative ability.37 ...
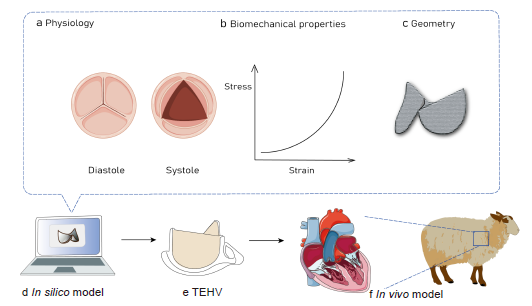
... Computational modelling, particularly when integrated with experimental studies, can substantially accelerate our comprehension of inter-species differences in TEHV adaptation,22 differential remodelling and functionality,100 and the interaction between immune systems-mediated and mechanical stress-driven regenerative processes. Computational modelling of valve mechanics101 and corresponding tissue remodelling provides a powerful approach to predict the consequences of changes in valve design to help inform the rational selection of scaffold parameters to fabricate TEHVs102 (Figure 3). Computational modelling helps designing TEHVs and consistently predicts valve in vivo tissue remodelling and long-term functionality in a large animal model. These findings indicate that integrating computational simulation into tissue engineering approaches can lead to predictable clinical outcomes and the promotion of clinical effectiveness22, 103-119 (Table 3). ...
... 22, 103-119 (Table 3). ...
... Advantages and disadvantages of animal models and bioreactors used in tissue-engineered heart valve studies.
| Model | Application | Objective | Year | Reference |
| Computational modelling | Tissue-engineered heart valve | Guiding tissue-engineered heart valve design for long-term in vivo performance in a translational sheep model | 2018 | 22 |
| Tissue-engineered vascular graft | Identifying optimal design parameters to save development time and costs while improving clinical outcomes | 2019 | 103 |
| Bioprosthetic heart valve | Investigating the impacts of bovine and porcine pericardium tissues with different thicknesses and tissue mechanical properties in bioprosthetic heart valve applications | 2019
2017
2016 | 104–106 |
| Tissue-engineered heart valve | Integrating computational simulation into tissue-engineering approaches can lead to more successful and predictable clinical outcomes | 2018 | 107–109 |
| Biomechanical model | Aortic stenosis | Providing the results of the numerical simulation of the valve function | 2020 | 110 |
| Finite element models | Congenital bicuspid aortic valve | Quantifying aortic valve and root biomechanical alterations associated with bicuspid geometry | 2010 | 111 |
| Calcific aortic valve disease | Studying the calcification progression in aortic valves | 2017 | 112 |
| Bioprosthetic heart valve | Comparing tensile properties of xenopericardium to choose tissue more appropriate for bioprosthetic heart valve tissue | 2020 | 113 |
| Three-dimensional bioprinting | Heart valve | Using computational fluid dynamics, digital image processing, artificial intelligence, and continuum mechanics during their optimisation and implementation to mimic the original and understand valvular problems | 2019
2018 | 114, 115 |
| Geometric model | Functional tri-leaflet aortic valves | Establishing a list of geometric guidelines to ensure safe operation of the valve during the cardiac cycle | 2006 | 116 |
| Numeric model | Aortic root | Studying the correlation between intraoperative effective height and diastolic coaptation | 2013 | 117 |
| Neural network material model | Simulation of the aortic heart valve | Providing an efficient computational analysis framework with increased physical and functional realism for the simulation of native and replacement tri-leaflet heart valves | 2021
2020 | 118, 119 |
The short-term functionality of TEHVs was demonstrated to be excellent in many pre-clinical studies, but leaflet retraction often leads to valvular insufficiency in medium-term follow-up. Many factors that contribute to leaflet retraction such as cellular traction,120 remodelling of the collagen network,121, 122 and contraction of the matrix components. Computational models are necessary to increase our understanding of the underlying mechanisms, and predict the risk for the development of valvular insufficiency.123 ...
... Emmert et al.22 adopted computational modelling of valve mechanics and corresponding tissue remodelling to provide a powerful approach to predict the consequences of changes in valve design on the overall outcome.124 For example, the mechanical behaviour of TEHVs can influence α-smooth muscle actin expression that leads to leaflet retraction and cardiovascular tissue remodelling.125 The use of computational modelling predicted that a more physiological valve geometry126 in combination with a relatively large coaptation area would considerably increase the radial stretch of TEHVs and hence potentially reduce the development of retraction.127 Loerakker et al.123 also used a computational approach to predict the remodelling process in TEHVs subjected under dynamic pulmonary and aortic pressure conditions, and improve assessment of the risk of valvular insufficiency. In addition, they also investigated the importance of intrinsic cell contractility on the valvular matrix remodelling process in the end. Additionally, the model predicted that valvular insufficiency is unlikely because the blood pressure is high enough to prevent the development of leaflet retraction.123 Another study with a computational modelling design indicated that TEHV geometry can significantly influence the host cell response by determining the infiltration and presence of macrophages and α-smooth muscle actin-positive cells, which play a crucial role in orchestrating TEHV remodelling.128 ...
In vivo remodeling and structural characterization of fibrin-based tissue-engineered heart valves in the adult sheep model
2
2009
... Animal models used in TEHV studies.
| Species | TEHV manufacture | Surgical method | Functionality assessment and post-mortem analysis | Reference |
| Small animal model |
| Mouse | Sulfonic polymer hybrid extracellular matrix | Subdermal | Histological and immunohistochemical analysis | 18 |
| Rat | Glut-fixed porcine aortic valve | Subdermal | Histological and quantitative analysis | 19 |
| Electrospinning polycaprolactone scaffold | Subdermal | Scanning electron microscopic and histological analysis | 20 |
| Allogeneic aortic conduit grafts | End-to-side anastomosis with infrarenal aorta | Histological and immunohistochemical analysis, quantitative real-time polymerase chain reaction | 21 |
| Rabbit | Poly urethane scaffold | Subdermal | Macroscopic and histological analysis | 13 |
| Large animal model |
| Adult sheep | Poly(glycolic acid) scaffold | Pulmonary valve replacement via transcatheter-based jugular | Intracardiac echocardiography, cardiac magnetic resonance imaging, computed tomography, histological and immunohistochemical analysis | 22 |
| Polyvinylidene fluoride scaffold | Left anterolateral thoracotomy | Inspected for thrombotic deposits, light microscopic analysis, electron microscopic analysis or processed for extracellular matrix assay | 23 |
Juvenile sheep
(13–17 kg) | Tissue-engineered porcine pulmonary valved conduit | Off-pump cardiopulmonary bypass/left anterolateral thoracotomy | Transthoracic ultrasound echocardiography, mortality, operating time | 24 |
| Foetal ovine | PCL scaffold | Transcatheter pulmonary valve replacement | Ultrasound (pulmonary valve annulus), foetal outcome | 25 |
| Yorkshire pigs (80 kg) | AZ31 magnesium alloy biodegradable stent frame scaffold-based polycarbonate urethane urea TEHV | Cardiopulmonary bypass in the pulmonary position | Echocardiography, biaxial mechanical testing, scanning electron microscopy | 26 |
| Porcine | Decellularised porcine pulmonary heart valves | Median sternotomy or limited lateral thoracotomy, then injection of TEHV | Echocardiographic examination, invasive pressure, angiographic measurements | 27 |
| Vietnamese pigs | Decellularised porcine aortic valves conduit | Xypho‐jugular incision, followed by median longitudinal sternotomy | Echocardiography, macroscopic inspection, metabolic labelling | 28 |
| Canine | Decellularised porcine pulmonary heart valves | Left anterolateral thoracotomy | Echocardiography, histology (haematoxylin and eosin, Masson) and immunohistochemistry, transmission electron microscopy | 29 |
| Non-human primate (Chacma Baboon) | Poly(glycolic acid) scaffold | A mini-sternotomy using an antegrade transapical approach | Transesophageal echocardiography, epicardial echocardiography, Pulmonary artery pressure measurement, biochemical analysis | 30 |
Note: TEHV: tissue-engineered heart valve. ...
... In addition to the above, there are also other options in terms of the age of the animals and the surgical approach. Wu et al.24 used lamb models to compare the outcomes of off-pump cardiopulmonary bypass (CPB) and left anterolateral thoracotomy in TEHV replacement. Flanagan et al.23 used left anterolateral thoracotomy and normothermic CPB to replace pulmonary valves. Zakko et al.25 used transcatheter valve replacement technology to successfully perform pulmonary valve replacement surgery on foetal ovine. Overall, these studies show that the ovine model is a very appropriate model to evaluate short-term complications and the long-term durability of implanted TEHVs. ...
An in vivo model of in situ implantation using pulmonary valved conduit in large animals under off-pump condition
2
2013
... Animal models used in TEHV studies.
| Species | TEHV manufacture | Surgical method | Functionality assessment and post-mortem analysis | Reference |
| Small animal model |
| Mouse | Sulfonic polymer hybrid extracellular matrix | Subdermal | Histological and immunohistochemical analysis | 18 |
| Rat | Glut-fixed porcine aortic valve | Subdermal | Histological and quantitative analysis | 19 |
| Electrospinning polycaprolactone scaffold | Subdermal | Scanning electron microscopic and histological analysis | 20 |
| Allogeneic aortic conduit grafts | End-to-side anastomosis with infrarenal aorta | Histological and immunohistochemical analysis, quantitative real-time polymerase chain reaction | 21 |
| Rabbit | Poly urethane scaffold | Subdermal | Macroscopic and histological analysis | 13 |
| Large animal model |
| Adult sheep | Poly(glycolic acid) scaffold | Pulmonary valve replacement via transcatheter-based jugular | Intracardiac echocardiography, cardiac magnetic resonance imaging, computed tomography, histological and immunohistochemical analysis | 22 |
| Polyvinylidene fluoride scaffold | Left anterolateral thoracotomy | Inspected for thrombotic deposits, light microscopic analysis, electron microscopic analysis or processed for extracellular matrix assay | 23 |
Juvenile sheep
(13–17 kg) | Tissue-engineered porcine pulmonary valved conduit | Off-pump cardiopulmonary bypass/left anterolateral thoracotomy | Transthoracic ultrasound echocardiography, mortality, operating time | 24 |
| Foetal ovine | PCL scaffold | Transcatheter pulmonary valve replacement | Ultrasound (pulmonary valve annulus), foetal outcome | 25 |
| Yorkshire pigs (80 kg) | AZ31 magnesium alloy biodegradable stent frame scaffold-based polycarbonate urethane urea TEHV | Cardiopulmonary bypass in the pulmonary position | Echocardiography, biaxial mechanical testing, scanning electron microscopy | 26 |
| Porcine | Decellularised porcine pulmonary heart valves | Median sternotomy or limited lateral thoracotomy, then injection of TEHV | Echocardiographic examination, invasive pressure, angiographic measurements | 27 |
| Vietnamese pigs | Decellularised porcine aortic valves conduit | Xypho‐jugular incision, followed by median longitudinal sternotomy | Echocardiography, macroscopic inspection, metabolic labelling | 28 |
| Canine | Decellularised porcine pulmonary heart valves | Left anterolateral thoracotomy | Echocardiography, histology (haematoxylin and eosin, Masson) and immunohistochemistry, transmission electron microscopy | 29 |
| Non-human primate (Chacma Baboon) | Poly(glycolic acid) scaffold | A mini-sternotomy using an antegrade transapical approach | Transesophageal echocardiography, epicardial echocardiography, Pulmonary artery pressure measurement, biochemical analysis | 30 |
Note: TEHV: tissue-engineered heart valve. ...
... In addition to the above, there are also other options in terms of the age of the animals and the surgical approach. Wu et al.24 used lamb models to compare the outcomes of off-pump cardiopulmonary bypass (CPB) and left anterolateral thoracotomy in TEHV replacement. Flanagan et al.23 used left anterolateral thoracotomy and normothermic CPB to replace pulmonary valves. Zakko et al.25 used transcatheter valve replacement technology to successfully perform pulmonary valve replacement surgery on foetal ovine. Overall, these studies show that the ovine model is a very appropriate model to evaluate short-term complications and the long-term durability of implanted TEHVs. ...
Development of tissue engineered heart valves for percutaneous transcatheter delivery in a fetal ovine model
2
2020
... Animal models used in TEHV studies.
| Species | TEHV manufacture | Surgical method | Functionality assessment and post-mortem analysis | Reference |
| Small animal model |
| Mouse | Sulfonic polymer hybrid extracellular matrix | Subdermal | Histological and immunohistochemical analysis | 18 |
| Rat | Glut-fixed porcine aortic valve | Subdermal | Histological and quantitative analysis | 19 |
| Electrospinning polycaprolactone scaffold | Subdermal | Scanning electron microscopic and histological analysis | 20 |
| Allogeneic aortic conduit grafts | End-to-side anastomosis with infrarenal aorta | Histological and immunohistochemical analysis, quantitative real-time polymerase chain reaction | 21 |
| Rabbit | Poly urethane scaffold | Subdermal | Macroscopic and histological analysis | 13 |
| Large animal model |
| Adult sheep | Poly(glycolic acid) scaffold | Pulmonary valve replacement via transcatheter-based jugular | Intracardiac echocardiography, cardiac magnetic resonance imaging, computed tomography, histological and immunohistochemical analysis | 22 |
| Polyvinylidene fluoride scaffold | Left anterolateral thoracotomy | Inspected for thrombotic deposits, light microscopic analysis, electron microscopic analysis or processed for extracellular matrix assay | 23 |
Juvenile sheep
(13–17 kg) | Tissue-engineered porcine pulmonary valved conduit | Off-pump cardiopulmonary bypass/left anterolateral thoracotomy | Transthoracic ultrasound echocardiography, mortality, operating time | 24 |
| Foetal ovine | PCL scaffold | Transcatheter pulmonary valve replacement | Ultrasound (pulmonary valve annulus), foetal outcome | 25 |
| Yorkshire pigs (80 kg) | AZ31 magnesium alloy biodegradable stent frame scaffold-based polycarbonate urethane urea TEHV | Cardiopulmonary bypass in the pulmonary position | Echocardiography, biaxial mechanical testing, scanning electron microscopy | 26 |
| Porcine | Decellularised porcine pulmonary heart valves | Median sternotomy or limited lateral thoracotomy, then injection of TEHV | Echocardiographic examination, invasive pressure, angiographic measurements | 27 |
| Vietnamese pigs | Decellularised porcine aortic valves conduit | Xypho‐jugular incision, followed by median longitudinal sternotomy | Echocardiography, macroscopic inspection, metabolic labelling | 28 |
| Canine | Decellularised porcine pulmonary heart valves | Left anterolateral thoracotomy | Echocardiography, histology (haematoxylin and eosin, Masson) and immunohistochemistry, transmission electron microscopy | 29 |
| Non-human primate (Chacma Baboon) | Poly(glycolic acid) scaffold | A mini-sternotomy using an antegrade transapical approach | Transesophageal echocardiography, epicardial echocardiography, Pulmonary artery pressure measurement, biochemical analysis | 30 |
Note: TEHV: tissue-engineered heart valve. ...
... In addition to the above, there are also other options in terms of the age of the animals and the surgical approach. Wu et al.24 used lamb models to compare the outcomes of off-pump cardiopulmonary bypass (CPB) and left anterolateral thoracotomy in TEHV replacement. Flanagan et al.23 used left anterolateral thoracotomy and normothermic CPB to replace pulmonary valves. Zakko et al.25 used transcatheter valve replacement technology to successfully perform pulmonary valve replacement surgery on foetal ovine. Overall, these studies show that the ovine model is a very appropriate model to evaluate short-term complications and the long-term durability of implanted TEHVs. ...
In vivo functional assessment of a novel degradable metal and elastomeric scaffold-based tissue engineered heart valve
3
2019
... Animal models used in TEHV studies.
| Species | TEHV manufacture | Surgical method | Functionality assessment and post-mortem analysis | Reference |
| Small animal model |
| Mouse | Sulfonic polymer hybrid extracellular matrix | Subdermal | Histological and immunohistochemical analysis | 18 |
| Rat | Glut-fixed porcine aortic valve | Subdermal | Histological and quantitative analysis | 19 |
| Electrospinning polycaprolactone scaffold | Subdermal | Scanning electron microscopic and histological analysis | 20 |
| Allogeneic aortic conduit grafts | End-to-side anastomosis with infrarenal aorta | Histological and immunohistochemical analysis, quantitative real-time polymerase chain reaction | 21 |
| Rabbit | Poly urethane scaffold | Subdermal | Macroscopic and histological analysis | 13 |
| Large animal model |
| Adult sheep | Poly(glycolic acid) scaffold | Pulmonary valve replacement via transcatheter-based jugular | Intracardiac echocardiography, cardiac magnetic resonance imaging, computed tomography, histological and immunohistochemical analysis | 22 |
| Polyvinylidene fluoride scaffold | Left anterolateral thoracotomy | Inspected for thrombotic deposits, light microscopic analysis, electron microscopic analysis or processed for extracellular matrix assay | 23 |
Juvenile sheep
(13–17 kg) | Tissue-engineered porcine pulmonary valved conduit | Off-pump cardiopulmonary bypass/left anterolateral thoracotomy | Transthoracic ultrasound echocardiography, mortality, operating time | 24 |
| Foetal ovine | PCL scaffold | Transcatheter pulmonary valve replacement | Ultrasound (pulmonary valve annulus), foetal outcome | 25 |
| Yorkshire pigs (80 kg) | AZ31 magnesium alloy biodegradable stent frame scaffold-based polycarbonate urethane urea TEHV | Cardiopulmonary bypass in the pulmonary position | Echocardiography, biaxial mechanical testing, scanning electron microscopy | 26 |
| Porcine | Decellularised porcine pulmonary heart valves | Median sternotomy or limited lateral thoracotomy, then injection of TEHV | Echocardiographic examination, invasive pressure, angiographic measurements | 27 |
| Vietnamese pigs | Decellularised porcine aortic valves conduit | Xypho‐jugular incision, followed by median longitudinal sternotomy | Echocardiography, macroscopic inspection, metabolic labelling | 28 |
| Canine | Decellularised porcine pulmonary heart valves | Left anterolateral thoracotomy | Echocardiography, histology (haematoxylin and eosin, Masson) and immunohistochemistry, transmission electron microscopy | 29 |
| Non-human primate (Chacma Baboon) | Poly(glycolic acid) scaffold | A mini-sternotomy using an antegrade transapical approach | Transesophageal echocardiography, epicardial echocardiography, Pulmonary artery pressure measurement, biochemical analysis | 30 |
Note: TEHV: tissue-engineered heart valve. ...
... Pig models have been used to assess the functionality and durability of implanted TEHVs. In one innovative study, Coyan and colleagues26 employed AZ31 biodegradable magnesium alloy as a frame scaffold, then applied a polycarbonate urethane urea coating to the surface. The Yorkshire pigs then underwent open TEHV implantation during CPB in the pulmonary position. Echocardiography, biaxial mechanical testing, and scanning electron microscopy all showed that the grafts retained their mechanical strength and ultrastructure, without platelet activation or thrombosis.26 Decellularised porcine pulmonary valves have also been used in piglet models. Schlegal et al.27 successfully implanted decellularised TEHVs in juvenile pigs under fluoroscopic guidance. The results of angiography and epicardial echocardiography revealed no trans- or paravalvular leakage, and the haemodynamic parameters were stable.27 Diminutive pigs such as Vietnamese pigs have been considered to be a suitable model for assessment of novel TEHVs in the right ventricular outflow tract,42 and an intra-operative protocol has been developed.43 Gallo and colleagues44 implanted decellularised aortic valves in Vietnamese pigs for 15 months, and the results of haemodynamic analysis and regeneration were satisfactory, demonstrating the potential of the grafts. Pigs are also the models of choice for transcatheter aortic valve replacement, in the same way as sheep.45 The surgical access for transcatheter aortic valve replacement in pigs and sheep may be via the femoral or jugular vein.46-48 However, the jury is still out regarding which species allows more accurate evaluation of the functionality of the graft. At present, anatomic studies have shown that sheep have long necks and thus if the catheter is long, the sheep may be a better model.49 Moreover, the size of a sheep is smaller and more similar to a human compared to a pig. However, there is a remarkable analytical difficulty associated with this model at the protein level because of the lack of appropriate antibodies. ...
... 26 Decellularised porcine pulmonary valves have also been used in piglet models. Schlegal et al.27 successfully implanted decellularised TEHVs in juvenile pigs under fluoroscopic guidance. The results of angiography and epicardial echocardiography revealed no trans- or paravalvular leakage, and the haemodynamic parameters were stable.27 Diminutive pigs such as Vietnamese pigs have been considered to be a suitable model for assessment of novel TEHVs in the right ventricular outflow tract,42 and an intra-operative protocol has been developed.43 Gallo and colleagues44 implanted decellularised aortic valves in Vietnamese pigs for 15 months, and the results of haemodynamic analysis and regeneration were satisfactory, demonstrating the potential of the grafts. Pigs are also the models of choice for transcatheter aortic valve replacement, in the same way as sheep.45 The surgical access for transcatheter aortic valve replacement in pigs and sheep may be via the femoral or jugular vein.46-48 However, the jury is still out regarding which species allows more accurate evaluation of the functionality of the graft. At present, anatomic studies have shown that sheep have long necks and thus if the catheter is long, the sheep may be a better model.49 Moreover, the size of a sheep is smaller and more similar to a human compared to a pig. However, there is a remarkable analytical difficulty associated with this model at the protein level because of the lack of appropriate antibodies. ...
Injectable tissue engineered pulmonary heart valve implantation into the pig model: A feasibility study
3
2015
... Animal models used in TEHV studies.
| Species | TEHV manufacture | Surgical method | Functionality assessment and post-mortem analysis | Reference |
| Small animal model |
| Mouse | Sulfonic polymer hybrid extracellular matrix | Subdermal | Histological and immunohistochemical analysis | 18 |
| Rat | Glut-fixed porcine aortic valve | Subdermal | Histological and quantitative analysis | 19 |
| Electrospinning polycaprolactone scaffold | Subdermal | Scanning electron microscopic and histological analysis | 20 |
| Allogeneic aortic conduit grafts | End-to-side anastomosis with infrarenal aorta | Histological and immunohistochemical analysis, quantitative real-time polymerase chain reaction | 21 |
| Rabbit | Poly urethane scaffold | Subdermal | Macroscopic and histological analysis | 13 |
| Large animal model |
| Adult sheep | Poly(glycolic acid) scaffold | Pulmonary valve replacement via transcatheter-based jugular | Intracardiac echocardiography, cardiac magnetic resonance imaging, computed tomography, histological and immunohistochemical analysis | 22 |
| Polyvinylidene fluoride scaffold | Left anterolateral thoracotomy | Inspected for thrombotic deposits, light microscopic analysis, electron microscopic analysis or processed for extracellular matrix assay | 23 |
Juvenile sheep
(13–17 kg) | Tissue-engineered porcine pulmonary valved conduit | Off-pump cardiopulmonary bypass/left anterolateral thoracotomy | Transthoracic ultrasound echocardiography, mortality, operating time | 24 |
| Foetal ovine | PCL scaffold | Transcatheter pulmonary valve replacement | Ultrasound (pulmonary valve annulus), foetal outcome | 25 |
| Yorkshire pigs (80 kg) | AZ31 magnesium alloy biodegradable stent frame scaffold-based polycarbonate urethane urea TEHV | Cardiopulmonary bypass in the pulmonary position | Echocardiography, biaxial mechanical testing, scanning electron microscopy | 26 |
| Porcine | Decellularised porcine pulmonary heart valves | Median sternotomy or limited lateral thoracotomy, then injection of TEHV | Echocardiographic examination, invasive pressure, angiographic measurements | 27 |
| Vietnamese pigs | Decellularised porcine aortic valves conduit | Xypho‐jugular incision, followed by median longitudinal sternotomy | Echocardiography, macroscopic inspection, metabolic labelling | 28 |
| Canine | Decellularised porcine pulmonary heart valves | Left anterolateral thoracotomy | Echocardiography, histology (haematoxylin and eosin, Masson) and immunohistochemistry, transmission electron microscopy | 29 |
| Non-human primate (Chacma Baboon) | Poly(glycolic acid) scaffold | A mini-sternotomy using an antegrade transapical approach | Transesophageal echocardiography, epicardial echocardiography, Pulmonary artery pressure measurement, biochemical analysis | 30 |
Note: TEHV: tissue-engineered heart valve. ...
... Pig models have been used to assess the functionality and durability of implanted TEHVs. In one innovative study, Coyan and colleagues26 employed AZ31 biodegradable magnesium alloy as a frame scaffold, then applied a polycarbonate urethane urea coating to the surface. The Yorkshire pigs then underwent open TEHV implantation during CPB in the pulmonary position. Echocardiography, biaxial mechanical testing, and scanning electron microscopy all showed that the grafts retained their mechanical strength and ultrastructure, without platelet activation or thrombosis.26 Decellularised porcine pulmonary valves have also been used in piglet models. Schlegal et al.27 successfully implanted decellularised TEHVs in juvenile pigs under fluoroscopic guidance. The results of angiography and epicardial echocardiography revealed no trans- or paravalvular leakage, and the haemodynamic parameters were stable.27 Diminutive pigs such as Vietnamese pigs have been considered to be a suitable model for assessment of novel TEHVs in the right ventricular outflow tract,42 and an intra-operative protocol has been developed.43 Gallo and colleagues44 implanted decellularised aortic valves in Vietnamese pigs for 15 months, and the results of haemodynamic analysis and regeneration were satisfactory, demonstrating the potential of the grafts. Pigs are also the models of choice for transcatheter aortic valve replacement, in the same way as sheep.45 The surgical access for transcatheter aortic valve replacement in pigs and sheep may be via the femoral or jugular vein.46-48 However, the jury is still out regarding which species allows more accurate evaluation of the functionality of the graft. At present, anatomic studies have shown that sheep have long necks and thus if the catheter is long, the sheep may be a better model.49 Moreover, the size of a sheep is smaller and more similar to a human compared to a pig. However, there is a remarkable analytical difficulty associated with this model at the protein level because of the lack of appropriate antibodies. ...
... 27 Diminutive pigs such as Vietnamese pigs have been considered to be a suitable model for assessment of novel TEHVs in the right ventricular outflow tract,42 and an intra-operative protocol has been developed.43 Gallo and colleagues44 implanted decellularised aortic valves in Vietnamese pigs for 15 months, and the results of haemodynamic analysis and regeneration were satisfactory, demonstrating the potential of the grafts. Pigs are also the models of choice for transcatheter aortic valve replacement, in the same way as sheep.45 The surgical access for transcatheter aortic valve replacement in pigs and sheep may be via the femoral or jugular vein.46-48 However, the jury is still out regarding which species allows more accurate evaluation of the functionality of the graft. At present, anatomic studies have shown that sheep have long necks and thus if the catheter is long, the sheep may be a better model.49 Moreover, the size of a sheep is smaller and more similar to a human compared to a pig. However, there is a remarkable analytical difficulty associated with this model at the protein level because of the lack of appropriate antibodies. ...
Canine model for long-term evaluation of prosthetic mitral valves
2
1986
... Animal models used in TEHV studies.
| Species | TEHV manufacture | Surgical method | Functionality assessment and post-mortem analysis | Reference |
| Small animal model |
| Mouse | Sulfonic polymer hybrid extracellular matrix | Subdermal | Histological and immunohistochemical analysis | 18 |
| Rat | Glut-fixed porcine aortic valve | Subdermal | Histological and quantitative analysis | 19 |
| Electrospinning polycaprolactone scaffold | Subdermal | Scanning electron microscopic and histological analysis | 20 |
| Allogeneic aortic conduit grafts | End-to-side anastomosis with infrarenal aorta | Histological and immunohistochemical analysis, quantitative real-time polymerase chain reaction | 21 |
| Rabbit | Poly urethane scaffold | Subdermal | Macroscopic and histological analysis | 13 |
| Large animal model |
| Adult sheep | Poly(glycolic acid) scaffold | Pulmonary valve replacement via transcatheter-based jugular | Intracardiac echocardiography, cardiac magnetic resonance imaging, computed tomography, histological and immunohistochemical analysis | 22 |
| Polyvinylidene fluoride scaffold | Left anterolateral thoracotomy | Inspected for thrombotic deposits, light microscopic analysis, electron microscopic analysis or processed for extracellular matrix assay | 23 |
Juvenile sheep
(13–17 kg) | Tissue-engineered porcine pulmonary valved conduit | Off-pump cardiopulmonary bypass/left anterolateral thoracotomy | Transthoracic ultrasound echocardiography, mortality, operating time | 24 |
| Foetal ovine | PCL scaffold | Transcatheter pulmonary valve replacement | Ultrasound (pulmonary valve annulus), foetal outcome | 25 |
| Yorkshire pigs (80 kg) | AZ31 magnesium alloy biodegradable stent frame scaffold-based polycarbonate urethane urea TEHV | Cardiopulmonary bypass in the pulmonary position | Echocardiography, biaxial mechanical testing, scanning electron microscopy | 26 |
| Porcine | Decellularised porcine pulmonary heart valves | Median sternotomy or limited lateral thoracotomy, then injection of TEHV | Echocardiographic examination, invasive pressure, angiographic measurements | 27 |
| Vietnamese pigs | Decellularised porcine aortic valves conduit | Xypho‐jugular incision, followed by median longitudinal sternotomy | Echocardiography, macroscopic inspection, metabolic labelling | 28 |
| Canine | Decellularised porcine pulmonary heart valves | Left anterolateral thoracotomy | Echocardiography, histology (haematoxylin and eosin, Masson) and immunohistochemistry, transmission electron microscopy | 29 |
| Non-human primate (Chacma Baboon) | Poly(glycolic acid) scaffold | A mini-sternotomy using an antegrade transapical approach | Transesophageal echocardiography, epicardial echocardiography, Pulmonary artery pressure measurement, biochemical analysis | 30 |
Note: TEHV: tissue-engineered heart valve. ...
... Since the first studies on dogs by William Harvey in the 17th century, the use of canine models in the field of cardiovascular research has been developed. Because of their moderate size —between 10 and 30 kg — and their docile character, dogs have become appropriate animal models to work with. Compared with other large animals, their thinner skin makes implantation of catheters easier, and allows convenient imaging. In addition, the advantage of dogs in TEHV studies is the lower risk of post-operative infection.28 However, obtaining and maintenance of dog models are notably more expensive than small animal models, which should be taken into consideration when long-term studies are designed. Although Institutional Animal Care and Use Committee guidelines vary from agency to agency, the use of dogs as animal models is often difficult to obtain approval compared with other large animal models. In addition, the number of animals that can be used is limited, even though the use of dogs is reasonable and appropriately approved. These issues should be considered by reviewing specific institutional policies before designing studies.50 ...
The surface microstructure of cusps and leaflets in rabbit and mouse heart valves
3
2014
... Animal models used in TEHV studies.
| Species | TEHV manufacture | Surgical method | Functionality assessment and post-mortem analysis | Reference |
| Small animal model |
| Mouse | Sulfonic polymer hybrid extracellular matrix | Subdermal | Histological and immunohistochemical analysis | 18 |
| Rat | Glut-fixed porcine aortic valve | Subdermal | Histological and quantitative analysis | 19 |
| Electrospinning polycaprolactone scaffold | Subdermal | Scanning electron microscopic and histological analysis | 20 |
| Allogeneic aortic conduit grafts | End-to-side anastomosis with infrarenal aorta | Histological and immunohistochemical analysis, quantitative real-time polymerase chain reaction | 21 |
| Rabbit | Poly urethane scaffold | Subdermal | Macroscopic and histological analysis | 13 |
| Large animal model |
| Adult sheep | Poly(glycolic acid) scaffold | Pulmonary valve replacement via transcatheter-based jugular | Intracardiac echocardiography, cardiac magnetic resonance imaging, computed tomography, histological and immunohistochemical analysis | 22 |
| Polyvinylidene fluoride scaffold | Left anterolateral thoracotomy | Inspected for thrombotic deposits, light microscopic analysis, electron microscopic analysis or processed for extracellular matrix assay | 23 |
Juvenile sheep
(13–17 kg) | Tissue-engineered porcine pulmonary valved conduit | Off-pump cardiopulmonary bypass/left anterolateral thoracotomy | Transthoracic ultrasound echocardiography, mortality, operating time | 24 |
| Foetal ovine | PCL scaffold | Transcatheter pulmonary valve replacement | Ultrasound (pulmonary valve annulus), foetal outcome | 25 |
| Yorkshire pigs (80 kg) | AZ31 magnesium alloy biodegradable stent frame scaffold-based polycarbonate urethane urea TEHV | Cardiopulmonary bypass in the pulmonary position | Echocardiography, biaxial mechanical testing, scanning electron microscopy | 26 |
| Porcine | Decellularised porcine pulmonary heart valves | Median sternotomy or limited lateral thoracotomy, then injection of TEHV | Echocardiographic examination, invasive pressure, angiographic measurements | 27 |
| Vietnamese pigs | Decellularised porcine aortic valves conduit | Xypho‐jugular incision, followed by median longitudinal sternotomy | Echocardiography, macroscopic inspection, metabolic labelling | 28 |
| Canine | Decellularised porcine pulmonary heart valves | Left anterolateral thoracotomy | Echocardiography, histology (haematoxylin and eosin, Masson) and immunohistochemistry, transmission electron microscopy | 29 |
| Non-human primate (Chacma Baboon) | Poly(glycolic acid) scaffold | A mini-sternotomy using an antegrade transapical approach | Transesophageal echocardiography, epicardial echocardiography, Pulmonary artery pressure measurement, biochemical analysis | 30 |
Note: TEHV: tissue-engineered heart valve. ...
... The rabbit is the largest of small animal models, so that larger grafts can be implanted for study. Ye and colleagues29 used scanning electron microscopy to characterise the surface of rabbit heart valves. Based on the results, they developed a microstructural model of a heart valve to investigate the best geometric parameters of the rough surface of the valves to improve the replacement of heart valves.29 In addition, like rats, rabbit subdermal models have been used in TEHV studies. Hayashida et al.13 manufactured an autologous heart valve using a poly-urethane scaffold and implanted it in a rabbit subdermal model for 4 weeks, after which the graft was harvested for macroscopic and histologic analyses to evaluate its mechanical properties and microstructure. The results proved the good biocompatibility of the polymer scaffold. ...
... 29 In addition, like rats, rabbit subdermal models have been used in TEHV studies. Hayashida et al.13 manufactured an autologous heart valve using a poly-urethane scaffold and implanted it in a rabbit subdermal model for 4 weeks, after which the graft was harvested for macroscopic and histologic analyses to evaluate its mechanical properties and microstructure. The results proved the good biocompatibility of the polymer scaffold. ...
Off-the-shelf human decellularized tissue-engineered heart valves in a non-human primate model
4
2013
... The rapid development of TEHVs and in situ tissue-engineering triggered the studies on animals. Although animal models are essential to determine the clinical potential of TEHVs, because they are complete organisms that can mimic human physiology to some extent, there are still no ideal animal models or international consensus on standards. To further verify the safety and efficacy of TEHVs, several appropriate pre-clinical animal models, including large and small animals whose hearts are similar to that of humans, were translated into the preclinic. Briefly, small animals are used for in vivo assessments such as subcutaneous implantation,13, 18-21 while large animal models are used to test functionality and re-cellularisation in situ22-30 (Table 1). ...
... Animal models used in TEHV studies.
| Species | TEHV manufacture | Surgical method | Functionality assessment and post-mortem analysis | Reference |
| Small animal model |
| Mouse | Sulfonic polymer hybrid extracellular matrix | Subdermal | Histological and immunohistochemical analysis | 18 |
| Rat | Glut-fixed porcine aortic valve | Subdermal | Histological and quantitative analysis | 19 |
| Electrospinning polycaprolactone scaffold | Subdermal | Scanning electron microscopic and histological analysis | 20 |
| Allogeneic aortic conduit grafts | End-to-side anastomosis with infrarenal aorta | Histological and immunohistochemical analysis, quantitative real-time polymerase chain reaction | 21 |
| Rabbit | Poly urethane scaffold | Subdermal | Macroscopic and histological analysis | 13 |
| Large animal model |
| Adult sheep | Poly(glycolic acid) scaffold | Pulmonary valve replacement via transcatheter-based jugular | Intracardiac echocardiography, cardiac magnetic resonance imaging, computed tomography, histological and immunohistochemical analysis | 22 |
| Polyvinylidene fluoride scaffold | Left anterolateral thoracotomy | Inspected for thrombotic deposits, light microscopic analysis, electron microscopic analysis or processed for extracellular matrix assay | 23 |
Juvenile sheep
(13–17 kg) | Tissue-engineered porcine pulmonary valved conduit | Off-pump cardiopulmonary bypass/left anterolateral thoracotomy | Transthoracic ultrasound echocardiography, mortality, operating time | 24 |
| Foetal ovine | PCL scaffold | Transcatheter pulmonary valve replacement | Ultrasound (pulmonary valve annulus), foetal outcome | 25 |
| Yorkshire pigs (80 kg) | AZ31 magnesium alloy biodegradable stent frame scaffold-based polycarbonate urethane urea TEHV | Cardiopulmonary bypass in the pulmonary position | Echocardiography, biaxial mechanical testing, scanning electron microscopy | 26 |
| Porcine | Decellularised porcine pulmonary heart valves | Median sternotomy or limited lateral thoracotomy, then injection of TEHV | Echocardiographic examination, invasive pressure, angiographic measurements | 27 |
| Vietnamese pigs | Decellularised porcine aortic valves conduit | Xypho‐jugular incision, followed by median longitudinal sternotomy | Echocardiography, macroscopic inspection, metabolic labelling | 28 |
| Canine | Decellularised porcine pulmonary heart valves | Left anterolateral thoracotomy | Echocardiography, histology (haematoxylin and eosin, Masson) and immunohistochemistry, transmission electron microscopy | 29 |
| Non-human primate (Chacma Baboon) | Poly(glycolic acid) scaffold | A mini-sternotomy using an antegrade transapical approach | Transesophageal echocardiography, epicardial echocardiography, Pulmonary artery pressure measurement, biochemical analysis | 30 |
Note: TEHV: tissue-engineered heart valve. ...
... Baboons have been used as an ideal animal model for assessment of the functionality of TEHVs. During one study in 2013 by Weber et al.30 decellularised PGA scaffold TEHVs were implanted into the pulmonary valve of baboons by minimally-invasive surgery under fluoroscopic guidance, then non-invasive imaging technologies were used to assess the position and in vivo functionality. The results revealed that the morphology and functionality of the graft were retained for more than 8 weeks. Macroscopic outcomes revealed the initial contraction of leaflets, and no formation of thrombus. Transmission electron microscopy showed re-cellularisation on the surface of the scaffolds. Quantitative DNA analysis showed that cell density was similar to that of natural valves in primates.30 The success of the off-the-shelf decellularised TEHVs in preclinical models provided encouraging evidence that decellularised TEHVs may be a potential clinical alternative. Nevertheless, the ethical considerations and higher cost of purchase and management limit the use of NHPs in studies of TEHVs. On all accounts, small animal models are widely used for their easy approach and economic advantages. The general subdermal approach is used for assessment in chronic immunological response and calcification of TEHV. An ideal immunological response to TEHV subdermal implantation has three periods. In the hyperacute process, protein absorption occurs on the surface of TEHV. Then acute and chronic inflammations occur in turn. In the acute phase, invaded monocytes differentiate into macrophages, an ideal non-immunogenic TEHV will not induce invasion of massive neutrophils. The pro-inflammation M1 subtype is observed in the initial week, then in the chronic period, the macrophages shift towards the anti-inflammation M2 subtype. Eventually, mesenchymal progenitor cells migrate from surrounding tissue, differentiate into valvular interstitial cells, and produce new matrix.56 In conclusion, inflammatory cells infiltration in the acute inflammation period may not mean poor biocompatibility, because the remodelling generally occurs after inchoate inflammation. Nevertheless, pro-inflammation cells found on the graft after months to years mean adverse chronic response, which leads to calcification and failure of TEHVs. ...
... 30 The success of the off-the-shelf decellularised TEHVs in preclinical models provided encouraging evidence that decellularised TEHVs may be a potential clinical alternative. Nevertheless, the ethical considerations and higher cost of purchase and management limit the use of NHPs in studies of TEHVs. On all accounts, small animal models are widely used for their easy approach and economic advantages. The general subdermal approach is used for assessment in chronic immunological response and calcification of TEHV. An ideal immunological response to TEHV subdermal implantation has three periods. In the hyperacute process, protein absorption occurs on the surface of TEHV. Then acute and chronic inflammations occur in turn. In the acute phase, invaded monocytes differentiate into macrophages, an ideal non-immunogenic TEHV will not induce invasion of massive neutrophils. The pro-inflammation M1 subtype is observed in the initial week, then in the chronic period, the macrophages shift towards the anti-inflammation M2 subtype. Eventually, mesenchymal progenitor cells migrate from surrounding tissue, differentiate into valvular interstitial cells, and produce new matrix.56 In conclusion, inflammatory cells infiltration in the acute inflammation period may not mean poor biocompatibility, because the remodelling generally occurs after inchoate inflammation. Nevertheless, pro-inflammation cells found on the graft after months to years mean adverse chronic response, which leads to calcification and failure of TEHVs. ...
An experimental analysis of the curative action of penicillin in acute bacterial infections. III. The effect of suppuration upon the antibacterial action of the drug
1
1956
... The history of using small animals as models to explore valvular heart disease can be traced back to the 1950s.31 Small animal models have been popular and widely used since the surgical approach and assessing methods were accepted by researchers, for their easy approach and economic advantages. ...
Differences in xenoreactive immune response and patterns of calcification of porcine and bovine tissues in α-Gal knock-out and wild-type mouse implantation models
2
2015
... Due to their small size, assessment of TEHVs in mice is much more difficult than in other animal models. This animal model is typically used to evaluate the development of cardiac tissue and differentiation of valves, but ways of appraising the functionality of TEHVs in mice is challenging. Subdermal implantation models are the most commonly-used models at present. Mice have unique advantages including convenience in gene editing, availability of a great variety of immunodeficient strains, and multiple types of antibodies. Kim et al.32 implanted porcine and bovine pericardium, aortic valve and aortic wall into the subcutaneous tissue of wild-type and α-gal knock-out mice. Enzyme-linked immunosorbent assay analyses were used to measure anti-α-gal antibody titres before and after implantation. Histopathology and quantification of calcification revealed that immunoreaction to α-gal may cause more severe calcification in α-gal knock-out mice.32 Bioprosthetic heart valves (BHVs) have also been subcutaneously implanted in mouse models. Guo and his colleagues18 used sulphonic monomers to crosslink with decellularised ECM to obtain hybrid ECM. Then a mouse subdermal model was used to evaluate immune responses and calcification. The results indicated that BHVs crosslinked with sulphonic polymer hybrid ECM exhibited better biocompatibility compared with those crosslinked with glutaraldehyde.18 These studies reveal a potential mechanism of BHV calcification and provide novel perspectives in TEHV design. Although, mice models have unique advantages above, the single subdermal approach and difference of cardiovascular physiology still limit the use of mice. Otherwise, we discover that the back area of mice is so small that if multi-type grafts are subcutaneously implanted in mouse models, the grafts may affect each other, which reduces the veracity of the result. ...
... 32 Bioprosthetic heart valves (BHVs) have also been subcutaneously implanted in mouse models. Guo and his colleagues18 used sulphonic monomers to crosslink with decellularised ECM to obtain hybrid ECM. Then a mouse subdermal model was used to evaluate immune responses and calcification. The results indicated that BHVs crosslinked with sulphonic polymer hybrid ECM exhibited better biocompatibility compared with those crosslinked with glutaraldehyde.18 These studies reveal a potential mechanism of BHV calcification and provide novel perspectives in TEHV design. Although, mice models have unique advantages above, the single subdermal approach and difference of cardiovascular physiology still limit the use of mice. Otherwise, we discover that the back area of mice is so small that if multi-type grafts are subcutaneously implanted in mouse models, the grafts may affect each other, which reduces the veracity of the result. ...
Suitability of the rat subdermal model for tissue engineering of heart valves
1
2014
... Rat subdermal models have been proven to be an ideal model for assessment of immunoreaction and biological compatibility after implantation.33 Lovekamp and colleagues19 implanted tissue-engineered valve samples in juvenile rats through a small dorsal incision and subdermal pocket to evaluate calcification in vivo. Histological analysis and quantitative results showed extensive areas of calcification in tissue samples.19 Jana et al.20 employed a tri-layered nanofibrous substrate manufactured through electrospinning to imitate the structure of a heart valve. Then the TEHV sample was implanted subcutaneously into a rat model for 2 months. The results showed that cells infiltrated into the structure and glycosaminoglycans and elastin were observed, demonstrating good regeneration.20 ...
Acceleration of autologous in vivo recellularization of decellularized aortic conduits by fibronectin surface coating
2
2013
... In addition, other experimental methods have been developed such as implantation into the systemic circulation. Assmann and his colleagues34 implanted decellularised aortic conduits by fibronectin surface coating into the systemic circulation of Wistar rats for 8 weeks. Compared with controls, decellularised scaffold coated with bioactive proteins accelerated autologous re-cellularisation.34 Nevertheless, due to the small size, the graft was not able to open and close to simulate the function of valves in human haemodynamics as in large animal models. ...
... 34 Nevertheless, due to the small size, the graft was not able to open and close to simulate the function of valves in human haemodynamics as in large animal models. ...
The use of animal models in developing the discipline of cardiovascular tissue engineering: a review
1
2004
... The ovine model is the gold standard animal model in translational research into TEHV replacement that satisfies the requirements of the U.S. Food and Drug Administration.35 The similarity of the size of the heart valves and the animal’s cardiovascular physiology to those of the human make the sheep an ideal model for use in TEHV studies. These species also have the advantages of a long neck, which means that the carotid artery and jugular vein—the most common surgical approach for transcatheter replacement of TEHVs—are easily accessible. In addition, sheep are fast-maturing animals and adult individuals do not grow so that they can be used in assessment of long-term functionality and durability of the grafts. TEHVs manufactured from polymer or decellularised scaffolds have been used in adult sheep models. Emmert and colleagues22 used computer modelling to design PGA polymer scaffold TEHVs, and implanted them into the pulmonary valves of sheep using minimally-invasive percutaneous trans-jugular access, then used intracardiac echocardiography, computed tomography (CT), and magnetic resonance imaging to evaluate the functionality of the graft. After sacrifice, immunohistochemistry and other methods were used to assess the remodelling of the valve.22 The outcome suggested that TEHVs based on computer modelling can effectively overcome valve insufficiency due to the contraction of leaflets and adverse tissue remodelling.36 Shinoka et al.37 constructed TEHVs from PGA biodegradable scaffold seeded with fibroblasts and endothelial cells and implanted them into pulmonary valves of sheep. After 8 weeks, transplanted autologous cells generated ECM on the scaffold, and showed good regenerative ability.37 ...
Transcatheter implantation of homologous “off-the-shelf” tissue-engineered heart valves with self-repair capacity: long-term functionality and rapid in vivo remodeling in sheep
1
2014
... The ovine model is the gold standard animal model in translational research into TEHV replacement that satisfies the requirements of the U.S. Food and Drug Administration.35 The similarity of the size of the heart valves and the animal’s cardiovascular physiology to those of the human make the sheep an ideal model for use in TEHV studies. These species also have the advantages of a long neck, which means that the carotid artery and jugular vein—the most common surgical approach for transcatheter replacement of TEHVs—are easily accessible. In addition, sheep are fast-maturing animals and adult individuals do not grow so that they can be used in assessment of long-term functionality and durability of the grafts. TEHVs manufactured from polymer or decellularised scaffolds have been used in adult sheep models. Emmert and colleagues22 used computer modelling to design PGA polymer scaffold TEHVs, and implanted them into the pulmonary valves of sheep using minimally-invasive percutaneous trans-jugular access, then used intracardiac echocardiography, computed tomography (CT), and magnetic resonance imaging to evaluate the functionality of the graft. After sacrifice, immunohistochemistry and other methods were used to assess the remodelling of the valve.22 The outcome suggested that TEHVs based on computer modelling can effectively overcome valve insufficiency due to the contraction of leaflets and adverse tissue remodelling.36 Shinoka et al.37 constructed TEHVs from PGA biodegradable scaffold seeded with fibroblasts and endothelial cells and implanted them into pulmonary valves of sheep. After 8 weeks, transplanted autologous cells generated ECM on the scaffold, and showed good regenerative ability.37 ...
Tissue-engineered heart valves. Autologous valve leaflet replacement study in a lamb model
2
1996
... The ovine model is the gold standard animal model in translational research into TEHV replacement that satisfies the requirements of the U.S. Food and Drug Administration.35 The similarity of the size of the heart valves and the animal’s cardiovascular physiology to those of the human make the sheep an ideal model for use in TEHV studies. These species also have the advantages of a long neck, which means that the carotid artery and jugular vein—the most common surgical approach for transcatheter replacement of TEHVs—are easily accessible. In addition, sheep are fast-maturing animals and adult individuals do not grow so that they can be used in assessment of long-term functionality and durability of the grafts. TEHVs manufactured from polymer or decellularised scaffolds have been used in adult sheep models. Emmert and colleagues22 used computer modelling to design PGA polymer scaffold TEHVs, and implanted them into the pulmonary valves of sheep using minimally-invasive percutaneous trans-jugular access, then used intracardiac echocardiography, computed tomography (CT), and magnetic resonance imaging to evaluate the functionality of the graft. After sacrifice, immunohistochemistry and other methods were used to assess the remodelling of the valve.22 The outcome suggested that TEHVs based on computer modelling can effectively overcome valve insufficiency due to the contraction of leaflets and adverse tissue remodelling.36 Shinoka et al.37 constructed TEHVs from PGA biodegradable scaffold seeded with fibroblasts and endothelial cells and implanted them into pulmonary valves of sheep. After 8 weeks, transplanted autologous cells generated ECM on the scaffold, and showed good regenerative ability.37 ...
... 37 ...
Predictive capabilities of various constitutive models for arterial tissue
1
2018
... This species is widely used as a surgical model and also for translational researches.38, 39 Due to its resemblance to humans in terms of cardiovascular anatomy and physiology, pigs can be used in assessment of heart valves. However, the sensitivity of the species’ hearts presents difficulties with anaesthesia, and the tendency of pigs to easily contract infection makes them need specific sterile methods and more nursing care.40 In addition, CPB used in porcine surgery may lead to chronic arterial occlusion and cardiac death.41 Further, their large size and rapid growth rate can also lead to insufficiency of the graft, which affects the long-term assessment, so that miniature pigs have become popular for translational medical research in recent years. ...
Swine as models in biomedical research and toxicology testing
1
2012
... This species is widely used as a surgical model and also for translational researches.38, 39 Due to its resemblance to humans in terms of cardiovascular anatomy and physiology, pigs can be used in assessment of heart valves. However, the sensitivity of the species’ hearts presents difficulties with anaesthesia, and the tendency of pigs to easily contract infection makes them need specific sterile methods and more nursing care.40 In addition, CPB used in porcine surgery may lead to chronic arterial occlusion and cardiac death.41 Further, their large size and rapid growth rate can also lead to insufficiency of the graft, which affects the long-term assessment, so that miniature pigs have become popular for translational medical research in recent years. ...
Development and evaluation of a swine model to assess the preclinical safety of mechanical heart valves
1
2000
... This species is widely used as a surgical model and also for translational researches.38, 39 Due to its resemblance to humans in terms of cardiovascular anatomy and physiology, pigs can be used in assessment of heart valves. However, the sensitivity of the species’ hearts presents difficulties with anaesthesia, and the tendency of pigs to easily contract infection makes them need specific sterile methods and more nursing care.40 In addition, CPB used in porcine surgery may lead to chronic arterial occlusion and cardiac death.41 Further, their large size and rapid growth rate can also lead to insufficiency of the graft, which affects the long-term assessment, so that miniature pigs have become popular for translational medical research in recent years. ...
Hibernating myocardium: chronically adapted to ischemia but vulnerable to sudden death
1
2004
... This species is widely used as a surgical model and also for translational researches.38, 39 Due to its resemblance to humans in terms of cardiovascular anatomy and physiology, pigs can be used in assessment of heart valves. However, the sensitivity of the species’ hearts presents difficulties with anaesthesia, and the tendency of pigs to easily contract infection makes them need specific sterile methods and more nursing care.40 In addition, CPB used in porcine surgery may lead to chronic arterial occlusion and cardiac death.41 Further, their large size and rapid growth rate can also lead to insufficiency of the graft, which affects the long-term assessment, so that miniature pigs have become popular for translational medical research in recent years. ...
The Vietnamese pig as a translational animal model to evaluate tissue engineered heart valves: promising early experience
1
2017
... Pig models have been used to assess the functionality and durability of implanted TEHVs. In one innovative study, Coyan and colleagues26 employed AZ31 biodegradable magnesium alloy as a frame scaffold, then applied a polycarbonate urethane urea coating to the surface. The Yorkshire pigs then underwent open TEHV implantation during CPB in the pulmonary position. Echocardiography, biaxial mechanical testing, and scanning electron microscopy all showed that the grafts retained their mechanical strength and ultrastructure, without platelet activation or thrombosis.26 Decellularised porcine pulmonary valves have also been used in piglet models. Schlegal et al.27 successfully implanted decellularised TEHVs in juvenile pigs under fluoroscopic guidance. The results of angiography and epicardial echocardiography revealed no trans- or paravalvular leakage, and the haemodynamic parameters were stable.27 Diminutive pigs such as Vietnamese pigs have been considered to be a suitable model for assessment of novel TEHVs in the right ventricular outflow tract,42 and an intra-operative protocol has been developed.43 Gallo and colleagues44 implanted decellularised aortic valves in Vietnamese pigs for 15 months, and the results of haemodynamic analysis and regeneration were satisfactory, demonstrating the potential of the grafts. Pigs are also the models of choice for transcatheter aortic valve replacement, in the same way as sheep.45 The surgical access for transcatheter aortic valve replacement in pigs and sheep may be via the femoral or jugular vein.46-48 However, the jury is still out regarding which species allows more accurate evaluation of the functionality of the graft. At present, anatomic studies have shown that sheep have long necks and thus if the catheter is long, the sheep may be a better model.49 Moreover, the size of a sheep is smaller and more similar to a human compared to a pig. However, there is a remarkable analytical difficulty associated with this model at the protein level because of the lack of appropriate antibodies. ...
Tissue-engineered heart valves: intra-operative protocol
1
2013
... Pig models have been used to assess the functionality and durability of implanted TEHVs. In one innovative study, Coyan and colleagues26 employed AZ31 biodegradable magnesium alloy as a frame scaffold, then applied a polycarbonate urethane urea coating to the surface. The Yorkshire pigs then underwent open TEHV implantation during CPB in the pulmonary position. Echocardiography, biaxial mechanical testing, and scanning electron microscopy all showed that the grafts retained their mechanical strength and ultrastructure, without platelet activation or thrombosis.26 Decellularised porcine pulmonary valves have also been used in piglet models. Schlegal et al.27 successfully implanted decellularised TEHVs in juvenile pigs under fluoroscopic guidance. The results of angiography and epicardial echocardiography revealed no trans- or paravalvular leakage, and the haemodynamic parameters were stable.27 Diminutive pigs such as Vietnamese pigs have been considered to be a suitable model for assessment of novel TEHVs in the right ventricular outflow tract,42 and an intra-operative protocol has been developed.43 Gallo and colleagues44 implanted decellularised aortic valves in Vietnamese pigs for 15 months, and the results of haemodynamic analysis and regeneration were satisfactory, demonstrating the potential of the grafts. Pigs are also the models of choice for transcatheter aortic valve replacement, in the same way as sheep.45 The surgical access for transcatheter aortic valve replacement in pigs and sheep may be via the femoral or jugular vein.46-48 However, the jury is still out regarding which species allows more accurate evaluation of the functionality of the graft. At present, anatomic studies have shown that sheep have long necks and thus if the catheter is long, the sheep may be a better model.49 Moreover, the size of a sheep is smaller and more similar to a human compared to a pig. However, there is a remarkable analytical difficulty associated with this model at the protein level because of the lack of appropriate antibodies. ...
Physiological performance of a detergent decellularized heart valve implanted for 15 months in Vietnamese pigs: surgical procedure, follow-up, and explant inspection
1
2012
... Pig models have been used to assess the functionality and durability of implanted TEHVs. In one innovative study, Coyan and colleagues26 employed AZ31 biodegradable magnesium alloy as a frame scaffold, then applied a polycarbonate urethane urea coating to the surface. The Yorkshire pigs then underwent open TEHV implantation during CPB in the pulmonary position. Echocardiography, biaxial mechanical testing, and scanning electron microscopy all showed that the grafts retained their mechanical strength and ultrastructure, without platelet activation or thrombosis.26 Decellularised porcine pulmonary valves have also been used in piglet models. Schlegal et al.27 successfully implanted decellularised TEHVs in juvenile pigs under fluoroscopic guidance. The results of angiography and epicardial echocardiography revealed no trans- or paravalvular leakage, and the haemodynamic parameters were stable.27 Diminutive pigs such as Vietnamese pigs have been considered to be a suitable model for assessment of novel TEHVs in the right ventricular outflow tract,42 and an intra-operative protocol has been developed.43 Gallo and colleagues44 implanted decellularised aortic valves in Vietnamese pigs for 15 months, and the results of haemodynamic analysis and regeneration were satisfactory, demonstrating the potential of the grafts. Pigs are also the models of choice for transcatheter aortic valve replacement, in the same way as sheep.45 The surgical access for transcatheter aortic valve replacement in pigs and sheep may be via the femoral or jugular vein.46-48 However, the jury is still out regarding which species allows more accurate evaluation of the functionality of the graft. At present, anatomic studies have shown that sheep have long necks and thus if the catheter is long, the sheep may be a better model.49 Moreover, the size of a sheep is smaller and more similar to a human compared to a pig. However, there is a remarkable analytical difficulty associated with this model at the protein level because of the lack of appropriate antibodies. ...
Steps toward the percutaneous replacement of atrioventricular valves an experimental study
1
2005
... Pig models have been used to assess the functionality and durability of implanted TEHVs. In one innovative study, Coyan and colleagues26 employed AZ31 biodegradable magnesium alloy as a frame scaffold, then applied a polycarbonate urethane urea coating to the surface. The Yorkshire pigs then underwent open TEHV implantation during CPB in the pulmonary position. Echocardiography, biaxial mechanical testing, and scanning electron microscopy all showed that the grafts retained their mechanical strength and ultrastructure, without platelet activation or thrombosis.26 Decellularised porcine pulmonary valves have also been used in piglet models. Schlegal et al.27 successfully implanted decellularised TEHVs in juvenile pigs under fluoroscopic guidance. The results of angiography and epicardial echocardiography revealed no trans- or paravalvular leakage, and the haemodynamic parameters were stable.27 Diminutive pigs such as Vietnamese pigs have been considered to be a suitable model for assessment of novel TEHVs in the right ventricular outflow tract,42 and an intra-operative protocol has been developed.43 Gallo and colleagues44 implanted decellularised aortic valves in Vietnamese pigs for 15 months, and the results of haemodynamic analysis and regeneration were satisfactory, demonstrating the potential of the grafts. Pigs are also the models of choice for transcatheter aortic valve replacement, in the same way as sheep.45 The surgical access for transcatheter aortic valve replacement in pigs and sheep may be via the femoral or jugular vein.46-48 However, the jury is still out regarding which species allows more accurate evaluation of the functionality of the graft. At present, anatomic studies have shown that sheep have long necks and thus if the catheter is long, the sheep may be a better model.49 Moreover, the size of a sheep is smaller and more similar to a human compared to a pig. However, there is a remarkable analytical difficulty associated with this model at the protein level because of the lack of appropriate antibodies. ...
Transapical aortic valve implantation: an animal feasibility study
1
2006
... Pig models have been used to assess the functionality and durability of implanted TEHVs. In one innovative study, Coyan and colleagues26 employed AZ31 biodegradable magnesium alloy as a frame scaffold, then applied a polycarbonate urethane urea coating to the surface. The Yorkshire pigs then underwent open TEHV implantation during CPB in the pulmonary position. Echocardiography, biaxial mechanical testing, and scanning electron microscopy all showed that the grafts retained their mechanical strength and ultrastructure, without platelet activation or thrombosis.26 Decellularised porcine pulmonary valves have also been used in piglet models. Schlegal et al.27 successfully implanted decellularised TEHVs in juvenile pigs under fluoroscopic guidance. The results of angiography and epicardial echocardiography revealed no trans- or paravalvular leakage, and the haemodynamic parameters were stable.27 Diminutive pigs such as Vietnamese pigs have been considered to be a suitable model for assessment of novel TEHVs in the right ventricular outflow tract,42 and an intra-operative protocol has been developed.43 Gallo and colleagues44 implanted decellularised aortic valves in Vietnamese pigs for 15 months, and the results of haemodynamic analysis and regeneration were satisfactory, demonstrating the potential of the grafts. Pigs are also the models of choice for transcatheter aortic valve replacement, in the same way as sheep.45 The surgical access for transcatheter aortic valve replacement in pigs and sheep may be via the femoral or jugular vein.46-48 However, the jury is still out regarding which species allows more accurate evaluation of the functionality of the graft. At present, anatomic studies have shown that sheep have long necks and thus if the catheter is long, the sheep may be a better model.49 Moreover, the size of a sheep is smaller and more similar to a human compared to a pig. However, there is a remarkable analytical difficulty associated with this model at the protein level because of the lack of appropriate antibodies. ...
Percutaneous aortic valve replacement: an experimental study. I. Studies on implantation
0
2002
Transapical approach for sutureless stent-fixed aortic valve implantation: experimental results
1
2006
... Pig models have been used to assess the functionality and durability of implanted TEHVs. In one innovative study, Coyan and colleagues26 employed AZ31 biodegradable magnesium alloy as a frame scaffold, then applied a polycarbonate urethane urea coating to the surface. The Yorkshire pigs then underwent open TEHV implantation during CPB in the pulmonary position. Echocardiography, biaxial mechanical testing, and scanning electron microscopy all showed that the grafts retained their mechanical strength and ultrastructure, without platelet activation or thrombosis.26 Decellularised porcine pulmonary valves have also been used in piglet models. Schlegal et al.27 successfully implanted decellularised TEHVs in juvenile pigs under fluoroscopic guidance. The results of angiography and epicardial echocardiography revealed no trans- or paravalvular leakage, and the haemodynamic parameters were stable.27 Diminutive pigs such as Vietnamese pigs have been considered to be a suitable model for assessment of novel TEHVs in the right ventricular outflow tract,42 and an intra-operative protocol has been developed.43 Gallo and colleagues44 implanted decellularised aortic valves in Vietnamese pigs for 15 months, and the results of haemodynamic analysis and regeneration were satisfactory, demonstrating the potential of the grafts. Pigs are also the models of choice for transcatheter aortic valve replacement, in the same way as sheep.45 The surgical access for transcatheter aortic valve replacement in pigs and sheep may be via the femoral or jugular vein.46-48 However, the jury is still out regarding which species allows more accurate evaluation of the functionality of the graft. At present, anatomic studies have shown that sheep have long necks and thus if the catheter is long, the sheep may be a better model.49 Moreover, the size of a sheep is smaller and more similar to a human compared to a pig. However, there is a remarkable analytical difficulty associated with this model at the protein level because of the lack of appropriate antibodies. ...
Vascular access animal models used in research
1
2019
... Pig models have been used to assess the functionality and durability of implanted TEHVs. In one innovative study, Coyan and colleagues26 employed AZ31 biodegradable magnesium alloy as a frame scaffold, then applied a polycarbonate urethane urea coating to the surface. The Yorkshire pigs then underwent open TEHV implantation during CPB in the pulmonary position. Echocardiography, biaxial mechanical testing, and scanning electron microscopy all showed that the grafts retained their mechanical strength and ultrastructure, without platelet activation or thrombosis.26 Decellularised porcine pulmonary valves have also been used in piglet models. Schlegal et al.27 successfully implanted decellularised TEHVs in juvenile pigs under fluoroscopic guidance. The results of angiography and epicardial echocardiography revealed no trans- or paravalvular leakage, and the haemodynamic parameters were stable.27 Diminutive pigs such as Vietnamese pigs have been considered to be a suitable model for assessment of novel TEHVs in the right ventricular outflow tract,42 and an intra-operative protocol has been developed.43 Gallo and colleagues44 implanted decellularised aortic valves in Vietnamese pigs for 15 months, and the results of haemodynamic analysis and regeneration were satisfactory, demonstrating the potential of the grafts. Pigs are also the models of choice for transcatheter aortic valve replacement, in the same way as sheep.45 The surgical access for transcatheter aortic valve replacement in pigs and sheep may be via the femoral or jugular vein.46-48 However, the jury is still out regarding which species allows more accurate evaluation of the functionality of the graft. At present, anatomic studies have shown that sheep have long necks and thus if the catheter is long, the sheep may be a better model.49 Moreover, the size of a sheep is smaller and more similar to a human compared to a pig. However, there is a remarkable analytical difficulty associated with this model at the protein level because of the lack of appropriate antibodies. ...
Large mammalian animal models of heart disease
1
2016
... Since the first studies on dogs by William Harvey in the 17th century, the use of canine models in the field of cardiovascular research has been developed. Because of their moderate size —between 10 and 30 kg — and their docile character, dogs have become appropriate animal models to work with. Compared with other large animals, their thinner skin makes implantation of catheters easier, and allows convenient imaging. In addition, the advantage of dogs in TEHV studies is the lower risk of post-operative infection.28 However, obtaining and maintenance of dog models are notably more expensive than small animal models, which should be taken into consideration when long-term studies are designed. Although Institutional Animal Care and Use Committee guidelines vary from agency to agency, the use of dogs as animal models is often difficult to obtain approval compared with other large animal models. In addition, the number of animals that can be used is limited, even though the use of dogs is reasonable and appropriately approved. These issues should be considered by reviewing specific institutional policies before designing studies.50 ...
Minimally immunogenic decellularized porcine valve provides in situ recellularization as a stentless bioprosthetic valve
2
2007
... Due to the restrictions above, developing studies for the assessment of TEHVs implanted in dogs is extraordinarily hard. Iwai and colleagues51 tested the functionality of decellularised porcine pulmonary valves in dogs for 6 months. Left anterolateral thoracotomy and normothermic CPB were performed for the implantation. Echocardiography demonstrated that the functionality of the grafts was good, without thickening of the leaflets, regurgitation or thrombosis. Spontaneous re-endothelialisation of the leaflet surface was observed through haematoxylin and eosin staining, confirming regeneration of the structure.51 Nevertheless, the size of dogs and their hearts impose restrictions on further use. ...
... 51 Nevertheless, the size of dogs and their hearts impose restrictions on further use. ...
Transcriptome analysis of non human primate-induced pluripotent stem cell-derived cardiomyocytes in 2D monolayer culture vs. 3D engineered heart tissue
2
2021
... Due to their similarity to humans in terms of genome, cardiovascular anatomy and physiology, NHPs are considered to be attractive animal models for preclinical research to simulate many diseases such as enteric viral infection,52 human immunodeficiency virus vaccine,53 multiple sclerosis,54 and myocardial infarction. Yang et al.52 used NHP models to analyse the effect of induced pluripotent stem cell-derived cardiomyocytes in two-dimensional monolayer and three-dimensional engineered heart tissue. Moreover, NHPs, represented mostly by rhesus monkeys and baboons, have the advantage of an immunological response similar to humans, which makes NHPs a better choice for studies of the biocompatibility of TEHVs in xenograft implantation.55 ...
... 52 used NHP models to analyse the effect of induced pluripotent stem cell-derived cardiomyocytes in two-dimensional monolayer and three-dimensional engineered heart tissue. Moreover, NHPs, represented mostly by rhesus monkeys and baboons, have the advantage of an immunological response similar to humans, which makes NHPs a better choice for studies of the biocompatibility of TEHVs in xenograft implantation.55 ...
Accelerating HIV vaccine development using non-human primate models
1
2019
... Due to their similarity to humans in terms of genome, cardiovascular anatomy and physiology, NHPs are considered to be attractive animal models for preclinical research to simulate many diseases such as enteric viral infection,52 human immunodeficiency virus vaccine,53 multiple sclerosis,54 and myocardial infarction. Yang et al.52 used NHP models to analyse the effect of induced pluripotent stem cell-derived cardiomyocytes in two-dimensional monolayer and three-dimensional engineered heart tissue. Moreover, NHPs, represented mostly by rhesus monkeys and baboons, have the advantage of an immunological response similar to humans, which makes NHPs a better choice for studies of the biocompatibility of TEHVs in xenograft implantation.55 ...
Non-human primate models of multiple sclerosis
1
2001
... Due to their similarity to humans in terms of genome, cardiovascular anatomy and physiology, NHPs are considered to be attractive animal models for preclinical research to simulate many diseases such as enteric viral infection,52 human immunodeficiency virus vaccine,53 multiple sclerosis,54 and myocardial infarction. Yang et al.52 used NHP models to analyse the effect of induced pluripotent stem cell-derived cardiomyocytes in two-dimensional monolayer and three-dimensional engineered heart tissue. Moreover, NHPs, represented mostly by rhesus monkeys and baboons, have the advantage of an immunological response similar to humans, which makes NHPs a better choice for studies of the biocompatibility of TEHVs in xenograft implantation.55 ...
Xenotransplantation: current status in preclinical research
1
2019
... Due to their similarity to humans in terms of genome, cardiovascular anatomy and physiology, NHPs are considered to be attractive animal models for preclinical research to simulate many diseases such as enteric viral infection,52 human immunodeficiency virus vaccine,53 multiple sclerosis,54 and myocardial infarction. Yang et al.52 used NHP models to analyse the effect of induced pluripotent stem cell-derived cardiomyocytes in two-dimensional monolayer and three-dimensional engineered heart tissue. Moreover, NHPs, represented mostly by rhesus monkeys and baboons, have the advantage of an immunological response similar to humans, which makes NHPs a better choice for studies of the biocompatibility of TEHVs in xenograft implantation.55 ...
Foreign body reaction to biomaterials
1
2008
... Baboons have been used as an ideal animal model for assessment of the functionality of TEHVs. During one study in 2013 by Weber et al.30 decellularised PGA scaffold TEHVs were implanted into the pulmonary valve of baboons by minimally-invasive surgery under fluoroscopic guidance, then non-invasive imaging technologies were used to assess the position and in vivo functionality. The results revealed that the morphology and functionality of the graft were retained for more than 8 weeks. Macroscopic outcomes revealed the initial contraction of leaflets, and no formation of thrombus. Transmission electron microscopy showed re-cellularisation on the surface of the scaffolds. Quantitative DNA analysis showed that cell density was similar to that of natural valves in primates.30 The success of the off-the-shelf decellularised TEHVs in preclinical models provided encouraging evidence that decellularised TEHVs may be a potential clinical alternative. Nevertheless, the ethical considerations and higher cost of purchase and management limit the use of NHPs in studies of TEHVs. On all accounts, small animal models are widely used for their easy approach and economic advantages. The general subdermal approach is used for assessment in chronic immunological response and calcification of TEHV. An ideal immunological response to TEHV subdermal implantation has three periods. In the hyperacute process, protein absorption occurs on the surface of TEHV. Then acute and chronic inflammations occur in turn. In the acute phase, invaded monocytes differentiate into macrophages, an ideal non-immunogenic TEHV will not induce invasion of massive neutrophils. The pro-inflammation M1 subtype is observed in the initial week, then in the chronic period, the macrophages shift towards the anti-inflammation M2 subtype. Eventually, mesenchymal progenitor cells migrate from surrounding tissue, differentiate into valvular interstitial cells, and produce new matrix.56 In conclusion, inflammatory cells infiltration in the acute inflammation period may not mean poor biocompatibility, because the remodelling generally occurs after inchoate inflammation. Nevertheless, pro-inflammation cells found on the graft after months to years mean adverse chronic response, which leads to calcification and failure of TEHVs. ...
Microfluidically supported biochip design for culture of endothelial cell layers with improved perfusion conditions
2
2015
... Heart valves in our bodies encounter a variety of complex mechanical forces. During each cardiac cycle, native valves are continuously subjected to highly-complex tension, flexure, pressure, and shear stress forces as a result of blood flow, for example, aortic valve leaflets experience peak fluid-induced shear stresses of approximately 64-71 dyn/cm2 during systole.57 To better understand each component’s contribution to cellular behaviour and implementation, these mechanical forces must be separated. In an engineered heart valve tissue context, mechanical stimuli, particularly those that incorporate fluid-induced shear stress, have been shown to enhance progenitor cell differentiation pathways,58 regulate ECM remodelling,59 impact the behaviour of valvular endothelial cells behaviour60 or endothelial mesenchymal transformation,61 and induce morphological remodelling in cells cytoskeleton.57 ...
... 57 ...
Differentiation and distribution of marrow stem cells in flex-flow environments demonstrate support of the valvular phenotype
2
2015
... Heart valves in our bodies encounter a variety of complex mechanical forces. During each cardiac cycle, native valves are continuously subjected to highly-complex tension, flexure, pressure, and shear stress forces as a result of blood flow, for example, aortic valve leaflets experience peak fluid-induced shear stresses of approximately 64-71 dyn/cm2 during systole.57 To better understand each component’s contribution to cellular behaviour and implementation, these mechanical forces must be separated. In an engineered heart valve tissue context, mechanical stimuli, particularly those that incorporate fluid-induced shear stress, have been shown to enhance progenitor cell differentiation pathways,58 regulate ECM remodelling,59 impact the behaviour of valvular endothelial cells behaviour60 or endothelial mesenchymal transformation,61 and induce morphological remodelling in cells cytoskeleton.57 ...
... In contrast to laminar flow and pulsatile flow bioreactors designed to stimulate engineered tissues before, Engelmayr et al.91 designed the flexure, stretch, and flow bioreactor which allows to control fluid flow and specimen deformation independently. This novel feature is essential to the differentiation between the effects of flow and strain-mediated stimuli. Elsewhere, flexure, stretch, and flow bioreactors have also verified that coupled mechanical stimuli significantly promote ECM production; in particular, the combination of steady flow with cyclic flexure.58 A pressure-controlled bioreactor and a laminar-flow bioreactor were used to analyse the effects of pressure and shear stress independently, to understand the individual and synergistic effects of the two forces by measuring endothelin-1 and nitric oxide release from human umbilical vein endothelial cells.92 Using a bioreactor with non-physiologic conditioning parameters for TEHVs has led to inconsistent results, such as hypoxia and high cyclic pressures which may increase cellular migration and infiltration into the valve leaflet tissue and interstitial cellular repopulation.93 ...
The in vitro development of autologous fibrin-based tissue-engineered heart valves through optimised dynamic conditioning
1
2007
... Heart valves in our bodies encounter a variety of complex mechanical forces. During each cardiac cycle, native valves are continuously subjected to highly-complex tension, flexure, pressure, and shear stress forces as a result of blood flow, for example, aortic valve leaflets experience peak fluid-induced shear stresses of approximately 64-71 dyn/cm2 during systole.57 To better understand each component’s contribution to cellular behaviour and implementation, these mechanical forces must be separated. In an engineered heart valve tissue context, mechanical stimuli, particularly those that incorporate fluid-induced shear stress, have been shown to enhance progenitor cell differentiation pathways,58 regulate ECM remodelling,59 impact the behaviour of valvular endothelial cells behaviour60 or endothelial mesenchymal transformation,61 and induce morphological remodelling in cells cytoskeleton.57 ...
Shear type and magnitude affect aortic valve endothelial cell morphology, orientation, and differentiation
1
2021
... Heart valves in our bodies encounter a variety of complex mechanical forces. During each cardiac cycle, native valves are continuously subjected to highly-complex tension, flexure, pressure, and shear stress forces as a result of blood flow, for example, aortic valve leaflets experience peak fluid-induced shear stresses of approximately 64-71 dyn/cm2 during systole.57 To better understand each component’s contribution to cellular behaviour and implementation, these mechanical forces must be separated. In an engineered heart valve tissue context, mechanical stimuli, particularly those that incorporate fluid-induced shear stress, have been shown to enhance progenitor cell differentiation pathways,58 regulate ECM remodelling,59 impact the behaviour of valvular endothelial cells behaviour60 or endothelial mesenchymal transformation,61 and induce morphological remodelling in cells cytoskeleton.57 ...
Low shear stress-induced endothelial mesenchymal transformation via the down-regulation of TET2
1
2021
... Heart valves in our bodies encounter a variety of complex mechanical forces. During each cardiac cycle, native valves are continuously subjected to highly-complex tension, flexure, pressure, and shear stress forces as a result of blood flow, for example, aortic valve leaflets experience peak fluid-induced shear stresses of approximately 64-71 dyn/cm2 during systole.57 To better understand each component’s contribution to cellular behaviour and implementation, these mechanical forces must be separated. In an engineered heart valve tissue context, mechanical stimuli, particularly those that incorporate fluid-induced shear stress, have been shown to enhance progenitor cell differentiation pathways,58 regulate ECM remodelling,59 impact the behaviour of valvular endothelial cells behaviour60 or endothelial mesenchymal transformation,61 and induce morphological remodelling in cells cytoskeleton.57 ...
In vitro hydrodynamic evaluation of a scaffold for heart valve tissue engineering
1
2019
... In order to simulate complicated cardiovascular systems and the clinical application conditions for functional TEHVs, the pulsatile-flow testing apparatus was designed with blood pumps, vessels and valves to measure the transvalvular pressure, the regurgitation fraction, and the effective orifice area at a certain heart rate and flow rate to evaluate the risk of transcatheter heart valve substitution.62, 63 ...
Cardiac valve bioreactor for physiological conditioning and hydrodynamic performance assessment
2
2019
... In order to simulate complicated cardiovascular systems and the clinical application conditions for functional TEHVs, the pulsatile-flow testing apparatus was designed with blood pumps, vessels and valves to measure the transvalvular pressure, the regurgitation fraction, and the effective orifice area at a certain heart rate and flow rate to evaluate the risk of transcatheter heart valve substitution.62, 63 ...
... Alongside the rapid development of TEHVs, bioreactors have also been improved to better mimic physiological haemodynamics;97, 98 the value of bioreactor conditioning lies in developing not only the mechanical properties but also the biological properties of a valve including the capacity for remodelling, repair, and growth.99 These biological properties enable improvements in durability, growth, and blood compatibility that TEHVs promise to deliver to the clinic. However, disadvantages also exist, for example, extra time and cost are associated with the bioreactor-conditioning of a TEHV. The contamination risk is also significant, which means that a good aseptic technique is critical and antibiotics should be added to the culture medium when possible.63 ...
Pulsatile flow pump based on an iterative controlled piston pump actuator as an in-vitro cardiovascular flow model
1
2020
... The major advantages are that it does not involve humans or animals with no ethical issues, and it saves a lot of money compared to in vivo experiments.64 Otherwise, pulse-flow bioreactors can also elucidate the mechanism of the cellular responses65 and the interactions between cells and scaffold materials under different mechanical and biochemical conditions, as indicated by a large number of publications from their advent to the present day.66, 67 But they often generate complex and ill-defined mechanical conditioning, which cannot be readily controlled. ...
Adaptable pulsatile flow generated from stem cell-derived cardiomyocytes using quantitative imaging-based signal transduction
1
2020
... The major advantages are that it does not involve humans or animals with no ethical issues, and it saves a lot of money compared to in vivo experiments.64 Otherwise, pulse-flow bioreactors can also elucidate the mechanism of the cellular responses65 and the interactions between cells and scaffold materials under different mechanical and biochemical conditions, as indicated by a large number of publications from their advent to the present day.66, 67 But they often generate complex and ill-defined mechanical conditioning, which cannot be readily controlled. ...
Neurofilament 68 and neurofilament 200 protein levels decrease after traumatic brain injury
1
1994
... The major advantages are that it does not involve humans or animals with no ethical issues, and it saves a lot of money compared to in vivo experiments.64 Otherwise, pulse-flow bioreactors can also elucidate the mechanism of the cellular responses65 and the interactions between cells and scaffold materials under different mechanical and biochemical conditions, as indicated by a large number of publications from their advent to the present day.66, 67 But they often generate complex and ill-defined mechanical conditioning, which cannot be readily controlled. ...
Design and hydrodynamic evaluation of a novel pulsatile bioreactor for biologically active heart valves
2
2004
... The major advantages are that it does not involve humans or animals with no ethical issues, and it saves a lot of money compared to in vivo experiments.64 Otherwise, pulse-flow bioreactors can also elucidate the mechanism of the cellular responses65 and the interactions between cells and scaffold materials under different mechanical and biochemical conditions, as indicated by a large number of publications from their advent to the present day.66, 67 But they often generate complex and ill-defined mechanical conditioning, which cannot be readily controlled. ...
... Bioreactors and computers can be combined to create computational fluid dynamics technology which enables a better understanding of hydrodynamic parameters that influence cell function and remodelling of tissue engineering constructs.94 Williams et al.95, 96 employed a computational approach to establish a range of oscillatory shear stresses that may optimize in vitro valvular tissue growth and demonstrated that the fluid-oscillating effect in combination with a physiologically-relevant shear stress magnitude contributes to the de novo formation of stem cell-derived heart valve tissues. A pulsatile bioreactor has been designed to be controlled by a closed-loop feedback computer system that can change automatically to simulate both physiologic and non-physiologic haemodynamic conditions. The result showed that the valve tissues change as the modulations of pulsatile pressure and/or flow waveforms are changed.67 ...
Cells, scaffolds and bioreactors for tissue-engineered heart valves: a journey from basic concepts to contemporary developmental innovations
1
2011
... Thus, a more controlled mechanical conditioning has been studied that can mimic physiological haemodynamic conditions, including the cyclic stretching, flexure, laminar flow, oscillatory shear stress, and pressure that native valves are exposed to and to sufficiently reproduce these environments in an in vitro setting to grow engineered heart valves68, 69 (Figure 2). A good example of bioreactor with subtle control is Ibidi pump system, a typical commercial bioreactor, which can exert and accurately regulate a variety of shear forces on cells or valvular tissues, including laminar oscillatory flow, as well as large-scale use. Therefore, it is widely used in mechanical stimulation related researches.70-72 ...
A novel bioreactor for mechanobiological studies of engineered heart valve tissue formation under pulmonary arterial physiological flow conditions
1
2014
... Thus, a more controlled mechanical conditioning has been studied that can mimic physiological haemodynamic conditions, including the cyclic stretching, flexure, laminar flow, oscillatory shear stress, and pressure that native valves are exposed to and to sufficiently reproduce these environments in an in vitro setting to grow engineered heart valves68, 69 (Figure 2). A good example of bioreactor with subtle control is Ibidi pump system, a typical commercial bioreactor, which can exert and accurately regulate a variety of shear forces on cells or valvular tissues, including laminar oscillatory flow, as well as large-scale use. Therefore, it is widely used in mechanical stimulation related researches.70-72 ...
The increased adhesion of tumor cells to endothelial cells after irradiation can be reduced by FAK-inhibition
1
2019
... Thus, a more controlled mechanical conditioning has been studied that can mimic physiological haemodynamic conditions, including the cyclic stretching, flexure, laminar flow, oscillatory shear stress, and pressure that native valves are exposed to and to sufficiently reproduce these environments in an in vitro setting to grow engineered heart valves68, 69 (Figure 2). A good example of bioreactor with subtle control is Ibidi pump system, a typical commercial bioreactor, which can exert and accurately regulate a variety of shear forces on cells or valvular tissues, including laminar oscillatory flow, as well as large-scale use. Therefore, it is widely used in mechanical stimulation related researches.70-72 ...
Effect of intermittent shear stress on corneal epithelial cells using an in vitro flow culture model
0
2018
Flow-induced transcriptomic remodeling of endothelial cells derived from human induced pluripotent stem cells
1
2020
... Thus, a more controlled mechanical conditioning has been studied that can mimic physiological haemodynamic conditions, including the cyclic stretching, flexure, laminar flow, oscillatory shear stress, and pressure that native valves are exposed to and to sufficiently reproduce these environments in an in vitro setting to grow engineered heart valves68, 69 (Figure 2). A good example of bioreactor with subtle control is Ibidi pump system, a typical commercial bioreactor, which can exert and accurately regulate a variety of shear forces on cells or valvular tissues, including laminar oscillatory flow, as well as large-scale use. Therefore, it is widely used in mechanical stimulation related researches.70-72 ...
Cyclic mechanical preconditioning improves engineered muscle contraction
1
2008
... Mechanical stretch is one of the physical cues from an organismal level to a subcellular level, which is associated with many physiological and pathological processes such as beating heart or bone remodelling processes. It is worth noting that mechanical stretch, as an important novel pretreatment method in tissue engineering, has been widely used. For example, cyclic stretch preconditioning improves engineered muscle contraction.73 In addition, proper mechanical stimulation may promote the regeneration of cell‐based ligaments and improve the success rate of transplantation.74 Ku et al.75 used a Flexcell bioreactor to stretch valve cells and mesenchymal stem cells and showed that 14% stretching upregulated expression of the type I collagen gene and increased collagen synthesis. Syedain and Tranquillo76 also developed a controlled cyclic stretch bioreactor for TEHVs that resulted in improving tensile and compositional properties. To sum up, the mechanical stretch may promote tissue regeneration by influencing cell biological characteristics. ...
Effects of mechanical stretch on cell proliferation and matrix formation of mesenchymal stem cell and anterior cruciate ligament fibroblast
1
2016
... Mechanical stretch is one of the physical cues from an organismal level to a subcellular level, which is associated with many physiological and pathological processes such as beating heart or bone remodelling processes. It is worth noting that mechanical stretch, as an important novel pretreatment method in tissue engineering, has been widely used. For example, cyclic stretch preconditioning improves engineered muscle contraction.73 In addition, proper mechanical stimulation may promote the regeneration of cell‐based ligaments and improve the success rate of transplantation.74 Ku et al.75 used a Flexcell bioreactor to stretch valve cells and mesenchymal stem cells and showed that 14% stretching upregulated expression of the type I collagen gene and increased collagen synthesis. Syedain and Tranquillo76 also developed a controlled cyclic stretch bioreactor for TEHVs that resulted in improving tensile and compositional properties. To sum up, the mechanical stretch may promote tissue regeneration by influencing cell biological characteristics. ...
Collagen synthesis by mesenchymal stem cells and aortic valve interstitial cells in response to mechanical stretch
1
2006
... Mechanical stretch is one of the physical cues from an organismal level to a subcellular level, which is associated with many physiological and pathological processes such as beating heart or bone remodelling processes. It is worth noting that mechanical stretch, as an important novel pretreatment method in tissue engineering, has been widely used. For example, cyclic stretch preconditioning improves engineered muscle contraction.73 In addition, proper mechanical stimulation may promote the regeneration of cell‐based ligaments and improve the success rate of transplantation.74 Ku et al.75 used a Flexcell bioreactor to stretch valve cells and mesenchymal stem cells and showed that 14% stretching upregulated expression of the type I collagen gene and increased collagen synthesis. Syedain and Tranquillo76 also developed a controlled cyclic stretch bioreactor for TEHVs that resulted in improving tensile and compositional properties. To sum up, the mechanical stretch may promote tissue regeneration by influencing cell biological characteristics. ...
Controlled cyclic stretch bioreactor for tissue-engineered heart valves
1
2009
... Mechanical stretch is one of the physical cues from an organismal level to a subcellular level, which is associated with many physiological and pathological processes such as beating heart or bone remodelling processes. It is worth noting that mechanical stretch, as an important novel pretreatment method in tissue engineering, has been widely used. For example, cyclic stretch preconditioning improves engineered muscle contraction.73 In addition, proper mechanical stimulation may promote the regeneration of cell‐based ligaments and improve the success rate of transplantation.74 Ku et al.75 used a Flexcell bioreactor to stretch valve cells and mesenchymal stem cells and showed that 14% stretching upregulated expression of the type I collagen gene and increased collagen synthesis. Syedain and Tranquillo76 also developed a controlled cyclic stretch bioreactor for TEHVs that resulted in improving tensile and compositional properties. To sum up, the mechanical stretch may promote tissue regeneration by influencing cell biological characteristics. ...
The independent role of cyclic flexure in the early in vitro development of an engineered heart valve tissue
3
2005
... In vivo, cyclic flexure plays a vital role on heart valve deformation, the flexure bioreactor is necessary to evaluate candidate TEHV scaffolds and constructs. Engelmayr et al.77 stimulated ovine vascular smooth muscle cells by using a bioreactor with unidirectional cyclic three-point flexure at a physiological frequency and amplitude. The result showed that, compared to static groups, flexure had independent effects on TEHV cell and ECM development and even on the mechanical properties of TEHV scaffolds.77 Another study has showed that the mechanical properties of PGA/poly(L-lactic acid) fibre scaffolds have changed under dynamic flexure.78 In a word, the flexure bioreactor can investigate the underlying mechanisms of which flexure influences tissue development and produce quantitative and qualitative changes in the mechanical properties of TEHV scaffolds. ...
... 77 Another study has showed that the mechanical properties of PGA/poly(L-lactic acid) fibre scaffolds have changed under dynamic flexure.78 In a word, the flexure bioreactor can investigate the underlying mechanisms of which flexure influences tissue development and produce quantitative and qualitative changes in the mechanical properties of TEHV scaffolds. ...
... In vascular tissue engineering, unidirectional laminar shear stress has favourable effects on endothelial progenitor cells,79-81 inducing an antithrombotic and anti-atherosclerotic phenotype and, promoting endothelial cell survival.82 Furthermore, cyclic flexure and laminar flow can synergistically promote tissue formation, underlying a rational design of in vitro preconditioning programs for tissue engineering.77 ...
A novel bioreactor for the dynamic flexural stimulation of tissue engineered heart valve biomaterials
2
2003
... In vivo, cyclic flexure plays a vital role on heart valve deformation, the flexure bioreactor is necessary to evaluate candidate TEHV scaffolds and constructs. Engelmayr et al.77 stimulated ovine vascular smooth muscle cells by using a bioreactor with unidirectional cyclic three-point flexure at a physiological frequency and amplitude. The result showed that, compared to static groups, flexure had independent effects on TEHV cell and ECM development and even on the mechanical properties of TEHV scaffolds.77 Another study has showed that the mechanical properties of PGA/poly(L-lactic acid) fibre scaffolds have changed under dynamic flexure.78 In a word, the flexure bioreactor can investigate the underlying mechanisms of which flexure influences tissue development and produce quantitative and qualitative changes in the mechanical properties of TEHV scaffolds. ...
... To identify mechanical stimuli physiologically relevant to cells and biomaterials78 in the dynamic flexure and laminar flow experinced by TEHVs,87 Engelmayr et al.88, 89 designed a flex-stretch-flow bioreactor and seeded bone marrow-derived mesenchymal stem cells onto PGA and poly(L-lactic acid) scaffolds. They found that, after 4 days of static culture and 3 weeks of dynamic incubation, cyclic flexure and laminar flow synergistically accelerated bone marrow-derived mesenchymal stem cell-mediated tissue formation. Compared to the effect of static conditions, the aortic valve leaflets produced more collagen exposing to oscillatory and laminar flow environment, glycosaminoglycans and elastin, which suggested that fluid flow is important for the remodelling of valve ECM.90 ...
Shear stress: an essential driver of endothelial progenitor cells
1
2018
... In vascular tissue engineering, unidirectional laminar shear stress has favourable effects on endothelial progenitor cells,79-81 inducing an antithrombotic and anti-atherosclerotic phenotype and, promoting endothelial cell survival.82 Furthermore, cyclic flexure and laminar flow can synergistically promote tissue formation, underlying a rational design of in vitro preconditioning programs for tissue engineering.77 ...
Shear stress-induced activation of Tie2-dependent signaling pathway enhances reendothelialization capacity of early endothelial progenitor cells
0
2012
Blood flow forces in shaping the vascular system: a focus on endothelial cell behavior
1
2020
... In vascular tissue engineering, unidirectional laminar shear stress has favourable effects on endothelial progenitor cells,79-81 inducing an antithrombotic and anti-atherosclerotic phenotype and, promoting endothelial cell survival.82 Furthermore, cyclic flexure and laminar flow can synergistically promote tissue formation, underlying a rational design of in vitro preconditioning programs for tissue engineering.77 ...
Laminar shear stress-provoked cytoskeletal changes are mediated by epigenetic reprogramming of TIMP1 in human primary smooth muscle cells
1
2019
... In vascular tissue engineering, unidirectional laminar shear stress has favourable effects on endothelial progenitor cells,79-81 inducing an antithrombotic and anti-atherosclerotic phenotype and, promoting endothelial cell survival.82 Furthermore, cyclic flexure and laminar flow can synergistically promote tissue formation, underlying a rational design of in vitro preconditioning programs for tissue engineering.77 ...
Physiologically relevant fluid-induced oscillatory shear stress stimulation of mesenchymal stem cells enhances the engineered valve matrix phenotype
1
2020
... Gonzalez et al.83 seeded human bone-marrow-derived mesenchymal stem cells onto synthetic, biodegradable scaffolds and cultured them under physiologically-relevant oscillatory shear stresses for 2 weeks. The result showed that the expression of endothelial cell markers augmented and the level of the activated α-smooth muscle phenotype was reduced. Studies also showed that oscillatory shear stress determined the direction of endothelial progenitor cell differentiation, such as facilitating the transition of endothelial progenitor cells into mesenchymal cells by increasing expression of the Kir2.1 ion channel84 and reactive oxygen species/protein kinase Cζ/p53 pathway have played an important role in it.85 In conclusion, oscillatory shear stresses alone are significant to the development of TEHV. ...
Oscillating shear stress mediates mesenchymal transdifferentiation of EPCs by the Kir2.1 channel
1
2020
... Gonzalez et al.83 seeded human bone-marrow-derived mesenchymal stem cells onto synthetic, biodegradable scaffolds and cultured them under physiologically-relevant oscillatory shear stresses for 2 weeks. The result showed that the expression of endothelial cell markers augmented and the level of the activated α-smooth muscle phenotype was reduced. Studies also showed that oscillatory shear stress determined the direction of endothelial progenitor cell differentiation, such as facilitating the transition of endothelial progenitor cells into mesenchymal cells by increasing expression of the Kir2.1 ion channel84 and reactive oxygen species/protein kinase Cζ/p53 pathway have played an important role in it.85 In conclusion, oscillatory shear stresses alone are significant to the development of TEHV. ...
Oscillatory shear stress induces the transition of EPCs into mesenchymal cells through ROS/PKCζ/p53 pathway
1
2020
... Gonzalez et al.83 seeded human bone-marrow-derived mesenchymal stem cells onto synthetic, biodegradable scaffolds and cultured them under physiologically-relevant oscillatory shear stresses for 2 weeks. The result showed that the expression of endothelial cell markers augmented and the level of the activated α-smooth muscle phenotype was reduced. Studies also showed that oscillatory shear stress determined the direction of endothelial progenitor cell differentiation, such as facilitating the transition of endothelial progenitor cells into mesenchymal cells by increasing expression of the Kir2.1 ion channel84 and reactive oxygen species/protein kinase Cζ/p53 pathway have played an important role in it.85 In conclusion, oscillatory shear stresses alone are significant to the development of TEHV. ...
Design and efficacy of a single-use bioreactor for heart valve tissue engineering
1
2017
... In a TEHV framework, fluid-induced shear stresses can induce stem cell differentiation. Using a bioreactor as a tool for seeding with cyclic waveforms tuned to various negative and positive chamber pressures showed that subjecting the system to positive pressure improved infiltration of human mesenchymal stem cells into the aortic valve leaflet, while retaining gene expression within the mesenchymal stem cell-valve interstitial cell phenotype lineage.86 ...
Cardiovascular tissue engineering: a new laminar flow chamber for in vitro improvement of mechanical tissue properties
1
2002
... To identify mechanical stimuli physiologically relevant to cells and biomaterials78 in the dynamic flexure and laminar flow experinced by TEHVs,87 Engelmayr et al.88, 89 designed a flex-stretch-flow bioreactor and seeded bone marrow-derived mesenchymal stem cells onto PGA and poly(L-lactic acid) scaffolds. They found that, after 4 days of static culture and 3 weeks of dynamic incubation, cyclic flexure and laminar flow synergistically accelerated bone marrow-derived mesenchymal stem cell-mediated tissue formation. Compared to the effect of static conditions, the aortic valve leaflets produced more collagen exposing to oscillatory and laminar flow environment, glycosaminoglycans and elastin, which suggested that fluid flow is important for the remodelling of valve ECM.90 ...
Cyclic flexure and laminar flow synergistically accelerate mesenchymal stem cell-mediated engineered tissue formation: Implications for engineered heart valve tissues
1
2006
... To identify mechanical stimuli physiologically relevant to cells and biomaterials78 in the dynamic flexure and laminar flow experinced by TEHVs,87 Engelmayr et al.88, 89 designed a flex-stretch-flow bioreactor and seeded bone marrow-derived mesenchymal stem cells onto PGA and poly(L-lactic acid) scaffolds. They found that, after 4 days of static culture and 3 weeks of dynamic incubation, cyclic flexure and laminar flow synergistically accelerated bone marrow-derived mesenchymal stem cell-mediated tissue formation. Compared to the effect of static conditions, the aortic valve leaflets produced more collagen exposing to oscillatory and laminar flow environment, glycosaminoglycans and elastin, which suggested that fluid flow is important for the remodelling of valve ECM.90 ...
The role of organ level conditioning on the promotion of engineered heart valve tissue development in-vitro using mesenchymal stem cells
1
2010
... To identify mechanical stimuli physiologically relevant to cells and biomaterials78 in the dynamic flexure and laminar flow experinced by TEHVs,87 Engelmayr et al.88, 89 designed a flex-stretch-flow bioreactor and seeded bone marrow-derived mesenchymal stem cells onto PGA and poly(L-lactic acid) scaffolds. They found that, after 4 days of static culture and 3 weeks of dynamic incubation, cyclic flexure and laminar flow synergistically accelerated bone marrow-derived mesenchymal stem cell-mediated tissue formation. Compared to the effect of static conditions, the aortic valve leaflets produced more collagen exposing to oscillatory and laminar flow environment, glycosaminoglycans and elastin, which suggested that fluid flow is important for the remodelling of valve ECM.90 ...
Effect of side-specific valvular shear stress on the content of extracellular matrix in aortic valves
1
2018
... To identify mechanical stimuli physiologically relevant to cells and biomaterials78 in the dynamic flexure and laminar flow experinced by TEHVs,87 Engelmayr et al.88, 89 designed a flex-stretch-flow bioreactor and seeded bone marrow-derived mesenchymal stem cells onto PGA and poly(L-lactic acid) scaffolds. They found that, after 4 days of static culture and 3 weeks of dynamic incubation, cyclic flexure and laminar flow synergistically accelerated bone marrow-derived mesenchymal stem cell-mediated tissue formation. Compared to the effect of static conditions, the aortic valve leaflets produced more collagen exposing to oscillatory and laminar flow environment, glycosaminoglycans and elastin, which suggested that fluid flow is important for the remodelling of valve ECM.90 ...
A novel flex-stretch-flow bioreactor for the study of engineered heart valve tissue mechanobiology
1
2008
... In contrast to laminar flow and pulsatile flow bioreactors designed to stimulate engineered tissues before, Engelmayr et al.91 designed the flexure, stretch, and flow bioreactor which allows to control fluid flow and specimen deformation independently. This novel feature is essential to the differentiation between the effects of flow and strain-mediated stimuli. Elsewhere, flexure, stretch, and flow bioreactors have also verified that coupled mechanical stimuli significantly promote ECM production; in particular, the combination of steady flow with cyclic flexure.58 A pressure-controlled bioreactor and a laminar-flow bioreactor were used to analyse the effects of pressure and shear stress independently, to understand the individual and synergistic effects of the two forces by measuring endothelin-1 and nitric oxide release from human umbilical vein endothelial cells.92 Using a bioreactor with non-physiologic conditioning parameters for TEHVs has led to inconsistent results, such as hypoxia and high cyclic pressures which may increase cellular migration and infiltration into the valve leaflet tissue and interstitial cellular repopulation.93 ...
Hydrostatic pressure and shear stress affect endothelin-1 and nitric oxide release by endothelial cells in bioreactors
1
2014
... In contrast to laminar flow and pulsatile flow bioreactors designed to stimulate engineered tissues before, Engelmayr et al.91 designed the flexure, stretch, and flow bioreactor which allows to control fluid flow and specimen deformation independently. This novel feature is essential to the differentiation between the effects of flow and strain-mediated stimuli. Elsewhere, flexure, stretch, and flow bioreactors have also verified that coupled mechanical stimuli significantly promote ECM production; in particular, the combination of steady flow with cyclic flexure.58 A pressure-controlled bioreactor and a laminar-flow bioreactor were used to analyse the effects of pressure and shear stress independently, to understand the individual and synergistic effects of the two forces by measuring endothelin-1 and nitric oxide release from human umbilical vein endothelial cells.92 Using a bioreactor with non-physiologic conditioning parameters for TEHVs has led to inconsistent results, such as hypoxia and high cyclic pressures which may increase cellular migration and infiltration into the valve leaflet tissue and interstitial cellular repopulation.93 ...
Non-physiologic bioreactor processing conditions for heart valve tissue engineering
1
2019
... In contrast to laminar flow and pulsatile flow bioreactors designed to stimulate engineered tissues before, Engelmayr et al.91 designed the flexure, stretch, and flow bioreactor which allows to control fluid flow and specimen deformation independently. This novel feature is essential to the differentiation between the effects of flow and strain-mediated stimuli. Elsewhere, flexure, stretch, and flow bioreactors have also verified that coupled mechanical stimuli significantly promote ECM production; in particular, the combination of steady flow with cyclic flexure.58 A pressure-controlled bioreactor and a laminar-flow bioreactor were used to analyse the effects of pressure and shear stress independently, to understand the individual and synergistic effects of the two forces by measuring endothelin-1 and nitric oxide release from human umbilical vein endothelial cells.92 Using a bioreactor with non-physiologic conditioning parameters for TEHVs has led to inconsistent results, such as hypoxia and high cyclic pressures which may increase cellular migration and infiltration into the valve leaflet tissue and interstitial cellular repopulation.93 ...
Computational fluid dynamics for improved bioreactor design and 3D culture
1
2008
... Bioreactors and computers can be combined to create computational fluid dynamics technology which enables a better understanding of hydrodynamic parameters that influence cell function and remodelling of tissue engineering constructs.94 Williams et al.95, 96 employed a computational approach to establish a range of oscillatory shear stresses that may optimize in vitro valvular tissue growth and demonstrated that the fluid-oscillating effect in combination with a physiologically-relevant shear stress magnitude contributes to the de novo formation of stem cell-derived heart valve tissues. A pulsatile bioreactor has been designed to be controlled by a closed-loop feedback computer system that can change automatically to simulate both physiologic and non-physiologic haemodynamic conditions. The result showed that the valve tissues change as the modulations of pulsatile pressure and/or flow waveforms are changed.67 ...
A “sweet-spot” for fluid-induced oscillations in the conditioning of stem cell-based engineered heart valve tissues
1
2017
... Bioreactors and computers can be combined to create computational fluid dynamics technology which enables a better understanding of hydrodynamic parameters that influence cell function and remodelling of tissue engineering constructs.94 Williams et al.95, 96 employed a computational approach to establish a range of oscillatory shear stresses that may optimize in vitro valvular tissue growth and demonstrated that the fluid-oscillating effect in combination with a physiologically-relevant shear stress magnitude contributes to the de novo formation of stem cell-derived heart valve tissues. A pulsatile bioreactor has been designed to be controlled by a closed-loop feedback computer system that can change automatically to simulate both physiologic and non-physiologic haemodynamic conditions. The result showed that the valve tissues change as the modulations of pulsatile pressure and/or flow waveforms are changed.67 ...
Computational simulations predict a key role for oscillatory fluid shear stress in de novo valvular tissue formation
1
2014
... Bioreactors and computers can be combined to create computational fluid dynamics technology which enables a better understanding of hydrodynamic parameters that influence cell function and remodelling of tissue engineering constructs.94 Williams et al.95, 96 employed a computational approach to establish a range of oscillatory shear stresses that may optimize in vitro valvular tissue growth and demonstrated that the fluid-oscillating effect in combination with a physiologically-relevant shear stress magnitude contributes to the de novo formation of stem cell-derived heart valve tissues. A pulsatile bioreactor has been designed to be controlled by a closed-loop feedback computer system that can change automatically to simulate both physiologic and non-physiologic haemodynamic conditions. The result showed that the valve tissues change as the modulations of pulsatile pressure and/or flow waveforms are changed.67 ...
Preclinical testing of tissue-engineered heart valves re-endothelialized under simulated physiological conditions
1
2006
... Alongside the rapid development of TEHVs, bioreactors have also been improved to better mimic physiological haemodynamics;97, 98 the value of bioreactor conditioning lies in developing not only the mechanical properties but also the biological properties of a valve including the capacity for remodelling, repair, and growth.99 These biological properties enable improvements in durability, growth, and blood compatibility that TEHVs promise to deliver to the clinic. However, disadvantages also exist, for example, extra time and cost are associated with the bioreactor-conditioning of a TEHV. The contamination risk is also significant, which means that a good aseptic technique is critical and antibiotics should be added to the culture medium when possible.63 ...
In vitro re-endothelialization of detergent decellularized heart valves under simulated physiological dynamic conditions
1
2006
... Alongside the rapid development of TEHVs, bioreactors have also been improved to better mimic physiological haemodynamics;97, 98 the value of bioreactor conditioning lies in developing not only the mechanical properties but also the biological properties of a valve including the capacity for remodelling, repair, and growth.99 These biological properties enable improvements in durability, growth, and blood compatibility that TEHVs promise to deliver to the clinic. However, disadvantages also exist, for example, extra time and cost are associated with the bioreactor-conditioning of a TEHV. The contamination risk is also significant, which means that a good aseptic technique is critical and antibiotics should be added to the culture medium when possible.63 ...
Acrylate-based materials for heart valve scaffold engineering
1
2017
... Alongside the rapid development of TEHVs, bioreactors have also been improved to better mimic physiological haemodynamics;97, 98 the value of bioreactor conditioning lies in developing not only the mechanical properties but also the biological properties of a valve including the capacity for remodelling, repair, and growth.99 These biological properties enable improvements in durability, growth, and blood compatibility that TEHVs promise to deliver to the clinic. However, disadvantages also exist, for example, extra time and cost are associated with the bioreactor-conditioning of a TEHV. The contamination risk is also significant, which means that a good aseptic technique is critical and antibiotics should be added to the culture medium when possible.63 ...
Differential outcomes of venous and arterial tissue engineered vascular grafts highlight the importance of coupling long-term implantation studies with computational modeling
1
2019
... Computational modelling, particularly when integrated with experimental studies, can substantially accelerate our comprehension of inter-species differences in TEHV adaptation,22 differential remodelling and functionality,100 and the interaction between immune systems-mediated and mechanical stress-driven regenerative processes. Computational modelling of valve mechanics101 and corresponding tissue remodelling provides a powerful approach to predict the consequences of changes in valve design to help inform the rational selection of scaffold parameters to fabricate TEHVs102 (Figure 3). Computational modelling helps designing TEHVs and consistently predicts valve in vivo tissue remodelling and long-term functionality in a large animal model. These findings indicate that integrating computational simulation into tissue engineering approaches can lead to predictable clinical outcomes and the promotion of clinical effectiveness22, 103-119 (Table 3). ...
Dynamic and fluid-structure interaction simulations of bioprosthetic heart valves using parametric design with T-splines and Fung-type material models
1
2015
... Computational modelling, particularly when integrated with experimental studies, can substantially accelerate our comprehension of inter-species differences in TEHV adaptation,22 differential remodelling and functionality,100 and the interaction between immune systems-mediated and mechanical stress-driven regenerative processes. Computational modelling of valve mechanics101 and corresponding tissue remodelling provides a powerful approach to predict the consequences of changes in valve design to help inform the rational selection of scaffold parameters to fabricate TEHVs102 (Figure 3). Computational modelling helps designing TEHVs and consistently predicts valve in vivo tissue remodelling and long-term functionality in a large animal model. These findings indicate that integrating computational simulation into tissue engineering approaches can lead to predictable clinical outcomes and the promotion of clinical effectiveness22, 103-119 (Table 3). ...
Simulation of long-term fatigue damage in bioprosthetic heart valves: effects of leaflet and stent elastic properties
0
2014
Optimization of tissue-engineered vascular graft design using computational modeling
2
2019
... Computational modelling, particularly when integrated with experimental studies, can substantially accelerate our comprehension of inter-species differences in TEHV adaptation,22 differential remodelling and functionality,100 and the interaction between immune systems-mediated and mechanical stress-driven regenerative processes. Computational modelling of valve mechanics101 and corresponding tissue remodelling provides a powerful approach to predict the consequences of changes in valve design to help inform the rational selection of scaffold parameters to fabricate TEHVs102 (Figure 3). Computational modelling helps designing TEHVs and consistently predicts valve in vivo tissue remodelling and long-term functionality in a large animal model. These findings indicate that integrating computational simulation into tissue engineering approaches can lead to predictable clinical outcomes and the promotion of clinical effectiveness22, 103-119 (Table 3). ...
... Advantages and disadvantages of animal models and bioreactors used in tissue-engineered heart valve studies.
| Model | Application | Objective | Year | Reference |
| Computational modelling | Tissue-engineered heart valve | Guiding tissue-engineered heart valve design for long-term in vivo performance in a translational sheep model | 2018 | 22 |
| Tissue-engineered vascular graft | Identifying optimal design parameters to save development time and costs while improving clinical outcomes | 2019 | 103 |
| Bioprosthetic heart valve | Investigating the impacts of bovine and porcine pericardium tissues with different thicknesses and tissue mechanical properties in bioprosthetic heart valve applications | 2019
2017
2016 | 104–106 |
| Tissue-engineered heart valve | Integrating computational simulation into tissue-engineering approaches can lead to more successful and predictable clinical outcomes | 2018 | 107–109 |
| Biomechanical model | Aortic stenosis | Providing the results of the numerical simulation of the valve function | 2020 | 110 |
| Finite element models | Congenital bicuspid aortic valve | Quantifying aortic valve and root biomechanical alterations associated with bicuspid geometry | 2010 | 111 |
| Calcific aortic valve disease | Studying the calcification progression in aortic valves | 2017 | 112 |
| Bioprosthetic heart valve | Comparing tensile properties of xenopericardium to choose tissue more appropriate for bioprosthetic heart valve tissue | 2020 | 113 |
| Three-dimensional bioprinting | Heart valve | Using computational fluid dynamics, digital image processing, artificial intelligence, and continuum mechanics during their optimisation and implementation to mimic the original and understand valvular problems | 2019
2018 | 114, 115 |
| Geometric model | Functional tri-leaflet aortic valves | Establishing a list of geometric guidelines to ensure safe operation of the valve during the cardiac cycle | 2006 | 116 |
| Numeric model | Aortic root | Studying the correlation between intraoperative effective height and diastolic coaptation | 2013 | 117 |
| Neural network material model | Simulation of the aortic heart valve | Providing an efficient computational analysis framework with increased physical and functional realism for the simulation of native and replacement tri-leaflet heart valves | 2021
2020 | 118, 119 |
The short-term functionality of TEHVs was demonstrated to be excellent in many pre-clinical studies, but leaflet retraction often leads to valvular insufficiency in medium-term follow-up. Many factors that contribute to leaflet retraction such as cellular traction,120 remodelling of the collagen network,121, 122 and contraction of the matrix components. Computational models are necessary to increase our understanding of the underlying mechanisms, and predict the risk for the development of valvular insufficiency.123 ...
Evaluation of transcatheter heart valve biomaterials: Computational modeling using bovine and porcine pericardium
1
2019
... Advantages and disadvantages of animal models and bioreactors used in tissue-engineered heart valve studies.
| Model | Application | Objective | Year | Reference |
| Computational modelling | Tissue-engineered heart valve | Guiding tissue-engineered heart valve design for long-term in vivo performance in a translational sheep model | 2018 | 22 |
| Tissue-engineered vascular graft | Identifying optimal design parameters to save development time and costs while improving clinical outcomes | 2019 | 103 |
| Bioprosthetic heart valve | Investigating the impacts of bovine and porcine pericardium tissues with different thicknesses and tissue mechanical properties in bioprosthetic heart valve applications | 2019
2017
2016 | 104–106 |
| Tissue-engineered heart valve | Integrating computational simulation into tissue-engineering approaches can lead to more successful and predictable clinical outcomes | 2018 | 107–109 |
| Biomechanical model | Aortic stenosis | Providing the results of the numerical simulation of the valve function | 2020 | 110 |
| Finite element models | Congenital bicuspid aortic valve | Quantifying aortic valve and root biomechanical alterations associated with bicuspid geometry | 2010 | 111 |
| Calcific aortic valve disease | Studying the calcification progression in aortic valves | 2017 | 112 |
| Bioprosthetic heart valve | Comparing tensile properties of xenopericardium to choose tissue more appropriate for bioprosthetic heart valve tissue | 2020 | 113 |
| Three-dimensional bioprinting | Heart valve | Using computational fluid dynamics, digital image processing, artificial intelligence, and continuum mechanics during their optimisation and implementation to mimic the original and understand valvular problems | 2019
2018 | 114, 115 |
| Geometric model | Functional tri-leaflet aortic valves | Establishing a list of geometric guidelines to ensure safe operation of the valve during the cardiac cycle | 2006 | 116 |
| Numeric model | Aortic root | Studying the correlation between intraoperative effective height and diastolic coaptation | 2013 | 117 |
| Neural network material model | Simulation of the aortic heart valve | Providing an efficient computational analysis framework with increased physical and functional realism for the simulation of native and replacement tri-leaflet heart valves | 2021
2020 | 118, 119 |
The short-term functionality of TEHVs was demonstrated to be excellent in many pre-clinical studies, but leaflet retraction often leads to valvular insufficiency in medium-term follow-up. Many factors that contribute to leaflet retraction such as cellular traction,120 remodelling of the collagen network,121, 122 and contraction of the matrix components. Computational models are necessary to increase our understanding of the underlying mechanisms, and predict the risk for the development of valvular insufficiency.123 ...
Computational methods for the aortic heart valve and its replacements
1
2017
... Considering the rapid advancement of studies relevant to computational simulations for the design of BHV in recent years, appropriate models also can be selected to examine the role of different aspects of tissue-engineered valvular function, for example, using structural mechanics, computational fluid dynamics, and coupled fluid-structure interaction models can analyse the geometry of the valvular apparatus, accurate time-evolving biomaterial models, and accurate boundary conditions over the cardiac cycle, thus providing information regarding optimal geometries of the TEHVs that would be valuable for clinical diagnosis and treatment.105 ...
Characterization of three-dimensional anisotropic heart valve tissue mechanical properties using inverse finite element analysis
1
2016
... Advantages and disadvantages of animal models and bioreactors used in tissue-engineered heart valve studies.
| Model | Application | Objective | Year | Reference |
| Computational modelling | Tissue-engineered heart valve | Guiding tissue-engineered heart valve design for long-term in vivo performance in a translational sheep model | 2018 | 22 |
| Tissue-engineered vascular graft | Identifying optimal design parameters to save development time and costs while improving clinical outcomes | 2019 | 103 |
| Bioprosthetic heart valve | Investigating the impacts of bovine and porcine pericardium tissues with different thicknesses and tissue mechanical properties in bioprosthetic heart valve applications | 2019
2017
2016 | 104–106 |
| Tissue-engineered heart valve | Integrating computational simulation into tissue-engineering approaches can lead to more successful and predictable clinical outcomes | 2018 | 107–109 |
| Biomechanical model | Aortic stenosis | Providing the results of the numerical simulation of the valve function | 2020 | 110 |
| Finite element models | Congenital bicuspid aortic valve | Quantifying aortic valve and root biomechanical alterations associated with bicuspid geometry | 2010 | 111 |
| Calcific aortic valve disease | Studying the calcification progression in aortic valves | 2017 | 112 |
| Bioprosthetic heart valve | Comparing tensile properties of xenopericardium to choose tissue more appropriate for bioprosthetic heart valve tissue | 2020 | 113 |
| Three-dimensional bioprinting | Heart valve | Using computational fluid dynamics, digital image processing, artificial intelligence, and continuum mechanics during their optimisation and implementation to mimic the original and understand valvular problems | 2019
2018 | 114, 115 |
| Geometric model | Functional tri-leaflet aortic valves | Establishing a list of geometric guidelines to ensure safe operation of the valve during the cardiac cycle | 2006 | 116 |
| Numeric model | Aortic root | Studying the correlation between intraoperative effective height and diastolic coaptation | 2013 | 117 |
| Neural network material model | Simulation of the aortic heart valve | Providing an efficient computational analysis framework with increased physical and functional realism for the simulation of native and replacement tri-leaflet heart valves | 2021
2020 | 118, 119 |
The short-term functionality of TEHVs was demonstrated to be excellent in many pre-clinical studies, but leaflet retraction often leads to valvular insufficiency in medium-term follow-up. Many factors that contribute to leaflet retraction such as cellular traction,120 remodelling of the collagen network,121, 122 and contraction of the matrix components. Computational models are necessary to increase our understanding of the underlying mechanisms, and predict the risk for the development of valvular insufficiency.123 ...
Computer modelling to personalize bioengineered heart valves
2
2018
... Advantages and disadvantages of animal models and bioreactors used in tissue-engineered heart valve studies.
| Model | Application | Objective | Year | Reference |
| Computational modelling | Tissue-engineered heart valve | Guiding tissue-engineered heart valve design for long-term in vivo performance in a translational sheep model | 2018 | 22 |
| Tissue-engineered vascular graft | Identifying optimal design parameters to save development time and costs while improving clinical outcomes | 2019 | 103 |
| Bioprosthetic heart valve | Investigating the impacts of bovine and porcine pericardium tissues with different thicknesses and tissue mechanical properties in bioprosthetic heart valve applications | 2019
2017
2016 | 104–106 |
| Tissue-engineered heart valve | Integrating computational simulation into tissue-engineering approaches can lead to more successful and predictable clinical outcomes | 2018 | 107–109 |
| Biomechanical model | Aortic stenosis | Providing the results of the numerical simulation of the valve function | 2020 | 110 |
| Finite element models | Congenital bicuspid aortic valve | Quantifying aortic valve and root biomechanical alterations associated with bicuspid geometry | 2010 | 111 |
| Calcific aortic valve disease | Studying the calcification progression in aortic valves | 2017 | 112 |
| Bioprosthetic heart valve | Comparing tensile properties of xenopericardium to choose tissue more appropriate for bioprosthetic heart valve tissue | 2020 | 113 |
| Three-dimensional bioprinting | Heart valve | Using computational fluid dynamics, digital image processing, artificial intelligence, and continuum mechanics during their optimisation and implementation to mimic the original and understand valvular problems | 2019
2018 | 114, 115 |
| Geometric model | Functional tri-leaflet aortic valves | Establishing a list of geometric guidelines to ensure safe operation of the valve during the cardiac cycle | 2006 | 116 |
| Numeric model | Aortic root | Studying the correlation between intraoperative effective height and diastolic coaptation | 2013 | 117 |
| Neural network material model | Simulation of the aortic heart valve | Providing an efficient computational analysis framework with increased physical and functional realism for the simulation of native and replacement tri-leaflet heart valves | 2021
2020 | 118, 119 |
The short-term functionality of TEHVs was demonstrated to be excellent in many pre-clinical studies, but leaflet retraction often leads to valvular insufficiency in medium-term follow-up. Many factors that contribute to leaflet retraction such as cellular traction,120 remodelling of the collagen network,121, 122 and contraction of the matrix components. Computational models are necessary to increase our understanding of the underlying mechanisms, and predict the risk for the development of valvular insufficiency.123 ...
... Although TEHVs have a good application prospect, the mechanical and biological characteristics that can meet the standard for in vivo application have not been fully studied, so appropriate experimental models are needed for simulation in order to reach the standard of clinical application as soon as possible.107 ...
The root problem of heart valve engineering
0
2018
Taking bioengineered heart valves from faulty to functional
1
2018
... Advantages and disadvantages of animal models and bioreactors used in tissue-engineered heart valve studies.
| Model | Application | Objective | Year | Reference |
| Computational modelling | Tissue-engineered heart valve | Guiding tissue-engineered heart valve design for long-term in vivo performance in a translational sheep model | 2018 | 22 |
| Tissue-engineered vascular graft | Identifying optimal design parameters to save development time and costs while improving clinical outcomes | 2019 | 103 |
| Bioprosthetic heart valve | Investigating the impacts of bovine and porcine pericardium tissues with different thicknesses and tissue mechanical properties in bioprosthetic heart valve applications | 2019
2017
2016 | 104–106 |
| Tissue-engineered heart valve | Integrating computational simulation into tissue-engineering approaches can lead to more successful and predictable clinical outcomes | 2018 | 107–109 |
| Biomechanical model | Aortic stenosis | Providing the results of the numerical simulation of the valve function | 2020 | 110 |
| Finite element models | Congenital bicuspid aortic valve | Quantifying aortic valve and root biomechanical alterations associated with bicuspid geometry | 2010 | 111 |
| Calcific aortic valve disease | Studying the calcification progression in aortic valves | 2017 | 112 |
| Bioprosthetic heart valve | Comparing tensile properties of xenopericardium to choose tissue more appropriate for bioprosthetic heart valve tissue | 2020 | 113 |
| Three-dimensional bioprinting | Heart valve | Using computational fluid dynamics, digital image processing, artificial intelligence, and continuum mechanics during their optimisation and implementation to mimic the original and understand valvular problems | 2019
2018 | 114, 115 |
| Geometric model | Functional tri-leaflet aortic valves | Establishing a list of geometric guidelines to ensure safe operation of the valve during the cardiac cycle | 2006 | 116 |
| Numeric model | Aortic root | Studying the correlation between intraoperative effective height and diastolic coaptation | 2013 | 117 |
| Neural network material model | Simulation of the aortic heart valve | Providing an efficient computational analysis framework with increased physical and functional realism for the simulation of native and replacement tri-leaflet heart valves | 2021
2020 | 118, 119 |
The short-term functionality of TEHVs was demonstrated to be excellent in many pre-clinical studies, but leaflet retraction often leads to valvular insufficiency in medium-term follow-up. Many factors that contribute to leaflet retraction such as cellular traction,120 remodelling of the collagen network,121, 122 and contraction of the matrix components. Computational models are necessary to increase our understanding of the underlying mechanisms, and predict the risk for the development of valvular insufficiency.123 ...
A biomechanical model of the pathological aortic valve: simulation of aortic stenosis
1
2020
... Advantages and disadvantages of animal models and bioreactors used in tissue-engineered heart valve studies.
| Model | Application | Objective | Year | Reference |
| Computational modelling | Tissue-engineered heart valve | Guiding tissue-engineered heart valve design for long-term in vivo performance in a translational sheep model | 2018 | 22 |
| Tissue-engineered vascular graft | Identifying optimal design parameters to save development time and costs while improving clinical outcomes | 2019 | 103 |
| Bioprosthetic heart valve | Investigating the impacts of bovine and porcine pericardium tissues with different thicknesses and tissue mechanical properties in bioprosthetic heart valve applications | 2019
2017
2016 | 104–106 |
| Tissue-engineered heart valve | Integrating computational simulation into tissue-engineering approaches can lead to more successful and predictable clinical outcomes | 2018 | 107–109 |
| Biomechanical model | Aortic stenosis | Providing the results of the numerical simulation of the valve function | 2020 | 110 |
| Finite element models | Congenital bicuspid aortic valve | Quantifying aortic valve and root biomechanical alterations associated with bicuspid geometry | 2010 | 111 |
| Calcific aortic valve disease | Studying the calcification progression in aortic valves | 2017 | 112 |
| Bioprosthetic heart valve | Comparing tensile properties of xenopericardium to choose tissue more appropriate for bioprosthetic heart valve tissue | 2020 | 113 |
| Three-dimensional bioprinting | Heart valve | Using computational fluid dynamics, digital image processing, artificial intelligence, and continuum mechanics during their optimisation and implementation to mimic the original and understand valvular problems | 2019
2018 | 114, 115 |
| Geometric model | Functional tri-leaflet aortic valves | Establishing a list of geometric guidelines to ensure safe operation of the valve during the cardiac cycle | 2006 | 116 |
| Numeric model | Aortic root | Studying the correlation between intraoperative effective height and diastolic coaptation | 2013 | 117 |
| Neural network material model | Simulation of the aortic heart valve | Providing an efficient computational analysis framework with increased physical and functional realism for the simulation of native and replacement tri-leaflet heart valves | 2021
2020 | 118, 119 |
The short-term functionality of TEHVs was demonstrated to be excellent in many pre-clinical studies, but leaflet retraction often leads to valvular insufficiency in medium-term follow-up. Many factors that contribute to leaflet retraction such as cellular traction,120 remodelling of the collagen network,121, 122 and contraction of the matrix components. Computational models are necessary to increase our understanding of the underlying mechanisms, and predict the risk for the development of valvular insufficiency.123 ...
Biomechanical implications of the congenital bicuspid aortic valve: a finite element study of aortic root function from in vivo data
1
2010
... Advantages and disadvantages of animal models and bioreactors used in tissue-engineered heart valve studies.
| Model | Application | Objective | Year | Reference |
| Computational modelling | Tissue-engineered heart valve | Guiding tissue-engineered heart valve design for long-term in vivo performance in a translational sheep model | 2018 | 22 |
| Tissue-engineered vascular graft | Identifying optimal design parameters to save development time and costs while improving clinical outcomes | 2019 | 103 |
| Bioprosthetic heart valve | Investigating the impacts of bovine and porcine pericardium tissues with different thicknesses and tissue mechanical properties in bioprosthetic heart valve applications | 2019
2017
2016 | 104–106 |
| Tissue-engineered heart valve | Integrating computational simulation into tissue-engineering approaches can lead to more successful and predictable clinical outcomes | 2018 | 107–109 |
| Biomechanical model | Aortic stenosis | Providing the results of the numerical simulation of the valve function | 2020 | 110 |
| Finite element models | Congenital bicuspid aortic valve | Quantifying aortic valve and root biomechanical alterations associated with bicuspid geometry | 2010 | 111 |
| Calcific aortic valve disease | Studying the calcification progression in aortic valves | 2017 | 112 |
| Bioprosthetic heart valve | Comparing tensile properties of xenopericardium to choose tissue more appropriate for bioprosthetic heart valve tissue | 2020 | 113 |
| Three-dimensional bioprinting | Heart valve | Using computational fluid dynamics, digital image processing, artificial intelligence, and continuum mechanics during their optimisation and implementation to mimic the original and understand valvular problems | 2019
2018 | 114, 115 |
| Geometric model | Functional tri-leaflet aortic valves | Establishing a list of geometric guidelines to ensure safe operation of the valve during the cardiac cycle | 2006 | 116 |
| Numeric model | Aortic root | Studying the correlation between intraoperative effective height and diastolic coaptation | 2013 | 117 |
| Neural network material model | Simulation of the aortic heart valve | Providing an efficient computational analysis framework with increased physical and functional realism for the simulation of native and replacement tri-leaflet heart valves | 2021
2020 | 118, 119 |
The short-term functionality of TEHVs was demonstrated to be excellent in many pre-clinical studies, but leaflet retraction often leads to valvular insufficiency in medium-term follow-up. Many factors that contribute to leaflet retraction such as cellular traction,120 remodelling of the collagen network,121, 122 and contraction of the matrix components. Computational models are necessary to increase our understanding of the underlying mechanisms, and predict the risk for the development of valvular insufficiency.123 ...
A strain-based finite element model for calcification progression in aortic valves
1
2017
... Advantages and disadvantages of animal models and bioreactors used in tissue-engineered heart valve studies.
| Model | Application | Objective | Year | Reference |
| Computational modelling | Tissue-engineered heart valve | Guiding tissue-engineered heart valve design for long-term in vivo performance in a translational sheep model | 2018 | 22 |
| Tissue-engineered vascular graft | Identifying optimal design parameters to save development time and costs while improving clinical outcomes | 2019 | 103 |
| Bioprosthetic heart valve | Investigating the impacts of bovine and porcine pericardium tissues with different thicknesses and tissue mechanical properties in bioprosthetic heart valve applications | 2019
2017
2016 | 104–106 |
| Tissue-engineered heart valve | Integrating computational simulation into tissue-engineering approaches can lead to more successful and predictable clinical outcomes | 2018 | 107–109 |
| Biomechanical model | Aortic stenosis | Providing the results of the numerical simulation of the valve function | 2020 | 110 |
| Finite element models | Congenital bicuspid aortic valve | Quantifying aortic valve and root biomechanical alterations associated with bicuspid geometry | 2010 | 111 |
| Calcific aortic valve disease | Studying the calcification progression in aortic valves | 2017 | 112 |
| Bioprosthetic heart valve | Comparing tensile properties of xenopericardium to choose tissue more appropriate for bioprosthetic heart valve tissue | 2020 | 113 |
| Three-dimensional bioprinting | Heart valve | Using computational fluid dynamics, digital image processing, artificial intelligence, and continuum mechanics during their optimisation and implementation to mimic the original and understand valvular problems | 2019
2018 | 114, 115 |
| Geometric model | Functional tri-leaflet aortic valves | Establishing a list of geometric guidelines to ensure safe operation of the valve during the cardiac cycle | 2006 | 116 |
| Numeric model | Aortic root | Studying the correlation between intraoperative effective height and diastolic coaptation | 2013 | 117 |
| Neural network material model | Simulation of the aortic heart valve | Providing an efficient computational analysis framework with increased physical and functional realism for the simulation of native and replacement tri-leaflet heart valves | 2021
2020 | 118, 119 |
The short-term functionality of TEHVs was demonstrated to be excellent in many pre-clinical studies, but leaflet retraction often leads to valvular insufficiency in medium-term follow-up. Many factors that contribute to leaflet retraction such as cellular traction,120 remodelling of the collagen network,121, 122 and contraction of the matrix components. Computational models are necessary to increase our understanding of the underlying mechanisms, and predict the risk for the development of valvular insufficiency.123 ...
Comparison of tensile properties of xenopericardium from three animal species and finite element analysis for bioprosthetic heart valve tissue
1
2020
... Advantages and disadvantages of animal models and bioreactors used in tissue-engineered heart valve studies.
| Model | Application | Objective | Year | Reference |
| Computational modelling | Tissue-engineered heart valve | Guiding tissue-engineered heart valve design for long-term in vivo performance in a translational sheep model | 2018 | 22 |
| Tissue-engineered vascular graft | Identifying optimal design parameters to save development time and costs while improving clinical outcomes | 2019 | 103 |
| Bioprosthetic heart valve | Investigating the impacts of bovine and porcine pericardium tissues with different thicknesses and tissue mechanical properties in bioprosthetic heart valve applications | 2019
2017
2016 | 104–106 |
| Tissue-engineered heart valve | Integrating computational simulation into tissue-engineering approaches can lead to more successful and predictable clinical outcomes | 2018 | 107–109 |
| Biomechanical model | Aortic stenosis | Providing the results of the numerical simulation of the valve function | 2020 | 110 |
| Finite element models | Congenital bicuspid aortic valve | Quantifying aortic valve and root biomechanical alterations associated with bicuspid geometry | 2010 | 111 |
| Calcific aortic valve disease | Studying the calcification progression in aortic valves | 2017 | 112 |
| Bioprosthetic heart valve | Comparing tensile properties of xenopericardium to choose tissue more appropriate for bioprosthetic heart valve tissue | 2020 | 113 |
| Three-dimensional bioprinting | Heart valve | Using computational fluid dynamics, digital image processing, artificial intelligence, and continuum mechanics during their optimisation and implementation to mimic the original and understand valvular problems | 2019
2018 | 114, 115 |
| Geometric model | Functional tri-leaflet aortic valves | Establishing a list of geometric guidelines to ensure safe operation of the valve during the cardiac cycle | 2006 | 116 |
| Numeric model | Aortic root | Studying the correlation between intraoperative effective height and diastolic coaptation | 2013 | 117 |
| Neural network material model | Simulation of the aortic heart valve | Providing an efficient computational analysis framework with increased physical and functional realism for the simulation of native and replacement tri-leaflet heart valves | 2021
2020 | 118, 119 |
The short-term functionality of TEHVs was demonstrated to be excellent in many pre-clinical studies, but leaflet retraction often leads to valvular insufficiency in medium-term follow-up. Many factors that contribute to leaflet retraction such as cellular traction,120 remodelling of the collagen network,121, 122 and contraction of the matrix components. Computational models are necessary to increase our understanding of the underlying mechanisms, and predict the risk for the development of valvular insufficiency.123 ...
Quest for cardiovascular interventions: precise modeling and 3D printing of heart valves
1
2019
... Advantages and disadvantages of animal models and bioreactors used in tissue-engineered heart valve studies.
| Model | Application | Objective | Year | Reference |
| Computational modelling | Tissue-engineered heart valve | Guiding tissue-engineered heart valve design for long-term in vivo performance in a translational sheep model | 2018 | 22 |
| Tissue-engineered vascular graft | Identifying optimal design parameters to save development time and costs while improving clinical outcomes | 2019 | 103 |
| Bioprosthetic heart valve | Investigating the impacts of bovine and porcine pericardium tissues with different thicknesses and tissue mechanical properties in bioprosthetic heart valve applications | 2019
2017
2016 | 104–106 |
| Tissue-engineered heart valve | Integrating computational simulation into tissue-engineering approaches can lead to more successful and predictable clinical outcomes | 2018 | 107–109 |
| Biomechanical model | Aortic stenosis | Providing the results of the numerical simulation of the valve function | 2020 | 110 |
| Finite element models | Congenital bicuspid aortic valve | Quantifying aortic valve and root biomechanical alterations associated with bicuspid geometry | 2010 | 111 |
| Calcific aortic valve disease | Studying the calcification progression in aortic valves | 2017 | 112 |
| Bioprosthetic heart valve | Comparing tensile properties of xenopericardium to choose tissue more appropriate for bioprosthetic heart valve tissue | 2020 | 113 |
| Three-dimensional bioprinting | Heart valve | Using computational fluid dynamics, digital image processing, artificial intelligence, and continuum mechanics during their optimisation and implementation to mimic the original and understand valvular problems | 2019
2018 | 114, 115 |
| Geometric model | Functional tri-leaflet aortic valves | Establishing a list of geometric guidelines to ensure safe operation of the valve during the cardiac cycle | 2006 | 116 |
| Numeric model | Aortic root | Studying the correlation between intraoperative effective height and diastolic coaptation | 2013 | 117 |
| Neural network material model | Simulation of the aortic heart valve | Providing an efficient computational analysis framework with increased physical and functional realism for the simulation of native and replacement tri-leaflet heart valves | 2021
2020 | 118, 119 |
The short-term functionality of TEHVs was demonstrated to be excellent in many pre-clinical studies, but leaflet retraction often leads to valvular insufficiency in medium-term follow-up. Many factors that contribute to leaflet retraction such as cellular traction,120 remodelling of the collagen network,121, 122 and contraction of the matrix components. Computational models are necessary to increase our understanding of the underlying mechanisms, and predict the risk for the development of valvular insufficiency.123 ...
Engineering a 3D-bioprinted model of human heart valve disease using nanoindentation-based biomechanics
1
2018
... Advantages and disadvantages of animal models and bioreactors used in tissue-engineered heart valve studies.
| Model | Application | Objective | Year | Reference |
| Computational modelling | Tissue-engineered heart valve | Guiding tissue-engineered heart valve design for long-term in vivo performance in a translational sheep model | 2018 | 22 |
| Tissue-engineered vascular graft | Identifying optimal design parameters to save development time and costs while improving clinical outcomes | 2019 | 103 |
| Bioprosthetic heart valve | Investigating the impacts of bovine and porcine pericardium tissues with different thicknesses and tissue mechanical properties in bioprosthetic heart valve applications | 2019
2017
2016 | 104–106 |
| Tissue-engineered heart valve | Integrating computational simulation into tissue-engineering approaches can lead to more successful and predictable clinical outcomes | 2018 | 107–109 |
| Biomechanical model | Aortic stenosis | Providing the results of the numerical simulation of the valve function | 2020 | 110 |
| Finite element models | Congenital bicuspid aortic valve | Quantifying aortic valve and root biomechanical alterations associated with bicuspid geometry | 2010 | 111 |
| Calcific aortic valve disease | Studying the calcification progression in aortic valves | 2017 | 112 |
| Bioprosthetic heart valve | Comparing tensile properties of xenopericardium to choose tissue more appropriate for bioprosthetic heart valve tissue | 2020 | 113 |
| Three-dimensional bioprinting | Heart valve | Using computational fluid dynamics, digital image processing, artificial intelligence, and continuum mechanics during their optimisation and implementation to mimic the original and understand valvular problems | 2019
2018 | 114, 115 |
| Geometric model | Functional tri-leaflet aortic valves | Establishing a list of geometric guidelines to ensure safe operation of the valve during the cardiac cycle | 2006 | 116 |
| Numeric model | Aortic root | Studying the correlation between intraoperative effective height and diastolic coaptation | 2013 | 117 |
| Neural network material model | Simulation of the aortic heart valve | Providing an efficient computational analysis framework with increased physical and functional realism for the simulation of native and replacement tri-leaflet heart valves | 2021
2020 | 118, 119 |
The short-term functionality of TEHVs was demonstrated to be excellent in many pre-clinical studies, but leaflet retraction often leads to valvular insufficiency in medium-term follow-up. Many factors that contribute to leaflet retraction such as cellular traction,120 remodelling of the collagen network,121, 122 and contraction of the matrix components. Computational models are necessary to increase our understanding of the underlying mechanisms, and predict the risk for the development of valvular insufficiency.123 ...
Geometric modeling of functional trileaflet aortic valves: development and clinical applications
1
2006
... Advantages and disadvantages of animal models and bioreactors used in tissue-engineered heart valve studies.
| Model | Application | Objective | Year | Reference |
| Computational modelling | Tissue-engineered heart valve | Guiding tissue-engineered heart valve design for long-term in vivo performance in a translational sheep model | 2018 | 22 |
| Tissue-engineered vascular graft | Identifying optimal design parameters to save development time and costs while improving clinical outcomes | 2019 | 103 |
| Bioprosthetic heart valve | Investigating the impacts of bovine and porcine pericardium tissues with different thicknesses and tissue mechanical properties in bioprosthetic heart valve applications | 2019
2017
2016 | 104–106 |
| Tissue-engineered heart valve | Integrating computational simulation into tissue-engineering approaches can lead to more successful and predictable clinical outcomes | 2018 | 107–109 |
| Biomechanical model | Aortic stenosis | Providing the results of the numerical simulation of the valve function | 2020 | 110 |
| Finite element models | Congenital bicuspid aortic valve | Quantifying aortic valve and root biomechanical alterations associated with bicuspid geometry | 2010 | 111 |
| Calcific aortic valve disease | Studying the calcification progression in aortic valves | 2017 | 112 |
| Bioprosthetic heart valve | Comparing tensile properties of xenopericardium to choose tissue more appropriate for bioprosthetic heart valve tissue | 2020 | 113 |
| Three-dimensional bioprinting | Heart valve | Using computational fluid dynamics, digital image processing, artificial intelligence, and continuum mechanics during their optimisation and implementation to mimic the original and understand valvular problems | 2019
2018 | 114, 115 |
| Geometric model | Functional tri-leaflet aortic valves | Establishing a list of geometric guidelines to ensure safe operation of the valve during the cardiac cycle | 2006 | 116 |
| Numeric model | Aortic root | Studying the correlation between intraoperative effective height and diastolic coaptation | 2013 | 117 |
| Neural network material model | Simulation of the aortic heart valve | Providing an efficient computational analysis framework with increased physical and functional realism for the simulation of native and replacement tri-leaflet heart valves | 2021
2020 | 118, 119 |
The short-term functionality of TEHVs was demonstrated to be excellent in many pre-clinical studies, but leaflet retraction often leads to valvular insufficiency in medium-term follow-up. Many factors that contribute to leaflet retraction such as cellular traction,120 remodelling of the collagen network,121, 122 and contraction of the matrix components. Computational models are necessary to increase our understanding of the underlying mechanisms, and predict the risk for the development of valvular insufficiency.123 ...
Aortic root numeric model: correlation between intraoperative effective height and diastolic coaptation
1
2013
... Advantages and disadvantages of animal models and bioreactors used in tissue-engineered heart valve studies.
| Model | Application | Objective | Year | Reference |
| Computational modelling | Tissue-engineered heart valve | Guiding tissue-engineered heart valve design for long-term in vivo performance in a translational sheep model | 2018 | 22 |
| Tissue-engineered vascular graft | Identifying optimal design parameters to save development time and costs while improving clinical outcomes | 2019 | 103 |
| Bioprosthetic heart valve | Investigating the impacts of bovine and porcine pericardium tissues with different thicknesses and tissue mechanical properties in bioprosthetic heart valve applications | 2019
2017
2016 | 104–106 |
| Tissue-engineered heart valve | Integrating computational simulation into tissue-engineering approaches can lead to more successful and predictable clinical outcomes | 2018 | 107–109 |
| Biomechanical model | Aortic stenosis | Providing the results of the numerical simulation of the valve function | 2020 | 110 |
| Finite element models | Congenital bicuspid aortic valve | Quantifying aortic valve and root biomechanical alterations associated with bicuspid geometry | 2010 | 111 |
| Calcific aortic valve disease | Studying the calcification progression in aortic valves | 2017 | 112 |
| Bioprosthetic heart valve | Comparing tensile properties of xenopericardium to choose tissue more appropriate for bioprosthetic heart valve tissue | 2020 | 113 |
| Three-dimensional bioprinting | Heart valve | Using computational fluid dynamics, digital image processing, artificial intelligence, and continuum mechanics during their optimisation and implementation to mimic the original and understand valvular problems | 2019
2018 | 114, 115 |
| Geometric model | Functional tri-leaflet aortic valves | Establishing a list of geometric guidelines to ensure safe operation of the valve during the cardiac cycle | 2006 | 116 |
| Numeric model | Aortic root | Studying the correlation between intraoperative effective height and diastolic coaptation | 2013 | 117 |
| Neural network material model | Simulation of the aortic heart valve | Providing an efficient computational analysis framework with increased physical and functional realism for the simulation of native and replacement tri-leaflet heart valves | 2021
2020 | 118, 119 |
The short-term functionality of TEHVs was demonstrated to be excellent in many pre-clinical studies, but leaflet retraction often leads to valvular insufficiency in medium-term follow-up. Many factors that contribute to leaflet retraction such as cellular traction,120 remodelling of the collagen network,121, 122 and contraction of the matrix components. Computational models are necessary to increase our understanding of the underlying mechanisms, and predict the risk for the development of valvular insufficiency.123 ...
Isogeometric finite element-based simulation of the aortic heart valve: Integration of neural network structural material model and structural tensor fiber architecture representations
1
2021
... Advantages and disadvantages of animal models and bioreactors used in tissue-engineered heart valve studies.
| Model | Application | Objective | Year | Reference |
| Computational modelling | Tissue-engineered heart valve | Guiding tissue-engineered heart valve design for long-term in vivo performance in a translational sheep model | 2018 | 22 |
| Tissue-engineered vascular graft | Identifying optimal design parameters to save development time and costs while improving clinical outcomes | 2019 | 103 |
| Bioprosthetic heart valve | Investigating the impacts of bovine and porcine pericardium tissues with different thicknesses and tissue mechanical properties in bioprosthetic heart valve applications | 2019
2017
2016 | 104–106 |
| Tissue-engineered heart valve | Integrating computational simulation into tissue-engineering approaches can lead to more successful and predictable clinical outcomes | 2018 | 107–109 |
| Biomechanical model | Aortic stenosis | Providing the results of the numerical simulation of the valve function | 2020 | 110 |
| Finite element models | Congenital bicuspid aortic valve | Quantifying aortic valve and root biomechanical alterations associated with bicuspid geometry | 2010 | 111 |
| Calcific aortic valve disease | Studying the calcification progression in aortic valves | 2017 | 112 |
| Bioprosthetic heart valve | Comparing tensile properties of xenopericardium to choose tissue more appropriate for bioprosthetic heart valve tissue | 2020 | 113 |
| Three-dimensional bioprinting | Heart valve | Using computational fluid dynamics, digital image processing, artificial intelligence, and continuum mechanics during their optimisation and implementation to mimic the original and understand valvular problems | 2019
2018 | 114, 115 |
| Geometric model | Functional tri-leaflet aortic valves | Establishing a list of geometric guidelines to ensure safe operation of the valve during the cardiac cycle | 2006 | 116 |
| Numeric model | Aortic root | Studying the correlation between intraoperative effective height and diastolic coaptation | 2013 | 117 |
| Neural network material model | Simulation of the aortic heart valve | Providing an efficient computational analysis framework with increased physical and functional realism for the simulation of native and replacement tri-leaflet heart valves | 2021
2020 | 118, 119 |
The short-term functionality of TEHVs was demonstrated to be excellent in many pre-clinical studies, but leaflet retraction often leads to valvular insufficiency in medium-term follow-up. Many factors that contribute to leaflet retraction such as cellular traction,120 remodelling of the collagen network,121, 122 and contraction of the matrix components. Computational models are necessary to increase our understanding of the underlying mechanisms, and predict the risk for the development of valvular insufficiency.123 ...
The prediction model of warfarin individual maintenance dose for patients undergoing heart valve replacement, based on the back propagation neural network
2
2020
... Computational modelling, particularly when integrated with experimental studies, can substantially accelerate our comprehension of inter-species differences in TEHV adaptation,22 differential remodelling and functionality,100 and the interaction between immune systems-mediated and mechanical stress-driven regenerative processes. Computational modelling of valve mechanics101 and corresponding tissue remodelling provides a powerful approach to predict the consequences of changes in valve design to help inform the rational selection of scaffold parameters to fabricate TEHVs102 (Figure 3). Computational modelling helps designing TEHVs and consistently predicts valve in vivo tissue remodelling and long-term functionality in a large animal model. These findings indicate that integrating computational simulation into tissue engineering approaches can lead to predictable clinical outcomes and the promotion of clinical effectiveness22, 103-119 (Table 3). ...
... Advantages and disadvantages of animal models and bioreactors used in tissue-engineered heart valve studies.
| Model | Application | Objective | Year | Reference |
| Computational modelling | Tissue-engineered heart valve | Guiding tissue-engineered heart valve design for long-term in vivo performance in a translational sheep model | 2018 | 22 |
| Tissue-engineered vascular graft | Identifying optimal design parameters to save development time and costs while improving clinical outcomes | 2019 | 103 |
| Bioprosthetic heart valve | Investigating the impacts of bovine and porcine pericardium tissues with different thicknesses and tissue mechanical properties in bioprosthetic heart valve applications | 2019
2017
2016 | 104–106 |
| Tissue-engineered heart valve | Integrating computational simulation into tissue-engineering approaches can lead to more successful and predictable clinical outcomes | 2018 | 107–109 |
| Biomechanical model | Aortic stenosis | Providing the results of the numerical simulation of the valve function | 2020 | 110 |
| Finite element models | Congenital bicuspid aortic valve | Quantifying aortic valve and root biomechanical alterations associated with bicuspid geometry | 2010 | 111 |
| Calcific aortic valve disease | Studying the calcification progression in aortic valves | 2017 | 112 |
| Bioprosthetic heart valve | Comparing tensile properties of xenopericardium to choose tissue more appropriate for bioprosthetic heart valve tissue | 2020 | 113 |
| Three-dimensional bioprinting | Heart valve | Using computational fluid dynamics, digital image processing, artificial intelligence, and continuum mechanics during their optimisation and implementation to mimic the original and understand valvular problems | 2019
2018 | 114, 115 |
| Geometric model | Functional tri-leaflet aortic valves | Establishing a list of geometric guidelines to ensure safe operation of the valve during the cardiac cycle | 2006 | 116 |
| Numeric model | Aortic root | Studying the correlation between intraoperative effective height and diastolic coaptation | 2013 | 117 |
| Neural network material model | Simulation of the aortic heart valve | Providing an efficient computational analysis framework with increased physical and functional realism for the simulation of native and replacement tri-leaflet heart valves | 2021
2020 | 118, 119 |
The short-term functionality of TEHVs was demonstrated to be excellent in many pre-clinical studies, but leaflet retraction often leads to valvular insufficiency in medium-term follow-up. Many factors that contribute to leaflet retraction such as cellular traction,120 remodelling of the collagen network,121, 122 and contraction of the matrix components. Computational models are necessary to increase our understanding of the underlying mechanisms, and predict the risk for the development of valvular insufficiency.123 ...
Computational model predicts cell orientation in response to a range of mechanical stimuli
1
2014
... The short-term functionality of TEHVs was demonstrated to be excellent in many pre-clinical studies, but leaflet retraction often leads to valvular insufficiency in medium-term follow-up. Many factors that contribute to leaflet retraction such as cellular traction,120 remodelling of the collagen network,121, 122 and contraction of the matrix components. Computational models are necessary to increase our understanding of the underlying mechanisms, and predict the risk for the development of valvular insufficiency.123 ...
A physically motivated constitutive model for cell-mediated compaction and collagen remodeling in soft tissues
1
2014
... The short-term functionality of TEHVs was demonstrated to be excellent in many pre-clinical studies, but leaflet retraction often leads to valvular insufficiency in medium-term follow-up. Many factors that contribute to leaflet retraction such as cellular traction,120 remodelling of the collagen network,121, 122 and contraction of the matrix components. Computational models are necessary to increase our understanding of the underlying mechanisms, and predict the risk for the development of valvular insufficiency.123 ...
Predicting and understanding collagen remodeling in human native heart valves during early development
1
2018
... The short-term functionality of TEHVs was demonstrated to be excellent in many pre-clinical studies, but leaflet retraction often leads to valvular insufficiency in medium-term follow-up. Many factors that contribute to leaflet retraction such as cellular traction,120 remodelling of the collagen network,121, 122 and contraction of the matrix components. Computational models are necessary to increase our understanding of the underlying mechanisms, and predict the risk for the development of valvular insufficiency.123 ...
A computational analysis of cell-mediated compaction and collagen remodeling in tissue-engineered heart valves
3
2016
... The short-term functionality of TEHVs was demonstrated to be excellent in many pre-clinical studies, but leaflet retraction often leads to valvular insufficiency in medium-term follow-up. Many factors that contribute to leaflet retraction such as cellular traction,120 remodelling of the collagen network,121, 122 and contraction of the matrix components. Computational models are necessary to increase our understanding of the underlying mechanisms, and predict the risk for the development of valvular insufficiency.123 ...
... Emmert et al.22 adopted computational modelling of valve mechanics and corresponding tissue remodelling to provide a powerful approach to predict the consequences of changes in valve design on the overall outcome.124 For example, the mechanical behaviour of TEHVs can influence α-smooth muscle actin expression that leads to leaflet retraction and cardiovascular tissue remodelling.125 The use of computational modelling predicted that a more physiological valve geometry126 in combination with a relatively large coaptation area would considerably increase the radial stretch of TEHVs and hence potentially reduce the development of retraction.127 Loerakker et al.123 also used a computational approach to predict the remodelling process in TEHVs subjected under dynamic pulmonary and aortic pressure conditions, and improve assessment of the risk of valvular insufficiency. In addition, they also investigated the importance of intrinsic cell contractility on the valvular matrix remodelling process in the end. Additionally, the model predicted that valvular insufficiency is unlikely because the blood pressure is high enough to prevent the development of leaflet retraction.123 Another study with a computational modelling design indicated that TEHV geometry can significantly influence the host cell response by determining the infiltration and presence of macrophages and α-smooth muscle actin-positive cells, which play a crucial role in orchestrating TEHV remodelling.128 ...
... 123 Another study with a computational modelling design indicated that TEHV geometry can significantly influence the host cell response by determining the infiltration and presence of macrophages and α-smooth muscle actin-positive cells, which play a crucial role in orchestrating TEHV remodelling.128 ...
Modeling the role of oscillator flow and dynamic mechanical conditioning on dense connective tissue formation in mesenchymal stem cell-derived heart valve tissue engineering
1
2013
... Emmert et al.22 adopted computational modelling of valve mechanics and corresponding tissue remodelling to provide a powerful approach to predict the consequences of changes in valve design on the overall outcome.124 For example, the mechanical behaviour of TEHVs can influence α-smooth muscle actin expression that leads to leaflet retraction and cardiovascular tissue remodelling.125 The use of computational modelling predicted that a more physiological valve geometry126 in combination with a relatively large coaptation area would considerably increase the radial stretch of TEHVs and hence potentially reduce the development of retraction.127 Loerakker et al.123 also used a computational approach to predict the remodelling process in TEHVs subjected under dynamic pulmonary and aortic pressure conditions, and improve assessment of the risk of valvular insufficiency. In addition, they also investigated the importance of intrinsic cell contractility on the valvular matrix remodelling process in the end. Additionally, the model predicted that valvular insufficiency is unlikely because the blood pressure is high enough to prevent the development of leaflet retraction.123 Another study with a computational modelling design indicated that TEHV geometry can significantly influence the host cell response by determining the infiltration and presence of macrophages and α-smooth muscle actin-positive cells, which play a crucial role in orchestrating TEHV remodelling.128 ...
A triphasic constrained mixture model of engineered tissue formation under in vitro dynamic mechanical conditioning
1
2016
... Emmert et al.22 adopted computational modelling of valve mechanics and corresponding tissue remodelling to provide a powerful approach to predict the consequences of changes in valve design on the overall outcome.124 For example, the mechanical behaviour of TEHVs can influence α-smooth muscle actin expression that leads to leaflet retraction and cardiovascular tissue remodelling.125 The use of computational modelling predicted that a more physiological valve geometry126 in combination with a relatively large coaptation area would considerably increase the radial stretch of TEHVs and hence potentially reduce the development of retraction.127 Loerakker et al.123 also used a computational approach to predict the remodelling process in TEHVs subjected under dynamic pulmonary and aortic pressure conditions, and improve assessment of the risk of valvular insufficiency. In addition, they also investigated the importance of intrinsic cell contractility on the valvular matrix remodelling process in the end. Additionally, the model predicted that valvular insufficiency is unlikely because the blood pressure is high enough to prevent the development of leaflet retraction.123 Another study with a computational modelling design indicated that TEHV geometry can significantly influence the host cell response by determining the infiltration and presence of macrophages and α-smooth muscle actin-positive cells, which play a crucial role in orchestrating TEHV remodelling.128 ...
Improved geometry of decellularized tissue engineered heart valves to prevent leaflet retraction
1
2016
... Emmert et al.22 adopted computational modelling of valve mechanics and corresponding tissue remodelling to provide a powerful approach to predict the consequences of changes in valve design on the overall outcome.124 For example, the mechanical behaviour of TEHVs can influence α-smooth muscle actin expression that leads to leaflet retraction and cardiovascular tissue remodelling.125 The use of computational modelling predicted that a more physiological valve geometry126 in combination with a relatively large coaptation area would considerably increase the radial stretch of TEHVs and hence potentially reduce the development of retraction.127 Loerakker et al.123 also used a computational approach to predict the remodelling process in TEHVs subjected under dynamic pulmonary and aortic pressure conditions, and improve assessment of the risk of valvular insufficiency. In addition, they also investigated the importance of intrinsic cell contractility on the valvular matrix remodelling process in the end. Additionally, the model predicted that valvular insufficiency is unlikely because the blood pressure is high enough to prevent the development of leaflet retraction.123 Another study with a computational modelling design indicated that TEHV geometry can significantly influence the host cell response by determining the infiltration and presence of macrophages and α-smooth muscle actin-positive cells, which play a crucial role in orchestrating TEHV remodelling.128 ...
Effects of valve geometry and tissue anisotropy on the radial stretch and coaptation area of tissue-engineered heart valves
1
2013
... Emmert et al.22 adopted computational modelling of valve mechanics and corresponding tissue remodelling to provide a powerful approach to predict the consequences of changes in valve design on the overall outcome.124 For example, the mechanical behaviour of TEHVs can influence α-smooth muscle actin expression that leads to leaflet retraction and cardiovascular tissue remodelling.125 The use of computational modelling predicted that a more physiological valve geometry126 in combination with a relatively large coaptation area would considerably increase the radial stretch of TEHVs and hence potentially reduce the development of retraction.127 Loerakker et al.123 also used a computational approach to predict the remodelling process in TEHVs subjected under dynamic pulmonary and aortic pressure conditions, and improve assessment of the risk of valvular insufficiency. In addition, they also investigated the importance of intrinsic cell contractility on the valvular matrix remodelling process in the end. Additionally, the model predicted that valvular insufficiency is unlikely because the blood pressure is high enough to prevent the development of leaflet retraction.123 Another study with a computational modelling design indicated that TEHV geometry can significantly influence the host cell response by determining the infiltration and presence of macrophages and α-smooth muscle actin-positive cells, which play a crucial role in orchestrating TEHV remodelling.128 ...
Geometry influences inflammatory host cell response and remodeling in tissue-engineered heart valves in-vivo
1
2020
... Emmert et al.22 adopted computational modelling of valve mechanics and corresponding tissue remodelling to provide a powerful approach to predict the consequences of changes in valve design on the overall outcome.124 For example, the mechanical behaviour of TEHVs can influence α-smooth muscle actin expression that leads to leaflet retraction and cardiovascular tissue remodelling.125 The use of computational modelling predicted that a more physiological valve geometry126 in combination with a relatively large coaptation area would considerably increase the radial stretch of TEHVs and hence potentially reduce the development of retraction.127 Loerakker et al.123 also used a computational approach to predict the remodelling process in TEHVs subjected under dynamic pulmonary and aortic pressure conditions, and improve assessment of the risk of valvular insufficiency. In addition, they also investigated the importance of intrinsic cell contractility on the valvular matrix remodelling process in the end. Additionally, the model predicted that valvular insufficiency is unlikely because the blood pressure is high enough to prevent the development of leaflet retraction.123 Another study with a computational modelling design indicated that TEHV geometry can significantly influence the host cell response by determining the infiltration and presence of macrophages and α-smooth muscle actin-positive cells, which play a crucial role in orchestrating TEHV remodelling.128 ...