Biomaterials Translational ›› 2023, Vol. 4 ›› Issue (2): 67-84.doi: 10.12336/biomatertransl.2023.02.003
• REVIEW • Previous Articles Next Articles
Monchupa Kingsak1, Thongpon Meethong1, Jinnawat Jongkhumkrong1, Li Cai2, Qian Wang1,*( )
)
Received:2023-04-25
Revised:2023-05-26
Accepted:2023-06-13
Online:2023-06-28
Published:2023-06-28
Contact:
Qian Wang,About author:Qian Wang,Wang263@mailbox.sc.edu.| Genome type | Virus | Enveloped | Cell entry receptors | Aberrant oncogenic signalling pathway | References |
|---|---|---|---|---|---|
| Name of the OV | |||||
| DNA | Adenovirus | N | CAR, integrins | PKR, Rb and p16 | |
| Herpesvirus | Y | HVEM | PKR, Rb and p16 | ||
| Parvovirus H1 | N | Sialic acid, galectin-1 | - | ||
| Vaccinia virus | Y | - | RAS, PKR, Rb and p16, IFN-1 | ||
| RNA | Coxsackievirus | N | ICAM-1 (CD54), DAF (CD55) | - | |
| Poliovirus | N | CD155 | - | ||
| Measles virus | Y | CD46 | - | ||
| Vesicular stomatitis virus | Y | LDLR | IFN-1 | ||
| Sindbis virus | Y | LAMR | - | ||
| Echovirus | N | Integrin a2b1 | - | ||
| Reovirus | N | - | RAS, PKR, Rb and p16 | ||
| Newcastle disease virus | Y | Sialic acid | Bcl-xL, IFN-1 |
Table 1. The cell entry receptor and the aberrant oncogenic signalling pathway that OVs utilise to preferentially enter and replicate in cancer cells
| Genome type | Virus | Enveloped | Cell entry receptors | Aberrant oncogenic signalling pathway | References |
|---|---|---|---|---|---|
| Name of the OV | |||||
| DNA | Adenovirus | N | CAR, integrins | PKR, Rb and p16 | |
| Herpesvirus | Y | HVEM | PKR, Rb and p16 | ||
| Parvovirus H1 | N | Sialic acid, galectin-1 | - | ||
| Vaccinia virus | Y | - | RAS, PKR, Rb and p16, IFN-1 | ||
| RNA | Coxsackievirus | N | ICAM-1 (CD54), DAF (CD55) | - | |
| Poliovirus | N | CD155 | - | ||
| Measles virus | Y | CD46 | - | ||
| Vesicular stomatitis virus | Y | LDLR | IFN-1 | ||
| Sindbis virus | Y | LAMR | - | ||
| Echovirus | N | Integrin a2b1 | - | ||
| Reovirus | N | - | RAS, PKR, Rb and p16 | ||
| Newcastle disease virus | Y | Sialic acid | Bcl-xL, IFN-1 |
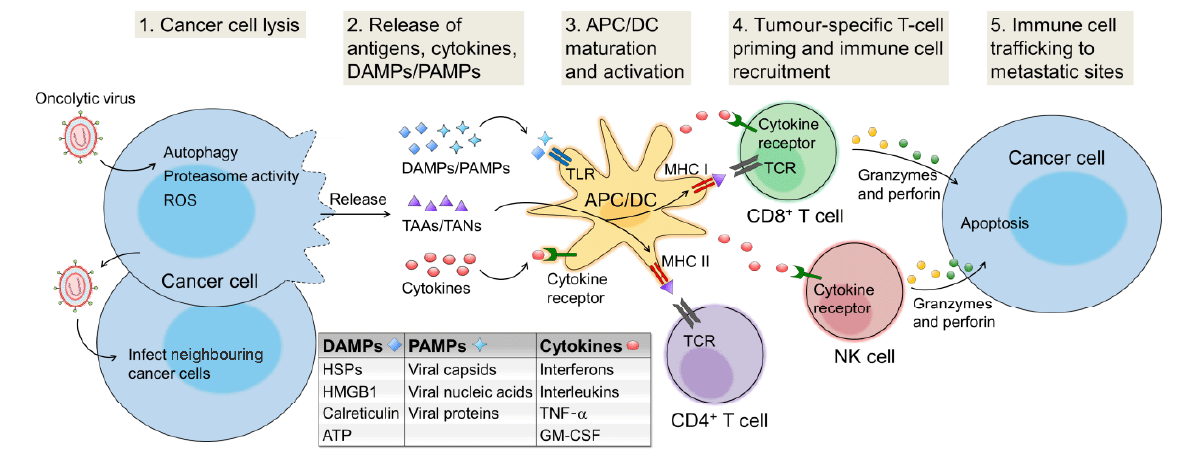
Figure 1. The generalised overview of OV-induced anti-tumour immunity. Initially, OVs infect primary cancer cells and cause direct oncolysis via inducing autophagy, increasing proteasome activity, and upregulating ROS caused by ER stress and genotoxic stress upon the infection. Subsequently, DAMPs and PAMPs trigger TLR, a major sub-family of the PRRs, and activate APCs. APC/DC uptake TAAs/TANs and express them to immune cells such as CD8+ T cells and CD4+ T cells through MHC I - TCR and MHC II - TCR interactions, respectively. The releasing cytokines and chemokines recruit both innate immune cells such as neutrophils, macrophages, NK cells, and DC, and adaptive immune cells such as T cells and B cells to the infected sites. In addition, APC helps stimulating and manipulating CD8+ T cells and NK cells to release granzymes and perforin causing apoptosis of cancer cells. Furthermore, cytotoxic T lymphocytes can migrate to a distant tumour, recognize tumour antigens, and kill cancer cells.9, 14, 46, 48, 59, 60 APC: antigen presenting cells; ATP: Adenosine triphosphate; DAMPs: damage-associated molecular patterns; DC: dendritic cells; ER: endoplasmic reticulum; GM-CSF: granulocyte macrophage colony-stimulating factor; HSP: heat shock protein; HMGB1: high mobility group protein; MHC: major histocompatibility complex; NK cell: natural killer cell; OV: oncolytic virus; PAMPs: pathogen-associated molecular patterns; PRR: pattern recognition receptor; ROS: reactive oxygen species; TAAs: tumour-associated antigens; TANs: tumour-associated neoantigens; TCR: T cell receptor; TLR: Toll-like receptor; TNF-α: tumour necrosis factor-α. Adapted from Kaufman et al.14
| Genetic modification | Purpose/aim | Example of OVs | References | |
|---|---|---|---|---|
| Gene deletion | Gene insertion | |||
| ICP34.5 | Selectively replicate in cancer cells, which have impaired PKR activity | Herpesvirus | ||
| ICP6 | LacZ | Selectively replicate in cancer cells, which have sufficient level of host RR and p16INK4A tumour suppressor inactivation, avoid RR encoding | Herpesvirus | |
| E1A | Restrict viral proliferation in healthy tissue | Adenovirus | ||
| g34.5 | Restrict viral proliferation in healthy tissue and reduce neurovirulence | Herpesvirus | ||
| a47 | Increase anti-tumour immunity | Herpesvirus | ||
| TK | Selectively replicate in cancer cells | Vaccinia virus | ||
| GM-CSF | Increase anti-tumour immunity | Herpesvirus, vaccinia virus, and adenovirus | ||
| Endostatin | Destroy tumour vasculature and enhance therapeutic efficiency | Herpesvirus | ||
| TSP1 | Destroy tumour vasculature and enhance therapeutic efficiency | Herpesvirus | ||
| GLAF-2 | Increases anti-angiogenic and anti-tumour properties | Vaccinia virus | ||
Table 2. Summary of important genetic engineering in OVs
| Genetic modification | Purpose/aim | Example of OVs | References | |
|---|---|---|---|---|
| Gene deletion | Gene insertion | |||
| ICP34.5 | Selectively replicate in cancer cells, which have impaired PKR activity | Herpesvirus | ||
| ICP6 | LacZ | Selectively replicate in cancer cells, which have sufficient level of host RR and p16INK4A tumour suppressor inactivation, avoid RR encoding | Herpesvirus | |
| E1A | Restrict viral proliferation in healthy tissue | Adenovirus | ||
| g34.5 | Restrict viral proliferation in healthy tissue and reduce neurovirulence | Herpesvirus | ||
| a47 | Increase anti-tumour immunity | Herpesvirus | ||
| TK | Selectively replicate in cancer cells | Vaccinia virus | ||
| GM-CSF | Increase anti-tumour immunity | Herpesvirus, vaccinia virus, and adenovirus | ||
| Endostatin | Destroy tumour vasculature and enhance therapeutic efficiency | Herpesvirus | ||
| TSP1 | Destroy tumour vasculature and enhance therapeutic efficiency | Herpesvirus | ||
| GLAF-2 | Increases anti-angiogenic and anti-tumour properties | Vaccinia virus | ||
| Virus type | Name (published year) | Cancer | Location | Patients number | Measurement | Route of administration | Study conclusion |
|---|---|---|---|---|---|---|---|
| Herpes virus | G207 | Glioblastoma | USA | 6 | Correlated gene analysis | Intratumoural stereotactic injection | The results have shown approximately 500 tumour-associated genes expression correlating to the patient's survival rate. The enhancement of T-cell and IFN production also affected the immune system. In the longest survival patient, there was the highest T-cell-related gene expression. |
| OrienX010 | Melanoma | China | 26 | Safety, tolerability, efficacy, and phase II dose level | Intratumoural injection | The oncolytic OrienX010 was safe and well-tolerated in patients with melanoma. This therapeutic method exhibited significant anti-tumour activity. The recommended dose for phase II clinical trials without severe AEs was 10 mL of 8 × 107 pfu/mL every 2 weeks. | |
| Adenovirus | ICOVIR-5 | Cutaneous and uveal melanoma | Spain | 12 | Toxicity and efficacy | Intravenous injection | Tumour targeting is possible but more efficient tumour debulking is needed via oncolysis due to the immune system leading to low anti-tumour efficacy. The toxicity was very short without inflammatory response syndrome. |
| DNX-2401 | Malignant glioma | USA | 25 | Safety, efficacy, and biologic effects | Intravenous injection | 25% of patients with single DNX-2401 survived more than 3 years and 95% decrease in tumour volumes was observed in three patients. This is due to oncolytic effects and emerging immune-mediated anti-glioma response | |
| Coxsackie virus | CVA21 | AML | UK | 16 | Anti-tumour ability and cellular mechanism responsible | Intravenous delivery | CVA21 can activate the immune system for anti-tumour activity comprising cytokine-mediated bystander killing, enhancing of natural killer cell-mediated cellular cytotoxicity and tumour-specific cytotoxic T lymphocytes. Type I IFN and NK cell activation was observed. Moreover, the crucial mediators are ICAM-1 and plasmacytoid dendritic cells. |
| CVA21 | Non‐muscle invasive bladder cancer (NMIBC) | UK | 15 | Safety, MTD, evidence of viral replication, induction of inflammatory cytokines, anti-tumour activity, and viral-induced changes in resected tissue | Intravesical administration | All patients showed no sign of toxicity in both virus and virus with subtherapeutic dose mitomycin C. Inflammation of NMIBC tissues was observed with the increasing immune checkpoint inhibitory genes (PD-L1 and LAG3) and Th1-associated chemokines. | |
| Measles virus | MV-NIS | Myeloma | USA | 32 | MTD, | Intravenous delivery | The maximum tolerated dose of the patient with MV-NIS was not reached. Phase II with TCID50 1011 will be evaluated. MV-NIS is capable of replicating before being cleared by the immune system. |
| Poliovirus | PVSRIPO | Melanoma | USA | 12 | Safety and tolerability | Intratumoural injection | The study showed well-tolerated with no SAEs or DLTs |
| PVSRIPO | Melanoma | USA | 12 | Immunologic effects in the TME | Intratumoural injection | Patients with lerapolturev and anti-PD-1 therapy have a median PFS of 2.3 years and had higher CD8+ T cell infiltrates in prelerapolturev tumour biopsies. | |
| Vaccinia virus | GL-ONC1 | Peritoneal cancer | Germany | 9 | Safety assessment, MTD, anti-tumour activity, viral replication, clinical efficacy, and biological effects in real-time study | intraperitoneal injection | GL-ONC1 administration into the peritoneal cavity was tolerated in advanced stage peritoneal carcinomatosis patients. There were limited efficient tumour cell infection, virus replication, and oncolysis. |
| ACAM2000 | AML | USA | 26 | safety and feasibility | Intravenous, intratumoural, and intraperitoneal injections | ACAM2000 treatment delivered by autologous adipose SVF cells in AML patients was safe and well tolerated. Many patients showed great signals of anti-cancer effect. | |
| Olvi-Vec | Platinum-resistant/refractory ovarian cancer (PRROC) | USA | 12 | Safety, adverse events assessments, | intraperitoneal injection | Intraperitoneal Olvi-Vec oncolytic viral therapy illustrated well safety, clinical activities, and immune activation in PRROC patients. | |
| Reovirus | PD-L1 with pelareorep and pembrolizumab | PDAC | USA | 11 | Safety, DLT, tumour response, reovirus replication, and immune analysis | Intravenous injection | Chemotherapy of pelareorep and pembrolizumab showed no toxicity and provided great efficacy. The pelareorep and anti-PD-1 therapy evaluation was ongoing. |
| Reovirus | Metastatic CRC | USA | 8 | Immune response, cytokine expression pattern in peripheral circulation, exosomal and cellular microRNA levels, and effects of reovirus on leukocyte transcriptome | Intravenous infusion | Reovirus as an oncolytic agent provided multi-layered effects in tumour patients. Reovirus can function in immune stimulants, including immuno-chemo-therapeutic drugs and an oncolytic agent efficacy. Reovirus caused lysis of tumour cells, and facilitator of immune-mediated recognition. | |
| Parvovirus | H-1PV | Glioblastoma | Germany | Safety and tolerability, virus distribution, and MTD | Intratumoural or intravenous injection | H-1PV treatment was safe and well tolerated, and no reached MTD. The virus could cross the blood-brain/tumour barrier and spread through the tumour. | |
| Seneca Valley virus | NTX-010 with cyclophosphamide | Relapsed/refractory neuroblastoma, rhabdomyosarcoma, carcinoid tumour, and adrenocorticocarcinoma | USA | 13 | MTD and recommended phase II dose | Intravenous injection | NTX-010 is well tolerable at the dose levels in relapsed/refractory solid tumours pediatric patients. The addition of cyclophosphamide showed limited applicability. |
Table 3. Summary of major oncolytic virus recently under clinical trials: phase I study
| Virus type | Name (published year) | Cancer | Location | Patients number | Measurement | Route of administration | Study conclusion |
|---|---|---|---|---|---|---|---|
| Herpes virus | G207 | Glioblastoma | USA | 6 | Correlated gene analysis | Intratumoural stereotactic injection | The results have shown approximately 500 tumour-associated genes expression correlating to the patient's survival rate. The enhancement of T-cell and IFN production also affected the immune system. In the longest survival patient, there was the highest T-cell-related gene expression. |
| OrienX010 | Melanoma | China | 26 | Safety, tolerability, efficacy, and phase II dose level | Intratumoural injection | The oncolytic OrienX010 was safe and well-tolerated in patients with melanoma. This therapeutic method exhibited significant anti-tumour activity. The recommended dose for phase II clinical trials without severe AEs was 10 mL of 8 × 107 pfu/mL every 2 weeks. | |
| Adenovirus | ICOVIR-5 | Cutaneous and uveal melanoma | Spain | 12 | Toxicity and efficacy | Intravenous injection | Tumour targeting is possible but more efficient tumour debulking is needed via oncolysis due to the immune system leading to low anti-tumour efficacy. The toxicity was very short without inflammatory response syndrome. |
| DNX-2401 | Malignant glioma | USA | 25 | Safety, efficacy, and biologic effects | Intravenous injection | 25% of patients with single DNX-2401 survived more than 3 years and 95% decrease in tumour volumes was observed in three patients. This is due to oncolytic effects and emerging immune-mediated anti-glioma response | |
| Coxsackie virus | CVA21 | AML | UK | 16 | Anti-tumour ability and cellular mechanism responsible | Intravenous delivery | CVA21 can activate the immune system for anti-tumour activity comprising cytokine-mediated bystander killing, enhancing of natural killer cell-mediated cellular cytotoxicity and tumour-specific cytotoxic T lymphocytes. Type I IFN and NK cell activation was observed. Moreover, the crucial mediators are ICAM-1 and plasmacytoid dendritic cells. |
| CVA21 | Non‐muscle invasive bladder cancer (NMIBC) | UK | 15 | Safety, MTD, evidence of viral replication, induction of inflammatory cytokines, anti-tumour activity, and viral-induced changes in resected tissue | Intravesical administration | All patients showed no sign of toxicity in both virus and virus with subtherapeutic dose mitomycin C. Inflammation of NMIBC tissues was observed with the increasing immune checkpoint inhibitory genes (PD-L1 and LAG3) and Th1-associated chemokines. | |
| Measles virus | MV-NIS | Myeloma | USA | 32 | MTD, | Intravenous delivery | The maximum tolerated dose of the patient with MV-NIS was not reached. Phase II with TCID50 1011 will be evaluated. MV-NIS is capable of replicating before being cleared by the immune system. |
| Poliovirus | PVSRIPO | Melanoma | USA | 12 | Safety and tolerability | Intratumoural injection | The study showed well-tolerated with no SAEs or DLTs |
| PVSRIPO | Melanoma | USA | 12 | Immunologic effects in the TME | Intratumoural injection | Patients with lerapolturev and anti-PD-1 therapy have a median PFS of 2.3 years and had higher CD8+ T cell infiltrates in prelerapolturev tumour biopsies. | |
| Vaccinia virus | GL-ONC1 | Peritoneal cancer | Germany | 9 | Safety assessment, MTD, anti-tumour activity, viral replication, clinical efficacy, and biological effects in real-time study | intraperitoneal injection | GL-ONC1 administration into the peritoneal cavity was tolerated in advanced stage peritoneal carcinomatosis patients. There were limited efficient tumour cell infection, virus replication, and oncolysis. |
| ACAM2000 | AML | USA | 26 | safety and feasibility | Intravenous, intratumoural, and intraperitoneal injections | ACAM2000 treatment delivered by autologous adipose SVF cells in AML patients was safe and well tolerated. Many patients showed great signals of anti-cancer effect. | |
| Olvi-Vec | Platinum-resistant/refractory ovarian cancer (PRROC) | USA | 12 | Safety, adverse events assessments, | intraperitoneal injection | Intraperitoneal Olvi-Vec oncolytic viral therapy illustrated well safety, clinical activities, and immune activation in PRROC patients. | |
| Reovirus | PD-L1 with pelareorep and pembrolizumab | PDAC | USA | 11 | Safety, DLT, tumour response, reovirus replication, and immune analysis | Intravenous injection | Chemotherapy of pelareorep and pembrolizumab showed no toxicity and provided great efficacy. The pelareorep and anti-PD-1 therapy evaluation was ongoing. |
| Reovirus | Metastatic CRC | USA | 8 | Immune response, cytokine expression pattern in peripheral circulation, exosomal and cellular microRNA levels, and effects of reovirus on leukocyte transcriptome | Intravenous infusion | Reovirus as an oncolytic agent provided multi-layered effects in tumour patients. Reovirus can function in immune stimulants, including immuno-chemo-therapeutic drugs and an oncolytic agent efficacy. Reovirus caused lysis of tumour cells, and facilitator of immune-mediated recognition. | |
| Parvovirus | H-1PV | Glioblastoma | Germany | Safety and tolerability, virus distribution, and MTD | Intratumoural or intravenous injection | H-1PV treatment was safe and well tolerated, and no reached MTD. The virus could cross the blood-brain/tumour barrier and spread through the tumour. | |
| Seneca Valley virus | NTX-010 with cyclophosphamide | Relapsed/refractory neuroblastoma, rhabdomyosarcoma, carcinoid tumour, and adrenocorticocarcinoma | USA | 13 | MTD and recommended phase II dose | Intravenous injection | NTX-010 is well tolerable at the dose levels in relapsed/refractory solid tumours pediatric patients. The addition of cyclophosphamide showed limited applicability. |
| Virus type | Name (published year) | Cancer | Location | Patient number | Measurement | Route of administration | Study conclusion | ||
|---|---|---|---|---|---|---|---|---|---|
| Herpes virus | OH2 | Various | China | 40 | Safety and tolerability | Intratumoural injection | This phase I/II study showed that the oncolytic virus OH2 was safe and well-tolerated in patients with solid tumours. The durability of anti-tumour activity was significantly remarkable in patients with metastatic esophageal and rectal cancer. | ||
| G47Δ | Glioblastoma | Japan | 13 | Safety and tumour response | Intratumoural injection | Study showed safety of G47Δ up to 1 × 109 pfu/dose for two doses within 14 days. It could cause immediate infiltration of lymphocytes that directed towards tumour cells. Three of 13 patients had long-term survival (> 46 months) from the delayed effect of anti-tumour immunity. | |||
| 19 | Efficacy | Intratumoural injection | The study showed the overall response in 2 years, partial response in 1 patient and stable disease in 18 patients. The number of tumour-infiltrating CD4+/CD8+ lymphocytes and persistent low numbers of Foxp3+ cells increased which was evidenced by biopsies. It also showed that G47Δ was safe for oncolytic cancer therapy. | ||||||
| Herpes virus | T-VEC | Breast cancer | USA | 35 | Efficacy, overall response rate ORR, rates of local overall response/disease, control rate, PFS, and OS | Intratumoural injection | In patients with inoperable locoregional recurrence of breast cancer, intratumoural T-VEC as monotherapy was not therapeutically desirable owing to uncontrolled disease progression. | ||
| STS | USA | 30 | Safety, tolerability, and efficacy | Intratumoural injection | The incorporation of TVEC and EBRT provided safety and good tolerability towards STS treatment. These can also increase the immune response without necrosis. The result also evidenced that Caspase-3 could be a biomarker relating to a positive effect of TVEC. | ||||
| Adenovirus | CG0070 | NMIBC | USA | 45 | Safety and efficacy in patients with high-risk BCG-unresponsive NMIBC | Intravesical injection | The toxicity of virotherapy was relatively low. There was 47% CR of patients with high-risk BCG-unresponsive NMIBC, 58% CR of patients with CIS, and 50% of patients with CIS-containing tumours. | ||
| Coxsackie virus | V937 | Melanoma | USA | 57 | Efficacy and safety in patients with unresectable stage IIIC or IV melanoma | Intratumoural injection | V937 was well tolerated and warrants further investigation for treatment of patients with unresectable melanoma without additional toxicities. The primary efficacy endpoint was 38.6% and durable response rate was 21.1%. 12-month PFS was 32.9% and 12-month OS was 75.4% | ||
| Vaccinia virus | JX-594 | Soft-tissue sarcoma | France | 20 | The 6-month non-progression rate, efficacy, immune response, and therapeutic potential | Intravenous injection | The administration of JX-594 oncolytic virus was safe in advanced STS patients. The role of immune-oncology agent combination and the patient population identification who received benefit from this approach were questions from major interest. | ||
| Reovirus | FOLFOX/BEV with pelareorep | Metastatic colorectal cancer | Canada | 103 | PFS, OS, ORR, and correlative analyses. | Intravenous injection | FOLFOX/BEV with pelareorep was increased ORR, but PFS was reduced. Reduction of treatment intensity with standard agents provided the lack of pelareorep benefit. | ||
| Pelareorep (reolysin) with pemetrexed or docetaxel | NSCLC | Canada | 166 | PFS, OS, ORR, and exploratory translational analyses. | Intravenous injection | No improvement of PFS in NSCLC patients was demonstrated in pelareorep chemotherapy. | |||
| Paclitaxel/Pelareorep | mBC | Canada | 81 | PFS, response rate, OS, circulating tumour cell counts, safety, and exploratory correlative analyses | Intravenous injection | This randomised phase II study of pelareorep and paclitaxel was not different in PFS for treated mBC patients. Pelareorep/paclitaxel combination revealed longer OS. | |||
| Parvovirus | H-1PV (ParvOryx) | metastatic PDAC | Germany | 7 | Safety, clinical efficacy, virus pharmacokinetics, shedding, and immune response | Intravenous injection | No environmental risks were indicated immune modulation once ParvOryx injection. H-1PV was systematic clinical development with immunomodulatory compounds. | ||
| Seneca Valley virus | NTX-010 | ES SCLC | USA | 50 | PFS, prespecified interim analysis for futility, viral clearance, and the development of neutralizing antibodies | Intravenous injection | NTX-010 treatment had no benefit with ES SCLC patients. Persistence of NTX-010 was related a short PFS. There was no outcome improvement of NTX010 treatment in ES SCLC patients after platinum-based chemotherapy. | ||
Table 4. Summary of major oncolytic virus recently under clinical trials: phase II study
| Virus type | Name (published year) | Cancer | Location | Patient number | Measurement | Route of administration | Study conclusion | ||
|---|---|---|---|---|---|---|---|---|---|
| Herpes virus | OH2 | Various | China | 40 | Safety and tolerability | Intratumoural injection | This phase I/II study showed that the oncolytic virus OH2 was safe and well-tolerated in patients with solid tumours. The durability of anti-tumour activity was significantly remarkable in patients with metastatic esophageal and rectal cancer. | ||
| G47Δ | Glioblastoma | Japan | 13 | Safety and tumour response | Intratumoural injection | Study showed safety of G47Δ up to 1 × 109 pfu/dose for two doses within 14 days. It could cause immediate infiltration of lymphocytes that directed towards tumour cells. Three of 13 patients had long-term survival (> 46 months) from the delayed effect of anti-tumour immunity. | |||
| 19 | Efficacy | Intratumoural injection | The study showed the overall response in 2 years, partial response in 1 patient and stable disease in 18 patients. The number of tumour-infiltrating CD4+/CD8+ lymphocytes and persistent low numbers of Foxp3+ cells increased which was evidenced by biopsies. It also showed that G47Δ was safe for oncolytic cancer therapy. | ||||||
| Herpes virus | T-VEC | Breast cancer | USA | 35 | Efficacy, overall response rate ORR, rates of local overall response/disease, control rate, PFS, and OS | Intratumoural injection | In patients with inoperable locoregional recurrence of breast cancer, intratumoural T-VEC as monotherapy was not therapeutically desirable owing to uncontrolled disease progression. | ||
| STS | USA | 30 | Safety, tolerability, and efficacy | Intratumoural injection | The incorporation of TVEC and EBRT provided safety and good tolerability towards STS treatment. These can also increase the immune response without necrosis. The result also evidenced that Caspase-3 could be a biomarker relating to a positive effect of TVEC. | ||||
| Adenovirus | CG0070 | NMIBC | USA | 45 | Safety and efficacy in patients with high-risk BCG-unresponsive NMIBC | Intravesical injection | The toxicity of virotherapy was relatively low. There was 47% CR of patients with high-risk BCG-unresponsive NMIBC, 58% CR of patients with CIS, and 50% of patients with CIS-containing tumours. | ||
| Coxsackie virus | V937 | Melanoma | USA | 57 | Efficacy and safety in patients with unresectable stage IIIC or IV melanoma | Intratumoural injection | V937 was well tolerated and warrants further investigation for treatment of patients with unresectable melanoma without additional toxicities. The primary efficacy endpoint was 38.6% and durable response rate was 21.1%. 12-month PFS was 32.9% and 12-month OS was 75.4% | ||
| Vaccinia virus | JX-594 | Soft-tissue sarcoma | France | 20 | The 6-month non-progression rate, efficacy, immune response, and therapeutic potential | Intravenous injection | The administration of JX-594 oncolytic virus was safe in advanced STS patients. The role of immune-oncology agent combination and the patient population identification who received benefit from this approach were questions from major interest. | ||
| Reovirus | FOLFOX/BEV with pelareorep | Metastatic colorectal cancer | Canada | 103 | PFS, OS, ORR, and correlative analyses. | Intravenous injection | FOLFOX/BEV with pelareorep was increased ORR, but PFS was reduced. Reduction of treatment intensity with standard agents provided the lack of pelareorep benefit. | ||
| Pelareorep (reolysin) with pemetrexed or docetaxel | NSCLC | Canada | 166 | PFS, OS, ORR, and exploratory translational analyses. | Intravenous injection | No improvement of PFS in NSCLC patients was demonstrated in pelareorep chemotherapy. | |||
| Paclitaxel/Pelareorep | mBC | Canada | 81 | PFS, response rate, OS, circulating tumour cell counts, safety, and exploratory correlative analyses | Intravenous injection | This randomised phase II study of pelareorep and paclitaxel was not different in PFS for treated mBC patients. Pelareorep/paclitaxel combination revealed longer OS. | |||
| Parvovirus | H-1PV (ParvOryx) | metastatic PDAC | Germany | 7 | Safety, clinical efficacy, virus pharmacokinetics, shedding, and immune response | Intravenous injection | No environmental risks were indicated immune modulation once ParvOryx injection. H-1PV was systematic clinical development with immunomodulatory compounds. | ||
| Seneca Valley virus | NTX-010 | ES SCLC | USA | 50 | PFS, prespecified interim analysis for futility, viral clearance, and the development of neutralizing antibodies | Intravenous injection | NTX-010 treatment had no benefit with ES SCLC patients. Persistence of NTX-010 was related a short PFS. There was no outcome improvement of NTX010 treatment in ES SCLC patients after platinum-based chemotherapy. | ||
| Virus type | Name (published year) | Cancer | Location | Patients number | Measurement | Route of administration | Study conclusion |
|---|---|---|---|---|---|---|---|
| Herpes virus | T-VEC | Melanoma | UK | 437 | Efficacy | Intratumoural delivery | Patients with early metastatic melanoma (stage IIIB-IVM1a) had a high CR rate and durability with T-VEC administration. The results still showed the well-tolerated ability of T-VEC and also exposed the association between the virus and the survival rate. |
| T-VEC | Melanoma | USA | 41 | Safety | Intralesional injection | The results showed consistent safety as previous research of T-VEC. Only influenza-like symptoms were observed which are mild or moderate AEs |
Table 5. Summary of major oncolytic virus recently under clinical trials: phase III study
| Virus type | Name (published year) | Cancer | Location | Patients number | Measurement | Route of administration | Study conclusion |
|---|---|---|---|---|---|---|---|
| Herpes virus | T-VEC | Melanoma | UK | 437 | Efficacy | Intratumoural delivery | Patients with early metastatic melanoma (stage IIIB-IVM1a) had a high CR rate and durability with T-VEC administration. The results still showed the well-tolerated ability of T-VEC and also exposed the association between the virus and the survival rate. |
| T-VEC | Melanoma | USA | 41 | Safety | Intralesional injection | The results showed consistent safety as previous research of T-VEC. Only influenza-like symptoms were observed which are mild or moderate AEs |
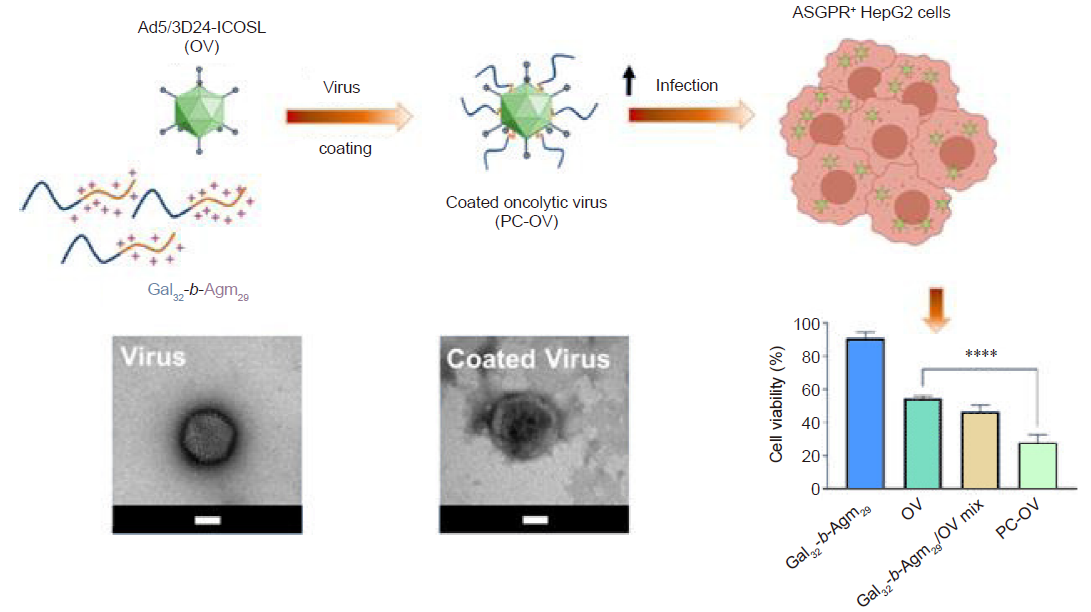
Figure 2. The design of a high potential therapeutic polymer-coated oncolytic viruses (PC-OVs) delivery system which is coated by electrostatic polygalactosyl-b-agmatyl (Gal32-b-Agm29) diblock copolymer with asialoglycoprotein receptor (ASGPR) for diagnosis of hepatocellular carcinoma in human hepatoma cell line HepG2 as a model. ****P < 0.0001. Reprinted from Garofalo et al.113 OV: oncolytic virus; PC: polymer-coated.
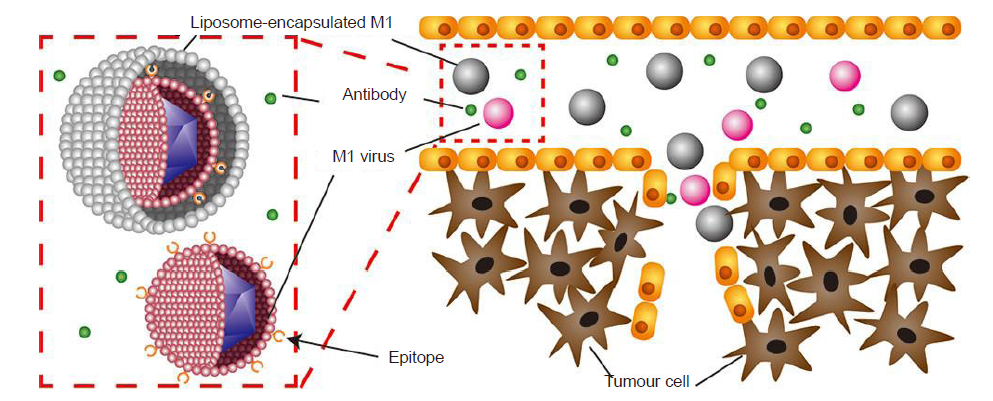
Figure 3. Schematic illustration of the fabrication of a liposome-encapsulated M1 virus platform (M-LPO) for tumour therapy in LoVo and Hep 3B cell lines. Reprinted with permission from Wang et al.120 Copyright 2019 American Chemical Society.
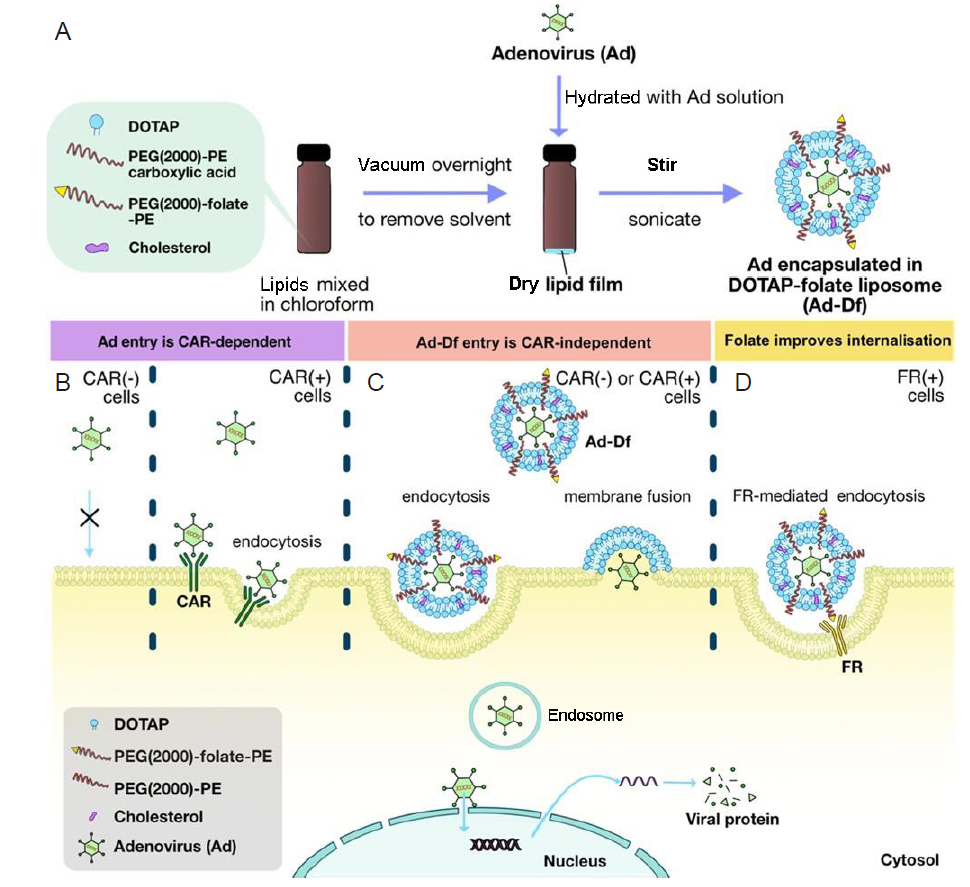
Figure 4. (A) The synthesis of adenovirus encapsulating cationic 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP)-folate liposomes (Ad-Df). (B) The cellular penetration approaches of Ad-Df viral platform in various coxsackievirus and adenovirus receptor (CAR)-deficient cell lines. (C) Ad-Df is capable of entering the cells via endocytosis during no expression of CAR of the target cells, leading to transfect CAR-positive and -negative cells. (D) The cellular uptake into FR-positive cells can be enhanced by Ad-Df containing folate-conjugated lipid through FR-mediated endocytosis. Reprinted with permission from Huang et al.122 Copyright 2022 American Chemical Society. FR: Folate receptor; PEG(2000)-PE: 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(poly-ethylene glycol)-2000].
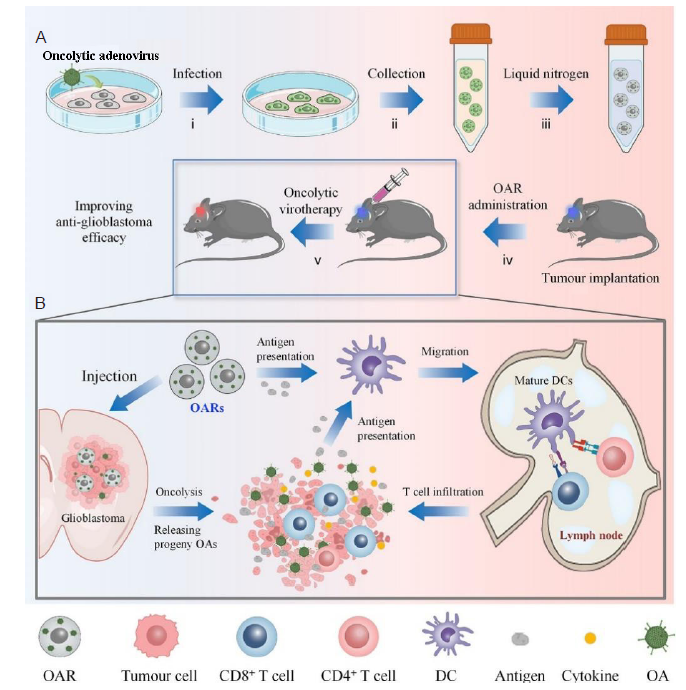
Figure 7. (A) Illustration of the preparation and in vivo experimental processes of cryo-shocked cancer cells as oncolytic adenovirus reservoir (OARs) for glioblastoma immunotherapy in a mouse glioblastoma model. (B) The administration mechanism of OARs via intratumoural injection. Reprinted with permission from Liu et al.135 Copyright 2022 American Chemical Society. DC: dendritic cell; OA: oncolytic adenovirus.
| 1. | Sung, H.; Ferlay, J.; Siegel, R. L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021, 71, 209-249. |
| 2. |
Siegel, R. L.; Miller, K. D.; Fuchs, H. E.; Jemal, A. Cancer statistics, 2022. CA Cancer J Clin. 2022, 72, 7-33.
doi: 10.3322/caac.v72.1 URL |
| 3. | Mondal, J.; Panigrahi, A. K.; Khuda-Bukhsh, A. R. Conventional chemotherapy: problems and scope for combined therapies with certain herbal products and dietary supplements. Austin J Mol Cell Biol. 2014, 1, 10. |
| 4. | Carvalho, A.; Fernandes, A. R.; Baptista, P. V. Chapter 10 - Nanoparticles as delivery systems in cancer therapy: focus on gold nanoparticles and drugs. In Applications of targeted nano drugs and delivery systems, Mohapatra, S. S.; Ranjan, S.; Dasgupta, N.; Mishra, R. K.; Thomas, S., eds.; Elsevier: 2019; pp 257-295. |
| 5. |
Chidambaram, M.; Manavalan, R.; Kathiresan, K. Nanotherapeutics to overcome conventional cancer chemotherapy limitations. J Pharm Pharm Sci. 2011, 14, 67-77.
doi: 10.18433/J30C7D URL |
| 6. |
Choi, C. H. ABC transporters as multidrug resistance mechanisms and the development of chemosensitizers for their reversal. Cancer Cell Int. 2005, 5, 30.
doi: 10.1186/1475-2867-5-30 URL |
| 7. |
Rahman, M. M.; McFadden, G. Oncolytic viruses: newest frontier for cancer immunotherapy. Cancers (Basel). 2021, 13, 5452.
doi: 10.3390/cancers13215452 URL |
| 8. |
Truong, C. S.; Yoo, S. Y. Oncolytic vaccinia virus in lung cancer vaccines. Vaccines. 2022, 10, 240.
doi: 10.3390/vaccines10020240 URL |
| 9. | Tian, Y.; Xie, D.; Yang, L. Engineering strategies to enhance oncolytic viruses in cancer immunotherapy. Signal Transduct Target Ther. 2022, 7, 117. |
| 10. |
Lemos de Matos, A.; Franco, L. S.; McFadden, G. Oncolytic viruses and the immune system: the dynamic duo. Mol Ther Methods Clin Dev. 2020, 17, 349-358.
doi: 10.1016/j.omtm.2020.01.001 URL |
| 11. |
Filley, A. C.; Dey, M. Immune system, friend or foe of oncolytic virotherapy? Front Oncol. 2017, 7, 106.
doi: 10.3389/fonc.2017.00106 URL |
| 12. |
Maroun, J.; Muñoz-Alía, M.; Ammayappan, A.; Schulze, A.; Peng, K. W.; Russell, S. Designing and building oncolytic viruses. Future Virol. 2017, 12, 193-213.
doi: 10.2217/fvl-2016-0129 URL |
| 13. |
Jin, K. T.; Du, W. L.; Liu, Y. Y.; Lan, H. R.; Si, J. X.; Mou, X. Z. Oncolytic virotherapy in solid tumors: the challenges and achievements. Cancers (Basel). 2021, 13, 588.
doi: 10.3390/cancers13040588 URL |
| 14. |
Kaufman, H. L.; Kohlhapp, F. J.; Zloza, A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov. 2015, 14, 642-662.
doi: 10.1038/nrd4663 |
| 15. | Martini, V.; D'Avanzo, F.; Maggiora, P. M.; Varughese, F. M.; Sica, A.; Gennari, A. Oncolytic virotherapy: new weapon for breast cancer treatment. Ecancermedicalscience. 2020, 14, 1149. |
| 16. |
You, Z.; Fischer, D. C.; Tong, X.; Hasenburg, A.; Aguilar-Cordova, E.; Kieback, D. G. Coxsackievirus-adenovirus receptor expression in ovarian cancer cell lines is associated with increased adenovirus transduction efficiency and transgene expression. Cancer Gene Ther. 2001, 8, 168-175.
doi: 10.1038/sj.cgt.7700284 |
| 17. |
Hensen, L. C. M.; Hoeben, R. C.; Bots, S. T. F. Adenovirus receptor expression in cancer and its multifaceted role in oncolytic adenovirus therapy. Int J Mol Sci. 2020, 21, 6828.
doi: 10.3390/ijms21186828 URL |
| 18. |
Cheng, T.; Bai, J.; Chung, C. S.; Chen, Y.; Fallon, E. A.; Ayala, A. Herpes virus entry mediator (HVEM) expression promotes inflammation/organ injury in response to experimental indirect-acute lung injury. Shock. 2019, 51, 487-494.
doi: 10.1097/SHK.0000000000001174 URL |
| 19. | Tang, M.; Cao, X.; Li, Y.; Li, G. Q.; He, Q. H.; Li, S. J.; Chen, J.; Xu, G. L.; Zhang, K. Q. High expression of herpes virus entry mediator is associated with poor prognosis in clear cell renal cell carcinoma. Am J Cancer Res. 2019, 9, 975-987. |
| 20. |
Ferreira, T.; Kulkarni, A.; Bretscher, C.; Richter, K.; Ehrlich, M.; Marchini, A. Oncolytic H-1 parvovirus enters cancer cells through clathrin-mediated endocytosis. Viruses. 2020, 12, 1199.
doi: 10.3390/v12101199 URL |
| 21. |
Nettelbeck, D. M.; Leber, M. F.; Altomonte, J.; Angelova, A.; Beil, J.; Berchtold, S.; Delic, M.; Eberle, J.; Ehrhardt, A.; Engeland, C. E.; Fechner, H.; Geletneky, K.; Goepfert, K.; Holm, P. S.; Kochanek, S.; Kreppel, F.; Krutzke, L.; Kühnel, F.; Lang, K. S.; Marchini, A.; Moehler, M.; Mühlebach, M. D.; Naumann, U.; Nawroth, R.; Nüesch, J.; Rommelaere, J.; Lauer, U. M.; Ungerechts, G. Virotherapy in Germany-recent activities in virus engineering, preclinical development, and clinical studies. Viruses. 2021, 13, 1420.
doi: 10.3390/v13081420 URL |
| 22. |
Ferreira, T.; Kulkarni, A.; Bretscher, C.; Nazarov, P. V.; Hossain, J. A.; Ystaas, L. A. R.; Miletic, H.; Röth, R.; Niesler, B.; Marchini, A. Oncolytic H-1 parvovirus hijacks galectin-1 to enter cancer cells. Viruses. 2022, 14, 1018.
doi: 10.3390/v14051018 URL |
| 23. |
Bretscher, C.; Marchini, A. H-1 parvovirus as a cancer-killing agent: past, present, and future. Viruses. 2019, 11, 562.
doi: 10.3390/v11060562 URL |
| 24. |
Marchini, A.; Bonifati, S.; Scott, E. M.; Angelova, A. L.; Rommelaere, J. Oncolytic parvoviruses: from basic virology to clinical applications. Virol J. 2015, 12, 6.
doi: 10.1186/s12985-014-0223-y URL |
| 25. | Bradley, S.; Jakes, A. D.; Harrington, K.; Pandha, H.; Melcher, A.; Errington-Mais, F. Applications of coxsackievirus A21 in oncology. Oncolytic Virother. 2014, 3, 47-55. |
| 26. |
Au, G. G.; Lincz, L. F.; Enno, A.; Shafren, D. R. Oncolytic coxsackievirus A21 as a novel therapy for multiple myeloma. Br J Haematol. 2007, 137, 133-141.
doi: 10.1111/bjh.2007.137.issue-2 URL |
| 27. | Li, Y. C.; Zhou, Q.; Song, Q. K.; Wang, R. B.; Lyu, S.; Guan, X.; Zhao, Y. J.; Wu, J. P. Overexpression of an immune checkpoint (CD155) in breast cancer associated with prognostic significance and exhausted tumor-infiltrating lymphocytes: a cohort study. J Immunol Res. 2020, 2020, 3948928. |
| 28. |
Lupo, K. B.; Matosevic, S. CD155 immunoregulation as a target for natural killer cell immunotherapy in glioblastoma. J Hematol Oncol. 2020, 13, 76.
doi: 10.1186/s13045-020-00913-2 |
| 29. |
Masson, D.; Jarry, A.; Baury, B.; Blanchardie, P.; Laboisse, C.; Lustenberger, P.; Denis, M. G. Overexpression of the CD155 gene in human colorectal carcinoma. Gut. 2001, 49, 236-240.
doi: 10.1136/gut.49.2.236 URL |
| 30. |
Huang, D. W.; Huang, M.; Lin, X. S.; Huang, Q. CD155 expression and its correlation with clinicopathologic characteristics, angiogenesis, and prognosis in human cholangiocarcinoma. Onco Targets Ther. 2017, 10, 3817-3825.
doi: 10.2147/OTT URL |
| 31. | Elvington, M.; Liszewski, M. K.; Atkinson, J. P. CD46 and oncologic interactions: friendly fire against cancer. Antibodies (Basel). 2020, 9, 59. |
| 32. |
Russell, L.; Peng, K. W. The emerging role of oncolytic virus therapy against cancer. Chin Clin Oncol. 2018, 7, 16.
doi: 10.21037/cco URL |
| 33. |
Nikolic, J.; Belot, L.; Raux, H.; Legrand, P.; Gaudin, Y.; A, A. A. Structural basis for the recognition of LDL-receptor family members by VSV glycoprotein. Nat Commun. 2018, 9, 1029.
doi: 10.1038/s41467-018-03432-4 |
| 34. |
Zhang, Y.; Nagalo, B. M. Immunovirotherapy based on recombinant vesicular stomatitis virus: where are we? Front Immunol. 2022, 13, 898631.
doi: 10.3389/fimmu.2022.898631 URL |
| 35. |
Martignone, S.; Ménard, S.; Bufalino, R.; Cascinelli, N.; Pellegrini, R.; Tagliabue, E.; Andreola, S.; Rilke, F.; Colnaghi, M. I. Prognostic significance of the 67-kilodalton laminin receptor expression in human breast carcinomas. J Natl Cancer Inst. 1993, 85, 398-402.
doi: 10.1093/jnci/85.5.398 URL |
| 36. | Fontanini, G.; Vignati, S.; Chiné, S.; Lucchi, M.; Mussi, A.; Angeletti, C. A.; Ménard, S.; Castronovo, V.; Bevilacqua, G. 67-Kilodalton laminin receptor expression correlates with worse prognostic indicators in non-small cell lung carcinomas. Clin Cancer Res. 1997, 3, 227-231. |
| 37. |
Sanjuán, X.; Fernández, P. L.; Miquel, R.; Muñoz, J.; Castronovo, V.; Ménard, S.; Palacín, A.; Cardesa, A.; Campo, E. Overexpression of the 67-kD laminin receptor correlates with tumour progression in human colorectal carcinoma. J Pathol. 1996, 179, 376-380.
doi: 10.1002/(ISSN)1096-9896 URL |
| 38. |
Taraboletti, G.; Belotti, D.; Giavazzi, R.; Sobel, M. E.; Castronovo, V. Enhancement of metastatic potential of murine and human melanoma cells by laminin receptor peptide G: attachment of cancer cells to subendothelial matrix as a pathway for hematogenous metastasis. J Natl Cancer Inst. 1993, 85, 235-240.
doi: 10.1093/jnci/85.3.235 URL |
| 39. |
van den Brule, F. A.; Buicu, C.; Berchuck, A.; Bast, R. C.; Deprez, M.; Liu, F. T.; Cooper, D. N.; Pieters, C.; Sobel, M. E.; Castronovo, V. Expression of the 67-kD laminin receptor, galectin-1, and galectin-3 in advanced human uterine adenocarcinoma. Hum Pathol. 1996, 27, 1185-1191.
doi: 10.1016/S0046-8177(96)90313-5 URL |
| 40. |
Xing, L.; Huhtala, M.; Pietiäinen, V.; Käpylä, J.; Vuorinen, K.; Marjomäki, V.; Heino, J.; Johnson, M. S.; Hyypiä, T.; Cheng, R. H. Structural and functional analysis of integrin alpha2I domain interaction with echovirus 1. J Biol Chem. 2004, 279, 11632-11638.
doi: 10.1074/jbc.M312441200 URL |
| 41. |
Haley, E. S.; Au, G. G.; Carlton, B. R.; Barry, R. D.; Shafren, D. R. Regional administration of oncolytic Echovirus 1 as a novel therapy for the peritoneal dissemination of gastric cancer. J Mol Med (Berl). 2009, 87, 385-399.
doi: 10.1007/s00109-008-0433-0 URL |
| 42. |
García-Romero, N.; Palacín-Aliana, I.; Esteban-Rubio, S.; Madurga, R.; Rius-Rocabert, S.; Carrión-Navarro, J.; Presa, J.; Cuadrado-Castano, S.; Sánchez-Gómez, P.; García-Sastre, A.; Nistal-Villan, E.; Ayuso-Sacido, A. Newcastle disease virus (NDV) oncolytic activity in human glioma tumors is dependent on CDKN2A-type I IFN gene cluster codeletion. Cells. 2020, 9, 1405.
doi: 10.3390/cells9061405 URL |
| 43. | Keshavarz, M.; Nejad, A. S. M.; Esghaei, M.; Bokharaei-Salim, F.; Dianat-Moghadam, H.; Keyvani, H.; Ghaemi, A. Oncolytic Newcastle disease virus reduces growth of cervical cancer cell by inducing apoptosis. Saudi J Biol Sci. 2020, 27, 47-52. |
| 44. |
Yuan, P.; Swanson, K. A.; Leser, G. P.; Paterson, R. G.; Lamb, R. A.; Jardetzky, T. S. Structure of the Newcastle disease virus hemagglutinin-neuraminidase (HN) ectodomain reveals a four-helix bundle stalk. Proc Natl Acad Sci U S A. 2011, 108, 14920-14925.
doi: 10.1073/pnas.1111691108 URL |
| 45. |
Chu, Z.; Gao, X.; Liu, H.; Ma, J.; Wang, C.; Lu, K.; Han, Q.; Wang, Y.; Wang, C.; Adam, F. E. A.; Wang, X.; Xiao, S.; Yang, Z. Newcastle disease virus selectively infects dividing cells and promotes viral proliferation. Vet Res. 2019, 50, 27.
doi: 10.1186/s13567-019-0644-0 |
| 46. |
Jhawar, S. R.; Thandoni, A.; Bommareddy, P. K.; Hassan, S.; Kohlhapp, F. J.; Goyal, S.; Schenkel, J. M.; Silk, A. W.; Zloza, A. Oncolytic viruses-natural and genetically engineered cancer immunotherapies. Front Oncol. 2017, 7, 202.
doi: 10.3389/fonc.2017.00202 URL |
| 47. |
Katze, M. G.; He, Y.; Gale, M., Jr. Viruses and interferon: a fight for supremacy. Nat Rev Immunol. 2002, 2, 675-687.
doi: 10.1038/nri888 |
| 48. |
Santos Apolonio, J.; Lima de Souza Gonçalves, V.; Cordeiro Santos, M. L.; Silva Luz, M.; Silva Souza, J. V.; Rocha Pinheiro, S. L.; de Souza, W. R.; Sande Loureiro, M.; de Melo, F. F. Oncolytic virus therapy in cancer: a current review. World J Virol. 2021, 10, 229-255.
doi: 10.5501/wjv.v10.i5.229 URL |
| 49. |
Samuel, C. E. Antiviral actions of interferons. Clin Microbiol Rev. 2001, 14, 778-809, table of contents.
doi: 10.1128/CMR.14.4.778-809.2001 URL |
| 50. | Fernandes, J. Oncogenes: the passport for viral oncolysis through PKR inhibition. Biomark Cancer. 2016, 8, 101-110. |
| 51. |
Balachandran, S.; Kim, C. N.; Yeh, W. C.; Mak, T. W.; Bhalla, K.; Barber, G. N. Activation of the dsRNA-dependent protein kinase, PKR, induces apoptosis through FADD-mediated death signaling. EMBO J. 1998, 17, 6888-6902.
doi: 10.1093/emboj/17.23.6888 URL |
| 52. | Nakayama, Y.; Plisch, E. H.; Sullivan, J.; Thomas, C.; Czuprynski, C. J.; Williams, B. R.; Suresh, M. Role of PKR and Type I IFNs in viral control during primary and secondary infection. PLoS Pathog. 2010, 6, e1000966. |
| 53. |
Cook, M.; Chauhan, A. Clinical application of oncolytic viruses: a systematic review. Int J Mol Sci. 2020, 21, 7505.
doi: 10.3390/ijms21207505 URL |
| 54. |
Ahlander, J.; Bosco, G. The RB/E2F pathway and regulation of RNA processing. Biochem Biophys Res Commun. 2009, 384, 280-283.
doi: 10.1016/j.bbrc.2009.04.107 URL |
| 55. |
Topacio, B. R.; Zatulovskiy, E.; Cristea, S.; Xie, S.; Tambo, C. S.; Rubin, S. M.; Sage, J.; Kõivomägi, M.; Skotheim, J. M. Cyclin D-Cdk4,6 drives cell-cycle progression via the retinoblastoma protein's C-terminal helix. Mol Cell. 2019, 74:758-770.e4.
doi: 10.1016/j.molcel.2019.03.020 URL |
| 56. |
Fernández-Medarde, A.; Santos, E. Ras in cancer and developmental diseases. Genes Cancer. 2011, 2, 344-358.
doi: 10.1177/1947601911411084 URL |
| 57. |
Mansour, M.; Palese, P.; Zamarin, D. Oncolytic specificity of Newcastle disease virus is mediated by selectivity for apoptosis-resistant cells. J Virol. 2011, 85, 6015-6023.
doi: 10.1128/JVI.01537-10 URL |
| 58. | Aurelian, L. Oncolytic viruses as immunotherapy: progress and remaining challenges. Onco Targets Ther. 2016, 9, 2627-2637. |
| 59. |
Chiocca, E. A.; Rabkin, S. D. Oncolytic viruses and their application to cancer immunotherapy. Cancer Immunol Res. 2014, 2, 295-300.
doi: 10.1158/2326-6066.CIR-14-0015 URL |
| 60. |
Bommareddy, P. K.; Shettigar, M.; Kaufman, H. L. Integrating oncolytic viruses in combination cancer immunotherapy. Nat Rev Immunol. 2018, 18, 498-513.
doi: 10.1038/s41577-018-0014-6 |
| 61. |
Lawler, S. E.; Speranza, M. C.; Cho, C. F.; Chiocca, E. A. Oncolytic viruses in cancer treatment: a review. JAMA Oncol. 2017, 3, 841-849.
doi: 10.1001/jamaoncol.2016.2064 URL |
| 62. |
Fukuhara, H.; Ino, Y.; Todo, T. Oncolytic virus therapy: a new era of cancer treatment at dawn. Cancer Sci. 2016, 107, 1373-1379.
doi: 10.1111/cas.2016.107.issue-10 URL |
| 63. |
Uchihashi, T.; Nakahara, H.; Fukuhara, H.; Iwai, M.; Ito, H.; Sugauchi, A.; Tanaka, M.; Kogo, M.; Todo, T. Oncolytic herpes virus G47Δ injected into tongue cancer swiftly traffics in lymphatics and suppresses metastasis. Mol Ther Oncolytics. 2021, 22, 388-398.
doi: 10.1016/j.omto.2021.06.008 URL |
| 64. | Woller, N.; Gürlevik, E.; Ureche, C. I.; Schumacher, A.; Kühnel, F. Oncolytic viruses as anticancer vaccines. Front Oncol. 2014, 4, 188. |
| 65. |
Todo, T.; Martuza, R. L.; Rabkin, S. D.; Johnson, P. A. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc Natl Acad Sci U S A. 2001, 98, 6396-6401.
doi: 10.1073/pnas.101136398 URL |
| 66. |
Goldstein, D. J.; Weller, S. K. Herpes simplex virus type 1-induced ribonucleotide reductase activity is dispensable for virus growth and DNA synthesis: isolation and characterization of an ICP6 lacZ insertion mutant. J Virol. 1988, 62, 196-205.
doi: 10.1128/jvi.62.1.196-205.1988 URL |
| 67. |
Gholami, S.; Marano, A.; Chen, N. G.; Aguilar, R. J.; Frentzen, A.; Chen, C. H.; Lou, E.; Fujisawa, S.; Eveno, C.; Belin, L.; Zanzonico, P.; Szalay, A.; Fong, Y. A novel vaccinia virus with dual oncolytic and anti-angiogenic therapeutic effects against triple-negative breast cancer. Breast Cancer Res Treat. 2014, 148, 489-499.
doi: 10.1007/s10549-014-3180-7 URL |
| 68. |
Howells, A.; Marelli, G.; Lemoine, N. R.; Wang, Y. Oncolytic viruses-interaction of virus and tumor cells in the battle to eliminate cancer. Front Oncol. 2017, 7, 195.
doi: 10.3389/fonc.2017.00195 URL |
| 69. | Bulcha, J. T.; Wang, Y.; Ma, H.; Tai, P. W. L.; Gao, G. Viral vector platforms within the gene therapy landscape. Signal Transduct Target Ther. 2021, 6, 53. |
| 70. | Bezeljak, U. Cancer gene therapy goes viral: viral vector platforms come of age. Radiol Oncol. 2022, 56, 1-13. |
| 71. |
Cross, D.; Burmester, J. K. Gene therapy for cancer treatment: past, present and future. Clin Med Res. 2006, 4, 218-227.
doi: 10.3121/cmr.4.3.218 URL |
| 72. | Ginn, S. L.; Amaya, A. K.; Alexander, I. E.; Edelstein, M.; Abedi, M. R. Gene therapy clinical trials worldwide to 2017: an update. J Gene Med. 2018, 20, e3015. |
| 73. |
Miller, K. E.; Cassady, K. A.; Roth, J. C.; Clements, J.; Schieffer, K. M.; Leraas, K.; Miller, A. R.; Prasad, N.; Leavenworth, J. W.; Aban, I. B.; Whitley, R. J.; Gillespie, G. Y.; Mardis, E. R.; Markert, J. M. Immune activity and response differences of oncolytic viral therapy in recurrent glioblastoma: gene expression analyses of a phase IB study. Clin Cancer Res. 2022, 28, 498-506.
doi: 10.1158/1078-0432.CCR-21-2636 URL |
| 74. | Cui, C.; Wang, X.; Lian, B.; Ji, Q.; Zhou, L.; Chi, Z.; Si, L.; Sheng, X.; Kong, Y.; Yu, J.; Li, S.; Mao, L.; Tang, B.; Dai, J.; Yan, X.; Bai, X.; Andtbacka, R.; Guo, J. OrienX010, an oncolytic virus, in patients with unresectable stage IIIC-IV melanoma: a phase Ib study. J Immunother Cancer. 2022, 10, e004307. |
| 75. |
García, M.; Moreno, R.; Gil-Martin, M.; Cascallò, M.; de Olza, M. O.; Cuadra, C.; Piulats, J. M.; Navarro, V.; Domenech, M.; Alemany, R.; Salazar, R. A phase 1 trial of oncolytic adenovirus ICOVIR-5 administered intravenously to cutaneous and uveal melanoma patients. Hum Gene Ther. 2019, 30, 352-364.
doi: 10.1089/hum.2018.107 URL |
| 76. |
Lang, F. F.; Conrad, C.; Gomez-Manzano, C.; Yung, W. K. A.; Sawaya, R.; Weinberg, J. S.; Prabhu, S. S.; Rao, G.; Fuller, G. N.; Aldape, K. D.; Gumin, J.; Vence, L. M.; Wistuba, I.; Rodriguez-Canales, J.; Villalobos, P. A.; Dirven, C. M. F.; Tejada, S.; Valle, R. D.; Alonso, M. M.; Ewald, B.; Peterkin, J. J.; Tufaro, F.; Fueyo, J. Phase I study of DNX-2401 (Delta-24-RGD) oncolytic adenovirus: replication and immunotherapeutic effects in recurrent malignant glioma. J Clin Oncol. 2018, 36, 1419-1427.
doi: 10.1200/JCO.2017.75.8219 URL |
| 77. |
Müller, L. M. E.; Holmes, M.; Michael, J. L.; Scott, G. B.; West, E. J.; Scott, K. J.; Parrish, C.; Hall, K.; Stäble, S.; Jennings, V. A.; Cullen, M.; McConnell, S.; Langton, C.; Tidswell, E. L.; Shafren, D.; Samson, A.; Harrington, K. J.; Pandha, H.; Ralph, C.; Kelly, R. J.; Cook, G.; Melcher, A. A.; Errington-Mais, F. Plasmacytoid dendritic cells orchestrate innate and adaptive anti-tumor immunity induced by oncolytic coxsackievirus A21. J Immunother Cancer. 2019, 7, 164.
doi: 10.1186/s40425-019-0632-y |
| 78. |
Annels, N. E.; Mansfield, D.; Arif, M.; Ballesteros-Merino, C.; Simpson, G. R.; Denyer, M.; Sandhu, S. S.; Melcher, A. A.; Harrington, K. J.; Davies, B.; Au, G.; Grose, M.; Bagwan, I.; Fox, B.; Vile, R.; Mostafid, H.; Shafren, D.; Pandha, H. S. Phase I trial of an ICAM-1-targeted immunotherapeutic-coxsackievirus A21 (CVA21) as an oncolytic agent against non muscle-invasive bladder cancer. Clin Cancer Res. 2019, 25, 5818-5831.
doi: 10.1158/1078-0432.CCR-18-4022 URL |
| 79. |
Dispenzieri, A.; Tong, C.; LaPlant, B.; Lacy, M. Q.; Laumann, K.; Dingli, D.; Zhou, Y.; Federspiel, M. J.; Gertz, M. A.; Hayman, S.; Buadi, F.; O'Connor, M.; Lowe, V. J.; Peng, K. W.; Russell, S. J. Phase I trial of systemic administration of Edmonston strain of measles virus genetically engineered to express the sodium iodide symporter in patients with recurrent or refractory multiple myeloma. Leukemia. 2017, 31, 2791-2798.
doi: 10.1038/leu.2017.120 URL |
| 80. |
Beasley, G. M.; Nair, S. K.; Farrow, N. E.; Landa, K.; Selim, M. A.; Wiggs, C. A.; Jung, S. H.; Bigner, D. D.; True Kelly, A.; Gromeier, M.; Salama, A. K. Phase I trial of intratumoral PVSRIPO in patients with unresectable, treatment-refractory melanoma. J Immunother Cancer. 2021, 9, e002203.
doi: 10.1136/jitc-2020-002203 URL |
| 81. | Beasley, G. M.; Brown, M. C.; Farrow, N. E.; Landa, K.; Al-Rohil, R. N.; Selim, M. A.; Therien, A. D.; Jung, S. H.; Gao, J.; Boczkowski, D.; Holl, E. K.; Salama, A. K. S.; Bigner, D. D.; Gromeier, M.; Nair, S. K. Multimodality analysis confers a prognostic benefit of a T-cell infiltrated tumor microenvironment and peripheral immune status in patients with melanoma. J Immunother Cancer. 2022, 10, e005052. |
| 82. |
Lauer, U. M.; Schell, M.; Beil, J.; Berchtold, S.; Koppenhöfer, U.; Glatzle, J.; Königsrainer, A.; Möhle, R.; Nann, D.; Fend, F.; Pfannenberg, C.; Bitzer, M.; Malek, N. P. Phase I study of oncolytic vaccinia virus GL-ONC1 in patients with peritoneal carcinomatosis. Clin Cancer Res. 2018, 24, 4388-4398.
doi: 10.1158/1078-0432.CCR-18-0244 URL |
| 83. |
Minev, B. R.; Lander, E.; Feller, J. F.; Berman, M.; Greenwood, B. M.; Minev, I.; Santidrian, A. F.; Nguyen, D.; Draganov, D.; Killinc, M. O.; Vyalkova, A.; Kesari, S.; McClay, E.; Carabulea, G.; Marincola, F. M.; Butterfield, L. H.; Szalay, A. A. First-in-human study of TK-positive oncolytic vaccinia virus delivered by adipose stromal vascular fraction cells. J Transl Med. 2019, 17, 271.
doi: 10.1186/s12967-019-2011-3 |
| 84. |
Manyam, M.; Stephens, A. J.; Kennard, J. A.; LeBlanc, J.; Ahmad, S.; Kendrick, J. E.; Holloway, R. W. A phase 1b study of intraperitoneal oncolytic viral immunotherapy in platinum-resistant or refractory ovarian cancer. Gynecol Oncol. 2021, 163, 481-489.
doi: 10.1016/j.ygyno.2021.10.069 URL |
| 85. |
Mahalingam, D.; Wilkinson, G. A.; Eng, K. H.; Fields, P.; Raber, P.; Moseley, J. L.; Cheetham, K.; Coffey, M.; Nuovo, G.; Kalinski, P.; Zhang, B.; Arora, S. P.; Fountzilas, C. Pembrolizumab in combination with the oncolytic virus pelareorep and chemotherapy in patients with advanced pancreatic adenocarcinoma: a phase ib study. Clin Cancer Res. 2020, 26, 71-81.
doi: 10.1158/1078-0432.CCR-19-2078 URL |
| 86. |
Parakrama, R.; Fogel, E.; Chandy, C.; Augustine, T.; Coffey, M.; Tesfa, L.; Goel, S.; Maitra, R. Immune characterization of metastatic colorectal cancer patients post reovirus administration. BMC Cancer. 2020, 20, 569.
doi: 10.1186/s12885-020-07038-2 |
| 87. |
Geletneky, K.; Hajda, J.; Angelova, A. L.; Leuchs, B.; Capper, D.; Bartsch, A. J.; Neumann, J. O.; Schöning, T.; Hüsing, J.; Beelte, B.; Kiprianova, I.; Roscher, M.; Bhat, R.; von Deimling, A.; Brück, W.; Just, A.; Frehtman, V.; Löbhard, S.; Terletskaia-Ladwig, E.; Fry, J.; Jochims, K.; Daniel, V.; Krebs, O.; Dahm, M.; Huber, B.; Unterberg, A.; Rommelaere, J. Oncolytic H-1 parvovirus shows safety and signs of immunogenic activity in a first phase I/IIa glioblastoma trial. Mol Ther. 2017, 25, 2620-2634.
doi: 10.1016/j.ymthe.2017.08.016 URL |
| 88. | Burke, M. J.; Ahern, C.; Weigel, B. J.; Poirier, J. T.; Rudin, C. M.; Chen, Y.; Cripe, T. P.; Bernhardt, M. B.; Blaney, S. M. Phase I trial of Seneca Valley Virus (NTX-010) in children with relapsed/refractory solid tumors: a report of the Children's Oncology Group. Pediatr Blood Cancer. 2015, 62, 743-750. |
| 89. |
Zhang, B.; Huang, J.; Tang, J.; Hu, S.; Luo, S.; Luo, Z.; Zhou, F.; Tan, S.; Ying, J.; Chang, Q.; Zhang, R.; Geng, C.; Wu, D.; Gu, X.; Liu, B. Intratumoral OH2, an oncolytic herpes simplex virus 2, in patients with advanced solid tumors: a multicenter, phase I/II clinical trial. J Immunother Cancer. 2021, 9, e002224.
doi: 10.1136/jitc-2020-002224 URL |
| 90. |
Todo, T.; Ino, Y.; Ohtsu, H.; Shibahara, J.; Tanaka, M. A phase I/II study of triple-mutated oncolytic herpes virus G47∆ in patients with progressive glioblastoma. Nat Commun. 2022, 13, 4119.
doi: 10.1038/s41467-022-31262-y |
| 91. |
Todo, T.; Ito, H.; Ino, Y.; Ohtsu, H.; Ota, Y.; Shibahara, J.; Tanaka, M. Intratumoral oncolytic herpes virus G47∆ for residual or recurrent glioblastoma: a phase 2 trial. Nat Med. 2022, 28, 1630-1639.
doi: 10.1038/s41591-022-01897-x |
| 92. | Monga, V.; Miller, B. J.; Tanas, M.; Boukhar, S.; Allen, B.; Anderson, C.; Stephens, L.; Hartwig, S.; Varga, S.; Houtman, J.; Wang, L.; Zhang, W.; Jaber, O.; Thomason, J.; Kuehn, D.; Rajput, M.; Metz, C.; Zamba, K. D.; Mott, S.; Abanonu, C.; Bhatia, S.; Milhem, M. Intratumoral talimogene laherparepvec injection with concurrent preoperative radiation in patients with locally advanced soft-tissue sarcoma of the trunk and extremities: phase IB/II trial. J Immunother Cancer. 2021, 9, e003119. |
| 93. |
Kai, M.; Marx, A. N.; Liu, D. D.; Shen, Y.; Gao, H.; Reuben, J. M.; Whitman, G.; Krishnamurthy, S.; Ross, M. I.; Litton, J. K.; Lim, B.; Ibrahim, N.; Kogawa, T.; Ueno, N. T. A phase II study of talimogene laherparepvec for patients with inoperable locoregional recurrence of breast cancer. Sci Rep. 2021, 11, 22242.
doi: 10.1038/s41598-021-01473-2 |
| 94. |
Packiam, V. T.; Lamm, D. L.; Barocas, D. A.; Trainer, A.; Fand, B.; Davis, R. L., 3rd; Clark, WL.; Kroeger, M.; Dumbadze, I.; Chamie, K.; Kader, A. K.; Curran, D.; Gutheil, J.; Kuan, A.; Yeung, A. W.; Steinberg, G. D. An open label, single-arm, phase II multicenter study of the safety and efficacy of CG0070 oncolytic vector regimen in patients with BCG-unresponsive non-muscle-invasive bladder cancer: Interim results. Urol Oncol. 2018, 36, 440-447.
doi: 10.1016/j.urolonc.2017.07.005 URL |
| 95. |
Andtbacka, R. H. I.; Curti, B.; Daniels, G. A.; Hallmeyer, S.; Whitman, E. D.; Lutzky, J.; Spitler, L. E.; Zhou, K.; Bommareddy, P. K.; Grose, M.; Wang, M.; Wu, C.; Kaufman, H. L. Clinical responses of oncolytic coxsackievirus A21 (V937) in patients with unresectable melanoma. J Clin Oncol. 2021, 39, 3829-3838.
doi: 10.1200/JCO.20.03246 URL |
| 96. |
Toulmonde, M.; Cousin, S.; Kind, M.; Guegan, J. P.; Bessede, A.; Le Loarer, F.; Perret, R.; Cantarel, C.; Bellera, C.; Italiano, A. Randomized phase 2 trial of intravenous oncolytic virus JX-594 combined with low-dose cyclophosphamide in patients with advanced soft-tissue sarcoma. J Hematol Oncol. 2022, 15, 149.
doi: 10.1186/s13045-022-01370-9 |
| 97. |
Jonker, D. J.; Tang, P. A.; Kennecke, H.; Welch, S. A.; Cripps, M. C.; Asmis, T.; Chalchal, H.; Tomiak, A.; Lim, H.; Ko, Y. J.; Chen, E. X.; Alcindor, T.; Goffin, J. R.; Korpanty, G. J.; Feilotter, H.; Tsao, M. S.; Theis, A.; Tu, D.; Seymour, L. A randomized phase II study of FOLFOX6/Bevacizumab with or without pelareorep in patients with metastatic colorectal cancer: IND.210, a Canadian Cancer Trials Group Trial. Clin Colorectal Cancer. 2018, 17:231-239.e7.
doi: 10.1016/j.clcc.2018.03.001 URL |
| 98. |
Bradbury, P. A.; Morris, D. G.; Nicholas, G.; Tu, D.; Tehfe, M.; Goffin, J. R.; Shepherd, F. A.; Gregg, R. W.; Rothenstein, J.; Lee, C.; Kuruvilla, S.; Keith, B. D.; Torri, V.; Blais, N.; Hao, D.; Korpanty, G. J.; Goss, G.; Melosky, B. L.; Mates, M.; Leighl, N.; Ayoub, J. P.; Sederias, J.; Feilotter, H.; Seymour, L.; Laurie, S. A. Canadian Cancer Trials Group CCTG IND211: A randomized trial of pelareorep Reolysin in patients with previously treated advanced or metastatic non-small cell lung cancer receiving standard salvage therapy. Lung Cancer. 2018, 120, 142-148.
doi: 10.1016/j.lungcan.2018.03.005 URL |
| 99. |
Bernstein, V.; Ellard, S. L.; Dent, S. F.; Tu, D.; Mates, M.; Dhesy-Thind, S. K.; Panasci, L.; Gelmon, K. A.; Salim, M.; Song, X.; Clemons, M.; Ksienski, D.; Verma, S.; Simmons, C.; Lui, H.; Chi, K.; Feilotter, H.; Hagerman, L. J.; Seymour, L. A randomized phase II study of weekly paclitaxel with or without pelareorep in patients with metastatic breast cancer: final analysis of Canadian Cancer Trials Group IND.213. Breast Cancer Res Treat. 2018, 167, 485-493.
doi: 10.1007/s10549-017-4538-4 URL |
| 100. |
Hajda, J.; Leuchs, B.; Angelova, A. L.; Frehtman, V.; Rommelaere, J.; Mertens, M.; Pilz, M.; Kieser, M.; Krebs, O.; Dahm, M.; Huber, B.; Engeland, C. E.; Mavratzas, A.; Hohmann, N.; Schreiber, J.; Jäger, D.; Halama, N.; Sedlaczek, O.; Gaida, M. M.; Daniel, V.; Springfeld, C.; Ungerechts, G. Phase 2 trial of oncolytic H-1 parvovirus therapy shows safety and signs of immune system activation in patients with metastatic pancreatic ductal adenocarcinoma. Clin Cancer Res. 2021, 27, 5546-5556.
doi: 10.1158/1078-0432.CCR-21-1020 URL |
| 101. |
Schenk, E. L.; Mandrekar, S. J.; Dy, G. K.; Aubry, M. C.; Tan, A. D.; Dakhil, S. R.; Sachs, B. A.; Nieva, J. J.; Bertino, E.; Lee Hann, C.; Schild, S. E.; Wadsworth, T. W.; Adjei, A. A.; Molina, J. R. A Randomized Double-Blind Phase II Study of the Seneca Valley Virus (NTX-010) versus Placebo for Patients with Extensive-Stage SCLC (ES SCLC) who were stable or responding after at least four cycles of platinum-based chemotherapy: North Central Cancer Treatment Group (Alliance) N0923 Study. J Thorac Oncol. 2020, 15, 110-119.
doi: 10.1016/j.jtho.2019.09.083 URL |
| 102. |
Andtbacka, R. H. I.; Collichio, F.; Harrington, K. J.; Middleton, M. R.; Downey, G.; Öhrling, K.; Kaufman, H. L. Final analyses of OPTiM: a randomized phase III trial of talimogene laherparepvec versus granulocyte-macrophage colony-stimulating factor in unresectable stage III-IV melanoma. J Immunother Cancer. 2019, 7, 145.
doi: 10.1186/s40425-019-0623-z |
| 103. |
Chesney, J.; Awasthi, S.; Curti, B.; Hutchins, L.; Linette, G.; Triozzi, P.; Tan, M. C. B.; Brown, R. E.; Nemunaitis, J.; Whitman, E.; Windham, C.; Lutzky, J.; Downey, G. F.; Batty, N.; Amatruda, T. Phase IIIb safety results from an expanded-access protocol of talimogene laherparepvec for patients with unresected, stage IIIB-IVM1c melanoma. Melanoma Res. 2018, 28, 44-51.
doi: 10.1097/CMR.0000000000000399 URL |
| 104. |
Chung, Y. H.; Cai, H.; Steinmetz, N. F. Viral nanoparticles for drug delivery, imaging, immunotherapy, and theranostic applications. Adv Drug Deliv Rev. 2020, 156, 214-235.
doi: 10.1016/j.addr.2020.06.024 URL |
| 105. | Steinmetz, N. F. Biological and evolutionary concepts for nanoscale engineering: Viruses as natural nanoparticles have great potential for a wide range of nanoscale products. EMBO Rep. 2019, 20, e48806. |
| 106. |
Bai, Y.; Hui, P.; Du, X.; Su, X. Updates to the antitumor mechanism of oncolytic virus. Thoracic cancer. 2019, 10, 1031-1035.
doi: 10.1111/tca.2019.10.issue-5 URL |
| 107. |
Pesonen, S.; Kangasniemi, L.; Hemminki, A. Oncolytic adenoviruses for the treatment of human cancer: focus on translational and clinical data. Mol Pharm. 2011, 8, 12-28.
doi: 10.1021/mp100219n URL |
| 108. |
Barnard, A. S. Nanohazards: knowledge is our first defence. Nat Mater. 2006, 5, 245-248.
doi: 10.1038/nmat1615 |
| 109. |
Ran, L.; Tan, X.; Li, Y.; Zhang, H.; Ma, R.; Ji, T.; Dong, W.; Tong, T.; Liu, Y.; Chen, D.; Yin, X.; Liang, X.; Tang, K.; Ma, J.; Zhang, Y.; Cao, X.; Hu, Z.; Qin, X.; Huang, B. Delivery of oncolytic adenovirus into the nucleus of tumorigenic cells by tumor microparticles for virotherapy. Biomaterials. 2016, 89, 56-66.
doi: 10.1016/j.biomaterials.2016.02.025 URL |
| 110. |
Hong, J.; Yun, C. O. Overcoming the limitations of locally administered oncolytic virotherapy. BMC Biomed Eng. 2019, 1, 17.
doi: 10.1186/s42490-019-0016-x |
| 111. |
Francini, N.; Cochrane, D.; Illingworth, S.; Purdie, L.; Mantovani, G.; Fisher, K.; Seymour, L. W.; Spain, S. G.; Alexander, C. Polyvalent diazonium polymers provide efficient protection of oncolytic adenovirus enadenotucirev from neutralizing antibodies while maintaining biological activity in vitro and in vivo. Bioconjug Chem. 2019, 30, 1244-1257.
doi: 10.1021/acs.bioconjchem.9b00189 URL |
| 112. |
Green, N. K.; Hale, A.; Cawood, R.; Illingworth, S.; Herbert, C.; Hermiston, T.; Subr, V.; Ulbrich, K.; van Rooijen, N.; Seymour, L. W.; Fisher, K. D. Tropism ablation and stealthing of oncolytic adenovirus enhances systemic delivery to tumors and improves virotherapy of cancer. Nanomedicine (Lond). 2012, 7, 1683-1695.
doi: 10.2217/nnm.12.50 URL |
| 113. |
Garofalo, M.; Bellato, F.; Magliocca, S.; Malfanti, A.; Kuryk, L.; Rinner, B.; Negro, S.; Salmaso, S.; Caliceti, P.; Mastrotto, F. Polymer coated oncolytic adenovirus to selectively target hepatocellular carcinoma cells. Pharmaceutics. 2021, 13, 949.
doi: 10.3390/pharmaceutics13070949 URL |
| 114. |
Semashko, V. V.; Pudovkin, M. S.; Cefalas, A. C.; Zelenikhin, P. V.; Gavriil, V. E.; Nizamutdinov, A. S.; Kollia, Z.; Ferraro, A.; Sarantopoulou, E. Tiny rare-earth fluoride nanoparticles activate tumour cell growth via electrical polar interactions. Nanoscale Res Lett. 2018, 13, 370.
doi: 10.1186/s11671-018-2775-z |
| 115. |
Choi, Y. J.; Kang, S. J.; Kim, Y. J.; Lim, Y. B.; Chung, H. W. Comparative studies on the genotoxicity and cytotoxicity of polymeric gene carriers polyethylenimine (PEI) and polyamidoamine (PAMAM) dendrimer in Jurkat T-cells. Drug Chem Toxicol. 2010, 33, 357-366.
doi: 10.3109/01480540903493507 URL |
| 116. |
Yang, C.; Cheng, W.; Teo, P. Y.; Engler, A. C.; Coady, D. J.; Hedrick, J. L.; Yang, Y. Y. Mitigated cytotoxicity and tremendously enhanced gene transfection efficiency of PEI through facile one-step carbamate modification. Adv Healthc Mater. 2013, 2, 1304-1308.
doi: 10.1002/adhm.v2.10 URL |
| 117. |
Aoyama, K.; Kuroda, S.; Morihiro, T.; Kanaya, N.; Kubota, T.; Kakiuchi, Y.; Kikuchi, S.; Nishizaki, M.; Kagawa, S.; Tazawa, H.; Fujiwara, T. Liposome-encapsulated plasmid DNA of telomerase-specific oncolytic adenovirus with stealth effect on the immune system. Sci Rep. 2017, 7, 14177.
doi: 10.1038/s41598-017-14717-x |
| 118. |
Record, M.; Silvente-Poirot, S.; Poirot, M.; Wakelam, M. J. O. Extracellular vesicles: lipids as key components of their biogenesis and functions. J Lipid Res. 2018, 59, 1316-1324.
doi: 10.1194/jlr.E086173 URL |
| 119. |
Huang, H.; Sun, M.; Liu, M.; Pan, S.; Liu, P.; Cheng, Z.; Li, J.; Xu, H.; Liu, F.; Pang, Z. Full encapsulation of oncolytic virus using hybrid erythroctye-liposome membranes for augmented anti-refractory tumor effectiveness. Nano Today. 2022, 47, 101671.
doi: 10.1016/j.nantod.2022.101671 URL |
| 120. |
Wang, Y.; Huang, H.; Zou, H.; Tian, X.; Hu, J.; Qiu, P.; Hu, H.; Yan, G. Liposome encapsulation of oncolytic virus M1 to reduce immunogenicity and immune clearance in vivo. Mol Pharm. 2019, 16, 779-785.
doi: 10.1021/acs.molpharmaceut.8b01046 URL |
| 121. | Lin, Y.; Zhang, H.; Liang, J.; Li, K.; Zhu, W.; Fu, L.; Wang, F.; Zheng, X.; Shi, H.; Wu, S.; Xiao, X.; Chen, L.; Tang, L.; Yan, M.; Yang, X.; Tan, Y.; Qiu, P.; Huang, Y.; Yin, W.; Su, X.; Hu, H.; Hu, J.; Yan, G. Identification and characterization of alphavirus M1 as a selective oncolytic virus targeting ZAP-defective human cancers. Proc Natl Acad Sci U S A. 2014, 111, E4504-4512. |
| 122. |
Huang, C. H.; Dong, T.; Phung, A. T.; Shah, J. R.; Larson, C.; Sanchez, A. B.; Blair, S. L.; Oronsky, B.; Trogler, W. C.; Reid, T.; Kummel, A. C. Full remission of CAR-deficient tumors by DOTAP-folate liposome encapsulation of adenovirus. ACS Biomater Sci Eng. 2022, 8, 5199-5209.
doi: 10.1021/acsbiomaterials.2c00966 URL |
| 123. |
Garofalo, M.; Saari, H.; Somersalo, P.; Crescenti, D.; Kuryk, L.; Aksela, L.; Capasso, C.; Madetoja, M.; Koskinen, K.; Oksanen, T.; Mäkitie, A.; Jalasvuori, M.; Cerullo, V.; Ciana, P.; Yliperttula, M. Antitumor effect of oncolytic virus and paclitaxel encapsulated in extracellular vesicles for lung cancer treatment. J Control Release. 2018, 283, 223-234.
doi: 10.1016/j.jconrel.2018.05.015 URL |
| 124. |
Garofalo, M.; Villa, A.; Rizzi, N.; Kuryk, L.; Rinner, B.; Cerullo, V.; Yliperttula, M.; Mazzaferro, V.; Ciana, P. Extracellular vesicles enhance the targeted delivery of immunogenic oncolytic adenovirus and paclitaxel in immunocompetent mice. J Control Release. 2019, 294, 165-175.
doi: 10.1016/j.jconrel.2018.12.022 URL |
| 125. |
Chyzy, A.; Tomczykowa, M.; Plonska-Brzezinska, M. E. Hydrogels as potential nano-, micro- and macro-scale systems for controlled drug delivery. Materials (Basel). 2020, 13, 188.
doi: 10.3390/ma13010188 URL |
| 126. |
Le, T. M. D.; Jung, B. K.; Li, Y.; Duong, H. T. T.; Nguyen, T. L.; Hong, J. W.; Yun, C. O.; Lee, D. S. Physically crosslinked injectable hydrogels for long-term delivery of oncolytic adenoviruses for cancer treatment. Biomater Sci. 2019, 7, 4195-4207.
doi: 10.1039/C9BM00992B URL |
| 127. |
Deng, S.; Iscaro, A.; Zambito, G.; Mijiti, Y.; Minicucci, M.; Essand, M.; Lowik, C.; Muthana, M.; Censi, R.; Mezzanotte, L.; Di Martino, P. Development of a new hyaluronic acid based redox-responsive nanohydrogel for the encapsulation of oncolytic viruses for cancer immunotherapy. Nanomaterials (Basel). 2021, 11, 144.
doi: 10.3390/nano11010144 URL |
| 128. |
Hadryś, A.; Sochanik, A.; McFadden, G.; Jazowiecka-Rakus, J. Mesenchymal stem cells as carriers for systemic delivery of oncolytic viruses. Eur J Pharmacol. 2020, 874, 172991.
doi: 10.1016/j.ejphar.2020.172991 URL |
| 129. |
Jazowiecka-Rakus, J.; Sochanik, A.; Rusin, A.; Hadryś, A.; Fidyk, W.; Villa, N.; Rahman, M. M.; Chmielik, E.; Franco, L. S.; McFadden, G. Myxoma virus-loaded mesenchymal stem cells in experimental oncolytic therapy of murine pulmonary melanoma. Mol Ther Oncolytics. 2020, 18, 335-350.
doi: 10.1016/j.omto.2020.07.003 URL |
| 130. |
Fares, J.; Ahmed, A. U.; Ulasov, I. V.; Sonabend, A. M.; Miska, J.; Lee-Chang, C.; Balyasnikova, I. V.; Chandler, J. P.; Portnow, J.; Tate, M. C.; Kumthekar, P.; Lukas, R. V.; Grimm, S. A.; Adams, A. K.; Hébert, C. D.; Strong, T. V.; Amidei, C.; Arrieta, V. A.; Zannikou, M.; Horbinski, C.; Zhang, H.; Burdett, K. B.; Curiel, D. T.; Sachdev, S.; Aboody, K. S.; Stupp, R.; Lesniak, M. S. Neural stem cell delivery of an oncolytic adenovirus in newly diagnosed malignant glioma: a first-in-human, phase 1, dose-escalation trial. Lancet Oncol. 2021, 22, 1103-1114.
doi: 10.1016/S1470-2045(21)00245-X URL |
| 131. |
Mooney, R.; Majid, A. A.; Batalla-Covello, J.; Machado, D.; Liu, X.; Gonzaga, J.; Tirughana, R.; Hammad, M.; Lesniak, M. S.; Curiel, D. T.; Aboody, K. S. Enhanced delivery of oncolytic adenovirus by neural stem cells for treatment of metastatic ovarian cancer. Mol Ther Oncolytics. 2019, 12, 79-92.
doi: 10.1016/j.omto.2018.12.003 URL |
| 132. | Hammad, M.; Cornejo, Y. R.; Batalla-Covello, J.; Majid, A. A.; Burke, C.; Liu, Z.; Yuan, Y. C.; Li, M.; Dellinger, T. H.; Lu, J.; Chen, N. G.; Fong, Y.; Aboody, K. S.; Mooney, R. Neural stem cells improve the delivery of oncolytic chimeric orthopoxvirus in a metastatic ovarian cancer model. Mol Ther Oncolytics. 2020, 18, 326-334. |
| 133. | Guo, Y.; Zhang, Z.; Xu, X.; Xu, Z.; Wang, S.; Huang, D.; Li, Y.; Mou, X.; Liu, F.; Xiang, C. Menstrual blood-derived stem cells as delivery vehicles for oncolytic adenovirus virotherapy for colorectal cancer. Stem Cells Dev. 2019, 28, 882-896. |
| 134. | Santos, J.; Heiniö, C.; Quixabeira, D.; Zafar, S.; Clubb, J.; Pakola, S.; Cervera-Carrascon, V.; Havunen, R.; Kanerva, A.; Hemminki, A. Systemic delivery of oncolytic adenovirus to tumors using tumor-infiltrating lymphocytes as carriers. Cells. 2021, 10, 978. |
| 135. | Liu, X.; Xu, J.; Yao, T.; Ding, J.; Li, S.; Su, R.; Zhang, H.; Li, H.; Yue, Q.; Gao, X. Cryo-shocked cancer cells as an oncolytic adenovirus reservoir for glioblastoma immunotherapy. ACS Appl Mater Interfaces. 2023, 15, 67-76. |
| 136. | Breitbach, C. J.; Arulanandam, R.; De Silva, N.; Thorne, S. H.; Patt, R.; Daneshmand, M.; Moon, A.; Ilkow, C.; Burke, J.; Hwang, T. H.; Heo, J.; Cho, M.; Chen, H.; Angarita, F. A.; Addison, C.; McCart, J. A.; Bell, J. C.; Kirn, D. H. Oncolytic vaccinia virus disrupts tumor-associated vasculature in humans. Cancer Res. 2013, 73, 1265-1275. |
| [1] | Yiqiang Hu, Yuan Xiong, Ranyang Tao, Hang Xue, Lang Chen, Ze Lin, Adriana C. Panayi, Bobin Mi, Guohui Liu. Advances and perspective on animal models and hydrogel biomaterials for diabetic wound healing [J]. Biomaterials Translational, 2022, 3(3): 188-200. |
| [2] | Hongtao Yang, Wenjiao Lin, Yufeng Zheng. Advances and perspective on the translational medicine of biodegradable metals [J]. Biomaterials Translational, 2021, 2(3): 177-187. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||