Introduction
When natural teeth are missing, a prosthesis is used to restore masticatory function. Options include a fixed denture, removable denture, or dental implant. Considering the disadvantages of fixed and removable dentures, such as normal tooth damage and strong foreign body sensation, dental implants are the current standard for the replacement of missing teeth. Implants have been widely applied in a variety of cases including surgical replacement of lost teeth or restoration of oral function.1 Although different types of implants have been developed previously, endosteal implants are currently the most widely used.2
However, endosteal implants still have some shortcomings. For example, in the event of inflammation developing around the implant, a dental implant may lose stability due to the small contact area between the implant and the bone, which is the main reason for dental implant failure.3 Some surgical complications such as destruction of the tooth groove nerve or maxillary sinus will also occur because the dental implant is implanted in the jaw. Moreover, a significant factor that limits the clinical application of an endosteal implant is the restrictive necessity for alveolar bone mechanical support.4 Successful implant placement requires sufficient alveolar bone volume in order to ensure implant stability and osseointegration, but the extraction of teeth will result in loss of alveolar ridge width and height within three years.5 This bone loss is exacerbated if the tooth is removed traumatically or if there are pre-existing endodontic or periodontal pathologies. Eventually, the height of the alveolar ridge will be insufficient to contain the length of the implant.
To overcome these problems, this study puts forward a novel dental implant design: the sub-scaffold dental implant system (SDIS), which is implanted just below the mucous membranes, without destroying the jaw. This implant system is composed of a metal implant and an osteogenic scaffold. The metal implant is used to attach the dentures, while the osteogenic scaffold will eventually transform into bone on the alveolar ridge, augmenting vertical bone at the same time. Vertical ridge augmentation remains a challenge in reconstruction of the atrophic maxilla and mandible. Various biomaterials and techniques have been developed to solve this problem including onlay bone grafts, guided bone regeneration, bone splitting for ridge expansion, distraction osteogenesis, revascularised flaps and sinus floor elevation via a lateral approach.6 Every surgical procedure has its advantages and disadvantages and it is difficult to demonstrate that one surgical procedure offers better outcomes than another.7, 8 Autografts are considered to be the ‘gold standard’ grafting material for reconstruction of the vertical ridge.9 However, they have certain drawbacks, including the necessity for a second stage surgery, the high morbidity and blood loss at the donor site, high resorption rate of the graft and limited bone availability.10
The use of bone formation materials instead of autografts is a better choice to achieve vertical ridge augmentation.11, 12 Recently, some studies have reported augmentation of the alveolar ridge by using osteogenic materials including tricalcium phosphate,13-15 hydroxyapatite,16 hydrogel17, 18 and anorganic bovine bone.19, 20 However, most of these methods required the addition of biomolecules or rapid establishment of a blood supply from the jaw by creating bone defects, which would not be suitable for actual clinical application or would result in additional trauma.
In our dental implant design, new bone is expected to be formed outside of the alveolar ridge without creating any bone defect. This requires the biomaterial to have excellent bone-forming ability. Micro-nano bioactive glass (MNBG) is a good choice for this application due to its enhanced biocompatibility, osteoconductive and osteoinductive properties.21 It has been widely applied in the clinic as a bone filler, bone repair material and adjuvant in bone grafts.22 The gene activation function is one of its great features which distinguishes it from other bone repair materials. When it is in contact with body fluids, ions (Si, Ca, P, etc.) can be quickly released from bioactive glass, which activate osteogenesis-related signalling pathways.23 In our current work, the SDIS was developed by fabricating an MNBG scaffold and metal implant. The SDISs were implanted into the sub-epicranial aponeuroses of Sprague-Dawley rats. After testing the denture repair effect, the presence of new bone formation was further investigated by micro-CT, scanning electron microscopy (SEM) and histology.
Methods
Fabrication of SDIS
Preparation of metal implants by selective laser melting
The SDIS was made up of two parts: a metal implant and an osteogenic scaffold. The metal implant was fabricated through a selective laser melting process. To fabricate the metal implants, 316 L stainless steel powders provided by Renishaw PLC (Wotton-under-Edge, UK), with a mean diameter of 30 μm, were used as raw material and the metal implant models were designed by computer-aided design (CAD). Selective laser melting was performed using EVOProject (Renishaw PLC) with an SPI red POWER 200 W ytterbium fibre laser, an automatic powder layering system, an argon gas protection system and a process control system. The surface topography of the stainless steel implant was tested by SEM (DSM 982-Gemini, Zeiss, Oberkochen, Germany).
Fabrication of MNBG scaffolds
MNBG microspheres, with a molar ratio of SiO2:CaO:P2O5 = 80:15:5, were synthesised by the sol-gel co-template method according to our previous reports.24 The initial paste for three-dimensional (3D) printing was prepared by mixing bioactive glass microspheres together with 10% polyvinyl alcohol (PVA) solution in a mass ratio of 1:1. The scaffolds were fabricated using a 4th generation 3D-Bioplotter system (EnvisionTEC GmbH, Gladbeck, Germany) under the guidance of supporting computer workstations. The desired scaffold models (10 mm × 10 mm × 1.8 mm) were designed by CAD. The paste was extruded through a conical plastic nozzle with an interior diameter of 200 µm with an interval between strands of 400 μm at room temperature. After drying at 30°C for two days, the scaffolds were cut into 8 mm diameter discs. Finally, a 1.8 mm diameter hole was made in the centre of each disc using a dental drill, which was used to accommodate the metal implant. The morphology of the scaffolds was observed using SEM.
Animal model
Six Sprague-Dawley rats (male, 200-250 g) were purchased from the Laboratory Animal Centre, South China Medical College and used as animal models to test the effect of implanting SDISs. All animal procedures were performed following a protocol approved by the Animal Care Committee of Guangdong Pharmaceutical University (approval No. 2017370) and adequate measures were taken to minimise pain and discomfort to the animals.
Surgical procedure
SDISs were implanted under the epicranial aponeurosis of the skull. Before animal surgery, the SDISs were assembled together and sterilised by gamma irradiation. After general anaesthesia with 10% chloral hydrate, the hair was shaved from the head of each rat. Then the cutaneous surface was disinfected with povidone iodine solution prior to the operation. A 1 cm long full depth incision was made on the calvaria in a coronal direction and the epicranial aponeurosis was separated from the bone surface by tunnelling dissection. One SDIS was inserted into the subgaleal space, which was then closed with sutures.
Evaluation of the repair effect
At 6 weeks after surgery, a 0.5 cm long full-depth incision was made at the most prominent part of the skull, and the metal implant was exposed by blunt dissection. A stainless steel connecting rod (diameter 0.8 mm, length 4 mm) was used to connect the implant and a false tooth. One end of the connecting rod was inserted into a hole in the metal implant. Then, a plastic tooth was fixed onto the other end of the connecting rod.
A further 6 weeks after this procedure, all the animals were sacrificed using an overdose of sodium pentobarbital. The SDIS and surrounding bone tissue of the skull were harvested. All the tissues were fixed in 10% phosphate-buffered formalin for 5 days before analysis.
Micro-CT analysis
Specimens were examined by a micro-CT (ZKKS-MCT-SharpII, Zhongkekaisheng Medical Technology Co., Ltd., Guangzhou, China) operated at a voltage of 60 kVp and an electric current of 67 mA. The voxel size after reconstruction was 25 μm × 25 μm × 25 μm. Based on the micro-CT results, three-dimensional images were reconstructed by MIMICS® (interactive medical image control system; Materialise Co., Leuven, Belgium). Different substances were distinguished according to their different densities which were indicated by different colours.
Histological examination
Following micro-CT scanning, the samples were cut into two halves on the periphery of the metal implant. The metal-free part of each sample was decalcified in 10% ethylenediaminetetraacetic acid for 4 weeks and then embedded in paraffin parallel to the cut surface. Serial cross-sections of decalcified samples were sectioned for Masson’s trichrome staining according to the manufacturer’s instructions. Images were acquired using a Zeiss Axion light microscope (Axioskop 40 FL, Zeiss) and a colour video camera (Soft Imaging System, Muenster, Germany). All images were captured at 5× magnification using ImagePro software (Media Cybernetics, Rockville, MD, USA) and merged to give a composite of the whole slice.
Statistical analysis
Results are expressed as mean ± standard deviation (SD). Statistical analysis was carried out using one-way analysis of variance. A P value less than 0.05 was considered to be statistically significant.
Results
Characterization of SDIS
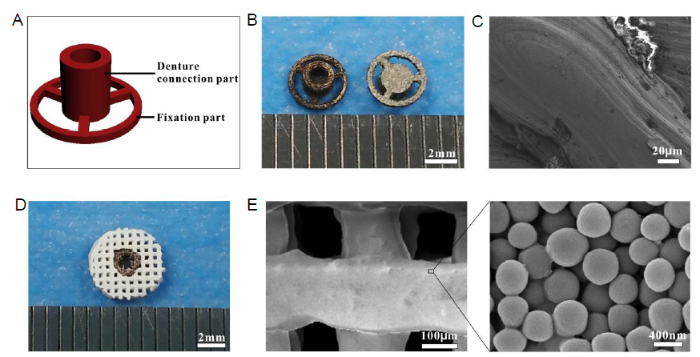
The SDIS consisted of two parts: a metal implant and an MNBG scaffold. The metal implant was composed of two parts with different functions, as shown in the CAD model (Figure 1A). The denture connection part was used to connect to the upper denture through the centre hole, which was similar to the neck of an endosteal implant. The fixation part was placed in contact with the newly-formed bone when SDIS was implanted and acted to fix the implant in place due to the large extension area. Figure 1B shows a digital image of two different surfaces of the metal implant. By comparing with the CAD model, it can be seen that the metal implant maintained the designed shape after the selective laser melting process. From the SEM image (Figure 1C), some print traces could be found on the surface of the metal implant. Figure 1D shows a digital image of the complete assembled SDIS. The MNBG scaffold was manufactured by the 3D printing method, then a hole with a diameter of 1.8 mm was created in the centre of the disc, which would match the denture connection part of the metal implant. An SEM image of the bioactive glass scaffold is shown in Figure 1E and the enlarged part of this image confirmed that the scaffold was composed of MNBG microspheres.
Figure 1.
Figure 1.
Composition and characterization of the SDIS. (A) A CAD model of the metal implant which consists of two parts: a denture connection part and a fixation part. (B) Digital photos of the two opposite surfaces of the metal implant, created by the SLM process. (C) SEM image of the metal implant. (D) Digital photo of the SDIS created by assembling the metal implant and MNBG scaffold together. (E) SEM image of the MNBG scaffold. Scale bars: 2 mm in B and D, 20 μm in C, 200 μm in E, 400 nm in enlarge part. CAD: computer-aided design; MNBG: micro-nano bioactive glass; SDIS: scaffold dental implant system; SEM: scanning electron microscopy; SLM: selective laser melting.
Clinical observations and denture repair effect
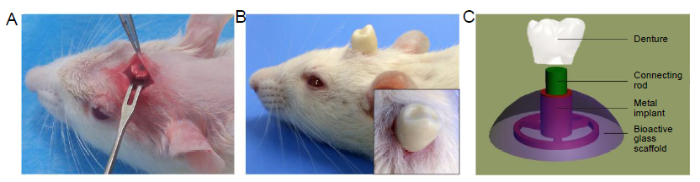
The SDIS was implanted into the sub-epicranial aponeurosis of Sprague-Dawley rats as shown in Figure 2A. All animals used in this experiment survived for 6 weeks after implantation. SDISs were closely attached to the skull without any movement for one week. The ultimate purpose of this study was to develop a method to repair missing teeth using this dental implant design. The reparative effect of the SDIS was tested as shown in Figure 2B. The denture had firmly bonded to the connecting rod without any movement. The repair principle was shown in Figure 2C, which was similar to the endosteal implant. One end of the connecting rod was fixed into the centre hole of the metal implant, and the other end was fixed into the dental crown model.
Figure 2.
Figure 2.
(A) Surgical placement of the SDIS implanted into the sub-epicranial aponeurosis; (B) Digital image of the repair effect and (insert) close-up of the top view; (C) Schematic illustration of the SDIS: the centre hole of the metal implant and the denture are joined together by a connecting rod. SDIS: scaffold dental implant system.
New bone formation
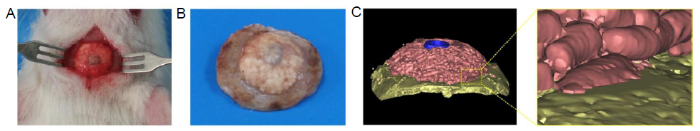
Photographs of the SDIS and surrounding tissue are shown in Figure 3A and B. The residual materials had been combined closely with the skull without any mobility, and some blood vessels could be found on the surface of the residual bioactive glass scaffold. Figure 3C shows a 3D-reconstructed image, using micro-CT analysis, of the SDIS together with surrounding tissues and residual MNBG scaffold after implantation for 6 weeks. Some short rod-shaped scaffold fibres can be recognised on the surface of the residual material. The implant material had been closely integrated with the skull, as shown in the enlarged image.
Figure 3.
Figure 3.
(A) Digital photo of the reparative effect. (B) Residual SDIS and surrounding bone tissue at week 6. (C) Micro-CT analysis of 3-dimensional reconstructed images of SDIS and surrounding tissue after implantation for 6 weeks. A magnified image of the join between implant and bone, showed good integration. Pink indicates residual bioactive glass scaffold, blue indicates the metal implant, and yellow indicates the skull. SDIS: scaffold dental implant system.
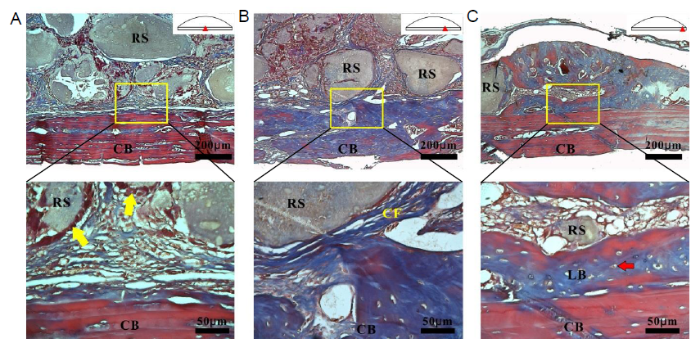
Since cortical bone has no blood vessels connecting with the bioactive glass scaffolds, the direction of colonisation of the soft tissue was from the periphery of the scaffolds to the interior. Therefore, different osteogenic stages could be observed at the interface between the bioactive glass scaffold and the cortical bone. The outcome of histological analysis after staining with Masson’s trichrome is shown in Figure 4. In the central area, the scaffold gaps were filled with collagen-rich connective tissues alone, as the cells migrated at the last stage (Figure 4A). A white blank line could be observed between connective tissues and pericranium. Moreover, in some places, connective tissues had connected with the pericranium. In the area between the edge and the centre (Figure 4B), the MNBG scaffold was completely surrounded by collagen fibres, and in some cases the pericraniums had begun to be absorbed. In the edge area (Figure 4C), typical morphology of pre-lamellar bone was observed in close contact with the cortical bone surface. Osteocytes (red arrow) were present in the pre-lamellar bone. A small amount of connective tissue and residual MNBG scaffolds had been completely surrounded by newly-formed bone.
Figure 4.
Figure 4.
Histological analysis of the MNBG scaffolds and cortical bone after Masson’s trichrome staining. (A, C) Centre and edge areas. (B) Area between edge and centre, as shown in the schematic diagram. The yellow boxes show the areas which are enlarged below. The yellow arrows indicate osteoblasts and the red arrow indicates an osteocyte. Scale bars: 200 μm (upper panel), 50 μm (lower panel). CB: cortical bone; CF: collagen fibres; LB: pre-lamellar bone; MNBG: micro-nano bioactive glass; RS: residual scaffolds.
Discussion
After the loss of natural teeth, the alveolar bone heals and is covered by cortical bone. Due to the lack of mechanical stimulation, the alveolar ridge is gradually resorbed and becomes flat. In this experiment, the animal model provides a similar microenvironment to the alveolar ridge when natural tooth loss occurs. Moreover, the epicranial aponeurosis also exhibits similar tenacity to that of the alveolar ridge mucosa, so that MNBG scaffolds are subjected to a large pressing force. Consequently the animal model is consistent with the actual alveolar ridge atrophy which has been described in some reports.25
In order to repair missing teeth, different types of implants have been used in the clinic since the advent of dental implants. These include endosteal implants, subperiosteal implants, endodontic implants and stable transosteal implants.26, 27 Although each type of implant has been popular for a period of time, endosteal implants (i.e., nail type implants) have become widely accepted in recent years. An endosteal implant is fixed into the alveolar ridge by making a hole which matches it. In this work, we designed a novel dental implant: the SDIS. Compared with traditional nail endosteal implants, the SDIS has the following advantages: (i) the surgical procedure is performed below the mucosa and does not destroy alveolar bone; (ii) due to the large contact area with the bone, the fixation part of the metal implant can act to disperse stress; (iii) this design can simultaneously replace multiple missing teeth by connecting the fixation parts together. In practical clinical application, the SDIS can be personalized manufacturing. Firstly, the morphology of the alveolar is needed to be reconstructed by using CT. Then the metal implants and BG scaffolds are fabricated by making full use of the advantages of 3D printing personalized manufacturing. Finally, the SDIS is installed on the surface of the alveolar after incising the mucoperiosteum of the alveolar.
Moreover, the most important advantage of the SDIS is that it can augment the alveolar ridge when the MNBG scaffold transforms into new bone. Currently, vertical bone augmentation for dental implant placement is one of the most challenging problems in implantology. Although several biomaterials have been used for alveolar ridge augment, such as tricalcium phosphate,28 hydroxyapatite16 and hydrogels,17 the repair effect is still not satisfactory. Most of these studies require the creation of a bone defect (as the material has only bone conductive properties)29 or the addition of biomolecules.18, 30 In this study, vertical bone augmentation was achieved by using bioactive glass alone without adding any biomolecules.
The bone augmentation observed can be attributed to the osteoinductive property of bioactive glass.31 Osteoinduction usually refers to the ability to form ectopic bone which means the material can be converted into new bone in non-bone sites (such as muscle and subcutaneous sites).32 Whether bioactive glass has an osteoinductive property is a topic which has caused long-term controversy. Yuan et al.33 reported that bone formation was found in pores of Bioglass® implanted in thigh muscles of dogs after 3 months. Miri et al.34 injected dense collagen-Bioglass® hybrid gel scaffolds subcutaneously into adult rats, but only mineralised regions could be found and no bone formation was observed. Some other studies reported that they achieved ectopic bone formation with bioactive glass by adding BMP-235 or osteogenic cells.36
Urist et al.37 thought that bone induction factors, osteogenic stem cells and a suitable osteogenic environment were the three conditions necessary for osteoinduction. In fact, osteoinduction in rodent subcutaneous models is a rare occurrence due to the lack of osteogenic stem cells at this site.38 Previous studies also demonstrated that bioactive glass had no ectopic osteogenic potential in subcutaneous tissue,39 but new bone could be found when bioactive glass was implanted subcutaneously together with osteogenic cells.36 In our research, bioactive glass was kept in close contact with the pericranium which contains osteogenic stem cells.40 The dissolution products of bioactive glass (Si, Ca and P) induced osteogenic differentiation of bone-marrow-derived adult stem cells (mesenchymal stem cells) into osteoblast-like cells, and the resulting cells produced mineralised matrix. This osteogenesis mechanism has been proven by in vitro experiments, which demonstrated that bioactive glass stimulates osteoprogenitor cells at the genetic level.22
In this experiment, the SDIS was placed directly onto the surface of the skull without any retention measures. However, the implants had unable to move after implanted for one week. This process could be divided into two stages: soft tissue combination and bone combination. In the early stage of implantation, the bioactive glass began to dissolve and combined with the soft tissue, which contributed to the formation of a hydroxycarbonate apatite layer on the surface of the bioactive glass.41 With the formation of new bone, the pericranium was gradually resorbed and the newly-formed bone was directly connected to the cortical bone. This was confirmed by histological analysis which showed that new bone could be found at the edge of the scaffolds after 6 weeks. A similar study was also reported by Hench et al.41 who demonstrated that bioactive glass could form a strong bond with bone.
In this study, a novel dental implant, the SDIS, was successfully developed which was composed of a metal implant and an MNBG scaffold. The metal implant was used to connect dentures while the MNBG scaffold was used to form new bone on the alveolar ridge and augment the vertical bone at the same time. We implanted SDISs in the sub-epicranial aponeurosis of Sprague-Dawley rats. After 6 weeks the SDIS was combined closely with the skull without any mobility and the denture could bear certain lateral forces. In addition, active osteogenesis could be observed without the addition of any biomolecules or destruction of the alveolar ridge. The osteogenic stem cells in the pericranium may play a key role in mediating the osteoinductivity of MNBG scaffolds.
Author contributions
Conceptualization, methodology and original draft: FZ; study design: LS; data curation, investigation and validation: FZ, ZY and LL; Project administration: XC; funding acquisition: DC, XC and LS; manuscript review & editing: FZ and XC. All authors approved the final version of this manuscript.
Financial support
This study was financially supported by the National Key Research and Development Program of China (No. 2018YFC1106300), the China Postdoctoral Science Foundation (No. 2020M672732), the Natural Science Foundation of Guangdong Province of China (No. 2019A1515110480), the Medical Scientific Research Foundation of Guangdong Province of China (No. A2020107) and the Beijing Municipal Health Commission of China (Nos. BMHC-2019-9, BMHC-2018-4, PXM2020_026275_000002).
Acknowledgement
None.
Conflicts of interest statement
The authors declare no competing financial interest.
Data sharing statement
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Reference
Survival rates and complication behaviour of tooth implant-supported, fixed dental prostheses: A systematic review and meta-analysis
DOI:10.1016/j.jdent.2019.07.005
URL
PMID:31306691
[Cited within: 1]

OBJECTIVE: To assess the survival and complication rates of tooth-implant supported fixed dental prostheses (T-I FDPs). SOURCES: An electronic search in MEDLINE/PubMed, Cochrane Library, and Embase was conducted using MeSH terms to identify randomised controlled trials (RCTs) or prospective studies with an observation period of at least 3 years, including at least 10 participants. STUDY SELECTION: Included studies were qualitatively assessed. Survival rates of T-I FDPs and implants as well as technical and biological complications were obtained. Failure and complication rates were pooled by weighting each rate in inverse proportion to its variance. DATA: A total of eight studies were considered for qualitative analysis; seven studies with a minimum follow-up of five years were included for quantitative analysis. Estimated survival rates of T-I FDPs were 90.8% (95% CI: 86.4-93.8%) after five years and 82.5% (95% CI: 74.7-88.0%) after 10 years. Implant survival estimates were 94.8% (90.9-97.0%) and 89.8% (82.7-99.4%) after 5 and 10 years, respectively. From a total of 185 T-I FDPs, 21 (11.4%) minor and 23 (12.4%) major biological complications were observed, whereas 23 (12.4%) minor and three (1.6%) major technical complications occurred. CONCLUSIONS: Due to the lack of well-designed studies exceeding a 10-year follow-up, prognosis for the long -term can hardly be given. Considering the inclusion criteria of this systematic review, T-I FDP-supported fixed dental prostheses show acceptable survival rates after five and 10 years. Rigidly constructed T-I FDPs should be preferred. With regard to the available data, these conclusions are valid only for three- to four-unit T-I FDPs. CLINICAL SIGNIFICANCE: Tooth-implant supported fixed dental prostheses are a recommendable treatment option in partial dentition. Based on the current literature, they should be rigidly constructed with a maximum number of four units.
Dental implants in growing patients: a systematic review
DOI:10.1016/j.bjoms.2019.04.011
URL
PMID:31076220
[Cited within: 1]

The aim of this systematic review (for which we followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines) was to provide an overview of the protocols and clinical outcomes of dental implants placed in growing jaws. We searched the MEDLINE/PubMed, Embase, Scopus, and Science Direct databases in October and November, 2017. A total of 3492 studies were identified, and all the studies reporting the outcomes of dental implants placed during the growth phase were included in the study. After duplicates had been removed, 2133 studies were screened based on their titles and abstracts, and 162 were selected for reading. Finally, 28 studies were included in the review. Overall, 493 dental implants were placed in 147 patients aged from 3-18 years old with follow-up being from 1-20 years. The most common disorders seen that were associated with missing teeth were ectodermal dysplasia and dental trauma. The main complications reported were the infraocclusion positioning of dental implants in the maxillary arch and the rotation of dental implants in the mandibular arch. Dental implants were indicated for the anterior regions of the maxilla and mandible in patients over 10 years old, and placement of maxillary implants in a more coronal position was recommended. Consultations and adjustments to prostheses were required until growth had ceased. In growing jaws, dental implants require positional modifications, and they should be considered only under special circumstances.
Dental implant complications
DOI:10.1053/j.sult.2015.09.007
URL
PMID:26589696
[Cited within: 1]

Dental implants have increased in the last few decades thus increasing the number of complications. Since many of these complications are easily diagnosed on postsurgical images, it is important for radiologists to be familiar with them and to be able to recognize and diagnose them. Radiologists should also have a basic understanding of their treatment. In a pictorial fashion, this article will present the basic complications of dental implants which we have divided into three general categories: biomechanical overload, infection or inflammation, and other causes. Examples of implant fracture, loosening, infection, inflammation from subgingival cement, failure of bone and soft tissue preservation, injury to surround structures, and other complications will be discussed as well as their common imaging appearances and treatment. Lastly, we will review pertinent dental anatomy and important structures that are vital for radiologists to evaluate in postoperative oral cavity imaging.
Alveolar ridge resorption after tooth extraction: A consequence of a fundamental principle of bone physiology
DOI:10.1177/1758736012456543
URL
PMID:22924065
[Cited within: 1]

It is well established that tooth extraction is followed by a reduction of the buccolingual as well as the apicocoronal dimension of the alveolar ridge. Different measures have been taken to avoid this bone modelling process, such as immediate implant placement and bone grafting, but in most cases with disappointing results. One fundamental principle of bone physiology is the adaptation of bone mass and bone structure to the levels and frequencies of strain. In the present article, it is shown that the reduction of the alveolar ridge dimensions after tooth extraction is a natural consequence of this physiological principle.
Mechanical aspects of dental implants and osseointegration: A narrative review
URL PMID:32090904 [Cited within: 1]
Human studies of vertical and horizontal alveolar ridge augmentation comparing different types of bone graft materials: a systematic review
DOI:10.1563/aaid-joi-D-17-00053
URL
PMID:29135351
[Cited within: 1]

Alveolar ridge augmentation can be completed with various types of bone augmentation materials (autogenous, allograft, xenograft, and alloplast). Currently, autogenous bone is labeled as the
Bone augmentation procedures in implant dentistry
URL PMID:19885448 [Cited within: 1]
Bone augmentation techniques
DOI:10.1902/jop.2007.060048
URL
PMID:17335361
[Cited within: 1]

BACKGROUND: The advent of osseointegration and advances in biomaterials and techniques have contributed to increased application of dental implants in the restoration of partial and completely edentulous patients. Often, in these patients, soft and hard tissue defects result from a variety of causes, such as infection, trauma, and tooth loss. These create an anatomically less favorable foundation for ideal implant placement. For prosthetic-driven dental implant therapy, reconstruction of the alveolar bone through a variety of regenerative surgical procedures has become predictable; it may be necessary prior to implant placement or simultaneously at the time of implant surgery to provide a restoration with a good long-term prognosis. Regenerative procedures are used for socket preservation, sinus augmentation, and horizontal and vertical ridge augmentation. METHODS: A broad overview of the published findings in the English literature related to various bone augmentation techniques is outlined. A comprehensive computer-based search was performed using various databases that include Medline and PubMed. A total of 267 papers were considered, with non-peer-reviewed articles eliminated as much as possible. RESULTS: The techniques for reconstruction of bony defects that are reviewed in this paper include the use of particulate bone grafts and bone graft substitutes, barrier membranes for guided bone regeneration, autogenous and allogenic block grafts, and the application of distraction osteogenesis. CONCLUSIONS: Many different techniques exist for effective bone augmentation. The approach is largely dependent on the extent of the defect and specific procedures to be performed for the implant reconstruction. It is most appropriate to use an evidenced-based approach when a treatment plan is being developed for bone augmentation cases.
Bone autografts & allografts placed simultaneously with dental implants in rabbits
DOI:10.1016/j.jcms.2017.11.006
URL
PMID:29198577
[Cited within: 1]

OBJECTIVE: This study compared stability, removal torque, bone implant contact (BIC) and area (BA) of implants installed simultaneously with onlay autografts or allografts in rabbits' tibias. MATERIAL AND METHODS: Total of 18 rabbits were used in this study. Fresh frozen allografts were obtained from six animals at T(-6). Two implants with autogenous grafts (Group 1) or allografts (Group 2) were simultaneously inserted into both sides of the tibiae in a vertical periimplant defect model at T0. The resonance frequency (ISQ) was measured in implant proximal epiphysis on the day of installation of T0 and T18 (18 weeks post-surgery). At T18 the removal torque was assessed at the distal implants, the implants' proximal epiphysis and surrounding bone were harvested to perform histomorphometric analysis. The BIC and BA within the limits of the implants threads were evaluated. RESULTS: The ISQ revealed a statistically significant difference between T0 and T18 in each group (p = 0.024, p = 0.003). The removal torque indicates that there was no significant difference between the two groups (p = 0.47). No significant differences were observed between the groups regarding both BIC (p = 0.3713) and the BA (p = 0.3883). CONCLUSION: Both grafts and implants demonstrated the same stability, torque removal and the BIC and BA.
Dental implants placed in resorbed alveolar ridges reconstructed with iliac crest autogenous onlay grafts: a 26-year median follow-up retrospective study
DOI:10.1016/j.jcms.2019.02.002
URL
PMID:30797661
[Cited within: 1]

PURPOSE: To evaluate the long-term outcome of dental implants placed with a staged procedure in resorbed alveolar ridges reconstructed with iliac crest autogenous onlay grafts. MATERIALS AND METHODS: All consecutive patients treated with iliac crest onlay bone grafts and dental implants were retrospectively evaluated. During the appointment, clinical and radiological examinations were conducted to assess implant survival. A survived implant was defined as an implant still stable and in function at the follow-up visit. Implant survival was estimated at the implant level using Kaplan-Meier analyses. The cumulative survival rate was estimated using a life-table analysis. Subgroup analyses were performed for age, position, and type of retention using the log-rank test. A p-value of <0.05 was considered statistically significant. RESULTS: The cohort consisted of 21 female subjects receiving a total of 140 rough-surface titanium implants. Of them, 128 survived and 12 failed, yielding a cumulative survival rate of 91.1% over a median survival time of 312 months. Implants supporting cement-retained prostheses exhibithed lower survival rate compared to screw-retained restorations (p = 0.001). CONCLUSION: Implants placed in bone augmented with iliac crest onlay grafts showed high long-term survival rates. Cement-retained restorations were more prone to develop implant failures.
3D-printed scaffolds and biomaterials: review of alveolar bone augmentation and periodontal regeneration applications
DOI:10.1155/2016/1239842
URL
PMID:27366149
[Cited within: 1]

To ensure a successful dental implant therapy, the presence of adequate vertical and horizontal alveolar bone is fundamental. However, an insufficient amount of alveolar ridge in both dimensions is often encountered in dental practice due to the consequences of oral diseases and tooth loss. Although postextraction socket preservation has been adopted to lessen the need for such invasive approaches, it utilizes bone grafting materials, which have limitations that could negatively affect the quality of bone formation. To overcome the drawbacks of routinely employed grafting materials, bone graft substitutes such as 3D scaffolds have been recently investigated in the dental field. In this review, we highlight different biomaterials suitable for 3D scaffold fabrication, with a focus on
Bone replacement materials and techniques used for achieving vertical alveolar bone augmentation
DOI:10.3390/ma8062953 URL [Cited within: 1]
Mesenchymal stem cells combined with barrier domes enhance vertical bone formation
DOI:10.1111/jcpe.12044
URL
PMID:23278529
[Cited within: 1]

AIM: To enhance vertical bone formation in a rat calvarium following combination of guided bone regeneration (GBR) and transplantation of bone marrow derived mesenchymal stem cells (bmMSC). MATERIALS AND METHODS: Gold domes (7 mm radius, 5 mm height) were filled with 5 x 10(5) bmMSC or osteogenic transformed bmMSC (otMSC) that were isolated from tibia of inbred rats and mixed with betaTCP. Domes filled with betaTCP served as control. Rats were sacrificed after 3 months. New bone formation was analysed by histology and histomorphometry. RESULTS: In all rats hard tissue filled the space under the dome. In the lower part of the specimens the newly formed mature bone was continuous with the original calvaria, whereas the upper (distal) part of the augmented tissue contained residual scaffold surrounded by connective tissue. Histomorphometric analysis revealed that cell transplantation doubled vertical bone height: (bmMSC 4.13 +/- 0.5 mm, otMSC 4.14 +/- 0.3 mm betaTCP 2.29 +/- 0.22 mm, p </= 0.001). Bone area fraction (%) was significantly increased following transplantation of otMSC (47.2 +/- 2.5%) when compared with bmMSC (37.3 +/- 3.35%) and with betaTCP (31.09 +/- 2.7%) (p </= 0.031 versus bmMSC, p </= 0.0004 versus control) CONCLUSION: In a rat calvaria model transplantation of both otMSC and bmMSC, when combined with GBR significantly enhanced bone formation.
Osseointegration of dental implants in 3D-printed synthetic onlay grafts customized according to bone metabolic activity in recipient site
DOI:10.1016/j.biomaterials.2014.03.050
URL
PMID:24726538

Onlay grafts made of monolithic microporous monetite bioresorbable bioceramics have the capacity to conduct bone augmentation. However, there is heterogeneity in the graft behaviour in vivo that seems to correlate with the host anatomy. In this study, we sought to investigate the metabolic activity of the regenerated bone in monolithic monetite onlays by using positron emission tomography-computed tomography (PET-CT) in rats. This information was used to optimize the design of monetite onlays with different macroporous architecture that were then fabricated using a 3D-printing technique. In vivo, bone augmentation was attempted with these customized onlays in rabbits. PET-CT findings demonstrated that bone metabolism in the calvarial bone showed higher activity in the inferior and lateral areas of the onlays. Histological observations revealed higher bone volume (up to 47%), less heterogeneity and more implant osseointegration (up to 38%) in the augmented bone with the customized monetite onlays. Our results demonstrated for the first time that it is possible to achieve osseointegration of dental implants in bone augmented with 3D-printed synthetic onlays. It was also observed that designing the macropore geometry according to the bone metabolic activity was a key parameter in increasing the volume of bone augmented within monetite onlays.
Graded porous β-tricalcium phosphate scaffolds enhance bone regeneration in mandible augmentation
DOI:10.1097/SCS.0000000000001383
URL
PMID:25675019
[Cited within: 1]

Bone augmentation requires scaffold to promote forming of natural bone structure. Currently, most of the reported bone scaffolds are porous solids with uniform pores. The aim of the current study is to evaluate the effect of a graded porous beta-tricalcium phosphate scaffolds on alveolar bone augmentation. Three groups of scaffolds were fabricated by a template-casting method: (1) graded porous scaffolds with large pores in the center and small pores at the periphery, (2) scaffolds with large uniform pores, and (3) scaffolds with small uniform pores. Bone augmentation on rabbit mandible was investigated by microcomputed tomography, sequential fluorescent labeling, and histologic examination 3 months after implantation.The result presents that all the scaffold groups maintain their augmented bone height after 3-month observation, whereas the autografting group presents an obvious bone resorption. Microcomputed tomography reveals that the graded porous group has significantly greater volume of new bone (P < 0.05) and similar bone density compared with the uniform pores groups. Bone substance distributes unevenly in all the 3 experimental groups. Greater bone volume can be observed in the area closer to the bone bed. The sequential fluorescent labeling observation reveals robust bone regeneration in the first month and faster bone growth in the graded porous scaffold group than that in the large porous scaffold group. Histologic examinations confirm bone structure in the aspect of distribution, activity, and maturity. We conclude that graded porous designed biodegradable beta-tricalcium phosphate scaffolds are beneficial to promote bone augmentation in the aspect of bone volume.
Two dimensional alveolar ridge augmentation using particulate hydroxyapatite and collagen membrane: A case report
DOI:10.1016/j.jobcr.2014.01.002
URL
PMID:25737935
[Cited within: 2]

BACKGROUND: Ridge augmentation procedures require bone regeneration outside of the existing bony walls or housing and are therefore often considered to be the most challenging surgical procedures. The bony deficiencies can be managed with GBR techniques involving bone grafting material and membrane while vertical augmentation may require the use of space-creating support mechanisms. Non-degradable membranes have been used for ridge augmentation with encouraging results however; requirement of second surgery for its removal and associated infection on exposure may compromise the desired results. These problems can be overcome by employing resorbable collagen membranes. Different bone graft materials are also used in combination with resorbable membranes, for prevention of membrane collapse and maintenance of space, as they lack sufficient rigidity. Particulate hydroxyapatite bone graft may be better alternative, because it treats the underlying bone defect to restore the natural support of the tissue architecture. Moreover, its use avoids potential donor site complications associated with autogenous block grafts. METHOD: Patient described in this report presented with missing right maxillary incisor with ridge deficiency. A treatment approach involving localised ridge augmentation with particulate hydroxyapatite and collagen membrane was used. RESULT: Six month post-operative periapical radiograph demonstrated a significant vertical bone fill. CONCLUSION: The clinical and radiographic findings of the present case suggests that HA in conjunction with a resorbable collagen membrane may be an acceptable alternative to the autogenous block graft and non-resorbable membrane in the treatment of compromised alveolar ridge deficiencies.
Synthetic biodegradable hydrogel delivery of demineralized bone matrix for bone augmentation in a rat model
DOI:10.1016/j.actbio.2014.07.011
URL
PMID:25046637
[Cited within: 2]

There exists a strong clinical need for a more capable and robust method to achieve bone augmentation, and a system with fine-tuned delivery of demineralized bone matrix (DBM) has the potential to meet that need. As such, the objective of the present study was to investigate a synthetic biodegradable hydrogel for the delivery of DBM for bone augmentation in a rat model. Oligo(poly(ethylene glycol) fumarate) (OPF) constructs were designed and fabricated by varying the content of rat-derived DBM particles (either 1:3, 1:1 or 3:1 DBM:OPF weight ratio on a dry basis) and using two DBM particle size ranges (50-150 or 150-250 mum). The physical properties of the constructs and the bioactivity of the DBM were evaluated. Selected formulations (1:1 and 3:1 with 50-150 mum DBM) were evaluated in vivo compared to an empty control to investigate the effect of DBM dose and construct properties on bone augmentation. Overall, 3:1 constructs with higher DBM content achieved the greatest volume of bone augmentation, exceeding 1:1 constructs and empty implants by 3- and 5-fold, respectively. As such, we have established that a synthetic, biodegradable hydrogel can function as a carrier for DBM, and that the volume of bone augmentation achieved by the constructs correlates directly to the DBM dose.
Tissue response to composite hydrogels for vertical bone augmentation in the rat
DOI:10.1002/jbm.a.34878
URL
PMID:23894052
[Cited within: 2]

The objective of the present study was to develop a preclinical animal model for evaluating bone augmentation and to examine the level of bone augmentation induced by hydrogel composites. Design criteria outlined for the development of the animal model included rigid immobilization of bilateral implants apposed to the parietal bone of the rat, while avoiding the calvarial sutures. The animal model was evaluated through the implantation of hydrogel composites of oligo(poly(ethylene glycol) fumarate) (OPF) and gelatin microparticles releasing bone morphogenetic protein-2 (BMP-2). The BMP-2 release profile was varied and compared to the implantation of a material control without BMP-2. Each hydrogel composite was implanted within a polypropylene cassette, which was immobilized to the calvarial bone using screws, and empty cassettes were implanted as a control. The design criteria for the animal model were realized; however, the level of bone augmentation did not vary between any of the groups after 4 weeks. Osteoclastic bone resorption occurred to a higher extent in groups releasing BMP-2, but the cause could not be elucidated. In conclusion, a promising bone augmentation model was established in the rat; however, refinement of the hydrogel composites was suggested to optimize the constructs for bone augmentation applications.
Natural graft tissues and synthetic biomaterials for periodontal and alveolar bone reconstructive applications: a review
URL PMID:28593053 [Cited within: 1]
Bone grafting materials in dentoalveolar reconstruction: A comprehensive review
DOI:10.1016/j.jdsr.2018.09.003
URL
PMID:30733842

Bone deficits of the jaws are often attributed to accidents, surgical removal of benign lesions or malignant neoplasms, congenital abnormalities, periodontal inflammation, tooth abscess or extraction and finally jaw atrophy due to advanced age or general disease. These bone defects require rehabilitation for a variety of reasons, e.g. maintaining the normal anatomic outline, eliminating empty space, aesthetic restoration and placing dental implants. Today, several techniques have been developed to eliminate these bone deformities including bone grafting, guided bone regeneration, distraction osteogenesis, use of growth factors and stem cells. Bone grafts consist of materials of natural or synthetic origin, implanted into the bone defect site, documented to possess bone healing properties. Currently, a variety of bone restorative materials with different characteristics are available, possesing different properties. Despite years of effort the 'perfect' bone reconstruction material has not yet been developed, a further effort is required to make this objective feasible. The aim of this article is to provide a contemporary and comprehensive overview of the grafting materials that can be applied in dentoalveolar reconstruction, discussing their properties, advantages and disadvantages, enlightening the present and the future perspectives in the field of bone regeneration.
The effects of morphology on physicochemical properties, bioactivity and biocompatibility of micro-/nano-bioactive glasses
DOI:10.1016/j.apt.2018.04.017 URL [Cited within: 1]
Review of bioactive glass: from Hench to hybrids
DOI:10.1016/j.actbio.2012.08.023
URL
PMID:22922331
[Cited within: 2]

Bioactive glasses are reported to be able to stimulate more bone regeneration than other bioactive ceramics but they lag behind other bioactive ceramics in terms of commercial success. Bioactive glass has not yet reached its potential but research activity is growing. This paper reviews the current state of the art, starting with current products and moving onto recent developments. Larry Hench's 45S5 Bioglass(R) was the first artificial material that was found to form a chemical bond with bone, launching the field of bioactive ceramics. In vivo studies have shown that bioactive glasses bond with bone more rapidly than other bioceramics, and in vitro studies indicate that their osteogenic properties are due to their dissolution products stimulating osteoprogenitor cells at the genetic level. However, calcium phosphates such as tricalcium phosphate and synthetic hydroxyapatite are more widely used in the clinic. Some of the reasons are commercial, but others are due to the scientific limitations of the original Bioglass 45S5. An example is that it is difficult to produce porous bioactive glass templates (scaffolds) for bone regeneration from Bioglass 45S5 because it crystallizes during sintering. Recently, this has been overcome by understanding how the glass composition can be tailored to prevent crystallization. The sintering problems can also be avoided by synthesizing sol-gel glass, where the silica network is assembled at room temperature. Process developments in foaming, solid freeform fabrication and nanofibre spinning have now allowed the production of porous bioactive glass scaffolds from both melt- and sol-gel-derived glasses. An ideal scaffold for bone regeneration would share load with bone. Bioceramics cannot do this when the bone defect is subjected to cyclic loads, as they are brittle. To overcome this, bioactive glass polymer hybrids are being synthesized that have the potential to be tough, with congruent degradation of the bioactive inorganic and the polymer components. Key to this is creating nanoscale interpenetrating networks, the organic and inorganic components of which have covalent coupling between them, which involves careful control of the chemistry of the sol-gel process. Bioactive nanoparticles can also now be synthesized and their fate tracked as they are internalized in cells. This paper reviews the main developments in the field of bioactive glass and its variants, covering the importance of control of hierarchical structure, synthesis, processing and cellular response in the quest for new regenerative synthetic bone grafts. The paper takes the reader from Hench's Bioglass 45S5 to new hybrid materials that have tailorable mechanical properties and degradation rates.
Regenerating bone with bioactive glass scaffolds: A review of in vivo studies in bone defect models
DOI:10.1016/j.actbio.2017.08.030
URL
PMID:28844964
[Cited within: 1]

Large bone defects resulting from fractures and disease are a medical concern, being often unable to heal spontaneously by the body's repair mechanisms. Bone tissue engineering (BTE) is a promising approach for treating bone defects through providing a template to guide osseous regeneration. 3D scaffolds with microstructure mimicking host bone are necessary in common BTE strategies. Bioactive glasses (BGs) attract researchers' attention as BTE scaffolds as they are osteoconductive and osteoinductive in certain formulations. In vivo animal models allow understanding and evaluation of materials' performance in the complex physiological environment, being an inevitable step before clinical trials. The aim of this paper is to review for the first time published research investigating the in vivo osseous regenerative capacity of 3D BG scaffolds in bone defect animal models, to better understand and evaluate the progress and future outlook of the use of such scaffolds in BTE. The literature analysis reveals that the regenerative capacity of BG scaffolds depends on several factors; including BG composition, fabrication method, scaffold microstructure and pore characteristics, in addition to scaffold pretreatment and whether or not the scaffolds are loaded with growth factors. In addition, animal species selected, defect size and implantation time affect the scaffold in vivo behavior and outcomes. The review of the literature also makes clear the difficulty encountered to compare different types of bioactive glass scaffolds in their bone forming ability. Even considering such limitations of the current state-of-the-art, results generated from animal bone defect models provide an essential source of information to guide the design of BG scaffolds in future. STATEMENT OF SIGNIFICANCE: Bioactive glasses are at the centre of increasing research efforts in bone tissue engineering as the number of research groups around the world carrying out research on this type of biomaterials continues to increase. However, there are no previous reviews in literature which specifically cover investigations of the performance of bioactive glass scaffolds in bone defect animal models. This is the topic of the present review, in which we have analysed comprehensively all available literature in the field. The review thus fills a gap in the biomaterials literature providing a broad platform of information for researchers interested in bioactive glasses in general and specifically in the outcomes of in vivo models. Bioactive glass scaffolds of different compositions tested in relevant bone defect models are covered.
3D printing nanoscale bioactive glass scaffolds enhance osteoblast migration and extramembranous osteogenesis through stimulating immunomodulation
DOI:10.1002/adhm.201800361
URL
PMID:29952135
[Cited within: 1]

Bioactive glass (BG) can repair bone defects, however, it is not clear whether BG has the ability for bone augmentation without making any bone defect. Unlike the intramembranous osteogenesis in bone defect repair, the extramembranous osteogenesis occurs outside the cortical bone and the osteoprogenitor cells show the reversed migration. Herein, nanoscale bioactive glass scaffolds (BGSs) are fabricated, and their role and immunomodulation-related mechanism in the extramembranous osteogenesis are investigated. The in vitro migration and differentiation of calvaria preosteoblasts are studied by culturing with peripheral macrophage-conditioned medium after stimulating with BGSs. The results indicate that the proinflammatory environment significantly promotes preosteoblast migration, but has limited effect on osteogenic differentiation. However, the anti-inflammatory environment and BGSs significantly increase the osteogenic differentiation of preosteoblasts. The in vivo extramembranous osteogenesis evaluation shows that the active osteogenesis is observed near the skull. The osteoblasts derived from the reverse migration of cranial cells can be confirmed by comparing with the scaffolds implanted in back subcutaneous which is just colonized by fibrous tissue. This study may bring a fresh perspective for BG in bone regeneration and explore the osteogenic immunomodulation of peripheral macrophages in a nonosteogenic environment.
Controlling bone graft substitute microstructure to improve bone augmentation
DOI:10.1002/adhm.201600052
URL
PMID:27214877
[Cited within: 1]

Vertical bone augmentation procedures are frequently carried out to allow successful placement of dental implants in otherwise atrophic ridges and represent one of the most common bone grafting procedures currently performed. Onlay autografting is one of the most prevalent and predictable techniques to achieve this; however, there are several well documented complications and drawbacks associated with it and synthetic alternatives are being sought. Monetite is a bioresorbable dicalcium phosphate with osteoconductive and osteoinductive potential that has been previously investigated for onlay bone grafting and it is routinely made by autoclaving brushite to simultaneously sterilize and phase convert. In this study, monetite disc-shaped grafts are produced by both wet and dry heating methods which alter their physical properties such as porosity, surface area, and mechanical strength. Histological observations after 12 weeks of onlay grafting on rabbit calvaria reveal higher bone volume (38%) in autoclaved monetite grafts in comparison with the dry heated monetite grafts (26%). The vertical bone height gained is similar for both the types of monetite grafts (up to 3.2 mm). However, it is observed that the augmented bone height is greater in the lateral than the medial areas of both types of monetite grafts. It is also noted that the higher porosity of autoclaved monetite grafts increases the bioresorbability, whereas the dry heated monetite grafts having lower porosity but higher surface area resorb to a significantly lesser extent. This study provides information regarding two types of monetite onlay grafts prepared with different physical properties that can be further investigated for clinical vertical bone augmentation applications.
An evaluation of early bone changes after the insertion of mental endosseous implants into the jaws of rhesus monkeys
DOI:10.1016/0030-4220(71)90321-5 URL PMID:4999108 [Cited within: 1]
Materials and designs for implant dentistry
URL PMID:779858 [Cited within: 1]
Craniofacial vertical bone augmentation: a comparison between 3D printed monolithic monetite blocks and autologous onlay grafts in the rabbit
DOI:10.1016/j.biomaterials.2009.07.049
URL
PMID:19695698
[Cited within: 1]

Onlay autografting is amongst the most predictable techniques for craniofacial vertical bone augmentation, however, complications related to donor site surgery are common and synthetic alternatives to onlay autografts are desirable. Recent studies have shown that the acidic calcium phosphates, brushite and monetite, are osteoconductive, osteoinductive and resorb faster in vivo than hydroxyapatite. Moreover, they can be 3D printed allowing precise host bone-implant conformation. The objectives of this study were to confirm that craniofacial screw fixation of 3D printed monetite blocks was possible and to compare the resulting vertical bone augmentation with autograft. 3D printed monolithic monetite onlay implants were fixed with osteosynthesis screws on the calvarial bone surface of New Zealand rabbits. After 8 weeks, integration between the implant and the calvarial bone surface was observed in all cases. Histomorphometry revealed that 42% of the monetite was resorbed and that the new bone formed within the implant occupied 43% of its volume, sufficient for immediate dental implant placement. Bone tissue within the autologous onlay occupied 60% of the volume. We observed that patterns of regeneration within the implants differed throughout the material and propose that this was due to the anatomy and blood supply pattern in the region. Rapid prototyped monetite being resorbable osteoconductive and osteoinductive would appear to be a promising biomaterial for many bone regeneration strategies.
Dose-dependent effect of adipose-derived adult stem cells on vertical bone regeneration in rabbit calvarium
DOI:10.1016/j.biomaterials.2010.01.066
URL
PMID:20170950
[Cited within: 1]

Previous in vivo studies have shown a limited potential for vertical bone regeneration using osteoconductive scaffolds alone. In the present study, we investigated whether the association of adipose-derived adult stem cells (ASCs) with anorganic bovine bone (ABB) scaffold improved bone formation and implant osseointegration in a vertical guided bone regeneration model. Two pre-formed titanium domes were placed on the calvaria of 12 rabbits. Four treatment modalities were evenly distributed among the 24 domes: ABB alone, and ABB containing 3 x 10(5), 3 x 10(6), or 3 x 10(7) cells/graft. After 1 month, the domes were removed and one titanium implant was placed into each augmented site. One month after the second operation, the animals were killed and biopsy specimens were examined by histomorphometric and micro-CT analyses. Results indicated that at all concentrations, the ASC-loaded groups showed significantly more new bone formation and higher mean values of bone-implant contact and bone density inside threads than the ABB group. Furthermore, ASCs demonstrated a dose-response relationship, with the highest dose chosen inducing more robust bone regeneration. This study suggests that the delivery of ASCs on ABB might effectively increase vertical bone regeneration and implant osseointegration, versus ABB alone.
Vertical alveolar ridge augmentation with beta-tricalcium phosphate and autologous osteoblasts in canine mandible
DOI:10.1016/j.biomaterials.2008.12.067
URL
PMID:19147220
[Cited within: 1]

A tissue-engineered bone has become a viable alternative to autologous bone for bone augmentation in atrophy alveolar ridge. The aim of the present study was to evaluate porous beta-tricalcium phosphate (beta-TCP) combined with autologous osteoblasts to augment edentulous alveolar ridge in a canine model. Autologous osteoblasts were expanded and combined with beta-TCP scaffold to fabricate a tissue-engineered bone. 12 bilateral alveolar ridge augmentation surgeries were carried out in 6 beagle dogs with the following 3 groups: beta-TCP/osteoblasts, beta-TCP alone and autogenous iliac bone control (n=4 per group). Sequential fluorescent labeling and radiographs were used to compare new bone formation and mineralization in each group. 24 weeks later, animals were sacrificed and non-decalcified and decalcified sections were evaluated histologically and histomorphometrically. Results indicated that the tissue-engineered bone dramatically enhanced new bone formation and mineralization, increase the new bone area, and maintain the height and thickness of the augmented alveolar ridge when compared with beta-TCP alone group. More importantly, the tissue-engineered bone achieved an elevated bone height and thickness comparable to that of autogenous iliac bone graft. This study demonstrated the potential of porous beta-TCP as a substrate for autogenous osteoblasts in bone tissue engineering for alveolar ridge augmentation.
Bioactivity and osteoinductivity of glasses and glassceramics and their material determinants
DOI:10.1016/j.ceramint.2016.06.077 URL [Cited within: 1]
Osteoinductive biomaterials: current knowledge of properties, experimental models and biological mechanisms
DOI:10.22203/ecm.v021a31
URL
PMID:21604242
[Cited within: 1]

In the past thirty years, a number of biomaterials have shown the ability to induce bone formation when implanted at heterotopic sites, an ability known as osteoinduction. Such biomaterials--osteoinductive biomaterials--hold great potential for the development of new therapies in bone regeneration. Although a variety of well characterised osteoinductive biomaterials have so far been reported in the literature, scientists still lack fundamental understanding of the biological mechanism underlying the phenomenon by which they induce bone formation. This is further complicated by the observations that larger animal models are required for research, since limited, if any, bone induction by biomaterials is observed in smaller animals, including particularly rodents. Besides interspecies variation, variations among individuals of the same species have been observed. Furthermore, comparing different studies and drawing general conclusions is challenging, as these usually differ not only in the physico-chemical and structural properties of the biomaterials, but also in animal model, implantation site and duration of the study. Despite these limitations, the knowledge of material properties relevant for osteoinduction to occur has tremendously increased in the past decades. Here we review the properties of osteoinductive biomaterials, in the light of the model and the conditions under which they were tested. Furthermore, we give an insight into the biological processes governing osteoinduction by biomaterials and our view on the future perspectives in this research field.
Bone induction by porous glass ceramic made from Bioglass (45S5)
URL PMID:11319740 [Cited within: 1]
Ectopic bone formation in rapidly fabricated acellular injectable dense collagen-Bioglass hybrid scaffolds via gel aspiration-ejection
DOI:10.1016/j.biomaterials.2016.01.047
URL
PMID:26871889
[Cited within: 1]

Gel aspiration-ejection (GAE) has recently been introduced as an effective technique for the rapid production of injectable dense collagen (IDC) gel scaffolds with tunable collagen fibrillar densities (CFDs) and microstructures. Herein, a GAE system was applied for the advanced production and delivery of IDC and IDC-Bioglass((R)) (IDC-BG) hybrid gel scaffolds for potential bone tissue engineering applications. The efficacy of GAE in generating mineralizable IDC-BG gels (from an initial 75-25 collagen-BG ratio) produced through needle gauge numbers 8G (3.4 mm diameter and 6 wt% CFD) and 14G (1.6 mm diameter and 14 wt% CFD) was investigated. Second harmonic generation (SHG) imaging of as-made gels revealed an increase in collagen fibril alignment with needle gauge number. In vitro mineralization of IDC-BG gels was confirmed where carbonated hydroxyapatite was detected as early as day 1 in simulated body fluid, which progressively increased up to day 14. In vivo mineralization of, and host response to, acellular IDC and IDC-BG gel scaffolds were further investigated following subcutaneous injection in adult rats. Mineralization, neovascularization and cell infiltration into the scaffolds was enhanced by the addition of BG and at day 21 post injection, there was evidence of remodelling of granulation tissue into woven bone-like tissue in IDC-BG. SHG imaging of explanted scaffolds indicated collagen fibril remodelling through cell infiltration and mineralization over time. In sum, the results suggest that IDC-BG hybrid gels have osteoinductive properties and potentially offer a novel therapeutic approach for procedures requiring the injectable delivery of a malleable and dynamic bone graft that mineralizes under physiological conditions.
Bioinspired trimodal macro/micro/nano-porous scaffolds loading rhBMP-2 for complete regeneration of critical size bone defect
DOI:10.1016/j.actbio.2015.12.006
URL
PMID:26689464
[Cited within: 1]

UNLABELLED: Critical size bone defects raise great demands for efficient bone substitutes. Mimicking the hierarchical porous architecture and specific biological cues of natural bone has been considered as an effective strategy to facilitate bone regeneration. Herein, a trimodal macro/micro/nano-porous scaffold loaded with recombinant human bone morphogenetic protein-2 (rhBMP-2) was developed. With mesoporous bioactive glass (MBG) as matrix, a trimodal MBG scaffold (TMS) with enhanced compressive strength (4.28 MPa, porosity of 80%) was prepared by a
Ectopic bone formation in and soft-tissue response to P(CL/DLLA)/bioactive glass composite scaffolds
DOI:10.1111/clr.12051
URL
PMID:23106633
[Cited within: 2]

OBJECTIVES: To characterize biological response to subcutaneously implanted macroporous poly(epsilon-caprolactone/D,L-lactide)-based scaffolds, and to evaluate the effect of bioactive glass (BAG) filler and osteogenic cells to the tissue response and ectopic bone formation. MATERIAL AND METHODS: In the first part of this study, six different scaffold types were screened in a rat subcutaneous implantation model. The polymer scaffolds with 70/30 caprolactone/lactide ratio and corresponding composites with < 45 mum BAG filler size were chosen for the further ectopic bone formation assay. The scaffolds were loaded with differentiating bone marrow stromal cells and implanted subcutaneously in syngeneic rats. RESULTS: With plain scaffolds, only mild foreign body reaction with no signs of gross inflammation was observed after 4 weeks of implantation. Furthermore, the scaffolds were fully invaded by well-vascularized soft connective tissue. Overall, all the tested scaffold types showed an appropriate host response. With cell-seeded scaffolds, several loci of immature mineralizing tissue and small amounts of mature bone were observed after 4 weeks. The incidence of mature bone formation was two and four in polymer scaffolds and composites, respectively (n = 8). After twelve weeks, mature bone was observed in only one polymer scaffold but in seven composites (n = 8). Excluding bone formation, the host response was considered similar to that with cell-free scaffolds. CONCLUSIONS: Plain scaffolds supported the ingrowth of well-vascularized fibroconnective tissue. Furthermore, cell seeded composites with BAG filler showed enhanced ectopic bone formation in comparison with corresponding neat polymer scaffolds.
The bone induction principle
URL PMID:4870495 [Cited within: 1]
Osteoinduction of bone grafting materials for bone repair and regeneration
URL PMID:26163110 [Cited within: 1]
The effect of current used bone substitution materials and platelet-rich plasma on periosteal cells by ectopic site implantation: an in-vivo pilot study
DOI:10.1016/j.jcms.2011.07.012
URL
PMID:21872487
[Cited within: 1]

The aim of this study was to investigate de novo bone formation following ectopic site implantation of bone substitutes covered by periosteum, with and without the application of autologous platelet-rich plasma (PRP). Twenty-four weeks after subcutaneous implantation of various bone substitutes (bovine hydroxyapatite (bHAP), phycogenic hydroxyapatite (pHAP), and bioglass (BG)) in 35 mini-pigs, bone regeneration rates were compared microradiographically and histologically. Without PRP, bHAP showed a mean de novo bone formation of 32.41%+/-29.99, in contrast to the other substitute materials where no mineralization could be detected. In combination with PRP, in the bHAP (63.61%+/-12.98; p+/-0.03) and pHAP (34.37+/-29.38; p=0.015) group, significantly higher de novo bone formation was ascertained than without PRP. No ossification could be detected in the BG group. In conclusion, bHAP and pHAP bone substitutes in combination with PRP showed a significant positive effect on periosteal cells by de novo bone formation after ectopic, subcutaneous, low-vascular site implantation.
Promoting in vivo early angiogenesis with sub-micrometer strontium-contained bioactive microspheres through modulating macrophage phenotypes
DOI:10.1016/j.biomaterials.2018.06.004
URL
PMID:29908343
[Cited within: 1]

Early vascularization capacity of biomaterials plays an essential role in efficient wound healing and tissue regeneration, especially in large tissue tension implanting position such as bone augmentation. Strontium-contained silica-based bioactive materials have shown the role of promoting angiogenesis by stimulating osteoblasts to secrete angiogenesis related cytokines. However, osteoblasts have little effect on early angiogenesis due to the inflammatory reaction of implantation site. Here, for the first time, we found that the monodispersed strontium-contained bioactive glasses microspheres (SrBGM) could significantly promote the early angiogenesis through regulating macrophage phenotypes. After being stimulated with SrBGM in vitro, RAW cells (macrophages) presented a trend towards to M2 phenotype and expressed high level of platelet-derived growth factor-BB (PDGF-BB). Moreover, the RAW conditioned medium of SrBGM significantly enhanced the angiogenic capacity of HUVECs. The in vivo early vascularization studies showed that significant new vessels were observed at the center of SrBGM-based scaffolds after implantation for 1 week in a bone defect model of rats, suggesting their enhanced early vascularization. Due to the efficient vascularization, the in vivo new bone formation was promoted significantly. Our study may provide a novel strategy to promote the early vascularization of biomaterials through modulating the microphage phenotypes, which has wide applications in various tissue regeneration and wound healing.
Bonding mechanisms at the interface of ceramic prosthetic materials
DOI:10.1002/(ISSN)1097-4636 URL [Cited within: 2]