Introduction
Bone damage can have many causes, including fractures, cancer, and obesity- and age-related stresses. Severe bone damage may be irreparable by natural processes alone.1 In these cases, bone grafts are required for proper healing. The demand for bone grafts is expected to keep rising in future because of the increase in the geriatric population.2
The current gold standard for bone transplantation is autografts, for which bone is taken from the patient and re-implanted at the trauma site. Taking bone directly from the patient has great advantages: it provides a mechanically-matched scaffold with osteogenic cells that can help grow new bone, is full of growth factors and other signalling molecules, and is fully biocompatible with osteoconductivity3 and osteoinductivity.4 Osteoconductivity is bone formation along the surface of an implanted bone graft scaffold by bone-forming cells, specifically osteoblasts and their progenitors, that migrate from nearby bone tissue; whereas osteoinductivity is bone formation through the recruitment and stimulation of immature cells in a connective tissue to develop new bone with/without a scaffold.5
However, autografts have significant drawbacks. Because the bone is taken from the patient, there is only a limited quantity available which may not be enough to fill the defect. It also adds an extra surgical site, and patients are likely to suffer from severe pain,6 increased recovery time, and donor site morbidity.7
Different bone graft substitutes have been developed to replace autografts. Allografts, bone taken from living human or cadaveric donors, are a widely used alternative.8 Allografts have success rates similar to autografts9 but do occasionally fail. The failure point tends to be the allograft-host junction point. New bone formation does not extend into the allograft very well, usually less than 5 mm even in the second year after implantation.10 There are also still other drawbacks, such as the risk of rejection and disease transmission.11, 12 Additionally, while much more abundant than autografts, the supply remains limited.13
Xenografts are produced from animal bones but carry similar risks to allografts, with the extra added risk of animal-borne diseases. Moreover, commercial xenografts are commonly subjected to sterilisation by heat treatment14 because of the prion risk. Bio-OssTM xenografts are treated at a relatively low 300°C, while Cerabone® products are calcinated at temperatures of up to 1250°C. Both treatments damage bone constituents, harm osteoinductivity, and change the scaffold structure by increasing the hydroxyapatite (HA) crystal size.14 The severe heat treatment of Cerabone® destroys all organic compounds and even partially degrades HA into other compounds.15 Some research has shown that xenografts perform poorly, finding evidence of graft loosening and a lack of incorporation.16 These results are controversial, as other researchers have found good results with xenografts.17, 18 In practice, however, xenografts are rarely used.13
These issues create a demand for other alternatives, and artificial grafts could be the solution. Autografts and allografts are both osteoconductive and osteoinductive. An artificial allograft should aim to mimic both of these properties to achieve the best results, which makes materials that are chemically similar to bone an obvious choice. HA, the main component of natural bone, is naturally a popular choice in bone-implant research.19 However, natural bone mineral is not pure hydroxyapatite as it may contain carbonate, magnesium or fluorine replacement in its crystal structure, therefore the composition can be different from synthetic hydroxyapatite. Hydroxyapatite is osteoconductive and biocompatible, but has a very low biodegradation rate.19 Hydroxyapatite is observed to be osteoinductive in vivo in primates,20 possibly by adsorption of osteoinductive agents after implantation.
Biocompatibility is a general term to describe a biomaterial that is compatible with host tissue without causing toxicity, irritation, inflammation or an immune response. For regulatory control, it is a standard required by the International Organisation of Standardisation (ISO) as ISO 10993.21
Calcium carbonate is much more easily resorbed by the body than HA.13 Biodegradation is the process by which the implanted materials are broken down during host physiologic and metabolic activities. A low biodegradation rate is suboptimal, since the scaffold should ideally be broken down and replaced by natural bone to avoid unexpected risks such as infection leading to biofilm formation.22 However, rapid biodegradation is also not desired where new bone growth may not be able to keep up, leaving open areas and lowering the mechanical strength of the scaffold.
Although many alternatives are being developed, according to a market report, bone allografts remain the mainstream product for bone grafting. The revenue share of bone allografts accounted for over 57.0% in 2020, which is the largest segment within the market for bone grafts and substitutes.23 A realistic target for newly-developed bone grafts should be a product with an inorganic/organic composition similar to allografts which can overcome the drawbacks of bone allografts such as resource limitation, host tissue rejection and disease transmission.
The main databases used for this review include PubMed, Medline, Web of Science, United States Patent and Trademark Office (www.uspto.gov), Clinicaltrials.gov, and Google Scholar.
Conventional Bone Allograft Preparation
Bone matrix consists of a mixture of organic and inorganic materials. The inorganic component, which makes up about 65% of the matrix, consists mainly of highly substituted HA, a calcium and phosphate-rich compound. The organic components of the matrix are mainly collagen type I (90%), along with other non-collagenous compounds such as osteocalcin, proteins, glycans and lipids.24 Allografts, being derived from human bone, are highly osteoconductive, as well as biocompatible and osteoinductive.25
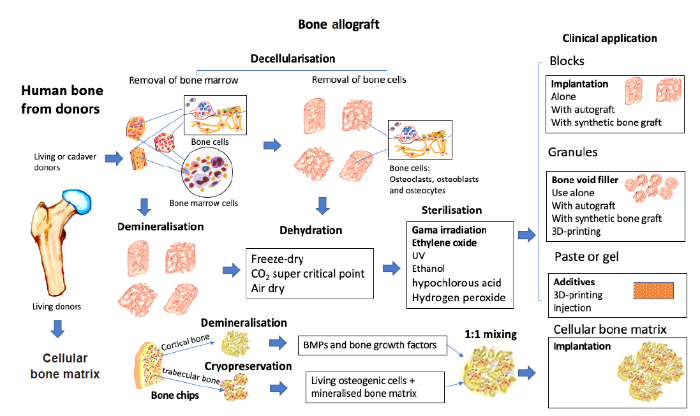
Bone allografts are typically processed within bone tissue banks. Unlike autografts, allografts are freeze-dried, decellularised and sterilised to reduce the risk of rejection and infection, respectively. These procedures remove cells, proteins, and potentially even collagen, lowering the bone-forming ability of the allograft.1, 25 Thus, some vital growth factors are removed, resulting in little to no osteoinductivity. The mechanical strength of bone allografts is reduced, and the bone integration slower than that of bone autografts. The current allograft manufacturing processes are summarised in Figure 1.
Figure 1.
Figure 1.
The conventional manufacturing processes and clinical applications of bone allografts. 3D: three-dimensional; UV: ultraviolet.
The main concerns of allograft use are the risk of disease transmission and the limited availability. A report showed that in the UK, the availability of bone allografts greatly exceeded the demand.8 This may be the case in the UK where tissue banks are well developed, but worldwide there are still shortages of bone allografts. Nonetheless, the availability of human bone allografts is not the highest priority, given that bone allografts account for 57% of the market share, as mentioned above. Therefore, removing immunogenic components, deactivation of pathogens and sterilisation of allograft bone are more important.
Bone allograft cleaning/decellularisation
Bone allografts are stored frozen or processed directly to small pieces under aseptic or clean-room conditions. Bone tissue contains lipids, bone marrow and bone cells such as osteoblasts, osteocytes and osteoclasts. Bone marrow cells are particularly immunogenic due to their enriched histocompatibility antigens. These cells, along with lipids, need to be removed during the cleaning process.
Bone allografts can be cleaned with a combination of physical, chemical and sterilisation processes. Physical cleaning processes include temperature control (4–65°C), sonication, water jet washes, high hydrostatic pressure and oscillating atmospheric pressure. Chemical-based cleaning processes include the use of alcohol, hydrogen peroxide, detergent (polyoxyethylene-23 lauryl ether, Triton X-100, and Tergitol NP40), and other solutions that produce hypochlorous acid.
Ethanol and detergents are bactericidal to some bacteria, but cannot completely sterilise and their penetration into tissue is very limited. Many detergents and chemicals inhibit key proteins that induce bone formation, such as bone morphogenetic proteins (BMPs), impacting the performance of the allograft after implantation. Hydrogen peroxide cleans bone tissue and acts as a bleach to whiten bone grafts. However, the decolourised bone can still contain immunogenic bone marrow tissue, and moreover, hydrogen peroxide can inhibit essential osteoinductive components of bone allografts.
The main limitation of conventional bone cleaning processes is that viruses, bacteria and fungi may still be present in the graft. Some combinational protocols have been developed, such as the “Allowash” from Life Net Tissue Bank, Biocleanse from Regeneration Technologies, Inc. and other patented methods such as supercritical fluid (U.S. Patent No. 5,725,579), and explosive decompression (U.S. Patent No. 5,288,462). After bone cleaning, further sterilisation is still required to deactivate pathogens. Bone allograft cleaning processes are summarised in Table 1.
Table 1 Allograft bone cleaning processes
| Bone clean | Methods/materials | Source |
|---|---|---|
| Physical process | Sonication | U.S. Patent No. 5,797,871 |
| Pressurised flow | U.S. Patent No 5,513,662 | |
| High pressure washing with agitation and liquid stream | U.S. Patent No. 5,333,625 | |
| Agitation/shaker | the University of Miami Tissue Bank | |
| Oscillating atmospheric pressure | U.S. Patent No. 6,652,818 | |
| Chemical process | Ethanol | KR101272958B1 |
| Polyoxyethylene-23 lauryl ether | Allowash XG® | |
| (n-Butyl) phosphate, betadyne, TritonX-100/TNBP | BioCleanse® | |
| 0.5% to 5% chlorhexidine gluconate | U.S. Patent No. 10,004,819 | |
| Sodium hypochlorite and hydrogen peroxide | U.S. Patent No. 7,507,254 | |
| Combinational | Sonication + detergent; water and alcohol | U.S. Patent application 20080188939 |
| Supercritical fluid | U.S. Patent No. 5,725,579 | |
| Explosive decompression | U.S. Patent No. 5,288,462 |
There are many different decellularisation methods, and some can be used in conjunction with others. A review by Blaudez et al.26 outlined that there are three main approaches: chemical, enzymatic and mechanical. Table 2 below outlines the more common techniques that have been trialled, each with its own advantages and disadvantages.
Table 2 Decellularisation methods for bone grafts*
| Method | Advantages | Drawbacks | |
|---|---|---|---|
| Chemical | SDS | Complete removal of cellular components | Damages ECM: • Collagen disruption • GAG reduction |
| Triton TnBP | Good preservation of the ECM | Poor cell removal efficiency | |
| Enzymatic | DNAse | • Not damaging to the ECM • Very efficient in DNA debris elimination | Difficult to wash off tissues. Works only in combination with treatment disrupting cell membranes |
| Trypsin | Efficient cell surface removal | Prolonged exposure can disrupt ECM | |
| EDTA | Disrupts cell adhesion to ECM | Inefficient alone, often combined with trypsin | |
| Mechanical | Freeze/thaw | Efficient disruption of cell membranes | Does not efficiently remove cellular components & can damage ECM |
| Pressure | Increases chemical exposure and debris removal in tissues | High pressures can affect ECM integrity |
Note: *Adapted from Blaudez et al.26 DNAse: deoxyribonuclease; ECM: extracellular matrix; EDTA: ethylenediamine tetraacetic acid; SDS: sodium dodecyl sulfate; TnBP: tri-n-butyl phosphate.
A combination of sonication, wash/centrifuge cycles, and treatments with ethanol and hydrogen peroxide has proved much more effective than any single method, leading to a 99.89% decrease in DNA. This was possibly caused by the mechanical effect of the sonication helping to clear DNA residue more effectively, revealing empty marrow cavities, a rough surface structure and distinct trabecular structures. However, these multi-treated scaffolds still retained more DNA and showed a somewhat lower cell viability when re-seeded compared to commercial allografts.27
Tutoplast®, a commercial allograft that has been found to perform well,27, 28 is processed in multiple steps. Processing starts with delipidation using acetone and sonication, osmotic treatment with saline, and oxidative treatment with 3% hydrogen peroxide to remove any fats and bone marrow.29 Serial dehydration is used to dehydrate the allografts. This method is considered to preserve the mineral matrix better than freeze-dried bone.29 A similar multi-step method may be advisable for artificial allografts as well.
Demineralised bone matrix
The therapeutic applications of DBM are derived from the discovery of BMPs in 1965 by Urist, who found that DBM implanted subcutaneously or intramuscularly could induce bone formation. This led to the identification of BMPs, which are now manufactured to be used alone or in combination with carriers for bone regeneration.32 However, DBM contains other extracellular bone matrix proteins, such as collagen type I and some collagen type IV and X, and can induce bone formation at very low concentrations of BMPs. One report showed that levels of the various BMPs per gram of human DBM were BMP-2: 22.4 ± 12.1 ng, BMP-4: 5.45 ± 2.05 ng, and BMP-7: 85.1 ± 34.6 ng.33
Due to the high efficiency of DBM-induced bone formation, human recombinant BMPs have been used with carriers to replace DBM in clinical practice. However, the effective doses of recombinant BMPs applied are excessively high. For example, the dose at which BMP-7 induces bone formation in animal models is 2.33 mg/g, which is 27,000-fold more than the physiological concentration of BMP-7 in DBM.34 The clinically-used doses of BMPs for spinal fusion are between 4.2 mg and 40 mg,35 which are equivalent to the concentration of BMP-2 in 187-1786 kg human DBM. The use of high doses of BMPs is not without risks: it is thought to be connected to a higher cancer incidence, though there is no conclusive evidence to support this.35 DBM provides a more physiological environment to induce bone regeneration than recombinant BMPs in carriers. However, it has the same disadvantages as allograft bone: limited resources and the risk of disease transmission, with lower structural strength. Additional Table 1 shows some commercially-available DBM products.36, 37
Sterilisation
Before a bone allograft or human DBM can be implanted into the host, they should be completely sterile. Contamination of grafts has led to severe, sometimes even fatal, infections in the past.38 Standard chemical solution sterilisation only sterilises surfaces where the graft tissue and solution are brought into contact. The deep part of a graft may be insufficiently penetrated, allowing pathogenic organisms to survive in the centre. The desired sterilisation process should effectively inactivate a wide range of bacteria, viruses and fungi without residual toxicity, yet retain the scaffold’s biomechanical strength and osteogenicity. Various sterilisation methods can be used, such as gamma irradiation, ethylene oxide gas treatment, thermal treatment with moist heat, beta-propiolactone, and treatment with antibiotics.39
Gamma irradiation with cobalt-60 is widely used for commercial allografts and bone banks, often in amounts from 17 to 35 kGy with 25 Gy often, but not always, considered a standard.29, 39-42 It is safe and effective at removing a variety of pathogens.39 One disadvantage of gamma irradiation is that it damages polypeptide chains. In allografts, this means that collagen specifically is targeted, reducing the mechanical properties of the graft proportionally to the amount of radiation.40, 42 High kGy gamma radiation is therefore not ideal, but too low may be insufficiently disinfecting. One solution posed is to treat the scaffolds with radioprotectants. This preserves the mechanical properties, and the radioprotectant used has been found not to be cytotoxic.43 Still, concerns remain over the solution’s safety for in-vivo use.40
Another important thing to consider is how sterilisation methods affect the osteogenicity of the implant by damaging or removing osteogenic compounds such as BMPs. Gamma irradiation, since it damages polypeptide chains, will damage BMPs and thus harm the osteoinductivity of the implant.44 This effect could be further exacerbated by the fact that BMPs primarily bind to the collagen/gelatine in grafts,40 which are also damaged by gamma radiation. In contrast, in another study TGF-β was shown to be unaffected by gamma irradiation.45 This difference could potentially be caused or exacerbated by the differing radiation intensities used, at 25 and 16–19 kGy, respectively.
Another effective sterilisation method is ethylene oxide gas treatment. During ethylene oxide treatment the ethylene oxide gas is converted into ethylene glycol, a cytotoxic and carcinogenic compound.39, 46 These compounds can be kept within US Food and Drug Administration (FDA)-approved levels45 but have been shown to be cytotoxic even in approved amounts.46
Unlike gamma irradiation, ethylene oxide gas does not directly target BMPs and has been shown to preserve the osteogenicity of collagen-BMP implants more than 25 kGy gamma irradiation. The temperature used is very important, as BMPs are heat-labile: the loss of osteogenicity in collagen-BMP implants has been estimated to be about one third after fumigation at 29°C47 while treatment at 37°C or 55°C proved more damaging compared to 29°C.44 A temperature of 29°C is thought to be the lowest temperature at which safe sterilisation can be achieved,44 since the sterilising capacity of ethylene oxide decreases by 50% for every 10°C decrease in temperature.48 These results are somewhat controversial. Some studies found similar or worse degradation of bone-forming capacity,49-51 while other studies found that it was relatively unaffected.52, 53 This is likely to be caused in part by the sterilisation being a function of temperature, humidity, gas concentration and exposure time-all variables that are not standardised. Particle size has also been shown to make a difference,51 and even the fat content of the scaffold is hypothesised to influence the damage rate.52 TGF-β has been shown to be almost fully inactivated by ethylene oxide gas when fumigated at 60°C;45 lower temperatures are likely to be less damaging.
Implants treated with ethylene oxide were found to produce inflammatory responses upon intraarticular transplantation in one study, though only in soft-tissue allografts for anterior cruciate ligament replacements and the results were not reproducible. Therefore, ethylene oxide sterilisation is likely safe and is a very effective tissue sterilisation method.
Cellular bone matrices
Previous reports have described that bone marrow and bone cells in an allograft are a source of immunogens that should be removed during tissue processing. In contradiction to these findings, a new method that uses cells from living donors, termed ‘cellular bone matrices’) has been developed recently is and widely commercially available (Table 3).54, 55 It is estimated that 17% of all bone grafts in the USA are cellular bone matrices.
Table 3 Commercial cellular bone matrices and their basic characteristics*
| Graft name | Vendor | Components | Cell count | Cell viability |
|---|---|---|---|---|
| Trinity ELITE | Orthofix Medical | Cancellous bone containing viable cells and demineralised bone | ≥ 500000 cells/mL, of which > 100000 cells/mL are osteogenic cells | ≥ 70% |
| Vivigen | DePuy Synthes | Corticocancellous chips containing lineage-committed bone cells and demineralised bone particulate | > 16000 cells/mL | 96% |
| Cellentra | Zimmer-Biomet | Cancellous bone containing viable cells and demineralised cortical bone | ≥ 250000 cells/mL in the cancellous tissue | ≥ 70% |
| Osteocel Pro | NuVasive | Cryopreserved viable cancellous matrix and ground demineralised bone matrix | Average of 3 million cells/mL | > 85% on average |
| Bio4 | Stryker | A cryopreserved viable bone matrix product that contains native matrix, endogenous osteoblasts and mesenchymal stem cells, and osteoinductive and angiogenic growth factors | On average, ≥ 600000 cells/mL | ≥ 70% |
| Map3 | RTI Surgical | Cortical cancellous bone chips, demineralised bone matrix and multipotent adult progenitor cell-class cells | ≥ 50000 viable cells/mL of implant | Not available |
| Allostem | Allosource | Allogenic adult adipose-derived mesenchymal stem cells combined with partially demineralised allograft bone. | 66255 viable cells/mL | Not available |
By definition, cellular bone matrices are allogenic or synthetic bone grafts containing live bone osteogenic cells. These materials are believed to replace autografts as they contain three components that are essential to bone formation: an osteoconductive scaffold for cell attachment and growth, extracellular growth factors with promoted osteogenic properties for cell proliferation and differentiation, and osteogenic cells that can differentiate into osteoblastic cells and are essential for bone regeneration, remodelling and maturation.55, 56
Although demineralised bone matrices are widely accepted to induce bone formation after implantation, the benefit of the viable bone‐forming cells or mesenchymal stem cells (MSCs) in cellular bone matrices is not universally acknowledged.57
First of all, cellular bone matrices contain bone marrow cells within the trabecular bone chips, which are difficult to separate and remove from MSCs. Bone marrow cells cause a host immune response and rejection, which has previously happened in bone marrow transplantation.
Secondly, many cells may not survive after cryopreservation, even if the cells are still viable at the time of thawing.
Finally, it is unclear whether living cells can survive under low oxygen and nutrient deprivation conditions after implantation.55
Due to differences in preparation methods, there are certain differences amongst currently-available cellular bone matrices in regard to their donor status, carrier matrix, cell sources and viability. The effects of these products in spinal fusion are still highly inconclusive. Nonetheless, commercial cellular bone matrices have been widely used for spinal fusion, non-union and fracture repair, and thus have paved the way for future cytotherapy.
Bone Tissue Engineering
Bone tissue engineering is believed to be a solution to replace autografts. This technique utilises synthetic organic/inorganic biomaterials as scaffolds as well as human autologous/allogenic osteogenic stem cells to seed the scaffolds with, and involves growing these in different bioreactors which mimic physiological conditions to produce bone-like tissue in vitro. The end product is expected to contain three key autograft components: a calcified bone-like matrix to conduct osteogenic cell attachment, a growth factor-rich environment to induce osteogenesis, and living stem cells with great proliferation potential to support new bone formation.
A major challenge in bone tissue engineering for transplantation is how the cells grown in vitro can survive in the body under the hypoxic conditions present before full vascularisation, and if there are any risk factors associated with the implantation of in vitro amplified and differentiated cells back into the body. Bone void fillers such as allografts are regulated as class II medical devices, whereas transplantation of living cells is regulated under ‘cell tissue’ as class III implants. Therefore the regulation, quality assurance and clinical evaluation are at very different levels.
Synthetic biomaterials as bone tissue engineering scaffolds
The purpose of bone tissue engineering is to create bone-like tissue in vitro and transplant the engineered tissue to regenerate bone defects. Living tissue is rich in extracellular matrix. Therefore, synthetic biomaterials are selected as scaffolds for stem cells to attach to, allowing them to proliferate and differentiate to form bone-like tissue.
Presently available bone tissue engineering scaffolds can be classified as the following types: (1) polymers; (2) ceramics/cement/calcium salts; (3) natural or natural mimetic materials; and (4) metals or alloys. Some commercially-available synthetic biomaterials for bone grafting and bone tissue engineering are listed in Table 4.
Table 4 Commercial bone grafts and their basic characteristics*
| Brand Name | Composition | Manufacturer | Price | |
|---|---|---|---|---|
| HA, HA/TCP, TCP | Edbone | Implant synthetic bone - 75% hydroxyapatite & 25% β-tricalcium phosphate - Straumann/Megagen/Nobelbiocare/Dio | Ethoss Regeneration Ltd. | 0.5 g, £39.86 |
| BiceraTM | Composed of HAP (60%) and β-tricalcium phosphate (40%) bioceramic which is similar to bone mineral | Hannox International Corporation | n.a. | |
| MASTERGRAFT® | β-Tricalcium phosphate (85%) and hydroxyapatite (15%) | Medtronic Sofamor Danek USA, Inc. | n.a. | |
| Syntoss | Dental implant synthetic bone graft β-tricalcium phosphate material block | Dental solution Israel | £46.80 | |
| Kuraray | Bone graft materials (synthetic bone substitute, bioresorbable bone graft substitute β-tricalcium phosphate) | Biomaterial Department Japan | n.a. | |
| chronOS® | A synthetic β-tricalcium phosphate bone void filler which is radiopaque, resorbable and osteoconductive | DePuy Synthes | n.a | |
| G Graft | Bone graft material hydroxyapatite with collagen implant dental | Rohit Enterprises, India | £32.87/mL | |
| Ostoden | Synthetic calcium phosphate based bone graft device | Ammdent | £22.14 | |
| MBCP® | Synthetic bone graft substitute bioactive calcium phosphate | Biomatlante SAS, France | n.a | |
| Silicated calcium phosphate | Osteo3 | A synthetic bone graft substitute, silicated calcium phosphate | OssDsign | n.a. |
| ActifuseTM | Silicated calcium phosphate | ApaTech Limited | n.a | |
| Coral | Corebone | Bio-active coral core bone graft sterile granules | Global Dental Transfer, Israel | n.a |
| BoneMedik | A porous, interconnected coral structure which is compatible with human bone structure | Meta Biomed Co. Ltd. | n.a. | |
| Xeno-graft, Bovine | Zenoss | Bovine bone graft material in blocks | Dental solution Israel | £56.40 |
| OSTEONTM II | Bone void filler, synthetic bone graft + collagen type 1 (bovine) | Genoss | 0.25g/ £57.28 | |
| Biotechmat | Dental bone graft - Xenograft (Bovine) - similar to Bioss | Technology in Biomaterials | £62.02 | |
| Natural | Bio-Oss | Dental Bio Oss collagen bone graft material, natural bone grafting materials | Geistlich UK | £154.51 |
Note: HAP: hydroxyapatite; n.a.: not applicable; TCP: β-tricalcium phosphate.
To mimic autografts, the biomaterials used have to be biodegradable. Non-biodegradable and slowly biodegradable products, such as polymethyl methacrylate, polyether-ether-ketone, alumina and HA ceramics are not suitable. Secondly, the mechanical properties of the scaffolds should be similar to those of bone tissue; scaffolds with low mechanical strength, such as collagen, gelatine, poly(lactic-co-glycolic acid) and bioglass, or scaffolds that are too rigid, such as metals or alloys, are also not suitable. Thirdly, the scaffold materials have to be biocompatible so that they both support osteogenic stem cell growth and differentiation in vitro and avoid local inflammatory or foreign body response. To this end, polylactic acid and polyglycolic acid may also have limited use. Finally, human trabecular bone has an interconnected porous and vascularised structure ranging from 50% to 90% porosity.58 It is highly beneficial for grafts to mimic this structure, as it allows nutrients and cells to reach every part of the scaffold. There are many ways to create a porous structure, many dependent on the materials used. Examples include gas foaming, sintering, fibre bonding, solvent casting and particulate leaching, membrane lamination, and melt moulding.59 These methods all have significant drawbacks, such as a lack of pore interconnectivity, temperature requirements, or difficulty regulating pore size.
Three-dimensional (3D) printing poses a potential solution to many of these issues, offering the ability to create scaffolds with consistent pore structure and size. However, it is still a significant challenge to obtain a suitable scaffold to meet all the criteria of autografts. The material used has to be suited to at least one 3D printing method, each of which has its own drawbacks in the form of temperature requirements, material limitations, or limited structure layouts. There are, therefore, still some constraints to the material production that have to be worked around.
Mesenchymal stem cells
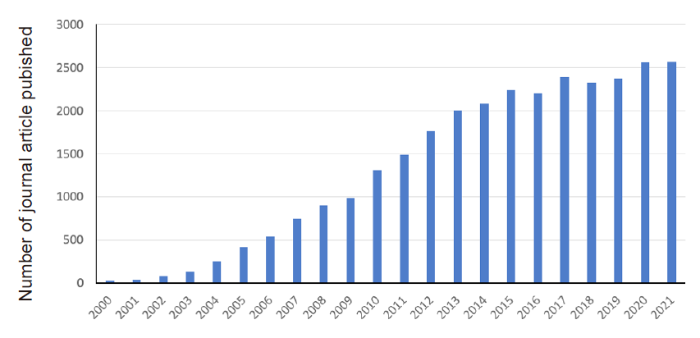
Figure 2.
Figure 2.
The number of journal article publications with the keywords ‘mesenchymal stem cells’ in their titles between 2000–2021 in PubMed index.
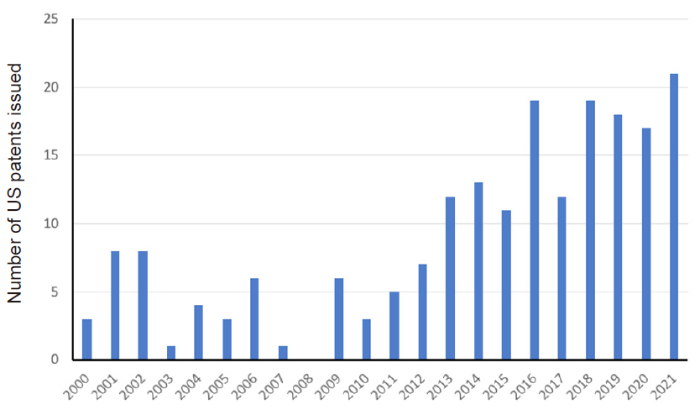
Figure 3.
Figure 3.
The US patents granted with the keywords ‘mesenchymal stem cells’ in the title.
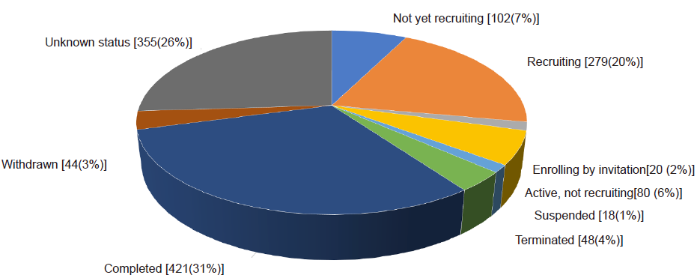
Figure 4.
Figure 4.
The number of clinical trials registered in the ClinicalTrials.gov with the keywords ‘mesenchymal stem cells’ in the title.
Since 2000, 29,435 journal articles in PubMed were titled with the keywords ‘mesenchymal stem cells’, a steady increase from 24 articles in the year 2000 to 2571 in 2021 (Figure 2). Specifically, over 2000 articles have been published each year since 2013. MSCs are believed to be the most promising cells for tissue and cytotherapy.
Normally the number of patents issued and clinical trials can indicate the commercialisation of potential products. There have been in total 206 patents issued by the US Patent Office (https://patft.uspto.gov/netahtml/PTO/search-bool.html) titled with the keywords of ‘mesenchymal stem cells’ (Figure 3). More than ten patents on MSCs were issued by the US Patent Office each year from 2013.
By searching clinical trials registered at the US National Library of Medicine (clinicaltrials.gov) that currently contains 401,145 research studies in 50 states in the US and 220 countries all over the world, we identified 1389 studies on ‘MSCs’ (Figure 4).62-64 Among these clinical trials, 421 studies were completed, yet only 10 products were approved for clinical application worldwide (Table 5) and no products were approved by the FDA in the US.
Table 5 Mesenchymal stem cell products with regulatory approval
| Mesenchymal stem cell product (company) | Approval granted (year) | Indication | Product type |
|---|---|---|---|
| Queencell (Anterogen Co. Ltd.) | South Korea (2010) | Subcutaneous tissue defects | Autologous human AT-MSC |
| Cellgram-AMI (Pharmicell Co. Ltd.) | South Korea (2011) | Acute myocardial infarction | Autologous human BM-MSC |
| Cartistem (Medipost Co. Ltd.) | South Korea (2012) | Knee articular cartilage defects | Allogeneic human UC-MSC |
| Cupistem (Anterogen Co. Ltd.) | South Korea (2012) | Crohn’s fistula | Autologous human BM-MSC |
| Prochymal, remestemcel-L (Osiris Therapeutics Inc., Mesoblast Ltd.) | Canada (2012) New Zealand (2012) | Graft versus host disease | Allogeneic human BM-MSC |
| Neuronata-R (Corestem Inc.) | South Korea (2014) | Amyotrophic lateral sclerosis | Autologous human BM-MSC |
| Temcell HS (JCR Pharmaceuticals) | Japan (2015) | Graft versus host disease | Allogeneic human BM-MSC |
| Stempeucel (Stempeutics Research PVT) | India (2016) | Critical limb ischemia | Allogeneic human BM-MSC |
| Alofisel (TiGenix NV/Takeda) | Europe (2018) | Complex perianal fistulas in Crohn’s disease | Allogeneic human AT-MSC |
| Stemirac (Nipro Corp) | Japan (2018) | Spinal cord injury | Autologous human BM-MSC |
Note: Data are from Wright et al.,62 Pereira Chilima et al.63 and Levy et al.64 AT-MSC: adipose tissue mesenchymal stem cells; BM-MSC: bone marrow mesenchymal stem cells.
The limitation of the concept of bone tissue engineering
There is still a long way to go before bone tissue engineering products containing living cells can be commercialised as a routine clinical procedure. As mentioned above, the current bone graft market is dominated by allografts. This may remain the same unless bone tissue engineering products can achieve the same efficacy/safety measures but overcome the drawbacks of bone allografts at an equivalent cost.
The high costs of bone tissue engineering products come from the additional aseptic tissue culture facilities needed, the maintenance of living cells during and after transplantation, and the constant monitoring of the cells for contamination/mutation. With the additional high costs, there has not been evidence to demonstrate that implanted cells have long-term and better osteogenic capacity compared to local osteogenic progenitor cells at the site of a bone defect.
The concept of bone tissue engineering may well be proven in research, but similar to the concept of MSCs, difficulties remain in manufacturing and commercialising bone tissue engineering products to benefit patients and society.
Potential Artificial Allograft Manufacturing Processes
With unparalleled huge public and private funding support and extensive research over the past 20 years, stem cell banks have been established in almost all major hospitals/tissue banks worldwide. The cryopreservation of the cell banks is a burden on the resources of healthcare systems where finances are already tight. At the same time, technologies for the use of stem cells have been rapidly developed. We are at a turning point to translate these technologies into socioeconomic benefits.
Since autografts are currently the golden standard, an ideal bone graft could be manufactured to resemble an autograft, with living cells embedded inside. Such a product has clear advantages; the living cells inside potentially allow for bone regeneration unmatched by allografts or artificial alternatives. However, bone tissue engineering using autologous osteogenic stem cells is not yet to be approved for clinical application, as mentioned above.
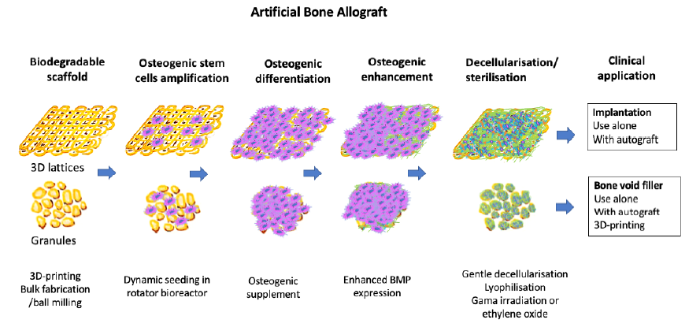
We therefore believe that the way forward is to step back to create an ‘artificial bone allograft’ that is substantially equivalent to natural bone allografts and can be manufactured and marketed.
Artificial bone allografts aim to overcome the key drawbacks of conventional allografts: scarcity, host immune response and potential contamination. Accordingly, the mass production process, decellularisation and sterilisation techniques are discussed.
Selection of scaffolds and stem cells
To mimic a bone allograft, the scaffold used for in vitro bone tissue engineering requires a synthetic composition similar to that of natural bone matrix. Compared with ceramics, polymers and metal, a mixture of HA and calcium carbonate creates a much more suitable material that, depending on the ratio used, can have a biodegradation rate compatible with the growth rate of new bone.19, 65
Corals are made of calcium carbonate, and some species naturally have a structure similar to human trabecular bone, including mechanical properties.66 Coral calcium carbonate can be partially hydrothermally converted into HA to create coralline HA and calcium carbonate with an adjustable resorption rate.66, 67 Coralline HA has been widely used in bone implant research and has been shown to be a suitable material for bone grafts.68, 69 However, a considerable disadvantage is that corals are endangered; therefore, it is a poor choice to solve allograft scarcity despite its otherwise suitable properties.
HA and calcium carbonate scaffolds are suited to extrusion 3D printing,19 which easily lends itself to larger-scale production as scaffolds can be produced almost continuously and with consistent shape and quality.
There are a number of requirements for the selection of the cells for mass production of allografts. The cells need to be able to differentiate into bone cells, and cells that have great proliferation capacity and are suitable for multiple subpassages are desired. Osteoprogenitors are therefore an obvious choice. Bone marrow stromal stem cells, also termed mesenchymal stem cells (MSCs), are a group of pluripotent, heterogenous cells that may differentiate into different lineages, including bone.70 They are reasonably popular in bone research,71, 72 being relatively accessible. Autologous bone marrow MSCs are the most used stem cells for in vitro bone tissue engineering.73 They are considered an accessible source,74 are known to express osteogenic markers,75 and can differentiate into osteocytes,76 A disadvantage is the process of obtaining them for the purpose of artificial allograft, which requires bone marrow aspiration,74 and ethics requirement.
Ideally, cells should be derived from medical waste as to be both abundant and ethical. Mesenchymal stem cells can be isolated from umbilical cord matrix, umbilical cord blood, and adipose tissue, all sources that are more suitable from an ethics perspective. MSCs isolated from different sources behave somewhat differently, and ideally their osteogenic capacity should at least match that of bone marrow MSCs. Comparative studies between bone marrow MSCs, umbilical cord blood MSCs and adipose tissue MSCs found that all three types are able to osteogenically differentiate77 and that the osteogenic differentiation capacity was similar between the three.78 In the case of adipose tissue MSCs this is not entirely uncontroversial; at least one study did find that their osteogenic capacity is lower than that of bone marrow MSCs.79 Differences were found in the expression of mesenchymal stem cell markers, with umbilical cord blood MSCs showing lower expression of CD105 and adipose tissue MSCs having reduced CD106 expression.77 Umbilical cord blood MSCs were shown to have the highest expansion potential, allowing subculture for at least 10 passages, while adipose tissue MSCs showed much lower senescence in earlier passages.77 A disadvantage of umbilical cord blood MSCs is that they are difficult to isolate compared to alternatives, with many studies failing to isolate MSCs from a large percentage of their samples.78, 80 A solution relying on only umbilical cord blood MSCs may therefore be less suitable, though there may be merit in using them in conjunction with umbilical cord matrix MSCs to avoid waste.
Umbilical cord matrix MSCs are somewhat less osteogenic than bone marrow MSCs.75, 81 For both umbilical cord matrix and adipose tissue MSCs, a slightly lower osteogenic capacity compared to bone marrow MSCs may be a solvable problem. The osteogenic differentiation capacity of umbilical cord matrix MSCs was shown to be retained better in monolayer cultures82 and is affected by which (mixture of) parts of the cord are used for MSC isolation.83 The proliferation rate of adipose tissue MSCs was shown to be affected by the tissue harvesting method,84 and the osteogenic capacity is affected by the MSC harvesting method85 and donor age.86 Undoubtedly, there is still room to optimize the osteogenic potential of both MSC types. The osteogenicity could also be enhanced through optimising other culture conditions, for example by altering the chemical concentrations in osteogenic medium. A lot remains unclear about the factors that affect osteogenicity, and further research is warranted.
Bioreactors for mass production
As we intend to create a product as close to natural allografts as possible, it makes sense to try and mimic in-vivo conditions to simulate natural cell-cell and cell-extracellular matrix interactions as closely as possible. Bioreactors are suitable for the bulk culture of MSC-seeded scaffolds.
Grafts are commonly seeded with cells before being transferred to a bioreactor. Grafts can be seeded directly by soaking them in a cell suspension or by pipetting cells onto the graft.72
In large grafts, liquid penetration into the centre may become an issue as air bubbles become trapped.87 Scaffolds can be submerged and then placed in a vacuum to remove air from the scaffold, although some air is resorbed when the vacuum is released.87 Hasegawa et al.87 describe a syringe method where the scaffolds are submerged inside a syringe, negative pressure is applied, and the syringes are shaken to remove the air. The number of cells, alkaline phosphatase activity and osteocalcin content of artificial allografts were compared in these four methods. The syringe method led to the best results, followed by the pipetting and vacuum methods.
Soaking consistently performed worse than any other method. Histological analysis showed much better bone growth in the centre of syringe scaffolds compared with soaked scaffolds eight weeks after implantation in mice.87 It must be noted that the syringe method is relatively labour-intensive and thus more suitable for small batches. The pipetting method or combining the vacuum method with a shaking step is worth looking into for mass production.
There is no clear consensus on how many cells should be seeded onto a scaffold. Various studies suggest that seeding more cells onto scaffolds is not necessarily beneficial as it leads to reduced proliferation,72, 88 while others found that collagen and glycosaminoglycan production were still increased in samples seeded with a higher number of cells.89 Numbers too low, on the other hand, may inhibit cell penetration into the scaffold.90 The number of cells required is likely dependent on many parameters, including the seeding method, cell type, and scaffold material and structure, and should be optimised for the particular combination used.90
There are several factors to consider when deciding what type of bioreactor to use. The cells should be seeded uniformly so that new bone is also grown uniformly after scaffold implantation. There should also be a way for nutrients to reach the centre of scaffolds, something that can be challenging even in smaller scaffolds.91 Nutrient flow in static cultures has been shown to halt even as low as 1 millimetre deep into grafts.92 Furthermore, static culture also depletes any oxygen and carbon dioxide in the medium.93 Static cultures are therefore entirely unsuitable, and bioreactors with a flow mechanism are a necessity.
One popular choice for bone graft culture is perfusion bioreactors. Some studies even go as far as to say they are essential for grafts of clinically relevant size.94 These bioreactors use pressure to force a flow of medium through the grafts. Their main advantage is thus that nutrients will reach every part of the graft, even in the centre. An additional advantage is that bone formation is encouraged by mechanical pressure.95, 96 Thus, the perfusion bioreactor actively encourages bone growth. Despite these advantages, perfusion bioreactors are not suitable for mass production because of their extremely low capacity. Needing a separate well for every graft, perfusion bioreactor capacity is significantly harder to scale up compared to other types of bioreactors.
Rotating wall vessel (RWV) bioreactors have the scaffolds sitting in a horizontal spinning cylinder. The rotation speed of the cylinder and the upwards hydrodynamic force can be balanced so that the scaffolds remain in suspension forever, never touching the edges. This movement allows for sufficient nutrient flow while reducing shear stresses.96 RWV bioreactors are useful for bone culture,95 constructs cultured in RWV bioreactors showed more natural chemical and mechanical properties compared to those cultured in spinning flasks.91 While theoretically easier to scale up than perfusion bioreactors, commercial RWV bioreactors still have low maximum capacities.
Bioreactor types that are more suited to bulk production are spinning and rocking bioreactors. The scaffolds float freely in medium, and flow is created by a stirring or rocking motion. Spinning bioreactors significantly outperform static bioreactors when measuring collagen and glycosaminoglycan synthesis.
The mixing intensity (eddy velocity, ranging from 0.31 to 1.24 cm/s) made no significant difference. Increasing the mixing intensity led to higher collagen and glycosaminoglycan production,93 which could be caused by increased nutrient flow and/or increased mechanical stress,96 but increased shear stress caused these to be leached into the medium instead of remaining in the scaffold.93 Spinning bioreactors lead to uniform cell distribution into grafts,44 and their overall performance is comparable to RWV bioreactors.97 Because of the lack of pressure, nutrients may not reach the centre of large grafts as effectively as in perfusion reactors. Many commercial allografts are sold as small chips instead of large blocks, which could be a potential solution if the nutrient flow poses a problem.
Improving osteogenicity of stem cells
Bone allografts are expected to contain osteogenic compounds, including BMPs, RunX2, TGF-β, stromal cell-derived factor 1, insulin-like growth factor 1, and many others.98 These compounds have long been proven to be beneficial: bovine collagen scaffolds enhanced with BMP-7 and implanted in humans were shown to reduce the incidence of postoperative osteomyelitis compared to autografts.99 Seeding of DBM scaffolds with TGF-β1 before implantation in dogs was shown to accelerate bone repair,31 and collagen and DBM scaffolds seeded with BMP-9 were significantly more osteoinductive than controls when implanted in rabbits.100
Scaffolds can be enhanced by soaking them in known growth factors, such as TGF-β and BMPs.31, 69 Notably, xenogeneic BMPs are immunogenic69 and recombinant variants are more suitable for use in bone grafts. However, clinically-used human recombinant BMPs have been used at excessively high doses.35 Either way, mimicking the exact number and concentration of compounds found in allografts would be highly expensive and very difficult, if not impossible, as there are many different compounds with their own ideal concentrations.
A more practical and efficient manner involves the creation of scaffolds that are then seeded with stem cells. When stem cells undergo osteogenic differentiation they can produce osteogenic compounds that stay in the scaffold even after decellularization, enhancing the osteoinductive capacity of the artificial allografts and increasing their similarity to natural allografts.
Previous research proved that certain compounds, such as retinoic acid, improve osteogenic differentiation and enhance BMP-7 expression both in vitro and in vivo.101
Icariin, a flavonoid extracted from a herb, can increase BMP-4 expression of MC3T3-E1 cells tenfold over control cells102 and increases BMP-2 expression in the same cell line.103 These effects were confirmed by further in vitro and in vivo experiments where icariin upregulated BMP-2 expression and enhanced bone regeneration at the suggested dose of 30 µg/mL.104 As both retinoic acid and icariin enhance osteogenesis, we may assume that BMPs and other growth factors are expressed simultaneously in the engineered products.
Another approach is to specifically enhance stem cell BMP expression through genetic modification of stem cells. After gene transfection through different vectors, the transfected cells can express BMPs at a concentration of ng/mL for weeks to months.105, 106 However, there are safety concerns about viral vectors as a tool for BMP delivery in clinical applications. Non-viral vectors are therefore developed for this purpose.106-108
In producing artificial allografts where the cells are removed before transplantation, the vectors used are less of a concern. Regulatory guidelines need to be followed to ensure the safety of the product.
Decellularisation of artificial bone grafts
Apart from cellular bone matrix, conventionally used bone allografts do not contain living cells. Most cell contents are removed in the decellularisation process in order to prevent the risk of rejection or disease transfer while preserving the osteogenic compounds.109 This method has also been used to improve allografts.110
Both allografts and xenografts are decellularised before use to prevent any immune responses. The extracellular matrix is often spared from the immune response; it also aids in cell proliferation and differentiation during the healing process and provides a structure and substrate for cell adhesion. Therefore, the aim of decellularisation methods is to maximise the removal of cellular components while minimising loss of the extracellular matrix and other desired components such as BMPs. A further benefit of decellularisation is that the graft material is far easier to store and transport. This also benefits patients as the product can be collected and stored for later use, driving down costs in the long run and expanding the operation window.109
Since in vitro engineered artificial bone allografts do not contain bone marrow or blood vessels/lipids, they are expected to require a relatively simple decellularisation process compared to human allografts and xenografts (Table 2). Minimal steps should be needed, such as freeze-thawing, sonication or carbon dioxide super critical fluid process. However, more research evidence is required to validate a standard procedure.
Sterilisation of artificial bone grafts
Artificial bone allografts are expected to be produced under aseptic conditions, which are much less likely to be contaminated by harmful microbes. The benefit of using stem cells and in vitro tissue engineering is to produce extracellular matrix and growth factors that may enhance bone regeneration after implantation. Commonly-used sterilisation methods, such as autoclaving, ethanol, hypochlorous acid, hydrogen peroxide and antibiotics, are not suitable to sterilise the materials produced.
Sterilisation by gamma irradiation may damage organic compounds such as collagen and BMPs, whereas ethylene oxide gas does not directly affect BMPs, and has been shown to retain the osteogenicity of collagen - BMP implants under more than 25 kGy gamma radiation;45 lower temperatures may potentially be less damaging. Ethylene oxide gas is expected to be a more suitable sterilisation method for artificial bone allografts.
Safety assurance and regulatory considerations
All bone graft substitutes are subject to regulatory control before clinical application. The process varies by country and region. The FDA provides a typical example of regulatory control.
By definition, artificial bone allografts are intended to fill bone voids or gaps caused by trauma or surgery, where the bone defects are not intrinsic to the bony structural stability. According to FDA special control guidance CRF §888.3045111 and CFR §872.3930,112 they can be classified as “Class II devices.” Therefore, premarket notification procedures and special controls in combination with general controls are sufficient to provide reasonable assurance of the bone graft’s safety and effectiveness.
Whether stem cells used with biomaterials will be treated as biological products according to FDA guidance is an interesting question. Clearly, the cells used for artificial allografts are removed at the endpoint, so the cells do not contribute a primary mode of action for the combination product. However, a product evaluation from the Centre for Biologics Evaluation and Research and the Office of Cellular, Tissue and Gene Therapies may be necessary.
If a bone graft contains drugs, it will be regulated as a Class III device by the FDA and therefore be subject to premarket approval. For example, human DBM combined with other biomaterials is regulated under Class III device regulations.
To register a bone graft through premarket notification or FDA 510(k) it will need to address the issues covered in the special control guidance to meet the recommendations of the guidance, or in some other way to provide equivalent assurances of safety and effectiveness.
A full biocompatibility evaluation will include cytotoxicity (ISO 10993-5); sensitisation (ISO 10993-10), irritation or intracutaneous reactivity (ISO 10993-10); acute systemic toxicity (ISO 10993-11); pyrogenicity; subacute/subchronic toxicity (ISO 10993-11); genotoxicity (ISO 10993-5); implantation (ISO 10993-6); chronic toxicity (per ISO 10993-11); and carcinogenicity (ISO 10993-5).113
It is important to know that according to 510(k),60 if the raw materials, manufacturing process, sterilisation, and packaging of a bone graft are all identical to those of another cleared product in the U.S. market, no additional biocompatibility testing is necessary. Even clinical trials for 510(k) bone grafts may not be required unless different technology and formulation have been used, indications of use have been changed, or concerns have been raised during biocompatibility tests.
Conclusion and Future Perspective
Bone grafts harvested from patients themselves or autografts work well but are scarce, and a substitute is needed. Bone allografts remain the most suitable bone graft substitute for bone autografts and currently dominate the market; however, bone allografts are a limited resource and there is a risk of host immune response/rejection and disease transmission. As mentioned above, there is currently difficulty in the clinical application and regulatory approval of stem cell therapy, gene therapy, and bone tissue engineering containing living cells. Commonly used artificial bone graft substitutes such as HA ceramics, bioglasses, biopolymers and metals each only share a relatively small market size. Therefore, the replacement for a bone allograft should be substantially similar to an allograft itself. We propose that artificial bone allografts can be an immediate replacement for conventional bone allografts (Figure 5).
Figure 5.
Figure 5.
The proposed manufacturing process. Scaffold is produced, then seeded with cells. When these cells osteogenically differentiate they will express growth factors such as BMP, which will remain on the scaffold after decellularisation. 3D: three-dimensional; BMP: bone morphogenic proteins.
Artificial bone allografts utilise well-established bone tissue engineering technology with selected optimal scaffolds and osteogenic stem cells to produce an extracellular matrix/growth factor-rich and biodegradable artificial bone graft. The prospective manufacturing process shown in Figure 5 can be as follows:
1. Scaffold selection: biodegradable porous scaffold composed of amorphous hydroxyapatite, calcium carbonate, collagen/gelatine and fabricated at near body temperature, ideally through 3D printing.
2. Stem cell selection: allogenic osteogenic stem cells from bone marrow, adipose tissue, periosteum or umbilical cord matrix, ideally those from young donors without the need for any extra procedure and without ethical concerns, such as umbilical cord matrix.
3. Mass production: rotating, spinning or shaking bioreactors with environmental control of gas, pH, nutrition and metabolism monitoring.
4. Osteogenicity enhancement: intrinsic BMPs and other osteogenic components produced by stem cells through addition of drugs, supplements or genetic modification.
5. Decellularisation: gentle decellularisation by using freeze-thaw cycles, sonication or supercritical point processes.
6. Sterilisation: ethylene oxide sterilisation of dried end products.
7. Regulatory approval: FDA 510(k) class II or III.
8. Application: bone void fillers to non-weightbearing bone, spinal fusion or dental application.
The above processes are technically feasible and ready. However, the translation from a prospective concept to manufacturing an end product for regulatory approval and clinical application is a difficult journey. The future success of the proposed artificial bone allograft will showcase the attainment of biomaterial translation.
There are a number of limitations of this perspective review. First of all, the concept and technologies described in this review to produce artificial allograft are already well developed and published in different sources of literature. The only gap is that there is not an artificial allograft end product in the market place. Secondly, there is a wide range of choices to select scaffolds, stem cells, osteogenic enhancement, decellularization and sterilisation technology, yet there is not any solid evidence of an optimal and standard manufacturing process. Finally, although there are certain drawbacks of current bone allografts, they still dominate the market. To replace bone allograft in clinical practice using artificial allograft, it is important to demonstrate substantial social-economic benefit that artificial allograft is over conventional bone allografts
Author contributions
Original concept, figure preparation and manuscript revision: ZX; online search, and manuscript draft and literature review: ZX, ES, AG; tables preparation: ZX, AG. All authors read and approved the final version of the manuscript.
Financial support
The work was partially supported by the ‘Health Technology Centre, HTC’ Accelerate project of Swansea University Medical School and the Institute for Innovative Materials, Processing and Numerical Technologies (IMPACT) project, Swansea University.
Conflicts of interest statement
The authors declare no competing interests.
Open access statement
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additiond Table 1. Commercially-available demineralised bone matrix*
| Source company | Product | DBM (%) | Carrier | Form | Indication |
|---|---|---|---|---|---|
| Allosource | AlloFuse | 36 (putty), 29 (gel) | Reverse phase medium | Putty, gel | Bone void filler/bone graft extender |
| Alphatech | AlphaGRAFT | 80 | Acellular matrix | Paste | Bone void filler |
| Amend Surgical | NanoFUSE | ND | 45S5 bioactive glass | Putty | Bone void filler |
| Bactarin International | OsteoSelect | 74 | Carboxymethylcellulose, phosphate-buffered saline | Putty | Bone void filler |
| Osteosponge | 100 | No carrier | Block, Disc, SC, strip filler | Bone void filler | |
| Exactech | Altiva | ND | Gelatine | Paste | Bone void filler |
| Optefil | 24 | Gelatine | Paste | Bone void filler | |
| Opteform | ND | Cortical and cancellous bone chips suspended in collagen-gelatine | Paste | Bone void filler | |
| Integra | Accell Connexus | 70 | Poloxamer reverse phase medium | Putty | Bone void filler/ bone graft extender |
| Accell Evo3 | 70 | Poloxamer reverse phase medium and cancellous bone chips | Putty | Bone void filler | |
| Accell TBM | 100 | No carrier | Strip | Bone void filler/ bone graft extender | |
| DynaGraft III | ND | Poloxamer reverse phase medium | Putty, gel | Bone void filler/ bone graft extender | |
| OrthoBlast | ND | Poloxamer reverse phase medium | Paste | Bone void filler/bone | |
| OrthoBlast II | ND | Poloxamer reverse phase medium | Putty, paste | Bone void filler/bone | |
| Lattice Biologics | H-GENIN | 100 | No carrier | Putty, crush‐mix, spongeous blocks, powder | Nonmanipulated substance |
| LifeNet Health | Optium | ND | Glycerol | Putty, gel | Bone void filler |
| Medtronic | DBX | 31 (putty), 26 (paste), 35 (mix), 45 (strip) | Hyaluronic acid | Putty, paste, mix, strip | Bone void filler |
| GRAFTON | Unpublished | Gel, flex, putty, matrix, CRUNCH®, orthoblend, strips, paste | Bone void filler/ bone graft extender | ||
| Osteofil | 24 | Collagen | Paste, strip | Bone void filler | |
| Progenix Plus | 60 | Collagen type 1 (bovine) and sodium alginate | Putty | Bone void filler/ bone graft extender/bone graft substitute | |
| Progenix Putty | 70 | Collagen type 1 (bovine) and sodium alginate | Putty | Bone void filler/ bone graft extender/bone graft substitute | |
| VIAGRAF | ND | Glycerol | Paste, strip | Bone void filler | |
| Osteotech | Grafton | 17 to 31 | Glycerol | Paste, strip | Bone void filler/ bone graft extender/bone graft substitute |
| Penta Biomedical | BioSet | 24 | Porcine gelatine | Paste, strip, disc, with or without cancellous bone chips | Bone void filler |
| RTI Surgical®, Inc. | BioSet DBM | 24 by weight | Porcine gelatine | Paste, strip, disc, with or without cancellous chips | Bone void filler |
| Stryker | AlloCraft | 80 | Acellular matrix | Paste | Bone void filler |
| Wright Medical | Allomatrix | 40 to 86 | Calcium sulphate | Paste | Bone void filler/bone graft extender |
| PRO‐STIM | 40 | Calcium sulphate and calcium phosphate | Paste, putty | Bone void filler | |
| Zimmer | Puros® DBM | 10000 | No carrier | Putty, putty with cortico‐cancellous chips | Non 510(k) regulated - non-manipulated substance |
| Zimmer Biomet | InterGro | 40 (putty), 35 (paste) | Lecithin | Putty, paste | Bone void filler/bone graft extender |
| NHS Blood & Transplant: Tissue and Eye services | DBM Powder | 100 | No carrier | Particle size-250-710 mm jar | Light and electrostatic - To be mixed with blood to enhance osteogenesis |
| DBM Paste | 55 | Glycerol (v/v) | Paste.1, 5, 10 mL blunt ended syringe | Bone void filler | |
| DBM Putty | 65 | Glycerol (v/v) | Putty.1, 5, 10 mL blunt ended syringe | Bone void filler |
Note: *Adapted from Shehadi and Elzein36 and Zhang et al.37 DBM: demineralised bone matrix; ND: no data; SC: subchondral.
Reference
Bone regeneration: current concepts and future directions
DOI:10.1186/1741-7015-9-66 URL [Cited within: 2]
Bone grafting, orthopaedic biomaterials, and the clinical need for bone engineering
DOI:10.1243/09544119JEIM770 URL [Cited within: 1]
Investigating the osteoinductive potential of a decellularized xenograft bone substitute
DOI:10.1159/000503280 URL [Cited within: 1]
Osteoinduction, osteoconduction and osseointegration
Reconsidering osteoconduction in the era of additive manufacturing
DOI:10.1089/ten.teb.2019.0047 URL [Cited within: 1]
Results of 10-year follow-up of the iliac donor site of graft patients
DOI:10.1177/0300060514550351 URL [Cited within: 1]
Donor site evaluation after osteochondral autograft transplantation for capitellar osteochondritis dissecans
DOI:10.1177/0363546519871064 URL [Cited within: 1]
Bone allograft in the U.K.: perceptions and realities
DOI:10.5301/hipint.5000018 URL [Cited within: 2]
Impaction bone grafting in revision hip surgery: past, present and future
DOI:10.1007/s10561-009-9147-y URL [Cited within: 1]
Retrieved human allografts : a clinicopathological study
DOI:10.2106/00004623-200107000-00001 URL [Cited within: 1]
Transmission of the hepatitis-C virus by tissue transplantation
DOI:10.2106/00004623-199502000-00007 URL [Cited within: 1]
Transmission of human immunodeficiency virus type 1 from a seronegative organ and tissue donor
DOI:10.1056/NEJM199203123261102 URL [Cited within: 1]
Bone substitutes in orthopaedic surgery: from basic science to clinical practice
DOI:10.1007/s10856-014-5240-2 URL [Cited within: 3]
Purification processes of xenogeneic bone substitutes and their impact on tissue reactions and regeneration
The influence of sintering temperature on the properties of compacted bovine hydroxyapatite
DOI:10.1016/j.msec.2009.01.007 URL [Cited within: 1]
Poor results after augmenting autograft with xenograft (Surgibone) in hip revision surgery: a report of 27 cases
DOI:10.1080/17453670510041547 URL [Cited within: 1]
Iliac crest versus artificial bone grafts in 250 cervical fusions
DOI:10.1007/BF01400873 URL [Cited within: 1]
Cervical Interbody fusion using calf bone
DOI:10.1016/0090-3019(82)90010-6 URL [Cited within: 1]
Three-dimensional biofabrication of an aragonite-enriched self-hardening bone graft substitute and assessment of its osteogenicity in vitro and in vivo
Osteoinduction in porous hydroxyapatite implanted in heterotopic sites of different animal models
DOI:10.1016/0142-9612(96)80752-6 URL [Cited within: 1]
Biocompatibility assessments for medical devices - evolving regulatory considerations
DOI:10.1080/17434440.2017.1280392 URL [Cited within: 1]
Biofilm formation on nanostructured hydroxyapatite-coated titanium
DOI:10.1002/jbm.a.34757 URL [Cited within: 1]
Cell biology of bone
DOI:10.1016/S0950-351X(88)80006-5 URL [Cited within: 1]
The choice between allograft or demineralized bone matrix is not unambiguous in trauma surgery
An overview of decellularisation techniques of native tissues and tissue engineered products for bone, ligament and tendon regeneration
DOI:10.1016/j.ymeth.2019.08.002 URL [Cited within: 2]
Evaluation of bone allograft processing methods: Impact on decellularization efficacy, biocompatibility and mesenchymal stem cell functionality
DOI:10.1371/journal.pone.0218404 URL [Cited within: 2]
Comparison of six bone-graft substitutes regarding to cell seeding efficiency, metabolism and growth behaviour of human mesenchymal stem cells (MSC) in vitro
DOI:10.1016/j.injury.2010.02.017 URL [Cited within: 1]
Evaluation of bone formation after grafting with deproteinized bovine bone and mineralized allogenic bone
DOI:10.1097/ID.0000000000000185 URL [Cited within: 3]
Demineralized bone matrix in bone repair: history and use
DOI:10.1016/j.addr.2012.06.008 URL [Cited within: 1]
Use of a graft of demineralized bone matrix along with TGF-β1 leads to an early bone repair in dogs
DOI:10.1292/jvms.10-0155 URL [Cited within: 3]
Urist and the discovery of bone morphogenetic proteins
DOI:10.1007/s00264-017-3402-9 URL [Cited within: 1]
Bone morphogenetic protein concentration in human demineralized bone matrix
The slow release of BMP-7 at a low dose accelerates dental implant healing in an osteopenic environment
A comprehensive assessment of the risk of bone morphogenetic protein use in spinal fusion surgery and postoperative cancer diagnosis
DOI:10.3171/2014.10.SPINE14338 URL [Cited within: 3]
Review of commercially available demineralized bone matrix products for spinal fusions: A selection paradigm
DOI:10.4103/sni.sni_155_17 URL [Cited within: 2]
Demineralized bone matrix carriers and their clinical applications: an overview
DOI:10.1111/os.v11.5 URL [Cited within: 2]
Clostridium infections associated with musculoskeletal-tissue allografts
DOI:10.1056/NEJMoa023222 URL [Cited within: 1]
Radiation sterilization of tissue allografts: a review
DOI:10.4329/wjr.v8.i4.355 URL [Cited within: 4]
Sterilization of allograft bone: effects of gamma irradiation on allograft biology and biomechanics
DOI:10.1007/s10561-006-9020-1 URL [Cited within: 3]
Sterilization by gamma radiation impairs the tensile fatigue life of cortical bone by two orders of magnitude
DOI:10.1016/j.orthres.2005.03.003 URL [Cited within: 2]
Effective use of optimized, high-dose (50 kGy) gamma irradiation for pathogen inactivation of human bone allografts
DOI:10.1016/j.biomaterials.2004.06.028 URL [Cited within: 1]
Effect of sterilization on bone morphogenetic protein
DOI:10.1002/(ISSN)1554-527X URL [Cited within: 4]
The effect of sterilization on transforming growth factor beta isolated from demineralized human bone
DOI:10.1046/j.1537-2995.1993.33893342752.x URL [Cited within: 4]
Residual gas concentration and fibroblast toxicity
DOI:10.3109/17453679408994621 URL [Cited within: 2]
Influence of ethylene oxide sterilization on the activity of native reindeer bone morphogenetic protein
DOI:10.1007/s00264-003-0524-z URL [Cited within: 1]
Ethylene oxide sterilization kinetics
Effect of sterilization on osteoinduction. Comparison of five methods in demineralized rat bone
DOI:10.3109/17453678809149340 URL [Cited within: 1]
Dose-dependent reduction of bone inductive properties by ethylene oxide
Ethene oxide and bone induction. Controversy remains
DOI:10.3109/17453679809117622 URL [Cited within: 2]
Ethylene oxide does not extinguish the osteoinductive capacity of demineralized bone. A reappraisal in rats
DOI:10.3109/17453679709003989 URL [Cited within: 2]
Ethylene oxide gas sterilization does not reduce the osteoinductive potential of demineralized bone in rats
DOI:10.1097/00001665-199505000-00004 URL [Cited within: 1]
Comparing cellular bone matrices for posterolateral spinal fusion in a rat model
Cellular bone matrices: viable stem cell-containing bone graft substitutes
DOI:10.1016/j.spinee.2014.05.024 URL [Cited within: 4]
The components of bone and what they can teach us about regeneration
DOI:10.3390/ma11010014 URL [Cited within: 1]
A comparison of commercially available demineralized bone matrices with and without human mesenchymal stem cells in a rodent spinal fusion model
DOI:10.3171/2015.12.SPINE15737 URL [Cited within: 1]
Mechanical basis of bone strength: influence of bone material, bone structure and muscle action
Development of biodegradable bone graft substitutes using 3D printing
A brief history of the development of stromal stem cells (stem cells of the skeleton)
Orthopaedic tissue engineering and stem cells - an unfulfilled promise
Therapeutic use of mesenchymal stromal cells: the need for inclusive characterization guidelines to accommodate all tissue sources and species
DOI:10.3389/fcell.2021.632717 URL [Cited within: 2]
Impact of allogeneic stem cell manufacturing decisions on cost of goods, process robustness and reimbursement
DOI:10.1016/j.bej.2018.04.017 URL [Cited within: 1]
Shattering barriers toward clinically meaningful MSC therapies
Characterization of a biodegradable coralline hydroxyapatite/calcium carbonate composite and its clinical implementation
DOI:10.1088/1748-6041/8/6/065007 URL [Cited within: 1]
Coralline hydroxyapatite is a suitable bone graft substitute in an intra-articular goat defect model
The induction of bone formation by coral-derived calcium carbonate/hydroxyapatite constructs
DOI:10.1016/j.biomaterials.2008.10.065 URL [Cited within: 1]
Comparative study of the osteogenic ability of four different ceramic constructs in an ectopic large animal model
DOI:10.1002/term.v10.3 URL [Cited within: 1]
The use of a coral composite implant containing bone morphogenetic protein to repair a segmental tibial defect in sheep
DOI:10.1007/s002640050149 URL [Cited within: 3]
The role of bone marrow stromal cells in blood diseases and clinical significance as a crucial part of the hematopoietic microenvironment
DOI:10.21037/aob URL [Cited within: 1]
In vivo bone formation by human bone marrow stromal cells: effect of carrier particle size and shape
DOI:10.1002/(ISSN)1097-0290 URL [Cited within: 1]
Does seeding density affect in vitro mineral nodules formation in novel composite scaffolds?
Bone tissue engineering using polyetherketoneketone scaffolds combined with autologous mesenchymal stem cells in a sheep calvarial defect model
DOI:10.1016/j.jcms.2016.04.012 URL [Cited within: 1]
Human marrow-derived mesenchymal progenitor cells: isolation, culture expansion, and analysis of differentiation
DOI:10.1385/MB:20:3 URL [Cited within: 2]
Osteogenic potential of human umbilical cord mesenchymal stem cells on coralline hydroxyapatite/calcium carbonate microparticles
Clinical trials with mesenchymal stem cells: an update
DOI:10.3727/096368915X689622 URL [Cited within: 1]
Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue
DOI:10.1634/stemcells.2005-0342 URL [Cited within: 3]
Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue
DOI:10.3181/0712-RM-356 URL [Cited within: 2]
Adipose-derived and bone marrow mesenchymal stem cells: a donor-matched comparison
DOI:10.1186/s13287-018-0914-1 URL [Cited within: 1]
The umbilical cord matrix is a better source of mesenchymal stem cells (MSC) than the umbilical cord blood
DOI:10.1042/CBI20090414 URL [Cited within: 1]
A comprehensive characterisation of large-scale expanded human bone marrow and umbilical cord mesenchymal stem cells
DOI:10.1186/s13287-019-1202-4 URL [Cited within: 1]
Superior osteogenic capacity for bone tissue engineering of fetal compared with perinatal and adult mesenchymal stem cells
DOI:10.1634/stemcells.2008-0456 URL [Cited within: 1]
Isolation and characterisation of mesenchymal stem cells from different regions of the human umbilical cord
Adipose-derived mesenchymal stem cells from liposuction and resected fat are feasible sources for regenerative medicine
DOI:10.1186/s40001-017-0258-9 URL [Cited within: 1]
Isolation of adipose-derived stem cells: a comparison among different methods
DOI:10.1007/s10529-013-1425-x URL [Cited within: 1]
Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation
DOI:10.1186/1479-5876-12-8 URL [Cited within: 1]
Efficient cell-seeding into scaffolds improves bone formation
DOI:10.1177/0022034510370022 URL [Cited within: 4]
Evaluating 3D bone tissue engineered constructs with different seeding densities using the alamarBlue assay and the effect on in vivo bone formation
DOI:10.1023/A:1021139415528 URL [Cited within: 1]
Dynamic cell seeding of polymer scaffolds for cartilage tissue engineering
DOI:10.1021/bp970120j URL [Cited within: 1]
Cultivation of human bone marrow stromal cells on three-dimensional scaffolds of mineralized collagen: influence of seeding density on colonization, proliferation and osteogenic differentiation
DOI:10.1002/term.v2:7 URL [Cited within: 2]
The role of bioreactors in tissue engineering
DOI:10.1016/j.tibtech.2003.12.001 URL [Cited within: 2]
Bioreactor cultivation of functional bone grafts
Effects of mixing intensity on tissue-engineered cartilage
DOI:10.1002/(ISSN)1097-0290 URL [Cited within: 3]
Bioreactor cultivation of anatomically shaped human bone grafts
Bioreactor-based bone tissue engineering
Continuum modelling of in vitro tissue engineering: a review
Mathematical and computational models for bone tissue engineering in bioreactor systems
Prospective review of mesenchymal stem cells differentiation into osteoblasts
DOI:10.1111/os.2017.9.issue-1 URL [Cited within: 1]
Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions
Effect of recombinant human bone morphogenic protein 9 (rhBMP9) loaded onto bone grafts versus barrier membranes on new bone formation in a rabbit calvarial defect model
DOI:10.1002/jbm.a.v105.10 URL [Cited within: 1]
Regulation of BMP-7 expression by retinoic acid and prostaglandin E(2)
DOI:10.1002/(ISSN)1097-4652 URL [Cited within: 1]
Icariin induces osteogenic differentiation in vitro in a BMP- and Runx2-dependent manner
DOI:10.1016/j.bbrc.2008.02.054 URL [Cited within: 1]
Icariin stimulates MC3T3-E1 cell proliferation and differentiation through up-regulation of bone morphogenetic protein-2
In vitro and in vivo osteogenesis induced by icariin and bone morphogenetic protein-2: a dynamic observation
DOI:10.3389/fphar.2020.01058 URL [Cited within: 1]
BMP-2 gene delivery in cell-loaded and cell-free constructs for bone regeneration
BMP-2 gene delivery-based bone regeneration in dentistry
DOI:10.3390/pharmaceutics11080393 URL [Cited within: 2]
Bone formation by heterodimers through non-viral gene delivery of BMP-2/6 and BMP-2/7
Protein expression following non-viral delivery of plasmid DNA coding for basic FGF and BMP-2 in a rat ectopic model
DOI:10.1016/j.biomaterials.2012.01.031 URL [Cited within: 1]
Decellularized orthopaedic tissue-engineered grafts: biomaterial scaffolds synthesised by therapeutic cells
DOI:10.1039/C8BM00772A URL [Cited within: 2]
21 CFR 888.3045 - Resorbable calcium salt bone void filler device
21 CFR 872.3930 - Bone grafting material
Co-transfection with BMP2 and FGF2 via chitosan nanoparticles potentiates osteogenesis in human adipose-derived stromal cells in vitro