Biomaterials Translational ›› 2022, Vol. 3 ›› Issue (1): 65-80.doi: 10.12336/biomatertransl.2022.01.007
• REVIEW • Previous Articles Next Articles
Emma Steijvers, Armaan Ghei, Zhidao Xia*( )
)
Received:2022-02-07
Revised:2022-03-11
Accepted:2022-03-14
Online:2022-03-28
Published:2022-03-28
Contact:
Zhidao Xia
E-mail:z.xia@swansea.ac.uk
About author:Zhidao Xia, z.xia@swansea.ac.uk.Steijvers, E.; Ghei, A.; Xia, Z. Manufacturingartificial bone allografts: a perspective. Biomater Transl. 2022, 3(1), 65-80.
| Bone clean | Methods/materials | Source |
|---|---|---|
| Physical process | Sonication | U.S. Patent No. 5,797,871 |
| Pressurised flow | U.S. Patent No 5,513,662 | |
| High pressure washing with agitation and liquid stream | U.S. Patent No. 5,333,625 | |
| Agitation/shaker | the University of Miami Tissue Bank | |
| Oscillating atmospheric pressure | U.S. Patent No. 6,652,818 | |
| Chemical process | Ethanol | KR101272958B1 |
| Polyoxyethylene-23 lauryl ether | Allowash XG® | |
| (n-Butyl) phosphate, betadyne, TritonX-100/TNBP | BioCleanse® | |
| 0.5% to 5% chlorhexidine gluconate | U.S. Patent No. 10,004,819 | |
| Sodium hypochlorite and hydrogen peroxide | U.S. Patent No. 7,507,254 | |
| Combinational | Sonication + detergent; water and alcohol | U.S. Patent application 20080188939 |
| Supercritical fluid | U.S. Patent No. 5,725,579 | |
| Explosive decompression | U.S. Patent No. 5,288,462 |
Table 1 Allograft bone cleaning processes
| Bone clean | Methods/materials | Source |
|---|---|---|
| Physical process | Sonication | U.S. Patent No. 5,797,871 |
| Pressurised flow | U.S. Patent No 5,513,662 | |
| High pressure washing with agitation and liquid stream | U.S. Patent No. 5,333,625 | |
| Agitation/shaker | the University of Miami Tissue Bank | |
| Oscillating atmospheric pressure | U.S. Patent No. 6,652,818 | |
| Chemical process | Ethanol | KR101272958B1 |
| Polyoxyethylene-23 lauryl ether | Allowash XG® | |
| (n-Butyl) phosphate, betadyne, TritonX-100/TNBP | BioCleanse® | |
| 0.5% to 5% chlorhexidine gluconate | U.S. Patent No. 10,004,819 | |
| Sodium hypochlorite and hydrogen peroxide | U.S. Patent No. 7,507,254 | |
| Combinational | Sonication + detergent; water and alcohol | U.S. Patent application 20080188939 |
| Supercritical fluid | U.S. Patent No. 5,725,579 | |
| Explosive decompression | U.S. Patent No. 5,288,462 |
| Method | Advantages | Drawbacks | |
|---|---|---|---|
| Chemical | SDS | Complete removal of cellular components | Damages ECM: • Collagen disruption • GAG reduction |
| Triton TnBP | Good preservation of the ECM | Poor cell removal efficiency | |
| Enzymatic | DNAse | • Not damaging to the ECM • Very efficient in DNA debris elimination | Difficult to wash off tissues. Works only in combination with treatment disrupting cell membranes |
| Trypsin | Efficient cell surface removal | Prolonged exposure can disrupt ECM | |
| EDTA | Disrupts cell adhesion to ECM | Inefficient alone, often combined with trypsin | |
| Mechanical | Freeze/thaw | Efficient disruption of cell membranes | Does not efficiently remove cellular components & can damage ECM |
| Pressure | Increases chemical exposure and debris removal in tissues | High pressures can affect ECM integrity |
Table 2 Decellularisation methods for bone grafts*
| Method | Advantages | Drawbacks | |
|---|---|---|---|
| Chemical | SDS | Complete removal of cellular components | Damages ECM: • Collagen disruption • GAG reduction |
| Triton TnBP | Good preservation of the ECM | Poor cell removal efficiency | |
| Enzymatic | DNAse | • Not damaging to the ECM • Very efficient in DNA debris elimination | Difficult to wash off tissues. Works only in combination with treatment disrupting cell membranes |
| Trypsin | Efficient cell surface removal | Prolonged exposure can disrupt ECM | |
| EDTA | Disrupts cell adhesion to ECM | Inefficient alone, often combined with trypsin | |
| Mechanical | Freeze/thaw | Efficient disruption of cell membranes | Does not efficiently remove cellular components & can damage ECM |
| Pressure | Increases chemical exposure and debris removal in tissues | High pressures can affect ECM integrity |
| Graft name | Vendor | Components | Cell count | Cell viability |
|---|---|---|---|---|
| Trinity ELITE | Orthofix Medical | Cancellous bone containing viable cells and demineralised bone | ≥ 500000 cells/mL, of which > 100000 cells/mL are osteogenic cells | ≥ 70% |
| Vivigen | DePuy Synthes | Corticocancellous chips containing lineage-committed bone cells and demineralised bone particulate | > 16000 cells/mL | 96% |
| Cellentra | Zimmer-Biomet | Cancellous bone containing viable cells and demineralised cortical bone | ≥ 250000 cells/mL in the cancellous tissue | ≥ 70% |
| Osteocel Pro | NuVasive | Cryopreserved viable cancellous matrix and ground demineralised bone matrix | Average of 3 million cells/mL | > 85% on average |
| Bio4 | Stryker | A cryopreserved viable bone matrix product that contains native matrix, endogenous osteoblasts and mesenchymal stem cells, and osteoinductive and angiogenic growth factors | On average, ≥ 600000 cells/mL | ≥ 70% |
| Map3 | RTI Surgical | Cortical cancellous bone chips, demineralised bone matrix and multipotent adult progenitor cell-class cells | ≥ 50000 viable cells/mL of implant | Not available |
| Allostem | Allosource | Allogenic adult adipose-derived mesenchymal stem cells combined with partially demineralised allograft bone. | 66255 viable cells/mL | Not available |
Table 3 Commercial cellular bone matrices and their basic characteristics*
| Graft name | Vendor | Components | Cell count | Cell viability |
|---|---|---|---|---|
| Trinity ELITE | Orthofix Medical | Cancellous bone containing viable cells and demineralised bone | ≥ 500000 cells/mL, of which > 100000 cells/mL are osteogenic cells | ≥ 70% |
| Vivigen | DePuy Synthes | Corticocancellous chips containing lineage-committed bone cells and demineralised bone particulate | > 16000 cells/mL | 96% |
| Cellentra | Zimmer-Biomet | Cancellous bone containing viable cells and demineralised cortical bone | ≥ 250000 cells/mL in the cancellous tissue | ≥ 70% |
| Osteocel Pro | NuVasive | Cryopreserved viable cancellous matrix and ground demineralised bone matrix | Average of 3 million cells/mL | > 85% on average |
| Bio4 | Stryker | A cryopreserved viable bone matrix product that contains native matrix, endogenous osteoblasts and mesenchymal stem cells, and osteoinductive and angiogenic growth factors | On average, ≥ 600000 cells/mL | ≥ 70% |
| Map3 | RTI Surgical | Cortical cancellous bone chips, demineralised bone matrix and multipotent adult progenitor cell-class cells | ≥ 50000 viable cells/mL of implant | Not available |
| Allostem | Allosource | Allogenic adult adipose-derived mesenchymal stem cells combined with partially demineralised allograft bone. | 66255 viable cells/mL | Not available |
| Brand Name | Composition | Manufacturer | Price | |
|---|---|---|---|---|
| HA, HA/TCP, TCP | Edbone | Implant synthetic bone - 75% hydroxyapatite & 25% β-tricalcium phosphate - Straumann/Megagen/Nobelbiocare/Dio | Ethoss Regeneration Ltd. | 0.5 g, £39.86 |
| BiceraTM | Composed of HAP (60%) and β-tricalcium phosphate (40%) bioceramic which is similar to bone mineral | Hannox International Corporation | n.a. | |
| MASTERGRAFT® | β-Tricalcium phosphate (85%) and hydroxyapatite (15%) | Medtronic Sofamor Danek USA, Inc. | n.a. | |
| Syntoss | Dental implant synthetic bone graft β-tricalcium phosphate material block | Dental solution Israel | £46.80 | |
| Kuraray | Bone graft materials (synthetic bone substitute, bioresorbable bone graft substitute β-tricalcium phosphate) | Biomaterial Department Japan | n.a. | |
| chronOS® | A synthetic β-tricalcium phosphate bone void filler which is radiopaque, resorbable and osteoconductive | DePuy Synthes | n.a | |
| G Graft | Bone graft material hydroxyapatite with collagen implant dental | Rohit Enterprises, India | £32.87/mL | |
| Ostoden | Synthetic calcium phosphate based bone graft device | Ammdent | £22.14 | |
| MBCP® | Synthetic bone graft substitute bioactive calcium phosphate | Biomatlante SAS, France | n.a | |
| Silicated calcium phosphate | Osteo3 | A synthetic bone graft substitute, silicated calcium phosphate | OssDsign | n.a. |
| ActifuseTM | Silicated calcium phosphate | ApaTech Limited | n.a | |
| Coral | Corebone | Bio-active coral core bone graft sterile granules | Global Dental Transfer, Israel | n.a |
| BoneMedik | A porous, interconnected coral structure which is compatible with human bone structure | Meta Biomed Co. Ltd. | n.a. | |
| Xeno-graft, Bovine | Zenoss | Bovine bone graft material in blocks | Dental solution Israel | £56.40 |
| OSTEONTM II | Bone void filler, synthetic bone graft + collagen type 1 (bovine) | Genoss | 0.25g/ £57.28 | |
| Biotechmat | Dental bone graft - Xenograft (Bovine) - similar to Bioss | Technology in Biomaterials | £62.02 | |
| Natural | Bio-Oss | Dental Bio Oss collagen bone graft material, natural bone grafting materials | Geistlich UK | £154.51 |
Table 4 Commercial bone grafts and their basic characteristics*
| Brand Name | Composition | Manufacturer | Price | |
|---|---|---|---|---|
| HA, HA/TCP, TCP | Edbone | Implant synthetic bone - 75% hydroxyapatite & 25% β-tricalcium phosphate - Straumann/Megagen/Nobelbiocare/Dio | Ethoss Regeneration Ltd. | 0.5 g, £39.86 |
| BiceraTM | Composed of HAP (60%) and β-tricalcium phosphate (40%) bioceramic which is similar to bone mineral | Hannox International Corporation | n.a. | |
| MASTERGRAFT® | β-Tricalcium phosphate (85%) and hydroxyapatite (15%) | Medtronic Sofamor Danek USA, Inc. | n.a. | |
| Syntoss | Dental implant synthetic bone graft β-tricalcium phosphate material block | Dental solution Israel | £46.80 | |
| Kuraray | Bone graft materials (synthetic bone substitute, bioresorbable bone graft substitute β-tricalcium phosphate) | Biomaterial Department Japan | n.a. | |
| chronOS® | A synthetic β-tricalcium phosphate bone void filler which is radiopaque, resorbable and osteoconductive | DePuy Synthes | n.a | |
| G Graft | Bone graft material hydroxyapatite with collagen implant dental | Rohit Enterprises, India | £32.87/mL | |
| Ostoden | Synthetic calcium phosphate based bone graft device | Ammdent | £22.14 | |
| MBCP® | Synthetic bone graft substitute bioactive calcium phosphate | Biomatlante SAS, France | n.a | |
| Silicated calcium phosphate | Osteo3 | A synthetic bone graft substitute, silicated calcium phosphate | OssDsign | n.a. |
| ActifuseTM | Silicated calcium phosphate | ApaTech Limited | n.a | |
| Coral | Corebone | Bio-active coral core bone graft sterile granules | Global Dental Transfer, Israel | n.a |
| BoneMedik | A porous, interconnected coral structure which is compatible with human bone structure | Meta Biomed Co. Ltd. | n.a. | |
| Xeno-graft, Bovine | Zenoss | Bovine bone graft material in blocks | Dental solution Israel | £56.40 |
| OSTEONTM II | Bone void filler, synthetic bone graft + collagen type 1 (bovine) | Genoss | 0.25g/ £57.28 | |
| Biotechmat | Dental bone graft - Xenograft (Bovine) - similar to Bioss | Technology in Biomaterials | £62.02 | |
| Natural | Bio-Oss | Dental Bio Oss collagen bone graft material, natural bone grafting materials | Geistlich UK | £154.51 |
| Mesenchymal stem cell product (company) | Approval granted (year) | Indication | Product type |
|---|---|---|---|
| Queencell (Anterogen Co. Ltd.) | South Korea (2010) | Subcutaneous tissue defects | Autologous human AT-MSC |
| Cellgram-AMI (Pharmicell Co. Ltd.) | South Korea (2011) | Acute myocardial infarction | Autologous human BM-MSC |
| Cartistem (Medipost Co. Ltd.) | South Korea (2012) | Knee articular cartilage defects | Allogeneic human UC-MSC |
| Cupistem (Anterogen Co. Ltd.) | South Korea (2012) | Crohn’s fistula | Autologous human BM-MSC |
| Prochymal, remestemcel-L (Osiris Therapeutics Inc., Mesoblast Ltd.) | Canada (2012) New Zealand (2012) | Graft versus host disease | Allogeneic human BM-MSC |
| Neuronata-R (Corestem Inc.) | South Korea (2014) | Amyotrophic lateral sclerosis | Autologous human BM-MSC |
| Temcell HS (JCR Pharmaceuticals) | Japan (2015) | Graft versus host disease | Allogeneic human BM-MSC |
| Stempeucel (Stempeutics Research PVT) | India (2016) | Critical limb ischemia | Allogeneic human BM-MSC |
| Alofisel (TiGenix NV/Takeda) | Europe (2018) | Complex perianal fistulas in Crohn’s disease | Allogeneic human AT-MSC |
| Stemirac (Nipro Corp) | Japan (2018) | Spinal cord injury | Autologous human BM-MSC |
Table 5 Mesenchymal stem cell products with regulatory approval
| Mesenchymal stem cell product (company) | Approval granted (year) | Indication | Product type |
|---|---|---|---|
| Queencell (Anterogen Co. Ltd.) | South Korea (2010) | Subcutaneous tissue defects | Autologous human AT-MSC |
| Cellgram-AMI (Pharmicell Co. Ltd.) | South Korea (2011) | Acute myocardial infarction | Autologous human BM-MSC |
| Cartistem (Medipost Co. Ltd.) | South Korea (2012) | Knee articular cartilage defects | Allogeneic human UC-MSC |
| Cupistem (Anterogen Co. Ltd.) | South Korea (2012) | Crohn’s fistula | Autologous human BM-MSC |
| Prochymal, remestemcel-L (Osiris Therapeutics Inc., Mesoblast Ltd.) | Canada (2012) New Zealand (2012) | Graft versus host disease | Allogeneic human BM-MSC |
| Neuronata-R (Corestem Inc.) | South Korea (2014) | Amyotrophic lateral sclerosis | Autologous human BM-MSC |
| Temcell HS (JCR Pharmaceuticals) | Japan (2015) | Graft versus host disease | Allogeneic human BM-MSC |
| Stempeucel (Stempeutics Research PVT) | India (2016) | Critical limb ischemia | Allogeneic human BM-MSC |
| Alofisel (TiGenix NV/Takeda) | Europe (2018) | Complex perianal fistulas in Crohn’s disease | Allogeneic human AT-MSC |
| Stemirac (Nipro Corp) | Japan (2018) | Spinal cord injury | Autologous human BM-MSC |
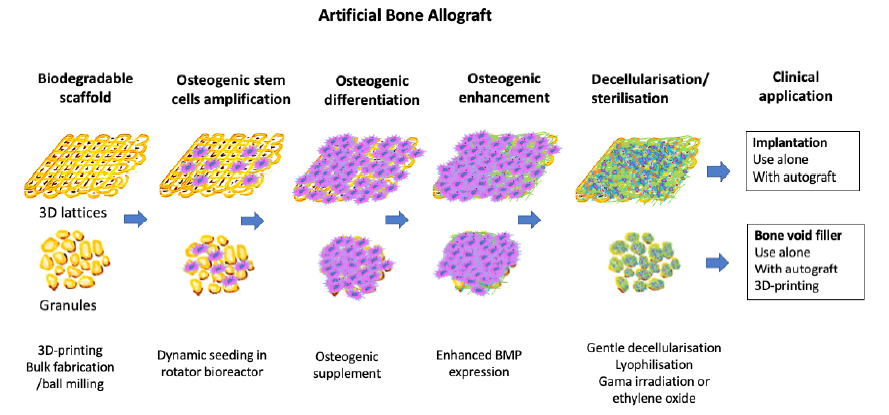
Figure 5. The proposed manufacturing process. Scaffold is produced, then seeded with cells. When these cells osteogenically differentiate they will express growth factors such as BMP, which will remain on the scaffold after decellularisation. 3D: three-dimensional; BMP: bone morphogenic proteins.
| Source company | Product | DBM (%) | Carrier | Form | Indication |
|---|---|---|---|---|---|
| Allosource | AlloFuse | 36 (putty), 29 (gel) | Reverse phase medium | Putty, gel | Bone void filler/bone graft extender |
| Alphatech | AlphaGRAFT | 80 | Acellular matrix | Paste | Bone void filler |
| Amend Surgical | NanoFUSE | ND | 45S5 bioactive glass | Putty | Bone void filler |
| Bactarin International | OsteoSelect | 74 | Carboxymethylcellulose, phosphate-buffered saline | Putty | Bone void filler |
| Osteosponge | 100 | No carrier | Block, Disc, SC, strip filler | Bone void filler | |
| Exactech | Altiva | ND | Gelatine | Paste | Bone void filler |
| Optefil | 24 | Gelatine | Paste | Bone void filler | |
| Opteform | ND | Cortical and cancellous bone chips suspended in collagen-gelatine | Paste | Bone void filler | |
| Integra | Accell Connexus | 70 | Poloxamer reverse phase medium | Putty | Bone void filler/ bone graft extender |
| Accell Evo3 | 70 | Poloxamer reverse phase medium and cancellous bone chips | Putty | Bone void filler | |
| Accell TBM | 100 | No carrier | Strip | Bone void filler/ bone graft extender | |
| DynaGraft III | ND | Poloxamer reverse phase medium | Putty, gel | Bone void filler/ bone graft extender | |
| OrthoBlast | ND | Poloxamer reverse phase medium | Paste | Bone void filler/bone | |
| OrthoBlast II | ND | Poloxamer reverse phase medium | Putty, paste | Bone void filler/bone | |
| Lattice Biologics | H-GENIN | 100 | No carrier | Putty, crush‐mix, spongeous blocks, powder | Nonmanipulated substance |
| LifeNet Health | Optium | ND | Glycerol | Putty, gel | Bone void filler |
| Medtronic | DBX | 31 (putty), 26 (paste), 35 (mix), 45 (strip) | Hyaluronic acid | Putty, paste, mix, strip | Bone void filler |
| GRAFTON | Unpublished | Gel, flex, putty, matrix, CRUNCH®, orthoblend, strips, paste | Bone void filler/ bone graft extender | ||
| Osteofil | 24 | Collagen | Paste, strip | Bone void filler | |
| Progenix Plus | 60 | Collagen type 1 (bovine) and sodium alginate | Putty | Bone void filler/ bone graft extender/bone graft substitute | |
| Progenix Putty | 70 | Collagen type 1 (bovine) and sodium alginate | Putty | Bone void filler/ bone graft extender/bone graft substitute | |
| VIAGRAF | ND | Glycerol | Paste, strip | Bone void filler | |
| Osteotech | Grafton | 17 to 31 | Glycerol | Paste, strip | Bone void filler/ bone graft extender/bone graft substitute |
| Penta Biomedical | BioSet | 24 | Porcine gelatine | Paste, strip, disc, with or without cancellous bone chips | Bone void filler |
| RTI Surgical®, Inc. | BioSet DBM | 24 by weight | Porcine gelatine | Paste, strip, disc, with or without cancellous chips | Bone void filler |
| Stryker | AlloCraft | 80 | Acellular matrix | Paste | Bone void filler |
| Wright Medical | Allomatrix | 40 to 86 | Calcium sulphate | Paste | Bone void filler/bone graft extender |
| PRO‐STIM | 40 | Calcium sulphate and calcium phosphate | Paste, putty | Bone void filler | |
| Zimmer | Puros® DBM | 10000 | No carrier | Putty, putty with cortico‐cancellous chips | Non 510(k) regulated - non-manipulated substance |
| Zimmer Biomet | InterGro | 40 (putty), 35 (paste) | Lecithin | Putty, paste | Bone void filler/bone graft extender |
| NHS Blood & Transplant: Tissue and Eye services | DBM Powder | 100 | No carrier | Particle size-250-710 mm jar | Light and electrostatic - To be mixed with blood to enhance osteogenesis |
| DBM Paste | 55 | Glycerol (v/v) | Paste.1, 5, 10 mL blunt ended syringe | Bone void filler | |
| DBM Putty | 65 | Glycerol (v/v) | Putty.1, 5, 10 mL blunt ended syringe | Bone void filler |
Additiond Table 1. Commercially-available demineralised bone matrix*
| Source company | Product | DBM (%) | Carrier | Form | Indication |
|---|---|---|---|---|---|
| Allosource | AlloFuse | 36 (putty), 29 (gel) | Reverse phase medium | Putty, gel | Bone void filler/bone graft extender |
| Alphatech | AlphaGRAFT | 80 | Acellular matrix | Paste | Bone void filler |
| Amend Surgical | NanoFUSE | ND | 45S5 bioactive glass | Putty | Bone void filler |
| Bactarin International | OsteoSelect | 74 | Carboxymethylcellulose, phosphate-buffered saline | Putty | Bone void filler |
| Osteosponge | 100 | No carrier | Block, Disc, SC, strip filler | Bone void filler | |
| Exactech | Altiva | ND | Gelatine | Paste | Bone void filler |
| Optefil | 24 | Gelatine | Paste | Bone void filler | |
| Opteform | ND | Cortical and cancellous bone chips suspended in collagen-gelatine | Paste | Bone void filler | |
| Integra | Accell Connexus | 70 | Poloxamer reverse phase medium | Putty | Bone void filler/ bone graft extender |
| Accell Evo3 | 70 | Poloxamer reverse phase medium and cancellous bone chips | Putty | Bone void filler | |
| Accell TBM | 100 | No carrier | Strip | Bone void filler/ bone graft extender | |
| DynaGraft III | ND | Poloxamer reverse phase medium | Putty, gel | Bone void filler/ bone graft extender | |
| OrthoBlast | ND | Poloxamer reverse phase medium | Paste | Bone void filler/bone | |
| OrthoBlast II | ND | Poloxamer reverse phase medium | Putty, paste | Bone void filler/bone | |
| Lattice Biologics | H-GENIN | 100 | No carrier | Putty, crush‐mix, spongeous blocks, powder | Nonmanipulated substance |
| LifeNet Health | Optium | ND | Glycerol | Putty, gel | Bone void filler |
| Medtronic | DBX | 31 (putty), 26 (paste), 35 (mix), 45 (strip) | Hyaluronic acid | Putty, paste, mix, strip | Bone void filler |
| GRAFTON | Unpublished | Gel, flex, putty, matrix, CRUNCH®, orthoblend, strips, paste | Bone void filler/ bone graft extender | ||
| Osteofil | 24 | Collagen | Paste, strip | Bone void filler | |
| Progenix Plus | 60 | Collagen type 1 (bovine) and sodium alginate | Putty | Bone void filler/ bone graft extender/bone graft substitute | |
| Progenix Putty | 70 | Collagen type 1 (bovine) and sodium alginate | Putty | Bone void filler/ bone graft extender/bone graft substitute | |
| VIAGRAF | ND | Glycerol | Paste, strip | Bone void filler | |
| Osteotech | Grafton | 17 to 31 | Glycerol | Paste, strip | Bone void filler/ bone graft extender/bone graft substitute |
| Penta Biomedical | BioSet | 24 | Porcine gelatine | Paste, strip, disc, with or without cancellous bone chips | Bone void filler |
| RTI Surgical®, Inc. | BioSet DBM | 24 by weight | Porcine gelatine | Paste, strip, disc, with or without cancellous chips | Bone void filler |
| Stryker | AlloCraft | 80 | Acellular matrix | Paste | Bone void filler |
| Wright Medical | Allomatrix | 40 to 86 | Calcium sulphate | Paste | Bone void filler/bone graft extender |
| PRO‐STIM | 40 | Calcium sulphate and calcium phosphate | Paste, putty | Bone void filler | |
| Zimmer | Puros® DBM | 10000 | No carrier | Putty, putty with cortico‐cancellous chips | Non 510(k) regulated - non-manipulated substance |
| Zimmer Biomet | InterGro | 40 (putty), 35 (paste) | Lecithin | Putty, paste | Bone void filler/bone graft extender |
| NHS Blood & Transplant: Tissue and Eye services | DBM Powder | 100 | No carrier | Particle size-250-710 mm jar | Light and electrostatic - To be mixed with blood to enhance osteogenesis |
| DBM Paste | 55 | Glycerol (v/v) | Paste.1, 5, 10 mL blunt ended syringe | Bone void filler | |
| DBM Putty | 65 | Glycerol (v/v) | Putty.1, 5, 10 mL blunt ended syringe | Bone void filler |
| 1. |
Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P. V. Bone regeneration: current concepts and future directions. BMC Med. 2011, 9, 66.
doi: 10.1186/1741-7015-9-66 URL |
| 2. |
Brydone, A. S.; Meek, D.; Maclaine, S. Bone grafting, orthopaedic biomaterials, and the clinical need for bone engineering. Proc Inst Mech Eng H. 2010, 224, 1329-1343.
doi: 10.1243/09544119JEIM770 URL |
| 3. |
Bracey, D. N.; Jinnah, A. H.; Willey, J. S.; Seyler, T. M.; Hutchinson, I. D.; Whitlock, P. W.; Smith, T. L.; Danelson, K. A.; Emory, C. L.; Kerr, B. A. Investigating the osteoinductive potential of a decellularized xenograft bone substitute. Cells Tissues Organs. 2019, 207, 97-113.
doi: 10.1159/000503280 URL |
| 4. | Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur Spine J. 2001, 10 Suppl 2, S96-101. |
| 5. |
Weber, F. E. Reconsidering osteoconduction in the era of additive manufacturing. Tissue Eng Part B Rev. 2019, 25, 375-386.
doi: 10.1089/ten.teb.2019.0047 URL |
| 6. |
Qi, L.; Liu, Y.; Li, H.; Zhang, Y. Results of 10-year follow-up of the iliac donor site of graft patients. J Int Med Res. 2014, 42, 1348-1352.
doi: 10.1177/0300060514550351 URL |
| 7. |
Matsuura, T.; Hashimoto, Y.; Kinoshita, T.; Nishino, K.; Nishida, Y.; Takigami, J.; Katsuda, H.; Shimada, N. Donor site evaluation after osteochondral autograft transplantation for capitellar osteochondritis dissecans. Am J Sports Med. 2019, 47, 2836-2843.
doi: 10.1177/0363546519871064 URL |
| 8. |
Lomas, R.; Chandrasekar, A.; Board, T. N. Bone allograft in the U.K.: perceptions and realities. Hip Int. 2013, 23, 427-433.
doi: 10.5301/hipint.5000018 URL |
| 9. |
McNamara, I. R. Impaction bone grafting in revision hip surgery: past, present and future. Cell Tissue Bank. 2010, 11, 57-73.
doi: 10.1007/s10561-009-9147-y URL |
| 10. |
Enneking, W. F.; Campanacci, D. A. Retrieved human allografts : a clinicopathological study. J Bone Joint Surg Am. 2001, 83, 971-986.
doi: 10.2106/00004623-200107000-00001 URL |
| 11. |
Conrad, E. U.; Gretch, D. R.; Obermeyer, K. R.; Moogk, M. S.; Sayers, M.; Wilson, J. J.; Strong, D. M. Transmission of the hepatitis-C virus by tissue transplantation. J Bone Joint Surg Am. 1995, 77, 214-224.
doi: 10.2106/00004623-199502000-00007 URL |
| 12. |
Simonds, R. J.; Holmberg, S. D.; Hurwitz, R. L.; Coleman, T. R.; Bottenfield, S.; Conley, L. J.; Kohlenberg, S. H.; Castro, K. G.; Dahan, B. A.; Schable, C. A.; et al. Transmission of human immunodeficiency virus type 1 from a seronegative organ and tissue donor. N Engl J Med. 1992, 326, 726-732.
doi: 10.1056/NEJM199203123261102 URL |
| 13. |
Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone substitutes in orthopaedic surgery: from basic science to clinical practice. J Mater Sci Mater Med. 2014, 25, 2445-2461.
doi: 10.1007/s10856-014-5240-2 URL |
| 14. | Perić Kačarević, Z.; Kavehei, F.; Houshmand, A.; Franke, J.; Smeets, R.; Rimashevskiy, D.; Wenisch, S.; Schnettler, R.; Jung, O.; Barbeck, M. Purification processes of xenogeneic bone substitutes and their impact on tissue reactions and regeneration. Int J Artif Organs. 2018, 41, 789-800. |
| 15. |
Herliansyah, M. K.; Hamdi, M.; Ide-Ektessabi, A.; Wildan, M. W.; Toque, J. A. The influence of sintering temperature on the properties of compacted bovine hydroxyapatite. Mater Sci Eng C. 2009, 29, 1674-1680.
doi: 10.1016/j.msec.2009.01.007 URL |
| 16. |
Charalambides, C.; Beer, M.; Cobb, A. G. Poor results after augmenting autograft with xenograft (Surgibone) in hip revision surgery: a report of 27 cases. Acta Orthop. 2005, 76, 544-549.
doi: 10.1080/17453670510041547 URL |
| 17. |
Savolainen, S.; Usenius, J. P.; Hernesniemi, J. Iliac crest versus artificial bone grafts in 250 cervical fusions. Acta Neurochir (Wien). 1994, 129, 54-57.
doi: 10.1007/BF01400873 URL |
| 18. |
Siqueira, E. B.; Kranzler, L. I. Cervical Interbody fusion using calf bone. Surg Neurol. 1982, 18, 37-39.
doi: 10.1016/0090-3019(82)90010-6 URL |
| 19. | Shi, Y.; He, R.; Deng, X.; Shao, Z.; Deganello, D.; Yan, C.; Xia, Z. Three-dimensional biofabrication of an aragonite-enriched self-hardening bone graft substitute and assessment of its osteogenicity in vitro and in vivo. Biomater Transl. 2020, 1, 69-81. |
| 20. |
Ripamonti, U. Osteoinduction in porous hydroxyapatite implanted in heterotopic sites of different animal models. Biomaterials. 1996, 17, 31-35.
doi: 10.1016/0142-9612(96)80752-6 URL |
| 21. |
Reeve, L.; Baldrick, P. Biocompatibility assessments for medical devices - evolving regulatory considerations. Expert Rev Med Devices. 2017, 14, 161-167.
doi: 10.1080/17434440.2017.1280392 URL |
| 22. |
Westas, E.; Gillstedt, M.; Lönn-Stensrud, J.; Bruzell, E.; Andersson, M. Biofilm formation on nanostructured hydroxyapatite-coated titanium. J Biomed Mater Res A. 2014, 102, 1063-1070.
doi: 10.1002/jbm.a.34757 URL |
| 23. | Grand View Research Inc. Bone grafts and substitutes market size, share & trends analysis report by material type (allograft, synthetic), by application (spinal fusion, foot & ankle, joint reconstruction), by region, segment forecasts, 2022-2030. https://www.grandviewresearch.com/industry-analysis/bone-grafts-substitutes-market. Accessed Marth 7, 2022. |
| 24. |
Martin, T. J.; Ng, K. W.; Nicholson, G. C. Cell biology of bone. Baillieres Clin Endocrinol Metab. 1988, 2, 1-29.
doi: 10.1016/S0950-351X(88)80006-5 URL |
| 25. | Brink, O. The choice between allograft or demineralized bone matrix is not unambiguous in trauma surgery. Injury. 2021, 52 Suppl 2, S23-s28. |
| 26. |
Blaudez, F.; Ivanovski, S.; Hamlet, S.; Vaquette, C. An overview of decellularisation techniques of native tissues and tissue engineered products for bone, ligament and tendon regeneration. Methods. 2020, 171, 28-40.
doi: 10.1016/j.ymeth.2019.08.002 URL |
| 27. |
Rasch, A.; Naujokat, H.; Wang, F.; Seekamp, A.; Fuchs, S.; Klüter, T. Evaluation of bone allograft processing methods: Impact on decellularization efficacy, biocompatibility and mesenchymal stem cell functionality. PLoS One. 2019, 14, e0218404.
doi: 10.1371/journal.pone.0218404 URL |
| 28. |
Seebach, C.; Schultheiss, J.; Wilhelm, K.; Frank, J.; Henrich, D. Comparison of six bone-graft substitutes regarding to cell seeding efficiency, metabolism and growth behaviour of human mesenchymal stem cells (MSC) in vitro. Injury. 2010, 41, 731-738.
doi: 10.1016/j.injury.2010.02.017 URL |
| 29. |
Moon, K. N.; Kim, S. G.; Oh, J. S.; Kim, C. S.; Lim, S. C.; Jeong, M. A. Evaluation of bone formation after grafting with deproteinized bovine bone and mineralized allogenic bone. Implant Dent. 2015, 24, 101-105.
doi: 10.1097/ID.0000000000000185 URL |
| 30. |
Gruskin, E.; Doll, B. A.; Futrell, F. W.; Schmitz, J. P.; Hollinger, J. O. Demineralized bone matrix in bone repair: history and use. Adv Drug Deliv Rev. 2012, 64, 1063-1077.
doi: 10.1016/j.addr.2012.06.008 URL |
| 31. |
Servin-Trujillo, M. A.; Reyes-Esparza, J. A.; Garrido-Fariña, G.; Flores-Gazca, E.; Osuna-Martinez, U.; Rodriguez-Fragoso, L. Use of a graft of demineralized bone matrix along with TGF-β1 leads to an early bone repair in dogs. J Vet Med Sci. 2011, 73, 1151-1161.
doi: 10.1292/jvms.10-0155 URL |
| 32. |
Grgurevic, L.; Pecina, M.; Vukicevic, S. Marshall R. Urist and the discovery of bone morphogenetic proteins. Int Orthop. 2017, 41, 1065-1069.
doi: 10.1007/s00264-017-3402-9 URL |
| 33. | McDonald, N. M.; Woodell-May, J. E.; Pietrzak, W. S. Bone morphogenetic protein concentration in human demineralized bone matrix. In 51st Annual Meeting of the Orthopaedic Research Society, 2005. |
| 34. | Hunziker, E. B.; Liu, Y.; Muff, M.; Haegi, T.; Shintani, N.; Lippuner, K. The slow release of BMP-7 at a low dose accelerates dental implant healing in an osteopenic environment. Eur Cell Mater. 2021, 41, 170-183. |
| 35. |
Cahill, K. S.; McCormick, P. C.; Levi, A. D. A comprehensive assessment of the risk of bone morphogenetic protein use in spinal fusion surgery and postoperative cancer diagnosis. J Neurosurg Spine. 2015, 23, 86-93.
doi: 10.3171/2014.10.SPINE14338 URL |
| 36. |
Shehadi, J. A.; Elzein, S. M. Review of commercially available demineralized bone matrix products for spinal fusions: A selection paradigm. Surg Neurol Int. 2017, 8, 203.
doi: 10.4103/sni.sni_155_17 URL |
| 37. |
Zhang, H.; Yang, L.; Yang, X. G.; Wang, F.; Feng, J. T.; Hua, K. C.; Li, Q.; Hu, Y. C. Demineralized bone matrix carriers and their clinical applications: an overview. Orthop Surg. 2019, 11, 725-737.
doi: 10.1111/os.v11.5 URL |
| 38. |
Kainer, M. A.; Linden, J. V.; Whaley, D. N.; Holmes, H. T.; Jarvis, W. R.; Jernigan, D. B.; Archibald, L. K. Clostridium infections associated with musculoskeletal-tissue allografts. N Engl J Med. 2004, 350, 2564-2571.
doi: 10.1056/NEJMoa023222 URL |
| 39. |
Singh, R.; Singh, D.; Singh, A. Radiation sterilization of tissue allografts: a review. World J Radiol. 2016, 8, 355-369.
doi: 10.4329/wjr.v8.i4.355 URL |
| 40. |
Nguyen, H.; Morgan, D. A.; Forwood, M. R. Sterilization of allograft bone: effects of gamma irradiation on allograft biology and biomechanics. Cell Tissue Bank. 2007, 8, 93-105.
doi: 10.1007/s10561-006-9020-1 URL |
| 41. | American Association of Tissue Banks. Standards for Tissue Banking. 14th ed.; 2016. |
| 42. |
Akkus, O.; Belaney, R. M. Sterilization by gamma radiation impairs the tensile fatigue life of cortical bone by two orders of magnitude. J Orthop Res. 2005, 23, 1054-1058.
doi: 10.1016/j.orthres.2005.03.003 URL |
| 43. |
Grieb, T. A.; Forng, R. Y.; Stafford, R. E.; Lin, J.; Almeida, J.; Bogdansky, S.; Ronholdt, C.; Drohan, W. N.; Burgess, W. H. Effective use of optimized, high-dose (50 kGy) gamma irradiation for pathogen inactivation of human bone allografts. Biomaterials. 2005, 26, 2033-2042.
doi: 10.1016/j.biomaterials.2004.06.028 URL |
| 44. |
Ijiri, S.; Yamamuro, T.; Nakamura, T.; Kotani, S.; Notoya, K. Effect of sterilization on bone morphogenetic protein. J Orthop Res. 1994, 12, 628-636.
doi: 10.1002/(ISSN)1554-527X URL |
| 45. |
Puolakkainen, P. A.; Ranchalis, J. E.; Strong, D. M.; Twardzik, D. R. The effect of sterilization on transforming growth factor beta isolated from demineralized human bone. Transfusion. 1993, 33, 679-685.
doi: 10.1046/j.1537-2995.1993.33893342752.x URL |
| 46. |
Arizono, T.; Iwamoto, Y.; Okuyama, K.; Sugioka, Y. Ethylene oxide sterilization of bone grafts. Residual gas concentration and fibroblast toxicity. Acta Orthop Scand. 1994, 65, 640-642.
doi: 10.3109/17453679408994621 URL |
| 47. |
Pekkarinen, T.; Hietala, O.; Lindholm, T. S.; Jalovaara, P. Influence of ethylene oxide sterilization on the activity of native reindeer bone morphogenetic protein. Int Orthop. 2004, 28, 97-101.
doi: 10.1007/s00264-003-0524-z URL |
| 48. | Ernst, R. R. Ethylene oxide sterilization kinetics. Biotechnol Bioeng Symp. 1974, 0, 865-878. |
| 49. |
Munting, E.; Wilmart, J. F.; Wijne, A.; Hennebert, P.; Delloye, C. Effect of sterilization on osteoinduction. Comparison of five methods in demineralized rat bone. Acta Orthop Scand. 1988, 59, 34-38.
doi: 10.3109/17453678809149340 URL |
| 50. | Aspenberg, P.; Johnsson, E.; Thorngren, K. G. Dose-dependent reduction of bone inductive properties by ethylene oxide. J Bone Joint Surg Br. 1990, 72, 1036-1037. |
| 51. |
Aspenberg, P.; Lindqvist, S. B. Ethene oxide and bone induction. Controversy remains. Acta Orthop Scand. 1998, 69, 173-176.
doi: 10.3109/17453679809117622 URL |
| 52. |
Zhang, Q.; Cornu, O.; Delloye, C. Ethylene oxide does not extinguish the osteoinductive capacity of demineralized bone. A reappraisal in rats. Acta Orthop Scand. 1997, 68, 104-108.
doi: 10.3109/17453679709003989 URL |
| 53. |
Solheim, E.; Pinholt, E. M.; Bang, G.; Sudmann, E. Ethylene oxide gas sterilization does not reduce the osteoinductive potential of demineralized bone in rats. J Craniofac Surg. 1995, 6, 195-198.
doi: 10.1097/00001665-199505000-00004 URL |
| 54. | Lin, C.; Zhang, N.; Waldorff, E. I.; Punsalan, P.; Wang, D.; Semler, E.; Ryaby, J. T.; Yoo, J.; Johnstone, B. Comparing cellular bone matrices for posterolateral spinal fusion in a rat model. JOR Spine. 2020, 3, e1084. |
| 55. |
Skovrlj, B.; Guzman, J. Z.; Al Maaieh, M.; Cho, S. K.; Iatridis, J. C.; Qureshi, S. A. Cellular bone matrices: viable stem cell-containing bone graft substitutes. Spine J. 2014, 14, 2763-2772.
doi: 10.1016/j.spinee.2014.05.024 URL |
| 56. |
Le, B. Q.; Nurcombe, V.; Cool, S. M.; van Blitterswijk, C. A.; de Boer, J.; LaPointe, V. L. S. The components of bone and what they can teach us about regeneration. Materials (Basel). 2017, 11, 14.
doi: 10.3390/ma11010014 URL |
| 57. |
Hayashi, T.; Lord, E. L.; Suzuki, A.; Takahashi, S.; Scott, T. P.; Phan, K.; Tian, H.; Daubs, M. D.; Shiba, K.; Wang, J. C. A comparison of commercially available demineralized bone matrices with and without human mesenchymal stem cells in a rodent spinal fusion model. J Neurosurg Spine. 2016, 25, 133-137.
doi: 10.3171/2015.12.SPINE15737 URL |
| 58. | Hart, N. H.; Nimphius, S.; Rantalainen, T.; Ireland, A.; Siafarikas, A.; Newton, R. U. Mechanical basis of bone strength: influence of bone material, bone structure and muscle action. J Musculoskelet Neuronal Interact. 2017, 17, 114-139. |
| 59. | Xia, Z.; Shi, Y.; He, H.; Pan, Y.; Liu, C. Development of biodegradable bone graft substitutes using 3D printing. In Developments and applications of calcium phosphate bone cements, Liu, C.; He, H., eds.; Springer Singapore: Singapore, 2018; pp 517-545. |
| 60. | Triffitt, J. T. A brief history of the development of stromal stem cells (stem cells of the skeleton). Biomater Transl. 2021, 2, 287-293. |
| 61. | Triffitt, J. T. Orthopaedic tissue engineering and stem cells - an unfulfilled promise. Biomater Transl. 2021, 2, 89-90. |
| 62. |
Wright, A.; Arthaud-Day, M. L.; Weiss, M. L. Therapeutic use of mesenchymal stromal cells: the need for inclusive characterization guidelines to accommodate all tissue sources and species. Front Cell Dev Biol. 2021, 9, 632717.
doi: 10.3389/fcell.2021.632717 URL |
| 63. |
Pereira Chilima, T. D.; Moncaubeig, F.; Farid, S. S. Impact of allogeneic stem cell manufacturing decisions on cost of goods, process robustness and reimbursement. Biochem Eng J. 2018, 137, 132-151.
doi: 10.1016/j.bej.2018.04.017 URL |
| 64. | Levy, O.; Kuai, R.; Siren, E. M. J.; Bhere, D.; Milton, Y.; Nissar, N.; De Biasio, M.; Heinelt, M.; Reeve, B.; Abdi, R.; Alturki, M.; Fallatah, M.; Almalik, A.; Alhasan, A. H.; Shah, K.; Karp, J. M. Shattering barriers toward clinically meaningful MSC therapies. Sci Adv. 2020, 6, eaba6884. |
| 65. |
Fu, K.; Xu, Q.; Czernuszka, J.; Triffitt, J. T.; Xia, Z. Characterization of a biodegradable coralline hydroxyapatite/calcium carbonate composite and its clinical implementation. Biomed Mater. 2013, 8, 065007.
doi: 10.1088/1748-6041/8/6/065007 URL |
| 66. | Koëter, S.; Tigchelaar, S. J.; Farla, P.; Driessen, L.; van Kampen, A.; Buma, P. Coralline hydroxyapatite is a suitable bone graft substitute in an intra-articular goat defect model. J Biomed Mater Res B Appl Biomater. 2009, 90, 116-122. |
| 67. |
Ripamonti, U.; Crooks, J.; Khoali, L.; Roden, L. The induction of bone formation by coral-derived calcium carbonate/hydroxyapatite constructs. Biomaterials. 2009, 30, 1428-1439.
doi: 10.1016/j.biomaterials.2008.10.065 URL |
| 68. |
Viateau, V.; Manassero, M.; Sensébé, L.; Langonné, A.; Marchat, D.; Logeart-Avramoglou, D.; Petite, H.; Bensidhoum, M. Comparative study of the osteogenic ability of four different ceramic constructs in an ectopic large animal model. J Tissue Eng Regen Med. 2016, 10, E177-187.
doi: 10.1002/term.v10.3 URL |
| 69. |
Gao, T. J.; Lindholm, T. S.; Kommonen, B.; Ragni, P.; Paronzini, A.; Lindholm, T. C.; Jalovaara, P.; Urist, M. R. The use of a coral composite implant containing bone morphogenetic protein to repair a segmental tibial defect in sheep. Int Orthop. 1997, 21, 194-200.
doi: 10.1007/s002640050149 URL |
| 70. |
Liang, X.; Song, E. The role of bone marrow stromal cells in blood diseases and clinical significance as a crucial part of the hematopoietic microenvironment. Ann Blood. 2020, 5, 2.
doi: 10.21037/aob URL |
| 71. |
Mankani, M. H.; Kuznetsov, S. A.; Fowler, B.; Kingman, A.; Robey, P. G. In vivo bone formation by human bone marrow stromal cells: effect of carrier particle size and shape. Biotechnol Bioeng. 2001, 72, 96-107.
doi: 10.1002/(ISSN)1097-0290 URL |
| 72. | Zhou, Y. F.; Sae-Lim, V.; Chou, A. M.; Hutmacher, D. W.; Lim, T. M. Does seeding density affect in vitro mineral nodules formation in novel composite scaffolds? J Biomed Mater Res A. 2006, 78, 183-193. |
| 73. |
Adamzyk, C.; Kachel, P.; Hoss, M.; Gremse, F.; Modabber, A.; Hölzle, F.; Tolba, R.; Neuss, S.; Lethaus, B. Bone tissue engineering using polyetherketoneketone scaffolds combined with autologous mesenchymal stem cells in a sheep calvarial defect model. J Craniomaxillofac Surg. 2016, 44, 985-994.
doi: 10.1016/j.jcms.2016.04.012 URL |
| 74. |
Caterson, E. J.; Nesti, L. J.; Danielson, K. G.; Tuan, R. S. Human marrow-derived mesenchymal progenitor cells: isolation, culture expansion, and analysis of differentiation. Mol Biotechnol. 2002, 20, 245-256.
doi: 10.1385/MB:20:3 URL |
| 75. | Day, A. G. E.; Francis, W. R.; Fu, K.; Pieper, I. L.; Guy, O.; Xia, Z. Osteogenic potential of human umbilical cord mesenchymal stem cells on coralline hydroxyapatite/calcium carbonate microparticles. Stem Cells Int. 2018, 2018, 4258613. |
| 76. |
Squillaro, T.; Peluso, G.; Galderisi, U. Clinical trials with mesenchymal stem cells: an update. Cell Transplant. 2016, 25, 829-848.
doi: 10.3727/096368915X689622 URL |
| 77. |
Kern, S.; Eichler, H.; Stoeve, J.; Klüter, H.; Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006, 24, 1294-1301.
doi: 10.1634/stemcells.2005-0342 URL |
| 78. |
Rebelatto, C. K.; Aguiar, A. M.; Moretão, M. P.; Senegaglia, A. C.; Hansen, P.; Barchiki, F.; Oliveira, J.; Martins, J.; Kuligovski, C.; Mansur, F.; Christofis, A.; Amaral, V. F.; Brofman, P. S.; Goldenberg, S.; Nakao, L. S.; Correa, A. Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Exp Biol Med (Maywood). 2008, 233, 901-913.
doi: 10.3181/0712-RM-356 URL |
| 79. |
Mohamed-Ahmed, S.; Fristad, I.; Lie, S. A.; Suliman, S.; Mustafa, K.; Vindenes, H.; Idris, S. B. Adipose-derived and bone marrow mesenchymal stem cells: a donor-matched comparison. Stem Cell Res Ther. 2018, 9, 168.
doi: 10.1186/s13287-018-0914-1 URL |
| 80. |
Zeddou, M.; Briquet, A.; Relic, B.; Josse, C.; Malaise, M. G.; Gothot, A.; Lechanteur, C.; Beguin, Y. The umbilical cord matrix is a better source of mesenchymal stem cells (MSC) than the umbilical cord blood. Cell Biol Int. 2010, 34, 693-701.
doi: 10.1042/CBI20090414 URL |
| 81. |
Mennan, C.; Garcia, J.; Roberts, S.; Hulme, C.; Wright, K. A comprehensive characterisation of large-scale expanded human bone marrow and umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2019, 10, 99.
doi: 10.1186/s13287-019-1202-4 URL |
| 82. |
Zhang, Z. Y.; Teoh, S. H.; Chong, M. S.; Schantz, J. T.; Fisk, N. M.; Choolani, M. A.; Chan, J. Superior osteogenic capacity for bone tissue engineering of fetal compared with perinatal and adult mesenchymal stem cells. Stem Cells. 2009, 27, 126-137.
doi: 10.1634/stemcells.2008-0456 URL |
| 83. | Mennan, C.; Wright, K.; Bhattacharjee, A.; Balain, B.; Richardson, J.; Roberts, S. Isolation and characterisation of mesenchymal stem cells from different regions of the human umbilical cord. Biomed Res Int. 2013, 2013, 916136. |
| 84. |
Schneider, S.; Unger, M.; van Griensven, M.; Balmayor, E. R. Adipose-derived mesenchymal stem cells from liposuction and resected fat are feasible sources for regenerative medicine. Eur J Med Res. 2017, 22, 17.
doi: 10.1186/s40001-017-0258-9 URL |
| 85. |
Markarian, C. F.; Frey, G. Z.; Silveira, M. D.; Chem, E. M.; Milani, A. R.; Ely, P. B.; Horn, A. P.; Nardi, N. B.; Camassola, M. Isolation of adipose-derived stem cells: a comparison among different methods. Biotechnol Lett. 2014, 36, 693-702.
doi: 10.1007/s10529-013-1425-x URL |
| 86. |
Choudhery, M. S.; Badowski, M.; Muise, A.; Pierce, J.; Harris, D. T. Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. J Transl Med. 2014, 12, 8.
doi: 10.1186/1479-5876-12-8 URL |
| 87. |
Hasegawa, T.; Miwa, M.; Sakai, Y.; Niikura, T.; Lee, S. Y.; Oe, K.; Iwakura, T.; Kurosaka, M.; Komori, T. Efficient cell-seeding into scaffolds improves bone formation. J Dent Res. 2010, 89, 854-859.
doi: 10.1177/0022034510370022 URL |
| 88. |
Wilson, C. E.; Dhert, W. J.; Van Blitterswijk, C. A.; Verbout, A. J.; De Bruijn, J. D. Evaluating 3D bone tissue engineered constructs with different seeding densities using the alamarBlue assay and the effect on in vivo bone formation. J Mater Sci Mater Med. 2002, 13, 1265-1269.
doi: 10.1023/A:1021139415528 URL |
| 89. |
vunjak-novakovic, g.; obradovic, b.; martin, i.; bursac, p. m.; langer, r.; freed, l. e. Dynamic cell seeding of polymer scaffolds for cartilage tissue engineering. Biotechnol Prog. 1998, 14, 193-202.
doi: 10.1021/bp970120j URL |
| 90. |
Lode, A.; Bernhardt, A.; Gelinsky, M. Cultivation of human bone marrow stromal cells on three-dimensional scaffolds of mineralized collagen: influence of seeding density on colonization, proliferation and osteogenic differentiation. J Tissue Eng Regen Med. 2008, 2, 400-407.
doi: 10.1002/term.v2:7 URL |
| 91. |
Martin, I.; Wendt, D.; Heberer, M. The role of bioreactors in tissue engineering. Trends Biotechnol. 2004, 22, 80-86.
doi: 10.1016/j.tibtech.2003.12.001 URL |
| 92. | Grayson, W. L.; Bhumiratana, S.; Cannizzaro, C.; Vunjak-Novakovic, G. Bioreactor cultivation of functional bone grafts. Methods Mol Biol. 2011, 698, 231-241. |
| 93. |
Gooch, K. J.; Kwon, J. H.; Blunk, T.; Langer, R.; Freed, L. E.; Vunjak-Novakovic, G. Effects of mixing intensity on tissue-engineered cartilage. Biotechnol Bioeng. 2001, 72, 402-407.
doi: 10.1002/(ISSN)1097-0290 URL |
| 94. | Temple, J. P.; Yeager, K.; Bhumiratana, S.; Vunjak-Novakovic, G.; Grayson, W. L. Bioreactor cultivation of anatomically shaped human bone grafts. Methods Mol Biol. 2014, 1202, 57-78. |
| 95. | Botchwey, E.; Ferrante, E.; Humphrey, J. Bioreactor-based bone tissue engineering. J Biomech. 2006, 39, S218. |
| 96. | O’Dea, D. R.; Byrne, H. M.; Waters, S. L. Continuum modelling of in vitro tissue engineering: a review. In Computational modeling in tissue engineering, Geris, L., ed. Springer Berlin Heidelberg: Berlin, Heidelberg, 2013; pp 229-266. |
| 97. | Burova, I.; Wall, I.; Shipley, R. J. Mathematical and computational models for bone tissue engineering in bioreactor systems. J Tissue Eng. 2019, 10, 2041731419827922. |
| 98. |
Garg, P.; Mazur, M. M.; Buck, A. C.; Wandtke, M. E.; Liu, J.; Ebraheim, N. A. Prospective review of mesenchymal stem cells differentiation into osteoblasts. Orthop Surg. 2017, 9, 13-19.
doi: 10.1111/os.2017.9.issue-1 URL |
| 99. | Friedlaender, G. E.; Perry, C. R.; Cole, J. D.; Cook, S. D.; Cierny, G.; Muschler, G. F.; Zych, G. A.; Calhoun, J. H.; LaForte, A. J.; Yin, S. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am. 2001, 83-A Suppl 1, S151-158. |
| 100. |
Fujioka-Kobayashi, M.; Kobayashi, E.; Schaller, B.; Mottini, M.; Miron, R. J.; Saulacic, N. Effect of recombinant human bone morphogenic protein 9 (rhBMP9) loaded onto bone grafts versus barrier membranes on new bone formation in a rabbit calvarial defect model. J Biomed Mater Res A. 2017, 105, 2655-2661.
doi: 10.1002/jbm.a.v105.10 URL |
| 101. |
Paralkar, V. M.; Grasser, W. A.; Mansolf, A. L.; Baumann, A. P.; Owen, T. A.; Smock, S. L.; Martinovic, S.; Borovecki, F.; Vukicevic, S.; Ke, H. Z.; Thompson, D. D. Regulation of BMP-7 expression by retinoic acid and prostaglandin E(2). J Cell Physiol. 2002, 190, 207-217.
doi: 10.1002/(ISSN)1097-4652 URL |
| 102. |
Zhao, J.; Ohba, S.; Shinkai, M.; Chung, U. I.; Nagamune, T. Icariin induces osteogenic differentiation in vitro in a BMP- and Runx2-dependent manner. Biochem Biophys Res Commun. 2008, 369, 444-448.
doi: 10.1016/j.bbrc.2008.02.054 URL |
| 103. | Cao, H.; Ke, Y.; Zhang, Y.; Zhang, C. J.; Qian, W.; Zhang, G. L. Icariin stimulates MC3T3-E1 cell proliferation and differentiation through up-regulation of bone morphogenetic protein-2. Int J Mol Med. 2012, 29, 435-439. |
| 104. |
Xie, L.; Liu, N.; Xiao, Y.; Liu, Y.; Yan, C.; Wang, G.; Jing, X. In vitro and in vivo osteogenesis induced by icariin and bone morphogenetic protein-2: a dynamic observation. Front Pharmacol. 2020, 11, 1058.
doi: 10.3389/fphar.2020.01058 URL |
| 105. | Loozen, L. D.; Kruyt, M. C.; Kragten, A. H. M.; Schoenfeldt, T.; Croes, M.; Oner, C. F.; Dhert, W. J. A.; Alblas, J. BMP-2 gene delivery in cell-loaded and cell-free constructs for bone regeneration. PLoS One. 2019, 14, e0220028. |
| 106. |
Park, S. Y.; Kim, K. H.; Kim, S.; Lee, Y. M.; Seol, Y. J. BMP-2 gene delivery-based bone regeneration in dentistry. Pharmaceutics. 2019, 11, 393.
doi: 10.3390/pharmaceutics11080393 URL |
| 107. | Loozen, L. D.; Vandersteen, A.; Kragten, A. H.; Öner, F. C.; Dhert, W. J.; Kruyt, M. C.; Alblas, J. Bone formation by heterodimers through non-viral gene delivery of BMP-2/6 and BMP-2/7. Eur Cell Mater. 2018, 35, 195-208. |
| 108. |
Rose, L. C.; Kucharski, C.; Uludağ, H. Protein expression following non-viral delivery of plasmid DNA coding for basic FGF and BMP-2 in a rat ectopic model. Biomaterials. 2012, 33, 3363-3374.
doi: 10.1016/j.biomaterials.2012.01.031 URL |
| 109. |
Nie, X.; Wang, D. A. Decellularized orthopaedic tissue-engineered grafts: biomaterial scaffolds synthesised by therapeutic cells. Biomater Sci. 2018, 6, 2798-2811.
doi: 10.1039/C8BM00772A URL |
| 110. | Shi, Y. Allografts combined with tissue derived stem cells for bone healing. Patent No. US20100124776A1. 2017. |
| 111. | Office of the Federal Register. 21 CFR 888.3045 - Resorbable calcium salt bone void filler device. National Archives and Records Administration. 2010. |
| 112. | Office of the Federal Register. 21 CFR 872.3930 - Bone grafting material. National Archives and Records Administration. 2012. |
| 113. | ISO 10993-1:2018. Biological evaluation of medical devices — Part 1: Evaluation and testing within a risk management process. |
| 114. | Hu, Y.; Zhao, Q. W.; Wang, Z. C.; Fang, Q. Q.; Zhu, H.; Hong, D. S.; Liang, X. G.; Lou, D.; Tan, W. Q. Co-transfection with BMP2 and FGF2 via chitosan nanoparticles potentiates osteogenesis in human adipose-derived stromal cells in vitro. J Int Med Res. 2021, 49, 300060521997679. |
| [1] | Xuechen Zhang, Ana Justo Caetano, Paul T. Sharpe, Ana Angelova Volponi. Oral stem cells, decoding and mapping the resident cells populations [J]. Biomaterials Translational, 2022, 3(1): 24-30. |
| [2] | Deepika Arora, Pamela Gehron Robey. Recent updates on the biological basis of heterogeneity in bone marrow stromal cells/skeletal stem cells [J]. Biomaterials Translational, 2022, 3(1): 3-16. |
| [3] | Suzanne M. Watt. The long and winding road: homeostatic and disordered haematopoietic microenvironmental niches: a narrative review [J]. Biomaterials Translational, 2022, 3(1): 31-54. |
| [4] | Shuqin Cao, Quan Yuan. An update of nanotopographical surfaces in modulating stem cell fate: a narrative review [J]. Biomaterials Translational, 2022, 3(1): 55-64. |
| [5] | Ke Hu, Yuxuan Li, Zunxiang Ke, Hongjun Yang, Chanjun Lu, Yiqing Li, Yi Guo, Weici Wang. History, progress and future challenges of artificial blood vessels: a narrative review [J]. Biomaterials Translational, 2022, 3(1): 81-98. |
| [6] | Trivia P. Frazier, Katie Hamel, Xiying Wu, Emma Rogers, Haley Lassiter, Jordan Robinson, Omair Mohiuddin, Michael Henderson, Jeffrey M. Gimble. Adipose-derived cells: building blocks of three-dimensional microphysiological systems [J]. Biomaterials Translational, 2021, 2(4): 301-306. |
| [7] | Yizhong Peng, Jinye Li, Hui Lin, Shuo Tian, Sheng Liu, Feifei Pu, Lei Zhao, Kaige Ma, Xiangcheng Qing, Zengwu Shao. Endogenous repair theory enriches construction strategies for orthopaedic biomaterials: a narrative review [J]. Biomaterials Translational, 2021, 2(4): 343-360. |
| [8] | Xirui Jing, Qiuyue Ding, Qinxue Wu, Weijie Su, Keda Yu, Yanlin Su, Bing Ye, Qing Gao, Tingfang Sun, Xiaodong Guo. Magnesium-based materials in orthopaedics: material properties and animal models [J]. Biomaterials Translational, 2021, 2(3): 197-213. |
| [9] | Kamolrat Metavarayuth, Esteban Villarreal, Hui Wang, Qian Wang. Surface topography and free energy regulate osteogenesis of stem cells: effects of shape-controlled gold nanoparticles [J]. Biomaterials Translational, 2021, 2(2): 165-173. |
| [10] | Yizhong Peng, Xiangcheng Qing, Hongyang Shu, Shuo Tian, Wenbo Yang, Songfeng Chen, Hui Lin, Xiao Lv, Lei Zhao, Xi Chen, Feifei Pu, Donghua Huang, Xu Cao, Zengwu Shao. Proper animal experimental designs for preclinical research of biomaterials for intervertebral disc regeneration [J]. Biomaterials Translational, 2021, 2(2): 91-142. |
| [11] | Pingli Wu, Yangyang Liang, Guoming Sun. Engineering immune-responsive biomaterials for skin regeneration [J]. Biomaterials Translational, 2021, 2(1): 61-71. |
| [12] | Yiqing Wang, Xiangyu Chu, Bing Wang. Recombinant adeno-associated virus-based gene therapy combined with tissue engineering for musculoskeletal regenerative medicine [J]. Biomaterials Translational, 2021, 2(1): 19-29. |
| [13] | Isak Jatoi, Jingyu Fan. A biomaterials viewpoint for the 2020 SARS-CoV-2 vaccine development [J]. Biomaterials Translational, 2021, 2(1): 30-42. |
| [14] | Maryam Tamaddon, Helena Gilja, Ling Wang, J. Miguel Oliveira, Xiaodan Sun, Rongwei Tan, Chaozong Liu. Osteochondral scaffolds for early treatment of cartilage defects in osteoarthritic joints: from bench to clinic [J]. Biomaterials Translational, 2020, 1(1): 3-17. |
| [15] | Xiaowen Xu, Jie Song. Segmental long bone regeneration guided by degradable synthetic polymeric scaffolds [J]. Biomaterials Translational, 2020, 1(1): 33-45. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||