Complex evolution of epidemiology of vascular diseases, including increased disease burden: from 2000 to 2015
1
2014
... Cardiovascular disease is the leading cause of morbidity and mortality in the world, of which coronary and peripheral artery vascular diseases comprise the largest proportion.1, 2 Vascular grafts are mainly used for the surgical treatment of vascular diseases that require new long-term revascularisation, including abdominal aortic aneurysms, coarctation of the aorta and chronic haemodialysis access. For this purpose, a single vascular graft is taken as an autologous graft from the patient, which is commonly the saphenous vein from the leg or the internal thoracic artery from the chest wall.3 However, autologous blood vessel availability is limited and requires invasive harvesting techniques. Moreover, with passage of time, the quality of autologous blood vessels can be difficult to guarantee, and the incidence of postoperative complications is higher.4, 5 Therefore, it is of great clinical significance to obtain vascular prosthetics that can function as blood vessels in order to restore blood flow around a blockage or replace damaged blood vessels.6 Currently, various types of materials are used to prepare artificial vascular grafts, including synthetic polymers, natural materials, or a mixture of types. In addition, there is increasing interest in regard to the development of tissue-engineered vascular grafts (TEVG) among various other approaches, which are currently under investigation.7 A tissue-engineered vessel can be grown, remodelled, and repaired in vivo without the necessity for autograft surgery, producing demonstrable benefits. Vascular graft failures are generally related to thrombosis, intimal hyperplasia, atherosclerosis, or infection.8, 9 Moreover, ideal artificial vascular grafts have good biocompatibility and blood compatibility which will last until the completion of endothelialisation.10 Any degradation in vivo is balanced by new tissue formation, replacing lost material with native proteins.11 The most promising materials provide mechanical support equal to autogenous blood vessels to sustain blood pressure load and promote tissue regeneration in vivo with long-term patency.12, 13 Furthermore, materials possessing the capacity for scaled production, sterilisation, and storage are preferred, enabling them to conform to existing clinical applications. Different anatomic locations for artificial blood vessels have different primary design requirements: for small-calibre or low-flow vessels, a non-thrombogenic luminal surface may be more important than for large-diameter vessels, which, in contrast, call for greater wall tensile properties to improve mechanical durability. Accordingly, this review attempts to outline the problems associated with the use of artificial blood vessels in vivo and describe past research efforts. In addition, current state-of-the-art technologies involved in the creation of artificial blood vessels, including advances in materials, fabrication techniques, various methods of surface modification, as well as preclinical and clinical applications, are discussed. Furthermore, the evaluation of grafts both in vivo and in vitro, mechanical properties, challenges, and directions for further research are also elaborated. Here, we searched related articles in the electronic databases of PubMed and Web of Science from inception to January 2022, using the terms “artificial blood vessels”, “vascular graft”, “vascular prosthesis”, and related keywords. ...
Sustainable Development Goals and the future of cardiovascular health. A statement from the Global Cardiovascular Disease Taskforce
1
2014
... Cardiovascular disease is the leading cause of morbidity and mortality in the world, of which coronary and peripheral artery vascular diseases comprise the largest proportion.1, 2 Vascular grafts are mainly used for the surgical treatment of vascular diseases that require new long-term revascularisation, including abdominal aortic aneurysms, coarctation of the aorta and chronic haemodialysis access. For this purpose, a single vascular graft is taken as an autologous graft from the patient, which is commonly the saphenous vein from the leg or the internal thoracic artery from the chest wall.3 However, autologous blood vessel availability is limited and requires invasive harvesting techniques. Moreover, with passage of time, the quality of autologous blood vessels can be difficult to guarantee, and the incidence of postoperative complications is higher.4, 5 Therefore, it is of great clinical significance to obtain vascular prosthetics that can function as blood vessels in order to restore blood flow around a blockage or replace damaged blood vessels.6 Currently, various types of materials are used to prepare artificial vascular grafts, including synthetic polymers, natural materials, or a mixture of types. In addition, there is increasing interest in regard to the development of tissue-engineered vascular grafts (TEVG) among various other approaches, which are currently under investigation.7 A tissue-engineered vessel can be grown, remodelled, and repaired in vivo without the necessity for autograft surgery, producing demonstrable benefits. Vascular graft failures are generally related to thrombosis, intimal hyperplasia, atherosclerosis, or infection.8, 9 Moreover, ideal artificial vascular grafts have good biocompatibility and blood compatibility which will last until the completion of endothelialisation.10 Any degradation in vivo is balanced by new tissue formation, replacing lost material with native proteins.11 The most promising materials provide mechanical support equal to autogenous blood vessels to sustain blood pressure load and promote tissue regeneration in vivo with long-term patency.12, 13 Furthermore, materials possessing the capacity for scaled production, sterilisation, and storage are preferred, enabling them to conform to existing clinical applications. Different anatomic locations for artificial blood vessels have different primary design requirements: for small-calibre or low-flow vessels, a non-thrombogenic luminal surface may be more important than for large-diameter vessels, which, in contrast, call for greater wall tensile properties to improve mechanical durability. Accordingly, this review attempts to outline the problems associated with the use of artificial blood vessels in vivo and describe past research efforts. In addition, current state-of-the-art technologies involved in the creation of artificial blood vessels, including advances in materials, fabrication techniques, various methods of surface modification, as well as preclinical and clinical applications, are discussed. Furthermore, the evaluation of grafts both in vivo and in vitro, mechanical properties, challenges, and directions for further research are also elaborated. Here, we searched related articles in the electronic databases of PubMed and Web of Science from inception to January 2022, using the terms “artificial blood vessels”, “vascular graft”, “vascular prosthesis”, and related keywords. ...
Vein grafting your way out of trouble: examining the utility and efficacy of vein grafts in microsurgery
1
2015
... Cardiovascular disease is the leading cause of morbidity and mortality in the world, of which coronary and peripheral artery vascular diseases comprise the largest proportion.1, 2 Vascular grafts are mainly used for the surgical treatment of vascular diseases that require new long-term revascularisation, including abdominal aortic aneurysms, coarctation of the aorta and chronic haemodialysis access. For this purpose, a single vascular graft is taken as an autologous graft from the patient, which is commonly the saphenous vein from the leg or the internal thoracic artery from the chest wall.3 However, autologous blood vessel availability is limited and requires invasive harvesting techniques. Moreover, with passage of time, the quality of autologous blood vessels can be difficult to guarantee, and the incidence of postoperative complications is higher.4, 5 Therefore, it is of great clinical significance to obtain vascular prosthetics that can function as blood vessels in order to restore blood flow around a blockage or replace damaged blood vessels.6 Currently, various types of materials are used to prepare artificial vascular grafts, including synthetic polymers, natural materials, or a mixture of types. In addition, there is increasing interest in regard to the development of tissue-engineered vascular grafts (TEVG) among various other approaches, which are currently under investigation.7 A tissue-engineered vessel can be grown, remodelled, and repaired in vivo without the necessity for autograft surgery, producing demonstrable benefits. Vascular graft failures are generally related to thrombosis, intimal hyperplasia, atherosclerosis, or infection.8, 9 Moreover, ideal artificial vascular grafts have good biocompatibility and blood compatibility which will last until the completion of endothelialisation.10 Any degradation in vivo is balanced by new tissue formation, replacing lost material with native proteins.11 The most promising materials provide mechanical support equal to autogenous blood vessels to sustain blood pressure load and promote tissue regeneration in vivo with long-term patency.12, 13 Furthermore, materials possessing the capacity for scaled production, sterilisation, and storage are preferred, enabling them to conform to existing clinical applications. Different anatomic locations for artificial blood vessels have different primary design requirements: for small-calibre or low-flow vessels, a non-thrombogenic luminal surface may be more important than for large-diameter vessels, which, in contrast, call for greater wall tensile properties to improve mechanical durability. Accordingly, this review attempts to outline the problems associated with the use of artificial blood vessels in vivo and describe past research efforts. In addition, current state-of-the-art technologies involved in the creation of artificial blood vessels, including advances in materials, fabrication techniques, various methods of surface modification, as well as preclinical and clinical applications, are discussed. Furthermore, the evaluation of grafts both in vivo and in vitro, mechanical properties, challenges, and directions for further research are also elaborated. Here, we searched related articles in the electronic databases of PubMed and Web of Science from inception to January 2022, using the terms “artificial blood vessels”, “vascular graft”, “vascular prosthesis”, and related keywords. ...
Saphenous vein grafts in contemporary coronary artery bypass graft surgery
1
2020
... Cardiovascular disease is the leading cause of morbidity and mortality in the world, of which coronary and peripheral artery vascular diseases comprise the largest proportion.1, 2 Vascular grafts are mainly used for the surgical treatment of vascular diseases that require new long-term revascularisation, including abdominal aortic aneurysms, coarctation of the aorta and chronic haemodialysis access. For this purpose, a single vascular graft is taken as an autologous graft from the patient, which is commonly the saphenous vein from the leg or the internal thoracic artery from the chest wall.3 However, autologous blood vessel availability is limited and requires invasive harvesting techniques. Moreover, with passage of time, the quality of autologous blood vessels can be difficult to guarantee, and the incidence of postoperative complications is higher.4, 5 Therefore, it is of great clinical significance to obtain vascular prosthetics that can function as blood vessels in order to restore blood flow around a blockage or replace damaged blood vessels.6 Currently, various types of materials are used to prepare artificial vascular grafts, including synthetic polymers, natural materials, or a mixture of types. In addition, there is increasing interest in regard to the development of tissue-engineered vascular grafts (TEVG) among various other approaches, which are currently under investigation.7 A tissue-engineered vessel can be grown, remodelled, and repaired in vivo without the necessity for autograft surgery, producing demonstrable benefits. Vascular graft failures are generally related to thrombosis, intimal hyperplasia, atherosclerosis, or infection.8, 9 Moreover, ideal artificial vascular grafts have good biocompatibility and blood compatibility which will last until the completion of endothelialisation.10 Any degradation in vivo is balanced by new tissue formation, replacing lost material with native proteins.11 The most promising materials provide mechanical support equal to autogenous blood vessels to sustain blood pressure load and promote tissue regeneration in vivo with long-term patency.12, 13 Furthermore, materials possessing the capacity for scaled production, sterilisation, and storage are preferred, enabling them to conform to existing clinical applications. Different anatomic locations for artificial blood vessels have different primary design requirements: for small-calibre or low-flow vessels, a non-thrombogenic luminal surface may be more important than for large-diameter vessels, which, in contrast, call for greater wall tensile properties to improve mechanical durability. Accordingly, this review attempts to outline the problems associated with the use of artificial blood vessels in vivo and describe past research efforts. In addition, current state-of-the-art technologies involved in the creation of artificial blood vessels, including advances in materials, fabrication techniques, various methods of surface modification, as well as preclinical and clinical applications, are discussed. Furthermore, the evaluation of grafts both in vivo and in vitro, mechanical properties, challenges, and directions for further research are also elaborated. Here, we searched related articles in the electronic databases of PubMed and Web of Science from inception to January 2022, using the terms “artificial blood vessels”, “vascular graft”, “vascular prosthesis”, and related keywords. ...
Fabrication and characterization of chitosan nanoparticles and collagen-loaded polyurethane nanocomposite membrane coated with heparin for atrial septal defect (ASD) closure
1
2017
... Cardiovascular disease is the leading cause of morbidity and mortality in the world, of which coronary and peripheral artery vascular diseases comprise the largest proportion.1, 2 Vascular grafts are mainly used for the surgical treatment of vascular diseases that require new long-term revascularisation, including abdominal aortic aneurysms, coarctation of the aorta and chronic haemodialysis access. For this purpose, a single vascular graft is taken as an autologous graft from the patient, which is commonly the saphenous vein from the leg or the internal thoracic artery from the chest wall.3 However, autologous blood vessel availability is limited and requires invasive harvesting techniques. Moreover, with passage of time, the quality of autologous blood vessels can be difficult to guarantee, and the incidence of postoperative complications is higher.4, 5 Therefore, it is of great clinical significance to obtain vascular prosthetics that can function as blood vessels in order to restore blood flow around a blockage or replace damaged blood vessels.6 Currently, various types of materials are used to prepare artificial vascular grafts, including synthetic polymers, natural materials, or a mixture of types. In addition, there is increasing interest in regard to the development of tissue-engineered vascular grafts (TEVG) among various other approaches, which are currently under investigation.7 A tissue-engineered vessel can be grown, remodelled, and repaired in vivo without the necessity for autograft surgery, producing demonstrable benefits. Vascular graft failures are generally related to thrombosis, intimal hyperplasia, atherosclerosis, or infection.8, 9 Moreover, ideal artificial vascular grafts have good biocompatibility and blood compatibility which will last until the completion of endothelialisation.10 Any degradation in vivo is balanced by new tissue formation, replacing lost material with native proteins.11 The most promising materials provide mechanical support equal to autogenous blood vessels to sustain blood pressure load and promote tissue regeneration in vivo with long-term patency.12, 13 Furthermore, materials possessing the capacity for scaled production, sterilisation, and storage are preferred, enabling them to conform to existing clinical applications. Different anatomic locations for artificial blood vessels have different primary design requirements: for small-calibre or low-flow vessels, a non-thrombogenic luminal surface may be more important than for large-diameter vessels, which, in contrast, call for greater wall tensile properties to improve mechanical durability. Accordingly, this review attempts to outline the problems associated with the use of artificial blood vessels in vivo and describe past research efforts. In addition, current state-of-the-art technologies involved in the creation of artificial blood vessels, including advances in materials, fabrication techniques, various methods of surface modification, as well as preclinical and clinical applications, are discussed. Furthermore, the evaluation of grafts both in vivo and in vitro, mechanical properties, challenges, and directions for further research are also elaborated. Here, we searched related articles in the electronic databases of PubMed and Web of Science from inception to January 2022, using the terms “artificial blood vessels”, “vascular graft”, “vascular prosthesis”, and related keywords. ...
Redo bypass surgery to the infrapopliteal arteries for critical leg ischaemia
1
2001
... Cardiovascular disease is the leading cause of morbidity and mortality in the world, of which coronary and peripheral artery vascular diseases comprise the largest proportion.1, 2 Vascular grafts are mainly used for the surgical treatment of vascular diseases that require new long-term revascularisation, including abdominal aortic aneurysms, coarctation of the aorta and chronic haemodialysis access. For this purpose, a single vascular graft is taken as an autologous graft from the patient, which is commonly the saphenous vein from the leg or the internal thoracic artery from the chest wall.3 However, autologous blood vessel availability is limited and requires invasive harvesting techniques. Moreover, with passage of time, the quality of autologous blood vessels can be difficult to guarantee, and the incidence of postoperative complications is higher.4, 5 Therefore, it is of great clinical significance to obtain vascular prosthetics that can function as blood vessels in order to restore blood flow around a blockage or replace damaged blood vessels.6 Currently, various types of materials are used to prepare artificial vascular grafts, including synthetic polymers, natural materials, or a mixture of types. In addition, there is increasing interest in regard to the development of tissue-engineered vascular grafts (TEVG) among various other approaches, which are currently under investigation.7 A tissue-engineered vessel can be grown, remodelled, and repaired in vivo without the necessity for autograft surgery, producing demonstrable benefits. Vascular graft failures are generally related to thrombosis, intimal hyperplasia, atherosclerosis, or infection.8, 9 Moreover, ideal artificial vascular grafts have good biocompatibility and blood compatibility which will last until the completion of endothelialisation.10 Any degradation in vivo is balanced by new tissue formation, replacing lost material with native proteins.11 The most promising materials provide mechanical support equal to autogenous blood vessels to sustain blood pressure load and promote tissue regeneration in vivo with long-term patency.12, 13 Furthermore, materials possessing the capacity for scaled production, sterilisation, and storage are preferred, enabling them to conform to existing clinical applications. Different anatomic locations for artificial blood vessels have different primary design requirements: for small-calibre or low-flow vessels, a non-thrombogenic luminal surface may be more important than for large-diameter vessels, which, in contrast, call for greater wall tensile properties to improve mechanical durability. Accordingly, this review attempts to outline the problems associated with the use of artificial blood vessels in vivo and describe past research efforts. In addition, current state-of-the-art technologies involved in the creation of artificial blood vessels, including advances in materials, fabrication techniques, various methods of surface modification, as well as preclinical and clinical applications, are discussed. Furthermore, the evaluation of grafts both in vivo and in vitro, mechanical properties, challenges, and directions for further research are also elaborated. Here, we searched related articles in the electronic databases of PubMed and Web of Science from inception to January 2022, using the terms “artificial blood vessels”, “vascular graft”, “vascular prosthesis”, and related keywords. ...
Selection of different endothelialization modes and different seed cells for tissue-engineered vascular graft
1
2021
... Cardiovascular disease is the leading cause of morbidity and mortality in the world, of which coronary and peripheral artery vascular diseases comprise the largest proportion.1, 2 Vascular grafts are mainly used for the surgical treatment of vascular diseases that require new long-term revascularisation, including abdominal aortic aneurysms, coarctation of the aorta and chronic haemodialysis access. For this purpose, a single vascular graft is taken as an autologous graft from the patient, which is commonly the saphenous vein from the leg or the internal thoracic artery from the chest wall.3 However, autologous blood vessel availability is limited and requires invasive harvesting techniques. Moreover, with passage of time, the quality of autologous blood vessels can be difficult to guarantee, and the incidence of postoperative complications is higher.4, 5 Therefore, it is of great clinical significance to obtain vascular prosthetics that can function as blood vessels in order to restore blood flow around a blockage or replace damaged blood vessels.6 Currently, various types of materials are used to prepare artificial vascular grafts, including synthetic polymers, natural materials, or a mixture of types. In addition, there is increasing interest in regard to the development of tissue-engineered vascular grafts (TEVG) among various other approaches, which are currently under investigation.7 A tissue-engineered vessel can be grown, remodelled, and repaired in vivo without the necessity for autograft surgery, producing demonstrable benefits. Vascular graft failures are generally related to thrombosis, intimal hyperplasia, atherosclerosis, or infection.8, 9 Moreover, ideal artificial vascular grafts have good biocompatibility and blood compatibility which will last until the completion of endothelialisation.10 Any degradation in vivo is balanced by new tissue formation, replacing lost material with native proteins.11 The most promising materials provide mechanical support equal to autogenous blood vessels to sustain blood pressure load and promote tissue regeneration in vivo with long-term patency.12, 13 Furthermore, materials possessing the capacity for scaled production, sterilisation, and storage are preferred, enabling them to conform to existing clinical applications. Different anatomic locations for artificial blood vessels have different primary design requirements: for small-calibre or low-flow vessels, a non-thrombogenic luminal surface may be more important than for large-diameter vessels, which, in contrast, call for greater wall tensile properties to improve mechanical durability. Accordingly, this review attempts to outline the problems associated with the use of artificial blood vessels in vivo and describe past research efforts. In addition, current state-of-the-art technologies involved in the creation of artificial blood vessels, including advances in materials, fabrication techniques, various methods of surface modification, as well as preclinical and clinical applications, are discussed. Furthermore, the evaluation of grafts both in vivo and in vitro, mechanical properties, challenges, and directions for further research are also elaborated. Here, we searched related articles in the electronic databases of PubMed and Web of Science from inception to January 2022, using the terms “artificial blood vessels”, “vascular graft”, “vascular prosthesis”, and related keywords. ...
Evaluation of remodeling process in small-diameter cell-free tissue-engineered arterial graft
1
2015
... Cardiovascular disease is the leading cause of morbidity and mortality in the world, of which coronary and peripheral artery vascular diseases comprise the largest proportion.1, 2 Vascular grafts are mainly used for the surgical treatment of vascular diseases that require new long-term revascularisation, including abdominal aortic aneurysms, coarctation of the aorta and chronic haemodialysis access. For this purpose, a single vascular graft is taken as an autologous graft from the patient, which is commonly the saphenous vein from the leg or the internal thoracic artery from the chest wall.3 However, autologous blood vessel availability is limited and requires invasive harvesting techniques. Moreover, with passage of time, the quality of autologous blood vessels can be difficult to guarantee, and the incidence of postoperative complications is higher.4, 5 Therefore, it is of great clinical significance to obtain vascular prosthetics that can function as blood vessels in order to restore blood flow around a blockage or replace damaged blood vessels.6 Currently, various types of materials are used to prepare artificial vascular grafts, including synthetic polymers, natural materials, or a mixture of types. In addition, there is increasing interest in regard to the development of tissue-engineered vascular grafts (TEVG) among various other approaches, which are currently under investigation.7 A tissue-engineered vessel can be grown, remodelled, and repaired in vivo without the necessity for autograft surgery, producing demonstrable benefits. Vascular graft failures are generally related to thrombosis, intimal hyperplasia, atherosclerosis, or infection.8, 9 Moreover, ideal artificial vascular grafts have good biocompatibility and blood compatibility which will last until the completion of endothelialisation.10 Any degradation in vivo is balanced by new tissue formation, replacing lost material with native proteins.11 The most promising materials provide mechanical support equal to autogenous blood vessels to sustain blood pressure load and promote tissue regeneration in vivo with long-term patency.12, 13 Furthermore, materials possessing the capacity for scaled production, sterilisation, and storage are preferred, enabling them to conform to existing clinical applications. Different anatomic locations for artificial blood vessels have different primary design requirements: for small-calibre or low-flow vessels, a non-thrombogenic luminal surface may be more important than for large-diameter vessels, which, in contrast, call for greater wall tensile properties to improve mechanical durability. Accordingly, this review attempts to outline the problems associated with the use of artificial blood vessels in vivo and describe past research efforts. In addition, current state-of-the-art technologies involved in the creation of artificial blood vessels, including advances in materials, fabrication techniques, various methods of surface modification, as well as preclinical and clinical applications, are discussed. Furthermore, the evaluation of grafts both in vivo and in vitro, mechanical properties, challenges, and directions for further research are also elaborated. Here, we searched related articles in the electronic databases of PubMed and Web of Science from inception to January 2022, using the terms “artificial blood vessels”, “vascular graft”, “vascular prosthesis”, and related keywords. ...
Rapid endothelialization of off-the-shelf small diameter silk vascular grafts
1
2018
... Cardiovascular disease is the leading cause of morbidity and mortality in the world, of which coronary and peripheral artery vascular diseases comprise the largest proportion.1, 2 Vascular grafts are mainly used for the surgical treatment of vascular diseases that require new long-term revascularisation, including abdominal aortic aneurysms, coarctation of the aorta and chronic haemodialysis access. For this purpose, a single vascular graft is taken as an autologous graft from the patient, which is commonly the saphenous vein from the leg or the internal thoracic artery from the chest wall.3 However, autologous blood vessel availability is limited and requires invasive harvesting techniques. Moreover, with passage of time, the quality of autologous blood vessels can be difficult to guarantee, and the incidence of postoperative complications is higher.4, 5 Therefore, it is of great clinical significance to obtain vascular prosthetics that can function as blood vessels in order to restore blood flow around a blockage or replace damaged blood vessels.6 Currently, various types of materials are used to prepare artificial vascular grafts, including synthetic polymers, natural materials, or a mixture of types. In addition, there is increasing interest in regard to the development of tissue-engineered vascular grafts (TEVG) among various other approaches, which are currently under investigation.7 A tissue-engineered vessel can be grown, remodelled, and repaired in vivo without the necessity for autograft surgery, producing demonstrable benefits. Vascular graft failures are generally related to thrombosis, intimal hyperplasia, atherosclerosis, or infection.8, 9 Moreover, ideal artificial vascular grafts have good biocompatibility and blood compatibility which will last until the completion of endothelialisation.10 Any degradation in vivo is balanced by new tissue formation, replacing lost material with native proteins.11 The most promising materials provide mechanical support equal to autogenous blood vessels to sustain blood pressure load and promote tissue regeneration in vivo with long-term patency.12, 13 Furthermore, materials possessing the capacity for scaled production, sterilisation, and storage are preferred, enabling them to conform to existing clinical applications. Different anatomic locations for artificial blood vessels have different primary design requirements: for small-calibre or low-flow vessels, a non-thrombogenic luminal surface may be more important than for large-diameter vessels, which, in contrast, call for greater wall tensile properties to improve mechanical durability. Accordingly, this review attempts to outline the problems associated with the use of artificial blood vessels in vivo and describe past research efforts. In addition, current state-of-the-art technologies involved in the creation of artificial blood vessels, including advances in materials, fabrication techniques, various methods of surface modification, as well as preclinical and clinical applications, are discussed. Furthermore, the evaluation of grafts both in vivo and in vitro, mechanical properties, challenges, and directions for further research are also elaborated. Here, we searched related articles in the electronic databases of PubMed and Web of Science from inception to January 2022, using the terms “artificial blood vessels”, “vascular graft”, “vascular prosthesis”, and related keywords. ...
Bioengineering artificial blood vessels from natural materials
3
2021
... Cardiovascular disease is the leading cause of morbidity and mortality in the world, of which coronary and peripheral artery vascular diseases comprise the largest proportion.1, 2 Vascular grafts are mainly used for the surgical treatment of vascular diseases that require new long-term revascularisation, including abdominal aortic aneurysms, coarctation of the aorta and chronic haemodialysis access. For this purpose, a single vascular graft is taken as an autologous graft from the patient, which is commonly the saphenous vein from the leg or the internal thoracic artery from the chest wall.3 However, autologous blood vessel availability is limited and requires invasive harvesting techniques. Moreover, with passage of time, the quality of autologous blood vessels can be difficult to guarantee, and the incidence of postoperative complications is higher.4, 5 Therefore, it is of great clinical significance to obtain vascular prosthetics that can function as blood vessels in order to restore blood flow around a blockage or replace damaged blood vessels.6 Currently, various types of materials are used to prepare artificial vascular grafts, including synthetic polymers, natural materials, or a mixture of types. In addition, there is increasing interest in regard to the development of tissue-engineered vascular grafts (TEVG) among various other approaches, which are currently under investigation.7 A tissue-engineered vessel can be grown, remodelled, and repaired in vivo without the necessity for autograft surgery, producing demonstrable benefits. Vascular graft failures are generally related to thrombosis, intimal hyperplasia, atherosclerosis, or infection.8, 9 Moreover, ideal artificial vascular grafts have good biocompatibility and blood compatibility which will last until the completion of endothelialisation.10 Any degradation in vivo is balanced by new tissue formation, replacing lost material with native proteins.11 The most promising materials provide mechanical support equal to autogenous blood vessels to sustain blood pressure load and promote tissue regeneration in vivo with long-term patency.12, 13 Furthermore, materials possessing the capacity for scaled production, sterilisation, and storage are preferred, enabling them to conform to existing clinical applications. Different anatomic locations for artificial blood vessels have different primary design requirements: for small-calibre or low-flow vessels, a non-thrombogenic luminal surface may be more important than for large-diameter vessels, which, in contrast, call for greater wall tensile properties to improve mechanical durability. Accordingly, this review attempts to outline the problems associated with the use of artificial blood vessels in vivo and describe past research efforts. In addition, current state-of-the-art technologies involved in the creation of artificial blood vessels, including advances in materials, fabrication techniques, various methods of surface modification, as well as preclinical and clinical applications, are discussed. Furthermore, the evaluation of grafts both in vivo and in vitro, mechanical properties, challenges, and directions for further research are also elaborated. Here, we searched related articles in the electronic databases of PubMed and Web of Science from inception to January 2022, using the terms “artificial blood vessels”, “vascular graft”, “vascular prosthesis”, and related keywords. ...
... Over the last two decades, significant progress has been made in developing various types of tissue-engineered vascular substitutes. Tissue engineering technology combines cells, tissue scaffold, and engineering in order to generate vascular grafts.25 Particularly, creation of small-diameter artificial blood vessels has progressed well in terms of employing tissue engineering techniques through different approaches, such as artificial blood vessels using biodegradable polymers as scaffolds or consisting of decellularised vascular tissue.26 Meanwhile, novel approaches have been used to construct TEVGs for clinical use and these have been demonstrated to have many advantages, such as three-dimensional (3D) bioprinting, which allows for the use of autologous cells alone without any scaffolding in the preparation of different sized small-diameter artificial blood vessels. Nevertheless, few comprehensive studies have been conducted pertaining to artificial small-calibre vascular grafts that possess long-term patency in vivo. In addition, an ideal tissue-engineered vascular graft has yet to be made clinically available.27 Evaluating the next generation of grafts encompasses resolving complex and multifaceted issues via a combination of mechanical engineering, vascular biology, and immunoregulation, which continue to pose a great challenge.10 ...
... Advantages and disadvantages of artificial blood vessels in different materials
| Vessel type | Materials | Advantages | Disadvantages | References |
| Synthetic polymers | ePTFE
(Gore-Tex),
PET (Dacron), PU, PCL, PLCL, PLA, PGS. | Excellent mechanical properties.
Easy availability.
Mass production.
Easy surgical suturing.
Preventing vascular burst.
Can be stored for
off-the-shelf use. | Causes thrombosis, intimal hyperplasia,
calcification, and chronic inflammation.
No growth potential.
Poor haemocompatibility.
Compliance mismatch. | 46, 47, 49, 51, 72 |
| Natural biomaterials | Silk fibroin, collagen, elastin, chitosan,
bacterial cellulose | Excellent biocompatibility.
Enhanced biological signalling.
Tunable mechanical properties. | Weak mechanical strength.
Causes vascular graft dilation and aneurysms.
Easy degradation.
Overly complex designs.
Difficulty in translation. | 10, 52-57, 66 |
| Decellularised vessels | Animal artery, umbilical artery, umbilical vein | Low immunogenicity.
Preserved extracellular matrix, meso- and microvasculature. | Increased thrombogenicity.
Host immune response.
Difficulty in precise recellularisation.
Calcification. | 67-69 |
Note: ePTFE: expanded polytetrafluoroethylene; PCL: polycaprolactone; PET: poly(ethylene terephthalate); PGS: poly(glycerol sebacate); PLA: polylactic acid; PLCL: poly(L-lactide-co-caprolactone); PU: polyurethane. ...
Effects of age-related shifts in cellular function and local microenvironment upon the innate immune response to implants
1
2017
... Cardiovascular disease is the leading cause of morbidity and mortality in the world, of which coronary and peripheral artery vascular diseases comprise the largest proportion.1, 2 Vascular grafts are mainly used for the surgical treatment of vascular diseases that require new long-term revascularisation, including abdominal aortic aneurysms, coarctation of the aorta and chronic haemodialysis access. For this purpose, a single vascular graft is taken as an autologous graft from the patient, which is commonly the saphenous vein from the leg or the internal thoracic artery from the chest wall.3 However, autologous blood vessel availability is limited and requires invasive harvesting techniques. Moreover, with passage of time, the quality of autologous blood vessels can be difficult to guarantee, and the incidence of postoperative complications is higher.4, 5 Therefore, it is of great clinical significance to obtain vascular prosthetics that can function as blood vessels in order to restore blood flow around a blockage or replace damaged blood vessels.6 Currently, various types of materials are used to prepare artificial vascular grafts, including synthetic polymers, natural materials, or a mixture of types. In addition, there is increasing interest in regard to the development of tissue-engineered vascular grafts (TEVG) among various other approaches, which are currently under investigation.7 A tissue-engineered vessel can be grown, remodelled, and repaired in vivo without the necessity for autograft surgery, producing demonstrable benefits. Vascular graft failures are generally related to thrombosis, intimal hyperplasia, atherosclerosis, or infection.8, 9 Moreover, ideal artificial vascular grafts have good biocompatibility and blood compatibility which will last until the completion of endothelialisation.10 Any degradation in vivo is balanced by new tissue formation, replacing lost material with native proteins.11 The most promising materials provide mechanical support equal to autogenous blood vessels to sustain blood pressure load and promote tissue regeneration in vivo with long-term patency.12, 13 Furthermore, materials possessing the capacity for scaled production, sterilisation, and storage are preferred, enabling them to conform to existing clinical applications. Different anatomic locations for artificial blood vessels have different primary design requirements: for small-calibre or low-flow vessels, a non-thrombogenic luminal surface may be more important than for large-diameter vessels, which, in contrast, call for greater wall tensile properties to improve mechanical durability. Accordingly, this review attempts to outline the problems associated with the use of artificial blood vessels in vivo and describe past research efforts. In addition, current state-of-the-art technologies involved in the creation of artificial blood vessels, including advances in materials, fabrication techniques, various methods of surface modification, as well as preclinical and clinical applications, are discussed. Furthermore, the evaluation of grafts both in vivo and in vitro, mechanical properties, challenges, and directions for further research are also elaborated. Here, we searched related articles in the electronic databases of PubMed and Web of Science from inception to January 2022, using the terms “artificial blood vessels”, “vascular graft”, “vascular prosthesis”, and related keywords. ...
Dynamic straining combined with fibrin gel cell seeding improves strength of tissue-engineered small-diameter vascular grafts
2
2009
... Cardiovascular disease is the leading cause of morbidity and mortality in the world, of which coronary and peripheral artery vascular diseases comprise the largest proportion.1, 2 Vascular grafts are mainly used for the surgical treatment of vascular diseases that require new long-term revascularisation, including abdominal aortic aneurysms, coarctation of the aorta and chronic haemodialysis access. For this purpose, a single vascular graft is taken as an autologous graft from the patient, which is commonly the saphenous vein from the leg or the internal thoracic artery from the chest wall.3 However, autologous blood vessel availability is limited and requires invasive harvesting techniques. Moreover, with passage of time, the quality of autologous blood vessels can be difficult to guarantee, and the incidence of postoperative complications is higher.4, 5 Therefore, it is of great clinical significance to obtain vascular prosthetics that can function as blood vessels in order to restore blood flow around a blockage or replace damaged blood vessels.6 Currently, various types of materials are used to prepare artificial vascular grafts, including synthetic polymers, natural materials, or a mixture of types. In addition, there is increasing interest in regard to the development of tissue-engineered vascular grafts (TEVG) among various other approaches, which are currently under investigation.7 A tissue-engineered vessel can be grown, remodelled, and repaired in vivo without the necessity for autograft surgery, producing demonstrable benefits. Vascular graft failures are generally related to thrombosis, intimal hyperplasia, atherosclerosis, or infection.8, 9 Moreover, ideal artificial vascular grafts have good biocompatibility and blood compatibility which will last until the completion of endothelialisation.10 Any degradation in vivo is balanced by new tissue formation, replacing lost material with native proteins.11 The most promising materials provide mechanical support equal to autogenous blood vessels to sustain blood pressure load and promote tissue regeneration in vivo with long-term patency.12, 13 Furthermore, materials possessing the capacity for scaled production, sterilisation, and storage are preferred, enabling them to conform to existing clinical applications. Different anatomic locations for artificial blood vessels have different primary design requirements: for small-calibre or low-flow vessels, a non-thrombogenic luminal surface may be more important than for large-diameter vessels, which, in contrast, call for greater wall tensile properties to improve mechanical durability. Accordingly, this review attempts to outline the problems associated with the use of artificial blood vessels in vivo and describe past research efforts. In addition, current state-of-the-art technologies involved in the creation of artificial blood vessels, including advances in materials, fabrication techniques, various methods of surface modification, as well as preclinical and clinical applications, are discussed. Furthermore, the evaluation of grafts both in vivo and in vitro, mechanical properties, challenges, and directions for further research are also elaborated. Here, we searched related articles in the electronic databases of PubMed and Web of Science from inception to January 2022, using the terms “artificial blood vessels”, “vascular graft”, “vascular prosthesis”, and related keywords. ...
... Mechanical properties of natural vessels and some artificial blood vessels.
| Burst pressure (mmHg) | Compliance (%/100 mmHg) |
| Values for artificial blood vessels12, 91 | >1000 | 10-20 |
| Dog femoral artery118 | 2895 ± 263 | 10.3 ± 2.3 |
| Human internal mammary artery119 | 3196 ± 1264 | 11.5 ± 3.9 |
Biodegradable chitosan vascular grafts
(with 4 mm inner diameter)118 | 1688 ± 236 | 5.7 ± 1.3 |
| Tissue-engineered blood vessels13 | 3490 ± 892 | 3.4 ± 1.6 |
| Expanded polytetrafluoroethylene grafts119 | – | 0.51 |
| Silk fibroin grafts119 | – | 1.90 |
Another consideration is individual variability, signifying the variation in the mechanical properties of blood vessels from vessel to vessel and between individuals. In future, vascular tissue engineering may ultimately involve tailoring the specific mechanical properties of a graft to the intended implantation site. A far greater understanding of vascular biomechanics, interactions between the graft and the native vessels, and how to engineer the mechanical properties of a vascular graft would be required. ...
Mechanical properties of completely autologous human tissue engineered blood vessels compared to human saphenous vein and mammary artery
2
2009
... Cardiovascular disease is the leading cause of morbidity and mortality in the world, of which coronary and peripheral artery vascular diseases comprise the largest proportion.1, 2 Vascular grafts are mainly used for the surgical treatment of vascular diseases that require new long-term revascularisation, including abdominal aortic aneurysms, coarctation of the aorta and chronic haemodialysis access. For this purpose, a single vascular graft is taken as an autologous graft from the patient, which is commonly the saphenous vein from the leg or the internal thoracic artery from the chest wall.3 However, autologous blood vessel availability is limited and requires invasive harvesting techniques. Moreover, with passage of time, the quality of autologous blood vessels can be difficult to guarantee, and the incidence of postoperative complications is higher.4, 5 Therefore, it is of great clinical significance to obtain vascular prosthetics that can function as blood vessels in order to restore blood flow around a blockage or replace damaged blood vessels.6 Currently, various types of materials are used to prepare artificial vascular grafts, including synthetic polymers, natural materials, or a mixture of types. In addition, there is increasing interest in regard to the development of tissue-engineered vascular grafts (TEVG) among various other approaches, which are currently under investigation.7 A tissue-engineered vessel can be grown, remodelled, and repaired in vivo without the necessity for autograft surgery, producing demonstrable benefits. Vascular graft failures are generally related to thrombosis, intimal hyperplasia, atherosclerosis, or infection.8, 9 Moreover, ideal artificial vascular grafts have good biocompatibility and blood compatibility which will last until the completion of endothelialisation.10 Any degradation in vivo is balanced by new tissue formation, replacing lost material with native proteins.11 The most promising materials provide mechanical support equal to autogenous blood vessels to sustain blood pressure load and promote tissue regeneration in vivo with long-term patency.12, 13 Furthermore, materials possessing the capacity for scaled production, sterilisation, and storage are preferred, enabling them to conform to existing clinical applications. Different anatomic locations for artificial blood vessels have different primary design requirements: for small-calibre or low-flow vessels, a non-thrombogenic luminal surface may be more important than for large-diameter vessels, which, in contrast, call for greater wall tensile properties to improve mechanical durability. Accordingly, this review attempts to outline the problems associated with the use of artificial blood vessels in vivo and describe past research efforts. In addition, current state-of-the-art technologies involved in the creation of artificial blood vessels, including advances in materials, fabrication techniques, various methods of surface modification, as well as preclinical and clinical applications, are discussed. Furthermore, the evaluation of grafts both in vivo and in vitro, mechanical properties, challenges, and directions for further research are also elaborated. Here, we searched related articles in the electronic databases of PubMed and Web of Science from inception to January 2022, using the terms “artificial blood vessels”, “vascular graft”, “vascular prosthesis”, and related keywords. ...
... Mechanical properties of natural vessels and some artificial blood vessels.
| Burst pressure (mmHg) | Compliance (%/100 mmHg) |
| Values for artificial blood vessels12, 91 | >1000 | 10-20 |
| Dog femoral artery118 | 2895 ± 263 | 10.3 ± 2.3 |
| Human internal mammary artery119 | 3196 ± 1264 | 11.5 ± 3.9 |
Biodegradable chitosan vascular grafts
(with 4 mm inner diameter)118 | 1688 ± 236 | 5.7 ± 1.3 |
| Tissue-engineered blood vessels13 | 3490 ± 892 | 3.4 ± 1.6 |
| Expanded polytetrafluoroethylene grafts119 | – | 0.51 |
| Silk fibroin grafts119 | – | 1.90 |
Another consideration is individual variability, signifying the variation in the mechanical properties of blood vessels from vessel to vessel and between individuals. In future, vascular tissue engineering may ultimately involve tailoring the specific mechanical properties of a graft to the intended implantation site. A far greater understanding of vascular biomechanics, interactions between the graft and the native vessels, and how to engineer the mechanical properties of a vascular graft would be required. ...
The novel hybrid polycarbonate polyurethane / polyester three-layered large-diameter artificial blood vessel
1
2022
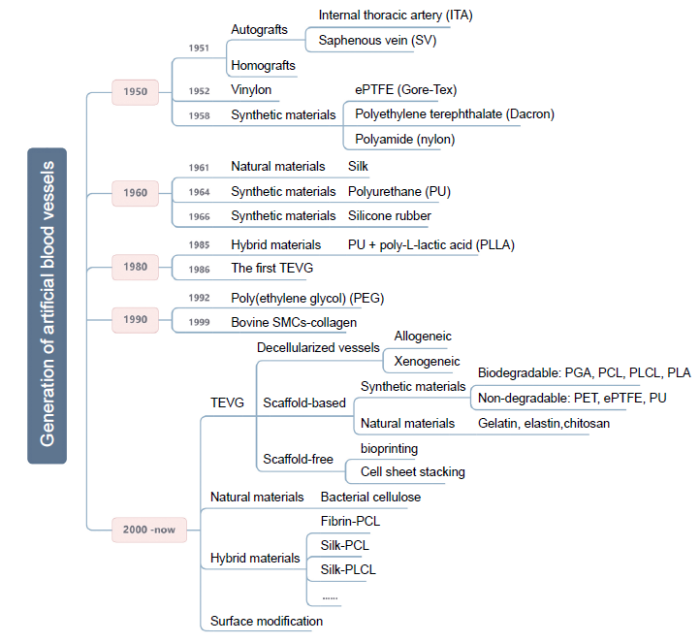
... Researchers have attempted to ameliorate the need for artificial vascular grafts in the past, as shown in Figure 1. Specifically, Austrian-German surgeon Payr experimented around 1900 with a thin tube made from absorbable magnesium, which marked the first time that a vascular prosthesis was used in humans. However, the patient died of pneumonia 3 days later due to the formation of fibrotic tissue induced by the magnesium.14 Dubost et al.15 utilised a cadaveric aortic allograft to perform the first successful aneurysm resection and graft implantation on March 29, 1951. In 1954, Blakemore and Voorhees16 were the first to treat 10 patients with a synthetic arterial substitute, laying the foundation for the rapid development of subsequent artificial blood vessels. Then, in 1958, De Bakey et al.17 presented the Dacron vascular prosthesis as an alternative to cadaveric allografts, which are prone to late complications. Most researchers have subsequently focused on various other synthetic materials, of which Dacron, polyurethane, and expanded polytetrafluoroethylene (ePTFE) have proven to be the most viable in vascular surgery.18 However, such materials are not suitable for use as implants when the diameter of the vessel is smaller than 6 mm due to the high risk of thrombus formation and compliance mismatch.18, 19 A type of hybrid graft, prepared from polyurethane and poly-L-lactic acid, was developed in 1985 and has been demonstrated to ensure the fast development of a complete new arterial wall, possessing strength, compliance, and thromboresistance equal to normal arterial wall tissue.20 Eventually, techniques have become more efficient and have led to novel developments. In 1986, the first tissue-engineered blood vessel construct was produced by Weinberg and Bell.21 Bovine endothelial cells (ECs), fibroblasts, and smooth muscle cells (SMCs) were cultured in a collagen matrix and shaped into tubes, realising tissue architectures analogous to natural blood vessels. However, the mechanical properties of the constructs were poor and required the support of a Dacron mesh. Around this time, Argentinian surgeon Parodi, in collaboration with the Argentinian radiologist Palmaz, experimented with stents made of stainless steel and Dacron tube grafts in canine models and developed an endovascular technique for abdominal aorta repair. In 1990, the first successful human endovascular aneurysm repair was performed.22 Additionally, the first completely autologous engineered arteries were implanted into porcine recipients in 1999. These vessels, which were 3 mm in diameter and 5–6 cm in length, demonstrated patency and satisfactory mechanical durability for 4 weeks.23, 24 ...
Resection of an aneurysm of the abdominal aorta: reestablishment of the continuity by a preserved human arterial graft, with result after five months
1
1952
... Researchers have attempted to ameliorate the need for artificial vascular grafts in the past, as shown in Figure 1. Specifically, Austrian-German surgeon Payr experimented around 1900 with a thin tube made from absorbable magnesium, which marked the first time that a vascular prosthesis was used in humans. However, the patient died of pneumonia 3 days later due to the formation of fibrotic tissue induced by the magnesium.14 Dubost et al.15 utilised a cadaveric aortic allograft to perform the first successful aneurysm resection and graft implantation on March 29, 1951. In 1954, Blakemore and Voorhees16 were the first to treat 10 patients with a synthetic arterial substitute, laying the foundation for the rapid development of subsequent artificial blood vessels. Then, in 1958, De Bakey et al.17 presented the Dacron vascular prosthesis as an alternative to cadaveric allografts, which are prone to late complications. Most researchers have subsequently focused on various other synthetic materials, of which Dacron, polyurethane, and expanded polytetrafluoroethylene (ePTFE) have proven to be the most viable in vascular surgery.18 However, such materials are not suitable for use as implants when the diameter of the vessel is smaller than 6 mm due to the high risk of thrombus formation and compliance mismatch.18, 19 A type of hybrid graft, prepared from polyurethane and poly-L-lactic acid, was developed in 1985 and has been demonstrated to ensure the fast development of a complete new arterial wall, possessing strength, compliance, and thromboresistance equal to normal arterial wall tissue.20 Eventually, techniques have become more efficient and have led to novel developments. In 1986, the first tissue-engineered blood vessel construct was produced by Weinberg and Bell.21 Bovine endothelial cells (ECs), fibroblasts, and smooth muscle cells (SMCs) were cultured in a collagen matrix and shaped into tubes, realising tissue architectures analogous to natural blood vessels. However, the mechanical properties of the constructs were poor and required the support of a Dacron mesh. Around this time, Argentinian surgeon Parodi, in collaboration with the Argentinian radiologist Palmaz, experimented with stents made of stainless steel and Dacron tube grafts in canine models and developed an endovascular technique for abdominal aorta repair. In 1990, the first successful human endovascular aneurysm repair was performed.22 Additionally, the first completely autologous engineered arteries were implanted into porcine recipients in 1999. These vessels, which were 3 mm in diameter and 5–6 cm in length, demonstrated patency and satisfactory mechanical durability for 4 weeks.23, 24 ...
The use of tubes constructed from vinyon N cloth in bridging arterial defects; experimental and clinical
1
1954
... Researchers have attempted to ameliorate the need for artificial vascular grafts in the past, as shown in Figure 1. Specifically, Austrian-German surgeon Payr experimented around 1900 with a thin tube made from absorbable magnesium, which marked the first time that a vascular prosthesis was used in humans. However, the patient died of pneumonia 3 days later due to the formation of fibrotic tissue induced by the magnesium.14 Dubost et al.15 utilised a cadaveric aortic allograft to perform the first successful aneurysm resection and graft implantation on March 29, 1951. In 1954, Blakemore and Voorhees16 were the first to treat 10 patients with a synthetic arterial substitute, laying the foundation for the rapid development of subsequent artificial blood vessels. Then, in 1958, De Bakey et al.17 presented the Dacron vascular prosthesis as an alternative to cadaveric allografts, which are prone to late complications. Most researchers have subsequently focused on various other synthetic materials, of which Dacron, polyurethane, and expanded polytetrafluoroethylene (ePTFE) have proven to be the most viable in vascular surgery.18 However, such materials are not suitable for use as implants when the diameter of the vessel is smaller than 6 mm due to the high risk of thrombus formation and compliance mismatch.18, 19 A type of hybrid graft, prepared from polyurethane and poly-L-lactic acid, was developed in 1985 and has been demonstrated to ensure the fast development of a complete new arterial wall, possessing strength, compliance, and thromboresistance equal to normal arterial wall tissue.20 Eventually, techniques have become more efficient and have led to novel developments. In 1986, the first tissue-engineered blood vessel construct was produced by Weinberg and Bell.21 Bovine endothelial cells (ECs), fibroblasts, and smooth muscle cells (SMCs) were cultured in a collagen matrix and shaped into tubes, realising tissue architectures analogous to natural blood vessels. However, the mechanical properties of the constructs were poor and required the support of a Dacron mesh. Around this time, Argentinian surgeon Parodi, in collaboration with the Argentinian radiologist Palmaz, experimented with stents made of stainless steel and Dacron tube grafts in canine models and developed an endovascular technique for abdominal aorta repair. In 1990, the first successful human endovascular aneurysm repair was performed.22 Additionally, the first completely autologous engineered arteries were implanted into porcine recipients in 1999. These vessels, which were 3 mm in diameter and 5–6 cm in length, demonstrated patency and satisfactory mechanical durability for 4 weeks.23, 24 ...
Clinical application of a new flexible knitted dacron arterial substitute
1
1958
... Researchers have attempted to ameliorate the need for artificial vascular grafts in the past, as shown in Figure 1. Specifically, Austrian-German surgeon Payr experimented around 1900 with a thin tube made from absorbable magnesium, which marked the first time that a vascular prosthesis was used in humans. However, the patient died of pneumonia 3 days later due to the formation of fibrotic tissue induced by the magnesium.14 Dubost et al.15 utilised a cadaveric aortic allograft to perform the first successful aneurysm resection and graft implantation on March 29, 1951. In 1954, Blakemore and Voorhees16 were the first to treat 10 patients with a synthetic arterial substitute, laying the foundation for the rapid development of subsequent artificial blood vessels. Then, in 1958, De Bakey et al.17 presented the Dacron vascular prosthesis as an alternative to cadaveric allografts, which are prone to late complications. Most researchers have subsequently focused on various other synthetic materials, of which Dacron, polyurethane, and expanded polytetrafluoroethylene (ePTFE) have proven to be the most viable in vascular surgery.18 However, such materials are not suitable for use as implants when the diameter of the vessel is smaller than 6 mm due to the high risk of thrombus formation and compliance mismatch.18, 19 A type of hybrid graft, prepared from polyurethane and poly-L-lactic acid, was developed in 1985 and has been demonstrated to ensure the fast development of a complete new arterial wall, possessing strength, compliance, and thromboresistance equal to normal arterial wall tissue.20 Eventually, techniques have become more efficient and have led to novel developments. In 1986, the first tissue-engineered blood vessel construct was produced by Weinberg and Bell.21 Bovine endothelial cells (ECs), fibroblasts, and smooth muscle cells (SMCs) were cultured in a collagen matrix and shaped into tubes, realising tissue architectures analogous to natural blood vessels. However, the mechanical properties of the constructs were poor and required the support of a Dacron mesh. Around this time, Argentinian surgeon Parodi, in collaboration with the Argentinian radiologist Palmaz, experimented with stents made of stainless steel and Dacron tube grafts in canine models and developed an endovascular technique for abdominal aorta repair. In 1990, the first successful human endovascular aneurysm repair was performed.22 Additionally, the first completely autologous engineered arteries were implanted into porcine recipients in 1999. These vessels, which were 3 mm in diameter and 5–6 cm in length, demonstrated patency and satisfactory mechanical durability for 4 weeks.23, 24 ...
Biomaterials for vascular tissue engineering
2
2010
... Researchers have attempted to ameliorate the need for artificial vascular grafts in the past, as shown in Figure 1. Specifically, Austrian-German surgeon Payr experimented around 1900 with a thin tube made from absorbable magnesium, which marked the first time that a vascular prosthesis was used in humans. However, the patient died of pneumonia 3 days later due to the formation of fibrotic tissue induced by the magnesium.14 Dubost et al.15 utilised a cadaveric aortic allograft to perform the first successful aneurysm resection and graft implantation on March 29, 1951. In 1954, Blakemore and Voorhees16 were the first to treat 10 patients with a synthetic arterial substitute, laying the foundation for the rapid development of subsequent artificial blood vessels. Then, in 1958, De Bakey et al.17 presented the Dacron vascular prosthesis as an alternative to cadaveric allografts, which are prone to late complications. Most researchers have subsequently focused on various other synthetic materials, of which Dacron, polyurethane, and expanded polytetrafluoroethylene (ePTFE) have proven to be the most viable in vascular surgery.18 However, such materials are not suitable for use as implants when the diameter of the vessel is smaller than 6 mm due to the high risk of thrombus formation and compliance mismatch.18, 19 A type of hybrid graft, prepared from polyurethane and poly-L-lactic acid, was developed in 1985 and has been demonstrated to ensure the fast development of a complete new arterial wall, possessing strength, compliance, and thromboresistance equal to normal arterial wall tissue.20 Eventually, techniques have become more efficient and have led to novel developments. In 1986, the first tissue-engineered blood vessel construct was produced by Weinberg and Bell.21 Bovine endothelial cells (ECs), fibroblasts, and smooth muscle cells (SMCs) were cultured in a collagen matrix and shaped into tubes, realising tissue architectures analogous to natural blood vessels. However, the mechanical properties of the constructs were poor and required the support of a Dacron mesh. Around this time, Argentinian surgeon Parodi, in collaboration with the Argentinian radiologist Palmaz, experimented with stents made of stainless steel and Dacron tube grafts in canine models and developed an endovascular technique for abdominal aorta repair. In 1990, the first successful human endovascular aneurysm repair was performed.22 Additionally, the first completely autologous engineered arteries were implanted into porcine recipients in 1999. These vessels, which were 3 mm in diameter and 5–6 cm in length, demonstrated patency and satisfactory mechanical durability for 4 weeks.23, 24 ...
... 18, 19 A type of hybrid graft, prepared from polyurethane and poly-L-lactic acid, was developed in 1985 and has been demonstrated to ensure the fast development of a complete new arterial wall, possessing strength, compliance, and thromboresistance equal to normal arterial wall tissue.20 Eventually, techniques have become more efficient and have led to novel developments. In 1986, the first tissue-engineered blood vessel construct was produced by Weinberg and Bell.21 Bovine endothelial cells (ECs), fibroblasts, and smooth muscle cells (SMCs) were cultured in a collagen matrix and shaped into tubes, realising tissue architectures analogous to natural blood vessels. However, the mechanical properties of the constructs were poor and required the support of a Dacron mesh. Around this time, Argentinian surgeon Parodi, in collaboration with the Argentinian radiologist Palmaz, experimented with stents made of stainless steel and Dacron tube grafts in canine models and developed an endovascular technique for abdominal aorta repair. In 1990, the first successful human endovascular aneurysm repair was performed.22 Additionally, the first completely autologous engineered arteries were implanted into porcine recipients in 1999. These vessels, which were 3 mm in diameter and 5–6 cm in length, demonstrated patency and satisfactory mechanical durability for 4 weeks.23, 24 ...
What is the proper role of polytetrafluoroethylene grafts in infrainguinal reconstruction?
1
1989
... Researchers have attempted to ameliorate the need for artificial vascular grafts in the past, as shown in Figure 1. Specifically, Austrian-German surgeon Payr experimented around 1900 with a thin tube made from absorbable magnesium, which marked the first time that a vascular prosthesis was used in humans. However, the patient died of pneumonia 3 days later due to the formation of fibrotic tissue induced by the magnesium.14 Dubost et al.15 utilised a cadaveric aortic allograft to perform the first successful aneurysm resection and graft implantation on March 29, 1951. In 1954, Blakemore and Voorhees16 were the first to treat 10 patients with a synthetic arterial substitute, laying the foundation for the rapid development of subsequent artificial blood vessels. Then, in 1958, De Bakey et al.17 presented the Dacron vascular prosthesis as an alternative to cadaveric allografts, which are prone to late complications. Most researchers have subsequently focused on various other synthetic materials, of which Dacron, polyurethane, and expanded polytetrafluoroethylene (ePTFE) have proven to be the most viable in vascular surgery.18 However, such materials are not suitable for use as implants when the diameter of the vessel is smaller than 6 mm due to the high risk of thrombus formation and compliance mismatch.18, 19 A type of hybrid graft, prepared from polyurethane and poly-L-lactic acid, was developed in 1985 and has been demonstrated to ensure the fast development of a complete new arterial wall, possessing strength, compliance, and thromboresistance equal to normal arterial wall tissue.20 Eventually, techniques have become more efficient and have led to novel developments. In 1986, the first tissue-engineered blood vessel construct was produced by Weinberg and Bell.21 Bovine endothelial cells (ECs), fibroblasts, and smooth muscle cells (SMCs) were cultured in a collagen matrix and shaped into tubes, realising tissue architectures analogous to natural blood vessels. However, the mechanical properties of the constructs were poor and required the support of a Dacron mesh. Around this time, Argentinian surgeon Parodi, in collaboration with the Argentinian radiologist Palmaz, experimented with stents made of stainless steel and Dacron tube grafts in canine models and developed an endovascular technique for abdominal aorta repair. In 1990, the first successful human endovascular aneurysm repair was performed.22 Additionally, the first completely autologous engineered arteries were implanted into porcine recipients in 1999. These vessels, which were 3 mm in diameter and 5–6 cm in length, demonstrated patency and satisfactory mechanical durability for 4 weeks.23, 24 ...
Arterial wall regeneration in small-caliber vascular grafts in rats. Neoendothelial healing and prostacyclin production
1
1985
... Researchers have attempted to ameliorate the need for artificial vascular grafts in the past, as shown in Figure 1. Specifically, Austrian-German surgeon Payr experimented around 1900 with a thin tube made from absorbable magnesium, which marked the first time that a vascular prosthesis was used in humans. However, the patient died of pneumonia 3 days later due to the formation of fibrotic tissue induced by the magnesium.14 Dubost et al.15 utilised a cadaveric aortic allograft to perform the first successful aneurysm resection and graft implantation on March 29, 1951. In 1954, Blakemore and Voorhees16 were the first to treat 10 patients with a synthetic arterial substitute, laying the foundation for the rapid development of subsequent artificial blood vessels. Then, in 1958, De Bakey et al.17 presented the Dacron vascular prosthesis as an alternative to cadaveric allografts, which are prone to late complications. Most researchers have subsequently focused on various other synthetic materials, of which Dacron, polyurethane, and expanded polytetrafluoroethylene (ePTFE) have proven to be the most viable in vascular surgery.18 However, such materials are not suitable for use as implants when the diameter of the vessel is smaller than 6 mm due to the high risk of thrombus formation and compliance mismatch.18, 19 A type of hybrid graft, prepared from polyurethane and poly-L-lactic acid, was developed in 1985 and has been demonstrated to ensure the fast development of a complete new arterial wall, possessing strength, compliance, and thromboresistance equal to normal arterial wall tissue.20 Eventually, techniques have become more efficient and have led to novel developments. In 1986, the first tissue-engineered blood vessel construct was produced by Weinberg and Bell.21 Bovine endothelial cells (ECs), fibroblasts, and smooth muscle cells (SMCs) were cultured in a collagen matrix and shaped into tubes, realising tissue architectures analogous to natural blood vessels. However, the mechanical properties of the constructs were poor and required the support of a Dacron mesh. Around this time, Argentinian surgeon Parodi, in collaboration with the Argentinian radiologist Palmaz, experimented with stents made of stainless steel and Dacron tube grafts in canine models and developed an endovascular technique for abdominal aorta repair. In 1990, the first successful human endovascular aneurysm repair was performed.22 Additionally, the first completely autologous engineered arteries were implanted into porcine recipients in 1999. These vessels, which were 3 mm in diameter and 5–6 cm in length, demonstrated patency and satisfactory mechanical durability for 4 weeks.23, 24 ...
A blood vessel model constructed from collagen and cultured vascular cells
1
1986
... Researchers have attempted to ameliorate the need for artificial vascular grafts in the past, as shown in Figure 1. Specifically, Austrian-German surgeon Payr experimented around 1900 with a thin tube made from absorbable magnesium, which marked the first time that a vascular prosthesis was used in humans. However, the patient died of pneumonia 3 days later due to the formation of fibrotic tissue induced by the magnesium.14 Dubost et al.15 utilised a cadaveric aortic allograft to perform the first successful aneurysm resection and graft implantation on March 29, 1951. In 1954, Blakemore and Voorhees16 were the first to treat 10 patients with a synthetic arterial substitute, laying the foundation for the rapid development of subsequent artificial blood vessels. Then, in 1958, De Bakey et al.17 presented the Dacron vascular prosthesis as an alternative to cadaveric allografts, which are prone to late complications. Most researchers have subsequently focused on various other synthetic materials, of which Dacron, polyurethane, and expanded polytetrafluoroethylene (ePTFE) have proven to be the most viable in vascular surgery.18 However, such materials are not suitable for use as implants when the diameter of the vessel is smaller than 6 mm due to the high risk of thrombus formation and compliance mismatch.18, 19 A type of hybrid graft, prepared from polyurethane and poly-L-lactic acid, was developed in 1985 and has been demonstrated to ensure the fast development of a complete new arterial wall, possessing strength, compliance, and thromboresistance equal to normal arterial wall tissue.20 Eventually, techniques have become more efficient and have led to novel developments. In 1986, the first tissue-engineered blood vessel construct was produced by Weinberg and Bell.21 Bovine endothelial cells (ECs), fibroblasts, and smooth muscle cells (SMCs) were cultured in a collagen matrix and shaped into tubes, realising tissue architectures analogous to natural blood vessels. However, the mechanical properties of the constructs were poor and required the support of a Dacron mesh. Around this time, Argentinian surgeon Parodi, in collaboration with the Argentinian radiologist Palmaz, experimented with stents made of stainless steel and Dacron tube grafts in canine models and developed an endovascular technique for abdominal aorta repair. In 1990, the first successful human endovascular aneurysm repair was performed.22 Additionally, the first completely autologous engineered arteries were implanted into porcine recipients in 1999. These vessels, which were 3 mm in diameter and 5–6 cm in length, demonstrated patency and satisfactory mechanical durability for 4 weeks.23, 24 ...
From ebers to EVARs: a historical perspective on aortic surgery
1
2013
... Researchers have attempted to ameliorate the need for artificial vascular grafts in the past, as shown in Figure 1. Specifically, Austrian-German surgeon Payr experimented around 1900 with a thin tube made from absorbable magnesium, which marked the first time that a vascular prosthesis was used in humans. However, the patient died of pneumonia 3 days later due to the formation of fibrotic tissue induced by the magnesium.14 Dubost et al.15 utilised a cadaveric aortic allograft to perform the first successful aneurysm resection and graft implantation on March 29, 1951. In 1954, Blakemore and Voorhees16 were the first to treat 10 patients with a synthetic arterial substitute, laying the foundation for the rapid development of subsequent artificial blood vessels. Then, in 1958, De Bakey et al.17 presented the Dacron vascular prosthesis as an alternative to cadaveric allografts, which are prone to late complications. Most researchers have subsequently focused on various other synthetic materials, of which Dacron, polyurethane, and expanded polytetrafluoroethylene (ePTFE) have proven to be the most viable in vascular surgery.18 However, such materials are not suitable for use as implants when the diameter of the vessel is smaller than 6 mm due to the high risk of thrombus formation and compliance mismatch.18, 19 A type of hybrid graft, prepared from polyurethane and poly-L-lactic acid, was developed in 1985 and has been demonstrated to ensure the fast development of a complete new arterial wall, possessing strength, compliance, and thromboresistance equal to normal arterial wall tissue.20 Eventually, techniques have become more efficient and have led to novel developments. In 1986, the first tissue-engineered blood vessel construct was produced by Weinberg and Bell.21 Bovine endothelial cells (ECs), fibroblasts, and smooth muscle cells (SMCs) were cultured in a collagen matrix and shaped into tubes, realising tissue architectures analogous to natural blood vessels. However, the mechanical properties of the constructs were poor and required the support of a Dacron mesh. Around this time, Argentinian surgeon Parodi, in collaboration with the Argentinian radiologist Palmaz, experimented with stents made of stainless steel and Dacron tube grafts in canine models and developed an endovascular technique for abdominal aorta repair. In 1990, the first successful human endovascular aneurysm repair was performed.22 Additionally, the first completely autologous engineered arteries were implanted into porcine recipients in 1999. These vessels, which were 3 mm in diameter and 5–6 cm in length, demonstrated patency and satisfactory mechanical durability for 4 weeks.23, 24 ...
Techview: medical technology. Replacement a.rteries made to order
1
1999
... Researchers have attempted to ameliorate the need for artificial vascular grafts in the past, as shown in Figure 1. Specifically, Austrian-German surgeon Payr experimented around 1900 with a thin tube made from absorbable magnesium, which marked the first time that a vascular prosthesis was used in humans. However, the patient died of pneumonia 3 days later due to the formation of fibrotic tissue induced by the magnesium.14 Dubost et al.15 utilised a cadaveric aortic allograft to perform the first successful aneurysm resection and graft implantation on March 29, 1951. In 1954, Blakemore and Voorhees16 were the first to treat 10 patients with a synthetic arterial substitute, laying the foundation for the rapid development of subsequent artificial blood vessels. Then, in 1958, De Bakey et al.17 presented the Dacron vascular prosthesis as an alternative to cadaveric allografts, which are prone to late complications. Most researchers have subsequently focused on various other synthetic materials, of which Dacron, polyurethane, and expanded polytetrafluoroethylene (ePTFE) have proven to be the most viable in vascular surgery.18 However, such materials are not suitable for use as implants when the diameter of the vessel is smaller than 6 mm due to the high risk of thrombus formation and compliance mismatch.18, 19 A type of hybrid graft, prepared from polyurethane and poly-L-lactic acid, was developed in 1985 and has been demonstrated to ensure the fast development of a complete new arterial wall, possessing strength, compliance, and thromboresistance equal to normal arterial wall tissue.20 Eventually, techniques have become more efficient and have led to novel developments. In 1986, the first tissue-engineered blood vessel construct was produced by Weinberg and Bell.21 Bovine endothelial cells (ECs), fibroblasts, and smooth muscle cells (SMCs) were cultured in a collagen matrix and shaped into tubes, realising tissue architectures analogous to natural blood vessels. However, the mechanical properties of the constructs were poor and required the support of a Dacron mesh. Around this time, Argentinian surgeon Parodi, in collaboration with the Argentinian radiologist Palmaz, experimented with stents made of stainless steel and Dacron tube grafts in canine models and developed an endovascular technique for abdominal aorta repair. In 1990, the first successful human endovascular aneurysm repair was performed.22 Additionally, the first completely autologous engineered arteries were implanted into porcine recipients in 1999. These vessels, which were 3 mm in diameter and 5–6 cm in length, demonstrated patency and satisfactory mechanical durability for 4 weeks.23, 24 ...
Functional arteries grown in vitro
1
1999
... Researchers have attempted to ameliorate the need for artificial vascular grafts in the past, as shown in Figure 1. Specifically, Austrian-German surgeon Payr experimented around 1900 with a thin tube made from absorbable magnesium, which marked the first time that a vascular prosthesis was used in humans. However, the patient died of pneumonia 3 days later due to the formation of fibrotic tissue induced by the magnesium.14 Dubost et al.15 utilised a cadaveric aortic allograft to perform the first successful aneurysm resection and graft implantation on March 29, 1951. In 1954, Blakemore and Voorhees16 were the first to treat 10 patients with a synthetic arterial substitute, laying the foundation for the rapid development of subsequent artificial blood vessels. Then, in 1958, De Bakey et al.17 presented the Dacron vascular prosthesis as an alternative to cadaveric allografts, which are prone to late complications. Most researchers have subsequently focused on various other synthetic materials, of which Dacron, polyurethane, and expanded polytetrafluoroethylene (ePTFE) have proven to be the most viable in vascular surgery.18 However, such materials are not suitable for use as implants when the diameter of the vessel is smaller than 6 mm due to the high risk of thrombus formation and compliance mismatch.18, 19 A type of hybrid graft, prepared from polyurethane and poly-L-lactic acid, was developed in 1985 and has been demonstrated to ensure the fast development of a complete new arterial wall, possessing strength, compliance, and thromboresistance equal to normal arterial wall tissue.20 Eventually, techniques have become more efficient and have led to novel developments. In 1986, the first tissue-engineered blood vessel construct was produced by Weinberg and Bell.21 Bovine endothelial cells (ECs), fibroblasts, and smooth muscle cells (SMCs) were cultured in a collagen matrix and shaped into tubes, realising tissue architectures analogous to natural blood vessels. However, the mechanical properties of the constructs were poor and required the support of a Dacron mesh. Around this time, Argentinian surgeon Parodi, in collaboration with the Argentinian radiologist Palmaz, experimented with stents made of stainless steel and Dacron tube grafts in canine models and developed an endovascular technique for abdominal aorta repair. In 1990, the first successful human endovascular aneurysm repair was performed.22 Additionally, the first completely autologous engineered arteries were implanted into porcine recipients in 1999. These vessels, which were 3 mm in diameter and 5–6 cm in length, demonstrated patency and satisfactory mechanical durability for 4 weeks.23, 24 ...
Concise review: patency of small-diameter tissue-engineered vascular grafts: a meta-analysis of preclinical trials
1
2019
... Over the last two decades, significant progress has been made in developing various types of tissue-engineered vascular substitutes. Tissue engineering technology combines cells, tissue scaffold, and engineering in order to generate vascular grafts.25 Particularly, creation of small-diameter artificial blood vessels has progressed well in terms of employing tissue engineering techniques through different approaches, such as artificial blood vessels using biodegradable polymers as scaffolds or consisting of decellularised vascular tissue.26 Meanwhile, novel approaches have been used to construct TEVGs for clinical use and these have been demonstrated to have many advantages, such as three-dimensional (3D) bioprinting, which allows for the use of autologous cells alone without any scaffolding in the preparation of different sized small-diameter artificial blood vessels. Nevertheless, few comprehensive studies have been conducted pertaining to artificial small-calibre vascular grafts that possess long-term patency in vivo. In addition, an ideal tissue-engineered vascular graft has yet to be made clinically available.27 Evaluating the next generation of grafts encompasses resolving complex and multifaceted issues via a combination of mechanical engineering, vascular biology, and immunoregulation, which continue to pose a great challenge.10 ...
Bioengineered human acellular vessels for dialysis access in patients with end-stage renal disease: two phase 2 single-arm trials
3
2016
... Over the last two decades, significant progress has been made in developing various types of tissue-engineered vascular substitutes. Tissue engineering technology combines cells, tissue scaffold, and engineering in order to generate vascular grafts.25 Particularly, creation of small-diameter artificial blood vessels has progressed well in terms of employing tissue engineering techniques through different approaches, such as artificial blood vessels using biodegradable polymers as scaffolds or consisting of decellularised vascular tissue.26 Meanwhile, novel approaches have been used to construct TEVGs for clinical use and these have been demonstrated to have many advantages, such as three-dimensional (3D) bioprinting, which allows for the use of autologous cells alone without any scaffolding in the preparation of different sized small-diameter artificial blood vessels. Nevertheless, few comprehensive studies have been conducted pertaining to artificial small-calibre vascular grafts that possess long-term patency in vivo. In addition, an ideal tissue-engineered vascular graft has yet to be made clinically available.27 Evaluating the next generation of grafts encompasses resolving complex and multifaceted issues via a combination of mechanical engineering, vascular biology, and immunoregulation, which continue to pose a great challenge.10 ...
... Cell-assembled grafts have been a dominant research topic over the past decade, where efforts following translational work indicate that TEVGs could be assembled entirely from human cells.91 Cell-assembly strategies involving culturing a range of human cell types (SMCs, fibroblasts, and induced pluripotent stem cells) for long periods of time to generate grafts are complex but the resulting grafts have the greatest degree of biomimicry.92 According to a study by Dahl et al.,81 employing human allogeneic or canine SMCs grown on a degradable polymer scaffold has been shown to be a feasible method of engineering a TEVG, in which grafts in baboons were demonstrated to retain their patency for up to 6 months. These grafts have shown great potential in preclinical evaluation. However, in a Phase II study, the final results were suboptimal as only 28% of the grafts remained open at 12 months.26 ...
... Common animal models for evaluation of artificial blood vessels
in vivo.
| Animal model | Characteristics | Application inner diameter (mm) | Common length (mm) | Longest implantation period | Implantation site | References |
| Sheep | Similar cardiovascular physiology, endothelialisation mechanisms and thrombogenicity mechanisms to humans.
Suitable size and long-term studies possible.Higher incidence of hypercoagulability. | 4–6 | 80–100 | 9 months | Carotid artery | 48, 74 |
| 4–6 | 60–100 | 3 months | Arteriovenous graft | 129 |
| Pig | Similar vascular physiology and anatomy to humans.
Well established as a model for assessing vascular grafts.
Mount an extensive immune response to implanted tissues. | 3–6 | 30–100 | 6 months | Iliac artery | 130 |
| 3–6 | 10–100 | 4 weeks | Carotid artery | 52 |
| Dog | Lack of spontaneous endothelialisation and immune response restricts study lengths.
Ease of accessing vessel due to thin skin.
Thrombogenicity mechanisms and vessel viscoelastic properties differ from humans. | 3–6 | 30–50 | 6 months | Abdominal artery | 131 |
| 3–6 | 30–50 | 1 year | Carotid artery | 119 |
| Baboon | Physiology and cardiovascular anatomy are the most similar to humans.
Suitable for a wide range of non-invasive imaging techniques adapted from humans.
High cost and ethical concerns associated with using primates in medical research. | 3–6 | 30–50 | 6 months | Arteriovenous graft | 26 |
| Rabbit | Similar endothelialisation rates and thrombogenicity mechanisms to humans.
Demanding higher anticoagulant function.
Limited to short-term studies. | 1–4 | 5–30 | 12 months | Carotid artery | 94, 132 |
| 1–4 | 5–30 | 2 weeks | Femoral artery | 133 |
| 1–4 | 5–30 | 3 months | Abdominal artery | 134 |
| Rat | Large sample size.
Wide variety of transgenic lines.
Allows exploration of genetic/molecular mechanisms.
Ideal for biocompatibility and cell infiltration studies. | 1–3 | 5–30 | 18 months | Abdominal artery | 73, 135 |
| 1–3 | 5–10 | 12 weeks | Carotid artery | 136 |
| Mouse | 0.5–1 | 3–10 | 6 months | Carotid artery | 137 |
Rats and mice are widely used because they are inexpensive to acquire, easy to maintain, and can be used to evaluate larger sample sizes.135-137 Furthermore, the availability of various transgenic strains makes it possible to examine the molecular mechanisms involved in graft remodelling.138 However, differences in their circulatory system with respect to humans limit their utility in short-term studies to only very small grafts (< 2 mm diameter). Rabbits are also suitable for artificial blood vessel assessments although they are limited to short-term studies.132-134 Their physiology is similar to that of humans in terms of endothelialisation rates and thrombogenicity, and they can accept clinically-relevant graft diameters (1–4 mm). Dogs differ from humans in terms of thrombogenicity and represent a more stringent model for the investigations because of their lack of spontaneous endothelialisation.119, 131 Moreover, they can provide a range of implantation site options with varied vessel sizes, although their immune response can restrict study durations. Both pigs and sheep demonstrate similarities to human physiology and they are appropriate for studying thrombogenicity, calcification, and long-term graft patency.129, 130 The cardiovascular physiology of non-human primates, such as baboons, is most similar to that of humans compared to dogs, pigs or sheep. Their structural similarity allows for various imaging techniques and testing methods that are typically applied in humans to be used on them. Despite such advantages, they are rarely used for vascular graft evaluation due to their high cost and ethical concerns.81, 91 The optimal model for studying all of the performance criteria associated with artificial vascular grafts has yet to be elucidated. An immunodeficient pig model, the operational immunodeficient pig, has been reported to be a large animal model that allows for long-term accommodation of artificial human vascular grafts to properly evaluate the clinical efficacy of artificial vascular grafts.139 ...
Regenerative implants for cardiovascular tissue engineering
1
2014
... Over the last two decades, significant progress has been made in developing various types of tissue-engineered vascular substitutes. Tissue engineering technology combines cells, tissue scaffold, and engineering in order to generate vascular grafts.25 Particularly, creation of small-diameter artificial blood vessels has progressed well in terms of employing tissue engineering techniques through different approaches, such as artificial blood vessels using biodegradable polymers as scaffolds or consisting of decellularised vascular tissue.26 Meanwhile, novel approaches have been used to construct TEVGs for clinical use and these have been demonstrated to have many advantages, such as three-dimensional (3D) bioprinting, which allows for the use of autologous cells alone without any scaffolding in the preparation of different sized small-diameter artificial blood vessels. Nevertheless, few comprehensive studies have been conducted pertaining to artificial small-calibre vascular grafts that possess long-term patency in vivo. In addition, an ideal tissue-engineered vascular graft has yet to be made clinically available.27 Evaluating the next generation of grafts encompasses resolving complex and multifaceted issues via a combination of mechanical engineering, vascular biology, and immunoregulation, which continue to pose a great challenge.10 ...
Saphenous vein versus PTFE for above-knee femoropopliteal bypass. A review of the literature
1
2004
... Vascular blood vessels made of synthetic polymers have been widely applied in cardiovascular disease treatment. Nevertheless, failures continue to occur, and failure to replicate key elements of the natural vasculature (Figure 2) along with the mismatch of biomechanical properties may further cause multiple complications.28, 29 Accordingly, it is essential to understand the most common failure modes in order to facilitate improvements in the materials’ design (Figure 3). Vessel graft failures are most commonly caused by restenosis and thrombus formation and always occur secondary to the progression of atherosclerotic disease or the development of intimal hyperplasia.30 In addition, infection also serves as the principal cause of late failure in grafts, in which the inflammatory response to infection can promote restenosis.31 In one case, an aneurysm developed in a Dacron prosthesis implanted in the femoral artery, with this complication occurring about five and a half years after insertion.32 ...
The fate of large-diameter Dacron® vascular grafts in surgical practice: are we really satisfied?
1
2013
... Vascular blood vessels made of synthetic polymers have been widely applied in cardiovascular disease treatment. Nevertheless, failures continue to occur, and failure to replicate key elements of the natural vasculature (Figure 2) along with the mismatch of biomechanical properties may further cause multiple complications.28, 29 Accordingly, it is essential to understand the most common failure modes in order to facilitate improvements in the materials’ design (Figure 3). Vessel graft failures are most commonly caused by restenosis and thrombus formation and always occur secondary to the progression of atherosclerotic disease or the development of intimal hyperplasia.30 In addition, infection also serves as the principal cause of late failure in grafts, in which the inflammatory response to infection can promote restenosis.31 In one case, an aneurysm developed in a Dacron prosthesis implanted in the femoral artery, with this complication occurring about five and a half years after insertion.32 ...
The ideal small arterial substitute: a search for the Holy Grail?
1
1998
... Vascular blood vessels made of synthetic polymers have been widely applied in cardiovascular disease treatment. Nevertheless, failures continue to occur, and failure to replicate key elements of the natural vasculature (Figure 2) along with the mismatch of biomechanical properties may further cause multiple complications.28, 29 Accordingly, it is essential to understand the most common failure modes in order to facilitate improvements in the materials’ design (Figure 3). Vessel graft failures are most commonly caused by restenosis and thrombus formation and always occur secondary to the progression of atherosclerotic disease or the development of intimal hyperplasia.30 In addition, infection also serves as the principal cause of late failure in grafts, in which the inflammatory response to infection can promote restenosis.31 In one case, an aneurysm developed in a Dacron prosthesis implanted in the femoral artery, with this complication occurring about five and a half years after insertion.32 ...
Infection of hemodialysis arteriovenous grafts
1
2010
... Vascular blood vessels made of synthetic polymers have been widely applied in cardiovascular disease treatment. Nevertheless, failures continue to occur, and failure to replicate key elements of the natural vasculature (Figure 2) along with the mismatch of biomechanical properties may further cause multiple complications.28, 29 Accordingly, it is essential to understand the most common failure modes in order to facilitate improvements in the materials’ design (Figure 3). Vessel graft failures are most commonly caused by restenosis and thrombus formation and always occur secondary to the progression of atherosclerotic disease or the development of intimal hyperplasia.30 In addition, infection also serves as the principal cause of late failure in grafts, in which the inflammatory response to infection can promote restenosis.31 In one case, an aneurysm developed in a Dacron prosthesis implanted in the femoral artery, with this complication occurring about five and a half years after insertion.32 ...
Aneurysm occurring in a femoral artery Dacron prosthesis five and one-half years after insertion
1
1962
... Vascular blood vessels made of synthetic polymers have been widely applied in cardiovascular disease treatment. Nevertheless, failures continue to occur, and failure to replicate key elements of the natural vasculature (Figure 2) along with the mismatch of biomechanical properties may further cause multiple complications.28, 29 Accordingly, it is essential to understand the most common failure modes in order to facilitate improvements in the materials’ design (Figure 3). Vessel graft failures are most commonly caused by restenosis and thrombus formation and always occur secondary to the progression of atherosclerotic disease or the development of intimal hyperplasia.30 In addition, infection also serves as the principal cause of late failure in grafts, in which the inflammatory response to infection can promote restenosis.31 In one case, an aneurysm developed in a Dacron prosthesis implanted in the femoral artery, with this complication occurring about five and a half years after insertion.32 ...
Monocyte adhesion to human vein grafts: a marker for occult intraoperative injury?
1
2001
... Preventing vascular graft occlusion due to endothelial hyperplasia would enable grafts to maintain their patency for a long period. There are three main causes of intimal hyperplasia, namely, intravascular SMC migration from the media to the intima, hyperproliferation of fibroblasts and SMCs, and extracellular matrix (ECM) deposition. A strong relationship is known to exist between haemodynamic factors and the development of intimal hyperplasia, which may develop in the graft vessel or the native vessel around the anastomosis.33 In addition, multiple other factors, such as mismatch of vessel diameter, trauma during surgery, and low blood flow may also serve as causes.34-36 Damage or lack of ECs lining the graft lumen results in thrombosis, which can cause the adherence of blood proteins as well as the activation of clotting mechanisms.37 The progression of atherosclerosis limits the long-term utility of grafts.38 Atheroma formation in artificial grafts is comparable to that in native arteries. Specifically, the development of atherosclerotic plaques is caused by the invasion of monocytes into the vascular neointima to form macrophages and eventually foam cells.39 Graft infections are more common in synthetic grafts, and foreign bodies are susceptible to bacterial colonisation. Such infections induce chronic inflammation, where bacteria release toxins that interfere with graft healing and may eventually result in sepsis, anastomotic failure or even rupture.40-42 ...
Increased blood flow inhibits neointimal hyperplasia in endothelialized vascular grafts
1
1991
... Preventing vascular graft occlusion due to endothelial hyperplasia would enable grafts to maintain their patency for a long period. There are three main causes of intimal hyperplasia, namely, intravascular SMC migration from the media to the intima, hyperproliferation of fibroblasts and SMCs, and extracellular matrix (ECM) deposition. A strong relationship is known to exist between haemodynamic factors and the development of intimal hyperplasia, which may develop in the graft vessel or the native vessel around the anastomosis.33 In addition, multiple other factors, such as mismatch of vessel diameter, trauma during surgery, and low blood flow may also serve as causes.34-36 Damage or lack of ECs lining the graft lumen results in thrombosis, which can cause the adherence of blood proteins as well as the activation of clotting mechanisms.37 The progression of atherosclerosis limits the long-term utility of grafts.38 Atheroma formation in artificial grafts is comparable to that in native arteries. Specifically, the development of atherosclerotic plaques is caused by the invasion of monocytes into the vascular neointima to form macrophages and eventually foam cells.39 Graft infections are more common in synthetic grafts, and foreign bodies are susceptible to bacterial colonisation. Such infections induce chronic inflammation, where bacteria release toxins that interfere with graft healing and may eventually result in sepsis, anastomotic failure or even rupture.40-42 ...
Improving vascular grafts: the importance of mechanical and haemodynamic properties
0
2000
Intimal hyperplasia and hemodynamic factors in arterial bypass and arteriovenous grafts: a review
1
2003
... Preventing vascular graft occlusion due to endothelial hyperplasia would enable grafts to maintain their patency for a long period. There are three main causes of intimal hyperplasia, namely, intravascular SMC migration from the media to the intima, hyperproliferation of fibroblasts and SMCs, and extracellular matrix (ECM) deposition. A strong relationship is known to exist between haemodynamic factors and the development of intimal hyperplasia, which may develop in the graft vessel or the native vessel around the anastomosis.33 In addition, multiple other factors, such as mismatch of vessel diameter, trauma during surgery, and low blood flow may also serve as causes.34-36 Damage or lack of ECs lining the graft lumen results in thrombosis, which can cause the adherence of blood proteins as well as the activation of clotting mechanisms.37 The progression of atherosclerosis limits the long-term utility of grafts.38 Atheroma formation in artificial grafts is comparable to that in native arteries. Specifically, the development of atherosclerotic plaques is caused by the invasion of monocytes into the vascular neointima to form macrophages and eventually foam cells.39 Graft infections are more common in synthetic grafts, and foreign bodies are susceptible to bacterial colonisation. Such infections induce chronic inflammation, where bacteria release toxins that interfere with graft healing and may eventually result in sepsis, anastomotic failure or even rupture.40-42 ...
Reprinted article “Pathophysiology of vein graft failure: a review”
1
2011
... Preventing vascular graft occlusion due to endothelial hyperplasia would enable grafts to maintain their patency for a long period. There are three main causes of intimal hyperplasia, namely, intravascular SMC migration from the media to the intima, hyperproliferation of fibroblasts and SMCs, and extracellular matrix (ECM) deposition. A strong relationship is known to exist between haemodynamic factors and the development of intimal hyperplasia, which may develop in the graft vessel or the native vessel around the anastomosis.33 In addition, multiple other factors, such as mismatch of vessel diameter, trauma during surgery, and low blood flow may also serve as causes.34-36 Damage or lack of ECs lining the graft lumen results in thrombosis, which can cause the adherence of blood proteins as well as the activation of clotting mechanisms.37 The progression of atherosclerosis limits the long-term utility of grafts.38 Atheroma formation in artificial grafts is comparable to that in native arteries. Specifically, the development of atherosclerotic plaques is caused by the invasion of monocytes into the vascular neointima to form macrophages and eventually foam cells.39 Graft infections are more common in synthetic grafts, and foreign bodies are susceptible to bacterial colonisation. Such infections induce chronic inflammation, where bacteria release toxins that interfere with graft healing and may eventually result in sepsis, anastomotic failure or even rupture.40-42 ...
Coronary bypass graft fate: long-term angiographic study
1
1991
... Preventing vascular graft occlusion due to endothelial hyperplasia would enable grafts to maintain their patency for a long period. There are three main causes of intimal hyperplasia, namely, intravascular SMC migration from the media to the intima, hyperproliferation of fibroblasts and SMCs, and extracellular matrix (ECM) deposition. A strong relationship is known to exist between haemodynamic factors and the development of intimal hyperplasia, which may develop in the graft vessel or the native vessel around the anastomosis.33 In addition, multiple other factors, such as mismatch of vessel diameter, trauma during surgery, and low blood flow may also serve as causes.34-36 Damage or lack of ECs lining the graft lumen results in thrombosis, which can cause the adherence of blood proteins as well as the activation of clotting mechanisms.37 The progression of atherosclerosis limits the long-term utility of grafts.38 Atheroma formation in artificial grafts is comparable to that in native arteries. Specifically, the development of atherosclerotic plaques is caused by the invasion of monocytes into the vascular neointima to form macrophages and eventually foam cells.39 Graft infections are more common in synthetic grafts, and foreign bodies are susceptible to bacterial colonisation. Such infections induce chronic inflammation, where bacteria release toxins that interfere with graft healing and may eventually result in sepsis, anastomotic failure or even rupture.40-42 ...
An immunocytochemical analysis of rapidly progressive atherosclerosis in human vein grafts
1
1992
... Preventing vascular graft occlusion due to endothelial hyperplasia would enable grafts to maintain their patency for a long period. There are three main causes of intimal hyperplasia, namely, intravascular SMC migration from the media to the intima, hyperproliferation of fibroblasts and SMCs, and extracellular matrix (ECM) deposition. A strong relationship is known to exist between haemodynamic factors and the development of intimal hyperplasia, which may develop in the graft vessel or the native vessel around the anastomosis.33 In addition, multiple other factors, such as mismatch of vessel diameter, trauma during surgery, and low blood flow may also serve as causes.34-36 Damage or lack of ECs lining the graft lumen results in thrombosis, which can cause the adherence of blood proteins as well as the activation of clotting mechanisms.37 The progression of atherosclerosis limits the long-term utility of grafts.38 Atheroma formation in artificial grafts is comparable to that in native arteries. Specifically, the development of atherosclerotic plaques is caused by the invasion of monocytes into the vascular neointima to form macrophages and eventually foam cells.39 Graft infections are more common in synthetic grafts, and foreign bodies are susceptible to bacterial colonisation. Such infections induce chronic inflammation, where bacteria release toxins that interfere with graft healing and may eventually result in sepsis, anastomotic failure or even rupture.40-42 ...
Vascular prosthetic graft infection: epidemiology, bacteriology, pathogenesis and treatment
1
2002
... Preventing vascular graft occlusion due to endothelial hyperplasia would enable grafts to maintain their patency for a long period. There are three main causes of intimal hyperplasia, namely, intravascular SMC migration from the media to the intima, hyperproliferation of fibroblasts and SMCs, and extracellular matrix (ECM) deposition. A strong relationship is known to exist between haemodynamic factors and the development of intimal hyperplasia, which may develop in the graft vessel or the native vessel around the anastomosis.33 In addition, multiple other factors, such as mismatch of vessel diameter, trauma during surgery, and low blood flow may also serve as causes.34-36 Damage or lack of ECs lining the graft lumen results in thrombosis, which can cause the adherence of blood proteins as well as the activation of clotting mechanisms.37 The progression of atherosclerosis limits the long-term utility of grafts.38 Atheroma formation in artificial grafts is comparable to that in native arteries. Specifically, the development of atherosclerotic plaques is caused by the invasion of monocytes into the vascular neointima to form macrophages and eventually foam cells.39 Graft infections are more common in synthetic grafts, and foreign bodies are susceptible to bacterial colonisation. Such infections induce chronic inflammation, where bacteria release toxins that interfere with graft healing and may eventually result in sepsis, anastomotic failure or even rupture.40-42 ...
Prosthetic vascular graft infection: a multi-center review of surgical management
0
2007
Complications of arteriovenous hemodialysis access: recognition and management
1
2008
... Preventing vascular graft occlusion due to endothelial hyperplasia would enable grafts to maintain their patency for a long period. There are three main causes of intimal hyperplasia, namely, intravascular SMC migration from the media to the intima, hyperproliferation of fibroblasts and SMCs, and extracellular matrix (ECM) deposition. A strong relationship is known to exist between haemodynamic factors and the development of intimal hyperplasia, which may develop in the graft vessel or the native vessel around the anastomosis.33 In addition, multiple other factors, such as mismatch of vessel diameter, trauma during surgery, and low blood flow may also serve as causes.34-36 Damage or lack of ECs lining the graft lumen results in thrombosis, which can cause the adherence of blood proteins as well as the activation of clotting mechanisms.37 The progression of atherosclerosis limits the long-term utility of grafts.38 Atheroma formation in artificial grafts is comparable to that in native arteries. Specifically, the development of atherosclerotic plaques is caused by the invasion of monocytes into the vascular neointima to form macrophages and eventually foam cells.39 Graft infections are more common in synthetic grafts, and foreign bodies are susceptible to bacterial colonisation. Such infections induce chronic inflammation, where bacteria release toxins that interfere with graft healing and may eventually result in sepsis, anastomotic failure or even rupture.40-42 ...
Fast-degrading elastomer enables rapid remodeling of a cell-free synthetic graft into a neoartery
1
2012
... Biodegradable materials are regarded as an auspicious choice for use as artificial blood vessels as the graft will be replaced by tissue ingrowth through the pores of the graft. However, this kind of reconstruction depends on the counterbalance between the degradation of the materials, ingrowth of host cells, and replacement of the materials by host tissue.43, 44 Moreover, aneurysms or even vascular rupture may develop if the degradation rate of the degradable material does not match the rate of vascular tissue regeneration. Another such issue is that SMCs may over-proliferate, causing restenosis in the later period after implantation while the endodermis of the artificial blood vessel grafts is not complete.45 These problems make it difficult for grafts to completely carry out model vascular tissue regeneration and adequately preserve biocompatibility in vivo. ...
In situ tissue regeneration using a novel tissue-engineered, small-caliber vascular graft without cell seeding
1
2008
... Biodegradable materials are regarded as an auspicious choice for use as artificial blood vessels as the graft will be replaced by tissue ingrowth through the pores of the graft. However, this kind of reconstruction depends on the counterbalance between the degradation of the materials, ingrowth of host cells, and replacement of the materials by host tissue.43, 44 Moreover, aneurysms or even vascular rupture may develop if the degradation rate of the degradable material does not match the rate of vascular tissue regeneration. Another such issue is that SMCs may over-proliferate, causing restenosis in the later period after implantation while the endodermis of the artificial blood vessel grafts is not complete.45 These problems make it difficult for grafts to completely carry out model vascular tissue regeneration and adequately preserve biocompatibility in vivo. ...
Atherosclerosis and the arterial smooth muscle cell: Proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis
1
1973
... Biodegradable materials are regarded as an auspicious choice for use as artificial blood vessels as the graft will be replaced by tissue ingrowth through the pores of the graft. However, this kind of reconstruction depends on the counterbalance between the degradation of the materials, ingrowth of host cells, and replacement of the materials by host tissue.43, 44 Moreover, aneurysms or even vascular rupture may develop if the degradation rate of the degradable material does not match the rate of vascular tissue regeneration. Another such issue is that SMCs may over-proliferate, causing restenosis in the later period after implantation while the endodermis of the artificial blood vessel grafts is not complete.45 These problems make it difficult for grafts to completely carry out model vascular tissue regeneration and adequately preserve biocompatibility in vivo. ...
Untangling the co-effects of oriented nanotopography and sustained anticoagulation in a biomimetic intima on neovessel remodeling
3
2020
... Synthetic biomaterials are produced from polymers derived by chemical synthesis. They have exact chemical structures and allow controlled adjustment to produce excellent mechanical properties. Owing to the simplicity of carrying out polymer synthesis, its mass reproducibility, and rapid availability, polymer-based synthetic vascular grafts have become an attractive option.46, 47 Most of these grafts have been commercially available since the 1970s and are often made of ePTFE, polyethylene terephthalate (e.g., Dacron), or polyurethane, which are all non-degradable synthetic materials. Particularly, Dacron and ePTFE have been involved in manufacture of large-diameter artificial vascular grafts for over 50 years.48 ...
... Advantages and disadvantages of artificial blood vessels in different materials
| Vessel type | Materials | Advantages | Disadvantages | References |
| Synthetic polymers | ePTFE
(Gore-Tex),
PET (Dacron), PU, PCL, PLCL, PLA, PGS. | Excellent mechanical properties.
Easy availability.
Mass production.
Easy surgical suturing.
Preventing vascular burst.
Can be stored for
off-the-shelf use. | Causes thrombosis, intimal hyperplasia,
calcification, and chronic inflammation.
No growth potential.
Poor haemocompatibility.
Compliance mismatch. | 46, 47, 49, 51, 72 |
| Natural biomaterials | Silk fibroin, collagen, elastin, chitosan,
bacterial cellulose | Excellent biocompatibility.
Enhanced biological signalling.
Tunable mechanical properties. | Weak mechanical strength.
Causes vascular graft dilation and aneurysms.
Easy degradation.
Overly complex designs.
Difficulty in translation. | 10, 52-57, 66 |
| Decellularised vessels | Animal artery, umbilical artery, umbilical vein | Low immunogenicity.
Preserved extracellular matrix, meso- and microvasculature. | Increased thrombogenicity.
Host immune response.
Difficulty in precise recellularisation.
Calcification. | 67-69 |
Note: ePTFE: expanded polytetrafluoroethylene; PCL: polycaprolactone; PET: poly(ethylene terephthalate); PGS: poly(glycerol sebacate); PLA: polylactic acid; PLCL: poly(L-lactide-co-caprolactone); PU: polyurethane. ...
... The mechanical performance of artificial blood vessels is an important design requirement to ensure that they can withstand a certain pressure generated by circulating blood flowing in the blood vessel. The most fundamental performance criteria include adequate mechanical strength to retain structural integrity and resist permanent deformation. The compliance of the graft and the manner in which it deforms under pressure are also important, as adverse biological responses are associated with a compliance mismatch between native and prosthetic vascular grafts. In terms of mechanical considerations, the core issue lies in achieving high strength while retaining compliance. Tensile strength results indicated that a nanofibre-oriented structure offers a higher elastic modulus than a non-oriented structure. Likewise, other properties including tensile strength, elongation at breakage, and burst pressure were also found to be significantly enhanced by an oriented alignment of nanofibres. Consequently, the oriented structure of vascular grafts may very much enhance their mechanical properties.46 An ideal artificial blood vessel should also have appropriate micropores, which can be represented by water permeability. The size and porosity of the micropores have an effect on cell culture.118 Table 3 shows several mechanical properties of natural arteries and various common artificial blood vessels.119 ...
Hybrid small-diameter vascular grafts: Anti-expansion effect of electrospun poly ε-caprolactone on heparin-coated decellularized matrices
5
2016
... Synthetic biomaterials are produced from polymers derived by chemical synthesis. They have exact chemical structures and allow controlled adjustment to produce excellent mechanical properties. Owing to the simplicity of carrying out polymer synthesis, its mass reproducibility, and rapid availability, polymer-based synthetic vascular grafts have become an attractive option.46, 47 Most of these grafts have been commercially available since the 1970s and are often made of ePTFE, polyethylene terephthalate (e.g., Dacron), or polyurethane, which are all non-degradable synthetic materials. Particularly, Dacron and ePTFE have been involved in manufacture of large-diameter artificial vascular grafts for over 50 years.48 ...
... Vascular grafts based on biodegradable materials may be more appropriate for use in clinical applications, especially for the generation of small-diameter blood vessels. Regenerated vascular tissue guarantees the same function as autologous blood vessels when the grafts degrade.47, 49 Polycaprolactone (PCL) is an US Food and Drug Administration (FDA)-approved biodegradable polymer that has been extensively applied to manufacture tissue engineering scaffolds, particularly its nanofibre form via electrospinning.50 ...
... As previously discussed, synthetic polymers have disadvantages in terms of their use in grafts as they often cause thrombosis, intimal hyperplasia, calcification, and have poor haemocompatibility and regenerative potential, although they exhibit excellent mechanical properties.47 Few natural materials have adequate mechanical properties for use as stand-alone materials. Natural materials have excellent biocompatibility; however, they are prone to causing vascular graft dilation and aneurysms (Table 1).66-69 In such cases, interest in hybrid materials has increased, since they constitute a reliable combination of natural and synthetic polymers and possess greater strength. Several pre-clinical studies have mixed natural materials with synthetic polymer materials in order to prepare artificial small-calibre vascular grafts. The properties of such composite materials have always been shown to be improved and balanced.70-72 Grafts made from PCL, a widely-used synthetic polymer in vascular graft research, typically degrade by 70–80% of their original weight by 18 months after implantation, which has been demonstrated in an abdominal aorta rat model.73 However, the quality of the regenerated tissue is insufficient in the long term and the tissue formed by collagen, myofibroblasts, and macrophages at earlier time points does not persist, which is detrimental to the long-term performance of a vascular graft.73 The addition of chitosan to PCL grafts has been shown to improve remodelling, resulting in a lumen fully covered with endothelium and organised contractile SMCs, which simultaneously increase the resistance to thrombosis in sheep models. Moreover, only 9.1 ± 5.4% of the original PCL/chitosan grafts have been shown to remain after the 6-month evaluation period, indicating that chitosan addition greatly accelerates degradation.74 Hybrid PCL collagen grafts have also been demonstrated to reduce the accumulation of oxidised lipid species and increase contractile SMCs when implanted in vivo.75 ...
... Advantages and disadvantages of artificial blood vessels in different materials
| Vessel type | Materials | Advantages | Disadvantages | References |
| Synthetic polymers | ePTFE
(Gore-Tex),
PET (Dacron), PU, PCL, PLCL, PLA, PGS. | Excellent mechanical properties.
Easy availability.
Mass production.
Easy surgical suturing.
Preventing vascular burst.
Can be stored for
off-the-shelf use. | Causes thrombosis, intimal hyperplasia,
calcification, and chronic inflammation.
No growth potential.
Poor haemocompatibility.
Compliance mismatch. | 46, 47, 49, 51, 72 |
| Natural biomaterials | Silk fibroin, collagen, elastin, chitosan,
bacterial cellulose | Excellent biocompatibility.
Enhanced biological signalling.
Tunable mechanical properties. | Weak mechanical strength.
Causes vascular graft dilation and aneurysms.
Easy degradation.
Overly complex designs.
Difficulty in translation. | 10, 52-57, 66 |
| Decellularised vessels | Animal artery, umbilical artery, umbilical vein | Low immunogenicity.
Preserved extracellular matrix, meso- and microvasculature. | Increased thrombogenicity.
Host immune response.
Difficulty in precise recellularisation.
Calcification. | 67-69 |
Note: ePTFE: expanded polytetrafluoroethylene; PCL: polycaprolactone; PET: poly(ethylene terephthalate); PGS: poly(glycerol sebacate); PLA: polylactic acid; PLCL: poly(L-lactide-co-caprolactone); PU: polyurethane. ...
... Scaffold-based techniques use macromolecular structures to support cells during the regeneration process and are prefabrication strategies that are relatively simple to implement. Except for decellularised vessels, many methods fail to mimic the structure of the natural ECM. Electrospinning technology is a promising technique that may be used in the production of TEVGs, which can address this problem. It has been reported that nanofibres produced via electrospinning technology can simulate the composition of the ECM by loading various drugs and growth factors.47 Moreover, it is also the only method used to prepare nano-scale polymer material fibres directly and continuously by applying a high voltage to materials dissolved in volatile solvents so as to create dry fibres that can be collected to form conduits. Therefore, it has been extensively applied in tissue engineering, especially to create small-diameter blood vessel grafts. Additionally, the surfaces of artificial blood vessel grafts prepared by electrospinning have good specific surface areas and high porosities.87 This method facilitates fine control of fibre size and deposition and allows for blending of materials. New methods for simultaneous spinning of multiple components88 and derivative techniques, such as gel spinning89 and conjugate electrospinning technology, have been developed in order to optimise the technique. Small-calibre blood vessel grafts have been constructed by combining both electrospinning technology and freeze-drying technology, providing insight into the fusion of multiple processes and further improvement of artificial blood vessel fabrication.90 ...
In vivo endothelialization and neointimal hyperplasia assessment after angioplasty of sheep carotid artery with a novel polycarbonate polyurethane patch
4
2019
... Synthetic biomaterials are produced from polymers derived by chemical synthesis. They have exact chemical structures and allow controlled adjustment to produce excellent mechanical properties. Owing to the simplicity of carrying out polymer synthesis, its mass reproducibility, and rapid availability, polymer-based synthetic vascular grafts have become an attractive option.46, 47 Most of these grafts have been commercially available since the 1970s and are often made of ePTFE, polyethylene terephthalate (e.g., Dacron), or polyurethane, which are all non-degradable synthetic materials. Particularly, Dacron and ePTFE have been involved in manufacture of large-diameter artificial vascular grafts for over 50 years.48 ...
... After graft transplantation, inflammatory and mesenchymal cells entering the artificial blood vessel wall and endothelial cells covering the endo-surface of artificial blood vessels can promote graft healing. Early endothelialisation of artificial vessels is crucial for the suppression of thrombosis and maintenance of patency.105 ECs can provide a suitable interface between blood and surrounding tissue while maintaining a balance between haemostasis and thrombosis. It is essential to endothelialise the inner surface of artificial blood vessels to prevent thrombosis and intimal hyperplasia.48, 109 There are three ways to improve endothelialisation of the grafts’ inner surface: trans-anastomotic ingrowth of the innate arteries’ endothelia, transmural ingrowth of the capillaries at the implantation sites, and haematogenous seeding of endothelia from the circulatory system.48, 94 ...
... 48, 94 ...
... Common animal models for evaluation of artificial blood vessels
in vivo.
| Animal model | Characteristics | Application inner diameter (mm) | Common length (mm) | Longest implantation period | Implantation site | References |
| Sheep | Similar cardiovascular physiology, endothelialisation mechanisms and thrombogenicity mechanisms to humans.
Suitable size and long-term studies possible.Higher incidence of hypercoagulability. | 4–6 | 80–100 | 9 months | Carotid artery | 48, 74 |
| 4–6 | 60–100 | 3 months | Arteriovenous graft | 129 |
| Pig | Similar vascular physiology and anatomy to humans.
Well established as a model for assessing vascular grafts.
Mount an extensive immune response to implanted tissues. | 3–6 | 30–100 | 6 months | Iliac artery | 130 |
| 3–6 | 10–100 | 4 weeks | Carotid artery | 52 |
| Dog | Lack of spontaneous endothelialisation and immune response restricts study lengths.
Ease of accessing vessel due to thin skin.
Thrombogenicity mechanisms and vessel viscoelastic properties differ from humans. | 3–6 | 30–50 | 6 months | Abdominal artery | 131 |
| 3–6 | 30–50 | 1 year | Carotid artery | 119 |
| Baboon | Physiology and cardiovascular anatomy are the most similar to humans.
Suitable for a wide range of non-invasive imaging techniques adapted from humans.
High cost and ethical concerns associated with using primates in medical research. | 3–6 | 30–50 | 6 months | Arteriovenous graft | 26 |
| Rabbit | Similar endothelialisation rates and thrombogenicity mechanisms to humans.
Demanding higher anticoagulant function.
Limited to short-term studies. | 1–4 | 5–30 | 12 months | Carotid artery | 94, 132 |
| 1–4 | 5–30 | 2 weeks | Femoral artery | 133 |
| 1–4 | 5–30 | 3 months | Abdominal artery | 134 |
| Rat | Large sample size.
Wide variety of transgenic lines.
Allows exploration of genetic/molecular mechanisms.
Ideal for biocompatibility and cell infiltration studies. | 1–3 | 5–30 | 18 months | Abdominal artery | 73, 135 |
| 1–3 | 5–10 | 12 weeks | Carotid artery | 136 |
| Mouse | 0.5–1 | 3–10 | 6 months | Carotid artery | 137 |
Rats and mice are widely used because they are inexpensive to acquire, easy to maintain, and can be used to evaluate larger sample sizes.135-137 Furthermore, the availability of various transgenic strains makes it possible to examine the molecular mechanisms involved in graft remodelling.138 However, differences in their circulatory system with respect to humans limit their utility in short-term studies to only very small grafts (< 2 mm diameter). Rabbits are also suitable for artificial blood vessel assessments although they are limited to short-term studies.132-134 Their physiology is similar to that of humans in terms of endothelialisation rates and thrombogenicity, and they can accept clinically-relevant graft diameters (1–4 mm). Dogs differ from humans in terms of thrombogenicity and represent a more stringent model for the investigations because of their lack of spontaneous endothelialisation.119, 131 Moreover, they can provide a range of implantation site options with varied vessel sizes, although their immune response can restrict study durations. Both pigs and sheep demonstrate similarities to human physiology and they are appropriate for studying thrombogenicity, calcification, and long-term graft patency.129, 130 The cardiovascular physiology of non-human primates, such as baboons, is most similar to that of humans compared to dogs, pigs or sheep. Their structural similarity allows for various imaging techniques and testing methods that are typically applied in humans to be used on them. Despite such advantages, they are rarely used for vascular graft evaluation due to their high cost and ethical concerns.81, 91 The optimal model for studying all of the performance criteria associated with artificial vascular grafts has yet to be elucidated. An immunodeficient pig model, the operational immunodeficient pig, has been reported to be a large animal model that allows for long-term accommodation of artificial human vascular grafts to properly evaluate the clinical efficacy of artificial vascular grafts.139 ...
A self-renewing, tissue-engineered vascular graft for arterial reconstruction
2
2008
... Vascular grafts based on biodegradable materials may be more appropriate for use in clinical applications, especially for the generation of small-diameter blood vessels. Regenerated vascular tissue guarantees the same function as autologous blood vessels when the grafts degrade.47, 49 Polycaprolactone (PCL) is an US Food and Drug Administration (FDA)-approved biodegradable polymer that has been extensively applied to manufacture tissue engineering scaffolds, particularly its nanofibre form via electrospinning.50 ...
... Advantages and disadvantages of artificial blood vessels in different materials
| Vessel type | Materials | Advantages | Disadvantages | References |
| Synthetic polymers | ePTFE
(Gore-Tex),
PET (Dacron), PU, PCL, PLCL, PLA, PGS. | Excellent mechanical properties.
Easy availability.
Mass production.
Easy surgical suturing.
Preventing vascular burst.
Can be stored for
off-the-shelf use. | Causes thrombosis, intimal hyperplasia,
calcification, and chronic inflammation.
No growth potential.
Poor haemocompatibility.
Compliance mismatch. | 46, 47, 49, 51, 72 |
| Natural biomaterials | Silk fibroin, collagen, elastin, chitosan,
bacterial cellulose | Excellent biocompatibility.
Enhanced biological signalling.
Tunable mechanical properties. | Weak mechanical strength.
Causes vascular graft dilation and aneurysms.
Easy degradation.
Overly complex designs.
Difficulty in translation. | 10, 52-57, 66 |
| Decellularised vessels | Animal artery, umbilical artery, umbilical vein | Low immunogenicity.
Preserved extracellular matrix, meso- and microvasculature. | Increased thrombogenicity.
Host immune response.
Difficulty in precise recellularisation.
Calcification. | 67-69 |
Note: ePTFE: expanded polytetrafluoroethylene; PCL: polycaprolactone; PET: poly(ethylene terephthalate); PGS: poly(glycerol sebacate); PLA: polylactic acid; PLCL: poly(L-lactide-co-caprolactone); PU: polyurethane. ...
Small-diameter hybrid vascular grafts composed of polycaprolactone and polydioxanone fibers
1
2017
... Vascular grafts based on biodegradable materials may be more appropriate for use in clinical applications, especially for the generation of small-diameter blood vessels. Regenerated vascular tissue guarantees the same function as autologous blood vessels when the grafts degrade.47, 49 Polycaprolactone (PCL) is an US Food and Drug Administration (FDA)-approved biodegradable polymer that has been extensively applied to manufacture tissue engineering scaffolds, particularly its nanofibre form via electrospinning.50 ...
Long-term results of PTFE grafts
2
2015
... Synthetic polymers endow strength to a biomaterial. However, they generally have poor cellular compatibility, with the majority of the degradation products having been shown to elicit adverse immune responses in vivo. They also have limited resistance to thrombus formation on the surface, which leads to obstruction of the grafts.51 In this regard, in order to further enhance the potency of synthetic polymers, a tactical approach must be adopted to modify their surface. ...
... Advantages and disadvantages of artificial blood vessels in different materials
| Vessel type | Materials | Advantages | Disadvantages | References |
| Synthetic polymers | ePTFE
(Gore-Tex),
PET (Dacron), PU, PCL, PLCL, PLA, PGS. | Excellent mechanical properties.
Easy availability.
Mass production.
Easy surgical suturing.
Preventing vascular burst.
Can be stored for
off-the-shelf use. | Causes thrombosis, intimal hyperplasia,
calcification, and chronic inflammation.
No growth potential.
Poor haemocompatibility.
Compliance mismatch. | 46, 47, 49, 51, 72 |
| Natural biomaterials | Silk fibroin, collagen, elastin, chitosan,
bacterial cellulose | Excellent biocompatibility.
Enhanced biological signalling.
Tunable mechanical properties. | Weak mechanical strength.
Causes vascular graft dilation and aneurysms.
Easy degradation.
Overly complex designs.
Difficulty in translation. | 10, 52-57, 66 |
| Decellularised vessels | Animal artery, umbilical artery, umbilical vein | Low immunogenicity.
Preserved extracellular matrix, meso- and microvasculature. | Increased thrombogenicity.
Host immune response.
Difficulty in precise recellularisation.
Calcification. | 67-69 |
Note: ePTFE: expanded polytetrafluoroethylene; PCL: polycaprolactone; PET: poly(ethylene terephthalate); PGS: poly(glycerol sebacate); PLA: polylactic acid; PLCL: poly(L-lactide-co-caprolactone); PU: polyurethane. ...
Three-layered silk fibroin tubular scaffold for the repair and regeneration of small caliber blood vessels: from design to in vivo pilot tests
3
2019
... In addition to the above-mentioned synthetic materials for vascular grafts, natural biomaterials are also widely used. They are usually extracted from plants or animals and include proteins, polysaccharides and proteoglycans. In general, classical components of mammalian ECM, such as collagen and elastin, as well as non-mammalian macromolecules, such as silk, cellulose, and chitosan, have all been shown to be of great value for use as materials for artificial blood vessels.52-54 The aforementioned biomaterials have better biocompatibility compared to that of synthetic materials, while some have been found to have beneficial properties for vascular applications when engineered using appropriate manufacturing techniques.55, 56 The physical properties of natural polymers have improved with the advancement of new separation, purification and manufacturing processes so that it is feasible and increasingly attractive to use them alone. ...
... Advantages and disadvantages of artificial blood vessels in different materials
| Vessel type | Materials | Advantages | Disadvantages | References |
| Synthetic polymers | ePTFE
(Gore-Tex),
PET (Dacron), PU, PCL, PLCL, PLA, PGS. | Excellent mechanical properties.
Easy availability.
Mass production.
Easy surgical suturing.
Preventing vascular burst.
Can be stored for
off-the-shelf use. | Causes thrombosis, intimal hyperplasia,
calcification, and chronic inflammation.
No growth potential.
Poor haemocompatibility.
Compliance mismatch. | 46, 47, 49, 51, 72 |
| Natural biomaterials | Silk fibroin, collagen, elastin, chitosan,
bacterial cellulose | Excellent biocompatibility.
Enhanced biological signalling.
Tunable mechanical properties. | Weak mechanical strength.
Causes vascular graft dilation and aneurysms.
Easy degradation.
Overly complex designs.
Difficulty in translation. | 10, 52-57, 66 |
| Decellularised vessels | Animal artery, umbilical artery, umbilical vein | Low immunogenicity.
Preserved extracellular matrix, meso- and microvasculature. | Increased thrombogenicity.
Host immune response.
Difficulty in precise recellularisation.
Calcification. | 67-69 |
Note: ePTFE: expanded polytetrafluoroethylene; PCL: polycaprolactone; PET: poly(ethylene terephthalate); PGS: poly(glycerol sebacate); PLA: polylactic acid; PLCL: poly(L-lactide-co-caprolactone); PU: polyurethane. ...
... Common animal models for evaluation of artificial blood vessels
in vivo.
| Animal model | Characteristics | Application inner diameter (mm) | Common length (mm) | Longest implantation period | Implantation site | References |
| Sheep | Similar cardiovascular physiology, endothelialisation mechanisms and thrombogenicity mechanisms to humans.
Suitable size and long-term studies possible.Higher incidence of hypercoagulability. | 4–6 | 80–100 | 9 months | Carotid artery | 48, 74 |
| 4–6 | 60–100 | 3 months | Arteriovenous graft | 129 |
| Pig | Similar vascular physiology and anatomy to humans.
Well established as a model for assessing vascular grafts.
Mount an extensive immune response to implanted tissues. | 3–6 | 30–100 | 6 months | Iliac artery | 130 |
| 3–6 | 10–100 | 4 weeks | Carotid artery | 52 |
| Dog | Lack of spontaneous endothelialisation and immune response restricts study lengths.
Ease of accessing vessel due to thin skin.
Thrombogenicity mechanisms and vessel viscoelastic properties differ from humans. | 3–6 | 30–50 | 6 months | Abdominal artery | 131 |
| 3–6 | 30–50 | 1 year | Carotid artery | 119 |
| Baboon | Physiology and cardiovascular anatomy are the most similar to humans.
Suitable for a wide range of non-invasive imaging techniques adapted from humans.
High cost and ethical concerns associated with using primates in medical research. | 3–6 | 30–50 | 6 months | Arteriovenous graft | 26 |
| Rabbit | Similar endothelialisation rates and thrombogenicity mechanisms to humans.
Demanding higher anticoagulant function.
Limited to short-term studies. | 1–4 | 5–30 | 12 months | Carotid artery | 94, 132 |
| 1–4 | 5–30 | 2 weeks | Femoral artery | 133 |
| 1–4 | 5–30 | 3 months | Abdominal artery | 134 |
| Rat | Large sample size.
Wide variety of transgenic lines.
Allows exploration of genetic/molecular mechanisms.
Ideal for biocompatibility and cell infiltration studies. | 1–3 | 5–30 | 18 months | Abdominal artery | 73, 135 |
| 1–3 | 5–10 | 12 weeks | Carotid artery | 136 |
| Mouse | 0.5–1 | 3–10 | 6 months | Carotid artery | 137 |
Rats and mice are widely used because they are inexpensive to acquire, easy to maintain, and can be used to evaluate larger sample sizes.135-137 Furthermore, the availability of various transgenic strains makes it possible to examine the molecular mechanisms involved in graft remodelling.138 However, differences in their circulatory system with respect to humans limit their utility in short-term studies to only very small grafts (< 2 mm diameter). Rabbits are also suitable for artificial blood vessel assessments although they are limited to short-term studies.132-134 Their physiology is similar to that of humans in terms of endothelialisation rates and thrombogenicity, and they can accept clinically-relevant graft diameters (1–4 mm). Dogs differ from humans in terms of thrombogenicity and represent a more stringent model for the investigations because of their lack of spontaneous endothelialisation.119, 131 Moreover, they can provide a range of implantation site options with varied vessel sizes, although their immune response can restrict study durations. Both pigs and sheep demonstrate similarities to human physiology and they are appropriate for studying thrombogenicity, calcification, and long-term graft patency.129, 130 The cardiovascular physiology of non-human primates, such as baboons, is most similar to that of humans compared to dogs, pigs or sheep. Their structural similarity allows for various imaging techniques and testing methods that are typically applied in humans to be used on them. Despite such advantages, they are rarely used for vascular graft evaluation due to their high cost and ethical concerns.81, 91 The optimal model for studying all of the performance criteria associated with artificial vascular grafts has yet to be elucidated. An immunodeficient pig model, the operational immunodeficient pig, has been reported to be a large animal model that allows for long-term accommodation of artificial human vascular grafts to properly evaluate the clinical efficacy of artificial vascular grafts.139 ...
A hybrid vascular graft harnessing the superior mechanical properties of synthetic fibers and the biological performance of collagen filaments
1
2021
... The presence of natural materials is known to improve biological function according to consensus; therefore, various coatings based on adding natural materials can be designed. The addition of an elastin coating to grafts has been shown to reduce platelet adhesion and increase EC attachment.65 Interestingly, the proportion of SMCs in a contractile phenotype was also increased in elastin-containing grafts, suggesting their usefulness for limiting neointimal hyperplasia.110-112 Moreover, collagen has been shown to improve EC viability, attachment, and migration when incorporated into poly(lactic acid) knitted and poly(vinyl alcohol) vascular scaffolds in vitro.53, 113 Similarly, a polymer blend of recombinant human collagen peptides and PCL gelatine have been shown to enhance the adhesion of human umbilical vein ECs (HUVECs) to the lumen, which adds further support for favourable vascular cell signalling provided by collagen sequences.114 ...
Coral-derived collagen fibers for engineering aligned tissues
1
2021
... In addition to the above-mentioned synthetic materials for vascular grafts, natural biomaterials are also widely used. They are usually extracted from plants or animals and include proteins, polysaccharides and proteoglycans. In general, classical components of mammalian ECM, such as collagen and elastin, as well as non-mammalian macromolecules, such as silk, cellulose, and chitosan, have all been shown to be of great value for use as materials for artificial blood vessels.52-54 The aforementioned biomaterials have better biocompatibility compared to that of synthetic materials, while some have been found to have beneficial properties for vascular applications when engineered using appropriate manufacturing techniques.55, 56 The physical properties of natural polymers have improved with the advancement of new separation, purification and manufacturing processes so that it is feasible and increasingly attractive to use them alone. ...
In vitro construction of artificial blood vessels using spider silk as a supporting matrix
1
2020
... In addition to the above-mentioned synthetic materials for vascular grafts, natural biomaterials are also widely used. They are usually extracted from plants or animals and include proteins, polysaccharides and proteoglycans. In general, classical components of mammalian ECM, such as collagen and elastin, as well as non-mammalian macromolecules, such as silk, cellulose, and chitosan, have all been shown to be of great value for use as materials for artificial blood vessels.52-54 The aforementioned biomaterials have better biocompatibility compared to that of synthetic materials, while some have been found to have beneficial properties for vascular applications when engineered using appropriate manufacturing techniques.55, 56 The physical properties of natural polymers have improved with the advancement of new separation, purification and manufacturing processes so that it is feasible and increasingly attractive to use them alone. ...
Bioactive potential of natural biomaterials: identification, retention and assessment of biological properties
1
2021
... In addition to the above-mentioned synthetic materials for vascular grafts, natural biomaterials are also widely used. They are usually extracted from plants or animals and include proteins, polysaccharides and proteoglycans. In general, classical components of mammalian ECM, such as collagen and elastin, as well as non-mammalian macromolecules, such as silk, cellulose, and chitosan, have all been shown to be of great value for use as materials for artificial blood vessels.52-54 The aforementioned biomaterials have better biocompatibility compared to that of synthetic materials, while some have been found to have beneficial properties for vascular applications when engineered using appropriate manufacturing techniques.55, 56 The physical properties of natural polymers have improved with the advancement of new separation, purification and manufacturing processes so that it is feasible and increasingly attractive to use them alone. ...
Novel silk fibroin/elastin wound dressings
2
2012
... Silk fibroin extracted from natural silk is extensively used in the field of biomedicine because of its special biodegradability and biocompatibility. Moreover, it has been demonstrated to promote faster endothelialisation of the vascular graft compared to ePTFE.57 Collagen is the most bountiful protein in higher animals and may be easily obtained in large quantities from pigs, sheep, and fish. In addition, gelatine can also be obtained cheaply, as it can be sourced from hydrolysed collagen. Likewise, elastin, a crucial constituent of the vascular ECM, is also derived from animals, though in smaller quantities.58 ...
... Advantages and disadvantages of artificial blood vessels in different materials
| Vessel type | Materials | Advantages | Disadvantages | References |
| Synthetic polymers | ePTFE
(Gore-Tex),
PET (Dacron), PU, PCL, PLCL, PLA, PGS. | Excellent mechanical properties.
Easy availability.
Mass production.
Easy surgical suturing.
Preventing vascular burst.
Can be stored for
off-the-shelf use. | Causes thrombosis, intimal hyperplasia,
calcification, and chronic inflammation.
No growth potential.
Poor haemocompatibility.
Compliance mismatch. | 46, 47, 49, 51, 72 |
| Natural biomaterials | Silk fibroin, collagen, elastin, chitosan,
bacterial cellulose | Excellent biocompatibility.
Enhanced biological signalling.
Tunable mechanical properties. | Weak mechanical strength.
Causes vascular graft dilation and aneurysms.
Easy degradation.
Overly complex designs.
Difficulty in translation. | 10, 52-57, 66 |
| Decellularised vessels | Animal artery, umbilical artery, umbilical vein | Low immunogenicity.
Preserved extracellular matrix, meso- and microvasculature. | Increased thrombogenicity.
Host immune response.
Difficulty in precise recellularisation.
Calcification. | 67-69 |
Note: ePTFE: expanded polytetrafluoroethylene; PCL: polycaprolactone; PET: poly(ethylene terephthalate); PGS: poly(glycerol sebacate); PLA: polylactic acid; PLCL: poly(L-lactide-co-caprolactone); PU: polyurethane. ...
Isolation of intact elastin fibers devoid of microfibrils
1
2005
... Silk fibroin extracted from natural silk is extensively used in the field of biomedicine because of its special biodegradability and biocompatibility. Moreover, it has been demonstrated to promote faster endothelialisation of the vascular graft compared to ePTFE.57 Collagen is the most bountiful protein in higher animals and may be easily obtained in large quantities from pigs, sheep, and fish. In addition, gelatine can also be obtained cheaply, as it can be sourced from hydrolysed collagen. Likewise, elastin, a crucial constituent of the vascular ECM, is also derived from animals, though in smaller quantities.58 ...
In vivo application of tissue-engineered blood vessels of bacterial cellulose as small arterial substitutes: proof of concept?
1
2014
... One shortcoming of natural polymers is their easy degradation. Therefore, finding new materials that are not easily degraded in natural systems may act as an effective solution. Bacterial cellulose (BC) is a polysaccharide produced by microorganisms, primarily Komagataeibacter xylinus, which is resistant to degradation in the human body due to the absence of cellulases.59 The potential of using BC materials alone to fabricate vascular grafts has been demonstrated because of their unique properties including high crystallinity, purity, good water retention, adequate mechanical properties, and enhanced biocompatibility.60 Long-term (9 months) preclinical studies in sheep have verified that BC confers high stability and low thrombogenicity in vivo and, according to the degree of cellular infiltration and ECM deposition, the strength of grafts may increase over time.61 Li et al.62 showed that utilising the shape-memory property of BC membranes can serve as an approach to promptly fabricate multi-layered artificial blood vessels. Notably, small-calibre bacterial nanocellulose (BNC) conduits composed of a nanofibril architecture possess greater potential in the creation of small-calibre vascular grafts. This is especially true of BNC hydrogel and air-dried BNC due to their three-dimensional network structure, which is similar to that of natural ECM, as well as their capacity to regulate cell growth, proliferation, and differentiation.63 It has been reported that air-dried BNC conduits remain patent after replacement of the carotid artery in rabbits 46 days later, exhibit superior properties, and possess manifestations convenient for surgical applications as small-diameter blood vessels.64 However, further development of naturally-derived graft substitutes in the future has continued to be limited by their weak mechanical strength, the complexity of design, difficulties in out-of-lab translation, and the presence of complications following implantation.65 Further efforts in regard to in vitro and in vivo studies are therefore indispensable so as to improve grafts made from natural biomaterials. ...
Current progress on the production, modification, and applications of bacterial cellulose
1
2020
... One shortcoming of natural polymers is their easy degradation. Therefore, finding new materials that are not easily degraded in natural systems may act as an effective solution. Bacterial cellulose (BC) is a polysaccharide produced by microorganisms, primarily Komagataeibacter xylinus, which is resistant to degradation in the human body due to the absence of cellulases.59 The potential of using BC materials alone to fabricate vascular grafts has been demonstrated because of their unique properties including high crystallinity, purity, good water retention, adequate mechanical properties, and enhanced biocompatibility.60 Long-term (9 months) preclinical studies in sheep have verified that BC confers high stability and low thrombogenicity in vivo and, according to the degree of cellular infiltration and ECM deposition, the strength of grafts may increase over time.61 Li et al.62 showed that utilising the shape-memory property of BC membranes can serve as an approach to promptly fabricate multi-layered artificial blood vessels. Notably, small-calibre bacterial nanocellulose (BNC) conduits composed of a nanofibril architecture possess greater potential in the creation of small-calibre vascular grafts. This is especially true of BNC hydrogel and air-dried BNC due to their three-dimensional network structure, which is similar to that of natural ECM, as well as their capacity to regulate cell growth, proliferation, and differentiation.63 It has been reported that air-dried BNC conduits remain patent after replacement of the carotid artery in rabbits 46 days later, exhibit superior properties, and possess manifestations convenient for surgical applications as small-diameter blood vessels.64 However, further development of naturally-derived graft substitutes in the future has continued to be limited by their weak mechanical strength, the complexity of design, difficulties in out-of-lab translation, and the presence of complications following implantation.65 Further efforts in regard to in vitro and in vivo studies are therefore indispensable so as to improve grafts made from natural biomaterials. ...
Patency and in vivo compatibility of bacterial nanocellulose grafts as small-diameter vascular substitute
1
2018
... One shortcoming of natural polymers is their easy degradation. Therefore, finding new materials that are not easily degraded in natural systems may act as an effective solution. Bacterial cellulose (BC) is a polysaccharide produced by microorganisms, primarily Komagataeibacter xylinus, which is resistant to degradation in the human body due to the absence of cellulases.59 The potential of using BC materials alone to fabricate vascular grafts has been demonstrated because of their unique properties including high crystallinity, purity, good water retention, adequate mechanical properties, and enhanced biocompatibility.60 Long-term (9 months) preclinical studies in sheep have verified that BC confers high stability and low thrombogenicity in vivo and, according to the degree of cellular infiltration and ECM deposition, the strength of grafts may increase over time.61 Li et al.62 showed that utilising the shape-memory property of BC membranes can serve as an approach to promptly fabricate multi-layered artificial blood vessels. Notably, small-calibre bacterial nanocellulose (BNC) conduits composed of a nanofibril architecture possess greater potential in the creation of small-calibre vascular grafts. This is especially true of BNC hydrogel and air-dried BNC due to their three-dimensional network structure, which is similar to that of natural ECM, as well as their capacity to regulate cell growth, proliferation, and differentiation.63 It has been reported that air-dried BNC conduits remain patent after replacement of the carotid artery in rabbits 46 days later, exhibit superior properties, and possess manifestations convenient for surgical applications as small-diameter blood vessels.64 However, further development of naturally-derived graft substitutes in the future has continued to be limited by their weak mechanical strength, the complexity of design, difficulties in out-of-lab translation, and the presence of complications following implantation.65 Further efforts in regard to in vitro and in vivo studies are therefore indispensable so as to improve grafts made from natural biomaterials. ...
Construction of small-diameter vascular graft by shape-memory and self-rolling bacterial cellulose membrane
2
2017
... One shortcoming of natural polymers is their easy degradation. Therefore, finding new materials that are not easily degraded in natural systems may act as an effective solution. Bacterial cellulose (BC) is a polysaccharide produced by microorganisms, primarily Komagataeibacter xylinus, which is resistant to degradation in the human body due to the absence of cellulases.59 The potential of using BC materials alone to fabricate vascular grafts has been demonstrated because of their unique properties including high crystallinity, purity, good water retention, adequate mechanical properties, and enhanced biocompatibility.60 Long-term (9 months) preclinical studies in sheep have verified that BC confers high stability and low thrombogenicity in vivo and, according to the degree of cellular infiltration and ECM deposition, the strength of grafts may increase over time.61 Li et al.62 showed that utilising the shape-memory property of BC membranes can serve as an approach to promptly fabricate multi-layered artificial blood vessels. Notably, small-calibre bacterial nanocellulose (BNC) conduits composed of a nanofibril architecture possess greater potential in the creation of small-calibre vascular grafts. This is especially true of BNC hydrogel and air-dried BNC due to their three-dimensional network structure, which is similar to that of natural ECM, as well as their capacity to regulate cell growth, proliferation, and differentiation.63 It has been reported that air-dried BNC conduits remain patent after replacement of the carotid artery in rabbits 46 days later, exhibit superior properties, and possess manifestations convenient for surgical applications as small-diameter blood vessels.64 However, further development of naturally-derived graft substitutes in the future has continued to be limited by their weak mechanical strength, the complexity of design, difficulties in out-of-lab translation, and the presence of complications following implantation.65 Further efforts in regard to in vitro and in vivo studies are therefore indispensable so as to improve grafts made from natural biomaterials. ...
... Notably, large-diameter (inner diameter > 8 mm) artificial blood vessels made of ePTFE, Dacron, silk, polyurethane, or other polymers have been successfully used in clinics for conditions such as coarctation of the aorta, aneurysm of the thoracic aorta, and aortic dissection.76 To date, treatment of abdominal aortic aneurysms primarily comprises two different surgical procedures: endovascular placement of an aortic stent graft and open surgical repair of abdominal aortic aneurysms. Most current stent-grafts are based on self-expandable nitinol stents made of Dacron or ePTFE fabric.77, 78 However, in most cases they fail to replace vessels of a small diameter (inner diameter < 6 mm) due to thrombosis, atherosclerosis, infections, or neointimal hyperplasia62 (Table 2). In view of the limitations of current vascular substitutes for small-diameter arteries, TEVGs may be an attractive potential solution. ...
Physicochemical properties and in vitro biocompatibility of three bacterial nanocellulose conduits for blood vessel applications
1
2020
... One shortcoming of natural polymers is their easy degradation. Therefore, finding new materials that are not easily degraded in natural systems may act as an effective solution. Bacterial cellulose (BC) is a polysaccharide produced by microorganisms, primarily Komagataeibacter xylinus, which is resistant to degradation in the human body due to the absence of cellulases.59 The potential of using BC materials alone to fabricate vascular grafts has been demonstrated because of their unique properties including high crystallinity, purity, good water retention, adequate mechanical properties, and enhanced biocompatibility.60 Long-term (9 months) preclinical studies in sheep have verified that BC confers high stability and low thrombogenicity in vivo and, according to the degree of cellular infiltration and ECM deposition, the strength of grafts may increase over time.61 Li et al.62 showed that utilising the shape-memory property of BC membranes can serve as an approach to promptly fabricate multi-layered artificial blood vessels. Notably, small-calibre bacterial nanocellulose (BNC) conduits composed of a nanofibril architecture possess greater potential in the creation of small-calibre vascular grafts. This is especially true of BNC hydrogel and air-dried BNC due to their three-dimensional network structure, which is similar to that of natural ECM, as well as their capacity to regulate cell growth, proliferation, and differentiation.63 It has been reported that air-dried BNC conduits remain patent after replacement of the carotid artery in rabbits 46 days later, exhibit superior properties, and possess manifestations convenient for surgical applications as small-diameter blood vessels.64 However, further development of naturally-derived graft substitutes in the future has continued to be limited by their weak mechanical strength, the complexity of design, difficulties in out-of-lab translation, and the presence of complications following implantation.65 Further efforts in regard to in vitro and in vivo studies are therefore indispensable so as to improve grafts made from natural biomaterials. ...
Implantation of air-dried bacterial nanocellulose conduits in a small-caliber vascular prosthesis rabbit model
1
2021
... One shortcoming of natural polymers is their easy degradation. Therefore, finding new materials that are not easily degraded in natural systems may act as an effective solution. Bacterial cellulose (BC) is a polysaccharide produced by microorganisms, primarily Komagataeibacter xylinus, which is resistant to degradation in the human body due to the absence of cellulases.59 The potential of using BC materials alone to fabricate vascular grafts has been demonstrated because of their unique properties including high crystallinity, purity, good water retention, adequate mechanical properties, and enhanced biocompatibility.60 Long-term (9 months) preclinical studies in sheep have verified that BC confers high stability and low thrombogenicity in vivo and, according to the degree of cellular infiltration and ECM deposition, the strength of grafts may increase over time.61 Li et al.62 showed that utilising the shape-memory property of BC membranes can serve as an approach to promptly fabricate multi-layered artificial blood vessels. Notably, small-calibre bacterial nanocellulose (BNC) conduits composed of a nanofibril architecture possess greater potential in the creation of small-calibre vascular grafts. This is especially true of BNC hydrogel and air-dried BNC due to their three-dimensional network structure, which is similar to that of natural ECM, as well as their capacity to regulate cell growth, proliferation, and differentiation.63 It has been reported that air-dried BNC conduits remain patent after replacement of the carotid artery in rabbits 46 days later, exhibit superior properties, and possess manifestations convenient for surgical applications as small-diameter blood vessels.64 However, further development of naturally-derived graft substitutes in the future has continued to be limited by their weak mechanical strength, the complexity of design, difficulties in out-of-lab translation, and the presence of complications following implantation.65 Further efforts in regard to in vitro and in vivo studies are therefore indispensable so as to improve grafts made from natural biomaterials. ...
Development of small-diameter elastin-silk fibroin vascular grafts
2
2020
... One shortcoming of natural polymers is their easy degradation. Therefore, finding new materials that are not easily degraded in natural systems may act as an effective solution. Bacterial cellulose (BC) is a polysaccharide produced by microorganisms, primarily Komagataeibacter xylinus, which is resistant to degradation in the human body due to the absence of cellulases.59 The potential of using BC materials alone to fabricate vascular grafts has been demonstrated because of their unique properties including high crystallinity, purity, good water retention, adequate mechanical properties, and enhanced biocompatibility.60 Long-term (9 months) preclinical studies in sheep have verified that BC confers high stability and low thrombogenicity in vivo and, according to the degree of cellular infiltration and ECM deposition, the strength of grafts may increase over time.61 Li et al.62 showed that utilising the shape-memory property of BC membranes can serve as an approach to promptly fabricate multi-layered artificial blood vessels. Notably, small-calibre bacterial nanocellulose (BNC) conduits composed of a nanofibril architecture possess greater potential in the creation of small-calibre vascular grafts. This is especially true of BNC hydrogel and air-dried BNC due to their three-dimensional network structure, which is similar to that of natural ECM, as well as their capacity to regulate cell growth, proliferation, and differentiation.63 It has been reported that air-dried BNC conduits remain patent after replacement of the carotid artery in rabbits 46 days later, exhibit superior properties, and possess manifestations convenient for surgical applications as small-diameter blood vessels.64 However, further development of naturally-derived graft substitutes in the future has continued to be limited by their weak mechanical strength, the complexity of design, difficulties in out-of-lab translation, and the presence of complications following implantation.65 Further efforts in regard to in vitro and in vivo studies are therefore indispensable so as to improve grafts made from natural biomaterials. ...
... The presence of natural materials is known to improve biological function according to consensus; therefore, various coatings based on adding natural materials can be designed. The addition of an elastin coating to grafts has been shown to reduce platelet adhesion and increase EC attachment.65 Interestingly, the proportion of SMCs in a contractile phenotype was also increased in elastin-containing grafts, suggesting their usefulness for limiting neointimal hyperplasia.110-112 Moreover, collagen has been shown to improve EC viability, attachment, and migration when incorporated into poly(lactic acid) knitted and poly(vinyl alcohol) vascular scaffolds in vitro.53, 113 Similarly, a polymer blend of recombinant human collagen peptides and PCL gelatine have been shown to enhance the adhesion of human umbilical vein ECs (HUVECs) to the lumen, which adds further support for favourable vascular cell signalling provided by collagen sequences.114 ...
Electrospun scaffolds for tissue engineering of vascular grafts
2
2014
... As previously discussed, synthetic polymers have disadvantages in terms of their use in grafts as they often cause thrombosis, intimal hyperplasia, calcification, and have poor haemocompatibility and regenerative potential, although they exhibit excellent mechanical properties.47 Few natural materials have adequate mechanical properties for use as stand-alone materials. Natural materials have excellent biocompatibility; however, they are prone to causing vascular graft dilation and aneurysms (Table 1).66-69 In such cases, interest in hybrid materials has increased, since they constitute a reliable combination of natural and synthetic polymers and possess greater strength. Several pre-clinical studies have mixed natural materials with synthetic polymer materials in order to prepare artificial small-calibre vascular grafts. The properties of such composite materials have always been shown to be improved and balanced.70-72 Grafts made from PCL, a widely-used synthetic polymer in vascular graft research, typically degrade by 70–80% of their original weight by 18 months after implantation, which has been demonstrated in an abdominal aorta rat model.73 However, the quality of the regenerated tissue is insufficient in the long term and the tissue formed by collagen, myofibroblasts, and macrophages at earlier time points does not persist, which is detrimental to the long-term performance of a vascular graft.73 The addition of chitosan to PCL grafts has been shown to improve remodelling, resulting in a lumen fully covered with endothelium and organised contractile SMCs, which simultaneously increase the resistance to thrombosis in sheep models. Moreover, only 9.1 ± 5.4% of the original PCL/chitosan grafts have been shown to remain after the 6-month evaluation period, indicating that chitosan addition greatly accelerates degradation.74 Hybrid PCL collagen grafts have also been demonstrated to reduce the accumulation of oxidised lipid species and increase contractile SMCs when implanted in vivo.75 ...
... Advantages and disadvantages of artificial blood vessels in different materials
| Vessel type | Materials | Advantages | Disadvantages | References |
| Synthetic polymers | ePTFE
(Gore-Tex),
PET (Dacron), PU, PCL, PLCL, PLA, PGS. | Excellent mechanical properties.
Easy availability.
Mass production.
Easy surgical suturing.
Preventing vascular burst.
Can be stored for
off-the-shelf use. | Causes thrombosis, intimal hyperplasia,
calcification, and chronic inflammation.
No growth potential.
Poor haemocompatibility.
Compliance mismatch. | 46, 47, 49, 51, 72 |
| Natural biomaterials | Silk fibroin, collagen, elastin, chitosan,
bacterial cellulose | Excellent biocompatibility.
Enhanced biological signalling.
Tunable mechanical properties. | Weak mechanical strength.
Causes vascular graft dilation and aneurysms.
Easy degradation.
Overly complex designs.
Difficulty in translation. | 10, 52-57, 66 |
| Decellularised vessels | Animal artery, umbilical artery, umbilical vein | Low immunogenicity.
Preserved extracellular matrix, meso- and microvasculature. | Increased thrombogenicity.
Host immune response.
Difficulty in precise recellularisation.
Calcification. | 67-69 |
Note: ePTFE: expanded polytetrafluoroethylene; PCL: polycaprolactone; PET: poly(ethylene terephthalate); PGS: poly(glycerol sebacate); PLA: polylactic acid; PLCL: poly(L-lactide-co-caprolactone); PU: polyurethane. ...
Detergent-enzymatic decellularization of swine blood vessels: insight on mechanical properties for vascular tissue engineering
2
2013
... Advantages and disadvantages of artificial blood vessels in different materials
| Vessel type | Materials | Advantages | Disadvantages | References |
| Synthetic polymers | ePTFE
(Gore-Tex),
PET (Dacron), PU, PCL, PLCL, PLA, PGS. | Excellent mechanical properties.
Easy availability.
Mass production.
Easy surgical suturing.
Preventing vascular burst.
Can be stored for
off-the-shelf use. | Causes thrombosis, intimal hyperplasia,
calcification, and chronic inflammation.
No growth potential.
Poor haemocompatibility.
Compliance mismatch. | 46, 47, 49, 51, 72 |
| Natural biomaterials | Silk fibroin, collagen, elastin, chitosan,
bacterial cellulose | Excellent biocompatibility.
Enhanced biological signalling.
Tunable mechanical properties. | Weak mechanical strength.
Causes vascular graft dilation and aneurysms.
Easy degradation.
Overly complex designs.
Difficulty in translation. | 10, 52-57, 66 |
| Decellularised vessels | Animal artery, umbilical artery, umbilical vein | Low immunogenicity.
Preserved extracellular matrix, meso- and microvasculature. | Increased thrombogenicity.
Host immune response.
Difficulty in precise recellularisation.
Calcification. | 67-69 |
Note: ePTFE: expanded polytetrafluoroethylene; PCL: polycaprolactone; PET: poly(ethylene terephthalate); PGS: poly(glycerol sebacate); PLA: polylactic acid; PLCL: poly(L-lactide-co-caprolactone); PU: polyurethane. ...
... One type of currently-used artificial vascular vessel that has demonstrated satisfactory preclinical and clinical results is made by decellularising allograft and xenograft vessels, such as arteries from swine or bovine models or the human umbilical artery.67, 81 Decellularisation is a tool that can be used in the generation of acellular scaffolds for tissue engineering, which completely removes all cellular and nuclear material from a tissue in order to reduce harmful immune reactions while preserving the natural ECM. It has already been used in various tissues with positive outcomes.82, 83 This outcome can be achieved through physical, chemical, or enzymatic methods.84 The resultant acellular scaffolds represent a great substrate that can promote cell adhesion, differentiation, and proliferation since specific ECM components and structures are key to tissue regeneration and remodelling.85 Moreover, decellularised scaffolds must have certain mechanical properties to avoid a mismatch with those of native tissues, which is key for blood vessels in tissue engineering. This can reduce immunogenicity but may also result in lower patency, which has been attributed to increased thrombogenicity, host immune response, and increased calcification.68, 69 Such grafts may have a clinical role, though they have not yet been widely used. Complete removal of tissue antigens remains a major issue, and the diversity of source materials makes it difficult to achieve manufacturing reproducibility.86 ...
The Shelhigh No-React bovine internal mammary artery: a questionable alternative conduit in coronary bypass surgery?
1
2008
... One type of currently-used artificial vascular vessel that has demonstrated satisfactory preclinical and clinical results is made by decellularising allograft and xenograft vessels, such as arteries from swine or bovine models or the human umbilical artery.67, 81 Decellularisation is a tool that can be used in the generation of acellular scaffolds for tissue engineering, which completely removes all cellular and nuclear material from a tissue in order to reduce harmful immune reactions while preserving the natural ECM. It has already been used in various tissues with positive outcomes.82, 83 This outcome can be achieved through physical, chemical, or enzymatic methods.84 The resultant acellular scaffolds represent a great substrate that can promote cell adhesion, differentiation, and proliferation since specific ECM components and structures are key to tissue regeneration and remodelling.85 Moreover, decellularised scaffolds must have certain mechanical properties to avoid a mismatch with those of native tissues, which is key for blood vessels in tissue engineering. This can reduce immunogenicity but may also result in lower patency, which has been attributed to increased thrombogenicity, host immune response, and increased calcification.68, 69 Such grafts may have a clinical role, though they have not yet been widely used. Complete removal of tissue antigens remains a major issue, and the diversity of source materials makes it difficult to achieve manufacturing reproducibility.86 ...
Rapid degradation and subsequent endovascular salvage of upper extremity cryogenic allograft bypass
3
2019
... As previously discussed, synthetic polymers have disadvantages in terms of their use in grafts as they often cause thrombosis, intimal hyperplasia, calcification, and have poor haemocompatibility and regenerative potential, although they exhibit excellent mechanical properties.47 Few natural materials have adequate mechanical properties for use as stand-alone materials. Natural materials have excellent biocompatibility; however, they are prone to causing vascular graft dilation and aneurysms (Table 1).66-69 In such cases, interest in hybrid materials has increased, since they constitute a reliable combination of natural and synthetic polymers and possess greater strength. Several pre-clinical studies have mixed natural materials with synthetic polymer materials in order to prepare artificial small-calibre vascular grafts. The properties of such composite materials have always been shown to be improved and balanced.70-72 Grafts made from PCL, a widely-used synthetic polymer in vascular graft research, typically degrade by 70–80% of their original weight by 18 months after implantation, which has been demonstrated in an abdominal aorta rat model.73 However, the quality of the regenerated tissue is insufficient in the long term and the tissue formed by collagen, myofibroblasts, and macrophages at earlier time points does not persist, which is detrimental to the long-term performance of a vascular graft.73 The addition of chitosan to PCL grafts has been shown to improve remodelling, resulting in a lumen fully covered with endothelium and organised contractile SMCs, which simultaneously increase the resistance to thrombosis in sheep models. Moreover, only 9.1 ± 5.4% of the original PCL/chitosan grafts have been shown to remain after the 6-month evaluation period, indicating that chitosan addition greatly accelerates degradation.74 Hybrid PCL collagen grafts have also been demonstrated to reduce the accumulation of oxidised lipid species and increase contractile SMCs when implanted in vivo.75 ...
... Advantages and disadvantages of artificial blood vessels in different materials
| Vessel type | Materials | Advantages | Disadvantages | References |
| Synthetic polymers | ePTFE
(Gore-Tex),
PET (Dacron), PU, PCL, PLCL, PLA, PGS. | Excellent mechanical properties.
Easy availability.
Mass production.
Easy surgical suturing.
Preventing vascular burst.
Can be stored for
off-the-shelf use. | Causes thrombosis, intimal hyperplasia,
calcification, and chronic inflammation.
No growth potential.
Poor haemocompatibility.
Compliance mismatch. | 46, 47, 49, 51, 72 |
| Natural biomaterials | Silk fibroin, collagen, elastin, chitosan,
bacterial cellulose | Excellent biocompatibility.
Enhanced biological signalling.
Tunable mechanical properties. | Weak mechanical strength.
Causes vascular graft dilation and aneurysms.
Easy degradation.
Overly complex designs.
Difficulty in translation. | 10, 52-57, 66 |
| Decellularised vessels | Animal artery, umbilical artery, umbilical vein | Low immunogenicity.
Preserved extracellular matrix, meso- and microvasculature. | Increased thrombogenicity.
Host immune response.
Difficulty in precise recellularisation.
Calcification. | 67-69 |
Note: ePTFE: expanded polytetrafluoroethylene; PCL: polycaprolactone; PET: poly(ethylene terephthalate); PGS: poly(glycerol sebacate); PLA: polylactic acid; PLCL: poly(L-lactide-co-caprolactone); PU: polyurethane. ...
... One type of currently-used artificial vascular vessel that has demonstrated satisfactory preclinical and clinical results is made by decellularising allograft and xenograft vessels, such as arteries from swine or bovine models or the human umbilical artery.67, 81 Decellularisation is a tool that can be used in the generation of acellular scaffolds for tissue engineering, which completely removes all cellular and nuclear material from a tissue in order to reduce harmful immune reactions while preserving the natural ECM. It has already been used in various tissues with positive outcomes.82, 83 This outcome can be achieved through physical, chemical, or enzymatic methods.84 The resultant acellular scaffolds represent a great substrate that can promote cell adhesion, differentiation, and proliferation since specific ECM components and structures are key to tissue regeneration and remodelling.85 Moreover, decellularised scaffolds must have certain mechanical properties to avoid a mismatch with those of native tissues, which is key for blood vessels in tissue engineering. This can reduce immunogenicity but may also result in lower patency, which has been attributed to increased thrombogenicity, host immune response, and increased calcification.68, 69 Such grafts may have a clinical role, though they have not yet been widely used. Complete removal of tissue antigens remains a major issue, and the diversity of source materials makes it difficult to achieve manufacturing reproducibility.86 ...
A bilayered elastomeric scaffold for tissue engineering of small diameter vascular grafts
1
2010
... As previously discussed, synthetic polymers have disadvantages in terms of their use in grafts as they often cause thrombosis, intimal hyperplasia, calcification, and have poor haemocompatibility and regenerative potential, although they exhibit excellent mechanical properties.47 Few natural materials have adequate mechanical properties for use as stand-alone materials. Natural materials have excellent biocompatibility; however, they are prone to causing vascular graft dilation and aneurysms (Table 1).66-69 In such cases, interest in hybrid materials has increased, since they constitute a reliable combination of natural and synthetic polymers and possess greater strength. Several pre-clinical studies have mixed natural materials with synthetic polymer materials in order to prepare artificial small-calibre vascular grafts. The properties of such composite materials have always been shown to be improved and balanced.70-72 Grafts made from PCL, a widely-used synthetic polymer in vascular graft research, typically degrade by 70–80% of their original weight by 18 months after implantation, which has been demonstrated in an abdominal aorta rat model.73 However, the quality of the regenerated tissue is insufficient in the long term and the tissue formed by collagen, myofibroblasts, and macrophages at earlier time points does not persist, which is detrimental to the long-term performance of a vascular graft.73 The addition of chitosan to PCL grafts has been shown to improve remodelling, resulting in a lumen fully covered with endothelium and organised contractile SMCs, which simultaneously increase the resistance to thrombosis in sheep models. Moreover, only 9.1 ± 5.4% of the original PCL/chitosan grafts have been shown to remain after the 6-month evaluation period, indicating that chitosan addition greatly accelerates degradation.74 Hybrid PCL collagen grafts have also been demonstrated to reduce the accumulation of oxidised lipid species and increase contractile SMCs when implanted in vivo.75 ...
A multi-layered vascular scaffold with symmetrical structure by bi-directional gradient electrospinning
0
2015
Bilayered scaffold for engineering cellularized blood vessels
2
2010
... As previously discussed, synthetic polymers have disadvantages in terms of their use in grafts as they often cause thrombosis, intimal hyperplasia, calcification, and have poor haemocompatibility and regenerative potential, although they exhibit excellent mechanical properties.47 Few natural materials have adequate mechanical properties for use as stand-alone materials. Natural materials have excellent biocompatibility; however, they are prone to causing vascular graft dilation and aneurysms (Table 1).66-69 In such cases, interest in hybrid materials has increased, since they constitute a reliable combination of natural and synthetic polymers and possess greater strength. Several pre-clinical studies have mixed natural materials with synthetic polymer materials in order to prepare artificial small-calibre vascular grafts. The properties of such composite materials have always been shown to be improved and balanced.70-72 Grafts made from PCL, a widely-used synthetic polymer in vascular graft research, typically degrade by 70–80% of their original weight by 18 months after implantation, which has been demonstrated in an abdominal aorta rat model.73 However, the quality of the regenerated tissue is insufficient in the long term and the tissue formed by collagen, myofibroblasts, and macrophages at earlier time points does not persist, which is detrimental to the long-term performance of a vascular graft.73 The addition of chitosan to PCL grafts has been shown to improve remodelling, resulting in a lumen fully covered with endothelium and organised contractile SMCs, which simultaneously increase the resistance to thrombosis in sheep models. Moreover, only 9.1 ± 5.4% of the original PCL/chitosan grafts have been shown to remain after the 6-month evaluation period, indicating that chitosan addition greatly accelerates degradation.74 Hybrid PCL collagen grafts have also been demonstrated to reduce the accumulation of oxidised lipid species and increase contractile SMCs when implanted in vivo.75 ...
... Advantages and disadvantages of artificial blood vessels in different materials
| Vessel type | Materials | Advantages | Disadvantages | References |
| Synthetic polymers | ePTFE
(Gore-Tex),
PET (Dacron), PU, PCL, PLCL, PLA, PGS. | Excellent mechanical properties.
Easy availability.
Mass production.
Easy surgical suturing.
Preventing vascular burst.
Can be stored for
off-the-shelf use. | Causes thrombosis, intimal hyperplasia,
calcification, and chronic inflammation.
No growth potential.
Poor haemocompatibility.
Compliance mismatch. | 46, 47, 49, 51, 72 |
| Natural biomaterials | Silk fibroin, collagen, elastin, chitosan,
bacterial cellulose | Excellent biocompatibility.
Enhanced biological signalling.
Tunable mechanical properties. | Weak mechanical strength.
Causes vascular graft dilation and aneurysms.
Easy degradation.
Overly complex designs.
Difficulty in translation. | 10, 52-57, 66 |
| Decellularised vessels | Animal artery, umbilical artery, umbilical vein | Low immunogenicity.
Preserved extracellular matrix, meso- and microvasculature. | Increased thrombogenicity.
Host immune response.
Difficulty in precise recellularisation.
Calcification. | 67-69 |
Note: ePTFE: expanded polytetrafluoroethylene; PCL: polycaprolactone; PET: poly(ethylene terephthalate); PGS: poly(glycerol sebacate); PLA: polylactic acid; PLCL: poly(L-lactide-co-caprolactone); PU: polyurethane. ...
Long term performance of polycaprolactone vascular grafts in a rat abdominal aorta replacement model
3
2012
... As previously discussed, synthetic polymers have disadvantages in terms of their use in grafts as they often cause thrombosis, intimal hyperplasia, calcification, and have poor haemocompatibility and regenerative potential, although they exhibit excellent mechanical properties.47 Few natural materials have adequate mechanical properties for use as stand-alone materials. Natural materials have excellent biocompatibility; however, they are prone to causing vascular graft dilation and aneurysms (Table 1).66-69 In such cases, interest in hybrid materials has increased, since they constitute a reliable combination of natural and synthetic polymers and possess greater strength. Several pre-clinical studies have mixed natural materials with synthetic polymer materials in order to prepare artificial small-calibre vascular grafts. The properties of such composite materials have always been shown to be improved and balanced.70-72 Grafts made from PCL, a widely-used synthetic polymer in vascular graft research, typically degrade by 70–80% of their original weight by 18 months after implantation, which has been demonstrated in an abdominal aorta rat model.73 However, the quality of the regenerated tissue is insufficient in the long term and the tissue formed by collagen, myofibroblasts, and macrophages at earlier time points does not persist, which is detrimental to the long-term performance of a vascular graft.73 The addition of chitosan to PCL grafts has been shown to improve remodelling, resulting in a lumen fully covered with endothelium and organised contractile SMCs, which simultaneously increase the resistance to thrombosis in sheep models. Moreover, only 9.1 ± 5.4% of the original PCL/chitosan grafts have been shown to remain after the 6-month evaluation period, indicating that chitosan addition greatly accelerates degradation.74 Hybrid PCL collagen grafts have also been demonstrated to reduce the accumulation of oxidised lipid species and increase contractile SMCs when implanted in vivo.75 ...
... 73 The addition of chitosan to PCL grafts has been shown to improve remodelling, resulting in a lumen fully covered with endothelium and organised contractile SMCs, which simultaneously increase the resistance to thrombosis in sheep models. Moreover, only 9.1 ± 5.4% of the original PCL/chitosan grafts have been shown to remain after the 6-month evaluation period, indicating that chitosan addition greatly accelerates degradation.74 Hybrid PCL collagen grafts have also been demonstrated to reduce the accumulation of oxidised lipid species and increase contractile SMCs when implanted in vivo.75 ...
... Common animal models for evaluation of artificial blood vessels
in vivo.
| Animal model | Characteristics | Application inner diameter (mm) | Common length (mm) | Longest implantation period | Implantation site | References |
| Sheep | Similar cardiovascular physiology, endothelialisation mechanisms and thrombogenicity mechanisms to humans.
Suitable size and long-term studies possible.Higher incidence of hypercoagulability. | 4–6 | 80–100 | 9 months | Carotid artery | 48, 74 |
| 4–6 | 60–100 | 3 months | Arteriovenous graft | 129 |
| Pig | Similar vascular physiology and anatomy to humans.
Well established as a model for assessing vascular grafts.
Mount an extensive immune response to implanted tissues. | 3–6 | 30–100 | 6 months | Iliac artery | 130 |
| 3–6 | 10–100 | 4 weeks | Carotid artery | 52 |
| Dog | Lack of spontaneous endothelialisation and immune response restricts study lengths.
Ease of accessing vessel due to thin skin.
Thrombogenicity mechanisms and vessel viscoelastic properties differ from humans. | 3–6 | 30–50 | 6 months | Abdominal artery | 131 |
| 3–6 | 30–50 | 1 year | Carotid artery | 119 |
| Baboon | Physiology and cardiovascular anatomy are the most similar to humans.
Suitable for a wide range of non-invasive imaging techniques adapted from humans.
High cost and ethical concerns associated with using primates in medical research. | 3–6 | 30–50 | 6 months | Arteriovenous graft | 26 |
| Rabbit | Similar endothelialisation rates and thrombogenicity mechanisms to humans.
Demanding higher anticoagulant function.
Limited to short-term studies. | 1–4 | 5–30 | 12 months | Carotid artery | 94, 132 |
| 1–4 | 5–30 | 2 weeks | Femoral artery | 133 |
| 1–4 | 5–30 | 3 months | Abdominal artery | 134 |
| Rat | Large sample size.
Wide variety of transgenic lines.
Allows exploration of genetic/molecular mechanisms.
Ideal for biocompatibility and cell infiltration studies. | 1–3 | 5–30 | 18 months | Abdominal artery | 73, 135 |
| 1–3 | 5–10 | 12 weeks | Carotid artery | 136 |
| Mouse | 0.5–1 | 3–10 | 6 months | Carotid artery | 137 |
Rats and mice are widely used because they are inexpensive to acquire, easy to maintain, and can be used to evaluate larger sample sizes.135-137 Furthermore, the availability of various transgenic strains makes it possible to examine the molecular mechanisms involved in graft remodelling.138 However, differences in their circulatory system with respect to humans limit their utility in short-term studies to only very small grafts (< 2 mm diameter). Rabbits are also suitable for artificial blood vessel assessments although they are limited to short-term studies.132-134 Their physiology is similar to that of humans in terms of endothelialisation rates and thrombogenicity, and they can accept clinically-relevant graft diameters (1–4 mm). Dogs differ from humans in terms of thrombogenicity and represent a more stringent model for the investigations because of their lack of spontaneous endothelialisation.119, 131 Moreover, they can provide a range of implantation site options with varied vessel sizes, although their immune response can restrict study durations. Both pigs and sheep demonstrate similarities to human physiology and they are appropriate for studying thrombogenicity, calcification, and long-term graft patency.129, 130 The cardiovascular physiology of non-human primates, such as baboons, is most similar to that of humans compared to dogs, pigs or sheep. Their structural similarity allows for various imaging techniques and testing methods that are typically applied in humans to be used on them. Despite such advantages, they are rarely used for vascular graft evaluation due to their high cost and ethical concerns.81, 91 The optimal model for studying all of the performance criteria associated with artificial vascular grafts has yet to be elucidated. An immunodeficient pig model, the operational immunodeficient pig, has been reported to be a large animal model that allows for long-term accommodation of artificial human vascular grafts to properly evaluate the clinical efficacy of artificial vascular grafts.139 ...
Tissue-engineered small diameter arterial vascular grafts from cell-free nanofiber PCL/chitosan scaffolds in a sheep model
2
2016
... As previously discussed, synthetic polymers have disadvantages in terms of their use in grafts as they often cause thrombosis, intimal hyperplasia, calcification, and have poor haemocompatibility and regenerative potential, although they exhibit excellent mechanical properties.47 Few natural materials have adequate mechanical properties for use as stand-alone materials. Natural materials have excellent biocompatibility; however, they are prone to causing vascular graft dilation and aneurysms (Table 1).66-69 In such cases, interest in hybrid materials has increased, since they constitute a reliable combination of natural and synthetic polymers and possess greater strength. Several pre-clinical studies have mixed natural materials with synthetic polymer materials in order to prepare artificial small-calibre vascular grafts. The properties of such composite materials have always been shown to be improved and balanced.70-72 Grafts made from PCL, a widely-used synthetic polymer in vascular graft research, typically degrade by 70–80% of their original weight by 18 months after implantation, which has been demonstrated in an abdominal aorta rat model.73 However, the quality of the regenerated tissue is insufficient in the long term and the tissue formed by collagen, myofibroblasts, and macrophages at earlier time points does not persist, which is detrimental to the long-term performance of a vascular graft.73 The addition of chitosan to PCL grafts has been shown to improve remodelling, resulting in a lumen fully covered with endothelium and organised contractile SMCs, which simultaneously increase the resistance to thrombosis in sheep models. Moreover, only 9.1 ± 5.4% of the original PCL/chitosan grafts have been shown to remain after the 6-month evaluation period, indicating that chitosan addition greatly accelerates degradation.74 Hybrid PCL collagen grafts have also been demonstrated to reduce the accumulation of oxidised lipid species and increase contractile SMCs when implanted in vivo.75 ...
... Common animal models for evaluation of artificial blood vessels
in vivo.
| Animal model | Characteristics | Application inner diameter (mm) | Common length (mm) | Longest implantation period | Implantation site | References |
| Sheep | Similar cardiovascular physiology, endothelialisation mechanisms and thrombogenicity mechanisms to humans.
Suitable size and long-term studies possible.Higher incidence of hypercoagulability. | 4–6 | 80–100 | 9 months | Carotid artery | 48, 74 |
| 4–6 | 60–100 | 3 months | Arteriovenous graft | 129 |
| Pig | Similar vascular physiology and anatomy to humans.
Well established as a model for assessing vascular grafts.
Mount an extensive immune response to implanted tissues. | 3–6 | 30–100 | 6 months | Iliac artery | 130 |
| 3–6 | 10–100 | 4 weeks | Carotid artery | 52 |
| Dog | Lack of spontaneous endothelialisation and immune response restricts study lengths.
Ease of accessing vessel due to thin skin.
Thrombogenicity mechanisms and vessel viscoelastic properties differ from humans. | 3–6 | 30–50 | 6 months | Abdominal artery | 131 |
| 3–6 | 30–50 | 1 year | Carotid artery | 119 |
| Baboon | Physiology and cardiovascular anatomy are the most similar to humans.
Suitable for a wide range of non-invasive imaging techniques adapted from humans.
High cost and ethical concerns associated with using primates in medical research. | 3–6 | 30–50 | 6 months | Arteriovenous graft | 26 |
| Rabbit | Similar endothelialisation rates and thrombogenicity mechanisms to humans.
Demanding higher anticoagulant function.
Limited to short-term studies. | 1–4 | 5–30 | 12 months | Carotid artery | 94, 132 |
| 1–4 | 5–30 | 2 weeks | Femoral artery | 133 |
| 1–4 | 5–30 | 3 months | Abdominal artery | 134 |
| Rat | Large sample size.
Wide variety of transgenic lines.
Allows exploration of genetic/molecular mechanisms.
Ideal for biocompatibility and cell infiltration studies. | 1–3 | 5–30 | 18 months | Abdominal artery | 73, 135 |
| 1–3 | 5–10 | 12 weeks | Carotid artery | 136 |
| Mouse | 0.5–1 | 3–10 | 6 months | Carotid artery | 137 |
Rats and mice are widely used because they are inexpensive to acquire, easy to maintain, and can be used to evaluate larger sample sizes.135-137 Furthermore, the availability of various transgenic strains makes it possible to examine the molecular mechanisms involved in graft remodelling.138 However, differences in their circulatory system with respect to humans limit their utility in short-term studies to only very small grafts (< 2 mm diameter). Rabbits are also suitable for artificial blood vessel assessments although they are limited to short-term studies.132-134 Their physiology is similar to that of humans in terms of endothelialisation rates and thrombogenicity, and they can accept clinically-relevant graft diameters (1–4 mm). Dogs differ from humans in terms of thrombogenicity and represent a more stringent model for the investigations because of their lack of spontaneous endothelialisation.119, 131 Moreover, they can provide a range of implantation site options with varied vessel sizes, although their immune response can restrict study durations. Both pigs and sheep demonstrate similarities to human physiology and they are appropriate for studying thrombogenicity, calcification, and long-term graft patency.129, 130 The cardiovascular physiology of non-human primates, such as baboons, is most similar to that of humans compared to dogs, pigs or sheep. Their structural similarity allows for various imaging techniques and testing methods that are typically applied in humans to be used on them. Despite such advantages, they are rarely used for vascular graft evaluation due to their high cost and ethical concerns.81, 91 The optimal model for studying all of the performance criteria associated with artificial vascular grafts has yet to be elucidated. An immunodeficient pig model, the operational immunodeficient pig, has been reported to be a large animal model that allows for long-term accommodation of artificial human vascular grafts to properly evaluate the clinical efficacy of artificial vascular grafts.139 ...
Gelatin coating promotes in situ endothelialization of electrospun polycaprolactone vascular grafts
1
2021
... As previously discussed, synthetic polymers have disadvantages in terms of their use in grafts as they often cause thrombosis, intimal hyperplasia, calcification, and have poor haemocompatibility and regenerative potential, although they exhibit excellent mechanical properties.47 Few natural materials have adequate mechanical properties for use as stand-alone materials. Natural materials have excellent biocompatibility; however, they are prone to causing vascular graft dilation and aneurysms (Table 1).66-69 In such cases, interest in hybrid materials has increased, since they constitute a reliable combination of natural and synthetic polymers and possess greater strength. Several pre-clinical studies have mixed natural materials with synthetic polymer materials in order to prepare artificial small-calibre vascular grafts. The properties of such composite materials have always been shown to be improved and balanced.70-72 Grafts made from PCL, a widely-used synthetic polymer in vascular graft research, typically degrade by 70–80% of their original weight by 18 months after implantation, which has been demonstrated in an abdominal aorta rat model.73 However, the quality of the regenerated tissue is insufficient in the long term and the tissue formed by collagen, myofibroblasts, and macrophages at earlier time points does not persist, which is detrimental to the long-term performance of a vascular graft.73 The addition of chitosan to PCL grafts has been shown to improve remodelling, resulting in a lumen fully covered with endothelium and organised contractile SMCs, which simultaneously increase the resistance to thrombosis in sheep models. Moreover, only 9.1 ± 5.4% of the original PCL/chitosan grafts have been shown to remain after the 6-month evaluation period, indicating that chitosan addition greatly accelerates degradation.74 Hybrid PCL collagen grafts have also been demonstrated to reduce the accumulation of oxidised lipid species and increase contractile SMCs when implanted in vivo.75 ...
Long-term dilatation of polyester and expanded polytetrafluoroethylene tube grafts after open repair of infrarenal abdominal aortic aneurysms
1
2011
... Notably, large-diameter (inner diameter > 8 mm) artificial blood vessels made of ePTFE, Dacron, silk, polyurethane, or other polymers have been successfully used in clinics for conditions such as coarctation of the aorta, aneurysm of the thoracic aorta, and aortic dissection.76 To date, treatment of abdominal aortic aneurysms primarily comprises two different surgical procedures: endovascular placement of an aortic stent graft and open surgical repair of abdominal aortic aneurysms. Most current stent-grafts are based on self-expandable nitinol stents made of Dacron or ePTFE fabric.77, 78 However, in most cases they fail to replace vessels of a small diameter (inner diameter < 6 mm) due to thrombosis, atherosclerosis, infections, or neointimal hyperplasia62 (Table 2). In view of the limitations of current vascular substitutes for small-diameter arteries, TEVGs may be an attractive potential solution. ...
Population-based long-term outcomes of open versus endovascular aortic repair of ruptured abdominal aortic aneurysms
1
2020
... Notably, large-diameter (inner diameter > 8 mm) artificial blood vessels made of ePTFE, Dacron, silk, polyurethane, or other polymers have been successfully used in clinics for conditions such as coarctation of the aorta, aneurysm of the thoracic aorta, and aortic dissection.76 To date, treatment of abdominal aortic aneurysms primarily comprises two different surgical procedures: endovascular placement of an aortic stent graft and open surgical repair of abdominal aortic aneurysms. Most current stent-grafts are based on self-expandable nitinol stents made of Dacron or ePTFE fabric.77, 78 However, in most cases they fail to replace vessels of a small diameter (inner diameter < 6 mm) due to thrombosis, atherosclerosis, infections, or neointimal hyperplasia62 (Table 2). In view of the limitations of current vascular substitutes for small-diameter arteries, TEVGs may be an attractive potential solution. ...
Endovascular grafts for abdominal aortic aneurysm
1
2016
... Notably, large-diameter (inner diameter > 8 mm) artificial blood vessels made of ePTFE, Dacron, silk, polyurethane, or other polymers have been successfully used in clinics for conditions such as coarctation of the aorta, aneurysm of the thoracic aorta, and aortic dissection.76 To date, treatment of abdominal aortic aneurysms primarily comprises two different surgical procedures: endovascular placement of an aortic stent graft and open surgical repair of abdominal aortic aneurysms. Most current stent-grafts are based on self-expandable nitinol stents made of Dacron or ePTFE fabric.77, 78 However, in most cases they fail to replace vessels of a small diameter (inner diameter < 6 mm) due to thrombosis, atherosclerosis, infections, or neointimal hyperplasia62 (Table 2). In view of the limitations of current vascular substitutes for small-diameter arteries, TEVGs may be an attractive potential solution. ...
Multifunctional silk-heparin biomaterials for vascular tissue engineering applications
1
2014
... A TEVG is made using appropriate materials and processes that possess high tissue compatibility, leading to a lack of rejection and maintaining long-term patency following transplantation.79 Due to these advantages, tissue engineering is frequently used for the construction of artificial blood vessels. Both synthetic and natural biodegradable materials have been tried in vascular tissue engineering. The scaffolds are considered to possess high porosity as well as a microstructure that can deliver, align, and maintain cell connections in favour of angiogenesis.80 The scaffold material required for TEVG creation should be biodegradable and nonimmunogenic. Furthermore, material processing techniques often affect the function of 3D scaffolds, as the same material composition with distinct micro/nano-scale structures can eventually perform different functions. To date, a number of fabrication techniques have been adopted to create clinically-viable TEVGs which vary widely in terms of materials, manufacturing methods, cell sources, and culture protocols. In a word, these strategies can be broadly classified into three common approaches: decellularised natural matrix methods; scaffold-based approaches using synthetic or natural materials; and scaffold-free processes. ...
Tissue engineering strategies for the induction of angiogenesis using biomaterials
1
2018
... A TEVG is made using appropriate materials and processes that possess high tissue compatibility, leading to a lack of rejection and maintaining long-term patency following transplantation.79 Due to these advantages, tissue engineering is frequently used for the construction of artificial blood vessels. Both synthetic and natural biodegradable materials have been tried in vascular tissue engineering. The scaffolds are considered to possess high porosity as well as a microstructure that can deliver, align, and maintain cell connections in favour of angiogenesis.80 The scaffold material required for TEVG creation should be biodegradable and nonimmunogenic. Furthermore, material processing techniques often affect the function of 3D scaffolds, as the same material composition with distinct micro/nano-scale structures can eventually perform different functions. To date, a number of fabrication techniques have been adopted to create clinically-viable TEVGs which vary widely in terms of materials, manufacturing methods, cell sources, and culture protocols. In a word, these strategies can be broadly classified into three common approaches: decellularised natural matrix methods; scaffold-based approaches using synthetic or natural materials; and scaffold-free processes. ...
Readily available tissue-engineered vascular grafts
3
2011
... One type of currently-used artificial vascular vessel that has demonstrated satisfactory preclinical and clinical results is made by decellularising allograft and xenograft vessels, such as arteries from swine or bovine models or the human umbilical artery.67, 81 Decellularisation is a tool that can be used in the generation of acellular scaffolds for tissue engineering, which completely removes all cellular and nuclear material from a tissue in order to reduce harmful immune reactions while preserving the natural ECM. It has already been used in various tissues with positive outcomes.82, 83 This outcome can be achieved through physical, chemical, or enzymatic methods.84 The resultant acellular scaffolds represent a great substrate that can promote cell adhesion, differentiation, and proliferation since specific ECM components and structures are key to tissue regeneration and remodelling.85 Moreover, decellularised scaffolds must have certain mechanical properties to avoid a mismatch with those of native tissues, which is key for blood vessels in tissue engineering. This can reduce immunogenicity but may also result in lower patency, which has been attributed to increased thrombogenicity, host immune response, and increased calcification.68, 69 Such grafts may have a clinical role, though they have not yet been widely used. Complete removal of tissue antigens remains a major issue, and the diversity of source materials makes it difficult to achieve manufacturing reproducibility.86 ...
... Cell-assembled grafts have been a dominant research topic over the past decade, where efforts following translational work indicate that TEVGs could be assembled entirely from human cells.91 Cell-assembly strategies involving culturing a range of human cell types (SMCs, fibroblasts, and induced pluripotent stem cells) for long periods of time to generate grafts are complex but the resulting grafts have the greatest degree of biomimicry.92 According to a study by Dahl et al.,81 employing human allogeneic or canine SMCs grown on a degradable polymer scaffold has been shown to be a feasible method of engineering a TEVG, in which grafts in baboons were demonstrated to retain their patency for up to 6 months. These grafts have shown great potential in preclinical evaluation. However, in a Phase II study, the final results were suboptimal as only 28% of the grafts remained open at 12 months.26 ...
... Rats and mice are widely used because they are inexpensive to acquire, easy to maintain, and can be used to evaluate larger sample sizes.135-137 Furthermore, the availability of various transgenic strains makes it possible to examine the molecular mechanisms involved in graft remodelling.138 However, differences in their circulatory system with respect to humans limit their utility in short-term studies to only very small grafts (< 2 mm diameter). Rabbits are also suitable for artificial blood vessel assessments although they are limited to short-term studies.132-134 Their physiology is similar to that of humans in terms of endothelialisation rates and thrombogenicity, and they can accept clinically-relevant graft diameters (1–4 mm). Dogs differ from humans in terms of thrombogenicity and represent a more stringent model for the investigations because of their lack of spontaneous endothelialisation.119, 131 Moreover, they can provide a range of implantation site options with varied vessel sizes, although their immune response can restrict study durations. Both pigs and sheep demonstrate similarities to human physiology and they are appropriate for studying thrombogenicity, calcification, and long-term graft patency.129, 130 The cardiovascular physiology of non-human primates, such as baboons, is most similar to that of humans compared to dogs, pigs or sheep. Their structural similarity allows for various imaging techniques and testing methods that are typically applied in humans to be used on them. Despite such advantages, they are rarely used for vascular graft evaluation due to their high cost and ethical concerns.81, 91 The optimal model for studying all of the performance criteria associated with artificial vascular grafts has yet to be elucidated. An immunodeficient pig model, the operational immunodeficient pig, has been reported to be a large animal model that allows for long-term accommodation of artificial human vascular grafts to properly evaluate the clinical efficacy of artificial vascular grafts.139 ...
Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: a multicentre cohort study
1
2009
... One type of currently-used artificial vascular vessel that has demonstrated satisfactory preclinical and clinical results is made by decellularising allograft and xenograft vessels, such as arteries from swine or bovine models or the human umbilical artery.67, 81 Decellularisation is a tool that can be used in the generation of acellular scaffolds for tissue engineering, which completely removes all cellular and nuclear material from a tissue in order to reduce harmful immune reactions while preserving the natural ECM. It has already been used in various tissues with positive outcomes.82, 83 This outcome can be achieved through physical, chemical, or enzymatic methods.84 The resultant acellular scaffolds represent a great substrate that can promote cell adhesion, differentiation, and proliferation since specific ECM components and structures are key to tissue regeneration and remodelling.85 Moreover, decellularised scaffolds must have certain mechanical properties to avoid a mismatch with those of native tissues, which is key for blood vessels in tissue engineering. This can reduce immunogenicity but may also result in lower patency, which has been attributed to increased thrombogenicity, host immune response, and increased calcification.68, 69 Such grafts may have a clinical role, though they have not yet been widely used. Complete removal of tissue antigens remains a major issue, and the diversity of source materials makes it difficult to achieve manufacturing reproducibility.86 ...
Acellular cardiac extracellular matrix as a scaffold for tissue engineering: in vitro cell support, remodeling, and biocompatibility
1
2010
... One type of currently-used artificial vascular vessel that has demonstrated satisfactory preclinical and clinical results is made by decellularising allograft and xenograft vessels, such as arteries from swine or bovine models or the human umbilical artery.67, 81 Decellularisation is a tool that can be used in the generation of acellular scaffolds for tissue engineering, which completely removes all cellular and nuclear material from a tissue in order to reduce harmful immune reactions while preserving the natural ECM. It has already been used in various tissues with positive outcomes.82, 83 This outcome can be achieved through physical, chemical, or enzymatic methods.84 The resultant acellular scaffolds represent a great substrate that can promote cell adhesion, differentiation, and proliferation since specific ECM components and structures are key to tissue regeneration and remodelling.85 Moreover, decellularised scaffolds must have certain mechanical properties to avoid a mismatch with those of native tissues, which is key for blood vessels in tissue engineering. This can reduce immunogenicity but may also result in lower patency, which has been attributed to increased thrombogenicity, host immune response, and increased calcification.68, 69 Such grafts may have a clinical role, though they have not yet been widely used. Complete removal of tissue antigens remains a major issue, and the diversity of source materials makes it difficult to achieve manufacturing reproducibility.86 ...
Extracellular matrix as a biological scaffold material: Structure and function
1
2009
... One type of currently-used artificial vascular vessel that has demonstrated satisfactory preclinical and clinical results is made by decellularising allograft and xenograft vessels, such as arteries from swine or bovine models or the human umbilical artery.67, 81 Decellularisation is a tool that can be used in the generation of acellular scaffolds for tissue engineering, which completely removes all cellular and nuclear material from a tissue in order to reduce harmful immune reactions while preserving the natural ECM. It has already been used in various tissues with positive outcomes.82, 83 This outcome can be achieved through physical, chemical, or enzymatic methods.84 The resultant acellular scaffolds represent a great substrate that can promote cell adhesion, differentiation, and proliferation since specific ECM components and structures are key to tissue regeneration and remodelling.85 Moreover, decellularised scaffolds must have certain mechanical properties to avoid a mismatch with those of native tissues, which is key for blood vessels in tissue engineering. This can reduce immunogenicity but may also result in lower patency, which has been attributed to increased thrombogenicity, host immune response, and increased calcification.68, 69 Such grafts may have a clinical role, though they have not yet been widely used. Complete removal of tissue antigens remains a major issue, and the diversity of source materials makes it difficult to achieve manufacturing reproducibility.86 ...
Extracellular matrix bioscaffolds in tissue remodeling and morphogenesis
1
2016
... One type of currently-used artificial vascular vessel that has demonstrated satisfactory preclinical and clinical results is made by decellularising allograft and xenograft vessels, such as arteries from swine or bovine models or the human umbilical artery.67, 81 Decellularisation is a tool that can be used in the generation of acellular scaffolds for tissue engineering, which completely removes all cellular and nuclear material from a tissue in order to reduce harmful immune reactions while preserving the natural ECM. It has already been used in various tissues with positive outcomes.82, 83 This outcome can be achieved through physical, chemical, or enzymatic methods.84 The resultant acellular scaffolds represent a great substrate that can promote cell adhesion, differentiation, and proliferation since specific ECM components and structures are key to tissue regeneration and remodelling.85 Moreover, decellularised scaffolds must have certain mechanical properties to avoid a mismatch with those of native tissues, which is key for blood vessels in tissue engineering. This can reduce immunogenicity but may also result in lower patency, which has been attributed to increased thrombogenicity, host immune response, and increased calcification.68, 69 Such grafts may have a clinical role, though they have not yet been widely used. Complete removal of tissue antigens remains a major issue, and the diversity of source materials makes it difficult to achieve manufacturing reproducibility.86 ...
In vivo performance of decellularized vascular grafts: a review article
1
2018
... One type of currently-used artificial vascular vessel that has demonstrated satisfactory preclinical and clinical results is made by decellularising allograft and xenograft vessels, such as arteries from swine or bovine models or the human umbilical artery.67, 81 Decellularisation is a tool that can be used in the generation of acellular scaffolds for tissue engineering, which completely removes all cellular and nuclear material from a tissue in order to reduce harmful immune reactions while preserving the natural ECM. It has already been used in various tissues with positive outcomes.82, 83 This outcome can be achieved through physical, chemical, or enzymatic methods.84 The resultant acellular scaffolds represent a great substrate that can promote cell adhesion, differentiation, and proliferation since specific ECM components and structures are key to tissue regeneration and remodelling.85 Moreover, decellularised scaffolds must have certain mechanical properties to avoid a mismatch with those of native tissues, which is key for blood vessels in tissue engineering. This can reduce immunogenicity but may also result in lower patency, which has been attributed to increased thrombogenicity, host immune response, and increased calcification.68, 69 Such grafts may have a clinical role, though they have not yet been widely used. Complete removal of tissue antigens remains a major issue, and the diversity of source materials makes it difficult to achieve manufacturing reproducibility.86 ...
Electrospinning and electrospun nanofibers: methods, materials, and applications
1
2019
... Scaffold-based techniques use macromolecular structures to support cells during the regeneration process and are prefabrication strategies that are relatively simple to implement. Except for decellularised vessels, many methods fail to mimic the structure of the natural ECM. Electrospinning technology is a promising technique that may be used in the production of TEVGs, which can address this problem. It has been reported that nanofibres produced via electrospinning technology can simulate the composition of the ECM by loading various drugs and growth factors.47 Moreover, it is also the only method used to prepare nano-scale polymer material fibres directly and continuously by applying a high voltage to materials dissolved in volatile solvents so as to create dry fibres that can be collected to form conduits. Therefore, it has been extensively applied in tissue engineering, especially to create small-diameter blood vessel grafts. Additionally, the surfaces of artificial blood vessel grafts prepared by electrospinning have good specific surface areas and high porosities.87 This method facilitates fine control of fibre size and deposition and allows for blending of materials. New methods for simultaneous spinning of multiple components88 and derivative techniques, such as gel spinning89 and conjugate electrospinning technology, have been developed in order to optimise the technique. Small-calibre blood vessel grafts have been constructed by combining both electrospinning technology and freeze-drying technology, providing insight into the fusion of multiple processes and further improvement of artificial blood vessel fabrication.90 ...
Coaxial electrospinning multicomponent functional controlled-release vascular graft: Optimization of graft properties
1
2017
... Scaffold-based techniques use macromolecular structures to support cells during the regeneration process and are prefabrication strategies that are relatively simple to implement. Except for decellularised vessels, many methods fail to mimic the structure of the natural ECM. Electrospinning technology is a promising technique that may be used in the production of TEVGs, which can address this problem. It has been reported that nanofibres produced via electrospinning technology can simulate the composition of the ECM by loading various drugs and growth factors.47 Moreover, it is also the only method used to prepare nano-scale polymer material fibres directly and continuously by applying a high voltage to materials dissolved in volatile solvents so as to create dry fibres that can be collected to form conduits. Therefore, it has been extensively applied in tissue engineering, especially to create small-diameter blood vessel grafts. Additionally, the surfaces of artificial blood vessel grafts prepared by electrospinning have good specific surface areas and high porosities.87 This method facilitates fine control of fibre size and deposition and allows for blending of materials. New methods for simultaneous spinning of multiple components88 and derivative techniques, such as gel spinning89 and conjugate electrospinning technology, have been developed in order to optimise the technique. Small-calibre blood vessel grafts have been constructed by combining both electrospinning technology and freeze-drying technology, providing insight into the fusion of multiple processes and further improvement of artificial blood vessel fabrication.90 ...
Fabricating mechanically improved silk-based vascular grafts by solution control of the gel-spinning process
1
2020
... Scaffold-based techniques use macromolecular structures to support cells during the regeneration process and are prefabrication strategies that are relatively simple to implement. Except for decellularised vessels, many methods fail to mimic the structure of the natural ECM. Electrospinning technology is a promising technique that may be used in the production of TEVGs, which can address this problem. It has been reported that nanofibres produced via electrospinning technology can simulate the composition of the ECM by loading various drugs and growth factors.47 Moreover, it is also the only method used to prepare nano-scale polymer material fibres directly and continuously by applying a high voltage to materials dissolved in volatile solvents so as to create dry fibres that can be collected to form conduits. Therefore, it has been extensively applied in tissue engineering, especially to create small-diameter blood vessel grafts. Additionally, the surfaces of artificial blood vessel grafts prepared by electrospinning have good specific surface areas and high porosities.87 This method facilitates fine control of fibre size and deposition and allows for blending of materials. New methods for simultaneous spinning of multiple components88 and derivative techniques, such as gel spinning89 and conjugate electrospinning technology, have been developed in order to optimise the technique. Small-calibre blood vessel grafts have been constructed by combining both electrospinning technology and freeze-drying technology, providing insight into the fusion of multiple processes and further improvement of artificial blood vessel fabrication.90 ...
Construction and performance evaluation of Hep/silk-PLCL composite nanofiber small-caliber artificial blood vessel graft
2
2020
... Scaffold-based techniques use macromolecular structures to support cells during the regeneration process and are prefabrication strategies that are relatively simple to implement. Except for decellularised vessels, many methods fail to mimic the structure of the natural ECM. Electrospinning technology is a promising technique that may be used in the production of TEVGs, which can address this problem. It has been reported that nanofibres produced via electrospinning technology can simulate the composition of the ECM by loading various drugs and growth factors.47 Moreover, it is also the only method used to prepare nano-scale polymer material fibres directly and continuously by applying a high voltage to materials dissolved in volatile solvents so as to create dry fibres that can be collected to form conduits. Therefore, it has been extensively applied in tissue engineering, especially to create small-diameter blood vessel grafts. Additionally, the surfaces of artificial blood vessel grafts prepared by electrospinning have good specific surface areas and high porosities.87 This method facilitates fine control of fibre size and deposition and allows for blending of materials. New methods for simultaneous spinning of multiple components88 and derivative techniques, such as gel spinning89 and conjugate electrospinning technology, have been developed in order to optimise the technique. Small-calibre blood vessel grafts have been constructed by combining both electrospinning technology and freeze-drying technology, providing insight into the fusion of multiple processes and further improvement of artificial blood vessel fabrication.90 ...
... As the most effective and routine anticoagulant for clinical cardiovascular surgery, heparin inhibits the occurrence of acute thrombosis following vascular transplantation.107 Heparin helps to promote the release of tissue factor inhibitors, synergistic anticoagulants, and plasminogen activators from ECs. Moreover, heparin has been shown to promote the growth of ECs and inhibit smooth muscle proliferation after vascular injury. In this regard, immobilised heparin can significantly influence the adherence and activation of platelets and enhance fibrinolysis.108 Heparinised silk fibroin can significantly enhance anticoagulation at the initial stage of implantation. Furthermore, Kuang et al.90 introduced a biodegradable graft made of core poly(L-lactide-co-caprolactone)-based nanofibres coated with heparin/silk gel as a shell layer, and demonstrated that the graft was unobstructed for over 8 months via in vivo animal experiments, providing a useful model for remodelling vascular structures. ...
Human tissue-engineered blood vessels for adult arterial revascularization
3
2006
... Cell-assembled grafts have been a dominant research topic over the past decade, where efforts following translational work indicate that TEVGs could be assembled entirely from human cells.91 Cell-assembly strategies involving culturing a range of human cell types (SMCs, fibroblasts, and induced pluripotent stem cells) for long periods of time to generate grafts are complex but the resulting grafts have the greatest degree of biomimicry.92 According to a study by Dahl et al.,81 employing human allogeneic or canine SMCs grown on a degradable polymer scaffold has been shown to be a feasible method of engineering a TEVG, in which grafts in baboons were demonstrated to retain their patency for up to 6 months. These grafts have shown great potential in preclinical evaluation. However, in a Phase II study, the final results were suboptimal as only 28% of the grafts remained open at 12 months.26 ...
... Mechanical properties of natural vessels and some artificial blood vessels.
| Burst pressure (mmHg) | Compliance (%/100 mmHg) |
| Values for artificial blood vessels12, 91 | >1000 | 10-20 |
| Dog femoral artery118 | 2895 ± 263 | 10.3 ± 2.3 |
| Human internal mammary artery119 | 3196 ± 1264 | 11.5 ± 3.9 |
Biodegradable chitosan vascular grafts
(with 4 mm inner diameter)118 | 1688 ± 236 | 5.7 ± 1.3 |
| Tissue-engineered blood vessels13 | 3490 ± 892 | 3.4 ± 1.6 |
| Expanded polytetrafluoroethylene grafts119 | – | 0.51 |
| Silk fibroin grafts119 | – | 1.90 |
Another consideration is individual variability, signifying the variation in the mechanical properties of blood vessels from vessel to vessel and between individuals. In future, vascular tissue engineering may ultimately involve tailoring the specific mechanical properties of a graft to the intended implantation site. A far greater understanding of vascular biomechanics, interactions between the graft and the native vessels, and how to engineer the mechanical properties of a vascular graft would be required. ...
... Rats and mice are widely used because they are inexpensive to acquire, easy to maintain, and can be used to evaluate larger sample sizes.135-137 Furthermore, the availability of various transgenic strains makes it possible to examine the molecular mechanisms involved in graft remodelling.138 However, differences in their circulatory system with respect to humans limit their utility in short-term studies to only very small grafts (< 2 mm diameter). Rabbits are also suitable for artificial blood vessel assessments although they are limited to short-term studies.132-134 Their physiology is similar to that of humans in terms of endothelialisation rates and thrombogenicity, and they can accept clinically-relevant graft diameters (1–4 mm). Dogs differ from humans in terms of thrombogenicity and represent a more stringent model for the investigations because of their lack of spontaneous endothelialisation.119, 131 Moreover, they can provide a range of implantation site options with varied vessel sizes, although their immune response can restrict study durations. Both pigs and sheep demonstrate similarities to human physiology and they are appropriate for studying thrombogenicity, calcification, and long-term graft patency.129, 130 The cardiovascular physiology of non-human primates, such as baboons, is most similar to that of humans compared to dogs, pigs or sheep. Their structural similarity allows for various imaging techniques and testing methods that are typically applied in humans to be used on them. Despite such advantages, they are rarely used for vascular graft evaluation due to their high cost and ethical concerns.81, 91 The optimal model for studying all of the performance criteria associated with artificial vascular grafts has yet to be elucidated. An immunodeficient pig model, the operational immunodeficient pig, has been reported to be a large animal model that allows for long-term accommodation of artificial human vascular grafts to properly evaluate the clinical efficacy of artificial vascular grafts.139 ...
Extracellular matrix for small-diameter vascular grafts
1
2020
... Cell-assembled grafts have been a dominant research topic over the past decade, where efforts following translational work indicate that TEVGs could be assembled entirely from human cells.91 Cell-assembly strategies involving culturing a range of human cell types (SMCs, fibroblasts, and induced pluripotent stem cells) for long periods of time to generate grafts are complex but the resulting grafts have the greatest degree of biomimicry.92 According to a study by Dahl et al.,81 employing human allogeneic or canine SMCs grown on a degradable polymer scaffold has been shown to be a feasible method of engineering a TEVG, in which grafts in baboons were demonstrated to retain their patency for up to 6 months. These grafts have shown great potential in preclinical evaluation. However, in a Phase II study, the final results were suboptimal as only 28% of the grafts remained open at 12 months.26 ...
Cell adhesion on nanofibrous polytetrafluoroethylene (nPTFE)
1
2007
... Stretch moulding is a traditional manufacturing technique for preparing ePTFE artificial blood vessels, which has achieved wide commercial application. The process involves extruding the raw material polymer polytetrafluoroethylene resin to form a preform that is longitudinally arranged into a fibrous shape, stretched at a high speed and then cooled in order to obtain a formed product.93 The process of each link has a great influence on the structure and mechanical properties of ePTFE. Porosity can affect the mechanical strength and biocompatibility of ePTFE artificial blood vessels and is key to affecting the performance of ePTFE.94 ...
Prosthetic vascular grafts: wrong models, wrong questions and no healing
3
2007
... Stretch moulding is a traditional manufacturing technique for preparing ePTFE artificial blood vessels, which has achieved wide commercial application. The process involves extruding the raw material polymer polytetrafluoroethylene resin to form a preform that is longitudinally arranged into a fibrous shape, stretched at a high speed and then cooled in order to obtain a formed product.93 The process of each link has a great influence on the structure and mechanical properties of ePTFE. Porosity can affect the mechanical strength and biocompatibility of ePTFE artificial blood vessels and is key to affecting the performance of ePTFE.94 ...
... After graft transplantation, inflammatory and mesenchymal cells entering the artificial blood vessel wall and endothelial cells covering the endo-surface of artificial blood vessels can promote graft healing. Early endothelialisation of artificial vessels is crucial for the suppression of thrombosis and maintenance of patency.105 ECs can provide a suitable interface between blood and surrounding tissue while maintaining a balance between haemostasis and thrombosis. It is essential to endothelialise the inner surface of artificial blood vessels to prevent thrombosis and intimal hyperplasia.48, 109 There are three ways to improve endothelialisation of the grafts’ inner surface: trans-anastomotic ingrowth of the innate arteries’ endothelia, transmural ingrowth of the capillaries at the implantation sites, and haematogenous seeding of endothelia from the circulatory system.48, 94 ...
... Common animal models for evaluation of artificial blood vessels
in vivo.
| Animal model | Characteristics | Application inner diameter (mm) | Common length (mm) | Longest implantation period | Implantation site | References |
| Sheep | Similar cardiovascular physiology, endothelialisation mechanisms and thrombogenicity mechanisms to humans.
Suitable size and long-term studies possible.Higher incidence of hypercoagulability. | 4–6 | 80–100 | 9 months | Carotid artery | 48, 74 |
| 4–6 | 60–100 | 3 months | Arteriovenous graft | 129 |
| Pig | Similar vascular physiology and anatomy to humans.
Well established as a model for assessing vascular grafts.
Mount an extensive immune response to implanted tissues. | 3–6 | 30–100 | 6 months | Iliac artery | 130 |
| 3–6 | 10–100 | 4 weeks | Carotid artery | 52 |
| Dog | Lack of spontaneous endothelialisation and immune response restricts study lengths.
Ease of accessing vessel due to thin skin.
Thrombogenicity mechanisms and vessel viscoelastic properties differ from humans. | 3–6 | 30–50 | 6 months | Abdominal artery | 131 |
| 3–6 | 30–50 | 1 year | Carotid artery | 119 |
| Baboon | Physiology and cardiovascular anatomy are the most similar to humans.
Suitable for a wide range of non-invasive imaging techniques adapted from humans.
High cost and ethical concerns associated with using primates in medical research. | 3–6 | 30–50 | 6 months | Arteriovenous graft | 26 |
| Rabbit | Similar endothelialisation rates and thrombogenicity mechanisms to humans.
Demanding higher anticoagulant function.
Limited to short-term studies. | 1–4 | 5–30 | 12 months | Carotid artery | 94, 132 |
| 1–4 | 5–30 | 2 weeks | Femoral artery | 133 |
| 1–4 | 5–30 | 3 months | Abdominal artery | 134 |
| Rat | Large sample size.
Wide variety of transgenic lines.
Allows exploration of genetic/molecular mechanisms.
Ideal for biocompatibility and cell infiltration studies. | 1–3 | 5–30 | 18 months | Abdominal artery | 73, 135 |
| 1–3 | 5–10 | 12 weeks | Carotid artery | 136 |
| Mouse | 0.5–1 | 3–10 | 6 months | Carotid artery | 137 |
Rats and mice are widely used because they are inexpensive to acquire, easy to maintain, and can be used to evaluate larger sample sizes.135-137 Furthermore, the availability of various transgenic strains makes it possible to examine the molecular mechanisms involved in graft remodelling.138 However, differences in their circulatory system with respect to humans limit their utility in short-term studies to only very small grafts (< 2 mm diameter). Rabbits are also suitable for artificial blood vessel assessments although they are limited to short-term studies.132-134 Their physiology is similar to that of humans in terms of endothelialisation rates and thrombogenicity, and they can accept clinically-relevant graft diameters (1–4 mm). Dogs differ from humans in terms of thrombogenicity and represent a more stringent model for the investigations because of their lack of spontaneous endothelialisation.119, 131 Moreover, they can provide a range of implantation site options with varied vessel sizes, although their immune response can restrict study durations. Both pigs and sheep demonstrate similarities to human physiology and they are appropriate for studying thrombogenicity, calcification, and long-term graft patency.129, 130 The cardiovascular physiology of non-human primates, such as baboons, is most similar to that of humans compared to dogs, pigs or sheep. Their structural similarity allows for various imaging techniques and testing methods that are typically applied in humans to be used on them. Despite such advantages, they are rarely used for vascular graft evaluation due to their high cost and ethical concerns.81, 91 The optimal model for studying all of the performance criteria associated with artificial vascular grafts has yet to be elucidated. An immunodeficient pig model, the operational immunodeficient pig, has been reported to be a large animal model that allows for long-term accommodation of artificial human vascular grafts to properly evaluate the clinical efficacy of artificial vascular grafts.139 ...
Recent Trends in the Fabrication of Starch Nanofibers: Electrospinning and Non-electrospinning Routes and Their Applications in Biotechnology
1
2019
... Electrospinning is a process in which a polymer solution or melt is used to form a small jet of polymer under the action of a high-voltage electrostatic field for spinning. Electrospinning enables the direct and continuous preparation of polymer nanofibres.95 Electrospinning has the following technical advantages: (1) it can increase the porosity of blood vessels to meet the requirements of cell growth; (2) it has a large specific surface area, which is conducive to cell adhesion and growth; (3) the diameter of the fibres produced is similar to the size of natural extracellular matrix or cells, hence, it can mimic the structure of human extracellular matrix to the greatest extent; and (4) it can load growth factors and induce cell adhesion, proliferation and differentiation. ...
Albumin-coated knitted Dacron aortic prosthses. Study of postoperative inflammatory reactions
1
2002
... Braiding is the process of weaving multiple filaments in a specific way to prepare artificial blood vessels.96 Preparation of artificial blood vessels by braiding technology improves elasticity and compression resistance, and enhances the compliance of the blood vessels. Due to the easy displacement and damage of the edge of a braided graft, its clinical application has been greatly hindered. Therefore, the braiding technique is often used to improve the strength of anastomosis while ensuring the flexibility of the inner layer material through the design of a multi-layer artificial blood vessel. ...
Personalized development of human organs using 3D printing technology
1
2016
... 3D printing technology appeared in the mid-1990s and is based on digital model files, using powdered metal or plastic materials to construct objects through a layer-by-layer printing method.97 Melchiorri et al.98 fabricated a vascular graft adapted to the curvature of the patient’s anatomy by using poly(propylene fumarate). Although it has an adjustable shaping feature, few studies have focused on the direct preparation of artificial blood vessels by 3D printing. Specifically, most research has focused on the preparation of in vitro vascular abrasives to assist in the production of inner layer materials of artificial blood vessels and the fabrication of organ chips in order to provide support for the study of material patency.99 ...
3D-printed biodegradable polymeric vascular grafts
1
2016
... 3D printing technology appeared in the mid-1990s and is based on digital model files, using powdered metal or plastic materials to construct objects through a layer-by-layer printing method.97 Melchiorri et al.98 fabricated a vascular graft adapted to the curvature of the patient’s anatomy by using poly(propylene fumarate). Although it has an adjustable shaping feature, few studies have focused on the direct preparation of artificial blood vessels by 3D printing. Specifically, most research has focused on the preparation of in vitro vascular abrasives to assist in the production of inner layer materials of artificial blood vessels and the fabrication of organ chips in order to provide support for the study of material patency.99 ...
Current progress in 3D printing for cardiovascular tissue engineering
1
2015
... 3D printing technology appeared in the mid-1990s and is based on digital model files, using powdered metal or plastic materials to construct objects through a layer-by-layer printing method.97 Melchiorri et al.98 fabricated a vascular graft adapted to the curvature of the patient’s anatomy by using poly(propylene fumarate). Although it has an adjustable shaping feature, few studies have focused on the direct preparation of artificial blood vessels by 3D printing. Specifically, most research has focused on the preparation of in vitro vascular abrasives to assist in the production of inner layer materials of artificial blood vessels and the fabrication of organ chips in order to provide support for the study of material patency.99 ...
Characterization and preparation of bio-tubular scaffolds for fabricating artificial vascular grafts by combining electrospinning and a 3D printing system
1
2015
... In addition to the above-mentioned preparation methods, the production of artificial blood vessels also includes technologies such as gas foaming, hydrogel technology and freezing-casting. However, it is often difficult for a single processing method to meet the various performance requirements of artificial blood vessels, and different processing methods are often used in combination. The most common example is the combination of electrospinning and weaving techniques. Electrospinning produces filaments that adapt to cell growth and migration, while weaving improves overall vascular elasticity and compliance. In addition, Lee et al.100 prepared a nanofibre hybrid artificial blood vessel material by electrospinning and 3D printing techniques, which exhibited excellent mechanical properties and good patency. ...
In vitro engineering of vascularized tissue surrogates
1
2013
... Thus far, commercialised vascular grafts are generally only used as structural substitutes and rarely have bioactivity or biofunctions. Hence, various strategies have been used to optimise the structure of engineered tissues.101 In recent years, novel fabrication techniques of preparing artificial blood vessel grafts have been combined with chemical grafting, surface modification, and coating technologies to improve the patency rate of artificial blood vessels in early transplantation, especially in small-calibre grafts.102 Most studies have focused on surface modification of materials so as to enhance haemocompatibility and facilitate cell adhesion and growth.103, 104 Therefore, the lifespan and effectiveness of a graft can be greatly extended by improving these two properties. ...
In vitro fabrication of functional three-dimensional tissues with perfusable blood vessels
1
2013
... Thus far, commercialised vascular grafts are generally only used as structural substitutes and rarely have bioactivity or biofunctions. Hence, various strategies have been used to optimise the structure of engineered tissues.101 In recent years, novel fabrication techniques of preparing artificial blood vessel grafts have been combined with chemical grafting, surface modification, and coating technologies to improve the patency rate of artificial blood vessels in early transplantation, especially in small-calibre grafts.102 Most studies have focused on surface modification of materials so as to enhance haemocompatibility and facilitate cell adhesion and growth.103, 104 Therefore, the lifespan and effectiveness of a graft can be greatly extended by improving these two properties. ...
Macromolecular approaches to prevent thrombosis and intimal hyperplasia following percutaneous coronary intervention
1
2014
... Thus far, commercialised vascular grafts are generally only used as structural substitutes and rarely have bioactivity or biofunctions. Hence, various strategies have been used to optimise the structure of engineered tissues.101 In recent years, novel fabrication techniques of preparing artificial blood vessel grafts have been combined with chemical grafting, surface modification, and coating technologies to improve the patency rate of artificial blood vessels in early transplantation, especially in small-calibre grafts.102 Most studies have focused on surface modification of materials so as to enhance haemocompatibility and facilitate cell adhesion and growth.103, 104 Therefore, the lifespan and effectiveness of a graft can be greatly extended by improving these two properties. ...
Enzyme-functionalized vascular grafts catalyze in-situ release of nitric oxide from exogenous NO prodrug
2
2015
... Thus far, commercialised vascular grafts are generally only used as structural substitutes and rarely have bioactivity or biofunctions. Hence, various strategies have been used to optimise the structure of engineered tissues.101 In recent years, novel fabrication techniques of preparing artificial blood vessel grafts have been combined with chemical grafting, surface modification, and coating technologies to improve the patency rate of artificial blood vessels in early transplantation, especially in small-calibre grafts.102 Most studies have focused on surface modification of materials so as to enhance haemocompatibility and facilitate cell adhesion and growth.103, 104 Therefore, the lifespan and effectiveness of a graft can be greatly extended by improving these two properties. ...
... Notably, increasingly complex surface coatings have been studied for their ability to promote endothelialisation through significant biological signalling while simultaneously having low thrombogenicity. Wang et al.104 showed that functional vascular grafts with in-situ catalysis of nitric oxide release based on enzyme prodrug therapy are helpful for localised and on-demand nitric oxide release. This approach illustrates the effective inhibition of thrombus formation in vivo as well as the enhancement of vascular tissue regeneration and remodelling on the grafts, which may be useful for the development of novel cell-free vascular grafts in treatment of vascular disease. Surface modification of polyurethane with hydrophilic molecules improves haemocompatibility; however, this may limit initial vascular EC adhesion.115 The protein Dickkopf-3 plays key roles in inducing the directional migration of vascular progenitor cells and promoting tissue regeneration. Issa Bhaloo et al.116 proposed the construction of artificial blood vessels with the function of delivering Dickkopf-3. In addition, it has been demonstrated that histone deacetylase 7 mRNA in vascular progenitor cells can be selectively translated to produce a polypeptide containing seven amino acids. This polypeptide induces the migration and differentiation of vascular progenitor cells into vascular ECs, thereby effectively promoting the endothelialisation of artificial blood vessels.117 To date, various small animal model experiments have been conducted to improve endothelialisation on artificial vascular grafts with specific peptides and biomolecules, which has shown some potential; however, further development in larger preclinical models is lacking. ...
Human blood vessel-derived endothelial progenitors for endothelialization of small diameter vascular prosthesis
2
2009
... The surface can be either cellular or biochemical, however, it should inhibit protein and cell adsorption, prevent blood coagulation contact activation, inhibit platelet adhesion and activation, and thus avoid thrombosis in the arterial system. A study has shown that platelet adhesion is reduced when ePTFE tubes are coated with a defined mix of extracellular matrix components. In addition, the amount of fibronectin adhering to it was also observed to be reduced after blood flow assay, which indicates a decrease in its thrombogenic nature.105 One researcher used albumin as a coating and found that it can significantly decrease platelet adhesion compared to other plasma proteins and achieve a thromboresistant effect.106 ...
... After graft transplantation, inflammatory and mesenchymal cells entering the artificial blood vessel wall and endothelial cells covering the endo-surface of artificial blood vessels can promote graft healing. Early endothelialisation of artificial vessels is crucial for the suppression of thrombosis and maintenance of patency.105 ECs can provide a suitable interface between blood and surrounding tissue while maintaining a balance between haemostasis and thrombosis. It is essential to endothelialise the inner surface of artificial blood vessels to prevent thrombosis and intimal hyperplasia.48, 109 There are three ways to improve endothelialisation of the grafts’ inner surface: trans-anastomotic ingrowth of the innate arteries’ endothelia, transmural ingrowth of the capillaries at the implantation sites, and haematogenous seeding of endothelia from the circulatory system.48, 94 ...
Immobilization of proteins on poly(methyl methacrylate) films
1
1993
... The surface can be either cellular or biochemical, however, it should inhibit protein and cell adsorption, prevent blood coagulation contact activation, inhibit platelet adhesion and activation, and thus avoid thrombosis in the arterial system. A study has shown that platelet adhesion is reduced when ePTFE tubes are coated with a defined mix of extracellular matrix components. In addition, the amount of fibronectin adhering to it was also observed to be reduced after blood flow assay, which indicates a decrease in its thrombogenic nature.105 One researcher used albumin as a coating and found that it can significantly decrease platelet adhesion compared to other plasma proteins and achieve a thromboresistant effect.106 ...
Pathogenesis of heparin-induced thrombocytopenia
1
2020
... As the most effective and routine anticoagulant for clinical cardiovascular surgery, heparin inhibits the occurrence of acute thrombosis following vascular transplantation.107 Heparin helps to promote the release of tissue factor inhibitors, synergistic anticoagulants, and plasminogen activators from ECs. Moreover, heparin has been shown to promote the growth of ECs and inhibit smooth muscle proliferation after vascular injury. In this regard, immobilised heparin can significantly influence the adherence and activation of platelets and enhance fibrinolysis.108 Heparinised silk fibroin can significantly enhance anticoagulation at the initial stage of implantation. Furthermore, Kuang et al.90 introduced a biodegradable graft made of core poly(L-lactide-co-caprolactone)-based nanofibres coated with heparin/silk gel as a shell layer, and demonstrated that the graft was unobstructed for over 8 months via in vivo animal experiments, providing a useful model for remodelling vascular structures. ...
Heparin after angioplasty: an unresolved issue?
1
1993
... As the most effective and routine anticoagulant for clinical cardiovascular surgery, heparin inhibits the occurrence of acute thrombosis following vascular transplantation.107 Heparin helps to promote the release of tissue factor inhibitors, synergistic anticoagulants, and plasminogen activators from ECs. Moreover, heparin has been shown to promote the growth of ECs and inhibit smooth muscle proliferation after vascular injury. In this regard, immobilised heparin can significantly influence the adherence and activation of platelets and enhance fibrinolysis.108 Heparinised silk fibroin can significantly enhance anticoagulation at the initial stage of implantation. Furthermore, Kuang et al.90 introduced a biodegradable graft made of core poly(L-lactide-co-caprolactone)-based nanofibres coated with heparin/silk gel as a shell layer, and demonstrated that the graft was unobstructed for over 8 months via in vivo animal experiments, providing a useful model for remodelling vascular structures. ...
Use of arterial patch to improve re-endothelialization in a sheep model of open carotid endarterectomy. An incentive to use internal thoracic artery as an on-lay patch following coronary endarterecomy?
1
2009
... After graft transplantation, inflammatory and mesenchymal cells entering the artificial blood vessel wall and endothelial cells covering the endo-surface of artificial blood vessels can promote graft healing. Early endothelialisation of artificial vessels is crucial for the suppression of thrombosis and maintenance of patency.105 ECs can provide a suitable interface between blood and surrounding tissue while maintaining a balance between haemostasis and thrombosis. It is essential to endothelialise the inner surface of artificial blood vessels to prevent thrombosis and intimal hyperplasia.48, 109 There are three ways to improve endothelialisation of the grafts’ inner surface: trans-anastomotic ingrowth of the innate arteries’ endothelia, transmural ingrowth of the capillaries at the implantation sites, and haematogenous seeding of endothelia from the circulatory system.48, 94 ...
The role of endothelial cells and their progenitors in intimal hyperplasia
1
2010
... The presence of natural materials is known to improve biological function according to consensus; therefore, various coatings based on adding natural materials can be designed. The addition of an elastin coating to grafts has been shown to reduce platelet adhesion and increase EC attachment.65 Interestingly, the proportion of SMCs in a contractile phenotype was also increased in elastin-containing grafts, suggesting their usefulness for limiting neointimal hyperplasia.110-112 Moreover, collagen has been shown to improve EC viability, attachment, and migration when incorporated into poly(lactic acid) knitted and poly(vinyl alcohol) vascular scaffolds in vitro.53, 113 Similarly, a polymer blend of recombinant human collagen peptides and PCL gelatine have been shown to enhance the adhesion of human umbilical vein ECs (HUVECs) to the lumen, which adds further support for favourable vascular cell signalling provided by collagen sequences.114 ...
Strategies and techniques to enhance the in situ endothelialization of small-diameter biodegradable polymeric vascular grafts
0
2013
Electrochemical fabrication of a biomimetic elastin-containing bi-layered scaffold for vascular tissue engineering
1
2018
... The presence of natural materials is known to improve biological function according to consensus; therefore, various coatings based on adding natural materials can be designed. The addition of an elastin coating to grafts has been shown to reduce platelet adhesion and increase EC attachment.65 Interestingly, the proportion of SMCs in a contractile phenotype was also increased in elastin-containing grafts, suggesting their usefulness for limiting neointimal hyperplasia.110-112 Moreover, collagen has been shown to improve EC viability, attachment, and migration when incorporated into poly(lactic acid) knitted and poly(vinyl alcohol) vascular scaffolds in vitro.53, 113 Similarly, a polymer blend of recombinant human collagen peptides and PCL gelatine have been shown to enhance the adhesion of human umbilical vein ECs (HUVECs) to the lumen, which adds further support for favourable vascular cell signalling provided by collagen sequences.114 ...
Bioconjugation of a collagen-mimicking peptide onto poly(vinyl alcohol) encourages endothelialization while minimizing thrombosis
1
2020
... The presence of natural materials is known to improve biological function according to consensus; therefore, various coatings based on adding natural materials can be designed. The addition of an elastin coating to grafts has been shown to reduce platelet adhesion and increase EC attachment.65 Interestingly, the proportion of SMCs in a contractile phenotype was also increased in elastin-containing grafts, suggesting their usefulness for limiting neointimal hyperplasia.110-112 Moreover, collagen has been shown to improve EC viability, attachment, and migration when incorporated into poly(lactic acid) knitted and poly(vinyl alcohol) vascular scaffolds in vitro.53, 113 Similarly, a polymer blend of recombinant human collagen peptides and PCL gelatine have been shown to enhance the adhesion of human umbilical vein ECs (HUVECs) to the lumen, which adds further support for favourable vascular cell signalling provided by collagen sequences.114 ...
Porous bilayer vascular grafts fabricated from electrospinning of the recombinant human collagen (RHC) peptide-based blend
1
2021
... The presence of natural materials is known to improve biological function according to consensus; therefore, various coatings based on adding natural materials can be designed. The addition of an elastin coating to grafts has been shown to reduce platelet adhesion and increase EC attachment.65 Interestingly, the proportion of SMCs in a contractile phenotype was also increased in elastin-containing grafts, suggesting their usefulness for limiting neointimal hyperplasia.110-112 Moreover, collagen has been shown to improve EC viability, attachment, and migration when incorporated into poly(lactic acid) knitted and poly(vinyl alcohol) vascular scaffolds in vitro.53, 113 Similarly, a polymer blend of recombinant human collagen peptides and PCL gelatine have been shown to enhance the adhesion of human umbilical vein ECs (HUVECs) to the lumen, which adds further support for favourable vascular cell signalling provided by collagen sequences.114 ...
Surface modification and endothelialization of polyurethane for vascular tissue engineering applications: a review
1
2016
... Notably, increasingly complex surface coatings have been studied for their ability to promote endothelialisation through significant biological signalling while simultaneously having low thrombogenicity. Wang et al.104 showed that functional vascular grafts with in-situ catalysis of nitric oxide release based on enzyme prodrug therapy are helpful for localised and on-demand nitric oxide release. This approach illustrates the effective inhibition of thrombus formation in vivo as well as the enhancement of vascular tissue regeneration and remodelling on the grafts, which may be useful for the development of novel cell-free vascular grafts in treatment of vascular disease. Surface modification of polyurethane with hydrophilic molecules improves haemocompatibility; however, this may limit initial vascular EC adhesion.115 The protein Dickkopf-3 plays key roles in inducing the directional migration of vascular progenitor cells and promoting tissue regeneration. Issa Bhaloo et al.116 proposed the construction of artificial blood vessels with the function of delivering Dickkopf-3. In addition, it has been demonstrated that histone deacetylase 7 mRNA in vascular progenitor cells can be selectively translated to produce a polypeptide containing seven amino acids. This polypeptide induces the migration and differentiation of vascular progenitor cells into vascular ECs, thereby effectively promoting the endothelialisation of artificial blood vessels.117 To date, various small animal model experiments have been conducted to improve endothelialisation on artificial vascular grafts with specific peptides and biomolecules, which has shown some potential; however, further development in larger preclinical models is lacking. ...
Binding of Dickkopf-3 to CXCR7 enhances vascular progenitor cell migration and degradable graft regeneration
1
2018
... Notably, increasingly complex surface coatings have been studied for their ability to promote endothelialisation through significant biological signalling while simultaneously having low thrombogenicity. Wang et al.104 showed that functional vascular grafts with in-situ catalysis of nitric oxide release based on enzyme prodrug therapy are helpful for localised and on-demand nitric oxide release. This approach illustrates the effective inhibition of thrombus formation in vivo as well as the enhancement of vascular tissue regeneration and remodelling on the grafts, which may be useful for the development of novel cell-free vascular grafts in treatment of vascular disease. Surface modification of polyurethane with hydrophilic molecules improves haemocompatibility; however, this may limit initial vascular EC adhesion.115 The protein Dickkopf-3 plays key roles in inducing the directional migration of vascular progenitor cells and promoting tissue regeneration. Issa Bhaloo et al.116 proposed the construction of artificial blood vessels with the function of delivering Dickkopf-3. In addition, it has been demonstrated that histone deacetylase 7 mRNA in vascular progenitor cells can be selectively translated to produce a polypeptide containing seven amino acids. This polypeptide induces the migration and differentiation of vascular progenitor cells into vascular ECs, thereby effectively promoting the endothelialisation of artificial blood vessels.117 To date, various small animal model experiments have been conducted to improve endothelialisation on artificial vascular grafts with specific peptides and biomolecules, which has shown some potential; however, further development in larger preclinical models is lacking. ...
Histone deacetylase 7-derived peptides play a vital role in vascular repair and regeneration
1
2018
... Notably, increasingly complex surface coatings have been studied for their ability to promote endothelialisation through significant biological signalling while simultaneously having low thrombogenicity. Wang et al.104 showed that functional vascular grafts with in-situ catalysis of nitric oxide release based on enzyme prodrug therapy are helpful for localised and on-demand nitric oxide release. This approach illustrates the effective inhibition of thrombus formation in vivo as well as the enhancement of vascular tissue regeneration and remodelling on the grafts, which may be useful for the development of novel cell-free vascular grafts in treatment of vascular disease. Surface modification of polyurethane with hydrophilic molecules improves haemocompatibility; however, this may limit initial vascular EC adhesion.115 The protein Dickkopf-3 plays key roles in inducing the directional migration of vascular progenitor cells and promoting tissue regeneration. Issa Bhaloo et al.116 proposed the construction of artificial blood vessels with the function of delivering Dickkopf-3. In addition, it has been demonstrated that histone deacetylase 7 mRNA in vascular progenitor cells can be selectively translated to produce a polypeptide containing seven amino acids. This polypeptide induces the migration and differentiation of vascular progenitor cells into vascular ECs, thereby effectively promoting the endothelialisation of artificial blood vessels.117 To date, various small animal model experiments have been conducted to improve endothelialisation on artificial vascular grafts with specific peptides and biomolecules, which has shown some potential; however, further development in larger preclinical models is lacking. ...
Mechanical properties of biodegradable small-diameter chitosan artificial vascular prosthesis
3
2012
... The mechanical performance of artificial blood vessels is an important design requirement to ensure that they can withstand a certain pressure generated by circulating blood flowing in the blood vessel. The most fundamental performance criteria include adequate mechanical strength to retain structural integrity and resist permanent deformation. The compliance of the graft and the manner in which it deforms under pressure are also important, as adverse biological responses are associated with a compliance mismatch between native and prosthetic vascular grafts. In terms of mechanical considerations, the core issue lies in achieving high strength while retaining compliance. Tensile strength results indicated that a nanofibre-oriented structure offers a higher elastic modulus than a non-oriented structure. Likewise, other properties including tensile strength, elongation at breakage, and burst pressure were also found to be significantly enhanced by an oriented alignment of nanofibres. Consequently, the oriented structure of vascular grafts may very much enhance their mechanical properties.46 An ideal artificial blood vessel should also have appropriate micropores, which can be represented by water permeability. The size and porosity of the micropores have an effect on cell culture.118 Table 3 shows several mechanical properties of natural arteries and various common artificial blood vessels.119 ...
... Mechanical properties of natural vessels and some artificial blood vessels.
| Burst pressure (mmHg) | Compliance (%/100 mmHg) |
| Values for artificial blood vessels12, 91 | >1000 | 10-20 |
| Dog femoral artery118 | 2895 ± 263 | 10.3 ± 2.3 |
| Human internal mammary artery119 | 3196 ± 1264 | 11.5 ± 3.9 |
Biodegradable chitosan vascular grafts
(with 4 mm inner diameter)118 | 1688 ± 236 | 5.7 ± 1.3 |
| Tissue-engineered blood vessels13 | 3490 ± 892 | 3.4 ± 1.6 |
| Expanded polytetrafluoroethylene grafts119 | – | 0.51 |
| Silk fibroin grafts119 | – | 1.90 |
Another consideration is individual variability, signifying the variation in the mechanical properties of blood vessels from vessel to vessel and between individuals. In future, vascular tissue engineering may ultimately involve tailoring the specific mechanical properties of a graft to the intended implantation site. A far greater understanding of vascular biomechanics, interactions between the graft and the native vessels, and how to engineer the mechanical properties of a vascular graft would be required. ...
...
118 1688 ± 236 | 5.7 ± 1.3 | | Tissue-engineered blood vessels13 | 3490 ± 892 | 3.4 ± 1.6 |
| Expanded polytetrafluoroethylene grafts119 | – | 0.51 |
| Silk fibroin grafts119 | – | 1.90 |
Another consideration is individual variability, signifying the variation in the mechanical properties of blood vessels from vessel to vessel and between individuals. In future, vascular tissue engineering may ultimately involve tailoring the specific mechanical properties of a graft to the intended implantation site. A far greater understanding of vascular biomechanics, interactions between the graft and the native vessels, and how to engineer the mechanical properties of a vascular graft would be required. ...
Small-diameter silk vascular grafts (3 mm diameter) with a double-raschel knitted silk tube coated with silk fibroin sponge
6
2013
... The mechanical performance of artificial blood vessels is an important design requirement to ensure that they can withstand a certain pressure generated by circulating blood flowing in the blood vessel. The most fundamental performance criteria include adequate mechanical strength to retain structural integrity and resist permanent deformation. The compliance of the graft and the manner in which it deforms under pressure are also important, as adverse biological responses are associated with a compliance mismatch between native and prosthetic vascular grafts. In terms of mechanical considerations, the core issue lies in achieving high strength while retaining compliance. Tensile strength results indicated that a nanofibre-oriented structure offers a higher elastic modulus than a non-oriented structure. Likewise, other properties including tensile strength, elongation at breakage, and burst pressure were also found to be significantly enhanced by an oriented alignment of nanofibres. Consequently, the oriented structure of vascular grafts may very much enhance their mechanical properties.46 An ideal artificial blood vessel should also have appropriate micropores, which can be represented by water permeability. The size and porosity of the micropores have an effect on cell culture.118 Table 3 shows several mechanical properties of natural arteries and various common artificial blood vessels.119 ...
... Mechanical properties of natural vessels and some artificial blood vessels.
| Burst pressure (mmHg) | Compliance (%/100 mmHg) |
| Values for artificial blood vessels12, 91 | >1000 | 10-20 |
| Dog femoral artery118 | 2895 ± 263 | 10.3 ± 2.3 |
| Human internal mammary artery119 | 3196 ± 1264 | 11.5 ± 3.9 |
Biodegradable chitosan vascular grafts
(with 4 mm inner diameter)118 | 1688 ± 236 | 5.7 ± 1.3 |
| Tissue-engineered blood vessels13 | 3490 ± 892 | 3.4 ± 1.6 |
| Expanded polytetrafluoroethylene grafts119 | – | 0.51 |
| Silk fibroin grafts119 | – | 1.90 |
Another consideration is individual variability, signifying the variation in the mechanical properties of blood vessels from vessel to vessel and between individuals. In future, vascular tissue engineering may ultimately involve tailoring the specific mechanical properties of a graft to the intended implantation site. A far greater understanding of vascular biomechanics, interactions between the graft and the native vessels, and how to engineer the mechanical properties of a vascular graft would be required. ...
...
119 – | 0.51 | | Silk fibroin grafts119 | – | 1.90 |
Another consideration is individual variability, signifying the variation in the mechanical properties of blood vessels from vessel to vessel and between individuals. In future, vascular tissue engineering may ultimately involve tailoring the specific mechanical properties of a graft to the intended implantation site. A far greater understanding of vascular biomechanics, interactions between the graft and the native vessels, and how to engineer the mechanical properties of a vascular graft would be required. ...
...
119 – | 1.90 | Another consideration is individual variability, signifying the variation in the mechanical properties of blood vessels from vessel to vessel and between individuals. In future, vascular tissue engineering may ultimately involve tailoring the specific mechanical properties of a graft to the intended implantation site. A far greater understanding of vascular biomechanics, interactions between the graft and the native vessels, and how to engineer the mechanical properties of a vascular graft would be required. ...
... Common animal models for evaluation of artificial blood vessels
in vivo.
| Animal model | Characteristics | Application inner diameter (mm) | Common length (mm) | Longest implantation period | Implantation site | References |
| Sheep | Similar cardiovascular physiology, endothelialisation mechanisms and thrombogenicity mechanisms to humans.
Suitable size and long-term studies possible.Higher incidence of hypercoagulability. | 4–6 | 80–100 | 9 months | Carotid artery | 48, 74 |
| 4–6 | 60–100 | 3 months | Arteriovenous graft | 129 |
| Pig | Similar vascular physiology and anatomy to humans.
Well established as a model for assessing vascular grafts.
Mount an extensive immune response to implanted tissues. | 3–6 | 30–100 | 6 months | Iliac artery | 130 |
| 3–6 | 10–100 | 4 weeks | Carotid artery | 52 |
| Dog | Lack of spontaneous endothelialisation and immune response restricts study lengths.
Ease of accessing vessel due to thin skin.
Thrombogenicity mechanisms and vessel viscoelastic properties differ from humans. | 3–6 | 30–50 | 6 months | Abdominal artery | 131 |
| 3–6 | 30–50 | 1 year | Carotid artery | 119 |
| Baboon | Physiology and cardiovascular anatomy are the most similar to humans.
Suitable for a wide range of non-invasive imaging techniques adapted from humans.
High cost and ethical concerns associated with using primates in medical research. | 3–6 | 30–50 | 6 months | Arteriovenous graft | 26 |
| Rabbit | Similar endothelialisation rates and thrombogenicity mechanisms to humans.
Demanding higher anticoagulant function.
Limited to short-term studies. | 1–4 | 5–30 | 12 months | Carotid artery | 94, 132 |
| 1–4 | 5–30 | 2 weeks | Femoral artery | 133 |
| 1–4 | 5–30 | 3 months | Abdominal artery | 134 |
| Rat | Large sample size.
Wide variety of transgenic lines.
Allows exploration of genetic/molecular mechanisms.
Ideal for biocompatibility and cell infiltration studies. | 1–3 | 5–30 | 18 months | Abdominal artery | 73, 135 |
| 1–3 | 5–10 | 12 weeks | Carotid artery | 136 |
| Mouse | 0.5–1 | 3–10 | 6 months | Carotid artery | 137 |
Rats and mice are widely used because they are inexpensive to acquire, easy to maintain, and can be used to evaluate larger sample sizes.135-137 Furthermore, the availability of various transgenic strains makes it possible to examine the molecular mechanisms involved in graft remodelling.138 However, differences in their circulatory system with respect to humans limit their utility in short-term studies to only very small grafts (< 2 mm diameter). Rabbits are also suitable for artificial blood vessel assessments although they are limited to short-term studies.132-134 Their physiology is similar to that of humans in terms of endothelialisation rates and thrombogenicity, and they can accept clinically-relevant graft diameters (1–4 mm). Dogs differ from humans in terms of thrombogenicity and represent a more stringent model for the investigations because of their lack of spontaneous endothelialisation.119, 131 Moreover, they can provide a range of implantation site options with varied vessel sizes, although their immune response can restrict study durations. Both pigs and sheep demonstrate similarities to human physiology and they are appropriate for studying thrombogenicity, calcification, and long-term graft patency.129, 130 The cardiovascular physiology of non-human primates, such as baboons, is most similar to that of humans compared to dogs, pigs or sheep. Their structural similarity allows for various imaging techniques and testing methods that are typically applied in humans to be used on them. Despite such advantages, they are rarely used for vascular graft evaluation due to their high cost and ethical concerns.81, 91 The optimal model for studying all of the performance criteria associated with artificial vascular grafts has yet to be elucidated. An immunodeficient pig model, the operational immunodeficient pig, has been reported to be a large animal model that allows for long-term accommodation of artificial human vascular grafts to properly evaluate the clinical efficacy of artificial vascular grafts.139 ...
... Rats and mice are widely used because they are inexpensive to acquire, easy to maintain, and can be used to evaluate larger sample sizes.135-137 Furthermore, the availability of various transgenic strains makes it possible to examine the molecular mechanisms involved in graft remodelling.138 However, differences in their circulatory system with respect to humans limit their utility in short-term studies to only very small grafts (< 2 mm diameter). Rabbits are also suitable for artificial blood vessel assessments although they are limited to short-term studies.132-134 Their physiology is similar to that of humans in terms of endothelialisation rates and thrombogenicity, and they can accept clinically-relevant graft diameters (1–4 mm). Dogs differ from humans in terms of thrombogenicity and represent a more stringent model for the investigations because of their lack of spontaneous endothelialisation.119, 131 Moreover, they can provide a range of implantation site options with varied vessel sizes, although their immune response can restrict study durations. Both pigs and sheep demonstrate similarities to human physiology and they are appropriate for studying thrombogenicity, calcification, and long-term graft patency.129, 130 The cardiovascular physiology of non-human primates, such as baboons, is most similar to that of humans compared to dogs, pigs or sheep. Their structural similarity allows for various imaging techniques and testing methods that are typically applied in humans to be used on them. Despite such advantages, they are rarely used for vascular graft evaluation due to their high cost and ethical concerns.81, 91 The optimal model for studying all of the performance criteria associated with artificial vascular grafts has yet to be elucidated. An immunodeficient pig model, the operational immunodeficient pig, has been reported to be a large animal model that allows for long-term accommodation of artificial human vascular grafts to properly evaluate the clinical efficacy of artificial vascular grafts.139 ...
Silk constructs for delivery of musculoskeletal therapeutics
1
2012
... Biocompatibility of biomaterials is also the most basic property that should be considered when designing viable grafts, ensuring that the material has good haemocompatibility and a low likelihood of immune rejection due to problems such as allergic reactions. Several studies have shown that properly degummed and sterilised silk products have better biocompatibility than virgin silk.120 They support cell attachment, migration, proliferation, differentiation, cell-cell interactions and have the ability to promote tissue repair. ECs are derived from their progenitor cells, which are characterised by the expression of CD133.121 Biocompatibility can be evaluated by culturing HUVECs in contact with biomaterials for a period of time and observing platelet adhesion under a scanning electron microscope. Moreover, when evaluating haemocompatibility, plasma re-calcification can be used as a measure of contact phase activation and the efficacy of heparinisation following blood contact with biomaterials.122 The adhesion morphology of HUVECs can be observed by electron microscopy and performing qualitative detection of HUVECs.123 Evaluation of biocompatibility involves determining whether EPCs can proliferate well in vitro when designing tissue-engineered blood vessels. Contemporary approaches rely on in situ endothelialisation of vascular grafts, which provides an optimal native haemocompatible surface and reduces rapid failure from blood clotting; however, ensuring the biocompatibility of biomaterials remains a primary concern for tissue engineers. ...
Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors
1
2000
... Biocompatibility of biomaterials is also the most basic property that should be considered when designing viable grafts, ensuring that the material has good haemocompatibility and a low likelihood of immune rejection due to problems such as allergic reactions. Several studies have shown that properly degummed and sterilised silk products have better biocompatibility than virgin silk.120 They support cell attachment, migration, proliferation, differentiation, cell-cell interactions and have the ability to promote tissue repair. ECs are derived from their progenitor cells, which are characterised by the expression of CD133.121 Biocompatibility can be evaluated by culturing HUVECs in contact with biomaterials for a period of time and observing platelet adhesion under a scanning electron microscope. Moreover, when evaluating haemocompatibility, plasma re-calcification can be used as a measure of contact phase activation and the efficacy of heparinisation following blood contact with biomaterials.122 The adhesion morphology of HUVECs can be observed by electron microscopy and performing qualitative detection of HUVECs.123 Evaluation of biocompatibility involves determining whether EPCs can proliferate well in vitro when designing tissue-engineered blood vessels. Contemporary approaches rely on in situ endothelialisation of vascular grafts, which provides an optimal native haemocompatible surface and reduces rapid failure from blood clotting; however, ensuring the biocompatibility of biomaterials remains a primary concern for tissue engineers. ...
Plasma recalcification as a measure of contact phase activation and heparinization efficacy after contact with biomaterials
1
1994
... Biocompatibility of biomaterials is also the most basic property that should be considered when designing viable grafts, ensuring that the material has good haemocompatibility and a low likelihood of immune rejection due to problems such as allergic reactions. Several studies have shown that properly degummed and sterilised silk products have better biocompatibility than virgin silk.120 They support cell attachment, migration, proliferation, differentiation, cell-cell interactions and have the ability to promote tissue repair. ECs are derived from their progenitor cells, which are characterised by the expression of CD133.121 Biocompatibility can be evaluated by culturing HUVECs in contact with biomaterials for a period of time and observing platelet adhesion under a scanning electron microscope. Moreover, when evaluating haemocompatibility, plasma re-calcification can be used as a measure of contact phase activation and the efficacy of heparinisation following blood contact with biomaterials.122 The adhesion morphology of HUVECs can be observed by electron microscopy and performing qualitative detection of HUVECs.123 Evaluation of biocompatibility involves determining whether EPCs can proliferate well in vitro when designing tissue-engineered blood vessels. Contemporary approaches rely on in situ endothelialisation of vascular grafts, which provides an optimal native haemocompatible surface and reduces rapid failure from blood clotting; however, ensuring the biocompatibility of biomaterials remains a primary concern for tissue engineers. ...
Evaluation of a simple off-the-shelf bi-layered vascular scaffold based on poly(L-lactide-co-ε-caprolactone)/silk fibroin in vitro and in vivo
3
2019
... Biocompatibility of biomaterials is also the most basic property that should be considered when designing viable grafts, ensuring that the material has good haemocompatibility and a low likelihood of immune rejection due to problems such as allergic reactions. Several studies have shown that properly degummed and sterilised silk products have better biocompatibility than virgin silk.120 They support cell attachment, migration, proliferation, differentiation, cell-cell interactions and have the ability to promote tissue repair. ECs are derived from their progenitor cells, which are characterised by the expression of CD133.121 Biocompatibility can be evaluated by culturing HUVECs in contact with biomaterials for a period of time and observing platelet adhesion under a scanning electron microscope. Moreover, when evaluating haemocompatibility, plasma re-calcification can be used as a measure of contact phase activation and the efficacy of heparinisation following blood contact with biomaterials.122 The adhesion morphology of HUVECs can be observed by electron microscopy and performing qualitative detection of HUVECs.123 Evaluation of biocompatibility involves determining whether EPCs can proliferate well in vitro when designing tissue-engineered blood vessels. Contemporary approaches rely on in situ endothelialisation of vascular grafts, which provides an optimal native haemocompatible surface and reduces rapid failure from blood clotting; however, ensuring the biocompatibility of biomaterials remains a primary concern for tissue engineers. ...
... High-resolution ultrasound may be used in order to evaluate the efficacy of a graft, such as the flatness of the mouth, the vascular lumen, velocity of the blood flow in the lumen, and spectral waveform of the artificial blood vessel grafts after implantation, which can also eventually be used to assess vascular patency following implantation by contrasting with the ultrasound image taken before surgery.140 Additionally, micro-computed tomographic angiography may also be used to evaluate the patency of grafts and the specific situation of artificial blood vessels in vivo. Immunofluorescence staining to evaluate the expression of specific molecules, such as CD31 and α-smooth muscle actin, can be used to verify biomimetic remodelling of endothelial and smooth muscle layers.123 Common evaluation methods, including tissue section staining, are shown in Figure 4. ...
... Materials are the basis of artificial blood vessels and also directly affect their performance. The best way to mix synthetic polymers and natural materials so as to maximise their advantages should be highlighted in further study. 123, 146, 147 The selection of appropriate materials will help to improve the requisite tissue mechanics, providing good biocompatibility, anti-thrombogenicity and long-term patency rate of blood vessels. A number of people have developed methods to improve materials, mainly by synthesising new materials, combining multiple materials together, improving the properties of materials through chemical modification and using decellularised heterogeneous blood vessels. Currently, many promising structures are multicomponent systems that have been created through the addition of natural surface coatings to basic synthetic materials. Grafts with three or more components are also increasingly common (e.g., heparin/silk/poly(L-lactide-co-caprolactone). Future development trends may include changing from synthetic materials to natural materials, or even hybrid materials, in order to produce better biocompatibility and improve the long-term patency rate. ...
Endothelialization and patency of RGD-functionalized vascular grafts in a rabbit carotid artery model
1
2012
... In order to meet the demand to reasonably test the performance of various types of artificial blood vessels, it is necessary to establish a viable and stable animal model. Transplanting and facilitating postoperative observations may be easily performed if the appropriate animal models exist, which are able to simulate applications in the environment of artificial blood vessels. Currently, commonly-used vascular in vivo animal models are pigs, sheep, dogs, rabbits, and rats.124-126 The carotid, abdominal, and femoral arteries in these animal models are the most chosen surgical sites. Moreover, different options are available based on the animals’ size, according to the calibre of the blood vessel. Rat abdominal aortic replacement and rabbit carotid replacement are viable animal models for in vivo validation of small-calibre vessels. In addition, specific criteria, such as implantability, mechanical performance, biocompatibility, thrombogenicity, and haemodynamics, must be considered.127, 128 Achieving balance with model-specific factors should also be taken into consideration, such as animal availability, ease of handling, ease of performing the implantation surgery, study duration, compatible methods of graft evaluation, and cost. The characteristics of common animal models are summarised in Table 4.129-137 ...
Appropriate density of PCL nano-fiber sheath promoted muscular remodeling of PGS/PCL grafts in arterial circulation
0
2016
Tissue engineering of acellular vascular grafts capable of somatic growth in young lambs
1
2016
... In order to meet the demand to reasonably test the performance of various types of artificial blood vessels, it is necessary to establish a viable and stable animal model. Transplanting and facilitating postoperative observations may be easily performed if the appropriate animal models exist, which are able to simulate applications in the environment of artificial blood vessels. Currently, commonly-used vascular in vivo animal models are pigs, sheep, dogs, rabbits, and rats.124-126 The carotid, abdominal, and femoral arteries in these animal models are the most chosen surgical sites. Moreover, different options are available based on the animals’ size, according to the calibre of the blood vessel. Rat abdominal aortic replacement and rabbit carotid replacement are viable animal models for in vivo validation of small-calibre vessels. In addition, specific criteria, such as implantability, mechanical performance, biocompatibility, thrombogenicity, and haemodynamics, must be considered.127, 128 Achieving balance with model-specific factors should also be taken into consideration, such as animal availability, ease of handling, ease of performing the implantation surgery, study duration, compatible methods of graft evaluation, and cost. The characteristics of common animal models are summarised in Table 4.129-137 ...
Animal models for vascular tissue-engineering
1
2013
... In order to meet the demand to reasonably test the performance of various types of artificial blood vessels, it is necessary to establish a viable and stable animal model. Transplanting and facilitating postoperative observations may be easily performed if the appropriate animal models exist, which are able to simulate applications in the environment of artificial blood vessels. Currently, commonly-used vascular in vivo animal models are pigs, sheep, dogs, rabbits, and rats.124-126 The carotid, abdominal, and femoral arteries in these animal models are the most chosen surgical sites. Moreover, different options are available based on the animals’ size, according to the calibre of the blood vessel. Rat abdominal aortic replacement and rabbit carotid replacement are viable animal models for in vivo validation of small-calibre vessels. In addition, specific criteria, such as implantability, mechanical performance, biocompatibility, thrombogenicity, and haemodynamics, must be considered.127, 128 Achieving balance with model-specific factors should also be taken into consideration, such as animal availability, ease of handling, ease of performing the implantation surgery, study duration, compatible methods of graft evaluation, and cost. The characteristics of common animal models are summarised in Table 4.129-137 ...
Animal models for the assessment of novel vascular conduits
1
2010
... In order to meet the demand to reasonably test the performance of various types of artificial blood vessels, it is necessary to establish a viable and stable animal model. Transplanting and facilitating postoperative observations may be easily performed if the appropriate animal models exist, which are able to simulate applications in the environment of artificial blood vessels. Currently, commonly-used vascular in vivo animal models are pigs, sheep, dogs, rabbits, and rats.124-126 The carotid, abdominal, and femoral arteries in these animal models are the most chosen surgical sites. Moreover, different options are available based on the animals’ size, according to the calibre of the blood vessel. Rat abdominal aortic replacement and rabbit carotid replacement are viable animal models for in vivo validation of small-calibre vessels. In addition, specific criteria, such as implantability, mechanical performance, biocompatibility, thrombogenicity, and haemodynamics, must be considered.127, 128 Achieving balance with model-specific factors should also be taken into consideration, such as animal availability, ease of handling, ease of performing the implantation surgery, study duration, compatible methods of graft evaluation, and cost. The characteristics of common animal models are summarised in Table 4.129-137 ...
A novel hybrid silk fibroin/polyurethane arteriovenous graft for hemodialysis: proof-of-concept animal study in an ovine model
3
2020
... In order to meet the demand to reasonably test the performance of various types of artificial blood vessels, it is necessary to establish a viable and stable animal model. Transplanting and facilitating postoperative observations may be easily performed if the appropriate animal models exist, which are able to simulate applications in the environment of artificial blood vessels. Currently, commonly-used vascular in vivo animal models are pigs, sheep, dogs, rabbits, and rats.124-126 The carotid, abdominal, and femoral arteries in these animal models are the most chosen surgical sites. Moreover, different options are available based on the animals’ size, according to the calibre of the blood vessel. Rat abdominal aortic replacement and rabbit carotid replacement are viable animal models for in vivo validation of small-calibre vessels. In addition, specific criteria, such as implantability, mechanical performance, biocompatibility, thrombogenicity, and haemodynamics, must be considered.127, 128 Achieving balance with model-specific factors should also be taken into consideration, such as animal availability, ease of handling, ease of performing the implantation surgery, study duration, compatible methods of graft evaluation, and cost. The characteristics of common animal models are summarised in Table 4.129-137 ...
... Common animal models for evaluation of artificial blood vessels
in vivo.
| Animal model | Characteristics | Application inner diameter (mm) | Common length (mm) | Longest implantation period | Implantation site | References |
| Sheep | Similar cardiovascular physiology, endothelialisation mechanisms and thrombogenicity mechanisms to humans.
Suitable size and long-term studies possible.Higher incidence of hypercoagulability. | 4–6 | 80–100 | 9 months | Carotid artery | 48, 74 |
| 4–6 | 60–100 | 3 months | Arteriovenous graft | 129 |
| Pig | Similar vascular physiology and anatomy to humans.
Well established as a model for assessing vascular grafts.
Mount an extensive immune response to implanted tissues. | 3–6 | 30–100 | 6 months | Iliac artery | 130 |
| 3–6 | 10–100 | 4 weeks | Carotid artery | 52 |
| Dog | Lack of spontaneous endothelialisation and immune response restricts study lengths.
Ease of accessing vessel due to thin skin.
Thrombogenicity mechanisms and vessel viscoelastic properties differ from humans. | 3–6 | 30–50 | 6 months | Abdominal artery | 131 |
| 3–6 | 30–50 | 1 year | Carotid artery | 119 |
| Baboon | Physiology and cardiovascular anatomy are the most similar to humans.
Suitable for a wide range of non-invasive imaging techniques adapted from humans.
High cost and ethical concerns associated with using primates in medical research. | 3–6 | 30–50 | 6 months | Arteriovenous graft | 26 |
| Rabbit | Similar endothelialisation rates and thrombogenicity mechanisms to humans.
Demanding higher anticoagulant function.
Limited to short-term studies. | 1–4 | 5–30 | 12 months | Carotid artery | 94, 132 |
| 1–4 | 5–30 | 2 weeks | Femoral artery | 133 |
| 1–4 | 5–30 | 3 months | Abdominal artery | 134 |
| Rat | Large sample size.
Wide variety of transgenic lines.
Allows exploration of genetic/molecular mechanisms.
Ideal for biocompatibility and cell infiltration studies. | 1–3 | 5–30 | 18 months | Abdominal artery | 73, 135 |
| 1–3 | 5–10 | 12 weeks | Carotid artery | 136 |
| Mouse | 0.5–1 | 3–10 | 6 months | Carotid artery | 137 |
Rats and mice are widely used because they are inexpensive to acquire, easy to maintain, and can be used to evaluate larger sample sizes.135-137 Furthermore, the availability of various transgenic strains makes it possible to examine the molecular mechanisms involved in graft remodelling.138 However, differences in their circulatory system with respect to humans limit their utility in short-term studies to only very small grafts (< 2 mm diameter). Rabbits are also suitable for artificial blood vessel assessments although they are limited to short-term studies.132-134 Their physiology is similar to that of humans in terms of endothelialisation rates and thrombogenicity, and they can accept clinically-relevant graft diameters (1–4 mm). Dogs differ from humans in terms of thrombogenicity and represent a more stringent model for the investigations because of their lack of spontaneous endothelialisation.119, 131 Moreover, they can provide a range of implantation site options with varied vessel sizes, although their immune response can restrict study durations. Both pigs and sheep demonstrate similarities to human physiology and they are appropriate for studying thrombogenicity, calcification, and long-term graft patency.129, 130 The cardiovascular physiology of non-human primates, such as baboons, is most similar to that of humans compared to dogs, pigs or sheep. Their structural similarity allows for various imaging techniques and testing methods that are typically applied in humans to be used on them. Despite such advantages, they are rarely used for vascular graft evaluation due to their high cost and ethical concerns.81, 91 The optimal model for studying all of the performance criteria associated with artificial vascular grafts has yet to be elucidated. An immunodeficient pig model, the operational immunodeficient pig, has been reported to be a large animal model that allows for long-term accommodation of artificial human vascular grafts to properly evaluate the clinical efficacy of artificial vascular grafts.139 ...
... Rats and mice are widely used because they are inexpensive to acquire, easy to maintain, and can be used to evaluate larger sample sizes.135-137 Furthermore, the availability of various transgenic strains makes it possible to examine the molecular mechanisms involved in graft remodelling.138 However, differences in their circulatory system with respect to humans limit their utility in short-term studies to only very small grafts (< 2 mm diameter). Rabbits are also suitable for artificial blood vessel assessments although they are limited to short-term studies.132-134 Their physiology is similar to that of humans in terms of endothelialisation rates and thrombogenicity, and they can accept clinically-relevant graft diameters (1–4 mm). Dogs differ from humans in terms of thrombogenicity and represent a more stringent model for the investigations because of their lack of spontaneous endothelialisation.119, 131 Moreover, they can provide a range of implantation site options with varied vessel sizes, although their immune response can restrict study durations. Both pigs and sheep demonstrate similarities to human physiology and they are appropriate for studying thrombogenicity, calcification, and long-term graft patency.129, 130 The cardiovascular physiology of non-human primates, such as baboons, is most similar to that of humans compared to dogs, pigs or sheep. Their structural similarity allows for various imaging techniques and testing methods that are typically applied in humans to be used on them. Despite such advantages, they are rarely used for vascular graft evaluation due to their high cost and ethical concerns.81, 91 The optimal model for studying all of the performance criteria associated with artificial vascular grafts has yet to be elucidated. An immunodeficient pig model, the operational immunodeficient pig, has been reported to be a large animal model that allows for long-term accommodation of artificial human vascular grafts to properly evaluate the clinical efficacy of artificial vascular grafts.139 ...
Vascular replacement using a layered elastin-collagen vascular graft in a porcine model: one week patency versus one month occlusion
2
2015
... Common animal models for evaluation of artificial blood vessels
in vivo.
| Animal model | Characteristics | Application inner diameter (mm) | Common length (mm) | Longest implantation period | Implantation site | References |
| Sheep | Similar cardiovascular physiology, endothelialisation mechanisms and thrombogenicity mechanisms to humans.
Suitable size and long-term studies possible.Higher incidence of hypercoagulability. | 4–6 | 80–100 | 9 months | Carotid artery | 48, 74 |
| 4–6 | 60–100 | 3 months | Arteriovenous graft | 129 |
| Pig | Similar vascular physiology and anatomy to humans.
Well established as a model for assessing vascular grafts.
Mount an extensive immune response to implanted tissues. | 3–6 | 30–100 | 6 months | Iliac artery | 130 |
| 3–6 | 10–100 | 4 weeks | Carotid artery | 52 |
| Dog | Lack of spontaneous endothelialisation and immune response restricts study lengths.
Ease of accessing vessel due to thin skin.
Thrombogenicity mechanisms and vessel viscoelastic properties differ from humans. | 3–6 | 30–50 | 6 months | Abdominal artery | 131 |
| 3–6 | 30–50 | 1 year | Carotid artery | 119 |
| Baboon | Physiology and cardiovascular anatomy are the most similar to humans.
Suitable for a wide range of non-invasive imaging techniques adapted from humans.
High cost and ethical concerns associated with using primates in medical research. | 3–6 | 30–50 | 6 months | Arteriovenous graft | 26 |
| Rabbit | Similar endothelialisation rates and thrombogenicity mechanisms to humans.
Demanding higher anticoagulant function.
Limited to short-term studies. | 1–4 | 5–30 | 12 months | Carotid artery | 94, 132 |
| 1–4 | 5–30 | 2 weeks | Femoral artery | 133 |
| 1–4 | 5–30 | 3 months | Abdominal artery | 134 |
| Rat | Large sample size.
Wide variety of transgenic lines.
Allows exploration of genetic/molecular mechanisms.
Ideal for biocompatibility and cell infiltration studies. | 1–3 | 5–30 | 18 months | Abdominal artery | 73, 135 |
| 1–3 | 5–10 | 12 weeks | Carotid artery | 136 |
| Mouse | 0.5–1 | 3–10 | 6 months | Carotid artery | 137 |
Rats and mice are widely used because they are inexpensive to acquire, easy to maintain, and can be used to evaluate larger sample sizes.135-137 Furthermore, the availability of various transgenic strains makes it possible to examine the molecular mechanisms involved in graft remodelling.138 However, differences in their circulatory system with respect to humans limit their utility in short-term studies to only very small grafts (< 2 mm diameter). Rabbits are also suitable for artificial blood vessel assessments although they are limited to short-term studies.132-134 Their physiology is similar to that of humans in terms of endothelialisation rates and thrombogenicity, and they can accept clinically-relevant graft diameters (1–4 mm). Dogs differ from humans in terms of thrombogenicity and represent a more stringent model for the investigations because of their lack of spontaneous endothelialisation.119, 131 Moreover, they can provide a range of implantation site options with varied vessel sizes, although their immune response can restrict study durations. Both pigs and sheep demonstrate similarities to human physiology and they are appropriate for studying thrombogenicity, calcification, and long-term graft patency.129, 130 The cardiovascular physiology of non-human primates, such as baboons, is most similar to that of humans compared to dogs, pigs or sheep. Their structural similarity allows for various imaging techniques and testing methods that are typically applied in humans to be used on them. Despite such advantages, they are rarely used for vascular graft evaluation due to their high cost and ethical concerns.81, 91 The optimal model for studying all of the performance criteria associated with artificial vascular grafts has yet to be elucidated. An immunodeficient pig model, the operational immunodeficient pig, has been reported to be a large animal model that allows for long-term accommodation of artificial human vascular grafts to properly evaluate the clinical efficacy of artificial vascular grafts.139 ...
... Rats and mice are widely used because they are inexpensive to acquire, easy to maintain, and can be used to evaluate larger sample sizes.135-137 Furthermore, the availability of various transgenic strains makes it possible to examine the molecular mechanisms involved in graft remodelling.138 However, differences in their circulatory system with respect to humans limit their utility in short-term studies to only very small grafts (< 2 mm diameter). Rabbits are also suitable for artificial blood vessel assessments although they are limited to short-term studies.132-134 Their physiology is similar to that of humans in terms of endothelialisation rates and thrombogenicity, and they can accept clinically-relevant graft diameters (1–4 mm). Dogs differ from humans in terms of thrombogenicity and represent a more stringent model for the investigations because of their lack of spontaneous endothelialisation.119, 131 Moreover, they can provide a range of implantation site options with varied vessel sizes, although their immune response can restrict study durations. Both pigs and sheep demonstrate similarities to human physiology and they are appropriate for studying thrombogenicity, calcification, and long-term graft patency.129, 130 The cardiovascular physiology of non-human primates, such as baboons, is most similar to that of humans compared to dogs, pigs or sheep. Their structural similarity allows for various imaging techniques and testing methods that are typically applied in humans to be used on them. Despite such advantages, they are rarely used for vascular graft evaluation due to their high cost and ethical concerns.81, 91 The optimal model for studying all of the performance criteria associated with artificial vascular grafts has yet to be elucidated. An immunodeficient pig model, the operational immunodeficient pig, has been reported to be a large animal model that allows for long-term accommodation of artificial human vascular grafts to properly evaluate the clinical efficacy of artificial vascular grafts.139 ...
Rapid endothelialization and thin luminal layers in vascular grafts using silk fibroin
2
2016
... Common animal models for evaluation of artificial blood vessels
in vivo.
| Animal model | Characteristics | Application inner diameter (mm) | Common length (mm) | Longest implantation period | Implantation site | References |
| Sheep | Similar cardiovascular physiology, endothelialisation mechanisms and thrombogenicity mechanisms to humans.
Suitable size and long-term studies possible.Higher incidence of hypercoagulability. | 4–6 | 80–100 | 9 months | Carotid artery | 48, 74 |
| 4–6 | 60–100 | 3 months | Arteriovenous graft | 129 |
| Pig | Similar vascular physiology and anatomy to humans.
Well established as a model for assessing vascular grafts.
Mount an extensive immune response to implanted tissues. | 3–6 | 30–100 | 6 months | Iliac artery | 130 |
| 3–6 | 10–100 | 4 weeks | Carotid artery | 52 |
| Dog | Lack of spontaneous endothelialisation and immune response restricts study lengths.
Ease of accessing vessel due to thin skin.
Thrombogenicity mechanisms and vessel viscoelastic properties differ from humans. | 3–6 | 30–50 | 6 months | Abdominal artery | 131 |
| 3–6 | 30–50 | 1 year | Carotid artery | 119 |
| Baboon | Physiology and cardiovascular anatomy are the most similar to humans.
Suitable for a wide range of non-invasive imaging techniques adapted from humans.
High cost and ethical concerns associated with using primates in medical research. | 3–6 | 30–50 | 6 months | Arteriovenous graft | 26 |
| Rabbit | Similar endothelialisation rates and thrombogenicity mechanisms to humans.
Demanding higher anticoagulant function.
Limited to short-term studies. | 1–4 | 5–30 | 12 months | Carotid artery | 94, 132 |
| 1–4 | 5–30 | 2 weeks | Femoral artery | 133 |
| 1–4 | 5–30 | 3 months | Abdominal artery | 134 |
| Rat | Large sample size.
Wide variety of transgenic lines.
Allows exploration of genetic/molecular mechanisms.
Ideal for biocompatibility and cell infiltration studies. | 1–3 | 5–30 | 18 months | Abdominal artery | 73, 135 |
| 1–3 | 5–10 | 12 weeks | Carotid artery | 136 |
| Mouse | 0.5–1 | 3–10 | 6 months | Carotid artery | 137 |
Rats and mice are widely used because they are inexpensive to acquire, easy to maintain, and can be used to evaluate larger sample sizes.135-137 Furthermore, the availability of various transgenic strains makes it possible to examine the molecular mechanisms involved in graft remodelling.138 However, differences in their circulatory system with respect to humans limit their utility in short-term studies to only very small grafts (< 2 mm diameter). Rabbits are also suitable for artificial blood vessel assessments although they are limited to short-term studies.132-134 Their physiology is similar to that of humans in terms of endothelialisation rates and thrombogenicity, and they can accept clinically-relevant graft diameters (1–4 mm). Dogs differ from humans in terms of thrombogenicity and represent a more stringent model for the investigations because of their lack of spontaneous endothelialisation.119, 131 Moreover, they can provide a range of implantation site options with varied vessel sizes, although their immune response can restrict study durations. Both pigs and sheep demonstrate similarities to human physiology and they are appropriate for studying thrombogenicity, calcification, and long-term graft patency.129, 130 The cardiovascular physiology of non-human primates, such as baboons, is most similar to that of humans compared to dogs, pigs or sheep. Their structural similarity allows for various imaging techniques and testing methods that are typically applied in humans to be used on them. Despite such advantages, they are rarely used for vascular graft evaluation due to their high cost and ethical concerns.81, 91 The optimal model for studying all of the performance criteria associated with artificial vascular grafts has yet to be elucidated. An immunodeficient pig model, the operational immunodeficient pig, has been reported to be a large animal model that allows for long-term accommodation of artificial human vascular grafts to properly evaluate the clinical efficacy of artificial vascular grafts.139 ...
... Rats and mice are widely used because they are inexpensive to acquire, easy to maintain, and can be used to evaluate larger sample sizes.135-137 Furthermore, the availability of various transgenic strains makes it possible to examine the molecular mechanisms involved in graft remodelling.138 However, differences in their circulatory system with respect to humans limit their utility in short-term studies to only very small grafts (< 2 mm diameter). Rabbits are also suitable for artificial blood vessel assessments although they are limited to short-term studies.132-134 Their physiology is similar to that of humans in terms of endothelialisation rates and thrombogenicity, and they can accept clinically-relevant graft diameters (1–4 mm). Dogs differ from humans in terms of thrombogenicity and represent a more stringent model for the investigations because of their lack of spontaneous endothelialisation.119, 131 Moreover, they can provide a range of implantation site options with varied vessel sizes, although their immune response can restrict study durations. Both pigs and sheep demonstrate similarities to human physiology and they are appropriate for studying thrombogenicity, calcification, and long-term graft patency.129, 130 The cardiovascular physiology of non-human primates, such as baboons, is most similar to that of humans compared to dogs, pigs or sheep. Their structural similarity allows for various imaging techniques and testing methods that are typically applied in humans to be used on them. Despite such advantages, they are rarely used for vascular graft evaluation due to their high cost and ethical concerns.81, 91 The optimal model for studying all of the performance criteria associated with artificial vascular grafts has yet to be elucidated. An immunodeficient pig model, the operational immunodeficient pig, has been reported to be a large animal model that allows for long-term accommodation of artificial human vascular grafts to properly evaluate the clinical efficacy of artificial vascular grafts.139 ...
Steady-state behavior and endothelialization of a silk-based small-caliber scaffold in vivo transplantation
2
2019
... Common animal models for evaluation of artificial blood vessels
in vivo.
| Animal model | Characteristics | Application inner diameter (mm) | Common length (mm) | Longest implantation period | Implantation site | References |
| Sheep | Similar cardiovascular physiology, endothelialisation mechanisms and thrombogenicity mechanisms to humans.
Suitable size and long-term studies possible.Higher incidence of hypercoagulability. | 4–6 | 80–100 | 9 months | Carotid artery | 48, 74 |
| 4–6 | 60–100 | 3 months | Arteriovenous graft | 129 |
| Pig | Similar vascular physiology and anatomy to humans.
Well established as a model for assessing vascular grafts.
Mount an extensive immune response to implanted tissues. | 3–6 | 30–100 | 6 months | Iliac artery | 130 |
| 3–6 | 10–100 | 4 weeks | Carotid artery | 52 |
| Dog | Lack of spontaneous endothelialisation and immune response restricts study lengths.
Ease of accessing vessel due to thin skin.
Thrombogenicity mechanisms and vessel viscoelastic properties differ from humans. | 3–6 | 30–50 | 6 months | Abdominal artery | 131 |
| 3–6 | 30–50 | 1 year | Carotid artery | 119 |
| Baboon | Physiology and cardiovascular anatomy are the most similar to humans.
Suitable for a wide range of non-invasive imaging techniques adapted from humans.
High cost and ethical concerns associated with using primates in medical research. | 3–6 | 30–50 | 6 months | Arteriovenous graft | 26 |
| Rabbit | Similar endothelialisation rates and thrombogenicity mechanisms to humans.
Demanding higher anticoagulant function.
Limited to short-term studies. | 1–4 | 5–30 | 12 months | Carotid artery | 94, 132 |
| 1–4 | 5–30 | 2 weeks | Femoral artery | 133 |
| 1–4 | 5–30 | 3 months | Abdominal artery | 134 |
| Rat | Large sample size.
Wide variety of transgenic lines.
Allows exploration of genetic/molecular mechanisms.
Ideal for biocompatibility and cell infiltration studies. | 1–3 | 5–30 | 18 months | Abdominal artery | 73, 135 |
| 1–3 | 5–10 | 12 weeks | Carotid artery | 136 |
| Mouse | 0.5–1 | 3–10 | 6 months | Carotid artery | 137 |
Rats and mice are widely used because they are inexpensive to acquire, easy to maintain, and can be used to evaluate larger sample sizes.135-137 Furthermore, the availability of various transgenic strains makes it possible to examine the molecular mechanisms involved in graft remodelling.138 However, differences in their circulatory system with respect to humans limit their utility in short-term studies to only very small grafts (< 2 mm diameter). Rabbits are also suitable for artificial blood vessel assessments although they are limited to short-term studies.132-134 Their physiology is similar to that of humans in terms of endothelialisation rates and thrombogenicity, and they can accept clinically-relevant graft diameters (1–4 mm). Dogs differ from humans in terms of thrombogenicity and represent a more stringent model for the investigations because of their lack of spontaneous endothelialisation.119, 131 Moreover, they can provide a range of implantation site options with varied vessel sizes, although their immune response can restrict study durations. Both pigs and sheep demonstrate similarities to human physiology and they are appropriate for studying thrombogenicity, calcification, and long-term graft patency.129, 130 The cardiovascular physiology of non-human primates, such as baboons, is most similar to that of humans compared to dogs, pigs or sheep. Their structural similarity allows for various imaging techniques and testing methods that are typically applied in humans to be used on them. Despite such advantages, they are rarely used for vascular graft evaluation due to their high cost and ethical concerns.81, 91 The optimal model for studying all of the performance criteria associated with artificial vascular grafts has yet to be elucidated. An immunodeficient pig model, the operational immunodeficient pig, has been reported to be a large animal model that allows for long-term accommodation of artificial human vascular grafts to properly evaluate the clinical efficacy of artificial vascular grafts.139 ...
... Rats and mice are widely used because they are inexpensive to acquire, easy to maintain, and can be used to evaluate larger sample sizes.135-137 Furthermore, the availability of various transgenic strains makes it possible to examine the molecular mechanisms involved in graft remodelling.138 However, differences in their circulatory system with respect to humans limit their utility in short-term studies to only very small grafts (< 2 mm diameter). Rabbits are also suitable for artificial blood vessel assessments although they are limited to short-term studies.132-134 Their physiology is similar to that of humans in terms of endothelialisation rates and thrombogenicity, and they can accept clinically-relevant graft diameters (1–4 mm). Dogs differ from humans in terms of thrombogenicity and represent a more stringent model for the investigations because of their lack of spontaneous endothelialisation.119, 131 Moreover, they can provide a range of implantation site options with varied vessel sizes, although their immune response can restrict study durations. Both pigs and sheep demonstrate similarities to human physiology and they are appropriate for studying thrombogenicity, calcification, and long-term graft patency.129, 130 The cardiovascular physiology of non-human primates, such as baboons, is most similar to that of humans compared to dogs, pigs or sheep. Their structural similarity allows for various imaging techniques and testing methods that are typically applied in humans to be used on them. Despite such advantages, they are rarely used for vascular graft evaluation due to their high cost and ethical concerns.81, 91 The optimal model for studying all of the performance criteria associated with artificial vascular grafts has yet to be elucidated. An immunodeficient pig model, the operational immunodeficient pig, has been reported to be a large animal model that allows for long-term accommodation of artificial human vascular grafts to properly evaluate the clinical efficacy of artificial vascular grafts.139 ...
Submillimeter diameter poly(vinyl alcohol) vascular graft patency in rabbit model
1
2016
... Common animal models for evaluation of artificial blood vessels
in vivo.
| Animal model | Characteristics | Application inner diameter (mm) | Common length (mm) | Longest implantation period | Implantation site | References |
| Sheep | Similar cardiovascular physiology, endothelialisation mechanisms and thrombogenicity mechanisms to humans.
Suitable size and long-term studies possible.Higher incidence of hypercoagulability. | 4–6 | 80–100 | 9 months | Carotid artery | 48, 74 |
| 4–6 | 60–100 | 3 months | Arteriovenous graft | 129 |
| Pig | Similar vascular physiology and anatomy to humans.
Well established as a model for assessing vascular grafts.
Mount an extensive immune response to implanted tissues. | 3–6 | 30–100 | 6 months | Iliac artery | 130 |
| 3–6 | 10–100 | 4 weeks | Carotid artery | 52 |
| Dog | Lack of spontaneous endothelialisation and immune response restricts study lengths.
Ease of accessing vessel due to thin skin.
Thrombogenicity mechanisms and vessel viscoelastic properties differ from humans. | 3–6 | 30–50 | 6 months | Abdominal artery | 131 |
| 3–6 | 30–50 | 1 year | Carotid artery | 119 |
| Baboon | Physiology and cardiovascular anatomy are the most similar to humans.
Suitable for a wide range of non-invasive imaging techniques adapted from humans.
High cost and ethical concerns associated with using primates in medical research. | 3–6 | 30–50 | 6 months | Arteriovenous graft | 26 |
| Rabbit | Similar endothelialisation rates and thrombogenicity mechanisms to humans.
Demanding higher anticoagulant function.
Limited to short-term studies. | 1–4 | 5–30 | 12 months | Carotid artery | 94, 132 |
| 1–4 | 5–30 | 2 weeks | Femoral artery | 133 |
| 1–4 | 5–30 | 3 months | Abdominal artery | 134 |
| Rat | Large sample size.
Wide variety of transgenic lines.
Allows exploration of genetic/molecular mechanisms.
Ideal for biocompatibility and cell infiltration studies. | 1–3 | 5–30 | 18 months | Abdominal artery | 73, 135 |
| 1–3 | 5–10 | 12 weeks | Carotid artery | 136 |
| Mouse | 0.5–1 | 3–10 | 6 months | Carotid artery | 137 |
Rats and mice are widely used because they are inexpensive to acquire, easy to maintain, and can be used to evaluate larger sample sizes.135-137 Furthermore, the availability of various transgenic strains makes it possible to examine the molecular mechanisms involved in graft remodelling.138 However, differences in their circulatory system with respect to humans limit their utility in short-term studies to only very small grafts (< 2 mm diameter). Rabbits are also suitable for artificial blood vessel assessments although they are limited to short-term studies.132-134 Their physiology is similar to that of humans in terms of endothelialisation rates and thrombogenicity, and they can accept clinically-relevant graft diameters (1–4 mm). Dogs differ from humans in terms of thrombogenicity and represent a more stringent model for the investigations because of their lack of spontaneous endothelialisation.119, 131 Moreover, they can provide a range of implantation site options with varied vessel sizes, although their immune response can restrict study durations. Both pigs and sheep demonstrate similarities to human physiology and they are appropriate for studying thrombogenicity, calcification, and long-term graft patency.129, 130 The cardiovascular physiology of non-human primates, such as baboons, is most similar to that of humans compared to dogs, pigs or sheep. Their structural similarity allows for various imaging techniques and testing methods that are typically applied in humans to be used on them. Despite such advantages, they are rarely used for vascular graft evaluation due to their high cost and ethical concerns.81, 91 The optimal model for studying all of the performance criteria associated with artificial vascular grafts has yet to be elucidated. An immunodeficient pig model, the operational immunodeficient pig, has been reported to be a large animal model that allows for long-term accommodation of artificial human vascular grafts to properly evaluate the clinical efficacy of artificial vascular grafts.139 ...
In vivo biocompatibility and hemocompatibility of a polytetrafluoroethylene small diameter vascular graft modified with sulfonated silk fibroin
2
2017
... Common animal models for evaluation of artificial blood vessels
in vivo.
| Animal model | Characteristics | Application inner diameter (mm) | Common length (mm) | Longest implantation period | Implantation site | References |
| Sheep | Similar cardiovascular physiology, endothelialisation mechanisms and thrombogenicity mechanisms to humans.
Suitable size and long-term studies possible.Higher incidence of hypercoagulability. | 4–6 | 80–100 | 9 months | Carotid artery | 48, 74 |
| 4–6 | 60–100 | 3 months | Arteriovenous graft | 129 |
| Pig | Similar vascular physiology and anatomy to humans.
Well established as a model for assessing vascular grafts.
Mount an extensive immune response to implanted tissues. | 3–6 | 30–100 | 6 months | Iliac artery | 130 |
| 3–6 | 10–100 | 4 weeks | Carotid artery | 52 |
| Dog | Lack of spontaneous endothelialisation and immune response restricts study lengths.
Ease of accessing vessel due to thin skin.
Thrombogenicity mechanisms and vessel viscoelastic properties differ from humans. | 3–6 | 30–50 | 6 months | Abdominal artery | 131 |
| 3–6 | 30–50 | 1 year | Carotid artery | 119 |
| Baboon | Physiology and cardiovascular anatomy are the most similar to humans.
Suitable for a wide range of non-invasive imaging techniques adapted from humans.
High cost and ethical concerns associated with using primates in medical research. | 3–6 | 30–50 | 6 months | Arteriovenous graft | 26 |
| Rabbit | Similar endothelialisation rates and thrombogenicity mechanisms to humans.
Demanding higher anticoagulant function.
Limited to short-term studies. | 1–4 | 5–30 | 12 months | Carotid artery | 94, 132 |
| 1–4 | 5–30 | 2 weeks | Femoral artery | 133 |
| 1–4 | 5–30 | 3 months | Abdominal artery | 134 |
| Rat | Large sample size.
Wide variety of transgenic lines.
Allows exploration of genetic/molecular mechanisms.
Ideal for biocompatibility and cell infiltration studies. | 1–3 | 5–30 | 18 months | Abdominal artery | 73, 135 |
| 1–3 | 5–10 | 12 weeks | Carotid artery | 136 |
| Mouse | 0.5–1 | 3–10 | 6 months | Carotid artery | 137 |
Rats and mice are widely used because they are inexpensive to acquire, easy to maintain, and can be used to evaluate larger sample sizes.135-137 Furthermore, the availability of various transgenic strains makes it possible to examine the molecular mechanisms involved in graft remodelling.138 However, differences in their circulatory system with respect to humans limit their utility in short-term studies to only very small grafts (< 2 mm diameter). Rabbits are also suitable for artificial blood vessel assessments although they are limited to short-term studies.132-134 Their physiology is similar to that of humans in terms of endothelialisation rates and thrombogenicity, and they can accept clinically-relevant graft diameters (1–4 mm). Dogs differ from humans in terms of thrombogenicity and represent a more stringent model for the investigations because of their lack of spontaneous endothelialisation.119, 131 Moreover, they can provide a range of implantation site options with varied vessel sizes, although their immune response can restrict study durations. Both pigs and sheep demonstrate similarities to human physiology and they are appropriate for studying thrombogenicity, calcification, and long-term graft patency.129, 130 The cardiovascular physiology of non-human primates, such as baboons, is most similar to that of humans compared to dogs, pigs or sheep. Their structural similarity allows for various imaging techniques and testing methods that are typically applied in humans to be used on them. Despite such advantages, they are rarely used for vascular graft evaluation due to their high cost and ethical concerns.81, 91 The optimal model for studying all of the performance criteria associated with artificial vascular grafts has yet to be elucidated. An immunodeficient pig model, the operational immunodeficient pig, has been reported to be a large animal model that allows for long-term accommodation of artificial human vascular grafts to properly evaluate the clinical efficacy of artificial vascular grafts.139 ...
... Rats and mice are widely used because they are inexpensive to acquire, easy to maintain, and can be used to evaluate larger sample sizes.135-137 Furthermore, the availability of various transgenic strains makes it possible to examine the molecular mechanisms involved in graft remodelling.138 However, differences in their circulatory system with respect to humans limit their utility in short-term studies to only very small grafts (< 2 mm diameter). Rabbits are also suitable for artificial blood vessel assessments although they are limited to short-term studies.132-134 Their physiology is similar to that of humans in terms of endothelialisation rates and thrombogenicity, and they can accept clinically-relevant graft diameters (1–4 mm). Dogs differ from humans in terms of thrombogenicity and represent a more stringent model for the investigations because of their lack of spontaneous endothelialisation.119, 131 Moreover, they can provide a range of implantation site options with varied vessel sizes, although their immune response can restrict study durations. Both pigs and sheep demonstrate similarities to human physiology and they are appropriate for studying thrombogenicity, calcification, and long-term graft patency.129, 130 The cardiovascular physiology of non-human primates, such as baboons, is most similar to that of humans compared to dogs, pigs or sheep. Their structural similarity allows for various imaging techniques and testing methods that are typically applied in humans to be used on them. Despite such advantages, they are rarely used for vascular graft evaluation due to their high cost and ethical concerns.81, 91 The optimal model for studying all of the performance criteria associated with artificial vascular grafts has yet to be elucidated. An immunodeficient pig model, the operational immunodeficient pig, has been reported to be a large animal model that allows for long-term accommodation of artificial human vascular grafts to properly evaluate the clinical efficacy of artificial vascular grafts.139 ...
Long-term evaluation of vascular grafts with circumferentially aligned microfibers in a rat abdominal aorta replacement model
2
2018
... Common animal models for evaluation of artificial blood vessels
in vivo.
| Animal model | Characteristics | Application inner diameter (mm) | Common length (mm) | Longest implantation period | Implantation site | References |
| Sheep | Similar cardiovascular physiology, endothelialisation mechanisms and thrombogenicity mechanisms to humans.
Suitable size and long-term studies possible.Higher incidence of hypercoagulability. | 4–6 | 80–100 | 9 months | Carotid artery | 48, 74 |
| 4–6 | 60–100 | 3 months | Arteriovenous graft | 129 |
| Pig | Similar vascular physiology and anatomy to humans.
Well established as a model for assessing vascular grafts.
Mount an extensive immune response to implanted tissues. | 3–6 | 30–100 | 6 months | Iliac artery | 130 |
| 3–6 | 10–100 | 4 weeks | Carotid artery | 52 |
| Dog | Lack of spontaneous endothelialisation and immune response restricts study lengths.
Ease of accessing vessel due to thin skin.
Thrombogenicity mechanisms and vessel viscoelastic properties differ from humans. | 3–6 | 30–50 | 6 months | Abdominal artery | 131 |
| 3–6 | 30–50 | 1 year | Carotid artery | 119 |
| Baboon | Physiology and cardiovascular anatomy are the most similar to humans.
Suitable for a wide range of non-invasive imaging techniques adapted from humans.
High cost and ethical concerns associated with using primates in medical research. | 3–6 | 30–50 | 6 months | Arteriovenous graft | 26 |
| Rabbit | Similar endothelialisation rates and thrombogenicity mechanisms to humans.
Demanding higher anticoagulant function.
Limited to short-term studies. | 1–4 | 5–30 | 12 months | Carotid artery | 94, 132 |
| 1–4 | 5–30 | 2 weeks | Femoral artery | 133 |
| 1–4 | 5–30 | 3 months | Abdominal artery | 134 |
| Rat | Large sample size.
Wide variety of transgenic lines.
Allows exploration of genetic/molecular mechanisms.
Ideal for biocompatibility and cell infiltration studies. | 1–3 | 5–30 | 18 months | Abdominal artery | 73, 135 |
| 1–3 | 5–10 | 12 weeks | Carotid artery | 136 |
| Mouse | 0.5–1 | 3–10 | 6 months | Carotid artery | 137 |
Rats and mice are widely used because they are inexpensive to acquire, easy to maintain, and can be used to evaluate larger sample sizes.135-137 Furthermore, the availability of various transgenic strains makes it possible to examine the molecular mechanisms involved in graft remodelling.138 However, differences in their circulatory system with respect to humans limit their utility in short-term studies to only very small grafts (< 2 mm diameter). Rabbits are also suitable for artificial blood vessel assessments although they are limited to short-term studies.132-134 Their physiology is similar to that of humans in terms of endothelialisation rates and thrombogenicity, and they can accept clinically-relevant graft diameters (1–4 mm). Dogs differ from humans in terms of thrombogenicity and represent a more stringent model for the investigations because of their lack of spontaneous endothelialisation.119, 131 Moreover, they can provide a range of implantation site options with varied vessel sizes, although their immune response can restrict study durations. Both pigs and sheep demonstrate similarities to human physiology and they are appropriate for studying thrombogenicity, calcification, and long-term graft patency.129, 130 The cardiovascular physiology of non-human primates, such as baboons, is most similar to that of humans compared to dogs, pigs or sheep. Their structural similarity allows for various imaging techniques and testing methods that are typically applied in humans to be used on them. Despite such advantages, they are rarely used for vascular graft evaluation due to their high cost and ethical concerns.81, 91 The optimal model for studying all of the performance criteria associated with artificial vascular grafts has yet to be elucidated. An immunodeficient pig model, the operational immunodeficient pig, has been reported to be a large animal model that allows for long-term accommodation of artificial human vascular grafts to properly evaluate the clinical efficacy of artificial vascular grafts.139 ...
... Rats and mice are widely used because they are inexpensive to acquire, easy to maintain, and can be used to evaluate larger sample sizes.135-137 Furthermore, the availability of various transgenic strains makes it possible to examine the molecular mechanisms involved in graft remodelling.138 However, differences in their circulatory system with respect to humans limit their utility in short-term studies to only very small grafts (< 2 mm diameter). Rabbits are also suitable for artificial blood vessel assessments although they are limited to short-term studies.132-134 Their physiology is similar to that of humans in terms of endothelialisation rates and thrombogenicity, and they can accept clinically-relevant graft diameters (1–4 mm). Dogs differ from humans in terms of thrombogenicity and represent a more stringent model for the investigations because of their lack of spontaneous endothelialisation.119, 131 Moreover, they can provide a range of implantation site options with varied vessel sizes, although their immune response can restrict study durations. Both pigs and sheep demonstrate similarities to human physiology and they are appropriate for studying thrombogenicity, calcification, and long-term graft patency.129, 130 The cardiovascular physiology of non-human primates, such as baboons, is most similar to that of humans compared to dogs, pigs or sheep. Their structural similarity allows for various imaging techniques and testing methods that are typically applied in humans to be used on them. Despite such advantages, they are rarely used for vascular graft evaluation due to their high cost and ethical concerns.81, 91 The optimal model for studying all of the performance criteria associated with artificial vascular grafts has yet to be elucidated. An immunodeficient pig model, the operational immunodeficient pig, has been reported to be a large animal model that allows for long-term accommodation of artificial human vascular grafts to properly evaluate the clinical efficacy of artificial vascular grafts.139 ...
Slow degrading poly(glycerol sebacate) derivatives improve vascular graft remodeling in a rat carotid artery interposition model
1
2020
... Common animal models for evaluation of artificial blood vessels
in vivo.
| Animal model | Characteristics | Application inner diameter (mm) | Common length (mm) | Longest implantation period | Implantation site | References |
| Sheep | Similar cardiovascular physiology, endothelialisation mechanisms and thrombogenicity mechanisms to humans.
Suitable size and long-term studies possible.Higher incidence of hypercoagulability. | 4–6 | 80–100 | 9 months | Carotid artery | 48, 74 |
| 4–6 | 60–100 | 3 months | Arteriovenous graft | 129 |
| Pig | Similar vascular physiology and anatomy to humans.
Well established as a model for assessing vascular grafts.
Mount an extensive immune response to implanted tissues. | 3–6 | 30–100 | 6 months | Iliac artery | 130 |
| 3–6 | 10–100 | 4 weeks | Carotid artery | 52 |
| Dog | Lack of spontaneous endothelialisation and immune response restricts study lengths.
Ease of accessing vessel due to thin skin.
Thrombogenicity mechanisms and vessel viscoelastic properties differ from humans. | 3–6 | 30–50 | 6 months | Abdominal artery | 131 |
| 3–6 | 30–50 | 1 year | Carotid artery | 119 |
| Baboon | Physiology and cardiovascular anatomy are the most similar to humans.
Suitable for a wide range of non-invasive imaging techniques adapted from humans.
High cost and ethical concerns associated with using primates in medical research. | 3–6 | 30–50 | 6 months | Arteriovenous graft | 26 |
| Rabbit | Similar endothelialisation rates and thrombogenicity mechanisms to humans.
Demanding higher anticoagulant function.
Limited to short-term studies. | 1–4 | 5–30 | 12 months | Carotid artery | 94, 132 |
| 1–4 | 5–30 | 2 weeks | Femoral artery | 133 |
| 1–4 | 5–30 | 3 months | Abdominal artery | 134 |
| Rat | Large sample size.
Wide variety of transgenic lines.
Allows exploration of genetic/molecular mechanisms.
Ideal for biocompatibility and cell infiltration studies. | 1–3 | 5–30 | 18 months | Abdominal artery | 73, 135 |
| 1–3 | 5–10 | 12 weeks | Carotid artery | 136 |
| Mouse | 0.5–1 | 3–10 | 6 months | Carotid artery | 137 |
Rats and mice are widely used because they are inexpensive to acquire, easy to maintain, and can be used to evaluate larger sample sizes.135-137 Furthermore, the availability of various transgenic strains makes it possible to examine the molecular mechanisms involved in graft remodelling.138 However, differences in their circulatory system with respect to humans limit their utility in short-term studies to only very small grafts (< 2 mm diameter). Rabbits are also suitable for artificial blood vessel assessments although they are limited to short-term studies.132-134 Their physiology is similar to that of humans in terms of endothelialisation rates and thrombogenicity, and they can accept clinically-relevant graft diameters (1–4 mm). Dogs differ from humans in terms of thrombogenicity and represent a more stringent model for the investigations because of their lack of spontaneous endothelialisation.119, 131 Moreover, they can provide a range of implantation site options with varied vessel sizes, although their immune response can restrict study durations. Both pigs and sheep demonstrate similarities to human physiology and they are appropriate for studying thrombogenicity, calcification, and long-term graft patency.129, 130 The cardiovascular physiology of non-human primates, such as baboons, is most similar to that of humans compared to dogs, pigs or sheep. Their structural similarity allows for various imaging techniques and testing methods that are typically applied in humans to be used on them. Despite such advantages, they are rarely used for vascular graft evaluation due to their high cost and ethical concerns.81, 91 The optimal model for studying all of the performance criteria associated with artificial vascular grafts has yet to be elucidated. An immunodeficient pig model, the operational immunodeficient pig, has been reported to be a large animal model that allows for long-term accommodation of artificial human vascular grafts to properly evaluate the clinical efficacy of artificial vascular grafts.139 ...
Novel bioresorbable vascular graft with sponge-type scaffold as a small-diameter arterial graft
3
2016
... In order to meet the demand to reasonably test the performance of various types of artificial blood vessels, it is necessary to establish a viable and stable animal model. Transplanting and facilitating postoperative observations may be easily performed if the appropriate animal models exist, which are able to simulate applications in the environment of artificial blood vessels. Currently, commonly-used vascular in vivo animal models are pigs, sheep, dogs, rabbits, and rats.124-126 The carotid, abdominal, and femoral arteries in these animal models are the most chosen surgical sites. Moreover, different options are available based on the animals’ size, according to the calibre of the blood vessel. Rat abdominal aortic replacement and rabbit carotid replacement are viable animal models for in vivo validation of small-calibre vessels. In addition, specific criteria, such as implantability, mechanical performance, biocompatibility, thrombogenicity, and haemodynamics, must be considered.127, 128 Achieving balance with model-specific factors should also be taken into consideration, such as animal availability, ease of handling, ease of performing the implantation surgery, study duration, compatible methods of graft evaluation, and cost. The characteristics of common animal models are summarised in Table 4.129-137 ...
... Common animal models for evaluation of artificial blood vessels
in vivo.
| Animal model | Characteristics | Application inner diameter (mm) | Common length (mm) | Longest implantation period | Implantation site | References |
| Sheep | Similar cardiovascular physiology, endothelialisation mechanisms and thrombogenicity mechanisms to humans.
Suitable size and long-term studies possible.Higher incidence of hypercoagulability. | 4–6 | 80–100 | 9 months | Carotid artery | 48, 74 |
| 4–6 | 60–100 | 3 months | Arteriovenous graft | 129 |
| Pig | Similar vascular physiology and anatomy to humans.
Well established as a model for assessing vascular grafts.
Mount an extensive immune response to implanted tissues. | 3–6 | 30–100 | 6 months | Iliac artery | 130 |
| 3–6 | 10–100 | 4 weeks | Carotid artery | 52 |
| Dog | Lack of spontaneous endothelialisation and immune response restricts study lengths.
Ease of accessing vessel due to thin skin.
Thrombogenicity mechanisms and vessel viscoelastic properties differ from humans. | 3–6 | 30–50 | 6 months | Abdominal artery | 131 |
| 3–6 | 30–50 | 1 year | Carotid artery | 119 |
| Baboon | Physiology and cardiovascular anatomy are the most similar to humans.
Suitable for a wide range of non-invasive imaging techniques adapted from humans.
High cost and ethical concerns associated with using primates in medical research. | 3–6 | 30–50 | 6 months | Arteriovenous graft | 26 |
| Rabbit | Similar endothelialisation rates and thrombogenicity mechanisms to humans.
Demanding higher anticoagulant function.
Limited to short-term studies. | 1–4 | 5–30 | 12 months | Carotid artery | 94, 132 |
| 1–4 | 5–30 | 2 weeks | Femoral artery | 133 |
| 1–4 | 5–30 | 3 months | Abdominal artery | 134 |
| Rat | Large sample size.
Wide variety of transgenic lines.
Allows exploration of genetic/molecular mechanisms.
Ideal for biocompatibility and cell infiltration studies. | 1–3 | 5–30 | 18 months | Abdominal artery | 73, 135 |
| 1–3 | 5–10 | 12 weeks | Carotid artery | 136 |
| Mouse | 0.5–1 | 3–10 | 6 months | Carotid artery | 137 |
Rats and mice are widely used because they are inexpensive to acquire, easy to maintain, and can be used to evaluate larger sample sizes.135-137 Furthermore, the availability of various transgenic strains makes it possible to examine the molecular mechanisms involved in graft remodelling.138 However, differences in their circulatory system with respect to humans limit their utility in short-term studies to only very small grafts (< 2 mm diameter). Rabbits are also suitable for artificial blood vessel assessments although they are limited to short-term studies.132-134 Their physiology is similar to that of humans in terms of endothelialisation rates and thrombogenicity, and they can accept clinically-relevant graft diameters (1–4 mm). Dogs differ from humans in terms of thrombogenicity and represent a more stringent model for the investigations because of their lack of spontaneous endothelialisation.119, 131 Moreover, they can provide a range of implantation site options with varied vessel sizes, although their immune response can restrict study durations. Both pigs and sheep demonstrate similarities to human physiology and they are appropriate for studying thrombogenicity, calcification, and long-term graft patency.129, 130 The cardiovascular physiology of non-human primates, such as baboons, is most similar to that of humans compared to dogs, pigs or sheep. Their structural similarity allows for various imaging techniques and testing methods that are typically applied in humans to be used on them. Despite such advantages, they are rarely used for vascular graft evaluation due to their high cost and ethical concerns.81, 91 The optimal model for studying all of the performance criteria associated with artificial vascular grafts has yet to be elucidated. An immunodeficient pig model, the operational immunodeficient pig, has been reported to be a large animal model that allows for long-term accommodation of artificial human vascular grafts to properly evaluate the clinical efficacy of artificial vascular grafts.139 ...
... Rats and mice are widely used because they are inexpensive to acquire, easy to maintain, and can be used to evaluate larger sample sizes.135-137 Furthermore, the availability of various transgenic strains makes it possible to examine the molecular mechanisms involved in graft remodelling.138 However, differences in their circulatory system with respect to humans limit their utility in short-term studies to only very small grafts (< 2 mm diameter). Rabbits are also suitable for artificial blood vessel assessments although they are limited to short-term studies.132-134 Their physiology is similar to that of humans in terms of endothelialisation rates and thrombogenicity, and they can accept clinically-relevant graft diameters (1–4 mm). Dogs differ from humans in terms of thrombogenicity and represent a more stringent model for the investigations because of their lack of spontaneous endothelialisation.119, 131 Moreover, they can provide a range of implantation site options with varied vessel sizes, although their immune response can restrict study durations. Both pigs and sheep demonstrate similarities to human physiology and they are appropriate for studying thrombogenicity, calcification, and long-term graft patency.129, 130 The cardiovascular physiology of non-human primates, such as baboons, is most similar to that of humans compared to dogs, pigs or sheep. Their structural similarity allows for various imaging techniques and testing methods that are typically applied in humans to be used on them. Despite such advantages, they are rarely used for vascular graft evaluation due to their high cost and ethical concerns.81, 91 The optimal model for studying all of the performance criteria associated with artificial vascular grafts has yet to be elucidated. An immunodeficient pig model, the operational immunodeficient pig, has been reported to be a large animal model that allows for long-term accommodation of artificial human vascular grafts to properly evaluate the clinical efficacy of artificial vascular grafts.139 ...
Tissue engineering on matrix: future of autologous tissue replacement
1
2011
... Rats and mice are widely used because they are inexpensive to acquire, easy to maintain, and can be used to evaluate larger sample sizes.135-137 Furthermore, the availability of various transgenic strains makes it possible to examine the molecular mechanisms involved in graft remodelling.138 However, differences in their circulatory system with respect to humans limit their utility in short-term studies to only very small grafts (< 2 mm diameter). Rabbits are also suitable for artificial blood vessel assessments although they are limited to short-term studies.132-134 Their physiology is similar to that of humans in terms of endothelialisation rates and thrombogenicity, and they can accept clinically-relevant graft diameters (1–4 mm). Dogs differ from humans in terms of thrombogenicity and represent a more stringent model for the investigations because of their lack of spontaneous endothelialisation.119, 131 Moreover, they can provide a range of implantation site options with varied vessel sizes, although their immune response can restrict study durations. Both pigs and sheep demonstrate similarities to human physiology and they are appropriate for studying thrombogenicity, calcification, and long-term graft patency.129, 130 The cardiovascular physiology of non-human primates, such as baboons, is most similar to that of humans compared to dogs, pigs or sheep. Their structural similarity allows for various imaging techniques and testing methods that are typically applied in humans to be used on them. Despite such advantages, they are rarely used for vascular graft evaluation due to their high cost and ethical concerns.81, 91 The optimal model for studying all of the performance criteria associated with artificial vascular grafts has yet to be elucidated. An immunodeficient pig model, the operational immunodeficient pig, has been reported to be a large animal model that allows for long-term accommodation of artificial human vascular grafts to properly evaluate the clinical efficacy of artificial vascular grafts.139 ...
Development of an immunodeficient pig model allowing long-term accommodation of artificial human vascular tubes
1
2019
... Rats and mice are widely used because they are inexpensive to acquire, easy to maintain, and can be used to evaluate larger sample sizes.135-137 Furthermore, the availability of various transgenic strains makes it possible to examine the molecular mechanisms involved in graft remodelling.138 However, differences in their circulatory system with respect to humans limit their utility in short-term studies to only very small grafts (< 2 mm diameter). Rabbits are also suitable for artificial blood vessel assessments although they are limited to short-term studies.132-134 Their physiology is similar to that of humans in terms of endothelialisation rates and thrombogenicity, and they can accept clinically-relevant graft diameters (1–4 mm). Dogs differ from humans in terms of thrombogenicity and represent a more stringent model for the investigations because of their lack of spontaneous endothelialisation.119, 131 Moreover, they can provide a range of implantation site options with varied vessel sizes, although their immune response can restrict study durations. Both pigs and sheep demonstrate similarities to human physiology and they are appropriate for studying thrombogenicity, calcification, and long-term graft patency.129, 130 The cardiovascular physiology of non-human primates, such as baboons, is most similar to that of humans compared to dogs, pigs or sheep. Their structural similarity allows for various imaging techniques and testing methods that are typically applied in humans to be used on them. Despite such advantages, they are rarely used for vascular graft evaluation due to their high cost and ethical concerns.81, 91 The optimal model for studying all of the performance criteria associated with artificial vascular grafts has yet to be elucidated. An immunodeficient pig model, the operational immunodeficient pig, has been reported to be a large animal model that allows for long-term accommodation of artificial human vascular grafts to properly evaluate the clinical efficacy of artificial vascular grafts.139 ...
A spectrum of Doppler waveforms in the carotid and vertebral arteries. AJR Am
1
2003
... High-resolution ultrasound may be used in order to evaluate the efficacy of a graft, such as the flatness of the mouth, the vascular lumen, velocity of the blood flow in the lumen, and spectral waveform of the artificial blood vessel grafts after implantation, which can also eventually be used to assess vascular patency following implantation by contrasting with the ultrasound image taken before surgery.140 Additionally, micro-computed tomographic angiography may also be used to evaluate the patency of grafts and the specific situation of artificial blood vessels in vivo. Immunofluorescence staining to evaluate the expression of specific molecules, such as CD31 and α-smooth muscle actin, can be used to verify biomimetic remodelling of endothelial and smooth muscle layers.123 Common evaluation methods, including tissue section staining, are shown in Figure 4. ...
Review: tissue engineering of small-diameter vascular grafts and their in vivo evaluation in large animals and humans
1
2021
... As previously discussed, the rise in patients with cardiovascular disease requiring replacement therapies has created a large demand for suitable vascular grafts. From an initial simple synthetic blood vessel substitute to tissue-engineered vascular grafts, much progress has recently been made, and tissue engineering techniques offer potential ideas for overcoming current vascular prosthesis replacement challenges and addressing future organ regeneration.141 Many scientists have focused on various fields related to artificial blood vessels, such as structural simulation, material improvement, performance optimisation, and tissue engineering. ...
Cellularized small-caliber tissue-engineered vascular grafts: looking for the ultimate gold standard
1
2021
... Normal human arteries consist of three structural layers. The intima, media and adventitia closely fit together to carry blood flow, and this structure is closely related to the physiological function of blood vessels. In order to simulate the multi-layered structure of natural blood vessels as closely as possible, researchers have improved the manufacturing process and used advanced technologies such as electrospinning, thermal-induced phase separation, microfluidics, stretch forming, and weaving technology .142, 143 Searching for the best fabrication techniques that can be effectively used to ameliorate issues related to the failure of artificial blood vessels has garnered much interest in research . 3D bioprinting technology is able to rotate and print along a rod template to obtain multiple orders of magnitude of complex three-dimensional structures.144, 145 By including a variety of modifications of the microenvironment, the overall structure of an artificial blood vessel can be made closer to the structure of the human body, which may result in better biological properties. ...
Surface modification and endothelialization of biomaterials as potential scaffolds for vascular tissue engineering applications
1
2015
... Normal human arteries consist of three structural layers. The intima, media and adventitia closely fit together to carry blood flow, and this structure is closely related to the physiological function of blood vessels. In order to simulate the multi-layered structure of natural blood vessels as closely as possible, researchers have improved the manufacturing process and used advanced technologies such as electrospinning, thermal-induced phase separation, microfluidics, stretch forming, and weaving technology .142, 143 Searching for the best fabrication techniques that can be effectively used to ameliorate issues related to the failure of artificial blood vessels has garnered much interest in research . 3D bioprinting technology is able to rotate and print along a rod template to obtain multiple orders of magnitude of complex three-dimensional structures.144, 145 By including a variety of modifications of the microenvironment, the overall structure of an artificial blood vessel can be made closer to the structure of the human body, which may result in better biological properties. ...
A 3D bioprinting system to produce human-scale tissue constructs with structural integrity
1
2016
... Normal human arteries consist of three structural layers. The intima, media and adventitia closely fit together to carry blood flow, and this structure is closely related to the physiological function of blood vessels. In order to simulate the multi-layered structure of natural blood vessels as closely as possible, researchers have improved the manufacturing process and used advanced technologies such as electrospinning, thermal-induced phase separation, microfluidics, stretch forming, and weaving technology .142, 143 Searching for the best fabrication techniques that can be effectively used to ameliorate issues related to the failure of artificial blood vessels has garnered much interest in research . 3D bioprinting technology is able to rotate and print along a rod template to obtain multiple orders of magnitude of complex three-dimensional structures.144, 145 By including a variety of modifications of the microenvironment, the overall structure of an artificial blood vessel can be made closer to the structure of the human body, which may result in better biological properties. ...
Research and development of 3D printed vasculature constructs
1
2018
... Normal human arteries consist of three structural layers. The intima, media and adventitia closely fit together to carry blood flow, and this structure is closely related to the physiological function of blood vessels. In order to simulate the multi-layered structure of natural blood vessels as closely as possible, researchers have improved the manufacturing process and used advanced technologies such as electrospinning, thermal-induced phase separation, microfluidics, stretch forming, and weaving technology .142, 143 Searching for the best fabrication techniques that can be effectively used to ameliorate issues related to the failure of artificial blood vessels has garnered much interest in research . 3D bioprinting technology is able to rotate and print along a rod template to obtain multiple orders of magnitude of complex three-dimensional structures.144, 145 By including a variety of modifications of the microenvironment, the overall structure of an artificial blood vessel can be made closer to the structure of the human body, which may result in better biological properties. ...
Silk fibroin for vascular regeneration
1
2017
... Materials are the basis of artificial blood vessels and also directly affect their performance. The best way to mix synthetic polymers and natural materials so as to maximise their advantages should be highlighted in further study. 123, 146, 147 The selection of appropriate materials will help to improve the requisite tissue mechanics, providing good biocompatibility, anti-thrombogenicity and long-term patency rate of blood vessels. A number of people have developed methods to improve materials, mainly by synthesising new materials, combining multiple materials together, improving the properties of materials through chemical modification and using decellularised heterogeneous blood vessels. Currently, many promising structures are multicomponent systems that have been created through the addition of natural surface coatings to basic synthetic materials. Grafts with three or more components are also increasingly common (e.g., heparin/silk/poly(L-lactide-co-caprolactone). Future development trends may include changing from synthetic materials to natural materials, or even hybrid materials, in order to produce better biocompatibility and improve the long-term patency rate. ...
Transcatheter-delivered expandable bioresorbable polymeric graft with stenting capacity induces vascular regeneration
1
2020
... Materials are the basis of artificial blood vessels and also directly affect their performance. The best way to mix synthetic polymers and natural materials so as to maximise their advantages should be highlighted in further study. 123, 146, 147 The selection of appropriate materials will help to improve the requisite tissue mechanics, providing good biocompatibility, anti-thrombogenicity and long-term patency rate of blood vessels. A number of people have developed methods to improve materials, mainly by synthesising new materials, combining multiple materials together, improving the properties of materials through chemical modification and using decellularised heterogeneous blood vessels. Currently, many promising structures are multicomponent systems that have been created through the addition of natural surface coatings to basic synthetic materials. Grafts with three or more components are also increasingly common (e.g., heparin/silk/poly(L-lactide-co-caprolactone). Future development trends may include changing from synthetic materials to natural materials, or even hybrid materials, in order to produce better biocompatibility and improve the long-term patency rate. ...