Biomaterials Translational ›› 2023, Vol. 4 ›› Issue (4): 280-290.doi: 10.12336/biomatertransl.2023.04.007
• RESEARCH ARTICLE • Previous Articles Next Articles
Ziwei Tao1#, Ziyang Yuan2#, Dong Zhou3#, Lang Qin1, Lan Xiao4, Shihao Zhang1, Changsheng Liu1,*( ), Jinzhong Zhao2,*(
), Jinzhong Zhao2,*( ), Yulin Li1,5,*(
), Yulin Li1,5,*( )
)
Received:2023-10-20
Revised:2023-11-14
Accepted:2023-11-25
Online:2023-12-27
Published:2023-12-28
Contact:
Yulin Li, yulinli@ecust.edu.cn;Changsheng Liu, liucs@ecust.edu.cn;Jinzhong Zhao, jzzhao@sjtu.edu.cn.
About author:#Author equally.
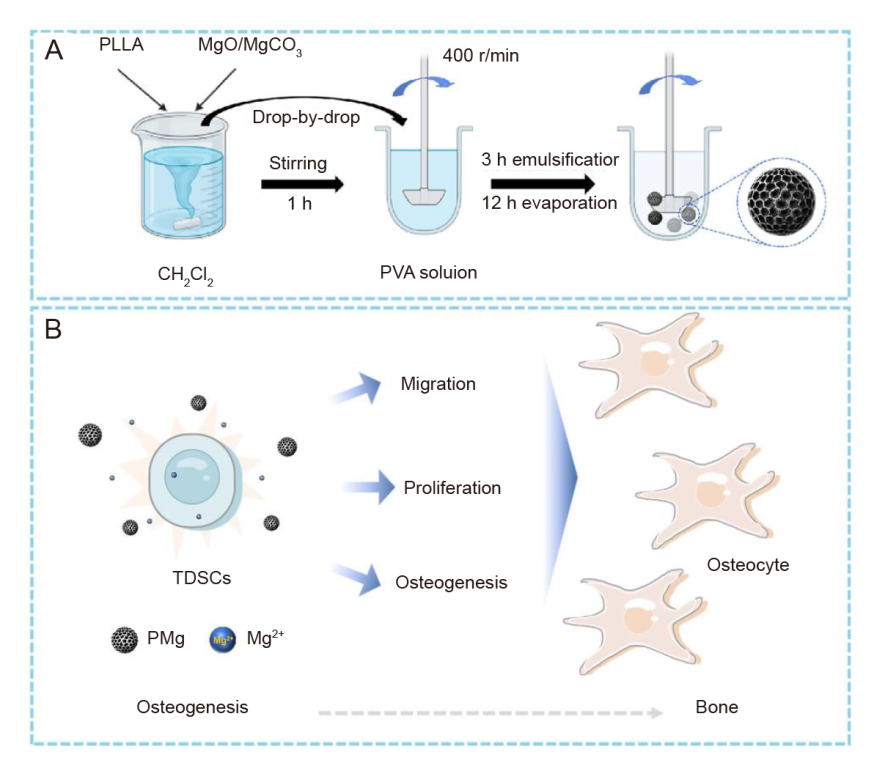
Figure 1. Schematic preparation of poly(L-lactic acid) microsphere loaded with magnesium oxide (MgO)/magnesium carbonate (MgCO3) microspheres (PMg) and influence of PMg on tendon-derived stem cells (TDSCs). (A) PMg microspheres were obtained in the form of oil in water, dichloromethane (CH2Cl2) is the oil phase, and polyvinyl alcohol (PVA) is the water phase, the oil phase and the water phase are incompatible, so an oil-in-water system is formed, and during stirring, CH2Cl2 gradually evaporates, and poly(L-lactic acid) microsphere (PM) and PMg are formed. (B) PMg microspheres can maintain the continuing release of Mg2+ to promote the TDSC migration, proliferation, osteogenesis. Created with BioRender.com.
| Gene | Primer sequence (5′-3′) |
|---|---|
| Arg-1 | Forward: ATC AAC ACT CCG CTG ACA ACC |
| Reverse: ATC TCG CAA GCC GAT GTA CAC | |
| Runx-2 | Forward: CGA ACA GAG CAA CAT CTC C |
| Reverse: GTC AGT GCC TTC CTT GG | |
| OCN | Forward: ACA AGT CCC ACA CAG CAA C |
| Reverse: CCA GGT CAG AGA GGC AGA | |
| GAPDH | Forward: CAA GAA GGT GGT GAA GCA G |
| Reverse: CAA AGG TGG AAG AAT GGG |
Table 1. The primer sequence for polymerase chain reaction
| Gene | Primer sequence (5′-3′) |
|---|---|
| Arg-1 | Forward: ATC AAC ACT CCG CTG ACA ACC |
| Reverse: ATC TCG CAA GCC GAT GTA CAC | |
| Runx-2 | Forward: CGA ACA GAG CAA CAT CTC C |
| Reverse: GTC AGT GCC TTC CTT GG | |
| OCN | Forward: ACA AGT CCC ACA CAG CAA C |
| Reverse: CCA GGT CAG AGA GGC AGA | |
| GAPDH | Forward: CAA GAA GGT GGT GAA GCA G |
| Reverse: CAA AGG TGG AAG AAT GGG |
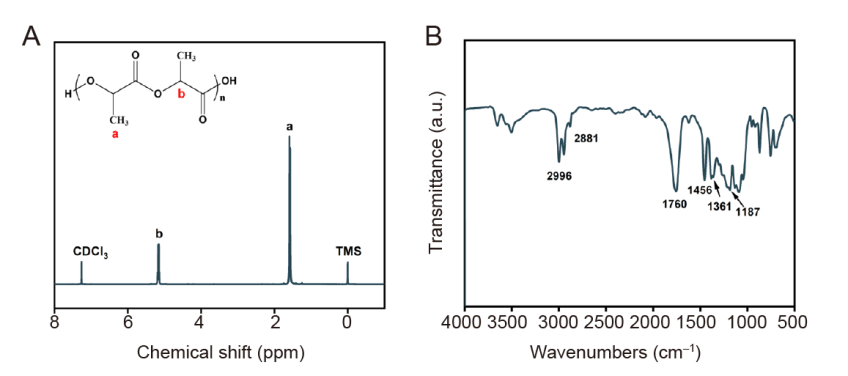
Figure 2. Chemical structure and microstructure analysis of poly(L-lactic acid) (PLLA). (A) Nuclear magnetic resonance spectroscopy, PLLA is a typical structure of polylactide, and the chemical structure of the polymer can be clearly analysed by measuring the chemical shift value of hydrogen. (B) Fourier transformed infrared spectrophotometer, the infrared visible light spectrum can clearly reflect the characteristic functional group structure in the polymer, and the polymer can be detected by the characteristic absorption peak. a.u.: arbitrary unit.
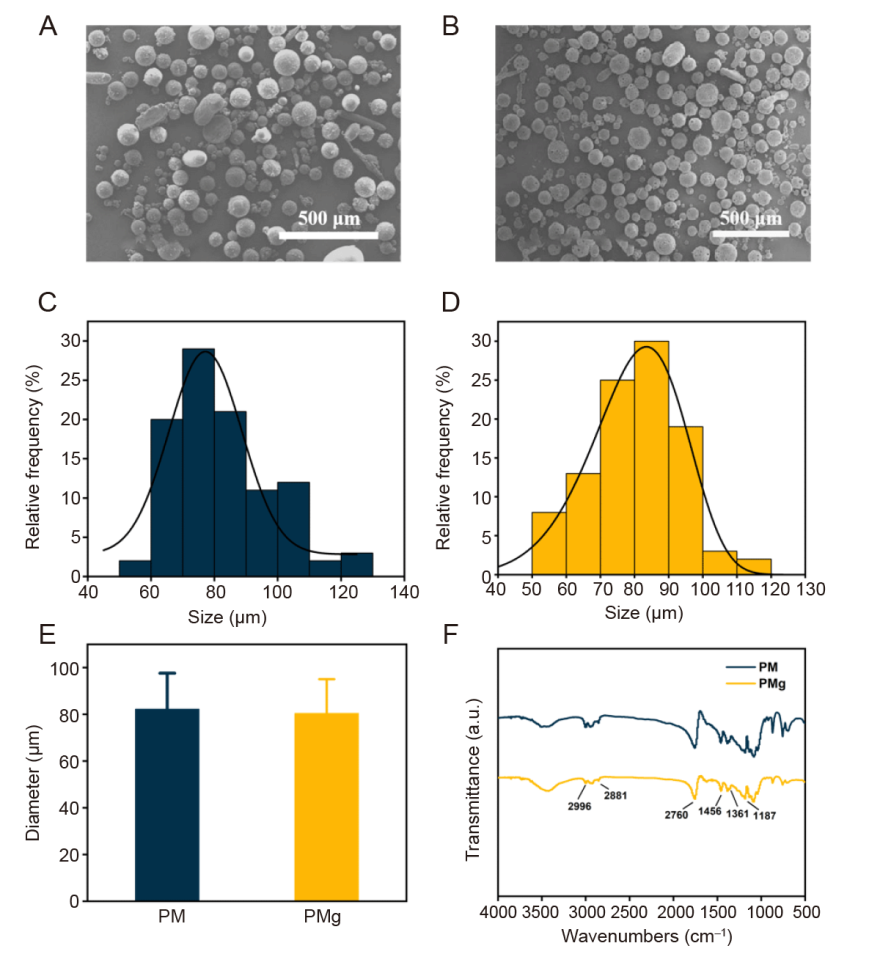
Figure 3. Characterisation of poly(L-lactic acid) microsphere (PM) and poly(L-lactic acid) microsphere loaded with magnesium oxide (MgO)/magnesium carbonate (MgCO3) (PMg). (A, B) Scanning electron micrograph image of PM (A) and PMg (B). (C, D) Particle size distribution of PM (C) and PMg (D). (E) Particle size of PM and PMg. Data are expressed as mean ± SD. (F) Fourier transformed infrared spectrophotometer spectra of PM and PMg. a.u.: arbitrary unit.
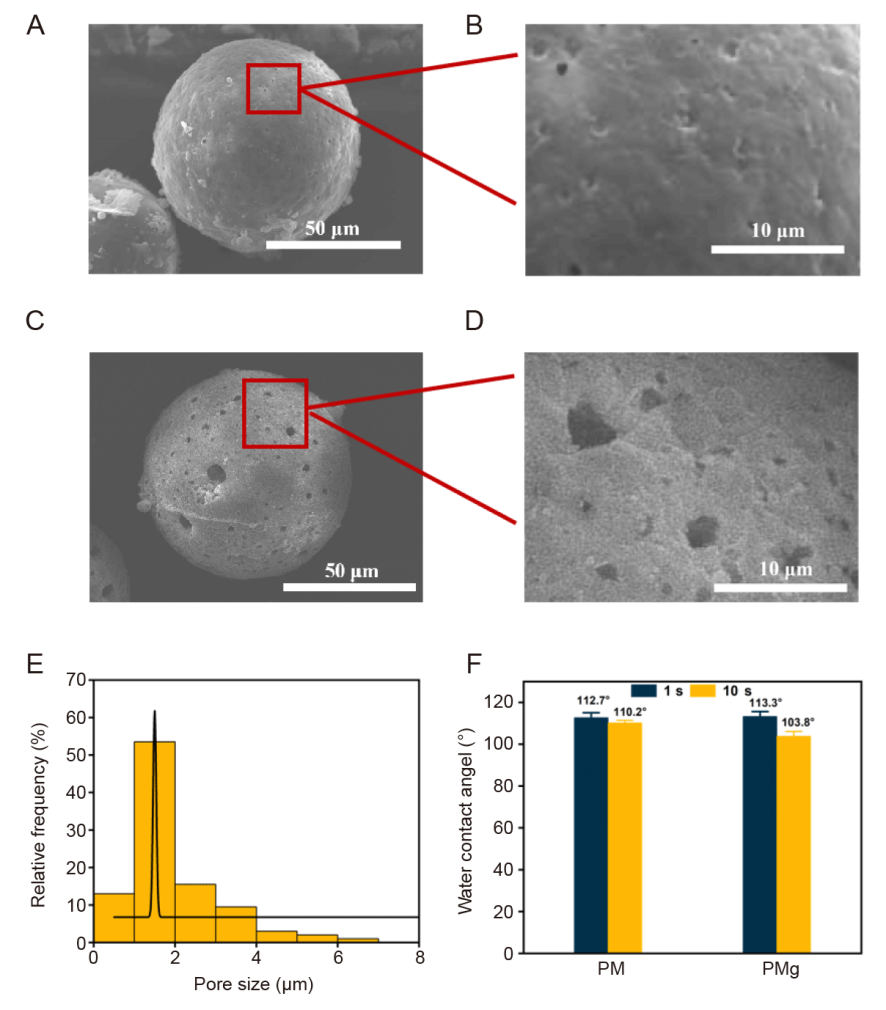
Figure 4. Enlarged microscopic images of poly(L-lactic acid) microsphere (PM) and poly(L-lactic acid) microsphere loaded with magnesium oxide (MgO)/magnesium carbonate (MgCO3) (PMg). (A-D) PM surface is smooth and without porous structure, and pores appeared on the surface of PMg, which was attributed to the generation of CO2 from MgCO3. Scale bars: 50 μm (A, C), 10 μm (B, D). (E) The distribution of pore size on the surface of PMg. (F) The contact angle test of PM and PMg. PMg is more hydrophilic and absorbent compared to PM. Data are expressed as mean ± SD.

Figure 5. In vitro degradation behaviours of poly(L-lactic acid) microsphere (PM) and poly(L-lactic acid) microsphere loaded with magnesium oxide (MgO)/magnesium carbonate (MgCO3) (PMg). (A) Accelerated degradation of PMg compared to PM, shows that MgO and MgCO3 accelerate the rate of molecular weight degradation of microspheres. (B) The degradation environment pH of PMg is stable in the neutral range and does not interfere with the body’s typical acidic and alkaline environment. (C) No sudden release of Mg2+ occurred during the 30-day degradation cycle. Data are expressed as mean ± SD, and were analysed by Student’s unpaired t-test. (D) The scanning electron micrograph images of PM before degradation. (E) On the 30th day of degradation, scanning electron micrograph image showed that there was a significant increase in the number of “craters” on the surface of PM. (F) Enlarged scanning electron micrograph images at 30 days of PM degradation (G) The scanning electron micrograph images of PMg before degradation. (H) At 30 days of degradation, the electron micrographs showed that the pores on the surface of PMg were significantly enlarged due to corrosion. (I) Enlarged scanning electron micrograph images at 30 days of PMg degradation. Scale bars: 50 μm (D, E, G, H), 10 μm (F, I).
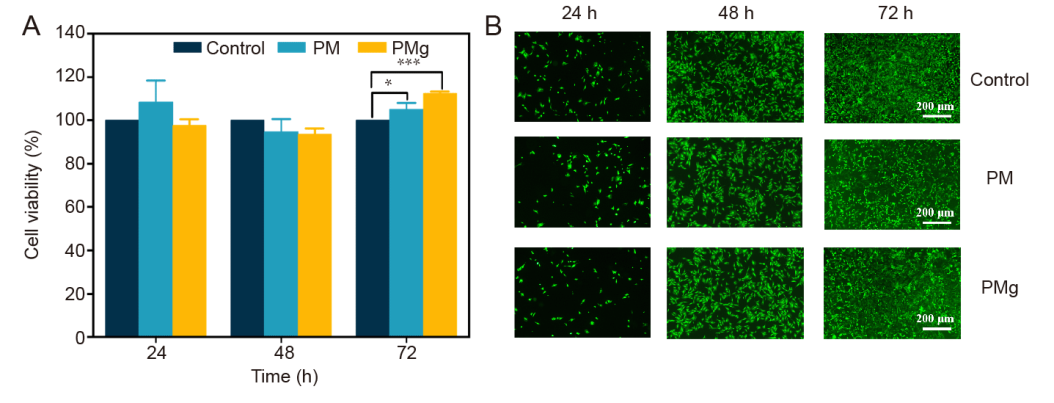
Figure 6. Cytocompatibility evaluation and live-dead staining of poly(L-lactic acid) microsphere (PM) and poly(L-lactic acid) microsphere loaded with magnesium oxide (MgO)/magnesium carbonate (MgCO3) (PMg). (A) More than 80% of cell viability of tendon-derived stem cells (TDSCs) on PM and PMg, which were normalised by control group. Data are expressed as the mean ± SD (n = 3). *P < 0.05, ***P < 0.001 (one-way analysis of variance followed by Dunnett’s multiple comparisons test). (B) Cytocompatibility of TDSCs on PM and PMg. The live/dead staining assay demonstrated that PM and PMg exhibits no cytotoxicity to cells. TDSCs cultured with osteogenic medium were used as control. Scale bars: 200 μm.
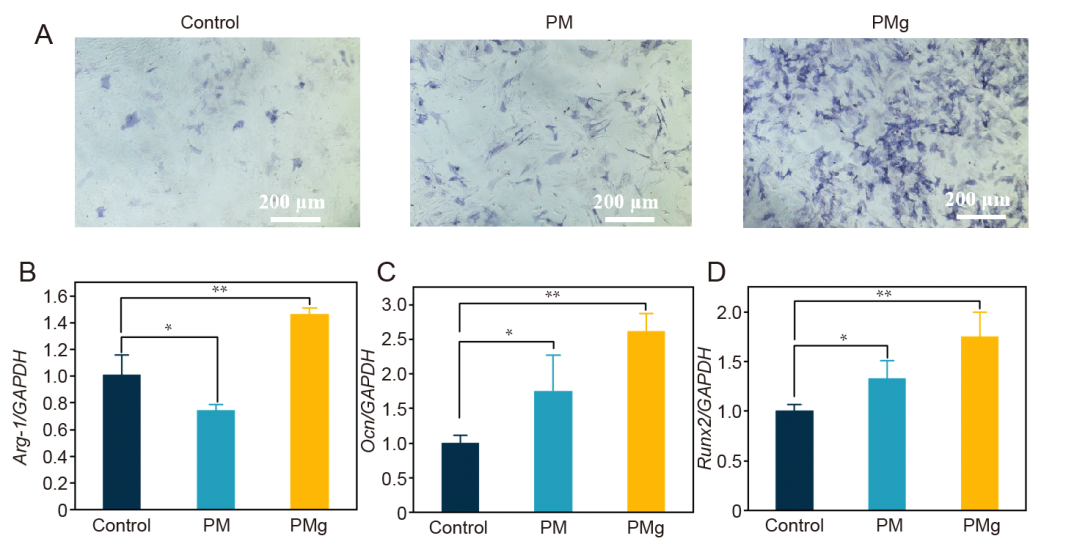
Figure 7. Osteogenic capacity evaluation of poly(L-lactic acid) microsphere (PM) and poly(L-lactic acid) (PLLA) microsphere loaded with magnesium oxide (MgO)/magnesium carbonate (MgCO3) (PMg). (A) The level of alkaline phosphatase (ALP) was more obvious in PMg compared to PM. PMg has more osteogenic properties compared to PM, while PLLA material has some osteogenic properties compared to the control. (B) The expression of anti-inflammatory gene (arginase-1, Arg-1) was more obvious in PMg compared to PM. (C, D) The expression of osteogenic differentiation gene (osteocalcin, Ocn) and (Runx2) was more obvious in PMg compared to PM, while PLLA material can promote osteogenic differentiation of tendon-derived stem cells (TDSCs) compared to the control. RAW264.7 cells were induced with lipopolysaccharides as controls in B and TDSCs cultured with osteogenic medium were as controls in C and D. Data (normalised by control group) are expressed as the mean ± SD (n = 3). *P < 0.05, **P < 0.01 (one-way analysis of variance followed by Dunnett’s multiple comparisons test). GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
| 1. | Cho, E. R.; Kang, S. W.; Kim, B. S. Poly(lactic-co-glycolic acid) microspheres as a potential bulking agent for urological injection therapy: preliminary results. J Biomed Mater Res B Appl Biomater. 2005, 72, 166-172. |
| 2. |
Gao, Q.; Duan, L.; Feng, X.; Xu, W. Superiority of poly(l-lactic acid) microspheres as dermal fillers. Chin Chem Lett. 2021, 32, 577-582.
doi: 10.1016/j.cclet.2020.03.071 URL |
| 3. | Kang, S. W.; Cho, E. R.; Jeon, O.; Kim, B. S. The effect of microsphere degradation rate on the efficacy of polymeric microspheres as bulking agents: an 18-month follow-up study. J Biomed Mater Res B Appl Biomater. 2007, 80, 253-259. |
| 4. |
Zhang, M.; Tang, Y.; Zhu, Z.; Zhao, H.; Yao, J.; Sun, D. Paclitaxel and etoposide-loaded Poly (lactic-co-glycolic acid) microspheres fabricated by coaxial electrospraying for dual drug delivery. J Biomater Sci Polym Ed. 2018, 29, 1949-1963.
doi: 10.1080/09205063.2018.1485816 URL |
| 5. |
Yu, C.; Zhu, W.; He, Z.; Xu, J.; Fang, F.; Gao, Z.; Ding, W.; Wang, Y.; Wang, J.; Wang, J.; Huang, A.; Cheng, A.; Wei, Y.; Ai, S. ATP-triggered drug release system based on ZIF-90 loaded porous poly(lactic-co-glycolic acid) microspheres. Colloids Surf Physicochem Eng Aspects. 2021, 615, 126255.
doi: 10.1016/j.colsurfa.2021.126255 URL |
| 6. |
Shi, X. D.; Sun, P. J.; Gan, Z.H. Preparation of porous polylactide microspheres and their application in tissue engineering. Chin J Polym Sci. 2018, 36, 712-719.
doi: 10.1007/s10118-018-2079-x |
| 7. | Seyyed Nasrollah, S. A.; Karimi-Soflou, R.; Karkhaneh, A. Photo-click crosslinked hydrogel containing MgO2-loaded PLGA microsphere with concurrent magnesium and oxygen release for bone tissue engineering. Mater Today Chem. 2023, 28, 101389. |
| 8. |
Yuan, X.; Lin, S.; Zhao, K.; Han, Y. Emulsion-ultrasonic spray method to prepare polylactic acid microspheres. Mater Lett. 2022, 309, 131461.
doi: 10.1016/j.matlet.2021.131461 URL |
| 9. |
Zeng, Y.; Li, X.; Liu, X.; Yang, Y.; Zhou, Z.; Fan, J.; Jiang, H. PLLA porous microsphere-reinforced silk-based scaffolds for auricular cartilage regeneration. ACS Omega. 2021, 6, 3372-3383.
doi: 10.1021/acsomega.0c05890 URL |
| 10. |
Lin, A.; Liu, S.; Xiao, L.; Fu, Y.; Liu, C.; Li, Y. Controllable preparation of bioactive open porous microspheres for tissue engineering. J Mater Chem B. 2022, 10, 6464-6471.
doi: 10.1039/D2TB01198K URL |
| 11. |
Ali, W.; Ali, H.; Gillani, S.; Zinck, P.; Souissi, S. Polylactic acid synthesis, biodegradability, conversion to microplastics and toxicity: a review. Environ Chem Lett. 2023, 21, 1761-1786.
doi: 10.1007/s10311-023-01564-8 |
| 12. |
Amiryaghoubi, N.; Fathi, M.; Barar, J.; Omidian, H.; Omidi, Y. Hybrid polymer-grafted graphene scaffolds for microvascular tissue engineering and regeneration. Eur Polym J. 2023, 193, 112095.
doi: 10.1016/j.eurpolymj.2023.112095 URL |
| 13. | Polyák, P.; Nagy, K.; Vértessy, B.; Pukánszky, B. Self-regulating degradation technology for the biodegradation of poly(lactic acid). EnvironTechnol Innov. 2023, 29, 103000. |
| 14. |
Bee, S. L.; Hamid, Z. A. A.; Mariatti, M.; Yahaya, B. H.; Lim, K.; Bee, S. T.; Sin, L. T. Approaches to improve therapeutic efficacy of biodegradable PLA/PLGA microspheres: a review. Polym Rev. 2018, 58, 495-536.
doi: 10.1080/15583724.2018.1437547 URL |
| 15. |
Sahini, M. G. Polylactic acid (PLA)-based materials: a review on the synthesis and drug delivery applications. Emergent Mater. 2023, 6, 1461-1479.
doi: 10.1007/s42247-023-00551-7 |
| 16. |
Dodda, J. M.; Azar, M. G.; Bělský, P.; Šlouf, M.; Gajdošová, V.; Kasi, P. B.; Anerillas, L. O.; Kovářík, T. Bioresorbable films of polycaprolactone blended with poly(lactic acid) or poly(lactic-co-glycolic acid). Int J Biol Macromol. 2023, 248, 126654.
doi: 10.1016/j.ijbiomac.2023.126654 URL |
| 17. |
Bogdanova, A.; Pavlova, E.; Polyanskaya, A.; Volkova, M.; Biryukova, E.; Filkov, G.; Trofimenko, A.; Durymanov, M.; Klinov, D.; Bagrov, D. Acceleration of electrospun PLA degradation by addition of gelatin. Int J Mol Sci. 2023, 24, 3535.
doi: 10.3390/ijms24043535 URL |
| 18. |
Chen, Y.; Geever, L. M.; Killion, J. A.; Lyons, J. G.; Higginbotham, C. L.; Devine, D. M. Review of multifarious applications of poly (lactic acid). Polym Plast Technol Eng. 2016, 55, 1057-1075.
doi: 10.1080/03602559.2015.1132465 URL |
| 19. |
Swetha, T. A.; Ananthi, V.; Bora, A.; Sengottuvelan, N.; Ponnuchamy, K.; Muthusamy, G.; Arun, A. A review on biodegradable polylactic acid (PLA) production from fermentative food waste - Its applications and degradation. Int J Biol Macromol. 2023, 234, 123703.
doi: 10.1016/j.ijbiomac.2023.123703 URL |
| 20. |
Brunšek, R.; Kopitar, D.; Schwarz, I.; Marasović, P. Biodegradation properties of cellulose fibers and PLA biopolymer. Polymers (Basel). 2023, 15, 3532.
doi: 10.3390/polym15173532 URL |
| 21. |
Shahdan, D.; Rosli, N. A.; Chen, R. S.; Ahmad, S.; Gan, S. Strategies for strengthening toughened poly(lactic acid) blend via natural reinforcement with enhanced biodegradability: A review. Int J Biol Macromol. 2023, 251, 126214.
doi: 10.1016/j.ijbiomac.2023.126214 URL |
| 22. |
Niu, Y.; Stadler, F. J.; Fu, M. Biomimetic electrospun tubular PLLA/gelatin nanofiber scaffold promoting regeneration of sciatic nerve transection in SD rat. Mater Sci Eng C Mater Biol Appl. 2021, 121, 111858.
doi: 10.1016/j.msec.2020.111858 URL |
| 23. |
Hou, Y.; Zhang, R.; Cheng, H.; Wang, Y.; Zhang, Q.; Zhang, L.; Wang, L.; Li, R.; Wu, X.; Li, B. Mg2+-doped carbon dots synthesized based on Lycium ruthenicum in cell imaging and promoting osteogenic differentiation in vitro. Colloids Surf Physicochem Eng Aspects. 2023, 656, 130264.
doi: 10.1016/j.colsurfa.2022.130264 URL |
| 24. |
Zhu, S.; Dai, Q.; Yao, L.; Wang, Z.; He, Z.; Li, M.; Wang, H.; Li, Q.; Gao, H.; Cao, X. Engineered multifunctional nanocomposite hydrogel dressing to promote vascularization and anti-inflammation by sustained releasing of Mg2+ for diabetic wounds. Compos B Eng. 2022, 231, 109569.
doi: 10.1016/j.compositesb.2021.109569 URL |
| 25. |
Zhang, X.; Huang, P.; Jiang, G.; Zhang, M.; Yu, F.; Dong, X.; Wang, L.; Chen, Y.; Zhang, W.; Qi, Y.; Li, W.; Zeng, H. A novel magnesium ion-incorporating dual-crosslinked hydrogel to improve bone scaffold-mediated osteogenesis and angiogenesis. Mater Sci Eng C Mater Biol Appl. 2021, 121, 111868.
doi: 10.1016/j.msec.2021.111868 URL |
| 26. |
Zhu, Y.; Zhao, S.; Cheng, L.; Lin, Z.; Zeng, M.; Ruan, Z.; Sun, B.; Luo, Z.; Tang, Y.; Long, H. Mg2+-mediated autophagy-dependent polarization of macrophages mediates the osteogenesis of bone marrow stromal stem cells by interfering with macrophage-derived exosomes containing miR-381. J Orthop Res. 2022, 40, 1563-1576.
doi: 10.1002/jor.v40.7 URL |
| 27. |
Faisal, S.; Abdullah; Jan, H.; Shah, S. A.; Shah, S.; Rizwan, M.; Zaman, N.; Hussain, Z.; Uddin, M. N.; Bibi, N.; Khattak, A.; Khan, W.; Iqbal, A.; Idrees, M.; Masood, R. Bio-catalytic activity of novel mentha arvensis intervened biocompatible magnesium oxide nanomaterials. Catalysts. 2021, 11, 780.
doi: 10.3390/catal11070780 URL |
| 28. |
Li, J.; Khalid, A.; Verma, R.; Abraham, A.; Qazi, F.; Dong, X.; Liang, G.; Tomljenovic-Hanic, S. Silk fibroin coated magnesium oxide nanospheres: a biocompatible and biodegradable tool for noninvasive bioimaging applications. Nanomaterials (Basel). 2021, 11, 695.
doi: 10.3390/nano11030695 URL |
| 29. |
Welch, K.; Latifzada, M. A.; Frykstrand, S.; Strømme, M. Investigation of the antibacterial effect of mesoporous magnesium carbonate. ACS Omega. 2016, 1, 907-914.
doi: 10.1021/acsomega.6b00124 URL |
| 30. |
Palominos, N.; Castillo, A.; Guerrero, L.; Borja, R.; Huiliñir, C. Coupling of anaerobic digestion and struvite precipitation in the same reactor: effect of zeolite and bischofite as Mg2+ source. Front Environ Sci. 2021, 9, 706730.
doi: 10.3389/fenvs.2021.706730 URL |
| 31. |
Hornak, J. Synthesis, properties, and selected technical applications of magnesium oxide nanoparticles: a review. Int J Mol Sci. 2021, 22, 12752.
doi: 10.3390/ijms222312752 URL |
| 32. | Lin, Z.; Shen, D.; Zhou, W.; Zheng, Y.; Kong, T.; Liu, X.; Wu, S.; Chu, P. K.; Zhao, Y.; Wu, J.; Cheung, K. M. C.; Yeung, K. W. K. Regulation of extracellular bioactive cations in bone tissue microenvironment induces favorable osteoimmune conditions to accelerate in situ bone regeneration. Bioact Mater. 2021, 6, 2315-2330. |
| 33. |
Fan, D.; De Rosa, E.; Murphy, M. B.; Peng, Y.; Smid, C. A.; Chiappini, C.; Liu, X.; Simmons, P.; Weiner, B. K.; Ferrari, M.; Tasciotti, E. Mesoporous silicon-PLGA composite microspheres for the double controlled release of biomolecules for orthopedic tissue engineering. Adv Funct Mater. 2012, 22, 282-293.
doi: 10.1002/adfm.v22.2 URL |
| 34. |
Yuan, Z.; Wei, P.; Huang, Y.; Zhang, W.; Chen, F.; Zhang, X.; Mao, J.; Chen, D.; Cai, Q.; Yang, X. Injectable PLGA microspheres with tunable magnesium ion release for promoting bone regeneration. Acta Biomater. 2019, 85, 294-309.
doi: 10.1016/j.actbio.2018.12.017 URL |
| 35. |
Wang, L.; Li, Y.; Jiang, S.; Zhang, Z.; Zhao, S.; Song, Y.; Liu, J.; Tan, F. Alginate hydrogels containing different concentrations of magnesium-containing poly(lactic-co-glycolic acid) microspheres for bone tissue engineering. Biomed Mater. 2023, 18, 055022.
doi: 10.1088/1748-605X/ace9a5 |
| 36. | Yuan, X.; Yang, W.; Fu, Y.; Tao, Z.; Xiao, L.; Zheng, Q.; Wu, D.; Zhang, M.; Li, L.; Lu, Z.; Wu, Y.; Gao, J.; Li, Y. Four-arm polymer-guided formation of curcumin-loaded flower-like porous microspheres as injectable cell carriers for diabetic wound healing. Adv Healthc Mater. 2023, e2301486. |
| 37. |
Hong, Y.; Gao, C.; Shi, Y.; Shen, J. Preparation of porous polylactide microspheres by emulsion-solvent evaporation based on solution induced phase separation. Polym Adv Technol. 2005, 16, 622-627.
doi: 10.1002/pat.v16:8 URL |
| 38. |
Lee, H.; Nguyen, T. T.; Kim, M.; Jeong, J. H.; Park, J. B. The effects of biodegradable poly(lactic-co-glycolic acid)-based microspheres loaded with quercetin on stemness, viability and osteogenic differentiation potential of stem cell spheroids. J Periodontal Res. 2018, 53, 801-815.
doi: 10.1111/jre.2018.53.issue-5 URL |
| 39. |
Yang, S.; Liu, H.; Huang, H.; Zhang, Z. Fabrication of superparamagnetic magnetite/poly(styrene-co-12-acryloxy-9-octadecenoic acid) nanocomposite microspheres with controllable structure. J Colloid Interface Sci. 2009, 338, 584-590.
doi: 10.1016/j.jcis.2009.07.007 URL |
| 40. |
Pan, X.; Gao, M.; Wang, Y.; He, Y.; Si, T.; Sun, Y. Poly(lactic acid)-aspirin microspheres prepared via the traditional and improved solvent evaporation methods and its application performances. Chin J Chem Eng. 2023, 60, 194-204.
doi: 10.1016/j.cjche.2023.01.002 URL |
| 41. | Schneider, C. A.; Rasband, W. S.; Eliceiri, K. W. NIH image to ImageJ: 25 years of image analysis. Nat Methods. 2012, 9, 671-675. |
| 42. |
Rui, Y. F.; Lui, P. P.; Li, G.; Fu, S. C.; Lee, Y. W.; Chan, K. M. Isolation and characterization of multipotent rat tendon-derived stem cells. Tissue Eng Part A. 2010, 16, 1549-1558.
doi: 10.1089/ten.tea.2009.0529 URL |
| 43. |
Livak, K. J.; Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001, 25, 402-408.
doi: 10.1006/meth.2001.1262 URL |
| 44. |
Liu, S.; Chen, W.; Xiao, L.; Zhao, Z.; Liu, F.; Lu, S.; Chen, C.; Luo, W.; Jiang, L.; Li, Y. Robust osteoconductive β-tricalcium phosphate/L-poly(lactic acid) membrane via orientation-strengthening technology. ACS Biomater Sci Eng. 2023, 9, 5293-5303.
doi: 10.1021/acsbiomaterials.3c00617 URL |
| 45. | Yuan, X.; Yang, W.; Fu, Y.; Tao, Z.; Xiao, L.; Zheng, Q.; Wu, D.; Zhang, M.; Li, L.; Lu, Z.; Wu, Y.; Gao, J.; Li, Y. Four-arm polymer-guided formation of curcumin-loaded flower-like porous microspheres as injectable cell carriers for diabetic wound healing. Adv Healthc Mater. 2023, 12, e2301486. |
| 46. |
Lai, X. L.; Yang, W.; Wang, Z.; Shi, D. W.; Liu, Z. Y.; Yang, M. B. Enhancing crystallization rate and melt strength of PLLA with four-arm PLLA grafted silica: the effect of molecular weight of the grafting PLLA chains. J Appl Polym Sci. 2018, 135, 45675.
doi: 10.1002/app.v135.2 URL |
| 47. | Lee, J. Y.; Kim, S. E.; Yun, Y. P.; Choi, S. W.; Jeon, D. I.; Kim, H. J.; Park, K.; Song, H. R. Osteogenesis and new bone formation of alendronate-immobilized porous PLGA microspheres in a rat calvarial defect model. J Ind Eng Chem. 2017, 52, 277-286. |
| 48. |
Capuana, E.; Lopresti, F.; Ceraulo, M.; La Carrubba, V. Poly-l-lactic acid (PLLA)-based biomaterials for regenerative medicine: a review on processing and applications. Polymers (Basel). 2022, 14, 1153.
doi: 10.3390/polym14061153 URL |
| 49. |
Zan, J.; Qian, G.; Deng, F.; Zhang, J.; Zeng, Z.; Peng, S.; Shuai, C. Dilemma and breakthrough of biodegradable poly-l-lactic acid in bone tissue repair. J Mater Res Technol. 2022, 17, 2369-2387.
doi: 10.1016/j.jmrt.2022.01.164 URL |
| 50. | Feng, P.; Shen, S.; Shuai, Y.; Peng, S.; Shuai, C.; Chen, S. PLLA grafting draws GO from PGA phase to the interface in PLLA/PGA bone scaffold owing enhanced interfacial interaction. Sustain Mater Technol. 2023, 35, e00566. |
| 51. |
Yao, H.; Wang, J.; Deng, Y.; Li, Z.; Wei, J. Osteogenic and antibacterial PLLA membrane for bone tissue engineering. Int J Biol Macromol. 2023, 247, 125671.
doi: 10.1016/j.ijbiomac.2023.125671 URL |
| 52. |
Han, X.; Zhou, X.; Qiu, K.; Feng, W.; Mo, H.; Wang, M.; Wang, J.; He, C. Strontium-incorporated mineralized PLLA nanofibrous membranes for promoting bone defect repair. Colloids Surf B Biointerfaces. 2019, 179, 363-373.
doi: 10.1016/j.colsurfb.2019.04.011 URL |
| 53. |
Baek, S. W.; Kim, D. S.; Song, D. H.; Lee, S.; Lee, J. K.; Park, S. Y.; Kim, J. H.; Kim, T. H.; Park, C. G.; Han, D. K. PLLA composites combined with delivery system of bioactive agents for anti-inflammation and re-endothelialization. Pharmaceutics. 2022, 14, 2661.
doi: 10.3390/pharmaceutics14122661 URL |
| 54. |
Shuai, Y. A tumor-microenvironment-activated nanoplatform of modified SnFe2O4 nanozyme in scaffold for enhanced PTT/PDT tumor therapy. Heliyon. 2023, 9, e18019.
doi: 10.1016/j.heliyon.2023.e18019 URL |
| 55. |
Li, B.; Yang, H.; Cheng, K.; Song, H.; Zou, J.; Li, C.; Xiao, W.; Liu, Z.; Liao, X. Development of magnetic poly(L-lactic Acid) nanofibrous microspheres for transporting and delivering targeted cells. Colloids Surf B Biointerfaces. 2023, 223, 113175.
doi: 10.1016/j.colsurfb.2023.113175 URL |
| 56. |
Wang, Y.; Zhao, L.; Zhou, L.; Chen, C.; Chen, G. Sequential release of vascular endothelial growth factor-A and bone morphogenetic protein-2 from osteogenic scaffolds assembled by PLGA microcapsules: A preliminary study in vitro. Int J Biol Macromol. 2023, 232, 123330.
doi: 10.1016/j.ijbiomac.2023.123330 URL |
| [1] | Jin Yang, Kanwal Fatima, Xiaojun Zhou, Chuanglong He. Meticulously engineered three-dimensional-printed scaffold with microarchitecture and controlled peptide release for enhanced bone regeneration [J]. Biomaterials Translational, 2024, 5(1): 69-83. |
| [2] | Gen Wang, Zhangqin Yuan, Li Yu, Yingkang Yu, Pinghui Zhou, Genglei Chu, Huan Wang, Qianping Guo, Caihong Zhu, Fengxuan Han, Song Chen, Bin Li. Mechanically conditioned cell sheets cultured on thermo-responsive surfaces promote bone regeneration [J]. Biomaterials Translational, 2023, 4(1): 27-40. |
| [3] | Yang Zhao, Qing Sun, Bo Huo. Focal adhesion regulates osteogenic differentiation of mesenchymal stem cells and osteoblasts [J]. Biomaterials Translational, 2021, 2(4): 312-322. |
| [4] | Qingchuan Wang, Weidan Wang, Yanfang Li, Weirong Li, Lili Tan, Ke Yang. Biofunctional magnesium coating of implant materials by physical vapour deposition [J]. Biomaterials Translational, 2021, 2(3): 248-256. |
| [5] | Kamolrat Metavarayuth, Esteban Villarreal, Hui Wang, Qian Wang. Surface topography and free energy regulate osteogenesis of stem cells: effects of shape-controlled gold nanoparticles [J]. Biomaterials Translational, 2021, 2(2): 165-173. |
| [6] | Yunsong Shi, Ruijun He, Xiangyu Deng, Zengwu Shao, Davide Deganello, Chunze Yan, Zhidao Xia. Three-dimensional biofabrication of an aragonite-enriched self-hardening bone graft substitute and assessment of its osteogenicity in vitro and in vivo [J]. Biomaterials Translational, 2020, 1(1): 69-81. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||