Biomaterials Translational ›› 2024, Vol. 5 ›› Issue (1): 69-83.doi: 10.12336/biomatertransl.2024.01.007
• RESEARCH ARTICLES • Previous Articles Next Articles
Jin Yang1,2, Kanwal Fatima1,2, Xiaojun Zhou1,2, Chuanglong He1,2,*( )
)
Received:2023-12-06
Revised:2024-02-08
Accepted:2024-02-29
Online:2024-03-28
Published:2024-03-28
Contact:
Chuanglong He, 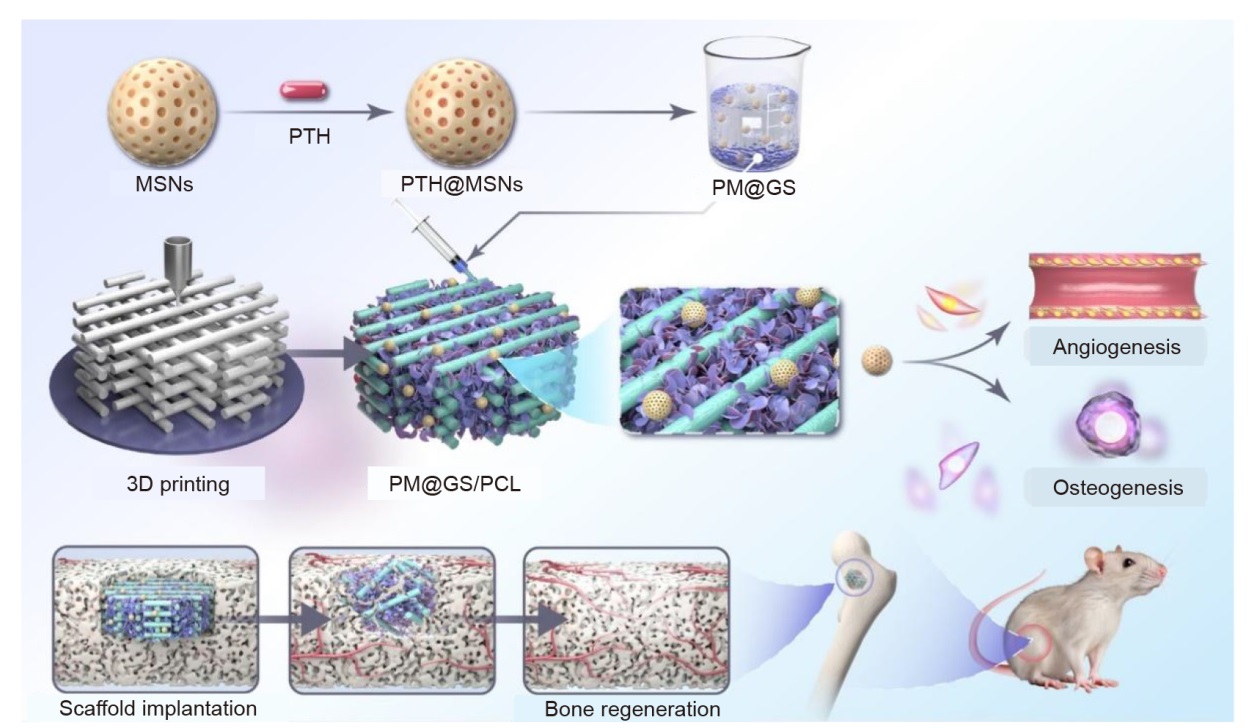
Figure 1. Schematic illustration of the construction of PM@GS/PCL composite scaffold and the process of promoting bone regeneration in a rat femoral defect model. 3D: three-dimensional; GelMA: methacrylate gelatin; MSNs: mesoporous silica nanoparticles; PCL: polycaprolactone; PM: PTH@MSNs; PM@GS/PCL: PTH@MSNs/GelMA/SFMA/PCL; PM@GS: PTH@MSNs/GelMA/SFMA; PTH: parathyroid hormone (1–34); PTH@MSNs: PTH-loaded MSNs; SFMA: methacrylated silk fibroin.
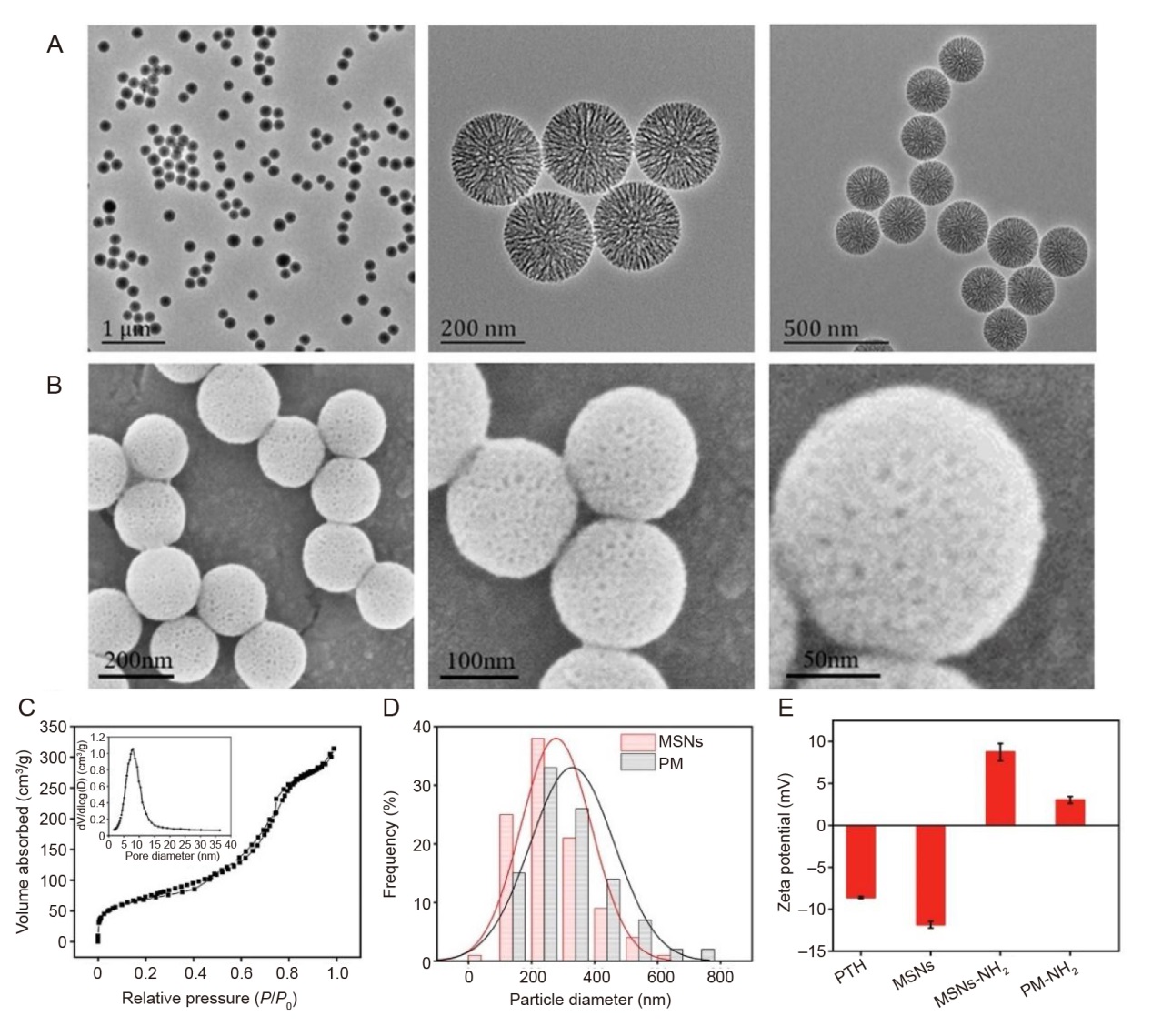
Figure 2. Characterisation of MSNs and PMs. (A) TEM images of MSNs at various magnifications. Scale bars: 1 μm (left), 200 nm (middle) and 500 nm (right). (B) SEM images of MSNs at various magnifications. The nanoparticles of MSNs exhibited a homogeneous spherical shape with mesoporous structure. Scale bars: 200 nm (left), 100 nm (middle) and 50 nm (right). (C) The nitrogen absorption-desorption isotherm curves of MSNs and the pore size distribution inside. (D) Size distribution of MSNs and PMs. (E) Zeta potential results of different samples. MSNs: mesoporous silica nanoparticles; P: the equilibrium adsorption pressure of the gas; P0: the saturated vapour pressure of the gas at the adsorption temperature; PM: PTH@MSNs; PTH: parathyroid hormone (1–34); SEM: scanning electron microscope; TEM: transmission electron microscope.
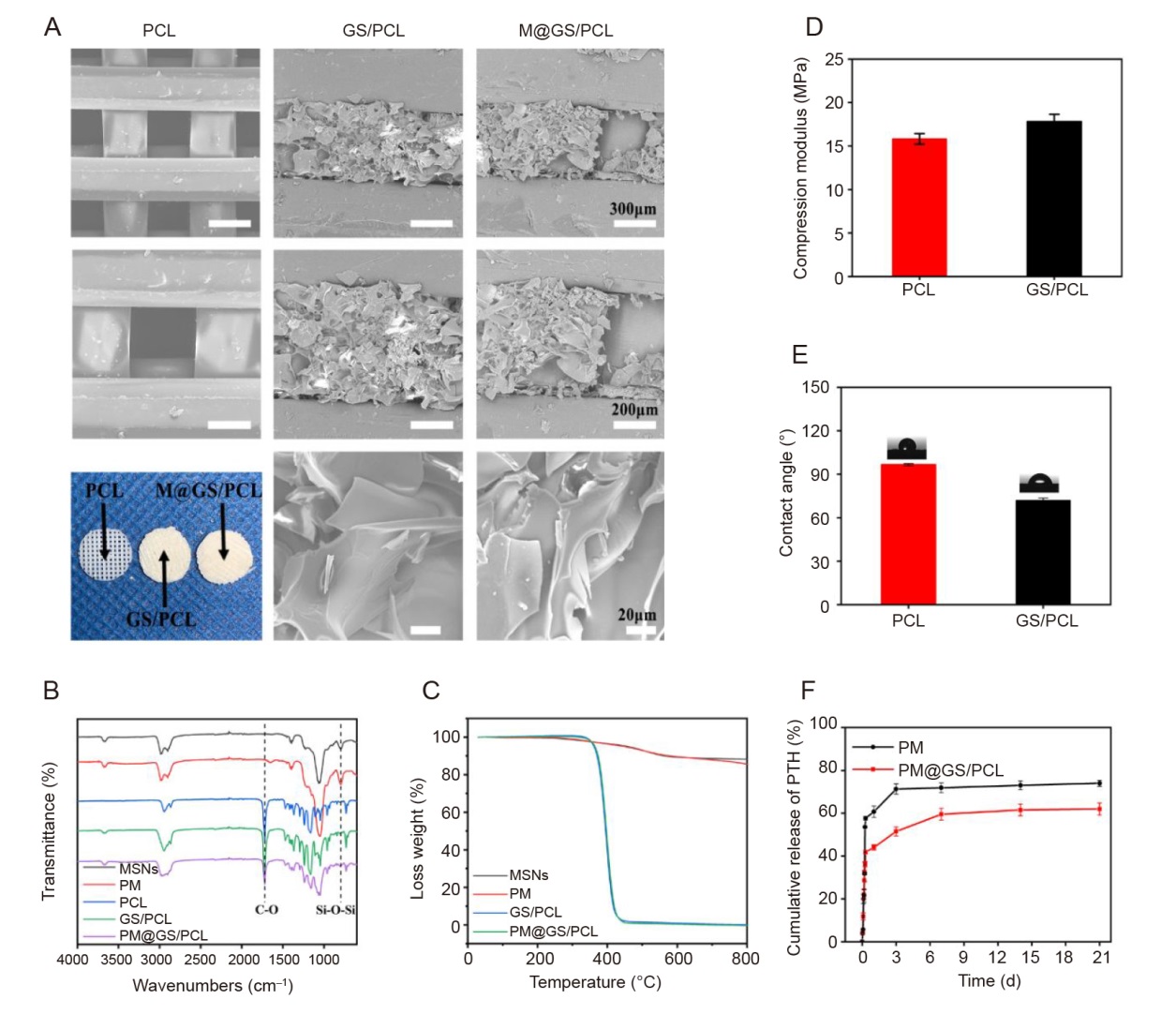
Figure 3. Characterisation of composite scaffolds. (A) SEM images of PCL, GS/PCL and M@GS/PCL at various magnifications and representative photographs of composite scaffolds. Scale bars: 300 μm (upper), 200 μm (middle) and 20 μm (lower). (B) ATR-FTIR spectra of MSNs and different composite scaffolds. (C) Thermogravimetric curves of MSNs, PTH@MSNs and different composite scaffolds. (D) Compressive modulus of PCL and GS/PCL scaffolds. (E) Water contact angle of PCL and GS/PCL scaffolds. (F) The release curves of PTH from PTH@MSNs and PM@GS/PCL in PBS. Data in D-F are presented as the mean ± SD. ATR-FTIR: attenuated total reflectance-Fourier transform infrared; GelMA: methacrylate gelatin; GS: GelMA/SFMA composite hydrogel; M@GS/PCL: MSNs@GelMA/SFMA/PCL; MSNs: mesoporous silica nanoparticles; PBS: phosphate-buffered saline; PCL: polycaprolactone; PM: PTH@MSNs; PM@GS/PCL: PTH@MSNs/GelMA/SFMA/PCL; PTH: parathyroid hormone (1–34); SEM: scanning electron microscope; SFMA: methacrylated silk fibroin.
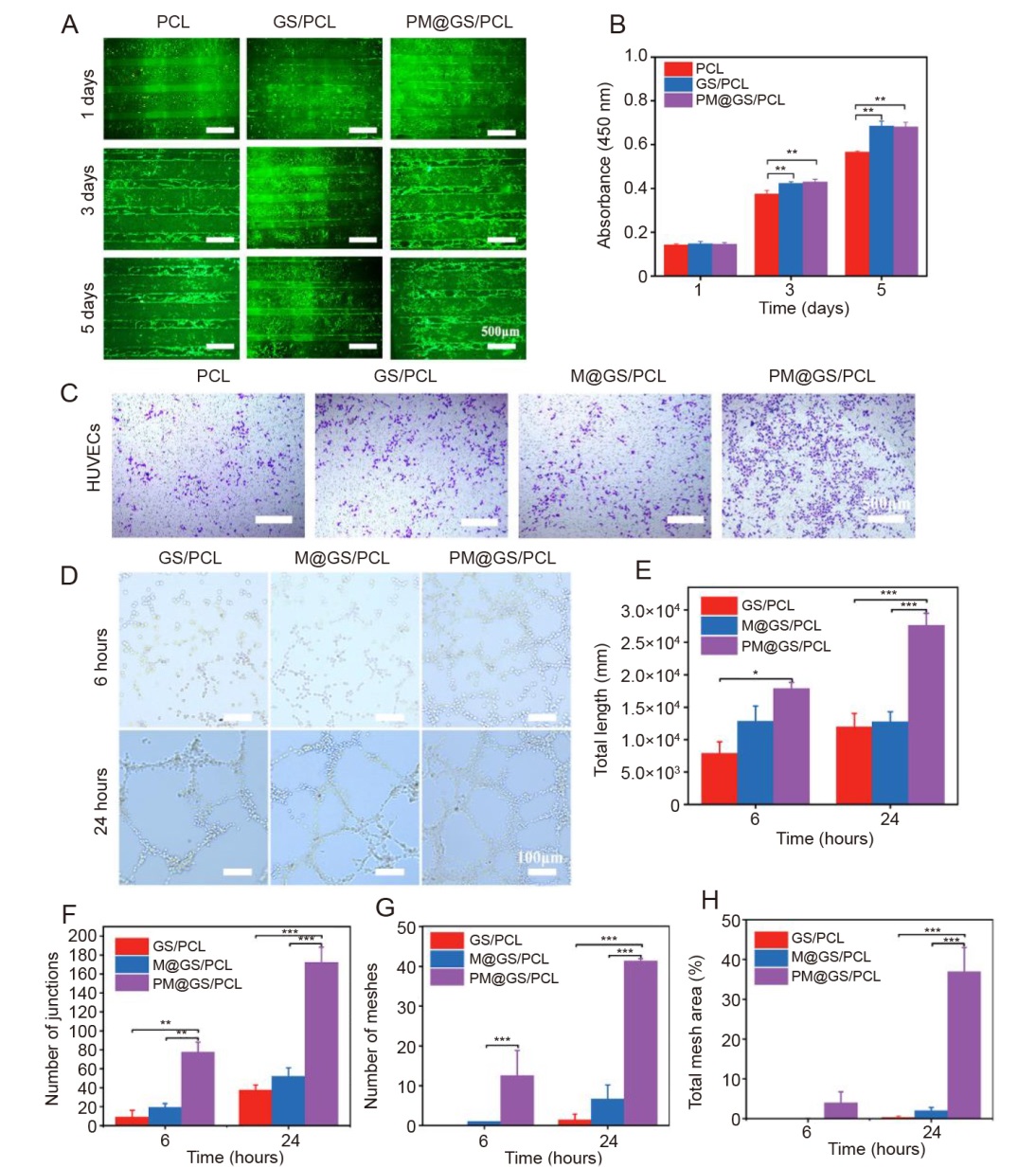
Figure 4. Impact of composite scaffolds on cell proliferation, migration and angiogenic activities. (A) BMSC proliferation on various scaffolds using a fluorescence microscope on days 1, 3 and 5. Viable cells were stained with Calcein-AM (green), while the dead cells were stained with PI (red). Scale bars: 500 μm. (B) Quantitative analysis of BMSCs proliferation with different scaffolds on days 1, 3 and 5 by CCK-8 assay. (C) Transwell migration assay for HUVECs at 24 hours using crystal violet staining. Scale bars: 500 μm. (D) Tube formation of HUVECs after 6 and 24 hours of incubation. Scale bars: 100 μm. (E–H) Quantitative assessment of angiogenic parameters, including total length, number of junctions, number of meshes, and total mesh area. Data are presented as the mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 (one-way analysis of variance followed by Tukey’s post hoc test). BMSC: bone marrow-derived mesenchymal stem cell; CCK-8: cell counting kit-8; GS/PCL: GelMA/SFMA/PCL; GelMA: methacrylate gelatin; HUVEC: human umbilical vein endothelial cell; M@GS/PCL: MSNs@GelMA/SFMA/PCL; MSNs: mesoporous silica nanoparticles; PCL: polycaprolactone; PI: propidium iodide; PM@GS/PCL: PTH@MSNs/GelMA/SFMA/PCL; PTH: parathyroid hormone (1–34); SFMA: methacrylated silk fibroin.

Figure 5. In vitro osteogenic potential of scaffold extracts. (A) Representative images of ALP staining of BMSCs cultured with the conditioned medium from different scaffolds on days 7 and 14. Scale bars: 500 μm. (B) Representative images of ARS staining of BMSCs cultured with extracts from different scaffolds to observe the mineral matrix formation on days 14 and 21. Scale bars: 500 μm. (C) Quantitative result of ALP activity on days 7 and 14. (D) Quantitative result of ARS on days 14 and 21. (E–G) Expression of osteogenesis-related genes in BMSCs after incubation with different scaffold extracts for 7 and 14 days, including Runx2 (E), OCN (F), and OPN (G). Data are presented as the mean ± SD. *P < 0.05, **P < 0.01 (one-way analysis of variance followed by Tukey’s post hoc test). ALP: alkaline phosphatase; ARS: Alizarin red S; BMSC: bone marrow-derived mesenchymal stem cell; GS/PCL: GelMA/SFMA/PCL; GelMA: methacrylate gelatin; M@GS/PCL: MSNs@GelMA/SFMA/PCL; MSNs: mesoporous silica nanoparticles; OCN: osteocalcin; OPN: osteopontin; PCL: polycaprolactone; PM@GS/PCL: PTH@MSNs/GelMA/SFMA/PCL; PTH: parathyroid hormone (1–34); Runx2: runt-related transcription factor 2; SFMA: methacrylated silk fibroin.
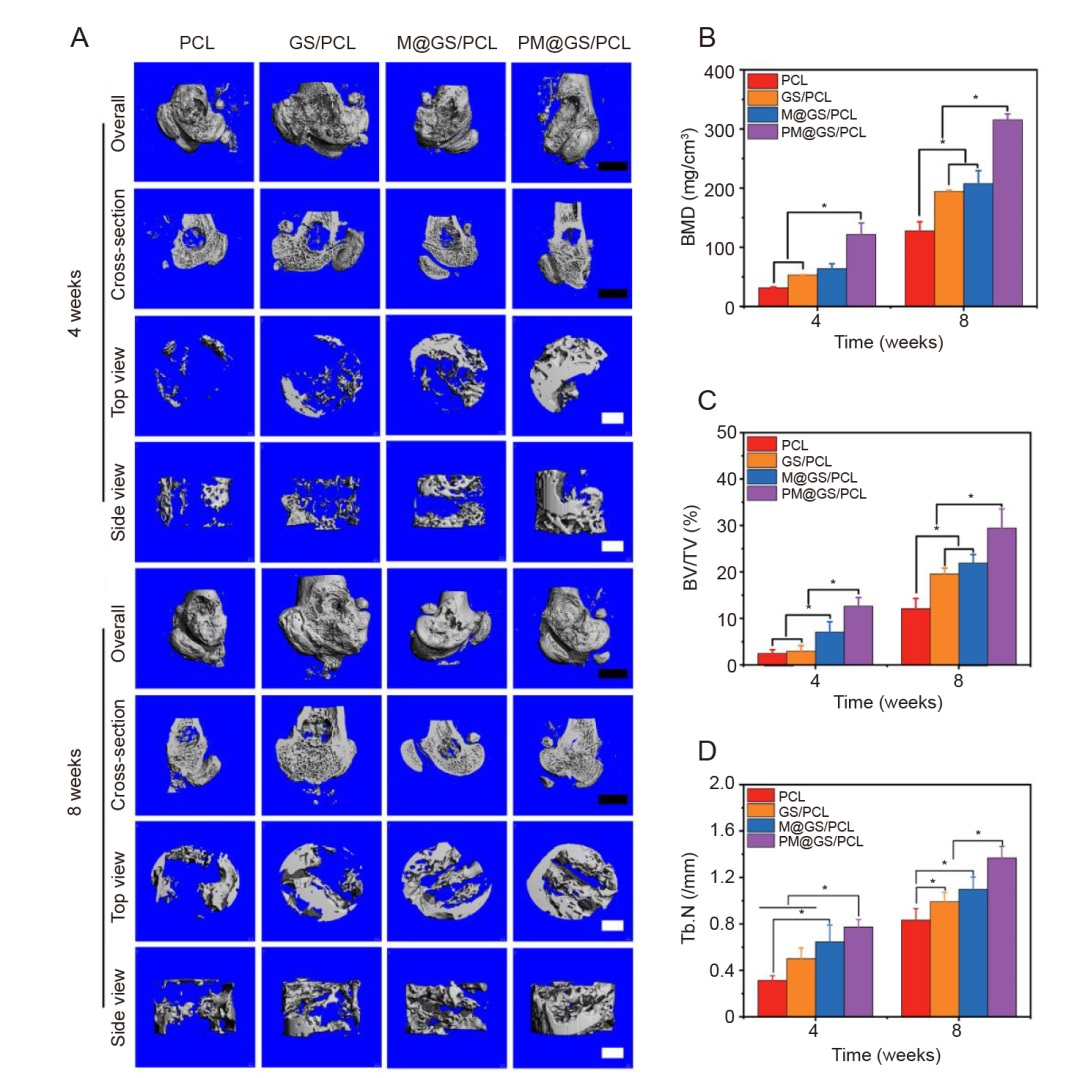
Figure 6. Bone regeneration in rat femoral condyle defect model with a diameter of 3 mm. (A) 3D reconstructed micro-CT images after scaffold implantation for 4 and 8 weeks, including overall, cross-section, top view and side view. Black scale bars: 1 mm; white scale bars: 500 μm. (B) BMD, (C) BV/TV and (D) Tb. N analysis of newly formed bone tissues obtained from micro-CT results. Data are presented as the mean ± SD. *P < 0.05 (one-way analysis of variance followed by Tukey’s post hoc test). 3D: three-dimensional; BMD: bone mineral density; BV/TV: ratio of new bone volume to total volume; CT: computed tomography; GS/PCL: GelMA/SFMA/PCL; GelMA: methacrylate gelatin; M@GS/PCL: MSNs@GelMA/SFMA/PCL; MSNs: mesoporous silica nanoparticles; PCL: polycaprolactone; PM@GS/PCL: PTH@MSNs/GelMA/SFMA/PCL; PTH: parathyroid hormone (1–34); SFMA: methacrylated silk fibroin; Tb. N: trabecular number.
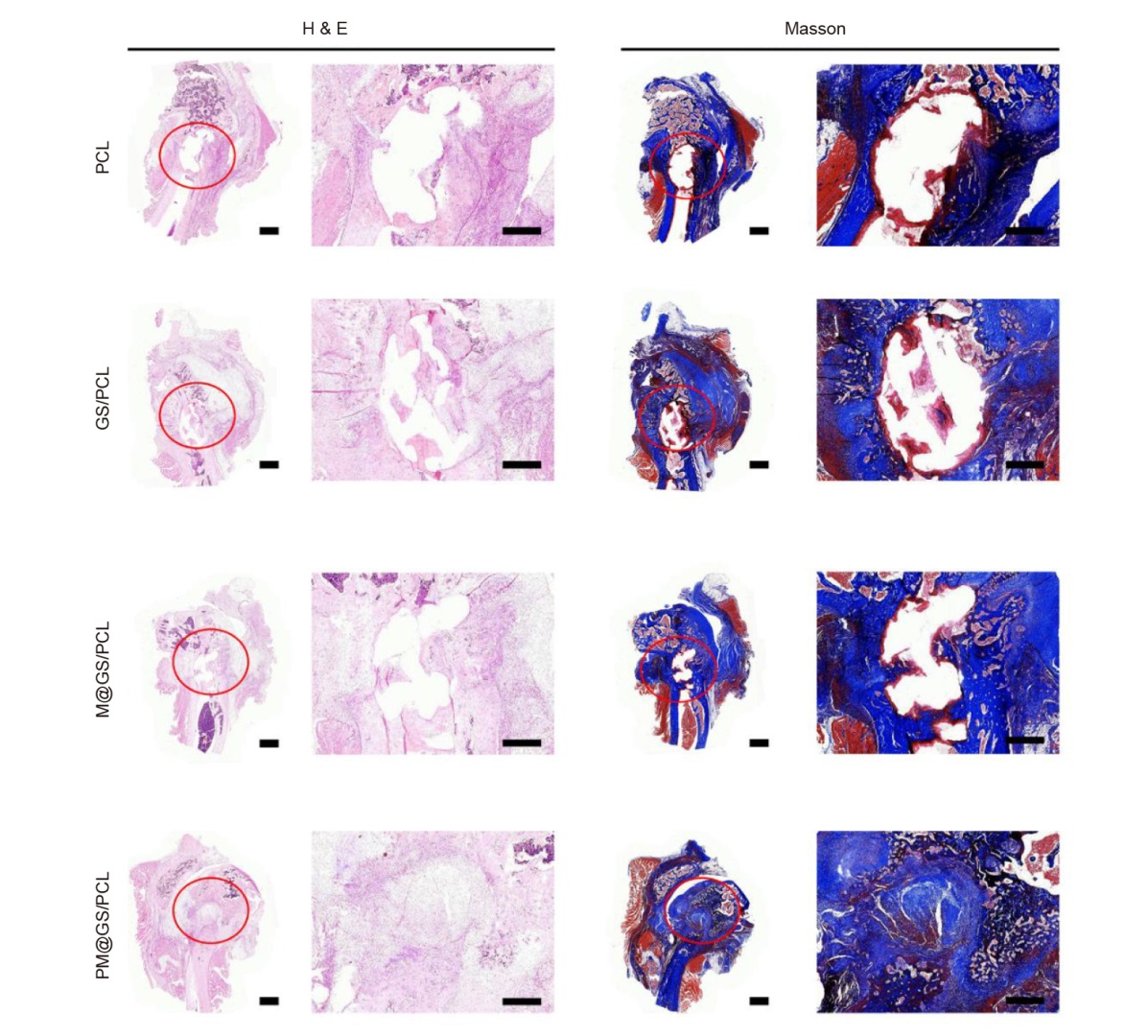
Figure 7. Histological analysis of femoral condyle defect at 8 weeks including H&E staining and Masson’s trichrome staining. Red circles indicate the areas of bone defect. Scale bars: 1 mm. GS/PCL: GelMA/SFMA/PCL; GelMA: methacrylate gelatin; H&E: haematoxylin and eosin; M@GS/PCL: MSNs@GelMA/SFMA/PCL; MSNs: mesoporous silica nanoparticles; PCL: polycaprolactone; PM@GS/PCL: PTH@MSNs/GelMA/SFMA/PCL; PTH: parathyroid hormone (1–34); SFMA: methacrylated silk fibroin.
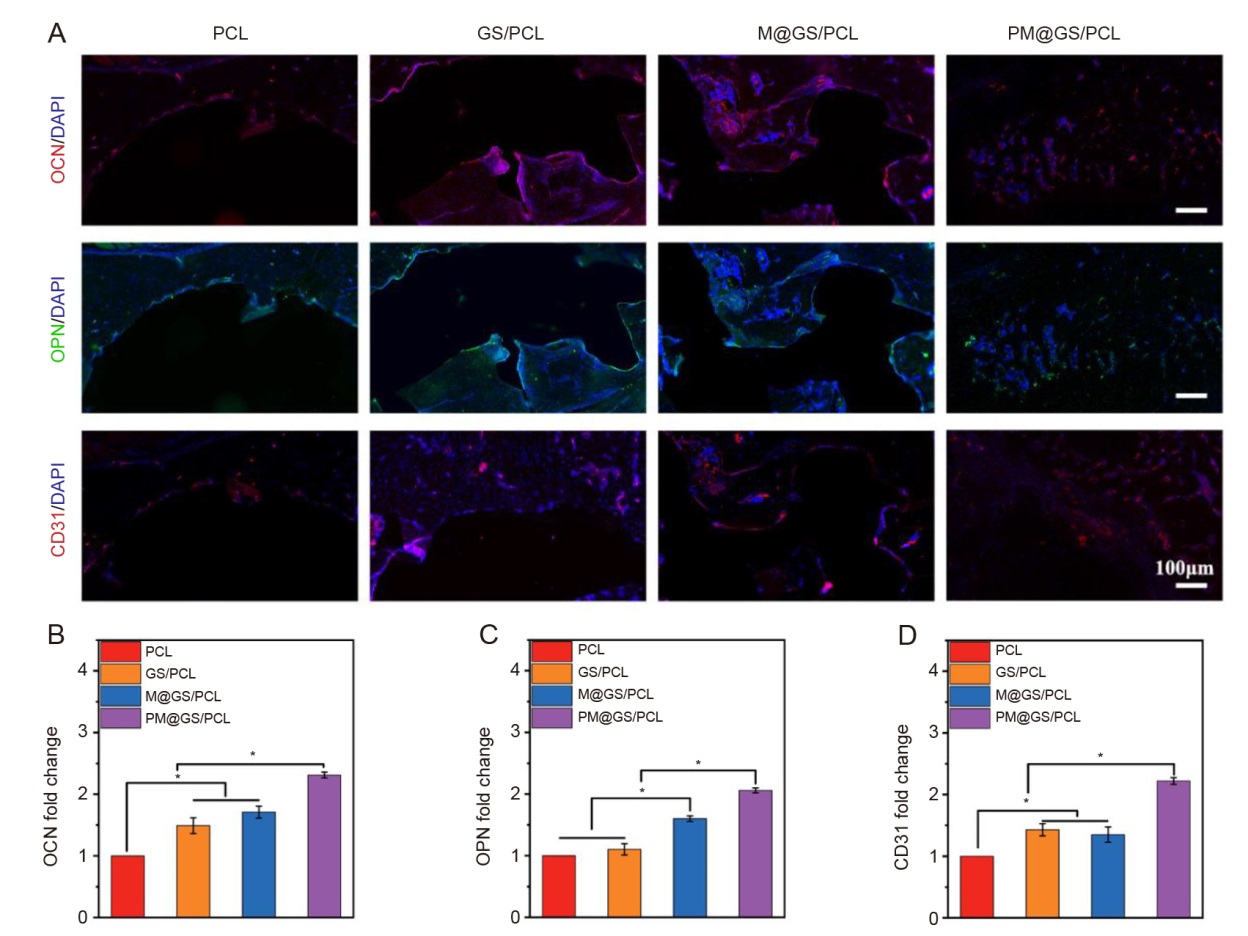
Figure 8. Assessment of osteogenic potential of various scaffolds using immunofluorescence staining. (A) Fluorescence images of OCN (red), OPN (green), and CD31 (red) after implantation for 8 weeks. The nuclei were stained with DAPI (blue). (B–D) Quantification of OCN, OPN and CD31 expression. Data are presented as the mean ± SD (one-way analysis of variance followed by Tukey’s post hoc test). *P < 0.05. CD31: platelet endothelial cell adhesion molecule-1; DAPI: 4′,6-diamidino-2-phenylindole; GS/PCL: GelMA/SFMA/PCL; GelMA: methacrylate gelatin; M@GS/PCL: MSNs@GelMA/SFMA/PCL; MSNs: mesoporous silica nanoparticles; OCN: osteocalcin; OPN: osteopontin; PCL: polycaprolactone; PM@GS/PCL: PTH@MSNs/GelMA/SFMA/PCL; PTH: parathyroid hormone (1–34); SFMA: methacrylated silk fibroin.
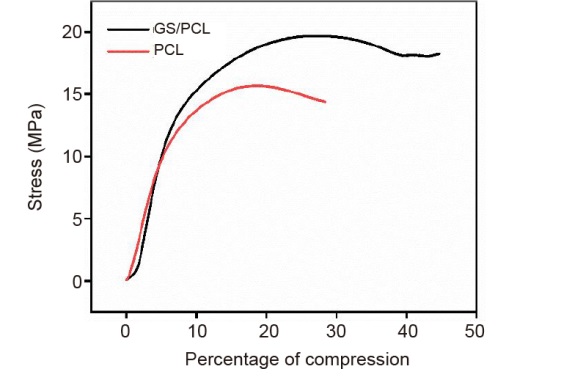
Additional Figure 1. Compressive stress-strain curve of PCL and GS/PCL scaffolds. GelMA: methacrylate gelatin; GS/PCL: GelMA/SFMA/PCL; PCL: polycaprolactone; SFMA: methacrylated silk fibroin.
Additional Figure 2. Confocal microscopy images of BMSCs treated with MSNs (A) and fluorescein isothiocyanate-labelled MSNs (green; B) for 48 hours. The cytoskeleton was stained with Alexa Fluor 568 phalloidin (red), and the nucleus was stained with DAPI (blue). Scale bar: 20 μm. BMSCs: bone marrow-derived mesenchymal stem cells; DAPI: 4′,6-diamidino-2-phenylindole; MSNs: mesoporous silica nanoparticles.
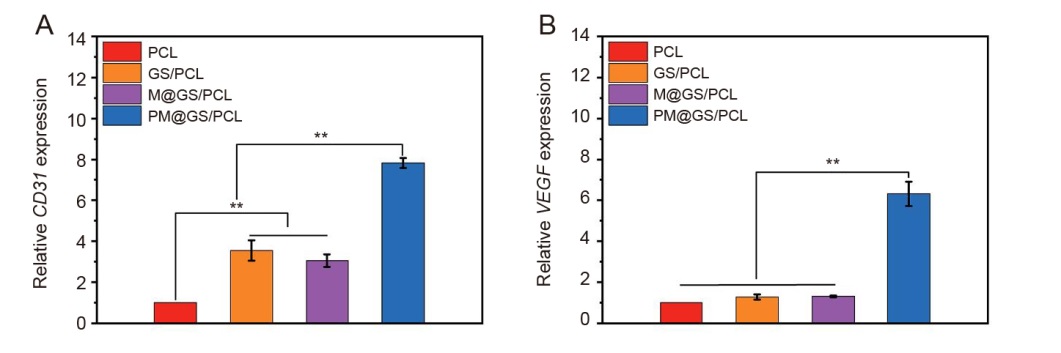
Additional Figure 3. Effect of different scaffold extracts on the expression of angiogenesis-related genes in HUVECs at 48 hours. (A) CD31; (B) VEGF. Data are presented as the mean ± SD. **P < 0.01 (one-way analysis of variance followed by Tukey’s post hoc test). CD31: platelet endothelial cell adhesion molecule-1; GelMA: methacrylate gelatin; HUVECs: human umbilical vein endothelial cells; MSNs: mesoporous silica nanoparticles; PCL: polycaprolactone; GS/PCL: GelMA/SFMA/PCL; M@GS/PCL: MSNs/GelMA/SFMA/PCL; PM@GS/PCL: PTH@MSNs/GelMA/SFMA/PCL; PTH: parathyroid hormone (1–34); SFMA: methacrylated silk fibroin; VEGF: vascular endothelial growth factor.
| Gene | Primer sequence |
|---|---|
| BMSCs | |
| Runx2 | Forward: 5′-CAG TAT GAG AGT AGG TGT CCC GC-3′ |
| Reverse: 5′-AAG AGG GGT AAG ACT GGT CAT AGG-3′ | |
| OCN | Forward: 5'-CAA CCC CAA TTG TGA CGA GC-3′ |
| Reverse: 5'-GGC AAC ACA TGC CCT AAA CG-3′ | |
| OPN | Forward: 5′-GTC TTC CCG TTG CTG TCC TGA-3′ |
| Reverse: 5′-TGA GCT GCC AGA ATC AGT CAC T-3′ | |
| GAPDH | Forward: 5′-GAT GAA CAG TAT CCC GAT GCC A-3′ |
| Reverse: 5′-GGT GGA AGA ATG GGA GTT GCT-3′ | |
| HUVECs | |
| CD31 | Forward: 5′-CTG GCC CAG GAG TTT CCA GA-3′ |
| Reverse: 5′-GTT GCC ACT GTG CTC CAC CA-3′ | |
| VEGF | Forward: 5′-ACC GGC TCT GAC CAG GAG TT-3′ |
| Reverse: 5′-CGC CCA GGC TCC TGA ATC TT-3′ | |
| GAPDH | Forward: 5′-CAT GCC ATC ACT GCC ACC CA-3′ |
| Reverse: 5′-TGA CCT TGC CCA CAG CCT TG-3′ |
Additional Table 1. Primers for real-time polymerase chain reaction analysis in BMSCs and HUVECs
| Gene | Primer sequence |
|---|---|
| BMSCs | |
| Runx2 | Forward: 5′-CAG TAT GAG AGT AGG TGT CCC GC-3′ |
| Reverse: 5′-AAG AGG GGT AAG ACT GGT CAT AGG-3′ | |
| OCN | Forward: 5'-CAA CCC CAA TTG TGA CGA GC-3′ |
| Reverse: 5'-GGC AAC ACA TGC CCT AAA CG-3′ | |
| OPN | Forward: 5′-GTC TTC CCG TTG CTG TCC TGA-3′ |
| Reverse: 5′-TGA GCT GCC AGA ATC AGT CAC T-3′ | |
| GAPDH | Forward: 5′-GAT GAA CAG TAT CCC GAT GCC A-3′ |
| Reverse: 5′-GGT GGA AGA ATG GGA GTT GCT-3′ | |
| HUVECs | |
| CD31 | Forward: 5′-CTG GCC CAG GAG TTT CCA GA-3′ |
| Reverse: 5′-GTT GCC ACT GTG CTC CAC CA-3′ | |
| VEGF | Forward: 5′-ACC GGC TCT GAC CAG GAG TT-3′ |
| Reverse: 5′-CGC CCA GGC TCC TGA ATC TT-3′ | |
| GAPDH | Forward: 5′-CAT GCC ATC ACT GCC ACC CA-3′ |
| Reverse: 5′-TGA CCT TGC CCA CAG CCT TG-3′ |
| 1. | Hao, S.; Wang, M.; Yin, Z.; Jing, Y.; Bai, L.; Su, J. Microenvironment-targeted strategy steers advanced bone regeneration. Mater Today Bio. 2023, 22, 100741. |
| 2. | Ma, H.; Yang, C.; Ma, Z.; Wei, X.; Younis, M. R.; Wang, H.; Li, W.; Wang, Z.; Wang, W.; Luo, Y.; Huang, P.; Wang, J. Multiscale hierarchical architecture-based bioactive scaffolds for versatile tissue engineering. Adv Healthc Mater. 2022, 11, e2102837. |
| 3. | Wang, W.; Yeung, K. W. K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact Mater. 2017, 2, 224-247. |
| 4. |
Spetzger, U.; Vougioukas, V.; Schipper, J. Materials and techniques for osseous skull reconstruction. Minim Invasive Ther Allied Technol. 2010, 19, 110-121.
doi: 10.3109/13645701003644087 URL |
| 5. | Yang, Y.; Wang, X.; Qian, H.; Cheng, L. Titanium-based sonosensitizers for sonodynamic cancer therapy. Appl Mater Today. 2021, 25, 101215. |
| 6. |
Sedghi, R.; Shaabani, A.; Sayyari, N. Electrospun triazole-based chitosan nanofibers as a novel scaffolds for bone tissue repair and regeneration. Carbohydr Polym. 2020, 230, 115707.
doi: 10.1016/j.carbpol.2019.115707 URL |
| 7. |
Dong, J.; Li, Y.; Lin, P.; Leeflang, M. A.; van Asperen, S.; Yu, K.; Tümer, N.; Norder, B.; Zadpoor, A. A.; Zhou, J. Solvent-cast 3D printing of magnesium scaffolds. Acta Biomater. 2020, 114, 497-514.
doi: 10.1016/j.actbio.2020.08.002 URL |
| 8. |
Tang, Y.; Lin, S.; Yin, S.; Jiang, F.; Zhou, M.; Yang, G.; Sun, N.; Zhang, W.; Jiang, X. In situ gas foaming based on magnesium particle degradation: a novel approach to fabricate injectable macroporous hydrogels. Biomaterials. 2020, 232, 119727.
doi: 10.1016/j.biomaterials.2019.119727 URL |
| 9. |
Shahbazarab, Z.; Teimouri, A.; Chermahini, A. N.; Azadi, M. Fabrication and characterization of nanobiocomposite scaffold of zein/chitosan/nanohydroxyapatite prepared by freeze-drying method for bone tissue engineering. Int J Biol Macromol. 2018, 108, 1017-1027.
doi: 10.1016/j.ijbiomac.2017.11.017 URL |
| 10. | Jian, Z.; Zhuang, T.; Qinyu, T.; Liqing, P.; Kun, L.; Xujiang, L.; Diaodiao, W.; Zhen, Y.; Shuangpeng, J.; Xiang, S.; Jingxiang, H.; Shuyun, L.; Libo, H.; Peifu, T.; Qi, Y.; Quanyi, G. 3D bioprinting of a biomimetic meniscal scaffold for application in tissue engineering. Bioact Mater. 2021, 6, 1711-1726. |
| 11. |
Ligon, S. C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D printing and customized additive manufacturing. Chem Rev. 2017, 117, 10212-10290.
doi: 10.1021/acs.chemrev.7b00074 URL |
| 12. |
Ali, M. A.; Hu, C.; Yttri, E. A.; Panat, R. Recent advances in 3D printing of biomedical sensing devices. Adv Funct Mater. 2022, 32, 2107671.
doi: 10.1002/adfm.v32.9 URL |
| 13. |
Feng, Y.; Zhu, S.; Mei, D.; Li, J.; Zhang, J.; Yang, S.; Guan, S. Application of 3D printing technology in bone tissue engineering: a review. Curr Drug Deliv. 2021, 18, 847-861.
doi: 10.2174/1567201817999201113100322 URL |
| 14. |
Kong, B.; Zhao, Y. 3D bioprinting for biomedical applications. BME Front. 2023, 4, 0010.
doi: 10.34133/bmef.0010 URL |
| 15. |
Dai, X.; Shao, Y.; Tian, X.; Cao, X.; Ye, L.; Gao, P.; Cheng, H.; Wang, X. Fusion between glioma stem cells and mesenchymal stem cells promotes malignant progression in 3D-bioprinted models. ACS Appl Mater Interfaces. 2022, 14, 35344-35356.
doi: 10.1021/acsami.2c06658 URL |
| 16. |
Gao, X.; Xu, Z.; Liu, G.; Wu, J. Polyphenols as a versatile component in tissue engineering. Acta Biomater. 2021, 119, 57-74.
doi: 10.1016/j.actbio.2020.11.004 URL |
| 17. |
Ojha, A. K.; Rajasekaran, R.; Hansda, A. K.; Singh, A.; Dutta, A.; Seesala, V. S.; Das, S.; Dogra, N.; Sharma, S.; Goswami, R.; Chaudhury, K.; Dhara, S. Biodegradable multi-layered silk fibroin-PCL stent for the management of cervical atresia: in vitro cytocompatibility and extracellular matrix remodeling in vivo. ACS Appl Mater Interfaces. 2023, 15, 39099-39116.
doi: 10.1021/acsami.3c06585 URL |
| 18. |
Yang, X.; Wang, Y.; Zhou, Y.; Chen, J.; Wan, Q. The application of polycaprolactone in three-dimensional printing scaffolds for bone tissue engineering. Polymers (Basel). 2021, 13, 2754.
doi: 10.3390/polym13162754 URL |
| 19. |
Neufurth, M.; Wang, X.; Wang, S.; Steffen, R.; Ackermann, M.; Haep, N. D.; Schröder, H. C.; Müller, W. E. G. 3D printing of hybrid biomaterials for bone tissue engineering: Calcium-polyphosphate microparticles encapsulated by polycaprolactone. Acta Biomater. 2017, 64, 377-388.
doi: 10.1016/j.actbio.2017.09.031 URL |
| 20. |
Rodríguez-Merchán, E. C. Bone healing materials in the treatment of recalcitrant nonunions and bone defects. Int J Mol Sci. 2022, 23, 3352.
doi: 10.3390/ijms23063352 URL |
| 21. |
Nitzsche, B.; Rong, W. W.; Goede, A.; Hoffmann, B.; Scarpa, F.; Kuebler, W. M.; Secomb, T. W.; Pries, A. R. Coalescent angiogenesis-evidence for a novel concept of vascular network maturation. Angiogenesis. 2022, 25, 35-45.
doi: 10.1007/s10456-021-09824-3 |
| 22. | Koushik, T. M.; Miller, C. M.; Antunes, E. Bone tissue engineering scaffolds: function of multi-material hierarchically structured scaffolds. Adv Healthc Mater. 2023, 12, e2202766. |
| 23. | Xiong, Y.; Mi, B. B.; Lin, Z.; Hu, Y. Q.; Yu, L.; Zha, K. K.; Panayi, A. C.; Yu, T.; Chen, L.; Liu, Z. P.; Patel, A.; Feng, Q.; Zhou, S. H.; Liu, G. H. The role of the immune microenvironment in bone, cartilage, and soft tissue regeneration: from mechanism to therapeutic opportunity. Mil Med Res. 2022, 9, 65. |
| 24. | Xu, Y.; Xu, C.; He, L.; Zhou, J.; Chen, T.; Ouyang, L.; Guo, X.; Qu, Y.; Luo, Z.; Duan, D. Stratified-structural hydrogel incorporated with magnesium-ion-modified black phosphorus nanosheets for promoting neuro-vascularized bone regeneration. Bioact Mater. 2022, 16, 271-284. |
| 25. | Marrella, A.; Lee, T. Y.; Lee, D. H.; Karuthedom, S.; Syla, D.; Chawla, A.; Khademhosseini, A.; Jang, H. L. Engineering vascularized and innervated bone biomaterials for improved skeletal tissue regeneration. Mater Today (Kidlington). 2018, 21, 362-376. |
| 26. | Huang, B.; Chen, M.; Tian, J.; Zhang, Y.; Dai, Z.; Li, J.; Zhang, W. Oxygen-carrying and antibacterial fluorinated nano-hydroxyapatite incorporated hydrogels for enhanced bone regeneration. Adv Healthc Mater. 2022, 11, e2102540. |
| 27. |
Carmeliet, P. Angiogenesis in health and disease. Nat Med. 2003, 9, 653-660.
doi: 10.1038/nm0603-653 |
| 28. |
Wang, J.; Wang, H.; Wang, Y.; Liu, Z.; Li, Z.; Li, J.; Chen, Q.; Meng, Q.; Shu, W. W.; Wu, J.; Xiao, C.; Han, F.; Li, B. Endothelialized microvessels fabricated by microfluidics facilitate osteogenic differentiation and promote bone repair. Acta Biomater. 2022, 142, 85-98.
doi: 10.1016/j.actbio.2022.01.055 URL |
| 29. |
Gong, L.; Yang, Z.; Zhang, F.; Gao, W. Cytokine conjugates to elastin-like polypeptides. Adv Drug Deliv Rev. 2022, 190, 114541.
doi: 10.1016/j.addr.2022.114541 URL |
| 30. |
Paschold, A.; Voigt, B.; Hause, G.; Kohlmann, T.; Rothemund, S.; Binder, W. H. Modulating the fibrillization of parathyroid-hormone (PTH) peptides: azo-switches as reversible and catalytic entities. Biomedicines. 2022, 10, 1512.
doi: 10.3390/biomedicines10071512 URL |
| 31. |
Martin, T. J.; Sims, N. A.; Seeman, E. Physiological and pharmacological roles of PTH and PTHrP in bone using their shared receptor, PTH1R. Endocr Rev. 2021, 42, 383-406.
doi: 10.1210/endrev/bnab005 URL |
| 32. | Huang, J.; Lin, D.; Wei, Z.; Li, Q.; Zheng, J.; Zheng, Q.; Cai, L.; Li, X.; Yuan, Y.; Li, J. Parathyroid hormone derivative with reduced osteoclastic activity promoted bone regeneration via synergistic bone remodeling and angiogenesis. Small. 2020, 16, e1905876. |
| 33. |
Jiang, L.; Zhang, W.; Wei, L.; Zhou, Q.; Yang, G.; Qian, N.; Tang, Y.; Gao, Y.; Jiang, X. Early effects of parathyroid hormone on vascularized bone regeneration and implant osseointegration in aged rats. Biomaterials. 2018, 179, 15-28.
doi: 10.1016/j.biomaterials.2018.06.035 URL |
| 34. | Liu, S.; Han, Z.; Hao, J. N.; Zhang, D.; Li, X.; Cao, Y.; Huang, J.; Li, Y. Engineering of a NIR-activable hydrogel-coated mesoporous bioactive glass scaffold with dual-mode parathyroid hormone derivative release property for angiogenesis and bone regeneration. Bioact Mater. 2023, 26, 1-13. |
| 35. | Zhao, Y.; Kang, H.; Wu, X.; Zhuang, P.; Tu, R.; Goto, T.; Li, F.; Dai, H. Multifunctional scaffold for osteoporotic pathophysiological microenvironment improvement and vascularized bone defect regeneration. Adv Healthc Mater. 2023, 12, e2203099. |
| 36. |
Mirkhalaf, M.; Men, Y.; Wang, R.; No, Y.; Zreiqat, H. Personalized 3D printed bone scaffolds: A review. Acta Biomater. 2023, 156, 110-124.
doi: 10.1016/j.actbio.2022.04.014 URL |
| 37. | Hull, S. M.; Brunel, L. G.; Heilshorn, S. C. 3D bioprinting of cell-laden hydrogels for improved biological functionality. Adv Mater. 2022, 34, e2103691. |
| 38. |
Sun, L.; Wang, X.; Gong, F.; Yin, K.; Zhu, W.; Yang, N.; Bai, S.; Liao, F.; Shao, M.; Cheng, L. Silicon nanowires decorated with platinum nanoparticles were applied for photothermal-enhanced sonodynamic therapy. Theranostics. 2021, 11, 9234-9242.
doi: 10.7150/thno.58755 URL |
| 39. |
Sun, P.; Zhang, Q.; Nie, W.; Zhou, X.; Chen, L.; Du, H.; Yang, S.; You, Z.; He, J.; He, C. Biodegradable mesoporous silica nanocarrier bearing angiogenic QK peptide and dexamethasone for accelerating angiogenesis in bone regeneration. ACS Biomater Sci Eng. 2019, 5, 6766-6778.
doi: 10.1021/acsbiomaterials.9b01521 URL |
| 40. |
Yang, J.; Li, Z.; Li, S.; Zhang, Q.; Zhou, X.; He, C. Tunable metacrylated silk fibroin-based hybrid bioinks for the bioprinting of tissue engineering scaffolds. Biomater Sci. 2023, 11, 1895-1909.
doi: 10.1039/D2BM01978G URL |
| 41. |
Li, Z.; Li, S.; Yang, J.; Ha, Y.; Zhang, Q.; Zhou, X.; He, C. 3D bioprinted gelatin/gellan gum-based scaffold with double-crosslinking network for vascularized bone regeneration. Carbohydr Polym. 2022, 290, 119469.
doi: 10.1016/j.carbpol.2022.119469 URL |
| 42. | Schneider, C. A.; Rasband, W. S.; Eliceiri, K. W. NIH image to ImageJ: 25 years of image analysis. Nat Methods. 2012, 9, 671-675. |
| 43. |
Zhou, X.; Liu, P.; Nie, W.; Peng, C.; Li, T.; Qiang, L.; He, C.; Wang, J. Incorporation of dexamethasone-loaded mesoporous silica nanoparticles into mineralized porous biocomposite scaffolds for improving osteogenic activity. Int J Biol Macromol. 2020, 149, 116-126.
doi: 10.1016/j.ijbiomac.2020.01.237 URL |
| 44. | Mora-Raimundo, P.; Lozano, D.; Benito, M.; Mulero, F.; Manzano, M.; Vallet-Regí, M. Osteoporosis remission and new bone formation with mesoporous silica nanoparticles. Adv Sci (Weinh). 2021, 8, e2101107. |
| 45. |
Woodruff, M. A.; Hutmacher, D. W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog Polym Sci. 2010, 35, 1217-1256.
doi: 10.1016/j.progpolymsci.2010.04.002 URL |
| 46. |
Liang, K.; Zhao, C.; Song, C.; Zhao, L.; Qiu, P.; Wang, S.; Zhu, J.; Gong, Z.; Liu, Z.; Tang, R.; Fang, X.; Zhao, Y. In situ biomimetic mineralization of bone-like hydroxyapatite in hydrogel for the acceleration of bone regeneration. ACS Appl Mater Interfaces. 2023, 15, 292-308.
doi: 10.1021/acsami.2c16217 URL |
| 47. |
Vadana, M.; Cecoltan, S.; Ciortan, L.; Macarie, R. D.; Mihaila, A. C.; Tucureanu, M. M.; Gan, A. M.; Simionescu, M.; Manduteanu, I.; Droc, I.; Butoi, E. Parathyroid hormone induces human valvular endothelial cells dysfunction that impacts the osteogenic phenotype of valvular interstitial cells. Int J Mol Sci. 2022, 23, 3776.
doi: 10.3390/ijms23073776 URL |
| [1] | Huaxin Yang, Mengjia Zheng, Yuyue Zhang, Chaochang Li, Joseph Ho Chi Lai, Qizheng Zhang, Kannie WY Chan, Hao Wang, Xin Zhao, Zijiang Yang, Chenjie Xu. Enhanced angiogenesis in porous poly(ε-caprolactone) scaffolds fortified with methacrylated hyaluronic acid hydrogel after subcutaneous transplantation [J]. Biomaterials Translational, 2024, 5(1): 59-68. |
| [2] | Long Bai, Peiran Song, Jiacan Su. Bioactive elements manipulate bone regeneration [J]. Biomaterials Translational, 2023, 4(4): 248-269. |
| [3] | Ziwei Tao, Ziyang Yuan, Dong Zhou, Lang Qin, Lan Xiao, Shihao Zhang, Changsheng Liu, Jinzhong Zhao, Yulin Li. Fabrication of magnesium-doped porous polylactic acid microsphere for bone regeneration [J]. Biomaterials Translational, 2023, 4(4): 280-290. |
| [4] | Gen Wang, Zhangqin Yuan, Li Yu, Yingkang Yu, Pinghui Zhou, Genglei Chu, Huan Wang, Qianping Guo, Caihong Zhu, Fengxuan Han, Song Chen, Bin Li. Mechanically conditioned cell sheets cultured on thermo-responsive surfaces promote bone regeneration [J]. Biomaterials Translational, 2023, 4(1): 27-40. |
| [5] | Andrew Tai, Euphemie Landao-Bassonga, Ziming Chen, Minh Tran, Brent Allan, Rui Ruan, Dax Calder, Mithran Goonewardene, Hien Ngo, Ming Hao Zheng. Systematic evaluation of three porcine-derived collagen membranes for guided bone regeneration [J]. Biomaterials Translational, 2023, 4(1): 41-50. |
| [6] | Yang Zhao, Qing Sun, Bo Huo. Focal adhesion regulates osteogenic differentiation of mesenchymal stem cells and osteoblasts [J]. Biomaterials Translational, 2021, 2(4): 312-322. |
| [7] | Xirui Jing, Qiuyue Ding, Qinxue Wu, Weijie Su, Keda Yu, Yanlin Su, Bing Ye, Qing Gao, Tingfang Sun, Xiaodong Guo. Magnesium-based materials in orthopaedics: material properties and animal models [J]. Biomaterials Translational, 2021, 2(3): 197-213. |
| [8] | Qingchuan Wang, Weidan Wang, Yanfang Li, Weirong Li, Lili Tan, Ke Yang. Biofunctional magnesium coating of implant materials by physical vapour deposition [J]. Biomaterials Translational, 2021, 2(3): 248-256. |
| [9] | Kamolrat Metavarayuth, Esteban Villarreal, Hui Wang, Qian Wang. Surface topography and free energy regulate osteogenesis of stem cells: effects of shape-controlled gold nanoparticles [J]. Biomaterials Translational, 2021, 2(2): 165-173. |
| [10] | Yunsong Shi, Ruijun He, Xiangyu Deng, Zengwu Shao, Davide Deganello, Chunze Yan, Zhidao Xia. Three-dimensional biofabrication of an aragonite-enriched self-hardening bone graft substitute and assessment of its osteogenicity in vitro and in vivo [J]. Biomaterials Translational, 2020, 1(1): 69-81. |
| [11] | Jishan Yuan, Panita Maturavongsadit, Zhihui Zhou, Bin Lv, Yuan Lin, Jia Yang, Jittima Amie Luckanagul. Hyaluronic acid-based hydrogels with tobacco mosaic virus containing cell adhesive peptide induce bone repair in normal and osteoporotic rats [J]. Biomaterials Translational, 2020, 1(1): 89-98. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||