Biomaterials Translational ›› 2020, Vol. 1 ›› Issue (1): 3-17.doi: 10.3877/cma.j.issn.2096-112X.2020.01.002
• REVIEW • Previous Articles Next Articles
Maryam Tamaddon1, Helena Gilja1, Ling Wang2, J. Miguel Oliveira3,4,5, Xiaodan Sun6, Rongwei Tan7, Chaozong Liu1,*( )
)
Received:2020-06-30
Revised:2020-09-14
Accepted:2020-09-25
Online:2020-12-28
Published:2020-12-28
Contact:
Chaozong Liu
E-mail:chaozong.liu@ucl.ac.uk
Tamaddon, M.; Gilja, H.; Wang, L.; Oliveira, J.; Sun, X.; Tan, R.; Liu, C. Osteochondral scaffolds for early treatment of cartilage defects in osteoarthritic joints: from bench to clinic. Biomater Transl. 2020, 1(1), 3-17.
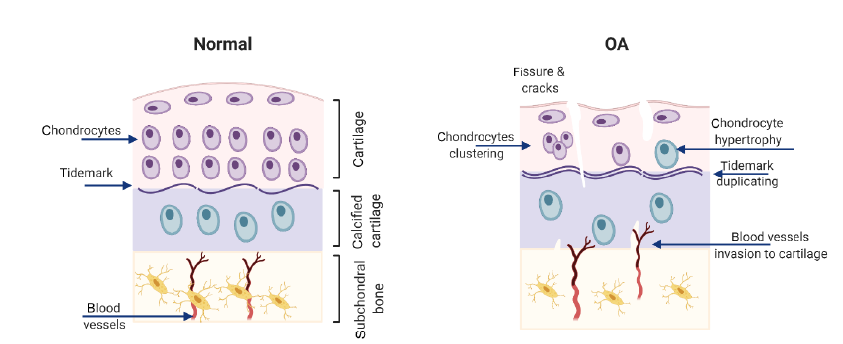
Figure 1. Changes in the osteochondral unit in osteoarthritic joints. Cartilage thinning, blood vessels infiltration into cartilage and subchondral plate thickening with the progress of osteoarthritis (OA). Created with BioRender.com.
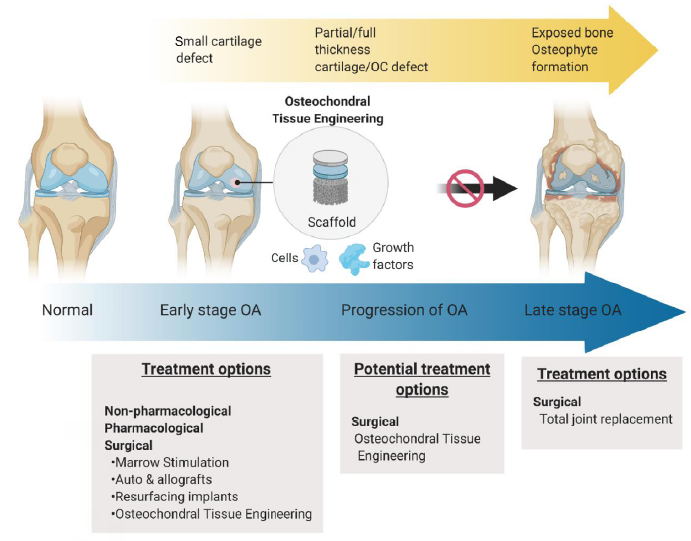
Figure 2. Osteoarthritis (OA) progression and treatment options: non-pharmacological and pharmacological therapies can be used for the treatment of mild and non-acute OA; when the cartilage and bone loss at the joint has significantly impacted the quality of life of the patient, and non-surgical treatments are no longer effective the current state of the art in terms of surgical intervention is a joint replacement operation. Osteochondral (OC) scaffold (with or without addition of cells, such as chondrocytes or stem cells and growth factors such as transforming growth factor-β) seeks to repair and regenerate the local cartilage defects at an early stage to stop or delay the progression of OA to avoid the use of joint replacements. Adapted from Tamaddon et al.12
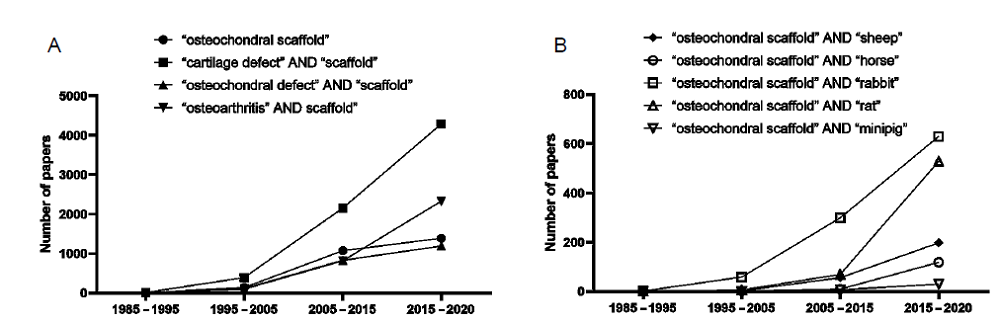
Figure 3. A literature search on scaffolds for cartilage repair was performed using Science Direct Databases from their inception to date for publications in English. The literature search showing: (A) the increasing number of researches on scaffolds for cartilage and osteochondral defects, and (B) in vivo performance evaluation of osteochondral scaffolds using animal models.
| Grade | Description of changes |
|---|---|
| Grade 0 | No changes |
| Grade I | Doubtful narrowing of the joint space and possible osteophytic lipping |
| Grade II | Definite osteophytes and possible narrowing of the joint space |
| Grade III | Moderate multiple osteophytes, definite narrowing of the joint space, and some sclerosis, and possible deformity of the bone ends |
| Grade IV | Large osteophytes marked narrowing of the joint space, severe sclerosis, and definite deformity of the bone ends |
Table 1 Kellgren and Lawrence grading scoring system based on radiography.
| Grade | Description of changes |
|---|---|
| Grade 0 | No changes |
| Grade I | Doubtful narrowing of the joint space and possible osteophytic lipping |
| Grade II | Definite osteophytes and possible narrowing of the joint space |
| Grade III | Moderate multiple osteophytes, definite narrowing of the joint space, and some sclerosis, and possible deformity of the bone ends |
| Grade IV | Large osteophytes marked narrowing of the joint space, severe sclerosis, and definite deformity of the bone ends |
| Grade | Description of changes | |
|---|---|---|
| Grade 0 | Normal | - |
| Grade 1 | Nearly normal | Superficial lesions. Soft indentation and/or superficial fissures and cracks |
| Grade 2 | Abnormal | Lesions extending down to < 50% of cartilage depth |
| Grade 3 | Severely abnormal | Cartilage defects extending down > 50% of cartilage depth as well as down to calcified layer and down to but not through the subchondral bone. Blisters are included in this Grade |
| Grade 4 | Severely abnormal | Osteochondral injuries, lesions extending to the subchondral bone plate or deeper into the trabecular bone |
Table 2 International Cartilage Repair Society grading of cartilage.
| Grade | Description of changes | |
|---|---|---|
| Grade 0 | Normal | - |
| Grade 1 | Nearly normal | Superficial lesions. Soft indentation and/or superficial fissures and cracks |
| Grade 2 | Abnormal | Lesions extending down to < 50% of cartilage depth |
| Grade 3 | Severely abnormal | Cartilage defects extending down > 50% of cartilage depth as well as down to calcified layer and down to but not through the subchondral bone. Blisters are included in this Grade |
| Grade 4 | Severely abnormal | Osteochondral injuries, lesions extending to the subchondral bone plate or deeper into the trabecular bone |
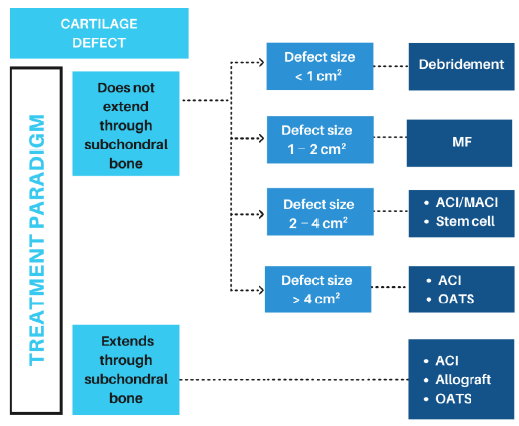
Figure 4. Cartilage/OCD treatment algorithm according to the condition and size of the defect. ACI: autologous chondrocyte implantation; MACI: matrix-assisted autologous chondrocyte implantation; MF: microfracture; OATS: osteochondral autograft transfer system.
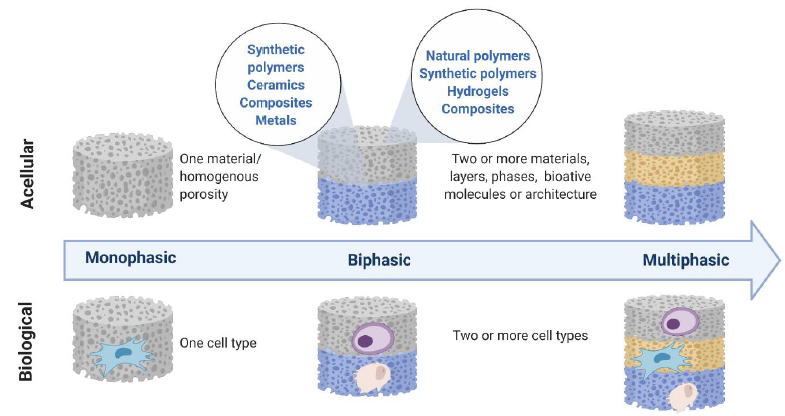
Figure 5. Evolution of osteochondral scaffolds from monophasic to bi- and multi-phasic states seek to recapitulate the zonal property of osteochondral tissue, concept adapted from Jeon et al.50 with permission from Elsevier.
| Structure | Device | Materials | References |
|---|---|---|---|
| Biphasic | Agili-CTM (CartiHeal, Tel Aviv, Israel) | Aragonite-hyaluronate biphasic scaffold. Thin hyaluronate covered cartilage phase, overlying a thick bone phase | Kon et al. |
| TruFitTM (Smith and Nephew, USA) | Chondral phase: PLGA, osseous phase: calcium sulfate and PGA fibres | Carmont et al. | |
| Chondro-mimetic (Collagen Solutions, UK) | Chondral phase: Collagen-GAG; | Getgood et al. | |
| Osseous layer: Collagen, GAG, CP | |||
| Biphasic | Chondral phase: PLGA; | Chiang et al. | |
| Osseous phase: PLGA-TCP, made by particulate leaching method | |||
| Chondro-Gide | Bilayered collagen I/III scaffold | Kusano et al. | |
| Multiphasic | MaioRegen® (Fin-Ceramica Faenza S.p.A., Italy) | Chondral phase: Type I equine collagen; | Kon et al. |
| Intermediate phase: Type I collagen (60%) and Mg-enriched HA (40%); | |||
| Osseous phase: A mineralised blend of type I collagen (30%) and 70% of Mg-enriched HA |
Table 3 Osteochondral scaffolds for repair of cartilage damages.
| Structure | Device | Materials | References |
|---|---|---|---|
| Biphasic | Agili-CTM (CartiHeal, Tel Aviv, Israel) | Aragonite-hyaluronate biphasic scaffold. Thin hyaluronate covered cartilage phase, overlying a thick bone phase | Kon et al. |
| TruFitTM (Smith and Nephew, USA) | Chondral phase: PLGA, osseous phase: calcium sulfate and PGA fibres | Carmont et al. | |
| Chondro-mimetic (Collagen Solutions, UK) | Chondral phase: Collagen-GAG; | Getgood et al. | |
| Osseous layer: Collagen, GAG, CP | |||
| Biphasic | Chondral phase: PLGA; | Chiang et al. | |
| Osseous phase: PLGA-TCP, made by particulate leaching method | |||
| Chondro-Gide | Bilayered collagen I/III scaffold | Kusano et al. | |
| Multiphasic | MaioRegen® (Fin-Ceramica Faenza S.p.A., Italy) | Chondral phase: Type I equine collagen; | Kon et al. |
| Intermediate phase: Type I collagen (60%) and Mg-enriched HA (40%); | |||
| Osseous phase: A mineralised blend of type I collagen (30%) and 70% of Mg-enriched HA |
| No. | Study title | Identifier No. | Submission date | Status/sponsor | Conditions | Intervention/follow up/results |
|---|---|---|---|---|---|---|
| 1 | Clinical and Radiological Results of Osteochondral (OC) Repair Using MaioRegen in Knee and Ankle Surgery | NCT02345564 | 11-Sep-14 | Unknown/Barmherzige Brüder Eisenstadt | OC lesion of talus degenerative lesion of articular cartilage of knee; size 2-4 cm2 | MaioRegen/18 months/no results posted |
| 2 | A Prospective, Post-marketing Registry on the Use of ChondroMimetic for the Repair of OCDs | NCT01209390 | 6-Aug-10 | Terminated (slow recruitment rate)/TiGenix n.v. | OCDs knee, less than 12 mm diameter and 8 mm depth | Chondromimetic device/6, 12, 24, 36 months/no results posted |
| 3 | Study for the Treatment of Knee Chondral and OC Lesions | NCT01282034 | 21-Jan-11 | Completed Feb 2016/FinCeramica Faenza Spa | Chondral and OC knee lesions; Grades III/IV outerbridge, 2-9 cm2 | MaioRegen/6, 12, 24 months/no results posted |
| 4 | Repair of articular OCD | NCT01409447 | 3-Aug-11 | Unknown/National Taiwan University Hospital | Osteochondritis dissecans knee, less than 3 cm | BiPhasic/-/no results posted |
| 5 | Chondrofix OC allograft prospective study | NCT01410136 | 2-Aug-11 | Terminated/Zimmer Orthobiologics, Inc. | Articular cartilage disorder, degeneration, defect and acute injury; Up to two cartilage lesion, each measuring less than 8 cm2 | Chondrofix OC allograft/24 up to 60 months/no results posted |
| 6 | Agili-CTM Implant Performance Evaluation in the Repair of Cartilage and OCDs | NCT02423629 | 3-Dec-14 | Completed/Cartiheal (2009) Ltd. | Cartilage or OCD of the knee, ICRS Grade III or above, 1-7 cm2 | Agili-C/6, 12, 18, 24 months/no results posted |
| 7 | BiPhasic Cartilage Repair Implant (BiCRI) IDE Clinical Trial - Taiwan | NCT01477008 | 14-Nov-11 | Active, not recruiting/BioGend Therapeutics Co. Ltd. | Chondral and OCD of femoral condyles and trochlea; ICRS grade 3-4 lesion, Outerbridge grade 4, or OCD grades 3-4, 12.5 mm diameter with one implant or if larger with two implants | BiPhasic Cartilage Implant/preop, 6 weeks, 3, 6 and 12 months/no results posted |
| 8 | Evaluation of the Agili-C Biphasic Implant in the Knee Joint | NCT01471236 | 10-Nov-11 | Completed/Cartiheal (2009) Ltd. | Cartilage diseases, osteochondritis dissecans, less than 2 cm2 and 3 mm depth | Agili-C/3, 6, 9, 12, 18, 24 months/no results posted |
| 9 | Pivotal Study to Evaluate the Safety and Efficacy of GelrinC for Treatment of Cartilage Defects | NCT03262909 | 17-Aug-17 | Recruiting/Regentis Biomaterials | Knee joint cartilage defects, ICRS III or IV, lesion size between 1 and 5 cm2 post debridement, less than or equal to 2.5 cm in diameter | GelrinC/24 months/no results posted |
Table 4 Osteochondral scaffolds in clinical studies registered with Clinicaltrial.gov.
| No. | Study title | Identifier No. | Submission date | Status/sponsor | Conditions | Intervention/follow up/results |
|---|---|---|---|---|---|---|
| 1 | Clinical and Radiological Results of Osteochondral (OC) Repair Using MaioRegen in Knee and Ankle Surgery | NCT02345564 | 11-Sep-14 | Unknown/Barmherzige Brüder Eisenstadt | OC lesion of talus degenerative lesion of articular cartilage of knee; size 2-4 cm2 | MaioRegen/18 months/no results posted |
| 2 | A Prospective, Post-marketing Registry on the Use of ChondroMimetic for the Repair of OCDs | NCT01209390 | 6-Aug-10 | Terminated (slow recruitment rate)/TiGenix n.v. | OCDs knee, less than 12 mm diameter and 8 mm depth | Chondromimetic device/6, 12, 24, 36 months/no results posted |
| 3 | Study for the Treatment of Knee Chondral and OC Lesions | NCT01282034 | 21-Jan-11 | Completed Feb 2016/FinCeramica Faenza Spa | Chondral and OC knee lesions; Grades III/IV outerbridge, 2-9 cm2 | MaioRegen/6, 12, 24 months/no results posted |
| 4 | Repair of articular OCD | NCT01409447 | 3-Aug-11 | Unknown/National Taiwan University Hospital | Osteochondritis dissecans knee, less than 3 cm | BiPhasic/-/no results posted |
| 5 | Chondrofix OC allograft prospective study | NCT01410136 | 2-Aug-11 | Terminated/Zimmer Orthobiologics, Inc. | Articular cartilage disorder, degeneration, defect and acute injury; Up to two cartilage lesion, each measuring less than 8 cm2 | Chondrofix OC allograft/24 up to 60 months/no results posted |
| 6 | Agili-CTM Implant Performance Evaluation in the Repair of Cartilage and OCDs | NCT02423629 | 3-Dec-14 | Completed/Cartiheal (2009) Ltd. | Cartilage or OCD of the knee, ICRS Grade III or above, 1-7 cm2 | Agili-C/6, 12, 18, 24 months/no results posted |
| 7 | BiPhasic Cartilage Repair Implant (BiCRI) IDE Clinical Trial - Taiwan | NCT01477008 | 14-Nov-11 | Active, not recruiting/BioGend Therapeutics Co. Ltd. | Chondral and OCD of femoral condyles and trochlea; ICRS grade 3-4 lesion, Outerbridge grade 4, or OCD grades 3-4, 12.5 mm diameter with one implant or if larger with two implants | BiPhasic Cartilage Implant/preop, 6 weeks, 3, 6 and 12 months/no results posted |
| 8 | Evaluation of the Agili-C Biphasic Implant in the Knee Joint | NCT01471236 | 10-Nov-11 | Completed/Cartiheal (2009) Ltd. | Cartilage diseases, osteochondritis dissecans, less than 2 cm2 and 3 mm depth | Agili-C/3, 6, 9, 12, 18, 24 months/no results posted |
| 9 | Pivotal Study to Evaluate the Safety and Efficacy of GelrinC for Treatment of Cartilage Defects | NCT03262909 | 17-Aug-17 | Recruiting/Regentis Biomaterials | Knee joint cartilage defects, ICRS III or IV, lesion size between 1 and 5 cm2 post debridement, less than or equal to 2.5 cm in diameter | GelrinC/24 months/no results posted |
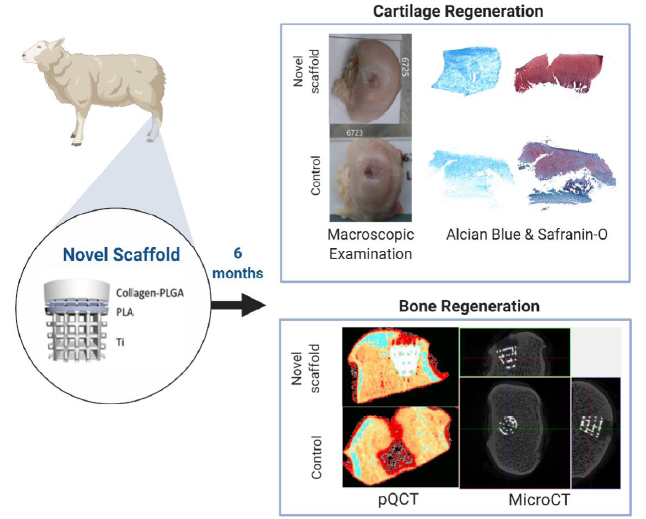
Figure 6. The novel multilayer osteochondral scaffold developed at University College London has achieved improved stable fixation via good bone integration which provides a strong support to the overlying cartilage healthy growth. Six months in vivo studies showed a hyaline-like cartilage formation, over 90% cartilage fill, and improved subchondral bone regeneration in a large OCD of sheep knee. OCD: osteochondral defect; PLA: polylactic acid; PLGA: polylactic acid; pQCT: peripheral quantitative computed tomography; Ti: titanium.
| 1. |
Hunter, D. J.; Schofield, D.; Callander, E. The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol. 2014,10, 437-441.
doi: 10.1038/nrrheum.2014.44 URL pmid: 24662640 |
| 2. |
Loeser, R. F.; Goldring, S. R.; Scanzello, C. R.; Goldring, M. B. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012,64, 1697-1707.
doi: 10.1002/art.34453 URL pmid: 22392533 |
| 3. |
Lories, R. J.; Luyten, F. P. The bone-cartilage unit in osteoarthritis. Nat Rev Rheumatol. 2011,7, 43-49.
URL pmid: 21135881 |
| 4. |
Longley, R.; Ferreira, A. M.; Gentile, P. Recent approaches to the manufacturing of biomimetic multi-phasic scaffolds for osteochondral regeneration. Int J Mol Sci. 2018,19, 1755.
doi: 10.3390/ijms19061755 URL |
| 5. |
Goldring, S. R.; Goldring, M. B. Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage-bone crosstalk. Nat Rev Rheumatol. 2016,12, 632-644.
doi: 10.1038/nrrheum.2016.148 URL pmid: 27652499 |
| 6. |
Williams, F. M. K.; Spector, T. D. Osteoarthritis. Medicine. 2006,34, 364-368.
doi: 10.1053/j.mpmed.2006.06.011 URL |
| 7. |
Roberts, S.; Weightman, B.; Urban, J.; Chappell, D. Mechanical and biochemical properties of human articular cartilage in osteoarthritic femoral heads and in autopsy specimens. J Bone Joint Surg Br. 1986,68, 278-288.
doi: 10.1302/0301-620X.68B2.3958016 URL pmid: 3958016 |
| 8. |
Findlay, D. M. Vascular pathology and osteoarthritis. Rheumatology (Oxford). 2007,46, 1763-1768.
doi: 10.1093/rheumatology/kem191 URL |
| 9. |
Findlay, D. M.; Kuliwaba, J. S. Bone-cartilage crosstalk: a conversation for understanding osteoarthritis. Bone Res. 2016,4, 16028.
doi: 10.1038/boneres.2016.28 URL pmid: 27672480 |
| 10. |
Burr, D. B.; Gallant, M. A. Bone remodelling in osteoarthritis. Nat Rev Rheumatol. 2012,8, 665-673.
doi: 10.1038/nrrheum.2012.130 URL pmid: 22868925 |
| 11. |
Goldring, M. B.; Goldring, S. R. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann N Y Acad Sci. 2010,1192, 230-237.
doi: 10.1111/j.1749-6632.2009.05240.x URL pmid: 20392241 |
| 12. |
Tamaddon, M.; Wang, L.; Liu, Z.; Liu, C. Osteochondral tissue repair in osteoarthritic joints: clinical challenges and opportunities in tissue engineering. Bio-design and manufacturing. 2018,1, 101-114.
doi: 10.1007/s42242-018-0015-0 URL pmid: 30533248 |
| 13. |
Luyten, F. P.; Denti, M.; Filardo, G.; Kon, E.; Engebretsen, L. Definition and classification of early osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. 2012,20, 401-406.
doi: 10.1007/s00167-011-1743-2 URL pmid: 22068268 |
| 14. |
Madry, H.; Kon, E.; Condello, V.; Peretti, G. M.; Steinwachs, M.; Seil, R.; Berruto, M.; Engebretsen, L.; Filardo, G.; Angele, P. Early osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. 2016,24, 1753-1762.
doi: 10.1007/s00167-016-4068-3 URL pmid: 27000393 |
| 15. |
Kellgren, J. H.; Lawrence, J. S. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957,16, 494-502.
doi: 10.1136/ard.16.4.494 URL pmid: 13498604 |
| 16. |
Kohn, M. D.; Sassoon, A. A.; Fernando, N. D. Classifications in brief: Kellgren-Lawrence classification of osteoarthritis. Clin Orthop Relat Res. 2016,474, 1886-1893.
doi: 10.1007/s11999-016-4732-4 URL pmid: 26872913 |
| 17. |
Casula, V.; Hirvasniemi, J.; Lehenkari, P.; Ojala, R.; Haapea, M.; Saarakkala, S.; Lammentausta, E.; Nieminen, M. T. Association between quantitative MRI and ICRS arthroscopic grading of articular cartilage. Knee Surg Sports Traumatol Arthrosc. 2016,24, 2046-2054.
doi: 10.1007/s00167-014-3286-9 URL pmid: 25209205 |
| 18. | Brittberg, M.; Winalski, C. S. Evaluation of cartilage injuries and repair. J Bone Joint Surg Am. 2003, 85-A Suppl 2, 58-69. |
| 19. |
Nelson, A. E.; Allen, K. D.; Golightly, Y. M.; Goode, A. P.; Jordan, J. M. A systematic review of recommendations and guidelines for the management of osteoarthritis: The chronic osteoarthritis management initiative of the U.S. bone and joint initiative. Semin Arthritis Rheum. 2014,43, 701-712.
doi: 10.1016/j.semarthrit.2013.11.012 URL pmid: 24387819 |
| 20. |
Giannini, S.; Buda, R.; Grigolo, B.; Vannini, F. Autologous chondrocyte transplantation in osteochondral lesions of the ankle joint. Foot Ankle Int. 2001,22, 513-517.
URL pmid: 11475462 |
| 21. |
Giannini, S.; Vannini, F. Operative treatment of osteochondral lesions of the talar dome: current concepts review. Foot Ankle Int. 2004,25, 168-175.
doi: 10.1177/107110070402500311 URL pmid: 15006340 |
| 22. | Rothrauff, B. B.; Murawski, C. D.; Angthong, C.; Becher, C.; Nehrer, S.; Niemeyer, P.; Sullivan, M.; Valderrabano, V.; Walther, M.; Ferkel, R. D. Scaffold-based therapies: Proceedings of the International Consensus Meeting on Cartilage Repair of the Ankle. Foot Ankle Int. 2018,39, 41s-47s. |
| 23. |
Vannini, F.; Filardo, G.; Kon, E.; Roffi, A.; Marcacci, M.; Giannini, S. Scaffolds for cartilage repair of the ankle joint: The impact on surgical practice. Foot Ankle Surg. 2013,19, 2-8.
doi: 10.1016/j.fas.2012.07.001 URL pmid: 23337268 |
| 24. |
Yousefi, A. M.; Hoque, M. E.; Prasad, R. G.; Uth, N. Current strategies in multiphasic scaffold design for osteochondral tissue engineering: A review. J Biomed Mater Res A. 2015,103, 2460-2481.
doi: 10.1002/jbm.a.35356 URL pmid: 25345589 |
| 25. |
Tamaddon, M.; Liu, C. Enhancing biological and biomechanical fixation of osteochondral scaffold: a grand challenge. Adv Exp Med Biol. 2018,1059, 255-298.
doi: 10.1007/978-3-319-76735-2_12 URL pmid: 29736578 |
| 26. |
Yan, L.; Oliveira, J. M.; Oliveira, A. L.; Reis, R. L. Current concepts and challenges in osteochondral tissue engineering and regenerative medicine. ACS Biomater Sci Eng. 2015. doi: 10.1021/ab500038y.
doi: 10.1021/acsbiomaterials.0c01189 URL pmid: 33320636 |
| 27. |
Hirahara, A. M.; Mueller, K. W. Jr. BioCartilage: A new biomaterial to treat chondral lesions. Sports Med Arthrosc Rev. 2015,23, 143-148.
doi: 10.1097/JSA.0000000000000071 URL pmid: 26225574 |
| 28. |
Melton, J. T.; Wilson, A. J.; Chapman-Sheath, P.; Cossey A. J. TruFit CB bone plug: chondral repair, scaffold design, surgical technique and early experiences. Expert Rev Med Devices. 2010,7, 333-341.
doi: 10.1586/erd.10.15 URL pmid: 20420556 |
| 29. |
Ye, K.; Di Bella, C.; Myers, D. E.; Choong, P. F. The osteochondral dilemma: review of current management and future trends. ANZ J Surg. 2014,84, 211-217.
doi: 10.1111/ans.12108 URL pmid: 23458285 |
| 30. |
Salzmann, G. M.; Niemeyer, P.; Steinwachs, M.; Kreuz, P. C.; Südkamp, N. P.; Mayr, H. O. Cartilage repair approach and treatment characteristics across the knee joint: a European survey. Arch Orthop Trauma Surg. 2011,131, 283-291.
doi: 10.1007/s00402-010-1047-x URL pmid: 20082085 |
| 31. |
Kreuz, P. C.; Erggelet, C.; Steinwachs, M. R.; Krause, S. J.; Lahm, A.; Niemeyer, P.; Ghanem, N.; Uhl, M.; Südkamp, N. Is microfracture of chondral defects in the knee associated with different results in patients aged 40 years or younger? Arthroscopy. 2006,22, 1180-1186.
doi: 10.1016/j.arthro.2006.06.020 URL pmid: 17084294 |
| 32. |
Kreuz, P. C.; Steinwachs, M. R.; Erggelet, C.; Krause, S. J.; Konrad, G.; Uhl, M.; Südkamp, N. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006,14, 1119-1125.
doi: 10.1016/j.joca.2006.05.003 URL pmid: 16815714 |
| 33. |
Salzmann, G. M.; Sah, B.; Südkamp, N. P.; Niemeyer, P. Clinical outcome following the first-line, single lesion microfracture at the knee joint. Arch Orthop Trauma Surg. 2013,133, 303-310.
doi: 10.1007/s00402-012-1660-y URL pmid: 23224561 |
| 34. |
Angele, P.; Niemeyer, P.; Steinwachs, M.; Filardo, G.; Gomoll, A. H.; Kon, E.; Zellner, J.; Madry, H. Chondral and osteochondral operative treatment in early osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2016,24, 1743-1752.
doi: 10.1007/s00167-016-4047-8 URL pmid: 26922057 |
| 35. |
Bentley, G.; Bhamra, J. S.; Gikas, P. D.; Skinner, J. A.; Carrington, R.; Briggs, T. W. Repair of osteochondral defects in joints how to achieve success. Injury. 2013,44 Suppl 1, S3-10.
doi: 10.1016/S0020-1383(13)70003-2 URL pmid: 23351867 |
| 36. |
Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994,331, 889-895.
doi: 10.1056/NEJM199410063311401 URL pmid: 8078550 |
| 37. |
Knutsen, G.; Drogset, J. O.; Engebretsen, L.; Grøntvedt, T.; Isaksen, V.; Ludvigsen, T. C.; Roberts, S.; Solheim, E.; Strand, T.; Johansen, O. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007,89, 2105-2112.
doi: 10.2106/JBJS.G.00003 URL pmid: 17908884 |
| 38. |
Nixon, A. J.; Sparks, H. D.; Begum, L.; McDonough, S.; Scimeca, M. S.; Moran, N.; Matthews, G. L. Matrix-induced autologous chondrocyte implantation (MACI) using a cell-seeded collagen membrane improves cartilage healing in the equine model. J Bone Joint Surg Am. 2017,99, 1987-1998.
doi: 10.2106/JBJS.16.00603 URL pmid: 29206788 |
| 39. |
Bartlett, W.; Skinner, J. A.; Gooding, C. R.; Carrington, R. W.; Flanagan, A. M.; Briggs, T. W.; Bentley, G. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005,87, 640-645.
doi: 10.1302/0301-620X.87B5.15905 URL pmid: 15855365 |
| 40. |
Behrens, P.; Bitter, T.; Kurz, B.; Russlies, M. Matrix-associated autologous chondrocyte transplantation/implantation (MACT/MACI)--5-year follow-uP. Knee. 2006,13, 194-202.
doi: 10.1016/j.knee.2006.02.012 URL pmid: 16632362 |
| 41. |
Ventura, A.; Memeo, A.; Borgo, E.; Terzaghi, C.; Legnani, C.; Albisetti, W. Repair of osteochondral lesions in the knee by chondrocyte implantation using the MACI® technique. Knee Surg Sports Traumatol Arthrosc. 2012,20, 121-126.
doi: 10.1007/s00167-011-1575-0 URL pmid: 21681599 |
| 42. | Oliveira, J.; Pina, S.; Reis, R. L.; Roman, J. S. Osteochondral Tissue Engineering: Challenges, Current Strategies, and Technological Advances. Springer International Publishing. 2018. |
| 43. | NICE. Autologous chondrocyte implantation for treating symptomatic articular cartilage defects of the knee. Report No. Technology appraisal guidance [TA477]. 2017. |
| 44. |
Filardo, G.; Kon, E.; Roffi, A.; Di Martino, A.; Marcacci, M. Scaffold-based repair for cartilage healing: a systematic review and technical note. Arthroscopy. 2013,29, 174-186.
doi: 10.1016/j.arthro.2012.05.891 URL pmid: 23159494 |
| 45. |
Filardo, G.; Kon, E.; Perdisa, F.; Tetta, C.; Di Martino, A.; Marcacci, M. Arthroscopic mosaicplasty: long-term outcome and joint degeneration progression. Knee. 2015,22, 36-40.
doi: 10.1016/j.knee.2014.10.001 URL pmid: 25482347 |
| 46. |
Filardo, G.; Kon, E.; Perdisa, F.; Balboni, F.; Marcacci, M. Autologous osteochondral transplantation for the treatment of knee lesions: results and limitations at two years’ follow-uP. Int OrthoP. 2014,38, 1905-1912.
doi: 10.1007/s00264-014-2322-1 URL pmid: 24663398 |
| 47. |
Hangody, L.; Dobos, J.; Baló, E.; Pánics, G.; Hangody, L. R.; Berkes, I. Clinical experiences with autologous osteochondral mosaicplasty in an athletic population: a 17-year prospective multicenter study. Am J Sports Med. 2010,38, 1125-1133.
doi: 10.1177/0363546509360405 URL pmid: 20360608 |
| 48. | Shasha, N.; Krywulak, S.; Backstein, D.; Pressman, A.; Gross, A. E. Long-term follow-up of fresh tibial osteochondral allografts for failed tibial plateau fractures. J Bone Joint Surg Am. 2003, 85-A Suppl 2, 33-39. |
| 49. |
Berruto, M.; Delcogliano, M.; de Caro, F.; Carimati, G.; Uboldi, F.; Ferrua, P.; Ziveri, G.; De Biase, C. F. Treatment of large knee osteochondral lesions with a biomimetic scaffold: results of a multicenter study of 49 patients at 2-year follow-uP. Am J Sports Med. 2014,42, 1607-1617.
doi: 10.1177/0363546514530292 URL pmid: 24778267 |
| 50. |
Jeon, J. E.; Vaquette, C.; Klein, T. J.; Hutmacher, D. W. Perspectives in multiphasic osteochondral tissue engineering. Anat Rec (Hoboken). 2014,297, 26-35.
doi: 10.1002/ar.v297.1 URL |
| 51. |
Gotterbarm, T.; Richter, W.; Jung, M.; Berardi Vilei, S.; Mainil-Varlet, P.; Yamashita, T.; Breusch, S. J. An in vivo study of a growth-factor enhanced, cell free, two-layered collagen-tricalcium phosphate in deep osteochondral defects. Biomaterials. 2006,27, 3387-3395.
doi: 10.1016/j.biomaterials.2006.01.041 URL pmid: 16488472 |
| 52. |
Ahn, S.; Yoon, H.; Kim, G.; Kim, Y.; Lee, S.; Chun, W. Designed three-dimensional collagen scaffolds for skin tissue regeneration. Tissue Eng Part C Methods. 2010,16, 813-820.
doi: 10.1089/ten.tec.2009.0511 URL pmid: 20001740 |
| 53. |
Marquass, B.; Somerson, J. S.; Hepp, P.; Aigner, T.; Schwan, S.; Bader, A.; Josten, C.; Zscharnack, M.; Schulz, R. M. A novel MSC-seeded triphasic construct for the repair of osteochondral defects. J Orthop Res. 2010,28, 1586-1599.
doi: 10.1002/jor.21173 URL pmid: 20973061 |
| 54. |
Sartori, M.; Pagani, S.; Ferrari, A.; Costa, V.; Carina, V.; Figallo, E.; Maltarello, M. C.; Martini, L.; Fini, M.; Giavaresi, G. A new bi-layered scaffold for osteochondral tissue regeneration: In vitro and in vivo preclinical investigations. Mater Sci Eng C Mater Biol Appl. 2017,70, 101-111.
doi: 10.1016/j.msec.2016.08.027 URL pmid: 27770869 |
| 55. | Crovace, A. M.; Giancamillo, A. D.; Gervaso, F.; Mangiavini, L.; Zani, D.; Scalera, F.; Palazzo, B.; Izzo, D.; Agnoletto, M.; Domenicucci, M.; Sosio, C.; Sannino, A.; Giancamillo, M. D.; Peretti, G. M. Evaluation of in vivo response of three biphasic scaffolds for osteochondral tissue regeneration in a sheep model. Vet Sci. 2019,6, 90. |
| 56. |
Liu, X.; Wei, Y.; Xuan, C.; Liu, L.; Lai, C.; Chai, M.; Zhang, Z.; Wang, L.; Shi, X. A biomimetic biphasic osteochondral scaffold with layer-specific release of stem cell differentiation inducers for the reconstruction of osteochondral defects. Adv Healthc Mater. 2020,e2000076.
doi: 10.1002/adhm.202001728 URL pmid: 33305535 |
| 57. |
Filardo, G.; Perdisa, F.; Gelinsky, M.; Despang, F.; Fini, M.; Marcacci, M.; Parrilli, A. P.; Roffi, A.; Salamanna, F.; Sartori, M.; Schütz, K.; Kon, E. Novel alginate biphasic scaffold for osteochondral regeneration: an in vivo evaluation in rabbit and sheep models. J Mater Sci Mater Med. 2018,29, 74.
doi: 10.1007/s10856-018-6074-0 URL pmid: 29804259 |
| 58. |
Vainieri, M. L.; Lolli, A.; Kops, N.; D’Atri, D.; Eglin, D.; Yayon, A.; Alini, M.; Grad, S.; Sivasubramaniyan, K.; van Osch, G. Evaluation of biomimetic hyaluronic-based hydrogels with enhanced endogenous cell recruitment and cartilage matrix formation. Acta Biomater. 2020,101, 293-303.
doi: 10.1016/j.actbio.2019.11.015 URL pmid: 31726249 |
| 59. |
Frenkel, S. R.; Bradica, G.; Brekke, J. H.; Goldman, S. M.; Ieska, K.; Issack, P.; Bong, M. R.; Tian, H.; Gokhale, J.; Coutts, R. D.; Kronengold, R. T. Regeneration of articular cartilage--evaluation of osteochondral defect repair in the rabbit using multiphasic implants. Osteoarthritis Cartilage. 2005,13, 798-807.
doi: 10.1016/j.joca.2005.04.018 URL pmid: 15967685 |
| 60. |
Feng, X.; Xu, P.; Shen, T.; Zhang, Y.; Ye, J.; Gao, C. Influence of pore architectures of silk fibroin/collagen composite scaffolds on the regeneration of osteochondral defects in vivo. J Mater Chem B. 2020,8, 391-405.
doi: 10.1039/c9tb01558b URL pmid: 31599917 |
| 61. |
Pérez-Silos, V.; Moncada-Saucedo, N. K.; Peña-Martínez, V.; Lara-Arias, J.; Marino-Martínez, I. A.; Camacho, A.; Romero-Díaz, V. J.; Lara Banda, M.; García-Ruiz, A.; Soto-Dominguez, A.; Rodriguez-Rocha, H.; López-Serna, N.; Tuan, R. S.; Lin, H.; Fuentes-Mera, L.; A cellularized biphasic implant based on a bioactive silk fibroin promotes integration and tissue organization during osteochondral defect repair in a porcine model. Int J Mol Sci. 2019,20, 5145.
doi: 10.3390/ijms20205145 URL |
| 62. |
Shao, X. X.; Hutmacher, D. W.; Ho, S. T.; Goh, J. C.; Lee, E. H. Evaluation of a hybrid scaffold/cell construct in repair of high-load-bearing osteochondral defects in rabbits. Biomaterials. 2006,27, 1071-1080.
doi: 10.1016/j.biomaterials.2005.07.040 URL pmid: 16129483 |
| 63. |
Zheng, P.; Hu, X.; Lou, Y.; Tang, K. A rabbit model of osteochondral regeneration using three-dimensional printed polycaprolactone-hydroxyapatite scaffolds coated with umbilical cord blood mesenchymal stem cells and chondrocytes. Med Sci Monit. 2019,25, 7361-7369.
doi: 10.12659/MSM.915441 URL pmid: 31570688 |
| 64. |
Cui, W.; Wang, Q.; Chen, G.; Zhou, S.; Chang, Q.; Zuo, Q.; Ren, K.; Fan, W. Repair of articular cartilage defects with tissue-engineered osteochondral composites in pigs. J Biosci Bioeng. 2011,111, 493-500.
doi: 10.1016/j.jbiosc.2010.11.023 URL pmid: 21208828 |
| 65. |
Reyes, R.; Delgado, A.; Sánchez, E.; Fernández, A.; Hernández, A.; Evora, C. Repair of an osteochondral defect by sustained delivery of BMP-2 or TGFβ1 from a bilayered alginate-PLGA scaffold. J Tissue Eng Regen Med. 2014,8, 521-533.
doi: 10.1002/term.1549 URL pmid: 22733683 |
| 66. |
Qi, Y.; Du, Y.; Li, W.; Dai, X.; Zhao, T.; Yan, W. Cartilage repair using mesenchymal stem cell (MSC) sheet and MSCs-loaded bilayer PLGA scaffold in a rabbit model. Knee Surg Sports Traumatol Arthrosc. 2014,22, 1424-1433.
doi: 10.1007/s00167-012-2256-3 URL pmid: 23108680 |
| 67. |
Duan, P.; Pan, Z.; Cao, L.; Gao, J.; Yao, H.; Liu, X.; Guo, R.; Liang, X.; Dong, J.; Ding, J. Restoration of osteochondral defects by implanting bilayered poly(lactide-co-glycolide) porous scaffolds in rabbit joints for 12 and 24 weeks. J Orthop Translat. 2019,19, 68-80.
doi: 10.1016/j.jot.2019.04.006 URL pmid: 31844615 |
| 68. |
Niederauer, G. G.; Slivka, M. A.; Leatherbury, N. C.; Korvick, D. L.; Harroff, H. H.; Ehler, W. C.; Dunn, C. J.; Kieswetter, K. Evaluation of multiphase implants for repair of focal osteochondral defects in goats. Biomaterials. 2000,21, 2561-2574.
doi: 10.1016/s0142-9612(00)00124-1 URL pmid: 11071606 |
| 69. |
Kumbhar, J. V.; Jadhav, S. H.; Bodas, D. S.; Barhanpurkar-Naik, A.; Wani, M. R.; Paknikar, K. M.; Rajwade, J. M. In vitro and in vivo studies of a novel bacterial cellulose-based acellular bilayer nanocomposite scaffold for the repair of osteochondral defects. Int J Nanomedicine. 2017,12, 6437-6459.
doi: 10.2147/IJN.S137361 URL pmid: 28919746 |
| 70. |
Gotterbarm, T.; Breusch, S. J.; Jung, M.; Streich, N.; Wiltfang, J.; Berardi Vilei, S.; Richter, W.; Nitsche, T. Complete subchondral bone defect regeneration with a tricalcium phosphate collagen implant and osteoinductive growth factors: a randomized controlled study in Göttingen minipigs. J Biomed Mater Res B Appl Biomater. 2014,102, 933-942.
doi: 10.1002/jbm.b.33074 URL pmid: 24259283 |
| 71. |
O’Brien, F. J. Biomaterials & scaffolds for tissue engineering. Mater Today. 2011,14, 88-95.
doi: 10.1016/S1369-7021(11)70058-X URL |
| 72. |
Tamaddon, M.; Samizadeh, S.; Wang, L.; Blunn, G.; Liu, C. Intrinsic osteoinductivity of porous titanium scaffold for bone tissue engineering. Int J Biomater. 2017,2017, 5093063.
doi: 10.1155/2017/5093063 URL pmid: 28814954 |
| 73. |
Chang, Y. S.; Gu, H. O.; Kobayashi, M.; Oka, M. Comparison of the bony ingrowth into an osteochondral defect and an artificial osteochondral composite device in load-bearing joints. Knee. 1998,5, 205-213.
doi: 10.1016/S0968-0160(97)10001-1 URL |
| 74. |
Duan, X.; Zhu, X.; Dong, X.; Yang, J.; Huang, F.; Cen, S.; Leung, F.; Fan, H.; Xiang, Z. Repair of large osteochondral defects in a beagle model with a novel type I collagen/glycosaminoglycan-porous titanium biphasic scaffold. Mater Sci Eng C Mater Biol Appl. 2013,33, 3951-3957.
doi: 10.1016/j.msec.2013.05.040 URL pmid: 23910301 |
| 75. |
Bal, B. S.; Rahaman, M. N.; Jayabalan, P.; Kuroki, K.; Cockrell, M. K.; Yao, J. Q.; Cook, J. L. In vivo outcomes of tissue-engineered osteochondral grafts. J Biomed Mater Res B Appl Biomater. 2010,93, 164-174.
doi: 10.1002/jbm.b.31571 URL pmid: 20091911 |
| 76. |
Mrosek, E. H.; Schagemann, J. C.; Chung, H. W.; Fitzsimmons, J. S.; Yaszemski, M. J.; Mardones, R. M.; O’Driscoll, S. W.; Reinholz, G. G. Porous tantalum and poly-epsilon-caprolactone biocomposites for osteochondral defect repair: preliminary studies in rabbits. J Orthop Res. 2010,28, 141-148.
doi: 10.1002/jor.20983 URL pmid: 19743507 |
| 77. |
Kang, H.; Zeng, Y.; Varghese, S. Functionally graded multilayer scaffolds for in vivo osteochondral tissue engineering. Acta Biomater. 2018,78, 365-377.
doi: 10.1016/j.actbio.2018.07.039 URL pmid: 30031911 |
| 78. |
Kon, E.; Filardo, G.; Shani, J.; Altschuler, N.; Levy, A.; Zaslav, K.; Eisman, J. E.; Robinson, D. Osteochondral regeneration with a novel aragonite-hyaluronate biphasic scaffold: up to 12-month follow-up study in a goat model. J Orthop Surg Res. 2015,10, 81.
doi: 10.1186/s13018-015-0211-y URL pmid: 26018574 |
| 79. |
Carmont, M. R.; Carey-Smith, R.; Saithna, A.; Dhillon, M.; Thompson, P.; Spalding, T. Delayed incorporation of a TruFit plug: perseverance is recommended. Arthroscopy. 2009,25, 810-814.
doi: 10.1016/j.arthro.2009.01.023 URL pmid: 19560648 |
| 80. | Spalding, T.; Carey-Smith, R.; Carmont, M.; Dunn, K. TruFit plugs for articular cartilage repair in the knee: 2 year experience, results and MRI appearances (SS-59). Arthroscopy. 2009,25, e32-e33. |
| 81. |
Dhollander, A. A.; Liekens, K.; Almqvist, K. F.; Verdonk, R.; Lambrecht, S.; Elewaut, D.; Verbruggen, G.; Verdonk, P. C. A pilot study of the use of an osteochondral scaffold plug for cartilage repair in the knee and how to deal with early clinical failures. Arthroscopy. 2012,28, 225-233.
doi: 10.1016/j.arthro.2011.07.017 URL pmid: 22014478 |
| 82. |
Joshi, N.; Reverte-Vinaixa, M.; Díaz-Ferreiro, E. W.; Domínguez-Oronoz, R. Synthetic resorbable scaffolds for the treatment of isolated patellofemoral cartilage defects in young patients: magnetic resonance imaging and clinical evaluation. Am J Sports Med. 2012,40, 1289-1295.
doi: 10.1177/0363546512441585 URL pmid: 22491793 |
| 83. |
Pearce, C. J.; Gartner, L. E.; Mitchell, A.; Calder, J. D. Synthetic osteochondral grafting of ankle osteochondral lesions. Foot Ankle Surg. 2012,18, 114-118.
doi: 10.1016/j.fas.2011.04.001 URL pmid: 22443998 |
| 84. |
Bekkers, J. E.; Bartels, L. W.; Vincken, K. L.; Dhert, W. J.; Creemers, L. B.; Saris, D. B. Articular cartilage evaluation after TruFit plug implantation analyzed by delayed gadolinium-enhanced MRI of cartilage (dGEMRIC). Am J Sports Med. 2013,41, 1290-1295.
doi: 10.1177/0363546513483536 URL pmid: 23585485 |
| 85. |
Getgood, A. M.; Kew, S. J.; Brooks, R.; Aberman, H.; Simon, T.; Lynn, A. K.; Rushton, N. Evaluation of early-stage osteochondral defect repair using a biphasic scaffold based on a collagen-glycosaminoglycan biopolymer in a caprine model. Knee. 2012,19, 422-430.
doi: 10.1016/j.knee.2011.03.011 URL pmid: 21620711 |
| 86. |
Chiang, H.; Liao, C. J.; Hsieh, C. H.; Shen, C. Y.; Huang, Y. Y.; Jiang, C. C. Clinical feasibility of a novel biphasic osteochondral composite for matrix-associated autologous chondrocyte implantation. Osteoarthritis Cartilage. 2013,21, 589-598.
doi: 10.1016/j.joca.2013.01.004 URL pmid: 23333470 |
| 87. |
Kusano, T.; Jakob, R. P.; Gautier, E.; Magnussen, R. A.; Hoogewoud, H.; Jacobi, M. Treatment of isolated chondral and osteochondral defects in the knee by autologous matrix-induced chondrogenesis (AMIC). Knee Surg Sports Traumatol Arthrosc. 2012,20, 2109-2115.
doi: 10.1007/s00167-011-1840-2 URL pmid: 22198419 |
| 88. |
Gille, J.; Behrens, P.; Volpi, P.; de Girolamo, L.; Reiss, E.; Zoch, W.; Anders, S. Outcome of autologous matrix induced chondrogenesis (AMIC) in cartilage knee surgery: data of the AMIC Registry. Arch Orthop Trauma Surg. 2013,133, 87-93.
doi: 10.1007/s00402-012-1621-5 URL pmid: 23070222 |
| 89. |
Dhollander, A.; Moens, K.; Van der Maas, J.; Verdonk, P.; Almqvist, K. F.; Victor, J. Treatment of patellofemoral cartilage defects in the knee by autologous matrix-induced chondrogenesis (AMIC). Acta Orthop Belg. 2014,80, 251-259.
URL pmid: 25090800 |
| 90. |
Kon, E.; Filardo, G.; Perdisa, F.; Di Martino, A.; Busacca, M.; Balboni, F.; Sessa, A.; Marcacci, M. A one-step treatment for chondral and osteochondral knee defects: clinical results of a biomimetic scaffold implantation at 2 years of follow-uP. J Mater Sci Mater Med. 2014,25, 2437-2444.
doi: 10.1007/s10856-014-5188-2 URL pmid: 24599553 |
| 91. |
Kon, E.; Filardo, G.; Perdisa, F.; Venieri, G.; Marcacci, M. Clinical results of multilayered biomaterials for osteochondral regeneration. J Exp OrthoP. 2014,1, 10.
doi: 10.1186/s40634-014-0010-0 URL pmid: 26914755 |
| 92. |
Kon, E.; Filardo, G.; Di Martino, A.; Busacca, M.; Moio, A.; Perdisa, F.; Marcacci, M. Clinical results and MRI evolution of a nano-composite multilayered biomaterial for osteochondral regeneration at 5 years. Am J Sports Med. 2014,42, 158-165.
doi: 10.1177/0363546513505434 URL pmid: 24114751 |
| 93. | Marcacci, M.; Filardo, G.; Kon, E. Treatment of cartilage lesions: what works and why? Injury. 2013,44 Suppl 1, S11-15. |
| 94. |
Kon, E.; Delcogliano, M.; Filardo, G.; Busacca, M.; Di Martino, A.; Marcacci, M. Novel nano-composite multilayered biomaterial for osteochondral regeneration: a pilot clinical trial. Am J Sports Med. 2011,39, 1180-1190.
doi: 10.1177/0363546510392711 URL pmid: 21310939 |
| 95. |
Kon, E.; Delcogliano, M.; Filardo, G.; Pressato, D.; Busacca, M.; Grigolo, B.; Desando, G.; Marcacci, M. A novel nano-composite multi-layered biomaterial for treatment of osteochondral lesions: technique note and an early stability pilot clinical trial. Injury. 2010,41, 693-701.
doi: 10.1016/j.injury.2009.11.014 URL pmid: 20035935 |
| 96. |
Kon, E.; Delcogliano, M.; Filardo, G.; Fini, M.; Giavaresi, G.; Francioli, S.; Martin, I.; Pressato, D.; Arcangeli, E.; Quarto, R.; Sandri, M.; Marcacci, M. Orderly osteochondral regeneration in a sheep model using a novel nano-composite multilayered biomaterial. J Orthop Res. 2010,28, 116-124.
doi: 10.1002/jor.20958 URL pmid: 19623663 |
| 97. | Young, R. Orthopaedics this week. https://ryortho.com/2019/05/getting-cartilage-repair-right-after-25-years/. Accessed by May 30, 2019. |
| 98. |
Kon, E.; Drobnic, M.; Davidson, P. A.; Levy, A.; Zaslav, K. R.; Robinson, D. Chronic posttraumatic cartilage lesion of the knee treated with an acellular osteochondral-regenerating implant: case history with rehabilitation guidelines. J Sport Rehabil. 2014,23, 270-275.
doi: 10.1123/jsr.2013-0054 URL pmid: 24231791 |
| 99. | Collagen solutions plc positive eight-year results of ChondroMimetic® cartilage repair clinical study. https://ir.collagensolutions.com/content/news/2018/210218. Accessed by February 21, 2018. |
| 100. |
Degen, R. M.; Tetreault, D.; Mahony, G. T.; Williams, R. J. Acute delamination of commercially available decellularized osteochondral allograft plugs: a report of two cases. Cartilage. 2016,7, 316-321.
doi: 10.1177/1947603515626973 URL pmid: 27688840 |
| 101. |
Farr, J.; Gracitelli, G. C.; Shah, N.; Chang, E. Y.; Gomoll, A. H. High failure rate of a decellularized osteochondral allograft for the treatment of cartilage lesions. Am J Sports Med. 2016,44, 2015-2022.
doi: 10.1177/0363546516645086 URL pmid: 27179056 |
| 102. |
Bishop, M. E.; Seigo, M. A.; Hadley, C. J.; Freedman, K. B. Failure after osteochondral allograft transplantation with the chondrofix implant: a report of two cases. JBJS Case Connect. 2018,8, e86.
doi: 10.2106/JBJS.CC.17.00311 URL pmid: 30601769 |
| 103. |
Trattnig, S.; Ohel, K.; Mlynarik, V.; Juras, V.; Zbyn, S.; Korner, A. Morphological and compositional monitoring of a new cell-free cartilage repair hydrogel technology - GelrinC by MR using semi-quantitative MOCART scoring and quantitative T2 index and new zonal T2 index calculation. Osteoarthritis Cartilage. 2015,23, 2224-2232.
doi: 10.1016/j.joca.2015.07.007 URL pmid: 26187572 |
| 104. |
Kon, E.; Filardo, G.; Brittberg, M.; Busacca, M.; Condello, V.; Engebretsen, L.; Marlovits, S.; Niemeyer, P.; Platzer, P.; Posthumus, M.; Verdonk, P.; Verdonk, R.; Victor, J.; van der Merwe, W.; Widuchowski, W.; Zorzi, C.; Marcacci, M. A multilayer biomaterial for osteochondral regeneration shows superiority vs microfractures for the treatment of osteochondral lesions in a multicentre randomized trial at 2 years. Knee Surg Sports Traumatol Arthrosc. 2018,26, 2704-2715.
doi: 10.1007/s00167-017-4707-3 URL pmid: 28913600 |
| 105. |
Delcogliano, M.; de Caro, F.; Scaravella, E.; Ziveri, G.; De Biase, C. F.; Marotta, D.; Marenghi, P.; Delcogliano, A. Use of innovative biomimetic scaffold in the treatment for large osteochondral lesions of the knee. Knee Surg Sports Traumatol Arthrosc. 2014,22, 1260-1269.
doi: 10.1007/s00167-013-2717-3 URL pmid: 24146051 |
| 106. |
Christensen, B. B.; Foldager, C. B.; Jensen, J.; Jensen, N. C.; Lind, M. Poor osteochondral repair by a biomimetic collagen scaffold: 1- to 3-year clinical and radiological follow-uP. Knee Surg Sports Traumatol Arthrosc. 2016,24, 2380-2387.
doi: 10.1007/s00167-015-3538-3 URL pmid: 25691368 |
| 107. |
Albano, D.; Martinelli, N.; Bianchi, A.; Messina, C.; Malerba, F.; Sconfienza, L. M. Clinical and imaging outcome of osteochondral lesions of the talus treated using autologous matrix-induced chondrogenesis technique with a biomimetic scaffold. BMC Musculoskelet Disord. 2017,18, 306.
doi: 10.1186/s12891-017-1679-x URL pmid: 28720091 |
| 108. |
Williams, R. J.; Gamradt, S. C. Articular cartilage repair using a resorbable matrix scaffold. Instr Course Lect. 2008,57, 563-571.
URL pmid: 18399610 |
| 109. | Saithna, A.; Arbuthnot, J.; Almazedi, B.; Spalding, T. Does acl reconstruction with accelerated rehabilitation influence the outcome of concomitant meniscal repair? Orthop Proc. 2010, 92-B, 423-423. |
| 110. |
Verhaegen, J.; Clockaerts, S.; Van Osch, G. J.; Somville, J.; Verdonk, P.; Mertens, P. TruFit Plug for Repair of Osteochondral Defects-Where Is the Evidence? Systematic Review of Literature. Cartilage. 2015,6, 12-19.
doi: 10.1177/1947603514548890 URL pmid: 26069706 |
| 111. |
Madry, H.; van Dijk, C. N.; Mueller-Gerbl, M. The basic science of the subchondral bone. Knee Surg Sports Traumatol Arthrosc. 2010,18, 419-433.
doi: 10.1007/s00167-010-1054-z URL pmid: 20119671 |
| 112. |
Flachsmann, E. R.; Broom, N. D.; Oloyede, A. A biomechanical investigation of unconstrained shear failure of the osteochondral region under impact loading. Clin Biomech (Bristol, Avon). 1995,10, 156-165.
doi: 10.1016/0268-0033(95)93706-Y URL |
| 113. |
Radin, E. L.; Rose, R. M. Role of subchondral bone in the initiation and progression of cartilage damage. Clin Orthop Relat Res. 1986, 34-40.
doi: 10.1097/00003086-199707000-00006 URL pmid: 9224237 |
| 114. |
Brix, M.; Kaipel, M.; Kellner, R.; Schreiner, M.; Apprich, S.; Boszotta, H.; Windhager, R.; Domayer, S.; Trattnig, S. Successful osteoconduction but limited cartilage tissue quality following osteochondral repair by a cell-free multilayered nano-composite scaffold at the knee. Int OrthoP. 2016,40, 625-632.
URL pmid: 26803322 |
| 115. |
Guilak, F.; Butler, D. L.; Goldstein, S. A. Functional tissue engineering: the role of biomechanics in articular cartilage repair. Clin Orthop Relat Res. 2001, S295-305.
URL pmid: 11603713 |
| 116. |
Setton, L. A.; Elliott, D. M.; Mow, V. C. Altered mechanics of cartilage with osteoarthritis: human osteoarthritis and an experimental model of joint degeneration. Osteoarthritis Cartilage. 1999,7, 2-14.
doi: 10.1053/joca.1998.0170 URL pmid: 10367011 |
| 117. | Pal, S. Mechanical properties of biological materials. In Design of Artificial Human Joints & Organs, Pal, S., ed. Springer US: Boston, MA, 2014; pp 23-40. |
| 118. | Kubicek, M.; Florian, Z. Stress strain analysis of knee joint. Eng Mech. 2009,16, 315-322. |
| 119. | Liu, C; Blunn, G. Osteochondral scaffold.WO Patent publication No. WO 2017/118863 A1. World Intellectual Property Organization International Bureau. |
| 120. |
Getgood, A.; Henson, F.; Skelton, C.; Brooks, R.; Guehring, H.; Fortier, L. A.; Rushton, N. Osteochondral tissue engineering using a biphasic collagen/GAG scaffold containing rhFGF18 or BMP-7 in an ovine model. J Exp OrthoP. 2014,1, 13.
doi: 10.1186/s40634-014-0013-x URL pmid: 26914758 |
| 121. |
Levingstone, T. J.; Ramesh, A.; Brady, R. T.; Brama, P. A. J.; Kearney, C.; Gleeson, J. P.; O’Brien, F. J. Cell-free multi-layered collagen-based scaffolds demonstrate layer specific regeneration of functional osteochondral tissue in caprine joints. Biomaterials. 2016,87, 69-81.
URL pmid: 26901430 |
| [1] | Xirui Jing, Qiuyue Ding, Qinxue Wu, Weijie Su, Keda Yu, Yanlin Su, Bing Ye, Qing Gao, Tingfang Sun, Xiaodong Guo. Magnesium-based materials in orthopaedics: material properties and animal models [J]. Biomaterials Translational, 2021, 2(3): 197-213. |
| [2] | Yiqing Wang, Xiangyu Chu, Bing Wang. Recombinant adeno-associated virus-based gene therapy combined with tissue engineering for musculoskeletal regenerative medicine [J]. Biomaterials Translational, 2021, 2(1): 19-29. |
| [3] | Xing Yang, Yuanyuan Li, Xujie Liu, Wei He, Qianli Huang, Qingling Feng. Nanoparticles and their effects on differentiation of mesenchymal stem cells [J]. Biomaterials Translational, 2020, 1(1): 58-68. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||