Biomaterials Translational ›› 2021, Vol. 2 ›› Issue (1): 19-29.doi: 10.3877/cma.j.issn.2096-112X.2021.01.004
• REVIEW • Previous Articles Next Articles
Yiqing Wang, Xiangyu Chu†, Bing Wang*( )
)
Received:2020-09-09
Revised:2021-01-10
Accepted:2021-01-11
Online:2021-03-31
Published:2021-03-28
Contact:
Bing Wang
E-mail:bingwang@pitt.edu
Wang, Y.; Chu, X.; Wang, B. Recombinant adeno-associated virus-based gene therapy combined with tissue engineering for musculoskeletal regenerative medicine. Biomater Transl. 2021, 2(1), 19-29.
| Serotype | Primary target tissues | Host tested | References |
|---|---|---|---|
| rAAV1 | Central nervous system, liver | Mouse | |
| Muscle, diaphragm | Human | ||
| rAAV2 | Joints, liver, brain | Mouse | |
| Brain, liver, muscle | Human | ||
| rAAV5 | Brain, lung, eye | Mouse | |
| Joints | Monkey | ||
| Lung, brain, eye | Human | ||
| rAAV6 | Heart | Mouse | |
| Liver | Human | ||
| rAAV6.2 | Liver | Mouse | |
| rAAV7 | Brain, central nervous system | Mouse | |
| Brain, eye | Monkey | ||
| Liver | Human | ||
| rAAV8 | Kidney, brain, liver, lung | Mouse | |
| Liver, eye | Human | ||
| rAAV9 | Heart, liver, skeletal muscle | Mouse | |
| Heart, liver, muscle, brain, central nervous system, lung, eye | Human | ||
| rAAVrh.10 | Brain, liver | Human |
Table 1 Common rAAV serotypes for gene delivery
| Serotype | Primary target tissues | Host tested | References |
|---|---|---|---|
| rAAV1 | Central nervous system, liver | Mouse | |
| Muscle, diaphragm | Human | ||
| rAAV2 | Joints, liver, brain | Mouse | |
| Brain, liver, muscle | Human | ||
| rAAV5 | Brain, lung, eye | Mouse | |
| Joints | Monkey | ||
| Lung, brain, eye | Human | ||
| rAAV6 | Heart | Mouse | |
| Liver | Human | ||
| rAAV6.2 | Liver | Mouse | |
| rAAV7 | Brain, central nervous system | Mouse | |
| Brain, eye | Monkey | ||
| Liver | Human | ||
| rAAV8 | Kidney, brain, liver, lung | Mouse | |
| Liver, eye | Human | ||
| rAAV9 | Heart, liver, skeletal muscle | Mouse | |
| Heart, liver, muscle, brain, central nervous system, lung, eye | Human | ||
| rAAVrh.10 | Brain, liver | Human |
| Polymer category | Type of scaffold | Source | Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| Natural | Porous-based scaffolds | Gelatine, collagen, polysaccharides | 1. Biocompatible, biodegradable 2. Low toxicity and inflammation 3. Functionally similar to extracellular matrix | 1. Low bearing capacity | |
| Hydrogel-based scaffolds | Fibrin glue, fibrin sealant, collagen, gelatine, hyaluronic acid | 1. Biodegradable 2. Water-soluble 3. Easily controlled architecture 4. Functionally-similar to extracellular matrix | 1. Poor mechanical properties | ||
| Synthetic | Porous-based scaffolds | Polyester urethane urea, polyester ether urethane urea, polycaprolactone, poly-L-lactic acid | 1. Strong mechanical properties 2. Easily manipulated 3. Versatile shape, toughness, and stability | 1. Low bioactivity 2. Slow degradation 3. Contain acid by-products | |
| Hydrogel-based scaffolds | Poly(ethylene oxide), poly(propylene oxide) | 1. Water-soluble 2. Better mechanical strength | 1. Slow degradation 2. Compromised flexibility |
Table 2 Commonly-used gene- and cell-activated biomaterials
| Polymer category | Type of scaffold | Source | Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| Natural | Porous-based scaffolds | Gelatine, collagen, polysaccharides | 1. Biocompatible, biodegradable 2. Low toxicity and inflammation 3. Functionally similar to extracellular matrix | 1. Low bearing capacity | |
| Hydrogel-based scaffolds | Fibrin glue, fibrin sealant, collagen, gelatine, hyaluronic acid | 1. Biodegradable 2. Water-soluble 3. Easily controlled architecture 4. Functionally-similar to extracellular matrix | 1. Poor mechanical properties | ||
| Synthetic | Porous-based scaffolds | Polyester urethane urea, polyester ether urethane urea, polycaprolactone, poly-L-lactic acid | 1. Strong mechanical properties 2. Easily manipulated 3. Versatile shape, toughness, and stability | 1. Low bioactivity 2. Slow degradation 3. Contain acid by-products | |
| Hydrogel-based scaffolds | Poly(ethylene oxide), poly(propylene oxide) | 1. Water-soluble 2. Better mechanical strength | 1. Slow degradation 2. Compromised flexibility |
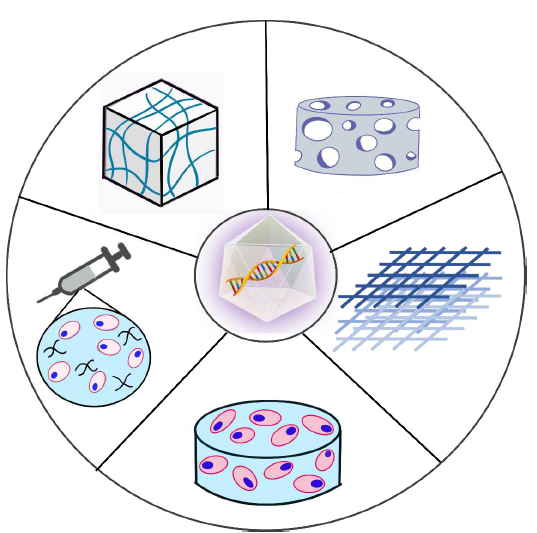
Figure 2. Schematic representation of gene-activated biomaterial scaffolds for delivering rAAV vectors. Starting from the top left in a counter-clockwise order: rAAV vectors can be incorporated into preformed hydrogel-based scaffold,88 or as a mixture containing cells, polymers, and viral particles for direct injection,70 or by transfecting stem cells which have been incorporated into a scaffold.58 An rAAV vector can also be incorporated into a porous or fibrous-based scaffold individually or with stem cells.40, 78, 89 rAAV: recombinant adeno-associated virus.
| Gene | AAV serotype | Scaffold | Biomaterial source | Clinical application | Reference |
|---|---|---|---|---|---|
| BMP-2 | AAV6 | Hydrogel | Gelatine | Cranial bone formation | |
| AAV6 | Porous | PLLA | Bone formation | ||
| AAV2.5 | Porous | PCL | Femoral bone formation | ||
| SOX9 | AAV2 | Micelles | PEO-PPO-PEO copolymer | Cartilage repair | |
| AAV2 | Films | PCL | Cartilage repair | ||
| TGF-β | AAV2 | Micelles | PEO-PPO copolymer | Cartilage repair | |
| VEGF | AAV2 & AAV9 | Fibrous | PEUU & PEEUU | Cardiac tissue regeneration | |
| AAV2 | Matrix | Collagen & glycosaminoglycan | Muscle regeneration |
Table 3 Biomaterial-mediated AAV gene delivery for musculoskeletal tissue repair
| Gene | AAV serotype | Scaffold | Biomaterial source | Clinical application | Reference |
|---|---|---|---|---|---|
| BMP-2 | AAV6 | Hydrogel | Gelatine | Cranial bone formation | |
| AAV6 | Porous | PLLA | Bone formation | ||
| AAV2.5 | Porous | PCL | Femoral bone formation | ||
| SOX9 | AAV2 | Micelles | PEO-PPO-PEO copolymer | Cartilage repair | |
| AAV2 | Films | PCL | Cartilage repair | ||
| TGF-β | AAV2 | Micelles | PEO-PPO copolymer | Cartilage repair | |
| VEGF | AAV2 & AAV9 | Fibrous | PEUU & PEEUU | Cardiac tissue regeneration | |
| AAV2 | Matrix | Collagen & glycosaminoglycan | Muscle regeneration |
| 1. |
GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018, 392, 1789-1858.
doi: 10.1016/S0140-6736(18)32279-7 URL pmid: 30496104 |
| 2. |
Langer, R.; Vacanti, J. P. Tissue engineering. Science. 1993, 260, 920-926.
doi: 10.1126/science.8493529 URL pmid: 8493529 |
| 3. |
Narayanan, G.; Vernekar, V. N.; Kuyinu, E. L.; Laurencin, C. T. Poly (lactic acid)-based biomaterials for orthopaedic regenerative engineering. Adv Drug Deliv Rev. 2016, 107, 247-276.
doi: 10.1016/j.addr.2016.04.015 URL pmid: 27125191 |
| 4. |
Grol, M. W.; Lee, B. H. Gene therapy for repair and regeneration of bone and cartilage. Curr Opin Pharmacol. 2018, 40, 59-66.
doi: 10.1016/j.coph.2018.03.005 URL pmid: 29621661 |
| 5. |
Mesure, B.; Menu, P.; Venkatesan, J. K.; Cucchiarini, M.; Velot, É. Biomaterials and gene therapy: A smart combination for msc musculoskeletal engineering. Curr Stem Cell Res Ther. 2019, 14, 337-343.
doi: 10.2174/1574888X14666181205121658 URL pmid: 30516113 |
| 6. | Yazici, C.; Schwarz, E. M. Regenerative therapy for the musculoskeletal system using recombinant adeno-associated viral vectors. In Advances in gene delivery for bone allografts. Cucchiarini, M.; Madry, H., eds. Research Signpost: Thiruvananthapuram. 2011. pp 109-126. |
| 7. | Gaharwar, A. K.; Singh, I.; Khademhosseini, A. Engineered biomaterials for in situ tissue regeneration. Nat Rev Mater. 2020, 5, 686-705. |
| 8. |
Bulaklak, K.; Xiao, X. Therapeutic advances in musculoskeletal AAV targeting approaches. Curr Opin Pharmacol. 2017, 34, 56-63.
URL pmid: 28743034 |
| 9. |
Xiao, X.; Li, J.; Samulski, R. J. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Virol. 1996, 70, 8098-8108.
doi: 10.1128/JVI.70.11.8098-8108.1996 URL pmid: 8892935 |
| 10. |
Kruzik, A.; Fetahagic, D.; Hartlieb, B.; Dorn, S.; Koppensteiner, H.; Horling, F. M.; Scheiflinger, F.; Reipert, B. M.; de la Rosa, M. Prevalence of anti-adeno-associated virus immune responses in international cohorts of healthy donors. Mol Ther Methods Clin Dev. 2019, 14, 126-133.
doi: 10.1016/j.omtm.2019.05.014 URL pmid: 31338384 |
| 11. |
Wang, D.; Tai, P. W. L.; Gao, G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat Rev Drug Discov. 2019, 18, 358-378.
doi: 10.1038/s41573-019-0012-9 URL pmid: 30710128 |
| 12. |
Naso, M. F.; Tomkowicz, B.; Perry, W. L. 3rd; Strohl, W. R. Adeno-associated virus (AAV) as a vector for gene therapy. Biodrugs. 2017, 31, 317-334.
doi: 10.1007/s40259-017-0234-5 URL pmid: 28669112 |
| 13. |
Levy, H. C.; Hulvey, D.; Adamson-Small, L.; Jn-Simon, N.; Prima, V.; Rivkees, S.; Hobbs, J. A. Improved cell-specificity of adeno-associated viral vectors for medullary thyroid carcinoma using calcitonin gene regulatory elements. PLoS One. 2020, 15, e0228005.
doi: 10.1371/journal.pone.0228005 URL pmid: 32027681 |
| 14. |
Li, C.; Samulski, R. J. Engineering adeno-associated virus vectors for gene therapy. Nat Rev Genet. 2020, 21, 255-272.
doi: 10.1038/s41576-019-0205-4 URL pmid: 32042148 |
| 15. |
Hickey, D. G.; Edwards, T. L.; Barnard, A. R.; Singh, M. S.; de Silva, S. R.; McClements, M. E.; Flannery, J. G.; Hankins, M. W.; MacLaren, R. E. Tropism of engineered and evolved recombinant AAV serotypes in the rd1 mouse and ex vivo primate retina. Gene Ther. 2017, 24, 787-800.
doi: 10.1038/gt.2017.85 URL pmid: 28872643 |
| 16. |
Muraine, L.; Bensalah, M.; Dhiab, J.; Cordova, G.; Arandel, L.; Marhic, A.; Chapart, M.; Vasseur, S.; Benkhelifa-Ziyyat, S.; Bigot, A.; Butler-Browne, G.; Mouly, V.; Negroni, E.; Trollet, C. Transduction efficiency of adeno-associated virus serotypes after local injection in mouse and human skeletal muscle. Hum Gene Ther. 2020, 31, 233-240.
doi: 10.1089/hum.2019.173 URL pmid: 31880951 |
| 17. |
Pacak, C. A.; Mah, C. S.; Thattaliyath, B. D.; Conlon, T. J.; Lewis, M. A.; Cloutier, D. E.; Zolotukhin, I.; Tarantal, A. F.; Byrne, B. J. Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo. Circ Res. 2006, 99, e3-9.
URL pmid: 16873720 |
| 18. |
Liu, Z.; Klose, K.; Neuber, S.; Jiang, M.; Gossen, M.; Stamm, C. Comparative analysis of adeno-associated virus serotypes for gene transfer in organotypic heart slices. J Transl Med. 2020, 18, 437.
doi: 10.1186/s12967-020-02605-4 URL pmid: 33208161 |
| 19. |
Majowicz, A.; Salas, D.; Zabaleta, N.; Rodríguez-Garcia, E.; González-Aseguinolaza, G.; Petry, H.; Ferreira, V. Successful repeated hepatic gene delivery in mice and non-human primates achieved by sequential administration of AAV5(ch) and AAV1. Mol Ther. 2017, 25, 1831-1842.
doi: 10.1016/j.ymthe.2017.05.003 URL pmid: 28596114 |
| 20. |
Lee, S. H.; Kim, S.; Lee, N.; Lee, J.; Yu, S. S.; Kim, J. H.; Kim, S. Intrathecal delivery of recombinant AAV1 encoding hepatocyte growth factor improves motor functions and protects neuromuscular system in the nerve crush and SOD1-G93A transgenic mouse models. Acta Neuropathol Commun. 2019, 7, 96.
doi: 10.1186/s40478-019-0737-z URL pmid: 31189468 |
| 21. | Clinical intramuscular gene transfer of rAAV1.CMV.huFollistatin344 trial to patients with duchenne muscular dystrophy. https://clinicaltrials.gov/ct2/show/NCT02354781. Accessed by January, 2015. |
| 22. |
Corti, M.; Liberati, C.; Smith, B. K.; Lawson, L. A.; Tuna, I. S.; Conlon, T. J.; Coleman, K. E.; Islam, S.; Herzog, R. W.; Fuller, D. D.; Collins, S. W.; Byrne, B. J. Safety of intradiaphragmatic delivery of adeno-associated virus-mediated alpha-glucosidase (rAAV1-CMV-hGAA) gene therapy in children affected by Pompe disease. Hum Gene Ther Clin Dev. 2017, 28, 208-218.
doi: 10.1089/humc.2017.146 URL pmid: 29160099 |
| 23. |
Zhou, X.; Shen, L.; Liu, L.; Wang, C.; Qi, W.; Zhao, A.; Wu, X.; Li, B. Preclinical safety evaluation of recombinant adeno-associated virus 2 vector encoding human tumor necrosis factor receptor-immunoglobulin Fc fusion gene. Hum Vaccin Immunother. 2016, 12, 732-739.
doi: 10.1080/21645515.2015.1090070 URL pmid: 26837862 |
| 24. |
Chen, Z.; Fan, G.; Li, A.; Yuan, J.; Xu, T. rAAV2-retro enables extensive and high-efficient transduction of lower motor neurons following intramuscular injection. Mol Ther Methods Clin Dev. 2020, 17, 21-33.
doi: 10.1016/j.omtm.2019.11.006 URL pmid: 31890738 |
| 25. | AAV2-GDNF for advanced Parkinson’s disease. https://clinicaltrials.gov/ct2/show/NCT01621581. Accessed by March 13, 2013. |
| 26. | Efficacy and safety of BIIB111 for the treatment of choroideremia (STAR). https://clinicaltrials.gov/ct2/show/NCT03496012. Accessed by December 11, 2017. |
| 27. | Safety and dose escalation study of AAV2-hCHM in subjects with CHM (Choroideremia) gene mutations. https://clinicaltrials.gov/ct2/show/NCT02341807. Accessed by January, 2015. |
| 28. | Long-term safety and efficacy follow-up of AAV2-REP1 for the treatment of choroideremia (SOLSTICE) (SOLSTICE). https://clinicaltrials.gov/ct2/show/NCT03584165. Accessed by June 4, 2018. |
| 29. |
Haggerty, D. L.; Grecco, G. G.; Reeves, K. C.; Atwood, B. Adeno-associated viral vectors in neuroscience research. Mol Ther Methods Clin Dev. 2020, 17, 69-82.
doi: 10.1016/j.omtm.2019.11.012 URL pmid: 31890742 |
| 30. |
Li, J.; Wen, A. M.; Potla, R.; Benshirim, E.; Seebarran, A.; Benz, M. A.; Henry, O. Y. F.; Matthews, B. D.; Prantil-Baun, R.; Gilpin, S. E.; Levy, O.; Ingber, D. E. AAV-mediated gene therapy targeting TRPV4 mechanotransduction for inhibition of pulmonary vascular leakage. APL Bioeng. 2019, 3, 046103.
doi: 10.1063/1.5122967 URL |
| 31. |
Aalbers, C. J.; Bevaart, L.; Loiler, S.; de Cortie, K.; Wright, J. F.; Mingozzi, F.; Tak, P. P.; Vervoordeldonk, M. J. Preclinical potency and biodistribution studies of an AAV 5 vector expressing human interferon-β (ART-I02) for local treatment of patients with rheumatoid arthritis. PLoS One. 2015, 10, e0130612.
doi: 10.1371/journal.pone.0130612 URL pmid: 26107769 |
| 32. | Six month lead-in study to evaluate prospective efficacy and safety data of current fix prophylaxis replacement therapy in adult hemophilia B subjects (FIX:C≤2%) or current FVIII Prophylaxis replacement therapy in adult hemophilia a subjects (FVIII:C≤1%). https://clinicaltrials.gov/ct2/show/NCT03587116. Accessed by July 26, 2018. |
| 33. | Study to evaluate the efficacy and safety of PF-07055480 in moderately severe to severe hemophilia a adults (AFFINE). https://clinicaltrials.gov/ct2/show/NCT04370054. Accessed by August 18, 2020. |
| 34. |
Rubin, J. D.; Nguyen, T. V.; Allen, K. L.; Ayasoufi, K.; Barry, M. A. Comparison of gene delivery to the kidney by adenovirus, adeno-associated virus, and lentiviral vectors after intravenous and direct kidney injections. Hum Gene Ther. 2019, 30, 1559-1571.
doi: 10.1089/hum.2019.127 URL pmid: 31637925 |
| 35. |
Taymans, J. M.; Vandenberghe, L. H.; Haute, C. V.; Thiry, I.; Deroose, C. M.; Mortelmans, L.; Wilson, J. M.; Debyser, Z.; Baekelandt, V. Comparative analysis of adeno-associated viral vector serotypes 1, 2, 5, 7, and 8 in mouse brain. Hum Gene Ther. 2007, 18, 195-206.
doi: 10.1089/hum.2006.178 URL pmid: 17343566 |
| 36. |
Shao, W.; Pei, X.; Cui, C.; Askew, C.; Dobbins, A.; Chen, X.; Abajas, Y. L.; Gerber, D. A.; Samulski, R. J.; Nichols, T. C.; Li, C. Superior human hepatocyte transduction with adeno-associated virus vector serotype 7. Gene Ther. 2019, 26, 504-514.
doi: 10.1038/s41434-019-0104-5 URL pmid: 31570819 |
| 37. |
Chen, V. P.; Gao, Y.; Geng, L.; Steele, M.; Jenks, N.; Peng, K. W.; Brimijoin, S. Systemic safety of a recombinant AAV8 vector for human cocaine hydrolase gene therapy: A good laboratory practice preclinical study in mice. Hum Gene Ther. 2020, 31, 70-79.
doi: 10.1089/hum.2019.233 URL pmid: 31650869 |
| 38. |
Nathwani, A. C.; Reiss, U. M.; Tuddenham, E. G.; Rosales, C.; Chowdary, P.; McIntosh, J.; Della Peruta, M.; Lheriteau, E.; Patel, N.; Raj, D.; Riddell, A.; Pie, J.; Rangarajan, S.; Bevan, D.; Recht, M.; Shen, Y. M.; Halka, K. G.; Basner-Tschakarjan, E.; Mingozzi, F.; High, K. A.; Allay, J.; Kay, M. A.; Ng, C. Y.; Zhou, J.; Cancio, M.; Morton, C. L.; Gray, J. T.; Srivastava, D.; Nienhuis, A. W.; Davidoff, A. M. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med. 2014, 371, 1994-2004.
URL pmid: 25409372 |
| 39. | Safety and efficacy of a single subretinal injection of rAAV.hCNGA3 in patients with CNGA3-linked achromatopsia. https://clinicaltrials.gov/ct2/show/NCT02610582. Accessed by November, 2015. |
| 40. |
Gu, X.; Matsumura, Y.; Tang, Y.; Roy, S.; Hoff, R.; Wang, B.; Wagner, W. R. Sustained viral gene delivery from a micro-fibrous, elastomeric cardiac patch to the ischemic rat heart. Biomaterials. 2017, 133, 132-143.
doi: 10.1016/j.biomaterials.2017.04.015 URL pmid: 28433936 |
| 41. |
Pattali, R.; Mou, Y.; Li, X. J. AAV9 Vector: a Novel modality in gene therapy for spinal muscular atrophy. Gene Ther. 2019, 26, 287-295.
doi: 10.1038/s41434-019-0085-4 URL pmid: 31243392 |
| 42. | AAVRh.10 administered to children with late infantile neuronal ceroid lipofuscinosis. https://clinicaltrials.gov/ct2/show/NCT01414985. Accessed by April 15, 2010. |
| 43. | Study of AAVrh10-h.SGSH gene therapy in patients with mucopolysaccharidosis type IIIA (MPS IIIA) (AAVance). https://clinicaltrials.gov/ct2/show/NCT03612869. Accessed by December 17, 2018. |
| 44. |
Wang, B.; Li, J.; Fu, F. H.; Chen, C.; Zhu, X.; Zhou, L.; Jiang, X.; Xiao, X. Construction and analysis of compact muscle-specific promoters for AAV vectors. Gene Ther. 2008, 15, 1489-1499.
doi: 10.1038/gt.2008.104 URL pmid: 18563184 |
| 45. | Charbord, P. Mesenchymal stem cell characterization. In Regenerative medicine and cell therapy. Stoltz, J. F., ed. IOS Press. 2012. pp 27-35. |
| 46. | Murphy, M.; Curtin, C.; Duffy, G.; Kavanagh, C.; Barry, F. Mesenchymal stem cells in regenerative medicine. In Regenerative medicine and cell therapy. Stoltz, J. F., ed. IOS Press. 2012. pp 51-61. |
| 47. |
Caplan, A. I. Mesenchymal stem cells. J Orthop Res. 1991, 9, 641-650.
doi: 10.1002/(ISSN)1554-527X URL |
| 48. |
Tuan, R. S.; Boland, G.; Tuli, R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res Ther. 2003, 5, 32-45.
doi: 10.1186/ar614 URL pmid: 12716446 |
| 49. | Colombini, A.; Perucca Orfei, C.; Kouroupis, D.; Ragni, E.; De Luca, P.; ViganÒ, M.; Correa, D.; de Girolamo, L. Mesenchymal stem cells in the treatment of articular cartilage degeneration: New biological insights for an old-timer cell. Cytotherapy. 2019, 21, 1179-1197. |
| 50. |
Qi, Y.; Feng, G.; Yan, W. Mesenchymal stem cell-based treatment for cartilage defects in osteoarthritis. Mol Biol Rep. 2012, 39, 5683-5689.
doi: 10.1007/s11033-011-1376-z URL pmid: 22183306 |
| 51. |
Huang, S.; Xu, L.; Zhang, Y.; Sun, Y.; Li, G. Systemic and local administration of allogeneic bone marrow-derived mesenchymal stem cells promotes fracture healing in rats. Cell Transplant. 2015, 24, 2643-2655.
doi: 10.3727/096368915X687219 URL pmid: 25647659 |
| 52. |
Maxson, S.; Lopez, E. A.; Yoo, D.; Danilkovitch-Miagkova, A.; Leroux, M. A. Concise review: Role of mesenchymal stem cells in wound repair. Stem Cells Transl Med. 2012, 1, 142-149.
doi: 10.5966/sctm.2011-0018 URL pmid: 23197761 |
| 53. |
Lee, D. E.; Ayoub, N.; Agrawal, D. K. Mesenchymal stem cells and cutaneous wound healing: novel methods to increase cell delivery and therapeutic efficacy. Stem Cell Res Ther. 2016, 7, 37.
doi: 10.1186/s13287-016-0303-6 URL pmid: 26960535 |
| 54. |
Einhorn, T. A.; Majeska, R. J.; Mohaideen, A.; Kagel, E. M.; Bouxsein, M. L.; Turek, T. J.; Wozney, J. M. A single percutaneous injection of recombinant human bone morphogenetic protein-2 accelerates fracture repair. J Bone Joint Surg Am. 2003, 85, 1425-1435.
doi: 10.2106/00004623-200308000-00002 URL pmid: 12925621 |
| 55. |
Bond, A. M.; Bhalala, O. G.; Kessler, J. A. The dynamic role of bone morphogenetic proteins in neural stem cell fate and maturation. Dev Neurobiol. 2012, 72, 1068-1084.
URL pmid: 22489086 |
| 56. |
Xu, H. H.; Liu, S. H.; Guo, Q. F.; Liu, Q. H.; Li, X. Y. Osteogenesis induced in goat bone marrow progenitor cells by recombinant adenovirus coexpressing bone morphogenetic protein 2 and basic fibroblast growth factor. Braz J Med Biol Res. 2013, 46, 809-814.
doi: 10.1590/1414-431X20132929 URL pmid: 24068195 |
| 57. | Zhang, C.; Meng, C.; Guan, D.; Ma, F. BMP2 and VEGF165 transfection to bone marrow stromal stem cells regulate osteogenic potential in vitro. Medicine (Baltimore). 2018, 97, e9787. |
| 58. |
Lin, H.; Tang, Y.; Lozito, T. P.; Oyster, N.; Wang, B.; Tuan, R. S. Efficient in vivo bone formation by BMP-2 engineered human mesenchymal stem cells encapsulated in a projection stereolithographically fabricated hydrogel scaffold. Stem Cell Res Ther. 2019, 10, 254.
doi: 10.1186/s13287-019-1350-6 URL pmid: 31412905 |
| 59. |
Evans, C. H.; Ghivizzani, S. C.; Robbins, P. D. Gene delivery to joints by intra-articular injection. Hum Gene Ther. 2018, 29, 2-14.
doi: 10.1089/hum.2017.181 URL pmid: 29160173 |
| 60. |
Nidetz, N. F.; McGee, M. C.; Tse, L. V.; Li, C.; Cong, L.; Li, Y.; Huang, W. Adeno-associated viral vector-mediated immune responses: Understanding barriers to gene delivery. Pharmacol Ther. 2020, 207, 107453.
URL pmid: 31836454 |
| 61. |
Ronzitti, G.; Gross, D. A.; Mingozzi, F. Human immune responses to adeno-associated virus (AAV) vectors. Front Immunol. 2020, 11, 670.
doi: 10.3389/fimmu.2020.00670 URL pmid: 32362898 |
| 62. | Buck, T. M.; Wijnholds, J. Recombinant Adeno-Associated Viral Vectors (rAAV)-Vector Elements in Ocular Gene Therapy Clinical Trials and Transgene Expression and Bioactivity Assays. Int J Mol Sci. 2020, 21, 4197. |
| 63. | O’Brien, F. J. Biomaterials & scaffolds for tissue engineering. Mater Today. 2011, 14, 88-95. |
| 64. | Pina, S.; Ribeiro, V. P.; Marques, C. F.; Maia, F. R.; Silva, T. H.; Reis, R. L.; Oliveira, J. M. Scaffolding strategies for tissue engineering and regenerative medicine applications. Materials (Basel). 2019, 12, 1824. |
| 65. |
BaoLin, G.; Ma, P. X. Synthetic biodegradable functional polymers for tissue engineering: a brief review. Sci China Chem. 2014, 57, 490-500.
doi: 10.1007/s11426-014-5086-y URL pmid: 25729390 |
| 66. |
D’Mello, S.; Atluri, K.; Geary, S. M.; Hong, L.; Elangovan, S.; Salem, A. K. Bone regeneration using gene-activated matrices. AAPS J. 2017, 19, 43-53.
doi: 10.1208/s12248-016-9982-2 URL pmid: 27655418 |
| 67. |
Yuan, T.; Zhang, L.; Li, K.; Fan, H.; Fan, Y.; Liang, J.; Zhang, X. Collagen hydrogel as an immunomodulatory scaffold in cartilage tissue engineering. J Biomed Mater Res B Appl Biomater. 2014, 102, 337-344.
doi: 10.1002/jbm.b.33011 URL pmid: 24000202 |
| 68. |
Mano, J. F.; Silva, G. A.; Azevedo, H. S.; Malafaya, P. B.; Sousa, R. A.; Silva, S. S.; Boesel, L. F.; Oliveira, J. M.; Santos, T. C.; Marques, A. P.; Neves, N. M.; Reis, R. L. Natural origin biodegradable systems in tissue engineering and regenerative medicine: present status and some moving trends. J R Soc Interface. 2007, 4, 999-1030.
URL pmid: 17412675 |
| 69. |
Cen, L.; Liu, W.; Cui, L.; Zhang, W.; Cao, Y. Collagen tissue engineering: development of novel biomaterials and applications. Pediatr Res. 2008, 63, 492-496.
doi: 10.1203/PDR.0b013e31816c5bc3 URL pmid: 18427293 |
| 70. |
Sun, K.; Lin, H.; Tang, Y.; Xiang, S.; Xue, J.; Yin, W.; Tan, J.; Peng, H.; Alexander, P. G.; Tuan, R. S.; Wang, B. Injectable BMP-2 gene-activated scaffold for the repair of cranial bone defect in mice. Stem Cells Transl Med. 2020, 9, 1631-1642.
doi: 10.1002/sctm.19-0315 URL pmid: 32785966 |
| 71. | Bambole, V.; Yakhmi, J. V. Chapter 14 - Tissue engineering: Use of electrospinning technique for recreating physiological functions. In Nanobiomaterials in soft tissue engineering. Grumezescu, A. M., ed. William Andrew Publishing. 2016. pp 387-455. |
| 72. |
Lee, H. H.; Haleem, A. M.; Yao, V.; Li, J.; Xiao, X.; Chu, C. R. Release of bioactive adeno-associated virus from fibrin scaffolds: effects of fibrin glue concentrations. Tissue Eng Part A. 2011, 17, 1969-1978.
URL pmid: 21449684 |
| 73. |
Gao, X.; Usas, A.; Tang, Y.; Lu, A.; Tan, J.; Schneppendahl, J.; Kozemchak, A. M.; Wang, B.; Cummins, J. H.; Tuan, R. S.; Huard, J. A comparison of bone regeneration with human mesenchymal stem cells and muscle-derived stem cells and the critical role of BMP. Biomaterials. 2014, 35, 6859-6870.
doi: 10.1016/j.biomaterials.2014.04.113 URL pmid: 24856105 |
| 74. |
Gao, X.; Usas, A.; Lu, A.; Tang, Y.; Wang, B.; Chen, C. W.; Li, H.; Tebbets, J. C.; Cummins, J. H.; Huard, J. BMP2 is superior to BMP4 for promoting human muscle-derived stem cell-mediated bone regeneration in a critical-sized calvarial defect model. Cell Transplant. 2013, 22, 2393-2408.
doi: 10.3727/096368912X658854 URL pmid: 23244588 |
| 75. |
Lin, H.; Tang, Y.; Lozito, T. P.; Oyster, N.; Kang, R. B.; Fritch, M. R.; Wang, B.; Tuan, R. S. Projection stereolithographic fabrication of BMP-2 gene-activated matrix for bone tissue engineering. Sci Rep. 2017, 7, 11327.
doi: 10.1038/s41598-017-11051-0 URL pmid: 28900122 |
| 76. |
Venkatesan, J. K.; Falentin-Daudré, C.; Leroux, A.; Migonney, V.; Cucchiarini, M. Biomaterial-Guided recombinant adeno-associated virus delivery from poly(sodium styrene sulfonate)-grafted poly(ε-caprolactone) films to target human bone marrow aspirates. Tissue Eng Part A. 2020, 26, 450-459.
URL pmid: 31680637 |
| 77. |
Salehi, S.; Czugala, M.; Stafiej, P.; Fathi, M.; Bahners, T.; Gutmann, J. S.; Singer, B. B.; Fuchsluger, T. A. Poly (glycerol sebacate)-poly (ε-caprolactone) blend nanofibrous scaffold as intrinsic bio- and immunocompatible system for corneal repair. Acta Biomater. 2017, 50, 370-380.
doi: 10.1016/j.actbio.2017.01.013 URL pmid: 28069498 |
| 78. |
Xue, J.; Lin, H.; Bean, A.; Tang, Y.; Tan, J.; Tuan, R. S.; Wang, B. One-step fabrication of bone morphogenetic protein-2 gene-activated porous poly-l-lactide scaffold for bone induction. Mol Ther Methods Clin Dev. 2017, 7, 50-59.
doi: 10.1016/j.omtm.2017.08.008 URL pmid: 29018836 |
| 79. |
Rey-Rico, A.; Venkatesan, J. K.; Frisch, J.; Schmitt, G.; Monge-Marcet, A.; Lopez-Chicon, P.; Mata, A.; Semino, C.; Madry, H.; Cucchiarini, M. Effective and durable genetic modification of human mesenchymal stem cells via controlled release of rAAV vectors from self-assembling peptide hydrogels with a maintained differentiation potency. Acta Biomater. 2015, 18, 118-127.
doi: 10.1016/j.actbio.2015.02.013 URL pmid: 25712390 |
| 80. |
Patenaude, M.; Campbell, S.; Kinio, D.; Hoare, T. Tuning gelation time and morphology of injectable hydrogels using ketone-hydrazide cross-linking. Biomacromolecules. 2014, 15, 781-790.
doi: 10.1021/bm401615d URL pmid: 24432725 |
| 81. |
Wang, Y.; Adokoh, C. K.; Narain, R. Recent development and biomedical applications of self-healing hydrogels. Expert Opin Drug Deliv. 2018, 15, 77-91.
doi: 10.1080/17425247.2017.1360865 URL pmid: 28771375 |
| 82. |
Shen, H.; Lin, H.; Sun, A. X.; Song, S.; Wang, B.; Yang, Y.; Dai, J.; Tuan, R. S. Acceleration of chondrogenic differentiation of human mesenchymal stem cells by sustained growth factor release in 3D graphene oxide incorporated hydrogels. Acta Biomater. 2020, 105, 44-55.
doi: 10.1016/j.actbio.2020.01.048 URL pmid: 32035282 |
| 83. |
Lin, H.; Cheng, A. W.; Alexander, P. G.; Beck, A. M.; Tuan, R. S. Cartilage tissue engineering application of injectable gelatin hydrogel with in situ visible-light-activated gelation capability in both air and aqueous solution. Tissue Eng Part A. 2014, 20, 2402-2411.
doi: 10.1089/ten.TEA.2013.0642 URL pmid: 24575844 |
| 84. | Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for biomedical applications: Their characteristics and the mechanisms behind them. Gels. 2017, 3, 6. |
| 85. |
Ahmed, E. M. Hydrogel: Preparation, characterization, and applications: A review. J Adv Res. 2015, 6, 105-121.
doi: 10.1016/j.jare.2013.07.006 URL pmid: 25750745 |
| 86. |
Madrigal, J. L.; Shams, S.; Stilhano, R. S.; Silva, E. A. Characterizing the encapsulation and release of lentivectors and adeno-associated vectors from degradable alginate hydrogels. Biomater Sci. 2019, 7, 645-656.
doi: 10.1039/c8bm01218k URL pmid: 30534722 |
| 87. |
Rey-Rico, A.; Madry, H.; Cucchiarini, M. Hydrogel-based controlled delivery systems for articular cartilage repair. Biomed Res Int. 2016, 2016, 1215263.
doi: 10.1155/2016/1215263 URL pmid: 27642587 |
| 88. | Alvarez-Rivera, F.; Rey-Rico, A.; Venkatesan, J. K.; Diaz-Gomez, L.; Cucchiarini, M.; Concheiro, A.; Alvarez-Lorenzo, C. Controlled release of rAAV vectors from APMA-functionalized contact lenses for corneal gene therapy. Pharmaceutics. 2020, 12, 335. |
| 89. |
Jackman, S. L.; Chen, C. H.; Chettih, S. N.; Neufeld, S. Q.; Drew, I. R.; Agba, C. K.; Flaquer, I.; Stefano, A. N.; Kennedy, T. J.; Belinsky, J. E.; Roberston, K.; Beron, C. C.; Sabatini, B. L.; Harvey, C. D.; Regehr, W. G. Silk fibroin films facilitate single-step targeted expression of optogenetic proteins. Cell Rep. 2018, 22, 3351-3361.
doi: 10.1016/j.celrep.2018.02.081 URL pmid: 29562189 |
| 90. |
Gamie, Z.; Tran, G. T.; Vyzas, G.; Korres, N.; Heliotis, M.; Mantalaris, A.; Tsiridis, E. Stem cells combined with bone graft substitutes in skeletal tissue engineering. Expert Opin Biol Ther. 2012, 12, 713-729.
doi: 10.1517/14712598.2012.679652 URL pmid: 22500826 |
| 91. |
Bruder, S. P.; Fink, D. J.; Caplan, A. I. Mesenchymal stem cells in bone development, bone repair, and skeletal regeneration therapy. J Cell Biochem. 1994, 56, 283-294.
doi: 10.1002/jcb.240560303 URL pmid: 7876320 |
| 92. |
Dupont, K. M.; Boerckel, J. D.; Stevens, H. Y.; Diab, T.; Kolambkar, Y. M.; Takahata, M.; Schwarz, E. M.; Guldberg, R. E. Synthetic scaffold coating with adeno-associated virus encoding BMP2 to promote endogenous bone repair. Cell Tissue Res. 2012, 347, 575-588.
doi: 10.1007/s00441-011-1197-3 URL pmid: 21695398 |
| 93. |
Chen, Z.; Bozec, A.; Ramming, A.; Schett, G. Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nat Rev Rheumatol. 2019, 15, 9-17.
doi: 10.1038/s41584-018-0109-2 URL pmid: 30341437 |
| 94. | Lin, Y. J.; Anzaghe, M.; Schülke, S. Update on the pathomechanism, diagnosis, and treatment options for rheumatoid arthritis. Cells. 2020, 9, 880. |
| 95. |
Bek, S.; Bojesen, A. B.; Nielsen, J. V.; Sode, J.; Bank, S.; Vogel, U.; Andersen, V. Systematic review and meta-analysis: pharmacogenetics of anti-TNF treatment response in rheumatoid arthritis. Pharmacogenomics J. 2017, 17, 403-411.
doi: 10.1038/tpj.2017.26 URL pmid: 28607508 |
| 96. |
Watson Levings, R. S.; Smith, A. D.; Broome, T. A.; Rice, B. L.; Gibbs, E. P.; Myara, D. A.; Hyddmark, E. V.; Nasri, E.; Zarezadeh, A.; Levings, P. P.; Lu, Y.; White, M. E.; Dacanay, E. A.; Foremny, G. B.; Evans, C. H.; Morton, A. J.; Winter, M.; Dark, M. J.; Nickerson, D. M.; Colahan, P. T.; Ghivizzani, S. C. Self-complementary adeno-associated virus-mediated interleukin-1 receptor antagonist gene delivery for the treatment of osteoarthritis: Test of efficacy in an equine model. Hum Gene Ther Clin Dev. 2018, 29, 101-112.
doi: 10.1089/humc.2017.143 URL pmid: 29869535 |
| 97. | Roseti, L.; Desando, G.; Cavallo, C.; Petretta, M.; Grigolo, B. Articular cartilage regeneration in osteoarthritis. Cells. 2019, 8, 1305. |
| 98. |
Tao, K.; Frisch, J.; Rey-Rico, A.; Venkatesan, J. K.; Schmitt, G.; Madry, H.; Lin, J.; Cucchiarini, M. Co-overexpression of TGF-β and SOX9 via rAAV gene transfer modulates the metabolic and chondrogenic activities of human bone marrow-derived mesenchymal stem cells. Stem Cell Res Ther. 2016, 7, 20.
doi: 10.1186/s13287-016-0280-9 URL pmid: 26830674 |
| 99. |
Cucchiarini, M.; Madry, H. Biomaterial-guided delivery of gene vectors for targeted articular cartilage repair. Nat Rev Rheumatol. 2019, 15, 18-29.
doi: 10.1038/s41584-018-0125-2 URL pmid: 30514957 |
| 100. |
Yang, R.; Chen, F.; Guo, J.; Zhou, D.; Luan, S. Recent advances in polymeric biomaterials-based gene delivery for cartilage repair. Bioact Mater. 2020, 5, 990-1003.
doi: 10.1016/j.bioactmat.2020.06.004 URL pmid: 32671293 |
| 101. |
Rey-Rico, A.; Venkatesan, J. K.; Schmitt, G.; Concheiro, A.; Madry, H.; Alvarez-Lorenzo, C.; Cucchiarini, M. rAAV-mediated overexpression of TGF-β via vector delivery in polymeric micelles stimulates the biological and reparative activities of human articular chondrocytes in vitro and in a human osteochondral defect model. Int J Nanomedicine. 2017, 12, 6985-6996.
doi: 10.2147/IJN.S144579 URL pmid: 29033566 |
| 102. | Rey-Rico, A.; Venkatesan, J. K.; Schmitt, G.; Speicher-Mentges, S.; Madry, H.; Cucchiarini, M. Effective remodelling of human osteoarthritic cartilage by sox9 gene transfer and overexpression upon delivery of rAAV vectors in polymeric micelles. Mol Pharm. 2018, 15, 2816-2826. |
| 103. | Venkatesan, J. K.; Meng, W.; Rey-Rico, A.; Schmitt, G.; Speicher-Mentges, S.; Falentin-Daudré, C.; Leroux, A.; Madry, H.; Migonney, V.; Cucchiarini, M. Enhanced chondrogenic differentiation activities in human bone marrow aspirates via sox9 overexpression mediated by pNaSS-grafted PCL film-guided rAAV gene transfer. Pharmaceutics. 2020, 12, 280. |
| 104. |
Rybalko, V. Y.; Pham, C. B.; Hsieh, P. L.; Hammers, D. W.; Merscham-Banda, M.; Suggs, L. J.; Farrar, R. P. Controlled delivery of SDF-1α and IGF-1: CXCR4(+) cell recruitment and functional skeletal muscle recovery. Biomater Sci. 2015, 3, 1475-1486.
doi: 10.1039/c5bm00233h URL pmid: 26247892 |
| 105. |
Blumenthal, B.; Golsong, P.; Poppe, A.; Heilmann, C.; Schlensak, C.; Beyersdorf, F.; Siepe, M. Polyurethane scaffolds seeded with genetically engineered skeletal myoblasts: a promising tool to regenerate myocardial function. Artif Organs. 2010, 34, E46-54.
doi: 10.1111/j.1525-1594.2009.00937.x URL pmid: 20420589 |
| 106. |
Moimas, S.; Manasseri, B.; Cuccia, G.; Stagno d’Alcontres, F.; Geuna, S.; Pattarini, L.; Zentilin, L.; Giacca, M.; Colonna, M. R. AAV vector encoding human VEGF165-transduced pectineus muscular flaps increase the formation of new tissue through induction of angiogenesis in an in vivo chamber for tissue engineering: A technique to enhance tissue and vessels in microsurgically engineered tissue. J Tissue Eng. 2015, 6, 2041731415611717.
doi: 10.1177/2041731415592075 URL pmid: 26977283 |
| 107. |
Wang, B.; Li, J.; Xiao, X. Adeno-associated virus vector carrying human minidystrophin genes effectively ameliorates muscular dystrophy in mdx mouse model. Proc Natl Acad Sci U S A. 2000, 97, 13714-13719.
doi: 10.1073/pnas.240335297 URL pmid: 11095710 |
| 108. |
Wang, B.; Li, J.; Fu, F. H.; Xiao, X. Systemic human minidystrophin gene transfer improves functions and life span of dystrophin and dystrophin/utrophin-deficient mice. J Orthop Res. 2009, 27, 421-426.
doi: 10.1002/jor.20781 URL pmid: 18973234 |
| 109. |
Tang, Y.; Reay, D. P.; Salay, M. N.; Mi, M. Y.; Clemens, P. R.; Guttridge, D. C.; Robbins, P. D.; Huard, J.; Wang, B. Inhibition of the IKK/NF-κB pathway by AAV gene transfer improves muscle regeneration in older mdx mice. Gene Ther. 2010, 17, 1476-1483.
doi: 10.1038/gt.2010.110 URL pmid: 20720575 |
| 110. |
Yang, Q.; Tang, Y.; Imbrogno, K.; Lu, A.; Proto, J. D.; Chen, A.; Guo, F.; Fu, F. H.; Huard, J.; Wang, B. AAV-based shRNA silencing of NF-κB ameliorates muscle pathologies in mdx mice. Gene Ther. 2012, 19, 1196-1204.
doi: 10.1038/gt.2011.207 URL pmid: 22278411 |
| 111. |
Yin, X.; Tang, Y.; Li, J.; Dzuricky, A. T.; Pu, C.; Fu, F.; Wang, B. Genetic ablation of P65 subunit of NF-κB in mdx mice to improve muscle physiological function. Muscle Nerve. 2017, 56, 759-767.
doi: 10.1002/mus.25517 URL pmid: 27997693 |
| [1] | Xirui Jing, Qiuyue Ding, Qinxue Wu, Weijie Su, Keda Yu, Yanlin Su, Bing Ye, Qing Gao, Tingfang Sun, Xiaodong Guo. Magnesium-based materials in orthopaedics: material properties and animal models [J]. Biomaterials Translational, 2021, 2(3): 197-213. |
| [2] | Kamolrat Metavarayuth, Esteban Villarreal, Hui Wang, Qian Wang. Surface topography and free energy regulate osteogenesis of stem cells: effects of shape-controlled gold nanoparticles [J]. Biomaterials Translational, 2021, 2(2): 165-173. |
| [3] | Maryam Tamaddon, Helena Gilja, Ling Wang, J. Miguel Oliveira, Xiaodan Sun, Rongwei Tan, Chaozong Liu. Osteochondral scaffolds for early treatment of cartilage defects in osteoarthritic joints: from bench to clinic [J]. Biomaterials Translational, 2020, 1(1): 3-17. |
| [4] | Xing Yang, Yuanyuan Li, Xujie Liu, Wei He, Qianli Huang, Qingling Feng. Nanoparticles and their effects on differentiation of mesenchymal stem cells [J]. Biomaterials Translational, 2020, 1(1): 58-68. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||