Introduction
Musculoskeletal disorders (MSDs) are conditions caused by injuries or diseases affecting the human musculoskeletal system. They range from genetic muscle diseases such as Duchenne muscular dystrophy (DMD) to bone disorders such as osteoporosis, as well as disorders of the joints, including osteoarthritis and rheumatoid arthritis. MSDs are common diseases affecting people worldwide that often cause inflammation, pain, and disabilities in millions of patients.1 Current clinical treatments focus more on stopping the progression of the symptoms such as swelling and pain in order to restore the functions of impaired musculoskeletal systems; however, current approaches to regeneration in those affected by MSDs have met with limited success clinically.
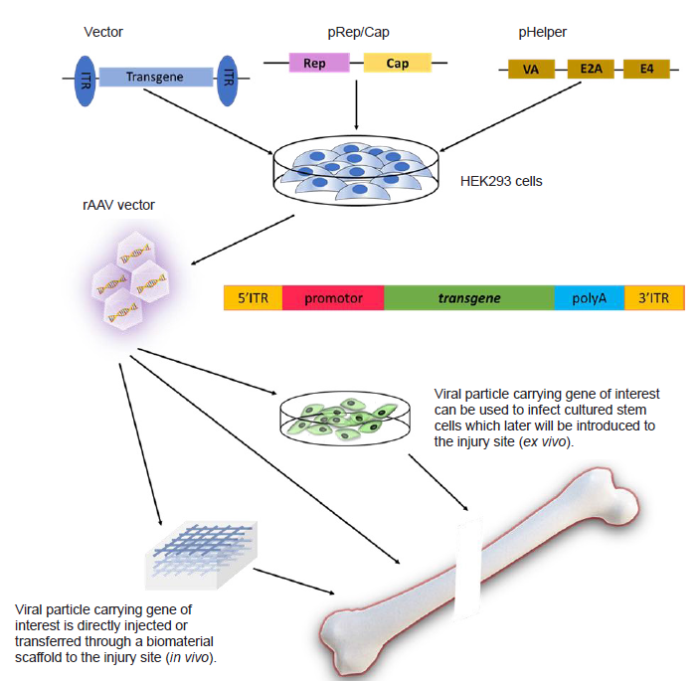
As a multidisciplinary field which combines engineering and life sciences to improve or replace biological tissues, tissue engineering is dedicated to restoring, maintaining or improving tissue functions.2 Traditional tissue engineering aims to combine cells or bioactive molecules with biomaterials, resulting in new tissue formation within the host environment. Approaches to tissue engineering often involve using stem cells that have regenerative properties in combination with biomaterials, which have the correct scaffolding geometry to provide mechanical support and modulate cellular activity.3 In recent years, gene therapy delivering transgenes to impaired tissue has provided another promising treatment for tissue regeneration in MSDs. Recombinant adeno-associated virus (rAAV) serving as a gene therapy vector has been used extensively in the treatment of MSDs and injuries.4 As shown in Figure 1, the rAAV-based gene therapy approach to tissue regeneration has two routes: (1) viral particles carrying a therapeutic gene can be injected directly into the site of injury (in vivo); (2) viral particles can be used to infect cells, which will later be introduced to the injury site (ex vivo).
Figure 1.
Figure 1.
Schematic diagram of rAAV-based gene and cell therapy for bone defect repair. rAAV: recombinant adeno-associated virus.
Since the above approaches for gene transfer are designed to treat MSDs, recently, the combination of gene therapy with tissue engineering has shown great potential in tissue regeneration.5 To be more specific, rAAV vectors carrying therapeutic genes can be loaded onto appropriate biomaterials to provide stable, dose- and time-dependent gene expression.6 Stem cells engineered by ex vivo gene transfer can also be loaded onto biomaterials and implanted into the site of injured tissue.7
In this review, we will discuss recent progress in tissue engineering, particularly the combination of rAAV-based gene therapy and tissue engineering for the regeneration of musculoskeletal tissues, such as bone, cartilage, muscles and joints. We will introduce advances in rAAV-mediated gene therapy and regeneration in tissue engineering. Requirements for appropriate biomaterials that optimize outcomes will also be discussed. The goal of this review is to introduce rAAV-mediated gene therapy, tissue engineering, and their application in MSDs.
Development of Recombinant Adeno-Associated Virus Vectors
An rAAV is produced by transfecting mammalian cells with several plasmids carrying the therapeutic genes and other components needed for viral assembly. In most scenarios, HEK 293 cells (expressing E1A and E1B) are transfected together with three additional plasmids: the vector that carries the gene of interest flanked by two internal terminal repeats, the RepCap plasmid that carries Rep and Cap genes, and the pHelper plasmid which provides other genes that are necessary for the replication process (Figure 1). As an efficient gene vector, the rAAV has been used extensively in the treatment of musculoskeletal diseases and injuries.8 Many favourable factors contribute to the preference for selecting rAAV vectors from among all the other viral particles available as gene delivery vehicles. To begin with, rAAVs are capable of infecting a large variety of host cells, including both dividing and quiescent cells.9 In addition, rAAVs produce long-term transgene expression, a low immune response after infection, and lack toxicity in humans.10 These unique advantages make rAAV-mediated gene therapy a promising strategy for injured tissue that is not able to undergo rapid regeneration. The use of rAAVs has been approved by the U.S. Food and Drug Administration for over 300 clinical studies on human subjects due to their good safety record and high efficiency.11 However, there are some limitations associated with the use of rAAV vectors. For example, the low capacity of the gene expression cassette restricts the choice of transgenes. The wild-type AAV consists of a regular icosahedral particle with a small size (diameter = 20 nm) and short viral genome, usually around 4.7 kb; thus, the genes of interest cannot exceed this specific length (< 5 kb).12 Owing to its ability to infect a large range of host cells, eliciting non-specific gene expression can also be a potential danger for the host organisms. Hence, the design of the tissue- or cell-specific rAAV gene delivery system is an important safety issue in clinical trials.11, 13
Based on their biological characteristics, several strategies have been used to enhance the application of rAAV vectors in gene therapy. A number of serotypes have been identified since the discovery of AAV (AAV1-12).14 Previous studies demonstrated that each serotype of rAAV has specific cellular transduction characteristics in different cell types due to its unique tissue tropism.15, 16 Therefore, increased efficiency in the delivery of a transgene can be achieved by selecting appropriate serotypes for different tissues (Table 1).11, 14, 16-43 Serotypes 6 and 9, for example, show the highest transduction level in myocardium.17, 18 The correct administration approach also increases transduction efficiency; for instance, serotypes 1 and 2 have the highest efficiency for local delivery into muscles; while serotypes 6, 8 and 9 are more attuned for systemic gene delivery to the entire body. It should be noted that the same serotype of rAAV shows different affinities in the same tissues of different animal models.
Table 1 Common rAAV serotypes for gene delivery
| Serotype | Primary target tissues | Host tested | References |
|---|---|---|---|
| rAAV1 | Central nervous system, liver | Mouse | 16, 19, 20 |
| Muscle, diaphragm | Human | 21, 22 | |
| rAAV2 | Joints, liver, brain | Mouse | 23, 24 |
| Brain, liver, muscle | Human | 11, 14, 25-28 | |
| rAAV5 | Brain, lung, eye | Mouse | 29, 30 |
| Joints | Monkey | 31 | |
| Lung, brain, eye | Human | 11, 14 | |
| rAAV6 | Heart | Mouse | 18 |
| Liver | Human | 32, 33 | |
| rAAV6.2 | Liver | Mouse | 34 |
| rAAV7 | Brain, central nervous system | Mouse | 35 |
| Brain, eye | Monkey | 11, 14 | |
| Liver | Human | 36 | |
| rAAV8 | Kidney, brain, liver, lung | Mouse | 34, 37 |
| Liver, eye | Human | 38, 39 | |
| rAAV9 | Heart, liver, skeletal muscle | Mouse | 16, 17, 40 |
| Heart, liver, muscle, brain, central nervous system, lung, eye | Human | 11, 14, 41 | |
| rAAVrh.10 | Brain, liver | Human | 42, 43 |
Note: rAAV: recombinant adeno-associated virus.
Besides using appropriate serotypes of rAAV, applying tissue-specific promotors can control the expression of the target gene and enhance transduction into the host cells.11 In addition, using universal promotors such as the promoters of cytomegalovirus, chicken beta-actin, and elongation factor 1-alpha are more prone to inactivation, building up toxicity, and non-specific transgene expression, while tissue-specific promotors increase safety and allow specific gene expression within different tissues. The muscle creatine kinase promoter has specificity for and activation in muscles, allowing the gene of interest to be expressed only in mature muscle cells and muscle fibres such as cardiac and skeletal muscles.44 Taken together, these findings demonstrate that selection of tissue-specific promoters along with the application of different serotypes can increase the specificity and efficiency of gene therapy.
Tissue Engineering and Regenerative Medicine
Stem cell-based therapy
The paradigm of tissue engineering is composed of cells, signalling molecules and scaffolds.2 Many researchers choose stem cells, such as mesenchymal stem cells (MSCs), as the best candidate for tissue regeneration because of their unique properties, including clonogenicity and self-renewal.45 Under different signalling pathways, they have the potential to differentiate into specialized cells. This pluripotentiality or multipotentiality gives rise to new opportunities for tissue repair, by delivering stem cells into the site of injury and controlling their fate by directing their differentiation into desired phenotypes.46 Bone marrow-derived MSCs (BMSCs) possess the potential to differentiate into bone, cartilage, tendon and connective tissues.47, 48 Owing to additional advantages such as their ready availability, ease of isolation and avoidance of allogeneic responses,48 MSCs have been used extensively in animal models for MSDs, including cartilage repair,49, 50 bone formation,51 and epidermal healing.52, 53
Ex vivo gene therapy and tissue engineering
Growth factors play crucial roles in the proliferation and differentiation of stem cells. In contrast to embryonic stem cells, adult stem cells can only be found in certain parts of the body compartment, and have limited proliferation and differentiation capacities.46 To overcome this restraint, genetic modification of stem cells via gene transfer enables a better therapeutic approach that promotes tissue repair through overexpression of growth factors. Osteoinductive proteins such as bone morphogenetic proteins (BMPs) stimulate the osteogenic differentiation of MSCs into bone-forming cells.54, 55 Viral vectors have been used extensively in preclinical studies to deliver osteoinductive BMP genes, since long-term expression of growth factors is required for successful bone formation.56, 57 Lin et al.58 transduced human MSCs (hMSCs) with the bone morphogenetic protein 2 (BMP-2) gene using a lentiviral vector. Lenti-BMP-2-transduced hMSCs were introduced into hydrogel scaffolds, which were capable of fitting different shapes of defects and were transplanted into severe combined immunodeficient (SCID) mice. Sustained higher expression of osteogenic genes was detected in the Lenti-BMP2 gene group compared with controls. Microcomputed tomographic imaging indicated bone formation as early as 14 days after implantation.58
In summary, efficient in vivo bone formation can be achieved by encapsulating hMSCs that express the BMP-2 gene in a projection stereolithographically-fabricated hydrogel scaffold.
In vivo gene therapy and tissue engineering
In addition to genetically modifying stem cells, viral vectors containing the gene of interest can be applied to the damaged site via direct injection. rAAV vectors are commonly used in intra-articular administration to block articular inflammation, promoting anabolic activities via the introduction of growth and critical factors.59 rAAV vectors remain a superior gene delivery strategy in vivo because of long-term expression and absence of an immune response.60 Whether blocking inflammation or delivering growth factors, administration of any agents via rAAV raises potential safety concerns which should be noted.61 Viral vectors should be controlled temporally and spatially, otherwise, uncontrolled prolonged expression of vector DNA might interfere with the cells’ normal function, even leading to a harmful immune response elicited by the host. Therefore, it is particularly important to develop a regulated rAAV expression system to avoid potential side effects. This requirement can be achieved by selecting appropriate serotypes, tissue-specific promotors, administration approaches, and adapted biomaterials.61, 62
Controlling the release of rAAV gene delivery by biomaterials
As the third component of tissue engineering, appropriate scaffolds provide the structural and biophysical support for cell growth and tissue regeneration.7, 63 While the chemical composition determines mechanical maintenance, more advanced scaffold architecture should be able to mimic the natural extracellular matrix of the tissue, providing the optimal biochemical environment for cell infiltration and for the developing functional tissues.63, 64
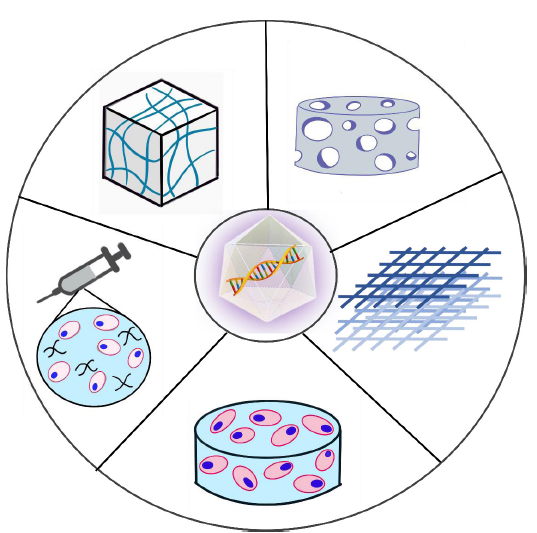
A wide variety of polymers, both natural and synthetic based, has been used in the field of tissue engineering. Several characteristics of the scaffold are necessary, and are shared among all of the material choices: biodegradability, biocompatibility, mechanical properties similar to the site of impairment, and ease of manufacture.63, 65 In the last decades, gene-activated matrix technology, which combines gene therapy and tissue engineering, has emerged as a novel therapeutic approach. Specifically, growth factors and signalling molecules are incorporated into the biomaterial scaffolds in the form of plasmid DNA instead of proteins. The genes of interest are then transcribed and translated within the endogenous damaged cells, in such a way as to achieve sustained gene expression and promote regeneration; however the efficiency of gene transfer is low.66 Given the benefits of viral vector-mediated gene therapy, incorporating viral vectors into an engineered biomaterial can potentiate the effect of therapeutic genes further. Such a gene- or cell-activated biomaterial is able to provide a more promising alternative to the traditional gene-activated matrix technology. When applying gene therapy to tissue engineering, additional requirements need to be met to effectively mediate gene vehicle-based gene transfer. Biomaterials that act to control gene expression should maintain a high and prolonged concentration of transgene at the site of interest while minimizing the dose needed for gene transfer. Two common strategies of biomaterial-mediated gene transfer are achieved by encapsulating viral vector during the fabrication process of the biomaterials or incorporating vectors within the preformed construct. Various biomaterial scaffolds are utilized in combination with gene therapy. Table 2 summarizes the differences in composition and architecture along with the benefits and disadvantages each possesses.67-79 Figure 2 covers approaches of incorporating rAAV vectors into different scaffolds.
Table 2 Commonly-used gene- and cell-activated biomaterials
| Polymer category | Type of scaffold | Source | Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| Natural | Porous-based scaffolds | Gelatine, collagen, polysaccharides | 1. Biocompatible, biodegradable 2. Low toxicity and inflammation 3. Functionally similar to extracellular matrix | 1. Low bearing capacity | 67-71 |
| Hydrogel-based scaffolds | Fibrin glue, fibrin sealant, collagen, gelatine, hyaluronic acid | 1. Biodegradable 2. Water-soluble 3. Easily controlled architecture 4. Functionally-similar to extracellular matrix | 1. Poor mechanical properties | 58, 72-75 | |
| Synthetic | Porous-based scaffolds | Polyester urethane urea, polyester ether urethane urea, polycaprolactone, poly-L-lactic acid | 1. Strong mechanical properties 2. Easily manipulated 3. Versatile shape, toughness, and stability | 1. Low bioactivity 2. Slow degradation 3. Contain acid by-products | 40, 65, 76-78 |
| Hydrogel-based scaffolds | Poly(ethylene oxide), poly(propylene oxide) | 1. Water-soluble 2. Better mechanical strength | 1. Slow degradation 2. Compromised flexibility | 79 |
Figure 2.
Figure 2.
Schematic representation of gene-activated biomaterial scaffolds for delivering rAAV vectors. Starting from the top left in a counter-clockwise order: rAAV vectors can be incorporated into preformed hydrogel-based scaffold,88 or as a mixture containing cells, polymers, and viral particles for direct injection,70 or by transfecting stem cells which have been incorporated into a scaffold.58 An rAAV vector can also be incorporated into a porous or fibrous-based scaffold individually or with stem cells.40, 78, 89 rAAV: recombinant adeno-associated virus.
Collagen is the most abundant protein in the body; and it is also one of the most studied natural polymers due to its natural properties.67-69 It functions similarly to the extracellular matrix, which provides structural support while improving cell growth and tissue repair simultaneously by modulating cell adhesion, proliferation, and differentiation. Natural polymers form microenvironments that promote tissue regeneration; at the same time, porous-based scaffolds provide a large surface area-to-volume ratio for cell infiltration and nutrient delivery. However, the lack of mechanical strength, in particular, low bearing capacity is one of the biggest challenges in applying them to functional scaffolds.64, 71 Synthetic polymers, on the other hand, provide stronger mechanical support with versatile structures, toughness, and stabilities. In the study conducted by Gu et al.40 an rAAV encoding a transgene (rAAV-CMV-GFP or rAAV-CMV-VEGF) was encapsulated into fibrous scaffolds composed of polyester urethane urea and polyester ether urethane urea to form an elastic epicardial patch. Cells seeded onto the rAAV-containing scaffolds showed higher and more sustained transgene expression compared with the direct rAAV injection group; thus demonstrating that controlled release of rAAV vectors using biomaterials could achieve a more localized and efficient gene delivery system, with the synthetic polymers providing structural and mechanical improvement in ischemic cardiomyopathy using an rAAV-CMV-VEGF gene construct. The polyester urethane urea matrix not only had good mechanical properties, but also served as a therapeutic gene delivery system, indicating that such a strategy could also be applied to repair of tendon and ligament post traumatic injury.
Due to their unique properties, hydrogel-based scaffolds have gained extensive attention in the field of tissue engineering over past decades.80-83 Hydrogels, formed by crosslinking natural or synthetic polymers with liquid, have a high water content which increases hydrophilicity and stability.84, 85 Holding a large amount of water in the structure increases their resemblance to the natural extracellular matrix, while at the same time, their highly hydrophilic nature makes hydrogels suitable for drug and gene vector delivery; it also facilitates movement of viral vectors encapsulated in hydrogels via diffusion.72, 79, 86 Rey-Rico et al.87 explained the many additional benefits and strategies of using hydrogel to deliver gene vectors in their recently-published review. As shown in Figure 2, an rAAV could be loaded onto a preformed hydrogel construct by incubation;88 or by transfecting stem cells which would then be incorporated into the construct.58, 89 An injectable solution containing cells, hydrogel polymers and virus particles is also a promising approach to delivery of stem cells and viral particles together.70
Application of Gene-Activated Biomaterials for Musculoskeletal Regeneration
Gene-activated biomaterials for bone healing
Over recent decades substantial research has shown positive therapeutic effects of cell- or gene-based therapy in promoting bone healing.4, 90, 91 Successful conversion of osteogenic progenitor cells into osteoblastic cells has been achieved by the overexpression of osteo-inductive genes. Current biomaterial-guided gene transfer provides a new promising approach to increasing gene transfer efficiency. Dupont et al.92 implanted a self-complementary rAAV (scrAAV) vector-coated poly(ε-caprolactone) scaffold carrying the BMP-2 gene into immunocompromised rats with femoral defects. The results demonstrated that defects treated with scrAAV2.5-BMP delivery in vivo showed increased production of BMP and higher mineral formation.
In ex vivo gene transfer therapy, hMSCs transduced with an rAAV-BMP construct are seeded within biomaterials to stimulate cell proliferation. In a study conducted by Sun et al.70 a gene-activated hydrogel scaffold carrying both rAAV vector and hBMSC simultaneously was developed, such that hBMSCs were transduced with rAAV6-BMP-2 in vivo after transplantation to obtain a temporally- and spatially-controlled release of rAAV particles. The results showed that the concentration of rAAV particles encapsulated within scaffolds decreased significantly more slowly compared with direct viral injection, indicating that the hydrogel had an extended release effect in delivering the rAAV vector. Analysis via microcomputed tomographic images, measurement of new bone volume, and bone mineral density demonstrated increased osteogenic capacity of the hBMSCs encapsulated in BMP-2 gene-activated scaffolds; higher levels of bone formation were observed as early as 6 weeks post implantation. It should be noted that the experimental group treated with hydrogel loaded with both the rAAV vector and hBMSCs had a higher expression level of BMP-2 and osteogenic-related genes (OCN and ALP) compared with the control (hydrogel loaded with modified hBMSCs in vitro). Taken together, these results suggest that a scaffold fabricated with an rAAV gene vector is an effective gene delivery system in bone tissue engineering that is able to control expression of the BMP-2 gene and acts to provide sustained and localized signals needed by hBMSCs for proliferation and differentiation into osteogenic cells, enhancing bone formation.
Gene-Activated Biomaterials for Cartilage Regeneration
Rheumatoid arthritis is an autoinflammatory disease affecting a large number of people worldwide. Osteoarthritis is another type of joint disease caused by cartilage degeneration. Early treatments for both rheumatoid arthritis and osteoarthritis include the administration of glucocorticoids to reduce pain and inflammation; however, no effective treatments have been developed to reverse their pathogenesis and progression. Gene therapy provides a potential therapeutic option for arthritis. By modifying gene expression, gene therapy could decrease chronic inflammation via the administration of inhibitors of proinflammatory cytokines, such as interleukin-1 receptor antagonist, TNF-α inhibitor and anti-inflammatory cytokines (interleukin-4, interleukin-10).93-96 As a treatment for osteoarthritis, gene transfer of growth factors could reduce cartilage degeneration and improve chondrocyte proliferation, which is needed for cartilage repair.59, 97, 98 rAAV-mediated gene therapy in combination with tissue engineering offers a more powerful therapeutic approach to delivery that achieves sustained overexpression of growth factors, and circumvents their characteristic of rapid degradation.99, 100 In 2017, Rey-Rico et al.101 applied a poly (ethylene oxide) (PEO) and poly (propylene oxide) (PPO) copolymer solution with rAAV vectors carrying the transforming growth factor-β (TGF-β) gene to human OA chondrocytes. Administration of rAAV-hTGF-β in combination with polymers led to increased expression of TGF-β and a higher level of type-II collagen deposition. The same research group also modified expression of SRY-box transcription factor 9 (SOX9), a DNA binding protein known to regulate skeletal and cartilage production, via PEO-PPO-PEO polymeric micelles coated with rAAV-FLAG-hSOX9. rAAV-mediated SOX9 gene expression was demonstrated to produce an increase in cell proliferation and improved cartilage remodelling ability.102 Under a similar approach, Venkatesan et al.103 coated rAAV-FLAG-hSOX9 onto pNaSS-grafted poly(ε-caprolactone) films. In the study, rAAV-mediated overexpression of the SOX9 gene via pNass-grated poly(ε-caprolactone) film induced type-II collagen formation and promoted more pronounced chondrogenic differentiation activities compared with any other treatments tested.
Gene-Activated Biomaterials for Skeletal Muscle Regeneration
Even though muscle has an inherent regenerative capacity, many conditions can prevent cells from achieving a full functional recovery. Therefore, studies have attempted to improve muscular growth and prevent formation of fibrous scar tissue which is known to hinder normal function. Critical factors supporting myogenesis have been incorporated into biomaterials to increase the half-life of proteins, in an attempt to increase regeneration of skeletal muscle.104 However, the delivery of genetically-engineered myoblasts via biomaterials offers a more promising effect on muscle regeneration. In a study performed by Blumenthal et al.105 myoblasts overexpressing growth factors were seeded onto polyurethane scaffolds and then transplanted onto damaged myocardium; a successful angiogenic effect was observed, indicating that biomaterial-mediated ex vivo gene therapy could be a potential strategy for cardiac tissue regeneration. The application of biomaterials could offer protection to the rAAV gene transfer system and ultimately enhance muscle repair. To achieve a more efficient and prolonged effect, research conducted by Moimas et al.106 used rAAV-mediated gene transfer in addition to a tissue scaffold. In this study, an rAAV vector encoding vascular endothelial growth factor in combination with a collagen-glycosaminoglycan template was applied onto a pectineus muscle flap to induce angiogenesis and muscle formation. The result indicated that rAAV-based gene therapy in combination with biomaterials is a promising tool to enhance muscle formation.
Muscular dystrophy is a large family of heterogeneous disorders caused by genetic defects in genes encoding muscle cells, preventing muscle from functioning properly. DMD is one of the most prevalent yet lethal muscular dystrophies, affecting 1 in every 3500 live male births. DMD is caused by X-linked genetic mutations of the dystrophin gene, resulting in the losses of structural and functional integrity of cardiac and skeletal muscle cells. Gene therapy offers a promising treatment, such as delivery of the functional micro (or mini)-dystrophin gene by rAAV vectors. In recent decades, our lab has dedicated to developing rAAV-based mini-dystrophin gene replacement therapy to ameliorate the pathology and restore muscle functions through intramuscular or systemic administration.107, 108 After intraperitoneal injection of rAAV-mini-dystrophin into a severe DMD murine model-10-day-old dystrophin/utrophin double knockout mice-strong mini-dystrophin expression was observed, with restored muscle structural integrity in major skeletal muscles, extending the life-span of treated mice. However, one of the biggest challenges of gene therapy, the host immune response against an rAAV vector, results in diminished transfer efficiency and curtailed transgene expression that is associated with chronic inflammation in dystrophic muscle. Therefore, an rAAV-based gene transfer approach has also been applied to reducing inflammation through inhibition of nuclear factor-κB in DMD animal models, improving muscle pathologies and physiological function.109-111 This combined gene therapy approach, with gene replacement and anti-inflammatory agents, may achieve a synergistic effect on the treatment of genetic muscle disorders. We expect that using a biomaterial scaffold to control the release of viral vector may potentially be beneficial in treating DMD, but more preclinical research will be needed in the near future. Table 3 summarises recent progress in combining AAV gene therapy with biomaterials to treat musculoskeletal disorders.40, 70, 78, 92, 101-103, 106
Table 3 Biomaterial-mediated AAV gene delivery for musculoskeletal tissue repair
| Gene | AAV serotype | Scaffold | Biomaterial source | Clinical application | Reference |
|---|---|---|---|---|---|
| BMP-2 | AAV6 | Hydrogel | Gelatine | Cranial bone formation | 70 |
| AAV6 | Porous | PLLA | Bone formation | 78 | |
| AAV2.5 | Porous | PCL | Femoral bone formation | 92 | |
| SOX9 | AAV2 | Micelles | PEO-PPO-PEO copolymer | Cartilage repair | 102 |
| AAV2 | Films | PCL | Cartilage repair | 103 | |
| TGF-β | AAV2 | Micelles | PEO-PPO copolymer | Cartilage repair | 101 |
| VEGF | AAV2 & AAV9 | Fibrous | PEUU & PEEUU | Cardiac tissue regeneration | 40 |
| AAV2 | Matrix | Collagen & glycosaminoglycan | Muscle regeneration | 106 |
Note: BMP-2: bone morphogenetic protein 2; PCL: poly(ε-caprolactone); PEEUU: polyester ether urethane urea; PEO: poly(ethylene oxide); PEUU: polyester urethane urea; PLLA: poly-L-lactic acid; PPO: poly(propylene oxide); rAAV: recombinant adeno-associated virus; SOX9: SRY-box transcription factor 9; TGF-β: transforming growth factor-β; VEGF: vascular endothelial growth factor.
Conclusion and Perspectives
By introducing therapeutic genes through in vivo or ex vivo routes, rAAV-based gene therapy is a promising strategy for treating musculoskeletal diseases and promoting tissue regeneration; yet regulating AAV expression both temporally and spatially is particularly important to avoid an unwanted immune response and achieve high efficiency. Tissue engineering is a field that combines stem cells, bioactive molecules, and scaffolding materials to improve or replace biological tissues. Long-term expression of growth or critical factors is often required for optimal repair and functional restoration. Recent studies have shown that the combination of gene therapy and tissue engineering could circumvent the barriers to both therapies. To be more specific, the viral delivery system could enhance the expression of bioactive factors; in addition, scaffold-mediated gene delivery increases the duration and localization of the transgene that achieves an in situ therapeutic effect. Choosing the best serotypes and promotors would overcome some current obstacles such as low efficiency of transgene expression; at the same time, more suitable designs of scaffold architecture and biomaterial chemical composition will increase the complementarity between gene and tissue-engineering therapy. In future, we expect to see more multidisciplinary translational research leading to potential application in clinical settings.
Author contributions
YW wrote and edited the manuscript; BW and XC supervised and helped to draft the manuscript. BW designed the structure of the review. All authors read and approved the final manuscript.
Financial support
None.
Acknowledgement
None.
Conflicts of interest statement
Bing Wang is an Editorial Board member of Biomaterials Translational.
Data sharing statement
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Reference
Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017
DOI:10.1016/S0140-6736(18)32279-7
URL
PMID:30496104
[Cited within: 1]

BACKGROUND: The Global Burden of Diseases, Injuries, and Risk Factors Study 2017 (GBD 2017) includes a comprehensive assessment of incidence, prevalence, and years lived with disability (YLDs) for 354 causes in 195 countries and territories from 1990 to 2017. Previous GBD studies have shown how the decline of mortality rates from 1990 to 2016 has led to an increase in life expectancy, an ageing global population, and an expansion of the non-fatal burden of disease and injury. These studies have also shown how a substantial portion of the world's population experiences non-fatal health loss with considerable heterogeneity among different causes, locations, ages, and sexes. Ongoing objectives of the GBD study include increasing the level of estimation detail, improving analytical strategies, and increasing the amount of high-quality data. METHODS: We estimated incidence and prevalence for 354 diseases and injuries and 3484 sequelae. We used an updated and extensive body of literature studies, survey data, surveillance data, inpatient admission records, outpatient visit records, and health insurance claims, and additionally used results from cause of death models to inform estimates using a total of 68 781 data sources. Newly available clinical data from India, Iran, Japan, Jordan, Nepal, China, Brazil, Norway, and Italy were incorporated, as well as updated claims data from the USA and new claims data from Taiwan (province of China) and Singapore. We used DisMod-MR 2.1, a Bayesian meta-regression tool, as the main method of estimation, ensuring consistency between rates of incidence, prevalence, remission, and cause of death for each condition. YLDs were estimated as the product of a prevalence estimate and a disability weight for health states of each mutually exclusive sequela, adjusted for comorbidity. We updated the Socio-demographic Index (SDI), a summary development indicator of income per capita, years of schooling, and total fertility rate. Additionally, we calculated differences between male and female YLDs to identify divergent trends across sexes. GBD 2017 complies with the Guidelines for Accurate and Transparent Health Estimates Reporting. FINDINGS: Globally, for females, the causes with the greatest age-standardised prevalence were oral disorders, headache disorders, and haemoglobinopathies and haemolytic anaemias in both 1990 and 2017. For males, the causes with the greatest age-standardised prevalence were oral disorders, headache disorders, and tuberculosis including latent tuberculosis infection in both 1990 and 2017. In terms of YLDs, low back pain, headache disorders, and dietary iron deficiency were the leading Level 3 causes of YLD counts in 1990, whereas low back pain, headache disorders, and depressive disorders were the leading causes in 2017 for both sexes combined. All-cause age-standardised YLD rates decreased by 3.9% (95% uncertainty interval [UI] 3.1-4.6) from 1990 to 2017; however, the all-age YLD rate increased by 7.2% (6.0-8.4) while the total sum of global YLDs increased from 562 million (421-723) to 853 million (642-1100). The increases for males and females were similar, with increases in all-age YLD rates of 7.9% (6.6-9.2) for males and 6.5% (5.4-7.7) for females. We found significant differences between males and females in terms of age-standardised prevalence estimates for multiple causes. The causes with the greatest relative differences between sexes in 2017 included substance use disorders (3018 cases [95% UI 2782-3252] per 100 000 in males vs s1400 [1279-1524] per 100 000 in females), transport injuries (3322 [3082-3583] vs 2336 [2154-2535]), and self-harm and interpersonal violence (3265 [2943-3630] vs 5643 [5057-6302]). INTERPRETATION: Global all-cause age-standardised YLD rates have improved only slightly over a period spanning nearly three decades. However, the magnitude of the non-fatal disease burden has expanded globally, with increasing numbers of people who have a wide spectrum of conditions. A subset of conditions has remained globally pervasive since 1990, whereas other conditions have displayed more dynamic trends, with different ages, sexes, and geographies across the globe experiencing varying burdens and trends of health loss. This study emphasises how global improvements in premature mortality for select conditions have led to older populations with complex and potentially expensive diseases, yet also highlights global achievements in certain domains of disease and injury. FUNDING: Bill & Melinda Gates Foundation.
Tissue engineering
DOI:10.1126/science.8493529
URL
PMID:8493529
[Cited within: 2]

The loss or failure of an organ or tissue is one of the most frequent, devastating, and costly problems in human health care. A new field, tissue engineering, applies the principles of biology and engineering to the development of functional substitutes for damaged tissue. This article discusses the foundations and challenges of this interdisciplinary field and its attempts to provide solutions to tissue creation and repair.
Poly (lactic acid)-based biomaterials for orthopaedic regenerative engineering
DOI:10.1016/j.addr.2016.04.015
URL
PMID:27125191
[Cited within: 1]

Regenerative engineering converges tissue engineering, advanced materials science, stem cell science, and developmental biology to regenerate complex tissues such as whole limbs. Regenerative engineering scaffolds provide mechanical support and nanoscale control over architecture, topography, and biochemical cues to influence cellular outcome. In this regard, poly (lactic acid) (PLA)-based biomaterials may be considered as a gold standard for many orthopaedic regenerative engineering applications because of their versatility in fabrication, biodegradability, and compatibility with biomolecules and cells. Here we discuss recent developments in PLA-based biomaterials with respect to processability and current applications in the clinical and research settings for bone, ligament, meniscus, and cartilage regeneration.
Gene therapy for repair and regeneration of bone and cartilage
DOI:10.1016/j.coph.2018.03.005
URL
PMID:29621661
[Cited within: 2]

Gene therapy refers to the use of viral and non-viral vectors to deliver nucleic acids to tissues of interest using direct (in vivo) or transduced cell-mediated (ex vivo) approaches. Over the past few decades, strategies have been adopted to express therapeutic transgenes at sites of injury to promote or facilitate repair of bone and cartilage. Targets of interest have typically included secreted proteins such as growth factors and anti-inflammatory mediators; however, work has also begun to focus intracellularly on signaling components, transcription factors and small, regulatory nucleic acids such as microRNAs (miRNAs). In recent years, a number of single therapeutic gene approaches (termed 'monotherapies') have proven effective in preclinical models of disease, and several are being evaluated in clinical trials. In particular, an ex vivo TGF-beta1 gene therapy was approved in Korea in 2017 for treatment of moderate-to-severe osteoarthritis (OA). The ability to utilize viral vectors for context-specific and combinatorial gene therapy is also being investigated, and these strategies are likely to be important in more robustly addressing the complexities of tissue repair and regeneration in skeletal disease. In this review, we provide an overview of viral gene therapies being developed for treatment of bone and cartilage pathologies, with an emphasis on emerging combinatorial strategies as well as those targeting intracellular mediators such as miRNAs.
Biomaterials and gene therapy: A smart combination for msc musculoskeletal engineering
DOI:10.2174/1574888X14666181205121658
URL
PMID:30516113
[Cited within: 1]

Musculoskeletal pathologies, especially those affecting bones and joints, remain a challenge for regenerative medicine. The main difficulties affecting bone tissue engineering are the size of the defects, the need for blood vessels and the synthesis of appropriate matrix elements in the engineered tissue. Indeed, the cartilage is an avascular tissue and consequently has limited regenerative abilities. Thanks to their self-renewal, plasticity and immunomodulatory properties, mesenchymal stem cells (MSCs) became a central player in tissue engineering, and have already been shown to be able to differentiate towards chondrogenic or osteogenic phenotypes. Whether synthetic (e.g. tricalcium phosphate) or from natural sources (e.g. hyaluronic acid), biomaterials can be shaped to fit into bone and cartilage defects to ensure mechanical resistance and may also be designed to control cell spatial distribution or differentiation. Soluble factors are classically used to promote cell differentiation and to stimulate extracellular matrix synthesis to achieve the desired tissue production. But as they have a limited lifetime, transfection using plasmid DNA or transduction via a viral vector of therapeutic genes to induce the cell secretion of these factors allows to have more lasting effects. Also, the chondrocyte phenotype may be difficult to control over time, with for example the production of hypertrophic or osteogenic markers that is undesirable in hyaline cartilage. Thus, tissue regeneration strategies became more elaborate, with an attempt at associating the benefits of MSCs, biomaterials, and gene therapy to achieve a proper tissue repair. This minireview focuses on in vitro and in vivo studies combining biomaterials and gene therapy associated with MSCs for bone and cartilage engineering.
Regenerative therapy for the musculoskeletal system using recombinant adeno-associated viral vectors
Engineered biomaterials for in situ tissue regeneration
Therapeutic advances in musculoskeletal AAV targeting approaches
URL PMID:28743034 [Cited within: 1]
Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector
DOI:10.1128/JVI.70.11.8098-8108.1996
URL
PMID:8892935
[Cited within: 1]

Muscle-directed gene transfer is being considered for the treatment of several metabolic diseases, including hemophilia and Duchene's muscular dystrophy. Previous efforts to target this tissue for somatic delivery with various vector systems have resulted in transient expression due to silencing of the transgene or to an immune response against the vector-transduced cells. We introduced recombinant adeno-associated virus vector (rAAV) carrying a lacZ reporter into muscle tissue of immunocompetent mice. The lacZ reporter gene was efficiently transduced and expressed with no evidence of a cellular immune response. Moreover, gene expression persisted for more than 1.5 years. Molecular characterization of rAAV vector DNA suggests a mechanism for persistence, since vector episomes convert to high-molecular-weight genomic DNA. These data provide the first report for establishing long-term gene transduction into mammalian muscle cells in vivo without the need for immune modulation of the organism.
Prevalence of anti-adeno-associated virus immune responses in international cohorts of healthy donors
DOI:10.1016/j.omtm.2019.05.014
URL
PMID:31338384
[Cited within: 1]

Preexisting immunity against adeno-associated virus (AAV) is a major challenge facing AAV gene therapy, resulting in the exclusion of patients from clinical trials. Accordingly, proper assessment of anti-AAV immunity is necessary for understanding clinical data and for product development. Previous studies on anti-AAV prevalence lack method standardization, rendering the assessment of prevalence difficult. Addressing this need, we used clinical assays that were validated according to guidelines for a comprehensive characterization of anti-AAV1, -AAV2, -AAV5, and -AAV8 immunity in large international cohorts of healthy donors and patients with hemophilia B. Here, we report a higher than expected average prevalence for anti-AAV8 ( approximately 40%) and anti-AAV5 ( approximately 30%) neutralizing antibodies (NAbs), which is supported by strongly correlating anti-AAV IgG antibody titers. A similar anti-AAV8 NAb prevalence was observed in hemophilia B patients. In addition, a high co-prevalence of NAbs against other serotypes makes switching to gene therapy using another serotype difficult. As anti-AAV T cell responses are believed to influence transduction, we characterized anti-AAV T cell responses using interleukin-2 (IL-2) and interferon-gamma (IFN-gamma) ELISpot assays, revealing a similar prevalence of IFN-gamma responses ( approximately 20%) against different serotypes that did not correlate with NAbs. These data, along with the long-term stability of NAbs, emphasize the need to develop strategies to circumvent anti-AAV immunity.
Adeno-associated virus vector as a platform for gene therapy delivery
DOI:10.1038/s41573-019-0012-9
URL
PMID:30710128
[Cited within: 8]

Adeno-associated virus (AAV) vectors are the leading platform for gene delivery for the treatment of a variety of human diseases. Recent advances in developing clinically desirable AAV capsids, optimizing genome designs and harnessing revolutionary biotechnologies have contributed substantially to the growth of the gene therapy field. Preclinical and clinical successes in AAV-mediated gene replacement, gene silencing and gene editing have helped AAV gain popularity as the ideal therapeutic vector, with two AAV-based therapeutics gaining regulatory approval in Europe or the United States. Continued study of AAV biology and increased understanding of the associated therapeutic challenges and limitations will build the foundation for future clinical success.
Adeno-associated virus (AAV) as a vector for gene therapy
DOI:10.1007/s40259-017-0234-5
URL
PMID:28669112
[Cited within: 1]

There has been a resurgence in gene therapy efforts that is partly fueled by the identification and understanding of new gene delivery vectors. Adeno-associated virus (AAV) is a non-enveloped virus that can be engineered to deliver DNA to target cells, and has attracted a significant amount of attention in the field, especially in clinical-stage experimental therapeutic strategies. The ability to generate recombinant AAV particles lacking any viral genes and containing DNA sequences of interest for various therapeutic applications has thus far proven to be one of the safest strategies for gene therapies. This review will provide an overview of some important factors to consider in the use of AAV as a vector for gene therapy.
Improved cell-specificity of adeno-associated viral vectors for medullary thyroid carcinoma using calcitonin gene regulatory elements
DOI:10.1371/journal.pone.0228005
URL
PMID:32027681
[Cited within: 1]

Engineering adeno-associated virus vectors for gene therapy
DOI:10.1038/s41576-019-0205-4
URL
PMID:32042148
[Cited within: 6]

Adeno-associated virus (AAV) vector-mediated gene delivery was recently approved for the treatment of inherited blindness and spinal muscular atrophy, and long-term therapeutic effects have been achieved for other rare diseases, including haemophilia and Duchenne muscular dystrophy. However, current research indicates that the genetic modification of AAV vectors may further facilitate the success of AAV gene therapy. Vector engineering can increase AAV transduction efficiency (by optimizing the transgene cassette), vector tropism (using capsid engineering) and the ability of the capsid and transgene to avoid the host immune response (by genetically modifying these components), as well as optimize the large-scale production of AAV.
Tropism of engineered and evolved recombinant AAV serotypes in the rd1 mouse and ex vivo primate retina
DOI:10.1038/gt.2017.85
URL
PMID:28872643
[Cited within: 1]

There is much debate on the adeno-associated virus (AAV) serotype that best targets specific retinal cell types and the route of surgical delivery-intravitreal or subretinal. This study compared three of the most efficacious AAV vectors known to date in a mouse model of retinal degeneration (rd1 mouse) and macaque and human retinal explants. Green fluorescent protein (GFP) driven by a ubiquitous promoter was packaged into three AAV capsids: AAV2/8(Y733F), AAV2/2(quad Y-F) and AAV2/2(7m8). Overall, AAV2/2(7m8) transduced the largest area of retina and resulted in the highest level of GFP expression, followed by AAV2/2(quad Y-F) and AAV2/8(Y733F). AAV2/2(7m8) and AAV2/2(quad Y-F) both resulted in similar patterns of transduction whether they were injected intravitreally or subretinally. AAV2/8(Y733F) transduced a significantly smaller area of retina when injected intravitreally compared with subretinally. Retinal ganglion cells, horizontal cells and retinal pigment epithelium expressed relatively high levels of GFP in the mouse retina, whereas amacrine cells expressed low levels of GFP and bipolar cells were infrequently transduced. Cone cells were the most frequently transduced cell type in macaque retina explants, whereas Muller cells were the predominant transduced cell type in human retinal explants. Of the AAV serotypes tested, AAV2/2(7m8) was the most effective at transducing a range of cell types in degenerate mouse retina and macaque and human retinal explants.
Transduction efficiency of adeno-associated virus serotypes after local injection in mouse and human skeletal muscle
DOI:10.1089/hum.2019.173
URL
PMID:31880951
[Cited within: 4]

The adeno-associated virus (AAV) vector is an efficient tool for gene delivery in skeletal muscle. AAV-based therapies show promising results for treatment of various genetic disorders, including muscular dystrophy. These dystrophies represent a heterogeneous group of diseases affecting muscles and typically characterized by progressive skeletal muscle wasting and weakness and the development of fibrosis. The tropism of each AAV serotype has been extensively studied using systemic delivery routes, but very few studies have compared their transduction efficiency through direct intramuscular injection. Yet, in some muscular dystrophies, where only a few muscles are primarily affected, a local intramuscular injection to target these muscles would be the most appropriate route. A comprehensive comparison between different recombinant AAV (rAAV) serotypes is therefore needed. In this study, we investigated the transduction efficiency of rAAV serotypes 1-10 by local injection in skeletal muscle of control C57BL/6 mice. We used a CMV-nls-LacZ reporter cassette allowing nuclear expression of LacZ to easily localize targeted cells. Detection of beta-galactosidase activity on muscle cryosections demonstrated that rAAV serotypes 1, 7, 8, 9, and 10 were more efficient than the others, with rAAV9 being the most efficient in mice. Furthermore, using a model of human muscle xenograft in immunodeficient mice, we observed that in human muscle, rAAV8 and rAAV9 had similar transduction efficiency. These findings demonstrate for the first time that the human muscle xenograft can be used to evaluate AAV-based therapeutical approaches in a human context.
Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo
URL PMID:16873720 [Cited within: 2]
Comparative analysis of adeno-associated virus serotypes for gene transfer in organotypic heart slices
DOI:10.1186/s12967-020-02605-4
URL
PMID:33208161
[Cited within: 2]

BACKGROUND: Vectors derived from adeno-associated viruses (AAVs) are widely used for gene transfer both in vitro and in vivo and have gained increasing interest as shuttle systems to deliver therapeutic genes to the heart. However, there is little information on their tissue penetration and cytotoxicity, as well as the optimal AAV serotype for transferring genes to diseased hearts. Therefore, we aimed to establish an organotypic heart slice culture system for mouse left ventricular (LV) myocardium and use this platform to analyze gene transfer efficiency, cell tropism, and toxicity of different AAV serotypes. METHODS: LV tissue slices, 300 microm thick, were prepared from 15- to 17-day-old transgenic alpha-myosin heavy-chain-mCherry mice using a vibrating microtome. Tissue slice viability in air-liquid culture was evaluated by calcein-acetoxymethyl ester staining, mCherry fluorescence intensity, and the tetrazolium assay. Four recombinant AAV serotypes (1, 2, 6, 8) expressing green fluorescent protein (GFP) under the CAG promoter were added to the slice surface. Gene transfer efficiency was quantified as the number of GFP-positive cells per slice. AAV cell tropism was examined by comparing the number of GFP-positive cardiomyocytes (CMs) and fibroblasts within heart slices. RESULTS: Slices retained viability in in vitro culture for at least 5 days. After adding AAV particles, AAV6-infected slices showed the highest number of GFP-expressing cells, almost exclusively CMs. Slice incubation with AAV1, 2, and 8 resulted in fewer GFP-positive cells, with AAV2 having the lowest gene transfer efficiency. None of the AAV serotypes tested caused significant cytotoxicity when compared to non-infected control slices. CONCLUSIONS: We have established a readily available mouse organotypic heart slice culture model and provided evidence that AAV6 may be a promising gene therapy vector for heart failure and other cardiac diseases.
Successful repeated hepatic gene delivery in mice and non-human primates achieved by sequential administration of AAV5(ch) and AAV1
DOI:10.1016/j.ymthe.2017.05.003
URL
PMID:28596114
[Cited within: 1]

In the gene therapy field, re-administration of adeno-associated virus (AAV) is an important topic because a decrease in therapeutic protein expression might occur over time. However, an efficient re-administration with the same AAV serotype is impossible due to serotype-specific, anti-AAV neutralizing antibodies (NABs) that are produced after initial AAV treatment. To address this issue, we explored the feasibility of using chimeric AAV serotype 5 (AAV5(ch)) and AAV1 for repeated liver-targeted gene delivery. To develop a relevant model, we immunized animals with a high dose of AAV5(ch)-human secreted embryonic alkaline phosphatase (hSEAP) that generates high levels of anti-AAV5(ch) NAB. Secondary liver transduction with the same dose of AAV1-human factor IX (hFIX) in the presence of high levels of anti-AAV5(ch) NAB proved to be successful because expression/activity of both reporter transgenes was observed. This is the first time that two different transgenes are shown to be produced by non-human primate (NHP) liver after sequential administration of clinically relevant doses of both AAV5(ch) and AAV1. The levels of transgene proteins achieved after delivery with AAV5(ch) and AAV1 illustrate the possibility of both serotypes for liver targeting. Furthermore, transgene DNA and RNA biodistribution patterns provided insight into the potential cause of decrease or loss of transgene protein expression over time in NHPs.
Intrathecal delivery of recombinant AAV1 encoding hepatocyte growth factor improves motor functions and protects neuromuscular system in the nerve crush and SOD1-G93A transgenic mouse models
DOI:10.1186/s40478-019-0737-z
URL
PMID:31189468
[Cited within: 1]

Amyotrophic lateral sclerosis (ALS) is a fatal neuromuscular disease resulting from motor neuron degeneration that causes muscle weakness, paralysis, and eventually respiratory failure. We investigated whether recombinant adeno-associated virus encoding human hepatocyte growth factor (rAAV-HGF) could generate beneficial effects in two mouse models with neuromuscular problems when intrathecally delivered to the subarachnoid space. We chose AAV serotype 1 (rAAV1) based on the expression levels and distribution of HGF protein in the lumbar spinal cord (LSC). After a single intrathecal (IT) injection of rAAV1-HGF, the protein level of HGF in the LSC peaked on day 14 and thereafter gradually decreased over the next 14 weeks. rAAV1-HGF was initially tested in the mouse nerve crush model. IT injection of rAAV1-HGF improved mouse hindlimb strength and rotarod performance, while histological analyses showed that the length of regenerated axons was increased and the structure of the neuromuscular junction (NMJ) was restored. rAAV1-HGF was also evaluated in the SOD1-G93A transgenic (TG) mouse model. Again, rAAV1-HGF not only improved motor performance but also increased the survival rate. Moreover, the number and diameter of spinal motor neurons (SMNs) were increased, and the shape of the NMJs restored. Data from in vitro motor cortical culture experiments indicated that treatment with recombinant HGF protein (rHGF) increased the axon length of corticospinal motor neurons (CSMNs). When cultures were treated with an ERK inhibitor, the effects of HGF on axon elongation, protein aggregation, and oxidative stress were suppressed, indicating that ERK phosphorylation played an important role(s). Taken together, our results suggested that HGF might play an important role(s) in delaying disease progression in the SOD1-G93A TG mouse model by reducing oxidative stress through the control of ERK phosphorylation.
Clinical intramuscular gene transfer of rAAV1.CMV.huFollistatin344 trial to patients with duchenne muscular dystrophy
https://clinicaltrials.gov/ct2/show/NCT02354781. Accessed by January,
Safety of intradiaphragmatic delivery of adeno-associated virus-mediated alpha-glucosidase (rAAV1-CMV-hGAA) gene therapy in children affected by Pompe disease
DOI:10.1089/humc.2017.146
URL
PMID:29160099
[Cited within: 1]

A first-in-human trial of diaphragmatic gene therapy (AAV1-CMV-GAA) to treat respiratory and neural dysfunction in early-onset Pompe disease was conducted. The primary objective of this study was to assess the safety of rAAV1-CMV-hGAA vector delivered to the diaphragm muscle of Pompe disease subjects with ventilatory insufficiency. Safety was assessed by measurement of change in serum chemistries and hematology, urinalysis, and immune response to GAA and AAV, as well as change in level of health. The data demonstrate that the AAV treatment was safe and there were no adverse events related to the study agent. Adverse events related to the study procedure were observed in subjects with lower baseline neuromuscular function. All adverse events were resolved before the end of the study, except for one severe adverse event determined not to be related to either the study agent or the study procedure. In addition, an anti-capsid and anti-transgene antibody response was observed in all subjects who received rAAV1-CMV-hGAA, except for subjects who received concomitant immunomodulation to manage reaction to enzyme replacement therapy, as per their standard of care. This observation is significant for future gene therapy studies and serves to establish a clinically relevant approach to blocking immune responses to both the AAV capsid protein and transgene product.
Preclinical safety evaluation of recombinant adeno-associated virus 2 vector encoding human tumor necrosis factor receptor-immunoglobulin Fc fusion gene
DOI:10.1080/21645515.2015.1090070
URL
PMID:26837862
[Cited within: 1]

Recombinant adeno-associated virus (rAAV) 2 vector gene therapy offers promise for the healing of Rheumatoid arthritis. To support the clinical development of the candidate gene therapeutic product in China, a comprehensive preclinical safety assessment of rAAV2 encoding human TNF receptor-immunoglobulin Fc fusion gene (rAAV2/human TNFR:Fc), were conducted in 3 species of experimental animals. No abnormal findings were observed in mice following single intravenous administration with test article. Compared with the control group, no differences in mean body weight, food consumption in rats and monkeys following the repeated intraarticular administration with rAAV2/human TNFR:Fc. There were also no significant adverse effects due to treatment noted by clinical chemistry, hematology and pathology assessments. After intraarticular administration with rAAV2/human TNFR:Fc, the vector DNA initially distributed to spleen, lymph nodes, and joint synovium. The vector DNA cleared rapidly as it could be detected mainly at the site of injection by 91 d post-administration (182 d for monkey). Taken together, localized delivery of rAAV2/human TNFR:Fc showed no significant toxicity in mice, rats, and monkeys, which support the planned clinical evaluation of this product.
rAAV2-retro enables extensive and high-efficient transduction of lower motor neurons following intramuscular injection
DOI:10.1016/j.omtm.2019.11.006
URL
PMID:31890738
[Cited within: 1]

The motor system controls muscle movement through lower motor neurons in the spinal cord and brainstem. Lower motor neurons are efferent neurons in the central nervous system (CNS) characterized by axonal projections that reach specific targets in the periphery. Lower motor neuron lesions result in the denervation and dysfunction of peripheral skeletal muscle. Great progress has been made to develop therapeutic strategies to transduce lower motor neurons with genes. However, the widespread distribution of lower motor neurons makes their specific, extensive, and efficient transduction a challenge. In this study, we demonstrated that, compared to the other tested recombinant adeno-associated virus (rAAV) serotypes, rAAV2-retro mediated the most efficient retrograde transduction of lower motor neurons in the spinal cord following intramuscular injection in neonatal mice. A single injection of rAAV2-retro in a single muscle enabled the efficient and extensive transduction of lower motor neurons in the spinal cord and brainstem rather than transducing only the lower motor neurons connected to the injected muscle. rAAV2-retro achieved the extensive transduction of lower motor neurons by the cerebrospinal fluid pathway. Our work suggests that gene delivery via the intramuscular injection of rAAV2-retro represents a promising tool in the development of gene therapy strategies for motor neuron diseases.
AAV2-GDNF for advanced Parkinson’s disease
https://clinicaltrials.gov/ct2/show/NCT01621581. Accessed by March 13,
Safety and dose escalation study of AAV2-hCHM in subjects with CHM (Choroideremia) gene mutations
https://clinicaltrials.gov/ct2/show/NCT02341807. Accessed by January,
Long-term safety and efficacy follow-up of AAV2-REP1 for the treatment of choroideremia (SOLSTICE) (SOLSTICE)
https://clinicaltrials.gov/ct2/show/NCT03584165. Accessed by June 4,
Adeno-associated viral vectors in neuroscience research
DOI:10.1016/j.omtm.2019.11.012
URL
PMID:31890742
[Cited within: 1]

Adeno-associated viral vectors (AAVs) are increasingly useful preclinical tools in neuroscience research studies for interrogating cellular and neurocircuit functions and mapping brain connectivity. Clinically, AAVs are showing increasing promise as viable candidates for treating multiple neurological diseases. Here, we briefly review the utility of AAVs in mapping neurocircuits, manipulating neuronal function and gene expression, and activity labeling in preclinical research studies as well as AAV-based gene therapies for diseases of the nervous system. This review highlights the vast potential that AAVs have for transformative research and therapeutics in the neurosciences.
AAV-mediated gene therapy targeting TRPV4 mechanotransduction for inhibition of pulmonary vascular leakage
DOI:10.1063/1.5122967 URL [Cited within: 1]
Preclinical potency and biodistribution studies of an AAV 5 vector expressing human interferon-β (ART-I02) for local treatment of patients with rheumatoid arthritis
DOI:10.1371/journal.pone.0130612
URL
PMID:26107769
[Cited within: 1]

INTRODUCTION: Proof of concept for local gene therapy for the treatment of arthritis with immunomodulatory cytokine interferon beta (IFN-beta) has shown promising results in animal models of rheumatoid arthritis (RA). For the treatment of RA patients, we engineered a recombinant adeno-associated serotype 5 vector (rAAV5) encoding human (h)IFN-beta under control of a nuclear factor kappaB promoter (ART-I02). METHODS: The potency of ART-I02 in vitro as well as biodistribution in vivo in arthritic animals was evaluated to characterize the vector prior to clinical application. ART-I02 expression and bioactivity after transduction was evaluated in fibroblast-like synoviocytes (FLS) from different species. Biodistribution of the vector after local injection was assessed in a rat adjuvant arthritis model through qPCR analysis of vector DNA. In vivo imaging was used to investigate transgene expression and kinetics in a mouse collagen induced arthritis model. RESULTS: Transduction of RA FLS in vitro with ART-I02 resulted in high expression levels of bioactive hIFN-beta. Transduction of FLS from rhesus monkeys, rodents and rabbits with ART-I02 showed high transgene expression, and hIFN-beta proved bioactive in FLS from rhesus monkeys. Transgene expression and bioactivity in RA FLS were unaltered in the presence of methotrexate. In vivo, vector biodistribution analysis in rats after intra-articular injection of ART-I02 demonstrated that the majority of vector DNA remained in the joint (>93%). In vivo imaging in mice confirmed local expression of rAAV5 in the knee joint region and demonstrated rapid detectable and sustained expression up until 7 weeks. CONCLUSIONS: These data show that hIFN-beta produced by RA FLS transduced with ART-I02 is bioactive and that intra-articular delivery of rAAV5 drives expression of a therapeutic transgene in the joint, with only limited biodistribution of vector DNA to other tissues, supporting progress towards a phase 1 clinical trial for the local treatment of arthritis in patients with RA.
Six month lead-in study to evaluate prospective efficacy and safety data of current fix prophylaxis replacement therapy in adult hemophilia B subjects (FIX:C≤2%) or current FVIII Prophylaxis replacement therapy in adult hemophilia a subjects (FVIII:C≤1%)
https://clinicaltrials.gov/ct2/show/NCT03587116. Accessed by July 26,
Study to evaluate the efficacy and safety of PF-07055480 in moderately severe to severe hemophilia a adults (AFFINE)
https://clinicaltrials.gov/ct2/show/NCT04370054. Accessed by August 18,
Comparison of gene delivery to the kidney by adenovirus, adeno-associated virus, and lentiviral vectors after intravenous and direct kidney injections
DOI:10.1089/hum.2019.127
URL
PMID:31637925
[Cited within: 2]

There are many kidney diseases that might be addressed by gene therapy. However, gene delivery to kidney cells is inefficient. This is due, in part, to the fact that the kidney excludes molecules above 50 kDa and that most gene delivery vectors are megaDaltons in mass. We compared the ability of adeno-associated virus (AAV), adenovirus (Ad), and lentiviral (LV) vectors to deliver genes to renal cells. When vectors were delivered by the intravenous (IV) route in mice, weak luciferase activity was observed in the kidney with substantially more in the liver. When gene delivery was observed in the kidney, expression was primarily in the glomerulus. To avoid these limitations, vectors were injected directly into the kidney by retrograde ureteral (RU) and subcapsular (SC) injections in mice. Small AAV vectors transduced the kidney, but also leaked from the organ and mediated higher levels of transduction in off-target tissues. Comparison of AAV2, 6.2, 8, and rh10 vectors by direct kidney injection demonstrated highest delivery by AAV6.2 and 8. Larger Ad and LV vectors transduced kidney cells and mediated less off-target tissue transduction. These data demonstrate the utility of direct kidney injections to circumvent the kidney size exclusion barrier. They also identify the effects of vector size on on-target and off-target transduction. This lays the foundation for the use of different vector platforms for gene therapy of diverse kidney diseases.
Comparative analysis of adeno-associated viral vector serotypes 1, 2, 5, 7, and 8 in mouse brain
DOI:10.1089/hum.2006.178
URL
PMID:17343566
[Cited within: 1]

Recombinant adeno-associated virus serotype 2 (rAAV2) vectors have been shown to deliver genes effectively to neurons in the brain, retina, and spinal cord. The characterization of new AAV serotypes revealed different patterns of transduction in a diverse array of tissues (Gao, G., Vandenberghe, L.H., and Wilson, J.M. [2005]. Curr. Gene Ther. 5, 285-297). Here, we extensively compare the neural tropism of human-derived rAAVs (types 2/1, 2, and 2/5) with nonhuman primate-derived rAAVs (types 2/7 and 2/8) in adult mouse brain. Mice were injected with rAAV type 2/1, 2, 2/5, 2/7, or 2/8 via the caudate-putamen and substantia nigra. Intrahippocampal injections were also performed for rAAV2/7 and rAAV2/8. In all regions injected, the vectors transduced neurons almost exclusively. Retrograde transduction of all rAAV pseudotypes was also observed in particular CNS areas. At high titers, all rAAV pseudotypes transduced comparable brain volumes in all targeted regions except for rAAV2, which transduced much smaller brain volumes. A dose-range comparison of intrastriatally injected rAAV types 2/5, 2/7, and 2/8 highlighted that the transduction efficiency, as determined by transduced volume and biophotonic imaging of green fluorescent protein expression intensity, was significantly higher for rAAV2/5 and rAAV2/7 compared with rAAV2/8 at low titers, whereas all three serotypes performed equally well at higher doses. These results demonstrate the use and efficiency of both human- and nonhuman primate-derived rAAV vectors for disease modeling and their potential for gene therapy.
Superior human hepatocyte transduction with adeno-associated virus vector serotype 7
DOI:10.1038/s41434-019-0104-5
URL
PMID:31570819
[Cited within: 1]

Although therapeutic outcomes have been achieved in hemophilia patients after delivery of clotting factor genes to the liver using adeno-associated virus (AAV) vectors, it is well known that the preclinical results generated from hemophilia animal models have not been directly predictive of successful translation in humans. To address this discrepancy humanized mouse models have recently been used to predict AAV transduction efficiency for human hepatocytes. In this study we evaluated AAV vector transduction from several serotypes in human liver hepatocytes xenografted into chimeric mice. After systemic administration of AAV vectors encoding a GFP transgene in humanized mice, the liver was harvested for either immunohistochemistry staining or flow cytometry assay for AAV human hepatocyte transduction analysis. We observed that AAV7 consistently transduced human hepatocytes more efficiently than other serotypes in both immunohistochemistry assay and flow cytometry analysis. To better assess the future application of AAV7 for systemic administration in the treatment of hemophilia or other liver diseases, we analyzed the prevalence of neutralizing antibodies (NAbs) to AAV7 in sera from healthy subjects and patients with hemophilia. In the general population, the prevalence of NAbs to AAV7 was lower than that of AAV2 or AAV3B. However, a higher prevalence of AAV7 NAbs was found in patients with hemophilia. In summary, results from this study suggest that AAV7 vectors should be considered as an effective vehicle for human liver targeting in future clinical trials.
Systemic safety of a recombinant AAV8 vector for human cocaine hydrolase gene therapy: A good laboratory practice preclinical study in mice
DOI:10.1089/hum.2019.233
URL
PMID:31650869
[Cited within: 1]

Cocaine addiction continues to impose major burdens on affected individuals and broader society but is highly resistant to medical treatment or psychotherapy. This study was undertaken with the goal of Food and Drug Administration (FDA) permission for a first-in-human clinical trial of a gene therapy for treatment-seeking cocaine users to become and remain abstinent. The approach was based on intravenous administration of AAV8-hCocH, an adeno-associated viral vector encoding a modified plasma enzyme that metabolizes cocaine into harmless by-products. To assess systemic safety, we conducted
Long-term safety and efficacy of factor IX gene therapy in hemophilia B
URL PMID:25409372 [Cited within: 1]
Safety and efficacy of a single subretinal injection of rAAV.hCNGA3 in patients with CNGA3-linked achromatopsia
https://clinicaltrials.gov/ct2/show/NCT02610582. Accessed by November,
Sustained viral gene delivery from a micro-fibrous, elastomeric cardiac patch to the ischemic rat heart
DOI:10.1016/j.biomaterials.2017.04.015
URL
PMID:28433936
[Cited within: 6]

Biodegradable and elastomeric patches have been applied to the surface of infarcted hearts as temporary mechanical supports to effectively alter adverse left ventricular remodeling processes. In this report, recombinant adeno-associated virus (AAV), known for its persistent transgene expression and low pathogenicity, was incorporated into elastomeric polyester urethane urea (PEUU) and polyester ether urethane urea (PEEUU) and processed by electrospinning into two formats (solid fibers and core-sheath fibers) designed to influence the controlled release behavior. The extended release of AAV encoding green fluorescent protein (GFP) was assessed in vitro. Sustained and localized viral particle delivery was achieved over 2 months in vitro. The biodegradable cardiac patches with or without AAV-GFP were implanted over rat left ventricular lesions three days following myocardial infarction to evaluate the transduction effect of released viral vectors. AAV particles were directly injected into the infarcted hearts as a control. Cardiac function and remodeling were significantly improved for 12 weeks after patch implantation compared to AAV injection. More GFP genes was expressed in the AAV patch group than AAV injection group, with both alpha-SMA positive cells and cardiac troponin T positive cells transduced in the patch group. Overall, the extended release behavior, prolonged transgene expression, and elastomeric mechanical properties make the AAV-loaded scaffold an attractive option for cardiac tissue engineering where both gene delivery and appropriate mechanical support are desired.
AAV9 Vector: a Novel modality in gene therapy for spinal muscular atrophy
DOI:10.1038/s41434-019-0085-4
URL
PMID:31243392
[Cited within: 1]

Spinal muscular atrophy (SMA), the leading genetic cause of infant mortality, is characterized by the deterioration of alpha motor neurons in the brainstem and spinal cord. Currently, there is no cure for SMA, which calls for an urgent need to explore affordable and effective therapies and to maximize patients' independence and quality of life. Adeno-associated virus (AAV) vector, one of the most promising and well-investigated vehicles for delivering transgenes, is a compelling candidate for gene therapy. Some of the hallmarks of AAVs are their nonpathogenicity, inability to incur an immune response, potential to achieve robust transgene expression, and varied tropism for several tissues of the body. Recently, these features were harnessed in a clinical trial conducted by AveXis in SMA patients, where AAV9 was employed as a vehicle for one-time administration of the SMN gene, the causative gene in SMA. The trial demonstrated remarkable improvements in motor milestones and rates of survival in the patients. This review focuses on the advent of SMA gene therapy and summarizes different preclinical studies that were conducted leading up to the AAV9-SMA trial in SMA patients.
10 administered to children with late infantile neuronal ceroid lipofuscinosis
https://clinicaltrials.gov/ct2/show/NCT01414985. Accessed by April 15,
Study of AAVrh10-h.SGSH gene therapy in patients with mucopolysaccharidosis type IIIA (MPS IIIA) (AAVance)
https://clinicaltrials.gov/ct2/show/NCT03612869. Accessed by December 17,
Construction and analysis of compact muscle-specific promoters for AAV vectors
DOI:10.1038/gt.2008.104
URL
PMID:18563184
[Cited within: 1]

Adeno-associated viral (AAV) vectors have been broadly used for gene transfer in vivo for various applications. However, AAV precludes the use of most of the original large-sized tissue-specific promoters for expression of transgenes. Efforts are made to develop highly compact, active and yet tissue-specific promoters for use in AAV vectors. In this study, we further abbreviated the muscle creatine kinase (MCK) promoter by ligating a double or triple tandem of MCK enhancer (206-bp) to its 87-bp basal promoter, generating the dMCK (509-bp) and tMCK (720-bp) promoters. The dMCK promoter is shorter but stronger than some previously developed MCK-based promoters such as the enh358MCK (584-bp) and CK6 (589-bp) in vitro in C2C12 myotubes and in vivo in skeletal muscles. The tMCK promoter is the strongest that we tested here, more active than the promiscuous cytomegalovirus (CMV) promoter. Furthermore, both the dMCK and tMCK promoters are essentially inactive in nonmuscle cell lines as well as in the mouse liver (>200-fold weaker than the CMV promoter). The dMCK promoter was further tested in a few lines of transgenic mice. Expression of LacZ or minidystrophin gene was detected in skeletal muscles throughout the body, but was weak in the diaphragm, and undetectable in the heart and other tissues. Similar to other miniature MCK promoters, the dMCK promoter also shows preference for fast-twitch myofibers. As a result, we further examined a short, synthetic muscle promoter C5-12 (312-bp). It is active in both skeletal and cardiac muscles but lacks apparent preference on myofiber types. Combination of a MCK enhancer to promoter C5-12 has increased its strength in muscle by two- to threefold. The above-mentioned compact muscle-specific promoters are well suited for AAV vectors in muscle-directed gene therapy studies.
Mesenchymal stem cells
DOI:10.1002/(ISSN)1554-527X URL [Cited within: 1]
Adult mesenchymal stem cells and cell-based tissue engineering
DOI:10.1186/ar614
URL
PMID:12716446
[Cited within: 2]

The identification of multipotential mesenchymal stem cells (MSCs) derived from adult human tissues, including bone marrow stroma and a number of connective tissues, has provided exciting prospects for cell-based tissue engineering and regeneration. This review focuses on the biology of MSCs, including their differentiation potentials in vitro and in vivo, and the application of MSCs in tissue engineering. Our current understanding of MSCs lags behind that of other stem cell types, such as hematopoietic stem cells. Future research should aim to define the cellular and molecular fingerprints of MSCs and elucidate their endogenous role(s) in normal and abnormal tissue functions.
Mesenchymal stem cells in the treatment of articular cartilage degeneration: New biological insights for an old-timer cell
Mesenchymal stem cell-based treatment for cartilage defects in osteoarthritis
DOI:10.1007/s11033-011-1376-z
URL
PMID:22183306
[Cited within: 1]

Osteoarthritis (OA) is a common disorder and the restoration of the diseased articular cartilage in patients with OA is still a challenge for researchers and clinicians. Currently, a variety of experimental strategies have investigated whether mesenchymal stem cells (MSCs) instead of chondrocytes can be used for the regeneration and maintenance of articular cartilage in OA. MSCs can modulate the immune response of individuals and positively influence the microenvironment of the stem cells already present in the diseased tissue. Through direct cell-cell interaction or the secretion of various factors, MSCs can initiate endogenous regenerative activities in the OA joint. Targeted gene-modified MSC-based therapy might further enhance the cartilage regeneration in OA. Conventionally, delivery of MSCs was attained by graft of engineered constructs derived from cell-seeded scaffolds. However, intra-articular MSCs transplantation without scaffolds is a more attractive option for OA treatment. This article briefly summarizes the current knowledge about MSC-based therapy for prevention or treatment of OA, discussing the direct intra-articular injection of MSCs for the treatment of OA in animal models and in clinical applications, as well as potential future strategies for OA treatment.
Systemic and local administration of allogeneic bone marrow-derived mesenchymal stem cells promotes fracture healing in rats
DOI:10.3727/096368915X687219
URL
PMID:25647659
[Cited within: 1]

Mesenchymal stem cells (MSCs) are immune privileged and a cell source for tissue repair. Previous studies showed that there is systemic mobilization of osteoblastic precursors to the fracture site. We hypothesized that both systemic and local administration of allogeneic MSCs may promote fracture healing. Bone marrow-derived MSCs and skin fibroblasts were isolated from GFP Sprague-Dawley rats, cultured, and characterized. Closed transverse femoral fracture with internal fixation was established in 48 adult male Sprague-Dawley rats, which were randomly assigned into four groups receiving PBS injection, MSC systemic injection, fibroblast systemic injection, and MSC fracture site injection; 2 x 10(6) cells were injected at 4 days after fracture. All animals were sacrificed at 5 weeks after fracture; examinations included weekly radiograph, micro-CT, mechanical testing, histology, immunohistochemistry, and double immunofluorescence. The callus size of MSC injection groups was significantly larger among all the groups. Radiographs and 3D reconstruction images showed that the fracture gaps united in the MSC injected groups, while gaps were still seen in the fibroblast and PBS injection groups. The mechanical properties were significantly higher in the MSC injection groups than those in the fibroblast and PBS groups, but no difference was found between the MSC local and systemic injection groups. Immunohistochemistry and double immunofluorescence demonstrated that GFP-positive MSCs were present in the callus in the MSC injection groups at 5 weeks after fracture, and some differentiated into osteoblasts. Quantitative analysis revealed the number of GFP-positive cells in the callus in the MSC systemic injection group was significantly lower than that of the MSC local injection group. The proportion of GFP osteoblasts in GFP-positive cells in the MSC systemic injection group was significantly lower than that of the MSC local injection group. These findings provide critical insight for developing MSC-based therapies, and systemic injection of allogeneic MSCs may be a novel treatment method for promoting fracture repair.
Concise review: Role of mesenchymal stem cells in wound repair
DOI:10.5966/sctm.2011-0018
URL
PMID:23197761
[Cited within: 1]

Wound healing requires a coordinated interplay among cells, growth factors, and extracellular matrix proteins. Central to this process is the endogenous mesenchymal stem cell (MSC), which coordinates the repair response by recruiting other host cells and secreting growth factors and matrix proteins. MSCs are self-renewing multipotent stem cells that can differentiate into various lineages of mesenchymal origin such as bone, cartilage, tendon, and fat. In addition to multilineage differentiation capacity, MSCs regulate immune response and inflammation and possess powerful tissue protective and reparative mechanisms, making these cells attractive for treatment of different diseases. The beneficial effect of exogenous MSCs on wound healing was observed in a variety of animal models and in reported clinical cases. Specifically, they have been successfully used to treat chronic wounds and stimulate stalled healing processes. Recent studies revealed that human placental membranes are a rich source of MSCs for tissue regeneration and repair. This review provides a concise summary of current knowledge of biological properties of MSCs and describes the use of MSCs for wound healing. In particular, the scope of this review focuses on the role MSCs have in each phase of the wound-healing process. In addition, characterization of MSCs containing skin substitutes is described, demonstrating the presence of key growth factors and cytokines uniquely suited to aid in wound repair.
Mesenchymal stem cells and cutaneous wound healing: novel methods to increase cell delivery and therapeutic efficacy
DOI:10.1186/s13287-016-0303-6
URL
PMID:26960535
[Cited within: 1]

Mesenchymal stem cells (MSCs) (also known as multipotent mesenchymal stromal cells) possess the capacity for self-renewal and multi-lineage differentiation, and their ability to enhance cutaneous wound healing has been well characterized. Acting via paracrine interactions, MSCs accelerate wound closure, increase angiogenesis, promote resolution of wound inflammation, favorably regulate extracellular matrix remodeling, and encourage regeneration of skin with normal architecture and function. A number of studies have employed novel methods to amplify the delivery and efficacy of MSCs. Non-traditional sources of MSCs, including Wharton's jelly and medical waste material, have shown efficacy comparable to that of traditional sources, such as bone marrow and adipose tissue. The potential of alternative methods to both introduce MSCs into wounds and increase migration of MSCs into wound areas has also been demonstrated. Taking advantage of the associations between MSCs with M2 macrophages and microRNA, methods to enhance the immunomodulatory capacity of MSCs have shown success. New measures to enhance angiogenic capabilities have also exhibited effectiveness, often demonstrated by increased levels of proangiogenic vascular endothelial growth factor. Finally, hypoxia has been shown to have strong wound-healing potential in terms of increasing MSC efficacy. We have critically reviewed the results of the novel studies that show promise for the continued development of MSC-based wound-healing therapies and provide direction for continued research in this field.
A single percutaneous injection of recombinant human bone morphogenetic protein-2 accelerates fracture repair
DOI:10.2106/00004623-200308000-00002
URL
PMID:12925621
[Cited within: 1]

The dynamic role of bone morphogenetic proteins in neural stem cell fate and maturation
URL PMID:22489086 [Cited within: 1]
Osteogenesis induced in goat bone marrow progenitor cells by recombinant adenovirus coexpressing bone morphogenetic protein 2 and basic fibroblast growth factor
DOI:10.1590/1414-431X20132929
URL
PMID:24068195
[Cited within: 1]

Bone morphogenetic protein 2 (BMP2) and basic fibroblast growth factor (bFGF) have been shown to exhibit a synergistic effect to promote bone repair and healing. In this study, we constructed a novel adenovirus with high coexpression of BMP2 and bFGF and evaluated its effect on osteogenic differentiation of goat bone marrow progenitor cells (BMPCs). Recombinant adenovirus Ad-BMP2-bFGF was constructed by using the T2A sequence. BMPCs were isolated from goats by density gradient centrifugation and adherent cell culture, and were then infected with Ad-BMP2-bFGF or Ad-BMP2. Expression of BMP2 and bFGF was detected by ELISA, and alkaline phosphatase (ALP) activity was detected by an ALP assay kit. In addition, von Kossa staining and immunocytochemical staining of collagen II were performed on BMPCs 21 days after infection. There was a high coexpression of BMP2 and bFGF in BMPCs infected with Ad-BMP2-bFGF. Twenty-one days after infection, ALP activity was significantly higher in BMPCs infected with Ad-BMP2-bFGF than in those infected with Ad-BMP2. Larger and more mineralized calcium nodules, as well as stronger collagen II staining, were observed in BMPCs infected with Ad-BMP2-bFGF than in those infected with Ad-BMP2. In summary, we developed a novel adenovirus vector Ad-BMP2-bFGF for simultaneous high coexpression of BMP2 and bFGF, which could induce BMPCs to differentiate efficiently into osteoblasts.
BMP2 and VEGF165 transfection to bone marrow stromal stem cells regulate osteogenic potential in vitro
Efficient in vivo bone formation by BMP-2 engineered human mesenchymal stem cells encapsulated in a projection stereolithographically fabricated hydrogel scaffold
DOI:10.1186/s13287-019-1350-6
URL
PMID:31412905
[Cited within: 5]

BACKGROUND: Stem cell-based bone tissue engineering shows promise for bone repair but faces some challenges, such as insufficient osteogenesis and limited architecture flexibility of the cell-delivery scaffold. METHODS: In this study, we first used lentiviral constructs to transduce ex vivo human bone marrow-derived stem cells with human bone morphogenetic protein-2 (BMP-2) gene (BMP-hBMSCs). We then introduced these cells into a hydrogel scaffold using an advanced visible light-based projection stereolithography (VL-PSL) technology, which is compatible with concomitant cell encapsulation and amenable to computer-aided architectural design, to fabricate scaffolds fitting local physical and structural variations in different bones and defects. RESULTS: The results showed that the BMP-hBMSCs encapsulated within the scaffolds had high viability with sustained BMP-2 gene expression and differentiated toward an osteogenic lineage without the supplement of additional BMP-2 protein. In vivo bone formation efficacy was further assessed using an intramuscular implantation model in severe combined immunodeficiency (SCID) mice. Microcomputed tomography (micro-CT) imaging indicated rapid bone formation by the BMP-hBMSC-laden constructs as early as 14 days post-implantation. Histological examination revealed a mature trabecular bone structure with considerable vascularization. Through tracking of the implanted cells, we also found that BMP-hBMSC were directly involved in the new bone formation. CONCLUSIONS: The robust, self-driven osteogenic capability and computer-designed architecture of the construct developed in this study should have potential applications for customized clinical repair of large bone defects or non-unions.
Gene delivery to joints by intra-articular injection
DOI:10.1089/hum.2017.181
URL
PMID:29160173
[Cited within: 2]

Most forms of arthritis are incurable, difficult to treat, and a major cause of disability in Western countries. Better local treatment of arthritis is impaired by the pharmacokinetics of the joint that make it very difficult to deliver drugs to joints at sustained, therapeutic concentrations. This is especially true of biologic drugs, such as proteins and RNA, many of which show great promise in preclinical studies. Gene transfer provides a strategy for overcoming this limitation. The basic concept is to deliver cDNAs encoding therapeutic products by direct intra-articular injection, leading to sustained, endogenous synthesis of the gene products within the joint. Proof of concept has been achieved for both in vivo and ex vivo gene delivery using a variety of vectors, genes, and cells in several different animal models. There have been a small number of clinical trials for rheumatoid arthritis (RA) and osteoarthritis (OA) using retrovirus vectors for ex vivo gene delivery and adeno-associated virus (AAV) for in vivo delivery. AAV is of particular interest because, unlike other viral vectors, it is able to penetrate deep within articular cartilage and transduce chondrocytes in situ. This property is of particular importance in OA, where changes in chondrocyte metabolism are thought to be fundamental to the pathophysiology of the disease. Authorities in Korea have recently approved the world's first arthritis gene therapy. This targets OA by the injection of allogeneic chondrocytes that have been transduced with a retrovirus carrying transforming growth factor-beta1 cDNA. Phase III studies are scheduled to start in the United States soon. Meanwhile, two additional Phase I trials are listed on Clinicaltrials.gov , both using AAV. One targets RA by transferring interferon-beta, and the other targets OA by transferring interleukin-1 receptor antagonist. The field is thus gaining momentum and promises to improve the treatment of these common and debilitating diseases.
Adeno-associated viral vector-mediated immune responses: Understanding barriers to gene delivery
URL PMID:31836454 [Cited within: 1]
Human immune responses to adeno-associated virus (AAV) vectors
DOI:10.3389/fimmu.2020.00670
URL
PMID:32362898
[Cited within: 2]

Recombinant adeno-associated virus (rAAV) vectors are one of the most promising in vivo gene delivery tools. Several features make rAAV vectors an ideal platform for gene transfer. However, the high homology with the parental wild-type virus, which often infects humans, poses limitations in terms of immune responses associated with this vector platform. Both humoral and cell-mediated immunity to wild-type AAV have been documented in healthy donors, and, at least in the case of anti-AAV antibodies, have been shown to have a potentially high impact on the outcome of gene transfer. While several factors can contribute to the overall immunogenicity of rAAV vectors, vector design and the total vector dose appear to be responsible of immune-mediated toxicities. While preclinical models have been less than ideal in predicting the outcome of gene transfer in humans, the current preclinical body of evidence clearly demonstrates that rAAV vectors can trigger both innate and adaptive immune responses. Data gathered from clinical trials offers key learnings on the immunogenicity of AAV vectors, highlighting challenges as well as the potential strategies that could help unlock the full therapeutic potential of in vivo gene transfer.
Recombinant Adeno-Associated Viral Vectors (rAAV)-Vector Elements in Ocular Gene Therapy Clinical Trials and Transgene Expression and Bioactivity Assays
Biomaterials & scaffolds for tissue engineering
Scaffolding strategies for tissue engineering and regenerative medicine applications
Synthetic biodegradable functional polymers for tissue engineering: a brief review
DOI:10.1007/s11426-014-5086-y
URL
PMID:25729390
[Cited within: 2]

Scaffolds play a crucial role in tissue engineering. Biodegradable polymers with great processing flexibility are the predominant scaffolding materials. Synthetic biodegradable polymers with well-defined structure and without immunological concerns associated with naturally derived polymers are widely used in tissue engineering. The synthetic biodegradable polymers that are widely used in tissue engineering, including polyesters, polyanhydrides, polyphosphazenes, polyurethane, and poly (glycerol sebacate) are summarized in this article. New developments in conducting polymers, photoresponsive polymers, amino-acid-based polymers, enzymatically degradable polymers, and peptide-activated polymers are also discussed. In addition to chemical functionalization, the scaffold designs that mimic the nano and micro features of the extracellular matrix (ECM) are presented as well, and composite and nanocomposite scaffolds are also reviewed.
Bone regeneration using gene-activated matrices
DOI:10.1208/s12248-016-9982-2
URL
PMID:27655418
[Cited within: 1]

Gene delivery to bone is a potential therapeutic strategy for directed, sustained, and regulated protein expression. Tissue engineering strategies for bone regeneration include delivery of proteins, genes (viral and non-viral-mediated delivery), and/or cells to the bone defect site. In addition, biomimetic scaffolds and scaffolds incorporating bone anabolic agents greatly enhance the bone repair process. Regional gene therapy has the potential of enhancing bone defect healing and bone regeneration by delivering osteogenic genes locally to the osseous lesions, thereby reducing systemic toxicity and the need for using supraphysiological dosages of therapeutic proteins. By implanting gene-activated matrices (GAMs), sustained gene expression and continuous osteogenic protein production in situ can be achieved in a way that stimulates osteogenesis and bone repair within osseous defects. Critical parameters substantially affecting the therapeutic efficacy of gene therapy include the choice of osteogenic transgene(s), selection of non-viral or viral vectors, the wound environment, and the selection of ex vivo and in vivo gene delivery strategies, such as GAMs. It is critical for gene therapy applications that clinically beneficial amounts of proteins are synthesized endogenously within and around the lesion in a sustained manner. It is therefore necessary that reliable and reproducible methods of gene delivery be developed and tested for their efficacy and safety before translating into clinical practice. Practical considerations such as the age, gender, and systemic health of patients and the nature of the disease process also need to be taken into account in order to personalize the treatments and progress towards developing a clinically applicable gene therapy for healing bone defects. This review discusses tissue engineering strategies to regenerate bone with specific focus on non-viral gene delivery systems.
Collagen hydrogel as an immunomodulatory scaffold in cartilage tissue engineering
DOI:10.1002/jbm.b.33011
URL
PMID:24000202
[Cited within: 3]

A collagen type I hydrogel was constructed and used as the scaffold for cartilage tissue engineering. Neonatal rabbit chondrocytes were seeded into the hydrogel, and the constructs were cultured in vitro for 7, 14, and 28 days. The immunomodulatory effect of the hydrogel on seeded chondrocytes was carefully investigated. The expressions of major histocompatibility complex classes I and II of seeded chondrocytes increased with the time, which indicated that the immunogenicity also increased with the time. Meanwhile, the properly designed collagen type I hydrogel could prompt the chondrogenesis of engineered cartilage. The extracellular matrix (ECM) synthesis ability of seeded chondrocytes and the accumulated ECM in the constructs continuously increased with the culture time. Both the isolation and protection, which come from formed ECM and hydrogel scaffold, can effectively control the adverse immunogenicity of seeded chondrocytes and even help to lessen the immunogenicity of the whole engineered cartilage. As the result, the levels of mixed lymphocyte chondrocyte reactions of seed cells and the constructs decreased gradually. The stimulation on allogeneic lymphocytes of the whole constructs was obviously lower than that of the retrieved cells from the constructs. Therefore, properly designed collagen type I hydrogel can give certain immunogenicity-reducing effects on engineered cartilage based on chondrocytes, and it may be a potential immunomodulatory biomaterial in tissue engineering.
Natural origin biodegradable systems in tissue engineering and regenerative medicine: present status and some moving trends
Collagen tissue engineering: development of novel biomaterials and applications
DOI:10.1203/PDR.0b013e31816c5bc3
URL
PMID:18427293
[Cited within: 1]

Scientific investigations involving collagen have inspired tissue engineering and design of biomaterials since collagen fibrils and their networks primarily regulate and define most tissues. The collagen networks form a highly organized, three-dimensional architecture to entrap other ingredients. Biomaterials are expected to function as cell scaffolds to replace native collagen-based extracellular matrix. The composition and properties of biomaterials used as scaffold for tissue engineering significantly affect the regeneration of neo-tissues and influence the conditions of collagen engineering. The complex scenario of collagen characteristics, types, fibril arrangement, and collagen structure-related functions (in a variety of connective tissues including bone, cartilage, tendon, skin and cornea) are addressed in this review. Discussion will focus on nanofibrillar assemblies and artificial synthetic peptides that mimic either the fibrillar structure or the elemental components of type I collagen as illustrated by their preliminary applications in tissue engineering. Conventional biomaterials used as scaffolds in engineering collagen-containing tissues are also discussed. The design of novel biomaterials and application of conventional biomaterials will facilitate development of additional novel tissue engineering bioproducts by refining the currently available techniques. The field of tissue engineering will ultimately be advanced by increasing control of collagen in native tissue and by continual manipulation of biomaterials.
Injectable BMP-2 gene-activated scaffold for the repair of cranial bone defect in mice
DOI:10.1002/sctm.19-0315
URL
PMID:32785966
[Cited within: 5]

Tissue engineering using adult human mesenchymal stem cells (MSCs) seeded within biomaterial scaffolds has shown the potential to enhance bone healing. Recently, we have developed an injectable, biodegradable methacrylated gelatin-based hydrogel, which was especially effective in producing scaffolds in situ and allowed the delivery of high viable stem cells and gene vehicles. The well-demonstrated benefits of recombinant adeno-associated viral (rAAV) vector, including long-term gene transfer efficiency and relative safety, combination of gene and cell therapies has been developed in both basic and translational research to support future bone tissue regeneration clinical trials. In this study, we have critically assessed the applicability of single-step visible light (VL) photocrosslinking fabrication of gelatin scaffold to deliver rAAV encoding human bone morphogenetic protein-2 (BMP-2) gene to address the need for sustained BMP-2 presence localized within scaffolds for the repair of cranial bone defect in mouse model. In this method, rAAV-BMP-2 and human bone marrow-derived MSCs (hBMSCs) were simultaneously included into gelatin scaffolds during scaffold formation by VL illumination. We demonstrated that the subsequent release of rAAV-BMP-2 constructs from the scaffold matrix, which resulted in efficient in situ expression of BMP-2 gene by hBMSCs seeded within the scaffolds, and thus induced their osteogenic differentiation without the supplement of exogenous BMP-2. The reparative capacity of this novel stem cell-seeded and gene-activated scaffolds was further confirmed in the cranial defect in the severe combined immunodeficiency mice, revealed by imaging, histology, and immunohistochemistry at 6 weeks after cranial defect treatment.
Release of bioactive adeno-associated virus from fibrin scaffolds: effects of fibrin glue concentrations
URL PMID:21449684 [Cited within: 2]
A comparison of bone regeneration with human mesenchymal stem cells and muscle-derived stem cells and the critical role of BMP
DOI:10.1016/j.biomaterials.2014.04.113
URL
PMID:24856105

Adult multipotent stem cells have been isolated from a variety of human tissues including human skeletal muscle, which represent an easily accessible source of stem cells. It has been shown that human skeletal muscle-derived stem cells (hMDSCs) are muscle-derived mesenchymal stem cells capable of multipotent differentiation. Although hMDSCs can undergo osteogenic differentiation and form bone when genetically modified to express BMP2; it is still unclear whether hMDSCs are as efficient as human bone marrow mesenchymal stem cells (hBMMSCs) for bone regeneration. The current study aimed to address this question by performing a parallel comparison between hMDSCs and hBMMSCs to evaluate their osteogenic and bone regeneration capacities. Our results demonstrated that hMDSCs and hBMMSCs had similar osteogenic-related gene expression profiles and had similar osteogenic differentiation capacities in vitro when transduced to express BMP2. Both the untransduced hMDSCs and hBMMSCs formed very negligible amounts of bone in the critical sized bone defect model when using a fibrin sealant scaffold; however, when genetically modified with lenti-BMP2, both populations successfully regenerated bone in the defect area. No significant differences were found in the newly formed bone volumes and bone defect coverage between the hMDSC and hBMMSC groups. Although both cell types formed mature bone tissue by 6 weeks post-implantation, the newly formed bone in the hMDSCs group underwent quicker remodelling than the hBMMSCs group. In conclusion, our results demonstrated that hMDSCs are as efficient as hBMMSCs in terms of their bone regeneration capacity; however, both cell types required genetic modification with BMP in order to regenerate bone in vivo.
BMP2 is superior to BMP4 for promoting human muscle-derived stem cell-mediated bone regeneration in a critical-sized calvarial defect model
DOI:10.3727/096368912X658854
URL
PMID:23244588

Muscle-derived cells have been successfully isolated using a variety of different methods and have been shown to possess multilineage differentiation capacities, including an ability to differentiate into articular cartilage and bone in vivo; however, the characterization of human muscle-derived stem cells (hMDSCs) and their bone regenerative capacities have not been fully investigated. Genetic modification of these cells may enhance their osteogenic capacity, which could potentially be applied to bone regenerative therapies. We found that hMDSCs, isolated by the preplate technique, consistently expressed the myogenic marker CD56, the pericyte/endothelial cell marker CD146, and the mesenchymal stem cell markers CD73, CD90, CD105, and CD44 but did not express the hematopoietic stem cell marker CD45, and they could undergo osteogenic, chondrogenic, adipogenic, and myogenic differentiation in vitro. In order to investigate the osteoinductive potential of hMDSCs, we constructed a retroviral vector expressing BMP4 and GFP and a lentiviral vector expressing BMP2. The BMP4-expressing hMDSCs were able to undergo osteogenic differentiation in vitro and exhibited enhanced mineralization compared to nontransduced cells; however, when transplanted into a calvarial defect, they failed to regenerate bone. Local administration of BMP4 protein and cell pretreatment with N-acetylcysteine (NAC), which improves cell survival, did not enhance the osteogenic capacity of the retro-BMP4-transduced cells. In contrast, lenti-BMP2-transduced hMDSCs not only exhibited enhanced in vitro osteogenic differentiation but also induced robust bone formation and nearly completely healed a critical-sized calvarial defect in CD-1 nude mice 6 weeks following transplantation. Herovici's staining of the regenerated bone demonstrated that the bone matrix contained a large amount of type I collagen. Our findings indicated that the hMDSCs are likely mesenchymal stem cells of muscle origin and that BMP2 is more efficient than BMP4 in promoting the bone regenerative capacity of the hMDSCs in vivo.
Projection stereolithographic fabrication of BMP-2 gene-activated matrix for bone tissue engineering
DOI:10.1038/s41598-017-11051-0
URL
PMID:28900122
[Cited within: 1]

Currently, sustained in vivo delivery of active bone morphogenetic protein-2 (BMP-2) protein to responsive target cells, such as bone marrow-derived mesenchymal stem cells (BMSCs), remains challenging. Ex vivo gene transfer method, while efficient, requires additional operation for cell culture and therefore, is not compatible with point-of-care treatment. In this study, two lentiviral gene constructs - (1) Lv-BMP/GFP, containing human BMP-2 and green fluorescent protein (GFP) gene (BMP group); or (2) Lv-GFP, containing GFP gene (GFP group) - were incorporated with human BMSCs into a solution of photocrosslinkable gelatin, which was then subjected to visible light-based projection stereolithographic printing to form a scaffold with desired architectures. Upon in vitro culture, compared to the GFP group, cells from BMP group showed >1,000-fold higher BMP-2 release, and the majority of them stained intensely for alkaline phosphatase activity. Real-time RT-PCR also showed dramatically increased expression of osteogenesis marker genes only in the BMP group. 3.5 months post-implantation into SCID mice, the micro-computed tomography imaging showed detectable mineralized areas only in the BMP group, which was restricted within the scaffolds. Alizarin red staining and immunohistochemistry of GFP and osteocalcin further indicated that the grafted hBMSCs, not host cells, contributed primarily to the newly formed bone.
Biomaterial-Guided recombinant adeno-associated virus delivery from poly(sodium styrene sulfonate)-grafted poly(ε-caprolactone) films to target human bone marrow aspirates
URL PMID:31680637 [Cited within: 1]
Poly (glycerol sebacate)-poly (ε-caprolactone) blend nanofibrous scaffold as intrinsic bio- and immunocompatible system for corneal repair
DOI:10.1016/j.actbio.2017.01.013
URL
PMID:28069498

A major challenge in corneal tissue engineering and lamellar corneal transplantation is to develop synthetic scaffolds able to simulate the optical and mechanical properties of the native cornea. As a carrier, the graft scaffolds should provide the basis for anchorage, repair and regeneration. Although quite a number of scaffolds have been engineered to date, they have not been able to simultaneously recapitulate chemical, mechanical, and structural properties of the corneal extracellular matrix (ECM). Here, we examined different compositions of elastomeric biodegradable poly (glycerol sebacate) (PGS)-poly (epsilon-caprolactone) (PCL) nanofibrous scaffolds with respect to their cyto- and immunocompatibility. These scaffolds were semi-transparent with well-defined mechanical properties and direct positive effects on viability of human corneal endothelial cells (HCEC) and human conjunctival epithelial cells (HCjEC). Moreover, within 3days HCEC established monolayers with the hexagonal morphology typical for this cell type. All PGS-PCL mixtures analyzed did not trigger effects in granulocytes, naive and activated peripheral blood mononuclear cells (PBMCs). However, scaffolds with a higher content of PGS-PCL ratio showed the best cell organization, cyto- and immunocompatibility. Subsequently, this PGS-PCL composition could be used for further development of clinical constructs to support corneal tissue repair. STATEMENT OF SIGNIFICANCE: In corneal tissue engineering a major challenge is the development of synthetic scaffolds with similar properties to native cornea. In our recent works, we introduced the biodegradable, polymeric nanofibrous scaffolds with similar optical and mechanical properties for corneal regeneration and here we examined the cyto- and immunocompatibility of biodegradable nanofibrous scaffolds in contact to white blood cells. Directing the alignment of human corneal cells by nanofibrous scaffolds and high viability of cells was detected by forming of endothelium monolayer with hexagonal morphology on the nanofibrous scaffold. In addition, our results for the first time show that these nanofibrous scaffolds did not trigger effects in white blood cells. These results highlight the considerable translational potential of the nanofibrous scaffolds to clinical applications.
One-step fabrication of bone morphogenetic protein-2 gene-activated porous poly-l-lactide scaffold for bone induction
DOI:10.1016/j.omtm.2017.08.008
URL
PMID:29018836
[Cited within: 4]

Bone morphogenetic protein 2 (BMP2) is an efficacious inducer for the osteogenesis of mesenchymal stem cells (MSCs). Conventional applications of BMP2 have involved either the direct incorporation of BMP2 protein or ex vivo BMP2 gene transfer into stem cells prior to their transplantation. These approaches are able to promote bone formation to some extent; however, they are hampered by either the lack of stability and sustainability of BMP2 protein or the time-consuming and cost-prohibitive in vitro cell culture procedure. To overcome these limitations, we have developed a gene-activated poly-L-lactide acid (PLLA) scaffold with the encapsulation of recombinant adeno-associated viral (AAV) vector encoding a full-length cDNA of human BMP2 using an ice-based microparticle porogenization method that was recently developed. Results showed continuous release of AAV particles from the micropores of scaffolds for up to 1 week, subsequently transducing embedded human MSCs and producing functional BMP2. MSCs within scaffolds underwent efficacious osteogenesis, on the basis of osteoinductive gene expression and osteogenic differentiation, which resulted in robust new bone formation in vivo at 4 weeks. These findings show the potential of the technology toward developing clinical applications of a rapid, cost-effective, and potentially point-of-care approach for the repair of bone defects.
Effective and durable genetic modification of human mesenchymal stem cells via controlled release of rAAV vectors from self-assembling peptide hydrogels with a maintained differentiation potency
DOI:10.1016/j.actbio.2015.02.013
URL
PMID:25712390
[Cited within: 3]

Controlling the release of recombinant adeno-associated virus (rAAV) vectors from biocompatible materials is a novel, attractive approach to increase the residence time and effectiveness of a gene carrier at a defined target site. Self-assembling peptides have an ability to form stable hydrogels and encapsulate cells upon exposure to physiological pH and ionic strength. Here, we examined the capacity of the peptide hydrogel RAD16-I in a pure (RAD) form or combined with hyaluronic acid (RAD-HA) to release rAAV vectors as a means to genetically modify primary human bone marrow-derived mesenchymal stem cells (hMSCs), a potent source of cells for regenerative medicine. Specifically, we demonstrate the ability of the systems to efficiently encapsulate and release rAAV vectors in a sustained, controlled manner for the effective transduction of hMSCs (up to 80%) without deleterious effects on cell viability (up to 100%) or on their potential for chondrogenic differentiation over time (up to 21days). The present study demonstrates that RAD16-I is an advantageous material with tunable properties to control the release of rAAV vectors as a promising tool to develop new, improved therapeutic approaches for tissue engineering in vivo.
Tuning gelation time and morphology of injectable hydrogels using ketone-hydrazide cross-linking
DOI:10.1021/bm401615d
URL
PMID:24432725
[Cited within: 1]

Injectable, covalently in situ forming hydrogels based on poly(N-isopropylacrylamide) have been designed on the basis of mixing hydrazide-functionalized nucleophilic precursor polymers with electrophilic precursor polymers functionalized with a combination of ketone (slow reacting) and aldehyde (fast reacting) functional groups. By tuning the ratio of aldehyde:ketone functional groups as well as the total number of ketone groups in the electrophilic precursor polymer, largely independent control over hydrogel properties including gelation time (from seconds to hours), degradation kinetics (from hours to months), optical transmission (from 1 to 85%), and mechanics (over nearly 1 order of magnitude) can be achieved. In addition, ketone-functionalized precursor polymers exhibit improved cytocompatibility at even extremely high concentrations relative to polymers functionalized with aldehyde groups, even at 4-fold higher functional group densities. Overall, increasing the ketone content of the precursor copolymers can result in in situ-gellable hydrogels with improved transparency and biocompatibility and equivalent mechanics and stimuli-responsiveness while only modestly sacrificing the speed of gel formation.
Recent development and biomedical applications of self-healing hydrogels
DOI:10.1080/17425247.2017.1360865
URL
PMID:28771375

INTRODUCTION: Hydrogels are of special importance, owing to their high-water content and various applications in biomedical and bio-engineering research. Self-healing properties is a common phenomenon in living organisms. Their endowed property of being able to self-repair after physical/chemical/mechanical damage to fully or partially its original properties demonstrates their prospective therapeutic applications. Due to complicated preparation and selection of suitable materials, the application of many host-guest supramolecular polymeric hydrogels are so limited. Thus, the design and construction of self-repairing material are highly desirable for effectively increase in the lifetime of a functional material. However, recent advances in the field of materials science and bioengineering and nanotechnology have led to the design of biologically relevant self-healing hydrogels for therapeutic applications. This review focuses on the recent development of self-healing hydrogels for biomedical application. AREAS COVERED: The strategies of making self-healing hydrogels and their healing mechanisms are discussed. The significance of self-healing hydrogel for biomedical application is also highlighted in areas such as 3D/4D printing, cell/drug delivery, as well as soft actuators. EXPERT OPINION: Materials that have the ability to self-repair damage and regain the desired mechanical properties, have been found to be excellent candidate materials for a range of biomedical uses especially if their unique characteristics are similar to that of soft-tissues. Self-healing hydrogels have been synthesized and shown to exhibit similar characteristics as human tissues, however, significant improvement is required in the fabrication process from inexpensive and nontoxic/non-hazardous materials and techniques, and, in addition, further fine-tuning of the self-healing properties are needed for specific biomedical uses.
Acceleration of chondrogenic differentiation of human mesenchymal stem cells by sustained growth factor release in 3D graphene oxide incorporated hydrogels
DOI:10.1016/j.actbio.2020.01.048
URL
PMID:32035282

Damaged articular cartilage has limited self-healing capabilities, leading to degeneration that affects millions of people. Although cartilage tissue engineering is considered a promising approach for treatment, robust and long-term chondrogenesis within a 3-dimensional (3D) scaffold remains a major challenge for complete regeneration. Most current approaches involve incorporation of transforming growth factor-beta (TGF-beta) into the scaffold, but have limited utility owing to the short functional half-life and/or rapid clearance of TGF-beta. In this study, we have tested the incorporation of graphene oxide nanosheets (GO) within a photopolymerizable poly-D, l-lactic acid/polyethylene glycol (PDLLA) hydrogel, for its applicability in sustained release of the chondroinductive growth factor TGF-beta3. We found that with GO incorporation, the hydrogel scaffold (GO/PDLLA) exhibited enhanced initial mechanical strength, i.e., increased compressive modulus, and supported long-term, sustained release of TGF-beta3 for up to 4 weeks. In addition, human bone marrow-derived mesenchymal stem cells (hBMSCs) seeded within TGF-beta3 loaded GO/PDLLA hydrogels displayed high cell viability and improved chondrogenesis in a TGF-beta3 concentration-dependent manner. hBMSCs cultured in GO/PDLLA also demonstrated significantly higher chondrogenic gene expression, including aggrecan, collagen type II and SOX9, and cartilage matrix production when compared to cultures maintained in GO-free scaffolds containing equivalent amounts of TGF-beta3. Upon subcutaneous implantation in vivo, hBMSC-seeded TGF-beta3-GO/PDLLA hydrogel constructs displayed considerably greater cartilage matrix than their TGF-beta3/PDLLA counterparts without GO. Taken together, these findings support the potential application of GO in optimizing TGF-beta3 induced hBMSC chondrogenesis for cartilage tissue engineering. STATEMENT OF SIGNIFICANCE: In this work, we have developed a graphene oxide (GO) incorporated, photocrosslinked PDLLA hybrid hydrogel for localized delivery and sustained release of loaded TGF-beta3 to seeded cells. The incorporation of GO in PDLLA hydrogel suppressed the burst release of TGF-beta3, and significantly prolonged the retention time of the TGF-beta3 initially loaded in the hydrogel. Additionally, the GO improved the initial compressive strength of the hydrogel. Both in vitro analyses and in vivo implantation results showed that the GO/PDLLA constructs seeded with human mesenchymal stem cells (hMSCs) showed significantly higher cartilage formation, compared to GO-free scaffolds containing equivalent amount of TGF-beta3. Findings from this work suggest the potential application of the GO-TGF/PDLLA hydrogel as a functional scaffold for hMSC-based cartilage tissue engineering.
Cartilage tissue engineering application of injectable gelatin hydrogel with in situ visible-light-activated gelation capability in both air and aqueous solution
DOI:10.1089/ten.TEA.2013.0642
URL
PMID:24575844
[Cited within: 1]

Chondroprogenitor cells encapsulated in a chondrogenically supportive, three-dimensional hydrogel scaffold represents a promising, regenerative approach to articular cartilage repair. In this study, we have developed an injectable, biodegradable methacrylated gelatin (mGL)-based hydrogel capable of rapid gelation via visible light (VL)-activated crosslinking in air or aqueous solution. The mild photocrosslinking conditions permitted the incorporation of cells during the gelation process. Encapsulated human-bone-marrow-derived mesenchymal stem cells (hBMSCs) showed high, long-term viability (up to 90 days) throughout the scaffold. To assess the applicability of the mGL hydrogel for cartilage tissue engineering, we have evaluated the efficacy of chondrogenesis of the encapsulated hBMSCs, using hBMSCs seeded in agarose as control. The ability of hBMSC-laden mGL constructs to integrate with host tissues after implantation was further investigated utilizing an in vitro cartilage repair model. The results showed that the mGL hydrogel, which could be photopolymerized in air and aqueous solution, supports hBMSC growth and TGF-beta3-induced chondrogenesis. Compared with agarose, mGL constructs laden with hBMSCs are mechanically stronger with time, and integrate well with native cartilage tissue upon implantation based on push-out mechanical testing. VL-photocrosslinked mGL scaffold thus represents a promising scaffold for cell-based repair and resurfacing of articular cartilage defects.
Hydrogels for biomedical applications: Their characteristics and the mechanisms behind them
Hydrogel: Preparation, characterization, and applications: A review
DOI:10.1016/j.jare.2013.07.006
URL
PMID:25750745
[Cited within: 1]

Hydrogel products constitute a group of polymeric materials, the hydrophilic structure of which renders them capable of holding large amounts of water in their three-dimensional networks. Extensive employment of these products in a number of industrial and environmental areas of application is considered to be of prime importance. As expected, natural hydrogels were gradually replaced by synthetic types due to their higher water absorption capacity, long service life, and wide varieties of raw chemical resources. Literature on this subject was found to be expanding, especially in the scientific areas of research. However, a number of publications and technical reports dealing with hydrogel products from the engineering points of view were examined to overview technological aspects covering this growing multidisciplinary field of research. The primary objective of this article is to review the literature concerning classification of hydrogels on different bases, physical and chemical characteristics of these products, and technical feasibility of their utilization. It also involved technologies adopted for hydrogel production together with process design implications, block diagrams, and optimized conditions of the preparation process. An innovated category of recent generations of hydrogel materials was also presented in some details.
Characterizing the encapsulation and release of lentivectors and adeno-associated vectors from degradable alginate hydrogels
DOI:10.1039/c8bm01218k
URL
PMID:30534722
[Cited within: 1]

Gene therapy using viral vectors has been licensed for clinical use both in the European Union and the United States. Lentivectors (LV) and adeno-associated vectors (AAV) are two promising and FDA approved gene-therapy viral vectors. Many future applications of these vectors will benefit from targeting specific regions of interest within the body. Therefore, building on the early success of these vectors may depend on finding effective delivery systems to localize therapeutic administration. Degradable alginate hydrogels have been tested as appealing delivery vehicles for the controlled delivery of vector payloads. In this study, we compare the ability of two different degradable alginate hydrogel formulations to efficiently deliver LV and AAV. We propose that release rates of viral vectors are dependent on the physical properties of both the hydrogels and vectors. Here, we demonstrate that the initial strength and degradation rate of alginate hydrogels provides levers of control for tuning LV release but do not provide control in the release of AAV. While both alginate formulations used showed sustained release of both LV and AAV, LV release was shown to be dependent on alginate hydrogel degradation, while AAV release was largely governed by diffusive mechanisms. Altogether, this study demonstrates alginate's use as a possible delivery platform for LV and, for the first time, AAV - highlighting the potential of injectable degradable alginate hydrogels to be used as a versatile delivery tool in gene therapy applications.
Hydrogel-based controlled delivery systems for articular cartilage repair
DOI:10.1155/2016/1215263
URL
PMID:27642587
[Cited within: 1]

Delivery of bioactive factors is a very valuable strategy for articular cartilage repair. Nevertheless, the direct supply of such biomolecules is limited by several factors including rapid degradation, the need for supraphysiological doses, the occurrence of immune and inflammatory responses, and the possibility of dissemination to nontarget sites that may impair their therapeutic action and raise undesired effects. The use of controlled delivery systems has the potential of overcoming these hurdles by promoting the temporal and spatial presentation of such factors in a defined target. Hydrogels are promising materials to develop delivery systems for cartilage repair as they can be easily loaded with bioactive molecules controlling their release only where required. This review exposes the most recent technologies on the design of hydrogels as controlled delivery platforms of bioactive molecules for cartilage repair.
Controlled release of rAAV vectors from APMA-functionalized contact lenses for corneal gene therapy
Silk fibroin films facilitate single-step targeted expression of optogenetic proteins
DOI:10.1016/j.celrep.2018.02.081
URL
PMID:29562189
[Cited within: 2]

Optical methods of interrogating neural circuits have emerged as powerful tools for understanding how the brain drives behaviors. Optogenetic proteins are widely used to control neuronal activity, while genetically encoded fluorescent reporters are used to monitor activity. These proteins are often expressed by injecting viruses, which frequently leads to inconsistent experiments due to misalignment of expression and optical components. Here, we describe how silk fibroin films simplify optogenetic experiments by providing targeted delivery of viruses. Films composed of silk fibroin and virus are applied to the surface of implantable optical components. After surgery, silk releases the virus to transduce nearby cells and provide localized expression around optical fibers and endoscopes. Silk films can also be used to express genetically encoded sensors in large cortical regions by using cranial windows coated with a silk/virus mixture. The ease of use and improved performance provided by silk make this a promising approach for optogenetic studies.
Stem cells combined with bone graft substitutes in skeletal tissue engineering
DOI:10.1517/14712598.2012.679652
URL
PMID:22500826
[Cited within: 1]

INTRODUCTION: Bone grafting is used to repair large bone defects and autograft is recognised as producing the best clinical outcome, which is partly due to its cellular component. When autograft is unavailable, allograft and bone graft substitutes can be used; however, they rely on the host bed to provide cellular osteogenic activity. AREAS COVERED: Bone graft substitutes have the potential to benefit from the addition of stem cells aimed at enhancing the rate and quality of defect repair. Mesenchymal stem cells (MSCs) can be isolated from bone marrow or periosteum and culture expanded. Other sources of primary cells include muscle, adipose tissue, human umbilical cord and the pluripotent embryonic stem cells (ESCs). EXPERT OPINION: MSCs isolated from bone marrow have been the best characterised approach for osteogenic differentiation. Their use with synthetic scaffolds such as hydroxyapatite and tricalcium phosphate has produced promising clinical results. MSCs derived from adipose tissue, muscle or human umbilical cord cells combined with various scaffolds are an attractive option. Further in vivo and clinical investigation of their potential is required. Pluripotent ESCs have a theoretical advantage over MSCs; however, purification, cell-specific differentiation, effective delivery vehicles-scaffolds and teratogenesis control are still under in vitro and in vivo evaluation.
Mesenchymal stem cells in bone development, bone repair, and skeletal regeneration therapy
DOI:10.1002/jcb.240560303
URL
PMID:7876320
[Cited within: 1]

Bone formation in the embryo, and during adult fracture repair and remodeling, involves the progeny of a small number of cells called mesenchymal stem cells (MSCs). These cells continuously replicate themselves, while a portion become committed to mesenchymal cell lineages such as bone, cartilage, tendon, ligament, and muscle. The differentiation of these cells, within each lineage, is a complex multistep pathway involving discrete cellular transitions much like that which occurs during hematopoiesis. Progression from one stage to the next depends on the presence of specific bioactive factors, nutrients, and other environmental cues whose exquisitely controlled contributions orchestrate the entire differentiation phenomenon. An understanding of the cellular and molecular events of osteogenic differentiation of MSCs provides the foundation for the emergence of a new therapeutic technology for cell therapy. The isolation and in vitro mitotic expansion of autologous human MSCs will support the development of novel protocols for the treatment of many clinically challenging conditions. For example, local bone defects can be repaired through site-directed delivery of MSCs in an appropriate carrier vehicle. Generalized conditions, such as osteoporosis, may be treatable by systemic administration of culture-expanded autologous MSCs or through biopharmaceutical regimens based on the discovery of critical regulatory molecules in the differentiation process. With this in mind, we can begin to explore therapeutic options that have never before been available.
Synthetic scaffold coating with adeno-associated virus encoding BMP2 to promote endogenous bone repair
DOI:10.1007/s00441-011-1197-3
URL
PMID:21695398
[Cited within: 3]

Biomaterial scaffolds functionalized to stimulate endogenous repair mechanisms via the incorporation of osteogenic cues offer a potential alternative to bone grafting for the treatment of large bone defects. We first quantified the ability of a self-complementary adeno-associated viral vector encoding bone morphogenetic protein 2 (scAAV2.5-BMP2) to enhance human stem cell osteogenic differentiation in vitro. In two-dimensional culture, scAAV2.5-BMP2-transduced human mesenchymal stem cells (hMSCs) displayed significant increases in BMP2 production and alkaline phosphatase activity compared with controls. hMSCs and human amniotic-fluid-derived stem cells (hAFS cells) seeded on scAAV2.5-BMP2-coated three-dimensional porous polymer Poly(epsilon-caprolactone) (PCL) scaffolds also displayed significant increases in BMP2 production compared with controls during 12 weeks of culture, although only hMSC-seeded scaffolds displayed significantly increased mineral formation. PCL scaffolds coated with scAAV2.5-BMP2 were implanted into critically sized immunocompromised rat femoral defects, both with or without pre-seeding of hMSCs, representing ex vivo and in vivo gene therapy treatments, respectively. After 12 weeks, defects treated with acellular scAAV2.5-BMP2-coated scaffolds displayed increased bony bridging and had significantly higher bone ingrowth and mechanical properties compared with controls, whereas defects treated with scAAV2.5-BMP2 scaffolds pre-seeded with hMSCs failed to display significant differences relative to controls. When pooled, defect treatment with scAAV2.5-BMP2-coated scaffolds, both with or without inclusion of pre-seeded hMSCs, led to significant increases in defect mineral formation at all time points and increased mechanical properties compared with controls. This study thus presents a novel acellular bone-graft-free endogenous repair therapy for orthotopic tissue-engineered bone regeneration.
Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis
DOI:10.1038/s41584-018-0109-2
URL
PMID:30341437
[Cited within: 1]

Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by a failure of spontaneous resolution of inflammation. Although the pro-inflammatory cytokines and mediators that trigger RA have been the focus of intense investigations, the regulatory and anti-inflammatory cytokines responsible for the suppression and resolution of disease in a context-dependent manner have been less well characterized. However, knowledge of the pathways that control the suppression and resolution of inflammation in RA is clinically relevant and conceptually important for understanding the pathophysiology of the disease and for the development of treatments that enable long-term remission. Cytokine-mediated processes such as the activation of T helper 2 cells by IL-4 and IL-13, the resolution of inflammation by IL-9, IL-5-induced eosinophil expansion, IL-33-mediated macrophage polarization, the production of IL-10 by regulatory B cells and IL-27-mediated suppression of lymphoid follicle formation are all involved in governing the regulation and resolution of inflammation in RA. By better understanding these immune-regulatory signalling pathways, new therapeutic strategies for RA can be envisioned that aim to balance and resolve, rather than suppress, inflammation.
Update on the pathomechanism, diagnosis, and treatment options for rheumatoid arthritis
Systematic review and meta-analysis: pharmacogenetics of anti-TNF treatment response in rheumatoid arthritis
DOI:10.1038/tpj.2017.26
URL
PMID:28607508

Rheumatoid arthritis (RA) is a chronic inflammatory disease that affects ~1% of the Caucasian population. Over the last decades, the availability of biological drugs targeting the proinflammatory cytokine tumour necrosis factor alpha, anti-TNF drugs, has improved the treatment of patients with RA. However, one-third of the patients do not respond to the treatment. We wanted to evaluate the status of pharmacogenomics of anti-TNF treatment. We performed a PubMed literature search and all studies reporting original data on associations between genetic variants and anti-TNF treatment response in RA patients were included and results evaluated by meta-analysis. In total, 25 single nucleotide polymorphisms were found to be associated with anti-TNF treatment response in RA (19 from genome-wide association studies and 6 from the meta-analyses), and these map to genes involved in T cell function, NFkappaB and TNF signalling pathways (including CTCN5, TEC, PTPRC, FCGR2A, NFKBIB, FCGR2A, IRAK3). Explorative prediction analyses found that biomarkers for clinical treatment selection are not yet available.
Self-complementary adeno-associated virus-mediated interleukin-1 receptor antagonist gene delivery for the treatment of osteoarthritis: Test of efficacy in an equine model
DOI:10.1089/humc.2017.143
URL
PMID:29869535
[Cited within: 1]

The authors are investigating self-complementary adeno-associated virus (scAAV) as a vector for intra-articular gene-delivery of interleukin-1 receptor antagonist (IL-1Ra), and its therapeutic capacity in the treatment of osteoarthritis (OA). To model gene transfer on a scale proportional to the human knee, a frequent site of OA incidence, studies were focused on the joints of the equine forelimb. Using AAV2.5 capsid and equine IL-1Ra as a homologous transgene, a functional ceiling dose of approximately 5 x 10(12) viral genomes was previously identified, which elevated the steady state levels of eqIL-1Ra in synovial fluids by >40-fold over endogenous production for at least 6 months. Here, using an osteochondral fragmentation model of early OA, the functional capacity of scAAV.IL-1Ra gene-delivery was examined in equine joints over a period of 12 weeks. In the disease model, transgenic eqIL-1Ra expression was several fold higher than seen previously in healthy joints, and correlated directly with the severity of joint pathology at the time of treatment. Despite wide variation in expression, the steady-state eqIL-1Ra in synovial fluids exceeded that of IL-1 by >400-fold in all animals, and a consistent treatment effect was observed. This included a 30-40% reduction in lameness and approximately 25% improvement in total joint pathology by both magnetic resonance imaging and arthroscopic assessments, which included reduced joint effusion and synovitis, and improved repair of the osteochondral lesion. No vector-related increase in eqIL-1Ra levels in blood or urine was noted. Cumulatively, these studies in the equine model indicate scAAV.IL-1Ra administration is reasonably safe and capable of sustained therapeutic IL-1Ra production intra-articularly in joints of human scale. This profile supports consideration for human testing in OA.
Articular cartilage regeneration in osteoarthritis
Co-overexpression of TGF-β and SOX9 via rAAV gene transfer modulates the metabolic and chondrogenic activities of human bone marrow-derived mesenchymal stem cells
DOI:10.1186/s13287-016-0280-9
URL
PMID:26830674
[Cited within: 1]

BACKGROUND: Articular cartilage has a limited potential for self-healing. Transplantation of genetically modified progenitor cells like bone marrow-derived mesenchymal stem cells (MSCs) is an attractive strategy to improve the intrinsic repair capacities of damaged articular cartilage. METHODS: In this study, we examined the potential benefits of co-overexpressing the pleiotropic transformation growth factor beta (TGF-beta) with the cartilage-specific transcription factor SOX9 via gene transfer with recombinant adeno-associated virus (rAAV) vectors upon the biological activities of human MSCs (hMSCs). Freshly isolated hMSCs were transduced over time with separate rAAV vectors carrying either TGF-beta or sox9 in chondrogenically-induced aggregate cultures to evaluate the efficacy and duration of transgene expression and to monitor the effects of rAAV-mediated genetic modification upon the cellular activities (proliferation, matrix synthesis) and chondrogenic differentiation potency compared with control conditions (lacZ treatment, sequential transductions). RESULTS: Significant, prolonged TGF-beta/sox9 co-overexpression was achieved in chondrogenically-induced hMSCs upon co-transduction via rAAV for up to 21 days, leading to enhanced proliferative, biosynthetic, and chondrogenic activities relative to control treatments, especially when co-applying the candidate vectors at the highest vector doses tested. Optimal co-administration of TGF-beta with sox9 also advantageously reduced hypertrophic differentiation of the cells in the conditions applied here. CONCLUSION: The present findings demonstrate the possibility of modifying MSCs by combined therapeutic gene transfer as potent future strategies for implantation in clinically relevant animal models of cartilage defects in vivo.
Biomaterial-guided delivery of gene vectors for targeted articular cartilage repair
DOI:10.1038/s41584-018-0125-2
URL
PMID:30514957
[Cited within: 1]

Articular cartilage defects are prevalent and are potentially involved in the initiation of osteoarthritis, yet the lack of efficient therapeutic options to treat cartilage defects represents a substantial challenge. Molecular treatments that require the delivery of therapeutic gene vectors are often less effective that specific, targeted approaches, and the scientific evidence for acellular biomaterial-assisted procedures is limited. Controlled delivery of gene vectors using biocompatible materials is emerging as a novel strategy for the sustained and tuneable release of gene therapies in a spatiotemporally precise manner, thereby reducing intra-articular vector spread and possible loss of the therapeutic gene product. Controlled, biomaterial-guided delivery of gene vectors could be used to enhance intrinsic mechanisms of cartilage repair while affording protection against potentially damaging host immune responses that might counteract the gene therapy component. This Review provides an overview of advances in gene vector-loaded biomaterials for articular cartilage repair. Such systems enable the sustained release of gene therapies while maintaining transduction efficacy. Strategies that harness these properties are likely to result in improved in situ cartilage tissue regeneration that could be safely translated into clinical applications in the near future.
Recent advances in polymeric biomaterials-based gene delivery for cartilage repair
DOI:10.1016/j.bioactmat.2020.06.004
URL
PMID:32671293
[Cited within: 1]

Untreated articular cartilage damage normally results in osteoarthritis and even disability that affects millions of people. However, both the existing surgical treatment and tissue engineering approaches are unable to regenerate the original structures of articular cartilage durably, and new strategies for integrative cartilage repair are needed. Gene therapy provides local production of therapeutic factors, especially guided by biomaterials can minimize the diffusion and loss of the genes or gene complexes, achieve accurate spatiotemporally release of gene products, thus provideing long-term treatment for cartilage repair. The widespread application of gene therapy requires the development of safe and effective gene delivery vectors and supportive gene-activated matrices. Among them, polymeric biomaterials are particularly attractive due to their tunable physiochemical properties, as well as excellent adaptive performance. This paper reviews the recent advances in polymeric biomaterial-guided gene delivery for cartilage repair, with an emphasis on the important role of polymeric biomaterials in delivery systems.
rAAV-mediated overexpression of TGF-β via vector delivery in polymeric micelles stimulates the biological and reparative activities of human articular chondrocytes in vitro and in a human osteochondral defect model
DOI:10.2147/IJN.S144579
URL
PMID:29033566
[Cited within: 3]

Recombinant adeno-associated virus (rAAV) vectors are clinically adapted vectors to durably treat human osteoarthritis (OA). Controlled delivery of rAAV vectors via polymeric micelles was reported to enhance the temporal and spatial presentation of the vectors into their targets. Here, we tested the feasibility of delivering rAAV vectors via poly (ethylene oxide) (PEO) and poly (propylene oxide) (PPO) (poloxamer and poloxamine) polymeric micelles as a means to overexpress the therapeutic factor transforming growth factor-beta (TGF-beta) in human OA chondrocytes and in experimental human osteochondral defects. Application of rAAV-human transforming growth factor-beta using such micelles increased the levels of TGF-beta transgene expression compared with free vector treatment. Overexpression of TGF-beta with these systems resulted in higher proteoglycan deposition and increased cell numbers in OA chondrocytes. In osteochondral defect cultures, a higher deposition of type-II collagen and reduced hypertrophic events were noted. Delivery of therapeutic rAAV vectors via PEO-PPO-PEO micelles may provide potential tools to remodel human OA cartilage.
Effective remodelling of human osteoarthritic cartilage by sox9 gene transfer and overexpression upon delivery of rAAV vectors in polymeric micelles
Enhanced chondrogenic differentiation activities in human bone marrow aspirates via sox9 overexpression mediated by pNaSS-grafted PCL film-guided rAAV gene transfer
Controlled delivery of SDF-1α and IGF-1: CXCR4(+) cell recruitment and functional skeletal muscle recovery
DOI:10.1039/c5bm00233h
URL
PMID:26247892
[Cited within: 1]

Therapeutic delivery of regeneration-promoting biological factors directly to the site of injury has demonstrated its efficacy in various injury models. Several reports describe improved tissue regeneration following local injection of tissue specific growth factors, cytokines and chemokines. Evidence exists that combined cytokine/growth factor treatment is superior for optimizing tissue repair by targeting different aspects of the regeneration response. The purpose of this study was to evaluate the therapeutic potential of the controlled delivery of stromal cell-derived factor-1alpha (SDF-1alpha) alone or in combination with insulin-like growth factor-I (SDF-1alpha/IGF-I) for the treatment of tourniquet-induced ischemia/reperfusion injury (TK-I/R) of skeletal muscle. We hypothesized that SDF-1alpha will promote sustained stem cell recruitment to the site of muscle injury, while IGF-I will induce progenitor cell differentiation to effectively restore muscle contractile function after TK-I/R injury while concurrently reducing apoptosis. Utilizing a novel poly-ethylene glycol PEGylated fibrin gel matrix (PEG-Fib), we incorporated SDF-1alpha alone (PEG-Fib/SDF-1alpha) or in combination with IGF-I (PEG-Fib/SDF-1alpha/IGF-I) for controlled release at the site of acute muscle injury. Despite enhanced cell recruitment and revascularization of the regenerating muscle after SDF-1alpha treatment, functional analysis showed no benefit from PEG-Fib/SDF-1alpha therapy, while dual delivery of PEG-Fib/SDF-1alpha/IGF-I resulted in IGF-I-mediated improvement of maximal force recovery and SDF-1alpha-driven in vivo neovasculogenesis. Histological data supported functional data, as well as highlighted the important differences in the regeneration process among treatment groups. This study provides evidence that while revascularization may be necessary for maximizing muscle force recovery, without modulation of other effects of inflammation it is insufficient.
Polyurethane scaffolds seeded with genetically engineered skeletal myoblasts: a promising tool to regenerate myocardial function
DOI:10.1111/j.1525-1594.2009.00937.x
URL
PMID:20420589
[Cited within: 1]

In animal models, intramyocardial injection of primary skeletal myoblasts is supposed to promote tissue regeneration and to improve cardiac function after myocardial infarction. The usage of genetically engineered myoblasts overexpressing the paracrine factors involved in tissue repair is believed to enhance these effects. However, cell therapy via injection is always accompanied by a high death rate of the injected cells. Here, we describe the construction of a growth factor-producing myoblast-seeded scaffold to overcome this limitation. Skeletal myoblasts were isolated and expanded from newborn Lewis rats. Cells were seeded on polyurethane (PU) scaffolds (Artelon) and transfected with DNA of VEGF-A, HGF, SDF-1, or Akt1 using the lipid-based Metafectene Pro method. Overexpression was verified by ELISA, RT-PCR (VEGF-A, HGF, and SDF-1) and Western blot analysis (Akt1). The seeded scaffolds were transplanted onto damaged myocardium of Lewis rats 2 weeks after myocardial infarction. Six weeks later, their therapeutic potential in vivo was analyzed by measurement of infarction size and capillary density. Primary rat skeletal myoblasts seeded on PU scaffolds were efficiently transfected, achieving transfection rates of 20%. In vitro, we noted a significant increase in expression of VEGF-A, HGF, SDF-1, and Akt1 after transfection. In vivo, transplantation of growth factor-producing myoblast-seeded scaffolds resulted in enhanced angiogenesis (VEGF-A, HGF, and Akt1) or a reduced infarction zone (SDF-1 and Akt1) in the ischemically damaged myocardium. In summary, we constructed a growth factor-producing myoblast-seeded scaffold which combines the beneficial potential of stem cell transplantation with the promising effects of gene-therapeutic approaches. Because this matrix also allows us to circumvent previous cell application drawbacks, it may represent a promising tool for tissue regeneration and the re-establishment of cardiac function after myocardial infarction.
AAV vector encoding human VEGF165-transduced pectineus muscular flaps increase the formation of new tissue through induction of angiogenesis in an in vivo chamber for tissue engineering: A technique to enhance tissue and vessels in microsurgically engineered tissue
DOI:10.1177/2041731415592075
URL
PMID:26977283
[Cited within: 3]

Adeno-associated virus vector carrying human minidystrophin genes effectively ameliorates muscular dystrophy in mdx mouse model
DOI:10.1073/pnas.240335297
URL
PMID:11095710
[Cited within: 1]

Systemic human minidystrophin gene transfer improves functions and life span of dystrophin and dystrophin/utrophin-deficient mice
DOI:10.1002/jor.20781
URL
PMID:18973234
[Cited within: 1]

Duchenne muscular dystrophy (DMD) is the most common and lethal genetic muscle disease, caused by mutations in the dystrophin gene. No efficacious treatment is currently available. Here we report AAV vector systemic delivery and therapeutic benefits of the functional human minidystrophin gene in a severe and more reliable DMD mouse model, the dystrophin/utrophin double deficiency mouse (dys-/-:utrn-/-, dKO). These mice show many pathologic and phenotypic signs typical of DMD in humans including kyphosis and shorter life span, all of which are not seen in the mdx mice due to their utrophin upregulation that partially compensates the loss of dystrophin functions and leads to mild phenotypes. The therapeutic value of this new approach was demonstrated in both mdx and dKO murine models, in which we observed highly efficient minidystrophin gene expression, ameliorated muscle pathologies, improvement in growth and motility, inhibition of spine and limb deformation, and prolongation of life span.
Inhibition of the IKK/NF-κB pathway by AAV gene transfer improves muscle regeneration in older mdx mice
DOI:10.1038/gt.2010.110
URL
PMID:20720575
[Cited within: 1]

The IkappaB kinase (IKKalpha, beta and the regulatory subunit IKKgamma) complex regulates nuclear factor of kappaB (NF-kappaB) transcriptional activity, which is upregulated in many chronic inflammatory diseases. NF-kappaB signaling promotes inflammation and limits muscle regeneration in Duchenne muscular dystrophy (DMD), resulting in fibrotic and fatty tissue replacement of muscle that exacerbates the wasting process in dystrophic muscles. Here, we examined whether dominant-negative forms of IKKalpha (IKKalpha-dn) and IKKbeta (IKKbeta-dn) delivered by adeno-associated viral (AAV) vectors to the gastrocnemius (GAS) and tibialis anterior (TA) muscles of 1, 2 and 11-month-old mdx mice, a murine DMD model, block NF-kappaB activation and increase muscle regeneration. At 1 month post-treatment, the levels of nuclear NF-kappaB in locally treated muscle were decreased by gene transfer with either AAV-CMV-IKKalpha-dn or AAV-CMV-IKKbeta-dn, but not by IKK wild-type controls (IKKalpha and beta) or phosphate-buffered saline (PBS). Although treatment with AAV-IKKalpha-dn or AAV-IKKbeta-dn vectors had no significant effect on muscle regeneration in young mdx mice treated at 1 and 2 months of age and collected 1 month later, treatment of old (11 months) mdx with AAV-CMV-IKKalpha-dn or AAV-CMV-IKKbeta-dn significantly increased levels of muscle regeneration. In addition, there was a significant decrease in myofiber necrosis in the AAV-IKKalpha-dn- and AAV-IKKbeta-dn-treated mdx muscle in both young and old mice. These results demonstrate that inhibition of IKKalpha or IKKbeta in dystrophic muscle reduces the adverse effects of NF-kappaB signaling, resulting in a therapeutic effect. Moreover, these results clearly demonstrate the therapeutic benefits of inhibiting NF-kappaB activation by AAV gene transfer in dystrophic muscle to promote regeneration, particularly in older mdx mice, and block necrosis.
AAV-based shRNA silencing of NF-κB ameliorates muscle pathologies in mdx mice
DOI:10.1038/gt.2011.207
URL
PMID:22278411

Chronic inflammation, promoted by an upregulated NF-kappa B (NF-kappaB) pathway, has a key role in Duchenne muscular dystrophy (DMD) patients' pathogenesis. Blocking the NF-kappaB pathway has been shown to be a viable approach to diminish chronic inflammation and necrosis in the dystrophin-defective mdx mouse, a murine DMD model. In this study, we used the recombinant adeno-associated virus serotype 9 (AAV9) carrying an short hairpin RNA (shRNA) specifically targeting the messenger RNA of NF-kappaB/p65 (p65-shRNA), the major subunit of NF-kappaB associated with chronic inflammation in mdx mice. We examined whether i.m. AAV9-mediated delivery of p65-shRNA could decrease NF-kappaB activation, allowing for amelioration of muscle pathologies in 1- and 4-month-old mdx mice. At 1 month after treatment, NF-kappaB/p65 levels were significantly decreased by AAV gene transfer of p65-shRNA in the two ages of treatment groups, with necrosis significantly decreased compared with controls. Quantitative analysis revealed that central nucleation (CN) of the myofibers of p65-shRNA-treated 1-month-old mdx muscles was reduced from 67 to 34%, but the level of CN was not significantly decreased in treated 4-month-old mdx mice. Moreover, delivery of the p65-shRNA enhanced the capacity of myofiber regeneration in old mdx mice treated at 4 months of age when the dystrophic myofibers were most exhausted; however, such p65 silencing diminished the myofiber regeneration in young mdx mice treated at 1 month of age. Taken together, these findings demonstrate that the AAV-mediated delivery of p65-shRNA has the capacity to ameliorate muscle pathologies in mdx mice by selectively reducing NF-kappaB/p65 activity.
Genetic ablation of P65 subunit of NF-κB in mdx mice to improve muscle physiological function
DOI:10.1002/mus.25517
URL
PMID:27997693
[Cited within: 1]

INTRODUCTION: Duchenne muscular dystrophy (DMD) is a genetic muscle disease characterized by dystrophin deficiency. Beyond gene replacement, the question of whether ablation of the p65 gene of nuclear factor-kappa B (NF-kappaB) in DMD can improve muscle physiology function is unknown. In this study, we investigated muscle physiological improvement in mdx mice (DMD model) with a genetic reduction of NF-kappaB. METHODS: Muscle physiological function and histology were studied in 2-month-old mdx/p65(+/-) , wild-type, mdx, and human minidystrophin gene transgenic mdx (TghDeltaDys/mdx) mice. RESULTS: Improved muscle physiological function was found in mdx/p65(+/-) mice when compared with mdx mice; however, it was similar to TghDeltaDys/mdx mice. The results indicate that genetic reduction of p65 levels diminished chronic inflammation in dystrophic muscle, thus leading to amelioration of muscle pathology and improved muscle physiological function. CONCLUSIONS: The results show that inhibition of NF-kappaB may be a promising therapy when combined with gene therapy for DMD. Muscle Nerve 56: 759-767, 2017.