Biomaterials Translational ›› 2021, Vol. 2 ›› Issue (3): 272-284.doi: 10.12336/biomatertransl.2021.03.009
• RESEARCH ARTICLE • Previous Articles
Jing Long1, Bin Teng2, Wei Zhang1, Long Li1,3, Ming Zhang3, Yingqi Chen3, Zhenyu Yao1, Xiangbo Meng1, Xinluan Wang1, Ling Qin1,4, Yuxiao Lai1,2,5,*( )
)
Received:2021-06-08
Revised:2021-07-31
Accepted:2021-08-20
Online:2021-09-28
Published:2021-09-28
Contact:
Yuxiao Lai
E-mail:yx.lai@siat.ac.cn
About author:Yuxiao Lai, yx.lai@siat.ac.cn.†Present Addresses: Centre for Translational Medicine and Research and Development, Institute of Biomedical and Health Engineering, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, Guangdong Province, China.
Long, J.; Teng, B.; Zhang, W.; Li, L.; Zhang, M.; Chen, Y.; Yao, Z.; Meng, X.; Wang, X.; Qin, L.; Lai, Y. Preclinical evaluation of acute systemic toxicity of magnesium incorporated poly (lactic-co-glycolic acid) porous scaffolds by three-dimensional printing. Biomater Transl. 2021, 2(3), 272-284.
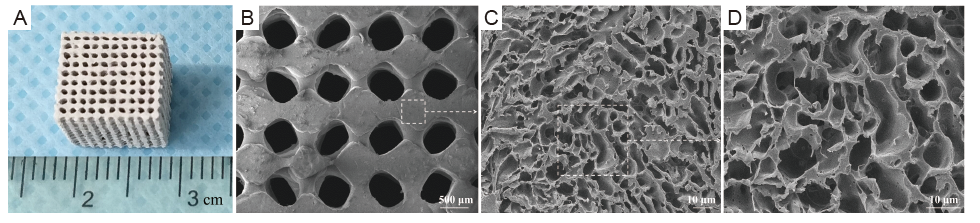
Figure 1. Macrograph image and scanning electron microscopy micrographs of 3D-printed PLGA/β-TCP/Mg composite porous scaffolds. (A) Macroscopic image of a fabricated scaffold. (B-D) Transverse section at a magnification of PT10M scaffold by SEM (original magnifications 30×, 500×, and 1000×). Scale bars: 500 μm in B, 10 μm in C, and D. 3D: three-dimensional; Mg: magnesium; PT10M: PLGA/β-TCP/10 wt% Mg porous composite scaffolds; PLGA: poly(lactic-co-glycolic acid); β-TCP: beta-tricalcium phosphate.
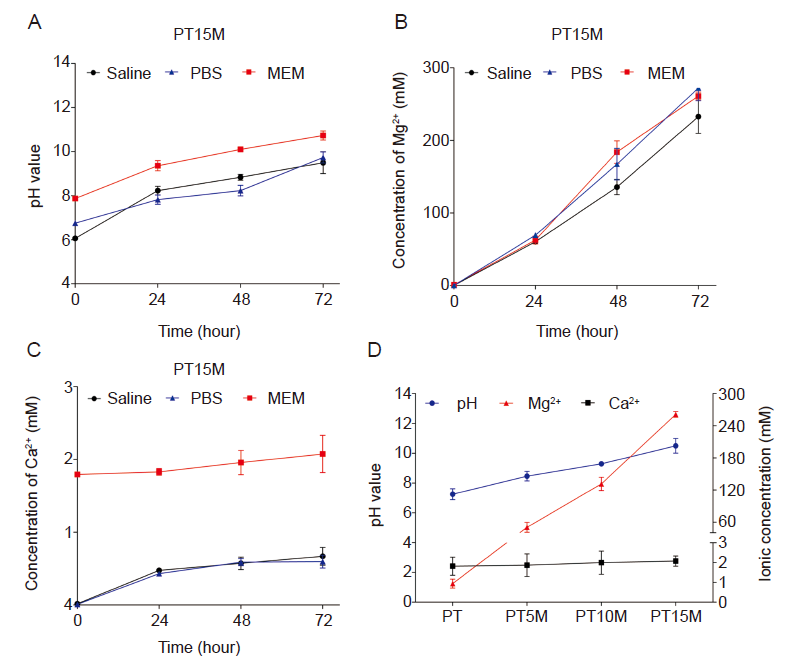
Figure 2. In vitro immersion degradation behaviour of scaffolds. (A) Changes in the pH value of different solutions used to soak the PT15M scaffold at 37°C for 24, 48, and 72 hours. (B, C) The concentrations of Mg ions (B) and Ca ions (C) released into serum-free MEM after incubation with PT15M at 37°C for 24, 48, and 72 hours. In the test results, MEM has a higher baseline as it contains calcium chloride. (D) The pH value, Mg, and Ca ion concentrations of MEM incubated with PT, PT5M, PT10M, and PT15M scaffolds at 37°C for 72 hours. Data are expressed as the mean ± SD. (n = 3 and experiments were repeated by twice). Ca: calcium; MEM: serum-free minimum essential medium; Mg: magnesium; PT: poly(lactic-co-glycolic acid)/beta-tricalcium phosphate porous composite scaffolds; PT5M: poly(lactic-co-glycolic acid)/beta-tricalcium phosphate/5 wt% Mg porous composite scaffolds; PT10M: poly(lactic-co-glycolic acid)/beta-tricalcium phosphate/10 wt% Mg porous composite scaffolds; PT15M: poly(lactic-co-glycolic acid)/beta-tricalcium phosphate/15 wt% Mg porous composite scaffolds.
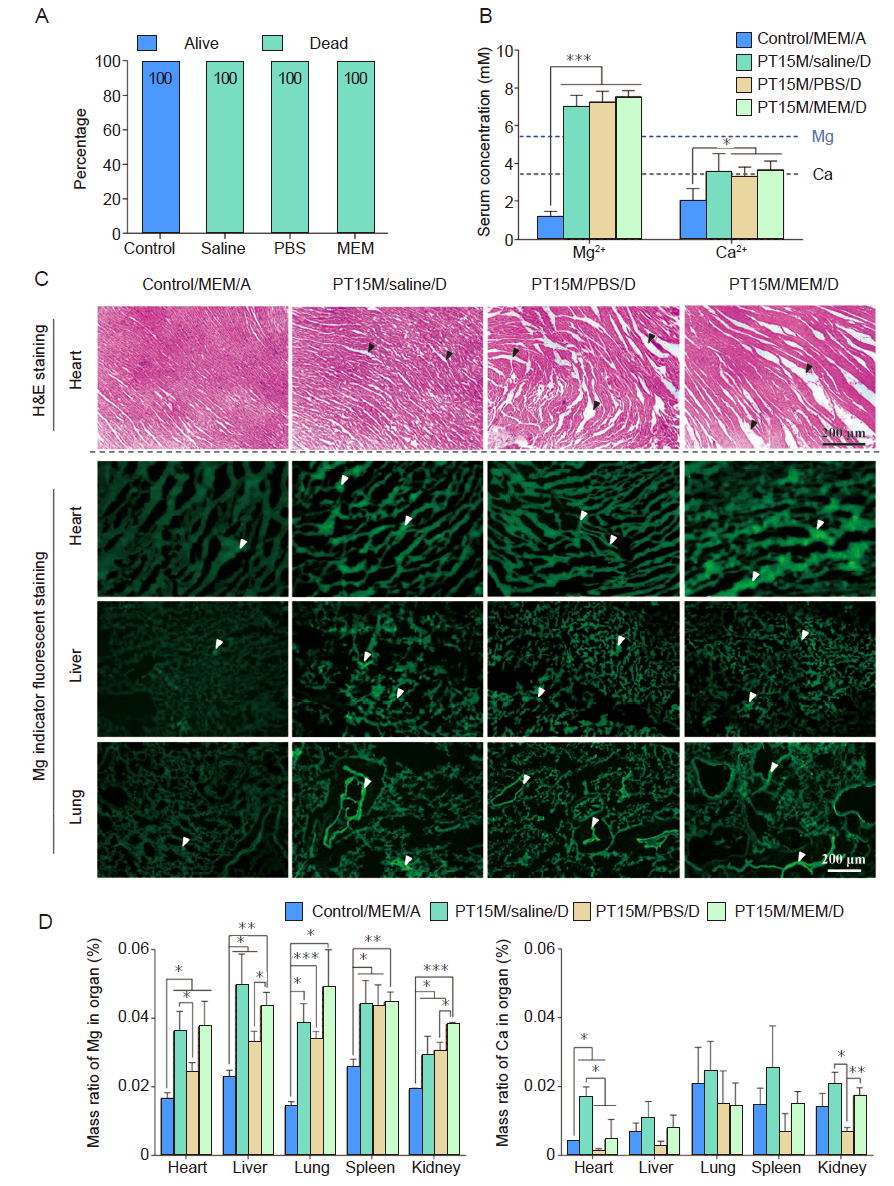
Figure 3. In vivo acute toxicity results in mice after intraperitoneal injection of different extract solutions. Each extract solution was prepared by soaking the PT15M scaffold in the solution at 37°C for 72 hours. (A) The percentage of live and dead animals. For those mice that survived or dead, corresponding to the extract medium they were injected, we grouped them into four groups which were control/MEM/alive (Control), PT15M/saline/dead, PT15M/PBS/dead, and PT15M/MEM/dead groups. (B) Mg and Ca ion concentrations of serum in mice, 1 hour after intraperitoneal injection of extract solution. (C) H&E staining of heart, and Mg indicator fluorescent staining (green) of heart, liver, and lung. Black arrows indicate loosened cardiac tissues, and white arrows indicate enrichment sites of Mg ions in tissues. Scale bars: 200 μm. (D) The mass ratios of Mg and Ca in different organs. The control group was injected with MEM that was not incubated with any scaffolds. Data are expressed as the mean ± SD (n = 10, 5 male and 5 female). *P < 0.05, **P < 0.01, ***P < 0.001 (one-way analysis of variance followed by Tukey’s post hoc test). “A” stands for alive mice, and “D” means dead mice. Ca: calcium; H&E: haematoxylin and eosin; MEM: serum-free minimum essential medium; Mg: magnesium; PBS: phosphate-buffered saline; PT15M: poly(lactic-co-glycolic acid)/beta-tricalcium phosphate/15 wt% Mg porous composite scaffolds; Saline: normal saline.
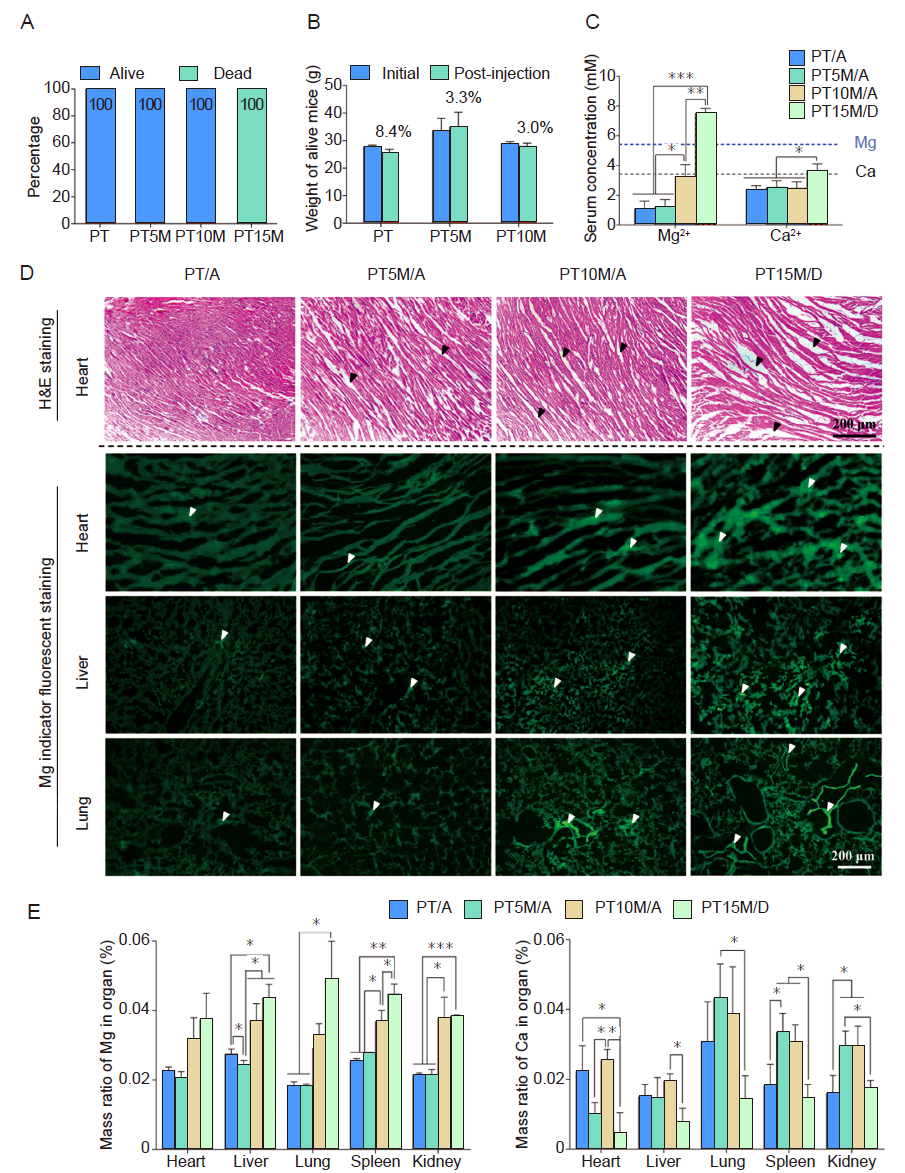
Figure 4. In vivo acute toxicity results of mice after intraperitoneal injection of different Mg content scaffold extract. Extract MEM solutions were prepared by soaking PT, PT5M, PT10M, and PT15M samples in MEM at 37°C for 72 hours. (A) The percentage of live and dead mice 1 hour after intraperitoneal injection of extract MEM. For those mice that survived or dead, corresponding to the scaffold extract they were injected, we grouped them into four groups which were PT/alive (PT/A), PT5M/alive (PT5M/A), PT10M/alive (PT5M/A), and PT15M/dead (PT5M/D) groups. (B) The weight change of live mice 72 hours after intraperitoneal injection of extract MEM. (C) Mg and Ca ion concentrations in serum 1 hour after intraperitoneal injection of extract MEM. Scale bars: 200 μm. (D) H&E staining of heart, and Mg indicator fluorescent staining of heart, liver, and lung. Black arrows indicate loosened cardiac tissues, and white arrows indicate enrichment sites of Mg ions in tissues. (E) The mass ratios of Mg and Ca in different organs at 1 hour after intraperitoneal injection of extract MEM. Data are expressed as the mean ± SD (n = 10-12, 5-6 male and 5-6 female). *P < 0.05, **P < 0.01, ***P < 0.001 (one-way analysis of variance followed by Tukey’s post hoc test). “A” stands for alive mice, and “D” means dead mice. Ca: calcium; H&E: haematoxylin and eosin; MEM: serum-free minimum essential medium; Mg: magnesium; PT: poly(lactic-co-glycolic acid)/beta-tricalcium phosphate porous composite scaffolds; PT5M: poly(lactic-co-glycolic acid)/beta-tricalcium phosphate/5 wt% Mg porous composite scaffolds; PT10M: poly(lactic-co-glycolic acid)/beta-tricalcium phosphate/10 wt% Mg porous composite scaffolds; PT15M: poly(lactic-co-glycolic acid)/beta-tricalcium phosphate/15 wt% Mg porous composite scaffolds.
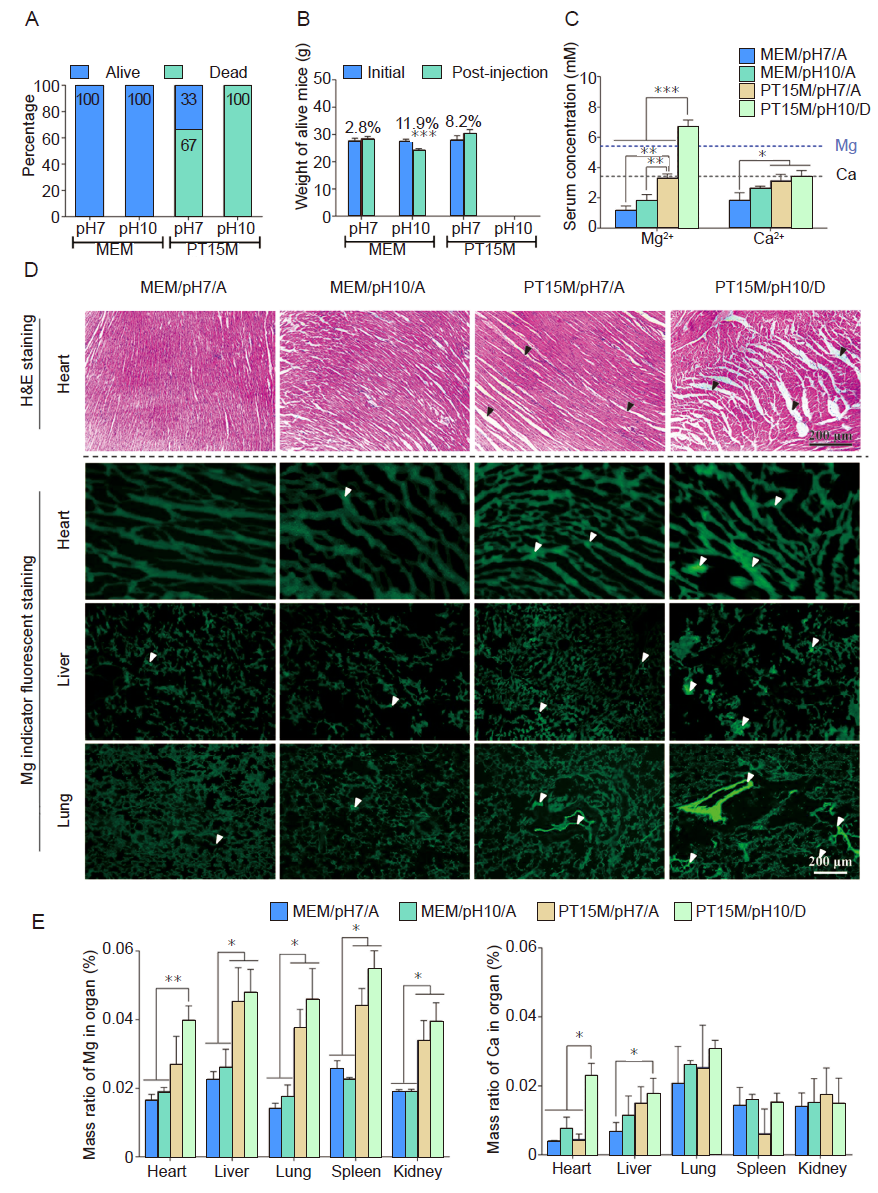
Figure 5. In vivo acute toxicity results in mice after intraperitoneal injection of extract MEM with different pH values (7.0 and 10.0). Extract MEM was prepared by soaking PT15M at 37°C for 72 hours. For those mice that survived or dead, corresponding to the different pH values of scaffold extract they were injected, we grouped them into four groups which were MEM/pH7/alive (MEM/pH7/A), MEM/pH10/alive (MEM/pH10/A), PT15M/pH7/alive (PT15M/pH7/A), and PT15M/pH7/dead (PT15M/pH7/D) groups. (A) The percentage of live and dead animals at 1 hour after intraperitoneal injection of extract MEM. (B) The weight change of live mice at 72 hours after intraperitoneal injection of extract MEM. (C) Mg and Ca ion concentrations of serum in mice 1 hour after intraperitoneal injection of extract MEM. (D) H&E staining of heart, and Mg indicator fluorescent staining of heart, liver, and lung. Black arrows indicate loosened cardiac tissues, and white arrows indicate enrichment sites of Mg ions in tissues. Scale bars: 200 μm. (E) The mass ratios of Mg and Ca in different organs at 1 hour after intraperitoneal injection with extract MEM. Data are expressed as the mean ± SD (n = 10, 5 male and 5 female). *P < 0.05, **P < 0.01, ***P < 0.001 (one-way analysis of variance followed by Tukey’s post hoc test). “A” stands for alive mice, and “D” means dead mice. Ca: calcium; H&E: haematoxylin and eosin; MEM: serum-free minimum essential medium; Mg: magnesium; PT15M: poly(lactic-co-glycolic acid)/beta-tricalcium phosphate/15 wt% Mg porous composite scaffolds.
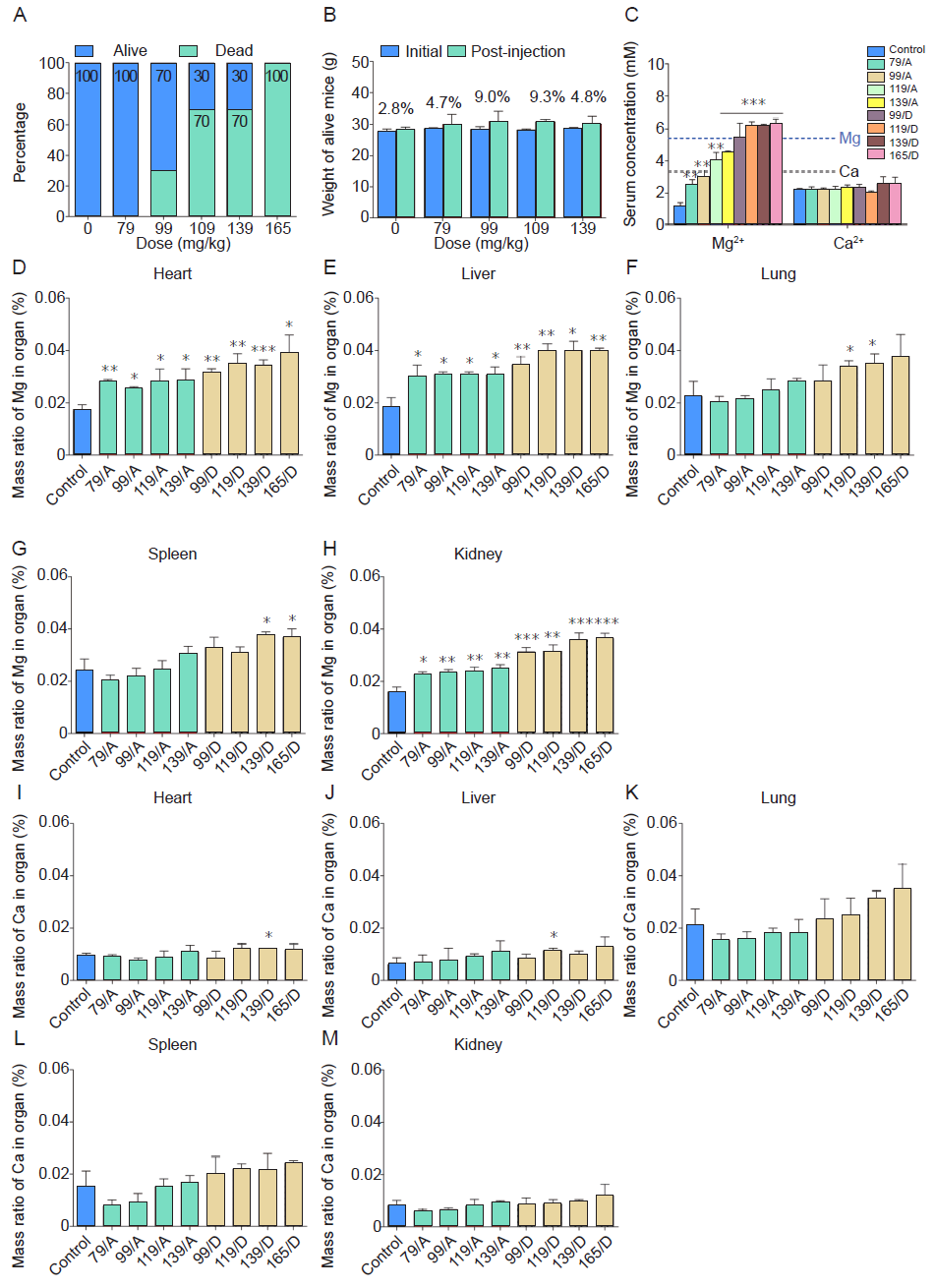
Figure 6. In vivo median lethal dosing results of mice after intraperitoneal injection of different concentrations of diluted extract MEM. The extract MEM was prepared by soaking the PT15M scaffold in MEM at 37 °C for 72 hours. (A) The percentage of live and dead animals. For those mice that survived or dead, corresponding to the different magnesium concentrations of MEM solutions they were injected, we grouped them into nine groups which were control group (serum-free MEM), 79 mg/kg Mg/A, 99 mg/kg Mg/A, 119 mg/kg Mg/A, 119 mg/kg Mg/D, 139 mg/kg Mg/D, and 165 mg/kg Mg/D groups. (B) The weight of the live animals. The x-axis indicates Mg content in extract solution versus the weight of the mice. (C) Mg and Ca ion concentrations in the serum of mice at 1 hour after intraperitoneal injection. (D﹣H) The mass ratios of Mg in different organs. (I-M) The mass ratios of Ca in different organs. The control group was injected with MEM with a pH value of 7.0. Data are expressed as the mean ± SD (n = 10, 5 male and 5 female). *P < 0.05, **P < 0.01, ***P < 0.001 (one-way analysis of variance followed by Tukey’s post hoc test). “A” stands for alive mice, and “D” means dead mice. Ca: calcium; MEM: serum-free minimum essential medium; Mg: magnesium.
| Dose (mg/kg) | Time | Dyspnea | Prostration | Convulsion | Ptosis | Salivation | Piloerection | Diuresis | Edema | Erythema |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 day | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ |
| 3 day | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | |
| 79 | 1 day | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ |
| 3 day | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | |
| 99 | 1 day | ﹣ | + | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ |
| 3 day | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | + | ﹣ | ﹣ | |
| 119 | 1 day | ﹣ | + | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ |
| 3 day | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | + | ﹣ | ﹣ | |
| 139 | 1 day | + | + | + | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ |
| 3 day | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | + | ﹣ | ﹣ | |
| 165 | 1 day | + | + | + | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ |
| 3 day | NA | NA | NA | NA | NA | NA | NA | NA | NA |
Table 1 The clinical signs and observed symptoms for mice after intraperitoneal injection of different ratio diluted extract MEM.
| Dose (mg/kg) | Time | Dyspnea | Prostration | Convulsion | Ptosis | Salivation | Piloerection | Diuresis | Edema | Erythema |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 day | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ |
| 3 day | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | |
| 79 | 1 day | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ |
| 3 day | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | |
| 99 | 1 day | ﹣ | + | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ |
| 3 day | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | + | ﹣ | ﹣ | |
| 119 | 1 day | ﹣ | + | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ |
| 3 day | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | + | ﹣ | ﹣ | |
| 139 | 1 day | + | + | + | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ |
| 3 day | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | + | ﹣ | ﹣ | |
| 165 | 1 day | + | + | + | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ | ﹣ |
| 3 day | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Group | Reagent | Male mice (n) | Female mice (n) | Death rate (%) |
|---|---|---|---|---|
| Control | MEM | 5 | 5 | 0 |
| PT15M | MEM | 5 | 5 | 100 |
| PT15M | PBS | 5 | 5 | 100 |
| PT15M | Saline | 5 | 5 | 100 |
Additional Table 1 Verified the difference between different extract solutions effect on acute systemic toxicity in vivo.
| Group | Reagent | Male mice (n) | Female mice (n) | Death rate (%) |
|---|---|---|---|---|
| Control | MEM | 5 | 5 | 0 |
| PT15M | MEM | 5 | 5 | 100 |
| PT15M | PBS | 5 | 5 | 100 |
| PT15M | Saline | 5 | 5 | 100 |
| Group | Reagent | Male mice (n) | Female mice (n) | Death rate (%) |
|---|---|---|---|---|
| PT | MEM | 5 | 5 | 0 |
| PT5M | MEM | 5 | 5 | 0 |
| PT10M | MEM | 5 | 5 | 0 |
| PT15M | MEM | 6 | 6 | 100 |
Additional Table 2 Determined Mg effect of in vivo acute toxicity based on various Mg content scaffolds.
| Group | Reagent | Male mice (n) | Female mice (n) | Death rate (%) |
|---|---|---|---|---|
| PT | MEM | 5 | 5 | 0 |
| PT5M | MEM | 5 | 5 | 0 |
| PT10M | MEM | 5 | 5 | 0 |
| PT15M | MEM | 6 | 6 | 100 |
| Group | Reagent | Male mice (n) | Female mice (n) | Death rate (%) |
|---|---|---|---|---|
| MEM (pH 7.0) | MEM | 5 | 5 | 0 |
| MEM (pH 10.0) | MEM | 5 | 5 | 0 |
| PT15M (pH 7.0) | MEM | 5 | 5 | 67 |
| PT15M (pH 10.0) | MEM | 5 | 5 | 100 |
Additional Table 3 Effect of pH value on in vivo acute systemic toxicity.
| Group | Reagent | Male mice (n) | Female mice (n) | Death rate (%) |
|---|---|---|---|---|
| MEM (pH 7.0) | MEM | 5 | 5 | 0 |
| MEM (pH 10.0) | MEM | 5 | 5 | 0 |
| PT15M (pH 7.0) | MEM | 5 | 5 | 67 |
| PT15M (pH 10.0) | MEM | 5 | 5 | 100 |
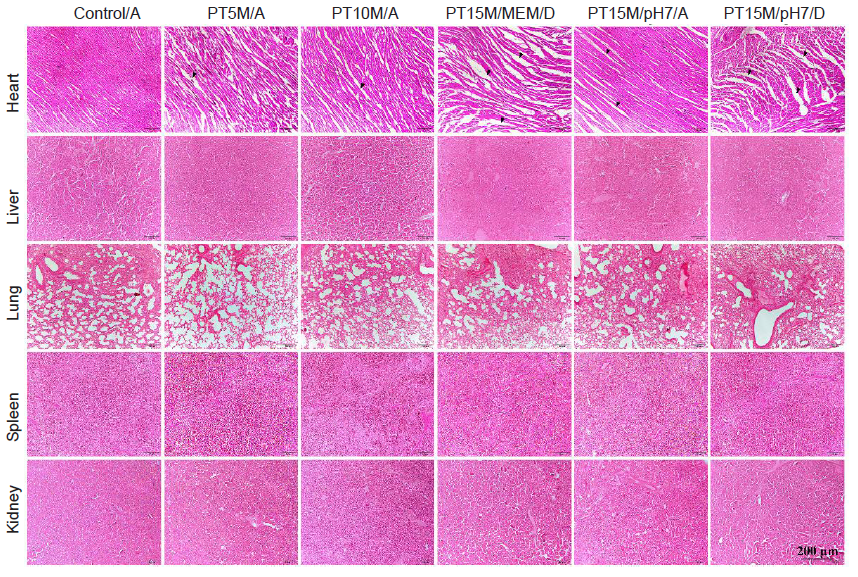
Additional Figure 1. Haematoxylin and eosin staining of heart, liver, lung, spleen, and kidney 1 hour after intraperitoneal injection of extract MEM. The extract MEM was incubated with scaffolds at 37°C for 72 hours. The results showed that the wavy appearance of the myocardium was only apparent in PT15M/pH7/D extract-treated dead mice, while the myocardium in the MEM, PT5M/A, PT10M/A, and PT15M/pH7/A groups remained normal. There were no abnormalities in the liver, lung, spleen, or kidney in any groups. Black arrows indicate loosened cardiac tissues. Scale bars: 200 μm. “A” stands for alive mice, and “D” means dead mice. MEM: serum-free minimum essential medium; Mg: magnesium; PT5M: poly(lactic-co-glycolic acid)/beta-tricalcium phosphate/5 wt% Mg porous composite scaffolds; PT10M: poly(lactic-co-glycolic acid)/beta-tricalcium phosphate/10 wt% Mg porous composite scaffolds; PT15M: poly(lactic-co-glycolic acid)/beta-tricalcium phosphate/15 wt% Mg porous composite scaffolds.
| [1] |
Al Maini, M.; Adelowo, F.; Al Saleh, J.; Al Weshahi, Y.; Burmester, G. R.; Cutolo, M.; Flood, J.; March, L.; McDonald-Blumer, H.; Pile, K.; Pineda, C.; Thorne, C.; Kvien, T. K. The global challenges and opportunities in the practice of rheumatology: white paper by the World Forum on Rheumatic and Musculoskeletal Diseases. Clin Rheumatol. 2015, 34, 819-829.
doi: 10.1007/s10067-014-2841-6 URL |
| [2] |
Zhang, Y.; Xu, J.; Ruan, Y. C.; Yu, M. K.; O’Laughlin, M.; Wise, H.; Chen, D.; Tian, L.; Shi, D.; Wang, J.; Chen, S.; Feng, J. Q.; Chow, D. H.; Xie, X.; Zheng, L.; Huang, L.; Huang, S.; Leung, K.; Lu, N.; Zhao, L.; Li, H.; Zhao, D.; Guo, X.; Chan, K.; Witte, F.; Chan, H. C.; Zheng, Y.; Qin, L. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat Med. 2016, 22, 1160-1169.
doi: 10.1038/nm.4162 URL |
| [3] |
Henkel, J.; Woodruff, M. A.; Epari, D. R.; Steck, R.; Glatt, V.; Dickinson, I. C.; Choong, P. F.; Schuetz, M. A.; Hutmacher, D. W. Bone regeneration based on tissue engineering conceptions - a 21st century perspective. Bone Res. 2013, 1, 216-248.
doi: 10.4248/BR201303002 URL |
| [4] |
Brown, A.; Zaky, S.; Ray, H., Jr, ., Sfeir, C. Porous magnesium/PLGA composite scaffolds for enhanced bone regeneration following tooth extraction. Acta Biomater. 2015, 11, 543-553.
doi: 10.1016/j.actbio.2014.09.008 URL |
| [5] |
Li, X.; Chu, C. L.; Liu, L.; Liu, X. K.; Bai, J.; Guo, C.; Xue, F.; Lin, P. H.; Chu, P. K. Biodegradable poly-lactic acid based-composite reinforced unidirectionally with high-strength magnesium alloy wires. Biomaterials. 2015, 49, 135-144.
doi: 10.1016/j.biomaterials.2015.01.060 URL |
| [6] |
Xu, T. O.; Kim, H. S.; Stahl, T.; Nukavarapu, S. P. Self-neutralizing PLGA/magnesium composites as novel biomaterials for tissue engineering. Biomed Mater. 2018, 13, 035013.
doi: 10.1088/1748-605X/aaaa29 URL |
| [7] |
Wu, Y. H.; Li, N.; Cheng, Y.; Zheng, Y. F.; Han, Y. In vitro study on biodegradable AZ31 magnesium alloy fibers reinforced PLGA composite. J Mater Sci Technol. 2013, 29, 545-550.
doi: 10.1016/j.jmst.2013.03.004 URL |
| [8] |
Chen, Y.; Ye, S. H.; Sato, H.; Zhu, Y.; Shanov, V.; Tiasha, T.; D’Amore, A.; Luketich, S.; Wan, G.; Wagner, W. R. Hybrid scaffolds of Mg alloy mesh reinforced polymer/extracellular matrix composite for critical-sized calvarial defect reconstruction. J Tissue Eng Regen Med. 2018, 12, 1374-1388.
doi: 10.1002/term.v12.6 URL |
| [9] |
Yu, W.; Li, R.; Long, J.; Chen, P.; Hou, A.; Li, L.; Sun, X.; Zheng, G.; Meng, H.; Wang, Y.; Wang, A.; Sui, X.; Guo, Q.; Tao, S.; Peng, J.; Qin, L.; Lu, S.; Lai, Y. Use of a three-dimensional printed polylactide-coglycolide/tricalcium phosphate composite scaffold incorporating magnesium powder to enhance bone defect repair in rabbits. J Orthop Translat. 2019, 16, 62-70.
doi: 10.1016/j.jot.2018.07.007 URL |
| [10] |
Lin, Z.; Wu, J.; Qiao, W.; Zhao, Y.; Wong, K. H. M.; Chu, P. K.; Bian, L.; Wu, S.; Zheng, Y.; Cheung, K. M. C.; Leung, F.; Yeung, K. W. K. Precisely controlled delivery of magnesium ions thru sponge-like monodisperse PLGA/nano-MgO-alginate core-shell microsphere device to enable in-situ bone regeneration. Biomaterials. 2018, 174, 1-16.
doi: 10.1016/j.biomaterials.2018.05.011 URL |
| [11] |
Wang, Y.; Fu, P.; Wang, N.; Peng, L.; Kang, B.; Zeng, H.; Yuan, G.; Ding, W. Challenges and solutions for the additive manufacturing of biodegradable magnesium implants. Engineering. 2020, 6, 1267-1275.
doi: 10.1016/j.eng.2020.02.015 URL |
| [12] |
Lai, Y.; Li, Y.; Cao, H.; Long, J.; Wang, X.; Li, L.; Li, C.; Jia, Q.; Teng, B.; Tang, T.; Peng, J.; Eglin, D.; Alini, M.; Grijpma, D. W.; Richards, G.; Qin, L. Osteogenic magnesium incorporated into PLGA/TCP porous scaffold by 3D printing for repairing challenging bone defect. Biomaterials. 2019, 197, 207-219.
doi: 10.1016/j.biomaterials.2019.01.013 URL |
| [13] | National Standard of the People’s Republic of China. GB/T 16886.11-2011. Biological evaluation of medical devices-Part 5: Tests for in vitro cytotoxicity. China Standards Press: Beijing. 2011. |
| [14] | National Standard of the People’s Republic of China. GB/T 16886.12-2017. Biological evaluation of medical devices-Part 12: Sample preparation and reference materials. China Standards Press: Beijing. 2017. |
| [15] |
Ma, R.; Lai, Y. X.; Li, L.; Tan, H. L.; Wang, J. L.; Li, Y.; Tang, T. T.; Qin, L. Bacterial inhibition potential of 3D rapid-prototyped magnesium-based porous composite scaffolds an in vitro efficacy study. Sci Rep. 2015, 5, 13775.
doi: 10.1038/srep13775 URL |
| [16] |
Li, L.; Long, J.; Li, L.; Cao, H.; Tang, T.; Xi, X.; Qin, L.; Lai, Y.; Wang, X. Quantitative determination of residual 1,4-dioxane in three-dimensional printed bone scaffold. J Orthop Translat. 2018, 13, 58-67.
doi: 10.1016/j.jot.2017.06.004 URL |
| [17] | National Standard of the People’s Republic of China. GB/T 8813-2008. Rigid cellular plastics - Determination of compression properties. China Standards Press: Beijing. 2008. |
| [18] |
Ramakrishnan, M. A. Determination of 50% endpoint titer using a simple formula. World J Virol. 2016, 5, 85-86.
doi: 10.5501/wjv.v5.i2.85 URL |
| [19] |
Long, J.; Zhang, W.; Chen, Y.; Teng, B.; Liu, B.; Li, H.; Yao, Z.; Wang, D.; Li, L.; Yu, X. F.; Qin, L.; Lai, Y. Multifunctional magnesium incorporated scaffolds by 3D-printing for comprehensive postsurgical management of osteosarcoma. Biomaterials. 2021, 275, 120950.
doi: 10.1016/j.biomaterials.2021.120950 URL |
| [20] |
Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current status on clinical applications of magnesium-based orthopaedic implants: A review from clinical translational perspective. Biomaterials. 2017, 112, 287-302.
doi: 10.1016/j.biomaterials.2016.10.017 URL |
| [21] | Kieboom, B. C.; Niemeijer, M. N.; Leening, M. J.; van den Berg, M. E.; Franco, O. H.; Deckers, J. W.; Hofman, A.; Zietse, R.; Stricker, B. H.; Hoorn, E. J. Serum magnesium and the risk of death from coronary heart disease and sudden cardiac death. J Am Heart Assoc. 2016, 5, e002707. |
| [22] |
Gerard, S. K.; Hernandez, C.; Khayam-Bashi, H. Extreme hypermagnesemia caused by an overdose of magnesium-containing cathartics. Ann Emerg Med. 1988, 17, 728-731.
doi: 10.1016/S0196-0644(88)80624-3 URL |
| [23] |
Bazydlo, L. A. L.; Needham, M.; Harris, N. S. Calcium, magnesium, and phosphate. Lab Med. 2014, 45, e44-e50.
doi: 10.1309/LMGLMZ8CIYMFNOGX URL |
| [24] |
Li, C.; Pisignano, D.; Zhao, Y.; Xue, J. Advances in medical applications of additive manufacturing. Engineering. 2020, 6, 1222-1231.
doi: 10.1016/j.eng.2020.02.018 URL |
| [25] |
Hirata, M.; Murata, H.; Takeshita, H.; Sakabe, T.; Tsuji, Y.; Kubo, T. Use of purified beta-tricalcium phosphate for filling defects after curettage of benign bone tumours. Int Orthop. 2006, 30, 510-513.
doi: 10.1007/s00264-006-0156-1 URL |
| [26] | Bucholz, R. W. Nonallograft osteoconductive bone graft substitutes. Clin Orthop Relat Res. 2002, 44-52. |
| [27] |
Diez-Escudero, A.; Espanol, M.; Beats, S.; Ginebra, M. P. In vitro degradation of calcium phosphates: Effect of multiscale porosity, textural properties and composition. Acta Biomater. 2017, 60, 81-92.
doi: 10.1016/j.actbio.2017.07.033 URL |
| [28] |
Kang, Y.; Yao, Y.; Yin, G.; Huang, Z.; Liao, X.; Xu, X.; Zhao, G. A study on the in vitro degradation properties of poly(L-lactic acid)/beta-tricalcuim phosphate (PLLA/beta-TCP) scaffold under dynamic loading. Med Eng Phys. 2009, 31, 589-594.
doi: 10.1016/j.medengphy.2008.11.014 URL |
| [29] | Witte, F. Reprint of: The history of biodegradable magnesium implants: a review. Acta Biomater. 2015, 23 Suppl, S28-40. |
| [30] |
Zhao, D.; Brown, A.; Wang, T.; Yoshizawa, S.; Sfeir, C.; Heineman, W. R. In vivo quantification of hydrogen gas concentration in bone marrow surrounding magnesium fracture fixation hardware using an electrochemical hydrogen gas sensor. Acta Biomater. 2018, 73, 559-566.
doi: 10.1016/j.actbio.2018.04.032 URL |
| [31] | Zheng, Y. F.; Gu, X. N.; Witte, F. Biodegradable metals. Mater Sci Eng RRep. 2014, 77, 1-34. |
| [32] | Glasdam, S. M.; Glasdam, S.; Peters, G. H. The importance of magnesium in the human body: a systematic literature review. Adv Clin Chem. 2016, 73, 169-193. |
| [33] | Al Alawi, A. M.; Majoni, S. W.; Falhammar, H. Magnesium and human health: perspectives and research directions. Int J Endocrinol. 2018, 2018, 9041694. |
| [34] |
Beto, J. A. The role of calcium in human aging. Clin Nutr Res. 2015, 4, 1-8.
doi: 10.7762/cnr.2015.4.1.1 URL |
| [1] | Hongtao Yang, Wenjiao Lin, Yufeng Zheng. Advances and perspective on the translational medicine of biodegradable metals [J]. Biomaterials Translational, 2021, 2(3): 177-187. |
| [2] | Ying Luo, Jue Wang, Michael Tim Yun Ong, Patrick Shu-hang Yung, Jiali Wang, Ling Qin. Update on the research and development of magnesium-based biodegradable implants and their clinical translation in orthopaedics [J]. Biomaterials Translational, 2021, 2(3): 188-196. |
| [3] | Xirui Jing, Qiuyue Ding, Qinxue Wu, Weijie Su, Keda Yu, Yanlin Su, Bing Ye, Qing Gao, Tingfang Sun, Xiaodong Guo. Magnesium-based materials in orthopaedics: material properties and animal models [J]. Biomaterials Translational, 2021, 2(3): 197-213. |
| [4] | Yu Lu, Subodh Deshmukh, Ian Jones, Yu-Lung Chiu. Biodegradable magnesium alloys for orthopaedic applications [J]. Biomaterials Translational, 2021, 2(3): 214-235. |
| [5] | Jialin Niu, Hua Huang, Jia Pei, Zhaohui Jin, Shaokang Guan, Guangyin Yuan. Research and development strategy for biodegradable magnesium-based vascular stents: a review [J]. Biomaterials Translational, 2021, 2(3): 236-247. |
| [6] | Qingchuan Wang, Weidan Wang, Yanfang Li, Weirong Li, Lili Tan, Ke Yang. Biofunctional magnesium coating of implant materials by physical vapour deposition [J]. Biomaterials Translational, 2021, 2(3): 248-256. |
| [7] | Aditya Joshi, George Dias, Mark P. Staiger. In silico modelling of the corrosion of biodegradable magnesium-based biomaterials: modelling approaches, validation and future perspectives [J]. Biomaterials Translational, 2021, 2(3): 257-271. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||