Biomaterials Translational ›› 2023, Vol. 4 ›› Issue (3): 166-179.doi: 10.12336/biomatertransl.2023.03.005
• REVIEW • Previous Articles Next Articles
Jiawei Ying, Haiyu Yu, Liangliang Cheng, Junlei Li, Bin Wu, Liqun Song, Pinqiao Yi, Haiyao Wang, Lingpeng Liu, Dewei Zhao*( )
)
Received:2022-11-16
Revised:2023-08-03
Accepted:2023-08-30
Online:2023-09-28
Published:2023-09-28
Contact:
*Dewei Zhao, Ying, J.; Yu, H.; Cheng, L.; Li, J.; Wu, B.; Song, L.; Yi, P.; Wang, H.; Liu, L.; Zhao D. Research progress and clinical translation of three-dimensional printed porous tantalum in orthopaedics. Biomater Transl. 2023, 4(3), 166-179.
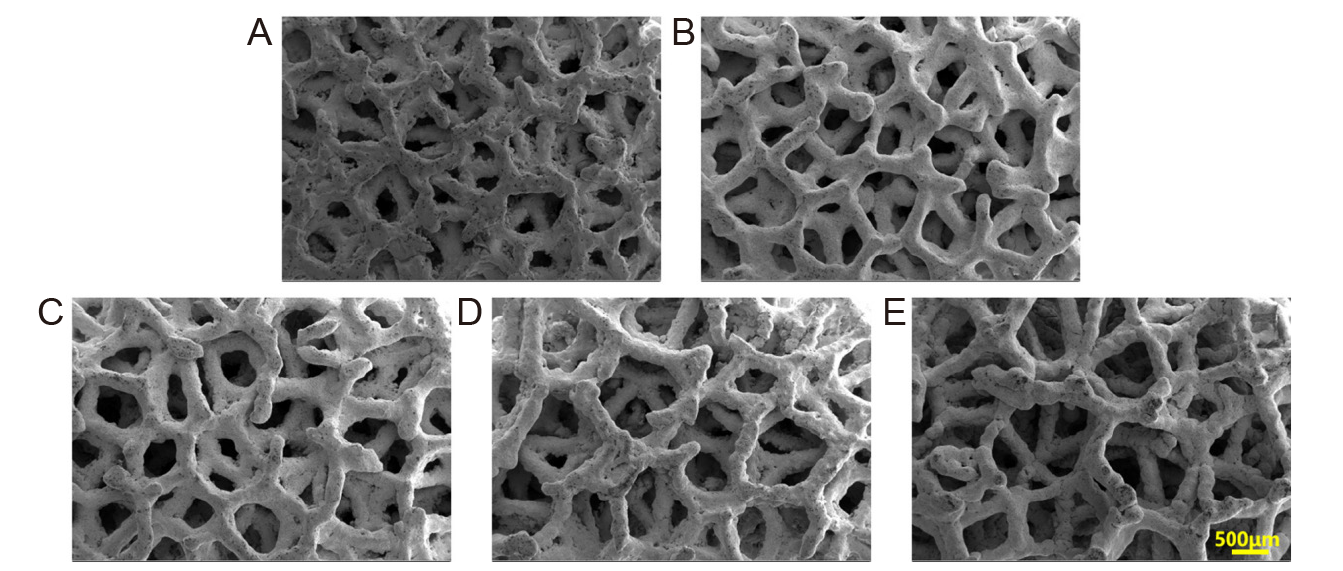
Figure 1. Scanning electron microscopic images of selective laser melting–fabricated porous Ta scaffolds with different porosities. (A–E) 60%, 65%, 70%, 75%, and 80%. Reprinted from Gao et al.9 Scale bar: 500 μm.
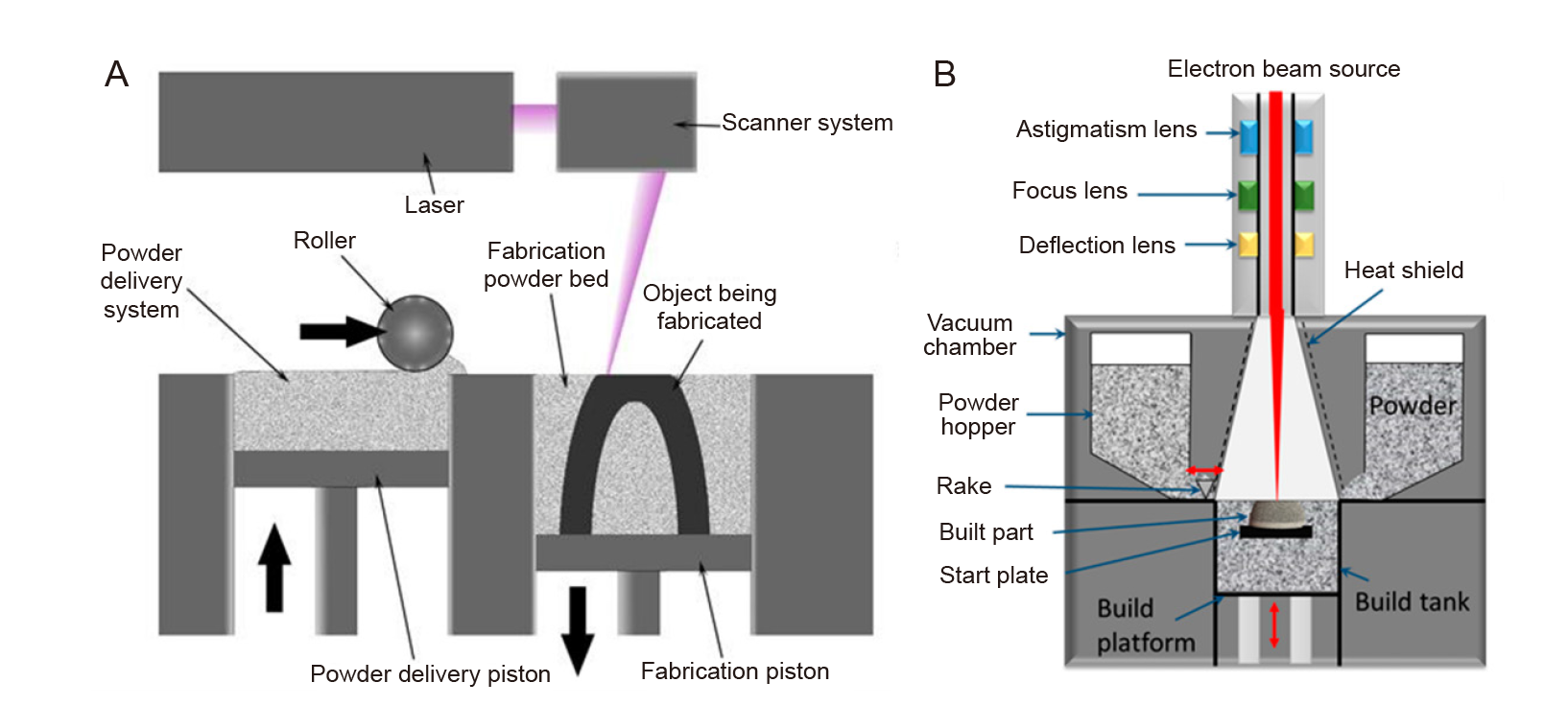
Figure 2. (A) Schematic of the selective laser melting process. Reprinted from Kamran and Farid.19 (B) An electron beam melting machine. Reprinted from Azam et al.20
| Manufacturing method | Porosity (%) | Elastic modulus (GPa) | Compressive strength (MPa) | Compressive yield strength (MPa) | Reference | |
|---|---|---|---|---|---|---|
| Cancellous bone | 50–90 | 0.01–3.0 | – | 2–12 | ||
| Ta | SLM | 79.7 ± 0.2 | 1.22 ± 0.07 | 3.61 ± 0.4 | 12.7 ± 0.6 | |
| 38–65 | 2–20 | – | – | |||
| 68.3 ± 1.1 | 2.34 ± 0.2 | 78.54 ± 9.1 | – | |||
| LENS | 27–55 | 1.5–20 | – | – | ||
| EBM | 75–85 | – | – | 6.8–24 | ||
| LMLMC | 35.48–50 | 2.8–9.0 | 56–480 | – |
Table 1. Mechanical properties of 3D–printed porous Ta
| Manufacturing method | Porosity (%) | Elastic modulus (GPa) | Compressive strength (MPa) | Compressive yield strength (MPa) | Reference | |
|---|---|---|---|---|---|---|
| Cancellous bone | 50–90 | 0.01–3.0 | – | 2–12 | ||
| Ta | SLM | 79.7 ± 0.2 | 1.22 ± 0.07 | 3.61 ± 0.4 | 12.7 ± 0.6 | |
| 38–65 | 2–20 | – | – | |||
| 68.3 ± 1.1 | 2.34 ± 0.2 | 78.54 ± 9.1 | – | |||
| LENS | 27–55 | 1.5–20 | – | – | ||
| EBM | 75–85 | – | – | 6.8–24 | ||
| LMLMC | 35.48–50 | 2.8–9.0 | 56–480 | – |
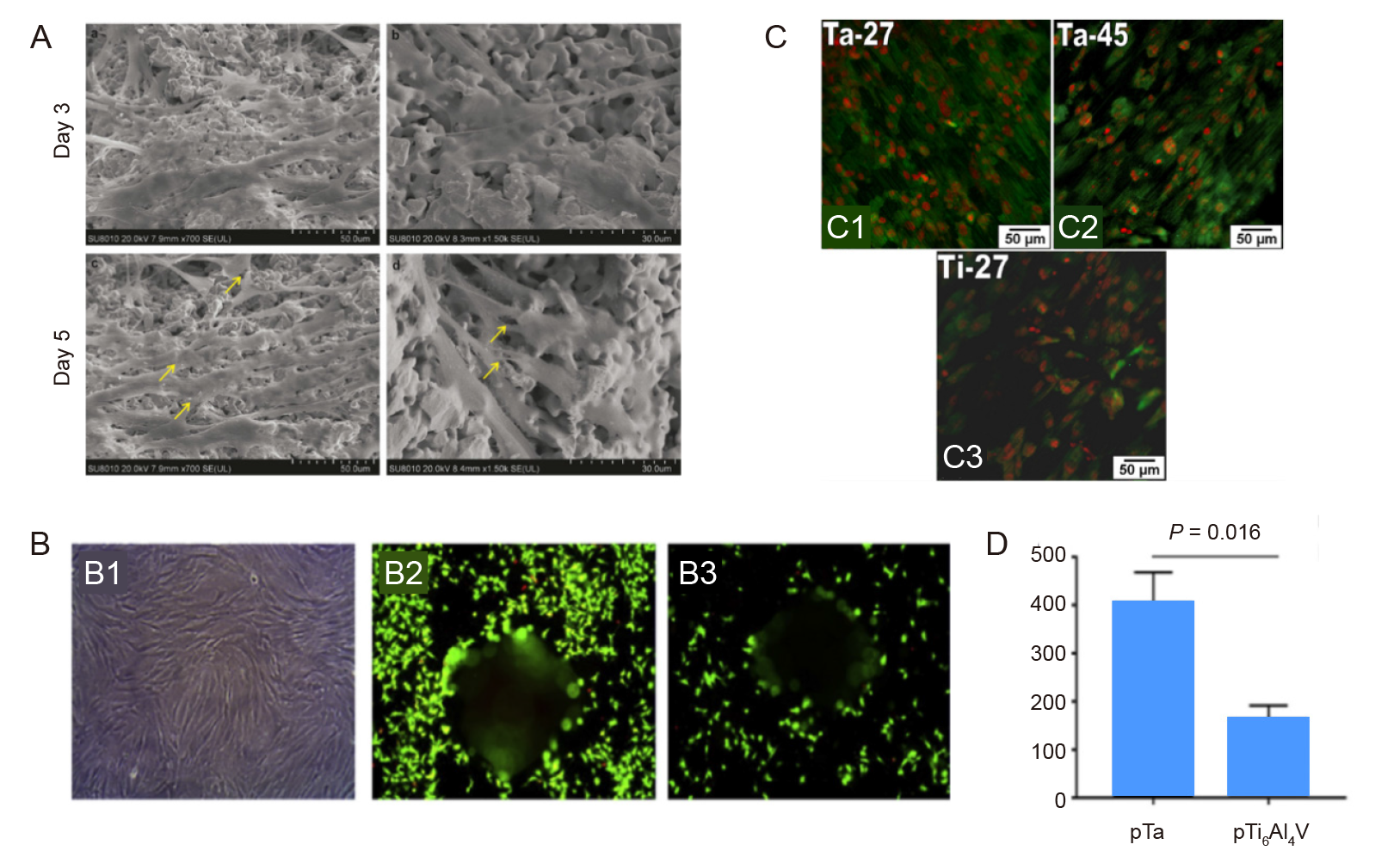
Figure 3. Cell adhesion and proliferation properties on porous Ta. (A) Morphology of mesenchymal stem cells (yellow arrows) cultured for 3 and 5 days. Reprinted from Wang et al.15 (B) Light (B1) and fluorescence microscopic images of live–dead–stained bone marrow mesenchymal stem cells incubated on porous Ta (B2) and Ti6Al4V (B3) for 1 day, and quantification of the adherent cells (B4). Reprinted from Dox et al.33(C) Confocal micrographs of vinculin expression on porous Ta with porosities of 27% (C1) and 45% (C2) and on porous Ti with 27% porosity (C3). Reprinted from Balla et al.32 Copyright ? 2010 Acta Materialia Inc. Scale bars: 50 μm. Ta: tantalum; Ti: titanium.
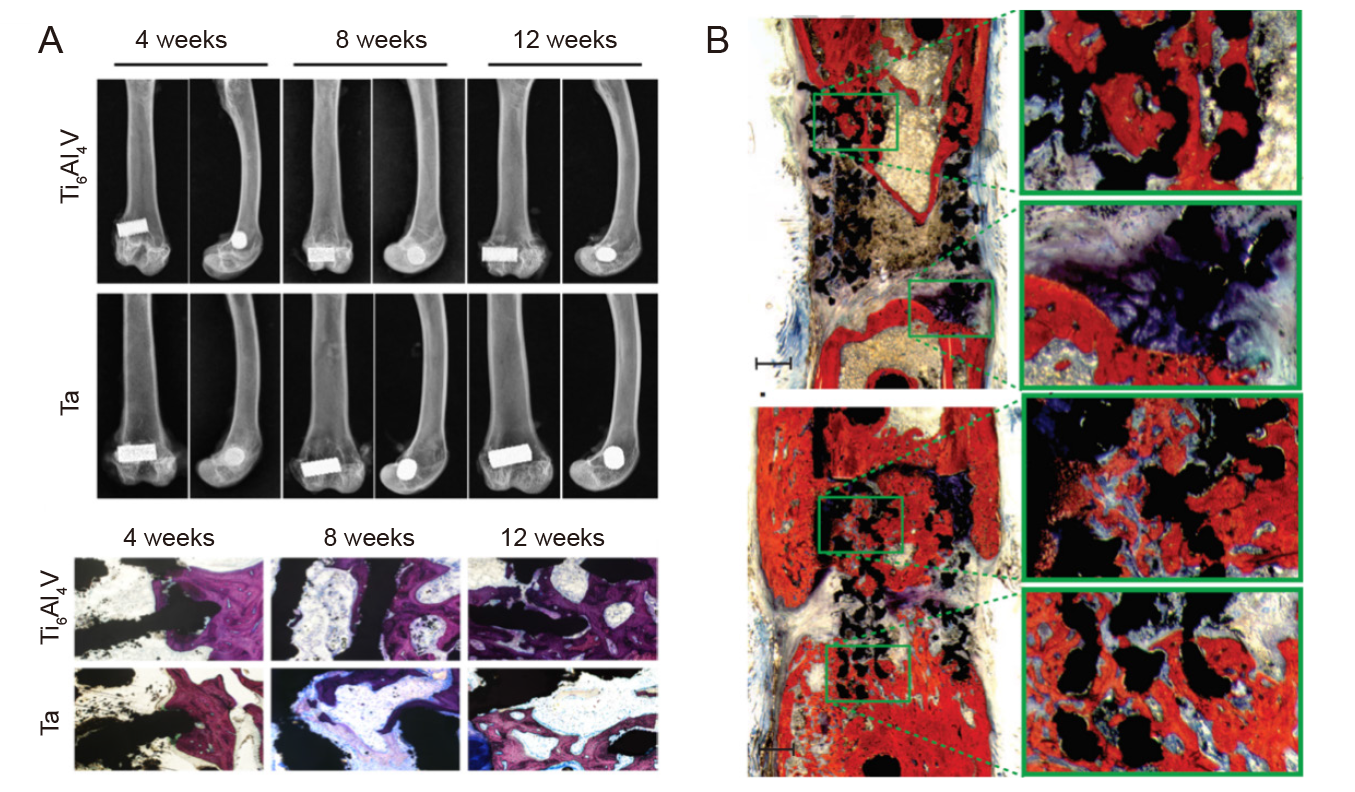
Figure 4. Osseointegration of porous tantalum (Ta) scaffolds. (A) Radiographic and histological images of porous Ta and Ti6Al4V implants at 4, 8, and 12 weeks. Reprinted from Guo et al.14 (B) Histological images of SLM porous Ta after 12 weeks in vivo. Reprinted from Wauthle et al.18 Copyright ? 2014 Acta Materialia Inc.
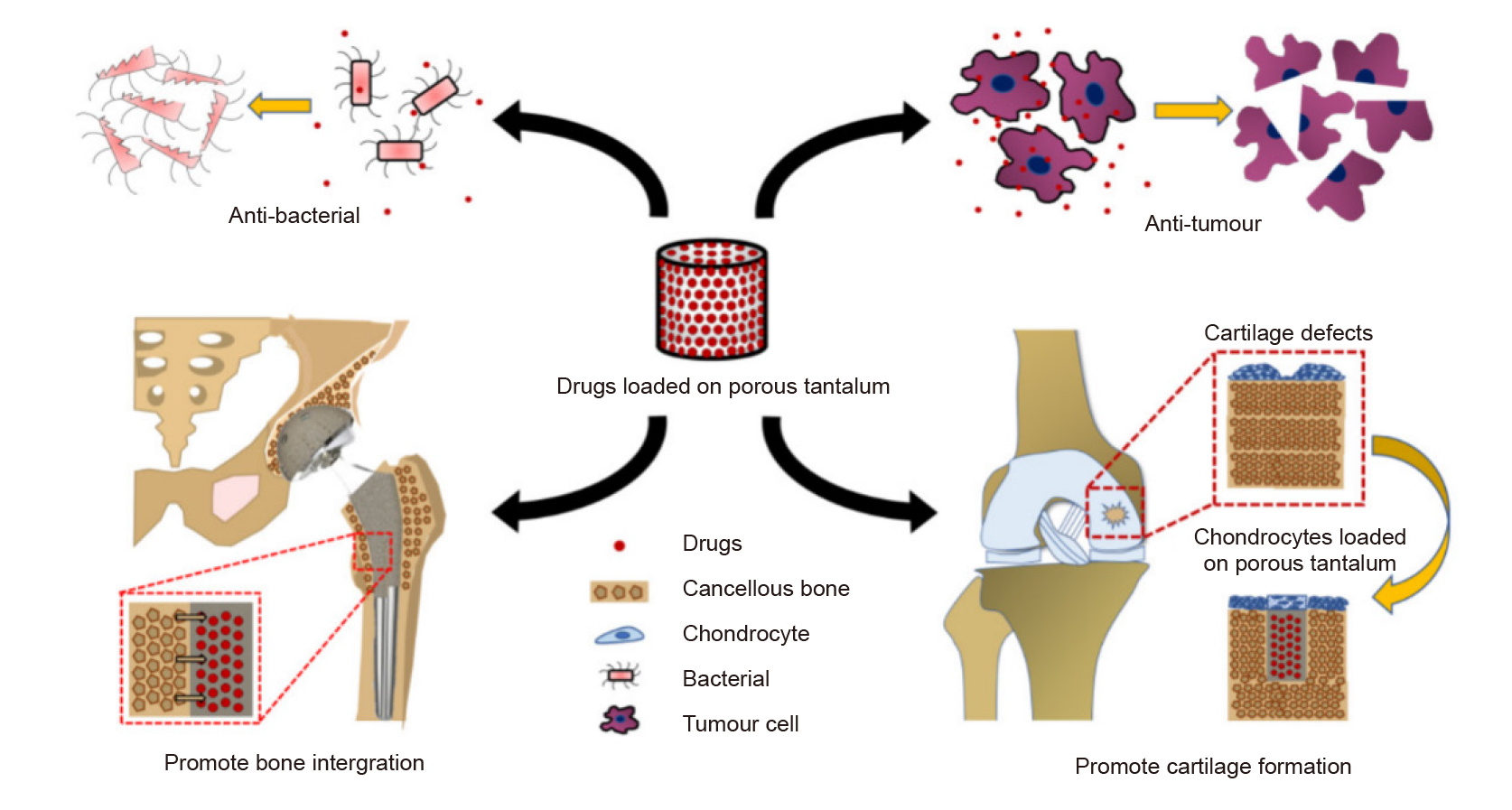
Figure 5. Drugs or cells loaded onto porous tantalum (Ta) for different treatments. Copyright 2021 from Hua et al.70 Reproduced by permission of Taylor and Francis Group, LLC, a division of Informapic.
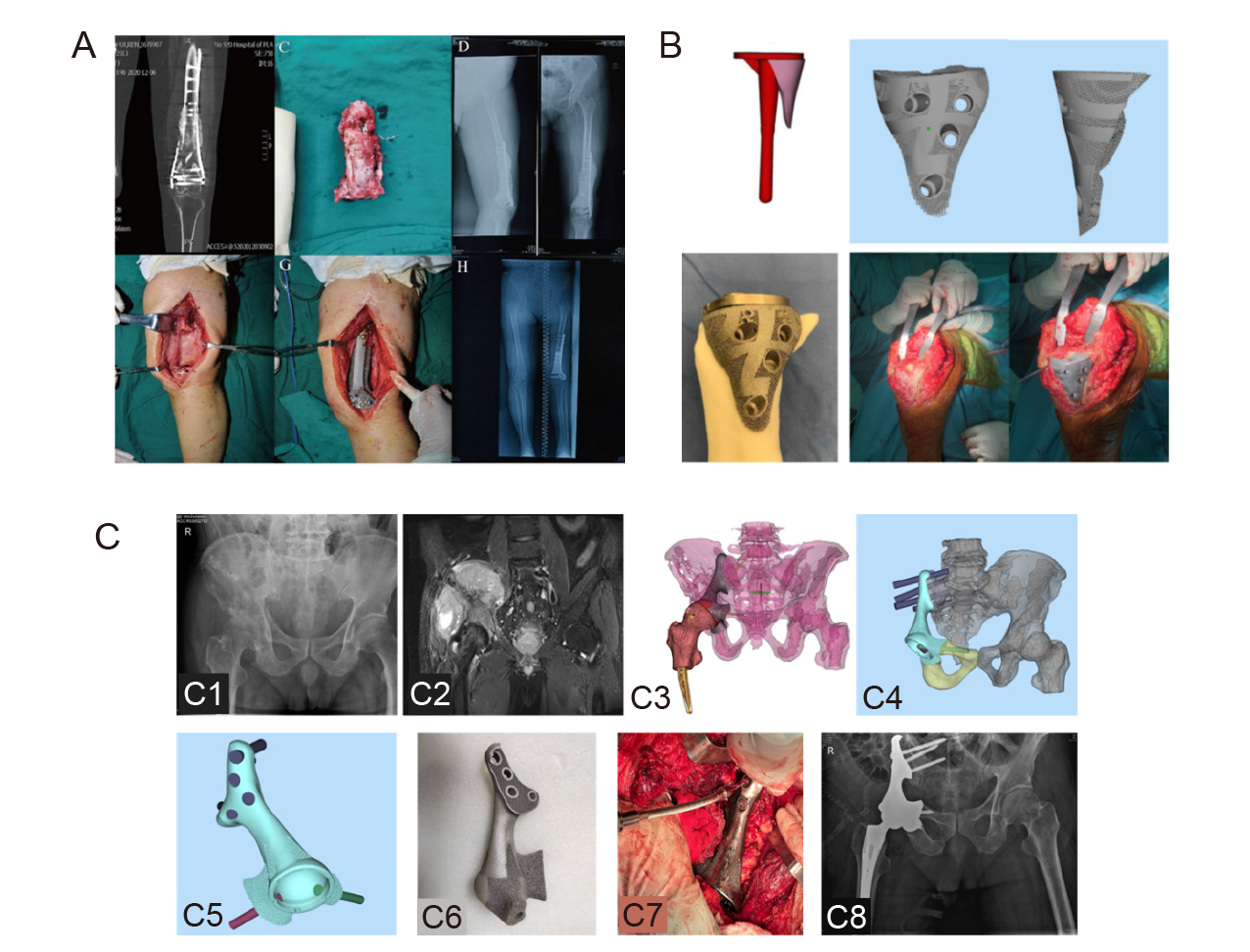
Figure 6. Clinical translation of 3D–printed porous Ta. (A) The clinical application of customized 3D–printed porous Ta scaffolds combined with Masquelet’s induced membrane technique to reconstruct an infected segmental femoral defect. Reprinted from Wu et al.72 (B) Knee reconstruction using 3D–printed porous Ta augmentation in the treatment of a Charcot joint. Reprinted from Hua et al.73 (C) After pelvic tumour resection, hemi–pelvic replacement surgery was performed using 3D–printed porous Ta implants. (C1) Anteroposterior X–ray of the patient’s hip joint showed an uneven density of the right iliac crest. (C2) Coronal MRI showed the extent of tumour invasion. (C3) Preoperative simulation of tumour resection and reconstruction range and location. (C4) Hemi–pelvic prosthesis design to restore the pelvic ring structure. (C5) Lateral view of the hemi–pelvic prosthesis. (C6) 3D–printed hemi–pelvic prosthesis. (C7) Intraoperative prosthesis implantation. (C8) X–ray at 6 months after surgery. C was from the authors’ original study. 3D: three–dimensional; MRI: magnetic resonance imaging; Ta: tantalum.
| 1. | Li, J. J.; Ebied, M.; Xu, J.; Zreiqat, H. Current approaches to bone tissue engineering: the interface between biology and engineering. Adv Healthc Mater. 2018, 7, e1701061. |
| 2. | Ghassemi, T.; Shahroodi, A.; Ebrahimzadeh, M. H.; Mousavian, A.; Movaffagh, J.; Moradi, A. Current concepts in scaffolding for bone tissue engineering. Arch Bone Jt Surg. 2018, 6, 90-99. |
| 3. |
Asri, R. I. M.; Harun, W. S. W.; Samykano, M.; Lah, N. A. C.; Ghani, S. A. C.; Tarlochan, F.; Raza, M. R. Corrosion and surface modification on biocompatible metals: A review. Mater Sci Eng C Mater Biol Appl. 2017, 77, 1261-1274.
doi: 10.1016/j.msec.2017.04.102 URL |
| 4. |
Han, Q.; Wang, C.; Chen, H.; Zhao, X.; Wang, J. Porous tantalum and titanium in orthopedics: a review. ACS Biomater Sci Eng. 2019, 5, 5798-5824.
doi: 10.1021/acsbiomaterials.9b00493 URL |
| 5. |
Cardonne, S. M.; Kumar, P.; Michaluk, C. A.; Schwartz, H. D. Tantalum and its alloys. Int J Refract Met Hard Mater. 1995, 13, 187-194.
doi: 10.1016/0263-4368(95)94023-R URL |
| 6. | Zhang, L.; Haddouti, E. M.; Beckert, H.; Biehl, R.; Pariyar, S.; Rüwald, J. M.; Li, X.; Jaenisch, M.; Burger, C.; Wirtz, D. C.; Kabir, K.; Schildberg, F. A. Investigation of cytotoxicity, oxidative stress, and inflammatory responses of tantalum nanoparticles in THP-1-derived macrophages. Mediators Inflamm. 2020, 2020, 3824593. |
| 7. |
Boyan, B. D.; Lotz, E. M.; Schwartz, Z. * Roughness and hydrophilicity as osteogenic biomimetic surface properties. Tissue Eng Part A. 2017, 23, 1479-1489.
doi: 10.1089/ten.tea.2017.0048 URL |
| 8. |
Chang, Y. Y.; Huang, H. L.; Chen, H. J.; Lai, C. H.; Wen, C. Y. Antibacterial properties and cytocompatibility of tantalum oxide coatings. Surf Coat Technol. 2014, 259, 193-198.
doi: 10.1016/j.surfcoat.2014.03.061 URL |
| 9. |
Gao, H.; Yang, J.; Jin, X.; Qu, X.; Zhang, F.; Zhang, D.; Chen, H.; Wei, H.; Zhang, S.; Jia, W.; Yue, B.; Li, X. Porous tantalum scaffolds: FABRICATION, structure, properties, and orthopedic applications. Mater Des. 2021, 210, 110095.
doi: 10.1016/j.matdes.2021.110095 URL |
| 10. |
Wang, Z.; Wang, C.; Li, C.; Qin, Y.; Zhong, L.; Chen, B.; Li, Z.; Liu, H.; Chang, F.; Wang, J. Analysis of factors influencing bone ingrowth into three-dimensional printed porous metal scaffolds: a review. J Alloys Compd. 2017, 717, 271-285.
doi: 10.1016/j.jallcom.2017.05.079 URL |
| 11. |
Biemond, J. E.; Aquarius, R.; Verdonschot, N.; Buma, P. Frictional and bone ingrowth properties of engineered surface topographies produced by electron beam technology. Arch Orthop Trauma Surg. 2011, 131, 711-718.
doi: 10.1007/s00402-010-1218-9 URL |
| 12. |
Markhoff, J.; Wieding, J.; Weissmann, V.; Pasold, J.; Jonitz-Heincke, A.; Bader, R. Influence of different three-dimensional open porous titanium scaffold designs on human osteoblasts behavior in static and dynamic cell investigations. Materials (Basel). 2015, 8, 5490-5507.
doi: 10.3390/ma8085259 URL |
| 13. |
Wang, H.; Su, K.; Su, L.; Liang, P.; Ji, P.; Wang, C. Comparison of 3D-printed porous tantalum and titanium scaffolds on osteointegration and osteogenesis. Mater Sci Eng C Mater Biol Appl. 2019, 104, 109908.
doi: 10.1016/j.msec.2019.109908 URL |
| 14. |
Guo, Y.; Xie, K.; Jiang, W.; Wang, L.; Li, G.; Zhao, S.; Wu, W.; Hao, Y. In vitro and in vivo study of 3D-printed porous tantalum scaffolds for repairing bone defects. ACS Biomater Sci Eng. 2019, 5, 1123-1133.
doi: 10.1021/acsbiomaterials.8b01094 URL |
| 15. |
Wang, X.; Zhu, Z.; Xiao, H.; Luo, C.; Luo, X.; Lv, F.; Liao, J.; Huang, W. Three-dimensional, multiscale, and interconnected trabecular bone mimic porous tantalum scaffold for bone tissue engineering. ACS Omega. 2020, 5, 22520-22528.
doi: 10.1021/acsomega.0c03127 URL |
| 16. |
Keaveny, T. M.; Morgan, E. F.; Niebur, G. L.; Yeh, O. C. Biomechanics of trabecular bone. Annu Rev Biomed Eng. 2001, 3, 307-333.
doi: 10.1146/bioeng.2001.3.issue-1 URL |
| 17. |
Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005, 26, 5474-5491.
doi: 10.1016/j.biomaterials.2005.02.002 URL |
| 18. |
Wauthle, R.; van der Stok, J.; Amin Yavari, S.; Van Humbeeck, J.; Kruth, J. P.; Zadpoor, A. A.; Weinans, H.; Mulier, M.; Schrooten, J. Additively manufactured porous tantalum implants. Acta Biomater. 2015, 14, 217-225.
doi: 10.1016/j.actbio.2014.12.003 URL |
| 19. | Kamran, S.; Farid, A. Microstructure-tailored stainless steels with high mechanical performance at elevated temperature. In Stainless Steels and Alloys, Zoia, D., ed. IntechOpen: Rijeka, 2018; p Ch. 7. |
| 20. |
Azam, F. I.; Abdul Rani, A. M.; Altaf, K.; Rao, T. V. V. L. N.; Zaharin, H. A. An in-depth review on direct additive manufacturing of metals. IOP Conf Ser Mater Sci Eng. 2018, 328, 012005.
doi: 10.1088/1757-899X/328/1/012005 URL |
| 21. |
Liu, Y.; Bao, C.; Wismeijer, D.; Wu, G. The physicochemical/biological properties of porous tantalum and the potential surface modification techniques to improve its clinical application in dental implantology. Mater Sci Eng C Mater Biol Appl. 2015, 49, 323-329.
doi: 10.1016/j.msec.2015.01.007 URL |
| 22. |
Yang, J.; Jin, X.; Gao, H.; Zhang, D.; Chen, H.; Zhang, S.; Li, X. Additive manufacturing of trabecular tantalum scaffolds by laser powder bed fusion: Mechanical property evaluation and porous structure characterization. Mater Charact. 2020, 170, 110694.
doi: 10.1016/j.matchar.2020.110694 URL |
| 23. |
Zardiackas, L. D.; Parsell, D. E.; Dillon, L. D.; Mitchell, D. W.; Nunnery, L. A.; Poggie, R. Structure, metallurgy, and mechanical properties of a porous tantalum foam. J Biomed Mater Res. 2001, 58, 180-187.
doi: 10.1002/(ISSN)1097-4636 URL |
| 24. |
Stiehl, J. B. Trabecular metal in hip reconstructive surgery. Orthopedics. 2005, 28, 662-670.
doi: 10.3928/0147-7447-20050701-13 URL |
| 25. |
Yang, M.; Ma, H.; Shen, Z.; Huang, Z.; Tian, Q.; Tian, J. Dissimilar material welding of tantalum foil and Q235 steel plate using improved explosive welding technique. Mater Des. 2020, 186, 108348.
doi: 10.1016/j.matdes.2019.108348 URL |
| 26. |
Qian, H.; Lei, T.; Lei, P.; Hu, Y. Additively manufactured tantalum implants for repairing bone defects: a systematic review. Tissue Eng Part B Rev. 2021, 27, 166-180.
doi: 10.1089/ten.teb.2020.0134 URL |
| 27. |
Tang, H. P.; Yang, K.; Jia, L.; He, W. W.; Yang, L.; Zhang, X. Z. Tantalum bone implants printed by selective electron beam manufacturing (SEBM) and their clinical applications. JOM. 2020, 72, 1016-1021.
doi: 10.1007/s11837-020-04016-8 |
| 28. |
Chen, C.; Li, Y.; Zhang, M.; Wang, X.; Zhang, C.; Jing, H. Effect of laser processing parameters on mechanical properties of porous tantalum fabricated by laser multi-layer micro-cladding. Rapid Prototyp J. 2017, 23, 758-770.
doi: 10.1108/RPJ-05-2014-0068 URL |
| 29. |
Maccauro, G.; Iommetti, P. R.; Muratori, F.; Raffaelli, L.; Manicone, P. F.; Fabbriciani, C. An overview about biomedical applications of micron and nano size tantalum. Recent Pat Biotechnol. 2009, 3, 157-165.
doi: 10.2174/187220809789389153 URL |
| 30. |
Cheng, X.; Wan, Q.; Pei, X. Graphene family materials in bone tissue regeneration: perspectives and challenges. Nanoscale Res Lett. 2018, 13, 289.
doi: 10.1186/s11671-018-2694-z |
| 31. | Wei, X.; Zuo, X.; Yang, B. In Sequential recommendation based on long-term and short-term user behavior with self-attention, Knowledge Science, Engineering and Management, Cham, 2019// Douligeris, C.; Karagiannis, D.; Apostolou, D., eds. Springer International Publishing: Cham, 2019; pp 72-83. |
| 32. |
Balla, V. K.; Bodhak, S.; Bose, S.; Bandyopadhyay, A. Porous tantalum structures for bone implants: fabrication, mechanical and in vitro biological properties. Acta Biomater. 2010, 6, 3349-3359.
doi: 10.1016/j.actbio.2010.01.046 URL |
| 33. |
Dou, X.; Wei, X.; Liu, G.; Wang, S.; Lv, Y.; Li, J.; Ma, Z.; Zheng, G.; Wang, Y.; Hu, M.; Yu, W.; Zhao, D. Effect of porous tantalum on promoting the osteogenic differentiation of bone marrow mesenchymal stem cells in vitro through the MAPK/ERK signal pathway. J Orthop Translat. 2019, 19, 81-93.
doi: 10.1016/j.jot.2019.03.006 URL |
| 34. |
Gee, E. C. A.; Eleotério, R.; Bowker, L. M.; Saithna, A.; Hunt, J. A. The influence of tantalum on human cell lineages important for healing in soft-tissue reattachment surgery: an in-vitro analysis. J Exp Orthop. 2019, 6, 40.
doi: 10.1186/s40634-019-0210-8 |
| 35. | Lu, M.; Xu, S.; Lei, Z. X.; Lu, D.; Cao, W.; Huttula, M.; Hou, C. H.; Du, S. H.; Chen, W.; Dai, S. W.; Li, H. M.; Jin, D. D. Application of a novel porous tantalum implant in rabbit anterior lumbar spine fusion model: in vitro and in vivo experiments. Chin Med J (Engl). 2019, 132, 51-62. |
| 36. |
Wei, X.; Liu, B.; Liu, G.; Yang, F.; Cao, F.; Dou, X.; Yu, W.; Wang, B.; Zheng, G.; Cheng, L.; Ma, Z.; Zhang, Y.; Yang, J.; Wang, Z.; Li, J.; Cui, D.; Wang, W.; Xie, H.; Li, L.; Zhang, F.; Lineaweaver, W. C.; Zhao, D. Mesenchymal stem cell-loaded porous tantalum integrated with biomimetic 3D collagen-based scaffold to repair large osteochondral defects in goats. Stem Cell Res Ther. 2019, 10, 72.
doi: 10.1186/s13287-019-1176-2 |
| 37. |
Wang, Y.; Wei, R.; Subedi, D.; Jiang, H.; Yan, J.; Li, J. Tantalum fusion device in anterior cervical discectomy and fusion for treatment of cervical degeneration disease: a systematic review and meta-analysis. Clin Spine Surg. 2020, 33, 111-119.
doi: 10.1097/BSD.0000000000000875 URL |
| 38. |
Temponi, E. F.; Souza, P. E. A.; Souto, G. R.; Magalhães, L. M. D.; Dutra, W. O.; Gollob, K. J.; Silva, T. A.; Soares, R. V. Effect of porous tantalum on the biological response of human peripheral mononuclear cells exposed to Porphyromonas gingivalis. J Investig Clin Dent. 2019, 10, e12472.
doi: 10.1111/jicd.v10.4 URL |
| 39. |
Sagomonyants, K. B.; Hakim-Zargar, M.; Jhaveri, A.; Aronow, M. S.; Gronowicz, G. Porous tantalum stimulates the proliferation and osteogenesis of osteoblasts from elderly female patients. J Orthop Res. 2011, 29, 609-616.
doi: 10.1002/jor.v29.4 URL |
| 40. |
Wang, Q.; Zhang, H.; Li, Q.; Ye, L.; Gan, H.; Liu, Y.; Wang, H.; Wang, Z. Biocompatibility and osteogenic properties of porous tantalum. Exp Ther Med. 2015, 9, 780-786.
doi: 10.3892/etm.2015.2208 URL |
| 41. |
Jonitz, A.; Lochner, K.; Lindner, T.; Hansmann, D.; Marrot, A.; Bader, R. Oxygen consumption, acidification and migration capacity of human primary osteoblasts within a three-dimensional tantalum scaffold. J Mater Sci Mater Med. 2011, 22, 2089-2095.
doi: 10.1007/s10856-011-4384-6 URL |
| 42. |
Wang, F.; Li, C.; Zhang, S.; Liu, H. Tantalum coated on titanium dioxide nanotubes by plasma spraying enhances cytocompatibility for dental implants. Surf Coat Technol. 2020, 382, 125161.
doi: 10.1016/j.surfcoat.2019.125161 URL |
| 43. | Qian, H.; Lei, T.; Ye, Z.; Hu, Y.; Lei, P. From the performance to the essence: the biological mechanisms of how tantalum contributes to osteogenesis. Biomed Res Int. 2020, 2020, 5162524. |
| 44. |
Wang, X.; Ning, B.; Pei, X. Tantalum and its derivatives in orthopedic and dental implants: Osteogenesis and antibacterial properties. Colloids Surf B Biointerfaces. 2021, 208, 112055.
doi: 10.1016/j.colsurfb.2021.112055 URL |
| 45. |
Horandghadim, N.; Khalil-Allafi, J.; Urgen, M. Effect of Ta(2)O(5) content on the osseointegration and cytotoxicity behaviors in hydroxyapatite-Ta(2)O(5) coatings applied by EPD on superelastic NiTi alloys. Mater Sci Eng C Mater Biol Appl. 2019, 102, 683-695.
doi: 10.1016/j.msec.2019.05.005 URL |
| 46. |
Mei, S.; Yang, L.; Pan, Y.; Wang, D.; Wang, X.; Tang, T.; Wei, J. Influences of tantalum pentoxide and surface coarsening on surface roughness, hydrophilicity, surface energy, protein adsorption and cell responses to PEEK based biocomposite. Colloids Surf B Biointerfaces. 2019, 174, 207-215.
doi: 10.1016/j.colsurfb.2018.10.081 URL |
| 47. |
Li, R.; Liu, G.; Yang, L.; Qing, Y.; Tang, X.; Guo, D.; Zhang, K.; Qin, Y. Tantalum boride as a biocompatible coating to improve osteogenesis of the bionano interface. J Biomed Mater Res A. 2020, 108, 1726-1735.
doi: 10.1002/jbm.a.v108.8 URL |
| 48. |
Rouwkema, J.; Rivron, N. C.; van Blitterswijk, C. A. Vascularization in tissue engineering. Trends Biotechnol. 2008, 26, 434-441.
doi: 10.1016/j.tibtech.2008.04.009 URL |
| 49. |
Cheng, S.; Ke, J.; Yao, M.; Shao, H.; Zhou, J.; Wang, M.; Ji, X.; Zhong, G.; Peng, F.; Ma, L.; Zhang, Y. Improved osteointegration and angiogenesis of strontium-incorporated 3D-printed tantalum scaffold via bioinspired polydopamine coating. J Mater Sci Technol. 2021, 69, 106-118.
doi: 10.1016/j.jmst.2020.08.017 URL |
| 50. |
Zhang, Y.; Zheng, Y.; Li, Y.; Wang, L.; Bai, Y.; Zhao, Q.; Xiong, X.; Cheng, Y.; Tang, Z.; Deng, Y.; Wei, S. Tantalum nitride-decorated titanium with enhanced resistance to microbiologically induced corrosion and mechanical property for dental application. PLoS One. 2015, 10, e0130774.
doi: 10.1371/journal.pone.0130774 URL |
| 51. |
Zhu, Y.; Gu, Y.; Qiao, S.; Zhou, L.; Shi, J.; Lai, H. Bacterial and mammalian cells adhesion to tantalum-decorated micro-/nano-structured titanium. J Biomed Mater Res A. 2017, 105, 871-878.
doi: 10.1002/jbm.v105.3 URL |
| 52. | Tokarski, A. T.; Novack, T. A.; Parvizi, J. Is tantalum protective against infection in revision total hip arthroplasty? Bone Joint J. 2015, 97-B, 45-49. |
| 53. | Subramani, K.; Jung, R. E.; Molenberg, A.; Hammerle, C. H. Biofilm on dental implants: a review of the literature. Int J Oral Maxillofac Implants. 2009, 24, 616-626. |
| 54. |
Harrison, P. L.; Harrison, T.; Stockley, I.; Smith, T. J. Does tantalum exhibit any intrinsic antimicrobial or antibiofilm properties? Bone Joint J. 2017, 99-B, 1153-1156.
doi: 10.1302/0301-620X.99B9.BJJ-2016-1309.R1 URL |
| 55. |
Wahl, P.; Sprecher, C. M.; Brüning, C.; Meier, C.; Milz, S.; Gautier, E.; Fintan Moriarty, T. Successful bony integration of a porous tantalum implant despite longlasting and ongoing infection: Histologic workup of an explanted shoulder prosthesis. J Biomed Mater Res B Appl Biomater. 2018, 106, 2924-2931.
doi: 10.1002/jbm.b.v106.8 URL |
| 56. |
Yang, C.; Li, J.; Zhu, C.; Zhang, Q.; Yu, J.; Wang, J.; Wang, Q.; Tang, J.; Zhou, H.; Shen, H. Advanced antibacterial activity of biocompatible tantalum nanofilm via enhanced local innate immunity. Acta Biomater. 2019, 89, 403-418.
doi: 10.1016/j.actbio.2019.03.027 URL |
| 57. |
Matharu, G. S.; Judge, A.; Murray, D. W.; Pandit, H. G. Do Trabecular metal acetabular components reduce the risk of rerevision after revision THA performed for periprosthetic joint infection? A study using the NJR data set. Clin Orthop Relat Res. 2019, 477, 1382-1389.
doi: 10.1097/CORR.0000000000000570 URL |
| 58. |
Wang, Q.; Zhang, H.; Gan, H.; Wang, H.; Li, Q.; Wang, Z. Application of combined porous tantalum scaffolds loaded with bone morphogenetic protein 7 to repair of osteochondral defect in rabbits. Int Orthop. 2018, 42, 1437-1448.
doi: 10.1007/s00264-018-3800-7 |
| 59. | Bandyopadhyay, A.; Mitra, I.; Shivaram, A.; Dasgupta, N.; Bose, S. Direct comparison of additively manufactured porous titanium and tantalum implants towards in vivo osseointegration. Addit Manuf. 2019, 28, 259-266. |
| 60. |
Wang, Q.; Qiao, Y.; Cheng, M.; Jiang, G.; He, G.; Chen, Y.; Zhang, X.; Liu, X. Tantalum implanted entangled porous titanium promotes surface osseointegration and bone ingrowth. Sci Rep. 2016, 6, 26248.
doi: 10.1038/srep26248 |
| 61. |
Wei, X.; Zhao, D.; Wang, B.; Wang, W.; Kang, K.; Xie, H.; Liu, B.; Zhang, X.; Zhang, J.; Yang, Z. Tantalum coating of porous carbon scaffold supplemented with autologous bone marrow stromal stem cells for bone regeneration in vitro and in vivo. Exp Biol Med (Maywood). 2016, 241, 592-602.
doi: 10.1177/1535370216629578 URL |
| 62. | Fraser, D.; Mendonca, G.; Sartori, E.; Funkenbusch, P.; Ercoli, C.; Meirelles, L. Bone response to porous tantalum implants in a gap-healing model. Clin Oral Implants Res. 2019, 30, 156-168. |
| 63. |
Hacking, S. A.; Bobyn, J. D.; Toh, K.; Tanzer, M.; Krygier, J. J. Fibrous tissue ingrowth and attachment to porous tantalum. J Biomed Mater Res. 2000, 52, 631-638.
doi: 10.1002/(ISSN)1097-4636 URL |
| 64. |
Bobyn, J. D.; Stackpool, G. J.; Hacking, S. A.; Tanzer, M.; Krygier, J. J. Characteristics of bone ingrowth and interface mechanics of a new porous tantalum biomaterial. J Bone Joint Surg Br. 1999, 81, 907-914.
doi: 10.1302/0301-620X.81B5.0810907 URL |
| 65. |
Sautet, P.; Parratte, S.; Mékidèche, T.; Abdel, M. P.; Flécher, X.; Argenson, J. N.; Ollivier, M. Antibiotic-loaded tantalum may serve as an antimicrobial delivery agent. Bone Joint J. 2019, 101-B, 848-851.
doi: 10.1302/0301-620X.101B7.BJJ-2018-1206.R1 URL |
| 66. | Guo, X.; Chen, M.; Feng, W.; Liang, J.; Zhao, H.; Tian, L.; Chao, H.; Zou, X. Electrostatic self-assembly of multilayer copolymeric membranes on the surface of porous tantalum implants for sustained release of doxorubicin. Int J Nanomedicine. 2011, 6, 3057-3064. |
| 67. |
Tanzer, M.; Karabasz, D.; Krygier, J. J.; Cohen, R.; Bobyn, J. D. The Otto Aufranc Award: bone augmentation around and within porous implants by local bisphosphonate elution. Clin Orthop Relat Res. 2005, 441, 30-39.
doi: 10.1097/01.blo.0000194728.62996.2d URL |
| 68. |
Zhou, R.; Xu, W.; Chen, F.; Qi, C.; Lu, B. Q.; Zhang, H.; Wu, J.; Qian, Q. R.; Zhu, Y. J. Amorphous calcium phosphate nanospheres/polylactide composite coated tantalum scaffold: facile preparation, fast biomineralization and subchondral bone defect repair application. Colloids Surf B Biointerfaces. 2014, 123, 236-245.
doi: 10.1016/j.colsurfb.2014.09.021 URL |
| 69. | Mrosek, E. H.; Schagemann, J. C.; Chung, H. W.; Fitzsimmons, J. S.; Yaszemski, M. J.; Mardones, R. M.; O’Driscoll, S. W.; Reinholz, G. G. Porous tantalum and poly-epsilon-caprolactone biocomposites for osteochondral defect repair: preliminary studies in rabbits. J Orthop Res. 2010, 28, 141-148. |
| 70. |
Hua, L.; Lei, T.; Qian, H.; Zhang, Y.; Hu, Y.; Lei, P. 3D-printed porous tantalum: recent application in various drug delivery systems to repair hard tissue defects. Expert Opin Drug Deliv. 2021, 18, 625-634.
doi: 10.1080/17425247.2021.1860015 URL |
| 71. |
Rodeo, S. A.; Delos, D.; Weber, A.; Ju, X.; Cunningham, M. E.; Fortier, L.; Maher, S. What’s new in orthopaedic research. J Bone Joint Surg Am. 2010, 92, 2491-2501.
doi: 10.2106/JBJS.J.01174 URL |
| 72. |
Wu, Y.; Shi, X.; Zi, S.; Li, M.; Chen, S.; Zhang, C.; Xu, Y. The clinical application of customized 3D-printed porous tantalum scaffolds combined with Masquelet’s induced membrane technique to reconstruct infective segmental femoral defect. J Orthop Surg Res. 2022, 17, 479.
doi: 10.1186/s13018-022-03371-3 |
| 73. |
Hua, L.; Lei, P.; Hu, Y. Knee Reconstruction Using 3D-Printed Porous Tantalum Augment in the Treatment of Charcot Joint. Orthop Surg. 2022, 14, 3125-3128.
doi: 10.1111/os.v14.11 URL |
| 74. | Cheng, L.; Zhao, D.; Yang, L.; Li, J.; Ma, Z.; Wang, Z.; Tian, F.; Tian, S. The application of 3D printed customized porous tantalum acetabular patch for adult DDH hip reconstruction. Zhonghua Guke Zazhi. 2018, 38, 650-657. |
| 75. | Veillette, C. J.; Mehdian, H.; Schemitsch, E. H.; McKee, M. D. Survivorship analysis and radiographic outcome following tantalum rod insertion for osteonecrosis of the femoral head. J Bone Joint Surg Am. 2006, 88 Suppl 3, 48-55. |
| 76. |
Liu, G.; Wang, J.; Yang, S.; Xu, W.; Ye, S.; Xia, T. Effect of a porous tantalum rod on early and intermediate stages of necrosis of the femoral head. Biomed Mater. 2010, 5, 065003.
doi: 10.1088/1748-6041/5/6/065003 URL |
| 77. |
Liu, Z. H.; Guo, W. S.; Li, Z. R.; Cheng, L. M.; Zhang, Q. D.; Yue, D. B.; Shi, Z. C.; Wang, B. L.; Sun, W.; Zhang, N. F. Porous tantalum rods for treating osteonecrosis of the femoral head. Genet Mol Res. 2014, 13, 8342-8352.
doi: 10.4238/2014.October.20.10 URL |
| 78. |
Zhang, X.; Wang, J.; Xiao, J.; Shi, Z. Early failures of porous tantalum osteonecrosis implants: a case series with retrieval analysis. Int Orthop. 2016, 40, 1827-1834.
doi: 10.1007/s00264-015-3087-x URL |
| 79. |
Tanzer, M.; Bobyn, J. D.; Krygier, J. J.; Karabasz, D. Histopathologic retrieval analysis of clinically failed porous tantalum osteonecrosis implants. J Bone Joint Surg Am. 2008, 90, 1282-1289.
doi: 10.2106/JBJS.F.00847 URL |
| 80. |
Zhao, D.; Zhang, Y.; Wang, W.; Liu, Y.; Li, Z.; Wang, B.; Yu, X. Tantalum rod implantation and vascularized iliac grafting for osteonecrosis of the femoral head. Orthopedics. 2013, 36, 789-795.
doi: 10.3928/01477447-20130523-26 URL |
| 81. | Zhao, D.; Liu, B.; Wang, B.; Yang, L.; Xie, H.; Huang, S.; Zhang, Y.; Wei, X. Autologous bone marrow mesenchymal stem cells associated with tantalum rod implantation and vascularized iliac grafting for the treatment of end-stage osteonecrosis of the femoral head. Biomed Res Int. 2015, 2015, 240506. |
| 82. |
Sculco, T. P. The acetabular component: an elliptical monoblock alternative. J Arthroplasty. 2002, 17, 118-120.
doi: 10.1054/arth.2002.32690 URL |
| 83. |
Macheras, G.; Kateros, K.; Kostakos, A.; Koutsostathis, S.; Danomaras, D.; Papagelopoulos, P. J. Eight- to ten-year clinical and radiographic outcome of a porous tantalum monoblock acetabular component. J Arthroplasty. 2009, 24, 705-709.
doi: 10.1016/j.arth.2008.06.020 URL |
| 84. | Macheras, G. A.; Papagelopoulos, P. J.; Kateros, K.; Kostakos, A. T.; Baltas, D.; Karachalios, T. S. Radiological evaluation of the metal-bone interface of a porous tantalum monoblock acetabular component. J Bone Joint Surg Br. 2006, 88, 304-309. |
| 85. |
Garbuz, D. S. Revision total hip: a novel modular cementless acetabular system for reconstruction of severe acetabular bone loss. Oper Tech Orthop. 2004, 14, 117-120.
doi: 10.1053/j.oto.2004.04.007 URL |
| 86. |
Sporer, S. M.; Paprosky, W. G. The use of a trabecular metal acetabular component and trabecular metal augment for severe acetabular defects. J Arthroplasty. 2006, 21, 83-86.
doi: 10.1016/j.arth.2006.05.008 URL |
| 87. |
Malkani, A. L.; Price, M. R.; Crawford, C. H.,3rd; Baker, D. L. Acetabular component revision using a porous tantalum biomaterial: a case series. J Arthroplasty. 2009, 24, 1068-1073.
doi: 10.1016/j.arth.2008.07.008 URL |
| 88. |
Löchel, J.; Janz, V.; Hipfl, C.; Perka, C.; Wassilew, G. I. Reconstruction of acetabular defects with porous tantalum shells and augments in revision total hip arthroplasty at ten-year follow-up. Bone Joint J. 2019, 101-b, 311-316.
doi: 10.1302/0301-620X.101B3.BJJ-2018-0959.R1 URL |
| 89. |
Hu, B.; Chen, Y.; Zhu, H.; Wu, H.; Yan, S. Cementless porous tantalum monoblock tibia vs cemented modular tibia in primary total knee arthroplasty: a meta-analysis. J Arthroplasty. 2017, 32, 666-674.
doi: 10.1016/j.arth.2016.09.011 URL |
| 90. |
Hayakawa, K.; Date, H.; Tsujimura, S.; Nojiri, S.; Yamada, H.; Nakagawa, K. Mid-term results of total knee arthroplasty with a porous tantalum monoblock tibial component. Knee. 2014, 21, 199-203.
doi: 10.1016/j.knee.2013.06.004 URL |
| 91. | Wang, F.; Chen, H.; Yang, P.; Muheremu, A.; He, P.; Fan, H.; Yang, L. Three-dimensional printed porous tantalum prosthesis for treating inflammation after total knee arthroplasty in one-stage surgery - a case report. J Int Med Res. 2020, 48, 300060519891280. |
| 92. |
De Martino, I.; D’Apolito, R.; Sculco, P. K.; Poultsides, L. A.; Gasparini, G. Total knee arthroplasty using cementless porous tantalum monoblock tibial component: a minimum 10-year follow-up. J Arthroplasty. 2016, 31, 2193-2198.
doi: 10.1016/j.arth.2016.03.057 URL |
| 93. | Sambaziotis, C.; Lovy, A. J.; Koller, K. E.; Bloebaum, R. D.; Hirsh, D. M.; Kim, S. J. Histologic retrieval analysis of a porous tantalum metal implant in an infected primary total knee arthroplasty. J Arthroplasty. 2012, 27, 1413.e5-9. |
| 94. |
Levine, B.; Sporer, S.; Della Valle, C. J.; Jacobs, J. J.; Paprosky, W. Porous tantalum in reconstructive surgery of the knee: a review. J Knee Surg. 2007, 20, 185-194.
doi: 10.1055/s-0030-1248041 URL |
| 95. |
Potter, G. D.,3rd; Abdel, M. P.; Lewallen, D. G.; Hanssen, A. D. Midterm results of porous tantalum femoral cones in revision total knee arthroplasty. J Bone Joint Surg Am. 2016, 98, 1286-1291.
doi: 10.2106/JBJS.15.00874 URL |
| 96. |
Kamath, A. F.; Lewallen, D. G.; Hanssen, A. D. Porous tantalum metaphyseal cones for severe tibial bone loss in revision knee arthroplasty: a five to nine-year follow-up. J Bone Joint Surg Am. 2015, 97, 216-223.
doi: 10.2106/JBJS.N.00540 URL |
| 97. |
Rodríguez-Merchán, E. C.; Gómez-Cardero, P.; Encinas-Ullán, C. A. Management of bone loss in revision total knee arthroplasty: therapeutic options and results. EFORT Open Rev. 2021, 6, 1073-1086.
doi: 10.1302/2058-5241.6.210007 URL |
| 98. |
Kamath, A. F.; Gee, A. O.; Nelson, C. L.; Garino, J. P.; Lotke, P. A.; Lee, G. C. Porous tantalum patellar components in revision total knee arthroplasty minimum 5-year follow-up. J Arthroplasty. 2012, 27, 82-87.
doi: 10.1016/j.arth.2011.04.024 URL |
| 99. |
Hanc, M.; Fokter, S. K.; Vogrin, M.; Molicnik, A.; Recnik, G. Porous tantalum in spinal surgery: an overview. Eur J Orthop Surg Traumatol. 2016, 26, 1-7.
doi: 10.1007/s00590-015-1654-x URL |
| 100. |
Fernández-Fairen, M.; Alvarado, E.; Torres, A. Eleven-year follow-up of two cohorts of patients comparing stand-alone porous tantalum cage versus autologous bone graft and plating in anterior cervical fusions. World Neurosurg. 2019, 122, e156-e167.
doi: 10.1016/j.wneu.2018.09.160 URL |
| 101. |
Mastronardi, L.; Roperto, R.; Cacciotti, G.; Calvosa, F. Anterior cervical fusion with stand-alone trabecular metal cages to treat cervical myelopathy caused by degenerative disk disease. observations in 88 cases with minimum 12-month follow-up. J Neurol Surg A Cent Eur Neurosurg. 2018, 79, 496-501.
doi: 10.1055/s-0038-1642008 URL |
| 102. |
Lebhar, J.; Kriegel, P.; Chatellier, P.; Breton, Y.; Ropars, M.; Huten, D. Tantalum implants for posterior lumbar interbody fusion: a safe method at medium-term follow-up? Orthop Traumatol Surg Res. 2020, 106, 269-274.
doi: 10.1016/j.otsr.2019.10.028 URL |
| 103. |
Li, N.; Hu, W. Q.; Xin, W. Q.; Li, Q. F.; Tian, P. Comparison between porous tantalum metal implants and autograft in anterior cervical discectomy and fusion: a meta-analysis. J Comp Eff Res. 2019, 8, 511-521.
doi: 10.2217/cer-2018-0107 URL |
| 104. |
Adukia, V.; Mangwani, J.; Issac, R.; Hussain, S.; Parker, L. Current concepts in the management of ankle arthritis. J Clin Orthop Trauma. 2020, 11, 388-398.
doi: 10.1016/j.jcot.2020.03.020 URL |
| 105. |
Horisberger, M.; Paul, J.; Wiewiorski, M.; Henninger, H. B.; Khalifa, M. S.; Barg, A.; Valderrabano, V. Commercially available trabecular metal ankle interpositional spacer for tibiotalocalcaneal arthrodesis secondary to severe bone loss of the ankle. J Foot Ankle Surg. 2014, 53, 383-387.
doi: 10.1053/j.jfas.2013.11.004 URL |
| 106. |
Tiusanen, H.; Kormi, S.; Kohonen, I.; Saltychev, M. Results of trabecular-metal total ankle arthroplasties with transfibular approach. Foot Ankle Int. 2020, 41, 411-418.
doi: 10.1177/1071100719894929 URL |
| 107. |
Sundet, M.; Johnsen, E.; Eikvar, K. H.; Eriksen, M. L. Retrograde nailing, trabecular metal implant and use of bone marrow aspirate concentrate after failed ankle joint replacement. Foot Ankle Surg. 2021, 27, 123-128.
doi: 10.1016/j.fas.2020.03.003 URL |
| 108. |
Onggo, J. R.; Nambiar, M.; Phan, K.; Hickey, B.; Galvin, M.; Bedi, H. Outcome after total ankle arthroplasty with a minimum of five years follow-up: a systematic review and meta-analysis. Foot Ankle Surg. 2020, 26, 556-563.
doi: 10.1016/j.fas.2019.07.006 URL |
| 109. | Epperson, R. T.; Barg, A.; Williams, D. L.; Saltzman, C. L. Histological analysis of a retrieved porous tantalum total ankle replacement: a case report. JBJS Case Connect. 2020, 10, e0379. |
| 110. |
Zhao, D. W.; Ma, Z. J.; Wang, T. N.; Liu, B. Y. Biocompatible porous tantalum metal plates in the treatment of tibial fracture. Orthop Surg. 2019, 11, 325-329.
doi: 10.1111/os.2019.11.issue-2 URL |
| 111. |
Li, F.; Jiang, C. Trabecular metal shoulder prosthesis in the treatment of complex proximal humeral fractures. Int Orthop. 2013, 37, 2259-2264.
doi: 10.1007/s00264-013-2061-8 URL |
| 112. |
Chen, R. E.; Mannava, S.; Miller, R. J.; Voloshin, I. Comparison of mid-term outcomes of total shoulder arthroplasty for B2 and A glenoids treated with trabecular metal glenoid components. Semin Arthroplasty. 2020, 30, 326-332.
doi: 10.1053/j.sart.2020.09.008 URL |
| 113. |
Sasanuma, H.; Iijima, Y.; Saito, T.; Kanaya, Y.; Yano, Y.; Fukushima, T.; Nakama, S.; Takeshita, K. Clinical results of reverse shoulder arthroplasty for comminuted proximal humerus fractures in elderly patients: a comparison between nonporous stems versus trabecular metal stems. JSES Int. 2020, 4, 952-958.
doi: 10.1016/j.jseint.2020.08.010 URL |
| [1] | Elena Giusto, Gordon Blunn, Roberta Ferro de Godoy, Chaozong Liu, Catherine Pendegrass. Optimising soft tissue in-growth in vivo in additive layer manufactured osseointegrated transcutaneous implants [J]. Biomaterials Translational, 2022, 3(4): 243-249. |
| [2] | Changning Sun, Jianfeng Kang, Chuncheng Yang, Jibao Zheng, Yanwen Su, Enchun Dong, Yingjie Liu, Siqi Yao, Changquan Shi, Huanhao Pang, Jiankang He, Ling Wang, Chaozong Liu, Jianhua Peng, Liang Liu, Yong Jiang, Dichen Li. Additive manufactured polyether-ether-ketone implants for orthopaedic applications: a narrative review [J]. Biomaterials Translational, 2022, 3(2): 116-133. |
| [3] | Seyed Ataollah Naghavi, Changning Sun, Mahbubeh Hejazi, Maryam Tamaddon, Jibao Zheng, Leilei Wang, Chenrui Zhang, Swastina Nath Varma, Dichen Li, Mehran Moazen, Ling Wang, Chaozong Liu. On the mechanical aspect of additive manufactured polyether-ether-ketone scaffold for repair of large bone defects [J]. Biomaterials Translational, 2022, 3(2): 142-151. |
| [4] | Ying Luo, Jue Wang, Michael Tim Yun Ong, Patrick Shu-hang Yung, Jiali Wang, Ling Qin. Update on the research and development of magnesium-based biodegradable implants and their clinical translation in orthopaedics [J]. Biomaterials Translational, 2021, 2(3): 188-196. |
| [5] | Jing Long, Bin Teng, Wei Zhang, Long Li, Ming Zhang, Yingqi Chen, Zhenyu Yao, Xiangbo Meng, Xinluan Wang, Ling Qin, Yuxiao Lai. Preclinical evaluation of acute systemic toxicity of magnesium incorporated poly(lactic-co-glycolic acid) porous scaffolds by three-dimensional printing [J]. Biomaterials Translational, 2021, 2(3): 272-284. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||