Introduction
Trauma, cancer, aging and genetic diseases can result in bone lesions and bone defects. An important step in the repair of such defects is to provide suitable scaffolds to retain mechanical and functional integrity for bone regeneration. Hydroxyapatite (HA) is the most studied biomaterial used as a bone graft substitute, with its clinical application dating back to the 1970s.1-3
Porous HA has now replaced the dense form due to its better integration into bone. After HA is implanted into bone defects, new bone grows into the pores.4, 5 However, HA has a low biodegradation rate.2, 3, 6 Another widely-studied inorganic material is calcium carbonate, which is low cost, safe, accessible, biocompatible, bioresorptive and osteoconductive.7, 8 Calcium carbonate has three crystalline polymorphs, namely calcite, aragonite and vaterite.9, 10 The differences in the morphological forms of calcium carbonate may be related to their synthetic conditions. Calcite is the stable form and exists as a trigonal crystalline form in nature. Vaterite is the least stable and exists as a hexagonal crystalline form. In contact with water, vaterite slowly dissolves and recrystallises to a stable form.11
Aragonite occurs as the orthorhombic form and has been the exclusive focus of research attention due to its biocompatible properties.12 Aragonite is biocompatible and can be integrated into and replaced by bone.13, 14 However, due to its fast biodegradation rate, aragonite alone is not suitable as a bone graft.
Our previous study demonstrated that a partially-converted coralline HA/calcium carbonate can be completely biodegraded with great osteogenic capacity.15 Since coral is an endangered species and a limited resource, a composite bone graft created by addition of aragonite into porous HA may enhance the biodegradation of HA whilst retaining ideal osteogenic capacity. However, it is a technical challenge to fabricate porous HA/aragonite using conventional methods.
There are a variety of techniques for fabricating porous bone scaffolds. Traditional technologies include chemical foaming16 and foam-gel technology,17, 18 solvent casting and particle leaching technology,19 freeze drying20 as well as thermally-induced phase separation.21 Pore magnitude, shape, and interconnectivity, however, are not completely controllable using these techniques. Furthermore, an ideal bone graft should be biocompatible and biodegradable. It should also have suitable mechanical properties. The interconnected porous structure of bone grafts allows vascularization, cell spreading and effective transport of nutrients, oxygen, and waste, as well as growth factors. This process favours continuous ingrowth of bone tissue from the surface into the inner part of the scaffold.22 However, scaffolds with porosity designed for particular defects are hard to fabricate using most of these techniques.23-26
Additive manufacturing, also termed three-dimensional (3D)-printing, can be one solution to the design and fabrication of such bone scaffolds.27-29 Various additive manufacturing processing techniques allow the building of complex form scaffolds directly from a computer-aided design model in stereolithography file format.30 At present, a variety of additive manufacturing techniques involving stereolithography, fused deposition modelling, and selective laser sintering have been developed for tissue engineering applications.22, 29, 31 However, the products fabricated using these techniques may suffer from the effects of the high temperatures involved, which prevent the incorporation of bioactive molecules.28
The use of low temperature 3D-printing of calcium phosphate cements has been demonstrated in the literature.32, 33 The binders used for calcium phosphate powders are acidic, which pose issues for biocompatibility.27, 28 In recent work, collagen or hydrogel has been used to develop 3D-printed scaffolds, either alone or combined with other materials.34, 35 However, crosslinking is required to improve the mechanical strength and accuracy of 3D-printed scaffolds.35, 36 In recent years, a new form of HA called HA cement has been developed for treating cranial defects.32 Fast-setting calcium phosphate cements such as tetracalcium phosphate (TTCP) and dicalcium phosphate anhydrous (DCPA) use a sodium phosphate solution as the liquid phase and the setting time is around 5 minutes.32, 37
Natural bone forms at body temperature (37°C) and is a composite consisting of HA, calcium carbonate and other minerals deposited onto an organic extracellular matrix. The crystalline structure and composition differ from those of artificial calcium phosphate ceramics such as HA, β-tricalcium phosphate (β-TCP) and their hybrid scaffolds (HA/TCP), which take longer than natural bone to degrade after implantation.6 We hypothesise that to fabricate HA under near-physiological conditions (37°C) in combination with calcium carbonate may not only improve the mechanical strength of the scaffolds without crosslinking, but will also enhance the osteogenic and biodegradation properties.
In this study, we report a novel HA/aragonite bone graft substitute fabricated by low temperature 3D bio-printing on a 3D bio-plotter®. The effect of printing parameters on the porosity and compression strength was analysed. The cytotoxicity and osteogenic capacity of the HA/aragonite was assessed in vitro using human umbilical cord matrix mesenchymal stem cells. Soft tissue responses to HA/aragonite were evaluated in vivo after implantation between the tibia and the tibialis anterior muscle in a rat model.
Methods
Preparation of HA/aragonite
TTCP/DCPA paste
TTCP (Shanghai Rebone Biomaterials Co., Ltd., Shanghai, China), DCPA (Shanghai Rebone Biomaterials Co., Ltd.) and aragonite powders (Sigma-Aldrich, St. Louis, MO, USA) were ground together for 20 minutes using a pestle and mortar. The molar ratio of TTCP/DCPA was 1:1, according to the equation
In brief, after being ground, 10 g of the combined powders were mixed with 5 g carrier liquid consisting of a gelatine solution in water. The paste was loaded into a 3D-Bioplotter® (Envisiontec GmbH, Gladbeck, Germany) through cartridges and printed within 30 minutes.
Design and optimization of parameters for bioplotting
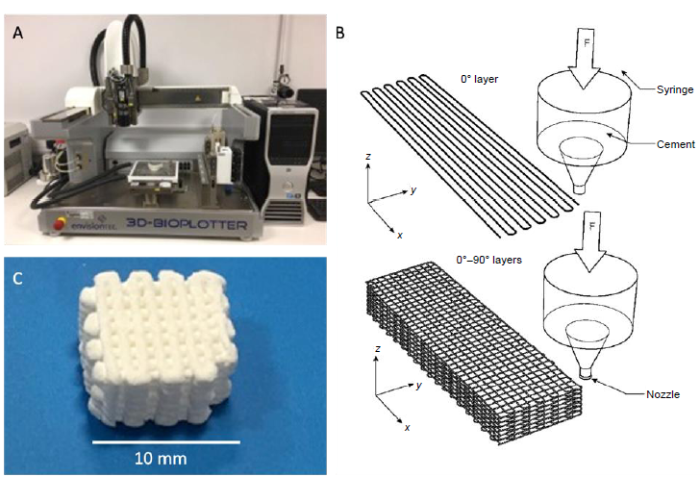
In this study computer-aided design software (Visual Machines, Envisiontec GmbH) was used to design the grid-like structure of HA/aragonite, which was formed into 10 mm × 10 mm × 5 mm blocks (Figure 1). The paste was printed by a 3D-Bioplotter® (Envision Developer, Envisiontec GmbH), using a 0.4 mm nozzle, with layer height of 0.4 mm.
Figure 1.
Figure 1.
(A) Bioplotter®. (B) The design of hydroxyapatite/aragonite. (C) The end product of fabrication. Scale bar: 10 mm.
To optimise the bioplotting process, a multi-level experimental trial was performed to analyse the effect of printing pressure, printing speed and distance between printed strands, and in total nine tests were performed. To compare the differently-printed 3D bio-printed bone graft samples, the resulting mechanical properties, specifically the compressive strength and the porosity, were assessed.
Characterization of HA/aragonite
X-ray powder diffraction of HA/aragonite and CaCO3 was performed using an X’pert3 powder X-ray diffractometer (Malvern PANalytical B.V., Almelo, Netherlands) with a Pixel detector. The dataset was collected using Cu Kα radiation in the 2θ range of 9°-36° at a scan speed of 3°/min.
Fourier transform infrared (FTIR) spectroscopy of HA/aragonite was carried out using a VERTEX 70 (Bruker, Kontich, Belgium). Chemical changes to the micro-structure were scrutinised by analysing the absorbances and positions of the infrared bands.
The surface morphology of HA/aragonite was observed by scanning electron microscopy (Sirion 200 and Quanta 200 FEI; FEI Company, Hillsboro, OR, USA) following platinum sputter coating for 300 seconds to avoid charging.
A universal material testing machine (Wuhan Guoliang Instrument Co. Ltd., Wuhan, China) was used to carry out the compression loading properties with a test standard GB/T4740-1999.38 The porosity of HA/aragonite was tested by Archimedes’ method.
The theoretical porosity of the HA/aragonite (φ) can be calculated from equation (2)
Where m1 is the dry weight of the HA/aragonite, m2 is the weight of the HA/aragonite soaked in water, v is the volume of the HA/aragonite, and ρ is the density of water.
Thermal analysis of the HA/aragonite was performed using a thermogravimetric analyser (Pyris 1; PerkinElmer, Waltham, MA, USA) in the range of 0-1000°C at a heating rate of 10 K/min.
In vitro assessments
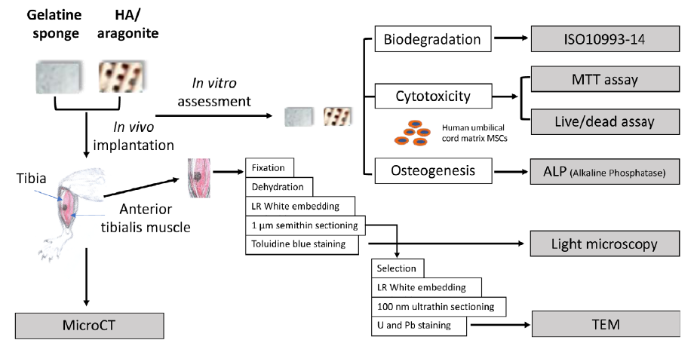
The design of the in vitro tests is shown in Figure 2.
Figure 2.
Figure 2.
Experimental design of in vitro and in vivo tests. The blocks of HA/aragonite were sterilised by autoclaving at 121°C, 15 psi (1 psi = 6894.76 Pa) for 30 minutes. Further in vitro and in vivo tests were designed to compare between HA/aragonite and clinically-applied gelatine sponge. HA: hydroxyapatite; U & Pb: uranyl acetate and lead citrate staining.
Cytotoxicity
Test samples of HA/aragonite and gelatine sponge were diced into 2 mm × 2 mm × 2 mm size cubes and soaked in Dulbecco’s modified Eagle medium/F12 (DMEM/F12 medium; Life Technology; Thermo Fisher Scientific, Waltham, MA, USA) overnight.
Human umbilical cord matrix mesenchymal stem cells were isolated from umbilical cord of healthy pregnancies during normal deliveries at the end of gestation with informed consent (South Wales REC No. 11/WA/0040). Umbilical cords were washed with DMEM/F12, diced into 2 mm2, explanted into T25 culture flasks containing 0.5 mL fetal bovine serum (FBS) for 24 hours then transferred to complete culture medium which was DMEM/F12 supplemented with 10% FBS, and 1% penicillin/streptomycin (Life Technology, Thermo Fisher Scientific) in a 5% CO2 atmosphere at 37°C. After 2 weeks, adherent cells were trypsinised for subpassage. Cells at passage three were seeded onto each of the test samples at 5 × 104 cells/well in a 96-well plate, while negative control cultures containing the same number of cells alone were cultured as a monolayer in wells of a 96-well plate without scaffolds. At 1, 3 and 7 days, the medium was replaced with 100 μL medium containing 10 μL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich). After incubating for 4 hours, the supernatant was carefully removed and 100 μL dimethyl sulfoxide was added to each well to dissolve the formazan crystals. The plates were wrapped in foil and incubated for 1 hour at room temperature. The supernatant was then transferred to a new 96-well plate, and the absorbance of each well was measured using a microplate reader at 570 nm wavelength (series No. 415-1387, BMG Labtech, Ortenberg, Germany).
Alkaline phosphatase assay
Alkaline phosphatase (ALP) activity of human umbilical cord matrix mesenchymal stem cells on HA/aragonite and control samples was quantified according to the manufacturer’s protocol (ab83369, Abcam, Cambridge, UK). In brief, cells at 5 × 104/well were seeded onto HA/aragonite, gelatine sponge or into blank wells and cultured for 3 days in DMEM/F12 supplemented with 10% FBS and 1% penicillin/streptomycin. The DMEM/F12 was then replaced by osteogenic medium containing dexamethasone (0.01 μM), β-glycerophosphate (0.1 M), ascorbic acid (0.5 mM), 10% FBS and 1% penicillin/streptomycin.
For ALP assay, HA/aragonite samples with attached cells were washed twice in cold phosphate-buffered saline, then cells were lysed in ALP assay buffer on ice for 2 hours with shaking, then processed according to the manufacturer’s protocol. ALP was quantified using a microplate reader (FLUOstar Omega; BMG LabTech) at an optical density of 405 nm. ALP activity (μmol/min/min or U/mL) was then calculated from the following equation
Where B is the amount of p-nitrophenol in the sample well, calculated from the standard curve; ΔT is the reaction time; V is the original sample volume added to each sample well and D is the sample dilution factor.
Degradation test
The degradation test was carried out following a standard testing method (ISO 10993-14).39 The HA/aragonite was ground into particles and the particle size was controlled to 355-415 μm by sieving. The particles were soaked in Tris-HCl buffer (pH 7.4) for 3, 7 or 14 days with a mixing speed of 120 r/min at 37°C. After 3, 7 or 14 days, the particles were filtered (0.2 μm) and washed twice with deionised water. The filtered particles were then dried in an oven for 24 hours to constant weight.
In vivo implantation
Six Sprague-Dawley male rats with a weight in the range of 200-250 g bought from Laboratory Animal Center, Huazhong University of Science and Technology (licence No. SCXK2016-0057) were employed in this study. The HA/aragonite scaffolds and control gelatine sponges were implanted juxtapositionally between the tibia and the anterior tibialis muscle (Figure 2) of six adult rats (three per group) to observe any soft/hard tissue reaction to the HA/aragonite in comparison with gelatine sponge controls.
The procedure was approved by the Animal Ethical Committee at Tongji Medical School, Huazhong University of Science and Technology on October 1, 2017 (IACUC No.738).
Six weeks after operation, the rats were euthanised and the implanted tissues were harvested and fixed immediately in 4% glutaraldehyde/0.1 M phosphate-buffered saline overnight, washed in deionised water and stored in 70% ethanol for MicroCT (Scanco VivaCT40; Scanco Medical, Bassersdorf, Switzerland), then processed for histology and transmission electron microscopy.
All samples were dehydrated and embedded into LR White resin (London Resin Company, Reading, UK) at 50°C for a minimum of 48 hours. Undecalcified 10 µm sections were obtained using a Leica RM2155 motorised microtome and stained with 1% toluidine blue in 50 mM Tris buffer pH 7.3. Stained sections were examined using an Olympus BX51 research light microscope (Olympus, Tokyo, Japan) and digital photomicrographs were captured using a Zeiss Axiocam and Axiovision software (Carl Zeiss Vision GmbH, Hallbergmoos, Germany).
The areas of interest identified by light microscopy were selected, post fixed in 1% osmium tetroxide and re-embedded in LR White resin, then 100 nm ultrathin sections were obtained using a glass knife on an Ultracut E ultramicrotome(Leica Microsystems Ltd, Wetzlar, Germany) stained with uranyl acetate and lead citrate, and examined in a Philips CM12 transmission electron microscope (FEI U.K. Ltd., Milton, UK) at 80 kV. Images were captured with a Megaview III camera and AnalySIS software (Soft Imaging System GmbH, Münster, Germany).
Statistical analysis
All data are presented as mean ± standard error (SE) and statistical analysis was performed by analysis of variance using GraphPad Prism 6 (GraphPad Software Inc., La Jolla, CA, USA). The differences between the two groups at each time point were analysed by two-way analysis of variance with the criterion α = 0.5.
Results
Optimization of 3D bio printing processing parameters
The effects of bioprinting parameters of pressure, printing speed and distance between strands on the compression strength and porosity of the end product are shown in Table 1. The results of analysis of the data are shown in Table 2, and the original calculation and data leading to the results in Table 2 can be referred to in the Additional Table 1.
Table 1 The effect of bioplotting parameters on hydroxyapatite/aragonite end products
| Sample No. | Factor | Compression (MPa) | Porosity (%) | ||
|---|---|---|---|---|---|
| (A) Pressure (MPa) | (B) Printing speed (mm/s) | (C) Distance between strands (mm) | |||
| 1 | 0.6 | 25 | 1 | 2.49 ± 0.14 | 37.0 ± 1.4 |
| 2 | 0.6 | 27 | 1.1 | 2.43 ± 0.23 | 37.5 ± 1.5 |
| 3 | 0.6 | 30 | 1.2 | 1.11 ± 0.07 | 42.8 ± 1.8 |
| 4 | 0.7 | 25 | 1.1 | 1.13 ± 0.11 | 41.1 ± 1.1 |
| 5 | 0.7 | 27 | 1.2 | 1.34 ± 0.09 | 30.2 ± 1.5 |
| 6 | 0.7 | 30 | 1 | 1.58 ± 0.13 | 40.7 ± 1.3 |
| 7 | 0.8 | 25 | 1.2 | 1.03 ± 0.08 | 41.8 ± 1.1 |
| 8 | 0.8 | 27 | 1 | 0.56 ± 0.05 | 40.2 ± 1.2 |
| 9 | 0.8 | 30 | 1.1 | 0.75 ± 0.07 | 35.5 ± 1.6 |
Note: Data are expressed as the mean ± SE.
Table 2 Range analysis of compression strength and porosity of hydroxyapatite/aragonite
| Factors | Pressure (MPa) | Printing speed (mm/s) | Distance between strands (mm) |
|---|---|---|---|
| Compression strength | |||
| k1 | 2.01 | 1.55 | 1.54 |
| k2 | 1.35 | 1.44 | 1.44 |
| k3 | 0.78 | 1.15 | 1.16 |
| R | 1.23 | 0.4 | 0.38 |
| Porosity | |||
| k1 | 39.1 | 40.0 | 39.3 |
| k2 | 27.3 | 36.0 | 38.0 |
| k3 | 39.2 | 39.7 | 38.3 |
| R | 11.9 | 3.0 | 1.0 |
Table 2 shows the range analysis of variance tests. Mean k1, k2 and k3 are the mean values of the sum of compression strength at the same level. For the same factor, range R is the average value difference between the maximum value and the minimum value of k at different levels.
In Table 2, k1, k2 and k3 are the mean values of the sum of compression strength at the same level, which can be calculated from Equation (4)
Where S1 is the average compression strength of pressure (factor A), S2 is the average compression strength of printing speed (factor B) and S3 is the average compression strength of distance between strands (factor C). For the same factor, range R is the average value difference between the maximum value and the minimum value of K at the different levels. According to the range analysis of compression strength, without any interaction between the three factors, the results of RA > RB > RC shown in Table 2 indicate that the printing pressure has the greatest effect on compression strength, printing speed takes second place, and distance between strands has the least effect. For printing pressure (factor A), kA1 > kA2 > kA3, which indicates that as the printing pressure increases, the compression strength decreases. For printing speed (factor B), kB1 > kB2 > kB3, indicating that as the printing speed increases, the compression strength decreases. For distance between strands (factor C), kC1 > kC2 > kC3, which means that as distance between strands increases, the compression strength decreases. According to these results, the best combination of the three factors should be A1B1C1, namely a printing pressure of 0.6 MPa, printing speed of 25 mm/s and distance between strands of 1 mm.
According to the results of the range analysis for porosity, without any interaction between the three factors, the result of R1 > R2 > R3, shown in Table 2, indicates that the printing pressure has the greatest effect, followed by printing speed in second place, while distance between strands has the least effect. For printing pressure (factor A), kA3 > kA1 > kA2, which means that as printing pressure increases, porosity decreases but porosity is the lowest when printing pressure reaches an intermediate value. For printing speed (factor B), kB1 > kB3 > kB2 which indicates that as the printing speed increases, the porosity decreases, but porosity is the lowest when printing speed reaches an intermediate value. For distance between strands (factor C), kC1 > kC3 > kC2, indicating that when distance between strands increases, porosity decreases, but porosity is the lowest when distance between strands reaches an intermediate value. According to these results, the best combination of the three factors should be A1B3C3, namely a printing pressure of 0.6 MPa, printing speed of 30 mm/s and distance between strands of 1.2 mm.
In general, the compression strength decreases as porosity increases. At a fixed printing pressure, the higher the printing speed the higher the porosity, which implies a lower compression strength. It can therefore be seen that the porosity exerts a lesser effect on compression strength compared with the printing pressure from 2. Following the above tests, for the in vivo and in vitro characterisation, samples were fabricated adopting processing parameters from test sample 1 in view of the resultant higher compressive strength.
Material characterization of HA/aragonite
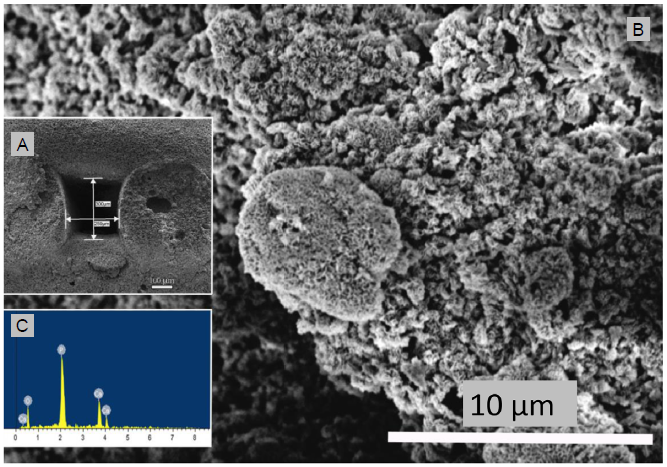
The surface structure, cross-section and X-ray spectroscopy analysis of HA/aragonite, visualised by scanning electron microscopy, are shown in Figure 3. The pore size was around 300 μm with some visible micro-pores on the surface and within the scaffolds.
Figure 3.
Figure 3.
(A) Scanning electron microscopic image of the cross section of hydroxyapatite/aragonite and measurement of pore size. (B) Higher magnification of the scanning electron microscopic image shown in A. (C) Energy dispersive X-ray spectroscopy analysis of the cross section of hydroxyapatite/aragonite. Scale bars: 100 μm in A, 10 μm in B.
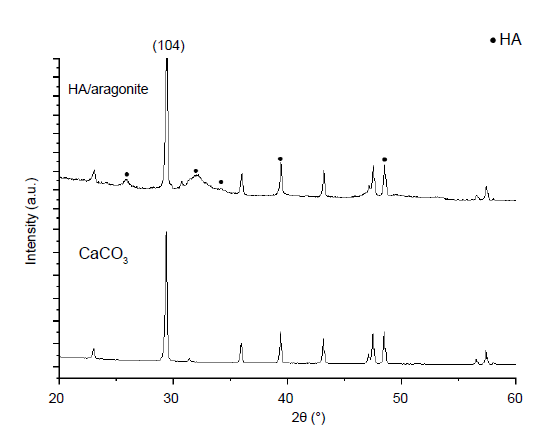
The results of X-ray diffraction analysis of HA/aragonite and calcium carbonate are shown in Figure 4. In Figure 4, the reflection peaks visible at a 2θ value of 104 in the calcium carbonate spectrum indicate that the calcium carbonate in the HA/aragonite belongs to aragonite.12, 13 In the X-ray diffraction patterns of the HA/aragonite, the diffraction peaks of calcium carbonate in the HA/aragonite are still visible. More importantly, the diffraction peaks of HA appear at 2θ values of 29.1°, 32.9°, 34.1°, 39.6° and 49.5° which correspond to (002), (211), (300), (130) and (213) planes, respectively. These diffraction peaks are in good agreement with the diffraction standard data of pure HA (JCPDS PDF#09-0432).40
Figure 4.
Figure 4.
X-ray diffraction analysis of hydroxyapatite/aragonite and calcium carbonate (CaCO3). The (104) in 2θ indicates that the CaCO3 in the HA/aragonite belongs to aragonite. HA: hydroxyapatite.
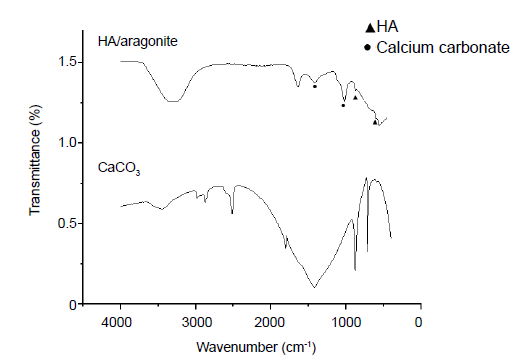
The FTIR spectra of HA/aragonite and calcium carbonate are shown in Figure 5. The FTIR spectra of the reference samples show absorptions at v1—963 cm-1, v3—1036 and 1095 cm-1, v4—568 and 600 cm-1 which are due to PO43- ions, while OH groups are visible at 630 cm-1.15, 41, 42 The FTIR spectra of the reference samples show absorptions at a ν3 peak of 1453.7 cm-1, a ν2 peak of 853.8 cm-1, a ν1 peak of 1083.8 cm-1 and ν4 peaks of 699.2 and 712.2 cm-1 corresponding to CO32-. The ν4 absorption peak of CO32- is a single peak for calcite calcium carbonate, but a double peak for aragonite calcium carbonate.40
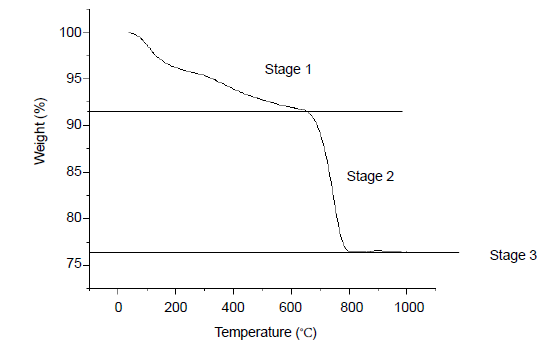
The results of thermogravimetric analysis of HA/aragonite are shown in Figure 6. The thermal decomposition process consists of three stages. In the first stage, as the temperature increases from 0°C to 200°C, gelatine in the HA/aragonite begins to decompose, resulting in weight loss. The decomposition products, CO2 and H2O, are emitted as gases. As the temperature continues to increase to 600°C, the trend of weight loss decreases until the weight reaches around 92%. At the second stage, as the temperature increases from 600°C to 800°C, CaCO3 in the HA/aragonite begins to dramatically decompose, resulting in massive weight loss. The decomposition product, CO2, is emitted as a gas. In the third stage, when the temperature increases to 1000°C, there is little weight loss and the residual weight tends to be stable because the residual components are CaO and HA with higher melting temperatures, which are stable at 1000°C, so no further weight loss will occur even as the temperature increases.43 The weight percentage of CaCO3 can be calculated from the following equation
Figure 5.
Figure 5.
Comparation of the Fourier-transform infrared spectra of HA/aragonite. and calcium carbonate. Triangle indicates that the peak corresponding to PO43-; dot indicates that the peak corresponding to CO32-.
Figure 6.
Figure 6.
Thermogravimetric analysis results of hydroxyapatite/aragonite. Stage 1, gelatine in the HA/aragonite begins to decompose; stage 2, aragonite begins to dramatically decompose; stage 3, residual components of CaO and HA are stable, there are no mass weight loss.
Thus, the weight percentage of CaCO3 is around 35%.
MTT and ALP assays
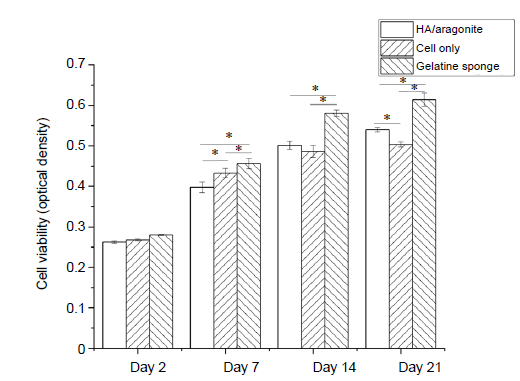
MTT assay demonstrated that there were no significant differences in cell viability between cells grown on HA/aragonite and cells alone at days 7 or 14 (Figure 7), indicating that HA/aragonite is nontoxic to human umbilical cord matrix mesenchymal cells. However, the result of MTT assay were significantly higher in the gelatine sponge group than the HA/aragonite group at days 7, 14 and 21.
Figure 7.
Figure 7.
Effect of HA/aragonite on the viability of human umbilical cord matrix mesenchymal stem cells detected by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Data are expressed as the mean ± SE. *P < 0.05 (two-way analysis of variance). HA: hydroxyapatite.
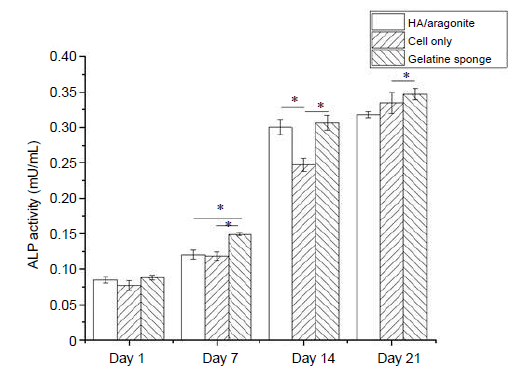
The osteogenic capacity of the HA/aragonite was confirmed by ALP assay. The ALP activity of cells seeded on HA/aragonite, on gelatine sponge and of cells alone is shown in Figure 8. After day 1, the ALP activity increased significantly on gelatine sponge, HA/aragonite and in cells alone between day 1 and day 14 (P < 0.05). However, ALP activity in the gelatine sponge group was higher than that of cells on HA/aragonite at days 7 and 21.
Figure 8.
Figure 8.
Effect of HA/aragonite on the ALP activity of human umbilical cord matrix mesenchymal stem cells. Data are expressed as the mean ± SE. *P < 0.05 (two-way analysis of variance. ALP: alkaline phosphatase; HA: hydroxyapatite.
In vitro degradation test of HA/aragonite
The degradation of the HA/aragonite was confirmed by degradation testing. The percentage degradation of the HA/aragonite was determined by weight loss which can be defined by equation
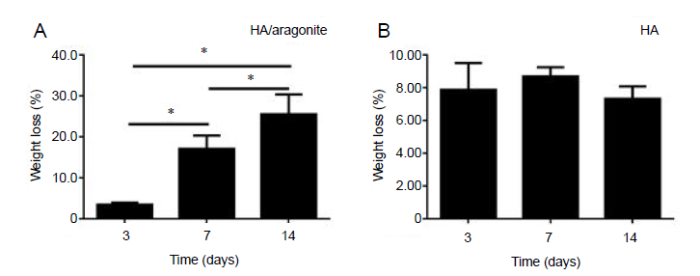
The weight loss of HA/aragonite at day 3 was around 3.5%, increasing to 25.58% after day 14. The percentage weight loss of HA/aragonite increased significantly over time, while the weight loss of HA alone showed no significant difference with increasing time (Figure 9).
Figure 9.
Figure 9.
(A, B) Weight loss percentage of HA/aragonite (A) and HA (B). Data are expressed as the mean ± SE. *P < 0.05 (two-way analysis of variance). HA: hydroxyapatite.
Juxtapositional implantation between tibia and tibialis anterior muscle in a rat model
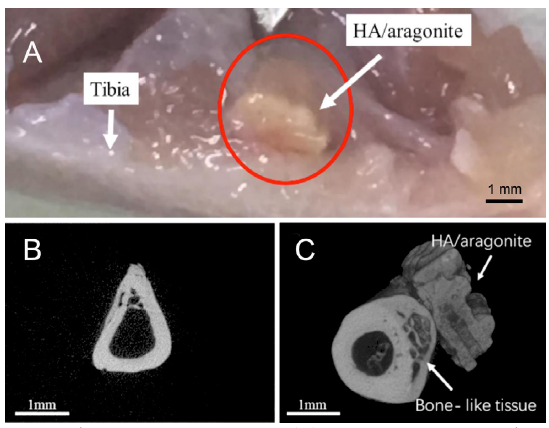
The position of the HA/aragonite implanted between the tibia and the tibialis anterior muscle in a rat is indicated in Figure 10A. The HA/aragonite was firmly integrated into the tibia after 6 weeks. Figure 10B shows a microCT image of a gelatine sponge implanted in a rat model, in which only the tibia is visible, with no mineralisation of the gelatine. Figure 10C shows HA/aragonite implanted in a rat model. The image shows that the HA/aragonite has the same density as bone, and HA/aragonite is firmly integrated with the indicated bone-like tissue.
Figure 10.
Figure 10.
Implantation of HA/aragonite in a rat model. (A) A specimen of the HA/aragonite (red circle) implanted between the tibia and the tibialis anterior muscle in a rat model. After 6 weeks the sample has become well integrated. (B) MicroCT of gelatine sponge implanted in a rat model. Only the tibia is visible, with no mineralised tissue formed in the sponge. (C) HA/aragonite implanted in a rat model. Interestingly, formation of bone-like tissue (arrow) can be seen between the tibia and the HA/aragonite. Scale bars: 1 mm. HA: hydroxyapatite.
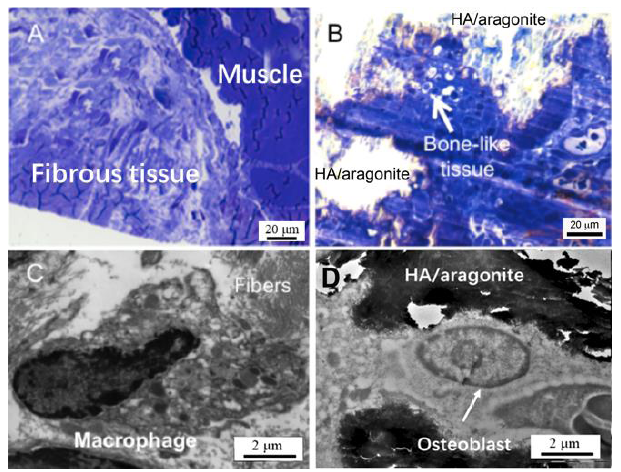
As shown in Figure 11A, light microscopy revealed that at 6 weeks after gelatine sponge implantation, fibrous tissue was formed between the tibia and the tibialis anterior muscle. The HA/aragonite samples implanted at the same time were covered mainly by connective tissue containing blood vessels, fibroblasts and some macrophages; in particular, there were patches of bone-like tissue formation and minimal inflammatory response, as shown in Figure 11B. The TEM results confirmed the findings of light microscopy. In the gelatine sponge implantation group (Figure 11C) macrophagic responses were observed with fibroblast infiltration for tissue regeneration; whereas within the patch of bone-like tissue in the HA/aragonite group (Figure 11D), typical osteoblast-like cells with calcified lacunae and canaliculi-like structure were observed, which confirmed that the marked area is likely to be new bone formation or callus formation.
Figure 11.
Figure 11.
(A) Light microscopy (1 mm section stained with Toluidine blue) revealed that implantation of a gelatine sponge resulted in formation of fibrous tissue between the tibia and the tibialis anterior muscle. (B) At 6 weeks after HA/aragonite implantation, the materials were covered by fibroblasts and macrophages; interestingly, there was a small patch of bone-like tissue formation. Transmission electron microscopy (100 nm section with uranyl acetate and lead citrate staining) observation confirmed the findings of light microscopy. In the control gelatine sponge implantation group (C), macrophagic responses were observed with fibroblast infiltration for tissue regeneration; whereas with implantation of the HA/aragonite (D), typical osteoblast-like cells with canaliculi-like structures within calcified lacunae were observed in the patch of bone-like tissue. Scale bars: 20 μm in A, B, 2 μm in C, D. HA: hydroxyapatite.
Discussion
The results of this study demonstrated the ability to fabricate a composite HA/aragonite bone graft substitute that supports osteogenesis in vitro and in vivo.
In this study, the purpose of adding aragonite into HA was to enhance the biodegradation of the bone graft. It is well known that HA is a bone graft material which is slow to degrade and its degradation period can be more than 100 months44 which is far beyond the bone remodelling cycle of between 6 and 24 months. In order to increase biodegradation, a more biodegradable ceramic, β-TCP is added into bone graft materials.44, 45 Even though HA/β-TCP is significantly more degradable than HA alone, the degradation period of HA/β-TCP is also rather long, reported to be more than 36 months.44, 45 It is therefore necessary to introduce more biodegradable bone graft materials, such as composites containing both HA and more soluble calcium carbonate, but apart from coralline HA, it is difficult to form HA composites containing calcium carbonate that also have the desired mechanical properties.
HA ceramics are normally sintered at between 1200-1300°C, whereas calcium carbonate undergoes thermal decomposition above 840°C:
Therefore it is not possible to synthesise HA/aragonite through sintering. It has been reported that when TTCP and DCPA are mixed in double-distilled water, the dissolved ions precipitate on the surface of the material and can self-set into various forms of HA.31 By adding calcium carbonate into this mixture, it may be possible to produce bulk self-setting HA/aragonite without pores.
The addition of gelatine solution formed a viscous and cohesive paste and created a strong combination between solid and liquid phases, thereby improving the injectability of the paste. The setting time of TTCP/DCPA is 6-10 minutes, but the addition of gelatine increases the setting time to around 30 minutes which makes the pastes ‘printable’ and allows the fabrication of desired features. The precipitated particles were crystalline in texture which is similar to the observations reported in previous publications.48 Strong evidence for the presence of HA was provided by the peak which appeared at around 961 cm-1. The ν4 absorption peak of CO32- was seen in the HA/aragonite, which indicated that calcium carbonate was present in the HA/aragonite after all the processing. The peaks had all shifted from the reference peaks but by a reasonable value. The absorption peak of HPO42- at 895 cm-1 caused by the decreasing TTCP/DCPA ratio did not appear in the HA/aragonite, indicating that there was no Ca9(HPO4)(PO4)5(OH) present in the HA/aragonite.49
The compression strength of cancellous bone is 1-12 MPa, and the porosity is 50-90%.50 The compression strength of the HA/aragonite we fabricated was 2.49 MPa and the highest porosity of HA/aragonite is 42.8%, indicating that the strength and porosity of HA/aragonite are comparable to those of cancellous bone. The pore size of HA/aragonite was around 280 μm, which is suitable for tissue ingrowth.51 As the porosity of the HA/aragonite increases, its compression strength decreases. Recent studies have reported the same issue,52 and consequently further research is needed to improve the mechanical strength.
In this study, significant weight loss was noted between successive time points, indicating that HA/aragonite is a degradable material, which confirmed the previous finding of the in vitro biodegradation of calcium carbonate.53
As a potential bone graft substitute, it is important that this new material is able to enhance osteogenesis and promote bone formation. It is well documented that HA and calcium carbonate, used either alone or combined, have demonstrated osteogenicity and bone conductivity.1, 4, 7, 8, 15 In the present study, HA/aragonite supported ALP activity of mesenchymal stem cells in vitro, which is a sign of enhanced osteogenicity.
HA/aragonite was compared with clinically-used gelatine sponges which consist of gelatine alone. Interestingly, gelatine sponges showed greater cell numbers and ALP activities in vitro compared with HA/aragonite. This may be due to the fact that gelatine sponges are much more porous than HA/aragonite, thus allowing more cell penetration and growth over the 3-week period of cell culture. However, after implantation, no bone formation was observed in the gelatine sponge group in vivo.
Conductive bone formation can only be observed in vivo. In order to observe the tissue response to HA/aragonite, this bone graft material was implanted between the tibialis anterior muscle and the tibia. No bone defects were created, as the initial plan was to exclude any potential adverse effect of this material. Unexpectedly, at the time the implants were harvested, the HA/aragonite was firmly integrated into the tibia. New bone formation between the HA/aragonite and the tibia was confirmed by microCT, with bony material bridging the implant to the tibia bone. The presence of bone-like tissue on the surface of the implants was also confirmed by both light and transmission electron microscopy.
Undifferentiated cells from muscles and blood vessel walls have osteogenic potential.54 The formation of bone on the surface of calcium phosphate scaffolds in intramuscular sites has been reported by several studies.55-57 The precipitation and dissolution of the material’s surface plays an important role in the intramuscular osteogenesis process.57, 58 In this study, the HA/aragonite formed a biodegradable bone graft. When HA/aragonite is implanted in the body, the process of interaction between the implant and the surrounding body fluid or tissue can be activated. The dissolution of aragonite should increase the concentration of Ca2+ on the surface of the material resulting in the formation of apatite-like phosphate by absorbing proteins and growth factors.57 The fast degradation rate of aragonite may be responsible for the early signs of osteogenesis, while other animal studies only showed signs of osteogenesis after 45 days.56, 59 In the current study, we not only observed small patches of bone-like tissue formation on the HA/aragonite when implanted in muscle, but also a large amount of visible bone formation between the HA/aragonite and the tibia.
Our results show that the HA/aragonite did not induce any adverse tissue responses after implantation between the tibia and the tibialis anterior muscle, but demonstrated conductive bone formation between the implant and the tibia. Further study is needed to understand the mechanism involved.
Within this study, there are a number of limitations. The first purpose of this study was to achieve the biofabrication of a novel HA/aragonite bone graft substitute using 3D bioprinting techniques. Even though the material fabrication process was successful, the ratio of raw materials in the formulation may not be optimal in terms of the mechanical strength, biodegradation and osteogenicity of the product. Secondly, the parameters used are designed for use in a 3D-Bioplotter® and may not be directly transferrable to other types of additive manufacturing devices. Thirdly, the favourable results of HA/aragonite in vitro and in vivo are still preliminary. More comprehensive and systematic studies are needed to explore the full potential of HA/aragonite on osteogenesis and biodegradation.
In summary, the 3D-bioprinted bone graft substitute HA/aragonite prepared in this study is a porous, biodegradable and nontoxic HA/calcium carbonate composite material with osteogenic capability. By controlling the printing parameters, product size and porosity were fabricated within the range desired for bone grafts. HA/aragonite can be printed with high resolution to achieve versatile geometries that are critical for fluid exchange and cellular ingrowth during bone healing. The mechanical strength and porosity of the HA/aragonite are comparable to those of cancellous bone, which allows implantation into a non-loading bone defect. In vitro and in vivo experiments were performed successfully showing suitability for bone formation. Since this material is formed around 37°C, there is great potential for the incorporation of bioactive particles to suit personalised application. Future studies will focus on further optimisation of the formulae to strengthen the mechanical properties with extensive evaluation of the effects on osteogenicity and biodegradation.
Author contributions
Literature search, methodology, instrument selection, refining of research ideas, day-to-day tests and processes of material development, investigation, data curation, statistical analysis, manuscript drafting and refining: YS; in vivo tests support: RH; in vivo tests support: XD; data collection: RH and XD; in vivo ethics approval, provision of test facilities and supervision of in vivo tests: ZS; additive manufacturing resources, instrument selection, validation, supervision: DD; manuscript revision and editing: DD, CY and ZX; instrument selection: CY and ZX; funding acquisition: CY and ZX; visualization: YS and ZX; study conceptualization and design, establishment and bioimaging of the in vivo model, statistical analysis interpretation, supervision, and project administration: ZX. All authors approved the final version of this manuscript.
Financial support
This study was supported by the Wuhan International Collaboration Project of China (No. 2017030209020252) and Wuhan Science and Technology Project of China (No. 2018010401011281).
Acknowledgement
We would like to thank for Dr. Christopher Von Ruhland, the Facility Lead (Electron and Light Microscopy), Central Biotechnology Services, Cardiff University for the sample preparation for light and electron microscopy; Dr. Hui Liu and Mr. Dahu Qi at Tongji Hospital, Huazhong University of Science and Technology for assistance with microCT and in vitro biodegradation analysis.
Conflicts of interest statement
The authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this study.
Data sharing statement
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional file
Additional Table 1. Original calculation and data leading to the results in Table 2.
Reference
Porous hydroxyapatite as a bone-graft substitute in metaphyseal defects. A histometric study
URL
PMID:3015975
[Cited within: 2]

Porous hydroxyapatite (Interpore 500) formed by conversion of the Porites goniopora coral exoskeleton has pores averaging 600 micrometers and pore interconnections averaging 260 micrometers in diameter. In the proximal tibial metaphysis of eight dogs, a defect one cubic centimeter in size was created unilaterally and was fitted with a block of Interpore 500. Both proximal tibial metaphyses were retrieved at two, four, six, and twelve months. Stained undecalcified sections were examined by light microscopy and quantitated by histometric methods. The implant-side specimens contained compact bone along the external surface and trabecular bone interiorly. The interior of these specimens was composed of 51.9 +/- 1.3 per cent soft tissue, 13.0 +/- 1.2 per cent bone, and 35.1 +/- 1.2 per cent Interpore 500 (mean and standard error). The interior of the normal specimens was composed of 79.7 +/- 1.4 per cent soft tissue and 20.2 +/- 1.4 per cent bone. The allocation of implant pore space between bone and soft tissue was proportional to that of bone and soft tissue in the normal tibiae. The stereological distribution of regenerated bone in the porous hydroxyapatite was also the same as in the normal tibiae. The appositional process of incorporation of the implant was confirmed by the finding that 66.5 per cent of the surface of the Interpore 500 was covered with bone ingrowth at twelve months.
Bone substitutes: an update
DOI:10.1016/j.injury.2005.07.029
URL
PMID:16188545
[Cited within: 1]

Autograft is considered ideal for grafting procedures, providing osteoinductive growth factors, osteogenic cells, and an osteoconductive scaffold. Limitations, however, exist regarding donor site morbidity and graft availability. Allograft on the other hand, posses the risk of disease transmission. Synthetic graft substitutes lack osteoinductive or osteogenic properties. Composite grafts combine scaffolding properties with biological elements to stimulate cell proliferation and differentiation and eventually osteogenesis. We present here an overview of bone grafts and graft substitutes available for clinical applications.
A review of bone substitutes
DOI:10.1016/j.coms.2007.06.002
URL
PMID:18088902
[Cited within: 2]

The use of bone grafts in the repair of defects has a long history of success, primarily with the use of autologous bone. With increasing technologic advances, researchers have been able to broaden the spectrum of grafting materials to allografts, xenografts, and synthetic materials, which provide the surgeon and patient with options, each with unique advantages. It is with the knowledge of each material that the clinician can present and suggest the best material and tailor treatment plans to fit each individual. In this article, we present an overview of the principles of bone grafting, the types of graft materials available, and an outlook to what the future holds in this area of medicine and dentistry.
The slow resorption with replacement by bone of a hydrothermally synthesized pure calcium-deficient hydroxyapatite
DOI:10.1016/j.biomaterials.2008.03.028
URL
PMID:18403011
[Cited within: 2]

A newly developed calcium-deficient hydroxyapatite composed of rod-shaped particles synthesized by the hydrothermal method (HHA) and stoichiometric hydroxyapatite (SHA) synthesized by the sintering method was used for in vivo implantation and in vitro culture systems to compare these biological responses. In the rabbit femur, implanted HHA was slowly resorbed and about 80% of the implant remained 24 weeks after implantation; however, up to 72 weeks after implantation, most of the implanted HHA was resorbed. The implanted SHA was unresorbed throughout the experimental period, but degradation by the invasion of newly formed bone was seen at 72 weeks after implantation. Bone histomorphometry showed that the volume of newly formed bone and the number of osteoclasts in the implanted region were significantly higher in HHA than in SHA 24 weeks after implantation. In vitro culture of C2C12 cells with the induction of osteoblastic phenotypes using recombinant bone morphogenetic protein-2 showed similar cell density and the induction of alkaline phosphatase activity between the cells on HHA and SHA discs. In vitro osteoclastogenesis of HHA and SHA discs using bone marrow macrophages and recombinant receptor activator of nuclear factor-kappaB ligand showed higher TRAP activity of osteoclasts cultured on HHA discs. These results showed that slow biodegradability did not always correlate to final replaceability in bone tissue, and suggested that the activity of osteoclasts correlated to the bone-forming activity of osteoblasts.
Bone remodeling and hydroxyapatite resorption in coated primary hip prostheses
DOI:10.1007/s11999-008-0559-y
URL
PMID:18855086
[Cited within: 1]

Hydroxyapatite coatings for THA promote bone ongrowth, but bone and coating are exposed to stress shielding-driven osteoclastic resorption. We asked: (1) if the resorption of hydroxyapatite coating and bone ongrowth correlated with demographics; (2) if the resorption related to the stem level; and (3) what happens to the implant-bone interface when all hydroxyapatite coating is resorbed? We recovered 13 femoral components from cadaveric specimens 3.3 to 11.2 years after uneventful primary THA. Three cross sections (proximal, medial, distal) of the hydroxyapatite-coated proximal implant sleeve were analyzed by measuring the percentage of residual hydroxyapatite and bone ongrowth on the implant perimeter. Hydroxyapatite resorption was independent of patient age but increased with time in vivo and mostly was gone after 8 years. Bone ongrowth was independent of time in vivo but decreased with aging patients. Only in the most proximal section did less residual hydroxyapatite correlate with less bone ongrowth. Hydroxyapatite resorption, which was more proximal than distal, showed no adverse effects on the implant-bone interface.
Biphasic calcium phosphate ceramics for bone reconstruction: A review of biological response
DOI:10.1016/j.actbio.2017.01.076
URL
PMID:28159720
[Cited within: 2]

Autologous bone graft is considered as the gold standard in bone reconstructive surgery. However, the quantity of bone available is limited and the harvesting procedure requires a second surgical site resulting in severe complications. Due to these limits, scientists and clinicians have considered alternatives to autologous bone graft. Calcium phosphates (CaPs) biomaterials including biphasic calcium phosphate (BCP) ceramics have proven efficacy in numerous clinical indications. Their specific physico-chemical properties (HA/TCP ratio, dual porosity and subsequent interconnected architecture) control (regulate/condition) the progressive resorption and the bone substitution process. By describing the most significant biological responses reported in the last 30years, we review the main events that made their clinical success. We also discuss about their exciting future applications as osteoconductive scaffold for delivering various bioactive molecules or bone cells in bone tissue engineering and regenerative medicine. STATEMENT OF SIGNIFICANCE: Nowadays, BCPs are definitely considered as the gold standard of bone substitutes in bone reconstructive surgery. Among the numerous clinical studies in literature demonstrating the performance of BCP, Passuti et al. and Randsford et al. studies largely contributed to the emergence of the BCPs. It could be interesting to come back to the main events that made their success and could explain their large adhesion from scientists to clinicians. This paper aims to review the most significant biological responses reported in the last 30years, of these BCP-based materials. We also discuss about their exciting future applications as osteoconductive scaffold for delivering various bioactive molecules or bone cells in bone tissue engineering and regenerative medicine.
Preparation of hierarchical hollow CaCO3 particles and the application as anticancer drug carrier
DOI:10.1021/ja8039585
URL
PMID:18980322
[Cited within: 2]

One-pot approach to couple the crystallization of CaCO(3) nanoparticles and the in situ symmetry-breaking assembly of these crystallites into hollow spherical shells was developed under the templating effect of a soluble starch. Further functional study using HP-a as an anticancer drug carrier (DOX) demonstrated its advantages for localizing drug release by the pH value-sensitive structure and enhancing cytotoxicity by increasing cellular uptake, perinuclear accumulation, and nuclear entry.
Calcium carbonate nanoparticles: synjournal, characterization and biocompatibility
DOI:10.1166/jnn.2011.4251
URL
PMID:22103092
[Cited within: 2]

The synthesis of nanoparticles and their functionalization to effectively utilize them in biological applications including drug delivery is currently a challenge. Calcium carbonate among many other inorganic nanosized particles offers promising results for such applications. We have synthesized calcium carbonate nanoparticles using polymer mediated growth technique, where one of the ions bound within polymer matrix and the other diffuses and reacts to form desired compound. The synthesized nanoparticles are characterized using X-ray diffraction, Scanning Electron Microscopy and spectroscopic techniques such as Fourier-Transform Infra-red spectroscopy and UV-Vis spectroscopy. The diameter of the calcium carbonate nanoparticles is estimated to be 39.8 nm and their biocompatibility studies showed no significant induction of oxidative stress or cell death even at higher concentrations (50 microg) upon exposure to HeLa and LE cells. Here, we report that the synthesized calcium carbonate nanosized particles using polymer mediated growth technique are biocompatible and can be safely used for biomedical applications.
Taking advantage of disorder: amorphous calcium carbonate and its roles in biomineralization
DOI:10.1002/adma.200300381 URL [Cited within: 1]
A pavement of pearl
DOI:10.1038/40010 URL [Cited within: 1]
Anticancer drug delivery system based on calcium carbonate particles loaded with a photosensitizer
DOI:10.1016/j.bpc.2013.07.006
URL
PMID:23932207
[Cited within: 1]

In photodynamic therapy (PDT), photosensitizers are required to arrive in high concentrations at selective targets like cancer cells avoiding toxicity in healthy tissue. In this work, we propose the application of porous calcium carbonate carriers in the form of polycrystalline vaterite for this task. We investigated the loading efficiency for the photosensitizer Photosens in vaterite micro- and nanocarriers. A possible release mechanism depending on the surrounding pH was studied, showing a fast degradation of the carriers in buffers below pH7. These results hold out the prospect of a novel PDT drug delivery system. Variation of particle size or additional coatings allow custom-design of workload release curves. An intrinsic cancer-sensitivity can be expected from the pH-dependent release in the acidic microenvironment of cancer tissue.
Templated growth of calcite, vaterite and aragonite crystals onself-assembled monolayers of substituted alkylthiols on gold
DOI:10.1039/a705859d URL [Cited within: 2]
Synthesis of nanosized calcium carbonate (aragonite) via a polyacrylamide inducing process
DOI:10.1016/j.powtec.2005.12.019 URL [Cited within: 2]
Characterisation of calcium carbonate and its polymorphs from cockle shells (Anadara granosa)
DOI:10.1016/j.powtec.2011.07.031 URL [Cited within: 1]
Characterization of a biodegradable coralline hydroxyapatite/calcium carbonate composite and its clinical implementation
DOI:10.1088/1748-6041/8/6/065007
URL
PMID:24288015
[Cited within: 2]

A partially converted, biodegradable coralline hydroxyapatite/calcium carbonate (CHACC) composite comprising a coral calcium carbonate scaffold enveloped by a thin layer of hydroxyapatite was used in the present study. The CHACC was characterized using powder x-ray diffraction, scanning electron microscopy and energy dispersive x-ray spectroscopy. The ability of the CHACC to promote conductive osteogenesis was assessed in vitro using human mesenchymal stem cells (hMSCs) and in vivo using an immunodeficient mouse model. The clinical performance of CHACC as a bone substitute to fill voids caused by excision of bone tumours was also observed in 16 patients. The CHACC was found to consist of two overlapping layers both morphologically and chemically. Hydroxyapatite formed a thin layer of nanocrystals on the surface and a thick rough crystal layer of around 30 microm in thickness enveloping the rock-like core calcium carbonate exoskeletal architecture. hMSCs cultured on CHACC in osteogenic medium demonstrated significant osteogenic differentiation. After subcutaneous implantation of CHACC incorporating osteogenically differentiated hMSCs and an anti-resorptive agent, risedronate, into an immunodeficient mouse model, bone formation was observed on the surface of the implants. Clinical application of CHACC alone in 16 patients for bone augmentation after tumour removal showed that after implantation, visible callus formation was observed at one month and clinical bone healing achieved at four months. The majority of the implanted CHACC was degraded in 18-24 months. In conclusion, CHACC appears to be an excellent biodegradable bone graft material. It biointegrates with the host, is osteoconductive, biodegradable and can be an attractive alternative to autogenous grafts.
Fabrication of in-situ foamed chitosan/β-TCP scaffolds for bone tissue engineering application
DOI:10.1016/j.matlet.2012.07.002 URL [Cited within: 1]
Highly degradable porous melt-derived bioactive glass foam scaffolds for bone regeneration
DOI:10.1016/j.actbio.2017.04.030
URL
PMID:28457960
[Cited within: 1]

A challenge in using bioactive melt-derived glass in bone regeneration is to produce scaffolds with interconnected pores while maintaining the amorphous nature of the glass and its associated bioactivity. Here we introduce a method for creating porous melt-derived bioactive glass foam scaffolds with low silica content and report in vitro and preliminary in vivo data. The gel-cast foaming process was adapted, employing temperature controlled gelation of gelatin, rather than the in situ acrylic polymerisation used previously. To form a 3D construct from melt derived glasses, particles must be fused via thermal processing, termed sintering. The original Bioglass(R) 45S5 composition crystallises upon sintering, altering its bioactivity, due to the temperature difference between the glass transition temperature and the crystallisation onset being small. Here, we optimised and compared scaffolds from three glass compositions, ICIE16, PSrBG and 13-93, which were selected due to their widened sintering windows. Amorphous scaffolds with modal pore interconnect diameters between 100-150microm and porosities of 75% had compressive strengths of 3.4+/-0.3MPa, 8.4+/-0.8MPa and 15.3+/-1.8MPa, for ICIE16, PSrBG and 13-93 respectively. These porosities and compressive strength values are within the range of cancellous bone, and greater than previously reported foamed scaffolds. Dental pulp stem cells attached to the scaffold surfaces during in vitro culture and were viable. In vivo, the scaffolds were found to regenerate bone in a rabbit model according to X-ray micro tomography imaging. STATEMENT OF SIGNIFICANCE: This manuscript describes a new method for making scaffolds from bioactive glasses using highly bioactive glass compositions. The glass compositions have lower silica content that those that have been previously made into amorphous scaffolds and they have been designed to have similar network connectivity to that of the original (and commercially used) 45S5 Bioglass. The aim was to match Bioglass' bioactivity. The scaffolds retain the amorphous nature of bioactive glass while having an open pore structure and compressive strength similar to porous bone (the original 45S5 Bioglass crystallises during sintering, which can cause reduced bioactivity or instability). The new scaffolds showed unexpectedly rapid bone regeneration in a rabbit model.
Interconnected porous hydroxyapatite ceramics for bone tissue engineering
A biodegradable porous composite scaffold of PGA/beta-TCP for bone tissue engineering
DOI:10.1016/j.bone.2009.09.031
URL
PMID:19800045
[Cited within: 1]

Polyglycolic acid (PGA) and beta-tricalcium phosphate (beta-TCP) each have many applications as tissue repair materials. In this study, three-dimensional (3D) porous composite scaffolds of PGA/beta-TCP (in 1:1 and 1:3 weight ratios) were fabricated using the solvent casting and particulate leaching method. PGA/beta-TCP scaffolds with high porosity, interconnected 3D pores and rough surfaces were obtained and were observed using scanning electron microscopy (SEM) and micro-computed tomography (micro-CT). The PGA/beta-TCP scaffolds were investigated during the repair of critical bone defects (3 mm diameter, 2 mm depth) in rat femoral medial-epicondyles, compared with hydroxylapatite (HAP) and no implant as controls. Quantitative imageology analysis (volume and density of new bone) and qualitative histological evaluations (hematoxylin and eosin staining; tartrate-resistant acid phosphatase-hematoxylin counterstaining) were characterized using in vivo micro-CT images and histological sections at 0, 14, 30 and 90 days after surgery. Significant differences of all variables were tested by multivariate analysis (P<0.05). The results showed that the bone reformation by using the PGA/beta-TCP scaffolds began within 14 days of surgery, and were healing well at 30 days after surgery. By 90 days after surgery, the bone replacement was almost completed and presented a healthy bone appearance. The new bone mineral densities (mg/cm(3)) with HAP, PGA/beta-TCP (1:1) and PGA/beta-TCP (1:3) at 90 days after surgery were: 390.4+/-18.1, 563.8+/-26.9 and 606.3+/-26.9, respectively. The new bone mineral density with the PGA/beta-TCP scaffold was higher than with HAP (P<0.001), and with the PGA/beta-TCP (1:3) scaffold was higher than with the PGA/beta-TCP (1:1) scaffold at each time examined (P<0.05). The biodegradation percents (%) of HAP, PGA/beta-TCP (1:1) and PGA/beta-TCP (1:3) at 90 days after surgery were: 35.1+/-5.5, 99.0+/-1.0 and 96.2+/-3.3, respectively. The biodegradation percents of the PGA/beta-TCP scaffolds were higher than HAP at each time examined (P<0.01), and matched the osteogenesis rates. The PGA/beta-TCP scaffolds were almost replaced by new growing bone within 90 days after surgery. Thus the PGA/beta-TCP composite scaffold, especially weight ratio 1:3, exhibited a strong ability for osteogenesis, mineralization and biodegradation for bone replacement.
Freeze-drying as a novel biofabrication method for achieving a controlled microarchitecture within large, complex natural biomaterial scaffolds
DOI:10.1002/adhm.v6.21 URL [Cited within: 1]
Approaches to fabricating multiple-layered vascular scaffolds using hybrid electrospinning and thermally induced phase separation methods
DOI:10.1021/acs.iecr.5b03462 URL [Cited within: 1]
Bone tissue engineering using 3D printing
DOI:10.1016/j.mattod.2013.11.017 URL [Cited within: 2]
3D printing for the design and fabrication of polymer-based gradient scaffolds
DOI:10.1016/j.actbio.2017.03.030
URL
PMID:28342878
[Cited within: 1]

To accurately mimic the native tissue environment, tissue engineered scaffolds often need to have a highly controlled and varied display of three-dimensional (3D) architecture and geometrical cues. Additive manufacturing in tissue engineering has made possible the development of complex scaffolds that mimic the native tissue architectures. As such, architectural details that were previously unattainable or irreproducible can now be incorporated in an ordered and organized approach, further advancing the structural and chemical cues delivered to cells interacting with the scaffold. This control over the environment has given engineers the ability to unlock cellular machinery that is highly dependent upon the intricate heterogeneous environment of native tissue. Recent research into the incorporation of physical and chemical gradients within scaffolds indicates that integrating these features improves the function of a tissue engineered construct. This review covers recent advances on techniques to incorporate gradients into polymer scaffolds through additive manufacturing and evaluate the success of these techniques. As covered here, to best replicate different tissue types, one must be cognizant of the vastly different types of manufacturing techniques available to create these gradient scaffolds. We review the various types of additive manufacturing techniques that can be leveraged to fabricate scaffolds with heterogeneous properties and discuss methods to successfully characterize them. STATEMENT OF SIGNIFICANCE: Additive manufacturing techniques have given tissue engineers the ability to precisely recapitulate the native architecture present within tissue. In addition, these techniques can be leveraged to create scaffolds with both physical and chemical gradients. This work offers insight into several techniques that can be used to generate graded scaffolds, depending on the desired gradient. Furthermore, it outlines methods to determine if the designed gradient was achieved. This review will help to condense the abundance of information that has been published on the creation and characterization of gradient scaffolds and to provide a single review discussing both methods for manufacturing gradient scaffolds and evaluating the establishment of a gradient.
Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size
DOI:10.1089/ten.TEB.2012.0437
URL
PMID:23672709

Tissue engineering applications commonly encompass the use of three-dimensional (3D) scaffolds to provide a suitable microenvironment for the incorporation of cells or growth factors to regenerate damaged tissues or organs. These scaffolds serve to mimic the actual in vivo microenvironment where cells interact and behave according to the mechanical cues obtained from the surrounding 3D environment. Hence, the material properties of the scaffolds are vital in determining cellular response and fate. These 3D scaffolds are generally highly porous with interconnected pore networks to facilitate nutrient and oxygen diffusion and waste removal. This review focuses on the various fabrication techniques (e.g., conventional and rapid prototyping methods) that have been employed to fabricate 3D scaffolds of different pore sizes and porosity. The different pore size and porosity measurement methods will also be discussed. Scaffolds with graded porosity have also been studied for their ability to better represent the actual in vivo situation where cells are exposed to layers of different tissues with varying properties. In addition, the ability of pore size and porosity of scaffolds to direct cellular responses and alter the mechanical properties of scaffolds will be reviewed, followed by a look at nature's own scaffold, the extracellular matrix. Overall, the limitations of current scaffold fabrication approaches for tissue engineering applications and some novel and promising alternatives will be highlighted.
Additive manufacturing (3D printing): A review of materials, methods, applications and challenges
DOI:10.1016/j.compositesb.2018.02.012 URL
Three-dimensional plotted scaffolds with controlled pore size gradients: Effect of scaffold geometry on mechanical performance and cell seeding efficiency
DOI:10.1016/j.actbio.2010.11.003
URL
PMID:21056125
[Cited within: 1]

Scaffolds produced by rapid prototyping (RP) techniques have proved their value for tissue engineering applications, due to their ability to produce predetermined forms and structures featuring fully interconnected pore architectures. Nevertheless, low cell seeding efficiency and non-uniform distribution of cells remain major limitations when using such types of scaffold. This can be mainly attributed to the inadequate pore architecture of scaffolds produced by RP and the limited efficiency of cell seeding techniques normally adopted. In this study we aimed at producing scaffolds with pore size gradients to enhance cell seeding efficiency and control the spatial organization of cells within the scaffold. Scaffolds based on blends of starch with poly(epsilon-caprolactone) featuring both homogeneously spaced pores (based on pore sizes of 0.75 and 0.1 mm) and pore size gradients (based on pore sizes of 0.1-0.75-0.1 and 0.75-0.1-0.75 mm) were designed and produced by three-dimensional plotting. The mechanical performance of the scaffolds was characterized using dynamic mechanical analysis (DMA) and conventional compression testing under wet conditions and subsequently characterized using scanning electron microscopy and micro-computed tomography. Osteoblast-like cells were seeded onto such scaffolds to investigate cell seeding efficiency and the ability to control the zonal distribution of cells upon seeding. Scaffolds featuring continuous pore size gradients were originally produced. These scaffolds were shown to have intermediate mechanical and morphological properties compared with homogenous pore size scaffolds. The pore size gradient scaffolds improved seeding efficiency from approximately 35% in homogeneous scaffolds to approximately 70% under static culture conditions. Fluorescence images of cross-sections of the scaffolds revealed that scaffolds with pore size gradients induce a more homogeneous distribution of cells within the scaffold.
3D printing of calcium phosphate ceramics for bone tissue engineering and drug delivery
DOI:10.1007/s10439-016-1678-3
URL
PMID:27324800
[Cited within: 2]

Additive manufacturing, also known as 3D printing, has emerged over the past 3 decades as a disruptive technology for rapid prototyping and manufacturing. Vat polymerization, powder bed fusion, material extrusion, and binder jetting are distinct technologies of additive manufacturing, which have been used in a wide variety of fields, including biomedical research and tissue engineering. The ability to print biocompatible, patient-specific geometries with controlled macro- and micro-pores, and to incorporate cells, drugs and proteins has made 3D-printing ideal for orthopaedic applications, such as bone grafting. Herein, we performed a systematic review examining the fabrication of calcium phosphate (CaP) ceramics by 3D printing, their biocompatibility in vitro, and their bone regenerative potential in vivo, as well as their use in localized delivery of bioactive molecules or cells. Understanding the advantages and limitations of the different 3D printing approaches, CaP materials, and bioactive additives through critical evaluation of in vitro and in vivo evidence of efficacy is essential for developing new classes of bone graft substitutes that can perform as well as autografts and allografts or even surpass the performance of these clinical standards.
3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration
DOI:10.1016/j.biomaterials.2014.01.064
URL
PMID:24529628
[Cited within: 2]

Low temperature 3D printing of calcium phosphate scaffolds holds great promise for fabricating synthetic bone graft substitutes with enhanced performance over traditional techniques. Many design parameters, such as the binder solution properties, have yet to be optimized to ensure maximal biocompatibility and osteoconductivity with sufficient mechanical properties. This study tailored the phosphoric acid-based binder solution concentration to 8.75 wt% to maximize cytocompatibility and mechanical strength, with a supplementation of Tween 80 to improve printing. To further enhance the formulation, collagen was dissolved into the binder solution to fabricate collagen-calcium phosphate composites. Reducing the viscosity and surface tension through a physiologic heat treatment and Tween 80, respectively, enabled reliable thermal inkjet printing of the collagen solutions. Supplementing the binder solution with 1-2 wt% collagen significantly improved maximum flexural strength and cell viability. To assess the bone healing performance, we implanted 3D printed scaffolds into a critically sized murine femoral defect for 9 weeks. The implants were confirmed to be osteoconductive, with new bone growth incorporating the degrading scaffold materials. In conclusion, this study demonstrates optimization of material parameters for 3D printed calcium phosphate scaffolds and enhancement of material properties by volumetric collagen incorporation via inkjet printing.
Powder-based 3D printing for bone tissue engineering
DOI:10.1016/j.biotechadv.2016.03.009
URL
PMID:27086202
[Cited within: 2]

Bone tissue engineered 3-D constructs customized to patient-specific needs are emerging as attractive biomimetic scaffolds to enhance bone cell and tissue growth and differentiation. The article outlines the features of the most common additive manufacturing technologies (3D printing, stereolithography, fused deposition modeling, and selective laser sintering) used to fabricate bone tissue engineering scaffolds. It concentrates, in particular, on the current state of knowledge concerning powder-based 3D printing, including a description of the properties of powders and binder solutions, the critical phases of scaffold manufacturing, and its applications in bone tissue engineering. Clinical aspects and future applications are also discussed.
Synjournal of hydroxyapatite nanopowders via sucrose-templated sol-gel method
DOI:10.1111/jace.2003.86.issue-6 URL [Cited within: 1]
Solid free-form fabrication technology and its application to bone tissue engineering
DOI:10.15283/ijsc.2010.3.2.85
URL
PMID:24855546
[Cited within: 2]

The development of scaffolds for use in cell-based therapies to repair damaged bone tissue has become a critical component in the field of bone tissue engineering. However, design of scaffolds using conventional fabrication techniques has limited further advancement, due to a lack of the required precision and reproducibility. To overcome these constraints, bone tissue engineers have focused on solid free-form fabrication (SFF) techniques to generate porous, fully interconnected scaffolds for bone tissue engineering applications. This paper reviews the potential application of SFF fabrication technologies for bone tissue engineering with respect to scaffold fabrication. In the near future, bone scaffolds made using SFF apparatus should become effective therapies for bone defects.
Calcium phosphate cements for bone engineering and their biological properties
DOI:10.1038/boneres.2017.56
URL
PMID:29354304
[Cited within: 3]

Calcium phosphate cements (CPCs) are frequently used to repair bone defects. Since their discovery in the 1980s, extensive research has been conducted to improve their properties, and emerging evidence supports their increased application in bone tissue engineering. Much effort has been made to enhance the biological performance of CPCs, including their biocompatibility, osteoconductivity, osteoinductivity, biodegradability, bioactivity, and interactions with cells. This review article focuses on the major recent developments in CPCs, including 3D printing, injectability, stem cell delivery, growth factor and drug delivery, and pre-vascularization of CPC scaffolds via co-culture and tri-culture techniques to enhance angiogenesis and osteogenesis.
Three-dimensional fabrication of thick and densely populated soft constructs with complex and actively perfused channel network
DOI:10.1016/j.actbio.2017.10.047
URL
PMID:29102798
[Cited within: 1]

One of the fundamental steps needed to design functional tissues and, ultimately organs is the ability to fabricate thick and densely populated tissue constructs with controlled vasculature and microenvironment. To date, bioprinting methods have been employed to manufacture tissue constructs with open vasculature in a square-lattice geometry, where the majority lacks the ability to be directly perfused. Moreover, it appears to be difficult to fabricate vascular tissue constructs targeting the stiffness of soft tissues such as the liver. Here we present a method for the fabrication of thick (e.g. 1cm) and densely populated (e.g. 10millioncells.mL(-1)) tissue constructs with a three-dimensional (3D) four arm branch network and stiffness in the range of soft tissues (1-10kPa), which can be directly perfused on a fluidic platform for long time periods (>14days). Specifically, we co-print a 3D four-arm branch using water-soluble Poly(vinyl alcohol) (PVA) as main material and Poly(lactic acid) (PLA) as the support structure. The PLA support structure was selectively removed, and the water soluble PVA structure was used for creating a 3D vascular network within a customized extracellular matrix (ECM) targeting the stiffness of the liver and with encapsulated hepatocellular carcinoma (HepG2) cells. These constructs were directly perfused with medium inducing the proliferation of HepG2 cells and the formation of spheroids. The highest spheroid density was obtained with perfusion, but overall the tissue construct displayed two distinct zones, one of rapid proliferation and one with almost no cell division and high cell death. The created model, therefore, simulate gradients in tissues of necrotic regions in tumors. This versatile method could represent a fundamental step in the fabrication of large functional and complex tissues and finally organs. STATEMENT OF SIGNIFICANCE: Vascularization within hydrogels with mechanical properties in the range of soft tissues remains a challenge. To date, bioprinting have been employed to manufacture tissue constructs with open vasculature in a square-lattice geometry that are most of the time not perfused. This study shows the creation of densely populated tissue constructs with a 3D four arm branch network and stiffness in the range of soft tissues, which can be directly perfused. The cells encapsulated within the construct showed proliferation as a function of the vasculature distance, and the control of the micro-environment induced the encapsulated cells to aggregate in spheroids in specific positions. This method could be used for modeling tumors and for fabricating more complex and densely populated tissue constructs with translational potential.
Bioprinting of 3D hydrogels
DOI:10.1039/c5lc90069g
URL
PMID:26066320
[Cited within: 2]

Three-dimensional (3D) bioprinting has recently emerged as an extension of 3D material printing, by using biocompatible or cellular components to build structures in an additive, layer-by-layer methodology for encapsulation and culture of cells. These 3D systems allow for cell culture in a suspension for formation of highly organized tissue or controlled spatial orientation of cell environments. The in vitro 3D cellular environments simulate the complexity of an in vivo environment and natural extracellular matrices (ECM). This paper will focus on bioprinting utilizing hydrogels as 3D scaffolds. Hydrogels are advantageous for cell culture as they are highly permeable to cell culture media, nutrients, and waste products generated during metabolic cell processes. They have the ability to be fabricated in customized shapes with various material properties with dimensions at the micron scale. 3D hydrogels are a reliable method for biocompatible 3D printing and have applications in tissue engineering, drug screening, and organ on a chip models.
Recent trends in bioinks for 3D printing
DOI:10.1186/s40824-018-0122-1
URL
PMID:29636985
[Cited within: 1]

Background: The worldwide demand for the organ replacement or tissue regeneration is increasing steadily. The advancements in tissue engineering and regenerative medicine have made it possible to regenerate such damaged organs or tissues into functional organ or tissue with the help of 3D bioprinting. The main component of the 3D bioprinting is the bioink, which is crucial for the development of functional organs or tissue structures. The bioinks used in 3D printing technology require so many properties which are vital and need to be considered during the selection. Combination of different methods and enhancements in properties are required to develop more successful bioinks for the 3D printing of organs or tissue structures. Main body: This review consists of the recent state-of-art of polymer-based bioinks used in 3D printing for applications in tissue engineering and regenerative medicine. The subsection projects the basic requirements for the selection of successful bioinks for 3D printing and developing 3D tissues or organ structures using combinations of bioinks such as cells, biomedical polymers and biosignals. Different bioink materials and their properties related to the biocompatibility, printability, mechanical properties, which are recently reported for 3D printing are discussed in detail. Conclusion: Many bioinks formulations have been reported from cell-biomaterials based bioinks to cell-based bioinks such as cell aggregates and tissue spheroids for tissue engineering and regenerative medicine applications. Interestingly, more tunable bioinks, which are biocompatible for live cells, printable and mechanically stable after printing are emerging with the help of functional polymeric biomaterials, their modifications and blending of cells and hydrogels. These approaches show the immense potential of these bioinks to produce more complex tissue/organ structures using 3D bioprinting in the future.
The rheological behavior of a fast-setting calcium phosphate bone cement and its dependence on deformation conditions
DOI:10.1016/j.jmbbm.2017.05.017
URL
PMID:28505594
[Cited within: 1]

Calcium phosphate cements are osteoconductive biomaterials that are widely used for bone repair and regeneration applications, including spinal fusion, vertebroplasty, khyphoplasty, cranioplasty and periodontal surgeries. The flow and deformation behavior (rheology) and injectability of the calcium phosphate bone cements to the treatment site are governed by the setting kinetics of the cement during which the initially flowable, viscous cement paste transforms into a rigid elastic solid. Here time-dependent development of the linear viscoelastic properties of a brushite-forming calcium phosphate cement are characterized and linked to the mechanism and kinetics of the setting reaction and to the injectability window available during the surgical applications of the cement. The setting kinetics is shown to be a function of the deformation conditions that are utilized in rheological characterization, emphasizing the intimate relationships between setting kinetics, particle to particle network formation and deformation history. Furthermore, the preshearing of the calcium phosphate cement prior to injection and temperature are shown to alter the kinetics of the setting reaction and thus to provide additional degrees of freedom for the tailoring of the rheological behavior and injectability of the calcium phosphate cement.
Standard test method for compressive resistance of ceramic materials
GB/T 4740-1984.
Biological evaluation of medical devices — Part 14: Identification and quantification of degradation products from ceramics
ISO 10993-14:2001.
Effect of reaction systems and surfactant additives on the morphology evolution of hydroxyapatite nanorods obtained via a hydrothermal route
DOI:10.1016/j.apsusc.2010.12.067 URL [Cited within: 2]
Synjournal and characterization of carbonate hydroxyapatite
DOI:10.1023/a:1008975507498
URL
PMID:15348939
[Cited within: 1]

Substituted apatite ceramics are of clinical interest as they offer the potential to improve the bioactive properties of implants. Carbonate hydroxyapatite (CHA) has been synthesized by an aqueous precipitation method and precipitates with two different levels of carbonate, processed as powders. Sintering experiments were performed to establish the influence of carbonate in significantly reducing the temperature required to prepare high-density ceramics when compared with stoichiometric hydroxyapatite (HA). High-temperature X-ray diffraction was used to characterize the phase stability of the apatites on sintering. Increasing carbonate content was shown to reduce the temperature at which decomposition occurred, to phases of CaO and beta-TCP. Mechanical testing, performed using biaxial flexure, showed that the CHA specimens had strengths similar to stoichiometric HA.
Rietveld refinements and spectroscopic studies of the structure of Ca-deficient apatite
DOI:10.1016/j.biomaterials.2004.04.038
URL
PMID:15475062
[Cited within: 1]

Nine samples of Ca-deficient apatite (Ca-def Ap) were prepared from suspensions of CaHPO4 (monetite) at 90 degrees C by raising the pH from approximately 4 through release of NH3 produced by the hydrolysis of urea. Products were dried at 100 degrees C for 24h and studied by chemical analyses, X-ray powder diffraction (XRPD) (and Rietveld analysis of this data), Ca/P ratio determination (quantitative phase analysis of samples after heating to 900 degrees C from Rietveld analysis of XRPD data), scanning electron microscopy, He pycknometry, 1H and 31P MAS NMR spectrometry and Fourier transform infrared and Raman spectroscopy. All samples contained apatite, but three also contained monetite. Infrared and Raman spectroscopy confirmed the presence of HPO4(2-) and absence of carbonate ions in the six monetite-free samples. Mean results for the six samples were: a = 9.4320(40), c = 6.8751(31) A; unit cell formula from chemical analysis neglecting protonation of phosphate ion, Ca(9.303(50))(PO4)6(OH)(0.606(99)).1.97(12)H2O; theoretical density 3.10 g cm(-3); experimental density (mean for three samples) 3.15 g cm(-3); and Ca/P mole ratio from chemical analysis and phase analysis after heating to 900 degrees C, 1.550(8) and 1.550(2), respectively. An earlier assignment of a line at 6 ppm in the 1H NMR spectrum of similar samples to HPO4(2-) ions could not be confirmed; hence no information about the HPO4(2-) ion content could be derived, in disagreement with the previous NMR study. A shoulder at approximately 0.9 ppm relative to 85 wt% H3PO4 in the 31P NMR spectrum was assigned to HPO4(2-) ions. Occupancies from the Rietveld structure refinements indicated preferential loss of Ca from Ca2 sites compared with Ca1, but the loss was substantially smaller than expected from chemical analyses. It is suggested that imperfect modelling of the structure in the refinement, particularly disorder associated with the Ca2 site, resulted in errors in Ca2 occupancies. The P-O bonds were slightly shorter than those in stoichiometric hydroxyapatite, rather than longer as might be expected from protonation of phosphate tetrahedra. However, consideration of known acid phosphate structures indicated that it was unlikely that the increase in P-O lengths would be sufficient to be detected. The observed decrease was tentatively assigned to the presence of Ca2+ ion vacancies.
Hydroxyapatite nanocomposites: synthesis, sintering and mechanical properties
DOI:10.1016/j.ceramint.2012.09.023 URL [Cited within: 1]
Comparison of hydroxyapatite and beta tricalcium phosphate as bone substitutes after excision of bone tumors
DOI:10.1002/jbm.b.30136
URL
PMID:15376187
[Cited within: 2]

Long-term results are reported in 23 patients and short-term results in 30 patients presenting with bone tumors treated by curettage or resection followed by implantation of hydroxyapatite (HA) or highly purified beta-tricalcium phosphate (beta-TCP), respectively. Mean follow-up was 97 and 26 months in cases involving HA implantation and beta-TCP implantation, respectively. Radiographs revealed HA incorporation into host bone in all but two cases; moreover, no obvious evidence of HA biodegradation was observed. A single patient exhibited late deformity following implantation of HA. All grafted beta-TCP was, at least partially, absorbed and replaced by newly formed bone. The mean period required for the disappearance of radiolucent zones between the ceramics and host bone was 17 weeks in HA and 9.7 weeks in beta-TCP. Highly purified beta-TCP appears to be advantageous relative to HA for surgical intervention in bone tumors consequent to the nature of remodeling and superior osteoconductivity.
Comparison of osteoconductivity and absorbability of beta-tricalcium phosphate and hydroxyapatite in clinical scenario of opening wedge high tibial osteotomy
DOI:10.1007/s10856-016-5795-1
URL
PMID:27757780
[Cited within: 2]

The purpose of this study was to compare the osteoconductivity, and absorbability of hydroxyapatite or beta-tricalcium phosphate in clinical scenario of opening wedge high tibial osteotomy Total 41 knees of 40 patients with follow up period of more than 1 year were enrolled. These patients were divided into two groups, Group I (22 knees, 21 patients) used hydroxyapatite and Group II (19 knees, 19 patients) used beta-tricalcium phosphate as a substitute in the opening gap. According to proven method, the osteoconductivity was assessed radiographically by the extent of new bone formation at osteotomy space and absorbability was evaluated by measuring the area occupied by substitute at immediate postoperative, postoperative 6 months and 1 year. Regarding preoperative demographic data, no significant differences were found between two groups. No statistically significant differences were found between two groups regarding lower limb alignment (mechanical femorotibial angle, weight-bearing line%) and posterior tibial slope at postoperative and final follow up radiographs. Concerning the osteoconductivity, there were no significant differences between two groups in any zone. However, the absorption rate was significantly greater in the Group II than in Group I at 6 months (Group I: 13.7 +/- 6.8, group II: 35.3 +/- 15.8, P = 0.001) and 1 year (Group I: 24.2 +/- 6.3, Group II: 49.6 +/- 14.3, P < 0.0001). The complications related to bone substitutes were not observed. Both hydroxyapatite and beta-tricalcium phosphate showed satisfactory gap healing without complications and can be successfully used as alternative healing materials in opening wedge high tibial osteotomy. Our study showed that beta-tricalcium phosphate has superior absorbability than hydroxyapatite. But osteoconductivity showed no significant difference.
Injectability of calcium phosphate pastes
DOI:10.1016/j.biomaterials.2004.05.010
URL
PMID:15522757
[Cited within: 1]

A theoretical model was developed to assess ways to improve the injectability of calcium phosphate pastes. The theoretical results were then compared to experimental data obtained on calcium phosphate slips. The theoretical approach predicted that the injectability of a cement paste could be improved by an increase of the liquid-to-powder ratio, and a decrease of the particle size and the plastic limit (PL) of the powder. The theoretical results were confirmed by experimental data. Interestingly, an increase of the viscosity of the mixing liquid with small additions of xanthan had a positive effect on the paste injectability. This effect could be due to a change of the PL of the powder or to the lubricating effect of the polymer.
Ionic modification of calcium phosphate cement viscosity. Part I: hypodermic injection and strength improvement of apatite cement
DOI:10.1016/j.biomaterials.2003.08.066
URL
PMID:14741634
[Cited within: 1]

A broadening of the indications for which calcium phosphate cements (CPC) can be used, for example, in the field of vertebroplasty, would require injectable and higher strength materials. Unmodified CPC are not injectable due to a filter-pressing effect during injection. In this work we demonstrated that an effective method for improving the injection properties of CPC was by the use of sodium citrate solution as a liquid component. Cement consisting of tetracalcium phosphate (TTCP) and monetite (DCPA) mixed with water up to a powder:liquid ratio (P:L) of 3.3 g/ml had an injectability of approximately 60%. The use of 500 mM trisodium citrate solution instead of water decreased the viscosity of the cement paste to a point, where complete injectability (>95%) through an 800 microm diameter hypodermic needle could be achieved at low loads. The reduction in water demand of the cement effected by the use of sodium citrate enabled high P:L mixes to be formed which were 400% stronger than cements made with water. The effect was less pronounced with compacted cements such that at 9 MPa applied pressure, 58% improvement was obtained and at 50 MPa 36% improvement was measured yielding a cement with a compressive strength of 154 MPa. The liquefying effect of sodium citrate was thought to derive from a strong increase in the surface charge of both the reactants and the product as determined by zeta-potential measurement.
Hydroxyapatite from cuttlefish bone: isolation, characterizations, and applications
DOI:10.1007/s12257-018-0169-9 URL [Cited within: 1]
Reaction of calcium phosphate cements with different amounts of tetracalcium phosphate and dicalcium phosphate anhydrous
DOI:10.1002/(sici)1097-4636(19990915)46:4<504::aid-jbm8>3.0.co;2-h
URL
PMID:10398011
[Cited within: 1]

Calcium phosphate cements (CPCs) with different amounts of tetracalcium phosphate (TTCP) and dicalcium phosphate anhydrous (DCPA) (TTCP/DCPA molar ratio from 0.25 to 2.00) were prepared to further understand the setting reaction and the factors that could influence the properties of CPCs. Quantitative X-ray diffraction patterns, Fourier transform IR spectra, and diametral tensile strength of the set mass were measured along with pH measurements of the CPC suspension. Calcium-deficient hydroxyapatite (d-HAP) with a calcium to phosphate molar ratio of approximately 1.5 was formed initially in the CPC setting consisting of an equimolar mixture of TTCP and DCPA. This gradually transformed into stoichiometric HA (s-HA) with increasing incubation time. The s-HA was formed in the initial stage when the CPC contained an excess amount of TTCP. In contrast, maturation to s-HAP was slow when the CPC contained excess amounts of DCPA. The highest mechanical strength of set CPC was associated with an equimolar mixture of TTCP and DCPA, and the mechanical strength decreased as the TTCP/DCPA molar ratio deviated from 1.00. We concluded, therefore, that the setting reaction and the nature of the resulting set mass are dependent on the molar ratios of TTCP and DCPA.
Porous hydroxyapatite for artificial bone applications
DOI:10.1016/j.stam.2006.11.017 URL [Cited within: 1]
Investigation of the mechanical properties and porosity relationships in selective laser-sintered polyhedral for functionally graded scaffolds
DOI:10.1016/j.actbio.2010.09.024
URL
PMID:20883840
[Cited within: 1]

An important requirement for a bone tissue engineering scaffold is a stiffness gradient that mimics that of native bone. Such scaffolds can be achieved by controlling their structure and porosity and are termed functionally graded scaffolds (FGS). Currently, the main challenges in FGS fabrication include the iterative and tedious design process as well as a heavy reliance on the user's CAD/CAM skills. This work aims to bring automated FGS production a step closer by providing a database that correlates scaffold porosity values and the corresponding compressive stiffness and integrating it into the design process. To achieve this goal, scaffolds with different structural configurations were designed using CASTS (Computer Aided System for Tissue Scaffolds), an in-house developed library system consisting of 13 different polyhedral units that can be assembled into scaffold structures. Polycaprolactone (PCL) was chosen as the scaffold material, while selective laser sintering, a powder-based rapid prototyping or additive manufacturing system was employed to fabricate the scaffolds. Mathematical relations correlating scaffold porosity and compressive stiffness readings were formulated and compiled. In addition, cytotoxicity assessment was conducted to evaluate the toxicity of the fabricated PCL scaffolds. Lastly, a brief demonstration of how the formulated relations are used in the FGS design process is presented.
In vitro degradation and cell response of calcium carbonate composite ceramic in comparison with other synthetic bone substitute materials
DOI:10.1016/j.msec.2015.02.019
URL
PMID:25746269
[Cited within: 1]

The robust calcium carbonate composite ceramics (CC/PG) can be acquired by fast sintering calcium carbonate at a low temperature (650 degrees C) using a biocompatible, degradable phosphate-based glass (PG) as sintering agent. In the present study, the in vitro degradation and cell response of CC/PG were assessed and compared with 4 synthetic bone substitute materials, calcium carbonate ceramic (CC), PG, hydroxyapatite (HA) and beta-tricalcium phosphate (beta-TCP) ceramics. The degradation rates in decreasing order were as follows: PG, CC, CC/PG, beta-TCP, and HA. The proliferation of rat bone mesenchymal stem cells (rMSCs) cultured on the CC/PG was comparable with that on CC and PG, but inferior to HA and beta-TCP. The alkaline phosphatase (ALP) activity of rMSCs on CC/PG was lower than PG, comparable with beta-TCP, but higher than HA. The rMSCs on CC/PG and PG had enhanced gene expression in specific osteogenic markers, respectively. Compared to HA and beta-TCP, the rMSCs on the CC/PG expressed relatively lower level of collagen I and runt-related transcription factor 2, but showed more considerable expression of osteopontin. Although CC, PG, HA, and beta-TCP possessed impressive performances in some specific aspects, they faced extant intrinsic drawbacks in either degradation rate or mechanical strength. Based on considerable compressive strength, moderate degradation rate, good cell response, and being free of obvious shortcoming, the CC/PG is promising as another choice for bone substitute materials.
Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation
DOI:10.1002/jcb.10435
URL
PMID:12616527
[Cited within: 1]

Fracture healing is a specialized post-natal repair process that recapitulates aspects of embryological skeletal development. While many of the molecular mechanisms that control cellular differentiation and growth during embryogenesis recur during fracture healing, these processes take place in a post-natal environment that is unique and distinct from those which exist during embryogenesis. This Prospect Article will highlight a number of central biological processes that are believed to be crucial in the embryonic differentiation and growth of skeletal tissues and review the functional role of these processes during fracture healing. Specific aspects of fracture healing that will be considered in relation to embryological development are: (1) the anatomic structure of the fracture callus as it evolves during healing; (2) the origins of stem cells and morphogenetic signals that facilitate the repair process; (3) the role of the biomechanical environment in controlling cellular differentiation during repair; (4) the role of three key groups of soluble factors, pro-inflammatory cytokines, the TGF-beta superfamily, and angiogenic factors, during repair; and (5) the relationship of the genetic components that control bone mass and remodeling to the mechanisms that control skeletal tissue repair in response to fracture.
Ectopic bone formation by microporous calcium phosphate ceramic particles in sheep muscles
DOI:10.1016/j.bone.2005.02.017
URL
PMID:15869915
[Cited within: 1]

Calcium phosphate ceramics are widely used in bone reconstructive surgery because of their osteconductive properties. However, these materials generally lack osteoinductive properties required to support bone healing in large defects. In this article, we study the osteoinductive potential of calcium phosphate ceramic particles implanted for 6 months into the dorsal muscles of eight adult female sheep. Microporous biphasic calcium phosphate (MBCP) granules of 1-2 mm composed of hydroxyapatite and beta-tricalcium phosphate (60/40) had macropores of 450 microm, micropores of 0.43 microm, and a specific surface area of 1.8 m(2)/g. After 6 months in the back muscles of sheep, the explants composed of MBCP granules were hard and encapsulated by normal muscle tissue. Ectopic bone formation with Haversian structures was observed in close contact with the MBCP granules in histological sections. Back-scattered electron microscopy and micro-computed tomography indicated that approximately 10% of well-mineralized bone with mature osteocytes had formed between or upon the granules. The ectopic bone showed trabeculae bridging the MBCP granules. Both the number and thickness of the trabeculae formed between the MBCP particles were comparable to those measured in spongious bone. The overall results therefore confirmed the presence of mature bone after intramuscular implantation of MBCP granules. The different hypotheses explaining ectopic bone formation induced by MBCP granules are discussed. Synthetic bone substitutes with osteoinductive properties could be used in bone reconstructive surgery.
Osteogenic responses to extraskeletally implanted synthetic porous calcium phosphate ceramics: an early stage histomorphological study in dogs
DOI:10.1023/a:1018540024082
URL
PMID:15348821
[Cited within: 1]

In this experiment, synthetic porous calcium phosphate ceramics (hydroxyapatite-tricalcium phosphate) were prepared and implanted in dorsal muscles of dogs. The purpose was to study the biological processes prior to and during the morphogenesis of bone in extraskeletally implanted porous calcium phosphate ceramics. Specimens were harvested after implantation for 7, 15, 30, 45, 60, 90 and 120 days. Decalcified and undecalcified sections were prepared for alkaline phosphatase (ALP) histochemical localization and comparative histological analysis. The results show that bone morphogenesis in the pore regions of the extraskeletally implanted ceramics follows a complex process involving clot formation, vascular invasion, granulation-like tissue formation, polymorphic cell aggregation, osteoblast differentiation and bone formation. The characteristic feature preceding bone formation was polymorphic cell aggregation on the pore inner surface and near the invading capillaries or small venules. These cells were of various sizes and shapes, and some of them were positive for ALP activity. ALP-positive cell aggregates were more numerous where capillaries or venules were close to the pore inner surface. Osteoblast differentiation occurred within the cell clusters aggregated on the pore inner surface and bone matrix was secreted in direct contact with the ceramics. During bone formation, capillaries or small venules were always found close to the developing fronts of the osseous nidi. It is suggested that those cells which first appeared near the invading vasculature, the cells which aggregated on the pore inner surface and those cells which finally differentiated into osteoblasts may be interrelated in some way.
Biological properties of calcium phosphate biomaterials for bone repair: a review
DOI:10.1039/C7RA11278E URL [Cited within: 3]
Osteoinduction by biomaterials physicochemical and structural influences
DOI:10.1002/jbm.a.30712
URL
PMID:16557498
[Cited within: 1]

Osteoinduction by biomaterials has been shown to be a real phenomenon by many investigators in the last decade. The exact mechanism of this phenomenon is, however, still largely unknown. This in vivo study in goats was performed to get insight into processes governing the phenomenon of osteoinduction by biomaterials and had four main goals: (i) to further investigate the influence of physicochemical properties and structure on biomaterial osteoinductive potential, (ii) to investigate the influence of implant size on the amount of induced bone, (iii) to investigate implantation site dependence, and (iv) to investigate changes occurring on the surface of the material after implantation. Intramuscular implantations of four different biphasic calcium phosphate ceramics, consisting of hydroxyapatite and beta-tricalcium phosphate and a carbonated apatite ceramic, indicated that, for a maximal osteoinductive potential, there is an optimal specific surface area for each material type. It was further shown that a decrease of the implant size with a half significantly decreased the relative amount of induced bone. In addition, subcutaneous implantation did not give rise to ectopic bone formation in any of the animals, while bone was induced in most animals intramuscularly. Analysis of the surfaces of the materials after subcutaneous implantation inside diffusion chambers indicated that the increased specific surface area leads to more surface reactivity, which is hypothesized to be essential for osteoinductivity by biomaterials.
Finite element representation of bone substitute remodelling in the jaw bone
DOI:10.1515/BMT.2008.039 URL [Cited within: 1]