Biomaterials Translational ›› 2022, Vol. 3 ›› Issue (2): 105-115.doi: 10.12336/biomatertransl.2022.02.004
• REVIEW • Previous Articles Next Articles
Melika Sahranavard1,*( ), Soulmaz Sarkari2, SeyedehMina Safavi2, Farnaz Ghorbani3
), Soulmaz Sarkari2, SeyedehMina Safavi2, Farnaz Ghorbani3
Received:2022-04-10
Revised:2022-05-11
Accepted:2022-06-01
Online:2022-06-28
Published:2022-06-28
Contact:
Melika Sahranavard
E-mail:Melika.sahra@yahoo.com
About author:Melika Sahranavard, Melika.sahra@yahoo.com.
Sahranavard, M;. Sarkari, S;. Safavi, S;. Ghorbani, F. Three-dimensional bio-printing of decellularized extracellular matrix-based bio-inks for cartilage regeneration: a systematic review. Biomater Transl. 2022, 3(2), 105-115.
| Advantages | Disadvantages | |
|---|---|---|
| Organ/tissue-derived dECM | Similarity to native ECM (architectural/mechanical) | Availability or lack thereof |
| Easy preparation at large scale | Present stem cell niche | |
| - | Large batch-to-batch differences | |
| Cell-derived dECM | Possibility of preparation in limited regions | Low similarity to native ECM |
| Present stem cells, cells | Difficult to preparation at large scale | |
| Small batch-to-batch differences | - |
Table 1. Differences between organ/tissue dECM and cell-derived dECM
| Advantages | Disadvantages | |
|---|---|---|
| Organ/tissue-derived dECM | Similarity to native ECM (architectural/mechanical) | Availability or lack thereof |
| Easy preparation at large scale | Present stem cell niche | |
| - | Large batch-to-batch differences | |
| Cell-derived dECM | Possibility of preparation in limited regions | Low similarity to native ECM |
| Present stem cells, cells | Difficult to preparation at large scale | |
| Small batch-to-batch differences | - |
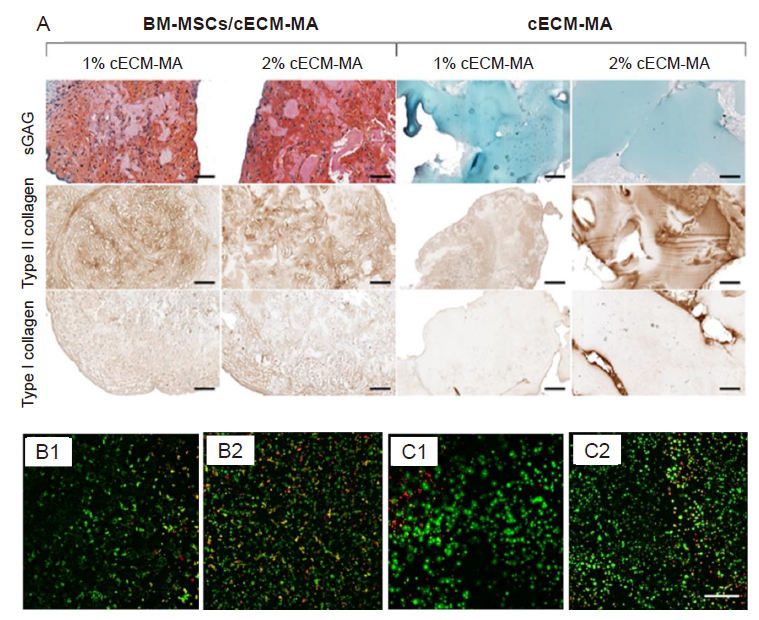
Figure 2. (A) The histological staining for type I and II collagens and sGAGs of cECM-MA bio-inks contained/free BM-MSCs. Cell contained bio-inks as expected showed higher type I and II collagens and sGAGs. Reprinted from Behan et al.82 (B, C) The cell viability of cis-5-norbornene-endo-2,3-dicarboxylic anhydride-modified PVA samples (B) and cis-5-norbornene-endo-2,3-dicarboxylic anhydride-modified PVA contained solubilized dECM (C) after 1 and 7 days. Reprinted from Setayeshmehr et al.85 Scale bars: 100 µm. BM-MSCs: Bone marrow-derived mesenchymal stem cells; cECM-MA: methacrylated cartilage ECM-based hydrogel/bio-ink; dECM: decellularized extracellular matrix; PVA: poly (vinyl alcohol); sGAGs: sulphated glycosaminoglycans.
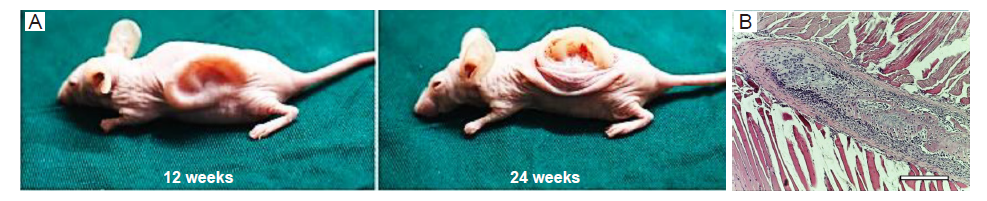
Figure 3. (A) The in-vivo auricular tissue regeneration on the back of nude mice after 12 and 24 weeks. Reprinted from Jia et al.90 (B) Microscopic observation of in-vivo investigation using decellularized extracellular matrix and mesenchymal stem cell bio-ink after 1 week. There was indirect osteogenesis in the muscle tissue near the scaffold. Scale bar: 800 μm. Reprinted from Isaeva et al.91
| 1. |
Lee, S.; Choi, J.; Youn, J.; Lee, Y.; Kim, W.; Choe, S.; Song, J.; Reis, R. L.; Khang, G. Development and evaluation of gellan gum/silk fibroin/chondroitin sulfate ternary injectable hydrogel for cartilage tissue engineering. Biomolecules. 2021, 11, 1184.
doi: 10.3390/biom11081184 URL |
| 2. |
Zhang, Y.; Liu, X.; Zeng, L.; Zhang, J.; Zuo, J.; Zou, J.; Ding, J.; Chen, X. Polymer fiber scaffolds for bone and cartilage tissue engineering. Adv Funct Mater. 2019, 29, 1903279.
doi: 10.1002/adfm.201903279 URL |
| 3. |
Kreller, T.; Distler, T.; Heid, S.; Gerth, S.; Detsch, R.; Boccaccini, A. R. Physico-chemical modification of gelatine for the improvement of 3D printability of oxidized alginate-gelatine hydrogels towards cartilage tissue engineering. Mater Des. 2021, 208, 109877.
doi: 10.1016/j.matdes.2021.109877 URL |
| 4. |
Gan, D.; Xu, T.; Xing, W.; Wang, M.; Fang, J.; Wang, K.; Ge, X.; Chan, C. W.; Ren, F.; Tan, H.; Lu, X. Mussel-inspired dopamine oligomer intercalated tough and resilient gelatin methacryloyl (GelMA) hydrogels for cartilage regeneration. J Mater Chem B. 2019, 7, 1716-1725.
doi: 10.1039/C8TB01664J URL |
| 5. | Ghorbani, F.; Zamanian, A.; Kermanian, F.; Shamoosi, A. A bioinspired 3D shape olibanum-collagen-gelatin scaffolds with tunable porous microstructure for efficient neural tissue regeneration. Biotechnol Prog. 2020, 36, e2918. |
| 6. |
Zhang, X.; Liu, Y.; Luo, C.; Zhai, C.; Li, Z.; Zhang, Y.; Yuan, T.; Dong, S.; Zhang, J.; Fan, W. Crosslinker-free silk/decellularized extracellular matrix porous bioink for 3D bioprinting-based cartilage tissue engineering. Mater Sci Eng C Mater Biol Appl. 2021, 118, 111388.
doi: 10.1016/j.msec.2020.111388 URL |
| 7. | Fu, L.; Li, P.; Li, H.; Gao, C.; Yang, Z.; Zhao, T.; Chen, W.; Liao, Z.; Peng, Y.; Cao, F.; Sui, X.; Liu, S.; Guo, Q. The application of bioreactors for cartilage tissue engineering: advances, limitations, and future perspectives. Stem Cells Int. 2021, 2021, 6621806. |
| 8. |
Sahranavard, M.; Zamanian, A.; Ghorbani, F.; Shahrezaee, M. H. A critical review on three dimensional-printed chitosan hydrogels for development of tissue engineering. Bioprinting. 2020, 17, e00063.
doi: 10.1016/j.bprint.2019.e00063 URL |
| 9. |
Lee, H.; Han, W.; Kim, H.; Ha, D. H.; Jang, J.; Kim, B. S.; Cho, D. W. Development of liver decellularized extracellular matrix bioink for three-dimensional cell printing-based liver tissue engineering. Biomacromolecules. 2017, 18, 1229-1237.
doi: 10.1021/acs.biomac.6b01908 URL |
| 10. |
Bandyopadhyay, A.; Mandal, B. B.; Bhardwaj, N. 3D bioprinting of photo-crosslinkable silk methacrylate (SilMA)-polyethylene glycol diacrylate (PEGDA) bioink for cartilage tissue engineering. J Biomed Mater Res A. 2022, 110, 884-898.
doi: 10.1002/jbm.a.37336 URL |
| 11. |
Choi, J. H.; Park, A.; Lee, W.; Youn, J.; Rim, M. A.; Kim, W.; Kim, N.; Song, J. E.; Khang, G. Preparation and characterization of an injectable dexamethasone-cyclodextrin complexes-loaded gellan gum hydrogel for cartilage tissue engineering. J Control Release. 2020, 327, 747-765.
doi: 10.1016/j.jconrel.2020.08.049 URL |
| 12. |
Ni, T.; Liu, M.; Zhang, Y.; Cao, Y.; Pei, R. 3D bioprinting of bone marrow mesenchymal stem cell-laden silk fibroin double network scaffolds for cartilage tissue repair. Bioconjug Chem. 2020, 31, 1938-1947.
doi: 10.1021/acs.bioconjchem.0c00298 URL |
| 13. |
Tsai, W. B.; Chen, W. T.; Chien, H. W.; Kuo, W. H.; Wang, M. J. Poly(dopamine) coating of scaffolds for articular cartilage tissue engineering. Acta Biomater. 2011, 7, 4187-4194.
doi: 10.1016/j.actbio.2011.07.024 URL |
| 14. |
Chen, Z.; Xiao, H.; Zhang, H.; Xin, Q.; Zhang, H.; Liu, H.; Wu, M.; Zuo, L.; Luo, J.; Guo, Q.; Ding, C.; Tan, H.; Li, J. Heterogenous hydrogel mimicking the osteochondral ECM applied to tissue regeneration. J Mater Chem B. 2021, 9, 8646-8658.
doi: 10.1039/D1TB00518A URL |
| 15. |
Xu, Y.; Shi, G.; Tang, J.; Cheng, R.; Shen, X.; Gu, Y.; Wu, L.; Xi, K.; Zhao, Y.; Cui, W.; Chen, L. ECM-inspired micro/nanofibers for modulating cell function and tissue generation. Sci Adv. 2020, 6, eabc2036.
doi: 10.1126/sciadv.abc2036 URL |
| 16. | Nam, S. Y.; Park, S. H. ECM based bioink for tissue mimetic 3D bioprinting. Adv Exp Med Biol. 2018, 1064, 335-353. |
| 17. |
Kim, B. S.; Das, S.; Jang, J.; Cho, D. W. Decellularized extracellular matrix-based bioinks for engineering tissue- and organ-specific microenvironments. Chem Rev. 2020, 120, 10608-10661.
doi: 10.1021/acs.chemrev.9b00808 URL |
| 18. | Adamski, M.; Fontana, G.; Gershlak, J. R.; Gaudette, G. R.; Le, H. D.; Murphy, W. L. Two methods for decellularization of plant tissues for tissue engineering applications. J Vis Exp. 2018, 57586. |
| 19. |
Porzionato, A.; Stocco, E.; Barbon, S.; Grandi, F.; Macchi, V.; De Caro, R. Tissue-engineered grafts from human decellularized extracellular matrices: a systematic review and future perspectives. Int J Mol Sci. 2018, 19, 4117.
doi: 10.3390/ijms19124117 URL |
| 20. |
Harris, A. F.; Lacombe, J.; Zenhausern, F. The emerging role of decellularized plant-based scaffolds as a new biomaterial. Int J Mol Sci. 2021, 22, 12347.
doi: 10.3390/ijms222212347 URL |
| 21. |
Kim, H. S.; Mandakhbayar, N.; Kim, H. W.; Leong, K. W.; Yoo, H. S. Protein-reactive nanofibrils decorated with cartilage-derived decellularized extracellular matrix for osteochondral defects. Biomaterials. 2021, 269, 120214.
doi: 10.1016/j.biomaterials.2020.120214 URL |
| 22. |
Crapo, P. M.; Gilbert, T. W.; Badylak, S. F. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011, 32, 3233-3243.
doi: 10.1016/j.biomaterials.2011.01.057 URL |
| 23. |
Kabirian, F.; Mozafari, M. Decellularized ECM-derived bioinks: prospects for the future. Methods. 2020, 171, 108-118.
doi: 10.1016/j.ymeth.2019.04.019 URL |
| 24. | Kheir, E.; Stapleton, T.; Shaw, D.; Jin, Z.; Fisher, J.; Ingham, E. Development and characterization of an acellular porcine cartilage bone matrix for use in tissue engineering. J Biomed Mater Res A. 2011, 99, 283-294. |
| 25. | Galliger, Z.; Panoskaltsis-Mortari, A. Tracheal cartilage isolation and decellularization. Methods Mol Biol. 2018, 1577, 155-160. |
| 26. |
Schneider, C.; Lehmann, J.; van Osch, G. J.; Hildner, F.; Teuschl, A.; Monforte, X.; Miosga, D.; Heimel, P.; Priglinger, E.; Redl, H.; Wolbank, S.; Nürnberger, S. Systematic comparison of protocols for the preparation of human articular cartilage for use as scaffold material in cartilage tissue engineering. Tissue Eng Part C Methods. 2016, 22, 1095-1107.
doi: 10.1089/ten.tec.2016.0380 URL |
| 27. |
Ravichandran, A.; Murekatete, B.; Moedder, D.; Meinert, C.; Bray, L. J. Photocrosslinkable liver extracellular matrix hydrogels for the generation of 3D liver microenvironment models. Sci Rep. 2021, 11, 15566.
doi: 10.1038/s41598-021-94990-z URL |
| 28. |
Reing, J. E.; Brown, B. N.; Daly, K. A.; Freund, J. M.; Gilbert, T. W.; Hsiong, S. X.; Huber, A.; Kullas, K. E.; Tottey, S.; Wolf, M. T.; Badylak, S. F. The effects of processing methods upon mechanical and biologic properties of porcine dermal extracellular matrix scaffolds. Biomaterials. 2010, 31, 8626-8633.
doi: 10.1016/j.biomaterials.2010.07.083 URL |
| 29. |
Wang, Z.; Li, Z.; Li, Z.; Wu, B.; Liu, Y.; Wu, W. Cartilaginous extracellular matrix derived from decellularized chondrocyte sheets for the reconstruction of osteochondral defects in rabbits. Acta Biomater. 2018, 81, 129-145.
doi: 10.1016/j.actbio.2018.10.005 URL |
| 30. |
Rahman, S.; Griffin, M.; Naik, A.; Szarko, M.; Butler, P. E. M. Optimising the decellularization of human elastic cartilage with trypsin for future use in ear reconstruction. Sci Rep. 2018, 8, 3097.
doi: 10.1038/s41598-018-20592-x URL |
| 31. |
O’Neill, J. D.; Anfang, R.; Anandappa, A.; Costa, J.; Javidfar, J.; Wobma, H. M.; Singh, G.; Freytes, D. O.; Bacchetta, M. D.; Sonett, J. R.; Vunjak-Novakovic, G. Decellularization of human and porcine lung tissues for pulmonary tissue engineering. Ann Thorac Surg. 2013, 96, 1046-1055; discussion 1055-1056.
doi: 10.1016/j.athoracsur.2013.04.022 URL |
| 32. |
Keane, T. J.; Swinehart, I. T.; Badylak, S. F. Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods. 2015, 84, 25-34.
doi: 10.1016/j.ymeth.2015.03.005 URL |
| 33. |
Bordbar, S.; Lotfi Bakhshaiesh, N.; Khanmohammadi, M.; Sayahpour, F. A.; Alini, M.; Baghaban Eslaminejad, M. Production and evaluation of decellularized extracellular matrix hydrogel for cartilage regeneration derived from knee cartilage. J Biomed Mater Res A. 2020, 108, 938-946.
doi: 10.1002/jbm.a.36871 URL |
| 34. |
Ghassemi, T.; Saghatoleslami, N.; Mahdavi-Shahri, N.; Matin, M. M.; Gheshlaghi, R.; Moradi, A. A comparison study of different decellularization treatments on bovine articular cartilage. J Tissue Eng Regen Med. 2019, 13, 1861-1871.
doi: 10.1002/term.2936 URL |
| 35. |
Zhou, J.; Fritze, O.; Schleicher, M.; Wendel, H. P.; Schenke-Layland, K.; Harasztosi, C.; Hu, S.; Stock, U. A. Impact of heart valve decellularization on 3-D ultrastructure, immunogenicity and thrombogenicity. Biomaterials. 2010, 31, 2549-2554.
doi: 10.1016/j.biomaterials.2009.11.088 URL |
| 36. | Tavassoli, A.; Matin, M. M.; Niaki, M. A.; Mahdavi-Shahri, N.; Shahabipour, F. Mesenchymal stem cells can survive on the extracellular matrix-derived decellularized bovine articular cartilage scaffold. Iran J Basic Med Sci. 2015, 18, 1221-1227. |
| 37. | Azhim, A.; Ono, T.; Fukui, Y.; Morimoto, Y.; Furukawa, K.; Ushida, T. Preparation of decellularized meniscal scaffolds using sonication treatment for tissue engineering. Annu Int Conf IEEE Eng Med Biol Soc. 2013, 2013, 6953-6956. |
| 38. |
Mendibil, U.; Ruiz-Hernandez, R.; Retegi-Carrion, S.; Garcia-Urquia, N.; Olalde-Graells, B.; Abarrategi, A. Tissue-specific decellularization methods: rationale and strategies to achieve regenerative compounds. Int J Mol Sci. 2020, 21, 5447.
doi: 10.3390/ijms21155447 URL |
| 39. |
Guimaraes, A. B.; Correia, A. T.; Alves, B. P.; Da Silva, R. S.; Martins, J. K.; Pêgo-Fernandes, P. M.; Xavier, N. S.; Dolhnikoff, M.; Cardoso, P. F. G. Evaluation of a physical-chemical protocol for porcine tracheal decellularization. Transplant Proc. 2019, 51, 1611-1613.
doi: 10.1016/j.transproceed.2019.01.042 URL |
| 40. |
Al-Qurayshi, Z.; Wafa, E. I.; Hoffman, H.; Chang, K.; Salem, A. K. Tissue-engineering the larynx: Effect of decellularization on human laryngeal framework and the cricoarytenoid joint. J Biomed Mater Res B Appl Biomater. 2021, 109, 2030-2040.
doi: 10.1002/jbm.b.34851 URL |
| 41. |
Singh, S.; Afara, I. O.; Tehrani, A. H.; Oloyede, A. Effect of decellularization on the load-bearing characteristics of articular cartilage matrix. Tissue Eng Regen Med. 2015, 12, 294-305.
doi: 10.1007/s13770-014-0083-y URL |
| 42. |
Khajavi, M.; Hajimoradloo, A.; Zandi, M.; Pezeshki-Modaress, M.; Bonakdar, S.; Zamani, A. Fish cartilage: a promising source of biomaterial for biological scaffold fabrication in cartilage tissue engineering. J Biomed Mater Res A. 2021, 109, 1737-1750.
doi: 10.1002/jbm.a.37169 URL |
| 43. |
Giraldo-Gomez, D. M.; Leon-Mancilla, B.; Del Prado-Audelo, M. L.; Sotres-Vega, A.; Villalba-Caloca, J.; Garciadiego-Cazares, D.; Piña-Barba, M. C. Trypsin as enhancement in cyclical tracheal decellularization: Morphological and biophysical characterization. Mater Sci Eng C Mater Biol Appl. 2016, 59, 930-937.
doi: 10.1016/j.msec.2015.10.094 URL |
| 44. | Keane, T. J.; Saldin, L. T.; Badylak, S. F. 4 - Decellularization of mammalian tissues:Preparing extracellular matrix bioscaffolds. In Characterisation and design of tissue scaffolds, Tomlins, P., ed. Woodhead Publishing: 2016; pp 75-103. |
| 45. | Zhang, X.; Chen, X.; Hong, H.; Hu, R.; Liu, J.; Liu, C. Decellularized extracellular matrix scaffolds: Recent trends and emerging strategies in tissue engineering. Bioact Mater. 2022, 10, 15-31. |
| 46. |
Li, J.; Cai, Z.; Cheng, J.; Wang, C.; Fang, Z.; Xiao, Y.; Feng, Z. G.; Gu, Y. Characterization of a heparinized decellularized scaffold and its effects on mechanical and structural properties. J Biomater Sci Polym Ed. 2020, 31, 999-1023.
doi: 10.1080/09205063.2020.1736741 URL |
| 47. |
Phan, N. V.; Wright, T.; Rahman, M. M.; Xu, J.; Coburn, J. M. In vitro biocompatibility of decellularized cultured plant cell-derived matrices. ACS Biomater Sci Eng. 2020, 6, 822-832.
doi: 10.1021/acsbiomaterials.9b00870 URL |
| 48. |
Zang, M.; Zhang, Q.; Chang, E. I.; Mathur, A. B.; Yu, P. Decellularized tracheal matrix scaffold for tissue engineering. Plast Reconstr Surg. 2012, 130, 532-540.
doi: 10.1097/PRS.0b013e31825dc084 URL |
| 49. |
Kang, H.; Peng, J.; Lu, S.; Liu, S.; Zhang, L.; Huang, J.; Sui, X.; Zhao, B.; Wang, A.; Xu, W.; Luo, Z.; Guo, Q. In vivo cartilage repair using adipose-derived stem cell-loaded decellularized cartilage ECM scaffolds. J Tissue Eng Regen Med. 2014, 8, 442-453.
doi: 10.1002/term.1538 URL |
| 50. |
Hayrapetyan, L.; Arestakesyan, H.; Margaryan, A.; Oganesyan, A.; Grigoryan, V.; Karapetyan, A. Comparison of articular and auricular cartilages: decellularization, cell proliferation rate, and infiltration in scaffolds. Res Biomed Eng. 2021, 37, 193-200.
doi: 10.1007/s42600-021-00141-8 URL |
| 51. |
Amirazad, H.; Dadashpour, M.; Zarghami, N. Application of decellularized bone matrix as a bioscaffold in bone tissue engineering. J Biol Eng. 2022, 16, 1.
doi: 10.1186/s13036-021-00282-5 URL |
| 52. |
Visscher, D. O.; Lee, H.; van Zuijlen, P. P. M.; Helder, M. N.; Atala, A.; Yoo, J. J.; Lee, S. J. A photo-crosslinkable cartilage-derived extracellular matrix bioink for auricular cartilage tissue engineering. Acta Biomater. 2021, 121, 193-203.
doi: 10.1016/j.actbio.2020.11.029 URL |
| 53. |
Pati, F.; Jang, J.; Ha, D. H.; Won Kim, S.; Rhie, J. W.; Shim, J. H.; Kim, D. H.; Cho, D. W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun. 2014, 5, 3935.
doi: 10.1038/ncomms4935 URL |
| 54. |
Shen, Y.; Xu, Y.; Yi, B.; Wang, X.; Tang, H.; Chen, C.; Zhang, Y. Engineering a highly biomimetic chitosan-based cartilage scaffold by using short fibers and a cartilage-decellularized matrix. Biomacromolecules. 2021, 22, 2284-2297.
doi: 10.1021/acs.biomac.1c00366 URL |
| 55. |
Tian, G.; Jiang, S.; Li, J.; Wei, F.; Li, X.; Ding, Y.; Yang, Z.; Sun, Z.; Zha, K.; Wang, F.; Huang, B.; Peng, L.; Wang, Q.; Tian, Z.; Yang, X.; Wang, Z.; Guo, Q.; Guo, W.; Liu, S. Cell-free decellularized cartilage extracellular matrix scaffolds combined with interleukin 4 promote osteochondral repair through immunomodulatory macrophages: In vitro and in vivo preclinical study. Acta Biomater. 2021, 127, 131-145.
doi: 10.1016/j.actbio.2021.03.054 URL |
| 56. |
Solarte David, V. A.; Güiza-Argüello, V. R.; Arango-Rodríguez, M. L.; Sossa, C. L.; Becerra-Bayona, S. M. Decellularized tissues for wound healing: towards closing the gap between scaffold design and effective extracellular matrix remodeling. Front Bioeng Biotechnol. 2022, 10, 821852.
doi: 10.3389/fbioe.2022.821852 URL |
| 57. |
Garreta, E.; Oria, R.; Tarantino, C.; Pla-Roca, M.; Prado, P.; Fernández-Avilés, F.; Campistol, J. M.; Samitier, J.; Montserrat, N. Tissue engineering by decellularization and 3D bioprinting. Mater Today. 2017, 20, 166-178.
doi: 10.1016/j.mattod.2016.12.005 URL |
| 58. |
Jang, J.; Park, H. J.; Kim, S. W.; Kim, H.; Park, J. Y.; Na, S. J.; Kim, H. J.; Park, M. N.; Choi, S. H.; Park, S. H.; Kim, S. W.; Kwon, S. M.; Kim, P. J.; Cho, D. W. 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials. 2017, 112, 264-274.
doi: 10.1016/j.biomaterials.2016.10.026 URL |
| 59. |
An, J.; Teoh, J. E. M.; Suntornnond, R.; Chua, C. K. Design and 3D printing of scaffolds and tissues. Engineering. 2015, 1, 261-268.
doi: 10.15302/J-ENG-2015061 URL |
| 60. |
Goodale, H. D. The progeny test as a means of evaluating the breeding potentialities of farm animals. Am Nat. 1933, 67, 481-499.
doi: 10.1086/280509 URL |
| 61. |
Dzobo, K.; Motaung, K.; Adesida, A. Recent trends in decellularized extracellular matrix bioinks for 3D printing: an updated review. Int J Mol Sci. 2019, 20, 4628.
doi: 10.3390/ijms20184628 URL |
| 62. |
Tottey, S.; Johnson, S. A.; Crapo, P. M.; Reing, J. E.; Zhang, L.; Jiang, H.; Medberry, C. J.; Reines, B.; Badylak, S. F. The effect of source animal age upon extracellular matrix scaffold properties. Biomaterials. 2011, 32, 128-136.
doi: 10.1016/j.biomaterials.2010.09.006 URL |
| 63. |
Aamodt, J. M.; Grainger, D. W. Extracellular matrix-based biomaterial scaffolds and the host response. Biomaterials. 2016, 86, 68-82.
doi: 10.1016/j.biomaterials.2016.02.003 URL |
| 64. | Johnson, T. D.; Dequach, J. A.; Gaetani, R.; Ungerleider, J.; Elhag, D.; Nigam, V.; Behfar, A.; Christman, K. L. Human versus porcine tissue sourcing for an injectable myocardial matrix hydrogel. Biomater Sci. 2014, 2014, 60283D. |
| 65. |
Pati, F.; Ha, D. H.; Jang, J.; Han, H. H.; Rhie, J. W.; Cho, D. W. Biomimetic 3D tissue printing for soft tissue regeneration. Biomaterials. 2015, 62, 164-175.
doi: 10.1016/j.biomaterials.2015.05.043 URL |
| 66. |
Assunção, M.; Dehghan-Baniani, D.; Yiu, C. H. K.; Später, T.; Beyer, S.; Blocki, A. Cell-derived extracellular matrix for tissue engineering and regenerative medicine. Front Bioeng Biotechnol. 2020, 8, 602009.
doi: 10.3389/fbioe.2020.602009 URL |
| 67. | Hoshiba, T. Cultured cell-derived decellularized extracellular matrix (cultured cell-derived dECM): Future applications and problems — a mini review. Curr Opin Biomed Eng. 2021, 17, 100256. |
| 68. | Antich, C.; Jiménez, G.; de Vicente, J.; López-Ruiz, E.; Chocarro-Wrona, C.; Griñán-Lisón, C.; Carrillo, E.; Montañez, E.; Marchal, J. A. Development of a biomimetic hydrogel based on predifferentiated mesenchymal stem-cell-derived ecm for cartilage tissue engineering. Adv Healthc Mater. 2021, 10, e2001847. |
| 69. |
Lee, S. E.; Park, Y. S. The role of bacterial cellulose in artificial blood vessels. Mol Cell Toxicol. 2017, 13, 257-261.
doi: 10.1007/s13273-017-0028-3 URL |
| 70. |
Sun, B.; Zhang, M.; Shen, J.; He, Z.; Fatehi, P.; Ni, Y. Applications of cellulose-based materials in sustained drug delivery systems. Curr Med Chem. 2019, 26, 2485-2501.
doi: 10.2174/0929867324666170705143308 URL |
| 71. |
Müller, F. A.; Müller, L.; Hofmann, I.; Greil, P.; Wenzel, M. M.; Staudenmaier, R. Cellulose-based scaffold materials for cartilage tissue engineering. Biomaterials. 2006, 27, 3955-3963.
doi: 10.1016/j.biomaterials.2006.02.031 URL |
| 72. |
Markstedt, K.; Mantas, A.; Tournier, I.; Martínez Ávila, H.; Hägg, D.; Gatenholm, P. 3D bioprinting human chondrocytes with nanocellulose-alginate bioink for cartilage tissue engineering applications. Biomacromolecules. 2015, 16, 1489-1496.
doi: 10.1021/acs.biomac.5b00188 URL |
| 73. |
Hong, H.; Seo, Y. B.; Kim, D. Y.; Lee, J. S.; Lee, Y. J.; Lee, H.; Ajiteru, O.; Sultan, M. T.; Lee, O. J.; Kim, S. H.; Park, C. H. Digital light processing 3D printed silk fibroin hydrogel for cartilage tissue engineering. Biomaterials. 2020, 232, 119679.
doi: 10.1016/j.biomaterials.2019.119679 URL |
| 74. |
Nuernberger, S.; Cyran, N.; Albrecht, C.; Redl, H.; Vécsei, V.; Marlovits, S. The influence of scaffold architecture on chondrocyte distribution and behavior in matrix-associated chondrocyte transplantation grafts. Biomaterials. 2011, 32, 1032-1040.
doi: 10.1016/j.biomaterials.2010.08.100 URL |
| 75. |
Cao, Y.; Cheng, P.; Sang, S.; Xiang, C.; An, Y.; Wei, X.; Yan, Y.; Li, P. 3D printed PCL/GelMA biphasic scaffold boosts cartilage regeneration using co-culture of mesenchymal stem cells and chondrocytes: in vivo study. Mater Des. 2021, 210, 110065.
doi: 10.1016/j.matdes.2021.110065 URL |
| 76. |
Uto, S.; Hikita, A.; Sakamoto, T.; Mori, D.; Yano, F.; Ohba, S.; Saito, T.; Takato, T.; Hoshi, K. Ear cartilage reconstruction combining induced pluripotent stem cell-derived cartilage and three-dimensional shape-memory scaffold. Tissue Eng Part A. 2021, 27, 604-617.
doi: 10.1089/ten.tea.2020.0106 URL |
| 77. |
Sommar, P.; Pettersson, S.; Ness, C.; Johnson, H.; Kratz, G.; Junker, J. P. Engineering three-dimensional cartilage- and bone-like tissues using human dermal fibroblasts and macroporous gelatine microcarriers. J Plast Reconstr Aesthet Surg. 2010, 63, 1036-1046.
doi: 10.1016/j.bjps.2009.02.072 URL |
| 78. | Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D. S. Polymeric scaffolds in tissue engineering application: a review. Int J Polym Sci. 2011, 2011, 290602. |
| 79. |
Izadifar, Z.; Chen, X.; Kulyk, W. Strategic design and fabrication of engineered scaffolds for articular cartilage repair. J Funct Biomater. 2012, 3, 799-838.
doi: 10.3390/jfb3040799 URL |
| 80. |
Keeney, M.; Lai, J. H.; Yang, F. Recent progress in cartilage tissue engineering. Curr Opin Biotechnol. 2011, 22, 734-740.
doi: 10.1016/j.copbio.2011.04.003 URL |
| 81. |
Yang, K.; Sun, J.; Wei, D.; Yuan, L.; Yang, J.; Guo, L.; Fan, H.; Zhang, X. Photo-crosslinked mono-component type II collagen hydrogel as a matrix to induce chondrogenic differentiation of bone marrow mesenchymal stem cells. J Mater Chem B. 2017, 5, 8707-8718.
doi: 10.1039/C7TB02348K URL |
| 82. |
Behan, K.; Dufour, A.; Garcia, O.; Kelly, D. Methacrylated cartilage ECM-based hydrogels as injectables and bioinks for cartilage tissue engineering. Biomolecules. 2022, 12, 216.
doi: 10.3390/biom12020216 URL |
| 83. |
Sun, B.; Han, Y.; Jiang, W.; Dai, K. 3D printing bioink preparation and application in cartilage tissue reconstruction in vitro. J Shanghai Jiaotong Univ (Sci). 2021, 26, 267-271.
doi: 10.1007/s12204-021-2292-6 URL |
| 84. |
Terpstra, M. L.; Li, J.; Mensinga, A.; de Ruijter, M.; van Rijen, M. H. P.; Androulidakis, C.; Galiotis, C.; Papantoniou, I.; Matsusaki, M.; Malda, J.; Levato, R. Bioink with cartilage-derived extracellular matrix microfibers enables spatial control of vascular capillary formation in bioprinted constructs. Biofabrication. 2022, 14, 034104.
doi: 10.1088/1758-5090/ac6282 URL |
| 85. |
Setayeshmehr, M.; Hafeez, S.; van Blitterswijk, C.; Moroni, L.; Mota, C.; Baker, M. B. Bioprinting via a dual-gel bioink based on poly(vinyl alcohol) and solubilized extracellular matrix towards cartilage engineering. Int J Mol Sci. 2021, 22, 3901.
doi: 10.3390/ijms22083901 URL |
| 86. |
Govindharaj, M.; Hashimi, N. A.; Soman, S. S.; Kanwar, S.; Vijayavenkataraman, S. 3D bioprinting of human mesenchymal stem cells in a novel tunic decellularized ECM bioink for cartilage tissue engineering. Materialia. 2022, 23, 101457.
doi: 10.1016/j.mtla.2022.101457 URL |
| 87. |
Wiggenhauser, P. S.; Schwarz, S.; Koerber, L.; Hoffmann, T. K.; Rotter, N. Addition of decellularized extracellular matrix of porcine nasal cartilage improves cartilage regenerative capacities of PCL-based scaffolds in vitro. J Mater Sci Mater Med. 2019, 30, 121.
doi: 10.1007/s10856-019-6323-x URL |
| 88. |
Zare, P.; Pezeshki-Modaress, M.; Davachi, S. M.; Chahsetareh, H.; Simorgh, S.; Asgari, N.; Haramshahi, M. A.; Alizadeh, R.; Bagher, Z.; Farhadi, M. An additive manufacturing-based 3D printed poly ε-caprolactone/alginate sulfate/extracellular matrix construct for nasal cartilage regeneration. J Biomed Mater Res A. 2022, 110, 1199-1209.
doi: 10.1002/jbm.a.37363 URL |
| 89. |
Jung, C. S.; Kim, B. K.; Lee, J.; Min, B. H.; Park, S. H. Development of printable natural cartilage matrix bioink for 3D printing of irregular tissue shape. Tissue Eng Regen Med. 2018, 15, 155-162.
doi: 10.1007/s13770-017-0104-8 URL |
| 90. | Jia, L.; Hua, Y.; Zeng, J.; Liu, W.; Wang, D.; Zhou, G.; Liu, X.; Jiang, H. Bioprinting and regeneration of auricular cartilage using a bioactive bioink based on microporous photocrosslinkable acellular cartilage matrix. Bioact Mater. 2022, 16, 66-81. |
| 91. |
Isaeva, E. V.; Beketov, E. E.; Demyashkin, G. A.; Yakovleva, N. D.; Arguchinskaya, N. V.; Kisel, A. A.; Lagoda, T. S.; Malakhov, E. P.; Smirnova, A. N.; Petriev, V. M.; Eremin, P. S.; Osidak, E. O.; Domogatsky, S. P.; Ivanov, S. A.; Shegay, P. V.; Kaprin, A. D. Cartilage formation in vivo using high concentration collagen-based bioink with MSC and decellularized ECM granules. Int J Mol Sci. 2022, 23, 2703.
doi: 10.3390/ijms23052703 URL |
| 92. |
Chen, W.; Xu, Y.; Li, Y.; Jia, L.; Mo, X.; Jiang, G.; Zhou, G. 3D printing electrospinning fiber-reinforced decellularized extracellular matrix for cartilage regeneration. Chem Eng J. 2020, 382, 122986.
doi: 10.1016/j.cej.2019.122986 URL |
| 93. |
Partington, L.; Mordan, N. J.; Mason, C.; Knowles, J. C.; Kim, H. W.; Lowdell, M. W.; Birchall, M. A.; Wall, I. B. Biochemical changes caused by decellularization may compromise mechanical integrity of tracheal scaffolds. Acta Biomater. 2013, 9, 5251-5261.
doi: 10.1016/j.actbio.2012.10.004 URL |
| 94. |
Tchoukalova, Y. D.; Hintze, J. M.; Hayden, R. E.; Lott, D. G. Tracheal decellularization using a combination of chemical, physical and bioreactor methods. Int J Artif Organs. 2017. doi: 10.5301/ijao.5000648.
doi: 10.5301/ijao.5000648 URL |
| [1] | Chavee Laomeephol, Helena Ferreira, Sorada Kanokpanont, Jittima Amie Luckanagul, Nuno M Neves, Siriporn Damrongsakkul. Osteogenic differentiation of encapsulated cells in dexamethasone–loaded phospholipid–induced silk fibroin hydrogels [J]. Biomaterials Translational, 2022, 3(3): 213-220. |
| [2] | Ricardo Donate, Maryam Tamaddon, Viviana Ribeiro, Mario Monzón, J. Miguel Oliveira, Chaozong Liu. Translation through collaboration: practice applied in BAMOS project in in vivo testing of innovative osteochondral scaffolds [J]. Biomaterials Translational, 2022, 3(2): 102-104. |
| [3] | Emma Steijvers, Armaan Ghei, Zhidao Xia. Manufacturing artificial bone allografts: a perspective [J]. Biomaterials Translational, 2022, 3(1): 65-80. |
| [4] | Ke Hu, Yuxuan Li, Zunxiang Ke, Hongjun Yang, Chanjun Lu, Yiqing Li, Yi Guo, Weici Wang. History, progress and future challenges of artificial blood vessels: a narrative review [J]. Biomaterials Translational, 2022, 3(1): 81-98. |
| [5] | Yizhong Peng, Jinye Li, Hui Lin, Shuo Tian, Sheng Liu, Feifei Pu, Lei Zhao, Kaige Ma, Xiangcheng Qing, Zengwu Shao. Endogenous repair theory enriches construction strategies for orthopaedic biomaterials: a narrative review [J]. Biomaterials Translational, 2021, 2(4): 343-360. |
| [6] | Xirui Jing, Qiuyue Ding, Qinxue Wu, Weijie Su, Keda Yu, Yanlin Su, Bing Ye, Qing Gao, Tingfang Sun, Xiaodong Guo. Magnesium-based materials in orthopaedics: material properties and animal models [J]. Biomaterials Translational, 2021, 2(3): 197-213. |
| [7] | Yiqing Wang, Xiangyu Chu, Bing Wang. Recombinant adeno-associated virus-based gene therapy combined with tissue engineering for musculoskeletal regenerative medicine [J]. Biomaterials Translational, 2021, 2(1): 19-29. |
| [8] | Maryam Tamaddon, Helena Gilja, Ling Wang, J. Miguel Oliveira, Xiaodan Sun, Rongwei Tan, Chaozong Liu. Osteochondral scaffolds for early treatment of cartilage defects in osteoarthritic joints: from bench to clinic [J]. Biomaterials Translational, 2020, 1(1): 3-17. |
| [9] | Xing Yang, Yuanyuan Li, Xujie Liu, Wei He, Qianli Huang, Qingling Feng. Nanoparticles and their effects on differentiation of mesenchymal stem cells [J]. Biomaterials Translational, 2020, 1(1): 58-68. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||