Biomaterials Translational ›› 2021, Vol. 2 ›› Issue (4): 343-360.doi: 10.12336/biomatertransl.2021.04.008
• REVIEW • Previous Articles Next Articles
Yizhong Peng, Jinye Li, Hui Lin, Shuo Tian, Sheng Liu, Feifei Pu, Lei Zhao, Kaige Ma, Xiangcheng Qing*( ), Zengwu Shao*(
), Zengwu Shao*( )
)
Received:2020-06-20
Revised:2020-10-29
Accepted:2021-11-19
Online:2021-12-28
Published:2021-12-28
Contact:
Xiangcheng Qing,Zengwu Shao
E-mail:353220817@qq.com;szwpro@163.com
About author:Zengwu Shao, szwpro@163.com; Xiangcheng Qing, 353220817@qq.com.Peng, Y.; Li, J.; Lin, H.; Tian, S.; Liu, S.; Pu, F.; Zhao, L.; Ma, K.; Qing, X.; Shao, Z. Endogenous repair theory enriches construction strategies for orthopaedic biomaterials: a narrative review. Biomater Transl. 2021, 2(4), 343-360.
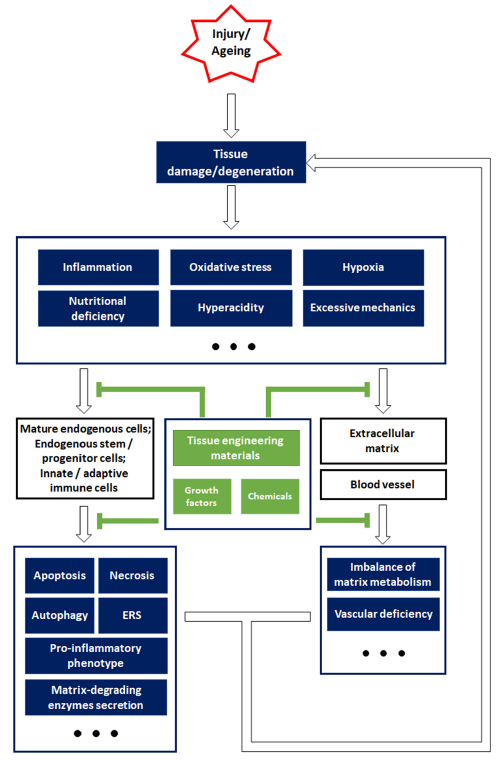
Figure 2. Schematic overview of the strategies for constructing biomaterials, inspired by endogenous repair failure that occurs owing to injury or ageing-related pathophysiological changes. Aberrant external impacts cause tissue damage, while ageing often leads to tissue degeneration. After tissue damage or degeneration, the resulting unfavourable microenvironment is characterized by inflammation, oxidative stress, hypoxia, insufficient nutrition, hyperacidity, and abnormal mechanical properties, which impose a great burden on the endogenous cells and non-cellular components. Specifically, mature endogenous cells and stem/progenitor cells typically suffer from cell death and endoplasmic reticulum stress (ERS), and secrete pro-inflammatory factors (interleukin 1β, interleukin 6, tumour necrosis factor α, etc.), while immune cells are also involved in aggravating the inflammation. In addition, the harsh environment also leads to an imbalance in the matrix metabolism and impairs the endothelial cells that are essential for angiogenesis. Cellular and non-cellular alterations in unfavourable environments contribute to endogenous repair failure. However, tissue engineering materials and other bioactive agents are efficient in relieving the pathological changes and their damaging impact on cells and extracellular components, which may help re-establish endogenous repair mechanisms and alleviate tissue damage or degeneration.
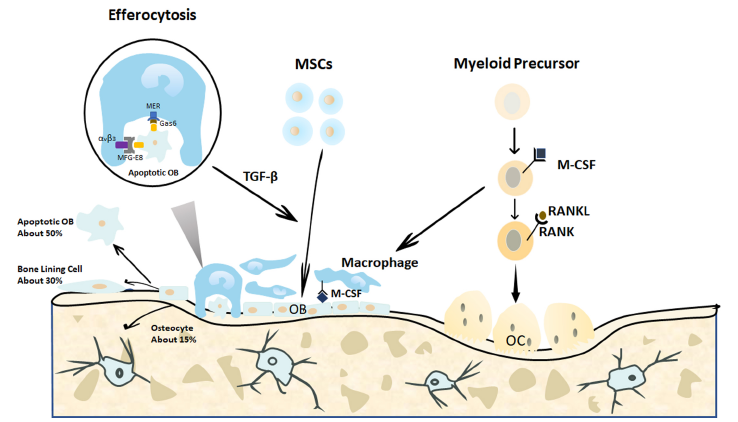
Figure 3. Endogenous cellular changes after bone fracture. When a bone is fractured, the MSCs migrate to the bone defect area and differentiate into osteoblasts to form and remodel the bone matrix. In the end, approximately 15% of the osteoblasts become embedded in the bone matrix as osteocytes, 30% of the osteoblasts become quiescent bone lining cells, and the remaining 40–70% of the osteoblasts are likely to undergo death by apoptosis. The apoptotic osteoblasts expressing certain signals are efficiently cleared by macrophages in a process called efferocytosis. This process is initiated by the expression of the apoptotic signals on osteoblasts and is activated by the binding of linking proteins, including MFG-E8 or Gas6, and macrophage proteins, such as αvβ3 or Mer. The efferocytosis-induced production of specific proteins, such as TGF-β, may promote continuous bone modelling by recruiting osteoblasts from progenitor cells.29 Gas6: growth arrest-specific 6; M-CSF: macrophage colony-stimulating factor; MER (tk): receptor tyrosine kinase MerTK; MFG-8: milk fat globule-epidermal growth factor 8; MSCs: mesenchymal stromal cells; OB: osteoblasts; OC: osteoclasts; RANK: receptor activator of nuclear factor-κB; RANKL: receptor activator of nuclear factor-κB ligand; TGF-β: transforming growth factor β; αvβ3: alpha-V beta-3 integrin.
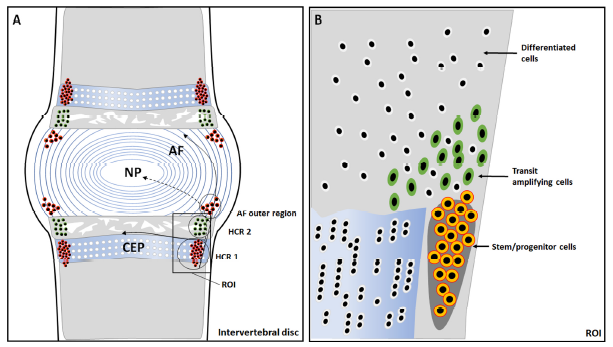
Figure 4. Anatomical structure of an intervertebral disc and identification of the stem cell niche and hypothetical migration paths. (A) The potential stem cell niche is in the perichondrium region adjacent to the epiphyseal plate and outer layers of the annulus fibrosus (AF). In the hypercellular region 1 (HCR 1), the cells are densely distributed, and this is where the stem cell niche is located; while in the hypercellular region 2 (HCR 2), the cells are relatively dispersed and morphologically mature. (B) A magnification of the region of interest shows slow cycling stem/progenitor cells (outlined in orange), the transit amplifying cells (outlined in green), and differentiated cells (outlined in white).153 CEP: cartilage endplate; NP: nucleus pulposus.
| How to maintain the viability and multi-lineage differentiation potential of endogenous stem cells in an injured tissue? |
| How to mobilise the endogenous stem cells to sufficiently proliferate and restore the decreased cell number? |
| How to enable the targeted migration of endogenous stem cells to damaged areas? |
| How to induce the targeted differentiation of endogenous stem cells into progenitors capable of regenerating desired cell types in vivo? |
| How to ensure that newly-generated cells integrate into the surrounding tissues and establish functional connectivity? |
Table 1 Challenges in endogenous repair.
| How to maintain the viability and multi-lineage differentiation potential of endogenous stem cells in an injured tissue? |
| How to mobilise the endogenous stem cells to sufficiently proliferate and restore the decreased cell number? |
| How to enable the targeted migration of endogenous stem cells to damaged areas? |
| How to induce the targeted differentiation of endogenous stem cells into progenitors capable of regenerating desired cell types in vivo? |
| How to ensure that newly-generated cells integrate into the surrounding tissues and establish functional connectivity? |
| 1. | National Science Foundation. The emergence of tissue engineering as a research field.http://www.nsf.gov/pubs/2004/nsf0450/emergence.htm. Accissed May 15, 2021. |
| 2. |
Langer, R.; Vacanti, J. P. Tissue engineering. Science. 1993, 260, 920-926.
doi: 10.1126/science.8493529 URL |
| 3. |
Jahromi, M.; Razavi, S.; Bakhtiari, A. The advances in nerve tissue engineering: From fabrication of nerve conduit to in vivo nerve regeneration assays. J Tissue Eng Regen Med. 2019, 13, 2077-2100.
doi: 10.1002/term.v13.11 URL |
| 4. |
Qasim, M.; Chae, D. S.; Lee, N. Y. Advancements and frontiers in nano-based 3D and 4D scaffolds for bone and cartilage tissue engineering. Int J Nanomedicine. 2019, 14, 4333-4351.
doi: 10.2147/IJN URL |
| 5. |
Frueh, F. S.; Menger, M. D.; Lindenblatt, N.; Giovanoli, P.; Laschke, M. W. Current and emerging vascularization strategies in skin tissue engineering. Crit Rev Biotechnol. 2017, 37, 613-625.
doi: 10.1080/07388551.2016.1209157 URL |
| 6. |
Goodarzi, H.; Jadidi, K.; Pourmotabed, S.; Sharifi, E.; Aghamollaei, H. Preparation and in vitro characterization of cross-linked collagen-gelatin hydrogel using EDC/NHS for corneal tissue engineering applications. Int J Biol Macromol. 2019, 126, 620-632.
doi: 10.1016/j.ijbiomac.2018.12.125 URL |
| 7. |
Berthiaume, F.; Maguire, T. J.; Yarmush, M. L. Tissue engineering and regenerative medicine: history, progress, and challenges. Annu Rev Chem Biomol Eng. 2011, 2, 403-430.
doi: 10.1146/chembioeng.2011.2.issue-1 URL |
| 8. |
Song, H. G.; Rumma, R. T.; Ozaki, C. K.; Edelman, E. R.; Chen, C. S. Vascular tissue engineering: progress, challenges, and clinical promise. Cell Stem Cell. 2018, 22, 340-354.
doi: 10.1016/j.stem.2018.02.009 URL |
| 9. |
Kim, H. D.; Amirthalingam, S.; Kim, S. L.; Lee, S. S.; Rangasamy, J.; Hwang, N. S. Biomimetic materials and fabrication approaches for bone tissue engineering. Adv Healthc Mater. 2017, 6, 1700612.
doi: 10.1002/adhm.v6.23 URL |
| 10. |
Katagiri, W.; Watanabe, J.; Toyama, N.; Osugi, M.; Sakaguchi, K.; Hibi, H. Clinical study of bone regeneration by conditioned medium from mesenchymal stem cells after maxillary sinus floor elevation. Implant Dent. 2017, 26, 607-612.
doi: 10.1097/ID.0000000000000618 URL |
| 11. |
Falanga, V.; Iwamoto, S.; Chartier, M.; Yufit, T.; Butmarc, J.; Kouttab, N.; Shrayer, D.; Carson, P. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007, 13, 1299-1312.
doi: 10.1089/ten.2006.0278 URL |
| 12. |
Yoshikawa, T.; Ueda, Y.; Miyazaki, K.; Koizumi, M.; Takakura, Y. Disc regeneration therapy using marrow mesenchymal cell transplantation: a report of two case studies. Spine (Phila Pa 1976). 2010, 35, E475-480.
doi: 10.1097/BRS.0b013e3181cd2cf4 URL |
| 13. |
Mendonça, M. V.; Larocca, T. F.; de Freitas Souza, B. S.; Villarreal, C. F.; Silva, L. F.; Matos, A. C.; Novaes, M. A.; Bahia, C. M.; de Oliveira Melo Martinez, A. C.; Kaneto, C. M.; Furtado, S. B.; Sampaio, G. P.; Soares, M. B.; dos Santos, R. R. Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell Res Ther. 2014, 5, 126.
doi: 10.1186/scrt516 URL |
| 14. |
Al-Najar, M.; Khalil, H.; Al-Ajlouni, J.; Al-Antary, E.; Hamdan, M.; Rahmeh, R.; Alhattab, D.; Samara, O.; Yasin, M.; Abdullah, A. A.; Al-Jabbari, E.; Hmaid, D.; Jafar, H.; Awidi, A. Intra-articular injection of expanded autologous bone marrow mesenchymal cells in moderate and severe knee osteoarthritis is safe: a phase I/II study. J Orthop Surg Res. 2017, 12, 190.
doi: 10.1186/s13018-017-0689-6 URL |
| 15. |
Pigott, J. H.; Ishihara, A.; Wellman, M. L.; Russell, D. S.; Bertone, A. L. Investigation of the immune response to autologous, allogeneic, and xenogeneic mesenchymal stem cells after intra-articular injection in horses. Vet Immunol Immunopathol. 2013, 156, 99-106.
doi: 10.1016/j.vetimm.2013.09.003 URL |
| 16. |
Pas, H. I.; Winters, M.; Haisma, H. J.; Koenis, M. J.; Tol, J. L.; Moen, M. H. Stem cell injections in knee osteoarthritis: a systematic review of the literature. Br J Sports Med. 2017, 51, 1125-1133.
doi: 10.1136/bjsports-2016-096793 URL |
| 17. |
Jin, J. Stem cell treatments. JAMA. 2017, 317, 330.
doi: 10.1001/jama.2016.17822 URL |
| 18. |
Sackett, S. D.; Brown, M. E.; Tremmel, D. M.; Ellis, T.; Burlingham, W. J.; Odorico, J. S. Modulation of human allogeneic and syngeneic pluripotent stem cells and immunological implications for transplantation. Transplant Rev (Orlando). 2016, 30, 61-70.
doi: 10.1016/j.trre.2016.02.001 URL |
| 19. |
Wells, J. M.; Watt, F. M. Diverse mechanisms for endogenous regeneration and repair in mammalian organs. Nature. 2018, 557, 322-328.
doi: 10.1038/s41586-018-0073-7 URL |
| 20. |
Du, S. H.; Feng, Y. Z.; Huang, Y. X.; Guo, X. S.; Xia, D. D. Comparison of pediatric forearm fracture fixation between single- and double-elastic stable intramedullary nailing. Am J Ther. 2016, 23, e730-736.
doi: 10.1097/MJT.0000000000000031 URL |
| 21. |
Majidinia, M.; Sadeghpour, A.; Yousefi, B. The roles of signaling pathways in bone repair and regeneration. J Cell Physiol. 2018, 233, 2937-2948.
doi: 10.1002/jcp.v233.4 URL |
| 22. |
Dekoninck, S.; Blanpain, C. Stem cell dynamics, migration and plasticity during wound healing. Nat Cell Biol. 2019, 21, 18-24.
doi: 10.1038/s41556-018-0237-6 URL |
| 23. |
Stenudd, M.; Sabelström, H.; Frisén, J. Role of endogenous neural stem cells in spinal cord injury and repair. JAMA Neurol. 2015, 72, 235-237.
doi: 10.1001/jamaneurol.2014.2927 URL |
| 24. |
Nocera, G.; Jacob, C. Mechanisms of Schwann cell plasticity involved in peripheral nerve repair after injury. Cell Mol Life Sci. 2020, 77, 3977-3989.
doi: 10.1007/s00018-020-03516-9 URL |
| 25. |
Li, W.; Li, L.; Hui, L. Cell plasticity in liver regeneration. Trends Cell Biol. 2020, 30, 329-338.
doi: 10.1016/j.tcb.2020.01.007 URL |
| 26. |
Li, C. J.; Cheng, P.; Liang, M. K.; Chen, Y. S.; Lu, Q.; Wang, J. Y.; Xia, Z. Y.; Zhou, H. D.; Cao, X.; Xie, H.; Liao, E. Y.; Luo, X. H. MicroRNA-188 regulates age-related switch between osteoblast and adipocyte differentiation. J Clin Invest. 2015, 125, 1509-1522.
doi: 10.1172/JCI77716 URL |
| 27. |
Adachi, J. D.; Lyles, K. W.; Colón-Emeric, C. S.; Boonen, S.; Pieper, C. F.; Mautalen, C.; Hyldstrup, L.; Recknor, C.; Nordsletten, L.; Moore, K. A.; Bucci-Rechtweg, C.; Su, G.; Eriksen, E. F.; Magaziner, J. S. Zoledronic acid results in better health-related quality of life following hip fracture: the HORIZON-recurrent fracture trial. Osteoporos Int. 2011, 22, 2539-2549.
doi: 10.1007/s00198-010-1514-9 URL |
| 28. |
Kalbasi Anaraki, P.; Patecki, M.; Tkachuk, S.; Kiyan, Y.; Haller, H.; Dumler, I. Urokinase receptor mediates osteoclastogenesis via M-CSF release from osteoblasts and the c-Fms/PI3K/Akt/NF-κB pathway in osteoclasts. J Bone Miner Res. 2015, 30, 379-388.
doi: 10.1002/jbmr.2350 URL |
| 29. |
Sinder, B. P.; Pettit, A. R.; McCauley, L. K. Macrophages: their emerging roles in bone. J Bone Miner Res. 2015, 30, 2140-2149.
doi: 10.1002/jbmr.2735 URL |
| 30. |
Alippe, Y.; Wang, C.; Ricci, B.; Xiao, J.; Qu, C.; Zou, W.; Novack, D. V.; Abu-Amer, Y.; Civitelli, R.; Mbalaviele, G. Bone matrix components activate the NLRP3 inflammasome and promote osteoclast differentiation. Sci Rep. 2017, 7, 6630.
doi: 10.1038/s41598-017-07014-0 URL |
| 31. |
Omari, S.; Makareeva, E.; Roberts-Pilgrim, A.; Mirigian, L.; Jarnik, M.; Ott, C.; Lippincott-Schwartz, J.; Leikin, S. Noncanonical autophagy at ER exit sites regulates procollagen turnover. Proc Natl Acad Sci U S A. 2018, 115, E10099-E10108.
doi: 10.1073/pnas.1814552115 URL |
| 32. |
Odkhuu, E.; Koide, N.; Haque, A.; Tsolmongyn, B.; Naiki, Y.; Hashimoto, S.; Komatsu, T.; Yoshida, T.; Yokochi, T. Inhibition of receptor activator of nuclear factor-κB ligand (RANKL)-induced osteoclast formation by pyrroloquinoline quinine (PQQ). Immunol Lett. 2012, 142, 34-40.
doi: 10.1016/j.imlet.2011.12.001 URL |
| 33. |
Roohani-Esfahani, S. I.; No, Y. J.; Lu, Z.; Ng, P. Y.; Chen, Y.; Shi, J.; Pavlos, N. J.; Zreiqat, H. A bioceramic with enhanced osteogenic properties to regulate the function of osteoblastic and osteocalastic cells for bone tissue regeneration. Biomed Mater. 2016, 11, 035018.
doi: 10.1088/1748-6041/11/3/035018 URL |
| 34. |
Atanga, E.; Dolder, S.; Dauwalder, T.; Wetterwald, A.; Hofstetter, W. TNFα inhibits the development of osteoclasts through osteoblast-derived GM-CSF. Bone. 2011, 49, 1090-1100.
doi: 10.1016/j.bone.2011.08.003 URL |
| 35. |
Ozaki, A.; Tsunoda, M.; Kinoshita, S.; Saura, R. Role of fracture hematoma and periosteum during fracture healing in rats: interaction of fracture hematoma and the periosteum in the initial step of the healing process. J Orthop Sci. 2000, 5, 64-70.
doi: 10.1007/s007760050010 URL |
| 36. |
Marcucci, G.; Beltrami, G.; Tamburini, A.; Body, J. J.; Confavreux, C. B.; Hadji, P.; Holzer, G.; Kendler, D.; Napoli, N.; Pierroz, D. D.; Rizzoli, R.; Brandi, M. L. Bone health in childhood cancer: review of the literature and recommendations for the management of bone health in childhood cancer survivors. Ann Oncol. 2019, 30, 908-920.
doi: 10.1093/annonc/mdz120 URL |
| 37. |
Alman, B. A.; Kelley, S. P.; Nam, D. Heal thyself: using endogenous regeneration to repair bone. Tissue Eng Part B Rev. 2011, 17, 431-436.
doi: 10.1089/ten.teb.2011.0189 URL |
| 38. |
Zhang, Y.; Hao, Z.; Wang, P.; Xia, Y.; Wu, J.; Xia, D.; Fang, S.; Xu, S. Exosomes from human umbilical cord mesenchymal stem cells enhance fracture healing through HIF-1α-mediated promotion of angiogenesis in a rat model of stabilized fracture. Cell Prolif. 2019, 52, e12570.
doi: 10.1111/cpr.2019.52.issue-2 URL |
| 39. |
Fan, Y.; Hanai, J. I.; Le, P. T.; Bi, R.; Maridas, D.; DeMambro, V.; Figueroa, C. A.; Kir, S.; Zhou, X.; Mannstadt, M.; Baron, R.; Bronson, R. T.; Horowitz, M. C.; Wu, J. Y.; Bilezikian, J. P.; Dempster, D. W.; Rosen, C. J.; Lanske, B. Parathyroid hormone directs bone marrow mesenchymal cell fate. Cell Metab. 2017, 25, 661-672.
doi: 10.1016/j.cmet.2017.01.001 URL |
| 40. |
Kajimura, D.; Lee, H. W.; Riley, K. J.; Arteaga-Solis, E.; Ferron, M.; Zhou, B.; Clarke, C. J.; Hannun, Y. A.; DePinho, R. A.; Guo, X. E.; Mann, J. J.; Karsenty, G. Adiponectin regulates bone mass via opposite central and peripheral mechanisms through FoxO1. Cell Metab. 2013, 17, 901-915.
doi: 10.1016/j.cmet.2013.04.009 URL |
| 41. |
Funamoto, T.; Sekimoto, T.; Murakami, T.; Kurogi, S.; Imaizumi, K.; Chosa, E. Roles of the endoplasmic reticulum stress transducer OASIS in fracture healing. Bone. 2011, 49, 724-732.
doi: 10.1016/j.bone.2011.06.012 URL |
| 42. |
Wang, J.; Yang, J.; Cheng, X.; Xiao, R.; Zhao, Y.; Xu, H.; Zhu, Y.; Yan, Z.; Ommati, M. M.; Manthari, R. K.; Wang, J. Calcium alleviates fluoride-induced bone damage by inhibiting endoplasmic reticulum stress and mitochondrial dysfunction. J Agric Food Chem. 2019, 67, 10832-10843.
doi: 10.1021/acs.jafc.9b04295 URL |
| 43. |
Park, J. K.; Jang, H.; Hwang, S.; Kim, E. J.; Kim, D. E.; Oh, K. B.; Kwon, D. J.; Koh, J. T.; Kimura, K.; Inoue, H.; Jang, W. G.; Lee, J. W. ER stress-inducible ATF3 suppresses BMP2-induced ALP expression and activation in MC3T3-E1 cells. Biochem Biophys Res Commun. 2014, 443, 333-338.
doi: 10.1016/j.bbrc.2013.11.121 URL |
| 44. |
Dai, P.; Mao, Y.; Sun, X.; Li, X.; Muhammad, I.; Gu, W.; Zhang, D.; Zhou, Y.; Ni, Z.; Ma, J.; Huang, S. Attenuation of oxidative stress-induced osteoblast apoptosis by curcumin is associated with preservation of mitochondrial functions and increased Akt-GSK3β signaling. Cell Physiol Biochem. 2017, 41, 661-677.
doi: 10.1159/000457945 URL |
| 45. |
Dimitriou, R.; Tsiridis, E.; Giannoudis, P. V. Current concepts of molecular aspects of bone healing. Injury. 2005, 36, 1392-1404.
doi: 10.1016/j.injury.2005.07.019 URL |
| 46. |
Li, L.; Yang, S.; Xu, L.; Li, Y.; Fu, Y.; Zhang, H.; Song, J. Nanotopography on titanium promotes osteogenesis via autophagy-mediated signaling between YAP and β-catenin. Acta Biomater. 2019, 96, 674-685.
doi: 10.1016/j.actbio.2019.07.007 URL |
| 47. |
Chen, X. J.; Shen, Y. S.; He, M. C.; Yang, F.; Yang, P.; Pang, F. X.; He, W.; Cao, Y. M.; Wei, Q. S. Polydatin promotes the osteogenic differentiation of human bone mesenchymal stem cells by activating the BMP2-Wnt/β-catenin signaling pathway. Biomed Pharmacother. 2019, 112, 108746.
doi: 10.1016/j.biopha.2019.108746 URL |
| 48. |
zur Nieden, N. I.; Price, F. D.; Davis, L. A.; Everitt, R. E.; Rancourt, D. E. Gene profiling on mixed embryonic stem cell populations reveals a biphasic role for beta-catenin in osteogenic differentiation. Mol Endocrinol. 2007, 21, 674-685.
doi: 10.1210/me.2005-0438 URL |
| 49. |
Yang, M.; Li, C. J.; Sun, X.; Guo, Q.; Xiao, Y.; Su, T.; Tu, M. L.; Peng, H.; Lu, Q.; Liu, Q.; He, H. B.; Jiang, T. J.; Lei, M. X.; Wan, M.; Cao, X.; Luo, X. H. MiR-497~195 cluster regulates angiogenesis during coupling with osteogenesis by maintaining endothelial Notch and HIF-1α activity. Nat Commun. 2017, 8, 16003.
doi: 10.1038/ncomms16003 URL |
| 50. |
Fasolino, I.; Raucci, M. G.; Soriente, A.; Demitri, C.; Madaghiele, M.; Sannino, A.; Ambrosio, L. Osteoinductive and anti-inflammatory properties of chitosan-based scaffolds for bone regeneration. Mater Sci Eng C Mater Biol Appl. 2019, 105, 110046.
doi: 10.1016/j.msec.2019.110046 URL |
| 51. |
Wang, Y.; Cao, L.; Liu, X. Ghrelin alleviates endoplasmic reticulum stress and inflammation-mediated reproductive dysfunction induced by stress. J Assist Reprod Genet. 2019, 36, 2357-2366.
doi: 10.1007/s10815-019-01589-5 URL |
| 52. |
Ohashi, E.; Kohno, K.; Arai, N.; Harashima, A.; Ariyasu, T.; Ushio, S. Adenosine N1-oxide exerts anti-inflammatory effects through the PI3K/Akt/GSK-3β signaling pathway and promotes osteogenic and adipocyte differentiation. Biol Pharm Bull. 2019, 42, 968-976.
doi: 10.1248/bpb.b18-00988 URL |
| 53. |
He, Y. Q.; Yang, H.; Shen, Y.; Zhang, J. H.; Zhang, Z. G.; Liu, L. L.; Song, H. T.; Lin, B.; Hsu, H. Y.; Qin, L. P.; Han, T.; Xin, H. L.; Zhang, Q. Y. Monotropein attenuates ovariectomy and LPS-induced bone loss in mice and decreases inflammatory impairment on osteoblast through blocking activation of NF-κB pathway. Chem Biol Interact. 2018, 291, 128-136.
doi: 10.1016/j.cbi.2018.06.015 URL |
| 54. |
Xu, L.; Zhang, L.; Wang, Z.; Li, C.; Li, S.; Li, L.; Fan, Q.; Zheng, L. Melatonin suppresses estrogen deficiency-induced osteoporosis and promotes osteoblastogenesis by inactivating the NLRP3 inflammasome. Calcif Tissue Int. 2018, 103, 400-410.
doi: 10.1007/s00223-018-0428-y URL |
| 55. |
Peng, Y.; Huang, D.; Li, J.; Liu, S.; Qing, X.; Shao, Z. Genipin-crosslinked decellularized annulus fibrosus hydrogels induces tissue-specific differentiation of bone mesenchymal stem cells and intervertebral disc regeneration. J Tissue Eng Regen Med. 2020, 14, 497-509.
doi: 10.1002/term.v14.3 URL |
| 56. |
Duan, H.; Song, W.; Zhao, W.; Gao, Y.; Yang, Z.; Li, X. Endogenous neurogenesis in adult mammals after spinal cord injury. Sci China Life Sci. 2016, 59, 1313-1318.
doi: 10.1007/s11427-016-0205-2 URL |
| 57. |
Salih, E.; Wang, J.; Mah, J.; Fluckiger, R. Natural variation in the extent of phosphorylation of bone phosphoproteins as a function of in vivo new bone formation induced by demineralized bone matrix in soft tissue and bony environments. Biochem J. 2002, 364, 465-474.
doi: 10.1042/bj20011272 URL |
| 58. | Wildemann, B.; Kadow-Romacker, A.; Haas, N. P.; Schmidmaier, G. Quantification of various growth factors in different demineralized bone matrix preparations. J Biomed Mater Res A. 2007, 81, 437-442. |
| 59. |
Holt, D. J.; Grainger, D. W. Demineralized bone matrix as a vehicle for delivering endogenous and exogenous therapeutics in bone repair. Adv Drug Deliv Rev. 2012, 64, 1123-1128.
doi: 10.1016/j.addr.2012.04.002 URL |
| 60. |
Pietrzak, W. S.; Woodell-May, J.; McDonald, N. Assay of bone morphogenetic protein-2, -4, and -7 in human demineralized bone matrix. J Craniofac Surg. 2006, 17, 84-90.
doi: 10.1097/01.scs.0000179745.91165.73 URL |
| 61. |
Hu, Q.; Liu, M.; Chen, G.; Xu, Z.; Lv, Y. Demineralized bone scaffolds with tunable matrix stiffness for efficient bone integration. ACS Appl Mater Interfaces. 2018, 10, 27669-27680.
doi: 10.1021/acsami.8b08668 URL |
| 62. |
Chen, G.; Dong, C.; Yang, L.; Lv, Y. 3D scaffolds with different stiffness but the same microstructure for bone tissue engineering. ACS Appl Mater Interfaces. 2015, 7, 15790-15802.
doi: 10.1021/acsami.5b02662 URL |
| 63. |
Ranly, D. M.; McMillan, J.; Keller, T.; Lohmann, C. H.; Meunch, T.; Cochran, D. L.; Schwartz, Z.; Boyan, B. D. Platelet-derived growth factor inhibits demineralized bone matrix-induced intramuscular cartilage and bone formation. A study of immunocompromised mice. J Bone Joint Surg Am. 2005, 87, 2052-2064.
doi: 10.2106/00004623-200509000-00019 URL |
| 64. |
Peel, S. A.; Hu, Z. M.; Clokie, C. M. In search of the ideal bone morphogenetic protein delivery system: in vitro studies on demineralized bone matrix, purified, and recombinant bone morphogenetic protein. J Craniofac Surg. 2003, 14, 284-291.
doi: 10.1097/00001665-200305000-00005 URL |
| 65. |
Gombotz, W. R.; Pankey, S. C.; Bouchard, L. S.; Ranchalis, J.; Puolakkainen, P. Controlled release of TGF-beta 1 from a biodegradable matrix for bone regeneration. J Biomater Sci Polym Ed. 1993, 5, 49-63.
doi: 10.1163/156856294X00644 URL |
| 66. | Moxham, J. P.; Kibblewhite, D. J.; Bruce, A. G.; Rigley, T.; Gillespy, T.3rd; Lane, J. Transforming growth factor-beta 1 in a guanidine-extracted demineralized bone matrix carrier rapidly closes a rabbit critical calvarial defect. J Otolaryngol. 1996, 25, 82-87. |
| 67. |
Del Rosario, C.; Rodríguez-Evora, M.; Reyes, R.; González-Orive, A.; Hernández-Creus, A.; Shakesheff, K. M.; White, L. J.; Delgado, A.; Evora, C. Evaluation of nanostructure and microstructure of bone regenerated by BMP-2-porous scaffolds. J Biomed Mater Res A. 2015, 103, 2998-3011.
doi: 10.1002/jbm.a.v103.9 URL |
| 68. |
Chen, B.; Lin, H.; Wang, J.; Zhao, Y.; Wang, B.; Zhao, W.; Sun, W.; Dai, J. Homogeneous osteogenesis and bone regeneration by demineralized bone matrix loading with collagen-targeting bone morphogenetic protein-2. Biomaterials. 2007, 28, 1027-1035.
doi: 10.1016/j.biomaterials.2006.10.013 URL |
| 69. |
Ho, S. S.; Murphy, K. C.; Binder, B. Y.; Vissers, C. B.; Leach, J. K. Increased survival and function of mesenchymal stem cell spheroids entrapped in instructive alginate hydrogels. Stem Cells Transl Med. 2016, 5, 773-781.
doi: 10.5966/sctm.2015-0211 URL |
| 70. |
Lu, Z.; Jiang, X.; Chen, M.; Feng, L.; Kang, Y. J. An oxygen-releasing device to improve the survival of mesenchymal stem cells in tissue engineering. Biofabrication. 2019, 11, 045012.
doi: 10.1088/1758-5090/ab332a URL |
| 71. |
Hosseinzadeh, A.; Kamrava, S. K.; Joghataei, M. T.; Darabi, R.; Shakeri-Zadeh, A.; Shahriari, M.; Reiter, R. J.; Ghaznavi, H.; Mehrzadi, S. Apoptosis signaling pathways in osteoarthritis and possible protective role of melatonin. J Pineal Res. 2016, 61, 411-425.
doi: 10.1111/jpi.12362 URL |
| 72. |
Lai, M.; Jin, Z.; Tang, Q.; Lu, M. Sustained release of melatonin from TiO(2) nanotubes for modulating osteogenic differentiation of mesenchymal stem cells in vitro. J Biomater Sci Polym Ed. 2017, 28, 1651-1664.
doi: 10.1080/09205063.2017.1342334 URL |
| 73. |
Hoemann, C. D.; Chen, G.; Marchand, C.; Tran-Khanh, N.; Thibault, M.; Chevrier, A.; Sun, J.; Shive, M. S.; Fernandes, M. J.; Poubelle, P. E.; Centola, M.; El-Gabalawy, H. Scaffold-guided subchondral bone repair: implication of neutrophils and alternatively activated arginase-1+ macrophages. Am J Sports Med. 2010, 38, 1845-1856.
doi: 10.1177/0363546510369547 URL |
| 74. | Deng, M.; Tan, J.; Hu, C.; Hou, T.; Peng, W.; Liu, J.; Yu, B.; Dai, Q.; Zhou, J.; Yang, Y.; Dong, R.; Ruan, C.; Dong, S.; Xu, J. Modification of PLGA scaffold by MSC-derived extracellular matrix combats macrophage inflammation to initiate bone regeneration via TGF-β-induced protein. Adv Healthc Mater. 2020, 9, e2000353. |
| 75. |
Knudson, C. B.; Knudson, W. Cartilage proteoglycans. Semin Cell Dev Biol. 2001, 12, 69-78.
doi: 10.1006/scdb.2000.0243 URL |
| 76. |
Pacifici, M.; Koyama, E.; Iwamoto, M.; Gentili, C. Development of articular cartilage: what do we know about it and how may it occur? Connect Tissue Res. 2000, 41, 175-184.
doi: 10.3109/03008200009005288 URL |
| 77. |
Huang, C. C.; Chiou, C. H.; Liu, S. C.; Hu, S. L.; Su, C. M.; Tsai, C. H.; Tang, C. H. Melatonin attenuates TNF-α and IL-1β expression in synovial fibroblasts and diminishes cartilage degradation: Implications for the treatment of rheumatoid arthritis. J Pineal Res. 2019, 66, e12560.
doi: 10.1111/jpi.2019.66.issue-3 URL |
| 78. |
Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J. P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011, 7, 33-42.
doi: 10.1038/nrrheum.2010.196 URL |
| 79. |
Liu, Y.; Zou, R.; Wang, Z.; Wen, C.; Zhang, F.; Lin, F. Exosomal KLF3-AS1 from hMSCs promoted cartilage repair and chondrocyte proliferation in osteoarthritis. Biochem J. 2018, 475, 3629-3638.
doi: 10.1042/BCJ20180675 URL |
| 80. |
Barbero, A.; Ploegert, S.; Heberer, M.; Martin, I. Plasticity of clonal populations of dedifferentiated adult human articular chondrocytes. Arthritis Rheum. 2003, 48, 1315-1325.
doi: 10.1002/(ISSN)1529-0131 URL |
| 81. |
Alsalameh, S.; Amin, R.; Gemba, T.; Lotz, M. Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum. 2004, 50, 1522-1532.
doi: 10.1002/(ISSN)1529-0131 URL |
| 82. | Hiraoka, K.; Grogan, S.; Olee, T.; Lotz, M. Mesenchymal progenitor cells in adult human articular cartilage. Biorh. 2006, 43, 447-454. |
| 83. |
Seol, D.; McCabe, D. J.; Choe, H.; Zheng, H.; Yu, Y.; Jang, K.; Walter, M. W.; Lehman, A. D.; Ding, L.; Buckwalter, J. A.; Martin, J. A. Chondrogenic progenitor cells respond to cartilage injury. Arthritis Rheum. 2012, 64, 3626-3637.
doi: 10.1002/art.34613 URL |
| 84. |
McCarthy, H. E.; Bara, J. J.; Brakspear, K.; Singhrao, S. K.; Archer, C. W. The comparison of equine articular cartilage progenitor cells and bone marrow-derived stromal cells as potential cell sources for cartilage repair in the horse. Vet J. 2012, 192, 345-351.
doi: 10.1016/j.tvjl.2011.08.036 URL |
| 85. | Ozbey, O.; Sahin, Z.; Acar, N.; Ustunel, I. Distribution of CD105 and CD166 positive cells in the proximal epiphysis of developing rat humerus. Histol Histopathol. 2010, 25, 1437-1445. |
| 86. |
Fickert, S.; Fiedler, J.; Brenner, R. E. Identification of subpopulations with characteristics of mesenchymal progenitor cells from human osteoarthritic cartilage using triple staining for cell surface markers. Arthritis Res Ther. 2004, 6, R422-432.
doi: 10.1186/ar1210 URL |
| 87. |
Churchill, J. L.; Krych, A. J.; Lemos, M. J.; Redd, M.; Bonner, K. F. A case series of successful repair of articular cartilage fragments in the knee. Am J Sports Med. 2019, 47, 2589-2595.
doi: 10.1177/0363546519865497 URL |
| 88. |
García-Arnandis, I.; Guillén, M. I.; Castejón, M. A.; Gomar, F.; Alcaraz, M. J. Haem oxygenase-1 down-regulates high mobility group box 1 and matrix metalloproteinases in osteoarthritic synoviocytes. Rheumatology (Oxford). 2010, 49, 854-861.
doi: 10.1093/rheumatology/kep463 URL |
| 89. |
Joos, H.; Wildner, A.; Hogrefe, C.; Reichel, H.; Brenner, R. E. Interleukin-1 beta and tumor necrosis factor alpha inhibit migration activity of chondrogenic progenitor cells from non-fibrillated osteoarthritic cartilage. Arthritis Res Ther. 2013, 15, R119.
doi: 10.1186/ar4299 URL |
| 90. |
Mishima, Y.; Lotz, M. Chemotaxis of human articular chondrocytes and mesenchymal stem cells. J Orthop Res. 2008, 26, 1407-1412.
doi: 10.1002/jor.20668 URL |
| 91. |
Schnabel, M.; Marlovits, S.; Eckhoff, G.; Fichtel, I.; Gotzen, L.; Vécsei, V.; Schlegel, J. Dedifferentiation-associated changes in morphology and gene expression in primary human articular chondrocytes in cell culture. Osteoarthritis Cartilage. 2002, 10, 62-70.
doi: 10.1053/joca.2001.0482 URL |
| 92. |
Miller, R. E.; Scanzello, C. R.; Malfait, A. M. An emerging role for Toll-like receptors at the neuroimmune interface in osteoarthritis. Semin Immunopathol. 2019, 41, 583-594.
doi: 10.1007/s00281-019-00762-3 URL |
| 93. |
Mills, C. D.; Kincaid, K.; Alt, J. M.; Heilman, M. J.; Hill, A. M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000, 164, 6166-6173.
doi: 10.4049/jimmunol.164.12.6166 URL |
| 94. |
Kraus, V. B.; McDaniel, G.; Huebner, J. L.; Stabler, T. V.; Pieper, C. F.; Shipes, S. W.; Petry, N. A.; Low, P. S.; Shen, J.; McNearney, T. A.; Mitchell, P. Direct in vivo evidence of activated macrophages in human osteoarthritis. Osteoarthritis Cartilage. 2016, 24, 1613-1621.
doi: 10.1016/j.joca.2016.04.010 URL |
| 95. |
Scanzello, C. R.; Goldring, S. R. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012, 51, 249-257.
doi: 10.1016/j.bone.2012.02.012 URL |
| 96. |
Wang, T.; He, C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018, 44, 38-50.
doi: 10.1016/j.cytogfr.2018.10.002 URL |
| 97. | Li, Y. S.; Luo, W.; Zhu, S. A.; Lei, G. H. T cells in osteoarthritis: alterations and beyond. Front Immunol. 2017, 8, 356. |
| 98. |
Glyn-Jones, S.; Palmer, A. J.; Agricola, R.; Price, A. J.; Vincent, T. L.; Weinans, H.; Carr, A. J. Osteoarthritis. Lancet. 2015, 386, 376-387.
doi: 10.1016/S0140-6736(14)60802-3 URL |
| 99. |
Imada, K.; Oka, H.; Kawasaki, D.; Miura, N.; Sato, T.; Ito, A. Anti-arthritic action mechanisms of natural chondroitin sulfate in human articular chondrocytes and synovial fibroblasts. Biol Pharm Bull. 2010, 33, 410-414.
doi: 10.1248/bpb.33.410 URL |
| 100. |
Cheleschi, S.; Fioravanti, A.; De Palma, A.; Corallo, C.; Franci, D.; Volpi, N.; Bedogni, G.; Giannotti, S.; Giordano, N. Methylsulfonylmethane and mobilee prevent negative effect of IL-1β in human chondrocyte cultures via NF-κB signaling pathway. Int Immunopharmacol. 2018, 65, 129-139.
doi: 10.1016/j.intimp.2018.10.004 URL |
| 101. |
Zhuang, C.; Wang, Y.; Zhang, Y.; Xu, N. Oxidative stress in osteoarthritis and antioxidant effect of polysaccharide from angelica sinensis. Int J Biol Macromol. 2018, 115, 281-286.
doi: 10.1016/j.ijbiomac.2018.04.083 URL |
| 102. |
Ertürk, C.; Altay, M. A.; Selek, S.; Koçyiğit, A. Paraoxonase-1 activity and oxidative status in patients with knee osteoarthritis and their relationship with radiological and clinical parameters. Scand J Clin Lab Invest. 2012, 72, 433-439.
doi: 10.3109/00365513.2012.687116 URL |
| 103. |
Courties, A.; Gualillo, O.; Berenbaum, F.; Sellam, J. Metabolic stress-induced joint inflammation and osteoarthritis. Osteoarthritis Cartilage. 2015, 23, 1955-1965.
doi: 10.1016/j.joca.2015.05.016 URL |
| 104. |
Lepetsos, P.; Papavassiliou, K. A.; Papavassiliou, A. G. Redox and NF-κB signaling in osteoarthritis. Free Radic Biol Med. 2019, 132, 90-100.
doi: 10.1016/j.freeradbiomed.2018.09.025 URL |
| 105. | Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S. A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J. T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018, 233, 6425-6440. |
| 106. |
Laskin, D. L.; Sunil, V. R.; Gardner, C. R.; Laskin, J. D. Macrophages and tissue injury: agents of defense or destruction? Annu Rev Pharmacol Toxicol. 2011, 51, 267-288.
doi: 10.1146/pharmtox.2011.51.issue-1 URL |
| 107. |
Ackerman, J. E.; Geary, M. B.; Orner, C. A.; Bawany, F.; Loiselle, A. E. Obesity/Type II diabetes alters macrophage polarization resulting in a fibrotic tendon healing response. PLoS One. 2017, 12, e0181127.
doi: 10.1371/journal.pone.0181127 URL |
| 108. |
Zhang, S.; Chuah, S. J.; Lai, R. C.; Hui, J. H. P.; Lim, S. K.; Toh, W. S. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018, 156, 16-27.
doi: 10.1016/j.biomaterials.2017.11.028 URL |
| 109. |
Dai, M.; Sui, B.; Xue, Y.; Liu, X.; Sun, J. Cartilage repair in degenerative osteoarthritis mediated by squid type II collagen via immunomodulating activation of M2 macrophages, inhibiting apoptosis and hypertrophy of chondrocytes. Biomaterials. 2018, 180, 91-103.
doi: 10.1016/j.biomaterials.2018.07.011 URL |
| 110. |
Chu, J.; Yan, B.; Zhang, J.; Peng, L.; Ao, X.; Zheng, Z.; Jiang, T.; Zhang, Z. Casticin attenuates osteoarthritis-related cartilage degeneration by inhibiting the ROS-mediated NF-κB signaling pathway in vitro and in vivo. Inflammation. 2020, 43, 810-820.
doi: 10.1007/s10753-019-01167-y URL |
| 111. |
Hu, S. L.; Wang, K.; Shi, Y. F.; Shao, Z. X.; Zhang, C. X.; Sheng, K. W.; Ge, Z. D.; Chen, J. X.; Wang, X. Y. Downregulating Akt/NF-κB signaling and its antioxidant activity with Loureirin A for alleviating the progression of osteoarthritis: In vitro and vivo studies. Int Immunopharmacol. 2020, 78, 105953.
doi: 10.1016/j.intimp.2019.105953 URL |
| 112. |
Gleghorn, J. P.; Jones, A. R.; Flannery, C. R.; Bonassar, L. J. Boundary mode lubrication of articular cartilage by recombinant human lubricin. J Orthop Res. 2009, 27, 771-777.
doi: 10.1002/jor.v27:6 URL |
| 113. |
Flannery, C. R.; Zollner, R.; Corcoran, C.; Jones, A. R.; Root, A.; Rivera-Bermúdez, M. A.; Blanchet, T.; Gleghorn, J. P.; Bonassar, L. J.; Bendele, A. M.; Morris, E. A.; Glasson, S. S. Prevention of cartilage degeneration in a rat model of osteoarthritis by intraarticular treatment with recombinant lubricin. Arthritis Rheum. 2009, 60, 840-847.
doi: 10.1002/art.v60:3 URL |
| 114. |
Sophia Fox, A. J.; Bedi, A.; Rodeo, S. A. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009, 1, 461-468.
doi: 10.1177/1941738109350438 URL |
| 115. |
Chuah, Y. J.; Peck, Y.; Lau, J. E.; Hee, H. T.; Wang, D. A. Hydrogel based cartilaginous tissue regeneration: recent insights and technologies. Biomater Sci. 2017, 5, 613-631.
doi: 10.1039/C6BM00863A URL |
| 116. |
Shields, K. J.; Beckman, M. J.; Bowlin, G. L.; Wayne, J. S. Mechanical properties and cellular proliferation of electrospun collagen type II. Tissue Eng. 2004, 10, 1510-1517.
doi: 10.1089/ten.2004.10.1510 URL |
| 117. |
Liu, S.; Wu, J.; Liu, X.; Chen, D.; Bowlin, G. L.; Cao, L.; Lu, J.; Li, F.; Mo, X.; Fan, C. Osteochondral regeneration using an oriented nanofiber yarn-collagen type I/hyaluronate hybrid/TCP biphasic scaffold. J Biomed Mater Res A. 2015, 103, 581-592.
doi: 10.1002/jbm.v103.2 URL |
| 118. |
Dong, C.; Lv, Y. Application of collagen scaffold in tissue engineering: recent advances and new perspectives. Polymers (Basel). 2016, 8, 42.
doi: 10.3390/polym8020042 URL |
| 119. |
Mohan, N.; Mohanan, P. V.; Sabareeswaran, A.; Nair, P. Chitosan-hyaluronic acid hydrogel for cartilage repair. Int J Biol Macromol. 2017, 104, 1936-1945.
doi: 10.1016/j.ijbiomac.2017.03.142 URL |
| 120. |
Pulkkinen, H. J.; Tiitu, V.; Valonen, P.; Jurvelin, J. S.; Lammi, M. J.; Kiviranta, I. Engineering of cartilage in recombinant human type II collagen gel in nude mouse model in vivo. Osteoarthritis Cartilage. 2010, 18, 1077-1087.
doi: 10.1016/j.joca.2010.05.004 URL |
| 121. |
Marquass, B.; Somerson, J. S.; Hepp, P.; Aigner, T.; Schwan, S.; Bader, A.; Josten, C.; Zscharnack, M.; Schulz, R. M. A novel MSC-seeded triphasic construct for the repair of osteochondral defects. J Orthop Res. 2010, 28, 1586-1599.
doi: 10.1002/jor.21173 URL |
| 122. |
Leone, G.; Volpato, M. D.; Nelli, N.; Lamponi, S.; Boanini, E.; Bigi, A.; Magnani, A. Continuous multilayered composite hydrogel as osteochondral substitute. J Biomed Mater Res A. 2015, 103, 2521-2530.
doi: 10.1002/jbm.a.v103.8 URL |
| 123. |
Mallick, S. P.; Singh, B. N.; Rastogi, A.; Srivastava, P. Design and evaluation of chitosan/poly(l-lactide)/pectin based composite scaffolds for cartilage tissue regeneration. Int J Biol Macromol. 2018, 112, 909-920.
doi: 10.1016/j.ijbiomac.2018.02.049 URL |
| 124. |
Duan, P.; Pan, Z.; Cao, L.; He, Y.; Wang, H.; Qu, Z.; Dong, J.; Ding, J. The effects of pore size in bilayered poly(lactide-co-glycolide) scaffolds on restoring osteochondral defects in rabbits. J Biomed Mater Res A. 2014, 102, 180-192.
doi: 10.1002/jbm.a.34683 URL |
| 125. |
Yang, J.; Zhang, Y. S.; Yue, K.; Khademhosseini, A. Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta Biomater. 2017, 57, 1-25.
doi: 10.1016/j.actbio.2017.01.036 URL |
| 126. | Peng, Y.; Qing, X.; Lin, H.; Huang, D.; Li, J.; Tian, S.; Liu, S.; Lv, X.; Ma, K.; Li, R.; Rao, Z.; Bai, Y.; Chen, S.; Lei, M.; Quan, D.; Shao, Z. Decellularized disc hydrogels for hBMSCs tissue-specific differentiation and tissue regeneration. Bioact Mater. 2021, 6, 3541-3556. |
| 127. | Antons, J.; Marascio, M. G.; Aeberhard, P.; Weissenberger, G.; Hirt-Burri, N.; Applegate, L. A.; Bourban, P. E.; Pioletti, D. P. Decellularised tissues obtained by a CO(2)-philic detergent and supercritical CO(2). Eur Cell Mater. 2018, 36, 81-95. |
| 128. |
Sun, Y.; Yan, L.; Chen, S.; Pei, M. Functionality of decellularized matrix in cartilage regeneration: A comparison of tissue versus cell sources. Acta Biomater. 2018, 74, 56-73.
doi: 10.1016/j.actbio.2018.04.048 URL |
| 129. |
Pati, F.; Jang, J.; Ha, D. H.; Won Kim, S.; Rhie, J. W.; Shim, J. H.; Kim, D. H.; Cho, D. W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun. 2014, 5, 3935.
doi: 10.1038/ncomms4935 URL |
| 130. |
Sutherland, A. J.; Beck, E. C.; Dennis, S. C.; Converse, G. L.; Hopkins, R. A.; Berkland, C. J.; Detamore, M. S. Decellularized cartilage may be a chondroinductive material for osteochondral tissue engineering. PLoS One. 2015, 10, e0121966.
doi: 10.1371/journal.pone.0121966 URL |
| 131. |
Almeida, H. V.; Eswaramoorthy, R.; Cunniffe, G. M.; Buckley, C. T.; O’Brien, F. J.; Kelly, D. J. Fibrin hydrogels functionalized with cartilage extracellular matrix and incorporating freshly isolated stromal cells as an injectable for cartilage regeneration. Acta Biomater. 2016, 36, 55-62.
doi: 10.1016/j.actbio.2016.03.008 URL |
| 132. |
Shintani, N.; Hunziker, E. B. Chondrogenic differentiation of bovine synovium: bone morphogenetic proteins 2 and 7 and transforming growth factor beta1 induce the formation of different types of cartilaginous tissue. Arthritis Rheum. 2007, 56, 1869-1879.
doi: 10.1002/(ISSN)1529-0131 URL |
| 133. |
Holland, T. A.; Bodde, E. W.; Cuijpers, V. M.; Baggett, L. S.; Tabata, Y.; Mikos, A. G.; Jansen, J. A. Degradable hydrogel scaffolds for in vivo delivery of single and dual growth factors in cartilage repair. Osteoarthritis Cartilage. 2007, 15, 187-197.
doi: 10.1016/j.joca.2006.07.006 URL |
| 134. |
Patil, A. S.; Sable, R. B.; Kothari, R. M. Role of insulin-like growth factors (IGFs), their receptors and genetic regulation in the chondrogenesis and growth of the mandibular condylar cartilage. J Cell Physiol. 2012, 227, 1796-1804.
doi: 10.1002/jcp.22905 URL |
| 135. |
Fischer, J.; Knoch, N.; Sims, T.; Rosshirt, N.; Richter, W. Time-dependent contribution of BMP, FGF, IGF, and HH signaling to the proliferation of mesenchymal stroma cells during chondrogenesis. J Cell Physiol. 2018, 233, 8962-8970.
doi: 10.1002/jcp.v233.11 URL |
| 136. |
Boushell, M. K.; Mosher, C. Z.; Suri, G. K.; Doty, S. B.; Strauss, E. J.; Hunziker, E. B.; Lu, H. H. Polymeric mesh and insulin-like growth factor 1 delivery enhance cell homing and graft-cartilage integration. Ann N Y Acad Sci. 2019, 1442, 138-152.
doi: 10.1111/nyas.2019.1442.issue-1 URL |
| 137. | Lacci, K. M.; Dardik, A. Platelet-rich plasma: support for its use in wound healing. Yale J Biol Med. 2010, 83, 1-9. |
| 138. |
Lindeboom, J. A.; Mathura, K. R.; Aartman, I. H.; Kroon, F. H.; Milstein, D. M.; Ince, C. Influence of the application of platelet-enriched plasma in oral mucosal wound healing. Clin Oral Implants Res. 2007, 18, 133-139.
doi: 10.1111/clr.2007.18.issue-1 URL |
| 139. |
Barbon, S.; Stocco, E.; Macchi, V.; Contran, M.; Grandi, F.; Borean, A.; Parnigotto, P. P.; Porzionato, A.; De Caro, R. Platelet-rich fibrin scaffolds for cartilage and tendon regenerative medicine: from bench to bedside. Int J Mol Sci. 2019, 20, 1701.
doi: 10.3390/ijms20071701 URL |
| 140. |
Chang, N. J.; Erdenekhuyag, Y.; Chou, P. H.; Chu, C. J.; Lin, C. C.; Shie, M. Y. Therapeutic effects of the addition of platelet-rich plasma to bioimplants and early rehabilitation exercise on articular cartilage repair. Am J Sports Med. 2018, 46, 2232-2241.
doi: 10.1177/0363546518780955 URL |
| 141. |
Anderson, J. M.; Rodriguez, A.; Chang, D. T. Foreign body reaction to biomaterials. Semin Immunol. 2008, 20, 86-100.
doi: 10.1016/j.smim.2007.11.004 URL |
| 142. |
Vasconcelos, D. M.; Gonçalves, R. M.; Almeida, C. R.; Pereira, I. O.; Oliveira, M. I.; Neves, N.; Silva, A. M.; Ribeiro, A. C.; Cunha, C.; Almeida, A. R.; Ribeiro, C. C.; Gil, A. M.; Seebach, E.; Kynast, K. L.; Richter, W.; Lamghari, M.; Santos, S. G.; Barbosa, M. A. Fibrinogen scaffolds with immunomodulatory properties promote in vivo bone regeneration. Biomaterials. 2016, 111, 163-178.
doi: 10.1016/j.biomaterials.2016.10.004 URL |
| 143. |
Revati, R.; Abdul Majid, M. S.; Ridzuan, M. J. M.; Normahira, M.; Mohd Nasir, N. F.; Rahman, Y. M.; Gibson, A. G. Mechanical, thermal and morphological characterisation of 3D porous Pennisetum purpureum/PLA biocomposites scaffold. Mater Sci Eng C Mater Biol Appl. 2017, 75, 752-759.
doi: 10.1016/j.msec.2017.02.127 URL |
| 144. |
Harrington, S.; Williams, J.; Rawal, S.; Ramachandran, K.; Stehno-Bittel, L. Hyaluronic acid/collagen hydrogel as an alternative to alginate for long-term immunoprotected islet transplantation. Tissue Eng Part A. 2017, 23, 1088-1099.
doi: 10.1089/ten.tea.2016.0477 URL |
| 145. |
Lu, H. T.; Chang, W. T.; Tsai, M. L.; Chen, C. H.; Chen, W. Y.; Mi, F. L. Development of injectable fucoidan and biological macromolecules hybrid hydrogels for intra-articular delivery of platelet-rich plasma. Mar Drugs. 2019, 17, 236.
doi: 10.3390/md17040236 URL |
| 146. |
Zhao, Y.; Wei, C.; Chen, X.; Liu, J.; Yu, Q.; Liu, Y.; Liu, J. Drug delivery system based on near-infrared light-responsive molybdenum disulfide nanosheets controls the high-efficiency release of dexamethasone to inhibit inflammation and treat osteoarthritis. ACS Appl Mater Interfaces. 2019, 11, 11587-11601.
doi: 10.1021/acsami.8b20372 URL |
| 147. |
Alini, M.; Eisenstein, S. M.; Ito, K.; Little, C.; Kettler, A. A.; Masuda, K.; Melrose, J.; Ralphs, J.; Stokes, I.; Wilke, H. J. Are animal models useful for studying human disc disorders/degeneration? Eur Spine J. 2008, 17, 2-19.
doi: 10.1007/s00586-007-0414-y URL |
| 148. | Daly, C.; Ghosh, P.; Jenkin, G.; Oehme, D.; Goldschlager, T. A review of animal models of intervertebral disc degeneration: pathophysiology, regeneration, and translation to the clinic. Biomed Res Int. 2016, 2016, 5952165. |
| 149. |
Ao, X.; Wang, L.; Shao, Y.; Chen, X.; Zhang, J.; Chu, J.; Jiang, T.; Zhang, Z.; Huang, M. Development and characterization of a novel bipedal standing mouse model of intervertebral disc and facet joint degeneration. Clin Orthop Relat Res. 2019, 477, 1492-1504.
doi: 10.1097/CORR.0000000000000712 URL |
| 150. |
Walter, B. A.; Korecki, C. L.; Purmessur, D.; Roughley, P. J.; Michalek, A. J.; Iatridis, J. C. Complex loading affects intervertebral disc mechanics and biology. Osteoarthritis Cartilage. 2011, 19, 1011-1018.
doi: 10.1016/j.joca.2011.04.005 URL |
| 151. | Hartman, R.; Patil, P.; Tisherman, R.; St Croix, C.; Niedernhofer, L. J.; Robbins, P. D.; Ambrosio, F.; Van Houten, B.; Sowa, G.; Vo, N. Age-dependent changes in intervertebral disc cell mitochondria and bioenergetics. Eur Cell Mater. 2018, 36, 171-183. |
| 152. |
Lyu, F. J.; Cheung, K. M.; Zheng, Z.; Wang, H.; Sakai, D.; Leung, V. Y. IVD progenitor cells: a new horizon for understanding disc homeostasis and repair. Nat Rev Rheumatol. 2019, 15, 102-112.
doi: 10.1038/s41584-018-0154-x URL |
| 153. |
Henriksson, H.; Thornemo, M.; Karlsson, C.; Hägg, O.; Junevik, K.; Lindahl, A.; Brisby, H. Identification of cell proliferation zones, progenitor cells and a potential stem cell niche in the intervertebral disc region: a study in four species. Spine (Phila Pa 1976). 2009, 34, 2278-2287.
doi: 10.1097/BRS.0b013e3181a95ad2 URL |
| 154. |
Henriksson, H. B.; Svala, E.; Skioldebrand, E.; Lindahl, A.; Brisby, H. Support of concept that migrating progenitor cells from stem cell niches contribute to normal regeneration of the adult mammal intervertebral disc: a descriptive study in the New Zealand white rabbit. Spine (Phila Pa 1976). 2012, 37, 722-732.
doi: 10.1097/BRS.0b013e318231c2f7 URL |
| 155. |
Sasaki, N.; Henriksson, H. B.; Runesson, E.; Larsson, K.; Sekiguchi, M.; Kikuchi, S.; Konno, S.; Rydevik, B.; Brisby, H. Physical exercise affects cell proliferation in lumbar intervertebral disc regions in rats. Spine (Phila Pa 1976). 2012, 37, 1440-1447.
doi: 10.1097/BRS.0b013e31824ff87d URL |
| 156. |
Shi, R.; Wang, F.; Hong, X.; Wang, Y. T.; Bao, J. P.; Cai, F.; Wu, X. T. The presence of stem cells in potential stem cell niches of the intervertebral disc region: an in vitro study on rats. Eur Spine J. 2015, 24, 2411-2424.
doi: 10.1007/s00586-015-4168-7 URL |
| 157. | Grunhagen, T.; Shirazi-Adl, A.; Fairbank, J. C.; Urban, J. P. Intervertebral disk nutrition: a review of factors influencing concentrations of nutrients and metabolites. Orthop Clin North Am. 2011, 42, 465-477, vii. |
| 158. |
Holm, S.; Maroudas, A.; Urban, J. P.; Selstam, G.; Nachemson, A. Nutrition of the intervertebral disc: solute transport and metabolism. Connect Tissue Res. 1981, 8, 101-119.
doi: 10.3109/03008208109152130 URL |
| 159. |
Risbud, M. V.; Shapiro, I. M. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014, 10, 44-56.
doi: 10.1038/nrrheum.2013.160 URL |
| 160. |
Li, Z.; Chen, S.; Ma, K.; Lv, X.; Lin, H.; Hu, B.; He, R.; Shao, Z. CsA attenuates compression-induced nucleus pulposus mesenchymal stem cells apoptosis via alleviating mitochondrial dysfunction and oxidative stress. Life Sci. 2018, 205, 26-37.
doi: 10.1016/j.lfs.2018.05.014 URL |
| 161. | Chen, S.; Deng, X.; Ma, K.; Zhao, L.; Huang, D.; Li, Z.; Shao, Z. Icariin improves the viability and function of cryopreserved human nucleus pulposus-derived mesenchymal stem cells. Oxid Med Cell Longev. 2018, 2018, 3459612. |
| 162. |
Patil, P.; Falabella, M.; Saeed, A.; Lee, D.; Kaufman, B.; Shiva, S.; Croix, C. S.; Van Houten, B.; Niedernhofer, L. J.; Robbins, P. D.; Lee, J.; Gwendolyn, S.; Vo, N. V. Oxidative stress-induced senescence markedly increases disc cell bioenergetics. Mech Ageing Dev. 2019, 180, 97-106.
doi: 10.1016/j.mad.2019.04.006 URL |
| 163. | Childs, B. G.; Gluscevic, M.; Baker, D. J.; Laberge, R. M.; Marquess, D.; Dananberg, J.; van Deursen, J. M. Senescent cells: an emerging target for diseases of ageing. Nat Rev Drug Discov. 2017, 16, 718-735. |
| 164. |
Huang, D.; Peng, Y.; Li, Z.; Chen, S.; Deng, X.; Shao, Z.; Ma, K. Compression-induced senescence of nucleus pulposus cells by promoting mitophagy activation via the PINK1/PARKIN pathway. J Cell Mol Med. 2020, 24, 5850-5864.
doi: 10.1111/jcmm.v24.10 URL |
| 165. |
Chen, D.; Xia, D.; Pan, Z.; Xu, D.; Zhou, Y.; Wu, Y.; Cai, N.; Tang, Q.; Wang, C.; Yan, M.; Zhang, J. J.; Zhou, K.; Wang, Q.; Feng, Y.; Wang, X.; Xu, H.; Zhang, X.; Tian, N. Metformin protects against apoptosis and senescence in nucleus pulposus cells and ameliorates disc degeneration in vivo. Cell Death Dis. 2016, 7, e2441.
doi: 10.1038/cddis.2016.334 URL |
| 166. |
Xia, C.; Zeng, Z.; Fang, B.; Tao, M.; Gu, C.; Zheng, L.; Wang, Y.; Shi, Y.; Fang, C.; Mei, S.; Chen, Q.; Zhao, J.; Lin, X.; Fan, S.; Jin, Y.; Chen, P. Mesenchymal stem cell-derived exosomes ameliorate intervertebral disc degeneration via anti-oxidant and anti-inflammatory effects. Free Radic Biol Med. 2019, 143, 1-15.
doi: 10.1016/j.freeradbiomed.2019.07.026 URL |
| 167. |
Nakamichi, R.; Ito, Y.; Inui, M.; Onizuka, N.; Kayama, T.; Kataoka, K.; Suzuki, H.; Mori, M.; Inagawa, M.; Ichinose, S.; Lotz, M. K.; Sakai, D.; Masuda, K.; Ozaki, T.; Asahara, H. Mohawk promotes the maintenance and regeneration of the outer annulus fibrosus of intervertebral discs. Nat Commun. 2016, 7, 12503.
doi: 10.1038/ncomms12503 URL |
| 168. |
Yao, M.; Zhang, J.; Li, Z.; Guo, S.; Zhou, X.; Zhang, W. Marein protects human nucleus pulposus cells against high glucose-induced injury and extracellular matrix degradation at least partly by inhibition of ROS/NF-κB pathway. Int Immunopharmacol. 2020, 80, 106126.
doi: 10.1016/j.intimp.2019.106126 URL |
| 169. |
Saraiya, M.; Nasser, R.; Zeng, Y.; Addya, S.; Ponnappan, R. K.; Fortina, P.; Anderson, D. G.; Albert, T. J.; Shapiro, I. M.; Risbud, M. V. Reversine enhances generation of progenitor-like cells by dedifferentiation of annulus fibrosus cells. Tissue Eng Part A. 2010, 16, 1443-1455.
doi: 10.1089/ten.tea.2009.0343 URL |
| 170. |
Tendulkar, G.; Chen, T.; Ehnert, S.; Kaps, H. P.; Nüssler, A. K. Intervertebral disc nucleus repair: hype or hope? Int J Mol Sci. 2019, 20, 3622.
doi: 10.3390/ijms20153622 URL |
| 171. |
Roberts, S.; Ayad, S.; Menage, P. J. Immunolocalisation of type VI collagen in the intervertebral disc. Ann Rheum Dis. 1991, 50, 787-791.
doi: 10.1136/ard.50.11.787 URL |
| 172. |
Newell, N.; Little, J. P.; Christou, A.; Adams, M. A.; Adam, C. J.; Masouros, S. D. Biomechanics of the human intervertebral disc: A review of testing techniques and results. J Mech Behav Biomed Mater. 2017, 69, 420-434.
doi: 10.1016/j.jmbbm.2017.01.037 URL |
| 173. |
Clouet, J.; Grimandi, G.; Pot-Vaucel, M.; Masson, M.; Fellah, H. B.; Guigand, L.; Cherel, Y.; Bord, E.; Rannou, F.; Weiss, P.; Guicheux, J.; Vinatier, C. Identification of phenotypic discriminating markers for intervertebral disc cells and articular chondrocytes. Rheumatology (Oxford). 2009, 48, 1447-1450.
doi: 10.1093/rheumatology/kep262 URL |
| 174. |
Li, J.; Liu, C.; Guo, Q.; Yang, H.; Li, B. Regional variations in the cellular, biochemical, and biomechanical characteristics of rabbit annulus fibrosus. PLoS One. 2014, 9, e91799.
doi: 10.1371/journal.pone.0091799 URL |
| 175. |
Guerin, H. A.; Elliott, D. M. Degeneration affects the fiber reorientation of human annulus fibrosus under tensile load. J Biomech. 2006, 39, 1410-1418.
doi: 10.1016/j.jbiomech.2005.04.007 URL |
| 176. | Klein, J. A.; Hukins, D. W. Collagen fibre orientation in the annulus fibrosus of intervertebral disc during bending and torsion measured by x-ray diffraction. Biochim Biophys Acta. 1982, 719, 98-101. |
| 177. |
Pearce, R. H.; Grimmer, B. J.; Adams, M. E. Degeneration and the chemical composition of the human lumbar intervertebral disc. J Orthop Res. 1987, 5, 198-205.
doi: 10.1002/(ISSN)1554-527X URL |
| 178. |
Hee, H. T.; Chuah, Y. J.; Tan, B. H.; Setiobudi, T.; Wong, H. K. Vascularization and morphological changes of the endplate after axial compression and distraction of the intervertebral disc. Spine (Phila Pa 1976). 2011, 36, 505-511.
doi: 10.1097/BRS.0b013e3181d32410 URL |
| 179. |
Growney Kalaf, E. A.; Flores, R.; Bledsoe, J. G.; Sell, S. A. Characterization of slow-gelling alginate hydrogels for intervertebral disc tissue-engineering applications. Mater Sci Eng C Mater Biol Appl. 2016, 63, 198-210.
doi: 10.1016/j.msec.2016.02.067 URL |
| 180. |
Li, Z.; Lang, G.; Chen, X.; Sacks, H.; Mantzur, C.; Tropp, U.; Mader, K. T.; Smallwood, T. C.; Sammon, C.; Richards, R. G.; Alini, M.; Grad, S. Polyurethane scaffold with in situ swelling capacity for nucleus pulposus replacement. Biomaterials. 2016, 84, 196-209.
doi: 10.1016/j.biomaterials.2016.01.040 URL |
| 181. |
Woiciechowsky, C.; Abbushi, A.; Zenclussen, M. L.; Casalis, P.; Krüger, J. P.; Freymann, U.; Endres, M.; Kaps, C. Regeneration of nucleus pulposus tissue in an ovine intervertebral disc degeneration model by cell-free resorbable polymer scaffolds. J Tissue Eng Regen Med. 2014, 8, 811-820.
doi: 10.1002/term.v8.10 URL |
| 182. |
Priyadarshani, P.; Li, Y.; Yang, S.; Yao, L. Injectable hydrogel provides growth-permissive environment for human nucleus pulposus cells. J Biomed Mater Res A. 2016, 104, 419-426.
doi: 10.1002/jbm.a.35580 URL |
| 183. |
Feng, G.; Jin, X.; Hu, J.; Ma, H.; Gupte, M. J.; Liu, H.; Ma, P. X. Effects of hypoxias and scaffold architecture on rabbit mesenchymal stem cell differentiation towards a nucleus pulposus-like phenotype. Biomaterials. 2011, 32, 8182-8189.
doi: 10.1016/j.biomaterials.2011.07.049 URL |
| 184. |
Zhou, X.; Wang, J.; Fang, W.; Tao, Y.; Zhao, T.; Xia, K.; Liang, C.; Hua, J.; Li, F.; Chen, Q. Genipin cross-linked type II collagen/chondroitin sulfate composite hydrogel-like cell delivery system induces differentiation of adipose-derived stem cells and regenerates degenerated nucleus pulposus. Acta Biomater. 2018, 71, 496-509.
doi: 10.1016/j.actbio.2018.03.019 URL |
| 185. |
Zhou, X.; Wang, J.; Huang, X.; Fang, W.; Tao, Y.; Zhao, T.; Liang, C.; Hua, J.; Chen, Q.; Li, F. Injectable decellularized nucleus pulposus-based cell delivery system for differentiation of adipose-derived stem cells and nucleus pulposus regeneration. Acta Biomater. 2018, 81, 115-128.
doi: 10.1016/j.actbio.2018.09.044 URL |
| 186. |
Huang, Y. Z.; Cai, J. Q.; Lv, F. J.; Xie, H. L.; Yang, Z. M.; Huang, Y. C.; Deng, L. Species variation in the spontaneous calcification of bone marrow-derived mesenchymal stem cells. Cytotherapy. 2013, 15, 323-329.
doi: 10.1016/j.jcyt.2012.11.011 URL |
| 187. |
Vadalà, G.; Sowa, G.; Hubert, M.; Gilbertson, L. G.; Denaro, V.; Kang, J. D. Mesenchymal stem cells injection in degenerated intervertebral disc: cell leakage may induce osteophyte formation. J Tissue Eng Regen Med. 2012, 6, 348-355.
doi: 10.1002/term.v6.5 URL |
| 188. |
Liu, C.; Zhu, C.; Li, J.; Zhou, P.; Chen, M.; Yang, H.; Li, B. The effect of the fibre orientation of electrospun scaffolds on the matrix production of rabbit annulus fibrosus-derived stem cells. Bone Res. 2015, 3, 15012.
doi: 10.1038/boneres.2015.12 URL |
| 189. | Yuan, C.; Wang, J.; Zhu, X.; Zheng, Y.; Huang, B.; Li, C.; Zhou, Y. Stress regulating osteogenic differentiation of human intervertebral disc cartilage endplate-derived stem cells. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2015, 29, 351-355. |
| 190. |
Lv, F.; Lu, M.; Cheung, K. M.; Leung, V. Y.; Zhou, G. Intrinsic properties of mesemchymal stem cells from human bone marrow, umbilical cord and umbilical cord blood comparing the different sources of MSC. Curr Stem Cell Res Ther. 2012, 7, 389-399.
doi: 10.2174/157488812804484611 URL |
| 191. |
Liu, C.; Jin, Z.; Ge, X.; Zhang, Y.; Xu, H. Decellularized annulus fibrosus matrix/chitosan hybrid hydrogels with basic fibroblast growth factor for annulus fibrosus tissue engineering. Tissue Eng Part A. 2019, 25, 1605-1613.
doi: 10.1089/ten.tea.2018.0297 URL |
| 192. |
Xu, J.; Liu, S.; Wang, S.; Qiu, P.; Chen, P.; Lin, X.; Fang, X. Decellularised nucleus pulposus as a potential biologic scaffold for disc tissue engineering. Mater Sci Eng C Mater Biol Appl. 2019, 99, 1213-1225.
doi: 10.1016/j.msec.2019.02.045 URL |
| 193. |
Illien-Jünger, S.; Pattappa, G.; Peroglio, M.; Benneker, L. M.; Stoddart, M. J.; Sakai, D.; Mochida, J.; Grad, S.; Alini, M. Homing of mesenchymal stem cells in induced degenerative intervertebral discs in a whole organ culture system. Spine (Phila Pa 1976). 2012, 37, 1865-1873.
doi: 10.1097/BRS.0b013e3182544a8a URL |
| 194. |
Baek, S. J.; Kang, S. K.; Ra, J. C. In vitro migration capacity of human adipose tissue-derived mesenchymal stem cells reflects their expression of receptors for chemokines and growth factors. Exp Mol Med. 2011, 43, 596-603.
doi: 10.3858/emm.2011.43.10.069 URL |
| 195. |
Lee, M. J.; Kim, J.; Kim, M. Y.; Bae, Y. S.; Ryu, S. H.; Lee, T. G.; Kim, J. H. Proteomic analysis of tumor necrosis factor-alpha-induced secretome of human adipose tissue-derived mesenchymal stem cells. J Proteome Res. 2010, 9, 1754-1762.
doi: 10.1021/pr900898n URL |
| 196. |
Zhang, H.; Yu, S.; Zhao, X.; Mao, Z.; Gao, C. Stromal cell-derived factor-1α-encapsulated albumin/heparin nanoparticles for induced stem cell migration and intervertebral disc regeneration in vivo. Acta Biomater. 2018, 72, 217-227.
doi: 10.1016/j.actbio.2018.03.032 URL |
| 197. |
Frapin, L.; Clouet, J.; Chédeville, C.; Moraru, C.; Samarut, E.; Henry, N.; André, M.; Bord, E.; Halgand, B.; Lesoeur, J.; Fusellier, M.; Guicheux, J.; Le Visage, C. Controlled release of biological factors for endogenous progenitor cell migration and intervertebral disc extracellular matrix remodelling. Biomaterials. 2020, 253, 120107.
doi: 10.1016/j.biomaterials.2020.120107 URL |
| 198. | Huang, D.; Peng, Y.; Ma, K.; Qing, X.; Deng, X.; Li, Z.; Shao, Z. Puerarin relieved compression-induced apoptosis and mitochondrial dysfunction in human nucleus pulposus mesenchymal stem cells via the PI3K/Akt pathway. Stem Cells Int. 2020, 2020, 7126914. |
| 199. |
Liang, C. Z.; Li, H.; Tao, Y. Q.; Peng, L. H.; Gao, J. Q.; Wu, J. J.; Li, F. C.; Hua, J. M.; Chen, Q. X. Dual release of dexamethasone and TGF-β3 from polymeric microspheres for stem cell matrix accumulation in a rat disc degeneration model. Acta Biomater. 2013, 9, 9423-9433.
doi: 10.1016/j.actbio.2013.08.019 URL |
| 200. |
Liu, J.; Tao, H.; Wang, H.; Dong, F.; Zhang, R.; Li, J.; Ge, P.; Song, P.; Zhang, H.; Xu, P.; Liu, X.; Shen, C. Biological behavior of human nucleus pulposus mesenchymal stem cells in response to changes in the acidic environment during intervertebral disc degeneration. Stem Cells Dev. 2017, 26, 901-911.
doi: 10.1089/scd.2016.0314 URL |
| 201. |
Huang, S.; Leung, V. Y.; Long, D.; Chan, D.; Lu, W. W.; Cheung, K. M.; Zhou, G. Coupling of small leucine-rich proteoglycans to hypoxic survival of a progenitor cell-like subpopulation in Rhesus Macaque intervertebral disc. Biomaterials. 2013, 34, 6548-6558.
doi: 10.1016/j.biomaterials.2013.05.027 URL |
| 202. |
Ni, L.; Liu, X.; Sochacki, K. R.; Ebraheim, M.; Fahrenkopf, M.; Shi, Q.; Liu, J.; Yang, H. Effects of hypoxia on differentiation from human placenta-derived mesenchymal stem cells to nucleus pulposus-like cells. Spine J. 2014, 14, 2451-2458.
doi: 10.1016/j.spinee.2014.03.028 URL |
| 203. | Zhang, Z.; Li, F.; Tian, H.; Guan, K.; Zhao, G.; Shan, J.; Ren, D. Differentiation of adipose-derived stem cells toward nucleus pulposus-like cells induced by hypoxia and a three-dimensional chitosan-alginate gel scaffold in vitro. Chin Med J (Engl). 2014, 127, 314-321. |
| 204. |
Ma, K.; Chen, S.; Li, Z.; Deng, X.; Huang, D.; Xiong, L.; Shao, Z. Mechanisms of endogenous repair failure during intervertebral disc degeneration. Osteoarthritis Cartilage. 2019, 27, 41-48.
doi: 10.1016/j.joca.2018.08.021 URL |
| 205. |
Liu, L.; DiGirolamo, C. M.; Navarro, P. A.; Blasco, M. A.; Keefe, D. L. Telomerase deficiency impairs differentiation of mesenchymal stem cells. Exp Cell Res. 2004, 294, 1-8.
doi: 10.1016/j.yexcr.2003.10.031 URL |
| 206. |
Mårtensson, K.; Chrysis, D.; Sävendahl, L. Interleukin-1beta and TNF-alpha act in synergy to inhibit longitudinal growth in fetal rat metatarsal bones. J Bone Miner Res. 2004, 19, 1805-1812.
doi: 10.1359/JBMR.040805 URL |
| 207. | Liang, H.; Chen, S.; Huang, D.; Deng, X.; Ma, K.; Shao, Z. Effect of compression loading on human nucleus pulposus-derived mesenchymal stem cells. Stem Cells Int. 2018, 2018, 1481243. |
| 208. |
Dahms, K.; Sharkova, Y.; Heitland, P.; Pankuweit, S.; Schaefer, J. R. Cobalt intoxication diagnosed with the help of Dr House. Lancet. 2014, 383, 574.
doi: 10.1016/S0140-6736(14)60037-4 URL |
| 209. |
Goodman, S. B.; Pajarinen, J.; Yao, Z.; Lin, T. Inflammation and bone repair: from particle disease to tissue regeneration. Front Bioeng Biotechnol. 2019, 7, 230.
doi: 10.3389/fbioe.2019.00230 URL |
| 210. |
Johnson, K. E.; Makanji, Y.; Temple-Smith, P.; Kelly, E. K.; Barton, P. A.; Al-Musawi, S. L.; Mueller, T. D.; Walton, K. L.; Harrison, C. A. Biological activity and in vivo half-life of pro-activin A in male rats. Mol Cell Endocrinol. 2016, 422, 84-92.
doi: 10.1016/j.mce.2015.12.007 URL |
| 211. |
Etulain, J. Platelets in wound healing and regenerative medicine. Platelets. 2018, 29, 556-568.
doi: 10.1080/09537104.2018.1430357 URL |
| 212. |
Zhu, Y.; Tan, J.; Zhu, H.; Lin, G.; Yin, F.; Wang, L.; Song, K.; Wang, Y.; Zhou, G.; Yi, W. Development of kartogenin-conjugated chitosan-hyaluronic acid hydrogel for nucleus pulposus regeneration. Biomater Sci. 2017, 5, 784-791.
doi: 10.1039/C7BM00001D URL |
| [1] | Trivia P. Frazier, Katie Hamel, Xiying Wu, Emma Rogers, Haley Lassiter, Jordan Robinson, Omair Mohiuddin, Michael Henderson, Jeffrey M. Gimble. Adipose-derived cells: building blocks of three-dimensional microphysiological systems [J]. Biomaterials Translational, 2021, 2(4): 301-306. |
| [2] | Xirui Jing, Qiuyue Ding, Qinxue Wu, Weijie Su, Keda Yu, Yanlin Su, Bing Ye, Qing Gao, Tingfang Sun, Xiaodong Guo. Magnesium-based materials in orthopaedics: material properties and animal models [J]. Biomaterials Translational, 2021, 2(3): 197-213. |
| [3] | Kamolrat Metavarayuth, Esteban Villarreal, Hui Wang, Qian Wang. Surface topography and free energy regulate osteogenesis of stem cells: effects of shape-controlled gold nanoparticles [J]. Biomaterials Translational, 2021, 2(2): 165-173. |
| [4] | Yizhong Peng, Xiangcheng Qing, Hongyang Shu, Shuo Tian, Wenbo Yang, Songfeng Chen, Hui Lin, Xiao Lv, Lei Zhao, Xi Chen, Feifei Pu, Donghua Huang, Xu Cao, Zengwu Shao. Proper animal experimental designs for preclinical research of biomaterials for intervertebral disc regeneration [J]. Biomaterials Translational, 2021, 2(2): 91-142. |
| [5] | Pingli Wu, Yangyang Liang, Guoming Sun. Engineering immune-responsive biomaterials for skin regeneration [J]. Biomaterials Translational, 2021, 2(1): 61-71. |
| [6] | Yiqing Wang, Xiangyu Chu, Bing Wang. Recombinant adeno-associated virus-based gene therapy combined with tissue engineering for musculoskeletal regenerative medicine [J]. Biomaterials Translational, 2021, 2(1): 19-29. |
| [7] | Isak Jatoi, Jingyu Fan. A biomaterials viewpoint for the 2020 SARS-CoV-2 vaccine development [J]. Biomaterials Translational, 2021, 2(1): 30-42. |
| [8] | Maryam Tamaddon, Helena Gilja, Ling Wang, J. Miguel Oliveira, Xiaodan Sun, Rongwei Tan, Chaozong Liu. Osteochondral scaffolds for early treatment of cartilage defects in osteoarthritic joints: from bench to clinic [J]. Biomaterials Translational, 2020, 1(1): 3-17. |
| [9] | Xing Yang, Yuanyuan Li, Xujie Liu, Wei He, Qianli Huang, Qingling Feng. Nanoparticles and their effects on differentiation of mesenchymal stem cells [J]. Biomaterials Translational, 2020, 1(1): 58-68. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||