Biomaterials Translational ›› 2022, Vol. 3 ›› Issue (3): 213-220.doi: 10.12336/biomatertransl.2022.03.005
• RESEARCH ARTICLE • Previous Articles Next Articles
Chavee Laomeephol1,2, Helena Ferreira3,4, Sorada Kanokpanont2,5,6, Jittima Amie Luckanagul1,7, Nuno M Neves3,4, Siriporn Damrongsakkul2,5,6,*( )
)
Received:2022-08-02
Revised:2022-09-06
Accepted:2022-09-16
Online:2022-09-28
Published:2022-09-28
Contact:
Siriporn Damrongsakkul
E-mail:Siriporn.D@chula.ac.th
About author:Siriporn Damrongsakkul, Siriporn.D@chula.ac.th.Laomeephol, C.; Ferreira, H.; Kanokpanont, S.; Luckanagul, J.; Neves, N.; Damrongsakkul, S. Osteogenic differentiation of encapsulated cells in dexamethasone-loaded phospholipid-induced silk fibroin hydrogels. Biomater Transl. 2022, 3(3), 213-220.
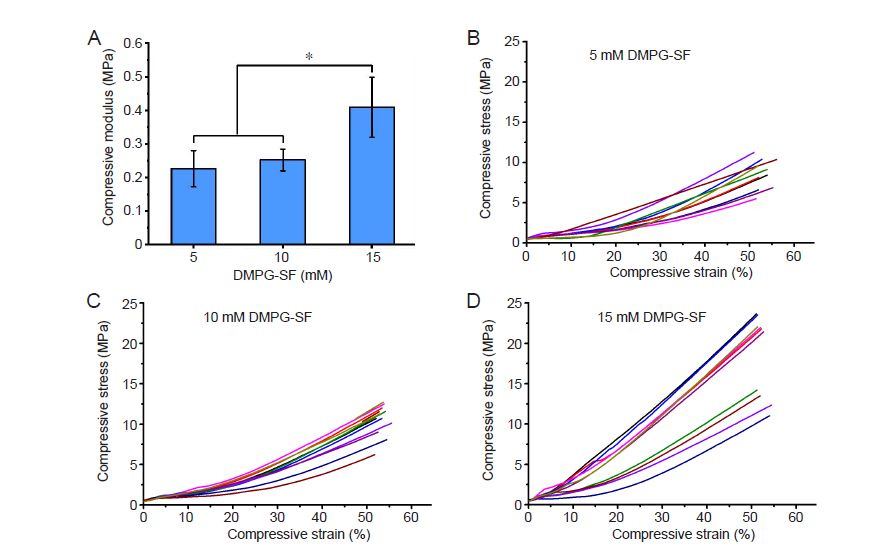
Figure 1. (A) Elastic modulus of the 5, 10 and 15 mM DMPG–3% (w/v) SF hydrogels determined by unconfined compression (mean ± SD, n = 10). *P ≤ 0.05 (one–way analysis of variance followed by Bonferroni post–hoc tests). (B–D) Compressive stress–strain plots of 5 (B), 10 (C), and 15 (D) mM DMPG–3% (w/v) SF hydrogels. DMPG: dimyristoyl glycerophosphoglycerol; SF: silk fibroin.
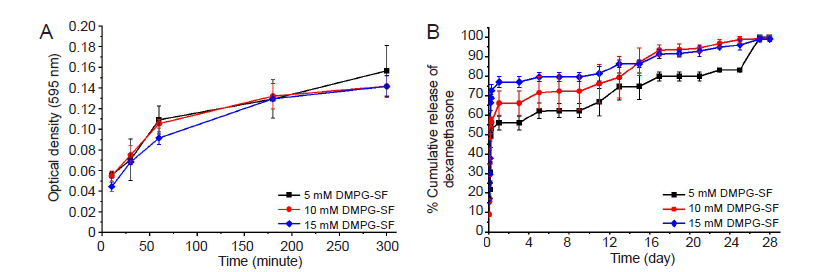
Figure 2. (A) Diffusivity of fetal bovine serum from the DMPG–SF hydrogels, determined from the protein released into the supernatants using Bradford protein assay. (B) Release profile of dexamethasone from 5, 10 and 15 mM DMPG/dexamethasone–SF hydrogels (containing 0.625, 1.25 and 1.875 mM dexamethasone, respectively) in 0.01 M phosphate–buffered saline (pH 7.4). Data are expressed as mean ± SD, and were analysed by one–way analysis of variance followed by Bonferroni post–hoc tests. The experiment was repeated three times. DMPG: dimyristoyl glycerophosphoglycerol; SF: silk fibroin.
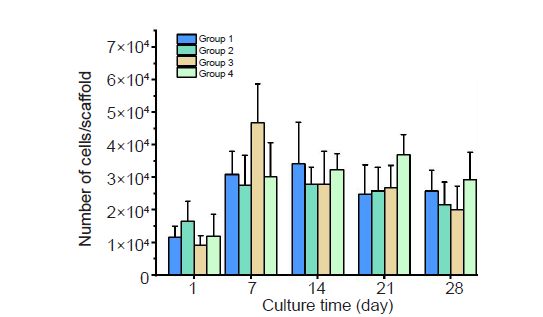
Figure 3. The number of SaOS–2 cells entrapped within DMPG–SF hydrogels cultured under different conditions. Group 1: cell–loaded DMPG–SF hydrogels cultured in proliferation medium; Group 2: cell–loaded DMPG–SF hydrogels cultured in osteogenic medium; Group 3: cell–loaded DMPG/Dex–SF hydrogels cultured in proliferation medium; Group 4: cell–loaded DMPG/Dex–SF hydrogels cultured in Dex–depleted osteogenic medium. Cell number was determined using Hoechst 33258 DNA quantification assay. Data are expressed as mean ± SD, and were analysed by one–way analysis of variance followed by Bonferroni post–hoc tests. The experiment was repeated three times. Dex: dexamethasone; DMPG: dimyristoyl glycerophosphoglycerol; SF: silk fibroin.
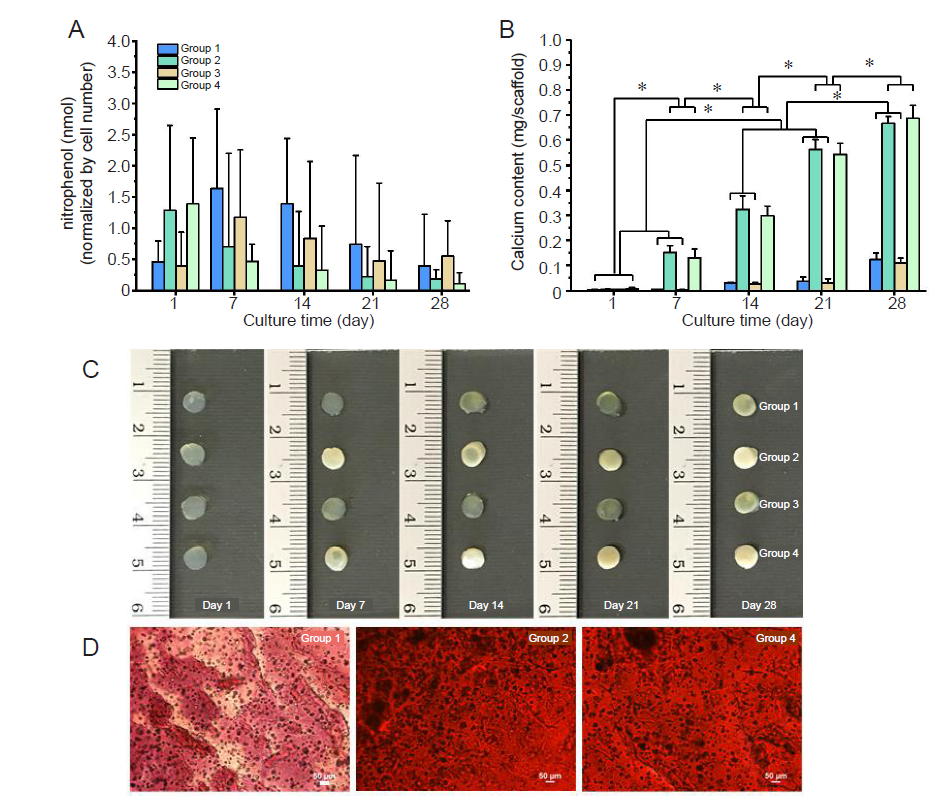
Figure 4. Osteogenic differentiation of encapsulated SaOS–2 cells in DMPG– or DMPG/Dex–SF hydrogels. Group 1: cell–loaded DMPG–SF hydrogels cultured in proliferation medium; Group 2: cell–loaded DMPG–SF hydrogels cultured in osteogenic medium; Group 3: cell–loaded DMPG/Dex–SF hydrogels cultured in proliferation medium; Group 4: cell–loaded DMPG/Dex–SF hydrogels cultured in Dex–depleted osteogenic medium. (A) Alkaline phosphatase activity of the encapsulated cells, represented as the concentration of nitrophenol standard normalised to the number of cells. (B) Calcium content deposited in the hydrogels. Data are expressed as mean ± SD. The experiment was repeated three times. *P ≤ 0.05 (one–way analysis of variance followed by Bonferroni post–hoc tests). (C) Appearance of the SaOS–2 cells encapsulated in hydrogels and cultured under different conditions at each time point. The opacity of Groups 2 and 4 hydrogels can be seen, implying high calcium deposition in the samples. (D) Microscopic analysis of alizarin red staining of calcium deposition within the cell–entrapped hydrogels cultured for 7 days. The orange–red stain indicates calcium mineralisation. Scale bars: 50 μm. DMPG: dimyristoyl glycerophosphoglycerol; Dex: dexamethasone; SF: silk fibroin.
| 1. | Murphy, C. M.; O’Brien, F. J.; Little, D. G.; Schindeler, A. Cell-scaffold interactions in the bone tissue engineering triad. Eur Cell Mater. 2013, 26, 120-132. |
| 2. |
Langer, R.; Vacanti, J. P. Tissue engineering. Science. 1993, 260, 920-926.
doi: 10.1126/science.8493529 URL |
| 3. |
Gaharwar, A. K.; Singh, I.; Khademhosseini, A. Engineered biomaterials for in situ tissue regeneration. Nat Rev Mater. 2020, 5, 686-705.
doi: 10.1038/s41578-020-0209-x URL |
| 4. | Murphy, A. R.; Romero, I. S. 8 - Biochemical and biophysical properties of native Bombyx mori silk for tissue engineering applications. In Silk Biomaterials for Tissue Engineering and Regenerative Medicine, Kundu, S. C., ed. Woodhead Publishing: 2014; pp 219-238. |
| 5. |
Kundu, B.; Rajkhowa, R.; Kundu, S. C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv Drug Deliv Rev. 2013, 65, 457-470.
doi: 10.1016/j.addr.2012.09.043 URL |
| 6. |
Bhattacharjee, P.; Kundu, B.; Naskar, D.; Kim, H. W.; Maiti, T. K.; Bhattacharya, D.; Kundu, S. C. Silk scaffolds in bone tissue engineering: An overview. Acta Biomater. 2017, 63, 1-17.
doi: 10.1016/j.actbio.2017.09.027 URL |
| 7. |
Nicodemus, G. D.; Bryant, S. J. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng Part B Rev. 2008, 14, 149-165.
doi: 10.1089/ten.teb.2007.0332 URL |
| 8. |
Matsumoto, A.; Chen, J.; Collette, A. L.; Kim, U. J.; Altman, G. H.; Cebe, P.; Kaplan, D. L. Mechanisms of silk fibroin sol-gel transitions. J Phys Chem B. 2006, 110, 21630-21638.
doi: 10.1021/jp056350v URL |
| 9. |
Wang, X.; Kluge, J. A.; Leisk, G. G.; Kaplan, D. L. Sonication-induced gelation of silk fibroin for cell encapsulation. Biomaterials. 2008, 29, 1054-1064.
doi: 10.1016/j.biomaterials.2007.11.003 URL |
| 10. |
Yucel, T.; Cebe, P.; Kaplan, D. L. Vortex-induced injectable silk fibroin hydrogels. Biophys J. 2009, 97, 2044-2050.
doi: 10.1016/j.bpj.2009.07.028 URL |
| 11. |
Wu, X.; Hou, J.; Li, M.; Wang, J.; Kaplan, D. L.; Lu, S. Sodium dodecyl sulfate-induced rapid gelation of silk fibroin. Acta Biomater. 2012, 8, 2185-2192.
doi: 10.1016/j.actbio.2012.03.007 URL |
| 12. |
Zhang, F.; Li, J.; Zhu, T.; Zhang, S.; Kundu, S. C.; Lu, S. Potential of biocompatible regenerated silk fibroin/sodium N-lauroyl sarcosinate hydrogels. J Biomater Sci Polym Ed. 2015, 26, 780-795.
doi: 10.1080/09205063.2015.1058576 URL |
| 13. | Choi, J.; McGill, M.; Raia, N. R.; Hasturk, O.; Kaplan, D. L. Silk hydrogels crosslinked by the Fenton reaction. Adv Healthc Mater. 2019, 8, e1900644. |
| 14. |
Laomeephol, C.; Ferreira, H.; Yodmuang, S.; Reis, R. L.; Damrongsakkul, S.; Neves, N. M. Exploring the gelation mechanisms and cytocompatibility of gold (III)-mediated regenerated and thiolated silk fibroin hydrogels. Biomolecules. 2020, 10, 466.
doi: 10.3390/biom10030466 URL |
| 15. |
Bhunia, B. K.; Mandal, B. B. Exploring gelation and physicochemical behavior of in situ bioresponsive silk hydrogels for disc degeneration therapy. ACS Biomater Sci Eng. 2019, 5, 870-886.
doi: 10.1021/acsbiomaterials.8b01099 URL |
| 16. |
Laomeephol, C.; Vasuratna, A.; Ratanavaraporn, J.; Kanokpanont, S.; Luckanagul, J. A.; Humenik, M.; Scheibel, T.; Damrongsakkul, S. Impacts of blended bombyx mori silk fibroin and recombinant spider silk fibroin hydrogels on cell growth. Polymers. 2021, 13, 4182.
doi: 10.3390/polym13234182 URL |
| 17. | Cui, X.; Soliman, B. G.; Alcala-Orozco, C. R.; Li, J.; Vis, M. A. M.; Santos, M.; Wise, S. G.; Levato, R.; Malda, J.; Woodfield, T. B. F.; Rnjak-Kovacina, J.; Lim, K. S. Rapid photocrosslinking of silk hydrogels with high cell density and enhanced shape fidelity. Adv Healthc Mater. 2020, 9, e1901667. |
| 18. |
Das, S.; Pati, F.; Choi, Y. J.; Rijal, G.; Shim, J. H.; Kim, S. W.; Ray, A. R.; Cho, D. W.; Ghosh, S. Bioprintable, cell-laden silk fibroin-gelatin hydrogel supporting multilineage differentiation of stem cells for fabrication of three-dimensional tissue constructs. Acta Biomater. 2015, 11, 233-246.
doi: 10.1016/j.actbio.2014.09.023 URL |
| 19. |
Partlow, B. P.; Hanna, C. W.; Rnjak-Kovacina, J.; Moreau, J. E.; Applegate, M. B.; Burke, K. A.; Marelli, B.; Mitropoulos, A. N.; Omenetto, F. G.; Kaplan, D. L. Highly tunable elastomeric silk biomaterials. Adv Funct Mater. 2014, 24, 4615-4624.
doi: 10.1002/adfm.201400526 URL |
| 20. |
Laomeephol, C.; Guedes, M.; Ferreira, H.; Reis, R. L.; Kanokpanont, S.; Damrongsakkul, S.; Neves, N. M. Phospholipid-induced silk fibroin hydrogels and their potential as cell carriers for tissue regeneration. J Tissue Eng Regen Med. 2020, 14, 160-172.
doi: 10.1002/term.2982 URL |
| 21. |
Laomeephol, C.; Ferreira, H.; Kanokpanont, S.; Neves, N. M.; Kobayashi, H.; Damrongsakkul, S. Dual-functional liposomes for curcumin delivery and accelerating silk fibroin hydrogel formation. Int J Pharm. 2020, 589, 119844.
doi: 10.1016/j.ijpharm.2020.119844 URL |
| 22. |
Langenbach, F.; Handschel, J. Effects of dexamethasone, ascorbic acid and β-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Res Ther. 2013, 4, 117.
doi: 10.1186/scrt328 URL |
| 23. |
Madamsetty, V. S.; Mohammadinejad, R.; Uzieliene, I.; Nabavi, N.; Dehshahri, A.; García-Couce, J.; Tavakol, S.; Moghassemi, S.; Dadashzadeh, A.; Makvandi, P.; Pardakhty, A.; Aghaei Afshar, A.; Seyfoddin, A. Dexamethasone: insights into pharmacological aspects, therapeutic mechanisms, and delivery systems. ACS Biomater Sci Eng. 2022, 8, 1763-1790.
doi: 10.1021/acsbiomaterials.2c00026 URL |
| 24. |
Rockwood, D. N.; Preda, R. C.; Yücel, T.; Wang, X.; Lovett, M. L.; Kaplan, D. L. Materials fabrication from Bombyx mori silk fibroin. Nat Protoc. 2011, 6, 1612-1631.
doi: 10.1038/nprot.2011.379 URL |
| 25. |
Monteiro, N.; Martins, A.; Ribeiro, D.; Faria, S.; Fonseca, N. A.; Moreira, J. N.; Reis, R. L.; Neves, N. M. On the use of dexamethasone-loaded liposomes to induce the osteogenic differentiation of human mesenchymal stem cells. J Tissue Eng Regen Med. 2015, 9, 1056-1066.
doi: 10.1002/term.1817 URL |
| 26. | Carvalho, A. F.; Gasperini, L.; Ribeiro, R. S.; Marques, A. P.; Reis, R. L. Control of osmotic pressure to improve cell viability in cell-laden tissue engineering constructs. J Tissue Eng Regen Med. 2018, 12, e1063-e1067. |
| 27. |
Ding, X.; Yang, G.; Zhang, W.; Li, G.; Lin, S.; Kaplan, D. L.; Jiang, X. Increased stem cells delivered using a silk gel/scaffold complex for enhanced bone regeneration. Sci Rep. 2017, 7, 2175.
doi: 10.1038/s41598-017-02053-z URL |
| 28. |
Labarca, C.; Paigen, K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980, 102, 344-352.
doi: 10.1016/0003-2697(80)90165-7 URL |
| 29. |
Connerty, H. V.; Briggs, A. R. Determination of serum calcium by means of orthocresolphthalein complexone. Am J Clin Pathol. 1966, 45, 290-296.
doi: 10.1093/ajcp/45.3.290 URL |
| 30. | Bucciarelli, A.; Motta, A. Use of Bombyx mori silk fibroin in tissue engineering: From cocoons to medical devices, challenges, and future perspectives. Biomater Adv. 2022, 139, 212982. |
| 31. |
Melke, J.; Midha, S.; Ghosh, S.; Ito, K.; Hofmann, S. Silk fibroin as biomaterial for bone tissue engineering. Acta Biomater. 2016, 31, 1-16.
doi: 10.1016/j.actbio.2015.09.005 URL |
| 32. | Leong, M. F.; Chian, K. S.; Mhaisalkar, P. S.; Ong, W. F.; Ratner, B. D. Effect of electrospun poly(D,L-lactide) fibrous scaffold with nanoporous surface on attachment of porcine esophageal epithelial cells and protein adsorption. J Biomed Mater Res A. 2009, 89, 1040-1048. |
| 33. | Anderson, H. C.; Hsu, H. H.; Raval, P.; Hunt, T. R.; Schwappach, J. R.; Morris, D. C.; Schneider, D. J. The mechanism of bone induction and bone healing by human osteosarcoma cell extracts. Clin Orthop Relat Res. 1995, 129-134. |
| 34. |
Acharya, C.; Ghosh, S. K.; Kundu, S. C. Silk fibroin protein from mulberry and non-mulberry silkworms: cytotoxicity, biocompatibility and kinetics of L929 murine fibroblast adhesion. J Mater Sci Mater Med. 2008, 19, 2827-2836.
doi: 10.1007/s10856-008-3408-3 URL |
| 35. |
Madden, P. W.; Lai, J. N.; George, K. A.; Giovenco, T.; Harkin, D. G.; Chirila, T. V. Human corneal endothelial cell growth on a silk fibroin membrane. Biomaterials. 2011, 32, 4076-4084.
doi: 10.1016/j.biomaterials.2010.12.034 URL |
| 36. |
Panchamanee, J.; Laomeephol, C.; Luckanagul, J. A.; Wang, Q.; Damrongsakkul, S. In vitro biological activities of the flexible and virus nanoparticle-decorated silk fibroin-based films. Int J Biol Macromol. 2022, 216, 437-445.
doi: 10.1016/j.ijbiomac.2022.07.011 URL |
| 37. |
Long, M. W. Osteogenesis and bone-marrow-derived cells. Blood Cells Mol Dis. 2001, 27, 677-690.
doi: 10.1006/bcmd.2001.0431 URL |
| [1] | Ricardo Donate, Maryam Tamaddon, Viviana Ribeiro, Mario Monzón, J. Miguel Oliveira, Chaozong Liu. Translation through collaboration: practice applied in BAMOS project in in vivo testing of innovative osteochondral scaffolds [J]. Biomaterials Translational, 2022, 3(2): 102-104. |
| [2] | Melika Sahranavard, Soulmaz Sarkari, SeyedehMina Safavi, Farnaz Ghorbani. Three-dimensional bio-printing of decellularized extracellular matrix-based bio-inks for cartilage regeneration: a systematic review [J]. Biomaterials Translational, 2022, 3(2): 105-115. |
| [3] | Emma Steijvers, Armaan Ghei, Zhidao Xia. Manufacturing artificial bone allografts: a perspective [J]. Biomaterials Translational, 2022, 3(1): 65-80. |
| [4] | Ke Hu, Yuxuan Li, Zunxiang Ke, Hongjun Yang, Chanjun Lu, Yiqing Li, Yi Guo, Weici Wang. History, progress and future challenges of artificial blood vessels: a narrative review [J]. Biomaterials Translational, 2022, 3(1): 81-98. |
| [5] | Yizhong Peng, Jinye Li, Hui Lin, Shuo Tian, Sheng Liu, Feifei Pu, Lei Zhao, Kaige Ma, Xiangcheng Qing, Zengwu Shao. Endogenous repair theory enriches construction strategies for orthopaedic biomaterials: a narrative review [J]. Biomaterials Translational, 2021, 2(4): 343-360. |
| [6] | Xirui Jing, Qiuyue Ding, Qinxue Wu, Weijie Su, Keda Yu, Yanlin Su, Bing Ye, Qing Gao, Tingfang Sun, Xiaodong Guo. Magnesium-based materials in orthopaedics: material properties and animal models [J]. Biomaterials Translational, 2021, 2(3): 197-213. |
| [7] | Yiqing Wang, Xiangyu Chu, Bing Wang. Recombinant adeno-associated virus-based gene therapy combined with tissue engineering for musculoskeletal regenerative medicine [J]. Biomaterials Translational, 2021, 2(1): 19-29. |
| [8] | Maryam Tamaddon, Helena Gilja, Ling Wang, J. Miguel Oliveira, Xiaodan Sun, Rongwei Tan, Chaozong Liu. Osteochondral scaffolds for early treatment of cartilage defects in osteoarthritic joints: from bench to clinic [J]. Biomaterials Translational, 2020, 1(1): 3-17. |
| [9] | Xing Yang, Yuanyuan Li, Xujie Liu, Wei He, Qianli Huang, Qingling Feng. Nanoparticles and their effects on differentiation of mesenchymal stem cells [J]. Biomaterials Translational, 2020, 1(1): 58-68. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||